User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
The Impact of Primary Tumor Site on Survival in Mycosis Fungoides
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
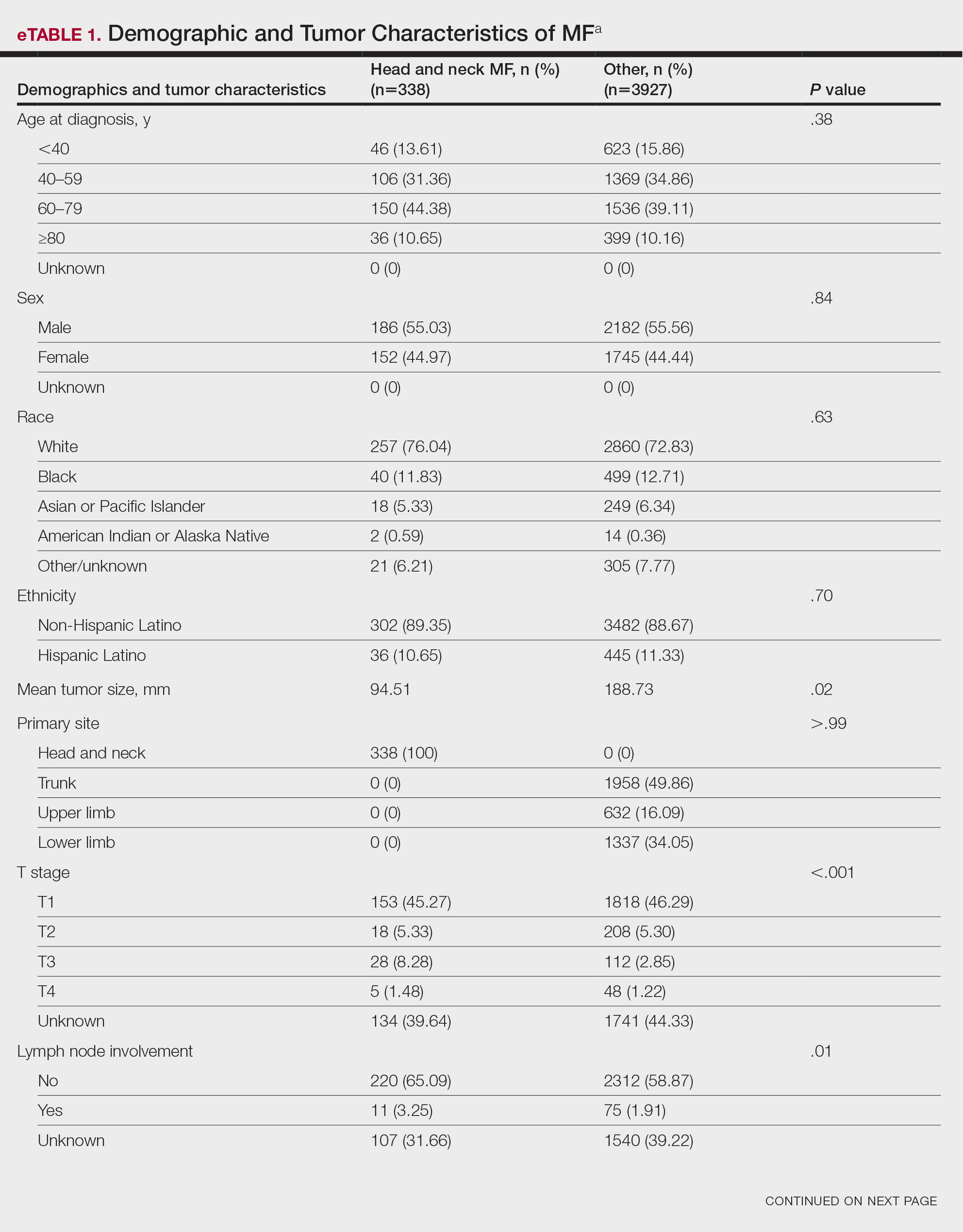
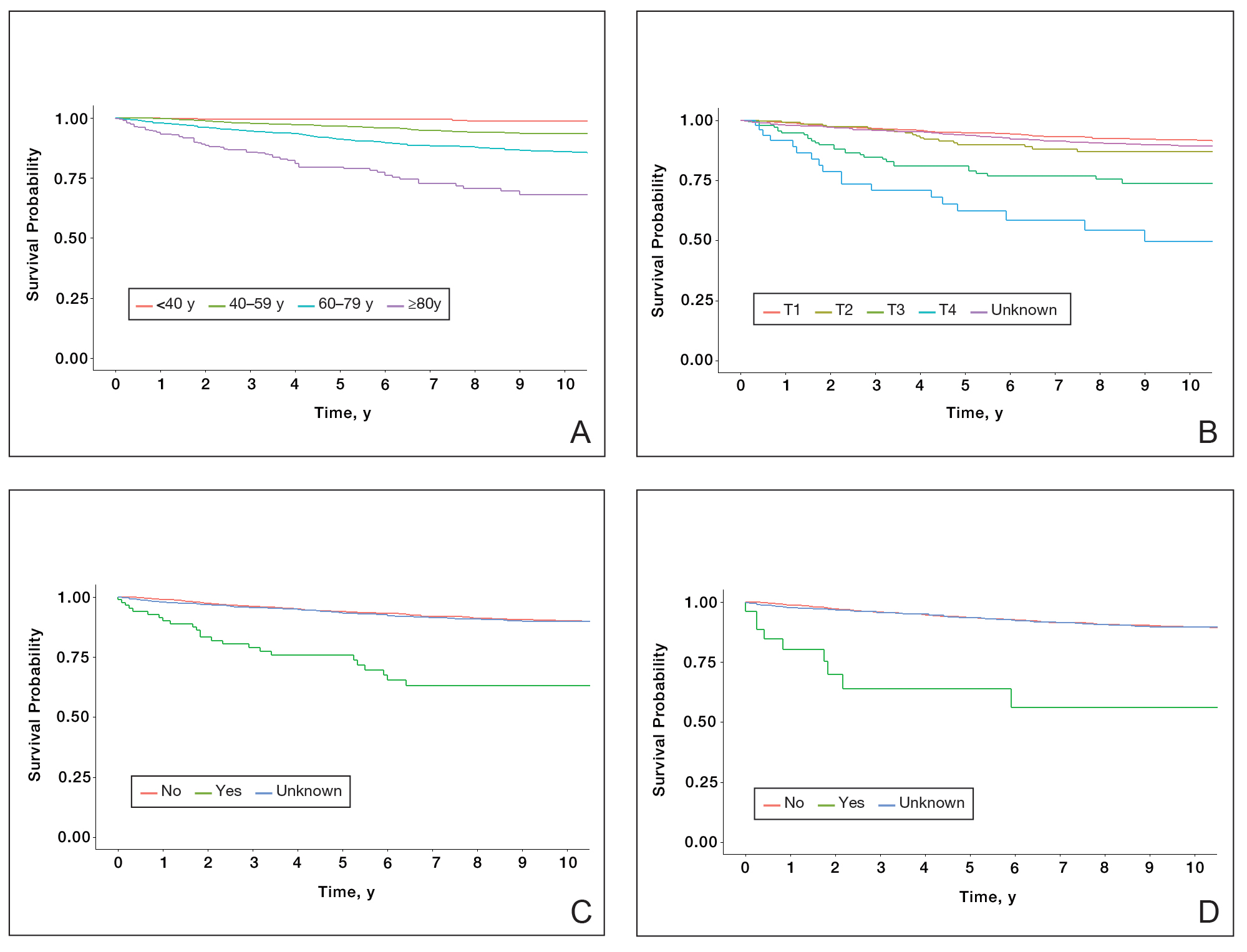
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.
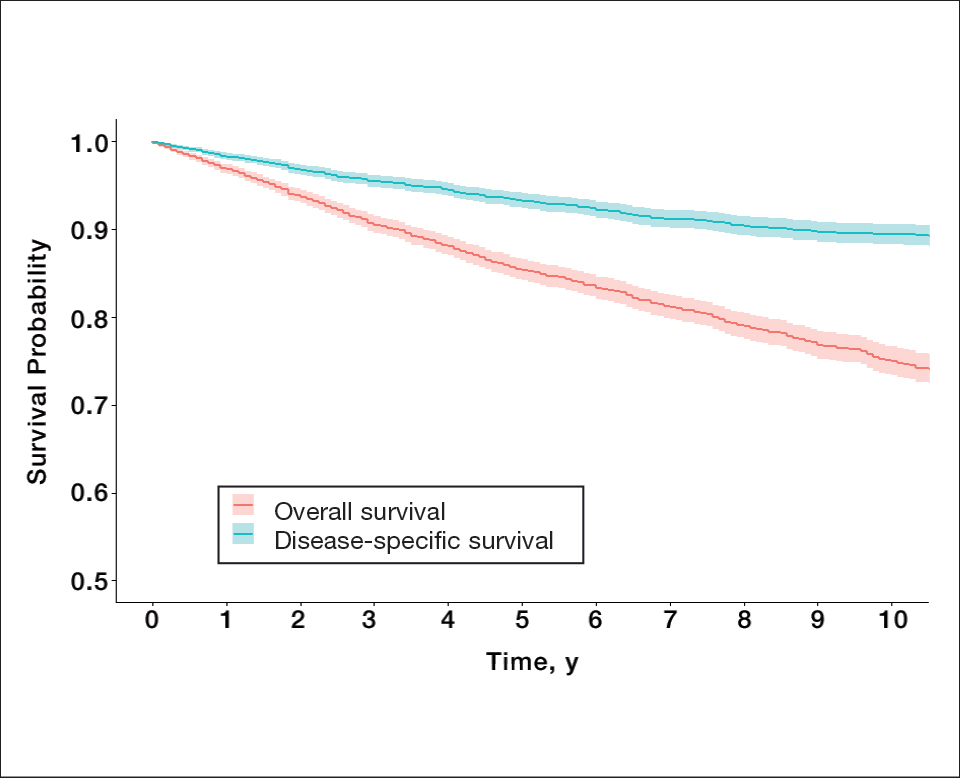
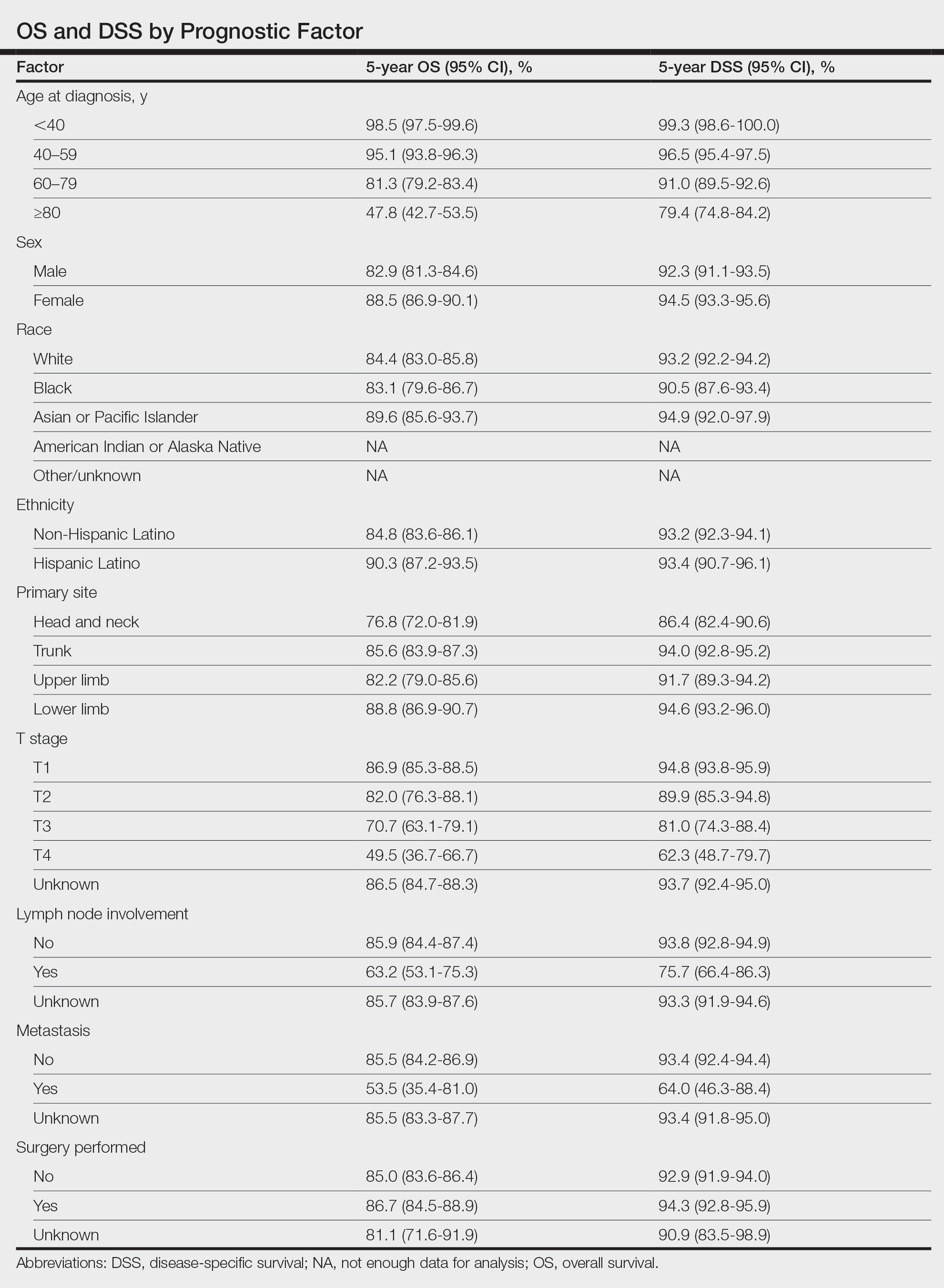
Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).
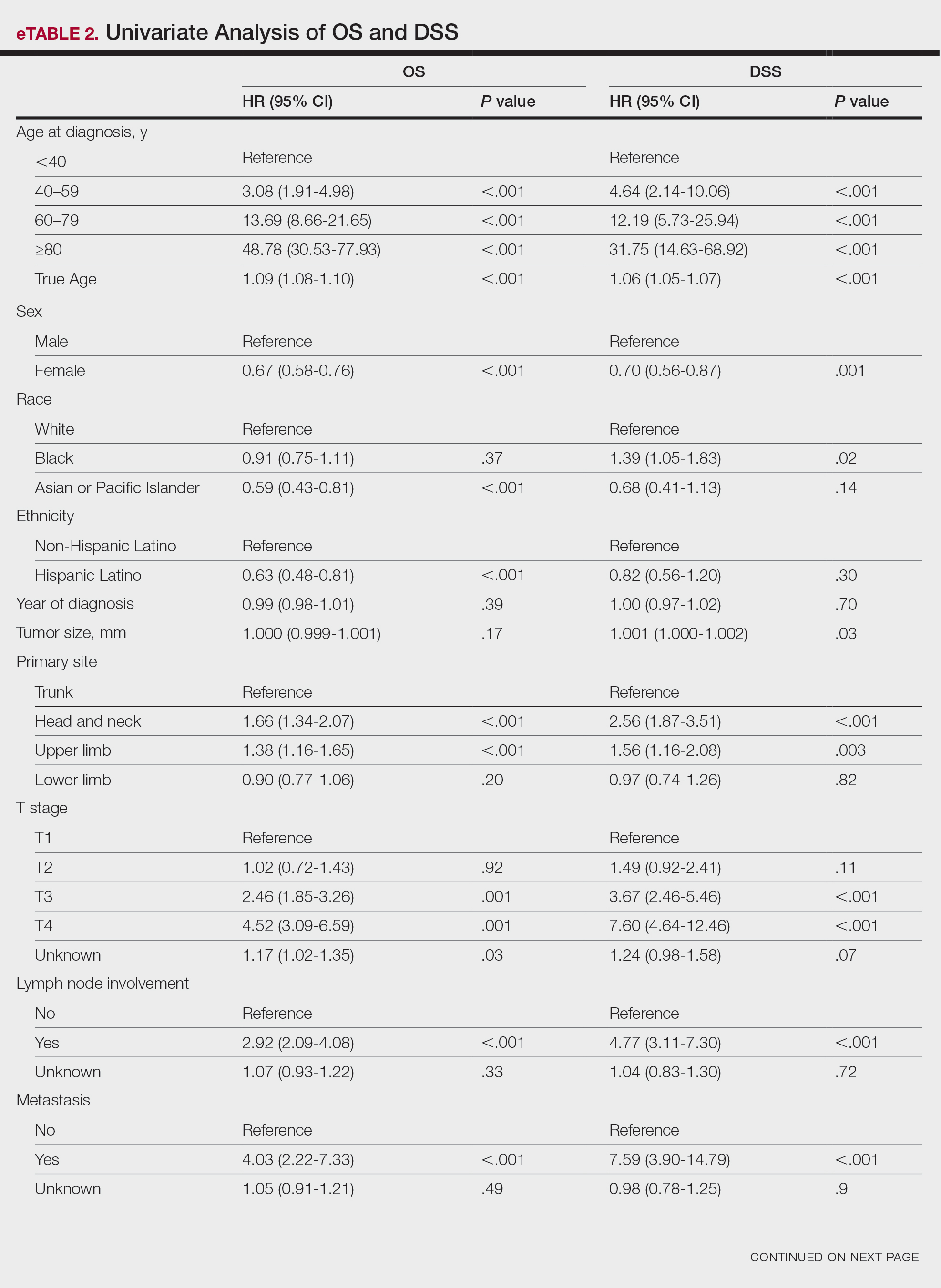
For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).
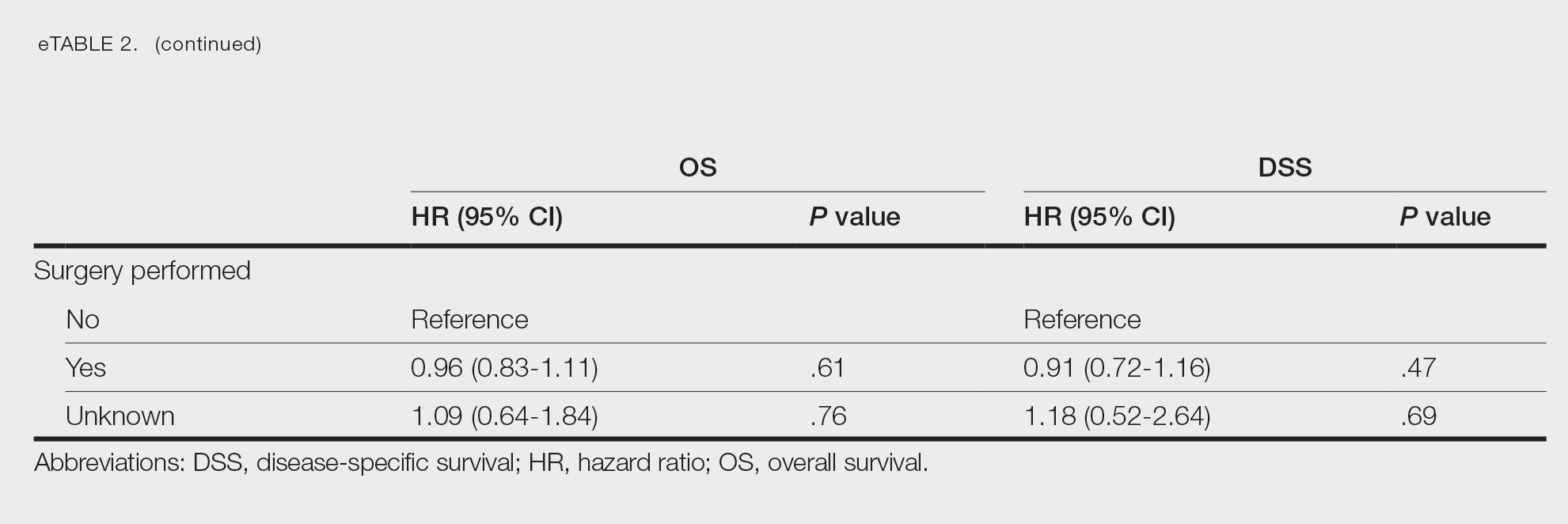
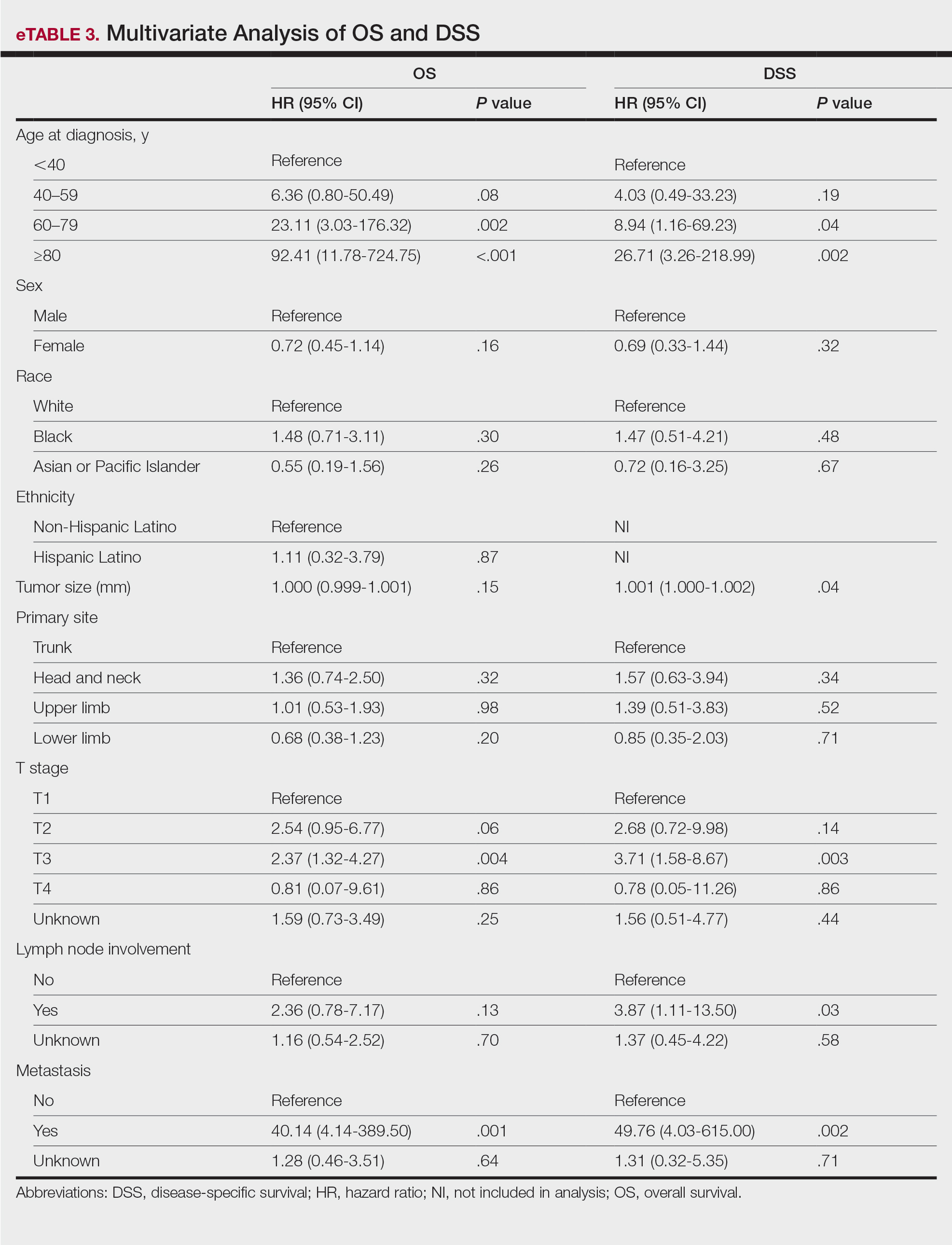
Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).
Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
Mycosis fungoides (MF), the most common cutaneous T-cell lymphoma (CTCL), is characterized by clonal proliferation of predominantly CD4+ T cells with localization to the skin.1 Mycosis fungoides typically affects older adults with a male to female ratio of 2:1 but also can occur in children and younger adults.2,3 Known as the great imitator, the manifestations of MF can be variable with considerable clinical and pathologic overlap with benign inflammatory skin diseases, rendering definitive diagnosis challenging.4-7 The early stages of classic MF manifest as pruritic erythematous patches and plaques with variable scaling that can progress in later stages to ulceration and tumors.8 Histopathologically, classic MF is characterized by epidermotropic proliferation of small- to intermediate-sized pleomorphic lymphocytes with cerebriform nuclei and a haloed appearance; intraepidermal nests of atypical lymphocytes known as Pautrier microabscesses occasionally are observed.5 Mycosis fungoides typically follows an indolent clinical course, with advanced-stage MF portending a poor prognosis.9,10 Current treatment is focused on halting disease progression, with topical therapies, phototherapy, and radiation therapy as the standard therapies for early-stage MF.11-13 For advanced-stage MF, treatment may include systemic therapies such as interferon alfa and oral retinoids along with chemotherapies for more refractive cases.14 Allogenic hematopoietic cell transplantation is the only curative treatment.11
Current staging guidelines for MF do not address anatomic location as there is little known about its impact on patient outcomes.11,15 Due to the indolent nature of MF leading to diagnostic challenges, the exact frequency of each primary disease site for MF also remains unclear, though the suggested incidence of MF of the head and neck ranges from 30% to 70%.16,17 Involvement of the head and neck16,18 or external ear and external auditory canal19 is associated with worse prognosis. The purpose of this study was to examine the impact of anatomic location of primary disease site on survival in MF.
Methods
The National Cancer Institute’s Surveillance, Epidemiology, and End Results (SEER) database includes patient records from 18 registries and encompasses approximately 48% of the US population.20 Using SEER*STAT software (version 8.4.0.1), we conducted a search of patients diagnosed with MF (International Classification of Diseases for Oncology, Third Edition [ICD-O-3] histologic code 9700/3 [mycosis fungoides]) between 2000 and 2019. For inclusion in the study, patients were required to have a known age, specified primary site, and a known cause of death (if applicable). Patients with known Sézary syndrome (SS)—an aggressive form of CTCL that is characterized by the presence of clonally related neoplastic T cells in the skin, lymph nodes, and peripheral blood—were not included because the World Health Organization/European Organisation for Research and Treatment of Cancer considers SS and MF to be separate entities1,15; SS does not necessarily arise from preexisting MF and is associated with markedly poorer survival. This study was exempt from institutional review board approval because the data were publicly available and anonymized.
Data Collection—For age at diagnosis, patients were divided into the following categories: younger than 40 years, 40 to 59 years, 60 to 79 years, and 80 years and older. Demographics, tumor characteristics, and surgical management (if applicable) were obtained for each patient. The designations of chemotherapy and radiation treatment in the SEER database are not reliable and prone to false negatives. As such, these were excluded from analysis.
The primary outcomes of interest were overall survival (OS) and disease-specific survival (DSS), which were calculated as time from MF diagnosis to death. Although OS included all patients who died of any cause, DSS only included patients who died of MF.
Statistical Analysis—Demographics (age, sex, race, ethnicity), tumor characteristics (tumor size, primary site, T stage, lymph node involvement, metastasis), and surgical management (if applicable) were summarized. Overall survival and DSS were calculated using Kaplan-Meier analysis. Univariate and multivariable Cox proportional hazards regression models were generated to determine which prognostic factors for MF were associated with poorer OS and DSS. Only statistically significant variables in the univariate analysis were used to construct the multivariable analysis. Hazard ratios (HRs) and their associated 95% CIs were reported. Incidence rates were calculated and age adjusted to the 2000 US standard population. The SEER JoinPoint Regression program was used to determine the annual percent change (APC)—change in incidence rate over time. P<.05 was considered statistically significant. All statistical analyses were conducted with R version 4.0.2.
Results
Patient Demographics and Tumor Characteristics—There were 4265 patients diagnosed with MF from 2000 to 2019. The overall incidence of MF was 2.55 per million (95% CI, 2.48-2.63) when age adjusted to the 2000 US standard population, which increased with time (mean APC, 0.97% per year; P=.01). The mean age at diagnosis was 56.4 years with a male to female ratio of 1.2:1. Males (3.07 per million; 95% CI, 2.94-3.20) had a higher incidence of MF than females (2.16 per million; 95% CI, 2.06-2.26), with incidence in females increasing over time (mean APC, 1.52% per year; P=.02) while incidence in males remained stable (mean APC, 1.09%; P=.37). Patients predominantly self-identified as White (73.08%). Patients with MF of the head and neck were more likely to have smaller tumors (P=.02), a more advanced T stage (P<.001), and lymph node involvement (P=.01) at the time of diagnosis. Additional demographics and tumor characteristics are summarized in eTable 1.


Survival Outcomes—The mean follow-up time was 86.9 months. The 5- and 10-year OS rates were 85.4% (95% CI, 84.2%-86.6%) and 75.0% (95% CI, 73.4%-76.7%), respectively (Figure 1)(Table). The 5- and 10-year DSS rates were 93.3% (95% CI, 92.4%-94.1%) and 89.5% (95% CI, 88.3%-90.6%), respectively. For OS, univariate analysis indicated that significant prognostic factors included increasing age (P<.001), female sex (P<.001), self-identifying as Asian or Pacific Islander (P<.001), self-identifying as Hispanic Latino (P<.001), primary tumor sites of either the head and neck or upper limb (P<.001), T3 or T4 staging (P=.001), lymph node involvement at the time of diagnosis (P<.001), and metastasis (P<.001).


For DSS, univariate analysis had similar risk factors with self-identifying as Black being an additional risk factor (P=.02), though self-identifying as Asian/Pacific Islander or Hispanic Latino were not significant nor was location on the lower limb. For recorded tumor size, the HR increased by 1.001 per each 1-mm increase in size (eTable 2).


Multivariate analysis showed age at diagnosis (60–79 years: HR, 23.11 [95% CI, 3.03-176.32]; P=.002; ≥80 years: HR, 92.41 [95% CI, 11.78-724.75]; P<.001), T3 staging (HR, 2.37 [95% CI, 1.32-4.27]; P=.004), and metastasis (HR, 40.14 [95% CI, 4.14-389.50]; P=.001) significantly influenced OS. For DSS, multivariate analysis indicated the significant prognostic factors were age at diagnosis (60–79 years: HR, 8.94 [95% CI, 1.16-69.23]; P=.04];≥80 years: HR, 26.71; [95% CI, 3.26-218.99]; P=.002), tumor size (HR, 1.001 [95% CI, 1.000-1.002]; P=.04), T3 staging (HR, 3.71 [95% CI, 1.58-8.67]; P=.003), lymph node involvement (HR, 3.87 [95% CI, 1.11-13.50]; P=.03) and metastasis (HR, 49.76 [95% CI, 4.03-615.00]; P=.002)(Figure 2). When controlling for the aforementioned factors, the primary disease site was not significant (eTable 3).


Comment
Although the prognostic significance of primary disease sites on various types of CTCLs has been examined, limited research exists on MF due to its rarity. For the 4265 patients with MF included in our study, statistically significant prognostic factors on multivariate analysis for DSS included age at diagnosis, tumor size, T staging, lymph node involvement, and presence of metastasis. For OS, only age at diagnosis, T staging, and presence of metastasis were statistically significant predictors. Although initially statistically significant on univariate analysis for both OS and DSS, tumor location was not significant when controlling for confounders.
Our population-based analysis found that 5- and 10-year OS for patients with head and neck MF were 85.4% and 75.0%, respectively, and 5- and 10-year DSS were 93.3% and 89.5%, respectively. Our 10-year OS survival rate of 75.0% was slightly worse than the 81.6% reported by Jung et al16 in a study of 39 cases of MF of the head and neck from the Asan Medical Center database. The difference in survival rate may not only be due to differences in sample size but also because the Asan Medical Center database had a higher proportion of Asian patients as a Korean registry. In our univariate analysis, Asian/Pacific Islander race was shown to be a statistically significant predictor of worse prognosis for OS (P<.001). When comparing survival in patients with head and neck MF vs all primary tumor sites, our OS rate for head and neck MF was more favorable than the 5-year OS of 75% reported by Agar et al21 in their analysis of 1502 patients with MF of all locations, though their cohort also included patients with SS, which is known to have a poorer prognosis. Additionally, our 10-year OS rate of 75.0% for patients with MF with a primary tumor site of the head and neck was slightly less favorable than the 81.0% reported by a prior analysis of the SEER database for MF of all locations,22 which initially may be suggestive of worse outcomes associated with MF originating from the head and neck.
Although MF originating in the head and neck region was found to be a statistically significant prognostic factor under univariate analysis (P<.001), tumor location was not significant upon adjusting for confounders in the multivariate analysis. These results are consistent with those reported in a multivariable analysis conducted by Jung et al,16 which compared 39 cases of head and neck MF to 85 cases without head and neck involvement. The investigators found that the head and neck as the primary site was a significant prognostic factor associated with worsened rates of OS when patients had stages IA to IIA (P=.009) and T2 stage tumors (P=.012) but not in either T1 stage or advanced stage IIB to IVB tumors.16 In contrast, a study by Su et al18 evaluating patients with MF from the National Cancer Database found that patients with MF originating in the head and neck region had similar survival compared with MF originating in the lower limbs after pairwise propensity matching. It previously has been postulated that primary MF lesions originating in the head and neck region have relatively higher frequencies of biological markers believed to be associated with more aggressive tumor behavior and poorer prognosis, such as histopathologic folliculotropism, T-cell receptor gene rearrangements, and large-cell transformations.16 However, MF typically is an indolent disease with advanced-stage MF following an aggressive disease course that often is refractory to treatment. A review from a single academic center noted that 5-year DSS was 97.3% for T1a but only 37.5% for T4.23 Similarly, a meta-analysis evaluating survival in patients with MF noted the 5-year OS for stage IB was 85.8% while for stage IVB it was only 23.3%.24 As such, having advanced-stage MF influences survival to a far greater extent than the presence of head and neck involvement alone. Accordingly, the significantly higher prevalence of advanced T stage disease and increased likelihood of lymph node involvement in MF lesions originating in the head and neck region (both P<.001) may explain why previous studies noted a poorer survival rate with head and neck involvement, as they did not have the sample size to adjust for these factors. Controlling for the above factors likely explains the nonsignificance of this region as a prognostic indicator in our multivariate analysis of OS and DSS.
Similar to MF originating in the head and neck region, the upper limb as a primary tumor site initially was found to be a significant predictor of both OS and DSS on univariate analysis but not on multivariate analysis. By contrast, Su et al18 found survival outcomes were worse for patients diagnosed with MF with the upper limb as the primary tumor site compared with the lower limb on multivariate Cox proportional hazards analysis but not on pairwise propensity score matching. The difference in our results compared with Su et al18 may be because the National Cancer Database only reports OS, while DSS may be more useful in determining prognostic factors associated with poorer survival, especially in an older patient population with greater comorbidities. Furthermore, the nonsignificance of the upper limb as a primary tumor site on further multivariate analysis may be due to similar reasonings as for the head and neck, including more advanced T staging and an anatomic location close to lymph nodes.
Study Limitations—The SEER database is a national registry, which lends itself to potential data heterogeneity in recording and miscoding. Additionally, there may be higher rates of unconfirmed or missing information given the retrospective nature of the SEER database; the database also does not delineate facility type, insurance status, or Charlson/Deyo comorbidity index as demographic factors, which could influence the multivariable analysis. Finally, the SEER database does not further demarcate subtypes of MF, such as the aggressive folliculotropic variant commonly seen in head and neck MF lesions, which precludes independent analysis of disease course by subtype.
Conclusion
Our study evaluated primary disease site as a prognostic factor for OS and DSS in patients with MF. Although head and neck and upper limb as primary disease sites were found to be significant on univariate analysis, they were found to be an insignificant prognostic variable for OS or DSS in our multivariable analysis, potentially due to the aggressive nature of advanced-stage MF and localization close to lymph nodes. Further research including a deeper dive into MF of all stages and subtypes is needed to fully investigate primary disease site as a prognostic indicator. Older age, larger tumor size, a higher T stage, lymph node involvement, and presence of metastasis were associated with worse DSS, and patients with these attributes should be counseled regarding expected disease course and prognosis.
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
- Willemze R, Jaffe ES, Burg G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105:3768-3785. doi:10.1182/blood-2004-09-3502
- Hwang ST, Janik JE, Jaffe ES, et al. Mycosis fungoides and Sézary syndrome. Lancet. 2008;371:945-957. doi:10.1016/S0140-6736(08)60420-1
- Jung JM, Lim DJ, Won CH, et al. Mycosis fungoides in children and adolescents: a systematic review. JAMA Dermatol. 2021;157:431-438. doi:10.1001/jamadermatol.2021.0083
- Hodak E, Amitay-Laish I. Mycosis fungoides: a great imitator. Clin Dermatol. 2019;37:255-267. doi:10.1016/j.clindermatol.2019.01.004
- Pimpinelli N, Olsen EA, Santucci M, et al. Defining early mycosis fungoides. J Am Acad Dermatol. 2005;53:1053-1063. doi:10.1016/j.jaad.2005.08.057
- Spieth K, Grundmann-Kollmann M, Runne U, et al. Mycosis-fungoides-type Cutaneous T cell lymphoma of the hands and soles: a variant causing delay in diagnosis and adequate treatment of patients with palmoplantar eczema. Dermatology. 2002;205:239-244. doi:10.1159/000065862
- Scarisbrick JJ, Quaglino P, Prince HM, et al. The PROCLIPI international registry of early-stage mycosis fungoides identifies substantial diagnostic delay in most patients. Br J Dermatol. 2019;181:350-357. doi:10.1111/bjd.17258
- Jawed SI, Myskowski PL, Horwitz S, et al. Primary cutaneous T-cell lymphoma (mycosis fungoides and Sézary syndrome): part i. diagnosis: clinical and histopathologic features and new molecular and biologic markers. J Am Acad Dermatol. 2014;70:205.e1-205.e16. doi:10.1016/j.jaad.2013.07.049
- Suh KS, Jang MS, Jung JH, et al. Clinical characteristics and long-term outcome of 223 patients with mycosis fungoides at a single tertiary center in Korea: a 29-year review. J Am Acad Dermatol. 2022;86:1275-1284. doi:10.1016/j.jaad.2021.06.860
- Kim YH, Liu HL, Mraz-Gernhard S, et al. Long-term outcome of 525 patients with mycosis fungoides and Sézary syndrome: clinical prognostic factors and risk for disease progression. Arch Dermatol. 2003;139:857-866. doi:10.1001/archderm.139.7.857
- Trautinger F, Eder J, Assaf C, et al. European Organisation for Research and Treatment of Cancer consensus recommendations for the treatment of mycosis fungoides/Sézary syndrome—update 2017. Eur J Cancer. 2017;77:57-74. doi:10.1016/j.ejca.2017.02.027
- Quaglino P, Prince HM, Cowan R, et al. Treatment of early-stage mycosis fungoides: results from the PROspective Cutaneous Lymphoma International Prognostic Index (PROCLIPI) study. Br J Dermatol. 2021;184:722-730. doi:10.1111/bjd.19252
- Specht L, Dabaja B, Illidge T, et al. Modern radiation therapy for primary cutaneous lymphomas: field and dose guidelines from the International Lymphoma Radiation Oncology Group. Int J Radiat Oncol Biol Phys. 2015;92:32-39. doi:10.1016/j.ijrobp.2015.01.008
- Alberti-Violetti S, Talpur R, Schlichte M, et al. Advanced-stagemycosis fungoides and Sézary syndrome: survival and response to treatment. Clin Lymphoma Myeloma Leuk. 2015;15:E105-E112. doi:10.1016/j.clml.2015.02.027
- Olsen E, Vonderheid E, Pimpinelli N, et al. Revisions to the staging and classification of mycosis fungoides and Sézary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC). Blood. 2007;110:1713-1722. doi:10.1182/blood-2007-03-055749
- Jung JM, Yoo H, Lim DJ, et al. Clinicoprognostic implications of head and neck involvement by mycosis fungoides: a retrospective cohort study. J Am Acad Dermatol. 2022;86:1258-1265. doi:10.1016/j.jaad.2021.03.056
- Brennan JA. The head and neck manifestations of mycosis fungoides. Laryngoscope. 1995;105(5, pt 1):478-480. doi:10.1288/00005537-199505000-00005
- Su C, Tang R, Bai HX, et al. Disease site as a prognostic factor for mycosis fungoides: an analysis of 2428 cases from the US National Cancer Database. Br J Haematol. 2019;185:592-595. doi:10.1111/bjh.15570
- Wilkinson AJ, Nader ME, Roberts D, et al. Survival outcomes of patients with mycosis fungoides involving the external ear and ear canal. Laryngoscope. 2023;133:1486-1491. doi:10.1002/lary.30377
- National Cancer Institute. Surveillance, Epidemiology, and End Results (SEER) surveillance research program. Published July 2021. Accessed March 14, 2024. https://seer.cancer.gov/about/factsheets/SEER_Overview.pdf
- Agar NS, Wedgeworth E, Crichton S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sézary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer Staging proposal. J Clin Oncol. 2010;28:4730-4739. doi:10.1200/JCO.2009.27.7665
- Vollmer RT. A review of survival in mycosis fungoides. Am J Clin Pathol. 2014;141:706-711. doi:10.1309/AJCPH2PHXFCX3BOX
- Desai M, Liu S, Parker S. Clinical characteristics, prognostic factors, and survival of 393 patients with mycosis fungoides and Sézary syndrome in the southeastern United States: a single-institution cohort. J Am Acad Dermatol. 2015;72:276-285. doi:10.1016/j.jaad.2014.10.019
- Mourad A, Gniadecki R. Overall survival in mycosis fungoides: a systematic review and meta-analysis. J Invest Dermatol. 2020;140:495-497.e5. doi:10.1016/j.jid.2019.07.712
Practice Points
- Mycosis fungoides (MF) is the most common cutaneous T-cell lymphoma.
- Because MF is associated with diagnostic challenges due to its indolent course, data regarding primary tumor site as a prognostic factor are limited.
- Although MF originating from the head and neck region did not appear to influence survival, it was found that patients who were older or who had a larger tumor size at diagnosis, a higher T stage, lymph node involvement, or presence of metastasis had poorer survival overall and may benefit from additional counseling regarding their prognosis.
Centrifugally Spreading Lymphocutaneous Sporotrichosis: A Rare Cutaneous Manifestation
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
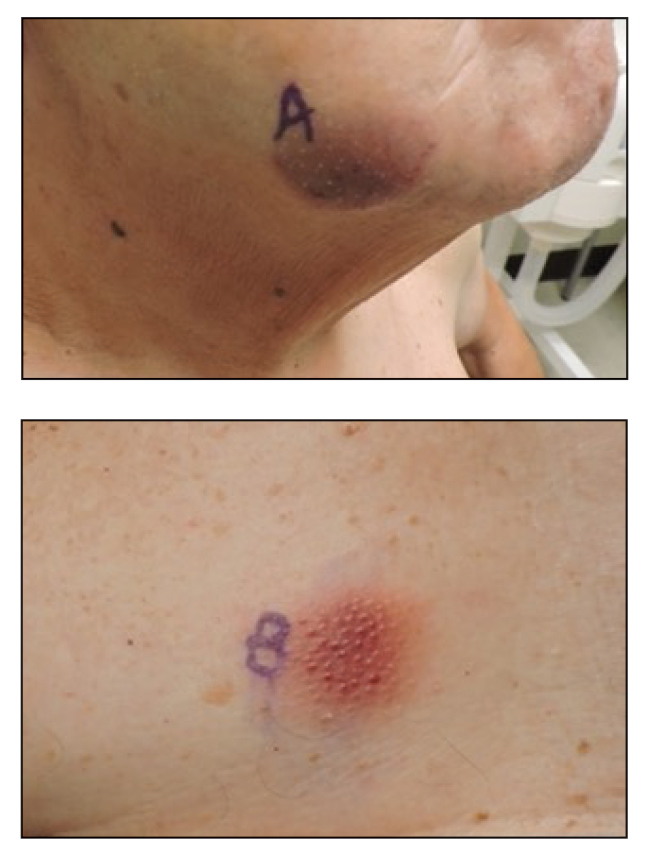
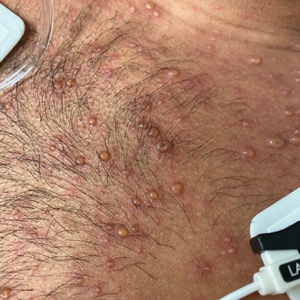
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).
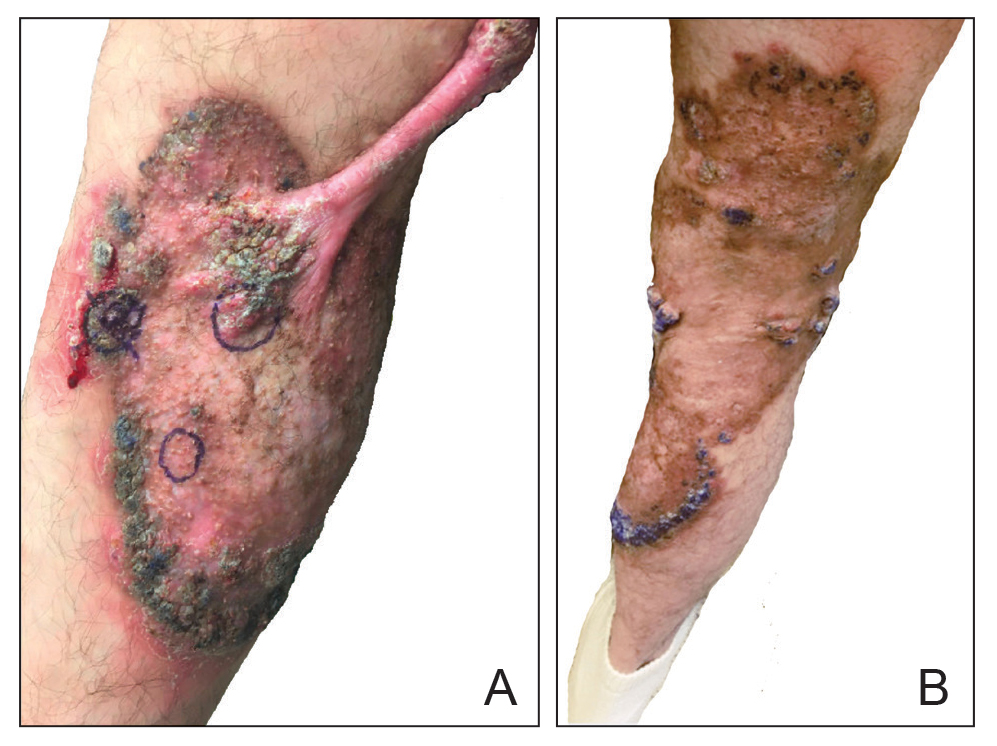
Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
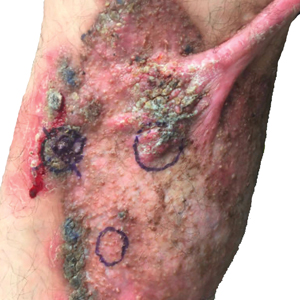
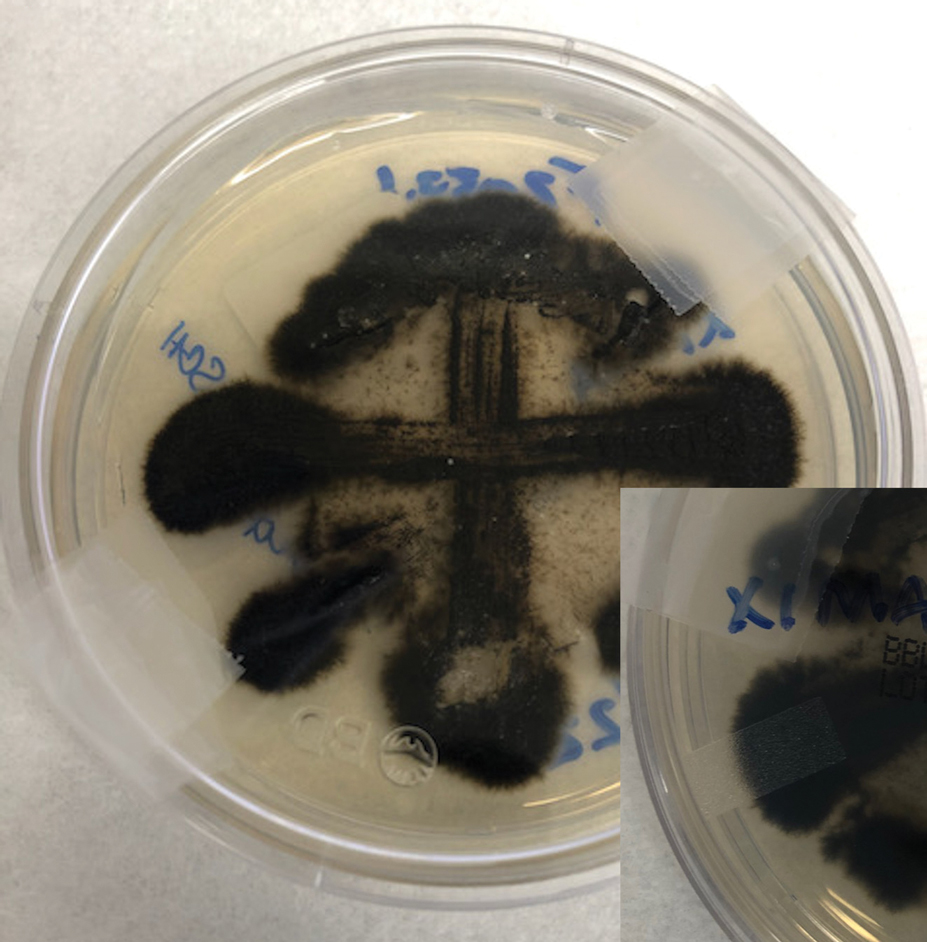
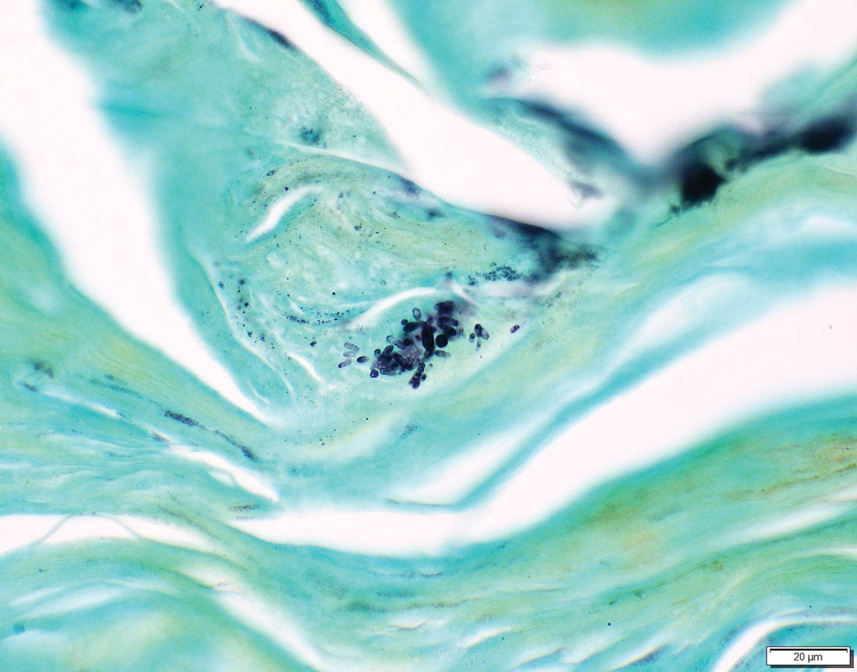
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.
The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.
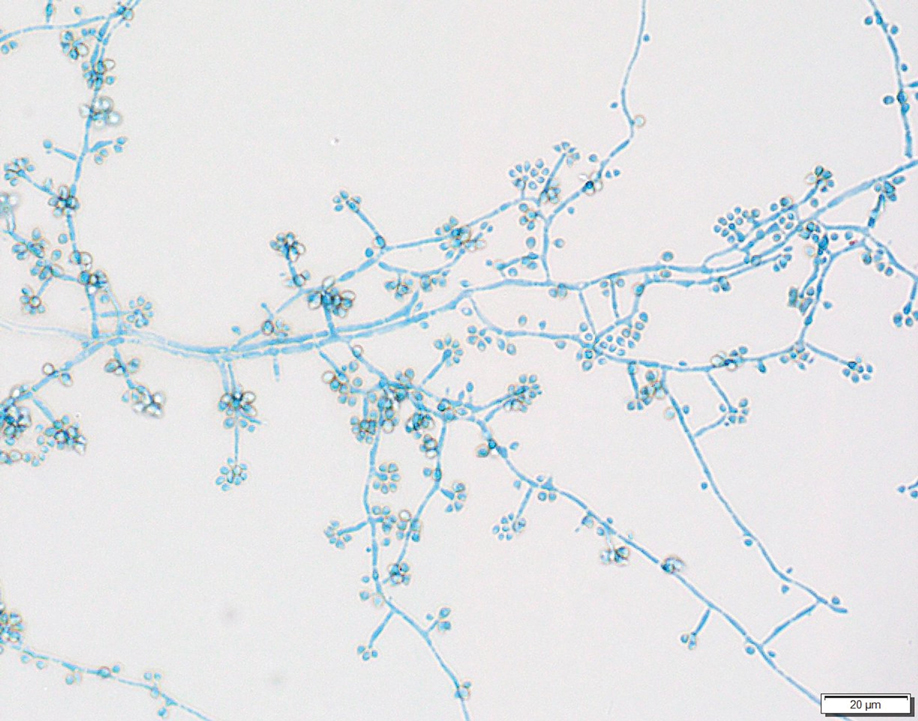
Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4
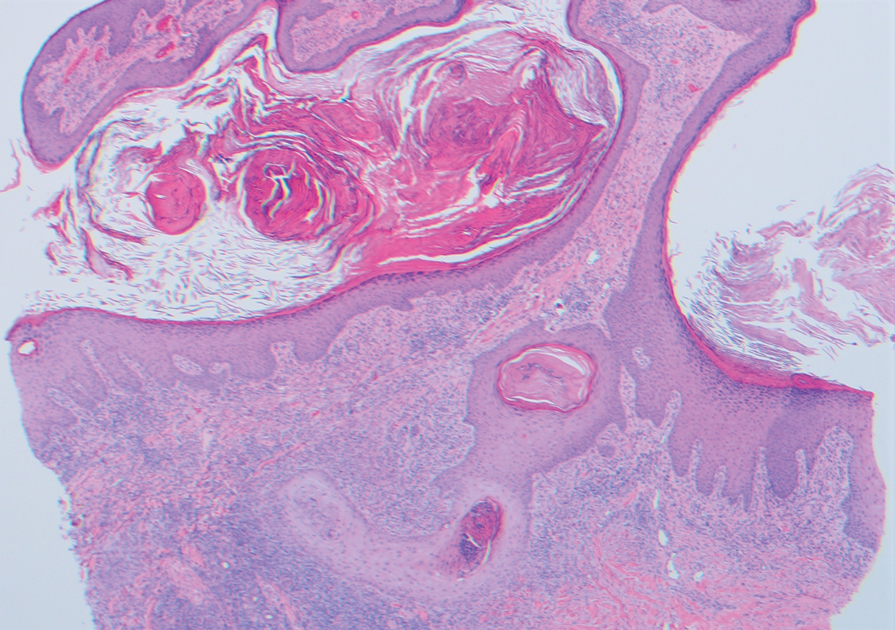
Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.
In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
To the Editor:
Sporotrichosis refers to a subacute to chronic fungal infection that usually involves the cutaneous and subcutaneous tissues and is caused by the introduction of Sporothrix, a dimorphic fungus, through the skin. We present a case of chronic atypical lymphocutaneous sporotrichosis.
A 46-year-old man presented to the outpatient dermatology clinic for follow-up for a rash on the right leg that spread to the thigh and became painful and pruritic. It initially developed 8 years prior to the current presentation after he sustained trauma to the leg from an electroshock weapon. One year prior to the current presentation, he had presented to the emergency department and was prescribed doxycycline 100 mg twice daily for 7 days as well as bacitracin ointment. He also was instructed to follow up with dermatology, but a lack of health insurance and other socioeconomic barriers prevented him from seeking dermatologic care. Nine months later, he again presented to the emergency department due to a motor vehicle accident. Computed tomography (CT) of the right leg revealed exophytic dermal masses, inflammatory stranding of the subcutaneous tissue, and right inguinal lymph nodes measuring up to 1.4 cm; there was no osteoarticular involvement. At that time, the patient was applying gentian violet to the skin lesions and taking hydroxyzine 50 mg 3 times daily as needed for pruritus with minimal relief. Financial support was provided for follow-up with dermatology, which occurred almost 5 months later.
At the current presentation, physical examination revealed a large annular plaque with verrucous, scaly, erythematous borders and a hypopigmented atrophic center extending from the medial aspect of the right leg to the posterior thigh. Numerous pink, scaly, crusted nodules were scattered primarily along the periphery, with some evidence of draining sinus tracts. In addition, a fibrotic pink linear plaque extended from the medial right leg to the popliteal fossa, consistent with a keloid. Violet staining along the periphery of the lesion also was appreciated secondary to the application of topical gentian violet (Figure 1).

Based on the chronic history and morphology, a diagnosis of a chronic fungal or atypical mycobacterial infection was favored. In particular, chromoblastomycosis, cutaneous tuberculosis (eg, scrofuloderma, lupus vulgaris, tuberculosis verrucosa cutis), and atypical mycobacterial infection were highest on the differential, as these conditions often exhibit annular, nodular, verrucous, and/or atrophic lesions. The nodularity, crusting, and draining sinus tracts also raised the possibility of mycetoma. Given the extension of the lesion from the lower to upper leg, a sporotrichoid infection also was considered but was thought to be less likely based on the annular configuration.
Two 4-mm punch biopsies were taken from a peripheral nodule—one for routine histology and another for bacterial, fungal, and mycobacterial cultures. An interferon-gamma release assay also was ordered to evaluate for immune responses indicative of prior Mycobacterium tuberculosis infection, but the patient did not obtain this for unknown reasons. Histology demonstrated pseudoepitheliomatous hyperplasia and necrotizing granulomas, which suggested an infectious etiology, but no organisms were identified on tissue staining and all cultures were negative for growth at 6 weeks. The patient was asked to return at that point, and 4 additional scouting biopsies were performed and sent for routine histology, M tuberculosis nucleic acid amplification testing, and microbiologic cultures (ie, bacterial, mycobacterial, fungal, nocardia, actinomycetes). Within 1 week, a filamentous organism with pigmentation visible on the front and back of a Sabouraud dextrose agar plate was identified on fungal culture (Figure 2). Microscopic evaluation of this mold with lactophenol blue stain revealed thin septate hyphae with conidiophores arising at right angles that bore clusters of microconidia (Figure 3). Sequencing analysis ultimately identified this organism as Sporothrix schenckii. Routine histology demonstrated pseudoepitheliomatous hyperplasia with scattered intraepidermal collections of neutrophils (Figure 4). The dermis showed a dense, superficial, and deep infiltrate composed of lymphocytes, histiocytes, and plasma cells with occasional neutrophils and eosinophils. A Grocott-Gomori methenamine-silver stain revealed a cluster of ovoid yeast forms within the stratum corneum (Figure 5). The patient was referred to infectious disease for follow-up and treatment.

The patient later visited a community clinic providing dermatologic care for patients without insurance. He was started on itraconazole 200 mg daily for a total of 6 months until dermatologic clearance of the cutaneous lesions was observed. He was followed by the clinic with laboratory tests including a liver function test. At follow-up 8 months later, a repeat biopsy was performed to ensure histologic clearance of the sporotrichosis, which revealed a dermal scar and no evidence of residual infection.

Sporothrix schenckii was first isolated in 1898 by Benjamin Schenck, a student at Johns Hopkins Medicine (Baltimore, Maryland), and identified by a mycologist as sporotricha.1 Species within the genus Sporothrix are unique in that the fungi are both dimorphic (growing as a mold at 25 °C but as a yeast at 37 °C) and dematiaceous (dark pigmentation from melanin is visible on inspection of the anterior and reverse sides of culture plates). Infection usually occurs when cutaneous or subcutaneous tissues are exposed to the fungus via microabrasions; activities thought to contribute to exposure include gardening, agricultural work, animal husbandry, and feline scratches.2 Although skin trauma frequently is considered the primary route of infection, patient recall is variable, with one study noting that only 37.7% of patients recalled trauma and another study similarly demonstrating a patient recall rate of 25%.3,4

Lymphocutaneous sporotrichosis is the most common presentation of the fungal infection,5 and clinical cases may be classified into 1 of 4 categories: (1) lymphangitic lesions—papules at the site of inoculation with spread along the lymphatic channels; (2) localized (fixed) cutaneous lesions—1 or 2 lesions at the inoculation site; (3) disseminated (multifocal) cutaneous lesions; and (4) extracutaneous lesions.6 Extracutaneous manifestations of this infection most notably have been reported as pulmonary disease through inhalation of conidia or through dissemination in immunocompromised hosts.7 Our patient’s infection was categorized as lymphangitic lesions due to spread from the lower to upper leg, albeit in a highly atypical, annular fashion. A review of systems was otherwise negative, and CT ruled out osteoarticular involvement.

In addition to socioeconomic barriers, several factors contributed to a delayed diagnosis in this patient including the annular presentation with central hypopigmentation and atrophy, negative initial microbiological cultures and lack of visualization of organisms on histopathology, and the consequent need for repeat biopsies. For lymphocutaneous sporotrichosis, the typical presentation consists of a papule or ulcerated nodule at the site of inoculation with subsequent linear spread along lymphatic channels. This classic sporotrichoid pattern is a key diagnostic clue for identifying sporotrichosis but was absent at the time our patient presented for medical care. Rather, the sporotrichoid spread seemed to have occurred in a centrifugal fashion up the leg. Few case reports have documented an annular presentation of lymphocutaneous sporotrichosis,8-13 and one report described central atrophy and hypopigmentation.10 Pain and pruritus, which were present in our patient, rarely are documented.9 Finally, the diagnosis of cutaneous fungal infections may require multiple biopsies due to the variable abundance of viable organisms in tissue specimens as well as the fastidious growth characteristics of these organisms. Furthermore, sensitivity often is low for both fungal and mycobacterial cultures, and cultures may take days to weeks to yield growth.14,15 For these reasons, empiric therapy and repeat biopsies often are pursued if clinical suspicion is high enough.16 Our patient returned for multiple scouting biopsies after the initial tissue culture was negative and was even considered for empiric treatment against Mycobacterium prior to positive fungal cultures.
Another unique aspect of our case was the presence of a keloid. It is difficult to know if this keloid was secondary to the trauma the patient sustained in the inciting incident or formed from the fungal infection. Interestingly, it has been hypothesized that fungal infections may contribute to keloid and hypertrophic scar formation.17 In a case series of 3 patients with either keloids or hypertrophic scars and concomitant tinea infection, there was notable improvement in the appearance of the scars 2 weeks after beginning itraconazole therapy.17 However, it is not yet known if a fungal infection can contribute to the pathogenesis of keloid formation.
As with other aspects of this case, the length of time the patient went without diagnosis and treatment was unusual and may help explain the atypical presentation. Although the incubation period for S schenckii can vary, most reports identify patients as seeking medical attention within 1 year of rash onset.18-20 In our case, the patient was not diagnosed until 8 years after his symptoms began, requiring multiple referrals, multiple health system touchpoints, and an institution-specific financial aid program. As such, this case also highlights the potential need for a multidisciplinary team approach when caring for patients with poor access to health care.
In conclusion, this case illustrates a unique presentation of lymphocutaneous sporotrichosis that may mimic other chronic infections and result in delayed diagnosis. Although lymphangitic sporotrichosis generally is recognized as having a linear distribution, mounting evidence from this report and others suggests an annular presentation also is possible. Pruritus or pain is rare but should not preclude a diagnosis of sporotrichosis if present. For patients with limited access to health care resources, it is especially important to involve multiple members of the health care team, including social workers and specialists, to prevent a protracted and severe course of disease.
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
- Schenck BR. On refractory subcutaneous abscesses caused by a fungus possibly related to the sporotricha. Bulletin of the Johns Hopkins Hospital. 1898;93:286-290.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654. doi:10.1128/CMR.00007-11
- Crevasse L, Ellner PD. An outbreak of sporotrichosis in florida. J Am Med Assoc. 1960;173:29-33. doi:10.1001/jama.1960.03020190031006
- Mayorga R, Cáceres A, Toriello C, et al. An endemic area of sporotrichosis in Guatemala [in French]. Sabouraudia. 1978;16:185-198.
- Morris-Jones R. Sporotrichosis. Clin Exp Dermatol. 2002;27:427-431. doi:10.1046/j.1365-2230.2002.01087.x
- Sampaio SA, Da Lacaz CS. Clinical and statistical studies on sporotrichosis in Sao Paulo (Brazil). Article in German. Hautarzt. 1959;10:490-493.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187. doi:10.1016/j.clindermatol.2006.05.006
- Williams BA, Jennings TA, Rushing EC, et al. Sporotrichosis on the face of a 7-year-old boy following a bicycle accident. Pediatr Dermatol. 2013;30:E246-E247. doi:10.1111/j.1525-1470.2011.01696.x
- Vaishampayan SS, Borde P. An unusual presentation of sporotrichosis. Indian J Dermatol. 2013;58:409. doi:10.4103/0019-5154.117350
- Qin J, Zhang J. Sporotrichosis. N Engl J Med. 2019;380:771. doi:10.1056/NEJMicm1809179
- Patel A, Mudenda V, Lakhi S, et al. A 27-year-old severely immunosuppressed female with misleading clinical features of disseminated cutaneous sporotrichosis. Case Rep Dermatol Med. 2016;2016:1-4. doi:10.1155/2016/9403690
- de Oliveira-Esteves ICMR, Almeida Rosa da Silva G, Eyer-Silva WA, et al. Rapidly progressive disseminated sporotrichosis as the first presentation of HIV infection in a patient with a very low CD4 cell count. Case Rep Infect Dis. 2017;2017:4713140. doi:10.1155/2017/4713140
- Singh S, Bachaspatimayum R, Meetei U, et al. Terbinafine in fixed cutaneous sporotrichosis: a case series. J Clin Diagnostic Res. 2018;12:FR01-FR03. doi:10.7860/JCDR/2018/25315.12223
- Guarner J, Brandt ME. Histopathologic diagnosis of fungal infections in the 21st century. Clin Microbiol Rev. 2011;24:247-280. doi:10.1128/CMR.00053-10
- Peters F, Batinica M, Plum G, et al. Bug or no bug: challenges in diagnosing cutaneous mycobacterial infections. J Ger Soc Dermatol. 2016;14:1227-1236. doi:10.1111/ddg.13001
- Khadka P, Koirala S, Thapaliya J. Cutaneous tuberculosis: clinicopathologic arrays and diagnostic challenges. Dermatol Res Pract. 2018;2018:7201973. doi:10.1155/2018/7201973
- Okada E, Maruyama Y. Are keloids and hypertrophic scars caused by fungal infection? . Plast Reconstr Surg. 2007;120:814-815. doi:10.1097/01.prs.0000278813.23244.3f
- Pappas PG, Tellez I, Deep AE, et al. Sporotrichosis in Peru: description of an area of hyperendemicity. Clin Infect Dis. 2000;30:65-70. doi:10.1086/313607
- McGuinness SL, Boyd R, Kidd S, et al. Epidemiological investigation of an outbreak of cutaneous sporotrichosis, Northern Territory, Australia. BMC Infect Dis. 2016;16:1-7. doi:10.1186/s12879-016-1338-0
- Rojas FD, Fernández MS, Lucchelli JM, et al. Cavitary pulmonary sporotrichosis: case report and literature review. Mycopathologia. 2017;182:1119-1123. doi:10.1007/s11046-017-0197-6
Practice Points
- An atypical presentation of lymphocutaneous sporotrichosis may pose challenges to timely diagnosis and treatment.
- Although lymphocutaneous sporotrichosis spreads most commonly in a linear fashion along lymphatic channels, an annular configuration is possible.
- Initial tissue cultures and histopathology of lymphocutaneous sporotrichosis may not yield a diagnosis, necessitating repeat biopsies when clinical suspicion is high.
Eosinophilic Pustular Folliculitis in the Setting of Untreated Chronic Lymphocytic Leukemia
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.
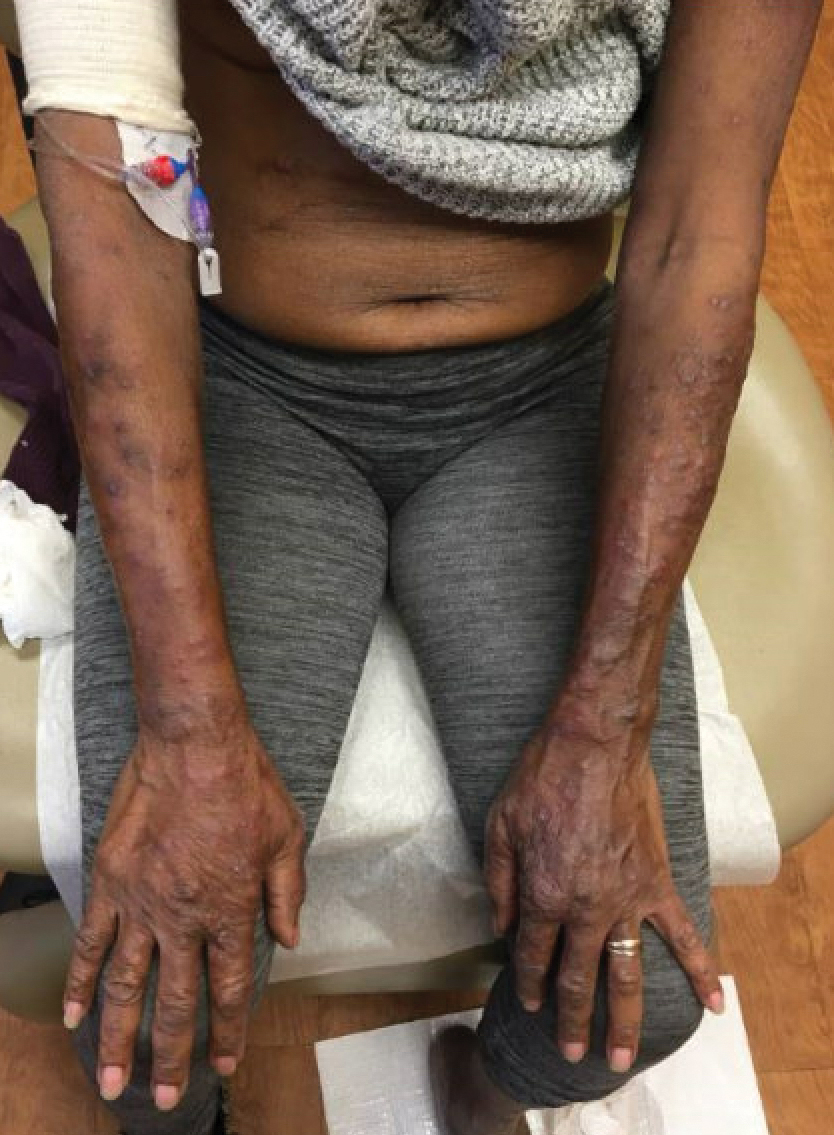
A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.
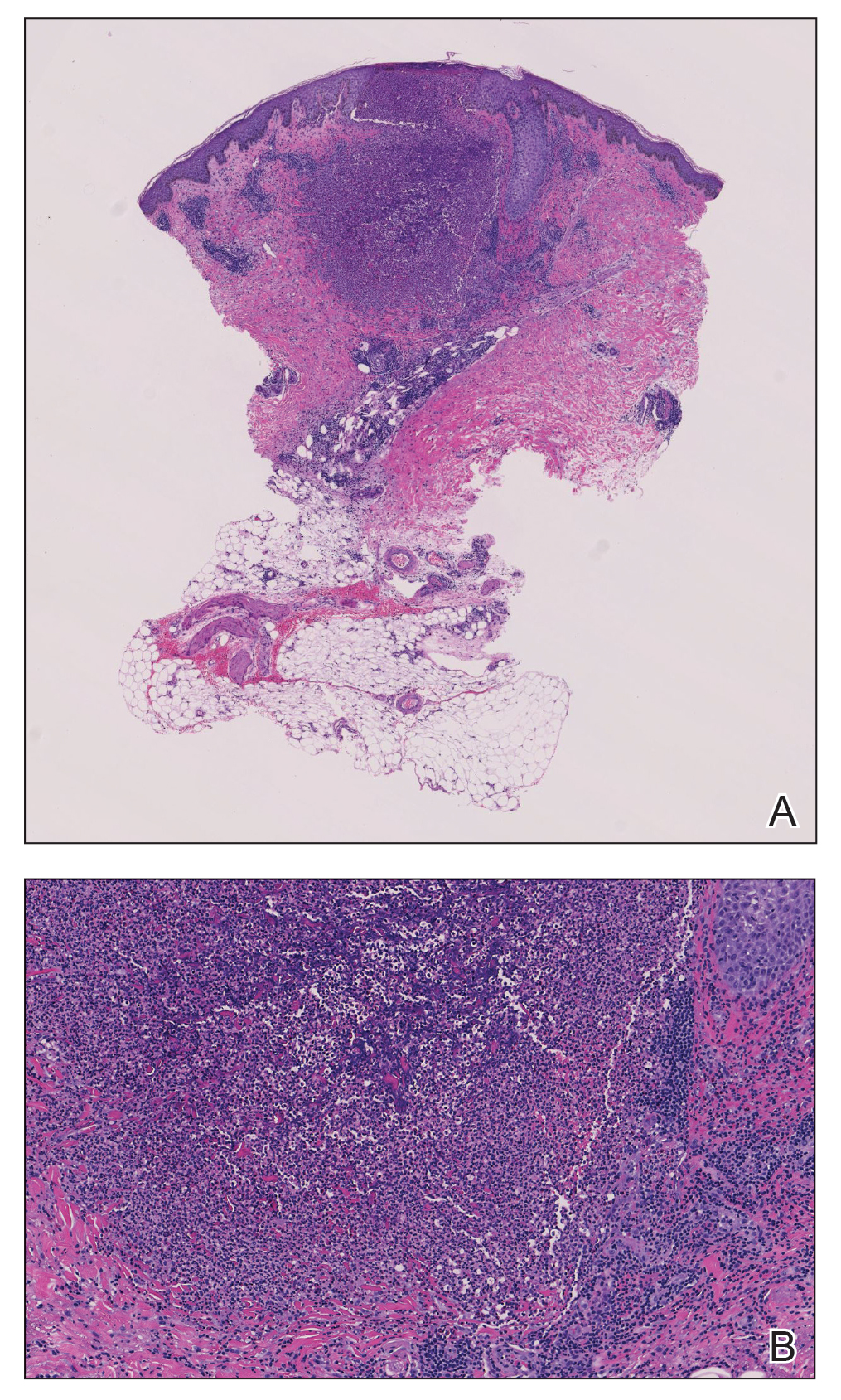
Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
To the Editor:
Eosinophilic pustular folliculitis (EPF) is a noninfectious dermatosis that typically manifests as recurrent follicular papulopustules that generally affect the face and occasionally the trunk and arms. There are several subtypes of EPF: classic EPF (Ofuji disease), infancy-associated EPF, and immunosuppression-associated EPF.1,2 We report a rare case of EPF in the setting of untreated chronic lymphocytic leukemia (CLL), a subtype of immunosuppression-associated EPF that has been associated with hematologic malignancy EPF (HM-EPF).3-5
A 69-year-old woman presented with diffusely scattered, pruritic, erythematous, erosive lesions on the back, arms, legs, and forehead (Figure 1) of 4 months’ duration, as well as an ulcerative lesion on the left third toe due to a suspected insect bite. She had a history of untreated CLL that was diagnosed 2 years prior. The patient was empirically started on clindamycin for presumed infection of the toe. A punch biopsy of the left wrist revealed superficial and deep dermal perivascular and interstitial inflammatory infiltrates composed of lymphocytes, histiocytes, and numerous eosinophils in association with edema and necrosis. Histopathology was overall most consistent with an exuberant arthropod reaction; however, at 2-week follow-up, the patient reported that the pustular lesions improved upon starting antibiotics, which raised concerns for a bacterial process. The patient initially was continued on clindamycin given subjective improvement but was later switched to daptomycin, as she developed clindamycin-resistant methicillin-resistant Staphylococcus aureus osteomyelitis from the necrotic toe.

A month later, the patient returned with new papules and pustules on the arms and trunk. A repeat biopsy showed notable dermal collections comprised predominantly of neutrophils and eosinophils as well as involvement of follicular structures by dense inflammation (Figure 2). Immunohistochemistry demonstrated a predominant population of small CD3+ T cells, which raised concern for cutaneous T-cell lymphoma. However, retention of CD5 expression made this less likely. Few scattered CD20+ B cells with limited CD23 reactivity and without CD5 co-expression were detected, which ruled out cutaneous involvement of the patient’s CLL. Bacterial culture and Grocott methenamine-silver, Gram, acid-fast bacilli, and periodic acid-Schiff stains were negative. Polymerase chain reaction testing for varicella-zoster virus and herpes simplex virus also were negative. Thus, a diagnosis of EPF secondary to CLL was favored, as an infectious process also was unlikely. The patient was started on triamcinolone cream 0.1% with gradual improvement.

Cases of HM-EPF predominantly have been reported in patients who have undergone chemotherapy, bone marrow transplantation, or hematopoietic stem cell transplantation. Furthermore, a vast majority of these cases have been reported in older males.3-16 In a retrospective study of more than 750 patients with established CLL, Agnew et al7 identified 125 different skin complications in 40 patients. Of this subset, only a small number (2/40) were associated with eosinophilic folliculitis, with 1 case noted in a middle-aged woman with a history of CLL treatment.7 Moreover, Motaparthi et al4 reported 3 additional cases of HM-EPF, with all patients identified as middle-aged men who were treated with chemotherapy for underlying CLL. Our patient represents a case of EPF in the context of untreated CLL in a woman.
Although topical corticosteroids remain the first-line treatment for EPF, a survey study conducted across 67 hospitals in Japan indicated that antibiotics were moderately or highly effective in 79% of EPF patients (n=143).17 This association may explain the subjective improvement reported by our patient upon starting clindamycin. Furthermore, in HIV-associated EPF, high-dose cetirizine, itraconazole, and metronidazole have been successful when topical therapies have failed.18 Although the precise pathogenesis of EPF is unknown, histopathologic features, clinical appearance, and identification of the accurate EPF subtype can still prove valuable in informing empiric treatment strategies. Consequently, the initial histopathologic diagnosis of an arthropod bite reaction in our patient highlights the importance of clinical correlation and additional ancillary studies in the determination of EPF vs other inflammatory dermatoses that manifest microscopically with lymphocytic infiltrates, prominent eosinophils, and follicular involvement.4 The histopathologic features of EPF demonstrate considerable overlap with eosinophilic dermatosis of hematologic malignancy (also known as eosinophilic dermatosis of myeloproliferative disease). It is suspected that eosinophilic dermatosis of hematologic malignancy and EPF may exist on a spectrum, and additional cases may improve categorization of these entities.19
In conclusion, this report adds to the medical practitioner’s awareness of EPF manifestations in patients with underlying CLL, an infrequently reported subtype of HM-EPF.
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
- Fujiyama T, Tokura Y. Clinical and histopathological differential diagnosis of eosinophilic pustular folliculitis. J Dermatol. 2013;40:419-423. doi:10.1111/1346-8138.12125
- Katoh M, Nomura T, Miyachi Y, et al. Eosinophilic pustular folliculitis: a review of the Japanese published works. J Dermatol. 2013;40:15-20. doi:10.1111/1346-8138.12008
- Takamura S, Teraki Y. Eosinophilic pustular folliculitis associated with hematological disorders: a report of two cases and review of Japanese literature. J Dermatol. 2016;43:432-435. doi: 10.1111/1346-8138.13088
- Motaparthi K, Kapil J, Hsu S. Eosinophilic folliculitis in association with chronic lymphocytic leukemia: a clinicopathologic series. JAAD Case Rep. 2017;3:263-268. doi:10.1016/j.jdcr.2017.03.007
- Lambert J, Berneman Z, Dockx P, et al. Eosinophilic pustular folliculitis and B-cell chronic lymphatic leukaemia. Dermatology. 1994;189(suppl 2):58-59. doi:10.1159/000246994
- Patrizi A, Chieregato C, Visani G, et al. Leukaemia-associated eosinophilic folliculitis (Ofuji’s disease). J Eur Acad Dermatol Venereol. 2004;18:596-598. doi:10.1111/j.1468-3083.2004.00982.x
- Agnew KL, Ruchlemer R, Catovsky D, et al. Cutaneous findings in chronic lymphocytic leukaemia. Br J Dermatol. 2004;150:1129-1135. doi:10.1111/j.1365-2133.2004.05982.x
- Zitelli K, Fernandes N, Adams BB. Eosinophilic folliculitis occurring after stem cell transplant for acute lymphoblastic leukemia: a case report and review. Int J Dermatol. 2015;54:785-789. doi:10.1111/j.1365-2133.2004.05982.x
- Goiriz R, Guhl-Millán G, Peñas PF, et al. Eosinophilic folliculitis following allogeneic peripheral blood stem cell transplantation: case report and review. J Cutan Pathol. 2007;34(suppl 1):33-36. doi:10.1111/j.1600-0560.2006.00725.x
- Bhandare PC, Ghodge RR, Bhobe MR, et al. Eosinophilic pustular folliculitis post chemotherapy in a patient of non-Hodgkins lymphoma: a case report. Indian J Dermatol. 2015;60:521. doi:10.4103/0019-5154.164432
- Sugaya M, Suga H, Miyagaki T, et al. Eosinophilic pustular folliculitis associated with Sézary syndrome. Clin Exp Dermatol. 2014;39:536-538. doi:10.1111/ced.12315
- Keida T, Hayashi N, Kawashima M. Eosinophilic pustular folliculitis following autologous peripheral blood stem-cell transplantation. J Dermatol. 2004;31:21-26. doi:10.1111/j.1346-8138.2004.tb00499.x
- Ota M, Shimizu T, Hashino S, et al. Eosinophilic folliculitis in a patient after allogeneic bone marrow transplantation: case report and review of the literature. Am J Hematol. 2004;76:295-296. doi:10.1002/ajh.20080
- Vassallo C, Ciocca O, Arcaini L, et al. Eosinophilic folliculitis occurring in a patient affected by Hodgkin lymphoma. Int J Dermatol. 2002;41:298-300. doi:10.1046/j.1365-4362.2002.01356_6.x
- Evans TR, Mansi JL, Bull R, et al. Eosinophilic folliculitis occurring after bone marrow autograft in a patient with non-Hodgkin’s lymphoma. Cancer. 1994;73:2512-2514. doi:10.1002/1097-0142(19940515)73:10<2512::aid-cncr2820731010>3.0.co;2-s
- Patrizi A, Di Lernia V, Neri I, et al. Eosinophilic pustular folliculitis (Ofuji’s disease) and non-Hodgkin lymphoma. Acta Derm Venereol. 1992;72:146-147.
- Ono S, Yamamoto Y, Otsuka A, et al. Evaluation of the effectiveness of antibiotics against eosinophilic pustular folliculitis. Case Rep Dermatol. 2013;5:144-147. doi:10.1159/000351330
- Ellis E, Scheinfeld N. Eosinophilic pustular folliculitis. Am J Clin Dermatol. 2004;5:189-197. doi:10.2165/00128071-200405030-00007
- Bailey CAR, Laurain DA, Sheinbein DM, et al. Eosinophilic folliculitis, eosinophilic dermatosis of hematologic malignancy and acneiform follicular mucinosis: two case reports and a review of the literature highlighting the spectrum of histopathology. J Cutan Pathol. 2021;48:439-450. doi:10.1111/cup.13932
Practice Points
- Eosinophilic pustular folliculitis (EPF) is associated with an immunosuppressed state, as in patients with underlying hematologic malignancy.
- Topical corticosteroids remain the first-line treatment for EPF; however, antimicrobial agents have been used with moderate success when topical therapies have failed.
Papulosquamous Dermatophytid Reaction in a Child With Tinea Capitis
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.
Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
To the Editor:
Tinea capitis is a common childhood infection seen worldwide and is more prevalent in children of African descent.1 Treatment can be effective; however, the diagnosis may be delayed due to variability in presentation, camouflage of scalp scale with ointment, and the diagnostic experience of the provider. A common complication of tinea capitis is the dermatophytid (id) reaction, which commonly manifests as multiple 1- to 2-mm monomorphic papules. We report a case of a papulosquamous variant of an id reaction secondary to tinea capitis.
An 8-year-old African American child presented with annular hyperpigmented patches on the face and trunk of several months’ duration. There was no preceding fever, illness, scalp pruritus, or alopecia according to the patient’s mother. The hyperpigmented patches persisted despite use of hydrocortisone and antifungal creams prescribed by a primary care provider. A fungal culture of a scalp specimen was negative. Physical examination during the initial dermatology visit revealed multiple annular hyperpigmented patches on the trunk and extremities. No plaques were evident; however, the mother reported that when the lesions first developed, they were raised and mildly pruritic. The patient was prescribed triamcinolone ointment 0.1% twice daily as needed for itching, and sun protection was emphasized.
At the follow-up visit weeks later, the patient’s mother reported that the ointment had helped the lesions resolve faster, but new lesions continued to appear. Physical examination at this visit was notable for scattered hyperpigmented patches, annular hyperpigmented plaques, and erythematous plaques on the trunk, arms, and legs, in addition to papulosquamous plaques and hyperpigmented patches on the forehead (Figure 1). Suspicion for tinea capitis was discussed, a repeat scalp fungal culture was performed, and oral terbinafine 250 mg once daily was started empirically. The culture was positive for Trichophyton tonsurans supporting the diagnosis of concomitant tinea capitis. The rash resolved with terbinafine, and annular patches of postinflammatory hyperpigmentation remained.

Dermatophytid reactions are immunologically mediated, disseminated, eczematous eruptions occurring after cutaneous infections or inflammatory skin conditions. Reactions occur days to weeks after exposure to antigens of dermatophytes causing tinea pedis or capitis.2
Common culprits include Microsporum canis and T tonsurans.3 Dermatophytid reactions with tinea capitis exhibit morphologic variability including a symmetric distribution of grouped or diffuse,4 pruritic, erythematous or flesh-colored, follicular papules on the trunk, with or without progression to the face, torso, upper extremities, and/or lower extremities.3 Other reported manifestations include erythema multiforme, erythema nodosum,3 or lupuslike lesions, and crops of dyshidrotic vesicles on the hands in the setting of Trichophyton mentagrophytes–induced tinea pedis.5
The papulosquamous variant id reaction should be considered in a wider differential that includes psoriasis, nummular eczema, and pityriasis rosea. Unlike psoriasis, the id reaction is not chronic and responds to systemic antifungal therapy. Nummular eczema can be ruled out, though not entirely, by a lack of personal or family history of atopy. The characteristic cleavage lines of pityriasis rosea on the trunk are absent in patients with an id reaction, and there would be no preceding illness or herald patches seen in the id reaction.
Tinea capitis may cause a variety of id manifestations, including the papulosquamous phenotype. This case addresses practice gaps that may lead to delayed diagnosis. It also highlights the importance of recognizing uncommon morphologies, performing repeat cultures of the scalp after a negative fungal culture, and lowering the threshold of suspicion for tinea capitis in the appropriate age group and demographic, specifically pediatric patients of African descent.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
- Sharma V, Silverberg NB, Howard R, et al. Do hair care practices affect the acquisition of tinea capitis? a case-control study. Arch Pediatr Adolesc Med. 2001;155:818-821.
- Cheng N, Rucker Wright D, Cohen BA. Dermatophytid in tinea capitis: rarely reported common phenomenon with clinical implications. Pediatrics. 2011;128:e453-e457.
- Mayser P. Dermatophyte: current situation [in German]. Hautarzt. 2017;68:316-323.
- Nowicki R. Allergic phenomena in the course of dermatomycoses [in Polish]. Pol Merkur Lekarski. 2003;14:532-534.
5. Boralevi F, Léauté-Labrèze C, Roul S, et al. Lupus-erythematosus-like eruption induced by Trichophyton mentagrophytes infection. Dermatology. 2003;206:303-306.
Practice Points
- Dermatophytid (id) reactions can manifest as papulosquamous eruptions after cutaneous infections or inflammatory skin conditions.
- High clinical suspicion for id reaction in patients of the appropriate age group and demographic—pediatric patients of African descent—is imperative for reaching the correct diagnosis.
- Repeat cultures of the scalp may be indicated in patients with high clinical probability for an id reaction despite a negative fungal culture or empiric systemic treatment.
The Role of Dermatology in Identifying and Reporting a Primary Varicella Outbreak
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).
Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).
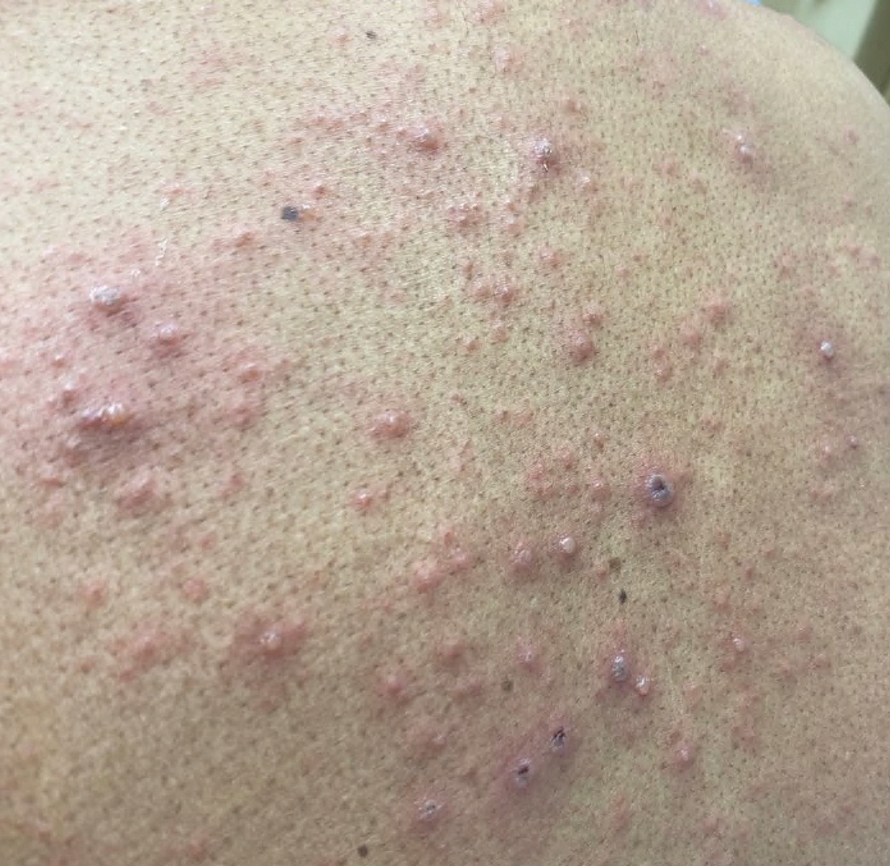
Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).
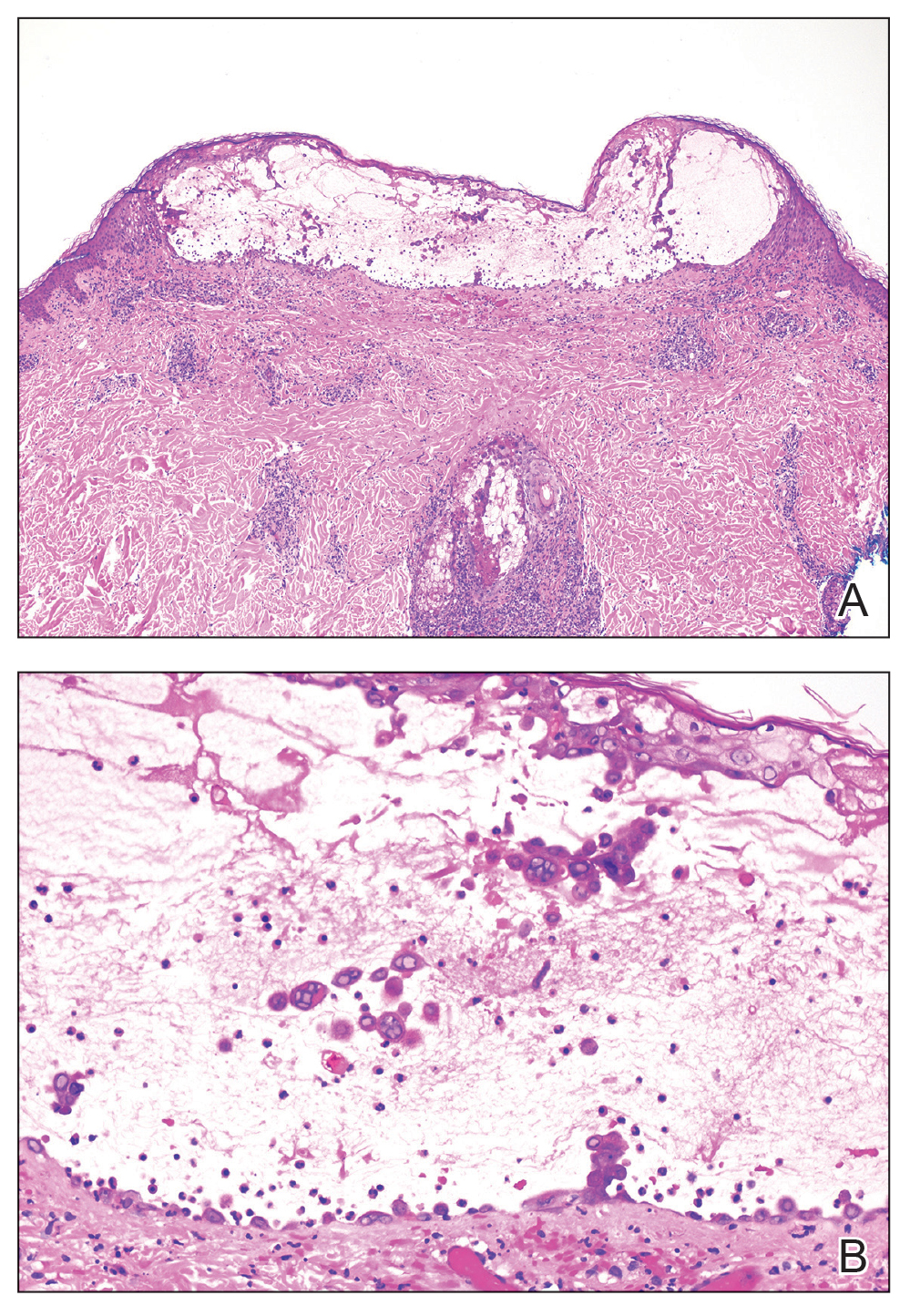
Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).
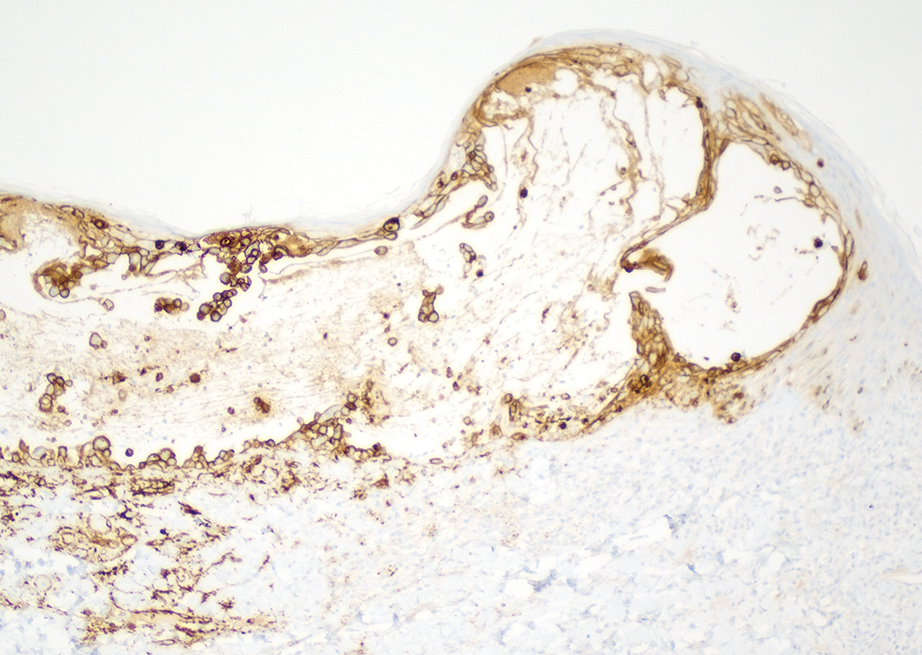
The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
To the Editor:
Cases of primary varicella-zoster virus (VZV) are relatively uncommon in the United States since the introduction of the varicella vaccine in 1995, with an overall decline in cases of more than 97%.1 Prior to the vaccine, 70% of hospitalizations occurred in children; subsequently, hospitalizations among the pediatric population (aged ≤20 years) declined by 97%. Compared to children, adults and immunocompromised patients with VZV infection may present with more severe disease and experience more complications.1
Most children in the United States are vaccinated against VZV, with 90.3% receiving at least 1 dose by 24 months of age.2 However, many countries do not implement universal varicella vaccination for infants.3 As a result, physicians should remember to include primary varicella in the differential when clinically correlated, especially when evaluating patients who have immigrated to the United States or who may be living in unvaccinated communities. We report 2 cases of primary VZV manifesting in adults to remind readers of the salient clinical features of this disease and how dermatologists play a critical role in early and accurate identification of diseases that can have wide-reaching public health implications.
A 26-year-old man with no relevant medical history presented to the emergency department with an itchy and painful rash of 5 days’ duration that began on the trunk and spread to the face, lips, feet, hands, arms, and legs. He also reported shortness of breath, cough, and chills, and he had a temperature of 100.8 °F (38.2 °C). Physical examination revealed numerous erythematous papules and vesiculopustules, some with central umbilication and some with overlying gold crusts (Figure 1).

Later that day, a 47-year-old man with no relevant medical history presented to the same emergency department with a rash along with self-reported fever and sore throat of 3 days’ duration. Physical examination found innumerable erythematous vesicopustules scattered on the face, scalp, neck, trunk, arms, and legs, some with a “dew drop on a rose petal” appearance and some with overlying hemorrhagic crust (Figure 2).

Although infection was of primary concern for the first patient, the presentation of the second patient prompted specific concern for primary VZV infection in both patients, who were placed on airborne and contact isolation precautions.
Skin biopsies from both patients showed acantholytic blisters, hair follicle necrosis, and marked dermal inflammation (Figure 3). Herpetic viral changes were seen in keratinocytes, with steel-grey nuclei, multinucleated keratinocytes, and chromatin margination. An immunostain for VZV was diffusely positive, and VZV antibody IgG was positive (Figure 4).

Upon additional questioning, both patients reported recent exposure to VZV-like illnesses in family members without a history of international travel. Neither of the patients was sure of their vaccination status or prior infection history. Both patients received intravenous acyclovir 10 mg/kg administered every 8 hours. Both patients experienced improvement and were discharged after 3 days on oral valacyclovir (1 g 3 times daily for a 7-day treatment course).

The similar presentation and timing of these 2 VZV cases caused concern for an unidentified community outbreak. The infection control team was notified; additionally, per hospital protocol the state health department was alerted as well as the clinicians and staff of the hospital with a request to be vigilant for further cases.
Despite high vaccination rates in the United States, outbreaks of varicella still occur, particularly among unvaccinated individuals, and a robust and efficient response is necessary to control the spread of such outbreaks.4 Many states, including Arkansas where our cases occurred, have laws mandating report of VZV cases to the department of health.5 Dermatologists play an important role in reporting cases, aiding in diagnosis through recognition of the physical examination findings, obtaining appropriate biopsy, and recommending additional laboratory testing.
Typical skin manifestations include a pruritic rash of macules, papules, vesicles, and crusted lesions distributed throughout the trunk, face, arms, and legs. Because new lesions appear over several days, they will be in different stages of healing, resulting in the simultaneous presence of papules, vesicles, and crusted lesions.6 This unique characteristic helps distinguish VZV from other skin diseases such as smallpox or mpox (monkeypox), which generally show lesions in similar stages of evolution.
Biopsy also can aid in identification. Viruses in the herpes family reveal similar histopathologic characteristics, including acantholysis and vesicle formation, intranuclear inclusions with margination of chromatin, multinucleation, and nuclear molding.7 Immunohistochemistry can be used to differentiate VZV from herpes simplex virus; however, neither microscopic examination nor immunohistochemistry distinguish primary VZV infection from herpes zoster (HZ).8
The mpox rash progresses more slowly than a VZV rash and has a centrifugal rather than central distribution that can involve the palms and soles. Lymphadenopathy is a characteristic finding in mpox.9 Rickettsialpox is distinguished from VZV primarily by the appearance of brown or black eschar after the original papulovesicular lesions dry out.10 Atypical hand, foot, and mouth disease can manifest in adults as widespread papulovesicular lesions. This form is associated with coxsackievirus A6 and may require direct fluorescent antibody assay or polymerase chain reaction of keratinocytes to rule out VZV.11
Herpes zoster occurs in older adults with a history of primary VZV.6 It manifests as vesicular lesions confined to 1 or 2 adjacent dermatomes vs the diffuse spread of VZV over the entire body. However, HZ can become disseminated in immunocompromised individuals, making it difficult to clinically distinguish from VZV.6 Serology can be helpful, as high IgM titers indicate an acute primary VZV infection. Subsequently increased IgG titers steadily wane over time and spike during reactivation.12
Dermatology and infectious disease consultations in our cases yielded a preliminary diagnosis through physical examination that was confirmed by biopsy and subsequent laboratory testing, which allowed for a swift response by the infection control team including isolation precautions to control a potential outbreak. Patients with VZV should remain in respiratory isolation until all lesions have crusted over.6
Individuals who had face-to-face indoor contact for at least 5 minutes or who shared a living space with an infected individual should be assessed for VZV immunity, which is defined as confirmed prior immunization or infection.5,13 Lack of VZV immunity requires postexposure prophylaxis—active immunization for the immunocompetent and passive immunization for the immunocompromised.13 Ultimately, no additional cases were reported in the community where our patients resided.
Immunocompetent children with primary VZV require supportive care only. Oral antiviral therapy is the treatment of choice for immunocompetent adults or anyone at increased risk for complications, while intravenous antivirals are recommended for the immunocompromised or those with VZV-related complications.14 A similar approach is used for HZ. Uncomplicated cases are treated with oral antivirals, and complicated cases (eg, HZ ophthalmicus) are treated with intravenous antivirals.15 Commonly used antivirals include acyclovir, valacyclovir, and famciclovir.14
Our cases highlight the ongoing risk for varicella outbreaks in unvaccinated or undervaccinated communities. Physician vigilance is necessary, and dermatology plays a particularly important role in swift and accurate detection of VZV, as demonstrated in our cases by the recognition of classic physical examination findings of erythematous and vesicular papules in each of the patients. Because primary VZV infection can result in life-threatening complications including hepatitis, encephalitis, and pancreatitis, prompt identification and initiation of therapy is important.6 Similarly, quick notification of public health officials about detected primary VZV cases is vital to containing potential community outbreaks.
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
- Centers for Disease Control and Prevention. Chickenpox (varicella) for healthcare professionals. Published October 21, 2022. Accessed March 6, 2024. https://www.cdc.gov/chickenpox/hcp/index.html#vaccination-impact
- National Center for Health Statistics. Immunization. Published June 13, 2023. Accessed March 6, 2024. https://www.cdc.gov/nchs/fastats/immunize.htm
- Lee YH, Choe YJ, Lee J, et al. Global varicella vaccination programs. Clin Exp Pediatr. 2022;65:555. doi:10.3345/CEP.2021.01564
- Leung J, Lopez AS, Marin M. Changing epidemiology of varicella outbreaks in the United States during the Varicella Vaccination Program, 1995–2019. J Infect Dis. 2022;226(suppl 4):S400-S406.
- Arkansas Department of Health. Rules Pertaining to Reportable Diseases. Published September 11, 2023. Accessed March 6, 2024. https://www.healthy.arkansas.gov/images/uploads/rules/ReportableDiseaseList.pdf
- Pergam S, Limaye A; The AST Infectious Diseases Community of Practice. Varicella zoster virus (VZV). Am J Transplant. 2009;9(suppl 4):S108-S115. doi:10.1111/J.1600-9143.2009.02901.X
- Hoyt B, Bhawan J. Histological spectrum of cutaneous herpes infections. Am J Dermatopathol. 2014;36:609-619. doi:10.1097/DAD.0000000000000148
- Oumarou Hama H, Aboudharam G, Barbieri R, et al. Immunohistochemical diagnosis of human infectious diseases: a review. Diagn Pathol. 2022;17. doi:10.1186/S13000-022-01197-5
- World Health Organization. Mpox (monkeypox). Published April 18, 2023. Accessed March 7, 2024. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- Akram SM, Jamil RT, Gossman W. Rickettsia akari (Rickettsialpox). StatPearls [Internet]. Updated May 8, 2023. Accessed February 29, 2024. https://www.ncbi.nlm.nih.gov/books/NBK448081/
- Lott JP, Liu K, Landry ML, et al. Atypical hand-foot-mouth disease associated with coxsackievirus A6 infection. J Am Acad Dermatol. 2013;69:736. doi:10.1016/J.JAAD.2013.07.024
- Petrun B, Williams V, Brice S. Disseminated varicella-zoster virus in an immunocompetent adult. Dermatol Online J. 2015;21. doi:10.5070/D3213022343
- Kimberlin D, Barnett E, Lynfield R, et al. Exposure to specific pathogens. In: Red Book: 2021-2024 Report of the Committee of Infectious Disease. 32nd ed. American Academy of Pediatrics; 2021:1007-1009.
- Treatment of varicella (chickenpox) infection. UpToDate [Internet]. Updated February 7, 2024. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-varicella-chickenpox-infection
- Treatment of herpes zoster in the immunocompetent host. UpToDate [Internet]. Updated November 29, 2023. Accessed March 6, 2024. https://www.uptodate.com/contents/treatment-of-herpes-zoster
Practice Points
- Primary varicella is a relatively infrequent occurrence since the introduction of vaccination, creating the need for a reminder on the importance of including it in the differential when clinically appropriate.
- When outbreaks do happen, typically among unvaccinated communities, swift identification via physical examination and histology is imperative to allow infection control teams and public health officials to quickly take action.
Asymptomatic Erythematous Plaque in an Outdoorsman
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
The Diagnosis: Erythema Migrans
The patient was clinically diagnosed with erythema migrans. He did not recall a tick bite but spent a lot of time outdoors. He was treated with 10 days of doxycycline 100 mg twice daily with complete resolution of the rash.
Lyme disease is a spirochete infection caused by the Borrelia burgdorferi sensu lato species complex and transmitted by the Ixodidae tick family. It is the most common tick-borne disease in the United States and mostly is reported in the northeastern and upper midwestern states during the warmer seasons, but it is prevalent worldwide. In geographic areas where Lyme disease is common, the incidence is approximately 40 cases per 100,000 individuals.1 Our patient resided in coastal South Carolina. Lyme disease is more commonly reported in White individuals. The skin lesions may be more difficult to discern and diagnose in patients with darker skin types, leading to delayed diagnosis and treatment.2,3
Patients may be diagnosed with early localized, early disseminated, or late Lyme disease. Erythema migrans is the early localized form of the disease and is classically described as an erythematous targetlike plaque with raised borders arising at the site of the tick bite 1 to 2 weeks later.4 However, many patients simply have a homogeneous erythematous plaque with raised advancing borders ranging in size from 5 to 68 cm.5 In a 2022 study of 69 patients with suspected Lyme disease, only 35 (50.7%) were determined to truly have acute Lyme disease.6 Of them, only 2 (5.7%) had the classic ringwithin- a-ring pattern. Most plaques were uniform, pink, oval-shaped lesions with well-demarcated borders.6
The rash may present with a burning sensation, or patients may experience no symptoms at all, which can lead to delayed diagnosis and progression to late disease. Patients may develop malaise, fever, headache, body aches, or joint pain. Early disseminated disease manifests similarly. Patients with disseminated disease also may develop more serious complications, including lymphadenopathy; cranial nerve palsies; ocular involvement; meningitis; or cardiac abnormalities such as myocarditis, pericarditis, or arrhythmia. Late disease most often causes arthritis of the large joints, though it also can have cardiac or neurologic manifestations. Some patients with chronic disease—the majority of whom were diagnosed in Europe—may develop acrodermatitis chronica atrophicans with edematous blue-red plaques that become atrophic and hyperpigmented fibrotic plaques over the course of years.
Allergic contact dermatitis to a plant more likely would cause itchy or painful, oozy, weepy, vesicular lesions arranged in a linear pattern. A dermatophyte infection likely would cause a scaly eruption. Although our patient presented with a sharply demarcated, raised, erythematous lesion, the distribution did not follow normal clothing lines and would be unusual for a photosensitive drug eruption. Cellulitis likely would be associated with tenderness or warmth to the touch. Finally, southern tick-associated rash illness, which is associated with Amblyomma americanum (lone star tick) bites, may appear with a similar rash but few systemic symptoms. It also can be treated with tetracycline antibiotics.7
Our case in South Carolina demonstrates the importance of keeping Lyme disease in the differential. Clinicians should remember to ask patients about their travel history. In endemic areas, patients with erythema migrans can be started on treatment without waiting for serology. Patients with early Lyme disease may or may not have positive serologies at the time of presentation.6 Guidelines for the treatment of Lyme disease have been revised in recent years to decrease patient antibiotic exposure by reducing the number of days of antibiotic therapy.8 A recent randomized controlled trial found no significant difference in recurrence for patients treated with 7 days of doxycycline compared with 14 days.9 We typically prescribe a 10-day course of doxycycline, which also is adequate for concurrent rickettsial disease. Patients who develop malarialike symptoms should be evaluated for babesiosis, which is treated with clindamycin.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
- Skar GL, Simonsen KA. Lyme disease. StatPearls [Internet]. Updated February 4, 2024. Accessed March 20, 2024. https://www.ncbi.nlm.nih.gov/books/NBK431066/
- Dennison R, Novak C, Rebman A, et al. Lyme disease with erythema migrans and seventh nerve palsy in an African-American man. Cureus. 2019;11:E6509.
- Bax CE, Clark AK, Oboite M, et al. A case of disseminated Lyme disease in a child with skin of color. Pediatr Dermatol. 2021;38 (suppl 2):140-141.
- Shah AS, Varatharaj Palraj BR. Multiple erythema migrans rashes characteristic of early disseminated lyme disease, before and after therapy. Mayo Clin Proc. 2019;94:172-173.
- Feder HM Jr, Abeles M, Bernstein M, et al. Diagnosis, treatment, and prognosis of erythema migrans and Lyme arthritis. Clin Dermatol. 2006;24:509-520.
- Schotthoefer AM, Green CB, Dempsey G, et al. The spectrum of erythema migrans in early Lyme disease: can we improve its recognition? Cureus. 2022;14:E30673.
- Strle F, Wormser GP. Early Lyme disease (erythema migrans) and its mimics (southern tick-associated rash illness and tick-associated rash illness). Infect Dis Clin North Am. 2022;36:523-539.
- Torbahn G, Hofmann H, Rücker G, et al. Efficacy and safety of antibiotic therapy in early cutaneous Lyme borreliosis: a network meta-analysis. JAMA Dermatol. 2018;154:1292-1303.
- Stupica D, Collinet-Adler S, Blagus R, et al. Treatment of erythema migrans with doxycycline for 7 days versus 14 days in Slovenia: a randomised open-label non-inferiority trial. Lancet Infect Dis. 2023;23:371-379.
A middle-aged man presented with a well-demarcated, hyperpigmented, erythematous patch with an annular erythematous border that extended from the mid-back to the lower back. The patient was otherwise asymptomatic. He was an avid gardener who resided in South Carolina and had recently adopted 2 puppies.
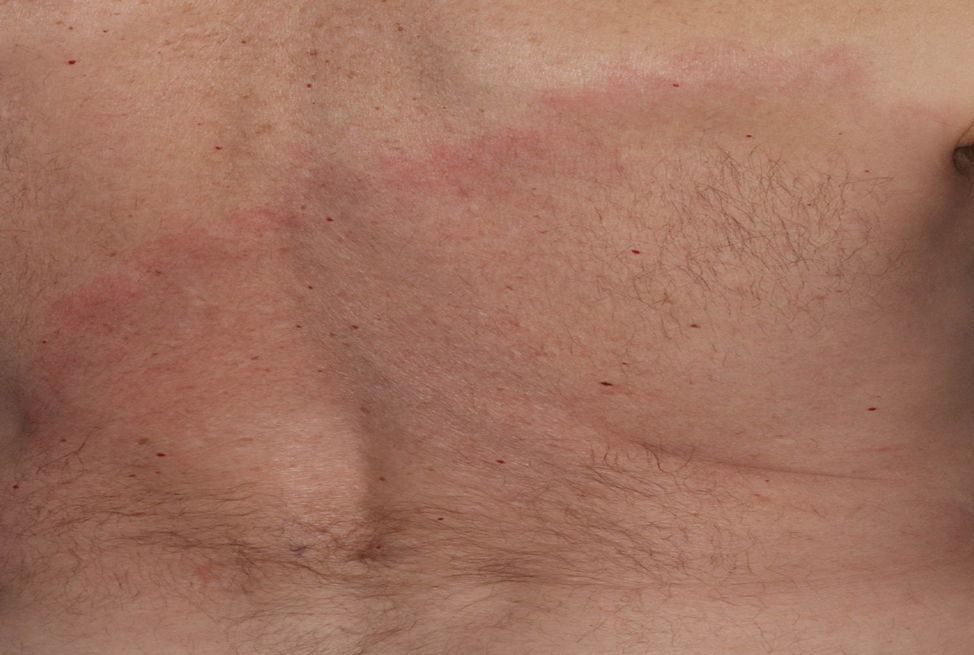
DermGPT Can Help Improve Your Office Productivity
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
For anyone (physicians included) concerned about whether generative artificial intelligence (AI) tools will take your job, the likely answer is no—but those who use generative AI will have an advantage, according to Faranak (Fara) Kamangar, MD, Department Chair, Palo Alto Medical Foundation, who presented on AI at the 2024 Annual Meeting of the American Academy of Dermatology, San Diego, California.
Dr. Kamangar is a dermatologist and inventor of DermGPT, an AI tool created specifically to help health care providers with clinical tasks to increase productivity. According to Dr. Kamangar, “For every 8 hours of scheduled patient time, ambulatory physicians spend more than 5 hours on the [electronic health record].” Her advice is to use AI when you can to complete clinical tasks and move on.
DermGPT utilizes a learned language model that is based on dermatology knowledge acquired from more than 3000 peer-reviewed articles and texts (eg, systematic literature reviews, other published sources in the field of dermatology). Search output includes citations so that users can confirm that the answers and sources are accurate. “Still, with all of these safeguards, all AI models can create inaccuracies and it is important to proofread all content,” says Dr. Kamangar.
During her presentation, Dr. Kamangar gave the following examples of potentially useful DermGPT prompts for dermatologists:
- Can you help me write a response to a denial letter to an insurance company for upadacitinib in a patient with atopic dermatitis?
- I am seeing a patient with blisters. What is the differential diagnosis?
- I am prescribing bimekizumab. What labs do I need to check?
Other potential time-saving uses for a dermatologist include:
- prior authorization letters
- coding support
- responses to common patient messages
- letters of recommendation
- information on new treatments.
In Dr. Kamangar’s practice, they have been able to save at least 30 to 60 minutes at the end of the day that is usually spent updating the electronic health record. “This allows us to complete the clinic day much earlier and does not leave work that will spill over to the next day,” she shares. “During my workday, I have [DermGPT] open on my computer, and as clinical tasks arise, if they require more information or assistance, I turn to DermGPT to help de-escalate the task from a moderate to difficult level to an easy task that can be easily managed.”
The next stage of health technology—AI—is here, and physicians are understandably cautious. Board-certified dermatologists, dermatology residents, fellows, and medical students can try DermGPT for free at https://www.dermgpt.com/.
Dr. Kamangar is the founder of DermGPT.
Melissa Sears is the Director, Editorial, of Cutis.
Skin Lesions on the Face and Chest
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.
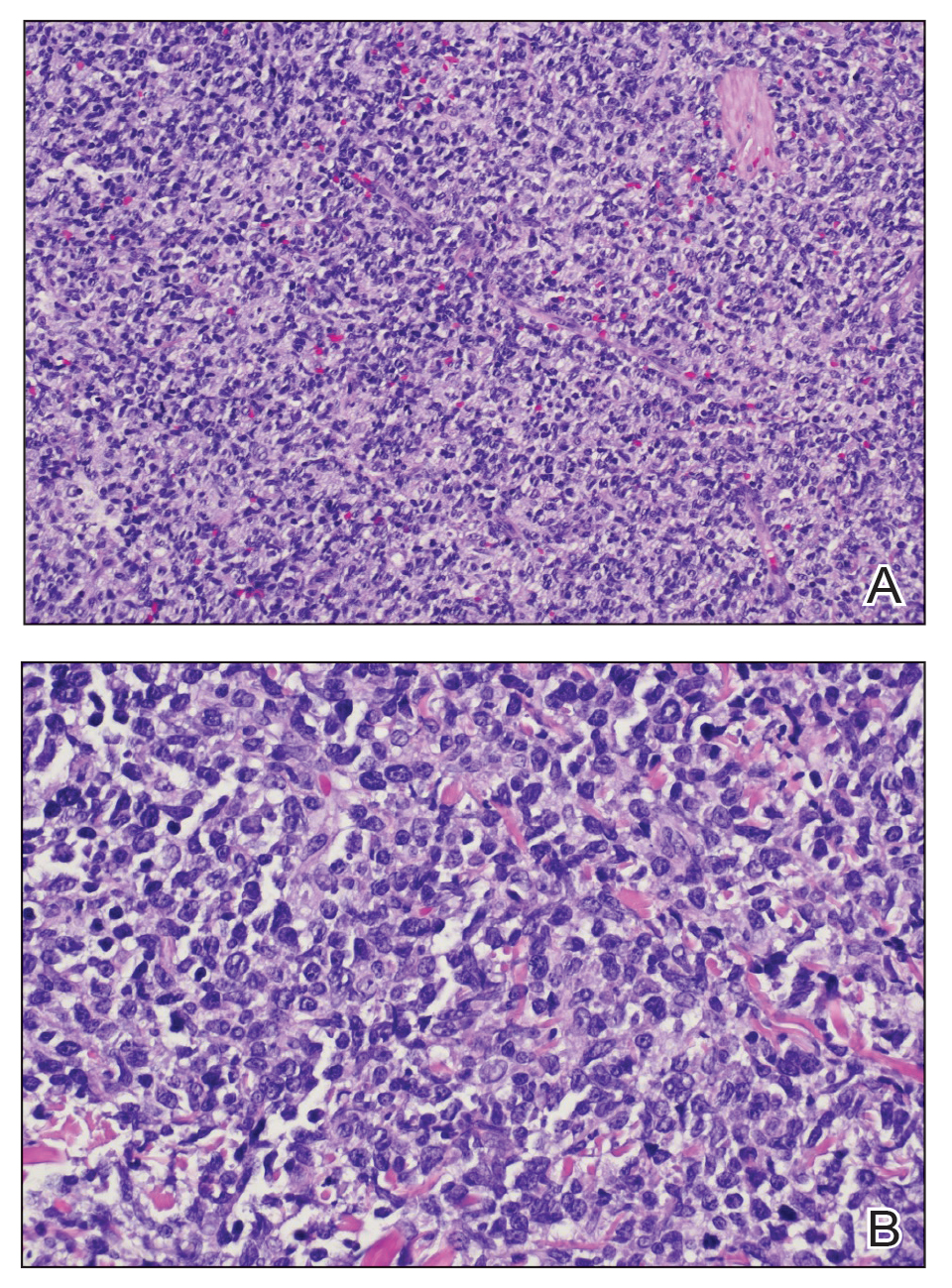
Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
The Diagnosis: Blastic Plasmacytoid Dendritic Cell Neoplasm
Cutaneous plasmacytoma initially was suspected because of the patient’s history of monoclonal gammopathy as well as angiosarcoma due to the purpuric vascular appearance of the lesions. However, histopathology revealed a pleomorphic cellular dermal infiltrate characterized by atypical cells with mediumlarge nuclei, fine chromatin, and small nucleoli; the cells also had little cytoplasm (Figure). The infiltrate did not involve the epidermis but extended into the subcutaneous tissue. Immunohistochemistry revealed that the cells were positive for CD45, CD43, CD4, CD7, CD56, CD123, CD33, T-cell leukemia/lymphoma protein 1, and CD68. The cells were negative for CD2, CD3, CD5, CD8, T-cell intracellular antigen 1, CD13, CD15, CD19, CD20, CD21, CD23, cyclin D1, Bcl-2, Bcl-6, CD10, PAX5, MUM1, lysozyme, myeloperoxidase, perforin, granzyme B, CD57, CD34, CD117, terminal deoxynucleotidyl transferase, activin receptorlike kinase 1 βF1, Epstein-Barr virus– encoded small RNA, CD30, CD163, and pancytokeratin. Thus, the clinical and histopathologic findings led to a diagnosis of blastic plasmacytoid dendritic cell neoplasm (BPDCN), a rare and aggressive hematologic malignancy.

Blastic plasmacytoid dendritic cell neoplasm affects males older than 60 years.1 It is characterized by the clonal proliferation of precursor plasmacytoid dendritic cells—otherwise known as professional type I interferonproducing cells or plasmacytoid monocytes—of myeloid origin. Plasmacytoid dendritic cells have been renamed on several occasions, reflecting uncertainties of their histogenesis. The diagnosis of BPDCN requires a biopsy showing the morphology of plasmacytoid dendritic blast cells and immunophenotypic criteria established by either immunohistochemistry or flow cytometry.2,3 Tumor cells morphologically show an immature blastic appearance, and the diagnosis rests upon the demonstration of CD4 and CD56, together with markers more restricted to plasmacytoid dendritic cells (eg, BDCA-2, CD123, T-cell leukemia/lymphoma protein 1, CD2AP, BCL11A) and negativity for lymphoid and myeloid lineage–associated antigens.1,4
Blastic plasmacytoid dendritic cell neoplasms account for less than 1% of all hematopoietic neoplasms. Cutaneous lesions occur in 64% of patients with the disease and often are the reason patients seek medical care.5 Clinical findings include numerous erythematous and violaceous papules, nodules, and plaques that resemble purpura or vasculitis. Cutaneous lesions can vary in size from a few millimeters to 10 cm and vary in color. Moreover, patients often present with bruiselike patches, disseminated lesions, or mucosal lesions.1 Extracutaneous involvement includes lymphadenopathy, splenomegaly, and cytopenia caused by bone marrow infiltration, which may be present at diagnosis or during disease progression. Bone marrow involvement often is present with thrombocytopenia, anemia, and neutropenia. One-third of patients with BPDCN have central nervous system involvement and no disease relapse.6 Other affected sites include the liver, lungs, tonsils, soft tissues, and eyes. Patients with BPDCN may present with a history of myeloid neoplasms, such as acute/chronic myeloid leukemia, chronic myelomonocytic leukemia, or myelodysplastic syndrome.4 Our case highlights the importance of skin biopsy for making the correct diagnosis, as BPDCN manifests with cutaneous lesions that are nonspecific for neoplastic or nonneoplastic etiologies.
Given the aggressive nature of BPDCN, along with its potential for acute leukemic transformation, treatment has been challenging due to both poor response rates and lack of consensus and treatment strategies. Historically, patients who have received high-dose acute leukemia–based chemotherapy followed by an allogeneic stem cell transplant during the first remission appeared to have the best outcomes.7 Conventional treatments have included surgical excision with radiation and various leukemia-based chemotherapy regimens, with hyper- CVAD (fractionated cyclophosphamide, vincristine, doxorubicin, dexamethasone-methotrexate, and cytarabine) being the most commonly used regimen.7,8 Venetoclax, a B-cell lymphoma 2 protein inhibitor, has shown promise when used in combination with hyper-CVAD. For older patients who may not tolerate aggressive chemotherapy, hypomethylating agents are preferred for their tolerability. Although tagraxofusp, a CD123-directed cytotoxin, has been utilized, Sapienza et al9 demonstrated an association with capillary leak syndrome.
Leukemia cutis is characterized by infiltration of the skin by malignant leukocytes, often associated with a prior diagnosis of systemic leukemia or myelodysplasia. Extramedullary accumulation of leukemic cells typically is referred to as myeloid sarcoma, while leukemia cutis serves as a general term for specific skin involvement.10 In rare instances, cutaneous lesions may manifest as the initial sign of systemic disease.
Cutaneous T-cell lymphomas comprise a diverse group of non-Hodgkin lymphomas that manifest as malignant monoclonal T-lymphocyte infiltration in the skin. Mycosis fungoides, Sézary syndrome, and primary cutaneous peripheral T-cell lymphomas are among the key subtypes. Histologically, differentiating these conditions from benign inflammatory disorders can be challenging due to subtle features such as haloed lymphocytes, epidermotropism, and Pautrier microabscesses seen in mycosis fungoides.11
Multiple myeloma involves monoclonal plasma cell proliferation, primarily affecting bone and bone marrow. Extramedullary plasmacytomas can occur outside these sites through hematogenous spread or adjacent infiltration, while metastatic plasmacytomas result from metastasis. Cutaneous plasmacytomas may arise from hematogenous dissemination or infiltration from neighboring structures.12
Extranodal natural killer/T-cell lymphoma, nasal type, manifests as aggressive mid-facial necrotizing lesions with extranodal involvement, notably in the nasal/paranasal area. These lesions can cause local destruction of cartilage, bone, and soft tissues and may progress through stages or arise de novo. Diagnostic challenges arise from the historical variety of terms used to describe extranodal natural killer/T-cell lymphoma, including midline lethal granuloma and lymphomatoid granulomatosis.13
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
- Cheng W, Yu TT, Tang AP, et al. Blastic plasmacytoid dendritic cell neoplasm: progress in cell origin, molecular biology, diagnostic criteria and therapeutic approaches. Curr Med Sci. 2021;41:405-419. doi:10.1007/s11596-021-2393-3
- Chang HJ, Lee MD, Yi HG, et al. A case of blastic plasmacytoid dendritic cell neoplasm initially mimicking cutaneous lupus erythematosus. Cancer Res Treat. 2010;42:239-243. doi:10.4143/crt.2010.42.4.239
- Garnache-Ottou F, Vidal C, Biichlé S, et al. How should we diagnose and treat blastic plasmacytoid dendritic cell neoplasm patients? Blood Adv. 2019;3:4238-4251. doi:10.1182/bloodadvances.2019000647
- Sweet K. Blastic plasmacytoid dendritic cell neoplasm. Curr Opin Hematol. 2020;27:103-107. doi:10.1097/moh.0000000000000569
- Julia F, Petrella T, Beylot-Barry M, et al. Blastic plasmacytoid dendritic cell neoplasm: clinical features in 90 patients. Br J Dermatol. 2013;169:579-586. doi:10.1111/bjd.12412
- Molina Castro D, Perilla Suárez O, Cuervo-Sierra J, et al. Blastic plasmacytoid dendritic cell neoplasm with central nervous system involvement: a case report. Cureus. 2022;14:e23888. doi:10.7759 /cureus.23888
- Grushchak S, Joy C, Gray A, et al. Novel treatment of blastic plasmacytoid dendritic cell neoplasm: a case report. Medicine (Baltimore). 2017;96:E9452.
- Lim MS, Lemmert K, Enjeti A. Blastic plasmacytoid dendritic cell neoplasm (BPDCN): a rare entity. BMJ Case Rep. 2016;2016:bcr2015214093. doi:10.1136/bcr-2015-214093
- Sapienza MR, Pileri A, Derenzini E, et al. Blastic plasmacytoid dendritic cell neoplasm: state of the art and prospects. Cancers (Basel). 2019;11:595. doi:10.3390/cancers11050595
- Wang CX, Pusic I, Anadkat MJ. Association of leukemia cutis with survival in acute myeloid leukemia. JAMA Dermatol. 2019;155:826. doi:10.1001/jamadermatol.2019.0052
- Ralfkiaer U, Hagedorn PH, Bangsgaard N, et al. Diagnostic micro RNA profiling in cutaneous T-cell lymphoma (CTCL). Blood. 2011;118: 5891-5900. doi:10.1182/blood-2011-06-358382
- Tsang DS, Le LW, Kukreti V. Treatment and outcomes for primary cutaneous extramedullary plasmacytoma: a case series. Curr Oncol. 2016;23:630-646. doi:10.3747/co.23.3288
- Lee J, Kim W, Park Y, et al. Nasal-type NK/T cell lymphoma: clinical features and treatment outcome. Br J Cancer. 2005;92:1226-1230. doi:10.1038/sj.bjc.6602502
A 79-year-old man presented to the dermatology clinic with multiple skin lesions of 4 months’ duration. The patient had a history of monoclonal gammopathy and reported no changes in medication, travel, or trauma. He reported tenderness only when trying to comb hair over the left occipital nodule. He denied fevers, night sweats, weight loss, or poor appetite. Physical examination revealed 4 concerning skin lesions: a 3×3-cm violaceous nodule with underlying ecchymosis on the right medial jaw (top), a 3×2.5-cm violaceous nodule on the posterior occiput, a pink plaque with 1-mm vascular papules on the right mid-chest (bottom), and a 4×2.5-cm oval pink patch on the left side of the lower back. Punch biopsies were performed on the right medial jaw nodule and right mid-chest plaque.