User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Rituximab Treatment and Improvement of Health-Related Quality of Life in Patients With Pemphigus
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
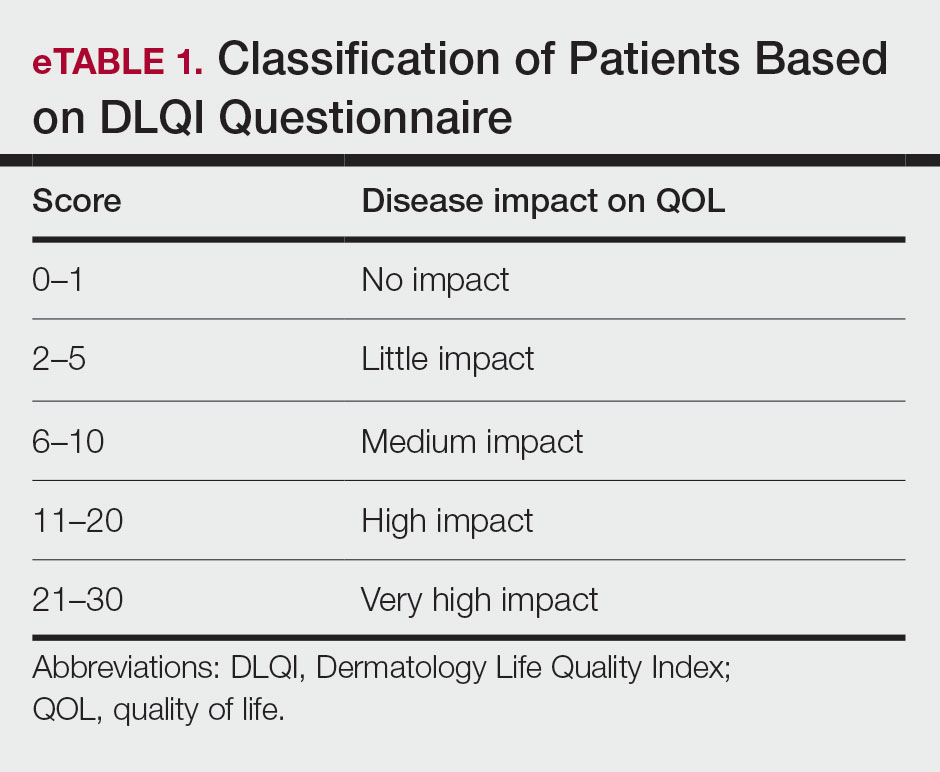
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.
Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
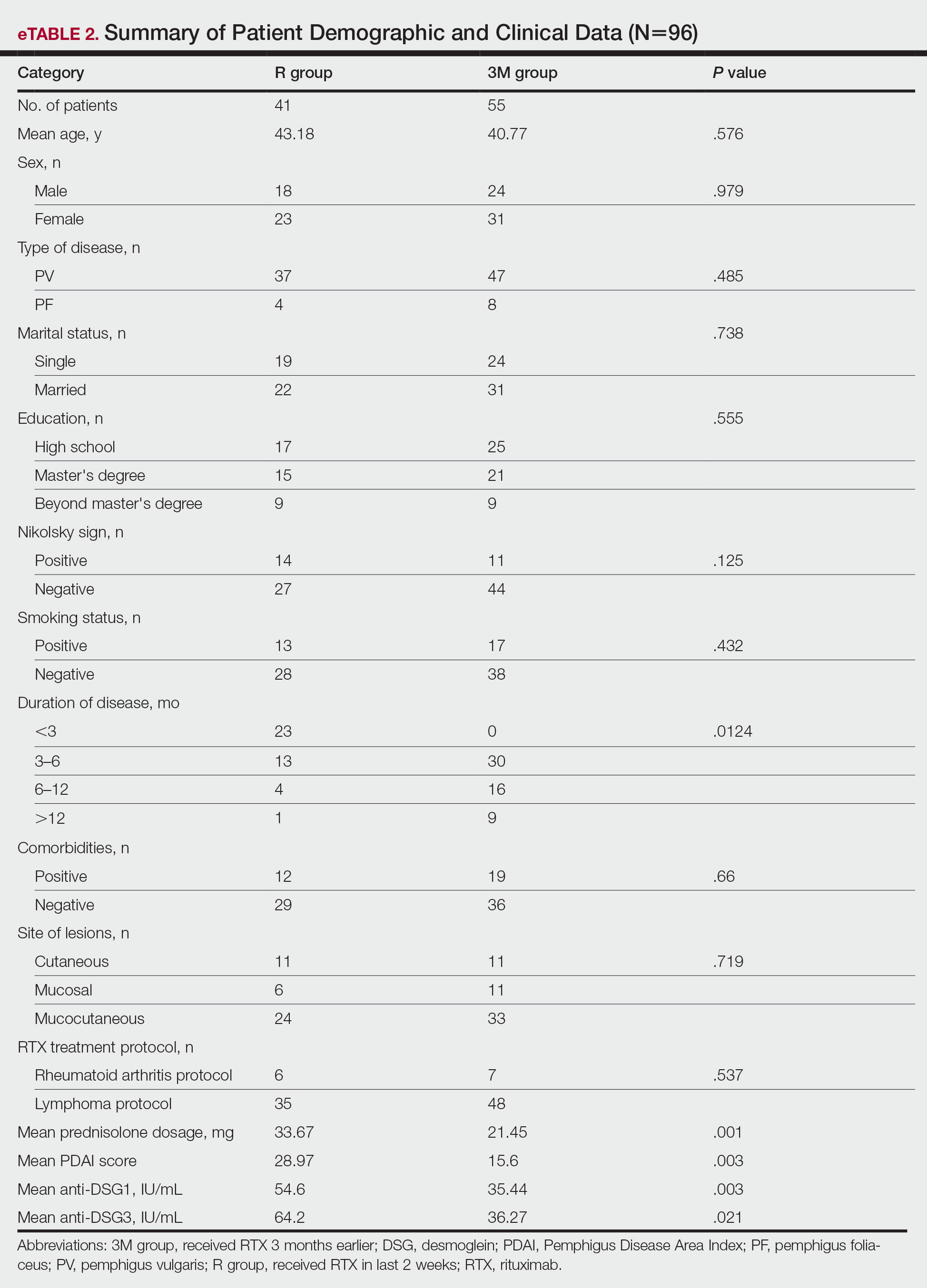
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.
Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).
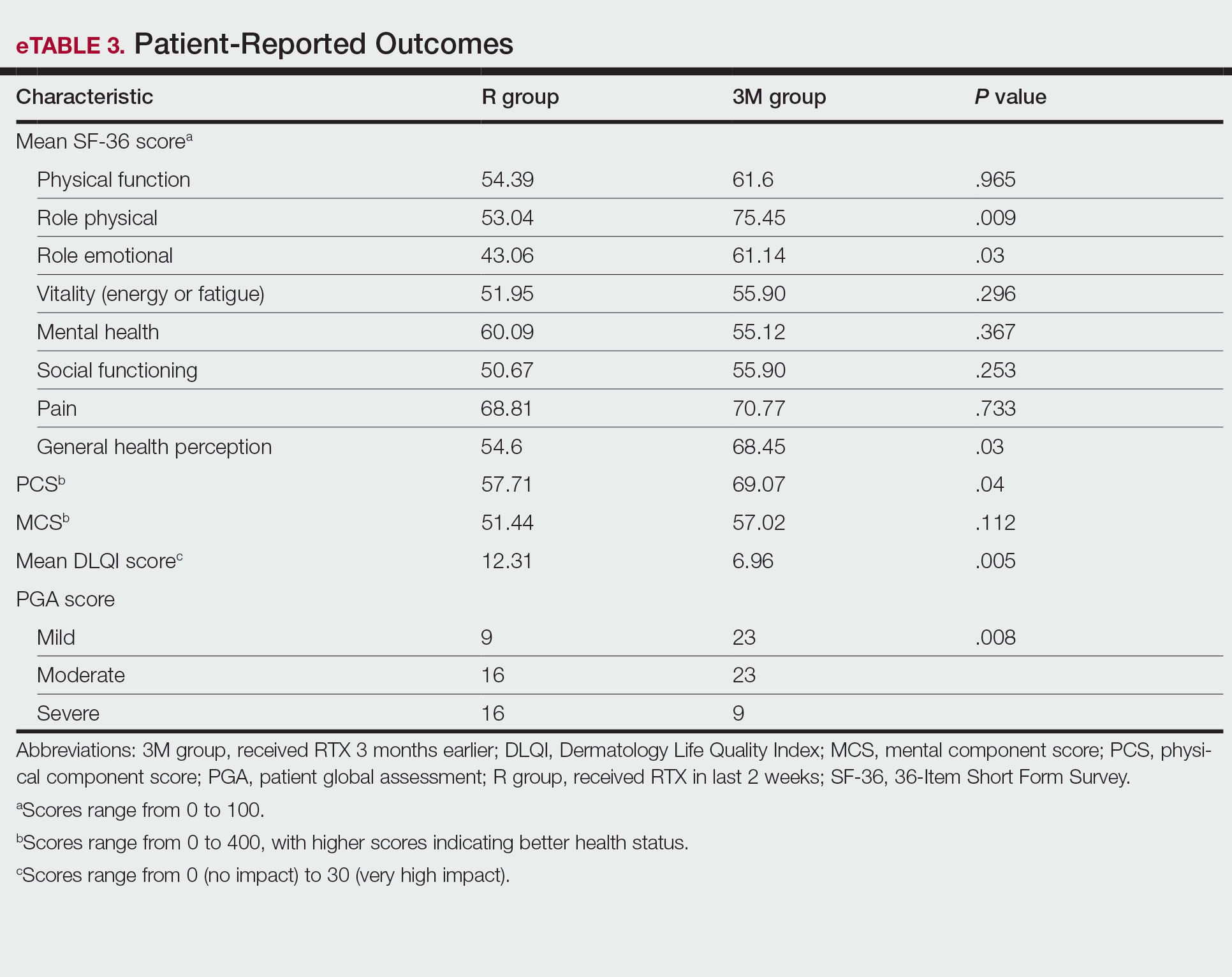
Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
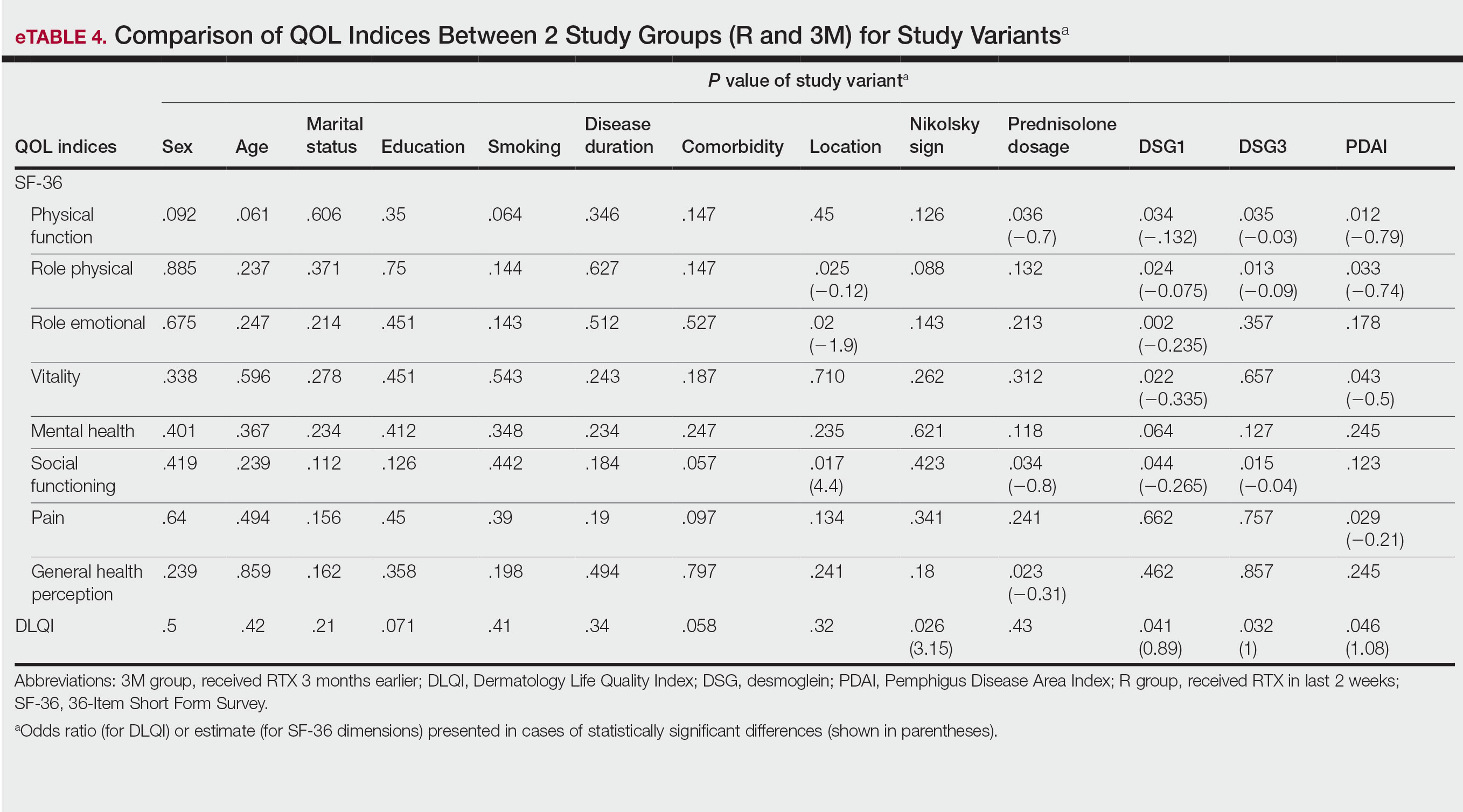
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.
COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
Pemphigus is a group of autoimmune blistering diseases characterized by the development of painful and flaccid blisters on the skin and/or mucous membranes. Pemphigus vulgaris (PV) and pemphigus foliaceus (PF) are 2 major subtypes and can be distinguished by the location of blister formation or the specificity of autoantibodies directed against different desmogleins.1,2 Although rare, pemphigus is considered a serious and life-threatening condition with a great impact on quality of life (QOL) due to disease symptoms (eg, painful lesions, physical appearance of skin lesions) as well as treatment complications (eg, adverse drug effects, cost of treatment).3-6 Moreover, the physical and psychological effects can lead to marked functional morbidity and work-related disability during patients’ productive years.7 Therefore, affected individuals usually have a remarkably compromised health-related quality of life (HRQOL).8 Effective treatments may considerably improve the QOL of patients with pemphigus.6
Despite the available treatment options, finding the best regimen for pemphigus remains a challenge. Corticosteroids are assumed to be the main treatment, though they have considerable side effects.9,10 Adjuvant therapies are used to suppress or modulate immune responses, leading to remission with the least possible need for corticosteroids. Finding an optimal steroid-sparing agent has been the aim of research, and biologic agents seem to be the best option.8 Rituximab (RTX), an anti-CD20 monoclonal antibody, has shown great promise in several studies of its clinical efficacy and has become a first-line treatment in new guidelines.11-14 Rituximab treatment has been associated with notable improvement in physician-assessed outcome measures with a favorable safety profile in patients with pemphigus.11-15 However, it is important to assess response to treatment from a patient’s perspective through the use of outcome-assessment measures that encompass patient-reported outcomes to reflect the complete patient experience and establish the overall impact of RTX as well as its likelihood of acceptance by patients with pemphigus.
In our study, we compared clinical outcomes and HRQOL through the use of disease-specific measures as well as comprehensive generic health status measures among patients with PV and PF who received RTX treatment 3 months earlier and those who received RTX in the last 2 weeks. The clinical relevance of the patient-reported outcomes is discussed.
MATERIALS AND METHODS
Study Design
We conducted a single-center cross-sectional study of 96 patients with pemphigus aged 18 to 65 years of either sex who were willing to participate in this study. Patients with a confirmed diagnosis of PV or PF who received RTX 3 months earlier or in the last 2 weeks were enrolled in the study. Patients were identified using Dermatry.ir, an archiving software that contains patients’ medical data. Exclusion criteria included lack of sufficient knowledge of the concepts of the questionnaires as well as age younger than 16 years. The study was conducted from October 2019 to April 2020 by the Autoimmune Bullous Disease Research Center at Razi Hospital in Tehran, Iran, which is the main dermatology-specific center and teaching hospital of Iran. The study protocol was approved by the relevant ethics committee.
Patients were categorized into 2 groups: (1) those who received RTX 3 months earlier (3M group); and (2) those who received RTX in the last 2 weeks (R group).
After an explanation of the study to participants, informed written consent was signed by each patient, and their personal data (eg, age, sex, education, marital status, smoking status), as well as clinical data (eg, type of pemphigus, duration of disease, site of onset, prednisolone dosage, presence of Nikolsky sign, anti-DSG1 and anti-DSG3 values, Pemphigus Disease Area Index [PDAI] score, RTX treatment protocol); any known comorbidities such as hypertension, diabetes mellitus, or morbid obesity; and any chronic pulmonary, cardiac, endocrinologic, renal, or hepatic condition, were collected and recorded in a predefined Case Record.
Patient-Reported Outcome Measures
The effect of RTX on QOL in patients with pemphigus was assessed using 2 HRQOL instruments: (1) a general health status indicator, the 36-Item Short Form Survey (SF-36), and (2) a validated, Persian version of a dermatology-specific questionnaire, Dermatology Life Quality Index (DLQI). The questionnaires were completed by each patient or by an assistant if needed.
The SF-36 is a widely used 36-item questionnaire measuring functional health and well-being across 8 domains—mental health, pain, physical function, role emotional, role physical, social functioning, vitality, and general health perception—with scores for each ranging from 0 to 100. The physical component scores (PCSs) and mental component scores (MCSs) were derived from these 8 subscales, each ranging from 0 to 400, with higher scores indicating better health status.6
The DLQI, one of the most frequently used QOL measures in dermatology, contains 10 questions, each referring to the prior week and classified in the following 6 subscales: symptoms and feelings, daily activities, leisure, personal relationships, work and school, and treatment.16 The total score ranges from 0 (no impact) to 30 (very high impact), with a higher score indicating a lower QOL (eTable 1). The minimal clinically important difference (MCD) for the DLQI was considered to be 2- to 5-point changes in prior studies.17,18 In this study, we used an MCD of a 5-point change or more between study groups.

Moreover, the patient general assessment (PGA) of disease severity was identified using a 3-point scale (1=mild, 2=moderate, 3=severe).
Statistical Analysis
Data were analyzed using SPSS Statistics version 23. P≤.05 was considered significant. Mean and SD were calculated for descriptive data. The t test, Fisher exact test, analysis of variance, multiple regression analysis, and logistic regression analysis were used to identify the relationship between variables.
RESULTS
Patient Characteristics
A total of 96 patients were enrolled in this study. The mean (SD) age of participants was 41.42 (15.1) years (range, 18–58 years). Of 96 patients whose data were included, 55 (57.29%) patients had received RTX 3 months earlier (3M group) and 41 (42.71%) received RTX in the last 2 weeks (R group). A summary of study patient characteristics in each group is provided in eTable 2. There was no significant difference between the 2 groups in terms of age, sex, type of pemphigus, marital status, education, positive Nikolsky sign, smoking status, existence of comorbidities, site of lesions, and RTX treatment protocol. However, a significant difference was found for duration of disease (P=.0124) and mean prednisolone dosage (P=.001) as well as severity of disease measured by PDAI score (P=.003) and anti-DSG1 (P=.003) and anti-DSG3 (P=.021) values.

Patient-Reported Outcomes
Physical and mental component scores are summarized in eTable 3. Generally, SF-36 scores were improved with RTX treatment in all dimensions except for mental health, though these differences were not statistically significant, with the greatest mean improvement in the role physical index (75.45 in the 3M group vs 53.04 in the R group; P=.009). Mean SF-36 PCS and MCS scores were higher in the 3M group vs the R group, though the difference in MCS score did not reach the level of significance (eTable 3).

Mean DLQI scores in the R and 3M groups were 12.31 and 6.96, respectively, indicating a considerable burden on HRQOL in both groups. However, a statistically significant difference between these values was seen that also was clinically meaningful, indicating a significant improvement of QOL in patients receiving RTX 3 months earlier (P=.005)(eTable 3).
The PGA scores indicated that patients in the 3M group were significantly more likely to report less severe disease vs the R group (P=.008)(eTable 3).
Multivariate Analysis—Effect of the patient characteristics and some disease features on indices of QOL was evaluated using the multiple linear regression model. eTable 4 shows the P values of those analyses.

COMMENT
Pemphigus is a chronic disabling disease with notable QOL impairment due to disease burden as well as the need for long-term use of immunosuppressive agents during the disease course. To study the effect of RTX on QOL of patients with pemphigus, we compared 2 sets of patients. Prior studies have shown that clinically significant effects of RTX take 4 to 12 weeks to appear.19,20 Therefore, we selected patients who received RTX 3 months earlier to measure their HRQOL indices and compare them with patients who had received RTX in the last 2 weeks as a control group to investigate the effect of RTX intrinsically, as this was the focus of this study.
In our study, one of the research tools was the DLQI. Healthy patients typically have an average score of 0.5.21 The mean DLQI score of the patients in R group was 12.31, which was similar to prior analysis8 and reflects a substantial burden of disease comparable to atopic dermatitis and psoriasis.21,22 In patients in the 3M group, the mean DLQI score was lower than the R group (6.96 vs 12.31), indicating a significant (P=.005) and clinically meaningful improvement in QOL of patients due to the dramatic therapeutic effect of RTX. However, this score indicated a moderate effect on HRQOL, even in the context of clinical improvement due to RTX treatment, which may reflect that the short duration of treatment in the 3M group was a limitation of this study. Although the 12-week treatment duration was comparable with other studies19,20 and major differences in objective measures of treatment efficacy were found in PDAI as well as anti-DSG1 and anti-DSG3 values, longer treatment duration may be needed for a more comprehensive assessment of the benefit of RTX on HRQOL indices in patients with pemphigus.
Based on results of the SF-36 questionnaire, PCS and MCS scores were not substantially impaired in the R group considering the fact that a mean score of 50 has been articulated as a normative value for all scales.23 These data demonstrated the importance of using a dermatologic-specific instrument such as the DLQI instead of a general questionnaire to assess QOL in patients with pemphigus. However, better indices were reported with RTX treatment in the 3 SF-36 domains—role physical (P=.009), role emotional (P=.03), and general health perception (P=.03)—with the role physical showing the greatest magnitude of mean change (75.45 in the 3M group vs 53.04 in the R group). Notably, PCS was impaired to a greater extent than MCS in patients in the R group and showed a greater magnitude of improvement after 3 months of treatment. These results could be explained by the fact that MCS can be largely changed in diseases with a direct effect on the central nervous system.23
Our results also revealed that the dose of corticosteroid correlated to HRQOL of patients with pemphigus who recently received RTX therapy. Indeed, it is more likely that patients on lower-dose prednisolone have a higher QOL, especially on physical function and social function dimensions of SF-36. This finding is highly expectable by less severe disease due to RTX treatment and also lower potential dose-dependent adverse effects of long-term steroid therapy.
One of the most striking findings of this study was the correlation of location of lesions to QOL indices. We found that the mucocutaneous phenotype was significantly correlated to greater improvement in role emotional, role physical, and social functioning scores due to RTX treatment compared with cutaneous or mucosal types (P=.02, P=.025, and P=.017, respectively). Although mucosal involvement of the disease can be the most burdensome feature because of its large impact on essential activities such as eating and speaking, cutaneous lesions with unpleasant appearance and undesirable symptoms may have a similar impact on QOL. Therefore, having both mucosal and cutaneous lesions causes a worsened QOL and decreased treatment efficacy vs having only one area involved. This may explain the greater improvement in some QOL indices with RTX treatment.
Limitations—Given the cross-sectional design of this study in which patients were observed at a single time point during their treatment course, it is not possible to establish a clear cause-effect relationship between variables. Moreover, we did not evaluate the impact of RTX or prednisolone adverse effects on QOL. Therefore, further prospective studies with longer treatment durations may help to validate our findings. In addition, MCDs for DLQI and SF-36 in pemphigus need to be determined and validated in future studies.
CONCLUSION
The results of our study demonstrated that patients with pemphigus may benefit from taking RTX, not only in terms of clinical improvement of their disease measured by objective indices such as PDAI and anti-DSG1 and anti-DSG3 values but also in several domains that are important to patients, including physical and mental health status (SF-36), HRQOL (DLQI), and overall disease severity (PGA). Rituximab administration in patients with pemphigus can lead to rapid and significant improvement in HRQOL as well as patient- and physician-assessed measures. Its favorable safety profile along with its impact on patients’ daily lives and mental health makes RTX a suitable treatment option for patients with pemphigus. Moreover, we recommend taking QOL indices into account while evaluating the efficacy of new medications to improve our insight into the patient experience and provide better patient adherence to treatment, which is an important issue for optimal control of chronic disorders.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
- Hammers CM, Stanley JR. Mechanisms of disease: pemphigus and bullous pemphigoid. Ann Rev Pathol. 2016;11:175-197.
- Kasperkiewicz M, Ellebrecht CT, Takahashi H, et al. Pemphigus. Nat Rev Dis Primers. 2017;3:17026.
- Mayrshofer F, Hertl M, Sinkgraven R, et al. Significant decrease in quality of life in patients with pemphigus vulgaris, result from the German Bullous Skin Disease (BSD) Study Group. J Dtsch Dermatol Ges. 2005;3:431-435.
- Terrab Z, Benckikhi H, Maaroufi A, et al. Quality of life and pemphigus. Ann Dermatol Venereol. 2005;132:321-328.
- Tabolli S, Mozzetta A, Antinone V, et al. The health impact of pemphigus vulgaris and pemphigus foliaceus assessed using the Medical Outcomes Study 36-item short form health survey questionnaire. Br J Dermatol. 2008;158:1029-1034.
- Paradisi A, Sampogna F, Di Pietro, C, et al. Quality-of-life assessment in patients with pemphigus using a minimum set of evaluation tools. J Am Acad Dermatol. 2009;60:261-269.
- Heelan K, Hitzig SL, Knowles S, et al. Loss of work productivity and quality of life in patients with autoimmune bullous dermatoses. J Cutan Med Surg. 2015;19:546-554.
- Ghodsi SZ, Chams-Davatchi C, Daneshpazhooh M, et al. Quality of life and psychological status of patients with pemphigus vulgaris using Dermatology Life Quality Index and General Health Questionnaires. J Dermatol. 2012;39:141-144.
- Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:2343.
- Mohammad-Javad N, Parvaneh H, Maryam G, et al. Randomized trial of tacrolimus 0.1% ointment versus triamcinolone acetonide 0.1% paste in the treatment of oral pemphigus vulgaris. Iranian J Dermatol. 2012;15:42-46.
- Lunardon L, Tsai KJ, Propert KJ, et al. Adjuvant rituximab therapy of pemphigus: a single-center experience with 31 patients. Arch Dermatol. 2012;148:1031-1036.
- Colliou N, Picard D, Caillot F, et al. Long-term remissions of severe pemphigus after rituximab therapy are associated with prolonged failure of desmoglein B cell response. Sci Transl Med. 2013;5:175ra30.
- Heelan K, Al-Mohammedi F, Smith MJ, et al. Durable remission of pemphigus with a fixed-dose rituximab protocol. JAMA Dermatol. 2014;150:703-708.
- Joly P, Maho-Vaillant M, Prost-Squarcioni C, et al. First-line rituximab combined with short-term prednisone versus prednisone alone for the treatment of pemphigus (Ritux3): a prospective, multicentre, parallel-group, open-label randomised trial. Lancet. 2017;389:2031-2040
- Aryanian Z, Balighi K, Daneshpazhooh M, et al. Rituximab exhibits a better safety profile when used as a first line of treatment for pemphigus vulgaris: a retrospective study. Int Immunopharmacol. 2021;96:107755.
- Aghai S, Sodaifi M, Jafari P, et al. DLQI scores in vitiligo: reliability and validity of the Persian version. BMC Dermatol. 2004;4:8.
- Schünemann HJ, Akl EA, Guyatt GH. Interpreting the results of patient reported outcome measures in clinical trials: the clinician’s perspective. Health Qual Life Outcomes. 2006;4:62.
- Quality of life questionnaires. Cardiff University website. Accessed December 16, 2022. http://sites.cardiff.ac.uk/dermatology/quality-oflife/dermatology-quality-of-life-index-dlqi/dlqi-instructions-foruse-and-scoring/
- Kanwar AJ, Tsuruta D, Vinay K, et al. Efficacy and safety of rituximab treatment in Indian pemphigus patients. J Eur Acad Dermatol Venereol. 2013;27:E17-E23.
- Ingen-Housz-Oro S, Valeyrie-Allanore L, Cosnes A, et al. First-line treatment of pemphigus vulgaris with a combination of rituximab and high-potency topical corticosteroids. JAMA Dermatol. 2015;151:200-203.
- Finlay AY, Khan GK. Dermatology Life Quality Index (DLQI): a simple practical measure for routine clinical use. Clin Exp Dermatol. 1994;19:210-216.
- Aghaei S, Moradi A, Ardekani GS. Impact of psoriasis on quality of life in Iran. Indian J Dermatol Venereol Leprol. 2009;75:220.
- Ware JE Jr, Sherbourne CD. The MOS 36-item Short-Form Health Survey (SF-36). 1. conceptual framework and item selection. Med Care. 1992;30:473-483.
PRACTICE POINTS
- Pemphigus is an autoimmune blistering disease that can negatively affect patients’ lives.
- Assessing the impact of treatment from a patient’s perspective using outcome assessment measures is important and relevant in trials of new pemphigus treatments including rituximab.
- Rituximab administration in pemphigus patients led to rapid and notable improvement in health-related quality of life and patient-assessed measures.
Cutaneous Manifestations in Hereditary Alpha Tryptasemia
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
Hereditary alpha tryptasemia (HaT), an autosomal-dominant disorder of tryptase overproduction, was first described in 2014 by Lyons et al.1 It has been associated with multiple dermatologic, allergic, gastrointestinal (GI) tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities. These multisystem concerns may include cutaneous flushing, chronic pruritus, urticaria, GI tract symptoms, arthralgia, and autonomic dysfunction.2 The diverse symptoms and the recent discovery of HaT make recognition of this disorder challenging. Currently, it also is believed that HaT is associated with an elevated risk for anaphylaxis and is a biomarker for severe symptoms in disorders with increased mast cell burden such as mastocytosis.3-5
Given the potential cutaneous manifestations and the fact that dermatologic symptoms may be the initial presentation of HaT, awareness and recognition of this condition by dermatologists are essential for diagnosis and treatment. This review summarizes the cutaneous presentations consistent with HaT and discusses various conditions that share overlapping dermatologic symptoms with HaT.
Background on HaT
Mast cells are known to secrete several vasoactive mediators including tryptase and histamine when activated by foreign substances, similar to IgE-mediated hypersensitivity reactions. In their baseline state, mast cells continuously secrete immature forms of tryptases called protryptases.6 These protryptases come in 2 forms: α and β. Although mature tryptase is acutely elevatedin anaphylaxis, persistently elevated total serum tryptase levels frequently are regarded as indicative of a systemic mast cell disorder such as systemic mastocytosis (SM).3 Despite the wide-ranging phenotype of HaT, all individuals with the disorder have an elevated basal serum tryptase level (>8 ng/mL). Hereditary alpha tryptasemia has been identified as another possible cause of persistently elevated levels.2,6
Genetics and Epidemiology of HaT—The humantryptase locus at chromosome 16p13.3 is composed of 4 paralog genes: TPSG1, TPSB2, TPSAB1, and TPSD1.4 Only TPSAB1 encodes for α-tryptase, while both TPSB2 and TPSAB1 encode for β-tryptase.4 Hereditary alpha tryptasemia is an autosomal-dominant disorder resulting from a copy number increase in the α-tryptase encoding sequence within the TPSAB1 gene. Despite the wide-ranging phenotype of HaT, all individuals identified with the disorder have a basal serum tryptase level greater than 8 ng/mL, with mean (SD) levels of 15 (5) ng/mL and 24 (6) ng/mL with gene duplication and triplication, respectively (reference range, 0–11.4 ng/mL).2,6 Hereditary alpha tryptasemia likely is common and largely undiagnosed, with a recently estimated prevalence of 5% in the United Kingdom7 and 5.6% in a cohort of 125 individuals from Italy, Slovenia, and the United States.5
Implications of Increased α-tryptase Levels—After an inciting stimulus, the active portions of α-protryptase and β-protryptase are secreted as tetramers by activated mast cells via degranulation. In vitro, β-tryptase homotetramers have been found to play a role in anaphylaxis, while α-homotetramers are nearly inactive.8,9 Recently, however, it has been discovered that α2β2 tetramers also can form and do so in a higher ratio in individuals with increased α-tryptase–encoding gene copies, such as those with HaT.8 These heterotetramers exhibit unique properties compared with the homotetramers and may stimulate epidermal growth factor–like module-containing mucinlike hormone receptor 2 and protease-activated receptor 2 (PAR2). Epidermal growth factor–like module-containing mucinlike hormone receptor 2 activation likely contributes to vibratory urticaria in patients, while activation of PAR2 may have a range of clinical effects, including worsening asthma, inflammatory bowel disease, pruritus, and the exacerbation of dermal inflammation and hyperalgesia.8,10 Thus, α- and β-tryptase tetramers can be considered mediators that may influence the severity of disorders in which mast cells are naturally prevalent and likely contribute to the phenotype of those with HaT.7 Furthermore, these characteristics have been shown to potentially increase in severity with increasing tryptase levels and with increased TPSAB1 duplications.1,2 In contrast, more than 25% of the population is deficient in α-tryptase without known deleterious effects.5
Cutaneous Manifestations of HaT
A case series reported by Lyons et al1 in 2014 detailed persistent elevated basal serum tryptase levels in 9 families with an autosomal-dominant pattern of inheritance. In this cohort, 31 of 33 (94%) affected individuals had a history of atopic dermatitis (AD), and 26 of 33 (79%) affected individuals reported symptoms consistent with mast cell degranulation, including urticaria; flushing; and/or crampy abdominal pain unprovoked or triggered by heat, exercise, vibration, stress, certain foods, or minor physical stimulation.1 A later report by Lyons et al2 in 2016 identified the TPSAB1 α-tryptase–encoding sequence copy number increase as the causative entity for HaT by examining a group of 96 patients from 35 families with frequent recurrent cutaneous flushing and pruritus, sometimes associated with urticaria and sleep disruption. Flushing and pruritus were found in 45% (33/73) of those with a TPSAB1 duplication and 80% (12/15) of those with a triplication (P=.022), suggesting a gene dose effect regarding α-tryptase encoding sequence copy number and these symptoms.2
A 2019 study further explored the clinical finding of urticaria in patients with HaT by specifically examining if vibration-induced urticaria was affected by TPSAB1 gene dosage.8 A cohort of 56 volunteers—35 healthy and 21 with HaT—underwent tryptase genotyping and cutaneous vibratory challenge. The presence of TPSAB1 was significantly correlated with induction of vibration-induced urticaria (P<.01), as the severity and prevalence of the urticarial response increased along with α- and β-tryptase gene ratios.8
Urticaria and angioedema also were seen in 51% (36/70) of patients in a cohort of HaT patients in the United Kingdom, in which 41% (29/70) also had skin flushing. In contrast to prior studies, these manifestations were not more common in patients with gene triplications or quintuplications than those with duplications.7 In another recent retrospective evaluation conducted at Brigham and Women’s Hospital (Boston, Massachusetts)(N=101), 80% of patients aged 4 to 85 years with confirmed diagnoses of HaT had skin manifestations such as urticaria, flushing, and pruritus.4
HaT and Mast Cell Activation Syndrome—In 2019, a Mast Cell Disorders Committee Work Group Report outlined recommendations for diagnosing and treating primary mast cell activation syndrome (MCAS), a disorder in which mast cells seem to be more easily activated. Mast cell activation syndrome is defined as a primary clinical condition in which there are episodic signs and symptoms of systemic anaphylaxis (Table) concurrently affecting at least 2 organ systems, resulting from secreted mast cell mediators.9,11 The 2019 report also touched on clinical criteria that lack precision for diagnosing MCAS yet are in use, including dermographism and several types of rashes.9 Episode triggers frequent in MCAS include hot water, alcohol, stress, exercise, infection, hormonal changes, and physical stimuli.

Hereditary alpha tryptasemia has been suggested to be a risk factor for MCAS, which also can be associated with SM and clonal MCAS.9 Patients with MCAS should be tested for increased α-tryptase gene copy number given the overlap in symptoms, the likely predisposition of those with HaT to develop MCAS, and the fact that these patients could be at an increased risk for anaphylaxis.4,7,9,11 However, the clinical phenotype for HaT includes allergic disorders affecting the skin as well as neuropsychiatric and connective tissue abnormalities that are distinctive from MCAS. Although HaT may be considered a heritable risk factor for MCAS, MCAS is only 1 potential phenotype associated with HaT.9
Implications of HaT
Hereditary alpha tryptasemia should be considered in all patients with basal tryptase levels greater than 8 ng/mL. Cutaneous symptoms are among the most common presentations for individuals with HaT and can include AD, chronic or episodic urticaria, pruritus, flushing, and angioedema. However, HaT is unique because of the coupling of these common dermatologic findings with other abnormalities, including abdominal pain and diarrhea, hypermobile joints, and autonomic dysfunction. Patients with HaT also may manifest psychiatric concerns of anxiety, depression, and chronic pain, all of which have been linked to this disorder.
It is unclear in HaT if the presence of extra-allelic copies of tryptase in an individual is directly pathogenic. The effects of increased basal tryptase and α2β2 tetramers have been shown to likely be responsible for some of the clinical features in these individuals but also may magnify other individual underlying disease(s) or diathesis in which mast cells are naturally abundant.8 In the skin, this increased mast cell activation and subsequent histamine release frequently are visible as dermatographia and urticaria. However, mast cell numbers also are known to be increased in both psoriatic and AD skin lesions,12 thus severe presentation of these diseases in conjunction with the other symptoms associated with mast cell activation should prompt suspicion for HaT.
Effects of HaT on Other Cutaneous Disease—Given the increase of mast cells in AD skin lesions and fact that 94% of patients in the 2014 Lyons et al1 study cited a history of AD, HaT may be a risk factor in the development of AD. Interestingly, in addition to the increased mast cells in AD lesions, PAR2+ nerve fibers also are increased in AD lesions and have been implicated in the nonhistaminergic pruritus experienced by patients with AD.12 Thus, given the proposed propensity for α2β2 tetramers to activate PAR2, it is possible this mechanism may contribute to severe pruritus in individuals with AD and concurrent HaT, as those with HaT express increased α2β2 tetramers. However, no study to date has directly compared AD symptoms in patients with concurrent HaT vs patients without it. Further research is needed on how HaT impacts other allergic and inflammatory skin diseases such as AD and psoriasis, but one may reasonably consider HaT when treating chronic inflammatory skin diseases refractory to typical interventions and/or severe presentations. Although HaT is an autosomal-dominant disorder, it is not detected by standard whole exome sequencing or microarrays. A commercial test is available, utilizing a buccal swab to test for TPSAB1 copy number.
HaT and Mast Cell Disorders—When evaluating someone with suspected HaT, it is important to screen for other symptoms of mast cell activation. For instance, in the GI tract increased mast cell activation results in activation of motor neurons and nociceptors and increases secretion and peristalsis with consequent bloating, abdominal pain, and diarrhea.10 Likewise, tryptase also has neuromodulatory effects that amplify the perception of pain and are likely responsible for the feelings of hyperalgesia reported in patients with HaT.13
There is substantial overlap in the clinical pictures of HaT and MCAS, and HaT is considered a heritable risk factor for MCAS. Consequently, any patient undergoing workup for MCAS also should be tested for HaT. Although HaT is associated with consistently elevated tryptase, MCAS is episodic in nature, and an increase in tryptase levels of at least 20% plus 2 ng/mL from baseline only in the presence of other symptoms reflective of mast cell activation (Table) is a prerequisite for diagnosis.9 Chronic signs and symptoms of atopy, chronic urticaria, and severe asthma are not indicative of MCAS but are frequently seen in HaT.
Another cause of persistently elevated tryptase levels is SM. Systemic mastocytosis is defined by aberrant clonal mast cell expansion and systemic involvement11 and can cause persistent symptoms, unlike MCAS alone. However, SM also can be associated with MCAS.9 Notably, a baseline serum tryptase level greater than 20 ng/mL—much higher than the threshold of greater than 8 ng/mL for suspicion of HaT—is seen in 75% of SM cases and is part of the minor diagnostic criteria for the disease.9,11 However, the 2016 study identifying increased TPSAB1 α-tryptase–encoding sequences as the causative entity for HaT by Lyons et al2 found the average (SD) basal serum tryptase level in individuals with α-tryptase–encoding sequence duplications to be 15 (5) ng/mL and 24 (6) ng/mL in those with triplications. Thus, there likely is no threshold for elevated baseline tryptase levels that would indicate SM over HaT as a more likely diagnosis. However, SM will present with new persistently elevated tryptase levels, whereas the elevation in HaT is believed to be lifelong.5 Also in contrast to HaT, SM can present with liver, spleen, and lymph node involvement; bone sclerosis; and cytopenia.11,14
Mastocytosis is much rarer than HaT, with an estimated prevalence of 9 cases per 100,000 individuals in the United States.11 Although HaT diagnostic testing is noninvasive, SM requires a bone marrow biopsy for definitive diagnosis. Given the likely much higher prevalence of HaT than SM and the patient burden of a bone marrow biopsy, HaT should be considered before proceeding with a bone marrow biopsy to evaluate for SM when a patient presents with persistent systemic symptoms of mast cell activation and elevated baseline tryptase levels. Furthermore, it also would be prudent to test for HaT in patients with known SM, as a cohort study by Lyons et al5 indicated that HaT is likely more common in those with SM (12.2% [10/82] of cohort with known SM vs 5.3% of 398 controls), and patients with concurrent SM and HaT were at a higher risk for severe anaphylaxis (RR=9.5; P=.007).
Studies thus far surrounding HaT have not evaluated timing of initial symptom onset or age of initial presentation for HaT. Furthermore, there is no guarantee that those with increased TPSAB1 copy number will be symptomatic, as there have been reports of asymptomatic individuals with HaT who had basal serum levels greater than 8 ng/mL.7 As research into HaT continues and larger cohorts are evaluated, questions surrounding timing of symptom onset and various factors that may make someone more likely to display a particular phenotype will be answered.
Treatment—Long-term prognosis for individuals with HaT is largely unknown. Unfortunately, there are limited data to support a single effective treatment strategy for managing HaT, and treatment has varied based on predominant symptoms. For cutaneous and GI tract symptoms, trials of maximal H1 and H2 antihistamines twice daily have been recommended.4 Omalizumab was reported to improve chronic urticaria in 3 of 3 patients, showing potential promise as a treatment.4 Mast cell stabilizers, such as oral cromolyn, have been used for severe GI symptoms, while some patients also have reported improvement with oral ketotifen.6 Other medications, such as tricyclic antidepressants, clemastine fumarate, and gabapentin, have been beneficial anecdotally.6 Given the lack of harmful effects seen in individuals who are α-tryptase deficient, α-tryptase inhibition is an intriguing target for future therapies.
Conclusion
Patients who present with a constellation of dermatologic, allergic, GI tract, neuropsychiatric, respiratory, autonomic, and connective tissue abnormalities consistent with HaT may receive a prompt diagnosis if the association is recognized. The full relationship between HaT and other chronic dermatologic disorders is still unknown. Ultimately, heightened interest and research into HaT will lead to more treatment options available for affected patients.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
1. Lyons JJ, Sun G, Stone KD, et al. Mendelian inheritance of elevated serum tryptase associated with atopy and connective tissue abnormalities. J Allergy Clin Immunol. 2014;133:1471-1474.
2. Lyons JJ, Yu X, Hughes JD, et al. Elevated basal serum tryptase identifies a multisystem disorder associated with increased TPSAB1 copy number. Nat Genet. 2016;48:1564-1569.
3. Schwartz L. Diagnostic value of tryptase in anaphylaxis and mastocytosis. Immunol Allergy Clin North Am. 2006;6:451-463.
4. Giannetti MP, Weller E, Bormans C, et al. Hereditary alpha-tryptasemia in 101 patients with mast cell activation–related symptomatology including anaphylaxis. Ann Allergy Asthma Immunol. 2021;126:655-660.
5. Lyons JJ, Chovanec J, O’Connell MP, et al. Heritable risk for severe anaphylaxis associated with increased α-tryptase–encoding germline copy number at TPSAB1. J Allergy Clin Immunol. 2020;147:622-632.
6. Lyons JJ. Hereditary alpha tryptasemia: genotyping and associated clinical features. Immunol Allergy Clin North Am. 2018;38:483-495.
7. Robey RC, Wilcock A, Bonin H, et al. Hereditary alpha-tryptasemia: UK prevalence and variability in disease expression. J Allergy Clin Immunol Pract. 2020;8:3549-3556.
8. Le QT, Lyons JJ, Naranjo AN, et al. Impact of naturally forming human α/β-tryptase heterotetramers in the pathogenesis of hereditary α-tryptasemia. J Exp Med. 2019;216:2348-2361.
9. Weiler CR, Austen KF, Akin C, et al. AAAAI Mast Cell Disorders Committee Work Group Report: mast cell activation syndrome (MCAS) diagnosis and management. J Allergy Clin Immunol. 2019;144:883-896.
10. Ramsay DB, Stephen S, Borum M, et al. Mast cells in gastrointestinal disease. Gastroenterol Hepatol (N Y). 2010;6:772-777.
11. Giannetti A, Filice E, Caffarelli C, et al. Mast cell activation disorders. Medicina (Kaunas). 2021;57:124.
12. Siiskonen H, Harvima I. Mast cells and sensory nerves contribute to neurogenic inflammation and pruritus in chronic skin inflammation. Front Cell Neurosci. 2019;13:422.
13. Varrassi G, Fusco M, Skaper SD, et al. A pharmacological rationale to reduce the incidence of opioid induced tolerance and hyperalgesia: a review. Pain Ther. 2018;7:59-75.
14. Núñez E, Moreno-Borque R, García-Montero A, et al. Serum tryptase monitoring in indolent systemic mastocytosis: association with disease features and patient outcome. PLoS One. 2013;8:E76116.
Practice Points
- Chronic or episodic urticaria, flushing, and pruritus are the most consistent cutaneous abnormalities associated with hereditary alpha tryptasemia (HaT), but HaT also may augment symptoms of other underlying inflammatory skin disorders, such as atopic dermatitis and psoriasis.
- Individuals with episodic dermatologic manifestations indicative of mast cell activation accompanied by symptoms affecting 1 or more organ systems should be evaluated for mast cell activation syndrome as well as HaT.
Hyperpigmented Papules on the Tongue of a Child
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
The Diagnosis: Pigmented Fungiform Papillae of the Tongue
Our patient’s hyperpigmentation was confined to the fungiform papillae, leading to a diagnosis of pigmented fungiform papillae of the tongue (PFPT). A biopsy was not performed, and reassurance was provided regarding the benign nature of this finding, which did not require treatment.
Pigmented fungiform papillae of the tongue is a benign, nonprogressive, asymptomatic pigmentary condition that is most common among patients with skin of color and typically develops within the second or third decade of life.1,2 The pathogenesis is unclear, but activation of subepithelial melanophages without evidence of inflammation has been implicated.2 Although no standard treatment exists, cosmetic improvement with the use of the Q-switched ruby laser has been reported.3,4 Clinically, PFPT presents as asymptomatic hyperpigmentation confined to the fungiform papillae along the anterior and lateral portions of the tongue.1,2
Pigmented fungiform papillae of the tongue typically is an isolated finding but rarely can be associated with hyperpigmentation of the nails (as in our patient) or gingiva.2 Three different clinical patterns of presentation have been described: (1) a single well-circumscribed collection of pigmented fungiform papillae, (2) few scattered pigmented fungiform papillae admixed with many nonpigmented fungiform papillae, or (3) pigmentation of all fungiform papillae on the dorsal aspect of the tongue.2,5,6 Pigmented fungiform papillae of the tongue is a clinical diagnosis based on visual recognition. Dermoscopic examination revealing a cobblestonelike or rose petal–like pattern may be helpful in diagnosing PFPT.2,5-7 Although not typically recommended in the evaluation of PFPT, a biopsy will reveal papillary structures with hyperpigmentation of basilar keratinocytes as well as melanophages in the lamina propria.8 The latter finding suggests a transient inflammatory process despite the hallmark absence of inflammation.5 Melanocytic neoplasia and exogenous granules of pigment typically are not seen.8
Other conditions that may present with dark-colored macules or papules on the tongue should be considered in the evaluation of a patient with these clinical findings. Black hairy tongue (BHT), or lingua villosa nigra, is a benign finding due to filiform papillae hypertrophy on the dorsum of the tongue.9 Food particle debris caught in BHT can lead to porphyrin production by chromogenic bacteria and fungi. These porphyrins result in discoloration ranging from brown-black to yellow and green occurring anteriorly to the circumvallate papillae while usually sparing the tip and lateral sides of the tongue. Dermoscopy can show thin discolored fibers with a hairy appearance. Although normal filiform papillae are less than 1-mm long, 3-mm long papillae are considered diagnostic of BHT.9 Treatment includes effective oral hygiene and desquamation measures, which can lead to complete resolution.10
Peutz-Jeghers syndrome is a rare genodermatosis that is characterized by focal hyperpigmentation and multiple gastrointestinal mucosal hamartomatous polyps. Peutz-Jeghers syndrome should be suspected in a patient with discrete, 1- to 5-mm, brown to black macules on the perioral or periocular skin, tongue, genitals, palms, soles, and buccal mucosa with a history of abdominal symptoms.11,12
Addison disease, or primary adrenal insufficiency, may present with brown hyperpigmentation on chronically sun-exposed areas; regions of friction or pressure; surrounding scar tissue; and mucosal surfaces such as the tongue, inner surface of the lip, and buccal and gingival mucosa.13 Addison disease is differentiated from PFPT by a more generalized hyperpigmentation due to increased melanin production as well as the presence of systemic symptoms related to hypocortisolism. The pigmentation seen on the buccal mucosa in Addison disease is patchy and diffuse, and histology reveals basal melanin hyperpigmentation with superficial dermal melanophages.13
Hereditary hemorrhagic telangiectasia is an inherited disorder featuring telangiectasia and generally appears in the third decade of life.14 Telangiectases classically are 1 to 3 mm in diameter with or without slight elevation. Dermoscopic findings include small red clots, lacunae, and serpentine or linear vessels arranged in a radial conformation surrounding a homogenous pink center.15 These telangiectases typically occur on the skin or mucosa, particularly the face, lips, tongue, nail beds, and nasal mucosa; however, any organ can be affected with arteriovenous malformations. Recurrent epistaxis occurs in more than half of patients with hereditary hemorrhagic telangiectasia.14 Histopathology reveals dilated vessels and lacunae near the dermoepidermal junction displacing the epidermis and papillary dermis.15 It is distinguished from PFPT by the vascular nature of the lesions and by the presence of other characteristic symptoms such as recurrent epistaxis and visceral arteriovenous malformations.
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
- Romiti R, Molina De Medeiros L. Pigmented fungiform papillae of the tongue. Pediatr Dermatol. 2010;27:398-399. doi:10.1111/j .1525-1470.2010.01183.x
- Chessa MA, Patrizi A, Sechi A, et al. Pigmented fungiform lingual papillae: dermoscopic and clinical features. J Eur Acad Dermatol Venereol. 2018;32:935-939. doi:10.1111/jdv.14809
- Rice SM, Lal K. Successful treatment of pigmented fungiform papillae of the tongue with Q-switched ruby laser. Dermatol Surg. 2022;48:368-369. doi:10.1097/DSS.0000000000003371
- Mizawa M, Makino T, Furukawa F, et al. Efficacy of Q-switched ruby laser treatment for pigmented fungiform papillae of the tongue. J Dermatol. 2022;49:E133-E134. doi:10.1111/1346-8138.16270
- Holzwanger JM, Rudolph RI, Heaton CL. Pigmented fungiform papillae of the tongue: a common variant of oral pigmentation. Int J Dermatol. 1974;13:403-408. doi:10.1111/j.1365-4362.1974. tb05073.x
- Mukamal LV, Ormiga P, Ramos-E-Silva M. Dermoscopy of the pigmented fungiform papillae of the tongue. J Dermatol. 2012;39:397-399. doi:10.1111/j.1346-8138.2011.01328.x
- Surboyo MDC, Santosh ABR, Hariyani N, et al. Clinical utility of dermoscopy on diagnosing pigmented papillary fungiform papillae of the tongue: a systematic review. J Oral Biol Craniofac Res. 2021;11:618-623. doi:10.1016/j.jobcr.2021.09.008
- Chamseddin B, Vandergriff T. Pigmented fungiform papillae of the tongue: a clinical and histologic description [published online September 15, 2019]. Dermatol Online J. 2019;25:13030/qt8674c519.
- Jayasree P, Kaliyadan F, Ashique KT. Black hairy tongue. JAMA Dermatol. 2022;158:573. doi:10.1001/jamadermatol.2021.5314
- Schlager E, St Claire C, Ashack K, et al. Black hairy tongue: predisposing factors, diagnosis, and treatment. Am J Clin Dermatol. 2017;18:563-569. doi:10.1007/s40257-017-0268-y
- Sandru F, Petca A, Dumitrascu MC, et al. Peutz-Jeghers syndrome: skin manifestations and endocrine anomalies (review). Exp Ther Med. 2021;22:1387. doi:10.3892/etm.2021.10823
- Shah KR, Boland CR, Patel M, et al. Cutaneous manifestations of gastrointestinal disease: part I. J Am Acad Dermatol. 2013;68:189.e1-210. doi:10.1016/j.jaad.2012.10.037
- Lee K, Lian C, Vaidya A, et al. Oral mucosal hyperpigmentation. JAAD Case Rep. 2020;6:993-995. doi:10.1016/j.jdcr.2020.08.013
- Haitjema T, Westermann CJ, Overtoom TT, et al. Hereditary hemorrhagic telangiectasia (Osler-Weber-Rendu disease): new insights in pathogenesis, complications, and treatment. Arch Intern Med. 1996;156:714-719.
- Tokoro S, Namiki T, Ugajin T, et al. Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber’s disease): detailed assessment of skin lesions by dermoscopy and ultrasound. Int J Dermatol. 2019;58:E224-E226. doi:10.1111/ijd.14578
A 9-year-old Black boy presented to the dermatology clinic for evaluation of dark spots on the tongue. The family first noted these spots 5 months prior and reported that they remained stable during that time. The patient’s medical history was notable for autism spectrum disorder and multiple food allergies. His family history was negative for similar oral pigmentation or other pigmentary anomalies. A review of systems was positive only for selective eating and rare nosebleeds. Physical examination revealed numerous dark brown, pinpoint papules across the dorsal aspect of the tongue. No hyperpigmentation of the buccal mucosae, lips, palms, or soles was identified. Several light brown streaks were present on the fingernails and toenails, consistent with longitudinal melanonychia. A prior complete blood cell count was within reference range.
Botanical Briefs: Daffodils (Narcissus Species)
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
Contact dermatitis is a common problem in the floral bulb industry and is considered an occupational disease. Daffodils (Narcissus species)(Figure) are thought to be the most common cause of irritant contact dermatitis among florists.1
Clinical Importance
Picking daffodils can start as early as October, when the flowers are still closed. The picker’s hand slides down the stem to snap the stalk at the base. This potentially traumatic maneuver to the web of the fingers leads to abrasions, which are irritated by the sap and cause granulomatous sores and paronychia. An experienced picker can pick 20,000 flowers a day, leading to extensive contact with sap.2
Eczematous or granulomatous rash on the arms also is seen as the sap irritates the wrist and forearm. The pickers often hold the flowers until a bunch of 10 has been collected. The 10 flowers are held together by a rubber band and stacked along the arm, the chin, and the axilla, causing the rash to extend to those areas. Sap also can be transferred by the hand to other parts of the body, such as the face. In men, sap can be transferred to the genitalia as the men urinate in the field.
Narcissus also can cause poisoning if ingested by humans or animals. Researchers who analyzed calls made to the New Zealand Natural Poisons Centre between 2003 and 2010 determined that daffodil was the 11th most common call for plant-related poisoning.3
Although the severity of plant poisoning often is low due to the small amount of plant material usually consumed, more severe poisoning can occur when the plant is eaten for medicinal purposes or mistaken for an edible plant.3 Vomiting, respiratory symptoms, abdominal pain, diarrhea, trembling, and convulsions can occur when daffodils are ingested. Death has been reported due to ingestion of the bulbs.4
In February 2010, 10 children aged 10 and 11 years and their 22-year-old guide presented to an emergency department in Israel after ingesting Narcissus bulbs, which were mistakenly believed to be the bulbs of onions.4 Eight children and the guide vomited. One child and the guide reported abdominal pain. All were discharged in stable condition after 4 hours of observation.4
Clinical Manifestations
Daffodil rash or lily rash was first described in 1910.5 The typical rash presents as dryness, fissures, scaling, and erythema of the fingertips, hands, and forearms, often with subungual hyperkeratosis. Vesicles and pustules may be seen. The rash may extend to other areas of the body, including the face.6
Prevention and Treatment
Use of protective gloves and clothing to avoid contact with the plant is recommended.2 Treatment includes stopping contact with the irritant, eye irrigation, and supportive measures (airway, breathing, and circulation). Activated charcoal can be helpful if used within 1 hour after ingestion but is contraindicated in vomiting patients.4
Identifying Features
The genus Narcissus is in the family Amaryllidaceae and contains ornamental plants, including daffodil (trumpet Narcissus, Narcissus pseudonarcissus), jonquil (Narcissus jonquilla), and poet’s narcissus (Narcissus poeticus). Most species are perennial; the plant emerges from a bulb in spring. Leaves originate from the base of the plant and range from 5-cm to 1.2-meters long, depending on the species. The flowers span a range of shapes and colors—from a trumpet (the daffodil) to a ringlike cup (poet’s Narcissus) and in yellow, white, and pink.7
Distribution and Plant Facts
Distribution—There are approximately 80 to 100 wild Narcissus species, which are found in southwestern Europe, North Africa, the Balkan Peninsula, Italy, and France. There are more than 27,000 Narcissus cultivars registered in the International Daffodil Register.8
Plant Facts—The daffodil is the national flower of Wales. It also is often used to depict hope and joy and is the symbol of cancer charities in many countries.9
The name Narcissus is believed to have originated from Greek mythology. A handsome youth, Narcissus, fell in love with his own reflection, for which the gods punished him by turning him into a flower.10
Another theory states that Narcissus is derived from the Greek word narkao (to benumb) due to its narcotic properties. When an open wound is subjected to an extract of the bulb, numbness of the entire nervous system is said to occur as well as paralysis of the heart. This narcotic effect led Socrates to refer to the Narcissus plant as the “chaplet of the infernal gods.”11
Narcissus is an important flower in various ethnic rituals. The Greeks often planted daffodils near tombs. In Muslim culture, white is believed to be the symbol of good and purity; Narcissus was one of the most common white-flowered plants found in Muslim graveyards.12
Medicinal Qualities and Uses—Narcissus species have been used as medicinal plants for a variety of ailments. For example, Narcissus tazetta contains flavonoids, alkaloids, saponins, tannins, cardiac glycosides, oil, steroids, terpenoids, and anthraquinones that contribute to its antibacterial, antifungal, antiviral, antimalarial, anticancer, antioxidant, dermatologic, cardiovascular, immunomodulatory, and acetylcholinesterase inhibitory effects.13 In a study, chloroform extracts from N tazetta bulbs were found to be more active than doxorubicin against hepatocellular and colon cancer cell lines.14
More than 500 alkaloids have been isolated from the Narcissus genus.15 In 2001, the US Food and Drug Administration approved one of the alkaloids, galantamine, for the treatment of mild to moderate stages of Alzheimer disease.16 Galantamine selectively and reversibly inhibits acetylcholinesterase, the enzyme believed responsible for neurodegeneration seen in Alzheimer disease. Plants are the main source of galantamine, despite the ability of pharmaceutical companies to synthesize the compound. Galantamine hydrobromide is sold by prescription (Razadyne [Janssen Pharmaceuticals, Inc]); generic formulations approved by the US Food and Drug Administration have been produced by more than 15 pharmaceutical companies.17,18
Irritant and Allergen
Sap found in the bulbs and hollow stems of Narcissus contains calcium oxalate crystals, or raphides. The minute, needle-shaped calcium oxalate crystals are believed to be a waste product of cellular metabolism.19 When the plant structure is compromised by pickers snapping the stalk, the sharp crystals penetrate the skin to cause an irritant contact dermatitis.
Relevant Research—A study used electron microscopy to characterize the structure of raphides from various plants,2 though not from Narcissus species; the structure of each raphide was then compared to the degree of irritation it produced. The researchers concluded that more elongated crystals (those containing barbs) produce a greater degree of irritation. Narcissus species are known to cause varying degrees of skin irritation: For example, N tazetta rarely causes skin irritation, whereas N pseudonarcissi (daffodil) tends to cause remarkably more skin irritation.2
Allergic reactions to and strong toxicity from Narcissus species are not well understood. In a study, only 2 alkaloids—homolycorine and masonin—produced a weakly positive reaction in patch tests on sensitized guinea pigs, which correlates with the finding of a different study, in which only 2 of 12 patients whose findings were examined over 14 years had a positive patch test for Narcissus.20,21
However, IgE-mediated allergies indicative of an allergic response to Narcissus have been reported. A study isolated an allergenic protein, narcin, from bulbs of N tazetta. Narcin is a 13-kDa protein with potent allergenic effects capable of inducing production of proinflammatory cytokines and increasing IgE levels in mononuclear cells in peripheral blood.22
More research is required to find and understand the compounds responsible for causing an allergic reaction to Narcissus.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
- Modi GM, Doherty CB, Katta R, et al. Irritant contact dermatitis from plants. Dermatitis. 2009;20:63-78. doi:10.2310/6620.2009.08051
- Julian CG, Bowers PW. The nature and distribution of daffodil pickers’ rash. Contact Dermatitis. 1997;37:259-262. doi:10.1111/j.1600-0536.1997.tb02461.x
- Slaughter RJ, Beasley DMG, Lambie BS, et al. Poisonous plants in New Zealand: a review of those that are most commonly enquired about to the National Poisons Centre. N Z Med J. 2012;125:87-118.
- Hussein A, Yassin A. Poisoning following ingestion of Narcissus tazetta bulbs by schoolchildren. Isr Med Assoc J. 2014;16:125-126.
- Hanks GR, ed. Narcissus and Daffodil: The Genus Narcissus. CRC Press; 2002. https://doi.org/10.1201/9780203219355
- McGovern TW. Botanical briefs: daffodils—Narcissus L. Cutis. 2000;65:130-132.
- The Editors of Encyclopaedia Britannica. Narcissus. Encyclopedia Britannica. Accessed December 13, 2022. https://www.britannica.com/plant/narcissus-plant
- M, A, D, et al. Alkaloids from Narcissus poeticus cv. Pink Parasol of various structural types and their biological activity. Arch Pharm Res. 2018;41:208-218. doi:10.1007/s12272-017-1000-4
- Crampton L. Beautiful daffodils: plant facts, toxicity, and a symbol of hope. Owlcation. April 19, 2022. Accessed December 13, 2022. https://owlcation.com/stem/Daffodils-Beautiful-Flowers-and-a-Symbol-of-Hope
- Rademaker M. Daffodil. DermNet. Published 1999. Accessed December 13, 2022. https://dermnetnz.org/topics/daffodil
- Grieve M. Narcissus. Accessed December 13, 2022. https://botanical.com/botanical/mgmh/n/narcis01.html
- Dafni A, Lev E, Beckmann S, et al. Ritual plants of Muslim graveyards in northern Israel. J Ethnobiolog Ethnomed. 2006;2:38. doi:10.1186/1746-4269-2-38
- Al-Snafi AE. Constituents and pharmacology of Narcissus tazetta. IOSR J Pharm. 2020;10:44-53.
- Shawky E, Abou-Donia AH, Darwish FA, et al. In vitro cytotoxicity of some Narcissus plants extracts. Nat Prod Res. 2015;29:363-365. doi:10.1080/14786419.2014.942302
- Havlasov J, M, Siatka T, et al. Chemical composition of bioactive alkaloid extracts from some Narcissus species and varieties and their biological activity. Nat Prod Commun. 2014;9:1151-1155.
- Pigni NB, S, V, et al. Alkaloids from Narcissus serotinus. J Nat Prod. 2012;75:1643-1647. doi:10.1021/np3003595
- Razadyne. Prescribing information. Janssen Pharmaceuticals, Inc; 2013. Accessed December 19, 2022. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/021169Orig1s032,021224Orig1s030,021615Orig1s023lbl.pdf
- Takos AM, Rook F. Towards a molecular understanding of the biosynthesis of amaryllidaceae alkaloids in support of their expanding medical use. Int J Mol Sci. 2013;14:11713-11741. doi:10.3390/ijms140611713
- Evans FJ, Schmidt RJ. Plants and plant products that induce contact dermatitis. Planta Med. 1980;38:289-316. doi:10.1055/s-2008-1074883
- Gude M, Hausen BM, Heitsch H, et al. An investigation of the irritant and allergenic properties of daffodils (Narcissus pseudonarcissus L., Amaryllidaceae). a review of daffodil dermatitis. Contact Dermatitis. 1988;19:1-10.
- Lamminpää A, Estlander T, Jolanki R, et al. Occupational allergic contact dermatitis caused by decorative plants. Contact Dermatitis. 1996;34:330-335.
- Sinha M, Singh A, Shokeen A, et al. Evidence of a novel allergenic protein Narcin in the bulbs of Narcissus tazetta. Int J Biochem Mol Biol. 2013;4:95-101.
Practice Points
- Narcissus species are thought to be the most common cause of irritant contact dermatitis among florists.
- Use of protective gloves and clothing to prevent Narcissus-induced contact dermatitis is recommended.
The Role of Dietary Antioxidants in Melanoma and Nonmelanoma Skin Cancer
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
Nonmelanoma skin cancer (NMSC) is the most common cancer in the United States, and cutaneous melanoma is projected to be the fifth most common form of cancer in 2022, with increasing incidence and high potential for mortality.1-3 Estimates indicate that 35% to 45% of all cancers in White patients are cutaneous, with 4% to 5% occurring in Hispanic patients, 2% to 4% in Asian patients, and 1% to 2% in Black patients.4 Of the keratinocyte carcinomas, basal cell carcinoma (BCC) is the most prevalent, projected to affect approximately 33% to 39% of White males and 23% to 28% of White females in the United States during their lifetimes. Squamous cell carcinoma (SCC) is the second most common skin malignancy, with a lifetime risk of 9% to 14% for White males and 4% to 9% for White females in the United States.5 The incidence of melanoma continues to increase, with approximately 99,780 new cases expected in the United States in 2022.1
UV-induced DNA damage plays a key role in the pathogenesis and development of various skin malignancies.6 UV radiation from sunlight or tanning devices causes photocarcinogenesis due to molecular and cellular effects, including the generation of reactive oxygen species, DNA damage due to the formation of cyclobutane pyrimidine dimers and pyrimidine-pyrimidone, melanogenesis, apoptosis, and the increased expression of harmful genes and proteins.6 The summation of this damage can result in skin malignancies, including NMSC and melanoma.6,7 Dietary antioxidants theoretically help prevent oxidative reactions from occurring within the body, and it has been suggested that intake of dietary antioxidants may decrease DNA damage and prevent tumorigenesis secondary to UV radiation.8 Antioxidants exist naturally in the body but can be acquired exogenously. Investigators have studied dietary antioxidants in preventing skin cancer formation with promising results in the laboratory setting.8-11 Recently, more robust human studies have been initiated to further delineate this relationship. We present clinical evidence of several frequently utilized antioxidant vitamins and their effects on melanoma and NMSC.
Antioxidants
Vitamin A—Vitamin A is a fat-soluble vitamin found in animal sources, including fish, liver, and eggs. Carotenoids, such as beta carotene, are provitamin A plant derivatives found in fruits and vegetables that are converted into biologically active retinol and retinoic acid.12 Retinols play a key role in cellular growth and differentiation and are thought to be protective against skin cancer via the inactivation of free radicals and immunologic enhancement due to their antiproliferative, antioxidative, and antiapoptotic effects.13-16 Animal studies have demonstrated this protective effect and the ability of retinoids to suppress carcinogenesis; however, human studies reveal conflicting results.17,18
Greenberg et al19 investigated the use of beta carotene in preventing the formation of NMSC. Patients (N=1805) were randomized to receive 50 mg of beta carotene daily or placebo. Over a 5-year period, there was no significant reduction in the occurrence of NMSC (relative risk [RR], 1.05; 95% CI, 0.91-1.22).19 Frieling et al20 conducted a similar randomized, double-blind, placebo-controlled trial investigating beta carotene for primary prevention of NMSC in 22,071 healthy male physicians. The study group received 50 mg of beta carotene every other day for 12 years’ duration, and there was no significant effect on the incidence of first NMSC development (RR, 0.98; 95% CI, 0.92-1.05).20
A case-control study by Naldi et al21 found an inverse association between vitamin A intake and development of melanoma. Study participants were stratified into quartiles based on level of dietary intake and found an odds ratio (OR) of 0.71 for beta carotene (95% CI, 0.50-1.02), 0.57 for retinol (95% CI, 0.39-0.83), and 0.51 for total vitamin A (95% CI, 0.35-0.75) when comparing the upper quartile of vitamin A intake to the lower quartile. Upper-quartile cutoff values of vitamin A intake were 214 µg/d for beta carotene, 149 µg/d for retinol, and 359 µg/d for total vitamin A.21 More recently, a meta-analysis by Zhang et al22 pooled data from 8 case-control studies and 2 prospective studies. Intake of retinol but not total vitamin A or beta carotene was associated with a reduced risk for development of melanoma (retinol: OR, 0.80; 95% CI, 0.69-0.92; total vitamin A: OR, 0.86; 95% CI, 0.59-1.25; beta carotene: OR, 0.87; 95% CI, 0.62-1.20).22 Feskanich et al23 demonstrated similar findings with use of food-frequency questionnaires in White women, suggesting that retinol intake from food combined with supplements may be protective for women who were otherwise at a low risk for melanoma based on nondietary factors. These factors included painful or blistering sunburns during childhood, history of more than 6 sunburns, more than 3 moles on the left arm, having red or blonde hair, and having a parent or sibling with melanoma (P=.01). However, this relationship did not hold true when looking at women at an intermediate or high risk for melanoma (P=.16 and P=.46).23
When looking at high-risk patients, such as transplant patients, oral retinoids have been beneficial in preventing NMSC.24-27 Bavinck et al24 investigated 44 renal transplant patients with a history of more than 10 NMSCs treated with 30 mg of acitretin daily vs placebo. Patients receiving oral retinoid supplementation developed fewer NMSCs over a 6-month treatment period (P=.01).24 Similarly, George et al25 investigated acitretin in renal transplant patients and found a statistically significant decrease in number of SCCs in patients on supplementation (P=.002). Solomon-Cohen et al26 performed a retrospective case-crossover study in solid organ transplant recipients and found that those treated with 10 mg of acitretin daily for 2 years had a significant reduction in the number of new keratinocyte carcinomas (P=.002). Other investigators have demonstrated similar results, and in 2006, Otley et al27 proposed standardized dosing of acitretin for chemoprevention in high-risk patients, including patients developing 5 to 10 NMSCs per year, solid organ transplant recipients, and those with syndromes associated with the development of NMSC.28,29 Overall, in the general population, vitamin A and related compounds have not demonstrated a significant association with decreased development of NMSC; however, oral retinoids have proven useful for high-risk patients. Furthermore, several studies have suggested a negative association between vitamin A levels and the incidence of melanoma, specifically in the retinol formulation.
Vitamin B3—Nicotinamide (also known as niacinamide) is a water-soluble form of vitamin B3 and is obtained from animal-based and plant-based foods, such as meat, fish, and legumes.30 Nicotinamide plays a key role in cellular metabolism, cellular signaling, and DNA repair, including protection from UV damage within keratinocytes.31,32 Early mouse models demonstrated decreased formation of skin tumors in mice treated with topical or oral nicotinamide.32,33 A number of human studies have revealed similar results.34-36
Chen et al34 conducted the ONTRAC study, a phase 3, double-blind, randomized controlled trial (RCT) looking at 386 participants with a history of at least 2 NMSCs in the preceding 5 years. At 12 months, those treated with 500 mg of nicotinamide twice daily demonstrated a statistically significant decreased rate of SCC formation (P=.05). A decreased incidence of BCC development was noted; however, this trend did not reach statistical significance (P=.12). Precancerous skin lesions also were found to be decreased in the treatment group, with 20% lower incidence of actinic keratoses (AKs) after 9 months of treatment (P<.001).34 Drago et al35 specifically studied the incidence of AKs in 38 transplant recipients—8 liver and 30 kidney—and found that previously noted AKs had decreased in size for 18 of 19 patients taking 500 mg of nicotinamide daily when originally photographed AKs were remeasured at 6-month follow-up, with 7 of these 18 patients demonstrating complete clinical regression. Of those on nicotinamide supplementation, no new AKs developed compared to the control group, which demonstrated increased size of AKs or development of new AKs in 91% of patients, with 7 AKs progressing into SCC.35
Nicotinamide has been demonstrated to be useful in preventing skin cancer in high-risk populations, such as transplant patients or those with a high incidence of NMSC.34,36 Despite promising results within the laboratory setting, nicotinamide’s effects on melanoma in humans remains less clear.31,37 Studies suggest that nicotinamide enhances tumor-infiltrating lymphocytes and DNA repair mechanisms in melanocytes, which may translate into nicotinamide, providing chemoprevention for melanoma, but research in human patients is limited.31,37
Vitamin B9—Folate, the natural form of vitamin B9, is a water-soluble compound that is found in many foods, especially green leafy vegetables, and often is supplemented because of its health benefits.38,39 In the skin, folic acid plays a key role in cellular replication and proliferation.38 Controversy exists regarding folate’s effects on cellular growth and turnover with respect to cancer incidence.38,40 Donnenfeld et al41 conducted a prospective study assessing dietary folic acid intake and development of NMSC. A total of 5880 participants completed dietary records throughout the first 2 years of the study. After an average follow-up period of 12.6 years, there was an overall increased incidence of skin cancer in those with increased dietary folate (P=.03). Furthermore, when striating by skin cancer type, there was an increased incidence of NMSC overall as well as BCC when analyzing by type of NMSC (P=.03 for NMSC; P=.05 for BCC). However, when stratifying by gender, these findings only held true for women.41 Similar effects were observed by Fung et al,42 who prospectively studied the intake of various vitamins in relationship to the development of BCC in women. During 12 years of follow-up, a positive association was observed between folate intake and BCC development (OR, 1.2; 95% CI, 1.10-1.31).42 Fung et al43 also investigated the role of several vitamins in the development of SCC and found that folate showed a negative association, which did not reach statistical significance (RR, 0.79; 95% CI, 0.56-1.11). Furthermore, Vollset et al40 conducted a meta-analysis comparing folic acid to placebo in the incidence of various types of cancer. The study excluded NMSC but reported no significant association between the development of melanoma and folic acid supplementation.40 In summary, the effects of folate have diverse consequences, potentially promoting the formation of NMSC, but studies suggest that an individual’s gender and other genetic and environmental factors also may play a role.
Vitamin C—Vitamin C (also known as ascorbic acid) is a water-soluble vitamin with antioxidant immune-mediating effects. It is found in various fruits and vegetables and serves as a cofactor for enzymes within the body playing a key role in immune function and collagen formation.44,45 It has been postulated that ascorbic acid can provide protection from UV radiation damage via its intracellular activity but conversely can contribute to oxidative damage.44 Multiple in vitro laboratory studies and animal models have demonstrated photoprotective effects of ascorbic acid.46-48 Despite these findings, minimal photoprotective effects have been found in the human population.
Kune et al49 performed a case-control study of 88 males with previously diagnosed NMSC undergoing surgical removal and investigated patients’ prior dietary habits. Patients with NMSC had a statistically significantly lower level of vitamin C–containing food in their diet than those without NMSC (P=.004).49 In addition, Vural et al50 analyzed plasma samples and blood cells of patients with AK and BCC and found a significant decrease in ascorbic acid levels in both the AK (P<.001) and BCC (P<.001) groups compared with controls. However, studies have found that consumption of certain dietary compounds can rapidly increase plasma concentration levels, which may serve as a major confounding variable in this study. Plasma concentrations of ascorbic acid and beta carotene were found to be significantly increased following consumption of a high-antioxidant diet for as short a duration as 2 weeks (P<.05).51 More recently, Heinen et al52 performed a prospective study on 1001 adults. In patients without a history of skin cancer, they found that vitamin C from food sources plus dietary supplements was positively associated with the development of BCC (P=.03).52 Similarly, Fung et al42 performed a study in women and found a positive association between vitamin C intake and the development of BCC (OR, 1.13; 95% CI, 1.03-1.23).
The relationship between vitamin C intake—either in dietary or supplemental form—and melanoma remains controversial. Mice-based studies found that high concentrations of orally administered vitamin C induce cytotoxicity in melanoma cell lines, but at low concentrations they promote tumor growth of malignant melanoma.53 Feskanich et al23 examined the relationship between vitamin C intake and melanoma development via food frequency questionnaires in White women and found that vitamin C was associated with a higher risk for melanoma (P=.05), and furthermore, a positive dose response with frequency of orange juice intake was observed (P=.008). Overall, despite promising laboratory studies, there is a lack of RCTs investigating the use of vitamin C supplementation for prevention of NMSC and melanoma in humans, and the oral benefits of vitamin C for chemoprevention remain unclear.
Vitamin D—Vitamin D is a fat-soluble vitamin that is found in fish, liver, egg, and cheese, and is endogenously produced when UV radiation from sun exposure interacts with the skin, triggering the synthesis of vitamin D.54 Vitamin D is biologically inactive and must be converted to its active form 1,25-dihydroxyvitamin D after entering the body. Vitamin D modulates many genes involved in cellular proliferation and differentiation.54 Vitamin D receptors are expressed on keratinocytes and melanocytes.55 Animal studies have demonstrated a potentially protective effect of vitamin D in the development of NMSC.56 In a mouse model, Ellison et al56 found that mice without vitamin D receptors developed skin tumors more rapidly than those with vitamin D receptors.
Unfortunately, these findings have not been demonstrated in humans, and studies have even reported an increased risk for development of NMSC in patients with normal or increased vitamin D levels compared with those with low levels of vitamin D.57-60 Eide et al57 studied 3223 patients seeking advice for low bone density by recording their vitamin D levels at the time of presentation and monitoring development of NMSC. Vitamin D levels greater than 15 ng/mL were positively associated with the development of NMSC (OR, 1.7; 95% CI, 1.04-2.7). This association held true for both SCC and BCC, with a higher risk estimated for SCC (OR, 3.2; 95% CI, 0.4-24.0 for SCC; OR, 1.7; 95% CI, 0.5-5.8 for BCC).57 An increased vitamin D serum level also was found to be significantly associated with a higher risk for BCC and melanoma by van der Pols et al.58 This prospective study looked at the incidence of skin cancer over 11 years. Study participants with vitamin D levels over 75 nmol/L more frequently developed BCC (P=.01) and melanoma (P=.05). In contrast, SCC was less frequently observed in participants with these high levels of vitamin D (P=.07).58 Furthermore, Park et al60 looked at vitamin D and skin cancer risk for men and women in the United States and found no association with risk for SCC or melanoma but a positive association with BCC (P=.05 for total vitamin D; P<.01 for dietary vitamin D). Additional studies have been performed with inconsistent results, and multiple authors suggest the possible confounding relationship between vitamin D levels and UV radiation exposure.59-62 Furthermore, some studies have even demonstrated a negative association between vitamin D and NMSC. Tang et al63 performed a retrospective case-control study in elderly males, investigating serum levels of vitamin D and patients’ self-reported history of NMSC, which demonstrated that higher levels of vitamin D were associated with a decreased risk for NMSC. Overall, the relationship between vitamin D and skin cancer development remains unclear for both melanoma and NMSC.
Vitamin E—Vitamin E is a fat-soluble vitamin that is found in plant-based oils, nuts, seeds, fruits, and vegetables.64 It works as an antioxidant to protect against free radicals and heighten immune function, and it also serves as a pro-oxidant.65,66 Vitamin E naturally exists in 8 chemical forms, of which gamma-tocopherol is the most frequently obtained form in the diet, and alpha-tocopherol is the most abundant form found in the body.64,65
Early animal studies demonstrated the inhibition of UV-induced damage in mice receiving vitamin E supplementation.67,68 Human studies have not consistently shown these effects. Vural et al50 investigated plasma samples and blood cells of patients with AKs and BCCs and reported a significant decrease in alpha-tocopherol levels in both the AK (P<.05) and BCC (P<.001) groups compared with controls. However, studies also have demonstrated a positive association between vitamin E intake and the development of BCC, including one by Fung et al,42 which found a significant association in women (OR, 1.15; 95% CI, 1.06-1.26).
Vitamin E has been found to inhibit melanin synthesis in the laboratory, suggesting a potentially protective effect in melanoma.69,70 However, in the study performed by Feskanich et al23 examining vitamin intake and melanoma incidence via food-frequency questionnaires, vitamin E was not associated with a lower risk for melanoma. Despite promising laboratory studies, the data surrounding the use of a vitamin E supplement for prevention of melanoma and NMSC in humans remains unclear.
Selenium—Selenium is a trace mineral found in plants, meat, and fish. It plays a key role in reproduction, hormone metabolism, DNA synthesis, and protection from oxidative damage.71 In mice studies, lack of selenium-containing proteins resulted in skin abnormalities, including the development of a hyperplastic epidermis and aberrant hair follicle morphogenesis with alopecia after birth, and numerous experimental studies have demonstrated a negative association between selenium intake and cancer.72,73 However, human studies have yielded alternative results.
The Nutritional Prevention of Cancer Study Group analyzed 1312 dermatology patients with a history of NMSC.74 The study population was obtained from 7 dermatology clinics with randomization to control for confounding variables. Study participants received either 200 μg of selenium daily or placebo.74 Baseline characteristics of each study group were overall balanced. Selenium intake was found to have no effect on the development of BCC (hazard ratio [HR], 1.09; 95% CI, 0.94-1.26) but an increased risk for developing SCC (HR, 1.25; 95% CI, 1.03-1.51) and total NMSC (HR, 1.17; 95% CI, 1.02-1.34).74,75 Similarly, Reid et al76 performed an RCT comparing patients treated with 400 μg/d of selenium to those treated with 200 μg/d of selenium. When compared with placebo, those treated with 200 μg/d of selenium had a statistically significantly increased incidence of NMSC (P=.006); however, those treated with 400 μg/d of selenium had no significant change in total incidence of NMSC (P=.51).76 Furthermore, Vinceti et al77 performed a review of 83 studies from the literature investigating the effect of dietary selenium, and from the RCTs, there was no beneficial effect of selenium in reducing cancer risk in general; however, some studies demonstrated an increased incidence of other types of cancer, including melanoma. Of the RCTs included in the study investigating NMSC incidence specifically, it was found that the incidence was not affected by selenium administration (RR, 1.16; 95% CI, 0.30-4.42; 2 studies, 2027 participants).77 Despite data from several studies demonstrating an increased risk for NMSC, the effects of selenium on the risk for NMSC and melanoma remain unclear.
Combination Antioxidant Studies
In addition to investigating the use of single antioxidants in skin cancer prevention, studies utilizing the combination of various antioxidants or other dietary minerals have been conducted. Hercberg et al78 performed a randomized, double-blinded, placebo-controlled trial of 13,017 adults (7876 women and 5141 men) receiving a combination of 120 mg vitamin C, 30 mg vitamin E, 100 μg selenium, 6 mg beta carotene, and 20 mg zinc. Study participants were followed for an average of 7.5 years, and the development of skin cancers were recorded. Overall, the incidence rate of skin cancer did not differ between the 2 treatment groups; however, when segregated by gender, the study found that there was an increased risk for developing skin cancer in women taking the antioxidant supplement combination compared with placebo (P=.03). This difference was not observed in the 2 treatment groups of male patients (P=.11). When looking specifically at NMSC, there was no difference between treatment groups for male or female patients (P=.39 for males; P=.15 for females). In contrast, there was a higher incidence of melanoma identified in female patients taking the combination antioxidant supplement (P=.01), but this was not seen within the male study population (P=.51).78 In addition, Chang et al79 performed a meta-analysis of 10 previously published RCTs. Analysis revealed that treatment with a variety of supplements, including vitamins A, C, E, and beta carotene, were found to have no preventative effects on the incidence of skin cancer development (RR, 0.98; CI, 0.98-1.03). Notable limitations to this study included the variability in protocols of the studies included in this meta-analysis, the limited number of RCTs investigating vitamin supplementation and the risk for skin cancer development, and the influence of dietary intake on study outcomes.79
Other Dietary Agents
Furocoumarins—Furocoumarins are botanical substances found in various fruits and plants, including many citrus products. Furocoumarins are activated by UV light radiation and can lead to development of a phototoxic eruption. Several studies have suggested a pharmacogenetic effect of furocoumarins.80 Sun et al80 collected dietary data from 47,453 men and 75,291 women on furocoumarin intake and correlation with the development of NMSC. Overall, the study suggested that the intake of furocoumarins may lead to an increase in the development of BCC (HR, 1.16; 95% CI, 1.11-1.21; P=.002); however, there was no significant association identified between total intake of furocoumarins in the risk for SCC or melanoma.80 Furthermore, Sakaki et al81 conducted a survey study looking at the consumption of citrus products and the development of NMSC. The group found that there was an increased risk for NMSC in those consuming an increased amount of citrus products (P=.007).81
Conclusion
Dietary antioxidants have been investigated for their potential role in the prevention of tumorigenesis. Specific antioxidant vitamins, such as vitamin A derivatives and niacinamide, have demonstrated clinical utility in the prevention of NMSC in high-risk populations. Retinol also has been associated with a reduced incidence of melanoma. Numerous antioxidants have demonstrated promising data within the laboratory setting; however, inconsistent results have been appreciated in humans. Furthermore, several research studies suggest that folate, vitamin D, and furocoumarins may be associated with an increased risk for skin cancer development; however, these studies are inconclusive, and dietary studies are challenging to conduct. Overall, RCTs investigating the role of antioxidants for chemoprevention are limited. Moreover, the study of dietary antioxidants and vitamins may be affected by various confounding variables that can be difficult to account for because of patients’ potentially poor recall of dietary intake and the effect of dietary intake in supplemental studies. Given the increasing prevalence of skin cancer worldwide, further research into the clinical utility of antioxidants in skin cancer prevention is warranted.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
- Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2022. CA Cancer J Clin. 2022;72:7-33.
- Global Burden of Disease Cancer Collaboration; Fitzmaurice C, Abate D, Abbasi N, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: a systematic analysis for the Global Burden of Disease Study. JAMA Oncol. 2019;5:1749-1768.
- Leiter U, Keim U, Garbe C. Epidemiology of skin cancer: update 2019. In: Reichrath J, ed. Sunlight, Vitamin D and Skin Cancer. Springer International Publishing; 2020:123-139.
- Bradford PT. Skin cancer in skin of color. Dermatol Nurs. 2009;21:170-177, 206; quiz 178.
- Miller DL, Weinstock MA. Nonmelanoma skin cancer in the United States: incidence. J Am Acad Dermatol. 1994;30:774-778.
- Young AR, Claveau J, Rossi AB. Ultraviolet radiation and the skin: photobiology and sunscreen photoprotection. J Am Acad Dermatol. 2017;76(3S1):S100-S109.
- Pleasance ED, Cheetham RK, Stephens PJ, et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature. 2010;463:191-196.
- Baek J, Lee MG. Oxidative stress and antioxidant strategies in dermatology. Redox Rep. 2016;21:164-169.
- Katta R, Brown DN. Diet and skin cancer: the potential role of dietary antioxidants in nonmelanoma skin cancer prevention. J Skin Cancer. 2015;2015:893149.
- Stoj V, Shahriari N, Shao K, et al. Nutrition and nonmelanoma skin cancers. Clin Dermatol. 2022;40:173-185.
- O’Connor EA, Evans CV, Ivlev I, et al. Vitamin and mineral supplements for the primary prevention of cardiovascular disease and cancer: updated evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2022;327:2334-2347.
- National Institutes of Health Office of Dietary Supplements. Vitamin A and carotenoids. fact sheet for health professionals. Updated June 15, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminA-HealthProfessional/
- Keller KL, Fenske NA. Uses of vitamins A, C, and E and related compounds in dermatology: a review. J Am Acad Dermatol. 1998;39:611-625.
- Wright TI, Spencer JM, Flowers FP. Chemoprevention of nonmelanoma skin cancer. J Am Acad Dermatol. 2006;54:933-946; quiz 947-950.
- Bushue N, Wan YJY. Retinoid pathway and cancer therapeutics. Adv Drug Deliv Rev. 2010;62:1285-1298.
- Stahl W, Sies H. β-Carotene and other carotenoids in protection from sunlight. Am J Clin Nutr. 2012;96:1179S-1184S.
- Bukhari MH, Qureshi SS, Niazi S, et al. Chemotherapeutic/chemopreventive role of retinoids in chemically induced skin carcinogenesis in albino mice. Int J Dermatol. 2007;46:1160-1165.
- Lambert LA, Wamer WG, Wei RR, et al. The protective but nonsynergistic effect of dietary beta-carotene and vitamin E on skin tumorigenesis in Skh mice. Nutr Cancer. 1994;21:1-12.
- Greenberg ER, Baron JA, Stukel TA, et al. A clinical trial of beta carotene to prevent basal-cell and squamous-cell cancers of the skin. The Skin Cancer Prevention Study Group. N Engl J Med. 1990;323:789-795.
- Frieling UM, Schaumberg DA, Kupper TS, et al. A randomized, 12-year primary-prevention trial of beta carotene supplementation for nonmelanoma skin cancer in the physician’s health study. Arch Dermatol. 2000;136:179-184.
- Naldi L, Gallus S, Tavani A, et al; Oncology Study Group of the Italian Group for Epidemiologic Research in Dermatology. Risk of melanoma and vitamin A, coffee and alcohol: a case-control study from Italy. Eur J Cancer Prev. 2004;13:503-508.
- Zhang YP, Chu RX, Liu H. Vitamin A intake and risk of melanoma: a meta-analysis. PloS One. 2014;9:e102527.
- Feskanich D, Willett WC, Hunter DJ, et al. Dietary intakes of vitamins A, C, and E and risk of melanoma in two cohorts of women. Br J Cancer. 2003;88:1381-1387.
- Bavinck JN, Tieben LM, Van der Woude FJ, et al. Prevention of skin cancer and reduction of keratotic skin lesions during acitretin therapy in renal transplant recipients: a double-blind, placebo-controlled study. J Clin Oncol. 1995;13:1933-1938.
- George R, Weightman W, Russ GR, et al. Acitretin for chemoprevention of non-melanoma skin cancers in renal transplant recipients. Australas J Dermatol. 2002;43:269-273.
- Solomon-Cohen E, Reiss-Huss S, Hodak E, et al. Low-dose acitretin for secondary prevention of keratinocyte carcinomas in solid-organ transplant recipients. Dermatology. 2022;238:161-166.
- Otley CC, Stasko T, Tope WD, et al. Chemoprevention of nonmelanoma skin cancer with systemic retinoids: practical dosing and management of adverse effects. Dermatol Surg. 2006;32:562-568.
- Kadakia KC, Barton DL, Loprinzi CL, et al. Randomized controlled trial of acitretin versus placebo in patients at high-risk for basal cell or squamous cell carcinoma of the skin (North Central Cancer Treatment Group Study 969251). Cancer. 2012;118:2128-2137.
- McKenna DB, Murphy GM. Skin cancer chemoprophylaxis in renal transplant recipients: 5 years of experience using low-dose acitretin. Br J Dermatol. 1999;140:656-660.
- National Institutes of Health Office of Dietary Supplements. Niacin: fact sheet for health professionals. Updated August 23, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Niacin-HealthProfessional/
- Malesu R, Martin AJ, Lyons JG, et al. Nicotinamide for skin cancer chemoprevention: effects of nicotinamide on melanoma in vitro and in vivo. Photochem Photobiol Sci. 2020;19:171-179.
- Gensler HL. Prevention of photoimmunosuppression and photocarcinogenesis by topical nicotinamide. Nutr Cancer. 1997;29:157-162.
- Gensler HL, Williams T, Huang AC, et al. Oral niacin prevents photocarcinogenesis and photoimmunosuppression in mice. Nutr Cancer. 1999;34:36-41.
- Chen AC, Martin AJ, Choy B, et al. A phase 3 randomized trial of nicotinamide for skin-cancer chemoprevention. N Engl J Med. 2015;373:1618-1626.
- Drago F, Ciccarese G, Cogorno L, et al. Prevention of non-melanoma skin cancers with nicotinamide in transplant recipients: a case-control study. Eur J Dermatol. 2017;27:382-385.
- Yélamos O, Halpern AC, Weinstock MA. Reply to “A phase II randomized controlled trial of nicotinamide for skin cancer chemoprevention in renal transplant recipients.” Br J Dermatol. 2017;176:551-552.
- Scatozza F, Moschella F, D’Arcangelo D, et al. Nicotinamide inhibits melanoma in vitro and in vivo. J Exp Clin Cancer Res. 2020;39:211.
- National Institutes of Health Office of Dietary Supplements. Folate: fact sheet for health professionals. Updated November 1, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Folate-HealthProfessional/
- Butzbach K, Epe B. Photogenotoxicity of folic acid. Free Radic Biol Med. 2013;65:821-827.
- Vollset SE, Clarke R, Lewington S, et al. Effects of folic acid supplementation on overall and site-specific cancer incidence during the randomised trials: meta-analyses of data on 50,000 individuals. Lancet. 2013;381:1029-1036.
- Donnenfeld M, Deschasaux M, Latino-Martel P, et al. Prospective association between dietary folate intake and skin cancer risk: results from the Supplémentation en Vitamines et Minéraux Antioxydants cohort. Am J Clin Nutr. 2015;102:471-478.
- Fung TT, Hunter DJ, Spiegelman D, et al. Vitamins and carotenoids intake and the risk of basal cell carcinoma of the skin in women (United States). Cancer Causes Control. 2002;13:221-230.
- Fung TT, Spiegelman D, Egan KM, et al. Vitamin and carotenoid intake and risk of squamous cell carcinoma of the skin. Int J Cancer. 2003;103:110-115.
- National Institutes of Health Office of Dietary Supplements. Vitamin C: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminC-HealthProfessional/
- Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24:248-255.
- Moison RMW, Beijersbergen van Henegouwen GMJ. Topical antioxidant vitamins C and E prevent UVB-radiation-induced peroxidation of eicosapentaenoic acid in pig skin. Radiat Res. 2002;157:402-409.
- Lin JY, Selim MA, Shea CR, et al. UV photoprotection by combination topical antioxidants vitamin C and vitamin E. J Am Acad Dermatol. 2003;48:866-874.
- Pauling L, Willoughby R, Reynolds R, et al. Incidence of squamous cell carcinoma in hairless mice irradiated with ultraviolet light in relation to intake of ascorbic acid (vitamin C) and of D, L-alpha-tocopheryl acetate (vitamin E). Int J Vitam Nutr Res Suppl. 1982;23:53-82.
- Kune GA, Bannerman S, Field B, et al. Diet, alcohol, smoking, serum beta-carotene, and vitamin A in male nonmelanocytic skin cancer patients and controls. Nutr Cancer. 1992;18:237-244.
- Vural P, Canbaz M, Selçuki D. Plasma antioxidant defense in actinic keratosis and basal cell carcinoma. J Eur Acad Dermatol Venereol. 1999;13:96-101.
- Record IR, Dreosti IE, McInerney JK. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br J Nutr. 2001;85:459-464.
- Heinen MM, Hughes MC, Ibiebele TI, et al. Intake of antioxidant nutrients and the risk of skin cancer. Eur J Cancer. 2007;43:2707-2716.
- Yang G, Yan Y, Ma Y, et al. Vitamin C at high concentrations induces cytotoxicity in malignant melanoma but promotes tumor growth at low concentrations. Mol Carcinog. 2017;56:1965-1976.
- National Institutes of Health Office of Dietary Supplements. Vitamin D: fact sheet for health professionals. Updated August 12, 2022. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminD-HealthProfessional/
- Reichrath J, Saternus R, Vogt T. Endocrine actions of vitamin D in skin: relevance for photocarcinogenesis of non-melanoma skin cancer, and beyond. Mol Cell Endocrinol. 2017;453:96-102.
- Ellison TI, Smith MK, Gilliam AC, et al. Inactivation of the vitamin D receptor enhances susceptibility of murine skin to UV-induced tumorigenesis. J Invest Dermatol. 2008;128:2508-2517.
- Eide MJ, Johnson DA, Jacobsen GR, et al. Vitamin D and nonmelanoma skin cancer in a health maintenance organization cohort. Arch Dermatol. 2011;147:1379-1384.
- van der Pols JC, Russell A, Bauer U, et al. Vitamin D status and skin cancer risk independent of time outdoors: 11-year prospective study in an Australian community. J Invest Dermatol. 2013;133:637-641.
- Caini S, Gnagnarella P, Stanganelli I, et al. Vitamin D and the risk of non-melanoma skin cancer: a systematic literature review and meta-analysis on behalf of the Italian Melanoma Intergroup. Cancers (Basel). 2021;13:4815.
- Park SM, Li T, Wu S, et al. Vitamin D intake and risk of skin cancer in US women and men. PLoS One. 2016;11:e0160308.
- Afzal S, Nordestgaard BG, Bojesen SE. Plasma 25-hydroxyvitamin D and risk of non-melanoma and melanoma skin cancer: a prospective cohort study. J Invest Dermatol. 2013;133:629-636.
- Asgari MM, Tang J, Warton ME, et al. Association of prediagnostic serum vitamin D levels with the development of basal cell carcinoma. J Invest Dermatol. 2010;130:1438-1443.
- Tang JY, Parimi N, Wu A, et al. Inverse association between serum 25(OH) vitamin D levels and non-melanoma skin cancer in elderly men. Cancer Causes Control. 2010;21:387-391.
- Keen MA, Hassan I. Vitamin E in dermatology. Indian Dermatol Online J. 2016;7:311-315.
- National Institutes of Health Office of Dietary Supplements. Vitamin E: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/VitaminE-HealthProfessional/
- Pearson P, Lewis SA, Britton J, et al. The pro-oxidant activity of high-dose vitamin E supplements in vivo. BioDrugs. 2006;20:271-273.
- Gerrish KE, Gensler HL. Prevention of photocarcinogenesis by dietary vitamin E. Nutr Cancer. 1993;19:125-133.
- McVean M, Liebler DC. Prevention of DNA photodamage by vitamin E compounds and sunscreens: roles of ultraviolet absorbance and cellular uptake. Mol Carcinog. 1999;24:169-176.
- Prasad KN, Cohrs RJ, Sharma OK. Decreased expressions of c-myc and H-ras oncogenes in vitamin E succinate induced morphologically differentiated murine B-16 melanoma cells in culture. Biochem Cell Biol. 1990;68:1250-1255.
- Funasaka Y, Komoto M, Ichihashi M. Depigmenting effect of alpha-tocopheryl ferulate on normal human melanocytes. Pigment Cell Res. 2000;13(suppl 8):170-174.
- National Institutes of Health Office of Dietary Supplements. Selenium: fact sheet for health professionals. Updated March 26, 2021. Accessed November 14, 2022. https://ods.od.nih.gov/factsheets/Selenium-HealthProfessional/
- Sengupta A, Lichti UF, Carlson BA, et al. Selenoproteins are essential for proper keratinocyte function and skin development. PLoS One. 2010;5:e12249.
- Das RK, Hossain SKU, Bhattacharya S. Diphenylmethyl selenocyanate inhibits DMBA-croton oil induced two-stage mouse skin carcinogenesis by inducing apoptosis and inhibiting cutaneous cell proliferation. Cancer Lett. 2005;230:90-101.
- Clark LC, Combs GF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1996;276:1957-1963.
- Duffield-Lillico AJ, Slate EH, Reid ME, et al. Selenium supplementation and secondary prevention of nonmelanoma skin cancer in a randomized trial. J Natl Cancer Inst. 2003;95:1477-1481.
- Reid ME, Duffield-Lillico AJ, Slate E, et al. The nutritional prevention of cancer: 400 mcg per day selenium treatment. Nutr Cancer. 2008;60:155-163.
- Vinceti M, Filippini T, Del Giovane C, et al. Selenium for preventing cancer. Cochrane Database Syst Rev. 2018;1:CD005195.
- Hercberg S, Ezzedine K, Guinot C, et al. Antioxidant supplementation increases the risk of skin cancers in women but not in men. J Nutr. 2007;137:2098-2105.
- Chang YJ, Myung SK, Chung ST, et al. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36-44.
- Sun W, Rice MS, Park MK, et al. Intake of furocoumarins and risk of skin cancer in 2 prospective US cohort studies. J Nutr. 2020;150:1535-1544.
- Sakaki JR, Melough MM, Roberts MB, et al. Citrus consumption and the risk of non-melanoma skin cancer in the Women’s Health Initiative. Cancers (Basel). 2021;13:2173.
Practice Points
- Melanoma and nonmelanoma skin cancer (NMSC) are 2 of the most frequently diagnosed cancers in the United States. UV radiation plays a key role in the pathogenesis of both.
- Dietary antioxidants may mechanistically decrease DNA damage caused by UV radiation and could play a potential role in the prevention or development of melanoma and NMSC.
Epidermal Growth Factor Receptor Inhibitor–Induced Symmetrical Drug-Related Intertriginous and Flexural Exanthema: Should You Discontinue the Offending Agent?
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.
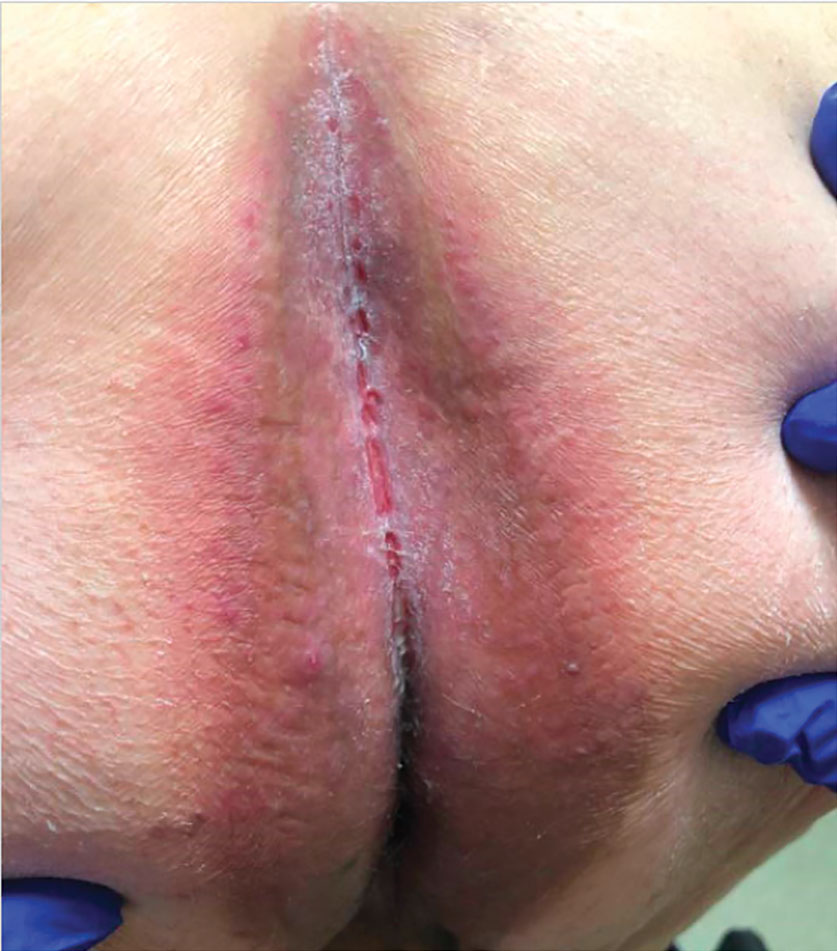
Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.
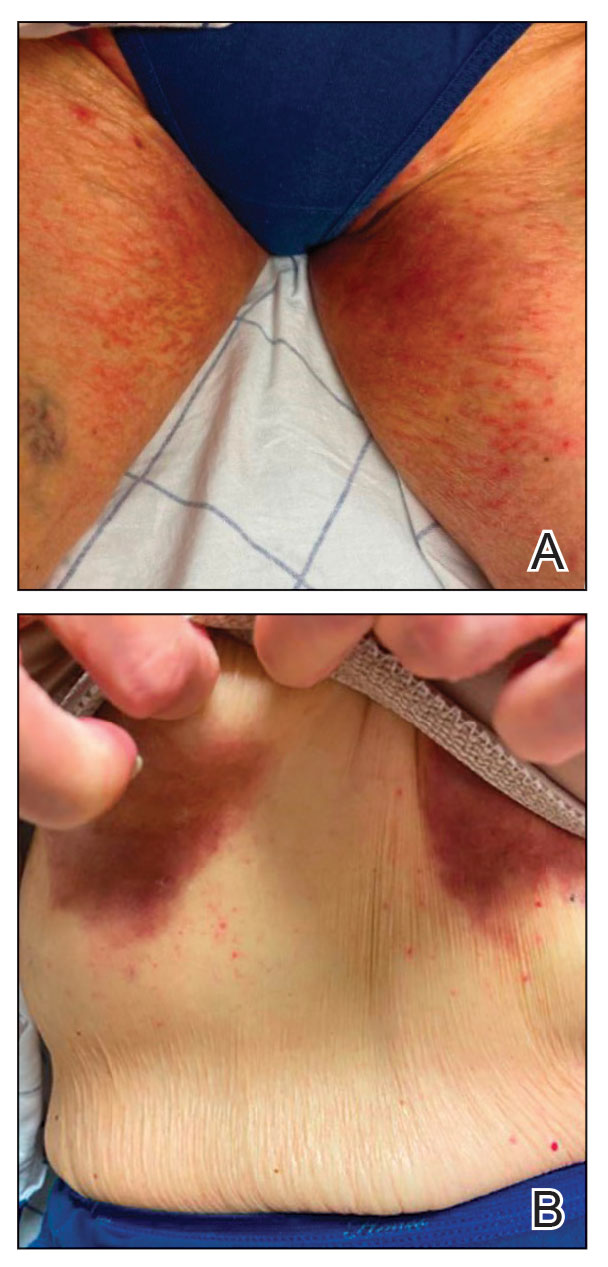
Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.
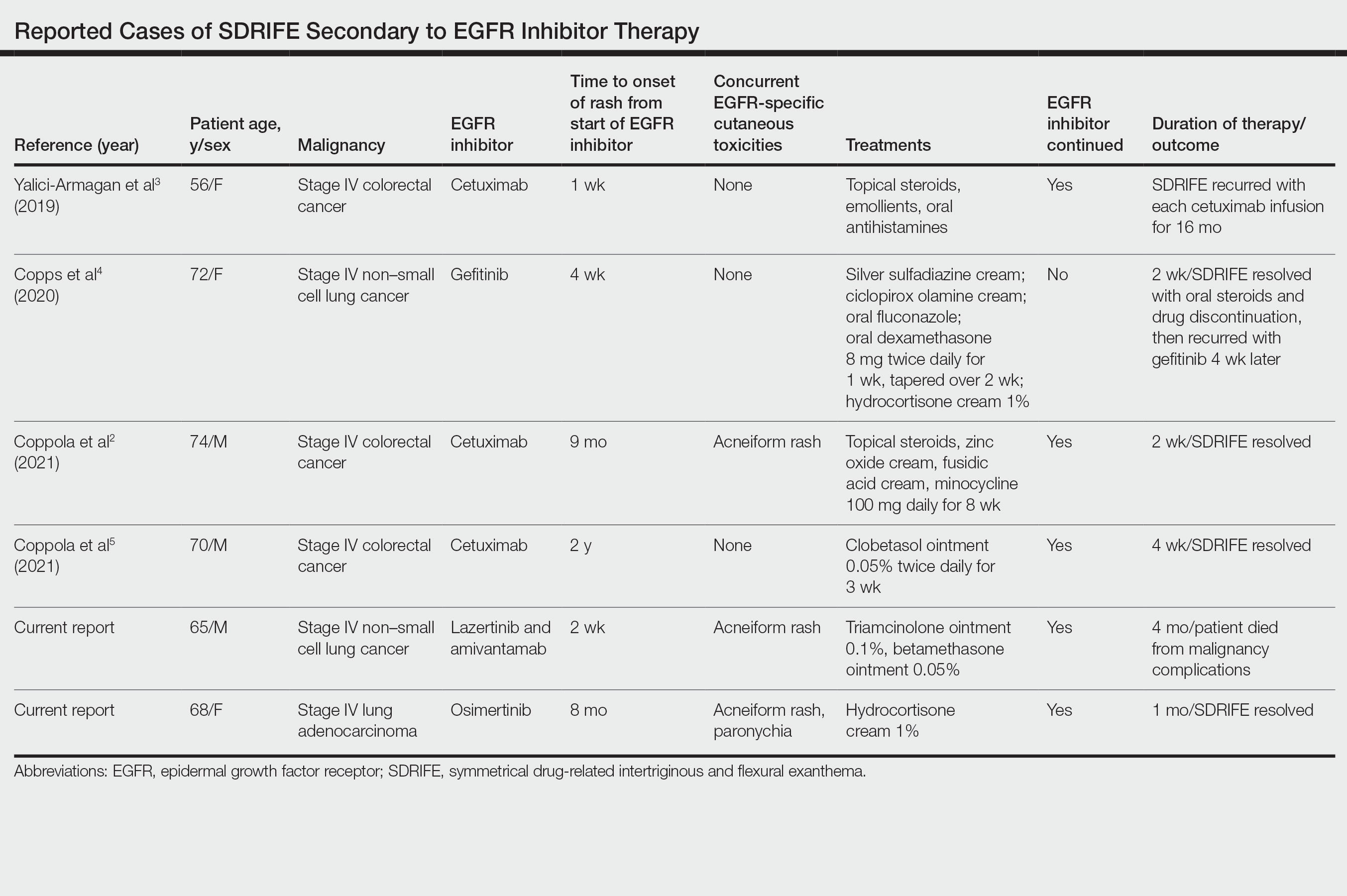
Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
Epidermal growth factor receptor (EGFR) inhibitors cause numerous cutaneous adverse events (AEs), including papulopustular eruptions, paronychia, acral fissures, xerosis, alopecia, and trichomegaly.1 Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon type IV hypersensitivity reaction reported most commonly in association with β-lactam antibiotics and other medications.2 Treatment of SDRIFE generally involves withdrawing the inciting medication; however, in SDRIFE secondary to oncologic therapies, medication withdrawal may not be feasible or desirable. We present 2 cases of SDRIFE secondary to EGFR inhibitors in which treatment was continued alongside supportive skin-directed therapies. We also review the literature.
Case Reports
Patient 1—A 65-year-old man with stage IV non–small cell lung cancer presented to the dermatology clinic with an eruption of 2 months’ duration that began in the periumbilical area and spread to the perianal area within 2 weeks of starting treatment with lazertinib and amivantamab. Physical examination was notable for Common Terminology Criteria for Adverse Events (CTCAE) Grade 2 periumbilical erythema and erosions as well as symmetric red-brown patches with linear erosions in the gluteal cleft (Figure 1) and Grade 2 facial papulopustular rash. Herpes simplex virus polymerase chain reaction and bacterial culture were negative. A skin biopsy from the left buttock revealed dermal edema and a perivascular lymphocytic infiltrate compatible with SDRIFE. Triamcinolone ointment 0.1% twice daily was initiated, then uptitrated to betamethasone ointment 0.05% twice daily with moderate improvement. The patient had a treatment interruption due to malignancy complications, at which time his skin improved, with recurrence of the eruption after treatment re-initiation. He resumed skin-directed treatment and was maintained on betamethasone ointment 0.05% and tacrolimus ointment 0.1% twice daily on alternating days. This treatment was continued for 4 months before the patient died from complications of the malignancy.

Patient 2—A 68-year-old woman with stage IV lung adenocarcinoma presented to the dermatology clinic with a rash of 3 weeks’ duration. Treatment with osimertinib was initiated 8 months prior to presentation, and there were no recent medication changes. Physical examination revealed CTCAE Grade 2 erythematous patches in the inguinal folds (Figure 2A), inframammary folds (Figure 2B), and on the nasal tip, as well as Grade 2 paronychia. The patient was managed with hydrocortisone cream 1% twice daily, and osimertinib was continued. At follow-up 4 weeks later, the erythema had faded to hyperpigmentation in affected areas with resolution of symptoms. No further treatment was required.

Comment
Supportive oncodermatologists and dermatology hospitalists should be aware of SDRIFE as an uncommon but increasingly recognized cutaneous AE of EGFR inhibitors. Other cases of SDRIFE secondary to EGFR inhibition are described in the Table.2-5 Although SDRIFE typically is treated by discontinuation of the offending agent, in all reported cases of EGFR inhibitor–associated SDRIFE the rash was CTCAE Grade 2, meaning that it did not interfere with instrumental activities of daily living. In 5 of 6 cases, EGFR therapy was continued while skin-directed therapies were used for symptom management.

Presentation of SDRIFE—Symmetrical drug-related intertriginous and flexural exanthema is characterized by a symmetric, sharply demarcated erythema in the inguinal, gluteal, or perianal area with at least 1 other flexural localization involved in the absence of systemic signs. It is observed most frequently at initial exposure or re-exposure to a medication. Onset typically is within a few hours to a few days after exposure to a medication.6 Interestingly, in this case series, half of reported SDRIFE cases developed 8 months or more after EGFR inhibitor initiation.
Pathophysiology of SDRIFE—The mechanism of SDRIFE has not been clearly elucidated; it generally is accepted to be a delayed-type hypersensitivity drug reaction, though other proposed pathophysiologic mechanisms for the distribution of SDRIFE include recall phenomenon or predisposing anatomic factors such as temperature, humidity, and apocrine or eccrine gland density.6,7 Epidermal growth factor receptor plays a critical role in regulating differentiation and proliferation of epidermal keratinocytes, hair follicles, and the sweat gland apparatus. Additionally, it has been hypothesized that EGFR inhibitor use may affect the microflora of the skin and that EGFR inhibitors directly affect the immune system, as demonstrated in an experiment showing EGFR inhibitor–treated mice had enhanced skin inflammation and contact hypersensitivity responses.8 How these disparate mechanisms may interact to produce SDRIFE and the reason for the notably delayed presentation of SDRIFE in half of the cases we reviewed is not known. Other delayed cutaneous AEs of EGFR inhibitor therapy, such as paronychia, are thought to be secondary to development of skin fragility and decreased keratinocyte proliferation with secondary infection.1 It is conceivable that a combination of proliferative, immunologic, and microbiome-related factors may each be playing a role in EGFR inhibitor–related SDRIFE.
Dermatology Inpatient Considerations—As seen in our cases, dermatologists can play a valuable role in diagnosing, grading, and managing cutaneous AEs associated with the administration of oncologic therapies. The array of cutaneous AEs has grown as cancer treatment options have expanded from conventional antimetabolite agents to kinase inhibitors and immune checkpoint inhibitors. Dermatologists may play an important role in differentiating the etiology of a skin finding (eg, infectious vs inflammatory) and can identify serious or dose-limiting reactions, such as Stevens-Johnson syndrome or drug reaction with eosinophilia and systemic symptoms (DRESS). If cutaneous AEs appear to occur secondary to administration of a chemotherapeutic agent, use of the National Cancer Institute CTCAE should be employed. For certain AEs (eg, alopecia, acneiform rashes, bullous dermatitis), specific grading has been developed based on a combination of body surface area involved, psychosocial impact, symptoms, and other associated morbidity.9
In management of chemotherapy-associated cutaneous AEs, dermatologists are likely to be the members of the health care team most comfortable with prescribing high-potency anti-inflammatory topical medications. Dermatologic consultation for management of cutaneous AEs has been shown to both reduce the need for systemic immunosuppression and limit interruptions in oncologic treatment.10
Conclusion
Epidermal growth factor receptor inhibitors commonly are prescribed for colorectal cancer, non–small cell lung cancer, and squamous cell carcinoma of the head and neck. They are associated with a variety of cutaneous AEs, including acneiform eruptions, paronychia, and xerosis, which rarely necessitate stopping EGFR inhibitor therapy. Our cases support an approach to managing EGFR inhibitor–related SDRIFE that does not involve discontinuation of the offending agent. Further studies are needed on the best supportive topical and systemic regimens for EGFR inhibitor–associated SDRIFE.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
- Hu JC, Sadeghi P, Pinter-Brown LC, et al. Cutaneous side effects of epidermal growth factor receptor inhibitors: clinical presentation, pathogenesis, and management. J Am Acad Dermatol. 2007;56:317-326.
- Coppola R, Santo B, Silipigni S, et al. Symmetrical drug-related intertriginous and flexural exanthema and acneiform eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:331-332.
- Yalici-Armagan B, Ayanoglu BT, Demirdag HG. Targeted tumour therapy induced papulopustular rash and other dermatologic side effects: a retrospective study. Cutan Ocul Toxicol. 2019;38:261-266.
- Copps B, Lacroix JP, Sasseville D. Symmetrical drug-related intertriginous and flexural exanthema secondary to epidermal growth factor receptor inhibitor gefitinib. JAAD Case Rep. 2020;6:172-175.
- Coppola R, Santo B, Ramella S, et al. Novel skin toxicity of epidermal growth factor receptor inhibitors: a case of intertrigo-like eruption in a patient with metastatic colorectal cancer treated with cetuximab. Clin Cancer Investig J. 2021;10:91-92.
- Häusermann P, Harr T, Bircher AJ. Baboon syndrome resulting from systemic drugs: is there strife between SDRIFE and allergic contact dermatitis syndrome? Contact Dermatitis. 2004;51:297-310.
- Wolf R, Orion E, Matz H. The baboon syndrome or intertriginous drug eruption: a report of eleven cases and a second look at its pathomechanism. Dermatol Online J. 2003;9:2.
- Mascia F, Mariani V, Girolomoni G, et al. Blockade of the EGF receptor induces a deranged chemokine expression in keratinocytes leading to enhanced skin inflammation. Am J Pathol. 2003;163:303-312.
- National Cancer Institute (U.S.). Common Terminology Criteria for Adverse Events: (CTCAE), Version 5.0. US Department of Health and Human Services; 2017. Accessed December 16, 2022. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/CTCAE_v5_Quick_Reference_8.5x11.pdf
- Chen ST, Molina GE, Lo JA, et al. Dermatology consultation reduces interruption of oncologic management among hospitalized patients with immune-related adverse events: a retrospective cohort study. J Am Acad Dermatol. 2020;82:994-996.
Practice Points
- Symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) is an uncommon but increasingly recognized cutaneous adverse event (AE) of epidermal growth factor receptor (EGFR) inhibitors.
- Epidermal growth factor receptor inhibitor–associated SDRIFE may be approached similarly to other EGFR inhibitor–related cutaneous AEs in that it may not require discontinuation of the offending agent.
Alopecia Areata in Skin of Color Patients: New Considerations Sparked by the Approval of Baricitinib
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
With the introduction of the first US Food and Drug Administration (FDA)–approved medication for alopecia areata (AA)—the Janus kinase (JAK) inhibitor, baricitinib—there is an important focus on this disease in the literature and for practicing dermatologists. Known by all as an autoimmune genetic disease that causes relapsing and remitting nonscarring hair loss, AA is a condition where the psychological burden has been less widely recognized. Patients with AA have reported lower health-related quality of life scores compared to patients with other skin conditions, including psoriasis or atopic dermatitis. In addition, a lesser amount of scalp coverage is negatively correlated to health-related quality of life scores.1 Patients with AA also have a 39% lifetime prevalence of major depressive disorder and generalized anxiety disorder.2 The treatment of AA has been a hodgepodge of topical, intralesional, and systemic agents, all with indirect immunosuppressive or anagen prolongation effects. Now that there is an approved therapy for AA with more treatments likely to be approved in the near future, there must be a focus on real-world outcomes. With the dawn of a new era in the treatment of AA as well as new information showcasing an altered prevalence of AA in skin of color, highlighting disparities among this population may help ease challenges dermatologic providers will face.
Efficacy of Baricitinib in Different Races and Ethnicities
How will patients of different races and ethnicities respond to this new treatment, and how will their emotional health be affected? The 2 phase 3 pivotal trials showing efficacy of baricitinib in AA included Black and Latino patients but not in a way that is representative of the US population.3 Until recently, the most commonly used prevalence of AA in the United States was from the NHANES I study completed between 1971 and 1974, which was between 0.1% and 0.2%4 with minimal focus on race and ethnicity. Recent studies suggest that there may be increased prevalence of this condition in Black patients in the United States. These new findings raise concern around access to care and treatment and the need to tailor psychosocial interventions for populations that may not currently have these supports.
A large cross-sectional study published in 2020 demonstrated that these data remained similar, with a lifetime prevalence of 0.21%.5 Of the 45,016 participants—representative of the US population based on the 2015 US Census—the average age of AA patients was 41.2 years, with 61.3% being White and not of Hispanic origin.5 In recent years, other studies have challenged the narrative that AA predominantly affects White patients.6-8 A different cross-sectional study utilizing National Alopecia Areata Registry data from 2002 to 2016 suggested that Black patients have greater odds of developing AA.6 In this study of 2645 cases of AA, the odds ratios of developing the condition were 1.36 for Blacks, 0.53 for Asians, and 0.83 for Hispanics compared with the referent White population. These results were consistent through the varying subtypes of AA.6 In a reply to these findings, Gonzalez and Fleischer7 analyzed data from the 2007 to 2016 National Ambulatory Medical Care Survey database with a focus on racial and ethnic prevalence of AA. This study concluded that Latino and non-White individuals had an increased likelihood of clinician visits for AA compared with White individuals.7
More evidence of the Black predominance of AA was demonstrated in a study published in 2018. In this large-scale study, 63,960 women from the Nurses’ Health Study (NHS) and 88,368 women from the Nurses’ Health Study II (NHSII) were included to examine prevalence of disease among these US women.8 Analysis showed increased odds of AA based on self-reported race in Black and Hispanic women. Lifetime incidence of AA was greater in Black women, with 2.63 and 5.23 in NHS and NHSII, respectively. It was hypothesized that hairstyling practices in Black and Hispanic women may cause AA to be more noticeable,8 which may drive patients to seek medical evaluation.
Feaster and McMichael9 published information on the epidemiology of AA in a busy hair loss clinic. This retrospective single-institution study of 265 pediatric and adult Black patients with AA seen over a 5-year period showed that patients aged 18 to 34 years were most likely to present for care, which accounted for 35.8% of the study population, followed by patients aged 10 to 17 years, which accounted for 15.1%. This study also found that females were the larger segment of AA patients, with an increased distribution of disease in young patients. Most of these patients (68.2%) had patchy hair loss, and the ophiasis pattern was seen in 15.1%.9 Although the pathogenesis of AA is linked to autoimmunity,10 the leading cause for these epidemiologic findings of increased prevalence in Black patients is still uncertain.
Baricitinib for AA
In June 2022, the FDA announced the first biologic drug approved for the treatment of AA—baricitinib. Baricitinib is an oral, selective, reversible inhibitor of JAK1 and JAK2.3 The phase 3 trials for baricitinib—BRAVE-AA1 (N=654) and BRAVE-AA2 (N=546)—were conducted between March 2019 and May 2020. In these double-blind, parallel-group, randomized, placebo-controlled trials, 33% of the patient population receiving baricitinib accomplished 80% or more scalp coverage at 36 weeks. The Severity of Alopecia Tool (SALT) score also decreased to 20 or less in 36 weeks. The BRAVE-AA1 and BRAVE-AA2 trials consisted of a total of 1200 patients, with only 98 identifying as Black. Of these 98 patients, 33 were randomly selected to receive placebo.3 With studies now suggesting that Black individuals have greater odds of AA compared with White individuals6 and Black patients being more likely to seek medical care for AA,7 the BRAVE-AA1 and BRAVE-AA2 study population did not allow for significant comparative data for Black patients. These studies did not document Latino patient involvement.3 Future studies in AA must recruit a diversified group of study participants to better reflect the patients with an increased likelihood of presenting with AA.
Other Treatments on the Horizon
Baricitinib likely will remain alone in its class for only a short time. Phase 3 trials have been completed for ritlecitinib, brepocitinib, and deuruxolitinib for AA. Ritlecitinib, an irreversible inhibitor of JAK3 and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) kinase family, has met all end points in a phase 2b/3 study.11 Brepocitinib is an oral tyrosine kinase 2/JAK1 inhibitor,12 and deuruxolitinib is an investigational JAK1/2 inhibitor for AA.13
Insurance Coverage Considerations and Health Care Disparities
Prior authorizations have been the initial step for many drugs in varying fields of medical practice. A study completed in 2016 suggested that insurance coverage for biologics used in the treatment of psoriasis was becoming increasingly difficult.14 Prior authorization requirement rates increased from 16% of patients in 2009 to 75% in 2014. The decision time also increased from 3.7 days in 2009 to 6.7 days in 2014. The most common reason for delay in decisions and denials was due to step therapy.14 Insurance companies wanted many patients to try less expensive treatment options prior to “stepping up” to more expensive treatments. Although this may be the case in the treatment of psoriasis, the role of step therapy is unclear for patients with AA because there is only 1 FDA-approved medication. This sets out an ambiguous future for our patients with AA and approval for baricitinib.
The time required for the correspondence between insurance companies, clinic staff, and patients for drug approval may delay treatments, and not all providers have enough staff to coordinate and perform this work. For Black patients, who may present more frequently and with more severe disease,7 this could lead to a health care disparity due to the likelihood of the increased need for biologic treatment. Because Black patients have an increased likelihood of being uninsured or underinsured,15 this further decreases the chances of the most severe AA patients receiving the most helpful medication for their condition.
Many pharmaceutical companies have drug cost assistance programs that aim to provide support covering expensive medications for patients unable to afford them. Although this is a good first step, treatment with any JAK inhibitor potentially can be lifelong. Regarding the social determinants of health, it is known that access to medications does not solely depend on cost. Transportation and access to qualified health professionals are among the issues that create barriers to health care. Instilling long-term practices to ensure equal access to JAK inhibitors and treatment of AA may be the cornerstone to treating AA with equity. Whether we require pharmaceutical companies to make sure all patients have equal access to medications or provide community resources to hairstylists and federally qualified health centers, raising awareness and advocating for and creating attainable access to treatment modalities is imperative to providing well-rounded care to a diverse population.
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
- Liu LY, King BA, Craiglow BG. Health-related quality of life (HRQoL) among patients with alopecia areata (AA): a systematic review. J Am Acad Dermatol. 2016;75:806-812.e3.
- Colón EA, Popkin MK, Callies AL, et al. Lifetime prevalence of psychiatric disorders in patients with alopecia areata. Compr Psychiatry. 1991;32:245-251.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Safavi K. Prevalence of alopecia areata in the First National Health and Nutrition Examination Survey. Arch Dermatol. 1992;128:702. doi:10.1001/archderm.1992.01680150136027
- Benigno M, Anastassopoulos KP, Mostaghimi A, et al. A large cross-sectional survey study of the prevalence of alopecia areata in the United States. Clin Cosmet Investig Dermatol. 2020;13:259-266.
- Lee H, Jung SJ, Patel AB, et al. Racial characteristics of alopecia areata in the United States. J Am Acad Dermatol. 2020;83:1064-1070.
- Gonzalez T, Fleischer AB Jr. Reply to: racial characteristics of alopecia areata in the United States [published online March 3, 2021]. J Am Acad Dermatol. 2021;84:E295-E296. doi:10.1016/j.jaad.2021.02.063
- Thompson JM, Park MK, Qureshi AA, et al. Race and alopecia areata amongst US women. J Investig Dermatol Symp Proc. 2018;19:S47-S50.
- Feaster B, McMichael AJ. Epidemiology of alopecia areata in Black patients: a retrospective chart review. J Am Acad Dermatol. 2022;87:1121-1123. doi.org/10.1016/j.jaad.2022.01.033
- Barahmani N, de Andrade M, Slusser JP, et al. Human leukocyte antigen class II alleles are associated with risk of alopecia areata. J Invest Dermatol. 2008;128:240-243.
- Xu H, Jesson MI, Seneviratne UI, et al. PF-06651600, a dual JAK3/TEC family kinase inhibitor. ACS Chem Biol. 2019;14:1235-1242.
- Fensome A, Ambler CM, Arnold E, et al. Dual inhibition of TYK2and JAK1 for the treatment of autoimmune diseases: discovery of((S)-2,2-difluorocyclopropyl)((1 R,5 S)-3-(2-((1-methyl-1 H-pyrazol-4-yl) amino)pyrimidin-4-yl)-3,8-diazabicyclo3.2.1octan-8-yl)methanone (PF-06700841). J Med Chem. 2018;61:8597-8612.
- King B, Mesinkovska N, Mirmirani P, et al. Phase 2 randomized, dose-ranging trial of CTP-543, a selective Janus kinase inhibitor, in moderate-to-severe alopecia areata [published online March 29, 2022]. J Am Acad Dermatol. 2022;87:306-313. doi:10.1016/j.jaad.2022.03.045
- Abdelnabi M, Patel A, Rengifo-Pardo M, et al. Insurance coverage of biologics for moderate-to-severe psoriasis: a retrospective, observational 5-year chart review. Am J Clin Dermatol. 2016;17:421-424. doi:10.1007/s40257-016-0194-4
- Office of the Assistant Secretary for Planning and Evaluation, U.S. Department of Health and Human Services. Health insurance coverage and access to care among black Americans: recent trends and key challenges (Issue Brief No. HP-2022-07). February 22, 2022. Accessed December 21, 2022. https://aspe.hhs.gov/sites/default/files/documents/08307d793263d5069fdd6504385e22f8/black-americans-coverages-access-ib.pdf
How to Optimize Wound Closure in Thin Skin
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.
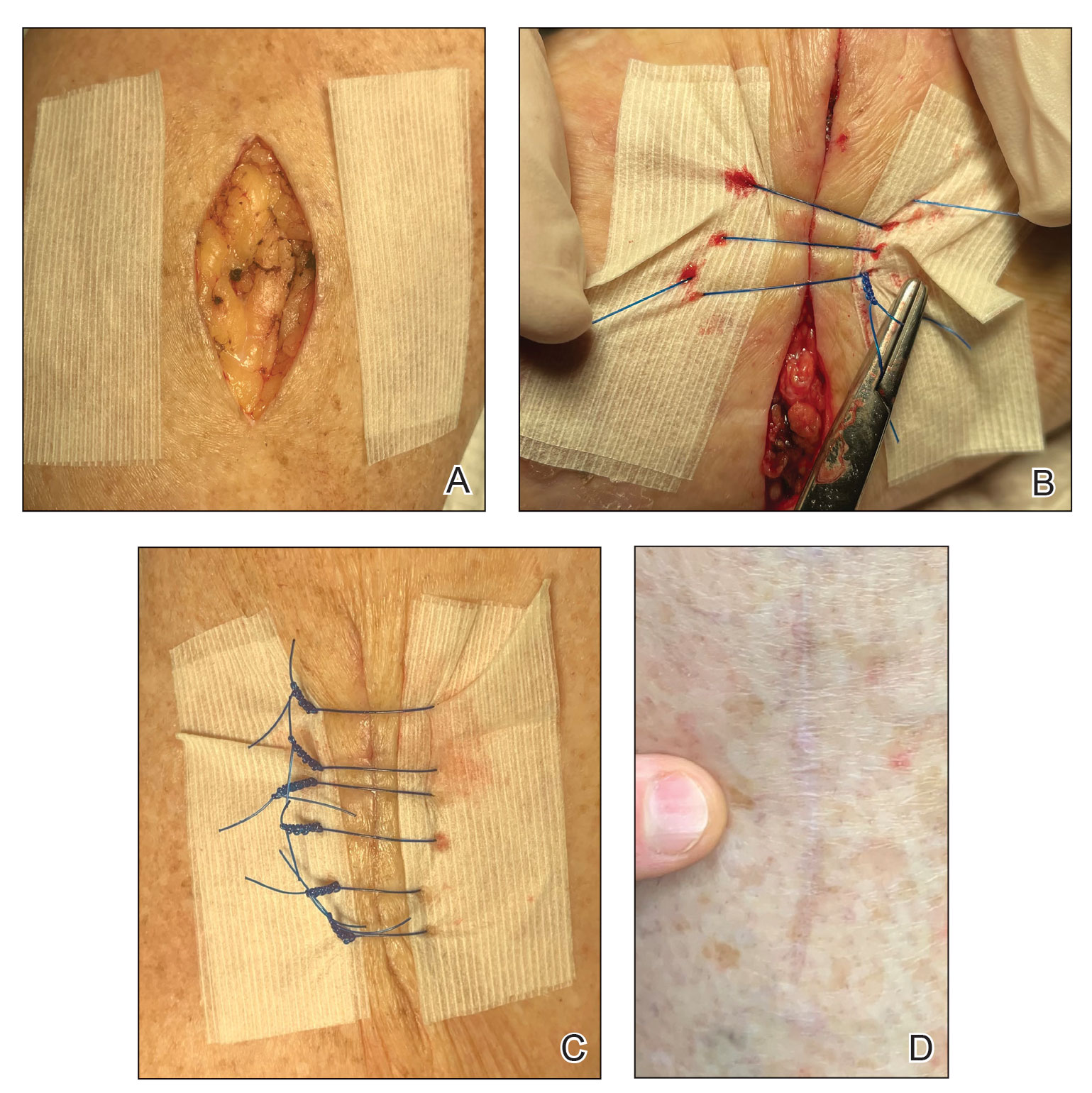
Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.

Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
Practice Gap
Cutaneous surgery involves many areas where skin is quite thin and fragile, which often is encountered in elderly patients; the forearms and lower legs are the most frequent locations for thin skin.1 Dermatologic surgeons frequently encounter these situations, making this a highly practical arena for technical improvements.
For many of these patients, there is little meaningful dermis for placement of subcutaneous sutures. Therefore, a common approach following surgery, particularly following Mohs micrographic surgery in which tumors and defects typically are larger, is healing by secondary intention.2 Although healing by secondary intention often is a reasonable option, we have found that maximizing the use of epidermal skin for primary closure can be an effective means of closing many such defects. Antimicrobial reinforced skin closure strips have been incorporated in wound closure for thin skin. However, earlier efforts involving reinforcement perpendicular to the wound lacked critical details or used a different technique.3
The Technique
We developed a novel effective closure technique that minimizes these problems. Our technique has been used on the wounds of hundreds of patients with satisfying results. Early on, we used multiple variations to optimize outcomes, including different sizes of sutures and reinforced skin closure strips, application of medical liquid adhesive, liquid adhesive, and varying postoperative dressings. For 3 years, we tracked outcomes in-house and gradually narrowed down our successes into a single, user-friendly paradigm.
Supplies—To perform this technique, required supplies include:
• 2-0 Polypropylene suture with a PS-2 needle, or the equivalent. Polyglactin or silk suture can be utilized if a less-rigid suture is desired; however, we primarily have used polypropylene for repairs with good results. Each repair requires at least 2 sutures.
• Reinforced skin closure strips (1×5 inches). This width affords increased strength.
• Conforming stretch bandage and elastic self-adherent wrap.
• Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum)(Johnson & Johnson).
• All usual surgical instruments and supplies, including paper tape and nonadherent gauze (surgeon dependent).
Step-by-step Technique—Close the wound using the following steps:
1. Once the defect is finalized following Mohs micrographic surgery or excision, excise the ellipse to be utilized for the closure and perform complete hemostasis.
2. Place 2 layers of reinforced skin closure strips—one on top of the other—along each side of the defect, leaving approximately 1 cm of uncovered skin between the wound edges and the reinforced skin closure strips (Figure, A).
3. Take a big-bite pulley suture about one-third of the way from one end of the ellipse, with both punctures passing through the reinforced skin closure strips. Leave that in place or have the assistant hold it and wait. Place a second suture immediately adjacent to the pulley suture. Once that suture is placed but still untied, have the assistant carefully pull the pulley suture outward away from the wound edge while you carefully bring the suture together and tie it off gently (Figure, B). Doing this utilizes the pulley ability of the suture to protect the skin from tearing and releases sufficient pressure on the single suture so that it can be easily tightened without risk to the fragile skin.
4. Repeat step 3, this time placing a pulley suture near the midline of the ellipse and the subsequent single suture adjacent to it.
5. Take pulley sutures repeatedly as in steps 3 and 4 until multiple sutures are secured in place. Replace the pulley sutures with single sutures because the double-pulley sutures in areas of lower vascularity tend to have, in our experience, a slightly increased incidence of focal necrosis in comparison to single sutures.
6. Make a concerted attempt to keep as much blood as possible off the reinforced skin closure strips throughout the procedure; the less dried blood on the reinforced skin closure strips, the cleaner and better the final closure (Figure, C).
7. Most of these cases involve the forearms and the legs below the knees. Because any increase in pressure or swelling on the wound can result in skin breakdown, postoperative dressing is critical. We use a layered approach; the following sequence can be modified to the preference of the surgeon: Polysporin (bacitracin zinc, polymyxin B sulfate, and petrolatum), nonadherent gauze, paper tape, conforming stretch bandage, and elastic self-adherent wrap. Minimizing swelling and infection are the primary goals. The wrap is left on for 1 week and should be kept dry.
8. Have the patient return to the office in 1 week. Unwrap the entire wound; trim back the reinforced skin closure strips; and have the patient utilize typical wound care at home thereafter consisting of cleaning and application of Polysporin or plain petrolatum, nonadherent gauze, and a paper-tape bandage. Because liquid adhesive is not utilized in this technique, the reinforced skin closure strips can be carefully removed without tearing skin. Leave sutures in for 3 weeks for arm procedures and 4 weeks for leg procedures, unless irritation develops or rapid suture overgrowth occurs in either location.
Complications
Most outcomes after using this technique are typical of optimized linear surgeries, with reduced scarring and complete wound healing (Figure, D). We seldom see complications but the following are possible:
• Bleeding occurs but rarely; the weeklong wrap likely provides great benefit.
• Infection is rare but does occur occasionally, as in any surgical procedure.
• Breakdown of the entire wound is rare; however, we occasionally see focal necrosis near 1 stitch—or rarely 2 stitches—that does not require intervention, apart from longer use of topical Polysporin or petrolatum alone to maximize healing by secondary intention in those small areas.• Despite simple suture placement far from the edge of the wound, wound inversion is seldom a problem because these taut closures have a tendency to expand slightly due to postoperative swelling.

Practice Implications
Any experienced dermatologic surgeon can perfect this technique for closing a wound in thin skin. Because wound closure in areas of fragile skin frequently is encountered in cutaneous surgery, we hope that utilizing this technique results in an optimal outcome for many patients.
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077
- Shuster S, Black MM, McVitie E. The influence of age and sex on skin thickness, skin collagen and density. Br J Dermatol. 1975;93:639-643. doi:10.1111/j.1365-2133.1975.tb05113.x
- Molina GE, Yu SH, Neel VA. Observations regarding infection risk in lower-extremity wound healing by second intention. Dermatol Surg. 2020;46:1342-1344. doi:10.1097/DSS.0000000000002094
- Davis M, Nakhdjevani A, Lidder S. Suture/Steri-Strip combination for the management of lacerations in thin-skinned individuals. J Emerg Med. 2011;40:322-323. doi:10.1016/j.jemermed.2010.05.077