User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Firm Exophytic Tumor on the Shin
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
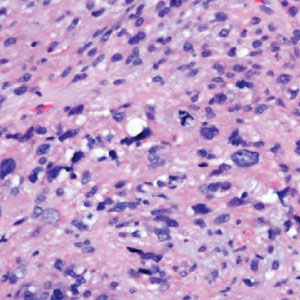
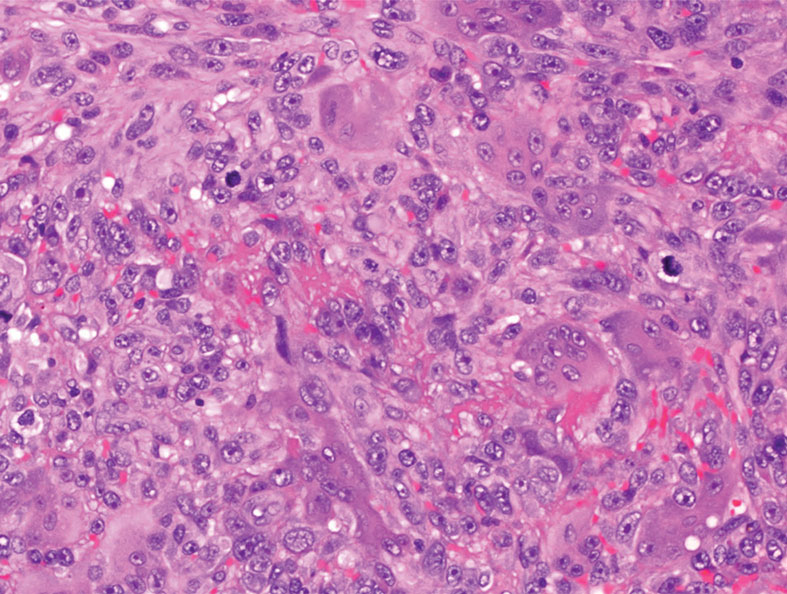
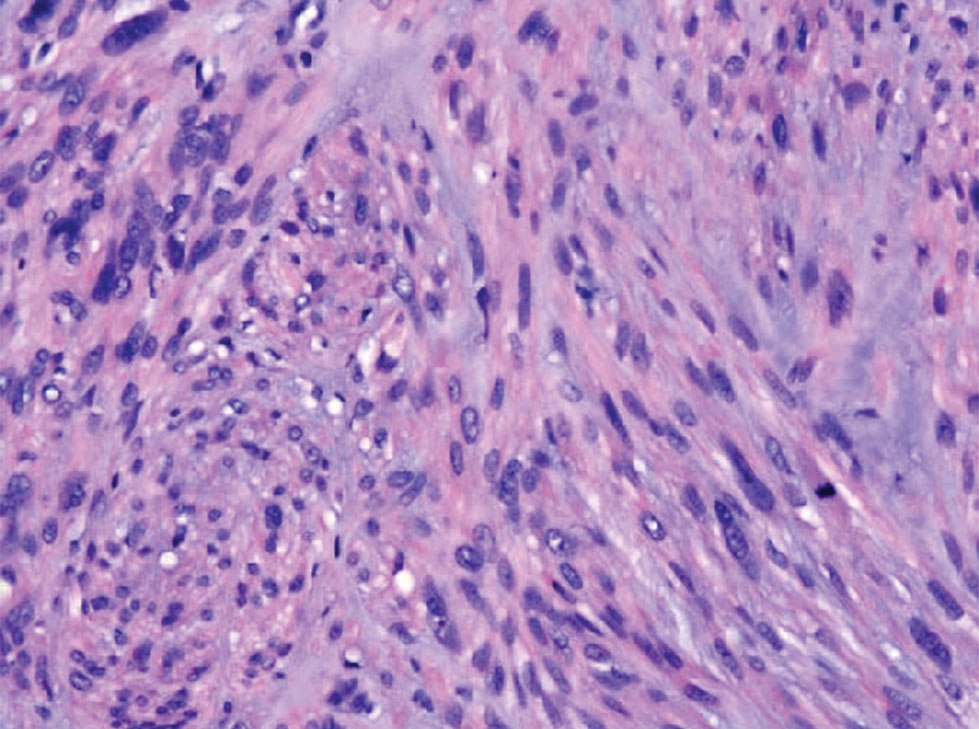
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.
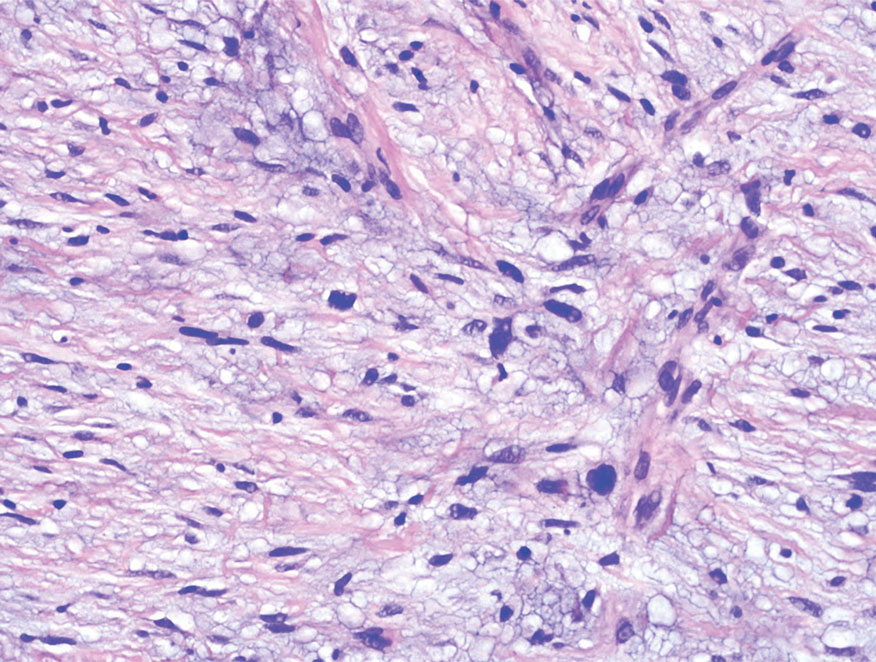
Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11
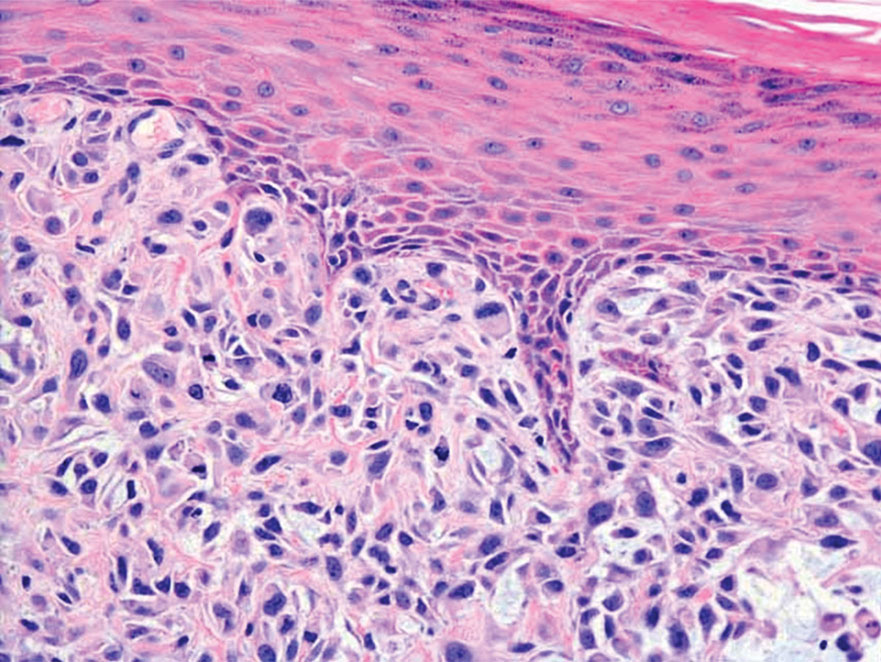
Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6
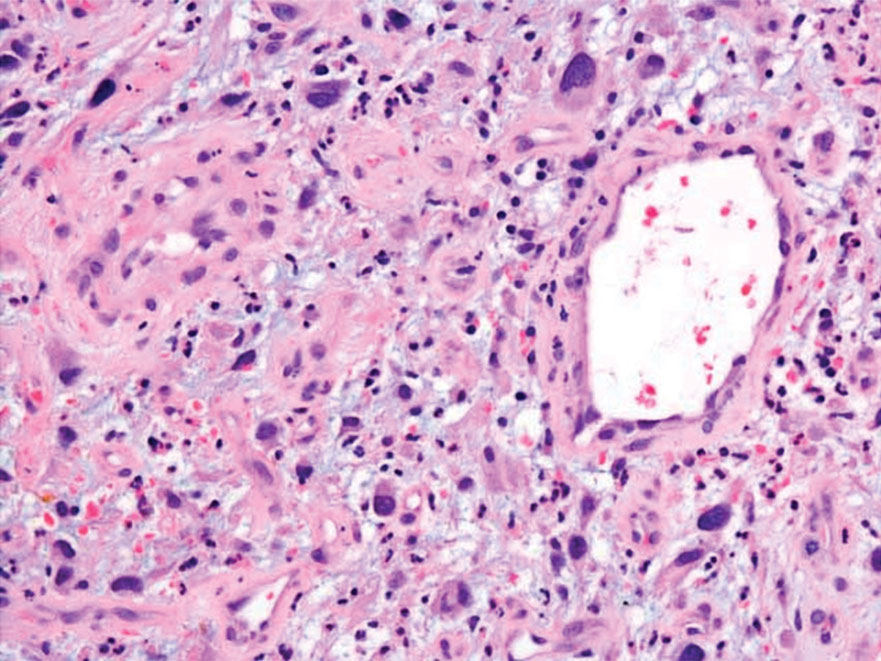
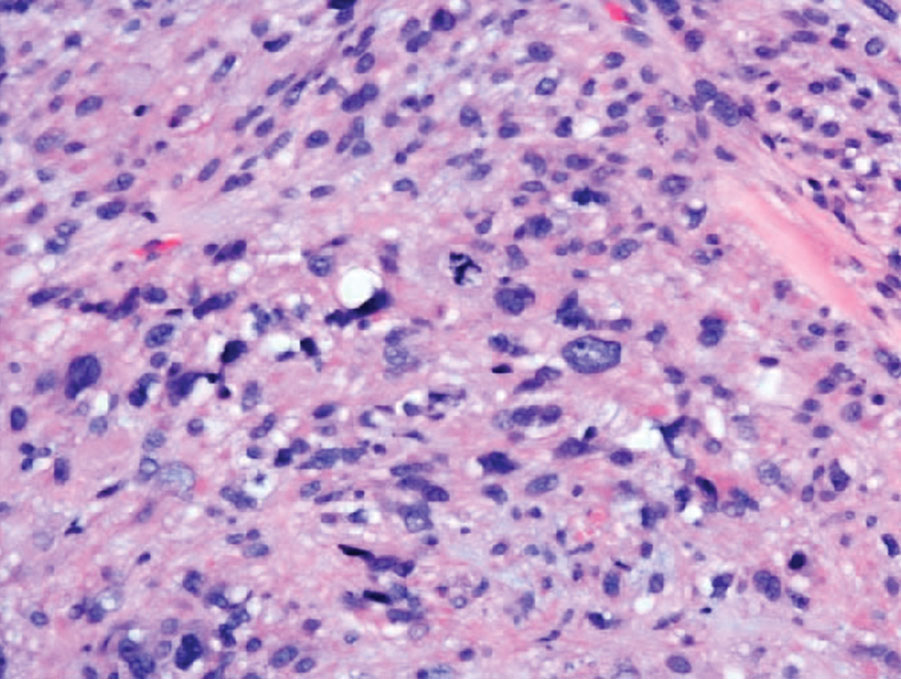
Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

The Diagnosis: Leiomyosarcoma
Cutaneous leiomyosarcomas are relatively rare neoplasms that favor the head, neck, and extremities of older adults.1 Dermal leiomyosarcomas originate from arrector pili and are locally aggressive, whereas subcutaneous leiomyosarcomas arise from vascular smooth muscle and metastasize in 30% to 60% of cases.2 Clinically, leiomyosarcomas present as solitary, firm, well-circumscribed nodules with possible ulceration and crusting.3 Histopathology of leiomyosarcoma shows fascicles of atypical spindle cells with blunt-ended nuclei and perinuclear glycogen vacuoles, variable atypia, and mitotic figures (quiz images). Definitive diagnosis is based on positive immunohistochemical staining for desmin and smooth muscle actin.4 Treatment entails complete removal via wide local excision or Mohs micrographic surgery.5
Atypical fibroxanthoma (AFX) is a malignant fibrohistiocytic neoplasm that arises in the dermis and preferentially affects the head and neck in older individuals.3 Atypical fibroxanthoma presents as a nonspecific, pinkred, sometimes ulcerated papule on sun-damaged skin that may clinically resemble a squamous cell carcinoma (SCC) or basal cell carcinoma.6 Histopathology shows pleomorphic spindle cells with hyperchromatic nuclei and abundant cytoplasm mixed with multinucleated giant cells and scattered mitotic figures (Figure 1). Immunohistochemistry is essential for distinguishing AFX from other spindle cell neoplasms. Atypical fibroxanthoma stains positively for vimentin, procollagen-1, CD10, and CD68 but is negative for S-100, human melanoma black 45, Melan-A, desmin, cytokeratin, p40, and p63.6 Treatment includes wide local excision or Mohs micrographic surgery.

Melanoma is an aggressive cancer with the propensity to metastasize. Both desmoplastic and spindle cell variants demonstrate atypical spindled melanocytes on histology, and desmoplasia is seen in the desmoplastic variant (Figure 2). In some cases, evaluation of the epidermis for melanoma in situ may aid in diagnosis.7 Clinical and prognostic features differ between the 2 variants. Desmoplastic melanomas usually present on the head and neck as scarlike nodules with a low rate of nodal involvement, while spindle cell melanomas can occur anywhere on the body, often are amelanotic, and are associated with widespread metastatic disease at the time of presentation.8 SOX10 (SRY-box transcription factor 10) and S-100 may be the only markers that are positive in desmoplastic melanoma.9,10 Treatment depends on the thickness of the lesion.11

Spindle cell SCC is a histologic variant of SCC characterized by spindled epithelial cells. Spindle cell SCC typically presents as an ulcerated or exophytic mass in sun-exposed areas or areas exposed to ionizing radiation, or in immunocompromised individuals. Histopathology shows spindled pleomorphic keratinocytes with elongated nuclei infiltrating the dermis and minimal keratinization (Figure 3).12 Immunohistochemistry is necessary to distinguish spindle cell SCC from other spindle cell tumors such as spindle cell melanoma, AFX, and leiomyosarcoma. Spindle cell SCC is positive for high-molecular-weight cytokeratin, p40, and p63. Mohs micrographic surgery provides the highest cure rate, and radiation therapy may be considered when clear surgical margins cannot be obtained.6

Undifferentiated pleomorphic sarcoma (UPS) (formerly known as malignant fibrous histiocytoma) describes tumors that resemble AFX but are more invasive. They commonly involve the soft tissue with a higher risk for both recurrence and metastasis than AFX.13 Histopathology shows marked cytologic pleomorphism, bizarre cellular forms, atypical mitoses, and ulceration (Figure 4).14 Diagnosis of UPS is by exclusion and is dependent on immunohistochemical studies. In contrast to AFX, UPS is more likely to be positive for LN-2 (CD74).6 Undifferentiated pleomorphic sarcoma has been treated with surgical excision in combination with chemical and radiation therapy, but due to limited data, optimal management is less clear compared to AFX.15 There is a substantial risk for local recurrence and metastasis, and the lungs are the most common sites of distant metastasis.13 In a study of 23 individuals with high-grade UPS, 5-year metastasis-free survival and local recurrence-free survival were 26% and 16%, respectively.10

- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
- Massi D, Franchi A, Alos L, et al. Primary cutaneous leiomyosarcoma: clinicopathological analysis of 36 cases. Histopathology. 2010;56: 251-262. doi:10.1111/j.1365-2559.2009.03471.x
- Ciurea ME, Georgescu CV, Radu CC, et al. Cutaneous leiomyosarcoma—case report [published online June 25, 2014]. J Med Life. 2014;7:270-273.
- Fleury LFF, Sanches JA. Primary cutaneous sarcomas. An Bras Dermatol. 2006;81:207-221. doi:10.1590/s0365-05962006000300002
- Murback NDN, de Castro BC, Takita LC, et al. Cutaneous leiomyosarcoma on the face. An Bras Dermatol. 2018;93:262-264. doi:10.1590 /abd1806-4841.20186715
- Winchester DS, Hocker TL, Brewer JD, et al. Leiomyosarcoma of the skin: clinical, histopathologic, and prognostic factors that influence outcomes. J Am Acad Dermatol. 2014;71:919-925. doi:10.1016/j .jaad.2014.07.020
- Hollmig ST, Sachdev R, Cockerell CJ, et al. Spindle cell neoplasms encountered in dermatologic surgery: a review. Dermatol Surg. 2012;38:825-850. doi:10.1111/j.1524-4725.2012.02296.x
- De Almeida LS, Requena L, Rütten A, et al. Desmoplastic malignant melanoma: a clinicopathologic analysis of 113 cases. Am J Dermatopathol. 2008;30:207-215. doi:10.1097/DAD.0B013E3181716E6B
- Weissinger SE, Keil P, Silvers DN, et al. A diagnostic algorithm to distinguish desmoplastic from spindle cell melanoma. Mod Pathol. 2014;27:524-534. doi:10.1038/modpathol.2013.162
- Ohsie SJ, Sarantopoulos GP, Cochran AJ, et al. Immunohistochemical characteristics of melanoma. J Cutan Pathol. 2008;35:433-444. doi:10.1111/j.1600-0560.2007.00891.x
- Delisca GO, Mesko NW, Alamanda VK, et al. MFH and highgrade undifferentiated pleomorphic sarcoma—what’s in a name? [published online September 12, 2014]. J Surg Oncol. 2015;111:173-177. doi:10.1002/jso.23787
- Baron PL, Nguyen CL. Malignant of melanoma. In: Holzheimer RG, Mannick JA, eds. Surgical Treatment: Evidence-Based and Problem- Oriented. Zuckschwerdt; 2001. https://www.ncbi.nlm.nih.gov/books /NBK6877
- Wernheden E, Trøstrup H, Pedersen Pilt A. Unusual presentation of cutaneous spindle cell squamous cell carcinoma: a case report. Case Rep Dermatol. 2020;12:70-75. doi:10.1159/000507358
- Ramsey JK, Chen JL, Schoenfield L, et al. Undifferentiated pleomorphic sarcoma metastatic to the orbit. Ophthal Plast Reconstr Surg. 2018;34:E193-E195. doi:10.1097/IOP.0000000000001240
- Winchester D, Lehman J, Tello T, et al. Undifferentiated pleomorphic sarcoma: factors predictive of adverse outcomes. J Am Acad Dermatol. 2018;79:853-859. doi:10.1016/j.jaad.2018.05.022
- Soleymani T, Tyler Hollmig S. Conception and management of a poorly understood spectrum of dermatologic neoplasms: atypical fibroxanthoma, pleomorphic dermal sarcoma, and undifferentiated pleomorphic sarcoma. Curr Treat Options Oncol. 2017;18:50. doi:10.1007 /s11864-017-0489-6
A 62-year-old man presented with a firm, exophytic, 2.8×1.5-cm tumor on the left shin of 6 to 7 years’ duration. An excisional biopsy was obtained for histopathologic evaluation.
Unique Treatment for Alopecia Areata Combining Epinephrine With an Intralesional Steroid
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
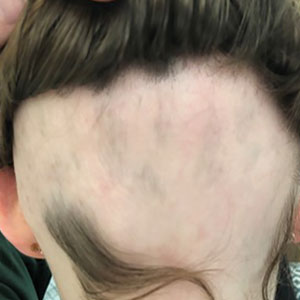
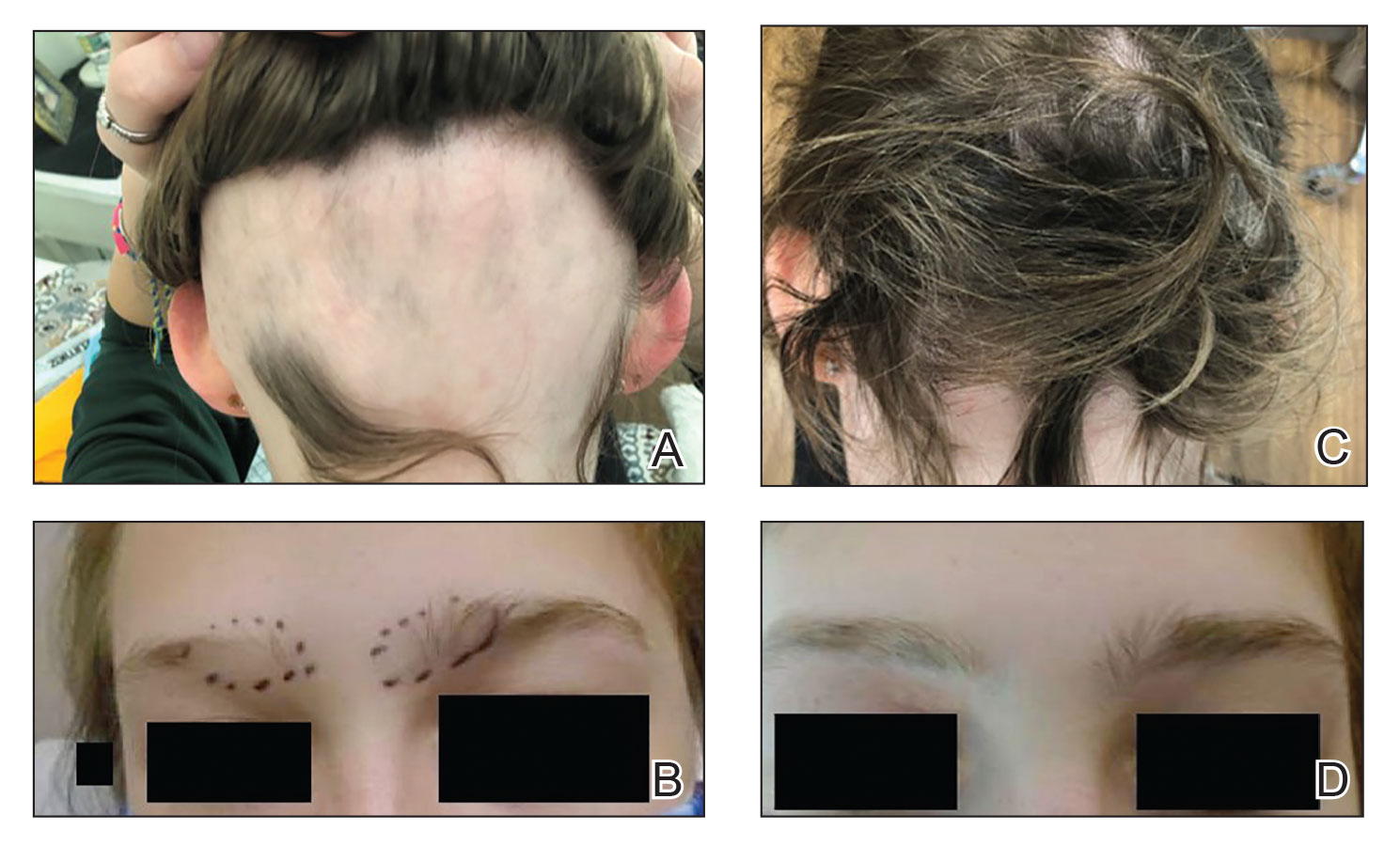
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.
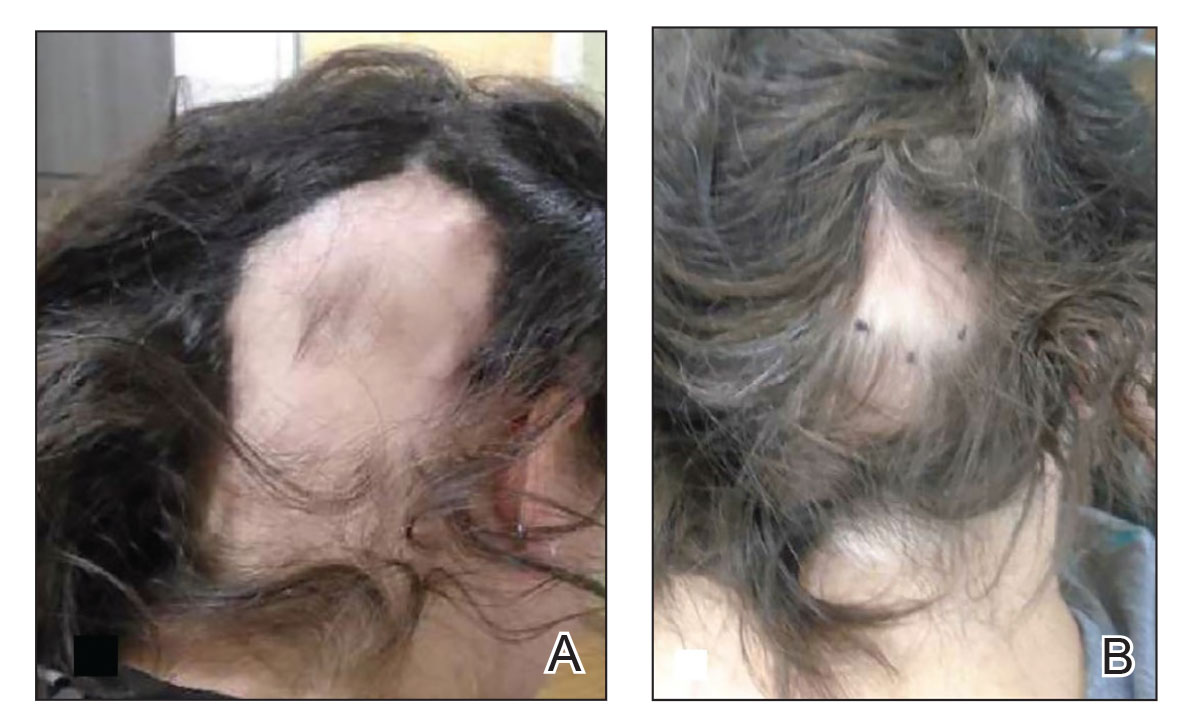
Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.
Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
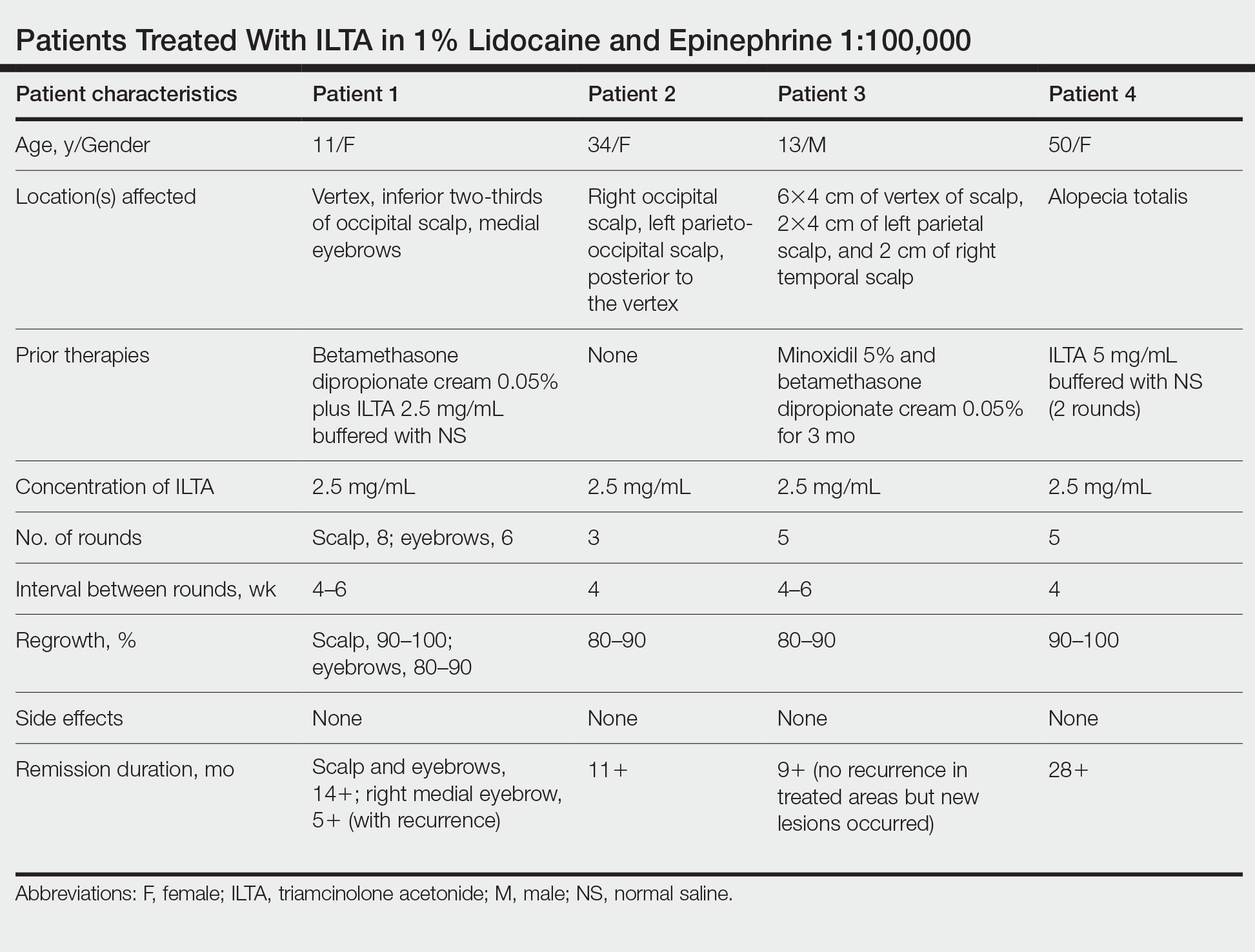
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.
Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
Alopecia areata (AA) is an autoimmune disorder characterized by transient hair loss with preservation of the hair follicle (HF). The lifetime incidence risk of AA is approximately 2%,1 with a mean age of onset of 25 to 36 years and with no clinically relevant significant differences between sex or ethnicity.2 Most commonly, it presents as round, well-demarcated patches of alopecia on the scalp and spontaneously resolves in nearly 30% of patients. However, severe disease is associated with younger age of presentation and can progress to a total loss of scalp or body hair—referred to as alopecia totalis and alopecia universalis, respectively—thus severely impacting quality of life.3,4
First-line treatment options for AA include potent topical steroids5,6 and intralesional (IL) steroids, most commonly IL triamcinolone acetonide (ILTA). Intralesional steroids have been found to be more effective than topicals in stimulating hair growth at the injection site.7,8 A recent systemic therapy—the Janus kinase inhibitor baricitinib—was approved by the US Food and Drug Administration for AA.9 Other systemic therapies such as oral corticosteroids have been studied in small trials with promising results.10 However, the risks of systemic therapies may outweigh the benefits.9,10
Another less common topical therapy is contact immunotherapy, which involves topical application of an unlicensed non–pharmaceutical-grade agent to areas affected with AA. It is reported to have a wide range of response rates (29%–87%).11
We report 2 cases of extensive AA that were treated with a novel combination regimen— 2.5 mg/mL of ILTA diluted with lidocaine 1% and epinephrine 1:100,000 in place of normal saline (NS)— which is a modification to an already widely used first-line treatment.
Case Reports
Patient 1—An 11-year-old girl presented with nonscarring alopecia of the vertex and occipital scalp. Three years prior she was treated with topical and IL corticosteroids by a different provider. Physical examination revealed almost complete alopecia involving the bottom two-thirds of the occipital scalp as well as the medial eyebrows (Figures 1A and 1B). Over the span of 1 year she was treated with betamethasone dipropionate cream 0.05% and several rounds of ILTA 2.5 mg/mL buffered with NS, with minimal improvement. A year after the initial presentation, the decision was made to initiate monthly injections of ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000. Some hair regrowth of the occipital scalp was noted by 3 months, with near-complete regrowth of the scalp hair and eyebrows by 7 months and 5 months, respectively (Figures 1C and 1D). During this period, the patient continued to develop new areas of alopecia of the scalp and eyebrows, which also were injected with this combination. In total, the patient received 8 rounds of IL injections 4 to 6 weeks apart in the scalp and 6 rounds in the eyebrows. The treated areas showed resolution over a follow-up period of 14 months, though there was recurrence at the right medial eyebrow at 5 months. No localized skin atrophy or other adverse effects were noted.

Patient 2—A 34-year-old woman who was otherwise healthy presented with previously untreated AA involving the scalp of 2 months’ duration. Physical examination revealed the following areas of nonscarring alopecia: a 10×10-cm area of the right occipital scalp with some regrowth; a 10×14-cm area of the left parieto-occipital scalp; and a 1-cm area posterior to the vertex (Figure 2A). Given the extensive involvement, the decision was made to initiate ILTA 2.5 mg/mL buffered with 1% lidocaine and epinephrine 1:100,000 once monthly. Appreciable hair regrowth was noted within 1 month, mostly on the parietal scalp. Substantial improvement was noted after 3 months in all affected areas of the hair-bearing scalp, with near-complete regrowth on the left occipital scalp and greater than 50% regrowth on the right occipital scalp (Figure 2B). No adverse effects were noted. She currently has no alopecia.

Comment
Alopecia Pathogenesis—The most widely adopted theory of AA etiology implicates an aberrant immune response. The HF, which is a dynamic “mini-organ” with its own immune and hormonal microenvironment, is considered an “immune-privileged site”—meaning it is less exposed to immune responses than most other body areas. It is hypothesized that AA results from a breakdown in this immune privilege, with the subsequent attack on the peribulbar part of the follicle by CD8+ T lymphocytes. This lymphocytic infiltrate induces apoptosis in the HF keratinocytes, resulting in inhibition of hair shaft production.12 Other theories suggest a link to the sympathetic-adrenal-medullary system and hypothalamic-pituitary-adrenal axis.13
Therapies for Alopecia—Topical and IL corticosteroids are the first-line therapies for localized AA in patients with less than 50% scalp involvement. Triamcinolone acetonide generally is the IL steroid of choice because it is widely available and less atrophogenic than other steroids. Unlike topicals, ILTA bypasses the epidermis when injected, achieving direct access to the HF.14
High-quality controlled studies regarding the use of ILTA in AA are scarce. A meta-analysis concluded that 5 mg/mL and 10 mg/mL of ILTA diluted in NS were equally effective (80.9% [P<.05] vs 76.4% [P<.005], respectively). Concentrations of less than 5 mg/mL of ILTA resulted in lower rates of hair regrowth (62.3%; P=.04).15 The role of diluents other than NS has not been studied.
Benefits of Epinephrine in ILTA Therapy—The role of epinephrine 1:100,000 is to decrease the rate of clearance of triamcinolone acetonide from the HF, allowing for a better therapeutic effect. Laser Doppler blood flowmeter studies have shown that epinephrine 1:100,000 injected in the scalp causes vasoconstriction, thereby decreasing the blood flow rate of clearance of other substances in the same solution.16 Also, a more gradual systemic absorption is achieved, decreasing systemic side effects such as osteoporosis.17
Another potential benefit of epinephrine has been suggested in animal studies that demonstrate the important role of the sympathetic nervous system in HF growth. In a mouse study, chemical sympathectomy led to diminished norepinephrine levels in the skin, accompanied by a decreased keratinocyte proliferation and hair growth. Conversely, norepinephrine was found to promote HF growth in an organotypic skin culture model.18 Topically applied isoproterenol, a panadrenergic receptor agonist, accelerated HF growth in an organotypic skin culture. It also has been shown that external light and temperature changes stimulate hair growth via the sympathetic nervous system, promoting anagen HF growth in cultured skin explants, further linking HF activity with sympathetic nerve activity.19
In our experience, cases of AA that at first failed ILTA 5 mg/mL in NS have been successfully treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000. One such case was alopecia totalis, though we do not have high-quality photographs to present for this report. The 2 cases presented here are the ones with the best photographs to demonstrate our outcomes. Both were treated with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000 administered using a 0.5-in long 30-gauge needle, with 0.05 to 0.1 mL per injection approximately 0.51-cm apart. The treatment intervals were 4 weeks, with a maximal dose of 20 mg per session. In addition to the 2 cases reported here, the Table includes 2 other patients in our practice who were successfully treated with this novel regimen.

Prior to adopting this combination regimen, our standard therapy for AA was 5 mg/mL ILTA buffered with NS. Instead of NS, we now use the widely available 1% lidocaine with epinephrine 1:100,000 and dilute the ILTA to 2.5 mg/mL. We postulate that epinephrine 1:100,000 enhances therapeutic efficacy via local vasoconstriction, thus keeping the ILTA in situ longer than NS. This effect allows for a lower concentration of ILTA (2.5 mg/mL) to be effective. Furthermore, epinephrine 1:100,000 may have an independent effect, as suggested in mouse studies.18
Our first case demonstrated the ophiasis subtype of AA (symmetric bandlike hair loss), which has a poorer prognosis and is less responsive to therapy.20 In this patient, prior treatment with topical corticosteroids and ILTA in NS failed to induce a response. After a series of injections with 2.5 mg/mL ILTA in 1% lidocaine and epinephrine 1:100,000, she entered remission. Our second case is one of alopecia subtotalis, which responded quickly, and the patient entered remission after just 3 months of treatment. These 2 cases are illustrative of the results that we regularly get and have come to expect with this treatment.
Conclusion
Our novel modified regimen of 2.5 mg/mL ILTA diluted with 1% lidocaine and epinephrine 1:100,000 has yielded a series of excellent outcomes in many of our most challenging AA cases without any untoward effects. Two cases are presented here. Higher-powered studies are needed to validate this new yet simple approach. A split-scalp or split-lesion study comparing ILTA with and without epinephrine 1:100,000 would be warranted for further investigation.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
- Mirzoyev SA, Schrum AG, Davis MDP, et al. Lifetime incidence risk of alopecia areata estimated at 2.1 percent by Rochester Epidemiology Project, 1990-2009. J Invest Dermatol. 2014;134:1141-1142.
- Villasante Fricke AC, Miteva M. Epidemiology and burden of alopecia areata: a systematic review. Clin Cosmet Investig Dermatol. 2015;8:397-403.
- Tosti A, Bellavista S, Iorizzo M. Alopecia areata: a long term follow-up study of 191 patients. J Am Acad Dermatol. 2006;55:438-441.
- Walker SA, Rothman S. A statistical study and consideration of endocrine influences. J Invest Dermatol. 1950;14:403-413.
- Charuwichitratana S, Wattanakrai P, Tanrattanakorn S. Randomized double-blind placebo-controlled trial in the treatment of alopecia areata with 0.25% desoximetasone cream. Arch Dermatol. 2000;136:1276-1277.
- Tosti A, Iorizzo M, Botta GL, et al. Efficacy and safety of a new clobetasol propionate 0.05% foam in alopecia areata: a randomized, double-blind placebo-controlled trial. J Eur Acad Dermatol Venereol. 2006;20:1243-1247.
- Kubeyinje EP. Intralesional triamcinolone acetonide in alopecia areata amongst 62 Saudi Arabs. East Afr Med J. 1994;71:674-675.
- Porter D, Burton JL. A comparison of intra-lesional triamcinolonehexacetonide and triamcinolone acetonide in alopecia areata. Br J Dermatol. 1971;85:272-273.
- King B, Ohyama M, Kwon O, et al. Two phase 3 trials of baricitinib for alopecia areata. N Engl J Med. 2022;386:1687-1699. doi:10.1056/NEJMoa2110343
- Lai VWY, Chen G, Gin D, et al. Systemic treatments for alopeciaareata: a systematic review. Australas J Dermatol. 2019;60:E1-E13. doi:10.1111/ajd.12913
- Rokhsar CK, Shupack JL, Vafai JJ, et al. Efficacy of topical sensitizers in the treatment of alopecia areata. J Am Acad Dermatol. 1998;39:751-761.
- Dainichi T, Kabashima K. Alopecia areata: what’s new in epidemiology, pathogenesis, diagnosis, and therapeutic options? J Dermatol Sci. 2017;86:3-12.
- Ito T. Recent advances in the pathogenesis of autoimmune hair loss disease alopecia areata. Clin Dev Immunol. 2013;2013:348546.
- Ramos PM, Anzai A, Duque-Estrada B, et al. Consensus on the treatment of alopecia areata—Brazilian Society of Dermatology. An Bras Dermatol. 2020;95(suppl 1):39-52.
- Yee BE, Tong Y, Goldenberg A, et al. Efficacy of different concentrations of intralesional triamcinolone acetonide for alopecia areata: a systematic review and meta-analysis. J Am Acad Dermatol. 2020;82:1018-1021.
- Na YC, Park R, Jeong HS, et al. Epinephrine vasoconstriction effect time in the scalp differs according to injection site and concentration. Dermatol Surg. 2016;42:1054-1060.
- Samrao A, Fu JM, Harris ST, et al. Bone mineral density in patients with alopecia areata treated with long-term intralesional corticosteroids. J Drugs Dermatol. 2013;12:E36-E40.
- Kong Y, Liu Y, Pan L, et al. Norepinephrine regulates keratinocyte proliferation to promote the growth of hair follicles. Cells Tissues Organs. 2015-2016;201:423-435.
- Fan SM, Chang YT, Chen CL, et al. External light activates hair follicle stem cells through eyes via an ipRGC-SCN-sympathetic neural pathway. Proc Natl Acad Sci U S A. 2018;115:E6880-E6889. Erratum appears in Proc Natl Acad Sci U S A. 2018;115:E12121.
- Spano F, Donovan JC. Alopecia areata: part 1: pathogenesis, diagnosis, and prognosis. Can Fam Physician. 2015;61:751-755.
Practice Points
- Patients with alopecia areata that is refractory to first-line treatments may benefit from intralesional triamcinolone acetonide (ILTA) diluted to 2.5 mg/mL in 1% lidocaine and epinephrine 1:100,000 in place of normal saline.
- Local vasoconstriction due to epinephrine may potentiate ILTA effects and play an independent role.
Differences in Underrepresented in Medicine Applicant Backgrounds and Outcomes in the 2020-2021 Dermatology Residency Match
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
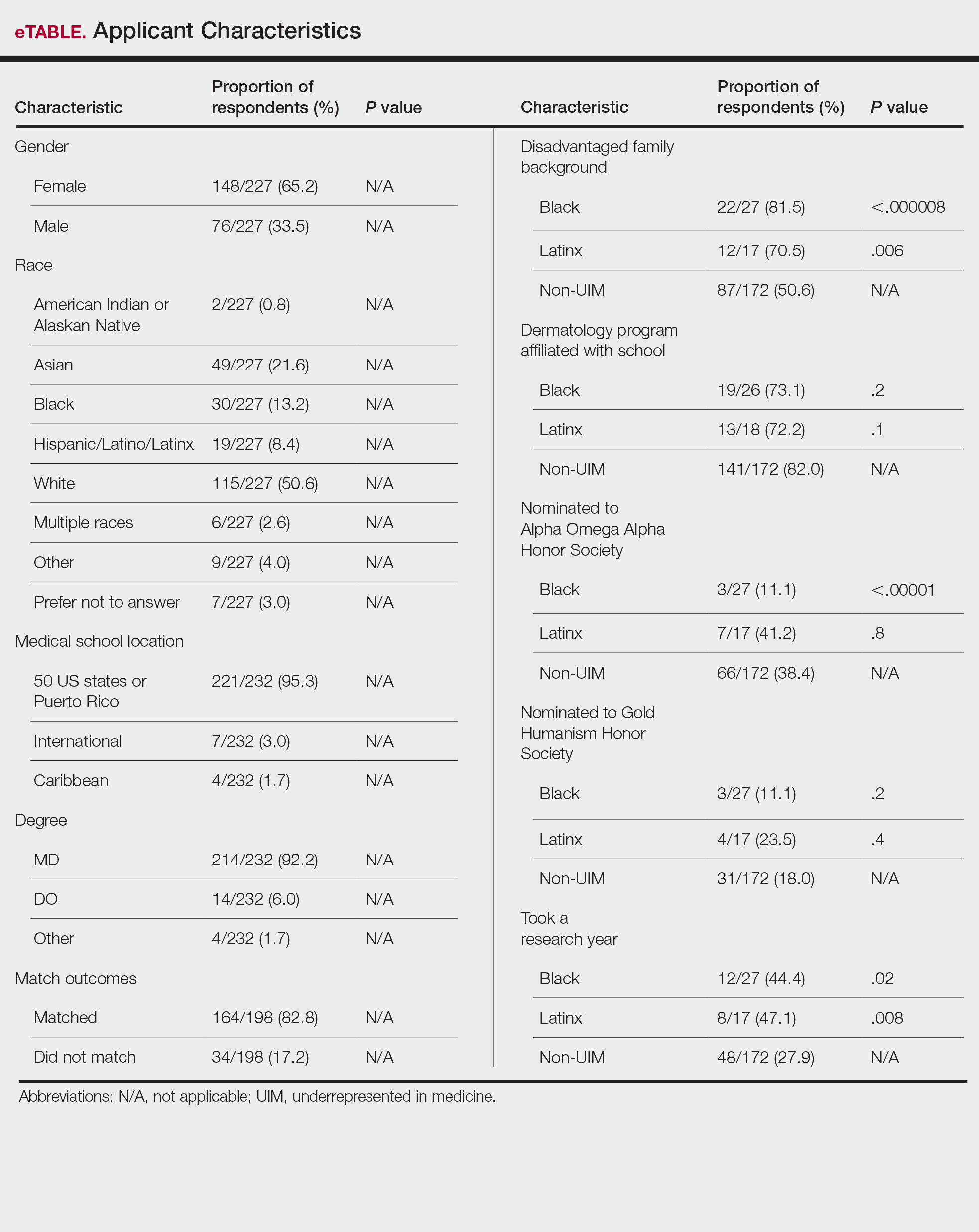
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.
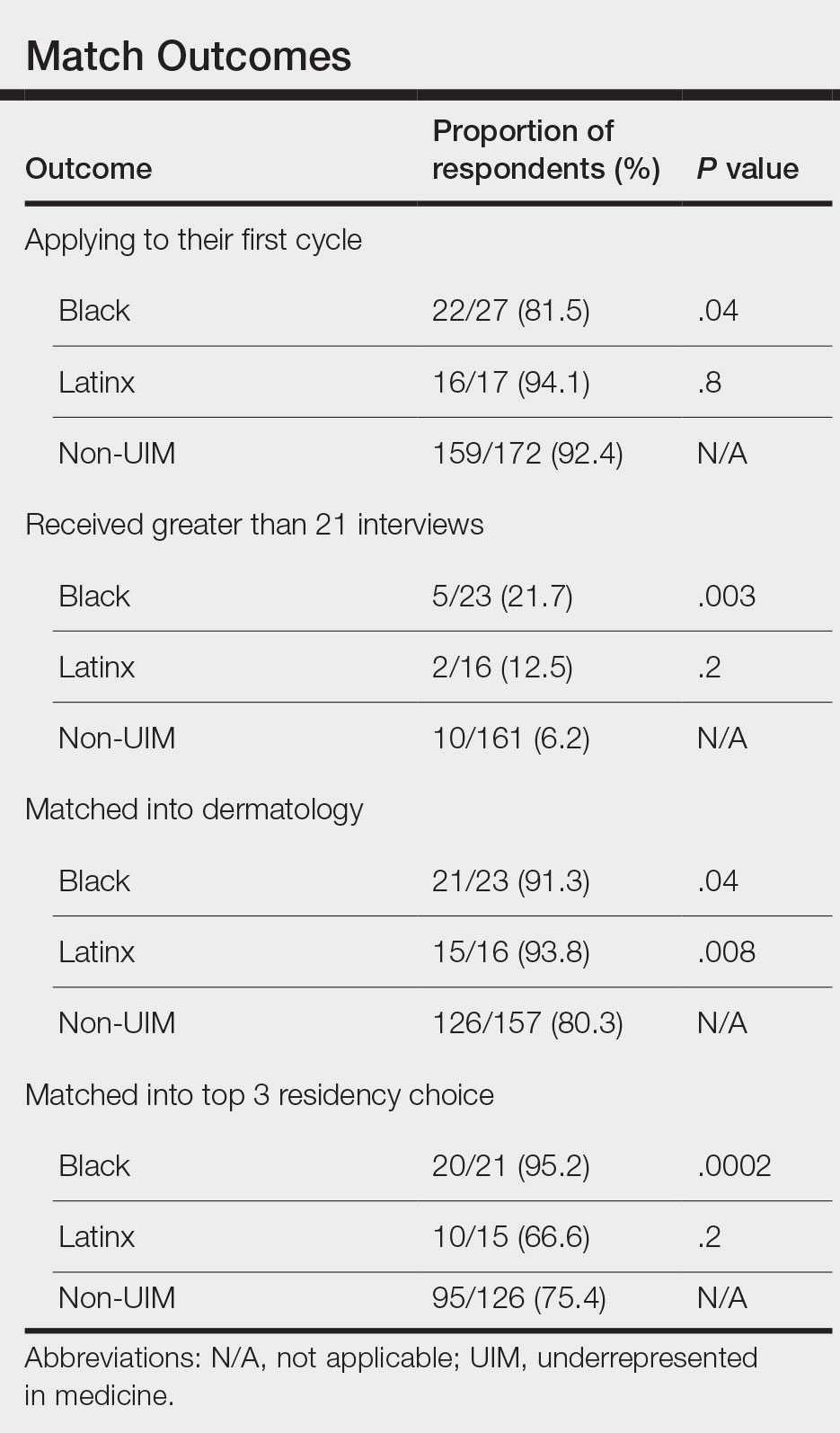
Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.
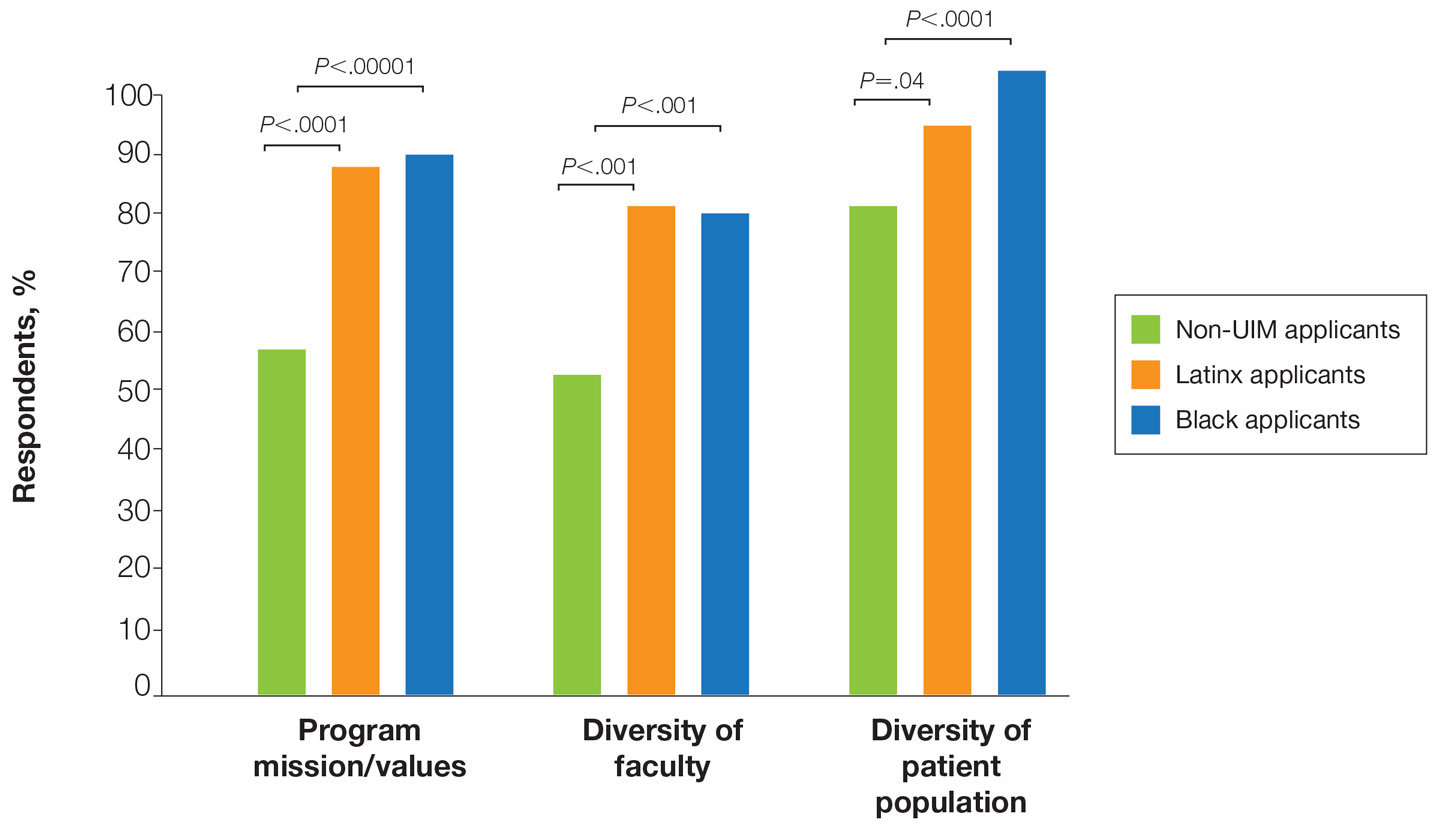
There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).
In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
Dermatology is one of the least diverse medical specialties with only 3% of dermatologists being Black and 4% Latinx.1 Leading dermatology organizations have called for specialty-wide efforts to improve diversity, with a particular focus on the resident selection process.2,3 Medical students who are underrepresented in medicine (UIM)(ie, those who identify as Black, Latinx, Native American, or Pacific Islander) face many potential barriers in applying to dermatology programs, including financial limitations, lack of support and mentorship, and less exposure to the specialty.1,2,4 The COVID-19 pandemic introduced additional challenges in the residency application process with limitations on clinical, research, and volunteer experiences; decreased opportunities for in-person mentorship and away rotations; and a shift to virtual recruitment. Although there has been increased emphasis on recruiting diverse candidates to dermatology, the COVID-19 pandemic may have exacerbated existing barriers for UIM applicants.
We surveyed dermatology residency program directors (PDs) and applicants to evaluate how UIM students approach and fare in the dermatology residency application process as well as the effects of COVID-19 on the most recent application cycle. Herein, we report the results of our surveys with a focus on racial differences in the application process.
Methods
We administered 2 anonymous online surveys—one to 115 PDs through the Association of Professors of Dermatology (APD) email listserve and another to applicants who participated in the 2020-2021 dermatology residency application cycle through the Dermatology Interest Group Association (DIGA) listserve. The surveys were distributed from March 29 through May 23, 2021. There was no way to determine the number of dermatology applicants on the DIGA listserve. The surveys were reviewed and approved by the University of Southern California (Los Angeles, California) institutional review board (approval #UP-21-00118).
Participants were not required to answer every survey question; response rates varied by question. Survey responses with less than 10% completion were excluded from analysis. Data were collected, analyzed, and stored using Qualtrics, a secure online survey platform. The test of equal or given proportions in R studio was used to determine statistically significant differences between variables (P<.05 indicated statistical significance).
Results
The PD survey received 79 complete responses (83.5% complete responses, 73.8% response rate) and the applicant survey received 232 complete responses (83.6% complete responses).
Applicant Characteristics—Applicant characteristics are provided in the eTable; 13.2% and 8.4% of participants were Black and Latinx (including those who identify as Hispanic/Latino), respectively. Only 0.8% of respondents identified as American Indian or Alaskan Native and were excluded from the analysis due to the limited sample size. Those who identified as White, Asian, multiple races, or other and those who preferred not to answer were considered non-UIM participants.
Differences in family background were observed in our cohort, with UIM candidates more likely to have experienced disadvantages, defined as being the first in their family to attend college/graduate school, growing up in a rural area, being a first-generation immigrant, or qualifying as low income. Underrepresented in medicine applicants also were less likely to have a dermatology program at their medical school (both Black and Latinx) and to have been elected to honor societies such as Alpha Omega Alpha and the Gold Humanism Honor Society (Black only).
Underrepresented in medicine applicants were more likely to complete a research gap year (eTable). Most applicants who took research years did so to improve their chances of matching, regardless of their race/ethnicity. For those who did not complete a research year, Black applicants (46.7%) were more likely to base that decision on financial limitations compared to non-UIMs (18.6%, P<.0001). Interestingly, in the PD survey, only 4.5% of respondents considered completion of a research year extremely or very important when compiling rank lists.

Application Process and Match Outcomes—The Table highlights differences in how UIM applicants approached the application process. Black but not Latinx applicants were less likely to be first-time applicants to dermatology compared to non-UIM applicants. Black applicants (8.3%) were significantly less likely to apply to more than 100 programs compared to non-UIM applicants (29.5%, P=.0002). Underrepresented in medicine applicants received greater numbers of interviews despite applying to fewer programs overall.

There also were differences in how UIM candidates approached their rank lists, with Black and Latinx applicants prioritizing diversity of patient populations and program faculty as well as program missions and values (Figure).

In our cohort, UIM candidates were more likely than non-UIM to match, and Black applicants were most likely to match at one of their top 3 choices (Table). In the PD survey, 77.6% of PDs considered contribution to diversity an important factor when compiling their rank lists.
Comment
Applicant Background—Dermatology is a competitive specialty with a challenging application process2 that has been further complicated by the COVID-19 pandemic. Our study elucidated how the 2020-2021 application cycle affected UIM dermatology applicants. Prior studies have found that UIM medical students were more likely to come from lower socioeconomic backgrounds; financial constraints pose a major barrier for UIM and low-income students interested in dermatology.4-6 We found this to be true in our cohort, as Black and Latinx applicants were significantly more likely to come from disadvantaged backgrounds (P<.000008 and P=.006, respectively). Additionally, we found that Black applicants were more likely than any other group to indicate financial concerns as their primary reason for not taking a research gap year.
Although most applicants who completed a research year did so to increase their chances of matching, a higher percentage of UIMs took research years compared to non-UIM applicants. This finding could indicate greater anxiety about matching among UIM applicants vs their non-UIM counterparts. Black students have faced discrimination in clinical grading,7 have perceived racial discrimination in residency interviews,8,9 and have shown to be less likely to be elected to medical school honor societies.10 We found that UIM applicants were more likely to pursue a research year compared to other applicants,11 possibly because they felt additional pressure to enhance their applications or because UIM candidates were less likely to have a home dermatology program. Expansion of mentorship programs, visiting student electives, and grants for UIMs may alleviate the need for these candidates to complete a research year and reduce disparities in the application process.
Factors Influencing Rank Lists for Applicants—In our cohort, UIMs were significantly more likely to rank diversity of patients (P<.0001 for Black applicants and P=.04 for Latinx applicants) and faculty (P<.001 for Black applicants and P<.001 for Latinx applicants) as important factors in choosing a residency program. Historically, dermatology has been disproportionately White in its physician workforce and patient population.1,12 Students with lower incomes or who identify as minorities cite the lack of diversity in dermatology as a considerable barrier to pursuing a career in the specialty.4,5 Service learning, pipeline programs aimed at early exposure to dermatology, and increased access to care for diverse patient populations are important measures to improve diversity in the dermatology workforce.13-15 Residency programs should consider how to incorporate these aspects into didactic and clinical curricula to better recruit diverse candidates to the field.
Equity in the Application Process—We found that Black applicants were more likely than non-UIM applicants to be reapplicants to dermatology; however, Black applicants in our study also were more likely to receive more interview invites, match into dermatology, and match into one of their top 3 programs. These findings are interesting, particularly given concerns about equity in the application process. It is possible that Black applicants who overcome barriers to applying to dermatology ultimately are more successful applicants. Recently, there has been an increased focus in the field on diversifying dermatology, which was further intensified last year.2,3 Indicative of this shift, our PD survey showed that most programs reported that applicants’ contributions to diversity were important factors in the application process. Additionally, an emphasis by PDs on a holistic review of applications coupled with direct advocacy for increased representation may have contributed to the increased match rates for UIM applicants reported in our survey.
Latinx Applicants—Our study showed differences in how Latinx candidates fared in the application process; although Latinx applicants were more likely than their non-Latinx counterparts to match into dermatology, they were less likely than non-Latinx applicants to match into one of their top 3 programs. Given that Latinx encompasses ethnicity, not race, there may be a difference in how intentional focus on and advocacy for increasing diversity in dermatology affected different UIM applicant groups. Both race and ethnicity are social constructs rather than scientific categorizations; thus, it is difficult in survey studies such as ours to capture the intersectionality present across and between groups. Lastly, it is possible that the respondents to our applicant survey are not representative of the full cohort of UIM applicants.
Study Limitations—A major limitation of our study was that we did not have a method of reaching all dermatology applicants. Although our study shows promising results suggestive of increased diversity in the last application cycle, release of the National Resident Matching Program results from 2020-2021 with racially stratified data will be imperative to assess equity in the match process for all specialties and to confirm the generalizability of our results.
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
- Pandya AG, Alexis AF, Berger TG, et al. Increasing racial and ethnic diversity in dermatology: a call to action. J Am Acad Dermatol. 2016;74:584-587. doi:10.1016/j.jaad.2015.10.044
- Chen A, Shinkai K. Rethinking how we select dermatology applicants—turning the tide. JAMA Dermatol. 2017;153:259-260. doi:10.1001/jamadermatol.2016.4683
- American Academy of Dermatology Association. Diversity In Dermatology: Diversity Committee Approved Plan 2021-2023. Published January 26, 2021. Accessed July 26, 2022. https://assets.ctfassets.net/1ny4yoiyrqia/xQgnCE6ji5skUlcZQHS2b/65f0a9072811e11afcc33d043e02cd4d/DEI_Plan.pdf
- Vasquez R, Jeong H, Florez-Pollack S, et al. What are the barriers faced by under-represented minorities applying to dermatology? a qualitative cross-sectional study of applicants applying to a large dermatologyresidency program. J Am Acad Dermatol. 2020;83:1770-1773. doi:10.1016/j.jaad.2020.03.067
- Jones VA, Clark KA, Patel PM, et al. Considerations for dermatology residency applicants underrepresented in medicine amid the COVID-19 pandemic. J Am Acad Dermatol. 2020;83:E247.doi:10.1016/j.jaad.2020.05.141
- Soliman YS, Rzepecki AK, Guzman AK, et al. Understanding perceived barriers of minority medical students pursuing a career in dermatology. JAMA Dermatol. 2019;155:252-254. doi:10.1001/jamadermatol.2018.4813
- Grbic D, Jones DJ, Case ST. The role of socioeconomic status in medical school admissions: validation of a socioeconomic indicator for use in medical school admissions. Acad Med. 2015;90:953-960. doi:10.1097/ACM.0000000000000653
- Low D, Pollack SW, Liao ZC, et al. Racial/ethnic disparities in clinical grading in medical school. Teach Learn Med. 2019;31:487-496. doi:10.1080/10401334.2019.1597724
- Ellis J, Otugo O, Landry A, et al. Interviewed while Black [published online November 11, 2020]. N Engl J Med. 2020;383:2401-2404. doi:10.1056/NEJMp2023999
- Anthony Douglas II, Hendrix J. Black medical student considerations in the era of virtual interviews. Ann Surg. 2021;274:232-233. doi:10.1097/SLA.0000000000004946
- Boatright D, Ross D, O’Connor P, et al. Racial disparities in medical student membership in the Alpha Omega Alpha honor society. JAMA Intern Med. 2017;177:659. doi:10.1001/jamainternmed.2016.9623
- Runge M, Renati S, Helfrich Y. 16146 dermatology residency applicants: how many pursue a dedicated research year or dual-degree, and do their stats differ [published online December 1, 2020]? J Am Acad Dermatol. doi:10.1016/j.jaad.2020.06.304
- Stern RS. Dermatologists and office-based care of dermatologic disease in the 21st century. J Investig Dermatol Symp Proc. 2004;9:126-130. doi:10.1046/j.1087-0024.2003.09108.x
- Oyesanya T, Grossberg AL, Okoye GA. Increasing minority representation in the dermatology department: the Johns Hopkins experience. JAMA Dermatol. 2018;154:1133-1134. doi:10.1001/jamadermatol.2018.2018
- Humphrey VS, James AJ. The importance of service learning in dermatology residency: an actionable approach to improve resident education and skin health equity. Cutis. 2021;107:120-122. doi:10.12788/cutis.0199
Practice Points
- Underrepresented in medicine (UIM) dermatology residency applicants (Black and Latinx) are more likely to come from disadvantaged backgrounds and to have financial concerns about the residency application process.
- When choosing a dermatology residency program, diversity of patients and faculty are more important to UIM dermatology residency applicants than to their non-UIM counterparts.
- Increased awareness of and focus on a holistic review process by dermatology residency programs may contribute to higher rates of matching among Black applicants in our study.
Audit Proof Your Mohs Note
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
In October 2020, Medicare released an updated guidance to reduce Mohs micrographic surgery (MMS) reimbursement issues,1 which initially was released in 2013. This guidance defines the latest performance and documentation requirements that Medicare requires for MMS. Understanding these requirements and making sure that your Mohs surgical reports have all the needed documentation details are critical because auditors from not only Medicare Administrative Contractors (MACs) but also private insurers and Medicare Advantage plans have adopted these standards and will deny payment for Mohs surgical codes if they are not met. This article provides a review of the updated Medicare requirements to make sure your MMS procedure notes are audit proof.
Notes Must Indicate Mohs Is the Most Appropriate Treatment
I review many of my colleagues’ Mohs notes and can tell you that some of the requirements laid out in the updated guidance typically are already reported by Mohs surgeons in their notes, including the location, number, and size of the lesion or lesions treated and the number of stages performed. However, there are some new requirements that often are not reported by Mohs surgeons that now need to be included. The guidance indicates the following:
The majority of skin cancers can be managed by simple excision or destruction techniques. The medical record of a patient undergoing MMS should clearly show that this procedure was chosen because of the complexity (eg, poorly defined clinical borders, possible deep invasion, prior irradiation), size or location (eg, maximum conservation of tumor-free tissue is important). Medicare will consider reimbursement for MMS for accepted diagnoses and indications, which you must document in the patient’s medical record as being appropriate for MMS and that MMS is the most appropriate choice for the treatment of a particular lesion.1
In my experience, most Mohs notes include some statement that the skin cancer treated is appropriate based on the Mohs appropriate use criteria (AUC) or the AUC score. However, notes should make clear not just that the lesion treated is “appropriate” for MMS but also that it is the most appropriate treatment (eg, why the lesion was not managed by standard excision or destruction technique).
Mohs Surgeon Must Perform the Surgery and Interpret Slides
The updated guidance clearly indicates that MMS may only be performed by a physician who is specifically trained and highly skilled in Mohs techniques and pathologic identification: “Medicare will only reimburse for MMS services when the Mohs surgeon acts as both surgeon and pathologist.”1 Mohs micrographic surgery codes may not be billed if preparation or interpretation of the pathology slides is performed by a physician other than the Mohs surgeon. Operative notes and pathology documentation in the patient’s medical record should clearly show that MMS was performed using an accepted MMS technique in which the physician acts in 2 integrated and distinct capacities—surgeon and pathologist—thereby confirming that the procedure meets the definition of the Current Procedural Terminology code(s).
Furthermore, the Mohs operative report should detail “the number of specimens per stage.”1 I interpret this statement to indicate that the Mohs surgeon should document the number of tissue blocks examined in each stage of Mohs surgery. For example, a statement in the notes such as “the specimen from the first Mohs stage was oriented, mapped, and divided into 4 blocks” should suffice to meet this requirement.
Histologic Description Must Be Included in Mohs Notes
Medicare will require the Mohs surgeon to document “the histology of the specimens taken. That description should include depth of invasion, pathological pattern, cell morphology, and, if present, perineural invasion or presence of scar tissue.”1 Although this histologic description requirement appears daunting, it is common for Mohs surgeons to indicate their pathologic findings on their Mohs map such as “NBCC” next to a red area to indicate “nodular basal cell carcinoma visualized.” A template-based system to translate typical pathologic findings can be employed to rapidly and accurately populate a Mohs note with histologic description such as “NBBC=nodular aggregates of palisaded basaloid epithelial tumor arising from the epidermis forming a palisade with a cleft forming from the adjacent mucinous stroma extending to the mid dermis. Centrally the nuclei become crowded with scattered mitotic figures and necrotic bodies evident.”
Recent Improvement for 1-Stage Mohs Surgeries
The most notable improvement in the
Final Thoughts
Overall, the updated Medicare guidance provides important details in the requirements for performance and documentation of Mohs surgery cases. However, additional critical information will be found in Mohs coverage policies and local coverage determinations (LCDs) from MACs and private insurers.2-4 Each LCD and insurer Mohs payment policy has unique wording and requirements. Coverage of MMS for specific malignant diagnoses, histologic subtypes, locations, and clinical scenarios varies between LCDs; most are based directly on the Mohs AUC, while others have a less specific coverage criteria. To understand the specific documentation and coverage requirements of the MAC for a particular region or private insurer, Mohs surgeons are encouraged to familiarize themselves with the Mohs surgery LCD of their local MAC and coverage policies of their insurers and to ensure their documentation substantiates these requirements. Making sure that your MMS documentation is accurate and complies with Medicare and insurer requirements will keep you out of hot water with auditors and allow reimbursement for this critical skin cancer procedure.
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
- Centers for Disease Control and Prevention. Guidance to reduce Mohs surgery reimbursement issues. MLN Matters. Published October 27, 2020. Accessed July 18, 2022. https://www.cms.gov/Outreach-and-Education/Medicare-Learning-Network-MLN/MLNMattersArticles/Downloads/SE1318.pdf
- Mohs micrographic surgery policy, professional. United Healthcare website. Accessed July 12, 2022. https://www.uhcprovider.com/content/dam/provider/docs/public/policies/comm-reimbursement/COMM-Mohs-Micrographic-Surgery-Policy.pdf#:~:text=This%20policy%20describes%20reimbursement%20guidelines%20for%20reporting%20Mohs,CCI%20Editing%20Policy%20and%20the%20Laboratory%20Services%20Policy.
- Clinical UM guideline—Mohs micrographic surgery. Anthem Insurance Companies website. Published October 6, 2021. Accessed July 27, 2022. https://www.anthem.com/dam/medpolicies/abcbs/active/guidelines/gl_pw_d085074.html
- Local coverage determinations. Centers for Medicare and Medicaid Services website. Updated July 12, 2022. Accessed July 12, 2022. https://www.cms.gov/Medicare/Coverage/DeterminationProcess/LCDs
Practice Points
- Medicare’s updated guidance for documentation of Mohs micrographic surgery (MMS) includes some new requirements that Mohs surgeons should ensure are implemented in their Mohs records.
- Per Medicare guidance, MMS records should include a justification of why MMS was the most appropriate treatment and a description of the histologic findings from the Mohs slides.
- One major improvement with the updated documentation requirements is that if no tumor is visualized in the first stage of MMS, then no histology description of the tumor is required.
Discrepancies in Skin Cancer Screening Reporting Among Patients, Primary Care Physicians, and Patient Medical Records
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”
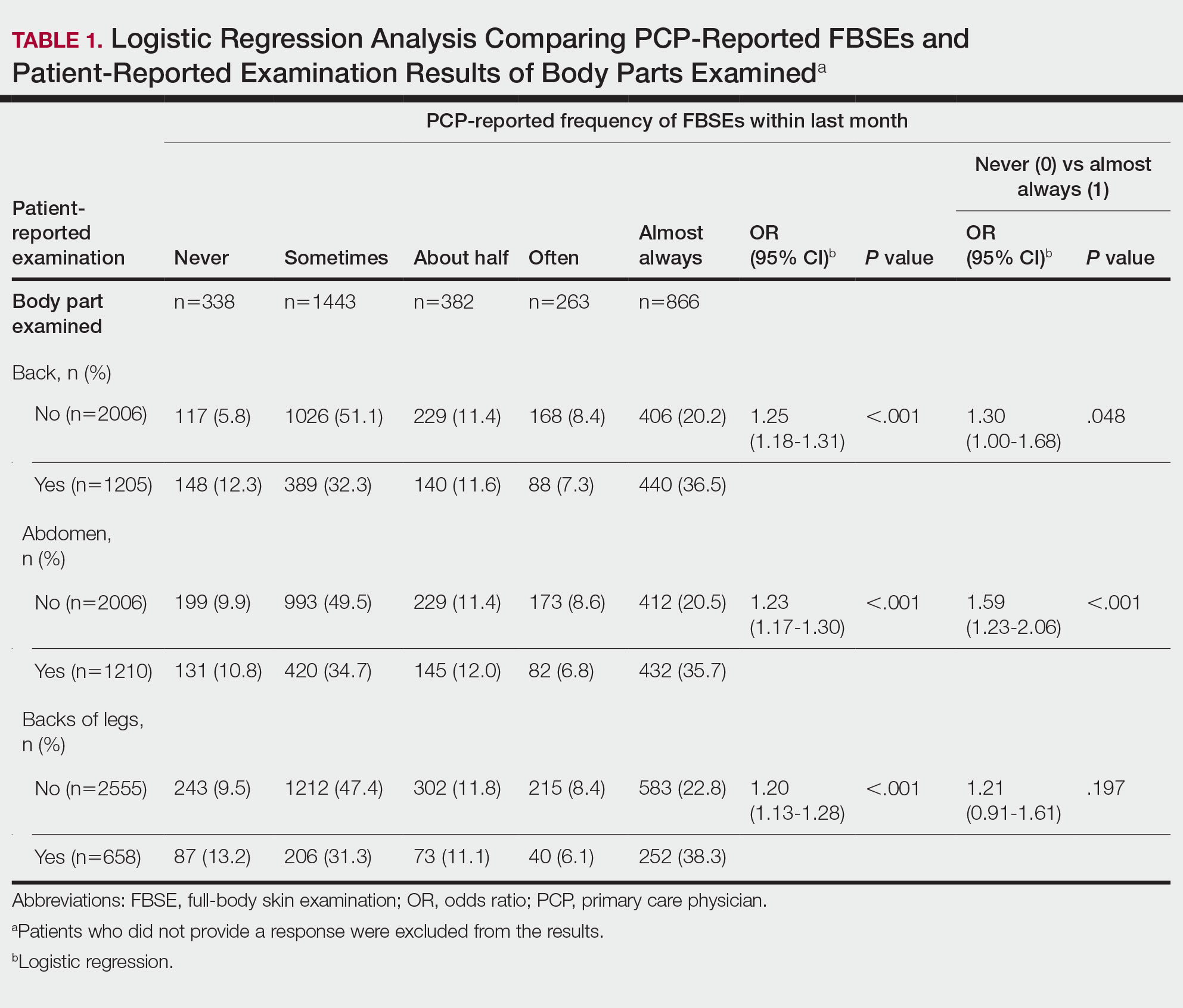
Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).
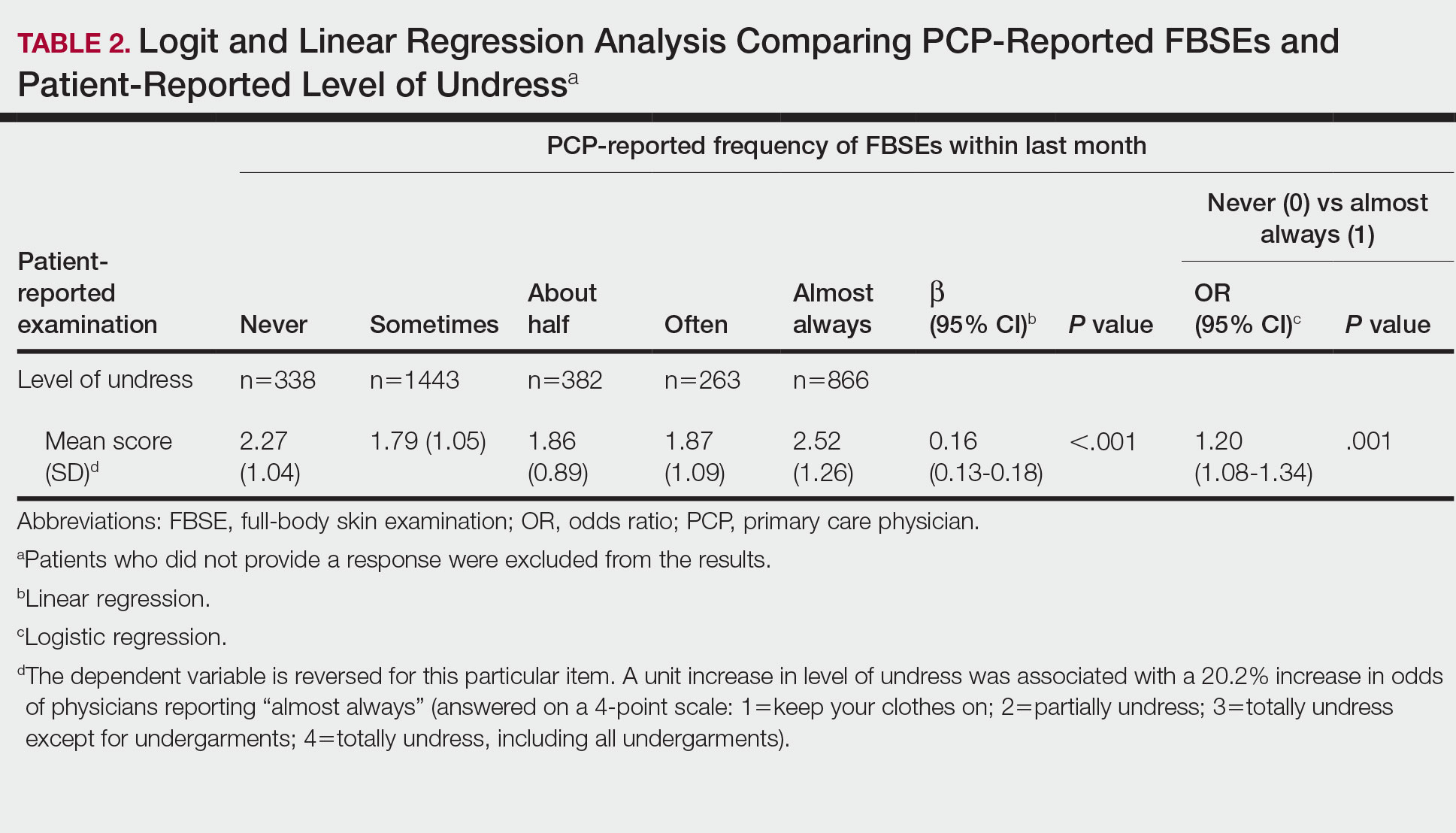
Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.
When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).
COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
Keratinocyte carcinoma (KC), or nonmelanoma skin cancer, is the most commonly diagnosed cancer in the United States.1 Basal cell carcinoma comprises the majority of all KCs.2,3 Squamous cell carcinoma is the second most common skin cancer, representing approximately 20% of KCs and accounting for the majority of KC-related deaths.4-7 Malignant melanoma represents the majority of all skin cancer–related deaths.8 The incidence of basal cell carcinoma, squamous cell carcinoma, and malignant melanoma in the United States is on the rise and carries substantial morbidity and mortality with notable social and economic burdens.1,8-10
Prevention is necessary to reduce skin cancer morbidity and mortality as well as rising treatment costs. The most commonly used skin cancer screening method among dermatologists is the visual full-body skin examination (FBSE), which is a noninvasive, safe, quick, and cost-effective method of early detection and prevention.11 To effectively confront the growing incidence and health care burden of skin cancer, primary care providers (PCPs) must join dermatologists in conducting FBSEs.12,13
Despite being the predominant means of secondary skin cancer prevention, the US Preventive Services Task Force (USPSTF) issued an I rating for insufficient evidence to assess the benefits vs harms of screening the adult general population by PCPs.14,15 A major barrier to studying screening is the lack of a standardized method for conducting and reporting FBSEs.13 Systematic thorough skin examination generally is not performed in the primary care setting.16-18
We aimed to investigate what occurs during an FBSE in the primary care setting and how often they are performed. We examined whether there was potential variation in the execution of the examination, what was perceived by the patient vs reported by the physician, and what was ultimately included in the medical record. Miscommunication between patient and provider regarding performance of FBSEs has previously been noted,17-19 and we sought to characterize and quantify that miscommunication. We hypothesized that there would be lower patient-reported FBSEs compared to physicians and patient medical records. We also hypothesized that there would be variability in how physicians screened for skin cancer.
METHODS
This study was cross-sectional and was conducted based on interviews and a review of medical records at secondary- and tertiary-level units (clinics and hospitals) across the United States. We examined baseline data from a randomized controlled trial of a Web-based skin cancer early detection continuing education course—the Basic Skin Cancer Triage curriculum. Complete details have been described elsewhere.12 This study was approved by the institutional review boards of the Providence Veterans Affairs Medical Center, Rhode Island Hospital, and Brown University (all in Providence, Rhode Island), as well as those of all recruitment sites.
Data were collected from 2005 to 2008 and included physician online surveys, patient telephone interviews, and patient medical record data abstracted by research assistants. Primary care providers included in the study were general internists, family physicians, or medicine-pediatrics practitioners who were recruited from 4 collaborating centers across the United States in the mid-Atlantic region, Ohio, Kansas, and southern California, and who had been in practice for at least a year. Patients were recruited from participating physician practices and selected by research assistants who traveled to each clinic for coordination, recruitment, and performance of medical record reviews. Patients were selected as having minimal risk of melanoma (eg, no signs of severe photodamage to the skin). Patients completed structured telephone surveys within 1 to 2 weeks of the office visit regarding the practices observed and clinical questions asked during their recent clinical encounter with their PCP.
Measures
Demographics—Demographic variables asked of physicians included age, sex, ethnicity, academic degree (MD vs DO), years in practice, training, and prior dermatology training. Demographic information asked of patients included age, sex, ethnicity, education, and household income.
Physician-Reported Examination and Counseling Variables—Physicians were asked to characterize their clinical practices, prompted by questions regarding performance of FBSEs: “Please think of a typical month and using the scale below, indicate how frequently you perform a total body skin exam during an annual exam (eg, periodic follow-up exam).” Physicians responded to 3 questions on a 5-point scale (1=never, 2=sometimes, 3=about half, 4=often, 5=almost always).
Patient-Reported Examination Variables—Patients also were asked to characterize the skin examination experienced in their clinical encounter with their PCP, including: “During your last visit, as far as you could tell, did your physician: (1) look at the skin on your back? (2) look at the skin on your belly area? (3) look at the skin on the back of your legs?” Patient responses were coded as yes, no, don’t know, or refused. Participants who refused were excluded from analysis; participants who responded are detailed in Table 1. In addition, patients also reported the level of undress with their physician by answering the following question: “During your last medical exam, did you: 1=keep your clothes on; 2=partially undress; 3=totally undress except for undergarments; 4=totally undress, including all undergarments?”

Patient Medical Record–Extracted Data—Research assistants used a structured abstract form to extract the information from the patient’s medical record and graded it as 0 (absence) or 1 (presence) from the medical record.
Statistical Analysis
Descriptive statistics included mean and standard deviation (SD) for continuous variables as well as frequency and percentage for categorical variables. Logit/logistic regression analysis was used to predict the odds of patient-reported outcomes that were binary with physician-reported variables as the predictor. Linear regression analysis was used to assess the association between 2 continuous variables. All analyses were conducted using SPSS version 24 (IBM).20 Significance criterion was set at α of .05.
RESULTS Demographics
The final sample included data from 53 physicians and 3343 patients. The study sample mean age (SD) was 50.3 (9.9) years for PCPs (n=53) and 59.8 (16.9) years for patients (n=3343). The physician sample was 36% female and predominantly White (83%). Ninety-one percent of the PCPs had an MD (the remaining had a DO degree), and the mean (SD) years practicing was 21.8 (10.6) years. Seventeen percent of PCPs were trained in internal medicine, 4% in internal medicine and pediatrics, and 79% family medicine; 79% of PCPs had received prior training in dermatology. The patient sample was 58% female, predominantly White (84%), non-Hispanic/Latinx (95%), had completed high school (94%), and earned more than $40,000 annually (66%).
Physician- and Patient-Reported FBSEs
Physicians reported performing FBSEs with variable frequency. Among PCPs who conducted FBSEs with greater frequency, there was a modest increase in the odds that patients reported a particular body part was examined (back: odds ratio [OR], 24.5% [95% CI, 1.18-1.31; P<.001]; abdomen: OR, 23.3% [95% CI, 1.17-1.30; P<.001]; backs of legs: OR, 20.4% [95% CI, 1.13-1.28; P<.001])(Table 1). The patient-reported level of undress during examination was significantly associated with physician-reported FBSE (β=0.16 [95% CI, 0.13-0.18; P<.001])(Table 2).

Because of the bimodal distribution of scores in the physician-reported frequency of FBSEs, particularly pertaining to the extreme points of the scale, we further repeated analysis with only the never and almost always groups (Table 1). Primary care providers who reported almost always for FBSE had 29.6% increased odds of patient-reported back examination (95% CI, 1.00-1.68; P=.048) and 59.3% increased odds of patient-reported abdomen examination (95% CI, 1.23-2.06; P<.001). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having never conducted an FBSE were 56%, 40%, and 26%, respectively. The raw percentages of patients who reported having their back, abdomen, and backs of legs examined when the PCP reported having almost always conducted an FBSE were 52%, 51%, and 30%, respectively. Raw percentages were calculated by dividing the number of "yes" responses by participants for each body part examined by thetotal number of participant responses (“yes” and “no”) for each respective body part. There was no significant change in odds of patient-reported backs of legs examined with PCP-reported never vs almost always conducting an FBSE. In addition, a greater patient-reported level of undress was associated with 20.2% increased odds of PCPs reporting almost always conducting an FBSE (95% CI, 1.08-1.34; P=.001).
FBSEs in Patient Medical Records
When comparing PCP-reported FBSE and report of FBSE in patient medical records, there was a 39.0% increased odds of the patient medical record indicating FBSE when physicians reported conducting an FBSE with greater frequency (95% CI, 1.30-1.48; P<.001)(eTable 1). When examining PCP-reported never vs almost always conducting an FBSE, a report of almost always was associated with 79.0% increased odds of the patient medical record indicating that an FBSE was conducted (95% CI, 1.28-2.49; P=.001). The raw percentage of the patient medical record indicating an FBSE was conducted when the PCP reported having never conducted an FBSE was 17% and 26% when the PCP reported having almost always conducted an FBSE.

When comparing the patient-reported body part examined with patient FBSE medical record documentation, an indication of yes for FBSE on the patient medical record was associated with a considerable increase in odds that patients reported a particular body part was examined (back: 91.4% [95% CI, 1.59-2.31; P<.001]; abdomen: 75.0% [95% CI, 1.45-2.11; P<.001]; backs of legs: 91.6% [95% CI, 1.56-2.36; P<.001])(eTable 2). The raw percentages of patients who reported having their back, abdomen, and backs of legs examined vs not examined when the patient medical record indicated an FBSE was completed were 24% vs 14%, 23% vs 15%, and 26% vs 16%, respectively. An increase in patient-reported level of undress was associated with a 57.0% increased odds of their medical record indicating an FBSE was conducted (95% CI, 1.45-1.70; P<.001).

COMMENT How PCPs Perform FBSEs Varies
We found that PCPs performed FBSEs with variable frequency, and among those who did, the patient report of their examination varied considerably (Table 1). There appears to be considerable ambiguity in each of these means of determining the extent to which the skin was inspected for skin cancer, which may render the task of improving such inspection more difficult. We asked patients whether their back, abdomen, and backs of legs were examined as an assessment of some of the variety of areas inspected during an FBSE. During a general well-visit appointment, a patient’s back and abdomen may be examined for multiple reasons. Patients may have misinterpreted elements of the pulmonary, cardiac, abdominal, or musculoskeletal examinations as being part of the FBSE. The back and abdomen—the least specific features of the FBSE—were reported by patients to be the most often examined. Conversely, the backs of the legs—the most specific feature of the FBSE—had the lowest odds of being examined (Table 1).
In addition to the potential limitations of patient awareness of physician activity, our results also could be explained by differences among PCPs in how they performed FBSEs. There is no standardized method of conducting an FBSE. Furthermore, not all medical students and residents are exposed to dermatology training. In our sample of 53 physicians, 79% had reported receiving dermatology training; however, we did not assess the extent to which they had been trained in conducting an FBSE and/or identifying malignant lesions. In an American survey of 659 medical students, more than two-thirds of students had never been trained or never examined a patient for skin cancer.21 In another American survey of 342 internal medicine, family medicine, pediatrics, and obstetrics/gynecology residents across 7 medical schools and 4 residency programs, more than three-quarters of residents had never been trained in skin cancer screening.22 Our findings reflect insufficient and inconsistent training in skin cancer screening and underscore the need for mandatory education to ensure quality FBSEs are performed in the primary care setting.
Frequency of PCPs Performing FBSEs
Similar to prior studies analyzing the frequency of FBSE performance in the primary care setting,16,19,23,24 more than half of our PCP sample reported sometimes to never conducting FBSEs. The percentage of physicians who reported conducting FBSEs in our sample was greater than the proportion reported by the National Health Interview Survey, in which only 8% of patients received an FBSE in the prior year by a PCP or obstetrician/gynecologist,16 but similar to a smaller patient study.19 In that study, 87% of patients, regardless of their skin cancer history, also reported that they would like their PCP to perform an FBSE regularly.19 Although some of our patient participants may have declined an FBSE, it is unlikely that that would have entirely accounted for the relatively low number of PCPs who reported frequently performing FBSEs.
Documentation in Medical Records of FBSEs
Compared to PCP self-reported performance of FBSEs, considerably fewer PCPs marked the patient medical record as having completed an FBSE. Among patients with medical records that indicated an FBSE had been conducted, they reported higher odds of all 3 body parts being examined, the highest being the backs of the legs. Also, when the patient medical record indicated an FBSE had been completed, the odds that the PCP reported an FBSE also were higher. The relatively low medical record documentation of FBSEs highlights the need for more rigorous enforcement of accurate documentation. However, among the cases that were recorded, it appeared that the content of the examinations was more consistent.
Benefits of PCP-Led FBSEs
Although the USPSTF issued an I rating for PCP-led FBSEs,14 multiple national medical societies, including the American Cancer Society,25 American Academy of Dermatology,26 and Skin Cancer Foundation,27 as well as international guidelines in Germany,28 Australia,29,30 and New Zealand,31 recommend regular FBSEs among the general or at-risk population; New Zealand and Australia have the highest incidence and prevalence of melanoma in the world.8 The benefits of physician-led FBSEs on detection of early-stage skin cancer, and in particular, melanoma detection, have been documented in numerous studies.30,32-38 However, the variability and often poor quality of skin screening may contribute in part to the just as numerous null results from prior skin screening studies,15 perpetuating the insufficient status of skin examinations by USPSTF standards.14 Our study underscores both the variability in frequency and content of PCP-administered FBSEs. It also highlights the need for standardization of screening examinations at the medical student, trainee, and physician level.
Study Limitations
The present study has several limitations. First, there was an unknown time lag between the FBSEs and physician self-reported surveys. Similarly, there was a variable time lag between the patient examination encounter and subsequent telephone survey. Both the physician and patient survey data may have been affected by recall bias. Second, patients were not asked directly whether an FBSE had been conducted. Furthermore, patients may not have appreciated whether the body part examined was part of the FBSE or another examination. Also, screenings often were not recorded in the medical record, assuming that the patient report and/or physician report was more accurate than the medical record.
Our study also was limited by demographics; our patient sample was largely comprised of White, educated, US adults, potentially limiting the generalizability of our findings. Conversely, a notable strength of our study was that our participants were recruited from 4 geographically diverse centers. Furthermore, we had a comparatively large sample size of patients and physicians. Also, the independent assessment of provider-reported examinations, objective assessment of medical records, and patient reports of their encounters provides a strong foundation for assessing the independent contributions of each data source.
CONCLUSION
Our study highlights the challenges future studies face in promoting skin cancer screening in the primary care setting. Our findings underscore the need for a standardized FBSE as well as clear clinical expectations regarding skin cancer screening that is expected of PCPs.
As long as skin cancer screening rates remain low in the United States, patients will be subject to potential delays and missed diagnoses, impacting morbidity and mortality.8 There are burgeoning resources and efforts in place to increase skin cancer screening. For example, free validated online training is available for early detection of melanoma and other skin cancers (https://www.visualdx.com/skin-cancer-education/).39-42 Future directions for bolstering screening numbers must focus on educating PCPs about skin cancer prevention and perhaps narrowing the screening population by age-appropriate risk assessments.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
- Rogers HW, Weinstock MA, Feldman SR, et al. Incidence estimate of nonmelanoma skin cancer (keratinocyte carcinomas) in the U.S. population, 2012. JAMA Dermatol. 2015;151:1081-1086.
- Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. 2015;88:167-179.
- Dourmishev LA, Rusinova D, Botev I. Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J. 2013;4:12-17.
- Thompson AK, Kelley BF, Prokop LJ, et al. Risk factors for cutaneous squamous cell carcinoma outcomes: a systematic review and meta-analysis. JAMA Dermatol. 2016;152:419-428.
- Motaparthi K, Kapil JP, Velazquez EF. Cutaneous squamous cell carcinoma: review of the eighth edition of the American Joint Committee on Cancer Staging Guidelines, Prognostic Factors, and Histopathologic Variants. Adv Anat Pathol. 2017;24:171-194.
- Barton V, Armeson K, Hampras S, et al. Nonmelanoma skin cancer and risk of all-cause and cancer-related mortality: a systematic review. Arch Dermatol Res. 2017;309:243-251.
- Weinstock MA, Bogaars HA, Ashley M, et al. Nonmelanoma skin cancer mortality. a population-based study. Arch Dermatol. 1991;127:1194-1197.
- Matthews NH, Li W-Q, Qureshi AA, et al. Epidemiology of melanoma. In: Ward WH, Farma JM, eds. Cutaneous Melanoma: Etiology and Therapy. Codon Publications; 2017:3-22.
- Cakir BO, Adamson P, Cingi C. Epidemiology and economic burden of nonmelanoma skin cancer. Facial Plast Surg Clin North Am. 2012;20:419-422.
- Guy GP, Machlin SR, Ekwueme DU, et al. Prevalence and costs of skin cancer treatment in the U.S., 2002-2006 and 2007-2011. Am J Prev Med. 2015;48:183-187.
- Losina E, Walensky RP, Geller A, et al. Visual screening for malignant melanoma: a cost-effectiveness analysis. Arch Dermatol. 2007;143:21-28.
- Markova A, Weinstock MA, Risica P, et al. Effect of a web-based curriculum on primary care practice: basic skin cancer triage trial. Fam Med. 2013;45:558-568.
- Johnson MM, Leachman SA, Aspinwall LG, et al. Skin cancer screening: recommendations for data-driven screening guidelines and a review of the US Preventive Services Task Force controversy. Melanoma Manag. 2017;4:13-37.
- Agency for Healthcare Research and Quality. Screening for skin cancer in adults: an updated systematic evidence review for the U.S. Preventive Services Task Force. November 30, 2015. Accessed July 25, 2022. http://uspreventiveservicestaskforce.org/Page/Document/draft-evidence-review159/skin-cancer-screening2
- Wernli KJ, Henrikson NB, Morrison CC, et al. Screening for skin cancer in adults: updated evidence report and systematic review forthe US Preventive Services Task Force. JAMA. 2016;316:436-447.
- LeBlanc WG, Vidal L, Kirsner RS, et al. Reported skin cancer screening of US adult workers. J Am Acad Dermatol. 2008;59:55-63.
- Federman DG, Concato J, Caralis PV, et al. Screening for skin cancer in primary care settings. Arch Dermatol. 1997;133:1423-1425.
- Kirsner RS, Muhkerjee S, Federman DG. Skin cancer screening in primary care: prevalence and barriers. J Am Acad Dermatol. 1999;41:564-566.
- Federman DG, Kravetz JD, Tobin DG, et al. Full-body skin examinations: the patient’s perspective. Arch Dermatol. 2004;140:530-534.
- IBM. IBM SPSS Statistics for Windows. IBM Corp; 2015.
- Moore MM, Geller AC, Zhang Z, et al. Skin cancer examination teaching in US medical education. Arch Dermatol. 2006;142:439-444.
- Wise E, Singh D, Moore M, et al. Rates of skin cancer screening and prevention counseling by US medical residents. Arch Dermatol. 2009;145:1131-1136.
- Lakhani NA, Saraiya M, Thompson TD, et al. Total body skin examination for skin cancer screening among U.S. adults from 2000 to 2010. Prev Med. 2014;61:75-80.
- Coups EJ, Geller AC, Weinstock MA, et al. Prevalence and correlates of skin cancer screening among middle-aged and older white adults in the United States. Am J Med. 2010;123:439-445.
- American Cancer Society. Cancer facts & figures 2016. Accessed March 13, 2022. https://cancer.org/research/cancerfactsstatistics/cancerfactsfigures2016/
- American Academy of Dermatology. Skin cancer incidence rates. Updated April 22, 2022. Accessed August 1, 2022. https://www.aad.org/media/stats-skin-cancer
- Skin Cancer Foundation. Skin cancer prevention. Accessed July 25, 2022. http://skincancer.org/prevention/sun-protection/prevention-guidelines
- Katalinic A, Eisemann N, Waldmann A. Skin cancer screening in Germany. documenting melanoma incidence and mortality from 2008 to 2013. Dtsch Arztebl Int. 2015;112:629-634.
- Cancer Council Australia. Position statement: screening and early detection of skin cancer. Published July 2014. Accessed July 25, 2022. https://dermcoll.edu.au/wp-content/uploads/2014/05/PosStatEarlyDetectSkinCa.pdf
- Royal Australian College of General Practitioners. Guidelines for Preventive Activities in General Practice. 9th ed. The Royal Australian College of General Practitioners; 2016. Accessed July 27, 2022. https://www.racgp.org.au/download/Documents/Guidelines/Redbook9/17048-Red-Book-9th-Edition.pdf
- Cancer Council Australia and Australian Cancer Network and New Zealand Guidelines Group. Clinical Practice Guidelines for the Management of Melanoma in Australia and New Zealand. The Cancer Council Australia and Australian Cancer Network, Sydney and New Zealand Guidelines Group, Wellington; 2008. Accessed July 27, 2022. https://www.health.govt.nz/system/files/documents/publications/melanoma-guideline-nov08-v2.pdf
- Swetter SM, Pollitt RA, Johnson TM, et al. Behavioral determinants of successful early melanoma detection: role of self and physician skin examination. Cancer. 2012;118:3725-3734.
- Terushkin V, Halpern AC. Melanoma early detection. Hematol Oncol Clin North Am. 2009;23:481-500, viii.
- Aitken JF, Elwood M, Baade PD, et al. Clinical whole-body skin examination reduces the incidence of thick melanomas. Int J Cancer. 2010;126:450-458.
- Aitken JF, Elwood JM, Lowe JB, et al. A randomised trial of population screening for melanoma. J Med Screen. 2002;9:33-37.
- Breitbart EW, Waldmann A, Nolte S, et al. Systematic skin cancer screening in Northern Germany. J Am Acad Dermatol. 2012;66:201-211.
- Janda M, Lowe JB, Elwood M, et al. Do centralised skin screening clinics increase participation in melanoma screening (Australia)? Cancer Causes Control. 2006;17:161-168.
- Aitken JF, Janda M, Elwood M, et al. Clinical outcomes from skin screening clinics within a community-based melanoma screening program. J Am Acad Dermatol. 2006;54:105-114.
- Eide MJ, Asgari MM, Fletcher SW, et al. Effects on skills and practice from a web-based skin cancer course for primary care providers. J Am Board Fam Med. 2013;26:648-657.
- Weinstock MA, Ferris LK, Saul MI, et al. Downstream consequences of melanoma screening in a community practice setting: first results. Cancer. 2016;122:3152-3156.
- Matthews NH, Risica PM, Ferris LK, et al. Psychosocial impact of skin biopsies in the setting of melanoma screening: a cross-sectional survey. Br J Dermatol. 2019;180:664-665.
- Risica PM, Matthews NH, Dionne L, et al. Psychosocial consequences of skin cancer screening. Prev Med Rep. 2018;10:310-316.
PRACTICE POINTS
- Dermatologists should be aware of the variability in practice and execution of full-body skin examinations (FBSEs) among primary care providers and offer comprehensive examinations for every patient.
- Variability in reporting and execution of FBSEs may impact the continued US Preventive Services Task Force I rating in their guidelines and promotion of skin cancer screening in the primary care setting.
How to Address Scar Pincushioning and Webbing of the Nasal Dorsum Using Surgical Defatting and Z-plasty
Practice Gap
Nonmelanoma skin cancer is the most common cancer, typically growing in sun-exposed areas. As such, the nasal area is a common site of onset, constituting approximately 25% of cases. Surgical excision of these cancers generally has a high cure rate.1
Although complete excision of the tumor is the primary goal of the dermatologic surgeon, achieving a cosmetically satisfactory scar also is important. As a prominent feature of the face, any irregularities to the nose are easily noticeable.2 The subsequent scar may exhibit features that are less than ideal and cause notable stress to the patient.
When a scar presents with several complications, using a single surgical technique may not sufficiently address all defects. As a result, it can be challenging for the surgeon to decide which combination of methods among the myriad of nonsurgical and surgical options for scar revision will produce the best cosmetic outcome.
Case and Technique
A 76-year-old man presented 1 year after he underwent Mohs micrographic surgery for squamous cell carcinoma on the nasal dorsum. The tumor cleared after 1 stage and was repaired using a bilateral V-Y advancement flap. Postoperatively, the patient developed pincushioning of the flap, atrophic scarring inferior to the flap, and webbing of the pivotal restraint point at the nasal root (Figures 1A and 1B). We opted to address the pincushioning and nasal root webbing by defatting the flap and performing Z-plasty, respectively.
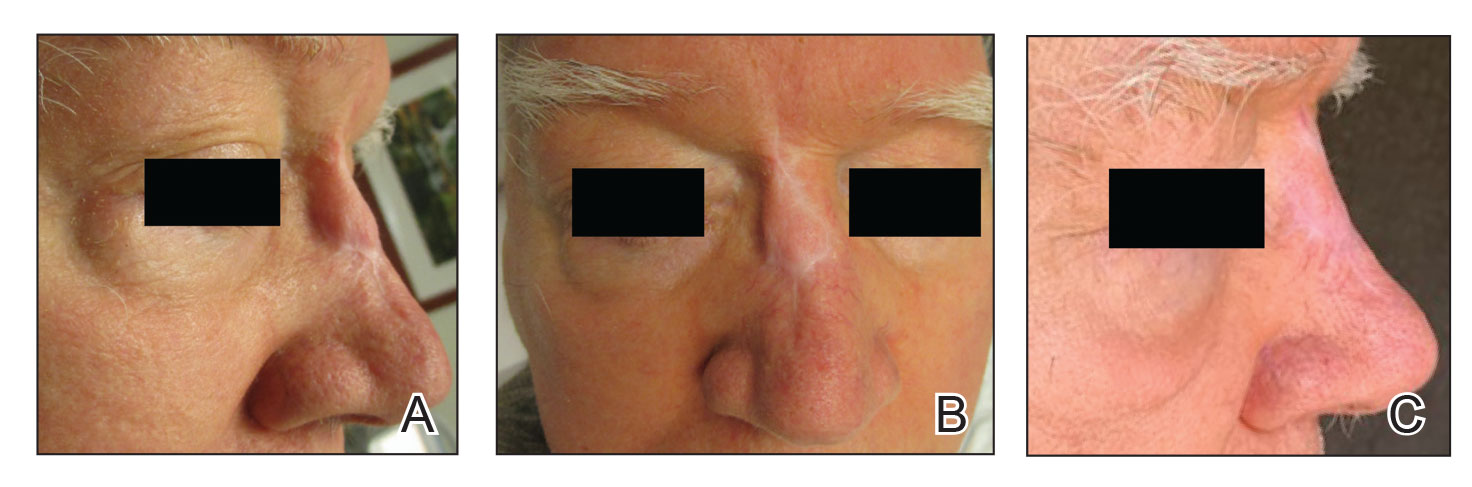
Pincushioning—Pincushioning of a flap arises due to contraction and lymphedema at the edge of the repair. It is seen more often in nasal repairs due to the limited availability of surrounding skin and changes in skin texture from rhinion to tip.3 To combat this in our patient, an incision was made around the site of the original flap, surrounding tissue was undermined, and the flap was reflected back. Subcutaneous tissue was removed with scissors. The flap was then laid back into the defect, and the subcutaneous tissue and dermis were closed with interrupted buried vertical mattress sutures. The epidermis was closed in a simple running fashion.
Webbing—Webbing of a scar also may develop from the contractile wound-healing process.4 Z-plasty commonly is used to camouflage a linear or contracted scar, increase skin availability in an area, or alter scar direction to better align with skin-tension lines.5,6 In our patient, we incised the webbing of the nasal root along the vertical scar. Two arms were drawn at each end of the scar at a 60° angle (Figure 2); the side arms were drawn equal in length and incised vertically. Full-thickness skin flaps were then undermined at the level of subcutaneous fat, creating 2 triangular flaps. Adequate undermining of the surrounding subcutaneous tissue was performed to achieve proper mobilization of the flaps, which allowed for flap transposition to occur without tension and therefore for proper redirection of the scar.6 The flaps were secured using buried vertical mattress sutures and simple running sutures. Using too many buried interrupted sutures can cause vascular compromise of the fragile tips of the Z and should be avoided.3
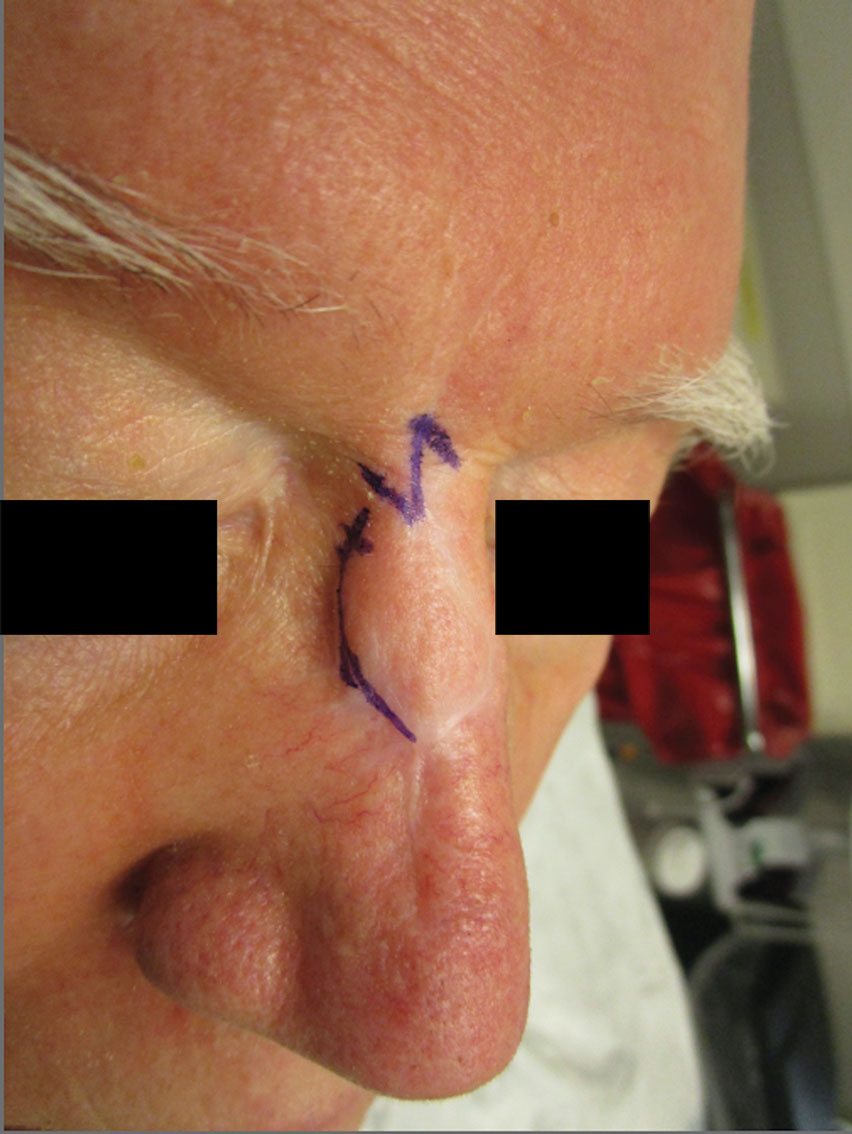
At 4-month postoperative follow-up, the cosmetic outcome was judged satisfactory (Figure 1C).
Practice Implications
In our patient, pincushioning of the flap was easily addressed by defatting the area. However, doing just this would not have sufficed and necessitated another surgical technique—the Z-plasty—which needed to be designed carefully. The larger the angle between the side arms and central limb, the greater directional change and scar length that is gained (Figure 3). As a result, longer limbs and a greater angle could advantageously break up the scar line but consequently would lengthen the scar considerably. Therefore, if the scar was longer or the skin was inelastic, multiple Z-plasty procedures may have been preferred.
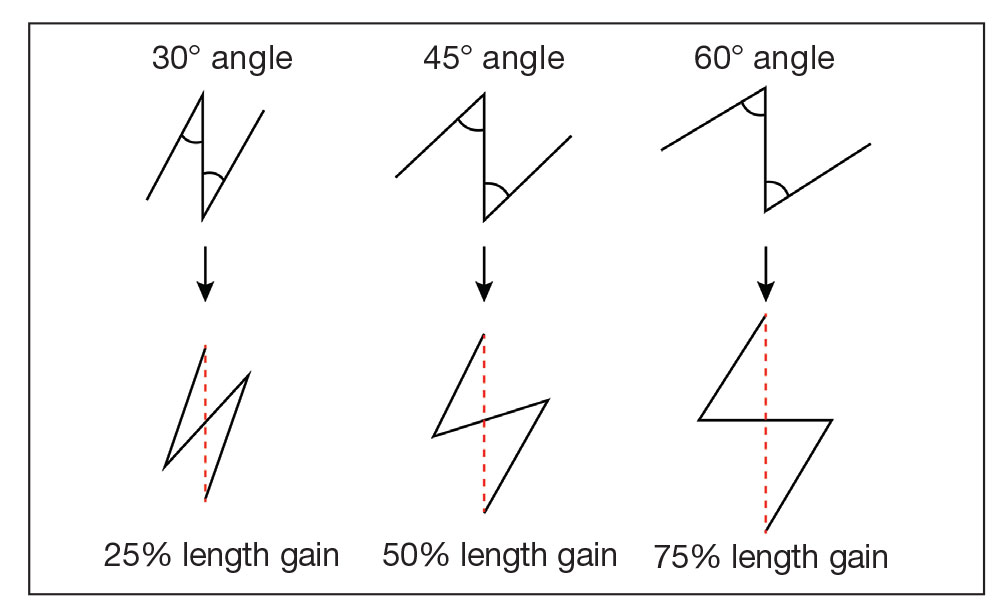
Additionally, for each central limb, both mirror-image options for peripheral arms were considered, with the optimal choice being the one that allowed for final scar lines to mimic relaxed skin-tension lines. Accuracy of the incisions was critical and was assessed by drawing a line between the free ends of the lateral limbs of the Z; this line should pass perpendicularly through the midpoint of the central limb. Last, as with other transposition flap options, Z-plasty has the potential to create a trapdoor or pincushion effect; we reduced this risk by wide undermining to establish an even contraction plate.6
When planning the revision, we considered multiple approaches to achieve the best aesthetic outcome in 1 stage. Had there been notable depression in the scar, we may have used a full-thickness skin graft. If the skin surface was lumpy and uneven, dermabrasion or a laser may have been utilized. Another consideration was to avoid using intralesional steroids, which could have made the already atrophied portions of the scar worse.
Overall, the surgical plan that we chose took into consideration the patient’s nasal anatomic structure, the combination of scar defects, the patient’s desires, and the tools available.
Final Thoughts
The ideal scar is inconspicuous, does not impair the function of surrounding structures, and blends well with adjacent skin.5 Consequently, the combination of pincushioning and webbing of a scar, especially in the nasal area, can pose a surgical challenge to the surgeon and can cause severe anxiety in the patient. In those circumstances, a single surgical technique is not likely to produce the revision with the best cosmetic outcome. Therefore, the synergy of 2 or more surgical techniques with proper planning and meticulous selection may be necessary. A broad knowledge of various scar revision techniques increases the surgeon’s capability to create the ideal scar.
Acknowledgment—The authors thank the case patient for granting permission to publish this information.
- Arginelli F, Salgarelli AC, Ferrari B, et al. Crescentic flap for the reconstruction of the nose after skin cancer resection. J Craniomaxillofac Surg. 2016;44:703-707. doi:10.1016/j.jcms.2016.02.008
- Helml G, von Gregory HF, Amr A, et al. One-stage nasal soft tissue reconstruction with local flaps. Facial Plast Surg. 2014;30:260-267. doi:10.1055/s-0034-1376871
- Woodard CR. Complications in facial flap surgery. Facial Plast Surg Clin North Am. 2013;21:599-604. doi:10.1016/j.fsc.2013.07.009
- Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001;17:263-272. doi:10.1055/s-2001-18827
- A, B, MA. Surgical principles for achieving a functional and cosmetically acceptable scar. Actas Dermosifiliogr. 2013;104:17-28. doi:10.1016/j.ad.2011.12.010
- Aasi SZ. Z-plasty made simple. Dermatol Res Pract. 2010;2010:982623. doi:10.1155/2010/982623
Practice Gap
Nonmelanoma skin cancer is the most common cancer, typically growing in sun-exposed areas. As such, the nasal area is a common site of onset, constituting approximately 25% of cases. Surgical excision of these cancers generally has a high cure rate.1
Although complete excision of the tumor is the primary goal of the dermatologic surgeon, achieving a cosmetically satisfactory scar also is important. As a prominent feature of the face, any irregularities to the nose are easily noticeable.2 The subsequent scar may exhibit features that are less than ideal and cause notable stress to the patient.
When a scar presents with several complications, using a single surgical technique may not sufficiently address all defects. As a result, it can be challenging for the surgeon to decide which combination of methods among the myriad of nonsurgical and surgical options for scar revision will produce the best cosmetic outcome.
Case and Technique
A 76-year-old man presented 1 year after he underwent Mohs micrographic surgery for squamous cell carcinoma on the nasal dorsum. The tumor cleared after 1 stage and was repaired using a bilateral V-Y advancement flap. Postoperatively, the patient developed pincushioning of the flap, atrophic scarring inferior to the flap, and webbing of the pivotal restraint point at the nasal root (Figures 1A and 1B). We opted to address the pincushioning and nasal root webbing by defatting the flap and performing Z-plasty, respectively.

Pincushioning—Pincushioning of a flap arises due to contraction and lymphedema at the edge of the repair. It is seen more often in nasal repairs due to the limited availability of surrounding skin and changes in skin texture from rhinion to tip.3 To combat this in our patient, an incision was made around the site of the original flap, surrounding tissue was undermined, and the flap was reflected back. Subcutaneous tissue was removed with scissors. The flap was then laid back into the defect, and the subcutaneous tissue and dermis were closed with interrupted buried vertical mattress sutures. The epidermis was closed in a simple running fashion.
Webbing—Webbing of a scar also may develop from the contractile wound-healing process.4 Z-plasty commonly is used to camouflage a linear or contracted scar, increase skin availability in an area, or alter scar direction to better align with skin-tension lines.5,6 In our patient, we incised the webbing of the nasal root along the vertical scar. Two arms were drawn at each end of the scar at a 60° angle (Figure 2); the side arms were drawn equal in length and incised vertically. Full-thickness skin flaps were then undermined at the level of subcutaneous fat, creating 2 triangular flaps. Adequate undermining of the surrounding subcutaneous tissue was performed to achieve proper mobilization of the flaps, which allowed for flap transposition to occur without tension and therefore for proper redirection of the scar.6 The flaps were secured using buried vertical mattress sutures and simple running sutures. Using too many buried interrupted sutures can cause vascular compromise of the fragile tips of the Z and should be avoided.3

At 4-month postoperative follow-up, the cosmetic outcome was judged satisfactory (Figure 1C).
Practice Implications
In our patient, pincushioning of the flap was easily addressed by defatting the area. However, doing just this would not have sufficed and necessitated another surgical technique—the Z-plasty—which needed to be designed carefully. The larger the angle between the side arms and central limb, the greater directional change and scar length that is gained (Figure 3). As a result, longer limbs and a greater angle could advantageously break up the scar line but consequently would lengthen the scar considerably. Therefore, if the scar was longer or the skin was inelastic, multiple Z-plasty procedures may have been preferred.

Additionally, for each central limb, both mirror-image options for peripheral arms were considered, with the optimal choice being the one that allowed for final scar lines to mimic relaxed skin-tension lines. Accuracy of the incisions was critical and was assessed by drawing a line between the free ends of the lateral limbs of the Z; this line should pass perpendicularly through the midpoint of the central limb. Last, as with other transposition flap options, Z-plasty has the potential to create a trapdoor or pincushion effect; we reduced this risk by wide undermining to establish an even contraction plate.6
When planning the revision, we considered multiple approaches to achieve the best aesthetic outcome in 1 stage. Had there been notable depression in the scar, we may have used a full-thickness skin graft. If the skin surface was lumpy and uneven, dermabrasion or a laser may have been utilized. Another consideration was to avoid using intralesional steroids, which could have made the already atrophied portions of the scar worse.
Overall, the surgical plan that we chose took into consideration the patient’s nasal anatomic structure, the combination of scar defects, the patient’s desires, and the tools available.
Final Thoughts
The ideal scar is inconspicuous, does not impair the function of surrounding structures, and blends well with adjacent skin.5 Consequently, the combination of pincushioning and webbing of a scar, especially in the nasal area, can pose a surgical challenge to the surgeon and can cause severe anxiety in the patient. In those circumstances, a single surgical technique is not likely to produce the revision with the best cosmetic outcome. Therefore, the synergy of 2 or more surgical techniques with proper planning and meticulous selection may be necessary. A broad knowledge of various scar revision techniques increases the surgeon’s capability to create the ideal scar.
Acknowledgment—The authors thank the case patient for granting permission to publish this information.
Practice Gap
Nonmelanoma skin cancer is the most common cancer, typically growing in sun-exposed areas. As such, the nasal area is a common site of onset, constituting approximately 25% of cases. Surgical excision of these cancers generally has a high cure rate.1
Although complete excision of the tumor is the primary goal of the dermatologic surgeon, achieving a cosmetically satisfactory scar also is important. As a prominent feature of the face, any irregularities to the nose are easily noticeable.2 The subsequent scar may exhibit features that are less than ideal and cause notable stress to the patient.
When a scar presents with several complications, using a single surgical technique may not sufficiently address all defects. As a result, it can be challenging for the surgeon to decide which combination of methods among the myriad of nonsurgical and surgical options for scar revision will produce the best cosmetic outcome.
Case and Technique
A 76-year-old man presented 1 year after he underwent Mohs micrographic surgery for squamous cell carcinoma on the nasal dorsum. The tumor cleared after 1 stage and was repaired using a bilateral V-Y advancement flap. Postoperatively, the patient developed pincushioning of the flap, atrophic scarring inferior to the flap, and webbing of the pivotal restraint point at the nasal root (Figures 1A and 1B). We opted to address the pincushioning and nasal root webbing by defatting the flap and performing Z-plasty, respectively.

Pincushioning—Pincushioning of a flap arises due to contraction and lymphedema at the edge of the repair. It is seen more often in nasal repairs due to the limited availability of surrounding skin and changes in skin texture from rhinion to tip.3 To combat this in our patient, an incision was made around the site of the original flap, surrounding tissue was undermined, and the flap was reflected back. Subcutaneous tissue was removed with scissors. The flap was then laid back into the defect, and the subcutaneous tissue and dermis were closed with interrupted buried vertical mattress sutures. The epidermis was closed in a simple running fashion.
Webbing—Webbing of a scar also may develop from the contractile wound-healing process.4 Z-plasty commonly is used to camouflage a linear or contracted scar, increase skin availability in an area, or alter scar direction to better align with skin-tension lines.5,6 In our patient, we incised the webbing of the nasal root along the vertical scar. Two arms were drawn at each end of the scar at a 60° angle (Figure 2); the side arms were drawn equal in length and incised vertically. Full-thickness skin flaps were then undermined at the level of subcutaneous fat, creating 2 triangular flaps. Adequate undermining of the surrounding subcutaneous tissue was performed to achieve proper mobilization of the flaps, which allowed for flap transposition to occur without tension and therefore for proper redirection of the scar.6 The flaps were secured using buried vertical mattress sutures and simple running sutures. Using too many buried interrupted sutures can cause vascular compromise of the fragile tips of the Z and should be avoided.3

At 4-month postoperative follow-up, the cosmetic outcome was judged satisfactory (Figure 1C).
Practice Implications
In our patient, pincushioning of the flap was easily addressed by defatting the area. However, doing just this would not have sufficed and necessitated another surgical technique—the Z-plasty—which needed to be designed carefully. The larger the angle between the side arms and central limb, the greater directional change and scar length that is gained (Figure 3). As a result, longer limbs and a greater angle could advantageously break up the scar line but consequently would lengthen the scar considerably. Therefore, if the scar was longer or the skin was inelastic, multiple Z-plasty procedures may have been preferred.

Additionally, for each central limb, both mirror-image options for peripheral arms were considered, with the optimal choice being the one that allowed for final scar lines to mimic relaxed skin-tension lines. Accuracy of the incisions was critical and was assessed by drawing a line between the free ends of the lateral limbs of the Z; this line should pass perpendicularly through the midpoint of the central limb. Last, as with other transposition flap options, Z-plasty has the potential to create a trapdoor or pincushion effect; we reduced this risk by wide undermining to establish an even contraction plate.6
When planning the revision, we considered multiple approaches to achieve the best aesthetic outcome in 1 stage. Had there been notable depression in the scar, we may have used a full-thickness skin graft. If the skin surface was lumpy and uneven, dermabrasion or a laser may have been utilized. Another consideration was to avoid using intralesional steroids, which could have made the already atrophied portions of the scar worse.
Overall, the surgical plan that we chose took into consideration the patient’s nasal anatomic structure, the combination of scar defects, the patient’s desires, and the tools available.
Final Thoughts
The ideal scar is inconspicuous, does not impair the function of surrounding structures, and blends well with adjacent skin.5 Consequently, the combination of pincushioning and webbing of a scar, especially in the nasal area, can pose a surgical challenge to the surgeon and can cause severe anxiety in the patient. In those circumstances, a single surgical technique is not likely to produce the revision with the best cosmetic outcome. Therefore, the synergy of 2 or more surgical techniques with proper planning and meticulous selection may be necessary. A broad knowledge of various scar revision techniques increases the surgeon’s capability to create the ideal scar.
Acknowledgment—The authors thank the case patient for granting permission to publish this information.
- Arginelli F, Salgarelli AC, Ferrari B, et al. Crescentic flap for the reconstruction of the nose after skin cancer resection. J Craniomaxillofac Surg. 2016;44:703-707. doi:10.1016/j.jcms.2016.02.008
- Helml G, von Gregory HF, Amr A, et al. One-stage nasal soft tissue reconstruction with local flaps. Facial Plast Surg. 2014;30:260-267. doi:10.1055/s-0034-1376871
- Woodard CR. Complications in facial flap surgery. Facial Plast Surg Clin North Am. 2013;21:599-604. doi:10.1016/j.fsc.2013.07.009
- Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001;17:263-272. doi:10.1055/s-2001-18827
- A, B, MA. Surgical principles for achieving a functional and cosmetically acceptable scar. Actas Dermosifiliogr. 2013;104:17-28. doi:10.1016/j.ad.2011.12.010
- Aasi SZ. Z-plasty made simple. Dermatol Res Pract. 2010;2010:982623. doi:10.1155/2010/982623
- Arginelli F, Salgarelli AC, Ferrari B, et al. Crescentic flap for the reconstruction of the nose after skin cancer resection. J Craniomaxillofac Surg. 2016;44:703-707. doi:10.1016/j.jcms.2016.02.008
- Helml G, von Gregory HF, Amr A, et al. One-stage nasal soft tissue reconstruction with local flaps. Facial Plast Surg. 2014;30:260-267. doi:10.1055/s-0034-1376871
- Woodard CR. Complications in facial flap surgery. Facial Plast Surg Clin North Am. 2013;21:599-604. doi:10.1016/j.fsc.2013.07.009
- Brissett AE, Sherris DA. Scar contractures, hypertrophic scars, and keloids. Facial Plast Surg. 2001;17:263-272. doi:10.1055/s-2001-18827
- A, B, MA. Surgical principles for achieving a functional and cosmetically acceptable scar. Actas Dermosifiliogr. 2013;104:17-28. doi:10.1016/j.ad.2011.12.010
- Aasi SZ. Z-plasty made simple. Dermatol Res Pract. 2010;2010:982623. doi:10.1155/2010/982623
Combatting Climate Change: 10 Interventions for Dermatologists to Consider for Sustainability
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
The impacts of anthropogenic climate change on human health are numerous and growing. The evidence that climate change is occurring due to the burning of fossil fuels is substantial, with a 2019 report elevating the data supporting anthropogenic climate change to a gold standard 5-sigma level of significance.1 In the peer-reviewed scientific literature, the consensus that humans are causing climate change is greater than 99%.2 Both the American Medical Association and the American College of Physicians have acknowledged the health impacts of climate change and importance for action. They encourage physicians to engage in environmentally sustainable practices and to advocate for effective climate change mitigation strategies.3,4 A survey of dermatologists also found that 99.3% (n=148) recognize climate change is occurring, and similarly high numbers are concerned about its health impacts.5
Notably, the health care industry must grapple not only with the health impacts of climate change but with the fact that the health care sector itself is responsible for a large amount of carbon emissions.6 The global health care industry as a whole produces enough carbon emissions to be ranked as the fifth largest emitting nation in the world.7 A quarter of these emissions are attributed to the US health care system.8,9 Climate science has shown we must limit CO2 emissions to avoid catastrophic climate change, with the sixth assessment report of the United Nations’ Intergovernmental Panel on Climate Change and the Paris Agreement targeting large emission reductions within the next decade.10 In August 2021, the US Department of Health and Human Services created the Office of Climate Change and Health Equity. Assistant Secretary for Health ADM Rachel L. Levine, MD, has committed to reducing the carbon emissions from the health care sector by 25% in the next decade, in line with scientific consensus regarding necessary changes.11
The dermatologic impacts of climate change are myriad. Rising temperatures, increasing air and water pollution, and stratospheric ozone depletion will lead to expanded geographic ranges of vector-borne diseases, worsening of chronic skin conditions such as atopic dermatitis/eczema and pemphigus, and increasing rates of skin cancer.12 For instance, warmer temperatures have allowed mosquitoes of the Aedes genus to infest new areas, leading to outbreaks of viral illnesses with cutaneous manifestations such as dengue, chikungunya, and Zika virus in previously nonindigenous regions.13 Rising temperatures also have been associated with an expanding geographic range of tick- and sandfly-borne illnesses such as Lyme disease, Rocky Mountain spotted fever, and cutaneous leishmaniasis.13,14 Additionally, short-term exposure to air pollution from wildfire smoke has been associated with an increased use of health care services by patients with atopic dermatitis.15 Increased levels of air pollutants also have been found to be associated with psoriasis flares as well as hyperpigmentation and wrinkle formation.16,17 Skin cancer incidence is predicted to rise due to increased UV radiation exposure secondary to stratospheric ozone depletion.18
Although the effects of climate change are significant and the magnitude of the climate crisis may feel overwhelming, it is essential to avoid doomerism and focus on meaningful impactful actions. Current CO2 emissions will remain in the atmosphere for hundreds to thousands of years, and the choices we make now commit future generations to live in a world shaped by our decisions. Importantly, there are impactful and low-cost, cost-effective, or cost-saving changes that can be made to mitigate the climate crisis. Herein, we provide 10 practical actionable interventions for dermatologists to help combat climate change.
10 Interventions for Dermatologists to Combat Climate Change
1. Consider switching to renewable sources of energy. Making this switch often is the most impactful decision a dermatologist can make to address climate change. The electricity sector is the largest source of greenhouse gas emissions in the US health care system, and dermatology outpatient practices in particular have been observed to have a higher peak energy consumption than most other specialties studied.19,20 Many dermatology practices—both privately owned and academic—can switch to renewable energy seamlessly through power purchase agreements (PPAs), which are contracts between power providers and private entities to install renewable energy equipment or source renewable energy from offsite sources at a fixed rate. Using PPAs instead of traditional fossil fuel energy can provide cost savings as well as protect buyers from electrical price volatility. Numerous health care systems utilize PPAs such as Kaiser Permanente, Cleveland Clinic, and Rochester Regional Health. Additionally, dermatologists can directly purchase renewable energy equipment and eventually receive a return on investment from substantially lowered electric bills. It is important to note that the cost of commercial solar energy systems has decreased 69% since 2010 with further cost reductions predicted.21,22
2. Reduce standby power consumption. This refers to the use of electricity by a device when it appears to be off or is not in use, which can lead to considerable energy consumption and subsequently a larger carbon footprint for your practice. Ensuring electronics such as phone chargers, light fixtures, television screens, and computers are switched off prior to the end of the workday can make a large difference; for instance, a single radiology department at the University of Maryland (College Park, Maryland) found that if clinical workstations were shut down when not in use after an 8-hour workday, it would save 83,866 kWh of energy and $9225.33 per year.23 Additionally, using power strips with an automatic shutoff feature to shut off power to devices not in use provides a more convenient way to reduce standby power.
3. Optimize thermostat settings. An analysis of energy consumption in 157,000 US health care facilities found that space heating and cooling accounted for 40% of their total energy consumption.24 Thus, ensuring your thermostat and heating/cooling systems are working efficiently can conserve a substantial amount of energy. For maximum efficiency, it is recommended to set air conditioners to 74 °F (24 °C) and heaters to 68 °F (20 °C) or employ smart thermostats to optimally adjust temperatures when the office is not in use.25 In addition, routinely replacing or cleaning air conditioner filters can lower energy consumption by 5% to 15%.26 Similarly, improving insulation and ruggedization of both homes and offices may reduce heating and cooling waste and limit costs and emissions as a result.
4. Offer bicycle racks and charging ports for electric vehicles. In the United States, transportation generates more greenhouse gas emissions than any other source, primarily due to the burning of fossil fuels to power automobiles, trains, and planes. Because bicycles do not consume any fossil fuels and the use of electric vehicles has been found to result in substantial air pollution health benefits, encouraging the use of both can make a considerable positive impact on our climate.27 Providing these resources not only allows those who already travel sustainably to continue to do so but also serves as a reminder to your patients that sustainability is important to you as their health care provider. As electric vehicle sales continue to climb, infrastructure to support their use, including charging stations, will grow in importance. A physician’s office that offers a car-charging station may soon have a competitive advantage over others in the area.
5. Ensure properly regulated medical waste management. Regulated medical waste (also known as infectious medical waste or red bag waste) refers to health care–generated waste unsuitable for disposal in municipal solid waste systems due to concern for the spread of infectious or pathogenic materials. This waste largely is disposed via incineration, which harms the environment in a multitude of ways—both through harmful byproducts and from the CO2 emissions required to ship the waste to special processing facilities.28 Incineration of regulated medical waste emits potent toxins such as dioxins and furans as well as particulate matter, which contribute to air pollution. Ensuring only materials with infectious potential (as defined by each state’s Environmental Protection Agency) are disposed in regulated medical waste containers can dramatically reduce the harmful effects of incineration. Additionally, limiting regulated medical waste can be very cost-effective, as its disposal is 5- to 10-times more expensive than that of unregulated medical waste.29 Simple nudge measures such as educating staff about what waste goes in which receptacle, placing signage over the red bag waste to prompt staff to pause to consider if use of that bin is required before utilizing, using weights or clasps to make opening red bag waste containers slightly harder, and positioning different trash receptacles in different parts of examination rooms may help reduce inappropriate use of red bag waste.
6. Consider virtual platforms when possible. Due to the COVID-19 pandemic, virtual meeting platforms saw a considerable increase in usage by dermatologists. Teledermatology for patient care became much more widely adopted, and traditionally in-person meetings turned virtual.30 The reduction in emissions from these changes was remarkable. A recent study looking at the environmental impact of 3 months of teledermatology visits early during the COVID-19 pandemic found that 1476 teledermatology appointments saved 55,737 miles of car travel, equivalent to 15.37 metric tons of CO2.31 Whether for patient care when appropriate, academic conferences and continuing medical education credit, or for interviews (eg, medical students, residents, other staff), use of virtual platforms can reduce unnecessary travel and therefore substantially reduce travel-related emissions. When travel is unavoidable, consider exploring validated vetted companies that offer carbon offsets to reduce the harmful environmental impact of high-emission flights.
7. Limit use of single-use disposable items. Although single-use items such as examination gloves or needles are necessary in a dermatology practice, there are many opportunities to incorporate reusable items in your workplace. For instance, you can replace plastic cutlery and single-use plates in kitchen or dining areas with reusable alternatives. Additionally, using reusable isolation gowns instead of their single-use counterparts can help reduce waste; a reusable isolation gown system for providers including laundering services was found to consume 28% less energy and emit 30% fewer greenhouse gases than a single-use isolation gown system.32 Similarly, opting for reusable instruments instead of single-use instruments when possible also can help reduce your practice’s carbon footprint. Carefully evaluating each part of your “dermatology visit supply chain” may offer opportunities to utilize additional cost-saving, environmentally friendly options; for example, an individually plastic-wrapped Dermablade vs a bulk-packaged blade for shave biopsies has a higher cost and worse environmental impact. A single gauze often is sufficient for shave biopsies, but many practices open a plastic container of bulk gauze, much of which results in waste that too often is inappropriately disposed of as regulated medical waste despite not being saturated in blood/body fluids.
8. Educate on the effects of climate change. Dermatologists and other physicians have the unique opportunity to teach members of their community every day through patient care. Physicians are trusted messengers, and appropriately counseling patients regarding the risks of climate change and its effects on their dermatologic health is in line with both American Medical Association and American College of Physicians guidelines.3,4 For instance, patients with Lyme disease in Canada or Maine were unheard of a few decades ago, but now they are common; flares of atopic dermatitis in regions adjacent to recent wildfires may be attributable to harmful particulate matter resulting from fossil-fueled climate change and record droughts. Educating medical trainees on the impacts of climate change is just as vital, as it is a topic that often is neglected in medical school and residency curricula.33
9. Install water-efficient toilets and faucets. Anthropogenic climate change has been shown to increase the duration and intensity of droughts throughout the world.34 Much of the western United States also is experiencing record droughts. One way in which dermatology practices can work to combat droughts is through the use of water-conserving toilets, faucets, and urinals. Using water fixtures with the US Environmental Protection Agency’s WaterSense label is a convenient way to do so. The WaterSense label helps identify water fixtures certified to use at least 20% less water as well as save energy and decrease water costs.
10. Advocate through local and national organizations. There are numerous ways in which dermatologists can advocate for action against climate change. Joining professional organizations focused on addressing the climate crisis can help you connect with fellow dermatologists and physicians. The Expert Resource Group on Climate Change and Environmental Issues affiliated with the American Academy of Dermatology (AAD) is one such organization with many opportunities to raise awareness within the field of dermatology. The AAD recently joined the Medical Society Consortium on Climate and Health, an organization providing opportunities for policy and media outreach as well as research on climate change. Advocacy also can mean joining your local chapter of Physicians for Social Responsibility or encouraging divestment from fossil fuel companies within your institution. Voicing support for climate change–focused lectures at events such as grand rounds and society meetings at the local, regional, and state-wide levels can help raise awareness. As the dermatologic effects of climate change grow, being knowledgeable of the views of future leaders in our specialty and country on this issue will become increasingly important.
Final Thoughts
In addition to the climate-friendly decisions one can make as a dermatologist, there are many personal lifestyle choices to consider. Small dietary changes such as limiting consumption of beef and minimizing food waste can have large downstream effects. Opting for transportation via train and limiting air travel are both impactful decisions in reducing CO2 emissions. Similarly, switching to an electric vehicle or vehicle with minimal emissions can work to reduce greenhouse gas accumulation. For additional resources, note the AAD has partnered with My Green Doctor, a nonprofit service for health care practices that includes practical cost-saving suggestions to support sustainability in physician practices.
A recent joint publication in more than 200 medical journals described climate change as the greatest threat to global public health.35 Climate change is having devastating effects on dermatologic health and will only continue to do so if not addressed now. Dermatologists have the opportunity to join with our colleagues in the house of medicine and to take action to fight climate change and mitigate the health impacts on our patients, the population, and future generations.
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
- Santer BD, Bonfils CJW, Fu Q, et al. Celebrating the anniversary of three key events in climate change science. Nat Clim Chang. 2019;9:180-182.
- Lynas M, Houlton BZ, Perry S. Greater than 99% consensus on human caused climate change in the peer-reviewed scientific literature. Environ Res Lett. 2021;16:114005.
- Crowley RA; Health and Public Policy Committee of the American College of Physicians. Climate change and health: a position paper of the American College of Physicians [published online April 19, 2016]. Ann Intern Med. 2016;164:608-610. doi:10.7326/M15-2766
- Global climate change and human health H-135.398. American Medical Association website. Updated 2019. Accessed July 13, 2022. https://policysearch.ama-assn.org/policyfinder/detail/climate%20change?uri=%2FAMADoc%2FHOD.xml-0-309.xml
- Mieczkowska K, Stringer T, Barbieri JS, et al. Surveying the attitudes of dermatologists regarding climate change. Br J Dermatol. 2022;186:748-750.
- Eckelman MJ, Sherman J. Environmental impacts of the U.S. health care system and effects on public health. PLoS One. 2016;11:e0157014. doi:10.1371/journal.pone.0157014
- Karliner J, Slotterback S, Boyd R, et al. Health care’s climate footprint: how the health sector contributes to the global climate crisis and opportunities for action. Health Care Without Harm website. Published September 2019. Accessed July 13, 2022. https://noharm-global.org/sites/default/files/documents-files/5961/HealthCaresClimateFootprint_090619.pdf
- Pichler PP, Jaccard IS, Weisz U, et al. International comparison of health care carbon footprints. Environ Res Lett. 2019;14:064004.
- Solomon CG, LaRocque RC. Climate change—a health emergency. N Engl J Med. 2019;380:209-211. doi:10.1056/NEJMp1817067
- IPCC, 2021: Summary for Policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, et al, eds. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press; 2021:3-32.
- Dzau VJ, Levine R, Barrett G, et al. Decarbonizing the U.S. Health Sector—a call to action [published online October 13, 2021]. N Engl J Med. 2021;385:2117-2119. doi:10.1056/NEJMp2115675
- Silva GS, Rosenbach M. Climate change and dermatology: an introduction to a special topic, for this special issue. Int J Womens Dermatol 2021;7:3-7.
- Coates SJ, Norton SA. The effects of climate change on infectious diseases with cutaneous manifestations. Int J Womens Dermatol. 2021;7:8-16. doi:10.1016/j.ijwd.2020.07.005
- Andersen LK, Davis MD. Climate change and the epidemiology of selected tick-borne and mosquito-borne diseases: update from the International Society of Dermatology Climate Change Task Force [published online October 1, 2016]. Int J Dermatol. 2017;56:252-259. doi:10.1111/ijd.13438
- Fadadu RP, Grimes B, Jewell NP, et al. Association of wildfire air pollution and health care use for atopic dermatitis and itch. JAMA Dermatol. 2021;157:658-666. doi:10.1001/jamadermatol.2021.0179
- Bellinato F, Adami G, Vaienti S, et al. Association between short-term exposure to environmental air pollution and psoriasis flare. JAMA Dermatol. 2022;158:375-381. doi:10.1001/jamadermatol.2021.6019
- Krutmann J, Bouloc A, Sore G, et al. The skin aging exposome [published online September 28, 2016]. J Dermatol Sci. 2017;85:152-161.
- Parker ER. The influence of climate change on skin cancer incidence—a review of the evidence. Int J Womens Dermatol. 2020;7:17-27. doi:10.1016/j.ijwd.2020.07.003
- Eckelman MJ, Huang K, Lagasse R, et al. Health care pollution and public health damage in the United States: an update. Health Aff (Millwood). 2020;39:2071-2079.
- Sheppy M, Pless S, Kung F. Healthcare energy end-use monitoring. US Department of Energy website. Published August 2014. Accessed July 13, 2022. https://www.energy.gov/sites/prod/files/2014/09/f18/61064.pdf
- Feldman D, Ramasamy V, Fu R, et al. U.S. solar photovoltaic system and energy storage cost benchmark: Q1 2020. Published January 2021. Accessed July 7, 2022. https://www.nrel.gov/docs/fy21osti/77324.pdf
- 22. Apostoleris H, Sgouridis S, Stefancich M, et al. Utility solar prices will continue to drop all over the world even without subsidies. Nat Energy. 2019;4:833-834.
- Prasanna PM, Siegel E, Kunce A. Greening radiology. J Am Coll Radiol. 2011;8:780-784. doi:10.1016/j.jacr.2011.07.017
- Bawaneh K, Nezami FG, Rasheduzzaman MD, et al. Energy consumption analysis and characterization of healthcare facilities in the United States. Energies. 2019;12:1-20. doi:10.3390/en12193775
- Blum S, Buckland M, Sack TL, et al. Greening the office: saving resources, saving money, and educating our patients [published online July 4, 2020]. Int J Womens Dermatol. 2020;7:112-116.
- Maintaining your air conditioner. US Department of Energy website. Accessed July 13, 2022. https://www.energy.gov/energysaver/maintaining-your-air-conditioner
- Choma EF, Evans JS, Hammitt JK, et al. Assessing the health impacts of electric vehicles through air pollution in the United States [published online August 25, 2020]. Environ Int. 2020;144:106015.
- Windfeld ES, Brooks MS. Medical waste management—a review [published online August 22, 2015]. J Environ Manage. 2015;1;163:98-108. doi:10.1016/j.jenvman.2015.08.013
- Fathy R, Nelson CA, Barbieri JS. Combating climate change in the clinic: cost-effective strategies to decrease the carbon footprint of outpatient dermatologic practice. Int J Womens Dermatol. 2020;7:107-111.
- Pulsipher KJ, Presley CL, Rundle CW, et al. Teledermatology application use in the COVID-19 era. Dermatol Online J. 2020;26:13030/qt1fs0m0tp.
- O’Connell G, O’Connor C, Murphy M. Every cloud has a silver lining: the environmental benefit of teledermatology during the COVID-19 pandemic [published online July 9, 2021]. Clin Exp Dermatol. 2021;46:1589-1590. doi:10.1111/ced.14795
- Vozzola E, Overcash M, Griffing E. Environmental considerations in the selection of isolation gowns: a life cycle assessment of reusable and disposable alternatives [published online April 11, 2018]. Am J Infect Control. 2018;46:881-886. doi:10.1016/j.ajic.2018.02.002
- Rabin BM, Laney EB, Philipsborn RP. The unique role of medical students in catalyzing climate change education [published online October 14, 2020]. J Med Educ Curric Dev. doi:10.1177/2382120520957653
- Chiang F, Mazdiyasni O, AghaKouchak A. Evidence of anthropogenic impacts on global drought frequency, duration, and intensity [published online May 12, 2021]. Nat Commun. 2021;12:2754. doi:10.1038/s41467-021-22314-w
- Atwoli L, Baqui AH, Benfield T, et al. Call for emergency action to limit global temperature increases, restore biodiversity, and protect health [published online September 5, 2021]. N Engl J Med. 2021;385:1134-1137. doi:10.1056/NEJMe2113200
Pigmented Papules on the Face, Neck, and Chest
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.
In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).
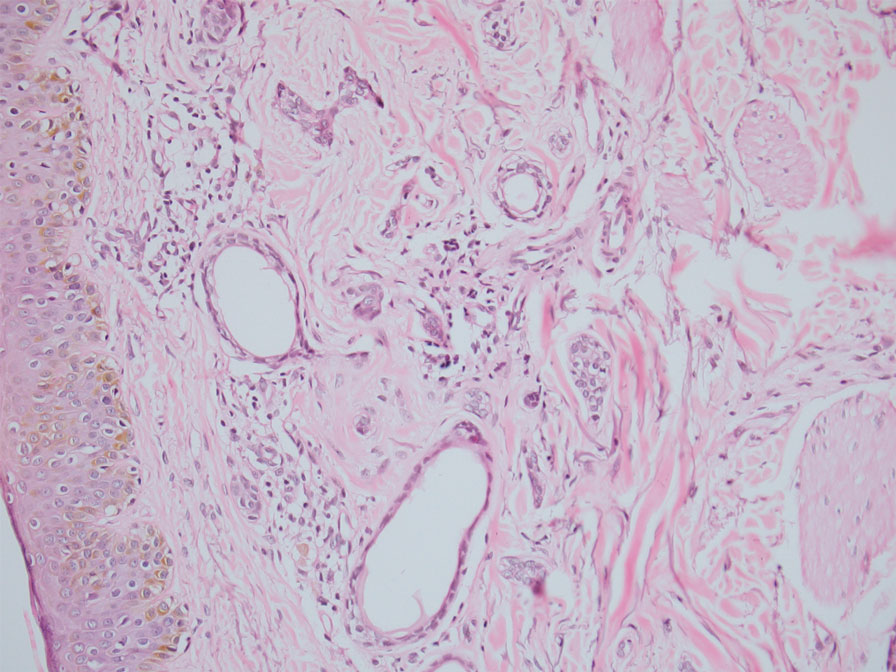
Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
The Diagnosis: Syringoma
Syringomas are benign adnexal tumors with distinct histopathologic features, including the characteristic comma- or tadpole-shaped tail comprised of dilated cystic eccrine ducts. Clinically, syringomas typically present predominantly in the periorbital region in adolescent girls. They may present as solitary or multiple lesions, and sites such as the genital area, palms, scalp, and chest rarely can be involved.1 Eruptive syringoma is a clinical subtype of syringoma that is seen on the face, neck, chest, and axillae that predominantly occurs in females with skin of color in countries such as Asia and Africa before or during puberty.2,3 Lesions appear as small, flesh-colored or slightly pigmented, flat-topped papules.3 The condition can be cosmetically disfiguring and difficult to treat, especially in patients with darker skin.

In our patient, dermoscopic evaluation revealed reticular light brown lines, structureless light brown areas, clustered brown dots, globules, and reticular vessels on a faint background (Figure 1A). Glittering yellow-whitish round structures over a fading pink-brown background also were seen at some sites (Figure 1B). Histologic examination of a neck lesion revealed an epidermis with focal acanthosis; the upper dermis had tumor islands and ducts with cells with round to vesicular nuclei and eosinophilic cytoplasm. A well-circumscribed tumor in the dermis composed of tubules of varying sizes lined by cuboidal cells was seen, consistent with syringoma (Figure 2).

Dermoscopic features of syringomas have not been widely studied. Hayashi et al4 reported the dermoscopic features of unilateral linear syringomas as a delicate and faint reticular pigmentation network and multiple hypopigmented areas. Sakiyama et al5 also defined an incomplete pigment network with faint erythema in 2 eruptive syringoma cases.
Treatment of this condition is for cosmetic reasons only, and there are no reports of long-term morbidity associated with the disease.6,7 Multiple therapeutic options are available but are associated with complications such as hyperpigmentation and sclerosis in patients with skin of color due to the dermal location of these syringomas. Management of syringomas includes topical and surgical methods, including topical retinoids such as tretinoin and atropine solution 1%; surgical methods include dermabrasion, excision, cryotherapy, electrocautery, electrofulguration, laser therapy, and chemical cautery. However, there is a substantial risk for recurrence with these treatment options. In a case series of 5 patients with periorbital syringomas, treatment using radiofrequency and a CO2 laser was performed with favorable outcomes, highlighting the use of combination therapies for treatment.8 Seo et al9 reported a retrospective case series of 92 patients with periorbital syringomas in which they treated one group with CO2 laser and the other with botulinum toxin A injection; CO2 laser combined with botulinum toxin A showed a greater effect than laser treatment alone. The differential diagnosis includes pigmented plane warts, sebaceous hyperplasia, eruptive xanthomas, and hidrocystomas. Pigmented plane warts characteristically present as flat-topped papules with small hemorrhagic dots or tiny pinpoint vessels on dermoscopy. In sebaceous hyperplasia, yellowish umbilicated papular lesions are seen with crown vessels on dermoscopy. Eruptive xanthomas usually are erythematous to yellow, dome-shaped papules that appear mainly over the extensor aspects of the extremities. Hidrocystoma presents as a solitary translucent larger syringomalike lesion commonly seen in the periorbital region and/or on the cheeks.
We report a case of widespread syringomas with multiple close mimickers such as pigmented plane warts; however, dermoscopy of the lesions helped to arrive at the diagnosis. Dermatologists should be aware of this condition and its benign nature to ensure correct diagnosis and appropriate treatment.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
- Williams K, Shinkai K. Evaluation and management of the patient with multiple syringomas: a systematic review of the literature. J Am Acad Dermatol. 2016;74:1234.e9-1240.e9.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Singh S, Tewari R, Gupta S. An unusual case of generalised eruptive syringoma in an adult male. Med J Armed Forces India. 2014;70:389-391.
- Hayashi Y, Tanaka M, Nakajima S, et al. Unilateral linear syringoma in a Japanese female: dermoscopic differentiation from lichen lanus linearis. Dermatol Rep. 2011;3:E42.
- Sakiyama M, Maeda M, Fujimoto N, et al. Eruptive syringoma localized in intertriginous areas. J Dtsch Dermatol Ges. 2014;12:72-73.
- Wang JI, Roenigk HH Jr. Treatment of multiple facial syringomas with the carbon dioxide (CO2) laser. Dermatol Surg. 1999;25:136-139.
- Tsunemi Y, Ihn H, Saeki H, et al. Generalized eruptive syringoma. Pediatr Dermatol. 2005;22:492-493.
- Hasson A, Farias MM, Nicklas C, et al. Periorbital syringoma treated with radiofrequency and carbon dioxide (CO2) laser in 5 patients. J Drugs Dermatol. 2012;11:879-880.
- Seo HM, Choi JY, Min J, et al. Carbon dioxide laser combined with botulinum toxin A for patients with periorbital syringomas [published online March 31, 2016]. J Cosmet Laser Ther. 2016;18:149-153.
A 46-year-old woman presented with multiple asymptomatic, flesh-colored, hyperpigmented papules on the face of 5 to 6 months’ duration that were progressively increasing in number. The lesions first appeared near the eyebrows and cheeks (top) and subsequently spread to involve the neck. She had no notable medical history. Cutaneous examination revealed multiple tan to brown papules over the periorbital, malar, and neck regions ranging in size from 1 to 5 mm. The lesions over the periorbital region were arranged in a linear pattern (bottom). Similar lesions also were present on the chest and arms. No other sites were involved, and systemic examination was normal.
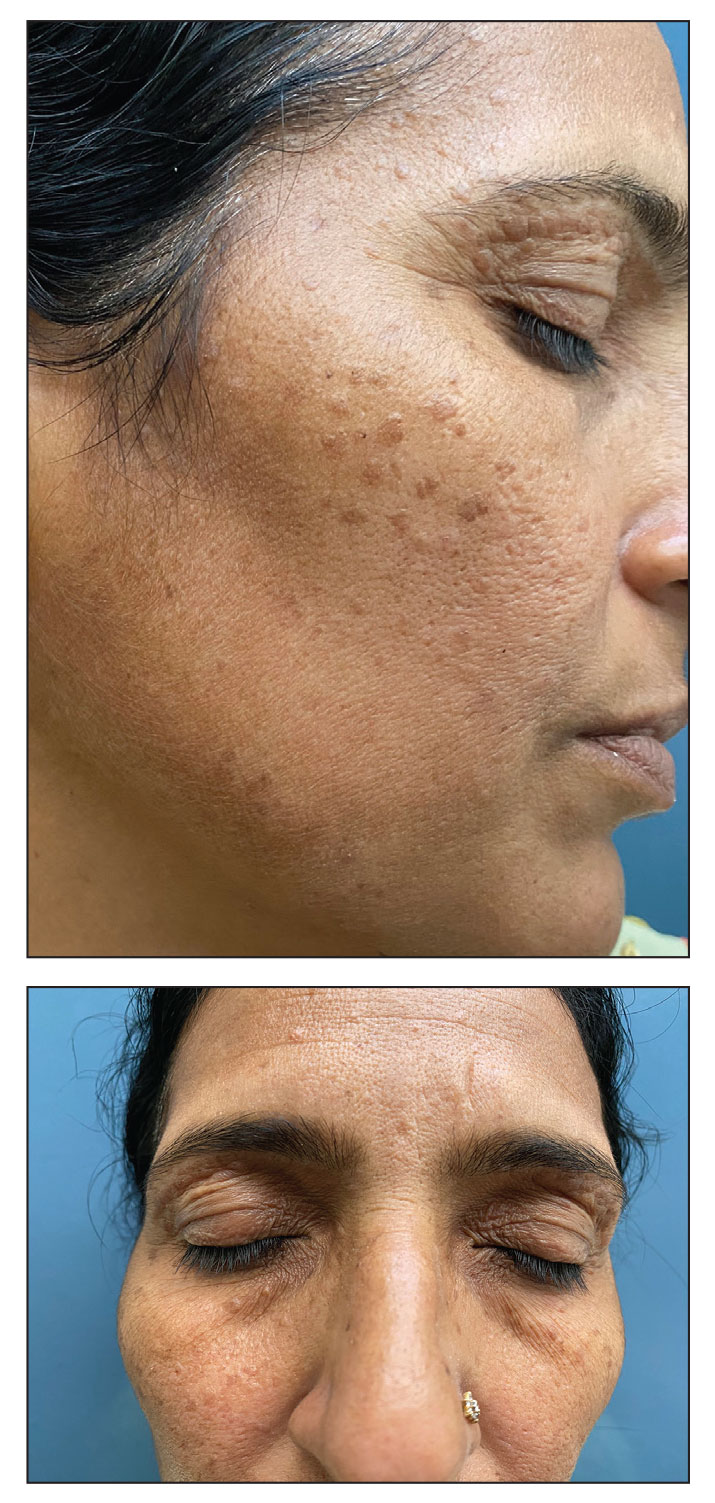
Nail Changes Associated With Thyroid Disease
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.
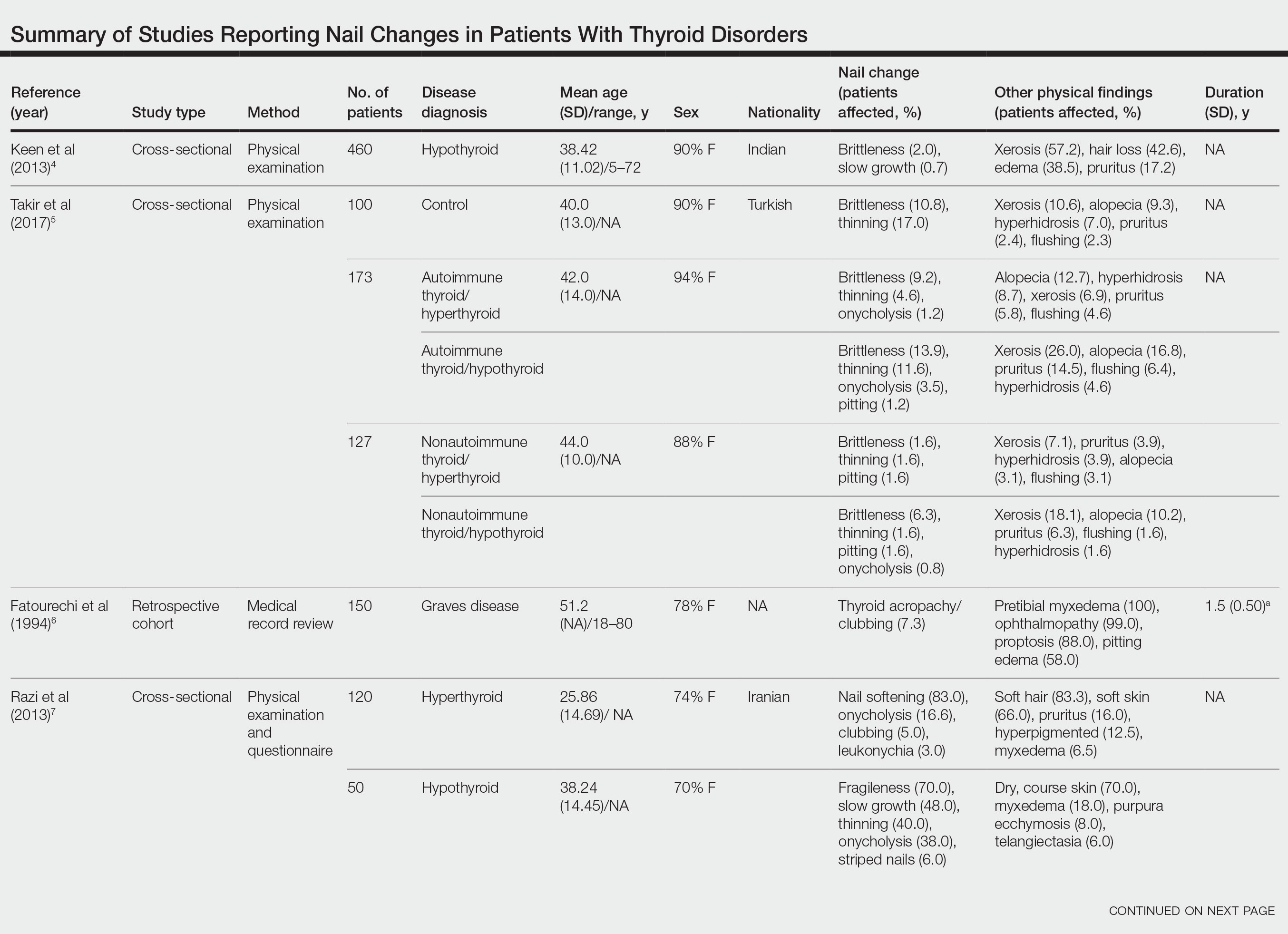
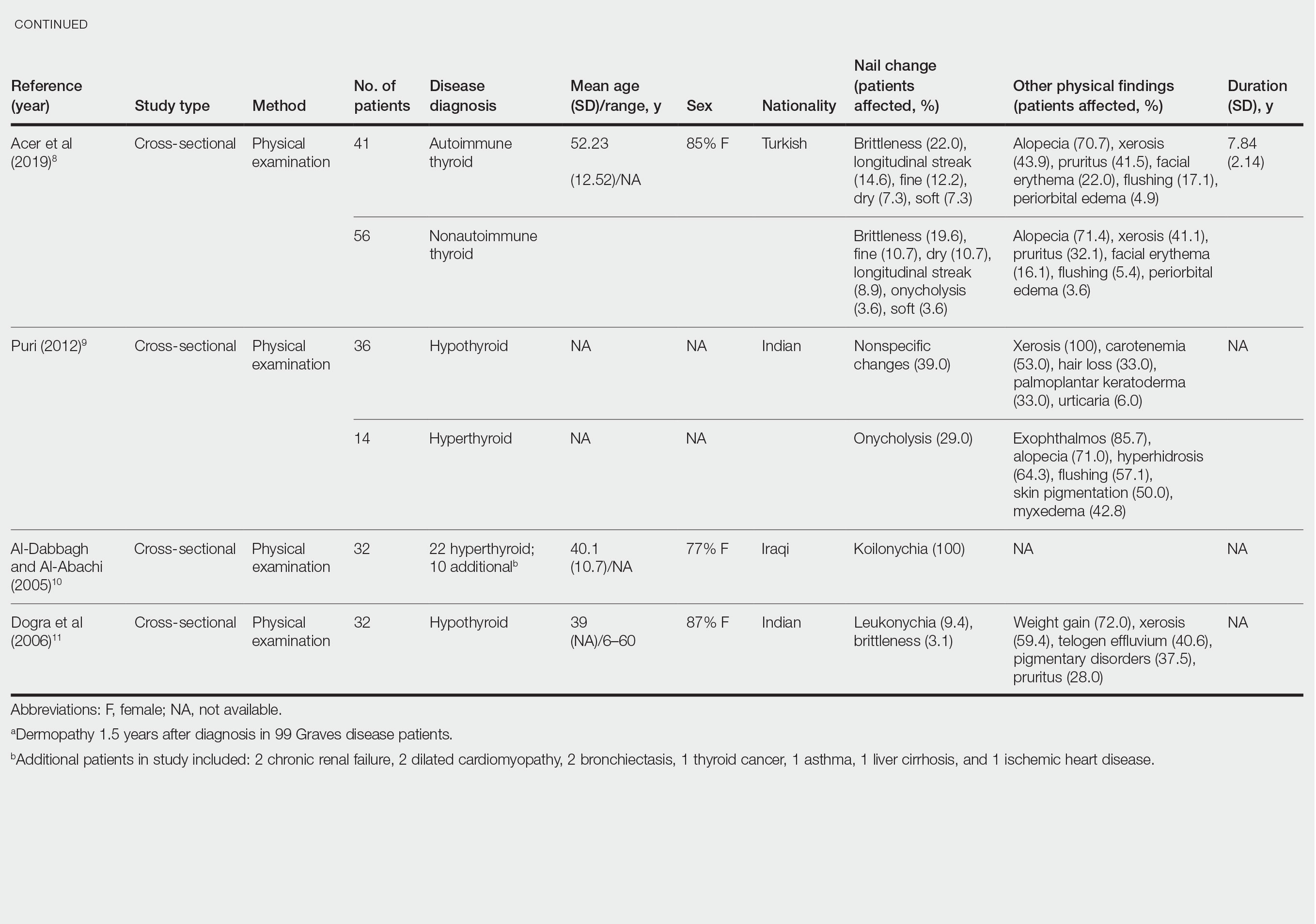
Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
The major classifications of thyroid disease include hyperthyroidism, which is seen in Graves disease, and hypothyroidism due to iodine deficiency and Hashimoto thyroiditis, which have potentially devastating health consequences. The prevalence of hyperthyroidism ranges from 0.2% to 1.3% in iodine-sufficient parts of the world, and the prevalence of hypothyroidism in the general population is 5.3% in Europe and 3.7% in the United States.1 Thyroid hormones physiologically potentiate α- and β-adrenergic receptors by increasing their sensitivity to catecholamines. Excess thyroid hormones manifest as tachycardia, increased cardiac output, increased body temperature, hyperhidrosis, and warm moist skin. Reduced sensitivity of adrenergic receptors to catecholamines from insufficient thyroid hormones results in a lower metabolic rate and decreases response to the sympathetic nervous system.2 Nail changes in thyroid patients have not been well studied.3 Our objectives were to characterize nail findings in patients with thyroid disease. Early diagnosis of thyroid disease and prompt referral for treatment may be instrumental in preventing serious morbidities and permanent sequelae.
Methods
PubMed, Scopus, Web of Science, and Google Scholar were searched for the terms nail + thyroid, nail + hyperthyroid, nail + hypothyroid, nail + Graves, and nail + Hashimoto on June 10, 2020, and then updated on November 18, 2020. All English-language articles were included. Non–English-language articles and those that did not describe clinical trials of nail changes in patients with thyroid disease were excluded. One study that utilized survey-based data for nail changes without corroboration with physical examination findings was excluded. Hypothyroidism/hyperthyroidism was defined by all authors as measurement of serum thyroid hormones triiodothyronine, thyroxine, and thyroid-stimulating hormone outside of the normal range. Eight studies were included in the final analysis. Patient demographics, thyroid disease type, physical examination findings, nail clinical findings, age at diagnosis, age at onset of nail changes, treatments/medications, and comorbidities were recorded and analyzed.
Results
Nail changes in patients with thyroid disease were reported in 8 studies (7 cross-sectional, 1 retrospective cohort) and are summarized in the Table.4-11 The mean age was 41.2 years (range, 5–80 years), with a higher representation of females (range, 70%–94% female). The most common nail changes in thyroid patients were koilonychia, clubbing, and nail brittleness. Other changes included onycholysis, thin nails, dryness, and changes in nail growth rate. Frequent physical findings were xerosis, pruritus, and alopecia.


Both koilonychia and clubbing were reported in patients with hyperthyroidism. In a study of 32 patients with koilonychia, 22 (68.8%) were diagnosed with hyperthyroidism.10 Nail clubbing affected 7.3% of Graves disease patients (n=150)6 and 5.0% of hyperthyroid patients (n=120).7 Dermopathy presented more than 1 year after diagnosis of Graves disease in 99 (66%) of 150 patients as a late manifestation of thyrotoxicosis.6 Additional physical features in patients with Graves disease (n=150) were pretibial myxedema (100%), ophthalmopathy (99.0%), and proptosis (88.0%). Non–Graves hyperthyroid patients showed physical features of soft hair (83.3%) and soft skin (66.0%).7
Nail brittleness was a frequently reported nail change in thyroid patients (4/8 studies, 50%), most often seen in 22% of autoimmune patients, 19.6% of nonautoimmune patients, 13.9% of hypothyroid patients, and 9.2% of hyperthyroid patients.5,8 For comparison, brittle nails presented in 10.8% of participants in a control group.5 Brittle nails in thyroid patients often are accompanied by other nail findings such as thinning, onycholysis, and pitting.
Among hypothyroid patients, nail changes included fragility (70%; n=50), slow growth (48%; n=50), thinning (40%; n=50), onycholysis (38%; n=50),7 and brittleness (13.9%; n=173).5 Less common nail changes in hypothyroid patients were leukonychia (9.4%; n=32), striped nails (6%; n=50), and pitting (1.2%; n=173).5,7,11 Among hyperthyroid patients, the most common nail changes were koilonychia (100%; n=22), softening (83%; n=120), onycholysis (29%; n=14), and brittleness (9.2%; n=173).5,7,9,10 Less common nail changes in hyperthyroid patients were clubbing (5%; n=120), thinning (4.6%; n=173), and leukonychia (3%; n=120).5,7
Additional cutaneous findings of thyroid disorder included xerosis, alopecia, pruritus, and weight change. Xerosis was most common in hypothyroid disease (57.2%; n=460).4 In 2 studies,8,9 alopecia affected approximately 70% of autoimmune, nonautoimmune, and hyperthyroid patients. Hair loss was reported in 42.6% (n=460)4 and 33.0% (n=36)9 of hypothyroid patients. Additionally, pruritus affected up to 28% (n=32)11 of hypothyroid and 16.0% (n=120)7 of hyperthyroid patients and was more common in autoimmune (41%) vs nonautoimmune (32%) thyroid patients.8 Weight gain was seen in 72% of hypothyroid patients (n=32),11 and soft hair and skin were reported in 83.3% and 66% of hyperthyroid patients (n=120), respectively.7 Flushing was a less common physical finding in thyroid patients (usually affecting <10%); however, it also was reported in 17.1% of autoimmune and 57.1% of hyperthyroid patients from 2 separate studies.8,9
Comment
There are limited data describing nail changes with thyroid disease. Singal and Arora3 reported in their clinical review of nail changes in systemic disease that koilonychia, onycholysis, and melanonychia are associated with thyroid disorders. We similarly found that koilonychia and onycholysis are associated with thyroid disorders without an association with melanonychia.
In his clinical review of thyroid hormone action on the skin, Safer12 described hypothyroid patients having coarse, dull, thin, and brittle nails, whereas in thyrotoxicosis, patients had shiny, soft, and concave nails with onycholysis; however, the author commented that there were limited data on the clinical findings in thyroid disorders. These nail findings are consistent with our results, but onycholysis was more common in hypothyroid patients than in hyperthyroid patients in our review. Fox13 reported on 30 cases of onycholysis, stating that it affected patients with hypothyroidism and improved with thyroid treatment. In a clinical review of 8 commonly seen nail abnormalities, Fowler et al14 reported that hyperthyroidism was associated with nail findings in 5% of cases and may result in onycholysis of the fourth and fifth nails or all nails. They also reported that onychorrhexis may be seen in patients with hypothyroidism, a finding that differed from our results.14
The mechanism of nail changes in thyroid disease has not been well studied. A protein/amino acid–deficiency state may contribute to the development of koilonychia. Hyperthyroid patients, who have high metabolic activity, may have hypoalbuminemia, leading to koilonychia.15 Hypothyroidism causes hypothermia from decreased metabolic rate and secondary compensatory vasoconstriction. Vasoconstriction decreases blood flow of nutrients and oxygen to cutaneous structures and may cause slow-growing, brittle nails. In hyperthyroidism, vasodilation alternatively may contribute to the fast-growing nails. Anti–thyroid-stimulating hormone receptor antibodies in Graves disease may increase the synthesis of hyaluronic acid and glycosaminoglycans from fibroblasts, keratinocytes, adipocytes, or endothelial cells in the dermis and may contribute to development of clubbing.16
Our review is subject to several limitations. We recorded nail findings as they were described in the original studies; however, we could not confirm the accuracy of these descriptions. In addition, some specific nail changes were not described in sufficient detail. In all but 1 study, dermatologists performed the physical examination. In the study by Al-Dabbagh and Al-Abachi,10 the physical examinations were performed by general medicine physicians, but they selected only for patients with koilonychia and did not assess for other skin findings. Fragile nails and brittle nails were described in hypothyroid and hyperthyroid patients, but these nail changes were not described in detail. There also were studies describing nail changes in thyroid patients; some studies had small numbers of patients, and many did not have a control group.
Conclusion
Nail changes may be early clinical presenting signs of thyroid disorders and may be the clue to prompt diagnosis of thyroid disease. Dermatologists should be mindful that fragile, slow-growing, thin nails and onycholysis are associated with hypothyroidism and that koilonychia, softening, onycholysis, and brittle nail changes may be seen in hyperthyroidism. Our review aimed to describe nail changes associated with thyroid disease to guide dermatologists on diagnosis and promote future research on dermatologic manifestations of thyroid disease. Future research is necessary to explore the association between koilonychia and hyperthyroidism as well as the association of nail changes with thyroid disease duration and severity.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
- Taylor PN, Albrecht D, Scholz A, et al. Global epidemiology of hyperthyroidism and hypothyroidism. Nat Rev Endocrinol. 2018;14:301-316.
- Lause M, Kamboj A, Faith EF. Dermatologic manifestations of endocrine disorders. Transl Pediatr. 2017;6:300-312.
- Singal A, Arora R. Nail as a window of systemic diseases. Indian Dermatol Online J. 2015;6:67-74.
- Keen MA, Hassan I, Bhat MH. A clinical study of the cutaneous manifestations of hypothyroidism in Kashmir Valley. Indian J Dermatol. 2013;58:326.
- Takir M, Özlü E, Köstek O, et al. Skin findings in autoimmune and nonautoimmune thyroid disease with respect to thyroid functional status and healthy controls. Turk J Med Sci. 2017;47:764-770.
- Fatourechi V, Pajouhi M, Fransway AF. Dermopathy of Graves disease (pretibial myxedema). review of 150 cases. Medicine (Baltimore). 1994;73:1-7.
- Razi A, Golforoushan F, Nejad AB, et al. Evaluation of dermal symptoms in hypothyroidism and hyperthyroidism. Pak J Biol Sci. 2013;16:541-544.
- Acer E, Ag˘aog˘lu E, Yorulmaz G, et al. Evaluation of cutaneous manifestations in patients under treatment with thyroid disease. Turkderm-Turk Arch Dermatol Venereol. 2019;54:46-50.
- Puri N. A study on cutaneous manifestations of thyroid disease. Indian J Dermatol. 2012;57:247-248.
- Al-Dabbagh TQ, Al-Abachi KG. Nutritional koilonychia in 32 Iraqi subjects. Ann Saudi Med. 2005;25:154-157.
- Dogra A, Dua A, Singh P. Thyroid and skin. Indian J Dermatol. 2006;51:96-99.
- Safer JD. Thyroid hormone action on skin. Dermatoendocrinol. 2011;3:211-215.
- Fox EC. Diseases of the nails: report of cases of onycholysis. Arch Derm Syphilol. 1940;41:98-112.
- Fowler JR, Stern E, English JC 3rd, et al. A hand surgeon’s guide to common onychodystrophies. Hand (N Y). 2014;9:24-28.
- Truswell AS. Nutritional factors in disease. In: Edwards CRW, Bouchier IAD, Haslett C, et al, eds. Davidson’s Principles and Practice of Medicine. 17th ed. Churchill Livingstone; 1995:554.
- Heymann WR. Cutaneous manifestations of thyroid disease. J Am Acad Dermatol. 1992;26:885-902.
Practice Points
- Koilonychia is associated with hyperthyroidism.
- Clubbing is a manifestation of thyroid acropachy in Graves disease and also affects other patients with hyperthyroidism.
- Onycholysis improves in patients with hypothyroidism treated with thyroid hormone replacement therapy.