User login
Enhanced disinfection of duodenoscopes did not reduce contamination
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Duodenoscopes had similar rates of contamination after double high-level disinfection, standard high-level disinfection, or standard high-level disinfection followed by ethylene oxide gas sterilization, a randomized, prospective study of 516 bacterial cultures of 18 duodenoscopes showed.
“Our results do not support the routine use of double high-level disinfection or ethylene oxide sterilization for duodenoscope reprocessing,” wrote Graham M. Snyder, MD, of Beth Israel Deaconess Medical Center and Harvard Medical School, Boston, and his associates. They stopped the study after 3 months because none of the duodenoscopes cultured multidrug-resistant organisms, the primary endpoint. “[We] found that in the nonoutbreak setting, duodenoscope contamination by multidrug-resistant organisms is extremely uncommon,” they wrote in the October issue of Gastroenterology (doi: 10.1053/j.gastro.2017.06.052). However, 16% of duodenoscopes cultured at least one colony-forming unit (CFU) after either standard high-level or double high-level disinfection, and 23% of duodenoscopes produced at least one CFU despite standard high-level disinfection followed by ethylene gas sterilization (P = .2), the investigators reported.
Outbreaks of carbapenem-resistant Enterobacteriaceae infections have been traced to duodenoscopes, even though they were reprocessed according to manufacturer instructions. In 2015, the Food and Drug Administration responded by warning that the design of duodenoscopes might preclude effective cleaning. Reasons for residual contamination remain uncertain, but biofilms, which are notoriously resistant to standard disinfection methods, might be a culprit, Dr. Snyder and his associates noted. Accordingly, some experts have suggested repeating the reprocessing cycle or adding ethylene oxide sterilization, but these measures are costly, time intensive, and not widely available. Furthermore, their efficacy “has never been systematically studied in a nonoutbreak setting,” the researchers wrote.
In response, they studied 516 cultures of elevator mechanisms and working channels from 18 reprocessed duodenoscopes (Olympus, model TJF-Q180). Immediately after use, each duodenoscope was manually wiped with enzymatic solution (EmPower), and then was manually reprocessed within an hour before undergoing automated reprocessing (System 83 Plus 9) with ortho-phthalaldehyde disinfectant (MetriCide OPA Plus) followed by ethanol flush. One-third of the duodenoscopes were randomly assigned to undergo double high-level disinfection with two automated reprocessing cycles, and another third underwent standard high-level disinfection followed by ethylene oxide gas sterilization (Steri-Vac sterilizer/aerator). All instruments were stored by hanging them vertically in an unventilated cabinet.
Multidrug-resistant organisms were cultured from 3% of rectal swabs and duodenal aspirates, but not from any of the cultures of duodenoscopes. Therefore, the study was stopped for futility. The enhanced disinfection methods failed to prevent contamination, compared with standard high-level disinfection, the researchers noted. Ten or more CFUs grew in 2% of duodenoscopes that underwent standard high-level disinfection, 4% of those that underwent double high-level disinfection, and 4% of those that underwent high-level disinfection followed by ethylene oxide sterilization (P = .4).
“There is no consensus on what parts of the standard high-level disinfection process should be repeated,” the investigators wrote. “It is uncertain if the addition of a second cycle of manual reprocessing might have improved the effectiveness of double high-level disinfection.”
Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
FROM GASTROENTEROLOGY
Key clinical point: Enhanced disinfection of duodenoscopes did not provide additional protection against contamination.
Major finding: No cultures were positive for multidrug-resistant organisms, but 16% of duodenoscopes had at least one colony-forming unit despite standard high-level disinfection or double high-level disinfection. Standard high-level disinfection followed by ethylene oxide gas failed to sterilize 23% of duodenoscopes (P = .2).
Data source: A single-center, prospective randomized study of 516 cultures of 18 duodenoscopes.
Disclosures: Funders included the American Society for Gastrointestinal Endoscopy and Beth Israel Deaconess Medical Center. The investigators reported having no conflicts of interest.
Light alcohol use did not affect liver fibrosis progression in HIV/HCV-coinfected women
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
This study highlights the importance of assessing alcohol consumption during routine practice (for example, with the Alcohol Use Disorders Identification Test) to classify patients’ level of use. HIV/HCV-coinfected women should be counseled to minimize alcohol consumption, and any patient with evidence of advanced hepatic fibrosis/cirrhosis should avoid alcohol use, given that the risk of liver complications, such as decompensated cirrhosis and hepatocellular carcinoma, associated with light or moderate use remains unknown in this group. However, some may be unable or unwilling to completely abstain from alcohol because of mental health or substance use disorders. This study suggests that light or moderate alcohol use by coinfected women is not associated with accelerated liver fibrosis progression, as measured by changes in the FIB-4 score.
Future studies should determine the effects of light and moderate alcohol consumption on changes in other noninvasive measures of liver fibrosis, such as transient elastography, and on rates of liver complications, such as hepatic decompensation and hepatocellular carcinoma, in HIV/HCV-coinfected men and women to confirm this study’s findings. Additional research also is needed to evaluate the effects of alcohol use categories on adherence to direct-acting antiviral therapy and HCV treatment response to examine whether these outcomes differ by HIV status and sex. These data will further help inform whether there is a “safe” level of alcohol intake in HIV/HCV patients.
This text is taken from a commentary published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix720). Vincent Lo Re III, MD, MSCE, is with the Center for Clinical Epidemiology and Biostatistics at the University of Pennsylvania, Philadelphia. He disclosed having received research grant support from the University of Pennsylvania, the National Cancer Institute, the National Institute of Allergy and Infectious Diseases, and AstraZeneca.
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
Women coinfected with HIV and hepatitis C virus who drank light and moderate amounts of alcohol did not experience significant progression of liver fibrosis, compared with those who drank heavily, results from a large cohort study found.
“Although heavy alcohol use is clearly detrimental to the health of patients with CHC [chronic hepatitis C], it is unclear whether consumption of smaller quantities of alcohol impact fibrosis progression,” researchers led by Erin M. Kelly, MD, wrote in a study published online Aug. 16 in Clinical Infectious Diseases (doi: 10.1093/cid/cix716). “Many patients with CHC consume alcohol and are unable or unwilling to completely abstain. Some studies have suggested a linear dose-response relationship for fibrosis progression even at lower quantities, while others have not clearly demonstrated a risk for fibrosis progression below 20-50 g of alcohol per day. HIV/HCV [hepatitis C virus]-coinfected patients have accelerated fibrosis progression, compared with HCV monoinfected individuals. Whether regular consumption of small quantities of alcohol further increase the rate of fibrosis progression is unknown.”
At baseline, the mean age of study participants was 40 years, their mean body mass index was 26 kg/m2, 17% had diabetes, and 11% had significant fibrosis, defined as a FIB-4 index of greater than 3.25. Nearly half (46%) reported no alcohol use; 26.8% reported light use; 7.1%, moderate use; and 19.7%, heavy use. The median FIB-4 scores at entry were similar between groups. On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively). On the other hand, those who reported very heavy drinking showed significant fibrosis acceleration, compared with abstainers (0.25 FIB-4 units/year), while those who reported drinking 8-14 drinks per week showed minimal acceleration of fibrosis progression (0.04 FIB-4 units/year).
“Of interest, despite WIHS research clinicians recommending limiting or avoiding alcohol at semiannual visits, most women who consumed alcohol at WIHS entry continued to have periods of alcohol use in follow-up,” Dr. Kelly and her associates wrote. “This suggests that, despite being enrolled in a long-term observational cohort study, patients did not change their drinking behaviors, limiting any potential bias in changing behaviors due to participation in a research study.”
The investigators acknowledged certain limitations of the study, including the fact that while current alcohol use was captured at study entry and at follow-up, lifetime alcohol exposure was not collected. “Women categorized as abstinent may have had a prior history of alcohol use,” they noted. “Some of these women may represent ‘sick abstainers’ that have ceased alcohol consumption due to the severity of their liver disease. This may explain the finding that entry fibrosis scores were similar between groups, when one would expect heavy users to have higher fibrosis scores, as compared to abstinent patients.”
The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development; the National Cancer Institute; the National Institute on Drug Abuse; the National Institute on Mental Health; and the University of California, San Francisco, Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
FROM CLINICAL INFECTIOUS DISEASES
Key clinical point:
Major finding: On multivariable analysis, no significant difference in fibrosis progression was observed in abstainers, compared with those who reported light and moderate alcohol use (0.004 and 0.006 FIB-4 units/year, respectively).
Data source: A cohort study of 686 participants in the multicenter Women’s Interagency HIV Study.
Disclosures: The study was supported by the National Institute of Allergy and Infectious Diseases, with additional cofunding from the National Institute of Child Health and Human Development, the National Cancer Institute, the National Institute on Drug Abuse, the National Institute on Mental Health, and the UCSF Liver Center Biostatistics Core. The researchers reported having no financial disclosures.
Pembrolizumab, nivolumab linked to 3% rate of neurologic events
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Neurologic symptoms have been and continue to be one of the most common reasons for admission to a cancer center. Neurotoxic chemotherapy, direct invasion of cancer, and other neurologic complications of treatment contribute to the substantial cross talk between oncologists and neurologists. Over the past 5 years, oncology has witnessed an explosion of new immunotherapeutics that are revolutionizing drug development and patient care in oncology today. In contrast to traditional chemotherapy, which targets rapidly dividing cancer cells and can lead to adverse effects in other organs with rapid cell turnover, immunotherapies target and activate the immune system, potentially leading to a wide range of inflammatory and immune-mediated adverse events, including those in the nervous system.
Only 5 of the 10 patients described by Kao et al. experienced nonneurologic immune-related adverse events, suggesting that neurologic complications may be the only defining symptom of an immune-related reaction. Consultation calls from the cancer center are all too familiar for neurologists, and this pattern appears likely to persist in the era of immunotherapy. The horizon of new checkpoint targets continues to expand, and combination therapies are beginning to emerge. Neurologists and oncologists need to be aware of the important checkpoints ahead in patient care.
Roy E. Strowd III, MD, is with the section on hematology and oncology, department of neurology and internal medicine, Wake Forest University, Winston-Salem, N.C. He reported having no conflicts of interest. These comments are excerpted from his editorial (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1916).
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Three percent of patients developed immune-related adverse neurologic events within 12 months of receiving nivolumab or pembrolizumab, according to the results of a single-center retrospective study.
These syndromes included myopathy, axonal thoracolumbar polyradiculopathy, severe demyelinating length-dependent peripheral neuropathy with axonal loss, a facial diplegic variant of Guillain-Barré syndrome, asymmetric vasculitic neuropathy, cerebellar ataxia with dysarthria, autoimmune retinopathy, bilateral internuclear ophthalmoplegia, and headache, reported Justin C. Kao, MD, of Mayo Clinic, Rochester, Minn., and his coinvestigators. Most patients improved after stopping treatment and starting corticosteroids, but one patient developed necrotizing myopathy and died after withdrawal of ventilator support.
Nivolumab and pembrolizumab, which inhibit the programmed death–1 (PD-1) receptor, are approved for treating metastatic melanoma, non–small-cell lung cancer, renal cell carcinoma, Hodgkin lymphoma, head and neck cancers, and urothelial carcinoma. In response to a surge in reports of neurologic events associated with anti–PD-1 therapy, the investigators searched the Mayo Clinic pharmacy database and identified 347 patients treated with pembrolizumab or nivolumab between 2014 and 2016. Ten patients (2.9%) developed neurologic complications within 12 months of anti–PD-1 exposure, including eight men and two women. The median age was 71 years. None of their neurologic symptoms could be directly attributed to other treatments or to metastatic disease. Most had mild to moderate disability, with modified Rankin Scale (mRS) scores of 2, and symptom severity peaked between 1 day and more than 3 months after starting anti–PD-1 treatment (JAMA Neurol. 2017 Sep 5. doi: 10.1001/jamaneurol.2017.1912).
Stopping anti–PD-1 treatment and starting high-dose corticosteroids led to substantial neurologic improvements (mRS scores, 0-3), except in the case of fatal necrotizing myopathy, the researchers said. That patient, who was receiving pembrolizumab for stage 4 melanoma, developed extraocular, bulbar, and proximal limb girdle weakness that worsened over a period of 3 weeks and did not respond to prednisone (80 mg daily) or to three sessions of plasmapheresis.
If a patient on anti–PD-1 therapy develops neurologic symptoms, clinicians should promptly stop treatment and pursue a full work-up, including electrodiagnostic studies and consideration of muscle or nerve biopsy to clarify underlying pathophysiologic mechanisms, the researchers said. “If the clinical examination demonstrates severe clinical deficits at onset or worsens despite medication discontinuation, additional immune suppressant treatment should be considered,” they said. They recommended prednisone (1 mg/kg) with a taper over a 1-month period. Intravenous immunoglobulin therapy or plasma exchange may be warranted if patients continue to worsen, they said.
The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
FROM JAMA NEUROLOGY
Key clinical point: Watch for immune-related adverse effects of nivolumab and pembrolizumab.
Major finding: Ten of 347 patients (2.9%) developed subacute neurologic immune-related adverse events, typically neuromuscular syndromes.
Data source: A single-center, retrospective cohort study of 347 patients who received pembrolizumab or nivolumab for metastatic melanoma or solid tumors.
Disclosures: The investigators did not report external funding sources. Mr. Kao had no disclosures. Two coinvestigators disclosed ties to the American Association of Neuromuscular & Electrodiagnostic Medicine, the American Academy of Neurology, the Continuum: Lifelong Learning in Neurology, Ionis Pharmaceuticals, Alnylam, and Oxford University Press. The remaining coinvestigators reported having no conflicts of interest.
Exenatide improved motor function in Parkinson’s patients with off-medication symptoms
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
The EXENATIDE-PD trial is an exciting peek into a potential new mechanism in treating Parkinson’s, but it must be viewed cautiously.
The baseline between-group differences are concerning, and although the authors tried to adjust for this discrepancy, a confounding effect for differences in concomitant dopaminergic therapy during the trial cannot be excluded.
It is also puzzling that only off-medication dyskinesias improved without any on-medication improvements or other benefits. The 12-week washout period also might have been too short to eliminate potentially long-lasting symptomatic effects of exenatide.
The DaTscan results are not completely reliable in this analysis because it has previously been shown that GLP-1 receptor stimulation in rodents inhibits the ability of cocaine to increase extracellular dopamine concentrations, which is associated with increased DAT surface expression in the forebrain lateral septum. If present in human beings, such a pharmacological mechanism could potentially account for the symptomatic motor effects of exenatide in Parkinson’s disease.
Nevertheless, the MDS-UPDRS part 3 improvements at 12 weeks do suggest that exenatide has symptomatic motor effects. It’s just not clear how the drug exerts those effects. Other potential symptomatic pharmacological mechanisms of exenatide could include improved functioning in surviving dopaminergic neurons or modified pharmacokinetics of dopaminergic treatments.
Whether exenatide acts as a novel symptomatic agent or has neuroprotective effects on the underlying Parkinson’s disease pathology remains unclear, but this study opens up a new therapeutic avenue in treatment of Parkinson’s disease.
Werner Poewe, MD, is professor of neurology and director of the department of neurology at Innsbruck (Austria) Medical University. Klaus Seppi, MD, is assistant professor of neurology there. Both reported a variety of financial relationships with companies that make drugs for Parkinson’s. Their comments are taken from an editorial accompanying the EXENATIDE-PD trial report (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]32101-3).
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
An anti-diabetes drug significantly improved motor function in patients with Parkinson’s disease who had off-medication symptoms despite dopaminergic therapy in a phase 2 trial.
Patients taking exenatide (Byetta), an agonist of the GLP-1 receptor, experienced a mean 2.5-point improvement in the part 3 motor score on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale (MDS-UPDRS) over 48 weeks, compared with a 1-point decline in patients taking placebo, Dilan Athauda, MBBS, and his colleagues reported (Lancet. 2017 Aug 3. doi: 10.1016/S0140-6736[17]31585-4).
The mechanism of action is unclear, the investigators noted. Dopamine transporter scanning with [123I]FP-CIT single photon emission CT (DaTscan) revealed a tantalizing hint of neuroprotection, as the rate of decline in dopaminergic neurons seemed to be slightly reduced among those taking the medication. However, it’s also possible that exenatide somehow altered the pharmacokinetics of levodopa and other dopaminergic drugs, making them more effective, Dr. Athauda and his associates said.
Still, the double-blinded study’s positive results are encouraging, and they replicate those of the team’s 2013 open-label trial (J Clin Invest. 2013 Jun 3;123[6]:2730-6), they asserted.
“Whether this drug acts as a novel symptomatic agent, influences compensatory responses or behaviors, or has neuroprotective effects on underlying pathology is unclear, but there is a strong indication that GLP-1 receptor agonists may have a useful role in future treatment of Parkinson’s disease,” the investigators wrote.
The study randomized 62 patients who had Parkinson’s with off-medication motor symptoms to weekly injections of either placebo or 2 mg subcutaneous exenatide for 48 weeks. A 12-week washout period followed. Despite randomization, there were some important baseline differences between the groups. Those taking exenatide were older (62 vs. 58 years) and had a higher score on the part 3 motor score of the MDS-UPDRS, the study’s primary endpoint (32.8 vs. 27.1). Exenatide users were also taking a lower mean dopaminergic drug dose (mean 774 mg vs. 826 mg levodopa equivalent).
Patients were assessed in clinic every 12 weeks, not only for the primary endpoint of dyskinesia off-medication, but for cognition, quality of life, mood, and nonmotor symptoms. All assessments were done in the morning, after at least 8 hours off levodopa or 36 hours off long-acting dopaminergic drugs.
Exenatide’s benefit in off-medication dyskinesias was apparent after the first 12 weeks of treatment, Dr. Athauda and his coauthors noted. The MDS-UPDRS score had decreased from 32.8 to 30.2 in the active group, and increased from 27.1 to 27.6 in the placebo group. Those taking exenatide held steady at that improvement for the entire 48 weeks, ending at 30.3 (2.3 points below baseline). Those taking placebo continued to decline, ending at 28.8 (1.7 points above baseline). The adjusted between-group difference was 4.3 points, in favor of exenatide (P = .0026).
At 60 weeks, after the 12-week washout period, patients who took exenatide were still doing better, reaching an adjusted between-group difference of –3.5 (P-= .0318).
However, off-medication dyskinesia was the only improvement noted in the trial. Exenatide did not affect any secondary endpoints, including any sections of the on-medication MDS-UPDRS.
The investigators noted that, during the 60 weeks, mean levodopa equivalent dosage increased more in the active group than in the placebo group (132 vs. 112 mg). This brought the active group up much closer to the placebo group’s dose than had been observed at baseline (906 vs. 942 mg).
Exenatide was generally well tolerated, with the exception of a mean 2.6-kg weight loss among those taking it. This was likely related to an increased incidence of gastrointestinal side effects. Weight returned to normal during the washout period.
There were three drop-outs, two in the placebo arm because of worsening anxiety and worsening dyskinesia and one in the exenatide arm because of asymptomatic hyperamylasemia.
The investigators also measured dopamine transporter availability via DaTscan to assess exenatide’s potential impact on dopaminergic neurons. Although areas of decreased binding declined in both groups, the exenatide group showed a signal of reduced rate of decline in the right and left putamen.
“However,” the authors noted, “because this signal was detectable only at uncorrected height thresholds of P = .0034 or less, these data would benefit from larger confirmatory studies or studies of patients at an earlier disease stage when the rate of change of DaTscan uptake is greater, making group differences more readily detectable.”
It won’t be easy to discover how exenatide exerts its benefit, the authors said. They pointed to a robust compendium of preclinical data suggesting that the drug reduces inflammation, promotes mitochondrial biogenesis, exerts neurotrophic effects, stimulates neurogenesis, and restores neuronal insulin signaling.
“Whether some or all of these mechanisms contributed to the clinical effects in our study cannot be definitively established, but one or several of these mechanisms could have acted in synergy to promote cell survival, preserve compensatory responses, and prevent maladaptive responses.”
The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures but several of his coauthors disclosed relationships with pharmaceutical companies.
msullivan@frontlinemedcom.com
On Twitter @alz_gal
FROM THE LANCET
Key clinical point:
Major finding: After 48 weeks, those taking the drug had a 4.3-point advantage over those taking placebo on the Movement Disorders Society Unified Parkinson’s Disease Rating Scale part 3 motor score.
Data source: The phase 2, double-blind, randomized, placebo-controlled study comprised 62 patients with moderate Parkinson’s.
Disclosures: The Michael J. Fox Foundation for Parkinson’s Research funded the study. Dr. Athauda had no financial disclosures; several of his coauthors disclosed relationships with pharmaceutical companies.
Adoption of robotic-assisted surgery uneven across specialties
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).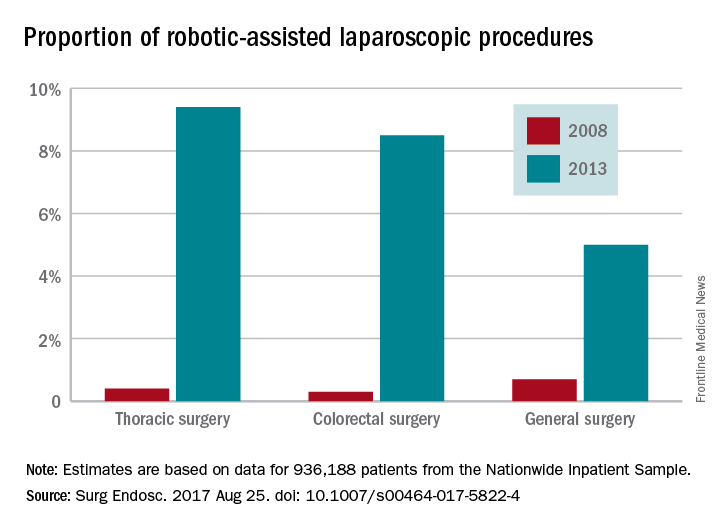
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
Robotic-assisted laparoscopy is on the rise but its spread is uneven across specialties and procedures, findings of a large national study of surgical technology show.
The trend favoring robotic-assisted surgery is especially apparent for urologic, gynecologic, and endocrinologic procedures, according to a study of data drawn from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample (HCUP-NIS) conducted by Yen-Yi Juo, MD, of George Washington University, Washington, and his colleagues (Surg Endosc. 2017 Aug 25. doi: 10.1007/s00464-017-5822-4).
While robotic-assisted surgery is gradually increasing, conventional laparoscopic procedures are declining, the data showed. The case volume of all traditional laparoscopic surgeries decreased by 39.4% between 2009 and 2013 while robotic-assisted laparoscopic procedures increased by 250% over a 6-year period, although the total number of robotic-assisted surgeries is still comparatively small. The study found that the total number of traditional laparoscopic procedures decreased from 956,304 to 737,615 from 2009 to 2013. From mid-2008 to mid-2013, robotic-assisted laparoscopic procedures increased from 17,720 to 33,530.
Patient characteristics such as age, sex, income level, type of insurance, presence of comorbidity, and type of hospital were all significant predictors of whether robotic-assisted surgery would be utilized during a laparoscopic procedure, study findings suggest. Additionally, 5 of the top 10 most common robotic-assisted procedures are performed by urologists.
The investigators noted that there were limitations on the study due to the lack of data on operative indication, disease severity, and postoperative complications in the HCUP-NIS database. In addition, “surgeons are known to preferentially select ‘safer’ patients during the initial adoption of new technology; this may lead to an overestimation of robotic procedure’s clinical benefit in our simple outcome measure.”
The investigators concluded that “although robotic-assisted technology has seen progressive increase in prevalence, its adoption is conspicuously uneven across specialties and procedures. ... The next step in this body of work is to identify specific perceived technical advantages by surgeons working in certain anatomic areas in order to optimize target procedures for the uptake of robotic technology in the future.”
The investigators reported no conflicts of interest.
FROM SURGICAL ENDOSCOPY
Key clinical point:
Major finding: Procedures performed with robotic assistance increased from 6.8% to 17% over a 5-year period.
Data source: Analysis of data from 936,188 patients from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database.
Disclosures: Investigators reported no conflicts of interest.
In California, medical vaccine exemptions tripled after personal belief exemption ban
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
The proportion of California kindergartners with medical exemptions from vaccination tripled after the state eliminated personal belief exemptions, a study has shown.
Furthermore, California counties that previously had the highest rates of personal belief exemptions now have the highest rates of medical exemptions, Paul L. Delamater, PhD, of the University of North Carolina at Chapel Hill, and his associates reported in a research letter in JAMA. Such trends undermine California Senate Bill 277, will “limit [the law’s] long-term benefits, if sustained,” and could lead to outbreaks of vaccine-preventable diseases in the near future, the researchers warned.
Beginning in fall 2016, SB 277 prohibited unvaccinated kindergartners from matriculating at a public or private school in California without a medical exemption from vaccination, having eliminated the personal belief exemption from the state’s school-entry vaccine requirements. But the new law also gave physicians broader discretion to grant the medical exemptions, prompting concerns that “vaccine-hesitant” parents might successfully obtain medical exemptions in lieu of personal belief exemptions. To explore that possibility, the researchers analyzed data from the California state health department on kindergarten enrollment, vaccination, and vaccination exemptions. These data covered about 95% of California kindergartners, Dr. Delamater and his associates noted (JAMA. 2017;318[9]:863-4).
At the county level, rates of medical exemptions strongly correlated with historic rates of personal belief exemptions (P less than .001). Thus, counties with the highest historic rates of personal belief exemptions had the largest increases in rates of medical exemptions in 2016, that is, a change of between –1.00% and 3.38% of kindergartners. Furthermore, vaccination rates among all elementary school-aged children in California are even lower because SB 277 permitted children who previously entered kindergarten with personal belief exemptions to continue attending school without receiving vaccines until seventh grade. “Because the largest increases in [medical exemption] percentage occurred in regions with high past–[personal belief exemption] use, portions of California may remain susceptible to vaccine-preventable disease outbreaks in the near future,” Dr. Delamater and his associates concluded.
The investigators reported having no conflicts of interest.
FROM JAMA
Key clinical point:
Major finding: In 2016, 0.51% of California kindergartners had medical exemptions, a threefold rise from 2015.
Data source: An analysis of reportable state health department data from 2001 to 2016.
Disclosures: The investigators reported having no conflicts of interest.
The Disease for Which There Is No Cure and Not Enough Conversation
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
If I simply let the title of this column stand alone, I suspect most readers of Federal Practitioner would fill in the blank with diseases, such as cancer, HIV, or even devastating genetic conditions, just as I would if presented with the statement without explication.
I read the sentence several weeks ago on a website for caregivers of patients with dementia while browsing for quite a different purpose, and it has haunted me ever since. As a consultation psychiatrist who has spent my career as a VA hospitalist, I am well aware of the sad reality of dementia, but against the backdrop of the aging veteran population, the poignancy of the human tragedy overwhelmed me.
Almost every day on the medical and surgical wards of the VA hospital where I have worked for nearly 2 decades, I see an aging veteran population. There are days when the average age of inpatients is pushing 70 years, and there are many patients in their 80s and 90s. The statistics show that my facility is by no means unique in the VA. Data from the American Community Survey Profile of veterans in 2015 indicate that the median age of veterans is 64 years whereas that of nonveterans is 41.1 The survey emphasized that this age factor has a rippling effect on many other demographic parameters, such as disability, income, and employment, all, in turn, impact the epidemiology of health and illness.1
It is not just age that increases the likelihood that a veteran will develop dementia: Research has identified several aspects of military service that raise the risk of being diagnosed with major neurocognitive disorder, the Diagnostic and Statistical Manual of Mental Disorders, 5th Edition designation for dementia. Many families, patients, and even a few health care professionals may not realize that major neurocognitive disorder is the new neuropsychiatric term for dementia.
Also, many health care professionals do not realize that dementia is the sixth leading cause of death in the U.S.2 Traumatic brain injury, posttraumatic stress disorder, and depression are identified as potential contributors to a higher incidence of dementia in service men and women often with onset at an earlier age.3 Given the prevalence of these comorbidities in persons who were in the military, the VA and DoD will face the medical and psychosocial challenges of providing not only clinical treatment, but also a range of social services for military personnel and veterans. Indeed, federal institutions like the GRECC (Geriatric Research Education and Clinical Center) already are engaged in cutting edge research, delivering high-quality medical treatment, and specialized geriatric and dementia care education and support.
Despite these impressive efforts, too often families ask me 2 crucial questions when a patient is already at a moderate or severe stage of the disease: Is there a cure, and will they get better with or without treatment? This lack of knowledge and understanding is by no means confined to federal health care.
A 2015 report from the Alzheimer’s Association found that 45% of patients with Alzheimer disease or their caregivers were not told about the diagnosis by the doctor.2 Doctors reported that they were more likely to have informed the family of a cancer diagnosis at least in part because they felt there were treatments available and in some cases a cure.
Families ask these questions of me and other health care professionals in the hope of finding guidance. Often the veteran has been hospitalized after behavioral disturbances or wandering have made it impossible to care for the loved elder at home. The family is faced with a double blow: learning the patient has an incurable terminal disease and having to make the decision to place a grandmother or father in a nursing facility. Granted this woeful decision may have to be made even when the family has been fully informed at the time of diagnosis, but it is more distressing when the decision is needed immediately based on safety.
Husbands and wives of 50 years or more and adult children, graying themselves, often ask the second question about improvement. Although treatments exist that can help relieve symptoms and slow progression temporarily, the inexorable and tragic course of the wiping away of memory cannot be reversed or halted.
Not surprisingly, practitioners avoid telling patients and families about a dementia diagnosis because those conversations are painful and difficult. However, the news is much less agonizing to hear when there is time to enjoy the good days that remain and to make arrangements for finances and families. For these important reasons, VA emphasizes shared decision making as the cornerstone of geriatric care. Yet there can be no shared decisions without the compassionate and truthful telling about the diagnosis and the prognosis.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.
1. U.S. Department of Veterans Affairs National Ce- nter for Veterans Analytics and Statistics. Profile of veterans: 2015 data from the American Community Survey. https://www.va.gov/vetdata/docs/Specia lReports/Profile_of_Veterans_2015.pdf. Published March 2017. Accessed August 22, 2017.
2. Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimers Dement. 2015;11(3):332-384.
3. Weiner MW, Friedl KE, Pacifico A, et al. Military risk factors for Alzheimer’s disease. Alzheimers Dement. 2013;9(4):445-451.
Optical Coherence Tomography in Dermatology
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
Optical coherence tomography (OCT) is a noninvasive imaging technique that is cleared by the US Food and Drug Administration as a 510(k) class II regulatory device to visualize biological tissues in vivo and in real time.1-3 In July 2017, OCT received 2 category III Current Procedural Terminology (CPT) codes from the American Medical Association—0470T and 0471T—enabling physicians to report and track the usage of this emerging imaging method.4 Category III CPT codes remain investigational and therefore are not easily reimbursed by insurance.5 The goal of OCT manufacturers and providers within the next 5 years is to upgrade to category I coding before the present codes are archived. Although documented advantages of OCT include its unique ability to effectively differentiate and monitor skin lesions throughout nonsurgical treatment as well as to efficiently delineate presurgical margins, additional research reporting its efficacy may facilitate the coding conversion and encourage greater usage of OCT technology. We present a brief review of OCT imaging in dermatology, including its indications and limitations.
RELATED VIDEO: Imaging Overview: Report From the Mount Sinai Fall Symposium
Types of OCT
Optical coherence tomography, based on the principle of low-coherence interferometry, uses infrared light to extract fine details from within highly scattering turbid media to visualize the subsurface of the skin.2 Since its introduction for use in dermatology, OCT has been used to study skin in both the research and clinical settings.2,3 Current OCT devices on the market are mobile and easy to use in a busy dermatology practice. The Table reviews the most commonly used noninvasive imaging tools for the skin, depicting the inverse relationship between penetration depth and cellular resolution as well as field of view discrepancies.2,6-8 Optical coherence tomography technology collects cross-sectional (vertical) images similar to histology and en face (horizontal) images similar to reflective confocal microscopy (RCM) of skin areas with adequate cellular resolution and without compromising penetration depth as well as a field of view comparable to the probe aperture contacting the skin.
RELATED VIDEO: Noninvasive Imaging: Report From the Mount Sinai Fall Symposium
Conventional OCT
Due to multiple simultaneous beams, conventional frequency-domain OCT (FD-OCT) provides enhanced lateral resolution of 7.5 to 15 µm and axial resolution of 5 to 10 µm with a field of view of 6.0×6.0 mm2 and depth of 1.5 to 2.0 mm.2,6,8 Conventional FD-OCT detects architectural details within tissue with better cellular clarity than high-frequency ultrasound and better depth than RCM, yet FD-OCT is not sufficient to distinguish individual cells.
Dynamic OCT
The recent development of dynamic OCT (D-OCT) software based on speckle-variance has the added ability to visualize the skin microvasculature and therefore detect blood vessels and their distribution within specific lesions. This angiographic variant of FD-OCT detects motion corresponding to blood flow in the images and may enhance diagnostic accuracy, particularly in the differentiation of nevi and malignant melanomas.8-11
High-Definition OCT
High-definition OCT (HD-OCT), a hybrid of RCM and FD-OCT, provides improved optical resolution of 3 μm for both lateral and axial imaging approaching a resolution similar to RCM making it possible to visualize individual cells, though at the expense of lower penetration depth of 0.5 to 1.0 mm and reduced field of view of 1.8×1.5 mm2 to FD-OCT. High-definition OCT combines 2 different views to produce a 3-dimensional image for additional data interpretation (Table).7,8,12
Current CPT Guidelines
Two category III CPT codes—0470T and 0471T—allow the medical community to collect and track the usage of the emerging OCT technology. Code 0470T is used for microstructural and morphological skin imaging, specifically acquisition, interpretation, and reading of the images. Code 0471T is used for each additional skin lesion imaged.4
Current Procedural Terminology category III codes remain investigational in contrast to the permanent category I codes. Reimbursement for CPT III codes is difficult because it is not generally an accepted service covered by insurance.5 The goal within the next 5 years is to convert to category I CPT codes, meanwhile the CPT III codes should encourage increased utilization of OCT technology.
Indications for OCT
Depiction of Healthy Versus Diseased Skin
Optical coherence tomography is a valuable tool in visualizing normal skin morphology including principal skin layers, namely the dermis, epidermis, and dermoepidermal junction, as well as structures such as hair follicles, blood vessels, and glands.2,13 The OCT images show architectural changes of the skin layers and can be used to differentiate abnormal from normal tissue in vivo.2
Diagnosis and Treatment Monitoring of Skin Cancers
Optical coherence tomography is well established for use in the diagnosis and management of nonmelanoma skin cancers and to determine clinical end points of nonsurgical treatment without the need for skin biopsy. Promising diagnostic criteria have been developed for nonmelanoma skin cancers including basal cell carcinoma (BCC) and squamous cell carcinoma, as well as premalignant actinic keratoses using FD-OCT and the newer D-OCT and HD-OCT devices.9-17 For example, FD-OCT offers improved diagnosis of lesions suspicious for BCC, the most common type of skin cancer, showing improved sensitivity (79%–96%) and specificity (75%–96%) when compared with clinical assessment and dermoscopy alone.12,14 Typical OCT features differentiating BCC from other lesions include hyporeflective ovoid nests with a dark rim and an alteration of the dermoepidermal junction. In addition to providing a good diagnostic overview of skin, OCT devices show promise in monitoring the effects of treatment on primary and recurrent lesions.14-16
In Vivo Excision Planning
Additionally, OCT is a helpful tool in delineating tumor margins prior to surgical resection to achieve optimal cosmesis. By detecting subclinical tumor extension, this preoperative technique has been shown to reduce the number of surgical stages. Pomerantz et al17 showed that mapping BCC tumor margins with OCT prior to Mohs micrographic surgery closely approximated the final surgical defects. Alawi et al18 showed that the OCT-defined lateral margins correctly indicated complete removal of tumors. These studies illustrate the ability of OCT to minimize the amount of skin excised without compromising the integrity of tumor-free borders. The use of ex vivo OCT to detect residual tumors is not recommended based on current studies.6,17,18
Diagnosis and Treatment Monitoring of Other Diseases
Further applications of OCT include diagnosis of noncancerous lesions such as nail conditions, scleroderma, psoriatic arthritis, blistering diseases, and vascular lesions, as well as assessment of skin moisture and hydration, burn depth, wound healing, skin atrophy, and UV damage.2 For example, Aldahan et al19 demonstrated the utility of D-OCT to identify structural and vascular features specific to nail psoriasis useful in the diagnosis and treatment monitoring of the condition.
Limitations of OCT
Resolution
Frequency-domain OCT enables the detection of architectural details within tissue, but its image resolution is not sufficient to distinguish individual cells, therefore restricting its use in evaluating pigmented benign and malignant lesions such as dysplastic nevi and melanomas. Higher-resolution RCM is superior for imaging these lesions, as its device can better evaluate microscopic structures. With the advent of D-OCT and HD-OCT, research is being conducted to assess their use in differentiating pigmented lesions.8,20 Schuh et al9 and Gambichler et al20 reported preliminary results indicating the utility of D-OCT and HD-OCT to differentiate dysplastic nevi from melanomas and melanoma in situ, respectively.
Depth Measurement
Another limitation is associated with measuring lesion depth for advanced tumors. Although the typical imaging depth of OCT is significantly deeper than most other noninvasive imaging modalities used on skin, imaging deep tumor margins and invasion is restricted.
Image Interpretation
Diagnostic imaging requires image interpretation leading to potential interobserver and intraobserver variation. Experienced observers in OCT more accurately differentiated normal from lesional skin compared to novices, which suggests that training could improve agreement.21,22
Reimbursement and Device Cost
Other practical limitations to widespread OCT utilization at this time include its initial laser device cost and lack of reimbursement. As such, large academic and research centers remain the primary sites to utilize these devices.
Future Directions
Optical coherence tomography complements other established noninvasive imaging tools allowing for real-time visualization of the skin without interfering with the tissue and offering images with a good balance of depth, resolution, and field of view. Although a single histology cut has superior cellular resolution to any imaging modality, OCT provides additional information that is not provided by a physical biopsy, given the multiple vertical sections of data. Optical coherence tomography is a useful diagnostic technique enabling patients to avoid unnecessary biopsies while increasing early lesion diagnosis. Furthermore, OCT helps to decrease repetitive biopsies throughout nonsurgical treatments. With the availability of newer technology such as D-OCT and HD-OCT, OCT will play an increasing role in patient management. Clinicians and researchers should work to convert from category III to category I CPT codes and obtain reimbursement for imaging, with the ultimate goal of increasing its use in clinical practice and improving patient care.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
- Michelson Diagnostics secures CPT codes for optical coherence tomography imaging of skin [press release]. Maidstone, Kent, United Kingdom: Michelson Diagnostics; July 14, 2017. https://vivosight.com/wp-content/uploads/2017/07/Press-Release-CPT-code-announcement-12-July-2017.pdf. Accessed August 17, 2017.
- Schmitz L, Reinhold U, Bierhoff E, et al. Optical coherence tomography: its role in daily dermatological practice. J Dtsch Dermatol Ges. 2013;11:499-507.
- Hibler BP, Qi Q, Rossi AM. Current state of imaging in dermatology. Semin Cutan Med Surg. 2016;35:2-8.
- Current Procedural Terminology 2018, Professional Edition. Chicago IL: American Medical Association; 2017.
- Current Procedural Terminology 2017, Professional Edition. Chicago IL: American Medical Association; 2016.
- Cheng HM, Guitera P. Systemic review of optical coherence tomography usage in the diagnosis and management of basal cell carcinoma. Br J Dermatol. 2015;173:1371-1380.
- Cao T, Tey HL. High-definition optical coherence tomography—an aid to clinical practice and research in dermatology. J Dtsch Dermatol Ges. 2015;13:886-890.
- Schwartz M, Siegel DM, Markowitz O. Commentary on the diagnostic utility of non-invasive imaging devices for field cancerization. Exp Dermatol. 2016;25:855-856.
- Schuh S, Holmes J, Ulrich M, et al. Imaging blood vessel morphology in skin: dynamic optical coherence tomography as a novel potential diagnostic tool in dermatology. Dermatol Ther. 2017;7:187-202.
- Themstrup L, Pellacani G, Welzel J, et al. In vivo microvascular imaging of cutaneous actinic keratosis, Bowen’s disease and squamous cell carcinoma using dynamic optical coherence tomography [published online May 14, 2017]. J Eur Acad Dermatol Venereol. doi:10.1111/jdv.14335.
- Markowitz O, Schwartz M, Minhas S, et al. DM. Speckle-variance optical coherence tomography: a novel approach to skin cancer characterization using vascular patterns. Dermatol Online J. 2016;18:22. pii:13030/qt7w10290r.
- Ulrich M, von Braunmuehl T, Kurzen H, et al. The sensitivity and specificity of optical coherence tomography for the assisted diagnosis of nonpigmented basal cell carcinoma: an observational study. Br J Dermatol. 2015;173:428-435.
- Hussain AA, Themstrup L, Jemec GB. Optical coherence tomography in the diagnosis of basal cell carcinoma. Arch Dermatol Res. 2015;307:1-10.
- Markowitz O, Schwartz M, Feldman E, et al. Evaluation of optical coherence tomography as a means of identifying earlier stage basal carcinomas while reducing the use of diagnostic biopsy. J Clin Aesthet Dermatol. 2015;8:14-20.
- Banzhaf CA, Themstrup L, Ring HC, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing imiquimod therapy. Skin Res Technol. 2014;20:170-176.
- Themstrup L, Banzhaf CA, Mogensen M, et al. Optical coherence tomography imaging of non-melanoma skin cancer undergoing photodynamic therapy reveals subclinical residual lesions. Photodiagnosis Photodyn Ther. 2014;11:7-12.
- Pomerantz R, Zell D, McKenzie G, et al. Optical coherence tomography used as a modality to delineate basal cell carcinoma prior to Mohs micrographic surgery. Case Rep Dermatol. 2011;3:212-218.
- Alawi SA, Kuck M, Wahrlich C, et al. Optical coherence tomography for presurgical margin assessment of non-melanoma skin cancer—a practical approach. Exp Dermatol. 2013;22:547-551.
- Aldahan AS, Chen LL, Fertig RM, et al. Vascular features of nail psoriasis using dynamic optical coherence tomography. Skin Appendage Disord. 2017;2:102-108.
- Gambichler T, Plura I, Schmid-Wendtner M, et al. High-definition optical coherence tomography of melanocytic skin lesions. J Biophotonics. 2015;8:681-686.
- Mogensen M, Joergensen TM, Nurnberg BM, et al. Assessment of optical coherence tomography imaging in the diagnosis of non-melanoma skin cancer and benign lesions versus normal skin: observer-blinded evaluation by dermatologists. Dermatol Surg. 2009;35:965-972.
- Olsen J, Themstrup L, De Carbalho N, et al. Diagnostic accuracy of optical coherence tomography in actinic keratosis and basal cell carcinoma. Photodiagnosis Photodyn Ther. 2016;16:44-49.
Practice Points
- Optical coherence tomography (OCT) technology has considerable utility in research and clinical settings given its high resolution, wide field of view, moderate penetration depth, straightforward image acquisition, and accessibility to anatomically challenging areas.
- Potential benefits of OCT include its ability to noninvasively diagnose and monitor nonmelanoma skin cancers as well as to delineate presurgical margins and elucidate the course and mechanism of action of skin conditions at the bedside.
- Limitations of OCT include device cost, lack of reimbursement, and training, as well as restricted ability to image advanced deep tumors and differentiate melanocytic lesions.
- Optical coherence tomography recently received 2 category III Current Procedural Terminology (CPT) codes to track its utilization in clinical practice and will hopefully receive category I CPT codes within the next 5 years.
ACS NSQIP pilot project IDs risks in older surgical patients
NEW YORK – The American College of Surgeons’ National Surgical Improvement Program Geriatric Surgery Pilot Project, which was initiated in 2014, is beginning to bear fruit.
Institutions participating in the project are generating data on geriatric-specific factors such as cognition and mobility that have been shown to add to standard risks associated with surgery in older adults.
“Before you operate at all, there is a decision, and often surgeons use this framework when deciding whether or not to operate: There is an isolated surgical problem, and I think we can fix that problem,” Julia R. Berian, MD, said at the ACS Quality and Safety Conference. “This fails to really incorporate the context of these older, complicated surgical patients.”
“We are facing a silver tsunami. The population is aging,” Emily Finlayson, MD, FACS, said during a separate presentation at the conference. “People are coming to us to decide, A, if they should have surgery, and B, how best to prepare for surgery.”
“As we know, from mounting evidence, surgical outcomes in frail older adults are pretty abysmal.” In addition to the physiologic vulnerabilities, “there is a lot of social isolation, depression, and anxiety that is underdiagnosed in this population,” Dr. Finlayson said. “In light of these incredibly high risks, we need to approach decision making in a slightly different way than we do with, say, a 40-year-old patient.”
Use data to guide interventions
The ACS NSQIP and the ACS Geriatric Task Force created the ACS NSQIP Geriatric Surgery Pilot Project in part to determine if including geriatric-specific preoperative variables and outcome measures in the NSQIP database would improve postoperative outcomes. Since its launch in January 2014, more than 30 hospitals have contributed data from over 30,000 surgical cases involving patients 65 years and older. The vast majority of cases involve orthopedic surgery or general surgery, with total hip and total knee arthroplasty, colectomy, spine surgery, and hip fracture procedures leading the list.
Cognition, function, mobility, and goals/decision making are the four main project domains. “The event rate for postoperative delirium overall was 12%; the functional decline was quite high at 43%; and the need for postoperative mobility aid was 30%,” said Dr. Berian, a fourth-year general surgery resident at the University of Chicago and an ACS Clinical Scholar, when presenting initial 3-year results.
“What we have learned from this experience is that these geriatric-specific risk factors do contribute to risk adjustment for traditional morbidity and mortality outcomes. In other words, we think they are very important to collect,” Dr. Berian said.
Cognitive impairment was associated only with prolonged ventilation, whereas surrogate consent for surgery correlated with any morbidity, reintubation, pneumonia, and more. Use of a mobility aid before surgery correlated with increased risk for a UTI, surgical site infection, sepsis, and other morbidities. A history of falls within the previous year was associated with higher risk of cardiac complications and mortality. Functional status, origin from home before surgery, and use of preoperative palliative care were not contributors to risk.
A second objective of the project is to create a platform for introducing interventions to improve outcomes in this population. Future plans include further validation of the pilot data and incorporation of the results into a geriatric-specific quality program.
Focus on potential solutions
Addressing a wide range of preoperative considerations in older adults may seem daunting, but “there are simple, low-tech things you can do,” said Dr. Finlayson, director of the University of California San Francisco Center for Surgery in Older Adults. Strategies include reviewing medications, providing adequate hydration “so they don’t come in as dry as a potato chip,” and removing earwax. “You might think they’re confused but they really cannot hear.”
Whenever possible, address the core vulnerabilities that put an older patient at higher risk, Dr. Finlayson said. Comorbidity, polypharmacy, incontinence, social isolation, depression and anxiety, as well as deficits in function, nutrition, and mobility can contribute.
Cognition is also critical. If you think an older patient is at risk of postoperative delirium, involve the family, Dr. Finlayson recommended. “We know if family members are at the bedside, the patient is less likely to get confused.” Clinicians at UCSF found this “very helpful” and even give families a sign-up sheet to assign shifts in the hospital.
“If you don’t think delirium is an important outcome to begin tracking in our registries, I want to point out that there are serious consequences for postop delirium,” Dr. Berian said. Delirium alone in surgical patients doubles the increased risk of prolonged length of stay, 1.5 times the risk for institutional discharge, and 2.3 times the risk for 30-day readmission (JAMA Surgery. 2015;150[12]:1134-40). “When you combine delirium with complications, those risks increase dramatically,” she added.
Take a team approach
Session moderator David A. Hoyt, MD, FACS, executive director of the American College of Surgeons, asked Dr. Finlayson how she convinced her colleagues to participate in the program at UCSF.
“We haven’t had any problems with buy-in in terms of recognizing the need,” she replied. “The challenge is a lot of surgeons feel like they don’t have the expertise or the time to slow down and learn how to do these assessments and optimization strategies.” She suggested involving geriatricians and other providers when possible. “You have to be very creative within your own system in terms of what kind of team you are going to put together.”
Elicit patient goals
Perhaps most importantly, you really need to individualize your approach, Dr. Finlayson said. Take the time to talk to these patients. “This isn’t just don’t smoke, lose weight, diet and exercise. It’s eliciting patient goals and tailoring an assessment of geriatric vulnerability,” she added. “It’s not one size fits all. It’s not just about fitness for surgery; it’s about what they want for the rest of their lives.”
Patient-driven goals are important, Dr. Berian said, because “older adults may prioritize quality of life over quantity of life.” She also noted that surgery could cure their disease, prolong life, and/or provide symptom relief, or it could cause loss of function and independence, delirium, cognitive loss, and/or premature death. “There was an interesting study … looking at outcomes that could be considered worse than death,” Dr. Berian said (JAMA Intern. Med. 2016;176[10]:1557-9). Bowel and bladder incontinence, being confused all the time, and relying on a feeding tube to live were among the outcomes the researchers examined.
Dr. Finlayson highlighted a high-touch, resource-intensive, and successful intervention in older patients in the United Kingdom (Age Ageing. 2007;36[6]:670-5). “The reduction in morbidity was incredibly dramatic.” The study shows if you truly have the resources to address these geriatric syndromes, you can really improve care in this population.
Dr. Berian had no relevant financial disclosures. Dr. Finlayson is a founding shareholder of Ooney, Inc.
NEW YORK – The American College of Surgeons’ National Surgical Improvement Program Geriatric Surgery Pilot Project, which was initiated in 2014, is beginning to bear fruit.
Institutions participating in the project are generating data on geriatric-specific factors such as cognition and mobility that have been shown to add to standard risks associated with surgery in older adults.
“Before you operate at all, there is a decision, and often surgeons use this framework when deciding whether or not to operate: There is an isolated surgical problem, and I think we can fix that problem,” Julia R. Berian, MD, said at the ACS Quality and Safety Conference. “This fails to really incorporate the context of these older, complicated surgical patients.”
“We are facing a silver tsunami. The population is aging,” Emily Finlayson, MD, FACS, said during a separate presentation at the conference. “People are coming to us to decide, A, if they should have surgery, and B, how best to prepare for surgery.”
“As we know, from mounting evidence, surgical outcomes in frail older adults are pretty abysmal.” In addition to the physiologic vulnerabilities, “there is a lot of social isolation, depression, and anxiety that is underdiagnosed in this population,” Dr. Finlayson said. “In light of these incredibly high risks, we need to approach decision making in a slightly different way than we do with, say, a 40-year-old patient.”
Use data to guide interventions
The ACS NSQIP and the ACS Geriatric Task Force created the ACS NSQIP Geriatric Surgery Pilot Project in part to determine if including geriatric-specific preoperative variables and outcome measures in the NSQIP database would improve postoperative outcomes. Since its launch in January 2014, more than 30 hospitals have contributed data from over 30,000 surgical cases involving patients 65 years and older. The vast majority of cases involve orthopedic surgery or general surgery, with total hip and total knee arthroplasty, colectomy, spine surgery, and hip fracture procedures leading the list.
Cognition, function, mobility, and goals/decision making are the four main project domains. “The event rate for postoperative delirium overall was 12%; the functional decline was quite high at 43%; and the need for postoperative mobility aid was 30%,” said Dr. Berian, a fourth-year general surgery resident at the University of Chicago and an ACS Clinical Scholar, when presenting initial 3-year results.
“What we have learned from this experience is that these geriatric-specific risk factors do contribute to risk adjustment for traditional morbidity and mortality outcomes. In other words, we think they are very important to collect,” Dr. Berian said.
Cognitive impairment was associated only with prolonged ventilation, whereas surrogate consent for surgery correlated with any morbidity, reintubation, pneumonia, and more. Use of a mobility aid before surgery correlated with increased risk for a UTI, surgical site infection, sepsis, and other morbidities. A history of falls within the previous year was associated with higher risk of cardiac complications and mortality. Functional status, origin from home before surgery, and use of preoperative palliative care were not contributors to risk.
A second objective of the project is to create a platform for introducing interventions to improve outcomes in this population. Future plans include further validation of the pilot data and incorporation of the results into a geriatric-specific quality program.
Focus on potential solutions
Addressing a wide range of preoperative considerations in older adults may seem daunting, but “there are simple, low-tech things you can do,” said Dr. Finlayson, director of the University of California San Francisco Center for Surgery in Older Adults. Strategies include reviewing medications, providing adequate hydration “so they don’t come in as dry as a potato chip,” and removing earwax. “You might think they’re confused but they really cannot hear.”
Whenever possible, address the core vulnerabilities that put an older patient at higher risk, Dr. Finlayson said. Comorbidity, polypharmacy, incontinence, social isolation, depression and anxiety, as well as deficits in function, nutrition, and mobility can contribute.
Cognition is also critical. If you think an older patient is at risk of postoperative delirium, involve the family, Dr. Finlayson recommended. “We know if family members are at the bedside, the patient is less likely to get confused.” Clinicians at UCSF found this “very helpful” and even give families a sign-up sheet to assign shifts in the hospital.
“If you don’t think delirium is an important outcome to begin tracking in our registries, I want to point out that there are serious consequences for postop delirium,” Dr. Berian said. Delirium alone in surgical patients doubles the increased risk of prolonged length of stay, 1.5 times the risk for institutional discharge, and 2.3 times the risk for 30-day readmission (JAMA Surgery. 2015;150[12]:1134-40). “When you combine delirium with complications, those risks increase dramatically,” she added.
Take a team approach
Session moderator David A. Hoyt, MD, FACS, executive director of the American College of Surgeons, asked Dr. Finlayson how she convinced her colleagues to participate in the program at UCSF.
“We haven’t had any problems with buy-in in terms of recognizing the need,” she replied. “The challenge is a lot of surgeons feel like they don’t have the expertise or the time to slow down and learn how to do these assessments and optimization strategies.” She suggested involving geriatricians and other providers when possible. “You have to be very creative within your own system in terms of what kind of team you are going to put together.”
Elicit patient goals
Perhaps most importantly, you really need to individualize your approach, Dr. Finlayson said. Take the time to talk to these patients. “This isn’t just don’t smoke, lose weight, diet and exercise. It’s eliciting patient goals and tailoring an assessment of geriatric vulnerability,” she added. “It’s not one size fits all. It’s not just about fitness for surgery; it’s about what they want for the rest of their lives.”
Patient-driven goals are important, Dr. Berian said, because “older adults may prioritize quality of life over quantity of life.” She also noted that surgery could cure their disease, prolong life, and/or provide symptom relief, or it could cause loss of function and independence, delirium, cognitive loss, and/or premature death. “There was an interesting study … looking at outcomes that could be considered worse than death,” Dr. Berian said (JAMA Intern. Med. 2016;176[10]:1557-9). Bowel and bladder incontinence, being confused all the time, and relying on a feeding tube to live were among the outcomes the researchers examined.
Dr. Finlayson highlighted a high-touch, resource-intensive, and successful intervention in older patients in the United Kingdom (Age Ageing. 2007;36[6]:670-5). “The reduction in morbidity was incredibly dramatic.” The study shows if you truly have the resources to address these geriatric syndromes, you can really improve care in this population.
Dr. Berian had no relevant financial disclosures. Dr. Finlayson is a founding shareholder of Ooney, Inc.
NEW YORK – The American College of Surgeons’ National Surgical Improvement Program Geriatric Surgery Pilot Project, which was initiated in 2014, is beginning to bear fruit.
Institutions participating in the project are generating data on geriatric-specific factors such as cognition and mobility that have been shown to add to standard risks associated with surgery in older adults.
“Before you operate at all, there is a decision, and often surgeons use this framework when deciding whether or not to operate: There is an isolated surgical problem, and I think we can fix that problem,” Julia R. Berian, MD, said at the ACS Quality and Safety Conference. “This fails to really incorporate the context of these older, complicated surgical patients.”
“We are facing a silver tsunami. The population is aging,” Emily Finlayson, MD, FACS, said during a separate presentation at the conference. “People are coming to us to decide, A, if they should have surgery, and B, how best to prepare for surgery.”
“As we know, from mounting evidence, surgical outcomes in frail older adults are pretty abysmal.” In addition to the physiologic vulnerabilities, “there is a lot of social isolation, depression, and anxiety that is underdiagnosed in this population,” Dr. Finlayson said. “In light of these incredibly high risks, we need to approach decision making in a slightly different way than we do with, say, a 40-year-old patient.”
Use data to guide interventions
The ACS NSQIP and the ACS Geriatric Task Force created the ACS NSQIP Geriatric Surgery Pilot Project in part to determine if including geriatric-specific preoperative variables and outcome measures in the NSQIP database would improve postoperative outcomes. Since its launch in January 2014, more than 30 hospitals have contributed data from over 30,000 surgical cases involving patients 65 years and older. The vast majority of cases involve orthopedic surgery or general surgery, with total hip and total knee arthroplasty, colectomy, spine surgery, and hip fracture procedures leading the list.
Cognition, function, mobility, and goals/decision making are the four main project domains. “The event rate for postoperative delirium overall was 12%; the functional decline was quite high at 43%; and the need for postoperative mobility aid was 30%,” said Dr. Berian, a fourth-year general surgery resident at the University of Chicago and an ACS Clinical Scholar, when presenting initial 3-year results.
“What we have learned from this experience is that these geriatric-specific risk factors do contribute to risk adjustment for traditional morbidity and mortality outcomes. In other words, we think they are very important to collect,” Dr. Berian said.
Cognitive impairment was associated only with prolonged ventilation, whereas surrogate consent for surgery correlated with any morbidity, reintubation, pneumonia, and more. Use of a mobility aid before surgery correlated with increased risk for a UTI, surgical site infection, sepsis, and other morbidities. A history of falls within the previous year was associated with higher risk of cardiac complications and mortality. Functional status, origin from home before surgery, and use of preoperative palliative care were not contributors to risk.
A second objective of the project is to create a platform for introducing interventions to improve outcomes in this population. Future plans include further validation of the pilot data and incorporation of the results into a geriatric-specific quality program.
Focus on potential solutions
Addressing a wide range of preoperative considerations in older adults may seem daunting, but “there are simple, low-tech things you can do,” said Dr. Finlayson, director of the University of California San Francisco Center for Surgery in Older Adults. Strategies include reviewing medications, providing adequate hydration “so they don’t come in as dry as a potato chip,” and removing earwax. “You might think they’re confused but they really cannot hear.”
Whenever possible, address the core vulnerabilities that put an older patient at higher risk, Dr. Finlayson said. Comorbidity, polypharmacy, incontinence, social isolation, depression and anxiety, as well as deficits in function, nutrition, and mobility can contribute.
Cognition is also critical. If you think an older patient is at risk of postoperative delirium, involve the family, Dr. Finlayson recommended. “We know if family members are at the bedside, the patient is less likely to get confused.” Clinicians at UCSF found this “very helpful” and even give families a sign-up sheet to assign shifts in the hospital.
“If you don’t think delirium is an important outcome to begin tracking in our registries, I want to point out that there are serious consequences for postop delirium,” Dr. Berian said. Delirium alone in surgical patients doubles the increased risk of prolonged length of stay, 1.5 times the risk for institutional discharge, and 2.3 times the risk for 30-day readmission (JAMA Surgery. 2015;150[12]:1134-40). “When you combine delirium with complications, those risks increase dramatically,” she added.
Take a team approach
Session moderator David A. Hoyt, MD, FACS, executive director of the American College of Surgeons, asked Dr. Finlayson how she convinced her colleagues to participate in the program at UCSF.
“We haven’t had any problems with buy-in in terms of recognizing the need,” she replied. “The challenge is a lot of surgeons feel like they don’t have the expertise or the time to slow down and learn how to do these assessments and optimization strategies.” She suggested involving geriatricians and other providers when possible. “You have to be very creative within your own system in terms of what kind of team you are going to put together.”
Elicit patient goals
Perhaps most importantly, you really need to individualize your approach, Dr. Finlayson said. Take the time to talk to these patients. “This isn’t just don’t smoke, lose weight, diet and exercise. It’s eliciting patient goals and tailoring an assessment of geriatric vulnerability,” she added. “It’s not one size fits all. It’s not just about fitness for surgery; it’s about what they want for the rest of their lives.”
Patient-driven goals are important, Dr. Berian said, because “older adults may prioritize quality of life over quantity of life.” She also noted that surgery could cure their disease, prolong life, and/or provide symptom relief, or it could cause loss of function and independence, delirium, cognitive loss, and/or premature death. “There was an interesting study … looking at outcomes that could be considered worse than death,” Dr. Berian said (JAMA Intern. Med. 2016;176[10]:1557-9). Bowel and bladder incontinence, being confused all the time, and relying on a feeding tube to live were among the outcomes the researchers examined.
Dr. Finlayson highlighted a high-touch, resource-intensive, and successful intervention in older patients in the United Kingdom (Age Ageing. 2007;36[6]:670-5). “The reduction in morbidity was incredibly dramatic.” The study shows if you truly have the resources to address these geriatric syndromes, you can really improve care in this population.
Dr. Berian had no relevant financial disclosures. Dr. Finlayson is a founding shareholder of Ooney, Inc.
AT THE ACS QUALITY AND SAFETY CONFERENCE
Incorporating New Atopic Dermatitis Medications in Your Practice
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.
What advice do you give your patients today?
There is more scientific data supporting educational intervention with an eczema action plan as the core of prevention and therapy. Early institution of emollient therapy is preventive of approximately half of atopic dermatitis (AD) cases. Application of emollients immediately after bathing is best for improvement of skin hydration. The art of medicine is deciding how to pick emollients with patients. It is important to avoid patient's allergens, but ultimately the choice comes down to cold weather petrolatum and warm weather thick lotions or creams.
Therapy must still be individually tailored. Head and neck disease is best treated with nonsteroidal agents including low-strength topical corticosteroids and calcineurin inhibitors that have a black box warning, both of which have a track record of efficacy in the care of AD. A newer option is crisaborole, a topical phosphodiesterase inhibitor, which is an alternative for childhood and adult AD. For the body, any of these agents can be used comfortably, but often a mixture of topical corticosteroids of various strengths is chosen to address different sites of disease. When topical corticosteroids fail, the usage of systemic agents or phototherapy may be appropriate. The new prescription injectable dupilumab is approved for adults with AD and therapies such as these will hopefully soon be available for children with severe disease who need intervention to improve their quality of life.
How have you integrated new medications? How do you deal with side effects?
For all the therapies that truly work for AD, there are still many patients with limited to poor response on standard regimens and I offer them newer options and I also review their old regimens. Many patients believe they will be cured in 1 to 2 weeks and stop ongoing care. Counseling on the recurrent and relapsing nature of AD is important. On the other hand, I have AD patients who believe they had or truly have steroid sensitivity including allergy or withdrawal syndromes. I have seen topical steroid atrophy in this setting due to lack of intermittent discontinuation. Other situations in which topical steroid side effects are common in my practice are in the application sites of the thigh and calf in teenaged girls and the chest in teenaged boys, sites where striae are not uncommon naturally during adolescence. In these settings, confirmation of allergy via patch testing may be helpful and offering nonsteroidal agents can allow for remission of disease. Side effects with nonsteroidal agents are common but usually mild including pruritus, burning, and stinging. It is common for these symptoms to dissipate with time; therefore, preemptive education is vital (ie, stopping and restarting a day later) as well as avoidance of application to recently washed skin and limited application initially. Steroid pretreatment sometimes aids in acceptance of a nonsteroidal agent.
What information do patients want to hear?
Patients and guardians believe there has to be a cure for AD and that it will be dietary in nature. They hope I will provide an avoidance diet that will rapidly clear the disease, which I wish was true. In reality, the nature of current research is such that long-term remissions and possible cure do lie on the horizon but today are not readily available. No one can bypass good skin care and the current treatment paradigm. Withdrawal diets may cause malnourishment in children and should not be undertaken without proof of allergy.
How do you deal with steroid phobia?
Steroid phobia has become a hot topic but has existed since the advent of topical agents. Steroid phobia can cause nonadherence and poor outcomes. In reality, many topical steroidal agents have good testing and approvals in younger children. Fear is a powerful motivator and hard to break. Therefore, parents/guardians may reasonably opt for nonsteroidal care, which is a fine option when it works. Although little data on real-world combination usage of nonsteroidal and steroidal agents exist, combinations in my practice often enhance clearance.
What patient resources do you recommend?
Quoting study data may be beneficial. One of my favorite studies is historic comparative data of hydrocortisone cream 1% and mometasone furoate cream 0.1% in 48 children with moderate to severe AD (Vernon et al). At completion of the study, mometasone performed better in clearance and the only patient who developed hypothalamic-pituitary-adrenal axis suppression was in the hydrocortisone arm. I use this study to explain to parents why a prescription-strength agent may produce better results with fewer side effects.
Online snake oils abound in AD and the sources for solid information I choose are the websites of the National Eczema Association as well as academic organizations such as the American Academy of Dermatology and the Society for Pediatric Dermatology. Membership in support groups and participation can help parents/guardians and children alike and allow access to early clinical trial data. I sometimes ask parents/guardians to review manufacturer websites to specifically look for quoted clinical trial data. Although all clinical trials are not equivalent, many better eczema care manufacturers have numerous clinical trials in support of their agents, which should give a parent some enhanced comfort level.
Suggested Readings
- Chiang C, Eichenfield LF. Quantitative assessment of combination bathing and moisturizing regimens on skin hydration in atopic dermatitis. Pediatr Dermatol. 2009;26:273-278.
- Juha'sz MLW, Curley RA, Rasmussen A, et al. Systematic review of the topical steroid addiction and steroid withdrawal phenomenon in children diagnosed with atopic dermatitis and treated with topical corticosteroids. J Dermatol Nurses Assoc. In press.
- Mueller SM, Itin P, Vogt DR, et al. Assessment of "corticophobia" as an indicator of non-adherence to topical corticosteroids: a pilot study. J Dermatolog Treat. 2017;28:104-111.
- Shirley M. Dupilumab: first global approval. Drugs. 2017;77:1115-1121.
- Silverberg NB, Durán-McKinster C. Special considerations for therapy of pediatric atopic dermatitis. Dermatol Clin. 2017;35:351-363.
- Simpson EL, Chalmers JR, Hanifin JM, et al. Emollient enhancement of the skin barrier from birth offers effective atopic dermatitis prevention. J Allergy Clin Immunol. 2014;134:818-823.
- Vernon HJ, Lane AT, Weston W. Comparison of mometasone furoate 0.1% cream and hydrocortisone 1.0% cream in the treatment of childhood atopic dermatitis. J Am Acad Dermatol. 1991;24:603-607.