User login
Case Studies in Toxicology: One Last Kick—Transverse Myelitis After an Overdose of Heroin via Insufflation
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 17-year-old adolescent girl with a history of depression and opioid dependence, for which she was taking buprenorphine until 2 weeks earlier, presented to the ED via emergency medical services (EMS) after her father found her lying on the couch unresponsive and with shallow respirations. Naloxone was administered by EMS and her mental status improved.
At presentation, the patient admitted to insufflation of an unknown amount of heroin and ingestion of 2 mg of alprazolam earlier in the day. She denied any past or current use of intravenous (IV) drugs. During monitoring, she began to complain of numbness in her legs and an inability to urinate. Examination revealed paralysis and decreased sensation of her bilateral lower extremities to the midthigh, with decreased rectal tone. Because of the patient’s history of drug use and temporal association with the heroin overdose, both neurosurgery and toxicology services were consulted.
What can cause lower extremity paralysis in a drug user?
The differential diagnosis for the patient at this point included toxin-induced myelopathy, Guillain-Barré syndrome, hypokalemic periodic paralysis, spinal compression, epidural abscess, cerebrovascular accident, spinal lesion, and spinal artery dissection or infarction.
Although Guillain-Barré syndrome presents with ascending paralysis, there is usually an antecedent respiratory or gastrointestinal infection. While epidural abscess with spinal compression is associated with IV drug use and can present similarly, the patient in this case denied IV use. In the absence of any risk factors, cerebrovascular accident and spinal artery dissection were also unlikely.
Case Continuation
A bladder catheter was placed due to the patient’s inability to urinate, and approximately 1 L of urine output was retrieved. Immediate magnetic resonance imaging (MRI) demonstrated increased T2 signal intensity and expansion of the distal thoracic cord and conus without mass lesion, consistent with transverse myelitis (TM).
What is transverse myelitis and why does it occur?
Transverse myelitis is an inflammatory demyelinating disorder that focally affects the spinal cord, resulting in a specific pattern of motor, sensory, and autonomic dysfunction.1 Signs and symptoms include paresthesia, paralysis of the extremities, and loss of bladder and bowel control. The level of the spinal cord affected determines the clinical effects. Demyelination typically occurs at the thoracic segment, producing findings in the legs, as well as bladder and bowel dysfunction.
The exact cause of TM is unknown, but the inflammation may result from a viral complication or an abnormal immune response. Infectious viral agents suspected of causing TM include varicella zoster, herpes simplex, cytomegalovirus, Epstein-Barr, influenza, human immunodeficiency virus, hepatitis A, and rubella. It has also been postulated that an autoimmune reaction is responsible for the condition.
In some individuals, TM represents the first manifestation of an underlying demyelinating disorder such as multiple sclerosis or neuromyelitis optica. A diagnosis of TM is made through patient history, physical examination, and characteristic findings on neuroimaging, specifically MRI.
Heroin use has long been associated with the development of TM, and is usually associated with IV administration of the drug after a period of abstinence.2 This association strengthens the basis for an immunologic etiology—an initial sensitization and subsequent reexposure causing the effects of TM. There have also been cases of TM coexisting with rhabdomyolysis due to the patient being found in a contorted position.3 Another theory of the etiology of heroin-associated TM is a reaction to a possible adulterant or contaminant in the heroin.4
What is the treatment and prognosis of transverse myelitis?
Since there is no cure for TM, treatment is directed at reducing inflammation in the spinal cord. Initial therapy generally includes corticosteroids. In patients with a minimal response to corticosteroids, plasma exchange can be attempted. There are also limited data to suggest a beneficial role for the use of IV immunoglobulin.5 In addition to treatment, general supportive care must also be optimized, such as the use of prophylaxis for thrombophlebitis due to immobility and physical therapy, if possible.
The prognosis of patients with TM is variable, and up to two thirds of patients will have moderate-to-severe residual neurological disability.6 Recovery is slow, with most patients beginning to show improvement within the first 2 to 12 weeks from treatment and supportive care. The recovery process can continue for 2 years. However, if no improvement is made within the first 3 to 6 months, recovery is unlikely.7 Cases of heroin-associated TM may have a more favorable prognosis.8
A majority of individuals will only experience this clinical entity once, but there are rare causes of recurrent or relapsing TM.7 In these situations, a search for underlying demyelinating diseases should be performed.
Case Conclusion
The patient was immediately started on IV corticosteroids, but as there was no improvement after 5 days, plasmapheresis was performed. She received 5 cycles of plasmapheresis and a 5-day course of IV immunoglobulin but still without any improvement. A repeat MRI of the thoracic spine was performed and raised the possibility of cord infarct, but infectious or inflammatory myelitis remained within differential consideration. The patient continued to make minimal improvement with physical therapy and, after a 3-week hospital course, she was transferred to inpatient rehabilitation for further care. Over the next 2 months, the loss of sensation and motor ability of her legs did not improve, but she did regain control of her bowels and bladder.
Dr Regina is a medical toxicology fellow in the department of emergency medicine at North Shore Long Island Jewish Health System, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.
- Pandit L. Transverse myelitis spectrum disorders. Neurol India. 2009;57(2):126-133.
- Richter RW, Rosenberg RN. Transverse myelitis associated with heroin addiction. JAMA. 1968;206(6):1255-1257.
- Sahni V, Garg D, Garg S, Agarwal SK, Singh NP. Unusual complications of heroin abuse: transverse myelitis, rhabdomyolysis, compartment syndrome, and ARF. Clin Toxicol (Phila). 2008;46(2):153-155.
- Schein PS, Yessayan L, Mayman CI. Acute transverse myelitis associated with intravenous opium. Neurology. 1971;21(1):101-102.
- Absoud M, Gadian J, Hellier J, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312.
- West TW. Transverse myelitis--a review of the presentation, diagnosis, and initial management. Discov Med. 2013;16(88):167-177.
- Transverse myelitis fact sheet. National Institute of Neurological Disorders and Stroke. http://www.ninds.nih.gov/disorders/transversemyelitis/detail_transversemyelitis.htm. Updated June 24, 2015. Accessed September 2, 2015.
- McGuire JL, Beslow LA, Finkel RS, Zimmerman RA, Henretig FM. A teenager with focal weakness. Pediatr Emerg Care. 2008;24(12):875-879.
Malpractice Counsel: Cervical Spine Injury
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
| An 83-year-old man presented to the ED via emergency medical services (EMS) with a chief complaint of neck pain. He was the restrained driver of a car that was struck from behind by another vehicle. The patient denied any head injury, loss of consciousness, chest pain, shortness of breath, or abdominal pain. His medical history was significant for hypertension and coronary artery disease, for which he was taking several medications. Regarding his social history, the patient denied alcohol consumption or cigarette smoking. |
The patient’s physical examination was unremarkable. His vital signs were normal, and there was no obvious external evidence of trauma. The posterior cervical spine was tender to palpation in the midline, but no step-off signs were appreciated. The neurological examination, including strength and sensation in all four extremities, was normal.
Since the patient’s only complaint was neck pain and his physical examination and history were otherwise normal, the emergency physician (EP) ordered radiographs of the cervical spine. The imaging studies were interpreted as showing advanced degenerative changes but no fractures, and the patient was prescribed an analgesic and discharged home.
When the patient woke up the next morning, he was unable to move his extremities, and returned to the same ED via EMS. He was placed in a cervical collar and found to have flaccid extremities on examination. A computed tomography (CT) scan of the cervical spine revealed a transverse fracture through the C6 vertebra. Radiology services also reviewed the cervical spine X-rays from the previous day, noting the presence of fracture.
The patient was taken to the operating room by neurosurgery services but remained paralyzed postoperatively. He never recovered from his injury and died 6 months later. His family sued the EP and the hospital for missed diagnosis of cervical spine fracture at the first ED presentation and the resulting paralysis. The case was settled for $1.3 million prior to trial.
Discussion
The evaluation of suspected cervical spine injury secondary to blunt trauma is a frequent and important skill practiced by EPs. Motor vehicle accidents are the most common cause of spinal cord injury in the United States (42%), followed by falls (27%), acts of violence (15%), and sports-related injuries (8%).1 A review by Sekon and Fehlings2 showed that 55% of all spinal injuries involve the cervical spine. Interestingly, the majority of cervical spine injuries occur at the upper or lower ends of the cervical spine; C2 vertebral fractures account for 33%, while C6 and C7 vertebral fractures account for approximately 50%.1
There are two commonly used criteria to clinically clear the cervical spine (ie, no imaging studies necessary) in blunt-trauma patients. The first is the National Emergency X-Radiography Use Study (NEXUS), which has a sensitivity of 99.6% of identifying cervical spine fractures.1 According to the NEXUS criteria, no imaging studies are required if: (1) there is no midline cervical spine tenderness; (2) there are no focal neurological deficits; (3) the patient exhibits a normal level of alertness; (4) the patient is not intoxicated; and (5) there is no distracting injury.1
The other set of criteria used to clear the cervical spine is the Canadian Cervical Spine Rule. In these criteria, a patient is considered at very low risk for cervical spine fracture in the following cases: (1) the patient is fully alert with a Glasgow Coma scale of 15; (2) the patient has no high-risk factors (ie, age >65 years, dangerous mechanism of injury, fall greater than five stairs, axial load to the head, high-speed vehicular crash, bicycle or motorcycle crash, or the presence of paresthesias in the extremities); (3) the patient has low-risk factors (eg, simple vehicle crash, sitting position in the ED, ambulatory at any time, delayed onset of neck pain, and the absence of midline cervical tenderness); and (4) the patient can actively rotate his or her neck 45 degrees to the left and to the right. The Canadian group found the above criteria to have 100% sensitivity for predicting the absence of cervical spine injury.1
The patient in this case failed both sets of criteria (ie, presence of cervical spine tenderness and age >65 years) and therefore required imaging. Historically, cervical spine X-ray (three views, anteroposterior, lateral, and odontoid; or five views, three views plus obliques) has been the imaging study of choice for such patients. Unfortunately, however, cervical spine radiographs have severe limitations in identifying spinal injury. In a large retrospective review, Woodring and Lee,3 found that the standard three-view cervical spine series failed to demonstrate 61% of all fractures and 36% of all subluxation and dislocations. Similarly, in a prospective study of 1,006 patients with 72 injuries, Diaz et al,4 found a 52.3% missed fracture rate when five-view radiographs were used to identify cervical spine injury. In addition, radiographic evaluation of elderly patients was found to be even more challenging in identifying cervical spine injury due to age-related degenerative changes.
Given the abovementioned limitations associated with radiographic imaging, CT scan of the cervical spine has become the imaging study of choice in moderate-to-severe risk patients with blunt cervical spine trauma. This modality has been shown to have a higher sensitivity and specificity for evaluating cervical spine injury compared to plain X-ray films, with CT detecting 97% to 100% of cervical spine fractures.5
In addition to demonstrating a higher sensitivity, CT also has the advantage of speed—especially when the patient is undergoing other CT studies (eg, head, abdomen, pelvis). While some clinicians criticize the higher cost of CT versus plain films, CT has been shown to decrease institutional costs (when settlement costs are taken into account) due to the reduction of the incidence of paralysis resulting from false-negative imaging studies.6
Forgotten Tourniquet
| A 33-year-old woman presented to the ED with a chief complaint of left-sided abdominal and flank pain. She described the onset of pain as abrupt, severe, and lasting approximately 3 hours in duration. She admitted to nausea, but no vomiting. She also denied a history of any previous similar symptoms or recent trauma. The patient’s medical history was unremarkable. Her last menstrual period began 3 days prior to presentation. Regarding social history, she denied any tobacco or alcohol use. |
The patient’s vital signs were: blood pressure, 138/82 mm Hg; heart rate, 102 beats/minute; respiratory rate, 18 breaths/minute; temperature 98.6˚F. Oxygen saturation was 99% on room air.
The patient appeared uncomfortable overall. The physical examination was remarkable only for mild left-sided costovertebral angle tenderness. Her abdomen was soft, nontender, and without guarding or rebound.
The EP ordered the placement of an intravenous (IV) line, through which the patient was administered normal saline and morphine and promethazine, respectively, for pain and nausea. A complete blood count, basic metabolic panel, urinalysis, and urine pregnancy test were ordered. All of the laboratory bloodwork results were normal, and the urine pregnancy test was negative. The urinalysis was remarkable for 50 to 100 red blood cells.
A noncontrast CT scan of the abdomen and pelvis revealed a 3-mm ureteral stone on the left side. When the patient returned from radiology services, her pain was significantly decreased and she felt much improved. She was diagnosed with a kidney stone and discharged home with an analgesic and a strainer, along with instructions to follow-up with urology services. The patient was in the ED for a total of 5 hours.
The plaintiff sued the EP and hospital, claiming that the tourniquet used to start the IV line and draw blood was never removed, which in turn caused nerve damage resulting in reflex sympathetic dystrophy and complex regional pain syndrome. The defense denied all of these allegations, and the ED personnel testified that the tourniquet was removed as soon as the IV was established. The defense cited the plaintiff’s medical records, which contained documentation that the tourniquet had been removed. The defense further argued that if the tourniquet had been left on as the patient alleged, she would have experienced obvious physical signs, such as swelling, redness, infiltration of fluids, pain, and numbness. A defense verdict was returned.
Discussion
It is very tempting to simply dismiss this case as absurd, with nothing to be learned from it. It does defy common sense that no one would have noticed the tourniquet or, at the very least, that the patient would not have spoken up about it during her stay in the ED. While the jury clearly came to the correct conclusion, it does highlight a real problem: forgotten tourniquets.
According to the Pennsylvania Patient Safety Advisory (PPSA), there were 125 reports of tourniquets being left on patients in Pennsylvania healthcare facilities in 1 year alone.1 In 5% of these cases, the tourniquet was discovered within a half hour of application. In approximately 66% of cases, the tourniquet was left on for up to 2 hours, and the remaining were left in place for 2 to 18 hours.
Few locations within the hospital are without risk for this type of accident. The PPSA further noted that approximately 30% of retained tourniquets occurred on medical/surgical units, 14% in the ED, and 14% on inpatient and ambulatory surgical services departments. Approximately 19% were discovered when patients were transferred from one department to another.1
In the analysis of these incidents, contributing factors to forgotten tourniquets included staff failing to follow proper procedures, inadequate staff proficiency, and staff distractions and/or interruptions.1 In addition, some patients appeared to be at increased risk of having a retained tourniquet than others. Sixty percent of 125 patients with a forgotten tourniquet were aged 70 years or older, whereas some patients were younger than age 2 years.1 Not surprisingly, patients who were unable to verbally communicate (eg, patients who were intubated, under anesthesia, had expressive aphasia, severe dementia), were at the highest risk.
In a review of recovery room incidents, Salman and Asfar2 identified two cases of forgotten tourniquets out of approximately 7,000 patients. Potential strategies to avoid this mistake include: (1) only documenting procedures after they have been completed (eg, tourniquet removal); (2) double-checking that the tourniquet has been removed prior to leaving patient bedside; and (3) the use of extra-long tourniquets so the ends are more clearly visible.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
Reference - Missed Cervical Spine Injury
- Looby S, Flanders A. Spine trauma. Radiol Clin North Am. 2011;49(1):129-163.
- Sekon LH, Fehlings MG. Epidemiology, demographics, and pathophysiology of acute spinal cord injury. Spine (Phila Pa 1976). 2001;26(24 Suppl):S2-S12.
- Woodring JH, Lee C. Limitations of cervical radiography in the evaluation of acute cervical trauma. J Trauma. 1993;34(1):32-39.
- Diaz JJ Jr, Gillman C, Morris JA Jr, May AK, Carrillo YM, Guy J. Are five-view plain films of the cervical spine unreliable? A prospective evaluation in blunt trauma patients with altered mental status. J Trauma. 2003;55(4):658-663.
- Parizel PM, Zijden T, Gaudino S, et al. Trauma of the spine and spinal cord: imagining strategies. Eur Spine J. 2010;19(Suppl 1):S8-S17.
- Grogan EL, Morris JA Jr, Dittus RS, et al. Cervical spine evaluation in urban trauma centers: lowering institutional costs and complications through helical CT scan. J Am Coll Surg. 2005;200(2):160-165.
Reference - Forgotten Tourniquet
- Pennsylvania Safety Advisory. Forgotten but not gone: tourniquets left on patients. PA PSRS Patient Saf Advis. 2005;2(2):19-21.
- Salman JM, Asfar SN. Recovery room incidents. Bas J Surg. 2007;24:3.
Hospitalists Can Improve Healthcare Value
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
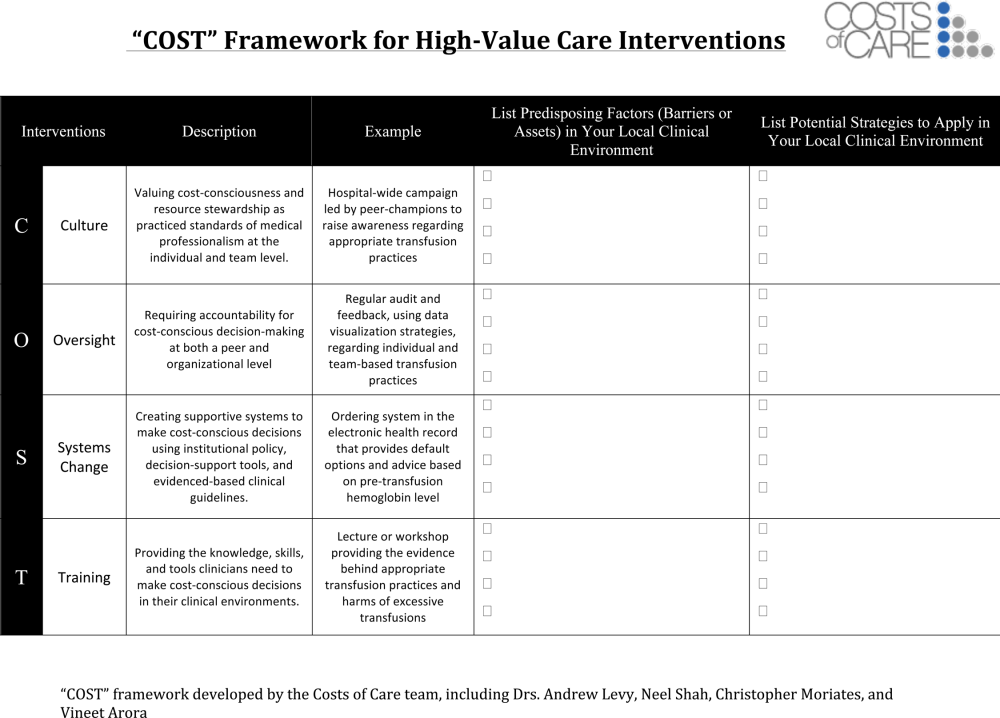
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.
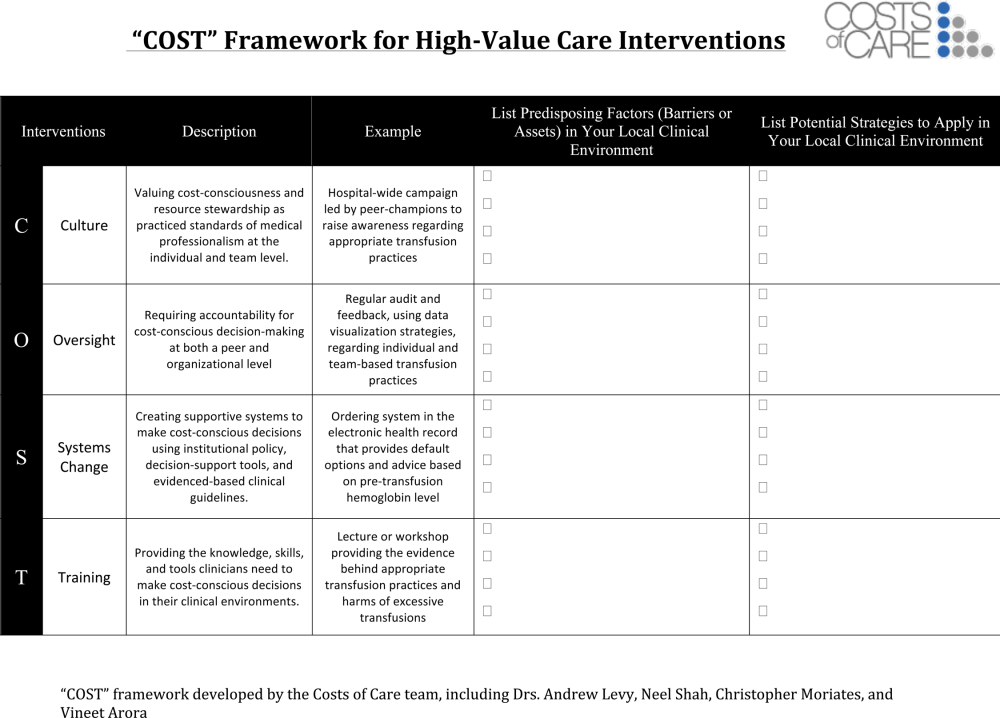
Launch High‐Value Care Programs
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.

Launch High‐Value Care Programs
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
As the nation considers how to reduce healthcare costs, hospitalists can play a crucial role in this effort because they control many healthcare services through routine clinical decisions at the point of care. In fact, the government, payers, and the public now look to hospitalists as essential partners for reining in healthcare costs.[1, 2] The role of hospitalists is even more critical as payers, including Medicare, seek to shift reimbursements from volume to value.[1] Medicare's Value‐Based Purchasing program has already tied a percentage of hospital payments to metrics of quality, patient satisfaction, and cost,[1, 3] and Health and Human Services Secretary Sylvia Burwell announced that by the end of 2018, the goal is to have 50% of Medicare payments tied to quality or value through alternative payment models.[4]
Major opportunities for cost savings exist across the care continuum, particularly in postacute and transitional care, and hospitalist groups are leading innovative models that show promise for coordinating care and improving value.[5] Individual hospitalists are also in a unique position to provide high‐value care for their patients through advocating for appropriate care and leading local initiatives to improve value of care.[6, 7, 8] This commentary article aims to provide practicing hospitalists with a framework to incorporate these strategies into their daily work.
DESIGN STRATEGIES TO COORDINATE CARE
As delivery systems undertake the task of population health management, hospitalists will inevitably play a critical role in facilitating coordination between community, acute, and postacute care. During admission, discharge, and the hospitalization itself, standardizing care pathways for common hospital conditions such as pneumonia and cellulitis can be effective in decreasing utilization and improving clinical outcomes.[9, 10] Intermountain Healthcare in Utah has applied evidence‐based protocols to more than 60 clinical processes, re‐engineering roughly 80% of all care that they deliver.[11] These types of care redesigns and standardization promise to provide better, more efficient, and often safer care for more patients. Hospitalists can play important roles in developing and delivering on these pathways.
In addition, hospital physician discontinuity during admissions may lead to increased resource utilization, costs, and lower patient satisfaction.[12] Therefore, ensuring clear handoffs between inpatient providers, as well as with outpatient providers during transitions in care, is a vital component of delivering high‐value care. Of particular importance is the population of patients frequently readmitted to the hospital. Hospitalists are often well acquainted with these patients, and the myriad of psychosocial, economic, and environmental challenges this vulnerable population faces. Although care coordination programs are increasing in prevalence, data on their cost‐effectiveness are mixed, highlighting the need for testing innovations.[13] Certainly, hospitalists can be leaders adopting and documenting the effectiveness of spreading interventions that have been shown to be promising in improving care transitions at discharge, such as the Care Transitions Intervention, Project RED (Re‐Engineered Discharge), or the Transitional Care Model.[14, 15, 16]
The University of Chicago, through funding from the Centers for Medicare and Medicaid Innovation, is testing the use of a single physician who cares for frequently admitted patients both in and out of the hospital, thereby reducing the costs of coordination.[5] This comprehensivist model depends on physicians seeing patients in the hospital and then in a clinic located in or near the hospital for the subset of patients who stand to benefit most from this continuity. This differs from the old model of having primary care providers (PCPs) see inpatients and outpatients because the comprehensivist's patient panel is enriched with only patients who are at high risk for hospitalization, and thus these physicians have a more direct focus on hospital‐related care and higher daily hospitalized patient censuses, whereas PCPs were seeing fewer and fewer of their patients in the hospital on a daily basis. Evidence concerning the effectiveness of this model is expected by 2016. Hospitalists have also ventured out of the hospital into skilled nursing facilities, specializing in long‐term care.[17] These physicians are helping provide care to the roughly 1.6 million residents of US nursing homes.[17, 18] Preliminary evidence suggests increased physician staffing is associated with decreased hospitalization of nursing home residents.[18]
ADVOCATE FOR APPROPRIATE CARE
Hospitalists can advocate for appropriate care through avoiding low‐value services at the point of care, as well as learning and teaching about value.
Avoiding Low‐Value Services at the Point of Care
The largest contributor to the approximately $750 billion in annual healthcare waste is unnecessary services, which includes overuse, discretionary use beyond benchmarks, and unnecessary choice of higher‐cost services.[19] Drivers of overuse include medical culture, fee‐for‐service payments, patient expectations, and fear of malpractice litigation.[20] For practicing hospitalists, the most substantial motivation for overuse may be a desire to reassure patients and themselves.[21] Unfortunately, patients commonly overestimate the benefits and underestimate the potential harms of testing and treatments.[22] However, clear communication with patients can reduce overuse, underuse, and misuse.[23]
Specific targets for improving appropriate resource utilization may be identified from resources such as Choosing Wisely lists, guidelines, and appropriateness criteria. The Choosing Wisely campaign has brought together an unprecedented number of medical specialty societies to issue top five lists of things that physicians and patients should question (
| Adult Hospital Medicine Recommendations | Pediatric Hospital Medicine Recommendations |
|---|---|
| 1. Do not place, or leave in place, urinary catheters for incontinence or convenience, or monitoring of output for noncritically ill patients (acceptable indications: critical illness, obstruction, hospice, perioperatively for <2 days or urologic procedures; use weights instead to monitor diuresis). | 1. Do not order chest radiographs in children with uncomplicated asthma or bronchiolitis. |
| 2. Do not prescribe medications for stress ulcer prophylaxis to medical inpatients unless at high risk for gastrointestinal complication. | 2. Do not routinely use bronchodilators in children with bronchiolitis. |
| 3. Avoid transfusing red blood cells just because hemoglobin levels are below arbitrary thresholds such as 10, 9, or even 8 mg/dL in the absence of symptoms. | 3. Do not use systemic corticosteroids in children under 2 years of age with an uncomplicated lower respiratory tract infection. |
| 4. Avoid overuse/unnecessary use of telemetry monitoring in the hospital, particularly for patients at low risk for adverse cardiac outcomes. | 4. Do not treat gastroesophageal reflux in infants routinely with acid suppression therapy. |
| 5. Do not perform repetitive complete blood count and chemistry testing in the face of clinical and lab stability. | 5. Do not use continuous pulse oximetry routinely in children with acute respiratory illness unless they are on supplemental oxygen. |
As an example of this strategy, 1 multi‐institutional group has started training medical students to augment the traditional subjective‐objective‐assessment‐plan (SOAP) daily template with a value section (SOAP‐V), creating a cognitive forcing function to promote discussion of high‐value care delivery.[28] Physicians could include brief thoughts in this section about why they chose a specific intervention, their consideration of the potential benefits and harms compared to alternatives, how it may incorporate the patient's goals and values, and the known and potential costs of the intervention. Similarly, Flanders and Saint recommend that daily progress notes and sign‐outs include the indication, day of administration, and expected duration of therapy for all antimicrobial treatments, as a mechanism for curbing antimicrobial overuse in hospitalized patients.[29] Likewise, hospitalists can also document whether or not a patient needs routine labs, telemetry, continuous pulse oximetry, or other interventions or monitoring. It is not yet clear how effective this type of strategy will be, and drawbacks include creating longer progress notes and requiring more time for documentation. Another approach would be to work with the electronic health record to flag patients who are scheduled for telemetry or other potentially wasteful practices to inspire a daily practice audit to question whether the patient still meets criteria for such care. This approach acknowledges that patient's clinical status changes, and overcomes the inertia that results in so many therapies being continued despite a need or indication.
Communicating With Patients Who Want Everything
Some patients may be more worried about not getting every possible test, rather than concerns regarding associated costs. This may oftentimes be related to patients routinely overestimating the benefits of testing and treatments while not realizing the many potential downstream harms.[22] The perception is that patient demands frequently drive overtesting, but studies suggest the demanding patient is actually much less common than most physicians think.[30]
The Choosing Wisely campaign features video modules that provide a framework and specific examples for physician‐patient communication around some of the Choosing Wisely recommendations (available at:
Clinicians can explain why they do not believe that a test will help a patient and can share their concerns about the potential harms and downstream consequences of a given test. In addition, Consumer Reports and other groups have created trusted resources for patients that provide clear information for the public about unnecessary testing and services.
Learn and Teach Value
Traditionally, healthcare costs have largely remained hidden from both the public and medical professionals.[31, 32] As a result, hospitalists are generally not aware of the costs associated with their care.[33, 34] Although medical education has historically avoided the topic of healthcare costs,[35] recent calls to teach healthcare value have led to new educational efforts.[35, 36, 37] Future generations of medical professionals will be trained in these skills, but current hospitalists should seek opportunities to improve their knowledge of healthcare value and costs.
Fortunately, several resources can fill this gap. In addition to Choosing Wisely and ACR appropriateness criteria discussed above, newer tools focus on how to operationalize these recommendations with patients. The American College of Physicians (ACP) has launched a high‐value care educational platform that includes clinical recommendations, physician resources, curricula and public policy recommendations, and patient resources to help them understand the benefits, harms, and costs of tests and treatments for common clinical issues (
In an effort to provide frontline clinicians with the knowledge and tools necessary to address healthcare value, we have authored a textbook, Understanding Value‐Based Healthcare.[38] To identify the most promising ways of teaching these concepts, we also host the annual Teaching Value & Choosing Wisely Challenge and convene the Teaching Value in Healthcare Learning Network (bit.ly/teachingvaluenetwork) through our nonprofit, Costs of Care.[39]
In addition, hospitalists can also advocate for greater price transparency to help improve cost awareness and drive more appropriate care. The evidence on the effect of transparent costs in the electronic ordering system is evolving. Historically, efforts to provide diagnostic test prices at time of order led to mixed results,[40] but recent studies show clear benefits in resource utilization related to some form of cost display.[41, 42] This may be because physicians care more about healthcare costs and resource utilization than before. Feldman and colleagues found in a controlled clinical trial at Johns Hopkins that providing the costs of lab tests resulted in substantial decreases of certain lab tests and yielded a net cost reduction (based on 2011 Medicare Allowable Rate) of more than $400,000 at the hospital level during the 6‐month intervention period.[41] A recent systematic review concluded that charge information changed ordering and prescribing behavior in the majority of studies.[42] Some hospitalist programs are developing dashboards for various quality and utilization metrics. Sharing ratings or metrics internally or publically is a powerful way to motivate behavior change.[43]
LEAD LOCAL VALUE INITIATIVES
Hospitalists are ideal leaders of local value initiatives, whether it be through running value‐improvement projects or launching formal high‐value care programs.
Conduct Value‐Improvement Projects
Hospitalists across the country have largely taken the lead on designing value‐improvement pilots, programs, and groups within hospitals. Although value‐improvement projects may be built upon the established structures and techniques for quality improvement, importantly these programs should also include expertise in cost analyses.[8] Furthermore, some traditional quality‐improvement programs have failed to result in actual cost savings[44]; thus, it is not enough to simply rebrand quality improvement with a banner of value. Value‐improvement efforts must overcome the cultural hurdle of more care as better care, as well as pay careful attention to the diplomacy required with value improvement, because reducing costs may result in decreased revenue for certain departments or even decreases in individuals' wages.
One framework that we have used to guide value‐improvement project design is COST: culture, oversight accountability, system support, and training.[45] This approach leverages principles from implementation science to ensure that value‐improvement projects successfully provide multipronged tactics for overcoming the many barriers to high‐value care delivery. Figure 1 includes a worksheet for individual clinicians or teams to use when initially planning value‐improvement project interventions.[46] The examples in this worksheet come from a successful project at the University of California, San Francisco aimed at improving blood utilization stewardship by supporting adherence to a restrictive transfusion strategy. To address culture, a hospital‐wide campaign was led by physician peer champions to raise awareness about appropriate transfusion practices. This included posters that featured prominent local physician leaders displaying their support for the program. Oversight was provided through regular audit and feedback. Each month the number of patients on the medicine service who received transfusion with a pretransfusion hemoglobin above 8 grams per deciliter was shared at a faculty lunch meeting and shown on a graph included in the quality newsletter that was widely distributed in the hospital. The ordering system in the electronic medical record was eventually modified to include the patient's pretransfusion hemoglobin level at time of transfusion order and to provide default options and advice based on whether or not guidelines would generally recommend transfusion. Hospitalists and resident physicians were trained through multiple lectures and informal teaching settings about the rationale behind the changes and the evidence that supported a restrictive transfusion strategy.

Launch High‐Value Care Programs
As value‐improvement projects grow, some institutions have created high‐value care programs and infrastructure. In March 2012, the University of California, San Francisco Division of Hospital Medicine launched a high‐value care program to promote healthcare value and clinician engagement.[8] The program was led by clinical hospitalists alongside a financial administrator, and aimed to use financial data to identify areas with clear evidence of waste, create evidence‐based interventions that would simultaneously improve quality while cutting costs, and pair interventions with cost awareness education and culture change efforts. In the first year of this program, 6 projects were launched targeting: (1) nebulizer to inhaler transitions,[47] (2) overuse of proton pump inhibitor stress ulcer prophlaxis,[48] (3) transfusions, (4) telemetry, (5) ionized calcium lab ordering, and (6) repeat inpatient echocardiograms.[8]
Similar hospitalist‐led groups have now formed across the country including the Johns Hopkins High‐Value Care Committee, Johns Hopkins Bayview Physicians for Responsible Ordering, and High‐Value Carolina. These groups are relatively new, and best practices and early lessons are still emerging, but all focus on engaging frontline clinicians in choosing targets and leading multipronged intervention efforts.
What About Financial Incentives?
Hospitalist high‐value care groups thus far have mostly focused on intrinsic motivations for decreasing waste by appealing to hospitalists' sense of professionalism and their commitment to improve patient affordability. When financial incentives are used, it is important that they are well aligned with internal motivations for clinicians to provide the best possible care to their patients. The Institute of Medicine recommends that payments are structured in a way to reward continuous learning and improvement in the provision of best care at lower cost.[19] In the Geisinger Health System in Pennsylvania, physician incentives are designed to reward teamwork and collaboration. For example, endocrinologists' goals are based on good control of glucose levels for all diabetes patients in the system, not just those they see.[49] Moreover, a collaborative approach is encouraged by bringing clinicians together across disciplinary service lines to plan, budget, and evaluate one another's performance. These efforts are partly credited with a 43% reduction in hospitalized days and $100 per member per month in savings among diabetic patients.[50]
Healthcare leaders, Drs. Tom Lee and Toby Cosgrove, have made a number of recommendations for creating incentives that lead to sustainable changes in care delivery[49]: avoid attaching large sums to any single target, watch for conflicts of interest, reward collaboration, and communicate the incentive program and goals clearly to clinicians.
In general, when appropriate extrinsic motivators align or interact synergistically with intrinsic motivation, it can promote high levels of performance and satisfaction.[51]
CONCLUSIONS
Hospitalists are now faced with a responsibility to reduce financial harm and provide high‐value care. To achieve this goal, hospitalist groups are developing innovative models for care across the continuum from hospital to home, and individual hospitalists can advocate for appropriate care and lead value‐improvement initiatives in hospitals. Through existing knowledge and new frameworks and tools that specifically address value, hospitalists can champion value at the bedside and ensure their patients get the best possible care at lower costs.
Disclosures: Drs. Moriates, Shah, and Arora have received grant funding from the ABIM Foundation, and royalties from McGraw‐Hill for the textbook Understanding Value‐Based Healthcare. The authors report no conflicts of interest.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
- , . Value‐based purchasing—national programs to move from volume to value. N Engl J Med. 2012;367(4):292–295.
- . Value‐driven health care: implications for hospitals and hospitalists. J Hosp Med. 2009;4(8):507–511.
- , . Hospital value‐based purchasing. J Hosp Med. 2013;8(5):271–277.
- . Setting value‐based payment goals—HHS efforts to improve U.S. health care. N Engl J Med. 2015;372(10):897–899.
- , . Redesigning care for patients at increased hospitalization risk: the Comprehensive Care Physician model. Health Aff Proj Hope. 2014;33(5):770–777.
- , , , et al. Choosing wisely in adult hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):486–492.
- , , . First, do no (financial) harm. JAMA. 2013;310(6):577–578.
- , , , . Development of a hospital‐based program focused on improving healthcare value. J Hosp Med. 2014;9(10):671–677.
- , , , et al. A controlled trial of a critical pathway for treatment of community‐acquired pneumonia. JAMA. 2000;283(6):749–755.
- , , , , . Evidence‐based care pathway for cellulitis improves process, clinical, and cost outcomes [published online July 28, 2015]. J Hosp Med. doi:10.1002/jhm.2433.
- . The Lean approach to health care: safety, quality, and cost. Institute of Medicine. Available at: http://nam.edu/perspectives‐2012‐the‐lean‐approach‐to‐health‐care‐safety‐quality‐and‐cost/. Accessed September 22, 2015.
- , , , et al. The impact of hospitalist discontinuity on hospital cost, readmissions, and patient satisfaction. J Gen Intern Med. 2014;29(7):1004–1008.
- Congressional Budget Office. Lessons from Medicare's Demonstration Projects on Disease Management, Care Coordination, and Value‐Based Payment. Available at: https://www.cbo.gov/publication/42860. Accessed April 26, 2015.
- , , , et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009;150(3):178–187.
- , , , . The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006;166(17):1822–1828.
- , , , et al. Comprehensive discharge planning and home follow‐up of hospitalized elders: a randomized clinical trial. JAMA. 1999;281(7):613–620.
- . “SNFists” at work: nursing home docs patterned after hospitalists. Mod Healthc. 2012;42(13):32–33.
- , , , . Nursing home physician specialists: a response to the workforce crisis in long‐term care. Ann Intern Med. 2009;150(6):411–413.
- Institute of Medicine. Best Care at Lower Cost: The Path to Continuously Learning Health Care in America. Washington, DC: National Academies Press; 2012.
- , . The perfect storm of overutilization. JAMA. 2008;299(23):2789–2791.
- , , , et al. Overuse of testing in preoperative evaluation and syncope: a survey of hospitalists. Ann Intern Med. 2015;162(2):100–108.
- , . Patients' expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015;175(2):274–286.
- , , , et al. Enhancing the Use and Quality of Colorectal Cancer Screening. Rockville, MD: Agency for Healthcare Research and Quality; 2010. Available at: http://www.ncbi.nlm.nih.gov/books/NBK44526. Accessed September 30, 2013.
- , , , et al. Choosing wisely in pediatric hospital medicine: five opportunities for improved healthcare value. J Hosp Med. 2013;8(9):479–485.
- . Teaching Choosing Wisely in medical education and training: the story of a pioneer. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/teaching‐choosing‐wisely‐in‐meded. Accessed March 29, 2014.
- American College of Radiology. ACR appropriateness criteria overview. November 2013. Available at: http://www.acr.org/∼/media/ACR/Documents/AppCriteria/Overview.pdf. Accessed March 4, 2014.
- American College of Cardiology Foundation. Appropriate use criteria: what you need to know. Available at: http://www.cardiosource.org/∼/media/Files/Science%20and%20Quality/Quality%20Programs/FOCUS/E1302_AUC_Primer_Update.ashx. Accessed March 4, 2014.
- , , , , . SOAP‐V: applying high‐value care during patient care. The Medical Professionalism Blog. Available at: http://blog.abimfoundation.org/soap‐v‐applying‐high‐value‐care‐during‐patient‐care. Accessed April 3, 2015.
- , . Why does antimicrobial overuse in hospitalized patients persist? JAMA Intern Med. 2014;174(5):661–662.
- . The myth of the demanding patient. JAMA Oncol. 2015;1(1):18–19.
- . The disruptive innovation of price transparency in health care. JAMA. 2013;310(18):1927–1928.
- United States Government Accountability Office. Health Care Price Transparency—Meaningful Price Information Is Difficult for Consumers to Obtain Prior to Receiving Care. Washington, DC: United States Government Accountability Office; 2011:43.
- , , . General pediatric attending physicians' and residents' knowledge of inpatient hospital finances. Pediatrics. 2013;131(6):1072–1080.
- , , . Hospitalists' awareness of patient charges associated with inpatient care. J Hosp Med. 2010;5(5):295–297.
- . Cost consciousness in patient care—what is medical education's responsibility? N Engl J Med. 2010;362(14):1253–1255.
- . Providing high‐value, cost‐conscious care: a critical seventh general competency for physicians. Ann Intern Med. 2011;155(6):386–388.
- , , , . Defining competencies for education in health care value: recommendations from the University of California, San Francisco Center for Healthcare Value Training Initiative. Acad Med. 2015;90(4):421–424.
- , , . Understanding Value‐Based Healthcare. New York: McGraw‐Hill; 2015.
- , , , . Wisdom of the crowd: bright ideas and innovations from the teaching value and choosing wisely challenge. Acad Med. 2015;90(5):624–628.
- , , , et al. Does the computerized display of charges affect inpatient ancillary test utilization? Arch Intern Med. 1997;157(21):2501–2508.
- , , , et al. Impact of providing fee data on laboratory test ordering: a controlled clinical trial. JAMA Intern Med. 2013;173(10):903–908.
- , , , . The effect of charge display on cost of care and physician practice behaviors: a systematic review. J Gen Intern Med. 2015;30(6):835–842.
- , , , , , . Closing the Quality Gap: Revisiting the State of the Science. Vol. 5. Public Reporting as a Quality Improvement Strategy. Rockville, MD: Agency for Healthcare Research and Quality; 2012.
- , , , . The savings illusion—why clinical quality improvement fails to deliver bottom‐line results. N Engl J Med. 2011;365(26):e48.
- , , , . Fostering value in clinical practice among future physicians: time to consider COST. Acad Med. 2014;89(11):1440.
- , , , , , . The Teaching Value Workshop. MedEdPORTAL Publications; 2014. Available at: https://www.mededportal.org/publication/9859. Accessed September 22, 2015.
- , , , , . “Nebs no more after 24”: a pilot program to improve the use of appropriate respiratory therapies. JAMA Intern Med. 2013;173(17):1647–1648.
- , , , et al. The development and implementation of a bundled quality improvement initiative to reduce inappropriate stress ulcer prophylaxis. ICU Dir. 2013;4(6):322–325.
- , . Engaging doctors in the health care revolution. Harvard Business Review. June 2014. Available at: http://hbr.org/2014/06/engaging‐doctors‐in‐the‐health‐care‐revolution/ar/1. Accessed July 30, 2014.
- , , . Geisinger Health System: achieving the potential of system integration through innovation, leadership, measurement, and incentives. June 2009. Available at: http://www.commonwealthfund.org/publications/case‐studies/2009/jun/geisinger‐health‐system‐achieving‐the‐potential‐of‐system‐integration. Accessed September 22, 2015.
- Motivational synergy: toward new conceptualizations of intrinsic and extrinsic motivation in the workplace. Hum Resource Manag 1993;3(3):185–201. Available at: http://www.hbs.edu/faculty/Pages/item.aspx?num=2500. Accessed July 31, 2014.
© 2015 Society of Hospital Medicine
Hand Hygiene Intervention in Japan
Healthcare‐associated infections are a major cause of illness and death in hospitalized patients, and preventing healthcare‐associated infection is a global challenge.[1] Worldwide, the prevalence of healthcare‐associated infections in developed and undeveloped countries ranges from 5.1% to 11.6% and 5.7% to 19.1%, respectively.[2] In the United States, roughly 2 million such infections occur annually, resulting in approximately 99,000 deaths[3] and estimated annual direct medical costs between $28.4 and $33.8 billion.[4] In Japan, nearly 9% of patients admitted to the intensive care unit (ICU) develop an infection during hospitalization,[5] and 5% of all patients hospitalized become infected with methicillin‐resistant Staphylococcus aureus.[6] The management of healthcare‐associated infections in Japan accounts for up to 5% of total annual healthcare costs, with an estimated $6.8 billion estimated to be potentially preventable.[7] In addition, healthcare‐associated infections are associated with increased length of stay in the hospital. Studies estimate surgical site infections extend length of stay by 9.7 days,[8] and bloodstream infections increase length of stay by 10 days.[9]
Improving hand hygiene practice for healthcare workers is considered a core strategy to decrease the incidence of healthcare‐associated infection.[6, 10] Specifically, the use of alcohol‐based hand rub is strongly recommended in acute care hospitals by both the World Health Organization (WHO) and the US Centers for Disease Control and Prevention.[11, 12] Improving hand hygiene adherence may reduce healthcare‐associated infection by 9% to 50%,[13, 14] and multiple studies have reported that greater use of alcohol‐based hand rubs results in significant reductions in healthcare‐associated infections.[14, 15]
Due to the difficulty in improving hand hygiene in various settings across the world, the WHO strategy for improving hand hygiene has been adopted and implemented by several studies in varying locations, such as Costa Rica, Italy, Mali, Pakistan, and Saudi Arabia.[16] Implementations of these multimodal strategies, following WHObased guidelines, have been shown to increase the level of hand hygiene adherence among healthcare workers and reduce infections at these locations.[14, 17, 18] This study expands upon that work by extending the same implementation strategy to assess the effectiveness of the introduction of alcohol‐based hand rub on hand hygiene practice at multiple hospitals in Japan.
In a previous article[19] we reported results from an observational study assessing healthcare worker hand hygiene adherence before touching the patient in 4 geographically diverse hospitals in Japan. The study reported that hand hygiene adherence in Japanese hospitals was lower than reported mean values from other international studies, and that greater adherence to hand hygiene should be encouraged. In this article, we present the results of a multimodal intervention intended to improve levels of healthcare worker hand hygiene in 3 of these hospitals.
METHODS
Participating Institutions
Three of the 4 hospitals participating in the prior observational study chose to participate in this intervention. Evaluation of hand hygiene practice was performed in at least 3 wards of each hospital including an inpatient surgical ward, an inpatient medicine ward, an ICU, or an emergency ward.
Table 1 lists the characteristics of the participating hospitals. Hospital A is a university‐affiliated, tertiary care medical center with 312 beds in East Japan. Although the hospital did not have an infection prevention unit or designated infection control nurses during the preintervention periods, the hospital hired a designated infection prevention nurse and established a department of infection prevention before this intervention in April 2012. Hospital B is a community‐based, tertiary care medical center with 428 beds, located in Midwest Japan. Although the facility had no infection control nurses at the outset of the study, a physician certified by the American Board of Internal Medicine and Infectious Diseases provided educational sessions of hand hygiene. Hospital B hired a designated infection prevention nurse and established a department of infection prevention in April 2012. Hospital C, located in Northern Japan, is a community‐based, tertiary care medical center with 562 beds. The department of infection prevention was established in 2010 and has 1 full‐time and 2 part‐time infection prevention nurses.
| Hospital A | Hospital B | Hospital C | ||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention | |
| ||||||
| Hospital characteristics | ||||||
| Location | East Japan | Midwest Japan | Northern Japan | |||
| Hospital type | University affiliated | Community based | Community based | |||
| Level of care | Tertiary care | Tertiary care | Tertiary care | |||
| Residency program | Yes | Yes | Yes | |||
| No. of beds | 250 | 312 | 428 | 428 | 550 | 562 |
| No. of employees | 398 | 475 | 1,035 | 1,263 | 1,500 | 1,568 |
| No. of physicians | 73 | 91 | 179 | 188 | 207 | 217 |
| No. of nurses | 172 | 210 | 410 | 540 | 616 | 800 |
| Infection control practice | ||||||
| Establishment of infection prevention units (year) | N/A | Yes (2012) | N/A | Yes (2012) | Yes (2010) | Yes |
| Employment of certified nurses in infection control (FTE) | 0 | 1 (1) | 0 | 1 (1) | 3 (1.5) | 3 (1.5) |
| Employment of ABIM‐IDcertified physician | 0 | 0 | 1 | 1 | 1 | 0 |
Role of the Funding Source
This study was unfunded. The prize for the contest was provided by an American collaborator (S.S.) who was not affiliated with any of the participating hospitals.
Intervention
In the prior preintervention study, hand hygiene adherence rates of healthcare workers were evaluated between July 2011 and November 2011.[19] To improve hand hygiene adherence in these facilities, we initiated a multimodal intervention based on WHO recommendations and the findings from the prior study. Each facility was provided the same guidance on how to improve hand hygiene adherence (Table 2) and encouraged to tailor the intervention to their local setting. As an added incentive, we initiated a contest, where the facility obtaining the highest hand hygiene adherence postintervention would win a trophy and 500,000 Japanese yen (approximately $5000 US dollars). The recommended strategies consisted of 15 components (Table 2): infrastructure (3 components), training and education (2 components), evaluation and feedback (5 components), reminder in the workplace (1 component), and institution safety climate (4 components). Of note, the participating institutions had already implemented a varying number of the intervention components prior to the start of the intervention. Each facility conducted a 6‐month intervention to improve hand hygiene adherence; however, the actual timing of interventions varied slightly by institution. Hospitals A and C conducted an intervention from October 2012 through March 2013, whereas hospital B's intervention was from April 2012 to September 2012. Details of the multimodal intervention performed at each participating hospital are shown in Table 3.
| Intervention Components | Description |
|---|---|
| 1. Infrastructure (3 components) | |
| Hand‐washing faucets for each room | At least 1 faucet and sink for each room was available. |
| Placement of alcohol hand rub at patient's room entrance | Alcohol hand rub was placed at all patient room entrances. |
| Portable alcohol hand rub distributed for each healthcare worker | Personal, portable alcohol hand rub dispensers were provided for healthcare workers who contact patients. |
| 2. Training/education (2 components) | |
| Educational resources | At least 1 physician or 1 nurse who provides educational sessions regarding hand hygiene practice was available. |
| Periodic seminars and lectures regarding hand hygiene education | Hospital‐wide hand hygiene seminar or educational activities were held during the intervention period. |
| 3. Evaluation and feedback (5 components) | |
| Evaluation of hand hygiene practice by direct observation | Hospitals utilize direct observation for healthcare worker's hand hygiene practice. |
| Evaluation of hand hygiene practice by monitoring the amount of alcohol hand rub consumption | Hospitals utilize the amount of alcohol hand rub consumption as a parameter for healthcare worker's hand hygiene practice. |
| Hand hygiene rate feedback at infection control committee | Hand hygiene adherence rate was reported and discussed at hospital infection control committee. |
| Hand hygiene rate feedback to the designated wards/units | Hand hygiene adherence rate was reported and discussed with healthcare workers at the designated wards/units where hand hygiene observation was performed. |
| Granting the award of top‐rated person of hand hygiene | Hospitals established the system to assess individual healthcare worker's hand hygiene adherence rate. |
| 4. Reminder in the workplace (1 components) | |
| Poster notification | Poster notification for hand hygiene practice was performed in the intervention period. |
| 5. Institutional safety climate (4 components) | |
| Commitment of hospital president or hospital executives | Hospital executives including the president agreed on the importance of hand hygiene practice and declared to healthcare workers to enhance hand hygiene practice during the intervention period. |
| Commitment of nurse managers and physician leaders | Commitment of improving hand hygiene practice by representative healthcare workers at the designated wards/units (eg, meeting by nurse manager or physician leaders at the designated wards/units and collaborative work with infection prevention services). |
| Meeting at the designated wards/units | A ward/unit‐level meeting or voluntary session for hands‐on hand hygiene practice by healthcare workers at the designated wards/units. |
| Identifying champions at the designated wards/units | An individual healthcare worker who contributed to improving hand hygiene practice was appointed. |
| Hospital A | Hospital Ba | Hospital C | ||||
|---|---|---|---|---|---|---|
| ||||||
| Intervention period | October 2012March 2013 | April 2012September 2012 | October 2012March 2013 | |||
| Evaluation of hand hygiene in the postintervention period | May 2013July 2013 | October 2012 | June 2013 | |||
| Suggested intervention components | Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention |
| No. of implemented components | 2/15 | 10/15 | 9/15 | 10/15 | 6/15 | 8/15 |
| Infrastructure (3 components) | ||||||
| Hand‐washing faucets for each room | No | No | Yes | Yes | Yes | Yes |
| Placement of alcohol hand rubs at patient's room entrance | Yes | Yes | Yes | Yes | Yes | Yes |
| Portable alcohol hand rub distributed for each healthcare worker | No | Yesb | No | Yesb | No | No |
| Training/education (2 components) | ||||||
| Educational resources | No | Yesb | Yes | Yesb | Yes | Yes |
| Periodic seminars and lectures regarding hand hygiene education | No | Yesb | Yes | Yes | Yes | Yes |
| Evaluation and feedback (5 components) | ||||||
| Evaluation of hand hygiene practice by direct observation | No | Yesb | Yes | Yes | No | No |
| Evaluation of hand hygiene practice by the amount of alcohol hand rub consumption | No | No | Yes | Yes | Yes | Yes |
| Hand hygiene rate feedback at infection control committee | No | Yesb | Yes | Yes | No | Yesb |
| Hand hygiene rate feedback to designated departments | No | Yesb | Yes | Yes | No | Yesb |
| Granting the award of top‐rated person | No | No | No | No | No | No |
| Reminders in the workplace (1 component) | ||||||
| Poster notification | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Institutional safety climate (4 components) | ||||||
| Commitment of hospital president or hospital executives | No | Yesb | No | No | No | No |
| Commitment of nurse managers and physicians leaders | No | Yesb | No | No | No | No |
| Meeting regarding hand hygiene practice by the designated wards/units | No | No | No | No | No | No |
| Identifying champions at the designated wards/units | No | No | No | No | No | No |
Observation of Hand Hygiene Practice
The same methods for hand hygiene observation used for the preintervention study was used for postintervention assessment. Ten distinct units across the 3 participating hospitals were evaluated for healthcare worker hand hygiene prior to patient contact. Three to 4 units were observed at each facility. One of the study authors (T.S.), a Japanese board‐certified infection control nurse, conducted all of the hand hygiene observations for both the preintervention and postintervention studies. Intraobserver variation was minimized by providing the same training outlined in the previous study.[19] Appropriate hand hygiene was defined as the use of soap and water or alcohol‐based hand rub before patient contact, which corresponds to the first moment of the WHO's 5 moments of hand hygiene.[11]
Hand hygiene practice prior to patient contact for each individual provider‐patient encounter was observed and recorded using the hand hygiene observation form adapted from a previous study by Saint et al.[6, 20] Identical to the preintervention study,[19] the form captured the following information: unit in which observations were performed, time of initiation and completion of observations, healthcare worker subgroup (physician or nurse), and the type of hand hygiene before patient contact (ie, hand washing with soap and water, use of alcohol‐based hand rub, or no hand hygiene). Unit physicians and nurses were informed that their clinical practices were going to be observed, but were not informed of the purpose of the observations (eg, hand hygiene adherence). To avoid interfering with clinical care delivery, the observer was given strict instructions to maintain a certain distance from the observed healthcare workers. The observer was instructed to leave immediately if asked for any reason by the unit staff or patients.
Statistical Analysis
Overall hand hygiene adherence rates were calculated and compared between the pre‐ and the postintervention periods. Comparison of hand hygiene adherence by healthcare worker subgroup and by hospital unit between the pre‐ and postintervention periods was also performed. Hand hygiene adherence rates were compared using JMP 9.0 and SAS 9.3 (SAS Institute Inc., Cary, NC). Comparison of hand hygiene adherence rates by observational periods was calculated by Pearson [2] tests, and 95% confidence intervals (CIs) were estimated using binomial distribution. Pearson correlations were used to determine the relationship of hand hygiene between physicians and nurses in the same unit. Two‐tailed P value0.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at the participating hospitals.
RESULTS
Data were collected from May 2013 to July 2013 in hospital A, in October 2012 in hospital B, and June 2013 in hospital C to ensure data were collected after the 6‐month intervention at each site. A total of 2982 observations of hand hygiene were performed in 10 distinct units across the 3 participating hospitals during the postintervention periods. Hand hygiene observations were performed during the day Monday through Friday between 8:30 am and 7:30 pm, with the majority occurring prior to 1:00 pm.
The overall postintervention hand hygiene adherence rate (in all 3 hospitals) was significantly higher at 32.7% (974/2982) adherence compared to 18.0% (482/2679) adherence in the preintervention period (P<0.001). An increased hand hygiene adherence rate in each participating hospital in the postintervention period was observed (Figure 1). Similar trends of higher overall hand hygiene adherence rates for both nurses and physicians in the postintervention period were seen. Use of alcohol‐based hand rub among those with appropriate hand hygiene was significantly higher, with 90.0% (880/974) using hand rub in the postintervention period versus 67.0% (322/482) in the preintervention period (P<0.001). Comparison of overall hand hygiene adherence rates by unit type and healthcare worker subgroup between the pre‐ and postintervention periods are shown in Table 4. Detailed comparisons of hand hygiene adherence rates for each hospital are available in the supplementary appendix. Although a significant improvement of hand hygiene practice was observed in the majority of participating units (6/10), there was a significant decline in hand hygiene practice in 2 units for nurses and 1 unit for physicians. Hand hygiene adherence rates by healthcare worker subgroups (both physicians and nurses) were significantly higher in the postintervention period than those in the preintervention period. Trends toward higher hand hygiene adherence rate of nurses in the postintervention period were observed (34.8% adherence for nurses compared to 30.4% adherence for physicians); the difference between nurses and physicians were not statistically significant (P=0.07).
| Ward/Unit | Healthcare Worker Subgroup | Preintervention Period | Postintervention Period | Improvement After Intervention (%) | P Value | ||
|---|---|---|---|---|---|---|---|
| No. of Observations | Hand hygiene Adherence (%) | No. of Observations | Hand Hygiene Adherence (%) | ||||
| |||||||
| All 3 hospitals | |||||||
| Surgery | Nurse | 455 | 20 | 480 | 40 | 20 | <0.001 |
| Physician | 424 | 18 | 448 | 43 | 25 | <0.001 | |
| All | 879 | 19 | 928 | 41 | 22 | <0.001 | |
| Medicine | Nurse | 455 | 23 | 508 | 39 | 16 | <0.001 |
| Physician | 435 | 15 | 452 | 33 | 18 | <0.001 | |
| All | 890 | 20 | 960 | 36 | 16 | <0.001 | |
| ICU | Nurse | 305 | 21 | 379 | 25 | 4 | 0.17 |
| Physician | 203 | 9 | 268 | 28 | 19 | <0.001 | |
| All | 508 | 16 | 647 | 26 | 10 | <0.001 | |
| ED | Nurse | 170 | 16 | 173 | 27 | 11 | 0.01 |
| Physician | 232 | 14 | 274 | 9 | ‐5 | 0.07 | |
| All | 402 | 15 | 447 | 16 | 1 | 0.64 | |
| All units | Nurse | 1385 | 21 | 1540 | 35 | 14 | <0.001 |
| Physician | 1294 | 15 | 1442 | 30 | 15 | <0.001 | |
| All | 2679 | 18 | 2982 | 33 | 15 | <0.001 | |
Hospital A achieved the highest postintervention adherence rates (39.9% adherence postintervention), as well as the greatest absolute improvement in hand hygiene (increase of 29.0%). There were significant improvements in 3 of the 4 participating units in hospital A, with the emergency department showing improvements only in the nurse subgroup. In hospital B, total hand hygiene adherence increased from 24.7% to 30.0% (P=0.01); however, this increase was mainly due to increase in hand hygiene adherence rates of nurses. There were significant increases in hand hygiene adherence rates for nurses in the medicine (+11%, P=0.04) and surgery wards (+14%, P=0.01), with nonsignificant increases for physicians (+10% medicine, P=0.07;+2% surgery, P=0.78). However, in the emergency department, nurses showed no significant improvement, and physicians had a significant decrease in adherence (15.7% preintervention vs 7.4% postintervention; P=0.02). In hospital C, total hand hygiene practice rates were significantly improved (from 18.9% to 26.5%; P<0.001); however, this was driven by improvements only in the surgical ward (14.6% preintervention to 42.3% postintervention; P<0.001). The rates for nurses declined significantly in both the medicine and ICU wards, leading to no observed improvements on those wards.
DISCUSSION
Our multicenter intervention study in Japan included observations from almost 3000 encounters between clinicians and patients. Before the intervention, the overall rate of hand hygiene adherence was 18%. After the multimodal intervention, the absolute increase in healthcare worker hand hygiene adherence was 15%. Although there was overall improvement, the adherence rates varied by hospital, with hospital A increasing by 29% and hospital B and C only attaining increases of 5% and 7%, respectively.
Despite the importance of hand hygiene of healthcare workers, it is challenging to increase hand hygiene adherence because it requires behavioral modification. Moreover, it remains uncertain what factors will affect healthcare worker behavior. We implemented pragmatic strategies to evaluate the efficacy of hand hygiene multimodal interventions based on internationally recognized WHO hand hygiene adherence strategies[11] and an institutional‐level contest with financial incentives. The findings in the current study help us understand not only how a multimodal intervention importantly improves hand hygiene adherence, but also what factors potentially make healthcare workers modify their behaviors.
In this study, we evaluated whether an institutional‐level contest with financial incentives contributed to improved hand hygiene adherence of healthcare workers. This study demonstrated improvement of hand hygiene practice after implementation of a multimodal hand hygiene intervention combined with an institutional‐level contest with financial incentives. The contest might have had a modest effect to help motivate the participating hospitals to improve their hand hygiene adherence rate. This is consistent with a previous study that demonstrated financial incentives were associated with modifying healthcare workers' hand hygiene practice.[21] However, we did not strictly standardize how the contest information was distributed in each participating institution and the objective assessment for changes in motivation by the contest was lacking in this study. Thus, changes in motivation by the contest with financial incentives likely varied by each participating institution. Further studies are needed to assess if this type of approach is worth pursuing.
We observed several noteworthy associations between the intervention components that were implemented at each facility and their improvement in hand hygiene adherence. Among the participating hospitals, hospital A was most successful with improving hand hygiene adherence, although all participating hospitals achieved a similar number of the 15 recommended intervention components during the intervention (8 to 10 per hospital). Interestingly, hospital A initiated the most new components during the intervention period (8 new components for a total of 10 out of 15), whereas hospital B and hospital C initiated only 1 or 2 new components during the intervention period. Hospital A also successfully involved hospital executives, and elicited the commitment of a nurse manager and physician leader. Consistent with a previous study,[22] we believe that involvement of hospital executives appears to be important to increase overall hand hygiene rate among healthcare workers.
In contrast, hospitals B and C did not involve senior executives or identify nurse or physician champions for all participating units. Based on the results in this study, we believe that the involvement of hospital executives is likely a key for the penetration of hospital‐wide hand hygiene culture among healthcare workers.
Although this study was unable to determine which components are precisely associated with improving hand hygiene adherence, the findings suggest initiating multiple intervention components at the same time may provide more motivation for change than initiating only 1 or 2 components at a time. It is also possible that certain intervention components were more beneficial than others. For example, hospital A, which achieved the most success, was the only hospital to obtain leadership support. Other studies have demonstrated that the presence of leadership appeared to play a key role in improving hand hygiene adherence.[23, 24] Moreover, a recent Japanese nationwide survey demonstrated higher safety centeredness was associated with regular use of standard infection prevention practice.[25] Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introduction of portable alcohol‐based hand rub) alone, but it depends on altering healthcare worker behavior.[26]
This study has several limitations. Because participating hospitals could tailor the specific interventions chosen for their facility, the improvement in hand hygiene adherence was likely multifactorial. We are unable in the existing study to determine a direct causal relationship between any of the individual intervention components and hand hygiene adherence. We are also unable to determine whether the improvements seen in hospital A were due to participation in the contest or due to the specific intervention components that were implemented. However, WHO hand hygiene guidelines point out that recognition of the importance of hand hygiene varies in different regions and countries, and the goal for hand hygiene interventions is to establish a culture of hand hygiene practice through pragmatic intervention strategies, frequent evaluation, and feedback to healthcare workers.[27] Thus, we prioritized pragmatic strategies to include in our intervention to promote hand hygiene adherence. Another limitation was the date of implementation of the multimodal intervention was slightly different at each facility. It was challenging to implement the intervention simultaneously across institutions due to competing priorities at each facility. Although the primary goal of hand hygiene is to reduce the burden of healthcare‐associated infection, we were unable to measure infection rates at the participating facilities. It is possible the presence of an external observer had an impact on the healthcare workers' behavior.[28] However, the healthcare workers were not informed as to what the observer was monitoring to minimize this potential effect. Lastly, the findings in this study provide immediate intervention effects but further study will be required to determine if these effects are sustainable.
Altering healthcare worker behavior is likely the key element to improve hand hygiene adherence, and behavioral modification may be achieved with the support of leadership at the unit and facility level. However, even though we found significant improvements in healthcare worker hand hygiene adherence after the intervention, the adherence rates are still relatively low compared to reported adherence rates from other countries,[29] suggesting further intervention is needed in this setting to optimize and hygiene practice. Because hand hygiene practice is a crucial strategy to prevent healthcare‐associated infections, every effort should be made to enhance the hand hygiene practice of healthcare workers.
Acknowledgements
The authors thank the International Ann Arbor Safety Collaborative (
Disclosure: Nothing to report.
- . Infection control—a problem for patient safety. N Engl J Med. 2003;348(7):651–656.
- World Health Organization. The burden of health care‐associated infection worldwide: a summary. Available at: http://www.who.int/gpsc/country_work/summary_20100430_en.pdf. Accessed October 6, 2014.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed April 20, 2015.
- , , . Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30–35.
- , , , et al. Improving healthcare worker hand hygiene adherence before patient contact: a before‐and‐after five‐unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429–433.
- . Economical efficiency of infection control. Antibiot Chemother (Northfield). 2004;20:635–638.
- , , , , , . Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397.
- , , , , , . Hospital‐acquired, laboratory‐confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158–162.
- . APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251–269.
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. Clean care is safer care: first global patient safety challenge. Geneva, Switzerland; 2009. Available at: http://www.who.int/gpsc/en/index.html. Accessed October 6, 2014.
- , ; Healthcare Infection Control Practices Advisory Committee, HICPAC SHEA APIC IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health‐care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51(RR‐16):1–45.
- National Patient Safety Agency. The economic case: implementing near‐patient alcohol hand rum in your trust. London, United Kingdom; 2004. Available at: http://www.npsa.nhs.uk/cleanyourhands/resource‐area/evidence‐base/?EntryId34=58433. Accessed October 9, 2014.
- , , , et al. Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–1312.
- , . Role of hand hygiene in healthcare‐associated infection prevention. J Hosp Infect. 2009;73(4):305–315.
- , , , et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: a quasi‐experimental study. Lancet Infect Dis. 2013;13(10):843–851.
- , , , et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited‐resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415–423.
- , , , . Bundling hand hygiene interventions and measurement to decrease health care‐associated infections. Am J Infect Control. 2012;40(4 suppl 1):S18–S27.
- , , , et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan [published online April 8, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000108.
- , , , et al. Marked variability in adherence to hand hygiene: a 5‐unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306–310.
- , , , et al. Sustained improvement in hand hygiene adherence: utilizing shared accountability and financial incentives. Infect Control Hosp Epidemiol. 2013;34(11):1129–1136.
- , , , . Status of the implementation of the World Health Organization multimodal hand hygiene strategy in United States of America health care facilities. Am J Infect Control. 2014;42(3):224–230.
- , , , et al. The effect of leadership on hand hygiene: assessing hand hygiene adherence prior to patient contact in 2 infectious disease units in Tuscany. Infect Control Hosp Epidemiol. 2014;35(3):313–316.
- , , , , , . Impact of a hospital‐wide hand hygiene initiative on healthcare‐associated infections: results of an interrupted time series. BMJ Qual Saf. 2012;21(12):1019–1026.
- , , , , , . Health care‐associated infection prevention in Japan: the role of safety culture. Am J Infect Control. 2014;42(8):888–893.
- , , . Why healthcare workers don't wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol. 2006;27(5):484–492.
- World Health Organization. Guide to implementation. A guide to the implementation of the WHO multimodal hand hygiene improvement strategy. Available at: http://whqlibdoc.who.int/hq/2009/WHO_IER_PSP_2009.02_eng.pdf. Accessed October 9, 2014.
- , , , et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746.
- , , , et al. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31(3):283–294.
Healthcare‐associated infections are a major cause of illness and death in hospitalized patients, and preventing healthcare‐associated infection is a global challenge.[1] Worldwide, the prevalence of healthcare‐associated infections in developed and undeveloped countries ranges from 5.1% to 11.6% and 5.7% to 19.1%, respectively.[2] In the United States, roughly 2 million such infections occur annually, resulting in approximately 99,000 deaths[3] and estimated annual direct medical costs between $28.4 and $33.8 billion.[4] In Japan, nearly 9% of patients admitted to the intensive care unit (ICU) develop an infection during hospitalization,[5] and 5% of all patients hospitalized become infected with methicillin‐resistant Staphylococcus aureus.[6] The management of healthcare‐associated infections in Japan accounts for up to 5% of total annual healthcare costs, with an estimated $6.8 billion estimated to be potentially preventable.[7] In addition, healthcare‐associated infections are associated with increased length of stay in the hospital. Studies estimate surgical site infections extend length of stay by 9.7 days,[8] and bloodstream infections increase length of stay by 10 days.[9]
Improving hand hygiene practice for healthcare workers is considered a core strategy to decrease the incidence of healthcare‐associated infection.[6, 10] Specifically, the use of alcohol‐based hand rub is strongly recommended in acute care hospitals by both the World Health Organization (WHO) and the US Centers for Disease Control and Prevention.[11, 12] Improving hand hygiene adherence may reduce healthcare‐associated infection by 9% to 50%,[13, 14] and multiple studies have reported that greater use of alcohol‐based hand rubs results in significant reductions in healthcare‐associated infections.[14, 15]
Due to the difficulty in improving hand hygiene in various settings across the world, the WHO strategy for improving hand hygiene has been adopted and implemented by several studies in varying locations, such as Costa Rica, Italy, Mali, Pakistan, and Saudi Arabia.[16] Implementations of these multimodal strategies, following WHObased guidelines, have been shown to increase the level of hand hygiene adherence among healthcare workers and reduce infections at these locations.[14, 17, 18] This study expands upon that work by extending the same implementation strategy to assess the effectiveness of the introduction of alcohol‐based hand rub on hand hygiene practice at multiple hospitals in Japan.
In a previous article[19] we reported results from an observational study assessing healthcare worker hand hygiene adherence before touching the patient in 4 geographically diverse hospitals in Japan. The study reported that hand hygiene adherence in Japanese hospitals was lower than reported mean values from other international studies, and that greater adherence to hand hygiene should be encouraged. In this article, we present the results of a multimodal intervention intended to improve levels of healthcare worker hand hygiene in 3 of these hospitals.
METHODS
Participating Institutions
Three of the 4 hospitals participating in the prior observational study chose to participate in this intervention. Evaluation of hand hygiene practice was performed in at least 3 wards of each hospital including an inpatient surgical ward, an inpatient medicine ward, an ICU, or an emergency ward.
Table 1 lists the characteristics of the participating hospitals. Hospital A is a university‐affiliated, tertiary care medical center with 312 beds in East Japan. Although the hospital did not have an infection prevention unit or designated infection control nurses during the preintervention periods, the hospital hired a designated infection prevention nurse and established a department of infection prevention before this intervention in April 2012. Hospital B is a community‐based, tertiary care medical center with 428 beds, located in Midwest Japan. Although the facility had no infection control nurses at the outset of the study, a physician certified by the American Board of Internal Medicine and Infectious Diseases provided educational sessions of hand hygiene. Hospital B hired a designated infection prevention nurse and established a department of infection prevention in April 2012. Hospital C, located in Northern Japan, is a community‐based, tertiary care medical center with 562 beds. The department of infection prevention was established in 2010 and has 1 full‐time and 2 part‐time infection prevention nurses.
| Hospital A | Hospital B | Hospital C | ||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention | |
| ||||||
| Hospital characteristics | ||||||
| Location | East Japan | Midwest Japan | Northern Japan | |||
| Hospital type | University affiliated | Community based | Community based | |||
| Level of care | Tertiary care | Tertiary care | Tertiary care | |||
| Residency program | Yes | Yes | Yes | |||
| No. of beds | 250 | 312 | 428 | 428 | 550 | 562 |
| No. of employees | 398 | 475 | 1,035 | 1,263 | 1,500 | 1,568 |
| No. of physicians | 73 | 91 | 179 | 188 | 207 | 217 |
| No. of nurses | 172 | 210 | 410 | 540 | 616 | 800 |
| Infection control practice | ||||||
| Establishment of infection prevention units (year) | N/A | Yes (2012) | N/A | Yes (2012) | Yes (2010) | Yes |
| Employment of certified nurses in infection control (FTE) | 0 | 1 (1) | 0 | 1 (1) | 3 (1.5) | 3 (1.5) |
| Employment of ABIM‐IDcertified physician | 0 | 0 | 1 | 1 | 1 | 0 |
Role of the Funding Source
This study was unfunded. The prize for the contest was provided by an American collaborator (S.S.) who was not affiliated with any of the participating hospitals.
Intervention
In the prior preintervention study, hand hygiene adherence rates of healthcare workers were evaluated between July 2011 and November 2011.[19] To improve hand hygiene adherence in these facilities, we initiated a multimodal intervention based on WHO recommendations and the findings from the prior study. Each facility was provided the same guidance on how to improve hand hygiene adherence (Table 2) and encouraged to tailor the intervention to their local setting. As an added incentive, we initiated a contest, where the facility obtaining the highest hand hygiene adherence postintervention would win a trophy and 500,000 Japanese yen (approximately $5000 US dollars). The recommended strategies consisted of 15 components (Table 2): infrastructure (3 components), training and education (2 components), evaluation and feedback (5 components), reminder in the workplace (1 component), and institution safety climate (4 components). Of note, the participating institutions had already implemented a varying number of the intervention components prior to the start of the intervention. Each facility conducted a 6‐month intervention to improve hand hygiene adherence; however, the actual timing of interventions varied slightly by institution. Hospitals A and C conducted an intervention from October 2012 through March 2013, whereas hospital B's intervention was from April 2012 to September 2012. Details of the multimodal intervention performed at each participating hospital are shown in Table 3.
| Intervention Components | Description |
|---|---|
| 1. Infrastructure (3 components) | |
| Hand‐washing faucets for each room | At least 1 faucet and sink for each room was available. |
| Placement of alcohol hand rub at patient's room entrance | Alcohol hand rub was placed at all patient room entrances. |
| Portable alcohol hand rub distributed for each healthcare worker | Personal, portable alcohol hand rub dispensers were provided for healthcare workers who contact patients. |
| 2. Training/education (2 components) | |
| Educational resources | At least 1 physician or 1 nurse who provides educational sessions regarding hand hygiene practice was available. |
| Periodic seminars and lectures regarding hand hygiene education | Hospital‐wide hand hygiene seminar or educational activities were held during the intervention period. |
| 3. Evaluation and feedback (5 components) | |
| Evaluation of hand hygiene practice by direct observation | Hospitals utilize direct observation for healthcare worker's hand hygiene practice. |
| Evaluation of hand hygiene practice by monitoring the amount of alcohol hand rub consumption | Hospitals utilize the amount of alcohol hand rub consumption as a parameter for healthcare worker's hand hygiene practice. |
| Hand hygiene rate feedback at infection control committee | Hand hygiene adherence rate was reported and discussed at hospital infection control committee. |
| Hand hygiene rate feedback to the designated wards/units | Hand hygiene adherence rate was reported and discussed with healthcare workers at the designated wards/units where hand hygiene observation was performed. |
| Granting the award of top‐rated person of hand hygiene | Hospitals established the system to assess individual healthcare worker's hand hygiene adherence rate. |
| 4. Reminder in the workplace (1 components) | |
| Poster notification | Poster notification for hand hygiene practice was performed in the intervention period. |
| 5. Institutional safety climate (4 components) | |
| Commitment of hospital president or hospital executives | Hospital executives including the president agreed on the importance of hand hygiene practice and declared to healthcare workers to enhance hand hygiene practice during the intervention period. |
| Commitment of nurse managers and physician leaders | Commitment of improving hand hygiene practice by representative healthcare workers at the designated wards/units (eg, meeting by nurse manager or physician leaders at the designated wards/units and collaborative work with infection prevention services). |
| Meeting at the designated wards/units | A ward/unit‐level meeting or voluntary session for hands‐on hand hygiene practice by healthcare workers at the designated wards/units. |
| Identifying champions at the designated wards/units | An individual healthcare worker who contributed to improving hand hygiene practice was appointed. |
| Hospital A | Hospital Ba | Hospital C | ||||
|---|---|---|---|---|---|---|
| ||||||
| Intervention period | October 2012March 2013 | April 2012September 2012 | October 2012March 2013 | |||
| Evaluation of hand hygiene in the postintervention period | May 2013July 2013 | October 2012 | June 2013 | |||
| Suggested intervention components | Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention |
| No. of implemented components | 2/15 | 10/15 | 9/15 | 10/15 | 6/15 | 8/15 |
| Infrastructure (3 components) | ||||||
| Hand‐washing faucets for each room | No | No | Yes | Yes | Yes | Yes |
| Placement of alcohol hand rubs at patient's room entrance | Yes | Yes | Yes | Yes | Yes | Yes |
| Portable alcohol hand rub distributed for each healthcare worker | No | Yesb | No | Yesb | No | No |
| Training/education (2 components) | ||||||
| Educational resources | No | Yesb | Yes | Yesb | Yes | Yes |
| Periodic seminars and lectures regarding hand hygiene education | No | Yesb | Yes | Yes | Yes | Yes |
| Evaluation and feedback (5 components) | ||||||
| Evaluation of hand hygiene practice by direct observation | No | Yesb | Yes | Yes | No | No |
| Evaluation of hand hygiene practice by the amount of alcohol hand rub consumption | No | No | Yes | Yes | Yes | Yes |
| Hand hygiene rate feedback at infection control committee | No | Yesb | Yes | Yes | No | Yesb |
| Hand hygiene rate feedback to designated departments | No | Yesb | Yes | Yes | No | Yesb |
| Granting the award of top‐rated person | No | No | No | No | No | No |
| Reminders in the workplace (1 component) | ||||||
| Poster notification | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Institutional safety climate (4 components) | ||||||
| Commitment of hospital president or hospital executives | No | Yesb | No | No | No | No |
| Commitment of nurse managers and physicians leaders | No | Yesb | No | No | No | No |
| Meeting regarding hand hygiene practice by the designated wards/units | No | No | No | No | No | No |
| Identifying champions at the designated wards/units | No | No | No | No | No | No |
Observation of Hand Hygiene Practice
The same methods for hand hygiene observation used for the preintervention study was used for postintervention assessment. Ten distinct units across the 3 participating hospitals were evaluated for healthcare worker hand hygiene prior to patient contact. Three to 4 units were observed at each facility. One of the study authors (T.S.), a Japanese board‐certified infection control nurse, conducted all of the hand hygiene observations for both the preintervention and postintervention studies. Intraobserver variation was minimized by providing the same training outlined in the previous study.[19] Appropriate hand hygiene was defined as the use of soap and water or alcohol‐based hand rub before patient contact, which corresponds to the first moment of the WHO's 5 moments of hand hygiene.[11]
Hand hygiene practice prior to patient contact for each individual provider‐patient encounter was observed and recorded using the hand hygiene observation form adapted from a previous study by Saint et al.[6, 20] Identical to the preintervention study,[19] the form captured the following information: unit in which observations were performed, time of initiation and completion of observations, healthcare worker subgroup (physician or nurse), and the type of hand hygiene before patient contact (ie, hand washing with soap and water, use of alcohol‐based hand rub, or no hand hygiene). Unit physicians and nurses were informed that their clinical practices were going to be observed, but were not informed of the purpose of the observations (eg, hand hygiene adherence). To avoid interfering with clinical care delivery, the observer was given strict instructions to maintain a certain distance from the observed healthcare workers. The observer was instructed to leave immediately if asked for any reason by the unit staff or patients.
Statistical Analysis
Overall hand hygiene adherence rates were calculated and compared between the pre‐ and the postintervention periods. Comparison of hand hygiene adherence by healthcare worker subgroup and by hospital unit between the pre‐ and postintervention periods was also performed. Hand hygiene adherence rates were compared using JMP 9.0 and SAS 9.3 (SAS Institute Inc., Cary, NC). Comparison of hand hygiene adherence rates by observational periods was calculated by Pearson [2] tests, and 95% confidence intervals (CIs) were estimated using binomial distribution. Pearson correlations were used to determine the relationship of hand hygiene between physicians and nurses in the same unit. Two‐tailed P value0.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at the participating hospitals.
RESULTS
Data were collected from May 2013 to July 2013 in hospital A, in October 2012 in hospital B, and June 2013 in hospital C to ensure data were collected after the 6‐month intervention at each site. A total of 2982 observations of hand hygiene were performed in 10 distinct units across the 3 participating hospitals during the postintervention periods. Hand hygiene observations were performed during the day Monday through Friday between 8:30 am and 7:30 pm, with the majority occurring prior to 1:00 pm.
The overall postintervention hand hygiene adherence rate (in all 3 hospitals) was significantly higher at 32.7% (974/2982) adherence compared to 18.0% (482/2679) adherence in the preintervention period (P<0.001). An increased hand hygiene adherence rate in each participating hospital in the postintervention period was observed (Figure 1). Similar trends of higher overall hand hygiene adherence rates for both nurses and physicians in the postintervention period were seen. Use of alcohol‐based hand rub among those with appropriate hand hygiene was significantly higher, with 90.0% (880/974) using hand rub in the postintervention period versus 67.0% (322/482) in the preintervention period (P<0.001). Comparison of overall hand hygiene adherence rates by unit type and healthcare worker subgroup between the pre‐ and postintervention periods are shown in Table 4. Detailed comparisons of hand hygiene adherence rates for each hospital are available in the supplementary appendix. Although a significant improvement of hand hygiene practice was observed in the majority of participating units (6/10), there was a significant decline in hand hygiene practice in 2 units for nurses and 1 unit for physicians. Hand hygiene adherence rates by healthcare worker subgroups (both physicians and nurses) were significantly higher in the postintervention period than those in the preintervention period. Trends toward higher hand hygiene adherence rate of nurses in the postintervention period were observed (34.8% adherence for nurses compared to 30.4% adherence for physicians); the difference between nurses and physicians were not statistically significant (P=0.07).

| Ward/Unit | Healthcare Worker Subgroup | Preintervention Period | Postintervention Period | Improvement After Intervention (%) | P Value | ||
|---|---|---|---|---|---|---|---|
| No. of Observations | Hand hygiene Adherence (%) | No. of Observations | Hand Hygiene Adherence (%) | ||||
| |||||||
| All 3 hospitals | |||||||
| Surgery | Nurse | 455 | 20 | 480 | 40 | 20 | <0.001 |
| Physician | 424 | 18 | 448 | 43 | 25 | <0.001 | |
| All | 879 | 19 | 928 | 41 | 22 | <0.001 | |
| Medicine | Nurse | 455 | 23 | 508 | 39 | 16 | <0.001 |
| Physician | 435 | 15 | 452 | 33 | 18 | <0.001 | |
| All | 890 | 20 | 960 | 36 | 16 | <0.001 | |
| ICU | Nurse | 305 | 21 | 379 | 25 | 4 | 0.17 |
| Physician | 203 | 9 | 268 | 28 | 19 | <0.001 | |
| All | 508 | 16 | 647 | 26 | 10 | <0.001 | |
| ED | Nurse | 170 | 16 | 173 | 27 | 11 | 0.01 |
| Physician | 232 | 14 | 274 | 9 | ‐5 | 0.07 | |
| All | 402 | 15 | 447 | 16 | 1 | 0.64 | |
| All units | Nurse | 1385 | 21 | 1540 | 35 | 14 | <0.001 |
| Physician | 1294 | 15 | 1442 | 30 | 15 | <0.001 | |
| All | 2679 | 18 | 2982 | 33 | 15 | <0.001 | |
Hospital A achieved the highest postintervention adherence rates (39.9% adherence postintervention), as well as the greatest absolute improvement in hand hygiene (increase of 29.0%). There were significant improvements in 3 of the 4 participating units in hospital A, with the emergency department showing improvements only in the nurse subgroup. In hospital B, total hand hygiene adherence increased from 24.7% to 30.0% (P=0.01); however, this increase was mainly due to increase in hand hygiene adherence rates of nurses. There were significant increases in hand hygiene adherence rates for nurses in the medicine (+11%, P=0.04) and surgery wards (+14%, P=0.01), with nonsignificant increases for physicians (+10% medicine, P=0.07;+2% surgery, P=0.78). However, in the emergency department, nurses showed no significant improvement, and physicians had a significant decrease in adherence (15.7% preintervention vs 7.4% postintervention; P=0.02). In hospital C, total hand hygiene practice rates were significantly improved (from 18.9% to 26.5%; P<0.001); however, this was driven by improvements only in the surgical ward (14.6% preintervention to 42.3% postintervention; P<0.001). The rates for nurses declined significantly in both the medicine and ICU wards, leading to no observed improvements on those wards.
DISCUSSION
Our multicenter intervention study in Japan included observations from almost 3000 encounters between clinicians and patients. Before the intervention, the overall rate of hand hygiene adherence was 18%. After the multimodal intervention, the absolute increase in healthcare worker hand hygiene adherence was 15%. Although there was overall improvement, the adherence rates varied by hospital, with hospital A increasing by 29% and hospital B and C only attaining increases of 5% and 7%, respectively.
Despite the importance of hand hygiene of healthcare workers, it is challenging to increase hand hygiene adherence because it requires behavioral modification. Moreover, it remains uncertain what factors will affect healthcare worker behavior. We implemented pragmatic strategies to evaluate the efficacy of hand hygiene multimodal interventions based on internationally recognized WHO hand hygiene adherence strategies[11] and an institutional‐level contest with financial incentives. The findings in the current study help us understand not only how a multimodal intervention importantly improves hand hygiene adherence, but also what factors potentially make healthcare workers modify their behaviors.
In this study, we evaluated whether an institutional‐level contest with financial incentives contributed to improved hand hygiene adherence of healthcare workers. This study demonstrated improvement of hand hygiene practice after implementation of a multimodal hand hygiene intervention combined with an institutional‐level contest with financial incentives. The contest might have had a modest effect to help motivate the participating hospitals to improve their hand hygiene adherence rate. This is consistent with a previous study that demonstrated financial incentives were associated with modifying healthcare workers' hand hygiene practice.[21] However, we did not strictly standardize how the contest information was distributed in each participating institution and the objective assessment for changes in motivation by the contest was lacking in this study. Thus, changes in motivation by the contest with financial incentives likely varied by each participating institution. Further studies are needed to assess if this type of approach is worth pursuing.
We observed several noteworthy associations between the intervention components that were implemented at each facility and their improvement in hand hygiene adherence. Among the participating hospitals, hospital A was most successful with improving hand hygiene adherence, although all participating hospitals achieved a similar number of the 15 recommended intervention components during the intervention (8 to 10 per hospital). Interestingly, hospital A initiated the most new components during the intervention period (8 new components for a total of 10 out of 15), whereas hospital B and hospital C initiated only 1 or 2 new components during the intervention period. Hospital A also successfully involved hospital executives, and elicited the commitment of a nurse manager and physician leader. Consistent with a previous study,[22] we believe that involvement of hospital executives appears to be important to increase overall hand hygiene rate among healthcare workers.
In contrast, hospitals B and C did not involve senior executives or identify nurse or physician champions for all participating units. Based on the results in this study, we believe that the involvement of hospital executives is likely a key for the penetration of hospital‐wide hand hygiene culture among healthcare workers.
Although this study was unable to determine which components are precisely associated with improving hand hygiene adherence, the findings suggest initiating multiple intervention components at the same time may provide more motivation for change than initiating only 1 or 2 components at a time. It is also possible that certain intervention components were more beneficial than others. For example, hospital A, which achieved the most success, was the only hospital to obtain leadership support. Other studies have demonstrated that the presence of leadership appeared to play a key role in improving hand hygiene adherence.[23, 24] Moreover, a recent Japanese nationwide survey demonstrated higher safety centeredness was associated with regular use of standard infection prevention practice.[25] Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introduction of portable alcohol‐based hand rub) alone, but it depends on altering healthcare worker behavior.[26]
This study has several limitations. Because participating hospitals could tailor the specific interventions chosen for their facility, the improvement in hand hygiene adherence was likely multifactorial. We are unable in the existing study to determine a direct causal relationship between any of the individual intervention components and hand hygiene adherence. We are also unable to determine whether the improvements seen in hospital A were due to participation in the contest or due to the specific intervention components that were implemented. However, WHO hand hygiene guidelines point out that recognition of the importance of hand hygiene varies in different regions and countries, and the goal for hand hygiene interventions is to establish a culture of hand hygiene practice through pragmatic intervention strategies, frequent evaluation, and feedback to healthcare workers.[27] Thus, we prioritized pragmatic strategies to include in our intervention to promote hand hygiene adherence. Another limitation was the date of implementation of the multimodal intervention was slightly different at each facility. It was challenging to implement the intervention simultaneously across institutions due to competing priorities at each facility. Although the primary goal of hand hygiene is to reduce the burden of healthcare‐associated infection, we were unable to measure infection rates at the participating facilities. It is possible the presence of an external observer had an impact on the healthcare workers' behavior.[28] However, the healthcare workers were not informed as to what the observer was monitoring to minimize this potential effect. Lastly, the findings in this study provide immediate intervention effects but further study will be required to determine if these effects are sustainable.
Altering healthcare worker behavior is likely the key element to improve hand hygiene adherence, and behavioral modification may be achieved with the support of leadership at the unit and facility level. However, even though we found significant improvements in healthcare worker hand hygiene adherence after the intervention, the adherence rates are still relatively low compared to reported adherence rates from other countries,[29] suggesting further intervention is needed in this setting to optimize and hygiene practice. Because hand hygiene practice is a crucial strategy to prevent healthcare‐associated infections, every effort should be made to enhance the hand hygiene practice of healthcare workers.
Acknowledgements
The authors thank the International Ann Arbor Safety Collaborative (
Disclosure: Nothing to report.
Healthcare‐associated infections are a major cause of illness and death in hospitalized patients, and preventing healthcare‐associated infection is a global challenge.[1] Worldwide, the prevalence of healthcare‐associated infections in developed and undeveloped countries ranges from 5.1% to 11.6% and 5.7% to 19.1%, respectively.[2] In the United States, roughly 2 million such infections occur annually, resulting in approximately 99,000 deaths[3] and estimated annual direct medical costs between $28.4 and $33.8 billion.[4] In Japan, nearly 9% of patients admitted to the intensive care unit (ICU) develop an infection during hospitalization,[5] and 5% of all patients hospitalized become infected with methicillin‐resistant Staphylococcus aureus.[6] The management of healthcare‐associated infections in Japan accounts for up to 5% of total annual healthcare costs, with an estimated $6.8 billion estimated to be potentially preventable.[7] In addition, healthcare‐associated infections are associated with increased length of stay in the hospital. Studies estimate surgical site infections extend length of stay by 9.7 days,[8] and bloodstream infections increase length of stay by 10 days.[9]
Improving hand hygiene practice for healthcare workers is considered a core strategy to decrease the incidence of healthcare‐associated infection.[6, 10] Specifically, the use of alcohol‐based hand rub is strongly recommended in acute care hospitals by both the World Health Organization (WHO) and the US Centers for Disease Control and Prevention.[11, 12] Improving hand hygiene adherence may reduce healthcare‐associated infection by 9% to 50%,[13, 14] and multiple studies have reported that greater use of alcohol‐based hand rubs results in significant reductions in healthcare‐associated infections.[14, 15]
Due to the difficulty in improving hand hygiene in various settings across the world, the WHO strategy for improving hand hygiene has been adopted and implemented by several studies in varying locations, such as Costa Rica, Italy, Mali, Pakistan, and Saudi Arabia.[16] Implementations of these multimodal strategies, following WHObased guidelines, have been shown to increase the level of hand hygiene adherence among healthcare workers and reduce infections at these locations.[14, 17, 18] This study expands upon that work by extending the same implementation strategy to assess the effectiveness of the introduction of alcohol‐based hand rub on hand hygiene practice at multiple hospitals in Japan.
In a previous article[19] we reported results from an observational study assessing healthcare worker hand hygiene adherence before touching the patient in 4 geographically diverse hospitals in Japan. The study reported that hand hygiene adherence in Japanese hospitals was lower than reported mean values from other international studies, and that greater adherence to hand hygiene should be encouraged. In this article, we present the results of a multimodal intervention intended to improve levels of healthcare worker hand hygiene in 3 of these hospitals.
METHODS
Participating Institutions
Three of the 4 hospitals participating in the prior observational study chose to participate in this intervention. Evaluation of hand hygiene practice was performed in at least 3 wards of each hospital including an inpatient surgical ward, an inpatient medicine ward, an ICU, or an emergency ward.
Table 1 lists the characteristics of the participating hospitals. Hospital A is a university‐affiliated, tertiary care medical center with 312 beds in East Japan. Although the hospital did not have an infection prevention unit or designated infection control nurses during the preintervention periods, the hospital hired a designated infection prevention nurse and established a department of infection prevention before this intervention in April 2012. Hospital B is a community‐based, tertiary care medical center with 428 beds, located in Midwest Japan. Although the facility had no infection control nurses at the outset of the study, a physician certified by the American Board of Internal Medicine and Infectious Diseases provided educational sessions of hand hygiene. Hospital B hired a designated infection prevention nurse and established a department of infection prevention in April 2012. Hospital C, located in Northern Japan, is a community‐based, tertiary care medical center with 562 beds. The department of infection prevention was established in 2010 and has 1 full‐time and 2 part‐time infection prevention nurses.
| Hospital A | Hospital B | Hospital C | ||||
|---|---|---|---|---|---|---|
| Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention | |
| ||||||
| Hospital characteristics | ||||||
| Location | East Japan | Midwest Japan | Northern Japan | |||
| Hospital type | University affiliated | Community based | Community based | |||
| Level of care | Tertiary care | Tertiary care | Tertiary care | |||
| Residency program | Yes | Yes | Yes | |||
| No. of beds | 250 | 312 | 428 | 428 | 550 | 562 |
| No. of employees | 398 | 475 | 1,035 | 1,263 | 1,500 | 1,568 |
| No. of physicians | 73 | 91 | 179 | 188 | 207 | 217 |
| No. of nurses | 172 | 210 | 410 | 540 | 616 | 800 |
| Infection control practice | ||||||
| Establishment of infection prevention units (year) | N/A | Yes (2012) | N/A | Yes (2012) | Yes (2010) | Yes |
| Employment of certified nurses in infection control (FTE) | 0 | 1 (1) | 0 | 1 (1) | 3 (1.5) | 3 (1.5) |
| Employment of ABIM‐IDcertified physician | 0 | 0 | 1 | 1 | 1 | 0 |
Role of the Funding Source
This study was unfunded. The prize for the contest was provided by an American collaborator (S.S.) who was not affiliated with any of the participating hospitals.
Intervention
In the prior preintervention study, hand hygiene adherence rates of healthcare workers were evaluated between July 2011 and November 2011.[19] To improve hand hygiene adherence in these facilities, we initiated a multimodal intervention based on WHO recommendations and the findings from the prior study. Each facility was provided the same guidance on how to improve hand hygiene adherence (Table 2) and encouraged to tailor the intervention to their local setting. As an added incentive, we initiated a contest, where the facility obtaining the highest hand hygiene adherence postintervention would win a trophy and 500,000 Japanese yen (approximately $5000 US dollars). The recommended strategies consisted of 15 components (Table 2): infrastructure (3 components), training and education (2 components), evaluation and feedback (5 components), reminder in the workplace (1 component), and institution safety climate (4 components). Of note, the participating institutions had already implemented a varying number of the intervention components prior to the start of the intervention. Each facility conducted a 6‐month intervention to improve hand hygiene adherence; however, the actual timing of interventions varied slightly by institution. Hospitals A and C conducted an intervention from October 2012 through March 2013, whereas hospital B's intervention was from April 2012 to September 2012. Details of the multimodal intervention performed at each participating hospital are shown in Table 3.
| Intervention Components | Description |
|---|---|
| 1. Infrastructure (3 components) | |
| Hand‐washing faucets for each room | At least 1 faucet and sink for each room was available. |
| Placement of alcohol hand rub at patient's room entrance | Alcohol hand rub was placed at all patient room entrances. |
| Portable alcohol hand rub distributed for each healthcare worker | Personal, portable alcohol hand rub dispensers were provided for healthcare workers who contact patients. |
| 2. Training/education (2 components) | |
| Educational resources | At least 1 physician or 1 nurse who provides educational sessions regarding hand hygiene practice was available. |
| Periodic seminars and lectures regarding hand hygiene education | Hospital‐wide hand hygiene seminar or educational activities were held during the intervention period. |
| 3. Evaluation and feedback (5 components) | |
| Evaluation of hand hygiene practice by direct observation | Hospitals utilize direct observation for healthcare worker's hand hygiene practice. |
| Evaluation of hand hygiene practice by monitoring the amount of alcohol hand rub consumption | Hospitals utilize the amount of alcohol hand rub consumption as a parameter for healthcare worker's hand hygiene practice. |
| Hand hygiene rate feedback at infection control committee | Hand hygiene adherence rate was reported and discussed at hospital infection control committee. |
| Hand hygiene rate feedback to the designated wards/units | Hand hygiene adherence rate was reported and discussed with healthcare workers at the designated wards/units where hand hygiene observation was performed. |
| Granting the award of top‐rated person of hand hygiene | Hospitals established the system to assess individual healthcare worker's hand hygiene adherence rate. |
| 4. Reminder in the workplace (1 components) | |
| Poster notification | Poster notification for hand hygiene practice was performed in the intervention period. |
| 5. Institutional safety climate (4 components) | |
| Commitment of hospital president or hospital executives | Hospital executives including the president agreed on the importance of hand hygiene practice and declared to healthcare workers to enhance hand hygiene practice during the intervention period. |
| Commitment of nurse managers and physician leaders | Commitment of improving hand hygiene practice by representative healthcare workers at the designated wards/units (eg, meeting by nurse manager or physician leaders at the designated wards/units and collaborative work with infection prevention services). |
| Meeting at the designated wards/units | A ward/unit‐level meeting or voluntary session for hands‐on hand hygiene practice by healthcare workers at the designated wards/units. |
| Identifying champions at the designated wards/units | An individual healthcare worker who contributed to improving hand hygiene practice was appointed. |
| Hospital A | Hospital Ba | Hospital C | ||||
|---|---|---|---|---|---|---|
| ||||||
| Intervention period | October 2012March 2013 | April 2012September 2012 | October 2012March 2013 | |||
| Evaluation of hand hygiene in the postintervention period | May 2013July 2013 | October 2012 | June 2013 | |||
| Suggested intervention components | Preintervention | Postintervention | Preintervention | Postintervention | Preintervention | Postintervention |
| No. of implemented components | 2/15 | 10/15 | 9/15 | 10/15 | 6/15 | 8/15 |
| Infrastructure (3 components) | ||||||
| Hand‐washing faucets for each room | No | No | Yes | Yes | Yes | Yes |
| Placement of alcohol hand rubs at patient's room entrance | Yes | Yes | Yes | Yes | Yes | Yes |
| Portable alcohol hand rub distributed for each healthcare worker | No | Yesb | No | Yesb | No | No |
| Training/education (2 components) | ||||||
| Educational resources | No | Yesb | Yes | Yesb | Yes | Yes |
| Periodic seminars and lectures regarding hand hygiene education | No | Yesb | Yes | Yes | Yes | Yes |
| Evaluation and feedback (5 components) | ||||||
| Evaluation of hand hygiene practice by direct observation | No | Yesb | Yes | Yes | No | No |
| Evaluation of hand hygiene practice by the amount of alcohol hand rub consumption | No | No | Yes | Yes | Yes | Yes |
| Hand hygiene rate feedback at infection control committee | No | Yesb | Yes | Yes | No | Yesb |
| Hand hygiene rate feedback to designated departments | No | Yesb | Yes | Yes | No | Yesb |
| Granting the award of top‐rated person | No | No | No | No | No | No |
| Reminders in the workplace (1 component) | ||||||
| Poster notification | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Institutional safety climate (4 components) | ||||||
| Commitment of hospital president or hospital executives | No | Yesb | No | No | No | No |
| Commitment of nurse managers and physicians leaders | No | Yesb | No | No | No | No |
| Meeting regarding hand hygiene practice by the designated wards/units | No | No | No | No | No | No |
| Identifying champions at the designated wards/units | No | No | No | No | No | No |
Observation of Hand Hygiene Practice
The same methods for hand hygiene observation used for the preintervention study was used for postintervention assessment. Ten distinct units across the 3 participating hospitals were evaluated for healthcare worker hand hygiene prior to patient contact. Three to 4 units were observed at each facility. One of the study authors (T.S.), a Japanese board‐certified infection control nurse, conducted all of the hand hygiene observations for both the preintervention and postintervention studies. Intraobserver variation was minimized by providing the same training outlined in the previous study.[19] Appropriate hand hygiene was defined as the use of soap and water or alcohol‐based hand rub before patient contact, which corresponds to the first moment of the WHO's 5 moments of hand hygiene.[11]
Hand hygiene practice prior to patient contact for each individual provider‐patient encounter was observed and recorded using the hand hygiene observation form adapted from a previous study by Saint et al.[6, 20] Identical to the preintervention study,[19] the form captured the following information: unit in which observations were performed, time of initiation and completion of observations, healthcare worker subgroup (physician or nurse), and the type of hand hygiene before patient contact (ie, hand washing with soap and water, use of alcohol‐based hand rub, or no hand hygiene). Unit physicians and nurses were informed that their clinical practices were going to be observed, but were not informed of the purpose of the observations (eg, hand hygiene adherence). To avoid interfering with clinical care delivery, the observer was given strict instructions to maintain a certain distance from the observed healthcare workers. The observer was instructed to leave immediately if asked for any reason by the unit staff or patients.
Statistical Analysis
Overall hand hygiene adherence rates were calculated and compared between the pre‐ and the postintervention periods. Comparison of hand hygiene adherence by healthcare worker subgroup and by hospital unit between the pre‐ and postintervention periods was also performed. Hand hygiene adherence rates were compared using JMP 9.0 and SAS 9.3 (SAS Institute Inc., Cary, NC). Comparison of hand hygiene adherence rates by observational periods was calculated by Pearson [2] tests, and 95% confidence intervals (CIs) were estimated using binomial distribution. Pearson correlations were used to determine the relationship of hand hygiene between physicians and nurses in the same unit. Two‐tailed P value0.05 was considered statistically significant. The study protocol was reviewed and approved by the ethics committees at the participating hospitals.
RESULTS
Data were collected from May 2013 to July 2013 in hospital A, in October 2012 in hospital B, and June 2013 in hospital C to ensure data were collected after the 6‐month intervention at each site. A total of 2982 observations of hand hygiene were performed in 10 distinct units across the 3 participating hospitals during the postintervention periods. Hand hygiene observations were performed during the day Monday through Friday between 8:30 am and 7:30 pm, with the majority occurring prior to 1:00 pm.
The overall postintervention hand hygiene adherence rate (in all 3 hospitals) was significantly higher at 32.7% (974/2982) adherence compared to 18.0% (482/2679) adherence in the preintervention period (P<0.001). An increased hand hygiene adherence rate in each participating hospital in the postintervention period was observed (Figure 1). Similar trends of higher overall hand hygiene adherence rates for both nurses and physicians in the postintervention period were seen. Use of alcohol‐based hand rub among those with appropriate hand hygiene was significantly higher, with 90.0% (880/974) using hand rub in the postintervention period versus 67.0% (322/482) in the preintervention period (P<0.001). Comparison of overall hand hygiene adherence rates by unit type and healthcare worker subgroup between the pre‐ and postintervention periods are shown in Table 4. Detailed comparisons of hand hygiene adherence rates for each hospital are available in the supplementary appendix. Although a significant improvement of hand hygiene practice was observed in the majority of participating units (6/10), there was a significant decline in hand hygiene practice in 2 units for nurses and 1 unit for physicians. Hand hygiene adherence rates by healthcare worker subgroups (both physicians and nurses) were significantly higher in the postintervention period than those in the preintervention period. Trends toward higher hand hygiene adherence rate of nurses in the postintervention period were observed (34.8% adherence for nurses compared to 30.4% adherence for physicians); the difference between nurses and physicians were not statistically significant (P=0.07).

| Ward/Unit | Healthcare Worker Subgroup | Preintervention Period | Postintervention Period | Improvement After Intervention (%) | P Value | ||
|---|---|---|---|---|---|---|---|
| No. of Observations | Hand hygiene Adherence (%) | No. of Observations | Hand Hygiene Adherence (%) | ||||
| |||||||
| All 3 hospitals | |||||||
| Surgery | Nurse | 455 | 20 | 480 | 40 | 20 | <0.001 |
| Physician | 424 | 18 | 448 | 43 | 25 | <0.001 | |
| All | 879 | 19 | 928 | 41 | 22 | <0.001 | |
| Medicine | Nurse | 455 | 23 | 508 | 39 | 16 | <0.001 |
| Physician | 435 | 15 | 452 | 33 | 18 | <0.001 | |
| All | 890 | 20 | 960 | 36 | 16 | <0.001 | |
| ICU | Nurse | 305 | 21 | 379 | 25 | 4 | 0.17 |
| Physician | 203 | 9 | 268 | 28 | 19 | <0.001 | |
| All | 508 | 16 | 647 | 26 | 10 | <0.001 | |
| ED | Nurse | 170 | 16 | 173 | 27 | 11 | 0.01 |
| Physician | 232 | 14 | 274 | 9 | ‐5 | 0.07 | |
| All | 402 | 15 | 447 | 16 | 1 | 0.64 | |
| All units | Nurse | 1385 | 21 | 1540 | 35 | 14 | <0.001 |
| Physician | 1294 | 15 | 1442 | 30 | 15 | <0.001 | |
| All | 2679 | 18 | 2982 | 33 | 15 | <0.001 | |
Hospital A achieved the highest postintervention adherence rates (39.9% adherence postintervention), as well as the greatest absolute improvement in hand hygiene (increase of 29.0%). There were significant improvements in 3 of the 4 participating units in hospital A, with the emergency department showing improvements only in the nurse subgroup. In hospital B, total hand hygiene adherence increased from 24.7% to 30.0% (P=0.01); however, this increase was mainly due to increase in hand hygiene adherence rates of nurses. There were significant increases in hand hygiene adherence rates for nurses in the medicine (+11%, P=0.04) and surgery wards (+14%, P=0.01), with nonsignificant increases for physicians (+10% medicine, P=0.07;+2% surgery, P=0.78). However, in the emergency department, nurses showed no significant improvement, and physicians had a significant decrease in adherence (15.7% preintervention vs 7.4% postintervention; P=0.02). In hospital C, total hand hygiene practice rates were significantly improved (from 18.9% to 26.5%; P<0.001); however, this was driven by improvements only in the surgical ward (14.6% preintervention to 42.3% postintervention; P<0.001). The rates for nurses declined significantly in both the medicine and ICU wards, leading to no observed improvements on those wards.
DISCUSSION
Our multicenter intervention study in Japan included observations from almost 3000 encounters between clinicians and patients. Before the intervention, the overall rate of hand hygiene adherence was 18%. After the multimodal intervention, the absolute increase in healthcare worker hand hygiene adherence was 15%. Although there was overall improvement, the adherence rates varied by hospital, with hospital A increasing by 29% and hospital B and C only attaining increases of 5% and 7%, respectively.
Despite the importance of hand hygiene of healthcare workers, it is challenging to increase hand hygiene adherence because it requires behavioral modification. Moreover, it remains uncertain what factors will affect healthcare worker behavior. We implemented pragmatic strategies to evaluate the efficacy of hand hygiene multimodal interventions based on internationally recognized WHO hand hygiene adherence strategies[11] and an institutional‐level contest with financial incentives. The findings in the current study help us understand not only how a multimodal intervention importantly improves hand hygiene adherence, but also what factors potentially make healthcare workers modify their behaviors.
In this study, we evaluated whether an institutional‐level contest with financial incentives contributed to improved hand hygiene adherence of healthcare workers. This study demonstrated improvement of hand hygiene practice after implementation of a multimodal hand hygiene intervention combined with an institutional‐level contest with financial incentives. The contest might have had a modest effect to help motivate the participating hospitals to improve their hand hygiene adherence rate. This is consistent with a previous study that demonstrated financial incentives were associated with modifying healthcare workers' hand hygiene practice.[21] However, we did not strictly standardize how the contest information was distributed in each participating institution and the objective assessment for changes in motivation by the contest was lacking in this study. Thus, changes in motivation by the contest with financial incentives likely varied by each participating institution. Further studies are needed to assess if this type of approach is worth pursuing.
We observed several noteworthy associations between the intervention components that were implemented at each facility and their improvement in hand hygiene adherence. Among the participating hospitals, hospital A was most successful with improving hand hygiene adherence, although all participating hospitals achieved a similar number of the 15 recommended intervention components during the intervention (8 to 10 per hospital). Interestingly, hospital A initiated the most new components during the intervention period (8 new components for a total of 10 out of 15), whereas hospital B and hospital C initiated only 1 or 2 new components during the intervention period. Hospital A also successfully involved hospital executives, and elicited the commitment of a nurse manager and physician leader. Consistent with a previous study,[22] we believe that involvement of hospital executives appears to be important to increase overall hand hygiene rate among healthcare workers.
In contrast, hospitals B and C did not involve senior executives or identify nurse or physician champions for all participating units. Based on the results in this study, we believe that the involvement of hospital executives is likely a key for the penetration of hospital‐wide hand hygiene culture among healthcare workers.
Although this study was unable to determine which components are precisely associated with improving hand hygiene adherence, the findings suggest initiating multiple intervention components at the same time may provide more motivation for change than initiating only 1 or 2 components at a time. It is also possible that certain intervention components were more beneficial than others. For example, hospital A, which achieved the most success, was the only hospital to obtain leadership support. Other studies have demonstrated that the presence of leadership appeared to play a key role in improving hand hygiene adherence.[23, 24] Moreover, a recent Japanese nationwide survey demonstrated higher safety centeredness was associated with regular use of standard infection prevention practice.[25] Consistent with a previous study, improving hand hygiene adherence cannot be simply achieved by improving infrastructure (eg, introduction of portable alcohol‐based hand rub) alone, but it depends on altering healthcare worker behavior.[26]
This study has several limitations. Because participating hospitals could tailor the specific interventions chosen for their facility, the improvement in hand hygiene adherence was likely multifactorial. We are unable in the existing study to determine a direct causal relationship between any of the individual intervention components and hand hygiene adherence. We are also unable to determine whether the improvements seen in hospital A were due to participation in the contest or due to the specific intervention components that were implemented. However, WHO hand hygiene guidelines point out that recognition of the importance of hand hygiene varies in different regions and countries, and the goal for hand hygiene interventions is to establish a culture of hand hygiene practice through pragmatic intervention strategies, frequent evaluation, and feedback to healthcare workers.[27] Thus, we prioritized pragmatic strategies to include in our intervention to promote hand hygiene adherence. Another limitation was the date of implementation of the multimodal intervention was slightly different at each facility. It was challenging to implement the intervention simultaneously across institutions due to competing priorities at each facility. Although the primary goal of hand hygiene is to reduce the burden of healthcare‐associated infection, we were unable to measure infection rates at the participating facilities. It is possible the presence of an external observer had an impact on the healthcare workers' behavior.[28] However, the healthcare workers were not informed as to what the observer was monitoring to minimize this potential effect. Lastly, the findings in this study provide immediate intervention effects but further study will be required to determine if these effects are sustainable.
Altering healthcare worker behavior is likely the key element to improve hand hygiene adherence, and behavioral modification may be achieved with the support of leadership at the unit and facility level. However, even though we found significant improvements in healthcare worker hand hygiene adherence after the intervention, the adherence rates are still relatively low compared to reported adherence rates from other countries,[29] suggesting further intervention is needed in this setting to optimize and hygiene practice. Because hand hygiene practice is a crucial strategy to prevent healthcare‐associated infections, every effort should be made to enhance the hand hygiene practice of healthcare workers.
Acknowledgements
The authors thank the International Ann Arbor Safety Collaborative (
Disclosure: Nothing to report.
- . Infection control—a problem for patient safety. N Engl J Med. 2003;348(7):651–656.
- World Health Organization. The burden of health care‐associated infection worldwide: a summary. Available at: http://www.who.int/gpsc/country_work/summary_20100430_en.pdf. Accessed October 6, 2014.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed April 20, 2015.
- , , . Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30–35.
- , , , et al. Improving healthcare worker hand hygiene adherence before patient contact: a before‐and‐after five‐unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429–433.
- . Economical efficiency of infection control. Antibiot Chemother (Northfield). 2004;20:635–638.
- , , , , , . Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397.
- , , , , , . Hospital‐acquired, laboratory‐confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158–162.
- . APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251–269.
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. Clean care is safer care: first global patient safety challenge. Geneva, Switzerland; 2009. Available at: http://www.who.int/gpsc/en/index.html. Accessed October 6, 2014.
- , ; Healthcare Infection Control Practices Advisory Committee, HICPAC SHEA APIC IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health‐care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51(RR‐16):1–45.
- National Patient Safety Agency. The economic case: implementing near‐patient alcohol hand rum in your trust. London, United Kingdom; 2004. Available at: http://www.npsa.nhs.uk/cleanyourhands/resource‐area/evidence‐base/?EntryId34=58433. Accessed October 9, 2014.
- , , , et al. Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–1312.
- , . Role of hand hygiene in healthcare‐associated infection prevention. J Hosp Infect. 2009;73(4):305–315.
- , , , et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: a quasi‐experimental study. Lancet Infect Dis. 2013;13(10):843–851.
- , , , et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited‐resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415–423.
- , , , . Bundling hand hygiene interventions and measurement to decrease health care‐associated infections. Am J Infect Control. 2012;40(4 suppl 1):S18–S27.
- , , , et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan [published online April 8, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000108.
- , , , et al. Marked variability in adherence to hand hygiene: a 5‐unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306–310.
- , , , et al. Sustained improvement in hand hygiene adherence: utilizing shared accountability and financial incentives. Infect Control Hosp Epidemiol. 2013;34(11):1129–1136.
- , , , . Status of the implementation of the World Health Organization multimodal hand hygiene strategy in United States of America health care facilities. Am J Infect Control. 2014;42(3):224–230.
- , , , et al. The effect of leadership on hand hygiene: assessing hand hygiene adherence prior to patient contact in 2 infectious disease units in Tuscany. Infect Control Hosp Epidemiol. 2014;35(3):313–316.
- , , , , , . Impact of a hospital‐wide hand hygiene initiative on healthcare‐associated infections: results of an interrupted time series. BMJ Qual Saf. 2012;21(12):1019–1026.
- , , , , , . Health care‐associated infection prevention in Japan: the role of safety culture. Am J Infect Control. 2014;42(8):888–893.
- , , . Why healthcare workers don't wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol. 2006;27(5):484–492.
- World Health Organization. Guide to implementation. A guide to the implementation of the WHO multimodal hand hygiene improvement strategy. Available at: http://whqlibdoc.who.int/hq/2009/WHO_IER_PSP_2009.02_eng.pdf. Accessed October 9, 2014.
- , , , et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746.
- , , , et al. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31(3):283–294.
- . Infection control—a problem for patient safety. N Engl J Med. 2003;348(7):651–656.
- World Health Organization. The burden of health care‐associated infection worldwide: a summary. Available at: http://www.who.int/gpsc/country_work/summary_20100430_en.pdf. Accessed October 6, 2014.
- , , , et al. Estimating health care‐associated infections and deaths in U.S. hospitals, 2002. Public Health Rep. 2007;122(2):160–166.
- . The direct medical costs of healthcare‐associated infections in U.S. hospitals and the benefits of prevention. Atlanta, GA: Centers for Disease Control and Prevention; 2009. Available at: http://www.cdc.gov/HAI/pdfs/hai/Scott_CostPaper.pdf. Accessed April 20, 2015.
- , , . Epidemiological approach to nosocomial infection surveillance data: the Japanese Nosocomial Infection Surveillance System. Environ Health Prev Med. 2008;13(1):30–35.
- , , , et al. Improving healthcare worker hand hygiene adherence before patient contact: a before‐and‐after five‐unit multimodal intervention in Tuscany. Qual Saf Health Care. 2009;18(6):429–433.
- . Economical efficiency of infection control. Antibiot Chemother (Northfield). 2004;20:635–638.
- , , , , , . Surgical site infection: incidence and impact on hospital utilization and treatment costs. Am J Infect Control. 2009;37(5):387–397.
- , , , , , . Hospital‐acquired, laboratory‐confirmed bloodstream infections: linking national surveillance data to clinical and financial hospital data to estimate increased length of stay and healthcare costs. J Hosp Infect. 2010;75(3):158–162.
- . APIC guideline for handwashing and hand antisepsis in health care settings. Am J Infect Control. 1995;23(4):251–269.
- World Health Organization. WHO Guidelines on Hand Hygiene in Health Care. Clean care is safer care: first global patient safety challenge. Geneva, Switzerland; 2009. Available at: http://www.who.int/gpsc/en/index.html. Accessed October 6, 2014.
- , ; Healthcare Infection Control Practices Advisory Committee, HICPAC SHEA APIC IDSA Hand Hygiene Task Force. Guideline for hand hygiene in health‐care settings. Recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Society for Healthcare Epidemiology of America/Association for Professionals in Infection Control/Infectious Diseases Society of America. MMWR Recomm Rep. 2002;51(RR‐16):1–45.
- National Patient Safety Agency. The economic case: implementing near‐patient alcohol hand rum in your trust. London, United Kingdom; 2004. Available at: http://www.npsa.nhs.uk/cleanyourhands/resource‐area/evidence‐base/?EntryId34=58433. Accessed October 9, 2014.
- , , , et al. Effectiveness of a hospital‐wide programme to improve compliance with hand hygiene. Infection Control Programme. Lancet. 2000;356(9238):1307–1312.
- , . Role of hand hygiene in healthcare‐associated infection prevention. J Hosp Infect. 2009;73(4):305–315.
- , , , et al. Global implementation of WHO's multimodal strategy for improvement of hand hygiene: a quasi‐experimental study. Lancet Infect Dis. 2013;13(10):843–851.
- , , , et al. Impact of the International Nosocomial Infection Control Consortium (INICC) multidimensional hand hygiene approach over 13 years in 51 cities of 19 limited‐resource countries from Latin America, Asia, the Middle East, and Europe. Infect Control Hosp Epidemiol. 2013;34(4):415–423.
- , , , . Bundling hand hygiene interventions and measurement to decrease health care‐associated infections. Am J Infect Control. 2012;40(4 suppl 1):S18–S27.
- , , , et al. Hand hygiene adherence among health care workers at Japanese hospitals: a multicenter observational study in Japan [published online April 8, 2014]. J Patient Saf. doi: 10.1097/PTS.0000000000000108.
- , , , et al. Marked variability in adherence to hand hygiene: a 5‐unit observational study in Tuscany. Am J Infect Control. 2009;37(4):306–310.
- , , , et al. Sustained improvement in hand hygiene adherence: utilizing shared accountability and financial incentives. Infect Control Hosp Epidemiol. 2013;34(11):1129–1136.
- , , , . Status of the implementation of the World Health Organization multimodal hand hygiene strategy in United States of America health care facilities. Am J Infect Control. 2014;42(3):224–230.
- , , , et al. The effect of leadership on hand hygiene: assessing hand hygiene adherence prior to patient contact in 2 infectious disease units in Tuscany. Infect Control Hosp Epidemiol. 2014;35(3):313–316.
- , , , , , . Impact of a hospital‐wide hand hygiene initiative on healthcare‐associated infections: results of an interrupted time series. BMJ Qual Saf. 2012;21(12):1019–1026.
- , , , , , . Health care‐associated infection prevention in Japan: the role of safety culture. Am J Infect Control. 2014;42(8):888–893.
- , , . Why healthcare workers don't wash their hands: a behavioral explanation. Infect Control Hosp Epidemiol. 2006;27(5):484–492.
- World Health Organization. Guide to implementation. A guide to the implementation of the WHO multimodal hand hygiene improvement strategy. Available at: http://whqlibdoc.who.int/hq/2009/WHO_IER_PSP_2009.02_eng.pdf. Accessed October 9, 2014.
- , , , et al. Compliance of health care workers with hand hygiene practices: independent advantages of overt and covert observers. PLoS One. 2013;8(1):e53746.
- , , , et al. Systematic review of studies on compliance with hand hygiene guidelines in hospital care. Infect Control Hosp Epidemiol. 2010;31(3):283–294.
© 2015 Society of Hospital Medicine
History of Sexual Abuse May Affect Risk for Chronic Migraine
VALENCIA, SPAIN—A history of childhood sexual abuse is nearly fourfold more common among patients with chronic migraine than in those with episodic migraine, according to research presented at the International Headache Congress. This association raises the possibility that prior sexual abuse is a contributing factor in the transformation from episodic migraine to chronic migraine, said Brad Torphy, MD, of Diamond Headache Clinic in Chicago.
“The clinical implication of these findings, and what I’m stressing, is the importance of intervention—such as psychological counseling—in episodic migraine patients who have a history of abuse,” he said in an interview. “The other key point is that patients who have episodic migraine may warrant more aggressive therapy, including going on preventive medications sooner, if they have a history of sexual abuse, because they’re probably at risk for progression to chronic migraine.”
Dr. Torphy presented a retrospective study of all new patients seen at the Diamond Headache Clinic during the final four months of last year. The new-patient questionnaire includes a section about past sexual abuse. Such a history was reported by six (4.4%) of the 135 patients with episodic migraine, compared with 30 (15.5%) of the 194 patients with chronic migraine.
Based upon his experience in the clinic, Dr. Torphy suspects that the true proportion of patients with a positive history for sexual abuse is considerably higher than the rates the new-patient questionnaire would suggest.
“A lot of factors would lead to that being a very low number,” Dr. Torphy continued. “It’s the patient’s first visit, and it’s a paper questionnaire, so patients may not be comfortable checking that box when they don’t know who’s going to see the results. I’ve had cases where patients shared with me only after two or three visits that, yes, I do have that history. I think it’s underreported across the board.”
In his review of the literature, he found that other investigators have tended either to lump together all kinds of abuse—physical, emotional, and sexual—in analyzing an association with migraine, or if they looked at sexual abuse in particular, it was in association with all types of chronic pain, not specifically migraine.
—Bruce Jancin
VALENCIA, SPAIN—A history of childhood sexual abuse is nearly fourfold more common among patients with chronic migraine than in those with episodic migraine, according to research presented at the International Headache Congress. This association raises the possibility that prior sexual abuse is a contributing factor in the transformation from episodic migraine to chronic migraine, said Brad Torphy, MD, of Diamond Headache Clinic in Chicago.
“The clinical implication of these findings, and what I’m stressing, is the importance of intervention—such as psychological counseling—in episodic migraine patients who have a history of abuse,” he said in an interview. “The other key point is that patients who have episodic migraine may warrant more aggressive therapy, including going on preventive medications sooner, if they have a history of sexual abuse, because they’re probably at risk for progression to chronic migraine.”
Dr. Torphy presented a retrospective study of all new patients seen at the Diamond Headache Clinic during the final four months of last year. The new-patient questionnaire includes a section about past sexual abuse. Such a history was reported by six (4.4%) of the 135 patients with episodic migraine, compared with 30 (15.5%) of the 194 patients with chronic migraine.
Based upon his experience in the clinic, Dr. Torphy suspects that the true proportion of patients with a positive history for sexual abuse is considerably higher than the rates the new-patient questionnaire would suggest.
“A lot of factors would lead to that being a very low number,” Dr. Torphy continued. “It’s the patient’s first visit, and it’s a paper questionnaire, so patients may not be comfortable checking that box when they don’t know who’s going to see the results. I’ve had cases where patients shared with me only after two or three visits that, yes, I do have that history. I think it’s underreported across the board.”
In his review of the literature, he found that other investigators have tended either to lump together all kinds of abuse—physical, emotional, and sexual—in analyzing an association with migraine, or if they looked at sexual abuse in particular, it was in association with all types of chronic pain, not specifically migraine.
—Bruce Jancin
VALENCIA, SPAIN—A history of childhood sexual abuse is nearly fourfold more common among patients with chronic migraine than in those with episodic migraine, according to research presented at the International Headache Congress. This association raises the possibility that prior sexual abuse is a contributing factor in the transformation from episodic migraine to chronic migraine, said Brad Torphy, MD, of Diamond Headache Clinic in Chicago.
“The clinical implication of these findings, and what I’m stressing, is the importance of intervention—such as psychological counseling—in episodic migraine patients who have a history of abuse,” he said in an interview. “The other key point is that patients who have episodic migraine may warrant more aggressive therapy, including going on preventive medications sooner, if they have a history of sexual abuse, because they’re probably at risk for progression to chronic migraine.”
Dr. Torphy presented a retrospective study of all new patients seen at the Diamond Headache Clinic during the final four months of last year. The new-patient questionnaire includes a section about past sexual abuse. Such a history was reported by six (4.4%) of the 135 patients with episodic migraine, compared with 30 (15.5%) of the 194 patients with chronic migraine.
Based upon his experience in the clinic, Dr. Torphy suspects that the true proportion of patients with a positive history for sexual abuse is considerably higher than the rates the new-patient questionnaire would suggest.
“A lot of factors would lead to that being a very low number,” Dr. Torphy continued. “It’s the patient’s first visit, and it’s a paper questionnaire, so patients may not be comfortable checking that box when they don’t know who’s going to see the results. I’ve had cases where patients shared with me only after two or three visits that, yes, I do have that history. I think it’s underreported across the board.”
In his review of the literature, he found that other investigators have tended either to lump together all kinds of abuse—physical, emotional, and sexual—in analyzing an association with migraine, or if they looked at sexual abuse in particular, it was in association with all types of chronic pain, not specifically migraine.
—Bruce Jancin
A New Biomarker for Episodic Migraine in Women?
Sphingolipid metabolism is altered in women with episodic migraine, according to a study published online ahead of print September 9 in Neurology. According to the study authors, serum sphingolipid panels may have the potential to differentiate episodic migraine presence and absence. “While more research is needed to confirm these initial findings, the possibility of discovering a new biomarker for migraine is exciting,” said lead author B. Lee Peterlin, DO, Director of Johns Hopkins Headache Research and Associate Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore.
Case–Control Study
In this study, 88 female participants, 52 with episodic migraine and 36 without, were evaluated on the basis of demographic and health-related criteria, including marital status, BMI, and neuronal functioning. The patients also submitted blood samples, which were tested for lipid concentrations among other things.
The findings suggest that migraineurs had a decreased de novo synthesis of ceramides, which, paired with an independent downstream increase in the conversion of ceramide metabolic products, resulted in a major deficit. Women with migraines had an average concentration of ceramide levels of 6,000 ng/mL, a 43% decrease when compared with controls, who had an average of 10,500 ng/mL of ceramide in their blood.
There was also a marked difference in sphingolipid concentrations in those with migraines, with some species increasing while others were decreased. Dr. Peterlin and colleagues used the difference in blood lipid levels to create a list of 10 sphingolipids thought to classify episodic migraine. In a later test, this constructed biomarker evaluative list was 100% effective in identifying women with and without episodic migraine for a small group of eight migraineurs and six controls.
“This study is an important contribution to our understanding of the pathophysiology of migraine and may have vast practical, clinical, and therapeutic implications if it is supported by further studies,” said Karl Ekbom, PhD, a neurologist at the Karolinska Institutet in Stockholm, in an accompanying commentary.
The study suggests that the presence of migraines is a neurologic disorder of sphingolipid dsymetabolism. With the positive identification of these biomarkers comes the potential of targeted drug therapies directed against the specific sphingolipid pathways involved.
—Adaeze Stephanie Onyechi
Suggested Reading
Peterlin BL, Mielke MM, Dickens AM, et al. Interictal, circulating sphingolipids in women with episodic migraine. Neurology. 2015 Sept 9 [Epub ahead of print].
Ekbom K. Comment: Altered sphingolipid metabolism—a marker for episodic migraine? Neurology. 2015 Sept 9 [Epub ahead of print].
Sphingolipid metabolism is altered in women with episodic migraine, according to a study published online ahead of print September 9 in Neurology. According to the study authors, serum sphingolipid panels may have the potential to differentiate episodic migraine presence and absence. “While more research is needed to confirm these initial findings, the possibility of discovering a new biomarker for migraine is exciting,” said lead author B. Lee Peterlin, DO, Director of Johns Hopkins Headache Research and Associate Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore.
Case–Control Study
In this study, 88 female participants, 52 with episodic migraine and 36 without, were evaluated on the basis of demographic and health-related criteria, including marital status, BMI, and neuronal functioning. The patients also submitted blood samples, which were tested for lipid concentrations among other things.
The findings suggest that migraineurs had a decreased de novo synthesis of ceramides, which, paired with an independent downstream increase in the conversion of ceramide metabolic products, resulted in a major deficit. Women with migraines had an average concentration of ceramide levels of 6,000 ng/mL, a 43% decrease when compared with controls, who had an average of 10,500 ng/mL of ceramide in their blood.
There was also a marked difference in sphingolipid concentrations in those with migraines, with some species increasing while others were decreased. Dr. Peterlin and colleagues used the difference in blood lipid levels to create a list of 10 sphingolipids thought to classify episodic migraine. In a later test, this constructed biomarker evaluative list was 100% effective in identifying women with and without episodic migraine for a small group of eight migraineurs and six controls.
“This study is an important contribution to our understanding of the pathophysiology of migraine and may have vast practical, clinical, and therapeutic implications if it is supported by further studies,” said Karl Ekbom, PhD, a neurologist at the Karolinska Institutet in Stockholm, in an accompanying commentary.
The study suggests that the presence of migraines is a neurologic disorder of sphingolipid dsymetabolism. With the positive identification of these biomarkers comes the potential of targeted drug therapies directed against the specific sphingolipid pathways involved.
—Adaeze Stephanie Onyechi
Sphingolipid metabolism is altered in women with episodic migraine, according to a study published online ahead of print September 9 in Neurology. According to the study authors, serum sphingolipid panels may have the potential to differentiate episodic migraine presence and absence. “While more research is needed to confirm these initial findings, the possibility of discovering a new biomarker for migraine is exciting,” said lead author B. Lee Peterlin, DO, Director of Johns Hopkins Headache Research and Associate Professor of Neurology at Johns Hopkins University School of Medicine in Baltimore.
Case–Control Study
In this study, 88 female participants, 52 with episodic migraine and 36 without, were evaluated on the basis of demographic and health-related criteria, including marital status, BMI, and neuronal functioning. The patients also submitted blood samples, which were tested for lipid concentrations among other things.
The findings suggest that migraineurs had a decreased de novo synthesis of ceramides, which, paired with an independent downstream increase in the conversion of ceramide metabolic products, resulted in a major deficit. Women with migraines had an average concentration of ceramide levels of 6,000 ng/mL, a 43% decrease when compared with controls, who had an average of 10,500 ng/mL of ceramide in their blood.
There was also a marked difference in sphingolipid concentrations in those with migraines, with some species increasing while others were decreased. Dr. Peterlin and colleagues used the difference in blood lipid levels to create a list of 10 sphingolipids thought to classify episodic migraine. In a later test, this constructed biomarker evaluative list was 100% effective in identifying women with and without episodic migraine for a small group of eight migraineurs and six controls.
“This study is an important contribution to our understanding of the pathophysiology of migraine and may have vast practical, clinical, and therapeutic implications if it is supported by further studies,” said Karl Ekbom, PhD, a neurologist at the Karolinska Institutet in Stockholm, in an accompanying commentary.
The study suggests that the presence of migraines is a neurologic disorder of sphingolipid dsymetabolism. With the positive identification of these biomarkers comes the potential of targeted drug therapies directed against the specific sphingolipid pathways involved.
—Adaeze Stephanie Onyechi
Suggested Reading
Peterlin BL, Mielke MM, Dickens AM, et al. Interictal, circulating sphingolipids in women with episodic migraine. Neurology. 2015 Sept 9 [Epub ahead of print].
Ekbom K. Comment: Altered sphingolipid metabolism—a marker for episodic migraine? Neurology. 2015 Sept 9 [Epub ahead of print].
Suggested Reading
Peterlin BL, Mielke MM, Dickens AM, et al. Interictal, circulating sphingolipids in women with episodic migraine. Neurology. 2015 Sept 9 [Epub ahead of print].
Ekbom K. Comment: Altered sphingolipid metabolism—a marker for episodic migraine? Neurology. 2015 Sept 9 [Epub ahead of print].
Sacubitril-valsartan and the evolution of heart failure care
Three decades ago, the only drugs we had for treating chronic heart failure were digitalis and loop diuretics. The mortality rate was very high, and heart transplantation was a newly developing treatment that could help only a very few patients.
The early 80s heralded new hope for patients with heart failure, with the introduction of angiotensin-converting enzyme (ACE) inhibitors1–5 and, later, beta-blockers. Beta-blockers were considered contraindicated in heart failure until new trials provided evidence of dramatic benefit such as better quality of life and longer survival.6–8 ACE inhibitors, along with beta-blockers, quickly became the standard of care for all patients with systolic heart failure.
The implantable cardioverter-defibrillator (ICD) required numerous clinical trials in ischemic and nonischemic cardiomyopathy to define its role.9,10 Cardiac resynchronization therapy did not arrive until 15 years ago and is now indicated in a specific niche of patients with left bundle branch block.11,12 Mineralocorticoid antagonists required three pivotal clinical trials before their important role in the treatment of systolic heart failure was defined.13–16
And in the current decade, the roles of ACE inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, mineralocorticoid antagonists, ICDs, and cardiac resynchronization therapy have been further defined, as reflected in the latest guidelines for the treatment of systolic heart failure.17
Guideline-directed medical therapy for systolic heart failure with the agents and devices mentioned above improves quality of life and extends survival. It was therefore hard to imagine that any new additive therapy could offer significant incremental improvement. However, more than 5 years ago, in an ambitious effort, the largest global clinical trial ever performed in chronic heart failure was launched with a novel agent.18
THE PARADIGM-HF TRIAL
In this issue of the Journal, Sabe et al19 describe the results of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial of the novel combination drug sacubitril-valsartan, designated LCZ696 during its development and now available as Entresto.20
The mean age of the 8,442 patients in PARADIGM-HF was 64, and 78% were men. Despite guideline-directed medical therapy (93% of the patients were receiving a beta-blocker, and 60% were receiving a mineralocorticoid receptor antagonist), patients had persistent symptoms and signs of heart failure, diminished health-related quality of life, reduced ejection fraction (mean 29%), and elevated n-terminal pro-B-type natriuretic peptide levels (median 1,608 pg/mL, interquartile range 886–3,221).
The investigators reported a remarkable 20% reduction in the primary outcome of death from cardiovascular causes or hospitalization for heart failure in the patients who received sacubitril-valsartan compared with enalapril.20
Sacubitril-valsartan was reviewed under a US Food and Drug Administration (FDA) program that provides expedited review of drugs that are intended to treat a serious disease or condition and that may provide a significant improvement over available therapy. It was also granted a fast-track designation, which supports FDA efforts to facilitate the development and expedite the review of drugs to treat serious and life-threatening conditions and fill an unmet medical need. The FDA approved sacubitril-valsartan on July 7, 2015, for use in place of an ACE inhibitor or ARB in patients with New York Heart Association class II, III, or IV heart failure with reduced ejection fraction.21
WHAT WE STILL NEED TO KNOW
The results of PARADIGM-HF are generalizable, and sacubitril-valsartan was well tolerated in patients whose blood pressure was acceptable and who were able to tolerate ACE inhibitors in target doses. More than 90% of patients were receiving a beta-blocker. The dosing of enalapril (target 10 mg twice a day) is the guideline-directed target dose, and ACE inhibition is considered the gold standard for heart failure with reduced ejection fraction. Sacubitril-valsartan vs enalapril was a very appropriate comparison.
Far fewer PARADIGM-HF patients outside the United States had an ICD than those in the United States, which is a common finding in global clinical trials. However, Desai et al reported that sacubitril-valsartan reduced rates of cardiovascular mortality both from worsening heart failure and from sudden cardiac death, independent of whether the patient had an ICD.22
Sacubitril-valsartan is taken twice a day, but most heart failure patients already take medications at several times during the day, so this should not pose a problem.
More information is needed on the use of this new drug in patients with New York Heart Association class IV symptoms, as only 60 patients with class IV symptoms were included in the PARADIGM-HF trial. Also, the efficacy of the drug in patients unable to tolerate a full dose will need to be analyzed.
PARADIGM-HF was conducted in stable, nonhospitalized patients with chronic heart failure; the use of the drug in new-onset heart failure and its initiation in hospitalized patients will require further study. In addition, the PARAGON-HF trial23 will examine the efficacy of sacubitril-valsartan in patients with heart failure and an ejection fraction of 45% or higher.
Sacubitril-valsartan ushers in a new era in heart failure treatment for patients with reduced ejection fraction and will certainly prompt quick revision of heart failure guidelines.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992; 327:669–677.
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999; 341:1882–1890.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 2007; 297:2502–2514.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Pitt B, White H, Nicolau J, et al; EPHESUS Investigators. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005; 46:425–431.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Sabe IA, Jacob MS, Taylor DO. A new class of drugs for systolic heart failure: The PARADIGM-HF study. Cleve Clin J Med 2015; 82:693–701.
- McMurray JJ, Packer M, Desai AS, Gong J, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- US Food and Drug Administration. FDA approves new drug to treat heart failure. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm. Accessed September 2, 2015.
- Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 2015; 36:1990–1997.
- ClinicalTrials.gov. Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF). https://clinicaltrials.gov/ct2/show/NCT01920711. Accessed September 2, 2015.
Three decades ago, the only drugs we had for treating chronic heart failure were digitalis and loop diuretics. The mortality rate was very high, and heart transplantation was a newly developing treatment that could help only a very few patients.
The early 80s heralded new hope for patients with heart failure, with the introduction of angiotensin-converting enzyme (ACE) inhibitors1–5 and, later, beta-blockers. Beta-blockers were considered contraindicated in heart failure until new trials provided evidence of dramatic benefit such as better quality of life and longer survival.6–8 ACE inhibitors, along with beta-blockers, quickly became the standard of care for all patients with systolic heart failure.
The implantable cardioverter-defibrillator (ICD) required numerous clinical trials in ischemic and nonischemic cardiomyopathy to define its role.9,10 Cardiac resynchronization therapy did not arrive until 15 years ago and is now indicated in a specific niche of patients with left bundle branch block.11,12 Mineralocorticoid antagonists required three pivotal clinical trials before their important role in the treatment of systolic heart failure was defined.13–16
And in the current decade, the roles of ACE inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, mineralocorticoid antagonists, ICDs, and cardiac resynchronization therapy have been further defined, as reflected in the latest guidelines for the treatment of systolic heart failure.17
Guideline-directed medical therapy for systolic heart failure with the agents and devices mentioned above improves quality of life and extends survival. It was therefore hard to imagine that any new additive therapy could offer significant incremental improvement. However, more than 5 years ago, in an ambitious effort, the largest global clinical trial ever performed in chronic heart failure was launched with a novel agent.18
THE PARADIGM-HF TRIAL
In this issue of the Journal, Sabe et al19 describe the results of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial of the novel combination drug sacubitril-valsartan, designated LCZ696 during its development and now available as Entresto.20
The mean age of the 8,442 patients in PARADIGM-HF was 64, and 78% were men. Despite guideline-directed medical therapy (93% of the patients were receiving a beta-blocker, and 60% were receiving a mineralocorticoid receptor antagonist), patients had persistent symptoms and signs of heart failure, diminished health-related quality of life, reduced ejection fraction (mean 29%), and elevated n-terminal pro-B-type natriuretic peptide levels (median 1,608 pg/mL, interquartile range 886–3,221).
The investigators reported a remarkable 20% reduction in the primary outcome of death from cardiovascular causes or hospitalization for heart failure in the patients who received sacubitril-valsartan compared with enalapril.20
Sacubitril-valsartan was reviewed under a US Food and Drug Administration (FDA) program that provides expedited review of drugs that are intended to treat a serious disease or condition and that may provide a significant improvement over available therapy. It was also granted a fast-track designation, which supports FDA efforts to facilitate the development and expedite the review of drugs to treat serious and life-threatening conditions and fill an unmet medical need. The FDA approved sacubitril-valsartan on July 7, 2015, for use in place of an ACE inhibitor or ARB in patients with New York Heart Association class II, III, or IV heart failure with reduced ejection fraction.21
WHAT WE STILL NEED TO KNOW
The results of PARADIGM-HF are generalizable, and sacubitril-valsartan was well tolerated in patients whose blood pressure was acceptable and who were able to tolerate ACE inhibitors in target doses. More than 90% of patients were receiving a beta-blocker. The dosing of enalapril (target 10 mg twice a day) is the guideline-directed target dose, and ACE inhibition is considered the gold standard for heart failure with reduced ejection fraction. Sacubitril-valsartan vs enalapril was a very appropriate comparison.
Far fewer PARADIGM-HF patients outside the United States had an ICD than those in the United States, which is a common finding in global clinical trials. However, Desai et al reported that sacubitril-valsartan reduced rates of cardiovascular mortality both from worsening heart failure and from sudden cardiac death, independent of whether the patient had an ICD.22
Sacubitril-valsartan is taken twice a day, but most heart failure patients already take medications at several times during the day, so this should not pose a problem.
More information is needed on the use of this new drug in patients with New York Heart Association class IV symptoms, as only 60 patients with class IV symptoms were included in the PARADIGM-HF trial. Also, the efficacy of the drug in patients unable to tolerate a full dose will need to be analyzed.
PARADIGM-HF was conducted in stable, nonhospitalized patients with chronic heart failure; the use of the drug in new-onset heart failure and its initiation in hospitalized patients will require further study. In addition, the PARAGON-HF trial23 will examine the efficacy of sacubitril-valsartan in patients with heart failure and an ejection fraction of 45% or higher.
Sacubitril-valsartan ushers in a new era in heart failure treatment for patients with reduced ejection fraction and will certainly prompt quick revision of heart failure guidelines.
Three decades ago, the only drugs we had for treating chronic heart failure were digitalis and loop diuretics. The mortality rate was very high, and heart transplantation was a newly developing treatment that could help only a very few patients.
The early 80s heralded new hope for patients with heart failure, with the introduction of angiotensin-converting enzyme (ACE) inhibitors1–5 and, later, beta-blockers. Beta-blockers were considered contraindicated in heart failure until new trials provided evidence of dramatic benefit such as better quality of life and longer survival.6–8 ACE inhibitors, along with beta-blockers, quickly became the standard of care for all patients with systolic heart failure.
The implantable cardioverter-defibrillator (ICD) required numerous clinical trials in ischemic and nonischemic cardiomyopathy to define its role.9,10 Cardiac resynchronization therapy did not arrive until 15 years ago and is now indicated in a specific niche of patients with left bundle branch block.11,12 Mineralocorticoid antagonists required three pivotal clinical trials before their important role in the treatment of systolic heart failure was defined.13–16
And in the current decade, the roles of ACE inhibitors, angiotensin II receptor blockers (ARBs), beta-blockers, mineralocorticoid antagonists, ICDs, and cardiac resynchronization therapy have been further defined, as reflected in the latest guidelines for the treatment of systolic heart failure.17
Guideline-directed medical therapy for systolic heart failure with the agents and devices mentioned above improves quality of life and extends survival. It was therefore hard to imagine that any new additive therapy could offer significant incremental improvement. However, more than 5 years ago, in an ambitious effort, the largest global clinical trial ever performed in chronic heart failure was launched with a novel agent.18
THE PARADIGM-HF TRIAL
In this issue of the Journal, Sabe et al19 describe the results of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial of the novel combination drug sacubitril-valsartan, designated LCZ696 during its development and now available as Entresto.20
The mean age of the 8,442 patients in PARADIGM-HF was 64, and 78% were men. Despite guideline-directed medical therapy (93% of the patients were receiving a beta-blocker, and 60% were receiving a mineralocorticoid receptor antagonist), patients had persistent symptoms and signs of heart failure, diminished health-related quality of life, reduced ejection fraction (mean 29%), and elevated n-terminal pro-B-type natriuretic peptide levels (median 1,608 pg/mL, interquartile range 886–3,221).
The investigators reported a remarkable 20% reduction in the primary outcome of death from cardiovascular causes or hospitalization for heart failure in the patients who received sacubitril-valsartan compared with enalapril.20
Sacubitril-valsartan was reviewed under a US Food and Drug Administration (FDA) program that provides expedited review of drugs that are intended to treat a serious disease or condition and that may provide a significant improvement over available therapy. It was also granted a fast-track designation, which supports FDA efforts to facilitate the development and expedite the review of drugs to treat serious and life-threatening conditions and fill an unmet medical need. The FDA approved sacubitril-valsartan on July 7, 2015, for use in place of an ACE inhibitor or ARB in patients with New York Heart Association class II, III, or IV heart failure with reduced ejection fraction.21
WHAT WE STILL NEED TO KNOW
The results of PARADIGM-HF are generalizable, and sacubitril-valsartan was well tolerated in patients whose blood pressure was acceptable and who were able to tolerate ACE inhibitors in target doses. More than 90% of patients were receiving a beta-blocker. The dosing of enalapril (target 10 mg twice a day) is the guideline-directed target dose, and ACE inhibition is considered the gold standard for heart failure with reduced ejection fraction. Sacubitril-valsartan vs enalapril was a very appropriate comparison.
Far fewer PARADIGM-HF patients outside the United States had an ICD than those in the United States, which is a common finding in global clinical trials. However, Desai et al reported that sacubitril-valsartan reduced rates of cardiovascular mortality both from worsening heart failure and from sudden cardiac death, independent of whether the patient had an ICD.22
Sacubitril-valsartan is taken twice a day, but most heart failure patients already take medications at several times during the day, so this should not pose a problem.
More information is needed on the use of this new drug in patients with New York Heart Association class IV symptoms, as only 60 patients with class IV symptoms were included in the PARADIGM-HF trial. Also, the efficacy of the drug in patients unable to tolerate a full dose will need to be analyzed.
PARADIGM-HF was conducted in stable, nonhospitalized patients with chronic heart failure; the use of the drug in new-onset heart failure and its initiation in hospitalized patients will require further study. In addition, the PARAGON-HF trial23 will examine the efficacy of sacubitril-valsartan in patients with heart failure and an ejection fraction of 45% or higher.
Sacubitril-valsartan ushers in a new era in heart failure treatment for patients with reduced ejection fraction and will certainly prompt quick revision of heart failure guidelines.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992; 327:669–677.
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999; 341:1882–1890.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 2007; 297:2502–2514.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Pitt B, White H, Nicolau J, et al; EPHESUS Investigators. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005; 46:425–431.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Sabe IA, Jacob MS, Taylor DO. A new class of drugs for systolic heart failure: The PARADIGM-HF study. Cleve Clin J Med 2015; 82:693–701.
- McMurray JJ, Packer M, Desai AS, Gong J, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- US Food and Drug Administration. FDA approves new drug to treat heart failure. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm. Accessed September 2, 2015.
- Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 2015; 36:1990–1997.
- ClinicalTrials.gov. Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF). https://clinicaltrials.gov/ct2/show/NCT01920711. Accessed September 2, 2015.
- Captopril Multicenter Research Group. A placebo-controlled trial of captopril in refractory chronic congestive heart failure. J Am Coll Cardiol 1983; 2:755–763.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- The SOLVD Investigators. Effect of enalapril on survival in patients with reduced left ventricular ejection fraction and congestive heart failure. N Engl J Med 1991; 325:293–302.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Pfeffer MA, Braunwald E, Moyé LA, et al. Effect of captopril on mortality and morbidity in patients with left ventricular dysfunction after myocardial infarction. Results of the survival and ventricular enlargement trial. The SAVE Investigators. N Engl J Med 1992; 327:669–677.
- Packer M, Coats AJ, Fowler MB, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Brophy JM, Joseph L, Rouleau JL. Beta-blockers in congestive heart failure. A Bayesian meta-analysis. Ann Intern Med 2001; 134:550–560.
- Buxton AE, Lee KL, Fisher JD, et al. A randomized study of the prevention of sudden death in patients with coronary artery disease. Multicenter Unsustained Tachycardia Trial Investigators. N Engl J Med 1999; 341:1882–1890.
- Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Abraham WT, Fisher WG, Smith AL, et al; MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med 2002; 346:1845–1853.
- McAlister FA, Ezekowitz J, Hooton N, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA 2007; 297:2502–2514.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Pitt B, White H, Nicolau J, et al; EPHESUS Investigators. Eplerenone reduces mortality 30 days after randomization following acute myocardial infarction in patients with left ventricular systolic dysfunction and heart failure. J Am Coll Cardiol 2005; 46:425–431.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Yancy CW, Jessup M, Bozkurt B, et al; American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e239.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Sabe IA, Jacob MS, Taylor DO. A new class of drugs for systolic heart failure: The PARADIGM-HF study. Cleve Clin J Med 2015; 82:693–701.
- McMurray JJ, Packer M, Desai AS, Gong J, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- US Food and Drug Administration. FDA approves new drug to treat heart failure. www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm. Accessed September 2, 2015.
- Desai AS, McMurray JJ, Packer M, et al. Effect of the angiotensin-receptor-neprilysin inhibitor LCZ696 compared with enalapril on mode of death in heart failure patients. Eur Heart J 2015; 36:1990–1997.
- ClinicalTrials.gov. Efficacy and Safety of LCZ696 Compared to Valsartan, on Morbidity and Mortality in Heart Failure Patients With Preserved Ejection Fraction (PARAGON-HF). https://clinicaltrials.gov/ct2/show/NCT01920711. Accessed September 2, 2015.
A new class of drugs for systolic heart failure: The PARADIGM-HF study
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
In a large phase trial, a combination drug that contains the angiotensin II receptor blocker (ARB) valsartan and the neprilysin inhibitor sacubitril was found to be superior to the angiotensin-converting enzyme (ACE) inhibitor enalapril in terms of important end points, including death and hospitalization for heart failure, in patients with heart failure with reduced ejection fraction.1
Recently approved by the US Food and Drug Administration, this combination drug, marketed under the brand name Entresto, represents a new drug class, angiotensin receptor-neprilysin inhibitors, or ARNIs.
This article is an overview of the Prospective Comparison of ARNI With ACEI to Determine Impact on Global Mortality and Morbidity in Heart Failure (PARADIGM-HF) trial1 and the implications it may have on the care of patients with chronic heart failure.
NEED FOR NEW HEART FAILURE DRUGS
Heart failure is a major public health problem, and the care of patients with heart failure is challenging.
Almost 6 million US adults have heart failure, and the prevalence is projected to increase in the next few decades as the population continues to age.2 Furthermore, the total healthcare cost for heart failure patients was almost $31 billion in 2012 and is projected to rise to $70 billion by 2030.2
The care of patients with severely decompensated heart failure has changed dramatically in the last few decades with advances in heart transplantation and mechanical support devices. But day-to-day management of patients with chronic mildly to moderately symptomatic heart failure continues to pose a clinical challenge.
The drugs currently available for these patients include beta-blockers, ACE inhibitors, ARBs, aldosterone antagonists, digoxin, diuretics, and vasodilators. But even with these drugs, the death and readmission rates of patients with heart failure with reduced ejection fraction remain high. More than 50% of patients with heart failure die within 5 years of diagnosis,3 and 25% of patients hospitalized with heart failure are readmitted within 30 days of discharge.2 Furthermore, death rates are higher in those patients who have a history of heart failure hospitalization.4
Although heart failure with preserved ejection fraction encompasses an important group of heart failure patients with high morbidity, the focus of this article will be on patients with heart failure with reduced ejection fraction.
Available drugs to date
The cornerstone drugs that lower the odds of death in patients with heart failure with reduced ejection fraction are ACE inhibitors, ARBs, beta-blockers, and mineralocorticoid antagonists.
ACE inhibitors were the first class of drugs shown to reduce the death rate in patients with heart failure with reduced ejection fraction. The landmark CONSENSUS trial,5 published in 1987, found that the death rate in patients who received enalapril was 27% lower than in those receiving placebo, an effect driven entirely by a reduction in progressive heart failure. Similarly, the SOLVD trial,6 published in 1991, showed a 26% reduction in heart failure hospitalization and a 16% lower rate of death with enalapril compared with placebo, an effect driven predominantly by a decrease in the progression of heart failure.
ARBs have also been shown to decrease the rate of death, although not by as much as ACE inhibitors. In the CHARM trial,7 compared with placebo, candesartan significantly decreased the risk of death from any cause, of death from cardiovascular causes, and of hospitalization related to heart failure.7
Beta-blockers. The MERIT-HF trial,8 published in 1999, was stopped early because fewer patients were dying in the group receiving metoprolol succinate than in the group receiving placebo (relative risk 0.66). Similarly, in 2001, the COPERNICUS trial9 reported a 34% reduction in deaths in patients receiving carvedilol in addition to an ACE inhibitor compared with those receiving an ACE inhibitor alone.
Mineralocorticoid receptor antagonists were found to be beneficial when added to standard therapy for chronic symptomatic heart failure in the RALES10 and EMPHASIS-HF11 trials.
Vasodilators (specifically, the combination of isosorbide dinitrate and hydralazine) were found to have benefit in terms of mortality when added to standard therapy in African American patients in the A-HeEFT trial.12
WHY INHIBIT BOTH ANGIOTENSIN AND NEPRILYSIN?
The renin-angiotensin-aldosterone system is a major focus in treating heart failure, as overactivity of this system plays a key role in the pathophysiology of this disease. Therefore, essential drugs for heart failure patients include those that inhibit overactivity of this system such as ACE inhibitors, ARBs, and aldosterone antagonists.
The natriuretic peptide system is another important pathway that can be targeted in patients with heart failure. Natriuretic peptides are key molecules that counteract heart failure, as they contribute to diuresis and vasodilation and protect against vascular remodeling.13 An increased understanding of the importance of this system in slowing the progression of heart failure has motivated evaluation of drugs such as nesiritide in patients with symptomatic heart failure. However, these drugs can cause hypotension and have limited bioavailability.14
Neprilysin is an endopeptidase—an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides.14 Drugs that inhibit neprilysin increase the levels of these peptides and thus counteract neurohormonal stimuli that lead to cardiac remodeling, sodium retention, and vasoconstriction.15
However, neprilysin also hydrolyzes angiotensin I to angiotensin (1–7), an inhibitor of angiotensin II. Thus, inhibition of neprilysin alone could lead to increased activity of angiotensin II and so have an overall neutral effect. To be beneficial, neprilysin inhibition needs to be combined with renin-angiotensin system inhibition. Furthermore, the benefit of renin-angiotensin-aldosterone system blockade may be amplified by up-regulation of the endogenous natriuretic peptide system.15
Omapatrilat, the most studied combination neprilysin inhibitor and ACE inhibitor, improved cardiac function and decreased cardiac mass in animal experiments.15 In addition, this drug showed promise in terms of blood pressure, heart failure readmissions, death, and preservation of renal function when compared with ACE inhibitors in patients with heart failure.15–17 But in clinical trials this drug posed a greater risk of hypotension, dizziness, and, its major shortcoming, an unacceptably high incidence of angioedema compared with ACE inhibitors.15,16,18 This higher risk of angioedema is thought to be from inhibition of three enzymes that break down bradykinin: ACE, neprilysin, and aminopeptidase P.19
ARNIs contain an angiotensin receptor blocker rather than an ACE inhibitor, and thus in theory they may be associated with a lower risk of angioedema.19 Sacubitril-valsartan, the first drug of this class, contains its two constitutive drugs in a one-to-one molecular ratio (Figure 1).
PARADIGM-HF investigated the benefit of this drug in patients with systolic heart failure.1
STUDY DESIGN AND OBJECTIVES
PARADIGM-HF was a double-blind, randomized controlled trial comparing sacubitril-valsartan and enalapril in patients with chronic systolic heart failure. As such, it was the first trial in recent years to study a new drug in comparison with a well-established heart failure drug rather than as an add-on strategy.1
Inclusion criteria
To be included in the PARADIGM-HF trial, patients had to have:
- A left ventricular ejection fraction of 40% or less (later changed to ≤ 35%)
- New York Heart Association class II, III, or IV symptoms
- A B-type natriuretic peptide (BNP) level of at least 150 pg/mL or an N-terminal proBNP (NT-proBNP) level of at least 600 pg/mL; for patients hospitalized for heart failure within the previous 12 months, the cut points were lower (BNP ≥ 100 pg/mL or NT-proBNP ≥ 400 pg/mL).
End points
The primary end point was the composite of cardiovascular death or first hospitalization for heart failure. Other outcomes assessed were time to death from any cause, the change from baseline in the Kansas City Cardiomyopathy Questionnaire (KCCQ) score at 8 months, time to new-onset atrial fibrillation, and the time to decline in renal function (defined as end-stage renal disease or a decrease in estimated glomerular filtration rate of at least 50% from randomization). All end points were blindly adjudicated by a clinical end points committee.
Two run-in periods
The study enrolled 10,521 patients from 1,043 centers in 47 countries, who entered the initial run-in period consisting of 2 weeks of treatment with enalapril at the study dosage (10 mg twice daily) in order to ensure no unacceptable side effects. At this point, 1,102 patients exited the study, leaving 9,419 who entered the second run-in period.
The second run-in period consisted of 4 weeks of treatment with sacubitril-valsartan, initially at half the study regimen (100 mg twice daily) and eventually at the full study dosage (200 mg twice daily). During the second run-in period, 977 participants left the study, leaving a total of 8,442 patients who underwent randomization. Forty-three patients were then excluded (6 because of invalid randomization and 37 because of four sites that closed because of major violations of good clinical practice).
Of those randomized, 4,187 patients were assigned to the sacubitril-valsartan treatment group and 4,212 were assigned to the enalapril group. The investigators used an intention-to-treat analysis for this study.
Most patients had NYHA class II symptoms
The randomized patients had a mean age of 64 years, 75% were men, 66% were white, and 58% were from Europe (only 7% were from North America). The mean left ventricular ejection fraction was about 30%, and 60% of the study participants had an ischemic cause for their cardiomyopathy. Although one of the inclusion criteria was New York Heart Association class II, III, or IV symptoms, about 5% of the patients had class I symptoms. Seventy percent had class II symptoms, 24% had class III, and less than 1% had class IV symptoms.
At the time of randomization, 78% of the patients were taking an ACE inhibitor and 93% were taking a beta-blocker, but only a little more than half were taking a mineralocorticoid antagonist and only about 15% had an implantable cardioverter-defibrillator.
STUDY OUTCOMES
This study was designed to detect a 15% lower risk of cardiovascular death in the sacubitril-valsartan group. It was projected to continue for at least 34 months but was stopped early because of an overwhelming benefit of the new drug at a median follow-up of 27 months.
Major findings
The primary composite outcome (cardiovascular death or first hospitalization for heart failure)1 occurred in 21.8% of the patients in the sacubitril-valsartan group vs 26.5% of patients in the enalapril group (hazard ratio [HR] 0.80, 95% confidence interval [CI] 0.73–0.87, P < .001). The number of patients who needed to be treated to prevent one occurrence of the primary composite outcome (100/absolute risk reduction) was only 21. The benefit was strong and consistent across both of the individual components of the composite outcome:
- Cardiovascular death 13.3% vs 16.5%, HR 0.80 (95% CI 0.71–0.89), P < .001
- First hospitalization for worsening heart failure 12.8% vs 15.6%, HR 0.79 (95% CI 0.71–0.89), P < .001.
Secondary outcomes. The sacubitril-valsartan group had a significantly lower rate of death from any cause (17.0% vs 19.8%, HR 0.84, 95% CI 0.76–0.93, P < .001) and a lower mean decrease in KCCQ clinical summary scores at 8 months (2.99 points vs 4.63 points, mean difference 1.64, 95% CI 0.63–2.65, P = .001). The KCCQ score measures subjective symptoms and physical limitations caused by heart failure; possible scores range from 0 to 100, with a higher score indicating better functional status. Notably, sacubitril-valsartan did not increase the KCCQ score in these patients; rather, sacubitril-valsartan recipients had a lower decrease in their scores than those in the enalapril group.
The incidence of new-onset atrial fibrillation was the same in both groups (3.1% and 3.1%).1 A decline in renal function (defined as end-stage renal disease, a decrease of 50% or more in estimated glomerular filtration rate from the value at randomization or a decrease in the estimated glomerular filtration rate of more than 30 mL/min/1.73 m2 to less than 60 mL/min/1.73 m2) occurred in 2.2% of the valsartan-sacubitril group and 2.6% of the enalapril group (P = .28).
The effects of the study drug on the primary composite outcome and on death from a cardiovascular cause were similar in all prespecified subgroups except for NYHA class: the reduction in the risk of the composite outcome was lower in sacubitril-valsartan recipients with NYHA I or II symptoms than in those with NYHA III or IV symptoms (P for interaction .03). However, there were no differences in the other prespecified subgroups, defined by age, sex, race, region, estimated glomerular filtration rate, diabetes, systolic blood pressure, ejection fraction, atrial fibrillation, NT-proBNP, hypertension, previous use of an ACE inhibitor, previous use of an aldosterone antagonist, previous heart failure hospitalization, and time since diagnosis of heart failure.
SAFETY: ANGIOEDEMA, HYPOTENSION, AND RENAL DYSFUNCTION
Angioedema
As noted above, the combination of neprilysin inhibitors and ACE inhibitors has been associated with an increased risk of angioedema. That was an important consideration before starting this study, which used a combination of a neprilysin inhibitor and an ARB in an attempt to avoid this serious side effect.
As it happened, there was no increased risk of significant angioedema with sacubitril-valsartan use compared with enalapril. Rates were similar to those in other studies, which showed a less than 1% risk of angioedema caused by ACE inhibitors.20,21 Only 19 patients (0.5%) in the sacubitril-valsartan group and 10 patients (0.2%) in the enalapril group experienced any angioedema. Of these, just three patients in the sacubitril-valsartan group and one patient in the enalapril group experienced angioedema that required hospitalization (P = .31). None of these patients had airway compromise due to angioedema.
Hypotension, cough, renal dysfunction, hyperkalemia
Other safety issues that were assessed included hypotension, worsening renal function, increase in potassium levels, and cough. Patients in the sacubitril-valsartan group were more likely to have symptomatic hypotension than patients in the enalapril group (14.0% vs 9.2%, P < .001); however, the authors noted that this was a rare cause of drug discontinuation.
Patients in the sacubitril-valsartan group were less likely to develop cough (11.3% vs 14.3%, P < .001), a serum creatinine level of 2.5 mg/dL or more (3.3% vs 4.5%, P = .007), or a serum potassium level of more than 6.0 mmol/L (11.3% vs 14.3%, P = .007).1
During the two run-in periods combined, 12% of the patients in the study withdrew because of adverse events, including cough, renal dysfunction, hyperkalemia, and symptomatic hypotension. During the enalapril run-in period, 591 patients (5.6%) withdrew due to adverse events, and 547 patients (5.8%) withdrew due to these events during the sacubitril-valsartan run-in period. After adjusting for the shorter time on enalapril, there was a higher rate of withdrawal because of adverse events from enalapril than from sacubitril-valsartan.
LOWER RISK OF CLINICAL PROGRESSION
In a separate paper,22 the PARADIGM-HF investigators reported that, among the survivors in the study, those who received sacubitril-valsartan fared better in terms of a number of markers of progression of heart failure, with lower rates of:
- Intensification of medical treatment for heart failure
- Emergency department visits for worsening heart failure
- Hospitalization for worsening heart failure
- Need for intensive care
- Need for intravenous inotropic agents
- Need for cardiac devices or heart transplants
- Worsening symptom scores
- Elevation of biomarkers of myocardial injury.
QUESTIONS AND CONCERNS
Although this study, which was funded by the manufacturer, showed consistent benefit for sacubitril-valsartan over enalapril, questions remain.
Are the findings generalizable?
Despite the study’s rigorous run-in period, 12% of patients dropped out because of adverse events, and thus the patients who completed the study may not be representative of the general population of heart failure patients. The authors included this double-level wash-out to ensure patient tolerance of both drugs. But in everyday practice, a significant number of patients may be unable to tolerate one of these drugs.
Moreover, after adjusting for the difference in the run-in periods, patients actually withdrew more often during the enalapril run-in period than during the sacubitril-valsartan run-in period. However, there may be overlap in tolerability in these two drugs, which both affect the renin-angiotensin-aldosterone system. Thus, the enalapril run-in period may have contributed to the lower tolerability of this drug compared with sacubitril-valsartan.
Were patients receiving the best possible therapy?
Another important point when considering how we treat heart failure patients in the United States is how few patients in this study were using cardiac implantable electronic devices. Only 15% of the patients in this study had an implantable cardioverter-defibrillator despite a mean left ventricular ejection fraction less than 30%. This likely reflects differences in practice internationally; however, based on American College of Cardiology, American Heart Association, and Heart Rhythm Society guidelines, these patients would have a class I indication for an implantable cardioverter-defibrillator for primary prevention of sudden cardiac death.23
Therefore, based on these recommendations, the patients in this study were not necessarily on optimal medical and device therapy and furthermore may not be representative of heart failure patients in the United States.
Was enalapril 10 mg twice a day a fair comparison?
Another concern about the results of this study relates to the dosages used in the two treatment groups. The sacubitril-valsartan formulation included full-dose valsartan, whereas the ACE inhibitor group received enalapril at less than a full dose.
Although the authors explained that the dose of enalapril chosen for the study was based on the one used in previous studies that showed survival benefit, this raises the question of whether the significant difference in outcomes in this trial was due to a greater inhibition of the renin-angiotensin-aldosterone system related to a higher dose of drug in the sacubitril-valsartan group.
What about black patients taking hydralazine-isosorbide?
Only about 5% of patients in the PARADIGM-HF trial were black. Based on the A-HeFT study results, black patients can be prescribed an ACE inhibitor as well as hydralazine and isosorbide dinitrate as tolerated to decrease the risk of death. Does sacubitril-valsartan offer benefit to these patients compared with a regimen of an ACE inhibitor, hydralazine, and isosorbide dinitrate?
Another concern is that the incidence of angioedema observed with ACE inhibitors and omapatrilat is higher in black patients.15,21 Thus, it would be prudent to investigate whether the risk of angioedema with sacubitril-valsartan would be higher if more black patients are studied.
IMPLICATIONS AND CONSIDERATIONS
In this study, sacubitril-valsartan showed impressive and consistent results, with an almost 20% decrease in the composite end point of heart failure hospitalization or cardiovascular death and a similar decrease in the composite outcomes with a very low number needed to treat (21 patients). It did not show a decrease in the incidence of new-onset atrial fibrillation; however, only 80 cases of atrial fibrillation were reported, so there may have been a lack of statistical power to detect a difference.
To avoid angioedema, wait at least 36 hours after stopping an ACE inhibitor. Sacubitril-valsartan was not associated with an increased risk of severe angioedema, and no patients experienced life-threatening angioedema. In the trial, the sacubitril-valsartan run-in period was started at least 24 hours after enalapril was stopped, and thus the authors recommended at least a 1-day washout period after discontinuing an ACE inhibitor to avoid angioedema in patients starting sacubitril-valsartan.
Hypotension is a concern. Although there was actually a decreased risk of renal dysfunction, hyperkalemia, and cough compared with enalapril, there was a significantly increased rate of symptomatic hypotension in the sacubitril-valsartan group, which raises the question of patient tolerance and physician comfort when prescribing and titrating this drug in clinical practice. This side effect will be an important consideration when attempting to titrate the drug to target doses.
Start treatment early. This trial largely consisted of patients with NYHA class II or III symptoms, with about 70% of patients with class II symptoms. Since this drug showed benefit in patients with mildly to moderately symptomatic heart failure, clinicians who are considering prescribing this drug should not wait until the patient is closer to end-stage disease. Patients with mildly symptomatic heart failure may be followed by a general cardiologist, internist, or both, and thus it is important to emphasize to the entire medical community the need to start this medication early on.
How much will it cost? Cost is a concern that could heavily weigh on the decision to prescribe this drug. Generic ACE inhibitors are relatively inexpensive, and it may difficult to switch from an affordable generic drug to a new drug that is likely to be much more expensive. Arguably, this drug may be cost-effective in the long run owing to a large decrease in heart failure readmissions. We await further analyses to evaluate this issue.
Will patients take a twice-a-day drug as prescribed? Most patients who are prescribed an ACE inhibitor take it just once a day, and switching from a daily to a twice-daily drug may present a challenge for some.
What about other outcomes? Based on this study, it is unclear what effect sacubitril-valsartan has on the incidence of fatal arrhythmias, sudden cardiac death, and pump failure. Furthermore, the effect on quality of life is still uncertain. Quality of life is an integral component in the evaluation of heart failure patients, and in this study the changes in KCCQ scores were not impressive. We hope to see further evaluations of this drug’s impact on quality of life of patients with heart failure. Furthermore, it would be interesting to study if this drug has any long-term effects on the need for advanced therapies such as left ventricular assist devices and orthotopic heart transplant.
What about patients with heart failure with preserved ejection fraction? This study included only patients with heart failure with reduced ejection fraction. However PARAMOUNT, a phase 2 study that evaluated the benefit of sacubitril-valsartan in patients with heart failure with preserved ejection fraction, has shown encouraging results.24 We look forward to further investigation of this agent in patients with heart failure with preserved ejection fraction.
Sacubitril-valsartan, the first ARNI to be studied in humans, has a dual action in that it enhances the activity of the natriuretic peptide system and inhibits that of the renin-angiotensin-aldosterone system. It is the first drug in over a decade to show mortality benefit in patients with chronic systolic heart failure when compared with an already well-established heart failure medication. It appears to decrease rates of mortality and heart failure hospitalization without increasing the risk of severe angioedema in patients with mild or moderate chronic systolic heart failure. Symptomatic hypotension and high cost may pose the largest barriers to the use of this new drug. And we have yet to see how the clinical community and patients with heart failure will respond to it.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Investigators and Committees. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med 2014; 371:993–1004.
- Dunlay SM, Pereira NL, Kushwaha SS. Contemporary strategies in the diagnosis and management of heart failure. Mayo Clin Proc 2014; 89:662–676.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bello NA, Claggett B, Desai AS, et al. Influence of previous heart failure hospitalization on cardiovascular events in patients with reduced and preserved ejection fraction. Circ Heart Fail 2014; 7:590–595.
- Effects of enalapril on mortality in severe congestive heart failure. Results of the Cooperative North Scandinavian Enalapril Survival Study (CONSENSUS). The CONSENSUS Trial Study Group. N Engl J Med 1987; 316:1429–1435.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure. The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Pfeffer MA, Swedberg K, Granger CB, et al; CHARM Investigators and Committees. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: the CHARM-Overall programme. Lancet 2003; 362:759–766.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Schreiner GF, Protter AA. B-type natriuretic peptide for the treatment of congestive heart failure. Curr Opin Pharmacol 2002; 2:142–147.
- von Lueder TG, Sangaralingham SJ, Wang BH, et al. Renin-angiotensin blockade combined with natriuretic peptide system augmentation: novel therapeutic concepts to combat heart failure. Circ Heart Fail 2013; 6:594–605.
- Abassi Z, Karram T, Ellaham S, Winaver J, Hoffman A. Implications of the natriuretic peptide system in the pathogenesis of heart failure: diagnostic and therapeutic importance. Pharmacol Ther 2004; 102:223–241.
- Packer M, Califf RM, Konstam MA, et al. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE). Circulation 2002; 106:920–926.
- Rouleau JL, Pfeffer MA, Stewart DJ, et al. Comparison of vasopeptidase inhibitor, omapatrilat, and lisinopril on exercise tolerance and morbidity in patients with heart failure: IMPRESS randomised trial. Lancet 2000; 356:615–620.
- Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens 2004; 17:103–111.
- McMurray JJ, Packer M, Desai AS, et al; PARADIGM-HF Committees and Investigators. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF). Eur J Heart Fail 2013; 15:1062–1073.
- Toh S, Reichman ME, Houstoun M, et al. Comparative risk for angioedema associated with the use of drugs that target the renin-angiotensin-aldosterone system. Arch Intern Med 2012; 172:1582–1589.
- Kostis JB, Kim HJ, Rusnak J, et al. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med 2005; 165:1637–1642.
- Packer M, McMurray JJV, Desai AS, et al; on behalf of the PARADIGM-HF Investigators and Coordinators. Angiotensin receptor neprilysin inhibition compared with enalapril on the risk of clinical progression in surviving patients with heart failure. Circulation 2015; 131:54–61.
- Epstein AE, Dimarco JP, Ellenbogen KA, et al; American College of Cardiology/American Heart Association Task Force on Practice; American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: executive summary. Heart Rhythm 2008; 5:934–955.
- Solomon SD, Zile M, Pieske B, et al; Prospective comparison of ARNI with ARB on Management Of Heart Failure with Preserved Ejection Fraction (PARAMOUNT) Investigators. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet 2012; 380:1387–1395.
KEY POINTS
- Neprilysin is an endogenous enzyme that degrades vasoactive peptides such as bradykinin and natriuretic peptides. Inhibition of neprilysin raises the levels of these peptides, leading to less cardiac remodeling, less sodium retention, and less vasoconstriction.
- Neprilysin inhibition must be combined with inhibition of the renin-angiotensin-aldosterone system, optimally with an angiotensin II receptor blocker.
- PARADIGM-HF showed a 20% reduction in the primary outcome of death from cardiovascular causes or hospitalization for heart failure with sacubitril-valsartan 200 mg twice daily vs enalapril 10 mg twice daily at a median follow-up of 27 months.
- The ultimate role of combined neprilysin and angiotensin receptor inhibitors remains to be determined.
Genitourinary manifestations of sickle cell disease
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
Sickle cell disease is a common genetic disorder in the United States that disproportionately affects people of African ancestry. The characteristic sickling of red blood cells under conditions of reduced oxygen tension leads to intravascular hemolysis and vaso-occlusive events, which in turn cause tissue ischemia-reperfusion injury affecting multiple organs, including the genitourinary system.1–3
In this paper, we review the genitourinary effects of sickle cell disease, focusing on sickle cell nephropathy, priapism, and renal medullary carcinoma.
THE WIDE-RANGING EFFECTS OF SICKLE CELL DISEASE
In the United States, sickle cell disease affects 1 of every 500 blacks and 1 of every 36,000 Hispanics.1 The term describes hemoglobinopathies associated with sickling of red blood cells.
Sickling of red blood cells results from a single base-pair change in the beta-globin gene from glutamic acid to valine at position 6, causing abnormal hemoglobin (hemoglobin S), which polymerizes under conditions of reduced oxygen tension and alters the biconcave disk shape into a rigid, irregular, unstable cell. The sickle-shaped cells are prone to intravascular hemolysis,2 causing intermittent vaso-occlusive events that result in tissue ischemia-reperfusion injury. Genitourinary problems include impaired ability to concentrate urine, hematuria, renal medullary carcinoma, and increased frequency of urinary tract infection.
SICKLE CELL NEPHROPATHY
The kidney is one of the most frequently affected organs in sickle cell disease. Renal manifestations begin to appear in early childhood, with impaired medullary concentrating ability and ischemic damage to the tubular cells caused by sickling within the vasa recta renis precipitated by the acidic, hypoxic, and hypertonic environment in the renal medulla.
As in early diabetic nephropathy, renal blood flow is enhanced and the glomerular filtration rate (GFR) is increased. Increased cardiac output as a result of anemia, localized release of prostaglandins, and a hypoxia-induced increase in nitric oxide synthesis all play a role in the increase in GFR.4,5
Oxidative stress, an increase in markers of inflammation, and local activation of the renin-angiotensin system contribute to renal damage in sickle cell disease.5–7 The resulting hyperfiltration injury leads to microalbuminuria, which occurs in 20% to 40% of children with sickle cell anemia8,9 and in as many as 60% of adults.
The glomerular lesions associated with sickle cell disease vary from glomerulopathy in the early stages to secondary focal segmental glomerulosclerosis, membranoproliferative glomerulonephritis, and glomerular thrombotic microangiopathy.10
Clinical presentations and workup
Clinical presentations are not limited to glomerular disease but include hyperchloremic metabolic acidosis and hyperkalemia resulting from defects in potassium secretion and renal acidification.
Hyperphosphatemia—a result of increased reabsorption of phosphorus, increased secretion of uric acid, and increased creatinine clearance—is seen in patients with sickle cell disease.11,12 About 10% of patients can develop an acute kidney injury as a result of volume depletion, rhabdomyolysis, renal vein thrombosis, papillary necrosis, and urinary tract obstruction secondary to blood clots.11,13
Up to 30% of adult patients with sickle cell disease develop chronic kidney disease. Predictors include severe anemia, hypertension, proteinuria, nephrotic syndrome, and microscopic hematuria.14 From 4% to 12% of patients go on to develop end-stage renal disease, but with a 1-year mortality rate three times higher than in patients without sickle cell disease.15
In general, patients with sickle cell anemia have blood pressures below those of age- and sex-matched individuals, but elevated blood pressure and low GFR are not uncommon in affected children. In a cohort of 48 children ages 3 to 18, 8.3% had an estimated GFR less than 90 mL/min/1.73 m2, and 16.7% had elevated blood pressure (prehypertension and hypertension).16
In patients with sickle cell disease, evaluation of proteinuria, hematuria, hypertension, and renal failure should take into consideration the unique renal physiologic and pathologic processes involved. Recent evidence17,18 suggests that the Chronic Kidney Disease Epidemiology Collaboration equation provides a better estimate of GFR than the Modification of Diet in Renal Disease and Cockcroft-Gault equations, although all three creatinine-based methods overestimate GFR in patients with sickle cell disease when compared with GFR measured with technetium-99m-labeled diethylenetriamine penta-acetic acid renal scanning.
Treatment options
Treatment of sickle cell nephropathy includes adequate fluid intake (given the loss of concentrating ability), adequate blood pressure control, use of angiotensin-converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARBs) in patients who have microalbuminuria or proteinuria (or both)9,11,19 and hydroxyurea. Treatment with enalapril has been shown to decrease proteinuria in patients with sickle cell nephropathy.9 In a cohort of children with sickle cell disease, four of nine patients treated with an ACE inhibitor developed hyperkalemia, leading to discontinuation of the drug in three patients.9
ACE inhibitors and ARBs must be used cautiously in these patients because they have defects in potassium secretion. Hydroxyurea has also been shown to decrease hyperfiltration and microalbuminuria in recent studies,20,21 and this could protect against the development of overt nephropathy.
Higher mortality rates have been reported in patients with sickle cell disease who developed end-stage renal disease than in patients with end-stage renal disease without sickle cell disease. Sickle cell disease also increases the risk of pulmonary hypertension and the vaso-occlusive complication known as acute chest syndrome, contributing to increased mortality rates. Of note, in a study that looked at the association between mortality rates and pre-end-stage care of renal disease using data from the Centers for Medicare and Medicaid Services, patients with sickle cell disease who had had predialysis nephrology care had lower mortality rates.15
Treatments for end-stage renal disease are also effective in patients with sickle cell disease and include hemodialysis, peritoneal dialysis, and renal transplantation. Data from the Organ Procurement and Transplantation Network and the United Network for Organ Sharing show that from 2000 to 2011, African American kidney recipients with sickle cell disease had better survival rates than patients who had undergone transplantation from 1988 to 1999, although rates of long-term survival and graft survival were lower than in transplant recipients with other diagnoses.22
It is important to note that complications as a result of vaso-occlusive events and thrombosis can lead to graft loss; therefore, sickle cell crisis after transplantation requires careful management.
Take-home messages
- Loss of urine-concentrating ability and hyperfiltration are the earliest pathologic changes in sickle cell disease.
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- ACE inhibitors or ARBs should be used with caution, given the heightened risk of hyperkalemia in sickle cell disease.
- Recent results with hydroxyurea in decreasing hyperfiltration and microalbuminuria are encouraging.
- Early referral for predialysis nephrologic care is needed in sickle cell patients with chronic kidney disease.
PRIAPISM IN SICKLE CELL DISEASE
Priapism was formerly defined as a full, painful erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm. But priapism is now recognized as two separate disorders—ischemic (veno-occlusive, low-flow) priapism and nonischemic (arterial, high-flow) priapism. The new definition includes both disorders: ie, a full or partial erection lasting more than 4 hours and unrelated to sexual stimulation or orgasm.
Ischemic priapism
Hematologic disorders are major contributors to ischemic priapism and include sickle cell disease, multiple myeloma, fat emboli (hyperalimentation),23 glucose-6-phosphate dehydrogenase deficiency, and hemoglobin Olmsted variant.24
Ischemic priapism is often seen in sickle cell disease and is considered an emergency. It is characterized by an abnormally rigid erection not involving the glans penis. Entrapment of blood in the corpora cavernosa leads to hypoxia, hypercarbia, and acidosis, which in turn leads to a painful compartment syndrome that, if untreated, results in smooth muscle necrosis and subsequent fibrosis. The results are a smaller penis and erectile dysfunction that is unresponsive to any treatment other than implantation of a penile prosthesis. However, scarring of the corpora cavernosa can make this procedure exceedingly difficult, requiring advanced techniques such as corporeal excavation.25
Men with a subtype of ischemic priapism called “stuttering” priapism26 suffer recurrent prolonged erections during sleep. The patient awakens with a painful erection that usually subsides, but sometimes only after several hours. Patients with this disorder suffer from sleep deprivation. Stuttering priapism may lead to full-blown ischemic priapism that does not resolve without intervention.
Nonischemic priapism
In nonischemic priapism, the corpora are engorged but not rigid. The condition results from unregulated arterial inflow and thus is not painful and does not result in damage to the corporeal smooth muscle.
Most cases of nonischemic priapism follow blunt perineal trauma or trauma associated with needle insertion into the corpora. This form of priapism is not associated with sickle cell disease. Because tissue damage does not occur, nonischemic or arterial priapism is not considered an emergency.
Treatment guidelines
Differentiating ischemic from nonischemic priapism is usually straightforward, based on the history, physical examination, corporeal blood gases, and duplex ultrasonography.27
Ischemic priapism is an emergency. After needle aspiration of blood from the corpora cavernosa, phenylephrine is diluted with normal saline to a concentration of 100 to 500 µg/mL and is injected in 1-mL amounts repeatedly at 3- to 5-minute intervals until the erection subsides or until a 1-hour time limit is reached. Blood pressure and pulse are monitored during these injections. If aspiration and phenylephrine irrigation fail, surgical shunting is performed.27
Measures to treat sickle cell disease (hydration, oxygen, exchange transfusions) may be employed simultaneously but should never delay aspiration and phenylephrine injections.25
As nonischemic priapism is not considered an emergency, management begins with observation. Patients eventually become dissatisfied with their constant partial erection, and they then present for treatment. Most cases resolve after selective catheterization of the internal pudendal artery and embolization of the fistula with absorbable material. If this fails, surgical exploration with ligation of the vessels leading to the fistula is indicated.
Prevalence in sickle cell trait vs sickle cell disease
Ischemic priapism is uncommon in men with sickle cell trait, but prevalence rates in men with sickle cell disease are as high as 42%.28 In a study of 130 men with sickle cell disease, 35% had a history of prolonged ischemic priapism, 72% had a history of stuttering priapism, and 75% of men with stuttering priapism had their first episode before age 20.29
Rates of erectile dysfunction increase with the duration of ischemic episodes and range from 20% to 90%.28,30 In childhood, sickle cell disease accounts for 63% of the cases of ischemic priapism, and in adults it accounts for 23% of cases.31
Take-home messages
- Sickle cell disease accounts for two-thirds of cases of ischemic priapism in children, and one-fourth of adult cases.
- Ischemic priapism is a medical emergency.
- Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease (hydration, oxygen, exchange transfusions).
OTHER UROLOGIC COMPLICATIONS OF SICKLE CELL DISEASE
Other urologic complications of sickle cell trait and sickle cell disease include microscopic hematuria, gross hematuria, and renal colic. A formal evaluation of any patient with persistent microscopic hematuria or gross hematuria should consist of urinalysis, computed tomography, and cystoscopy. This approach assesses the upper and lower genitourinary system for treatable causes. Renal ultrasonography can be used instead of computed tomography but tends to provide less information.
Special considerations
In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis. In papillary hypertrophy, friable blood vessels can rupture, resulting in microscopic and gross hematuria. In papillary necrosis, the papilla can slough off and become lodged in the ureter.
Nevertheless, hematuria and renal colic in patients with sickle cell disease or trait are most often attributable to common causes such as infection and stones. A finding of hydronephrosis in the absence of a stone, however, suggests obstruction due to a clot or a sloughed papilla. Ureteroscopy, fulguration, and ureteral stent placement can stop the bleeding and alleviate obstruction in these cases.
Renal medullary carcinoma
Another important reason to order imaging in patients with sickle cell disease or trait who present with urologic symptoms is to rule out renal medullary carcinoma, a rare but aggressive cancer that arises from the collecting duct epithelium. This cancer is twice as likely to occur in males than in females; it has been reported in patients ranging in age from 10 to 40, with a median age at presentation of 26.32
When patients present with symptomatic renal medullary cancer, in most cases the cancer has already metastasized.
On computed tomography, the tumor tends to occupy a central location in the kidney and appears to infiltrate and replace adjacent kidney tissue. Retroperitoneal lymphadenopathy and metastasis are common.
Treatment typically entails radical nephrectomy, chemotherapy, and in some circumstances, radiotherapy. Case reports have shown promising tumor responses to carboplatin and paclitaxel regimens.33,34 Also, a low threshold for imaging in patients with sickle cell disease and trait may increase the odds of early detection of this aggressive cancer.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
- Centers for Disease Control and Prevention (CDC). Sickle cell disease (SCD). Data and statistics. www.cdc.gov/ncbddd/sicklecell/data.html. Accessed August 18, 2015.
- Paulin L, Itano HA, Singer SJ, Wells IC. Sickle cell anemia, a molecular disease. Science 1949; 110:543–548.
- Powars DR, Chan LS, Hiti A, Ramicone E, Johnson C. Outcome of sickle cell anemia: a 4-decade observational study of 1056 patients. Medicine (Baltimore) 2005; 84:363–376.
- Haymann JP, Stankovic K, Levy P, et al. Glomerular hyperfiltration in adult sickle cell anemia: a frequent hemolysis associated feature. Clin J Am Soc Nephrol 2010; 5:756–761.
- da Silva GB Jr, Libório AB, Daher Ede F. New insights on pathophysiology, clinical manifestations, diagnosis, and treatment of sickle cell nephropathy. Ann Hematol 2011; 90:1371–1379.
- Emokpae MA, Uadia PO, Gadzama AA. Correlation of oxidative stress and inflammatory markers with the severity of sickle cell nephropathy. Ann Afr Med 2010; 9:141–146.
- Chirico EN, Pialoux V. Role of oxidative stress in the pathogenesis of sickle cell disease. IUBMB Life 2012; 64:72–80.
- Datta V, Ayengar JR, Karpate S, Chaturvedi P. Microalbuminuria as a predictor of early glomerular injury in children with sickle cell disease. Indian J Pediatr 2003; 70:307–309.
- Falk RJ, Scheinman J, Phillips G, Orringer E, Johnson A, Jennette JC. Prevalence and pathologic features of sickle cell nephropathy and response to inhibition of angiotensin-converting enzyme. N Engl J Med 1992; 326:910–915.
- Maigne G, Ferlicot S, Galacteros F, et al. Glomerular lesions in patients with sickle cell disease. Medicine (Baltimore) 2010; 89:18–27.
- Sharpe CC, Thein SL. Sickle cell nephropathy—a practical approach. Br J Haematol 2011; 155:287–297.
- Batlle D, Itsarayoungyuen K, Arruda JA, Kurtzman NA. Hyperkalemic hyperchloremic metabolic acidosis in sickle cell hemoglobinopathies. Am J Med 1982; 72:188–192.
- Sklar AH, Perez JC, Harp RJ, Caruana RJ. Acute renal failure in sickle cell anemia. Int J Artif Organs 1990; 13:347–351.
- Powars DR, Elliott-Mills DD, Chan L, et al. Chronic renal failure in sickle cell disease: risk factors, clinical course, and mortality. Ann Intern Med 1991; 115:614–620.
- McClellan AC, Luthi JC, Lynch JR, et al. High one year mortality in adults with sickle cell disease and end-stage renal disease. Br J Haematol 2012; 159:360–367.
- Bodas P, Huang A, O Riordan MA, Sedor JR, Dell KM. The prevalence of hypertension and abnormal kidney function in children with sickle cell disease—a cross sectional review. BMC Nephrol 2013; 14:237.
- Asnani MR, Lynch O, Reid ME. Determining glomerular filtration rate in homozygous sickle cell disease: utility of serum creatinine based estimating equations. PLoS One 2013; 8:e69922.
- Arlet JB, Ribeil JA, Chatellier G, et al. Determination of the best method to estimate glomerular filtration rate from serum creatinine in adult patients with sickle cell disease: a prospective observational cohort study. BMC Nephrol 2012; 13:83.
- McKie KT, Hanevold CD, Hernandez C, Waller JL, Ortiz L, McKie KM. Prevalence, prevention, and treatment of microalbuminuria and proteinuria in children with sickle cell disease. J Pediatr Hematol Oncol 2007; 29:140–144.
- Laurin LP, Nachman PH, Desai PC, Ataga KI, Derebail VK. Hydroxyurea is associated with lower prevalence of albuminuria in adults with sickle cell disease. Nephrol Dial Transplant 2014; 29:1211–1218.
- Aygun B, Mortier NA, Smeltzer MP, Shulkin BL, Hankins JS, Ware RE. Hydroxyurea treatment decreases glomerular hyperfiltration in children with sickle cell anemia. Am J Hematol 2013; 88:116–119.
- Huang E, Parke C, Mehrnia A, et al. Improved survival among sickle cell kidney transplant recipients in the recent era. Nephrol Dial Transplant 2013; 28:1039–1046.
- Klein EA, Montague DK, Steiger E. Priapism associated with the use of intravenous fat emulsion: case reports and postulated pathogenesis. J Urol May 1985; 133:857–859.
- Thuret I, Bardakdjian J, Badens C, et al. Priapism following splenectomy in an unstable hemoglobin: hemoglobin Olmsted beta 141 (H19) Leu-->Arg. Am J Hematol 1996; 51:133–136.
- Montague DK, Angermeier KW. Corporeal excavation: new technique for penile prosthesis implantation in men with severe corporeal fibrosis. Urology 2006; 67:1072–1075.
- Levey HR, Kutlu O, Bivalacqua TJ. Medical management of ischemic stuttering priapism: a contemporary review of the literature. Asian J Androl 2012; 14:156–163.
- Montague DK, Jarow J, Broderick GA, et al; Members of the Erectile Dysfunction Guideline Update Panel; American Urological Association. American Urological Association guideline on the management of priapism. J Urol 2003; 170:1318–1324.
- Emond AM, Holman R, Hayes RJ, Serjeant GR. Priapism and impotence in homozygous sickle cell disease. Arch Intern Med 1980; 140:1434–1437.
- Adeyoju AB, Olujohungbe AB, Morris J, et al. Priapism in sickle-cell disease; incidence, risk factors and complications—an international multicentre study. BJU Int 2002; 90:898–902.
- Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med 2004; 1:116–120.
- Nelson JH, 3rd, Winter CC. Priapism: evolution of management in 48 patients in a 22-year series. J Urol 1977; 117:455–458.
- Liu Q, Galli S, Srinivasan R, Linehan WM, Tsokos M, Merino MJ. Renal medullary carcinoma: molecular, immunohistochemistry, and morphologic correlation. Am J Surg Pathol 2013; 37:368–374.
- Gangireddy VG, Liles GB, Sostre GD, Coleman T. Response of metastatic renal medullary carcinoma to carboplatinum and Paclitaxel chemotherapy. Clin Genitourin Cancer 2012; 10:134–139.
- Walsh AM, Fiveash JB, Reddy AT, Friedman GK. Response to radiation in renal medullary carcinoma. Rare Tumors 2011; 3:e32.
KEY POINTS
- Microalbuminuria as seen in diabetic nephropathy is the earliest manifestation of sickle cell nephropathy, and the prevalence increases as these patients get older and live longer.
- Ischemic priapism is a medical emergency. Treatment with aspiration and phenylephrine injections should begin immediately and should not await treatment measures for sickle cell disease.
- In patients with sickle cell trait and sickle cell disease, chronic hypoxia and subsequent sickling of erythrocytes in the renal medulla can lead to papillary hypertrophy and papillary necrosis.
Do healthy patients need routine laboratory testing before elective noncardiac surgery?
A 63-year-old physician is referred for preoperative evaluation before arthroscopic repair of a torn medial meniscus. Her exercise tolerance was excellent before the knee injury, including running without cardiopulmonary symptoms. She is otherwise healthy except for hypertension that is well controlled on amlodipine. She has no known history of liver or kidney disease, bleeding disorder, recent illness, or complications with anesthesia. She inquires as to whether “routine blood testing” is needed before the procedure.
What laboratory studies, if any, should be ordered?
UNLIKELY TO BE OF BENEFIT
Preoperative laboratory testing is not necessary in this otherwise healthy, asymptomatic patient. In the absence of clinical indications, routine testing before elective, low-risk procedures often increases both the cost of care and the potential anxiety caused by abnormal results that provide no substantial benefit to the patient or the clinician.
Preoperative diagnostic tests should be ordered only to identify and optimize disorders that alter the likelihood of perioperative and postoperative adverse outcomes and to establish a baseline assessment. Yet clinicians often perceive that laboratory testing is required by their organization or by other providers.
A comprehensive history and physical examination are the cornerstones of the effective preoperative evaluation. Preferably, the history and examination should guide further testing rather than ordering a battery of standard tests for all patients. However, selective preoperative laboratory testing may be useful in certain situations, such as in patients undergoing high-risk procedures and those with known underlying conditions or factors that may affect operative management (Table 1).
Unfortunately, high-quality evidence for this selective approach is lacking. According to one observational study,1 when laboratory testing is appropriate, it is reasonable to use test results already obtained and normal within the preceding 4 months unless the patient has had an interim change in health status.
Definitions of risk stratification (eg, urgency of surgical procedure, graded risk according to type of operation) and tools such as the Revised Cardiac Risk Index can be found in the 2014 American College of Cardiology/American Heart Association guidelines2 and may be useful to distinguish healthy patients from those with significant comorbidities, as well as to distinguish low-risk, elective procedures from those that impart higher risk.
Professional societies and guidelines in many countries have criticized the habitual practice of extensive, nonselective laboratory testing.3–6 Yet despite lack of evidence of benefit, routine preoperative testing is still often done. At an estimated cost of more than $18 billion in the United States annually,7 preoperative testing deserves attention, especially in this time of ballooning healthcare costs and increased focus on effective and efficient care.
EVIDENCE AND GUIDELINES
Numerous studies have established that routine laboratory testing rarely changes the preoperative management of the patient or improves surgical outcomes. Narr et al8 found that 160 (4%) of 3,782 patients who underwent ambulatory surgery had abnormal test results, and only 10 required treatment. In this study, there was no association between abnormal test results and perioperative management or postoperative adverse events.
In a systematic review, Smetana and Macpherson9 noted that the incidence of laboratory test abnormalities that led to a change in management ranged from 0.1% to 2.6%. Notably, clinicians ignore 30% to 60% of abnormal preoperative laboratory results, a practice that may create additional medicolegal risk.7
Little evidence exists that helps in the development of guidelines for preoperative laboratory testing. Most guidelines are based on expert opinion, case series, and consensus. As an example of the heterogeneity this creates, the American Society of Anesthesiologists, the Ontario Preoperative Testing Group, and the Canadian Anesthesiologists’ Society provide different recommended indications for preoperative laboratory testing in patients with “advanced age” but do not define a clear minimum age for this cohort.10
However, one area that does have substantial data is cataract surgery. Patients in their usual state of health who are to undergo this procedure do not require preoperative testing, a claim supported by high-quality evidence including a 2012 Cochrane systematic review.11
Munro et al5 performed a systematic review of the evidence behind preoperative laboratory testing, concluding that the power of preoperative tests to predict adverse postoperative outcomes in asymptomatic patients is either weak or nonexistent. The National Institute for Health and Clinical Excellence guidelines of 2003,6 the Practice Advisory for Preanesthesia Evaluation of the American Society of Anesthesiologists of 2012,12 the Institute for Clinical Systems Improvement guideline of 2012,13 and a systematic review conducted by Johansson et al14 found no evidence from high-quality studies to support the claim that routine preoperative testing is beneficial in healthy adults undergoing noncardiac surgery, but that certain patient populations may benefit from selective testing.
A randomized controlled trial evaluated the elimination of preoperative testing in patients undergoing low-risk ambulatory surgery and found no difference in perioperative adverse events in the control and intervention arms.15 Similar studies achieved the same results.
The Choosing Wisely campaign
The American Board of Internal Medicine Foundation has partnered with medical specialty societies to create lists of common practice patterns that should be questioned and possibly discontinued. These lists are collectively called the Choosing Wisely campaign (www.choosingwisely.org). Avoiding routine preoperative laboratory testing in patients undergoing low-risk surgery without clinical indications can be found in the lists for the American Society of Anesthesiologists, the American Society for Clinical Pathology, and the Society of General Internal Medicine.
THE POSSIBLE HARMS OF TESTING
The prevalence of unrecognized disease that influences the risk of surgery in healthy patients is low, and thus the predictive value of abnormal test values in these patients is low. This leads to substantial false-positivity, which is of uncertain clinical significance and which may in turn cause a cascade of further testing. Not surprisingly, the probability of an abnormal test result increases dramatically with the number of tests ordered, a fact that magnifies the problem of false-positive results.
The costs and harms associated with testing are both direct and indirect. Direct effects include increased healthcare costs of further testing or potentially unnecessary treatment as well as risk associated with additional testing, though these are not common, as there is a low (< 3%) incidence of a change in preoperative management based on an abnormal test result. Likewise, normal results do not appear to substantially reduce the likelihood of postoperative complications.9
Indirect effects, which are particularly challenging to measure, may include time lost from employment to pursue further evaluation and anxiety surrounding abnormal results.
THE CLINICAL BOTTOM LINE
Based on over 2 decades of data, our 63-year-old patient should not undergo “routine” preoperative laboratory testing before her upcoming elective, low-risk, noncardiac procedure. Her hypertension is well controlled, and she is taking no medications that may lead to clinically significant metabolic derangements or significant changes in surgical outcome. There are no convincing clinical indications for further laboratory investigation. Further, the results are unlikely to affect the preoperative management and rate of adverse events; the direct and indirect costs may be substantial; and there is a small but tangible risk of harm.
Given the myriad factors that influence unnecessary preoperative testing, a focus on systems-level solutions is paramount. Key steps may include creation and adoption of clear and consistent guidelines, development of clinical care pathways, physician education and modification of practice, interdisciplinary communication and information sharing, economic analysis, and outcomes assessment.
- Macpherson DS, Snow R, Lofgren RP. Preoperative screening: value of previous tests. Ann Intern Med 1990; 113:969–973.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2014; 130:e278–e333.
- Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of medical testing for cataract surgery. N Engl J Med 2000; 342:168–175.
- The Swedish Council on Technology Assessment in Health Care (SBU). Preoperative routines. Stockholm, 1989.
- Munro J, Booth A, Nicholl J. Routine preoperative testing: a systematic review of the evidence. Health Technol Assess 1997; 1:1–62.
- National Institute for Health and Clinical Excellence (NICE). Preoperative tests: The use of routine preoperative tests for elective surgery. London: National Collaborating Centre for Acute Care, 2003.
- Roizen MF. More preoperative assessment by physicians and less by laboratory tests. N Engl J Med 2000; 342:204–205.
- Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc 1991; 66:155–159.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87:7–40.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg 2012; 256:518–528.
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev 2012; 3:CD007293.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Institute for Clinical Systems Improvement (ICSI). Health care guideline: preoperative evaluation. 10th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2012.
- Johansson T, Fritsch G, Flamm M, et al. Effectiveness of non-cardiac preoperative testing in non-cardiac elective surgery: a systematic review. Br J Anaesth 2013; 110:926–939.
- Chung F, Yuan H, Yin L, Vairavanathan S, Wong DT. Elimination of preoperative testing in ambulatory surgery. Anesth Analg 2009; 108:467–475.
A 63-year-old physician is referred for preoperative evaluation before arthroscopic repair of a torn medial meniscus. Her exercise tolerance was excellent before the knee injury, including running without cardiopulmonary symptoms. She is otherwise healthy except for hypertension that is well controlled on amlodipine. She has no known history of liver or kidney disease, bleeding disorder, recent illness, or complications with anesthesia. She inquires as to whether “routine blood testing” is needed before the procedure.
What laboratory studies, if any, should be ordered?
UNLIKELY TO BE OF BENEFIT
Preoperative laboratory testing is not necessary in this otherwise healthy, asymptomatic patient. In the absence of clinical indications, routine testing before elective, low-risk procedures often increases both the cost of care and the potential anxiety caused by abnormal results that provide no substantial benefit to the patient or the clinician.
Preoperative diagnostic tests should be ordered only to identify and optimize disorders that alter the likelihood of perioperative and postoperative adverse outcomes and to establish a baseline assessment. Yet clinicians often perceive that laboratory testing is required by their organization or by other providers.
A comprehensive history and physical examination are the cornerstones of the effective preoperative evaluation. Preferably, the history and examination should guide further testing rather than ordering a battery of standard tests for all patients. However, selective preoperative laboratory testing may be useful in certain situations, such as in patients undergoing high-risk procedures and those with known underlying conditions or factors that may affect operative management (Table 1).
Unfortunately, high-quality evidence for this selective approach is lacking. According to one observational study,1 when laboratory testing is appropriate, it is reasonable to use test results already obtained and normal within the preceding 4 months unless the patient has had an interim change in health status.
Definitions of risk stratification (eg, urgency of surgical procedure, graded risk according to type of operation) and tools such as the Revised Cardiac Risk Index can be found in the 2014 American College of Cardiology/American Heart Association guidelines2 and may be useful to distinguish healthy patients from those with significant comorbidities, as well as to distinguish low-risk, elective procedures from those that impart higher risk.
Professional societies and guidelines in many countries have criticized the habitual practice of extensive, nonselective laboratory testing.3–6 Yet despite lack of evidence of benefit, routine preoperative testing is still often done. At an estimated cost of more than $18 billion in the United States annually,7 preoperative testing deserves attention, especially in this time of ballooning healthcare costs and increased focus on effective and efficient care.
EVIDENCE AND GUIDELINES
Numerous studies have established that routine laboratory testing rarely changes the preoperative management of the patient or improves surgical outcomes. Narr et al8 found that 160 (4%) of 3,782 patients who underwent ambulatory surgery had abnormal test results, and only 10 required treatment. In this study, there was no association between abnormal test results and perioperative management or postoperative adverse events.
In a systematic review, Smetana and Macpherson9 noted that the incidence of laboratory test abnormalities that led to a change in management ranged from 0.1% to 2.6%. Notably, clinicians ignore 30% to 60% of abnormal preoperative laboratory results, a practice that may create additional medicolegal risk.7
Little evidence exists that helps in the development of guidelines for preoperative laboratory testing. Most guidelines are based on expert opinion, case series, and consensus. As an example of the heterogeneity this creates, the American Society of Anesthesiologists, the Ontario Preoperative Testing Group, and the Canadian Anesthesiologists’ Society provide different recommended indications for preoperative laboratory testing in patients with “advanced age” but do not define a clear minimum age for this cohort.10
However, one area that does have substantial data is cataract surgery. Patients in their usual state of health who are to undergo this procedure do not require preoperative testing, a claim supported by high-quality evidence including a 2012 Cochrane systematic review.11
Munro et al5 performed a systematic review of the evidence behind preoperative laboratory testing, concluding that the power of preoperative tests to predict adverse postoperative outcomes in asymptomatic patients is either weak or nonexistent. The National Institute for Health and Clinical Excellence guidelines of 2003,6 the Practice Advisory for Preanesthesia Evaluation of the American Society of Anesthesiologists of 2012,12 the Institute for Clinical Systems Improvement guideline of 2012,13 and a systematic review conducted by Johansson et al14 found no evidence from high-quality studies to support the claim that routine preoperative testing is beneficial in healthy adults undergoing noncardiac surgery, but that certain patient populations may benefit from selective testing.
A randomized controlled trial evaluated the elimination of preoperative testing in patients undergoing low-risk ambulatory surgery and found no difference in perioperative adverse events in the control and intervention arms.15 Similar studies achieved the same results.
The Choosing Wisely campaign
The American Board of Internal Medicine Foundation has partnered with medical specialty societies to create lists of common practice patterns that should be questioned and possibly discontinued. These lists are collectively called the Choosing Wisely campaign (www.choosingwisely.org). Avoiding routine preoperative laboratory testing in patients undergoing low-risk surgery without clinical indications can be found in the lists for the American Society of Anesthesiologists, the American Society for Clinical Pathology, and the Society of General Internal Medicine.
THE POSSIBLE HARMS OF TESTING
The prevalence of unrecognized disease that influences the risk of surgery in healthy patients is low, and thus the predictive value of abnormal test values in these patients is low. This leads to substantial false-positivity, which is of uncertain clinical significance and which may in turn cause a cascade of further testing. Not surprisingly, the probability of an abnormal test result increases dramatically with the number of tests ordered, a fact that magnifies the problem of false-positive results.
The costs and harms associated with testing are both direct and indirect. Direct effects include increased healthcare costs of further testing or potentially unnecessary treatment as well as risk associated with additional testing, though these are not common, as there is a low (< 3%) incidence of a change in preoperative management based on an abnormal test result. Likewise, normal results do not appear to substantially reduce the likelihood of postoperative complications.9
Indirect effects, which are particularly challenging to measure, may include time lost from employment to pursue further evaluation and anxiety surrounding abnormal results.
THE CLINICAL BOTTOM LINE
Based on over 2 decades of data, our 63-year-old patient should not undergo “routine” preoperative laboratory testing before her upcoming elective, low-risk, noncardiac procedure. Her hypertension is well controlled, and she is taking no medications that may lead to clinically significant metabolic derangements or significant changes in surgical outcome. There are no convincing clinical indications for further laboratory investigation. Further, the results are unlikely to affect the preoperative management and rate of adverse events; the direct and indirect costs may be substantial; and there is a small but tangible risk of harm.
Given the myriad factors that influence unnecessary preoperative testing, a focus on systems-level solutions is paramount. Key steps may include creation and adoption of clear and consistent guidelines, development of clinical care pathways, physician education and modification of practice, interdisciplinary communication and information sharing, economic analysis, and outcomes assessment.
A 63-year-old physician is referred for preoperative evaluation before arthroscopic repair of a torn medial meniscus. Her exercise tolerance was excellent before the knee injury, including running without cardiopulmonary symptoms. She is otherwise healthy except for hypertension that is well controlled on amlodipine. She has no known history of liver or kidney disease, bleeding disorder, recent illness, or complications with anesthesia. She inquires as to whether “routine blood testing” is needed before the procedure.
What laboratory studies, if any, should be ordered?
UNLIKELY TO BE OF BENEFIT
Preoperative laboratory testing is not necessary in this otherwise healthy, asymptomatic patient. In the absence of clinical indications, routine testing before elective, low-risk procedures often increases both the cost of care and the potential anxiety caused by abnormal results that provide no substantial benefit to the patient or the clinician.
Preoperative diagnostic tests should be ordered only to identify and optimize disorders that alter the likelihood of perioperative and postoperative adverse outcomes and to establish a baseline assessment. Yet clinicians often perceive that laboratory testing is required by their organization or by other providers.
A comprehensive history and physical examination are the cornerstones of the effective preoperative evaluation. Preferably, the history and examination should guide further testing rather than ordering a battery of standard tests for all patients. However, selective preoperative laboratory testing may be useful in certain situations, such as in patients undergoing high-risk procedures and those with known underlying conditions or factors that may affect operative management (Table 1).
Unfortunately, high-quality evidence for this selective approach is lacking. According to one observational study,1 when laboratory testing is appropriate, it is reasonable to use test results already obtained and normal within the preceding 4 months unless the patient has had an interim change in health status.
Definitions of risk stratification (eg, urgency of surgical procedure, graded risk according to type of operation) and tools such as the Revised Cardiac Risk Index can be found in the 2014 American College of Cardiology/American Heart Association guidelines2 and may be useful to distinguish healthy patients from those with significant comorbidities, as well as to distinguish low-risk, elective procedures from those that impart higher risk.
Professional societies and guidelines in many countries have criticized the habitual practice of extensive, nonselective laboratory testing.3–6 Yet despite lack of evidence of benefit, routine preoperative testing is still often done. At an estimated cost of more than $18 billion in the United States annually,7 preoperative testing deserves attention, especially in this time of ballooning healthcare costs and increased focus on effective and efficient care.
EVIDENCE AND GUIDELINES
Numerous studies have established that routine laboratory testing rarely changes the preoperative management of the patient or improves surgical outcomes. Narr et al8 found that 160 (4%) of 3,782 patients who underwent ambulatory surgery had abnormal test results, and only 10 required treatment. In this study, there was no association between abnormal test results and perioperative management or postoperative adverse events.
In a systematic review, Smetana and Macpherson9 noted that the incidence of laboratory test abnormalities that led to a change in management ranged from 0.1% to 2.6%. Notably, clinicians ignore 30% to 60% of abnormal preoperative laboratory results, a practice that may create additional medicolegal risk.7
Little evidence exists that helps in the development of guidelines for preoperative laboratory testing. Most guidelines are based on expert opinion, case series, and consensus. As an example of the heterogeneity this creates, the American Society of Anesthesiologists, the Ontario Preoperative Testing Group, and the Canadian Anesthesiologists’ Society provide different recommended indications for preoperative laboratory testing in patients with “advanced age” but do not define a clear minimum age for this cohort.10
However, one area that does have substantial data is cataract surgery. Patients in their usual state of health who are to undergo this procedure do not require preoperative testing, a claim supported by high-quality evidence including a 2012 Cochrane systematic review.11
Munro et al5 performed a systematic review of the evidence behind preoperative laboratory testing, concluding that the power of preoperative tests to predict adverse postoperative outcomes in asymptomatic patients is either weak or nonexistent. The National Institute for Health and Clinical Excellence guidelines of 2003,6 the Practice Advisory for Preanesthesia Evaluation of the American Society of Anesthesiologists of 2012,12 the Institute for Clinical Systems Improvement guideline of 2012,13 and a systematic review conducted by Johansson et al14 found no evidence from high-quality studies to support the claim that routine preoperative testing is beneficial in healthy adults undergoing noncardiac surgery, but that certain patient populations may benefit from selective testing.
A randomized controlled trial evaluated the elimination of preoperative testing in patients undergoing low-risk ambulatory surgery and found no difference in perioperative adverse events in the control and intervention arms.15 Similar studies achieved the same results.
The Choosing Wisely campaign
The American Board of Internal Medicine Foundation has partnered with medical specialty societies to create lists of common practice patterns that should be questioned and possibly discontinued. These lists are collectively called the Choosing Wisely campaign (www.choosingwisely.org). Avoiding routine preoperative laboratory testing in patients undergoing low-risk surgery without clinical indications can be found in the lists for the American Society of Anesthesiologists, the American Society for Clinical Pathology, and the Society of General Internal Medicine.
THE POSSIBLE HARMS OF TESTING
The prevalence of unrecognized disease that influences the risk of surgery in healthy patients is low, and thus the predictive value of abnormal test values in these patients is low. This leads to substantial false-positivity, which is of uncertain clinical significance and which may in turn cause a cascade of further testing. Not surprisingly, the probability of an abnormal test result increases dramatically with the number of tests ordered, a fact that magnifies the problem of false-positive results.
The costs and harms associated with testing are both direct and indirect. Direct effects include increased healthcare costs of further testing or potentially unnecessary treatment as well as risk associated with additional testing, though these are not common, as there is a low (< 3%) incidence of a change in preoperative management based on an abnormal test result. Likewise, normal results do not appear to substantially reduce the likelihood of postoperative complications.9
Indirect effects, which are particularly challenging to measure, may include time lost from employment to pursue further evaluation and anxiety surrounding abnormal results.
THE CLINICAL BOTTOM LINE
Based on over 2 decades of data, our 63-year-old patient should not undergo “routine” preoperative laboratory testing before her upcoming elective, low-risk, noncardiac procedure. Her hypertension is well controlled, and she is taking no medications that may lead to clinically significant metabolic derangements or significant changes in surgical outcome. There are no convincing clinical indications for further laboratory investigation. Further, the results are unlikely to affect the preoperative management and rate of adverse events; the direct and indirect costs may be substantial; and there is a small but tangible risk of harm.
Given the myriad factors that influence unnecessary preoperative testing, a focus on systems-level solutions is paramount. Key steps may include creation and adoption of clear and consistent guidelines, development of clinical care pathways, physician education and modification of practice, interdisciplinary communication and information sharing, economic analysis, and outcomes assessment.
- Macpherson DS, Snow R, Lofgren RP. Preoperative screening: value of previous tests. Ann Intern Med 1990; 113:969–973.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2014; 130:e278–e333.
- Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of medical testing for cataract surgery. N Engl J Med 2000; 342:168–175.
- The Swedish Council on Technology Assessment in Health Care (SBU). Preoperative routines. Stockholm, 1989.
- Munro J, Booth A, Nicholl J. Routine preoperative testing: a systematic review of the evidence. Health Technol Assess 1997; 1:1–62.
- National Institute for Health and Clinical Excellence (NICE). Preoperative tests: The use of routine preoperative tests for elective surgery. London: National Collaborating Centre for Acute Care, 2003.
- Roizen MF. More preoperative assessment by physicians and less by laboratory tests. N Engl J Med 2000; 342:204–205.
- Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc 1991; 66:155–159.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87:7–40.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg 2012; 256:518–528.
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev 2012; 3:CD007293.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Institute for Clinical Systems Improvement (ICSI). Health care guideline: preoperative evaluation. 10th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2012.
- Johansson T, Fritsch G, Flamm M, et al. Effectiveness of non-cardiac preoperative testing in non-cardiac elective surgery: a systematic review. Br J Anaesth 2013; 110:926–939.
- Chung F, Yuan H, Yin L, Vairavanathan S, Wong DT. Elimination of preoperative testing in ambulatory surgery. Anesth Analg 2009; 108:467–475.
- Macpherson DS, Snow R, Lofgren RP. Preoperative screening: value of previous tests. Ann Intern Med 1990; 113:969–973.
- Fleisher LA, Fleischmann KE, Auerbach AD, et al. 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation 2014; 130:e278–e333.
- Schein OD, Katz J, Bass EB, et al. The value of routine preoperative medical testing before cataract surgery. Study of medical testing for cataract surgery. N Engl J Med 2000; 342:168–175.
- The Swedish Council on Technology Assessment in Health Care (SBU). Preoperative routines. Stockholm, 1989.
- Munro J, Booth A, Nicholl J. Routine preoperative testing: a systematic review of the evidence. Health Technol Assess 1997; 1:1–62.
- National Institute for Health and Clinical Excellence (NICE). Preoperative tests: The use of routine preoperative tests for elective surgery. London: National Collaborating Centre for Acute Care, 2003.
- Roizen MF. More preoperative assessment by physicians and less by laboratory tests. N Engl J Med 2000; 342:204–205.
- Narr BJ, Hansen TR, Warner MA. Preoperative laboratory screening in healthy Mayo patients: cost-effective elimination of tests and unchanged outcomes. Mayo Clin Proc 1991; 66:155–159.
- Smetana GW, Macpherson DS. The case against routine preoperative laboratory testing. Med Clin North Am 2003; 87:7–40.
- Benarroch-Gampel J, Sheffield KM, Duncan CB, et al. Preoperative laboratory testing in patients undergoing elective, low-risk ambulatory surgery. Ann Surg 2012; 256:518–528.
- Keay L, Lindsley K, Tielsch J, Katz J, Schein O. Routine preoperative medical testing for cataract surgery. Cochrane Database Syst Rev 2012; 3:CD007293.
- Committee on Standards and Practice Parameters; Apfelbaum JL, Connis RT, Nickinovich DG, et al. Practice advisory for preanesthesia evaluation: an updated report by the American Society of Anesthesiologists Task Force on Preanesthesia Evaluation. Anesthesiology 2012; 116:522–538.
- Institute for Clinical Systems Improvement (ICSI). Health care guideline: preoperative evaluation. 10th ed. Bloomington, MN: Institute for Clinical Systems Improvement; 2012.
- Johansson T, Fritsch G, Flamm M, et al. Effectiveness of non-cardiac preoperative testing in non-cardiac elective surgery: a systematic review. Br J Anaesth 2013; 110:926–939.
- Chung F, Yuan H, Yin L, Vairavanathan S, Wong DT. Elimination of preoperative testing in ambulatory surgery. Anesth Analg 2009; 108:467–475.