User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
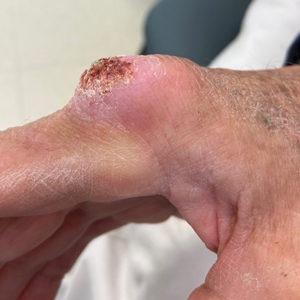
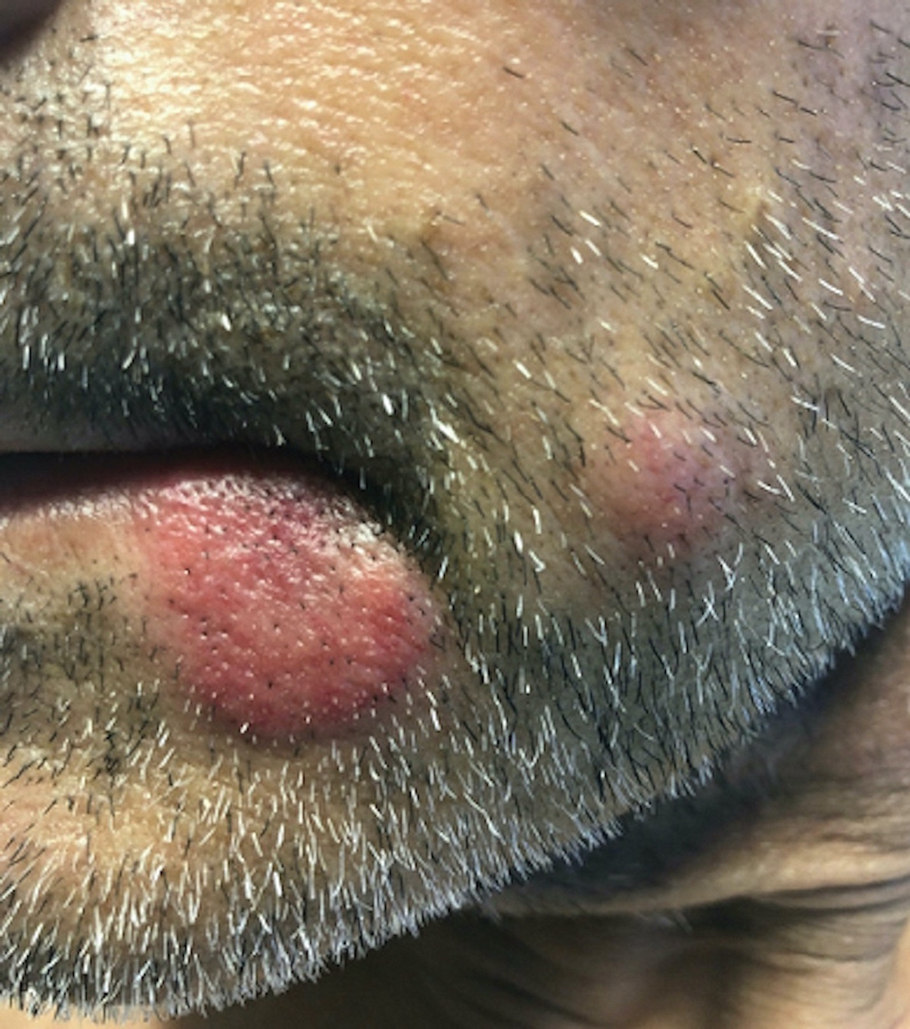
Atypical Keratotic Nodule on the Knuckle
The Diagnosis: Atypical Mycobacterial Infection
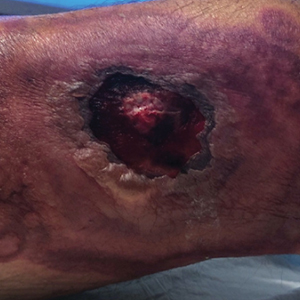
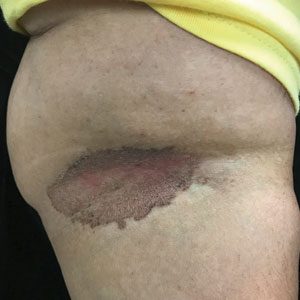
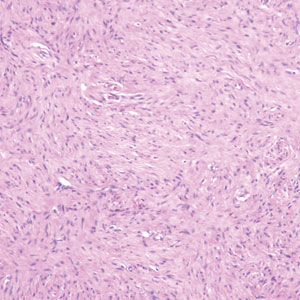
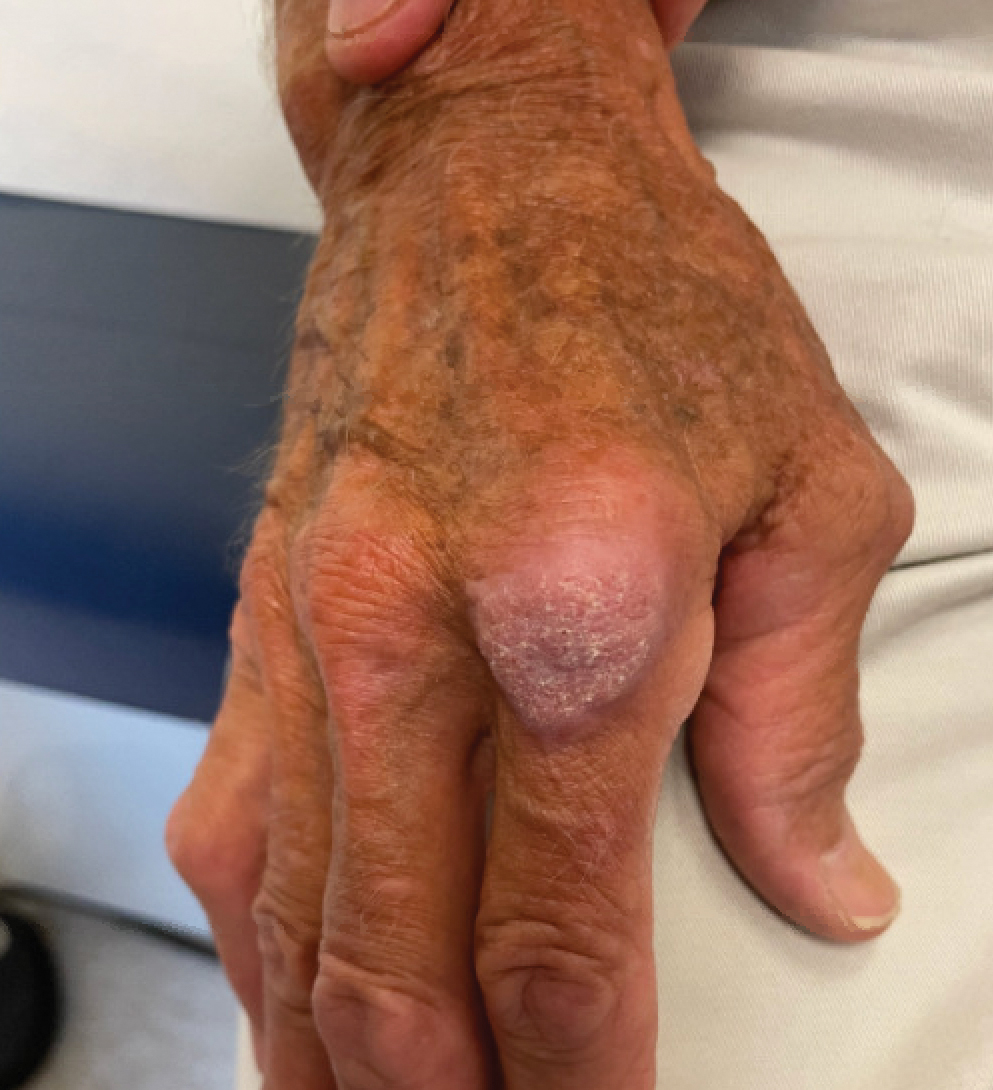
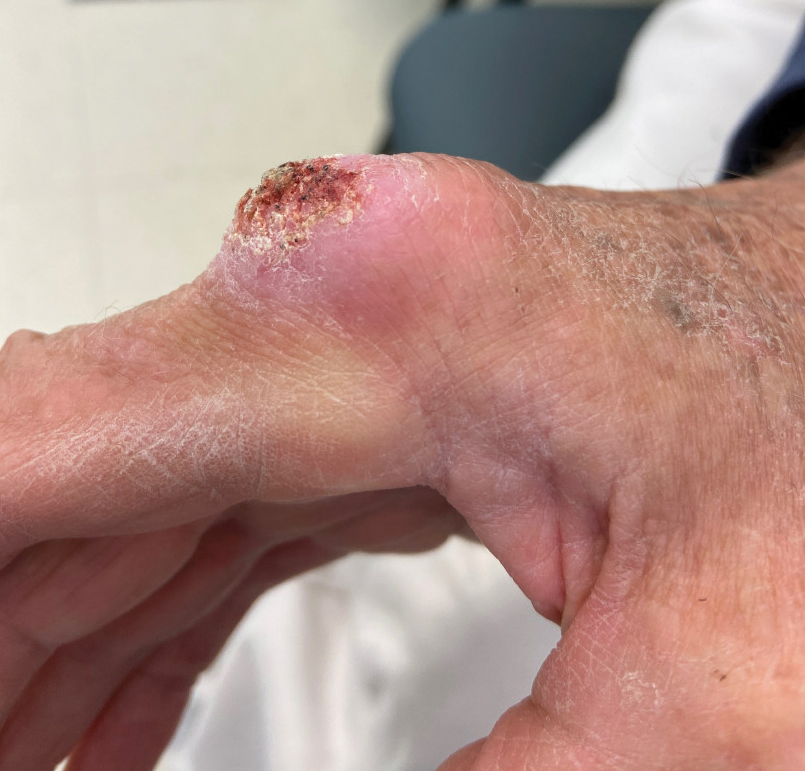
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.
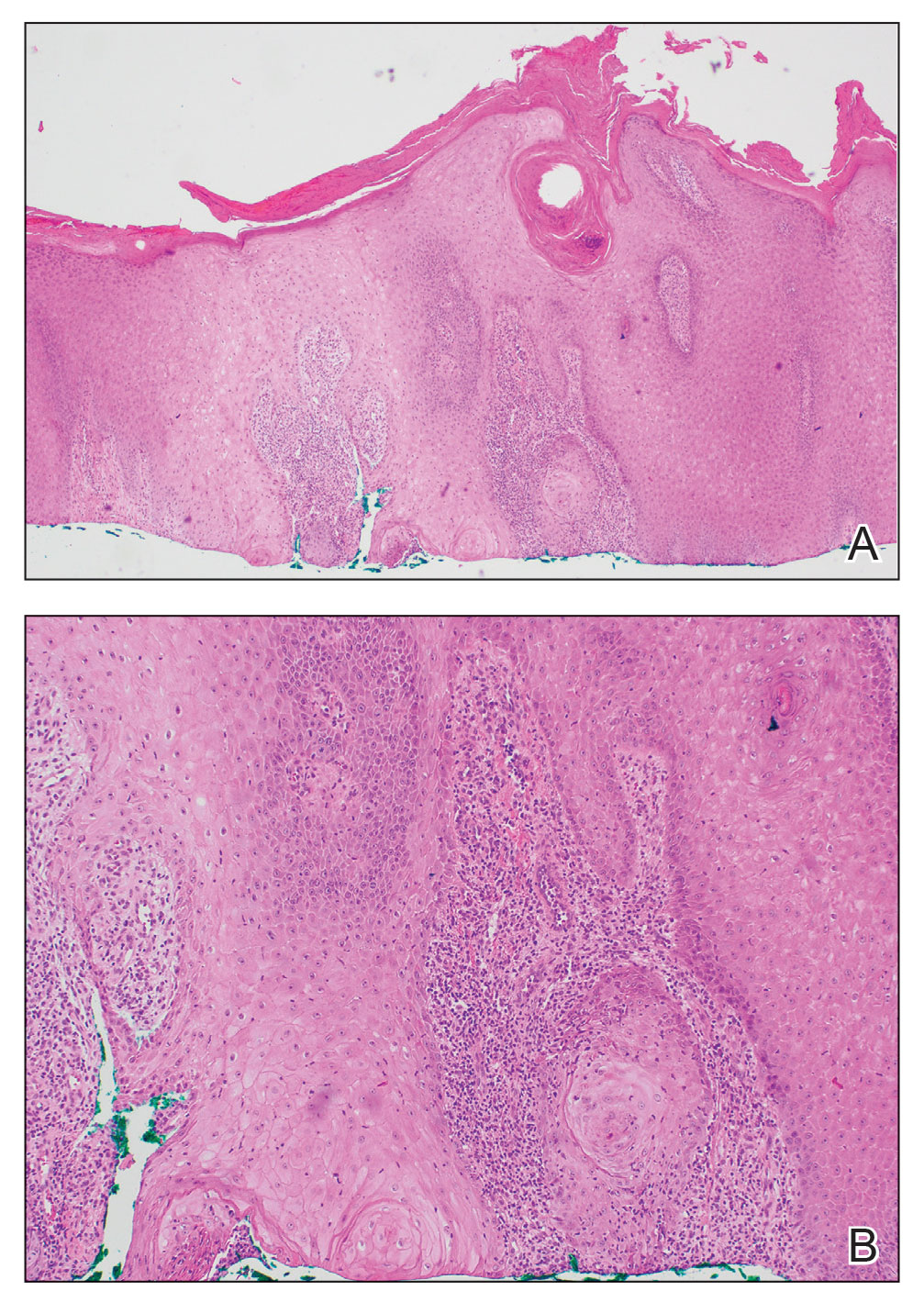
The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2
Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
The Diagnosis: Atypical Mycobacterial Infection
The history of rapid growth followed by shrinkage as well as the craterlike clinical appearance of our patient’s lesion were suspicious for the keratoacanthoma variant of squamous cell carcinoma (SCC). Periodic acid–Schiff green staining was negative for fungal or bacterial organisms, and the biopsy findings of keratinocyte atypia and irregular epidermal proliferation seemed to confirm our suspicion for well-differentiated SCC (Figure 1). Our patient subsequently was scheduled for Mohs micrographic surgery. Fortunately, a sample of tissue had been sent for panculture—bacterial, fungal, and mycobacterial—to rule out infectious etiologies, given the history of possible traumatic inoculation, and returned positive for Mycobacterium marinum infection prior to the surgery. Mohs surgery was canceled, and he was referred to an infectious disease specialist who started antibiotic treatment with azithromycin, ethambutol, and rifabutin. After 1 month of treatment the lesion substantially improved (Figure 2), further supporting the diagnosis of M marinum infection over SCC.

The differential diagnosis also included sporotrichosis, leishmaniasis, and chromoblastomycosis. Sporotrichosis lesions typically develop as multiple nodules and ulcers along a path of lymphatic drainage and can exhibit asteroid bodies and cigar-shaped yeast forms on histology. Chromoblastomycosis may display pseudoepitheliomatous hyperplasia and granulomatous inflammation; however, pathognomonic pigmented Medlar bodies also likely would be present.1 Leishmaniasis has a wide variety of presentations; however, it typically occurs in patients with exposure to endemic areas outside of the United States. Although leishmaniasis may demonstrate pseudoepitheliomatous hyperplasia, ulceration, and mixed inflammation on histology, it also likely would show amastigotes within dermal macrophages.2

Atypical mycobacterial infections initially may be misdiagnosed as SCC due to their tendency to induce irregular acanthosis in the form of pseudoepitheliomatous hyperplasia as well as mild keratinocyte atypia secondary to inflammation.3,4 Our case is unique because it occurred with M marinum infection specifically. The histopathologic findings of M marinum infections are variable and may additionally include granulomas, most commonly suppurative; intraepithelial abscesses; small vessel proliferation; dermal fibrosis; multinucleated giant cells; and transepidermal elimination.4,5 Periodic acid–Schiff, Ziehl-Neelsen (acid-fast bacilli), and Fite staining may be used to distinguish M marinum infection from SCC but have low sensitivities (approximately 30%). Culture remains the most reliable test, with a sensitivity of nearly 80%.5-7 In our patient, a Periodic acid–Schiff stain was obtained prior to receiving culture results, and acid-fast bacilli and Fite staining were added after the culture returned positive; however, all 3 stains failed to highlight any mycobacteria.
The primary risk factor for infection with M marinum is contact with aquatic environments or marine animals, and most cases involve the fingers or the hand.6 After we reached the diagnosis and further discussed the patient’s history, he recalled fishing for and cleaning raw shrimp around the time that he had a splinter. The Infectious Diseases Society of America recommends a treatment course extending 1 to 2 months after clinical symptoms resolve with ethambutol in addition to clarithromycin or azithromycin.8 If the infection is near a joint, rifampin should be empirically added to account for a potentially deeper infection. Imaging should be obtained to evaluate for joint space involvement, with magnetic resonance imaging being the preferred modality. If joint space involvement is confirmed, surgical debridement is indicated. Surgical debridement also is indicated for infections that fail to respond to antibiotic therapy.8
This case highlights M marinum infection as a potential mimicker of SCC, particularly if the biopsy is relatively superficial, as often occurs when obtained via the common shave technique. The distinction is critical, as M marinum infection is highly treatable and inappropriate surgery on the typical hand and finger locations may subject patients to substantial morbidity, such as the need for a skin graft, reduced mobility from scarring, or risk for serious wound infection.9 For superficial biopsies of an atypical squamous process, pathologists also may consider routinely recommending tissue culture, especially for hand and finger locations or when a history of local trauma is reported, instead of recommending complete excision or repeat biopsy alone.
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
- Elewski BE, Hughey LC, Hunt KM, et al. Fungal diseases. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1329-1363.
- Bravo FG. Protozoa and worms. In: Bolognia J, Schaffer JV, Cerroni L, eds. Dermatology. 4th ed. Elsevier; 2018:1470-1502.
- Zayour M, Lazova R. Pseudoepitheliomatous hyperplasia: a review. Am J Dermatopathol. 2011;33:112-122; quiz 123-126. doi:10.1097 /DAD.0b013e3181fcfb47
- Li JJ, Beresford R, Fyfe J, et al. Clinical and histopathological features of cutaneous nontuberculous mycobacterial infection: a review of 13 cases. J Cutan Pathol. 2017;44:433-443. doi:10.1111/cup.12903
- Abbas O, Marrouch N, Kattar MM, et al. Cutaneous non-tuberculous mycobacterial infections: a clinical and histopathological study of 17 cases from Lebanon. J Eur Acad Dermatol Venereol. 2011;25:33-42. doi:10.1111/j.1468-3083.2010.03684.x
- Johnson MG, Stout JE. Twenty-eight cases of Mycobacterium marinum infection: retrospective case series and literature review. Infection. 2015;43:655-662. doi:10.1007/s15010-015-0776-8
- Aubry A, Mougari F, Reibel F, et al. Mycobacterium marinum. Microbiol Spectr. 2017;5. doi:10.1128/microbiolspec.TNMI7-0038-2016
- Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367-416. doi:10.1164/rccm.200604-571ST
- Alam M, Ibrahim O, Nodzenski M, et al. Adverse events associated with Mohs micrographic surgery: multicenter prospective cohort study of 20,821 cases at 23 centers. JAMA Dermatol. 2013;149:1378-1385. doi:10.1001/jamadermatol.2013.6255
A 75-year-old man presented with a lesion on the knuckle of 5 months’ duration. He reported that the lesion initially grew very quickly before shrinking down to its current size. He denied any bleeding or pain but thought he may have had a splinter in the area around the time the lesion appeared. He reported spending a lot of time outdoors and noted several recent insect and tick bites. He also owned a boat and frequently went fishing. He previously had been treated for actinic keratoses but had no history of skin cancer and no family history of melanoma. Physical examination revealed a 2-cm erythematous nodule with central hyperkeratosis overlying the metacarpophalangeal joint of the right index finger. A shave biopsy was performed.
Feedback and Education in Dermatology Residency
A dermatology resident has more education and experience than a medical student or intern but less than a fellow or attending physician. Because of this position, residents have a unique opportunity to provide feedback and education to those with less knowledge and experience as a teacher and also to provide feedback to their more senior colleagues about their teaching effectiveness while simultaneously learning from them. The reciprocal exchange of information—from patients and colleagues in clinic, co-residents or attendings in lectures, or in other environments such as pathology at the microscope or skills during simulation training sessions—is the cornerstone of medical education. Being able to give effective feedback while also learning to accept it is one of the most vital skills a resident can learn to thrive in medical education.
The importance of feedback cannot be understated. The art of medicine involves the scientific knowledge needed to treat disease, as well as the social ability to educate, comfort, and heal those afflicted. Mastering this art takes a lifetime. The direct imparting of knowledge from those more experienced to those learning occurs via feedback. In addition, the desire to better oneself leads to more satisfaction with work and improved performance.1 The ability to give and receive feedback is vital for the field of dermatology and medicine in general.
Types and Implementation of Feedback
Feedback comes in many forms and can be classified via different characteristics such as formal vs informal, written vs spoken, real time vs delayed, and single observer vs pooled data. Each style of feedback has positive and negative aspects, and a feedback provider will need to weigh the pros and cons when deciding the most appropriate one. Although there is no one correct way to provide feedback, the literature shows that some forms of feedback may be more effective and better received than others. This can depend on the context of what is being evaluated.
Many dermatology residencies employ formal scheduled feedback as part of their curricula, ensuring that residents will receive feedback at preset time intervals and providing residency directors with information to assess improvement and areas where more growth is needed. The Accreditation Council for Graduate Medical Education provides a reference for programs on how to give this formal standardized feedback in The Milestones Guidebook.2 This feedback is a minimum required amount, with a survey of residents showing preference for frequent informal feedback sessions in addition to standardized formal feedback.3 Another study showed that residents want feedback that is confidential, in person, shortly after experiences, and specific to their actions.4 Medical students also voiced a need for frequent, transparent, and actionable feedback during protected, predetermined, and communicated times.5 Clearly, learners appreciate spoken intentional feedback as opposed to the traditional formal model of feedback.
Finally, a study was performed analyzing how prior generations of physician educators view millennial trainees.6 Because most current dermatology residents were born between 1981 and 1996, this study seemed to pinpoint thoughts toward teaching current residents. The study found that although negative judgments such as millennial entitlement (P<.001), impoliteness (P<.001), oversensitivity (P<.001), and inferior work ethic (P<.001) reached significance, millennial ideals of social justice (P<.001) and savviness with technology (P<.001) also were notable. Overall, millennials were thought to be good colleagues (P<.001), were equally competent to more experienced clinicians (P<.001), and would lead medicine to a good future (P=.039).6
Identifying and Maximizing the Impact of Feedback
In addition to how and when to provide feedback, there are discrepancies between attending and resident perception of what is considered feedback. This disconnect can be seen in a study of 122 respondents (67 residents and 55 attendings) that showed 31% of attendings reported giving feedback daily, as opposed to only 9% of residents who reported receiving daily feedback.4 When feedback is to be performed, it may be important to specifically announce the process so that it can be properly acknowledged.7
Beach8 provided a systematic breakdown of clinical teaching to those who may be unfamiliar with the process. This method is divided into preclinic, in-clinic, and postclinic strategies to maximize learning. The author recommended establishing the objectives of the rotation from the teacher’s perspective and inquiring about the objectives of the learner. Both perspectives should inform the lessons to be learned; for example, if a medical student expresses specific interest in psoriasis (a well-established part of a medical student curriculum), all efforts should be placed on arranging for that student to see those specific patients. Beach8 also recommended providing resources and creating a positive supportive learning environment to better utilize precious clinic time and create investment in all learning parties. The author recommended matching trainees during clinic to competence-specific challenges in clinical practice where appropriate technical skill is needed. Appropriate autonomy also is promoted, as it requires higher levels of learning and knowledge consolidation. Group discussions can be facilitated by asking questions of increasing levels of difficulty as experience increases. Finally, postclinic feedback should be timely and constructive.8
One technique discussed by Beach8 is the “1-minute preceptor plus” approach. In this approach, the teacher wants to establish 5 “micro-skills” by first getting a commitment, then checking for supportive evidence of this initial plan, teaching a general principle, reinforcing what was properly performed, and correcting errors. The “plus” comes from trying to take that lesson and apply it to a broader concept. Although this concept is meant to be used in a time-limited setting, it can be expanded to larger conversations. A common example could be made when residents teach rotating medical students through direct observation and supervision during clinic. In this hypothetical situation, the resident and medical student see a patient with erythematous silver-scaled plaques on the elbows and knees. During the patient encounter, the student then inquires about any personal history of cardiovascular disease, diabetes mellitus, and hypertension. After leaving the examination room, the medical student asserts the diagnosis is plaque psoriasis because of the physical examination findings and distribution of lesions. A discussion about the relationship between psoriasis and metabolic syndrome commences, emphasizing the pathophysiology of type 1 helper T-cell–mediated and type 17 helper T-cell–mediated inflammation with vascular damage and growth from inflammatory cytokines.9 The student subsequently is praised on inquiring about relevant comorbidities, and a relevant journal article is retrieved for the student’s future studies. Teaching points regarding the Koebner phenomenon, such as that it is not an instantaneous process and comes with a differential diagnosis, are then provided.
Situation-Behavior-Impact is another teaching method developed by the Center for Creative Leadership. In this technique, one will identify what specifically happened, how the learner responded, and what occurred because of the response.10 This technique is exemplified in the following mock conversation between an attending and their resident following a challenging patient situation: “When you walked into the room and asked the patient coming in for a follow-up appointment ‘What brings you in today?,’ they immediately tensed up and responded that you should already know and check your electronic medical record. This tension could be ameliorated by reviewing the patient’s medical record and addressing what they initially presented for, followed by inquiring if there are other skin problems they want to discuss afterwards.” By identifying the cause-and-effect relationship, helpful and unhelpful responses can be identified and ways to mitigate or continue behaviors can be brainstormed.
The Learning Process
Brodell et all11 outlined techniques to augment the education process that are specific to dermatology. They recommended learning general applicable concepts instead of contextless memorization, mnemonic devices to assist memory for associations and lists, and repetition and practice of learned material. For teaching, they divided techniques into Aristotelian or Socratic; Aristotelian teaching is the formal lecture style, whereas Socratic is conversation based. Both have a place in teaching—as fundamental knowledge grows via Aristotelian teaching, critical thinking can be enhanced via the Socratic method. The authors then outlined tips to create the most conducive learning environment for students.11
Feedback is a reciprocal process with information being given and received by both the teacher and the learner. This is paramount because perfecting the art of teaching is a career-long process and can only be achieved via correction of oversights and mistakes. A questionnaire-based study found that when critiquing the teacher, a combination of self-assessment with assessment from learners was effective in stimulating the greatest level of change in the teacher.12 This finding likely is because the educator was able to see the juxtaposition of how they think they performed with how students interpreted the same situation. Another survey-based study showed that of 68 attending physicians, 28 attendings saw utility in specialized feedback training; an additional 11 attendings agreed with online modules to improve their feedback skills. A recommendation that trainees receive training on the acceptance feedback also was proposed.13 Specialized training to give and receive feedback could be initiated for both attending and resident physicians to fully create an environment emphasizing improvement and teamwork.
Final Thoughts
The art of giving and receiving feedback is a deliberate process that develops with experience and training. Because residents are early in their medical career, being familiar with techniques such as those outlined in this article can enhance teaching and the reception of feedback. Residents are in a unique position, as residency itself is a time of dramatic learning and teaching. Providing feedback gives us a way to advance medicine and better ourselves by solidifying good habits and knowledge.
Acknowledgment—I thank Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and reviewing this article.
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Edgar L, McLean S, Hogan SO, et al. The Milestones Guidebook. Accreditation Council for Graduate Medical Education; 2020. Accessed December 12, 2022. https://www.acgme.org/globalassets/milestonesguidebook.pdf
- Wang JV, O’Connor M, McGuinn K, et al. Feedback practices in dermatology residency programs: building a culture for millennials. Clin Dermatol. 2019;37:282-283.
- Hajar T, Wanat KA, Fett N. Survey of resident physician and attending physician feedback perceptions: there is still work to be done. Dermatol Online J. 2020;25:13030/qt2sg354p6.
- Yoon J, Said JT, Thompson LL, et al. Medical student perceptions of assessment systems, subjectivity, and variability on introductory dermatology clerkships. Int J Womens Dermatol. 2021;7:232-330.
- Marka A, LeBoeuf MR, Vidal NY. Perspectives of dermatology faculty toward millennial trainees and colleagues: a national survey. Mayo Clin Proc Innov Qual Outcomes. 2021;5:65-71.
- Bernard AW, Kman NE, Khandelwal S. Feedback in the emergency medicine clerkship. West J Emerg Med. 2011;12:537-542.
- Beach RA. Strategies to maximise teaching in your next ambulatory clinic. Clin Teach. 2017;14:85-89.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases part I. epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Olbricht SM. What makes feedback productive? Cutis. 2016;98:222-223.
- Brodell RT, Wile MZ, Chren M, et al. Learning and teaching in dermatology: a practitioner’s guide. Arch Dermatol. 1996;132:946-952.
- Stalmeijer RE, Dolmans DHJM, Wolfhagen IHAP, et al. Combined student ratings and self-assessment provide useful feedback for clinical teachers. Adv in Health Sci Educ. 2010;15:315-328.
- Chelliah P, Srivastava D, Nijhawan RI. What makes giving feedback challenging? a survey of the Association of Professors of Dermatology (APD)[published online July 19, 2022]. Arch Dermatol Res. doi:10.1007/s00403-022-02370-y
A dermatology resident has more education and experience than a medical student or intern but less than a fellow or attending physician. Because of this position, residents have a unique opportunity to provide feedback and education to those with less knowledge and experience as a teacher and also to provide feedback to their more senior colleagues about their teaching effectiveness while simultaneously learning from them. The reciprocal exchange of information—from patients and colleagues in clinic, co-residents or attendings in lectures, or in other environments such as pathology at the microscope or skills during simulation training sessions—is the cornerstone of medical education. Being able to give effective feedback while also learning to accept it is one of the most vital skills a resident can learn to thrive in medical education.
The importance of feedback cannot be understated. The art of medicine involves the scientific knowledge needed to treat disease, as well as the social ability to educate, comfort, and heal those afflicted. Mastering this art takes a lifetime. The direct imparting of knowledge from those more experienced to those learning occurs via feedback. In addition, the desire to better oneself leads to more satisfaction with work and improved performance.1 The ability to give and receive feedback is vital for the field of dermatology and medicine in general.
Types and Implementation of Feedback
Feedback comes in many forms and can be classified via different characteristics such as formal vs informal, written vs spoken, real time vs delayed, and single observer vs pooled data. Each style of feedback has positive and negative aspects, and a feedback provider will need to weigh the pros and cons when deciding the most appropriate one. Although there is no one correct way to provide feedback, the literature shows that some forms of feedback may be more effective and better received than others. This can depend on the context of what is being evaluated.
Many dermatology residencies employ formal scheduled feedback as part of their curricula, ensuring that residents will receive feedback at preset time intervals and providing residency directors with information to assess improvement and areas where more growth is needed. The Accreditation Council for Graduate Medical Education provides a reference for programs on how to give this formal standardized feedback in The Milestones Guidebook.2 This feedback is a minimum required amount, with a survey of residents showing preference for frequent informal feedback sessions in addition to standardized formal feedback.3 Another study showed that residents want feedback that is confidential, in person, shortly after experiences, and specific to their actions.4 Medical students also voiced a need for frequent, transparent, and actionable feedback during protected, predetermined, and communicated times.5 Clearly, learners appreciate spoken intentional feedback as opposed to the traditional formal model of feedback.
Finally, a study was performed analyzing how prior generations of physician educators view millennial trainees.6 Because most current dermatology residents were born between 1981 and 1996, this study seemed to pinpoint thoughts toward teaching current residents. The study found that although negative judgments such as millennial entitlement (P<.001), impoliteness (P<.001), oversensitivity (P<.001), and inferior work ethic (P<.001) reached significance, millennial ideals of social justice (P<.001) and savviness with technology (P<.001) also were notable. Overall, millennials were thought to be good colleagues (P<.001), were equally competent to more experienced clinicians (P<.001), and would lead medicine to a good future (P=.039).6
Identifying and Maximizing the Impact of Feedback
In addition to how and when to provide feedback, there are discrepancies between attending and resident perception of what is considered feedback. This disconnect can be seen in a study of 122 respondents (67 residents and 55 attendings) that showed 31% of attendings reported giving feedback daily, as opposed to only 9% of residents who reported receiving daily feedback.4 When feedback is to be performed, it may be important to specifically announce the process so that it can be properly acknowledged.7
Beach8 provided a systematic breakdown of clinical teaching to those who may be unfamiliar with the process. This method is divided into preclinic, in-clinic, and postclinic strategies to maximize learning. The author recommended establishing the objectives of the rotation from the teacher’s perspective and inquiring about the objectives of the learner. Both perspectives should inform the lessons to be learned; for example, if a medical student expresses specific interest in psoriasis (a well-established part of a medical student curriculum), all efforts should be placed on arranging for that student to see those specific patients. Beach8 also recommended providing resources and creating a positive supportive learning environment to better utilize precious clinic time and create investment in all learning parties. The author recommended matching trainees during clinic to competence-specific challenges in clinical practice where appropriate technical skill is needed. Appropriate autonomy also is promoted, as it requires higher levels of learning and knowledge consolidation. Group discussions can be facilitated by asking questions of increasing levels of difficulty as experience increases. Finally, postclinic feedback should be timely and constructive.8
One technique discussed by Beach8 is the “1-minute preceptor plus” approach. In this approach, the teacher wants to establish 5 “micro-skills” by first getting a commitment, then checking for supportive evidence of this initial plan, teaching a general principle, reinforcing what was properly performed, and correcting errors. The “plus” comes from trying to take that lesson and apply it to a broader concept. Although this concept is meant to be used in a time-limited setting, it can be expanded to larger conversations. A common example could be made when residents teach rotating medical students through direct observation and supervision during clinic. In this hypothetical situation, the resident and medical student see a patient with erythematous silver-scaled plaques on the elbows and knees. During the patient encounter, the student then inquires about any personal history of cardiovascular disease, diabetes mellitus, and hypertension. After leaving the examination room, the medical student asserts the diagnosis is plaque psoriasis because of the physical examination findings and distribution of lesions. A discussion about the relationship between psoriasis and metabolic syndrome commences, emphasizing the pathophysiology of type 1 helper T-cell–mediated and type 17 helper T-cell–mediated inflammation with vascular damage and growth from inflammatory cytokines.9 The student subsequently is praised on inquiring about relevant comorbidities, and a relevant journal article is retrieved for the student’s future studies. Teaching points regarding the Koebner phenomenon, such as that it is not an instantaneous process and comes with a differential diagnosis, are then provided.
Situation-Behavior-Impact is another teaching method developed by the Center for Creative Leadership. In this technique, one will identify what specifically happened, how the learner responded, and what occurred because of the response.10 This technique is exemplified in the following mock conversation between an attending and their resident following a challenging patient situation: “When you walked into the room and asked the patient coming in for a follow-up appointment ‘What brings you in today?,’ they immediately tensed up and responded that you should already know and check your electronic medical record. This tension could be ameliorated by reviewing the patient’s medical record and addressing what they initially presented for, followed by inquiring if there are other skin problems they want to discuss afterwards.” By identifying the cause-and-effect relationship, helpful and unhelpful responses can be identified and ways to mitigate or continue behaviors can be brainstormed.
The Learning Process
Brodell et all11 outlined techniques to augment the education process that are specific to dermatology. They recommended learning general applicable concepts instead of contextless memorization, mnemonic devices to assist memory for associations and lists, and repetition and practice of learned material. For teaching, they divided techniques into Aristotelian or Socratic; Aristotelian teaching is the formal lecture style, whereas Socratic is conversation based. Both have a place in teaching—as fundamental knowledge grows via Aristotelian teaching, critical thinking can be enhanced via the Socratic method. The authors then outlined tips to create the most conducive learning environment for students.11
Feedback is a reciprocal process with information being given and received by both the teacher and the learner. This is paramount because perfecting the art of teaching is a career-long process and can only be achieved via correction of oversights and mistakes. A questionnaire-based study found that when critiquing the teacher, a combination of self-assessment with assessment from learners was effective in stimulating the greatest level of change in the teacher.12 This finding likely is because the educator was able to see the juxtaposition of how they think they performed with how students interpreted the same situation. Another survey-based study showed that of 68 attending physicians, 28 attendings saw utility in specialized feedback training; an additional 11 attendings agreed with online modules to improve their feedback skills. A recommendation that trainees receive training on the acceptance feedback also was proposed.13 Specialized training to give and receive feedback could be initiated for both attending and resident physicians to fully create an environment emphasizing improvement and teamwork.
Final Thoughts
The art of giving and receiving feedback is a deliberate process that develops with experience and training. Because residents are early in their medical career, being familiar with techniques such as those outlined in this article can enhance teaching and the reception of feedback. Residents are in a unique position, as residency itself is a time of dramatic learning and teaching. Providing feedback gives us a way to advance medicine and better ourselves by solidifying good habits and knowledge.
Acknowledgment—I thank Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and reviewing this article.
A dermatology resident has more education and experience than a medical student or intern but less than a fellow or attending physician. Because of this position, residents have a unique opportunity to provide feedback and education to those with less knowledge and experience as a teacher and also to provide feedback to their more senior colleagues about their teaching effectiveness while simultaneously learning from them. The reciprocal exchange of information—from patients and colleagues in clinic, co-residents or attendings in lectures, or in other environments such as pathology at the microscope or skills during simulation training sessions—is the cornerstone of medical education. Being able to give effective feedback while also learning to accept it is one of the most vital skills a resident can learn to thrive in medical education.
The importance of feedback cannot be understated. The art of medicine involves the scientific knowledge needed to treat disease, as well as the social ability to educate, comfort, and heal those afflicted. Mastering this art takes a lifetime. The direct imparting of knowledge from those more experienced to those learning occurs via feedback. In addition, the desire to better oneself leads to more satisfaction with work and improved performance.1 The ability to give and receive feedback is vital for the field of dermatology and medicine in general.
Types and Implementation of Feedback
Feedback comes in many forms and can be classified via different characteristics such as formal vs informal, written vs spoken, real time vs delayed, and single observer vs pooled data. Each style of feedback has positive and negative aspects, and a feedback provider will need to weigh the pros and cons when deciding the most appropriate one. Although there is no one correct way to provide feedback, the literature shows that some forms of feedback may be more effective and better received than others. This can depend on the context of what is being evaluated.
Many dermatology residencies employ formal scheduled feedback as part of their curricula, ensuring that residents will receive feedback at preset time intervals and providing residency directors with information to assess improvement and areas where more growth is needed. The Accreditation Council for Graduate Medical Education provides a reference for programs on how to give this formal standardized feedback in The Milestones Guidebook.2 This feedback is a minimum required amount, with a survey of residents showing preference for frequent informal feedback sessions in addition to standardized formal feedback.3 Another study showed that residents want feedback that is confidential, in person, shortly after experiences, and specific to their actions.4 Medical students also voiced a need for frequent, transparent, and actionable feedback during protected, predetermined, and communicated times.5 Clearly, learners appreciate spoken intentional feedback as opposed to the traditional formal model of feedback.
Finally, a study was performed analyzing how prior generations of physician educators view millennial trainees.6 Because most current dermatology residents were born between 1981 and 1996, this study seemed to pinpoint thoughts toward teaching current residents. The study found that although negative judgments such as millennial entitlement (P<.001), impoliteness (P<.001), oversensitivity (P<.001), and inferior work ethic (P<.001) reached significance, millennial ideals of social justice (P<.001) and savviness with technology (P<.001) also were notable. Overall, millennials were thought to be good colleagues (P<.001), were equally competent to more experienced clinicians (P<.001), and would lead medicine to a good future (P=.039).6
Identifying and Maximizing the Impact of Feedback
In addition to how and when to provide feedback, there are discrepancies between attending and resident perception of what is considered feedback. This disconnect can be seen in a study of 122 respondents (67 residents and 55 attendings) that showed 31% of attendings reported giving feedback daily, as opposed to only 9% of residents who reported receiving daily feedback.4 When feedback is to be performed, it may be important to specifically announce the process so that it can be properly acknowledged.7
Beach8 provided a systematic breakdown of clinical teaching to those who may be unfamiliar with the process. This method is divided into preclinic, in-clinic, and postclinic strategies to maximize learning. The author recommended establishing the objectives of the rotation from the teacher’s perspective and inquiring about the objectives of the learner. Both perspectives should inform the lessons to be learned; for example, if a medical student expresses specific interest in psoriasis (a well-established part of a medical student curriculum), all efforts should be placed on arranging for that student to see those specific patients. Beach8 also recommended providing resources and creating a positive supportive learning environment to better utilize precious clinic time and create investment in all learning parties. The author recommended matching trainees during clinic to competence-specific challenges in clinical practice where appropriate technical skill is needed. Appropriate autonomy also is promoted, as it requires higher levels of learning and knowledge consolidation. Group discussions can be facilitated by asking questions of increasing levels of difficulty as experience increases. Finally, postclinic feedback should be timely and constructive.8
One technique discussed by Beach8 is the “1-minute preceptor plus” approach. In this approach, the teacher wants to establish 5 “micro-skills” by first getting a commitment, then checking for supportive evidence of this initial plan, teaching a general principle, reinforcing what was properly performed, and correcting errors. The “plus” comes from trying to take that lesson and apply it to a broader concept. Although this concept is meant to be used in a time-limited setting, it can be expanded to larger conversations. A common example could be made when residents teach rotating medical students through direct observation and supervision during clinic. In this hypothetical situation, the resident and medical student see a patient with erythematous silver-scaled plaques on the elbows and knees. During the patient encounter, the student then inquires about any personal history of cardiovascular disease, diabetes mellitus, and hypertension. After leaving the examination room, the medical student asserts the diagnosis is plaque psoriasis because of the physical examination findings and distribution of lesions. A discussion about the relationship between psoriasis and metabolic syndrome commences, emphasizing the pathophysiology of type 1 helper T-cell–mediated and type 17 helper T-cell–mediated inflammation with vascular damage and growth from inflammatory cytokines.9 The student subsequently is praised on inquiring about relevant comorbidities, and a relevant journal article is retrieved for the student’s future studies. Teaching points regarding the Koebner phenomenon, such as that it is not an instantaneous process and comes with a differential diagnosis, are then provided.
Situation-Behavior-Impact is another teaching method developed by the Center for Creative Leadership. In this technique, one will identify what specifically happened, how the learner responded, and what occurred because of the response.10 This technique is exemplified in the following mock conversation between an attending and their resident following a challenging patient situation: “When you walked into the room and asked the patient coming in for a follow-up appointment ‘What brings you in today?,’ they immediately tensed up and responded that you should already know and check your electronic medical record. This tension could be ameliorated by reviewing the patient’s medical record and addressing what they initially presented for, followed by inquiring if there are other skin problems they want to discuss afterwards.” By identifying the cause-and-effect relationship, helpful and unhelpful responses can be identified and ways to mitigate or continue behaviors can be brainstormed.
The Learning Process
Brodell et all11 outlined techniques to augment the education process that are specific to dermatology. They recommended learning general applicable concepts instead of contextless memorization, mnemonic devices to assist memory for associations and lists, and repetition and practice of learned material. For teaching, they divided techniques into Aristotelian or Socratic; Aristotelian teaching is the formal lecture style, whereas Socratic is conversation based. Both have a place in teaching—as fundamental knowledge grows via Aristotelian teaching, critical thinking can be enhanced via the Socratic method. The authors then outlined tips to create the most conducive learning environment for students.11
Feedback is a reciprocal process with information being given and received by both the teacher and the learner. This is paramount because perfecting the art of teaching is a career-long process and can only be achieved via correction of oversights and mistakes. A questionnaire-based study found that when critiquing the teacher, a combination of self-assessment with assessment from learners was effective in stimulating the greatest level of change in the teacher.12 This finding likely is because the educator was able to see the juxtaposition of how they think they performed with how students interpreted the same situation. Another survey-based study showed that of 68 attending physicians, 28 attendings saw utility in specialized feedback training; an additional 11 attendings agreed with online modules to improve their feedback skills. A recommendation that trainees receive training on the acceptance feedback also was proposed.13 Specialized training to give and receive feedback could be initiated for both attending and resident physicians to fully create an environment emphasizing improvement and teamwork.
Final Thoughts
The art of giving and receiving feedback is a deliberate process that develops with experience and training. Because residents are early in their medical career, being familiar with techniques such as those outlined in this article can enhance teaching and the reception of feedback. Residents are in a unique position, as residency itself is a time of dramatic learning and teaching. Providing feedback gives us a way to advance medicine and better ourselves by solidifying good habits and knowledge.
Acknowledgment—I thank Warren R. Heymann, MD (Camden, New Jersey), for assisting in the creation of this topic and reviewing this article.
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Edgar L, McLean S, Hogan SO, et al. The Milestones Guidebook. Accreditation Council for Graduate Medical Education; 2020. Accessed December 12, 2022. https://www.acgme.org/globalassets/milestonesguidebook.pdf
- Wang JV, O’Connor M, McGuinn K, et al. Feedback practices in dermatology residency programs: building a culture for millennials. Clin Dermatol. 2019;37:282-283.
- Hajar T, Wanat KA, Fett N. Survey of resident physician and attending physician feedback perceptions: there is still work to be done. Dermatol Online J. 2020;25:13030/qt2sg354p6.
- Yoon J, Said JT, Thompson LL, et al. Medical student perceptions of assessment systems, subjectivity, and variability on introductory dermatology clerkships. Int J Womens Dermatol. 2021;7:232-330.
- Marka A, LeBoeuf MR, Vidal NY. Perspectives of dermatology faculty toward millennial trainees and colleagues: a national survey. Mayo Clin Proc Innov Qual Outcomes. 2021;5:65-71.
- Bernard AW, Kman NE, Khandelwal S. Feedback in the emergency medicine clerkship. West J Emerg Med. 2011;12:537-542.
- Beach RA. Strategies to maximise teaching in your next ambulatory clinic. Clin Teach. 2017;14:85-89.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases part I. epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Olbricht SM. What makes feedback productive? Cutis. 2016;98:222-223.
- Brodell RT, Wile MZ, Chren M, et al. Learning and teaching in dermatology: a practitioner’s guide. Arch Dermatol. 1996;132:946-952.
- Stalmeijer RE, Dolmans DHJM, Wolfhagen IHAP, et al. Combined student ratings and self-assessment provide useful feedback for clinical teachers. Adv in Health Sci Educ. 2010;15:315-328.
- Chelliah P, Srivastava D, Nijhawan RI. What makes giving feedback challenging? a survey of the Association of Professors of Dermatology (APD)[published online July 19, 2022]. Arch Dermatol Res. doi:10.1007/s00403-022-02370-y
- Crommelinck M, Anseel F. Understanding and encouraging feedback-seeking behavior: a literature review. Med Educ. 2013;47:232-241.
- Edgar L, McLean S, Hogan SO, et al. The Milestones Guidebook. Accreditation Council for Graduate Medical Education; 2020. Accessed December 12, 2022. https://www.acgme.org/globalassets/milestonesguidebook.pdf
- Wang JV, O’Connor M, McGuinn K, et al. Feedback practices in dermatology residency programs: building a culture for millennials. Clin Dermatol. 2019;37:282-283.
- Hajar T, Wanat KA, Fett N. Survey of resident physician and attending physician feedback perceptions: there is still work to be done. Dermatol Online J. 2020;25:13030/qt2sg354p6.
- Yoon J, Said JT, Thompson LL, et al. Medical student perceptions of assessment systems, subjectivity, and variability on introductory dermatology clerkships. Int J Womens Dermatol. 2021;7:232-330.
- Marka A, LeBoeuf MR, Vidal NY. Perspectives of dermatology faculty toward millennial trainees and colleagues: a national survey. Mayo Clin Proc Innov Qual Outcomes. 2021;5:65-71.
- Bernard AW, Kman NE, Khandelwal S. Feedback in the emergency medicine clerkship. West J Emerg Med. 2011;12:537-542.
- Beach RA. Strategies to maximise teaching in your next ambulatory clinic. Clin Teach. 2017;14:85-89.
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases part I. epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Olbricht SM. What makes feedback productive? Cutis. 2016;98:222-223.
- Brodell RT, Wile MZ, Chren M, et al. Learning and teaching in dermatology: a practitioner’s guide. Arch Dermatol. 1996;132:946-952.
- Stalmeijer RE, Dolmans DHJM, Wolfhagen IHAP, et al. Combined student ratings and self-assessment provide useful feedback for clinical teachers. Adv in Health Sci Educ. 2010;15:315-328.
- Chelliah P, Srivastava D, Nijhawan RI. What makes giving feedback challenging? a survey of the Association of Professors of Dermatology (APD)[published online July 19, 2022]. Arch Dermatol Res. doi:10.1007/s00403-022-02370-y
RESIDENT PEARLS
- Feedback between dermatology trainees and their educators should be provided in a private and constructive way soon after the observation was performed.
- One method to improve education and feedback in a residency program is a specialty course to improve giving and receiving feedback by both residents and attending physicians.
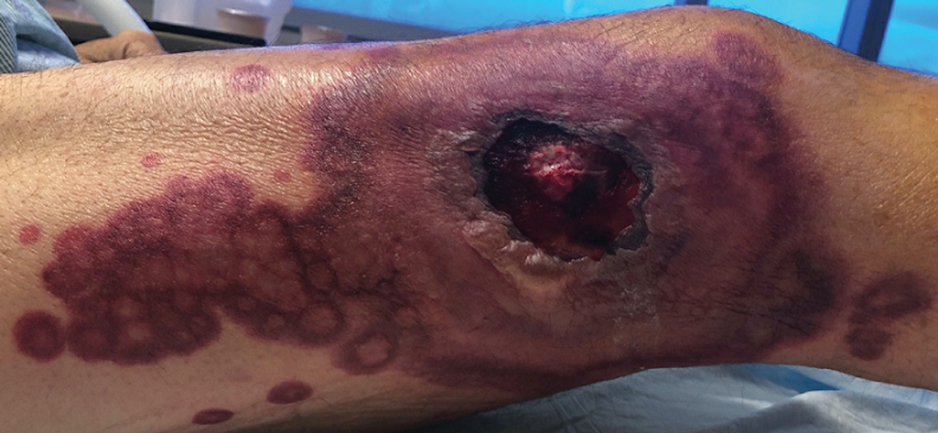
Violaceous-Purpuric Targetoid Macules and Patches With Bullae and Ulceration
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
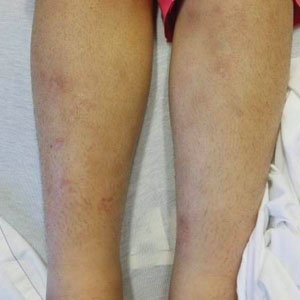
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.
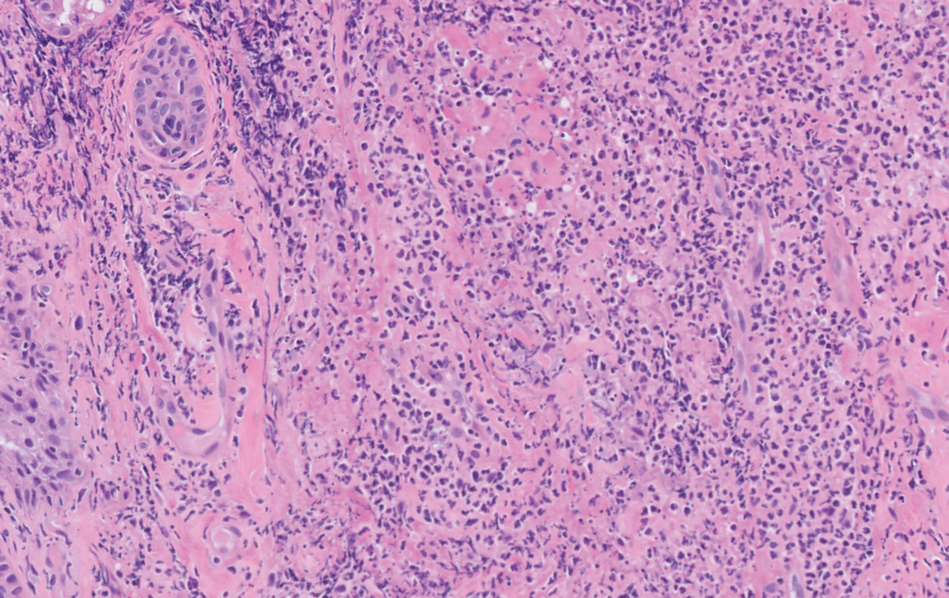
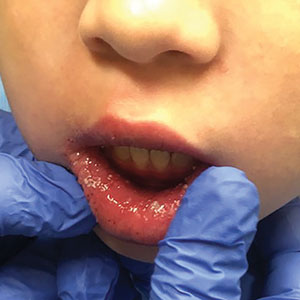
Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1
The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
The Diagnosis: Sweet Syndrome (Acute Febrile Neutrophilic Dermatosis)
A skin biopsy of the right lower extremity demonstrated diffuse interstitial, perivascular, and periadnexal neutrophilic dermal infiltrate in the reticular dermis (Figure 1), consistent with a diagnosis of Sweet syndrome without evidence of leukemia cutis or infection. The firm erythematous papulonodules with follicular accentuation on the face (Figure 2) also were confirmed as Sweet syndrome on histopathology. Concern for leukemic transformation was confirmed with bone biopsy revealing acute myeloid leukemia (AML). Our patient began a short course of prednisone, and the cutaneous lesions improved during hospitalization; however, he was lost to follow-up.

Sweet syndrome (also known as acute febrile neutrophilic dermatosis) is a rare inflammatory skin condition typically characterized by asymmetric, painful, erythematous to violaceous papules, plaques, or nodules involving the arms, face, and neck.1 It most commonly occurs in women and typically presents in patients aged 47 to 57 years. Although the pathogenesis of neutrophilic dermatoses is not completely understood, they are believed to be due to altered expression of inflammatory cytokines, irregular neutrophil function, and a genetic predisposition.2 There are 3 main categories of Sweet syndrome: classical (or idiopathic), drug induced, and malignancy associated.1 The lesions associated with Sweet syndrome vary from a few millimeters to several centimeters and may be annular or targetoid in the later stages. They also may form bullae and ulcerate. Fever, leukocytosis, and elevated acute-phase reactants also are common on presentation.1 Histopathologic analysis demonstrates an intense neutrophilic infiltrate within the reticular dermis with marked leukocytoclasia. Admixed within the neutrophil polymorphs are variable numbers of lymphocytes and histiocytes. Edema in the upper dermis also is characteristic.3 The exact pathogenesis of Sweet syndrome has yet to be elucidated but may involve a combination of cytokine dysregulation, hypersensitivity reactions, and genetics.4 Our case demonstrates 3 distinct morphologies of Sweet syndrome in a single patient, including classic edematous plaques, agminated targetoid plaques, and ulceration. Based on the clinical presentation, diagnostic workup for an undiagnosed malignancy was warranted, which confirmed AML. The malignancy-associated form of Sweet syndrome accounts for a substantial portion of cases, with approximately 21% of patients diagnosed with Sweet syndrome having an underlying malignancy, commonly a hematologic malignancy or myeloproliferative disorder with AML being the most common.1

The differential diagnosis for Sweet syndrome includes cutaneous small vessel vasculitis, which commonly presents with symmetric palpable purpura of the legs. Lesions may be round, port wine–colored plaques and even may form ulcers, vesicles, and targetoid lesions. However, skin biopsy shows polymorphonuclear infiltrate affecting postcapillary venules, fibrinoid deposits, and extravasation of red blood cells.5 Leukemia cutis describes any type of leukemia that manifests in the skin. It typically presents as violaceous or red-brown papules, nodules, and plaques most commonly on the legs. Histopathology varies by immunophenotype but generally demonstrates perivascular or periadnexal involvement or a diffuse, interstitial, or nodular infiltrate of the dermis or subcutis.6 Neutrophilic eccrine hidradenitis describes an aseptic neutrophilic infiltration around eccrine coils and glands. It may present as papules or plaques that usually are erythematous but also may be pigmented. Lesions can be asymptomatic or painful as in Sweet syndrome and are distributed proximally or on the distal extremities. Histopathologic examination demonstrates the degeneration of the eccrine gland and neutrophilic inflammatory infiltrates.7 Lastly, necrotizing fasciitis is a life-threatening infection of the deep soft tissue and fascia, classically caused by group A Streptococcus. The infected site may have erythema, tenderness, fluctuance, necrosis, and bullae.8 Although our patient had a fever, he did not display the tachycardia, hypotension, tachypnea, and rapid deterioration that is common in necrotizing fasciitis.
Sweet syndrome may present with various morphologies within the same patient. Painful, erythematous to violaceous papules, plaques, nodules, bullae, and ulcers may be seen. A workup for an underlying malignancy may be warranted based on clinical presentation. Most patients have a rapid and dramatic response to systemic corticosteroids.
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
- Cohen PR. Sweet’s syndrome—a comprehensive review of an acute febrile neutrophilic dermatosis. Orphanet J Rare Dis. 2007;2:34. doi:10.1186/1750-1172-2-34
- Nelson CA, Stephen S, Ashchyan HJ, et al. Neutrophilic dermatoses: pathogenesis, Sweet syndrome, neutrophilic eccrine hidradenitis, and Behçet disease. J Am Acad Dermatol. 2018;79:987-1006. doi:10.1016/J .JAAD.2017.11.064
- Pulido-Pérez A, Bergon-Sendin M, Sacks CA. Images in clinical medicine. N Engl J Med. 2020;16:382. doi:10.1056/NEJMicm1911025
- Marzano AV, Hilbrands L, Le ST, et al. Insights into the pathogenesis of Sweet’s syndrome. Front Immunol. 2019;10:414. doi:10.3389/fimmu.2019.00414
- Goeser MR, Laniosz V, Wetter DA. A practical approach to the diagnosis, evaluation, and management of cutaneous small-vessel vasculitis. Am J Clin Dermatol. 2014;15:299-306. doi:10.1007/s40257-014-0076-6
- Hee Cho-Vega J, Jeffrey Medeiros L, Prieto VG, et al. Leukemia cutis. Am J Clin Pathol. 2008;129:130-142. doi:10.1309/WYAC YWF6NGM3WBRT
- Bachmeyer C, Aractingi S. Neutrophilic eccrine hidradenitis. Clin Dermatol. 2000;18:319-330. doi:10.1016/S0738-081X(99)00123-6
- Shimizu T, Tokuda Y. Necrotizing fasciitis. Intern Med. 2010; 49:1051-1057. doi:10.2169/internalmedicine.49.2964
A 64-year-old man with long-standing myelofibrosis presented with neutropenic fevers as well as progressive painful lesions of 3 days’ duration on the legs. A bone marrow biopsy during this hospitalization demonstrated a recent progression of the patient’s myelofibrosis to acute myeloid leukemia. Physical examination revealed round to oval, violaceous, targetoid plaques. Within a week, new erythematous and nodular lesions appeared on the right arm and left vermilion border. The lesions on the legs enlarged, formed bullae, and ulcerated.
Juvenile Dermatomyositis–Associated Panniculitis
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.
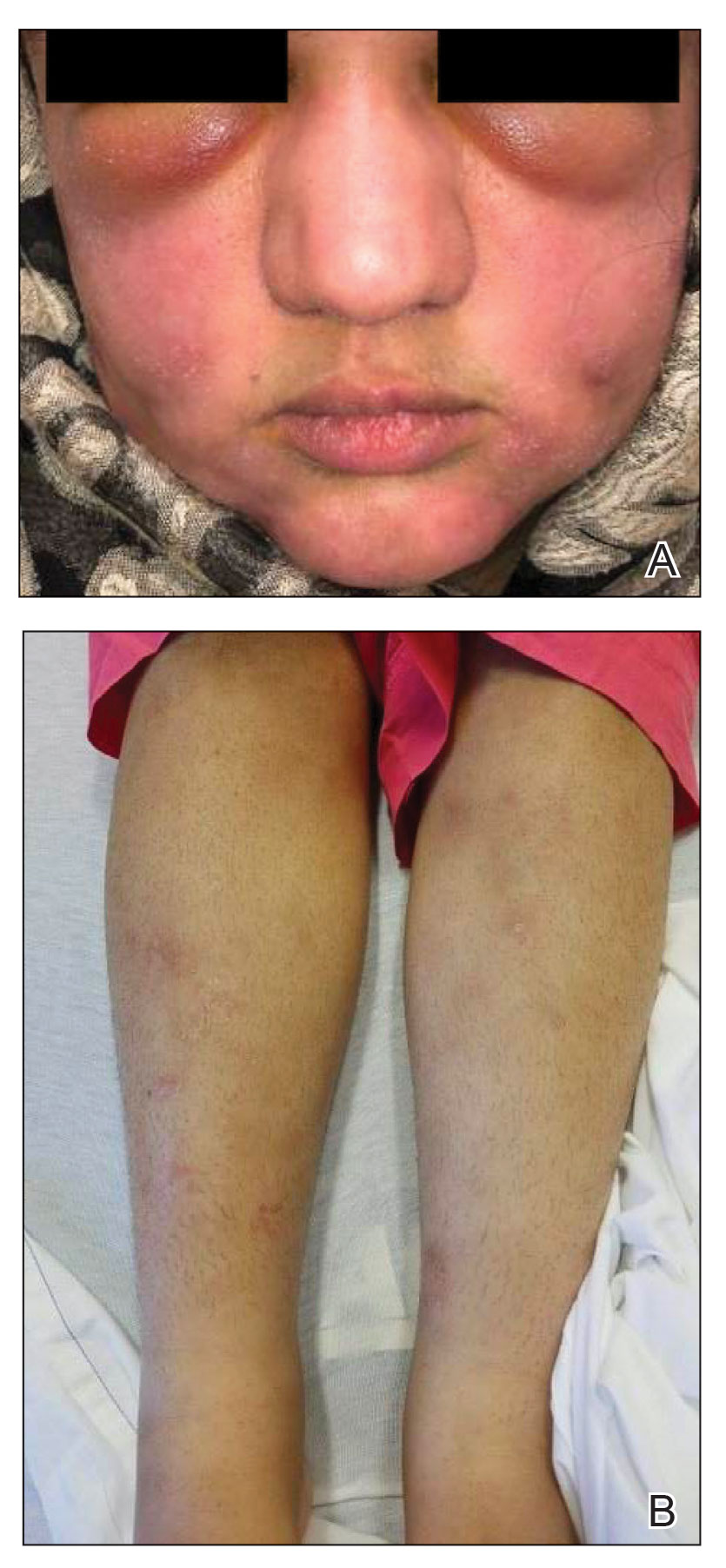
A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.
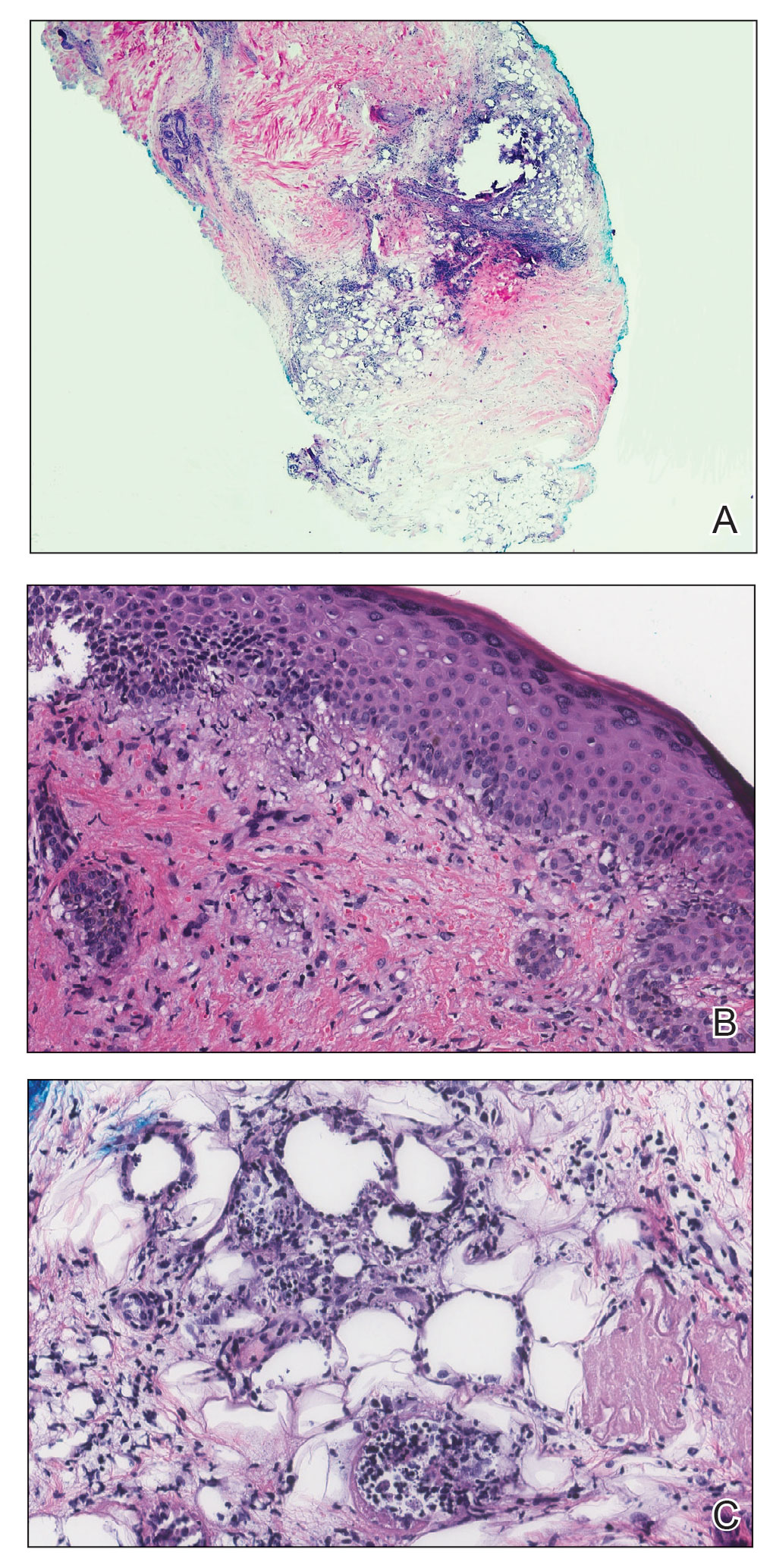
Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
To the Editor:
Juvenile dermatomyositis (JDM) is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin, among other organs. Since the recognition of dermatomyositis (DM) more than 100 years ago, a variety of clinical diagnostic criteria have been utilized. Classically, DM presents with muscle weakness and a pathognomonic cutaneous macular, violaceous, erythematous eruption. The juvenile variant is defined by onset prior to 16 years of age. Histologically, these entities are indistinguishable and demonstrate an interface dermatitis with epidermal atrophy. Clinically, JDM has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant.1 Panniculitis is a rare but serious complication in a subset of patients with DM and may represent a precursor to calcinosis cutis.2 We describe a case of JDM-associated panniculitis that was difficult to control with prednisone and rituximab.

A 21-year-old woman with fever, fatigue, muscle pain, and new-onset swelling of 2 weeks’ duration was admitted to the hospital. She had a 5-year history of intermittent muscle weakness and concomitant rash. Prior to presentation, she had been hospitalized twice for fever of unknown origin, and the source remained undetermined. Physical examination revealed prominent facial and periorbital edema. There was tender nonpitting edema present on all 4 extremities and hyperpigmented indurated nodules on the shins (Figure 1). A full laboratory and imaging workup was performed for autoantibodies and infectious etiologies. The complete blood cell count was notable for pancytopenia, and a thorough infectious workup was negative. Creatine kinase level was within reference range. A biopsy of the right shin was performed, and histopathology revealed a lobular panniculitis with fat necrosis and mixed inflammation with neutrophils with perieccrine involvement as well as an interface dermatitis (Figure 2). Periodic acid–Schiff, Grocott methenamine-silver, and Gram stains were negative. Myositis-specific antibody testing revealed anti-p155/140 autoantibodies, and magnetic resonance imaging did not reveal active myositis within the visualized muscles, consistent with stable nonprogressing DM. A diagnosis of JDM with panniculitis was made. The patient was started on oral prednisone. Subsequently, a trial of rituximab was initiated. Although the patient’s symptoms initially improved, the response was not sustained on rituximab, and the patient was continued on systemic steroids with initiation of cyclosporine.

Juvenile dermatomyositis is an autoimmune disorder with childhood onset that involves systemic inflammation of the muscles, skin, and internal organs. It often can present diagnostic and therapeutic challenges.2,3 Bohan and Peter4,5 clinical criteria may help identify potential patients with JDM, but magnetic resonance imaging, electromyography, and muscle biopsy often are required to confirm the diagnosis.6 Skin manifestations include heliotrope rash; V sign; shawl sign; Gottron papules; periorbital edema; and infrequently panniculitis, the subcutaneous inflammation of adipose tissue.3,7
Although panniculitis is found in approximately 10% of skin biopsies in patients with DM, our patient presented with anti-p155/140 antibodies.8-10 Fat involvement in these patients traditionally manifests as lipodystrophy. Panniculitis also may precede calcinosis cutis, a debilitating skin change that may occur in approximately 46% of patients with JDM and can cause severe morbidity.2,6,9
Subcutaneous edema rarely is described in DM-panniculitis, present in only 6% of 86 DM patients in one study.7 The pathophysiology of DM may be due to antibodies that target endothelial cells and activate complement, resulting in the membranolytic attack complex. This leads to microischemia, and microinfarction of the muscle fibers has been suggested to result in edema of the subcutaneous tissue in severe cases.7,11 Microinfarction has been found to be present 2.3 times more often in edematous DM compared with nonedematous DM.7 Subcutaneous edema may be an isolated presentation of DM that arises more quickly with severe disease activity. As such, recommendations have been made to consider edema in future classification schemes.7
Because of the severity of edematous and/or subcutaneous DM, aggressive therapy may be required. First-line therapy consists of corticosteroids with additional immunosuppressants and immunomodulatory agents if adequate response is not achieved.3,12 The effectiveness of rituximab in DM has been suggested.2,12,13 The Rituximab in Myositis (RIM) trial (N=200) was the first double-blind, placebo-controlled, phase 3 clinical trial to assess rituximab’s efficacy in refractory compared with early-onset inflammatory myopathies. Although outcomes were similar in both groups, 83% of patients overall, including the JDM subset, met the definition of improvement.12 In re-examining the RIM trial data and other cases using rituximab to treat inflammatory myopathies, an overall response rate of 78.3% was observed, with 52.1% of patients with DM reporting improvement in skin lesions (N=458, pooled from 48 studies).13 Further analysis of the RIM data revealed that panniculitis affected 10.4% of patients with JDM at baseline, which decreased to 6.8% at 36 weeks of rituximab therapy (N=48).12
As exhibited in our patient, subcutaneous tissue involvement, including calcinosis cutis and panniculitis, is seen more often in JDM than adult DM.2,6 However, panniculitis in anti-p155/140 patients is rare. Our patient also had antibody positivity, which likely predisposed her to a more severe course. Despite not having sustained improvement on rituximab, initiating aggressive therapy earlier in the disease course may be beneficial, and our patient continues with alternative therapies.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
- Jorizzo JL, Vleugels RA. Dermatomyositis. In: Bolognia J, Schaffer J, Cerroni L. Dermatology. 4th ed. Elsevier; 2019:681-687.
- Aggarwal R, Loganathan P, Koontz D, et al. Cutaneous improvement in refractory adult and juvenile dermatomyositis after treatment with rituximab. Rheumatology. 2016;56:247-254.
- Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol. 2018;32:1352-1359.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (first of two parts). N Engl J Med. 1975;292:344-347.
- Bohan A, Peter JB. Polymyositis and dermatomyositis (second of two parts). N Engl J Med. 1975;292:403-407.
- Sakurai N, Hino-Shishikura A, Nozawa T, et al. Clinical significance of subcutaneous fat and fascial involvement in juvenile dermatomyositis. Mod Rheumatol. 2019;29:808-813.
- Milisenda JC, Doti PI, Prieto-Gonzalez S, et al. Dermatomyositis presenting with severe subcutaneous edema: five additional cases and review of the literature. Semin Arthritis Rheum. 2014;44:228-233.
- Janis JF, Winkelmann RK. Histopathology of the skin in dermatomyositis: a histopathologic study of 55 cases. Arch Dermatol. 1968;97:640-650.
- van Dongen HM, van Vugt RM, Stoof TJ. Extensive persistent panniculitis in the context of dermatomyositis. J Clin Rheumatol. 2020;26:e187-e188.
- Gunawardena H, Wedderburn LR, North J, et al. Clinical associations of autoantibodies to a p155/140 kDa doublet protein in juvenile dermatomyositis. Rheumatology. 2008;47:324-328.
- Dalakas MC, Hohlfeld R. Polymyositis and dermatomyositis. Lancet. 2003;362:971-982.
- Oddis CV, Reed AM, Aggarwal R, et al. Rituximab in the treatment of refractory adult and juvenile dermatomyositis and adult polymyositis: a randomized, placebo-phase trial. Arthritis Rheum. 2013;65:314-324.
- Fasano S, Gordon P, Hajji R, et al. Rituximab in the treatment of inflammatory myopathies: a review. Rheumatology. 2016;56:26-36.
Practice Points
- Juvenile dermatomyositis is an autoimmune disorder with childhood onset that predominantly affects the muscles and skin.
- Juvenile dermatomyositis has a higher incidence of calcinosis cutis and is not associated with an increased risk for malignancy in contrast to the adult-onset variant, dermatomyositis (DM).
- Panniculitis is a rare but severe complication of DM, and this subset of DM may be challenging to treat, requiring aggressive therapy.
Skin Manifestations of Complex Regional Pain Syndrome
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.
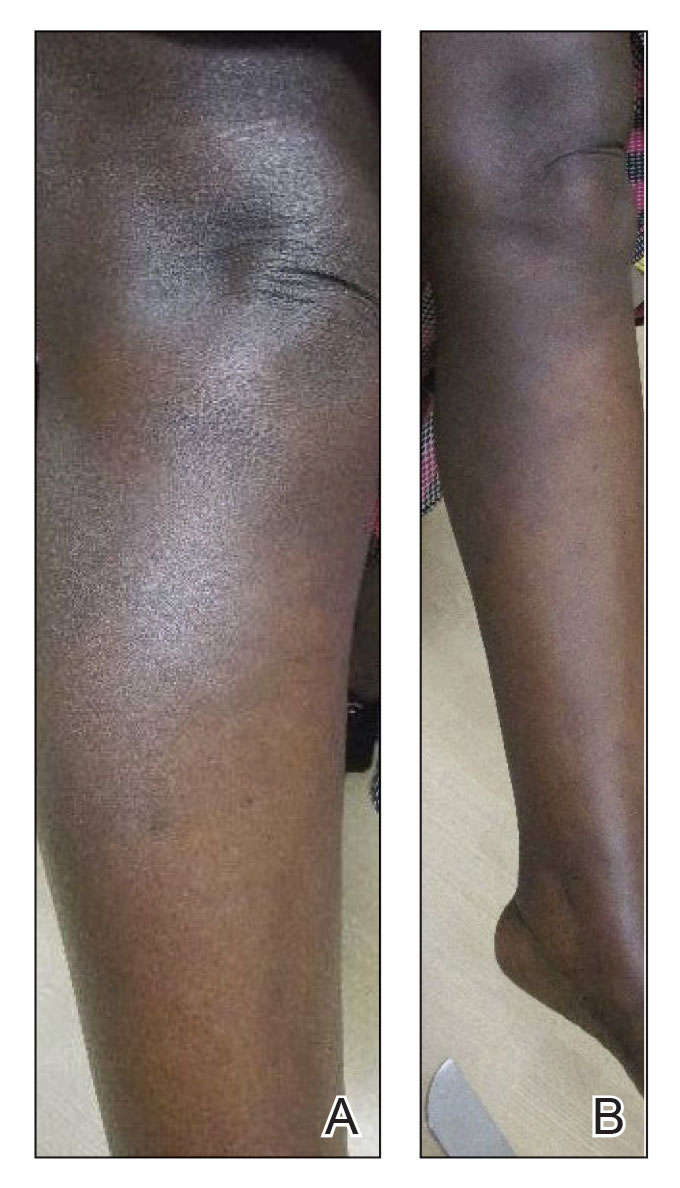
To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
To the Editor:
Complex regional pain syndrome (CRPS) is a neurologic condition characterized by chronic pain and sensory changes, including allodynia and hyperalgesia, that usually affect the extremities.1,2 The syndrome is defined by the International Association for the Study of Pain (IASP) as a condition that appears regionally after an injury, with a variety of symptoms that often exceed the expected clinical course both in magnitude and duration, causing impairment of motor function and variable progression.3
Although CRPS most often is described following minor peripheral trauma, other precipitating causes include surgery and vascular events.4 Additional features of the condition include autonomic dysfunction, edema, and trophic changes.1 Symptoms of CRPS traditionally present in 3 stages, with notable skin changes most often documented in stages II and III.2
Skin changes are a known manifestation of the syndrome, but reports in the dermatologic literature are scarce. Qureshi and Friedman5 identified only 23 articles in the dermatology literature since 1990 in which skin changes in CRPS were described. We present a patient with a diagnosis of CRPS who developed hyperpigmentation and sclerotic changes, including skin thickening, induration, and skin tightening.
A middle-aged Black woman presented to dermatology for evaluation of progressive hyperpigmentation, hyperhidrosis, and sclerotic changes to the skin. Approximately 3 years prior, the patient was given a diagnosis of CRPS of the hands and feet. Pain symptoms started approximately 3 years prior to the onset of symptoms. Symptoms started in the left hand and eventually spread to the right arm, left leg, and subsequently to the right leg. The first dermatologic change the patient noticed was tightening of the skin in the affected area that led to decreased mobility, which improved over time—partly on its own and partly with physical therapy.
A biopsy performed by an outside dermatologist at the initial presentation demonstrated sclerodermalike changes, which were treated with creams but without improvement. Scleroderma was later ruled out by the same dermatologist. Skin tightening improved over time, with complete resolution approximately 1 year after the onset of symptoms.
Upon presentation to our clinic, the patient reported continuing intermittent flares of CRPS; however, she said she was most concerned about diffuse hyperpigmentation, which spread to include the face, arms, abdomen, legs (Figure), and buttocks and persisted after skin tightening resolved.

To treat the hyperpigmentation, a decision was made to first focus on a localized area. Facial hyperpigmentation was chosen because it was of greatest concern to the patient. She was instructed to use azelaic acid gel 15% in the morning, tretinoin cream 0.05% at night, and sunscreen daily. The patient had mild improvement in hyperpigmentation after a 4-month period but has been inconsistent in follow-up. She continues to have intermittent flares of CRPS, which may interfere with her response to treatment. In addition to the aforementioned regimen of azelaic acid gel and tretinoin, she has continued to work with a pain specialist to better control the neurologic symptoms and pain associated with her CRPS.
Complex regional pain syndrome, a neurological condition characterized by chronic pain, affects women 3 times more often than men. The syndrome is more common in the fourth and fifth decades of life.1,2
There are 2 subtypes of CRPS. Type I (also known as reflex sympathetic dystrophy) is more common and occurs following minor trauma without peripheral nerve injury. Type II (otherwise known as causalgia) occurs following more notable trauma with injury to a peripheral nerve.1,6 Onset of symptoms most often is secondary to minor peripheral trauma. More common triggers include soft-tissue injury (40%); fractures and subsequent orthopedic surgery (25%); and visceral lesions, such as myocardial infarction and cerebral vascular accident (12%).5 Regardless of the inciting event, prolonged immobilization of a limb has been identified as an important predisposing factor. One study found that 47% of patients who received a diagnosis of CRPS previously underwent immobilization of the same limb.7
The pathogenesis of CRPS has not been fully elucidated. Possible explanations include central nervous system sensitization to thermal, mechanical, and pain stimuli; sympathetic dysfunction leading to vasomotor, pseudomotor, and trophic changes; and inflammatory cytokine release and microcirculatory dysfunction, causing tissue injury.1,2,6
The diagnosis of CRPS is a based on clinical findings. Using the Budapest Criteria established to define CRPS, a clinical diagnosis can be made when all of the following criteria are met: chronic continuing pain disproportionate to any inciting event; 1 or more reported symptoms from 3 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic; 1 or more sign at the time of evaluation in 2 or more of the categories of involvement including sensory, vasomotor, pseudomotor, edema, and motor or trophic.8 Dermatologic findings are a common presenting feature of CRPS and are included in the Budapest Criteria used for diagnosis. In a retrospective chart review (N=26), researchers found that vascular findings were the most common dermatologic manifestation of CRPS—edema in 58% of patients and erythema in 54%.9 Other common manifestations included dermatitis (35%), erythematous papules (23%), and cutaneous atrophy (23%). Hyperpigmentation, which was present in our patient, was seen in 8% of patients in the chart review.9
Complex regional pain syndrome progresses through 3 stages; dermatologic changes are present in each stage and are more severe in later stages. Stage I lasts 2 or 3 months and is characterized by onset of pain, usually burning type, accompanied by allodynia and hyperalgesia. Early vasomotor and pseudomotor changes, such as erythema and edema, may become apparent.1,2 Stage II lasts 3 to 6 months and is characterized by more severe edema and more obvious trophic changes. Functional limitations, such as limited range of motion and muscle weakness, begin to manifest. Stage III—the final and most severe stage—is characterized by obvious hair, skin, and nail changes, as well as functional limitations.1,2 The waxy thickened skin changes and hyperpigmentation observed in our patient are characteristic of stage III. Furthermore, our patient experienced decreased mobility and limited range of motion secondary to tightening of the skin, a characteristic motor change of late-stage CRPS. Although chronic pain and allodynia are the most common characteristics of CRPS, skin changes also can cause notable distress and early dermatologic manifestations can be a chief concern.
Dermatologic management is focused to address the specific skin changes of CRPS. However, traditional treatment of the common dermatologic findings of CRPS is difficult and often unsuccessful; instead, the most successful treatment of skin findings involves controlling the underlying CRPS.9 Current treatment options include removal of any nidus of tissue trauma, sympathetic neural blockade with a local anesthetic, spinal cord stimulation to interrupt dysregulated sympathetic innervation, and physiotherapy or occupational therapy to desensitize skin.1,10
Given the complexity of CRPS and the variability of its presentation, management of the syndrome and its associated dermatologic conditions often requires interdisciplinary care and coordination of multiple specialties. Dermatologists can play an important role in both identification of CRPS and co-management of affected patients. Early diagnosis of CRPS has been universally identified as a key prognostic factor. For that reason, dermatologists should be aware of CRPS and include the syndrome in the differential diagnosis when presented with severe cutaneous findings following trauma either with or without peripheral nerve damage, suggestive of CRPS.
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
- Sebastin SJ. Complex regional pain syndrome. Indian J Plast Surg. 2011;44:298-307. doi:10.4103/0970-0358.85351
- Kabani R, Brassard A. Dermatological findings in early detection of complex regional pain syndrome. JAMA Dermatol. 2014;150:640-642. doi:10.1001/jamadermatol.2013.7459
- Moseley L. What is complex regional pain syndrome – in plain English. International Association for the Study of Pain website. Published 2009. Accessed December 15, 2022. https://www.iasp-pain.org/publications/relief-news/article/what-is-complex-pain-syndrome-in-plain-english/
- Pak TJ, Martin GM, Magness JL, et al. Reflex sympathetic dystrophy. Review of 140 cases. Minn Med. 1970;53:507-512.
- Qureshi AA, Friedman AJ. Complex regional pain syndrome: what the dermatologist should know. J Drugs Dermatol. 2018;17:532-536.
- Gorodkin R. Complex regional pain syndrome. Rheumatology. 2016;55(suppl 1):i12.
- Araki E, Tanioka M, Miyachi Y, et al. A case of complex regional pain syndrome: an underdiagnosed condition in dermatology. Acta Derm Venereol. 2007;87:440-441. doi:10.2340/00015555-0281
- Pergolizzi JV, LeQuang JA, Nalamachu S, et al. The Budapest criteria for complex regional pain syndrome: the diagnostic challenge. Anaesthesiol Clin Sci Res. 2018;2:1-10. doi:10.35841/anesthesiology.2.1.1-10
- Sundaram S, Webster GF. Vascular diseases are the most common cutaneous manifestations of reflex sympathetic dystrophy. J Am Acad Dermatol. 2001;44:1050-1051. doi:10.1067/mjd.2001.114299
- Taylor RS, Van Buyten J-P, Buchser E. Spinal stimulation for complex regional pain syndrome: a systematic review of the clinical and cost-effectiveness literature and assessment of prognostic factors. Eur J Pain. 2006;10:91-101. doi:10.1016/j.ejpain.2005.02.004
PRACTICE POINTS
- Common dermatologic manifestations of complex regional pain syndrome (CRPS), which often are nonspecific and often the presenting symptoms of the syndrome, include allodynia, edema, erythema, hypopigmentation or hyperpigmentation, and petechiae.
- Diagnosis and management of CRPS are the most important steps in treating dermatologic manifestations of the syndrome.
Factors Influencing Patient Preferences for Phototherapy: A Survey Study
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
Phototherapy—particularly UVB phototherapy, which utilizes UVB rays of specific wavelengths within the UV spectrum—is indicated for a wide variety of dermatoses. In-office and at-home UVB treatments commonly are used, as are salon tanning and sunbathing. When selecting a form of phototherapy, patients are likely to consider safety, cost, effectiveness, insurance issues, and convenience. Research on patient preferences; the reasons for these preferences; and which options patients perceive to be the safest, most cost-effective, efficacious, and convenient is lacking. We aimed to assess the forms of phototherapy that patients would most consider using; the factors influencing patient preferences; and the forms patients perceived as the safest and most cost-effective, efficacious, and convenient.
Methods
Study Participants—We recruited 500 Amazon Mechanical Turk users who were 18 years or older to complete our REDCap-generated survey. The study was approved by the Wake Forest University institutional review board (Winston-Salem, North Carolina).
Evaluation—Participants were asked, “If you were diagnosed with a skin disease that benefited from UV therapy, which of the following forms of UV therapy would you consider choosing?” Participants were instructed to choose all of the forms they would consider using. Available options included in-office UV, at-home UV, home tanning, salon tanning, sunbathing, and other. Participants were asked to select which factors—from safety, cost, effectiveness, issues with insurance, convenience, and other—influenced their decision-making; which form of phototherapy they would most consider along with the factors that influenced their preference for this specific form of phototherapy; and which options they considered to be safest and most cost-effective, efficacious, and convenient. Participants were asked to provide basic sociodemographic information, level of education, income, insurance status (private, Medicare, Medicaid, Veterans Affairs, and uninsured), and distance from the nearest dermatologist.
Statistical Analysis—Descriptive and inferential statistics (χ2 test) were used to analyze the data with a significance set at P<.05.
Results
Five hundred participants completed the survey (Table 1).

Factors Influencing Patient Preferences—When asked to select all forms of phototherapy they would consider, 186 (37.2%) participants selected in-office UVB, 263 (52.6%) selected at-home UV, 141 (28.2%) selected home tanning, 117 (23.4%) selected salon tanning, 191 (38.2%) selected sunbathing, and 3 (0.6%) selected other. Participants who selected in-office UVB as an option were more likely to also select salon tanning (P<.012). No other relationship was found between the UVB options and the tanning options. When asked which factors influenced their phototherapy preferences, 295 (59%) selected convenience, 266 (53.2%) selected effectiveness, 220 (44%) selected safety, 218 (43.6%) selected cost, 72 (14.4%) selected issues with insurance, and 4 (0.8%) selected other. Forms of Phototherapy Patients Consider Using—When asked which form of phototherapy they would most consider using, 179 (35.8%) participants selected at-home UVB, 108 (21.6%) selected sunbathing, 92 (18.4%) selected in-office UVB, 62 (12.4%) selected home-tanning, 57 (11.4%) selected salon tanning, 1 (0.2%) selected other, and 1 participant provided no response (P<.001).
Reasons for Using Phototherapy—Of the 179 who selected at-home UVB, 125 (70%) cited convenience as a reason. Of the 108 who selected salon tanning as their top choice, 62 (57%) cited cost as a reason. Convenience (P<.001), cost (P<.001), and safety (P=.023) were related to top preference. Issues with insurance did not have a statistically significant relationship with the top preference. However, participant insurance type was related to top phototherapy preference (P=.021), with privately insured patients more likely to select in-office UVB, whereas those with Medicaid and Medicare were more likely to select home or salon tanning. Efficacy was not related to top preference. Furthermore, age, gender, education, income, and distance from nearest dermatologist were not related to top preference.
In-office UVB was perceived to be safest (P<.001) and most efficacious (P<.001). Meanwhile, at-home UVB was selected as most convenient (P<.001). Lastly, sunbathing was determined to be most cost-effective (P<.001)(Table 2). Cost-effectiveness had a relationship (P<.001) with the participant’s insurance, as those with private insurance were more likely to select at-home UVB, whereas those with Medicare or Medicaid were more likely to select the tanning options. Additionally, of the54 uninsured participants in the survey, 29 selected sunbathing as the most cost-effective option.

Comment
Phototherapy Treatment—UVB phototherapy at a wavelength of 290 to 320 nm (311–313 nm for narrowband UVB) is used to treat various dermatoses, including psoriasis and atopic dermatitis. UVB alters skin cytokines, induces apoptosis, promotes immunosuppression, causes DNA damage, and decreases the proliferation of dendritic cells and other cells of the innate immune system.1 In-office and at-home UV therapies make use of UVB wavelengths for treatment, while tanning and sunbathing contain not only UVB but also potentially harmful UVA rays. The wavelengths for indoor tanning devices include UVB at 280 to 315 nm and UVA at 315 to 400 nm, which are similar to those of the sun but with a different ratio of UVB to UVA and more intense total UV.2 When in-office and at-home UVB options are not available, various forms of tanning such as salon tanning and sunbathing may be alternatives that are widely used.3 One of the main reasons patients consider alternative phototherapy options is cost, as 1 in-office UVB treatment may cost $140, but a month of unlimited tanning may cost $30 or perhaps nothing if a patient has a gym membership with access to a tanning bed. Lack of insurance benefits covering phototherapy can exacerbate cost burden.4 However, tanning beds are associated with an increased risk for melanoma and nonmelanoma cancers.5,6 Additionally, all forms of phototherapy are associated with photoaging, but it is more intense with tanning and heliotherapy because of the presence of UVA, which penetrates deeper into the dermis.7 Meanwhile, for those who choose UVB therapy, deciding between an in-office and at-home UVB treatment could be a matter of convenience, as patients must consider long trips to the physician’s office; insurance status, as some insurances may not cover at-home UVB; or efficacy, which might be influenced by the presence of a physician or other medical staff. In many cases, patients may not be informed that at-home UVB is an option.
Patient Preferences—At-home UVB therapy was the most popular option in our study population, with most participants (52.6%) considering using it, and 35.9% choosing it as their top choice over all other phototherapy options. Safety, cost, and convenience were all found to be related to the option participants would most consider using. Prior analysis between at-home UVB and in-office UVB for the treatment of psoriasis determined that at-home UVB is as safe and cost-effective as in-office UVB without the inconvenience of the patient having to take time out of the week to visit the physician’s office,8,9 making at-home UVB an option dermatologists may strongly consider for patients who value safety, cost, and convenience. Oddly, efficacy was not related to the top preference, despite being the second highest–cited factor (53.2%) for which forms of phototherapy participants would consider using. For insurance coverage, those with Medicaid and Medicare selected the cheaper tanning options with higher-than-expected frequencies. Although problems with insurance were not related to the top preference, insurance status was related, suggesting that preferences are tied to cost. Of note, while the number of dermatologists that accept Medicare has increased in the last few years, there still remains an uneven distribution of phototherapy clinics. As of 2015, there were 19 million individuals who qualified for Medicare without a clinic within driving distance.10 This problem likely also exists for many Medicaid patients who may not qualify for at-home UVB. In this scenario, tanning or heliotherapy may be effective alternatives.
In-Office vs At-Home Options—Although in-office UVB was the option considered safest (26.2%) and most efficacious (26.8%), it was followed closely by at-home UVB in both categories (safest, 23.8%; most efficacious, 24.2%). Meanwhile, at-home UVB (40.2%) was chosen as the most convenient. Some patients consider tanning options over in-office UVB because of the inconvenience of traveling to an appointment.11 Therefore, at-home tanning may be a convenient alternative for these patients.
Considerations—Although our study was limited to an adult population, issues with convenience exist for the pediatric population as well, as children may need to miss multiple days of school each week to be treated in the office. For these pediatric patients, an at-home unit is preferable; however; issues with insurance coverage remain a challenge.12 Increasing insurance coverage of at-home units for the pediatric population therefore would be most prudent. However, when other options have been exhausted, including in-office UVB, tanning and sunbathing may be viable alternatives because of cost and convenience. In our study, sunbathing (33.2%) was considered the most cost-effective, likely because it does not require expensive equipment or a visit to a salon or physician’s office. Sunbathing has been effective in treating some dermatologic conditions, such as atopic dermatitis.13 However, it may only be effective during certain months and at different latitudes—conditions that make UVB sun rays more accessible—particularly when treating psoriasis.14 Furthermore, sunbathing may not be as cost-effective in patients with average-severity psoriasis compared with conventional psoriasis therapy because of the costs of travel to areas with sufficient UVB rays for treatment.15 Additionally, insurance status was related to which option was selected as the most cost-effective, as 29 (53.7%) of 54 uninsured participants chose sunbathing as the most cost-effective option, while only 92 (34.2%) of 269 privately insured patients selected sunbathing. Therefore, insurance status may be a factor for dermatologists to consider if a patient prefers a treatment that is cost-effective. Overall, dermatologists could perhaps consider guiding patients and optimizing their treatment plans based on the factors most important to the patients while understanding that costs and insurance status may ultimately determine the treatment option.
Limitations—Survey participants were recruited on Amazon Mechanical Turk, which could create sampling bias. Furthermore, these participants were representative of the general public and not exclusively patients on phototherapy, therefore representing the opinions of the general public and not those who may require phototherapy. Furthermore, given the nature of the survey, the study was limited to the adult population.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
- Totonchy MB, Chiu MW. UV-based therapy. Dermatol Clin. 2014;32:399-413, ix-x.
- Nilsen LT, Hannevik M, Veierød MB. Ultraviolet exposure from indoor tanning devices: a systematic review. Br J Dermatol. 2016;174:730-740.
- Su J, Pearce DJ, Feldman SR. The role of commercial tanning beds and ultraviolet A light in the treatment of psoriasis. J Dermatolog Treat. 2005;16:324-326.
- Anderson KL, Huang KE, Huang WW, et al. Dermatology residents are prescribing tanning bed treatment. Dermatol Online J. 2016;22:13030/qt19h4k7sx.
- Wehner MR, Shive ML, Chren MM, et al. Indoor tanning and non-melanoma skin cancer: systematic review and meta-analysis. BMJ. 2012;345:e5909.
- Boniol M, Autier P, Boyle P, et al. Cutaneous melanomaattributable to sunbed use: systematic review and meta-analysis. BMJ. 2012;345:E4757.
- Barros NM, Sbroglio LL, Buffara MO, et al. Phototherapy. An Bras Dermatol. 2021;96:397-407.
- Koek MB, Buskens E, van Weelden H, et al. Home versus outpatient ultraviolet B phototherapy for mild to severe psoriasis: pragmatic multicentre randomized controlled non-inferiority trial (PLUTO study). BMJ. 2009;338:b1542.
- Koek MB, Sigurdsson V, van Weelden H, et al. Cost effectiveness of home ultraviolet B phototherapy for psoriasis: economic evaluation of a randomized controlled trial (PLUTO study). BMJ. 2010;340:c1490.
- Tan SY, Buzney E, Mostaghimi A. Trends in phototherapy utilization among Medicare beneficiaries in the United States, 2000 to 2015. J Am Acad Dermatol. 2018;79:672-679.
- Felton S, Adinoff B, Jeon-Slaughter H, et al. The significant health threat from tanning bed use as a self-treatment for psoriasis. J Am Acad Dermatol. 2016;74:1015-1017.
- Juarez MC, Grossberg AL. Phototherapy in the pediatric population. Dermatol Clin. 2020;38:91-108.
- Autio P, Komulainen P, Larni HM. Heliotherapy in atopic dermatitis: a prospective study on climatotherapy using the SCORAD index. Acta Derm Venereol. 2002;82:436-440.
- Krzys´cin JW, Jarosławski J, Rajewska-Wie˛ch B, et al. Effectiveness of heliotherapy for psoriasis clearance in low and mid-latitudinal regions: a theoretical approach. J Photochem Photobiol B. 2012;115:35-41.
- Snellman E, Maljanen T, Aromaa A, et al. Effect of heliotherapy on the cost of psoriasis. Br J Dermatol. 1998;138:288-292.
Practice Points
- Patients have different priorities when selecting phototherapy, including safety, costs, effectiveness, insurance issues, and convenience.
- By offering and educating patients on all forms of phototherapy, dermatologists may help guide patients to their optimal treatment plan according to patient priorities.
Pyostomatitis Vegetans With Orofacial and Vulvar Granulomatosis in a Pediatric Patient
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.
The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.
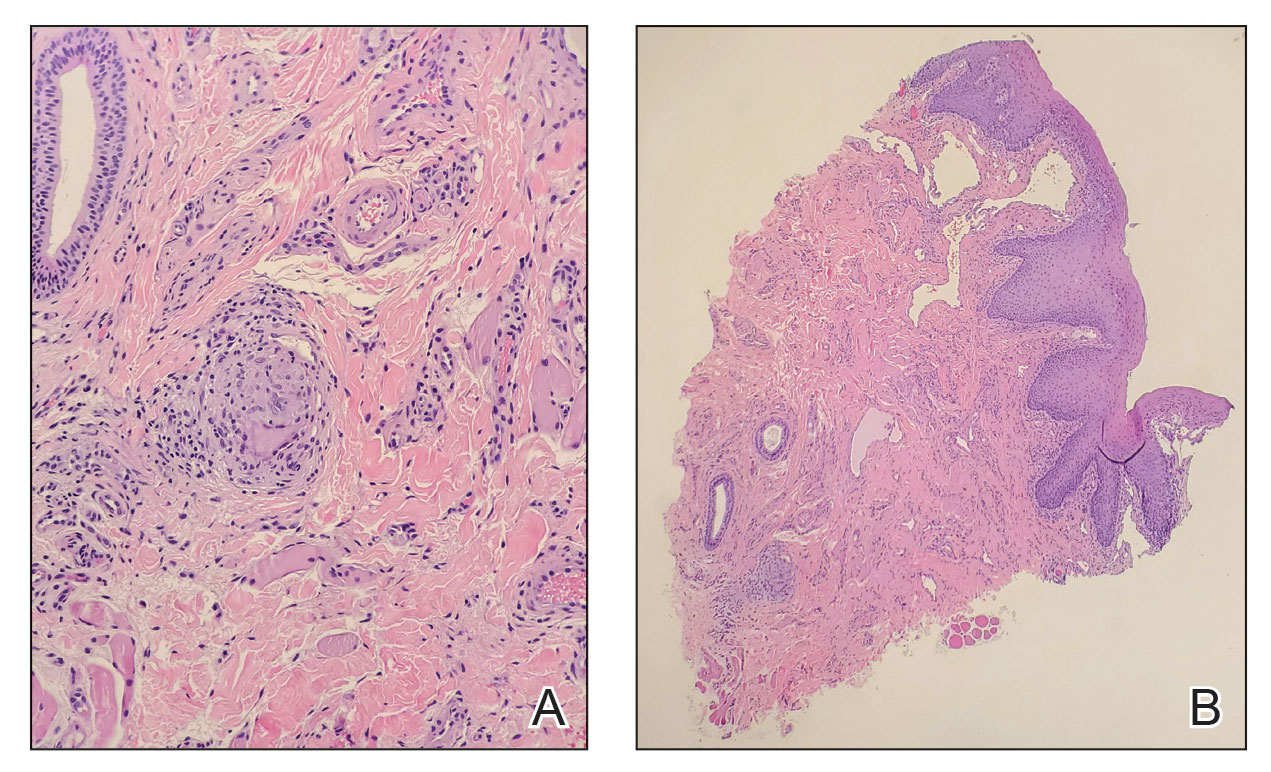
Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29
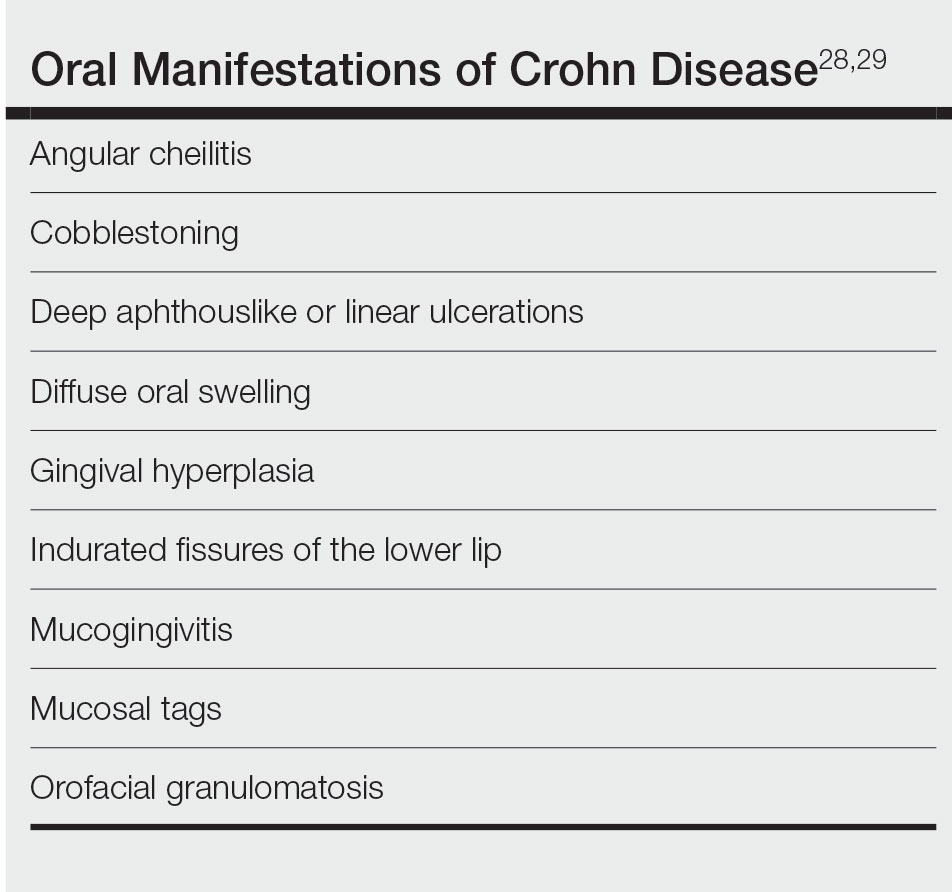
Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
Case Report
A 7-year-old girl who was otherwise healthy was referred by pediatric gastroenterology for evaluation of cutaneous Crohn disease (CD). The patient had a 4-year history of persistent lip swelling and a 3-year history of asymmetric erythematous labial swelling and perianal erythema with skin tags. She had been applying the calcineurin inhibitor tacrolimus ointment 0.03% 1 or 2 times daily to her lesions with minimal improvement. She did not have a medical history of recurrent or unusual infectious diseases. There was no family history of autoimmune disease.
The patient and her guardian reported intermittent perianal pain but denied constipation, diarrhea, abdominal pain, and blood in the stool. She denied throat and tongue swelling, dysphagia, dyspnea, drooling, facial paralysis, and eyelid edema. She was a well-nourished child whose height and weight percentiles tracked at 30% and 25%, respectively. Physical examination revealed confluent symmetric lip swelling with mild angular cheilitis. Multiple 1- to 2-mm white pustules with pinpoint erosions covered the upper and lower labial mucosa and extended onto the buccal mucosa (Figure 1). She had symmetric erythema and swelling of the left labia majora extending to and involving the left perianal mucosa. Three perianal erythematous skin tags and a perianal fissure were identified.

The patient had been assessed 2 years earlier by pediatric dermatology and gastroenterology with an extensive evaluation that favored a diagnosis of cutaneous CD because the combination of orofacial granulomatosis (OFG), vulvar edema, and perianal skin tags is strongly associated.1-3 Contact dermatitis affecting the mouth was considered; however, allergen testing did not demonstrate a trigger.
A trial of a benzoate- and cinnamon-free diet, which has been reported to improve OFG,4 did not provide symptomatic improvement. Topical corticosteroids and tacrolimus reduced the perioral erythema, but the swelling persisted. An infectious cause was considered; however, topical mupirocin had no effect, and amoxicillin resulted in oral candidiasis.
A perianal biopsy revealed a granulomatous dermatitis. Fungal and bacterial cultures were negative. Upper and lower gastrointestinal (GI) endoscopy and a fecal calprotectin assay were not suggestive of inflammatory bowel disease (IBD). A complete blood cell count and QuantiFERON-TB Gold test measuring the immune response to tuberculosis antigens were normal. Chronic granulomatous disease, RAG1/RAG2 deficiency, common variable immunodeficiency, and NOD2 defects were ruled out with normal tests of dihydrorhodamine, quantitative immunoglobulins, and toll-like receptors.
Because of the discomfort associated with the patient’s lesions, she was offered treatment with tumor necrosis factor α inhibitors, including infliximab and adalimumab. These agents had been offered since the onset of symptoms; however, her parents declined systemic medication unless she developed GI involvement. Instead, the tacrolimus concentration was increased to 0.1% applied to the lips, labia, and perianal area, and fluocinonide gel 0.05% applied nightly to the oral pustules was added.
Two months later the patient had notably fewer oral pustules and diminished erythema but only slightly reduced oral, vulvar, and perianal swelling. A trial of oral metronidazole, which has been reported to clear a patient with cutaneous CD,5 was discontinued by her parents after 6 weeks because of a lack of interval improvement.
One year later, a pre-existing perianal skin tag doubled in size and became exquisitely tender. The calprotectin level—previously within reference range at less than 16 μg/g—was now elevated at 149 μg/g (reference range, 1–120 μg/g) and increased to 336 μg/g 3 weeks later. Testing for C-reactive protein, zinc, and stool occult blood; a comprehensive metabolic panel; and a complete blood cell count were unremarkable.
Repeat upper and lower GI endoscopy did not suggest CD. A biopsy using direct immunofluorescence (DIF) was obtained to evaluate for pyostomatitis vegetans (PSV) and rule out
The captured biopsy did not demonstrate the intended pustule; instead, it included less-affected mucosa and was obtained during topical treatment when few pustules and erosions persisted. Pathologic analysis revealed noncaseating granulomas without an increase in microabscesses, neutrophils, or eosinophils (Figure 2). Direct immunofluorescence staining for IgG, IgA, and C3 and indirect immunofluorescence staining for desmoglein-1 and desmoglein-3 antibodies were negative. Although the biopsy did not capture the intended pustule, diagnosis of PV was made based on clinical features and the constellation of cutaneous findings associated with IBD.

Intralesional triamcinolone, which has been of benefit for pediatric patients with orofacial granulomatosis,1,6,7 was instituted and normalized the vulva and perianal mucosa; however, lip swelling improved only minimally.
Comment
Pyostomatitis vegetans is characterized by multiple white or yellow, friable, miliary pustules that rupture, leaving behind ulcerations and erosions that cause a varying degree of oral pain.8 The disorder can involve any area of the oral mucosa—most often the labia-attached gingiva, soft and hard palates, buccal mucosa, vestibule, and tonsillar areas—but often spares the floor of the mouth and tongue.8-11 The term pyostomatitis vegetans was proposed in 1949 by McCarthy12 when he noted in a patient who presented with the characteristic appearance of the oral mucosa, though cases of vaginal, nasal, and periocular involvement have been reported.8,13,14
Histopathology—Pyostomatitis vegetans displays pseudoepithelial hyperplasia with acanthosis, hyperkeratosis, and intraepithelial or subepithelial microabscesses (or both) with neutrophils and eosinophils.8,9,15 There are a few possible explanations for this patient’s lack of tissue eosinophilia. It has been theorized that the presence of granulomas could mask concurrent PSV16 or that tissue in PSV contains fewer eosinophils as the disorder progresses.11 The oral biopsy obtained from our patient did not capture a pustule, and the condition had noticeably improved with topical tacrolimus at the time of biopsy; therefore, neither neutrophils nor eosinophils were identified. Peripheral eosinophilia, which is present in 42% to 90% of cases of PSV,9,17 can be a diagnostic clue.18 However, PE is associated with IBD,24 which usually occurs with PSV, so the absence of peripheral eosinophilia in our patient may be explained by her lack of bowel disease.
Pathogenesis—The pathogenesis of PSV is unknown. A proposed etiology includes cross-reacting antigens in the bowel and skin secondary to IBD as well as an aberrant immune response to an unidentified factor.8 Pyostomatitis vegetans is considered by many to be the mucosal variant of pyodermatitis vegetans,9,15,19 a neutrophilic dermatosis characterized by asymmetric, crusted, erythematous papulopustules that extend peripherally and coalesce to form large vegetating plaques. These lesions commonly manifest in the axillary folds, groin, and scalp and can involve the face, trunk, and distal extremities.9,18 Infection has been suggested as a cause of PSV, though cultures for pathogenic bacteria, viruses, and fungi consistently show only normal flora.20 Zinc deficiency attributed to malabsorption from CD was reported in an adult with PSV.21 The PSV resolved after 6 weeks of zinc supplementation.
Differential Diagnosis—The main entity in the clinical differential diagnosis for PSV is PVH, which is considered a variant of pemphigus vulgaris. Pemphigus vegetans of Hallopeau presents with pustules and progresses to hyperpigmented vegetative plaques with peripheral hypertrophic granulation tissue.22 The clinical and histological presentation of PVH can be similar to PSV; in PVH, however, DIF demonstrates intercellular IgG and C3 due to circulating IgG autoantibodies specific for desmoglein 3, a cell adhesion molecule.22-24 In PSV, DIF typically is negative for IgG, IgA, and C3.8 Immunohistochemical findings of PSV may overlap with IgA pemphigus, IgG/IgA pemphigus, and IgG pemphigus, which has sparked debate if PSV is an autoimmune blistering disorder or a secondary finding of epithelial injury.9,18,24
Pyostomatitis vegetans is most prevalent in patients aged 20 to 59 years25 but can occur at any age.8,19 Overall, extraintestinal symptoms, including mucocutaneous findings, are common in pediatric patients—in 30% to 71% of children with CD and 21% to 22% of children with ulcerative colitis26—and can predate onset of GI symptoms in 6% of pediatric patients.27
Oral disease is common in CD; manifestations are listed in the Table.28,29 In a prospective study of 48 children with CD, 42% (20/48) had oral manifestations identified at diagnosis28; in a similar study of 25 children, researchers noted that 48% (12/25) had disease-specific oral lesions.29 None of these children recognized the oral findings prior to the onset of systemic symptoms.28 Pyostomatitis vegetans was the least common oral manifestation, reported in 1 of 73 patients in the 2 studies combined.28,29

Two recent articles that looked at PSV in pediatric and adolescent populations identified only 9 patients with PSV.24,30 Only 2 patients (siblings) had documented onset of PSV before 12 years of age,31 which suggests an underlying genetic predisposition in young children.
It has been reported that active or subclinical (ie, asymptomatic with positive endoscopic findings) IBD in adults precedes onset of PSV, which may be considered a sign of relapse.9,30 However, PSV is incredibly rare in children and adolescents and can be an early finding of IBD in children.16,31,32
Our patient has not developed GI involvement since her initial presentation 5 years prior, though another pediatric patient developed symptomatic CD 9 years after onset of OFG.5 A retrospective review of pediatric OFG without CD met criteria for CD at a median of 3.1 years (range, 0.4–6.9 years).33 Regrettably, the early presence of PSV has been associated with future progression to CD and a complicated disease course.12,34
Management—Pyoderma stomatitis vegetans is treated with management of underlying IBD,8 with scarce literature available regarding pediatric patients. Oral lesions have been treated with antiseptics and topical corticosteroids, though these have limited benefit.8 In an adult with IBD, topical tacrolimus initially cleared PSV; however, lesions recurred until mesalamine was initiated.35 Systemic steroids were effective in a 16-year-old patient with CD and PSV,12 but recurrence is common after corticosteroids are stopped.34
Some patients benefit from steroid-sparing medications, such as dapsone, azathioprine, sulfamethoxypyridazine, methotrexate, mycophenolate mofetil, and tumor necrosis factor α inhibitors such as infliximab and adalimumab.8,9,15,23,34,36 A 12-year-old patient with pyodermatitis–PSV without intestinal disease was treated with prednisone, dapsone, and azathioprine with improvement but not complete resolution of oral erosions after 18 weeks of treatment.32 A 15-year-old patient with CD and pyodermatitis–PSV did not show improvement on prednisone, dapsone, and azathioprine but rapidly responded to infliximab.23 Infliximab led to complete clearance of oral lesions in an adult with severe fistulizing CD who developed PSV.11 However, 2 adolescent patients with CD developed PSV while on adalimumab,6,34 though 1 did improve after increasing adalimumab from once to twice weekly.6
Conclusion
The case described here—PSV in a prepubertal 7-year-old with multiple cutaneous findings suggestive of CD, including OFG, perianal and vulvar edema with biopsy-proven noncaseating granulomas, anal skin tags, and an elevated calprotectin level, noted during a cutaneous flare without clinical or endoscopically identified underlying bowel involvement—is an extremely rare presentation. Literature regarding management of PSV primarily is found in the form of case reports and focuses on treating underlying IBD. In patients with intestinal disease, treatment with biologic therapy appears most effective.6,23
ADDENDUM
Interestingly, 3 years after the patient’s original presentation to our clinic, chromosomal sequencing analysis to assess for copy number variants and whole exome gene sequencing identified a variant of unknown significance in the heat shock protein family A member 1-like gene, HSPA1L, which has an unknown mode of inheritance, but the literature suggests that both truncating and missense variants could be associated with individuals with ulcerative colitis, CD, and IBD.37,38 Although we cannot use this information to render a molecular diagnosis, it is highly suspicious that this is the cause of her clinical findings. Additionally, the patient currently is aged 10 years with unchanged cutaneous findings and has not developed gastrointestinal findings of IBD.
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
- Tuxen AJ, Orchard D. Childhood and adolescent orofacial granulomatosis is strongly associated with Crohn’s disease and responds to intralesional corticosteroids. Australas J Dermatol. 2010;51:124-127. doi:10.1111/j.1440-0960.2010.00627.x
- Vaid RM, Cohen BA. Cutaneous Crohn’s disease in the pediatric population. Pediatr Dermatol. 2010;27:279-281. doi:10.1111/j.1525-1470.2010.01138.x
- van de Scheur MR, van der Waal RIF, van der Waal I, et al. Ano-genital granulomatosis: the counterpart of oro-facial granulomatosis. J Eur Acad Dermatol Venereol. 2003;17:184-189. doi:10.1046/j.1468-3083.2003.00573.x
- Campbell HE, Escudier MP, Patel P, et al. Review article: cinnamon- and benzoate-free diet as a primary treatment for orofacial granulomatosis. Aliment Pharmacol Ther. 2011;34:687-701. doi:10.1111/j.1365-2036.2011.04792.x
- Duhra P, Paul CJ. Metastatic Crohn’s disease responding to metronidazole. Br J Dermatol. 1988;119:87-91. doi:10.1111/j.1365-2133.1988.tb07107.x
- Katsanos KH, Torres J, Roda G, et al. Review article: non-malignant oral manifestations in inflammatory bowel diseases. Aliment Pharmacol Ther. 2015;42:40-60. doi:10.1111/apt.13217
- Schmitz BA, Unkel JH. Symptomatic oral Crohn’s disease in an adolescent. J Dent Child (Chic). 2018;85:66-69.
- Femiano F, Lanza A, Buonaiuto C, et al. Pyostomatitis vegetans: a review of the literature. Med Oral Patol Oral Cir Bucal. 2009;14:E114-E117.
- Clark LG, Tolkachjov SN, Bridges AG, et al. Pyostomatitis vegetans (PSV)–pyodermatitis vegetans (PDV): a clinicopathologic study of 7 cases at a tertiary referral center. J Am Acad Dermatol. 2016;75:578-584. doi:10.1016/j.jaad.2016.03.047
- Hansen LS, Silverman S Jr, Daniels TE. The differential diagnosis of pyostomatitis vegetans and its relation to bowel disease. Oral Surg Oral Med Oral Pathol. 1983;55:363-373. doi:10.1016/0030-4220(83)90191-3
- Cataldo E, Covino MC, Tesone PE. Pyostomatitis vegetans. Oral Surg Oral Med Oral Pathol. 1981;52:172-177. doi:10.1016/0030-4220(81)90316-9
- McCarthy FP. Pyostomatitis vegetans; report of three cases. Arch Derm Syphilol. 1949;60:750-764.
- Bens G, Laharie D, Beylot-Barry M, et al. Successful treatment with infliximab and methotrexate of pyostomatitis vegetans associated with Crohn’s disease. Br J Dermatol. 2003;149:181-184. doi:10.1046/j.1365-2133.2003.05385.x
- Leibovitch I, Ooi C, Huilgol SC, et al. Pyodermatitis–pyostomatitis vegetans of the eyelids: case report and review of the literature. Ophthalmology. 2005;112:1809-1813. doi:10.1016/j.ophtha.2005.04.027
- Ruiz-Roca JA, Berini-Aytés L, Gay-Escoda C. Pyostomatitis vegetans. report of two cases and review of the literature. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:447-454. doi:10.1016/j.tripleo.2003.08.022
- Molnár T, Farkas K, Nagy F, et al. Third case: another pediatric patient with pyostomatitis vegetans and oral granuloma as one of the initial symptoms of Crohn’s disease. Inflamm Bowel Dis. 2011;17:E122-E123. doi:10.1002/ibd.21791
- Leydhecker W, Lund OE. Eye involvement in pyostomatitis vegetans. Klin Monbl Augenheilkd Augenarztl Fortbild. 1962;141:595-602.
- Thornhill MH, Zakrzewska JM, Gilkes JJ. Pyostomatitis vegetans: report of three cases and review of the literature. J Oral Pathol Med. 1992;21:128-133. doi:10.1111/j.1600-0714.1992.tb00996.x
- Chaudhry SI, Philpot NS, Odell EW, et al. Pyostomatitis vegetans associated with asymptomatic ulcerative colitis: a case report. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87:327-330. doi:10.1016/s1079-2104(99)70217-9
- Konstantopoulou M, O’Dwyer EM, Steele JC, et al. Pyodermatitis–pyostomatitis vegetans complicated by methicillin-resistant Staphylococcus aureus infection. Clin Exp Dermatol. 2005;30:666-668. doi:10.1111/j.1365-2230.2005.01906.x
- Ficarra G, Cicchi P, Amorosi A, et al. Oral Crohn’s disease and pyostomatitis vegetans. an unusual association. Oral Surg Oral Med Oral Pathol. 1993;75:220-224. doi:10.1016/0030-4220(93)90097-n
- Markopoulos AK, Antoniades DZ, Zaraboukas T. Pemphigus vegetans of the oral cavity. Int J Dermatol. 2006;45:425-428. doi:10.1111/j.1365-4632.2004.02480.x
- Nico MMS, Hussein TP, Aoki V, et al. Pyostomatitis vegetans and its relation to inflammatory bowel disease, pyoderma gangrenosum, pyodermatitis vegetans, and pemphigus. J Oral Pathol Med. 2012;41:584-588. doi:10.1111/j.1600-0714.2012.01152.x
- Berzin D, Lahad A, Weiss B, et al. Inflammatory bowel disease presenting with pyodermatitis–pyostomatitis vegetans in a pediatric patient: a case report and review of the literature. Pediatr Dermatol. 2021;38:868-871. doi:10.1111/pde.14625
- Ballo FS, Camisa C, Allen CM. Pyostomatitis vegetans. report of a case and review of the literature. J Am Acad Dermatol. 1989;21:381-387.
- Greuter T, Bertoldo F, Rechner R, et al; Swiss IBD Cohort Study Group. Extraintestinal manifestations of pediatric inflammatory bowel disease: prevalence, presentation, and anti-TNF treatment. J Pediatr Gastroenterol Nutr. 2017;65:200-206. doi:10.1097/MPG.0000000000001455
- Jose FA, Garnett EA, Vittinghoff E, et al. Development of extraintestinal manifestations in pediatric patients with inflammatory bowel disease. Inflamm Bowel Dis. 2009;15:63-68. doi:10.1002/ibd.20604
- Harty S, Fleming P, Rowland M, et al. A prospective study of the oral manifestations of Crohn’s disease. Clin Gastroenterol Hepatol. 2005;3:886-891. doi:10.1016/s1542-3565(05)00424-6
- Pittock S, Drumm B, Fleming P, et al. The oral cavity in Crohn’s disease. J Pediatr. 2001;138:767-771. doi:10.1067/mpd.2001.113008
- Bardasi G, Romagnoli A, Foschini MP, et al. Pyostomatitis vegetans in a pediatric patient with ulcerative colitis: case report of a rare pediatric inflammatory bowel disease extraintestinal manifestation and review of the literature. Eur J Gastroenterol Hepatol. 2020;32:889-892. doi:10.1097/MEG.0000000000001723
- Mesquita Kde C, Costa IM. Case for diagnosis. An Bras Dermatol. 2012;87:929-931. doi:10.1590/s0365-05962012000600022
- Al-Rimawi HS, Hammad MM, Raweily EA, et al. Pyostomatitis vegetans in childhood. Eur J Pediatr. 1998;157:402-405. doi:10.1007/s004310050838
- Chen KL, Diiorio DA, Chiu YE, et al. Pediatric patients with orofacial granulomatosis likely to subsequently develop intestinal Crohn’s disease: brief report. Pediatr Dermatol. 2020;37:1162-1164. doi:10.1111/pde.14390
- Pazheri F, Alkhouri N, Radhakrishnan K. Pyostomatitis vegetans as an oral manifestation of Crohn’s disease in a pediatric patient. Inflamm Bowel Dis. 2010;16:2007. doi:10.1002/ibd.21245.
- Werchniak AE, Storm CA, Plunkett RW, et al. Treatment of pyostomatitis vegetans with topical tacrolimus. J Am Acad Dermatol. 2005;52:722-723. doi:10.1016/j.jaad.2004.11.041
- Stingeni L, Tramontana M, Bassotti G, et al. Pyodermatitis–pyostomatitis vegetans and antibullous pemphigoid antigen 180 autoantibodies: a casual association? Br J Dermatol. 2015;172:811-813. doi:10.1111/bjd.13297
- Takahashi S, Andreoletti G, Chen R, et al. De novo and rare mutations in the HSPA1L heat shock gene associated with inflammatory bowel disease. Genome Med. 2017;9:8. doi:10.1186/s13073-016-0394-9
- Crowley E, Warner N, Pan J, et al. Prevalence and clinical features of inflammatory bowel diseases associated with monogenic variants, identified by whole-exome sequencing in 1000 children at a single center. Gastroenterology. 2020;158:2208-2220. doi:10.1053/j .gastro.2020.02.023
Practice Points
- Pyostomatitis vegetans (PSV) is a rare manifestation of cutaneous Crohn disease in children and can precede the onset of bowel pathology.
- Although topical and intralesional corticosteroids were beneficial in our patient, systemic corticosteroids and tumor necrosis factor α inhibitors, including infliximab and adalimumab, used to treat underlying inflammatory bowel disease appear to be the most efficacious option for treating PSV.
Erythrasma
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.
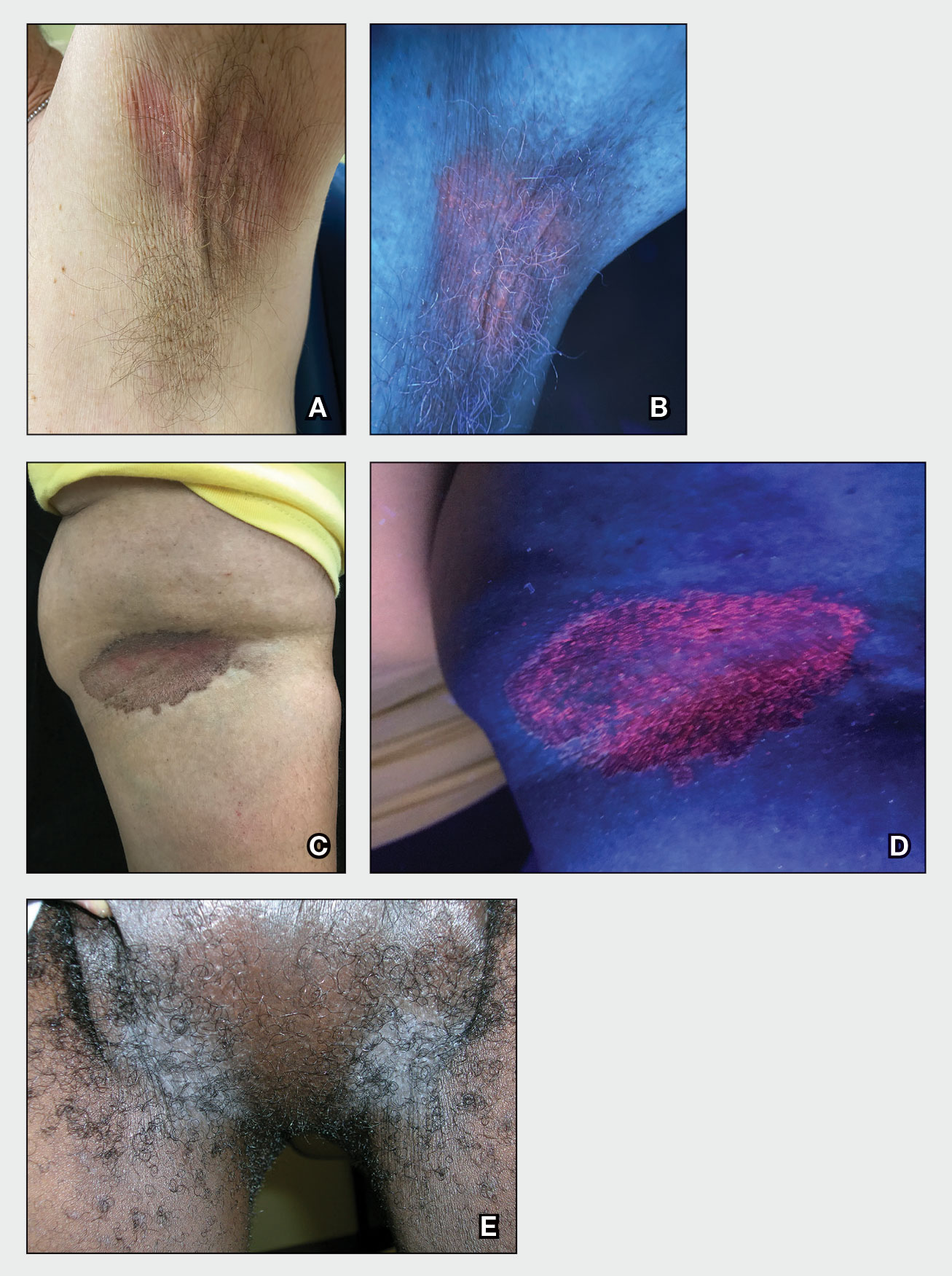
Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
THE COMPARISON
A and B Axilla of a 65-year-old White man with erythrasma showing a well-demarcated erythematous plaque with fine scale (A). Wood lamp examination of the area showed characteristic bright coral red fluorescence (B).
C and D A well-demarcated, red-brown plaque with fine scale in the antecubital fossa of an obese Hispanic woman (C). Wood lamp examination revealed bright coral red fluorescence (D).
E Hypopigmented patches in the groin with pruritus in a Black man. He also had erythrasma between the toes.
Erythrasma is a skin condition caused by acute or chronic infection of the outermost layer of the epidermis (stratum corneum) with Corynebacterium minutissimum. It has a predilection for intertriginous regions such as the axillae, groin, and interdigital spaces of the toes. It can be associated with pruritus or can be asymptomatic.

Epidemiology
Erythrasma typically affects adults, with greater prevalence among those residing in shared living facilities, such as dormitories or nursing homes, or in humid climates.1 It is a common disorder with an estimated prevalence of 17.6% of bacterial skin infections in elderly patients and 44% of diabetic interdigital toe space infections.2,3
Key clinical features
Erythrasma can manifest as red-brown hyperpigmented plaques with fine scale and little central clearing (Figures A and C) or as a hypopigmented patch (Figure E) with a sharply marginated, hyperpigmented border in patients with skin of color. In the interdigital toe spaces, the skin often is white and macerated. These findings may appear in patients of all skin tones.
Worth noting
• Corynebacterium minutissimum produces coproporphyrin III, which glows fluorescent red under Wood lamp examination (Figures B and D). A recent shower or bath may remove the fluorescent coproporphyrins and cause a false-negative result. The interdigital space between the fourth and fifth toes is a common location for C minutissimum; thus clinicians should consider examining these areas with a Wood lamp.
• Associated risk factors include obesity, immunosuppression, diabetes mellitus, and excessive sweating.1
• The differential diagnosis includes intertrigo, inverse psoriasis, confluent and reticulated papillomatosis (Gougerot-Carteaud syndrome), acanthosis nigricans, seborrheic dermatitis, and tinea pedis when present in the interdigital toe spaces. Plaques occurring in circular patterns may be mistaken for tinea corporis or pityriasis rotunda.
• There is a high prevalence of erythrasma in patients with inverse psoriasis, and it may exacerbate psoriatic plaques.4
• Treatment options include application of topical clindamycin or erythromycin to the affected area.1 Some patients have responded to topical mupiricin.2 For larger areas, a 1-g dose of clarithromycin5 or a 14-day course of erythromycin may be appropriate.1 Avoid prescribing clarithromycin to patients with preexisting heart disease due to its increased risk for cardiac events or death; consider other agents.
Health disparity highlight
Obesity, most prevalent in non-Hispanic Black adults (49.9%) and Hispanic adults (45.6%) followed by non- Hispanic White adults (41.4%),6 may cause velvety dark plaques on the neck called acanthosis nigricans. However, acute or chronic erythrasma also may cause hyperpigmentation of the body folds. Although the pathology of erythrasma is due to bacterial infection of the superficial layer of the stratum corneum, acanthosis nigricans is due to fibroblast proliferation and stimulation of epidermal keratinocytes likely from increased growth factors and insulinlike growth factor.7 If erythrasma is mistaken for acanthosis nigricans, the patient may be counseled inappropriately that the hyperpigmentation is something not easily resolved and subsequently left with an active treatable condition that adversely affects their quality of life.
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
- Groves JB, Nassereddin A, Freeman AM. Erythrasma. In: StatPearls. StatPearls Publishing; August 11, 2021. Accessed November 17, 2022. https://www.ncbi.nlm.nih.gov/books/NBK513352/
- Forouzan P, Cohen PR. Erythrasma revisited: diagnosis, differential diagnoses, and comprehensive review of treatment [published online September 30, 2020]. Cureus. 2020;12:E10733. doi:10.7759/cureus.10733
- Polat M, I˙lhan MN. Dermatological complaints of the elderly attending a dermatology outpatient clinic in Turkey: a prospective study over a one-year period. Acta Dermatovenerol Croat. 2015;23:277-281.
- Janeczek M, Kozel Z, Bhasin R, et al. High prevalence of erythrasma in patients with inverse psoriasis: a cross-sectional study. J Clin Aesthet Dermatol. 2020;13:12-14.
- Khan MJ. Interdigital pedal erythrasma treated with one-time dose of oral clarithromycin 1 g: two case reports [published online February 6, 2020]. Clin Case Rep. 2020;8:672-674. doi:10.1002/ccr3.2712
- Stierman B, Afful J, Carroll M, et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes. National Health Statistics Reports. Published June 14, 2021. Accessed November 17, 2022. https://stacks.cdc.gov/view/cdc/106273
- Brady MF, Rawla P. Acanthosis nigricans. In: StatPearls [Internet]. StatPearls Publishing; 2022. Updated October 9, 2022. Accessed November 30, 2022. https://www.ncbi.nlm.nih.gov/books/NBK431057
Mobile Enlarging Scalp Nodule
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6
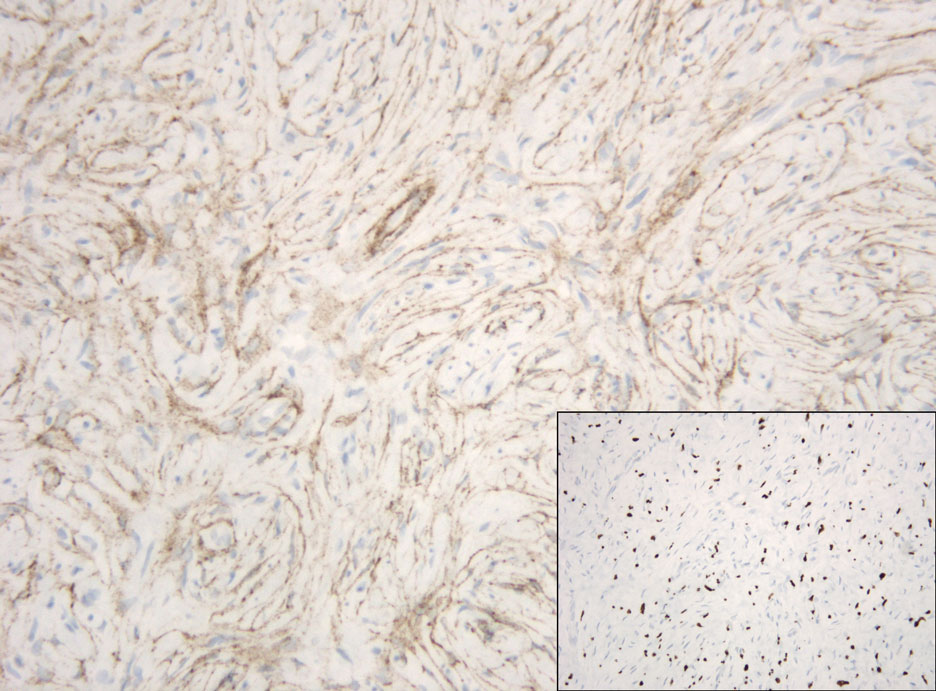
Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9
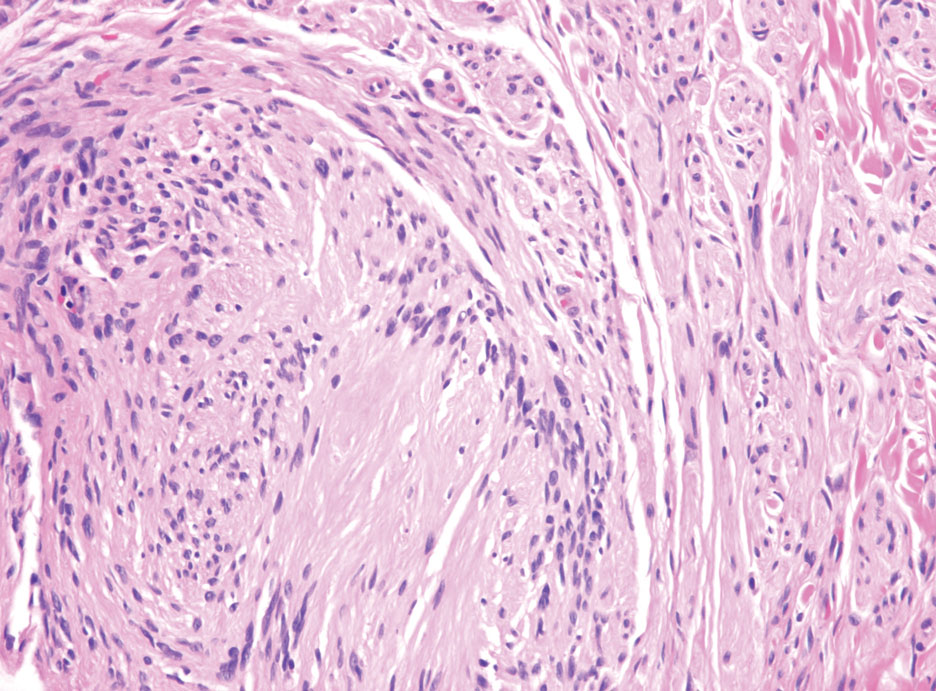
Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7
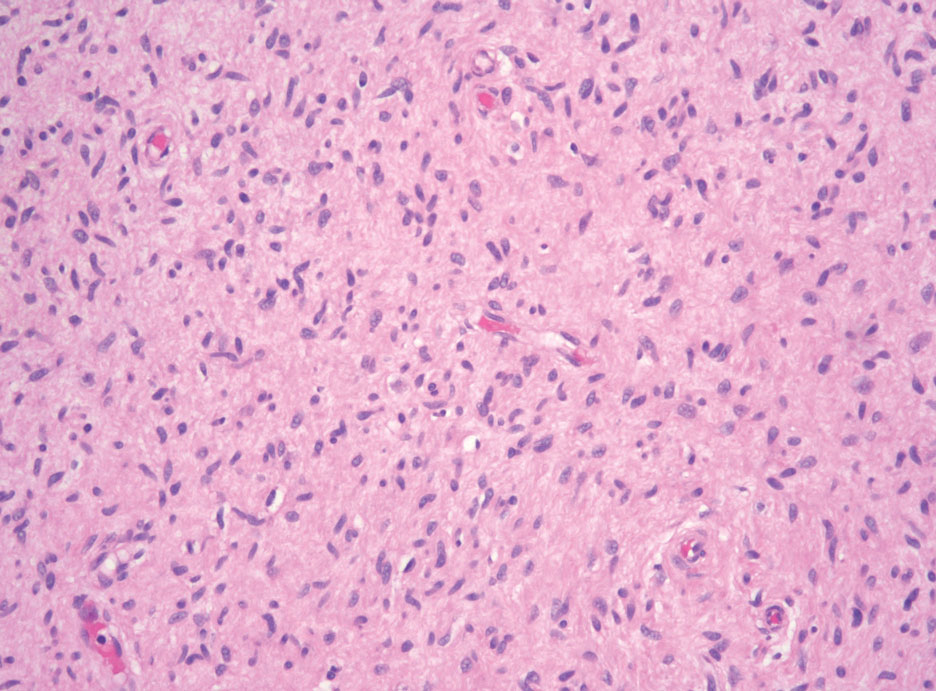
Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15
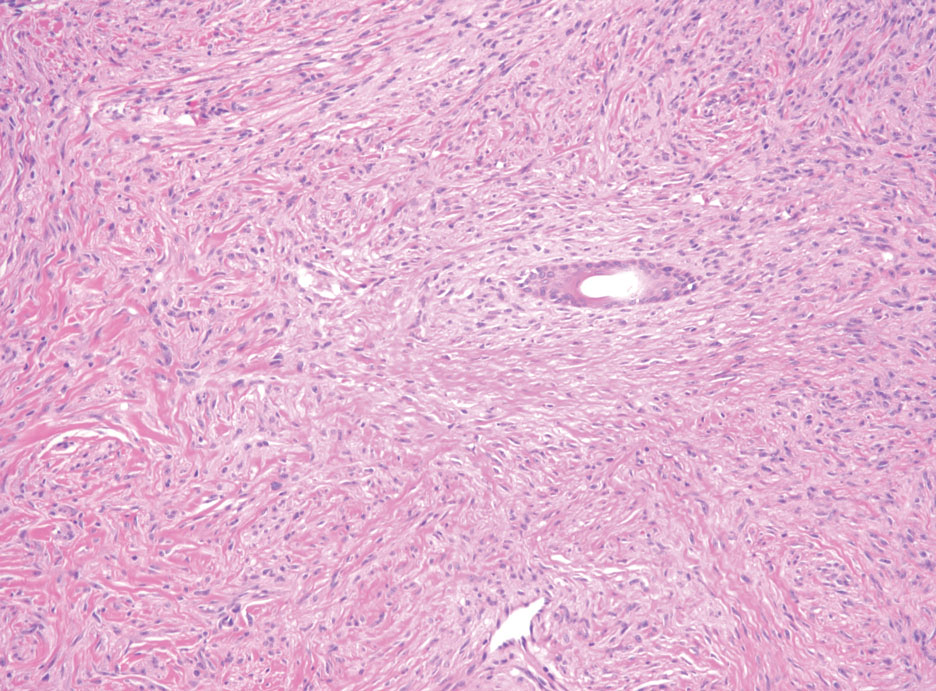
Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20
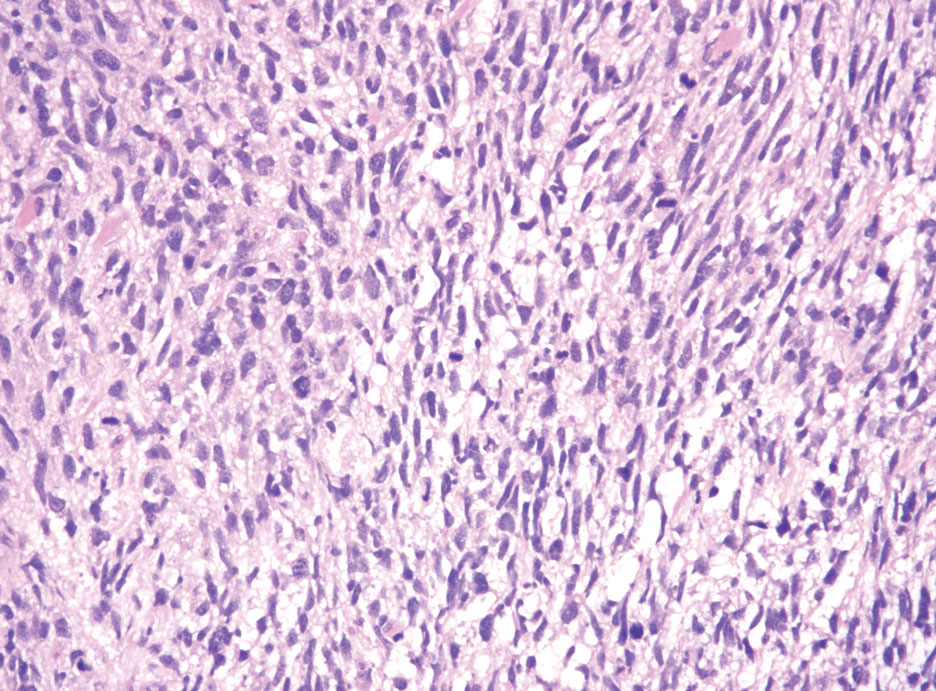
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

The Diagnosis: Hybrid Schwannoma-Perineurioma
Hybrid nerve sheath tumors are rare entities that display features of more than one nerve sheath tumor such as neurofibromas, schwannomas, and perineuriomas.1 These tumors often are found in the dermis or subcutaneous tissue of the extremities and abdomen2; however, cases of hybrid peripheral nerve sheath tumors have been reported in many anatomical locations without a gender predilection.3 The most common type of hybrid nerve sheath tumor is a schwannoma-perineurioma.3,4 Histologically, they are well-circumscribed lesions composed of both spindled Schwann cells with plump nuclei and spindled perineural cells with more elongated thin nuclei.5 Although the Schwann cell component tends to predominate, the 2 cell populations interdigitate, making it challenging to definitively distinguish them by hematoxylin and eosin staining alone.4 However, immunohistochemical (IHC) staining can be used to help distinguish the 2 separate cell populations. Staining for S-100 and SRY-box transcription factor 10 (SOX-10) will be positive in the Schwann cell component, and staining for epithelial membrane antigen, Claudin-1, or glucose transporter-1 (Figure 1) will be positive in the perineural component. Other hybrid forms of benign nerve sheath tumors include neurofibroma-schwannoma and neurofibromaperineurioma.4 Neurofibroma-schwannomas usually have a schwannoma component containing Antoni A areas with palisading Verocay bodies. The neurofibroma cells typically have wavy elongated nuclei, fibroblasts, and mucinous myxoid material.3 Neurofibroma-perineurioma is the least common hybrid tumor. These hybrid tumors have a plexiform neurofibroma appearance with areas of perineural differentiation, which can be difficult to identify on routine histology and typically will require IHC staining to appreciate. The neurofibroma component will stain positive for S-100 and negative for markers of perineural differentiation, including epithelial membrane antigen, glucose transporter-1, and Claudin-1.3 Although schwannoma-perineuriomas are benign sporadic tumors not associated with neurofibromatosis, neurofibromaschwannomas are associated with neurofibromatosis types 1 and 2 (NF1 and NF2). Neurofibroma-perineurioma tumors usually are associated with only NF1.3,6

Schwannomas typically present in middle-aged patients as tumors located on flexor surfaces.7 Although perineural cells can be seen at the periphery of a schwannoma forming a capsule, they do not interdigitate between the Schwann cells. Schwannomas are composed almost entirely of well-differentiated Schwann cells.1,4,8 Schwannomas classically are well-circumscribed, encapsulated, biphasic lesions with alternating compact areas (Antoni A) and loosely arranged areas (Antoni B). The spindled cells occasionally may display nuclear palisading within the Antoni A areas, known as Verocay bodies (Figure 2). Antoni B areas are more disorganized and hypocellular with variable macrophage infiltrate.1,4,8 The Schwann cells predominantly will have bland cytologic features, but scattered areas of degenerative nuclear atypia (also known as ancient change) may be present.4 Multiple schwannomas are associated with NF2 gene mutations and loss of merlin protein.8 There are different subtypes of schwannomas, including cellular and plexiform schwannomas.4 Because schwannomas are benign nerve sheath lesions, treatment typically consists of excision with careful dissection around the involved nerve.9

Neurofibromas are the most common peripheral nerve sheath tumors of the skin with no notable anatomic prediction, though one study found them to be more prevalent in the upper extremities.10 They typically present as sporadic solitary lesions, but multiple lesions may appear as superficial pedunculated growths that present in those aged 20 to 30 years.11 Microscopically, neurofibromas typically are not well circumscribed and have an infiltrative growth pattern. Neurofibromas are composed of cytologically bland spindled Schwann cells with thin wavy nuclei in a variable myxoid stroma (Figure 3). In addition to Schwann cells, neurofibromas contain other cell components, including fibroblasts, mast cells, perineurial-like cells, and residual axons.4 Neurofibromas typically are located in the dermis but may extend into the subcutaneous tissue. Clinically, the overlying skin may show hyperpigmentation.8 Neurofibromas can be localized, diffuse, or plexiform, with the majority being localized. Diffuse neurofibromas clinically have a raised plaque appearance. Treatment is unnecessary because these lesions are benign.7

Desmoplastic melanoma (DM) is another diagnosis in the differential for this case. Patients with DM are older compared to non-DM melanoma patients, with a male predilection.12 Desmoplastic melanomas are more likely to be located on the head and neck. In approximately one-third of cases, no in situ component will be identified, leading to confusion of the dermal lesion as a neural lesion or an area of scar formation. Microscopically, DM presents as a variable cellular infiltrative tumor composed of spindle cells with varying degrees of nuclear atypia. The spindled melanocytes are within a collagenous (desmoplastic) stroma (Figure 4).13 Desmoplastic melanoma has been described with a low mitotic index, leading to misdiagnosis with benign spindle cell neoplasms.14 The spindle cells should be positive for S-100 and SOX-10 with IHC staining. Unlike other melanomas, human melanoma black 45 and Melan-A often are negative or only focally positive. Treatment of DM is similar to non-DM in that wide local excision usually is employed. A systematic review evaluating sentinel lymph node biopsy (SLNB) recommended consideration of SLNB in mixed DM but not for pure DM, as rates of positive SLNB were much lower in the latter.15

Patients with malignant peripheral nerve sheath tumor (MPNST) usually present with an enlarging mass, pain, or neurologic symptoms. Most cases of MPNST are located on the trunk or extremities.16 Plexiform neurofibromas, especially in adults with NF1, have the potential to transform into an MPNST.4 In fact, MPNST is the most common malignancy in patients with NF1.17 Pediatric cancer survivors also are predisposed to MPNST, with a 40-fold increase in incidence compared to the general population.18 Transformation from schwannoma to MPNST is rare but has been reported.8 Histologically, spindle cells easily can be appreciated with a fasciculated growth pattern (Figure 5). Mitotic activity and tumor necrosis may be present. Diagnosis of these tumors historically has been challenging, though recent research has identified inactivation of polycomb repressive complex 2 in 70% to 90% of MPNSTs. Because of polycomb repressive complex 2 inactivation, there is loss of stone H3K27 trimethylation that can be capitalized on for MPNST diagnosis.19 Negative IHC staining for H3K27 trimethylation has been found to be highly specific for MPNST. Negative staining for different cytokeratin and melanoma markers can be helpful in differentiating it from carcinomas and melanoma. The only curative treatment for MPNST is complete excision, leaving patients with recurrent, refractory, and metastatic cases to be encouraged for enrollment in clinical trials. The 5-year survival rates for patients with MPNST reported in the literature range from 20% to 50%.20

- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
- Hornick JL, Bundock EA, Fletcher CD. Hybrid schwannoma /perineurioma: clinicopathologic analysis of 42 distinctive benign nerve sheath tumors. Am J Surg Pathol. 2009;33:1554-1561.
- Leung KCP, Chan E, Ng HYJ, et al. Novel case of hybrid perineuriomaneurofibroma of the orbit. Can J Ophthalmol. 2019;54:E283-E285.
- Ud Din N, Ahmad Z, Abdul-Ghafar J, et al. Hybrid peripheral nerve sheath tumors: report of five cases and detailed review of literature. BMC Cancer. 2017;17:349. doi:10.1186/s12885-017-3350-1
- Belakhoua SM, Rodriguez FJ. Diagnostic pathology of tumors of peripheral nerve. Neurosurgery. 2021;88:443-456.
- Michal M, Kazakov DV, Michal M. Hybrid peripheral nerve sheath tumors: a review. Cesk Patol. 2017;53:81-88.
- Harder A, Wesemann M, Hagel C, et al. Hybrid neurofibroma /schwannoma is overrepresented among schwannomatosis and neurofibromatosis patients. Am J Surg Pathol. 2012;36:702-709.
- Bhattacharyya AK, Perrin R, Guha A. Peripheral nerve tumors: management strategies and molecular insights. J Neurooncol. 2004;69:335-349.
- Pytel P, Anthony DC. Peripheral nerves and skeletal muscle. In: Kumar V, Abbas AK, Aster JC, eds. Robbins and Cotran Pathologic Basis of Disease. 10th ed. Elsevier/Saunders; 2015:1218-1239.
- Strike SA, Puhaindran ME. Nerve tumors of the upper extremity. Clin Plast Surg. 2019;46:347-350.
- Kim DH, Murovic JA, Tiel RL, et al. A series of 397 peripheral neural sheath tumors: 30-year experience at Louisiana State University Health Sciences Center. J Neurosurg. 2005;102:246-255.
- Pilavaki M, Chourmouzi D, Kiziridou A, et al. Imaging of peripheral nerve sheath tumors with pathologic correlation: pictorial review. Eur J Radiol. 2004;52:229-239.
- Murali R, Shaw HM, Lai K, et al. Prognostic factors in cutaneous desmoplastic melanoma: a study of 252 patients. Cancer. 2010; 116:4130-4138.
- Chen LL, Jaimes N, Barker CA, et al. Desmoplastic melanoma: a review. J Am Acad Dermatol. 2013;68:825-833.
- de Almeida LS, Requena L, Rutten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215.
- Dunne JA, Wormald JC, Steele J, et al. Is sentinel lymph node biopsy warranted for desmoplastic melanoma? a systematic review. J Plast Reconstr Aesthet Surg. 2017;70:274-280.
- Patel TD, Shaigany K, Fang CH, et al. Comparative analysis of head and neck and non-head and neck malignant peripheral nerve sheath tumors. Otolaryngol Head Neck Surg. 2016;154:113-120.
- Prudner BC, Ball T, Rathore R, et al. Diagnosis and management of malignant peripheral nerve sheath tumors: current practice and future perspectives. Neurooncol Adv. 2020;2(suppl 1):I40-I9.
- Bright CJ, Hawkins MM, Winter DL, et al. Risk of soft-tissue sarcoma among 69,460 five-year survivors of childhood cancer in Europe. J Natl Cancer Inst. 2018;110:649-660.
- Schaefer I-M, Fletcher CD, Hornick JL. Loss of H3K27 trimethylation distinguishes malignant peripheral nerve sheath tumors from histologic mimics. Mod Pathol. 2016;29:4-13.
- Kolberg M, Holand M, Agesen TH, et al. Survival meta-analyses for >1800 malignant peripheral nerve sheath tumor patients with and without neurofibromatosis type 1. Neuro Oncol. 2013;15:135-147.
A 50-year-old man presented with a 2.5-cm, subcutaneous, freely mobile nodule on the occipital scalp that first appeared 35 years prior but recently had started enlarging. Histologically the lesion was well circumscribed. Immunohistochemical staining was positive for SRY-box transcription factor 10 in some of the spindle cells, and staining for epithelial membrane antigen was positive in a separate population of intermixed spindle cells.
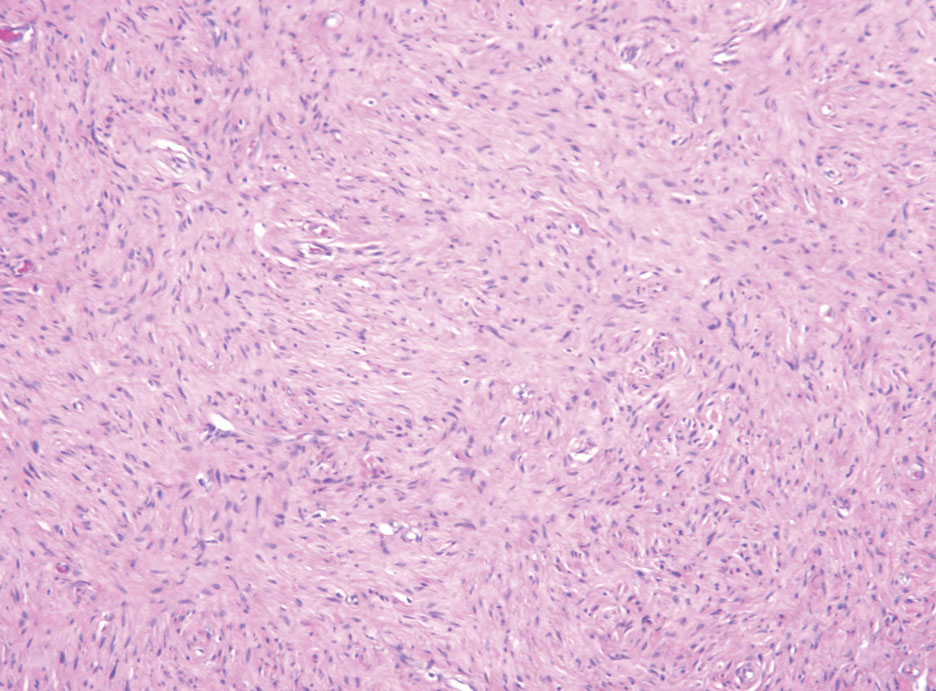
Janus Kinase Inhibitors in the Treatment of Atopic Dermatitis: Military Considerations
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
The atopic dermatitis (AD) therapeutic landscape is changing considerably with the advent of Janus kinase (JAK) inhibitors. Several JAK inhibitors recently have been approved by the US Food and Drug Administration, building off years of foundational research aimed at elucidating the downstream effects of the JAK–signal transducer and activator of transcription (STAT) pathway and its role in AD pathogenesis. Agents within this promising new class of drugs have performed well vs placebo in phase 2 and 3 clinical trials. This article reviews relevant trial efficacy and safety data of several JAK inhibitors as well as the implications of the use of these medications in AD patients, with specific considerations unique to active-duty military personnel.
Background on JAK Inhibitors
The hematopoietin superfamily of cytokine receptors encompasses a broad group that includes receptors for immune (eg, IL-2, IL-4, IFN-γ), hematopoietic (eg, erythropoietin, thrombopoietin, granulocyte-macrophage colony-stimulating factor), and nonimmune (eg, prolactin, leptin, growth hormone) cytokines. These cytokines signal via the JAK-STAT pathway. The hematopoietin family of cytokine receptors lacks intrinsic enzymatic activity, and as a result, they rely on JAK enzymes to transmit their signals intracellularly after cytokine binding to the receptor.1 Janus, of Roman mythology, was the god of doorways and archways and was commonly depicted with 2 heads. Janus kinases were named for their 2 “faces,” the kinase domain with its adjacent regulatory kinaselike domains.2 The binding of a cytokine to its receptor triggers engagement of the receptor by JAKs, leading to phosphorylation of both the JAKs and the receptor. Subsequent recruitment and phosphorylation of STAT proteins occurs. Following STAT phosphorylation, the STAT proteins dissociate, dimerize, and translocate to the nucleus, where they enact changes in cell behavior through transcriptional effects.1
Humans possess only 4 JAKs. Janus kinase 1, JAK2, and tyrosine kinase 2 are widely expressed, whereas JAK3 expression is largely limited to immune cells. Thus, there is notable overlap in the use of the 4 JAKs among the relatively larger number of various cytokines that utilize them to propagate intracellular signaling.1 Janus kinase 1 is important for signaling of receptors activated by a variety of interleukins, as well as IFN-α, IFN-β, and IFN-γ. Janus kinase 2 is important for signaling for the hormonelike cytokines erythropoietin, thrombopoietin, growth hormone, granulocyte-macrophage colony-stimulating factor, IL-3, and IL-5. Janus kinase 3 is important for hematopoietic cell proliferation and function.1
JAK Inhibitors and Atopic Dermatitis
Topical treatments, including corticosteroids and calcineurin inhibitors, are considered the standard-of-care therapy for most patients with AD; however, their clinical benefit often is limited by their anatomic use restrictions and local adverse events, including skin atrophy, striae, and application-site reactions such as stinging and burning.3 As a result, long-term application of these drugs, particularly in sensitive areas, is not recommended owing to safety/tolerability issues.3 Systemic immunomodulatory medications are indicated for patients with AD who do not achieve adequate disease control with topical treatments and/or phototherapy or for patients with severely impaired quality of life.4
Janus kinase inhibitors have several key benefits over biologics: oral and topical bioavailability, predictable pharmacokinetics, nonimmunogenicity, and dosing flexibility.4 Janus kinase 1 is central to the cell signaling of many cytokines involved in the pathogenesis of AD that comprise the T-helper lymphocytes type 2 axis: IL-4, IL-13, and thymic stromal lymphopoietin. Janus kinase signaling also may mediate itch responses by acting directly on sensory nerve fibers. Consequently, the substantial reduction in pruritus seen in many studies of JAK inhibitors is thought to be in part due to the effects on sensory nerve fibers in the skin and the blockade of early itch signaling in response to IL-4, IL-13, and IL-31.5
Abrocitinib is a JAK1 inhibitor with a similar side effect profile to upadacitinib. Both agents were approved by the FDA for the treatment of refractory moderate to severe AD on January 14, 2022.6 These are second-generation (also referred to as selective) oral JAK inhibitors with much greater inhibitory potency for JAK1 than for JAK2, JAK3, or tyrosine kinase 2, thereby reducing the risk for hematopoietic effects associated with JAK2 inhibition. The approval of abrocitinib stemmed from the phase 3 clinical trial JAK1 Atopic Dermatitis Efficacy and Safety (JADE)-MONO-1 (N=387),7 its replicate trial JADE-MONO-2 (N=391),8 and the JADE COMPARE trial.9 The JADE-MONO trials were multicenter, double-blind, placebo-controlled studies that enrolled patients 12 years and older with moderate to severe AD.7,8 Treatment groups consisted of 100-mg and 200-mg doses and were evaluated with the placebo group for their ability to achieve an investigator global assessment (IGA) score of 0 or 1 and eczema area and severity index 75 (EASI-75) at 12 weeks.7,8 Sixty-three percent of patients in the 200-mg group, 40% in the 100-mg group, and 12% in the placebo group reached the EASI-75 end point, and the differences in these response rates were statistically significant vs placebo (100 mg: 27.9% [95% CI, 17.4-38.3], P<.0001; 200 mg: 51.0% [95% CI, 40.5-61.5], P<.0001). Notably, 44% of patients using the 200-mg dose achieved almost complete or complete resolution of AD (IGA responders, improvement of ≥2 and IGA score of 0 or 1 at 12 weeks).7 In JADE-MONO-2, EASI-75 also was achieved significantly more frequently in the treatment groups compared with the placebo group at 12 weeks (200 mg: 61.0%; 100 mg: 44.5%; placebo: 10.4%; P<.001 vs placebo).8 Adjunctive therapy with topical corticosteroids was prohibited in both studies. A dose-dependent decrease in platelets was seen in both trials, as in the phase 2 trial that preceded them.10
The primary end point of the JADE COMPARE trial was to evaluate the efficacy of abrocitinib as compared with placebo at 12 weeks in adult patients with moderate to severe AD and in the setting of concomitant topical corticosteroid therapy.9 One of several secondary end points of this study compared the ability of dupilumab vs abrocitinib and placebo treatment groups to achieve itch reduction at 2 weeks, defined as 4-point improvement or more from baseline in the score on the Peak Pruritus Numerical Rating Scale (NRS), a well‐defined, reliable, sensitive, and valid scale for evaluating worst itch intensity in adults with moderate to severe AD.9,11 The primary end point was the same as in the other phase 3 studies and was met in the JADE COMPARE trial by all treatment arms. An EASI-75 was seen in 70.3% of patients treated with 200 mg of abrocitinib, 58.7% in the 100-mg abrocitinib group, 58.1% in the dupilumab group, and 27.1% in the placebo group (P<.001 for both abrocitinib doses vs placebo). Only the 200-mg dose of abrocitinib demonstrated superior itch response at week 2 compared with dupilumab (22.1% response rate difference [95% CI, 13.5-30.7; P<.001]). Both abrocitinib groups failed to demonstrate significant differences compared with dupilumab with respect to other secondary end points to include IGA response and EASI-75 at week 16.9
The most frequently reported treatment-associated adverse events were nausea, nasopharyngitis, upper respiratory tract infection, and headache, and the percentages were similar among trial groups.9 Acne was more frequently reported in the abrocitinib groups compared with placebo and the dupilumab group, and conjunctivitis was more frequently reported in the dupilumab group. Herpesvirus cutaneous infections were rare in the abrocitinib groups, as were other serious infections. No deaths, major adverse cardiovascular events (MACEs), or venous thromboembolic events (VTEs) occurred during the trial. Dose-dependent increases in creatinine phosphokinase were seen in the abrocitinib groups, whereas dose-dependent decreases were seen in platelet counts, with no patient demonstrating a platelet count below 75,000/mm3 during the study.9 Low-density lipoprotein cholesterol levels and high-density lipoprotein cholesterol levels increased in a dose-dependent manner as well, but the ratios of low-density lipoprotein to high-density lipoprotein were unchanged.9 The results of a phase 3, 92-week extension study, JADE EXTEND, were recently published and demonstrated a role for abrocitinib as a treatment for patients with moderate to severe AD, regardless of prior dupilumab response status.12
Upadacitinib, another selective JAK1 inhibitor, was approved following data from 2 replicate double-blind, phase 3, randomized, controlled trials—Measure Up 1 and Measure Up 2.13 Results demonstrated that monotherapy with once-daily upadacitinib 15 mg or 30 mg is an effective and well-tolerated treatment option for patients with moderate to severe AD vs placebo. All coprimary end points at week 16 were achieved in the upadacitinib groups in both trials. Acne, upper respiratory tract infections, nasopharyngitis, headache, and increase in serum creatinine phosphokinase levels were the most frequently reported adverse events. Rates of herpes zoster infection in upadacitinib groups were low.13
In the subsequent phase 3 AD Up trial, researchers evaluated the safety and efficacy of combination therapy with topical corticosteroids in patients aged 12 to 75 years.14 Upadacitinib groups again achieved the identical coprimary end points that were present in the Measure Up trials13 as well as all key secondary end points.14 Additionally, significant differences in secondary end points, such as a 4-point improvement in the Worst Pruritus NRS vs placebo, were noticed in both upadacitinib treatment groups as early as 1 week into the study (P<.0001), with maintenance of the effect through to week 16 (P<.0001).14 AD Up was followed by the Heads Up trial, a 24-week, phase 3, multicenter, double-blind, randomized, controlled trial comparing safety and efficacy of upadacitinib with dupilumab among 692 adults with moderate to severe AD.15 At week 16, a higher percentage of patients in the upadacitinib group achieved EASI-75 vs the dupilumab group (71.0% vs 61.1%, respectively; P=.006). The difference noted at week 2 was even more impressive, with 43.7% of patients in the upadacitinib treatment group achieving EASI-75 compared with 17.4% in the dupilumab group (P<.001). No new safety-related events were registered compared with the already available data for both drugs.15
Ruxolitinib (RUX) is a topical JAK1 and JAK2 inhibitor that was FDA approved in September 2021 for the treatment of AD.16 In a phase 2 clinical trial of 307 adult patients with 3% to 20% body surface area (BSA) affected with AD, significant reductions in itch NRS scores were observed within 36 hours after the first application of RUX cream 1.5% twice daily (-1.8 vs -0.2, P<.0001).17 These decreases were noted within the first 2 weeks of treatment for all the RUX cream regimens and were sustained through to week 8, the end of the double-blind period. At 4 weeks, change in itch from baseline was significantly reduced in the RUX 1.5% twice-daily group compared with the triamcinolone ointment 0.1% group (−4 vs −2.5, P=.003). During the open-label treatment period from 8 to 12 weeks, all patients who switched to RUX cream 1.5% twice daily noted further reductions in itch, and those who continued it demonstrated additional improvement.17
The recent FDA approval was further backed by positive phase 3 trial data from the TRuE-AD1 and TRuE-AD2 studies.18 Patients in these trials were aged 12 years and older and had AD for 2 or more years with an IGA score of 2 or 3 and 3% to 20% affected BSA. Patients were randomized to twice-daily RUX cream 0.75%, RUX cream 1.5%, or vehicle cream, and the primary end point was an IGA score of 0 or 1 and an improvement of 2 or more points from baseline at week 8. Significantly more patients achieved IGA treatment success with RUX cream 0.75% (TRuE-AD1, 50.0%; TRuE-AD2, 39.0%) and RUX cream 1.5% (TRuE-AD1, 53.8%; TRuE-AD2, 51.3%) vs vehicle (TRuE-AD1, 15.1%; TRuE-AD2, 7.6%; P<.0001) at week 8. The RUX groups experienced dramatically reduced itch compared with vehicle, with a mean reduction of approximately 3 points on the NRS at 8 weeks. Additionally, statistically significant itch reductions vs vehicle were reported within 12 hours of first application of RUX cream 1.5% (P<.05). Application-site reactions including stinging and burning occurred in less than 1% of patients, and none were considered clinically significant. Mean plasma concentrations of RUX were monitored during the phase 2 and 3 AD studies and did not lead to any clinically meaningful changes in hematologic parameters. The low bioavailability following topical application of RUX cream (6% in the TRuE-AD studies) allows for a targeted delivery of the active drug to lesional skin while reducing the safety issues associated with oral administration of JAK inhibitors.18
Baricitinib is a predominantly JAK1 and JAK2 inhibitor that was the first JAK inhibitor to be approved for the treatment of moderate to severe AD in the European Union and Japan.19 Although the FDA’s decision on baricitinib has lagged behind market competitors, in 2 phase 3 clinical trials, BREEZE-AD1 and BREEZE-AD2, baricitinib demonstrated benefit over placebo on clinically important measures of disease severity. The primary end point—the proportion of patients achieving an IGA score of 0 or 1 with an improvement of 2 or more points from baseline at week 16—was met by both tested doses of baricitinib (2 mg and 4 mg) vs placebo in BREEZE-AD1 (2 mg, P≤.05; 4 mg, P≤.001) and BREEZE-AD2 (2 mg, P≤.05; 4 mg, P≤.001). In addition, baricitinib 4 mg consistently demonstrated significant benefit over placebo on other clinically important measures of disease severity at week 16 to include itch (BREEZE-AD1 and BREEZE-AD2, P≤.001), sleep disturbance (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001), and skin pain (BREEZE-AD1, P≤.01; BREEZE-AD2, P≤.001). Nasopharyngitis, upper respiratory tract infections, creatine phosphokinase elevations, and headaches were the most frequently reported adverse events. During the 16-week treatment period in these trials, no deaths, MACEs, or VTEs occurred.19 Similar results were seen in a long-term extension study, BREEZE-AD3.20 The combination of baricitinib and topical corticosteroids were evaluated in 2 additional phase 3 trials, BREEZE-AD421 and BREEZE-AD7.22 Although only baricitinib 4 mg met the primary end point of EASI-75 at week 16 in both trials, both dosing regimens plus topical corticosteroids demonstrated notable reduction in multiple clinical and quality-of-life indices prior to week 2 when compared with placebo plus topical corticosteroids.22,23
AD in Military Service Members
Atopic dermatitis is a common condition in the general population, with a prevalence of 7.3% (95% CI, 5.9-8.8) in a recent study of American adults.24 Historically, the burden of AD that would be expected among active-duty military service members given the prevalence among the general population has not been observed, in part because of the disqualifying nature of AD for enlistment.25 The Department of Defense Instruction 6130.03, Volume 1, Medical Standards for Military Service: Appointment, Enlistment, or Induction stipulates that a history of AD or eczema after the twelfth birthday or history of residual or recurrent lesions in characteristic areas (ie, face, neck, antecubital or popliteal fossae, occasionally wrists and hands) is disqualifying.26 Specific military services possess additional standards that further define limits within the aforementioned Department of Defense instruction.25 Additionally, there are service-specific policies in place that mandate medical evaluation boards to determine fitness for continued service in the event the condition interferes with the member’s ability to perform their duties. Insection 4.2 of the U.S. Navy Aeromedical Reference and Waiver Guide, further restrictions for aviation personnel are delineated: “Depending on the location of lesions, there can be interference with the wearing of flight gear. The symptoms, particularly itching, can be distracting in flight. Patients with atopic dermatitis are more susceptible to contact dermatitis due to irritants found in a military environment.” Ultimately, the document stipulates that symptom severity and the requirement for therapy will determine the aeromedical disposition. It specifically states that “[p]atients controlled on topical therapy over small areas and patients who are asymptomatic on stable doses of loratadine (Claritin) OR fexofenadine (Allegra) may be considered for waiver,” and “intermittent use of topical steroids over a limited area is compatible with waiver.”27 It follows that limited use of topical JAK inhibitors, such as RUX, would be compatible with a waiver, given the favorable side effect profile and requirement for use in patients with 20% or lower affected BSA.16 This is just one example of duty-specific and service-specific medical standards that exist that could impact the use of both topical and oral JAK inhibitors.
Use of oral JAK inhibitors in active-duty service members is less ideal for multiple reasons. A large randomized safety clinical trial of patients with rheumatoid arthritis who received tofacitinib and methotrexate was required by the FDA to evaluate the risk of MACEs, malignancy, and infections associated with JAK inhibitor treatment. Data from this trial showed a dose-dependent increased risk for MACEs, all-cause mortality, and thrombosis at both doses of tofacitinib compared with tumor necrosis factor inhibitors and a non–dose-dependent increased risk for malignancy excluding nonmelanoma skin cancer.28 In contrast to the MACE and VTE data from patients with diseases other than AD treated with JAK inhibitors, there has been only 1 patient who developed a pulmonary embolism while being treated with baricitinib 4 mg.22,29 Downstream effects from the above study were label recommendations to reserve the medicines for patients who had an inadequate response or intolerance to 1 or more tumor necrosis factor blockers and to carefully consider risks vs benefits in patients, in particular current or prior smokers, those with other cardiovascular risk factors or a history of VTE, and those with a malignancy history other than already treated nonmelanoma skin cancer.28
There are consistent observations of laboratory abnormalities with JAK inhibitors, as discussed above, to include creatine phosphokinase elevation and cytopenias.30 Although existing data demonstrate that cytopenias are less of a concern in the AD population compared with the rheumatoid arthritis population, baseline and periodic laboratory monitoring are still recommended. In general, pretreatment laboratory assessment prior to initiating an oral JAK inhibitor should consist of a complete blood cell count with differential, complete metabolic panel, tuberculosis screening, chronic hepatitis panel, HIV screening, and a fasting lipid panel.2 The feasibility of obtaining these laboratory measurements in an operational setting or sea-going platform is limited, but many deployed locations and naval vessels possess the laboratory capability to perform a complete blood cell count and complete metabolic panel. Overall tolerability of oral JAK inhibitors in the treatment of AD appears favorable based on studies that were mostly 16 weeks in duration. Few recent longer-term studies have confirmed this side effect profile, but additional studies are needed.
Final Thoughts
Janus kinase inhibitors are a promising therapeutic class with multiple recently FDA-approved agents for the treatment of moderate to severe AD, with new agents on the horizon. Available efficacy data are promising and balanced by a favorable safety profile in clinical trials to date. The oral and topical bioavailability of JAK inhibitors makes them attractive alternatives to existing therapies. The rapidity of itch reduction and AD improvement demonstrated in multiple trials has the potential to decrease the length of limited-duty assignments, potentially returning treated service members to full-duty status more expeditiously. Other applications include use of these medications in scenarios where injectable medications are either unavailable or unsupported.
In the active-duty population, both the condition and/or the treatment may be duty limiting. Service members with AD who require more than topical treatment may require a medical evaluation board to determine if they are still fit to serve. The deployed environment routinely exacerbates AD and exposes service members to infections and environments where immunosuppression can create more risks than in the general population. Nonbiologic medications, which do not require refrigeration, are an exciting option for our patients with AD, including those actively serving or considering serving in the military. However, all factors in any patient’s life should be considered. Therefore, it is important for the nonmilitary dermatologist to work with local military physicians and the patient to determine the optimal treatment regimen to result in the best possible outcome.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
- Damsky W, Peterson D, Ramseier J, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J Allergy Clin Immunol. 2021;147:814-826.
- Gadina M, Le MT, Schwartz DM, et al. Janus kinases to jakinibs: from basic insights to clinical practice. Rheumatology (Oxford). 2019;58(suppl 1):i4-i6.
- Eichenfield LF, Tom WL, Berger TG, et al. Guidelines of care for the management of atopic dermatitis: section 2, management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71:116-132.
- Cartron AM, Nguyen TH, Roh YS, et al. Janus kinase inhibitors for atopic dermatitis: a promising treatment modality. Clin Exp Dermatol. 2021;46:820-824.
- Oetjen LK, Mack MR, Feng J, et al. Sensory neurons co-opt classical immune signaling pathways to mediate chronic itch. Cell. 2017;171:217-228.e13.
- U.S. FDA approves Pfizer’s CIBINQO® (abrocitinib) for adults with moderate-to-severe atopic dermatitis [press release]. January 14, 2022. Accessed November 18, 2022. https://www.pfizer.com/news/press-release/press-release-detail/us-fda-approves-pfizers-cibinqor-abrocitinib-adults
- Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396:255-266.
- Silverberg JI, Simpson EL, Thyssen JP, et al. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863-873.
- Bieber T, Simpson EL, Silverberg JI, et al. Abrocitinib versus placebo or dupilumab for atopic dermatitis. N Engl J Med. 2021;384:1101-1112.
- Gooderham MJ, Forman SB, Bissonnette R, et al. Efficacy and safety of oral Janus kinase 1 inhibitor abrocitinib for patients with atopic dermatitis: a phase 2 randomized clinical trial. JAMA Dermatol. 2019;155:1371-1379. Published correction appears in JAMA Dermatol. 2020;156:104.
- Yosipovitch G, Reaney M, Mastey V, et al. Peak Pruritus Numerical Rating Scale: psychometric validation and responder definition for assessing itch in moderate-to-severe atopic dermatitis. Br J Dermatol. 2019;181:761-769.
- Shi VY, Bhutani T, Fonacier L, et al. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND). J Am Acad Dermatol. 2022;87:351-358.
- Guttman-Yassky E, Teixeira HD, Simpson EL, et al. Once-daily upadacitinib versus placebo in adolescents and adults with moderate-to-severe atopic dermatitis (Measure Up 1 and Measure Up 2): results from two replicate double-blind, randomised controlled phase 3 trials. Lancet. 2021;397:2151-2168.
- Reich K, Teixeira HD, de Bruin-Weller M, et al. Safety and efficacy of upadacitinib in combination with topical corticosteroids in adolescents and adults with moderate-to-severe atopic dermatitis (AD Up): results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2021;397:2169-2181.
- Blauvelt A, Teixeira HD, Simpson EL, et al. Efficacy and safety of upadacitinib vs dupilumab in adults with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2021;157:1047-1055. Published correction appears in JAMA Dermatol. 2022;158:219.
- FDA approves Opzelura. Drugs.com. September 21, 2021. Accessed October 6, 2022. https://www.drugs.com/newdrugs/fda-approves-opzelura-ruxolitinib-cream-atopic-dermatitis-ad-5666.html
- Kim BS, Sun K, Papp K, et al. Effects of ruxolitinib cream on pruritus and quality of life in atopic dermatitis: results from a phase 2, randomized, doseranging, vehicle- and active-controlled study. J Am Acad Dermatol. 2020;82:1305-1313.
- Papp K, Szepietowski JC, Kircik L, et al. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863-872.
- Simpson EL, Lacour JP, Spelman L, et al. Baricitinib in patients with moderate-to-severe atopic dermatitis and inadequate response to topical corticosteroids: results from two randomized monotherapy phase III trials. Br J Dermatol. 2020;183:242-255.
- Silverberg JI, Simpson EL, Wollenberg A, et al. Long-term efficacy of baricitinib in adults with moderate to severe atopic dermatitis who were treatment responders or partial responders: an extension study of 2 randomized clinical trials. JAMA Dermatol. 2021;157:691-699.
- Lilly and Incyte announce top-line results from phase 3 study (BREEZE-AD4) of oral selective JAK inhibitor baricitinib in combination with topical corticosteroids in patients with moderate to severe atopic dermatitis not controlled with cyclosporine. January 27, 2020. Accessed November 18, 2022. https://investor.lilly.com/news-releases/news-release-details/lilly-and-incyte-announce-top-line-results-phase-3-study-breeze
- Reich K, Kabashima K, Peris K, et al. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333-1343.
- Wollenberg A, Nakahara T, Maari C, et al. Impact of baricitinib in combination with topical steroids on atopic dermatitis symptoms, quality of life and functioning in adult patients with moderate-to-severe atopic dermatitis from the BREEZE-AD7 phase 3 randomized trial. J Eur Acad Dermatol Venereol. 2021;35:1543-1552.
- Chiesa Fuxench ZC, Block JK, Boguniewicz M, et al. Atopic dermatitis in America study: a cross-sectional study examining the prevalence and disease burden of atopic dermatitis in the US adult population. J Invest Dermatol. 2019;139:583-590.
- Jeter J, Bowen C. Atopic dermatitis and implications for military service. Mil Med. 2019;184:E177-E182.
- Department of Defense. Medical standards for military service: appointment, enlistment, or induction. DoD Instruction 6130.03. Vol 1. May 6, 2022. Accessed November 18, 2022. https://www.esd.whs.mil/Portals/54/Documents/DD/issuances/dodi/613003_v1p.PDF?ver=9NsVi30gsHBBsRhMLcyVVQ%3d%3d
- Dermatitis. In: U.S. Navy Aeromedical Reference and Waiver Guide. Navy Medicine Operational Training Command and Naval Aerospace Medical Institute. August 11, 2021. Accessed November 18, 2022. https://www.med.navy.mil/Portals/62/Documents/NMFSC/NMOTC/NAMI/ARWG/Waiver%20Guide/ARWG%20COMPLETE_210811.pdf?ver=_pLPzFrtl8E2swFESnN4rA%3D%3D
- FDA requires warnings about increased risk of serious heart-related events, cancer, blood clots, and death for JAK inhibitors that treat certain chronic inflammatory conditions. FDA Drug Safety Podcast. U.S. Food and Drug Administration. Updated January 14, 2022. Accessed November 18, 2022. https://www.fda.gov/drugs/fda-drug-safety-podcasts/fda-requires-warnings-about-increased-risk-serious-heart-related-events-cancer-blood-clots-and-death
- Chang PH, Huang SF, Chang PS, et al. Safety considerations of systemic Janus kinase inhibitors in atopic dermatitis applications. J Dermatol. 2021;48:1631-1639.
- Wood H, Chandler A, Nezamololama N, et al. Safety of Janus kinase (JAK) inhibitors in the short-term treatment of atopic dermatitis. Int J Dermatol. 2022;61:746-754.
Practice Points
- Oral Janus kinase (JAK) inhibitors are novel therapies available for the treatment of atopic dermatitis (AD), with multiple recently approved agents within the class.
- Recommended laboratory monitoring during treatment with oral JAK inhibitors may limit the use of these medications in the active-duty military population or in those with special-duty assignments.
- The oral and topical bioavailability of these medications makes them a more feasible option for deploying service members or for those requiring flexible dosing.
- The rapid improvement in AD seen in multiple trials of oral JAK inhibitors suggests these agents could prove useful in management of acute AD flares, especially in military environments, where injectable agents are either unavailable or unsupported.