User login
Pediatric Dermatology Consult - September 2016
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
Onychomycosis
Onychomycosis is a nail infection caused by a variety of fungi, including dermatophytes, yeasts, and nondermatophyte molds. Tinea unguium refers specifically to nail infections caused by dermatophytes, which are the most common cause of onychomycosis; they cause 82% of cases in the U.S. hospital population.1
Onychomycosis is more prevalent in adults than in the pediatric population.2 A recent study showed that 3.22%, 0.40%, and 0.37% of adults have culture-proven dermatophyte, yeast, and nondermatophyte mold onychomycosis, respectively, while in the pediatric population, 0.14% have dermatophyte and 0.09% have yeast toenail onychomycosis.2
The likely reason for lower prevalence of onychomycosis in the pediatric population is the faster growth rate of pediatric nails, the smaller surface area susceptible to infection, and the absence of cumulative trauma and tinea pedis.2 Distal lateral subungual onychomycosis is the most common clinical pattern of onychomycosis.2
The diagnosis usually is based on strong clinical suspicion, but laboratory evidence to support a clinical diagnosis is ideal. Patients may be evaluated with a fungal culture, potassium hydroxide (KOH) preparation, or histologic evaluation of the nail clippings with periodic acid-Schiff (PAS) staining. A KOH preparation is highly specific for onychomycosis, but sensitivity depends on the specimen obtained. The histopathology of the nail clippings sample treated with a PAS stain demonstrates fungal elements and is the most sensitive test, but it does not identify the species.3 It takes a few days for the results and costs more than a KOH preparation.
Differential diagnosis
The differential for nail dystrophy in children includes trauma, fungal infection, congenital dystrophies, psoriasis, and lichen planus.4
Trauma can result in similar changes to onychomycosis, such as distal onycholysis. Unlike the most common type of onychomycosis, there is rarely distal thickening of the nail. Usually the morphology, history of trauma, and culture can be used to differentiate the two.
Congenital dystrophies often include diseases that have other clinical manifestations. Children exhibited nail alterations in diseases such as dystrophic epidermolysis bullosa, focal dermal hypoplasia, Turner syndrome, and Down syndrome.4
"Twenty-nail dystrophy," also known as trachyonychia, presents with longitudinal ridges, lost of luster, sandpaper-like rough appearance, and pitting. While the cause is not known, it may be associated with lichen planus, psoriasis, alopecia areata, and atopic dermatitis.
Nail psoriasis can present similarly to onychomycosis with subungual hyperkeratosis and onycholysis. However, distinguishing features for nail psoriasis include pitting, nail bed salmon patches (areas of yellow or pink discoloration), or "oil drop" discoloration, and other systemic findings such as cutaneous or joint findings.
Lichen planus is an inflammatory condition of unknown etiology that also can present with onycholysis with or without subungual hyperkeratosis when it involves the nail matrix. Its clinical characteristics include longitudinal ridging, nail plate thinning, and longitudinal fissuring.
Onychomadesis is the proximal separation of the nail plate from the nail matrix and bed. It is caused by temporary arrest of the nail matrix activity associated with a variety of systemic illnesses or drug exposure, and presents with "peeling" or shedding of the nail from the proximal portion of the plate. It has been noticed commonly with hand, foot, and mouth disease in children.
Etiology
The term dermatophytosis describes infections caused by members of the genera Microsporum, Trichophyton, and Epidermophyton. Trichophyton rubrum is the most common dermatophyte to cause onychomycosis.1 Risk factors for developing onychomycosis include older age, tinea pedis, psoriasis, diabetes, immunodeficiency, genetic predisposition, swimming, and living with family members who have onychomycosis.5 Tinea pedis is a major risk factor for the development of onychomycosis, with concurrent rates of the two diseases reported as high as 47%.6 Candida species may cause onychomycosis, while nondermatophyte molds (such as Acremonium, Alternaria, Aspergillus, Fusarium, Scytalidium, and Scopulariopsis species) are rarely true pathogens in immunocompetent children.2
Treatment
Onychomycosis may cause physical discomfort and pain, and may increase the risk for developing bacterial cellulitis, especially in patients with tinea pedis.7 Treatment options can include observation, if there is minimal discomfort, oral systemic antifungal medications, topical antifungal medications, and physical interventions.
While there is no systemic antifungal approved by the Food and Drug Administration for use in children, several systemic antifungals may be utilized off-label. Oral terbinafine or itraconazole are the most effective in achieving cure, with griseofulvin next most effective and fluconazole less so.8 The safety and effectiveness of these medications in children have not been established. With the exception of one case of ataxia with the use of itraconazole, adverse events for terbinafine and itraconazole treatment in children are limited to reports listed in the prescribing information and include: gastrointestinal side effects, urticaria, hepatotoxicity, neutropenia, thrombocytopenia, and cytochrome P450 enzyme inhibition.8 Terbinafine dosing for children is based on studies for tinea capitis and determined by weight: 10-20 kg, 62.5 mg/day; 20-40 kg, 125 mg/day; greater than 40 kg, 250 mg/day for 6 weeks.9 FDA prescribing information suggests a baseline liver function panel prior to initiation of the drug, but there are no recommendations on serial lab monitoring. Dosing of itraconazole in the pediatric population is not as well established.9
Topical antifungal agents can be used in pediatric nail infections that do not involve the nail matrix (lunula). Pediatric nails grow faster than adult nails and children have a thinner nail plate, which may allow better penetration of the drug, making children more likely to respond better to topical treatment.10 Topical therapy options for onychomycosis include ciclopirox and amorolfine nail lacquers, and bifonazole-urea; these require application for prolonged periods of time. Friedlander et al. showed that children with onychomycosis without nail matrix treated with ciclopirox 8% over 32 weeks had a 90% mycologic cure rate.11 Recently, new topical treatments (efinaconazole and tavaborole) became available for treatment of onychomycosis in adults and appear to be more effective.12,13 The data for these treatments in pediatric onychomycosis are being gathered, and the results will provide insight into the efficacy of these new formulations in the pediatric population.
Behavioral measures that may reduce risk of onychomycosis include: keeping feet cool and dry, wearing shoes in public areas, and avoidance of shared, unsterilized nail manicure equipment.5
References
- J Eur Acad Dermatol Venereol. 2014 Nov;28(11):1480-91.
- J Eur Acad Dermatol Venereol. 2015 Jun;29(6):1039-44.
- J Eur Acad Dermatol Venereol. 2011 Feb;25(2):235-7.
- Pediatric Dermatology 2001 Mar;18:107-9.
- J Drugs Dermatol. 2015 Oct;14(10 Suppl):s32-4.
- J Am Acad Dermatol. 1997 Mar;36(3 Pt 1):395-402.
- Dermatology. 2004;209(4):301-7.
- Pediatr Dermatol. 2013 May-Jun;30(3):294-302.
- Tinea Pedis and Tinea Unguium, in "Red Book: 2015 Report of the Committee on Infectious Diseases, 30th Edition (Elk Grove Village, IL: American Academy of Pediatrics, 2015; 784-6).
- Am J Clin Dermatol. 2014 Dec;15(6):489-502.
- Pediatr Dermatol. 2013 May-Jun;30(3):316-22.
- J Am Acad Dermatol. 2015 Jul;73(1):62-9.
- J Am Acad Dermatol. 2013 Apr;68(4):600-8.
Dr. Matiz is assistant professor of dermatology at Rady Children’s Hospital San Diego–University of California, San Diego, and Mr. Ginsberg is a research associate at the hospital. Dr. Matiz and Mr. Ginsberg said they have no relevant financial disclosures.
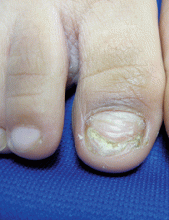
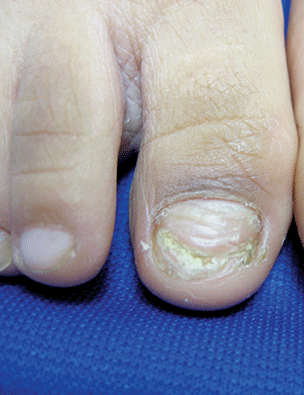
A 5-year-old boy presents to his physician for evaluation of "toenail issues" for at least 1 year. The family has noticed changes of the his right great toenail, which they thought might be due to "tight shoes," stating that the boy has been growing out of his shoes quickly. In the last 6 months, his mother has noted a "crumbly" nail with yellow discoloration. There has been no prior treatment, although his parents now are replacing his sneakers more regularly to allow him "room to grow." He has no history of toe swelling or pain. He is otherwise healthy, and he has no history of psoriasis or eczema. He has had no significant viral infections, although some children in his school did have hand, foot, and mouth disease several months ago. His mother states that her husband has athlete's foot, which has been treated with "creams and sprays." Physical exam The toenail of the right foot great toe has thickening of the distal part of the nail, with onycholysis (separation of the nail plate from the nail bed), yellow discoloration, and subungual debris. The right toe shows some chronic dystrophy. Other toenails appear normal, and the skin of the feet is otherwise unremarkable.
VIDEO: When geriatric depression turns psychotic
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
A geriatric patient who recently lost his wife presents with significant weight loss and appears disheveled. He speaks of reuniting with his wife as soon as possible. How do you quickly stabilize this patient who appears to be experiencing psychotic depression?
In this installment of Mental Health Consult, our panel members discuss their recommendations for triaging a 65-year-old recently widowed man with a history of prostate cancer but no prior history of psychosis.
Join our panel of experts from George Washington University, Washington, including Katalin Roth, MD, director of geriatrics and palliative medicine; April Barbour, MD, MPH, director of the division of general internal medicine; and Lorenzo Norris, MD, medical director of psychiatric and behavioral services, as they discuss how to effectively deal with a geriatric patient in crisis.
On Twitter @whitneymcknight
10 recommendations for the Cancer Moonshot
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
Responding to the Cancer Moonshot initiative, a panel of scientists, clinicians, patient advocates, and industry representatives has issued 10 recommendations for accelerating cancer research in an article published in Science.
The recommendations address:
• Development of a patient engagement network.
• Precise cataloging of tumor molecular changes.
• Analysis of samples already available from patients who have received the standard of care.
• Improvements for data sharing, access, and analysis.
• Development of models to understand how childhood cancers develop.
• Research to describe how fusion oncoproteins drive cancer development.
• Creation of a cancer immunotherapy clinical trials network.
• Systematic efforts to gather information on patient-reported outcomes.
• Implementation of evidence-based approaches to prevention.
The panel’s recommendations were presented to the National Cancer Advisory Board, the adviser to the National Cancer Institute. The ability to conduct research stemming from the panel’s recommendations will depend on whether, and how much, funding is approved by Congress.
Read the article here: http://science.sciencemag.org/content/early/2016/09/07/science.aai7862.full.
6 Tips for Community Hospitalists Initiating QI Projects
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.
The Society of Hospital Medicine asserts that one of the key principles of an effective hospital medicine group is demonstrating a commitment to continuous quality improvement (QI) and actively participating in initiatives directed at quality and patient safety.1 Large hospitalist groups expect their physicians to contribute to the QI initiatives of the hospitals they staff. But as any hospitalist practicing in a community setting can tell you, QI is much easier said than done.
Acknowledge, Overcome the Obstacles
One of the first hurdles hospitalists must overcome when initiating a QI program is finding the time in their schedule as well as obtaining the time commitment from group leadership and fellow clinicians.
“If a hospitalist has no dedicated time and is working clinically, it is difficult to find time to organize a study,” says Kenneth Epstein, MD, chief medical officer of Hospitalist Consultants, the hospitalist management division of ECI Healthcare Partners, in Traverse City, Mich.
However, many national hospitalist management groups, including ECI and IPC Healthcare of North Hollywood, Calif., expect their clinicians to be continuously engaged in QI projects relative to their facility.
Beyond time, an even tougher obstacle to surmount is a lack of training, according to Kerry Weiner, MD, IPC chief medical officer. He says that each of IPC’s clinical practice leaders must participate in a one-year training program that includes a QI project conducted within their facility and mentored by University of California, San Francisco faculty.
David Nash, MD, founding dean of Jefferson College of Population Health in Philadelphia, says The Joint Commission, as part of its accreditation process, requires hospitals to robustly review errors and “have a performance improvement system in place.” He believes the only way community hospitals can successfully undertake this effort is to make sure hospitalists have adequate training in quality and safety.
Training is available from SHM via its Quality and Safety Educators Academy as well as the American Association for Physician Leadership and the Institute for Healthcare Improvement. However, Dr. Nash recommends graduate-level programs in quality and safety available at several schools including Jefferson, Northwestern University in Chicago, and George Washington University in Washington, D.C.
Yet another hurdle is access to data. Many community hospitals have limited financial and human resources to collect accurate data to use for choosing an area to focus on and measuring improvement.
“Despite all the money invested in electronic medical records, finding timely and accurate data is still challenging,” says Jasen Gundersen, MD, president of Knoxville, Tenn.–based TeamHealth Acute Care Services. “The data may exist, but a community hospital may be limited when it comes to finding people to mine, configure, and analyze the data. Community hospitals tend to be focused on publically reported, whole-hospital data.
“If your project is not related to these metrics, you may have trouble getting quality department support.”
Dr. Weiner echoes that sentiment, noting most community hospitals “react to bad metrics, such as low HCAHPS scores. To get the most support possible,” he says, “design a QI program that people see as a genuine problem that needs to be fixed using their resources.”
Get Involved
Experience is another barrier to community-based QI projects. Dr. Gundersen believes that hospitalists who want to get involved in quality should first join a QI committee.
“One of the best ways to effect change in a hospital is to get to know the players—who’s who, who does what, and who is willing to help,” he says.
Arnu Mohan, MD, chief medical officer of hospital medicine at ApolloMD in Atlanta, agrees with gaining experience before setting out on your own.
“Joining a QI committee is almost never a bad idea,” Dr. Mohan says. “You’ll meet people who can support your work, get insight into the needs of the institution, be exposed to other work being done, and better understand the resources available.”
Choose Your Project Carefully
Dr. Gundersen recommends that before settling on a QI project, hospitalists should first consider what their career goals are.
“Ask yourself why you want to do it,” he says. “Do you have the ambition to become a medical director or chief quality officer? In that case, you need a few QI projects under your belt, and you want to choose a system-wide project. Or is there just something in your everyday life that frustrates you so much you must fix it?”
If the project that compels the clinician is not aligned with the needs of the hospital, “it is worthy of a discussion to make sure you are working on the right project,” he adds. “Is the hospitalist off base, or does the administration need to pay more attention to what is happening on the floor?”
Obtain Buy-in
A QI project has a greater chance at being successful if the participants have a high level of interest in the initiative and there is visible support from the administration: high-level people making public statements, making appearances at QI team meetings, and diverting resources such as information technology and process mapping support to sustain the project. This will only happen if community-based hospitalists are successful at selling their project to the C-suite.
“When you approach senior management, you have only 15 minutes to get their attention about your project,” Dr. Weiner says. “You need to show them that you are bringing part of the solution and your idea will affect their bottom line.”
Jeff Brady, MD, director of the Center for Quality Improvement and Patient Safety, says organization commitment is key to any patient safety initiative.
“In addition to the active engagement of leaders who focus on safety and quality, an organization’s culture is another factor that can either enable or thwart progress toward improving the care they deliver,” he says. “AHRQ [the Agency for Healthcare Research and Quality] developed a collection of instruments—AHRQ Surveys on Patient Safety Culture—to help organizations assess and better understand facilitators and barriers their organizations may encounter as they work to improve safety and quality.”2
Politics also can be a factor. Dr. Gundersen points out that smaller hospitals typically are used to “doing things one way.”
“They may not be receptive to changes a QI program would initiate,” he says. “You have to figure out a way to enlist people to move the project forward. Your ability to drive and influence change may be your most important quality as a physician leader.”
Dr. Mohan believes that the best approach is to find a mentor who has worked on QI initiatives before and can champion your efforts.
“You will need the support of the hospital to access required data, change processes, and implement new tools,” he says. “Many hospitals will have a chief medical officer, chief quality officer, or director of QI who can serve as an important ally to mobilize resources on your behalf.”
Go Beyond Hospital Medicine
Even with administrative support, it is better to assemble a team than attempt to go it alone. Successful QI projects, Dr. Mohan says, tend to be team efforts.
“Finding a community of people who will support your work is critical,” he adds. “A multidisciplinary team, including areas such as nursing, therapy, and administration, that engages people who will complement one another increases the likelihood of success.
“That said, multidisciplinary teams have their challenges. They can be unwieldy to lead and without clear roles and responsibilities. I would recommend a group of two to five people who are passionate about the issue you are trying to solve. And be clear from the beginning what each person’s role is within the group.”
Support can also be found in areas outside of the medical staff.
“Key people in other hospital departments can assist with supplying data, financial solutions, and institutional support,” Dr. Mohan says. “These people may be in various departments, such as quality improvement and case management.
“In the current era of value-based purchasing, where Medicare reimbursement is tied to quality metrics, it’s advantageous to show potential financial impact of the QI initiative on hospital revenue, so assistance by the CFO or others in finance may be helpful.”
Dr. Gundersen suggests hospitalists seek out a “lateral mentor,” someone in a department outside the medical staff who is looking for change and can offer resources.
“For example, physicians are looking for quality improvement, and those in the finance department are looking for good economic return. Physicians can explain medical reasons things need to be done, and the finance people can explain the impact of these choices,” he says. “Working together, they can improve both quality and the bottom line.”
Lateral mentoring also is an effective way to meet the challenge of obtaining accurate data, as it opens up the potential to mine data from various departments.
“At different institutions, data may reside in different departments,” Dr. Epstein says. “For example, patient satisfaction may reside with the CMO, core measures or readmissions may reside with the quality management department, and length of stay may be the purview of the finance department.”
Connections in other departments could be the source of your best data, according to Dr. Epstein.
Consider Incentives, Penalties
In addition to buy-in from administration and professionals in other departments, hospitalists also need the commitment of fellow clinicians. Dr. Weiner believes the only way to do this is through financial incentives.
“In a community setting, start with a meaningful reward for improvement. It must be enough that the hospitalist makes the QI project a priority,” he says.
Dr. Weiner also recommends a small penalty for non-participation.
“Most providers realize QI is just good practice, but for some individuals, you need a consequence. It must be part of the system so it isn’t personal,” Dr. Weiner says. “One way is to mandate that if you do not participate, not only do you not get any of the incentive pay, you might lose some of a productivity bonus. You need to be creative when thinking about how to promote QI.”
In the community hospital setting, Dr. Weiner says, practicality ultimately rules.
“The community hospital has real problems to deal with, so don’t make your project pie-in-the-sky,” he says. “Tie it to the bottom line of the hospital if you can. That’s where you start.” TH
Maybelle Cowan-Lincoln is a freelance writer in New Jersey.
References
- Cawley P, Deitelzweig S, Flores L. The key principles and characteristics of an effective hospital medicine group: as assessment guide for hospitals and hospitalists. J Hosp Med. 2014;9:123-128.
- Surveys on patient safety culture. AHRQ website. Accessed October 12, 2015.
- AHRQ Quality Indicators Toolkit for Hospitals: fact sheet. AHRQ website. Accessed October 10, 2015.
- Practice facilitation handbook. AHRQ website. Accessed on September 25, 2015.
- 5. SHM signature programs. SHM website. Accessed October 10, 2015.
Health Official Warns Zika Could Spread across U.S. Gulf
(Reuters) - One of the top U.S. public health officials on Sunday warned that the mosquito-borne Zika virus could extend its reach across the U.S. Gulf Coast after officials last week confirmed it as active in the popular tourist destination of Miami Beach.
The possibility of transmission in Gulf States such as Louisiana and Texas will likely fuel concerns that the virus, which has been shown to cause microcephaly, could spread across the continental United States, even though officials have played down such an outcome.
Concern has mounted since confirmation that Zika has expanded into a second region of the tourist hub of Miami-Dade County in Florida. Miami's Wynwood arts neighborhood last month became the site of the first locally transmitted cases of Zika in the continental United States.
"It would not be surprising we would see additional cases perhaps in other Gulf Coast states," Dr. Anthony Fauci, director of the allergy and infectious diseases unit of the National Institutes of Health (NIH), said in an interview on Sunday morning with ABC News.
Fauci noted that record flooding this month in Louisiana - which has killed at least 13 people and damaged some 60,000 homes damaged - has boosted the likelihood Zika will spread into that state.
"There's going to be a lot of problems getting rid of standing water" that could stymie the mosquito control efforts that are the best way to control Zika's spread, he said.
The connection between Zika and microcephaly first came to light last fall in Brazil, which has now confirmed 1,835 cases of microcephaly that it considers to be related to Zika infections in the mothers.
On Friday, Florida Governor Rick Scott confirmed that state health officials had identified five cases of Zika believed to be contracted in Miami Beach.
The U.S. Centers for Disease Control and Prevention told pregnant women they should avoid the trendy area and suggested those especially worried about exposure might consider avoiding all of Miami-Dade County.
NIH's Fauci on Sunday said the conditions of most of the country make it unlikely there would be a "diffuse, broad outbreak," even though officials need to prepare for that possibility.
He compared it with diseases such as dengue, which is endemic in certain tropical and subtropical regions of the world but rarely occurs in the continental United States. In Miami's Wynwood area, experts have seen "substantial" knockdowns of mosquito populations.
Still, its containment is more complicated because Zika can be sexually transmitted, Fauci said.
"This is something that could hang around for a year or two," he said.
The World Health Organization has said there is strong scientific consensus that Zika can also cause Guillain-Barre syndrome.
(c) Copyright Thomson Reuters 2016.
(Reuters) - One of the top U.S. public health officials on Sunday warned that the mosquito-borne Zika virus could extend its reach across the U.S. Gulf Coast after officials last week confirmed it as active in the popular tourist destination of Miami Beach.
The possibility of transmission in Gulf States such as Louisiana and Texas will likely fuel concerns that the virus, which has been shown to cause microcephaly, could spread across the continental United States, even though officials have played down such an outcome.
Concern has mounted since confirmation that Zika has expanded into a second region of the tourist hub of Miami-Dade County in Florida. Miami's Wynwood arts neighborhood last month became the site of the first locally transmitted cases of Zika in the continental United States.
"It would not be surprising we would see additional cases perhaps in other Gulf Coast states," Dr. Anthony Fauci, director of the allergy and infectious diseases unit of the National Institutes of Health (NIH), said in an interview on Sunday morning with ABC News.
Fauci noted that record flooding this month in Louisiana - which has killed at least 13 people and damaged some 60,000 homes damaged - has boosted the likelihood Zika will spread into that state.
"There's going to be a lot of problems getting rid of standing water" that could stymie the mosquito control efforts that are the best way to control Zika's spread, he said.
The connection between Zika and microcephaly first came to light last fall in Brazil, which has now confirmed 1,835 cases of microcephaly that it considers to be related to Zika infections in the mothers.
On Friday, Florida Governor Rick Scott confirmed that state health officials had identified five cases of Zika believed to be contracted in Miami Beach.
The U.S. Centers for Disease Control and Prevention told pregnant women they should avoid the trendy area and suggested those especially worried about exposure might consider avoiding all of Miami-Dade County.
NIH's Fauci on Sunday said the conditions of most of the country make it unlikely there would be a "diffuse, broad outbreak," even though officials need to prepare for that possibility.
He compared it with diseases such as dengue, which is endemic in certain tropical and subtropical regions of the world but rarely occurs in the continental United States. In Miami's Wynwood area, experts have seen "substantial" knockdowns of mosquito populations.
Still, its containment is more complicated because Zika can be sexually transmitted, Fauci said.
"This is something that could hang around for a year or two," he said.
The World Health Organization has said there is strong scientific consensus that Zika can also cause Guillain-Barre syndrome.
(c) Copyright Thomson Reuters 2016.
(Reuters) - One of the top U.S. public health officials on Sunday warned that the mosquito-borne Zika virus could extend its reach across the U.S. Gulf Coast after officials last week confirmed it as active in the popular tourist destination of Miami Beach.
The possibility of transmission in Gulf States such as Louisiana and Texas will likely fuel concerns that the virus, which has been shown to cause microcephaly, could spread across the continental United States, even though officials have played down such an outcome.
Concern has mounted since confirmation that Zika has expanded into a second region of the tourist hub of Miami-Dade County in Florida. Miami's Wynwood arts neighborhood last month became the site of the first locally transmitted cases of Zika in the continental United States.
"It would not be surprising we would see additional cases perhaps in other Gulf Coast states," Dr. Anthony Fauci, director of the allergy and infectious diseases unit of the National Institutes of Health (NIH), said in an interview on Sunday morning with ABC News.
Fauci noted that record flooding this month in Louisiana - which has killed at least 13 people and damaged some 60,000 homes damaged - has boosted the likelihood Zika will spread into that state.
"There's going to be a lot of problems getting rid of standing water" that could stymie the mosquito control efforts that are the best way to control Zika's spread, he said.
The connection between Zika and microcephaly first came to light last fall in Brazil, which has now confirmed 1,835 cases of microcephaly that it considers to be related to Zika infections in the mothers.
On Friday, Florida Governor Rick Scott confirmed that state health officials had identified five cases of Zika believed to be contracted in Miami Beach.
The U.S. Centers for Disease Control and Prevention told pregnant women they should avoid the trendy area and suggested those especially worried about exposure might consider avoiding all of Miami-Dade County.
NIH's Fauci on Sunday said the conditions of most of the country make it unlikely there would be a "diffuse, broad outbreak," even though officials need to prepare for that possibility.
He compared it with diseases such as dengue, which is endemic in certain tropical and subtropical regions of the world but rarely occurs in the continental United States. In Miami's Wynwood area, experts have seen "substantial" knockdowns of mosquito populations.
Still, its containment is more complicated because Zika can be sexually transmitted, Fauci said.
"This is something that could hang around for a year or two," he said.
The World Health Organization has said there is strong scientific consensus that Zika can also cause Guillain-Barre syndrome.
(c) Copyright Thomson Reuters 2016.
Emerging Cataract Surgery Practice Patterns in the Veterans Health Administration
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
The rates of cataract surgery, the most commonly performed ophthalmic procedure in the U.S., have increased in the past few decades with an estimated rate of 1,100 surgeries per 100,000 people in 2011.1,2 Several emerging practices have the potential to radically impact the efficacy, safety, and cost of cataract surgery.3-5 These practices include femtosecond laser-assisted cataract surgery, intracameral antibiotics, and bilateral same-day cataract surgery.
The femtosecond laser is capable of producing precise incisions in the cornea for access by surgical instruments and reduction of astigmatism. Laser pulses also can create a perfectly round incision of the anterior lens capsule, which surrounds and supports the crystalline lens, and make incisions into the cataractous lens to facilitate disassembly for easy removal of fragments.
Placement of antibiotics internally into the anterior chamber, the space between the crystalline lens and the posterior cornea (intracameral space), is a more direct method to prevent bacterial infection within the eye (endophthalmitis), compared with current external methods, including injections under the conjunctiva (subconjunctival) and/or use of antibiotic drops directly onto the eye surface (topical).6
Routine cataract surgery is typically staged, with a period of time between sequential surgeries of 1 week or more to allow for observation of infection (delayed sequential surgery). In view of the very low rate of infection and the impact of staged surgery on patients, including additional visits and copays, some surgeons have begun to perform bilateral surgery (immediate sequential bilateral surgery, using separate patient safety checklists, surgical preps, instruments, and medications) on the same day for patients with significant cataracts in both eyes to promote rapid restoration of binocular vision as well reduce the number of patient visits.
The extent of adaptation of femtosecond laser surgery, intracameral antibiotics, and immediate sequential bilateral surgery in the U.S. is currently unknown.7,8 To provide an updated snapshot of these cataract surgery practices, the authors report on the results of a brief survey administered to ophthalmology section chiefs in the VHA, the largest integrated health care system and the largest provider of health care training in the U.S.
Methods
Following institutional review board approval from the Providence VA Medical Center, the office of the National Program Director of VA Ophthalmology provided a list of all VHA ophthalmology section chiefs and their contact information. The study targeted section chiefs because they are responsible for all eye surgery performed at their respective VAMCs. The survey queried the section chiefs on femtosecond laser-assisted cataract surgery, intracameral antibiotics, immediate sequential bilateral cataract surgery, and resident training at their institutions (Table).
The survey was administered using the web-based Research Electronic Data Capture (REDCap) software.9 The initial survey was e-mailed in April 2015, followed by 2 reminder e-mails 1 week apart and then 2 phone calls 1 week apart to nonresponders.
The survey responses were stored anonymously in the REDCap database and analyzed using descriptive statistics.
Results
The original list from the office of the National Program Director included 114 ophthalmology section chiefs (excluding one of the authors). After follow-up phone calls, 9 individuals were identified who were not ophthalmologists (eg, optometrists or nonophthalmic surgeons) or who were incorrectly listed as section chiefs, and 9 were duplicates from institutions that were represented twice on the contact list. These 18 individuals, none of whom had responded to the survey, were removed from the eligible sample. Hence, the analysis included 86% (95/111) of the VAMCs where cataract surgery is performed.10 Sixty-five responses were received for an overall response rate of 68% (65/96), including 1 ophthalmologist who responded to the survey twice.
Most section chiefs (86%, 56/65) trained ophthalmology residents at their respective medical centers (Table). Eleven VAMCs (17%) offered femtosecond laser-assisted cataract surgery; 8 of those 11 (73%) also offered resident training in this surgery. At 12 VAMCs (18%), cataract surgeons used intracameral antibiotics, which included vancomycin (4), cefuroxime (4), moxifloxacin (3), and unspecified (1); at 10 of these VAMCs (83%), surgeons used intracameral and postoperative topical antibiotics concomitantly; 8 VAMCs (67%) compounded the intracameral antibiotics—either in the hospital pharmacy (5) or within the operating room (3). The 2 most common reasons cited for not using intracameral antibiotics were risk of dilution error (28%; 15/53) and a lack of evidence for use (25%; 13/53). Only 2 medical centers (3.1%) offered immediate sequential bilateral cataract surgery.
Discussion
This survey provides updated information on the role of emerging cataract surgery practices in the VHA. These trends may impact future U.S. cataract surgery practice patterns given the large number of ophthalmology residents who receive training in the VHA.
Only 17% of VAMCs offered femtosecond laser-assisted cataract surgery. Reasons for this low rate may include (a) the high cost of the femtosecond laser units (the lowest average cost of a laser is $400,000, while the average costs of services can be $40,000 or more per year); and (b) the lack of evidence that a femtosecond laser improves cataract surgery outcomes relative to standard phacoemulsification.4,11-15 Another potential barrier to procurement of femtosecond lasers is the emphasis within VHA to increase access to care for the many newly enrolled veterans, which this technology does not address. However, most of the VAMCs with a femtosecond laser unit offered resident training in this technique, confirming early reports on the potential for incorporating femtosecond laser-assisted cataract surgery into ophthalmic graduate medical education.16
In 2007, the multicenter, prospective, randomized European Society of Cataract and Refractive Surgery Endophthalmitis Study demonstrated that intracameral cefuroxime was associated with a 5-fold decrease in the risk of postoperative endophthalmitis.17 In 2011, a statement from the American Society of Cataract and Refractive Surgery (ASCRS) Cataract Clinical Committee noted that the method of antibiotic prophylaxis with the strongest evidence base is “a direct intracameral bolus at the conclusion of surgery.”18 However, surgeons used intracameral antibiotics in only 19% of VAMCs. Although this is a higher rate than those reported in older surveys of VHA ophthalmologists (14%)7 and ASCRS members (15%), it is still significantly lower than the 74% reported in a recent survey of the European Society of Cataract and Refractive Surgeons.3,8
The most common reasons given for not using intracameral antibiotics included risk of a dilution error when preparing the antibiotics and lack of evidence supporting their effectiveness. Less common reasons included risk of contamination, lack of pharmacy approval, and increasing bacterial resistance to commonly used antibiotics. Most of these concerns have been previously cited as barriers to the adoption of intracameral antibiotics.19 The availability of a prepackaged intracameral antibiotic (eg, cefuroxime in Europe) would help address the risks of compounding dilution errors and contamination in the U.S.6 The publication of 3 large observational studies in 2016 has also significantly strengthened the evidence base supporting the use of intracameral antibiotics.20-22
Only 2 VAMCs (3%) offered immediate sequential bilateral cataract surgery. The advocates of this practice have touted its potential cost savings, patient convenience, and the opportunity for more rapid visual rehabilitation.23 Recently, several multicenter, randomized clinical trials have reported similar refractive outcomes, complication rates, and patient satisfaction for immediate and delayed bilateral cataract surgery.24,25 Hence, it is possible that rates of immediate sequential bilateral cataract surgery may increase in the VHA over the next few years.
Strengths/Limitations
A strength of this survey is its high response rate (67.7%), which exceeds the 53% and 33% rates reported in previous surveys of cataract surgery practice patterns among VHA ophthalmologistsand ASCRS members, respectively.7,8 Another strength is lack of financial incentive for adaptation of any new practices by VHA surgeons, suggesting that these decisions have been made to improve patient safety, quality of care, and/or resident education. A limitation of this study is that its findings may not be generalizable to ophthalmologists practicing in the private sector or in teaching hospitals outside the VHA.
Conclusion
This study suggests that femtosecond laser-assisted cataract surgery, intracameral antibiotics, and immediate sequential bilateral cataract surgery have limited roles in VHA cataract surgery. More research and clinical experience are needed to understand the barriers to more widespread acceptance and to assess the impact of these emerging practices on cataract surgery in the U.S.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
1. Lindstrom R. Thoughts on cataract surgery: 2015. http://www.reviewofophthalmology.com/content/t/surgical_education/c/53422/. Published March 9, 2015. Accessed June 23, 2016.
2. Gollogly HE, Hodge DO, St Sauver JL, Erie JC. Increasing incidence of cataract surgery: population-based study. J Cataract Refract Surg. 2013;39(9):1383-1389.
3. Barry P. Adoption of intracameral antibiotic prophylaxis of endophthalmitis following cataract surgery: update on the ESCRS Endophthalmitis Study. J Cataract Refract Surg. 2014;40(1):138-142.
4. Quiñones A, Gleitsmann K, Freeman M, et al. Benefits and Harms of Femtosecond Laser Assisted Cataract Surgery: A Systematic Review. VA-ESP Project #05-225; 2013. Washington, DC: Department of Veterans Affairs; 2013.
5. Naseri A, McLeod S. Benefits of and barriers to immediate sequential cataract surgery. JAMA Ophthalmol. 2014;132(11):1362-1363.
6. Brage-Mele R, Chang DF, Henderson BA, Mamalis N, Talley-Rostov A, Vasavada A; ASCRS Clinical Cataract Committee. Intracameral antibiotics: safety, efficacy, and preparation. J Cataract Refract Surg. 2014;40(12):2134-2142.
7. Greenberg PB, Havnaer A, Oetting TA, Garcia-Ferrer FJ. Cataract surgery practice patterns in the United States Veterans Health Administration. J Cataract Refract Surg. 2012;38(4):705-709.
8. Chang DF, Braga-Mele R, Mamalis N, et al; ASCRS Clinical Cataract Committee. Prophylaxis of postoperative endophthalmitis after cataract surgery: results of the 2007 ASCRS member survey. J Cataract Refract Surg. 2007;33(10):1801-1805.
9. Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377-381.
10. French DD, Margo CE, Campbell RR. Do ophthalmology training programs affect corrective procedure rates after cataract surgery? Am J Med Qual. 2013;28(3):250-255.
11. Donaldson KE, Braga-Mele R, Cabot F, et al; ASCRS Refractive Cataract Surgery Subcommittee. Femtosecond laser-assisted cataract surgery. J Cataract Refract Surg. 2013;39(11):1753-1763.
12. Abouzeid H, Ferrini W. Femtosecond-laser assisted cataract surgery: a review. Acta Ophthalmol. 2014;92(7):597-603.
13. Chen H, Hyatt T, Afshari N. Visual and refractive outcomes of laser cataract surgery. Curr Opin Ophthalmol. 2014;25(1):49-53.
14. Yu Y, Chen X, Hua H, Wu M, Lai K, Yao K. Comparative outcomes of femtosecond laser-assisted cataract surgery and manual phacoemusification: a six-month follow-up. Clin Experiment Ophthalmol. 2016;44(6):472-480.
15. Ewe SY, Abell RG, Oakley CL, et al. A comparative cohort study of visual outcomes in femtosecond laser-assisted versus phacoemulsification cataract surgery. Ophthalmology. 2016;123(1):178-182.
16. Cohen MN, Intili A, Ni N, Blecher MH. Femtosecond laser-assisted cataract surgery in residency training. Curr Opin Ophthalmol. 2015;26(1):56-60.
17. Endophthalmitis Study Group, European Society of Cataract & Refractive Surgeons. Prophylaxis of postoperative endophthalmitis following cataract surgery: results of the ESCRS multicenter study and identification of risk factors. J Cataract Refract Surg. 2007;33(6):978-988.
18. Packer M, Chang DF, Dewey SH, et al; ASCRS Cataract Clinical Committee. Prevention, diagnosis, and management of acute postoperative bacterial endophthalmitis. J Cataract Refract Surg. 2011;37(9):1699-1714.
19. Schimel AM, Alfonso EC, Flynn HW Jr. Endophthalmitis prophylaxis for cataract surgery: are intracameral antibiotics necessary? JAMA Ophthalmol. 2014;132(11):1269-1270.
20. Herrinton LJ, Shorstein NH, Paschal JF, et al. Comparative effectiveness of antibiotic prophylaxis in cataract surgery. Ophthalmology. 2016;123(2):287-294.
21. Haripriya A, Chang DF, Namburar S, Smita A, Ravindran RD. Efficacy of intracameral moxifloxacin endophthalmitis prophylaxis at Aravind Eye Hospital. Ophthalmology. 2016;123(2):302-308.
22. Jabbarvand M, Hashemian H, Khodaparast M, Jouhari M, Tabatabaei A, Rezaei S. Endophthalmitis occurring after cataract surgery: outcomes of more than 480 000 cataract surgeries, epidemiologic features, and risk factors. Ophthalmology. 2016;123(2):295-301.
23. Neel ST. A cost and policy analysis comparing immediate sequential cataract surgery and delayed sequential cataract surgery from the physician perspective in the United States. JAMA Ophthalmol. 2014;132(11):1359-1362.
24. Sarikkola AU, Uusitalo RJ, Hellstedt T, Ess SL, Leivo T, Kivelä T. Simultaneous bilateral versus sequential bilateral cataract surgery: Helsinki Simultaneous Bilateral Cataract Surgery Study Report 1. J Cataract Refract Surg. 2011;37(6):992-1002.
25. Serrano-Aguilar P, Ramallo-Fariña Y, Cabrera-Hernández JM, et al. Immediately sequential versus delayed sequential bilateral cataract surgery: safety and effectiveness. J Cataract Refract Surg. 2012;38(10):1734-1742.
Combo could provide cure for CML, team says

Preclinical research suggests that combining a BCL2 inhibitor with a BCR-ABL tyrosine kinase inhibitor (TKI) can eradicate leukemia stem cells (LSCs) in chronic myeloid leukemia (CML).
In mouse models of CML, combining the TKI nilotinib with the BCL2 inhibitor venetoclax enhanced antileukemic activity and decreased numbers of long-term LSCs.
The 2-drug combination exhibited similar activity in samples from patients with blast crisis CML.
“Our results demonstrate that . . . employing combined blockade of BCL-2 and BCR-ABL has the potential for curing CML and significantly improving outcomes for patients with blast crisis, and, as such, warrants clinical testing,” said Michael Andreeff, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Andreeff and his colleagues reported these results in Science Translational Medicine. The study was funded by National Institutes of Health, the Paul and Mary Haas Chair in Genetics, and Abbvie Inc., the company developing venetoclax.
The researchers noted that, although BCR-ABL TKIs have proven effective against CML, they rarely eliminate CML stem cells.
“It is believed that TKIs do not eliminate residual stem cells because they are not dependent on BCR-ABL signaling,” said study author Bing Carter, PhD, also of MD Anderson Cancer Center. “Hence, cures of CML with TKIs are rare.”
Dr Carter has worked for several years on eliminating residual CML stem cells, which could mean CML patients would no longer require long-term treatment with TKIs. Based on the current study, she and her colleagues believe that combining a TKI with a BCL-2 inhibitor may be a solution.
The researchers found that targeting both BCL-2 and BCR-ABL with venetoclax and nilotinib, respectively, exerted “potent antileukemic activity” and prolonged survival in BCR-ABL transgenic mice.
After stopping treatment, the median survival was 34.5 days for control mice, 70 days for mice treated with nilotinib alone (P=0.2146), 115 days for mice treated with venetoclax alone (P=0.0079), and 168 days for mice treated with nilotinib and venetoclax in combination (P=0.0002).
Subsequent experiments in mice showed that nilotinib alone did not significantly affect the frequency of long-term LSCs, although venetoclax alone did. Treatment with both drugs reduced the frequency of long-term LSCs even more than venetoclax alone.
Finally, the researchers tested venetoclax, nilotinib, and the combination in cells from 6 patients with blast crisis CML, all of whom had failed treatment with at least 1 TKI.
The team found that venetoclax and nilotinib had a synergistic apoptotic effect on bulk and stem/progenitor CML cells.
The researchers said these results suggest that combined inhibition of BCL-2 and BCR-ABL tyrosine kinase has the potential to significantly improve the depth of response and cure rates of chronic phase and blast crisis CML.
“This combination strategy may also apply to other malignancies that depend on kinase signaling for progression and maintenance,” Dr Andreeff added. ![]()

Preclinical research suggests that combining a BCL2 inhibitor with a BCR-ABL tyrosine kinase inhibitor (TKI) can eradicate leukemia stem cells (LSCs) in chronic myeloid leukemia (CML).
In mouse models of CML, combining the TKI nilotinib with the BCL2 inhibitor venetoclax enhanced antileukemic activity and decreased numbers of long-term LSCs.
The 2-drug combination exhibited similar activity in samples from patients with blast crisis CML.
“Our results demonstrate that . . . employing combined blockade of BCL-2 and BCR-ABL has the potential for curing CML and significantly improving outcomes for patients with blast crisis, and, as such, warrants clinical testing,” said Michael Andreeff, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Andreeff and his colleagues reported these results in Science Translational Medicine. The study was funded by National Institutes of Health, the Paul and Mary Haas Chair in Genetics, and Abbvie Inc., the company developing venetoclax.
The researchers noted that, although BCR-ABL TKIs have proven effective against CML, they rarely eliminate CML stem cells.
“It is believed that TKIs do not eliminate residual stem cells because they are not dependent on BCR-ABL signaling,” said study author Bing Carter, PhD, also of MD Anderson Cancer Center. “Hence, cures of CML with TKIs are rare.”
Dr Carter has worked for several years on eliminating residual CML stem cells, which could mean CML patients would no longer require long-term treatment with TKIs. Based on the current study, she and her colleagues believe that combining a TKI with a BCL-2 inhibitor may be a solution.
The researchers found that targeting both BCL-2 and BCR-ABL with venetoclax and nilotinib, respectively, exerted “potent antileukemic activity” and prolonged survival in BCR-ABL transgenic mice.
After stopping treatment, the median survival was 34.5 days for control mice, 70 days for mice treated with nilotinib alone (P=0.2146), 115 days for mice treated with venetoclax alone (P=0.0079), and 168 days for mice treated with nilotinib and venetoclax in combination (P=0.0002).
Subsequent experiments in mice showed that nilotinib alone did not significantly affect the frequency of long-term LSCs, although venetoclax alone did. Treatment with both drugs reduced the frequency of long-term LSCs even more than venetoclax alone.
Finally, the researchers tested venetoclax, nilotinib, and the combination in cells from 6 patients with blast crisis CML, all of whom had failed treatment with at least 1 TKI.
The team found that venetoclax and nilotinib had a synergistic apoptotic effect on bulk and stem/progenitor CML cells.
The researchers said these results suggest that combined inhibition of BCL-2 and BCR-ABL tyrosine kinase has the potential to significantly improve the depth of response and cure rates of chronic phase and blast crisis CML.
“This combination strategy may also apply to other malignancies that depend on kinase signaling for progression and maintenance,” Dr Andreeff added. ![]()

Preclinical research suggests that combining a BCL2 inhibitor with a BCR-ABL tyrosine kinase inhibitor (TKI) can eradicate leukemia stem cells (LSCs) in chronic myeloid leukemia (CML).
In mouse models of CML, combining the TKI nilotinib with the BCL2 inhibitor venetoclax enhanced antileukemic activity and decreased numbers of long-term LSCs.
The 2-drug combination exhibited similar activity in samples from patients with blast crisis CML.
“Our results demonstrate that . . . employing combined blockade of BCL-2 and BCR-ABL has the potential for curing CML and significantly improving outcomes for patients with blast crisis, and, as such, warrants clinical testing,” said Michael Andreeff, MD, of the University of Texas MD Anderson Cancer Center in Houston.
Dr Andreeff and his colleagues reported these results in Science Translational Medicine. The study was funded by National Institutes of Health, the Paul and Mary Haas Chair in Genetics, and Abbvie Inc., the company developing venetoclax.
The researchers noted that, although BCR-ABL TKIs have proven effective against CML, they rarely eliminate CML stem cells.
“It is believed that TKIs do not eliminate residual stem cells because they are not dependent on BCR-ABL signaling,” said study author Bing Carter, PhD, also of MD Anderson Cancer Center. “Hence, cures of CML with TKIs are rare.”
Dr Carter has worked for several years on eliminating residual CML stem cells, which could mean CML patients would no longer require long-term treatment with TKIs. Based on the current study, she and her colleagues believe that combining a TKI with a BCL-2 inhibitor may be a solution.
The researchers found that targeting both BCL-2 and BCR-ABL with venetoclax and nilotinib, respectively, exerted “potent antileukemic activity” and prolonged survival in BCR-ABL transgenic mice.
After stopping treatment, the median survival was 34.5 days for control mice, 70 days for mice treated with nilotinib alone (P=0.2146), 115 days for mice treated with venetoclax alone (P=0.0079), and 168 days for mice treated with nilotinib and venetoclax in combination (P=0.0002).
Subsequent experiments in mice showed that nilotinib alone did not significantly affect the frequency of long-term LSCs, although venetoclax alone did. Treatment with both drugs reduced the frequency of long-term LSCs even more than venetoclax alone.
Finally, the researchers tested venetoclax, nilotinib, and the combination in cells from 6 patients with blast crisis CML, all of whom had failed treatment with at least 1 TKI.
The team found that venetoclax and nilotinib had a synergistic apoptotic effect on bulk and stem/progenitor CML cells.
The researchers said these results suggest that combined inhibition of BCL-2 and BCR-ABL tyrosine kinase has the potential to significantly improve the depth of response and cure rates of chronic phase and blast crisis CML.
“This combination strategy may also apply to other malignancies that depend on kinase signaling for progression and maintenance,” Dr Andreeff added. ![]()
Blood sample collection, storage impacts protein levels

Photo by Graham Colm
Factors related to blood sample collection and storage can have a substantial impact on the biomolecular composition of the sample, according to research published in EBioMedicine.
The study showed that freezer storage time and the month and season during which a blood sample is collected can affect protein concentrations.
In fact, researchers said these factors should be considered covariates of the same importance as the sample provider’s age or gender.
“This discovery will change the way the entire world works with biobank blood,” said study author Stefan Enroth, PhD, of Uppsala University in Sweden.
“All research on, and analysis of, biobank blood going forward should also take into account what we have discovered—namely, the time aspect. It is completely new.”
As part of their research on uterine cancer, Dr Enroth and his colleagues looked at plasma samples collected from 1988 to 2014. There were 380 samples from 106 women between the ages of 29 and 73.
The researchers looked at the duration of sample storage, the women’s chronological age at sample collection, and the season and month of the year the sample was collected, assessing the impact of these factors on the abundance levels of 108 proteins.
When studying the impact of storage time, the researchers used only samples from 50-year-old women in order to isolate the time effect. The team found that storage time affected 18 proteins and explained 4.8% to 34.9% of the variance observed.
The women’s chronological age at the time of sample collection, after the adjustment for storage time, affected 70 proteins and explained 1.1% to 33.5% of the variance.
“We suspected that we’d find an influence from storage time, but we thought it would be much less,” said study author Ulf Gyllensten, PhD, of Uppsala University.
“It has now been demonstrated that storage time can be a factor at least as important as the age of the individual at sampling.”
The other major finding of the study is that protein levels vary depending on the season or month in which the samples were taken.
The researchers said results in the month analysis corresponded with the seasonal analysis, so they hypothesized that sunlight hours at the time of sampling could explain some of the variance they observed in plasma protein abundance levels.
The team found the number of sunlight hours affected 36 proteins and explained up to 4.5% of the variance observed after adjusting for storage time and age.
The researchers said these results suggest that information on the sample handling history should be regarded as “equally prominent covariates” as age or gender. Therefore, the information should be included in epidemiological studies involving protein levels. ![]()

Photo by Graham Colm
Factors related to blood sample collection and storage can have a substantial impact on the biomolecular composition of the sample, according to research published in EBioMedicine.
The study showed that freezer storage time and the month and season during which a blood sample is collected can affect protein concentrations.
In fact, researchers said these factors should be considered covariates of the same importance as the sample provider’s age or gender.
“This discovery will change the way the entire world works with biobank blood,” said study author Stefan Enroth, PhD, of Uppsala University in Sweden.
“All research on, and analysis of, biobank blood going forward should also take into account what we have discovered—namely, the time aspect. It is completely new.”
As part of their research on uterine cancer, Dr Enroth and his colleagues looked at plasma samples collected from 1988 to 2014. There were 380 samples from 106 women between the ages of 29 and 73.
The researchers looked at the duration of sample storage, the women’s chronological age at sample collection, and the season and month of the year the sample was collected, assessing the impact of these factors on the abundance levels of 108 proteins.
When studying the impact of storage time, the researchers used only samples from 50-year-old women in order to isolate the time effect. The team found that storage time affected 18 proteins and explained 4.8% to 34.9% of the variance observed.
The women’s chronological age at the time of sample collection, after the adjustment for storage time, affected 70 proteins and explained 1.1% to 33.5% of the variance.
“We suspected that we’d find an influence from storage time, but we thought it would be much less,” said study author Ulf Gyllensten, PhD, of Uppsala University.
“It has now been demonstrated that storage time can be a factor at least as important as the age of the individual at sampling.”
The other major finding of the study is that protein levels vary depending on the season or month in which the samples were taken.
The researchers said results in the month analysis corresponded with the seasonal analysis, so they hypothesized that sunlight hours at the time of sampling could explain some of the variance they observed in plasma protein abundance levels.
The team found the number of sunlight hours affected 36 proteins and explained up to 4.5% of the variance observed after adjusting for storage time and age.
The researchers said these results suggest that information on the sample handling history should be regarded as “equally prominent covariates” as age or gender. Therefore, the information should be included in epidemiological studies involving protein levels. ![]()

Photo by Graham Colm
Factors related to blood sample collection and storage can have a substantial impact on the biomolecular composition of the sample, according to research published in EBioMedicine.
The study showed that freezer storage time and the month and season during which a blood sample is collected can affect protein concentrations.
In fact, researchers said these factors should be considered covariates of the same importance as the sample provider’s age or gender.
“This discovery will change the way the entire world works with biobank blood,” said study author Stefan Enroth, PhD, of Uppsala University in Sweden.
“All research on, and analysis of, biobank blood going forward should also take into account what we have discovered—namely, the time aspect. It is completely new.”
As part of their research on uterine cancer, Dr Enroth and his colleagues looked at plasma samples collected from 1988 to 2014. There were 380 samples from 106 women between the ages of 29 and 73.
The researchers looked at the duration of sample storage, the women’s chronological age at sample collection, and the season and month of the year the sample was collected, assessing the impact of these factors on the abundance levels of 108 proteins.
When studying the impact of storage time, the researchers used only samples from 50-year-old women in order to isolate the time effect. The team found that storage time affected 18 proteins and explained 4.8% to 34.9% of the variance observed.
The women’s chronological age at the time of sample collection, after the adjustment for storage time, affected 70 proteins and explained 1.1% to 33.5% of the variance.
“We suspected that we’d find an influence from storage time, but we thought it would be much less,” said study author Ulf Gyllensten, PhD, of Uppsala University.
“It has now been demonstrated that storage time can be a factor at least as important as the age of the individual at sampling.”
The other major finding of the study is that protein levels vary depending on the season or month in which the samples were taken.
The researchers said results in the month analysis corresponded with the seasonal analysis, so they hypothesized that sunlight hours at the time of sampling could explain some of the variance they observed in plasma protein abundance levels.
The team found the number of sunlight hours affected 36 proteins and explained up to 4.5% of the variance observed after adjusting for storage time and age.
The researchers said these results suggest that information on the sample handling history should be regarded as “equally prominent covariates” as age or gender. Therefore, the information should be included in epidemiological studies involving protein levels. ![]()
Change may improve efficacy of malaria vaccine

Photo by Caitlin Kleiboer
Results of a phase 2 trial suggest that changing the dosing schedule can improve the efficacy of the malaria vaccine candidate RTS,S/AS01 (Mosquirix).
Researchers tested RTS,S/AS01 in 46 malaria-naïve US adults, using the controlled human malaria infection model (CHMI).
About 87% of subjects who received the modified dosing regimen were protected from malaria, compared to 63% of subjects who received the standard dosing schedule.
Jason Regules, MD, of the US Army Medical Research Institute of Infectious Diseases in Frederick, Maryland, and his colleagues reported these results in the Journal of Infectious Diseases.
The study was funded by GlaxoSmithKline, the US Military Infectious Disease Research Program, and the PATH Malaria Vaccine Initiative. RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative.
RTS,S/AS01 has been tested in trials of young children in Africa, and early results seemed promising. But long-term follow-up in a phase 2 study and a phase 3 study suggested the vaccine’s efficacy wanes over time.
Therefore, Dr Regules and his colleagues sought to determine if a novel immunization schedule—specifically, delaying RTS,S/AS01 administration and reducing dosage of the third vaccination, as well as any following booster dose—would significantly increase the vaccine’s ability to protect against infection.
The researchers evaluated RTS,S/AS01 in 46 malaria-naïve adults. First, the team immunized the subjects according to 2 regimens:
- A 0-, 1-, 7-month schedule with a fractional third dose (Fx017M)
- A 0-, 1-, 2-month schedule (012M, the current standard).
Following the third vaccination, subjects were exposed to malaria-causing parasites using CHMI, and the researchers evaluated the extent to which each regimen protected against infection.
During follow-up, the team assessed the efficacy of an additional fractional dose, or booster, in protecting against a second CHMI.
Twenty-six of the 30 subjects—86.7%—who received the Fx017M regimen and 10 of the 16—62.5%—who received the 012M regimen were protected from infection following the first CHMI.
In addition to providing more protection from malaria infection, the Fx017M regimen delayed infection longer than the 012M regimen.
About 90% of the Fx017M group who received a fourth fractional booster dose and underwent the second CHMI were protected from infection.
Four out of 5 subjects from both vaccination groups who were infected during the first CHMI were protected against the second, after receiving the fourth (fractional) dose of RTS,S/AS01.
The subjects did not report any serious health events as a result of receiving the vaccinations, and no safety concerns were associated with reducing dosages.
“With these results in hand, we are planning additional studies in the United States and Africa that will seek to further refine the dosing and schedule for maximum impact and to see whether these early stage results in American adults will translate into similarly high efficacy in sub-Saharan Africa, a region that bears much of the malaria disease burden,” said study author Ashley J. Birkett, PhD, director of PATH’s Malaria Vaccine Initiative.
“The results of these planned studies won’t be available for several years, however. It therefore remains critical that the pilot implementation for the recommended pediatric regimen of RTS,S/AS01, being led by the World Health Organization, moves forward as soon as possible. We need to help protect as many children as we can, as soon as we can, while we continue to pursue eradication—the only truly sustainable solution to malaria.” ![]()

Photo by Caitlin Kleiboer
Results of a phase 2 trial suggest that changing the dosing schedule can improve the efficacy of the malaria vaccine candidate RTS,S/AS01 (Mosquirix).
Researchers tested RTS,S/AS01 in 46 malaria-naïve US adults, using the controlled human malaria infection model (CHMI).
About 87% of subjects who received the modified dosing regimen were protected from malaria, compared to 63% of subjects who received the standard dosing schedule.
Jason Regules, MD, of the US Army Medical Research Institute of Infectious Diseases in Frederick, Maryland, and his colleagues reported these results in the Journal of Infectious Diseases.
The study was funded by GlaxoSmithKline, the US Military Infectious Disease Research Program, and the PATH Malaria Vaccine Initiative. RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative.
RTS,S/AS01 has been tested in trials of young children in Africa, and early results seemed promising. But long-term follow-up in a phase 2 study and a phase 3 study suggested the vaccine’s efficacy wanes over time.
Therefore, Dr Regules and his colleagues sought to determine if a novel immunization schedule—specifically, delaying RTS,S/AS01 administration and reducing dosage of the third vaccination, as well as any following booster dose—would significantly increase the vaccine’s ability to protect against infection.
The researchers evaluated RTS,S/AS01 in 46 malaria-naïve adults. First, the team immunized the subjects according to 2 regimens:
- A 0-, 1-, 7-month schedule with a fractional third dose (Fx017M)
- A 0-, 1-, 2-month schedule (012M, the current standard).
Following the third vaccination, subjects were exposed to malaria-causing parasites using CHMI, and the researchers evaluated the extent to which each regimen protected against infection.
During follow-up, the team assessed the efficacy of an additional fractional dose, or booster, in protecting against a second CHMI.
Twenty-six of the 30 subjects—86.7%—who received the Fx017M regimen and 10 of the 16—62.5%—who received the 012M regimen were protected from infection following the first CHMI.
In addition to providing more protection from malaria infection, the Fx017M regimen delayed infection longer than the 012M regimen.
About 90% of the Fx017M group who received a fourth fractional booster dose and underwent the second CHMI were protected from infection.
Four out of 5 subjects from both vaccination groups who were infected during the first CHMI were protected against the second, after receiving the fourth (fractional) dose of RTS,S/AS01.
The subjects did not report any serious health events as a result of receiving the vaccinations, and no safety concerns were associated with reducing dosages.
“With these results in hand, we are planning additional studies in the United States and Africa that will seek to further refine the dosing and schedule for maximum impact and to see whether these early stage results in American adults will translate into similarly high efficacy in sub-Saharan Africa, a region that bears much of the malaria disease burden,” said study author Ashley J. Birkett, PhD, director of PATH’s Malaria Vaccine Initiative.
“The results of these planned studies won’t be available for several years, however. It therefore remains critical that the pilot implementation for the recommended pediatric regimen of RTS,S/AS01, being led by the World Health Organization, moves forward as soon as possible. We need to help protect as many children as we can, as soon as we can, while we continue to pursue eradication—the only truly sustainable solution to malaria.” ![]()

Photo by Caitlin Kleiboer
Results of a phase 2 trial suggest that changing the dosing schedule can improve the efficacy of the malaria vaccine candidate RTS,S/AS01 (Mosquirix).
Researchers tested RTS,S/AS01 in 46 malaria-naïve US adults, using the controlled human malaria infection model (CHMI).
About 87% of subjects who received the modified dosing regimen were protected from malaria, compared to 63% of subjects who received the standard dosing schedule.
Jason Regules, MD, of the US Army Medical Research Institute of Infectious Diseases in Frederick, Maryland, and his colleagues reported these results in the Journal of Infectious Diseases.
The study was funded by GlaxoSmithKline, the US Military Infectious Disease Research Program, and the PATH Malaria Vaccine Initiative. RTS,S/AS01 is being developed by GlaxoSmithKline and the PATH Malaria Vaccine Initiative.
RTS,S/AS01 has been tested in trials of young children in Africa, and early results seemed promising. But long-term follow-up in a phase 2 study and a phase 3 study suggested the vaccine’s efficacy wanes over time.
Therefore, Dr Regules and his colleagues sought to determine if a novel immunization schedule—specifically, delaying RTS,S/AS01 administration and reducing dosage of the third vaccination, as well as any following booster dose—would significantly increase the vaccine’s ability to protect against infection.
The researchers evaluated RTS,S/AS01 in 46 malaria-naïve adults. First, the team immunized the subjects according to 2 regimens:
- A 0-, 1-, 7-month schedule with a fractional third dose (Fx017M)
- A 0-, 1-, 2-month schedule (012M, the current standard).
Following the third vaccination, subjects were exposed to malaria-causing parasites using CHMI, and the researchers evaluated the extent to which each regimen protected against infection.
During follow-up, the team assessed the efficacy of an additional fractional dose, or booster, in protecting against a second CHMI.
Twenty-six of the 30 subjects—86.7%—who received the Fx017M regimen and 10 of the 16—62.5%—who received the 012M regimen were protected from infection following the first CHMI.
In addition to providing more protection from malaria infection, the Fx017M regimen delayed infection longer than the 012M regimen.
About 90% of the Fx017M group who received a fourth fractional booster dose and underwent the second CHMI were protected from infection.
Four out of 5 subjects from both vaccination groups who were infected during the first CHMI were protected against the second, after receiving the fourth (fractional) dose of RTS,S/AS01.
The subjects did not report any serious health events as a result of receiving the vaccinations, and no safety concerns were associated with reducing dosages.
“With these results in hand, we are planning additional studies in the United States and Africa that will seek to further refine the dosing and schedule for maximum impact and to see whether these early stage results in American adults will translate into similarly high efficacy in sub-Saharan Africa, a region that bears much of the malaria disease burden,” said study author Ashley J. Birkett, PhD, director of PATH’s Malaria Vaccine Initiative.
“The results of these planned studies won’t be available for several years, however. It therefore remains critical that the pilot implementation for the recommended pediatric regimen of RTS,S/AS01, being led by the World Health Organization, moves forward as soon as possible. We need to help protect as many children as we can, as soon as we can, while we continue to pursue eradication—the only truly sustainable solution to malaria.” ![]()
How AML suppresses hematopoiesis

Exosomes shed by acute myeloid leukemia (AML) cells carry microRNAs that directly impair hematopoiesis, according to preclinical research published in Science Signaling.
Previous research suggested that AML exosomes can suppress residual hematopoietic stem and progenitor cell (HSPC) function indirectly through stromal reprogramming of niche retention factors.
The new study indicates that AML exosomes can block hematopoiesis by delivering microRNAs that directly suppress blood production when taken up by HSPCs.
Noah Hornick, of Oregon Health & Science University in Portland, and his colleagues conducted this study, isolating exosomes from cultures of human AML cells and from the plasma of mice with AML.
The researchers found these exosomes were enriched in 2 microRNAs—miR-150 and miR-155.
When cultured with HSPCs, the exosomes suppressed the expression of the transcription factor c-MYB, which is involved in HSPC proliferation and differentiation.
Blocking the function of miR-155 prevented AML cells or their exosomes from reducing c-MYB abundance and inhibiting the proliferation of cultured HSPCs.
Using a method called RISC-Trap, the researchers identified other targets of microRNAs in AML exosomes, from which they predicted protein networks that could be disrupted in cells taking up the exosomes.
The team said this study suggests that interfering with exosome-delivered microRNAs in the bone marrow or restoring the abundance of their targets may enhance AML patients’ ability to produce healthy blood cells. ![]()

Exosomes shed by acute myeloid leukemia (AML) cells carry microRNAs that directly impair hematopoiesis, according to preclinical research published in Science Signaling.
Previous research suggested that AML exosomes can suppress residual hematopoietic stem and progenitor cell (HSPC) function indirectly through stromal reprogramming of niche retention factors.
The new study indicates that AML exosomes can block hematopoiesis by delivering microRNAs that directly suppress blood production when taken up by HSPCs.
Noah Hornick, of Oregon Health & Science University in Portland, and his colleagues conducted this study, isolating exosomes from cultures of human AML cells and from the plasma of mice with AML.
The researchers found these exosomes were enriched in 2 microRNAs—miR-150 and miR-155.
When cultured with HSPCs, the exosomes suppressed the expression of the transcription factor c-MYB, which is involved in HSPC proliferation and differentiation.
Blocking the function of miR-155 prevented AML cells or their exosomes from reducing c-MYB abundance and inhibiting the proliferation of cultured HSPCs.
Using a method called RISC-Trap, the researchers identified other targets of microRNAs in AML exosomes, from which they predicted protein networks that could be disrupted in cells taking up the exosomes.
The team said this study suggests that interfering with exosome-delivered microRNAs in the bone marrow or restoring the abundance of their targets may enhance AML patients’ ability to produce healthy blood cells. ![]()

Exosomes shed by acute myeloid leukemia (AML) cells carry microRNAs that directly impair hematopoiesis, according to preclinical research published in Science Signaling.
Previous research suggested that AML exosomes can suppress residual hematopoietic stem and progenitor cell (HSPC) function indirectly through stromal reprogramming of niche retention factors.
The new study indicates that AML exosomes can block hematopoiesis by delivering microRNAs that directly suppress blood production when taken up by HSPCs.
Noah Hornick, of Oregon Health & Science University in Portland, and his colleagues conducted this study, isolating exosomes from cultures of human AML cells and from the plasma of mice with AML.
The researchers found these exosomes were enriched in 2 microRNAs—miR-150 and miR-155.
When cultured with HSPCs, the exosomes suppressed the expression of the transcription factor c-MYB, which is involved in HSPC proliferation and differentiation.
Blocking the function of miR-155 prevented AML cells or their exosomes from reducing c-MYB abundance and inhibiting the proliferation of cultured HSPCs.
Using a method called RISC-Trap, the researchers identified other targets of microRNAs in AML exosomes, from which they predicted protein networks that could be disrupted in cells taking up the exosomes.
The team said this study suggests that interfering with exosome-delivered microRNAs in the bone marrow or restoring the abundance of their targets may enhance AML patients’ ability to produce healthy blood cells. ![]()