User login
Update on Guidelines and Technology in the Diagnosis and Treatment of Trichomonas vaginalis
The Centers for Disease Control and Prevention (CDC) published an update to the Sexually Transmitted Diseases Treatment Guidelines in June 2015. This supplement covers the specific changes related to the diagnosis and treatment of Trichomonas vaginalis infection.
Click here to download the PDF.
Sharon L. Hillier, PhD
Professor
Departments of Obstetrics, Gynecology and
Reproductive Sciences and Microbiology
and Molecular Genetics
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Claire Danby, MD, MSc
Assistant Professor
Tufts University School of Medicine
Boston, Massachusetts
Department of Obstetrics and Gynecology
Maine Medical Center
Portland, Maine
Paul Nyirjesy, MD
Professor
Departments of Obstetrics and Gynecology and Medicine
Director, Drexel Vaginitis Center
Drexel University College of Medicine
Philadelphia, Pennsylvania
Maria Trent, MD, MPH
Associate Professor of Pediatrics
Division of General Pediatrics and Adolescent Medicine
Johns Hopkins School of Medicine
Baltimore, Maryland
Disclosures: Dr. Hillier reports that she is a consultant to Perrigo and Symbiomix and has an ongoing relationship with Becton Dickinson, Cepheid, and Hologic. Dr. Danby reports no financial relationships relevant to this article. Dr. Nyirjesy reports that he is a consultant to Hologic and has received a research grant from Becton Dickinson. Dr. Trent reports that she is the Principal Investigator of an unrestricted research grant to Johns Hopkins University (employer) from Hologic, Inc.
The Centers for Disease Control and Prevention (CDC) published an update to the Sexually Transmitted Diseases Treatment Guidelines in June 2015. This supplement covers the specific changes related to the diagnosis and treatment of Trichomonas vaginalis infection.
Click here to download the PDF.
Sharon L. Hillier, PhD
Professor
Departments of Obstetrics, Gynecology and
Reproductive Sciences and Microbiology
and Molecular Genetics
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Claire Danby, MD, MSc
Assistant Professor
Tufts University School of Medicine
Boston, Massachusetts
Department of Obstetrics and Gynecology
Maine Medical Center
Portland, Maine
Paul Nyirjesy, MD
Professor
Departments of Obstetrics and Gynecology and Medicine
Director, Drexel Vaginitis Center
Drexel University College of Medicine
Philadelphia, Pennsylvania
Maria Trent, MD, MPH
Associate Professor of Pediatrics
Division of General Pediatrics and Adolescent Medicine
Johns Hopkins School of Medicine
Baltimore, Maryland
Disclosures: Dr. Hillier reports that she is a consultant to Perrigo and Symbiomix and has an ongoing relationship with Becton Dickinson, Cepheid, and Hologic. Dr. Danby reports no financial relationships relevant to this article. Dr. Nyirjesy reports that he is a consultant to Hologic and has received a research grant from Becton Dickinson. Dr. Trent reports that she is the Principal Investigator of an unrestricted research grant to Johns Hopkins University (employer) from Hologic, Inc.
The Centers for Disease Control and Prevention (CDC) published an update to the Sexually Transmitted Diseases Treatment Guidelines in June 2015. This supplement covers the specific changes related to the diagnosis and treatment of Trichomonas vaginalis infection.
Click here to download the PDF.
Sharon L. Hillier, PhD
Professor
Departments of Obstetrics, Gynecology and
Reproductive Sciences and Microbiology
and Molecular Genetics
University of Pittsburgh School of Medicine
Pittsburgh, Pennsylvania
Claire Danby, MD, MSc
Assistant Professor
Tufts University School of Medicine
Boston, Massachusetts
Department of Obstetrics and Gynecology
Maine Medical Center
Portland, Maine
Paul Nyirjesy, MD
Professor
Departments of Obstetrics and Gynecology and Medicine
Director, Drexel Vaginitis Center
Drexel University College of Medicine
Philadelphia, Pennsylvania
Maria Trent, MD, MPH
Associate Professor of Pediatrics
Division of General Pediatrics and Adolescent Medicine
Johns Hopkins School of Medicine
Baltimore, Maryland
Disclosures: Dr. Hillier reports that she is a consultant to Perrigo and Symbiomix and has an ongoing relationship with Becton Dickinson, Cepheid, and Hologic. Dr. Danby reports no financial relationships relevant to this article. Dr. Nyirjesy reports that he is a consultant to Hologic and has received a research grant from Becton Dickinson. Dr. Trent reports that she is the Principal Investigator of an unrestricted research grant to Johns Hopkins University (employer) from Hologic, Inc.
Number of U.S. tuberculosis cases increased in 2015
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
For the first time in 20 years, incidence of tuberculosis in the United States increased slightly in 2015, according to investigators from the Centers for Disease Control and Prevention.
In 2015, 9,563 cases of TB were reported in the United States, up 1.7% from the 9,406 cases reported in 2014. Texas saw the largest total increase in TB cases, going from 1,269 cases in 2014 to 1,334 cases in 2015, followed by South Carolina and Michigan, which both had 25 more TB cases in 2015 than in 2014. Vermont saw the largest relative increase, going from two cases in 2014 to seven cases in 2015, an increase of 250%.
Among U.S.-born patients, the largest number of TB cases were reported in black non-Hispanics, although the incidence rate was highest in Native Hawaiian/other Pacific Islanders at 8.4/100,000 people. For foreign-born patients, Mexico was the most common origin country, followed by the Philippines, India, Vietnam, and China. Incidence rate was significantly higher for patients from Asian countries than from any other region.
“Resuming declines in TB incidence will require more comprehensive public health approaches, both globally and domestically. These include increasing case detection and cure rates globally, reducing TB transmission in institutional settings such as health care settings and correctional facilities, and increasing detection and treatment of preexisting latent TB infection among the U.S. populations most affected by TB,” the CDC investigators said.
Find the full report in the MMWR (doi: 10.15585/mmwr.mm6511a2).
FROM THE MMWR
Endoscopic techniques provide organ-sparing options for myotomy, resection, and more
Minimally invasive endoscopic procedures are transforming gastrointestinal surgery by offering organ-sparing approaches for a range of pathologies. At the 2016 AGA Tech Summit, Dr. David Rattner updated attendees on three of these techniques – endoscopic submucosal dissection (ESD), peroral endoscopic myotomy (POEM), and endoscopic full-thickness resection (EFTR).
“It is likely that endoluminal treatments, combined with better understanding of molecular pathology, will decrease the need for radical surgical procedures for some common gastrointestinal conditions,” he said in an interview before the meeting, which is sponsored by the AGA Center for GI Innovation and Technology. Dr. Rattner is chief of the division of gastrointestinal and general surgery at Massachusetts General Hospital in Boston.
Dr. Jose Martinez put transorifice surgery in historical perspective and pointed the way forward in an accompanying presentation. “The collaboration with physicians and industry has really started to come along with the natural orifice approach,” said Dr. Martinez, professor of surgery at the University of Miami Health System.
Developed in the 1980s and substantially refined in the mid-2000s, ESD pioneered the endoscopic resection of entire intramucosal tumors without the need for open surgery. In a recent meta-analysis, ESD achieved significantly higher rates of en bloc resection than endoscopic mucosal resection (EMR), with significantly lower rates of local recurrence and comparable complication rates (World J Gastroenterol. 2014;20:8282-7).
ESD involves resecting the mucosa and much of the submucosa, while conserving the muscular layer of the esophagus. In contrast, during POEM, the surgeon intentionally divides the muscular layer, while the intact mucosa provides the barrier between the mediastinum or peritoneum and the esophageal lumen. The defining feature of POEM is the creation of a submucosal tunnel to access the muscle layer for myotomy without incising the skin.
POEM has been found highly successful for treating esophageal achalasia. In fact, in a recently published series of 500 cases, the success rate was 100%, even though the cohort included elderly patients and those with sigmoid esophagus and complex treatment histories (J Am Coll Surg. 2015;221:256-64). Two months post-treatment, average Eckardt scores had decreased by an average of 5.0, and lower esophageal sphincter pressures had fallen by nearly 50%. The adverse event rate was 3%, there were no fatalities, and improvements persisted 3 years later. “Nationwide, numerous centers have incorporated this technique and gotten significant positive results,” said Dr. Martinez.
“POEM will be shown to be as effective as any other currently existing therapy for the treatment of achalasia,” emphasized Dr. Rattner. Indeed, its success has sparked investigations of submucosal tunneling for other indications, such as tumor resection and pyloromyotomy for gastroparesis (World J Gastroenterol. 2014;20:17746-55).
Another cutting-edge technique is EFTR, described as a powerful tool not only for acquiring diagnostic tissue, but also for sparing some patients from surgery. Compared with ESD, it has the potential for higher diagnostic yield of full-thickness specimens, such as in cases of nonlifting adenomas, adenomas at difficult anatomic locations, T1-carcinomas, or submucosal colorectal tumors. For years, a lack of safe techniques and devices kept EFTR from entering routine clinical practice. But intensive research on natural orifice translumenal endoscopic surgery (NOTES) has helped propel innovations such as over-the-scope clips for closing incisions, and smaller, more maneuverable, over-the-scope full-thickness resection devices. Large-scale trials of EFTR are lacking, but in a recent report of 19 consecutive colonic submucosal tumors, EFTR enabled the removal of the entire tumor with capsule intact in 18 instances (Endoscopy. 2013;45[09]:770-3). Notably, colonic wall defects could be closed endoscopically in 16 of 18 cases.
“We are still a ways away from being able to perform EFTR on a routine basis, but new technologies are under development that will make this possible,” said Dr. Rattner. Agreeing that the future of transorifice surgery is bright and growing, Dr. Martinez said, “Strictures, obstructions, bleeding, foreign bodies are now fully resolved through endoscopic approaches.”
Minimally invasive endoscopic procedures are transforming gastrointestinal surgery by offering organ-sparing approaches for a range of pathologies. At the 2016 AGA Tech Summit, Dr. David Rattner updated attendees on three of these techniques – endoscopic submucosal dissection (ESD), peroral endoscopic myotomy (POEM), and endoscopic full-thickness resection (EFTR).
“It is likely that endoluminal treatments, combined with better understanding of molecular pathology, will decrease the need for radical surgical procedures for some common gastrointestinal conditions,” he said in an interview before the meeting, which is sponsored by the AGA Center for GI Innovation and Technology. Dr. Rattner is chief of the division of gastrointestinal and general surgery at Massachusetts General Hospital in Boston.
Dr. Jose Martinez put transorifice surgery in historical perspective and pointed the way forward in an accompanying presentation. “The collaboration with physicians and industry has really started to come along with the natural orifice approach,” said Dr. Martinez, professor of surgery at the University of Miami Health System.
Developed in the 1980s and substantially refined in the mid-2000s, ESD pioneered the endoscopic resection of entire intramucosal tumors without the need for open surgery. In a recent meta-analysis, ESD achieved significantly higher rates of en bloc resection than endoscopic mucosal resection (EMR), with significantly lower rates of local recurrence and comparable complication rates (World J Gastroenterol. 2014;20:8282-7).
ESD involves resecting the mucosa and much of the submucosa, while conserving the muscular layer of the esophagus. In contrast, during POEM, the surgeon intentionally divides the muscular layer, while the intact mucosa provides the barrier between the mediastinum or peritoneum and the esophageal lumen. The defining feature of POEM is the creation of a submucosal tunnel to access the muscle layer for myotomy without incising the skin.
POEM has been found highly successful for treating esophageal achalasia. In fact, in a recently published series of 500 cases, the success rate was 100%, even though the cohort included elderly patients and those with sigmoid esophagus and complex treatment histories (J Am Coll Surg. 2015;221:256-64). Two months post-treatment, average Eckardt scores had decreased by an average of 5.0, and lower esophageal sphincter pressures had fallen by nearly 50%. The adverse event rate was 3%, there were no fatalities, and improvements persisted 3 years later. “Nationwide, numerous centers have incorporated this technique and gotten significant positive results,” said Dr. Martinez.
“POEM will be shown to be as effective as any other currently existing therapy for the treatment of achalasia,” emphasized Dr. Rattner. Indeed, its success has sparked investigations of submucosal tunneling for other indications, such as tumor resection and pyloromyotomy for gastroparesis (World J Gastroenterol. 2014;20:17746-55).
Another cutting-edge technique is EFTR, described as a powerful tool not only for acquiring diagnostic tissue, but also for sparing some patients from surgery. Compared with ESD, it has the potential for higher diagnostic yield of full-thickness specimens, such as in cases of nonlifting adenomas, adenomas at difficult anatomic locations, T1-carcinomas, or submucosal colorectal tumors. For years, a lack of safe techniques and devices kept EFTR from entering routine clinical practice. But intensive research on natural orifice translumenal endoscopic surgery (NOTES) has helped propel innovations such as over-the-scope clips for closing incisions, and smaller, more maneuverable, over-the-scope full-thickness resection devices. Large-scale trials of EFTR are lacking, but in a recent report of 19 consecutive colonic submucosal tumors, EFTR enabled the removal of the entire tumor with capsule intact in 18 instances (Endoscopy. 2013;45[09]:770-3). Notably, colonic wall defects could be closed endoscopically in 16 of 18 cases.
“We are still a ways away from being able to perform EFTR on a routine basis, but new technologies are under development that will make this possible,” said Dr. Rattner. Agreeing that the future of transorifice surgery is bright and growing, Dr. Martinez said, “Strictures, obstructions, bleeding, foreign bodies are now fully resolved through endoscopic approaches.”
Minimally invasive endoscopic procedures are transforming gastrointestinal surgery by offering organ-sparing approaches for a range of pathologies. At the 2016 AGA Tech Summit, Dr. David Rattner updated attendees on three of these techniques – endoscopic submucosal dissection (ESD), peroral endoscopic myotomy (POEM), and endoscopic full-thickness resection (EFTR).
“It is likely that endoluminal treatments, combined with better understanding of molecular pathology, will decrease the need for radical surgical procedures for some common gastrointestinal conditions,” he said in an interview before the meeting, which is sponsored by the AGA Center for GI Innovation and Technology. Dr. Rattner is chief of the division of gastrointestinal and general surgery at Massachusetts General Hospital in Boston.
Dr. Jose Martinez put transorifice surgery in historical perspective and pointed the way forward in an accompanying presentation. “The collaboration with physicians and industry has really started to come along with the natural orifice approach,” said Dr. Martinez, professor of surgery at the University of Miami Health System.
Developed in the 1980s and substantially refined in the mid-2000s, ESD pioneered the endoscopic resection of entire intramucosal tumors without the need for open surgery. In a recent meta-analysis, ESD achieved significantly higher rates of en bloc resection than endoscopic mucosal resection (EMR), with significantly lower rates of local recurrence and comparable complication rates (World J Gastroenterol. 2014;20:8282-7).
ESD involves resecting the mucosa and much of the submucosa, while conserving the muscular layer of the esophagus. In contrast, during POEM, the surgeon intentionally divides the muscular layer, while the intact mucosa provides the barrier between the mediastinum or peritoneum and the esophageal lumen. The defining feature of POEM is the creation of a submucosal tunnel to access the muscle layer for myotomy without incising the skin.
POEM has been found highly successful for treating esophageal achalasia. In fact, in a recently published series of 500 cases, the success rate was 100%, even though the cohort included elderly patients and those with sigmoid esophagus and complex treatment histories (J Am Coll Surg. 2015;221:256-64). Two months post-treatment, average Eckardt scores had decreased by an average of 5.0, and lower esophageal sphincter pressures had fallen by nearly 50%. The adverse event rate was 3%, there were no fatalities, and improvements persisted 3 years later. “Nationwide, numerous centers have incorporated this technique and gotten significant positive results,” said Dr. Martinez.
“POEM will be shown to be as effective as any other currently existing therapy for the treatment of achalasia,” emphasized Dr. Rattner. Indeed, its success has sparked investigations of submucosal tunneling for other indications, such as tumor resection and pyloromyotomy for gastroparesis (World J Gastroenterol. 2014;20:17746-55).
Another cutting-edge technique is EFTR, described as a powerful tool not only for acquiring diagnostic tissue, but also for sparing some patients from surgery. Compared with ESD, it has the potential for higher diagnostic yield of full-thickness specimens, such as in cases of nonlifting adenomas, adenomas at difficult anatomic locations, T1-carcinomas, or submucosal colorectal tumors. For years, a lack of safe techniques and devices kept EFTR from entering routine clinical practice. But intensive research on natural orifice translumenal endoscopic surgery (NOTES) has helped propel innovations such as over-the-scope clips for closing incisions, and smaller, more maneuverable, over-the-scope full-thickness resection devices. Large-scale trials of EFTR are lacking, but in a recent report of 19 consecutive colonic submucosal tumors, EFTR enabled the removal of the entire tumor with capsule intact in 18 instances (Endoscopy. 2013;45[09]:770-3). Notably, colonic wall defects could be closed endoscopically in 16 of 18 cases.
“We are still a ways away from being able to perform EFTR on a routine basis, but new technologies are under development that will make this possible,” said Dr. Rattner. Agreeing that the future of transorifice surgery is bright and growing, Dr. Martinez said, “Strictures, obstructions, bleeding, foreign bodies are now fully resolved through endoscopic approaches.”
AT THE 2016 AGA TECH SUMMIT
The ‘worried well’ and the ‘walking wounded’: How will we know them?; ‘Struggling with inner demons’
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
The ‘worried well’ and the ‘walking wounded’: How will we know them?
One of Dr. Henry A. Nasrallah’s resolutions (16 New Year’s resolutions for psychiatrists in 2016, From the Editor, January 2016, p. 23,24) stated that a significant percentage of one’s practice should be dedicated to the sickest patients, followed by the statement, “There are enough non-physician mental health professionals to handle the walking wounded and worried well.”
Who are the “walking wounded” and the “worried well”? These are commonly used terms, but who falls into these categories? I think it is important to get a sense of who is in these groups, because my takeaway from this editorial is that it is acceptable to let the walking wounded and worried well be treated by lesser-trained clinicians. Do these terms refer to a diagnostic group? Level of functioning? Severity of symptoms? Or severity plus chronicity? Level of suffering? Ability to “fake” looking less severe?
I wonder, am I a walking wounded or worried well? Are some of my friends, or my family members? When I see a patient, I ask myself if he (she) might be in that category.
Susan Fredriksen, MD
Private Practice
Hayesville, North Carolina
Dr. Nasrallah responds
I use those terms to refer to persons who have psychiatric symptoms but are not disabled socially or vocationally. They deserve a full psychiatric evaluation when they initially seek help, but generally do well with various types of psychotherapy, including cognitive-behavioral therapy, interpersonal therapy, psychodynamic therapy, or dialectic behavior therapy. There are many well-trained psychologists and licensed therapists who can administer those therapies as well as, or better than, some psychiatrists.
I recommended that psychiatrists dedicate a significant percentage (more than 50%) of their practice to more severely ill patients (those with psychosis, bipolar disorder, major depressive disorder, panic disorders, obsessive-compulsive disorder, posttraumatic stress disorder, etc.) because we are the only mental health professionals who can competently integrate biopsychosocial treatments for these patients and administer pharmacotherapeutic agents in addition to non-drug approaches. The supply of psychiatrists is short, and the number of seriously ill patients who need the medical expertise we can provide is large.
Henry A. Nasrallah, MD
Professor and Chair
Department of Psychiatry
Saint Louis University School of Medicine
St. Louis, Missouri
‘Struggling with inner demons’
I would hope that Dr. Nasrallah would understand that the use of the metaphor, “struggling with inner demons,” does not suggest “stupid” (Stop blaming ‘demons’ for bizarre delusions or behavior!, From the Editor, February 2016, p. 19,20,22). A celebrity, or any other person, might be struggling with intense, conflicting emotions that create chaos and distress. I would shudder if I read in The New York Times, “Well known actor’s divorce and drug use clearly leading to hypertrophied amygdala.” The term inner demons does not necessarily imply medieval superstition, but rather a well-established use of creative language.
Ron Samarian, MD
Chief, Department of Psychiatry
William Beaumont Hospital
Royal Oak, Michigan
Chair, Oakland University
William Beaumont Medical School
Rochester, Michigan
Dr. Nasrallah responds
Dr. Samarian missed the reason for my umbrage with the “inner demons” metaphor. As a psychiatrist, educator, and researcher, I am exquisitely sensitive to the poor understanding of mental illness and the rampant stigma associated with psychiatric disorders despite the incredible neurobiologic advances. Thus, I regard the metaphor that employs words like “demons” when describing intense struggles with emotional upheavals and stress as having an unfortunate connotation to the obsolete beliefs that abnormal behavior, thoughts, or mood are due to the devil and his nefarious demons.
I would welcome a metaphor that describes a depressed person as having a shrunken hippocampus, which would regrow with antidepressant or electroconvulsive therapy, because that’s the biologic truth and has no misleading connotations; the same with Dr. Samarian’s example of a hypertrophied amygdala in a person with chronic stress.
U.S. flu activity may be waning
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.
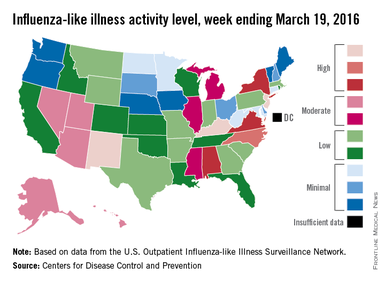
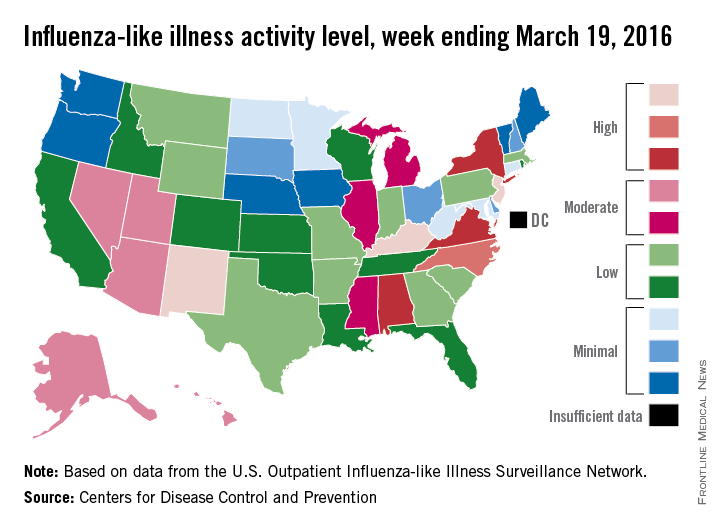
For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.

For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
The 2015-2016 U.S. flu season may have reached its peak. The proportion of outpatient visits for influenza-like illness (ILI) dropped to 3.2% for the week ending March 19, according to the Centers for Disease Control and Prevention.
The drop came after 9 consecutive weeks without a decrease, as the proportion of outpatient visits for ILI topped out at 3.7%, the CDC reported. The national baseline is 2.1%.

For the week ending March 19, three states – Kentucky, New Jersey, and New Mexico – were at level 10 on the CDC’s 1-10 scale of ILI activity, compared with seven the week before. Other states in the “high” range for the week were North Carolina at level 9 and Alabama, New York, and Virginia at level 8, according to data from the CDC’s Influenza-like Illness Surveillance Network (ILINet).
The CDC also reported a cumulative rate of 18.2 laboratory-confirmed influenza-associated hospitalizations per 100,000 population for the 2015-2016 flu season.
Two flu-related pediatric deaths were reported during the most recent week, one of which occurred during the week ending March 5. That brings the total to 30 reported for the 2015-2016 season. For the three previous flu seasons, the pediatric death totals were 148 (2014-2015), 111 (2013-2014), and 171 (2012-2013), according to the CDC report.
Forget EHRs—Let us get back to the practice of medicine
I completely agree with Dr. Selinger in his letter, “I will click those boxes, but first, I will care for my patient” (J Fam Pract. 2015;64:762). I graduated from medical school in 1969 and enjoyed the actual “laying on of hands” that characterized medicine at that time. Now that electronic health records (EHRs) are mandated, much of our time is spent as data entry personnel, rather than as physicians. Personally, I couldn’t stand it; I went into medicine to care for patients, not computers. I left medicine, as I am sure many of my fellow physicians have.
How did we allow EHRs to enter our field?
I am sure that there are many people who believe that EHRs allow us to be more efficient and to meet “the rules.” But to that I say, “Baloney!” Let us return to the true practice of medicine.
Deborah R. Ishida, MD
Beverly Hills, Calif
I completely agree with Dr. Selinger in his letter, “I will click those boxes, but first, I will care for my patient” (J Fam Pract. 2015;64:762). I graduated from medical school in 1969 and enjoyed the actual “laying on of hands” that characterized medicine at that time. Now that electronic health records (EHRs) are mandated, much of our time is spent as data entry personnel, rather than as physicians. Personally, I couldn’t stand it; I went into medicine to care for patients, not computers. I left medicine, as I am sure many of my fellow physicians have.
How did we allow EHRs to enter our field?
I am sure that there are many people who believe that EHRs allow us to be more efficient and to meet “the rules.” But to that I say, “Baloney!” Let us return to the true practice of medicine.
Deborah R. Ishida, MD
Beverly Hills, Calif
I completely agree with Dr. Selinger in his letter, “I will click those boxes, but first, I will care for my patient” (J Fam Pract. 2015;64:762). I graduated from medical school in 1969 and enjoyed the actual “laying on of hands” that characterized medicine at that time. Now that electronic health records (EHRs) are mandated, much of our time is spent as data entry personnel, rather than as physicians. Personally, I couldn’t stand it; I went into medicine to care for patients, not computers. I left medicine, as I am sure many of my fellow physicians have.
How did we allow EHRs to enter our field?
I am sure that there are many people who believe that EHRs allow us to be more efficient and to meet “the rules.” But to that I say, “Baloney!” Let us return to the true practice of medicine.
Deborah R. Ishida, MD
Beverly Hills, Calif
Screening for parasitic infections: One doctor’s experience
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
Soin, et al, reported an interesting case of strongyloidiasis in a refugee in their Photo Rounds article, “Rash, diarrhea, and eosinophilia” (J Fam Pract. 2015;64:655-658). They mentioned the importance of having a high degree of suspicion for parasitic infections among refugees. Indeed, health screenings for refugees are necessary and should include testing for parasitoses. However, there are several other issues to consider.
First, a single screening may not be effective. Thus, results should be verified with repeat screening tests. In my experience in Thailand, a single screening of migrants from nearby Indochinese countries failed to detect several infectious cases, including tuberculosis, malaria, and intestinal parasite infections. To optimize early detection and infection control, a repeated check-up system is needed. It should be noted, however, that a false-negative result for strongyloidiasis is not common from a stool examination or immunological test.1
Second, the mentioned symptoms of “rash, diarrhea, and eosinophilia” can be due to several etiologies and may have been caused by a completely separate illness. Or the findings might have been due to a forgotten condition, such as post-dengue infection illness.2
Finally, the existence of strongyloidiasis in the case presented by Soin, et al, could have been an incidental finding without a relationship to the exact pathology.
Viroj Wiwanitkit, MD
Bangkok, Thailand
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
1. Rodriguez EA, Abraham T, Williams FK. Severe strongyloidiasis with negative serology after corticosteroid treatment. Am J Case Rep. 2015;16:95-98.
2. Wiwanitkit V. Dengue fever: diagnosis and treatment. Expert Rev Anti Infect Ther. 2010;8:841-845.
An unconscious bias in this EHR study?
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
Like many physicians, I struggle with looking at my patients while they are talking and getting the stories that they tell me transcribed as accurately and completely as possible. After I read the article, “EHR use and patient satisfaction: What we learned” by Farber, et al, (J Fam Pract. 2015;64:687-696), I was struck by something.
Of the 126 patients chosen for the research, the educational level breakdown included 75% with at least some college education and 28% with postgraduate education. A study performed by the National Center for Veterans Analysis and Statistics published in 2015 has different statistics.1 Although a similar percentage had at least some college education, only 10.5% of the men and 12.4% of the women had postgraduate education.
In my practice, most of my patients who have worked with computers empathize with the amount of time that I spend looking at the screen. Those with less education are less agreeable. Since the patients were picked by their physicians to take part in the study, I wonder if there was an unconscious bias present.
Holly Leeds, MD
Auburn, Calif
1. National Center for Veterans Analysis and Statistics. Profile of Veterans: 2013. US Department of Veterans Affairs Web site. Available at: http://www.va.gov/vetdata/docs/SpecialReports/Profile_of_Veterans_2013.pdf. Accessed March 21, 2016.
Author's response:
Dr. Leeds brings up an interesting issue. It is possible that there is an unconscious bias on the part of physicians who participated in this study. Although the demographics are fairly similar to those that she cites, the veterans in our study were somewhat more educated.
If less well-educated subjects participated, this would make the data more impressive, in terms of less satisfaction with physicians who more readily focus their eyes on computer screens rather than on their patients. The fact that we did find this association is important for physicians who use EHR systems.
Neil J. Farber, MD, FACP
San Diego, Calif
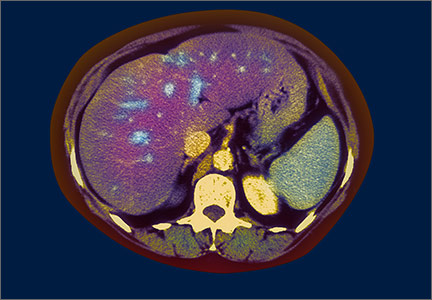
Non-alcoholic fatty liver disease: What’s in our arsenal?
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
› Screen patients with non-alcoholic fatty liver disease (NAFLD) for type 2 diabetes mellitus. A
› Treat components of the metabolic syndrome to improve the clinical outcome in patients with NAFLD. A
› Consider liver-directed pharmacotherapy, such as antioxidants (eg, vitamin E), insulin sensitizers, bile acid sequestrants, and pentoxifylline, to treat severe NAFLD. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE › A 39-year-old Hispanic man with a body mass index (BMI) of 35 kg/m2, type 2 diabetes mellitus (T2DM), and hypertension is referred for evaluation of abnormal liver function tests (LFTs) and fatty liver on ultrasound. He is taking metformin and lisinopril, and a patient alcohol screening survey is negative. LFT results reveal the following: alanine aminotransferase (ALT) 27 IU/dL; aspartate aminotransferase (AST) 43 IU/dL; albumin 4.2 g/dL; gamma glutamyl transferase 22 u/L; alkaline phosphatase 51 IU/L; and total bilirubin 0.3 mg/dL. Lactate dehydrogenase and prothrombin time are normal.
Results of his liver screen are as follows: hepatitis B surface antigen, hepatitis C antibody, antimitochondrial antibody, and anti-smooth muscle antibody are negative, and iron, transferrin saturation, and ceruloplasmin are in normal range. Antinuclear antibody (1:20 dilution) is weakly positive, and alpha-1 antitrypsin (264 mg/dL) and serum ferritin (300 ng/mL) are mildly increased.
The patient undergoes a liver biopsy that shows grade 2 steatosis, grade 1 lobular inflammation, few ballooned hepatocytes, and stage 1 fibrosis. Based on these clinical findings, he is given a diagnosis of non-alcoholic fatty liver disease (NAFLD).
NAFLD is the most frequent cause of chronic liver disease both in the United States and globally.1 In fact, a number of long-term epidemiologic studies report that nearly one-third of the US population has the disease.2 The spectrum of NAFLD ranges from simple steatosis to non-alcoholic steatohepatitis (NASH) to cirrhosis. Of patients with NAFLD, 10% to 30% have the more severe form—NASH—and about 10% of those with NASH progress to cirrhosis and other liver-related complications.3
People with NAFLD consume no alcohol, or only a modest amount (ie, weekly intake <140 g in women and <210 g in men). Typically, they are asymptomatic with normal or mildly abnormal LFTs discovered as part of a preventive health screening. In patients with simple hepatic steatosis alone, serum ALT levels are higher than serum AST levels. (In contrast, patients with alcoholic liver injury and NASH with progressive fibrosis have higher serum AST than ALT levels.) A serum hepatitis panel and liver screen are negative for other explanations of chronic liver disease.
NAFLD is strongly associated with obesity, insulin resistance/T2DM, and hyperlipidemia, all of which are components of metabolic syndrome. Obesity, particularly central obesity, is highly predictive of hepatic steatosis and disease progression.4 T2DM occurs 5 to 9 times more frequently in people with NAFLD than in the general population,5 and, conversely, nearly 66% of patients with T2DM have NAFLD.6,7 Furthermore, nearly 70% of patients with T2DM develop fatty liver and its consequences, including NASH, fibrosis, cirrhosis, and hepatocellular carcinoma.5,7
4 therapeutic strategies. Based on our current understanding of the pathogenesis of NAFLD, there are 4 main therapeutic avenues: lifestyle modification, liver-directed pharmacotherapy, management of metabolic syndrome, and surveillance of the complications of cirrhosis. The review that follows explores the evidence to date for each.
Take steps to reduce weight and increase physical activity
The primary objective with NAFLD is to right the imbalance between calorie intake and utilization so as to reverse the obesity and insulin resistance underlying the disease.
Target carbohydrates. Current data clearly suggest that energy intake is significantly higher in patients with NAFLD than in those without the disease.8 Thus, reducing dietary carbohydrate and overall energy intake is beneficial to preventing and halting the progression of liver damage. Increased intake of high fructose corn syrup may be at least partially to blame; research has linked the substance to the occurrence of obesity, metabolic syndrome, and NAFLD.9
The optimal diet to treat NAFLD is not known because of the difficulties inherent to performing well-designed dietary intervention trials and ensuring long-term compliance. At least one study reported that a Mediterranean diet helped reduce hepatic steatosis and improve insulin sensitivity in nondiabetic individuals.10 Generally, patients should avoid saturated fats, simple carbohydrates, and sweetened drinks, and they should be instructed to restrict calories to cause weight loss of about .5 kg to 1 kg per week until the target weight is achieved.11
Current observational studies indicate that prudent calorie restriction combined with increased physical activity is the best strategy for achieving and sustaining optimum body weight; severe calorie restriction is likely to cause skeletal muscle loss that can aggravate NAFLD.
Encourage exercise. Aerobic exercise improves skeletal muscle insulin sensitivity—the primary underlying mechanism that causes NAFLD.12 Although the optimum duration and intensity of exercise is not known, several randomized controlled trials (RCTs) found that moderately intense training, high-intensity training, and/or resistance training improved hepatic steatosis and insulin resistance, but an effect on ALT was inconsistent.13 (None of these studies included histology as an outcome measure.)
Given the multitude of benefits of aerobic exercise, there is no question that patients with NAFLD should try to increase their physical activity and incorporate exercise into their daily routine.
Hold off on pharmacologic weight loss. Orlistat, an enteric lipase inhibitor, causes malabsorption of dietary fat, which leads to weight loss. Although one study demonstrated that orlistat improves ALT and steatosis in patients with NAFLD, a subsequent RCT concluded that orlistat with caloric restriction and vitamin E (800 IU/d) did not enhance weight loss over caloric restriction and vitamin E alone.14 Additionally, in patients with weight loss >9% of body weight, histologic improvement occurred independent of orlistat.14 Therefore, orlistat is not currently recommended for weight loss in patients with NAFLD.
Keep bariatric surgery on your radar. Bariatric-metabolic surgery provides the most reliable method for achieving sustained weight loss in morbidly obese individuals with NAFLD. Commonly used surgical procedures are associated with reduced steatosis and lobular inflammatory changes, but reports are conflicting regarding fibrosis.15
The majority of published data indicate that bariatric surgery improves the histologic and metabolic changes associated with NAFLD and has potential as a treatment option for patients with morbid obesity and NAFLD. However, the timing and type of surgery that is most effective, and whether bariatric surgery can cure the disease, remain unanswered questions. Long-term follow-up and RCTs are needed to address these issues. As a result, no definitive recommendations regarding bariatric surgery as a treatment for NAFLD can be made at this time.15
Liver-directed pharmacotherapy: Evidence is lacking for many agents
Lifestyle modification remains the mainstay of therapy for NAFLD because of its efficacy and lack of adverse effects. But low compliance rates often make pharmacotherapy necessary to reduce the health burden related to NAFLD. Despite the success rate of pharmacologic agents that focus on insulin resistance and lipid metabolism and that have antioxidant properties, the long-term safety and efficacy of many of these agents is largely unknown. Furthermore, the FDA has not approved any pharmacologic agents specifically for the treatment of NAFLD. Here’s what we know:
Vitamin E. Five RCTs have evaluated the antioxidant vitamin E in patients with NASH. The best study published to date found that 96 weeks of therapy with 800 IU/d vitamin E was associated with a 42% improvement in hepatic histology, compared with 19% improvement in the placebo group.16 Vitamin E was also associated with improved serum ALT.
Although vitamin E seems to be a promising agent for the treatment of NASH, concerns exist about its long-term safety because of an increased risk of all-cause mortality and hemorrhagic stroke.17 In addition, because the optimal dose and duration of treatment is unknown and because studies have not evaluated the supplement in patients who have diabetes and NASH, vitamin E is not currently considered to be a standard therapy for NASH.
Insulin sensitizers. Because insulin resistance is believed to be the underlying mechanism for the development and progression of NAFLD, a compelling rationale exists for the use of insulin sensitizers in the management of the disease. Metformin, an activator of adenosine monophosphate-activated protein kinase, and the thiazolidinediones (pioglitazone and rosiglitazone) are the most extensively studied agents in clinical trials. A number of studies looking at the effects of metformin on NAFLD found that liver function, steatosis, and insulin sensitivity improved;18 however, a recent meta-analysis found that metformin failed to improve liver histology.19
Similarly, although clinical trials have shown that thiazolidinediones improve liver enzymes, inflammatory markers, and hepatic steatosis, questions surround their long-term safety.20 The largest placebo-controlled trial on this issue to date—PIVENS (pioglitazone vs vitamin E vs placebo)—found that pioglitazone was beneficial in improving hepatic histology.16 However, the well-recognized adverse effects of pioglitazone (eg, upper respiratory tract infection, edema, and hypoglycemia) may temper its utility.
Clinical trials involving newer antidiabetic agents, such as dipeptidyl peptidase-4 (DPP4) inhibitors and glucagon-like peptide-1 (GLP1) analogues, indicate that such agents improve insulin resistance, steatosis, and inflammation.21 However, these drugs are not considered to be routine therapy because of limited data and the lack of long-term benefits.
Bile acid regulatory agents. Ursodeoxycholic acid (UDCA), a bile acid with antiapoptotic and cytoprotective properties, is used as a hepatoprotectant in NAFLD. Although early studies showed no significant differences in LFT results between UDCA-treated and untreated groups, recent RCTs indicate that UDCA improves ALT and serum fibrosis.22,23 The FLINT trial, a recent multicenter RCT involving obeticholic acid, found that UDCA was associated with improvement in histologic outcomes, although long-term benefits and safety—especially with regard to worsening hyperlipidemia—are questionable.24
Pentoxifylline. Researchers have evaluated pentoxifylline, a hepatoprotectant with anti-tumor necrosis factor effect, in the treatment of NAFLD.25 In fact, pooled results from 5 well-designed studies indicate that pentoxifylline significantly reduces ALT and AST and improves steatosis, lobular inflammation, and fibrosis.26 Although these data suggest that pentoxifylline holds promise as a therapeutic option, the lack of large multicenter studies and FDA approval temper its utility in the management of NASH at this time.
Cholesterol-lowering agents. Statins inhibit hydroxymethylglutaryl-coenzyme A (HMG-CoA) reductase in the liver and have anti-inflammatory and anti-fibrogenic properties. They have been used in patients with NAFLD, primarily because of their cardiovascular benefit. Two RCTs with high risk of bias and a small number of participants found statin therapy to be associated with improved serum transaminases and ultrasound findings; however, liver biopsies were not performed in either of these studies.27
Lowering cholesterol using an absorption inhibitor, such as ezetimibe, was associated with improvement in liver histology in a single RCT.28 Even though statins are not considered to be a treatment for NAFLD, they can be used to safely lower plasma cholesterol in patients with the disease.
Renin-angiotensin system (RAS) inhibitors. Research in animals indicates that activation of the renin-angiotensin system contributes to the pathogenesis of NAFLD, but data on the benefits of angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) in patients with NAFLD are limited, conflicting, and derived largely from retrospective29 and pilot prospective studies.
Based on currently published literature, RAS inhibitors are not considered an NAFLD treatment. However, because cardiovascular disease is a major cause of death in patients with NAFLD, the renal and cardiovascular protection offered by these agents likely lowers mortality in patients with the disease.
Probiotics. The use of probiotics in the treatment of NAFLD is based on the premise that alterations in intestinal microbes and the inflammatory response may improve the disease. Three RCTs involving different formulations of probiotics, synbiotics, or placebo, showed improvement in serum liver markers and insulin resistance, but did not include histologic outcome measures.30 Furthermore, the long-term consequences of altered gut flora are presently unknown. As such, the available evidence does not support the use of probiotics for the treatment of NAFLD.
Polyunsaturated fatty acids (PUFA). Clearly, omega-3 fatty acids have beneficial effects on cardiometabolic risk factors and positively impact lipid metabolism and insulin sensitivity. In addition, a few studies have reported improvement in non-histologic outcome measures of NAFLD, but 2 high-quality RCTs found no benefit of fish oil-based PUFA on histology.31,32 Thus, current evidence does not support recommending PUFA supplementation for the treatment of NAFLD.
Chinese herbal medicines. At least 56 trials have looked at 75 different Chinese herbal medicines in varying formulations, dosages, routes of administration, and durations of treatment, using various controlled interventions.33 No trial reported primary outcomes, such as hepatic-related mortality, morbidity, or health care quality of life. Although a large number of the trials reported some positive effects on various biochemical or radiologic measures, the high risk of bias and the limited number of trials testing individual herbal medicines leave efficacy and safety open to question. As such, no Chinese herbal medicines are regarded as treatment for NAFLD at this time.
Target components of metabolic syndrome
Management of the components of metabolic syndrome remains one of the safest and most effective ways to manage NAFLD. Therefore, screening for and treating T2DM, hypertension, and dyslipidemia are priorities. Although obstructive sleep apnea (OSA) is not part of metabolic syndrome, the condition frequently coexists with metabolic syndrome because both entities have obesity as a risk factor.
T2DM. Screen all patients with NAFLD for T2DM and vice-versa because, as noted earlier, patients with diabetes have more severe and progressive NAFLD, and a high proportion of patients with NAFLD have T2DM.5,6 Although research has not shown metformin to improve histology in NASH, metformin is recommended as a first-line agent for the treatment of T2DM because it aids in weight loss and lowers diabetes-related mortality.34
Pioglitazone is considered a second-line agent. Despite its beneficial effects on insulin sensitivity and hepatic histology, there are concerns about the adverse effects of thiazolidinediones. GLP1 analogues, which improve liver enzymes and reduce hepatic steatosis, are considered third-line agents.
Hypertension. Because approximately 70% of patients with NAFLD have hypertension,35 it is imperative to screen patients for the condition. If blood pressure is >140/90 mm Hg, patients should be managed according to hypertension guidelines. ACE inhibitors or ARBs are recommended as first-line therapy, since blocking the renin-angiotensin system potentially reduces hepatic fibrosis,36 and ARBs may lower transaminases and improve insulin sensitivity in NAFLD.
Dyslipidemia. Treatment of dyslipidemia is essential to lowering cardiovascular mortality in patients with NAFLD. Even though elevated transaminases occur with NAFLD, this should not preclude starting therapy to lower triglycerides to <150 mg/dL and total cholesterol to <200 mg/dL.
OSA. Because of the high prevalence of OSA in patients with NAFLD, physicians should have a high index of suspicion and screen this population for sleep disorders. OSA is associated with an increased risk of NAFLD and advanced fibrosis in NASH.37 Treatment of OSA improves quality of life and controls blood pressure in patients with NAFLD, but it’s currently unclear whether targeting sleep disorders can slow the progression of fibrosis in NAFLD.
Concentrate on the complications of cirrhosis
Patients with NASH cirrhosis, like those with cirrhosis of other etiologies, are at risk for complications, including hepatic encephalopathy, ascites, hepatorenal syndrome, and esophageal variceal hemorrhage. Surveillance to detect these include an annual liver ultrasound, an alpha-fetoprotein test every 6 months, esophagogastroduodenoscopy for varices, and an assessment for liver transplantation. For more on these complications, see, “Cirrhosis complications: Keeping them under control,” J Fam Pract. 2015;64:338-342. NAFLD-associated cirrhosis is the third most frequent indication for liver transplantation in the United States and may become the most frequent indication in the next decade.38
CASE › Because the patient’s liver biopsy showed early NASH, we recommended that he aggressively pursue lifestyle modification, including regular physical activity and dietary changes. Additionally, we discussed optimization of glycemic control and continued use of lisinopril for control of hypertension. On follow-up 6 months later, he had lost weight and his BMI was 32 kg/m2. In addition, his transaminase levels had improved, but they had not normalized.
We recommended that he continue the same measures, with follow-up every 6 months to ensure compliance with lifestyle modifications and with diabetes and hypertension control.
CORRESPONDENCE
Jaividhya Dasarathy, MD, Metro Health Medical Center, 2500 Metro Health Drive, Cleveland, OH 44109; jdasarathy@metrohealth.org.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.
1. Bedogni G, Miglioli L, Masutti F, et al. Prevalence of and risk factors for nonalcoholic fatty liver disease: the Dionysos nutrition and liver study. Hepatology. 2005;42:44-52.
2. Lazo M, Hernaez R, Eberhardt MS, et al. Prevalence of non-alcoholic fatty liver disease in the United States: the Third National Health and Nutrition Examination Survey, 1988-1994. Am J Epidemiol. 2013;178:38-45.
3. Williams CD, Stengel J, Asike MI, et al. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124-131.
4. Wong VW, Wong GL, Choi PC, et al. Disease progression of non-alcoholic fatty liver disease: a prospective study with paired liver biopsies at 3 years. Gut. 2010;59:969-974.
5. Anstee QM, Targher G, Day CP. Progression of NAFLD to diabetes mellitus, cardiovascular disease or cirrhosis. Nat Rev Gastroenterol Hepatol. 2013;10:330-344.
6 Targher G, Bertolini L, Rodella S, et al. Nonalcoholic fatty liver disease is independently associated with an increased incidence of cardiovascular events in type 2 diabetic patients. Diabetes Care. 2007;30:2119-2121.
7. Stefan N, Häring HU. The metabolically benign and malignant fatty liver. Diabetes. 2011;60:2011-2017.
8. Capristo E, Miele L, Forgione A, et al. Nutritional aspects in patients with non-alcoholic steatohepatitis (NASH). Eur Rev Med Pharmacol Sci. 2005;9:265-268.
9. Raben A, Vasilaras TH, Møller AC, et al. Sucrose compared with artificial sweeteners: different effects on ad libitum food intake and body weight after 10 wk of supplementation in overweight subjects. Am J Clin Nutr. 2002;76:721-729.
10. Ryan MC, Itsiopoulos C, Thodis T, et al. The Mediterranean diet improves hepatic steatosis and insulin sensitivity in individuals with non-alcoholic fatty liver disease. J Hepatol. 2013;59:138-143.
11. Centre for Public Health Excellence at NICE. Obesity: The Prevention, Identification, Assessment and Management of Overweight and Obesity in Adults and Children. London: National Institute for Health and Clinical Excellence; 2006.
12. Kirwan JP, Solomon TP, Wojta DM, et al. Effects of 7 days of exercise training on insulin sensitivity and responsiveness in type 2 diabetes mellitus. Am J Physiol Endocrinol Metab. 2009;297:E151-E156.
13. Keating SE, Hackett DA, George J, et al. Exercise and non-alcoholic fatty liver disease: a systematic review and meta-analysis. J Hepatol. 2012;57:157-166.
14. Harrison SA, Fecht W, Brunt EM, et al. Orlistat for overweight subjects with nonalcoholic steatohepatitis: A randomized, prospective trial. Hepatology. 2009;49:80-86.
15. Chavez-Tapia NC, Tellez-Avila FI, Barrientos-Gutierrez T, et al. Bariatric surgery for non-alcoholic steatohepatitis in obese patients. Cochrane Database Syst Rev. 2010:CD007340.
16. Sanyal AJ, Chalasani N, Kowdley KV, et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. N Engl J Med. 2010;362:1675-1685.
17. Schurks M, Glynn RJ, Rist PM, et al. Effects of vitamin E on stroke subtypes: meta-analysis of randomised controlled trials. BMJ. 2010;341:c5702.
18. Han Y, Shi JP, Ma AL, et al. Randomized, vitamin E-controlled trial of bicyclol plus metformin in non-alcoholic fatty liver disease patients with impaired fasting glucose. Clin Drug Investig. 2014;34:1-7.
19. Li Y, Liu L, Wang B, et al. Metformin in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Biomed Rep. 2013;1:57-64.
20. Belfort R, Harrison SA, Brown K, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297-2307.
21. Olaywi M, Bhatia T, Anand S, et al. Novel anti-diabetic agents in non-alcoholic fatty liver disease: a mini-review. Hepatobiliary Pancreat Dis Int. 2013;12:584-588.
22. Troisi G, Crisciotti F, Gianturco V, et al. The treatment with ursodeoxycholic acid in elderly patients affected by NAFLD and metabolic syndrome: a case-control study. Clin Ter. 2013;164:203-207.
23. Ratziu V, de Ledinghen V, Oberti F, et al. A randomized controlled trial of high-dose ursodeoxycholic acid for nonalcoholic steatohepatitis. J Hepatol. 2011;54:1011-1019.
24. Neuschwander-Tetri BA, Loomba R, Sanyal AJ, et al. Farnesoid X nuclear receptor ligand obeticholic acid for non-cirrhotic, non-alcoholic steatohepatitis (FLINT): a multicentre, randomised, placebo-controlled trial. Lancet. 2015;385:946.
25. Zein CO, Yerian LM, Gogate P, et al. Pentoxifylline improves nonalcoholic steatohepatitis: a randomized placebo-controlled trial. Hepatology. 2011;54:1610-1619.
26. Du J, Ma YY, Yu CH, et al. Effects of pentoxifylline on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2014;20:569-577.
27. Eslami L, Merat S, Malekzadeh R, et al. Statins for non-alcoholic fatty liver disease and non-alcoholic steatohepatitis. Cochrane Database Syst Rev. 2013;12:CD008623.
28. akeshita Y, Takamura T, Honda M, et al. The effects of ezetimibe on non-alcoholic fatty liver disease and glucose metabolism: a randomised controlled trial. Diabetologia. 2014;57:878-890.
29. Goh GB, Pagadala MR, Dasarathy J, et al. Renin-angiotensin system and fibrosis in non-alcoholic fatty liver disease. Liver Int. 2015;35:979-985.
30. Ma YY, Li L, Yu CH, et al. Effects of probiotics on nonalcoholic fatty liver disease: a meta-analysis. World J Gastroenterol. 2013;19:6911-6918.
31. Dasarathy S, Dasarathy J, Khiyami A, et al. Double-blind randomized placebo-controlled clinical trial of omega 3 fatty acids for the treatment of diabetic patients with nonalcoholic steatohepatitis. J Clin Gastroenterol. 2015;49:137-144.
32. Sanyal AJ, Abdelmalek MF, Suzuki A, et al. No significant effects of ethyl-eicosapentanoic acid on histologic features of nonalcoholic steatohepatitis in a phase 2 trial. Gastroenterology. 2014;147:377-384.
33. Liu ZL, Xie LZ, Zhu J, et al. Herbal medicines for fatty liver diseases. Cochrane Database Syst Rev. 2013;8:CD009059.
34. National Collaborating Centre for Chronic Conditions. Type 2 Diabetes: National Clinical Guideline for Management in Primary and Secondary Care (Update). London: Royal College of Physicians; 2008.
35. Goh GB, Pagadala MR, Dasarathy J, et al. Clinical spectrum of non-alcoholic fatty liver disease in diabetic and non-diabetic patients. BBA Clin. 2014;3:141-145.
36. Georgescu EF, Ionescu R, Niculescu M. Angiotensin-receptor blockers as therapy for mild-to-moderate hypertension-associated non-alcoholic steatohepatitis. World J Gastroenterol. 2009;15:942-954.
37. Musso G, Cassader M, Olivetti C, et al. Association of obstructive sleep apnoea with the presence and severity of non-alcoholic fatty liver disease. A systematic review and meta-analysis. Obes Rev. 2013;14:417-431.
38. Charlton MR, Burns JM, Pedersen RA, et al. Frequency and outcomes of liver transplantation for nonalcoholic steatohepatitis in the United States. Gastroenterology. 2011;141:1249-1253.