User login
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
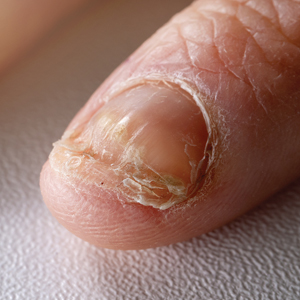
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
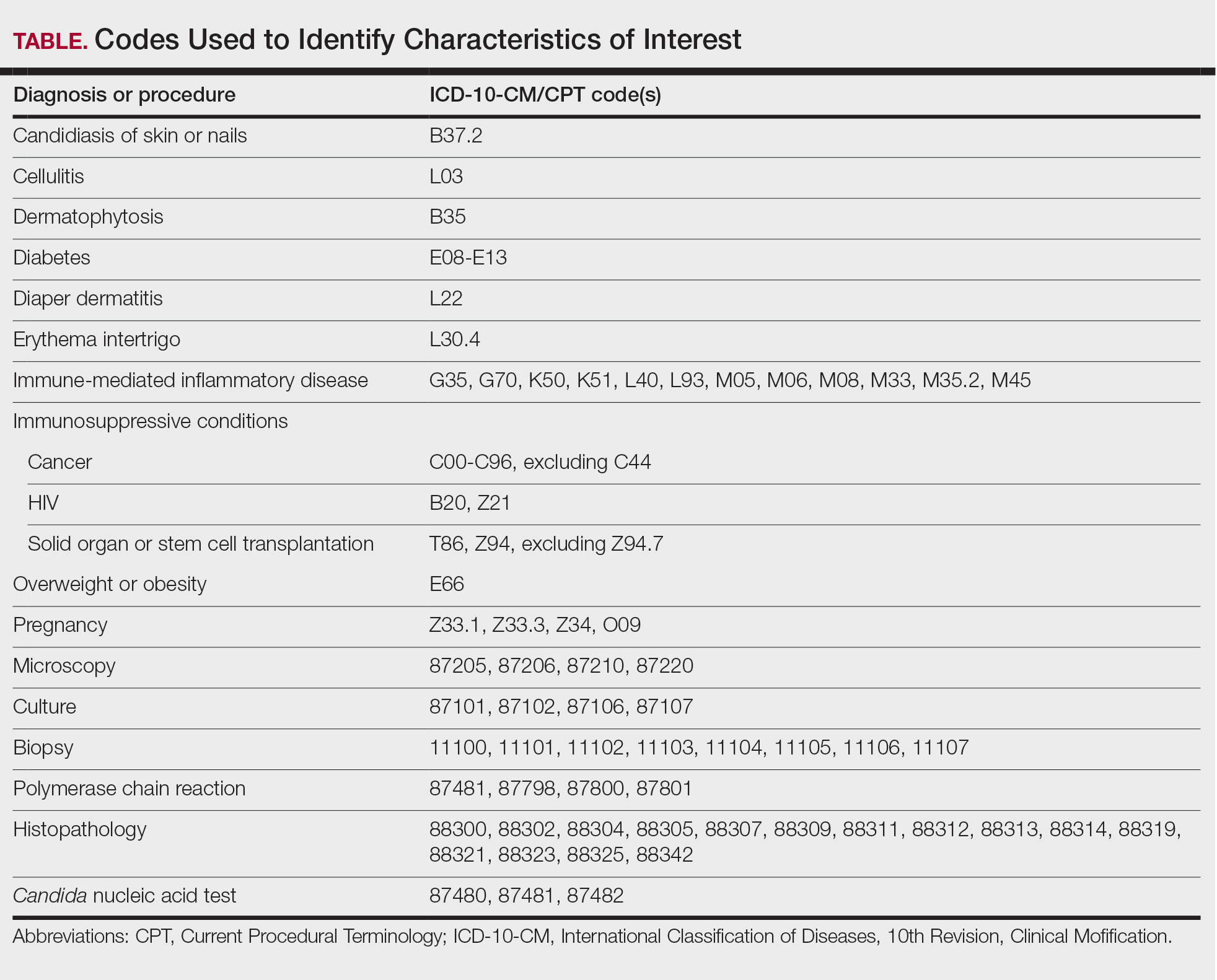
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.
Results
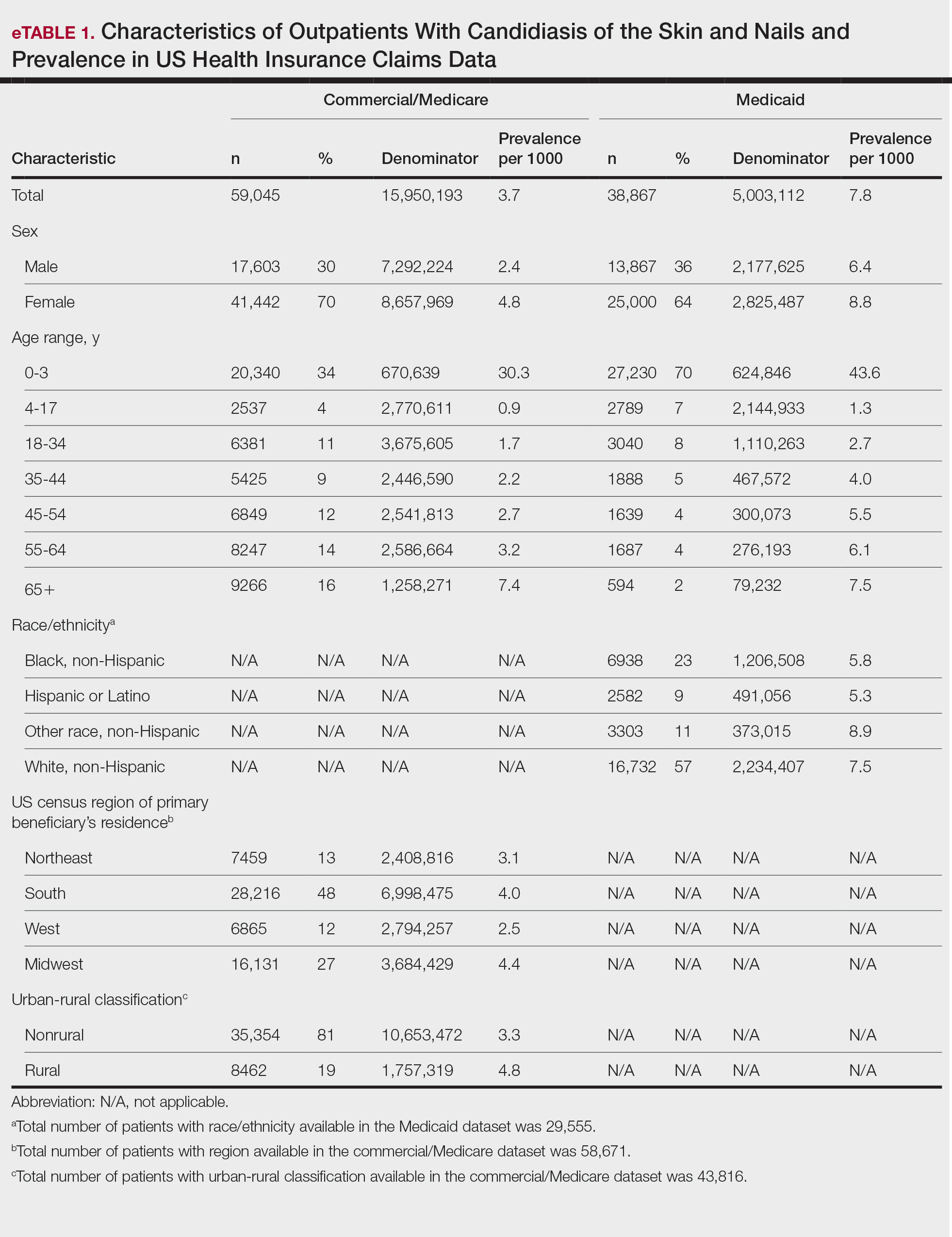
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).
Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).

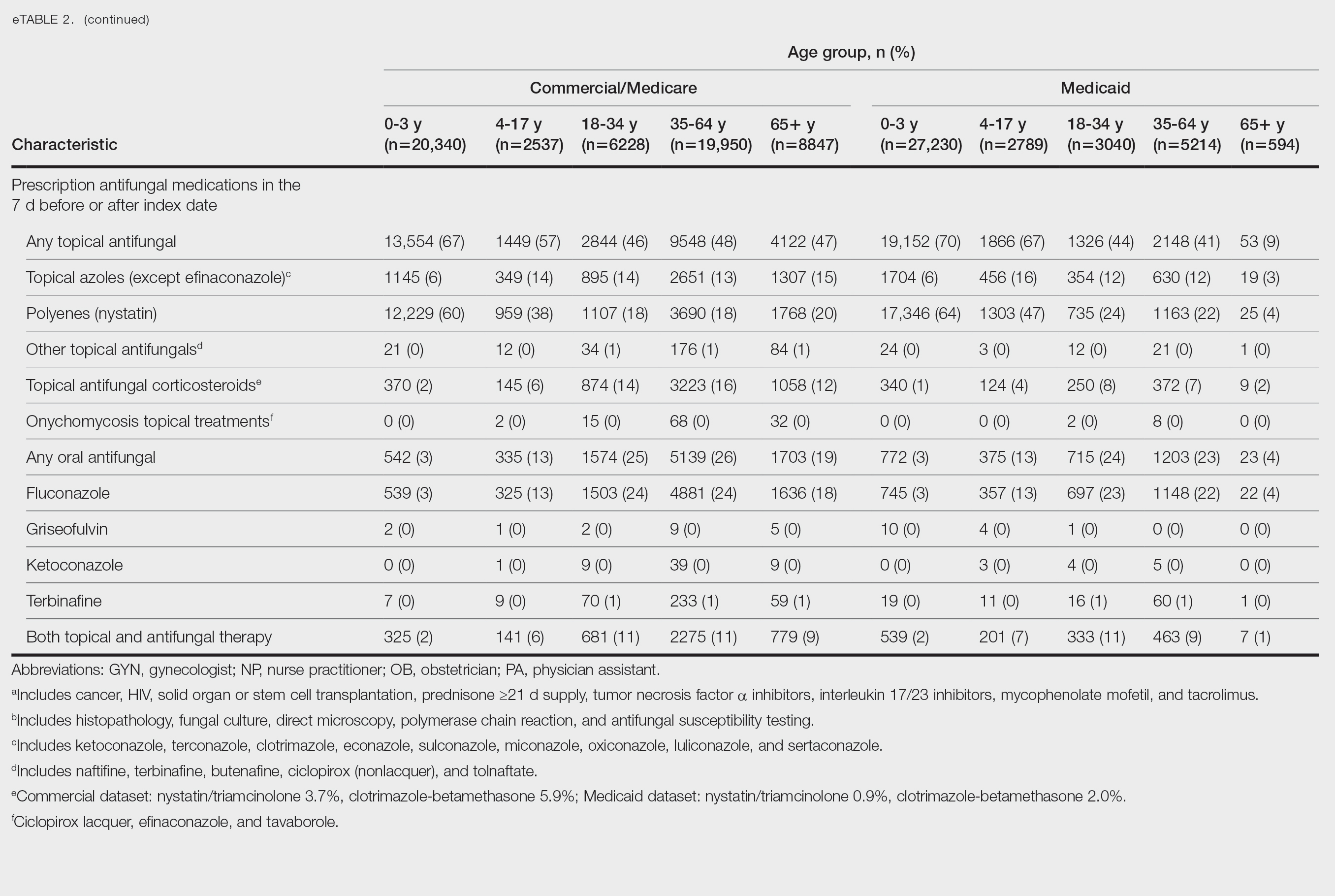
Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
Candida is a common commensal organism of human skin and mucous membranes. Candidiasis of the skin and nails is caused by overgrowth of Candida species due to excess skin moisture, skin barrier disruption, or immunosuppression. Candidiasis of the skin manifests as red, moist, itchy patches that develop particularly in skin folds. Nail involvement is associated with onycholysis (separation of the nail plate from the nail bed) and subungual debris.1 Data on the prevalence of candidiasis of the skin and nails in the United States are scarce. In this study, we evaluated the prevalence, characteristics, and treatment practices of candidiasis of the skin and nails using data from 2 large US health insurance claims databases.
Methods
We used the 2023 Merative MarketScan Commercial, Medicare Supplemental, and Multi-State Medicaid Databases (https://www.merative.com/documents/merative-marketscan-research-databases) to identify outpatients with the International Classification of Diseases, 10th Revision, Clinical Modification (ICD-10-CM) code B37.2 for candidiasis of the skin and nails. The Commercial and Medicare Supplemental databases include health insurance claims data submitted by large employers and health plans for more than 19 million patients throughout the United States, and the Multi-State Medicaid database includes similar data from more than 5 million patients across several geographically dispersed states. The index date for each patient corresponded with their first qualifying diagnosis of skin and nail candidiasis during January 1, 2023, to December 31, 2023. Inclusion in the study required continuous insurance enrollment from 30 days prior to 7 days after the index date, resulting in exclusion of 7% of commercial/Medicare patients and 8% of Medicaid patients. Prevalence per 1000 outpatients was calculated, with stratification by demographic characteristics.
We examined selected diagnoses made on or within 30 days before the index date, diagnostic testing performed within the 7 days before or after the index date after using specific Current Procedural Terminology codes, and outpatient antifungal and combination antifungal-corticosteroid prescriptions made within 7 days before or after the index date (Table). Race/ethnicity data are unavailable in the commercial/Medicare database, and geographic data are unavailable in the Medicaid database.

Results
The prevalence of skin and nail candidiasis was 3.7 per 1000 commercial/Medicare outpatients and 7.8 per 1000 Medicaid outpatients (eTable 1). Prevalence was highest among patients aged 0 to 3 years (commercial/Medicare, 30.3 per 1000; Medicaid, 43.6 per 1000), followed by patients 65 years or older (commercial/Medicare, 7.4 per 1000; Medicaid, 7.5 per 1000). Prevalence was higher among females compared with males (commercial/Medicare, 4.8 vs 2.4 per 1000, respectively; Medicaid, 8.8 vs 6.4 per 1000, respectively). Among Medicaid patients, prevalence was highest among those of other race, non-Hispanic (8.9 per 1000) and White non-Hispanic patients (7.5 per 1000). In the commercial/Medicare dataset, prevalence was highest in patients residing in the Midwest (4.4 per 1000) and the South (4.0 per 1000).

Diaper dermatitis was listed as a concurrent diagnosis among 51% of patients aged 0 to 3 years in both datasets (eTable 2). Diabetes (commercial/Medicare, 32%; Medicaid, 36%) and immunosuppressive conditions (commercial/Medicare, 10%; Medicaid, 7%) were most frequent among patients aged 65 years or older. Obesity was most commonly listed as a concurrent diagnosis among patients aged 35 to 64 years (commercial/Medicare, 17%; Medicaid, 23%).


Patients aged 18 to 34 years had the highest rates of diagnostic testing in the 7 days before or after the index date (commercial/Medicare, 9%; Medicaid, 10%). Topical antifungal medications (primarily nystatin) were most frequently prescribed for patients aged 0 to 3 years (commercial/Medicare, 67%; Medicaid, 70%). Topical combination antifungal-corticosteroid medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (16%) and for patients aged 18 to 34 years in the Medicaid dataset (8%). Topical onychomycosis treatments were prescribed for fewer than 1% of patients in both datasets. Oral antifungal medications were most frequently prescribed for patients aged 35 to 64 years in the commercial/Medicare dataset (26%) and for patients aged 18 to 34 years in the Medicaid dataset (24%). Fewer than 11% of patients across all age groups in both datasets were prescribed both topical and oral antifungal medications.
Comment
Our analysis provides preliminary insight into the prevalence of skin and nail candidiasis in the United States based on health insurance claims data. Higher prevalence of skin and nail candidiasis among patients with Medicaid compared with those with commercial/Medicare health insurance is consistent with previous studies showing increased rates of other superficial fungal infections (eg, dermatophytosis) among patients of lower socioeconomic status.2 This finding could reflect differences in underlying health status or reduced access to health care, which could delay treatment or follow-up care and potentially lead to prolonged exposure to conditions favoring the development of candidiasis.
In both the commercial/Medicare health insurance and Medicaid datasets, prevalence of diagnosis codes for candidiasis of the skin and nails was highest among infants and toddlers. Diaper dermatitis also was observed in more than half of patients aged 0 to 3 years; this is a well-established risk factor for cutaneous candidiasis, as immature skin barrier function and prolonged exposure to moisture and occlusion facilitate fungal overgrowth.3 In adults, diabetes and obesity were among the most frequent comorbidities observed; both conditions are recognized risk factors for superficial candidiasis due to their impact on immune function and skin integrity.4
In both study cohorts, diagnostic testing in the 7 days before or after the index date was infrequent (≤10%), consistent with most cases being diagnosed clinically.5 Topical antifungals, especially nystatin, were most frequently prescribed for young children, while oral antifungals were more frequently prescribed for adults; nystatin is one of the most well-studied topical treatments for cutaneous candidiasis, and oral fluconazole is the primary systemic treatment for cutaneous candidiasis.1 In our study, the ICD-10-CM code B37.2 appeared to be used primarily for diagnosis of skin rather than nail infections based on the low proportions of patients who received treatment that was onychomycosis specific.
Our study was limited by potential misclassification inherent to data based on diagnosis codes; incomplete capture of underlying conditions given the short continuous enrollment criteria; and lack of information about affected body site(s) and laboratory results, including data identifying the Candida species. A previous study found that Candida parapsilosis and Candida albicans were the most common species involved in candidiasis of the skin and nails and that one-third of isolates exhibited low sensitivity to commonly used antifungals.6 For nails, Candida species are sometimes contaminants rather than pathogens.
Conclusion
Our findings provide a baseline understanding of the epidemiology of candidiasis of the skin and nails in the United States. The growing threat of antifungal resistance, particularly among non-albicans Candida species, underscores the need for appropriate use of antifungals.7 Future epidemiologic studies about laboratory-confirmed candidiasis of the skin and nails to understand causative species and drug resistance would be useful, as would further investigation into disparities.
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
- Taudorf EH, Jemec GBE, Hay RJ, et al. Cutaneous candidiasis—an evidence-based review of topical and systemic treatments to inform clinical practice. J Eur Acad Dermatol Venereol. 2019;33:1863-1873. doi:10.1111/jdv.15782
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. eClinicalMedicine. 2023;66:102325. doi:10.1016/j.eclinm.2023.102325
- Benitez Ojeda AB, Mendez MD. Diaper dermatitis. StatPearls [Internet]. Updated July 3, 2023. Accessed January 14, 2026. https://www.ncbi.nlm.nih.gov/books/NBK559067/
- Shahabudin S, Azmi NS, Lani MN, et al. Candida albicans skin infection in diabetic patients: an updated review of pathogenesis and management. Mycoses. 2024;67:E13753. doi:10.1111/myc.13753
- Kalra MG, Higgins KE, Kinney BS. Intertrigo and secondary skin infections. Am Fam Physician. 2014;89:569-573.
- Ranđelovic M, Ignjatovic A, Đorđevic M, et al. Superficial candidiasis: cluster analysis of species distribution and their antifungal susceptibility in vitro. J Fungi (Basel). 2025;11:338.
- Hay R. Therapy of skin, hair and nail fungal infections. J Fungi (Basel). 2018;4:99. doi:10.3390/jof4030099
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Retrospective Analysis of Prevalence and Treatment Patterns of Skin and Nail Candidiasis From US Health Insurance Claims Data
Practice Points
- Candidiasis of the skin or nails is a common outpatient condition that is most frequently diagnosed in infants, toddlers, and adults aged 65 years or older.
- Most cases are diagnosed clinically without diagnostic testing and treated with topical antifungals, but increased attention to formal diagnosis and treatment may be warranted given the emergence of antifungal-resistant Candida species.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
With increasing reports of terbinafine resistance, how has your strategy for treating dermatophyte onychomycosis evolved?
DR. LIPNER: Most cases of onychomycosis are not resistant to terbinafine, so for a patient newly diagnosed with onychomycosis, my approach involves evaluating the severity of disease, number of nails affected, comorbid conditions, and concomitant medications and then discussing the risks and benefits of oral vs topical treatment. If a patient’s onychomycosis previously did not resolve with oral terbinafine, I would test for terbinafine resistance. If positive, I would treat with itraconazole for more severe cases and efinaconazole for mild to moderate cases.
Are there any new systemic or topical antifungals for onychomycosis that dermatologists should be aware of?
DR. LIPNER: There have been no new US Food and Drug Administration–approved antifungals for onychomycosis since 2014 (efinaconazole and tavaborole). For most patients, our current antifungals generally have good efficacy. For treatment failures, I would recommend reconfirming the diagnosis and testing for terbinafine resistance.
When do you choose oral antifungal therapy vs topical/combination therapy?
DR. LIPNER: almost never prescribe combination antifungal therapy because monotherapy alone is usually effective, and there is no obvious benefit to combination therapy. If treatment is working (or not working), it is hard to know which agent (if any) is effective. The one time I would use combination therapy (eg, oral terbinafine and topical efinaconazole) would be if the patient has distal lateral subungual onychomycosis and a dermatophytoma. Oral terbinafine would generally be most effective for distal lateral subungual onychomycosis, and topical efinaconazole would likely be most effective for dermatophytoma.
What is the role of adjunctive therapies in onychomycosis?
DR. LIPNER: Debridement can be effective for patients with very thick nails, combined with oral or topical antifungals. Nail avulsion generally is not helpful and should be avoided because it causes permanent shortening of the nail bed. Devices (eg, lasers, photodynamic therapy) are not subject to the same stringent endpoints as medication-based approvals. Because studies to date are small and have different efficacy endpoints, I do not use devices for treatment of onychomycosis.
How do you counsel patients about expectations and timelines for onychomycosis therapy and cure vs improvement?
DR. LIPNER: Oral treatments for toenail onychomycosis are generally given for 3-month courses, but patients should be counseled that the nail could take up to 12 to 18 months to fully grow out and look normal. If patients also have mechanical nail dystrophy, the fungus may be cured with antifungal therapy, but the nail may look better but not perfect, so it is important to manage long-term expectations.
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Treating Dermatophyte Onychomycosis: Clinical Insights From Dr. Shari R. Lipner
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
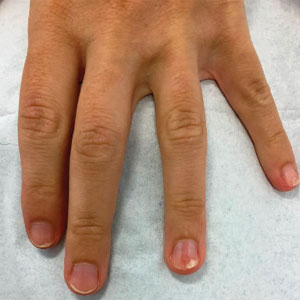
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
Practice Gap
Postoperative care after nail biopsies can be challenging for patients due to the bulky dressing that must remain in place for 48 hours.1 The dressing can restrict daily activities such as bathing, washing dishes, and other household tasks. A common solution is to cover the hand with a plastic bag secured with tape during water-related activities, but efficacy is variable. In one study, 23 participants tested this method by holding a paper towel with their hand covered by a plastic bag and measuring the weight of the paper towel before and after submersion of the hand in water.2 Any saturation of the paper towel was defined as failure; the failure rate was 52.2% (12/23) with motion (rotating the arm at the elbow for 30 seconds clockwise, counterclockwise, and left to right) and 60.9% (14/23) without motion. There was an average of 5.50 g of moisture accumulation without motion and 4.51 g with motion, with failure occurring most often immediately following submersion of the hand. Furthermore, the plastic bag with tape method was rated poorly by all 23 participants based on efficacy and comfort.2
In the same study, participants also reported that removal of the adhesive tape was unpleasant and irritating,2 which suggests these same complaints may apply to use of a waterproof bandage, another potential option for coverage of the wound dressing. As an alternative, we propose the use of a removable waterproof arm cast protector following nail surgery that allows patients to continue their regular activities while keeping the dressing dry and intact to allow for optimal wound healing.
The Technique
Our technique involves the use of a removable waterproof arm cast protector that is sealed with a thick rubber cuff, allowing patients to perform regular daily activities such as bathing, washing dishes, cleaning, and doing laundry without the wound dressing underneath becoming wet (Figure). Cast protectors made of flexible latex-free plastic are readily available and can slide on and off the arm as needed. We recommend that patients purchase the cast protector prior to undergoing surgery. There are options to fit most adults, with the opening generally accommodating arm diameters of 2 to 7 inches. These reusable cast protectors are available via popular online retailers and typically cost patients $10 to $15.

Practice Implications
In our experience, using a reusable waterproof cast protector following nail surgery is effective at keeping wound dressings dry and provides a practical solution for bathing and other activities involving water exposure. It is durable and easy to use, especially when compared to a plastic bag and waterproof tape. However, some patients find the waterproof seal uncomfortable, especially when worn for extended periods of time. According to online product feedback, limitations of the cast protector include potential leakage with prolonged immersion in water, swimming, or high-pressure water exposure. The cast protector should not be worn for more than 30 minutes, as it can restrict blood flow, and condensation from prolonged use may dampen the dressing. While we have not encountered allergic contact dermatitis associated with the use of cast protectors for this purpose in our practice, patients should be cautioned of this potential risk. While these cast protectors generally can accommodate a range of arm diameters, they may not fit all hand sizes or shapes and may reduce dexterity for motor tasks. Additionally, the patient must purchase the protector ahead of surgery.
Our technique involving the use of a waterproof arm cast protector is an affordable solution that allows patients to keep their wound dressing dry while continuing to perform regular daily activities. The cast protector also can be used following other dermatologic procedures (eg, biopsy, Mohs micrographic surgery) that involve the hand and lower arm when waterproof protection may be necessary.
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
- Ricardo JW, Lipner SR. How we do it: pressure-padded dressing with self-adherent elastic wrap for wound care after nail surgery. Dermatol Surg. 2021;47:442–444. doi:10.1097/DSS.0000000000002371
- Kwan S, Santoro A, Cheesman Q, et al. Efficacy of waterproof cast protectors and their ability to keep casts dry. J Hand Surg Am. 2023;48:803–809. doi:10.1016/j.jhsa.2022.05.006
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Waterproof Cast Protector Keeps Wound Dressing Intact Following Nail Surgery
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
To the Editor:
Residency applicants, especially in competitive specialties such as dermatology, face major financial barriers due to the high costs of applications, interviews, and away rotations.1 While several studies have examined application costs of other specialties, few have analyzed expenses associated with dermatology applications.1,2 There are no data examining costs following the start of the COVID-19 pandemic in 2020; thus, our study evaluated dermatology application cost trends from 2021 to 2024 and compared them to other specialties to identify strategies to reduce the financial burden on applicants.
Self-reported total application costs, application fees, interview expenses, and away rotation costs from 2021 to 2024 were collected from the Texas Seeking Transparency in Application to Residency (STAR) database powered by the UT Southwestern Medical Center (Dallas, Texas).3 The mean total application expenses per year were compared among specialties, and an analysis of variance was used to determine if the differences were statistically significant.
The number of applicants who recorded information in the Texas STAR database was 110 in 2021, 163 in 2022, 136 in 2023, and 129 in 2024.3 The total dermatology application expenses increased from $2805 in 2021 to $6231 in 2024; interview costs increased from $404 in 2021 to $911 in 2024; and away rotation costs increased from $850 in 2021 to $3812 in 2024 (all P<.05)(Table). There was no significant change in application fees during the study period ($2176 in 2021 to $2125 in 2024 [P=.58]). Dermatology had the fourth highest average total cost over the study period compared to all other specialties, increasing from $2250 in 2021 to $5250 in 2024, following orthopedic surgery ($2250 in 2021 to $6750 in 2024), plastic surgery ($2250 in 2021 to $9750 in 2024), and neurosurgery ($1750 in 2021 to $11,250 in 2024).

Our study found that dermatology residency application costs have increased significantly from 2021 to 2024, primarily driven by rising interview and away rotation expenses (both P<.05). This trend places dermatology among the most expensive fields to apply to for residency. A cross-sectional survey of dermatology residency program directors identified away rotations as one of the top 5 selection criteria, underscoring their importance in the matching process.4 In addition, a cross-sectional analysis of 345 dermatology residents found that 26.2% matched at institutions where they had mentors, including those they connected with through away rotations.5,6 Overall, the high cost of away rotations partially may reflect the competitive nature of the specialty, as building connections at programs may enhance the chances of matching. These costs also can vary based on geography, as rotating in high-cost urban centers can be more expensive than in rural areas; however, rural rotations may be less common due to limited program availability and applicant preferences. For example, nearly 50% of 2024 Electronic Residency Application Service applicants indicated a preference for urban settings, while fewer than 5% selected rural settings.7 Additionally, the high costs associated with applying to residency programs and completing away rotations can disproportionately impact students from rural backgrounds and underrepresented minorities, who may have fewer financial resources.
In our study, the lower application-related expenses in 2021 (during the pandemic) compared to those of 2024 (postpandemic) likely stem from the Association of American Medical Colleges’ recommendation to conduct virtual interviews during the pandemic.8 In 2024, some dermatology programs returned to in-person interviews, with some applicants consequently incurring higher costs related to travel, lodging, and other associated expenses.8 A cost-analysis study of 4153 dermatology applicants from 2016 to 2021 found that the average application costs were $1759 per applicant during the pandemic, when virtual interviews replaced in-person ones, whereas costs were $8476 per applicant during periods with in-person interviews and no COVID-19 restrictions.2 However, we did not observe a significant change in application fees over our study period, likely because the pandemic did not affect application numbers. A cross-sectional analysis of dermatology applicants during the pandemic similarly reported reductions in application-related expenses during the period when interviews were conducted virtually,9 supporting the trend observed in our study. Overall, our findings taken together with other studies highlight the pandemic’s role in reducing expenses and underscore the potential for exploring additional cost-saving measures.
Implementing strategies to reduce these financial burdens—including virtual interviews, increasing student funding for away rotations, and limiting the number of applications individual students can submit—could help alleviate socioeconomic disparities. The new signaling system for residency programs aims to reduce the number of applications submitted, as applicants typically receive interviews only from the limited number of programs they signal, reducing overall application costs. However, our data from the Texas STAR database suggest that application numbers remained relatively stable from 2021 to 2024, indicating that, despite signaling, many applicants still may apply broadly in hopes of improving their chances in an increasingly competitive field. Although a definitive solution to reducing the financial burden on dermatology applicants remains elusive, these strategies can raise awareness and encourage important dialogues.
Limitations of our study include the voluntary nature of the Texas STAR survey, leading to potential voluntary response bias, as well as the small sample size. Students who choose to submit cost data may differ systematically from those who do not; for example, students who match may be more likely to report their outcomes, while those who do not match may be less likely to participate, potentially introducing selection bias. In addition, general awareness of the Texas STAR survey may vary across institutions and among students, further limiting the number of students who participate. Additionally, 2021 was the only presignaling year included, making it difficult to assess longer-term trends. Despite these limitations, the Texas STAR database remains a valuable resource for analyzing general residency application expenses and trends, as it offers comprehensive data from more than 100 medical schools and includes many variables.3
In conclusion, our study found that total dermatology residency application costs have increased significantly from 2021 to 2024 (all P<.05), making dermatology among the most expensive specialties for applying. This study sets the foundation for future survey-based research for applicants and program directors on strategies to alleviate financial burdens.
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
- Mansouri B, Walker GD, Mitchell J, et al. The cost of applying to dermatology residency: 2014 data estimates. J Am Acad Dermatol. 2016;74:754-756. doi:10.1016/j.jaad.2015.10.049
- Gorgy M, Shah S, Arbuiso S, et al. Comparison of cost changes due to the COVID-19 pandemic for dermatology residency applications in the USA. Clin Exp Dermatol. 2022;47:600-602. doi:10.1111/ced.15001<.li>
- UT Southwestern. Texas STAR. 2024. Accessed November 5, 2025. https://www.utsouthwestern.edu/education/medical-school/about-the-school/student-affairs/texas-star.html
- Baldwin K, Weidner Z, Ahn J, et al. Are away rotations critical for a successful match in orthopaedic surgery? Clin Orthop Relat Res. 2009;467:3340-3345. doi:10.1007/s11999-009-0920-9
- Yeh C, Desai AD, Wilson BN, et al. Cross-sectional analysis of scholarly work and mentor relationships in matched dermatology residency applicants. J Am Acad Dermatol. 2022;86:1437-1439. doi:10.1016/j.jaad.2021.06.861
- Gorouhi F, Alikhan A, Rezaei A, et al. Dermatology residency selection criteria with an emphasis on program characteristics: a national program director survey. Dermatol Res Pract. 2014;2014:692760. doi:10.1155/2014/692760
- Association of American Medical Colleges. Decoding geographic and setting preferences in residency selection. January 18, 2024. Accessed October 27, 2025. https://www.aamc.org/services/eras-institutions/geographic-preferences
- Association of American Medical Colleges. Virtual interviews: tips for program directors. Updated May 14, 2020. https://med.stanford.edu/content/dam/sm/gme/program_portal/pd/pd_meet/2019-2020/8-6-20-Virtual_Interview_Tips_for_Program_Directors_05142020.pdf
- Williams GE, Zimmerman JM, Wiggins CJ, et al. The indelible marks on dermatology: impacts of COVID-19 on dermatology residency match using the Texas STAR database. Clin Dermatol. 2023;41:215-218. doi:10.1016/j.clindermatol.2022.12.001
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
Cost Analysis of Dermatology Residency Applications From 2021 to 2024 Using the Texas Seeking Transparency in Application to Residency Database
PRACTICE POINTS
- Dermatology application costs increased from 2021 to 2024, largely due to expenses related to away rotations and, in some cases, a return to in-person interviews.
- Away rotations play a critical role in the dermatology match; however, they also contribute substantially to financial burden.
- The cost-saving impact of virtual interviews during the COVID-19 pandemic highlights a meaningful opportunity for future cost reduction.
- Further interventions are needed to meaningfully reduce financial burden and promote equity.
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
Use of artificial intelligence (AI) in dermatology has increased over the past decade, likely driven by advances in deep learning algorithms, computing hardware, and machine learning.1 Studies comparing the performance of AI algorithms to dermatologists in classifying skin disorders have shown conflicting results.2,3 In this study, we aimed to analyze AI tools used for diagnosing and classifying skin disease and evaluate the role of dermatologists in the creation of AI technology. We also investigated the number of clinical images used in datasets to train AI programs and compared tools that were created with dermatologist input to those created without dermatologist/clinician involvement.
Methods
A search of PubMed articles indexed for MEDLINE using the terms machine learning, artificial intelligence, and dermatology was conducted on September 18, 2022. Articles were included if they described full-length trials; used machine learning for diagnosis of or screening for dermatologic conditions; and used dermoscopic or gross image datasets of the skin, hair, or nails. Articles were categorized into 4 groups based on the conditions covered: chronic wounds, inflammatory skin diseases, mixed conditions, and pigmented skin lesions. Algorithms were sorted into 4 categories: convolutional/convoluted neural network, deep learning model/deep neural network, AI/artificial neural network, and other. Details regarding Fitzpatrick skin type and skin of color (SoC) inclusion in the articles or AI algorithm datasets were recorded. Univariate and multivariate analyses were performed using Microsoft Excel and SAS Studio 3.8. Sensitivity and specificity were calculated for all included AI technology. Sensitivity, specificity, and the number of clinical images were compared among the included articles using analysis of variance and t tests (α=0.05; P<.05 indicated statistical significance).
Results
Our search yielded 1016 articles, 58 of which met the inclusion criteria. Overall, 25.9% (15/58) of the articles utilized AI to diagnose or classify mixed skin diseases; 22.4% (13/58) for pigmented skin lesions; 19.0% (11/58) for wounds; 17.2% (10/58) for inflammatory skin diseases; and 5.2% (3/58) each for acne, psoriasis, and onychomycosis. Overall, 24.0% (14/58) of articles provided information about Fitzpatrick skin type, and 58.7% (34/58) included clinical images depicting SoC. Furthermore, we found that only 20.7% (12/58) of articles on deep learning models included descriptions of patient ethnicity or race in at least 1 dataset, and only 10.3% (6/58) of studies included any information about skin tone in the dataset. Studies with a dermatologist as the last author (most likely to be supervising the project) were more likely to include clinical images depicting SoC than those without (82.6% [19/23] and 16.7% [3/18], respectively [P=.0411]).
The mean (SD) number of clinical images in the study articles was 28,422 (84,050). Thirty-seven (63.8%) of the study articles included gross images, 17 (29.3%) used dermoscopic images, and 4 (6.9%) used both. Twenty-seven (46.6%) articles used convolutional/convoluted neural networks, 15 (25.9%) used deep learning model/deep neural networks, 8 (13.8%) used other algorithms, 6 (10.3%) used AI/artificial neural network, and 2 (3.4%) used fuzzy algorithms. Most studies were conducted in China (29.3% [17/58]), Germany (12.1% [7/58]), India (10.3% [6/58]), multiple nations (10.3% [6/58]), and the United States (10.3% [6/58]). Overall, 82.8% (48/58) of articles included at least 1 dermatologist coauthor. Sensitivity of the AI models was 0.85, and specificity was 0.85. The average percentage of images in the dataset correctly identified by a physician was 76.87% vs 81.62% of images correctly identified by AI. Average agreement between AI and physician assessment was 77.98%, defined as AI and physician both having the same diagnosis.
Articles authored by dermatologists contained more clinical images than those without dermatologists in key authorship roles (P<.0001)(eTable). Psoriasis-related algorithms had the fewest (mean [SD]: 3173 [4203]), and pigmented skin lesions had the most clinical images (mean [SD]: 53,19l [155,579]).

Comment
Our results indicated that AI studies with dermatologist authors had significantly more images in their datasets (ie, the set of clinical images of skin lesions used to train AI algorithms in diagnosing or classifying lesions) than those with nondermatologist authors (P<.0001)(eTable). Similarly, in a study of AI technology for skin cancer diagnosis, AI studies with dermatologist authors (ie, included in the development of the AI algorithm) had more images than studies without dermatologist authors.1 Deep learning textbooks have suggested that 5000 clinical images or training input per output category are needed to produce acceptable algorithm performance, and more than 10 million are needed to produce results superior to human performance.4-10 Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists1; therefore, dermatologist involvement in AI development is necessary to facilitate collection of larger image datasets and optimal performance for image diagnosis/classification tasks.
We found that 20.7% of articles on deep learning models included descriptions of patient ethnicity or race, and only 10.3% of studies included any information about skin tone in the dataset. Furthermore, American investigators primarily trained models using clinical images of patients with lighter skin tones, whereas Chinese investigators exclusively included images depicting darker skin tones. Similarly, in a study of 52 cutaneous imaging deep learning articles, only 17.3% (9/52) reported race and/or Fitzpatrick skin type, and only 7.7% (4/52) of articles included both.2,6,8 Therefore, dermatologists are needed to contribute images representing diverse populations and collaborate in AI research studies, as their involvement is necessary to ensure the accuracy of AI models in classifying lesions or diagnosing skin lesions across all skin types.
Our search was limited to PubMed, and real-world applications could not be evaluated.
Conclusion
In summary, we found that AI studies with dermatologist authors used larger numbers of clinical images in their datasets and more images representing diverse skin types than studies without. Therefore, we advocate for greater involvement of dermatologists in AI research, which might result in better patient outcomes by improving diagnostic accuracy.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
- Zakhem GA, Fakhoury JW, Motosko CC, et al. Characterizing the role of dermatologists in developing artificial intelligence for assessment of skin cancer. J Am Acad Dermatol. 2021;85:1544-1556.
- Daneshjou R, Vodrahalli K, Novoa RA, et al. Disparities in dermatology AI performance on a diverse, curated clinical image set. Sci Adv. 2022;8:eabq6147.
- Wu E, Wu K, Daneshjou R, et al. How medical AI devices are evaluated: limitations and recommendations from an analysis of FDA approvals. Nat Med. 2021;27:582-584.
- Murphree DH, Puri P, Shamim H, et al. Deep learning for dermatologists: part I. Fundamental concepts. J Am Acad Dermatol. 2022;87:1343-1351.
- Goodfellow I, Bengio Y, Courville A. Deep Learning. The MIT Press; 2016.
- Kim YH, Kobic A, Vidal NY. Distribution of race and Fitzpatrick skin types in data sets for deep learning in dermatology: a systematic review. J Am Acad Dermatol. 2022;87:460-461.
- Liu Y, Jain A, Eng C, et al. A deep learning system for differential diagnosis of skin diseases. Nat Med. 2020;26:900-908.
- Zhu CY, Wang YK, Chen HP, et al. A deep learning based framework for diagnosing multiple skin diseases in a clinical environment. Front Med (Lausanne). 2021;8:626369.
- Capurro N, Pastore VP, Touijer L, et al. A deep learning approach to direct immunofluorescence pattern recognition in autoimmune bullous diseases. Br J Dermatol. 2024;191:261-266.
- Han SS, Park I, Eun Chang S, et al. Augmented intelligence dermatology: deep neural networks empower medical professionals in diagnosing skin cancer and predicting treatment options for 134 skin disorders. J Invest Dermatol. 2020;140:1753-1761.
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
The Role of Dermatologists in Developing AI Tools for Diagnosis and Classification of Skin Disease
Practice Points
- Artificial intelligence (AI) technology is emerging as a valuable tool in diagnosing and classifying dermatologic conditions.
- Despite advances in AI for dermatologic image analysis, the creation of these models often has been directed by nondermatologists.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48
Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.
A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
Sporotrichosis is an implantation mycosis that classically manifests as a localized skin and subcutaneous fungal infection but may disseminate to other parts of the body.1 It is caused by several species within the Sporothrix genus2 and is associated with varying clinical manifestations, geographic distributions, virulence profiles, and antifungal susceptibility patterns.3,4 Transmission of the fungus can involve inoculation from wild or domestic animals (eg, cats).5,6 Occupations such as landscaping and gardening or elements in the environment (eg, soil, plant fragments) also can be sources of exposure.7,8
Sporotrichosis is recognized by the World Health Organization as a neglected tropical disease that warrants global advocacy to prevent infections and improve patient outcomes.9,10 It carries substantial stigma and socioeconomic burden.11,12 Diagnostics, species identification, and antifungal susceptibility testing often are limited, particularly in resource-limited settings.13 In this article, we outline steps to diagnose and manage sporotrichosis to improve care for affected patients globally.
Epidemiology
Sporotrichosis occurs worldwide but is most common in tropical and subtropical regions.14,15 Outbreaks and clusters of sporotrichosis have been observed across North, Central, and South America as well as in southern Africa and Asia. The estimated annual incidence is 40,000 cases worldwide,16-20 but global case counts likely are underestimated due to limited surveillance data and diagnostic capability.21
On the Asian subcontinent, Sporothrix globosa is the predominant causative species of sporotrichosis, typically via contaminated plant material22; however, at least 1 outbreak has been associated with severe flooding.23 In Africa, infections are most commonly caused by Sporothrix schenckii sensu stricto through a similar transmission route. Across Central America, S schenckii sensu stricto is the predominant causative species; however, Sporothrix brasiliensis is the predominant species in some countries in South America, particularly Brazil.20
Data describing the current geographic distribution and prevalence of sporotrichosis in the United States are limited. Historically, the disease was reported most commonly in Midwestern states and was associated with outbreaks related to handling Sphagnum moss.24,25 Epidemiologic studies using health insurance data indicate an average annual incidence of 2.0 cases per million individuals in the United States, with a higher prevalence among women and a median age at diagnosis of 54 years.26 A review of sporotrichosis-associated hospitalizations across the United States from 2000 to 2013 indicated an average hospitalization rate of 0.35 cases per 1 million individuals; rates were higher (0.45 cases per million) in the West and lower (0.15 per million) in the Northeast and in men (0.40 per million).27 Type 2 diabetes, immune-mediated inflammatory disease, and chronic obstructive pulmonary disease are associated with an increased risk for infection and hospitalization.27
Causative Organisms
Sporothrix species are thermally dimorphic fungi that can grow as mold in the environment and as yeast in human tissue. Sporothrix brasiliensis is the only thermodimorphic fungus known to be transmitted directly in its yeast form.28 In other species, inoculation usually occurs after contact with contaminated soil or plant material during gardening, carpentry, or agricultural practices.7
Zoonotic transmission of sporotrichosis from animals to humans has been reported from a range of domestic and wild animals and birds but historically has been rare.5,7,29,30 Recently, the importance of both cat-to-cat (epizootic) and cat-to-human (zoonotic) transmission of S brasiliensis has been recognized, with infection typically following traumatic inoculation after a scratch or bite; less frequently, transmission occurs due to exposure to respiratory droplets or contact with feline exudates.5,29,31 Sporothrix brasiliensis is responsible for zoonotic epidemics in South America, primarily in Brazil. Transmission occurs among humans, cats, and canines, with felines serving as the primary vector.32 Transmission of this species is particularly common in stray and unneutered male cats that exhibit aggressive behaviors.33 This species also is thought to be the most virulent Sporothrix species.21
Sporothrix brasiliensis can persist on nondisinfected inanimate surfaces, which suggests that fomite transmission can lead to human infection.31 The epidemiology of sporotrichosis has transformed in regions where S brasiliensis circulates, with epidemic spread resulting in thousands of cases, whereas in other areas without S brasilinesis, sporotrichosis predominantly occurs sporadically with rare clusters.1,2,7,15
Sporotrichosis has been the subject of a taxonomic debate in the mycology community.21 Sporothrix schenckii sensu lato originally was believed to be the sole fungal pathogen causing sporotrichosis34 but was later divided into S schenckii sensu stricto, Sporothrix globosa, and S brasiliensis.35 More than 60 distinct species now have been described within the Sporothrix genus,36,37 but the primary species causing human sporotrichosis include S schenckii sensu stricto, S brasiliensis, S globosa, Sporothrix mexicana, and Sporothrix luriei.35 Both S schenckii and S brasiliensis have greater virulence than other Sporothrix species4; however, S schenckii causes infections that typically are localized and are milder, while S brasiliensis can lead to more atypical, severe, and disseminated infections38,39 and can spread epidemically.
Clinical Manifestations
Sporotrichosis has 4 main clinical presentations: cutaneous lymphatic, fixed cutaneous, cutaneous or systemic disseminated, and extracutaneous.40,41 The most common clinical manifestation is the cutaneous lymphatic form, which predominantly affects the hands and forearms in adults and the face in children.7 The primary lesion usually manifests as a unilateral papule, nodule, or pustule that may ulcerate (sporotrichotic chancre), but multiple sites of inoculation are possible. Subsequent lesions may appear in a linear distribution along a regional lymphatic path (sporotrichoid spread). Systemic symptoms and regional lymphadenopathy are uncommon and usually are mild.
The second most common clinical manifestation is the fixed cutaneous form, typically affecting the face, neck, trunk, or legs with a single papule, nodule, or verrucous lesion with no lymphangitic spread.7 Usually confined to the inoculation site, the primary lesion may be accompanied by satellite lesions and often presents a diagnostic challenge.
Disseminated sporotrichosis (either cutaneous or systemic) is rare. Disseminated cutaneous sporotrichosis manifests with multiple noncontiguous skin lesions caused by lymphatic and possible hematogenous spread. Lesions may include a combination of papules, pustules, follicular eruptions, crusted plaques, and ulcers that may mimic other systemic infections. Immunoreactive changes such as erythema nodosum, erythema multiforme, or arthritis may accompany skin lesions, most commonly with S brasiliensis infections. Nearly 10% of S brasiliensis infections involve the ocular adnexa, and Parinaud oculoglandular syndrome is commonly described in cases reported in Brazil.42,43 Disseminated disease usually occurs in immunocompromised hosts; however, despite a focus on HIV co-infection,8,44 prior epidemiologic research has suggested that diabetes and alcoholism are the most common predisposing factors.45 Systemic disseminated sporotrichosis by definition affects at least 2 body systems, most commonly the central nervous system, lungs, and musculoskeletal system (including joints and bone marrow).45
Extracutaneous sporotrichosis is rare and often is difficult to diagnose. Risk factors include chronic obstructive pulmonary disease, alcoholism, use of steroid medications, AIDS, solid organ transplantation, and use of tumor necrosis factor α inhibitors. It usually affects bony structures through hematogenous spread in immunocompromised hosts and is associated with a high risk for osteomyelitis due to delayed diagnosis.2
Clinical progression of sporotrichosis usually is slow, and lesions may persist for months or years if untreated. Sporotrichosis should always be considered for atypical, persistent, or treatment-resistant manifestations of nodular or ulcerated skin lesions in endemic regions or acute illness with these symptoms following exposure. Preventing secondary bacterial infection is an important consideration as it can exacerbate disease severity, extend the treatment duration, prolong hospitalization, and increase mortality risk.46
Diagnosis
In regions endemic for S brasiliensis, it may be acceptable to commence treatment on clinical suspicion without a definitive diagnosis,21 but caution is necessary, as lesions easily can be mistaken for other conditions such as Mycobacterium marinum infections (sporotrichoid lesions) or cutaneous leishmaniasis. Limited availability of molecular diagnostic tools in routine clinical laboratories affects the diagnosis of sporotrichosis and species identification. Direct microscopy on a 10% to 30% potassium hydroxide wet mount has low diagnostic sensitivity and is not recommended47; findings typically include cigar-shaped yeast cells (eFigure 1). Biopsy and histopathology also are useful, although in many infections (other than those due to S brasiliensis) there are very few detectable organisms in the tissue. Fluorescent staining of fungi with optical brighteners (eg, Calcofluor, Blankophor) is a useful technique with high sensitivity in clinical specimens on histopathologic and direct examination.48

Fungal culture has higher sensitivity and specificity than microscopy and is the gold-standard approach for diagnosis of sporotrichosis (eFigure 2); however, culture cannot differentiate between Sporothrix species and may take more than a month to yield a positive result.7 No reliable serologic test for sporotrichosis has been validated, and a standardized antigen assay currently is unavailable.49 Serology may be more useful for patients who present with systemic disease or have persistently negative culture results despite a high index of suspicion.

A recent study evaluated the effectiveness of a lateral flow assay for detecting anti-Sporothrix antibodies, demonstrating the potential for its use as a rapid diagnostic test.50 Investigating different molecular methods to increase the sensitivity and specificity of diagnosis and distinguish Sporothrix species has been a focus of recent research, with a preference for polymerase chain reaction (PCR)–based genotypic methods.13,51 Recent advances in diagnostic testing include the development of multiplex PCR,52 culture-independent PCR techniques,53 and matrix-assisted laser desorption/ionization–time of flight mass spectrometry,54 each with varying clinical and practical applicability. Specialized testing can be beneficial for patients who have a poor therapeutic response to standard treatment, guide antifungal treatment choices, and identify epidemiologic disease and transmission patterns.21
Although rarely performed, antifungal susceptibility testing may be useful in guiding therapy to improve patient outcomes, particularly in the context of treatment failure, which has been documented with isolates exhibiting high minimal inhibitory concentrations (MICs) to first-line therapy and a poor clinical response.55,56 Proposed mechanisms of resistance include increased cellular melanin production, which protects against oxidative stress and reduces antifungal activity.56 Antifungal susceptibility profiles for therapeutics vary across Sporothrix species; for example, S brasiliensis generally shows lower MICs to itraconazole and terbinafine compared with S schenckii and S globosa, and S schenckii has shown a high MIC to itraconazole, as reflected in MIC distribution studies and epidemiologic cutoff values for antifungal agents.55,57-59 However, specific breakpoints for different Sporothrix species have not been determined.60 Robust clinical studies are needed to determine the correlation of in vitro MICs to clinical outcomes to assess the utility of antifungal susceptibility testing for Sporothrix species.
Management
Treatment of sporotrichosis is guided by clinical presentation, host immune status, and species identification. Management can be challenging in cases with an atypical or delayed diagnosis and limited access to molecular testing methods. Itraconazole is the first-line therapy for management of cutaneous sporotrichosis. It is regarded as safe, effective, well tolerated, and easily administered, with doses ranging from 100 mg in mild cases to 400 mg (with daily or twice-daily dosing).61 Treatment usually is for 3 to 6 months and should continue for 1 month after complete clinical resolution is achieved62; however, some cases of S brasiliensis infection require longer treatment, and complex or disseminated cases may require therapy for up to 12 months.61 Itraconazole is contraindicated in pregnancy and has many drug interactions (through cytochrome P450 inhibition) that may preclude administration, particularly in elderly populations. Therapeutic drug monitoring is recommended for prolonged or high-dose therapy, with periodic liver function testing to reduce the risk for toxicity. Itraconazole should be administered with food, and concurrent use of antacids or proton pump inhibitors should be avoided.61
Oral terbinafine (250 mg daily) can be considered as an effective alternative to treat cutaneous disease.63 Particularly in resource-limited settings, potassium iodide is an affordable and effective treatment for cutaneous sporotrichosis, administered as a saturated oral solution,64 but due to adverse effects such as severe nausea, the daily dose should be increased slowly each day to ensure tolerance.
Amphotericin B is the treatment of choice for severe and treatment-resistant cases of sporotrichosis as well as for immunocompromised patients.21,61 In patients with HIV, a longer treatment course is recommended with oversight from an infectious diseases specialist and usually is followed by a 12-month course of itraconazole after completion of initial therapy.61 Surgical excision infrequently is recommended but can be used in combination with another treatment modality and may be useful with a slow or incomplete response to medical therapy. Thermotherapy involves direct application of heat to cutaneous lesions and may be considered for small and localized lesions, particularly if antifungal agents are contraindicated or poorly tolerated.61 Public health measures include promoting case detection through practitioner education and patient awareness in endemic regions, as well as zoonotic control of infected animals to manage sporotrichosis.
Final Thoughts
Sporotrichosis is a fungal infection with growing public health significance. While the global disease burden is unknown, rising case numbers and geographic spread likely reflect a complex interaction between humans, the environment, and animals, exemplified by the spread of feline-associated infection due to S brasiliensis in South America.28 Cases of S brasiliensis infection after importation of an affected cat have been detected outside South America, and clinicians should be alert for introduction to the United States. Strengthening genotypic and phenotypic diagnostic capabilities will allow species identification and guide treatment and management. Disease surveillance and operational research will inform public health approaches to control sporotrichosis worldwide.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
- Queiroz-Telles F, Nucci M, Colombo AL, et al. Mycoses of implantation in Latin America: an overview of epidemiology, clinical manifestations, diagnosis and treatment. Med Mycol. 2011;49:225-236.
- Orofino-Costa R, de Macedo PM, Rodrigues AM, et al. Sporotrichosis: an update on epidemiology, etiopathogenesis, laboratory and clinical therapeutics. An Bras Dermatol. 2017;92:606-620.
- Almeida-Paes R, de Oliveira MM, Freitas DF, et al. Sporotrichosis in Rio de Janeiro, Brazil: Sporothrix brasiliensis is associated with atypical clinical presentations. PLoS Negl Trop Dis. 2014;8:E3094.
- Arrillaga-Moncrieff I, Capilla J, Mayayo E, et al. Different virulence levels of the species of Sporothrix in a murine model. Clin Microbiol Infect. 2009;15:651-655.
- de Lima Barros MB, Schubach TM, Gutierrez-Galhardo MC, et al. Sporotrichosis: an emergent zoonosis in Rio de Janeiro. Mem Inst Oswaldo Cruz. 2001;96:777-779.
- Bao F, Huai P, Chen C, et al. An outbreak of sporotrichosis associated with tying crabs. JAMA Dermatol. 2025;161:883-885.
- de Lima Barros MB, de Almeida Paes R, Schubach AO. Sporothrix schenckii and sporotrichosis. Clin Microbiol Rev. 2011;24:633-654.
- Queiroz-Telles F, Buccheri R, Benard G. Sporotrichosis in immunocompromised hosts. J Fungi. 2019;5:8.
- World Health Organization. Generic Framework for Control, Elimination and Eradication of Neglected Tropical Diseases. World Health Organization; 2016.
- Smith DJ, Soebono H, Parajuli N, et al. South-East Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561.
- Winck GR, Raimundo RL, Fernandes-Ferreira H, et al. Socioecological vulnerability and the risk of zoonotic disease emergence in Brazil. Sci Adv. 2022;8:eabo5774.
- Jenks JD, Prattes J, Wurster S, et al. Social determinants of health as drivers of fungal disease. EClinicalMedicine. 2023;66:102325.
- Rodrigues AM, Gonçalves SS, de Carvalho JA, et al. Current progress on epidemiology, diagnosis, and treatment of sporotrichosis and their future trends. J Fungi. 2022;8:776.
- Evans EGV, Ashbee HR, Frankland JC, et al. Tropical mycoses: hazards to travellers. In: Evans EGV, Ashbee HR, eds. Tropical Mycology. Vol 2. CABI Publishing; 2002:145-163.
- Matute DR, Teixeira MM. Sporothrix is neglected among the neglected. PLoS Pathog. 2025;21:E1012898.
- Matruchot L. Sur un nouveau groupe de champignons pathogenes, agents des sporotrichoses. Comptes Rendus De L’Académie Des Sci. 1910;150:543-545.
- Dangerfield LF. Sporotriehosis among miners on the Witwatersrand gold mines. S Afr Med J. 1941;15:128-131.
- Fukushiro R. Epidemiology and ecology of sporotrichosis in Japan. Zentralbl Bakteriol Mikrobiol Hyg. 1984;257:228-233.
- Dixon DM, Salkin IF, Duncan RA, et al. Isolation and characterization of Sporothrix schenckii from clinical and environmental sources associated with the largest US epidemic of sporotrichosis. J Clin Microbiol. 1991;29:1106-1113.
- dos Santos AR, Misas E, Min B, et al. Emergence of zoonotic sporotrichosis in Brazil: a genomic epidemiology study. Lancet Microbe. 2024;5:E282-E290.
- Schechtman RC, Falcão EM, Carard M, et al. Sporotrichosis: hyperendemic by zoonotic transmission, with atypical presentations, hypersensitivity reactions and greater severity. An Bras Dermatol. 2022;97:1-13.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Sporothrix species causing outbreaks in animals and humans driven by animal-animal transmission. PLoS Pathog. 2016;12:E1005638.
- Li HY, Song J, Zhang Y. Epidemiological survey of sporotrichosis in Zhaodong, Heilongjiang. Chin J Dermatol. 1995;28:401-402.
- Hajjeh R, McDonnell S, Reef S, et al. Outbreak of sporotrichosis among tree nursery workers. J Infect Dis. 1997;176:499-504.
- Coles FB, Schuchat A, Hibbs JR, et al. A multistate outbreak of sporotrichosis associated with sphagnum moss. Am J Epidemiol. 1992;136:475-487.
- Benedict K, Jackson BR. Sporotrichosis cases in commercial insurance data, United States, 2012-2018. Emerg Infect Dis. 2020;26:2783-2785.
- Gold JAW, Derado G, Mody RK, et al. Sporotrichosis-associated hospitalizations, United States, 2000-2013. Emerg Infect Dis. 2016;22:1817-1820.
- Rossow JA, Queiroz-Telles F, Caceres DH, et al. A One Health approach to combatting Sporothrix brasiliensis: narrative review of an emerging zoonotic fungal pathogen in South America. J Fungi. 2020;6:247-274.
- Madrid IM, Mattei AS, Fernandes CG, et al. Epidemiological findings and laboratory evaluation of sporotrichosis: a description of 103 cases in cats and dogs in southern Brazil. Mycopathologia. 2012;173:265-273.
- Fichman V, Gremião ID, Mendes-Júnior AA, et al. Sporotrichosis transmitted by a cockatiel (Nymphicus hollandicus). J Eur Acad Dermatol Venereol. 2018;32:E157-E158.
- Cognialli RC, Queiroz-Telles F, Cavanaugh AM, et al. New insights on transmission of Sporothrix brasiliensis. Mycoses. 2025;68:E70047.
- Bastos FA, De Farias MR, Gremião ID, et al. Cat-transmitted sporotrichosis by Sporothrix brasiliensis: focus on its potential transmission routes and epidemiological profile. Med Mycol. 2025;63.
- Gremiao ID, Menezes RC, Schubach TM, et al. Feline sporotrichosis: epidemiological and clinical aspects. Med Mycol. 2015;53:15-21.
- Hektoen L, Perkins CF. Refractory subcutaneous abscesses caused by Sporothrix schenckii: a new pathogenic fungus. J Exp Med. 1900;5:77-89.
- Marimon R, Cano J, Gené J, et al. Sporothrix brasiliensis, S. globosa, and S. mexicana, three new Sporothrix species of clinical interest. J Clin Microbiol. 2007;45:3198-3206.
- Rodrigues AM, Della Terra PP, Gremião ID, et al. The threat of emerging and re-emerging pathogenic Sporothrix species. Mycopathologia. 2020;185:813-842.
- Morgado DS, Castro R, Ribeiro-Alves M, et al. Global distribution of animal sporotrichosis: a systematic review of Sporothrix sp. identified using molecular tools. Curr Res Microbial Sci. 2022;3:100140.
- de Lima IM, Ferraz CE, Lima-Neto RG, et al. Case report: Sweet syndrome in patients with sporotrichosis: a 10-case series. Am J Trop Med Hyg. 2020;103:2533-2538.
- Xavier MO, Bittencourt LR, da Silva CM, et al. Atypical presentation of sporotrichosis: report of three cases. Rev Soc Bras Med Trop. 2013;46:116-118.
- Ramos-e-Silva M, Vasconcelos C, Carneiro S, et al. Sporotrichosis. Clin Dermatol. 2007;25:181-187.
- Sampaio SA, Lacaz CS. Klinische und statische Untersuchungen uber Sporotrichose in Sao Paulo. Der Hautarzt. 1959;10:490-493.
- Arinelli A, Aleixo L, Freitas DF, et al. Ocular manifestations of sporotrichosis in a hyperendemic region in Brazil: description of a series of 120 cases. Ocul Immunol Inflamm. 2023;31:329-337.
- Cognialli RC, Cáceres DH, Bastos FA, et al. Rising incidence of Sporothrix brasiliensis infections, Curitiba, Brazil, 2011-2022. Emerg Infect Dis. 2023;29:1330-1339.
- Freitas DF, Valle AC, da Silva MB, et al. Sporotrichosis: an emerging neglected opportunistic infection in HIV-infected patients in Rio de Janeiro, Brazil. PLoS Negl Trop Dis. 2014;8:E3110.
- Bonifaz A, Tirado-Sánchez A. Cutaneous disseminated and extracutaneous sporotrichosis: current status of a complex disease. J Fungi. 2017;3:6.
- Falcão EM, de Lima Filho JB, Campos DP, et al. Hospitalizações e óbitos relacionados à esporotricose no Brasil (1992-2015). Cad Saude Publica. 2019;35:4.
- Mahajan VK, Burkhart CG. Sporotrichosis: an overview and therapeutic options. Dermatol Res Pract. 2014;2014:32-44.
- Hamer EC, Moore CB, Denning DW. Comparison of two fluorescent whiteners, Calcofluor and Blankophor, for the detection of fungal elements in clinical specimens in the diagnostic laboratory. Clin Microbiol Infect. 2006;12:181-184.
- Bernardes-Engemann AR, Orofino Costa RC, Miguens BP, et al. Development of an enzyme-linked immunosorbent assay for the serodiagnosis of several clinical forms of sporotrichosis. Med Mycol. 2005;43:487-493.
- Cognialli R, Bloss K, Weiss I, et al. A lateral flow assay for the immunodiagnosis of human cat-transmitted sporotrichosis. Mycoses. 2022;65:926-934.
- Rodrigues AM, de Hoog GS, de Camargo ZP. Molecular diagnosis of pathogenic Sporothrix species. PLoS Negl Trop Dis. 2015;9:E0004190.
- Della Terra PP, Gonsales FF, de Carvalho JA, et al. Development and evaluation of a multiplex qPCR assay for rapid diagnostics of emerging sporotrichosis. Transbound Emerg Dis. 2022;69.
- Kano R, Nakamura Y, Watanabe S, et al. Identification of Sporothrix schenckii based on sequences of the chitin synthase 1 gene. Mycoses. 2001;44:261-265.
- Oliveira MM, Santos C, Sampaio P, et al. Development and optimization of a new MALDI-TOF protocol for identification of the Sporothrix species complex. Res Microbiol. 2015;166:102-110.
- Bernardes-Engemann AR, Tomki GF, Rabello VBS, et al. Sporotrichosis caused by non-wild type Sporothrix brasiliensis strains. Front Cell Infect Microbiol. 2022;12:893501.
- Waller SB, Dalla Lana DF, Quatrin PM, et al. Antifungal resistance on Sporothrix species: an overview. Braz J Microbiol. 2021;52:73-80.
- Marimon R, Serena C, Gene J. In vitro antifungal susceptibilities of five species of sporothrix. Antimicrob Agents Chemother. 2008;52:732-734.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts (M27, 4th edition). 4th ed. Clinical and Laboratory Standards Institute (CLSI); 2017.
- Clinical and Laboratory Standards Institute (CLSI). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi (Approved Standard, M38, 3rd edition). Clinical and Laboratory Standards Institute (CLSI); 2017
- Oliveira DC, Lopes PG, Spader TB, et al. Antifungal susceptibilities of Sporothrix albicans, S. brasiliensis, and S. luriei of the S. schenckii complex identified in Brazil. J Clin Microbiol. 2011;49:3047-3049.
- Kauffman CA, Bustamante B, Chapman SW, et al. Clinical practice guidelines for the management of sporotrichosis: 2007 update by the Infectious Diseases Society of America. Clin Infect Dis. 2007;45:1255-1265.
- Thompson GR, Le T, Chindamporn A, et al. Global guideline for the diagnosis and management of the endemic mycoses: an initiative of the European Confederation of Medical Mycology in cooperation with the International Society for Human and Animal Mycology. Lancet Infect Dis. 2021;21:E364-E374.
- Francesconi G, Valle AC, Passos S, et al. Terbinafine (250 mg/day): an effective and safe treatment of cutaneous sporotrichosis. J Eur Acad Dermatol Venereol. 2009;23:1273-1276.
- Macedo PM, Lopes-Bezerra LM, Bernardes-Engemann AR, et al. New posology of potassium iodide for the treatment of cutaneous sporotrichosis: study of efficacy and safety in 102 patients. J Eur Acad Dermatol Venereol. 2015;29:719-724.
Approach to Diagnosing and Managing Sporotrichosis
Approach to Diagnosing and Managing Sporotrichosis
Practice Points
- Sporotrichosis is an implantation mycosis that is considered a neglected tropical disease warranting global advocacy to prevent infections and improve patient outcomes.
- Common diagnostic methods such as microscopy may have a low sensitivity for confirming sporotrichosis. Culture from lesional tissue or pus is considered the gold standard for diagnosis.
Longitudinal Erythronychia Manifesting With Pain and Cold Sensitivity
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
The Diagnosis: Glomangiomyoma
The nail unit excision specimen showed collections of cuboidal cells and spindled cells within the corium that were consistent with a diagnosis of a glomangiomyoma, a rare glomus tumor variant (Figure). Glomus tumors are benign neoplasms comprising glomus bodies, which are arteriovenous anastomoses involved in thermoregulation.1 They develop in areas densely populated by glomus bodies, including the fingers, toes, and subungual areas. Glomus tumors most commonly develop in middle-aged women.2 Clinically, they manifest with a characteristic triad of intense pain, point tenderness, and cold sensitivity and may appear as reddish-pink or blue macules under the nail plate and/or longitudinal erythronychia.2-6 The presence of multiple glomus tumors is associated with neurofibromatosis type 1.7

Advanced imaging including ultrasonography and magnetic resonance imaging (MRI) may help confirm the diagnosis but may not be cost effective, as excision with histopathology is needed to relieve symptoms and render a definitive diagnosis. Radiography is highly insensitive in identifying bone erosions associated with glomus tumors.8 With ultrasonography, glomus tumors appear hypoechoic; with Doppler ultrasonography, they appear hypervascular. With MRI, glomus tumors appear as well-defined nodular lesions with hypointense signal intensity on T1-weighted sequence and hyperintense signal intensity on T2-weighted sequence, with strong enhancement using gadolinium-based contrast.9,10 On histopathology, a glomus tumor appears as a nodular tumor with sheets of oval-nucleated cells arranged in multicellular layers surrounding blood vessels and are immunoreactive for α-smooth muscle actin, muscle-specific actin, and type IV collagen.11,12
There are several glomus tumor variants. The most common is a solid glomus tumor, which predominantly is composed of glomus cells, followed by glomangioma, which mainly is composed of blood vessels. Glomangiomyoma, which mostly is composed of smooth muscle cells, is the rarest variant.13
While glomus tumors are common in the subungual areas, it is an uncommon location for glomangiomyomas, which have been reported in the nail unit in only 7 prior case reports identified through searches of PubMed and Google Scholar using the terms glomangiomyoma, glomangiomyoma nail, and subungual glomangiomyoma (Table).13-19 Glomangiomyomas more commonly are described in solid organs, including the stomach, kidney, pancreas, and bladder.16 The mean age of patients with subungual glomangiomyomas, including our patient, was 40.4 years (range, 3-61 years), with the majority being female (75.0% [6/8]). Most patients presented with fingernail involvement (75.0% [6/8]), nail dystrophy (eg, nail plate thinning, longitudinal grooves, splinter hemorrhages, longitudinal erythronychia)(62.5% [5/8]), and intermittent pain and/or point tenderness in the affected nail (75.0% [6/8]).13-19 Notably, only our patient had longitudinal erythronychia as a clinical feature, and only one other case described MRI findings, which included a lobulated mass with intense contrast and distal phalanx destruction.18 One patient was a 3-year-old girl with a family history of generalized multiple glomangiomyomas. Although subungual glomangiomyoma was not confirmed on histopathology, the diagnosis in this patient was presumed based on her family history.13 On histopathology, glomangiomyomas are composed of oval-nucleated cells surrounding blood vessels. These oval-nucleated cells then gradually transition to smooth muscle cells.20

A myxoid cyst is composed of a pseudocyst, which lacks a cyst lining, and is a result of synovial fluid from the distal interphalangeal joint entering the pseudocyst space.2 It typically manifests with a longitudinal groove in the nail plate. A flesh-colored nodule may be appreciated between the cuticle and the distal interphalangeal joint.2 The depth of the longitudinal groove may vary depending on the volume of synovial fluid within the myxoid cyst.21 In a series of 35 cases of subungual myxoid cysts, none manifested with longitudinal erythronychia. Due to their composition, myxoid cysts can be distinguished easily from solid tumors of the nail unit via transillumination.22 Pain is a much less common with myxoid cysts vs glomus tumors, as the filling of the pseudocyst space with synovial fluid typically is gradual, allowing the surrounding tissue to accommodate and adapt over time.21 In equivocal cases, MRI or high-resolution ultrasonography may be used to distinguish myxoid cysts and glomus tumors.8 Histopathology shows accumulation of mucin in the dermis with surrounding fibrous stroma.23
Subungual neuromas are painful benign tumors that develop due to disorganized neural proliferation following disruption to peripheral nerves secondary to trauma or surgery. In 3 case reports, subungual neuromas manifested as painful subungual nodules, with proximal nail plate ridging, or onycholysis.24-26 Since neuromas have only rarely been described in the subungual region, reports of MRI and ultrasonography findings are unknown. Histopathology is needed to distinguish neuromas from glomus tumors. Histopathology shows an acapsular structure consisting of disorganized spindle-cell proliferation and nerve fibers arranged in a tangle of fascicles within fibrotic tissue.25 On immunochemistry, spindle cells typically are positive for cellular antigen protein S100.26
Leiomyomas are benign neoplasms derived from smooth muscle, typically localized to the uterus or gastrointestinal tract, and have been described rarely in the nail unit.27,28 It is hypothesized that subungual leiomyomas originate from the vascular smooth muscle in the subcutaneous layer of the nail unit.28 Like glomus tumors, leiomyomas of the subungual region often manifest with pain and longitudinal erythronychia.27-30 Subungual leiomyomas may be distinguished from glomus tumors via advanced imaging techniques, including ultrasonography and MRI. Cutaneous leiomyomas have been described with mild to moderate internal low flow vascularity on Doppler ultrasonography, while glomus tumors typically reveal high internal vascularity.28 Biopsy with histopathology is needed for definitive diagnosis. On histopathology, leiomyomas demonstrate bland-appearing spindle-shaped cells with elongated nuclei arranged in fascicles.27 They typically are positive for α-smooth muscle actin and caldesmon on immunostaining.
Eccrine spiradenomas are benign adnexal tumors likely of apocrine origin with limited case reports in the literature.31,32 Clinically, eccrine spiradenomas involving the nail unit may manifest with longitudinal nail splitting of the nail or as a papule on the proximal nail fold, with associated tenderness.31,32 In a report of a 50-year-old woman with a histopathologically confirmed eccrine spiradenoma manifesting with longitudinal splitting of the nail and pain in the proximal nail fold, the mass appeared hypoechoic on ultrasonography with increased intramass vascularity on Doppler, while MRI showed an intensely enhancing lesion.31 These imaging features, combined with a classically manifesting feature of pain, make eccrine spiradenomas difficult to distinguish from glomus tumors; therefore, histopathologic examination can provide a definitive diagnosis, and surgical excision is used for treatment.31 On histopathology, these tumors are well circumscribed and composed of both small dark basaloid cells with peripheral compact nuclei and larger cells with central pale nuclei, which may be arranged in tubules.31,32
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
- Gombos Z, Zhang PJ. Glomus tumor. Arch Pathol Lab Med. 2008;132: 1448-1452. doi:10.5858/2008-132-1448-gt
- Hare AQ, Rich P. Nail tumors. Dermatol Clin. 2021;39:281-292. doi:10.1016/j.det.2020.12.007
- Hazani R, Houle JM, Kasdan ML, et al. Glomus tumors of the hand. Eplasty. 2008;8:E48.
- Hwang JK, Lipner SR. Blue nail discoloration: literature review and diagnostic algorithms. Am J Clin Dermatol. 2023;24:419-441. doi:10.1007/s40257-023-00768-6
- Lipner SR, Scher RK. Longitudinal erythronychia of the fingernail. JAMA Dermatol. 2016;152:1271-1272. doi:10.1001/jamadermatol.2016.2747
- Jellinek NJ, Lipner SR. Longitudinal erythronychia: retrospective single-center study evaluating differential diagnosis and the likelihood of malignancy. Dermatol Surg. 2016;42:310-319. doi:10.1097 /DSS.0000000000000594
- Lipner SR, Scher RK. Subungual glomus tumors: underrecognized clinical findings in neurofibromatosis 1. J Am Acad Dermatol. 2021;84:E269. doi:10.1016/j.jaad.2020.08.129
- Dhami A, Vale SM, Richardson ML, et al. Comparing ultrasound with magnetic resonance imaging in the evaluation of subungual glomus tumors and subungual myxoid cysts. Skin Appendage Disord. 2023;9:262-267. doi:10.1159/000530397
- Baek HJ, Lee SJ, Cho KH, et al. Subungual tumors: clinicopathologic correlation with US and MR imaging findings. Radiographics. 2010;30:1621-1636. doi:10.1148/rg.306105514
- Patel T, Meena V, Meena P. Hand and foot glomus tumors: significance of MRI diagnosis followed by histopathological assessment. Cureus. 2022;14:E30038. doi:10.7759/cureus.30038
- Mravic M, LaChaud G, Nguyen A, et al. Clinical and histopathological diagnosis of glomus tumor: an institutional experience of 138 cases. Int J Surg Pathol. 2015;23:181-188. doi:10.1177/1066896914567330
- Folpe AL, Fanburg-Smith JC, Miettinen M, et al. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25:1-12. doi:10.1097/00000478-200101000-00001
- Calduch L, Monteagudo C, Martínez-Ruiz E, et al. Familial generalized multiple glomangiomyoma: report of a new family, with immunohistochemical and ultrastructural studies and review of the literature. Pediatr Dermatol. 2002;19:402-408. doi:10.1046/j.1525-1470.2002.00114.x
- Mentzel T, Hügel H, Kutzner H. CD34-positive glomus tumor: clinicopathologic and immunohistochemical analysis of six cases with myxoid stromal changes. J Cutan Pathol. 2002;29:421-425. doi:10.1034 /j.1600-0560.2002.290706.x
- Kang TW, Lee KH, Park CJ. A case of subungual glomangiomyoma with myxoid stromal change. Korean J Dermatol. 2008;46:550-553.
- Wollstein A, Wollstein R. Subungual glomangiomyoma—a case report. Hand Surg. 2012;17:271-273. doi:10.1142/S021881041272032X
- Aqil N, Gallouj S, Moustaide K, et al. Painful tumors in a patient with neurofibromatosis type 1: a case report. J Med Case Rep. 2018;12:319. doi:10.1186/s13256-018-1847-0
- Demirdag HG, Akay BN, Kirmizi A, et al. Subungual glomangiomyoma. J Am Podiatr Med Assoc. 2020;110:Article_13. doi:10.7547/19-051
- Vega SML, Ruiz SJA, Ramírez CS, et al. Subungual glomangiomyoma: a case report. Dermatol Cosmet Med Quir. 2022;20:258-262.
- Chalise S, Jha A, Neupane PR. Glomangiomyoma of uncertain malignant potential in the urinary bladder: a case report. JNMA J Nepal Med Assoc. 2021;59:719-722. doi:10.31729/jnma.5388
- de Berker D, Goettman S, Baran R. Subungual myxoid cysts: clinical manifestations and response to therapy. J Am Acad Dermatol. 2002;46:394-398. doi:10.1067/mjd.2002.119652
- Gupta MK, Lipner SR. Transillumination for improved diagnosis of digital myxoid cysts. Cutis. 2020;105:82.
- Fernandez-Flores A, Saeb-Lima M. Mucin as a diagnostic clue in dermatopathology. J Cutan Pathol. 2016;43:1005-1016. doi:10.1111/cup.12782
- Choi R, Kim SR, Glusac EJ, et al. Subungual neuroma masquerading as green nail syndrome. JAAD Case Rep. 2022;20:17-19. doi:10.1016 /j.jdcr.2021.11.025
- Rashid RM, Rashid RM, Thomas V. Subungal traumatic neuroma. J Am Acad Dermatol. 2010;63:E7-E8. doi:10.1016/j.jaad.2010.01.028
- Whitehouse HJ, Urwin R, Stables G. Traumatic subungual neuroma. Clin Exp Dermatol. 2018;43:65-66. doi:10.1111/ced.13247
- Lipner SR, Ko D, Husain S. Subungual leiyomyoma presenting as erythronychia: case report and review of the literature. J Drugs Dermatol. 2019;18:465-467.
- Taleb E, Saldías C, Gonzalez S, et al. Sonographic characteristics of leiomyomatous tumors of skin and nail: a case series. Dermatol Pract Concept. 2022;12:e2022082. doi:10.5826/dpc.1203a82
- Baran R, Requena L, Drapé JL. Subungual angioleiomyoma masquerading as a glomus tumour. Br J Dermatol. 2000;142:1239-1241. doi:10.1046/ j.1365-2133.2000.03560.x
- Watabe D, Sakurai E, Mori S, et al. Subungual angioleiomyoma. Indian J Dermatol Venereol Leprol. 2017;83:74-75. doi:10.4103/0378-6323 .185045
- Jha AK, Sinha R, Kumar A, et al. Spiradenoma causing longitudinal splitting of the nail. Clin Exp Dermatol. 2016;41:754-756. doi:10.1111 /ced.12886
- Leach BC, Graham BS. Papular lesion of the proximal nail fold. eccrine spiradenoma. Arch Dermatol. 2004;140:1003-1008. doi:10.1001 /archderm.140.8.1003-a
A 38-year-old woman presented to our nail specialty clinic with a red line and associated pain on the left fourth fingernail of 2 and 3 years’ duration, respectively. The patient described the pain as throbbing, with sensitivity to pressure and cold. She noted that the nail grew slowly and would sometimes split at the distal edge. She did not recall any discrete trauma to the digit or nail. The patient was right-handed, making the symptoms less likely to be due to overuse from daily activities. She had received no prior treatment for these symptoms.
The patient’s medical history included iron deficiency as well as acne and eczema. She had no personal or family history of skin cancer. Physical examination of the affected digit and nail revealed a longitudinal red line and distal onycholysis. With contact dermoscopy, the red line blanched. Pressure applied using a #11 scalpel blade elicited pinpoint tenderness (positive Love test), and application of an ice pack caused pain (positive cold test). A radiograph of the left hand was negative for bone erosions, and magnetic resonance imaging showed a 0.3-cm subungual lesion at the level of the fourth distal phalanx. An excision of the nail unit was performed.
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.
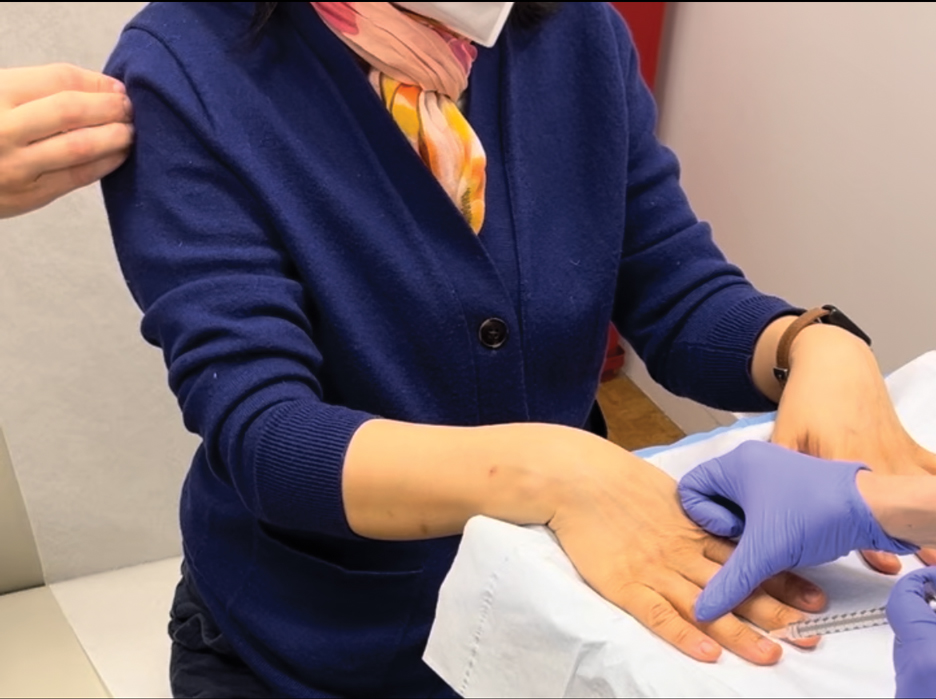
This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
Practice Gap
Pain during minimally invasive dermatologic procedures such as lidocaine injections, cryotherapy, nail unit injections, and cosmetic procedures including neurotoxin injections can cause patient discomfort leading to procedural anxiety, poor compliance with treatment regimens, and avoidance of necessary care. Current solutions to manage pain during dermatologic procedures present several limitations; for example, topical anesthetics seldom alleviate procedural pain,1 particularly in sensitive areas (eg, nail unit, face) or for patients with a needle phobia. Additionally, topical anesthetics often require up to 2 hours to take effect, making them impractical for quick outpatient procedures. Other pain reduction strategies including vibration devices or cold sprays2,3 can be effective but are an added expense to the physician or clinic, which may preclude their use in resource-limited settings. Psychological distraction techniques such as deep breathing require active patient participation and might reinforce pain expectations and increase patient anxiety.4 Given these challenges, there is a need for effective, affordable, nonpharmacologic pain reduction strategies that can be integrated seamlessly into clinical practice to enhance the patient experience.
The Technique
Tapping is a simple noninvasive distraction technique that may alleviate procedural pain by exploiting the gate control theory of pain.5 According to this theory, tactile stimuli activate mechanoreceptors that send inhibitory signals to the spinal cord, effectively closing the gate to pain transmission. Unlike the Helfer skin tap technique,6 which involves 15 preinjection taps and 3 postinjection taps directly on the injection site, our approach targets distant bony prominences. This modification allows for immediate needle insertion without interfering with the sterile field or increasing the risk for needlestick injuries from tapping near the injection site. Bony sites such as the shoulder or knee are ideal for this technique due to their high density and rigidity that efficiently transmit tactile stimuli––similar to how sound travels faster through solids than through liquids or gases.7
To implement this technique in practice, we first stabilize the injection site to reduce movement from tapping. This can be done by stabilizing the injection site (eg, resting the hand on an instrument stand during a nail unit injection). A second person—such as a medical assistant, medical student, resident, or even the patient’s family member—taps at a distant site at least an arm’s length away from the injection site (Figure). The tapping pressure should be firm enough for the patient to feel the vibration but not forceful enough that it becomes unpleasant or disrupts the injection area. Tapping starts just before needle insertion and continues through the injection. No warning is given to the patient, as the surprise element may help distract them from pain. Varying the rhythm, intensity, or location of the tapping can enhance its distracting effect.

This tapping technique can be effectively combined with other pain reduction strategies in a multimodal approach; for example, when used concurrently with topical anesthetics, both the central (tapping) and peripheral (anesthetic) pain pathways are addressed, potentially yielding additive effects. For patients with a needle phobia, pairing tapping with cognitive distraction (eg, talkesthesia) may further reduce anxiety. In our nail specialty clinic at Weill Cornell Medicine (New York, New York), we often combine tapping with cold sprays and talkesthesia, which improves patient comfort without prolonging the visit. Importantly, the technique enables seamless integration with most pharmacologic and nonpharmacologic methods, eliminating the need for additional patient education or procedure time.
Practice Implications
The tapping technique described here is free, easy to implement, and requires no additional resources aside from another person to tap the patient during the procedure. It can be used for a wide range of dermatologic procedures, including biopsies, intralesional injections, and cosmetic treatments, including neurotoxin injections. The minimal learning curve and ease of integration into procedural workflows make this technique a valuable tool for dermatologists aiming to improve patient comfort without disrupting workflow. In our practice, we have observed that tapping reduces self-reported pain and helps ease anxiety, particularly in patients with a needle phobia. Its simplicity and accessibility make it a valuable addition to a wide range of dermatologic procedures. Prospective studies investigating patient-reported outcomes could help establish this technique’s role in clinical practice.
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
- Navarro-Rodriguez JM, Suarez-Serrano C, Martin-Valero R, et al. Effectiveness of topical anesthetics in pain management for dermal injuries: a systematic review. J Clin Med. 2021;10:2522. doi:10.3390/jcm10112522
- Lipner SR. Pain-minimizing strategies for nail surgery. Cutis. 2018;101:76-77.
- Ricardo JW, Lipner SR. Air cooling for improved analgesia during local anesthetic infiltration for nail surgery. J Am Acad Dermatol. 2021;84:e231-e232. doi:10.1016/j.jaad.2019.11.032
- Hill RC, Chernoff KA, Lipner SR. A breath of fresh air: use of deep breathing technique to minimize pain with nail injections. J Am Acad Dermatol. 2024;90:e163. doi:10.1016/j.jaad.2023.10.043
- Mendell LM. Constructing and deconstructing the gate theory of pain. Pain. 2014;155:210-216. doi:10.1016/j.pain.2013.12.010
- Jyoti G, Arora S, Sharma B. Helfer Skin Tap Tech Technique for the IM injection pain among adult patients. Nursing & Midwifery Research Journal. 2018;14:18-30. doi:10.1177/0974150X20180304
- Iowa State University. Nondestructive Evaluation Physics: Sound. Published 2021. Accessed July 31, 2025. https://www.nde-ed.org/Physics/Sound/speedinmaterials.xhtml
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
Tapping Into Relief: A Distraction Technique to Reduce Pain During Dermatologic Procedures
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.
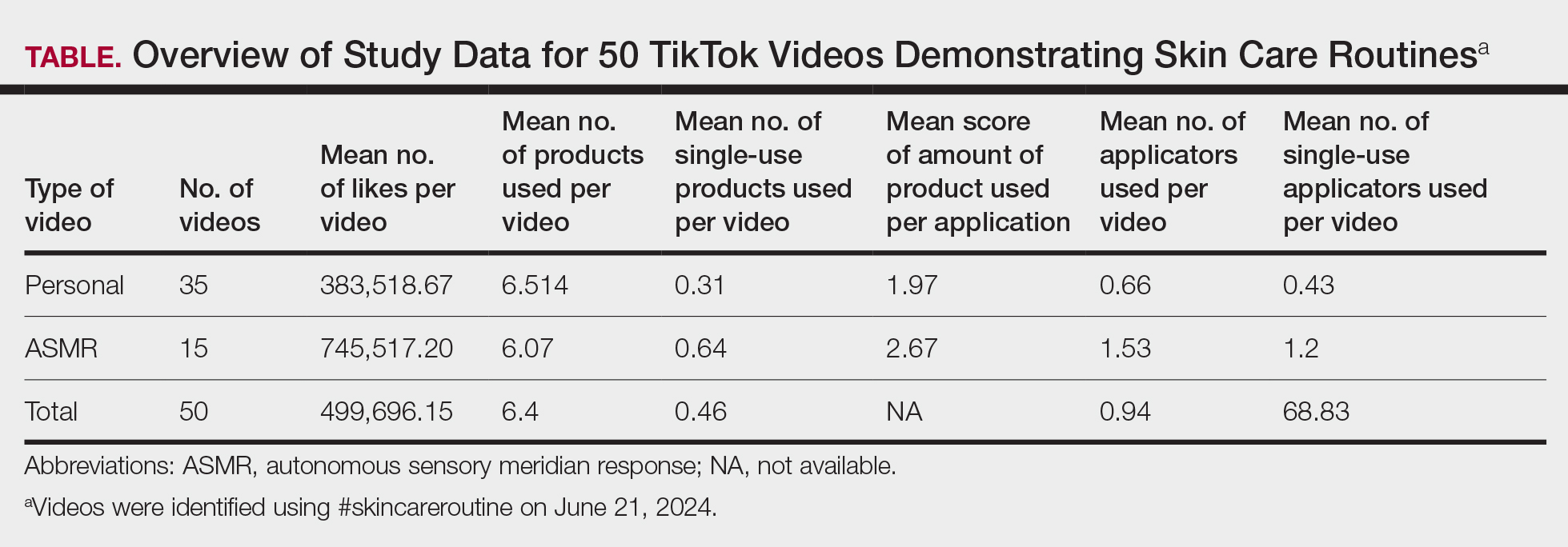
Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
To the Editor:
The popularity of the social media platform TikTok, which is known for its short-form videos, has surged in recent years. Viral videos demonstrating skin care routines reach millions of viewers,1 showcasing specific products, detailing beauty regimens, and setting fads that many users eagerly follow. These trends often influence consumer behavior—in 2023, viral videos using the tag #TikTokMadeMeBuy lead to a 14% growth in the sale of skin care products.2 However, they also encourage purchasing decisions that may escalate environmental waste through plastic packaging and single-use products. In this study, we analyzed videos on TikTok to assess the environmental impact of trending skin care routines. By examining the types of products promoted, their packaging, and the frequency with which they appear in viral content, we aimed to investigate how these trends, which may be imitated by users, impact the environment.
A search of TikTok videos using #skincareroutine was conducted on June 21, 2024. Sponsored content, non–English language videos, videos without demonstrated skin care routines, and videos showing makeup routines were excluded from our analysis. Data collected from each video included username, date posted, number of likes, total number of skin care products used, number of single-use skin care products used, average amount of product used, number of skin care applicators used, and number of single-use applicators used. Single-use items, defined as those intended for one-time use and subsequent disposal, were identified visually by packaging, manufacturer intent, and common consumer usage patterns. The amount of product used per application was graded on a scale of 1 to 3 (1=pea-sized amount or less; 2=single full pump/spray; 3=multiple pumps/sprays). Videos were categorized as personal (ie, skin care routine walk-throughs by the creator) or autonomous sensory meridian response (ASMR)(focused on product sounds and aesthetics).3 A Mann-Whitney U test was utilized to statistically compare the 2 groups. Statistical analysis was performed using Microsoft Excel (α=0.05).
A total of 50 videos met the inclusion criteria and were included in the analysis. The average number of likes per video was 499,696.15, with skin care routines featuring an average of 6.4 unique products (Table). There was a weak positive correlation (r=0.1809) between the number of skin care products used and the number of likes. A total of 320 products were used across the videos, 23 of which were single-use (7.2%).On average, single-use skin care items were used 0.46 times per routine, comprising a mean 7.99% of total products per video. The average score for the amount of product used per application was 2.18. There was no difference in personal vs ASMR videos with regard to the total number of skin care products used or the average amount of product used per application (P>.05). Thirty-three (70.2%) of the 47 applicators used across all videos were single-use. An average of 0.94 applicators per routine were utilized, with a mean 68.83% being single-use applicators. Common single-use products were toner wipes and eye patches, and single-use applicators included cotton pads and plastic spatulas.

Our findings indicated a prevalence of multiple products and large amount of product used in trending skin care routines, suggesting a shift toward multistep skin care. This implies a high rate of product consumption that may accelerate the carbon footprint associated with skin care products,3 which could contribute to climate change and environmental degradation. Consumers also may feel compelled to purchase and discard numerous partially used products in order to keep up with the latest trends, exacerbating the environmental impact. Furthermore, the utilization of single-use products and applicators contributes to increased plastic waste, pollution, and resource depletion. Single-use items often are difficult to recycle due to their mixed materials and small size,4,5 and therefore they can accumulate in landfills and oceans. This impact can be mitigated by switching to reusable applicators, refillable packaging, and biodegradable materials.
The substantial average number of likes per video indicates high engagement with skin care content among TikTok users. The continued popularity of complex multistep skin care routines, despite a weak correlation between the number of skin care products used and the number of likes per video, likely stems from factors such as aesthetic appeal, ASMR effects, and creators’ established followings, which may drive user engagement to contribute to unsustainable consumption patterns. Factors such as presentation style, aesthetics, or creators’ pre-existing online following may have a major impact on how well a video performs on TikTok. The similarity between personal and ASMR videos, particularly in the number of products used and the amount applied, suggests that both formats employ common approaches to meet audience expectations and align with promotional trends, relying more on sensory and aesthetic strategies than substantive differences in skin care routines.
Our use of only one tag in our search as well as the subjective quantity scale limits the generalizability of these findings to broader TikTok skin care content.
Overall, our study underscores the role of brands and social media influencers in skin care education and promotion of sustainable practices. The extensive number of products used and generous application of each product in skin care routines demonstrated in TikTok videos may mislead viewers into believing that using more product improves outcomes, when often, less is more. We recommend that dermatologists counsel patients about informed skin care regimens that prioritize individual needs over social media fads.
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
- Pagani K, Lukac D, Martinez R, et al. Slugging: TikTokTM as a source of a viral “harmless” beauty trend. Clin Dermatol. 2022;40:810-812. doi:10.1016/j.clindermatol.2022.08.005
- Stern C. TikTok drives $31.7B in beauty sales: how viral trends are shaping the future of cosmetics. CosmeticsDesign. August 20, 2024. Accessed June 24, 2025. https://www.cosmeticsdesign.com/Article/2024/08/20/tiktok-drives-31.7b-in-beauty-sales-how-viral-trends-are-shaping-the-future-of-cosmetics/
- Fountain C. ASMR content saw huge growth on YouTube, but now creators are flocking to TikTok instead. Business Insider. July 4, 2022. Accessed June 24, 2025. https://www.businessinsider.com/asmr-tiktok-instead-of-youtube-growth-subscribers-2022-7
- Rathore S, Schuler B, Park J. Life cycle assessment of multiple dispensing systems used for cosmetic product packaging. Packaging Technol Sci. 2023;36:533-547. doi:10.1002/pts.2729
- Shaw S. How to actually recycle your empty beauty products. CNN Underscored. Updated April 17, 2024. Accessed June 24, 2025. https://www.cnn.com/cnn-underscored/beauty/how-to-recycle-beauty-products
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
A Cross-Sectional Analysis of TikTok Skin Care Routines and the Associated Environmental Impact
PRACTICE POINTS
- Social media platforms are increasingly influential in shaping consumer skin care habits, particularly among younger demographics.
- Dermatologists should be aware of the aesthetic-driven nature of online skin care trends when advising patients on product use.
- Viral skin care routines often feature multiple products and applicators, potentially encouraging excessive product use and waste.
Approach to Diagnosing and Managing Implantation Mycoses
Approach to Diagnosing and Managing Implantation Mycoses
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.
Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26
Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32
Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36
Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.


Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26

Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32


Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36

Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
Implantation mycoses such as chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are a diverse group of fungal diseases that occur when a break in the skin allows the entry of the causative fungus. These diseases disproportionately affect individuals in low- and middle-income countries causing substantial disability, decreased quality of life, and severe social stigma.1-3 Timely diagnosis and appropriate treatment are critical.
Chromoblastomycosis and mycetoma are designated as neglected tropical diseases, but research to improve their management is sparse, even compared to other neglected tropical diseases.4,5 Since there are no global diagnostic and treatment guidelines to date, we outline steps to diagnose and manage chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma.
Chromoblastomycosis
Chromoblastomycosis is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Chromoblastomycosis is distinguished from subcutaneous phaeohyphomycosis by microscopically visualizing the characteristic thick-walled, single, or multicellular clusters of pigmented fungal cells (also known as medlar bodies, muriform cells, or sclerotic bodies).6 In phaeohyphomycosis, short hyphae and pseudohyphae plus some single cells typically are seen.
Epidemiology—Globally, the distribution and burden of chromoblastomycosis are relatively unknown. Infections are more common in tropical and subtropical areas but can be acquired anywhere. A literature review conducted in 2021 identified 7740 cases of chromoblastomycosis, mostly reported in South America, Africa, Central America and Mexico, and Asia.7 Most of the patients were male, and the median age was 52 years. One study found an incidence of 14.7 per 1,000,000 patients in the United States for both chromoblastomycosis and phaeohyphomycotic abscesses (which included both skin and brain abscesses).8 Most patients were aged 65 years or older, with a higher incidence in males. Geographically, the incidence was highest in the Northeast followed by the South; patients in rural areas also had higher incidence of disease.8
Causative Organisms—Causative species cannot reliably distinguish between chromoblastomycosis and subcutaneous phaeohyphomycosis, as some species overlap. Cladophialophora carrionii, Fonsecaea species, Phialophora verrucosa species complex, and Rhinocladiella aquaspersa most commonly cause chromoblastomycosis.9,10
Clinical Manifestations—Chromoblastomycosis initially manifests as a solitary erythematous macule at a site of trauma (often not recalled by the patient) that can evolve to a smooth pink papule and may progress to 1 of 5 morphologies: nodular, verrucous, tumorous, cicatricial, or plaque.6 Patients may present with more than one morphology, particularly in long-standing or advanced disease. Lesions commonly manifest on the arms and legs in otherwise healthy individuals in environments (eg, rural, agricultural) that have more opportunities for injury and exposure to the causative fungi. Affected individuals often have small black specks on the lesion surface that are visible with the naked eye.6
Diagnosis—Common differential diagnoses include cutaneous blastomycosis, fixed sporotrichosis, warty tuberculosis nocardiosis, cutaneous leishmaniasis, human papillomavirus (HPV) infection, podoconiosis, lymphatic filariasis, cutaneous tuberculosis, and psoriasis.6 Squamous cell carcinoma is both a differential diagnosis as well as a potential complication of the disease.11
Potassium hydroxide preparation with skin scapings or a biopsy from the lesion has high sensitivity and quick turnaround times. There often is a background histopathologic reaction of pseudoepitheliomatous hyperplasia. Examining samples taken from areas with the visible small black dots on the skin surface can increase the likelihood of detecting fungal elements (Figure 1). Clinicians also may choose to obtain a 6- to 8-mm deep skin biopsy from the lesion and splice it in half, with one sample sent for histopathology and the other for culture (Figure 2). Skin scrapings can be sent for culture instead. In the case of verrucous lesions, biopsy is preferred if feasible.


Treatment should not be delayed while awaiting the culture results if infection is otherwise confirmed by direct microscopy or histopathology. The treatment approach remains similar regardless of the causative species. If the culture results are positive, the causative genus can be identified by the microscopic morphology; however, molecular diagnostic tools are needed for accurate species identification.12,13
Antifungal Susceptibility Testing—For most dematiaceous fungi, interpreting minimum inhibitory concentrations (MICs) is challenging due to a lack of data from multicenter studies. One report examined sequential isolates of Fonsecaea pedrosoi and demonstrated both high MIC values and clinical resistance to itraconazole in some cases, likely from treatment pressure.14 Clinical Laboratory Standards Institute–approved epidemiologic cutoff values (ECVs) are established for F pedrosoi for commonly used antifungals including itraconazole (0.5 µg/mL), terbinafine (0.25 µg/mL), and posaconazole (0.5 µg/mL).15 Clinicians may choose to obtain sequential isolates for any causative fungi in recalcitrant disease to monitor for increases in MIC.
Management—In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. If antifungals are needed, itraconazole is the most commonly prescribed agent, typically at a dose of 100 to 200 mg twice daily. Terbinafine also has been used first-line at a dose of 250 to 500 mg per day. Voriconazole and posaconazole also may be suitable options for first-line or for refractory disease treatment. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy usually is several months, but many patients require years of therapy until resolution of lesions.
Clinicians can consider combination therapy with an antifungal and a topical immunomodulator such as imiquimod (applied topically 3 times per week); this combination can be considered in refractory disease and even upon initial diagnosis, especially in severe disease.17,18 Nonpharmacologic interventions such as cryotherapy, heat, and light-based therapies have been used, but outcome data are scarce.19-23
Subcutaneous Phaeohyphomycosis
Subcutaneous phaeohyphomycosis also is caused by dematiaceous fungi that typically affect the skin and subcutaneous tissue. Subcutaneous phaeohyphomycosis is distinguished from chromoblastomycosis by short hyphae and hyphal fragments usually seen microscopically instead of visualizing thick-walled, single, or multicellular clusters of pigmented fungal cells.6
Epidemiology—Globally, the burden and distribution of phaeohyphomycosis, including its cutaneous manifestations, are not well understood. Infections are more common in tropical and subtropical areas but can be acquired anywhere. Phaeohyphomycosis is a generic term used to describe infections caused by pigmented hyphal fungi that can manifest on the skin (subcutaneous phaeohyphomycosis) but also can affect deep structures including the brain (systemic phaeohyphomycosis).24
Causative Organisms—Alternaria, Bipolaris, Cladosporium, Curvularia, Exophiala, and Exserohilum species most commonly cause subcutaneous phaeohyphomycosis. Alternaria infections manifesting with skin lesions often are referred to as cutaneous alternariosis.25
Clinical Manifestations—The most common skin manifestation of phaeohyphomycosis is a subcutaneous cyst (cystic phaeohyphomycosis)(Figure 2). Subcutaneous phaeohyphomycosis also may manifest with nodules or plaques (Figure 3). Phaeohyphomycosis appears to occur more commonly in individuals who are immunosuppressed, those in whom T-cell function is affected, in congenital immunodeficiency states (eg, individuals with CARD9 mutations).26

Diagnosis—Culture is the gold standard for confirming phaeohyphomycosis.27 For cystic phaeohyphomycosis, clinicians can consider aspiration of the cyst for direct microscopic examination and culture. Histopathology may be utilized but can have lower sensitivity in showing dematiaceous hyphae and granulomatous inflammation; using the Masson-Fontana stain for melanin can be helpful. Molecular diagnostic tools including metagenomics applied directly to the tissue may be useful but are likely to have lower sensitivity than culture and require specialist diagnostic facilities.
Management—The approaches to managing chromoblastomycosis and subcutaneous phaeohyphomycosis are similar, though the preferred agents often differ. In early-stage disease, excision of the skin nodule may be curative, although concomitant treatment for several months with an antifungal is advised. In localized forms, itraconazole usually is used, but in those cases associated with immunodeficiency states, voriconazole may be necessary. Fluconazole does not have good activity against dematiaceous fungi and should be avoided.16 Topical antifungals will not reach the site of infection in adequate concentrations. Topical corticosteroids can make the disease worse and should be avoided. The duration of therapy may be substantially longer for chromoblastomycosis (months to years) compared to subcutaneous phaeohyphomycosis (weeks to months), although in immunocompromised individuals treatment may be even more prolonged.
Mycetoma
Mycetoma is caused by one of several different types of fungi (eumycetoma) and bacteria (actinomycetoma) that lead to progressively debilitating yet painless subcutaneous tumorlike lesions. The lesions usually manifest on the arms and legs but can occur anywhere.
Epidemiology—Little is known about the true global burden of mycetoma, but it occurs more frequently in low-income communities in rural areas.28 A retrospective review identified 19,494 cases published from 1876 to 2019, with cases reported in 102 countries.29 The countries with the highest numbers of cases are Sudan and Mexico, where there is more information on the distribution of the disease. Cases often are reported in what is known as the mycetoma belt (between latitudes 15° south and 30° north) but are increasingly identified outside this region.28 Young men aged 20 to 40 years are most commonly affected.
In the United States, mycetoma is uncommon, but clinicians can encounter locally acquired and travel-associated cases; hence, taking a good travel history is essential. One study specifically evaluating eumycetoma found a prevalence of 5.2 per 1,000,000 patients.8 Women and those aged 65 years or older had a higher incidence. Incidence was similar across US regions, but a higher incidence was reported in nonrural areas.8
Causative Organisms—More than 60 different species of fungi can cause eumycetoma; most cases are caused by Madurella mycetomatis, Trematosphaeria grisea (formerly Madurella grisea); Pseudallescheria boydii species complex, and Falciformispora (formerly Leptosphaeria) senegalensis.30 Actinomycetoma commonly is caused by Nocardia species (Nocardia brasiliensis, Nocardia asteroides, Nocardia otitidiscaviarum, Nocardia transvalensis, Nocardia harenae, and Nocardia takedensis), Streptomyces somaliensis, and Actinomadura species (Actinomadura madurae, Actinomadura pelletieri).31
Clinical Manifestations—Mycetoma is a chronic granulomatous disease with a progressive inflammatory reaction (Figures 4 and 5). Over the course of years, mycetoma progresses from small nodules to large, bone-invasive, mutilating lesions. Mycetoma manifests as a triad of painless firm subcutaneous masses, formation of multiple sinuses within the masses, and a purulent or seropurulent discharge containing sandlike visible particles (grains) that can be white, yellow, red, or black.28 Lesions usually are painless in early disease and are slowly progressive. Large lesion size, bone destruction, secondary bacterial infections, and actinomycetoma may lead to higher likelihood of pain.32


Diagnosis—Other conditions that could manifest with the same triad seen in mycetoma such as botryomycosis should be included in the differential. Other differential diagnoses include foreign body granuloma, filariasis, mycobacterial infection, skeletal tuberculosis, and yaws.
Proper treatment requires an accurate diagnosis that distinguishes actinomycetoma from eumycetoma.33 Culturing of grains obtained from deep lesion aspirates enables identification of the causative organism (Figure 6). The color of the grains may provide clues to their etiology: black grains are caused by fungus, red grains by a bacterium (A pelletieri), and pale (yellow or white) grains can be caused by either one.31Nocardia mycetoma grains are very small and usually cannot be appreciated with the naked eye. Histopathology of deep biopsy specimens (biopsy needle or surgical biopsy) stained with hematoxylin and eosin can diagnose actinomycetoma and eumycetoma. Punch biopsies often are not helpful, as the inflammatory mass is too deeply located. Deep surgical biopsy is preferred; however, species identification cannot be made without culture. Molecular tests for certain causative organisms of mycetoma have been developed but are not readily available.34,35 Currently, no serologic tests can diagnose mycetoma reliably. Ultrasonography can be used to diagnose mycetoma and, with appropriate training, distinguish between actinomycetoma and eumycetoma; it also can be combined with needle aspiration for taking grain samples.36

Treatment—Treatment of mycetoma depends on identification of the causal etiology and requires long-term and expensive drug regimens. It is not possible to determine the causative organism clinically. Actinomycetoma generally responds to medical treatment, and surgery rarely is needed. The current first-line treatment is co-trimoxazole (trimethoprim/sulfamethoxazole) in combination with amoxicillin and clavulanate acid or co-trimoxazole and amikacin for refractory disease; linezolid also may be a promising option for refractory disease.37
Eumycetoma is less responsive to medical therapies, and recurrence is common. Current recommended therapy is itraconazole for 9 to 12 months; however, cure rates ranging from 26% to 75% in combination with surgery have been reported, and fungi often can still be cultured from lesions posttreatment.38,39 Surgical excision often is used following 6 months of treatment with itraconazole to obtain better outcomes. Amputation may be required if the combination of antifungals and surgical excision fails. Fosravuconazole has shown promise in one clinical trial, but it is not approved in most countries, including the United States.39
Final Thoughts
Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma can cause devastating disease. Patients with these conditions often are unable to carry out daily activities and experience stigma and discrimination. Limited diagnostic and treatment options hamper the ability of clinicians to respond appropriately to suspect and confirmed disease. Effectively examining the skin is the starting point for diagnosing and managing these diseases and can help clinicians to care for patients and prevent severe disease.
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
- Smith DJ, Soebono H, Parajuli N, et al. South-east Asia regional neglected tropical disease framework: improving control of mycetoma, chromoblastomycosis, and sporotrichosis. Lancet Reg Health Southeast Asia. 2025;35:100561. doi:10.1016/j.lansea.2025.100561
- Abbas M, Scolding PS, Yosif AA, et al. The disabling consequences of mycetoma. PLoS Negl Trop Dis. 2018;12:E0007019. doi:10.1371/journal.pntd.0007019
- Siregar GO, Harianja M, Rinonce HT, et al. Chromoblastomycosis: a case series from Sumba, eastern Indonesia. Clin Exp Dermatol. Published online March 8, 2025. doi:10.1093/ced/llaf111
- World Health Organization. Ending the neglect to attain the Sustainable Development Goals: a road map for neglected tropical diseases 2021-2030. Published January 28, 2021. Accessed May 5, 2024. https://www.who.int/publications/i/item/9789240010352
- Impact Global Health. The G-FINDER 2024 neglected disease R&D report. Impact Global Health. Published January 30, 2025. Accessed January 12, 2025. https://cdn.impactglobalhealth.org/media/G-FINDER%202024_Full%20report-1.pdf
- Queiroz-Telles F, de Hoog S, Santos DWCL, et al. Chromoblastomycosis. Clin Microbiol Rev. 2017;30:233-276. doi:10.1128/CMR.00032-16
- Santos DWCL, de Azevedo CMPS, Vicente VA, et al. The global burden of chromoblastomycosis. PLoS Negl Trop Dis. 2021;15:E0009611. doi:10.1371/journal.pntd.0009611
- Gold JAW, Smith DJ, Benedict K, et al. Epidemiology of implantation mycoses in the United States: an analysis of commercial insurance claims data, 2017 to 2021. J Am Acad Dermatol. 2023;89:427-430. doi:10.1016/j.jaad.2023.04.048
- Smith DJ, Queiroz-Telles F, Rabenja FR, et al. A global chromoblastomycosis strategy and development of the global chromoblastomycosis working group. PLoS Negl Trop Dis. 2024;18:e0012562. doi:10.1371/journal.pntd.0012562
- Heath CP, Sharma PC, Sontakke S, et al. The brief case: hidden in plain sight—exophiala jeanselmei subcutaneous phaeohyphomycosis of hand masquerading as a hematoma. J Clin Microbiol. 2024;62:E01068-24. doi:10.1128/jcm.01068-24
- Azevedo CMPS, Marques SG, Santos DWCL, et al. Squamous cell carcinoma derived from chronic chromoblastomycosis in Brazil. Clin Infect Dis. 2015;60:1500-1504. doi:10.1093/cid/civ104
- Sun J, Najafzadeh MJ, Gerrits van den Ende AHG, et al. Molecular characterization of pathogenic members of the genus Fonsecaea using multilocus analysis. PloS One. 2012;7:E41512. doi:10.1371/journal.pone.0041512
- Najafzadeh MJ, Sun J, Vicente V, et al. Fonsecaea nubica sp. nov, a new agent of human chromoblastomycosis revealed using molecular data. Med Mycol. 2010;48:800-806. doi:10.3109/13693780903503081
- Andrade TS, Castro LGM, Nunes RS, et al. Susceptibility of sequential Fonsecaea pedrosoi isolates from chromoblastomycosis patients to antifungal agents. Mycoses. 2004;47:216-221. doi:10.1111/j.1439-0507.2004.00984.x
- Smith DJ, Melhem MSC, Dirven J, et al. Establishment of epidemiological cutoff values for Fonsecaea pedrosoi, the primary etiologic agent of chromoblastomycosis, and eight antifungal medications. J Clin Microbiol. Published online April 4, 2025. doi:10.1128/jcm.01903-24
- Revankar SG, Sutton DA. Melanized fungi in human disease. Clin Microbiol Rev. 2010;23:884-928. doi:10.1128/CMR.00019-10
- de Sousa M da GT, Belda W, Spina R, et al. Topical application of imiquimod as a treatment for chromoblastomycosis. Clin Infect Dis. 2014;58:1734-1737. doi:10.1093/cid/ciu168
- Logan C, Singh M, Fox N, et al. Chromoblastomycosis treated with posaconazole and adjunctive imiquimod: lending innate immunity a helping hand. Open Forum Infect Dis. Published online March 14, 2023. doi:10.1093/ofid/ofad124
- Castro LGM, Pimentel ERA, Lacaz CS. Treatment of chromomycosis by cryosurgery with liquid nitrogen: 15 years’ experience. Int J Dermatol. 2003;42:408-412. doi:10.1046/j.1365-4362.2003.01532.x
- Tagami H, Ohi M, Aoshima T, et al. Topical heat therapy for cutaneous chromomycosis. Arch Dermatol. 1979;115:740-741.
- Lyon JP, Pedroso e Silva Azevedo C de M, Moreira LM, et al. Photodynamic antifungal therapy against chromoblastomycosis. Mycopathologia. 2011;172:293-297. doi:10.1007/s11046-011-9434-6
- Kinbara T, Fukushiro R, Eryu Y. Chromomycosis—report of two cases successfully treated with local heat therapy. Mykosen. 1982;25:689-694. doi:10.1111/j.1439-0507.1982.tb01944.x
- Yang Y, Hu Y, Zhang J, et al. A refractory case of chromoblastomycosis due to Fonsecaea monophora with improvement by photodynamic therapy. Med Mycol. 2012;50:649-653. doi:10.3109/13693786.2012.655258
- Sánchez-Cárdenas CD, Isa-Pimentel M, Arenas R. Phaeohyphomycosis: a review. Microbiol Res. 2023;14:1751-1763. doi:10.3390/microbiolres14040120
- Guillet J, Berkaoui I, Gargala G, et al. Cutaneous alternariosis. Mycopathologia. 2024;189:81. doi:10.1007/s11046-024-00888-5
- Wang X, Wang W, Lin Z, et al. CARD9 mutations linked to subcutaneous phaeohyphomycosis and TH17 cell deficiencies. J Allergy Clin Immunol. 2014;133:905-908. doi:10.1016/j.jaci.2013.09.033
- Revankar SG, Baddley JW, Chen SCA, et al. A mycoses study group international prospective study of phaeohyphomycosis: an analysis of 99 proven/probable cases. Open Forum Infect Dis. 2017;4:ofx200. doi:10.1093/ofid/ofx200
- Zijlstra EE, van de Sande WWJ, Welsh O, et al. Mycetoma: a unique neglected tropical disease. Lancet Infect Dis. 2016;16:100-112. doi:10.1016/S1473-3099(15)00359-X
- Emery D, Denning DW. The global distribution of actinomycetoma and eumycetoma. PLoS Negl Trop Dis. 2020;14:E0008397. doi:10.1371/journal.pntd.0008397
- van de Sande WWJ, Fahal AH. An updated list of eumycetoma causative agents and their differences in grain formation and treatment response. Clin Microbiol Rev. Published online May 2024. doi:10.1128/cmr.00034-23
- Nenoff P, van de Sande WWJ, Fahal AH, et al. Eumycetoma and actinomycetoma—an update on causative agents, epidemiology, pathogenesis, diagnostics and therapy. J Eur Acad Dermatol Venereol. 2015;29:1873-1883. doi:10.1111/jdv.13008
- El-Amin SO, El-Amin RO, El-Amin SM, et al. Painful mycetoma: a study to understand the risk factors in patients visiting the Mycetoma Research Centre (MRC) in Khartoum, Sudan. Trans R Soc Trop Med Hyg. 2025;119:145-151. doi:10.1093/trstmh/trae093
- Ahmed AA, van de Sande W, Fahal AH. Mycetoma laboratory diagnosis: review article. PLoS Negl Trop Dis. 2017;11:e0005638. doi:10.1371/journal.pntd.0005638
- Siddig EE, Ahmed A, Hassan OB, et al. Using a Madurella mycetomatis specific PCR on grains obtained via noninvasive fine needle aspirated material is more accurate than cytology. Mycoses. Published online February 5, 2023. doi:10.1111/myc.13572
- Konings M, Siddig E, Eadie K, et al. The development of a multiplex recombinase polymerase amplification reaction to detect the most common causative agents of eumycetoma. Eur J Clin Microbiol Infect Dis. Published online April 30, 2025. doi:10.1007/s10096-025-05134-4
- Siddig EE, El Had Bakhait O, El nour Hussein Bahar M, et al. Ultrasound-guided fine-needle aspiration cytology significantly improved mycetoma diagnosis. J Eur Acad Dermatol Venereol. 2022;36:1845-1850. doi:10.1111/jdv.18363
- Bonifaz A, García-Sotelo RS, Lumbán-Ramirez F, et al. Update on actinomycetoma treatment: linezolid in the treatment of actinomycetomas due to Nocardia spp and Actinomadura madurae resistant to conventional treatments. Expert Rev Anti Infect Ther. 2025;23:79-89. doi:10.1080/14787210.2024.2448723
- Chandler DJ, Bonifaz A, van de Sande WWJ. An update on the development of novel antifungal agents for eumycetoma. Front Pharmacol. 2023;14:1165273. doi:10.3389/fphar.2023.1165273
- Fahal AH, Siddig Ahmed E, Mubarak Bakhiet S, et al. Two dose levels of once-weekly fosravuconazole versus daily itraconazole, in combination with surgery, in patients with eumycetoma in Sudan: a randomised, double-blind, phase 2, proof-of-concept superiority trial. Lancet Infect Dis. 2024;24:1254-1265. doi:10.1016/S1473-3099(24)00404-3
Approach to Diagnosing and Managing Implantation Mycoses
Approach to Diagnosing and Managing Implantation Mycoses
Practice Points
- Chromoblastomycosis, subcutaneous phaeohyphomycosis, and mycetoma are implantation mycoses that cause substantial morbidity, decreased quality of life, and social stigma.
- Consider obtaining a biopsy of suspected chromoblastomycosis and subcutaneous phaeohyphomycosis to confirm infection while sending half of the sample for culture for organism identification.
- Distinguishing between actinomycetoma (caused by filamentous bacteria) and eumycetoma (caused by fungi) is critical for appropriate mycetoma treatment.