User login
American Heart Association (AHA): Scientific Sessions 2016
Bone marrow cells prove limb-saving for some
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
NEW ORLEANS – Treatment with autologous bone marrow cells can avert amputation in selected patients who have critical limb ischemia and are not candidates for revascularization surgery, based on results from the randomized phase III MOBILE trial.
Critical limb ischemia, the end stage of peripheral arterial disease, accounts for more than 53,000 major limb amputations in the United States annually, noted principal investigator Dr. Michael P. Murphy, director of the Vascular and Cardiac Center for Adult Stem Cell Therapy at Indiana University, Indianapolis.
“About 30% of our patient population with critical limb ischemia have no options for the standard of care, such as surgical bypass, due to absence of a surgical target or chronic total occlusion, which mitigates an endovascular approach,” he said at the American Heart Association scientific sessions. “Thus, these no-option critical limb ischemia patients represent an unmet medical need for a novel agent that may promote limb salvage and prevent amputation and its associated disabilities.”
In the trial, investigators randomized 155 affected limbs (in 152 patients) 3:1 to receive double-blinded treatment with injections of concentrated autologous bone marrow aspirate or placebo.
Amputation-free survival 1 year after treatment, the trial’s primary endpoint, was not significantly better in the aspirate group than in the placebo group, according to the researchers. However, the aspirate reduced the risk of major amputation by 73% in the subgroup with less severe (Rutherford class 4) disease and by 67% in the subgroup of patients who did not have diabetes.
“Personally, I would recommend cell therapy for my Rutherford 4 patients and my nondiabetic Rutherford 5 patients,” Dr. Murphy said. “And one would say even for the Rutherford 5 diabetics, there were no safety concerns and there is hope for improvement rather than amputation – it might be worth the roll of the dice.”
The investigators are preparing a manuscript based on the full data and working with Biomet, the trial sponsor, to apply for Food and Drug Administration approval of the product for the treatment of critical limb ischemia, he said.
Lessons learned
A session attendee asked whether stronger demonstration of benefit in a larger trial is needed to justify the use and cost of such new cellular therapies.
“If we could go back and do it all over again, we would do things differently knowing what we know now,” Dr. Murphy replied, noting that the investigators had to stick to a trial design created more than a decade ago.
“We discovered that the event rate in patients with critical limb ischemia in the control group is actually 30% and not 40% because medical management has changed,” he said. “Secondly, we would do a 1:1 randomization and most likely would increase our sample size to 200, and we probably would have seen a difference overall. That difference overall would have been provided by, of course, increasing the Rutherford 4 and Rutherford 5 nondiabetics, which would overshadow the increased amputation rate in the diabetic group.”
“I think from this [experience], we can launch a much larger study, if there were funding for it, and look at autologous versus allogeneic cells in this domain,” Dr. Murphy concluded.
Identifying patients who benefit
Session panelist Roberto Bolli, MD, chief of the division of cardiovascular medicine at the University of Louisville (Ky.), commended the MOBILE trial for its study design and prespecified subgroup analyses.
“The whole problem we are coping with, not just in this trial, but in other studies, is that patients are different and some patients may respond better than others, or some patients may not respond at all. And really, we still don’t understand why some patients respond or not – it may have to do with their immune system, their regenerative capacity, as well as the type of cells that are being used,” he said.
“Even though it was in its entirety a negative study, it’s very important because it identified a possible target for future trials, which is patients without diabetes in Rutherford class 4, which may benefit from therapy,” Dr. Bolli concluded.
Trial details
Patients were enrolled in MOBILE from 24 U.S. centers. All had critical limb ischemia, were ineligible for revascularization, had an ankle-brachial index of less than 0.60 or a toe-brachial index of less than 0.40, and had Rutherford 4 disease (rest pain) or Rutherford 5 disease (tissue loss).
They received concentrated autologous bone marrow aspirate or placebo by intramuscular injection at 35-40 sites in the affected limb.
Results showed that the groups did not differ significantly with respect to the rates of adverse events or serious adverse events overall, Dr. Murphy reported. The aspirate group had lower rates of respiratory failure and fever; they also had a higher rate of anemia (68.9% vs. 36.1%, P less than .001) as expected, from the aspiration procedure, but with no associated complications.
The 1-year rate of amputation-free survival events, reflecting both major amputations and death, was lower with the aspirate, at 20.2%, than with placebo, at 30.5%, but not significantly so (P = .224). Findings were similar for major amputation in the entire trial population (16.0% vs. 22.2%, P = .392). However, this outcome was less common with the aspirate among patients with Rutherford 4 disease (7.7% vs. 26.3%, P = .041) and nondiabetic patients (10.0% vs. 27.7%, P = .046).
“Looking at these data, it became apparent to us that the Rutherford 5 diabetic was the outlying group,” said Dr. Murphy, who disclosed that he had no relevant conflicts of interest.
Considering all other patients – Rutherford 4 regardless of diabetes status and nondiabetic Rutherford 5 – the aspirate was associated with a dramatically lower rate of amputation compared with the placebo (9.6% vs. 26.7%, P = .021; hazard ratio, 0.33). In this subset, the number needed to treat with bone marrow aspirate to prevent a single amputation was just 6.
The aspirate and placebo groups did not differ with respect to ankle-brachial index, toe-brachial index, or 6-minute walk test distance in the entire trial population. Transcutaneous oxygen pressure (TcP02), an indicator of microvascular perfusion in the subcutaneous tissue of the ischemic limb, increased by 59% in the aspirate group but by only 7% in the placebo group.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: The 52-week rate of amputation-free survival events was 20.2% with the aspirate and 30.5% with placebo (P = .224).
Data source: A randomized phase III trial among 152 patients with critical limb ischemia who were not candidates for revascularization surgery (MOBILE trial).
Disclosures: Dr. Murphy disclosed that he had no relevant conflicts of interest. The trial was sponsored by Biomet Biologics, LLC.
VIDEO: HeartMate 3 LVAD solves pump thrombosis
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
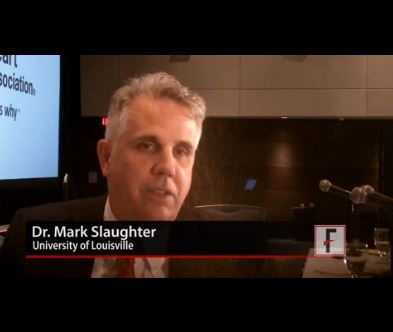
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
By eliminating all episodes of pump thrombosis during 6-month follow-up, the HeartMate 3 appeared to resolve one of the major issues that has stood in the way of patients and physicians feeling comfortable with left ventricular assist devices. The smaller size of the HeartMate 3 pump and its ability to be placed with minimally invasive and fairly rapid surgery is another big advance, putting this device on par with the rival pump, the HeartWare HVAD.
But the performance of the HeartMate 3 left ventricular assist device (LVAD) in MOMENTUM 3 also highlighted the shortcomings that still remain for these devices: the unchanged rates of stroke, gastrointestinal bleeds, and infections with HeartMate 3, compared with HeartMate II in this trial, and similar 6-month survival rates in the two arms of the study.
The HeartMate 3 can be implanted without sternotomy, using an 8 cm incision on the lateral chest wall, resulting in a shorter postoperative stay and fewer perisurgical adverse events. Despite the less invasive surgery and absence of pump thrombosis, some patients and physicians will remain hesitant to use an LVAD unless it is unavoidable because of concern about strokes. Until further design and procedural refinements change the rate of serious strokes and other adverse events, LVADs will not be fully competitive with heart transplantation.
The competition between HeartMate and the HeartWare devices will help drive this field forward, leading to further improvements in outcomes and expanded LVAD use.
Mark Slaughter, MD, is professor of surgery and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). He was an investigator in MOMENTUM 3, he has been a consultant to EvaHeart and Oregon Heart, and he has received research support from Carmat and HeartWare. He made these comments as designated discussant for the report and in a video interview.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
NEW ORLEANS – HeartMate 3, the latest left ventricular assist device in the HeartMate line, appears to have solved the problem of pump thrombosis, a complication that has dogged ventricular pumps since the issue leapt into medical awareness about 3 years ago (New Engl J Med. 2014 Jan 2;370:33-40).
During 6 months of follow-up, none of 152 heart failure patients assigned to receive a HeartMate 3 left ventricular assist device (LVAD) developed suspected or confirmed pump thrombosis, compared with 14 patients (10%) having pump thrombosis out of 138 recipients of the prior-generation HeartMate II LVAD who served as the control group for the study.
“Three years ago, when the issue of pump thrombosis was first revealed, there was a lot of consternation and some drop in LVAD use, especially as destination therapy. We think that seeing no pump thrombosis whatsoever will give people renewed confidence in this technology,” said Dr. Mehra, professor of medicine at Harvard Medical School and medical director of the Heart and Vascular Center of Brigham and Women’s Hospital, both in Boston.
Pump thrombosis has also been a problem for the patients who have received a competitor LVAD, the HeartWare HVAD device (Circulation. 2015 Nov 10;132[suppl 3]:A19675), approved for U.S. use as bridge to transplant. HeartMate II is approved for both bridge to transplant and for destination therapy.
In addition to apparently eliminating pump thrombosis, HeartMate 3’s size and potential implantation approach should make its placement during routine use as quick and minimally invasive as the HeartWare device, features that should further help broader use of HeartMate 3, commented Mark Slaughter, MD, professor and chairman of cardiovascular and thoracic surgery at the University of Louisville (Ky.). But Dr. Slaughter and others were also quick to highlight the shortcomings that remain with both devices that will continue to hamper a broader role for LVAD treatment of patients with advanced heart failure.
“We thought that if there was less pump thrombosis we’d see less stroke, but that is not what the data suggest. It’s the big puzzle we need to figure out before we see widespread acceptance of this treatment,” Dr. Sweitzer said.
“This will not shift LVAD use substantially,” commented Christopher B. Granger, MD, a professor of medicine and a heart failure specialist at Duke University, Durham, N.C. “Reducing the need for reoperation is good for the field, and is an incremental advance, but it is not transformational,” he said in an interview.
The MOMENTUM 3 (Multicenter Study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy with HeartMate 3) trial randomized 294 patients at 69 U.S. centers. The study’s primary endpoint of 6-month survival free from disabling stroke or reoperation to repair or replace the LVAD occurred in 86% of 152 patients who received a HeartMate 3 and 77% of 142 patients randomized to HeartMate II, a statistical difference that met the prespecified criteria for both noninferiority and superiority. Concurrently with Dr. Mehra’s report at the meeting, a journal article appeared online (New Engl J Med. 2016 Nov 16. doi: 10.1056/NEJMoa1610426). He stated that as far as he understood, St. Jude would submit the 6-month data he reported to the Food and Drug Administration in an application for marketing approval for HeartMate 3.
“I agree that there are still morbid evens [with HeartMate 3] that need to be surmounted, but this is a confidence-building step in the right direction,” Dr. Mehra said.
mzoler@frontlinemedcom.com
On Twitter @mitchelzoler
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: During 6 months, suspected or confirmed pump thrombosis occurred in no HeartMate 3 patients and in 10% of HeartMate II recipients.
Data source: The MOMENTUM 3 trial, which randomized 294 patients at 69 U.S. centers.
Disclosures: MOMENTUM 3 was sponsored by St. Jude, the company developing the HeartMate 3 LVAD. Dr. Mehra has received travel reimbursements from St. Jude and has been a consultant to Medtronic, Stealth, and Teva. Dr. Sweitzer was an investigator in MOMENTUM 3 and has been a consultant to Acorda and Medtronic and received research support from Bayer, Corvia, and Novartis. Dr. Granger has been a consultant to Boehringer Ingelheim, and received research support from Medtronic and several other drug and device companies. Dr. Slaughter was an investigator in MOMENTUM 3, has been a consultant to EvaHeart and Oregon Heart, and has received research support from Carmat and HeartWare.
Mixed findings on iron repletion in HF
NEW ORLEANS – Oral iron supplementation was declared ineffective for the treatment of iron deficiency in patients with heart failure with reduced ejection fraction in one major randomized trial while intravenous ferric carboxymaltose improved exercise capacity and quality of life in another study presented at the American Heart Association scientific sessions.
Iron deficiency is present in roughly half of patients with heart failure with reduced ejection fraction (HFrEF). In affected patients it’s associated with diminished functional capacity and poor quality of life, and is an independent predictor of mortality. Thus, it has emerged as a potential therapeutic target in HFrEF.
The IRONOUT HF (Oral Iron Repletion Effects on Oxygen UpTake in Heart Failure) trial was a multicenter, randomized, double-blind, placebo-controlled clinical trial conducted by the National Heart, Lung, and Blood Institute’s Clinical Heart Failure Network investigators. It included 225 patients with HFrEF and iron deficiency who were randomized to 16 weeks of oral iron polysaccharide at 150 mg b.i.d. or matching placebo.
The primary endpoint was change from baseline to 16 weeks in exercise capacity, as measured via peak oxygen uptake during cardiopulmonary exercise testing. The results were no better in the iron-supplemented group than in placebo-treated controls. Nor was there any benefit for oral iron therapy in terms of quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire or any of numerous other secondary endpoints, according to Dr. Lewis, head of the heart failure section and director of the cardiopulmonary exercise testing laboratory at Massachusetts General Hospital, Boston.
Participants’ mean baseline hemoglobin level was 12.6 g/dL, but whether patients were anemic at baseline or not made no difference in terms of outcomes. Rates of venous congestion and bleeding were low during the trial.
The problem, the investigators found, was that high-dose oral iron only minimally repleted iron stores. Transferrin saturation increased by a paltry absolute 3% after 16 weeks of twice-daily therapy. Serum ferritin levels increased only 1/20th as much as after intravenous iron ferric carboxymaltose therapy in the earlier positive FAIR-HF (Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure) trial (N Engl J Med. 2009 Dec 17;361:2436-48).
Levels of hepcidin were elevated in study participants. And the higher the level of hepcidin – which Dr. Lewis called “a massive regulator of iron bioavailability and absorption” – the more refractory patients were to oral iron repletion.
“Hepcidin was invented by nature to protect against iron from stimulating growth of bacteria. And when it’s elevated, taking iron orally just has no chance of success,” explained Dr. Anker, professor of cardiology at the University of Gottingen (Germany).
Dr. Anker, who chaired the positive FAIR-HF trial of intravenous iron, said IRONOUT HF was a very well-conducted and definitive clinical trial of oral iron supplementation in HFrEF.
“It’s very simple: Oral iron does not work in patients with chronic heart failure. That’s the take home message. It’s true for peak VO2 [oxygen consumption], for 6-minute walk distance, for symptoms, for quality of life, and even for surrogate markers like NT-proBNP [N-terminal pro b-type natriuretic peptide]. If iron doesn’t get into the body, it’s really difficult to [imagine] that the iron that doesn’t get into the body can exert an effect,” he said.
The primary endpoint in EFFECT-HF was assessor-blinded change in peak VO2 from baseline to week 24. The control group experienced a decrease in peak VO2 over time such that there was a significant difference of 1.0 mL/kg per minute between the two groups.
The ferric carboxymaltose recipients also did significantly better in terms of secondary endpoints including improvement in New York Heart Association functional class, self-reported Patient Global Assessment score, and quality-of-life measures, reported Dr. van Veldhuisen, professor and chairman of cardiology at University Medical Center Groningen (the Netherlands).
Session moderator Clyde Yancy, MD, commented that intravenous iron is not ready for prime time use in clinical practice for several reasons. The open-label EFFECT-HF trial, like the earlier positive double-blind FAIR-HF and CONFIRM-HF IV ferric carboxymaltose trials, was too modest in size to be convincing, especially since this is an expensive and intrusive therapy.
“The endpoint of peak VO2, although a very powerful endpoint, is still one for which there may be some subjectivity, and so we need a more definitive endpoint to be absolutely certain about the potential benefit of the administration of ferric carboxymaltose,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
Discussant Adrian Hernandez, MD, of the Duke Clinical Research Institute in Durham, N.C., said he considers peak VO2 an important endpoint.
“When you talk to patients, exercise capacity is an outcome that matters to them,” he said. “They often comment that what matters to them is living longer with a better quality of life, free of worsening heart failure, and having improvement in everyday functional status. So the cardiopulmonary exercise test is not just a surrogate endpoint; it’s a measure of functional outcome that matters to patients,” he said.
Still, like Dr. Yancy, Dr. Hernandez said he thinks it’s time to have larger, longer, definitive trials with clinical endpoints in order to understand the role of intravenous iron. Both cardiologists applauded Dr. Anker’s announcement that such a trial, known as FAIR-HF2, is now getting started.
The IRONOUT HF trial was funded by the National Institutes of Health. Dr. Lewis reported receiving research support from a handful of pharmaceutical and medical device companies. Dr. van Velduisen reported serving as a scientific adviser to Vifor Pharma, which sponsored the EFFECT-HF trial. Dr. Anker, who was an EFFECT-HF investigator, serves as a consultant to Vifor and several other companies. Dr. Hernandez was an IRONOUT HF investigator and reported receiving research grants from a handful of pharmaceutical companies.
NEW ORLEANS – Oral iron supplementation was declared ineffective for the treatment of iron deficiency in patients with heart failure with reduced ejection fraction in one major randomized trial while intravenous ferric carboxymaltose improved exercise capacity and quality of life in another study presented at the American Heart Association scientific sessions.
Iron deficiency is present in roughly half of patients with heart failure with reduced ejection fraction (HFrEF). In affected patients it’s associated with diminished functional capacity and poor quality of life, and is an independent predictor of mortality. Thus, it has emerged as a potential therapeutic target in HFrEF.
The IRONOUT HF (Oral Iron Repletion Effects on Oxygen UpTake in Heart Failure) trial was a multicenter, randomized, double-blind, placebo-controlled clinical trial conducted by the National Heart, Lung, and Blood Institute’s Clinical Heart Failure Network investigators. It included 225 patients with HFrEF and iron deficiency who were randomized to 16 weeks of oral iron polysaccharide at 150 mg b.i.d. or matching placebo.
The primary endpoint was change from baseline to 16 weeks in exercise capacity, as measured via peak oxygen uptake during cardiopulmonary exercise testing. The results were no better in the iron-supplemented group than in placebo-treated controls. Nor was there any benefit for oral iron therapy in terms of quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire or any of numerous other secondary endpoints, according to Dr. Lewis, head of the heart failure section and director of the cardiopulmonary exercise testing laboratory at Massachusetts General Hospital, Boston.
Participants’ mean baseline hemoglobin level was 12.6 g/dL, but whether patients were anemic at baseline or not made no difference in terms of outcomes. Rates of venous congestion and bleeding were low during the trial.
The problem, the investigators found, was that high-dose oral iron only minimally repleted iron stores. Transferrin saturation increased by a paltry absolute 3% after 16 weeks of twice-daily therapy. Serum ferritin levels increased only 1/20th as much as after intravenous iron ferric carboxymaltose therapy in the earlier positive FAIR-HF (Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure) trial (N Engl J Med. 2009 Dec 17;361:2436-48).
Levels of hepcidin were elevated in study participants. And the higher the level of hepcidin – which Dr. Lewis called “a massive regulator of iron bioavailability and absorption” – the more refractory patients were to oral iron repletion.
“Hepcidin was invented by nature to protect against iron from stimulating growth of bacteria. And when it’s elevated, taking iron orally just has no chance of success,” explained Dr. Anker, professor of cardiology at the University of Gottingen (Germany).
Dr. Anker, who chaired the positive FAIR-HF trial of intravenous iron, said IRONOUT HF was a very well-conducted and definitive clinical trial of oral iron supplementation in HFrEF.
“It’s very simple: Oral iron does not work in patients with chronic heart failure. That’s the take home message. It’s true for peak VO2 [oxygen consumption], for 6-minute walk distance, for symptoms, for quality of life, and even for surrogate markers like NT-proBNP [N-terminal pro b-type natriuretic peptide]. If iron doesn’t get into the body, it’s really difficult to [imagine] that the iron that doesn’t get into the body can exert an effect,” he said.
The primary endpoint in EFFECT-HF was assessor-blinded change in peak VO2 from baseline to week 24. The control group experienced a decrease in peak VO2 over time such that there was a significant difference of 1.0 mL/kg per minute between the two groups.
The ferric carboxymaltose recipients also did significantly better in terms of secondary endpoints including improvement in New York Heart Association functional class, self-reported Patient Global Assessment score, and quality-of-life measures, reported Dr. van Veldhuisen, professor and chairman of cardiology at University Medical Center Groningen (the Netherlands).
Session moderator Clyde Yancy, MD, commented that intravenous iron is not ready for prime time use in clinical practice for several reasons. The open-label EFFECT-HF trial, like the earlier positive double-blind FAIR-HF and CONFIRM-HF IV ferric carboxymaltose trials, was too modest in size to be convincing, especially since this is an expensive and intrusive therapy.
“The endpoint of peak VO2, although a very powerful endpoint, is still one for which there may be some subjectivity, and so we need a more definitive endpoint to be absolutely certain about the potential benefit of the administration of ferric carboxymaltose,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
Discussant Adrian Hernandez, MD, of the Duke Clinical Research Institute in Durham, N.C., said he considers peak VO2 an important endpoint.
“When you talk to patients, exercise capacity is an outcome that matters to them,” he said. “They often comment that what matters to them is living longer with a better quality of life, free of worsening heart failure, and having improvement in everyday functional status. So the cardiopulmonary exercise test is not just a surrogate endpoint; it’s a measure of functional outcome that matters to patients,” he said.
Still, like Dr. Yancy, Dr. Hernandez said he thinks it’s time to have larger, longer, definitive trials with clinical endpoints in order to understand the role of intravenous iron. Both cardiologists applauded Dr. Anker’s announcement that such a trial, known as FAIR-HF2, is now getting started.
The IRONOUT HF trial was funded by the National Institutes of Health. Dr. Lewis reported receiving research support from a handful of pharmaceutical and medical device companies. Dr. van Velduisen reported serving as a scientific adviser to Vifor Pharma, which sponsored the EFFECT-HF trial. Dr. Anker, who was an EFFECT-HF investigator, serves as a consultant to Vifor and several other companies. Dr. Hernandez was an IRONOUT HF investigator and reported receiving research grants from a handful of pharmaceutical companies.
NEW ORLEANS – Oral iron supplementation was declared ineffective for the treatment of iron deficiency in patients with heart failure with reduced ejection fraction in one major randomized trial while intravenous ferric carboxymaltose improved exercise capacity and quality of life in another study presented at the American Heart Association scientific sessions.
Iron deficiency is present in roughly half of patients with heart failure with reduced ejection fraction (HFrEF). In affected patients it’s associated with diminished functional capacity and poor quality of life, and is an independent predictor of mortality. Thus, it has emerged as a potential therapeutic target in HFrEF.
The IRONOUT HF (Oral Iron Repletion Effects on Oxygen UpTake in Heart Failure) trial was a multicenter, randomized, double-blind, placebo-controlled clinical trial conducted by the National Heart, Lung, and Blood Institute’s Clinical Heart Failure Network investigators. It included 225 patients with HFrEF and iron deficiency who were randomized to 16 weeks of oral iron polysaccharide at 150 mg b.i.d. or matching placebo.
The primary endpoint was change from baseline to 16 weeks in exercise capacity, as measured via peak oxygen uptake during cardiopulmonary exercise testing. The results were no better in the iron-supplemented group than in placebo-treated controls. Nor was there any benefit for oral iron therapy in terms of quality of life, as assessed by the Kansas City Cardiomyopathy Questionnaire or any of numerous other secondary endpoints, according to Dr. Lewis, head of the heart failure section and director of the cardiopulmonary exercise testing laboratory at Massachusetts General Hospital, Boston.
Participants’ mean baseline hemoglobin level was 12.6 g/dL, but whether patients were anemic at baseline or not made no difference in terms of outcomes. Rates of venous congestion and bleeding were low during the trial.
The problem, the investigators found, was that high-dose oral iron only minimally repleted iron stores. Transferrin saturation increased by a paltry absolute 3% after 16 weeks of twice-daily therapy. Serum ferritin levels increased only 1/20th as much as after intravenous iron ferric carboxymaltose therapy in the earlier positive FAIR-HF (Ferinject Assessment in Patients With Iron Deficiency and Chronic Heart Failure) trial (N Engl J Med. 2009 Dec 17;361:2436-48).
Levels of hepcidin were elevated in study participants. And the higher the level of hepcidin – which Dr. Lewis called “a massive regulator of iron bioavailability and absorption” – the more refractory patients were to oral iron repletion.
“Hepcidin was invented by nature to protect against iron from stimulating growth of bacteria. And when it’s elevated, taking iron orally just has no chance of success,” explained Dr. Anker, professor of cardiology at the University of Gottingen (Germany).
Dr. Anker, who chaired the positive FAIR-HF trial of intravenous iron, said IRONOUT HF was a very well-conducted and definitive clinical trial of oral iron supplementation in HFrEF.
“It’s very simple: Oral iron does not work in patients with chronic heart failure. That’s the take home message. It’s true for peak VO2 [oxygen consumption], for 6-minute walk distance, for symptoms, for quality of life, and even for surrogate markers like NT-proBNP [N-terminal pro b-type natriuretic peptide]. If iron doesn’t get into the body, it’s really difficult to [imagine] that the iron that doesn’t get into the body can exert an effect,” he said.
The primary endpoint in EFFECT-HF was assessor-blinded change in peak VO2 from baseline to week 24. The control group experienced a decrease in peak VO2 over time such that there was a significant difference of 1.0 mL/kg per minute between the two groups.
The ferric carboxymaltose recipients also did significantly better in terms of secondary endpoints including improvement in New York Heart Association functional class, self-reported Patient Global Assessment score, and quality-of-life measures, reported Dr. van Veldhuisen, professor and chairman of cardiology at University Medical Center Groningen (the Netherlands).
Session moderator Clyde Yancy, MD, commented that intravenous iron is not ready for prime time use in clinical practice for several reasons. The open-label EFFECT-HF trial, like the earlier positive double-blind FAIR-HF and CONFIRM-HF IV ferric carboxymaltose trials, was too modest in size to be convincing, especially since this is an expensive and intrusive therapy.
“The endpoint of peak VO2, although a very powerful endpoint, is still one for which there may be some subjectivity, and so we need a more definitive endpoint to be absolutely certain about the potential benefit of the administration of ferric carboxymaltose,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
Discussant Adrian Hernandez, MD, of the Duke Clinical Research Institute in Durham, N.C., said he considers peak VO2 an important endpoint.
“When you talk to patients, exercise capacity is an outcome that matters to them,” he said. “They often comment that what matters to them is living longer with a better quality of life, free of worsening heart failure, and having improvement in everyday functional status. So the cardiopulmonary exercise test is not just a surrogate endpoint; it’s a measure of functional outcome that matters to patients,” he said.
Still, like Dr. Yancy, Dr. Hernandez said he thinks it’s time to have larger, longer, definitive trials with clinical endpoints in order to understand the role of intravenous iron. Both cardiologists applauded Dr. Anker’s announcement that such a trial, known as FAIR-HF2, is now getting started.
The IRONOUT HF trial was funded by the National Institutes of Health. Dr. Lewis reported receiving research support from a handful of pharmaceutical and medical device companies. Dr. van Velduisen reported serving as a scientific adviser to Vifor Pharma, which sponsored the EFFECT-HF trial. Dr. Anker, who was an EFFECT-HF investigator, serves as a consultant to Vifor and several other companies. Dr. Hernandez was an IRONOUT HF investigator and reported receiving research grants from a handful of pharmaceutical companies.
EXPERT ANALYSIS FROM THE AHA SCIENTIFIC SESSIONS
PPIs may boost ischemic stroke risk
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
A growing number of retrospective studies have associated proton pump inhibitors with a host of serious adverse effects. These include chronic kidney disease, dementia, osteoporosis, cardiovascular events, pneumonia, enteric infections, and others. The authors of this large, retrospective Danish study have now added ischemic stroke to the list.
Nonetheless, this study should serve as wake-up call to closely examine the risks and benefits of ongoing PPI use for each individual patient. For example, guidelines clearly advocate the use of PPIs in patients at high risk for peptic ulcer disease (for example, use of aspirin and warfarin together), and these patients should continue PPIs unless more convincing evidence of serious side effects emerge. On the other hand, several studies have shown that many patients with uncomplicated gastroesophageal reflux disease symptoms can achieve symptom control with substitution of histamine2 blockers, p.r.n. dosing of PPIs, or without acid-reducing medications entirely. Still, many patients with confirmed pathologic acid reflux are likely to require ongoing PPIs. For patients who continue PPIs, they should use the lowest effective dose.
Surely, physicians will be discussing PPI adverse effects with increasing numbers of patients. Until higher-quality evidence in the form of a randomized controlled trial emerges, physicians should get used to explaining the principles of epidemiology.
Jacob Kurlander, MD, is a clinical lecturer in the division of gastroenterology, University of Michigan, Ann Arbor. He has received research funding from Ironwood Pharmaceuticals.
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
NEW ORLEANS – The use of proton pump inhibitors (PPIs) was associated with significantly increased risk of having a first ischemic stroke in a large nationwide Danish cohort study, Thomas S. Sehested, MD, reported at the American Heart Association scientific sessions.
The relationship was dose dependent. At the lowest available dose of each of the four PPIs studied there was no significantly increased risk. At the intermediate doses of three of the four PPIs studied, the increased risk of ischemic stroke became statistically significant. And the highest dose of each drug was associated with the greatest ischemic stroke risk.
In Denmark, for instance, where most PPIs are prescription only and use is easily trackable, it’s estimated that, at any given time, 7% of the adult population is taking a PPI, often not as directed in the labeling.
The impetus for this study, Dr. Sehested explained, was the mounting evidence that PPIs may constitute an independent risk factor for acute MI and other cardiovascular events. For example, a recent meta-analysis of 17 randomized controlled trials totaling 7,540 participants published through mid-2015 concluded that the use of PPIs was associated with a 70% increase in cardiovascular risk (Neurogastroenterol Motil. 2016 Aug 30. doi: 10.1111/nmo.12926).
He reported on 245,676 Danes above age 30 who were free of prior MI or stroke when they underwent elective GI endoscopy during 1997-2012. After a 30-day postendoscopy grace period during which 1,476 patients had a first MI, stroke, or died of any cause, the final study population was 244,200, of whom 43.7% were PPI users during the grace period and beyond.
During a median 5.8 years of follow-up, 9,489 subjects (3.9%) had a first ischemic stroke. Because of the comprehensive nature of Denmark’s interlocking birth to death registries, there was virtually no loss to follow-up in this study.
The unadjusted incidence of ischemic stroke in PPI nonusers was 55.7 per 10,000 person-years, compared with 88.9 per 10,000 in PPI users.
The PPI users were slightly older than nonusers by roughly 3 years. They were also an absolute 5% more likely to be hypertensive and an absolute 1.7% more likely to be regular users of NSAIDs. All of these differences, while modest, were statistically significant because of the large patient numbers involved.
In a multivariate analysis adjusted for age, sex, calendar year, comorbid diabetes, hypertension, alcohol use disorder, heart failure, peptic ulcer, peripheral artery disease, kidney disease, aspirin, oral anticoagulants and other medications, and socioeconomic status, current users of PPIs were 19% more likely to have a first ischemic stroke than nonusers. That difference is statistically significant and clinically meaningful, Dr. Sehested said.
In contrast, when the same sort of nationwide analysis was repeated, comparing current users of histamine-2 receptor antagonists to nonusers of those drugs or PPIs, there was no difference in ischemic stroke risk between the two groups.
The message, according to Dr. Sehested, is that physicians should encourage more cautious use of PPIs. And especially in the United States, where most PPIs are available over the counter, it’s prudent during office visits to ask what nonprescription drugs a patient is taking.
Dr. Sehested presented his study findings in a session devoted to original research in cardiovascular epidemiology. Many top American epidemiologists were present in the audience, and several rose to congratulate him on his presentation of the latest elegant epidemiologic study to come out of Denmark, the only place in the world where this sort of nationwide comprehensive research is possible.
“Wow! I just love the work you do in Denmark. It’s really inspiring,” commented David Siscovick, MD, senior vice president for research at the New York Academy of Medicine and professor emeritus of medicine and epidemiology at the University of Washington in Seattle.
He had a question: “Did you deal with PPI starters and stoppers and compliance in any way?”
Dr. Sehested replied that he and his coinvestigators were able to see who was on a PPI at any given point in the study, and they accounted for that. One issue the researchers plan to examine but haven’t yet had a chance to, however, is the relationship between duration of PPI therapy and ischemic stroke risk. It’s likely that some patients had already been on a PPI for a lengthy time at elective endoscopy, which is when the study in its current form began.
“I think that would strengthen the study,” he said.
Comoderator Jorge Kizer, MD, of Albert Einstein College of Medicine in New York, commented, “Confounding by indication is clearly the elephant in the room. The guidelines actually recommend adding a PPI if a patient is on dual-antiplatelet therapy and has an NSAID added. Did you adjust for that? It would boost confidence that the results are actually due to the PPI.”
Dr. Sehested answered that the great majority of individuals with cardiovascular disease at baseline were excluded from the analysis.
“I don’t think we had that many on dual-antiplatelet therapy,” he added.
Preclinical studies suggest a possible mechanism by which PPIs may harm cardiovascular health. The drugs reduce nitric oxide synthase levels, with resultant endothelial dysfunction, he said.
Dr. Sehested is employed at the Danish Heart Foundation, which funded the study.
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Current use of a proton pump inhibitor was independently associated with a 19% increased risk of a first ischemic stroke, and the risk was greater at the top approved doses.
Data source: This retrospective nationwide Danish study involved 244,200 adults age 30 or older followed for a median of nearly 6 years following elective GI endoscopy.
Disclosures: The presenter is employed at the Danish Heart Foundation, which funded the study.
Allogeneic stem cells show promise for treating nonischemic dilated cardiomyopathy
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
NEW ORLEANS – Allogeneic stem cells appear to be a safe treatment option for nonischemic dilated cardiomyopathy and show somewhat greater efficacy than autologous stem cells, according to the results of the randomized POSEIDON-DCM trial.
“Nonischemic dilated cardiomyopathy is an incurable condition with significant genetic and immunologic underpinnings,” noted lead investigator Joshua M. Hare, MD, director of the Interdisciplinary Stem Cell Institute and professor of medicine at the University of Miami.
The phase I/II trial undertook a head-to-head comparison of allogeneic and autologous bone marrow–derived mesenchymal stem cells in 37 patients with nonischemic dilated cardiomyopathy.
Results presented at the American Heart Association scientific sessions and simultaneously published (J Am Coll Cardiol. 2016. doi: 10.1016/j.jacc.2016.11.009) showed that none of the patients in either group experienced a 30-day treatment-emergent serious adverse event, the trial’s primary endpoint.
The allogeneic group had a greater shift to a lesser inflammatory immune profile, and, at 12 months, a lower rate of major adverse cardiac events and more improvement in walk test distance. Additionally, half of patients in the allogeneic group no longer met the ejection fraction cutoff typically used to define dilated cardiomyopathy, compared with only about one-fifth of those in the autologous group.
“Immunomodulation may contribute to the efficacy of allogeneic human mesenchymal stem cells in nonischemic dilated cardiomyopathy, as we have shown suppression of immune activation to a greater degree with the allo versus auto cells,” Dr. Hare said.
“We argue that these data support the use of allogeneic mesenchymal stem cell therapy in future pivotal placebo-controlled clinical trials for this patient population, an important patient population with significant unmet need.”
Trial details
The patients enrolled in POSEIDON-DCM had left ventricular dysfunction due to nonischemic dilated cardiomyopathy and were randomized evenly to allogeneic or autologous stem cell therapy. Stem cells were delivered by transendocardial injection into 10 left ventricular sites using a catheter.
In the first 30 days after treatment, there were no treatment-emergent serious adverse events, defined as death, nonfatal myocardial infarction, stroke, hospitalization for worsening heart failure, cardiac perforation, pericardial tamponade, or sustained ventricular arrhythmias. “The 30-day safety and tolerability was excellent in both groups receiving either allogeneic or autologous therapy,” Dr. Hare said.
At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
In terms of efficacy, ejection fraction at 12 months had improved by a significant 8.0 Units in the allogeneic group and a nonsignificant 5.4 Units in the autologous group (P not significant for difference between groups). Roughly half of patients in the allogeneic group had achieved an ejection fraction of greater than 40%, compared with only two patients in the autologous group. “This is meaningful because the clinical definition of dilated cardiomyopathy typically uses an ejection fraction cutoff of 40%,” he noted.
The 6-minute walk test distance increased by a significant 37.0 m for the allogeneic group and by a nonsignificant 7.3 m for the autologous group (P = .0168 for difference between groups). Scores on the Minnesota Living With Heart Failure Questionnaire fell significantly in the former group and nonsignificantly in the latter group (P not significant for difference between groups).
Patients in the allogeneic group were more likely to have an improvement from baseline in New York Heart Association class (66.7% vs. 27.3%, P = .0527).
“An issue of concern in this field has been the formation of ectopic tissue with mesenchymal stem cells, so patients received whole-body CT scanning over 12 months,” Dr. Hare reported. “There was no ectopic tissue formation or tumor formation in any patient.”
In terms of biologic endpoints, two measures of endothelial function known to be suppressed in the setting of circulatory failure – endothelial progenitor cell colony-forming units and flow-mediated vasodilation – had increased significantly at 3 months in the allogeneic group only. Tumor necrosis factor–alpha levels fell by roughly 70% with allogeneic therapy versus 50% with autologous therapy (P = .05).
Both groups had a lessening of the immunosuppression that is common in heart failure, but benefit in several markers, such as the percentage of switched memory B cells, was greater with the allogeneic therapy. Additionally, there was a trend toward greater reduction of early T-cell activation in the allogeneic group.
“Of importance in the field of allogeneic cell therapy is [whether] the allogeneic cells mount a panel-reactive antigen [PRA],” commented Dr. Hare, who disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
Results showed that one patient in the allogeneic group developed a high-risk PRA, compared with none in the autologous group. Another four patients in the former group developed a moderate-risk PRA, compared with one in the latter group (P less than or equal to .05).
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: At 12 months, the allogeneic group had lower rates than the autologous group of major adverse cardiac events (20.3% vs. 57.1%, P = .0186) and all-cause rehospitalizations (28.2% vs. 70.0%, P = .0447).
Data source: A randomized phase I/II trial among 37 patients with nonischemic dilated cardiomyopathy (POSEIDON-DCM trial).
Disclosures: Dr. Hare disclosed that he has an ownership interest in and is a consultant or advisory board member for Vestion.
GLAGOV finds evolocumab plus statin drives unprecedented plaque regression
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
NEW ORLEANS – Adding the PCSK9 inhibitor evolocumab to maximum tolerated statin therapy in subjects with symptomatic coronary disease induced atheromatous plaque regression of a previously unheard-of scale in the phase III GLAGOV trial, Steven E. Nissen, MD, reported at the American Heart Association scientific sessions.
Over 18 months of follow-up, percent atheroma volume was unchanged in patients treated with maximum tolerated statin therapy. But in patients on maximum tolerated statin therapy plus evolocumab, mean atheroma volume decreased by 0.95%. A reduction in atheroma volume as small as 0.5% has been associated with a reduced rate of cardiovascular events in previous studies, according to GLAGOV principal investigator Stephen J. Nicholls, MBBS, PhD, of the University of Adelaide in Australia.
Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Among 423 patients on statin monotherapy, 47% had regression and 53% had progression of atheroma volume. In contrast, 64% of 423 patients on a statin plus evolocumab had atheroma regressions and 36% had atheroma progression.
What’s more, plaque regression was directly related to declines in LDL levels.
“We saw a linear relationship between lower LDL and greater regression with no tailing off of benefit at very low LDL levels. Down to 20 mg/dL, it’s continuous and linear,” the cardiologist said. “I thought there might be diminishing return at low LDL levels. We don’t see that.”
GLAGOV (Global Assessment of Plaque Regression with a PCSK9 antibody as Measured by Intravascular Ultrasound) was a double-blind, placebo-controlled, randomized trial including 846 evaluable patients with symptomatic CAD at 197 centers. All were on a stable maximum tolerated dose of a statin at baseline, at which point they underwent intravascular ultrasound (IVUS) and were assigned to subcutaneous evolocumab (Repatha) at 420 mg once monthly or placebo injections while continuing on their statin. At 18 months, participants had a follow-up IVUS of the originally imaged target vessel.
The mean LDL-cholesterol level was 93 mg/dL at the start of the study. With the addition of evolocumab, the mean LDL level dropped to 37 mg/dL; that’s a 60% further reduction below the level on statin alone.
The investigators conducted a post hoc exploratory subgroup analysis confined to 144 patients whose baseline LDL on statin monotherapy was less than 70 mg/dL. In this group, the addition of evolocumab caused the mean LDL level to plunge to 24 mg/dL. Mean atheroma volume in dual-treatment patients with a baseline LDL below 70 mg/dL decreased by 1.97% – double the reduction seen in the overall statin/evolocumab study arm. Further, 81% of patients on dual therapy who started out with an LDL below 70 mg/dL showed IVUS evidence of plaque regression, compared with 48% of those on statin monotherapy. For those whose baseline LDL level was below 70 mg/dL and who remained on statin monotherapy, there was no change in atheroma volume over time.
No signals of any safety concerns arose in the group on a statin plus evolocumab. Of particular interest, there was no increase in new-onset diabetes, neurocognitive dysfunction, or myalgia in patients given dual therapy, compared with patients on a statin alone.
Dr. Santos, of the University of Sao Paolo in Brazil, noted that GLAGOV wasn’t powered to provide evidence of long-term safety or of hard clinical endpoints such as acute MI. For those outcomes, physicians must await the soon-to-come results of the nearly 28,000-patient FOURIER clinical outcomes trial.
The GLAGOV trial was sponsored by Amgen, the maker of evolocumab (Repatha). Dr. Santos reported serving as a consultant to and paid researcher for the company. Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies.
Simultaneously with Dr. Nissen’s presentation in New Orleans, the GLAGOV results were published online (JAMA. 2016 Nov 15. doi: 10.1001/jama.2016.16951).
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: Total atheroma volume was reduced by 5.8 mm3 with dual therapy, compared with a nonsignificant 0.9-mm3 decrease in patients on statin monotherapy.
Data source: GLAGOV, a phase III, randomized, double-blind, 18-month intravascular ultrasound study of 846 evaluable patients with symptomatic CAD.
Disclosures: The GLAGOV trial was sponsored by Amgen, maker of evolocumab (Repatha). Dr. Nissen reported serving as a consultant to Amgen and several other companies; any resultant consultant fees are directly paid to charities. Dr. Nicholls reported receiving research support from and serving as a consultant to Amgen and several other drug companies. Dr. Santos reported serving as a consultant to and paid researcher for the company.
TRUE-AHF: Urgent vasodilator therapy in acute HF provides no long-term benefit
NEW ORLEANS – An investigational synthetic natriuretic peptide given early during hospitalization for acute decompensated heart failure didn’t produce any of the hoped-for intermediate- or long-term clinical benefits in the phase III TRUE-AHF study, Milton Packer, MD, reported at the American Heart Association scientific sessions.
The failure of this investigational vasodilator, ularitide, to influence downstream cardiovascular mortality or early readmission for heart failure closes the door on the once-promising hypothesis that myocardial microinjury occurring during ADHF is due to ventricular distension, observed Dr. Packer, the Distinguished Scholar in Cardiovascular Science at Baylor University Medical Center, Dallas. “Ularitide did exactly what we expected it to do while we were giving it: we caused intravascular decompression, we reduced cardiac wall stress, but we did not affect cardiac microinjury, and we didn’t change long-term cardiovascular mortality or any of our secondary endpoints, including and in particular the 30-day risk of rehospitalization for heart failure,” he said.
During a median follow-up of 15 months there were 236 cardiovascular deaths in the ularitide group and 225 in the control group, a nonsignificant difference. Nor were there any differences between the two groups in secondary endpoints including length of stay in the ICU during the index hospitalization, rehospitalization for ADHF within 30 days of discharge, or the composite of all-cause mortality or cardiovascular hospitalization within 6 months, which occurred in 40.7% of the ularitide group and 37.2% of controls.
The explanation for this lack of long-term benefit lies in the finding that myocardial microinjury wasn’t prevented by the rapid reduction of cardiac distension produced by ularitide. This was evident in the therapy’s inability to dampen the rise in high-sensitivity cardiac troponin T which occurred in the initial 48 hours of the study.
“The trial demonstrated the effects and safety of ularitide. However, to gain long-term benefits on hospitalizations and death in patients following a hospital admission for heart failure, physicians must focus on the drugs that patients take as an outpatient rather than the drugs they receive as an inpatient,” Dr. Packer concluded.
“Readmission rates remain stubbornly at 20% within 30 days and near 50% at 6 months. Acute decompensation is an inflection point in the natural history of heart failure with subsequent 1-year mortality rates consistently approximating 25%. Clearly there is something about the hospitalization that is a herald event which speaks to much worse outcomes, compared with chronic ambulatory heart failure,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
He agreed with Dr. Packer that in light of the TRUE-AHF results, what Dr. Yancy termed “the early injury hypothesis” isn’t worth further pursuit.
Ularitide thus joins a long list of failed therapies for ADHF. Treatments that have convincingly been shown to have no significant impact on mortality and at best only modest impact on morbidity include continuous IV infusion of loop diuretics in the DOSE trial; the arginine vasopressin antagonists, which failed to impress in EVEREST, SECRET, and TACTICS; nesiritide (Natrecor) in the ASCEND-HF trial; and levosimendan (Simdax), which proved disappointing in the SURVIVE and REVIVE II studies, according to Dr. Yancy.
The jury is still out on serelaxin, he added. The drug showed a favorable signal in the RELAX-AHF trial. The results of RELAX II are awaited with interest.
“Today we still don’t have an effective single intervention for acute decompensated heart failure other than process-of-care improvement,” the cardiologist noted.
What holds promise for improved long-term outcomes in ADHF at this point? Dr. Yancy said sacubitril/valsartan (Entresto) is intriguing based upon the results of the PARADIGM-HF trial (N Engl J Med. 2014;371:993-1004). But the drug needs to be studied prospectively in patients in the throes of ADHF before it can appropriately be recommended for the purpose of changing the natural history of this disorder, Dr. Yancy stressed.
Devices such as the implantable pulmonary catheter are under study as a promising means of altering the natural history of ADHF by identifying actionable signals of impending decompensation weeks beforehand, he added.
The TRUE-AHF trial was sponsored by Cardiorentis. Dr. Packer reported serving as a consultant to that company and more than a dozen other pharmaceutical and medical device companies.
bjancin@frontlinemedcom.com
NEW ORLEANS – An investigational synthetic natriuretic peptide given early during hospitalization for acute decompensated heart failure didn’t produce any of the hoped-for intermediate- or long-term clinical benefits in the phase III TRUE-AHF study, Milton Packer, MD, reported at the American Heart Association scientific sessions.
The failure of this investigational vasodilator, ularitide, to influence downstream cardiovascular mortality or early readmission for heart failure closes the door on the once-promising hypothesis that myocardial microinjury occurring during ADHF is due to ventricular distension, observed Dr. Packer, the Distinguished Scholar in Cardiovascular Science at Baylor University Medical Center, Dallas. “Ularitide did exactly what we expected it to do while we were giving it: we caused intravascular decompression, we reduced cardiac wall stress, but we did not affect cardiac microinjury, and we didn’t change long-term cardiovascular mortality or any of our secondary endpoints, including and in particular the 30-day risk of rehospitalization for heart failure,” he said.
During a median follow-up of 15 months there were 236 cardiovascular deaths in the ularitide group and 225 in the control group, a nonsignificant difference. Nor were there any differences between the two groups in secondary endpoints including length of stay in the ICU during the index hospitalization, rehospitalization for ADHF within 30 days of discharge, or the composite of all-cause mortality or cardiovascular hospitalization within 6 months, which occurred in 40.7% of the ularitide group and 37.2% of controls.
The explanation for this lack of long-term benefit lies in the finding that myocardial microinjury wasn’t prevented by the rapid reduction of cardiac distension produced by ularitide. This was evident in the therapy’s inability to dampen the rise in high-sensitivity cardiac troponin T which occurred in the initial 48 hours of the study.
“The trial demonstrated the effects and safety of ularitide. However, to gain long-term benefits on hospitalizations and death in patients following a hospital admission for heart failure, physicians must focus on the drugs that patients take as an outpatient rather than the drugs they receive as an inpatient,” Dr. Packer concluded.
“Readmission rates remain stubbornly at 20% within 30 days and near 50% at 6 months. Acute decompensation is an inflection point in the natural history of heart failure with subsequent 1-year mortality rates consistently approximating 25%. Clearly there is something about the hospitalization that is a herald event which speaks to much worse outcomes, compared with chronic ambulatory heart failure,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
He agreed with Dr. Packer that in light of the TRUE-AHF results, what Dr. Yancy termed “the early injury hypothesis” isn’t worth further pursuit.
Ularitide thus joins a long list of failed therapies for ADHF. Treatments that have convincingly been shown to have no significant impact on mortality and at best only modest impact on morbidity include continuous IV infusion of loop diuretics in the DOSE trial; the arginine vasopressin antagonists, which failed to impress in EVEREST, SECRET, and TACTICS; nesiritide (Natrecor) in the ASCEND-HF trial; and levosimendan (Simdax), which proved disappointing in the SURVIVE and REVIVE II studies, according to Dr. Yancy.
The jury is still out on serelaxin, he added. The drug showed a favorable signal in the RELAX-AHF trial. The results of RELAX II are awaited with interest.
“Today we still don’t have an effective single intervention for acute decompensated heart failure other than process-of-care improvement,” the cardiologist noted.
What holds promise for improved long-term outcomes in ADHF at this point? Dr. Yancy said sacubitril/valsartan (Entresto) is intriguing based upon the results of the PARADIGM-HF trial (N Engl J Med. 2014;371:993-1004). But the drug needs to be studied prospectively in patients in the throes of ADHF before it can appropriately be recommended for the purpose of changing the natural history of this disorder, Dr. Yancy stressed.
Devices such as the implantable pulmonary catheter are under study as a promising means of altering the natural history of ADHF by identifying actionable signals of impending decompensation weeks beforehand, he added.
The TRUE-AHF trial was sponsored by Cardiorentis. Dr. Packer reported serving as a consultant to that company and more than a dozen other pharmaceutical and medical device companies.
bjancin@frontlinemedcom.com
NEW ORLEANS – An investigational synthetic natriuretic peptide given early during hospitalization for acute decompensated heart failure didn’t produce any of the hoped-for intermediate- or long-term clinical benefits in the phase III TRUE-AHF study, Milton Packer, MD, reported at the American Heart Association scientific sessions.
The failure of this investigational vasodilator, ularitide, to influence downstream cardiovascular mortality or early readmission for heart failure closes the door on the once-promising hypothesis that myocardial microinjury occurring during ADHF is due to ventricular distension, observed Dr. Packer, the Distinguished Scholar in Cardiovascular Science at Baylor University Medical Center, Dallas. “Ularitide did exactly what we expected it to do while we were giving it: we caused intravascular decompression, we reduced cardiac wall stress, but we did not affect cardiac microinjury, and we didn’t change long-term cardiovascular mortality or any of our secondary endpoints, including and in particular the 30-day risk of rehospitalization for heart failure,” he said.
During a median follow-up of 15 months there were 236 cardiovascular deaths in the ularitide group and 225 in the control group, a nonsignificant difference. Nor were there any differences between the two groups in secondary endpoints including length of stay in the ICU during the index hospitalization, rehospitalization for ADHF within 30 days of discharge, or the composite of all-cause mortality or cardiovascular hospitalization within 6 months, which occurred in 40.7% of the ularitide group and 37.2% of controls.
The explanation for this lack of long-term benefit lies in the finding that myocardial microinjury wasn’t prevented by the rapid reduction of cardiac distension produced by ularitide. This was evident in the therapy’s inability to dampen the rise in high-sensitivity cardiac troponin T which occurred in the initial 48 hours of the study.
“The trial demonstrated the effects and safety of ularitide. However, to gain long-term benefits on hospitalizations and death in patients following a hospital admission for heart failure, physicians must focus on the drugs that patients take as an outpatient rather than the drugs they receive as an inpatient,” Dr. Packer concluded.
“Readmission rates remain stubbornly at 20% within 30 days and near 50% at 6 months. Acute decompensation is an inflection point in the natural history of heart failure with subsequent 1-year mortality rates consistently approximating 25%. Clearly there is something about the hospitalization that is a herald event which speaks to much worse outcomes, compared with chronic ambulatory heart failure,” said Dr. Yancy, professor of medicine and chief of cardiology at Northwestern University in Chicago.
He agreed with Dr. Packer that in light of the TRUE-AHF results, what Dr. Yancy termed “the early injury hypothesis” isn’t worth further pursuit.
Ularitide thus joins a long list of failed therapies for ADHF. Treatments that have convincingly been shown to have no significant impact on mortality and at best only modest impact on morbidity include continuous IV infusion of loop diuretics in the DOSE trial; the arginine vasopressin antagonists, which failed to impress in EVEREST, SECRET, and TACTICS; nesiritide (Natrecor) in the ASCEND-HF trial; and levosimendan (Simdax), which proved disappointing in the SURVIVE and REVIVE II studies, according to Dr. Yancy.
The jury is still out on serelaxin, he added. The drug showed a favorable signal in the RELAX-AHF trial. The results of RELAX II are awaited with interest.
“Today we still don’t have an effective single intervention for acute decompensated heart failure other than process-of-care improvement,” the cardiologist noted.
What holds promise for improved long-term outcomes in ADHF at this point? Dr. Yancy said sacubitril/valsartan (Entresto) is intriguing based upon the results of the PARADIGM-HF trial (N Engl J Med. 2014;371:993-1004). But the drug needs to be studied prospectively in patients in the throes of ADHF before it can appropriately be recommended for the purpose of changing the natural history of this disorder, Dr. Yancy stressed.
Devices such as the implantable pulmonary catheter are under study as a promising means of altering the natural history of ADHF by identifying actionable signals of impending decompensation weeks beforehand, he added.
The TRUE-AHF trial was sponsored by Cardiorentis. Dr. Packer reported serving as a consultant to that company and more than a dozen other pharmaceutical and medical device companies.
bjancin@frontlinemedcom.com
Key clinical point:
Major finding: Early administration of ularitide during hospitalization for acute decompensated heart failure failed to achieve any long-term clinical benefits.
Data source: The TRUE-AHF trial was a double-blind, placebo-controlled, randomized trial including 2,157 patients hospitalized for acute decompensated heart failure at 156 centers in 23 countries.
Disclosures: The study was sponsored by Cardiorentis. The presenter reported serving as a consultant to that company and more than a dozen other pharmaceutical and medical device companies.
VIDEO: Novel agent inclisiran dramatically lowers LDL
NEW ORLEANS – A single 300-mg injection of inclisiran, a novel non–monoclonal antibody PCSK9 inhibitor, plus statin therapy, achieved a mean 51% reduction in LDL cholesterol, compared with placebo plus a statin, an effect sustained for 90 days.
Moreover, a second subcutaneous 300-mg dose given at day 90 resulted in a 57% reduction in LDL sustained at day 180 with a highly favorable safety profile in the phase II ORION-1 study, Kausik K. Ray, MD, reported at the American Heart Association scientific sessions.
“The efficacy, safety and dosing profile of inclisiran are likely to ensure significant and durable reductions in LDL-C and thus could potentially impact cardiovascular outcomes,” said Dr. Ray, professor of public health at Imperial College London.
Inclisiran is a synthetic RNA interference agent. After injection it selectively goes to the liver, bypassing other tissues and thereby limiting toxicity. Inclisiran halts PCSK9 protein synthesis in the liver, with a resultant sharp, steep, and sustained drop in LDL levels.
Inclisiran achieves LDL lowering of a magnitude similar to that of the PCSK9-inhibiting monoclonal antibodies alirocumab (Praluent) and evolocumab (Repatha), but it will be less expensive to produce and far less costly to administer than the monoclonal antibodies. Dosing is planned to be two or three injections per year, rather than every 2 or 4 weeks, as with the PCSK9 inhibitor monoclonal antibodies, which cost a hefty $14,000 per year. And the sustained efficacy of a dose of inclisiran should make patient adherence to LDL-lowering therapy less of an issue, Dr. Ray continued.
ORION-1 (Inhibition of PCSK9 Synthesis Via RNA Interference) was a double-blind, randomized, placebo-controlled trial comprising 501 patients already on maximally tolerated statin therapy for atherosclerotic cardiovascular disease or at high risk. Nevertheless, their baseline LDL was 125-135 mg/dL. Participants were randomized to one of six doses of inclisiran or placebo. At 90 days they received a second dose. Dr. Ray presented 90- and 180-day outcomes.
This was primarily a safety and dose-ranging study. Side effects at 90 days were no different from placebo regardless of whether patients received 100, 200, 300, or 500 mg of inclisiran. There was no signal of a problem with myalgia or elevated liver enzymes. Mild injection site reactions lasting for more than 4 hours occurred in 1.5%-3.3% of inclisiran-treated patients, but not in a dose-dependent fashion.
One 300-mg dose of inclisiran, in addition to achieving a mean 51% reduction in LDL from baseline at day 90, resulted in a mean 28% reduction in total cholesterol, a modest 10% drop in triglycerides, a 9% increase in HDL, a 37% drop in Apo-B, and a median 23% reduction in Lp(a). Plasma PCSK9 levels plunged in parallel with LDL.
The mean drop in LDL from baseline at day 180 after two 300-mg doses of inclisiran spaced 90 days apart was 65 mg/dL. The LDL reduction ranged from a minimum of 26.5 mg/dL to a maximum of 122 mg/dL.
Dr. Ray’s presentation of the ORION-1 findings followed on the heels of publication of a positive but much smaller phase I study of inclisiran (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1609243).
A large phase II clinical trial with cardiovascular outcomes is planned.
Discussant Borge G. Nordestgaard, MD, could find no fault with ORION-1 or inclisiran’s performance to date.
“A 50%-60% reduction in LDL lasting for 3-6 months, with two or three injections per year – it seems fantastic. And there don’t seem to be side effects except for mild injection site reactions. It looks really, really encouraging, I must say,” said Dr. Nordestgaard, professor of genetic epidemiology at the University of Copenhagen.
“I think the key question is, will the very impressive LDL reduction that we see now be sustainable over time in the longer phase III trials,” he added.
Dr. Ray reported serving as a consultant to the Medicines Company, which together with Alnylam Pharmaceuticals sponsored ORION-1, and receiving research grants from and/or serving as a consultant to more than half a dozen other pharmaceutical companies.
Dr. Ray discussed his findings in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A single 300-mg injection of inclisiran, a novel non–monoclonal antibody PCSK9 inhibitor, plus statin therapy, achieved a mean 51% reduction in LDL cholesterol, compared with placebo plus a statin, an effect sustained for 90 days.
Moreover, a second subcutaneous 300-mg dose given at day 90 resulted in a 57% reduction in LDL sustained at day 180 with a highly favorable safety profile in the phase II ORION-1 study, Kausik K. Ray, MD, reported at the American Heart Association scientific sessions.
“The efficacy, safety and dosing profile of inclisiran are likely to ensure significant and durable reductions in LDL-C and thus could potentially impact cardiovascular outcomes,” said Dr. Ray, professor of public health at Imperial College London.
Inclisiran is a synthetic RNA interference agent. After injection it selectively goes to the liver, bypassing other tissues and thereby limiting toxicity. Inclisiran halts PCSK9 protein synthesis in the liver, with a resultant sharp, steep, and sustained drop in LDL levels.
Inclisiran achieves LDL lowering of a magnitude similar to that of the PCSK9-inhibiting monoclonal antibodies alirocumab (Praluent) and evolocumab (Repatha), but it will be less expensive to produce and far less costly to administer than the monoclonal antibodies. Dosing is planned to be two or three injections per year, rather than every 2 or 4 weeks, as with the PCSK9 inhibitor monoclonal antibodies, which cost a hefty $14,000 per year. And the sustained efficacy of a dose of inclisiran should make patient adherence to LDL-lowering therapy less of an issue, Dr. Ray continued.
ORION-1 (Inhibition of PCSK9 Synthesis Via RNA Interference) was a double-blind, randomized, placebo-controlled trial comprising 501 patients already on maximally tolerated statin therapy for atherosclerotic cardiovascular disease or at high risk. Nevertheless, their baseline LDL was 125-135 mg/dL. Participants were randomized to one of six doses of inclisiran or placebo. At 90 days they received a second dose. Dr. Ray presented 90- and 180-day outcomes.
This was primarily a safety and dose-ranging study. Side effects at 90 days were no different from placebo regardless of whether patients received 100, 200, 300, or 500 mg of inclisiran. There was no signal of a problem with myalgia or elevated liver enzymes. Mild injection site reactions lasting for more than 4 hours occurred in 1.5%-3.3% of inclisiran-treated patients, but not in a dose-dependent fashion.
One 300-mg dose of inclisiran, in addition to achieving a mean 51% reduction in LDL from baseline at day 90, resulted in a mean 28% reduction in total cholesterol, a modest 10% drop in triglycerides, a 9% increase in HDL, a 37% drop in Apo-B, and a median 23% reduction in Lp(a). Plasma PCSK9 levels plunged in parallel with LDL.
The mean drop in LDL from baseline at day 180 after two 300-mg doses of inclisiran spaced 90 days apart was 65 mg/dL. The LDL reduction ranged from a minimum of 26.5 mg/dL to a maximum of 122 mg/dL.
Dr. Ray’s presentation of the ORION-1 findings followed on the heels of publication of a positive but much smaller phase I study of inclisiran (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1609243).
A large phase II clinical trial with cardiovascular outcomes is planned.
Discussant Borge G. Nordestgaard, MD, could find no fault with ORION-1 or inclisiran’s performance to date.
“A 50%-60% reduction in LDL lasting for 3-6 months, with two or three injections per year – it seems fantastic. And there don’t seem to be side effects except for mild injection site reactions. It looks really, really encouraging, I must say,” said Dr. Nordestgaard, professor of genetic epidemiology at the University of Copenhagen.
“I think the key question is, will the very impressive LDL reduction that we see now be sustainable over time in the longer phase III trials,” he added.
Dr. Ray reported serving as a consultant to the Medicines Company, which together with Alnylam Pharmaceuticals sponsored ORION-1, and receiving research grants from and/or serving as a consultant to more than half a dozen other pharmaceutical companies.
Dr. Ray discussed his findings in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – A single 300-mg injection of inclisiran, a novel non–monoclonal antibody PCSK9 inhibitor, plus statin therapy, achieved a mean 51% reduction in LDL cholesterol, compared with placebo plus a statin, an effect sustained for 90 days.
Moreover, a second subcutaneous 300-mg dose given at day 90 resulted in a 57% reduction in LDL sustained at day 180 with a highly favorable safety profile in the phase II ORION-1 study, Kausik K. Ray, MD, reported at the American Heart Association scientific sessions.
“The efficacy, safety and dosing profile of inclisiran are likely to ensure significant and durable reductions in LDL-C and thus could potentially impact cardiovascular outcomes,” said Dr. Ray, professor of public health at Imperial College London.
Inclisiran is a synthetic RNA interference agent. After injection it selectively goes to the liver, bypassing other tissues and thereby limiting toxicity. Inclisiran halts PCSK9 protein synthesis in the liver, with a resultant sharp, steep, and sustained drop in LDL levels.
Inclisiran achieves LDL lowering of a magnitude similar to that of the PCSK9-inhibiting monoclonal antibodies alirocumab (Praluent) and evolocumab (Repatha), but it will be less expensive to produce and far less costly to administer than the monoclonal antibodies. Dosing is planned to be two or three injections per year, rather than every 2 or 4 weeks, as with the PCSK9 inhibitor monoclonal antibodies, which cost a hefty $14,000 per year. And the sustained efficacy of a dose of inclisiran should make patient adherence to LDL-lowering therapy less of an issue, Dr. Ray continued.
ORION-1 (Inhibition of PCSK9 Synthesis Via RNA Interference) was a double-blind, randomized, placebo-controlled trial comprising 501 patients already on maximally tolerated statin therapy for atherosclerotic cardiovascular disease or at high risk. Nevertheless, their baseline LDL was 125-135 mg/dL. Participants were randomized to one of six doses of inclisiran or placebo. At 90 days they received a second dose. Dr. Ray presented 90- and 180-day outcomes.
This was primarily a safety and dose-ranging study. Side effects at 90 days were no different from placebo regardless of whether patients received 100, 200, 300, or 500 mg of inclisiran. There was no signal of a problem with myalgia or elevated liver enzymes. Mild injection site reactions lasting for more than 4 hours occurred in 1.5%-3.3% of inclisiran-treated patients, but not in a dose-dependent fashion.
One 300-mg dose of inclisiran, in addition to achieving a mean 51% reduction in LDL from baseline at day 90, resulted in a mean 28% reduction in total cholesterol, a modest 10% drop in triglycerides, a 9% increase in HDL, a 37% drop in Apo-B, and a median 23% reduction in Lp(a). Plasma PCSK9 levels plunged in parallel with LDL.
The mean drop in LDL from baseline at day 180 after two 300-mg doses of inclisiran spaced 90 days apart was 65 mg/dL. The LDL reduction ranged from a minimum of 26.5 mg/dL to a maximum of 122 mg/dL.
Dr. Ray’s presentation of the ORION-1 findings followed on the heels of publication of a positive but much smaller phase I study of inclisiran (N Engl J Med. 2016 Nov 13. doi: 10.1056/NEJMoa1609243).
A large phase II clinical trial with cardiovascular outcomes is planned.
Discussant Borge G. Nordestgaard, MD, could find no fault with ORION-1 or inclisiran’s performance to date.
“A 50%-60% reduction in LDL lasting for 3-6 months, with two or three injections per year – it seems fantastic. And there don’t seem to be side effects except for mild injection site reactions. It looks really, really encouraging, I must say,” said Dr. Nordestgaard, professor of genetic epidemiology at the University of Copenhagen.
“I think the key question is, will the very impressive LDL reduction that we see now be sustainable over time in the longer phase III trials,” he added.
Dr. Ray reported serving as a consultant to the Medicines Company, which together with Alnylam Pharmaceuticals sponsored ORION-1, and receiving research grants from and/or serving as a consultant to more than half a dozen other pharmaceutical companies.
Dr. Ray discussed his findings in a video interview at the meeting.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
AT THE AHA SCIENTIFIC SESSIONS
Key clinical point:
Major finding: A single 300-mg subcutaneous injection of inclisiran quickly dropped elevated LDL-cholesterol levels by 51% and kept them there for 90 days.
Data source: ORION-1, a double-blind, randomized, placebo-controlled, phase II study conducted in 501 patients assigned to various doses of inclisiran or placebo on top of maximally tolerated statin therapy.
Disclosures: Dr. Ray reported serving as a consultant to the Medicines Company, which together with Alnylam Pharmaceuticals sponsored ORION-1, and receiving research grants from and/or serving as a consultant to more than half a dozen other pharmaceutical companies.
Interatrial shunt benefits sustained for 1 year in HFpEF patients
NEW ORLEANS – An interatrial septal shunt device continued to provide “sustained and meaningful clinical benefit” at 1-year follow-up for 64 patients who had heart failure with preserved ejection fraction (HFpEF), David M. Kaye, MD, PhD, reported at the American Heart Association scientific sessions.
The device is implanted via cardiac catheterization and is intended to reduce elevated left atrial pressure, particularly that associated with exertion, by allowing a small amount but not excessive left-to-right shunting. Patients showed improvements in 6-minute walk distance, New York Heart Association class, and HF-related quality of life scores at 6 months, and those effects persisted at the most recent (12-month) follow-up, he said in a presentation that was simultaneously published online in Circulation (2016 Nov 16).
Overall survival at 1 year was 95%. Three patients died (one from combined pneumonia and renal failure, one from a fatal stroke, and one from an undetermined cause) and one was lost to follow-up. Thirteen patients required 17 hospitalizations for heart failure.
Six-minute walk distance improved from 331 meters at baseline to 363 meters. NYHA classification improved dramatically, as did quality of life scores as assessed by the Minnesota Living with HF questionnaire.
All 48 devices that were evaluable on echocardiographic imaging remained patent, showing continued left-to-right shunting. Left ventricular ejection fraction remained unchanged while right ventricular ejection fraction was significantly elevated over baseline levels. “In conjunction, there were modest but stable reductions in LV end-diastolic volume index with a concomitant rise in RV end-diastolic index,” he said.
A subset of 18 study participants underwent heart catheterization during both rest and exercise so that hemodynamics could be assessed. Exercise time increased significantly, from 8.2 minutes at baseline to 9.7 minutes at 6 months and to 10.4 minutes at 1 year. Similarly, peak work capacity during supine cycling increased from 48 watts at baseline to 60 watts at 6 months and 55 watts at 1 year. These benefits occurred without any increase in pulmonary capillary wedge pressure.
Systemic blood pressure did not change over time, either at rest or during exercise. Left and right atrial volumes also remained unchanged.
Perhaps most importantly, Dr. Kaye said, right-sided cardiac output increased significantly, while left-sided cardiac output remained unchanged. There was no evidence of increased pulmonary pressure or pulmonary vascular resistance. This meant that patients could do more physical activity for a given level of left atrial pressure, he said.
Furthermore, 1-year mortality was lower in this trial, at 4.6%, than in the placebo groups of the I-PRESERVE trial in irbesartan (5.2%) and the U.S. group of the TOPCAT trial in spironolactone (7.7%), said Dr. Kaye, professor and chief of cardiology at the University of Arizona, Tuscon.
Device therapy could have an enormous impact in carefully selected patients with HFpEF, for whom there are no medical treatments, despite the nonrandomized nature of the trial.
REDUCE LAP-HF was funded by Corvia Medical, maker of the shunt device. Dr. Kaye is an unpaid member of Corvia’s scientific advisory group. Dr. Sweitzer is an investigator in the ongoing randomized trial of the interatrial shunt.
NEW ORLEANS – An interatrial septal shunt device continued to provide “sustained and meaningful clinical benefit” at 1-year follow-up for 64 patients who had heart failure with preserved ejection fraction (HFpEF), David M. Kaye, MD, PhD, reported at the American Heart Association scientific sessions.
The device is implanted via cardiac catheterization and is intended to reduce elevated left atrial pressure, particularly that associated with exertion, by allowing a small amount but not excessive left-to-right shunting. Patients showed improvements in 6-minute walk distance, New York Heart Association class, and HF-related quality of life scores at 6 months, and those effects persisted at the most recent (12-month) follow-up, he said in a presentation that was simultaneously published online in Circulation (2016 Nov 16).
Overall survival at 1 year was 95%. Three patients died (one from combined pneumonia and renal failure, one from a fatal stroke, and one from an undetermined cause) and one was lost to follow-up. Thirteen patients required 17 hospitalizations for heart failure.
Six-minute walk distance improved from 331 meters at baseline to 363 meters. NYHA classification improved dramatically, as did quality of life scores as assessed by the Minnesota Living with HF questionnaire.
All 48 devices that were evaluable on echocardiographic imaging remained patent, showing continued left-to-right shunting. Left ventricular ejection fraction remained unchanged while right ventricular ejection fraction was significantly elevated over baseline levels. “In conjunction, there were modest but stable reductions in LV end-diastolic volume index with a concomitant rise in RV end-diastolic index,” he said.
A subset of 18 study participants underwent heart catheterization during both rest and exercise so that hemodynamics could be assessed. Exercise time increased significantly, from 8.2 minutes at baseline to 9.7 minutes at 6 months and to 10.4 minutes at 1 year. Similarly, peak work capacity during supine cycling increased from 48 watts at baseline to 60 watts at 6 months and 55 watts at 1 year. These benefits occurred without any increase in pulmonary capillary wedge pressure.
Systemic blood pressure did not change over time, either at rest or during exercise. Left and right atrial volumes also remained unchanged.
Perhaps most importantly, Dr. Kaye said, right-sided cardiac output increased significantly, while left-sided cardiac output remained unchanged. There was no evidence of increased pulmonary pressure or pulmonary vascular resistance. This meant that patients could do more physical activity for a given level of left atrial pressure, he said.
Furthermore, 1-year mortality was lower in this trial, at 4.6%, than in the placebo groups of the I-PRESERVE trial in irbesartan (5.2%) and the U.S. group of the TOPCAT trial in spironolactone (7.7%), said Dr. Kaye, professor and chief of cardiology at the University of Arizona, Tuscon.
Device therapy could have an enormous impact in carefully selected patients with HFpEF, for whom there are no medical treatments, despite the nonrandomized nature of the trial.
REDUCE LAP-HF was funded by Corvia Medical, maker of the shunt device. Dr. Kaye is an unpaid member of Corvia’s scientific advisory group. Dr. Sweitzer is an investigator in the ongoing randomized trial of the interatrial shunt.
NEW ORLEANS – An interatrial septal shunt device continued to provide “sustained and meaningful clinical benefit” at 1-year follow-up for 64 patients who had heart failure with preserved ejection fraction (HFpEF), David M. Kaye, MD, PhD, reported at the American Heart Association scientific sessions.
The device is implanted via cardiac catheterization and is intended to reduce elevated left atrial pressure, particularly that associated with exertion, by allowing a small amount but not excessive left-to-right shunting. Patients showed improvements in 6-minute walk distance, New York Heart Association class, and HF-related quality of life scores at 6 months, and those effects persisted at the most recent (12-month) follow-up, he said in a presentation that was simultaneously published online in Circulation (2016 Nov 16).
Overall survival at 1 year was 95%. Three patients died (one from combined pneumonia and renal failure, one from a fatal stroke, and one from an undetermined cause) and one was lost to follow-up. Thirteen patients required 17 hospitalizations for heart failure.
Six-minute walk distance improved from 331 meters at baseline to 363 meters. NYHA classification improved dramatically, as did quality of life scores as assessed by the Minnesota Living with HF questionnaire.
All 48 devices that were evaluable on echocardiographic imaging remained patent, showing continued left-to-right shunting. Left ventricular ejection fraction remained unchanged while right ventricular ejection fraction was significantly elevated over baseline levels. “In conjunction, there were modest but stable reductions in LV end-diastolic volume index with a concomitant rise in RV end-diastolic index,” he said.
A subset of 18 study participants underwent heart catheterization during both rest and exercise so that hemodynamics could be assessed. Exercise time increased significantly, from 8.2 minutes at baseline to 9.7 minutes at 6 months and to 10.4 minutes at 1 year. Similarly, peak work capacity during supine cycling increased from 48 watts at baseline to 60 watts at 6 months and 55 watts at 1 year. These benefits occurred without any increase in pulmonary capillary wedge pressure.
Systemic blood pressure did not change over time, either at rest or during exercise. Left and right atrial volumes also remained unchanged.
Perhaps most importantly, Dr. Kaye said, right-sided cardiac output increased significantly, while left-sided cardiac output remained unchanged. There was no evidence of increased pulmonary pressure or pulmonary vascular resistance. This meant that patients could do more physical activity for a given level of left atrial pressure, he said.
Furthermore, 1-year mortality was lower in this trial, at 4.6%, than in the placebo groups of the I-PRESERVE trial in irbesartan (5.2%) and the U.S. group of the TOPCAT trial in spironolactone (7.7%), said Dr. Kaye, professor and chief of cardiology at the University of Arizona, Tuscon.
Device therapy could have an enormous impact in carefully selected patients with HFpEF, for whom there are no medical treatments, despite the nonrandomized nature of the trial.
REDUCE LAP-HF was funded by Corvia Medical, maker of the shunt device. Dr. Kaye is an unpaid member of Corvia’s scientific advisory group. Dr. Sweitzer is an investigator in the ongoing randomized trial of the interatrial shunt.
AT THE AHA SCIENTIFIC SESSIONS 2016
Key clinical point: An interatrial septal shunt device continued to provide sustained and meaningful clinical benefit at 1-year follow-up for 64 patients who had heart failure with preserved ejection fraction.
Major finding: Six-minute walk distance improved from 331 meters at baseline to 363 meters at 1 year, NYHA classification improved dramatically, and HF-related quality of life scores also improved.
Data source: REDUCE LAP-HF, a multicenter, prospective, open-label study involving 64 patients followed for 1 year after transcatheter implantation of a shunt device.
Disclosures: REDUCE LAP-HF was funded by Corvia Medical, maker of the shunt device. Dr. Kaye is an unpaid member of Corvia’s scientific advisory group.
VIDEO: Bariatric surgery may protect against heart failure
NEW ORLEANS – Results of a new 40,000-patient Swedish observational study provide the strongest evidence to date suggesting a causal relationship between bariatric surgery and reduced risk of heart failure, according to Johan Sundström, MD.
The study, which included patients drawn from two large Swedish national registries, demonstrated that bariatric surgery was associated with a 46% reduction in the incidence of heart failure during a median 4.1 years of follow-up, compared with an intensive lifestyle modification program for weight loss.
“These are observational data, but it’s a very large study population – and probably there will never be a large randomized trial of bariatric surgery versus weight loss through intensive lifestyle modification as a means of reducing the risk of heart failure,” Dr. Sundström, professor of epidemiology and a cardiologist at Uppsala (Sweden) University, said at the American Heart Association scientific sessions.
The study included 25,804 bariatric surgery patients in SOReg, the Scandinavian Obesity Surgery Registry, and a matched comparator group of 13,701 participants in a Swedish national registry of obese participants in a commercial Sweden-based intensive structural lifestyle modification program for weight loss called Itrim. The two groups were matched for baseline body mass index, which was a mean of 41.5 kg, and numerous other demographic factors and comorbid conditions. Participants weighed an average of 119 kg at baseline. None of the subjects had a history of heart failure.
The bariatric surgery group lost substantially more weight than did the lifestyle modification group: an average loss of about 35 kg after 1 year, which was 18.8 kg more than in the lifestyle modification group. After 2 years, the bariatric surgery group had an average of 22.6 kg more weight loss than did the comparison group.
The primary outcome was hospitalization for new-onset heart failure during a median 4.1 years of follow-up. Subjects were well below the age range when the incidence of heart failure accelerates – they averaged 41 years of age – but 73 of them did develop heart failure during follow-up. The incidence was 46% lower in the bariatric surgery patients. This supports the study hypothesis that bariatric surgery leads to a low incidence of new-onset heart failure, compared with intensive lifestyle modification because of its larger weight loss effect.
When Dr. Sundström and his coinvestigators combined the two study groups, they found that a 10-kg weight loss at 1 year was associated with a 23% reduction in the risk of heart failure during follow-up, irrespective of whether the weight loss was achieved surgically or through the lifestyle program.
“A great way of studying causality is to take away the exposure and note what happens to the outcome. If there’s a causal link, then if you take away the risk factor – in this case, obesity – the disease should go away,” he explained in a video interview.
The reduced risk of heart failure in the bariatric surgery patients wasn’t because of fewer acute MIs. Indeed, their acute MI rate during follow-up was similar to that of the lifestyle modification group. But bariatric surgery was associated with relative risk reductions of 35%-37% for atrial fibrillation or need for diabetes or blood pressure–lowering medications at 1 year – and atrial fibrillation, diabetes, and hypertension are all established risk factors for heart failure, Dr. Sundström noted.
The Itrim intensive lifestyle modification program entailed an initial very-low-energy diet for the first 3 months in order to achieve massive weight loss, followed by a 9-month maintenance program involving motivational counseling, exercise, behavioral therapy, and a restricted diet.
Dr. Sundström said he and his coinvestigators plan to continue the study and expand it to look at differences in additional cardiovascular endpoints as patients age.
The study was funded by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, Uppsala University, the Karolinska Institute, and the Swedish Research Council. Dr. Sundström reported serving as a scientific advisor to Itrim.
NEW ORLEANS – Results of a new 40,000-patient Swedish observational study provide the strongest evidence to date suggesting a causal relationship between bariatric surgery and reduced risk of heart failure, according to Johan Sundström, MD.
The study, which included patients drawn from two large Swedish national registries, demonstrated that bariatric surgery was associated with a 46% reduction in the incidence of heart failure during a median 4.1 years of follow-up, compared with an intensive lifestyle modification program for weight loss.
“These are observational data, but it’s a very large study population – and probably there will never be a large randomized trial of bariatric surgery versus weight loss through intensive lifestyle modification as a means of reducing the risk of heart failure,” Dr. Sundström, professor of epidemiology and a cardiologist at Uppsala (Sweden) University, said at the American Heart Association scientific sessions.
The study included 25,804 bariatric surgery patients in SOReg, the Scandinavian Obesity Surgery Registry, and a matched comparator group of 13,701 participants in a Swedish national registry of obese participants in a commercial Sweden-based intensive structural lifestyle modification program for weight loss called Itrim. The two groups were matched for baseline body mass index, which was a mean of 41.5 kg, and numerous other demographic factors and comorbid conditions. Participants weighed an average of 119 kg at baseline. None of the subjects had a history of heart failure.
The bariatric surgery group lost substantially more weight than did the lifestyle modification group: an average loss of about 35 kg after 1 year, which was 18.8 kg more than in the lifestyle modification group. After 2 years, the bariatric surgery group had an average of 22.6 kg more weight loss than did the comparison group.
The primary outcome was hospitalization for new-onset heart failure during a median 4.1 years of follow-up. Subjects were well below the age range when the incidence of heart failure accelerates – they averaged 41 years of age – but 73 of them did develop heart failure during follow-up. The incidence was 46% lower in the bariatric surgery patients. This supports the study hypothesis that bariatric surgery leads to a low incidence of new-onset heart failure, compared with intensive lifestyle modification because of its larger weight loss effect.
When Dr. Sundström and his coinvestigators combined the two study groups, they found that a 10-kg weight loss at 1 year was associated with a 23% reduction in the risk of heart failure during follow-up, irrespective of whether the weight loss was achieved surgically or through the lifestyle program.
“A great way of studying causality is to take away the exposure and note what happens to the outcome. If there’s a causal link, then if you take away the risk factor – in this case, obesity – the disease should go away,” he explained in a video interview.
The reduced risk of heart failure in the bariatric surgery patients wasn’t because of fewer acute MIs. Indeed, their acute MI rate during follow-up was similar to that of the lifestyle modification group. But bariatric surgery was associated with relative risk reductions of 35%-37% for atrial fibrillation or need for diabetes or blood pressure–lowering medications at 1 year – and atrial fibrillation, diabetes, and hypertension are all established risk factors for heart failure, Dr. Sundström noted.
The Itrim intensive lifestyle modification program entailed an initial very-low-energy diet for the first 3 months in order to achieve massive weight loss, followed by a 9-month maintenance program involving motivational counseling, exercise, behavioral therapy, and a restricted diet.
Dr. Sundström said he and his coinvestigators plan to continue the study and expand it to look at differences in additional cardiovascular endpoints as patients age.
The study was funded by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, Uppsala University, the Karolinska Institute, and the Swedish Research Council. Dr. Sundström reported serving as a scientific advisor to Itrim.
NEW ORLEANS – Results of a new 40,000-patient Swedish observational study provide the strongest evidence to date suggesting a causal relationship between bariatric surgery and reduced risk of heart failure, according to Johan Sundström, MD.
The study, which included patients drawn from two large Swedish national registries, demonstrated that bariatric surgery was associated with a 46% reduction in the incidence of heart failure during a median 4.1 years of follow-up, compared with an intensive lifestyle modification program for weight loss.
“These are observational data, but it’s a very large study population – and probably there will never be a large randomized trial of bariatric surgery versus weight loss through intensive lifestyle modification as a means of reducing the risk of heart failure,” Dr. Sundström, professor of epidemiology and a cardiologist at Uppsala (Sweden) University, said at the American Heart Association scientific sessions.
The study included 25,804 bariatric surgery patients in SOReg, the Scandinavian Obesity Surgery Registry, and a matched comparator group of 13,701 participants in a Swedish national registry of obese participants in a commercial Sweden-based intensive structural lifestyle modification program for weight loss called Itrim. The two groups were matched for baseline body mass index, which was a mean of 41.5 kg, and numerous other demographic factors and comorbid conditions. Participants weighed an average of 119 kg at baseline. None of the subjects had a history of heart failure.
The bariatric surgery group lost substantially more weight than did the lifestyle modification group: an average loss of about 35 kg after 1 year, which was 18.8 kg more than in the lifestyle modification group. After 2 years, the bariatric surgery group had an average of 22.6 kg more weight loss than did the comparison group.
The primary outcome was hospitalization for new-onset heart failure during a median 4.1 years of follow-up. Subjects were well below the age range when the incidence of heart failure accelerates – they averaged 41 years of age – but 73 of them did develop heart failure during follow-up. The incidence was 46% lower in the bariatric surgery patients. This supports the study hypothesis that bariatric surgery leads to a low incidence of new-onset heart failure, compared with intensive lifestyle modification because of its larger weight loss effect.
When Dr. Sundström and his coinvestigators combined the two study groups, they found that a 10-kg weight loss at 1 year was associated with a 23% reduction in the risk of heart failure during follow-up, irrespective of whether the weight loss was achieved surgically or through the lifestyle program.
“A great way of studying causality is to take away the exposure and note what happens to the outcome. If there’s a causal link, then if you take away the risk factor – in this case, obesity – the disease should go away,” he explained in a video interview.
The reduced risk of heart failure in the bariatric surgery patients wasn’t because of fewer acute MIs. Indeed, their acute MI rate during follow-up was similar to that of the lifestyle modification group. But bariatric surgery was associated with relative risk reductions of 35%-37% for atrial fibrillation or need for diabetes or blood pressure–lowering medications at 1 year – and atrial fibrillation, diabetes, and hypertension are all established risk factors for heart failure, Dr. Sundström noted.
The Itrim intensive lifestyle modification program entailed an initial very-low-energy diet for the first 3 months in order to achieve massive weight loss, followed by a 9-month maintenance program involving motivational counseling, exercise, behavioral therapy, and a restricted diet.
Dr. Sundström said he and his coinvestigators plan to continue the study and expand it to look at differences in additional cardiovascular endpoints as patients age.
The study was funded by the U.S. National Institute of Diabetes and Digestive and Kidney Diseases, Uppsala University, the Karolinska Institute, and the Swedish Research Council. Dr. Sundström reported serving as a scientific advisor to Itrim.
AT THE AHA SCIENTIFIC SESSIONS 2016
Key clinical point:
Major finding: The incidence of new-onset heart failure was 46% lower during follow-up after bariatric surgery than among participants in an intensive lifestyle modification program for weight loss.
Data source: This observational registry study followed nearly 26,000 Swedish bariatric surgery patients and 14,000 matched participants in a commercial intensive lifestyle modification program for a median of 4.1 years.
Disclosures: The study was funded by the U.S. National Institutes of Diabetes and Digestive and Kidney Diseases, Uppsala University, the Karolinska Institute, and the Swedish Research Council. The presenter reported serving as a scientific advisor to Itrim.