User login
Complex regional pain syndrome: Steps that FPs can take
USPSTF weighs in on postmenopausal hormone therapy to prevent chronic conditions
Resources
US Preventive Services Task Force. Final Recommendation Statement: Hormone Therapy in Postmenopausal Women: Primary Prevention of Chronic Conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/menopausal-hormone-therapy-preventive-medication1. Accessed January 5, 2018.
Resources
US Preventive Services Task Force. Final Recommendation Statement: Hormone Therapy in Postmenopausal Women: Primary Prevention of Chronic Conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/menopausal-hormone-therapy-preventive-medication1. Accessed January 5, 2018.
Resources
US Preventive Services Task Force. Final Recommendation Statement: Hormone Therapy in Postmenopausal Women: Primary Prevention of Chronic Conditions. Available at: https://www.uspreventiveservicestaskforce.org/Page/Document/RecommendationStatementFinal/menopausal-hormone-therapy-preventive-medication1. Accessed January 5, 2018.
Using oral and topical cosmeceuticals to prevent and treat skin aging, Part II
This month’s column resumes my review of prevention and treatment strategies for aging skin using oral and topical cosmeceutical agents.
Preventing and treating inflammation
Skin aging can result from inflammation through several mechanisms, including the formation of reactive oxygen species. Inflammation itself arises from myriad etiologic pathways, with multiple inflammatory mediators potentially involved, including histamines, cytokines, eicosanoids (for example, prostaglandins, thromboxanes, and leukotrienes), complement cascade components, kinins, fibrinopeptide enzymes, nuclear factor–kappa B, and free radicals.
Topically applied argan oil, caffeine, chamomile, feverfew, green tea, licorice extract, aloe, linoleic acid (found in high concentrations in argan oil and safflower oil), and niacinamide are among the anti-inflammatory ingredients that have been used successfully in topical skin care to reduce inflammation. The Food and Drug Administration does not allow cosmetics to make “anti-inflammatory” claims. For this reason, these products will state they have “soothing” effects or imply they improve of redness.
Oral polypodium leucotomos has been demonstrated to suppress the effect of UV radiation on COX-2 expression.2 Also, glycolic acid has exhibited the capacity to inhibit COX-2 signaling and other inflammatory mediators.3
Preventing and treating glycation
Glycation is produced by the Maillard reaction, a chemical reaction – particularly well known in cooking – between an amino acid and a sugar molecule that typically requires heat. This reaction was first described by Louis Camille Maillard in 1912 when he noted that amino acids can react with sugar to yield brown or golden-brown substances. It took until the 1980s for scientists to understand the importance of glycation in health.
When glycation occurs, sugar molecules attach to proteins, creating cross-linked proteins known as advanced glycation end products (or AGEs) and causing a series of chemical reactions. Glycation occurs in collagen fibers and results in the formation of cross-links that bind collagen fibers to each other, which leaves the skin stiffer. Glycosylated collagen is believed to be a factor in the appearance of aged skin.4 Glycation also can affect elastin: Recent research suggests that glycation can engender elastosis, which is elastin that is abnormally clumped together and presents more frequently in aged skin.
Several antiaging skin care products claim to treat glycation, but – unfortunately – glycation is not a reversible reaction. It must be prevented in the first place. Some studies suggest that antioxidants can prevent glycation, but it is more likely that they just divert the process down a different pathway that still leads to glycation. Reducing serum glucose levels is the optimum method of preventing glycation.5 Dietary intervention and oral metformin are recommended for lowering glycation.
REVERSING SKIN CELL AGING
Epidermal keratinocytes in aging
Young basal stem cells synthesize a plethora of new keratinocytes at a pace that leads to fast cell turnover and vigorous production of protective epidermal constituents. Old keratinocytes display less energy, show reduced responsiveness to cellular signals, and do not synthesize these protective components.6,7 Keratinocyte stem cell function declines over time while damage accumulates, as seen in a diminished response to growth factors, decreased keratinization, and impaired function.8
Dermal fibroblasts in cutaneous aging
Young fibroblasts produce key cellular constituents, including collagen, elastin, hyaluronic acid, and heparan sulfate. This production declines in older fibroblasts. Like aging keratinocytes, old fibroblasts lose energy and responsiveness to growth factors and other cellular signals.6,7
Rejuvenating aged skin with cosmeceuticals
Gene expression, growth factors, cytokines, chemokines, and receptor activation guide the function of keratinocytes and fibroblasts. To reverse or slow cellular skin aging, old keratinocytes and fibroblasts must be galvanized to respond to such signals or the signals must be enhanced.
Stimulating old keratinocytes and fibroblasts
Essential steps in stimulating aged keratinocytes and fibroblasts include: activating gene expression, adding growth factors, activating cytokines and chemokines, turning on receptors, and making cells more responsive to signals.
Influencing gene expression
Retinoids are known to affect collagen genes and increase activity of procollagen genes, thereby reducing the production of collagenase. Many studies have shown the efficacy of retinoids in treating aged skin and preventing cutaneous aging in both areas frequently exposed to the sun but also those that aren’t.9,10 Prescription retinoids (tretinoin, adapalene, tazarotene) and over-the-counter retinoids (retinol) are first-line options to treat and prevent aging by stimulating old keratinocytes and fibroblasts.10,11 However, exposing retinoic acid receptors to retinoids almost invariably leads to erythema and flaking in the first few weeks. Therefore, retinoids should be titrated slowly. Note that retinoid esters, such as retinyl palmitate and retinyl linoleate, do not penetrate well into the dermis;12 they also are not as effective as retinol, tretinoin, adapalene, and tazarotene. Compliance with retinoids is always an issue with patients. They should receive printed educational material about how to begin use and why it is important to use these products consistently.
Growth factors
The use of cosmetic formulations that contain growth factors can contribute to skin rejuvenation. There are various types of growth factors that have the capacity to stimulate old keratinocytes and fibroblasts to enhance function.17 Growth factors, which are inactive or vulnerable to degradation in their native, soluble form, can directly energize genes or act as a signaling mechanism. To exert their quintessential functions, growth factors must be transferred to the correct receptor site in order for the cell to respond to their signal.18
Heparan sulfate
Heparan sulfate (HS) plays a primary role in cell-to-cell communications. It increases cellular response to growth factors by facilitating the response of old, lazy fibroblasts to the cellular signals.18 HS binds, stores, and protects growth factors, which allows them to complete movement to their targets, and then presents them to the appropriate binding site.18,19 A topically applied analogue of HS has been demonstrated to rejuvenate aged skin.20
Stem cells
Stem cells included and pointedly marketed in cosmeceutical products are usually plant derived, are too large to penetrate the stratum corneum, display short shelf lives, and do not behave as human stem cells would. As a result, stem cells in cosmeceutical agents are essentially useless.
However, novel technologies have revealed ingredients that can incite native stem cells to repopulate the epidermis and dermis with young cells. Stem cells in skin include basal stem cells and 10 varieties of hair follicle stem cells. The LGR6+ hair follicle cells play a pivotal role in repopulating the epidermis after wounding has occurred.21,22 Aesthetic physicians have known for several years that inducing skin wounding with lasers, needles, and acidic peels leads to improvement in its appearance. Researchers have provided new data showing that wounding the skin prompts LGR6+ stem cells to repopulate the epidermis. Once wounding occurs, neutrophils release the peptide defensin, which stimulates the LGR6+ stem cells to repopulate the epidermis.23 Topical defensin that has been formulated to penetrate into hair follicles, where the LGR6+ stem cells reside, has been demonstrated to render a smoother, more youthful appearance to the skin.
Conclusion
It is important for practitioners to identify patients at risk for premature skin aging as early as possible and start them on an appropriate and consistent skin care regimen. This typically will include at least a daily sunscreen with an SPF 15 or higher, a nightly topical retinoid, and oral and topical antioxidants. The patient’s additional skin type proclivities (for example, dryness, inflammation, melanocyte activity) should guide the physician as to how to combine these baseline product types with cleansers, moisturizers, and formulations with hydroxy acids, growth factors, heparan sulfate, and defensin.
Several studies have revealed that patients exhibit poor compliance with recommended regimens.24 Informing patients about the need for skin protection and providing printed instructions can help to improve compliance.25 This can promote healthy lifestyle habits and compliance with scientifically proven antiaging therapies.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
1. Arch Dermatol Res. 2010 Jan;302(1):5-17.
2. Am J Pathol. 2009 Nov;175(5):1952-61.
3. J Dermatol Sci. 2017 Jun;86(3):238-48.
4. Eur J Dermatol. 2007 Jan-Feb;17(1):12-20.
5. “Advanced Glycation End Products (AGEs): Emerging Mediators of Skin Aging,” in Textbook of Aging Skin (Berlin: Springer, 2017, pp. 1675-86).
6. Mech Ageing Dev. 1986 Jul;35(2):185-98.
7. Exp Cell Res. 1996 Sep 15;227(2):252-5.
8. J Cutan Pathol. 2003 Jul;30(6):351-7.
9. PLoS One. 2015 Feb 6;10(2):e0117491.
10. Arch Dermatol. 2007 May;143(5):606-12.
11. JAMA. 1988 Jan 22-29;259(4):527-32.
12. J Invest Dermatol. 1997 Sep;109(3):301-5.
13. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.
14. J Am Acad Dermatol. 1996 Sep;35(3 Pt 1):388-91.
15. Dermatol Surg. 2001 May;27(5):429-33.
16. J Invest Dermatol. 1994 Aug;103(2):228-32.
17. Clin Cosmet Investig Dermatol. 2016 Nov 9;9:411-9.
18. Chem Biol Drug Des. 2008 Dec;72(6):455-82.
19. Front Immunol. 2013 Dec 18;4:470.
20. J Drugs Dermatol. 2015 Jul;14(7):669-74.
21. Science. 2010 Mar 12;327(5971):1385-9.
22. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
23. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
24. J Am Acad Dermatol. 2008 Jul;59(1):27-33.
25. J Am Acad Dermatol. 2013 Mar;68(3):364.e1-10.
This month’s column resumes my review of prevention and treatment strategies for aging skin using oral and topical cosmeceutical agents.
Preventing and treating inflammation
Skin aging can result from inflammation through several mechanisms, including the formation of reactive oxygen species. Inflammation itself arises from myriad etiologic pathways, with multiple inflammatory mediators potentially involved, including histamines, cytokines, eicosanoids (for example, prostaglandins, thromboxanes, and leukotrienes), complement cascade components, kinins, fibrinopeptide enzymes, nuclear factor–kappa B, and free radicals.
Topically applied argan oil, caffeine, chamomile, feverfew, green tea, licorice extract, aloe, linoleic acid (found in high concentrations in argan oil and safflower oil), and niacinamide are among the anti-inflammatory ingredients that have been used successfully in topical skin care to reduce inflammation. The Food and Drug Administration does not allow cosmetics to make “anti-inflammatory” claims. For this reason, these products will state they have “soothing” effects or imply they improve of redness.
Oral polypodium leucotomos has been demonstrated to suppress the effect of UV radiation on COX-2 expression.2 Also, glycolic acid has exhibited the capacity to inhibit COX-2 signaling and other inflammatory mediators.3
Preventing and treating glycation
Glycation is produced by the Maillard reaction, a chemical reaction – particularly well known in cooking – between an amino acid and a sugar molecule that typically requires heat. This reaction was first described by Louis Camille Maillard in 1912 when he noted that amino acids can react with sugar to yield brown or golden-brown substances. It took until the 1980s for scientists to understand the importance of glycation in health.
When glycation occurs, sugar molecules attach to proteins, creating cross-linked proteins known as advanced glycation end products (or AGEs) and causing a series of chemical reactions. Glycation occurs in collagen fibers and results in the formation of cross-links that bind collagen fibers to each other, which leaves the skin stiffer. Glycosylated collagen is believed to be a factor in the appearance of aged skin.4 Glycation also can affect elastin: Recent research suggests that glycation can engender elastosis, which is elastin that is abnormally clumped together and presents more frequently in aged skin.
Several antiaging skin care products claim to treat glycation, but – unfortunately – glycation is not a reversible reaction. It must be prevented in the first place. Some studies suggest that antioxidants can prevent glycation, but it is more likely that they just divert the process down a different pathway that still leads to glycation. Reducing serum glucose levels is the optimum method of preventing glycation.5 Dietary intervention and oral metformin are recommended for lowering glycation.
REVERSING SKIN CELL AGING
Epidermal keratinocytes in aging
Young basal stem cells synthesize a plethora of new keratinocytes at a pace that leads to fast cell turnover and vigorous production of protective epidermal constituents. Old keratinocytes display less energy, show reduced responsiveness to cellular signals, and do not synthesize these protective components.6,7 Keratinocyte stem cell function declines over time while damage accumulates, as seen in a diminished response to growth factors, decreased keratinization, and impaired function.8
Dermal fibroblasts in cutaneous aging
Young fibroblasts produce key cellular constituents, including collagen, elastin, hyaluronic acid, and heparan sulfate. This production declines in older fibroblasts. Like aging keratinocytes, old fibroblasts lose energy and responsiveness to growth factors and other cellular signals.6,7
Rejuvenating aged skin with cosmeceuticals
Gene expression, growth factors, cytokines, chemokines, and receptor activation guide the function of keratinocytes and fibroblasts. To reverse or slow cellular skin aging, old keratinocytes and fibroblasts must be galvanized to respond to such signals or the signals must be enhanced.
Stimulating old keratinocytes and fibroblasts
Essential steps in stimulating aged keratinocytes and fibroblasts include: activating gene expression, adding growth factors, activating cytokines and chemokines, turning on receptors, and making cells more responsive to signals.
Influencing gene expression
Retinoids are known to affect collagen genes and increase activity of procollagen genes, thereby reducing the production of collagenase. Many studies have shown the efficacy of retinoids in treating aged skin and preventing cutaneous aging in both areas frequently exposed to the sun but also those that aren’t.9,10 Prescription retinoids (tretinoin, adapalene, tazarotene) and over-the-counter retinoids (retinol) are first-line options to treat and prevent aging by stimulating old keratinocytes and fibroblasts.10,11 However, exposing retinoic acid receptors to retinoids almost invariably leads to erythema and flaking in the first few weeks. Therefore, retinoids should be titrated slowly. Note that retinoid esters, such as retinyl palmitate and retinyl linoleate, do not penetrate well into the dermis;12 they also are not as effective as retinol, tretinoin, adapalene, and tazarotene. Compliance with retinoids is always an issue with patients. They should receive printed educational material about how to begin use and why it is important to use these products consistently.
Growth factors
The use of cosmetic formulations that contain growth factors can contribute to skin rejuvenation. There are various types of growth factors that have the capacity to stimulate old keratinocytes and fibroblasts to enhance function.17 Growth factors, which are inactive or vulnerable to degradation in their native, soluble form, can directly energize genes or act as a signaling mechanism. To exert their quintessential functions, growth factors must be transferred to the correct receptor site in order for the cell to respond to their signal.18
Heparan sulfate
Heparan sulfate (HS) plays a primary role in cell-to-cell communications. It increases cellular response to growth factors by facilitating the response of old, lazy fibroblasts to the cellular signals.18 HS binds, stores, and protects growth factors, which allows them to complete movement to their targets, and then presents them to the appropriate binding site.18,19 A topically applied analogue of HS has been demonstrated to rejuvenate aged skin.20
Stem cells
Stem cells included and pointedly marketed in cosmeceutical products are usually plant derived, are too large to penetrate the stratum corneum, display short shelf lives, and do not behave as human stem cells would. As a result, stem cells in cosmeceutical agents are essentially useless.
However, novel technologies have revealed ingredients that can incite native stem cells to repopulate the epidermis and dermis with young cells. Stem cells in skin include basal stem cells and 10 varieties of hair follicle stem cells. The LGR6+ hair follicle cells play a pivotal role in repopulating the epidermis after wounding has occurred.21,22 Aesthetic physicians have known for several years that inducing skin wounding with lasers, needles, and acidic peels leads to improvement in its appearance. Researchers have provided new data showing that wounding the skin prompts LGR6+ stem cells to repopulate the epidermis. Once wounding occurs, neutrophils release the peptide defensin, which stimulates the LGR6+ stem cells to repopulate the epidermis.23 Topical defensin that has been formulated to penetrate into hair follicles, where the LGR6+ stem cells reside, has been demonstrated to render a smoother, more youthful appearance to the skin.
Conclusion
It is important for practitioners to identify patients at risk for premature skin aging as early as possible and start them on an appropriate and consistent skin care regimen. This typically will include at least a daily sunscreen with an SPF 15 or higher, a nightly topical retinoid, and oral and topical antioxidants. The patient’s additional skin type proclivities (for example, dryness, inflammation, melanocyte activity) should guide the physician as to how to combine these baseline product types with cleansers, moisturizers, and formulations with hydroxy acids, growth factors, heparan sulfate, and defensin.
Several studies have revealed that patients exhibit poor compliance with recommended regimens.24 Informing patients about the need for skin protection and providing printed instructions can help to improve compliance.25 This can promote healthy lifestyle habits and compliance with scientifically proven antiaging therapies.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
1. Arch Dermatol Res. 2010 Jan;302(1):5-17.
2. Am J Pathol. 2009 Nov;175(5):1952-61.
3. J Dermatol Sci. 2017 Jun;86(3):238-48.
4. Eur J Dermatol. 2007 Jan-Feb;17(1):12-20.
5. “Advanced Glycation End Products (AGEs): Emerging Mediators of Skin Aging,” in Textbook of Aging Skin (Berlin: Springer, 2017, pp. 1675-86).
6. Mech Ageing Dev. 1986 Jul;35(2):185-98.
7. Exp Cell Res. 1996 Sep 15;227(2):252-5.
8. J Cutan Pathol. 2003 Jul;30(6):351-7.
9. PLoS One. 2015 Feb 6;10(2):e0117491.
10. Arch Dermatol. 2007 May;143(5):606-12.
11. JAMA. 1988 Jan 22-29;259(4):527-32.
12. J Invest Dermatol. 1997 Sep;109(3):301-5.
13. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.
14. J Am Acad Dermatol. 1996 Sep;35(3 Pt 1):388-91.
15. Dermatol Surg. 2001 May;27(5):429-33.
16. J Invest Dermatol. 1994 Aug;103(2):228-32.
17. Clin Cosmet Investig Dermatol. 2016 Nov 9;9:411-9.
18. Chem Biol Drug Des. 2008 Dec;72(6):455-82.
19. Front Immunol. 2013 Dec 18;4:470.
20. J Drugs Dermatol. 2015 Jul;14(7):669-74.
21. Science. 2010 Mar 12;327(5971):1385-9.
22. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
23. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
24. J Am Acad Dermatol. 2008 Jul;59(1):27-33.
25. J Am Acad Dermatol. 2013 Mar;68(3):364.e1-10.
This month’s column resumes my review of prevention and treatment strategies for aging skin using oral and topical cosmeceutical agents.
Preventing and treating inflammation
Skin aging can result from inflammation through several mechanisms, including the formation of reactive oxygen species. Inflammation itself arises from myriad etiologic pathways, with multiple inflammatory mediators potentially involved, including histamines, cytokines, eicosanoids (for example, prostaglandins, thromboxanes, and leukotrienes), complement cascade components, kinins, fibrinopeptide enzymes, nuclear factor–kappa B, and free radicals.
Topically applied argan oil, caffeine, chamomile, feverfew, green tea, licorice extract, aloe, linoleic acid (found in high concentrations in argan oil and safflower oil), and niacinamide are among the anti-inflammatory ingredients that have been used successfully in topical skin care to reduce inflammation. The Food and Drug Administration does not allow cosmetics to make “anti-inflammatory” claims. For this reason, these products will state they have “soothing” effects or imply they improve of redness.
Oral polypodium leucotomos has been demonstrated to suppress the effect of UV radiation on COX-2 expression.2 Also, glycolic acid has exhibited the capacity to inhibit COX-2 signaling and other inflammatory mediators.3
Preventing and treating glycation
Glycation is produced by the Maillard reaction, a chemical reaction – particularly well known in cooking – between an amino acid and a sugar molecule that typically requires heat. This reaction was first described by Louis Camille Maillard in 1912 when he noted that amino acids can react with sugar to yield brown or golden-brown substances. It took until the 1980s for scientists to understand the importance of glycation in health.
When glycation occurs, sugar molecules attach to proteins, creating cross-linked proteins known as advanced glycation end products (or AGEs) and causing a series of chemical reactions. Glycation occurs in collagen fibers and results in the formation of cross-links that bind collagen fibers to each other, which leaves the skin stiffer. Glycosylated collagen is believed to be a factor in the appearance of aged skin.4 Glycation also can affect elastin: Recent research suggests that glycation can engender elastosis, which is elastin that is abnormally clumped together and presents more frequently in aged skin.
Several antiaging skin care products claim to treat glycation, but – unfortunately – glycation is not a reversible reaction. It must be prevented in the first place. Some studies suggest that antioxidants can prevent glycation, but it is more likely that they just divert the process down a different pathway that still leads to glycation. Reducing serum glucose levels is the optimum method of preventing glycation.5 Dietary intervention and oral metformin are recommended for lowering glycation.
REVERSING SKIN CELL AGING
Epidermal keratinocytes in aging
Young basal stem cells synthesize a plethora of new keratinocytes at a pace that leads to fast cell turnover and vigorous production of protective epidermal constituents. Old keratinocytes display less energy, show reduced responsiveness to cellular signals, and do not synthesize these protective components.6,7 Keratinocyte stem cell function declines over time while damage accumulates, as seen in a diminished response to growth factors, decreased keratinization, and impaired function.8
Dermal fibroblasts in cutaneous aging
Young fibroblasts produce key cellular constituents, including collagen, elastin, hyaluronic acid, and heparan sulfate. This production declines in older fibroblasts. Like aging keratinocytes, old fibroblasts lose energy and responsiveness to growth factors and other cellular signals.6,7
Rejuvenating aged skin with cosmeceuticals
Gene expression, growth factors, cytokines, chemokines, and receptor activation guide the function of keratinocytes and fibroblasts. To reverse or slow cellular skin aging, old keratinocytes and fibroblasts must be galvanized to respond to such signals or the signals must be enhanced.
Stimulating old keratinocytes and fibroblasts
Essential steps in stimulating aged keratinocytes and fibroblasts include: activating gene expression, adding growth factors, activating cytokines and chemokines, turning on receptors, and making cells more responsive to signals.
Influencing gene expression
Retinoids are known to affect collagen genes and increase activity of procollagen genes, thereby reducing the production of collagenase. Many studies have shown the efficacy of retinoids in treating aged skin and preventing cutaneous aging in both areas frequently exposed to the sun but also those that aren’t.9,10 Prescription retinoids (tretinoin, adapalene, tazarotene) and over-the-counter retinoids (retinol) are first-line options to treat and prevent aging by stimulating old keratinocytes and fibroblasts.10,11 However, exposing retinoic acid receptors to retinoids almost invariably leads to erythema and flaking in the first few weeks. Therefore, retinoids should be titrated slowly. Note that retinoid esters, such as retinyl palmitate and retinyl linoleate, do not penetrate well into the dermis;12 they also are not as effective as retinol, tretinoin, adapalene, and tazarotene. Compliance with retinoids is always an issue with patients. They should receive printed educational material about how to begin use and why it is important to use these products consistently.
Growth factors
The use of cosmetic formulations that contain growth factors can contribute to skin rejuvenation. There are various types of growth factors that have the capacity to stimulate old keratinocytes and fibroblasts to enhance function.17 Growth factors, which are inactive or vulnerable to degradation in their native, soluble form, can directly energize genes or act as a signaling mechanism. To exert their quintessential functions, growth factors must be transferred to the correct receptor site in order for the cell to respond to their signal.18
Heparan sulfate
Heparan sulfate (HS) plays a primary role in cell-to-cell communications. It increases cellular response to growth factors by facilitating the response of old, lazy fibroblasts to the cellular signals.18 HS binds, stores, and protects growth factors, which allows them to complete movement to their targets, and then presents them to the appropriate binding site.18,19 A topically applied analogue of HS has been demonstrated to rejuvenate aged skin.20
Stem cells
Stem cells included and pointedly marketed in cosmeceutical products are usually plant derived, are too large to penetrate the stratum corneum, display short shelf lives, and do not behave as human stem cells would. As a result, stem cells in cosmeceutical agents are essentially useless.
However, novel technologies have revealed ingredients that can incite native stem cells to repopulate the epidermis and dermis with young cells. Stem cells in skin include basal stem cells and 10 varieties of hair follicle stem cells. The LGR6+ hair follicle cells play a pivotal role in repopulating the epidermis after wounding has occurred.21,22 Aesthetic physicians have known for several years that inducing skin wounding with lasers, needles, and acidic peels leads to improvement in its appearance. Researchers have provided new data showing that wounding the skin prompts LGR6+ stem cells to repopulate the epidermis. Once wounding occurs, neutrophils release the peptide defensin, which stimulates the LGR6+ stem cells to repopulate the epidermis.23 Topical defensin that has been formulated to penetrate into hair follicles, where the LGR6+ stem cells reside, has been demonstrated to render a smoother, more youthful appearance to the skin.
Conclusion
It is important for practitioners to identify patients at risk for premature skin aging as early as possible and start them on an appropriate and consistent skin care regimen. This typically will include at least a daily sunscreen with an SPF 15 or higher, a nightly topical retinoid, and oral and topical antioxidants. The patient’s additional skin type proclivities (for example, dryness, inflammation, melanocyte activity) should guide the physician as to how to combine these baseline product types with cleansers, moisturizers, and formulations with hydroxy acids, growth factors, heparan sulfate, and defensin.
Several studies have revealed that patients exhibit poor compliance with recommended regimens.24 Informing patients about the need for skin protection and providing printed instructions can help to improve compliance.25 This can promote healthy lifestyle habits and compliance with scientifically proven antiaging therapies.
Dr. Baumann is a private practice dermatologist, researcher, author, and entrepreneur who practices in Miami. She founded the Cosmetic Dermatology Center at the University of Miami in 1997. Dr. Baumann wrote two textbooks: “Cosmetic Dermatology: Principles and Practice” (New York: McGraw-Hill, 2002) and “Cosmeceuticals and Cosmetic Ingredients” (New York: McGraw-Hill, 2014); she also authored a New York Times Best Seller for consumers, “The Skin Type Solution” (New York: Bantam Dell, 2006). Dr. Baumann has received funding for advisory boards and/or clinical research trials from Allergan, Evolus, Galderma, and Revance Therapeutics. She is the founder and CEO of Skin Type Solutions Franchise Systems LLC.
1. Arch Dermatol Res. 2010 Jan;302(1):5-17.
2. Am J Pathol. 2009 Nov;175(5):1952-61.
3. J Dermatol Sci. 2017 Jun;86(3):238-48.
4. Eur J Dermatol. 2007 Jan-Feb;17(1):12-20.
5. “Advanced Glycation End Products (AGEs): Emerging Mediators of Skin Aging,” in Textbook of Aging Skin (Berlin: Springer, 2017, pp. 1675-86).
6. Mech Ageing Dev. 1986 Jul;35(2):185-98.
7. Exp Cell Res. 1996 Sep 15;227(2):252-5.
8. J Cutan Pathol. 2003 Jul;30(6):351-7.
9. PLoS One. 2015 Feb 6;10(2):e0117491.
10. Arch Dermatol. 2007 May;143(5):606-12.
11. JAMA. 1988 Jan 22-29;259(4):527-32.
12. J Invest Dermatol. 1997 Sep;109(3):301-5.
13. J Am Acad Dermatol. 1996 Feb;34(2 Pt 1):187-95.
14. J Am Acad Dermatol. 1996 Sep;35(3 Pt 1):388-91.
15. Dermatol Surg. 2001 May;27(5):429-33.
16. J Invest Dermatol. 1994 Aug;103(2):228-32.
17. Clin Cosmet Investig Dermatol. 2016 Nov 9;9:411-9.
18. Chem Biol Drug Des. 2008 Dec;72(6):455-82.
19. Front Immunol. 2013 Dec 18;4:470.
20. J Drugs Dermatol. 2015 Jul;14(7):669-74.
21. Science. 2010 Mar 12;327(5971):1385-9.
22. Plast Reconstr Surg. 2014 Mar;133(3):579-90.
23. Plast Reconstr Surg. 2013 Nov;132(5):1159-71.
24. J Am Acad Dermatol. 2008 Jul;59(1):27-33.
25. J Am Acad Dermatol. 2013 Mar;68(3):364.e1-10.
Antipsychotics for obsessive-compulsive disorder: Weighing risks vs benefits
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of
Box
Antipsychotics for OCD: What the guidelines recommend
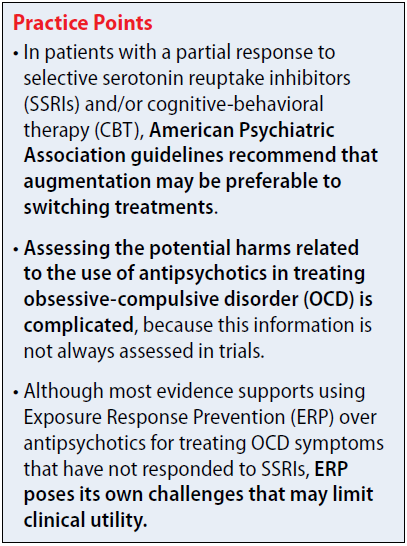
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of

Box
Antipsychotics for OCD: What the guidelines recommend
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
Mr. E, age 37, has a 20-year history of obsessive-compulsive disorder (OCD), with comorbid generalized anxiety disorder and hypertension. His medication regimen consists of

Box
Antipsychotics for OCD: What the guidelines recommend
The 2013 American Psychiatric Association (APA) obsessive-compulsive disorder (OCD) treatment guidelines include recommendations regarding the use of antipsychotics in patients who do not respond to first-line treatment with selective serotonin reuptake inhibitors (SSRIs) and/or cognitive-behavioral therapy (CBT). The APA recommends evaluating contributing factors, including comorbidities, family support, and ability to tolerate psychotherapy or maximum recommended drug doses, before augmenting or switching therapies.1
In patients with a partial response to SSRIs and/or CBT, the APA suggests that augmentation may be preferable to switching treatments. Augmentation strategies for SSRIs include antipsychotics or CBT with Exposure Response Prevention (ERP); augmentation strategies for CBT include SSRIs. Combining SSRIs and CBT may decrease the chance of relapse when medication is discontinued. If the patient has a partial response to ERP, intensification of therapy also can be considered based on patient-specific factors. In non-responders, switching therapies may be necessary. Alternative treatments including a different SSRI; an antidepressant from a difference class, such as clomipramine or mirtazapine; an antipsychotic; or CBT.
The 2006 National Institute for Health and Clinical Excellence guidelines for OCD recommend additional high-intensity CBT, adding an antipsychotic to an SSRI or clomipramine, or combining clomipramine with citalopram in non-responders. There is no guidance regarding the order in which these treatments should be trialed. Antipsychotics are recommended as an entire class, and there are no recommendations regarding dosing or long-term risks. These guidelines are based on limited evidence, including only 1 trial of quetiapine and 1 trial of olanzapine.2,3
Efficacy
The 2013 National Institute for Health Care and Excellence Evidence Update included a 2010 Cochrane Review of 11 randomized controlled trials (RCTs) of antipsychotics as adjunctive treatment to SSRIs.5 All trials were <6 months, and most were limited regarding quality aspects. Two trials found no statistically significant difference with olanzapine in efficacy measures (Y-BOCS mean difference [MD] −2.96; 95% confidence interval [CI] −7.41 to 1.22; effect size d = −2.96 [−7.14, 1.22]). Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was no significant difference between groups (n = 44, 1 RCT, odds ratio [OR] 0.76; 95% CI 0.17 to 3.29; effect size d = 0.76 [0.17, 3.29]). Studies found increased weight gain with olanzapine compared with antidepressant monotherapy.
Statistically significant differences were demonstrated with the addition of quetiapine to antidepressant monotherapy as shown in Y-BOCS score at endpoint (Y-BOCS MD −2.28; 95% CI −4.05 to −0.52; effect size d −2.28 [−4.05, −0.52]). Quetiapine also demonstrated benefit for depressive and anxiety symptoms. Among patients with no clinically significant change (defined as ≤35% reduction in Y-BOCS), there was a significant difference between groups (n = 80, 2 RCTs, OR 0.27; 95% CI 0.09 to 0.87; effect size d = 0.27 [0.09, 0.87]).
Adjunctive treatment with risperidone was superior to antidepressant monotherapy for participants without a significant response in OCD symptom severity of at least 25% with validated measures (OR 0.17; 95% CI 0.04 to 0.66; effect size d = 0.17 [0.04, 0.66]), and in depressive and anxiety symptoms. Mean reduction in Y-BOCS scores was not statistically significant with risperidone (MD −3.35; 95% CI −8.25 to 1.55; effect size d = −3.35 [−8.25, 1.55]).5
A 2014 meta-analysis by Veale et al3 included double-blind, randomized trials that examined atypical antipsychotics compared with placebo for adults with OCD that used an intention-to-treat analysis. Unlike the Cochrane Review, these studies used the Y-BOCS as a primary outcome measure. Participants had a Y-BOCS score of ≥16; had at least 1 appropriate trial of an SSRI or clomipramine (defined as the maximum dose tolerated for at least 8 weeks); and had to continue taking the SSRI or
Fourteen articles were included in the meta-analysis, but all had small sample sizes and no long-term follow-up data.3 Antipsychotics in the meta-analysis included risperidone (4 studies), quetiapine (5 studies), olanzapine (2 studies),
The overall difference in Y-BOCS score change between drug and placebo groups was 2.34 points, which had an overall effect size of d = 0.40. Those taking antipsychotics had approximately a 10% reduction in Y-BOCS score over time. The overall difference was statistically significant with risperidone (overall mean reduction of 3.89 points on the Y-BOCS; 95% CI 1.43 to 5.48; effect size of d = 0.53) and aripiprazole (difference in Y-BOCS outcome 0.1 scores of 6.29 points; effect size of d = 1.11). One trial of risperidone used a low dose (0.5 mg) and had a larger effect size than the studies that used moderate doses. The overall difference was not statistically significant for quetiapine (difference of Y-BOCS outcome scores of 0.81 points) or olanzapine (difference in Y-BOCS outcome scores of −0.19; indicating <1 point difference on the Y-BOCS).3
Studies included in the meta-analysis ranged in durations from 6 to 16 weeks; duration of ≥4 weeks did not make a difference in response. One study demonstrated a worsening of symptoms in the quetiapine group between weeks 4 and 12. Only 4 studies included most patients that had a previous trial of CBT. One study with an additional treatment arm evaluating CBT found that adding CBT was superior to adjunctive risperidone or placebo. Another study found that adding clomipramine or placebo to
Two studies included in the meta-analysis classified OCD symptoms by subtype, such as by dimensions of checking; symmetry, ordering, counting, and repeating; contamination and cleaning; and hoarding. Currently, no clinically significant predictor of outcome of antipsychotic therapy has been identified. Two studies included in the meta-analysis assessed patients with comorbid tic disorders and found no difference by treatment. One study demonstrated benefit of haloperidol in patients with comorbid tic disorders compared with those without comorbid tic disorders. Of note, none of the studies included in the meta-analysis excluded patients with hoarding characteristics, which generally indicate a worse prognosis with treatment.3
In 2015, Dold et al6 provided an update to a 2013 meta-analysis7 assessing antipsychotic augmentation of SSRIs in treatment-resistant OCD. This update included 2 new RCTs. The 2013 analysis7 concluded that risperidone should be considered first-line and is preferred over olanzapine and quetiapine. However, the update found the highest effect size for aripiprazole (d = −1.35), followed by haloperidol (d = −0.82), risperidone (d = −0.59), quetiapine (d = −0.50), olanzapine (d = −0.49), and paliperidone (d = −0.21).6,7
The 2015 update6 concluded that the antipsychotic doses used in trials were moderate and that there was no association between dose and treatment response, indicating that high doses of antipsychotics may not be more effective. Dold et al6 postulated that the antipsychotic doses required for treating OCD are similar to those used in treating major depressive disorder and lower than doses used in treating schizophrenia. The 2013 meta-analysis demonstrated that moderate doses of antipsychotics resulted in statistically significant efficacy (relative risk [RR] = 3.99, 95% CI 1.92 to 8.27), while low doses did not demonstrate statistical significance (RR = 1.06, 95% CI 0.45 to 2.53).6,7
The 2015 subgroup analysis update evaluated the duration of SSRI treatment prior to the antipsychotic augmentation phase, but did not demonstrate statistically significant efficacy for studies with <8 weeks’ duration of SSRI treatment, further highlighting the need for extended duration of treatment with an SSRI prior to augmentation.6
The 2013 meta-analysis discussed populations with comorbid tic disorders, including a study that found that patients with OCD and comorbid tic disorders benefit more from adjunctive antipsychotic therapy than those without the comorbidity. The 2015 update excluded trials that included patients with comorbid tic disorders to reduce bias, which did not affect the overall effect sizes of the data.6,7
In summary, efficacy has been demonstrated for risperidone and aripiprazole. There has been no benefit demonstrated with olanzapine and limited benefit with quetiapine. One study suggested worsening of symptoms with quetiapine the longer that treatment persisted.3,5-7
Safety
Assessing potential harms related to the use of antipsychotics in treating OCD is complicated, because this information is not always assessed in trials. Instead, researchers often focus on exploring potential benefits because long-term effects of antipsychotics, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects, are well documented.3
Trials included in the meta-analysis by Veale et al3 had a maximum duration of 16 weeks, so it is likely that many of the potential harms of antipsychotic use would not yet have been measurable. The authors cautioned that, although aripiprazole and risperidone demonstrated benefit, their benefit must be weighed against the potential physical risks of long-term antipsychotic use.3One study that was not included in the meta-analysis by Veale et al3 evaluated individuals who did not respond to a SSRI, and randomly assigned them to quetiapine, olanzapine, or risperidone plus CBT. At 1-year follow-up, 50% of participants receiving an antipsychotic had an increase of >10% in body mass index (BMI) and had higher fasting blood sugars compared with only 15.2% of participants with increased BMI in the comparison group (SSRI responders).3
Foa et al8 investigated long-term outcomes (ie, 6 months) of SSRI augmentation with ERP or risperidone in patients with OCD. Forty patients were randomized to receive risperidone, and 9 were considered responders. Only 8 chose to enter the maintenance phase, and of those participants, 5 did not complete the study. Two withdrew due to worsening depression, 2 withdrew due to intolerable adverse effects, and 1 was lost to follow-up. Unfortunately, there was no further discussion of what the intolerable adverse effects were.8
Patients with comorbid schizophrenia and OCD face additional risks. Lifetime prevalence rates of OCD are greater in persons with schizophrenia compared with the general population (26% vs 8%, respectively). Most studies have demonstrated poor prognosis and medication adherence among patients with comorbid schizophrenia and OCD. Fonseka et al9 assessed the risk of antipsychotic induction and exacerbation of OCD symptoms in patients with schizophrenia. Induction and exacerbation of OCD symptoms
Evidence of olanzapine induction and exacerbation of OCD symptoms is also limited to case reports and retrospective studies. However, some studies have estimated induction of OCD symptoms with olanzapine in 11% to 20% of patients.9 There is insufficient evidence to form conclusions regarding other antipsychotics. Fonseka et al9 recommends switching to an antipsychotic with lower 5HT-2 binding affinity or adding an SSRI, such as fluvoxamine, if induction or exacerbation of OCD symptoms occurs.
Consider long-term risks
The evidence for benefits with antipsychotics in treatment-resistant OCD is limited by different populations recruited, small sample sizes, and lack of long-term follow-up. Most evidence supports using ERP over antipsychotics for treating OCD symptoms that have not responded to SSRIs. However, ERP poses its own challenges that may limit clinical utility, such as economic and time restraints. Therefore, benefits with antipsychotics, such as risperidone and aripiprazole, must be weighed against potential long-term risks of treatment, including sedation, weight gain, metabolic syndrome, and extrapyramidal side effects.
Regarding Mr. E’s case, because he had been maximized on SSRI therapy for an adequate duration (escitalopram, 40 mg/d, for 12 weeks) and completed CBT with ERP with a partial response, adding risperidone, 0.5 mg at bedtime, was an appropriate treatment option that is supported by the available guidelines and evidence. The risperidone dose is reflective of the initial dosing strategies used in clinical trials. It is recommended to assess efficacy of treatment at 8 weeks with a validated measure, such as the Y-BOCS. A dose increase may be needed to achieve clinically significant symptom improvement, because moderate doses of risperidone have demonstrated efficacy in trials; however, high doses of risperidone are unlikely to provide additional benefit and increase the risk of adverse effects. If risperidone does not provide a clinically favorable risk–benefit ratio for Mr. E, aripiprazole is a potential alternative.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
1. American Psychiatric Association. Practice guideline for the treatment of patients with obsessive-compulsive disorder. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/ocd.pdf. Published July 2007. Accessed December 11, 2017.
2. National Institute for Health and Care Excellence (NICE). Obsessive compulsive disorder. http://arms.evidence.nhs.uk/resources/hub/1028833/attachment. Updated September 18, 2013. Accessed December 11, 2017.
3. Veale D, Miles S, Smallcombe N, et al. Atypical antipsychotic augmentation in SSRI treatment refractory obsessive-compulsive disorder: a systematic review and
4. Diagnostic and statistical manual of mental disorders, 5th ed. Washington, DC: American Psychiatric Publishing; 2013.
5. Komossa K, Depping AM, Meyer M, et al. Second-generation antipsychotics for obsessive compulsive disorder. Cochrane Database Syst Rev. 2010;12:1-44.
6. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: an update meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2015;18(9). doi: 10.1093/ijnp/pyv047.
7. Dold M, Aigner M, Lanzenberger R, et al. Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol. 2013;16(3):557-574.
8. Foa EB, Simpson HB, Rosenfield D, et al. Six-month outcomes from a randomized trial augmenting serotonin reuptake inhibitors with exposure and response prevention or risperidone in adults with obsessive-compulsive disorder. J Clin Psychiatry. 2015;76(4):440-446.
9. Fonseka TM, Richter MA, Muller DJ. Second generation antipsychotic-induced obsessive-compulsive symptoms in schizophrenia: a review of the experimental literature. Curr Psychiatry Rep. 2014;16(11):510.
Welcome to The New Gastroenterologist online!
Dear Colleagues,
It is with great excitement that I introduce the first e-newsletter version of The New Gastroenterologist! As more content in medicine, and life in general, is moving toward digital platforms, we at the AGA believe this transition will improve both content dissemination and accessibility to all our readers. In this new format, we will continue to provide articles on topics of importance to the early-career community, expand our offerings by including the new “In Focus” articles (concise overviews of GI topics) both digitally and in GI & Hepatology News print issues, as well as increase the use of multimedia resources, such as videos, to further enhance our content.
In this issue of The New Gastroenterologist, our In Focus article provides a practical overview of the management of chronic constipation. This article, written by Nitin Ahuja and James Reynolds from the Neurogastroenterology and Motility Program at the University of Pennsylvania, Philadelphia, addresses a common topic in our field, and can also be found in the February print issue of GI & Hepatology News. To complement this article, there is a corresponding video abstract that can be viewed.
Also in this issue, Richard Peek (Vanderbilt University, Nashville, Tenn.) – one of the Coeditors in Chief of Gastroenterology – provides a summary of the newly created 1-year editorial fellowship for the AGA’s flagship journal. This is a fantastic new opportunity and you can learn firsthand about the experience of the inaugural editorial fellow, Eric Shah (University of Michigan, Ann Arbor), in an accompanying video. Additionally, as helping patients make a successful transition from a pediatric GI practice to an adult GI practice can be very challenging, in this issue Manreet Kaur and Allyson Wyatt (Baylor College of Medicine, Houston) provide a primer on how to successfully aid in this transition.
Are you considering a career in hospital administration? If so, you will enjoy reading about pursuing a career in hospital administration from Brijen Shah, who is the chief medical officer of Mount Sinai Queens (Icahn School of Medicine at Mount Sinai, New York). Have you been to one of the AGA’s Regional Practice Skills Workshops? These workshops are sponsored by the AGA Trainee and Early Career Committee and held in a growing number of cities across the country. In this issue, Munish Ashat (University of Iowa, Iowa City) provides a recap of the workshop he attended, complete with many useful career pearls.
I hope that you also enjoy the other features in the new e-newsletter format of The New Gastroenterologist. I especially want to point out one of our new sections entitled “In Case You Missed It.” As we all undoubtedly experience information overload with so many new articles released each month, this section collects relevant articles from the numerous AGA publications and consolidates them to ensure you don’t miss any of this great content.
If you are interested in contributing to future issues of The New Gastroenterologist or if there are topics that would interest you, please let us know. You can contact me (bryson.katona@uphs.upenn.edu) or the managing editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It is with great excitement that I introduce the first e-newsletter version of The New Gastroenterologist! As more content in medicine, and life in general, is moving toward digital platforms, we at the AGA believe this transition will improve both content dissemination and accessibility to all our readers. In this new format, we will continue to provide articles on topics of importance to the early-career community, expand our offerings by including the new “In Focus” articles (concise overviews of GI topics) both digitally and in GI & Hepatology News print issues, as well as increase the use of multimedia resources, such as videos, to further enhance our content.
In this issue of The New Gastroenterologist, our In Focus article provides a practical overview of the management of chronic constipation. This article, written by Nitin Ahuja and James Reynolds from the Neurogastroenterology and Motility Program at the University of Pennsylvania, Philadelphia, addresses a common topic in our field, and can also be found in the February print issue of GI & Hepatology News. To complement this article, there is a corresponding video abstract that can be viewed.
Also in this issue, Richard Peek (Vanderbilt University, Nashville, Tenn.) – one of the Coeditors in Chief of Gastroenterology – provides a summary of the newly created 1-year editorial fellowship for the AGA’s flagship journal. This is a fantastic new opportunity and you can learn firsthand about the experience of the inaugural editorial fellow, Eric Shah (University of Michigan, Ann Arbor), in an accompanying video. Additionally, as helping patients make a successful transition from a pediatric GI practice to an adult GI practice can be very challenging, in this issue Manreet Kaur and Allyson Wyatt (Baylor College of Medicine, Houston) provide a primer on how to successfully aid in this transition.
Are you considering a career in hospital administration? If so, you will enjoy reading about pursuing a career in hospital administration from Brijen Shah, who is the chief medical officer of Mount Sinai Queens (Icahn School of Medicine at Mount Sinai, New York). Have you been to one of the AGA’s Regional Practice Skills Workshops? These workshops are sponsored by the AGA Trainee and Early Career Committee and held in a growing number of cities across the country. In this issue, Munish Ashat (University of Iowa, Iowa City) provides a recap of the workshop he attended, complete with many useful career pearls.
I hope that you also enjoy the other features in the new e-newsletter format of The New Gastroenterologist. I especially want to point out one of our new sections entitled “In Case You Missed It.” As we all undoubtedly experience information overload with so many new articles released each month, this section collects relevant articles from the numerous AGA publications and consolidates them to ensure you don’t miss any of this great content.
If you are interested in contributing to future issues of The New Gastroenterologist or if there are topics that would interest you, please let us know. You can contact me (bryson.katona@uphs.upenn.edu) or the managing editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Dear Colleagues,
It is with great excitement that I introduce the first e-newsletter version of The New Gastroenterologist! As more content in medicine, and life in general, is moving toward digital platforms, we at the AGA believe this transition will improve both content dissemination and accessibility to all our readers. In this new format, we will continue to provide articles on topics of importance to the early-career community, expand our offerings by including the new “In Focus” articles (concise overviews of GI topics) both digitally and in GI & Hepatology News print issues, as well as increase the use of multimedia resources, such as videos, to further enhance our content.
In this issue of The New Gastroenterologist, our In Focus article provides a practical overview of the management of chronic constipation. This article, written by Nitin Ahuja and James Reynolds from the Neurogastroenterology and Motility Program at the University of Pennsylvania, Philadelphia, addresses a common topic in our field, and can also be found in the February print issue of GI & Hepatology News. To complement this article, there is a corresponding video abstract that can be viewed.
Also in this issue, Richard Peek (Vanderbilt University, Nashville, Tenn.) – one of the Coeditors in Chief of Gastroenterology – provides a summary of the newly created 1-year editorial fellowship for the AGA’s flagship journal. This is a fantastic new opportunity and you can learn firsthand about the experience of the inaugural editorial fellow, Eric Shah (University of Michigan, Ann Arbor), in an accompanying video. Additionally, as helping patients make a successful transition from a pediatric GI practice to an adult GI practice can be very challenging, in this issue Manreet Kaur and Allyson Wyatt (Baylor College of Medicine, Houston) provide a primer on how to successfully aid in this transition.
Are you considering a career in hospital administration? If so, you will enjoy reading about pursuing a career in hospital administration from Brijen Shah, who is the chief medical officer of Mount Sinai Queens (Icahn School of Medicine at Mount Sinai, New York). Have you been to one of the AGA’s Regional Practice Skills Workshops? These workshops are sponsored by the AGA Trainee and Early Career Committee and held in a growing number of cities across the country. In this issue, Munish Ashat (University of Iowa, Iowa City) provides a recap of the workshop he attended, complete with many useful career pearls.
I hope that you also enjoy the other features in the new e-newsletter format of The New Gastroenterologist. I especially want to point out one of our new sections entitled “In Case You Missed It.” As we all undoubtedly experience information overload with so many new articles released each month, this section collects relevant articles from the numerous AGA publications and consolidates them to ensure you don’t miss any of this great content.
If you are interested in contributing to future issues of The New Gastroenterologist or if there are topics that would interest you, please let us know. You can contact me (bryson.katona@uphs.upenn.edu) or the managing editor of The New Gastroenterologist, Ryan Farrell (rfarrell@gastro.org).
Sincerely,
Bryson W. Katona, MD, PhD
Editor in Chief
Dr. Katona is an instructor of medicine in the division of gastroenterology at the University of Pennsylvania, Philadelphia.
Study eyed natural history of branch-duct intraductal papillary mucinous neoplasms
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
The appropriate management of branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs), a precursor cystic lesion to pancreatic cancer, has been a controversial issue since their initial description in 1982. Current national and international guidelines are primarily based on surgical series with potential selection bias and on observational studies with short surveillance periods. Consequently, there is limited information on the natural history and, more importantly, the malignant potential of BD-IPMNs.
The study by Youngmin Han and colleagues represents a comprehensive analysis of over 1,000 patients, each with at least 3 years of follow-up for a suspected BD-IPMN. In addition, the authors identified an optimal screening method for patients based on cyst size. Their data largely validates prior reports and will undoubtedly serve as the basis for future pancreatic cyst guidelines.
However, as the authors note, limitations of their study include its retrospective design and validation of their screening protocol. Moreover, several lingering questions remain for patients with BD-IPMNs: What is the best method of measuring a BD-IPMN (for example, CT, MRI, or endoscopic ultrasound)? How long should surveillance continue? And what is the role for cytopathology and ancillary studies, such as carcinoembryonic antigen testing, molecular testing, and testing for other pancreatic cyst biomarkers? At the risk of enouncing a cliché, “further studies are needed” to identify an optimal treatment algorithm and, considering the increasingly frequent detection of pancreatic cysts, a cost-effective approach to the evaluation of patients with BD-IPMNs.
Aatur D. Singhi, MD, PhD, is in the division of anatomic pathology in the department of pathology at the University of Pittsburgh Medical Center. He has no conflicts of interest.
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
Branch-duct intraductal papillary mucinous neoplasms (BD-IPMNs) grew at a median annual rate of 0.8 mm in a retrospective study of 1,369 patients.
While most of these cysts were “indolent and dormant,” some grew rapidly and developed “other worrisome features,” Youngmin Han, MS, of Seoul (South Korea) National University reported with his associates in the February issue of Gastroenterology. Therefore, clinicians should plan follow-up surveillance based on initial cyst size and growth rate, they concluded.
Based on their findings, the researchers recommended surgery for young, fit, asymptomatic patients who have BD-IPMNs with a diameter of least 30 mm or with thickened cyst walls or those who have a main pancreatic duct measuring 5-9 mm. Surgery also should be considered when patients have lymphadenopathy, high tumor marker levels, or an abrupt change in pancreatic duct caliber with distal pancreatic atrophy or a rapidly growing cyst, they said.
For asymptomatic patients whose cysts are under 10 mm and who do not have worrisome features, they recommended follow-up with CT or MRI at 6 months and then every 2 years after that. Cysts of 10-20 mm should be imaged at 6 months, at 12 months, and then every 1.5-2 years after that, they said. Patients with cyst diameters greater than 20 mm “should undergo MRI or CT or EUS [endoscopic ultrasound] every 6 months for 1 year and then annually thereafter, until the cyst size and features become stable,” they added. Patients whose cysts have a diameter of 30 mm or greater “should be closely monitored with MRI or CT or EUS every 6 months. Surgical resection can be considered in younger patients or those with other combined worrisome features.”
To characterize the natural history of BD-IPMN, the investigators evaluated clinical and imaging data collected between 2001 and 2016 from patients with classical features of BD-IPMN. Each patient included in the study provided 3 or more years of CT, MRI, EUS, and endoscopic retrograde cholangiopancreatography data. The researchers used regression models to estimate changes in sizes of cysts and main pancreatic ducts.
Median follow-up time was 61 months (range, 36-189 months). Cyst diameter averaged 12.8 mm (standard deviation, 6.5 mm) at baseline and 17 mm (SD, 9.2 mm) at final measurement. Larger baseline diameter was associated with faster growth (P = .046): Cysts measuring less than 10 mm at baseline grew at a median annual rate of 0.8 mm (SD, 1.1 mm), while those measuring at least 30 mm grew at a median annual rate of 1.2 mm (SD, 2.1 mm).
Worrisome features were present in 59 patients at baseline and emerged in another 150 patients during follow-up. At baseline, only 2.3% of cysts exceeded 30 mm in diameter, but 8.0% did at final measurement. Cyst wall thickening was found in 0.5% of patients at baseline and 3.7% of patients at final measurement. Main pancreatic ducts measured 5-9 mm in 1.9% of patients at baseline and in 5.6% of patients at final measurement. Additionally, the prevalence of mural nodules rose from 0.4% at baseline to 3.1% at final measurement.
Main pancreatic ducts averaged 1.8 mm (SD, 1.0 mm) at baseline and 2.4 mm (SD, 1.8 mm) at final measurement. Compared with the values seen with smaller cysts, larger baseline cyst diameter correlated significantly with larger main pancreatic ducts, more cases of cyst wall thickening, and more cases with mural nodules (P less than .001 for all comparisons).
The study was funded by a grant from Korean Health Technology R&D Project of Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
SOURCE: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
FROM GASTROENTEROLOGY
Key clinical point: Tailor the surveillance of BD-IPMNs based on initial diameter and the presence or absence of high-risk features.
Major finding: Median annual growth rate was 0.8 mm.
Data source: A retrospective study of 1,369 patients with BD-IPMNs.
Disclosures: The study was funded by a grant from the Korean Health Technology R&D Project of the Ministry of Health and Welfare, Republic of Korea. The investigators reported having no conflicts of interest.
Source: Han Y et al. Gastroenterology. 2018. doi: 10.1053/j.gastro.2017.10.013.
One in five Crohn’s disease patients have major complications after infliximab withdrawal
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
The option of stopping a biologic agent is an attractive prospect for most Crohn's disease (CD) patients in stable clinical remission. The STORI trial, published in 2012, was among the earliest and select few studies addressing withdrawal of biologic therapy in CD among patients in sustained clinical remission with combination therapy (infliximab and thiopurine/methotrexate) for at least 6 months. Almost 50% of patients experienced disease relapse within a year of stopping infliximab in the trial.
Reenaers et al. recently published long-term follow-up of the original STORI cohort. After a median follow-up time of 7 years; four out five patients previously in clinical remission with combination therapy experienced worsening disease activity following withdrawal of infliximab. While the majority (70%) were able to resume infliximab and recapture disease response without any untoward adverse effects; one in five patients experienced major disease-related complications such as complex perianal disease or need for abdominal surgery. Upper GI tract involvement, high white blood cell count, and low hemoglobin concentration were associated with increased likelihood of a major complication. Notably, median time to a major complication was almost 4 years.
These results are similar to long-term relapse rates reported in other studies of withdrawal of therapy in CD. While biomarkers such as C-reactive protein, fecal calprotectin, along with endoscopic disease activity are reliable predictors of short-term relapse; clinical factors such as family history of CD, disease extent, stricturing or penetrating disease, and cigarette smoking are more relevant predictors of long-term disease activity. It is important to consider both types of predictors when considering withdrawal of therapy in CD.
Lastly, while the majority of patients who relapse following withdrawal of a biologic agent will do so within a year or two, a subset may not experience disease-related complications for several years - underscoring the need for long-term follow-up.
Manreet Kaur, MD, is assistant professor in the division of gastroenterology and hepatology; medical director, Inflammatory Bowel Disease Center, and medical director, faculty group practice, Baylor College of Medicine, Houston.
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
About , according to research published in the February issue of Clinical Gastroenterology and Hepatology (doi: 10.1016/j.cgh.2017.09.061).
About 70% of patients remained free of both infliximab restart failure and major complications, said Catherine Reenaers, MD, PhD, of Centre Hospitalier Universitaire de Liège (Belgium), and her associates. Significant predictors of major complications included upper gastrointestinal disease at the time of infliximab withdrawal, white blood cell count of at least 5.0 x 109 per L, and hemoglobin level under 12.5 g per dL. “Patients with at least two of these factors had a more than 40% risk of major complication in the 7 years following infliximab withdrawal,” the researchers reported.
Little is known about long-term outcomes after patients with Crohn’s disease withdraw from infliximab. Therefore, Dr. Reenaers and her associates retrospectively studied 102 patients with Crohn’s disease who had received infliximab and an antimetabolite (azathioprine, mercaptopurine, or methotrexate) for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and then withdrew from infliximab. Patients were recruited from 19 centers in Belgium and France and were originally part of a prospective cohort study of infliximab withdrawal in Crohn’s disease (Gastroenterology. 2012;142[1]:63-70.e5).
About half of patients relapsed and restarted infliximab within 12 months, which is in line with other studies, the researchers noted. Over a median follow-up of 83 months (interquartile range, 71-93 months), 21% (95% confidence interval, 13.1%-30.3%) of patients had no complications, did not restart infliximab, and started no other biologics. In all, 70.2% of patients (95% CI, 60.2%-80.1%) had no major complications and did not fail to respond after restarting infliximab.
Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: 14 who required surgery and 4 who developed new complex perianal lesions. In a multivariable model, the strongest independent predictor of major complications was leukocytosis (hazard ratio, 10.5; 95% CI, 1.3-83; P less than .002), followed by upper gastrointestinal disease (HR, 5.8; 95% CI, 1.5-22) and low hemoglobin level (HR, 4.1; 95% CI, 1.5-21.8; P less than .01). The 13 patients who lacked these risk factors had no major complications of infliximab withdrawal. Among 72 patients who had at least one risk factor, 16.3% (95% CI, 7%-25%) developed major complications over 7 years. Strikingly, among 17 with at least two risk factors, 43% (95% CI, 17%-69%) developed major complications over 7 years, the researchers noted.
Complications emerged a median of 50 months (interquartile range, 41-73 months) after patients received their last infliximab infusion, highlighting the need for close long-term monitoring even if patients show no signs of early clinical relapse after infliximab withdrawal, the investigators said. “One strength of this cohort was the homogeneity of the population,” they stressed. “Most studies of anti–tumor necrosis factor withdrawal after clinical remission were limited by heterogeneous populations, variable lengths of infliximab treatment before discontinuation, and variable use of immunomodulators and corticosteroids. In [our] cohort, the population was homogenous, infliximab withdrawal was standardized, and the disease characteristics at the time of stopping were collected prospectively.” Although follow-up times varied, less than 5% of patients were followed for less than 3 years, they noted.
The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
SOURCE: Reenaers C et al. Clin Gastro Hepatol 2018 February (in press).
FROM CLINICAL GASTROENTEROLOGY AND HEPATOLOGY
Key clinical point: Over 7 years, about one in five patients with remitted Crohn’s disease developed a major complication after withdrawing from infliximab, despite remaining on an antimetabolite.
Major finding: Eighteen patients (19%; 95% CI, 10%-27%) developed major complications: Fourteen needed surgery and four developed new complex perianal lesions.
Data source: A cohort study of 102 patients with Crohn’s disease who had received infliximab and an antimetabolite for at least 12 months, had been in steroid-free clinical remission for at least 6 months, and who then withdrew from infliximab.
Disclosures: The researchers did not acknowledge external funding sources. Dr. Reenaers disclosed ties to AbbVie, Takeda, MSD, Mundipharma, Hospira, and Ferring.
Source: Reenaers C et al. Clin Gastroenterol Hepatol. 2018 February (in press).
APOE4: Elders with allele benefit from lifestyle changes
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
allele, Alina Solomon, MD, PhD, reported in JAMA Neurology.
“Whether such benefits are more pronounced in APOE4 carriers, compared with noncarriers, should be further investigated,” wrote Dr. Solomon of the Institute of Clinical Medicine/Neurology at the University of Eastern Finland, Kuopio, and her associates.
The investigators analyzed data of 1,109 participants in the multicenter Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), a randomized, controlled trial of at-risk individuals from the general population. Participants were aged 60-77 years, and 362 of them were carriers of the APOE4 allele.
Those randomized to the intervention group received targeted information about nutrition, instructions about physical exercise, and cognitive training – including group sessions led by a psychologist. The control group received “regular health advice” (JAMA Neurol. 2018 Jan 22. doi: 10.1001/jamaneurol.2017.4365).
After the interventions, participants underwent a battery of neuropsychological testing. Dr. Solomon and her associates found that the per-year difference between the intervention and control groups in the total score change was 0.037 (95% confidence interval, 0.001-0.073) among APOE4 carriers and 0.014 (95% CI, −0.011-0.039) among noncarriers.
Among other things, the findings stress the importance of early prevention strategies targeting simultaneously many risk factors that are modifiable, the investigators said.
To read the full story, click here.
FROM JAMA NEUROLOGY
Preparing from the Outside Looking In for Safely Transitioning Pediatric Inpatients to Home
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
The transition of children from hospital to home introduces a unique set of challenges to patients and families who may not be well-versed in the healthcare system. In addition to juggling the stress and worry of a sick child, which can inhibit the ability to understand complicated discharge instructions prior to leaving the hospital,1 caregivers need to navigate the medical system to ensure continued recovery. The responsibility to fill and administer medications, arrange follow up appointments, and determine when to seek care if the child’s condition changes are burdens we as healthcare providers expect caregivers to manage but may underestimate how frequently they are reliably completed.2-4
In this issue of the Journal of Hospital Medicine, the article by Rehm et al.5 adds to the growing body of evidence highlighting challenges that caregivers of children face upon discharge from the hospital. The multicenter, retrospective study of postdischarge encounters for over 12,000 patients discharged from 4 children’s hospitals aimed to evaluate the following: (1) various methods for hospital-initiated postdischarge contact of families, (2) the type and frequency of postdischarge issues, and (3) specific characteristics of pediatric patients most commonly affected by postdischarge issues.
Using standardized questions administered through telephone, text, or e-mail contact, postdischarge issues were identified in 25% of discharges across all hospitals. Notably, there was considerable variation of rates of postdischarge issues among hospitals (from 16% to 62.8%). The hospital with the highest rate of postdischarge issues identified had attending hospitalists calling families after discharge. Thus, postdischarge issues may be most easily identified by providers who are familiar with both the patient and the expected postdischarge care.
Often, postdischarge issues represented events that could be mitigated with intentional planning to better anticipate and address patient and family needs prior to discharge. The vast majority of postdischarge issues identified across all hospitals were related to appointments, accounting for 76.3% of postdischarge issues, which may be attributed to a variety of causes, from inadequate or unclear provider recommendations to difficulty scheduling the appointments. The most common medication postdischarge issue was difficulty filling prescriptions, accounting for 84.8% of the medication issues. “Other” postdischarge issues (12.7%) as reported by caregivers included challenges with understanding discharge instructions and concerns about changes in their child’s clinical status. Forty percent of included patients had a chronic care condition. Older children, patients with more medication classes, shorter length of stay, and neuromuscular chronic care conditions had higher odds of postdischarge issues. Although a high proportion of postdischarge issues suggests a systemic problem addressing the needs of patients and families after hospital discharge, these data likely underestimate the magnitude of the problem; as such, the need for improvement may be higher.
Postdischarge challenges faced by families are not unique to pediatrics. Pediatric and adult medical patients face similar rates of challenges after
Given the prevalence of postdischarge issues after both pediatric and adult hospitalizations, how should hospitalists proceed? Physicians and health systems should explore approaches to better prepare caregivers, perhaps using models akin to the Seamless Transitions and (Re)admissions Network model of enhanced communication, care coordination, and family engagement.10 Pediatric hospitalists can prepare children for discharge long before departure by delivering medications to patients prior to discharge,11,12 providing discharge instructions that are clear and readable,13,14 as well as utilizing admission-discharge teaching nurses,15 inpatient care managers,16,17 and pediatric nurse practitioners18 to aid transition.
While a variety of interventions show promise in securing a successful transition to home from the hospitalist vantage point, a partnership with primary care physicians (PCPs) in our communities is paramount. Though the evidence linking gaps in primary care after discharge and readmission rates remain elusive, effective partnerships with PCPs are important for ensuring discharge plans are carried out, which may ultimately lead to decreased rates of unanticipated adverse outcomes. Several adult studies note that no single intervention is likely to prevent issues after discharge, but interventions should include high-quality communication with and involvement of community partners.9,19,20 In practice, providing a high-quality, reliable handoff can be difficult given competing priorities of busy outpatient clinic schedules and inpatient bed capacity concerns, necessitating efficient discharge practices. Some of these challenges are amenable to quality improvement efforts to improve discharge communication.21 Innovative ideas include collaborating with PCPs earlier in the admission to design the care plan up front, including PCPs in weekly team meetings for patients with chronic care conditions,16,17 and using telehealth to communicate with PCPs.
Ensuring a safe transition to home is our responsibility as hospitalists, but the solutions to doing so reliably require multi-fold interventions that build teams within hospitals, innovative outreach to those patients recently discharged to ensure their well-being and mitigate postdischarge issues and broad community programs—including greater access to primary care—to meet our urgent imperative.
Disclosure
The authors declare no conflicts of interest. Dr. Auger’s research is funded by the Agency for Healthcare Research and Quality (1K08HS024735).
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
1. Solan LG, Beck AF, Brunswick SA, et al. The Family Perspective on Hospital to Home Transitions: A Qualitative Study. Pediatrics. 2015;136(6):e1539-1549. PubMed
2. Misky GJ, Wald HL, Coleman EA. Post-hospitalization transitions: Examining the effects of timing of primary care provider follow-up. J Hosp Med. 2010;5(7):392-397. PubMed
3. Yin HS, Johnson M, Mendelsohn AL, Abrams MA, Sanders LM, Dreyer BP. The health literacy of parents in the United States: a nationally representative study. Pediatrics. 2009;124 Suppl 3:S289-298. PubMed
4. Glick AF, Farkas JS, Nicholson J, et al. Parental Management of Discharge Instructions: A Systematic Review. Pediatrics. 2017. [Epub ahead of print]. PubMed
5. Rehm KP, Brittan MS, Stephens JR, et al. Issues Identified by Post-Discharge Contact after Pediatric Hospitalization: A Multi-site Study (published online ahead of print February 2, 2018) J Hosp Med. doi: 10.12788/jhm.2934
6. Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161-167. PubMed
7. Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013;8(8):421-427. PubMed
8. Auerbach AD, Kripalani S, Vasilevskis EE, et al. Preventability and Causes of Readmissions in a National Cohort of General Medicine Patients. JAMA Intern Med. 2016;176(4):484-493. PubMed
9. Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831-841. PubMed
10. Auger KA, Simon TD, Cooperberg D, et al. Summary of STARNet: Seamless Transitions and (Re)admissions Network. Pediatrics. 2015;135(1):164-175. PubMed
11. Hatoun J, Bair-Merritt M, Cabral H, Moses J. Increasing Medication Possession at Discharge for Patients With Asthma: The Meds-in-Hand Project. Pediatrics. 2016;137(3):e20150461. PubMed
12. White CM, Statile AM, White DL, et al. Using quality improvement to optimise paediatric discharge efficiency. BMJ Qual Saf. 2014;23(5):428-436. PubMed
13. Unaka N, Statile A, Jerardi K, et al. Improving the Readability of Pediatric Hospital Medicine Discharge Instructions. J Hosp Med. 2017;12(7):551-557. PubMed
14. Wu S, Tyler A, Logsdon T, et al. A Quality Improvement Collaborative to Improve the Discharge Process for Hospitalized Children. Pediatrics. 2016;138(2). PubMed
15. Blankenship JS, Winslow SA. Admission-discharge-teaching nurses: bridging the gap in today’s workforce. J Nurs Adm. 2003;33(1):11-13. PubMed
16. White CM, Thomson JE, Statile AM, et al. Development of a New Care Model for Hospitalized Children With Medical Complexity. Hosp Pediatr. 2017;7(7):410-414. PubMed
17. Statile AM, Schondelmeyer AC, Thomson JE, et al. Improving Discharge Efficiency in Medically Complex Pediatric Patients. Pediatrics. 2016;138(2). PubMed
18. Dunn K, Rogers J. Discharge Facilitation: An Innovative PNP Role. J Pediatr Health Care. 2016;30(5):499-505. PubMed
19. Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med. 2007;2(5):314-323. PubMed
20. Scott AM, Li J, Oyewole-Eletu S, et al. Understanding Facilitators and Barriers to Care Transitions: Insights from Project ACHIEVE Site Visits. Jt Comm J Qual Patient Saf. 2017;43(9):433-447. PubMed
21. Shen MW, Hershey D, Bergert L, Mallory L, Fisher ES, Cooperberg D. Pediatric hospitalists collaborate to improve timeliness of discharge communication. Hosp Pediatr. 2013;3(3):258-265. PubMed
© 2018 Society of Hospital Medicine
Accuracy Comparisons between Manual and Automated Respiratory Rate for Detecting Clinical Deterioration in Ward Patients
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
Respiratory rate is the most accurate vital sign for predicting adverse outcomes in ward patients.1,2 Though other vital signs are typically collected by using machines, respiratory rate is collected manually by caregivers counting the breathing rate. However, studies have shown significant discrepancies between a patient’s respiratory rate documented in the medical record, which is often 18 or 20, and the value measured by counting the rate over a full minute.3 Thus, despite the high accuracy of respiratory rate, it is possible that these values do not represent true patient physiology. It is unknown whether a valid automated measurement of respiratory rate would be more predictive than a manually collected respiratory rate for identifying patients who develop deterioration. The aim of this study was to compare the distribution and predictive accuracy of manually and automatically recorded respiratory rates.
METHODS
In this prospective cohort study, adult patients admitted to one oncology ward at the University of Chicago from April 2015 to May 2016 were approached for consent (Institutional Review Board #14-0682). Enrolled patients were fit with a cableless, FDA-approved respiratory pod device (Philips IntelliVue clResp Pod; Philips Healthcare, Andover, MA) that automatically recorded respiratory rate and heart rate every 15 minutes while they remained on the ward. Pod data were paired with vital sign data documented in the electronic health record (EHR) by taking the automated value closest, but prior to, the manual value up to a maximum of 4 hours. Automated and manual respiratory rate were compared by using the area under the receiver operating characteristic curve (AUC) for whether an intensive care unit (ICU) transfer occurred within 24 hours of each paired observation without accounting for patient-level clustering.
RESULTS
DISCUSSION
In this prospective cohort study, we found that manual respiratory rates were different than those collected from an automated system and, yet, were significantly more accurate for predicting ICU transfer. These results suggest that the predictive accuracy of respiratory rates documented in the EHR is due to more than just physiology. Our findings have important implications for the risk stratification of ward patients.
Though previous literature has suggested that respiratory rate is the most accurate predictor of deterioration, this may not be true.1 Respiratory rates manually recorded by clinical staff may contain information beyond pure physiology, such as a proxy of clinician concern, which may inflate the predictive value. Nursing staff may record standard respiratory rate values for patients that appear to be well (eg, 18) but count actual rates for those patients they suspect have a more severe disease, which is one possible explanation for our findings. In addition, automated assessments are likely to be more sensitive to intermittent fluctuations in respiratory rate associated with patient movement or emotion. This might explain the improved accuracy at higher rates for manually recorded vital signs.
Although limited by its small sample size, our results have important implications for patient monitoring and early warning scores designed to identify high-risk ward patients given that both simple scores and statistically derived models include respiratory rates as a predictor.4 As hospitals move to use newer technologies to automate vital sign monitoring and decrease nursing workload, our findings suggest that accuracy for identifying high-risk patients may be lost. Additional methods for capturing subjective assessments from clinical providers may be necessary and could be incorporated into risk scores.5 For example, the 7-point subjective Patient Acuity Rating has been shown to augment the Modified Early Warning Score for predicting ICU transfer, rapid response activation, or cardiac arrest within 24 hours.6
Manually recorded respiratory rate may include information beyond pure physiology, which inflates its predictive value. This has important implications for the use of automated monitoring technology in hospitals and the integration of these measurements into early warning scores.
Acknowledgments
The authors thank Pamela McCall, BSN, OCN for her assistance with study implementation, Kevin Ig-Izevbekhai and Shivraj Grewal for assistance with data collection, UCM Clinical Engineering for technical support, and Timothy Holper, MS, Julie Johnson, MPH, RN, and Thomas Sutton for assistance with data abstraction.
Disclosure
Dr. Churpek is supported by a career development award from the National Heart, Lung, and Blood Institute (K08 HL121080) and has received honoraria from Chest for invited speaking engagements. Dr. Churpek and Dr. Edelson have a patent pending (ARCD. P0535US.P2) for risk stratification algorithms for hospitalized patients. In addition, Dr. Edelson has received research support from Philips Healthcare (Andover, MA), research support from the American Heart Association (Dallas, TX) and Laerdal Medical (Stavanger, Norway), and research support from EarlySense (Tel Aviv, Israel). She has ownership interest in Quant HC (Chicago, IL), which is developing products for risk stratification of hospitalized patients. This study was supported by a grant from Philips Healthcare in Andover, MA. The sponsor had no role in data collection, interpretation of results, or drafting of the manuscript.
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
1. Churpek MM, Yuen TC, Huber MT, Park SY, Hall JB, Edelson DP. Predicting cardiac arrest on the wards: a nested case-control study. Chest. 2012;141(5):1170-1176. PubMed
2. Fieselmann JF, Hendryx MS, Helms CM, Wakefield DS. Respiratory rate predicts cardiopulmonary arrest for internal medicine inpatients. J Gen Intern Med. 1993;8(7):354-360. PubMed
3. Semler MW, Stover DG, Copland AP, et al. Flash mob research: a single-day, multicenter, resident-directed study of respiratory rate. Chest. 2013;143(6):1740-1744. PubMed
4. Churpek MM, Yuen TC, Edelson DP. Risk stratification of hospitalized patients on the wards. Chest. 2013;143(6):1758-1765. PubMed
5. Edelson DP, Retzer E, Weidman EK, et al. Patient acuity rating: quantifying clinical judgment regarding inpatient stability. J Hosp Med. 2011;6(8):475-479. PubMed
6. Patel AR, Zadravecz FJ, Young RS, Williams MV, Churpek MM, Edelson DP. The value of clinical judgment in the detection of clinical deterioration. JAMA Intern Med. 2015;175(3):456-458. PubMed
© 2018 Society of Hospital Medicine