User login
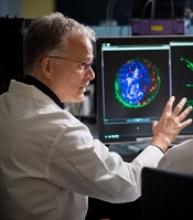
Team discovers mechanism of resistance in AML
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Researchers say they have uncovered a target to overcome drug resistance in acute myeloid leukemia (AML).
The team discovered how a linkage between 2 proteins enables AML cells to resist chemotherapy and showed that disrupting the linkage could render the cells vulnerable to treatment.
The researchers believe their discovery could lead to drugs to enhance chemotherapy in patients with AML and other cancers.
John Schuetz, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee, and his colleagues described this research in Nature Communications.
The team launched their experiments based on previous findings that a protein called ABCC4 was greatly elevated in aggressive cases of AML.
Dr Schuetz and his colleagues searched for other proteins that might interact with ABCC4 and enable its function. The team’s screening of candidate proteins yielded one, MPP1, which was also greatly increased in AML.
The researchers found the 2 proteins are connected, and the connection enables cells to assume the characteristics of highly proliferative leukemia cells.
These experiments involved genetically altering hematopoietic progenitor cells to have high MPP1 and ABCC4 levels. The cells were grown in culture and then replated to see if they would continue to grow, as such self-renewal is a hallmark of leukemia cells.
The researchers found that serial regrowth depended on the cells having high levels of both ABCC4 and MPP1.
“Typically, if you take normal progenitors and you replate, you could do that one time, maybe twice,” Dr Schuetz said. “But our big surprise was that overexpressing MPP1—analogous to what you would see in leukemia—allows those progenitors to self-renew, to be replated over and over, to form new colonies.”
The experiments also revealed that MPP1 and ABCC4 functioned at the cell membrane, where they could play a role in the machinery that would rid the leukemia cells of chemotherapy drugs.
“When we disrupted their interaction, ABCC4 moved off the membrane and the cells became more sensitive to drugs used in AML—drugs that are pumped out of the cell by ABCC4,” Dr Schuetz said.
By screening thousands of compounds, the researchers identified some that could disrupt the ABCC4-MPP1 connection. One, called Antimycin-A, reversed drug resistance in AML cell lines and in cells from AML patients.
Antimycin-A is too toxic to be used in chemotherapy, but the researchers believe identification of the compound should aid the search for other, less-toxic drugs to disrupt the ABCC4-MPP1 interaction.
The team’s findings could also enable clinicians to identify AML patients with high levels of ABCC4 and MPP1. In such patients, drugs that disrupt ABCC4-MPP1 might enhance the effectiveness of standard chemotherapy, Dr Schuetz said.
He also noted that other cancers, including breast and colon cancer and medulloblastoma, show high levels of both ABCC4 and MPP1. Chemotherapy for those cancers might also be enhanced by drugs that disrupt ABCC4-MPP1. ![]()
Price transparency of laboratory testing does not change provider ordering habits
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
Clinical question: Does price transparency of laboratory tests at the point of order entry affect provider ordering behavior?
Background: Up to 30% of laboratory testing may be unnecessary, and health systems are seeking ways to effectively influence provider ordering of tests to reduce costs and improve value to patients. Price transparency and cost displaying is one strategy that has had mixed results in influencing provider ordering and reducing the amount of unnecessary laboratory testing.
Setting: Three urban academic hospitals in Philadelphia.
Synopsis: Sixty inpatient laboratory tests were randomized to either display Medicare fees at the point of order entry or not. Changes in outcomes were followed for 1 year preintervention and 1 year post intervention. The population included 98,529 patients comprising 142,921 hospital admissions. Tests ordered per patient-day and Medicare-associated fees did not significantly change in the intervention group or the control group in the year after the intervention, compared to the year preintervention.
Bottom line: Displaying laboratory testing fees at the point of order entry did not lead to a significant change in provider ordering behavior or reduction in costs.
Citation: Sedrak MS, Myers JS, Small DS, et al. Effect of a price transparency intervention in the electronic health record on clinician ordering of inpatient laboratory tests: The PRICE randomized clinical trial. JAMA Intern Med. 2017 Jul 1;177(7):939-45.
Dr. Chung is hospitalist and assistant professor of medicine, Icahn School of Medicine of the Mount Sinai Health System.
FDA: Febuxostat may have increased heart-related death risk
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
lfranki@frontlinemedcom.com
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
lfranki@frontlinemedcom.com
The urate-lowering therapy febuxostat may have a higher risk of heart-related death than does another urate-lowering drug, allopurinol, according to a Safety Alert from the Food and Drug Administration.
The safety trial was commissioned after febuxostat was approved by the FDA in 2009. Clinical trials conducted pre-approval showed an increased risk of heart-related problems, compared with allopurinol, and the drug label already carries a warning about cardiovascular events.
“Once the final results from the manufacturer are received, the FDA will conduct a comprehensive review and will update the public with any new information,” the agency said in the Safety Alert.
lfranki@frontlinemedcom.com
Researchers say there is room for improvement in ObGyn opioid prescribing practices
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
Drug overdose is the leading cause of death for Americans under age 50. And the number of men and women dying from drug overdose shows no abating, with a sharp 17% increase in 2016 over 2015.1 The rate of fatal overdoses rose to nearly 20 people per 100,000 in 2016, according to the Centers for Disease Control and Prevention. The highest death rates are reported in West Virginia, New Hampshire, Kentucky, Ohio, and Rhode Island.2
Dr. Andrew Kolodny, director of opioid policy research at Brandeis University, told The New York Times that there are “roughly two groups of Americans that are getting addicted. We have an older group that is overdosing on pain medicine, and we have a younger group that is overdosing on black market opioids.”1
Are ObGyns contributing to the over-prescription of opioid pain medications? Investigators from Florida Hospital, the University of Florida, and Orlando VA Medical Center are researching the question, and preliminary findings indicate that, yes, in fact “gynecologists are overprescribing postoperative prescription opioids in all levels of gynecologic surgery,” including after laparotomies and major and minor minimally invasive surgeries. Their work thus far, a prospective cohort study involving 113 patients enrolled to date, was presented November 15, 2017, in Washington, DC, as part of the 46th AAGL Global Congress on MIGS. They found that, on average, patients were prescribed 29.6 (SD, 9.3) opioid tablets, and they had 19.1 (SD, 12.6) tablets left over after surgery. Not surprisingly, patients undergoing major minimally invasive surgery and laparotomy were prescribed larger amounts of opioids than those undergoing minor minimally invasive surgery, but the amount of pain medication left over was similar regardless of surgery type.
The researchers also asked patients if they were told how to dispose of their leftover opioids; only 3 patients reported being told what to do if they had leftover medication.
Too many pills prescribed
In separate research presented at the meeting, investigators from Tufts Medical Center and Lahey Hospital and Medical Center in Boston, Massachusetts, sought to determine opioid prescription practices and patient opioid use after benign hysterectomy. Using retrospective online physician and telephone patient surveys, they found that 51 gynecologists prescribed a median of 30 tabs of oxycodone or hydromorphone after abdominal hysterectomy and a median of 20 tabs after laparoscopic or vaginal hysterectomy. Nearly 65% (36/56) of women used less than half of the opioids they were prescribed, and 16.1% (9/56) used zero tabs. Opioid use was not found to be significantly different for women undergoing abdominal versus minimally invasive hysterectomy.
Managing pain expectations
It takes only 3 days of opioid use for a patient to be at risk for continued use (1 to 3 years) of opioids, said Georgine Lamvu, MD, MPH, CPE, in the educational session “Perioperative Management of the Chronic Pain Patient” at the AAGL meeting. And long-term opioid use is associated with addiction, misuse, and mortality.3 It is therefore crucial to understand how to prescribe pain medications and how to educate women on expectations of pain relief.
Dr. Lamvu and fellow presenters described a 4-step process to pain management: 1) assess—including taking a history and physical exam and providing a risk assessment; 2) check—for other medications that a patient may be taking and possible interactions, as well make sure that the patient is not obtaining opioids or benzodiazepines from other providers; 3) discuss—what pain expectations you have for the patient following surgery; and 4) observe—for clinical improvement, overuse, and misuse, and go slow with dose increases and consult support pain management teams when needed.
Overall, they recommended that surgeons perform a risk assessment (determine the risks and benefits of available therapies); educate themselves and their patients on the risks and benefits; and document the risk assessment, the final recommendations to the patient, and the education provided to the patient.
“We need to do a lot better job at educating ourselves and our patients about pain medications and pain management strategies,” said Dr. Lamvu. The presenters provided these key points for patient and family education:
- Opioids are not first-line or routine therapy for chronic pain, and for acute pain they are used only for severe pain and in short-duration amounts.
- Analgesia will not make you pain free. It only helps to alleviate some pain, and most pain medications take 1 to 2 hours to take effect.
- A 30% improvement in pain and function can be expected for most therapies.
- Recovery from surgical or acute traumatic injury is not immediate; it is expected to take 2 to 4 weeks.
- Do not take extra medication doses beyond what is prescribed, and tell all of your providers what medications you are taking.
- Do not stop taking opioids suddenly, instead taper the dose slowly as instructed by your provider.
- Dispose of excess drugs appropriately—by taking unused pills back to your provider or crushing the pills, placing them in a small amount of liquid, and putting them in the trash.
“The reality is that the majority of patients are not using as many pills as we give them,” said Dr. Lamvu. “Adequate pain control does not supersede patient safety or the responsibility that we have as providers to our society.”
Share your thoughts! Send your Letter to the Editor to rbarbieri@frontlinemedcom.com. Please include your name and the city and state in which you practice.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.
- Kaplan S. CDC reports a record jump in drug overdose deaths last year. The New York Times. November 3, 2017. https://www.nytimes.com/2017/11/03/health/deaths-drug-overdose-cdc.html. Accessed November 15, 2017.
- Drug overdose death data. Centers for Disease Control and Prevention website. https://www.cdc.gov/drugoverdose/data/statedeaths.html. Accessed November 15, 2017.
- Wapner J. CDC study finds opioid dependency begins within a few days of initial use. Newsweek. March 22, 2017. http://www.newsweek.com/cdc-opiate-addiction-572498. Accessed November 17, 2017.
Universal paternal Rh screening is cost effective in IVF
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
SAN ANTONIO – Implementing universal paternal Rh screening would be a cost-effective safety strategy among patients receiving in vitro fertilization, according to a model that used up-to-date data and accounted for ethnic variations in the prevalence of the Rh (D) antibody.
Using a universal Rh screening strategy for semen donors to Rh (D) negative women undergoing in vitro fertilization would result in a cost savings of $11.01 per patient, or $1,120,000 per 100,000 Rh negative IVF pregnancies, according to Pietro Bortoletto, MD, and his coauthors. Their findings were presented during a poster session at the annual meeting of the American Society for Reproductive Medicine.
If paternal Rh factor status is unknown, vaginal bleeding during pregnancy prompts maternal administration of anti-D immune globulin if the mother is Rh (D) negative to prevent hemolytic disease of the fetus and newborn. However, wrote Dr. Bortoletto and his colleagues, “in the IVF population, where paternity status is presumed to be certain, the Rh (D) status of the male partner can be used to triage Rh (D) negative women to more appropriate administration of anti-D globulin.”
To see whether a universal paternal Rh screening strategy would be cost effective, Dr. Bortoletto, a resident physician in obstetrics and gynecology at Harvard Medical School and Brigham and Women’s Hospital, both in Boston, and his collaborators constructed a decision tree to estimate cost savings. The model compared a universal paternal-screening strategy for Rh (D) negative women undergoing IVF with the current standard of practice, which does not involve routine Rh (D) screening for the sperm donor.
In constructing the model, the investigators drew on published data showing that first trimester bleeding is more common in women who undergo IVF: It occurs in one-third of these women, compared with about 20% of the general pregnant population.
They also established probability estimates of pregnancy loss before 20 weeks, with and without first trimester bleeding (0.34 and 0.18, respectively); third trimester bleeding, with and without first trimester bleeding (0.05 and 0.02); and trauma in pregnancy (0.08). They estimated the overall probability of the pregnancy producing an Rh positive neonate at 0.62.
An additional factor that Dr. Bortoletto and his collaborators took into account was the variable prevalence of Rh factor by ethnicity; in the United States, it’s most common in white men and least common in Asian men, with intermediate prevalence in African American and Hispanic men.
When paternal ethnicity was included in the analysis, savings were greatest with white sperm donors, at $1,889,000 per 100,000 Rh negative IVF pregnancies. The lower prevalence of Rh factor in Asian men meant that the strategy was not cost effective in this population since it would cost a net $2,323,000 per 100,000 Rh negative IVF pregnancies.
“A targeted screening approach, by paternal ethnicity, may be a targeted strategy for cost reduction,” wrote Dr. Bortoletto and his colleagues.
Figures for cost estimates were drawn from data from the Centers for Medicare & Medicaid (CMS) using 2017 dollars. The cost for tests to determine blood type and Rh status ranged from $6 to $11, so the investigators set the cost estimate of $8.20. The cost for an antibody screen was estimated at $5.25 (range, $4-$7).
The cost for a 300 mcg dose of anti-D immune globulin was estimated at $93.93 (range, $79-$109), and administration costs were $27.04 (range, $25-$28). Kleihauer-Betke testing to determine the amount of fetal blood in maternal circulation was pinned at $10.61 (range, $8-$14).
Even when the lowest end of the cost range of anti-D immune globulin was used, a universal screening model would still realize a cost savings of $820,000 per 100,000 Rh negative IVF pregnancies. When Rh screening cost was set at $11 – at the high end of the range – “the strategy still remained favorable at $981,000,” wrote Dr. Bortoletto and his colleagues.
“Universal paternal Rh screening provides a cost saving intervention by preventing nonindicated and costly administration of anti-D immune globulin in the IVF population presenting with bleeding or trauma in pregnancy,” they wrote.
Dr. Bortoletto reported no outside sources of funding and no conflicts of interest.
koakes@frontlinemedcom.com
On Twitter @karioakes
At ASRM 2017
Key clinical point:
Major finding: Universal screening would save $1,120,000 per 100,000 Rh negative IVF pregnancies.
Data source: Decision tree incorporating Rh (D) prevalence, bleeding risk, and cost data.
Disclosures: Dr. Bortoletto reported having no relevant disclosures and no outside funding.
Online Conference Library Available
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at info@edgecreekmedia.com. ■
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at info@edgecreekmedia.com. ■
For 44 years, the latest pharmacologic, radiologic, surgical, and endovascular techniques and technologies have been presented at the VEITHsymposium, along with discussions of when these treatments are indicated. Updates on clinical trials and opportunities for dialogue with experts in the field provide insight along with the latest results of the various treatment modalities.
This information is packed into a single meeting with as many short presentations as possible - some of them concurrent. Access has been facilitated by providing electronically archived material, including talks, slides, and panels after the meeting.
To avoid the conundrum of having to choose between concurrent sessions, meeting attendees can access them through this year’s online library, which can be found at www.veithondemand.com. For individuals unable to attend the meeting, the online library is CME accredited so that they may receive credit for their educational viewing experience.
VEITHsymposium has partnered with Edge Creek Media, Inc. to digitally capture the presentations from this year’s event and make them available online for cross-platform and mobile device viewing. Each webcast presentation will be produced with synchronous presenter audio, and slide content to reproduce the presentations.
The online library will contain over 1,200 webcast presentations and will include presentations from approximately 600 expert speakers. This library will recreate the remarkable educational experience of attending in person. It will also provide an invaluable resource of ongoing vascular information.
Edge Creek Media is a specialized multimedia production company that offers custom live event recording and webcast streaming solutions. Other core services include mobile app development, live event production, audio/video post production, and multimedia web development.
For more information, visit www.edgecreekmedia.com or contact the company at info@edgecreekmedia.com. ■
Stenting improved outcomes for treating chronic IVC obstruction
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
Stenting improved outcomes in patients with chronic obstruction of the inferior vena cava (IVC), according to a retrospective analysis of medical records from March 2010 to September 2015.
In the study, 19 of 20 patients with chronic IVC obstruction had successful stent placement, with most resulting in improved and sustained clinical outcomes. The study, conducted by Ole Jørgen Grøtta, MD, and colleagues, was reported in the European Journal of Vascular and Endovascular Surgery (2017;54:620-8).
Dr. Grøtta and colleagues investigated 11 men and 9 women patients, with a median age 43years, and with venography-verified chronic IVC obstruction. Most patients also were screened and found to be positive for thrombophilia. Median follow-up was 25 months (range, 3-70 months).
The researchers reported on 13 patients with IVC occlusion and 7 with stenosis. Patient clinical status and symptom severity was assessed according to standardized CEAP (clinical, etiology, anatomy and pathophysiology) classification and the venous clinical severity score (VCSS), respectively. Sixteen patients presented chronic venous disease (CVD) symptoms in their lower limbs; of these, nine exhibited symptoms of an acute thrombosis and, based on their symptoms, seven were assigned to a specific CEAP category, ranging from C3-C6. The additional four patients included in the study had been referred to receive endovascular treatment intervention based on symptoms consistent with physical activity–related reduced cardiac preload.
A total of 13 of 19 (68%) patients showed sustained and significant clinical improvement, seen as a shift from the baseline VCSS score of 8.5 down to 7.0 (P = .007) at final follow-up. This included all four of the patients identified as having reduced cardiac preload problems. The authors also reported that here were no periprocedural or long-term complications.
The need for an alternative treatment for patients with chronic obstruction of the IVC is exemplified by typically poor sustained clinical improvement outcomes and/or gradual clinical deterioration when using the traditional conservative approach of anticoagulants combined with compression stockings, according to the authors.
“The endovascular approach with stent placement for chronic IVC obstructions is a safe treatment alternative that should be offered to patients who otherwise have little opportunity for clinical improvement,” they concluded.
The authors reported that they had no conflicts of interest.
FROM THE EUROPEAN JOURNAL OF VASCULAR AND ENDOVASCULAR SURGERY
Key clinical point:
Major finding: Most patients showed a significant improvement from the baseline score of 8.5 score down to 7.0 (P = .007).
Data source: A retrospective analysis of 20 patients referred to the Norwegian National Unit for Reconstructive Deep Venous Surgery for stenting.
Disclosures: The authors reported they had no conflict of interest.
Day-Long Program to Provide Comprehensive Overview of Hemodialysis Issues
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
Optimizing care of dialysis patients will be the focus of a comprehensive program with five sessions, “New Developments in Vascular Access for Hemodialysis,” taking place all day Saturday.
“Chronic kidney disease (CKD) has become an epidemic in the United States. Medicare spending for patients with CKD ages 65 and older exceeded $50 billion in 2013 and represented 20% of all Medicare spending for this age group. This epidemic has been driven by the rise in diabetes, hypertension and obesity and has resulted in a staggering increase in the number of patients requiring hemodialysis,” stated program organizer Dr. Larry A. Scher, professor of clinical surgery at Albert Einstein College of Medicine and attending surgeon at Montefiore Medical Center.
“Providing functioning vascular access for these patients has become a significant challenge for vascular surgeons, transplant surgeons, interventional nephrologists, and interventional radiologists along with nephrologists, nurses, dialysis technicians, and others interested in optimizing the care of dialysis patients,” he continued. “These practitioners are the target audience for this program, which will address many important topics in hemodialysis access.”
There will be five sessions covering issues in the field, optimization of outcomes, political, economic and legal topics, new technologies and concepts, and updates on clinical challenges.
The first sessions will cover important issues in the field and outcome optimization. Experts will address topics such as fistula maturation, use of ultrasound for access planning and cannulation, importance of dialysis maturation, significance of dialysis blood flow, and the use of stent grafts and drug-eluting balloons. Other talks will address cognitive function in patients with chronic kidney disease, measuring cardiac output in the dialysis improve patient safety, review of significant contributions to the literature, and an update on the mission of Kidney Health International.
“We are honored to have Harald C. Ott, MD, principal investigator at the Ott Laboratory for Organ Engineering and Regeneration at Massachusetts General Hospital as our guest speaker,” said Dr. Scher. “There is a critical shortage of kidneys available for transplantation, and Dr. Ott has performed important research on reengineered organs.” His presentation will be on the revolution in renal replacement therapy, specifically the current status of the bio-artificial kidney.
“The talk should be of great interest to medical professionals interested in improving care for our patients with end-stage renal disease,” said Dr. Scher. Other session talks will discuss Medicare costs for patients on hemodialysis, changes in reimbursement for outpatient procedures, and training of vascular access surgeons.
“The segment on new technologies and concepts will present updated results of several important clinical trials, including efforts aimed at improving fistula maturation with elastase, sirolimus, and the VasQ device,” explained Dr. Scher. Results will be presented of trials of minimally invasive technologies for creating hemodialysis access. Also covered will be a unique sensor capable of providing remote monitoring of AV fistulas and grafts, as well as the RADAR technique, which emphasizes the importance of hemodynamics in arteriovenous fistula maturation.
“The final session will delve into clinical issues in hemodialysis access,” said Dr. Scher. “There will be several talks about achieving successful access in challenging patient populations including obese, elderly and hypercoagulable patients, as well as patients with implantable cardiac devices.” Subject areas will include the role of biologic grafts in hemodialysis access and management of dialysis access complications, including steal syndrome, high flow fistula, central venous stenosis, aneurysms, and infection.
“We have assembled an expert faculty that will offer a comprehensive overview of a wide-range of topics of interest to physicians and allied professionals who care for patients with end-stage renal disease,” said Dr. Scher. “Panel discussions will further enhance the program, allowing attendees to not only interact with faculty, but also discuss topics of interest and concern to their clinical practices.” ■
FDA approves obinutuzumab for follicular lymphoma
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
The Food and Drug Administration has approved obinutuzumab in combination with chemotherapy, followed by obinutuzumab alone in those who responded, for people with previously untreated advanced follicular lymphoma (stage II bulky, III or IV).
The most common adverse events associated with obinutuzumab were infusion reactions, low white blood cell count, upper respiratory tract infection, cough, constipation, and diarrhea. The most common significant adverse events are low white blood cell count, low white blood cell count with fever, and low platelet count.
Obinutuzumab is marketed as Gazyva by Genentech.
“Today’s Gazyva approval is an important advance for the thousands of people diagnosed each year with follicular lymphoma who hope to delay disease progression for as long as possible,” said Sarah Horning, MD, chief medical officer and head of global product development at Genentech, in the company press release.
Special Focus on Management of Superficial Vein Thrombosis
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■
The options for treatment and management of superficial vein thrombosis will be the focus of “Venous Imaging, Thrombophilia” on Saturday morning.
“This session will include talks on management of superficial vein thrombosis using direct oral anticoagulants, balancing anticoagulation with bleeding risk after surgery, and predicting patients at risk of post-thrombotic syndrome,” said Dr. Ian J. Franklin of the Imperial College and London Vascular Clinic. Dr. Franklin is co-moderator of the second half of the morning session. “There is much variation in practice in these areas, which will be addressed during the presentations,” he said.
“We have a fairly decent grasp regarding the optimal management of some aspects of venous disease,” added co-moderator Dr. Timothy K. Liem, professor of surgery at Oregon Health & Science University and codirector for quality at the Knight Cardiovascular Institute. “For example, in patients with proximal deep vein thrombosis or pulmonary embolism, the vast majority of clinicians would administer therapeutic anticoagulation for at least 3 months. However, when it comes to other very common venous problems and scenarios, such as superficial vein thrombosis (with or without the presence of venous reflux), there are still significant knowledge gaps with regard to optimal care. The same goes for perioperative management of anticoagulation and prevention of post-thrombotic syndrome,” he continued. “This has led to significant variability in the ways patients are treated. Attendees will learn more about these issues and ways to better manage their patients.”
Dr. Franklin and Dr. Liem will each be making several presentations.
“Trial evidence is consistent in showing that risk of venous thromboembolism (VTE) in patients with superficial vein thrombosis is reduced significantly by prolonged treatment with anticoagulants, but the number needed to prevent one VTE episode is more than 80,” explained Dr. Franklin. “This presents problems relating to cost and clinical effectiveness, which will be discussed in the session.” In one talk, Dr. Franklin will be discussing the grading of severity of venous thrombophlebitis and variation of treatment between primary and secondary care. He will also be covering treatment options: anticoagulation, compression, and follow-up.
Dr. Liem will be highlighting anticoagulation issues, looking at the use of direct oral anticoagulants in one talk and management of anticoagulation to avoid postoperative hemorrhage in another. “The presentations will allow attendees who specialize in venous disease to understand when to anticoagulate and when to administer compression for patients with superficial vein thrombosis,” he stated. “It will also allow these physicians to better identify patients who are at increased risk of developing post-thrombotic syndrome.” In addition, he noted, “we hope to provide a better understanding regarding optimal strategies for managing coagulation that minimize the risk of postoperative hemorrhage while reducing the risk for recurrent thromboembolism during surgery or other invasive procedures.”
Dr. Tomasz Urbanek of the Medical University of Silesia, Katowice, Poland, will present the final talk on the predictive factors of post-thrombotic syndrome. When asked how the session might influence the practices of those in attendance, Dr. Franklin replied, “Hopefully, it will result in more rational use of anticoagulation treatment for patients with superficial vein thrombosis, better use of direct oral anticoagulants as a treatment option, and safer surgery on anticoagulated patients.”
Dr. Liem concluded, “These sessions will have the goal of helping clinicians standardize as much of our care as possible.” Dr. Franklin added, “The take-home message is better risk stratification may help rationalize treatment.” ■