User login
Cutis is a peer-reviewed clinical journal for the dermatologist, allergist, and general practitioner published monthly since 1965. Concise clinical articles present the practical side of dermatology, helping physicians to improve patient care. Cutis is referenced in Index Medicus/MEDLINE and is written and edited by industry leaders.
ass lick
assault rifle
balls
ballsac
black jack
bleach
Boko Haram
bondage
causas
cheap
child abuse
cocaine
compulsive behaviors
cost of miracles
cunt
Daech
display network stats
drug paraphernalia
explosion
fart
fda and death
fda AND warn
fda AND warning
fda AND warns
feom
fuck
gambling
gfc
gun
human trafficking
humira AND expensive
illegal
ISIL
ISIS
Islamic caliphate
Islamic state
madvocate
masturbation
mixed martial arts
MMA
molestation
national rifle association
NRA
nsfw
nuccitelli
pedophile
pedophilia
poker
porn
porn
pornography
psychedelic drug
recreational drug
sex slave rings
shit
slot machine
snort
substance abuse
terrorism
terrorist
texarkana
Texas hold 'em
UFC
section[contains(@class, 'nav-hidden')]
section[contains(@class, 'nav-hidden active')
A peer-reviewed, indexed journal for dermatologists with original research, image quizzes, cases and reviews, and columns.
Adjunctive Use of Halobetasol Propionate–Tazarotene in Biologic-Experienced Patients With Psoriasis
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).
The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).
The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
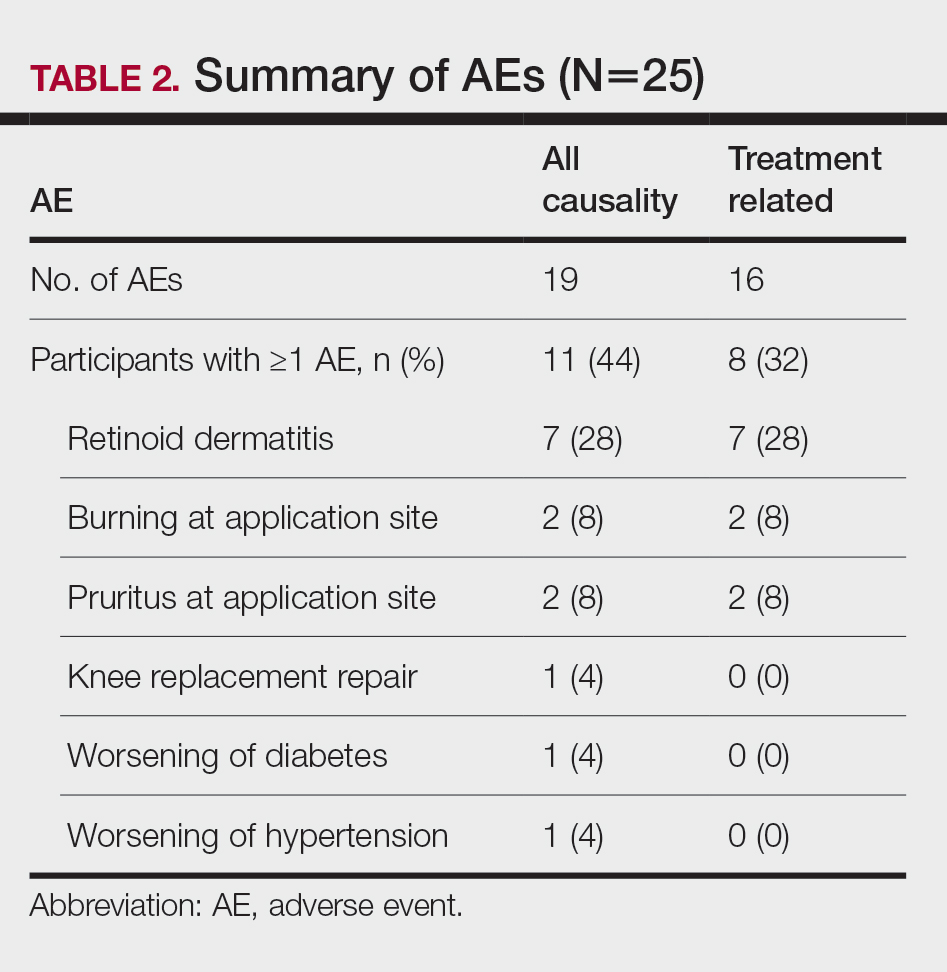
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.
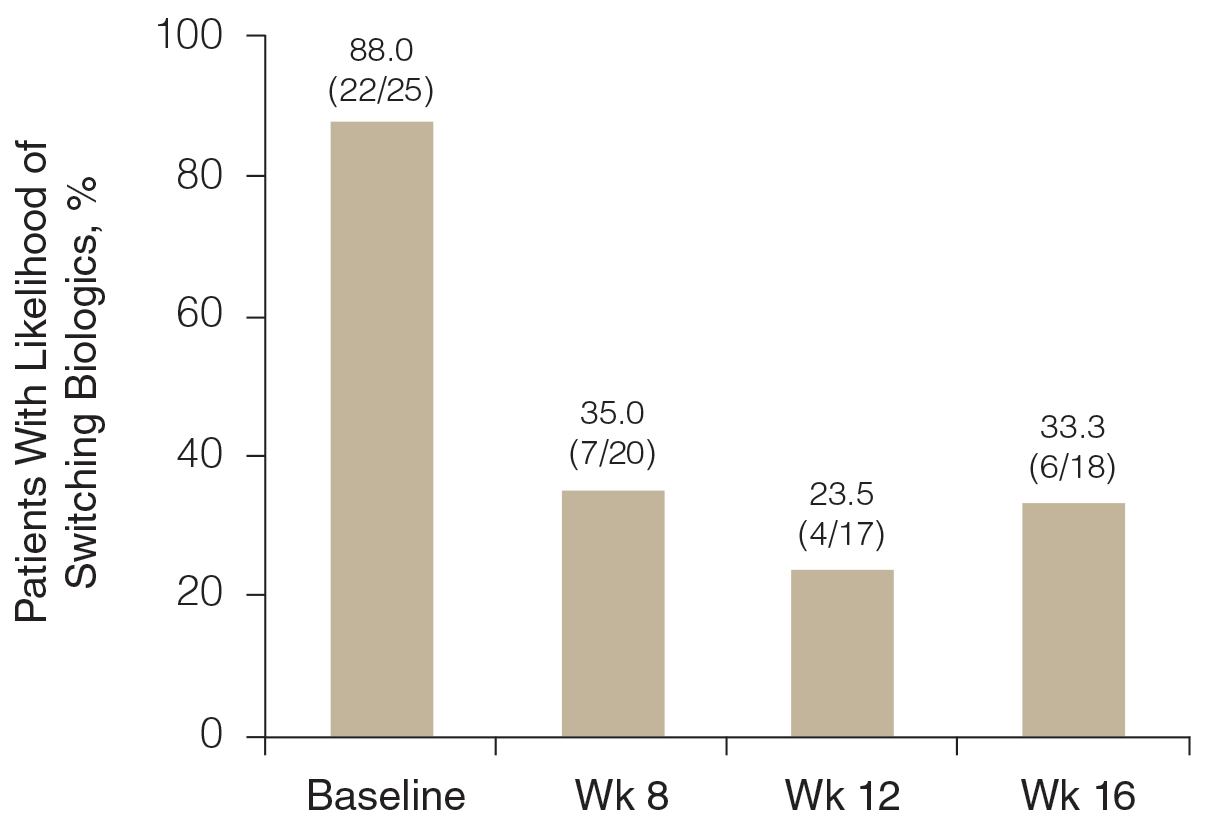
Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).
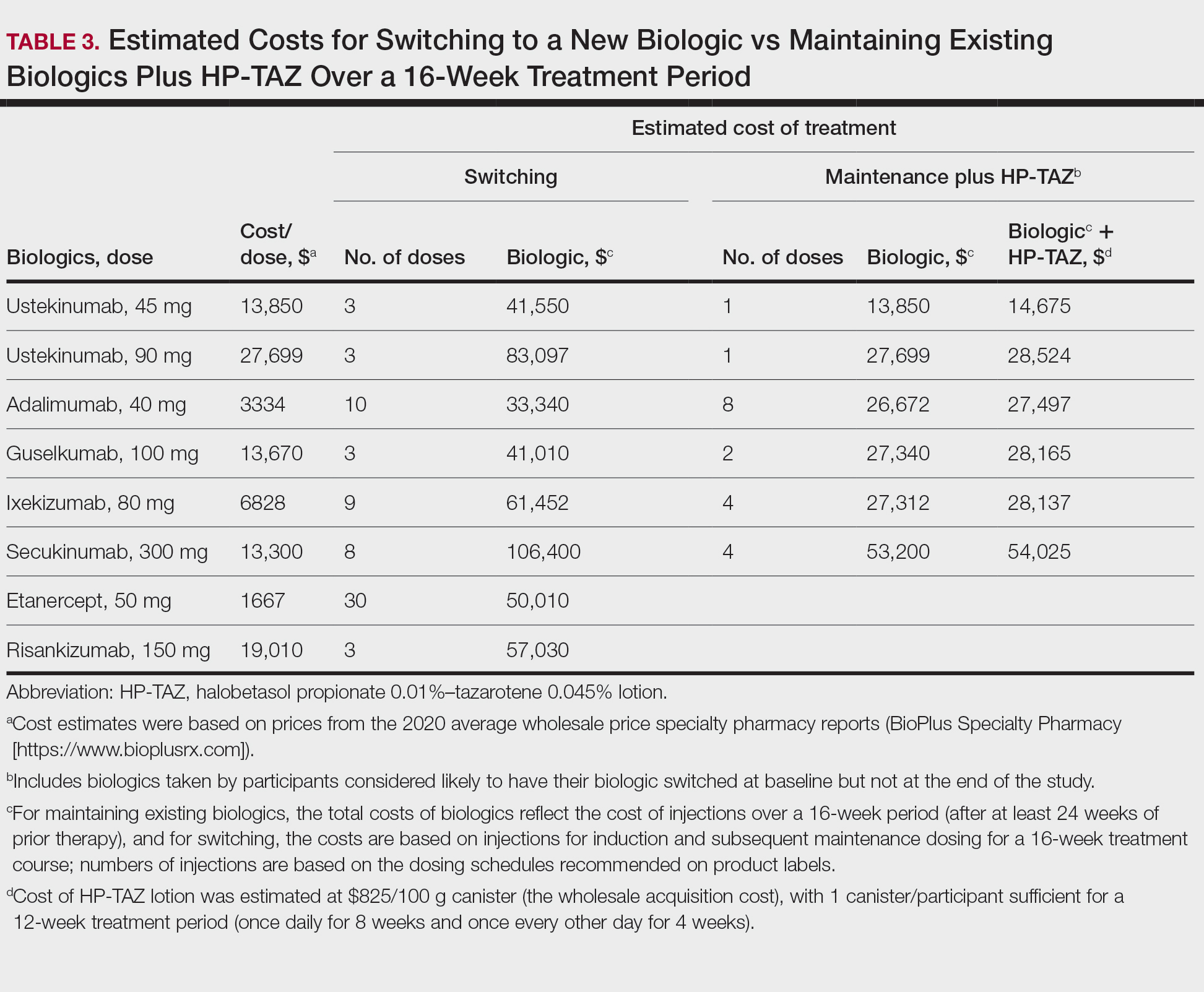
Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.
Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).
The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).
The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.
Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).
Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.
Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
Psoriasis is a common chronic immunologic skin disease that affects approximately 7.4 million adults in the United States1 and more than 100 million individuals worldwide.2 Patients with psoriasis have a potentially heightened risk for cardiometabolic diseases, psychiatric disorders, and psoriatic arthritis,3 as well as impaired quality of life (QOL).4 Psoriasis also is associated with increased health care costs5 and may result in substantial socioeconomic repercussions for affected patients.6,7
Psoriasis treatments focus on relieving symptoms and improving patient QOL. Systemic therapy has been the mainstay of treatment for moderate to severe psoriasis.8 Although topical therapy usually is applied to treat mild symptoms, it also can be used as an adjunct to enhance efficacy of other treatment approaches.9 The National Psoriasis Foundation (NPF) recommends a treat-to-target (TTT) strategy for plaque psoriasis, the most common form of psoriasis, with a target response of attaining affected body surface area (BSA) of 1% or lower at 3 months after treatment initiation, allowing for regular assessments of treatment responses.10
Not all patients with moderate to severe psoriasis can achieve a satisfactory response with systemic biologic monotherapy.11 Switching to a new biologic improves responses in some but not all cases12 and could be associated with new safety issues and additional costs. Combinations of biologics with phototherapy, nonbiologic systemic agents, or topical medications were found to be more effective than biologics alone,9,11 though long-term safety studies are needed for biologics combined with other systemic inverventions.11
A lotion containing a fixed combination of halobetasol propionate (HP) 0.01%, a corticosteroid, and tazarotene (TAZ) 0.045%, a retinoid, is indicated for plaque psoriasis in adults.13 Two randomized, controlled, phase 3 trials demonstrated the rapid and sustained efficacy of HP-TAZ in treating moderate to severe plaque psoriasis without any safety concerns.14,15 However, combining HP-TAZ lotion with biologics has not been examined yet, to our knowledge.
This open-label study evaluated the effectiveness and safety of adjunctive HP-TAZ lotion in adult patients with moderate to severe plaque psoriasis who were being treated with biologics in a real-world setting. Potential cost savings with the addition of topical HP-TAZ to ongoing biologics vs switching to a new biologic also were assessed.
Methods
Study Design and Participants—A single-center, institutional review board–approved, open-label study evaluated adjunctive therapy with HP 0.01%–TAZ 0.045% lotion in patients with psoriasis being treated with biologic agents. The study was conducted in accordance with the ethical principles of the Declaration of Helsinki and in compliance with Good Clinical Practices. All patients provided written informed consent before enrollment.
Male and nonpregnant female patients (aged ≥18 years)with moderate to severe chronic plaque psoriasis and a BSA of 2% to 10% who were being treated with biologics for at least 24 weeks at baseline were enrolled. Patients were excluded if they had used oral systemic medications for psoriasis (≤4 weeks), other topical antipsoriatic therapies (≤14 days), UVB phototherapy (≤2 weeks), and psoralen plus UVA phototherapy (≤4 weeks) prior to study initiation. Concomitant use of steroid-free topical emollients or low-potency topical steroids and appropriate interventions deemed necessary by the investigator were allowed.
Although participants maintained their prescribed biologics for the duration of the study, HP-TAZ lotion also was applied once daily for 8 weeks, followed by once every other day for an additional 4 weeks. Participants then continued with biologics only for the last 4 weeks of the study.
Study Outcome Measures—Disease severity and treatment efficacy were assessed by affected BSA, Physician Global Assessment (PGA) score, composite BSA×PGA score, and participant-reported Dermatology Life Quality Index (DLQI). The primary end point was the proportion of participants achieving a BSA of 0% to 1% (NPF TTT status) at week 8. Secondary end points included the proportions of participants with BSA of 0% to 1% at weeks 12 and 16; BSA×PGA score at weeks 8, 12, and 16; and improvements in BSA, PGA, and DLQI at weeks 8, 12, and 16.
Adverse events (AEs) that occurred after the signing of the informed consent and for the duration of the participant’s participation were recorded, regardless of causality. Physical examinations were performed at screening; baseline; and weeks 8, 12, and 16 to document any clinically significant abnormalities. Localized skin reactions were assessed for tolerability of the study drug, with any reaction requiring concomitant therapy recorded as an AE.
The likelihood of switching to a new biologic regimen was assessed by the investigator for each participant at baseline and weeks 8, 12, and 16. Participants with unacceptable responses to their treatments (BSA >3%) were reported as likely to be considered for switching biologics by the investigator.
Pharmacoeconomic Evaluation—Potential cost savings were evaluated for the addition of HP-TAZ lotion to ongoing biologics vs switching to a new biologic. Cost comparisons were made in participants for whom the investigator would likely have switched biologics at baseline but not at the end of the study. For maintaining the same biologic with adjunctive topical HP-TAZ, total cost was estimated by adding the cost for 12 weeks (once daily for 8 weeks and once every other day for 4 weeks) of the HP-TAZ lotion to that of 16-week maintenance dosing with the biologic. The projected cost for switching to a new biologic for 16 weeks of treatment was based on both induction and maintenance dosing as recommended in its product label. Prices were obtained from the 2020 average wholesale price specialty pharmacy reports (BioPlus Specialty Pharmacy Services [https://www.bioplusrx.com]).
Data Handling—Enrollment of approximately 25 participants was desired for the study. Data on disease severity and participant-reported outcomes were assessed using descriptive statistics. Adverse events were summarized descriptively by incidence, severity, and relationship to the study drug. All participants with data available at a measured time point were included in the analyses for that time point.
Results
Participant Disposition and Demographics—Twenty-five participants (15 male and 10 female) were included in the study (Table 1). Seven participants discontinued the study for the following reasons: AEs (n=4), patient choice (n=2), and noncompliance (n=1).
The average age of the participants was 50 years, the majority were White (76.0% [19/25]) andnon-Hispanic (88.0% [22/25]), and the mean duration of chronic plaque psoriasis was 18.9 years (Table 1). Participants had been receiving biologic monotherapy for at least 24 weeks prior to enrollment, most commonly ustekinumab (32.0% [8/25])(Table 1). None had achieved the NPF TTT status with their biologics. At baseline, mean (SD) affected BSA, PGA, BSA×PGA, and participant-reported DLQI were 4.16% (2.04%), 2.84 (0.55), 11.88 (6.39), and 4.00 (4.74), respectively.
Efficacy Assessment—Application of HP-TAZ lotion in addition to the participants’ existing biologic therapy reduced severity of the disease, as evidenced by the reductions in mean BSA, PGA, and BSA×PGA. After 8 weeks of once-daily concomitant HP-TAZ use with biologic, mean BSA and PGA dropped by approximately 40% and 37%, respectively (Figures 1A and 1B). A greater reduction (54%) was found for mean BSA×PGA (Figure 1C). Disease severity continued to improve when the application schedule for HP-TAZ was changed to once every other day for 4 weeks, as mean BSA, PGA, and BSA×PGA decreased further at week 12. These beneficial effects were sustained during the last 4 weeks of the study after HP-TAZ was discontinued, with reductions of 57%, 43%, and 70% from baseline for mean BSA, PGA, and BSA×PGA, respectively (Figure 1).
The proportion of participants who achieved NPF TTT status increased from 0% at baseline to 20.0% (5/20) at week 8 with once-daily use of HP-TAZ plus biologic for 8 weeks (Figure 2). At week 12, more participants (64.7% [11/17]) achieved the treatment goal after application of HP-TAZ once every other day with biologic for 4 weeks. Most participants maintained NPF TTT status after HP-TAZ was discontinued; at week 16, 50.0% (9/18) attained the NPF treatment goal (Figure 2).
The mean DLQI score decreased from 4.00 at baseline to 2.45 after 8 weeks of concomitant use of once-daily HP-TAZ with biologic, reflecting a 39% score reduction. An additional 4 weeks of adjunctive HP-TAZ applied once every other day with biologic further decreased the DLQI score to 2.18 at week 12. Mean DLQI remained similar (2.33) after another 4 weeks of biologics alone. The proportion of participants reporting a DLQI score of 0 to 1 increased from 40% (10/25) at baseline to 60% (12/20) at week 8 and 76.5% (13/17) at week 12 with adjunctive HP-TAZ lotion use with biologic. At week 16, a DLQI score of 0 to 1 was reported in 61.1% (11/18) of participants after receiving only biologics for 4 weeks.
Safety Assessment—A total of 19 AEs were reported in 11 participants during the study; 16 AEs were considered treatment related in 8 participants (Table 2). The most common AEs were retinoid dermatitis (28% [7/25]), burning at the application site (8% [2/25]), and pruritus at the application site (8% [2/25]), all of which were considered related to the treatment. Among all AEs, 12 were mild in severity, and the remaining 7 were moderate. Adverse events led to early study termination in 4 participants, all with retinoid dermatitis as the primary reason.
Likelihood of Switching Biologics—At baseline, almost 90% (22/25) of participants were rated as likely to switch biologics by the investigator due to unacceptable responses to their currently prescribed biologics (BSA >3%)(Figure 3). The likelihood was greatly reduced by concomitant HP-TAZ, as the proportion of participants defined as nonresponders to their biologic decreased to 35% (7/20) with 8-week adjunctive application of once-daily HP-TAZ with biologic and further decreased to 23.5% (4/17) with another 4 weeks of adjunctive HP-TAZ applied every other day plus biologic. At week 16, after 4 weeks of biologics alone, the proportion was maintained at 33.3% (6/18).
Pharmacoeconomics of Adding Topical HP-TAZ vs Switching Biologics—In the participants whom the investigator reported as likely to switch biologics at baseline, 9 had improvements in disease control such that switching biologics was no longer considered necessary for them at week 16. Potential cost savings with adjunctive therapy of HP-TAZ plus biologic vs switching biologics were therefore evaluated in these 9 participants, who were receiving ustekinumab, adalimumab, guselkumab, ixekizumab, and secukinumab during the study (Table 3). The estimated total cost of 16-week maintenance dosing of biologics plus adjunct HP-TAZ lotion ranged from $14,675 (ustekinumab 45 mg) to $54,025 (secukinumab 300 mg), while switching to other most commonly prescribed biologics for 16 weeks would cost an estimated $33,340 to $106,400 (induction and subsequent maintenance phases)(Table 3). Most biologic plus HP-TAZ combinations were estimated to cost less than $30,000, potentially saving $4816 to $91,725 compared with switching to any of the other 7 biologics (Table 3). The relatively more expensive maintenance combination containing secukinumab plus HP-TAZ ($54,025) appeared to be a less expensive option when compared with switching to ustekinumab (90 mg)($83,097), ixekizumab (80 mg)($61,452), or risankizumab (150 mg)($57,030) as an alternative biologic.
Comment
Adjunctive Use of HP-TAZ Lotion—In the present study, we showed that adjunctive HP-TAZ lotion improved biologic treatment response and reduced disease severity in participants with moderate to severe psoriasis whose symptoms could not be adequately controlled by 24 weeks or more of biologic monotherapy in a real-world setting. Disease activity decreased as evidenced by reductions in all assessed effectiveness variables, including BSA involvement, PGA score, composite BSA×PGA score, and participant-reported DLQI score. Half of the participants achieved NPF TTT status at the end of the study. The treatment was well tolerated with no unexpected safety concerns. Compared with switching to a new biologic, adding topical HP-TAZ to ongoing biologics appeared to be a more cost-effective approach to enhance treatment effects. Our results suggest that adjunctive use of HP-TAZ lotion may be a safe, effective, and economical option for patients with psoriasis who are failing their ongoing biologic monotherapy.
Treat-to-Target Status—The NPF-recommended target response to a treatment for plaque psoriasis is BSA of 1% or lower at 3 months postinitiation.10 Patients in the current study had major psoriasis activity at study entry despite being treated with a biologic for at least 24 weeks, as none had attained NPF TTT status at baseline. Because the time period of prior biologic treatment (at least 24 weeks) is much longer than the 3 months suggested by NPF, we believe that we were observing a true failure of the biologic rather than a slow onset of treatment effects in these patients at the time of enrollment. By week 12, with the addition of HP-TAZ lotion to the biologic, a high rate of participants achieved NPF TTT status (64.7%), with most participants being able to maintain this TTT status at study end after 4 weeks of biologic alone. Most participants also reported no impact of psoriasis on their QOL (DLQI, 0–116; 76.5%) at week 12. Improvements we found in disease control with adjunctive HP-TAZ lotion plus biologic support prior reports showing enhanced responses when a topical medication was added to a biologic.17,18 Reductions in psoriasis activity after 8 weeks of combined biologics plus once-daily HP-TAZ also are consistent with 2 phase 3 RCTs in which a monotherapy of HP-TAZ lotion used once daily for 8 weeks reduced BSA and DLQI.15 Notably, in the current study, disease severity continued to decrease when dosing of HP-TAZ was reduced to once every other day for 4 weeks, and the improvements were maintained even after the adjunct topical therapy was discontinued.
Safety Profile of HP-TAZ Lotion—The overall safety profile in our study also was consistent with that previously reported for HP-TAZ lotion,15,19-21 with no new safety signals observed. The combination treatment was well tolerated, with most reported AEs being mild in severity. Adverse events were mostly related to application-site reactions, the most common being dermatitis (28%), which was likely attributable to the TAZ component of the topical regimen.15
Likelihood of Switching Biologics—Reduced disease activity was reflected by a decrease in the percentage of participants the investigator considered likely to change biologics, which was 88.0% at baseline but only 33.3% at the end of the study. Although switching to a different biologic agent can improve treatment effect,22 it could lead to a substantial increase in health care costs and use of resources compared with no switch.5 We found that switching to one of the other most commonly prescribed biologics could incur $4816 to $91,725 in additional costs in most cases when compared with the combination strategy we evaluated over the 16-week treatment period. Therefore, concomitant use of HP-TAZ lotion with the ongoing biologics would be a potentially more economical alternative for patients to achieve acceptable responses or the NPF TTT goal. Moreover, combination with an adjunctive topical medication could avoid potential risks associated with switching, such as new AEs with new biologic regimens or disease flare during any washout period sometimes adopted for switching biologics.
Study Limitations—Our estimated costs were based on average wholesale prices and did not reflect net prices paid by patients or health plans due to the lack of known discount rates.
Conclusion
In this real-world study, patients with psoriasis that failed to respond to biologic monotherapy had improved disease control and QOL and reported no new safety concerns with adjunctive use of HP-TAZ lotion. Adding HP-TAZ to the ongoing biologics could be a more cost-effective option vs switching biologics for patients whose psoriasis symptoms could not be controlled with biologic monotherapy. Taken together, our results support the use of HP-TAZ lotion as an effective and safe adjunctive topical therapy in combination with biologics for psoriasis treatment.
Acknowledgments—We acknowledge the medical writing assistance provided by Hui Zhang, PhD, and Kathleen Ohleth, PhD, from Precise Publications LLC (Far Hills, New Jersey), which was funded by Ortho Dermatologics.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
- Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70:512-516.
- Global Report on Psoriasis. World Health Organization; 2016. Accessed January 11, 2022. https://apps.who.int/iris/handle/10665/204417
- Takeshita J, Grewal S, Langan SM, et al. Psoriasis and comorbid diseases: epidemiology. J Am Acad Dermatol. 2017;76:377-390.
- Moller AH, Erntoft S, Vinding GR, et al. A systematic literature review to compare quality of life in psoriasis with other chronic diseases using EQ-5D-derived utility values. Patient Relat Outcome Meas. 2015;6:167-177.
- Feldman SR, Tian H, Wang X, et al. Health care utilization and cost associated with biologic treatment patterns among patients with moderate to severe psoriasis: analyses from a large U.S. claims database. J Manag Care Spec Pharm. 2019;25:479-488.
- Thomsen SF, Skov L, Dodge R, et al. Socioeconomic costs and health inequalities from psoriasis: a cohort study. Dermatology. 2019;235:372-379.
- Fowler JF, Duh MS, Rovba L, et al. The impact of psoriasis on health care costs and patient work loss. J Am Acad Dermatol. 2008;59:772-780.
- Menter A, Gottlieb A, Feldman SR, et al. Guidelines of care for the management of psoriasis and psoriatic arthritis: section 1. overview of psoriasis and guidelines of care for the treatment of psoriasis with biologics. J Am Acad Dermatol. 2008;58:826-850.
- Bagel J, Gold LS. Combining topical psoriasis treatment to enhance systemic and phototherapy: a review of the literature. J Drugs Dermatol. 2017;16:1209-1222.
- Armstrong AW, Siegel MP, Bagel J, et al. From the Medical Board of the National Psoriasis Foundation: treatment targets for plaque psoriasis. J Am Acad Dermatol. 2017;76:290-298.
- Armstrong AW, Bagel J, Van Voorhees AS, et al. Combining biologic therapies with other systemic treatments in psoriasis: evidence-based, best-practice recommendations from the Medical Board of the National Psoriasis Foundation. JAMA Dermatol. 2015;151:432-438.
- Menter A, Strober BE, Kaplan DH, et al. Joint AAD-NPF guidelines of care for the management and treatment of psoriasis with biologics. J Am Acad Dermatol. 2019;80:1029-1072.
- Duobrii. Prescribing information. Bausch Health Companies Inc; 2019.
- Sugarman JL, Weiss J, Tanghetti EA, et al. Safety and efficacy of a fixed combination halobetasol and tazarotene lotion in the treatment of moderate-to-severe plaque psoriasis: a pooled analysis of two phase 3 studies. J Drugs Dermatol. 2018;17:855-861.
- Gold LS, Lebwohl MG, Sugarman JL, et al. Safety and efficacy of a fixed combination of halobetasol and tazarotene in the treatment of moderate-to-severe plaque psoriasis: results of 2 phase 3 randomized controlled trials. J Am Acad Dermatol. 2018;79:287-293.
- Finlay AY. Current severe psoriasis and the rule of tens. Br J Dermatol. 2005;152:861-867.
- Campione E, Mazzotta A, Paterno EJ, et al. Effect of calcipotriol on etanercept partial responder psoriasis vulgaris and psoriatic arthritis patients. Acta Derm Venereol. 2009;89:288-291.
- Bagel J, Zapata J, Nelson E. A prospective, open-label study evaluating adjunctive calcipotriene 0.005%/betamethasone dipropionate 0.064% foam in psoriasis patients with inadequate response to biologic therapy. J Drugs Dermatol. 2018;17:845-850.
- Sugarman JL, Gold LS, Lebwohl MG, et al. A phase 2, multicenter, double-blind, randomized, vehicle controlled clinical study to assess the safety and efficacy of a halobetasol/tazarotene fixed combination in the treatment of plaque psoriasis. J Drugs Dermatol. 2017;16:197-204.
- Lebwohl MG, Sugarman JL, Gold LS, et al. Long-term safety results from a phase 3 open-label study of a fixed combination halobetasol propionate 0.01% and tazarotene 0.045% lotion in moderate-to-severe plaque psoriasis. J Am Acad Dermatol. 2019;80:282-285.
- Bhatia ND, Pariser DM, Kircik L, et al. Safety and efficacy of a halobetasol 0.01%/tazarotene 0.045% fixed combination lotion in the treatment of moderate-to-severe plaque psoriasis: a comparison with halobetasol propionate 0.05% cream. J Clin Aesthet Dermatol. 2018;11:15-19.
- Wang TS, Tsai TF. Biologics switch in psoriasis. Immunotherapy. 2019;11:531-541.
Practice Points
- Although monotherapy with biologic agents is effective to treat psoriasis, some patients do not achieve a satisfactory response.
- Adjunctive therapy with halobetasol propionate (HP) 0.01%–tazarotene (TAZ) 0.045% lotion can improve responses to biologic treatment in patients whose psoriasis symptoms could not be adequately controlled by biologic monotherapy.
- Adjunctive use of HP-TAZ lotion in addition to biologics was well tolerated.
- Compared with switching to a new biologic regimen, adding a topical regimen of HP-TAZ lotion to ongoing biologics may be a more cost-effective approach to enhance treatment effects.
Guttate Psoriasis Following COVID-19 Infection
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
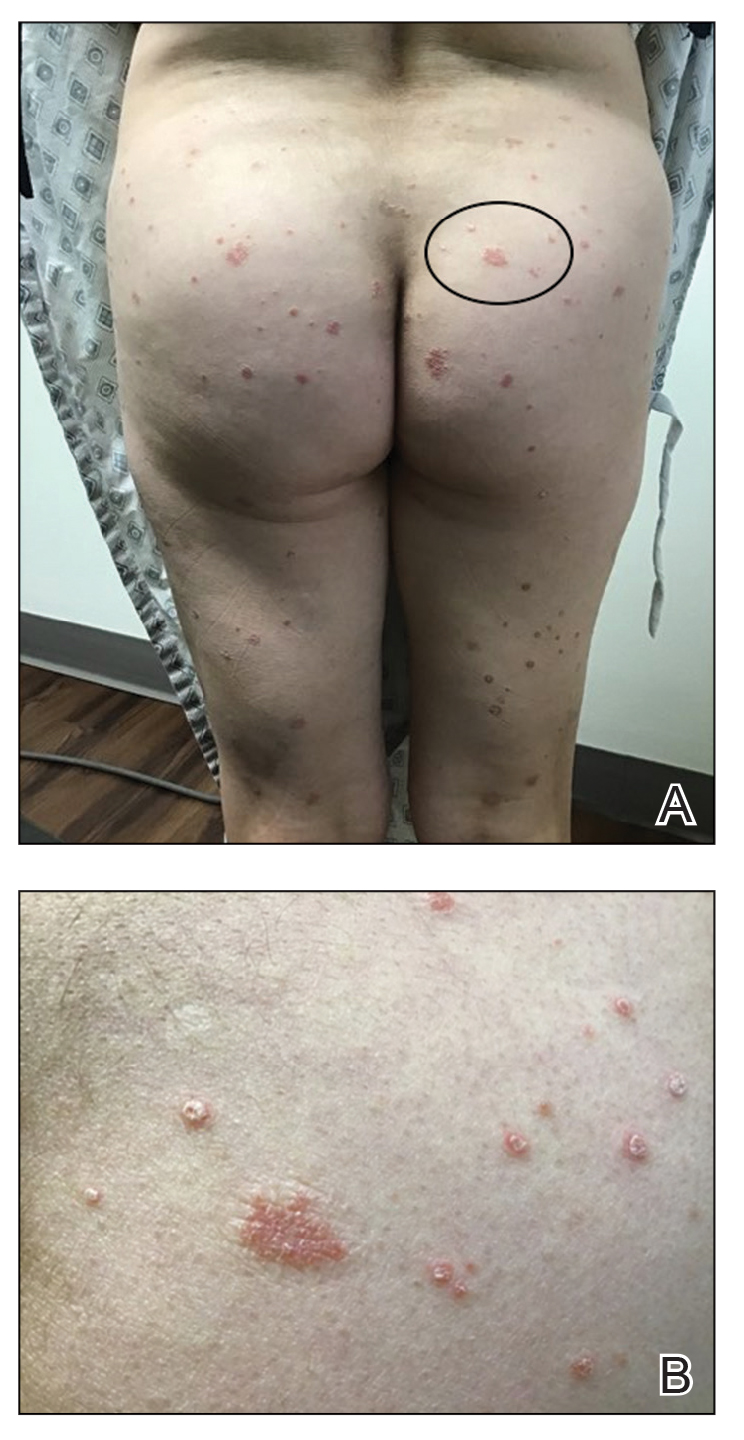
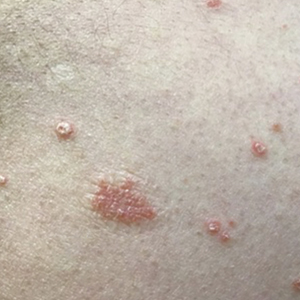
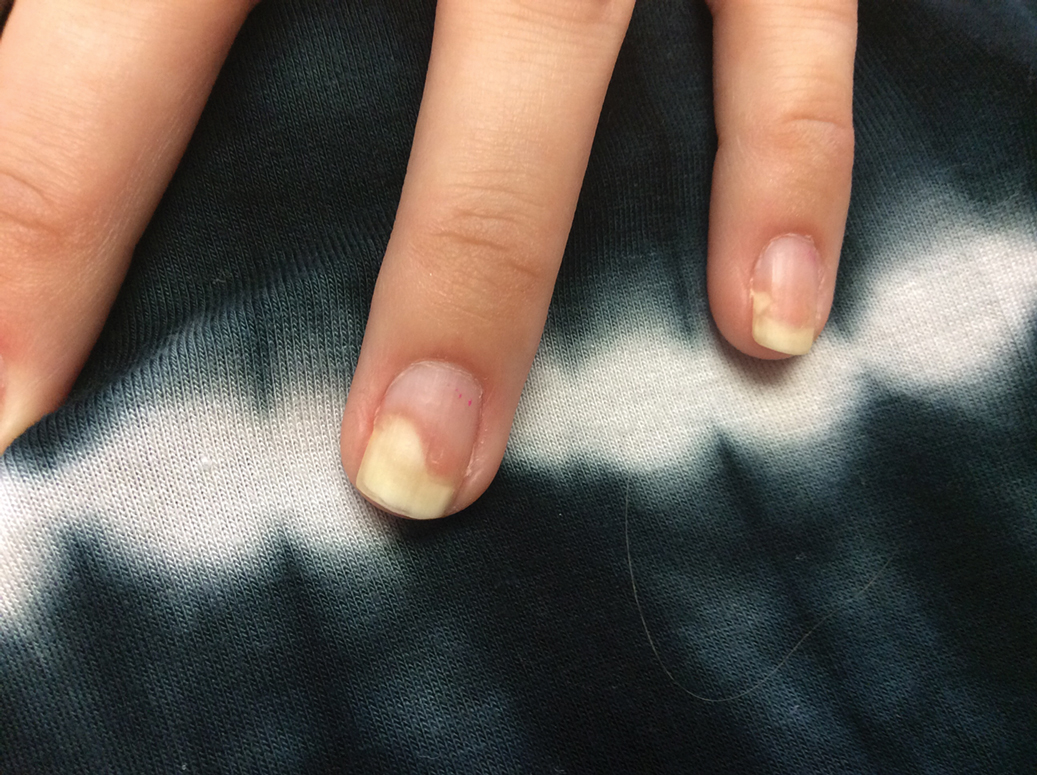
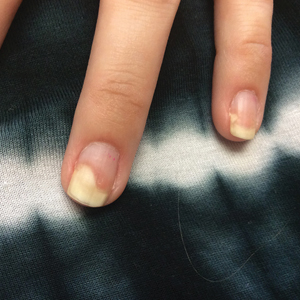
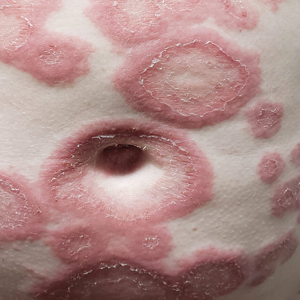
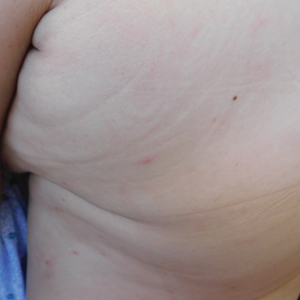
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.
The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.
The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
Psoriasis is an inflammatory skin condition affecting 1% to 5% of the world population. 1 Guttate psoriasis is a subgroup of psoriasis that most commonly presents as raindroplike, erythematous, silvery, scaly papules. There have been limited reports of guttate psoriasis caused by rhinovirus and COVID-19 infection, but a PubMed search of articles indexed for MEDLINE using the term COVID-19 guttate psoriasis yielded only 3 documented cases of a psoriatic flare secondary to SARS-CoV-2 infection. 1-4 Herein, we detail a case in which a patient with mild SARS-CoV-2 infection who did not have a personal or family history of psoriasis experienced a moderate psoriatic flare 3 weeks after diagnosis of COVID-19.
Case Report
A 55-year-old woman was diagnosed with COVID-19 after SARS-CoV-2 RNA was detected from a nasopharyngeal swab. She reported moderate fatigue but no other symptoms. At the time of infection, she was not taking medications and reported neither a personal nor family history of psoriasis.
Three weeks after the COVID-19 diagnosis, she reported erythematous scaly papules only on the trunk and backs of the legs. Two months after the COVID-19 diagnosis, she was evaluated in our practice and diagnosed with guttate psoriasis. The patient refused biopsy. Physical examination revealed that the affected body surface area had increased to 5%; erythematous, silvery, scaly papules were found on the trunk, anterior and posterior legs, and lateral thighs (Figure). At the time of evaluation, she did not report joint pain or nail changes.
The patient was treated with triamcinolone acetonide cream 0.1% twice daily for 2 to 4 weeks. The guttate psoriasis resolved.
Comment
A sudden psoriatic flare can be linked to dysregulation of the innate immune response. Guttate psoriasis and generalized plaque-type psoriasis are postulated to have similar pathogenetic mechanisms, but guttate psoriasis is the only type of psoriasis that originates from viral infection. Initially, viral RNA will stimulate the toll-like receptor 3 protein, leading to increased production of the pathogenic cytokine IL-36γ and pathogenic chemokine CXCL8 (also known as IL-8), both of which are biomarkers for psoriasis.1 Specifically, IL-36γ and CXCL8 are known to further stimulate the proinflammatory cascade during the innate immune response displayed in guttate psoriasis.5,6
Our patient had a mild case of COVID-19, and she first reported the erythematous and scaly papules 3 weeks after infection. Dysregulation of proinflammatory cytokines must have started in the initial stages—within 7 days—of the viral infection. Guttate psoriasis arises within 3 weeks of infection with other viral and bacterial triggers, most commonly with streptococcal infections.1
Rodríguez et al7 described a phenomenon in which both SARS-CoV-2 and Middle East respiratory syndrome, both caused by a coronavirus, can lead to a reduction of type I interferon, which in turn leads to failure of control of viral replication during initial stages of a viral infection. This triggers an increase in proinflammatory cytokines and chemokines, including IL‐36γ and CXCL8. This pathologic mechanism might apply to SARS-CoV-2, as demonstrated in our patient’s sudden psoriatic flare 3 weeks after the COVID-19 diagnosis. However, further investigation and quantification of the putatively involved cytokines is necessary for confirmation.
Conclusion
Psoriasis, a chronic inflammatory skin condition, has been linked predominantly to genetic and environmental factors. Guttate psoriasis as a secondary reaction after streptococcal tonsillar and respiratory infections has been reported.1
Our case is the fourth documented case of guttate psoriasis secondary to COVID-19 infection.2-4 However, it is the second documented case of a patient with a diagnosis of guttate psoriasis secondary to COVID-19 infection who had neither a personal nor family history of psoriasis.
Because SARS-CoV-2 is a novel virus, the long-term effects of COVID-19 remain unclear. We report this case and its findings to introduce a novel clinical manifestation of SARS-CoV-2 infection.
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
- Sbidian E, Madrange M, Viguier M, et al. Respiratory virus infection triggers acute psoriasis flares across different clinical subtypes and genetic backgrounds. Br J Dermatol. 2019;181:1304-1306. doi:10.1111/bjd.18203
- Gananandan K, Sacks B, Ewing I. Guttate psoriasis secondary to COVID-19. BMJ Case Rep. 2020;13:e237367. doi:10.1136/bcr-2020-237367
- Rouai M, Rabhi F, Mansouri N, et al. New-onset guttate psoriasis secondary to COVID-19. Clin Case Rep. 2021;9:e04542. doi:10.1002/ccr3.4542
- Agarwal A, Tripathy T, Kar BR. Guttate flare in a patient with chronic plaque psoriasis following COVID-19 infection: a case report. J Cosmet Dermatol. 2021;20:3064-3065. doi:10.1111/jocd.14396
- Madonna S, Girolomoni G, Dinarello CA, et al. The significance of IL-36 hyperactivation and IL-36R targeting in psoriasis. Int J Mol Sci. 2019;20:3318. doi:10.3390/ijms20133318
- Nedoszytko B, Sokołowska-Wojdyło M, Ruckemann-Dziurdzin´ska K, et al. Chemokines and cytokines network in the pathogenesis of the inflammatory skin diseases: atopic dermatitis, psoriasis and skin mastocytosis. Postepy Dermatol Alergol. 2014;31:84-91. doi:10.5114/pdia.2014.40920
- Rodríguez Y, Novelli L, Rojas M, et al. Autoinflammatory and autoimmune conditions at the crossroad of COVID-19. J Autoimmun. 2020;114:102506. doi:10.1016/j.jaut.2020.102506
Practice Points
- Guttate psoriasis is the only type of psoriasis that originates from viral infection.
- Dysregulation of proinflammatory cytokines during COVID-19 infection in our patient led to development of guttate psoriasis 3 weeks later.
Hairstyling Practices to Prevent Hair Damage and Alopecia in Women of African Descent
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
Central centrifugal cicatricial alopecia (CCCA), traction alopecia, and acquired proximal trichorrhexis nodosa are 3 forms of alopecia that disproportionately affect women of African descent.1 Central centrifugal cicatricial alopecia is characterized by a shiny smooth patch of hair loss over the vertex of the scalp that spreads centrifugally (Figure 1).1-4 Traction alopecia results from prolonged or repeated tension on the hair root that causes mechanical damage, hair loss, and shortening of hairs along the frontotemporal line (the so-called fringe sign)(Figure 2).1,3,5 Acquired proximal trichorrhexis nodosa, a result of trauma, is identified by a substantial number of hairs breaking off midshaft during a hair pull test.1 By understanding the unique structural properties and grooming methods of hair in women of African descent, physicians can better manage and stop the progression of hair loss before it becomes permanent.1,4,5
The characterization of hair between and within ethnic groups is challenging and lies on a spectrum.6,7 Many early studies broadly differentiated hair in 3 ethnic subgroups: African, Asian, and Caucasian6-8; older descriptions of hair texture also included terms such as straight, wavy, curly, and kinky.6 However, defining hair texture should be based on an approach that is more objective than an inaccurate ethnicity-based classification or the use of subjective, ill-defined, and overlapping descriptive terms.7 The segmentation tree analysis method (STAM) is an objective classification system that, when applied to hair, yields 8 curl-type groups (I=straight; VIII=tightly curly) based on curve diameter, curl index, number of waves, and twists.6-9 (We discuss the “tightly coiled” [group VII] through “tight, interwoven small curls” [group VIII] groups in the STAM classification of hair.)
Highly textured hair has been found to be more susceptible to breakage than other hair types because of an increased percentage of spirals and relatively fewer elastic fibers anchoring hair follicles to the dermis.1-4,10,11 In a cross-section, the hair shaft of individuals of African descent tends to be more elliptical and kidney shaped than the hair shaft of Asian individuals, which is round and has a large diameter, and the hair shaft of Caucasian individuals, which structurally lies between African and Asian hair.1,2,4,11 This axial asymmetry and section size contributes to points of lower tensile strength and increased fragility, which are exacerbated by everyday combing and grooming. Curvature of the hair follicle leads to the characteristic curly and spiral nature of African hair, which can lead to increased knotting.2,4
Practice Gap
Among women of African descent, a variety of hairstyles and hair treatments frequently are employed to allow for ease of management and self-expression.1 Many of these practices have been implicated as risk factors for alopecia. Simply advising patients to avoid tight hairstyles is ineffective because tension is subjective and difficult to quantify.5 Furthermore, it might be unreasonable to ask a patient to discontinue a hairstyle or treatment when they are unaware of less damaging alternatives.3,5
We provide an overview of hairstyles for patients who have highly textured hair so that physicians can better identify high-risk hairstyles and provide individualized recommendations for safer alternatives.1,3,5
Techniques for Hair Straightening
Traditional thermal straightening uses a hot comb or flat iron1,2,4,12 to temporarily disrupt hydrogen bonds within the hair shafts, which is reversible with exposure to moisture.1,2,4,5 Patients repeat this process every 1 or 2 weeks to offset the effects of normal perspiration and environmental humidity.5,12 Thermal straightening techniques can lead to increased fragility of the hair shaft and loss of tensile strength.11
Alternate methods of hair straightening use lye (sodium hydroxide) or nonlye (lithium and guanidine hydroxide) “relaxers” to permanently disrupt hydrogen and disulfide bonds in the hair shaft, which can damage and weaken hair.1-5,11,12 Touch-ups to the roots often are performed every 6 to 8 weeks.1,2
Chemical relaxers historically have been associated with CCCA but have not been definitively implicated as causative.2,3,4,13 Most studies have not demonstrated a statistically significant association between chemical relaxers and CCCA because, with a few exceptions,13 studies have either been based on surveys or have not employed trichoscopy or scalp biopsy. In one of those studies, patients with CCCA were determined to be 12.37 times more likely to have used a chemical relaxer in the past (P<.001).13 In another study of 39 women in Nigeria, those who had frequent and prolonged use of a chemical relaxer developed scarring alopecia more often than those who did not use a chemical relaxer (P<.0001). However, it is now known that the pathogenesis of CCCA may be related to an upregulation in genes implicated in fibroproliferative disorders (FPDs), a group of conditions characterized by aberrant wound healing, low-grade inflammation and irritation, and excessive fibrosis.14 They include systemic sclerosis, keloids, atherosclerosis, and uterine fibroids. The risk for certain FPDs is increased in individuals of African descent, and this increased risk is thought to be secondary to the protective effect that profibrotic alleles offer against helminths found in sub-Saharan Africa. A study of 5 patients with biopsy-proven CCCA found that there was increased expression of platelet-derived growth factor gene, PDGF; collagen I gene, COL I; collagen III gene, COL III; matrix metallopeptidase 1 gene, MMP1; matrix metallopeptidase 2 gene, MMP2; matrix metallopeptidase 7 gene, MMP7; and matrix metallopeptidase 9 gene, MMP9, in an affected scalp compared with an unaffected scalp.14 Still, chemical relaxers weaken the hair shaft and follicle structure, increasing the possibility of hair breakage and allowing for inflammation and trauma to render negative follicular effects.3,13
The following interventions can be recommended to patients who thermally or chemically treat their hair to prevent hair damage:
- Decrease the frequency of thermal straightening.
- Use lower heat settings on flat irons and blow-dryers.
- Thermally straighten only clean dry hair.
- Regularly trim split ends.
- Use moisturizing shampoos and conditioners.
- Have a trained professional apply a chemical relaxer, if affordable.
- Consider decreasing (1) the frequency of chemical relaxer touch-up (to every 8 to 10 weeks) and (2) the overall manipulation of hair. There is a fine balance between not treating often enough and treating too often: The transition point between chemically processed hair and grown-out roots is a high-tension breakage point.
- Apply a thick protective emollient (known as scalp basing) to the scalp before applying a relaxer1,5; this protects the scalp from irritation.
Techniques for Braids, Weaves, and Twists
Braids and cornrows, sewn-in or glued-on extensions and weaves, and twists are popular hairstyles. When applied improperly, however, they also can lead to alopecia.1-5,11,12 When braids are too tight, the patient might complain of headache. Characteristic tenting—hair pulled so tight that the scalp is raised—might be observed.3,5 Twists are achieved by interlocking 2 pieces of hair, which are held together by styling gel.1,4 When twists remain over many months, hair eventually knots or tangles into a permanent locking pattern (also known as dreadlocks, dreads, or locs).1,2,4 In some cases, the persistent weight of dreadlocks results in hair breakage.1,3,5
The following recommendations can be made to patients who style their hair with braids or cornrows, extensions or weaves, twists, or dreadlocks:
- Apply these styles with as little traction as possible.
- Change the direction in which braids and cornrows are styled frequently to avoid constant tension over the same areas.
- Opt for larger-diameter braids and twists.
- Leave these styles in place no longer than 2 or 3 months; consider removing extensions and weaves every 3 or 4 weeks.
- Remove extensions and weaves if they cause pain or irritation.
- Avoid the use of glue; opt for loosely sewn-in extensions and weaves.
- Consider the alternative of crochet braiding; this is a protective way to apply extensions to hair and can be worn straight, curly, braided, or twisted.5,12
Techniques for Other Hairstyling Practices
Low-hanging ponytails or buns, wigs, and natural hairstyles generally are considered safe when applied correctly.1,5 The following recommendations can be made to patients who have a low-hanging ponytail, bun, wig, or other natural hairstyle:
- Before a wig is applied, hold the hair against the scalp with a cotton, nylon, or satin wig cap and with clips, tapes, or bonds. Because satin does not cause constant friction or absorb moisture, it is the safest material for a wig cap.5
- Achieve a natural hairstyle by cutting off chemically processed hair and allowing hair to grow out.5
- Hair that has not been thermally or chemically processed better withstands the stresses of traction, pulling, and brushing.5
- For women with natural hair, wash hair at least every 2 weeks and moisturize frequently.5,12
- Caution patients that adding synthetic or human hair (ie, extensions, weaves) to any hairstyle to increase volume or length using glue or sewing techniques1-4,11 can cause problems. The extra weight and tension of extensions and weaves can lead to alopecia. Glue can trigger an irritant or allergic reaction, especially in women who have a latex allergy.1,4,5,11
Practice Implications
Women of African descent might be more susceptible to alopecia because of the distinctive structural properties of their hair and the various hair treatments and styles they often employ. Physicians should be knowledgeable when counseling these patients on their hair care practices. It also is important to understand that it might not be feasible for a patient to completely discontinue a hair treatment or style. In that situation, be prepared to make recommendations for safer hairstyling practices.
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
- Callender VD, McMichael AJ, Cohen GF. Medical and surgical therapies for alopecias in black women. Dermatol Ther. 2004;17:164-176. doi:10.1111/j.1396-0296.2004.04017.x
- Herskovitz I, Miteva M. Central centrifugal cicatricial alopecia: challenges and solutions. Clin Cosmet Investig Dermatol. 2016;9:175-181. doi:10.2147/CCID.S100816
- Tanus A, Oliveira CCC, Villarreal DJ, et al. Black women’s hair: the main scalp dermatoses and aesthetic practices in women of African ethnicity. An Bras Dermatol. 2015;90:450-465. doi:10.1590/abd1806-4841.20152845
- Gathers RC, Lim HW. Central centrifugal cicatricial alopecia: past, present, and future. J Am Acad Dermatol. 2009;60:660-668. doi:10.1016/j.jaad.2008.09.066
- Haskin A, Aguh C. All hairstyles are not created equal: what the dermatologist needs to know about black hairstyling practices and the risk of traction alopecia (TA). J Am Acad Dermatol. 2016;75:606-611. doi:10.1016/j.jaad.2016.02.1162
- Loussouarn G, Garcel A-L, Lozano I, et al. Worldwide diversity of hair curliness: a new method of assessment. Int J Dermatol. 2007;46(suppl 1):2-6. doi:10.1111/j.1365-4632.2007.03453.x
- De la Mettrie R, Saint-Léger D, Loussouarn G, et al. Shape variability and classification of human hair: a worldwide approach. Hum Biol. 2007;79:265-281. doi:10.1353/hub.2007.0045
- Takahashi T. Unique hair properties that emerge from combinations of multiple races. Cosmetics. 2019;6:36. https://doi.org/10.3390/cosmetics6020036
- Cloete E, Khumalo NP, Ngoepe MN. The what, why and how of curly hair: a review. Proc Math Phys Eng Sci. 2019;475:20190516. doi:10.1098/rspa.2019.0516
- Westgate GE, Ginger RS, Green MR. The biology and genetics of curly hair. Exp Dermatol. 2017;26:483-490. doi:10.1111/exd.13347
- McMichael AJ. Ethnic hair update: past and present. J Am Acad Dermatol. 2003;48(6 suppl):S127-S133. doi:10.1067/mjd.2003.278
- Roseborough IE, McMichael AJ. Hair care practices in African-American patients. Semin Cutan Med Surg. 2009;28:103-108. doi:10.1016/j.sder.2009.04.007
- Narasimman M, De Bedout V, Castillo DE, et al. Increased association between previous pregnancies and use of chemical relaxers in 74 women with central centrifugal cicatricial alopecia. Int J Trichology. 2020;12:176-181. doi:10.4103/ijt.ijt_37_20
- Aguh C, Dina Y, Talbot CC Jr, et al. Fibroproliferative genes are preferentially expressed in central centrifugal cicatricial alopecia. J Am Acad Dermatol. 2018;79:904-912.e901. doi:10.1016/j.jaad.2018.05.1257
Pencil-core Granuloma Forming 62 Years After Initial Injury
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.
An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.
Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.
An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.
Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
To the Editor:
Trauma from a pencil tip can sometimes result in a fragment of lead being left embedded within the skin. Pencil lead is composed of 66% graphite carbon, 26% aluminum silicate, and 8% paraffin.1,2 While the toxicity of these individual elements is low, paraffin can cause nonallergic foreign-body reactions, aluminum silicate can induce epithelioid granulomatous reactions, and graphite has been reported to cause chronic granulomatous reactions in the lungs of those who work with graphite.2 Penetrating trauma with a pencil can result in the formation of a cutaneous granulomatous reaction that can sometimes occur years to decades after the initial injury.3,4 Several cases of pencil-core granulomas have been published, with lag times between the initial trauma and lesion growth as long as 58 years.1-10 The pencil-core granuloma may simulate malignant melanoma, as it presents clinically as a growing, darkly pigmented lesion, thus prompting biopsy. We present a case of a pencil-core granuloma that began to grow 62 years after the initial trauma.
A 72-year-old woman was referred to our clinic for evaluation of a dark nodule on the forehead. The lesion had been present since the age of 10 years, reportedly from an accidental stabbing with a pencil. The lesion had been flat, stable, and asymptomatic since the trauma occurred; however, the patient reported that approximately 9 months prior to presentation, it had started growing and became painful. Physical examination revealed a 1.0-cm, round, bluish-black nodule on the right superior forehead (Figure 1A). No satellite lesions or local lymphadenopathy were noted on general examination.
An elliptical excision of the lesion with 1-cm margins revealed a bluish-black mass extending through the dermis, through the frontalis muscle, and into the periosteum and frontal bone (Figure 1B). A No. 15 blade was then used to remove the remaining pigment from the outer table of the frontal bone. Histopathologic findings demonstrated a sarcoidal granulomatous dermatitis associated with abundant, nonpolarizable, black, granular pigment consistent with carbon tattoo. This foreign material was readily identifiable in large extracellular deposits and also within histiocytes, including numerous multinucleated giant cells (Figure 2). Immunostaining for MART-1 and SOX-10 antigens failed to demonstrate a melanocytic proliferation. These findings were consistent with a sarcoidal foreign-body granulomatous reaction to carbon tattoo following traumatic graphite implantation.
Granulomatous reactions to carbon tattoo may be sarcoidal (foreign-body granulomatous dermatitis), palisading, or rarely tuberculoid (caseating). Sarcoidal granulomatous tattoo reactions may occur in patients with sarcoidosis due to koebnerization, and histology alone is not discriminatory; however, in our patient, the absence of underlying sarcoidosis or clinical or histologic findings of sarcoidosis outside of the site of the pencil-core granuloma excluded that possibility.11 Pencil-core granulomas are characterized by a delayed foreign-body reaction to retained fragments of lead often years following a penetrating trauma with a pencil. Previous reports have described various lag times from injury to lesion growth of up to 58 years.1-10 Our patient claimed to have noticed the lesion growing and becoming painful only after a 62-year lag time following the initial trauma. To our knowledge, this is the longest lag time between the initial pencil injury and induction of the foreign-body reaction reported in the literature. Clinically, the lesion appeared and behaved very similar to a melanoma, prompting further treatment and evaluation.
It has been suggested that the lag period between the initial trauma and the rapid growth of the lesion may correspond to the amount of time required for the breakdown of the pencil lead to a critical size followed by the dispersal of those particles within the interstitium, where they can induce a granulomatous reaction.1,2,9 One case described a patient who reported that the growth and clinical change of the pencil-core granuloma only started when the patient accidentally hit the area where the trauma had occurred 31 years prior.1 This additional trauma may have caused further mechanical breakdown of the lead to set off the tissue reaction. In our case, the patient did not recall any additional trauma to the head prior to the onset of growth of the nodule on the forehead.
Our case indicates that carbon tattoo may be a possible sequela of a penetrating injury from a pencil with retained pencil lead fragments; however, many of these carbon tattoos may remain stable throughout the remainder of the patient’s life.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
- Gormley RH, Kovach SJ III, Zhang PJ. Role for trauma in inducing pencil “lead” granuloma in the skin. J Am Acad Dermatol. 2010;62:1074-1075.
- Terasawa N, Kishimoto S, Kibe Y, et al. Graphite foreign body granuloma. Br J Dermatol. 1999;141:774-776.
- Fukunaga Y, Hashimoto I, Nakanishi H, et al. Pencil-core granuloma of the face: report of two rare cases. J Plast Reconstr Aesthet Surg. 2011;64:1235-1237.
- Aswani VH, Kim SL. Fifty-three years after a pencil puncture wound. Case Rep Dermatol. 2015;7:303-305.
- Taylor B, Frumkin A, Pitha JV. Delayed reaction to “lead” pencil simulating melanoma. Cutis. 1988;42:199-201.
- Granick MS, Erickson ER, Solomon MP. Pencil-core granuloma. Plast Reconstr Surg. 1992;89:136-138.
- Andreano J. Stump the experts. foreign body granuloma. J Dermatol Surg Oncol. 1992;18:277, 343.
- Yoshitatsu S, Takagi T. A case of giant pencil-core granuloma. J Dermatol. 2000;27:329-332.
- Hatano Y, Asada Y, Komada S, et al. A case of pencil core granuloma with an unusual temporal profile. Dermatology. 2000;201:151-153.
- Seitz IA, Silva BA, Schechter LS. Unusual sequela from a pencil stab wound reveals a retained graphite foreign body. Pediatr Emerg Care. 2014;30:568-570.
- Motaparthi K. Tattoo ink. In: Cockerell CJ, Hall BJ, eds. Nonneoplastic Dermatopathology. 2nd ed. Amirsys; 2016: 270.
Practice Points
- Pencil-core granulomas can arise even decades after the lead is embedded in the skin.
- It is important to biopsy to confirm the diagnosis, as pencil-core granulomas can very closely mimic melanomas.
Lower Leg Hyperpigmentation in MYH9-Related Disorder
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
To the Editor:
MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.1 We describe a case of lower leg hyperpigmentation secondary to hemosiderin deposition from MYH9-related disorder.
A 31-year-old woman with a history of MYH9-related disorder and mixed connective tissue disease presented to the outpatient dermatology clinic with asymptomatic brown patches on the lower legs (Figure) of 10 years’ duration. She also had epistaxis, hearing loss, renal disease, and menorrhagia secondary to MYH9-related disorder. The patient had been started on hydroxychloroquine 2 years earlier by rheumatology for mixed connective tissue disorder. A biopsy was not performed, given the risk of bleeding from thrombocytopenia. Ammonium lactate lotion was recommended for the leg patches. No further interventions were undertaken. At 6-month follow-up, hyperpigmentation on the lower legs was stable. The patient expressed no desire for cosmetic intervention.
Prior to discovery of a common gene, MYH9-related disorder was classified as 4 overlapping syndromes: May-Hegglin anomaly, Epstein syndrome, Fechtner syndrome, and Sebastian syndrome.2 More than 30 MYH9 mutations have been identified, all of which encode for myosin-9, a subunit of myosin IIA,1,3 that is a nonmuscle myosin needed for cell movement, shape, and cytokinesis. Although most cells use myosin IIA to IIC, certain cells, such as platelets and neutrophils, use myosin IIA exclusively.
In neutrophils of patients with MYH9-related disorder, nonfunctional myosin-9 clumps to form hallmark inclusion bodies, which are seen on the peripheral blood smear. Macrothrombocytopenia, another hallmark of MYH9-related disorder, also can be seen on the peripheral smear of all affected patients. Approximately 30%of patients develop clinical manifestations of the disorder (eg, bleeding, renal failure, hearing loss, presenile cataracts). Bleeding tendency usually is mild; epistaxis and menorrhagia are the most common hematologic manifestations.4
We attribute the lower leg hyperpigmentation in our patient to a severe phenotype of MYH9-related disorder. In addition to hyperpigmentation, our patient had menorrhagia requiring treatment with tranexamic acid, renal failure, and hearing loss, further pointing to a more severe phenotype. Furthermore, it is likely that our patient’s hyperpigmentation was made worse by hydroxychloroquine and a coexisting diagnosis of mixed connective tissue disease, which led to a propensity for increased vessel fragility in the setting of thrombocytopenia.
The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder. The presence of small red blood cells (RBCs) in microcytic anemia and large platelets of MYH9-related disorder can lead to a situation in which platelets travel near the center of the lumen of blood vessels, while RBCs travel to the periphery. This decrease in the platelet-endothelium interaction increases the risk for bleeding. Our patient’s hemoglobin level was within reference range, without evidence of iron-deficiency anemia. Correction of iron-deficiency anemia, if applicable, can prevent bleeding brought on by the mechanism of decreased platelet-endothelium interaction and avoid unnecessary antiplatelet medication because of misdiagnosis based on an erroneous platelet count.
The workup of MYH9-related disorder also should include audiography, ophthalmologic examination, and renal function testing for hearing loss, cataracts, and renal disease, respectively. Referral to genetics also may be warranted.
It also is of clinical interest that automated cell counters may underestimate the count of abnormally large platelets in MYH9-related disorder, counting them as RBCs or white blood cells. The platelet count in MYH9-related disorder may be underestimated by 4-fold or greater.4-7
Treatment of leg hyperpigmentation can prove challenging, given the location of dermal hemosiderin. Topical therapy likely is ineffective. Lasers and intense pulsed light therapy are treatment modalities to consider for the hyperpigmentation of MYH9-related disorder. There have been reports of improved cosmesis in dermal hemosiderin depositional disorders, such as venous stasis.4 Our patient was given ammonium lactate lotion to thicken collagen, possibly preventing future bleeding episodes.
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
- Pecci A, Canobbio I, Balduini A, et al. Pathogenetic mechanisms of hematological abnormalities of patients with MYH9 mutations. Hum Mol Genet. 2005;14:3169-3178. doi:10.1093/hmg/ddi344
- Seri M, Pecci A, Di Bari F, et al. MYH9-related disease: May-Hegglin anomaly, Sebastian syndrome, Fechtner syndrome, and Epstein syndrome are not distinct entities but represent a variable expression of a single illness. Medicine (Baltimore). 2003;82:203-215. doi:10.1097/01.md.0000076006.64510.5c
- Medline Plus. MYH9-related disorder. National Library of Medicine website. Updated August 18, 2020. Accessed January 21, 2022. https://ghr.nlm.nih.gov/condition/myh9-related-disorder#diagnosis
- Althaus K, Greinachar A. MYH9-related platelet disorders. Semin Thromb Hemost. 2009;35:189-203. doi:10.1055/s-0029-1220327
- Kunishima S, Hamaguchi M, Saito H. Differential expression of wild-type and mutant NMMHC-IIA polypeptides in blood cells suggests cell-specific regulation mechanisms in MYH9 disorders. Blood. 2008;111:3015-3023. doi:10.1182/blood-2007-10-116194
- Arrondel C, Vodovar N, Knebelmann B, et al. Expression of the nonmuscle myosin heavy chain IIA in the human kidney and screening for MYH9 mutations in Epstein and Fechtner syndromes. J Am Soc Nephrol. 2002;13:65-74. doi:10.1681/ASN.V13165
- Selleng K, Lubenow LE, Greinacher A, et al. Perioperative management of MYH9 hereditary macrothrombocytopenia (Fechtner syndrome). Eur J Haematol. 2007;79:263-268. doi:10.1111/j.1600-0609.2007.00913.x
Practice Points
- MYH9-related disorder is an autosomal-dominant disorder characterized by macrothrombocytopenia and neutrophil inclusions secondary to defective myosin-9.
- Leg hyperpigmentation can occur secondary to hemosiderin deposition from MYH9-related disorder.
- The workup of suspected MYH9-related disorder includes exclusion of iron-deficiency anemia, which can increase bleeding in patients with the disorder.
- Lasers and intense pulsed light therapy are modalities to consider for the hyperpigmentation of MYH9- related disorder.
Severe Acute Systemic Reaction After the First Injections of Ixekizumab
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
Case Report
A 39-year-old woman who was otherwise healthy presented with fatigue, malaise, a resolving rash, focal lymphadenopathy, increasing distal arthritis, dactylitis, resolving ecchymoses, and acute onycholysis of 1 week’s duration that developed 13 days after initiating ixekizumab. The patient had a history of psoriasis and psoriatic arthritis for more than 10 years. She had been successfully treated in the past for psoriasis with adalimumab for several years; however, adalimumab was discontinued after an episode of Clostridium difficile colitis. The patient had a negative purified protein derivative (tuberculin) test prior to starting biologics as she works in the health care field. Routine follow-up purified protein derivative (tuberculin) test was positive. She discontinued all therapy for psoriasis and psoriatic arthritis prior to being appropriately treated for 6 months under the care of infectious disease physicians. She then had several pregnancies and chose to restart biologic treatment after weaning her third child from breastfeeding, as her skin and joint disease were notably flaring.
Ustekinumab was chosen to shift treatment away from tumor necrosis factor (TNF) α inhibitors. The patient's condition was under relatively good control for 1 year; however, she experienced notable gastrointestinal tract upset (ie, intermittent diarrhea and constipation), despite multiple negative tests for C difficile. The patient was referred to see a gastroenterologist but never followed up. Due to long-term low-grade gastrointestinal problems, ustekinumab was discontinued, and the gastrointestinal symptoms resolved without treatment.
Given the side effects noted with TNF-α and IL-12/23 inhibitors and the fact that the patient’s cutaneous and joint disease were notable, the decision was made to start the IL-17A inhibitor ixekizumab. The patient administered 2 injections, one in each thigh. Within 12 hours, she experienced severe injection-site pain. The pain was so severe that it woke her from sleep the night of the first injections. She then developed severe pain in the right axilla that limited upper extremity mobility. Within 48 hours, she developed an erythematous, nonpruritic, nonscaly, mottled rash on the right breast that began to resolve within 24 hours without treatment. In addition, 3 days after the injections, she developed ecchymoses on the trunk and extremities without any identifiable trauma, severe acute onycholysis in several fingernails (Figure 1) and toenails, dactylitis such that she could not wear her wedding ring, and a flare of psoriatic arthritis in the fingers and ankles.
At the current presentation (2 weeks after the injections), the patient reported malaise, flulike symptoms, and low-grade intermittent fevers. Results from a hematology panel displayed leukopenia at 2.69×103/μL (reference range, 3.54–9.06×103/μL) and thrombocytopenia at 114×103/μL (reference range, 165–415×103/μL).1 Her most recent laboratory results before the ixekizumab injections displayed a white blood cell count level at 4.6×103/μL and platelet count at 159×103/μL. C-reactive protein and erythrocyte sedimentation rate were within reference range. A shave biopsy of an erythematous nodule on the proximal interphalangeal joint of the fourth finger on the right hand displayed spongiotic dermatitis with eosinophils (Figure 2).
Interestingly, the psoriatic plaques on the scalp, trunk, and extremities had nearly completely resolved after only the first 2 injections. However, given the side effects, the second dose of ixekizumab was held, repeat laboratory tests were ordered to ensure normalization of cytopenia, and the patient was transitioned to pulse-dose topical steroids to control the remaining psoriatic plaques.
One week after presentation (3 weeks after the initial injections), the patient’s systemic symptoms had almost completely resolved, and she denied any further concerns. Her fingernails and toenails, however, continued to show the changes of onycholysis noted at the visit.
Comment
Ixekizumab is a human IgG4 monoclonal antibody that binds to IL-17A, one of the cytokines involved in the pathogenesis of psoriasis. The monoclonal antibody prevents its attachment to the IL-17 receptor, which inhibits the release of further cytokines and chemokines, decreasing the inflammatory and immune response.2
Ixekizumab was approved by the US Food and Drug Administration for plaque psoriasis after 3 clinical trials—UNCOVER-1, UNCOVER-2, and UNCOVER-3—were performed. In UNCOVER-3, the most common side effects that occurred—nasopharyngitis, upper respiratory tract infection, injection-site reaction, arthralgia, headache, and infections (specifically candidiasis)—generally were well tolerated. More serious adverse events included cardiovascular and cerebrovascular events, inflammatory bowel disease, and nonmelanoma skin cancer.3
Notable laboratory abnormalities that have been documented from ixekizumab include elevated liver function tests (eg, alanine aminotransferase, aspartate aminotransferase, bilirubin, and alkaline phosphatase), as well as leukopenia, neutropenia, and thrombocytopenia.4 Although short-term thrombocytopenia, as described in our patient, provides an explanation for the bruising noted on observation, it is unusual to note such notable ecchymoses within days of the first injection.
Onycholysis has not been documented as a side effect of ixekizumab; however, it has been reported as an adverse event from other biologic medications. Sfikakis et al5 reported 5 patients who developed psoriatic skin lesions after treatment with 3 different anti-TNF biologics—infliximab, adalimumab, or etanercept—fo
The exact pathophysiology of these adverse events has not been clearly understood, but it has been proposed that anti-TNF biologics may initiate an autoimmune reaction in the skin and nails, leading to paradoxical psoriasis and nail changes such as onycholysis. Tumor necrosis factor may have a regulatory role in the skin that prevents autoreactive T cells, such as cutaneous lymphocyte antigen–expressing T cells that promote the formation of psoriasiform lesions. By inhibiting TNF, there can be an underlying activation of autoreactive T cells that leads to tissue destruction in the skin and nails.6 Anti-TNF biologics also could increase CXCR3, a chemokine receptor that allows autoreactive T cells to enter the skin and cause pathology.7
IL-17A and IL-17F also have been shown to upregulate the expression of TNF receptor II in synoviocytes,8 which demonstrates that IL-17 works in synergy with TNF-α to promote an inflammatory reaction.9 Due to the inhibitory effects of ixekizumab, psoriatic arthritis should theoretically improve. However, if there is an alteration in the inflammatory sequence, then the regulatory role of TNF could be suppressed and psoriatic arthritis could become exacerbated. Additionally, its associated symptoms, such as dactylitis, could develop, as seen in our patient.4 Because psoriatic arthritis is closely associated with nail changes of psoriasis, it is conceivable that acute arthritic flares and acute onycholysis are both induced by the same cytokine dysregulation. Further studies and a larger patient population need to be evaluated to determine the exact cause of the acute exacerbation of psoriatic arthritis with concomitant nail changes as noted in our patient.
Acute onycholysis (within 72 hours) is a rare side effect of ixekizumab. It can be postulated that our patient’s severe acute onycholysis associated with a flare of psoriatic arthritis could be due to idiosyncratic immune dysregulation, promoting the activity of autoreactive T cells. The pharmacologic effects of ixekizumab occur through the inhibition of IL-17. We propose that by inhibiting IL-17 with associated TNF alterations, an altered inflammatory cascade could promote an autoimmune reaction leading to the described pathology.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
- Kratz A, Pesce MA, Basner RC, et al. Laboratory values of clinical importance. In: Kasper D, Fauci A, Hauser S, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. McGraw-Hill; 2014.
- Ixekizumab. Package insert. Eli Lilly & Co; 2017.
- Gordon KB, Blauvelt A, Papp KA, et al. Phase 3 trials of ixekizumab in moderate-to-severe plaque psoriasis. N Engl J Med. 2016;375:345-356.
- Leonardi C, Matheson R, Zachariae C, et al. Anti-interleukin-17 monoclonal antibody ixekizumab in chronic plaque psoriasis. N Engl J Med. 2012;366:1190-1199.
- Sfikakis PP, Iliopoulos A, Elezoglou A, et al. Psoriasis induced by anti-tumor necrosis factor therapy: a paradoxical adverse reaction. Arthritis Rheum. 2005;52:2513-2518.
- Berg EL, Yoshino T, Rott LS, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461-1466.
- Flier J, Boorsma DM, van Beek PJ, et al. Differential expression of CXCR3 targeting chemokines CXCL10, CXCL9, and CXCL11 in different types of skin inflammation. J Pathol. 2001;194:398-405.
- Zrioual S, Ecochard R, Tournadre A, et al. Genome-wide comparison between IL-17A- and IL-17F-induced effects in human rheumatoid arthritis synoviocytes. J Immunol. 2009;182:3112-3120.
- Gaffen SL. The role of interleukin-17 in the pathogenesis of rheumatoid arthritis. Curr Rheumatol Rep. 2009;11:365-370.
Practice Points
- Psoriasis is an autoimmune disorder with a predominance of CD4+ and CD8+ T cells that release cytokines, such as tumor necrosis factor 11α and interleukins, which promote inflammation in the skin and joints and is associated with systemic inflammation predisposing patients to cardiovascular disease.
- Common adverse effects of most biologic medications for psoriasis include injection-site pain and rash, fever, malaise, back pain, urticaria and flushing, edema, dyspnea, and nausea.
- Ixekizumab is a humanized IL-17A antagonist intended for adults with moderate to severe psoriasis. Certain rare side effects specific to ixekizumab include inflammatory bowel disease, thrombocytopenia, severe injection-site reactions, and candidiasis.
- Acute onycholysis and acute exacerbation of arthritis/dactylitis are rare side effects of ixekizumab therapy.
Scleral Plaques in Nephrogenic Systemic Fibrosis
To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2
- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2
To the Editor:
A 44-year-old man with a history of systemic lupus erythematosus (SLE) complicated by lupus nephritis, end-stage renal disease, and antiphospholipid syndrome was evaluated for progressive skin tightening over the last 3 years, predominantly on the hands but also involving the feet, legs, and arms. Physical examination revealed multiple flesh-colored to hypopigmented, bound-down, indurated, fissured plaques over the distal upper and lower extremities, most prominent over the hands (Figure 1). Yellow plaques appeared on the lateral sclera of both eyes (Figure 2). A diagnosis of nephrogenic systemic fibrosis (NSF) was supported by typical findings on punch biopsy, including a proliferation of dermal fibroblasts with thickened collagen bundles and mucin deposition.
Nephrogenic systemic fibrosis, also known as nephrogenic fibrosing dermopathy, is characterized by fibrotic plaques and nodules that tend to be bilateral.1 The chronic course of this disease often is accompanied by flexion contractures. Yellow scleral plaques caused by calcium phosphate deposition are present in up to 75% of cases and are more specific to a diagnosis of NSF in patients younger than 45 years.1,2 A strong association exists between NSF and gadolinium contrast agents in patients with acute renal failure; our patient later confirmed multiple gadolinium exposures years prior. Deposits of gadolinium have even been found in NSF skin lesions.2
- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
- Stone JH. A Clinician’s Pearls & Myths in Rheumatology. Springer London; 2009.
- Barker-Griffith A, Goldberg J, Abraham JL. Ocular pathologic features and gadolinium deposition in nephrogenic systemic fibrosis. Arch Ophthalmol. 2011;129:661-663.
Practice Points
- It is important to examine the eyes in a patient with sclerotic skin changes on physical examination.
- The presence of yellow scleral plaques strongly is associated with a diagnosis of nephrogenic systemic fibrosis.
Diffuse Urticarial Rash in a Pregnant Patient
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
The Diagnosis: Pemphigoid Gestationis
A lesional biopsy showed a subepidermal split with eosinophils and neutrophils. Perilesional biopsy for direct immunofluorescence (DIF) showed linear deposition of 3+ C3 along the basement membrane zone. The clinical, histopathologic, and immunofluorescent findings were consistent with pemphigoid gestationis (PG). Prednisone 1 mg/kg daily was initiated. Her condition continued to worsen, and cyclosporine 250 mg daily was added while prednisone was tapered, with remission of disease.
Pemphigoid gestationis is an autoimmune bullous dermatosis that occurs in the second or third trimester of pregnancy, with an incidence of 1 in 50,000 to 60,000 pregnancies.1 In terms of pathogenesis, aberrant expression of major histocompatibility complex class II molecules on placental tissues causes the loss of immune tolerance of the placenta, which leads to the production of antibodies against the placental protein bullous pemphigoid 180.2 Bullous pemphigoid 180 also is a hemidesmosomal protein found in the skin of the mother; therefore, the presence of the circulating antibodies leads to separation at the dermoepidermal junction and vesiculation.
Pemphigoid gestationis is characterized by the sudden eruption of intensely pruritic urticarial papules and plaques, classically with periumbilical involvement. Tense vesicles and bullae can develop. Women with PG have an increased risk for development of Graves disease. Histopathology shows subepidermal vesiculation with a predominance of eosinophils. Direct immunofluorescence classically shows linear deposition of C3 along the basement membrane zone. Fetal complications include prematurity and small size for gestational age. Additionally, blisters can be seen in 5% to 10% of neonates due to placental transmission of autoantibodies.3
Frequently PG flares shortly postpartum. Pemphigoid gestationis resolves within 6 months postdelivery but frequently reoccurs in subsequent pregnancies. Mild disease can be treated with mid- to high-potency topical corticosteroids. Severe disease is managed with oral corticosteroids, most commonly prednisone. Refractory disease is managed with azathioprine, cyclosporine, intravenous immunoglobulin, or plasmapheresis.
The differential diagnosis of PG includes other pregnancy-associated dermatoses such as atopic eruption of pregnancy, impetigo herpetiformis, intrahepatic cholestasis of pregnancy, and polymorphous eruption of pregnancy. Atopic eruption of pregnancy is the most common dermatosis of pregnancy and is characterized by an eczematous eruption in patients with an atopic history, typically in the first trimester. Blisters are not seen, and DIF is negative. Impetigo herpetiformis, or pustular psoriasis of pregnancy, is a variant of generalized pustular psoriasis that occurs during pregnancy. Diffuse erythematous patches studded with pustules, rather than vesicles, are seen; DIF is negative. Intrahepatic cholestasis of pregnancy presents without primary skin findings and severe pruritus predominantly on the palms and soles, often with secondary excoriations. Polymorphous eruption of pregnancy presents as a polymorphous eruption of urticarial to erythematous papules and plaques commonly originating in striae. In contrast to PG, there is periumbilical sparing, vesiculation is rare, and DIF is negative.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
- Shornick JK, Bangert JL, Freeman RG, et al. Herpes gestationis: clinical and histologic features of twenty-eight cases. J Am Acad Dermatol. 1983;8:214-224.
- Sadik CD, Lima AL, Zillikens D. Pemphigoid gestationis: toward a better understanding of the etiopathogenesis. Clin Dermatol. 2016;34:378-382.
- Shornick JK, Black MM. Fetal risks in herpes gestationis. J Am Acad Dermatol. 1992;26:63-68.
A 29-year-old pregnant woman at 18 weeks and 5 days of gestation presented with a diffuse, pruritic, blistering rash of 5 weeks’ duration that started on the forearms and generalized to affect the trunk, legs, palms, and soles. Physical examination showed diffuse urticarial papules and plaques with small tense vesicles with an annular configuration on the abdomen and marked periumbilical involvement.
Pruritic Papules on the Trunk, Extremities, and Face
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
The Diagnosis: Gamasoidosis
An entomologist confirmed the specimen as an avian mite in either the Dermanyssus or Ornithonyssus genera (quiz image [bottom]). The patient was asked whether any bird had nested around her bedroom, and she affirmed that a woodpecker had nested outside her bedroom closet that spring. She subsequently discovered it had burrowed a hole into her closet wall. She and her husband removed the nest, and within 4 weeks, the eruption permanently cleared.
Gamasoidosis, or avian mite dermatitis, often is an overlooked, difficult-to-make diagnosis that is increasing in prevalence.1 Bird mites are ectoparasitic arthropods that are 0.3 to 1 mm in length. They have egg-shaped bodies with 4 pairs of legs; they are a translucent brown color before feeding and red after feeding.2 Although most avian mites cannot subsist off human blood, if the mites are without an avian host, such as after affected birds abandon their nests, the mites will bite humans.3 Studies have discovered the presence of mammalian erythrocytes in the digestive tracts of one species of bird mite, Dermanyssus gallinae, suggesting that at least one form of avian mite may feed off humans but typically cannot reproduce without an avian blood meal.4 Individuals with gamasoidosis often are exposed to avian mites by owning birds as pets, rearing chickens or messenger pigeons, or having bird’s nests around their bedrooms or air conditioning units.1
Most people who develop avian mite dermatitis are the only affected member of the family to develop pruritus and papules since the reaction requires both bites and hypersensitivity to them; however, there are cases of nuclear families all reacting to avian mite bites.2,4 As in this case, hypersensitivity to avian mite bites causes exquisitely pruritic 2- to 5-mm papules, vesicles, or urticarial lesions that may be diagnosed as papular urticaria or misdiagnosed as scabies. Although bird mites may carry bacteria such as Salmonella, Spirochaete, Rickettsia, and Pasteurella, they have not demonstrated an ability to pass these on to human vectors.5,6
Bird mites will spend most of their lives on avian hosts but can spread to humans through direct contact or through air.7 Mites can go through floors, walls, ceilings, or most commonly through ventilation or air conditioning units. Increasing urbanization, especially in warmer climates where avian mites thrive, has increased the prevalence of gamasoidosis.1
Avian mite dermatitis commonly can be mistaken for scabies, but the mites can be seen with the naked eye and cannot form burrows, unlike scabies.4,8 Avian mites usually are not found on human skin since they leave the host after feeding and move with surprising speed.8 Pediculosis corporis (body lice) results from an infestation of Pediculus humanus corporis. At 2- to 4-mm long, this louse is much larger than a bird mite. Body lice rarely are found on the skin but rather live and lay eggs on clothing, particularly along the seams. The body louse has an elongated body with 3 segments and short antennae. Pthirus pubis (pubic lice) measure 1.5 to 2.0 mm in adulthood and have wider, more crablike bodies compared to body or hair lice or avian mites. Lice, being insects, have 6 legs as opposed to mites, being arachnids, having 8 legs. Cheyletiella are 0.5-mm long, nonburrowing mites commonly found on cats, dogs, and rabbits. Cheyletiella blakei affects cats. They look somewhat similar to bird mites but have hooklike palps extending from their heads instead of antennae.
Antihistamines and topical corticosteroids may reduce discomfort from avian bites but are not curative.2,9 The most efficient way to treat gamasoidosis is to remove any affected birds or nearby bird’s nests, as the mites cannot survive more than a few weeks to months without feeding on an avian host.8 It also may be necessary to fumigate infested rooms.10
The diagnosis of avian mite dermatitis often is missed to the frustration of the patient and clinician alike. Becoming familiar with this bite reaction will help clinicians diagnose this dermatologic conundrum.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
- Wambier CG, de Farias Wambier SP. Gamasoidosis illustrated— from the nest to dermoscopy. An Bras Dermatol. 2012;87:926-927. doi:10.1590/s0365-05962012000600021
- Collgros H, Iglesias-Sancho M, Aldunce MJ, et al. Dermanyssus gallinae (chicken mite): an underdiagnosed environmental infestation. Clin Exp Dermatol. 2013;38:374-377.
- Akdemir C, Gülcan E, Tanritanir P. Case report: Dermanyssus gallinae in a patient with pruritus and skin lesions. Turkiye Parazitol Derg. 2009;33:242-244.
- Williams RW. An infestation of a human habitation by Dermanyssus gallinae (de Geer, 1778)(Acarina: Dermanyssidae) in New York resulting in sanguisugent attacks upon the occupants. Am J Trop Med Hyg. 1958;7:627-629.
- Walker A. The Arthropods of Humans and Domestic Animals. A Guide to Preliminary Identification. Chapman and Hall; 1994.
- Vaiente MC, Chauve C, Zenner L. Experimental infection of Salmonella enteritidis by the poultry red mite, Dermanyssus gallinae. Vet Parasitol. 2007;146:329-336.
- Regan AM, Metersky ML, Craven DE. Nosocomial dermatitis and pruritus caused by pigeon mite infestation. Arch Intern Med. 1987;147:2185-2187.
- Orton DI, Warren LJ, Wilkinson JD. Avian mite dermatitis. Clin Exp Dermatol. 2000;25:129-131.
- Bassini-Silva R, de Castro Jacinavicius F, Akashi Hernandes F, et al. Dermatitis in humans caused by Ornithonyssus bursa (Berlese 1888) (Mesostigmata: Macronyssidae) and new records from Brazil. Rev Bras Parasitol Vet. 2019;28:134-139.
- Watson CR. Human infestation with bird mites in Wollongong. Commun Dis Intell Q Rep. 2003;27:259-261.
A 69-year-old woman presented in early summer in southeastern Michigan with several itchy bumps (top) of 4 to 5 weeks’ duration that erupted and remitted over the trunk, extremities, and face. She had taken no new medications. She had an asymptomatic cat and no exposure to anyone else who had been itching. Physical examination revealed approximately a dozen 2- to 5-mm edematous papules on the trunk, arms, shins, thighs, and left cheek, as well as one 3-mm vesicle on the forearm. No burrows could be identified on physical examination. Lesions treated with betamethasone dipropionate cream 0.05% improved, but new lesions continued to arise. An exterminator was contacted but found no signs of bedbugs or other infestations. Later, the patient reported seeing 3 tiny black dots crawl across the screen of her cell phone as she read in bed. She was able to capture them on tape and bring them to her appointment. The specimens were approximately 1 mm in length (bottom).

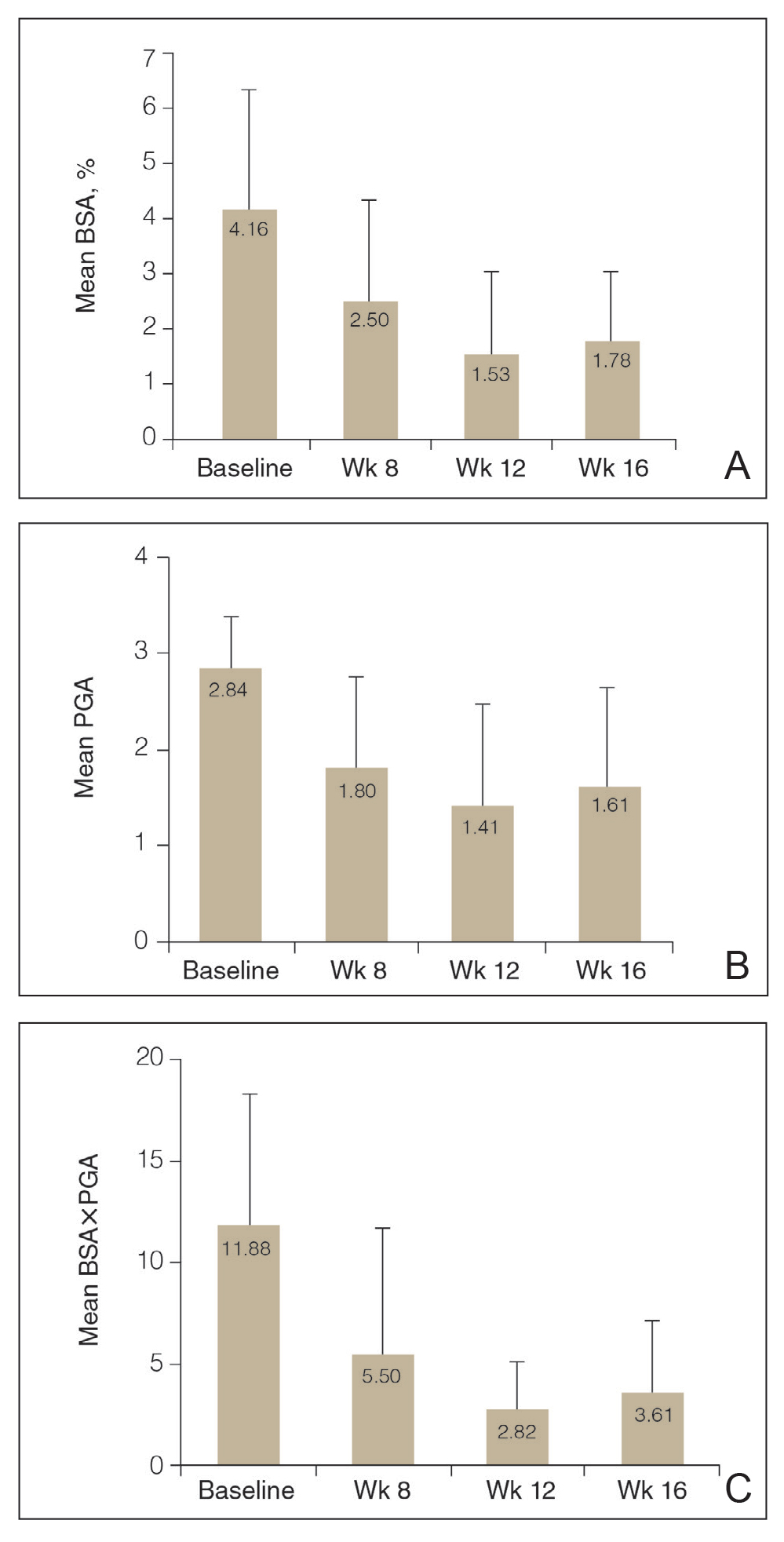
![Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16 Proportion of participants achieving National Psoriasis Foundation target-to-treat status (body surface area [BSA] ≤1%) at baseline and weeks 8, 12, and 16](https://cdn.mdedge.com/files/s3fs-public/Bagel_0222_2.JPG)