User login
A better approach to opioid prescribing in primary care
ABSTRACT
Purpose Primary care physicians are at the center of a national prescription opioid epidemic, with little training or knowledge about the management of patients on opioids for chronic noncancer pain (CNCP). We developed an electronic medical record (EMR)-based protocol and educational intervention to standardize documentation and management of patients prescribed opioids by primary care providers. Our objective was to evaluate provider adherence to this protocol, attitudes toward the management of these patients, and knowledge of opioid prescribing.
Methods We trained providers and select staff from 3 primary care practices at the Division of General Internal Medicine at the University of Pennsylvania in the use of a protocol for managing patients taking opioids for CNCP. The following served as measures of protocol adherence: 1) the provider used a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the problem list, 2) the provider ordered at least one urine drug screen (UDS) for the patient in the past year, and 3) the patient came in for at least one office visit every 6 months. We assessed physician and staff attitudes and knowledge with pre- and post-intervention surveys. Adherence to the protocol was linked to a monetary incentive.
Results Provider adherence to the protocol significantly improved measured outcomes. The number of UDSs ordered increased by 145%, and the diagnosis of chronic pain on the problem list increased by 424%. There was a statistically significant improvement in providers’ role adequacy, role support, and job satisfaction/role-related self-esteem when working with patients taking opioids. In addition, provider knowledge of proper management of these patients improved significantly. Eighty-nine percent of our physicians attained the monetary incentive.
Conclusions We developed a quality improvement intervention that addressed the need for better regulation of opioid prescribing, resulted in increased adherence to best-practice guidelines, and improved provider knowledge and attitudes.
Primary care physicians often express dissatisfaction with their competency in treating patients with opioids,1 and at our institution, this includes residents and faculty, as well. Their concern, combined with apprehension about patient safety and the potential for addiction, can hinder appropriate opioid management.1 We asked: Could a protocol that structures the intervention improve physician competence and performance in prescribing opioids and reduce patient risk?
Physician concerns are well-founded. Nonmedical use of prescription opioids is second only to smoking marijuana in the illicit use of drugs in the United States.2 Since 2003, more overdose deaths have involved opioid analgesics than heroin and cocaine combined, leading the Centers for Disease Control and Prevention to declare in 2012 that the problem was a “national epidemic.”3 The Washington State Medical Quality Assurance Commission now mandates extensive patient evaluation and documentation, the use of a Controlled Medication Agreement (CMA), and specific education requirements for physicians prescribing long-acting or high-dose opioids.4
Necessary adjustments going forward. As the nation moves toward more regulated prescribing of opioids, physicians will need to develop a consistent approach to this complicated task. Primary care doctors must be at the center of this effort, as they generate most opioid prescriptions for the treatment of CNCP. Currently, providers vary widely in their management of this condition,5-7 and recommended corrective steps include increased education8 and improved adherence to national guidelines. Our contention—and the basis of our study—was that a clinical protocol for opioid prescribing could improve the care that physicians and staff were providing to CNCP patients, as well as improve the satisfaction that clinicians felt in providing this care.
Our protocol intervention. Prior to our protocol intervention, no guidelines existed for managing patients on long-term opioid therapy in the clinical practices of the University of Pennsylvania Division of General Internal Medicine. Our providers, too, varied widely in their prescribing and management. Though regular urine drug screening is known to improve detection of opioid misuse and decrease the problem in patients treated for CNCP,9,10 a study reviewing opioid prescribing practices in our clinics from 2004 to 2007 showed that physicians ordered UDSs for only 8% of patients.11 Furthermore, only half of patients (49.8%) had regular office visits—even those at high risk for opioid misuse.11
|
Based on expert opinion and national best-practice guidelines, we created a division-wide quality improvement intervention for opioid prescribing. The protocol required standardized evaluation and documentation of a patient’s pain history and treatment plan, and the use of a UDS and a CMA, which is known to decrease emergency room visits and improve physician satisfaction, respectively.9,10 We trained attending physicians and staff on the protocol, and they in turn taught residents at their practice sites. The goal of this study was to determine whether this initiative would result in adherence to the protocol and improve provider and staff knowledge and satisfaction with management of patients prescribed opioids for CNCP.
METHODS
The intervention consisted of (1) the development of an EMR-based protocol to standardize documentation and management of patients with CNCP taking opioids; (2) instruction on using the protocol and on key components of opioid management; (3) collection of data; and (4) a monetary incentive for attending physicians to adhere to the protocol. We measured the impact of this intervention by assessing physician compliance with the protocol, provider satisfaction, and knowledge.
Protocol and process
We developed a division-wide protocol for managing primary-care patients with CNCP taking opioids, based on national guidelines, expert input, best practice data, and EMR capabilities (EpicCare Ambulatory Medical Record, version Summer 2009).
Health system experts from anesthesia, pain management, and psychiatry met regularly with our monthly workgroup to review the latest literature on UDSs and CMAs, and to assess best practices researched by the Center for Evidence Based Practice at our institution. We trained providers on the following steps:
• select patients who are taking opioids for CNCP (ie, receiving >2 opioid prescriptions in the 6 months prior to the intervention for a nonlimited pain condition)
• risk stratify these patients using the Opioid Risk Tool12
• follow high-risk patients monthly; low-to-moderate-risk patients every 3 to 6 months
• use a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the EMR problem list
• complete a standardized EMR “smart set” documenting evaluation and management in the overview section of the EMR’s chronic pain diagnosis module (TABLE 1)
• complete a CMA
• order a UDS at regular intervals (at least one per year; every 1-3 months in high-risk patients)
• designate one provider (in the EMR) to be responsible for opioid prescribing. Medical residents were encouraged to specify a “Continuity Attending” to maintain continuity of care when they were not in clinic.
Educational intervention
The principal investigator conducted 4 training sessions that were available to all attending physicians and staff, to review the protocol as well as information on best practices in opioid prescribing. One session was a Quality Improvement Grand Rounds for the division, and 3 sessions were open presentations within each participating practice. During all sessions, we taught the protocol, provided instruction on riskstratifying patients, reviewed the definition and prevalence of chronic pain, described the national opioid problem, detailed the components of proper documentation, and explained how to interpret and manage UDS results.
We trained categorical internal medicine interns for 1 hour during their mandatory clinical lecture series. Primary care track residents received 4 hours of training as part of their regular educational program.
Ongoing education for attending physicians occurred at 4 bimonthly opioid management case conferences, where difficult cases were presented to a rotating panel of experts from pain medicine, addiction psychiatry, and primary care. We held regular noon conferences on opioid management for residents.
Monetary incentive for physicians
Our division further aided our efforts by offering a monetary incentive ($1500) to attending physicians who achieved all 3 of the following measures of adherence with at least 80% of their chronic pain patients: at least one UDS in the past year, an office visit at least every 6 months, and a chronic pain diagnosis on the problem list in the EMR.
Data feedback
We gave providers a list of their patients receiving >2 opioid prescriptions over 6 months, and were able to exclude those patients treated for a limited pain condition. For the remainder of patients, physicians received quarterly individual reports on their adherence to the protocol.
Study population
Three internal medicine clinical practices of the University of Pennsylvania in Philadelphia took part in this initiative. We included all attending providers at these practices in the analysis assessing adherence to the protocol. Those who consented and completed a survey were included in the survey analysis. Providers were attending physicians and nurse practitioners. In Practice 1, primary care track residents are fully integrated into the practice and were included in the survey as their extended training was timed with our intervention. We did not survey residents at the other practices due to their variable schedules and inability to train as a group.
Staff included registered nurses, licensed practical nurses, medical assistants, and patient service representatives. Because nurses and medical assistants are responsible for medication refills, they received education specifically about this intervention. The remaining staff also received instruction, as they have personal interactions with patients at the provider visit, and thus their attitudes were important to measure. Participants completed surveys at the time of the educational sessions and again 9 months following implementation of the intervention. This was a one-year intervention, with 3 initial months of teaching; the study period therefore lasted 9 months. Since surveys were anonymous, we could not link results to specific individuals. However, we provided post-intervention surveys only to those who reported completing the initial survey.
Survey design and administration
The provider survey contained an attitude component and a knowledge component (TABLE 2). The attitude component consisted of 6 items taken from the Drug Problems Perceptions Questionnaire,13 to address role adequacy, support, and self-esteem, as well as job satisfaction (the words “drug users” were replaced with “patients on [chronic] opioids”). We created an additional 3 items to further explore these domains (items 1-3). Three additional items addressed provider access to EMR specific tools (items 10-12).
The knowledge survey consisted of multiple choice questions created by the study team, and it reflected best practice guidelines for opioid management for CNCP and knowledge of protocol elements. Items included the definition of chronic pain, opioid medications not included on the UDS, interpretation of UDS results, addiction risk, intervals for office visits for patients on chronic opioid therapy, and pain medication dose escalation.
The staff survey included similar attitude components and a modified knowledge portion regarding which patients should have a CMA, where to document a CMA in the EMR, addiction risk, intervals for office visits, and how to handle early prescription refill requests.
Evaluation and statistical analysis
To assess the impact of the intervention, we chose 2 measures of physician adherence with the protocol (UDS and chronic pain diagnosis) because of our ability to access these measures within our approved protocol.
Individual attitude survey questions were compared using paired t-tests. We averaged knowledge test scores, and also used the paired t-test to compare pre- and posttest averages. We used Stata 11.2 (StataCorp LP, 2009) to analyze survey data.
This study was sponsored by the Matthew Slap Research Award and approved by the University of Pennsylvania Institutional Review Board.
RESULTS
Practice demographics
The 3 practices are located within the same zip code, a few city blocks from one another. Despite geographic proximity of the practices, their populations differ racially and ethnically as well as in neighborhood income distributions (TABLE 3). In all 3 practices, the total number of patients prescribed >2 opioid medications declined during the year-long study period. Practice 3 had the sharpest decline in the number of patients prescribed chronic opioids, likely due to provider turnover during the study period. Practices 1 and 2 had the highest adherence to guidelines. The marked variability in adoption of guidelines likely reflects a number of factors: the difference in baseline opioid prescribing (highest in Practice 3), the presence of physician champions in Practices 1 and 2, and more intensive training of the primary care residents in Practice 1.
Protocol adherence
We measured provider adherence to the protocol by comparing data from the year before the intervention to the year following the start of the intervention for the number of UDSs ordered, the number of chronic pain diagnoses on patients’ EMR problem lists, and the number of office visits with CNCP patients. UDSs ordered increased by 145% across all 3 practices, with the largest improvement seen in Practice 1 (430%; P<.05). Documentation of a chronic pain diagnosis in the EMR problem list increased by 424% across practices, with the largest improvement seen again in Practice 1 (918%, P<.05) (TABLE 4). Based on this performance, 24 of 27 (89%) full time physicians qualified for the financial incentive. We chose not to include the third measure (number of office visits) for analysis, as we discovered that >90% of patients were seen at least every 6 months before the intervention.
Survey results
Before the protocol training, we surveyed 26 providers and 33 staff members. Nine months after the initiation of the protocol, 25 providers and 26 staff were again surveyed. Surveys were anonymous so we were unable to link knowledge gains to individuals.
Providers exhibited statistically significant improvement of attitude for role adequacy (item 5), role support (item 6), job satisfaction/role-related self-esteem (item 9), and access to EMR-specific tools (items 10-12) (TABLE 2). In addition, the knowledge test score increased by 15% (P<.05) in the postintervention survey.
Staff surveys showed statistically significant improvement of attitude for job satisfaction/role-related self-esteem. There was no improvement in knowledge for staff, which is likely due to variability in training.
DISCUSSION
More than 40% of opioid prescriptions in the United States are written by primary care physicians.14 Therefore, interventions that enhance provider knowledge, institute best practices, and support role-related self-esteem in opioid management are vital to our profession.
Through a straightforward protocol, we greatly increased the number of UDSs ordered (145%) and documentation of chronic pain on the problem list (424%). By increasing adherence to best practice standards, we believe this protocol will lead to improved management of patients with CNCP by providing objective urine data to guide a treatment plan, patient education with the CMA, and a documented evaluation and care plan.
In addition to fostering adherence to the protocol, our multicomponent intervention resulted in marked improvement of provider and staff attitudes toward patients taking opioids for CNCP (TABLE 2). Participants’ satisfaction in working with these patients improved significantly (27%), as did their confidence in knowing whom to ask for help with management (43%). After this intervention, physicians reported a nonstatistically significant but large reductions in the perception that patients on opioids create stress for the office (-20%), and that patients on opioids make their job harder (-18%). Knowledge about chronic opioid prescribing also improved significantly for providers (15%).
At all practices, the number of patients receiving opioids decreased, likely due to the protocol intervention.
Previous studies have shown low adoption of best practices in opioid management without a structured intervention.10 Our findings suggest that a multicomponent quality improvement intervention that combines education, financial incentive, and a structured protocol can positively impact provider and staff attitudes and adherence to best practices in caring for patients with CNCP taking opioid medications. We believe that similar interventions could be adapted by other primary care clinics with a comparable favorable impact on physician behavior, attitudes, and knowledge.
Limitations
Our findings may not apply to nonacademic practices, as we required training and the use of an EMR. Additionally, our urban patient populations may not be generalizable to rural, suburban, or other populations in the management of patients taking prescription opioids. Further, the monetary incentive, which was included in a yearly incentive package at our institution, may not be feasible at other sites.
We did not design this study to allow for practice-level comparisons or to assess patient level variables. Analysis of patient data on safety, aberrant behavior, abnormal UDS results, and the impact of the intervention on these outcomes was outside of the scope of this study. We were unable to determine whether physician turnover, particularly high in one practice, could be linked to the results.
Providers often neglected to indicate their level of training on surveys, and we were therefore unable to compare adherence and knowledge between residents and attending physicians. Additionally, we lacked approval to search individual charts to completely investigate the components of our protocol (for example, completion of a CMA or UDS). Lastly, we did not design the study to control for confounders on a provider level (such as age, gender, and years of experience). A more comprehensive review of these important variables is warranted to assess the degree to which division- or practice-level quality improvement interventions can affect provider and patient behavior change and enhance patient safety.
CORRESPONDENCE
Robin E. Canada, MD, Medical Arts Building, Suite 102, 38th and Market Sts, Philadelphia, PA 19104; robin.canada@uphs.upenn.edu
Acknowledgement
The authors gratefully acknowledge Judy Shea, PhD and Joanna Starrels, MD, who provided valuable comments in the development of this manuscript.
1. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
2. Hughes A, Sathe N, Spagnola K. (2009). State estimates of substance use from the 2006-2007 National Surveys on Drug Use and Health. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2009. NSDUH Series H-35, HHS Publication No. SMA 09-4362.
3. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses - a US epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10-13.
4. Washington State Department of Health- Medical Quality Assurance Commission. Rule-Making Order CR-103. University of Washington Web site. Available at: http://depts.washington.edu/anesth/education/forms/pain/WAC-Rules-CR-103P.pdf. Accessed February 1, 2012.
5. Leverence RR, Williams RL, Potter M, et al. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
6. Green CR, Wheeler JR, LaPorte F, et al. How well is chronic pain managed? Who does it well? Pain Med. 2002;3:56-65.
7. Webster BS, Cifuentes M, Verma S, et al. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: a multilevel analysis. Am J Ind Med. 2009;52:162-171.
8. Gunderson EW, Coffin PO, Chang N, et al. The interface between substance abuse and chronic pain management in primary care: a curriculum for medical residents. Subst Abus. 2009;30:253-260.
9. Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician. 2006;9:123-129.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
11. Starrels JL, Becker WC, Weiner MG, et al. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958-964.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid- treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
13. Watson H, Maclaren W, Kerr S. Staff attitudes toward working with drug users: development of the Drug Problems Perceptions Questionnaire. Addiction. 2007;102:206-215.
14. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981-1985.
ABSTRACT
Purpose Primary care physicians are at the center of a national prescription opioid epidemic, with little training or knowledge about the management of patients on opioids for chronic noncancer pain (CNCP). We developed an electronic medical record (EMR)-based protocol and educational intervention to standardize documentation and management of patients prescribed opioids by primary care providers. Our objective was to evaluate provider adherence to this protocol, attitudes toward the management of these patients, and knowledge of opioid prescribing.
Methods We trained providers and select staff from 3 primary care practices at the Division of General Internal Medicine at the University of Pennsylvania in the use of a protocol for managing patients taking opioids for CNCP. The following served as measures of protocol adherence: 1) the provider used a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the problem list, 2) the provider ordered at least one urine drug screen (UDS) for the patient in the past year, and 3) the patient came in for at least one office visit every 6 months. We assessed physician and staff attitudes and knowledge with pre- and post-intervention surveys. Adherence to the protocol was linked to a monetary incentive.
Results Provider adherence to the protocol significantly improved measured outcomes. The number of UDSs ordered increased by 145%, and the diagnosis of chronic pain on the problem list increased by 424%. There was a statistically significant improvement in providers’ role adequacy, role support, and job satisfaction/role-related self-esteem when working with patients taking opioids. In addition, provider knowledge of proper management of these patients improved significantly. Eighty-nine percent of our physicians attained the monetary incentive.
Conclusions We developed a quality improvement intervention that addressed the need for better regulation of opioid prescribing, resulted in increased adherence to best-practice guidelines, and improved provider knowledge and attitudes.
Primary care physicians often express dissatisfaction with their competency in treating patients with opioids,1 and at our institution, this includes residents and faculty, as well. Their concern, combined with apprehension about patient safety and the potential for addiction, can hinder appropriate opioid management.1 We asked: Could a protocol that structures the intervention improve physician competence and performance in prescribing opioids and reduce patient risk?
Physician concerns are well-founded. Nonmedical use of prescription opioids is second only to smoking marijuana in the illicit use of drugs in the United States.2 Since 2003, more overdose deaths have involved opioid analgesics than heroin and cocaine combined, leading the Centers for Disease Control and Prevention to declare in 2012 that the problem was a “national epidemic.”3 The Washington State Medical Quality Assurance Commission now mandates extensive patient evaluation and documentation, the use of a Controlled Medication Agreement (CMA), and specific education requirements for physicians prescribing long-acting or high-dose opioids.4
Necessary adjustments going forward. As the nation moves toward more regulated prescribing of opioids, physicians will need to develop a consistent approach to this complicated task. Primary care doctors must be at the center of this effort, as they generate most opioid prescriptions for the treatment of CNCP. Currently, providers vary widely in their management of this condition,5-7 and recommended corrective steps include increased education8 and improved adherence to national guidelines. Our contention—and the basis of our study—was that a clinical protocol for opioid prescribing could improve the care that physicians and staff were providing to CNCP patients, as well as improve the satisfaction that clinicians felt in providing this care.
Our protocol intervention. Prior to our protocol intervention, no guidelines existed for managing patients on long-term opioid therapy in the clinical practices of the University of Pennsylvania Division of General Internal Medicine. Our providers, too, varied widely in their prescribing and management. Though regular urine drug screening is known to improve detection of opioid misuse and decrease the problem in patients treated for CNCP,9,10 a study reviewing opioid prescribing practices in our clinics from 2004 to 2007 showed that physicians ordered UDSs for only 8% of patients.11 Furthermore, only half of patients (49.8%) had regular office visits—even those at high risk for opioid misuse.11
|
Based on expert opinion and national best-practice guidelines, we created a division-wide quality improvement intervention for opioid prescribing. The protocol required standardized evaluation and documentation of a patient’s pain history and treatment plan, and the use of a UDS and a CMA, which is known to decrease emergency room visits and improve physician satisfaction, respectively.9,10 We trained attending physicians and staff on the protocol, and they in turn taught residents at their practice sites. The goal of this study was to determine whether this initiative would result in adherence to the protocol and improve provider and staff knowledge and satisfaction with management of patients prescribed opioids for CNCP.
METHODS
The intervention consisted of (1) the development of an EMR-based protocol to standardize documentation and management of patients with CNCP taking opioids; (2) instruction on using the protocol and on key components of opioid management; (3) collection of data; and (4) a monetary incentive for attending physicians to adhere to the protocol. We measured the impact of this intervention by assessing physician compliance with the protocol, provider satisfaction, and knowledge.
Protocol and process
We developed a division-wide protocol for managing primary-care patients with CNCP taking opioids, based on national guidelines, expert input, best practice data, and EMR capabilities (EpicCare Ambulatory Medical Record, version Summer 2009).
Health system experts from anesthesia, pain management, and psychiatry met regularly with our monthly workgroup to review the latest literature on UDSs and CMAs, and to assess best practices researched by the Center for Evidence Based Practice at our institution. We trained providers on the following steps:
• select patients who are taking opioids for CNCP (ie, receiving >2 opioid prescriptions in the 6 months prior to the intervention for a nonlimited pain condition)
• risk stratify these patients using the Opioid Risk Tool12
• follow high-risk patients monthly; low-to-moderate-risk patients every 3 to 6 months
• use a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the EMR problem list
• complete a standardized EMR “smart set” documenting evaluation and management in the overview section of the EMR’s chronic pain diagnosis module (TABLE 1)
• complete a CMA
• order a UDS at regular intervals (at least one per year; every 1-3 months in high-risk patients)
• designate one provider (in the EMR) to be responsible for opioid prescribing. Medical residents were encouraged to specify a “Continuity Attending” to maintain continuity of care when they were not in clinic.
Educational intervention
The principal investigator conducted 4 training sessions that were available to all attending physicians and staff, to review the protocol as well as information on best practices in opioid prescribing. One session was a Quality Improvement Grand Rounds for the division, and 3 sessions were open presentations within each participating practice. During all sessions, we taught the protocol, provided instruction on riskstratifying patients, reviewed the definition and prevalence of chronic pain, described the national opioid problem, detailed the components of proper documentation, and explained how to interpret and manage UDS results.
We trained categorical internal medicine interns for 1 hour during their mandatory clinical lecture series. Primary care track residents received 4 hours of training as part of their regular educational program.
Ongoing education for attending physicians occurred at 4 bimonthly opioid management case conferences, where difficult cases were presented to a rotating panel of experts from pain medicine, addiction psychiatry, and primary care. We held regular noon conferences on opioid management for residents.
Monetary incentive for physicians
Our division further aided our efforts by offering a monetary incentive ($1500) to attending physicians who achieved all 3 of the following measures of adherence with at least 80% of their chronic pain patients: at least one UDS in the past year, an office visit at least every 6 months, and a chronic pain diagnosis on the problem list in the EMR.
Data feedback
We gave providers a list of their patients receiving >2 opioid prescriptions over 6 months, and were able to exclude those patients treated for a limited pain condition. For the remainder of patients, physicians received quarterly individual reports on their adherence to the protocol.
Study population
Three internal medicine clinical practices of the University of Pennsylvania in Philadelphia took part in this initiative. We included all attending providers at these practices in the analysis assessing adherence to the protocol. Those who consented and completed a survey were included in the survey analysis. Providers were attending physicians and nurse practitioners. In Practice 1, primary care track residents are fully integrated into the practice and were included in the survey as their extended training was timed with our intervention. We did not survey residents at the other practices due to their variable schedules and inability to train as a group.
Staff included registered nurses, licensed practical nurses, medical assistants, and patient service representatives. Because nurses and medical assistants are responsible for medication refills, they received education specifically about this intervention. The remaining staff also received instruction, as they have personal interactions with patients at the provider visit, and thus their attitudes were important to measure. Participants completed surveys at the time of the educational sessions and again 9 months following implementation of the intervention. This was a one-year intervention, with 3 initial months of teaching; the study period therefore lasted 9 months. Since surveys were anonymous, we could not link results to specific individuals. However, we provided post-intervention surveys only to those who reported completing the initial survey.
Survey design and administration
The provider survey contained an attitude component and a knowledge component (TABLE 2). The attitude component consisted of 6 items taken from the Drug Problems Perceptions Questionnaire,13 to address role adequacy, support, and self-esteem, as well as job satisfaction (the words “drug users” were replaced with “patients on [chronic] opioids”). We created an additional 3 items to further explore these domains (items 1-3). Three additional items addressed provider access to EMR specific tools (items 10-12).
The knowledge survey consisted of multiple choice questions created by the study team, and it reflected best practice guidelines for opioid management for CNCP and knowledge of protocol elements. Items included the definition of chronic pain, opioid medications not included on the UDS, interpretation of UDS results, addiction risk, intervals for office visits for patients on chronic opioid therapy, and pain medication dose escalation.
The staff survey included similar attitude components and a modified knowledge portion regarding which patients should have a CMA, where to document a CMA in the EMR, addiction risk, intervals for office visits, and how to handle early prescription refill requests.
Evaluation and statistical analysis
To assess the impact of the intervention, we chose 2 measures of physician adherence with the protocol (UDS and chronic pain diagnosis) because of our ability to access these measures within our approved protocol.
Individual attitude survey questions were compared using paired t-tests. We averaged knowledge test scores, and also used the paired t-test to compare pre- and posttest averages. We used Stata 11.2 (StataCorp LP, 2009) to analyze survey data.
This study was sponsored by the Matthew Slap Research Award and approved by the University of Pennsylvania Institutional Review Board.
RESULTS
Practice demographics
The 3 practices are located within the same zip code, a few city blocks from one another. Despite geographic proximity of the practices, their populations differ racially and ethnically as well as in neighborhood income distributions (TABLE 3). In all 3 practices, the total number of patients prescribed >2 opioid medications declined during the year-long study period. Practice 3 had the sharpest decline in the number of patients prescribed chronic opioids, likely due to provider turnover during the study period. Practices 1 and 2 had the highest adherence to guidelines. The marked variability in adoption of guidelines likely reflects a number of factors: the difference in baseline opioid prescribing (highest in Practice 3), the presence of physician champions in Practices 1 and 2, and more intensive training of the primary care residents in Practice 1.
Protocol adherence
We measured provider adherence to the protocol by comparing data from the year before the intervention to the year following the start of the intervention for the number of UDSs ordered, the number of chronic pain diagnoses on patients’ EMR problem lists, and the number of office visits with CNCP patients. UDSs ordered increased by 145% across all 3 practices, with the largest improvement seen in Practice 1 (430%; P<.05). Documentation of a chronic pain diagnosis in the EMR problem list increased by 424% across practices, with the largest improvement seen again in Practice 1 (918%, P<.05) (TABLE 4). Based on this performance, 24 of 27 (89%) full time physicians qualified for the financial incentive. We chose not to include the third measure (number of office visits) for analysis, as we discovered that >90% of patients were seen at least every 6 months before the intervention.
Survey results
Before the protocol training, we surveyed 26 providers and 33 staff members. Nine months after the initiation of the protocol, 25 providers and 26 staff were again surveyed. Surveys were anonymous so we were unable to link knowledge gains to individuals.
Providers exhibited statistically significant improvement of attitude for role adequacy (item 5), role support (item 6), job satisfaction/role-related self-esteem (item 9), and access to EMR-specific tools (items 10-12) (TABLE 2). In addition, the knowledge test score increased by 15% (P<.05) in the postintervention survey.
Staff surveys showed statistically significant improvement of attitude for job satisfaction/role-related self-esteem. There was no improvement in knowledge for staff, which is likely due to variability in training.
DISCUSSION
More than 40% of opioid prescriptions in the United States are written by primary care physicians.14 Therefore, interventions that enhance provider knowledge, institute best practices, and support role-related self-esteem in opioid management are vital to our profession.
Through a straightforward protocol, we greatly increased the number of UDSs ordered (145%) and documentation of chronic pain on the problem list (424%). By increasing adherence to best practice standards, we believe this protocol will lead to improved management of patients with CNCP by providing objective urine data to guide a treatment plan, patient education with the CMA, and a documented evaluation and care plan.
In addition to fostering adherence to the protocol, our multicomponent intervention resulted in marked improvement of provider and staff attitudes toward patients taking opioids for CNCP (TABLE 2). Participants’ satisfaction in working with these patients improved significantly (27%), as did their confidence in knowing whom to ask for help with management (43%). After this intervention, physicians reported a nonstatistically significant but large reductions in the perception that patients on opioids create stress for the office (-20%), and that patients on opioids make their job harder (-18%). Knowledge about chronic opioid prescribing also improved significantly for providers (15%).
At all practices, the number of patients receiving opioids decreased, likely due to the protocol intervention.
Previous studies have shown low adoption of best practices in opioid management without a structured intervention.10 Our findings suggest that a multicomponent quality improvement intervention that combines education, financial incentive, and a structured protocol can positively impact provider and staff attitudes and adherence to best practices in caring for patients with CNCP taking opioid medications. We believe that similar interventions could be adapted by other primary care clinics with a comparable favorable impact on physician behavior, attitudes, and knowledge.
Limitations
Our findings may not apply to nonacademic practices, as we required training and the use of an EMR. Additionally, our urban patient populations may not be generalizable to rural, suburban, or other populations in the management of patients taking prescription opioids. Further, the monetary incentive, which was included in a yearly incentive package at our institution, may not be feasible at other sites.
We did not design this study to allow for practice-level comparisons or to assess patient level variables. Analysis of patient data on safety, aberrant behavior, abnormal UDS results, and the impact of the intervention on these outcomes was outside of the scope of this study. We were unable to determine whether physician turnover, particularly high in one practice, could be linked to the results.
Providers often neglected to indicate their level of training on surveys, and we were therefore unable to compare adherence and knowledge between residents and attending physicians. Additionally, we lacked approval to search individual charts to completely investigate the components of our protocol (for example, completion of a CMA or UDS). Lastly, we did not design the study to control for confounders on a provider level (such as age, gender, and years of experience). A more comprehensive review of these important variables is warranted to assess the degree to which division- or practice-level quality improvement interventions can affect provider and patient behavior change and enhance patient safety.
CORRESPONDENCE
Robin E. Canada, MD, Medical Arts Building, Suite 102, 38th and Market Sts, Philadelphia, PA 19104; robin.canada@uphs.upenn.edu
Acknowledgement
The authors gratefully acknowledge Judy Shea, PhD and Joanna Starrels, MD, who provided valuable comments in the development of this manuscript.
ABSTRACT
Purpose Primary care physicians are at the center of a national prescription opioid epidemic, with little training or knowledge about the management of patients on opioids for chronic noncancer pain (CNCP). We developed an electronic medical record (EMR)-based protocol and educational intervention to standardize documentation and management of patients prescribed opioids by primary care providers. Our objective was to evaluate provider adherence to this protocol, attitudes toward the management of these patients, and knowledge of opioid prescribing.
Methods We trained providers and select staff from 3 primary care practices at the Division of General Internal Medicine at the University of Pennsylvania in the use of a protocol for managing patients taking opioids for CNCP. The following served as measures of protocol adherence: 1) the provider used a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the problem list, 2) the provider ordered at least one urine drug screen (UDS) for the patient in the past year, and 3) the patient came in for at least one office visit every 6 months. We assessed physician and staff attitudes and knowledge with pre- and post-intervention surveys. Adherence to the protocol was linked to a monetary incentive.
Results Provider adherence to the protocol significantly improved measured outcomes. The number of UDSs ordered increased by 145%, and the diagnosis of chronic pain on the problem list increased by 424%. There was a statistically significant improvement in providers’ role adequacy, role support, and job satisfaction/role-related self-esteem when working with patients taking opioids. In addition, provider knowledge of proper management of these patients improved significantly. Eighty-nine percent of our physicians attained the monetary incentive.
Conclusions We developed a quality improvement intervention that addressed the need for better regulation of opioid prescribing, resulted in increased adherence to best-practice guidelines, and improved provider knowledge and attitudes.
Primary care physicians often express dissatisfaction with their competency in treating patients with opioids,1 and at our institution, this includes residents and faculty, as well. Their concern, combined with apprehension about patient safety and the potential for addiction, can hinder appropriate opioid management.1 We asked: Could a protocol that structures the intervention improve physician competence and performance in prescribing opioids and reduce patient risk?
Physician concerns are well-founded. Nonmedical use of prescription opioids is second only to smoking marijuana in the illicit use of drugs in the United States.2 Since 2003, more overdose deaths have involved opioid analgesics than heroin and cocaine combined, leading the Centers for Disease Control and Prevention to declare in 2012 that the problem was a “national epidemic.”3 The Washington State Medical Quality Assurance Commission now mandates extensive patient evaluation and documentation, the use of a Controlled Medication Agreement (CMA), and specific education requirements for physicians prescribing long-acting or high-dose opioids.4
Necessary adjustments going forward. As the nation moves toward more regulated prescribing of opioids, physicians will need to develop a consistent approach to this complicated task. Primary care doctors must be at the center of this effort, as they generate most opioid prescriptions for the treatment of CNCP. Currently, providers vary widely in their management of this condition,5-7 and recommended corrective steps include increased education8 and improved adherence to national guidelines. Our contention—and the basis of our study—was that a clinical protocol for opioid prescribing could improve the care that physicians and staff were providing to CNCP patients, as well as improve the satisfaction that clinicians felt in providing this care.
Our protocol intervention. Prior to our protocol intervention, no guidelines existed for managing patients on long-term opioid therapy in the clinical practices of the University of Pennsylvania Division of General Internal Medicine. Our providers, too, varied widely in their prescribing and management. Though regular urine drug screening is known to improve detection of opioid misuse and decrease the problem in patients treated for CNCP,9,10 a study reviewing opioid prescribing practices in our clinics from 2004 to 2007 showed that physicians ordered UDSs for only 8% of patients.11 Furthermore, only half of patients (49.8%) had regular office visits—even those at high risk for opioid misuse.11
|
Based on expert opinion and national best-practice guidelines, we created a division-wide quality improvement intervention for opioid prescribing. The protocol required standardized evaluation and documentation of a patient’s pain history and treatment plan, and the use of a UDS and a CMA, which is known to decrease emergency room visits and improve physician satisfaction, respectively.9,10 We trained attending physicians and staff on the protocol, and they in turn taught residents at their practice sites. The goal of this study was to determine whether this initiative would result in adherence to the protocol and improve provider and staff knowledge and satisfaction with management of patients prescribed opioids for CNCP.
METHODS
The intervention consisted of (1) the development of an EMR-based protocol to standardize documentation and management of patients with CNCP taking opioids; (2) instruction on using the protocol and on key components of opioid management; (3) collection of data; and (4) a monetary incentive for attending physicians to adhere to the protocol. We measured the impact of this intervention by assessing physician compliance with the protocol, provider satisfaction, and knowledge.
Protocol and process
We developed a division-wide protocol for managing primary-care patients with CNCP taking opioids, based on national guidelines, expert input, best practice data, and EMR capabilities (EpicCare Ambulatory Medical Record, version Summer 2009).
Health system experts from anesthesia, pain management, and psychiatry met regularly with our monthly workgroup to review the latest literature on UDSs and CMAs, and to assess best practices researched by the Center for Evidence Based Practice at our institution. We trained providers on the following steps:
• select patients who are taking opioids for CNCP (ie, receiving >2 opioid prescriptions in the 6 months prior to the intervention for a nonlimited pain condition)
• risk stratify these patients using the Opioid Risk Tool12
• follow high-risk patients monthly; low-to-moderate-risk patients every 3 to 6 months
• use a standard diagnosis (chronic pain, ICD-9 code 338.29A) in the EMR problem list
• complete a standardized EMR “smart set” documenting evaluation and management in the overview section of the EMR’s chronic pain diagnosis module (TABLE 1)
• complete a CMA
• order a UDS at regular intervals (at least one per year; every 1-3 months in high-risk patients)
• designate one provider (in the EMR) to be responsible for opioid prescribing. Medical residents were encouraged to specify a “Continuity Attending” to maintain continuity of care when they were not in clinic.
Educational intervention
The principal investigator conducted 4 training sessions that were available to all attending physicians and staff, to review the protocol as well as information on best practices in opioid prescribing. One session was a Quality Improvement Grand Rounds for the division, and 3 sessions were open presentations within each participating practice. During all sessions, we taught the protocol, provided instruction on riskstratifying patients, reviewed the definition and prevalence of chronic pain, described the national opioid problem, detailed the components of proper documentation, and explained how to interpret and manage UDS results.
We trained categorical internal medicine interns for 1 hour during their mandatory clinical lecture series. Primary care track residents received 4 hours of training as part of their regular educational program.
Ongoing education for attending physicians occurred at 4 bimonthly opioid management case conferences, where difficult cases were presented to a rotating panel of experts from pain medicine, addiction psychiatry, and primary care. We held regular noon conferences on opioid management for residents.
Monetary incentive for physicians
Our division further aided our efforts by offering a monetary incentive ($1500) to attending physicians who achieved all 3 of the following measures of adherence with at least 80% of their chronic pain patients: at least one UDS in the past year, an office visit at least every 6 months, and a chronic pain diagnosis on the problem list in the EMR.
Data feedback
We gave providers a list of their patients receiving >2 opioid prescriptions over 6 months, and were able to exclude those patients treated for a limited pain condition. For the remainder of patients, physicians received quarterly individual reports on their adherence to the protocol.
Study population
Three internal medicine clinical practices of the University of Pennsylvania in Philadelphia took part in this initiative. We included all attending providers at these practices in the analysis assessing adherence to the protocol. Those who consented and completed a survey were included in the survey analysis. Providers were attending physicians and nurse practitioners. In Practice 1, primary care track residents are fully integrated into the practice and were included in the survey as their extended training was timed with our intervention. We did not survey residents at the other practices due to their variable schedules and inability to train as a group.
Staff included registered nurses, licensed practical nurses, medical assistants, and patient service representatives. Because nurses and medical assistants are responsible for medication refills, they received education specifically about this intervention. The remaining staff also received instruction, as they have personal interactions with patients at the provider visit, and thus their attitudes were important to measure. Participants completed surveys at the time of the educational sessions and again 9 months following implementation of the intervention. This was a one-year intervention, with 3 initial months of teaching; the study period therefore lasted 9 months. Since surveys were anonymous, we could not link results to specific individuals. However, we provided post-intervention surveys only to those who reported completing the initial survey.
Survey design and administration
The provider survey contained an attitude component and a knowledge component (TABLE 2). The attitude component consisted of 6 items taken from the Drug Problems Perceptions Questionnaire,13 to address role adequacy, support, and self-esteem, as well as job satisfaction (the words “drug users” were replaced with “patients on [chronic] opioids”). We created an additional 3 items to further explore these domains (items 1-3). Three additional items addressed provider access to EMR specific tools (items 10-12).
The knowledge survey consisted of multiple choice questions created by the study team, and it reflected best practice guidelines for opioid management for CNCP and knowledge of protocol elements. Items included the definition of chronic pain, opioid medications not included on the UDS, interpretation of UDS results, addiction risk, intervals for office visits for patients on chronic opioid therapy, and pain medication dose escalation.
The staff survey included similar attitude components and a modified knowledge portion regarding which patients should have a CMA, where to document a CMA in the EMR, addiction risk, intervals for office visits, and how to handle early prescription refill requests.
Evaluation and statistical analysis
To assess the impact of the intervention, we chose 2 measures of physician adherence with the protocol (UDS and chronic pain diagnosis) because of our ability to access these measures within our approved protocol.
Individual attitude survey questions were compared using paired t-tests. We averaged knowledge test scores, and also used the paired t-test to compare pre- and posttest averages. We used Stata 11.2 (StataCorp LP, 2009) to analyze survey data.
This study was sponsored by the Matthew Slap Research Award and approved by the University of Pennsylvania Institutional Review Board.
RESULTS
Practice demographics
The 3 practices are located within the same zip code, a few city blocks from one another. Despite geographic proximity of the practices, their populations differ racially and ethnically as well as in neighborhood income distributions (TABLE 3). In all 3 practices, the total number of patients prescribed >2 opioid medications declined during the year-long study period. Practice 3 had the sharpest decline in the number of patients prescribed chronic opioids, likely due to provider turnover during the study period. Practices 1 and 2 had the highest adherence to guidelines. The marked variability in adoption of guidelines likely reflects a number of factors: the difference in baseline opioid prescribing (highest in Practice 3), the presence of physician champions in Practices 1 and 2, and more intensive training of the primary care residents in Practice 1.
Protocol adherence
We measured provider adherence to the protocol by comparing data from the year before the intervention to the year following the start of the intervention for the number of UDSs ordered, the number of chronic pain diagnoses on patients’ EMR problem lists, and the number of office visits with CNCP patients. UDSs ordered increased by 145% across all 3 practices, with the largest improvement seen in Practice 1 (430%; P<.05). Documentation of a chronic pain diagnosis in the EMR problem list increased by 424% across practices, with the largest improvement seen again in Practice 1 (918%, P<.05) (TABLE 4). Based on this performance, 24 of 27 (89%) full time physicians qualified for the financial incentive. We chose not to include the third measure (number of office visits) for analysis, as we discovered that >90% of patients were seen at least every 6 months before the intervention.
Survey results
Before the protocol training, we surveyed 26 providers and 33 staff members. Nine months after the initiation of the protocol, 25 providers and 26 staff were again surveyed. Surveys were anonymous so we were unable to link knowledge gains to individuals.
Providers exhibited statistically significant improvement of attitude for role adequacy (item 5), role support (item 6), job satisfaction/role-related self-esteem (item 9), and access to EMR-specific tools (items 10-12) (TABLE 2). In addition, the knowledge test score increased by 15% (P<.05) in the postintervention survey.
Staff surveys showed statistically significant improvement of attitude for job satisfaction/role-related self-esteem. There was no improvement in knowledge for staff, which is likely due to variability in training.
DISCUSSION
More than 40% of opioid prescriptions in the United States are written by primary care physicians.14 Therefore, interventions that enhance provider knowledge, institute best practices, and support role-related self-esteem in opioid management are vital to our profession.
Through a straightforward protocol, we greatly increased the number of UDSs ordered (145%) and documentation of chronic pain on the problem list (424%). By increasing adherence to best practice standards, we believe this protocol will lead to improved management of patients with CNCP by providing objective urine data to guide a treatment plan, patient education with the CMA, and a documented evaluation and care plan.
In addition to fostering adherence to the protocol, our multicomponent intervention resulted in marked improvement of provider and staff attitudes toward patients taking opioids for CNCP (TABLE 2). Participants’ satisfaction in working with these patients improved significantly (27%), as did their confidence in knowing whom to ask for help with management (43%). After this intervention, physicians reported a nonstatistically significant but large reductions in the perception that patients on opioids create stress for the office (-20%), and that patients on opioids make their job harder (-18%). Knowledge about chronic opioid prescribing also improved significantly for providers (15%).
At all practices, the number of patients receiving opioids decreased, likely due to the protocol intervention.
Previous studies have shown low adoption of best practices in opioid management without a structured intervention.10 Our findings suggest that a multicomponent quality improvement intervention that combines education, financial incentive, and a structured protocol can positively impact provider and staff attitudes and adherence to best practices in caring for patients with CNCP taking opioid medications. We believe that similar interventions could be adapted by other primary care clinics with a comparable favorable impact on physician behavior, attitudes, and knowledge.
Limitations
Our findings may not apply to nonacademic practices, as we required training and the use of an EMR. Additionally, our urban patient populations may not be generalizable to rural, suburban, or other populations in the management of patients taking prescription opioids. Further, the monetary incentive, which was included in a yearly incentive package at our institution, may not be feasible at other sites.
We did not design this study to allow for practice-level comparisons or to assess patient level variables. Analysis of patient data on safety, aberrant behavior, abnormal UDS results, and the impact of the intervention on these outcomes was outside of the scope of this study. We were unable to determine whether physician turnover, particularly high in one practice, could be linked to the results.
Providers often neglected to indicate their level of training on surveys, and we were therefore unable to compare adherence and knowledge between residents and attending physicians. Additionally, we lacked approval to search individual charts to completely investigate the components of our protocol (for example, completion of a CMA or UDS). Lastly, we did not design the study to control for confounders on a provider level (such as age, gender, and years of experience). A more comprehensive review of these important variables is warranted to assess the degree to which division- or practice-level quality improvement interventions can affect provider and patient behavior change and enhance patient safety.
CORRESPONDENCE
Robin E. Canada, MD, Medical Arts Building, Suite 102, 38th and Market Sts, Philadelphia, PA 19104; robin.canada@uphs.upenn.edu
Acknowledgement
The authors gratefully acknowledge Judy Shea, PhD and Joanna Starrels, MD, who provided valuable comments in the development of this manuscript.
1. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
2. Hughes A, Sathe N, Spagnola K. (2009). State estimates of substance use from the 2006-2007 National Surveys on Drug Use and Health. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2009. NSDUH Series H-35, HHS Publication No. SMA 09-4362.
3. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses - a US epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10-13.
4. Washington State Department of Health- Medical Quality Assurance Commission. Rule-Making Order CR-103. University of Washington Web site. Available at: http://depts.washington.edu/anesth/education/forms/pain/WAC-Rules-CR-103P.pdf. Accessed February 1, 2012.
5. Leverence RR, Williams RL, Potter M, et al. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
6. Green CR, Wheeler JR, LaPorte F, et al. How well is chronic pain managed? Who does it well? Pain Med. 2002;3:56-65.
7. Webster BS, Cifuentes M, Verma S, et al. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: a multilevel analysis. Am J Ind Med. 2009;52:162-171.
8. Gunderson EW, Coffin PO, Chang N, et al. The interface between substance abuse and chronic pain management in primary care: a curriculum for medical residents. Subst Abus. 2009;30:253-260.
9. Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician. 2006;9:123-129.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
11. Starrels JL, Becker WC, Weiner MG, et al. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958-964.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid- treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
13. Watson H, Maclaren W, Kerr S. Staff attitudes toward working with drug users: development of the Drug Problems Perceptions Questionnaire. Addiction. 2007;102:206-215.
14. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981-1985.
1. Lin JJ, Alfandre D, Moore C. Physician attitudes toward opioid prescribing for patients with persistent noncancer pain. Clin J Pain. 2007;23:799-803.
2. Hughes A, Sathe N, Spagnola K. (2009). State estimates of substance use from the 2006-2007 National Surveys on Drug Use and Health. Rockville, MD: Office of Applied Studies, Substance Abuse and Mental Health Services Administration; 2009. NSDUH Series H-35, HHS Publication No. SMA 09-4362.
3. Centers for Disease Control and Prevention (CDC). CDC grand rounds: prescription drug overdoses - a US epidemic. MMWR Morb Mortal Wkly Rep. 2012;61:10-13.
4. Washington State Department of Health- Medical Quality Assurance Commission. Rule-Making Order CR-103. University of Washington Web site. Available at: http://depts.washington.edu/anesth/education/forms/pain/WAC-Rules-CR-103P.pdf. Accessed February 1, 2012.
5. Leverence RR, Williams RL, Potter M, et al. Chronic non-cancer pain: a siren for primary care—a report from the PRImary Care MultiEthnic Network (PRIME Net). J Am Board Fam Med. 2011;24:551-561.
6. Green CR, Wheeler JR, LaPorte F, et al. How well is chronic pain managed? Who does it well? Pain Med. 2002;3:56-65.
7. Webster BS, Cifuentes M, Verma S, et al. Geographic variation in opioid prescribing for acute, work-related, low back pain and associated factors: a multilevel analysis. Am J Ind Med. 2009;52:162-171.
8. Gunderson EW, Coffin PO, Chang N, et al. The interface between substance abuse and chronic pain management in primary care: a curriculum for medical residents. Subst Abus. 2009;30:253-260.
9. Manchikanti L, Manchukonda R, Pampati V, et al. Does random urine drug testing reduce illicit drug use in chronic pain patients receiving opioids? Pain Physician. 2006;9:123-129.
10. Starrels JL, Becker WC, Alford DP, et al. Systematic review: treatment agreements and urine drug testing to reduce opioid misuse in patients with chronic pain. Ann Intern Med. 2010;152:712-720.
11. Starrels JL, Becker WC, Weiner MG, et al. Low use of opioid risk reduction strategies in primary care even for high risk patients with chronic pain. J Gen Intern Med. 2011;26:958-964.
12. Webster LR, Webster RM. Predicting aberrant behaviors in opioid- treated patients: preliminary validation of the Opioid Risk Tool. Pain Med. 2005;6:432-442.
13. Watson H, Maclaren W, Kerr S. Staff attitudes toward working with drug users: development of the Drug Problems Perceptions Questionnaire. Addiction. 2007;102:206-215.
14. Okie S. A flood of opioids, a rising tide of deaths. N Engl J Med. 2010;363:1981-1985.
Teenager with shortness of breath and hypoxia
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
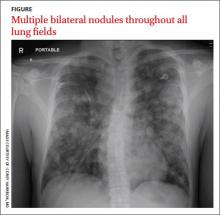
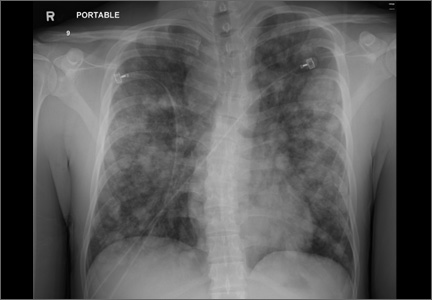
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
A 19-year-old male complaining of shortness of breath was transferred from our facility’s urgent care unit to our emergency department. He had a 2-week history of hemoptysis and vomiting, and over the previous week, he had developed mild hematemesis. His other symptoms included left thigh, flank, and upper quadrant pain; left chest pain exacerbated by exertion, light-headedness, and palpitations. He said that over the past 8 months, he’d been tired and lost some weight.
His blood pressure was 138/77 mm Hg, pulse was 142 beats per min, respiratory rate was 22 breaths per min, and oxygen saturation was 93% on room air. The physical exam revealed normal breath sounds and a diffusely tender abdomen. We ordered a chest X-ray (FIGURE).
WHAT IS YOUR DIAGNOSIS?
HOW WOULD YOU TREAT THIS PATIENT?
Diagnosis: Metastatic testicular cancer
The chest x-ray showed multiple bilateral discrete nodules throughout all of the lung fields. These findings, along with the age of the patient, prompted the radiologist to suspect metastatic testicular cancer. An examination of the patient’s scrotum revealed an 11-cm mass encompassing the patient’s left testicle. When asked about the mass, the patient acknowledged that it had been there for about 8 months.
A rare cancer seen in younger men
Although relatively uncommon, testicular cancer accounts for 1% to 2% of all tumors in men.1 If caught it is highly treatable.
Testicular cancer is classified into germ cell tumors (which our patient had) and sex cord-stromal tumors. Germ cell tumors are the most common malignancy in men ages 15 to 44 years, and have a 95% cure rate when identified early and promptly treated.2,3 Sex cordstromal tumors are more common in pediatric patients and are more often benign.2
Diagnosis usually is made clinically and pathologically at resection. Left untreated, testicular cancer spreads via the lymphatic system to the retroperitoneal lymph nodes and through the bloodstream to the lungs (predominantly),4 as well as to bone, the liver, and the brain. Metastatic testicular cancer to the lungs, liver, and retroperitoneum occurs in advanced disease and has a poor prognosis.4,5
Differential diagnosis includes pneumonia, septic emboli
The differential diagnosis includes atypical pneumonia, septic emboli (ie, endocarditis or Lemierre’s syndrome), or sarcoidosis. Patients with atypical pneumonia often present with a cough, fever, and malaise. Patients with septic emboli will have an x-ray that looks similar to that of our patient. Their signs and symptoms will include malaise, shortness of breath, hypoxia, tachycardia, and tachypnea. Risk factors and physical exam findings might include a history of intravenous drug abuse (endocarditis) or deep tissue neck infection (Lemierre’s syndrome). Sarcoidosis can be a challenging diagnosis without further study.
Successful treatment hinges on early detection
Treatment for testicular cancer often is successful if the condition is localized.
The choice of treatment depends on tumor type and stage. Options include orchiectomy, retroperitoneal lymph node dissection, chemotherapy, and radiation.2-5 After being diagnosed with testicular cancer 95% of patients live for 5 or more years.6 For localized testicular cancer, the 5-year survival rate is 99%.6
An eye toward prevention. The US Preventive Services Task Force recommends against screening with clinical examination or testicular self examination7; however, some clinicians support regular screening and self examinations.
When silence is deadly
Although physicians expect that patients will disclose obvious physical manifestations of disease, we know that this is not always the case. Patients often have barriers to care, including their own reluctance to share certain types of information with a provider.
Our patient. After we diagnosed metastatic testicular cancer in our patient, he was transferred to the medical intensive care unit. His overall clinical status declined and he died 14 days later.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.
1. Manecksha RP, Fitzpatrick JM. Epidemiology of testicular cancer. BJU Int. 2009;104(9 pt B):1329-1333.
2. Schultz KA, Schneider DT, Pashankar F, et al. Management of ovarian and testicular sex cord-stromal tumors in children and adolescents. J Pediatr Hematol Oncol. 2012;34 suppl 2:S55-S63.
3. Sohaib SA, Koh DM, Husband JE. The role of imaging in the diagnosis, staging, and management of testicular cancer. AJR Am J Roentgenol. 2008;191:387-395.
4. Viatori M. Testicular cancer. Semin Oncol Nurs. 2012;28:180-189.
5. Mannuel H, Mitikiri N, Khan M, et al. Testicular germ cell tumors: biology and clinical update. Curr Opin Oncol. 2012;24:266-271.
6. SEER Stat Fact Sheets: Testis Cancer. National Cancer Institute Web site. Available at: http://seer.cancer.gov/statfacts/html/testis.html. Accessed May 20, 2014.
7. Screening for testicular cancer. US Preventive Services Task Force Web site. Available at: http://www.uspreventiveservicestaskforce.org/uspstf10/testicular/testicuprs.htm. Accessed May 21, 2014.
Insulin before surgery
To the Editor: We appreciated the thoughtful 1-Minute Consult by Drs. Dobri and Lansang, “How should we manage insulin therapy before surgery?”1 We agree with them in regard to the benefits of perioperative control of blood glucose levels. However, we disagree in general with their assertion that the full dose of the patient’s home dose of basal insulin be administered while the patient is nil per os (NPO) before surgery, with a reduction to 75% of the home dose only if the patient has a history of hypoglycemia, a recommendation that did not differentiate between patients with type 1 and type 2 diabetes mellitus.
The RABBIT 2 Surgery trial,2 which showed superiority of basal-bolus insulin over sliding scale insulin in surgical patients with type 2 diabetes mellitus, also showed a surprisingly high rate of hypoglycemia—24 (23.1%) of 104 patients had blood glucose levels lower than 70 mg/dL, compared with a similar trial in nonsurgical patients in which 2 (3.1%) of 65 patients had a blood glucose level less than 60 mg/dL.3 The authors of the two studies explained2 that “differences in hypoglycemic events between the two trials could be in part explained by reduced nutritional intake in surgical patients…”
Although patients with well-controlled type 1 diabetes mellitus may tolerate their full dose of basal insulin while NPO, we contend that patients with type 2 diabetes mellitus should be prescribed a reduced dose of basal insulin while NPO, regardless of the dose distribution or the patient’s overall glycemic control. It is routine practice on our consult service to reduce the basal insulin dose in such patients by roughly half.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
To the Editor: We appreciated the thoughtful 1-Minute Consult by Drs. Dobri and Lansang, “How should we manage insulin therapy before surgery?”1 We agree with them in regard to the benefits of perioperative control of blood glucose levels. However, we disagree in general with their assertion that the full dose of the patient’s home dose of basal insulin be administered while the patient is nil per os (NPO) before surgery, with a reduction to 75% of the home dose only if the patient has a history of hypoglycemia, a recommendation that did not differentiate between patients with type 1 and type 2 diabetes mellitus.
The RABBIT 2 Surgery trial,2 which showed superiority of basal-bolus insulin over sliding scale insulin in surgical patients with type 2 diabetes mellitus, also showed a surprisingly high rate of hypoglycemia—24 (23.1%) of 104 patients had blood glucose levels lower than 70 mg/dL, compared with a similar trial in nonsurgical patients in which 2 (3.1%) of 65 patients had a blood glucose level less than 60 mg/dL.3 The authors of the two studies explained2 that “differences in hypoglycemic events between the two trials could be in part explained by reduced nutritional intake in surgical patients…”
Although patients with well-controlled type 1 diabetes mellitus may tolerate their full dose of basal insulin while NPO, we contend that patients with type 2 diabetes mellitus should be prescribed a reduced dose of basal insulin while NPO, regardless of the dose distribution or the patient’s overall glycemic control. It is routine practice on our consult service to reduce the basal insulin dose in such patients by roughly half.
To the Editor: We appreciated the thoughtful 1-Minute Consult by Drs. Dobri and Lansang, “How should we manage insulin therapy before surgery?”1 We agree with them in regard to the benefits of perioperative control of blood glucose levels. However, we disagree in general with their assertion that the full dose of the patient’s home dose of basal insulin be administered while the patient is nil per os (NPO) before surgery, with a reduction to 75% of the home dose only if the patient has a history of hypoglycemia, a recommendation that did not differentiate between patients with type 1 and type 2 diabetes mellitus.
The RABBIT 2 Surgery trial,2 which showed superiority of basal-bolus insulin over sliding scale insulin in surgical patients with type 2 diabetes mellitus, also showed a surprisingly high rate of hypoglycemia—24 (23.1%) of 104 patients had blood glucose levels lower than 70 mg/dL, compared with a similar trial in nonsurgical patients in which 2 (3.1%) of 65 patients had a blood glucose level less than 60 mg/dL.3 The authors of the two studies explained2 that “differences in hypoglycemic events between the two trials could be in part explained by reduced nutritional intake in surgical patients…”
Although patients with well-controlled type 1 diabetes mellitus may tolerate their full dose of basal insulin while NPO, we contend that patients with type 2 diabetes mellitus should be prescribed a reduced dose of basal insulin while NPO, regardless of the dose distribution or the patient’s overall glycemic control. It is routine practice on our consult service to reduce the basal insulin dose in such patients by roughly half.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Dobri GA, Lansang MC. How should we manage insulin therapy before surgery? Cleve Clin J Med 2013; 80:702–704.
- Umpierrez GE, Smiley D, Jacobs S, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
In reply: Insulin before surgery
In Reply: We appreciate the kind words of Drs. Ditch and Moore, as well as their opinion.
Our article was intentionally brief—a 1-Minute Consult—and so could not cover all specific situations we encounter in clinical practice. We meant only to provide a general approach in this matter.
Quite often before surgery, patients receive less basal insulin than needed, or none at all, rather than too much. It has to be borne in mind that perioperative hyperglycemia—not just hypoglycemia—is linked with poor outcomes in cardiac1 and noncardiac surgery.2,3
Through our scenarios and suggestions, we have taken steps to err on the side of preventing hypoglycemia while averting hyperglycemia, at the same time making it easy to calculate the dose. In a scenario in which the basal insulin dose is about the same as the total of the prandial boluses, we have not yet seen evidence that raises concern for hypoglycemia, maybe because many of the patients with type 2 diabetes seen in our institution for surgery take, in addition to insulin, oral agents or noninsulin injections (which are appropriately withheld before surgery), and have suboptimal glycemic control on their home regimen. But if a physician has concerns for hypoglycemia, a dose reduction should be made.
There were some differences between the RABBIT 2 trial in medical patients4 and the RABBIT 2 Surgery trial5 that would make the results not completely comparable. In RABBIT 2, the medical patients included were on diet alone or any combination of oral antidiabetic agents (not on insulin), and they were started on a total daily dose of insulin of either 0.4 or 0.5 U/kg/day, depending on the glucose level. In RABBIT 2 Surgery, patients who were on insulin at home with a total daily dose of 0.4 U/kg or less were also included, and the starting daily dose of insulin was 0.5 U/kg (unless they were older or had a high serum creatinine).
In view of all the above, we agree with Drs. Ditch and Moore that if there is concern for hypoglycemia, the clinician should reduce the insulin dose in the manner that evidence from the local practice suggests, without causing undue hyperglycemia and postsurgical complications.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–1021.
- King JT, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 2011; 253:158–165.
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33:1783–1788.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Umpierrez GE, Smiley D, Jacobs S, Peng L, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
In Reply: We appreciate the kind words of Drs. Ditch and Moore, as well as their opinion.
Our article was intentionally brief—a 1-Minute Consult—and so could not cover all specific situations we encounter in clinical practice. We meant only to provide a general approach in this matter.
Quite often before surgery, patients receive less basal insulin than needed, or none at all, rather than too much. It has to be borne in mind that perioperative hyperglycemia—not just hypoglycemia—is linked with poor outcomes in cardiac1 and noncardiac surgery.2,3
Through our scenarios and suggestions, we have taken steps to err on the side of preventing hypoglycemia while averting hyperglycemia, at the same time making it easy to calculate the dose. In a scenario in which the basal insulin dose is about the same as the total of the prandial boluses, we have not yet seen evidence that raises concern for hypoglycemia, maybe because many of the patients with type 2 diabetes seen in our institution for surgery take, in addition to insulin, oral agents or noninsulin injections (which are appropriately withheld before surgery), and have suboptimal glycemic control on their home regimen. But if a physician has concerns for hypoglycemia, a dose reduction should be made.
There were some differences between the RABBIT 2 trial in medical patients4 and the RABBIT 2 Surgery trial5 that would make the results not completely comparable. In RABBIT 2, the medical patients included were on diet alone or any combination of oral antidiabetic agents (not on insulin), and they were started on a total daily dose of insulin of either 0.4 or 0.5 U/kg/day, depending on the glucose level. In RABBIT 2 Surgery, patients who were on insulin at home with a total daily dose of 0.4 U/kg or less were also included, and the starting daily dose of insulin was 0.5 U/kg (unless they were older or had a high serum creatinine).
In view of all the above, we agree with Drs. Ditch and Moore that if there is concern for hypoglycemia, the clinician should reduce the insulin dose in the manner that evidence from the local practice suggests, without causing undue hyperglycemia and postsurgical complications.
In Reply: We appreciate the kind words of Drs. Ditch and Moore, as well as their opinion.
Our article was intentionally brief—a 1-Minute Consult—and so could not cover all specific situations we encounter in clinical practice. We meant only to provide a general approach in this matter.
Quite often before surgery, patients receive less basal insulin than needed, or none at all, rather than too much. It has to be borne in mind that perioperative hyperglycemia—not just hypoglycemia—is linked with poor outcomes in cardiac1 and noncardiac surgery.2,3
Through our scenarios and suggestions, we have taken steps to err on the side of preventing hypoglycemia while averting hyperglycemia, at the same time making it easy to calculate the dose. In a scenario in which the basal insulin dose is about the same as the total of the prandial boluses, we have not yet seen evidence that raises concern for hypoglycemia, maybe because many of the patients with type 2 diabetes seen in our institution for surgery take, in addition to insulin, oral agents or noninsulin injections (which are appropriately withheld before surgery), and have suboptimal glycemic control on their home regimen. But if a physician has concerns for hypoglycemia, a dose reduction should be made.
There were some differences between the RABBIT 2 trial in medical patients4 and the RABBIT 2 Surgery trial5 that would make the results not completely comparable. In RABBIT 2, the medical patients included were on diet alone or any combination of oral antidiabetic agents (not on insulin), and they were started on a total daily dose of insulin of either 0.4 or 0.5 U/kg/day, depending on the glucose level. In RABBIT 2 Surgery, patients who were on insulin at home with a total daily dose of 0.4 U/kg or less were also included, and the starting daily dose of insulin was 0.5 U/kg (unless they were older or had a high serum creatinine).
In view of all the above, we agree with Drs. Ditch and Moore that if there is concern for hypoglycemia, the clinician should reduce the insulin dose in the manner that evidence from the local practice suggests, without causing undue hyperglycemia and postsurgical complications.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–1021.
- King JT, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 2011; 253:158–165.
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33:1783–1788.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Umpierrez GE, Smiley D, Jacobs S, Peng L, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
- Furnary AP, Gao G, Grunkemeier GL, et al. Continuous insulin infusion reduces mortality in patients with diabetes undergoing coronary artery bypass grafting. J Thorac Cardiovasc Surg 2003; 125:1007–1021.
- King JT, Goulet JL, Perkal MF, Rosenthal RA. Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 2011; 253:158–165.
- Frisch A, Chandra P, Smiley D, et al. Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 2010; 33:1783–1788.
- Umpierrez GE, Smiley D, Zisman A, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes (RABBIT 2 trial). Diabetes Care 2007; 30:2181–2186.
- Umpierrez GE, Smiley D, Jacobs S, Peng L, et al. Randomized study of basal-bolus insulin therapy in the inpatient management of patients with type 2 diabetes undergoing general surgery (RABBIT 2 surgery). Diabetes Care 2011; 34:256–261.
A comment on a CME test question
To the Editor: Question 1 of the December 2013 CME test “Can an ARB be given to patients who have had angioedema on an ACE inhibitor?” presents the case of a 73-year-old woman with angioedema thought to be due to her taking enalapril; in addition, she takes hydrochlorothiazide. Her blood pressure is 118/72 mm Hg, and her heart rate is not specified. The question is what the next best step would be to manage her blood pressure medications. The “correct” answer is given as “substitute metoprolol for enalapril in her regimen.”
While this answer is the best choice given, I would take issue with it for two reasons. First, many elderly hypertension patients are overmedicated. With a blood pressure of 118/72 on two medications, it is entirely possible that she may not need to replace the enalapril with any other medication to maintain her pressure below the new JNC 8 threshold of 150/90 for the elderly, or even the 140/90 level specified in other guidelines.
I would recheck her pressure daily on her diuretic alone before adding back a second medication. If she does require a second blood pressure medication, JNC 8 (in agreement with other recent guidelines) recommends adding a calcium channel blocker. Beta-blockers are not recommended by any recent guidelines for first-line or second-line treatment of hypertension for elderly patients without special indications, such as tachyarrhythmias or history of myocardial infarction. No special indications for a beta-blocker were mentioned in this case. Indeed, elderly hypertensive patients often have slow-normal heart rates, or even mild resting bradycardia, which would make the addition of metoprolol contraindicated and potentially dangerous.
To the Editor: Question 1 of the December 2013 CME test “Can an ARB be given to patients who have had angioedema on an ACE inhibitor?” presents the case of a 73-year-old woman with angioedema thought to be due to her taking enalapril; in addition, she takes hydrochlorothiazide. Her blood pressure is 118/72 mm Hg, and her heart rate is not specified. The question is what the next best step would be to manage her blood pressure medications. The “correct” answer is given as “substitute metoprolol for enalapril in her regimen.”
While this answer is the best choice given, I would take issue with it for two reasons. First, many elderly hypertension patients are overmedicated. With a blood pressure of 118/72 on two medications, it is entirely possible that she may not need to replace the enalapril with any other medication to maintain her pressure below the new JNC 8 threshold of 150/90 for the elderly, or even the 140/90 level specified in other guidelines.
I would recheck her pressure daily on her diuretic alone before adding back a second medication. If she does require a second blood pressure medication, JNC 8 (in agreement with other recent guidelines) recommends adding a calcium channel blocker. Beta-blockers are not recommended by any recent guidelines for first-line or second-line treatment of hypertension for elderly patients without special indications, such as tachyarrhythmias or history of myocardial infarction. No special indications for a beta-blocker were mentioned in this case. Indeed, elderly hypertensive patients often have slow-normal heart rates, or even mild resting bradycardia, which would make the addition of metoprolol contraindicated and potentially dangerous.
To the Editor: Question 1 of the December 2013 CME test “Can an ARB be given to patients who have had angioedema on an ACE inhibitor?” presents the case of a 73-year-old woman with angioedema thought to be due to her taking enalapril; in addition, she takes hydrochlorothiazide. Her blood pressure is 118/72 mm Hg, and her heart rate is not specified. The question is what the next best step would be to manage her blood pressure medications. The “correct” answer is given as “substitute metoprolol for enalapril in her regimen.”
While this answer is the best choice given, I would take issue with it for two reasons. First, many elderly hypertension patients are overmedicated. With a blood pressure of 118/72 on two medications, it is entirely possible that she may not need to replace the enalapril with any other medication to maintain her pressure below the new JNC 8 threshold of 150/90 for the elderly, or even the 140/90 level specified in other guidelines.
I would recheck her pressure daily on her diuretic alone before adding back a second medication. If she does require a second blood pressure medication, JNC 8 (in agreement with other recent guidelines) recommends adding a calcium channel blocker. Beta-blockers are not recommended by any recent guidelines for first-line or second-line treatment of hypertension for elderly patients without special indications, such as tachyarrhythmias or history of myocardial infarction. No special indications for a beta-blocker were mentioned in this case. Indeed, elderly hypertensive patients often have slow-normal heart rates, or even mild resting bradycardia, which would make the addition of metoprolol contraindicated and potentially dangerous.
Stress ulcer prophylaxis
To the Editor: In the January 2014 issue, Eisa et al1 suggested that patients who require prolonged mechanical ventilatory support, ie, for more than 48 hours, should receive stress ulcer prophylaxis. This recommendation came from a study by Cook et al2 in 1994, which found a significant increase in the risk of gastrointestinal blood loss in this group of patients. Other studies have shown a different result. Zandstra et al3 found an extremely low rate of stress ulcer-related bleeding in this group in the absence of stress ulcer prophylaxis. Another study4 in critically ill patients also found no relationship between stress ulcer incidence and prolonged mechanical ventilatory support. Interestingly, that study found that prolonged use of a nasogastric tube is the major risk factor for developing a stress ulcer.4 The explanation for why newer studies did not demonstrate the relationship between mechanical ventilation and stress ulcer development may lie in the result of a meta-analysis by Marik et al,5 which showed that stress ulcer prophylaxis may not be required in a patient who receives early enteral nutrition. That practice was not common in the past, including at the time the original study was conducted.
According to current evidence, mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer. The medical community should start questioning the routine practice of stress ulcer prophylaxis in this group of patients. In addition, more studies have identified the adverse effects of acid-suppression therapy in this group of patients, and these effects likely make the harms outweigh the benefits. This notion was confirmed in the most recent meta-analysis by Krag et al.6 In summary, the practice of routine stress ulcer prophylaxis in all mechanically ventilated patients will likely change in the future, with more focus on patients who are at higher risk.
- Eisa N, Bazerbachi F, Alraiyes AH, Alraies MC. Do all hospitalized patients need stress ulcer prophylaxis? Cleve Clin J Med 2014; 81:23–25.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Krag M, Perner A, Wetterslev J, Wise MP, Hylander Møller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med 2014; 40:11–22.
To the Editor: In the January 2014 issue, Eisa et al1 suggested that patients who require prolonged mechanical ventilatory support, ie, for more than 48 hours, should receive stress ulcer prophylaxis. This recommendation came from a study by Cook et al2 in 1994, which found a significant increase in the risk of gastrointestinal blood loss in this group of patients. Other studies have shown a different result. Zandstra et al3 found an extremely low rate of stress ulcer-related bleeding in this group in the absence of stress ulcer prophylaxis. Another study4 in critically ill patients also found no relationship between stress ulcer incidence and prolonged mechanical ventilatory support. Interestingly, that study found that prolonged use of a nasogastric tube is the major risk factor for developing a stress ulcer.4 The explanation for why newer studies did not demonstrate the relationship between mechanical ventilation and stress ulcer development may lie in the result of a meta-analysis by Marik et al,5 which showed that stress ulcer prophylaxis may not be required in a patient who receives early enteral nutrition. That practice was not common in the past, including at the time the original study was conducted.
According to current evidence, mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer. The medical community should start questioning the routine practice of stress ulcer prophylaxis in this group of patients. In addition, more studies have identified the adverse effects of acid-suppression therapy in this group of patients, and these effects likely make the harms outweigh the benefits. This notion was confirmed in the most recent meta-analysis by Krag et al.6 In summary, the practice of routine stress ulcer prophylaxis in all mechanically ventilated patients will likely change in the future, with more focus on patients who are at higher risk.
To the Editor: In the January 2014 issue, Eisa et al1 suggested that patients who require prolonged mechanical ventilatory support, ie, for more than 48 hours, should receive stress ulcer prophylaxis. This recommendation came from a study by Cook et al2 in 1994, which found a significant increase in the risk of gastrointestinal blood loss in this group of patients. Other studies have shown a different result. Zandstra et al3 found an extremely low rate of stress ulcer-related bleeding in this group in the absence of stress ulcer prophylaxis. Another study4 in critically ill patients also found no relationship between stress ulcer incidence and prolonged mechanical ventilatory support. Interestingly, that study found that prolonged use of a nasogastric tube is the major risk factor for developing a stress ulcer.4 The explanation for why newer studies did not demonstrate the relationship between mechanical ventilation and stress ulcer development may lie in the result of a meta-analysis by Marik et al,5 which showed that stress ulcer prophylaxis may not be required in a patient who receives early enteral nutrition. That practice was not common in the past, including at the time the original study was conducted.
According to current evidence, mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer. The medical community should start questioning the routine practice of stress ulcer prophylaxis in this group of patients. In addition, more studies have identified the adverse effects of acid-suppression therapy in this group of patients, and these effects likely make the harms outweigh the benefits. This notion was confirmed in the most recent meta-analysis by Krag et al.6 In summary, the practice of routine stress ulcer prophylaxis in all mechanically ventilated patients will likely change in the future, with more focus on patients who are at higher risk.
- Eisa N, Bazerbachi F, Alraiyes AH, Alraies MC. Do all hospitalized patients need stress ulcer prophylaxis? Cleve Clin J Med 2014; 81:23–25.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Krag M, Perner A, Wetterslev J, Wise MP, Hylander Møller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med 2014; 40:11–22.
- Eisa N, Bazerbachi F, Alraiyes AH, Alraies MC. Do all hospitalized patients need stress ulcer prophylaxis? Cleve Clin J Med 2014; 81:23–25.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Krag M, Perner A, Wetterslev J, Wise MP, Hylander Møller M. Stress ulcer prophylaxis versus placebo or no prophylaxis in critically ill patients. A systematic review of randomised clinical trials with meta-analysis and trial sequential analysis. Intensive Care Med 2014; 40:11–22.
In reply: Stress ulcer prophylaxis
In Reply: We welcome the comments from Dr. Chongnarungsin on our article and the opportunity to further discuss our opinions.
In our paper, we discussed current recommendations for prophylaxis of stress ulcer-related bleeding in hospitalized patients and advocated against the blind administration of drugs without risk stratification.
The landmark trial that provides the most-cited definitions and the risk factors for clinically significant stress ulcer-related bleeding in critically ill patients was published in 1994 by Cook et al.1 In their multicenter prospective cohort study of 2,252 patients, the authors reported that prolonged mechanical ventilation is an important risk factor for clinically significant stress ulcer-related bleeding.
Another major prospective cohort study observed an incidence rate of clinically significant stress ulcer-related bleeding of 3.5%.2
Dr. Chongnarungsin cites another prospective cohort study of 183 patients from the same era,3 wherein the authors defined stress ulcer-related bleeding as bleeding requiring transfusion of packed red blood cells, found on endoscopy or on postmortem evaluation. This was in contrast to the 1994 study of Cook et al,1 who had a more rigorous and comprehensive definition for overt and clinically significant stress ulcer-related bleeding, applied by up to three independent adjudicators not involved in the patients’ care. Their definition not only entailed a more accurate transfusion-dependent bleeding criterion, but also included hemodynamic and laboratory criteria. As such, the “very low rate” of stress ulcer-related bleeding reported by Zandstra et al3 should be critically appraised. Of note, the authors in that study did not report the rates of patients who received early enteral feeding, and their patients received cefotaxime for digestive tract decontamination, an important confounder to the interpretation of the study results.
Indeed, the remarkable variation in estimates of the incidence of stress ulcer-related bleeding is probably related to the lack of a uniform definition. Even when rates of endoscopic and occult bleeding are set aside, agreement is lacking as to which category of bleeding is clinically significant.
Dr. Chongnarungsin also cites the study by Ellison et al4 of a cohort of 874 patients who had no previous gastrointestinal bleeding or peptic ulcer disease and who were enrolled in a multicenter randomized controlled trial of prophylactic intravenous immune globulin to prevent infections associated with an intensive care unit. In a secondary objective, the authors did not identify coagulopathy or prolonged mechanical ventilation as a principal risk factor for bleeding. The authors ascribed this discrepancy with previously published literature to their unique study population, which consisted predominantly of elderly men and rarely included trauma patients. In light of these unique peculiarities of their population, the lack of an association between prolonged mechanical ventilation and stress ulcer-related bleeding cannot be determined. Moreover, that study showed that prolonged nasogastric tube insertion was one of the risk factors for increased risk of gastrointestinal bleeding, and not the risk factor for development of stress ulcer as stated by Dr. Chongnarungsin.
The decrease in the incidence of stress ulcer-related bleeding in critically ill patients over the years could be attributed to an era effect, from advances in critical care medicine and prophylactic methods.5 We agree with Dr. Chongnarungsin that the increased introduction of early enteral feeding may have also contributed to the reduced incidence of stress ulcer-related bleeding.6 However, we think the conclusion that “mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer” is overelaborated, and we believe that strong evidence demonstrates this association.1,2
Alternatively, we recognize the lack of mortality-benefit evidence for stress ulcer prophylaxis. This notwithstanding, according to recent Surviving Sepsis Campaign guidelines, the use of stress ulcer prophylaxis is listed as a 1B recommendation (strong recommendation) for severely septic patients who require prolonged mechanical ventilation. In addition, the updated 2014 guidelines of the American Society of Health-System Pharmacists7 continue to recommend stress ulcer prophylaxis in the context of mechanical ventilation, with H2 receptor antagonists being the preferred first-line agents.8
It is important to acknowledge that these recommendations were endorsed despite the lack of obvious mortality benefit, and it is our opinion that large randomized controlled studies are needed to evaluate the risks and mortality benefit of these prophylaxis methods.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Cook DJ, Griffith LE, Walter SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001; 5:368–375.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Duerksen DR. Stress-related mucosal disease in critically ill patients. Best Pract Res Clin Gastroenterol 2003; 17:327–344.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Cohen H, editor. Stop stressing out: the new stress ulcer prophylaxis (SUP) guidelines are finally here! ASHP Midyear Clinical Meeting; 2013 11 Dec 2013; Orlando, FL.
In Reply: We welcome the comments from Dr. Chongnarungsin on our article and the opportunity to further discuss our opinions.
In our paper, we discussed current recommendations for prophylaxis of stress ulcer-related bleeding in hospitalized patients and advocated against the blind administration of drugs without risk stratification.
The landmark trial that provides the most-cited definitions and the risk factors for clinically significant stress ulcer-related bleeding in critically ill patients was published in 1994 by Cook et al.1 In their multicenter prospective cohort study of 2,252 patients, the authors reported that prolonged mechanical ventilation is an important risk factor for clinically significant stress ulcer-related bleeding.
Another major prospective cohort study observed an incidence rate of clinically significant stress ulcer-related bleeding of 3.5%.2
Dr. Chongnarungsin cites another prospective cohort study of 183 patients from the same era,3 wherein the authors defined stress ulcer-related bleeding as bleeding requiring transfusion of packed red blood cells, found on endoscopy or on postmortem evaluation. This was in contrast to the 1994 study of Cook et al,1 who had a more rigorous and comprehensive definition for overt and clinically significant stress ulcer-related bleeding, applied by up to three independent adjudicators not involved in the patients’ care. Their definition not only entailed a more accurate transfusion-dependent bleeding criterion, but also included hemodynamic and laboratory criteria. As such, the “very low rate” of stress ulcer-related bleeding reported by Zandstra et al3 should be critically appraised. Of note, the authors in that study did not report the rates of patients who received early enteral feeding, and their patients received cefotaxime for digestive tract decontamination, an important confounder to the interpretation of the study results.
Indeed, the remarkable variation in estimates of the incidence of stress ulcer-related bleeding is probably related to the lack of a uniform definition. Even when rates of endoscopic and occult bleeding are set aside, agreement is lacking as to which category of bleeding is clinically significant.
Dr. Chongnarungsin also cites the study by Ellison et al4 of a cohort of 874 patients who had no previous gastrointestinal bleeding or peptic ulcer disease and who were enrolled in a multicenter randomized controlled trial of prophylactic intravenous immune globulin to prevent infections associated with an intensive care unit. In a secondary objective, the authors did not identify coagulopathy or prolonged mechanical ventilation as a principal risk factor for bleeding. The authors ascribed this discrepancy with previously published literature to their unique study population, which consisted predominantly of elderly men and rarely included trauma patients. In light of these unique peculiarities of their population, the lack of an association between prolonged mechanical ventilation and stress ulcer-related bleeding cannot be determined. Moreover, that study showed that prolonged nasogastric tube insertion was one of the risk factors for increased risk of gastrointestinal bleeding, and not the risk factor for development of stress ulcer as stated by Dr. Chongnarungsin.
The decrease in the incidence of stress ulcer-related bleeding in critically ill patients over the years could be attributed to an era effect, from advances in critical care medicine and prophylactic methods.5 We agree with Dr. Chongnarungsin that the increased introduction of early enteral feeding may have also contributed to the reduced incidence of stress ulcer-related bleeding.6 However, we think the conclusion that “mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer” is overelaborated, and we believe that strong evidence demonstrates this association.1,2
Alternatively, we recognize the lack of mortality-benefit evidence for stress ulcer prophylaxis. This notwithstanding, according to recent Surviving Sepsis Campaign guidelines, the use of stress ulcer prophylaxis is listed as a 1B recommendation (strong recommendation) for severely septic patients who require prolonged mechanical ventilation. In addition, the updated 2014 guidelines of the American Society of Health-System Pharmacists7 continue to recommend stress ulcer prophylaxis in the context of mechanical ventilation, with H2 receptor antagonists being the preferred first-line agents.8
It is important to acknowledge that these recommendations were endorsed despite the lack of obvious mortality benefit, and it is our opinion that large randomized controlled studies are needed to evaluate the risks and mortality benefit of these prophylaxis methods.
In Reply: We welcome the comments from Dr. Chongnarungsin on our article and the opportunity to further discuss our opinions.
In our paper, we discussed current recommendations for prophylaxis of stress ulcer-related bleeding in hospitalized patients and advocated against the blind administration of drugs without risk stratification.
The landmark trial that provides the most-cited definitions and the risk factors for clinically significant stress ulcer-related bleeding in critically ill patients was published in 1994 by Cook et al.1 In their multicenter prospective cohort study of 2,252 patients, the authors reported that prolonged mechanical ventilation is an important risk factor for clinically significant stress ulcer-related bleeding.
Another major prospective cohort study observed an incidence rate of clinically significant stress ulcer-related bleeding of 3.5%.2
Dr. Chongnarungsin cites another prospective cohort study of 183 patients from the same era,3 wherein the authors defined stress ulcer-related bleeding as bleeding requiring transfusion of packed red blood cells, found on endoscopy or on postmortem evaluation. This was in contrast to the 1994 study of Cook et al,1 who had a more rigorous and comprehensive definition for overt and clinically significant stress ulcer-related bleeding, applied by up to three independent adjudicators not involved in the patients’ care. Their definition not only entailed a more accurate transfusion-dependent bleeding criterion, but also included hemodynamic and laboratory criteria. As such, the “very low rate” of stress ulcer-related bleeding reported by Zandstra et al3 should be critically appraised. Of note, the authors in that study did not report the rates of patients who received early enteral feeding, and their patients received cefotaxime for digestive tract decontamination, an important confounder to the interpretation of the study results.
Indeed, the remarkable variation in estimates of the incidence of stress ulcer-related bleeding is probably related to the lack of a uniform definition. Even when rates of endoscopic and occult bleeding are set aside, agreement is lacking as to which category of bleeding is clinically significant.
Dr. Chongnarungsin also cites the study by Ellison et al4 of a cohort of 874 patients who had no previous gastrointestinal bleeding or peptic ulcer disease and who were enrolled in a multicenter randomized controlled trial of prophylactic intravenous immune globulin to prevent infections associated with an intensive care unit. In a secondary objective, the authors did not identify coagulopathy or prolonged mechanical ventilation as a principal risk factor for bleeding. The authors ascribed this discrepancy with previously published literature to their unique study population, which consisted predominantly of elderly men and rarely included trauma patients. In light of these unique peculiarities of their population, the lack of an association between prolonged mechanical ventilation and stress ulcer-related bleeding cannot be determined. Moreover, that study showed that prolonged nasogastric tube insertion was one of the risk factors for increased risk of gastrointestinal bleeding, and not the risk factor for development of stress ulcer as stated by Dr. Chongnarungsin.
The decrease in the incidence of stress ulcer-related bleeding in critically ill patients over the years could be attributed to an era effect, from advances in critical care medicine and prophylactic methods.5 We agree with Dr. Chongnarungsin that the increased introduction of early enteral feeding may have also contributed to the reduced incidence of stress ulcer-related bleeding.6 However, we think the conclusion that “mechanical ventilation for more than 48 hours does not seem to increase the risk of stress ulcer” is overelaborated, and we believe that strong evidence demonstrates this association.1,2
Alternatively, we recognize the lack of mortality-benefit evidence for stress ulcer prophylaxis. This notwithstanding, according to recent Surviving Sepsis Campaign guidelines, the use of stress ulcer prophylaxis is listed as a 1B recommendation (strong recommendation) for severely septic patients who require prolonged mechanical ventilation. In addition, the updated 2014 guidelines of the American Society of Health-System Pharmacists7 continue to recommend stress ulcer prophylaxis in the context of mechanical ventilation, with H2 receptor antagonists being the preferred first-line agents.8
It is important to acknowledge that these recommendations were endorsed despite the lack of obvious mortality benefit, and it is our opinion that large randomized controlled studies are needed to evaluate the risks and mortality benefit of these prophylaxis methods.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Cook DJ, Griffith LE, Walter SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001; 5:368–375.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Duerksen DR. Stress-related mucosal disease in critically ill patients. Best Pract Res Clin Gastroenterol 2003; 17:327–344.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Cohen H, editor. Stop stressing out: the new stress ulcer prophylaxis (SUP) guidelines are finally here! ASHP Midyear Clinical Meeting; 2013 11 Dec 2013; Orlando, FL.
- Cook DJ, Fuller HD, Guyatt GH, et al. Risk factors for gastrointestinal bleeding in critically ill patients. Canadian Critical Care Trials Group. N Engl J Med 1994; 330:377–381.
- Cook DJ, Griffith LE, Walter SD, et al. The attributable mortality and length of intensive care unit stay of clinically important gastrointestinal bleeding in critically ill patients. Crit Care 2001; 5:368–375.
- Zandstra DF, Stoutenbeek CP. The virtual absence of stress-ulceration related bleeding in ICU patients receiving prolonged mechanical ventilation without any prophylaxis. A prospective cohort study. Intensive Care Med 1994; 20:335–340.
- Ellison RT, Perez-Perez G, Welsh CH, et al. Risk factors for upper gastrointestinal bleeding in intensive care unit patients: role of Helicobacter pylori. Federal Hyperimmune Immunoglobulin Therapy Study Group. Crit Care Med 1996; 24:1974–1981.
- Duerksen DR. Stress-related mucosal disease in critically ill patients. Best Pract Res Clin Gastroenterol 2003; 17:327–344.
- Marik PE, Vasu T, Hirani A, Pachinburavan M. Stress ulcer prophylaxis in the new millennium: a systematic review and meta-analysis. Crit Care Med 2010; 38:2222–2228.
- Cohen H, editor. Stop stressing out: the new stress ulcer prophylaxis (SUP) guidelines are finally here! ASHP Midyear Clinical Meeting; 2013 11 Dec 2013; Orlando, FL.
Albuminuria
To the Editor: Stephen et al1 have written a nice review of the implications of albuminuria. However, they are clearly incorrect when they state, “Most of the protein in the urine is albumin filtered from the plasma.”1 First, as they later point out in the article, the normal upper limit of protein excretion is about 150 mg/day, and only about 20 mg/day is normally albumin. Therefore, most of the protein in normally found in urine is not albumin, but instead is mostly a variety of globulins. Tamm-Horsfall mucoprotein or uromodulin is usually the protein found in highest concentration in normal urine.
- Stephen R, Jolly SE, Nally JV, Navaneethan SD. Albuminuria: When urine predicts kidney and cardiovascular disease. Cleve Clin J Med 2014; 81:41–50.
To the Editor: Stephen et al1 have written a nice review of the implications of albuminuria. However, they are clearly incorrect when they state, “Most of the protein in the urine is albumin filtered from the plasma.”1 First, as they later point out in the article, the normal upper limit of protein excretion is about 150 mg/day, and only about 20 mg/day is normally albumin. Therefore, most of the protein in normally found in urine is not albumin, but instead is mostly a variety of globulins. Tamm-Horsfall mucoprotein or uromodulin is usually the protein found in highest concentration in normal urine.
To the Editor: Stephen et al1 have written a nice review of the implications of albuminuria. However, they are clearly incorrect when they state, “Most of the protein in the urine is albumin filtered from the plasma.”1 First, as they later point out in the article, the normal upper limit of protein excretion is about 150 mg/day, and only about 20 mg/day is normally albumin. Therefore, most of the protein in normally found in urine is not albumin, but instead is mostly a variety of globulins. Tamm-Horsfall mucoprotein or uromodulin is usually the protein found in highest concentration in normal urine.
- Stephen R, Jolly SE, Nally JV, Navaneethan SD. Albuminuria: When urine predicts kidney and cardiovascular disease. Cleve Clin J Med 2014; 81:41–50.
- Stephen R, Jolly SE, Nally JV, Navaneethan SD. Albuminuria: When urine predicts kidney and cardiovascular disease. Cleve Clin J Med 2014; 81:41–50.
Think twice about nebulizers for asthma attacks
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
Stop ordering nebulizers to deliver beta-agonists to patients over age 2 with mild or moderate asthma exacerbations. A metered-dose inhaler (MDI) with a spacer produces the same benefits with fewer adverse effects.1
Strength of recommendation
A: Based on an updated Cochrane meta-analysis of 39 randomized controlled trials (RCTs).
Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
Illustrative case
A 6-year-old girl with a history of reactive airway disease comes to your office complaining of cough and wheezing. On exam, she has mild retractions, a respiratory rate of 35, and an oxygen saturation of 96% on room air. Her lung fields are diffusely wheezy. Her parents would like to keep her out of the hospital. How should you order her albuterol to decrease her wheezing and minimize adverse effects?
Asthma affects nearly 19 million adults and 7 million children in the United States.2 Asthma exacerbations are the third most common reason for hospitalization in children.2,3 Treatment usually requires multiple agents, including inhaled beta-agonists. These are most effective when delivered to the peripheral airways, which is a challenge during an asthma exacerbation because of airway swelling and rapid breathing. Two devices have been developed to effectively deliver medication to the peripheral airways: nebulizers and MDIs with a holding chamber (spacer).1
Several studies have demonstrated that for mild to moderate asthma exacerbations, administering a beta-agonist via an MDI with a spacer is as effective as using a nebulizer.4,5 Asthma treatment guidelines also state that spacers are either comparable to or preferred over nebulizers for beta-agonist administration in children and adults.6,7 However, based on our experience, physicians still frequently order nebulizer treatments for patients with asthma exacerbations, despite several advantages of MDIs with spacers. Notably, they cost less and don’t require maintenance or a power source. Physicians administered nebulizer therapy at more than 3.6 million emergency department (ED) visits in 2006.8
In this latest Cochrane review, Cates et al1 added 4 new studies to those included in their earlier Cochrane meta-analysis, and looked at what, if any, effect these studies had on our understanding of nebulizers vs MDIs with spacers.
STUDY SUMMARY: Outcomes with nebulizers are no better than those with spacers
This systematic review and meta-analysis pooled the results of RCTs comparing spacers to nebulizers for administering beta-agonists during acute, non-life-threatening asthma exacerbations.1 The authors reviewed studies conducted in EDs, hospitals, and outpatient settings that included children and adults. The primary outcomes were hospital admission rates and duration of hospital stay. Secondary outcomes included time spent in the ED, change in pulse rate, and incidence of tremor.
Cates et al1 analyzed 39 trials that included 1897 children and 729 adults and were conducted primarily in an ED or outpatient setting. The 4 new studies added 295 children and 58 adults to the researchers’ earlier meta-analysis. Studies involving adults and children were pooled separately. Most patients received multiple treatments with beta-agonists titrated to the individual’s response.
No differences in hospitalizations. Rates of hospital admissions did not differ between patients receiving beta-agonists via a spacer compared to a nebulizer in both adults (relative risk [RR]=.94; 95% confidence interval [CI], .61-1.43) and children (RR=.71; 95% CI, .47-1.08). Duration of hospital stay did not differ between the 2 delivery methods in adults (mean difference [MD]=-.60 days; 95% CI, -3.23 to 2.03) and children (MD=.33 days; 95% CI, -.10 to .76).
For kids, spacers meant less time in the ED. Duration in the ED was approximately half an hour shorter for children using spacers (MD=-33.48 minutes; 95% CI, -43.3 to -23.6, P<.001). There was no difference in time spent in the ED observed in adults (MD=1.75 minutes; 95% CI, -23.45 to 26.95). The rate of tremor was lower in children using spacers (RR=.64; 95% CI, .44-.95, P=.027), and was similar in adults (RR=1.12; 95% CI, .66-1.9). The rise in pulse rate was lower in children using spacers (MD=-5.41% change from baseline; 95% CI, -8.34 to -2.48; P<.001), and was similar in adults (MD=-1.23%; 95% CI, -4.06 to 1.60).
WHAT'S NEW: Additional evidence that spacers are as effective as nebulizers
This meta-analysis, which included 4 new studies, should finally dispel the myth that nebulizers deliver beta-agonists more effectively than MDIs with spacers. Additionally, in children, spacers are associated with lower rates of side effects, including tremor and elevated pulse rate.
CAVEATS: Most studies involving children were open label
Although most of the adult trials in this meta-analysis involved a double-dummy design, which allows for effective participant blinding, most of the studies involving children were open label. This open-label design might have been a source of reporting bias for symptom-related outcomes, but should not have affected hospital admission rates or duration of hospital stay.
In the double-dummy studies, adults received both a nebulizer and a spacer, which likely explains the similar time spent in the ED by the treatment and control groups.
CHALLENGES TO IMPLEMENTATION: Old habits are hard to break
Doctors may think that patients view nebulizers as more potent or more effective than spacers and thus be more likely to order them. Some patients may prefer nebulizers because of convenience or other factors.
Acknowledgement
The PURLs Surveillance System was supported in part by Grant Number UL1RR024999 from the National Center For Research Resources, a Clinical Translational Science Award to the University of Chicago. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center For Research Resources or the National Institutes of Health.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
1. Cates CJ, Welsh EJ, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2013;9:CD000052.
2. Barrett ML, Wier LM, Washington R. Trends in pediatric and adult hospital stays for asthma, 2000-2010. HCUP Statistical Brief #169. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb169-Asthma-Trends-Hospital-Stays.pdf. Published January 2014. Accessed March 18, 2014.
3. Pfuntner A, Wier LM, Stocks C. Most frequent conditions in US hospitals, 2011. HCUP Statistical Brief #162. Available at: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb162.pdf. Published September 2013. Accessed March 18, 2014.
4. Cates CJ, Crilly JA, Rowe BH. Holding chambers (spacers) versus nebulisers for beta-agonist treatment of acute asthma. Cochrane Database Syst Rev. 2006;(2):CD000052.
5. Turner MO, Patel A, Ginsburg S, et al. Bronchodilator delivery in acute airflow obstruction. A meta-analysis. Arch Intern Med. 1997;157:1736-1744.
6. Expert Panel Report 3 (EPR3): Guidelines for the diagnosis and management of asthma. National Heart, Lung, and Blood Institute Web site. Available at: www.nhlbi.nih.gov/guidelines/asthma/asthgdln.htm. Accessed March 18, 2014.
7. British guideline of the management of asthma: A national clinical guideline. British Thoracic Society Web site. Available at: https://www.brit-thoracic.org.uk/document-library/clinical-information/asthma/btssign-guideline-on-the-management-of-asthma/. Published May 2008. Revised January 2012. Accessed March 15, 2014.
8. Pitts SR, Niska RW, Xu J, et al. National Hospital Ambulatory Medical Care Survey: 2006 emergency department summary. Available at: http://www.cdc.gov/nchs/data/nhsr/nhsr007.pdf. Accessed May 8, 2014.
Copyright © 2014 Family Physicians Inquiries Network. All rights reserved.
Group finds a way to target MDSCs

Researchers say they’ve found a way to target myeloid-derived suppressor cells (MDSCs) while sparing other immune cells.
In preclinical experiments, the team showed they could deplete MDSCs—and shrink tumors—using peptide antibodies.
These “peptibodies” wiped out MDSCs in the blood, spleen, and tumor cells of mice without binding to other white blood cells or dendritic cells.
The researchers described this work in Nature Medicine.
“We’ve known about [MDSCs] blocking immune response for a decade but haven’t been able to shut them down for lack of an identified target,” said the paper’s senior author, Larry Kwak, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“This is the first demonstration of a molecule on these cells that allows us to make an antibody—in this case, a peptide—to bind to them and get rid of them. It’s a brand new immunotherapy target.”
Dr Kwak and his colleagues began this research by applying a peptide phage library to MDSCs, which allowed for a mass screening of candidate peptides that bind to the surface of MDSCs. This revealed 2 peptides, labeled G3 and H6, that bound only to MDSCs.
The researchers fused the 2 peptides to a portion of mouse immune globulin to generate experimental peptibodies called pep-G3 and pep-H6. Both peptibodies bound to both types of MDSCs—monocytic and granulocytic cells.
Dr Kwak and his colleagues then tested the peptibodies in 2 mouse models of thymic tumors, as well as models of melanoma and lymphoma. The team compared pep-G3 and pep-H6 to a control peptibody and an antibody against Gr-1.
Both pep-G3 and pep-H6 depleted monocytic and granulocytic MDSCs in the blood and spleens of all mice. But the Gr-1 antibody only worked against granulocytic MDSCs.
To see whether MDSC depletion would impede tumor growth, the researchers administered the peptibodies to mice with thymic tumors every other day for 2 weeks.
Mice treated with either pep-G3 or pep-H6 had tumors that were about half the size and weight of those in mice treated with the control peptibody or the Gr-1 antibody.
Lastly, the researchers analyzed surface proteins on the MDSCs and found that S100A9 and S100A8 are the likely binding targets for pep-G3 and pep-H6.
Dr Kwak and his colleagues said they are now working to extend these findings to human MDSCs. ![]()

Researchers say they’ve found a way to target myeloid-derived suppressor cells (MDSCs) while sparing other immune cells.
In preclinical experiments, the team showed they could deplete MDSCs—and shrink tumors—using peptide antibodies.
These “peptibodies” wiped out MDSCs in the blood, spleen, and tumor cells of mice without binding to other white blood cells or dendritic cells.
The researchers described this work in Nature Medicine.
“We’ve known about [MDSCs] blocking immune response for a decade but haven’t been able to shut them down for lack of an identified target,” said the paper’s senior author, Larry Kwak, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“This is the first demonstration of a molecule on these cells that allows us to make an antibody—in this case, a peptide—to bind to them and get rid of them. It’s a brand new immunotherapy target.”
Dr Kwak and his colleagues began this research by applying a peptide phage library to MDSCs, which allowed for a mass screening of candidate peptides that bind to the surface of MDSCs. This revealed 2 peptides, labeled G3 and H6, that bound only to MDSCs.
The researchers fused the 2 peptides to a portion of mouse immune globulin to generate experimental peptibodies called pep-G3 and pep-H6. Both peptibodies bound to both types of MDSCs—monocytic and granulocytic cells.
Dr Kwak and his colleagues then tested the peptibodies in 2 mouse models of thymic tumors, as well as models of melanoma and lymphoma. The team compared pep-G3 and pep-H6 to a control peptibody and an antibody against Gr-1.
Both pep-G3 and pep-H6 depleted monocytic and granulocytic MDSCs in the blood and spleens of all mice. But the Gr-1 antibody only worked against granulocytic MDSCs.
To see whether MDSC depletion would impede tumor growth, the researchers administered the peptibodies to mice with thymic tumors every other day for 2 weeks.
Mice treated with either pep-G3 or pep-H6 had tumors that were about half the size and weight of those in mice treated with the control peptibody or the Gr-1 antibody.
Lastly, the researchers analyzed surface proteins on the MDSCs and found that S100A9 and S100A8 are the likely binding targets for pep-G3 and pep-H6.
Dr Kwak and his colleagues said they are now working to extend these findings to human MDSCs. ![]()

Researchers say they’ve found a way to target myeloid-derived suppressor cells (MDSCs) while sparing other immune cells.
In preclinical experiments, the team showed they could deplete MDSCs—and shrink tumors—using peptide antibodies.
These “peptibodies” wiped out MDSCs in the blood, spleen, and tumor cells of mice without binding to other white blood cells or dendritic cells.
The researchers described this work in Nature Medicine.
“We’ve known about [MDSCs] blocking immune response for a decade but haven’t been able to shut them down for lack of an identified target,” said the paper’s senior author, Larry Kwak, MD, PhD, of The University of Texas MD Anderson Cancer Center in Houston.
“This is the first demonstration of a molecule on these cells that allows us to make an antibody—in this case, a peptide—to bind to them and get rid of them. It’s a brand new immunotherapy target.”
Dr Kwak and his colleagues began this research by applying a peptide phage library to MDSCs, which allowed for a mass screening of candidate peptides that bind to the surface of MDSCs. This revealed 2 peptides, labeled G3 and H6, that bound only to MDSCs.
The researchers fused the 2 peptides to a portion of mouse immune globulin to generate experimental peptibodies called pep-G3 and pep-H6. Both peptibodies bound to both types of MDSCs—monocytic and granulocytic cells.
Dr Kwak and his colleagues then tested the peptibodies in 2 mouse models of thymic tumors, as well as models of melanoma and lymphoma. The team compared pep-G3 and pep-H6 to a control peptibody and an antibody against Gr-1.
Both pep-G3 and pep-H6 depleted monocytic and granulocytic MDSCs in the blood and spleens of all mice. But the Gr-1 antibody only worked against granulocytic MDSCs.
To see whether MDSC depletion would impede tumor growth, the researchers administered the peptibodies to mice with thymic tumors every other day for 2 weeks.
Mice treated with either pep-G3 or pep-H6 had tumors that were about half the size and weight of those in mice treated with the control peptibody or the Gr-1 antibody.
Lastly, the researchers analyzed surface proteins on the MDSCs and found that S100A9 and S100A8 are the likely binding targets for pep-G3 and pep-H6.
Dr Kwak and his colleagues said they are now working to extend these findings to human MDSCs. ![]()