User login
Research Finds the Main Cause of Inferior Vena Cava Thrombosis
NEW YORK (Reuters Health) - In the absence of a congenital anomaly, the main cause of inferior vena cava (IVC) thrombosis is the presence of an unretrieved IVC filter, researchers report.
"Since IVC filter thrombosis is the main etiology for IVC thrombosis, physicians may want to ensure the absolute need for the filter before its placement," Dr. Mohamad Alkhouli from University of Rochester Medical Center, New York told Reuters Health by email. "A tracking system should be instituted to follow up with these patients, and the implanted IVC filter should be pulled out as soon as is safe and reasonable."
IVC thrombosis accompanies lower extremity deep vein thrombosis (DVT) in 4% or more of patients, leading to post-thrombotic syndrome (PTS) in up to 90% of patients, disabling venous claudication in 45%, pulmonary embolism in 30%, and venous ulceration in 15%, according to Dr. Alkhouli and colleagues, who reviewed the diagnosis and management of IVC thrombosis in a report online March 9th in JACC: Cardiovascular Interventions.
IVC filter placement rates are 25 times higher in the U.S. than in Europe, and late filter thrombosis has been reported in up to a third of patients, yet retrieval rates are consistently low.
Presenting symptoms of IVC thrombosis include leg heaviness, pain, swelling, and cramping, often preceded by nonspecific back and abdominal/pelvic pain. Because of the ambiguous symptoms and insidious onset, IVC thrombosis often goes undiagnosed until clot migration or embolization into the lungs and renal veins results in dyspnea and oliguria.
Lower extremity duplex ultrasound can be used to screen for IVC thrombosis, but appropriately timed CT and MRI are essential for diagnosis and assessment of the extent of thrombosis.
Once IVC thrombosis is diagnosed, the mainstay of treatment is anticoagulation, although specific guidelines are lacking.
In observational studies, thrombus removal with pharmacomechanical catheter directed thrombolysis (PMCT) has reduced the incidence of PTS and improved quality of life, but whether this is as safe as standard anticoagulation remains unclear.
While acute thrombosis may be amenable to PMCT and catheter-directed thrombolysis (CDT), the presence of a fibrotic component in patients who present late may require balloon venoplasty with or without stenting.
The available treatments work best when IVC thrombosis is recognized early, Dr. Alkhouli said.
Dr. Michael Jaff from Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, who wrote an editorial related to this report, told Reuters Health by email, "Limit placement of IVC filters to only absolute indications, and retrieve them as soon as possible."
Dr. Jaff explained, "There are three basic questions I ask myself when considering catheter-based intervention for IVC thrombosis: (1) How long has the patient had symptoms/signs suggestive of this, and how severe are they? (2) What is my estimation of bleeding risk? (3) Do I have an interventionist with skill and experience available to perform the intervention?"
He continued, "Regarding question 1, shorter duration of symptoms and more severe symptoms and signs prompt me to aggressively consider catheter-based intervention. Regarding question 2, if there is significant bleeding risk (for example, inflammatory bowel disease as the underlying culprit for the IVC thrombosis), I am reluctant to consider catheter-based intervention.Regarding question 3, don't consider this if your colleague has little experience performing this procedure or managing the complications of the procedure."
Dr. Xiao-Qiang Li from Second Affiliated Hospital of Soochow University in Suzhou, China, who recently described the experience with CDT combined with manual aspiration thrombectomy for acute inferior vena cava filter thrombosis, told Reuters Health by email, "As you see, no consensuses have been reached."
"For a new patient with acute DVT, if he or she has no contraindications for thrombolysis, especially with a life expectancy more than one year, we prefer to perform catheter-based interventions, including catheter-directed thrombolysis, pharmacomechanical thrombolysis, ultrasound-assisted catheter-directed thrombolysis and subsequent percutaneous transluminal angioplasty and stenting,"Dr. Li said."But, of note, anticoagulation is the basic treatment whatever catheter-based interventions are adopted."
NEW YORK (Reuters Health) - In the absence of a congenital anomaly, the main cause of inferior vena cava (IVC) thrombosis is the presence of an unretrieved IVC filter, researchers report.
"Since IVC filter thrombosis is the main etiology for IVC thrombosis, physicians may want to ensure the absolute need for the filter before its placement," Dr. Mohamad Alkhouli from University of Rochester Medical Center, New York told Reuters Health by email. "A tracking system should be instituted to follow up with these patients, and the implanted IVC filter should be pulled out as soon as is safe and reasonable."
IVC thrombosis accompanies lower extremity deep vein thrombosis (DVT) in 4% or more of patients, leading to post-thrombotic syndrome (PTS) in up to 90% of patients, disabling venous claudication in 45%, pulmonary embolism in 30%, and venous ulceration in 15%, according to Dr. Alkhouli and colleagues, who reviewed the diagnosis and management of IVC thrombosis in a report online March 9th in JACC: Cardiovascular Interventions.
IVC filter placement rates are 25 times higher in the U.S. than in Europe, and late filter thrombosis has been reported in up to a third of patients, yet retrieval rates are consistently low.
Presenting symptoms of IVC thrombosis include leg heaviness, pain, swelling, and cramping, often preceded by nonspecific back and abdominal/pelvic pain. Because of the ambiguous symptoms and insidious onset, IVC thrombosis often goes undiagnosed until clot migration or embolization into the lungs and renal veins results in dyspnea and oliguria.
Lower extremity duplex ultrasound can be used to screen for IVC thrombosis, but appropriately timed CT and MRI are essential for diagnosis and assessment of the extent of thrombosis.
Once IVC thrombosis is diagnosed, the mainstay of treatment is anticoagulation, although specific guidelines are lacking.
In observational studies, thrombus removal with pharmacomechanical catheter directed thrombolysis (PMCT) has reduced the incidence of PTS and improved quality of life, but whether this is as safe as standard anticoagulation remains unclear.
While acute thrombosis may be amenable to PMCT and catheter-directed thrombolysis (CDT), the presence of a fibrotic component in patients who present late may require balloon venoplasty with or without stenting.
The available treatments work best when IVC thrombosis is recognized early, Dr. Alkhouli said.
Dr. Michael Jaff from Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, who wrote an editorial related to this report, told Reuters Health by email, "Limit placement of IVC filters to only absolute indications, and retrieve them as soon as possible."
Dr. Jaff explained, "There are three basic questions I ask myself when considering catheter-based intervention for IVC thrombosis: (1) How long has the patient had symptoms/signs suggestive of this, and how severe are they? (2) What is my estimation of bleeding risk? (3) Do I have an interventionist with skill and experience available to perform the intervention?"
He continued, "Regarding question 1, shorter duration of symptoms and more severe symptoms and signs prompt me to aggressively consider catheter-based intervention. Regarding question 2, if there is significant bleeding risk (for example, inflammatory bowel disease as the underlying culprit for the IVC thrombosis), I am reluctant to consider catheter-based intervention.Regarding question 3, don't consider this if your colleague has little experience performing this procedure or managing the complications of the procedure."
Dr. Xiao-Qiang Li from Second Affiliated Hospital of Soochow University in Suzhou, China, who recently described the experience with CDT combined with manual aspiration thrombectomy for acute inferior vena cava filter thrombosis, told Reuters Health by email, "As you see, no consensuses have been reached."
"For a new patient with acute DVT, if he or she has no contraindications for thrombolysis, especially with a life expectancy more than one year, we prefer to perform catheter-based interventions, including catheter-directed thrombolysis, pharmacomechanical thrombolysis, ultrasound-assisted catheter-directed thrombolysis and subsequent percutaneous transluminal angioplasty and stenting,"Dr. Li said."But, of note, anticoagulation is the basic treatment whatever catheter-based interventions are adopted."
NEW YORK (Reuters Health) - In the absence of a congenital anomaly, the main cause of inferior vena cava (IVC) thrombosis is the presence of an unretrieved IVC filter, researchers report.
"Since IVC filter thrombosis is the main etiology for IVC thrombosis, physicians may want to ensure the absolute need for the filter before its placement," Dr. Mohamad Alkhouli from University of Rochester Medical Center, New York told Reuters Health by email. "A tracking system should be instituted to follow up with these patients, and the implanted IVC filter should be pulled out as soon as is safe and reasonable."
IVC thrombosis accompanies lower extremity deep vein thrombosis (DVT) in 4% or more of patients, leading to post-thrombotic syndrome (PTS) in up to 90% of patients, disabling venous claudication in 45%, pulmonary embolism in 30%, and venous ulceration in 15%, according to Dr. Alkhouli and colleagues, who reviewed the diagnosis and management of IVC thrombosis in a report online March 9th in JACC: Cardiovascular Interventions.
IVC filter placement rates are 25 times higher in the U.S. than in Europe, and late filter thrombosis has been reported in up to a third of patients, yet retrieval rates are consistently low.
Presenting symptoms of IVC thrombosis include leg heaviness, pain, swelling, and cramping, often preceded by nonspecific back and abdominal/pelvic pain. Because of the ambiguous symptoms and insidious onset, IVC thrombosis often goes undiagnosed until clot migration or embolization into the lungs and renal veins results in dyspnea and oliguria.
Lower extremity duplex ultrasound can be used to screen for IVC thrombosis, but appropriately timed CT and MRI are essential for diagnosis and assessment of the extent of thrombosis.
Once IVC thrombosis is diagnosed, the mainstay of treatment is anticoagulation, although specific guidelines are lacking.
In observational studies, thrombus removal with pharmacomechanical catheter directed thrombolysis (PMCT) has reduced the incidence of PTS and improved quality of life, but whether this is as safe as standard anticoagulation remains unclear.
While acute thrombosis may be amenable to PMCT and catheter-directed thrombolysis (CDT), the presence of a fibrotic component in patients who present late may require balloon venoplasty with or without stenting.
The available treatments work best when IVC thrombosis is recognized early, Dr. Alkhouli said.
Dr. Michael Jaff from Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, who wrote an editorial related to this report, told Reuters Health by email, "Limit placement of IVC filters to only absolute indications, and retrieve them as soon as possible."
Dr. Jaff explained, "There are three basic questions I ask myself when considering catheter-based intervention for IVC thrombosis: (1) How long has the patient had symptoms/signs suggestive of this, and how severe are they? (2) What is my estimation of bleeding risk? (3) Do I have an interventionist with skill and experience available to perform the intervention?"
He continued, "Regarding question 1, shorter duration of symptoms and more severe symptoms and signs prompt me to aggressively consider catheter-based intervention. Regarding question 2, if there is significant bleeding risk (for example, inflammatory bowel disease as the underlying culprit for the IVC thrombosis), I am reluctant to consider catheter-based intervention.Regarding question 3, don't consider this if your colleague has little experience performing this procedure or managing the complications of the procedure."
Dr. Xiao-Qiang Li from Second Affiliated Hospital of Soochow University in Suzhou, China, who recently described the experience with CDT combined with manual aspiration thrombectomy for acute inferior vena cava filter thrombosis, told Reuters Health by email, "As you see, no consensuses have been reached."
"For a new patient with acute DVT, if he or she has no contraindications for thrombolysis, especially with a life expectancy more than one year, we prefer to perform catheter-based interventions, including catheter-directed thrombolysis, pharmacomechanical thrombolysis, ultrasound-assisted catheter-directed thrombolysis and subsequent percutaneous transluminal angioplasty and stenting,"Dr. Li said."But, of note, anticoagulation is the basic treatment whatever catheter-based interventions are adopted."
Did You Commit to ‘Fight the Resistance’ at HM16?
If you missed HM16 but still want to show your support, visit www.FightTheResistance.org to review SHM’s recommendations for promoting antibiotic stewardship, download copies of our three posters, and submit your case study about how you’re fighting antibiotic resistance in your hospital.
Continue to check www.FightTheResistance.org and follow the #FightTheResistance hashtag on Twitter to learn about new SHM resources to help you continue the fight.
If you missed HM16 but still want to show your support, visit www.FightTheResistance.org to review SHM’s recommendations for promoting antibiotic stewardship, download copies of our three posters, and submit your case study about how you’re fighting antibiotic resistance in your hospital.
Continue to check www.FightTheResistance.org and follow the #FightTheResistance hashtag on Twitter to learn about new SHM resources to help you continue the fight.
If you missed HM16 but still want to show your support, visit www.FightTheResistance.org to review SHM’s recommendations for promoting antibiotic stewardship, download copies of our three posters, and submit your case study about how you’re fighting antibiotic resistance in your hospital.
Continue to check www.FightTheResistance.org and follow the #FightTheResistance hashtag on Twitter to learn about new SHM resources to help you continue the fight.
Jerome C. Siy, MD, SFHM Explores Hospital Medicine’s Global Reach
Hospitalist Jerome C. Siy, MD, SFHM, CHIE, is head of the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul, Minn., and chair of SHM’s Practice Management Committee. As a member of SHM for more than 15 years and recipient of SHM’s prestigious Award of Excellence for Clinical Excellence in 2009, Dr. Siy has been a driving force in advancing hospitalist practice and improving patient care in the U.S. and beyond.
Question: What led you to a career in hospital medicine?
Answer: After graduating from the Mayo Graduate School of Medical Education, I started in the med-peds residency program at the University of Minnesota and then transitioned to an internal medicine residency program. It was at the University of Minnesota that I recognized my intense passion for the care of acutely ill hospitalized patients. During my residency, I had the good fortune of finding exceptional hospitalist role models in my program. As I worked with them, my passion for working with hospitalized patients continued to grow; I realized that my ideal job was to work with this group of doctors that I so greatly admired. In 2000, I joined the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul and now proudly lead the department.
Q: When did you first get involved with SHM? What value does it bring to your daily practice?
A: When I first joined HealthPartners, Dr. Rusty Holman was our director. He was extremely active in the early days of SHM, when it was known as the National Association of Inpatient Physicians (NAIP). Our team at HealthPartners was fairly small, between 15 and 18 hospitalists, and identified early on with the hospital medicine specialty and NAIP. Many of them are still engaged with SHM 16 years later. As a team, we continue to encourage our entire group of over 90 practitioners, including our PAs and NPs, to get involved with SHM.
At SHM, chances to connect with people exist everywhere. So many hospitalists tell stories about how they went to an SHM meeting and ran into an old friend or medical school colleague that they didn’t realize was a hospitalist. That’s exactly why the SHM community is a fertile ground to build and expand upon ideas for your own program. For example, at HealthPartners, we are embarking on early work with telemedicine. With the network of hospitalists at SHM, I immediately knew colleagues who were working in hospital medicine and was able to visit some of them at their telemedicine specialty center.
Whether you join committees, give a joint lecture, or attend a session at an annual meeting with someone, you are opening yourself up to collaboration that will ultimately lead to better care for patients.
Q: What is one of the most unique or rewarding experiences you have had while practicing hospital medicine?
A: After 10 years at HealthPartners, I took a personal sabbatical to study Chinese in Taiwan for eight months. Even though I was away from my hospital, hospital medicine followed me overseas. While in Asia, I visited contacts in Taiwan and Japan. With the U.S. government just rolling out the Affordable Care Act, I wanted to gain a better understanding of how nationalized healthcare programs impacted care providers and care delivery.
While visiting the University of Osaka and one of the earliest hospital medicine groups at National Taiwan University Hospital, I had the opportunity to explore how a nation’s healthcare system impacted physicians and patients outside the U.S. A major takeaway for me was how important it is for physicians and care providers to be an active part of the healthcare system to create change that can influence the way they practice—and ultimately improve patient care.
In East Asia, whenever there was concern about evolving their healthcare models and the way providers take care of patients, physicians often felt limited in their potential impact. Culturally, senior physicians are the ones more apt to network and influence policy changes. The more physicians felt empowered to influence these senior leaders to address these issues with government officials, the better the chances of driving positive change.
As luck would have it, a hospitalist colleague invited me back to National Taiwan University Hospital in December 2015 to share my knowledge of how the specialty can continue to evolve, taking into consideration the challenges of navigating healthcare systems, physician engagement, and burnout. Even though many of their programs are relatively new, I stressed the fact that the more interactive you are with your healthcare system, the better your chances of engaging the right stakeholders and effectively influencing healthcare policy.
While their definition of burnout may differ slightly from ours in the U.S., they wanted to hear about what we experience in the U.S. and how we address it. I was able to share some techniques we are implementing at HealthPartners to minimize burnout and maximize engagement, including regular department meetings, during which there is an open forum for hot topics. This provides the care team with an avenue to express concerns or address important topics affecting their daily practice. We also have an internal website, where our team can access a repository of resources and a discussion board to share challenges, concerns, and best practices. I also emphasized the importance of professional development and investing in staff to improve their professional career and their patient care.
Q: What are some initiatives you are currently working on that you see having a substantial impact in hospital medicine?
A: As part of the Practice Management Committee at SHM, we are exploring opportunities in telemedicine, especially as it relates to rural care. Telemedicine could be the next big step to provide support for rural hospitalists and rural communities. Geographically speaking, the vast majority of the U.S. is rural, and SHM is poised to have a great impact on the clinicians serving these communities.
Another initiative the committee is working on is developing an update to co-management best practices. As hospital medicine has matured, its scope has changed dramatically, and so has the idea of co-management. We must truly embrace opportunities to improve care across specialties. This is especially important as we welcome younger physicians to the specialty who have not had the benefit of witnessing the evolution of the practice. They need to have a firm hold on the varied daily interactions of a hospitalist and their ultimate impact on outcomes.
At HealthPartners, we are actively addressing hospitalist engagement and burnout. We are mindful of how much our health systems have evolved and how much extra work they have asked us to accommodate. To be proactive, we are trying to be much more creative and innovative with our staffing model and our use of scarce resources in order to provide the best patient care possible. Over the last 16 years, we have worked hard to continue to develop our program but, more important, develop our patient care through the development of our physicians, NPs, and PAs. This includes developing a pathway for residents who wish to become hospitalists, introducing vigorous training for PAs during their student and post-graduate years, and providing staff with the professional development resources they need to expand their skills and knowledge base and stay up-to-date on the latest advances in medicine—whether that is through leadership development or skill straining like point-of-care ultrasound.
Hospital medicine has matured and grown, and our scope has changed dramatically to include observation care, research and academic medicine, telemedicine, palliative care, perioperative medicine, and more. At HealthPartners, we are embracing the opportunity to grow in scope and improve care across specialties.
Q: Given your experience in the U.S. and abroad, what words of advice would you give to medical students and residents considering a career in hospital medicine?
A: As you enter this career out of training, recognize that your potential impact is greater than you ever imagined in medical school. As you continue to grow in your career and make it your own, you will make lasting impacts on your patients and also be extremely creative in what you do. Just as the specialty continues to evolve, so will you. Keep an open mind and embrace the many opportunities that come your way. TH
Brett Radler is SHM’s communications coordinator.
Hospitalist Jerome C. Siy, MD, SFHM, CHIE, is head of the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul, Minn., and chair of SHM’s Practice Management Committee. As a member of SHM for more than 15 years and recipient of SHM’s prestigious Award of Excellence for Clinical Excellence in 2009, Dr. Siy has been a driving force in advancing hospitalist practice and improving patient care in the U.S. and beyond.
Question: What led you to a career in hospital medicine?
Answer: After graduating from the Mayo Graduate School of Medical Education, I started in the med-peds residency program at the University of Minnesota and then transitioned to an internal medicine residency program. It was at the University of Minnesota that I recognized my intense passion for the care of acutely ill hospitalized patients. During my residency, I had the good fortune of finding exceptional hospitalist role models in my program. As I worked with them, my passion for working with hospitalized patients continued to grow; I realized that my ideal job was to work with this group of doctors that I so greatly admired. In 2000, I joined the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul and now proudly lead the department.
Q: When did you first get involved with SHM? What value does it bring to your daily practice?
A: When I first joined HealthPartners, Dr. Rusty Holman was our director. He was extremely active in the early days of SHM, when it was known as the National Association of Inpatient Physicians (NAIP). Our team at HealthPartners was fairly small, between 15 and 18 hospitalists, and identified early on with the hospital medicine specialty and NAIP. Many of them are still engaged with SHM 16 years later. As a team, we continue to encourage our entire group of over 90 practitioners, including our PAs and NPs, to get involved with SHM.
At SHM, chances to connect with people exist everywhere. So many hospitalists tell stories about how they went to an SHM meeting and ran into an old friend or medical school colleague that they didn’t realize was a hospitalist. That’s exactly why the SHM community is a fertile ground to build and expand upon ideas for your own program. For example, at HealthPartners, we are embarking on early work with telemedicine. With the network of hospitalists at SHM, I immediately knew colleagues who were working in hospital medicine and was able to visit some of them at their telemedicine specialty center.
Whether you join committees, give a joint lecture, or attend a session at an annual meeting with someone, you are opening yourself up to collaboration that will ultimately lead to better care for patients.
Q: What is one of the most unique or rewarding experiences you have had while practicing hospital medicine?
A: After 10 years at HealthPartners, I took a personal sabbatical to study Chinese in Taiwan for eight months. Even though I was away from my hospital, hospital medicine followed me overseas. While in Asia, I visited contacts in Taiwan and Japan. With the U.S. government just rolling out the Affordable Care Act, I wanted to gain a better understanding of how nationalized healthcare programs impacted care providers and care delivery.
While visiting the University of Osaka and one of the earliest hospital medicine groups at National Taiwan University Hospital, I had the opportunity to explore how a nation’s healthcare system impacted physicians and patients outside the U.S. A major takeaway for me was how important it is for physicians and care providers to be an active part of the healthcare system to create change that can influence the way they practice—and ultimately improve patient care.
In East Asia, whenever there was concern about evolving their healthcare models and the way providers take care of patients, physicians often felt limited in their potential impact. Culturally, senior physicians are the ones more apt to network and influence policy changes. The more physicians felt empowered to influence these senior leaders to address these issues with government officials, the better the chances of driving positive change.
As luck would have it, a hospitalist colleague invited me back to National Taiwan University Hospital in December 2015 to share my knowledge of how the specialty can continue to evolve, taking into consideration the challenges of navigating healthcare systems, physician engagement, and burnout. Even though many of their programs are relatively new, I stressed the fact that the more interactive you are with your healthcare system, the better your chances of engaging the right stakeholders and effectively influencing healthcare policy.
While their definition of burnout may differ slightly from ours in the U.S., they wanted to hear about what we experience in the U.S. and how we address it. I was able to share some techniques we are implementing at HealthPartners to minimize burnout and maximize engagement, including regular department meetings, during which there is an open forum for hot topics. This provides the care team with an avenue to express concerns or address important topics affecting their daily practice. We also have an internal website, where our team can access a repository of resources and a discussion board to share challenges, concerns, and best practices. I also emphasized the importance of professional development and investing in staff to improve their professional career and their patient care.
Q: What are some initiatives you are currently working on that you see having a substantial impact in hospital medicine?
A: As part of the Practice Management Committee at SHM, we are exploring opportunities in telemedicine, especially as it relates to rural care. Telemedicine could be the next big step to provide support for rural hospitalists and rural communities. Geographically speaking, the vast majority of the U.S. is rural, and SHM is poised to have a great impact on the clinicians serving these communities.
Another initiative the committee is working on is developing an update to co-management best practices. As hospital medicine has matured, its scope has changed dramatically, and so has the idea of co-management. We must truly embrace opportunities to improve care across specialties. This is especially important as we welcome younger physicians to the specialty who have not had the benefit of witnessing the evolution of the practice. They need to have a firm hold on the varied daily interactions of a hospitalist and their ultimate impact on outcomes.
At HealthPartners, we are actively addressing hospitalist engagement and burnout. We are mindful of how much our health systems have evolved and how much extra work they have asked us to accommodate. To be proactive, we are trying to be much more creative and innovative with our staffing model and our use of scarce resources in order to provide the best patient care possible. Over the last 16 years, we have worked hard to continue to develop our program but, more important, develop our patient care through the development of our physicians, NPs, and PAs. This includes developing a pathway for residents who wish to become hospitalists, introducing vigorous training for PAs during their student and post-graduate years, and providing staff with the professional development resources they need to expand their skills and knowledge base and stay up-to-date on the latest advances in medicine—whether that is through leadership development or skill straining like point-of-care ultrasound.
Hospital medicine has matured and grown, and our scope has changed dramatically to include observation care, research and academic medicine, telemedicine, palliative care, perioperative medicine, and more. At HealthPartners, we are embracing the opportunity to grow in scope and improve care across specialties.
Q: Given your experience in the U.S. and abroad, what words of advice would you give to medical students and residents considering a career in hospital medicine?
A: As you enter this career out of training, recognize that your potential impact is greater than you ever imagined in medical school. As you continue to grow in your career and make it your own, you will make lasting impacts on your patients and also be extremely creative in what you do. Just as the specialty continues to evolve, so will you. Keep an open mind and embrace the many opportunities that come your way. TH
Brett Radler is SHM’s communications coordinator.
Hospitalist Jerome C. Siy, MD, SFHM, CHIE, is head of the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul, Minn., and chair of SHM’s Practice Management Committee. As a member of SHM for more than 15 years and recipient of SHM’s prestigious Award of Excellence for Clinical Excellence in 2009, Dr. Siy has been a driving force in advancing hospitalist practice and improving patient care in the U.S. and beyond.
Question: What led you to a career in hospital medicine?
Answer: After graduating from the Mayo Graduate School of Medical Education, I started in the med-peds residency program at the University of Minnesota and then transitioned to an internal medicine residency program. It was at the University of Minnesota that I recognized my intense passion for the care of acutely ill hospitalized patients. During my residency, I had the good fortune of finding exceptional hospitalist role models in my program. As I worked with them, my passion for working with hospitalized patients continued to grow; I realized that my ideal job was to work with this group of doctors that I so greatly admired. In 2000, I joined the Department of Hospital Medicine at HealthPartners in Minneapolis-St. Paul and now proudly lead the department.
Q: When did you first get involved with SHM? What value does it bring to your daily practice?
A: When I first joined HealthPartners, Dr. Rusty Holman was our director. He was extremely active in the early days of SHM, when it was known as the National Association of Inpatient Physicians (NAIP). Our team at HealthPartners was fairly small, between 15 and 18 hospitalists, and identified early on with the hospital medicine specialty and NAIP. Many of them are still engaged with SHM 16 years later. As a team, we continue to encourage our entire group of over 90 practitioners, including our PAs and NPs, to get involved with SHM.
At SHM, chances to connect with people exist everywhere. So many hospitalists tell stories about how they went to an SHM meeting and ran into an old friend or medical school colleague that they didn’t realize was a hospitalist. That’s exactly why the SHM community is a fertile ground to build and expand upon ideas for your own program. For example, at HealthPartners, we are embarking on early work with telemedicine. With the network of hospitalists at SHM, I immediately knew colleagues who were working in hospital medicine and was able to visit some of them at their telemedicine specialty center.
Whether you join committees, give a joint lecture, or attend a session at an annual meeting with someone, you are opening yourself up to collaboration that will ultimately lead to better care for patients.
Q: What is one of the most unique or rewarding experiences you have had while practicing hospital medicine?
A: After 10 years at HealthPartners, I took a personal sabbatical to study Chinese in Taiwan for eight months. Even though I was away from my hospital, hospital medicine followed me overseas. While in Asia, I visited contacts in Taiwan and Japan. With the U.S. government just rolling out the Affordable Care Act, I wanted to gain a better understanding of how nationalized healthcare programs impacted care providers and care delivery.
While visiting the University of Osaka and one of the earliest hospital medicine groups at National Taiwan University Hospital, I had the opportunity to explore how a nation’s healthcare system impacted physicians and patients outside the U.S. A major takeaway for me was how important it is for physicians and care providers to be an active part of the healthcare system to create change that can influence the way they practice—and ultimately improve patient care.
In East Asia, whenever there was concern about evolving their healthcare models and the way providers take care of patients, physicians often felt limited in their potential impact. Culturally, senior physicians are the ones more apt to network and influence policy changes. The more physicians felt empowered to influence these senior leaders to address these issues with government officials, the better the chances of driving positive change.
As luck would have it, a hospitalist colleague invited me back to National Taiwan University Hospital in December 2015 to share my knowledge of how the specialty can continue to evolve, taking into consideration the challenges of navigating healthcare systems, physician engagement, and burnout. Even though many of their programs are relatively new, I stressed the fact that the more interactive you are with your healthcare system, the better your chances of engaging the right stakeholders and effectively influencing healthcare policy.
While their definition of burnout may differ slightly from ours in the U.S., they wanted to hear about what we experience in the U.S. and how we address it. I was able to share some techniques we are implementing at HealthPartners to minimize burnout and maximize engagement, including regular department meetings, during which there is an open forum for hot topics. This provides the care team with an avenue to express concerns or address important topics affecting their daily practice. We also have an internal website, where our team can access a repository of resources and a discussion board to share challenges, concerns, and best practices. I also emphasized the importance of professional development and investing in staff to improve their professional career and their patient care.
Q: What are some initiatives you are currently working on that you see having a substantial impact in hospital medicine?
A: As part of the Practice Management Committee at SHM, we are exploring opportunities in telemedicine, especially as it relates to rural care. Telemedicine could be the next big step to provide support for rural hospitalists and rural communities. Geographically speaking, the vast majority of the U.S. is rural, and SHM is poised to have a great impact on the clinicians serving these communities.
Another initiative the committee is working on is developing an update to co-management best practices. As hospital medicine has matured, its scope has changed dramatically, and so has the idea of co-management. We must truly embrace opportunities to improve care across specialties. This is especially important as we welcome younger physicians to the specialty who have not had the benefit of witnessing the evolution of the practice. They need to have a firm hold on the varied daily interactions of a hospitalist and their ultimate impact on outcomes.
At HealthPartners, we are actively addressing hospitalist engagement and burnout. We are mindful of how much our health systems have evolved and how much extra work they have asked us to accommodate. To be proactive, we are trying to be much more creative and innovative with our staffing model and our use of scarce resources in order to provide the best patient care possible. Over the last 16 years, we have worked hard to continue to develop our program but, more important, develop our patient care through the development of our physicians, NPs, and PAs. This includes developing a pathway for residents who wish to become hospitalists, introducing vigorous training for PAs during their student and post-graduate years, and providing staff with the professional development resources they need to expand their skills and knowledge base and stay up-to-date on the latest advances in medicine—whether that is through leadership development or skill straining like point-of-care ultrasound.
Hospital medicine has matured and grown, and our scope has changed dramatically to include observation care, research and academic medicine, telemedicine, palliative care, perioperative medicine, and more. At HealthPartners, we are embracing the opportunity to grow in scope and improve care across specialties.
Q: Given your experience in the U.S. and abroad, what words of advice would you give to medical students and residents considering a career in hospital medicine?
A: As you enter this career out of training, recognize that your potential impact is greater than you ever imagined in medical school. As you continue to grow in your career and make it your own, you will make lasting impacts on your patients and also be extremely creative in what you do. Just as the specialty continues to evolve, so will you. Keep an open mind and embrace the many opportunities that come your way. TH
Brett Radler is SHM’s communications coordinator.
High costs limit CML patients’ access to TKIs

Photo courtesy of the CDC
A new study suggests that cost-sharing policies in the US create a barrier to the treatment of chronic myeloid leukemia (CML).
Researchers examined Medicare claims data and found that “Part D” (prescription drug plan) co-insurance policies for “specialty drugs” seem to be reducing or delaying the use of tyrosine kinase inhibitors (TKIs) in patients with CML.
The team reported these findings in the American Journal of Managed Care.
“High out-of-pocket costs for specialty drugs appear to pose a very real barrier to treatment,” said study author Jalpa A. Doshi, PhD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
While there is no standard definition for specialty drugs, the term typically refers to medications requiring special handling, administration, or monitoring. Most are aimed at treating chronic or life-threatening diseases.
Although specialty drugs typically tend to offer significant medical advances over non-specialty drugs, they are correspondingly more expensive. In 2014, such drugs accounted for less than 1% of prescriptions in the US but nearly a third of total prescription spending.
While insurers have been imposing higher cost-sharing requirements as part of their efforts to manage specialty drug spending, there has been limited information about the corresponding impact on patients.
“[I]t was particularly important to examine the extent to which the aggressive cost-sharing policies for specialty drugs seen under Medicare Part D, which are increasingly making their way into the private insurance market, adversely impact access to these treatments even for a condition like cancer,” Dr Doshi said.
So she and her colleagues examined the impact of specialty drug cost-sharing under the Medicare Part D prescription drug benefit on patients with CML. The team analyzed Medicare data on patients who were newly diagnosed with CML to examine whether and how quickly they initiated TKI treatment.
The researchers compared patients who were eligible for low-income subsidies and therefore faced nominal out-of-pocket costs to patients who faced average out-of-pocket costs of $2600 or more for their first 30-day TKI prescription fill.
Results showed that patients in the high-cost group were significantly less likely than the low-cost group to have a Part D claim for a TKI prescription within 6 months of their CML diagnosis. The rates were 45.3% and 66.9%, respectively (P<0.001).
Patients in the high cost-sharing group also took twice as long, on average, to initiate TKI treatment. The mean time to fill a TKI prescription was 50.9 days in the high-cost group and 23.7 days in the low-cost group (P<0.001).
“Medicare Part D was created to increase access to prescription drug treatment among beneficiaries, but our data suggest that current policies are interfering with that goal when it comes to specialty drugs,” Dr Doshi said.
She added that making Part D out-of-pocket costs more consistent and limiting them to more reasonable sums would help mitigate this negative impact.
Dr Doshi and her colleagues are now pursuing further studies of the impact of Part D cost-sharing policies in different disease areas. They hope to gain a better understanding of changes in drug access and of the long-range clinical outcomes and costs associated with any delays or interruptions in treatment.
“We need to know if the current aggressive cost-sharing arrangements have adverse long-term impacts on health and perhaps, paradoxically, increase overall spending due to complications of poorly controlled disease,” Dr Doshi said. ![]()

Photo courtesy of the CDC
A new study suggests that cost-sharing policies in the US create a barrier to the treatment of chronic myeloid leukemia (CML).
Researchers examined Medicare claims data and found that “Part D” (prescription drug plan) co-insurance policies for “specialty drugs” seem to be reducing or delaying the use of tyrosine kinase inhibitors (TKIs) in patients with CML.
The team reported these findings in the American Journal of Managed Care.
“High out-of-pocket costs for specialty drugs appear to pose a very real barrier to treatment,” said study author Jalpa A. Doshi, PhD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
While there is no standard definition for specialty drugs, the term typically refers to medications requiring special handling, administration, or monitoring. Most are aimed at treating chronic or life-threatening diseases.
Although specialty drugs typically tend to offer significant medical advances over non-specialty drugs, they are correspondingly more expensive. In 2014, such drugs accounted for less than 1% of prescriptions in the US but nearly a third of total prescription spending.
While insurers have been imposing higher cost-sharing requirements as part of their efforts to manage specialty drug spending, there has been limited information about the corresponding impact on patients.
“[I]t was particularly important to examine the extent to which the aggressive cost-sharing policies for specialty drugs seen under Medicare Part D, which are increasingly making their way into the private insurance market, adversely impact access to these treatments even for a condition like cancer,” Dr Doshi said.
So she and her colleagues examined the impact of specialty drug cost-sharing under the Medicare Part D prescription drug benefit on patients with CML. The team analyzed Medicare data on patients who were newly diagnosed with CML to examine whether and how quickly they initiated TKI treatment.
The researchers compared patients who were eligible for low-income subsidies and therefore faced nominal out-of-pocket costs to patients who faced average out-of-pocket costs of $2600 or more for their first 30-day TKI prescription fill.
Results showed that patients in the high-cost group were significantly less likely than the low-cost group to have a Part D claim for a TKI prescription within 6 months of their CML diagnosis. The rates were 45.3% and 66.9%, respectively (P<0.001).
Patients in the high cost-sharing group also took twice as long, on average, to initiate TKI treatment. The mean time to fill a TKI prescription was 50.9 days in the high-cost group and 23.7 days in the low-cost group (P<0.001).
“Medicare Part D was created to increase access to prescription drug treatment among beneficiaries, but our data suggest that current policies are interfering with that goal when it comes to specialty drugs,” Dr Doshi said.
She added that making Part D out-of-pocket costs more consistent and limiting them to more reasonable sums would help mitigate this negative impact.
Dr Doshi and her colleagues are now pursuing further studies of the impact of Part D cost-sharing policies in different disease areas. They hope to gain a better understanding of changes in drug access and of the long-range clinical outcomes and costs associated with any delays or interruptions in treatment.
“We need to know if the current aggressive cost-sharing arrangements have adverse long-term impacts on health and perhaps, paradoxically, increase overall spending due to complications of poorly controlled disease,” Dr Doshi said. ![]()

Photo courtesy of the CDC
A new study suggests that cost-sharing policies in the US create a barrier to the treatment of chronic myeloid leukemia (CML).
Researchers examined Medicare claims data and found that “Part D” (prescription drug plan) co-insurance policies for “specialty drugs” seem to be reducing or delaying the use of tyrosine kinase inhibitors (TKIs) in patients with CML.
The team reported these findings in the American Journal of Managed Care.
“High out-of-pocket costs for specialty drugs appear to pose a very real barrier to treatment,” said study author Jalpa A. Doshi, PhD, of the Perelman School of Medicine at the University of Pennsylvania in Philadelphia.
While there is no standard definition for specialty drugs, the term typically refers to medications requiring special handling, administration, or monitoring. Most are aimed at treating chronic or life-threatening diseases.
Although specialty drugs typically tend to offer significant medical advances over non-specialty drugs, they are correspondingly more expensive. In 2014, such drugs accounted for less than 1% of prescriptions in the US but nearly a third of total prescription spending.
While insurers have been imposing higher cost-sharing requirements as part of their efforts to manage specialty drug spending, there has been limited information about the corresponding impact on patients.
“[I]t was particularly important to examine the extent to which the aggressive cost-sharing policies for specialty drugs seen under Medicare Part D, which are increasingly making their way into the private insurance market, adversely impact access to these treatments even for a condition like cancer,” Dr Doshi said.
So she and her colleagues examined the impact of specialty drug cost-sharing under the Medicare Part D prescription drug benefit on patients with CML. The team analyzed Medicare data on patients who were newly diagnosed with CML to examine whether and how quickly they initiated TKI treatment.
The researchers compared patients who were eligible for low-income subsidies and therefore faced nominal out-of-pocket costs to patients who faced average out-of-pocket costs of $2600 or more for their first 30-day TKI prescription fill.
Results showed that patients in the high-cost group were significantly less likely than the low-cost group to have a Part D claim for a TKI prescription within 6 months of their CML diagnosis. The rates were 45.3% and 66.9%, respectively (P<0.001).
Patients in the high cost-sharing group also took twice as long, on average, to initiate TKI treatment. The mean time to fill a TKI prescription was 50.9 days in the high-cost group and 23.7 days in the low-cost group (P<0.001).
“Medicare Part D was created to increase access to prescription drug treatment among beneficiaries, but our data suggest that current policies are interfering with that goal when it comes to specialty drugs,” Dr Doshi said.
She added that making Part D out-of-pocket costs more consistent and limiting them to more reasonable sums would help mitigate this negative impact.
Dr Doshi and her colleagues are now pursuing further studies of the impact of Part D cost-sharing policies in different disease areas. They hope to gain a better understanding of changes in drug access and of the long-range clinical outcomes and costs associated with any delays or interruptions in treatment.
“We need to know if the current aggressive cost-sharing arrangements have adverse long-term impacts on health and perhaps, paradoxically, increase overall spending due to complications of poorly controlled disease,” Dr Doshi said. ![]()
iPSCs can differentiate into functional lymphocytes

Image from the Salk Institute
Researchers say they have generated induced pluripotent stem cells (iPSCs) that can differentiate into multiple lineages of functional lymphocytes.
The team noted that lymphohematopoietic stem cells (L-HSCs) generated from self-somatic cell-derived iPSCs could potentially be used to treat hematologic disorders, but no one has generated “truly functional” L-HSCs from iPSCs.
So the researchers set out to determine whether iPSCs have the inherent potential to generate multiple lineages of functional, terminally differentiated lymphocytes.
They described this work in Stem Cells and Development.
The researchers said they used tetraploid embryo complementation to provide a normal environment for the differentiation of L-HSCs from iPSCs and embryonic stem cells (ESCs). The team then compared lymphocytes derived from iPSCs, ESCs, and naïve isogenic C57BL/6 mice.
The researchers found that iPSC-derived lymphocytes expressed normal levels of major histocompatibility complex-I. Levels were comparable in iPSC-derived lymphocytes, ESC-derived lymphocytes, and lymphocytes from the control mice.
In addition, iPSC-derived lymphocytes were able to differentiate into multiple cell types—CD4+ T cells, CD8+ T cells, regulatory T cells, B cells, and natural killer cells.
Lymphocytes generated from iPSCs and lymphocytes generated from ESCs had the same capacity as lymphocytes from the control mice to proliferate and secrete chemical signals, such as cytokines.
All 3 types of lymphocytes proliferated under allogeneic stimulation but not under syngeneic stimulation. And the researchers found similar levels of IL-2, IL-4, IL-6, IL-10, IL-17, TNF, and IFN-γ in iPSC, ESC, and C57BL/6 lymphocyte culture supernatants.
The team also found that lymphocytes generated by iPSC-derived bone marrow cells could repopulate the hematopoietic systems of lethally irradiated recipient mice.
The iPSC bone marrow cells proved as effective as ESC-derived bone marrow cells and wild-type bone marrow cells. All 3 types of cells negated lymphocyte storage exhaustion in the spleen and peripheral blood.
In addition, there were no major phenotypic or behavioral abnormalities in any of the mice more than 1 month after cell transplantation.
The researchers said this work shows that truly functional lymphocytes can be generated from iPSCs, and it supports the clinical application of iPSC technology to develop treatments for hematologic disorders. ![]()

Image from the Salk Institute
Researchers say they have generated induced pluripotent stem cells (iPSCs) that can differentiate into multiple lineages of functional lymphocytes.
The team noted that lymphohematopoietic stem cells (L-HSCs) generated from self-somatic cell-derived iPSCs could potentially be used to treat hematologic disorders, but no one has generated “truly functional” L-HSCs from iPSCs.
So the researchers set out to determine whether iPSCs have the inherent potential to generate multiple lineages of functional, terminally differentiated lymphocytes.
They described this work in Stem Cells and Development.
The researchers said they used tetraploid embryo complementation to provide a normal environment for the differentiation of L-HSCs from iPSCs and embryonic stem cells (ESCs). The team then compared lymphocytes derived from iPSCs, ESCs, and naïve isogenic C57BL/6 mice.
The researchers found that iPSC-derived lymphocytes expressed normal levels of major histocompatibility complex-I. Levels were comparable in iPSC-derived lymphocytes, ESC-derived lymphocytes, and lymphocytes from the control mice.
In addition, iPSC-derived lymphocytes were able to differentiate into multiple cell types—CD4+ T cells, CD8+ T cells, regulatory T cells, B cells, and natural killer cells.
Lymphocytes generated from iPSCs and lymphocytes generated from ESCs had the same capacity as lymphocytes from the control mice to proliferate and secrete chemical signals, such as cytokines.
All 3 types of lymphocytes proliferated under allogeneic stimulation but not under syngeneic stimulation. And the researchers found similar levels of IL-2, IL-4, IL-6, IL-10, IL-17, TNF, and IFN-γ in iPSC, ESC, and C57BL/6 lymphocyte culture supernatants.
The team also found that lymphocytes generated by iPSC-derived bone marrow cells could repopulate the hematopoietic systems of lethally irradiated recipient mice.
The iPSC bone marrow cells proved as effective as ESC-derived bone marrow cells and wild-type bone marrow cells. All 3 types of cells negated lymphocyte storage exhaustion in the spleen and peripheral blood.
In addition, there were no major phenotypic or behavioral abnormalities in any of the mice more than 1 month after cell transplantation.
The researchers said this work shows that truly functional lymphocytes can be generated from iPSCs, and it supports the clinical application of iPSC technology to develop treatments for hematologic disorders. ![]()

Image from the Salk Institute
Researchers say they have generated induced pluripotent stem cells (iPSCs) that can differentiate into multiple lineages of functional lymphocytes.
The team noted that lymphohematopoietic stem cells (L-HSCs) generated from self-somatic cell-derived iPSCs could potentially be used to treat hematologic disorders, but no one has generated “truly functional” L-HSCs from iPSCs.
So the researchers set out to determine whether iPSCs have the inherent potential to generate multiple lineages of functional, terminally differentiated lymphocytes.
They described this work in Stem Cells and Development.
The researchers said they used tetraploid embryo complementation to provide a normal environment for the differentiation of L-HSCs from iPSCs and embryonic stem cells (ESCs). The team then compared lymphocytes derived from iPSCs, ESCs, and naïve isogenic C57BL/6 mice.
The researchers found that iPSC-derived lymphocytes expressed normal levels of major histocompatibility complex-I. Levels were comparable in iPSC-derived lymphocytes, ESC-derived lymphocytes, and lymphocytes from the control mice.
In addition, iPSC-derived lymphocytes were able to differentiate into multiple cell types—CD4+ T cells, CD8+ T cells, regulatory T cells, B cells, and natural killer cells.
Lymphocytes generated from iPSCs and lymphocytes generated from ESCs had the same capacity as lymphocytes from the control mice to proliferate and secrete chemical signals, such as cytokines.
All 3 types of lymphocytes proliferated under allogeneic stimulation but not under syngeneic stimulation. And the researchers found similar levels of IL-2, IL-4, IL-6, IL-10, IL-17, TNF, and IFN-γ in iPSC, ESC, and C57BL/6 lymphocyte culture supernatants.
The team also found that lymphocytes generated by iPSC-derived bone marrow cells could repopulate the hematopoietic systems of lethally irradiated recipient mice.
The iPSC bone marrow cells proved as effective as ESC-derived bone marrow cells and wild-type bone marrow cells. All 3 types of cells negated lymphocyte storage exhaustion in the spleen and peripheral blood.
In addition, there were no major phenotypic or behavioral abnormalities in any of the mice more than 1 month after cell transplantation.
The researchers said this work shows that truly functional lymphocytes can be generated from iPSCs, and it supports the clinical application of iPSC technology to develop treatments for hematologic disorders. ![]()
A better method for detecting amyloidosis?

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()

A novel molecular probe can detect amyloidosis at least as well as—and perhaps even better than—traditional methods, according to research published in Amyloid: The Journal of Protein Folding Disorders.
Investigators found that a luminescent conjugated oligothiophene, h-FTAA, allowed them to correctly identify amyloidosis in every sample tested.
But results also suggested h-FTAA may be more sensitive than traditional methods used to diagnose amyloidosis, as h-FTAA detected small amyloid deposits in samples that were previously determined to be amyloid-free.
The investigators said this suggests h-FTAA could be used to detect amyloidosis before symptoms present, leading to faster treatment.
“Given the sensitivity of the probe, we think this would make an excellent complement to traditional methods and could eventually be a replacement,” said study author Per Hammarström, PhD, of Linköping University in Sweden.
Dr Hammarström and his colleagues screened amyloid-containing tissues from 107 patients who had their amyloidosis verified by Congo red staining and/or immunohistochemistry, as well as tissues from 32 negative control cases.
The results showed that h-FTAA could detect amyloidosis with 100% sensitivity, identifying amyloid deposits in all 107 patients.
However, h-FTAA also detected microdeposits of amyloid-like protein aggregates in 5 of the control samples that were negative according to Congo red.
The investigators said they don’t know the clinical significance of these “false-positive” lesions. However, because h-FTAA fluorescence is 1 magnitude brighter than Congo red and because the staining is performed 4 magnitudes lower than the concentration of dye, the team believes these 5 cases may have been beyond detection by Congo red and h-FTAA may be a more sensitive technique.
They therefore concluded that h-FTAA could potentially be used as a complementary technique for accurate detection of amyloid in routine surgical pathology settings, for the detection of prodromal amyloidosis, and for the discovery of new amyloid-like protein aggregates. ![]()
Drug ‘not powerful enough’ to treat CTCL

mycosis fungoides
Results of a phase 2 trial suggest the drug APO866 is not suitable for the treatment of relapsed or refractory cutaneous T-cell lymphoma (CTCL).
Researchers said APO866 had “a reasonable toxic effect,” but it was “not powerful enough,” so the trial was stopped early.
Two of the 14 patients studied achieved a partial response during the trial, but there were no complete responses, and most patients withdrew from the study early.
The researchers described these results in a letter to JAMA Dermatology. The study was initially sponsored by Apoxis SA and later by TopoTarget A/S, which is now known as Onxeo after merging with BioAlliance Pharma.
According to Onxeo, APO866 is an injectable molecule that induces apoptosis by inhibiting the biosynthesis of NAD+ from niacinamide, which is essential for the cellular metabolism, protein modification, and calcium-dependent messenger synthesis.
In the phase 2 trial, researchers tested APO866 in 14 patients with relapsed or refractory CTCL. The patients were 19 to 83 years of age, and half were female.
Eight patients had mycosis fungoides, 3 had Sézary syndrome, 1 had CD30+ anaplastic large-cell lymphoma, 1 had poikilodermic mycosis fungoides, and 1 had CD30- nonepidermotropic CTCL. One patient had stage IB disease, 2 had stage IIA, 3 had stage IIB, and 8 had stage IVA.
The patients received a continuous intravenous infusion, via pump, of APO866 at 0.126 mg/m2/h over the course of 96 hours. Patients received this treatment every 28 days for a total of 3 cycles.
Five patients completed all 3 treatment cycles and had no major protocol violations. Nine patients discontinued treatment early due to consent withdrawal (n=2), early disease progression (n=5), or adverse events (AEs, n=2).
At week 8, 1 patient had achieved a partial response to treatment, 4 patients had stable disease, 5 had progressed, and 4 patients were not evaluable because they had withdrawn from the study.
At week 16, 1 patient had a partial response (not the same patient as at week 8), 4 patients had stable disease, and 9 patients were not evaluable due to withdrawal.
There were a total of 141 AEs, and 77 of these were considered related to APO866. Most patients (n=12) had mild to moderate AEs, but there were 7 serious AEs thought to be treatment-related. These included pyrexia, lymphopenia (n=2), spondylitis, Staphylococcal sepsis, rhabdomyolysis, and thrombocytopenia.
There were 4 deaths, but they were not considered drug-related.
The researchers said these results suggest APO866 should not be pursued as a treatment for CTCL. However, as the drug induces immunosuppression and has insulin-mimicking effects, it might prove useful for treating other conditions. ![]()

mycosis fungoides
Results of a phase 2 trial suggest the drug APO866 is not suitable for the treatment of relapsed or refractory cutaneous T-cell lymphoma (CTCL).
Researchers said APO866 had “a reasonable toxic effect,” but it was “not powerful enough,” so the trial was stopped early.
Two of the 14 patients studied achieved a partial response during the trial, but there were no complete responses, and most patients withdrew from the study early.
The researchers described these results in a letter to JAMA Dermatology. The study was initially sponsored by Apoxis SA and later by TopoTarget A/S, which is now known as Onxeo after merging with BioAlliance Pharma.
According to Onxeo, APO866 is an injectable molecule that induces apoptosis by inhibiting the biosynthesis of NAD+ from niacinamide, which is essential for the cellular metabolism, protein modification, and calcium-dependent messenger synthesis.
In the phase 2 trial, researchers tested APO866 in 14 patients with relapsed or refractory CTCL. The patients were 19 to 83 years of age, and half were female.
Eight patients had mycosis fungoides, 3 had Sézary syndrome, 1 had CD30+ anaplastic large-cell lymphoma, 1 had poikilodermic mycosis fungoides, and 1 had CD30- nonepidermotropic CTCL. One patient had stage IB disease, 2 had stage IIA, 3 had stage IIB, and 8 had stage IVA.
The patients received a continuous intravenous infusion, via pump, of APO866 at 0.126 mg/m2/h over the course of 96 hours. Patients received this treatment every 28 days for a total of 3 cycles.
Five patients completed all 3 treatment cycles and had no major protocol violations. Nine patients discontinued treatment early due to consent withdrawal (n=2), early disease progression (n=5), or adverse events (AEs, n=2).
At week 8, 1 patient had achieved a partial response to treatment, 4 patients had stable disease, 5 had progressed, and 4 patients were not evaluable because they had withdrawn from the study.
At week 16, 1 patient had a partial response (not the same patient as at week 8), 4 patients had stable disease, and 9 patients were not evaluable due to withdrawal.
There were a total of 141 AEs, and 77 of these were considered related to APO866. Most patients (n=12) had mild to moderate AEs, but there were 7 serious AEs thought to be treatment-related. These included pyrexia, lymphopenia (n=2), spondylitis, Staphylococcal sepsis, rhabdomyolysis, and thrombocytopenia.
There were 4 deaths, but they were not considered drug-related.
The researchers said these results suggest APO866 should not be pursued as a treatment for CTCL. However, as the drug induces immunosuppression and has insulin-mimicking effects, it might prove useful for treating other conditions. ![]()

mycosis fungoides
Results of a phase 2 trial suggest the drug APO866 is not suitable for the treatment of relapsed or refractory cutaneous T-cell lymphoma (CTCL).
Researchers said APO866 had “a reasonable toxic effect,” but it was “not powerful enough,” so the trial was stopped early.
Two of the 14 patients studied achieved a partial response during the trial, but there were no complete responses, and most patients withdrew from the study early.
The researchers described these results in a letter to JAMA Dermatology. The study was initially sponsored by Apoxis SA and later by TopoTarget A/S, which is now known as Onxeo after merging with BioAlliance Pharma.
According to Onxeo, APO866 is an injectable molecule that induces apoptosis by inhibiting the biosynthesis of NAD+ from niacinamide, which is essential for the cellular metabolism, protein modification, and calcium-dependent messenger synthesis.
In the phase 2 trial, researchers tested APO866 in 14 patients with relapsed or refractory CTCL. The patients were 19 to 83 years of age, and half were female.
Eight patients had mycosis fungoides, 3 had Sézary syndrome, 1 had CD30+ anaplastic large-cell lymphoma, 1 had poikilodermic mycosis fungoides, and 1 had CD30- nonepidermotropic CTCL. One patient had stage IB disease, 2 had stage IIA, 3 had stage IIB, and 8 had stage IVA.
The patients received a continuous intravenous infusion, via pump, of APO866 at 0.126 mg/m2/h over the course of 96 hours. Patients received this treatment every 28 days for a total of 3 cycles.
Five patients completed all 3 treatment cycles and had no major protocol violations. Nine patients discontinued treatment early due to consent withdrawal (n=2), early disease progression (n=5), or adverse events (AEs, n=2).
At week 8, 1 patient had achieved a partial response to treatment, 4 patients had stable disease, 5 had progressed, and 4 patients were not evaluable because they had withdrawn from the study.
At week 16, 1 patient had a partial response (not the same patient as at week 8), 4 patients had stable disease, and 9 patients were not evaluable due to withdrawal.
There were a total of 141 AEs, and 77 of these were considered related to APO866. Most patients (n=12) had mild to moderate AEs, but there were 7 serious AEs thought to be treatment-related. These included pyrexia, lymphopenia (n=2), spondylitis, Staphylococcal sepsis, rhabdomyolysis, and thrombocytopenia.
There were 4 deaths, but they were not considered drug-related.
The researchers said these results suggest APO866 should not be pursued as a treatment for CTCL. However, as the drug induces immunosuppression and has insulin-mimicking effects, it might prove useful for treating other conditions. ![]()
Patient with intractable nausea and vomiting
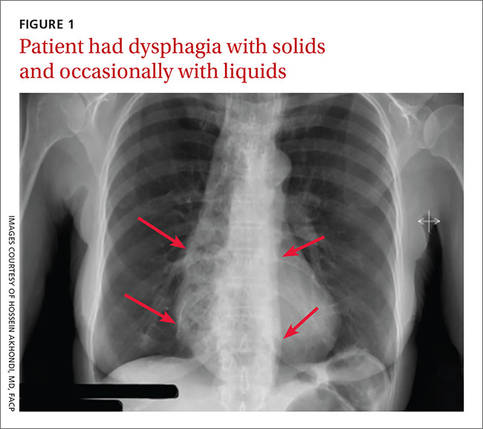
A 53-year-old African American woman was admitted to our hospital for intractable nausea and vomiting that she’d been experiencing for a month. She also reported dysphagia with solids and occasionally with liquids. She had no chest or abdominal pain, and no fever, bleeding, diarrhea, significant weight loss, or significant travel history. The patient was not taking any medication and her physical exam was normal. The patient’s complete blood count and electrolytes were normal. We ordered a chest x-ray (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Achalasia
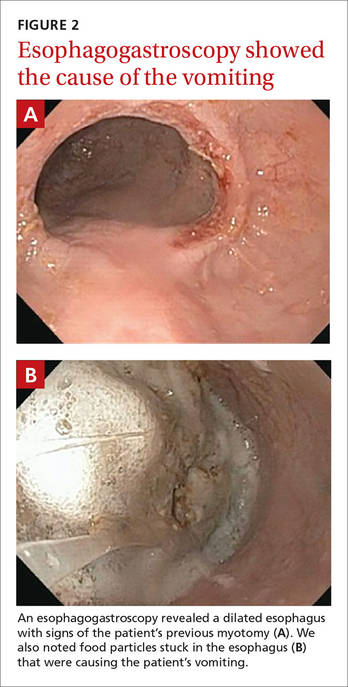
The radiologist who examined the x-ray noted a dilated esophagus (FIGURE 1, red arrows) with debris behind the heart shadow, which suggested achalasia. Upon further questioning, the patient reported a history of achalasia that had been treated with a myotomy 6 years ago. We performed an esophagogastroscopy, which showed a dilated esophagus with signs of the myotomy (FIGURE 2A), as well as food particles lodged in the esophagus (FIGURE 2B) that were causing the patient’s intractable vomiting.
Achalasia is a motor disorder of the esophagus smooth muscle in which the lower esophageal sphincter does not relax properly with swallowing, and the normal peristalsis of the esophagus body is replaced by abnormal contractions. Primary idiopathic achalasia is the most common form in the United States, but secondary forms caused by gastric carcinoma, lymphoma, or Chagas disease are also seen.1 The prevalence of achalasia is 1.6 per 100,000 in some populations.2 Symptoms can include dysphagia with solids and liquids, chest pain, and regurgitation.
A chest x-ray will show an absence of gastric air, and occasionally, as in this case, a tubular mass (the dilated esophagus) behind the heart and aorta. On fluoroscopy, the lower two-thirds of the esophagus does not have peristalsis and the terminal part has a bird beak appearance. Manometry will show normal or elevated pressure in the lower esophagus. Administration of cholinergic agonists will cause a marked increase in baseline pressure, as well as pain and regurgitation. Endoscopy can exclude secondary causes.
Narrowing the causes of dysphagia
The differential diagnosis is broad because there are 2 types of dysphagia: mechanical and neuromuscular.
Mechanical dysphagia is caused by a food bolus or foreign body, by intrinsic narrowing of the esophagus (from inflammation, esophageal webs, benign and malignant strictures, and tumors) or by extrinsic compression (from bone or thyroid abscesses or vascular tightening).
Neuromuscular dysphagia is either a swallowing reflex problem, a disorder of the pharyngeal and striated esophagus muscles, or an esophageal smooth muscle disorder.3 Close attention to the patient’s history and physical exam is key to zeroing in on the proper diagnosis.
On the other hand, food impaction in the esophagus almost always indicates certain etiologies. Benign esophageal stenosis caused by Schatzki rings (B rings) or by peptic strictures is the most common cause of food impaction, followed by esophageal webs, extrinsic compression, surgical anastomosis, esophagitis (eg, eosinophilic esophagitis), and motor disorders, such as achalasia.
First-line therapy is surgery; pharmacologic Tx is least effective
Treatment should be individualized by age, gender, and patient preference; however, there is no definitive treatment for this condition. First-line therapy includes graded pneumatic dilation or laparoscopic myotomy with a partial fundoplication.4 Botulinum toxin injection in the lower esophageal sphincter is recommended for patients who are not good candidates for surgery or dilation.5
Pharmacologic therapy, the least effective treatment option, is recommended for patients who are unwilling or unable to undergo myotomy and/or dilation and do not respond to botulinum toxin.6,7 Long-acting nitrates such as isosorbide, calcium channel blockers such as nifedipine, and phosphodiesterase-5 (PDE5) inhibitors such as sildenafil reduce lower esophageal sphincter tone and pressure.
Both nifedipine and isosorbide should be taken sublingually before meals (30 minutes and 10 minutes, respectively). The effects of nifedipine and isosorbide, however, are partial, and these agents do not provide complete relief from symptoms.6 PDE5 use has been limited and results are inconclusive.
Our patient. The food particles in the patient’s esophagus were removed during endoscopy, and she stopped vomiting completely. Based on the findings and clinical picture, the patient most likely suffered from mega-esophagus (an end-stage dilated malfunctioning esophagus). Our patient was discharged to follow-up with her gastroenterologist.
Because there is no definitive treatment for achalasia, the patient was counseled about the need for continuous monitoring and dietary precautions, including modification of food texture or change of fluid viscosity. Food may be chopped, minced, or pureed, and fluids may be thickened.
CORRESPONDENCE
Hossein Akhondi, MD, FACP, Georgetown University, 1010 Mass Ave, NW, Unit 904, Washington, DC 20001; h68akhond@hotmail.com.
1. Richter JE. Esophageal motility disorder achalasia. Curr Opin Otolaryngol Head Neck Surg. 2013;21:535-542.
2. O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806-5812.
3. Ott R, Bajbouj M, Feussner H, et al. [Dysphagia—what is important for primary diagnosis in private practice?]. MMW Fortschr Med. 2014;156:54-57.
4. Yaghoobi M. Treatment of patients with new diagnosis of achalasia: laparoscopic Heller’s myotomy may be more effective than pneumatic dilation. Gastrointest Endosc. 2014;80:360.
5. Blatnik JA, Ponsky JL. Advances in the treatment of achalasia. Curr Treat Options Gastroenterol. 2014;12:49-58.
6. Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-1249.
7. Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21-35.
A 53-year-old African American woman was admitted to our hospital for intractable nausea and vomiting that she’d been experiencing for a month. She also reported dysphagia with solids and occasionally with liquids. She had no chest or abdominal pain, and no fever, bleeding, diarrhea, significant weight loss, or significant travel history. The patient was not taking any medication and her physical exam was normal. The patient’s complete blood count and electrolytes were normal. We ordered a chest x-ray (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Achalasia
The radiologist who examined the x-ray noted a dilated esophagus (FIGURE 1, red arrows) with debris behind the heart shadow, which suggested achalasia. Upon further questioning, the patient reported a history of achalasia that had been treated with a myotomy 6 years ago. We performed an esophagogastroscopy, which showed a dilated esophagus with signs of the myotomy (FIGURE 2A), as well as food particles lodged in the esophagus (FIGURE 2B) that were causing the patient’s intractable vomiting.
Achalasia is a motor disorder of the esophagus smooth muscle in which the lower esophageal sphincter does not relax properly with swallowing, and the normal peristalsis of the esophagus body is replaced by abnormal contractions. Primary idiopathic achalasia is the most common form in the United States, but secondary forms caused by gastric carcinoma, lymphoma, or Chagas disease are also seen.1 The prevalence of achalasia is 1.6 per 100,000 in some populations.2 Symptoms can include dysphagia with solids and liquids, chest pain, and regurgitation.
A chest x-ray will show an absence of gastric air, and occasionally, as in this case, a tubular mass (the dilated esophagus) behind the heart and aorta. On fluoroscopy, the lower two-thirds of the esophagus does not have peristalsis and the terminal part has a bird beak appearance. Manometry will show normal or elevated pressure in the lower esophagus. Administration of cholinergic agonists will cause a marked increase in baseline pressure, as well as pain and regurgitation. Endoscopy can exclude secondary causes.
Narrowing the causes of dysphagia
The differential diagnosis is broad because there are 2 types of dysphagia: mechanical and neuromuscular.
Mechanical dysphagia is caused by a food bolus or foreign body, by intrinsic narrowing of the esophagus (from inflammation, esophageal webs, benign and malignant strictures, and tumors) or by extrinsic compression (from bone or thyroid abscesses or vascular tightening).
Neuromuscular dysphagia is either a swallowing reflex problem, a disorder of the pharyngeal and striated esophagus muscles, or an esophageal smooth muscle disorder.3 Close attention to the patient’s history and physical exam is key to zeroing in on the proper diagnosis.
On the other hand, food impaction in the esophagus almost always indicates certain etiologies. Benign esophageal stenosis caused by Schatzki rings (B rings) or by peptic strictures is the most common cause of food impaction, followed by esophageal webs, extrinsic compression, surgical anastomosis, esophagitis (eg, eosinophilic esophagitis), and motor disorders, such as achalasia.
First-line therapy is surgery; pharmacologic Tx is least effective
Treatment should be individualized by age, gender, and patient preference; however, there is no definitive treatment for this condition. First-line therapy includes graded pneumatic dilation or laparoscopic myotomy with a partial fundoplication.4 Botulinum toxin injection in the lower esophageal sphincter is recommended for patients who are not good candidates for surgery or dilation.5
Pharmacologic therapy, the least effective treatment option, is recommended for patients who are unwilling or unable to undergo myotomy and/or dilation and do not respond to botulinum toxin.6,7 Long-acting nitrates such as isosorbide, calcium channel blockers such as nifedipine, and phosphodiesterase-5 (PDE5) inhibitors such as sildenafil reduce lower esophageal sphincter tone and pressure.
Both nifedipine and isosorbide should be taken sublingually before meals (30 minutes and 10 minutes, respectively). The effects of nifedipine and isosorbide, however, are partial, and these agents do not provide complete relief from symptoms.6 PDE5 use has been limited and results are inconclusive.
Our patient. The food particles in the patient’s esophagus were removed during endoscopy, and she stopped vomiting completely. Based on the findings and clinical picture, the patient most likely suffered from mega-esophagus (an end-stage dilated malfunctioning esophagus). Our patient was discharged to follow-up with her gastroenterologist.
Because there is no definitive treatment for achalasia, the patient was counseled about the need for continuous monitoring and dietary precautions, including modification of food texture or change of fluid viscosity. Food may be chopped, minced, or pureed, and fluids may be thickened.
CORRESPONDENCE
Hossein Akhondi, MD, FACP, Georgetown University, 1010 Mass Ave, NW, Unit 904, Washington, DC 20001; h68akhond@hotmail.com.
A 53-year-old African American woman was admitted to our hospital for intractable nausea and vomiting that she’d been experiencing for a month. She also reported dysphagia with solids and occasionally with liquids. She had no chest or abdominal pain, and no fever, bleeding, diarrhea, significant weight loss, or significant travel history. The patient was not taking any medication and her physical exam was normal. The patient’s complete blood count and electrolytes were normal. We ordered a chest x-ray (FIGURE 1).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Achalasia
The radiologist who examined the x-ray noted a dilated esophagus (FIGURE 1, red arrows) with debris behind the heart shadow, which suggested achalasia. Upon further questioning, the patient reported a history of achalasia that had been treated with a myotomy 6 years ago. We performed an esophagogastroscopy, which showed a dilated esophagus with signs of the myotomy (FIGURE 2A), as well as food particles lodged in the esophagus (FIGURE 2B) that were causing the patient’s intractable vomiting.
Achalasia is a motor disorder of the esophagus smooth muscle in which the lower esophageal sphincter does not relax properly with swallowing, and the normal peristalsis of the esophagus body is replaced by abnormal contractions. Primary idiopathic achalasia is the most common form in the United States, but secondary forms caused by gastric carcinoma, lymphoma, or Chagas disease are also seen.1 The prevalence of achalasia is 1.6 per 100,000 in some populations.2 Symptoms can include dysphagia with solids and liquids, chest pain, and regurgitation.
A chest x-ray will show an absence of gastric air, and occasionally, as in this case, a tubular mass (the dilated esophagus) behind the heart and aorta. On fluoroscopy, the lower two-thirds of the esophagus does not have peristalsis and the terminal part has a bird beak appearance. Manometry will show normal or elevated pressure in the lower esophagus. Administration of cholinergic agonists will cause a marked increase in baseline pressure, as well as pain and regurgitation. Endoscopy can exclude secondary causes.
Narrowing the causes of dysphagia
The differential diagnosis is broad because there are 2 types of dysphagia: mechanical and neuromuscular.
Mechanical dysphagia is caused by a food bolus or foreign body, by intrinsic narrowing of the esophagus (from inflammation, esophageal webs, benign and malignant strictures, and tumors) or by extrinsic compression (from bone or thyroid abscesses or vascular tightening).
Neuromuscular dysphagia is either a swallowing reflex problem, a disorder of the pharyngeal and striated esophagus muscles, or an esophageal smooth muscle disorder.3 Close attention to the patient’s history and physical exam is key to zeroing in on the proper diagnosis.
On the other hand, food impaction in the esophagus almost always indicates certain etiologies. Benign esophageal stenosis caused by Schatzki rings (B rings) or by peptic strictures is the most common cause of food impaction, followed by esophageal webs, extrinsic compression, surgical anastomosis, esophagitis (eg, eosinophilic esophagitis), and motor disorders, such as achalasia.
First-line therapy is surgery; pharmacologic Tx is least effective
Treatment should be individualized by age, gender, and patient preference; however, there is no definitive treatment for this condition. First-line therapy includes graded pneumatic dilation or laparoscopic myotomy with a partial fundoplication.4 Botulinum toxin injection in the lower esophageal sphincter is recommended for patients who are not good candidates for surgery or dilation.5
Pharmacologic therapy, the least effective treatment option, is recommended for patients who are unwilling or unable to undergo myotomy and/or dilation and do not respond to botulinum toxin.6,7 Long-acting nitrates such as isosorbide, calcium channel blockers such as nifedipine, and phosphodiesterase-5 (PDE5) inhibitors such as sildenafil reduce lower esophageal sphincter tone and pressure.
Both nifedipine and isosorbide should be taken sublingually before meals (30 minutes and 10 minutes, respectively). The effects of nifedipine and isosorbide, however, are partial, and these agents do not provide complete relief from symptoms.6 PDE5 use has been limited and results are inconclusive.
Our patient. The food particles in the patient’s esophagus were removed during endoscopy, and she stopped vomiting completely. Based on the findings and clinical picture, the patient most likely suffered from mega-esophagus (an end-stage dilated malfunctioning esophagus). Our patient was discharged to follow-up with her gastroenterologist.
Because there is no definitive treatment for achalasia, the patient was counseled about the need for continuous monitoring and dietary precautions, including modification of food texture or change of fluid viscosity. Food may be chopped, minced, or pureed, and fluids may be thickened.
CORRESPONDENCE
Hossein Akhondi, MD, FACP, Georgetown University, 1010 Mass Ave, NW, Unit 904, Washington, DC 20001; h68akhond@hotmail.com.
1. Richter JE. Esophageal motility disorder achalasia. Curr Opin Otolaryngol Head Neck Surg. 2013;21:535-542.
2. O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806-5812.
3. Ott R, Bajbouj M, Feussner H, et al. [Dysphagia—what is important for primary diagnosis in private practice?]. MMW Fortschr Med. 2014;156:54-57.
4. Yaghoobi M. Treatment of patients with new diagnosis of achalasia: laparoscopic Heller’s myotomy may be more effective than pneumatic dilation. Gastrointest Endosc. 2014;80:360.
5. Blatnik JA, Ponsky JL. Advances in the treatment of achalasia. Curr Treat Options Gastroenterol. 2014;12:49-58.
6. Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-1249.
7. Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21-35.
1. Richter JE. Esophageal motility disorder achalasia. Curr Opin Otolaryngol Head Neck Surg. 2013;21:535-542.
2. O’Neill OM, Johnston BT, Coleman HG. Achalasia: a review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2013;19:5806-5812.
3. Ott R, Bajbouj M, Feussner H, et al. [Dysphagia—what is important for primary diagnosis in private practice?]. MMW Fortschr Med. 2014;156:54-57.
4. Yaghoobi M. Treatment of patients with new diagnosis of achalasia: laparoscopic Heller’s myotomy may be more effective than pneumatic dilation. Gastrointest Endosc. 2014;80:360.
5. Blatnik JA, Ponsky JL. Advances in the treatment of achalasia. Curr Treat Options Gastroenterol. 2014;12:49-58.
6. Vaezi MF, Pandolfino JE, Vela MF. ACG clinical guideline: diagnosis and management of achalasia. Am J Gastroenterol. 2013;108:1238-1249.
7. Vaezi MF, Richter JE. Current therapies for achalasia: comparison and efficacy. J Clin Gastroenterol. 1998;27:21-35.
Tips and algorithms to get your patient's BP to goal
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; steven_yarows@ihacares.com.
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; steven_yarows@ihacares.com.
The Systolic Blood Pressure Intervention Trial (SPRINT),1 a study of more than 9000 patients published late last year, was stopped prematurely when it became clear that those receiving intensive treatment (systolic target 120 mm Hg) had significantly lower rates of myocardial infarction (MI), stroke, cardiovascular death, and other severe heart disease than those getting standard treatment (systolic target 140 mm Hg). Participants had an elevated cardiovascular risk at baseline (age ≥75 years, history of cardiovascular disease [CVD], chronic kidney disease [CKD], or elevated 10-year Framingham CVD risk score ≥15%); those with diabetes, history of stroke, or polycystic kidney disease were excluded.
Although serious adverse events were not significantly different between the intensive and standard treatment groups, syncope, acute renal failure, electrolyte abnormalities, and hyponatremia were all statistically more common in the aggressively treated group. (To learn more, see “Is lower BP worth it in higher-risk patients with diabetes or coronary disease?” Clinical Inquiries, J Fam Pract. 2016;65:129-131.)
Taking an aggressive approach. This trial shined a light on an important topic in medicine—the aggressive treatment of hypertension. And while this article will not discuss the finer points of the SPRINT trial or the limitations of generalizing aggressive treatment to the broad population of patients with hypertension, it will outline important considerations for physicians who wish to aggressively treat hypertension. I offer recommendations based on my 34 years of clinical practice and experience as a co-investigator on a number of hypertension studies to help you better balance each patient’s risks (eg, age, frailty, fall risk) and potential benefits (prevention of stroke, MI, and congestive heart failure).
Taking an aggressive approach, however, starts with ensuring that the diagnosis and treatment are based on accurate measures.
How accurate are your BP readings?
Measuring BP in clinical practice is markedly different from measurements taken in a research setting.2 This can result in large, clinically significant differences in readings and adversely affect treatment decisions.
Quiet time, multiple readings
SPRINT used techniques similar to those followed by other hypertension outcomes studies I’ve been involved in—methods that are rare in medical practice. Each study participant sat quietly in a chair for 5 minutes prior to the first BP reading. In addition, the researchers used an automatic oscillatory BP device (Omron Healthcare, Lake Forest, Ill), recording the average of 3 readings.
Practices that compromise accuracy. In clinical practice, BP is rarely measured after the patient has had 5 minutes of rest in a quiet room. Nor are readings done in triplicate. Instead, BP is typically measured while patient and clinician are engaged in conversation, often using a BP cuff that is too small (in my experience, most Americans require a large cuff).
BP is usually taken shortly after the patient has walked, frequently with some difficulty, from the waiting area to the exam room. Often, too, patients are weighed before their BP is measured, a common source of concern that can lead to a short-term rise in pressure. (Conversely, rapid deflation during the auscultatory measurement [>2 mm Hg/sec] can have the opposite effect, resulting in under-reading the true value.)
Compounding matters is the failure to consider the approximately 20% of patients who develop White Coat Syndrome. Such individuals, who typically have elevated office measurements but normal out-of-office readings, may develop further hypotensive symptoms if their treatment is based solely on in-office findings. Overtreatment of frail patients who often have marked orthostatic hypotension is an additional concern.
How to get more accurate readings
It’s clear that taking the treatments that led to optimal outcomes in clinical trials and applying them to clinic patients based on their office measurements is likely to result in overtreatment, leading to hypotension and endangering patients. The following steps, however, can ensure more accurate readings and thus, a proper starting place for treatment.
Use an oscillatory device. I suggest that clinical practices switch to oscillatory digital devices like those used in virtually all clinical research studies I’ve been involved in for the past 20 years. There are oscillatory digital devices designed for medical offices that automatically record BP readings. However, these are much more expensive.2 The home oscillatory devices I’m referring to can be purchased for each exam room, with various sized cuffs.
Go slow, repeat as needed. Have the rooming staff or medical assistant measure BP only after the patient interview is complete. The patient should sit down, with both feet on the floor, legs uncrossed.
If the reading is elevated, the staffer should show the patient how to repeat the measurement, then prepare to leave the room, advising him or her to sit quietly for 3 to 5 minutes before doing so. This method is both practical and time efficient. Occasionally, oscillometric measures result in an extremely elevated diastolic reading; in such a case, I recommend that a clinician manually remeasure BP.
Incorporate home monitoring. Out-of-office readings are important, not only for the initial diagnosis of hypertension, but for clinical management of established hypertension, as well.3 Guidelines from both the US Preventive Services Task Force (USPSTF) and the National Institute for Health and Care Excellence (NICE) call for 24-hour ambulatory monitoring to establish a hypertension diagnosis.3,4 Accuracy is imperative, as this is commonly a lifelong diagnosis that should not be established based on a few, often inaccurately measured office readings.
Home monitoring improves BP control and correlates more closely with ambulatory monitoring than with office readings.5,6 I use an Excel spreadsheet (Microsoft, Bellevue, Wash.) to have patients send me their home BP readings, but commercially available software programs, if available, and smart phone apps may be used instead.
My preference is to have patients measure and record their BP at breakfast and dinnertime (always after a 3-to-5-minute rest) for a month after any change in the medication regimen (FIGURE), and then send the chart to the office. (There are other protocols for how often and how long to monitor home BP, but this is the format I use.) Adjustments in medications can be continued based on the home readings until the goal is reached.
I advise all patients I treat for hypertension to check their BP on the first day of each month and record the measurements for review at their next office visit.
What to consider for optimal treatment
Screening patients for concurrent disease and hypertensive end-organ damage, of course, should be routine for primary care physicians. Baseline tests should include a complete blood count, electrolytes with creatinine clearance, and an electrocardiogram. A review of a recent echocardiogram and spot urine for microalbuminuria will also be useful, if clinically indicated.
Cost, compliance, and concurrent disease. Generic drugs with a long half-life to ensure 24-hour coverage are the optimal choice due to both cost and compliance. Some agents may be chosen because they also treat concurrent disease—a beta-blocker for a patient with heart failure with reduced ejection fraction or migraines, an angiotensin-converting enzyme (ACE) inhibitor/angiotensin receptor blocker (ARB) for diabetes, a diuretic for fluid overload, or spironolactone for systolic congestive heart failure.
Single agent or combination?
Most single drugs lower BP by approximately 10 mm Hg systolic and 5 mm Hg diastolic, with 2-drug combinations lowering pressure by 20 mm Hg and 10 mm Hg, respectively.7 Amlodipine, chlorthalidone, and azilsartan medoxomil, all of which have long half-lives, are approximately 50% more potent than other antihypertensive agents.
When the target BP is a reduction ≥20/10 mm Hg, starting with dual drug therapy is often useful. In such cases, it is prudent not only to be sure that BP has been accurately measured, but to begin with half-tablet doses for several days to allow the patient to acclimate to the change in pressure. Beta-blockers, central sympatholytic drugs, direct vasodilators, and alpha antagonists are not considered first-, second-, or third-line agents.
Spironolactone, an aldosterone receptor antagonist, is very useful in resistant hypertension,8 defined as inadequate BP control despite a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and thiazide diuretic. (For more information, see "Resistant hypertension? Time to consider this fourth-line drug.") Patients on spironolactone require electrolyte monitoring due to the risks of hyponatremia and hyperkalemia, especially in combination with an ACE inhibitor or ARB.
I advocate monitoring such patients after one month, although every 2 weeks for at least the first 6 weeks of treatment is prudent for patients with CKD. Mild hyperkalemia (<5.5 mEq/L) or hyponatremia (>130 mEq/L) is well tolerated, but conditions associated with sudden dehydration, such as diarrhea or vomiting, can rapidly worsen these imbalances and be clinically significant.
Treatment algorithms can help
SPRINT and other hypertension trials have used algorithm-based drug additions to reach the desired goals. In SPRINT, one or more antihypertensive drug classes with the strongest evidence to prevent cardiovascular disease outcomes were initiated and adjusted at the discretion of the investigators. The initial drug classes were thiazide-type diuretics (chlorthalidone was preferred unless advanced CKD was present, and then loop diuretics), calcium channel blockers (amlodipine preferred), ACE inhibitors (lisinopril was preferred), and ARBs (losartan or azilsartan medoxomil preferred).
The algorithms in this article may be considered for the treatment of hypertension. They are based on my experience, as well as on guidance from the Seventh Report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure.9
ALGORITHM 1 is suitable for patients who initially need only 10/5 mm Hg lowering.10ALGORITHM 2A may be used for patients for whom you wish to lower BP by ≥20/10 mm Hg. I also recommend 2A for patients of Asian descent; that’s because ARBs are preferable to ACE inhibitors, which are associated with a high incidence of cough in this patient population. Either ALGORITHM 2A or 2B may be used for African-American patients with hypertension, as ACE inhibitors and ARBs alone are less effective for this group.
CORRESPONDENCE
Steven Yarows, MD, FACP, FASH, IHA Chelsea Family and Internal Medicine, 128 Van Buren St, Chelsea, MI 48118; steven_yarows@ihacares.com.
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.
1. SPRINT Research Group, Wright JT, Williamson JD, Whelton PK, et al. A randomized trial of intensive versus standard blood-pressure control. N Engl J Med. 2015;373:2103-2116.
2. Myers MG, Goodwin M, Dawes M, et al. Measurement of blood pressure in the office: recognizing the problem and proposing the solution. Hypertension. 2010;55:195-200.
3. Siu A. Screening for high blood pressure in adults: US Preventive Services Task Force Recommendation Statement. Ann Intern Med. 2015;163:778-786.
4. McCormack T, Krause T. Management of hypertension in adults in primary care: NICE guideline. Br J Gen Pract. 2012;62:163-164.
5. Cuspidi C, Meani S, Fusi V, et al. Home blood pressure measurement and its relationship with blood pressure control in a large selected hypertensive population. J Hum Hypertens. 2004;18:725–731.
6. Mansoor GA, White WB. Self-measured home blood pressure in predicting ambulatory hypertension. Am J Hypertens. 2004;17:1017-1022.
7. Law MR, Wald NJ, Morris JK, et al. Value of low-dose combination treatment with blood pressure lowering drugs: analysis of 354 randomised trials. BMJ. 2003:326:1427.
8. Bloch MJ, Basile JN. Ambulatory blood pressure monitoring to diagnose hypertension—an idea whose time has come. J Am Soc Hypertens. 2016;10:89-91.
9. National Institutes of Health. JNC 7 Express. The Seventh Report of the Joint Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Available at: https://www.nhlbi.nih.gov/files/docs/guidelines/express.pdf. Accessed February 26, 2016.
10. Roush GC, Ernst ME, Kostis JB, et al. Head-to-head comparisons of hydrochlorothiazide with chlorthalidone: antihypertensive and metabolic effects. Hypertension. 2015;65:1041-1046.
Resistant hypertension? Time to consider this fourth-line drug
When a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and a thiazide diuretic fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068.
Illustrative case
Willie S, a 56-year-old with chronic essential hypertension, has been on an optimally dosed 3-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than 3 months, but his blood pressure is still not at goal.
What is the best antihypertensive agent to add to his regimen?
Resistant hypertension—defined as inadequate blood pressure (BP) control despite a triple regimen of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic—affects an estimated 5% to 30% of those being treated for hypertension.1,2 Guidelines from the 8th Joint National Committee (JNC-8) on the management of high BP, released in 2014, recommend beta-blockers, alpha-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Hypertension guidelines from the UK’s National Institute for Health and Care Excellence, released in 2011, recommend an AA if BP targets have not been met with the triple regimen. This recommendation, however, is based on lower-quality evidence, without comparison with beta-blockers, alpha-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1200 participants (3 randomized controlled trials [RCTs], one nonrandomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5 In the 4 comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg (95% confidence interval [CI], 8.65-39.87; P=.002) and diastolic blood pressure (DBP) by 7.8 mm Hg (95% CI, 3.79-11.79; P=.0001) more than placebo. In the 11 single arm studies, AAs reduced SBP by 22.74 mm Hg (95% CI, 18.21-27.27; P <.00001), and DBP by 10.49 mm Hg (95% CI, 8.85–12.13; P <.00001).
The previous year, a randomized, placebo-controlled trial examined the effect of low-dose (25 mg) spironolactone compared with placebo in 161 patients with resistant hypertension.6 At 8 weeks, 73% of those receiving spironolactone reached a goal SBP <140 mm Hg vs 41% of patients on placebo (P=.001). The same proportion (73%) achieved a goal DBP <90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group (P=.223).
Ambulatory BP was likewise assessed and found to be significantly improved among those receiving spironolactone vs placebo, with a decrease in SBP of 9.8 mm Hg (95% CI, -14.2 to -5.4; P<.001), and a 3.2 mm Hg decline in DBP (95% CI, -5.9 to -0.5; P=.013).6
STUDY SUMMARY
First study to compare spironolactone with other drugs
The study by Williams et al—a double-blind, randomized placebo-controlled crossover trial conducted in the UK—was the first RCT to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already on triple therapy with an ACE inhibitor or ARB, a CCB, and a thiazide diuretic.1 The trial randomized 335 individuals with a mean age of 61.4 years (age range 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥140 mm Hg (or ≥135 mm Hg in those with diabetes) and home SBP (in 18 readings over 4 days) of ≥130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of 3 months on a maximally dosed triple regimen.
Diabetes prevalence was 14%; tobacco use was 7.8%; and average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Participants underwent 4, 12-week rotations
All participants began the trial with 4 weeks of placebo, followed by randomization to 12-week rotations of once daily oral treatment with 1) spironolactone 25 to 50 mg, 2) doxazosin modified release 4 to 8 mg, 3) bisoprolol 5 to 10 mg, and 4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on 4 consecutive days prior to the study visits on Weeks 6 and 12. Participants were required to have at least 6 BP measurements per each 6-week period in order to establish a valid average. Primary endpoints included: the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other 2 drugs, and the difference in home SBP between spironolactone and each of the other 2 drugs.
The results: Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (TABLE),1 and clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP <135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other 2 study drugs. However, spironolactone had a superior BP lowering effect throughout nearly the entire renin distribution of the cohort. The mean difference between spironolactone and placebo was -10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone, and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and 3 times less than for doxazosin.1
WHAT’S NEW
Evidence of spironolactone’s superiority
This is the first RCT to compare spironolactone with 2 other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets vs a beta-blocker or an alpha-blocker.
CAVEATS
Findings do not apply across the board
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the drug’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of initiating treatment and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61 years. Although smaller observational studies that included African American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions, lack of patient-oriented results
The evidence supporting this change in practice has been accumulating for the past few years. However, physicians treating patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). (NICE), National Institute for Health and Care Excellence. 2011. Available at: https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
When a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and a thiazide diuretic fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068.
Illustrative case
Willie S, a 56-year-old with chronic essential hypertension, has been on an optimally dosed 3-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than 3 months, but his blood pressure is still not at goal.
What is the best antihypertensive agent to add to his regimen?
Resistant hypertension—defined as inadequate blood pressure (BP) control despite a triple regimen of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic—affects an estimated 5% to 30% of those being treated for hypertension.1,2 Guidelines from the 8th Joint National Committee (JNC-8) on the management of high BP, released in 2014, recommend beta-blockers, alpha-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Hypertension guidelines from the UK’s National Institute for Health and Care Excellence, released in 2011, recommend an AA if BP targets have not been met with the triple regimen. This recommendation, however, is based on lower-quality evidence, without comparison with beta-blockers, alpha-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1200 participants (3 randomized controlled trials [RCTs], one nonrandomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5 In the 4 comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg (95% confidence interval [CI], 8.65-39.87; P=.002) and diastolic blood pressure (DBP) by 7.8 mm Hg (95% CI, 3.79-11.79; P=.0001) more than placebo. In the 11 single arm studies, AAs reduced SBP by 22.74 mm Hg (95% CI, 18.21-27.27; P <.00001), and DBP by 10.49 mm Hg (95% CI, 8.85–12.13; P <.00001).
The previous year, a randomized, placebo-controlled trial examined the effect of low-dose (25 mg) spironolactone compared with placebo in 161 patients with resistant hypertension.6 At 8 weeks, 73% of those receiving spironolactone reached a goal SBP <140 mm Hg vs 41% of patients on placebo (P=.001). The same proportion (73%) achieved a goal DBP <90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group (P=.223).
Ambulatory BP was likewise assessed and found to be significantly improved among those receiving spironolactone vs placebo, with a decrease in SBP of 9.8 mm Hg (95% CI, -14.2 to -5.4; P<.001), and a 3.2 mm Hg decline in DBP (95% CI, -5.9 to -0.5; P=.013).6
STUDY SUMMARY
First study to compare spironolactone with other drugs
The study by Williams et al—a double-blind, randomized placebo-controlled crossover trial conducted in the UK—was the first RCT to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already on triple therapy with an ACE inhibitor or ARB, a CCB, and a thiazide diuretic.1 The trial randomized 335 individuals with a mean age of 61.4 years (age range 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥140 mm Hg (or ≥135 mm Hg in those with diabetes) and home SBP (in 18 readings over 4 days) of ≥130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of 3 months on a maximally dosed triple regimen.
Diabetes prevalence was 14%; tobacco use was 7.8%; and average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Participants underwent 4, 12-week rotations
All participants began the trial with 4 weeks of placebo, followed by randomization to 12-week rotations of once daily oral treatment with 1) spironolactone 25 to 50 mg, 2) doxazosin modified release 4 to 8 mg, 3) bisoprolol 5 to 10 mg, and 4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on 4 consecutive days prior to the study visits on Weeks 6 and 12. Participants were required to have at least 6 BP measurements per each 6-week period in order to establish a valid average. Primary endpoints included: the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other 2 drugs, and the difference in home SBP between spironolactone and each of the other 2 drugs.
The results: Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (TABLE),1 and clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP <135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other 2 study drugs. However, spironolactone had a superior BP lowering effect throughout nearly the entire renin distribution of the cohort. The mean difference between spironolactone and placebo was -10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone, and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and 3 times less than for doxazosin.1
WHAT’S NEW
Evidence of spironolactone’s superiority
This is the first RCT to compare spironolactone with 2 other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets vs a beta-blocker or an alpha-blocker.
CAVEATS
Findings do not apply across the board
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the drug’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of initiating treatment and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61 years. Although smaller observational studies that included African American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions, lack of patient-oriented results
The evidence supporting this change in practice has been accumulating for the past few years. However, physicians treating patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
When a triple regimen of an ACE inhibitor or ARB, calcium channel blocker, and a thiazide diuretic fails to achieve the target blood pressure, try adding spironolactone.
Strength of recommendation
C: Based on a high-quality disease-oriented randomized controlled trial.1
Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059–2068.
Illustrative case
Willie S, a 56-year-old with chronic essential hypertension, has been on an optimally dosed 3-drug regimen of an ACE inhibitor, a calcium channel blocker, and a thiazide diuretic for more than 3 months, but his blood pressure is still not at goal.
What is the best antihypertensive agent to add to his regimen?
Resistant hypertension—defined as inadequate blood pressure (BP) control despite a triple regimen of angiotensin-converting enzyme (ACE) inhibitor or angiotensin receptor blocker (ARB), calcium channel blocker (CCB), and thiazide diuretic—affects an estimated 5% to 30% of those being treated for hypertension.1,2 Guidelines from the 8th Joint National Committee (JNC-8) on the management of high BP, released in 2014, recommend beta-blockers, alpha-blockers, or aldosterone antagonists (AAs) as equivalent choices for a fourth-line agent. The recommendation is based on expert opinion.3
Hypertension guidelines from the UK’s National Institute for Health and Care Excellence, released in 2011, recommend an AA if BP targets have not been met with the triple regimen. This recommendation, however, is based on lower-quality evidence, without comparison with beta-blockers, alpha-blockers, or other drug classes.4
More evidence since guideline’s release
A 2015 meta-analysis of 15 studies and a total of more than 1200 participants (3 randomized controlled trials [RCTs], one nonrandomized placebo-controlled comparative trial, and 11 single-arm observational studies) demonstrated the effectiveness of the AAs spironolactone and eplerenone on resistant hypertension.5 In the 4 comparative studies, AAs decreased office systolic blood pressure (SBP) by 24.3 mm Hg (95% confidence interval [CI], 8.65-39.87; P=.002) and diastolic blood pressure (DBP) by 7.8 mm Hg (95% CI, 3.79-11.79; P=.0001) more than placebo. In the 11 single arm studies, AAs reduced SBP by 22.74 mm Hg (95% CI, 18.21-27.27; P <.00001), and DBP by 10.49 mm Hg (95% CI, 8.85–12.13; P <.00001).
The previous year, a randomized, placebo-controlled trial examined the effect of low-dose (25 mg) spironolactone compared with placebo in 161 patients with resistant hypertension.6 At 8 weeks, 73% of those receiving spironolactone reached a goal SBP <140 mm Hg vs 41% of patients on placebo (P=.001). The same proportion (73%) achieved a goal DBP <90 mm Hg in the spironolactone group, compared with 63% of those in the placebo group (P=.223).
Ambulatory BP was likewise assessed and found to be significantly improved among those receiving spironolactone vs placebo, with a decrease in SBP of 9.8 mm Hg (95% CI, -14.2 to -5.4; P<.001), and a 3.2 mm Hg decline in DBP (95% CI, -5.9 to -0.5; P=.013).6
STUDY SUMMARY
First study to compare spironolactone with other drugs
The study by Williams et al—a double-blind, randomized placebo-controlled crossover trial conducted in the UK—was the first RCT to directly compare spironolactone with other medications for the treatment of resistant hypertension in adults already on triple therapy with an ACE inhibitor or ARB, a CCB, and a thiazide diuretic.1 The trial randomized 335 individuals with a mean age of 61.4 years (age range 18 to 79), 69% of whom were male; 314 were included in the intention-to-treat analysis.1
Enrollment criteria for resistant hypertension specified a clinic-recorded SBP of ≥140 mm Hg (or ≥135 mm Hg in those with diabetes) and home SBP (in 18 readings over 4 days) of ≥130 mm Hg.1 To ensure fidelity to treatment protocols, the investigators directly observed therapy, took tablet counts, measured serum ACE activity, and assessed BP measurement technique, with all participants adhering to a minimum of 3 months on a maximally dosed triple regimen.
Diabetes prevalence was 14%; tobacco use was 7.8%; and average weight was 93.5 kg (205.7 lbs).1 Because of the expected inverse relationship between plasma renin and response to AAs, plasma renin was measured at baseline to test whether resistant hypertension was primarily due to sodium retention.1
Participants underwent 4, 12-week rotations
All participants began the trial with 4 weeks of placebo, followed by randomization to 12-week rotations of once daily oral treatment with 1) spironolactone 25 to 50 mg, 2) doxazosin modified release 4 to 8 mg, 3) bisoprolol 5 to 10 mg, and 4) placebo.1 Six weeks after initiation of each study medication, participants were titrated to the higher dose. There was no washout period between cycles.
The primary outcome was mean SBP measured at home on 4 consecutive days prior to the study visits on Weeks 6 and 12. Participants were required to have at least 6 BP measurements per each 6-week period in order to establish a valid average. Primary endpoints included: the difference in home SBP between spironolactone and placebo, the difference in home SBP between spironolactone and the mean of the other 2 drugs, and the difference in home SBP between spironolactone and each of the other 2 drugs.
The results: Spironolactone lowered SBP more than placebo, doxazosin, and bisoprolol (TABLE),1 and clinic measurements were consistent with home BP readings.
Overall, 58% of participants achieved goal SBP <135 mm Hg on spironolactone, compared with 42% on doxazosin, 44% on bisoprolol, and 24% on placebo.1 The effectiveness of spironolactone on SBP reduction was shown to exhibit an inverse relationship to plasma renin levels, a finding that was not apparent with the other 2 study drugs. However, spironolactone had a superior BP lowering effect throughout nearly the entire renin distribution of the cohort. The mean difference between spironolactone and placebo was -10.2 mm Hg; compared with the other drugs, spironolactone lowered SBP, on average, by 5.64 mm Hg more than bisoprolol and doxazosin; 5.3 mm Hg more than doxazosin alone, and 5.98 mm Hg more than bisoprolol alone.
Only 1% of trial participants had to discontinue spironolactone due to adverse events—the same proportion of withdrawals as that for bisoprolol and placebo and 3 times less than for doxazosin.1
WHAT’S NEW
Evidence of spironolactone’s superiority
This is the first RCT to compare spironolactone with 2 other commonly used fourth-line antihypertensives—bisoprolol and doxazosin—in patients with resistant hypertension. The study demonstrated clear superiority of spironolactone in achieving carefully measured ambulatory and clinic-recorded BP targets vs a beta-blocker or an alpha-blocker.
CAVEATS
Findings do not apply across the board
Spironolactone is contraindicated in patients with severe renal impairment. Although multiple drug trials have demonstrated the drug’s safety and effectiveness, especially in patients with resistant hypertension, we should factor in the need for monitoring electrolytes and renal function within weeks of initiating treatment and periodically thereafter.7,8 In this study, spironolactone increased potassium levels, on average, by 0.45 mmol/L. No gynecomastia (typically seen in about 6% of men) was found in those taking spironolactone for a 12-week cycle.1
This single trial enrolled mostly Caucasian men with a mean age of 61 years. Although smaller observational studies that included African American patients have shown promising results for spironolactone, the question of external validity or applicability to a diverse population has yet to be decisively answered.9
CHALLENGES TO IMPLEMENTATION
Potential for adverse reactions, lack of patient-oriented results
The evidence supporting this change in practice has been accumulating for the past few years. However, physicians treating patients with resistant hypertension may have concerns about hyperkalemia, gynecomastia, and effects on renal function. More patient-oriented evidence is likewise needed to assist with the revision of guidelines and wider adoption of AAs by primary care providers.
ACKNOWLEDGEMENT
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). (NICE), National Institute for Health and Care Excellence. 2011. Available at: https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
1. Williams B, MacDonald TM, Morant S, et al. Spironolactone versus placebo, bisoprolol, and doxazosin to determine the optimal treatment for drug-resistant hypertension (PATHWAY-2): a randomised, double-blind, crossover trial. Lancet. 2015;386:2059-2068.
2. Rosa J, Widimsky P, Tousek P, et al. Randomized comparison of renal denervation versus intensified pharmacotherapy including spironolactone in true-resistant hypertension: six-month results from the Prague-15 Study. Hypertension. 2015;65:407-413.
3. James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. JAMA. 2014;311:507-520.
4. Hypertension in adults: diagnosis and management (Clinical Guideline CG127). (NICE), National Institute for Health and Care Excellence. 2011. Available at: https://www.nice.org.uk/guidance/cg127. Accessed March 4, 2016.
5. Dahal K, Kunwar S, Rijal J, et al. The effects of aldosterone antagonists in patients with resistant hypertension: a meta-analysis of randomized and nonrandomized studies. Am J Hypertens. 2015;28:1376-1385.
6. Václavík J, Sedlák R, Jarkovský J, et al. Effect of spironolactone in resistant arterial hypertension: a randomized, double-blind, placebo-controlled trial (ASPIRANT-EXT). Medicine (Baltimore). 2014;93:e162.
7. Wei L, Struthers AD, Fahey T, et al. Spironolactone use and renal toxicity: population based longitudinal analysis. BMJ. 2010;340:c1768.
8. Oxlund CS, Henriksen JE, Tarnow L, et al. Low dose spironolactone reduces blood pressure in patients with resistant hypertension and type 2 diabetes mellitus. J Hypertens. 2013;31:2094-2102.
9. Nishizaka M, Zaman MA, Calhoun DA. Efficacy of low-dose spironolactone in subjects with resistant hypertension. Am J Hypertens. 2003;16:925-930.
Copyright © 2016. The Family Physicians Inquiries Network. All rights reserved.