User login
Diabetes Hub contains news and clinical review articles for physicians seeking the most up-to-date information on the rapidly evolving options for treating and preventing Type 2 Diabetes in at-risk patients. The Diabetes Hub is powered by Frontline Medical Communications.
In T1 diabetes, CABG seems better than PCI
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with aggressive multivessel CAD and stable symptoms associated with diabetes or high SYNTAX score, the mechanisms of benefit of PCI and CABG are different, and this difference likely explains the superior results of CABG.
Better stents alone cannot change the superiority of CABG, compared with PCI for patients with aggressive CAD (diabetes or high SYNTAX score), because PCI addresses only a small portion of the coronary anatomy. This does not diminish the importance of continuing advances in stent technology, but rather, it puts into appropriate perspective what can be expected from these advances.
The findings of this important study help to better inform practice, and should influence decision-making for revascularization in patients with T1DM.
These remarks were taken from an editorial by Michael J. Domanski, MD, and Michael E. Farkouh, MD (J Am Coll Cardiol. 2017. doi: 10.1016/j.jacc.2017.07.781). Dr. Domanski is with the Peter Munk Cardiac Centre, Toronto, and the Heart and Stroke Richard Lewar Centre, University of Toronto. Dr. Farokouh is the director of clinical trials at the Peter Munk Cardiac Centre, University of Toronto.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
In patients with type 1 diabetes in need of multivessel revascularization, coronary artery bypass graft (CABG) may be a better choice than percutaneous coronary intervention (PCI), according to results from a new comparative study presented at the annual congress of the European Society of Cardiology by Martin J. Holzmann, PhD, of the Karolinska Institute, Stockholm.
The two procedures had similar mortality rates, but PCI patients fared worse with respect to mortality due to myocardial infarction and several cardiovascular outcomes.
Previous studies had also suggested better outcomes with CABG than with PCI, but they lumped together patients with type 1 and type 2 diabetes, while the current study focused only on patients with type 1 diabetes.
The study included patients in Sweden with type 1 diabetes who underwent CABG (683 patients) or PCI (1,863 patients) between 1995 and 2013. During follow-up, 44.6% of patients in the PCI group died, compared with 53.3% in the CABG group. After adjustment for between-group differences, however, there was no significant difference in mortality risk between the two groups.
However, assessments of cause-specific mortality told a different story. Subjects in the PCI group had a greater risk of death from coronary artery disease (hazard ratio, 1.45; 95% confidence interval, 1.21-1.74).
Subjects in the PCI group were also more likely to suffer myocardial infarction (HR, 1.47; 95% CI, 1.21-1.77) and were more than five times more likely to undergo repeat vascularization (adjusted HR, 5.64; 95% CI, 4.67-6.82). The CABG group had a higher 30-day stroke risk (1.9% vs. 0.8%), but there was no difference in long-term risk.
The two groups had similar risks of hospitalization for heart failure.
The researchers noted a large difference between the two groups with respect to risk during the first year of follow-up, which suggests that some patients underwent PCI because they were too ill to undergo CABG. This limitation is also suggested by the greater proportion of previous stroke, heart failure, active cancer, and end-stage renal disease in the PCI group. The researchers adjusted for these differences, but it remains possible that there were residual confounders.
No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
FROM THE ESC CONGRESS 2017
Key clinical point: Patients undergoing PCI had worse cardiovascular outcomes than those receiving CABG.
Major finding: The PCI group had a 45% increased risk of death due to myocardial infarction.
Data source: Observational study (n = 2,546).
Disclosures: No source of funding was disclosed. One of the authors has received consultancy honoraria from Actelion and Pfizer. Dr. Domanski and Dr. Farkouh report no relevant financial relationships.
Think beyond BMI to optimize bariatric patients presurgery
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
NEW YORK – A structured, four-pronged approach to get patients as fit and healthy as possible prior to bariatric surgery holds the potential to improve postoperative outcomes. In general, bariatric surgery patients are in a better position than most surgery candidates because of a longer preoperative period. During this time, surgeons can work with a multidisciplinary team to optimize any medical, nutritional, exercise-related, and mental health concerns.
“People focus on the size of our patients and the weight of our patients, but [body mass index] is only one factor. They can have many other comorbidities that are significant,” Dr. LaMasters said. Patients can present with cardiac and pulmonary issues, hypertension, sleep apnea, diabetes, asthma, reflux and “a very high incidence of anxiety and depression.”
“So we have a lot of challenges,” she added. “We take care of complex, high-risk patients, and our goal is to improve outcomes. Using presurgery optimization can be a key to that.”
Maximizing medical readiness
Multiple providers drive the medical intervention, Dr. LaMasters said, including surgeons and primary care doctors, as well as advanced practice providers, medical weight loss providers, and other specialists. “We do try to get patients to lose weight before surgery, but that’s not an absolute requirement. More important is adjustment of other risk factors like pulmonary risk factors, control of hypertension, treatment of sleep apnea, and control of hyperglycemia. We’d like to have their A1c [test results to be] under 8%. We want to start [proton pump inhibitors] early because there is a very high prevalence of reflux and gastritis in this population.”
Bariatric surgery patients “are uniquely positioned to have a substantial benefit from that ‘prehabilitation,’ but this only works if you have a multidisciplinary team,” Dr. LaMasters said at the American College of Surgeons Quality and Safety Conference. “Think of this as down-staging disease, like in a cancer model.”
“The message from this is there is an opportunity if we build it into the prehab phase of care. It’s a new way of thinking in surgery. You can change your results,” said session moderator David B. Hoyt, MD, FACS, Executive Director of the American College of Surgeons.
Nutritional know-how
Dietitians determine the second component – how to optimize nutrition before surgery. They focus on education, evaluation, setting goals, “and very importantly, supporting patients to attain those goals,” Dr. LaMasters said. Goals include increasing protein intake prior to surgery to a recommended 1.5 g/kg/day and starting nutritional supplements ahead of time.
Even though they typically consume an excess amount of calories, “many of our patients have baseline malnutrition,” Dr. LaMasters said. Establishing mindful behavior for meal planning, preparation, and eating is a potential solution, as is addressing any socioeconomic factors that can present challenges to healthy eating.
Emphasizing exercise
“The exercise piece is really key for our patients,” Dr. LaMasters said. Many candidates for bariatric surgery have mobility issues. “The first thing many say is ‘I can’t exercise.’ We instruct them that they can exercise. Our job is to find out what they can do – there are many different exercise modalities.”
A good baseline assessment is a 6-minute walk test to assess their distance limits, oxygen level, and any resulting symptoms.
“Our goal is to get them to walking – even those who can barely walk with a walker – for 5-10 minutes, six times a day,” Dr. LaMasters said. “We feel that is a minimum threshold to prevent blood clots after surgery.” Another recommendation is to get surgical candidates to do some activity 30 minutes a day, four times a week, at a minimum. “Eventually, after surgery and when they’ve lost weight and are healthier, the goal is going to be 1 hour, five days a week.”
Start the exercise program at least 4-8 weeks prior to surgery. Most studies show significant benefit if you start at least 4 weeks prior to surgery, Dr. LaMasters suggested. “In our own practice, we’ve seen if you can start a daily walking program even just 2 weeks prior to surgery, we see a significant benefit.”
Addressing anxiety or depression
The mental health piece is very important and should be guided by mental health providers on the multidisciplinary team, Dr. LaMasters said.
“Our patients have a high degree of stress in their lives, especially related to socioeconomic factors. A patient who does not have their anxiety or depression under control will not do as well after surgery.”
Optimization in other specialties
The benefits of a prehabilitation exercise program have been demonstrated across many other specialties, especially in colorectal surgery, cardiovascular surgery, and orthopedic surgery, Dr. LaMasters said. In randomized, controlled studies, this optimization is associated with decreased complications, mortality, and length of hospital stay.
“There is actually way less data from bariatric studies. I suggest to you that our bariatric surgery patients have similar comorbidities when compared with those other specialties – specialties that refer their patients to us for treatment,” Dr. LaMasters said.
In a study of cardiorespiratory fitness before bariatric surgery, other researchers found that the most serious postoperative complications occurred more often among patients who were less fit preoperatively (Chest. 2006 Aug;130[2]:517-25). These investigators measured peak oxygen consumption (VO2) preoperatively in 109 patients. “Each unit increase in peak VO2 rate was associated with 61% decrease in overall complications,” Dr. LaMasters said. “So a small increase in fitness led to a big decrease in complications.”
Other researchers compared optimization of exercise, nutrition, and psychological factors before and after surgery in 185 patients with colorectal cancer (Acta Oncol. 2017 Feb;56[2]:295-300). A control group received the interventions postoperatively. “They found a statistically significant difference in the prehabilitation group in increased functional capacity, with more than a 30-meter improvement in 6-minute walk test before surgery,” Dr. LaMasters said. Although the 6-minute walk test results decreased 4 weeks after surgery, as might be expected, by 8 weeks the prehabilitation patients performed better than controls – and even better than their own baseline, she added. “This model of optimization can be very well applied in bariatric surgery.”
“The goal is safe surgery with outstanding long-term outcomes,” Dr. LaMasters said. “It is really not enough in this era to ‘get a patient through surgery.’ We really need to optimize the risk factors we can and identify any areas where they will have additional needs after surgery,” she added. “This will allow us to have excellent outcomes in this complex patient population.”
Dr. LaMasters and Dr. Hoyt had no relevant financial disclosures.
AT THE ACS QUALITY & SAFETY CONFERENCE
Liraglutide approved for cardiovascular event reduction
The type 2 diabetes treatment Victoza (liraglutide) has been approved by the Food and Drug Administration for a new indication – to reduce the risk of several adverse cardiovascular events, according to a press release from Novo Nordisk.
FDA approval was based on results from the LEADER (NCT01179048) trial, where people with type 2 diabetes who received liraglutide were 13% less likely to experience cardiovascular death, nonfatal heart attack, or nonfatal stroke then type 2 diabetes patients who received a placebo, with an absolute risk reduction of 1.9%. Notably, risk of cardiovascular death was reduced by 22% in the liraglutide group, compared with the control group, and all-cause death was reduced by 15%.
“Today’s news is significant for millions of Americans living with type 2 diabetes because, even when controlled, diabetes puts patients at a greater risk for cardiovascular events. More treatment options like Victoza that address critical aspects of diabetes care beyond glucose lowering are essential to confront this pervasive issue,” Steve Marso, MD, medical director at the Cardiovascular Services HCA Midwest Health Heart and Vascular Institute and one of the primary investigators in LEADER said in the press release.
Find the full press release on the Novo Nordisk website.
The type 2 diabetes treatment Victoza (liraglutide) has been approved by the Food and Drug Administration for a new indication – to reduce the risk of several adverse cardiovascular events, according to a press release from Novo Nordisk.
FDA approval was based on results from the LEADER (NCT01179048) trial, where people with type 2 diabetes who received liraglutide were 13% less likely to experience cardiovascular death, nonfatal heart attack, or nonfatal stroke then type 2 diabetes patients who received a placebo, with an absolute risk reduction of 1.9%. Notably, risk of cardiovascular death was reduced by 22% in the liraglutide group, compared with the control group, and all-cause death was reduced by 15%.
“Today’s news is significant for millions of Americans living with type 2 diabetes because, even when controlled, diabetes puts patients at a greater risk for cardiovascular events. More treatment options like Victoza that address critical aspects of diabetes care beyond glucose lowering are essential to confront this pervasive issue,” Steve Marso, MD, medical director at the Cardiovascular Services HCA Midwest Health Heart and Vascular Institute and one of the primary investigators in LEADER said in the press release.
Find the full press release on the Novo Nordisk website.
The type 2 diabetes treatment Victoza (liraglutide) has been approved by the Food and Drug Administration for a new indication – to reduce the risk of several adverse cardiovascular events, according to a press release from Novo Nordisk.
FDA approval was based on results from the LEADER (NCT01179048) trial, where people with type 2 diabetes who received liraglutide were 13% less likely to experience cardiovascular death, nonfatal heart attack, or nonfatal stroke then type 2 diabetes patients who received a placebo, with an absolute risk reduction of 1.9%. Notably, risk of cardiovascular death was reduced by 22% in the liraglutide group, compared with the control group, and all-cause death was reduced by 15%.
“Today’s news is significant for millions of Americans living with type 2 diabetes because, even when controlled, diabetes puts patients at a greater risk for cardiovascular events. More treatment options like Victoza that address critical aspects of diabetes care beyond glucose lowering are essential to confront this pervasive issue,” Steve Marso, MD, medical director at the Cardiovascular Services HCA Midwest Health Heart and Vascular Institute and one of the primary investigators in LEADER said in the press release.
Find the full press release on the Novo Nordisk website.
BMI z scores fall short for tracking severe obesity
, based on data from nearly 7,000 children in the Bogalusa Heart Study.
The current parameters used in the Centers for Disease Control and Prevention growth charts for children with high body mass index (BMI) “can result in estimates that differ substantially from those that are observed and constrains the maximum BMI z that is attainable at a given sex and age,” wrote David S. Freedman, PhD, of the Centers for Disease Control and Prevention in Atlanta, and Gerald S. Berenson, MD, of Louisiana State University Health Sciences Center, New Orleans, (Pediatrics. 2017;140:e20171072).
The BMI adjusted z score (BMIaz) or the BMI expressed as a percentage of the 95th percentile (%BMIp95) “will provide more accurate information on body size over time among children with very high BMIs,” they said.
In children with severe obesity, BMI z was a weaker measure (r = 0.46) than were measures of %BMIp95 (r = 0.61) or BMIaz scores with no upper boundary (r = 0.65).
BMI z scores were weakest when applied to children younger than 10 years, with correlations of r = 0.36 for BMI z vs. correlations of 0.60 and 0.57 for BMIaz and %BMIp95, respectively.
The results were limited by several factors including the age of the data (40 years ago, when the prevalence of severe obesity was lower, 2% compared with approximately 6% now) and long intervals between exams in some cases (5 years or more), the researchers noted. However, the results suggest that BMI z values “can differ substantially from empirical estimates, have an effective upper limit, and are strongly influenced by sex and age,” they said. As an alternative, the researchers recommended that “very high BMIs should be should expressed as z scores on the basis of linear extrapolations of a fixed SD or as percentage of the CDC 95th percentile,” or using multilevel models that adjust for age and sex.
The researchers had no financial conflicts to disclose. The National Institute on Aging, the National Heart, Lung, and Blood Institute, and the National Institutes of Health funded the study.
The use of BMI z scores to assess and track severe obesity in children should be abandoned.
In the study by Freedman et al., BMI z scores, which are extrapolations of BMI measurements, did not correlate well with other measures of adiposity. Their use to assess severe obesity is problematic because large changes in weight and BMI are linked to small changes in BMI z or BMI percentiles.
William H. Dietz, MD, PhD, is at the Sumner M. Redstone Global Center for Prevention and Wellness, Milken Institute School of Public Health at George Washington University in Washington. He had no relevant financial disclosures, but disclosed that he serves on the scientific advisory board for Weight Watchers and is on the board of directors for the Partnership for a Healthier America. He discussed the article by Freedman et al. in an editorial (Pediatrics. 2017;140:e20172148).
The use of BMI z scores to assess and track severe obesity in children should be abandoned.
In the study by Freedman et al., BMI z scores, which are extrapolations of BMI measurements, did not correlate well with other measures of adiposity. Their use to assess severe obesity is problematic because large changes in weight and BMI are linked to small changes in BMI z or BMI percentiles.
William H. Dietz, MD, PhD, is at the Sumner M. Redstone Global Center for Prevention and Wellness, Milken Institute School of Public Health at George Washington University in Washington. He had no relevant financial disclosures, but disclosed that he serves on the scientific advisory board for Weight Watchers and is on the board of directors for the Partnership for a Healthier America. He discussed the article by Freedman et al. in an editorial (Pediatrics. 2017;140:e20172148).
The use of BMI z scores to assess and track severe obesity in children should be abandoned.
In the study by Freedman et al., BMI z scores, which are extrapolations of BMI measurements, did not correlate well with other measures of adiposity. Their use to assess severe obesity is problematic because large changes in weight and BMI are linked to small changes in BMI z or BMI percentiles.
William H. Dietz, MD, PhD, is at the Sumner M. Redstone Global Center for Prevention and Wellness, Milken Institute School of Public Health at George Washington University in Washington. He had no relevant financial disclosures, but disclosed that he serves on the scientific advisory board for Weight Watchers and is on the board of directors for the Partnership for a Healthier America. He discussed the article by Freedman et al. in an editorial (Pediatrics. 2017;140:e20172148).
, based on data from nearly 7,000 children in the Bogalusa Heart Study.
The current parameters used in the Centers for Disease Control and Prevention growth charts for children with high body mass index (BMI) “can result in estimates that differ substantially from those that are observed and constrains the maximum BMI z that is attainable at a given sex and age,” wrote David S. Freedman, PhD, of the Centers for Disease Control and Prevention in Atlanta, and Gerald S. Berenson, MD, of Louisiana State University Health Sciences Center, New Orleans, (Pediatrics. 2017;140:e20171072).
The BMI adjusted z score (BMIaz) or the BMI expressed as a percentage of the 95th percentile (%BMIp95) “will provide more accurate information on body size over time among children with very high BMIs,” they said.
In children with severe obesity, BMI z was a weaker measure (r = 0.46) than were measures of %BMIp95 (r = 0.61) or BMIaz scores with no upper boundary (r = 0.65).
BMI z scores were weakest when applied to children younger than 10 years, with correlations of r = 0.36 for BMI z vs. correlations of 0.60 and 0.57 for BMIaz and %BMIp95, respectively.
The results were limited by several factors including the age of the data (40 years ago, when the prevalence of severe obesity was lower, 2% compared with approximately 6% now) and long intervals between exams in some cases (5 years or more), the researchers noted. However, the results suggest that BMI z values “can differ substantially from empirical estimates, have an effective upper limit, and are strongly influenced by sex and age,” they said. As an alternative, the researchers recommended that “very high BMIs should be should expressed as z scores on the basis of linear extrapolations of a fixed SD or as percentage of the CDC 95th percentile,” or using multilevel models that adjust for age and sex.
The researchers had no financial conflicts to disclose. The National Institute on Aging, the National Heart, Lung, and Blood Institute, and the National Institutes of Health funded the study.
, based on data from nearly 7,000 children in the Bogalusa Heart Study.
The current parameters used in the Centers for Disease Control and Prevention growth charts for children with high body mass index (BMI) “can result in estimates that differ substantially from those that are observed and constrains the maximum BMI z that is attainable at a given sex and age,” wrote David S. Freedman, PhD, of the Centers for Disease Control and Prevention in Atlanta, and Gerald S. Berenson, MD, of Louisiana State University Health Sciences Center, New Orleans, (Pediatrics. 2017;140:e20171072).
The BMI adjusted z score (BMIaz) or the BMI expressed as a percentage of the 95th percentile (%BMIp95) “will provide more accurate information on body size over time among children with very high BMIs,” they said.
In children with severe obesity, BMI z was a weaker measure (r = 0.46) than were measures of %BMIp95 (r = 0.61) or BMIaz scores with no upper boundary (r = 0.65).
BMI z scores were weakest when applied to children younger than 10 years, with correlations of r = 0.36 for BMI z vs. correlations of 0.60 and 0.57 for BMIaz and %BMIp95, respectively.
The results were limited by several factors including the age of the data (40 years ago, when the prevalence of severe obesity was lower, 2% compared with approximately 6% now) and long intervals between exams in some cases (5 years or more), the researchers noted. However, the results suggest that BMI z values “can differ substantially from empirical estimates, have an effective upper limit, and are strongly influenced by sex and age,” they said. As an alternative, the researchers recommended that “very high BMIs should be should expressed as z scores on the basis of linear extrapolations of a fixed SD or as percentage of the CDC 95th percentile,” or using multilevel models that adjust for age and sex.
The researchers had no financial conflicts to disclose. The National Institute on Aging, the National Heart, Lung, and Blood Institute, and the National Institutes of Health funded the study.
FROM PEDIATRICS
Key clinical point: BMI z scores are weak trackers of severe obesity in children.
Major finding: Correlations were weaker for BMI z scores (r = 0.46) than for metrics of BMI using the 95th percentile or z scores with no upper bound (r = approximately 0.6) over 2.8 years.
Data source: The study is based on longitudinal data from 6,994 children participating in the Bogalusa Heart study, including 247 children with severe obesity.
Disclosures: The researchers had no relevant financial conflicts to disclose. The National Institute on Aging, the National Heart, Lung, and Blood Institute, and the National Institutes of Health funded the study.
Fewer severe hypoglycemia episodes seen over time in patients on tight control
Episodes of severe hypoglycemia became less frequent over a period of 26 years in patients with type 1 diabetes whose glucose was initially intensively managed to a hemoglobin A1c target of 7%, but, conversely, these problems increased in patients who had been managed to a conventional target of 9%, .
At the end of the 20-year-long Epidemiology of Diabetes Interventions and Complications (EDIC) study, which used an 8% HbA1c target for everyone, rates of severe hypoglycemia were 37 cases per 100 patient-years in patients managed intensively in the preceding Diabetes Control and Complications Trial (DCCT) – a significant decrease from the 61 cases per 100 patient-years observed when the DCCT concluded. However, rates of severe hypoglycemia also increased in the conventionally managed group, from 19 to 41 cases per 100 patient-years, according to Rose A. Gubitosi-Klug, MD, PhD, and her coauthors (Diab Care. 2017;40[8]:1010-6).
“The equalization of rates between the two original treatment groups is largely attributable to their similar HbA1c levels during EDIC,” wrote Dr. Gubitosi-Klug, who is division chief of pediatric endocrinology at University Hospital, Cleveland Medical Center, and her coauthors. “[There was] a 13%-15% rise in severe hypoglycemia risk for every 10% decrement in HbA1c.”
The DCCT enrolled 1,441 patients with type 1 diabetes from 1983 to 1989. They were either managed intensively, with a target HbA1c of 7%, or, conventionally, with a 9% target. Specifically, during the DCCT, participants in the intensive group had lower current and mean HbA1c levels (~2% mean difference; P less than .001) as well as higher insulin doses than those in the conventional group (mean difference, 0.04 units/kg per day; P less than .001). During the DCCT, pump use across all quarterly visits averaged 35.7% in the intensive group versus 0.7% in the conventional group and rose to 41% and 1.6%, respectively, by the DCCT closeout (P less than .001).
Almost everyone in the DCCT then enrolled in the EDIC study, which ran from 1995 to 2013. EDIC used an 8% HbA1c target. During EDIC, diabetes management has evolved dramatically, with the introduction of rapid- and long-acting insulins and improved insulin pumps and blood glucose meters. Dr. Gubitosi-Klug and her colleagues examined how rates of severe hypoglycemia have changed with those advances.
During the DCCT, intensively managed patients were three times more likely than those conventionally managed to experience an episode of severe hypoglycemia, including seizure or coma. During EDIC, the frequency of severe hypoglycemia increased in patients who had been conventionally managed but decreased among those who had been intensively managed.
When the DCCT ended with an average 6.5 years follow-up, 65% of the intensive group and 35% of the conventional group had experienced at least one episode of severe hypoglycemia. But when the 20-year EDIC study ended, about half of everyone in the group had experienced at least one episode. Many experienced multiple events. In the DCCT, 54% of the intensive group and 30% of the conventional group had experienced at least four episodes. In EDIC, 37% of the intensive group and 33% of the conventional group had experienced at least four.
But the repeat events seemed to occur in a subset of patients, with 14% in the DCCT experiencing about half of the study’s severe hypoglycemic events, and 7% in EDIC experiencing about a third of them in that study.
The biggest risk factor for severe hypoglycemia was similar for both groups. A first incident doubled the risk for another in the conventional therapy group and tripled it in the intensive therapy group.
“The current data support the clinical perception that a small subset of individuals is more susceptible to severe hypoglycemia,” with 7% of patients with 11 or more episodes during EDIC representing 32% all the events in that study.
These events impart a risk of serious consequences, the authors said. There were 51 major accidents during the 6.5 years of the DCCT and 143 during the 20 years of EDIC, and these were similar between treatment groups. Most of these were motor vehicle accidents, and hypoglycemia was the possible, probable, or principal cause of 18 of the 28 in the DCCT and 23 of the 54 in EDIC.
Nevertheless, the finding that intensively managed patients did better over the years is encouraging, the authors noted. “Advancements in the tools for diabetes management and additional clinical trials have also demonstrated the importance of educational programs to support intensive diabetes therapy. Thus, with increasing years of experience, participants have likely benefitted from tailored educational efforts provided by treating physicians and certified diabetes educators to minimize hypoglycemia.”
None of the study authors reported any financial conflicts.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Episodes of severe hypoglycemia became less frequent over a period of 26 years in patients with type 1 diabetes whose glucose was initially intensively managed to a hemoglobin A1c target of 7%, but, conversely, these problems increased in patients who had been managed to a conventional target of 9%, .
At the end of the 20-year-long Epidemiology of Diabetes Interventions and Complications (EDIC) study, which used an 8% HbA1c target for everyone, rates of severe hypoglycemia were 37 cases per 100 patient-years in patients managed intensively in the preceding Diabetes Control and Complications Trial (DCCT) – a significant decrease from the 61 cases per 100 patient-years observed when the DCCT concluded. However, rates of severe hypoglycemia also increased in the conventionally managed group, from 19 to 41 cases per 100 patient-years, according to Rose A. Gubitosi-Klug, MD, PhD, and her coauthors (Diab Care. 2017;40[8]:1010-6).
“The equalization of rates between the two original treatment groups is largely attributable to their similar HbA1c levels during EDIC,” wrote Dr. Gubitosi-Klug, who is division chief of pediatric endocrinology at University Hospital, Cleveland Medical Center, and her coauthors. “[There was] a 13%-15% rise in severe hypoglycemia risk for every 10% decrement in HbA1c.”
The DCCT enrolled 1,441 patients with type 1 diabetes from 1983 to 1989. They were either managed intensively, with a target HbA1c of 7%, or, conventionally, with a 9% target. Specifically, during the DCCT, participants in the intensive group had lower current and mean HbA1c levels (~2% mean difference; P less than .001) as well as higher insulin doses than those in the conventional group (mean difference, 0.04 units/kg per day; P less than .001). During the DCCT, pump use across all quarterly visits averaged 35.7% in the intensive group versus 0.7% in the conventional group and rose to 41% and 1.6%, respectively, by the DCCT closeout (P less than .001).
Almost everyone in the DCCT then enrolled in the EDIC study, which ran from 1995 to 2013. EDIC used an 8% HbA1c target. During EDIC, diabetes management has evolved dramatically, with the introduction of rapid- and long-acting insulins and improved insulin pumps and blood glucose meters. Dr. Gubitosi-Klug and her colleagues examined how rates of severe hypoglycemia have changed with those advances.
During the DCCT, intensively managed patients were three times more likely than those conventionally managed to experience an episode of severe hypoglycemia, including seizure or coma. During EDIC, the frequency of severe hypoglycemia increased in patients who had been conventionally managed but decreased among those who had been intensively managed.
When the DCCT ended with an average 6.5 years follow-up, 65% of the intensive group and 35% of the conventional group had experienced at least one episode of severe hypoglycemia. But when the 20-year EDIC study ended, about half of everyone in the group had experienced at least one episode. Many experienced multiple events. In the DCCT, 54% of the intensive group and 30% of the conventional group had experienced at least four episodes. In EDIC, 37% of the intensive group and 33% of the conventional group had experienced at least four.
But the repeat events seemed to occur in a subset of patients, with 14% in the DCCT experiencing about half of the study’s severe hypoglycemic events, and 7% in EDIC experiencing about a third of them in that study.
The biggest risk factor for severe hypoglycemia was similar for both groups. A first incident doubled the risk for another in the conventional therapy group and tripled it in the intensive therapy group.
“The current data support the clinical perception that a small subset of individuals is more susceptible to severe hypoglycemia,” with 7% of patients with 11 or more episodes during EDIC representing 32% all the events in that study.
These events impart a risk of serious consequences, the authors said. There were 51 major accidents during the 6.5 years of the DCCT and 143 during the 20 years of EDIC, and these were similar between treatment groups. Most of these were motor vehicle accidents, and hypoglycemia was the possible, probable, or principal cause of 18 of the 28 in the DCCT and 23 of the 54 in EDIC.
Nevertheless, the finding that intensively managed patients did better over the years is encouraging, the authors noted. “Advancements in the tools for diabetes management and additional clinical trials have also demonstrated the importance of educational programs to support intensive diabetes therapy. Thus, with increasing years of experience, participants have likely benefitted from tailored educational efforts provided by treating physicians and certified diabetes educators to minimize hypoglycemia.”
None of the study authors reported any financial conflicts.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
Episodes of severe hypoglycemia became less frequent over a period of 26 years in patients with type 1 diabetes whose glucose was initially intensively managed to a hemoglobin A1c target of 7%, but, conversely, these problems increased in patients who had been managed to a conventional target of 9%, .
At the end of the 20-year-long Epidemiology of Diabetes Interventions and Complications (EDIC) study, which used an 8% HbA1c target for everyone, rates of severe hypoglycemia were 37 cases per 100 patient-years in patients managed intensively in the preceding Diabetes Control and Complications Trial (DCCT) – a significant decrease from the 61 cases per 100 patient-years observed when the DCCT concluded. However, rates of severe hypoglycemia also increased in the conventionally managed group, from 19 to 41 cases per 100 patient-years, according to Rose A. Gubitosi-Klug, MD, PhD, and her coauthors (Diab Care. 2017;40[8]:1010-6).
“The equalization of rates between the two original treatment groups is largely attributable to their similar HbA1c levels during EDIC,” wrote Dr. Gubitosi-Klug, who is division chief of pediatric endocrinology at University Hospital, Cleveland Medical Center, and her coauthors. “[There was] a 13%-15% rise in severe hypoglycemia risk for every 10% decrement in HbA1c.”
The DCCT enrolled 1,441 patients with type 1 diabetes from 1983 to 1989. They were either managed intensively, with a target HbA1c of 7%, or, conventionally, with a 9% target. Specifically, during the DCCT, participants in the intensive group had lower current and mean HbA1c levels (~2% mean difference; P less than .001) as well as higher insulin doses than those in the conventional group (mean difference, 0.04 units/kg per day; P less than .001). During the DCCT, pump use across all quarterly visits averaged 35.7% in the intensive group versus 0.7% in the conventional group and rose to 41% and 1.6%, respectively, by the DCCT closeout (P less than .001).
Almost everyone in the DCCT then enrolled in the EDIC study, which ran from 1995 to 2013. EDIC used an 8% HbA1c target. During EDIC, diabetes management has evolved dramatically, with the introduction of rapid- and long-acting insulins and improved insulin pumps and blood glucose meters. Dr. Gubitosi-Klug and her colleagues examined how rates of severe hypoglycemia have changed with those advances.
During the DCCT, intensively managed patients were three times more likely than those conventionally managed to experience an episode of severe hypoglycemia, including seizure or coma. During EDIC, the frequency of severe hypoglycemia increased in patients who had been conventionally managed but decreased among those who had been intensively managed.
When the DCCT ended with an average 6.5 years follow-up, 65% of the intensive group and 35% of the conventional group had experienced at least one episode of severe hypoglycemia. But when the 20-year EDIC study ended, about half of everyone in the group had experienced at least one episode. Many experienced multiple events. In the DCCT, 54% of the intensive group and 30% of the conventional group had experienced at least four episodes. In EDIC, 37% of the intensive group and 33% of the conventional group had experienced at least four.
But the repeat events seemed to occur in a subset of patients, with 14% in the DCCT experiencing about half of the study’s severe hypoglycemic events, and 7% in EDIC experiencing about a third of them in that study.
The biggest risk factor for severe hypoglycemia was similar for both groups. A first incident doubled the risk for another in the conventional therapy group and tripled it in the intensive therapy group.
“The current data support the clinical perception that a small subset of individuals is more susceptible to severe hypoglycemia,” with 7% of patients with 11 or more episodes during EDIC representing 32% all the events in that study.
These events impart a risk of serious consequences, the authors said. There were 51 major accidents during the 6.5 years of the DCCT and 143 during the 20 years of EDIC, and these were similar between treatment groups. Most of these were motor vehicle accidents, and hypoglycemia was the possible, probable, or principal cause of 18 of the 28 in the DCCT and 23 of the 54 in EDIC.
Nevertheless, the finding that intensively managed patients did better over the years is encouraging, the authors noted. “Advancements in the tools for diabetes management and additional clinical trials have also demonstrated the importance of educational programs to support intensive diabetes therapy. Thus, with increasing years of experience, participants have likely benefitted from tailored educational efforts provided by treating physicians and certified diabetes educators to minimize hypoglycemia.”
None of the study authors reported any financial conflicts.
msullivan@frontlinemedcom.com
On Twitter @Alz_gal
FROM DIABETES CARE
Key clinical point:
Major finding: About half of the patients in the follow-up study, the Epidemiology of Diabetes Interventions and Complications trial, experienced at least one severe hypoglycemia event, regardless of their initial management.
Data source: The Diabetes Control and Complications Trial involving 1,441 patients, 97% of whom enrolled in the Epidemiology of Diabetes Interventions and Complications trial.
Disclosures: The authors had no financial disclosures.
Marijuana use triples risk of death from hypertension
The risk of death from hypertension is three times greater in adults who use marijuana, compared with nonusers, based on data from a retrospective study of 1,213 adults.
Changes in the legalization of marijuana may promote increased recreational use, but data on the long-term effects of marijuana use on cardiovascular and cerebrovascular mortality are limited, wrote Barbara A. Yankey, PhD, of Georgia State University, Atlanta, and her colleagues.
Data on 686 users and 527 nonusers were combined with the 2011 mortality data from the National Center for Health Statistics (Eur J Prev Cardiol. 2017 Aug 9; doi: 10.1177/2047487317723212).
Overall, marijuana users had a 3.42 times greater risk of death from hypertension than did nonusers (95% confidence interval, 1.20-9.79), and the risk increased by 1.04 for each year of use (95% CI, 1.00-1.07). The average duration of marijuana use was 11.5 years. At the time of study entry, the average age of the participants was 38 years, and the average body mass index was 29 kg/m2; 23% of marijuana users and 21% of nonusers had a prior diagnosis of hypertension.
Of the study participants, 20% used marijuana and smoked cigarettes, 16% used marijuana and were past smokers, 5% were past smokers, and 4% only smoked cigarettes. “In our study, increase in risk for hypertension, [heart disease], or cerebrovascular disease mortality associated with cigarette use was not significant,” the researchers wrote. They attributed this factor to the small sample size and noted that the dangers of cigarette smoking to the cardiovascular system are well established.
The study findings were limited by the relatively small sample size and potentially inaccurate data on the duration of marijuana use, the researchers said.
However, the results suggest that “cardiovascular risk associated with marijuana use may be greater than the cardiovascular risk already established for cigarette smoking,” and longitudinal studies are warranted, they concluded.
The researchers had no financial conflicts to disclose.
The risk of death from hypertension is three times greater in adults who use marijuana, compared with nonusers, based on data from a retrospective study of 1,213 adults.
Changes in the legalization of marijuana may promote increased recreational use, but data on the long-term effects of marijuana use on cardiovascular and cerebrovascular mortality are limited, wrote Barbara A. Yankey, PhD, of Georgia State University, Atlanta, and her colleagues.
Data on 686 users and 527 nonusers were combined with the 2011 mortality data from the National Center for Health Statistics (Eur J Prev Cardiol. 2017 Aug 9; doi: 10.1177/2047487317723212).
Overall, marijuana users had a 3.42 times greater risk of death from hypertension than did nonusers (95% confidence interval, 1.20-9.79), and the risk increased by 1.04 for each year of use (95% CI, 1.00-1.07). The average duration of marijuana use was 11.5 years. At the time of study entry, the average age of the participants was 38 years, and the average body mass index was 29 kg/m2; 23% of marijuana users and 21% of nonusers had a prior diagnosis of hypertension.
Of the study participants, 20% used marijuana and smoked cigarettes, 16% used marijuana and were past smokers, 5% were past smokers, and 4% only smoked cigarettes. “In our study, increase in risk for hypertension, [heart disease], or cerebrovascular disease mortality associated with cigarette use was not significant,” the researchers wrote. They attributed this factor to the small sample size and noted that the dangers of cigarette smoking to the cardiovascular system are well established.
The study findings were limited by the relatively small sample size and potentially inaccurate data on the duration of marijuana use, the researchers said.
However, the results suggest that “cardiovascular risk associated with marijuana use may be greater than the cardiovascular risk already established for cigarette smoking,” and longitudinal studies are warranted, they concluded.
The researchers had no financial conflicts to disclose.
The risk of death from hypertension is three times greater in adults who use marijuana, compared with nonusers, based on data from a retrospective study of 1,213 adults.
Changes in the legalization of marijuana may promote increased recreational use, but data on the long-term effects of marijuana use on cardiovascular and cerebrovascular mortality are limited, wrote Barbara A. Yankey, PhD, of Georgia State University, Atlanta, and her colleagues.
Data on 686 users and 527 nonusers were combined with the 2011 mortality data from the National Center for Health Statistics (Eur J Prev Cardiol. 2017 Aug 9; doi: 10.1177/2047487317723212).
Overall, marijuana users had a 3.42 times greater risk of death from hypertension than did nonusers (95% confidence interval, 1.20-9.79), and the risk increased by 1.04 for each year of use (95% CI, 1.00-1.07). The average duration of marijuana use was 11.5 years. At the time of study entry, the average age of the participants was 38 years, and the average body mass index was 29 kg/m2; 23% of marijuana users and 21% of nonusers had a prior diagnosis of hypertension.
Of the study participants, 20% used marijuana and smoked cigarettes, 16% used marijuana and were past smokers, 5% were past smokers, and 4% only smoked cigarettes. “In our study, increase in risk for hypertension, [heart disease], or cerebrovascular disease mortality associated with cigarette use was not significant,” the researchers wrote. They attributed this factor to the small sample size and noted that the dangers of cigarette smoking to the cardiovascular system are well established.
The study findings were limited by the relatively small sample size and potentially inaccurate data on the duration of marijuana use, the researchers said.
However, the results suggest that “cardiovascular risk associated with marijuana use may be greater than the cardiovascular risk already established for cigarette smoking,” and longitudinal studies are warranted, they concluded.
The researchers had no financial conflicts to disclose.
FROM THE EUROPEAN JOURNAL OF PREVENTIVE CARDIOLOGY
Key clinical point: A history of marijuana use significantly increases the risk of death from hypertension.
Major finding: Marijuana users had a 3.42 times higher risk of death from hypertension and a 1.04 times increased risk of death for each year of use.
Data source: A retrospective study of 1,213 adults aged 20 years and older using data from National Health and Nutrition Examination Survey and the National Center for Health Statistics.
Disclosures: The researchers had no financial conflicts to disclose.
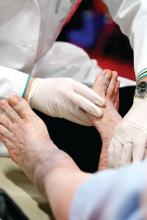
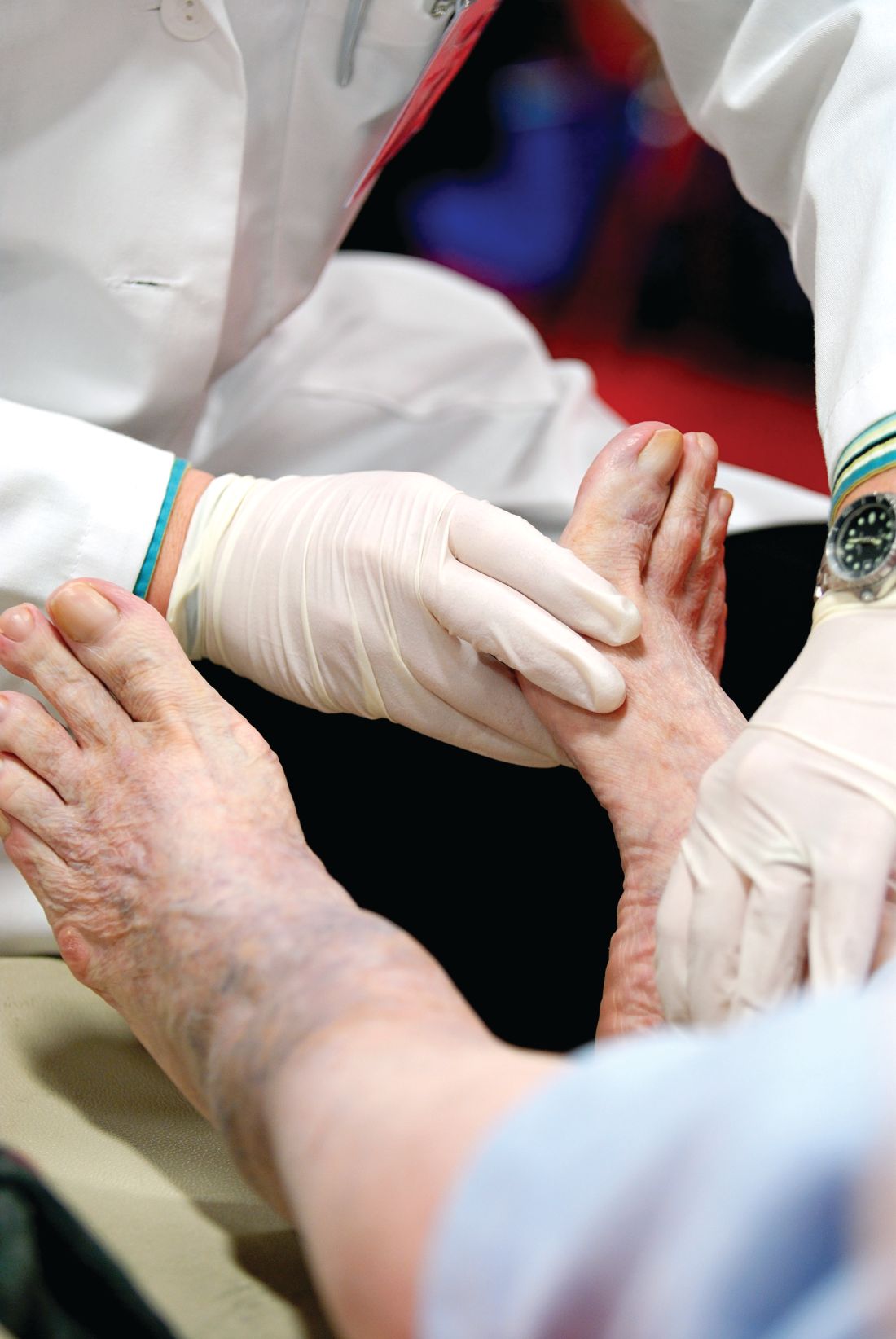
Neurologist to endocrinologists: Listen to your patients’ feet
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
SAN DIEGO – Listen to the feet of your patients, and don’t just focus on glucose control as a way to combat diabetic neuropathy, according to Eva L. Feldman, MD, PhD.
Regular foot exams are crucial for patients with diabetes because of the high risk of diabetic neuropathy (DN), Dr. Feldman, professor of neurology and director of the Program for Neurology Research and Discovery at University of Michigan, Ann Arbor, said in a presentation at the annual scientific sessions of the American Diabetes Association.
“Diabetic neuropathy has some important clinical consequences in a patient’s life. There’s clearly impaired function, a lower quality of life, and increased mortality. There’s also a high association between DN and cardiovascular disease and a high risk of amputation,” said Dr. Feldman, coauthor of the ADA’s new clinical guidelines for diabetic neuropathy (Diabetes Care. 2017;40[1]:136-54).
And in patients with type 2 diabetes, “diabetic neuropathy is not just hyperglycemia, which is what we’ve focused all our efforts on up until the past 5 years. There is also a role for dyslipidemia and other metabolic impairments,” she said.
In a follow-up interview, Dr. Feldman elaborated on a common misconception about DN, simple tools for foot exams by endocrinologists, and how to know when it’s time for a referral to a neurologist or podiatrist.
Q: What do you think physicians/endocrinologists misunderstand about diabetic neuropathy?
A: Commonly, physicians think if patients with diabetes do not complain of pain or numbness, they do not have DN. This simply isn’t correct. Over 80% of patients with DN have insensate feet – they simply do not have feeling in their feet. Physicians must examine a patient’s foot at least once a year to ensure the patient has not developed DN.
Q: What should endocrinologists understand about how diabetic neuropathy develops?
A: We know that excellent glucose control has a significant impact on DN in patients with type 1 diabetes. In patients with type 2 diabetes, we know that excellent glucose control plays a much less significant role. While it’s important, it must be coupled with control of other components of metabolic syndrome – elevated blood lipids, obesity, and hypertension.
A: Take a 126 Hz tuning fork and determine if the patient can feel vibration on the joint of the great toe for at least 10 seconds. Then take a 10-gram filament and a pin and determine if the patient can feel both of these instruments when they are applied to the joint of the great toe. Some physicians also take a 10-gram filament and apply it to the sole of the foot. I would not suggest using a pin on the sole of the foot.
Q: What else should they look for when they inspect feet? And how often would you recommend that endocrinologists do this per patient?
A: Inspection for callous formation, fissure formation, and fungal infections is important, and a foot exam should be done once yearly.
Q: What about testing whether patents can feel temperature?
A: The pin tests the same class of nerve fibers so this is routinely not done in an endocrinologist’s office.
Q: When should endocrinologists refer out for neuropathy?
A: Endocrinologists can treat DN by treating the diabetic condition and, in type 2 diabetes, the metabolic syndrome. Referral to a neurologist is indicated if there are atypical symptoms or signs, such as motor impairment that’s greater than sensory impairment, a significant asymmetry, or a very rapid onset. All patients with severe DN should be under the care of a podiatrist to prevent the development of nonhealing wounds and ulcers.
Q: What have you learned about how diabetic neuropathy affects the lives of patients?
A: DN definitely affects the quality of a patient’s life, not only in terms of work productivity and ability to perform activities of daily living. Quality of life and general enjoyment of life can frequently be adversely affected.
Q: How successful is treatment for diabetic neuropathy?
A: Treatment for pain can be very successful, and we have outlined a protocol in our recent ADA guidelines. For patients with uncontrollable pain, frequently a referral to a pain clinic is in order.
Dr. Feldman reports no relevant disclosures.
AT THE ADA ANNUAL SCIENTIFIC SESSIONS
Continuous glucose monitors aren’t just for abdomens anymore
Close to two-thirds of people with diabetes who wear a continuous glucose monitor position it on an area of the body other than the abdomen, the only site sanctioned by the device’s manufacturer and federal authorities. And that lack of compliance has resulted in no apparent ill effects, according to a new study of related social media.
Michelle L. Litchman, PhD, of the University of Utah in Salt Lake City, and her colleagues, examined nearly 3,000 online postings of photos of continuous glucose monitors (CGM) manufactured by Dexcom, currently the most popular brand of CGMs in the United States. The results of their study, presented at this year’s annual meeting of the American Association of Diabetes Educators, showed that about 74% of device-wearing patients place it on the upper arm, thigh, buttocks, or back, rather than the abdomen, as indicated by the Food and Drug Administration. The device is indicated for use on the abdomen in adults because that was the only location used for the clinical studies of the device, Dr. Litchman explained in an interview.
Overall, out of 2,923 Instagram posts concerning the Dexcom CGM device, Dr. Litchman and her fellow investigators culled 353 photos of the device being worn on the body. Of these, 26.1% indicated the device was placed according to FDA guidance, while 63.7% of the remaining photos depicted the device placed on the inner arm, the forearm, thigh, calf, buttock, or back. In just over 10% of the photos, the device’s location on the body was unclear. Dr. Litchman and her colleagues concluded that when the device was worn according to directions, the failure rate was 6.2%. When placed on the outer arm it had a 2.2% failure rate, and there was a 3.3% failure rate when the device was worn on the thigh.
Since the combined nonabdomen and abdomen failure rates were similar, Dr. Litchman suggested any noncompliance was simply pragmatism. Although the CGM is meant to be relocated weekly around the abdomen, that is also the area of the body typically used for insulin injections several times a day, often resulting in scar tissue build-up that lessens insulin absorption. “It boils down to how much real estate someone has for effective insulin administration,” Dr. Litchman noted. “Individuals with diabetes need to protect the sites where they will inject insulin for the rest of their lives.”
For tips on how to do this effectively, Dr. Litchman said people with diabetes increasingly turn to online communities.
“There are differences in the FDA-approved, by the book, information patients are given and what they do in real life. While some may view this as a threat, I like to see this as opportunity to learn from patients. Patients are finding successes outside of [official instructions], therefore, we should be seeking out this experiential evidence. This research is a start to better understanding the safety related to CGM use in sites other than the abdomen,” she said in the interview.
Dr. Litchman reported that she had no conflict of interest to disclose.
Close to two-thirds of people with diabetes who wear a continuous glucose monitor position it on an area of the body other than the abdomen, the only site sanctioned by the device’s manufacturer and federal authorities. And that lack of compliance has resulted in no apparent ill effects, according to a new study of related social media.
Michelle L. Litchman, PhD, of the University of Utah in Salt Lake City, and her colleagues, examined nearly 3,000 online postings of photos of continuous glucose monitors (CGM) manufactured by Dexcom, currently the most popular brand of CGMs in the United States. The results of their study, presented at this year’s annual meeting of the American Association of Diabetes Educators, showed that about 74% of device-wearing patients place it on the upper arm, thigh, buttocks, or back, rather than the abdomen, as indicated by the Food and Drug Administration. The device is indicated for use on the abdomen in adults because that was the only location used for the clinical studies of the device, Dr. Litchman explained in an interview.
Overall, out of 2,923 Instagram posts concerning the Dexcom CGM device, Dr. Litchman and her fellow investigators culled 353 photos of the device being worn on the body. Of these, 26.1% indicated the device was placed according to FDA guidance, while 63.7% of the remaining photos depicted the device placed on the inner arm, the forearm, thigh, calf, buttock, or back. In just over 10% of the photos, the device’s location on the body was unclear. Dr. Litchman and her colleagues concluded that when the device was worn according to directions, the failure rate was 6.2%. When placed on the outer arm it had a 2.2% failure rate, and there was a 3.3% failure rate when the device was worn on the thigh.
Since the combined nonabdomen and abdomen failure rates were similar, Dr. Litchman suggested any noncompliance was simply pragmatism. Although the CGM is meant to be relocated weekly around the abdomen, that is also the area of the body typically used for insulin injections several times a day, often resulting in scar tissue build-up that lessens insulin absorption. “It boils down to how much real estate someone has for effective insulin administration,” Dr. Litchman noted. “Individuals with diabetes need to protect the sites where they will inject insulin for the rest of their lives.”
For tips on how to do this effectively, Dr. Litchman said people with diabetes increasingly turn to online communities.
“There are differences in the FDA-approved, by the book, information patients are given and what they do in real life. While some may view this as a threat, I like to see this as opportunity to learn from patients. Patients are finding successes outside of [official instructions], therefore, we should be seeking out this experiential evidence. This research is a start to better understanding the safety related to CGM use in sites other than the abdomen,” she said in the interview.
Dr. Litchman reported that she had no conflict of interest to disclose.
Close to two-thirds of people with diabetes who wear a continuous glucose monitor position it on an area of the body other than the abdomen, the only site sanctioned by the device’s manufacturer and federal authorities. And that lack of compliance has resulted in no apparent ill effects, according to a new study of related social media.
Michelle L. Litchman, PhD, of the University of Utah in Salt Lake City, and her colleagues, examined nearly 3,000 online postings of photos of continuous glucose monitors (CGM) manufactured by Dexcom, currently the most popular brand of CGMs in the United States. The results of their study, presented at this year’s annual meeting of the American Association of Diabetes Educators, showed that about 74% of device-wearing patients place it on the upper arm, thigh, buttocks, or back, rather than the abdomen, as indicated by the Food and Drug Administration. The device is indicated for use on the abdomen in adults because that was the only location used for the clinical studies of the device, Dr. Litchman explained in an interview.
Overall, out of 2,923 Instagram posts concerning the Dexcom CGM device, Dr. Litchman and her fellow investigators culled 353 photos of the device being worn on the body. Of these, 26.1% indicated the device was placed according to FDA guidance, while 63.7% of the remaining photos depicted the device placed on the inner arm, the forearm, thigh, calf, buttock, or back. In just over 10% of the photos, the device’s location on the body was unclear. Dr. Litchman and her colleagues concluded that when the device was worn according to directions, the failure rate was 6.2%. When placed on the outer arm it had a 2.2% failure rate, and there was a 3.3% failure rate when the device was worn on the thigh.
Since the combined nonabdomen and abdomen failure rates were similar, Dr. Litchman suggested any noncompliance was simply pragmatism. Although the CGM is meant to be relocated weekly around the abdomen, that is also the area of the body typically used for insulin injections several times a day, often resulting in scar tissue build-up that lessens insulin absorption. “It boils down to how much real estate someone has for effective insulin administration,” Dr. Litchman noted. “Individuals with diabetes need to protect the sites where they will inject insulin for the rest of their lives.”
For tips on how to do this effectively, Dr. Litchman said people with diabetes increasingly turn to online communities.
“There are differences in the FDA-approved, by the book, information patients are given and what they do in real life. While some may view this as a threat, I like to see this as opportunity to learn from patients. Patients are finding successes outside of [official instructions], therefore, we should be seeking out this experiential evidence. This research is a start to better understanding the safety related to CGM use in sites other than the abdomen,” she said in the interview.
Dr. Litchman reported that she had no conflict of interest to disclose.
FROM AADE 2017
Key clinical point:
Major finding: Over one quarter of continuous glucose monitors are worn successfully in a non-FDA-approved location on the body.
Data source: Review of 353 photos posted online by persons with diabetes depicting them with the device worn elsewhere than the abdomen.
Disclosures: Dr. Litchman reported she had no relevant disclosures.
AADE: New standards for diabetes self-management programs
New standards for diabetes self-management education and support outline 10 key evidence-based standards for services that meet the Medicare diabetes self-management training regulations, although they do not guarantee coverage. The standards, produced by the American Association of Diabetes Educators in association with the American Diabetes Association, are an update to a similar document produced in 2014. The 2017 revision of the standards is the first to combine support and education to reflect the value of ongoing counsel for improved diabetes self-care, according to an accompanying statement (Diab Care. 2017 Jul 28. doi: 10.2337/dci17-0025).
The new document is full of good recommendations, but they do not cover some important areas. “I don’t disagree with any of them,” said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City School of Medicine (UMKC) and associate program director of the UMKC Endocrine Fellowship. But he pointed out that the standards did not include any mention of integrating patient care with other teams. “I think that’s unfortunate. Certainly in our small diabetes practice, we have certified diabetes educators, and all of our patients see them at some point. I hope in subsequent documents they’ll talk about that more,” said Dr. Hellman.
The standards focus on a sort of nuts-and-bolts approach and may be directed toward health care providers who operate in areas with relatively few resources to turn to for help. “It seems it’s answering what to do if you don’t have backup and support, and perhaps that is what it’s for, but the standards should be good in any setting, whether totally integrated or separate. I do think in the future they need to address that large group of people in an integrated setting, and also talk about people with behavioral health expertise. Both are very important,” said Dr. Hellman.
One recommendation he praised in particular was the call for oversight from a quality coordinator. The document calls for the quality coordinator to ensure that the standards are properly implemented, including evidence-based practice, service design, evaluation, and quality improvement. That’s a key consideration because many patients may have disabilities that interfere with comprehension, such as hearing loss or cognitive dysfunction. Such impediments may prevent patients from learning key skills, such as properly checking blood glucose. “That can have a profound effect on diabetes control,” said Dr. Hellman.
He pointed out that quality control can play a wider role in medicine. “Checking your own work isn’t something people always like to do, but it’s really essential. If you think you’re giving high quality care, why don’t you just check your work to see that it’s getting the results that you thought it would?” said Dr. Hellman.
The paper disclosed no sources of funding or conflict of interest information. Dr. Hellman reported having no financial disclosures.
From AADE: 10 standards
Diabetes self-management education and support service providers should:
• Adopt a mission statement and goals.
• Adopt ongoing input from stakeholders and experts to improve quality and participation.
• Analyze the needs of the communities they serve to ensure the best design, delivery method, and use of resources to meet their needs.
• Employ a quality coordinator to oversee services. This individual should be responsible for evidence-based practice, service design, evaluation, and continuous quality improvement.
• Include at least one registered nurse, registered dietitian nutritionist, or pharmacist with training and experience related to DSMES, or a health care professional with a certificate as a diabetes educator (CDE) or Board Certification in Advanced Diabetes Management (BC-ADM).
• Employ a curriculum that follows current evidence and practice guidelines, including a means for evaluating outcomes. The specific elements of the curriculum required will depend on the individual participant’s needs.
• Identify the needs of individual participants and be led by the participant, supported by diabetes self-management education and support team members. They should cooperatively develop an individualized diabetes self-management education and support plan.
• Provide options and resources for ongoing support that participants can choose.
• Monitor participants’ progress toward self-management goals and other outcomes.
• Have their quality control coordinators measure the impact and effectiveness of the diabetes self-management education and support services and determine potential improvements by systematically evaluating process and outcome data.
New standards for diabetes self-management education and support outline 10 key evidence-based standards for services that meet the Medicare diabetes self-management training regulations, although they do not guarantee coverage. The standards, produced by the American Association of Diabetes Educators in association with the American Diabetes Association, are an update to a similar document produced in 2014. The 2017 revision of the standards is the first to combine support and education to reflect the value of ongoing counsel for improved diabetes self-care, according to an accompanying statement (Diab Care. 2017 Jul 28. doi: 10.2337/dci17-0025).
The new document is full of good recommendations, but they do not cover some important areas. “I don’t disagree with any of them,” said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City School of Medicine (UMKC) and associate program director of the UMKC Endocrine Fellowship. But he pointed out that the standards did not include any mention of integrating patient care with other teams. “I think that’s unfortunate. Certainly in our small diabetes practice, we have certified diabetes educators, and all of our patients see them at some point. I hope in subsequent documents they’ll talk about that more,” said Dr. Hellman.
The standards focus on a sort of nuts-and-bolts approach and may be directed toward health care providers who operate in areas with relatively few resources to turn to for help. “It seems it’s answering what to do if you don’t have backup and support, and perhaps that is what it’s for, but the standards should be good in any setting, whether totally integrated or separate. I do think in the future they need to address that large group of people in an integrated setting, and also talk about people with behavioral health expertise. Both are very important,” said Dr. Hellman.
One recommendation he praised in particular was the call for oversight from a quality coordinator. The document calls for the quality coordinator to ensure that the standards are properly implemented, including evidence-based practice, service design, evaluation, and quality improvement. That’s a key consideration because many patients may have disabilities that interfere with comprehension, such as hearing loss or cognitive dysfunction. Such impediments may prevent patients from learning key skills, such as properly checking blood glucose. “That can have a profound effect on diabetes control,” said Dr. Hellman.
He pointed out that quality control can play a wider role in medicine. “Checking your own work isn’t something people always like to do, but it’s really essential. If you think you’re giving high quality care, why don’t you just check your work to see that it’s getting the results that you thought it would?” said Dr. Hellman.
The paper disclosed no sources of funding or conflict of interest information. Dr. Hellman reported having no financial disclosures.
From AADE: 10 standards
Diabetes self-management education and support service providers should:
• Adopt a mission statement and goals.
• Adopt ongoing input from stakeholders and experts to improve quality and participation.
• Analyze the needs of the communities they serve to ensure the best design, delivery method, and use of resources to meet their needs.
• Employ a quality coordinator to oversee services. This individual should be responsible for evidence-based practice, service design, evaluation, and continuous quality improvement.
• Include at least one registered nurse, registered dietitian nutritionist, or pharmacist with training and experience related to DSMES, or a health care professional with a certificate as a diabetes educator (CDE) or Board Certification in Advanced Diabetes Management (BC-ADM).
• Employ a curriculum that follows current evidence and practice guidelines, including a means for evaluating outcomes. The specific elements of the curriculum required will depend on the individual participant’s needs.
• Identify the needs of individual participants and be led by the participant, supported by diabetes self-management education and support team members. They should cooperatively develop an individualized diabetes self-management education and support plan.
• Provide options and resources for ongoing support that participants can choose.
• Monitor participants’ progress toward self-management goals and other outcomes.
• Have their quality control coordinators measure the impact and effectiveness of the diabetes self-management education and support services and determine potential improvements by systematically evaluating process and outcome data.
New standards for diabetes self-management education and support outline 10 key evidence-based standards for services that meet the Medicare diabetes self-management training regulations, although they do not guarantee coverage. The standards, produced by the American Association of Diabetes Educators in association with the American Diabetes Association, are an update to a similar document produced in 2014. The 2017 revision of the standards is the first to combine support and education to reflect the value of ongoing counsel for improved diabetes self-care, according to an accompanying statement (Diab Care. 2017 Jul 28. doi: 10.2337/dci17-0025).
The new document is full of good recommendations, but they do not cover some important areas. “I don’t disagree with any of them,” said Richard Hellman, MD, clinical professor of medicine at the University of Missouri–Kansas City School of Medicine (UMKC) and associate program director of the UMKC Endocrine Fellowship. But he pointed out that the standards did not include any mention of integrating patient care with other teams. “I think that’s unfortunate. Certainly in our small diabetes practice, we have certified diabetes educators, and all of our patients see them at some point. I hope in subsequent documents they’ll talk about that more,” said Dr. Hellman.
The standards focus on a sort of nuts-and-bolts approach and may be directed toward health care providers who operate in areas with relatively few resources to turn to for help. “It seems it’s answering what to do if you don’t have backup and support, and perhaps that is what it’s for, but the standards should be good in any setting, whether totally integrated or separate. I do think in the future they need to address that large group of people in an integrated setting, and also talk about people with behavioral health expertise. Both are very important,” said Dr. Hellman.
One recommendation he praised in particular was the call for oversight from a quality coordinator. The document calls for the quality coordinator to ensure that the standards are properly implemented, including evidence-based practice, service design, evaluation, and quality improvement. That’s a key consideration because many patients may have disabilities that interfere with comprehension, such as hearing loss or cognitive dysfunction. Such impediments may prevent patients from learning key skills, such as properly checking blood glucose. “That can have a profound effect on diabetes control,” said Dr. Hellman.
He pointed out that quality control can play a wider role in medicine. “Checking your own work isn’t something people always like to do, but it’s really essential. If you think you’re giving high quality care, why don’t you just check your work to see that it’s getting the results that you thought it would?” said Dr. Hellman.
The paper disclosed no sources of funding or conflict of interest information. Dr. Hellman reported having no financial disclosures.
From AADE: 10 standards
Diabetes self-management education and support service providers should:
• Adopt a mission statement and goals.
• Adopt ongoing input from stakeholders and experts to improve quality and participation.
• Analyze the needs of the communities they serve to ensure the best design, delivery method, and use of resources to meet their needs.
• Employ a quality coordinator to oversee services. This individual should be responsible for evidence-based practice, service design, evaluation, and continuous quality improvement.
• Include at least one registered nurse, registered dietitian nutritionist, or pharmacist with training and experience related to DSMES, or a health care professional with a certificate as a diabetes educator (CDE) or Board Certification in Advanced Diabetes Management (BC-ADM).
• Employ a curriculum that follows current evidence and practice guidelines, including a means for evaluating outcomes. The specific elements of the curriculum required will depend on the individual participant’s needs.
• Identify the needs of individual participants and be led by the participant, supported by diabetes self-management education and support team members. They should cooperatively develop an individualized diabetes self-management education and support plan.
• Provide options and resources for ongoing support that participants can choose.
• Monitor participants’ progress toward self-management goals and other outcomes.
• Have their quality control coordinators measure the impact and effectiveness of the diabetes self-management education and support services and determine potential improvements by systematically evaluating process and outcome data.
FROM DIABETES CARE
Risk of sexual dysfunction in diabetes is high, but treatments can help
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
SAN DIEGO –
This isn’t normal for men of that age, according to Hunter B. Wessells, MD.“It’s not just that they’re aging. It’s a 20-year acceleration of the aging process,” he said at the annual scientific sessions of the American Diabetes Association.
That’s not all. In some cases, men with diabetes may experience decreased libido that’s potentially caused by low testosterone, said Dr. Wessells, professor and Wilma Wise Nelson, Ole A. Nelson, and Mabel Wise Nelson Endowed Chair in Urology at the University of Washington, Seattle
Still, research findings offer useful insights into the frequency of sexual dysfunction in people with diabetes and the potential – and limitations – of available treatments, said Dr. Wessells.
In patients with well-controlled diabetes, “these conditions impact quality of life to a greater degree than complications like nephropathy, neuropathy, and retinopathy,” he said in an interview. “Thus, treatment of urological symptoms can be a high-yield endeavor.”
In both sexes, Dr. Wessells said, diabetes can disrupt the mechanism of desire, arousal, and orgasm by affecting a long list of bodily functions such as central nervous system stimulation, hormone activity, autonomic and somatic nerve activity, and processing of calcium ions and nitric acid.
In men, diabetes boosts the risk of erectile dysfunction to a larger extent than do related conditions such as obesity, heart disease, and depression. “But they are interrelated,” he said. “The primary mechanisms include the metabolic effects of high glucose, autonomic nerve damage, and microvascular disease.”
Low testosterone levels also can cause problems in patients with diabetes, he said. “Type 2 diabetes has greater effects on testosterone than type 1. It is most closely linked to weight in the type 1 population and affects only a small percentage.”
A 2017 systematic review and meta-analysis of 145 studies with more than 88,000 subjects (average age 55.8 ± 7.9 years) suggests that ED was more common in type 2 diabetes (66.3%) than type 1 diabetes (37.5%) after statistical adjustment to account for publication bias (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403).
A smaller analysis found that men with diabetes had almost four times the odds (odd ratio = 3.62) of ED compared with healthy controls (Diabet Med. 2017 Jul 18. doi: 10.1111/dme.13403). Phosphodiesterase-5 inhibitors – such as sildenafil, vardenafil, and tadalafil – are one option for men with diabetes and ED, Dr. Wessells said. “They work pretty well, but men with diabetes tend to have more severe ED. They’re going to get better, but will they get better enough to be normal? That’s the question.”
A 2007 Cochrane Library analysis found that men with diabetes and ED gained from PDE5 inhibitors overall (Cochrane Database Syst Rev. 2007 Jan 24[1]:CD002187. doi: 10.1002/14651858.CD002187.pub3).
“They’re not going to do as well as the general population,” Dr. Wessells said, “but we should try these as first-line agents in absence of things like severe unstable cardiovascular disease and other risk factors.”
Second-line therapies, typically offered by urologists, include penile prostheses and injection therapy, he said. A 2014 analysis of previous research found that men with diabetes were “more than 50% more likely to be prescribed secondary ED treatments over the 2-year observation period, and more than twice as likely to undergo penile prosthesis surgery” (Int J Impot Res. 2014 May-Jun;26[3]:112-5).
As for women, a 2009 study found that of 424 sexually active women with type 1 diabetes (97% of whom were white), 35% showed signs of female sexual dysfunction (FSD). Of those with FSD, problems included loss of libido (57%); problems with orgasm (51%), lubrication (47%), and/or arousal (38%); and pain (21%) (Diabetes Care. 2009 May;32[5]:780-5).
Only one drug, flibanserin (Addyi), is approved for FSD in the United States. Its impact on patients with diabetes is unknown, Dr. Wessells said, and the drug has the potential for significant adverse events.
The good news: Research is providing insight into which men and women are more likely to develop sexual dysfunction, Dr. Wessells said.
Age is important in both genders. For women, depression and being married appear to be risk factors, he said. “This needs more exploration to help us understand how to intervene.”
And in men, he said, ED is linked to jumps in hemoglobin A1c, while men on intensive glycemic therapy have a lower risk.
“Maybe we can find out who needs to be targeted for earlier intervention,” he said. This is especially important for men because ED becomes more likely to be irreversible after just a few years, he said.
Dr. Wessells reports no relevant disclosures.
EXPERT ANALYSIS AT THE ADA ANNUAL SCIENTIFIC SESSIONS