User login
Man, 32, With Severe Scrotal Pain and Swelling
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
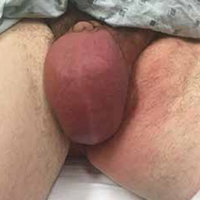
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
IN THIS ARTICLE
- Lab values for case patient
- Differential diagnoses
- Case outcome
A 32-year-old man presents to the urgent care center at a community hospital with severe scrotal pain and swelling of five days’ duration. What began as mild left scrotal discomfort is now causing increasing pain, swelling, hematuria, dysuria, low-grade fever, and nausea, prompting him to seek medical attention.
The patient, who is a pipefitter in a hospital, was at work when his symptoms began. He denies any history of scrotal trauma, and his review of systems is otherwise unremarkable. His medical history is significant for mild hypertension and morbid obesity, but he is not immunocompromised. Two months ago, he had an excision and repair of a left ureterocele, for which he was treated prophylactically with ciprofloxacin for one week. He has a 3–pack-year history of smoking and consumes three alcoholic beverages per week. He denies illicit drug use and has no report of sexually transmitted infection.
Upon arrival to urgent care, the patient appears to be in moderate distress, with a blood pressure (BP) of 111/79 mm Hg; pulse, 104 beats/min; respiratory rate, 18 breaths/min-1; temperature, 100.1°F; and SpO2, 94%. Physical exam reveals left scrotal erythema, severe tenderness upon palpation, marked scrotal edema, and a slight amount of foul-smelling discharge seeping from a pinpoint opening in the left perineum (see Figure 1a). Given his scrotal presentation, he is quickly transferred to a regional emergency department (ED) for a urology consult.
In the ED, lab testing yields significant findings (see Table 1). His ECG demonstrates sinus tachycardia at 126 beats/min without rhythm or ST changes. His urinalysis reveals a cloudy appearance, a protein level of 100 mg/dL, and trace leukocyte esterase.
Urgent CT with contrast is obtained; it shows significant soft-tissue inflammatory changes in the left groin and scrotum that extend into the left thigh. In addition, a collection of fluid is seen in the inferior aspect of the left scrotal wall, indicating a probable abscess. There is no free air or lymphadenopathy.
Given the patient’s worsening condition and his apparent advancement to a systemic inflammatory response syndrome, surgical consult is obtained. He is diagnosed with a scrotal abscess and cellulitis; two blood and two scrotal cultures are obtained, and the patient is empirically started on IV ampicillin and gentamicin.
Two hours later, he has a BP of 122/74 mm Hg; pulse, 112 beats/min; respiratory rate, 20 breaths/min-1; and temperature, 103.1°F. His genital inflammation has advanced to the perineum and the left lower abdomen. The purulent, bloody, foul-smelling drainage from the opening in the left perineum is increasingly apparent. The patient is taken emergently to surgery for an incision and drainage, along with exploration of the scrotal abscess. During surgery, the patient is discovered to have Fournier’s gangrene.
DISCUSSION
Fournier’s gangrene (FG) is a necrotizing fasciitis of the perineal, perianal, and/or genital areas involving the superficial and deep fascial planes while sparing the deep muscular structures and overlying skin.1 A rare but potentially fatal disease, FG spreads at a rate of up to 3 cm/h.2,3
Mortality rates range from 7.5% to 88%, with the highest mortality occurring within the first 96 hours of hospitalization.1,4-7 Mortality is often related to the onset of sepsis.4,5 Survival requires early recognition; immediate, aggressive surgical debridement of all necrotic tissue; and concomitant, early administration of appropriate antibiotics.1,4,5,8 Mortality risk and prognosis are improved in patients younger than 60 with localized disease and no toxicity, along with sterile blood cultures.1
Risk Factors
FG is most commonly seen in males between the ages of 50 and 70, with a 10:1 male-to-female ratio.3,9 Impaired immunity typically increases a patient’s susceptibility to FG, with type 2 diabetes having the highest incidence (85% of patients).1,4,6,8,10 Other conditions that can increase the risk for FG include obesity, alcoholism, cirrhosis, cardiac disease, tobacco use, peripheral vascular disease, malignancy, chronic steroid use, renal insufficiency, IV drug abuse, and HIV.1,4,6,8,9,11
Trauma frequently initiates the infectious process,with urogenital trauma (eg, placement of urethral instrumentation, surgery, and urinary tract infection) being the main cause of bacterial introduction.1,3 Localized infection causes the development of an obliterative endarteritis, resulting in subcutaneous vascular ischemia, necrosis, and bacterial proliferation.3,7,9
Presentation and Diagnosis
Presenting symptoms of FG include intense, abrupt genital pain that is disproportionate to the physical exam findings.9 This rapidly escalates to include extreme swelling, erythema, bullae, discolored skin, and tissue crepitus with eventual necrosis.2,10 Lab results typically show leukocytosis > 18.0 × 109/L.4 The testicle and spermatic cord are generally unaffected (as in this patient), due to the anatomic relationship between the various layers of fascia within the scrotum and the anterior abdominal wall, as well as the independent blood supply of the compartmentalized testicular tissue.1-3
During an exam of the acute scrotum, the differential diagnosis includes cellulitis, scrotal abscess, acute epididymitis, and testicular torsion, with scrotal abscess being most frequently diagnosed (57% of patients).9,11,12 The distinguishing features of these diagnoses can be found in Table 2. Necrotizing fasciitis in the form of FG tends to be an unexpected, rare finding usually only diagnosed during the surgical draining of an abscess.12
CT is the test of choice to detect FG and determine the extent of its spread by identifying subcutaneous air/gas within the involved fascial planes.10,13 However, an incisional biopsy with culture is needed to confirm the diagnosis.3,9 Most patients with FG require an average of four surgeries (eg, reconstruction, skin grafting, and possibly colostomy if the infection has entered the peritoneal cavity) in order to eradicate the disease and achieve the best functional and cosmetic outcome.4
Etiology
About 83% of FG cases are polymicrobial infections comprised of enterobacter, enterococci, Escherichia coli, group A streptococci, pseudomonas, and clostridium, with symptoms evolving two to four days following the initial insult.4,7,11,14,15 Monomicrobial infections are much less common, but the symptoms progress even more rapidly.15 Methicillin-resistant Staphylococcus aureus (MRSA) necrotizing fasciitis infections occur in about 3% of monomicrobial cases.12 MRSA emerged in the early 2000s as an additional causative pathogen for polymicrobial necrotizing fasciitis infections.12,14,15 Prior to that time, S aureus strains were almost uniformly susceptible to penicillinase-resistant ß lactams.12
A distinction should be made between health care-associated (HA) MRSA and community-acquired (CA) MRSA due to treatment considerations. HA-MRSA infections are contracted through previous health care exposure (within the past year) and are less resistant to treatment.16,17 In contrast, CA-MRSA, which comprises 29% of MRSA cases, causes infections in previously healthy young patients without prior health care contact within the past year.16 CA-MRSA strains are more robust than HA-MRSA strains and can cause sepsis and other invasive, rapidly progressive, and possibly life-threatening infections due to the amount of tissue destruction and necrosis.16,18 Transmission of CA-MRSA is often associated with crowded environments, frequent skin-to-skin contact, compromised skin integrity, contaminated items or surfaces, and lack of cleanliness.16 Over the years, CA-MRSA has developed resistance to multiple antimicrobials; providers should therefore consider CA-MRSA on initial evaluation of necrotizing infections, to ensure appropriate initiation of treatment.12,16
CASE CONTINUED
Extensive debridement was completed down to healthy tissue in all affected areas (see Figure 1b). The necrotizing fasciitis had spared the left testicle and spermatic cord, and a colostomy was not required.
The patient’s initial postoperative vital signs were unremarkable, except for his BP (86/54 mm Hg). The patient was taken postoperatively to the surgical intensive care unit (SICU) with the diagnosis of FG. Aggressive IV fluids were administered for resuscitation, and he was closely monitored for increasing sepsis. Metronidazole was added for anaerobic and gram-positive coverage. His postoperative lab results can also be found in Table 1.
His ECG showed a normal sinus rhythm without ST changes, and he denied any cardiac symptoms. His physical exam was significant for mild pallor, dry mucus membranes, and a left scrotal and pelvic packed dressing. He was given two units of packed red blood cells for acute postoperative blood-loss anemia. The preliminary tissue culture results showed gram-positive cocci consistent with a staphylococcal infection; his antibiotics were then changed to IV ampicillin/sulbactam and clindamycin.
Approximately five hours postoperatively, an ECG suddenly showed acute ST elevation in leads II, II, and aVF, with reciprocal changes. The patient was diagnosed with an acute myocardial infarction (AMI). He denied any chest pain, shortness of breath, or diaphoresis. The SICU team initiated aspirin therapy and immediately contacted cardiology for an emergent coronary angiogram.
The angiogram and cardiac catheterization revealed an elevated left ventricular end diastolic (LVED) volume, inferior wall hypokinesis, a low-normal ejection fraction, and a 30% lesion in the first diagonal of his left anterior descending artery. A postprocedure echocardiogram demonstrated left ventricular (LV) ejection fraction of 50%, with LV hypokinesis in the inferior base and mild left atrial enlargement. The patient was started on metoprolol for myocardial protection and recovery.
Complications
Perioperative complications of FG, including AMI, must be considered due to the physiologic stress on the body.19 Most patients with perioperative AMI after noncardiac surgery do not experience ischemic symptoms.20
Growing evidence suggests the pivotal role of acute inflammation (postoperatively or from infection) as a precipitating event in AMI.20,21 Chemical mediators, such as inflammatory cytokines, endotoxins, and nitric oxide, may play a role in the development of an AMI.22
If cardiovascular disease and/or significant cardiovascular risk factors (ie, older age, male, cigarette smoking, cardiac family history, acute kidney injury) are present, the risk for AMI increases in the first two days following surgery.21,23 Acute infections and sepsis also initiate or increase systemic inflammatory activity via these same chemical mediators.21
Most suspected infectious agents also produce coronary artery sheer stress and destabilization of vulnerable plaques, leading to plaque rupture and thrombosis.19,24 Proinflammatory cytokines promote enhanced platelet activation and contribute to this thrombotic environment.21,23 Thrombus leads to obstructed coronary blood flow, myocardial ischemia, and finally, infarction.21
A reversible myocardial depression, cardiomyopathy, or myocardial ischemia may occur in patients with acute systemic infection or sepsis when the myocardium is functionally and structurally injured by these inflammatory chemical mediators.19,22-24 Characteristics of such a cardiomyopathy include left ventricle dilation with a low filling pressure, an abnormal increase in LVED volume, and a depressed ejection fraction.22
An acute infectious or septic process can raise troponin levels in 43% to 85% of patients.22,24 Troponin biomarkers can assist in predicting myocardial injury and events after surgery with nearly absolute myocardial tissue specificity.20 Cardiovascular involvement caused by myocardial injury–related sepsis is observed in up to 70% of patients in the ICU for these reasons.23 Therefore, providers should consider measuring troponin biomarkers during such infectious and septic processes, as this team did for the case patient. The providers were able to diagnose his AMI early and institute appropriate treatment measures to avoid extensive myocardial tissue damage.
Several studies have already demonstrated a correlation between pneumococcal pneumonia and an increased risk for AMI, and the same mechanisms are presumed responsible for any severe acute infectious state.21 More research is needed to understand the pathophysiology of AMI in sepsis and acute systemic infections.23
OUTCOME FOR THE CASE PATIENT
On postoperative day 2, the patient’s vital signs and lab results were normal. Additional lab results included an A1C of 5.2%. His ECG showed a resolving ST-elevation myocardial infarction (STEMI). The surgical wound had initiation of early granulation tissue without any further signs of necrosis.
A postoperative acute STEMI was unexpected in this patient, as his only risk factors included being male, mild hypertension, obesity, and tobacco use. At the time of his initial elevated troponin level, he had no cardiac symptoms or ECG changes. This initial high troponin level may have been stress-induced from the acute infectious process, and his acute inferior wall STEMI may have been secondary to a transient thrombotic event. The STEMI may then have resolved on its own during the cardiac catheterization with the administration of heparin, IV fluids, blood products, aspirin, or dye infiltration, thus enhancing reperfusion of the coronary artery system.
The final tissue culture showed MRSA. Given his job and his history of a genitourinary procedure, as well as the less fulminant form of disease and relatively quick recovery, it was likely HA-MRSA (rather than CA-MRSA). Only clindamycin was used for treatment.
The wound continued to have decreasing erythema, a reduction in tenderness, and evidence of viable, pink granulation tissue. HIV testing was not completed during his admission. The remainder of the patient’s hospital course was unremarkable, and he was discharged home with wound care, urology, and cardiology follow-up services.
CONCLUSION
Multiple factors contribute to a delayed or mistaken diagnosis of FG; it may be overlooked in the initial working diagnoses because of its low incidence and manifestations similar to those of other soft-tissue infections (eg, cellulitis, scrotal abscess). The cutaneous signs of FG often lag behind the disease manifestation, with minimal or no external presence while extensive internal tissue destruction is occurring. Constant review of symptoms is required when treating patients with soft-tissue infections, and early signs—such as pain out of proportion to physical findings—should prompt a clinician to include FG in the differential.
Early diagnosis with prompt debridement and antibiotic therapy are crucial to patient survival. Detecting FG within the first 24 hours is critical. Further differentiation between CA-MRSA and HA-MRSA can assist in patient recovery and survival by guiding appropriate antibiotic therapy. Perioperative risk assessment and serial troponin biomarkers may identify patients in need of intensive monitoring and management postoperatively to avoid an AMI, since patients may not experience ischemic symptoms.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
1. Norton KS, Johnson LW, Perry T, et al. Management of Fournier’s gangrene: an eleven-year retrospective analysis of early recognition, diagnosis, and treatment. Am Surg. 2002;68(8):709-713.
2. Agostini T, Mori F, Perello R, et al. Successful combined approach to a severe Fournier’s gangrene. Indian J Plast Surg. 2014;47(1):132-136.
3. Cabrera G, March P. Fournier’s gangrene. Glendale, CA: Cinahl Information Systems; 2016.
4. Czymek R, Kujath P, Bruch HP, et al. Treatment, outcome and quality of life after Fournier’s gangrene: a multicentre study. Colorectal Dis. 2013;15(12):1529-1536.
5. Sugihara T, Yasunaga H, Horiguchi H, et al. Impact of surgical intervention timing on the case fatality rate for Fournier’s gangrene: an analysis of 379 cases. BJU Int. 2012;110(11c):E1096-1100.
6. Tuncel A, Keten T, Aslan Y, et al. Comparison of different scoring systems for outcome prediction in patients with Fournier’s gangrene: experience with 50 patients. Scand J Urol. 2014;48(4):393-399.
7. Taken K, Oncu MR, Ergun M, et al. Fournier’s gangrene: causes, presentation and survival of sixty-five patients. Pak J Med Sci. 2016;32(3):746-750.
8. Palvolgyi R, Kaji AH, Valeriano J, et al. Fournier’s gangrene: a model for early prediction. Am Surg. 2014;80(10):926-931.
9. Pais V, Santora T. Fournier gangrene. http://emedicine.medscape.com/article/2028899-overview. Accessed August 16, 2017.
10. Cottrill RR. A demonstration of clinical reasoning through a case of scrotal infection. Urol Nurs. 2013;33(1):33-37.
11. Summers A. Fournier’s gangrene. J Nurse Pract. 2014;10(8):582-587.
12. Miller LG, Perdreau-Remington F, Rieg G, et al. Necrotizing fasciitis caused by community-associated methicillin-resistant Staphylococcus aureus in Los Angeles. N Engl J Med. 2005;352(14):1445-1453.
13. Gupta N, Zinn K, Bansal I, Weinstein R. Fournier’s gangrene: ultrasound or computed tomography? A letter to the editor. Med Ultrason. 2014;16(4):389-390.
14. Bjurlin MA, O’Grady T, Kim DY, et al. Causative pathogens, antibiotic sensitivity, resistance patterns, and severity in a contemporary series of Fournier’s gangrene. Urol. 2013;81(4):752-758.
15. Goh T, Goh LG. Pitfalls in diagnosing necrotizing fasciitis. https://psnet.ahrq.gov/webmm/case/329/pitfalls-in-diagnos ing-necrotizing-fasciitis. Accessed August 16, 2017.
16. Kale P, Dhawan B. The changing face of community-acquired methicillin-resistant Staphylococcus aureus. Indian J Med Microbiol. 2016;34(3):275-285.
17. CDC. Necrotizing fasciitis. www.cdc.gov/Features/NecrotizingFasciitis/index.html. Accessed August 16, 2017.
18. Barnes BE, Sampson DA. A literature review on community-acquired methicillin-resistant Staphylococcus aureus in the United States: clinical information for primary care nurse practitioners. J Am Acad Nurse Pract. 2011;23(1):23-32.
19. Madjid M, Vela D, Khalili-Tabrizi H, et al. Systemic infections cause exaggerated local inflammation in atherosclerotic coronary arteries. Clues to the triggering effect of acute infections on acute coronary syndromes. Tex Heart Inst J. 2007;34(1):11-18.
20. Devereaux PJ, Chan MTV, Alonso-Coello PA, et al; VISION Study Investigators. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307(21):2295-2304.
21. Corrales-Medina VF, Fatemi O, Serpa J, et al. The association between Staphylococcus aureus bacteremia and acute myocardial infarction. Scand J Infect Dis. 2009;41(6-7):511-514.
22. Romero-Bermejo FJ, Ruiz-Bailen M, Gil-Cebrian J, Huertos-Ranchal MJ. Sepsis-induced cardiomyopathy. Curr Cardiol Rev. 2011;7(3):163-183.
23. Smilowitz NR, Gupta N, Guo Y, Bangalore S. Comparison of outcomes of patients with sepsis with versus without acute myocardial infarction and comparison of invasive versus noninvasive management of the patients with infarction. Am J Cardiol. 2016;117(7):1065-1071.
24. Mattson M. Sepsis and cardiac disease: improving outcomes through recognition and management. Prog Cardiovasc Nurs. 2009;24(4):199-201.
25. Papadakis MA, McPhee SJ. Current Medical Diagnosis & Treatment. 54th ed. New York, NY: McGraw Hill Education; 2015:137-138, 151-152, 937.
26. Eyre RC. Evaluation of the acute scrotum in adults. www.uptodate.com/contents/evaluation-of-the-acute-scrotum-in-adults. Accessed August 16, 2017.
Triple therapy reduces exacerbations in patients with symptomatic COPD
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.
Clinical Question: Does triple therapy (long-acting beta2-agonist, long-acting muscarinic antagonist, and inhaled corticosteroid) reduce exacerbations in patients with symptomatic chronic obstructive pulmonary disease (COPD)?
Background: Guidelines from GOLD and NICE recommend considering a step-up to triple therapy for patients with refractory COPD symptoms or exacerbations. However, it is unknown if this reduces the long term risk of exacerbations.
Study Design: A randomized controlled trial.
Synopsis: This study enrolled 2,691 patients with COPD, severe airflow restriction (FEV1 less than 50%), significant symptoms (CAT score greater than or equal to 10), and at least one exacerbation in the past year. Participants were randomized to a novel three-agent inhaler (containing an extrafine formulation of beclomethasone, formoterol, and glycopyrronium), an “open triple” regimen including beclomethasone/formoterol plus tiotropium, or to tiotropium alone.
During 52 weeks of treatment, the triple therapy regimens significantly reduced moderate to severe COPD exacerbations, compared with tiotropium alone, with annualized exacerbation rates of 0.46 (95% confidence interval, 0.41-0.51), 0.45 (0.39-0.52), and 0.57 (0.52-0.63), respectively. Rates of adverse events were similar between all three groups.
Bottom Line: Triple therapy was superior to tiotropium alone for reducing exacerbations in patients with symptomatic COPD. The two triple therapy regimens studied did not significantly differ in efficacy.
Citation: Vestbo J, Papi A, Corradi M, et al. Single inhaler extrafine triple therapy versus long-acting muscarinic antagonist therapy for chronic obstructive pulmonary disease (TRINITY): A double-blind, parallel group, randomized controlled trial. Lancet. 2017;389(10082):1919-29.
Dr. Troy is assistant professor in the University of Kentucky division of hospital medicine.
FDA Approves New Leukemia Treatments
The FDA has approved Besponsa (inotuzumab ozogamicin) for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), a rapidly progressing cancer affecting about 6,000 people each year. About 1 in 4 patients affected will die of the disease.
Inotuzumab ozogamicin is a targeted therapy “thought to work” by binding to B-cell ALL cancer cells that express the CD22 antigen, blocking the growth of cancerous cells. In a study of 326 patients with relapsed or refractory B-cell ALL who had received 1 or 2 prior treatments, 36% of 218 evaluated patients experienced complete remission for a median 8 months. Of the patients who received alternative chemotherapy, 17% experienced complete remission for a median 5 months.
A second drug, Vyxeos ( daunorubicin and cytarabine) liposome injection, is approved for adults with 2 types of acute myeloid leukemia (AML): newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC).
An estimated 8% to 10% of patients with AML develop t-AML as a complication of chemotherapy or radiation. AML-MRC is characterized by a history of certain blood disorders and other significant mutations within cancer cells. Patients with either disease have a low life expectancy. Vyxeos is a fixed-combination of daunorubicin and cytarabine. It’s the first approved treatment specifically for these patients, says Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
In a study of 309 patients with newly diagnosed t-AML or AML-MRC, those in the Vyxeos group lived longer: median survival, 9.56 months vs. 5.95 months in the patients who received separate treatments with daunorubicin and cytarabine.The third drug, Idhifa (enasidenib), is approved for adults with relapsed or refractory AML who have a mutation in the IDH2 gene. Idhifa is an isocitrate dehydrogenase-2 inhibitor that blocks several enzymes that promote cell growth.
The drug was studied in a single-arm trial of 199 patients. With a minimum of 6 months of treatment, 19% of patients experienced complete remission for a median of 8.2 months; 4% experienced complete remission with partial hematologic recovery for a median 9.6 months. Of the 157 patients who required blood or platelet transfusions due to AML at the start of the study, 34% no longer did after treatment with Idhifa.
Idhifa is approved for use with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect mutations in the IDH2 gene in blood or bone marrow.
Source:
FDA approves new treatment for adults with relapsed or refractory acute lymphoblastic leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 17,2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm572131.htm. Accessed August 31, 2017.
FDA approves new targeted treatment for relapsed or refractory acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 1, 2017. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm569421.htm. Accessed August 31, 2017.
FDA approves first treatment for certain types of poor-prognosis acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 3, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm569883.htm. Accessed August 31, 2017.
The FDA has approved Besponsa (inotuzumab ozogamicin) for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), a rapidly progressing cancer affecting about 6,000 people each year. About 1 in 4 patients affected will die of the disease.
Inotuzumab ozogamicin is a targeted therapy “thought to work” by binding to B-cell ALL cancer cells that express the CD22 antigen, blocking the growth of cancerous cells. In a study of 326 patients with relapsed or refractory B-cell ALL who had received 1 or 2 prior treatments, 36% of 218 evaluated patients experienced complete remission for a median 8 months. Of the patients who received alternative chemotherapy, 17% experienced complete remission for a median 5 months.
A second drug, Vyxeos ( daunorubicin and cytarabine) liposome injection, is approved for adults with 2 types of acute myeloid leukemia (AML): newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC).
An estimated 8% to 10% of patients with AML develop t-AML as a complication of chemotherapy or radiation. AML-MRC is characterized by a history of certain blood disorders and other significant mutations within cancer cells. Patients with either disease have a low life expectancy. Vyxeos is a fixed-combination of daunorubicin and cytarabine. It’s the first approved treatment specifically for these patients, says Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
In a study of 309 patients with newly diagnosed t-AML or AML-MRC, those in the Vyxeos group lived longer: median survival, 9.56 months vs. 5.95 months in the patients who received separate treatments with daunorubicin and cytarabine.The third drug, Idhifa (enasidenib), is approved for adults with relapsed or refractory AML who have a mutation in the IDH2 gene. Idhifa is an isocitrate dehydrogenase-2 inhibitor that blocks several enzymes that promote cell growth.
The drug was studied in a single-arm trial of 199 patients. With a minimum of 6 months of treatment, 19% of patients experienced complete remission for a median of 8.2 months; 4% experienced complete remission with partial hematologic recovery for a median 9.6 months. Of the 157 patients who required blood or platelet transfusions due to AML at the start of the study, 34% no longer did after treatment with Idhifa.
Idhifa is approved for use with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect mutations in the IDH2 gene in blood or bone marrow.
Source:
FDA approves new treatment for adults with relapsed or refractory acute lymphoblastic leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 17,2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm572131.htm. Accessed August 31, 2017.
FDA approves new targeted treatment for relapsed or refractory acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 1, 2017. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm569421.htm. Accessed August 31, 2017.
FDA approves first treatment for certain types of poor-prognosis acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 3, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm569883.htm. Accessed August 31, 2017.
The FDA has approved Besponsa (inotuzumab ozogamicin) for adults with relapsed or refractory B-cell precursor acute lymphoblastic leukemia (ALL), a rapidly progressing cancer affecting about 6,000 people each year. About 1 in 4 patients affected will die of the disease.
Inotuzumab ozogamicin is a targeted therapy “thought to work” by binding to B-cell ALL cancer cells that express the CD22 antigen, blocking the growth of cancerous cells. In a study of 326 patients with relapsed or refractory B-cell ALL who had received 1 or 2 prior treatments, 36% of 218 evaluated patients experienced complete remission for a median 8 months. Of the patients who received alternative chemotherapy, 17% experienced complete remission for a median 5 months.
A second drug, Vyxeos ( daunorubicin and cytarabine) liposome injection, is approved for adults with 2 types of acute myeloid leukemia (AML): newly diagnosed therapy-related AML (t-AML) or AML with myelodysplasia-related changes (AML-MRC).
An estimated 8% to 10% of patients with AML develop t-AML as a complication of chemotherapy or radiation. AML-MRC is characterized by a history of certain blood disorders and other significant mutations within cancer cells. Patients with either disease have a low life expectancy. Vyxeos is a fixed-combination of daunorubicin and cytarabine. It’s the first approved treatment specifically for these patients, says Richard Pazdur, MD, director of the FDA’s Oncology Center of Excellence.
In a study of 309 patients with newly diagnosed t-AML or AML-MRC, those in the Vyxeos group lived longer: median survival, 9.56 months vs. 5.95 months in the patients who received separate treatments with daunorubicin and cytarabine.The third drug, Idhifa (enasidenib), is approved for adults with relapsed or refractory AML who have a mutation in the IDH2 gene. Idhifa is an isocitrate dehydrogenase-2 inhibitor that blocks several enzymes that promote cell growth.
The drug was studied in a single-arm trial of 199 patients. With a minimum of 6 months of treatment, 19% of patients experienced complete remission for a median of 8.2 months; 4% experienced complete remission with partial hematologic recovery for a median 9.6 months. Of the 157 patients who required blood or platelet transfusions due to AML at the start of the study, 34% no longer did after treatment with Idhifa.
Idhifa is approved for use with a companion diagnostic, the RealTime IDH2 Assay, which is used to detect mutations in the IDH2 gene in blood or bone marrow.
Source:
FDA approves new treatment for adults with relapsed or refractory acute lymphoblastic leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 17,2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm572131.htm. Accessed August 31, 2017.
FDA approves new targeted treatment for relapsed or refractory acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 1, 2017. https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm569421.htm. Accessed August 31, 2017.
FDA approves first treatment for certain types of poor-prognosis acute myeloid leukemia [news release]. Silver Spring, MD: U.S. Food & Drug Administration; August 3, 2017. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm569883.htm. Accessed August 31, 2017.
Diabetes is a Strong Predictor of Dementia
Cardiovascular risk factors, such as diabetes and high blood pressure, increase the risk of dementia. That is not new information, but a long-term funded study by the National Institutes of Health found that not only is diabetes almost as strong a predictor of dementia as the APOE4 gene, but also prehypertension.
The researchers analyzed data on 15,744 participants aged 45 to 64 years in the Atherosclerosis Risk in Communities (ARIC) study. Over 25 years, the participants were examined 4 times, including being given cognitive tests during all but the first and third exams.
Over an average of 23 follow-up years, 1,516 people were diagnosed with dementia. During the time of the first exams, the risk of dementia increased most strongly with age, followed by the presence of APOE4. But as time went on, the link between cardiovascular risk factors and dementia became clearer. A separate study of an ARIC subgroup found that the presence of ≥ 1 vascular risk factor during midlife was associated with higher levels of beta amyloid, a protein that often accumulates in the brains of Alzheimer patients. The relationship was not affected by the presence of the APOE4 gene.
When the researchers reanalyzed the data according to who had a stroke, they found similar results: Diabetes, hypertension, prehypertension, and smoking raised the risk of dementia for people who had a stroke and those who had not.
“Our results contribute to a growing body of evidence linking midlife vascular health to dementia,” said study leader Rebecca Gottesman, MD, PhD, professor of neurology at Johns Hopkins University in Maryland. “These are modifiable risk factors. Our hope is that by addressing these types of factors early, people can reduce the chances that they will suffer from dementia later in life.”
Cardiovascular risk factors, such as diabetes and high blood pressure, increase the risk of dementia. That is not new information, but a long-term funded study by the National Institutes of Health found that not only is diabetes almost as strong a predictor of dementia as the APOE4 gene, but also prehypertension.
The researchers analyzed data on 15,744 participants aged 45 to 64 years in the Atherosclerosis Risk in Communities (ARIC) study. Over 25 years, the participants were examined 4 times, including being given cognitive tests during all but the first and third exams.
Over an average of 23 follow-up years, 1,516 people were diagnosed with dementia. During the time of the first exams, the risk of dementia increased most strongly with age, followed by the presence of APOE4. But as time went on, the link between cardiovascular risk factors and dementia became clearer. A separate study of an ARIC subgroup found that the presence of ≥ 1 vascular risk factor during midlife was associated with higher levels of beta amyloid, a protein that often accumulates in the brains of Alzheimer patients. The relationship was not affected by the presence of the APOE4 gene.
When the researchers reanalyzed the data according to who had a stroke, they found similar results: Diabetes, hypertension, prehypertension, and smoking raised the risk of dementia for people who had a stroke and those who had not.
“Our results contribute to a growing body of evidence linking midlife vascular health to dementia,” said study leader Rebecca Gottesman, MD, PhD, professor of neurology at Johns Hopkins University in Maryland. “These are modifiable risk factors. Our hope is that by addressing these types of factors early, people can reduce the chances that they will suffer from dementia later in life.”
Cardiovascular risk factors, such as diabetes and high blood pressure, increase the risk of dementia. That is not new information, but a long-term funded study by the National Institutes of Health found that not only is diabetes almost as strong a predictor of dementia as the APOE4 gene, but also prehypertension.
The researchers analyzed data on 15,744 participants aged 45 to 64 years in the Atherosclerosis Risk in Communities (ARIC) study. Over 25 years, the participants were examined 4 times, including being given cognitive tests during all but the first and third exams.
Over an average of 23 follow-up years, 1,516 people were diagnosed with dementia. During the time of the first exams, the risk of dementia increased most strongly with age, followed by the presence of APOE4. But as time went on, the link between cardiovascular risk factors and dementia became clearer. A separate study of an ARIC subgroup found that the presence of ≥ 1 vascular risk factor during midlife was associated with higher levels of beta amyloid, a protein that often accumulates in the brains of Alzheimer patients. The relationship was not affected by the presence of the APOE4 gene.
When the researchers reanalyzed the data according to who had a stroke, they found similar results: Diabetes, hypertension, prehypertension, and smoking raised the risk of dementia for people who had a stroke and those who had not.
“Our results contribute to a growing body of evidence linking midlife vascular health to dementia,” said study leader Rebecca Gottesman, MD, PhD, professor of neurology at Johns Hopkins University in Maryland. “These are modifiable risk factors. Our hope is that by addressing these types of factors early, people can reduce the chances that they will suffer from dementia later in life.”
Team creates rainbow of fluorescent dyes
Chemists have reported the creation of new fluorescent dyes that can be used in cells, tissues, and animals.
The scientists found that swapping out specific chemical building blocks in fluorescent molecules called rhodamines can generate dyes of nearly every color.
Such an expanded palette of dyes could help researchers better illuminate the inner workings of cells, said Luke Lavis, PhD, of the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia.
Dr Lavis and his colleagues used their new dyes to light up cell nuclei, label living brain tissue from fruit fly larvae, and highlight visual cortex neurons in mice that had tiny glass windows fitted into their skulls.
The team detailed their work in Nature Methods.
Dr Lavis noted that scientists used to concoct different dyes mostly by trial and error.
“Now, we’ve figured out the rules, and we can make almost any color,” he said.
Dr Lavis’s team focused their research on rhodamines because they’re especially bright and cell-permeable.
Chemists had been working with rhodamines for more than 100 years but created only a few dozen colors. Most were similar shades ranging from green to orange.
That’s because, until recently, making new rhodamines wasn’t easy. Scientists still used techniques from the earliest days of chemistry, boiling chemical ingredients in sulfuric acid. This forces the molecules to link together in a condensation reaction.
Mixing in different building blocks could yield new and unusual dyes, but ingredients had to be tough enough to survive the boiling acid bath. This didn’t leave a lot of options.
In 2011, Dr Lavis’s team developed a new way to tinker with rhodamines’ structure, under milder conditions. Using a reaction sparked by the metal palladium, the scientists could skip the acid step and construct dyes with more complicated building blocks than had been used before.
Four years later, the team revealed the Janelia Fluor dyes. The secret behind these dyes is a tiny, square-shaped appendage called an azetidine ring.
The scientists found that incorporating 4-membered azetidine rings into classic fluorophore structures elicited “substantial increases in brightness and photostability.” In fact, the Janelia Fluor dyes are up to 50 times brighter than other dyes.
Now, Dr Lavis’s group has figured out how to fine-tune their fluorescent dyes by tweaking rhodamines’ structure even further. The team showed that incorporating 3-substituted azetidine groups allowed them to tune spectral and chemical properties with “unprecedented precision.”
The dyes can be synthesized in a single step with inexpensive ingredients. The low cost has allowed Dr Lavis and his colleagues to share their work, shipping thousands of vials to hundreds of labs around the world. ![]()
Chemists have reported the creation of new fluorescent dyes that can be used in cells, tissues, and animals.
The scientists found that swapping out specific chemical building blocks in fluorescent molecules called rhodamines can generate dyes of nearly every color.
Such an expanded palette of dyes could help researchers better illuminate the inner workings of cells, said Luke Lavis, PhD, of the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia.
Dr Lavis and his colleagues used their new dyes to light up cell nuclei, label living brain tissue from fruit fly larvae, and highlight visual cortex neurons in mice that had tiny glass windows fitted into their skulls.
The team detailed their work in Nature Methods.
Dr Lavis noted that scientists used to concoct different dyes mostly by trial and error.
“Now, we’ve figured out the rules, and we can make almost any color,” he said.
Dr Lavis’s team focused their research on rhodamines because they’re especially bright and cell-permeable.
Chemists had been working with rhodamines for more than 100 years but created only a few dozen colors. Most were similar shades ranging from green to orange.
That’s because, until recently, making new rhodamines wasn’t easy. Scientists still used techniques from the earliest days of chemistry, boiling chemical ingredients in sulfuric acid. This forces the molecules to link together in a condensation reaction.
Mixing in different building blocks could yield new and unusual dyes, but ingredients had to be tough enough to survive the boiling acid bath. This didn’t leave a lot of options.
In 2011, Dr Lavis’s team developed a new way to tinker with rhodamines’ structure, under milder conditions. Using a reaction sparked by the metal palladium, the scientists could skip the acid step and construct dyes with more complicated building blocks than had been used before.
Four years later, the team revealed the Janelia Fluor dyes. The secret behind these dyes is a tiny, square-shaped appendage called an azetidine ring.
The scientists found that incorporating 4-membered azetidine rings into classic fluorophore structures elicited “substantial increases in brightness and photostability.” In fact, the Janelia Fluor dyes are up to 50 times brighter than other dyes.
Now, Dr Lavis’s group has figured out how to fine-tune their fluorescent dyes by tweaking rhodamines’ structure even further. The team showed that incorporating 3-substituted azetidine groups allowed them to tune spectral and chemical properties with “unprecedented precision.”
The dyes can be synthesized in a single step with inexpensive ingredients. The low cost has allowed Dr Lavis and his colleagues to share their work, shipping thousands of vials to hundreds of labs around the world. ![]()
Chemists have reported the creation of new fluorescent dyes that can be used in cells, tissues, and animals.
The scientists found that swapping out specific chemical building blocks in fluorescent molecules called rhodamines can generate dyes of nearly every color.
Such an expanded palette of dyes could help researchers better illuminate the inner workings of cells, said Luke Lavis, PhD, of the Howard Hughes Medical Institute’s Janelia Research Campus in Ashburn, Virginia.
Dr Lavis and his colleagues used their new dyes to light up cell nuclei, label living brain tissue from fruit fly larvae, and highlight visual cortex neurons in mice that had tiny glass windows fitted into their skulls.
The team detailed their work in Nature Methods.
Dr Lavis noted that scientists used to concoct different dyes mostly by trial and error.
“Now, we’ve figured out the rules, and we can make almost any color,” he said.
Dr Lavis’s team focused their research on rhodamines because they’re especially bright and cell-permeable.
Chemists had been working with rhodamines for more than 100 years but created only a few dozen colors. Most were similar shades ranging from green to orange.
That’s because, until recently, making new rhodamines wasn’t easy. Scientists still used techniques from the earliest days of chemistry, boiling chemical ingredients in sulfuric acid. This forces the molecules to link together in a condensation reaction.
Mixing in different building blocks could yield new and unusual dyes, but ingredients had to be tough enough to survive the boiling acid bath. This didn’t leave a lot of options.
In 2011, Dr Lavis’s team developed a new way to tinker with rhodamines’ structure, under milder conditions. Using a reaction sparked by the metal palladium, the scientists could skip the acid step and construct dyes with more complicated building blocks than had been used before.
Four years later, the team revealed the Janelia Fluor dyes. The secret behind these dyes is a tiny, square-shaped appendage called an azetidine ring.
The scientists found that incorporating 4-membered azetidine rings into classic fluorophore structures elicited “substantial increases in brightness and photostability.” In fact, the Janelia Fluor dyes are up to 50 times brighter than other dyes.
Now, Dr Lavis’s group has figured out how to fine-tune their fluorescent dyes by tweaking rhodamines’ structure even further. The team showed that incorporating 3-substituted azetidine groups allowed them to tune spectral and chemical properties with “unprecedented precision.”
The dyes can be synthesized in a single step with inexpensive ingredients. The low cost has allowed Dr Lavis and his colleagues to share their work, shipping thousands of vials to hundreds of labs around the world. ![]()
Direct Oral Anticoagulants or Warfarin for A-fib?
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
A 66-year-old man with a history of hypertension and type 2 diabetes is hospitalized for palpitations and dizziness and is diagnosed with atrial fibrillation (A-fib). His heart rate is successfully regulated with a ß-blocker. He has a CHA2DS2-VASc score of 3, making him a candidate for anticoagulation. Which agent should you start?
Thromboembolism in patients with A-fib often results in stroke and death, but appropriate use of antithrombotic therapy can reduce risk. Evidence-based guidelines recommend that patients with A-fib at intermediate or high risk for stroke (CHADS2 score ≥ 2, or prior history of cardioembolic stroke or transient ischemic attack) receive antithrombotic therapy with oral anticoagulation, rather than receive no therapy or therapy with antiplatelets.2,3
The American College of Chest Physicians also recommends use of the direct oral anticoagulant (DOAC) dabigatran instead of warfarin for those patients with nonvalvular A-fib with an estimated glomerular filtration rate ≥ 15 mL/min/1.73 m2.3
A meta-analysis of large randomized controlled trials (RCTs) investigated individual DOACs: dabigatran (a direct thrombin inhibitor) and the factor Xa inhibitors rivaroxaban, apixaban, and edoxaban. The results revealed similar or lower rates of ischemic stroke and major bleeding (except gastrointestinal bleeds; relative risk, 1.25) when compared with warfarin (at an international normalized ratio [INR] goal of 2-3).4 In addition, three separate meta-analyses that pooled results from large RCTs involving dabigatran, apixaban, and rivaroxaban also concluded that these medications significantly reduced incidence of embolic stroke and risk for major bleeds and hemorrhagic stroke, compared with warfarin.5-7
However, less is known about the comparative effectiveness and safety of the DOACs when they are used in clinical practice, and it is not clear which, if any, of these agents is superior to others. Moreover, only about half of the patients in the United States with A-fib who are eligible to take DOACs are currently managed with them.8
STUDY SUMMARY
Different DOACs, different benefits
This large cohort study used data from three Danish national databases to assess the effectiveness of three DOACs compared with warfarin. The nearly 62,000 patients had been recently diagnosed with A-fib without valvular disease or venous thromboembolism. Subjects were prescribed either standard doses of dabigatran (150 bid; N = 12,701), rivaroxaban (20 mg/d; N = 7,192), or apixaban (5 mg bid; N = 6,349) or dose-adjusted warfarin to an INR goal of 2 to 3 (N = 35,436). Patients were followed for an average of 1.9 years.
Ischemic stroke, systemic emboli. In the first year of observation, there were 1,702 reports of ischemic stroke or systemic emboli. The incidence of ischemic stroke or systemic embolism was the same or better for each of the three DOAC treatments than for warfarin (2.9-3.9 vs 3.3 events per 100 person-years, respectively). Ischemic stroke or systemic emboli events occurred less frequently in the rivaroxaban group than in the warfarin group at one year (hazard ratio [HR], 0.83) and after 2.5 years (HR, 0.80). The rates of ischemic stroke and systemic emboli for both apixaban and dabigatran were not significantly different than that for warfarin at either end-point.
Bleeding events (defined as intracranial, major gastrointestinal, and traumatic intracranial) were lower in the apixaban group (HR, 0.63) and dabigatran group (HR, 0.61) than in the warfarin group at one year. Significant reductions remained after 2.5 years. There was no difference in bleeding events between rivaroxaban and warfarin.
Risk for death. Compared with warfarin, the risk for death after one year of treatment was lower in the apixaban (HR, 0.65) and dabigatran (HR, 0.63) groups, and there was no significant difference in the rivaroxaban group (HR, 0.92).
WHAT’S NEW
No agent “has it all,” but DOACs have advantages
This comparative effectiveness and safety analysis reveals that all of the DOACs are at least as effective as warfarin in preventing ischemic stroke and systemic emboli, that rivaroxaban may be more effective, and that apixaban and dabigatran have a lower risk for bleeding than warfarin.
CAVEATS
Lacking INR data
This study was a nonrandomized cohort trial. And, while propensity weighting helps, the researchers were unable to completely control for underlying risk factors or unknown confounders.
INR data for patients on warfarin were not provided, so it is not clear how often patients were out of therapeutic range, which could affect the stroke and bleeding results in the warfarin group. This, however, is seen with routine use of warfarin. This study reflects the challenge of maintaining patients in warfarin’s narrow therapeutic range.
CHALLENGES TO IMPLEMENTATION
It comes down to cost
Cost could be a barrier, as health insurance coverage for DOACs varies. Patients with high-deductible health insurance plans, or who find themselves in the Medicare “donut hole,” may be at a particular disadvantage.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Copyright © 2017. The Family Physicians Inquiries Network. All rights reserved.
Reprinted with permission from the Family Physicians Inquiries Network and The Journal of Family Practice (2017;66[8]:518-519).
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
1. Larsen TB, Skjøth F, Nielsen PB, et al. Comparative effectiveness and safety of non-vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. 2016;353:i3189.
2. January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary. J Am Coll Cardiol. 2014;64:2246-2280.
3. You JJ, Singer DE, Howard PA, et al. Antithrombotic therapy for atrial fibrillation: Antithrombotic Therapy and Prevention of Thrombosis, 9th ed: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest. 2012; 141:e531S-e575S.
4. Ruff CT, Giugliano RP, Braunwald E, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962.
5. Dentali F, Riva N, Crowther M, et al. Efficacy and safety of the novel oral anticoagulants in atrial fibrillation: a systematic review and meta-analysis of the literature. Circulation. 2012;126:2381-2391.
6. Adam SS, McDuffie JR, Ortel TL, et al. Comparative effectiveness of warfarin and new oral anticoagulants for the management of atrial fibrillation and venous thromboembolism. Ann Intern Med. 2012;157:796-807.
7. Ntaios G, Papavasileiou V, Diener H, et al. Nonvitamin-K-antagonist oral anticoagulants in patients with atrial fibrillation and previous stroke or transient ischemic attack: a systematic review and meta-analysis of randomized controlled trials. Stroke. 2012;43:3298-3304.
8. Barnes GD, Lucas E, Alexander GC, et al. National trends in ambulatory oral anticoagulant use. Am J Med. 2015;128:1300-1305.
Paper-based diagnostic device is like ‘portable lab’
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()
Researchers say they have developed self-powered, paper-based electrochemical devices (SPEDs) that can provide sensitive diagnostics in low-resource settings and at the point of care.
The SPEDs can detect biomarkers in the blood and identify conditions such as anemia by performing electrochemical analyses that are powered by the user’s touch.
The devices produce color-coded test results that are easy for non-experts to understand.
“You could consider this a portable laboratory that is just completely made out of paper, is inexpensive, and can be disposed of through incineration,” said Ramses V. Martinez, PhD, of Purdue University in West Lafayette, Indiana.
“We hope these devices will serve untrained people located in remote villages or military bases to test for a variety of diseases without requiring any source of electricity, clean water, or additional equipment.”
Dr Martinez and his colleagues developed the SPEDs and described them in a paper published in Advanced Materials Technologies.
SPED testing is initiated by placing a pinprick of blood in a circular feature on the device, which is less than 2-inches square. The SPEDs also contain “self-pipetting test zones” that can be dipped into a sample instead of using a finger-prick test.
The top layer of each SPED is made of untreated cellulose paper with patterned hydrophobic domains that define channels that wick up blood samples for testing. These microfluidic channels allow for assays that change color to indicate specific test results.
The researchers also created a machine-vision diagnostic application to identify and quantify each of these colorimetric tests from a digital image of the SPED, perhaps taken with a cell phone. This provides rapid results for the user and allows for consultation with a remote expert if necessary.
The bottom layer of the SPED is a triboelectric generator (TEG), which generates the electric current necessary to run the diagnostic test by rubbing or pressing it.
An inexpensive, hand-held device called a potentiostat can be plugged into the SPED to automate the diagnostic tests so they can be performed by untrained users. The battery powering the potentiostat can be recharged using the TEG built into the SPEDs.
“To our knowledge, this work reports the first self-powered, paper-based devices capable of performing rapid, accurate, and sensitive electrochemical assays in combination with a low-cost, portable potentiostat that can be recharged using a paper-based TEG,” Dr Martinez said.
SPEDs can perform multiplexed analyses, enabling the detection of various targets for a range of point-of-care testing applications. In addition, the devices are compatible with mass-printing technologies, such as roll-to-roll printing or spray deposition. And the SPEDs can be used to power other electronic devices to facilitate telemedicine applications in resource-limited settings.
Dr Martinez and his colleagues used the SPEDs to detect biomarkers such as glucose, uric acid and L-lactate, ketones, and white blood cells, which indicate factors related to liver and kidney function, malnutrition, and anemia.
The researchers said future versions of the technology will contain several additional layers for more complex assays to detect diseases such as malaria, dengue fever, yellow fever, hepatitis, and HIV. ![]()