User login
TeamHealth Announces $1.6 Billion Acquisition of IPC Healthcare
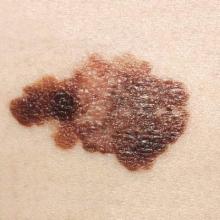
Eye Bleeds: Risk From Warfarin Transition?
NEW YORK (Reuters Health) - Patients transitioning from warfarin to rivaroxaban for atrial fibrillation, who are taking both drugs before dropping warfarin, may be at risk for spontaneous vitreous hemorrhage, according to a new case report.
Judy H. Jun and Dr. John C. Hwang, of Retina Associates of Orange County in Laguna Hills, California, report three such cases of vitreous hemorrhage in an article online June 25 in JAMA Ophthalmology.
"It doesn't look like it's a huge phenomenon, but we're the first to report the association," said Dr. Hwang, also of the University of Southern California Eye Institute and Keck School of Medicine in Los Angeles.
Two men in their 70s and one in his 80s all presented with a vitreous hemorrhage of the right eye, confirmed by B-scan ultrasonography. They all were taking warfarin and rivaroxaban (Xarelto, Janssen) in transition to taking just rivaroxaban. They stopped taking warfarin and continued the rivaroxaban.
The vitreous hemorrhages in the first two cases largely resolved by a month after presentation and the men's visual acuity returned. For the third man, the vitreous hemorrhage resolved by two months and his visual acuity had largely returned.
"In all cases, an acute vitreous hemorrhage occurred shortly after rivaroxaban treatment was initiated. The vitreous hemorrhage resolved spontaneously in all cases and subsequent clinical and angiographic assessments did not reveal any ocular pathology to account for the hemorrhage," the authors wrote.
"I think it's the double-whammying of the drugs, two anticoagulants at once," Dr. Hwang told Reuters. "All three patients were transitioning in treatment. At the time when they had the bleed, all of them were taking two medications. So we definitely think that the highest risk period for developing a bleeding is during this transition period when patients are stopping one medication and starting another one."
As the trend continues for switching patients from warfarin to rivaroxaban, so may the trend for patients to develop vitreous hemorrhage, he added, and clinicians should be aware of the potential risk and advise patients accordingly.
One study in 2013 found an association between use of aspirin, clopidogrel, and warfarin and vitreous hemorrhage (http://bit.ly/1Jl76YK). And a 2007 paper reported an association between warfarin and ocular bleeding (http://bit.ly/1Lx6BeC).
Janssen Pharmaceuticals issued the following statement in response to the JAMA Ophthalmology report: "Bleeding events are a known consequence of anticoagulation, and these types of bleeding events were identified in the clinical development program for Xarelto. We carefully monitor the safety performance of our medicine and Xarelto continues to perform as expected in the real-world setting, as evidenced by the 24 month results, in 39,000 patients, from our ongoing postmarketing safety surveillance program. We will continue to advance our real world data to ensure doctors and patients are equipped with the latest information about our medicine to optimize patient care."
In May, Janssen said at the American Geriatrics Society meeting that new data from their postmarketing study show that the safety profile of the drug in routine clinical practice is consistent with phase 3 clinical trial data, "which show a correlation between increased age and bleeding."
The authors of the case report made disclosures.
NEW YORK (Reuters Health) - Patients transitioning from warfarin to rivaroxaban for atrial fibrillation, who are taking both drugs before dropping warfarin, may be at risk for spontaneous vitreous hemorrhage, according to a new case report.
Judy H. Jun and Dr. John C. Hwang, of Retina Associates of Orange County in Laguna Hills, California, report three such cases of vitreous hemorrhage in an article online June 25 in JAMA Ophthalmology.
"It doesn't look like it's a huge phenomenon, but we're the first to report the association," said Dr. Hwang, also of the University of Southern California Eye Institute and Keck School of Medicine in Los Angeles.
Two men in their 70s and one in his 80s all presented with a vitreous hemorrhage of the right eye, confirmed by B-scan ultrasonography. They all were taking warfarin and rivaroxaban (Xarelto, Janssen) in transition to taking just rivaroxaban. They stopped taking warfarin and continued the rivaroxaban.
The vitreous hemorrhages in the first two cases largely resolved by a month after presentation and the men's visual acuity returned. For the third man, the vitreous hemorrhage resolved by two months and his visual acuity had largely returned.
"In all cases, an acute vitreous hemorrhage occurred shortly after rivaroxaban treatment was initiated. The vitreous hemorrhage resolved spontaneously in all cases and subsequent clinical and angiographic assessments did not reveal any ocular pathology to account for the hemorrhage," the authors wrote.
"I think it's the double-whammying of the drugs, two anticoagulants at once," Dr. Hwang told Reuters. "All three patients were transitioning in treatment. At the time when they had the bleed, all of them were taking two medications. So we definitely think that the highest risk period for developing a bleeding is during this transition period when patients are stopping one medication and starting another one."
As the trend continues for switching patients from warfarin to rivaroxaban, so may the trend for patients to develop vitreous hemorrhage, he added, and clinicians should be aware of the potential risk and advise patients accordingly.
One study in 2013 found an association between use of aspirin, clopidogrel, and warfarin and vitreous hemorrhage (http://bit.ly/1Jl76YK). And a 2007 paper reported an association between warfarin and ocular bleeding (http://bit.ly/1Lx6BeC).
Janssen Pharmaceuticals issued the following statement in response to the JAMA Ophthalmology report: "Bleeding events are a known consequence of anticoagulation, and these types of bleeding events were identified in the clinical development program for Xarelto. We carefully monitor the safety performance of our medicine and Xarelto continues to perform as expected in the real-world setting, as evidenced by the 24 month results, in 39,000 patients, from our ongoing postmarketing safety surveillance program. We will continue to advance our real world data to ensure doctors and patients are equipped with the latest information about our medicine to optimize patient care."
In May, Janssen said at the American Geriatrics Society meeting that new data from their postmarketing study show that the safety profile of the drug in routine clinical practice is consistent with phase 3 clinical trial data, "which show a correlation between increased age and bleeding."
The authors of the case report made disclosures.
NEW YORK (Reuters Health) - Patients transitioning from warfarin to rivaroxaban for atrial fibrillation, who are taking both drugs before dropping warfarin, may be at risk for spontaneous vitreous hemorrhage, according to a new case report.
Judy H. Jun and Dr. John C. Hwang, of Retina Associates of Orange County in Laguna Hills, California, report three such cases of vitreous hemorrhage in an article online June 25 in JAMA Ophthalmology.
"It doesn't look like it's a huge phenomenon, but we're the first to report the association," said Dr. Hwang, also of the University of Southern California Eye Institute and Keck School of Medicine in Los Angeles.
Two men in their 70s and one in his 80s all presented with a vitreous hemorrhage of the right eye, confirmed by B-scan ultrasonography. They all were taking warfarin and rivaroxaban (Xarelto, Janssen) in transition to taking just rivaroxaban. They stopped taking warfarin and continued the rivaroxaban.
The vitreous hemorrhages in the first two cases largely resolved by a month after presentation and the men's visual acuity returned. For the third man, the vitreous hemorrhage resolved by two months and his visual acuity had largely returned.
"In all cases, an acute vitreous hemorrhage occurred shortly after rivaroxaban treatment was initiated. The vitreous hemorrhage resolved spontaneously in all cases and subsequent clinical and angiographic assessments did not reveal any ocular pathology to account for the hemorrhage," the authors wrote.
"I think it's the double-whammying of the drugs, two anticoagulants at once," Dr. Hwang told Reuters. "All three patients were transitioning in treatment. At the time when they had the bleed, all of them were taking two medications. So we definitely think that the highest risk period for developing a bleeding is during this transition period when patients are stopping one medication and starting another one."
As the trend continues for switching patients from warfarin to rivaroxaban, so may the trend for patients to develop vitreous hemorrhage, he added, and clinicians should be aware of the potential risk and advise patients accordingly.
One study in 2013 found an association between use of aspirin, clopidogrel, and warfarin and vitreous hemorrhage (http://bit.ly/1Jl76YK). And a 2007 paper reported an association between warfarin and ocular bleeding (http://bit.ly/1Lx6BeC).
Janssen Pharmaceuticals issued the following statement in response to the JAMA Ophthalmology report: "Bleeding events are a known consequence of anticoagulation, and these types of bleeding events were identified in the clinical development program for Xarelto. We carefully monitor the safety performance of our medicine and Xarelto continues to perform as expected in the real-world setting, as evidenced by the 24 month results, in 39,000 patients, from our ongoing postmarketing safety surveillance program. We will continue to advance our real world data to ensure doctors and patients are equipped with the latest information about our medicine to optimize patient care."
In May, Janssen said at the American Geriatrics Society meeting that new data from their postmarketing study show that the safety profile of the drug in routine clinical practice is consistent with phase 3 clinical trial data, "which show a correlation between increased age and bleeding."
The authors of the case report made disclosures.
Recombinant vWF can safely halt bleeding in vWD
Results of a phase 3 trial suggest the recombinant von Willebrand factor (rvWF) product BAX 111 can safely control bleeding in patients with severe von Willebrand disease (vWD).
BAX 111, given with or without recombinant factor VIII (rFVIII), controlled bleeding episodes in 100% of patients treated.
None of the patients developed vWF or FVIII inhibitors, anti-VWF binding antibodies, or antibodies against host cell proteins.
There were 8 adverse events (AEs) associated with BAX 111, 2 of which were serious.
Investigators reported these results in Blood. The trial was sponsored by Baxalta, Inc., the company developing BAX 111.
Compared to other marketed vWF concentrates, BAX 111 contains a more consistent concentration of ultra-large-molecular-weight multimers. And the product is developed using a plasma- and albumin-free manufacturing method.
For the phase 3 trial, investigators set out to test BAX 111 in 49 patients who had received vWF concentrate treatment for at least 1 severe vWD-related bleed within the last 12 months. The patients’ median age was 37 (range, 18 to 64), and there were slightly more females (54.1%) than males (45.9%).
To observe BAX 111’s activity at varying doses and in different treatment scenarios, the investigators administered BAX 111 at 50 IU/kg or 80 IU/kg either alone or with rFVIII.
The majority of patients received as-needed treatment of BAX 111 at 40-60 IU/kg for regular bleeding episodes and up to 80 IU/kg for major bleeds. About 32% of treated subjects (7/22) also received an antifibrinolytic agent, such as tranexamic acid, during bleeding episodes.
In all, 22 patients experienced at least 1 bleeding episode that was treated with BAX 111. Seventeen of these patients had type 3 vWD, 4 had type 2A, and 1 had type 2N.
The investigators assessed 192 bleeding episodes treated with BAX 111 for hemostatic efficacy. Most bleeds (n=122) were minor, 61 were moderate, 7 were major/severe, and 2 were of unknown severity.
Efficacy
All 192 bleeds were treated successfully. When vWD specialists rated BAX 111’s ability to control bleeds on a scale of “excellent” to “no response,” they rated BAX 111 as “excellent” (96.9%) or “good” (3.1%).
One infusion of BAX 111 was sufficient in treating most bleeds (81.8%, n=157). One mucosal bleed (occurring in the genital tract and oral cavity simultaneously) required 4 infusions, which was the maximum number of infusions administered during the study.
The median doses of BAX 111 and rFVIII administered per bleed were 46.5 IU/kg and 33.6 IU/kg, respectively.
The activity of BAX 111 remained the same whether it was given with or without rFVIII. Patients who received BAX 111 alone experienced a rapid increase in their naturally produced FVIII.
In fact, within 6 hours of an infusion, patients had produced enough of the protein for proper clotting. This response was sustained through 72 hours post-infusion, suggesting that patients who receive BAX 111 are not likely to require additional rFVIII infusions.
Safety
There were 125 AEs in this trial, but only 8 were considered related to BAX 111. Six AEs (occurring in 4 patients) were not serious. These included mild infusion site paresthesia, moderate dysgeusia, moderate tachycardia, mild ECG T-wave inversion, mild generalized pruritus, and mild hot flush.
One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate. The symptoms improved after 10 minutes of oxygen treatment, and the subject fully recovered within 3 hours. The subject had a history of allergic responses to cryoprecipitate and a plasma-derived vWF concentrate.
None of the patients developed anti-VWF neutralizing or binding antibodies, FVIII neutralizing antibodies, or antibodies against recombinant furin, Chinese hamster ovary host cell proteins, or murine immunoglobulin G.
There were no clinical signs of thrombotic events and no clinically significant abnormal D-dimer values post-treatment.
“These efficacy and safety data . . . represent a major advance in our quest to develop an optimal treatment for people living with vWD,” said study investigator Bruce Ewenstein, MD, PhD, of Baxalta, Inc.
“As this product is specifically designed to be administered without FVIII, it will allow physicians to dose vWF and FVIII separately and precisely based on the needs of each individual patient. This treatment strategy has the potential to become the standard of care for patients with severe von Willebrand disease.” ![]()

Results of a phase 3 trial suggest the recombinant von Willebrand factor (rvWF) product BAX 111 can safely control bleeding in patients with severe von Willebrand disease (vWD).
BAX 111, given with or without recombinant factor VIII (rFVIII), controlled bleeding episodes in 100% of patients treated.
None of the patients developed vWF or FVIII inhibitors, anti-VWF binding antibodies, or antibodies against host cell proteins.
There were 8 adverse events (AEs) associated with BAX 111, 2 of which were serious.
Investigators reported these results in Blood. The trial was sponsored by Baxalta, Inc., the company developing BAX 111.
Compared to other marketed vWF concentrates, BAX 111 contains a more consistent concentration of ultra-large-molecular-weight multimers. And the product is developed using a plasma- and albumin-free manufacturing method.
For the phase 3 trial, investigators set out to test BAX 111 in 49 patients who had received vWF concentrate treatment for at least 1 severe vWD-related bleed within the last 12 months. The patients’ median age was 37 (range, 18 to 64), and there were slightly more females (54.1%) than males (45.9%).
To observe BAX 111’s activity at varying doses and in different treatment scenarios, the investigators administered BAX 111 at 50 IU/kg or 80 IU/kg either alone or with rFVIII.
The majority of patients received as-needed treatment of BAX 111 at 40-60 IU/kg for regular bleeding episodes and up to 80 IU/kg for major bleeds. About 32% of treated subjects (7/22) also received an antifibrinolytic agent, such as tranexamic acid, during bleeding episodes.
In all, 22 patients experienced at least 1 bleeding episode that was treated with BAX 111. Seventeen of these patients had type 3 vWD, 4 had type 2A, and 1 had type 2N.
The investigators assessed 192 bleeding episodes treated with BAX 111 for hemostatic efficacy. Most bleeds (n=122) were minor, 61 were moderate, 7 were major/severe, and 2 were of unknown severity.
Efficacy
All 192 bleeds were treated successfully. When vWD specialists rated BAX 111’s ability to control bleeds on a scale of “excellent” to “no response,” they rated BAX 111 as “excellent” (96.9%) or “good” (3.1%).
One infusion of BAX 111 was sufficient in treating most bleeds (81.8%, n=157). One mucosal bleed (occurring in the genital tract and oral cavity simultaneously) required 4 infusions, which was the maximum number of infusions administered during the study.
The median doses of BAX 111 and rFVIII administered per bleed were 46.5 IU/kg and 33.6 IU/kg, respectively.
The activity of BAX 111 remained the same whether it was given with or without rFVIII. Patients who received BAX 111 alone experienced a rapid increase in their naturally produced FVIII.
In fact, within 6 hours of an infusion, patients had produced enough of the protein for proper clotting. This response was sustained through 72 hours post-infusion, suggesting that patients who receive BAX 111 are not likely to require additional rFVIII infusions.
Safety
There were 125 AEs in this trial, but only 8 were considered related to BAX 111. Six AEs (occurring in 4 patients) were not serious. These included mild infusion site paresthesia, moderate dysgeusia, moderate tachycardia, mild ECG T-wave inversion, mild generalized pruritus, and mild hot flush.
One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate. The symptoms improved after 10 minutes of oxygen treatment, and the subject fully recovered within 3 hours. The subject had a history of allergic responses to cryoprecipitate and a plasma-derived vWF concentrate.
None of the patients developed anti-VWF neutralizing or binding antibodies, FVIII neutralizing antibodies, or antibodies against recombinant furin, Chinese hamster ovary host cell proteins, or murine immunoglobulin G.
There were no clinical signs of thrombotic events and no clinically significant abnormal D-dimer values post-treatment.
“These efficacy and safety data . . . represent a major advance in our quest to develop an optimal treatment for people living with vWD,” said study investigator Bruce Ewenstein, MD, PhD, of Baxalta, Inc.
“As this product is specifically designed to be administered without FVIII, it will allow physicians to dose vWF and FVIII separately and precisely based on the needs of each individual patient. This treatment strategy has the potential to become the standard of care for patients with severe von Willebrand disease.” ![]()

Results of a phase 3 trial suggest the recombinant von Willebrand factor (rvWF) product BAX 111 can safely control bleeding in patients with severe von Willebrand disease (vWD).
BAX 111, given with or without recombinant factor VIII (rFVIII), controlled bleeding episodes in 100% of patients treated.
None of the patients developed vWF or FVIII inhibitors, anti-VWF binding antibodies, or antibodies against host cell proteins.
There were 8 adverse events (AEs) associated with BAX 111, 2 of which were serious.
Investigators reported these results in Blood. The trial was sponsored by Baxalta, Inc., the company developing BAX 111.
Compared to other marketed vWF concentrates, BAX 111 contains a more consistent concentration of ultra-large-molecular-weight multimers. And the product is developed using a plasma- and albumin-free manufacturing method.
For the phase 3 trial, investigators set out to test BAX 111 in 49 patients who had received vWF concentrate treatment for at least 1 severe vWD-related bleed within the last 12 months. The patients’ median age was 37 (range, 18 to 64), and there were slightly more females (54.1%) than males (45.9%).
To observe BAX 111’s activity at varying doses and in different treatment scenarios, the investigators administered BAX 111 at 50 IU/kg or 80 IU/kg either alone or with rFVIII.
The majority of patients received as-needed treatment of BAX 111 at 40-60 IU/kg for regular bleeding episodes and up to 80 IU/kg for major bleeds. About 32% of treated subjects (7/22) also received an antifibrinolytic agent, such as tranexamic acid, during bleeding episodes.
In all, 22 patients experienced at least 1 bleeding episode that was treated with BAX 111. Seventeen of these patients had type 3 vWD, 4 had type 2A, and 1 had type 2N.
The investigators assessed 192 bleeding episodes treated with BAX 111 for hemostatic efficacy. Most bleeds (n=122) were minor, 61 were moderate, 7 were major/severe, and 2 were of unknown severity.
Efficacy
All 192 bleeds were treated successfully. When vWD specialists rated BAX 111’s ability to control bleeds on a scale of “excellent” to “no response,” they rated BAX 111 as “excellent” (96.9%) or “good” (3.1%).
One infusion of BAX 111 was sufficient in treating most bleeds (81.8%, n=157). One mucosal bleed (occurring in the genital tract and oral cavity simultaneously) required 4 infusions, which was the maximum number of infusions administered during the study.
The median doses of BAX 111 and rFVIII administered per bleed were 46.5 IU/kg and 33.6 IU/kg, respectively.
The activity of BAX 111 remained the same whether it was given with or without rFVIII. Patients who received BAX 111 alone experienced a rapid increase in their naturally produced FVIII.
In fact, within 6 hours of an infusion, patients had produced enough of the protein for proper clotting. This response was sustained through 72 hours post-infusion, suggesting that patients who receive BAX 111 are not likely to require additional rFVIII infusions.
Safety
There were 125 AEs in this trial, but only 8 were considered related to BAX 111. Six AEs (occurring in 4 patients) were not serious. These included mild infusion site paresthesia, moderate dysgeusia, moderate tachycardia, mild ECG T-wave inversion, mild generalized pruritus, and mild hot flush.
One patient experienced 2 simultaneous serious AEs—chest discomfort and increased heart rate. The symptoms improved after 10 minutes of oxygen treatment, and the subject fully recovered within 3 hours. The subject had a history of allergic responses to cryoprecipitate and a plasma-derived vWF concentrate.
None of the patients developed anti-VWF neutralizing or binding antibodies, FVIII neutralizing antibodies, or antibodies against recombinant furin, Chinese hamster ovary host cell proteins, or murine immunoglobulin G.
There were no clinical signs of thrombotic events and no clinically significant abnormal D-dimer values post-treatment.
“These efficacy and safety data . . . represent a major advance in our quest to develop an optimal treatment for people living with vWD,” said study investigator Bruce Ewenstein, MD, PhD, of Baxalta, Inc.
“As this product is specifically designed to be administered without FVIII, it will allow physicians to dose vWF and FVIII separately and precisely based on the needs of each individual patient. This treatment strategy has the potential to become the standard of care for patients with severe von Willebrand disease.” ![]()
FDA clears 7-day storage of platelets
The US Food and Drug Administration (FDA) has cleared a label change for the Fenwal Amicus system, a cell-separation device, to allow for 7-day storage of platelets collected via this system.
The Fenwal Amicus system is used to collect platelets, platelets with concurrent plasma and red cells, and mononuclear cells.
The label change means platelets collected via the Fenwal Amicus system can now be stored for 7 days instead of 5, but conditions apply.
“Our customers now have the ability to store and use 7-day platelets processed by Amicus to help meet an important need in transfusion medicine,” said Dean Gregory, of Fresenius Kabi North America, the company that manufactures the Fenwal Amicus system.
“We designed Amicus to help optimize apheresis programs for blood-collection professionals. With this new clearance, the Amicus system becomes an even more versatile choice.”
Previously, platelets were limited to a shelf life of 5 days due to the absence of appropriate bacterial testing methods and appropriate instructions for use.
The label change for the Fenwal Amicus system means blood centers that want to store apheresis-derived platelets for 7 days must label every product with a statement that the product must be tested with a bacterial detection device cleared by the FDA and labeled as a “safety measure.”
To support this requirement, Fresenius Kabi distributes the Verax Platelet PGD test, a rapid platelet PGD test that is cleared by the FDA as a “safety measure” for use in leukoreduced apheresis platelets 24 hours prior to transfusion.
For more information on the Verax Platelet PGD test, visit the Verax Biomedical website. For more information on the Fenwal Amicus system, visit the Fenwal website. ![]()

The US Food and Drug Administration (FDA) has cleared a label change for the Fenwal Amicus system, a cell-separation device, to allow for 7-day storage of platelets collected via this system.
The Fenwal Amicus system is used to collect platelets, platelets with concurrent plasma and red cells, and mononuclear cells.
The label change means platelets collected via the Fenwal Amicus system can now be stored for 7 days instead of 5, but conditions apply.
“Our customers now have the ability to store and use 7-day platelets processed by Amicus to help meet an important need in transfusion medicine,” said Dean Gregory, of Fresenius Kabi North America, the company that manufactures the Fenwal Amicus system.
“We designed Amicus to help optimize apheresis programs for blood-collection professionals. With this new clearance, the Amicus system becomes an even more versatile choice.”
Previously, platelets were limited to a shelf life of 5 days due to the absence of appropriate bacterial testing methods and appropriate instructions for use.
The label change for the Fenwal Amicus system means blood centers that want to store apheresis-derived platelets for 7 days must label every product with a statement that the product must be tested with a bacterial detection device cleared by the FDA and labeled as a “safety measure.”
To support this requirement, Fresenius Kabi distributes the Verax Platelet PGD test, a rapid platelet PGD test that is cleared by the FDA as a “safety measure” for use in leukoreduced apheresis platelets 24 hours prior to transfusion.
For more information on the Verax Platelet PGD test, visit the Verax Biomedical website. For more information on the Fenwal Amicus system, visit the Fenwal website. ![]()

The US Food and Drug Administration (FDA) has cleared a label change for the Fenwal Amicus system, a cell-separation device, to allow for 7-day storage of platelets collected via this system.
The Fenwal Amicus system is used to collect platelets, platelets with concurrent plasma and red cells, and mononuclear cells.
The label change means platelets collected via the Fenwal Amicus system can now be stored for 7 days instead of 5, but conditions apply.
“Our customers now have the ability to store and use 7-day platelets processed by Amicus to help meet an important need in transfusion medicine,” said Dean Gregory, of Fresenius Kabi North America, the company that manufactures the Fenwal Amicus system.
“We designed Amicus to help optimize apheresis programs for blood-collection professionals. With this new clearance, the Amicus system becomes an even more versatile choice.”
Previously, platelets were limited to a shelf life of 5 days due to the absence of appropriate bacterial testing methods and appropriate instructions for use.
The label change for the Fenwal Amicus system means blood centers that want to store apheresis-derived platelets for 7 days must label every product with a statement that the product must be tested with a bacterial detection device cleared by the FDA and labeled as a “safety measure.”
To support this requirement, Fresenius Kabi distributes the Verax Platelet PGD test, a rapid platelet PGD test that is cleared by the FDA as a “safety measure” for use in leukoreduced apheresis platelets 24 hours prior to transfusion.
For more information on the Verax Platelet PGD test, visit the Verax Biomedical website. For more information on the Fenwal Amicus system, visit the Fenwal website. ![]()
Group creates vascular graft that resists thrombosis
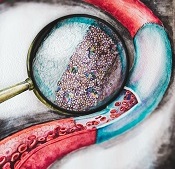
The enlarged section shows
the drug-loaded film that
coats the inside of the graft.
Image courtesy of ITMO
University/Yulia Chapurina
Researchers say they have developed a vascular graft that may be able to resist thrombosis indefinitely.
The team found they could trap urokinase-type plasminogen activator inside a porous, alumina sol–gel film, and this film retained its thrombolytic activity when applied to a polymer vascular graft.
Vladimir Vinogradov, PhD, of ITMO University in St. Petersburg, Russia, and his colleagues described this work in the Journal of Medicinal Chemistry.
The team synthesized a thin film made of densely packed aluminum oxide nanorods blended with molecules of the thrombolytic enzyme urokinase-type plasminogen activator.
When adhered to the inner surface of a polymer vascular graft, the film causes the parietal area of the graft to fill with a stable concentration of plasmin, which is capable of dissolving any clots that appear.
The researchers said the unique properties of the film arise from its structure, which represents a porous matrix, accommodating the plasminogen activator.
The matrix protects the plasminogen activator from the aggressive environment of the organism while also preserving the enzyme’s ability to interact with certain external agents through a system of pores.
In particular, the matrix lets in plasminogen. When plasminogen meets the plasminogen activator inside the matrix, clot-dissolving plasmin forms.
“In order to test how our improved vascular graft worked, we grew an artificial clot made of blood plasma mixed with thrombin and placed it inside the graft,” said Yulia Chapurina, also of ITMO University.
“The results of the experiment amazed us. Very soon, the clot started to dissolve and leak through the graft. In reality, our coating would destroy clots at the stage of formation, constantly ensuring an unobstructed blood flow in the graft.”
The researchers noted that the lifetime of drug-eluting grafts currently in use is often determined by the amount of drug stored within them. In essence, this means they can only postpone clot formation.
But the graft the researchers developed traps the drug inside a porous, protective shell, which, the team said, makes the lifetime of such a graft practically unlimited.
“Our approach is concept-based and can be applied not only to artificial blood vessels, but to any kind of implants,” Dr Vinogradov said. “You just need to take the right kind of drug.”
“For example, after the implantation of an artificial ureter, urease crystals often start to grow inside, and doctors do not know how to deal with this problem. It is possible to apply a similar drug-containing coating that dissolves urease. The same approach may be used for kidney or liver surgery, but these are plans for the future.” ![]()

The enlarged section shows
the drug-loaded film that
coats the inside of the graft.
Image courtesy of ITMO
University/Yulia Chapurina
Researchers say they have developed a vascular graft that may be able to resist thrombosis indefinitely.
The team found they could trap urokinase-type plasminogen activator inside a porous, alumina sol–gel film, and this film retained its thrombolytic activity when applied to a polymer vascular graft.
Vladimir Vinogradov, PhD, of ITMO University in St. Petersburg, Russia, and his colleagues described this work in the Journal of Medicinal Chemistry.
The team synthesized a thin film made of densely packed aluminum oxide nanorods blended with molecules of the thrombolytic enzyme urokinase-type plasminogen activator.
When adhered to the inner surface of a polymer vascular graft, the film causes the parietal area of the graft to fill with a stable concentration of plasmin, which is capable of dissolving any clots that appear.
The researchers said the unique properties of the film arise from its structure, which represents a porous matrix, accommodating the plasminogen activator.
The matrix protects the plasminogen activator from the aggressive environment of the organism while also preserving the enzyme’s ability to interact with certain external agents through a system of pores.
In particular, the matrix lets in plasminogen. When plasminogen meets the plasminogen activator inside the matrix, clot-dissolving plasmin forms.
“In order to test how our improved vascular graft worked, we grew an artificial clot made of blood plasma mixed with thrombin and placed it inside the graft,” said Yulia Chapurina, also of ITMO University.
“The results of the experiment amazed us. Very soon, the clot started to dissolve and leak through the graft. In reality, our coating would destroy clots at the stage of formation, constantly ensuring an unobstructed blood flow in the graft.”
The researchers noted that the lifetime of drug-eluting grafts currently in use is often determined by the amount of drug stored within them. In essence, this means they can only postpone clot formation.
But the graft the researchers developed traps the drug inside a porous, protective shell, which, the team said, makes the lifetime of such a graft practically unlimited.
“Our approach is concept-based and can be applied not only to artificial blood vessels, but to any kind of implants,” Dr Vinogradov said. “You just need to take the right kind of drug.”
“For example, after the implantation of an artificial ureter, urease crystals often start to grow inside, and doctors do not know how to deal with this problem. It is possible to apply a similar drug-containing coating that dissolves urease. The same approach may be used for kidney or liver surgery, but these are plans for the future.” ![]()

The enlarged section shows
the drug-loaded film that
coats the inside of the graft.
Image courtesy of ITMO
University/Yulia Chapurina
Researchers say they have developed a vascular graft that may be able to resist thrombosis indefinitely.
The team found they could trap urokinase-type plasminogen activator inside a porous, alumina sol–gel film, and this film retained its thrombolytic activity when applied to a polymer vascular graft.
Vladimir Vinogradov, PhD, of ITMO University in St. Petersburg, Russia, and his colleagues described this work in the Journal of Medicinal Chemistry.
The team synthesized a thin film made of densely packed aluminum oxide nanorods blended with molecules of the thrombolytic enzyme urokinase-type plasminogen activator.
When adhered to the inner surface of a polymer vascular graft, the film causes the parietal area of the graft to fill with a stable concentration of plasmin, which is capable of dissolving any clots that appear.
The researchers said the unique properties of the film arise from its structure, which represents a porous matrix, accommodating the plasminogen activator.
The matrix protects the plasminogen activator from the aggressive environment of the organism while also preserving the enzyme’s ability to interact with certain external agents through a system of pores.
In particular, the matrix lets in plasminogen. When plasminogen meets the plasminogen activator inside the matrix, clot-dissolving plasmin forms.
“In order to test how our improved vascular graft worked, we grew an artificial clot made of blood plasma mixed with thrombin and placed it inside the graft,” said Yulia Chapurina, also of ITMO University.
“The results of the experiment amazed us. Very soon, the clot started to dissolve and leak through the graft. In reality, our coating would destroy clots at the stage of formation, constantly ensuring an unobstructed blood flow in the graft.”
The researchers noted that the lifetime of drug-eluting grafts currently in use is often determined by the amount of drug stored within them. In essence, this means they can only postpone clot formation.
But the graft the researchers developed traps the drug inside a porous, protective shell, which, the team said, makes the lifetime of such a graft practically unlimited.
“Our approach is concept-based and can be applied not only to artificial blood vessels, but to any kind of implants,” Dr Vinogradov said. “You just need to take the right kind of drug.”
“For example, after the implantation of an artificial ureter, urease crystals often start to grow inside, and doctors do not know how to deal with this problem. It is possible to apply a similar drug-containing coating that dissolves urease. The same approach may be used for kidney or liver surgery, but these are plans for the future.” ![]()
T-cell activation is a dynamic process, study shows
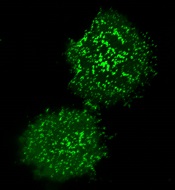
plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()

plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()

plasma membrane upon T-cell
activation. Image courtesy
of the Salk Institute
New research suggests that, contrary to previous assumptions, T-cell activation is a fluid process that relies on a dynamic protein network at the cell surface.
Previously, scientists thought T-cell activation was a static process in which molecules assemble within a T cell.
Instead, investigators discovered that molecules rapidly come and go, and the times at which these molecules arrive and depart affect the immune response.
“This is a completely new principle for how T-cell activity is controlled—whether it ignores or responds to a threat,” said Björn Lillemeier, PhD, of the Salk Institute in La Jolla, California.
He and his colleagues described this discovery in Nature Immunology.
They noted that the protein kinase ZAP-70 plays a central role in T-cell activation. Until now, scientists assumed that a silent form of ZAP-70 floats around inside the T cell until a threat is detected. The threat recruits ZAP-70 to the cell surface and activates it.
By analyzing mutant forms of ZAP-70, Dr Lillemeier’s group discovered that, instead of ZAP-70 binding the T-cell receptor firmly, it comes in contact with the receptor sporadically. Each time this happens, ZAP-70 has to adopt an unfavorable shape that forces it back inside the cell.
This cycle continues until a second molecule, Lck, helps ZAP-70 remain with the T-cell receptor. The prolonged stay at the cell surface activates ZAP-70 and prompts the T cell to attack invaders and diseased cells.
The investigators said this study suggests the steps underlying T-cell activation are more dynamic than the less mobile modes scientists had suspected before. And the research highlights how ZAP-70 and other molecules communicate in space and time, which is crucial for controlling the ultimate activity of a T cell.
By understanding this process, Dr Lillemeier said, “We might be able to encourage the immune system to be a little more sensitive in order to recognize and eliminate diseases.”
His team is now working to identify new principles that determine if T cells respond to a threat or stay quiet. They are also testing whether their findings could be applied across additional processes in T cells and other immune cells.
Because proteins have many of the same modular building blocks, Dr Lillemeier said that, in principle, any protein with structural characteristics comparable to those of ZAP-70 could be controlled by similar mechanisms. ![]()
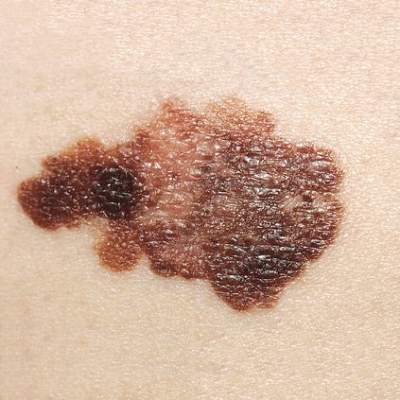
Melanoma twice as likely after CLL/SLL than other types of NHL
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma, according to a report published online Aug. 3 in Journal of Clinical Oncology.
Since patients with chronic lymphocytic leukemia/small lymphocytic lymphoma (CLL/SLL) have profound and prolonged immune dysfunction characterized by B-cell and T-cell defects, this finding suggests that immune perturbation may account for the excess of melanoma diagnoses observed in patients with non-Hodgkin lymphoma (NHL), said Dr. Clara J. K. Lam of the radiation epidemiology branch, National Cancer Institute, Bethesda Md., and her associates.
Although patients with NHL are known to be at increased risk for melanoma compared with the general population, the reasons remain unclear. Additional factors such as chemotherapy regimens and sunlight exposure likely complicate the picture, and no studies to date have been able to account for these confounders. To assess a large enough study sample to examine these issues, Dr. Lam and her associates analyzed data concerning 44,870 NHL survivors in the Surveillance, Epidemiology, and End Results (SEER) database. They focused on older patients aged 66-83 years at NHL diagnosis (mean age, 74 years) who were followed for at least 1 year (mean follow-up, 5.5 years), of whom 13,950 had CLL/SLL.
A total of 202 melanomas developed, and the median interval between NHL diagnosis and melanoma diagnosis was 3 years (range, 1-15 years). Nearly half of these melanomas occurred in patients with CLL/SLL rather than other types of NHL; 41% occurred on the face, head, or neck, and 43% were 1 mm or more in thickness. In contrast, among survivors of other NHL types, melanoma occurred most often on the trunk, and only 28% were 1 mm or more in thickness. This aligns with previous reports that melanomas arising after NHL tend to be more advanced and aggressive than those in the general population, the investigators said (J Clin Oncol. 2015 Aug 3. doi:10.1200/JCO.2014.60.2094).
Further analysis revealed that among patients with CLL/SLL, melanoma risk was significantly increased in those who received fludarabine rather than other treatments (HR, 1.90, 95% CI, 1.08 to 3.37)), with or without the addition of rituximab. In contrast, melanoma risks were unrelated to treatment among patients who had other types of NHL.
Similarly, patients with CLL/SLL who had T-cell-activating autoimmune disorders (such as Graves’ disease, localized scleroderma, psoriasis, chronic rheumatic heart disease, asthma, or skin-related conditions) either before or after diagnosis of their leukemia/lymphoma also had 2-4 times the risk of developing melanoma than that of patients without such autoimmune disorders. In contrast, melanoma risks were unrelated to autoimmune disorders in patients with other types of NHL. This finding underscores the importance of T-cell dysfunction as a contributor to melanoma risk after CLL/SLL, Dr. Lam and her associates said.
Taken together, their findings identify which survivors of NHL are at highest risk for developing melanoma and would benefit the most from undergoing regular full-skin examinations to facilitate early detection.
This study was limited in that it was confined to patients over age 65 with NHL. The results may not be generalizable to younger patients, Dr. Lam and her associates added.
FROM JOURNAL OF CLINICAL ONCOLOGY
Key clinical point: Survivors of chronic lymphocytic leukemia/small lymphocytic lymphoma are twice as likely to develop melanoma as are survivors of other types of non-Hodgkin lymphoma.
Major finding: A total of 202 melanomas developed during 5.5 years of follow-up, and nearly half occurred in patients with CLL/SLL rather than other types of NHL.
Data source: A large population-based study using SEER data to assess melanoma risk in 44,870 older survivors of non-Hodgkin lymphoma.
Disclosures: The National Cancer Institute supported the study. Dr. Lam and her associates reported having no relevant financial disclosures.
Anticoagulant therapy not contraindicated in brain metastases
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
Venous thromboembolism (VTE) is common in cancer patients with brain metastases but therapeutic anticoagulation does not increase the risk for intracranial hemorrhage, according to new data.
In a matched, retrospective cohort study of 293 cancer patients with brain metastases (104 treated with therapeutic enoxaparin and 189 controls), there were no differences at 1 year between the two groups for measurable (19% vs 21%; Gray test, P = .97; hazard ratio, 1.02; 90% confidence interval [CI], 0.66-1.59), significant (21% vs 22%; P = .87), and total (44% vs 37%; P = .13) intracranial hemorrhages.
“Reassuringly, the cumulative incidence of intracranial hemorrhage was not significantly different in those patients who received therapeutic enoxaparin compared with controls for all outcomes including measurable, total, and significant intracranial hemorrhages,” wrote Dr. Jessica Donato of Harvard Medical School, Boston, and colleagues (Blood 2015 Jul 23 doi:10.1182/blood-2015-02-626788).
The only covariate that was predictive of hemorrhage in this study was the combined group of renal cell carcinoma and melanoma, as the risk for intracranial hemorrhage was fourfold higher (adjusted hazard ratio, 3.98; 90% CI, 2.41-6.57; P less than .001) in those subgroups.
In an accompanying commentary in the same issue of Blood, Dr. Lisa Baumann Kreuziger of Medical College of Wisconsin, Milwaukee, wrote that although the study was well designed, “its retrospective nature creates inherent limitations.”
As an example, besides tumor type, the multivariable analysis did not identify other clinical factors that could guide clinicians when assessing the risk of intracranial hemorrhage, she wrote (Blood 2015 Jul 23. doi: 10.1182/blood-2015-06-648089]).
But in spite of the limitations, the study “further supports the statement from the 2014 American Society of Clinical Oncology Guidelines that brain metastases are not a contraindication to treatment of VTE with low-molecular-weight heparin,” she concluded.
FROM BLOOD
Key clinical point: Therapeutic anticoagulation does not increase the risk for intracranial hemorrhage in patients with brain metastases.
Major finding: There were no differences in the cumulative incidence of intracranial hemorrhage at 1 year in the enoxaparin and control cohorts for total (44% vs 37%; P = .13) intracranial hemorrhages.
Data source: A matched, retrospective cohort study of 293 cancer patients with brain metastases.
Disclosures: The Harvard Catalyst/The Harvard Clinical and Translational Science Center and a Dana-Farber/Harvard Cancer Center Core Grant supported the study. Dr. Zwicker received prior research funding from Sanofi, serves on advisory committees for Portola Pharmaceuticals and Merck, and receives consulting fees from Parexel. The remaining authors declared no financial conflicts.
Plasma tau level chronically elevated in TBI
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Researchers and funding agencies have been hoping that the recent development of ultrahigh-sensitivity technology to measure the extremely low levels of CNS-derived biomarkers in the blood would be a game changer for minor TBI, and it appears that our hopes are beginning to be borne out.
The usefulness of plasma tau as a marker of brain pathology in TBI, however, was relatively weak in this study, and it remains to be seen whether this marker will prove helpful in clinical practice. The between-group differences in mean plasma tau levels were small in this study, and the levels in individual samples overlapped substantially between affected patients and controls.
Dr. Elaine R. Peskind is with the Veterans Affairs Northwest Network Mental Illness Research, Education, and Clinical Center and the department of psychiatry and behavioral sciences at the University of Washington, both in Seattle. She reported having no relevant financial conflicts of interest. Dr. Peskind and her associates made these remarks in an editorial accompanying Dr. Olivera’s report (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1789).
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and appear to correlate with the severity of postconcussive symptoms, according to a report published Aug. 3 in JAMA Neurology.
If these findings are confirmed, this will be the first biomarker that is sensitive and specific to persistent traumatic brain injury–related symptoms. The results also suggest that “months to years after the primary brain injury, there may be a continuation of secondary injuries with residual axonal degeneration and blood-brain barrier disruptions in this population that may contribute to the maintenance of postconcussive disorder symptoms and affect symptom severity,” wrote Anlys Olivera, Ph.D., of the National Institute of Nursing Research, Bethesda, Md., and her associates.
Tau is a protein that stabilizes the structure of the axonal cytoskeleton. It is elevated in the cerebrospinal fluid and the peripheral blood (albeit in extremely low concentrations) of patients with severe traumatic brain injury (TBI), professional boxers, and athletes who sustain concussions. The extremely low levels of tau in the peripheral blood have been very difficult to measure until the recent development of an ultrahigh-sensitivity immunoassay technology. Using this innovation, the researchers were able to examine for the first time the associations between plasma tau levels and the frequency and severity of deployment-related TBIs.
Over a 2-year period, Dr. Olivera and her associates assessed tau levels in 70 members of the military who self-reported one or more TBIs and 28 control subjects without TBI who were matched for age, sex, race, time since deployment, and number of deployments. Almost all of those in the TBI group had been injured at least 18 months previously. The most common sources of TBI were blows to the head, exposure to blasts, vehicular crashes, and sports-related concussions.
Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL). Total tau also increased with increasing severity of the initial brain injury, with increasing numbers of TBIs, and increasing severity of present-day postconcussive symptoms. These associations, moreover, were independent of symptoms of posttraumatic brain disorder (PTSD) and depression, which were prevalent in the TBI group, the investigators said (JAMA Neurol. 2015 Aug 3 doi: 10.1001/jamaneurol.2015.1383).
Tau is not only a marker of brain injury; it also can contribute to secondary injury processes such as inflammation, which makes it a potential target for therapy. If the findings of this study are confirmed and extended to demonstrate a direct mechanistic relationship between TBI and tau aggregation, treatments such as the direct delivery of proteasomes “would be invaluable, considering the dearth of treatments for TBIs and chronic [postconcussive disorder] symptoms,” Dr. Olivera and her associates said.
Among the limitations cited by the investigators are lack of neuroimaging and neuropsychological data.
This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
FROM JAMA NEUROLOGY
Key clinical point: Peripheral plasma levels of the CNS protein tau are chronically elevated after traumatic brain injury and correlate with the severity of postconcussive symptoms.
Major finding: Total tau was significantly increased in the TBI group (mean level, 1.13 pg/mL), compared with the control group (0.63 pg/mL).
Data source: An observational case-control study assessing plasma tau levels in 70 military personnel with a history of TBI and 28 control subjects.
Disclosures: This study was supported by the National Institutes of Health’s National Institute of Nursing Research and the Center for Neuroscience and Regenerative Medicine, which is a collaborative program between the Department of Defense and the NIH. Dr. Olivera reported having no relevant financial disclosures. One of her associates reported ties to Quanterix, developer of the ultrahigh-sensitivity Simoa technology used in this study, which allows measurement of extremely low levels of tau and other CNS-derived biomarkers in the plasma or serum.
Direct oral anticoagulants may be inappropriate for frail elderly
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).
Caution must be used when prescribing direct oral anticoagulants to older patients, especially those in a frail condition, according to an opinion piece by Anne-Laure Sennesael and her associates.
In the case study, an 86-year-old woman weighing 55 kg was admitted to an emergency department with persistent epistaxis. The patient was taking 20 mg of rivaroxaban and 80 mg of aspirin daily. There was a history of peripheral arterial disease, and the patient had received a bioprosthetic heart implant 4 years prior. Creatinine clearance was 21 mL/min, hemoglobin was 9.4 g/dL, and prothrombin time Quick value was 30%.
Rivaroxaban was discontinued, and the patient was switched to a new anticoagulant, acenocoumarol. Aspirin also was discontinued, and the patient was discharged, reported Ms. Sennesael, of the Université catholique de Louvain in Brussels.
Use of direct oral anticoagulants (DOACs) are increasing rapidly, the authors write, mostly because doctors and patients view them as more convenient to use. However, in some patients, vitamin K antagonists (VKAs) remain the best course of treatment. In this case, the patient was receiving too much rivaroxaban in light of her low creatinine level. In addition, aspirin plus a DOAC is not appropriate, as it increases the risk of major bleeding significantly.
“A conservative strategy may be more valuable,” Ms. Sennesael and her associates wrote. “This kind of ‘less is more’ approach includes avoiding prescribing DOACs rather than VKAs without clear, compelling, evidence-based reasons; regularly reassessing renal function; monitoring for adverse effects; and reappraising aspirin prescription.”
Read the full study at JAMA Internal Medicine (doi:10.1001/jamainternmed.2015.3589).