User login
52-year-old man • syncopal episode • chest pain • mild lightheadedness • Dx?
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
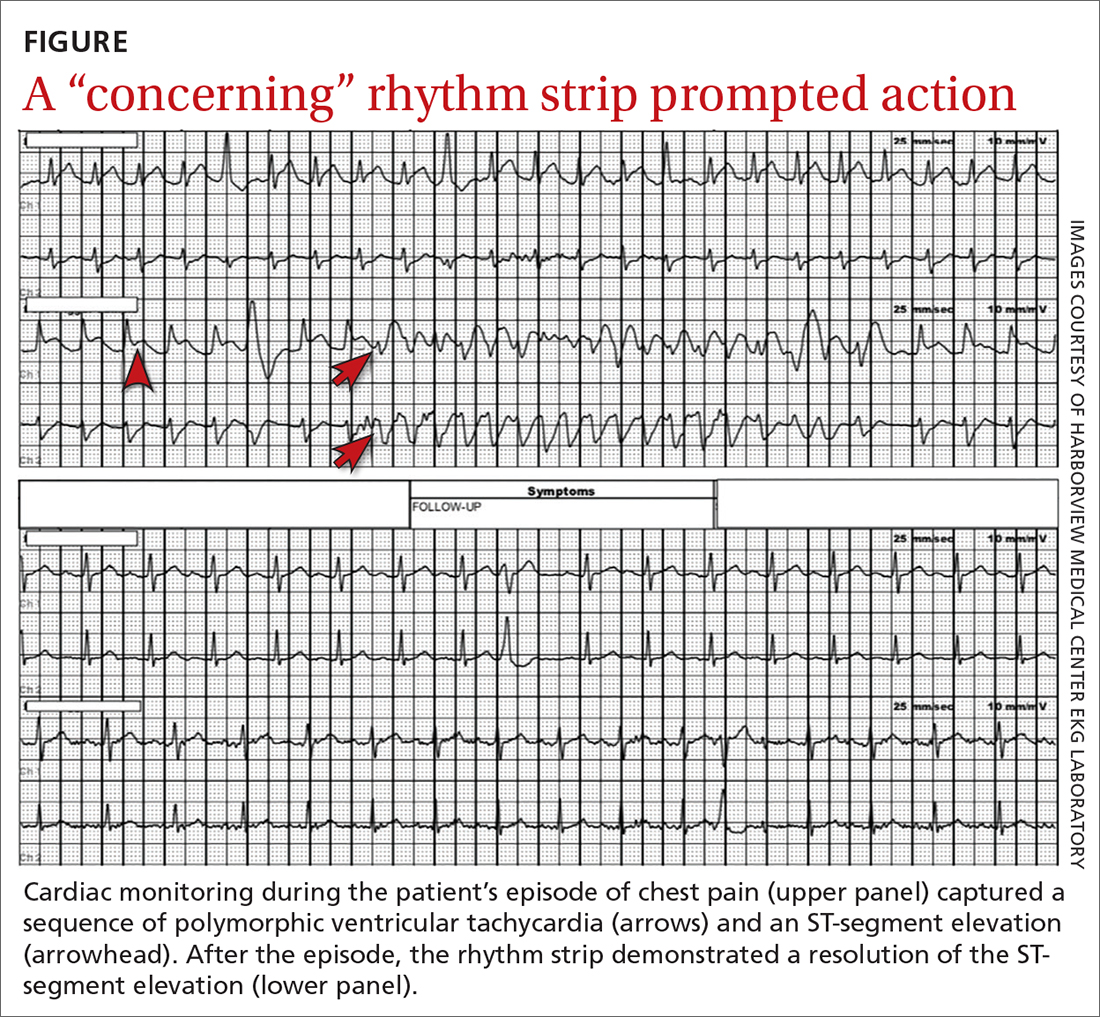
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.
THE DIAGNOSIS
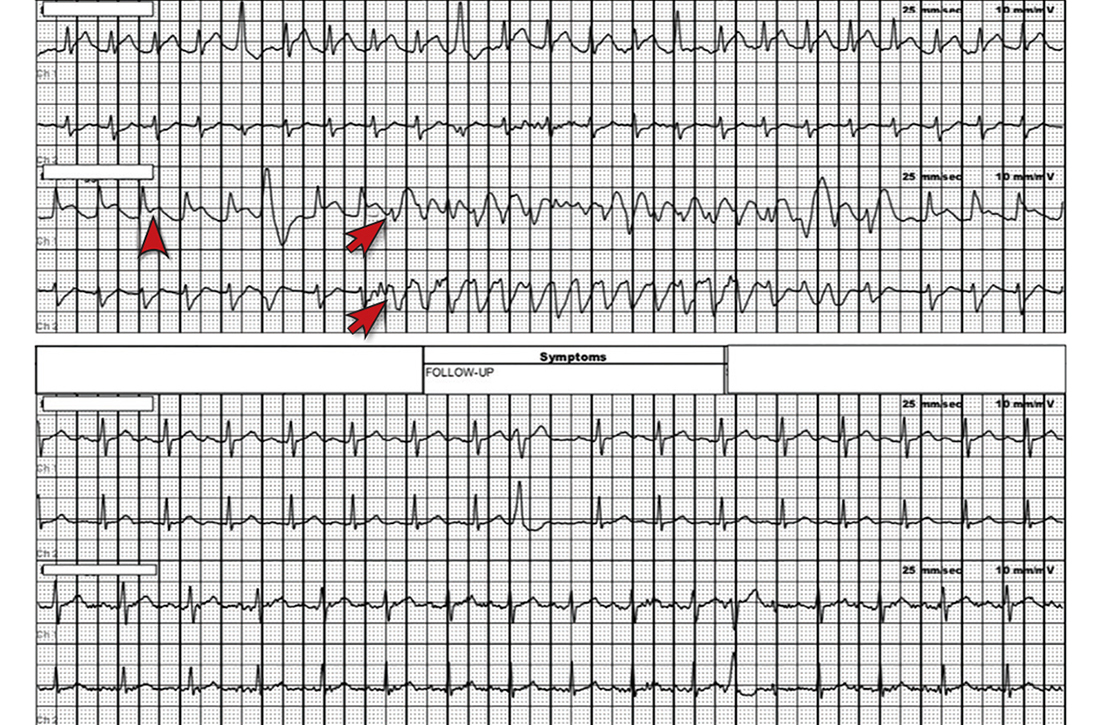
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; michen@u.washington.edu
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.
THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; michen@u.washington.edu
THE CASE
A 52-year-old man with a history of hypertension and gastroesophageal reflux disease (GERD) presented to the emergency department (ED) after an episode of syncope. He reported that the syncope occurred soon after he stood up to go to the kitchen to make dinner but was without prodrome or associated symptoms. He recalled little of the event, and the episode was unwitnessed. He had a few bruises on his arms but no significant injuries.
On questioning, he reported occasional palpitations but no changes in his normal exercise tolerance. His only medication was lisinopril 10 mg/d.
In the ED, his vital signs, physical exam (including orthostatic vital signs), basic labs (including troponin I), and a 12-lead EKG were normal. After a cardiology consultation, he was discharged home with a 30-day ambulatory rhythm monitor.
A few days later, while walking up and down some hills, he experienced about 15 seconds of chest pain accompanied by mild lightheadedness. Thinking it might be related to his GERD, he took some over-the-counter antacids when he returned home, since these had been effective for him in the past.
However, the rhythm monitoring company contacted the EKG lab to transmit a concerning strip (FIGURE). They also reported that the patient had been contacted and reported no further symptoms.
THE DIAGNOSIS
Most notable on the patient’s rhythm strip was a continuously varying QRS complex, which was indicative of polymorphic ventricular tachycardia and consistent with the patient’s syncope and other symptoms. Less obvious at first glance was an ST-segment elevation in the preceding beats. Comparison to a post-episode tracing (FIGURE) highlights the abnormality. Polymorphic ventricular tachycardia resolves in 1 of 2 ways: It will either stop on its own (causing syncope if it lasts more than a few seconds) or it will devolve into ventricular fibrillation, causing cardiac arrest.1
The combination of these findings and the clinical scenario prompted a recommendation that the patient report to the ED for admission (his wife drove him). He was admitted to the intensive care unit (ICU) for continuous telemetry monitoring, and a cardiac catheterization was ordered. The procedure revealed a 99% thrombotic mid-right coronary artery lesion, for which aspiration thrombectomy and uncomplicated stenting were performed.
Continue to: DISCUSSION
DISCUSSION
Guidelines from the American College of Cardiology/American Heart Association/Heart Rhythm Society recommend a detailed history and physical exam, as well as an EKG, for the initial evaluation of syncope.2 If this does not point to a diagnosis (and depending on the presentation and other factors), an ambulatory rhythm monitor can be considered. Other possible testing modalities include stress testing, resting transthoracic echocardiography, electrophysiologic testing, and cardiac magnetic resonance imaging or computed tomography.
Is the cause cardiac? The guidelines suggest that a cardiac cause of syncope is more likely if several of the following factors are present: age > 60 years; male sex; presence of known heart disease (acquired or congenital); brief prodrome (eg, palpitations) or no prodrome; exertional or supine syncope; 1 to 2 episodes; an abnormal cardiac exam; and a family history of premature sudden death.2 A noncardiac cause is suggested by other factors: younger age; no known cardiac disease; standing or a position change from supine to sitting/standing; prodrome; specific triggers (eg, dehydration, pain); and frequent and prolonged stereotypic episodes.2
While the guidelines do not specify the number of factors or endorse a specific scoring system, such tools have been developed. For example, the EGSYS (Evaluation of Guidelines in Syncope Study) Score assigns 1 point for each of 6 factors: palpitations; heart disease and/or abnormal EKG; effort syncope; supine syncope; precipitating or predisposing factors; and autonomic prodromes. A score ≥ 3 identified cardiac syncope with a sensitivity of 95%, but with a specificity of only 61%. In the derivation study, patients with a score ≥ 3 had higher mortality than those with a lower score (17 vs 3%; P < .001).3
Myocardial ischemia can trigger ventricular arrhythmias. In the GUSTO-1 trial of fibrinolytic therapy in patients with acute ST-segment elevation myocardial infarction (n = 40,895), the incidence of ventricular tachycardia or ventricular fibrillation was 10.2%.4 In a pooled analysis (4 trials; n = 26,416) of patients who were treated for non–ST-segment elevation or unstable angina-type acute coronary syndromes, the rate of these arrhythmias was markedly lower (2.1%).5 The risk of ventricular arrhythmia is one reason close monitoring (eg, continuous telemetry, ICU admission) is the standard of care for patients with acute coronary syndromes.
Our patient experienced syncope upon standing, which suggested a noncardiac cause (usually orthostatic hypotension). However, the history of palpitations increased the suspicion for a cardiac cause, and thus the rhythm monitor was ordered.
THE TAKEAWAY
This case was unusual in that ambulatory monitoring captured electrocardiographic evidence of myocardial ischemia leading directly to a ventricular arrhythmia. In the evaluation of syncope, a detailed history, physical exam, and a baseline 12-lead EKG can sometimes give clues to an arrhythmic cause of syncope (eg, Brugada syndrome, prior infarct pattern, prolonged QTc, bradycardia, heart block, arrhythmogenic right ventricular cardiomyopathy)—but prolonged rhythm monitoring is sometimes needed to identify a cause.
Michael A. Chen, MD, PhD, Harborview Medical Center, University of Washington School of Medicine, 325 9th Avenue, Box 359748 (Cardiology), Seattle, WA 98104; michen@u.washington.edu
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
1. Viskin S, Chorin E, Viskin D, et al. Polymorphic ventricular tachycardia: terminology, mechanism, diagnosis, and emergency therapy. Circulation. 2021;144:823-839. doi: 10.1161/CIRCULATIONAHA.121.055783
2. Shen W-K, Sheldon RS, Benditt DG, et al. 2017 ACC/AHA/HRS guideline for the evaluation and management of patients with syncope: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. J Am Coll Cardiol. 2017;70:620-663. doi: 10.1016/j.jacc.2017.03.002
3. Del Rosso A, Ungar A, Maggi R, et al. Clinical predictors of cardiac syncope at initial evaluation in patients referred urgently to a general hospital: the EGSYS score. Heart. 2008;94:1528-1529. doi: 10.1136/hrt.2008.143123
4. Newby KH, Thompson T, Stebbins A, et al. Sustained ventricular arrhythmias in patients receiving thrombolytic therapy: incidence and outcomes. The GUSTO Investigators. Circulation. 1998;98:2567-2573. doi: 10.1161/01.cir.98.23.2567
5. Al-Khatib SM, Granger CB, Huang Y, et al. Sustained ventricular arrhythmias among patients with acute coronary syndromes with no ST-segment elevation: incidence, predictors, and outcomes. Circulation. 2002;106:309-12. doi: 10.1161/01.cir.0000022692.49934.e3
How to screen for and treat teen alcohol use
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
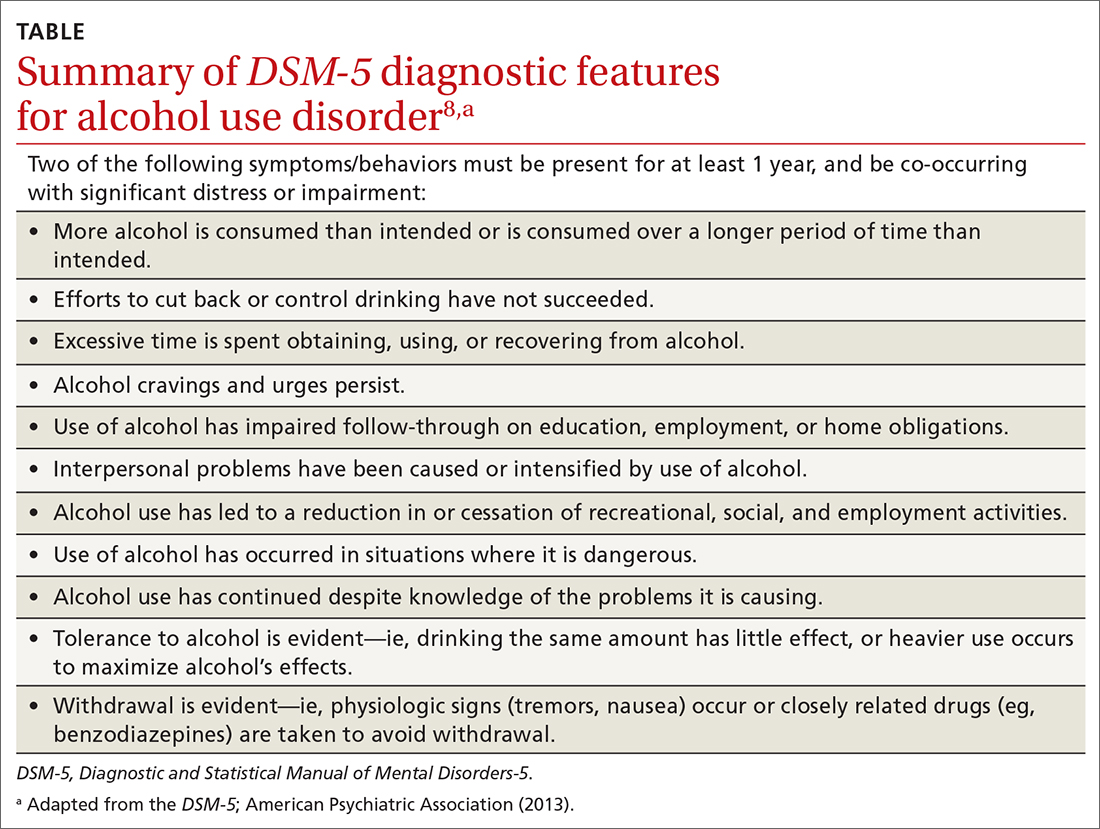
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
THE CASE
Paul F* is a 16-year-old White boy who lives with his mother and spends some weekends with his father who has shared custody. He recently presented to the clinic for treatment due to an arrest for disorderly conduct at school. He and a friend were found drinking liquor outside the school building when they were scheduled to be in class. Paul reported that he and his friends often drink at school and at extracurricular functions. He has been using alcohol for the past 2 years, with escalating consumption (5 or more drinks per episode) in the past year. Paul has been drinking most days of the week and has even driven under the influence at times. He said, “I just feel happier when I am drinking.” An accomplished soccer player recruited by colleges, Paul recently was suspended from the team due to his poor grades. His response was, “It’s stupid anyway. What’s the point of playing?”
●
* The patient’s name and some personal details have been changed to protect his identity.
Alcohol is the number 1 substance of abuse for adolescents, used more than tobacco or drugs.1-3 In 2007 and again in 2016, the Surgeon General of the United States issued reports to highlight this important topic,1,2 noting that early and repeated exposure to alcohol during this crucial time of brain development increases the risk for future problems, including addiction.2
Adolescent alcohol use is often underestimated by parents and physicians, including misjudging how much, how often, and how young children are when they begin to drink.1 Boys and girls tend to start drinking at similar ages (13.9 and 14.4 years, respectively),3 but as girls age, they tend to drink more and binge more.4 In 2019, 1 in 4 adolescents reported drinking and more than 4 million reported at least 1 episode of binge drinking in the prior month.4 These numbers have further ramifications: early drinking is associated with alcohol dependence, relapse, use of other substances, risky sexual behaviors, injurious behaviors, suicide, motor vehicle accidents, and dating violence.4-6
Diagnosing alcohol use disorder
The range of alcohol use includes consumption, bingeing, abuse, and dependence.7,8 Consumption is defined as the drinking of alcoholic beverages. Bingeing is the consumption of more than 5 drinks for men or 4 drinks for women in 2 hours, according to the National Institute on Alcohol Abuse and Alcoholism.7 However, the criterion is slightly different for the Substance Abuse and Mental Health Services Administration, which broadens the timeframe to “on the same occasion.”9 While previously known as separate disorders, alcohol abuse (or misuse) and alcohol dependence are now diagnostically classified together as alcohol use disorders (AUDs), per the Diagnostic and Statistical Manual of Mental Disorders-5 (DSM-5).8 AUD is further stratified as mild, moderate, or severe, depending on the number of criteria that are met by the patient (TABLE).8,10
Alcohol screening
Currently, the US Preventive Services Task Force (USPSTF) does not recommend screening adolescents ages 12 to 17 for AUD, and has instead issued an “I” statement (insufficient evidence).11 While the USPSTF recognizes the potential burdens of adolescent alcohol use, the potential harms of screening include “stigma, anxiety, labeling, discrimination, privacy concerns, and interference with the patient–clinician relationship.”11 The USPSTF also notes that it “did not find any evidence that specifically examined the harms of screening for alcohol use in adolescents.”11
This is at odds with recommendations from the American Academy of Pediatrics (AAP), which in 2011 released a policy statement advocating screening, brief intervention, and referral to treatment for adolescent substance use.12 In the United States, even though 83% of adolescents see a physician at least once each year,12,13 alcohol misuse screening still varies, occurring in the range of 50% to 86% of office visits.12 When screening does occur, it is often based on clinical impression only.12 Studies have shown that when a screening tool is not used, up to two-thirds of substance use disorders may be missed.12-15
Continue to: A full and complete biopsychosocial interview
A full and complete biopsychosocial interview with adolescents is a necessity, and should include queries about alcohol, drugs, and other substances. Acknowledgment of use should trigger further investigation into the substance use areas. Interviews may start with open-ended questions about alcohol use at home or at school before moving to more personalized and detailed questioning and use of screening tools.16
While various screening instruments exist, for the sake of brevity we provide as an example the Screening to Brief Intervention (S2BI) tool. It is an efficient, single-page tool that can help clinicians in their routine care of adolescents to quickly stratify the patient risk of substance use disorder as none/low, moderate, or severe.12 It can be found here: www.mcpap.com/pdf/S2Bi%20Toolkit.pdf (see page 10).
For all patients, but particularly for adolescents, confidentiality is important, and many specialty societies have created language to address this issue.12 Discuss confidentiality with both the adolescent patient and the patient’s caregiver simultaneously, with dialogue that includes: (a) the need to speak with adolescents alone during the office visit, (b) the benefits of confidentiality in the physician–patient relationship, and (c) the need to disclose selected information to keep patients safe.12 Describing the process for required disclosures is essential. Benefits of disclosure include further support for the adolescent patient as well as appropriate parental participation and support for possible referrals.12
Treating AUD
Treatment for AUD should be multifaceted. Screen for comorbid mood disorders, such as generalized anxiety,17,18 social anxiety,18 and depression,19 as well as for insomnia.18 Studies have demonstrated a strong link between insomnia and anxiety, and again between anxiety and AUD.17-19 Finally, screen for adverse childhood events such as trauma, victimization, and abuse.20 Addressing issues discovered in screening allows for more targeted and personalized treatment of AUD.
The National Institute on Drug Abuse categorizes evidence-based treatment into 3 areas: behavioral therapies, family therapies, and medications.21
Continue to: Behavioral therapies
Behavioral therapies can include group therapy, cognitive behavioral therapy (CBT), motivational enhancement therapy, 12-Step facilitation, and contingency management, in which small rewards or incentives are given for participation in treatment to reinforce positive behaviors.21
Family-based therapies, such as brief strategic family therapy, functional family therapy, and multisystem therapy recognize that adolescents exist in systems of families in communities, and that the patient’s success in treatment may be supported by these relationships.21
Some medications may achieve modest benefit for treatment of adolescents with AUD. Naltrexone, acamprosate, and disulfiram have all been used successfully to treat AUD in adults21; some physicians may choose to use these medications “off label” in adolescents. Bupropion has been used successfully in the treatment of nicotine use disorder,21 and a small study in 2005 showed some success with bupropion in treating adolescents with attention-deficit/hyperactivity disorder, comorbid depression, and substance use disorder.22 Naltrexone has also been studied in adolescents with opioid use disorder, although these were not large studies.23
Adolescents with serious, sustained issues with AUD may require more in-depth treatments such as an intensive outpatient program, a partial hospitalization program, or a residential treatment program.15 The least-restrictive environment is preferable.15 Families are generally included as part of the treatment and recovery process in those settings.21 Some patients may require detoxification prior to referral to residential treatment settings; the American Society of Addiction Medicine has published a comprehensive guideline on alcohol withdrawal.24
Paul’s family physician diagnosed his condition as AUD and referred him for CBT with a psychologist, who treated him for both the AUD and an underlying depressive disorder that was later identified. CBT focused on cognitive restructuring of depressive thoughts as well as support for continued abstinence from alcohol. The patient, with family support, declined antidepressant medication.
After 6 months of treatment, Paul and his parents were pleased with his progress. His grades improved to the point that he was permitted to play soccer again, and he was seriously looking at his future college options.
CORRESPONDENCE
Scott A. Fields, PhD, 3200 MacCorkle Avenue Southeast, 5th Floor, Robert C. Byrd Clinical Teaching Center, Department of Family Medicine, Charleston, WV 25304; sfields@hsc.wvu.edu
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
1. US Department of Health and Human Services. The Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2007.
2. US Department of Health and Human Services. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC; US Department of Health and Human Services, Office of the Surgeon General. 2016.
3. Hingson R, White A. New research findings since the 2007 Surgeon General’s Call to Action to Prevent and Reduce Underage Drinking: A review. J Stud Alcohol Drugs Suppl. 2014; 75:158-169.
4. National Institute on Alcohol Abuse and Alcoholism. Underage drinking. National Institute of Health. Accessed December 22, 2021. www.niaaa.nih.gov/publications/brochures-and-fact-sheets/underage-drinking.
5. Hingson R, Zha W, Iannotti R, et al. Physician advice to adolescents about drinking and other health behaviors. Pediatrics. 2013;131:249-257.
6. Schaus JF, Sole ML, McCoy TP, et al. Screening for high-risk drinking in a college student health center: characterizing students based on quantity, frequency, and harms. J Stud Alcohol Drugs Suppl. 2009;16:34-44.
7. National Institute on Alcohol Abuse and Alcoholism. Drinking levels defined. Accessed December 27, 2021. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
8. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (DSM-5). Arlington, VA; American Psychiatric Association. 2013.
9. Substance Abuse and Mental Health Services Administration. Bringing down binge drinking. Accessed December 27, 2021. www.samhsa.gov/sites/default/files/programs_campaigns/nation_prevention_week/data-binge-drinking.pdf
10. Grant BF, Goldstein RB, Saha TD, et al. Epidemiology of DSM-5 Alcohol Use Disorder: results from the National Epidemiologic Survey on Alcohol and Related Conditions III. JAMA Psychiatry. 2015;72:757-766.
11. USPSTF. Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force Recommendation Statement. JAMA. 2018;320:1899-1909.
12. Levy SJ, Williams JF, Committee on Substance Use and Prevention. Substance use screening, brief intervention, and referral to treatment. Pediatrics. 2016;138:e20161211.
13. MacKay AP, Duran CP. Adolescent Health in the United States. National Center for Health Statistics, Centers for Disease Control and Prevention. 2007.
14. Haller DM, Meynard A, Lefebvre D, et al. Effectiveness of training family physicians to deliver a brief intervention to address excessive substance use among young patients: a cluster randomized controlled trial. CMAJ. 2014;186:E263-E272.
15. Borus J, Parhami I, Levy S. Screening, brief intervention, and referral to treatment. Child Adolesc Psychiatric Clin N Am. 2016;25:579-601.
16. Knight J, Roberts T, Gabrielli J, et al. Adolescent alcohol and substance use and abuse. Performing preventive services: A bright futures handbook. Accessed December 22, 2021. American Academy of Pediatrics. https://ocfcpacourts.us/wp-content/uploads/2020/06/Adolescent_Alcohol_and_Substance_Abuse_001005.pdf
17. Dyer ML, Heron J, Hickman M, et al. Alcohol use in late adolescence and early adulthood: the role of generalized anxiety disorder and drinking to cope motives. Drug Alcohol Depend. 2019;204:107480.
18. Blumenthal H, Taylor DJ, Cloutier RM, et al. The links between social anxiety disorder, insomnia symptoms, and alcohol use disorders: findings from a large sample of adolescents in the United States. Behav Ther. 2019;50:50-59.
19. Pedrelli P, Shapero B, Archibald A, et al. Alcohol use and depression during adolescence and young adulthood: a summary and interpretation of mixed findings. Curr Addict Rep. 2016;3:91-97.
20. Davis JP, Dworkin ER, Helton J, et al. Extending poly-victimization theory: differential effects of adolescents’ experiences of victimization on substance use disorder diagnoses upon treatment entry. Child Abuse Negl. 2019; 89:165-177.
21. NIDA. Principles of adolescent substance use disorder treatment: a research-based guide. Accessed December 22, 2021. www.drugabuse.gov/publications/principles-adolescent-substance-use-disorder-treatment-research-based-guide
22. Solhkhah R, Wilens TE, Daly J, et al. Bupropion SR for the treatment of substance-abusing outpatient adolescents with attention-deficit/hyperactivity disorder and mood disorders. J Child Adolesc Psychopharmacol. 2005:15:777-786.
23. Camenga DR, Colon-Rivera HA, Muvvala SB. Medications for maintenance treatment of opioid use disorder in adolescents. J Stud Alcohol Drugs. 2019;80:393-402.
24. American Society of Addiction Medicine. The ASAM clinical practice guideline on alcohol withdrawal management. Accessed December 22, 2021. www.asam.org/quality-care/clinical-guidelines/alcohol-withdrawal-management-guideline
A practical guide to appendicitis evaluation and treatment
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
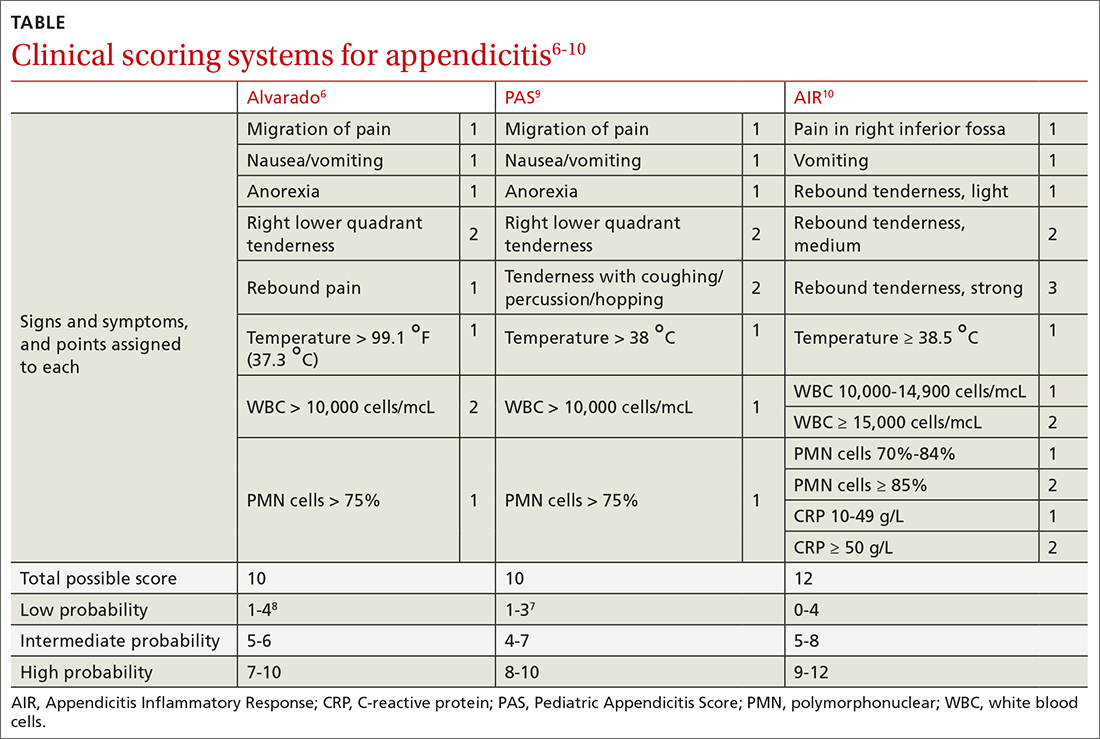
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; jessica.servey@usuhs.edu
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; jessica.servey@usuhs.edu
CASE
A 35-year-old man with a body mass index of 20 presented to the emergency department after 24 hours of abdominal pain that began in the periumbilical region and then migrated to the right lower quadrant. The pain was exacerbated during ambulation and was intense when the car transporting him to the hospital encountered bumps in the road. After his pain started, he had associated anorexia, followed by nausea and emesis. He reported fever and chills. On examination, his temperature was 100.8 °F (38.2 °C), and palpation of the right and left lower quadrants elicited right lower quadrant pain. Laboratory evaluation revealed a white blood cell (WBC) count of 14,000 cells/mcL with 85% neutrophils, C-reactive protein of 40 mg/L, and a negative urinalysis.
How would you proceed with this patient?
Acute appendicitis is the most common cause of abdominal pain resulting in the need for surgical treatment; lifetime risk of appendicitis is 6% to 7%.1 Appendicitis is caused by intraluminal obstruction in the appendix from enlarged lymphoid tissue or a fecalith. The obstruction leads to elevated intraluminal pressure due to persistent mucus and gas production by bacteria, ultimately leading to ischemia and perforation.1 Additionally, obstruction leads to bacterial overgrowth, most commonly colonic flora such as Escherichia coli, Bacteroides fragilis, Streptococcus viridans, Enterococcus sp., Pseudomonas aeruginosa, and Klebsiella pneumoniaei.1,2
The following review provides a look at how 3 clinical scoring systems compare in the identification of acute appendicitis and details which imaging studies you should order—and when. But first, we’ll quickly detail the relevant physical findings and lab values that point to a diagnosis of acute appendicitis.
Physical findings. The patient typically first experiences vague abdominal pain that then localizes to the right lower quadrant due to peritoneal inflammation. Anorexia and nausea typically follow the abdominal pain. On examination, the patient often appears ill and exhibits abdominal guarding due to peritonitis. Tachycardia and fever are common; however, the absence of either does not exclude appendicitis. Classically, on palpation, the patient will have pain at McBurney’s point (one-third the distance from the anterior iliac spine to the umbilicus). The exact point of maximal tenderness can differ because of the varying anatomy of the appendix (retrocecal, paracolic, pelvic, pre/post ileal, promontoric, or subcecal).1 Right lower quadrant pain, abdominal rigidity, and radiation of periumbilical pain to the right lower quadrant are the most accurate findings in adults to rule in appendicitis.3 For children, physical exam findings have the highest likelihood in predicting appendicitis and include a positive Obturator sign, positive Rovsing sign, or a positive Psoas sign, and absent or decreased bowel sounds.4
Laboratory studies can support a diagnosis of appendicitis but cannot exclude it. Leukocytosis with neutrophil predominance is present in 90% of cases.5 An elevated C-reactive protein level renders the highest diagnostic accuracy.5 Perform a pregnancy test for any woman of child-bearing age, to assist in the diagnosis and guide imaging choices for evaluation. Additional laboratory tests are not needed unless there are concerns about volume depletion.
Clinical scoring systems
Several clinical scoring systems (TABLE6-10) have been validated to aid clinicians in evaluating patients with possible appendicitis, to decrease unnecessary exposure to ionizing radiation from computed tomography (CT) scans, to identify and reassure patients with low likelihoods of appendicitis, and to conduct outpatient follow-up.
Continue to: The Alvarado score
The Alvarado score is the oldest scoring rule, developed in 1986; it entails 8 clinical and laboratory variables.6 Ebell et al altered the proposed cutoff values of the Alvarado score to be low risk (< 4), intermediate risk (4-8), and high risk (≥ 9), effectively improving the sensitivity and specificity rates.7
In a meta-analysis of the Alvarado score that included 42 studies of men, women, and children, the sensitivity for “ruling out” appendicitis with a cutoff of 5 points was 96% for men, 99% for women, and 99% for children.8 The accuracy of a high-risk score (> 7) for “ruling in” appendicitis was less with an overall specificity of 82%.8 The Alvarado score did seem to overestimate appendicitis in women in all score categories.8
The Pediatric Appendicitis Score (PAS) is similar to Alvarado and was prospectively validated in 1170 children in 2002 for more specific guidance in this age group.9 The PAS had excellent specificity in the study; those with a score of ≥ 6 had a high probability of appendicitis. In a study comparing Alvarado with PAS in 311 patients, insignificant differences were noted at a score of ≥ 7 for both tests (sensitivity 86% vs 89%, and specificity 59% vs 50%, respectively).11 No scoring system has been found to be sufficiently accurate for use in children 4 years of age and younger.12
The Appendicitis Inflammatory Response (AIR) Score was prospectively validated in 545 patients representing all age groups.10 Subsequently, in a larger prospective multicenter study of 3878 patients older than 5 years, the original cut points were altered, thereby improving test sensitivity and negative predictive value to 99% for those with low probability (0 to 3), and test specificity to 98% for those with high-probability (9 to 12).13 Compared with the Alvarado Score, the AIR Score has higher specificity for those in the high-probability range, and similar exclusion rates in the low-probability range.14
Caveats with clinical decision scores. These tools are accepted and often used. However, challenges that affect generalizability of study data include differences in patient selection for each study (undifferentiated abdominal pain vs appendicitis), prospective vs retrospective designs, and age and gender variations in the patient populations. Despite the numerous scoring systems developed, none can accurately be used to rule in appendicitis. They are best used to assist in ruling out appendicitis and to aid in deciding for or against imaging.
Continue to: A look at the imaging options
A look at the imaging options
Abdominal CT has sensitivity and specificity rates between 76% and 100% and 83% and 100%, respectively.15,20,21 Ultrasonography has sensitivity and specificity rates of 71% to 94% and 81% to 98%, respectively.15,20,21 Formal US is reliable to confirm appendicitis, but less so to rule out appendicitis. Special considerations for imagining in pregnant patients and children are discussed in a bit.
Timing of surgical consultation
Surgical consultation is paramount once the diagnosis of appendicitis is probable. Imaging is best obtained prior to surgical consultation to streamline evaluation and enhance decision- making. Typically, patients will be categorized as complicated or uncomplicated based on the presence or absence of perforation, a gangrenous appendix, an intra-abdominal abscess (IAA), or purulent peritonitis. Active continuous surgical involvement (co-management or assumption of care) is recommended in all cases of appendicitis, especially if nonoperative management is selected, given that some cases must convert to immediate operative treatment or may be selected for delayed future (interval) appendectomy.22
Management
Uncomplicated appendicitis
Prompt appendectomy has been the gold standard of care for uncomplicated acute appendicitis for 60 years. However, several studies have investigated an antibiotic-based strategy rather than surgical treatment for uncomplicated appendicitis.
Antibiotics vs appendectomy. In 2020, the CODA Collaborative published a randomized trial comparing a 10-day course of antibiotics with appendectomy in patients with uncomplicated appendicitis. In this multicenter study based in the United States, 1552 patients 18 years of age or older were randomized to receive antibiotics or undergo appendectomy (95% performed laparoscopically). The antibiotic treatment consisted of at least 24 hours of IV antibiotics, with or without admission to the hospital. Antibiotic choice was individualized according to guidelines for intra-abdominal infection published by the Infectious Diseases Society of America, with the most common IV medications being ertapenem, cefoxitin, or metronidazole plus one of the following: ceftriaxone, cefazolin, or levofloxacin. For the remaining 10 days, oral metronidazole plus ciprofloxacin or cefdinir were used.22
Continue to: The primary endpoint...
The primary endpoint was the European Quality of Life-5 Dimensions (EQ-5D) questionnaire, with secondary outcomes including appendectomy in the antibiotics group and complications through 90 days. Exclusion criteria included pregnancy, sepsis, peritonitis, recurrent appendicitis, severe phlegmon on imaging, or evidence of neoplasm.22
Antibiotics were noninferior to appendectomy for the 30-day study. However, antibiotics failed in 29%, who then proceeded to appendectomy by 90 days; these patients also accounted for 41% of those with an appendicolith. Overall complications were more common in the antibiotics group than in the appendectomy group (8.1 vs 3.5 per 100 participants; 95% CI, 1.3-3.98). Also more common in the antibiotic group were serious adverse events (4 vs 3 per 100 participants; hazard ratio [HR] = 1.29; 95% CI, 0.67-2.50). The presence of an appendicolith in the antibiotics group increased the conversion risk to appendectomy, as well as adverse events risk.22
The takeaway. Antibiotic treatment is a noninferior method to treat acute uncomplicated appendicitis. However, the informed consent process is important, given the ~30% failure rate. Patient factors such as continued access to care should help inform the decision.
Two main surgical approaches exist for appendectomy: open and minimally invasive. At this time, the minimally invasive options include laparoscopic, single incision laparoscopic surgery (SILS), and robotic appendectomy. A study comparing cost, availability, or complications of these options has not been conducted at this time.
A large Cochrane review of 67 studies examining open vs laparoscopic appendectomy in adults and children completed in 2018 revealed that the laparoscopic approach reduced early postoperative pain intensity and led to a shorter hospital stay, earlier return to work or usual activities, and a decrease in wound infections.23 The odds of IAA occurring with laparoscopic appendectomy increased by 65% compared with an open procedure; however, postoperative bowel obstruction and incisional hernias were less likely to occur.23 Additionally, following laparoscopic surgery, postoperative bowel obstruction and incisional hernias are less likely to occur. The laparoscopic approach is preferred due to overall increased patient satisfaction and a reduction in most, if not all, complications.
Continue to: Complicated appendicitis
Complicated appendicitis
Excluding patients with severe sepsis or purulent peritonitis requiring resuscitation and immediate surgical intervention of intra-abdominal infection, the approach to patients with complicated appendicitis varies between aggressive surgical intervention and nonoperative management.
In a 2007 meta-analysis reviewing nonsurgical treatment of appendiceal abscess/phlegmon, immediate surgery was associated with higher morbidity.24 Within the nonoperative management group 7.2% (CI, 4.0-10.5) required surgical intervention and 19.7% (CI, 11.0-28.3) required abscess drainage. Malignant disease was detected in 1.2% (CI, 0.6-1.7).24 Small subsequent studies concluded different results.25
Ultimately, the 2015 European Association of Endoscopic Surgery guidelines recommend a new systematic review; but with current data, initial nonoperative management is preferred.15 After initial nonoperative treatment, the only benefits from interval appendectomy are identification of an underlying malignancy (6% to 20%) and mitigating the risk of recurrent appendicitis (5% to 44%).15,25-30
Multiple single institutional series found increased neoplasm incidence (9% to 20%) in complicated appendicitis in patients 40 years and older.26-30 Prior to interval appendectomy in patients 45 years and older, ensuring they have an up-to-date screening colonoscopy is important. This is in line with 2021 US Preventive Services Task Force (Grade “B” recommendation), 2018 American Cancer Society (qualified recommendation), and 2021 American College of Gastroenterology (conditional recommendation) guidelines for colorectal cancer screening to start at age 45 in average-risk patients.31 Patients younger than 45 can consider screening through shared decision-making.
Special populations
Pregnant patients
In pregnancy, challenges exist with the presence of traditional signs and symptoms of appendicitis, with the most predictive sign being a WBC count higher than 18,000.32 The American College of Radiology’s (ACR) Appropriateness Criteria recommend US as the imaging modality of choice in pregnancy, with MRI as the best option when US is inconclusive.33 Two meta-analyses demonstrated high sensitivity (91.8%-96.6%) and specificity (95.9%-97.9%) of MRI in diagnosing appendicitis.34,35 CT scan is not the preferred initial imagining modality in pregnancy unless urgent information is needed and other modalities are insufficient or unavailable.36
Continue to: The most common...
The most common nonobstetric surgical intervention during pregnancy is appendectomy, at a rate of 6.3/10,000 person-years, which increases to 9.9/10,000 in the postpartum period.37 Two large population studies demonstrate the rate of appendicitis varies over the course of pregnancy, with the lowest rates in the third trimester,38,39 and a significant rebound lasting for 2 years postpartum.39 Peritonitis, septic shock, pneumonia, postoperative infection, and longer hospital stays occur more frequently in pregnant women than in nonpregnant women with appendicitis.40 Fetal loss is higher in the first trimester.32
In a 14-year review of 63,145 appendicitis cases, an increased risk of fetal loss and maternal death was noted across ages and ethnicities, with the largest risk of maternal death occurring in Hispanics and fetal death in non-Hispanic Blacks.41 In a large study of 1018 adverse events after appendectomy or cholecystectomy, the 3 most common events were preterm delivery (35.4%), preterm labor without preterm delivery (26.4%), and miscarriage (25.7%).42 The surgery itself was not a major risk factor for adverse events. Major risk factors included cervical incompetence (odds ratio [OR] = 24.3), preterm labor in current pregnancy (OR = 18.3), and presence of vulvovaginitis (OR = 5.2).42
Nonoperative management in pregnancy is not recommended; only 1 prospective trial has been done, with 20 patients, showing a 25% failure rate.43 Two meta-analyses published in 2019 highlight the potential increase of fetal loss with laparoscopic approaches to appendectomy.44,45 However, recently published literature demonstrates no significant maternal-fetal morbidity. Current guidelines of the Society of American Gastrointestinal and Endoscopic Surgeons agree that laparoscopy is the operative choice in pregnancy.36
Children
Acute appendicitis is the most common surgical emergency in children.4 Physical exam findings and laboratory results are not classic in this population, obtaining an accurate history can be challenging, and results of clinical scoring systems can be inconclusive.4 Additional serum biomarkers, procalcitonin and calprotectin, are gaining evidence for use in improving scoring systems to refine low-risk groups. Unavailability of timely, reliable biomarker testing in rural practice locations limits definitive recommendations at this time.46 ACR recommends no imaging in a pediatric patient whose risk of having appendicitis is low based on any of several scoring systems.47 For those assessed as having higher risk, US is the recommended initial modality,with CT with IV contrast or MRI without contrast equally recommended if the US is equivocal.47
Despite promising data from trials of nonoperative treatment for adults with appendicitis, no definitive evidence and recommendations are available for children. Two systematic reviews show nonoperative treatment is safe, with an efficacy rate of 76% to 82% at long-term follow-up,48,49 although the success of antibiotic regimens varies. Within the nonoperative treatment group, 16% of patients had appendectomy during the follow-up period, which varied from 8 weeks to 4 years.48 A randomized controlled trial is needed for final guidance.
Continue to: CASE
CASE
The patient had an Alvarado score of 9 (high probability) and an AIR score of 6 (intermediate probability). A CT with IV contrast showed a 9-mm fluid-filled appendix with periappendiceal fluid. During surgical consultation, he was offered laparoscopic appendectomy or nonoperative treatment with antibiotics. He opted for a preoperative dose of piperacillin-tazobactam 3.375 g IV and laparoscopic appendectomy. The patient was discharged home 6 hours after his procedure.
CORRESPONDENCE
Jessica Servey, MD, MHPE, 4301 Jones Bridge Road, Bethesda, MD 20814; jessica.servey@usuhs.edu
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
1. Prystowsky JB, Pugh CM, Nagle AP. Current problems in surgery. Appendicitis. Curr Probl Surg. 2005;42:688-742.
2. Song DW, Park BK, Suh SW, et al. Bacterial culture and antibiotic susceptibility in patients with acute appendicitis. Int J Colorectal Dis. 2018;33:441-447.
3. Wagner JM, McKinney WP, Carpenter JL. Does this patient have appendicitis? JAMA. 1996;276:1589-1594.
4. Benabbas R, Hanna M, Shah J, et al. Diagnostic accuracy of history, physical examination, laboratory tests, and point-of-care ultrasound for pediatric acute appendicitis in the emergency department: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:523-551.
5. Andersson RE. Meta-analysis of the clinical and laboratory diagnosis of appendicitis. Br J Surg. 2004;91:28-37.
6. Alvarado A. A practical score for the early diagnosis of acute appendicitis. Ann Emerg Med. 1986;15:557-564.
7. Ebell MH, Shinholser J. What are the most clinically useful cutoffs for the Alvarado and Pediatric Appendicitis Scores? A systematic review. Ann Emerg Med. 2014;64:365-372.e2.
8. Ohle R, O’Reilly F, O’Brien KK, et al. The Alvarado score for predicting acute appendicitis: a systematic review. BMC Med. 2011;9:139.
9. Samuel M. Pediatric appendicitis score. J Pediatr Surg. 2002;37:877-881.
10. Andersson M, Andersson RE. The appendicitis inflammatory response score: a tool for the diagnosis of acute appendicitis that outperforms the Alvarado score. World J Surg. 2008;32:1843-1849.
11. Pogorelić Z, Rak S, Mrklić I, et al. Prospective validation of Alvarado score and Pediatric Appendicitis Score for the diagnosis of acute appendicitis in children. Pediatr Emerg Care. 2015;31:164-168.
12. Rassi R, Muse F, Sánchez-Martínez J, et al. Diagnostic value of clinical prediction scores for acute appendicitis in children younger than 4 years. Eur J Pediatr Surg. 2021. [Online ahead of print]
13. Andersson M, Kolodziej B, Andersson RE. Validation of the Appendicitis Inflammatory Response (AIR) score. World J Surg. 2021;45:2081-2091.
14. Kollár D, McCartan DP, Bourke M, et al. Predicting acute appendicitis? A comparison of the Alvarado score, the Appendicitis Inflammatory Response Score and clinical assessment. World J Surg. 2015;39:104-109.
15. Gorter RR, Eker HH, Gorter-Stam MA, et al. Diagnosis and management of acute appendicitis. EAES consensus development conference 2015. Surg Endosc. 2016;30:4668-4690.
16. Matthew Fields J, Davis J, Alsup C, et al. Accuracy of point-of-care ultrasonography for diagnosing acute appendicitis: a systematic review and meta-analysis. Acad Emerg Med. 2017;24:1124-1136.
17. Sharif S, Skitch S, Vlahaki D, et al. Point-of-care ultrasound to diagnose appendicitis in a Canadian emergency department. CJEM. 2018;20:732-735.
18. Doniger SJ, Kornblith A. Point-of-care ultrasound integrated into a staged diagnostic algorithm for pediatric appendicitis. Pediatr Emerg Care. 2018;34:109-115.
19. Menon N, Kumar S, Keeler B, et al. A systematic review of point-of-care abdominal ultrasound scans performed by general surgeons. Surgeon. 2021. [Online ahead of print]
20. Doria AS, Moineddin R, Kellenberger CJ, et al. US or CT for diagnosis of appendicitis in children and adults? A meta-analysis. Radiology. 2006;241:83-94.
21. van Randen A, Laméris W, van Es HW, et al. A comparison of the accuracy of ultrasound and computed tomography in common diagnoses causing acute abdominal pain. Eur Radiol. 2011;21:1535-1545.
22. Flum DR, Davidson GH, Monsell SE, et al. A randomized trial comparing antibiotics with appendectomy for appendicitis. N Engl J Med. 2020;383:1907-1919.
23. Jaschinski T, Mosch CG, Eikermann M, et al. Laparoscopic versus open surgery for suspected appendicitis. Cochrane Database Syst Rev. 2018;11:CD001546.
24. Andersson RE, Petzold MG. Nonsurgical treatment of appendiceal abscess or phlegmon: a systematic review and meta-analysis. Ann Surg. 2007;246:741-748.
25. Deelder JD, Richir MC, Schoorl T, et al. How to treat an appendiceal inflammatory mass: operatively or nonoperatively? J Gastrointest Surg. 2014;18:641-645.
26. Carpenter SG, Chapital AB, Merritt MV, et al. Increased risk of neoplasm in appendicitis treated with interval appendectomy: single-institution experience and literature review. Am Surg. 2012;78:339-343.
27. Hayes D, Reiter S, Hagen E, et al. Is interval appendectomy really needed? A closer look at neoplasm rates in adult patients undergoing interval appendectomy after complicated appendicitis. Surg Endosc. 2021;35:3855-3860.
28. Peltrini R, Cantoni V, Green R, et al. Risk of appendiceal neoplasm after interval appendectomy for complicated appendicitis: a systematic review and meta-analysis. Surgeon. 2021. [Online ahead of print.]
29. Mällinen J, Rautio T, Grönroos J, et al. Risk of appendiceal neoplasm in periappendicular abscess in patients treated with interval appendectomy vs follow-up with magnetic resonance imaging: 1-year outcomes of the peri-appendicitis acuta randomized clinical trial. JAMA Surg. 2019;154:200-207.
30. Son J, Park YJ, Lee SR, et al. Increased risk of neoplasms in adult patients undergoing interval appendectomy. Ann Coloproctol. 2020;36:311-315.
31. Davidson KW, Barry MJ, Mangione CM, et al. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. JAMA. 2021;325:1965-1977.
32. Theilen LH, Mellnick VM, Shanks AL, et al. Acute appendicitis in pregnancy: predictive clinical factors and pregnancy outcomes. Am J Perinatol. 2017;34:523-528.
33. Garcia EM, Camacho MA, Karolyi DR, et al. ACR Appropriateness Criteria right lower quadrant pain-suspected appendicitis. J Am Coll Radiol. 2018;15:S373-s387.
34. Kave M, Parooie F, Salarzaei M. Pregnancy and appendicitis: a systematic review and meta-analysis on the clinical use of MRI in diagnosis of appendicitis in pregnant women. World J Emerg Surg. 2019;14:37.
35. Repplinger MD, Levy JF, Peethumnongsin E, et al. Systematic review and meta-analysis of the accuracy of MRI to diagnose appendicitis in the general population. J Magn Reson Imaging. 2016;43:1346-1354.
36. Pearl JP, Price RR, Tonkin AE, et al. SAGES guidelines for the use of laparoscopy during pregnancy. Surg Endosc. 2017;31:3767-3782.
37. Zingone F, Sultan AA, Humes DJ, et al. Risk of acute appendicitis in and around pregnancy: a population-based cohort study from England. Ann Surg. 2015;261:332-337.
38. Andersson RE, Lambe M. Incidence of appendicitis during pregnancy. Int J Epidemiol. 2001;30:1281-1285.
39. Moltubak E, Landerholm K, Blomberg M, et al. Major variation in the incidence of appendicitis before, during and after pregnancy: a population-based cohort study. World J Surg. 2020;44:2601-2608.
40. Abbasi N, Patenaude V, Abenhaim HA. Management and outcomes of acute appendicitis in pregnancy-population-based study of over 7000 cases. BJOG. 2014;121:1509-1514.
41. Dongarwar D, Taylor J, Ajewole V, et al. Trends in appendicitis among pregnant women, the risk for cardiac arrest, and maternal-fetal mortality. World J Surg. 2020;44:3999-4005.
42. Sachs A, Guglielminotti J, Miller R, et al. Risk factors and risk stratification for adverse obstetrical outcomes after appendectomy or cholecystectomy during pregnancy. JAMA Surg. 2017;152:436-441.
43. Joo JI, Park HC, Kim MJ, et al. Outcomes of antibiotic therapy for uncomplicated appendicitis in pregnancy. Am J Med. 2017;130:1467-1469.
44. Lee SH, Lee JY, Choi YY, Lee JG. Laparoscopic appendectomy versus open appendectomy for suspected appendicitis during pregnancy: a systematic review and updated meta-analysis. BMC Surg. 2019;19:41.
45. Frountzas M, Nikolaou C, Stergios K, et al. Is the laparoscopic approach a safe choice for the management of acute appendicitis in pregnant women? A meta-analysis of observational studies. Ann R Coll Surg Engl. 2019;101:235-248.
46. Di Saverio S, Podda M, De Simone B, et al. Diagnosis and treatment of acute appendicitis: 2020 update of the WSES Jerusalem guidelines. World J Emerg Surg. 2020;15:27.
47. Koberlein GC, Trout AT, Rigsby CK, et al. ACR Appropriateness Criteria suspected appendicitis-child. J Am Coll Radiol. 2019;16:S252-S263.
48. Maita S, Andersson B, Svensson JF, et al. Nonoperative treatment for nonperforated appendicitis in children: a systematic review and meta-analysis. Pediatr Surg Int. 2020;36:261-269.
49. Georgiou R, Eaton S, Stanton MP, et al. Efficacy and safety of nonoperative treatment for acute appendicitis: a meta-analysis. Pediatrics. 2017;139:e20163003.
PRACTICE RECOMMENDATIONS
› Use the Alvarado Score, Pediatric Appendicitis Score, or Appendicitis Inflammatory Response Score to help rule out appendicitis and thereby reduce unnecessary imaging. A
› Choose ultrasound first as the imaging procedure for children and pregnant women, followed by magnetic resonance imaging if needed, to reduce ionizing radiation in these populations. B
› Consider an antibiotic-based strategy under the care of a surgeon in lieu of immediate surgery for uncomplicated appendicitis. A
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
DKMS: Small nonprofit to world’s largest stem cell donor registry
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
When Mechtild Harf was diagnosed with acute leukemia in 1990, physicians told her and her husband Peter that a bone marrow transplant was her best hope for survival. Back then, her native Germany had only 3,000 registered donors, and none was a match.
“My dad just went crazy, you know, to save his wife,” recalled Katharina Harf, who was a young teen at the time of her mother’s diagnosis.
In the course of 1 year, the Harfs recruited more than 68,000 potential bone marrow donors, but their heroic efforts couldn’t save Mechtild.
“She unfortunately didn’t make it. She died because of leukemia,” Katharina said.
Although Mechtild Harf did not survive, her legacy lives on in the bone marrow and stem cell donor recruitment organization DKMS (Deutsche Knochenmarkspenderdatei, or German Bone Marrow Donor Center).
In May of 1991, Peter Harf and Gerhard Ehninger, MD, the hematologist who treated Mechtild, founded DKMS with the mission, as its website states, “to provide as many blood cancer patients as possible with a second chance at life.”
From its German roots, the nonprofit organization has extended its mission to the United States (where it was initially known as Delete Blood Cancer DKMS), Poland, the United Kingdom, Chile, and in 2021, to South Africa.
Three decades after her mother’s death, Katharina Harf serves as Executive Chairwoman of DKMS U.S., based in New York.
World’s largest registry
“DKMS has the largest number of unrelated donors of any organization in the world,” noted Richard E. Champlin, MD, chair of the department of stem cell transplantation and cellular therapy at the University of Texas MD Anderson Cancer Center in Houston.
“In a large fraction of our donor searches, we find matches that are in the DKMS registry,” he said in an interview,
Alexander Schmidt, MD, PhD, global chief medical officer for DKMS, said that approximately 25% of all registered donors worldwide were recruited by his organization, and 39% of all unrelated donor transplants are made with peripheral blood stem cell or bone marrow products, donated by volunteers who are recruited by DKMS.
Since its founding, DKMS has registered 7.1 million potential donors in Germany, who made a total of 80,000 stem cell donations. DKMS U.S., which began operations in 2004, has registered 1.1 million donors and enabled 4,700 donations.
Global partners
DKMS partners with donor centers and recruitment organizations in each country where it operates. In the United States, DKMS works with the National Marrow Donor Program (NMDP) and its “Be The Match” donor registry.
“DKMS donors, both those from DKMS in Germany and those from DKMS in the United States are also listed in the NMDP registry, to make it easier for US search coordinators to accept these donors,” Dr. Schmidt explained in an interview.
The international cooperation and coordination makes it possible for a donor in the UK, for example, to save a life of a patient in Germany, the U.S., Chile, India, or many other parts of the world – anywhere that can be reached in time for a patient in need to receive a stem cell donation.
Pandemic affects donations
But, as with just about every aspect of life, the COVID-19 pandemic has created enormous challenges for recruiters, donor centers, and stem cell transplant centers.
Dr. Schmidt said that decline in donations during the pandemic was less severe than initially feared, with a decrease of just 3.5% in 2020, compared with the prepandemic year of 2019. In contrast, though, the average annual growth rate for donations prior to the pandemic was about 4%.
“Nevertheless, at the beginning of the pandemic in March 2020, for a few days things looked quite terrible, because all the borders were closed and flights were canceled, and about 50% of all stem cell products go abroad, and between 20% and 25% go intercontinental,” Dr. Schmidt said.
However, close cooperation and coordination between donor centers and national health authorities soon resolved the problem and helped insure that the flow of life-saving donations could continue with minimal disruption, he noted.
“I don’t think we had any product that could not be delivered at the end of the day, due to the pandemic,” he told this news organization.
Workforce and clinical problems
Although the flow of donations within and between nations has continued, the COVID-19 pandemic has had profound negative effects on transplant centers, particularly during the wave of infections caused by the Omicron variant, according to a transplant expert.
“With this most recent strain and how transmissible it is, what we’re dealing with is mass workforce shortages,” said Yi-Bin Chen, MD, director of the bone marrow transplant program at Massachusetts General Hospital in Boston.
“On top of a short-staffed hospital, you then take a very transmissible variant and deplete it even more due to the need to quarantine,” he said in an interview.
Both Dr. Champlin and Dr. Chen said that on-again, off-again pandemic travel bans and donor illnesses have necessitated first obtaining products and cryopreserving them before starting the recipient on a conditioning regimen for the transplant.
“The problem is that, while you can preserve peripheral blood stem cells pretty reliably, cryopreserving bone marrow is a bit more difficult,” Dr. Chen said.
In addition, evidence from recent studies comparing stem cell sources suggest that outcomes are less good with cryopreserved products than with fresh products, and with peripheral blood stem cells compared with bone marrow.
“But you’ve got to make do. A transplant with a cryopreserved product is better than no transplant,” Dr. Chen said.
To make things even more frustrating, as the pandemic waxed and waned throughout 2020 and 2021, the recommendations from donor centers seesawed between using fresh or cryopreserved product, making it difficult to plan a transplant for an individual patient.
The Omicron wave has also resulted in a much higher rate of donor dropout than anticipated, making it that much harder to schedule a transplant, Dr. Chen noted.
‘Every patient saved’
The pandemic will eventually subside, however, while the need for stem cell transplantation to treat hematologic malignancies will continue.
DKMS recently launched special aid programs to improve access to stem cell transplants in developing nations by offering financial support, free HLA typing, and other services.
In addition to its core mission of recruiting donors, DKMS is dedicated to improving the quality and efficiency of stem cell transplants. For example, in 2017 scientists in DKMS’ Life Science Lab created an antibody test for donor cytomegalovirus (CMV) infection, using a simple buccal swab rather than a more invasive blood sample. CMV infections can compromise the integrity of stem cell grafts and could be fatal to immunocompromised transplant recipients.
The last word goes to Mechtild Harf’s daughter Katharina.
“My big dream is that every patient will be saved from blood cancer,” she said in a video posted on the DKMS website. “When they get sick, we have a solution for them, whether it’s because they need a donor, with research, building hospitals, providing them with the best medical care we can. I will just keep fighting and keep spreading the word, recruiting donors, raising money – all the things that it takes for us to delete blood cancer.”
“I have to believe that this dream will come true because otherwise, why dream, right?” she said.
Dr. Champlin was the recipient of a Mechtild Harf Science Award and is a member of the board of DKMS U.S. Dr. Schmidt is employed by DKMS. Dr. Chen reported having no relevant disclosures.
Fibroids: Growing management options for a prevalent problem
OBG Manag. 33(12). | doi 10.12788/obgm.0169
See Gastroenterology’s curated ‘Equity in GI’ journal collection
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Gastroenterology, an AGA journal, is proud to announce the release of a special collection of articles focused on the intersection of diversity, equity, and inclusion (DEI) within gastroenterology and hepatology. This curated collection, under the guidance of the journal’s new DEI section editor Chyke Doubeni, MBBS, MPH, includes original research, reviews, commentaries, and editorials on matters of health disparities, socioeconomic determinants of health outcomes, and population-based studies on disease incidence among races and ethnicities, among others. New articles are added to the collection as they are published.
View the special collection on Gastroenterology’s website, which is designed to help you quickly and easily look over the latest DEI articles and content of interest. Recent articles include the following:
- “How to incorporate health equity training into GI/hepatology fellowships,” by Jannel Lee-Allen, MD, and Brijen J. Shah, MD.
- “Disparities in preventable mortality from colorectal cancer: Are they the result of structural racism?” by Chyke A. Doubeni, MBBS, MPH; Kevin Selby, MD; and Theodore R. Levin, MD.
- “COVID-19 pediatric patients: GI symptoms, presentations and disparities by race/ethnicity in a large, multicenter U.S. study,” by Yusuf Ashktorab, MD; Anas Brim, MD; Antonio Pizuorno, MD; Vijay Gayam, MD; Sahar Nikdel, MD; and Hassan Brim, PhD.
View all of Gastroenterology’s curated article collections.
Closer post-ESD surveillance for early GI neoplasia warranted
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
The new AGA Clinical Practice Update on Surveillance After Pathologically Curative Endoscopic Submucosal Dissection of Early Gastrointestinal Neoplasia in the United States: Commentary offers advice regarding surveillance intervals using endoscopy and other relevant modalities after endoscopic removal of dysplastic lesions and early GI cancers with endoscopic submucosal dissection (ESD) which were deemed pathologically curative.
Main takeaway: Patients with malignant lesions removed by curative ESD possess a higher risk of lymph node metastasis and should be surveilled more closely than those with resection dysplasia not associated with lymphatic spread.
Busting three myths about planned giving
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
Gifts to charitable organizations, such as the AGA Research Foundation, in your future plans can ensure that your support for our mission to fund young investigators will continue even after your lifetime. See these three fast facts about planned giving.
- Planned gifts are complicated and confusing. They don’t have to be. There are many types of planned gifts: Most are simple and affordable, like a gift in your will or living trust. You just need to find the one that best meets your needs.
- Wills are only for older adults. Having a plan for the future is important – no matter your age. A will makes your wishes known and provides your loved ones with peace of mind.
- Planned gifts are only for the wealthy. Anyone can make a planned gift. Gifts of all sizes make a difference at the AGA Research Foundation. In fact, you may even be able to make a bigger impact than you thought possible when you make a planned gift.
For 2022, consider including a gift to the AGA Research Foundation in your will. You will help spark future discoveries in GI.
Want to learn more about including a gift to the AGA Research Foundation in your plans? Visit our website at https://gastro.planmylegacy.org or contact us at foundation@gastro.org.
Migraine Signs and Symptoms
COMMENT & CONTROVERSY
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.
HOW TO CHOOSE THE RIGHT VAGINAL MOISTURIZER OR LUBRICANT FOR YOUR PATIENT
JOHN PENNYCUFF, MD, MSPH, AND CHERYL IGLESIA, MD (JUNE 2021)
Which vaginal products to recommend
We applaud Drs. Pennycuff and Iglesia for providing education on lubricants and vaginal moisturizers in their recent article, and agree that ObGyns, urogynecologists, and primary care providers should be aware of the types of products available. However, the authors underplayed the health risks associated with the use of poor-quality lubricants and moisturizers.
Women often turn to lubricants or vaginal moisturizers because they experience vaginal dryness during intercourse, related to menopause, and from certain medications. Vaginal fluid is primarily composed of exudate from capillaries in the vaginal wall. During sexual arousal, blood flow to the vaginal wall increases, and in turn, this should increase exudate. But chronic inflammation can suppress these increases in vaginal blood flow, preventing adequate vaginal fluid production. One such cause of chronic inflammation is using hyperosmolar lubricants, as this has been shown to negatively affect the vaginal epithelium.1,2 In this way, use of hyperosmolar lubricants can actually worsen symptoms, creating a vicious circle of dryness, lubricant use, and worsening dryness.
In addition, hyperosmolar lubricants have been shown to reduce the epithelial barrier properties of the vaginal epithelium, increasing susceptibility to microbes associated with bacterial vaginosis and to true pathogens, including herpes simplex virus type 2.3 In fact, hyperosmolar lubricants are a serious enough problem that the World Health Organization has weighed in, recommending osmolality of personal lubricants be under 380 mOsm/kg to prevent damage to the vaginal epithelium.4
Appropriately acidic pH is just as critical as osmolality. Using products with a pH higher than 4.5 will reduce amounts of protective lactobacilli and other commensal vaginal bacteria, encouraging growth of opportunistic bacteria and yeast already present. This can lead to bacterial vaginosis, aerobic vaginitis, and candidiasis. Bacterial vaginosis can lead to other serious sequelae such as increased risk in acquisition of HIV infection and preterm birth in pregnancy. Unfortunately, much of the data cited in Drs. Pennycuff and Iglesia’s article were sourced from another study (by Edwards and Panay published in Climacteric in 2016), which measured product pH values with an inappropriately calibrated device; the study’s supplemental information stated that calibration was between 5 and 9, and so any measurement below 5 was invalid and subject to error. For example, the Good Clean Love lubricant is listed as having a pH of 4.7, but its pH is never higher than 4.4.
The products on the market that meet the dual criteria of appropriate pH and isotonicity to vaginal epithelial cells may be less well known to consumers. But this should not be a reason to encourage use of hyperosmolar products whose main selling point is that they are the “leading brand.” Educating women on their choices in personal lubricants should include a full discussion of product ingredients and properties, based upon the available literature to help them select a product that supports the health of their intimate tissues.
Members of the Scientific Advisory Board for the Sexual Health and Wellness Institute: Jill Krapf, MD, MEd, IF; Cathy Chung Hwa Yi, MD; Christine Enzmann, MD, PhD, NMCP; Susan Kellogg-Spadt, PhD, CRNP, IF, CSC, FCST; Betsy Greenleaf, DO, MBA; Elizabeth DuPriest, PhD
References
- Dezzutti CS, Brown ER, Moncla B, et al. Is wetter better? An evaluation of over-the-counter personal lubricants for safety and anti-HIV-1 activity. PLoS One. 2012;7:e48328. doi: 10.1371/journal .pone.0048328.
- Ayehunie S, Wang YY, Landry T, et al. Hyperosmolal vaginal lubricants markedly reduce epithelial barrier properties in a threedimensional vaginal epithelium model. Toxicol Rep. 2017;5:134-140. doi: 10.1016 /j.toxrep.2017.12.011.
- Moench TR, Mumper RJ, Hoen TE, et al. Microbicide excipients can greatly increase susceptibility to genital herpes transmission in the mouse. BMC Infect Dis. 2010;10:331. doi: 10.1186/1471 -2334-10-331.
- Use and procurement of additional lubricants for male and female condoms: WHO/UNFPA /FHI360 Advisory note. World Health Organization, 2012. http://apps.who.int/iris/bitstream /handle/10665/76580/WHO_RHR_12.33_eng .pdf?sequence=1. Accessed December 27, 2021.
Drs. Pennycuff And Iglesia Respond
We thank the members of the scientific advisory board for the Sexual Health and Wellness Institute for their thoughtful and insightful comments to our article. We agree with their comments on the importance of both pH and osmolality for vaginal moisturizers and lubricants. We also agree that selection of an incorrectly formulated product may lead to worsening of vulvovaginal symptoms as well as dysbiosis and all of its sequelae as the letter writers mentioned.
In writing the review article, we attempted to address the role that pH and osmolality play in vaginal moisturizers and lubricants and make clinicians more aware of the importance of these factors in product formulation. Our goal was to help to improve patient counseling. We tried to amass as much of the available literature as we could to act as a resource for practitioners, such as the table included in the article as well as the supplemental table included online. We hoped that by writing this article we would heighten awareness among female health practitioners about vaginal health products and encourage them to consider those products that may be better suited for their patients based on pH and osmolality.
While there remains a paucity of research on vaginal moisturizers and lubricants, there is even less consumer knowledge regarding ingredients and formulations of these products. We wholeheartedly agree with the scientific advisory board that we as health providers need to help educate women on the full spectrum of products available beyond the “leading brands.” Furthermore, we advocate that there be continued research on these products as well as more manufacturer transparency regarding not only the ingredients contained within these products but also the pH and osmolality. Simple steps such as these would ensure that providers could help counsel patients to make informed decisions regarding products for their pelvic health.
Continue to: DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES...
DISMANTLING RACISM IN YOUR PERSONAL AND PROFESSIONAL SPHERES
CASSANDRA CARBERRY, MD, MS; ANNETTA MADSEN, MD; OLIVIA CARDENAS-TROWERS, MD; OLUWATENIOLA BROWN, MD; MOIURI SIDDIQUE, MD; AND BLAIR WASHINGTON, MD, MHA (AUGUST 2021)
Dissenting opinion
“Race is real but it’s not biologic.” “Race is not based on genetic or biologic inheritance.” Am I the only one with a dissenting voice of opinion when it comes to these types of statements?
Scott Peters, MD
Oak Ridge, Tennessee
The Authors Respond
Thank you for your opinion, Dr. Peters. Although it is not completely clear what your question is, it seems that it concerns the validity of the idea that race is a social construct. We will address this question with the assumption that this letter was an effort to invite discussion and increase understanding.
The National Human Genome Research Institute describes race in this way: “Race is a fluid concept used to group people according to various factors, including ancestral background and social identity. Race is also used to group people that share a set of visible characteristics, such as skin color and facial features. Though these visible traits are influenced by genes, the vast majority of genetic variation exists within racial groups and not between them.”1
The understanding that race is a social construct has been upheld by numerous medical organizations. In August 2020, a Joint Statement was published by the American College of Obstetricians and Gynecologists, the American Board of Obstetricians and Gynecologists, and 22 other organizations representing our specialty. This document states: “Recognizing that race is a social construct, not biologically based, is important to understanding that racism, not race, impacts health care, health, and health outcomes.”2
This idea is also endorsed by the AMA, who in November 2020 adopted the following policies3:
- “Recognize that race is a social construct and is distinct from ethnicity, genetic ancestry, or biology
- Support ending the practice of using race as a proxy for biology or genetics in medical education, research, and clinical practice.”
There are numerous sources that further illuminate why race is a social construct. Here are a few:
- https://www.racepowerofanillusion .org/resources/
- https ://www.pewresearch.org /fact-tank/2020/02/25/the-changing -categories-the-u-s-has-used-to -measure-race/
- Roberts D. Fatal Invention: How Science, Politics and Big Business Re-create Race in the Twenty-First Century. The New Press. 2011.
- Yudell M, Roberts D, DeSalle R, et al. Science and society. Taking race out of human genetics. Science. 2016;351(6273):564-5. doi: 10.1126/science.aac4951.
References
- National Human Genome Research Institute. Race. https://www.genome.gov/genetic-glossary /Race. Accessed December 27, 2021.
- The American College of Obstetricians and Gynecologists. Joint Statement: Collective Action Addressing Racism. https://www.acog.org /news/news-articles/2020/08/joint-statementobstetrics-and-gynecology-collective-actionaddressing-racism.
- O’Reilly KB. AMA: Racism is a threat to public health. November 16, 2020. https://www.ama -assn.org/delivering-care/health-equity/ama -racism-threat-public-health. Accessed December 27, 2021.