User login
Reflectance confocal microscopy: The future looks bright
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
CHICAGO – The future looks bright for to rule out malignancy, Ann M. John, MD, asserted at the annual meeting of the American College of Mohs Surgery.
“With the advent of dermoscopy, dermatologists were able to elucidate both benign and malignant patterns to help further guide their decision to biopsy or not. This increased diagnostic accuracy of suspicious lesions by 30%, while reducing the benign to malignant ratio of biopsies performed from 18:1 to 4:1. However, there are still lesions that are equivocal on dermoscopy, as we all know, and for this, there’s reflectance confocal microscopy,” observed Dr. John, of Robert Wood Johnson Medical School, New Brunswick, N.J.
RCM is a device technology that’s been cleared by the Food and Drug Administration since 2008 for the imaging of clinically suspicious lesions. It employs laser scanning to assess the light-scattering properties of cells in the epidermis and dermis, generating images with resolution comparable to histology.
RCM took a back seat initially while American dermatologists were gradually coming to embrace dermoscopy, which their European colleagues had done years earlier. Now, with the availability of handheld RCM for use in the dermatology clinic, expect RCM to assume a growing role in daily practice.
To illustrate the power of RCM as a diagnostic aid, she presented a single-center retrospective study of 1,189 clinically suspicious skin lesions that were equivocal on dermoscopy and then assessed using RCM with 1 year of subsequent patient follow-up. Overall, 155 lesions were deemed positive for cancer or atypia by RCM, while 1,034 were determined to be benign. Of those 155, 46 lesions were considered false positives because of their benign appearance on histologic inspection of the biopsy sample. Only 2 of the 1,034 lesions identified as negative by RCM proved to be false negatives on the basis of clinical changes within 1 year.
The overall sensitivity and specificity of RCM was 98.2% and 99.8%, respectively, with a positive predictive value of 70.3% and a negative predictive value of 99.8%.
The entire RCM procedure takes a skilled technician 15-20 minutes per lesion. As a practical matter, other investigators have estimated that RCM results in a cost savings of about $308,000 per million health plan members per year by reducing the need for biopsies (Dermatol Clin. 2016 Oct;34[4]:367-75).
In addition to evaluating clinically suspicious lesions, other situations in which RCM offers practical value include its use directly before the first cut during Mohs surgery in order to determine the margins of atypia; ex vivo imaging of Mohs margins, which has been shown to be comparable with frozen sections in accuracy but takes only one-third of the time; and imaging of biopsied lesions in order to determine the diagnosis relatively quickly, Dr. John noted.
She reported having no financial conflicts regarding her study.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: The future looks bright for reflectance confocal microscopy in dermatology.
Major finding: The sensitivity and specificity of reflectance confocal microscopy for diagnosis of skin cancer in patients with equivocal dermoscopic findings was 98.2% and 99.8%, respectively.
Study details: This retrospective single center study included 1,189 clinically suspicious skin lesions with equivocal dermoscopy findings, which were then evaluated using reflectance confocal microscopy.
Disclosures: The presenter reported having no financial conflicts regarding her study.
Mohs underutilized for melanoma of head and neck
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.
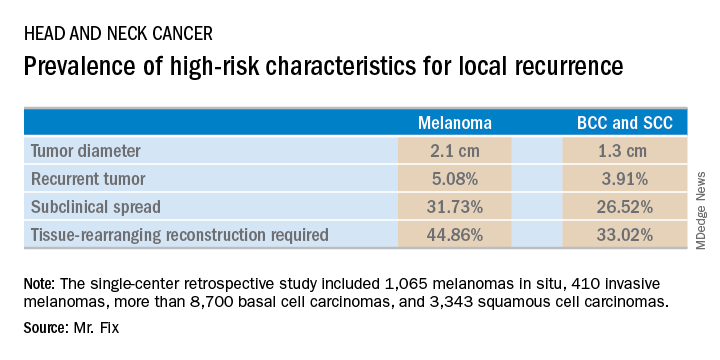
Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.

Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
CHICAGO – Contemporary national guidelines undervalue the benefits of Mohs micrographic surgery for patients with melanoma of the head and neck, William C. Fix asserted at the annual meeting of the American College of Mohs Surgery.

Mr. Fix, a medical student at the University of Pennsylvania, Philadelphia, presented a single-center retrospective study of 13,644 cases of head and neck skin cancer treated with Mohs micrographic surgery (MMS) for margin control. The cohort included 1,065 melanomas in situ, 410 invasive melanomas, more than 8,700 basal cell carcinomas, and 3,343 squamous cell carcinomas.
Mr. Fix and his coinvestigators undertook this observational study because they identified a gap in current guidelines for treatment of skin cancers of the head and neck. For example, the National Comprehensive Cancer Network recommends margin control at the time of primary surgery for BCCs and SCCs deemed at high risk for local recurrence and defines what those high-risk features are. For melanomas, however, the guidelines recommend wide local excision, even though that approach has roughly a 10% recurrence rate, compared with less than 1% for MMS.
Moreover, the 2012 appropriate use criteria for MMS put forth by the American Academy of Dermatology in concert with several other medical societies are unclear about invasive melanoma. As a result of this lack of guidance, the use of margin control in primary surgery for melanoma is applied in less than 4% of cases, according to Mr. Fix.
The University of Pennsylvania data he presented showed that melanomas of the head and neck were significantly more likely to be large in size, to be poorly defined, and to have other high-risk features for local recurrence than were the BCCs and SCCs. In a multivariate logistic regression analysis controlling for high-risk characteristics, melanomas were independently associated with a twofold increased likelihood of requiring flap reconstruction compared with BCCs and SCCs of the head and neck.
“We’ve shown that melanomas have high-risk features for local recurrence, possibly to a greater extent than BCCs and SCCs. These features help us triage resource use for BCC and SCC. Could these same features help us make decisions for melanomas?” he asked rhetorically.
Mr. Fix reported having no financial conflicts of interest regarding his study, which was conducted free of commercial support.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point: Margin control at the time of primary surgery for melanoma of the head and neck makes sense.
Major finding: Patients with a melanoma of the head and neck were twice as likely to require secondary flap reconstruction compared with patients with a basal cell carcinoma or squamous cell carcinoma of the head and neck.
Study details: A retrospective single-center study of 13,644 cases of skin cancer of the head and neck treated with Mohs surgery.
Disclosures: The presenter reported having no financial conflicts regarding the study, which was conducted free of commercial support.
Itraconazole for BCC: Does it work?
CHICAGO – Justin J. Leitenberger, MD, declared at the annual meeting of the American College of Mohs Surgery.
The oral antifungal inhibits the hedgehog signaling pathway, a key driver of BCC. And while itraconazole is not as potent an inhibitor of hedgehog pathway expression as, say vismodegib (Erivedge), the antifungal acts at a separate site on the pathway, making it an attractive candidate for combination therapy, explained Dr. Leitenberger of Oregon Health & Science University, Portland.
Dermatologists at Stanford University have led the way in exploring oral itraconazole as a treatment for BCC. Among a series of 29 patients with one or more large but nonadvanced BCCs, 19 were treated using oral itraconazole at 200 mg b.i.d. for 1 month or 100 mg b.i.d. for an average of 2.3 months. Hedgehog pathway expression was reduced by 65% in the itraconazole-treated patients, as compared with the 90% reduction which is achieved with vismodegib.
Of more direct clinical relevance, however, itraconazole also reduced tumor area by 24%. Four of eight patients with 57 tumors achieved a partial response, and the other four had stable disease (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
The Stanford group has also shown that combination therapy with oral itraconazole and intravenous arsenic trioxide reduces hedgehog pathway expression by 75%, up by an absolute 10% from itraconazole alone. The two agents inhibit the pathway at different sites.
Five patients with metastatic BCC who relapsed after treatment with vismodegib received intravenous arsenic trioxide for the first 5 days of every month, followed by oral itraconazole at 200 mg b.i.d. on days 6-28. Three patients completed three such cycles, while two discontinued early because of progressive disease or adverse events including a grade 3 infection and grade 2 transaminitis. All three patients who completed three treatment cycles achieved stable disease. The Stanford investigators speculated that concurrent continuous dosing might be required to obtain tumor shrinkage (JAMA Dermatol. 2016 Apr;152[4]:452-6).
Lots more work remains to be done in order to optimize combination therapy utilizing oral itraconazole for advanced BCC. Different dosing regimens and combinations of hedgehog pathway inhibitors need to be studied. Another important question is how effective itraconazole-based combinations will be in vismodegib-naive as compared with vismodegib-resistant patients, Dr. Leitenberger observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Justin J. Leitenberger, MD, declared at the annual meeting of the American College of Mohs Surgery.
The oral antifungal inhibits the hedgehog signaling pathway, a key driver of BCC. And while itraconazole is not as potent an inhibitor of hedgehog pathway expression as, say vismodegib (Erivedge), the antifungal acts at a separate site on the pathway, making it an attractive candidate for combination therapy, explained Dr. Leitenberger of Oregon Health & Science University, Portland.
Dermatologists at Stanford University have led the way in exploring oral itraconazole as a treatment for BCC. Among a series of 29 patients with one or more large but nonadvanced BCCs, 19 were treated using oral itraconazole at 200 mg b.i.d. for 1 month or 100 mg b.i.d. for an average of 2.3 months. Hedgehog pathway expression was reduced by 65% in the itraconazole-treated patients, as compared with the 90% reduction which is achieved with vismodegib.
Of more direct clinical relevance, however, itraconazole also reduced tumor area by 24%. Four of eight patients with 57 tumors achieved a partial response, and the other four had stable disease (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
The Stanford group has also shown that combination therapy with oral itraconazole and intravenous arsenic trioxide reduces hedgehog pathway expression by 75%, up by an absolute 10% from itraconazole alone. The two agents inhibit the pathway at different sites.
Five patients with metastatic BCC who relapsed after treatment with vismodegib received intravenous arsenic trioxide for the first 5 days of every month, followed by oral itraconazole at 200 mg b.i.d. on days 6-28. Three patients completed three such cycles, while two discontinued early because of progressive disease or adverse events including a grade 3 infection and grade 2 transaminitis. All three patients who completed three treatment cycles achieved stable disease. The Stanford investigators speculated that concurrent continuous dosing might be required to obtain tumor shrinkage (JAMA Dermatol. 2016 Apr;152[4]:452-6).
Lots more work remains to be done in order to optimize combination therapy utilizing oral itraconazole for advanced BCC. Different dosing regimens and combinations of hedgehog pathway inhibitors need to be studied. Another important question is how effective itraconazole-based combinations will be in vismodegib-naive as compared with vismodegib-resistant patients, Dr. Leitenberger observed.
He reported having no financial conflicts regarding his presentation.
CHICAGO – Justin J. Leitenberger, MD, declared at the annual meeting of the American College of Mohs Surgery.
The oral antifungal inhibits the hedgehog signaling pathway, a key driver of BCC. And while itraconazole is not as potent an inhibitor of hedgehog pathway expression as, say vismodegib (Erivedge), the antifungal acts at a separate site on the pathway, making it an attractive candidate for combination therapy, explained Dr. Leitenberger of Oregon Health & Science University, Portland.
Dermatologists at Stanford University have led the way in exploring oral itraconazole as a treatment for BCC. Among a series of 29 patients with one or more large but nonadvanced BCCs, 19 were treated using oral itraconazole at 200 mg b.i.d. for 1 month or 100 mg b.i.d. for an average of 2.3 months. Hedgehog pathway expression was reduced by 65% in the itraconazole-treated patients, as compared with the 90% reduction which is achieved with vismodegib.
Of more direct clinical relevance, however, itraconazole also reduced tumor area by 24%. Four of eight patients with 57 tumors achieved a partial response, and the other four had stable disease (J Clin Oncol. 2014 Mar 10;32[8]:745-51).
The Stanford group has also shown that combination therapy with oral itraconazole and intravenous arsenic trioxide reduces hedgehog pathway expression by 75%, up by an absolute 10% from itraconazole alone. The two agents inhibit the pathway at different sites.
Five patients with metastatic BCC who relapsed after treatment with vismodegib received intravenous arsenic trioxide for the first 5 days of every month, followed by oral itraconazole at 200 mg b.i.d. on days 6-28. Three patients completed three such cycles, while two discontinued early because of progressive disease or adverse events including a grade 3 infection and grade 2 transaminitis. All three patients who completed three treatment cycles achieved stable disease. The Stanford investigators speculated that concurrent continuous dosing might be required to obtain tumor shrinkage (JAMA Dermatol. 2016 Apr;152[4]:452-6).
Lots more work remains to be done in order to optimize combination therapy utilizing oral itraconazole for advanced BCC. Different dosing regimens and combinations of hedgehog pathway inhibitors need to be studied. Another important question is how effective itraconazole-based combinations will be in vismodegib-naive as compared with vismodegib-resistant patients, Dr. Leitenberger observed.
He reported having no financial conflicts regarding his presentation.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Cost-effective wound healing described with fetal bovine collagen matrix
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
CHICAGO – A novel, commercially available fetal bovine collagen matrix provides “an ideal wound healing environment” for outpatient treatment of partial and full thickness wounds, ulcers, burns, and surgical wounds, Katarina R. Kesty, MD, declared at the annual meeting of the American College of Mohs Surgery.
“. We applied this product to 46 patients over 10 months and have observed favorable healing times and good cosmesis,” said Dr. Kesty, a dermatology resident at Wake Forest University, Winston-Salem, N.C.
She shared the clinical experience she and her colleagues have accrued with this product, which is called PriMatrix and is manufactured by Integra LifeSciences. She also explained how to successfully code and bill for its use.
“In-office application of this product is cost-effective when compared to similar products applied in the operating room by plastic surgeons and other specialists,” Dr. Kesty noted.
How cost-effective? She provided one example of a patient with a 12.6-cm2 defect on the scalp repaired with fetal bovine collagen matrix. Upon application of the appropriate billing codes, this repair was reimbursed by Medicare to the tune of $1,208. In contrast, another patient at Wake Forest had a 16.6-cm2 Mohs defect on the scalp repaired in the operating room by an oculoplastic surgeon who used split thickness skin grafts. For this procedure, Medicare was billed $30,805.11, and the medical center received $9,241.53 in reimbursement.
“An office repair using this fetal bovine collagen matrix is much more cost-effective,” she observed. “It also saves the patient from the risks of general anesthesia or conscious sedation.”
PriMatrix is a porous acellular collagen matrix derived from fetal bovine dermis. It contains type I and type III collagen, with the latter being particularly effective at attracting growth factors, blood, and angiogenic cytokines in support of dermal regeneration and revascularization. The product is available in solid sheets, mesh, and fenestrated forms in a variety of sizes. It needs to be rehydrated for 1 minute in room temperature saline. It can then be cut to the size of the wound and secured to the wound bed, periosteum, fascia, or cartilage with sutures or staples. The site is then covered with a thick layer of petrolatum and a tie-over bolster.
Dr. Kesty and her dermatology colleagues have applied the matrix to surgical defects ranging in size from 0.2 cm2 to 70 cm2, with an average area of 19 cm2. They have utilized the mesh format most often in order to allow drainage. They found the average healing time when the matrix was applied to exposed bone, periosteum, or perichondrium was 13.8 weeks, compared with 10.8 weeks for subcutaneous wounds.
With the use of the fetal bovine collagen matrix, wounds less than 10 cm2 in size healed in an average of 9.3 weeks, those from 10 cm2 to 25 cm2 in size healed in an average of 10.4 weeks, and wounds larger than 25 cm2 healed in an average of 15.7 weeks.
Coding and reimbursement
PriMatrix has been available for outpatient office use and reimbursement by Medicare since January 2017. Successful reimbursement requires completion of a preauthorization form, which is typically approved on the same day by Medicare and other payers. The proper CPT codes are 1527x, signifying a skin substitute graft less than 100 cm2 in size; Q4110 times the number of 1-cm2 units of PriMatrix utilized; and, when appropriate, ICD10 code Z85.828, for personal history of nonmelanoma skin cancer.
Dr. Kesty reported no financial conflicts of interest.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Registry data provide evidence that Mohs surgery remains underutilized
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
CHICAGO – Analysis of U.S. national cancer registry data shows that, contrary to expectation, the , Sean Condon, MD, reported at the annual meeting of the American College of Mohs Surgery.
Ditto for the use of Mohs in patients with the rare cutaneous malignancies for which published evidence clearly demonstrates Mohs outperforms wide local excision, which is employed seven times more frequently than Mohs in such situations.
“Mohs utilization did not increase after the Affordable Care Act [ACA], despite new health insurance coverage for 20 million previously uninsured adults,” Dr. Condon said. “Surprisingly, after the ACA we actually saw a decrease in Mohs use for melanoma in situ.”
Indeed, his retrospective study of more than 25,000 patients in the National Cancer Institute’s SEER (Surveillance, Epidemiology, and End Results) registries showed that the proportion of patients with melanoma in situ treated with Mohs declined from 13.9% during 2008-2009 – prior to ACA implementation – to 12.3% in 2011-2013, after the ACA took effect. That’s a statistically significant 13% drop, even though numerous published studies have shown outcomes in melanoma in situ are better with Mohs, said the dermatologist, who conducted the study while completing a Mohs surgery fellowship at the Cleveland Clinic. He is now in private practice in Thousand Oaks, Calif.
His analysis included 19,013 patients treated in 2008-2014 for melanoma in situ and 6,309 others treated for rare cutaneous malignancies deemed appropriate for Mohs according to the criteria formally developed jointly by the American Academy of Dermatology, the American College of Mohs Surgery, the American Society for Dermatologic Surgery Association, and the American Society for Mohs Surgery (J Am Acad Dermatol. 2012 Oct;67[4]:531-50). These rare malignancies include adnexal carcinoma, Merkel cell carcinoma, dermatofibrosarcoma, extramammary Paget disease, sebaceous adenocarcinoma, and leiomyosarcoma.
“These rare cutaneous malignancies were historically treated with wide local excision. However, numerous studies have lately shown that lower recurrence rates were found with Mohs compared with wide local excision,” Dr. Condon noted.
Nonetheless, the proportion of the rare cutaneous malignancies treated using Mohs was unaffected by implementation of the ACA. Nor was it influenced one way or the other by publication of the joint Mohs appropriate use criteria in 2012: The Mohs-treated proportion of such cases was 15.25% in 2010-2011 and 14.6% in 2013-2014.
Similarly, even though the appropriate use criteria identified melanoma in situ as Mohs appropriate, the proportion of those malignancies treated via Mohs was the same before and after the 2012 release of the criteria.
“It’s commonly thought that Mohs is overused. However, our study and our data clearly identify that Mohs is being underutilized for melanoma in situ and for rare cutaneous malignancies. This represents a knowledge gap for other specialties regarding best-practice therapy,” Dr. Condon said.
He and his coinvestigators searched for socioeconomic predictors of Mohs utilization by matching the nationally representative SEER data with U.S. census data. They examined the impact of three metrics: insurance status, income, and poverty. They found that low-income patients and those in the highest quartile of poverty were significantly less likely to have Mohs surgery for their melanoma in situ and rare cutaneous malignancies throughout the study years. Lack of health insurance had no impact on Mohs utilization for melanoma in situ but was independently associated with decreased likelihood of Mohs for the rare cutaneous malignancies. White patients were 2-fold to 2.4-fold more likely to have Mohs surgery for their rare cutaneous malignancies than were black patients.
“One can conclude that Mohs micrographic surgery may be skewed toward more affluent patients, and lower socioeconomic status areas have less Mohs access. So our data from this study support a role for targeted education and improved patient access to Mohs,” Dr. Condon said.
He noted that because the SEER registries don’t track squamous or basal cell carcinomas, it’s unknown whether Mohs is also underutilized for the higher-risk forms of these most common of all skin cancers.
Dr. Condon reported having no financial conflicts regarding his study, conducted free of commercial support.
REPORTING FROM THE ACMS 50TH ANNUAL MEETING
Key clinical point: Mohs micrographic surgery remains seriously underutilized for the skin cancers for which it is most advantageous.
Major finding: The use of Mohs micrographic surgery to treat melanoma in situ declined significantly after passage of the Affordable Care Act.
Study details: This was a retrospective study of national SEER data on more than 25,000 patients treated for melanoma in situ or rare cutaneous malignancies during 2008-2014.
Disclosures: The presenter reported having no financial conflicts regarding his study, conducted free of commercial support.
Study pinpoints skin cancer risk factors after hematopoietic cell transplant
CHICAGO – The 10-year incidence rates for both squamous cell carcinoma and basal cell carcinoma arising after hematopoietic cell transplantation are impressively high at 17%-plus for each, but the malignancies occur on two very different timelines, according to Jeffrey F. Scott, MD, a fellow in micrographic surgery and dermatologic oncology at Case Western Reserve University in Cleveland.
Most of the squamous cell carcinomas (SCCs) in a large multicenter retrospective study developed within the first 5 years following hematopoietic cell transplantation (HCT), while the majority of the basal cell carcinomas (BCCs) occurred after that point, Dr. Scott reported at the annual meeting of the American College of Mohs Surgery.
He presented the results of the study, which included 876 HCT recipients followed for a mean of 6.1 years. The study objective was to pin down the risk factors for skin cancer after HCT, especially the patient-specific ones. This has become a pressing issue because the use of HCT is steadily growing, and the 5-year survival rate now exceeds 50%.
The transplant-specific risk factors have previously been fairly well described by others. They include the donor source, type of disease, the conditioning regimen, whether whole body irradiation was used, immunosuppression, graft versus host disease (GVHD), and others.
The patient-centric risk factors, in contrast, have not been well characterized. And it’s critical to thoroughly understand these risk factors in order to develop targeted prevention and surveillance strategies, Dr. Scott said.
“There remains a significant knowledge gap within our field. I would venture that the majority of this audience has treated a patient with skin cancer who has had a transplant,” he said. “Yet when a patient asks us, ‘Doc, what is my risk for skin cancer after my HCT?’ we’re really unable to give them an accurate and complete assessment of that risk. That’s because we’re missing the second major category of risk factors: the patient-specific risk factors.”
The reason for that, he added, is that the major population-based studies and national HCT registries are run by hematologists and oncologists, and they haven’t adequately captured the patient-specific skin cancer risk factors. But these are variables very familiar to dermatologists. They include skin phenotype, history of UV radiation exposure, and history of pre-HCT skin cancer.
Dr. Scott said the multicenter study he presented has two major advantages over prior studies: its large size and thorough followup. Nearly all 876 patients were followed by both an oncologist and a dermatologist at the same institution.
During followup, the HCT recipients collectively developed 63 SCCs, 55 BCCs, and 16 malignant melanomas. The 5- and 10-year incidence rates for SCC were 10.6% and 17.2%. For BCC, the 5- and 10-year rates were 5.7% and 17.6%. All 16 cases of melanoma occurred within 5 years after HCT.
In multivariate Cox proportional hazard analyses, photodamage documented on examination was independently associated with a 3.2-fold increased risk of post-HCT SCC and a 3.5-fold increased risk of BCC.
A pre-transplant history of BCC was associated with a 3.9-fold increased likelihood of developing a BCC afterwards. Similarly, a pre-HCT history of SCC conferred a 4.2-fold increased risk of post-transplant SCC and was also independently associated with a 6.6-fold increased risk of developing melanoma post-HCT.
Fitzpatrick skin types I and II were respectively associated with 9.3- and 7.2-fold increased risks of post-HCT nonmelanoma skin cancer, compared with skin types III-VI.
Acute GVHD wasn’t associated with an increased risk of nonmelanoma skin cancer after HCT. However, in an observation that hasn’t previously been reported by others, chronic GVHD with skin involvement was associated with a 2.7-fold increased likelihood of SCC post-HCT, Dr. Scott noted.
What’s next for Dr. Scott and his coinvestigators? “Our ultimate goal with this project is to develop an interactive risk assessment tool like the National Cancer Institute’s Breast Cancer Risk Assessment Tool that can be online and used by patients and providers to estimate their individualized risk of basal cell carcinoma, squamous cell carcinoma, and melanoma after HCT,” he said.
Dr. Scott reported having no financial conflicts related to the study.
CHICAGO – The 10-year incidence rates for both squamous cell carcinoma and basal cell carcinoma arising after hematopoietic cell transplantation are impressively high at 17%-plus for each, but the malignancies occur on two very different timelines, according to Jeffrey F. Scott, MD, a fellow in micrographic surgery and dermatologic oncology at Case Western Reserve University in Cleveland.
Most of the squamous cell carcinomas (SCCs) in a large multicenter retrospective study developed within the first 5 years following hematopoietic cell transplantation (HCT), while the majority of the basal cell carcinomas (BCCs) occurred after that point, Dr. Scott reported at the annual meeting of the American College of Mohs Surgery.
He presented the results of the study, which included 876 HCT recipients followed for a mean of 6.1 years. The study objective was to pin down the risk factors for skin cancer after HCT, especially the patient-specific ones. This has become a pressing issue because the use of HCT is steadily growing, and the 5-year survival rate now exceeds 50%.
The transplant-specific risk factors have previously been fairly well described by others. They include the donor source, type of disease, the conditioning regimen, whether whole body irradiation was used, immunosuppression, graft versus host disease (GVHD), and others.
The patient-centric risk factors, in contrast, have not been well characterized. And it’s critical to thoroughly understand these risk factors in order to develop targeted prevention and surveillance strategies, Dr. Scott said.
“There remains a significant knowledge gap within our field. I would venture that the majority of this audience has treated a patient with skin cancer who has had a transplant,” he said. “Yet when a patient asks us, ‘Doc, what is my risk for skin cancer after my HCT?’ we’re really unable to give them an accurate and complete assessment of that risk. That’s because we’re missing the second major category of risk factors: the patient-specific risk factors.”
The reason for that, he added, is that the major population-based studies and national HCT registries are run by hematologists and oncologists, and they haven’t adequately captured the patient-specific skin cancer risk factors. But these are variables very familiar to dermatologists. They include skin phenotype, history of UV radiation exposure, and history of pre-HCT skin cancer.
Dr. Scott said the multicenter study he presented has two major advantages over prior studies: its large size and thorough followup. Nearly all 876 patients were followed by both an oncologist and a dermatologist at the same institution.
During followup, the HCT recipients collectively developed 63 SCCs, 55 BCCs, and 16 malignant melanomas. The 5- and 10-year incidence rates for SCC were 10.6% and 17.2%. For BCC, the 5- and 10-year rates were 5.7% and 17.6%. All 16 cases of melanoma occurred within 5 years after HCT.
In multivariate Cox proportional hazard analyses, photodamage documented on examination was independently associated with a 3.2-fold increased risk of post-HCT SCC and a 3.5-fold increased risk of BCC.
A pre-transplant history of BCC was associated with a 3.9-fold increased likelihood of developing a BCC afterwards. Similarly, a pre-HCT history of SCC conferred a 4.2-fold increased risk of post-transplant SCC and was also independently associated with a 6.6-fold increased risk of developing melanoma post-HCT.
Fitzpatrick skin types I and II were respectively associated with 9.3- and 7.2-fold increased risks of post-HCT nonmelanoma skin cancer, compared with skin types III-VI.
Acute GVHD wasn’t associated with an increased risk of nonmelanoma skin cancer after HCT. However, in an observation that hasn’t previously been reported by others, chronic GVHD with skin involvement was associated with a 2.7-fold increased likelihood of SCC post-HCT, Dr. Scott noted.
What’s next for Dr. Scott and his coinvestigators? “Our ultimate goal with this project is to develop an interactive risk assessment tool like the National Cancer Institute’s Breast Cancer Risk Assessment Tool that can be online and used by patients and providers to estimate their individualized risk of basal cell carcinoma, squamous cell carcinoma, and melanoma after HCT,” he said.
Dr. Scott reported having no financial conflicts related to the study.
CHICAGO – The 10-year incidence rates for both squamous cell carcinoma and basal cell carcinoma arising after hematopoietic cell transplantation are impressively high at 17%-plus for each, but the malignancies occur on two very different timelines, according to Jeffrey F. Scott, MD, a fellow in micrographic surgery and dermatologic oncology at Case Western Reserve University in Cleveland.
Most of the squamous cell carcinomas (SCCs) in a large multicenter retrospective study developed within the first 5 years following hematopoietic cell transplantation (HCT), while the majority of the basal cell carcinomas (BCCs) occurred after that point, Dr. Scott reported at the annual meeting of the American College of Mohs Surgery.
He presented the results of the study, which included 876 HCT recipients followed for a mean of 6.1 years. The study objective was to pin down the risk factors for skin cancer after HCT, especially the patient-specific ones. This has become a pressing issue because the use of HCT is steadily growing, and the 5-year survival rate now exceeds 50%.
The transplant-specific risk factors have previously been fairly well described by others. They include the donor source, type of disease, the conditioning regimen, whether whole body irradiation was used, immunosuppression, graft versus host disease (GVHD), and others.
The patient-centric risk factors, in contrast, have not been well characterized. And it’s critical to thoroughly understand these risk factors in order to develop targeted prevention and surveillance strategies, Dr. Scott said.
“There remains a significant knowledge gap within our field. I would venture that the majority of this audience has treated a patient with skin cancer who has had a transplant,” he said. “Yet when a patient asks us, ‘Doc, what is my risk for skin cancer after my HCT?’ we’re really unable to give them an accurate and complete assessment of that risk. That’s because we’re missing the second major category of risk factors: the patient-specific risk factors.”
The reason for that, he added, is that the major population-based studies and national HCT registries are run by hematologists and oncologists, and they haven’t adequately captured the patient-specific skin cancer risk factors. But these are variables very familiar to dermatologists. They include skin phenotype, history of UV radiation exposure, and history of pre-HCT skin cancer.
Dr. Scott said the multicenter study he presented has two major advantages over prior studies: its large size and thorough followup. Nearly all 876 patients were followed by both an oncologist and a dermatologist at the same institution.
During followup, the HCT recipients collectively developed 63 SCCs, 55 BCCs, and 16 malignant melanomas. The 5- and 10-year incidence rates for SCC were 10.6% and 17.2%. For BCC, the 5- and 10-year rates were 5.7% and 17.6%. All 16 cases of melanoma occurred within 5 years after HCT.
In multivariate Cox proportional hazard analyses, photodamage documented on examination was independently associated with a 3.2-fold increased risk of post-HCT SCC and a 3.5-fold increased risk of BCC.
A pre-transplant history of BCC was associated with a 3.9-fold increased likelihood of developing a BCC afterwards. Similarly, a pre-HCT history of SCC conferred a 4.2-fold increased risk of post-transplant SCC and was also independently associated with a 6.6-fold increased risk of developing melanoma post-HCT.
Fitzpatrick skin types I and II were respectively associated with 9.3- and 7.2-fold increased risks of post-HCT nonmelanoma skin cancer, compared with skin types III-VI.
Acute GVHD wasn’t associated with an increased risk of nonmelanoma skin cancer after HCT. However, in an observation that hasn’t previously been reported by others, chronic GVHD with skin involvement was associated with a 2.7-fold increased likelihood of SCC post-HCT, Dr. Scott noted.
What’s next for Dr. Scott and his coinvestigators? “Our ultimate goal with this project is to develop an interactive risk assessment tool like the National Cancer Institute’s Breast Cancer Risk Assessment Tool that can be online and used by patients and providers to estimate their individualized risk of basal cell carcinoma, squamous cell carcinoma, and melanoma after HCT,” he said.
Dr. Scott reported having no financial conflicts related to the study.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point:
Major finding: Photodamage documented on examination more than triples the risk of developing nonmelanoma skin cancer after hematopoietic cell transplantation.
Study details: A multicenter retrospective study of 876 hematopoietic cell recipients followed for a mean of 6.1 years.
Disclosures: The presenter reported having no financial conflicts related to the study, which was conducted without commercial support.
Spotlight on nonmelanoma skin cancer’s true burden
CHICAGO – The true extent of the burden imposed by nonmelanoma skin cancer remains widely underappreciated by health policy makers, the public, employers, and nondermatologist physicians, Marta J. Van Beek, MD, asserted at the annual meeting of the American College of Mohs Surgery.
It’s very much in the interest of Mohs surgeons, as the experts in cutaneous malignancies, to get the accurate message out, she added.
Abundant evidence indicates there is an ongoing epidemic of nonmelanoma skin cancer (NMSC) in the United States – and it is associated with a surprising amount of morbidity and mortality, the dermatologic surgeon observed.
For example, while the American Academy of Dermatology’s 93-page Burden of Skin Disease report identified melanoma as the No. 1 cause of mortality because of skin disease – no surprise there – what may come as news to many is that NMSC was No. 2, accounting for 4,376 deaths in 2013, or 19% of the total. That’s more deaths than occurred because of wounds and burns.
And while the number of cases of NMSC is going up year after year as the population ages, it’s also the case that patients with complex NMSC are developing it at a younger and younger age. As documented in the AAD’s DataDerm registry encompassing more than 6 million patients seen by dermatologists during 2015-2017, well over 20,000 patients who underwent Mohs micrographic surgery for NMSC were aged 45-55 years, and another 60,000 were aged 55-65 years. That being said, Mohs surgery was used to treat 477,365 NMSCs in 318,933 patients included in DataDerm during 2015-2017, and in that population, basal cell carcinomas outnumbered squamous cell carcinomas 2:1.
An interesting aspect of the burden imparted by NMSC is that patients with NMSC have a higher risk of other types of cancer, and when they develop those other primary cancers they tend to do more poorly than cancer patients without a history of NMSC, Dr. Van Beek continued.
She cited a comprehensive study by investigators at the Medical University of South Carolina, Charleston, who concluded that the odds of developing a noncutaneous second primary malignancy were 27% greater in individuals with a history of NMSC than in those without such a history. The increased risk was statistically significant for 26 types of noncutaneous cancer, consistent in both men and women, and the younger a patient’s age at onset of NMSC, the stronger the association with noncutaneous cancers (Adv Cancer Res. 2016;130:257-91).
In a separate systematic review by some of the same investigators, patients with a history of squamous cell carcinoma were at a 30% increased risk of all-cause mortality and 117% greater cancer-specific mortality than those without a history of the disease. The associations were less potent for basal cell carcinoma (Arch Dermatol Res. 2017 May;309[4]:243-51).
“You are more likely to die of your nonskin cancer if you’ve ever had a skin cancer, regardless of what that other cancer is. This may mean that once you have a skin cancer, maybe that proves you have poor protoplasm that makes you more prone to other cancers, but even if that’s the case I think it demonstrates that nonmelanoma skin cancer has a substantial contribution to morbidity and mortality outside of what we normally think about,” Dr. Van Beek said.
Another underappreciated aspect of the burden of NMSC is what economists call lost opportunity cost. This isn’t the direct medical cost, but work time missed because of disease. In 2013, according to the AAD Burden of Skin Disease report, melanoma was responsible for $88 million worth of lost productivity, while for NMSC, the figure was $376 million.
“When you’re talking about the burden of disease, it’s important to actually talk to employers about how important it is to pay for the treatment of skin cancer because that keeps people at work and productive,” the dermatologist said.
Investigators for the World Health Organization’s Global Burden of Disease project estimate that the total years lost to disability for patients with NMSC are comparable with the figures for patients with thyroid, esophageal, or ovarian cancer, Dr. Van Beek noted.
Payers and health policy makers are unnerved by the growing utilization of Mohs surgery, she warned.
“This is really important: If you want to substantiate our utilization, you have to make policy makers understand that we are doing this because more people have skin cancer,” she emphasized.
Dr. Van Beek reported no financial conflicts regarding her presentation.
CHICAGO – The true extent of the burden imposed by nonmelanoma skin cancer remains widely underappreciated by health policy makers, the public, employers, and nondermatologist physicians, Marta J. Van Beek, MD, asserted at the annual meeting of the American College of Mohs Surgery.
It’s very much in the interest of Mohs surgeons, as the experts in cutaneous malignancies, to get the accurate message out, she added.
Abundant evidence indicates there is an ongoing epidemic of nonmelanoma skin cancer (NMSC) in the United States – and it is associated with a surprising amount of morbidity and mortality, the dermatologic surgeon observed.
For example, while the American Academy of Dermatology’s 93-page Burden of Skin Disease report identified melanoma as the No. 1 cause of mortality because of skin disease – no surprise there – what may come as news to many is that NMSC was No. 2, accounting for 4,376 deaths in 2013, or 19% of the total. That’s more deaths than occurred because of wounds and burns.
And while the number of cases of NMSC is going up year after year as the population ages, it’s also the case that patients with complex NMSC are developing it at a younger and younger age. As documented in the AAD’s DataDerm registry encompassing more than 6 million patients seen by dermatologists during 2015-2017, well over 20,000 patients who underwent Mohs micrographic surgery for NMSC were aged 45-55 years, and another 60,000 were aged 55-65 years. That being said, Mohs surgery was used to treat 477,365 NMSCs in 318,933 patients included in DataDerm during 2015-2017, and in that population, basal cell carcinomas outnumbered squamous cell carcinomas 2:1.
An interesting aspect of the burden imparted by NMSC is that patients with NMSC have a higher risk of other types of cancer, and when they develop those other primary cancers they tend to do more poorly than cancer patients without a history of NMSC, Dr. Van Beek continued.
She cited a comprehensive study by investigators at the Medical University of South Carolina, Charleston, who concluded that the odds of developing a noncutaneous second primary malignancy were 27% greater in individuals with a history of NMSC than in those without such a history. The increased risk was statistically significant for 26 types of noncutaneous cancer, consistent in both men and women, and the younger a patient’s age at onset of NMSC, the stronger the association with noncutaneous cancers (Adv Cancer Res. 2016;130:257-91).
In a separate systematic review by some of the same investigators, patients with a history of squamous cell carcinoma were at a 30% increased risk of all-cause mortality and 117% greater cancer-specific mortality than those without a history of the disease. The associations were less potent for basal cell carcinoma (Arch Dermatol Res. 2017 May;309[4]:243-51).
“You are more likely to die of your nonskin cancer if you’ve ever had a skin cancer, regardless of what that other cancer is. This may mean that once you have a skin cancer, maybe that proves you have poor protoplasm that makes you more prone to other cancers, but even if that’s the case I think it demonstrates that nonmelanoma skin cancer has a substantial contribution to morbidity and mortality outside of what we normally think about,” Dr. Van Beek said.
Another underappreciated aspect of the burden of NMSC is what economists call lost opportunity cost. This isn’t the direct medical cost, but work time missed because of disease. In 2013, according to the AAD Burden of Skin Disease report, melanoma was responsible for $88 million worth of lost productivity, while for NMSC, the figure was $376 million.
“When you’re talking about the burden of disease, it’s important to actually talk to employers about how important it is to pay for the treatment of skin cancer because that keeps people at work and productive,” the dermatologist said.
Investigators for the World Health Organization’s Global Burden of Disease project estimate that the total years lost to disability for patients with NMSC are comparable with the figures for patients with thyroid, esophageal, or ovarian cancer, Dr. Van Beek noted.
Payers and health policy makers are unnerved by the growing utilization of Mohs surgery, she warned.
“This is really important: If you want to substantiate our utilization, you have to make policy makers understand that we are doing this because more people have skin cancer,” she emphasized.
Dr. Van Beek reported no financial conflicts regarding her presentation.
CHICAGO – The true extent of the burden imposed by nonmelanoma skin cancer remains widely underappreciated by health policy makers, the public, employers, and nondermatologist physicians, Marta J. Van Beek, MD, asserted at the annual meeting of the American College of Mohs Surgery.
It’s very much in the interest of Mohs surgeons, as the experts in cutaneous malignancies, to get the accurate message out, she added.
Abundant evidence indicates there is an ongoing epidemic of nonmelanoma skin cancer (NMSC) in the United States – and it is associated with a surprising amount of morbidity and mortality, the dermatologic surgeon observed.
For example, while the American Academy of Dermatology’s 93-page Burden of Skin Disease report identified melanoma as the No. 1 cause of mortality because of skin disease – no surprise there – what may come as news to many is that NMSC was No. 2, accounting for 4,376 deaths in 2013, or 19% of the total. That’s more deaths than occurred because of wounds and burns.
And while the number of cases of NMSC is going up year after year as the population ages, it’s also the case that patients with complex NMSC are developing it at a younger and younger age. As documented in the AAD’s DataDerm registry encompassing more than 6 million patients seen by dermatologists during 2015-2017, well over 20,000 patients who underwent Mohs micrographic surgery for NMSC were aged 45-55 years, and another 60,000 were aged 55-65 years. That being said, Mohs surgery was used to treat 477,365 NMSCs in 318,933 patients included in DataDerm during 2015-2017, and in that population, basal cell carcinomas outnumbered squamous cell carcinomas 2:1.
An interesting aspect of the burden imparted by NMSC is that patients with NMSC have a higher risk of other types of cancer, and when they develop those other primary cancers they tend to do more poorly than cancer patients without a history of NMSC, Dr. Van Beek continued.
She cited a comprehensive study by investigators at the Medical University of South Carolina, Charleston, who concluded that the odds of developing a noncutaneous second primary malignancy were 27% greater in individuals with a history of NMSC than in those without such a history. The increased risk was statistically significant for 26 types of noncutaneous cancer, consistent in both men and women, and the younger a patient’s age at onset of NMSC, the stronger the association with noncutaneous cancers (Adv Cancer Res. 2016;130:257-91).
In a separate systematic review by some of the same investigators, patients with a history of squamous cell carcinoma were at a 30% increased risk of all-cause mortality and 117% greater cancer-specific mortality than those without a history of the disease. The associations were less potent for basal cell carcinoma (Arch Dermatol Res. 2017 May;309[4]:243-51).
“You are more likely to die of your nonskin cancer if you’ve ever had a skin cancer, regardless of what that other cancer is. This may mean that once you have a skin cancer, maybe that proves you have poor protoplasm that makes you more prone to other cancers, but even if that’s the case I think it demonstrates that nonmelanoma skin cancer has a substantial contribution to morbidity and mortality outside of what we normally think about,” Dr. Van Beek said.
Another underappreciated aspect of the burden of NMSC is what economists call lost opportunity cost. This isn’t the direct medical cost, but work time missed because of disease. In 2013, according to the AAD Burden of Skin Disease report, melanoma was responsible for $88 million worth of lost productivity, while for NMSC, the figure was $376 million.
“When you’re talking about the burden of disease, it’s important to actually talk to employers about how important it is to pay for the treatment of skin cancer because that keeps people at work and productive,” the dermatologist said.
Investigators for the World Health Organization’s Global Burden of Disease project estimate that the total years lost to disability for patients with NMSC are comparable with the figures for patients with thyroid, esophageal, or ovarian cancer, Dr. Van Beek noted.
Payers and health policy makers are unnerved by the growing utilization of Mohs surgery, she warned.
“This is really important: If you want to substantiate our utilization, you have to make policy makers understand that we are doing this because more people have skin cancer,” she emphasized.
Dr. Van Beek reported no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Cemiplimab impresses in advanced CSCC
CHICAGO – The investigational programmed cell death protein 1 checkpoint inhibitor cemiplimab proved highly effective for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in a phase 1 clinical trial, Michael R. Migden, MD, reported at the annual meeting of the American College of Mohs Surgery.
And this was no ordinary phase 1 study, he noted. Because there is no Food and Drug Administration–approved treatment for advanced cutaneous squamous cell carcinoma (CSCC), cemiplimab has been granted both Breakthrough Drug and Orphan Drug status by the FDA and the European Medicines Agency.
Given the likelihood that cemiplimab will receive expeditious regulatory approval to address this major unmet need, he offered his colleagues practical tips on its use, including information about the drug’s chief side effects as well as a heads-up regarding the importance of early recognition of the pseudoprogression phenomenon that can occur with the drug.
He predicted this fully human monoclonal antibody directed at programmed cell death protein 1 (PD-1) is going to be an important drug for Mohs surgeons.
“Immunotherapy is becoming increasingly relevant to micrographic surgery and dermatologic oncology practice and fellowship training. Care for larger, advanced CSCC falls within our scope of practice and we should play an essential role, inclusive of multidisciplinary care, in the management and follow-up of these patients,” asserted Dr. Migden, a dermatologic surgeon at the University of Texas MD Anderson Cancer Center, Houston.
The open-label, phase 1 study included seven patients with distant metastatic CSCC and nine with locally and/or regionally advanced disease. They were treated with 3 mg/kg IV cemiplimab every 2 weeks for 48 weeks, with Response Evaluation Criteria In Solid Tumors 1.1 criteria used for assessment of response status every 8 weeks. More than 80% of the tumors were located in the head and neck. The great majority of study participants had previously been treated with radiation therapy and systemic agents, to little effect.
The complete response rate at 48 weeks was 12.5%, with tumor clearance occurring as quickly as 14 weeks. Another 25% of patients had a partial response, for an overall response rate of 37.5%. But that’s not the full success story, as another 31% of patients had stable disease. Thus, 11 of 16 patients, or 69%, experienced disease control.
“A disease-control rate of nearly 70% is really important because these are patients with life-threatening tumors. To be able to hold them steady is a big deal,” Dr. Migden observed.
One-quarter of study participants experienced progressive disease. The remainder weren’t evaluated for various reasons.
The dermatologist pointed out that locally advanced disease was particularly responsive to cemiplimab, with four of nine affected patients experiencing complete or partial response, for an overall response rate of 44%. This is consistent with the preliminary results of the pivotal phase 2 study, in which the overall response rate in the 78 participants with unresectable, locally advanced CSCC was 46%.
The phase 2 trial also includes another 59 patients with metastatic CSCC on 3 mg/kg IV cemiplimab every 14 days, as well as 56 patients with metastatic disease assigned to flat-dose 350-mg IV cemiplimab every 21 days.
Treatment side effects
In the phase 1 study, immunotherapy with cemiplimab was far better tolerated than in traditional cancer chemotherapy. There were two grade 3 cases of elevated liver enzymes and one of arthralgia, but no significant fatigue or nausea and no hypothyroidism. However, judging from the cumulative experience accrued with the five PD-1 checkpoint inhibitors already approved for treatment of other cancers, one must be prepared to encounter hypothyroidism and other endocrinopathies, pneumonitis, hepatitis, and rashes.
“The clinician must have a very high index of suspicion for these immune-related adverse events and a low threshold to consult with colleagues in other specialties – pulmonary, endocrine, and medical oncology – for evaluation and management of these possible side effects. I tell all the patients who are on cemiplimab, ‘Any new anything – a slight cough, mild diarrhea – you’re coming in and you’re getting checked,’ ” according to Dr. Migden.
That being said, the majority of immune-related adverse events because of PD-1 inhibitors are mild to moderate. Of the few that reach grade 3 or above, most can be successfully managed by pausing or discontinuing anti–PD-1 therapy coupled with prompt initiation of immunosuppressive therapy, typically with high-dose steroids, he added.
Look sharp for pseudoprogression
Pseudoprogression is a phenomenon whereby immunotherapy results in inflammatory changes bringing about a temporary increase in tumor size that precedes tumor shrinkage. It’s uncommon, occurring in 3 of 16 patients in the phase 1 study. The mechanism probably involves tumor infiltration by massive numbers of activated T cells. And there is evidence from other PD-1 inhibitor studies in advanced cancers that pseudoprogression may actually be a marker for increased likelihood of survival beyond 1 year.
“Pseudoprogression is important to recognize because the patients you treat with cemiplimab can get worse before they get better,” the dermatologist explained. “So you don’t want to prematurely discontinue treatment because you’re misclassifying it as tumor progression.”
The rationale for anti-PD-1 therapy in CSCC
Tumors that express PD-1 bind to PD–ligand 1 on T cells, switching off T-cell mediated tumor destruction and thereby allowing the malignancy to thrive.
“Simplified, the strategy here is to interfere with the interaction at the T-cell off switch, either with an antibody to PD–ligand 1, such as atezolizumab [Tecentriq], or an antibody to the PD-1 receptor, where cemiplimab works. By turning off the off switch, we get a T cell fully on and attacking the tumor cell,” Dr. Migden said.
“The more the tumor mutation burden, the better immunotherapy works – and CSCC has the highest tumor mutation burden of any tumor type in the Cancer Genome Atlas, several times higher than melanoma. Interestingly, basal cell carcinoma has an even higher tumor mutation burden than CSCC, but it’s not part of the atlas,” he continued.
Although the proportion of CSCCs that are locally advanced hasn’t been well established, it’s clear that CSCC is the deadliest nonmelanoma skin cancer, accounting for 3,900-8,800 deaths annually in the United States.
The cemiplimab phase 1 and 2 clinical trials for CSCC were jointly sponsored by Regeneron and Sanofi. The monoclonal antibody is also being developed for treatment of myeloma and lung cancer. Dr. Migden reported receiving honoraria from Regeneron and Sanofi, as well as from Genentech, Lilly, Novartis, and Sun Pharmaceuticals.
CHICAGO – The investigational programmed cell death protein 1 checkpoint inhibitor cemiplimab proved highly effective for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in a phase 1 clinical trial, Michael R. Migden, MD, reported at the annual meeting of the American College of Mohs Surgery.
And this was no ordinary phase 1 study, he noted. Because there is no Food and Drug Administration–approved treatment for advanced cutaneous squamous cell carcinoma (CSCC), cemiplimab has been granted both Breakthrough Drug and Orphan Drug status by the FDA and the European Medicines Agency.
Given the likelihood that cemiplimab will receive expeditious regulatory approval to address this major unmet need, he offered his colleagues practical tips on its use, including information about the drug’s chief side effects as well as a heads-up regarding the importance of early recognition of the pseudoprogression phenomenon that can occur with the drug.
He predicted this fully human monoclonal antibody directed at programmed cell death protein 1 (PD-1) is going to be an important drug for Mohs surgeons.
“Immunotherapy is becoming increasingly relevant to micrographic surgery and dermatologic oncology practice and fellowship training. Care for larger, advanced CSCC falls within our scope of practice and we should play an essential role, inclusive of multidisciplinary care, in the management and follow-up of these patients,” asserted Dr. Migden, a dermatologic surgeon at the University of Texas MD Anderson Cancer Center, Houston.
The open-label, phase 1 study included seven patients with distant metastatic CSCC and nine with locally and/or regionally advanced disease. They were treated with 3 mg/kg IV cemiplimab every 2 weeks for 48 weeks, with Response Evaluation Criteria In Solid Tumors 1.1 criteria used for assessment of response status every 8 weeks. More than 80% of the tumors were located in the head and neck. The great majority of study participants had previously been treated with radiation therapy and systemic agents, to little effect.
The complete response rate at 48 weeks was 12.5%, with tumor clearance occurring as quickly as 14 weeks. Another 25% of patients had a partial response, for an overall response rate of 37.5%. But that’s not the full success story, as another 31% of patients had stable disease. Thus, 11 of 16 patients, or 69%, experienced disease control.
“A disease-control rate of nearly 70% is really important because these are patients with life-threatening tumors. To be able to hold them steady is a big deal,” Dr. Migden observed.
One-quarter of study participants experienced progressive disease. The remainder weren’t evaluated for various reasons.
The dermatologist pointed out that locally advanced disease was particularly responsive to cemiplimab, with four of nine affected patients experiencing complete or partial response, for an overall response rate of 44%. This is consistent with the preliminary results of the pivotal phase 2 study, in which the overall response rate in the 78 participants with unresectable, locally advanced CSCC was 46%.
The phase 2 trial also includes another 59 patients with metastatic CSCC on 3 mg/kg IV cemiplimab every 14 days, as well as 56 patients with metastatic disease assigned to flat-dose 350-mg IV cemiplimab every 21 days.
Treatment side effects
In the phase 1 study, immunotherapy with cemiplimab was far better tolerated than in traditional cancer chemotherapy. There were two grade 3 cases of elevated liver enzymes and one of arthralgia, but no significant fatigue or nausea and no hypothyroidism. However, judging from the cumulative experience accrued with the five PD-1 checkpoint inhibitors already approved for treatment of other cancers, one must be prepared to encounter hypothyroidism and other endocrinopathies, pneumonitis, hepatitis, and rashes.
“The clinician must have a very high index of suspicion for these immune-related adverse events and a low threshold to consult with colleagues in other specialties – pulmonary, endocrine, and medical oncology – for evaluation and management of these possible side effects. I tell all the patients who are on cemiplimab, ‘Any new anything – a slight cough, mild diarrhea – you’re coming in and you’re getting checked,’ ” according to Dr. Migden.
That being said, the majority of immune-related adverse events because of PD-1 inhibitors are mild to moderate. Of the few that reach grade 3 or above, most can be successfully managed by pausing or discontinuing anti–PD-1 therapy coupled with prompt initiation of immunosuppressive therapy, typically with high-dose steroids, he added.
Look sharp for pseudoprogression
Pseudoprogression is a phenomenon whereby immunotherapy results in inflammatory changes bringing about a temporary increase in tumor size that precedes tumor shrinkage. It’s uncommon, occurring in 3 of 16 patients in the phase 1 study. The mechanism probably involves tumor infiltration by massive numbers of activated T cells. And there is evidence from other PD-1 inhibitor studies in advanced cancers that pseudoprogression may actually be a marker for increased likelihood of survival beyond 1 year.
“Pseudoprogression is important to recognize because the patients you treat with cemiplimab can get worse before they get better,” the dermatologist explained. “So you don’t want to prematurely discontinue treatment because you’re misclassifying it as tumor progression.”
The rationale for anti-PD-1 therapy in CSCC
Tumors that express PD-1 bind to PD–ligand 1 on T cells, switching off T-cell mediated tumor destruction and thereby allowing the malignancy to thrive.
“Simplified, the strategy here is to interfere with the interaction at the T-cell off switch, either with an antibody to PD–ligand 1, such as atezolizumab [Tecentriq], or an antibody to the PD-1 receptor, where cemiplimab works. By turning off the off switch, we get a T cell fully on and attacking the tumor cell,” Dr. Migden said.
“The more the tumor mutation burden, the better immunotherapy works – and CSCC has the highest tumor mutation burden of any tumor type in the Cancer Genome Atlas, several times higher than melanoma. Interestingly, basal cell carcinoma has an even higher tumor mutation burden than CSCC, but it’s not part of the atlas,” he continued.
Although the proportion of CSCCs that are locally advanced hasn’t been well established, it’s clear that CSCC is the deadliest nonmelanoma skin cancer, accounting for 3,900-8,800 deaths annually in the United States.
The cemiplimab phase 1 and 2 clinical trials for CSCC were jointly sponsored by Regeneron and Sanofi. The monoclonal antibody is also being developed for treatment of myeloma and lung cancer. Dr. Migden reported receiving honoraria from Regeneron and Sanofi, as well as from Genentech, Lilly, Novartis, and Sun Pharmaceuticals.
CHICAGO – The investigational programmed cell death protein 1 checkpoint inhibitor cemiplimab proved highly effective for the treatment of locally advanced or metastatic cutaneous squamous cell carcinoma in a phase 1 clinical trial, Michael R. Migden, MD, reported at the annual meeting of the American College of Mohs Surgery.
And this was no ordinary phase 1 study, he noted. Because there is no Food and Drug Administration–approved treatment for advanced cutaneous squamous cell carcinoma (CSCC), cemiplimab has been granted both Breakthrough Drug and Orphan Drug status by the FDA and the European Medicines Agency.
Given the likelihood that cemiplimab will receive expeditious regulatory approval to address this major unmet need, he offered his colleagues practical tips on its use, including information about the drug’s chief side effects as well as a heads-up regarding the importance of early recognition of the pseudoprogression phenomenon that can occur with the drug.
He predicted this fully human monoclonal antibody directed at programmed cell death protein 1 (PD-1) is going to be an important drug for Mohs surgeons.
“Immunotherapy is becoming increasingly relevant to micrographic surgery and dermatologic oncology practice and fellowship training. Care for larger, advanced CSCC falls within our scope of practice and we should play an essential role, inclusive of multidisciplinary care, in the management and follow-up of these patients,” asserted Dr. Migden, a dermatologic surgeon at the University of Texas MD Anderson Cancer Center, Houston.
The open-label, phase 1 study included seven patients with distant metastatic CSCC and nine with locally and/or regionally advanced disease. They were treated with 3 mg/kg IV cemiplimab every 2 weeks for 48 weeks, with Response Evaluation Criteria In Solid Tumors 1.1 criteria used for assessment of response status every 8 weeks. More than 80% of the tumors were located in the head and neck. The great majority of study participants had previously been treated with radiation therapy and systemic agents, to little effect.
The complete response rate at 48 weeks was 12.5%, with tumor clearance occurring as quickly as 14 weeks. Another 25% of patients had a partial response, for an overall response rate of 37.5%. But that’s not the full success story, as another 31% of patients had stable disease. Thus, 11 of 16 patients, or 69%, experienced disease control.
“A disease-control rate of nearly 70% is really important because these are patients with life-threatening tumors. To be able to hold them steady is a big deal,” Dr. Migden observed.
One-quarter of study participants experienced progressive disease. The remainder weren’t evaluated for various reasons.
The dermatologist pointed out that locally advanced disease was particularly responsive to cemiplimab, with four of nine affected patients experiencing complete or partial response, for an overall response rate of 44%. This is consistent with the preliminary results of the pivotal phase 2 study, in which the overall response rate in the 78 participants with unresectable, locally advanced CSCC was 46%.
The phase 2 trial also includes another 59 patients with metastatic CSCC on 3 mg/kg IV cemiplimab every 14 days, as well as 56 patients with metastatic disease assigned to flat-dose 350-mg IV cemiplimab every 21 days.
Treatment side effects
In the phase 1 study, immunotherapy with cemiplimab was far better tolerated than in traditional cancer chemotherapy. There were two grade 3 cases of elevated liver enzymes and one of arthralgia, but no significant fatigue or nausea and no hypothyroidism. However, judging from the cumulative experience accrued with the five PD-1 checkpoint inhibitors already approved for treatment of other cancers, one must be prepared to encounter hypothyroidism and other endocrinopathies, pneumonitis, hepatitis, and rashes.
“The clinician must have a very high index of suspicion for these immune-related adverse events and a low threshold to consult with colleagues in other specialties – pulmonary, endocrine, and medical oncology – for evaluation and management of these possible side effects. I tell all the patients who are on cemiplimab, ‘Any new anything – a slight cough, mild diarrhea – you’re coming in and you’re getting checked,’ ” according to Dr. Migden.
That being said, the majority of immune-related adverse events because of PD-1 inhibitors are mild to moderate. Of the few that reach grade 3 or above, most can be successfully managed by pausing or discontinuing anti–PD-1 therapy coupled with prompt initiation of immunosuppressive therapy, typically with high-dose steroids, he added.
Look sharp for pseudoprogression
Pseudoprogression is a phenomenon whereby immunotherapy results in inflammatory changes bringing about a temporary increase in tumor size that precedes tumor shrinkage. It’s uncommon, occurring in 3 of 16 patients in the phase 1 study. The mechanism probably involves tumor infiltration by massive numbers of activated T cells. And there is evidence from other PD-1 inhibitor studies in advanced cancers that pseudoprogression may actually be a marker for increased likelihood of survival beyond 1 year.
“Pseudoprogression is important to recognize because the patients you treat with cemiplimab can get worse before they get better,” the dermatologist explained. “So you don’t want to prematurely discontinue treatment because you’re misclassifying it as tumor progression.”
The rationale for anti-PD-1 therapy in CSCC
Tumors that express PD-1 bind to PD–ligand 1 on T cells, switching off T-cell mediated tumor destruction and thereby allowing the malignancy to thrive.
“Simplified, the strategy here is to interfere with the interaction at the T-cell off switch, either with an antibody to PD–ligand 1, such as atezolizumab [Tecentriq], or an antibody to the PD-1 receptor, where cemiplimab works. By turning off the off switch, we get a T cell fully on and attacking the tumor cell,” Dr. Migden said.
“The more the tumor mutation burden, the better immunotherapy works – and CSCC has the highest tumor mutation burden of any tumor type in the Cancer Genome Atlas, several times higher than melanoma. Interestingly, basal cell carcinoma has an even higher tumor mutation burden than CSCC, but it’s not part of the atlas,” he continued.
Although the proportion of CSCCs that are locally advanced hasn’t been well established, it’s clear that CSCC is the deadliest nonmelanoma skin cancer, accounting for 3,900-8,800 deaths annually in the United States.
The cemiplimab phase 1 and 2 clinical trials for CSCC were jointly sponsored by Regeneron and Sanofi. The monoclonal antibody is also being developed for treatment of myeloma and lung cancer. Dr. Migden reported receiving honoraria from Regeneron and Sanofi, as well as from Genentech, Lilly, Novartis, and Sun Pharmaceuticals.
REPORTING FROM THE ACMS ANNUAL MEETING
Key clinical point:
Major finding: Disease control was achieved in 11 of 16 patients (69%).
Study details: The open-label, phase 1 study included seven patients with distant metastatic cutaneous squamous cell carcinoma and nine with locally and/or regionally advanced disease.
Disclosures: The cemiplimab phase 1 and 2 clinical trials for CSCC were jointly sponsored by Regeneron and Sanofi. Dr. Migden reported receiving honoraria from Regeneron and Sanofi as well as from Genentech, Lilly, Novartis, and Sun Pharmaceuticals.
Ropivacaine called top anesthesia for nail surgery
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
CHICAGO – Ropivacaine has a fast onset of action, longer duration than either lidocaine or bupivacaine, and it’s the only one of the three that’s inherently vasoconstrictive. For Brienne Cressey, MD, those features make ropivacaine the local anesthetic of choice in performing nail surgery.
“Local anesthesia is really key for nail surgery. If you don’t have good anesthesia it’s not a good experience for either the surgeon or the patient,” she observed at the annual meeting of the American College of Mohs Surgery.
Lidocaine has a fast onset – less than 1 minute – but a problematic short duration of 30-120 minutes. Bupivacaine has the disadvantage of a slow onset of up to 5 minutes, albeit with a longer duration of anesthesia at 2-4 hours. Ropivacaine has a fast onset, plus a duration of up to 8 hours. And unlike lidocaine and bupivacaine, which are vasodilatory, ropivacaine is vasoconstrictive.
“With lidocaine, you get a lot of blood right after you take off your tourniquet. With ropivacaine, you get really nice reperfusion, but it’s not too much. You take off the tourniquet, check to see you’ve got reperfusion, then you add a little ropivacaine – about 0.5 mL – on either side of the base of the distal phalanx. It stops the bleeding immediately and you can easily put on a pressure dressing. It’s a nice way to get the patient over the hump of those first hours of pain and lets them drive home in comfort,” explained Dr. Cressey, a dermatologist working in a group practice at Dermatology Professionals in East Greenwich, R.I.
Ropivacaine is less cardiotoxic than bupivacaine. And ropivacaine offers an additional advantage: Its pH is such that no buffering is necessary. “Ropivacaine doesn’t require any compounding. You can just use it at 1% straight out of the bottle. That’s what we do in our office, and we’ve had very good experience with it,” according to the dermatologist.
Achieving smooth sailing with local anesthesia
Dr. Cressey delivers ropivacaine slowly through a 30-gauge needle, which makes for a smaller, less painful puncture. She utilizes a topical cold spray, and places a vibrating machine as a distractant proximal to where she is injecting. She keeps the anesthetic at room temperature or warms it to body temperature in a water bath as another means of reducing the pain of injection.
The distal digital block
This is a cross between a traditional proximal digital block and a wing block. It works well for the second, third, and fourth digits, which are mostly volar dominant. The block bathes the volar nerve branch in anesthesia at the midline of the finger or toe.
Dr. Cressey begins by injecting ropivacaine proximal and lateral to the junction of the proximal nail fold and lateral nail fold. After creating a dermal wheal, she directs her needle perpendicularly downward toward the finger or toe pad, injecting 1-4 mL of anesthesia, depending upon digit size. Visible blanching will progress digitally. If resistance is encountered, it suggests the needle has penetrated a ligament or other fibrous tissue. Simply withdraw the needle and continue injecting.
“What’s nice about the distal digital block is you get an immediate effect, and there’s good hemostasis during the procedure as well,” she said.
Dr. Cressey reported no financial conflicts regarding her presentation.
EXPERT ANALYSIS FROM THE ACMS ANNUAL MEETING
Patidegib, the first topical hedgehog inhibitor, scores in Gorlin syndrome
CHICAGO – in a phase 2 study, and did so without causing the problematic adverse events that prompt many Gorlin patients to discontinue systemic hedgehog inhibitor drugs, Ervin Epstein Jr., MD, said at the annual meeting of the American College of Mohs Surgery.
He characterized patidegib, a small-molecule cyclopamine derivative, as “a Goldilocks drug, a topical hedgehog inhibitor with percutaneous absorption that’s just right: sufficient to have anti-hedgehog and anti–basal cell carcinoma efficacy, but not enough to cause systemic exposure or systemic adverse events.”
A complete response – tumor clinical disappearance – occurred in 25% of the BCCs in the two active treatment arms. In contrast, none of the BCCs in the control group cleared. Patients on patidegib developed one or more new surgically eligible BCCs after study week 2 at a rate of 0.4 tumors per patient, markedly less than the rate of 1.4 tumors per patient in controls.
“We think that prevention is really the place to go with this drug,” said Dr. Epstein, cofounder and chief medical officer at PellePharm, the company based in Menlo Park, Calif., that is developing patidegib.
Indeed, he envisions patidegib gel as lifetime therapy for Gorlin patients.
Tumor shrinkage was significantly greater with 2% patidegib than with the 4% concentration. But so was treatment adherence: Patients in the 2% patidegib arm missed on average just 2 days of therapy over the course of 6 months, while those in the 4% arm missed 50 days. Dr. Epstein attributed this discrepancy to a freak of randomization that can occur in such a small study: 5 of 6 patients in the 2% patidegib group were women, while most in the 4% arm were men. And the men were far less adherent to treatment, possibly because men are less accustomed to applying a product on their face daily, he noted.
In any case, it’s the 2% gel formulation that is moving on to a phase 3, double-blind, randomized trial. The 150-patient U.S. and European study, scheduled to start this summer, will have as its primary endpoint the rate of new BCCs over the course of 1 year. The Food and Drug Administration has granted topical patidegib Breakthrough Drug and Orphan Drug status.
Dr. Epstein said that, in the phase 2 study, BCC shrinkage occurred only in patients whose hedgehog pathway activity decreased after 6 weeks of topical therapy as evidenced by a reduction in the GLI1 mRNA biomarker. Of note, circulating blood levels of patidegib in study participants were more than 500-fold lower than when the drug is given orally. And of greatest importance, rates of the hallmark side effects of oral hedgehog inhibitor therapy that cause so many patients to discontinue therapy – muscle cramps, taste loss, and hair loss – were no different in patients on patidegib gel than in those on placebo.
“In the randomized trial of vismodegib [Erivedge], half of patients stopped taking it within 1 year despite good results. And indeed, when they stopped taking the drug, the basal cell carcinomas returned,” the dermatologist noted.
Dr. Epstein is an employee of PellePharm, which is developing patidegib. Dermatologists with patients with Gorlin syndrome who are interested in participating in the phase 3 trial can contact him at eepstein@pellepharm.com.
CHICAGO – in a phase 2 study, and did so without causing the problematic adverse events that prompt many Gorlin patients to discontinue systemic hedgehog inhibitor drugs, Ervin Epstein Jr., MD, said at the annual meeting of the American College of Mohs Surgery.
He characterized patidegib, a small-molecule cyclopamine derivative, as “a Goldilocks drug, a topical hedgehog inhibitor with percutaneous absorption that’s just right: sufficient to have anti-hedgehog and anti–basal cell carcinoma efficacy, but not enough to cause systemic exposure or systemic adverse events.”
A complete response – tumor clinical disappearance – occurred in 25% of the BCCs in the two active treatment arms. In contrast, none of the BCCs in the control group cleared. Patients on patidegib developed one or more new surgically eligible BCCs after study week 2 at a rate of 0.4 tumors per patient, markedly less than the rate of 1.4 tumors per patient in controls.
“We think that prevention is really the place to go with this drug,” said Dr. Epstein, cofounder and chief medical officer at PellePharm, the company based in Menlo Park, Calif., that is developing patidegib.
Indeed, he envisions patidegib gel as lifetime therapy for Gorlin patients.
Tumor shrinkage was significantly greater with 2% patidegib than with the 4% concentration. But so was treatment adherence: Patients in the 2% patidegib arm missed on average just 2 days of therapy over the course of 6 months, while those in the 4% arm missed 50 days. Dr. Epstein attributed this discrepancy to a freak of randomization that can occur in such a small study: 5 of 6 patients in the 2% patidegib group were women, while most in the 4% arm were men. And the men were far less adherent to treatment, possibly because men are less accustomed to applying a product on their face daily, he noted.
In any case, it’s the 2% gel formulation that is moving on to a phase 3, double-blind, randomized trial. The 150-patient U.S. and European study, scheduled to start this summer, will have as its primary endpoint the rate of new BCCs over the course of 1 year. The Food and Drug Administration has granted topical patidegib Breakthrough Drug and Orphan Drug status.
Dr. Epstein said that, in the phase 2 study, BCC shrinkage occurred only in patients whose hedgehog pathway activity decreased after 6 weeks of topical therapy as evidenced by a reduction in the GLI1 mRNA biomarker. Of note, circulating blood levels of patidegib in study participants were more than 500-fold lower than when the drug is given orally. And of greatest importance, rates of the hallmark side effects of oral hedgehog inhibitor therapy that cause so many patients to discontinue therapy – muscle cramps, taste loss, and hair loss – were no different in patients on patidegib gel than in those on placebo.
“In the randomized trial of vismodegib [Erivedge], half of patients stopped taking it within 1 year despite good results. And indeed, when they stopped taking the drug, the basal cell carcinomas returned,” the dermatologist noted.
Dr. Epstein is an employee of PellePharm, which is developing patidegib. Dermatologists with patients with Gorlin syndrome who are interested in participating in the phase 3 trial can contact him at eepstein@pellepharm.com.
CHICAGO – in a phase 2 study, and did so without causing the problematic adverse events that prompt many Gorlin patients to discontinue systemic hedgehog inhibitor drugs, Ervin Epstein Jr., MD, said at the annual meeting of the American College of Mohs Surgery.
He characterized patidegib, a small-molecule cyclopamine derivative, as “a Goldilocks drug, a topical hedgehog inhibitor with percutaneous absorption that’s just right: sufficient to have anti-hedgehog and anti–basal cell carcinoma efficacy, but not enough to cause systemic exposure or systemic adverse events.”
A complete response – tumor clinical disappearance – occurred in 25% of the BCCs in the two active treatment arms. In contrast, none of the BCCs in the control group cleared. Patients on patidegib developed one or more new surgically eligible BCCs after study week 2 at a rate of 0.4 tumors per patient, markedly less than the rate of 1.4 tumors per patient in controls.
“We think that prevention is really the place to go with this drug,” said Dr. Epstein, cofounder and chief medical officer at PellePharm, the company based in Menlo Park, Calif., that is developing patidegib.
Indeed, he envisions patidegib gel as lifetime therapy for Gorlin patients.
Tumor shrinkage was significantly greater with 2% patidegib than with the 4% concentration. But so was treatment adherence: Patients in the 2% patidegib arm missed on average just 2 days of therapy over the course of 6 months, while those in the 4% arm missed 50 days. Dr. Epstein attributed this discrepancy to a freak of randomization that can occur in such a small study: 5 of 6 patients in the 2% patidegib group were women, while most in the 4% arm were men. And the men were far less adherent to treatment, possibly because men are less accustomed to applying a product on their face daily, he noted.
In any case, it’s the 2% gel formulation that is moving on to a phase 3, double-blind, randomized trial. The 150-patient U.S. and European study, scheduled to start this summer, will have as its primary endpoint the rate of new BCCs over the course of 1 year. The Food and Drug Administration has granted topical patidegib Breakthrough Drug and Orphan Drug status.
Dr. Epstein said that, in the phase 2 study, BCC shrinkage occurred only in patients whose hedgehog pathway activity decreased after 6 weeks of topical therapy as evidenced by a reduction in the GLI1 mRNA biomarker. Of note, circulating blood levels of patidegib in study participants were more than 500-fold lower than when the drug is given orally. And of greatest importance, rates of the hallmark side effects of oral hedgehog inhibitor therapy that cause so many patients to discontinue therapy – muscle cramps, taste loss, and hair loss – were no different in patients on patidegib gel than in those on placebo.
“In the randomized trial of vismodegib [Erivedge], half of patients stopped taking it within 1 year despite good results. And indeed, when they stopped taking the drug, the basal cell carcinomas returned,” the dermatologist noted.
Dr. Epstein is an employee of PellePharm, which is developing patidegib. Dermatologists with patients with Gorlin syndrome who are interested in participating in the phase 3 trial can contact him at eepstein@pellepharm.com.
REPORTING FROM THE ACMS ANNUAL MEETING













