User login
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
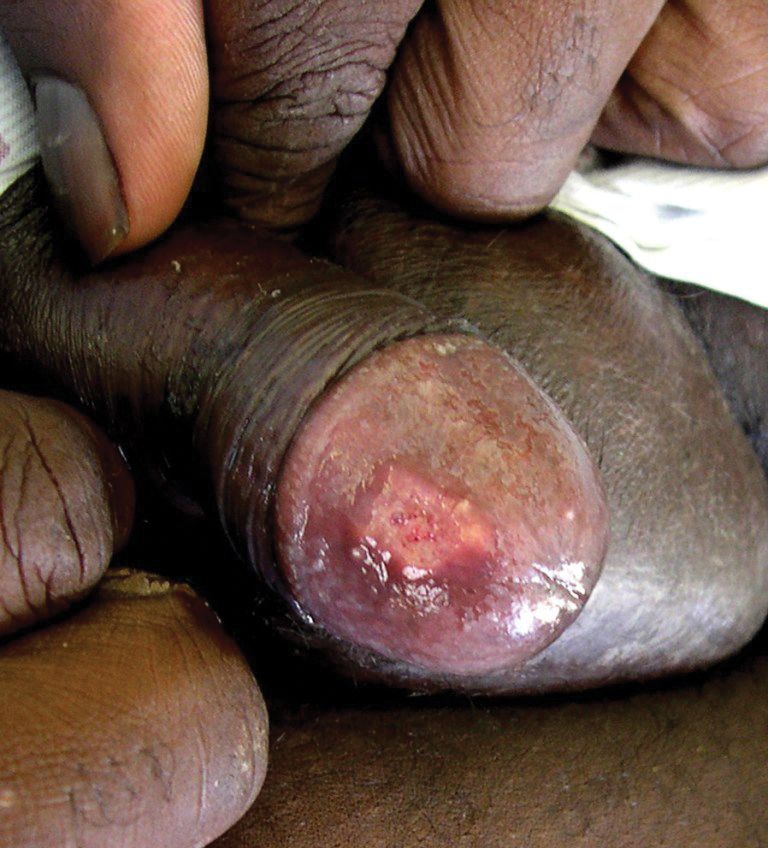
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
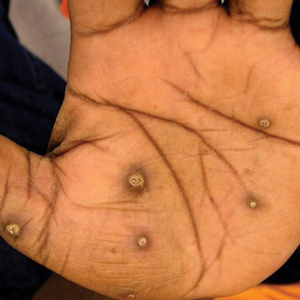
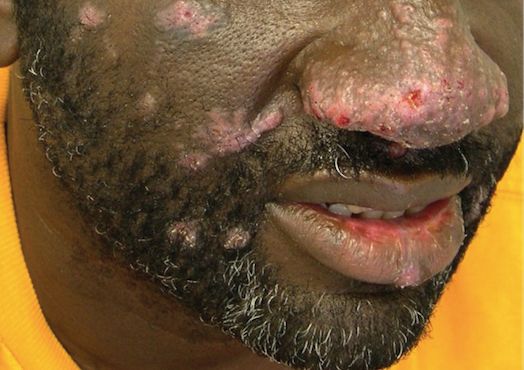
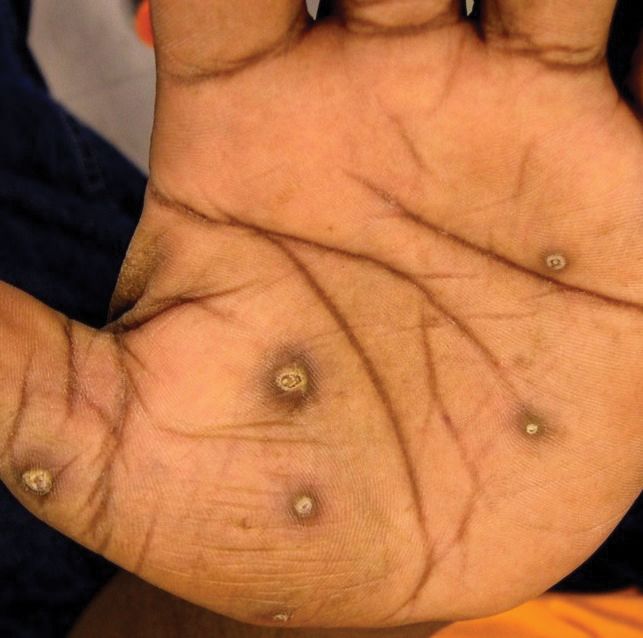
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).
A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
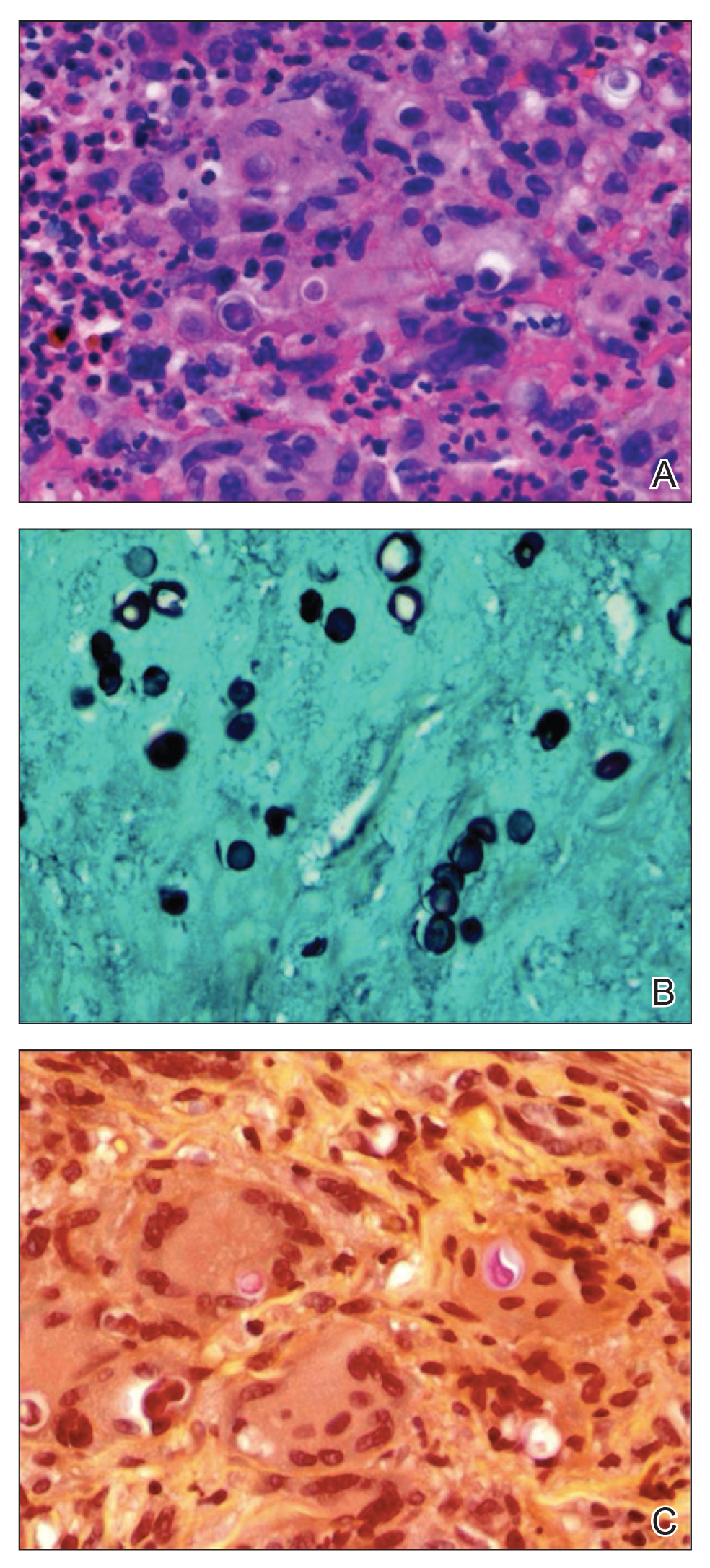
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.
The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).



A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.

The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
Blastomycosis is a systemic fungal infection that is endemic in the South Central, Midwest, and southeastern regions of the United States, as well as in provinces of Canada bordering the Great Lakes. After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period. The initial response at the infected site is suppurative, which progresses to granuloma formation. Blastomyces dermatitidis most commonly infects the lungs, followed by the skin, bones, prostate, and central nervous system (CNS). Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent.
We present the case of a 38-year-old man with a medical history of human immunodeficiency virus (HIV) infection and AIDS who reported a 3- to 4-week history of respiratory and cutaneous symptoms. Initial clinical impression favored secondary syphilis; however, after laboratory evaluation and lack of response to treatment for syphilis, further investigation revealed a diagnosis of widespread cutaneous North American blastomycosis.
Case Report
A 38-year-old man with a medical history of HIV infection and AIDS presented to the emergency department at a medical center in Minneapolis, Minnesota, with a cough; chest discomfort; and concomitant nonpainful, mildly pruritic papules and plaques of 3 to 4 weeks’ duration that initially appeared on the face and ears and spread to the trunk, arms, palms, legs, and feet. He had a nonpainful ulcer on the glans penis. Symptoms began while he was living in Atlanta, Georgia, before relocating to Minneapolis. A chest radiograph was negative.
The initial clinical impression favored secondary syphilis. Intramuscular penicillin G benzathine (2.4 million U) weekly for 3 weeks was initiated by the primary care team based on clinical suspicion alone without laboratory evidence of a positive rapid plasma reagin or VDRL test. Because laboratory evaluation and lack of response to treatment did not support syphilis, dermatology consultation was requested.
The patient had a history of crack cocaine abuse. He reported sexual activity with a single female partner while living in a halfway house in the Minneapolis–St. Paul area. Physical examination showed an age-appropriate man in no acute distress who was alert and oriented. He had well-demarcated papules and plaques on the forehead, ears, nose, cutaneous and mucosal lips, chest, back, arms, legs, palms, and soles. Many of the facial papules were pink, nonscaly, and concentrated around the nose and mouth; some were umbilicated (Figure 1). Trunk and extensor papules and plaques were well demarcated, oval, and scaly; some had erosions centrally and were excoriated. Palmar papules were round and had peripheral brown hyperpigmentation and central scale (Figure 2). A 1-cm, shallow, nontender, oval ulceration withraised borders was located on the glans penis under the foreskin (Figure 3).



A rapid plasma reagin test was nonreactive; a fluorescent treponemal antibody absorption test was negative. Chest radiograph, magnetic resonance imaging, and electroencephalogram were normal. In addition, spinal fluid drawn from a tap was negative on India ink and Gram stain preparations and was negative for cryptococcal antigen. In addition, spinal fluid was negative for fungal and bacterial growth, as were blood cultures.
Abnormal tests included a positive enzyme-linked immunosorbent assay and Western blot test for HIV, with an absolute CD4 count of 6 cells/mL and a viral load more than 100,000 copies/mL. Urine histoplasmosis antigen was markedly elevated. A potassium hydroxide preparation was performed on the skin of the right forearm, revealing broad-based budding yeast, later confirmed on skin and sputum cultures to be B dermatitidis.
Punch biopsy from the upper back revealed a mixed acute and granulomatous infiltrate with numerous yeast forms (Figure 4A) that were highlighted by Grocott-Gomori methenamine-silver (Figure 4B) and periodic acid–Schiff (Figure 4C) stains.

The patient was treated with intravenous amphotericin with improvement in skin lesions. A healing ointment and occlusive dressing were used on eroded skin lesions. The patient was discharged on oral itraconazole 200 mg twice daily for 6 months (for blastomycosis); oral sulfamethoxazole-trimethoprim 15 mg/kg/d every 8 hours for 21 days (for Pneumocystis carinii pneumonia prophylaxis); oral azithromycin 500 mg daily (for Mycobacterium avium-intracellulare prophylaxis); oral levetiracetam 500 mg every 12 hours (as an antiseizure agent); albuterol 90 µg per actuation; and healing ointment. He continues his chemical dependency program and is being followed by the neurology seizure clinic as well as the outpatient HIV infectious disease clinic for planned reinitiation of highly active antiretroviral therapy.
Comment
Diagnosis
Our patient had an interesting and dramatic presentation of widespread cutaneous North American blastomycosis that was initially considered to be secondary syphilis because of involvement of the palms and soles and the presence of the painless penile ulcer. In addition, the initial skin biopsy finding was considered morphologically consistent with Cryptococcus neoformans based on positive Grocott-Gomori methenamine-silver and periodic acid–Schiff stains and an equivocal mucicarmine stain. However, the potassium hydroxide preparation of skin and positive urine histoplasmosis antigen strongly suggested blastomycosis, which was confirmed by culture of B dermatitidis. The urine histoplasmosis antigen can cross-react with B dermatitidis and other mycoses (eg, Paracoccidioides brasiliensis and Penicillium marneffei); however, because the treatment of either of these mycoses is similar, the value of the test remains high.1
Skin tests and serologic markers are useful epidemiologic tools but are of inadequate sensitivity and specificity to be diagnostic for B dermatitidis. Diagnosis depends on direct examination of tissue or isolation of the fungus in culture.2
Source of Infection
The probable occult source of cutaneous infection was the lungs, given the natural history of disseminated blastomycosis; the history of cough and chest discomfort; the widespread nature of skin lesions; and the ultimate growth of rare yeast forms in sputum. Cutaneous infection generally is from disseminated disease and rarely from direct inoculation.
Unlike many other systemic dimorphic mycoses, blastomycosis usually occurs in healthy hosts and is frequently associated with point-source outbreak. Immunosuppressed patients typically develop infection following exposure to the organism, but reactivation also can occur. Blastomycosis is uncommon among HIV-infected individuals and is not recognized as an AIDS-defining illness.
In a review from Canada of 133 patients with blastomycosis, nearly half had an underlying medical condition but not one typically associated with marked immunosuppression.3 Only 2 of 133 patients had HIV infection. Overall mortality was 6.3%, and the average duration of symptoms before diagnosis was less in those who died vs those who survived the disease.3 In the setting of AIDS or other marked immunosuppression, disease usually is more severe, with multiple-system involvement, including the CNS, and can progress rapidly to death.2
Treatment
Therapy for blastomycosis is determined by the severity of the clinical presentation and consideration of the toxicities of the antifungal agent. There are no randomized, blinded trials comparing antifungal agents, and data on the treatment of blastomycosis in patients infected with HIV are limited. Amphotericin B 3 mg/kg every 24 hours is recommended in life-threatening systemic disease and CNS disease as well as in patients with immune suppression, including AIDS.4 In a retrospective study of 326 patients with blastomycosis, those receiving amphotericin B had a cure rate of 86.5% with a relapse rate of 3.9%; patients receiving ketoconazole had a cure rate of 81.7% with a relapse rate of 14%.4 Although data are limited, chronic suppressive therapy generally is recommended in patients with HIV who have been treated for blastomycosis. Fluconazole, itraconazole, and ketoconazole are all used as chronic suppressive therapy; however, given the higher relapse rate observed with ketoconazole, itraconazole is preferred. Because neither ketoconazole nor itraconazole penetrates the blood-brain barrier, these drugs are not recommended in cases of CNS involvement. Patients with CNS disease or intolerance to itraconazole should be treated with fluconazole for chronic suppression.3
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
- Wheat J, Wheat H, Connolly P, et al. Cross-reactivity in Histoplasma capsulatum variety capsulatum antigen assays of urine samples from patients with endemic mycoses. Clin Infect Dis. 1997;24:1169-1171.
- Pappas PG, Pottage JC, Powderly WG, et al. Blastomycosis in patients with the acquired immunodeficiency syndrome. Ann Intern Med. 1992;116:847-853.
- Crampton TL, Light RB, Berg GM, et al. Epidemiology and clinical spectrum of blastomycosis diagnosed at Manitoba hospitals. Clin Infect Dis. 2002;34:1310-1316. Cited by: Aberg JA. Blastomycosis and HIV. HIV In Site Knowledge Base Chapter. http://hivinsite.ucsf.edu/InSite?page=kb-05-02-09#SIX. Published April 2003. Updated January 2006. Accessed December 16, 2019.
- Chapman SW, Bradsher RW Jr, Campbell GD Jr, et al. Practice guidelines for the management of patients with blastomycosis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:679-683.
Practice Points
- Blastomycosis generally produces a pulmonary form of the disease and, to a lesser extent, extrapulmonary forms, such as cutaneous, osteoarticular, and genitourinary.
- Blastomycosis can be diagnosed by culture, direct visualization of the yeast in affected tissue, antigen testing, or a combination of these methods.
- After inhalation of Blastomyces dermatitidis spores, which are taken up by bronchopulmonary macrophages, there is an approximate 30- to 45-day incubation period.