User login
Psychiatry can promote growth amid chronic illness
Promoting resilience, benefit finding, and post-traumatic growth help patients and families cope well with chronic illness. A resilient family has good problem-solving skills and communication, and a shared belief system (Fam Process. 2003; 42:1-18). Benefit finding coexists with feelings of burden, grief, and loss, and post-traumatic growth can occur despite grief and loss. Convening family meetings, for example, is a way to provide a therapeutic space for families and an opportunity to reflect on ways that chronic illness has affected the family, both negatively and positively.
What is coping well?
Resilient families cope well by identifying and solving illness-related problems, communicating about symptoms, negotiating role changes, and developing new interests as a family and new ways of emotionally being together. In addition, resilient families are able to adapt or change their life goals or health behaviors, such as improving diet, increasing exercise, and stopping smoking. A family member may work fewer hours, for example, and older children may give up some childhood activities to take on caregiving responsibilities.
Families do not usually think about how they cope; they just get along the best they can. Families may not consider how each family member’s individual coping style meshes with the coping styles of other family members. Illness management often is punctuated by crises where change happens quickly, without the family having time to deliberate on what coping styles might work best. Family changes can become fixed, therefore, not by choice, but by happenstance.
The initial stage of coping is “assimilative.” This is when the impact of the illness is being understood and absorbed. Emotional coping occurs when distress is highest, for example, at the beginning of an illness – when uncertainty exists about the diagnosis. Another characteristic of emotional coping is that it is characterized by attempts to regulate negative emotions. For example, family members may blame themselves or others, engage in wishful thinking, or become avoidant.
At later stages of illness, coping becomes “accommodative” after attempts to change or cure the illness have been found to be ineffective. Emotional coping can then be replaced with a problem-based coping style, allowing a stressor to be discussed and a solution chosen from several alternatives. Reflective coping, a related concept, is the ability to generate and consider coping options, and to recognize the usefulness of a particular coping strategy in a particular situation.
Psychiatric imperatives
While providing psychotherapy with these couples, it is helpful to:
• Help families differentiate between emotional difficulties such as “I am the primary caregiver, and I feel overburdened,” and more general practical problems, such as “I am happy to be the primary caregiver, but I need some extra help.”
• Describe coping to the family. Coping is a dynamic process, and coping styles change over time. Each person copes in their own way, depending on their experiences of illness and expectations of living with illness. In a family, the experiences and behaviors of all the individuals influence the way the family unit functions as a whole.
• Promote a balance between acceptance and change.
• Encourage the family to talk about their experience with others who are experiencing the same stressors.
• Give the family a handout that outlines different coping styles to get the discussion started.
• Provide a therapeutic space for family members to think together about how they want to cope and what coping well means to their family.
Developing dyadic coping
When dyadic coping takes place, couples cope as a single entity. Dyadic coping research is relatively new; most studies have been conducted in the past 15 years. Couples who use positive dyadic coping employ joint problem solving, joint information seeking, sharing of feelings, mutual commitment, and relaxing together. Meanwhile, couples who use negative dyadic coping hide concerns from each other and avoid shared discussion. A systemic review of dyadic coping in couples with cancer found that couples using positive dyadic coping styles experienced higher relationship functioning (Br J Health Psychol. 2015 Feb;20[1]:85-114).
Couples with good dyadic coping view the illness as “our problem.” This approach necessitates a shared understanding of the illness. The couple usually has prior experience working together as a team, for example, parenting and dividing roles within the house. They are able to relax together and provide emotional support, such as mutual calming and expressions of solidarity. Talking together about one’s worries and needs allows couples to share the experience more adequately. Dyadic coping is reflected in the amount of “we” talk.
The person in the family who copes “best” may provide the model for developing the family coping style. In a study of 66 couples faced with the stress of forced relocation, nearly all the couples adapted to the stress “as a couple,” rather than “as individuals” (Fam Process. 1991 Sep;30[3]:347-61). At the 2-year follow-up, each husband and wife developed similar coping styles, with one individual’s coping ability driving the adjustment of his or her partner. The need to adjust together or to adapt as a couple lessened the stress of relocation. Adaptation occurred through the development of shared meaning of the relocation that emerged from conversation within the couples. In other words, the couple developed a shared worldview. The coping style of the person who coped best was the strongest predictor of adjustment for both members of the couple. For most couples, the style of the person who coped best dominated.
Questions to ask during therapy
While working with these couples, assess their motivation to develop dyadic coping, by asking:
• “Do either of you feel that the patient should do this alone?” If the answer is yes, it will be difficult, if not impossible, to move the couple to a dyadic coping style.
• “Do your efforts to work together result in greater conflict?”
• “How much do you want this to change?” This questions clarifies their motivation to work together.
• “When you think about problems related to your heart condition, to what extent do you view those as ‘our problem’ [shared by the patient and the spouse equally] or mainly as ‘your own problem?’ ”
• “When a problem related to your heart condition arises, to what extent do you and your partner work together to solve it?”
• “When you both talk about the illness, how much do you use ‘we-talk’?”
• “It is important that you both agree about what is causing the illness. Can I answer any questions that might help you reach this understanding?”
• “Are there times in the past where you have successfully solved difficult problems? How did you do that?”
• “How do you respond when your spouse becomes ill?”
• “What can your spouse do that will help you get better?”
• “Can you ask your spouse for help and support?”
• “Can you work on your spouse’s health problem together?”
‘Benefit finding’ and PTG
Benefit finding emerges later in the adjustment to chronic illness. For example, caregivers may develop a greater appreciation of their own health and ability to enjoy their own pursuits. Family connectedness is a frequent source of meaning, and a critical aspect of well-being and benefit finding. Seven factors make up benefit finding: compassion/empathy, spiritual growth, mindfulness, family relations growth, lifestyle gains, personal growth, and new opportunities (Psychol Health. 2009 Apr;24[4]:373-93). Benefit finding is associated with higher marital adjustment, improved life satisfaction, and a more positive affect, especially at high levels of stress.
Post-traumatic growth, or PTG, refers to positive changes that occur after traumatic life events. People who experience PTG are transformed by their struggles with adversity. It is the struggle after the trauma, not the trauma itself, that produces PTG. In contrast to resilience, PTG refers to changes that go beyond pretrauma levels of adaptation and beyond benefit finding. Relational benefits in the aftermath of a cancer diagnosis are well recognized. An instrument used to assess these outcomes is called the Post-traumatic Growth Inventory, or PTGI (J Trauma Stress. 1996 Jul;9:455-71).
Interventions for couples coping with cancer resulted in improvements in communication, dyadic coping, quality of life, psychosocial distress, sexual functioning, and marital satisfaction (Psychooncology. 2014 Apr;23[7]:731-9). PTG may, however, be more apparent in patients than spouses.
Potential interventions
Promoting dyadic coping is effective if the couple wants to engage in intervention. According to one study, a partner-assisted emotional disclosure improved relationship functioning and intimacy (J Marital Fam Ther. 2012 Jun;38 Suppl 1:284-95). Couples therapy improves relational functioning in couples coping with cancer, at 1-year follow-up (Psychooncology. 2009 Mar;18[3]:276-83). Most important, as a first step, the couple must agree that they want to develop dyadic coping. The concept of individual versus dyadic coping may be novel for couples, and it is worth spending time on this review before offering couples intervention.
A psychoeducational program also can teach dyadic coping. The Resilient Partners discussion group developed in collaboration with the Multiple Sclerosis Society focused on developing couples’ strengths in coping with multiple sclerosis (Rolland, J., McPheters, J., and Carbonell, E., 2008). This multifamily group program is based on the Family Systems Illness Model, which integrates the demands of multiple sclerosis over time within a family developmental framework. In a comparison of a couple skills intervention with a psychoeducation program, women in the couple skills intervention benefited more in terms of their relationship functioning (Ann Behav Med. 2012 Apr;43[2]:239-52).
Dr. John S. Rolland’s Family Systems Illness (FSI) model provides a framework for the psychoeducation, assessment, and intervention with families dealing with chronic illness. This model, developed in clinical experience with more than 1,000 families, views families as valued partners and resources, and emphasizes resilience and growth. The FSI model takes into account the interaction of an illness with the individual’s development and the family’s development, the multigenerational ways of coping with illness, the family’s health/illness belief system, available resources, and relationships between health care providers.
The PTGI includes five domains: improved relationships, new possibilities for one’s life, a greater appreciation for life, a greater sense of personal strength, and spiritual development. Several family oriented themes within the PTGI can be used by the psychiatrist to inquire about positive change. These themes are:
• Knowing that I can count on people in times of trouble.
• A sense of closeness with others.
• Having compassion for others.
• Putting effort into my relationships.
• I learned a great deal about how wonderful people are.
• I accept needing others.
To promote PTG, the psychiatrists can listen for accounts of the experience of growth, label the experience, decide when the patient is ready for more focused questioning, and recognize that a life narrative including the aftermath of a trauma has value. In order to get the patient to recognize PTG, the psychiatrist can state and ask: “You may have heard people say that they have found some benefit in their struggle with trauma. Given what has happened to you, do you think that is possible?” Another exchange might flow like this: “You mentioned last time that you noticed that you and your wife have grown closer since this happened. Can you tell me more about this closeness. What is it about this struggle that has produced this closeness?”
Conclusion
Strengths, resilience, and post-traumatic growth are distinct constructs that share conceptual overlap. Using these constructs, the psychiatrist can help the patients and their families move forward. At the time of diagnosis/trauma/bereavement, family and couple interventions provide support, education, and symptom management. Specific psychoeducational interventions or family therapy can be used if needed. As the illness progresses and the family moves from an assimilative stance to an accommodative stance, and as problem-solving moves from emotional problem solving to reflective problem solving, the possibility of benefit finding and PTG emerge. Most importantly, the psychiatrist can provide the family with a therapeutic space to consider their coping styles, and offer the family a path forward through discussion of dyadic coping and family growth.
Further reading
1. Tedeschi and Kilmer, “Assessing Strengths, Resilience, and Growth to Guide Clinical Interventions,” Professional Psychology: Research and Practice. 2005;36(3), p. 230-7.
2. Heru A.M., “Working With Families in Medical Settings,” (New York: Routledge), 2013.
3. Rolland J.S. “Families, Illness, & Disability: An Integrative Treatment Model,” (New York: Basic Books), 1994. New edition in press.
Thank you to Dr. Jennifer Caspari for assisting with resources for this article.
Dr. Heru is with the department of psychiatry at the University of Denver, Aurora. She has no conflicts of interest to disclose.
Promoting resilience, benefit finding, and post-traumatic growth help patients and families cope well with chronic illness. A resilient family has good problem-solving skills and communication, and a shared belief system (Fam Process. 2003; 42:1-18). Benefit finding coexists with feelings of burden, grief, and loss, and post-traumatic growth can occur despite grief and loss. Convening family meetings, for example, is a way to provide a therapeutic space for families and an opportunity to reflect on ways that chronic illness has affected the family, both negatively and positively.
What is coping well?
Resilient families cope well by identifying and solving illness-related problems, communicating about symptoms, negotiating role changes, and developing new interests as a family and new ways of emotionally being together. In addition, resilient families are able to adapt or change their life goals or health behaviors, such as improving diet, increasing exercise, and stopping smoking. A family member may work fewer hours, for example, and older children may give up some childhood activities to take on caregiving responsibilities.
Families do not usually think about how they cope; they just get along the best they can. Families may not consider how each family member’s individual coping style meshes with the coping styles of other family members. Illness management often is punctuated by crises where change happens quickly, without the family having time to deliberate on what coping styles might work best. Family changes can become fixed, therefore, not by choice, but by happenstance.
The initial stage of coping is “assimilative.” This is when the impact of the illness is being understood and absorbed. Emotional coping occurs when distress is highest, for example, at the beginning of an illness – when uncertainty exists about the diagnosis. Another characteristic of emotional coping is that it is characterized by attempts to regulate negative emotions. For example, family members may blame themselves or others, engage in wishful thinking, or become avoidant.
At later stages of illness, coping becomes “accommodative” after attempts to change or cure the illness have been found to be ineffective. Emotional coping can then be replaced with a problem-based coping style, allowing a stressor to be discussed and a solution chosen from several alternatives. Reflective coping, a related concept, is the ability to generate and consider coping options, and to recognize the usefulness of a particular coping strategy in a particular situation.
Psychiatric imperatives
While providing psychotherapy with these couples, it is helpful to:
• Help families differentiate between emotional difficulties such as “I am the primary caregiver, and I feel overburdened,” and more general practical problems, such as “I am happy to be the primary caregiver, but I need some extra help.”
• Describe coping to the family. Coping is a dynamic process, and coping styles change over time. Each person copes in their own way, depending on their experiences of illness and expectations of living with illness. In a family, the experiences and behaviors of all the individuals influence the way the family unit functions as a whole.
• Promote a balance between acceptance and change.
• Encourage the family to talk about their experience with others who are experiencing the same stressors.
• Give the family a handout that outlines different coping styles to get the discussion started.
• Provide a therapeutic space for family members to think together about how they want to cope and what coping well means to their family.
Developing dyadic coping
When dyadic coping takes place, couples cope as a single entity. Dyadic coping research is relatively new; most studies have been conducted in the past 15 years. Couples who use positive dyadic coping employ joint problem solving, joint information seeking, sharing of feelings, mutual commitment, and relaxing together. Meanwhile, couples who use negative dyadic coping hide concerns from each other and avoid shared discussion. A systemic review of dyadic coping in couples with cancer found that couples using positive dyadic coping styles experienced higher relationship functioning (Br J Health Psychol. 2015 Feb;20[1]:85-114).
Couples with good dyadic coping view the illness as “our problem.” This approach necessitates a shared understanding of the illness. The couple usually has prior experience working together as a team, for example, parenting and dividing roles within the house. They are able to relax together and provide emotional support, such as mutual calming and expressions of solidarity. Talking together about one’s worries and needs allows couples to share the experience more adequately. Dyadic coping is reflected in the amount of “we” talk.
The person in the family who copes “best” may provide the model for developing the family coping style. In a study of 66 couples faced with the stress of forced relocation, nearly all the couples adapted to the stress “as a couple,” rather than “as individuals” (Fam Process. 1991 Sep;30[3]:347-61). At the 2-year follow-up, each husband and wife developed similar coping styles, with one individual’s coping ability driving the adjustment of his or her partner. The need to adjust together or to adapt as a couple lessened the stress of relocation. Adaptation occurred through the development of shared meaning of the relocation that emerged from conversation within the couples. In other words, the couple developed a shared worldview. The coping style of the person who coped best was the strongest predictor of adjustment for both members of the couple. For most couples, the style of the person who coped best dominated.
Questions to ask during therapy
While working with these couples, assess their motivation to develop dyadic coping, by asking:
• “Do either of you feel that the patient should do this alone?” If the answer is yes, it will be difficult, if not impossible, to move the couple to a dyadic coping style.
• “Do your efforts to work together result in greater conflict?”
• “How much do you want this to change?” This questions clarifies their motivation to work together.
• “When you think about problems related to your heart condition, to what extent do you view those as ‘our problem’ [shared by the patient and the spouse equally] or mainly as ‘your own problem?’ ”
• “When a problem related to your heart condition arises, to what extent do you and your partner work together to solve it?”
• “When you both talk about the illness, how much do you use ‘we-talk’?”
• “It is important that you both agree about what is causing the illness. Can I answer any questions that might help you reach this understanding?”
• “Are there times in the past where you have successfully solved difficult problems? How did you do that?”
• “How do you respond when your spouse becomes ill?”
• “What can your spouse do that will help you get better?”
• “Can you ask your spouse for help and support?”
• “Can you work on your spouse’s health problem together?”
‘Benefit finding’ and PTG
Benefit finding emerges later in the adjustment to chronic illness. For example, caregivers may develop a greater appreciation of their own health and ability to enjoy their own pursuits. Family connectedness is a frequent source of meaning, and a critical aspect of well-being and benefit finding. Seven factors make up benefit finding: compassion/empathy, spiritual growth, mindfulness, family relations growth, lifestyle gains, personal growth, and new opportunities (Psychol Health. 2009 Apr;24[4]:373-93). Benefit finding is associated with higher marital adjustment, improved life satisfaction, and a more positive affect, especially at high levels of stress.
Post-traumatic growth, or PTG, refers to positive changes that occur after traumatic life events. People who experience PTG are transformed by their struggles with adversity. It is the struggle after the trauma, not the trauma itself, that produces PTG. In contrast to resilience, PTG refers to changes that go beyond pretrauma levels of adaptation and beyond benefit finding. Relational benefits in the aftermath of a cancer diagnosis are well recognized. An instrument used to assess these outcomes is called the Post-traumatic Growth Inventory, or PTGI (J Trauma Stress. 1996 Jul;9:455-71).
Interventions for couples coping with cancer resulted in improvements in communication, dyadic coping, quality of life, psychosocial distress, sexual functioning, and marital satisfaction (Psychooncology. 2014 Apr;23[7]:731-9). PTG may, however, be more apparent in patients than spouses.
Potential interventions
Promoting dyadic coping is effective if the couple wants to engage in intervention. According to one study, a partner-assisted emotional disclosure improved relationship functioning and intimacy (J Marital Fam Ther. 2012 Jun;38 Suppl 1:284-95). Couples therapy improves relational functioning in couples coping with cancer, at 1-year follow-up (Psychooncology. 2009 Mar;18[3]:276-83). Most important, as a first step, the couple must agree that they want to develop dyadic coping. The concept of individual versus dyadic coping may be novel for couples, and it is worth spending time on this review before offering couples intervention.
A psychoeducational program also can teach dyadic coping. The Resilient Partners discussion group developed in collaboration with the Multiple Sclerosis Society focused on developing couples’ strengths in coping with multiple sclerosis (Rolland, J., McPheters, J., and Carbonell, E., 2008). This multifamily group program is based on the Family Systems Illness Model, which integrates the demands of multiple sclerosis over time within a family developmental framework. In a comparison of a couple skills intervention with a psychoeducation program, women in the couple skills intervention benefited more in terms of their relationship functioning (Ann Behav Med. 2012 Apr;43[2]:239-52).
Dr. John S. Rolland’s Family Systems Illness (FSI) model provides a framework for the psychoeducation, assessment, and intervention with families dealing with chronic illness. This model, developed in clinical experience with more than 1,000 families, views families as valued partners and resources, and emphasizes resilience and growth. The FSI model takes into account the interaction of an illness with the individual’s development and the family’s development, the multigenerational ways of coping with illness, the family’s health/illness belief system, available resources, and relationships between health care providers.
The PTGI includes five domains: improved relationships, new possibilities for one’s life, a greater appreciation for life, a greater sense of personal strength, and spiritual development. Several family oriented themes within the PTGI can be used by the psychiatrist to inquire about positive change. These themes are:
• Knowing that I can count on people in times of trouble.
• A sense of closeness with others.
• Having compassion for others.
• Putting effort into my relationships.
• I learned a great deal about how wonderful people are.
• I accept needing others.
To promote PTG, the psychiatrists can listen for accounts of the experience of growth, label the experience, decide when the patient is ready for more focused questioning, and recognize that a life narrative including the aftermath of a trauma has value. In order to get the patient to recognize PTG, the psychiatrist can state and ask: “You may have heard people say that they have found some benefit in their struggle with trauma. Given what has happened to you, do you think that is possible?” Another exchange might flow like this: “You mentioned last time that you noticed that you and your wife have grown closer since this happened. Can you tell me more about this closeness. What is it about this struggle that has produced this closeness?”
Conclusion
Strengths, resilience, and post-traumatic growth are distinct constructs that share conceptual overlap. Using these constructs, the psychiatrist can help the patients and their families move forward. At the time of diagnosis/trauma/bereavement, family and couple interventions provide support, education, and symptom management. Specific psychoeducational interventions or family therapy can be used if needed. As the illness progresses and the family moves from an assimilative stance to an accommodative stance, and as problem-solving moves from emotional problem solving to reflective problem solving, the possibility of benefit finding and PTG emerge. Most importantly, the psychiatrist can provide the family with a therapeutic space to consider their coping styles, and offer the family a path forward through discussion of dyadic coping and family growth.
Further reading
1. Tedeschi and Kilmer, “Assessing Strengths, Resilience, and Growth to Guide Clinical Interventions,” Professional Psychology: Research and Practice. 2005;36(3), p. 230-7.
2. Heru A.M., “Working With Families in Medical Settings,” (New York: Routledge), 2013.
3. Rolland J.S. “Families, Illness, & Disability: An Integrative Treatment Model,” (New York: Basic Books), 1994. New edition in press.
Thank you to Dr. Jennifer Caspari for assisting with resources for this article.
Dr. Heru is with the department of psychiatry at the University of Denver, Aurora. She has no conflicts of interest to disclose.
Promoting resilience, benefit finding, and post-traumatic growth help patients and families cope well with chronic illness. A resilient family has good problem-solving skills and communication, and a shared belief system (Fam Process. 2003; 42:1-18). Benefit finding coexists with feelings of burden, grief, and loss, and post-traumatic growth can occur despite grief and loss. Convening family meetings, for example, is a way to provide a therapeutic space for families and an opportunity to reflect on ways that chronic illness has affected the family, both negatively and positively.
What is coping well?
Resilient families cope well by identifying and solving illness-related problems, communicating about symptoms, negotiating role changes, and developing new interests as a family and new ways of emotionally being together. In addition, resilient families are able to adapt or change their life goals or health behaviors, such as improving diet, increasing exercise, and stopping smoking. A family member may work fewer hours, for example, and older children may give up some childhood activities to take on caregiving responsibilities.
Families do not usually think about how they cope; they just get along the best they can. Families may not consider how each family member’s individual coping style meshes with the coping styles of other family members. Illness management often is punctuated by crises where change happens quickly, without the family having time to deliberate on what coping styles might work best. Family changes can become fixed, therefore, not by choice, but by happenstance.
The initial stage of coping is “assimilative.” This is when the impact of the illness is being understood and absorbed. Emotional coping occurs when distress is highest, for example, at the beginning of an illness – when uncertainty exists about the diagnosis. Another characteristic of emotional coping is that it is characterized by attempts to regulate negative emotions. For example, family members may blame themselves or others, engage in wishful thinking, or become avoidant.
At later stages of illness, coping becomes “accommodative” after attempts to change or cure the illness have been found to be ineffective. Emotional coping can then be replaced with a problem-based coping style, allowing a stressor to be discussed and a solution chosen from several alternatives. Reflective coping, a related concept, is the ability to generate and consider coping options, and to recognize the usefulness of a particular coping strategy in a particular situation.
Psychiatric imperatives
While providing psychotherapy with these couples, it is helpful to:
• Help families differentiate between emotional difficulties such as “I am the primary caregiver, and I feel overburdened,” and more general practical problems, such as “I am happy to be the primary caregiver, but I need some extra help.”
• Describe coping to the family. Coping is a dynamic process, and coping styles change over time. Each person copes in their own way, depending on their experiences of illness and expectations of living with illness. In a family, the experiences and behaviors of all the individuals influence the way the family unit functions as a whole.
• Promote a balance between acceptance and change.
• Encourage the family to talk about their experience with others who are experiencing the same stressors.
• Give the family a handout that outlines different coping styles to get the discussion started.
• Provide a therapeutic space for family members to think together about how they want to cope and what coping well means to their family.
Developing dyadic coping
When dyadic coping takes place, couples cope as a single entity. Dyadic coping research is relatively new; most studies have been conducted in the past 15 years. Couples who use positive dyadic coping employ joint problem solving, joint information seeking, sharing of feelings, mutual commitment, and relaxing together. Meanwhile, couples who use negative dyadic coping hide concerns from each other and avoid shared discussion. A systemic review of dyadic coping in couples with cancer found that couples using positive dyadic coping styles experienced higher relationship functioning (Br J Health Psychol. 2015 Feb;20[1]:85-114).
Couples with good dyadic coping view the illness as “our problem.” This approach necessitates a shared understanding of the illness. The couple usually has prior experience working together as a team, for example, parenting and dividing roles within the house. They are able to relax together and provide emotional support, such as mutual calming and expressions of solidarity. Talking together about one’s worries and needs allows couples to share the experience more adequately. Dyadic coping is reflected in the amount of “we” talk.
The person in the family who copes “best” may provide the model for developing the family coping style. In a study of 66 couples faced with the stress of forced relocation, nearly all the couples adapted to the stress “as a couple,” rather than “as individuals” (Fam Process. 1991 Sep;30[3]:347-61). At the 2-year follow-up, each husband and wife developed similar coping styles, with one individual’s coping ability driving the adjustment of his or her partner. The need to adjust together or to adapt as a couple lessened the stress of relocation. Adaptation occurred through the development of shared meaning of the relocation that emerged from conversation within the couples. In other words, the couple developed a shared worldview. The coping style of the person who coped best was the strongest predictor of adjustment for both members of the couple. For most couples, the style of the person who coped best dominated.
Questions to ask during therapy
While working with these couples, assess their motivation to develop dyadic coping, by asking:
• “Do either of you feel that the patient should do this alone?” If the answer is yes, it will be difficult, if not impossible, to move the couple to a dyadic coping style.
• “Do your efforts to work together result in greater conflict?”
• “How much do you want this to change?” This questions clarifies their motivation to work together.
• “When you think about problems related to your heart condition, to what extent do you view those as ‘our problem’ [shared by the patient and the spouse equally] or mainly as ‘your own problem?’ ”
• “When a problem related to your heart condition arises, to what extent do you and your partner work together to solve it?”
• “When you both talk about the illness, how much do you use ‘we-talk’?”
• “It is important that you both agree about what is causing the illness. Can I answer any questions that might help you reach this understanding?”
• “Are there times in the past where you have successfully solved difficult problems? How did you do that?”
• “How do you respond when your spouse becomes ill?”
• “What can your spouse do that will help you get better?”
• “Can you ask your spouse for help and support?”
• “Can you work on your spouse’s health problem together?”
‘Benefit finding’ and PTG
Benefit finding emerges later in the adjustment to chronic illness. For example, caregivers may develop a greater appreciation of their own health and ability to enjoy their own pursuits. Family connectedness is a frequent source of meaning, and a critical aspect of well-being and benefit finding. Seven factors make up benefit finding: compassion/empathy, spiritual growth, mindfulness, family relations growth, lifestyle gains, personal growth, and new opportunities (Psychol Health. 2009 Apr;24[4]:373-93). Benefit finding is associated with higher marital adjustment, improved life satisfaction, and a more positive affect, especially at high levels of stress.
Post-traumatic growth, or PTG, refers to positive changes that occur after traumatic life events. People who experience PTG are transformed by their struggles with adversity. It is the struggle after the trauma, not the trauma itself, that produces PTG. In contrast to resilience, PTG refers to changes that go beyond pretrauma levels of adaptation and beyond benefit finding. Relational benefits in the aftermath of a cancer diagnosis are well recognized. An instrument used to assess these outcomes is called the Post-traumatic Growth Inventory, or PTGI (J Trauma Stress. 1996 Jul;9:455-71).
Interventions for couples coping with cancer resulted in improvements in communication, dyadic coping, quality of life, psychosocial distress, sexual functioning, and marital satisfaction (Psychooncology. 2014 Apr;23[7]:731-9). PTG may, however, be more apparent in patients than spouses.
Potential interventions
Promoting dyadic coping is effective if the couple wants to engage in intervention. According to one study, a partner-assisted emotional disclosure improved relationship functioning and intimacy (J Marital Fam Ther. 2012 Jun;38 Suppl 1:284-95). Couples therapy improves relational functioning in couples coping with cancer, at 1-year follow-up (Psychooncology. 2009 Mar;18[3]:276-83). Most important, as a first step, the couple must agree that they want to develop dyadic coping. The concept of individual versus dyadic coping may be novel for couples, and it is worth spending time on this review before offering couples intervention.
A psychoeducational program also can teach dyadic coping. The Resilient Partners discussion group developed in collaboration with the Multiple Sclerosis Society focused on developing couples’ strengths in coping with multiple sclerosis (Rolland, J., McPheters, J., and Carbonell, E., 2008). This multifamily group program is based on the Family Systems Illness Model, which integrates the demands of multiple sclerosis over time within a family developmental framework. In a comparison of a couple skills intervention with a psychoeducation program, women in the couple skills intervention benefited more in terms of their relationship functioning (Ann Behav Med. 2012 Apr;43[2]:239-52).
Dr. John S. Rolland’s Family Systems Illness (FSI) model provides a framework for the psychoeducation, assessment, and intervention with families dealing with chronic illness. This model, developed in clinical experience with more than 1,000 families, views families as valued partners and resources, and emphasizes resilience and growth. The FSI model takes into account the interaction of an illness with the individual’s development and the family’s development, the multigenerational ways of coping with illness, the family’s health/illness belief system, available resources, and relationships between health care providers.
The PTGI includes five domains: improved relationships, new possibilities for one’s life, a greater appreciation for life, a greater sense of personal strength, and spiritual development. Several family oriented themes within the PTGI can be used by the psychiatrist to inquire about positive change. These themes are:
• Knowing that I can count on people in times of trouble.
• A sense of closeness with others.
• Having compassion for others.
• Putting effort into my relationships.
• I learned a great deal about how wonderful people are.
• I accept needing others.
To promote PTG, the psychiatrists can listen for accounts of the experience of growth, label the experience, decide when the patient is ready for more focused questioning, and recognize that a life narrative including the aftermath of a trauma has value. In order to get the patient to recognize PTG, the psychiatrist can state and ask: “You may have heard people say that they have found some benefit in their struggle with trauma. Given what has happened to you, do you think that is possible?” Another exchange might flow like this: “You mentioned last time that you noticed that you and your wife have grown closer since this happened. Can you tell me more about this closeness. What is it about this struggle that has produced this closeness?”
Conclusion
Strengths, resilience, and post-traumatic growth are distinct constructs that share conceptual overlap. Using these constructs, the psychiatrist can help the patients and their families move forward. At the time of diagnosis/trauma/bereavement, family and couple interventions provide support, education, and symptom management. Specific psychoeducational interventions or family therapy can be used if needed. As the illness progresses and the family moves from an assimilative stance to an accommodative stance, and as problem-solving moves from emotional problem solving to reflective problem solving, the possibility of benefit finding and PTG emerge. Most importantly, the psychiatrist can provide the family with a therapeutic space to consider their coping styles, and offer the family a path forward through discussion of dyadic coping and family growth.
Further reading
1. Tedeschi and Kilmer, “Assessing Strengths, Resilience, and Growth to Guide Clinical Interventions,” Professional Psychology: Research and Practice. 2005;36(3), p. 230-7.
2. Heru A.M., “Working With Families in Medical Settings,” (New York: Routledge), 2013.
3. Rolland J.S. “Families, Illness, & Disability: An Integrative Treatment Model,” (New York: Basic Books), 1994. New edition in press.
Thank you to Dr. Jennifer Caspari for assisting with resources for this article.
Dr. Heru is with the department of psychiatry at the University of Denver, Aurora. She has no conflicts of interest to disclose.
Lichen Striatus
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
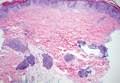
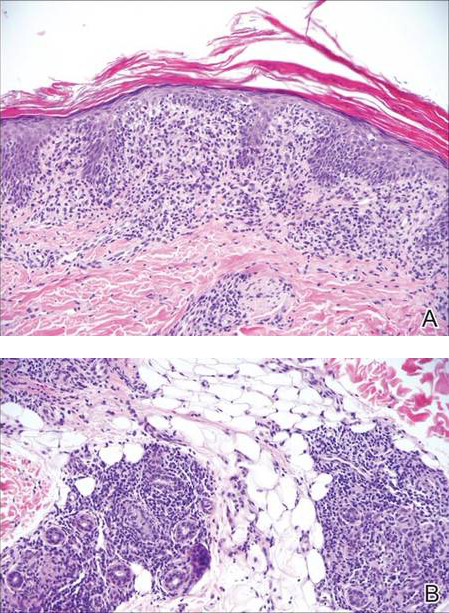
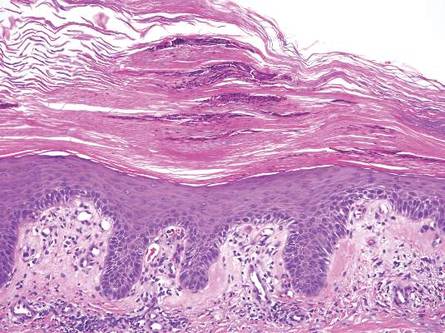
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.
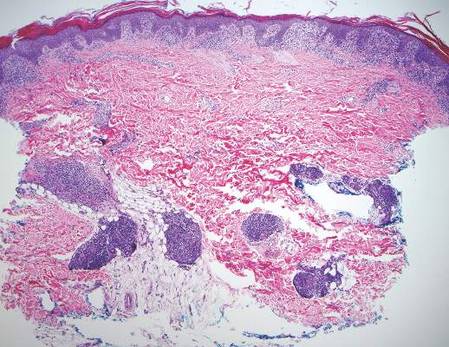
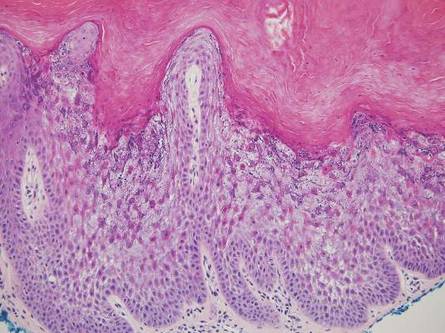
Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.
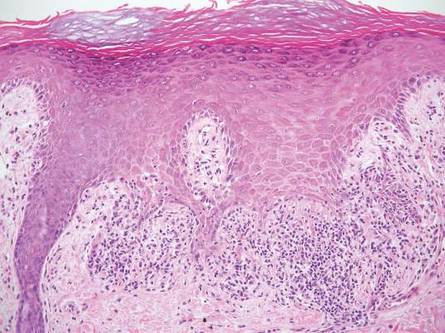
Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1
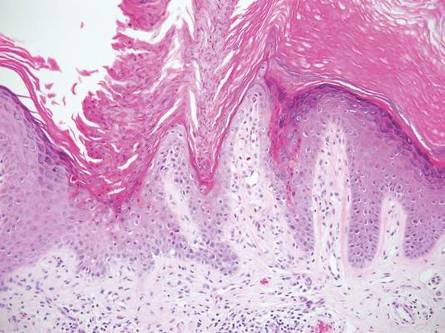
Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.
Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

Lichen striatus (LS) is a benign, uncommon, self-limited, linear inflammatory skin disorder that primarily affects children up to 15 years of age, most commonly around 2 to 3 years of age, and is seen more frequently in girls.1 It presents with a sudden eruption of asymptomatic small, flat-topped, lichenoid, scaly papules in a linear array on a single extremity. The lesions may be erythematous, flesh colored, or hypopigmented.1,2 Multiple lesions appear over days to weeks and coalesce into linear plaques in a continuous or interrupted pattern along the lines of Blaschko, indicating possible somatic mosaicism.1 Although typically asymptomatic, it may be pruritic. Most cases spontaneously resolve within 1 year.3 Recurrences are unusual. Digital involvement may result in onycholysis, longitudinal ridging, splitting, and nail loss.1 The underlying cause of LS may be an abnormal immunologic reaction or genetic predisposition that is precipitated by some trigger such as a viral infection, trauma, hypersensitivity reaction, vaccine, seasonal variation, medication, or pregnancy.1,2 An association with atopy has been described. Treatment is not necessary but options include topical steroids, topical retinoids, and topical calcineurin inhibitors.2
Histologically, findings in LS are somewhat variable but typically show a combination of spongiotic and lichenoid interface dermatitis with a perivascular and periadnexal lymphocytic infiltrate (Figure 1). Epidermal changes include intercellular and intracellular edema, focal spongiosis, lymphocytic exocytosis, parakeratosis, patchy hyperkeratosis, and keratinocyte necrosis (Figure 2A).1,3 The epidermis is normal or slightly acanthotic, and dyskeratotic keratinocytes can be found in the granular and horny layers or at the dermoepidermal junction.2 The lymphohistiocytic infiltrate in the superficial and deep dermis surrounds vascular plexuses and cutaneous adnexa such as eccrine glands and hair follicles.1 Perivascular lymphoid aggregates and eccrine coil involvement are particularly distinctive of LS (Figure 2B).4 Pigment incontinence also may be seen.


Another condition that distributes linearly along the lines of Blaschko is linear epidermolytic hyperkeratosis (EHK). Similar to LS, histology shows hyperkeratosis, focal parakeratosis, and acanthosis of the epidermis.5 However, EHK shows epidermolysis, acantholysis, and perinuclear vacuolization in spinous and granular layers (Figure 3).5 The lack of perivascular and periadnexal inflammation also can help differentiate EHK from LS.

Linear lichen planus (LLP), similar to LS, histologically shows a lichenoid lymphocytic bandlike infiltrate obscuring the dermoepidermal junction, vacuolization of the basal cell layer, and pigment incontinence.1,2 Although LS and LLP can have histologic overlap, the absence of adnexal or perieccrine lymphocytic inflammation can help distinguish the two.3 The histopathologic changes of intercellular edema or mild spongiosis, exocytosis, and parakeratosis present in LS also are typically absent in LLP. Linear lichen planus characteristically consists of wedge-shaped hypergranulosis and irregular acanthosis with saw-toothed rete ridges (Figure 4).2 In addition, lobular eosinophilic deposits known as cytoid or Civatte bodies representing degenerated keratinocytes can be visualized at the dermoepidermal junction in LLP.2 Immunofluorescence will highlight Civatte bodies with IgM, IgG, and C3, also helping to differentiate these 2 conditions.1

Linear porokeratosis can be mistaken for the linear lesion of LS. Both entities may reveal perivascular lymphocytes in the dermis, and porokeratosis can be lichenoid in the central portion of the lesion.6 However, porokeratosis is unique in that it contains a cornoid lamella, characterized by a thin column of tightly packed parakeratotic cells extending from an invagination of the epidermis through the adjacent stratum corneum (Figure 5).6 Beneath the cornoid lamella, the granular layer is either absent or markedly attenuated, and pyknotic keratinocytes with perinuclear edema are present in the spinous layer.6 The epidermis in the central portion of the porokeratotic lesion may be normal, hyperplastic, or atrophic with effacement of rete ridges.

Similar to LS, linear psoriasis follows lines of Blaschko clinically. However, it is distinguished by its characteristic psoriatic epidermal changes as well as its lack of lichenoid or perieccrine inflammation.3 Typical findings in linear psoriasis include hyperkeratosis, confluent parakeratosis with entrapped neutrophilic microabscesses, acanthosis with regular elongation of rete ridges, intraepidermal neutrophils, thinned suprapapillary plates, dilated capillaries in the tips of the dermal papillae, and a chronic dermal inflammatory infiltrate (Figure 6).4

- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
- Wang WL, Lazar A. Lichenoid and interface dermatitis. In: Calonje E, Brenn T, Lazar A, et al, eds. McKee’s Pathology of the Skin. 4th ed. London, England: Elsevier/Saunders; 2011:219-258.
- Shiohara T, Kano Y. Lichen planus and lichenoid dermatoses. In: Bolognia J, Jorizzo J, Schaffer J, eds. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:183-202.
- Zhang Y, McNutt NS. Lichen striatus. histological, immunohistochemical, and ultrastructural study of 37 cases. J Cutan Pathol. 2001;28:65-71.
- Johnson M, Walker D, Galloway W, et al. Interface dermatitis along Blaschko’s lines. J Cutan Pathol. 2014;41:950-954.
- Kumar P, Kumar R, Kumar Mandal RK, et al. Systematized linear epidermolytic hyperkeratosis. Dermatol Online J. 2014;20:21248.
- Requena L, Requena C, Cockerell C. Benign epidermal tumors and proliferations. In: Bolognia J, Jorizzo J, Schaffer J. Dermatology. 3rd ed. Philadelphia, PA: Elsevier/Saunders; 2012:1795-1815.
Subcision: The benefits of a classic technique
We’re always working toward medical breakthroughs so we can provide the most effective treatments for our patients with cutting-edge technology; however, there is a lot to be said about the techniques that have paved the way for new medical devices.
For certain conditions, the efficacy of classic procedures often cannot be matched by their modern successors. Subcision for treatment of deep depressed scars, for example, is often a more effective option than microneedling and can produce results with less healing time and fewer treatments, and at a more cost-effective price.
Both subcision and microneedling improve the appearance of scars by creating wounds in an effort to break up scar tissue and trigger collagen regrowth. Microneedling involves the use of a microneedling pen with several small needles that glide across the skin at different depths and speeds. Subcision is achieved with one larger gauge needle that is injected into scars at different angles and depths to break up scar tissue. Microneedling needles yield more epidermal damage than does subcision, causing more bleeding and ultimately lengthening the healing time.
The mechanism of subcising deeper scar tissue also seems to be more effective than that of microneedling. It often takes fewer subcision treatments than microneedling treatments to achieve comparable improvement of depressed scars. Microneedling needles are limited to penetrating at best 2.5 mm beneath the skin surface, while subcision allows the freedom to penetrate deeper into the dermis to reach deeper dermal scars. Subcising also creates larger channels within the scar tissue, which create more space for collagen regrowth, while microneedling does not.
A technique that has shown to improve treatment outcomes is the use of a 26- or 30-gauge needle, moving back and forth in a fanning pattern under the scar tissue while simultaneously injecting lidocaine or saline in those channels. The injection of a fluid component, particularly that of lidocaine, can both decrease the pain as well as inflate the scar in question, allowing more collagen regrowth and wound growth factors to fill the “gaps” created.
Unless scars have a significant epidermal component in addition to their dermal component, subcising the scar is a more effective and has faster healing times. Both procedures can cause bruising , edema, and erythema. However, the epidermal damage that can occur in microneedling has significantly more downtime.
In addition, subcision is a more cost-effective treatment than microneedling. The required materials for subcision are limited to materials that are readily used within practices: needles, syringes, saline, and lidocaine. Microneedling, on the other hand, requires purchase of expensive tools, including microneedling pens, sterile single-use microneedling tips, and protective sleeves for the device, in addition to topical skin care products to apply after the treatment to promote safe healing.
While microneedling is remarkably effective for treatment of superficial scars, fine lines, and hypopigmentation, subcision tends to be more effective for the treatment of deeper scars such as box-car acne scars.
We love new technology in our practices; however, sometimes our tried and true procedures may prove to be a better option in the appropriate patient.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
We’re always working toward medical breakthroughs so we can provide the most effective treatments for our patients with cutting-edge technology; however, there is a lot to be said about the techniques that have paved the way for new medical devices.
For certain conditions, the efficacy of classic procedures often cannot be matched by their modern successors. Subcision for treatment of deep depressed scars, for example, is often a more effective option than microneedling and can produce results with less healing time and fewer treatments, and at a more cost-effective price.
Both subcision and microneedling improve the appearance of scars by creating wounds in an effort to break up scar tissue and trigger collagen regrowth. Microneedling involves the use of a microneedling pen with several small needles that glide across the skin at different depths and speeds. Subcision is achieved with one larger gauge needle that is injected into scars at different angles and depths to break up scar tissue. Microneedling needles yield more epidermal damage than does subcision, causing more bleeding and ultimately lengthening the healing time.
The mechanism of subcising deeper scar tissue also seems to be more effective than that of microneedling. It often takes fewer subcision treatments than microneedling treatments to achieve comparable improvement of depressed scars. Microneedling needles are limited to penetrating at best 2.5 mm beneath the skin surface, while subcision allows the freedom to penetrate deeper into the dermis to reach deeper dermal scars. Subcising also creates larger channels within the scar tissue, which create more space for collagen regrowth, while microneedling does not.
A technique that has shown to improve treatment outcomes is the use of a 26- or 30-gauge needle, moving back and forth in a fanning pattern under the scar tissue while simultaneously injecting lidocaine or saline in those channels. The injection of a fluid component, particularly that of lidocaine, can both decrease the pain as well as inflate the scar in question, allowing more collagen regrowth and wound growth factors to fill the “gaps” created.
Unless scars have a significant epidermal component in addition to their dermal component, subcising the scar is a more effective and has faster healing times. Both procedures can cause bruising , edema, and erythema. However, the epidermal damage that can occur in microneedling has significantly more downtime.
In addition, subcision is a more cost-effective treatment than microneedling. The required materials for subcision are limited to materials that are readily used within practices: needles, syringes, saline, and lidocaine. Microneedling, on the other hand, requires purchase of expensive tools, including microneedling pens, sterile single-use microneedling tips, and protective sleeves for the device, in addition to topical skin care products to apply after the treatment to promote safe healing.
While microneedling is remarkably effective for treatment of superficial scars, fine lines, and hypopigmentation, subcision tends to be more effective for the treatment of deeper scars such as box-car acne scars.
We love new technology in our practices; however, sometimes our tried and true procedures may prove to be a better option in the appropriate patient.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
We’re always working toward medical breakthroughs so we can provide the most effective treatments for our patients with cutting-edge technology; however, there is a lot to be said about the techniques that have paved the way for new medical devices.
For certain conditions, the efficacy of classic procedures often cannot be matched by their modern successors. Subcision for treatment of deep depressed scars, for example, is often a more effective option than microneedling and can produce results with less healing time and fewer treatments, and at a more cost-effective price.
Both subcision and microneedling improve the appearance of scars by creating wounds in an effort to break up scar tissue and trigger collagen regrowth. Microneedling involves the use of a microneedling pen with several small needles that glide across the skin at different depths and speeds. Subcision is achieved with one larger gauge needle that is injected into scars at different angles and depths to break up scar tissue. Microneedling needles yield more epidermal damage than does subcision, causing more bleeding and ultimately lengthening the healing time.
The mechanism of subcising deeper scar tissue also seems to be more effective than that of microneedling. It often takes fewer subcision treatments than microneedling treatments to achieve comparable improvement of depressed scars. Microneedling needles are limited to penetrating at best 2.5 mm beneath the skin surface, while subcision allows the freedom to penetrate deeper into the dermis to reach deeper dermal scars. Subcising also creates larger channels within the scar tissue, which create more space for collagen regrowth, while microneedling does not.
A technique that has shown to improve treatment outcomes is the use of a 26- or 30-gauge needle, moving back and forth in a fanning pattern under the scar tissue while simultaneously injecting lidocaine or saline in those channels. The injection of a fluid component, particularly that of lidocaine, can both decrease the pain as well as inflate the scar in question, allowing more collagen regrowth and wound growth factors to fill the “gaps” created.
Unless scars have a significant epidermal component in addition to their dermal component, subcising the scar is a more effective and has faster healing times. Both procedures can cause bruising , edema, and erythema. However, the epidermal damage that can occur in microneedling has significantly more downtime.
In addition, subcision is a more cost-effective treatment than microneedling. The required materials for subcision are limited to materials that are readily used within practices: needles, syringes, saline, and lidocaine. Microneedling, on the other hand, requires purchase of expensive tools, including microneedling pens, sterile single-use microneedling tips, and protective sleeves for the device, in addition to topical skin care products to apply after the treatment to promote safe healing.
While microneedling is remarkably effective for treatment of superficial scars, fine lines, and hypopigmentation, subcision tends to be more effective for the treatment of deeper scars such as box-car acne scars.
We love new technology in our practices; however, sometimes our tried and true procedures may prove to be a better option in the appropriate patient.
Dr. Wesley and Dr. Talakoub are co-contributors to this column. Dr. Talakoub is in private practice in McLean, Va. Dr. Wesley practices dermatology in Beverly Hills, Calif. This month’s column is by Dr. Talakoub.
Nanoparticles deliver Aurora kinase inhibitor with increased safety and efficacy
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
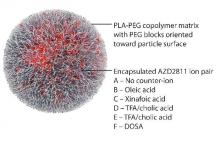
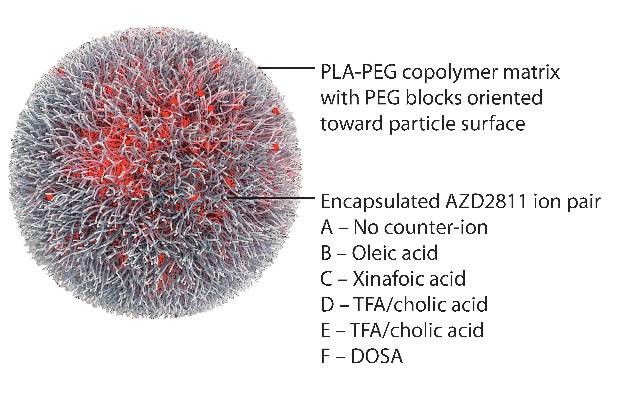
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
By encapsulating an Aurora B kinase inhibitor in Accurin particles, the researchers appear to have succeeded in enhancing the drug’s therapeutic activity and safety in mouse xenograft models. It remains to be seen how widely applicable this technology will be, and whether these results will be replicated in patients as the AZD1151-hQPA Accurin formulation heads toward first-in-human clinical trials. If the Accurin technology can solve the pharmacokinetic and toxicity issues for Aurora kinase inhibitors, it will likely also be applicable to other compounds that have encountered similar difficulties in clinical development and will add a formulation approach to address very meaningful and challenging issues that face many new molecules during clinical development.
David J. Bearss, Ph.D., is the chief executive officer of Tolero Pharmaceutical, Lehi, Utah. His remarks were part of an editorial accompanying the report in Science Translational Medicine (2016 Feb 10. doi: 10.1126/scitranslmed.aaf1417).
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Using nanoparticles to encapsulate an Aurora B kinase inhibitor improved the efficacy and tolerability of the drug and allowed less frequent dosing in preclinical models, according to researchers.
“The AZD2811 nanoparticles identified in this study have the potential to increase efficacy at tolerable doses using a more convenient dosing regimen, which may in turn extend the utility of Aurora B kinase inhibition to a broader range of hematological and solid tumor cancer indications,” wrote Susan Ashton of AstraZeneca, and her colleagues (Sci Transl Med. 2016 Feb 10. doi: 10.1126/scitranslmed.aad2355).
“The improved bone marrow profile observed with slow-releasing nanoparticles may enable efficacious combination treatments” with chemotherapy, radiotherapy, or poly(adenosine diphosphate–ribose) polymerase (PARP) inhibitors.
The study was undertaken because a free-drug version of the agent, known as AZD1152, had led to a significant improvement in the complete response rate of acute myeloid leukemia compared to standard of care in a phase II trial. Efficacy, however, was associated with major toxicities, including myelosuppression. Further, AZD1152 had to be administered as a 7-day continuous intravenous infusion.
By using the Accurin nanoparticle platform to vary drug release kinetics, the researchers devised a formulation to maximize the therapeutic effect of the kinase inhibitor while sparing healthy tissue. AZD1152 is a water-soluble prodrug of AZD2811, which the researchers used to develop their the nanoparticle formulation.
AZD2811 was encapsulated in polymeric nanoparticles termed Accurins, which are composed of block copolymers of poly-D,L-lactide (PLA) and poly(ethylene glycol) (PEG). Accurins accumulate in tumors, increasing the drug’s concentration and duration of exposure to the cancer cells. Organic acid counterions were used to increase encapsulation efficiency and decrease the release rate of AZD2811.
“We identified a formulation profile that could deliver active drug for more than 1 week, resulting in prolonged target inhibition in tumor tissue together with improved preclinical efficacy and therapeutic index over the AZD1152 prodrug in several animal models,” they wrote.
In nude rats bearing human colorectal adenocarcinoma SW620 xenografts, the nanoparticles inhibited kinase over a 96-hour time course, while the free drug resulted in complete enzyme recovery at 24 hours. Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity as evidenced by stable body weight. Nanoparticles were retained in the tumor xenografts for up to 6 days, while the free drug was undetected in tumors 24 hours after administration.
“Although we selected a lead formulation using a tumor model (SW620) that supported the AZD1152 program – and, as such, we had extensive comparator data from which to benchmark the tolerability, PD, and efficacy of candidate nanoparticles – the model is subject to the known limitations of xenografted human tumor cell lines in assessing therapeutic candidates in oncology. Moreover, although rat bone marrow is commonly used to model myelotoxicity in humans, interrogation of the nanoparticle dose and schedule in patients may be required to achieve optimal clinical results,” they concluded.
AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Key clinical point: Aurora B kinase inhibitor nanoparticles displayed accumulation and retention in tumors with improved efficacy and minimal bone marrow pathology in animal models.
Major finding: Nanoparticles inhibited tumor growth by over 90%, compared with 58% for the free drug at twice the dose, and showed little toxicity; the free drug was undetected in tumors 24 hours after administration, and nanoparticle-delivered drug was detectable up to 6 days.
Data sources: Nude rats and nude mice bearing human colorectal adenocarcinoma SW620 xenografts.
Disclosures: AstraZeneca funded the study. Dr. Ashton and several coauthors are current or former employees and shareholders of AstraZeneca or BIND. The companies are developing the drug and technologies.
Women with AF have a Higher Risk of Death and CVD
NEW YORK (Reuters Health) - Women with atrial fibrillation (AF) are at somewhat higher risk of death and cardiovascular disease (CVD) than men with the condition, a new systematic review and meta-analysis confirms.
"Physicians should be aware of this and they should also make sure they treat women as aggressively as men," Connor Emdin, a doctoral student at The George Institute for Global Health at the University of Oxford, U.K., told Reuters Health. "On average, women should probably be treated moreaggressively."
Smoking and diabetes are known to increase coronary heart disease risk more sharply for women than for men, Emdin and his team write in their report, online January 19 in The BMJ. Some studies have found that AF is more strongly associated with stroke and death in women than in men, but other studies have not, they add.
To better understand the relationship, the researchers looked at 30 studies including more than 4.3 million individuals. The ratio of relative risk for women compared to men with AF for all-cause mortality was 1.12. For stroke, the ratio was 1.99, while it was 1.93 for cardiovascular mortality, 1.55 for cardiac events, and 1.16 for heart failure. All increases were statistically significant.
While the CHADS2 score for estimating stroke risk in AF does not include female sex as a risk factor for stroke, Emdin noted, a more recent version, the CHA2DS2-VASc score, does. "Our results would support using risk scores which include female sex," he said.
AF is less prevalent among women than men, but thefindings confirm that it is more severe for them as well, Dr. Elsayed Soliman, director of the Epidemiological Cardiology Research Center at Wake Forest Baptist Medical Center in Wake Forest, North Carolina, told Reuters Health. Dr. Soliman was not involved in the new study.
"The article adds to this evidence that really women are different from men when it comes to cardiovascular disease and they need to be managed differently," Dr. Soliman said. He added that "we need to do more work to see what could bridge the gap in outcomes associated with atrial fibrillation."
NEW YORK (Reuters Health) - Women with atrial fibrillation (AF) are at somewhat higher risk of death and cardiovascular disease (CVD) than men with the condition, a new systematic review and meta-analysis confirms.
"Physicians should be aware of this and they should also make sure they treat women as aggressively as men," Connor Emdin, a doctoral student at The George Institute for Global Health at the University of Oxford, U.K., told Reuters Health. "On average, women should probably be treated moreaggressively."
Smoking and diabetes are known to increase coronary heart disease risk more sharply for women than for men, Emdin and his team write in their report, online January 19 in The BMJ. Some studies have found that AF is more strongly associated with stroke and death in women than in men, but other studies have not, they add.
To better understand the relationship, the researchers looked at 30 studies including more than 4.3 million individuals. The ratio of relative risk for women compared to men with AF for all-cause mortality was 1.12. For stroke, the ratio was 1.99, while it was 1.93 for cardiovascular mortality, 1.55 for cardiac events, and 1.16 for heart failure. All increases were statistically significant.
While the CHADS2 score for estimating stroke risk in AF does not include female sex as a risk factor for stroke, Emdin noted, a more recent version, the CHA2DS2-VASc score, does. "Our results would support using risk scores which include female sex," he said.
AF is less prevalent among women than men, but thefindings confirm that it is more severe for them as well, Dr. Elsayed Soliman, director of the Epidemiological Cardiology Research Center at Wake Forest Baptist Medical Center in Wake Forest, North Carolina, told Reuters Health. Dr. Soliman was not involved in the new study.
"The article adds to this evidence that really women are different from men when it comes to cardiovascular disease and they need to be managed differently," Dr. Soliman said. He added that "we need to do more work to see what could bridge the gap in outcomes associated with atrial fibrillation."
NEW YORK (Reuters Health) - Women with atrial fibrillation (AF) are at somewhat higher risk of death and cardiovascular disease (CVD) than men with the condition, a new systematic review and meta-analysis confirms.
"Physicians should be aware of this and they should also make sure they treat women as aggressively as men," Connor Emdin, a doctoral student at The George Institute for Global Health at the University of Oxford, U.K., told Reuters Health. "On average, women should probably be treated moreaggressively."
Smoking and diabetes are known to increase coronary heart disease risk more sharply for women than for men, Emdin and his team write in their report, online January 19 in The BMJ. Some studies have found that AF is more strongly associated with stroke and death in women than in men, but other studies have not, they add.
To better understand the relationship, the researchers looked at 30 studies including more than 4.3 million individuals. The ratio of relative risk for women compared to men with AF for all-cause mortality was 1.12. For stroke, the ratio was 1.99, while it was 1.93 for cardiovascular mortality, 1.55 for cardiac events, and 1.16 for heart failure. All increases were statistically significant.
While the CHADS2 score for estimating stroke risk in AF does not include female sex as a risk factor for stroke, Emdin noted, a more recent version, the CHA2DS2-VASc score, does. "Our results would support using risk scores which include female sex," he said.
AF is less prevalent among women than men, but thefindings confirm that it is more severe for them as well, Dr. Elsayed Soliman, director of the Epidemiological Cardiology Research Center at Wake Forest Baptist Medical Center in Wake Forest, North Carolina, told Reuters Health. Dr. Soliman was not involved in the new study.
"The article adds to this evidence that really women are different from men when it comes to cardiovascular disease and they need to be managed differently," Dr. Soliman said. He added that "we need to do more work to see what could bridge the gap in outcomes associated with atrial fibrillation."
Combo can produce durable remissions in PTCL

Photo by Larry Young
SAN FRANCISCO—A combination treatment regimen can produce durable remissions in patients newly diagnosed with peripheral T-cell lymphoma (PTCL), results of a phase 1 study suggest.
The patients received brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisolone (BV+CHP). In some cases, this was followed by BV monotherapy.
The estimated 3-year progression-free survival (PFS) for these patients was 52%, and the overall survival (OS) was 80%.
There was a high rate of peripheral neuropathy (73%), but most cases resolved or improved over time.
Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues presented these data as a poster at the 8th Annual T-cell Lymphoma Forum. The study was supported by Seattle Genetics and Millennium Pharmaceuticals.
The researchers presented data on 26 patients newly diagnosed with PTCL. Nineteen patients had systemic anaplastic large-cell lymphoma (ALCL; 16 ALK- and 3 ALK+), 2 had PTCL not otherwise specified, 2 had angioimmunoblastic T-cell lymphoma, 2 had adult T-cell leukemia/lymphoma, and 1 had enteropathy-associated T-cell lymphoma.
The patients’ median age was 56 (range, 21-82). Sixty-nine percent of patients had an IPI score of 2 or higher, and 73% had stage III/IV disease.
Treatment
The patients received BV+CHP every 3 weeks for 6 cycles. Those who achieved at least a partial remission could go on to receive up to 10 additional cycles of single-agent BV at 1.8 mg/kg every 3 weeks.
Twenty-three patients (88%) completed all 6 cycles of BV+CHP, and 21 patients (81%) went on to receive BV monotherapy, 11 of whom (42%) received all 10 cycles.
Fifteen patients (58%) discontinued treatment, 3 due to progressive disease, 3 due to investigator decision, 6 due to adverse events, and 3 due to patient decision.
After a median observation period of 38.7 months (range, 4.6 to 44.3), 77% of patients (n=20) remained on study.
Toxicity
The most common adverse events (occurring in at least 30% of patients) were nausea (69%), peripheral sensory neuropathy (69%), diarrhea (62%), fatigue (58%), alopecia (54%), dyspnea (46%), constipation (35%), myalgia (31%), peripheral edema (31%), chills (31%), anemia (31%), insomnia (31%), and febrile neutropenia.
The most common grade 3 or higher adverse events (occurring in at least 10% of patients) were febrile neutropenia (31%), neutropenia (23%), anemia (15%), and pulmonary embolism (12%).
There were 6 adverse events resulting in treatment discontinuation—peripheral sensory neuropathy (n=3), abdominal pain (n=1), asthenia (n=1), and peripheral motor neuropathy (n=1).
Seventy-three percent of patients (19/26) experienced peripheral neuropathy. Thirty-seven percent (n=7) had a complete resolution of neuropathy, and 58% (n=11) had some improvement. The median time to resolution was 1.3 months. Twelve patients (63%) had ongoing neuropathy at last follow-up, but most had grade 1 (n=10).
Response and survival
The objective response rate was 100%, and the complete response rate was 88% (n=23). One patient who had a partial response at the end of combination therapy achieved a complete response after going on to single-agent BV.
Twenty-one of the 26 patients are still alive—15 with ALCL and 6 with other PTCLs. Sixteen patients have not progressed—9 with ALCL and 5 with other PTCLs.
After progression, 5 patients received BV again, and 3 received stem cell transplants (2 allogeneic and 1 autologous).
The estimated 3-year PFS was 52%, and the estimated OS was 80%. The researchers noted that these rates compare favorably with the historical 3-year PFS and OS rates of 30% and 40%, respectively.
Researchers are currently conducting a phase 3 trial comparing BV+CHP with CHOP as frontline treatment of CD30+ mature T-cell lymphomas (ECHELON-2, NCT01777152). ![]()

Photo by Larry Young
SAN FRANCISCO—A combination treatment regimen can produce durable remissions in patients newly diagnosed with peripheral T-cell lymphoma (PTCL), results of a phase 1 study suggest.
The patients received brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisolone (BV+CHP). In some cases, this was followed by BV monotherapy.
The estimated 3-year progression-free survival (PFS) for these patients was 52%, and the overall survival (OS) was 80%.
There was a high rate of peripheral neuropathy (73%), but most cases resolved or improved over time.
Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues presented these data as a poster at the 8th Annual T-cell Lymphoma Forum. The study was supported by Seattle Genetics and Millennium Pharmaceuticals.
The researchers presented data on 26 patients newly diagnosed with PTCL. Nineteen patients had systemic anaplastic large-cell lymphoma (ALCL; 16 ALK- and 3 ALK+), 2 had PTCL not otherwise specified, 2 had angioimmunoblastic T-cell lymphoma, 2 had adult T-cell leukemia/lymphoma, and 1 had enteropathy-associated T-cell lymphoma.
The patients’ median age was 56 (range, 21-82). Sixty-nine percent of patients had an IPI score of 2 or higher, and 73% had stage III/IV disease.
Treatment
The patients received BV+CHP every 3 weeks for 6 cycles. Those who achieved at least a partial remission could go on to receive up to 10 additional cycles of single-agent BV at 1.8 mg/kg every 3 weeks.
Twenty-three patients (88%) completed all 6 cycles of BV+CHP, and 21 patients (81%) went on to receive BV monotherapy, 11 of whom (42%) received all 10 cycles.
Fifteen patients (58%) discontinued treatment, 3 due to progressive disease, 3 due to investigator decision, 6 due to adverse events, and 3 due to patient decision.
After a median observation period of 38.7 months (range, 4.6 to 44.3), 77% of patients (n=20) remained on study.
Toxicity
The most common adverse events (occurring in at least 30% of patients) were nausea (69%), peripheral sensory neuropathy (69%), diarrhea (62%), fatigue (58%), alopecia (54%), dyspnea (46%), constipation (35%), myalgia (31%), peripheral edema (31%), chills (31%), anemia (31%), insomnia (31%), and febrile neutropenia.
The most common grade 3 or higher adverse events (occurring in at least 10% of patients) were febrile neutropenia (31%), neutropenia (23%), anemia (15%), and pulmonary embolism (12%).
There were 6 adverse events resulting in treatment discontinuation—peripheral sensory neuropathy (n=3), abdominal pain (n=1), asthenia (n=1), and peripheral motor neuropathy (n=1).
Seventy-three percent of patients (19/26) experienced peripheral neuropathy. Thirty-seven percent (n=7) had a complete resolution of neuropathy, and 58% (n=11) had some improvement. The median time to resolution was 1.3 months. Twelve patients (63%) had ongoing neuropathy at last follow-up, but most had grade 1 (n=10).
Response and survival
The objective response rate was 100%, and the complete response rate was 88% (n=23). One patient who had a partial response at the end of combination therapy achieved a complete response after going on to single-agent BV.
Twenty-one of the 26 patients are still alive—15 with ALCL and 6 with other PTCLs. Sixteen patients have not progressed—9 with ALCL and 5 with other PTCLs.
After progression, 5 patients received BV again, and 3 received stem cell transplants (2 allogeneic and 1 autologous).
The estimated 3-year PFS was 52%, and the estimated OS was 80%. The researchers noted that these rates compare favorably with the historical 3-year PFS and OS rates of 30% and 40%, respectively.
Researchers are currently conducting a phase 3 trial comparing BV+CHP with CHOP as frontline treatment of CD30+ mature T-cell lymphomas (ECHELON-2, NCT01777152). ![]()

Photo by Larry Young
SAN FRANCISCO—A combination treatment regimen can produce durable remissions in patients newly diagnosed with peripheral T-cell lymphoma (PTCL), results of a phase 1 study suggest.
The patients received brentuximab vedotin plus cyclophosphamide, doxorubicin, and prednisolone (BV+CHP). In some cases, this was followed by BV monotherapy.
The estimated 3-year progression-free survival (PFS) for these patients was 52%, and the overall survival (OS) was 80%.
There was a high rate of peripheral neuropathy (73%), but most cases resolved or improved over time.
Steven Horwitz, MD, of Memorial Sloan Kettering Cancer Center in New York, New York, and his colleagues presented these data as a poster at the 8th Annual T-cell Lymphoma Forum. The study was supported by Seattle Genetics and Millennium Pharmaceuticals.
The researchers presented data on 26 patients newly diagnosed with PTCL. Nineteen patients had systemic anaplastic large-cell lymphoma (ALCL; 16 ALK- and 3 ALK+), 2 had PTCL not otherwise specified, 2 had angioimmunoblastic T-cell lymphoma, 2 had adult T-cell leukemia/lymphoma, and 1 had enteropathy-associated T-cell lymphoma.
The patients’ median age was 56 (range, 21-82). Sixty-nine percent of patients had an IPI score of 2 or higher, and 73% had stage III/IV disease.
Treatment
The patients received BV+CHP every 3 weeks for 6 cycles. Those who achieved at least a partial remission could go on to receive up to 10 additional cycles of single-agent BV at 1.8 mg/kg every 3 weeks.
Twenty-three patients (88%) completed all 6 cycles of BV+CHP, and 21 patients (81%) went on to receive BV monotherapy, 11 of whom (42%) received all 10 cycles.
Fifteen patients (58%) discontinued treatment, 3 due to progressive disease, 3 due to investigator decision, 6 due to adverse events, and 3 due to patient decision.
After a median observation period of 38.7 months (range, 4.6 to 44.3), 77% of patients (n=20) remained on study.
Toxicity
The most common adverse events (occurring in at least 30% of patients) were nausea (69%), peripheral sensory neuropathy (69%), diarrhea (62%), fatigue (58%), alopecia (54%), dyspnea (46%), constipation (35%), myalgia (31%), peripheral edema (31%), chills (31%), anemia (31%), insomnia (31%), and febrile neutropenia.
The most common grade 3 or higher adverse events (occurring in at least 10% of patients) were febrile neutropenia (31%), neutropenia (23%), anemia (15%), and pulmonary embolism (12%).
There were 6 adverse events resulting in treatment discontinuation—peripheral sensory neuropathy (n=3), abdominal pain (n=1), asthenia (n=1), and peripheral motor neuropathy (n=1).
Seventy-three percent of patients (19/26) experienced peripheral neuropathy. Thirty-seven percent (n=7) had a complete resolution of neuropathy, and 58% (n=11) had some improvement. The median time to resolution was 1.3 months. Twelve patients (63%) had ongoing neuropathy at last follow-up, but most had grade 1 (n=10).
Response and survival
The objective response rate was 100%, and the complete response rate was 88% (n=23). One patient who had a partial response at the end of combination therapy achieved a complete response after going on to single-agent BV.
Twenty-one of the 26 patients are still alive—15 with ALCL and 6 with other PTCLs. Sixteen patients have not progressed—9 with ALCL and 5 with other PTCLs.
After progression, 5 patients received BV again, and 3 received stem cell transplants (2 allogeneic and 1 autologous).
The estimated 3-year PFS was 52%, and the estimated OS was 80%. The researchers noted that these rates compare favorably with the historical 3-year PFS and OS rates of 30% and 40%, respectively.
Researchers are currently conducting a phase 3 trial comparing BV+CHP with CHOP as frontline treatment of CD30+ mature T-cell lymphomas (ECHELON-2, NCT01777152). ![]()
Drug could aid standard care for aTTP
Results of a phase 2 trial suggest an investigational agent may improve upon standard care for acquired thrombotic thrombocytopenic purpura (aTTP).
The agent, caplacizumab, is an anti-von Willebrand factor, humanized, single-variable-domain immunoglobulin that works by inhibiting the interaction between ultralarge von Willebrand factor multimers and platelets.
In the phase 2 TITAN trial, caplacizumab plus standard care induced a faster resolution of aTTP episodes when compared to placebo plus standard care. However, caplacizumab was also associated with a higher risk of bleeding.
Flora Peyvandi, MD, PhD, of the University of Milan in Italy, and her colleagues reported these results in The New England Journal of Medicine. The study was supported by Ablynx, the company developing caplacizumab.
“Caplacizumab has the potential to become an important new component in the standard of care for patients with acquired TTP,” Dr Peyvandi said. “The results from the phase 2 TITAN study showed that caplacizumab acts quickly to control the critical acute phase of the disease and protects patients until immunosuppressive treatments take effect.”
TITAN was a single-blinded, randomized, placebo-controlled study conducted at 56 centers around the world. The trial included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
Patients in the caplacizumab arm immediately received an intravenous bolus dose of caplacizumab at 10 mg and then a 10 mg subcutaneous dose of the drug daily until 30 days had elapsed after the final plasma exchange. Patients in the control arm received placebo at the same time points.
Response, recurrence, and relapse
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
One of the study’s secondary endpoints was exacerbation, which was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required reinitiation of daily exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively.
Another secondary endpoint was relapse, which was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. The investigators noted that 7 of the 8 patients had ADAMTS13 activity that remained below 10%, which suggests unresolved autoimmune activity.
Adverse events
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
The rate of bleeding-related AEs was 54% in the caplacizumab arm and 38% in the placebo arm. Of the 101 bleeding-related AEs, 84 (83%) were reported as mild, 14 (14%) as moderate, and 3 (3%) as severe.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
Caplacizumab development
The results of this trial will serve as the basis for filing for conditional approval of caplacizumab in Europe in the first half of 2017, according to Ablynx. The company is planning to file in the US in 2018.
Ablynx has started a phase 3 trial of caplacizumab known as the HERCULES study. In this double-blind, placebo-controlled study, investigators are evaluating the safety and efficacy of caplacizumab, in conjunction with the standard of care, in patients with aTTP.
The study is expected to enroll 92 patients at clinical sites across 17 countries. Recruitment is expected to be complete by the end of 2017. ![]()
Results of a phase 2 trial suggest an investigational agent may improve upon standard care for acquired thrombotic thrombocytopenic purpura (aTTP).
The agent, caplacizumab, is an anti-von Willebrand factor, humanized, single-variable-domain immunoglobulin that works by inhibiting the interaction between ultralarge von Willebrand factor multimers and platelets.
In the phase 2 TITAN trial, caplacizumab plus standard care induced a faster resolution of aTTP episodes when compared to placebo plus standard care. However, caplacizumab was also associated with a higher risk of bleeding.
Flora Peyvandi, MD, PhD, of the University of Milan in Italy, and her colleagues reported these results in The New England Journal of Medicine. The study was supported by Ablynx, the company developing caplacizumab.
“Caplacizumab has the potential to become an important new component in the standard of care for patients with acquired TTP,” Dr Peyvandi said. “The results from the phase 2 TITAN study showed that caplacizumab acts quickly to control the critical acute phase of the disease and protects patients until immunosuppressive treatments take effect.”
TITAN was a single-blinded, randomized, placebo-controlled study conducted at 56 centers around the world. The trial included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
Patients in the caplacizumab arm immediately received an intravenous bolus dose of caplacizumab at 10 mg and then a 10 mg subcutaneous dose of the drug daily until 30 days had elapsed after the final plasma exchange. Patients in the control arm received placebo at the same time points.
Response, recurrence, and relapse
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
One of the study’s secondary endpoints was exacerbation, which was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required reinitiation of daily exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively.
Another secondary endpoint was relapse, which was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. The investigators noted that 7 of the 8 patients had ADAMTS13 activity that remained below 10%, which suggests unresolved autoimmune activity.
Adverse events
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
The rate of bleeding-related AEs was 54% in the caplacizumab arm and 38% in the placebo arm. Of the 101 bleeding-related AEs, 84 (83%) were reported as mild, 14 (14%) as moderate, and 3 (3%) as severe.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
Caplacizumab development
The results of this trial will serve as the basis for filing for conditional approval of caplacizumab in Europe in the first half of 2017, according to Ablynx. The company is planning to file in the US in 2018.
Ablynx has started a phase 3 trial of caplacizumab known as the HERCULES study. In this double-blind, placebo-controlled study, investigators are evaluating the safety and efficacy of caplacizumab, in conjunction with the standard of care, in patients with aTTP.
The study is expected to enroll 92 patients at clinical sites across 17 countries. Recruitment is expected to be complete by the end of 2017. ![]()
Results of a phase 2 trial suggest an investigational agent may improve upon standard care for acquired thrombotic thrombocytopenic purpura (aTTP).
The agent, caplacizumab, is an anti-von Willebrand factor, humanized, single-variable-domain immunoglobulin that works by inhibiting the interaction between ultralarge von Willebrand factor multimers and platelets.
In the phase 2 TITAN trial, caplacizumab plus standard care induced a faster resolution of aTTP episodes when compared to placebo plus standard care. However, caplacizumab was also associated with a higher risk of bleeding.
Flora Peyvandi, MD, PhD, of the University of Milan in Italy, and her colleagues reported these results in The New England Journal of Medicine. The study was supported by Ablynx, the company developing caplacizumab.
“Caplacizumab has the potential to become an important new component in the standard of care for patients with acquired TTP,” Dr Peyvandi said. “The results from the phase 2 TITAN study showed that caplacizumab acts quickly to control the critical acute phase of the disease and protects patients until immunosuppressive treatments take effect.”
TITAN was a single-blinded, randomized, placebo-controlled study conducted at 56 centers around the world. The trial included 75 aTTP patients who were randomized to caplacizumab (n=36) or placebo (n=39), with all patients receiving the current standard of care (daily plasma exchange and immunosuppressive therapy).
Patients in the caplacizumab arm immediately received an intravenous bolus dose of caplacizumab at 10 mg and then a 10 mg subcutaneous dose of the drug daily until 30 days had elapsed after the final plasma exchange. Patients in the control arm received placebo at the same time points.
Response, recurrence, and relapse
The study’s primary endpoint was time to response (platelet count normalization). Patients in the caplacizumab arm had a 39% reduction in the median time to response compared to patients in the placebo arm (P=0.005).
Among the 69 patients who had not undergone a plasma-exchange session before enrollment, the median time to response was 3.0 days in the caplacizumab arm and 4.9 days in the placebo arm.
Among the 6 patients who did undergo a plasma-exchange session before enrollment, the median time to a response was 2.4 days in the caplacizumab arm and 4.3 days in the placebo arm.
The rate of confirmed response was 86.1% (n=31) in the caplacizumab arm and 71.8% (n=28) in the placebo arm.
One of the study’s secondary endpoints was exacerbation, which was defined as recurrent thrombocytopenia within 30 days of the end of daily plasma exchange that required reinitiation of daily exchange.
There were fewer exacerbations in the caplacizumab arm than the placebo arm—3 (8.3%) and 11 (28.2%), respectively.
Another secondary endpoint was relapse, which was defined as a TTP event occurring more than 30 days after the end of daily plasma exchange.
There were more relapses in the caplacizumab arm than the placebo arm—8 (22.2%) and 0, respectively. The investigators noted that 7 of the 8 patients had ADAMTS13 activity that remained below 10%, which suggests unresolved autoimmune activity.
Adverse events
There were 541 adverse events (AEs) in 34 of the 35 evaluable patients receiving caplacizumab (97%) and 522 AEs in all 37 evaluable patients receiving placebo (100%). TTP exacerbations and relapses were not included as AEs.
The rate of AEs thought to be related to the study drug was 17% in the caplacizumab arm and 11% in the placebo arm. The rate of AEs that were possibly related was 54% and 8%, respectively. And the rate of serious AEs was 37% and 32%, respectively.
The rate of bleeding-related AEs was 54% in the caplacizumab arm and 38% in the placebo arm. Of the 101 bleeding-related AEs, 84 (83%) were reported as mild, 14 (14%) as moderate, and 3 (3%) as severe.
There were no deaths in the caplacizumab arm and 2 in the placebo arm. One death was due to severe, refractory TTP, and the other was due to cerebral hemorrhage.
Caplacizumab development
The results of this trial will serve as the basis for filing for conditional approval of caplacizumab in Europe in the first half of 2017, according to Ablynx. The company is planning to file in the US in 2018.
Ablynx has started a phase 3 trial of caplacizumab known as the HERCULES study. In this double-blind, placebo-controlled study, investigators are evaluating the safety and efficacy of caplacizumab, in conjunction with the standard of care, in patients with aTTP.
The study is expected to enroll 92 patients at clinical sites across 17 countries. Recruitment is expected to be complete by the end of 2017. ![]()
Chemo linked to long-term learning problems in ALL survivors

Pediatric acute lymphoblastic leukemia (ALL) patients treated with chemotherapy alone are at risk for attention and learning problems that persist after treatment ends, according to a study published in the Journal of Clinical Oncology.
Researchers said this study is the largest assessment to date of neurocognitive outcomes in pediatric ALL survivors treated with intensive chemotherapy alone rather than in combination with cranial radiation.
“This is an important contribution to the literature because the smaller size and design of previous studies made examining the impact of treatment difficult,” said study author Lisa Jacola, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The findings underscore the need for neurocognitive and academic screening to be included as part of routine survivorship care for all pediatric ALL survivors.”
The study included patients enrolled in the St. Jude Total Therapy Study XV. They underwent neurocognitive assessments at the beginning of induction (n=142), end of maintenance (n=243), and 2 years after completing treatment (n=211).
The subjects completed standardized tests of overall intelligence, attention, learning, and academic performance. In addition, parents and other caregivers rated the subjects’ attention, learning, and behavior.
Two years after treatment completion, subjects performed as expected for their age (compared to national data) on measures of overall intelligence, learning, and memory. However, study subjects had significant attention deficits and a significantly greater frequency of learning problems (all P≤0.005).
The risk of such problems was greatest for survivors who were less than 5 years old when diagnosed with ALL and for those who received more intensive chemotherapy.
Specifically, the younger subjects had a greater risk of difficulties with attention, learning, working memory, and processing speed (all P≤0.05). And subjects who received higher-intensity, CNS-directed chemotherapy had a greater risk of difficulties in attention, processing speed, and academics (all P≤0.01).
Researchers also found that subjects with attention problems at the end of therapy had lower academic scores 2 years later (all P≤0.05).
“These findings provide additional evidence that neurocognitive functioning has improved in survivors of childhood ALL since cranial irradiation was replaced with intensified chemotherapy,” Dr Jacola said.
“But we also show these young people are at an elevated risk for attention problems that have real-world consequences, particularly for learning and school performance. Attention is a building block for learning, and, in this study, attention difficulties predicted academic problems later. If we know attention problems seen at the end of therapy continue and contribute to academic problems, then our goal is to intervene earlier to reduce or prevent such difficulties.” ![]()

Pediatric acute lymphoblastic leukemia (ALL) patients treated with chemotherapy alone are at risk for attention and learning problems that persist after treatment ends, according to a study published in the Journal of Clinical Oncology.
Researchers said this study is the largest assessment to date of neurocognitive outcomes in pediatric ALL survivors treated with intensive chemotherapy alone rather than in combination with cranial radiation.
“This is an important contribution to the literature because the smaller size and design of previous studies made examining the impact of treatment difficult,” said study author Lisa Jacola, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The findings underscore the need for neurocognitive and academic screening to be included as part of routine survivorship care for all pediatric ALL survivors.”
The study included patients enrolled in the St. Jude Total Therapy Study XV. They underwent neurocognitive assessments at the beginning of induction (n=142), end of maintenance (n=243), and 2 years after completing treatment (n=211).
The subjects completed standardized tests of overall intelligence, attention, learning, and academic performance. In addition, parents and other caregivers rated the subjects’ attention, learning, and behavior.
Two years after treatment completion, subjects performed as expected for their age (compared to national data) on measures of overall intelligence, learning, and memory. However, study subjects had significant attention deficits and a significantly greater frequency of learning problems (all P≤0.005).
The risk of such problems was greatest for survivors who were less than 5 years old when diagnosed with ALL and for those who received more intensive chemotherapy.
Specifically, the younger subjects had a greater risk of difficulties with attention, learning, working memory, and processing speed (all P≤0.05). And subjects who received higher-intensity, CNS-directed chemotherapy had a greater risk of difficulties in attention, processing speed, and academics (all P≤0.01).
Researchers also found that subjects with attention problems at the end of therapy had lower academic scores 2 years later (all P≤0.05).
“These findings provide additional evidence that neurocognitive functioning has improved in survivors of childhood ALL since cranial irradiation was replaced with intensified chemotherapy,” Dr Jacola said.
“But we also show these young people are at an elevated risk for attention problems that have real-world consequences, particularly for learning and school performance. Attention is a building block for learning, and, in this study, attention difficulties predicted academic problems later. If we know attention problems seen at the end of therapy continue and contribute to academic problems, then our goal is to intervene earlier to reduce or prevent such difficulties.” ![]()

Pediatric acute lymphoblastic leukemia (ALL) patients treated with chemotherapy alone are at risk for attention and learning problems that persist after treatment ends, according to a study published in the Journal of Clinical Oncology.
Researchers said this study is the largest assessment to date of neurocognitive outcomes in pediatric ALL survivors treated with intensive chemotherapy alone rather than in combination with cranial radiation.
“This is an important contribution to the literature because the smaller size and design of previous studies made examining the impact of treatment difficult,” said study author Lisa Jacola, PhD, of St. Jude Children’s Research Hospital in Memphis, Tennessee.
“The findings underscore the need for neurocognitive and academic screening to be included as part of routine survivorship care for all pediatric ALL survivors.”
The study included patients enrolled in the St. Jude Total Therapy Study XV. They underwent neurocognitive assessments at the beginning of induction (n=142), end of maintenance (n=243), and 2 years after completing treatment (n=211).
The subjects completed standardized tests of overall intelligence, attention, learning, and academic performance. In addition, parents and other caregivers rated the subjects’ attention, learning, and behavior.
Two years after treatment completion, subjects performed as expected for their age (compared to national data) on measures of overall intelligence, learning, and memory. However, study subjects had significant attention deficits and a significantly greater frequency of learning problems (all P≤0.005).
The risk of such problems was greatest for survivors who were less than 5 years old when diagnosed with ALL and for those who received more intensive chemotherapy.
Specifically, the younger subjects had a greater risk of difficulties with attention, learning, working memory, and processing speed (all P≤0.05). And subjects who received higher-intensity, CNS-directed chemotherapy had a greater risk of difficulties in attention, processing speed, and academics (all P≤0.01).
Researchers also found that subjects with attention problems at the end of therapy had lower academic scores 2 years later (all P≤0.05).
“These findings provide additional evidence that neurocognitive functioning has improved in survivors of childhood ALL since cranial irradiation was replaced with intensified chemotherapy,” Dr Jacola said.
“But we also show these young people are at an elevated risk for attention problems that have real-world consequences, particularly for learning and school performance. Attention is a building block for learning, and, in this study, attention difficulties predicted academic problems later. If we know attention problems seen at the end of therapy continue and contribute to academic problems, then our goal is to intervene earlier to reduce or prevent such difficulties.” ![]()
Low doses of iron may cause cell damage

Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()

Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()

Image courtesy of NIH
Low doses of iron can modify the vascular endothelium and induce a DNA-damage response, researchers have reported in PLOS ONE.
The team observed these phenomena in vitro and said the results must be confirmed via additional research.
However, the findings suggest a need to assess the amount of iron given in standard treatments and the effects this may have on the body, according to Claire Shovlin, PhD, of Imperial College London in the UK.
Dr Shovlin and her colleagues studied human endothelial cells, adding either placebo or an iron solution of 10 micromolar, which is a similar concentration to that seen in the blood after taking an iron tablet.
Within 10 minutes, cells treated with the iron solution had activated DNA repair systems, and these were still activated 6 hours later.
“We already knew that iron could be damaging to cells in very high doses,” Dr Shovlin said. “However, in this study, we found that when we applied the kinds of levels of iron you would find in the bloodstream after taking an iron tablet, this also seemed to be able to trigger cell damage—at least in the laboratory. In other words, cells seem more sensitive to iron than we previously thought.”
“This is very early stage research, and we need more work to confirm these findings and investigate what effects this may have on the body. We are still not sure how these laboratory findings translate to blood vessels in the body.”
“However, this study helps to open the conversation about how much iron people take. At the moment, each standard iron tablet contains almost 10 times the amount of iron men are recommended to eat each day, and these dosages haven’t changed for more than 50 years. This research suggests we may need to think more carefully about how much iron we give to people, and try and tailor the dose to the patient.”
Dr Shovlin and her colleagues initially started researching this area after finding that a small proportion of people using iron tablets for hereditary hemorrhagic telangiectasia, which causes abnormalities in the blood vessels, reported their nose bleeds worsened after iron treatment. ![]()
Let’s call a fungus a fungus
It seemed like a teachable moment. My student looked on as Laura took off her shoes and showed us livid, polycyclic plaques covering the dorsum of her left foot. The way her rash looked, bordering 10 obviously fungal toenails, left little doubt about the problem.
“I’m going to guess you’re using a steroid cream,” I said.
“Could I please tell you the whole story?” said Laura, with some impatience.
“Sure,” I said. I love whole stories.
So Laura told me hers, starting with her walk through the tall grass in the summer, followed by “poison ivy” that her primary care physician treated with “a cream.”
“Did the cream have hydrocortisone in it?”
“I think so,” she said. But that didn’t work, so her doctor prescribed another cream. That one seemed to help a bit, but then the rash got redder and itchier, so she got another cream. “I think it was called clobetasol,” Laura said.
“Several years ago,” Laura went on, “you said I had toenail fungus in my nails, but I didn’t want to take pills for it because it didn’t bother me enough.”
“Maybe now would be a good time,” I said.
After I had recommended oral and topical therapy (and stopped the clobetasol!), my student and I went into my office. Like most of my students, she is headed for a career in primary care, in her case, Family Medicine.
“What do you think?” I asked her. “How does this case reflect on the state of dermatology expertise in the primary care community?” We’ve been discussing this, because Laura’s was not the first such example, just the most egregious.
My student’s eyes widened. No need to belabor the point.
“The problem is not that Laura’s primary care physician made a mistake,” I said. “I make them too, like prescribing antifungal creams for eczema and steroid creams for fungi. The problem is not noticing that you’ve made the mistake – with the evidence literally staring you in the face – and then either fixing it, or else consulting someone else who can help you fix it.”
“I’m going to do a better job!” said my student, with feeling.
Perhaps she will. At least she will graduate medical school having learned that there is such a thing as nummular eczema and been told that not every round rash is a fungus. As with almost every 4th-year student who’s taken my elective for the last 35 years, she had little dermatology exposure until now beyond a couple of PowerPoint shows of exotic diseases. I had none either back in school, when dinosaurs roamed the earth.
After I graduated, my prestigious pediatric residency taught me a grand total of three dermatologic facts: 1. For tinea capitis, shine a Wood’s light on the scalp; 2. For pityriasis rosea, shine a Wood’s light on the body; and 3. If a groin wash involves the inguinal fold, it’s a yeast infection. I learned a lot, didn’t I?
Reflecting on Lesson #1, Trichophyton tonsurans, which doesn’t fluoresce, has predominated for half a century (and 90% of the time, the problem is seborrhea anyway). As for #2 and #3, never mind.
Decade after decade, the patients troop in: Eczemas treated as fungi, fungi treated with steroids, itchy rashes treated with permethrin, then treated again because the itch didn’t stop, because you can’t kill bugs that aren’t there.
Clinical dermatology is not rocket science. Eczema and fungus are so common that it is hardly possible not to encounter them in daily practice. Generations of providers come and go, yet the same clinical missteps persist.
Why are the common skin problems of ordinary patients not a priority in medical education? Why do so many practitioners keep doing the same things and not get better at doing them?
Perhaps such common problems just pass under the educational radar. Maybe these diseases aren’t sexy enough, their poor outcomes not consequential enough. Maybe the shoe just doesn’t pinch hard enough on these itchy, polycyclic plaques.
My students are very young and earnest. They mean to get out into the world and do a good job. Many challenges before them, which now include crushing, mind-numbing bureaucratic demands. Can we ask that, while they are busy clicking drop-down boxes on their EHR’s and mastering genomic medicine, they also treat eczema as eczema and fungus as fungus?
One hopes so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
It seemed like a teachable moment. My student looked on as Laura took off her shoes and showed us livid, polycyclic plaques covering the dorsum of her left foot. The way her rash looked, bordering 10 obviously fungal toenails, left little doubt about the problem.
“I’m going to guess you’re using a steroid cream,” I said.
“Could I please tell you the whole story?” said Laura, with some impatience.
“Sure,” I said. I love whole stories.
So Laura told me hers, starting with her walk through the tall grass in the summer, followed by “poison ivy” that her primary care physician treated with “a cream.”
“Did the cream have hydrocortisone in it?”
“I think so,” she said. But that didn’t work, so her doctor prescribed another cream. That one seemed to help a bit, but then the rash got redder and itchier, so she got another cream. “I think it was called clobetasol,” Laura said.
“Several years ago,” Laura went on, “you said I had toenail fungus in my nails, but I didn’t want to take pills for it because it didn’t bother me enough.”
“Maybe now would be a good time,” I said.
After I had recommended oral and topical therapy (and stopped the clobetasol!), my student and I went into my office. Like most of my students, she is headed for a career in primary care, in her case, Family Medicine.
“What do you think?” I asked her. “How does this case reflect on the state of dermatology expertise in the primary care community?” We’ve been discussing this, because Laura’s was not the first such example, just the most egregious.
My student’s eyes widened. No need to belabor the point.
“The problem is not that Laura’s primary care physician made a mistake,” I said. “I make them too, like prescribing antifungal creams for eczema and steroid creams for fungi. The problem is not noticing that you’ve made the mistake – with the evidence literally staring you in the face – and then either fixing it, or else consulting someone else who can help you fix it.”
“I’m going to do a better job!” said my student, with feeling.
Perhaps she will. At least she will graduate medical school having learned that there is such a thing as nummular eczema and been told that not every round rash is a fungus. As with almost every 4th-year student who’s taken my elective for the last 35 years, she had little dermatology exposure until now beyond a couple of PowerPoint shows of exotic diseases. I had none either back in school, when dinosaurs roamed the earth.
After I graduated, my prestigious pediatric residency taught me a grand total of three dermatologic facts: 1. For tinea capitis, shine a Wood’s light on the scalp; 2. For pityriasis rosea, shine a Wood’s light on the body; and 3. If a groin wash involves the inguinal fold, it’s a yeast infection. I learned a lot, didn’t I?
Reflecting on Lesson #1, Trichophyton tonsurans, which doesn’t fluoresce, has predominated for half a century (and 90% of the time, the problem is seborrhea anyway). As for #2 and #3, never mind.
Decade after decade, the patients troop in: Eczemas treated as fungi, fungi treated with steroids, itchy rashes treated with permethrin, then treated again because the itch didn’t stop, because you can’t kill bugs that aren’t there.
Clinical dermatology is not rocket science. Eczema and fungus are so common that it is hardly possible not to encounter them in daily practice. Generations of providers come and go, yet the same clinical missteps persist.
Why are the common skin problems of ordinary patients not a priority in medical education? Why do so many practitioners keep doing the same things and not get better at doing them?
Perhaps such common problems just pass under the educational radar. Maybe these diseases aren’t sexy enough, their poor outcomes not consequential enough. Maybe the shoe just doesn’t pinch hard enough on these itchy, polycyclic plaques.
My students are very young and earnest. They mean to get out into the world and do a good job. Many challenges before them, which now include crushing, mind-numbing bureaucratic demands. Can we ask that, while they are busy clicking drop-down boxes on their EHR’s and mastering genomic medicine, they also treat eczema as eczema and fungus as fungus?
One hopes so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.
It seemed like a teachable moment. My student looked on as Laura took off her shoes and showed us livid, polycyclic plaques covering the dorsum of her left foot. The way her rash looked, bordering 10 obviously fungal toenails, left little doubt about the problem.
“I’m going to guess you’re using a steroid cream,” I said.
“Could I please tell you the whole story?” said Laura, with some impatience.
“Sure,” I said. I love whole stories.
So Laura told me hers, starting with her walk through the tall grass in the summer, followed by “poison ivy” that her primary care physician treated with “a cream.”
“Did the cream have hydrocortisone in it?”
“I think so,” she said. But that didn’t work, so her doctor prescribed another cream. That one seemed to help a bit, but then the rash got redder and itchier, so she got another cream. “I think it was called clobetasol,” Laura said.
“Several years ago,” Laura went on, “you said I had toenail fungus in my nails, but I didn’t want to take pills for it because it didn’t bother me enough.”
“Maybe now would be a good time,” I said.
After I had recommended oral and topical therapy (and stopped the clobetasol!), my student and I went into my office. Like most of my students, she is headed for a career in primary care, in her case, Family Medicine.
“What do you think?” I asked her. “How does this case reflect on the state of dermatology expertise in the primary care community?” We’ve been discussing this, because Laura’s was not the first such example, just the most egregious.
My student’s eyes widened. No need to belabor the point.
“The problem is not that Laura’s primary care physician made a mistake,” I said. “I make them too, like prescribing antifungal creams for eczema and steroid creams for fungi. The problem is not noticing that you’ve made the mistake – with the evidence literally staring you in the face – and then either fixing it, or else consulting someone else who can help you fix it.”
“I’m going to do a better job!” said my student, with feeling.
Perhaps she will. At least she will graduate medical school having learned that there is such a thing as nummular eczema and been told that not every round rash is a fungus. As with almost every 4th-year student who’s taken my elective for the last 35 years, she had little dermatology exposure until now beyond a couple of PowerPoint shows of exotic diseases. I had none either back in school, when dinosaurs roamed the earth.
After I graduated, my prestigious pediatric residency taught me a grand total of three dermatologic facts: 1. For tinea capitis, shine a Wood’s light on the scalp; 2. For pityriasis rosea, shine a Wood’s light on the body; and 3. If a groin wash involves the inguinal fold, it’s a yeast infection. I learned a lot, didn’t I?
Reflecting on Lesson #1, Trichophyton tonsurans, which doesn’t fluoresce, has predominated for half a century (and 90% of the time, the problem is seborrhea anyway). As for #2 and #3, never mind.
Decade after decade, the patients troop in: Eczemas treated as fungi, fungi treated with steroids, itchy rashes treated with permethrin, then treated again because the itch didn’t stop, because you can’t kill bugs that aren’t there.
Clinical dermatology is not rocket science. Eczema and fungus are so common that it is hardly possible not to encounter them in daily practice. Generations of providers come and go, yet the same clinical missteps persist.
Why are the common skin problems of ordinary patients not a priority in medical education? Why do so many practitioners keep doing the same things and not get better at doing them?
Perhaps such common problems just pass under the educational radar. Maybe these diseases aren’t sexy enough, their poor outcomes not consequential enough. Maybe the shoe just doesn’t pinch hard enough on these itchy, polycyclic plaques.
My students are very young and earnest. They mean to get out into the world and do a good job. Many challenges before them, which now include crushing, mind-numbing bureaucratic demands. Can we ask that, while they are busy clicking drop-down boxes on their EHR’s and mastering genomic medicine, they also treat eczema as eczema and fungus as fungus?
One hopes so.
Dr. Rockoff practices dermatology in Brookline, Mass., and is a longtime contributor to Dermatology News. He serves on the clinical faculty at Tufts University, Boston, and has taught senior medical students and other trainees for 30 years.