User login
Stats show increase in cancer survival rates

Credit: NIH
New statistics suggest 10-year survival rates for cancer patients in England and Wales have more than doubled over a 40-year period.
And rates increased substantially for those with hematologic malignancies.
From 1971 to 2011, 10-year survival rates increased nearly 7-fold for patients with multiple myeloma and almost 6-fold for leukemia patients.
Rates nearly tripled for non-Hodgkin lymphoma patients and almost doubled for those with Hodgkin lymphoma.
These statistics were released by Cancer Research UK.
“These results come from detailed analysis of the survival of more than 7 million cancer patients diagnosed in England and Wales since the 1970s,” said Michel Coleman, BM BCh, head of Cancer Research UK’s Cancer Survival Group at the London School of Hygiene and Tropical Medicine.
“They show just how far we’ve come in improving cancer survival, but they also shine a spotlight on areas where much more needs to be done.”
The statistics include all adults (aged 15 to 99) diagnosed with cancer in England and Wales.
An analysis of the figures showed that, in 1971-1972, 24% of all cancer patients survived 10 years. By 2010-2011, that figure had increased to 50%.
For leukemia patients, 10-year survival increased from 8% in 1970-1971 to 46% in 2010-2011. For patients with multiple myeloma, it rose from 5% to 33%.
For patients with Hodgkin lymphoma, 10-year survival increased from 49% to 80%. And for non-Hodgkin lymphoma patients, it increased from 22% to 63%.
There were substantial increases in shorter-term survival rates (1-year and 5-year) as well. For details, see the Cancer Research UK website. ![]()

Credit: NIH
New statistics suggest 10-year survival rates for cancer patients in England and Wales have more than doubled over a 40-year period.
And rates increased substantially for those with hematologic malignancies.
From 1971 to 2011, 10-year survival rates increased nearly 7-fold for patients with multiple myeloma and almost 6-fold for leukemia patients.
Rates nearly tripled for non-Hodgkin lymphoma patients and almost doubled for those with Hodgkin lymphoma.
These statistics were released by Cancer Research UK.
“These results come from detailed analysis of the survival of more than 7 million cancer patients diagnosed in England and Wales since the 1970s,” said Michel Coleman, BM BCh, head of Cancer Research UK’s Cancer Survival Group at the London School of Hygiene and Tropical Medicine.
“They show just how far we’ve come in improving cancer survival, but they also shine a spotlight on areas where much more needs to be done.”
The statistics include all adults (aged 15 to 99) diagnosed with cancer in England and Wales.
An analysis of the figures showed that, in 1971-1972, 24% of all cancer patients survived 10 years. By 2010-2011, that figure had increased to 50%.
For leukemia patients, 10-year survival increased from 8% in 1970-1971 to 46% in 2010-2011. For patients with multiple myeloma, it rose from 5% to 33%.
For patients with Hodgkin lymphoma, 10-year survival increased from 49% to 80%. And for non-Hodgkin lymphoma patients, it increased from 22% to 63%.
There were substantial increases in shorter-term survival rates (1-year and 5-year) as well. For details, see the Cancer Research UK website. ![]()

Credit: NIH
New statistics suggest 10-year survival rates for cancer patients in England and Wales have more than doubled over a 40-year period.
And rates increased substantially for those with hematologic malignancies.
From 1971 to 2011, 10-year survival rates increased nearly 7-fold for patients with multiple myeloma and almost 6-fold for leukemia patients.
Rates nearly tripled for non-Hodgkin lymphoma patients and almost doubled for those with Hodgkin lymphoma.
These statistics were released by Cancer Research UK.
“These results come from detailed analysis of the survival of more than 7 million cancer patients diagnosed in England and Wales since the 1970s,” said Michel Coleman, BM BCh, head of Cancer Research UK’s Cancer Survival Group at the London School of Hygiene and Tropical Medicine.
“They show just how far we’ve come in improving cancer survival, but they also shine a spotlight on areas where much more needs to be done.”
The statistics include all adults (aged 15 to 99) diagnosed with cancer in England and Wales.
An analysis of the figures showed that, in 1971-1972, 24% of all cancer patients survived 10 years. By 2010-2011, that figure had increased to 50%.
For leukemia patients, 10-year survival increased from 8% in 1970-1971 to 46% in 2010-2011. For patients with multiple myeloma, it rose from 5% to 33%.
For patients with Hodgkin lymphoma, 10-year survival increased from 49% to 80%. And for non-Hodgkin lymphoma patients, it increased from 22% to 63%.
There were substantial increases in shorter-term survival rates (1-year and 5-year) as well. For details, see the Cancer Research UK website. ![]()
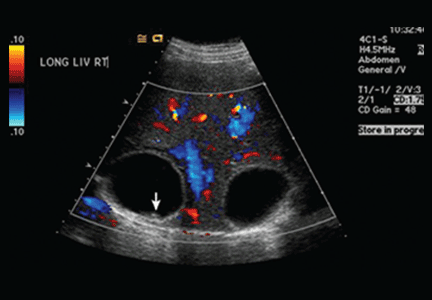
Liver transplant exceptions deserve fresh look
Room air hypoxemia was associated with greater post–liver transplant mortality in hepatopulmonary syndrome patients, according to Dr. David S. Goldberg and his colleagues.
On the other hand, HPS transplant candidates had overall decreased pretransplantation mortality compared with non-HPS patients, suggesting that "current exception policy might overprioritize waitlisted HPS patients," they wrote.
The report appears in the May 1 issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.005).
Dr. Goldberg of the University of Pennsylvania, Philadelphia, looked at all 973 liver transplant candidates from the United Network for Organ Sharing database who had at least one exception application for HPS approved between Feb. 27, 2002, and Dec. 14, 2012.
For comparison, the authors assessed 59,619 non-HPS adult waitlist candidates who registered for their first liver transplantation on or after Feb. 27, 2002.
Overall, there was a 1-year posttransplant survival rate of 91%, a 3-year survival rate of 81%, and a 5-year rate of 76% among the HPS cohort, the authors wrote. Those rates were comparable with the 1-year, 3-year, and 5-year posttransplant rates for non-HPS patients of 89%, 81%, and 74%.
However, looking at pretransplant survival, the authors found that a significantly greater proportion of non-HPS transplant candidates died on the waitlist, compared with HPS patients (20% vs. 9%; P less than .001). That translated to a hazard ratio of 0.82 among HPS patients for dying on the waitlist, compared with non-HPS patients (95% confidence interval, 0.70-0.96).
Next, the authors assessed the relationship between pretransplant room air oxygenation among HPS patients on posttransplant survival rates. They found that patients with pretransplant PaO2 (partial pressure of oxygen in arterial blood) levels of less than 50 mm Hg had a significantly higher posttransplant mortality, compared with patients with PaO2 levels between 50 and 59 mm Hg (HR = 1.56; 95% CI, 1.02-2.38).
Similarly, in a cubic spline model, transplant recipients with a PaO2 of less than 44.0 mm Hg had significantly increased posttransplantation mortality compared with recipients with a PaO2 of 44.1-54.0 mm Hg (HR = 1.58; 95% CI, 1.15-2.18).
"These data must be taken in context, as the 5-year posttransplantation patient survival in HPS patients with the lowest values of PaO2 is still at or above a threshold many would consider acceptable for a transplant recipient," the authors cautioned. "Therefore, the transplant community must decide what degree of hypoxemia makes a patient too high risk," they added.
The authors conceded several limitations. "First, we were unable to employ the strict criteria defining HPS used in prospective multicenter studies," they wrote.
"However, we are confident that most, if not all, of the patients had HPS based on the data documenting hypoxemia and shunting in nearly 90% of patients."
Nevertheless, "excellent posttransplantation outcomes in those with less severe hypoxemia suggest that it might be possible to optimize posttransplantation outcomes for patients with HPS without disadvantaging the broader transplant population," they wrote.
This could be accomplished by a review of current exception algorithms, and "by decreasing the initial number of exception points for HPS patients, while offering additional priority to those whose PaO2 values decline toward higher-risk values," they wrote.
Given the fact that this would increase the overall waitlist time, "an increase rather than decrease in data collected regarding these patients is needed to guide policy," they concluded.
The authors disclosed no conflicts of interest. Dr. Goldberg reported receiving funding from the National Institutes of Health, and the study was partially supported by the Health Resources and Services Administration.
This study is an important reminder of the need for ongoing evidence-based revision of the MELD-based liver allocation system. It has been clear since the adoption of MELD for the purpose of organ allocation that there are patients for whom the laboratory components of the MELD score do not accurately portray their disease-associated risk of death. Thus, there is widespread petitioning for MELD exception points for a number of conditions, including hepatopulmonary syndrome (HPS). While criteria for some such conditions are clearly defined, most prominently including hepatocellular carcinoma, for most other conditions practices vary by region and criteria are not always strictly adhered to.
The initial number of points granted for most conditions, including HPS, is arbitrary rather than based on objective analysis. This study suggests that while posttransplant outcomes are similar between patients with HPS exception points and those without points, pretransplant survival is superior in the HPS group, suggesting that perhaps a lower MELD score should be assigned to these patients.
While exceptions are currently a fundamental part of MELD-based liver allocation, greater standardization of MELD exception point criteria is urgently needed. Additional analysis of wait list survival and survival benefit models will be required to ensure that individual groups of patients are not being unjustly overprioritized.
Dr. Elizabeth C. Verna is assistant professor of medicine, Center for Liver Disease and Transplantation, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York. She disclosed no conflicts of interest.
This study is an important reminder of the need for ongoing evidence-based revision of the MELD-based liver allocation system. It has been clear since the adoption of MELD for the purpose of organ allocation that there are patients for whom the laboratory components of the MELD score do not accurately portray their disease-associated risk of death. Thus, there is widespread petitioning for MELD exception points for a number of conditions, including hepatopulmonary syndrome (HPS). While criteria for some such conditions are clearly defined, most prominently including hepatocellular carcinoma, for most other conditions practices vary by region and criteria are not always strictly adhered to.
The initial number of points granted for most conditions, including HPS, is arbitrary rather than based on objective analysis. This study suggests that while posttransplant outcomes are similar between patients with HPS exception points and those without points, pretransplant survival is superior in the HPS group, suggesting that perhaps a lower MELD score should be assigned to these patients.
While exceptions are currently a fundamental part of MELD-based liver allocation, greater standardization of MELD exception point criteria is urgently needed. Additional analysis of wait list survival and survival benefit models will be required to ensure that individual groups of patients are not being unjustly overprioritized.
Dr. Elizabeth C. Verna is assistant professor of medicine, Center for Liver Disease and Transplantation, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York. She disclosed no conflicts of interest.
This study is an important reminder of the need for ongoing evidence-based revision of the MELD-based liver allocation system. It has been clear since the adoption of MELD for the purpose of organ allocation that there are patients for whom the laboratory components of the MELD score do not accurately portray their disease-associated risk of death. Thus, there is widespread petitioning for MELD exception points for a number of conditions, including hepatopulmonary syndrome (HPS). While criteria for some such conditions are clearly defined, most prominently including hepatocellular carcinoma, for most other conditions practices vary by region and criteria are not always strictly adhered to.
The initial number of points granted for most conditions, including HPS, is arbitrary rather than based on objective analysis. This study suggests that while posttransplant outcomes are similar between patients with HPS exception points and those without points, pretransplant survival is superior in the HPS group, suggesting that perhaps a lower MELD score should be assigned to these patients.
While exceptions are currently a fundamental part of MELD-based liver allocation, greater standardization of MELD exception point criteria is urgently needed. Additional analysis of wait list survival and survival benefit models will be required to ensure that individual groups of patients are not being unjustly overprioritized.
Dr. Elizabeth C. Verna is assistant professor of medicine, Center for Liver Disease and Transplantation, Division of Digestive and Liver Diseases, Columbia University College of Physicians and Surgeons, New York. She disclosed no conflicts of interest.
Room air hypoxemia was associated with greater post–liver transplant mortality in hepatopulmonary syndrome patients, according to Dr. David S. Goldberg and his colleagues.
On the other hand, HPS transplant candidates had overall decreased pretransplantation mortality compared with non-HPS patients, suggesting that "current exception policy might overprioritize waitlisted HPS patients," they wrote.
The report appears in the May 1 issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.005).
Dr. Goldberg of the University of Pennsylvania, Philadelphia, looked at all 973 liver transplant candidates from the United Network for Organ Sharing database who had at least one exception application for HPS approved between Feb. 27, 2002, and Dec. 14, 2012.
For comparison, the authors assessed 59,619 non-HPS adult waitlist candidates who registered for their first liver transplantation on or after Feb. 27, 2002.
Overall, there was a 1-year posttransplant survival rate of 91%, a 3-year survival rate of 81%, and a 5-year rate of 76% among the HPS cohort, the authors wrote. Those rates were comparable with the 1-year, 3-year, and 5-year posttransplant rates for non-HPS patients of 89%, 81%, and 74%.
However, looking at pretransplant survival, the authors found that a significantly greater proportion of non-HPS transplant candidates died on the waitlist, compared with HPS patients (20% vs. 9%; P less than .001). That translated to a hazard ratio of 0.82 among HPS patients for dying on the waitlist, compared with non-HPS patients (95% confidence interval, 0.70-0.96).
Next, the authors assessed the relationship between pretransplant room air oxygenation among HPS patients on posttransplant survival rates. They found that patients with pretransplant PaO2 (partial pressure of oxygen in arterial blood) levels of less than 50 mm Hg had a significantly higher posttransplant mortality, compared with patients with PaO2 levels between 50 and 59 mm Hg (HR = 1.56; 95% CI, 1.02-2.38).
Similarly, in a cubic spline model, transplant recipients with a PaO2 of less than 44.0 mm Hg had significantly increased posttransplantation mortality compared with recipients with a PaO2 of 44.1-54.0 mm Hg (HR = 1.58; 95% CI, 1.15-2.18).
"These data must be taken in context, as the 5-year posttransplantation patient survival in HPS patients with the lowest values of PaO2 is still at or above a threshold many would consider acceptable for a transplant recipient," the authors cautioned. "Therefore, the transplant community must decide what degree of hypoxemia makes a patient too high risk," they added.
The authors conceded several limitations. "First, we were unable to employ the strict criteria defining HPS used in prospective multicenter studies," they wrote.
"However, we are confident that most, if not all, of the patients had HPS based on the data documenting hypoxemia and shunting in nearly 90% of patients."
Nevertheless, "excellent posttransplantation outcomes in those with less severe hypoxemia suggest that it might be possible to optimize posttransplantation outcomes for patients with HPS without disadvantaging the broader transplant population," they wrote.
This could be accomplished by a review of current exception algorithms, and "by decreasing the initial number of exception points for HPS patients, while offering additional priority to those whose PaO2 values decline toward higher-risk values," they wrote.
Given the fact that this would increase the overall waitlist time, "an increase rather than decrease in data collected regarding these patients is needed to guide policy," they concluded.
The authors disclosed no conflicts of interest. Dr. Goldberg reported receiving funding from the National Institutes of Health, and the study was partially supported by the Health Resources and Services Administration.
Room air hypoxemia was associated with greater post–liver transplant mortality in hepatopulmonary syndrome patients, according to Dr. David S. Goldberg and his colleagues.
On the other hand, HPS transplant candidates had overall decreased pretransplantation mortality compared with non-HPS patients, suggesting that "current exception policy might overprioritize waitlisted HPS patients," they wrote.
The report appears in the May 1 issue of Gastroenterology (doi:10.1053/j.gastro.2014.01.005).
Dr. Goldberg of the University of Pennsylvania, Philadelphia, looked at all 973 liver transplant candidates from the United Network for Organ Sharing database who had at least one exception application for HPS approved between Feb. 27, 2002, and Dec. 14, 2012.
For comparison, the authors assessed 59,619 non-HPS adult waitlist candidates who registered for their first liver transplantation on or after Feb. 27, 2002.
Overall, there was a 1-year posttransplant survival rate of 91%, a 3-year survival rate of 81%, and a 5-year rate of 76% among the HPS cohort, the authors wrote. Those rates were comparable with the 1-year, 3-year, and 5-year posttransplant rates for non-HPS patients of 89%, 81%, and 74%.
However, looking at pretransplant survival, the authors found that a significantly greater proportion of non-HPS transplant candidates died on the waitlist, compared with HPS patients (20% vs. 9%; P less than .001). That translated to a hazard ratio of 0.82 among HPS patients for dying on the waitlist, compared with non-HPS patients (95% confidence interval, 0.70-0.96).
Next, the authors assessed the relationship between pretransplant room air oxygenation among HPS patients on posttransplant survival rates. They found that patients with pretransplant PaO2 (partial pressure of oxygen in arterial blood) levels of less than 50 mm Hg had a significantly higher posttransplant mortality, compared with patients with PaO2 levels between 50 and 59 mm Hg (HR = 1.56; 95% CI, 1.02-2.38).
Similarly, in a cubic spline model, transplant recipients with a PaO2 of less than 44.0 mm Hg had significantly increased posttransplantation mortality compared with recipients with a PaO2 of 44.1-54.0 mm Hg (HR = 1.58; 95% CI, 1.15-2.18).
"These data must be taken in context, as the 5-year posttransplantation patient survival in HPS patients with the lowest values of PaO2 is still at or above a threshold many would consider acceptable for a transplant recipient," the authors cautioned. "Therefore, the transplant community must decide what degree of hypoxemia makes a patient too high risk," they added.
The authors conceded several limitations. "First, we were unable to employ the strict criteria defining HPS used in prospective multicenter studies," they wrote.
"However, we are confident that most, if not all, of the patients had HPS based on the data documenting hypoxemia and shunting in nearly 90% of patients."
Nevertheless, "excellent posttransplantation outcomes in those with less severe hypoxemia suggest that it might be possible to optimize posttransplantation outcomes for patients with HPS without disadvantaging the broader transplant population," they wrote.
This could be accomplished by a review of current exception algorithms, and "by decreasing the initial number of exception points for HPS patients, while offering additional priority to those whose PaO2 values decline toward higher-risk values," they wrote.
Given the fact that this would increase the overall waitlist time, "an increase rather than decrease in data collected regarding these patients is needed to guide policy," they concluded.
The authors disclosed no conflicts of interest. Dr. Goldberg reported receiving funding from the National Institutes of Health, and the study was partially supported by the Health Resources and Services Administration.
FROM GASTROENTEROLOGY
Major finding: Liver transplant recipients with hepatopulmonary syndrome have success similar to that of other transplant candidates, unless severe hypoxemia is present.
Data source: A retrospective cohort study of data submitted to the United Network for Organ Sharing.
Disclosures: The authors disclosed no conflicts of interest. Dr. Goldberg reported receiving funding from the National Institutes of Health, and the study was partially supported by the Health Resources and Services Administration.
Diagnosis and Management of Acute and Chronic Graft-versus-Host Disease
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Allogeneic hematopoietic stem cell transplantation (HSCT) is a potentially curative treatment option for several hematologic malignancies and other congenital diseases including immunodeficiencies or hemoglobinopathies. When the first allografts were performed, most patients given bone marrow (BM) from donors other than homozygotic twins developed skin, gut, and/or liver injury. This disease was defined by Billingham in 1966 as graft-versushost disease (GVHD). He also described 3 standard tenets for GVHD pathophysiology, which remain valid today even with rapid advances in this area: (1) donor graft must have immune-competent cells, (2) recipient must be incapable of rejecting the graft, and (3) recipient must have tissue antigens not present in the donor.
To read the full article in PDF:
Optimizing transitions of care to reduce rehospitalizations
You have spent several days checking on a patient hospitalized for an acute exacerbation of heart failure. You have straightened out her medications and diet and discussed a plan for follow-up with the patient and a family member, and now she is being wheeled out the door. What happens to her next?
Too often, not your desired plan. If she is going home, maybe she understands what she needs to do, maybe not. Maybe she will get your prescriptions filled and take the medications as directed, maybe not. If she is going to a nursing home, maybe the physician covering the nursing home will get your plan, maybe not. There is a good chance she will be back in the emergency room soon, all because of a poor transition of care.
Transitions of care are changes in the level, location, or providers of care as patients move within the health care system. These can be critical junctures in patients’ lives, and if poorly executed can result in many adverse effects—including rehospitalization.1
Although high rehospitalization rates gained national attention in 2009 after a analysis of Medicare data,2 health care providers have known about the lack of coordinated care transitions for more than 50 years.3 Despite some progress, improving care transitions remains a national challenge. As the health system evolves from a fee-for-service financial model to payment-for-value,4 it is especially important that health care providers improve care for patients by optimizing care transitions.
In this article, we summarize the factors contributing to poor care transitions, highlight programs that improve them, and discuss strategies for successful transitions.
TRANSITION PROBLEMS ARE COMMON
Transitions of care occur when patients move to short-term and long-term acute care hospitals, skilled nursing facilities, primary and specialty care offices, community health centers, rehabilitation facilities, home health agencies, hospice, and their own homes.5 Problems can arise at any of these transitions, but the risk is especially high when patients leave the hospital to receive care in another setting or at home.
In the past decade, one in five Medicare patients was rehospitalized within 30 days of discharge from the hospital,2 and up to 25% were rehospitalized after being discharged to a skilled nursing facility.6 Some diagnoses (eg, sickle cell anemia, gangrene) and procedures (eg, kidney transplantation, ileostomy) are associated with readmission rates of nearly one in three.7,8
The desire of policymakers to “bend the cost curve” of health care has led to efforts to enhance care coordination by improving transitions between care venues. Through the Patient Protection and Affordable Care Act, a number of federal initiatives are promoting strategies to improve care transitions and prevent readmissions after hospital discharge.
The Hospital Readmission Reduction Program9 drives much of this effort. In fiscal year 2013 (beginning October 1, 2012), more than 2,000 hospitals incurred financial penalties of up to 1% of total Medicare diagnosis-related group payments (about $280 million the first year) for excess readmissions.10 The penalty’s maximum rose to 2% in fiscal year 2014 and could increase to 3% in 2015. The total penalty for 2014 is projected to be $227 million, with 2,225 hospitals affected.11
The Centers for Medicare and Medicaid Innovation has committed hundreds of millions of dollars to Community-based Care Transitions Programs12 and more than $200 million to Hospital Engagement Networks13 to carry out the goals of the Partnership for Patients,14 aiming to reduce rehospitalizations and other adverse events.
At first, despite these efforts, readmission rates did not appear to change substantially.15 However, the Centers for Medicare and Medicaid Services reported that hospital readmission rates for Medicare fee-for-service beneficiaries declined in 2012 to 18.4%,16 although some believe that the reduction is related to an increase in the number of patients admitted for observation in recent years.17
TRANSITIONS ARE OFTEN POORLY COORDINATED
Although some readmissions are unavoidable—resulting from the inevitable progression of disease or worsening of chronic conditions18—they may also result from a fumbled transition between care settings. Our current system of care transition has serious deficiencies that endanger patients. Areas that need improvement include communication between providers, patient education about medications and treatments, monitoring of medication adherence and complications, follow-up of pending tests and procedures after discharge, and outpatient follow-up soon after discharge.19–21
Traditional health care does not have dependable mechanisms for coordinating care across settings; we are all ensconced in “silos” that generally keep the focus within individual venues.22 Lack of coordination blurs the lines of responsibility for patients in the period between discharge from one location and admission to another, leaving them confused about whom to contact for care, especially if symptoms worsen.23,24
Gaps in coordination are not surprising, given the complexity of the US health care system and the often remarkable number of physicians caring for an individual patient.5 Medicare beneficiaries see an average of two primary care physicians and five specialists during a 2-year period; patients with chronic conditions may see up to 16 physicians in 1 year.25 Coordinating care between so many providers in different settings, combined with possible patient factors such as disadvantaged socioeconomic status, lack of caregiver support, and inadequate health literacy, provides many opportunities for failures.
Research has identified several root causes behind most failed care transitions:
Poor provider communication
Multiple studies associate adverse events after discharge with a lack of timely communication between hospital and outpatient providers.26 One study estimated that 80% of serious medical errors involve miscommunication during the hand-off between medical providers.27 Discharge summaries often lack important information such as test results, hospital course, discharge medications, patient counseling, and follow-up plans. Most adverse drug events after hospital discharge result directly from breakdown in communication between hospital staff and patients or primary care physicians.28 Approximately 40% of patients have test results pending at the time of discharge and 10% of these require some action; yet outpatient physicians and patients are often unaware of them.21
Ineffective patient and caregiver education
The Institute of Medicine report, Crossing the Quality Chasm: A New Health System for the 21st Century,29 noted that patients leaving one setting for another receive little information on how to care for themselves, when to resume activities, what medication side effects to watch out for, and how to get answers to questions. Of particular concern is that patients and caregivers are sometimes omitted from transition planning and often must suddenly assume new self-care responsibilities upon going home that hospital staff managed before discharge. Too often, patients are discharged with inadequate understanding of their medical condition, self-care plan,23,24 and who should manage their care.30
Up to 36% of adults in the United States have inadequate health literacy (defined as the inability to understand basic health information needed to make appropriate decisions), hindering patient education efforts.31–33 Even if they understand, patients and their caregivers must be engaged or “activated” (ie, able and willing to manage one’s health) if we expect them to adhere to appropriate care and behaviors. A review found direct correlations between patient activation and healthy behavior, better health outcomes (eg, achieving normal hemoglobin A1c and cholesterol levels), and better care experiences.34 This review also noted that multiple studies have documented improved activation scores as a result of specific interventions.
No follow-up with primary care providers
The risk of hospital readmission is significantly lower for patients with chronic obstructive pulmonary disease or heart failure who receive follow-up within 7 days of discharge.35–38 Of Medicare beneficiaries readmitted to the hospital within 30 days of discharge in 2003–2004, half had no contact with an outpatient physician in the interval between their discharge and their readmission,2 and one in three adult patients discharged from a hospital to the community does not see a physician within 30 days of discharge.39 The dearth of primary care providers in many communities can make follow-up care difficult to coordinate.
Failure to address chronic conditions
Analyses of national data sets reveal that patients are commonly rehospitalized for conditions unrelated to their initial hospitalization. According to the Center for Studying Health System Change, more than a quarter of readmissions in the 30 days after discharge are for conditions unrelated to those identified in the index admission, the proportion rising to more than one-third at 1 year.39 Among Medicare beneficiaries readmitted within 30 days of discharge, the proportion readmitted for the same condition was just 35% after hospitalization for heart failure, 10% after hospitalization for acute myocardial infarction, and 22% after hospitalization for pneumonia.40
Lack of community support
Multiple social and environmental factors contribute to adverse postdischarge events.41–43 For socioeconomically disadvantaged patients, care-transition issues are compounded by insufficient access to outpatient care, lack of social support, and lack of transportation. Some studies indicate that between 40% to 50% of readmissions are linked to social problems and inadequate access to community resources.44–47 Psychosocial issues such as limited health literacy, poor self-management skills, inadequate social support, and living alone are associated with adverse outcomes, including readmission and death.48,49 Such factors may help explain high levels of “no-shows” to outpatient follow-up visits.
NATIONAL MODELS OF BEST PRACTICES
Efforts to reduce readmissions have traditionally focused on hospitals, but experts now recognize that multiple factors influence readmissions and must be comprehensively addressed. Several evidence-based models seek to improve patient outcomes with interventions aimed at care transitions:
Project BOOST
Project BOOST (Better Outcomes by Optimizing Safe Transitions)50 is a national initiative developed by the Society of Hospital Medicine to standardize and optimize the care of patients discharged from hospital to home. The program includes evidence-based clinical interventions that can easily be adopted by any hospital. Interventions are aimed at:
- Identifying patients at high risk on admission
- Targeting risk-specific situations
- Improving information flow between inpatient and outpatient providers
- Improving patient and caregiver education by using the teach-back method
- Achieving timely follow-up after discharge.
The program includes a year of technical support provided by a physician mentor.
Preliminary results from pilot sites showed a 14% reduction in 30-day readmission rates in units using BOOST compared with control units in the same hospital.51 Mentored implementation was recognized by the Joint Commission and the National Quality Forum with the 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality.52
Project RED
Project RED (Re-Engineered Discharge)53 evolved from efforts by Dr. Brian Jack and colleagues to re-engineer the hospital workflow process to improve patient safety and reduce rehospitalization rates at Boston Medical Center. The intervention has 12 mutually reinforcing components aimed at improving the discharge process.
In a randomized controlled trial, Project RED led to a 30% decrease in emergency department visits and readmissions within 30 days of discharge from a general medical service of an urban academic medical center.54 This study excluded patients admitted from a skilled nursing facility or discharged to one, but a recent study demonstrated that Project RED also led to a lower rate of hospital admission within 30 days of discharge from a skilled nursing facility.55
The STAAR initiative
The STAAR initiative (State Action on Avoidable Re-hospitalizations)56 was launched in 2009 by the Institute for Healthcare Improvement with the goal of reducing avoidable readmissions in the states of Massachusetts, Michigan, and Washington. Hospital teams focus on improving:
- Assessment of needs after hospital discharge
- Teaching and learning
- Real-time hand-off communication
- Timely follow-up after hospital discharge.
As yet, no published studies other than case reports show a benefit from STAAR.57
The Care Transitions Program
The Care Transitions Program,58 under the leadership of Dr. Eric Coleman, aims to empower patients and caregivers, who meet with a “transition coach.” The program provides assistance with medication reconciliation and self-management, a patient-centered record owned and maintained by the patient to facilitate cross-site information transfer, timely outpatient follow-up with primary or specialty care, a list of red flags to indicate a worsening condition, and instructions on proper responses.
A randomized controlled trial of the program demonstrated a reduction in hospital readmissions at 30, 90, and 180 days, and lower hospital costs at 90 and 180 days.59 This approach also proved effective in a real-world setting.60
The Transitional Care Model
Developed by Dr. Mary Naylor and colleagues, the Transitional Care Model61 also aims at patient and family empowerment, focusing on patients’ stated goals and priorities and ensuring patient engagement. In the program, a transitional care nurse has the job of enhancing patient and caregiver understanding, facilitating patient self-management, and overseeing medication management and transitional care.
A randomized controlled trial demonstrated improved outcomes after hospital discharge for elderly patients with complex medical illnesses, with overall reductions in medical costs through preventing or delaying rehospitalization.62 A subsequent real-world study validated this approach.63
The Bridge Model
The Illinois Transitional Care Consortium’s Bridge Model64 is for older patients discharged home after hospitalization. It is led by social workers (“bridge care coordinators”) who address barriers to implementing the discharge plan, coordinate resources, and intervene at three points: before discharge, 2 days after discharge, and 30 days after discharge.
An initial study showed no impact on the 30-day rehospitalization rate,65 but larger studies are under way with a modified version.
Guided Care
Developed at the Johns Hopkins Bloomberg School of Public Health, Guided Care66 involves nurses who work in partnership with physicians and others in primary care to provide patient-centered, cost-effective care to patients with multiple chronic conditions. Nurses conduct in-home assessments, facilitate care planning, promote patient self-management, monitor conditions, coordinate the efforts of all care professionals, and facilitate access to community resources.
A cluster-randomized controlled trial found that this program had mixed results, reducing the use of home health care but having little effect on the use of other health services in the short run. However, in the subgroup of patients covered by Kaiser-Permanente, those who were randomized to the program accrued, on average, 52% fewer skilled nursing facility days, 47% fewer skilled nursing facility admissions, 49% fewer hospital readmissions, and 17% fewer emergency department visits.67
The GRACE model
The GRACE model (Geriatric Resources for Assessment and Care of Elders)68 was developed to improve the quality of geriatric care, reduce excess health care use, and prevent long-term nursing home placement. Each patient is assigned a support team consisting of a nurse practitioner and a social worker who make home visits, coordinate health care and community services, and develop an individualized care plan.
In one study,69 GRACE reduced hospital admission rates for participants at high risk of hospitalization by 12% in the first year of the program and 44% in the second year. GRACE participants also reported higher quality of life compared with the control group.69
INTERACT tools
Led by Dr. Joseph Ouslander, INTERACT (Interventions to Reduce Acute Care Transfers)70 is a quality-improvement initiative for skilled nursing facilities, designed to facilitate the early identification, evaluation, documentation, and communication of changes in the status of residents. Visitors to its website can download a set of tools and strategies to help them manage conditions before they become serious enough to require a hospital transfer. The tools assist in promoting important communication among providers and enhancing advance-care planning.
A 6-month study in 25 nursing homes showed a 17% reduction in self-reported hospital admissions with this program compared with the same period the previous year.71
Additional home-based care interventions
Additional innovations are under way in home-based care.
The Home Health Quality Improvement National Campaign is a patient-centered movement to improve the quality of care received by patients residing at home.72 Through its Best Practices Intervention Packages, it offers evidence-based educational tools, resources, and interventions for reducing avoidable hospitalizations, improving medication management, and coordinating transitional care.
The Center for Medicare and Medicaid Innovation Independence at Home Demonstration73 is testing whether home-based comprehensive primary care can improve care and reduce hospitalizations for Medicare beneficiaries with multiple chronic conditions.
NO SINGLE INTERVENTION: MULTIPLE STRATEGIES NEEDED
A 2011 review found no single intervention that regularly reduced the 30-day risk of re-hospitalization.74 However, other studies have shown that multifaceted interventions can reduce 30-day readmission rates. Randomized controlled trials in short-stay, acute care hospitals indicate that improvement in the following areas can directly reduce hospital readmission rates:
- Comprehensive planning and risk assessment throughout hospitalization
- Quality of care during the initial admission
- Communication with patients, their caregivers, and their clinicians
- Patient education
- Predischarge assessment
- Coordination of care after discharge.
In randomized trials, successful programs reduced the 30-day readmission rates by 20% to 40%,54,62,75–79 and a 2011 meta-analysis of randomized clinical trials found evidence that interventions associated with discharge planning helped to reduce readmission rates.80
Methods developed by the national care transition models described above can help hospitals optimize patient transitions (Table 1). Although every model has its unique attributes, they have several strategies in common:
Engage a team of key stakeholders that may include patients and caregivers, hospital staff (physicians, nurses, case managers, social workers, and pharmacists), community physicians (primary care, medical homes, and specialists), advance practice providers (physician assistants and nurse practitioners), and postacute care facilities and services (skilled nursing facilities, home health agencies, assisted living residences, hospice, and rehabilitation facilities).
Develop a comprehensive transition plan throughout hospitalization that includes attention to factors that may affect self-care, such as health literacy, chronic conditions, medications, and social support.
Enhance medication reconciliation and management. Obtain the best possible medication history on admission, and ensure that patients understand changes in their medications, how to take each medicine correctly, and important side effects.
Institute daily interdisciplinary communication and care coordination by everyone on the health care team with an emphasis on the care plan, discharge planning, and safety issues.81
Standardize transition plans, procedures and forms. All discharging physicians should use a standard discharge summary template that includes pertinent diagnoses, active issues, a reconciled medication list with changes highlighted, results from important tests and consultations, pending test results, planned follow-up and required services, warning signs of a worsening condition, and actions needed if a problem arises.
Always send discharge summaries directly to the patient’s primary care physician or next care setting at the time of discharge.
Give the patient a discharge plan that is easy to understand. Enhance patient and family education using health literacy standards82 and interactive methods such as teach-back,83 in which patients demonstrate comprehension and skills required for self-care immediately after being taught. Such tools actively teach patients and caregivers to follow a care plan, including managing medications.
Follow up and coordinate support in a timely manner after a patient leaves the care setting. Follow-up visits should be arranged before discharge. Within 1 to 3 days after discharge, the patient should be called or visited by a case manager, social worker, nurse, or other health care provider.
CHALLENGES TO IMPROVING TRANSITIONS
Although several models demonstrated significant reductions of hospital readmissions in trials, challenges remain. Studies do not identify which features of the models are necessary or sufficient, or how applicable they are to different hospital and patient characteristics. A 2012 analysis84 of a program designed to reduce readmissions in three states identified key obstacles to successfully improving care transitions:
Collaborative relationships across settings are critical, but very difficult to achieve. It takes time to develop the relationships and trust among providers, and little incentive exists for skilled nursing facilities and physicians outside the hospital to engage in the process.
Infrastructure is lacking, as is experience to implement quality improvements.
We lack proof that models work on a large scale. Confusion exists about which readmissions are preventable and which are not. More evidence is needed to help guide hospitals’ efforts to improve transitions of care and reduce readmissions.
- Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc 2003; 51:549–555.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Rosenthal JM, Miller DB. Providers have failed to work for continuity. Hospitals 1979; 53:79–83.
- Gabow P, Halvorson G, Kaplan G. Marshaling leadership for high-value health care: an Institute of Medicine discussion paper. JAMA 2012; 308:239–240.
- Bonner A, Schneider CD, Weissman JS. Massachusetts State Quality Improvement Institute. Massachusetts Strategic Plan for Care Transitions. Massachusetts Executive Office of Health and Human Services, 2010. http://www.patientcarelink.org/uploadDocs/1/Strategic-Plan-for-Care-Transitions_2-11-2010-(2).pdf. Accessed April 7, 2014.
- Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood) 2010; 29:57–64.
- Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Diagnosis, 2010. HCUP Statistical Brief #153. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed April 7, 2014.
- Weiss AJ (Truven Health Analytics), Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Procedure, 2010. HCUP Statistical Brief #154. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb154.pdf. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Medicare To Penalize 2,217 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2012/August/13/medicare-hospitals-readmissions-penalties.aspx. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Armed With Bigger Fines, Medicare To Punish 2,225 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2013/August/02/readmission-penalties-medicare-hospitals-year-two.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Community-based Care Transitions Program. http://innovation.cms.gov/initiatives/CCTP/. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Hospital Engagement Networks (HENs). http://partnershipforpatients.cms.gov/about-the-partnership/hospital-engagement-networks/thehospitalengagementnetworks.html. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). About the Partnership for Patients. http://partnershipforpatients.cms.gov/about-the-partnership/about-thepartnershipforpatients.html. Accessed April 7, 2014.
- Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med 2012; 366:1606–615.
- Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N; Centers for Medicare & Medicaid Services (CMS). Medicare Readmission Rates Showed Meaningful Decline in 2012. http://www.cms.gov/mmrr/Briefs/B2013/mmrr-2013-003-02-b01.html. Accessed April 7, 2014.
- Office of Inspector General; US Department of Health and Human Services. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Report (OEI-02-12-00040). http://oig.hhs.gov/oei/reports/oei-02-12-00040.asp. Accessed April 7, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med 2007; 167:1305–1311.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005; 143:121–128.
- Coleman EA, Fox PD; HMO Workgroup on Care Management. Managing patient care transitions: a report of the HMO Care Management Workgroup. Health-plan 2004; 45:36–39.
- Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med 2004; 141:533–536.
- Snow V, Beck D, Budnitz T, et al; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med 2009; 24:971–976.
- Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med 2007; 356:1130–1139.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Solet DJ, Norvell JM, Rutan GH, Frankel RM. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med 2005; 80:1094–1099.
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2007; 2:314–323.
- National Research Council. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press, 2001.
- O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc 2010; 85:47–52.
- Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual 2013; 28:383–391.
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011; 155:97–107.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics, 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed April 7, 2014.
- Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013; 32:207–214.
- Lin CY, Barnato AE, Degenholtz HB. Physician follow-up visits after acute care hospitalization for elderly Medicare beneficiaries discharged to noninstitutional settings. J Am Geriatr Soc 2011; 59:1947–1954.
- Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010; 170:1664–1670.
- Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010; 303:1716–1722.
- van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med 2010; 5:398–405.
- Sommers A, Cunningham PJ. Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Research Brief No. 6. National Institute for Health Care Reform (NIHCR), 2011. www.nihcr.org/Reducing_Readmissions.html. Accessed April 7, 2014.
- Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309:355–363.
- Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med 2013; 28:269–282.
- Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med 2011; 11:49.
- Weissman JS, Stern RS, Epstein AM. The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals. Inquiry 1994; 31:163–172.
- Proctor EK, Morrow-Howell N, Li H, Dore P. Adequacy of home care and hospital readmission for elderly congestive heart failure patients. Health Soc Work 2000; 25:87–96.
- Kansagara D, Ramsay RS, Labby D, Saha S. Post-discharge intervention in vulnerable, chronically ill patients. J Hosp Med 2012; 7:124–130.
- Englander H, Kansagara D. Planning and designing the care transitions innovation (C-Train) for uninsured and Medicaid patients. J Hosp Med 2012; 7:524–529.
- Brown R, Peikes D, Chen A, Schore J. 15-site randomized trial of coordinated care in Medicare FFS. Health Care Financ Rev 2008; 30:5–25.
- Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Post-discharge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist 2008; 48:495–504.
- Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health 2009; 27:287–302.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. www.hospitalmedicine.org/BOOST. Accessed April 7, 2014.
- Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013; 8:421–427.
- Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf 2012; 38:301–310.
- Boston University Medical Center. Project RED: Re-Engineered Discharge. www.bu.edu/fammed/projectred/. Accessed April 7, 2014.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
- Berkowitz RE, Fang Z, Helfand BK, Jones RN, Schreiber R, Paasche-Orlow MK. Project ReEngineered Discharge (RED) lowers hospital readmissions of patients discharged from a skilled nursing facility. J Am Med Dir Assoc 2013; 14:736–740.
- Institute for Healthcare Improvement. STAAR: STate Action on Avoidable Re-hospitalizations. www.ihi.org/offerings/Initiatives/STAAR/Pages/default.aspx. Accessed April 7, 2014.
- Boutwell AE, Johnson MB, Rutherford P, et al. An early look at a four-state initiative to reduce avoidable hospital readmissions. Health Aff (Millwood) 2011; 30:1272–1280.
- University of Colorado Denver. The Care Transitions Program. www.caretransitions.org/. Accessed April 7, 2014.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Penn Nursing Science. Transitional Care Model. www.transitional-care.info/. Accessed April 7, 2014.
- Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med 1994; 120:999–1006.
- Stauffer BD, Fullerton C, Fleming N, et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med 2011; 171:1238–1243.
- The Illinois Transitional Care Consortium. The Bridge Model. www.transitionalcare.org/the-bridge-model. Accessed April 7, 2014.
- Altfeld SJ, Shier GE, Rooney M, et al. Effects of an enhanced discharge planning intervention for hospitalized older adults: a randomized trial. Gerontologist 2013; 53:430–440.
- Johns Hopkins Bloomberg School of Public Health. Guided Care. www.guidedcare.org. Accessed April 7, 2014.
- Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med 2011; 171:460–466.
- Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc 2006; 54:1136–1141.
- Bielaszka-DuVernay C. The ‘GRACE’ model: in-home assessments lead to better care for dual eligibles. Health Aff (Millwood) 2011; 30:431–434.
- Florida Atlantic University. INTERACT: Interventions to Reduce Acute Care Transfers. http://interact2.net/. Accessed April 7, 2014.
- Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. J Am Geriatr Soc 2011; 59:745–753.
- West Virginia Medical Institute. HHQI-BPIPs (Home Health Quality Improvement - Best Practices Intervention Packages). www.home-healthquality.org/Education/BPIPS.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Independence at Home Demonstration. http://innovation.cms.gov/initiatives/Independence-at-Home/. Accessed April 7, 2014.
- Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med 2011; 155:520–528.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009; 4:211–218.
- Garåsen H, Windspoll R, Johnsen R. Intermediate care at a community hospital as an alternative to prolonged general hospital care for elderly patients: a randomised controlled trial. BMC Public Health 2007; 7:68.
- Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc 2009; 57:395–402.
- Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc 2004; 52:1817–1825.
- Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood) 2011; 30:746–754.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med 2011; 171:678–684.
- Agency for Healthcare Research and Quality (AHRQ). Health Literacy Universal Precautions Toolkit. www.ahrq.gov/legacy/qual/literacy/. Accessed April 7, 2014.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Mittler JN, O’Hora JL, Harvey JB, Press MJ, Volpp KG, Scanlon DP. Turning readmission reduction policies into results: some lessons from a multistate initiative to reduce readmissions. Popul Health Manag 2013; 16:255–260.
You have spent several days checking on a patient hospitalized for an acute exacerbation of heart failure. You have straightened out her medications and diet and discussed a plan for follow-up with the patient and a family member, and now she is being wheeled out the door. What happens to her next?
Too often, not your desired plan. If she is going home, maybe she understands what she needs to do, maybe not. Maybe she will get your prescriptions filled and take the medications as directed, maybe not. If she is going to a nursing home, maybe the physician covering the nursing home will get your plan, maybe not. There is a good chance she will be back in the emergency room soon, all because of a poor transition of care.
Transitions of care are changes in the level, location, or providers of care as patients move within the health care system. These can be critical junctures in patients’ lives, and if poorly executed can result in many adverse effects—including rehospitalization.1
Although high rehospitalization rates gained national attention in 2009 after a analysis of Medicare data,2 health care providers have known about the lack of coordinated care transitions for more than 50 years.3 Despite some progress, improving care transitions remains a national challenge. As the health system evolves from a fee-for-service financial model to payment-for-value,4 it is especially important that health care providers improve care for patients by optimizing care transitions.
In this article, we summarize the factors contributing to poor care transitions, highlight programs that improve them, and discuss strategies for successful transitions.
TRANSITION PROBLEMS ARE COMMON
Transitions of care occur when patients move to short-term and long-term acute care hospitals, skilled nursing facilities, primary and specialty care offices, community health centers, rehabilitation facilities, home health agencies, hospice, and their own homes.5 Problems can arise at any of these transitions, but the risk is especially high when patients leave the hospital to receive care in another setting or at home.
In the past decade, one in five Medicare patients was rehospitalized within 30 days of discharge from the hospital,2 and up to 25% were rehospitalized after being discharged to a skilled nursing facility.6 Some diagnoses (eg, sickle cell anemia, gangrene) and procedures (eg, kidney transplantation, ileostomy) are associated with readmission rates of nearly one in three.7,8
The desire of policymakers to “bend the cost curve” of health care has led to efforts to enhance care coordination by improving transitions between care venues. Through the Patient Protection and Affordable Care Act, a number of federal initiatives are promoting strategies to improve care transitions and prevent readmissions after hospital discharge.
The Hospital Readmission Reduction Program9 drives much of this effort. In fiscal year 2013 (beginning October 1, 2012), more than 2,000 hospitals incurred financial penalties of up to 1% of total Medicare diagnosis-related group payments (about $280 million the first year) for excess readmissions.10 The penalty’s maximum rose to 2% in fiscal year 2014 and could increase to 3% in 2015. The total penalty for 2014 is projected to be $227 million, with 2,225 hospitals affected.11
The Centers for Medicare and Medicaid Innovation has committed hundreds of millions of dollars to Community-based Care Transitions Programs12 and more than $200 million to Hospital Engagement Networks13 to carry out the goals of the Partnership for Patients,14 aiming to reduce rehospitalizations and other adverse events.
At first, despite these efforts, readmission rates did not appear to change substantially.15 However, the Centers for Medicare and Medicaid Services reported that hospital readmission rates for Medicare fee-for-service beneficiaries declined in 2012 to 18.4%,16 although some believe that the reduction is related to an increase in the number of patients admitted for observation in recent years.17
TRANSITIONS ARE OFTEN POORLY COORDINATED
Although some readmissions are unavoidable—resulting from the inevitable progression of disease or worsening of chronic conditions18—they may also result from a fumbled transition between care settings. Our current system of care transition has serious deficiencies that endanger patients. Areas that need improvement include communication between providers, patient education about medications and treatments, monitoring of medication adherence and complications, follow-up of pending tests and procedures after discharge, and outpatient follow-up soon after discharge.19–21
Traditional health care does not have dependable mechanisms for coordinating care across settings; we are all ensconced in “silos” that generally keep the focus within individual venues.22 Lack of coordination blurs the lines of responsibility for patients in the period between discharge from one location and admission to another, leaving them confused about whom to contact for care, especially if symptoms worsen.23,24
Gaps in coordination are not surprising, given the complexity of the US health care system and the often remarkable number of physicians caring for an individual patient.5 Medicare beneficiaries see an average of two primary care physicians and five specialists during a 2-year period; patients with chronic conditions may see up to 16 physicians in 1 year.25 Coordinating care between so many providers in different settings, combined with possible patient factors such as disadvantaged socioeconomic status, lack of caregiver support, and inadequate health literacy, provides many opportunities for failures.
Research has identified several root causes behind most failed care transitions:
Poor provider communication
Multiple studies associate adverse events after discharge with a lack of timely communication between hospital and outpatient providers.26 One study estimated that 80% of serious medical errors involve miscommunication during the hand-off between medical providers.27 Discharge summaries often lack important information such as test results, hospital course, discharge medications, patient counseling, and follow-up plans. Most adverse drug events after hospital discharge result directly from breakdown in communication between hospital staff and patients or primary care physicians.28 Approximately 40% of patients have test results pending at the time of discharge and 10% of these require some action; yet outpatient physicians and patients are often unaware of them.21
Ineffective patient and caregiver education
The Institute of Medicine report, Crossing the Quality Chasm: A New Health System for the 21st Century,29 noted that patients leaving one setting for another receive little information on how to care for themselves, when to resume activities, what medication side effects to watch out for, and how to get answers to questions. Of particular concern is that patients and caregivers are sometimes omitted from transition planning and often must suddenly assume new self-care responsibilities upon going home that hospital staff managed before discharge. Too often, patients are discharged with inadequate understanding of their medical condition, self-care plan,23,24 and who should manage their care.30
Up to 36% of adults in the United States have inadequate health literacy (defined as the inability to understand basic health information needed to make appropriate decisions), hindering patient education efforts.31–33 Even if they understand, patients and their caregivers must be engaged or “activated” (ie, able and willing to manage one’s health) if we expect them to adhere to appropriate care and behaviors. A review found direct correlations between patient activation and healthy behavior, better health outcomes (eg, achieving normal hemoglobin A1c and cholesterol levels), and better care experiences.34 This review also noted that multiple studies have documented improved activation scores as a result of specific interventions.
No follow-up with primary care providers
The risk of hospital readmission is significantly lower for patients with chronic obstructive pulmonary disease or heart failure who receive follow-up within 7 days of discharge.35–38 Of Medicare beneficiaries readmitted to the hospital within 30 days of discharge in 2003–2004, half had no contact with an outpatient physician in the interval between their discharge and their readmission,2 and one in three adult patients discharged from a hospital to the community does not see a physician within 30 days of discharge.39 The dearth of primary care providers in many communities can make follow-up care difficult to coordinate.
Failure to address chronic conditions
Analyses of national data sets reveal that patients are commonly rehospitalized for conditions unrelated to their initial hospitalization. According to the Center for Studying Health System Change, more than a quarter of readmissions in the 30 days after discharge are for conditions unrelated to those identified in the index admission, the proportion rising to more than one-third at 1 year.39 Among Medicare beneficiaries readmitted within 30 days of discharge, the proportion readmitted for the same condition was just 35% after hospitalization for heart failure, 10% after hospitalization for acute myocardial infarction, and 22% after hospitalization for pneumonia.40
Lack of community support
Multiple social and environmental factors contribute to adverse postdischarge events.41–43 For socioeconomically disadvantaged patients, care-transition issues are compounded by insufficient access to outpatient care, lack of social support, and lack of transportation. Some studies indicate that between 40% to 50% of readmissions are linked to social problems and inadequate access to community resources.44–47 Psychosocial issues such as limited health literacy, poor self-management skills, inadequate social support, and living alone are associated with adverse outcomes, including readmission and death.48,49 Such factors may help explain high levels of “no-shows” to outpatient follow-up visits.
NATIONAL MODELS OF BEST PRACTICES
Efforts to reduce readmissions have traditionally focused on hospitals, but experts now recognize that multiple factors influence readmissions and must be comprehensively addressed. Several evidence-based models seek to improve patient outcomes with interventions aimed at care transitions:
Project BOOST
Project BOOST (Better Outcomes by Optimizing Safe Transitions)50 is a national initiative developed by the Society of Hospital Medicine to standardize and optimize the care of patients discharged from hospital to home. The program includes evidence-based clinical interventions that can easily be adopted by any hospital. Interventions are aimed at:
- Identifying patients at high risk on admission
- Targeting risk-specific situations
- Improving information flow between inpatient and outpatient providers
- Improving patient and caregiver education by using the teach-back method
- Achieving timely follow-up after discharge.
The program includes a year of technical support provided by a physician mentor.
Preliminary results from pilot sites showed a 14% reduction in 30-day readmission rates in units using BOOST compared with control units in the same hospital.51 Mentored implementation was recognized by the Joint Commission and the National Quality Forum with the 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality.52
Project RED
Project RED (Re-Engineered Discharge)53 evolved from efforts by Dr. Brian Jack and colleagues to re-engineer the hospital workflow process to improve patient safety and reduce rehospitalization rates at Boston Medical Center. The intervention has 12 mutually reinforcing components aimed at improving the discharge process.
In a randomized controlled trial, Project RED led to a 30% decrease in emergency department visits and readmissions within 30 days of discharge from a general medical service of an urban academic medical center.54 This study excluded patients admitted from a skilled nursing facility or discharged to one, but a recent study demonstrated that Project RED also led to a lower rate of hospital admission within 30 days of discharge from a skilled nursing facility.55
The STAAR initiative
The STAAR initiative (State Action on Avoidable Re-hospitalizations)56 was launched in 2009 by the Institute for Healthcare Improvement with the goal of reducing avoidable readmissions in the states of Massachusetts, Michigan, and Washington. Hospital teams focus on improving:
- Assessment of needs after hospital discharge
- Teaching and learning
- Real-time hand-off communication
- Timely follow-up after hospital discharge.
As yet, no published studies other than case reports show a benefit from STAAR.57
The Care Transitions Program
The Care Transitions Program,58 under the leadership of Dr. Eric Coleman, aims to empower patients and caregivers, who meet with a “transition coach.” The program provides assistance with medication reconciliation and self-management, a patient-centered record owned and maintained by the patient to facilitate cross-site information transfer, timely outpatient follow-up with primary or specialty care, a list of red flags to indicate a worsening condition, and instructions on proper responses.
A randomized controlled trial of the program demonstrated a reduction in hospital readmissions at 30, 90, and 180 days, and lower hospital costs at 90 and 180 days.59 This approach also proved effective in a real-world setting.60
The Transitional Care Model
Developed by Dr. Mary Naylor and colleagues, the Transitional Care Model61 also aims at patient and family empowerment, focusing on patients’ stated goals and priorities and ensuring patient engagement. In the program, a transitional care nurse has the job of enhancing patient and caregiver understanding, facilitating patient self-management, and overseeing medication management and transitional care.
A randomized controlled trial demonstrated improved outcomes after hospital discharge for elderly patients with complex medical illnesses, with overall reductions in medical costs through preventing or delaying rehospitalization.62 A subsequent real-world study validated this approach.63
The Bridge Model
The Illinois Transitional Care Consortium’s Bridge Model64 is for older patients discharged home after hospitalization. It is led by social workers (“bridge care coordinators”) who address barriers to implementing the discharge plan, coordinate resources, and intervene at three points: before discharge, 2 days after discharge, and 30 days after discharge.
An initial study showed no impact on the 30-day rehospitalization rate,65 but larger studies are under way with a modified version.
Guided Care
Developed at the Johns Hopkins Bloomberg School of Public Health, Guided Care66 involves nurses who work in partnership with physicians and others in primary care to provide patient-centered, cost-effective care to patients with multiple chronic conditions. Nurses conduct in-home assessments, facilitate care planning, promote patient self-management, monitor conditions, coordinate the efforts of all care professionals, and facilitate access to community resources.
A cluster-randomized controlled trial found that this program had mixed results, reducing the use of home health care but having little effect on the use of other health services in the short run. However, in the subgroup of patients covered by Kaiser-Permanente, those who were randomized to the program accrued, on average, 52% fewer skilled nursing facility days, 47% fewer skilled nursing facility admissions, 49% fewer hospital readmissions, and 17% fewer emergency department visits.67
The GRACE model
The GRACE model (Geriatric Resources for Assessment and Care of Elders)68 was developed to improve the quality of geriatric care, reduce excess health care use, and prevent long-term nursing home placement. Each patient is assigned a support team consisting of a nurse practitioner and a social worker who make home visits, coordinate health care and community services, and develop an individualized care plan.
In one study,69 GRACE reduced hospital admission rates for participants at high risk of hospitalization by 12% in the first year of the program and 44% in the second year. GRACE participants also reported higher quality of life compared with the control group.69
INTERACT tools
Led by Dr. Joseph Ouslander, INTERACT (Interventions to Reduce Acute Care Transfers)70 is a quality-improvement initiative for skilled nursing facilities, designed to facilitate the early identification, evaluation, documentation, and communication of changes in the status of residents. Visitors to its website can download a set of tools and strategies to help them manage conditions before they become serious enough to require a hospital transfer. The tools assist in promoting important communication among providers and enhancing advance-care planning.
A 6-month study in 25 nursing homes showed a 17% reduction in self-reported hospital admissions with this program compared with the same period the previous year.71
Additional home-based care interventions
Additional innovations are under way in home-based care.
The Home Health Quality Improvement National Campaign is a patient-centered movement to improve the quality of care received by patients residing at home.72 Through its Best Practices Intervention Packages, it offers evidence-based educational tools, resources, and interventions for reducing avoidable hospitalizations, improving medication management, and coordinating transitional care.
The Center for Medicare and Medicaid Innovation Independence at Home Demonstration73 is testing whether home-based comprehensive primary care can improve care and reduce hospitalizations for Medicare beneficiaries with multiple chronic conditions.
NO SINGLE INTERVENTION: MULTIPLE STRATEGIES NEEDED
A 2011 review found no single intervention that regularly reduced the 30-day risk of re-hospitalization.74 However, other studies have shown that multifaceted interventions can reduce 30-day readmission rates. Randomized controlled trials in short-stay, acute care hospitals indicate that improvement in the following areas can directly reduce hospital readmission rates:
- Comprehensive planning and risk assessment throughout hospitalization
- Quality of care during the initial admission
- Communication with patients, their caregivers, and their clinicians
- Patient education
- Predischarge assessment
- Coordination of care after discharge.
In randomized trials, successful programs reduced the 30-day readmission rates by 20% to 40%,54,62,75–79 and a 2011 meta-analysis of randomized clinical trials found evidence that interventions associated with discharge planning helped to reduce readmission rates.80
Methods developed by the national care transition models described above can help hospitals optimize patient transitions (Table 1). Although every model has its unique attributes, they have several strategies in common:
Engage a team of key stakeholders that may include patients and caregivers, hospital staff (physicians, nurses, case managers, social workers, and pharmacists), community physicians (primary care, medical homes, and specialists), advance practice providers (physician assistants and nurse practitioners), and postacute care facilities and services (skilled nursing facilities, home health agencies, assisted living residences, hospice, and rehabilitation facilities).
Develop a comprehensive transition plan throughout hospitalization that includes attention to factors that may affect self-care, such as health literacy, chronic conditions, medications, and social support.
Enhance medication reconciliation and management. Obtain the best possible medication history on admission, and ensure that patients understand changes in their medications, how to take each medicine correctly, and important side effects.
Institute daily interdisciplinary communication and care coordination by everyone on the health care team with an emphasis on the care plan, discharge planning, and safety issues.81
Standardize transition plans, procedures and forms. All discharging physicians should use a standard discharge summary template that includes pertinent diagnoses, active issues, a reconciled medication list with changes highlighted, results from important tests and consultations, pending test results, planned follow-up and required services, warning signs of a worsening condition, and actions needed if a problem arises.
Always send discharge summaries directly to the patient’s primary care physician or next care setting at the time of discharge.
Give the patient a discharge plan that is easy to understand. Enhance patient and family education using health literacy standards82 and interactive methods such as teach-back,83 in which patients demonstrate comprehension and skills required for self-care immediately after being taught. Such tools actively teach patients and caregivers to follow a care plan, including managing medications.
Follow up and coordinate support in a timely manner after a patient leaves the care setting. Follow-up visits should be arranged before discharge. Within 1 to 3 days after discharge, the patient should be called or visited by a case manager, social worker, nurse, or other health care provider.
CHALLENGES TO IMPROVING TRANSITIONS
Although several models demonstrated significant reductions of hospital readmissions in trials, challenges remain. Studies do not identify which features of the models are necessary or sufficient, or how applicable they are to different hospital and patient characteristics. A 2012 analysis84 of a program designed to reduce readmissions in three states identified key obstacles to successfully improving care transitions:
Collaborative relationships across settings are critical, but very difficult to achieve. It takes time to develop the relationships and trust among providers, and little incentive exists for skilled nursing facilities and physicians outside the hospital to engage in the process.
Infrastructure is lacking, as is experience to implement quality improvements.
We lack proof that models work on a large scale. Confusion exists about which readmissions are preventable and which are not. More evidence is needed to help guide hospitals’ efforts to improve transitions of care and reduce readmissions.
You have spent several days checking on a patient hospitalized for an acute exacerbation of heart failure. You have straightened out her medications and diet and discussed a plan for follow-up with the patient and a family member, and now she is being wheeled out the door. What happens to her next?
Too often, not your desired plan. If she is going home, maybe she understands what she needs to do, maybe not. Maybe she will get your prescriptions filled and take the medications as directed, maybe not. If she is going to a nursing home, maybe the physician covering the nursing home will get your plan, maybe not. There is a good chance she will be back in the emergency room soon, all because of a poor transition of care.
Transitions of care are changes in the level, location, or providers of care as patients move within the health care system. These can be critical junctures in patients’ lives, and if poorly executed can result in many adverse effects—including rehospitalization.1
Although high rehospitalization rates gained national attention in 2009 after a analysis of Medicare data,2 health care providers have known about the lack of coordinated care transitions for more than 50 years.3 Despite some progress, improving care transitions remains a national challenge. As the health system evolves from a fee-for-service financial model to payment-for-value,4 it is especially important that health care providers improve care for patients by optimizing care transitions.
In this article, we summarize the factors contributing to poor care transitions, highlight programs that improve them, and discuss strategies for successful transitions.
TRANSITION PROBLEMS ARE COMMON
Transitions of care occur when patients move to short-term and long-term acute care hospitals, skilled nursing facilities, primary and specialty care offices, community health centers, rehabilitation facilities, home health agencies, hospice, and their own homes.5 Problems can arise at any of these transitions, but the risk is especially high when patients leave the hospital to receive care in another setting or at home.
In the past decade, one in five Medicare patients was rehospitalized within 30 days of discharge from the hospital,2 and up to 25% were rehospitalized after being discharged to a skilled nursing facility.6 Some diagnoses (eg, sickle cell anemia, gangrene) and procedures (eg, kidney transplantation, ileostomy) are associated with readmission rates of nearly one in three.7,8
The desire of policymakers to “bend the cost curve” of health care has led to efforts to enhance care coordination by improving transitions between care venues. Through the Patient Protection and Affordable Care Act, a number of federal initiatives are promoting strategies to improve care transitions and prevent readmissions after hospital discharge.
The Hospital Readmission Reduction Program9 drives much of this effort. In fiscal year 2013 (beginning October 1, 2012), more than 2,000 hospitals incurred financial penalties of up to 1% of total Medicare diagnosis-related group payments (about $280 million the first year) for excess readmissions.10 The penalty’s maximum rose to 2% in fiscal year 2014 and could increase to 3% in 2015. The total penalty for 2014 is projected to be $227 million, with 2,225 hospitals affected.11
The Centers for Medicare and Medicaid Innovation has committed hundreds of millions of dollars to Community-based Care Transitions Programs12 and more than $200 million to Hospital Engagement Networks13 to carry out the goals of the Partnership for Patients,14 aiming to reduce rehospitalizations and other adverse events.
At first, despite these efforts, readmission rates did not appear to change substantially.15 However, the Centers for Medicare and Medicaid Services reported that hospital readmission rates for Medicare fee-for-service beneficiaries declined in 2012 to 18.4%,16 although some believe that the reduction is related to an increase in the number of patients admitted for observation in recent years.17
TRANSITIONS ARE OFTEN POORLY COORDINATED
Although some readmissions are unavoidable—resulting from the inevitable progression of disease or worsening of chronic conditions18—they may also result from a fumbled transition between care settings. Our current system of care transition has serious deficiencies that endanger patients. Areas that need improvement include communication between providers, patient education about medications and treatments, monitoring of medication adherence and complications, follow-up of pending tests and procedures after discharge, and outpatient follow-up soon after discharge.19–21
Traditional health care does not have dependable mechanisms for coordinating care across settings; we are all ensconced in “silos” that generally keep the focus within individual venues.22 Lack of coordination blurs the lines of responsibility for patients in the period between discharge from one location and admission to another, leaving them confused about whom to contact for care, especially if symptoms worsen.23,24
Gaps in coordination are not surprising, given the complexity of the US health care system and the often remarkable number of physicians caring for an individual patient.5 Medicare beneficiaries see an average of two primary care physicians and five specialists during a 2-year period; patients with chronic conditions may see up to 16 physicians in 1 year.25 Coordinating care between so many providers in different settings, combined with possible patient factors such as disadvantaged socioeconomic status, lack of caregiver support, and inadequate health literacy, provides many opportunities for failures.
Research has identified several root causes behind most failed care transitions:
Poor provider communication
Multiple studies associate adverse events after discharge with a lack of timely communication between hospital and outpatient providers.26 One study estimated that 80% of serious medical errors involve miscommunication during the hand-off between medical providers.27 Discharge summaries often lack important information such as test results, hospital course, discharge medications, patient counseling, and follow-up plans. Most adverse drug events after hospital discharge result directly from breakdown in communication between hospital staff and patients or primary care physicians.28 Approximately 40% of patients have test results pending at the time of discharge and 10% of these require some action; yet outpatient physicians and patients are often unaware of them.21
Ineffective patient and caregiver education
The Institute of Medicine report, Crossing the Quality Chasm: A New Health System for the 21st Century,29 noted that patients leaving one setting for another receive little information on how to care for themselves, when to resume activities, what medication side effects to watch out for, and how to get answers to questions. Of particular concern is that patients and caregivers are sometimes omitted from transition planning and often must suddenly assume new self-care responsibilities upon going home that hospital staff managed before discharge. Too often, patients are discharged with inadequate understanding of their medical condition, self-care plan,23,24 and who should manage their care.30
Up to 36% of adults in the United States have inadequate health literacy (defined as the inability to understand basic health information needed to make appropriate decisions), hindering patient education efforts.31–33 Even if they understand, patients and their caregivers must be engaged or “activated” (ie, able and willing to manage one’s health) if we expect them to adhere to appropriate care and behaviors. A review found direct correlations between patient activation and healthy behavior, better health outcomes (eg, achieving normal hemoglobin A1c and cholesterol levels), and better care experiences.34 This review also noted that multiple studies have documented improved activation scores as a result of specific interventions.
No follow-up with primary care providers
The risk of hospital readmission is significantly lower for patients with chronic obstructive pulmonary disease or heart failure who receive follow-up within 7 days of discharge.35–38 Of Medicare beneficiaries readmitted to the hospital within 30 days of discharge in 2003–2004, half had no contact with an outpatient physician in the interval between their discharge and their readmission,2 and one in three adult patients discharged from a hospital to the community does not see a physician within 30 days of discharge.39 The dearth of primary care providers in many communities can make follow-up care difficult to coordinate.
Failure to address chronic conditions
Analyses of national data sets reveal that patients are commonly rehospitalized for conditions unrelated to their initial hospitalization. According to the Center for Studying Health System Change, more than a quarter of readmissions in the 30 days after discharge are for conditions unrelated to those identified in the index admission, the proportion rising to more than one-third at 1 year.39 Among Medicare beneficiaries readmitted within 30 days of discharge, the proportion readmitted for the same condition was just 35% after hospitalization for heart failure, 10% after hospitalization for acute myocardial infarction, and 22% after hospitalization for pneumonia.40
Lack of community support
Multiple social and environmental factors contribute to adverse postdischarge events.41–43 For socioeconomically disadvantaged patients, care-transition issues are compounded by insufficient access to outpatient care, lack of social support, and lack of transportation. Some studies indicate that between 40% to 50% of readmissions are linked to social problems and inadequate access to community resources.44–47 Psychosocial issues such as limited health literacy, poor self-management skills, inadequate social support, and living alone are associated with adverse outcomes, including readmission and death.48,49 Such factors may help explain high levels of “no-shows” to outpatient follow-up visits.
NATIONAL MODELS OF BEST PRACTICES
Efforts to reduce readmissions have traditionally focused on hospitals, but experts now recognize that multiple factors influence readmissions and must be comprehensively addressed. Several evidence-based models seek to improve patient outcomes with interventions aimed at care transitions:
Project BOOST
Project BOOST (Better Outcomes by Optimizing Safe Transitions)50 is a national initiative developed by the Society of Hospital Medicine to standardize and optimize the care of patients discharged from hospital to home. The program includes evidence-based clinical interventions that can easily be adopted by any hospital. Interventions are aimed at:
- Identifying patients at high risk on admission
- Targeting risk-specific situations
- Improving information flow between inpatient and outpatient providers
- Improving patient and caregiver education by using the teach-back method
- Achieving timely follow-up after discharge.
The program includes a year of technical support provided by a physician mentor.
Preliminary results from pilot sites showed a 14% reduction in 30-day readmission rates in units using BOOST compared with control units in the same hospital.51 Mentored implementation was recognized by the Joint Commission and the National Quality Forum with the 2011 John M. Eisenberg Award for Innovation in Patient Safety and Quality.52
Project RED
Project RED (Re-Engineered Discharge)53 evolved from efforts by Dr. Brian Jack and colleagues to re-engineer the hospital workflow process to improve patient safety and reduce rehospitalization rates at Boston Medical Center. The intervention has 12 mutually reinforcing components aimed at improving the discharge process.
In a randomized controlled trial, Project RED led to a 30% decrease in emergency department visits and readmissions within 30 days of discharge from a general medical service of an urban academic medical center.54 This study excluded patients admitted from a skilled nursing facility or discharged to one, but a recent study demonstrated that Project RED also led to a lower rate of hospital admission within 30 days of discharge from a skilled nursing facility.55
The STAAR initiative
The STAAR initiative (State Action on Avoidable Re-hospitalizations)56 was launched in 2009 by the Institute for Healthcare Improvement with the goal of reducing avoidable readmissions in the states of Massachusetts, Michigan, and Washington. Hospital teams focus on improving:
- Assessment of needs after hospital discharge
- Teaching and learning
- Real-time hand-off communication
- Timely follow-up after hospital discharge.
As yet, no published studies other than case reports show a benefit from STAAR.57
The Care Transitions Program
The Care Transitions Program,58 under the leadership of Dr. Eric Coleman, aims to empower patients and caregivers, who meet with a “transition coach.” The program provides assistance with medication reconciliation and self-management, a patient-centered record owned and maintained by the patient to facilitate cross-site information transfer, timely outpatient follow-up with primary or specialty care, a list of red flags to indicate a worsening condition, and instructions on proper responses.
A randomized controlled trial of the program demonstrated a reduction in hospital readmissions at 30, 90, and 180 days, and lower hospital costs at 90 and 180 days.59 This approach also proved effective in a real-world setting.60
The Transitional Care Model
Developed by Dr. Mary Naylor and colleagues, the Transitional Care Model61 also aims at patient and family empowerment, focusing on patients’ stated goals and priorities and ensuring patient engagement. In the program, a transitional care nurse has the job of enhancing patient and caregiver understanding, facilitating patient self-management, and overseeing medication management and transitional care.
A randomized controlled trial demonstrated improved outcomes after hospital discharge for elderly patients with complex medical illnesses, with overall reductions in medical costs through preventing or delaying rehospitalization.62 A subsequent real-world study validated this approach.63
The Bridge Model
The Illinois Transitional Care Consortium’s Bridge Model64 is for older patients discharged home after hospitalization. It is led by social workers (“bridge care coordinators”) who address barriers to implementing the discharge plan, coordinate resources, and intervene at three points: before discharge, 2 days after discharge, and 30 days after discharge.
An initial study showed no impact on the 30-day rehospitalization rate,65 but larger studies are under way with a modified version.
Guided Care
Developed at the Johns Hopkins Bloomberg School of Public Health, Guided Care66 involves nurses who work in partnership with physicians and others in primary care to provide patient-centered, cost-effective care to patients with multiple chronic conditions. Nurses conduct in-home assessments, facilitate care planning, promote patient self-management, monitor conditions, coordinate the efforts of all care professionals, and facilitate access to community resources.
A cluster-randomized controlled trial found that this program had mixed results, reducing the use of home health care but having little effect on the use of other health services in the short run. However, in the subgroup of patients covered by Kaiser-Permanente, those who were randomized to the program accrued, on average, 52% fewer skilled nursing facility days, 47% fewer skilled nursing facility admissions, 49% fewer hospital readmissions, and 17% fewer emergency department visits.67
The GRACE model
The GRACE model (Geriatric Resources for Assessment and Care of Elders)68 was developed to improve the quality of geriatric care, reduce excess health care use, and prevent long-term nursing home placement. Each patient is assigned a support team consisting of a nurse practitioner and a social worker who make home visits, coordinate health care and community services, and develop an individualized care plan.
In one study,69 GRACE reduced hospital admission rates for participants at high risk of hospitalization by 12% in the first year of the program and 44% in the second year. GRACE participants also reported higher quality of life compared with the control group.69
INTERACT tools
Led by Dr. Joseph Ouslander, INTERACT (Interventions to Reduce Acute Care Transfers)70 is a quality-improvement initiative for skilled nursing facilities, designed to facilitate the early identification, evaluation, documentation, and communication of changes in the status of residents. Visitors to its website can download a set of tools and strategies to help them manage conditions before they become serious enough to require a hospital transfer. The tools assist in promoting important communication among providers and enhancing advance-care planning.
A 6-month study in 25 nursing homes showed a 17% reduction in self-reported hospital admissions with this program compared with the same period the previous year.71
Additional home-based care interventions
Additional innovations are under way in home-based care.
The Home Health Quality Improvement National Campaign is a patient-centered movement to improve the quality of care received by patients residing at home.72 Through its Best Practices Intervention Packages, it offers evidence-based educational tools, resources, and interventions for reducing avoidable hospitalizations, improving medication management, and coordinating transitional care.
The Center for Medicare and Medicaid Innovation Independence at Home Demonstration73 is testing whether home-based comprehensive primary care can improve care and reduce hospitalizations for Medicare beneficiaries with multiple chronic conditions.
NO SINGLE INTERVENTION: MULTIPLE STRATEGIES NEEDED
A 2011 review found no single intervention that regularly reduced the 30-day risk of re-hospitalization.74 However, other studies have shown that multifaceted interventions can reduce 30-day readmission rates. Randomized controlled trials in short-stay, acute care hospitals indicate that improvement in the following areas can directly reduce hospital readmission rates:
- Comprehensive planning and risk assessment throughout hospitalization
- Quality of care during the initial admission
- Communication with patients, their caregivers, and their clinicians
- Patient education
- Predischarge assessment
- Coordination of care after discharge.
In randomized trials, successful programs reduced the 30-day readmission rates by 20% to 40%,54,62,75–79 and a 2011 meta-analysis of randomized clinical trials found evidence that interventions associated with discharge planning helped to reduce readmission rates.80
Methods developed by the national care transition models described above can help hospitals optimize patient transitions (Table 1). Although every model has its unique attributes, they have several strategies in common:
Engage a team of key stakeholders that may include patients and caregivers, hospital staff (physicians, nurses, case managers, social workers, and pharmacists), community physicians (primary care, medical homes, and specialists), advance practice providers (physician assistants and nurse practitioners), and postacute care facilities and services (skilled nursing facilities, home health agencies, assisted living residences, hospice, and rehabilitation facilities).
Develop a comprehensive transition plan throughout hospitalization that includes attention to factors that may affect self-care, such as health literacy, chronic conditions, medications, and social support.
Enhance medication reconciliation and management. Obtain the best possible medication history on admission, and ensure that patients understand changes in their medications, how to take each medicine correctly, and important side effects.
Institute daily interdisciplinary communication and care coordination by everyone on the health care team with an emphasis on the care plan, discharge planning, and safety issues.81
Standardize transition plans, procedures and forms. All discharging physicians should use a standard discharge summary template that includes pertinent diagnoses, active issues, a reconciled medication list with changes highlighted, results from important tests and consultations, pending test results, planned follow-up and required services, warning signs of a worsening condition, and actions needed if a problem arises.
Always send discharge summaries directly to the patient’s primary care physician or next care setting at the time of discharge.
Give the patient a discharge plan that is easy to understand. Enhance patient and family education using health literacy standards82 and interactive methods such as teach-back,83 in which patients demonstrate comprehension and skills required for self-care immediately after being taught. Such tools actively teach patients and caregivers to follow a care plan, including managing medications.
Follow up and coordinate support in a timely manner after a patient leaves the care setting. Follow-up visits should be arranged before discharge. Within 1 to 3 days after discharge, the patient should be called or visited by a case manager, social worker, nurse, or other health care provider.
CHALLENGES TO IMPROVING TRANSITIONS
Although several models demonstrated significant reductions of hospital readmissions in trials, challenges remain. Studies do not identify which features of the models are necessary or sufficient, or how applicable they are to different hospital and patient characteristics. A 2012 analysis84 of a program designed to reduce readmissions in three states identified key obstacles to successfully improving care transitions:
Collaborative relationships across settings are critical, but very difficult to achieve. It takes time to develop the relationships and trust among providers, and little incentive exists for skilled nursing facilities and physicians outside the hospital to engage in the process.
Infrastructure is lacking, as is experience to implement quality improvements.
We lack proof that models work on a large scale. Confusion exists about which readmissions are preventable and which are not. More evidence is needed to help guide hospitals’ efforts to improve transitions of care and reduce readmissions.
- Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc 2003; 51:549–555.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Rosenthal JM, Miller DB. Providers have failed to work for continuity. Hospitals 1979; 53:79–83.
- Gabow P, Halvorson G, Kaplan G. Marshaling leadership for high-value health care: an Institute of Medicine discussion paper. JAMA 2012; 308:239–240.
- Bonner A, Schneider CD, Weissman JS. Massachusetts State Quality Improvement Institute. Massachusetts Strategic Plan for Care Transitions. Massachusetts Executive Office of Health and Human Services, 2010. http://www.patientcarelink.org/uploadDocs/1/Strategic-Plan-for-Care-Transitions_2-11-2010-(2).pdf. Accessed April 7, 2014.
- Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood) 2010; 29:57–64.
- Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Diagnosis, 2010. HCUP Statistical Brief #153. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed April 7, 2014.
- Weiss AJ (Truven Health Analytics), Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Procedure, 2010. HCUP Statistical Brief #154. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb154.pdf. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Medicare To Penalize 2,217 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2012/August/13/medicare-hospitals-readmissions-penalties.aspx. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Armed With Bigger Fines, Medicare To Punish 2,225 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2013/August/02/readmission-penalties-medicare-hospitals-year-two.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Community-based Care Transitions Program. http://innovation.cms.gov/initiatives/CCTP/. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Hospital Engagement Networks (HENs). http://partnershipforpatients.cms.gov/about-the-partnership/hospital-engagement-networks/thehospitalengagementnetworks.html. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). About the Partnership for Patients. http://partnershipforpatients.cms.gov/about-the-partnership/about-thepartnershipforpatients.html. Accessed April 7, 2014.
- Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med 2012; 366:1606–615.
- Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N; Centers for Medicare & Medicaid Services (CMS). Medicare Readmission Rates Showed Meaningful Decline in 2012. http://www.cms.gov/mmrr/Briefs/B2013/mmrr-2013-003-02-b01.html. Accessed April 7, 2014.
- Office of Inspector General; US Department of Health and Human Services. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Report (OEI-02-12-00040). http://oig.hhs.gov/oei/reports/oei-02-12-00040.asp. Accessed April 7, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med 2007; 167:1305–1311.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005; 143:121–128.
- Coleman EA, Fox PD; HMO Workgroup on Care Management. Managing patient care transitions: a report of the HMO Care Management Workgroup. Health-plan 2004; 45:36–39.
- Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med 2004; 141:533–536.
- Snow V, Beck D, Budnitz T, et al; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med 2009; 24:971–976.
- Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med 2007; 356:1130–1139.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Solet DJ, Norvell JM, Rutan GH, Frankel RM. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med 2005; 80:1094–1099.
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2007; 2:314–323.
- National Research Council. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press, 2001.
- O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc 2010; 85:47–52.
- Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual 2013; 28:383–391.
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011; 155:97–107.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics, 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed April 7, 2014.
- Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013; 32:207–214.
- Lin CY, Barnato AE, Degenholtz HB. Physician follow-up visits after acute care hospitalization for elderly Medicare beneficiaries discharged to noninstitutional settings. J Am Geriatr Soc 2011; 59:1947–1954.
- Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010; 170:1664–1670.
- Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010; 303:1716–1722.
- van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med 2010; 5:398–405.
- Sommers A, Cunningham PJ. Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Research Brief No. 6. National Institute for Health Care Reform (NIHCR), 2011. www.nihcr.org/Reducing_Readmissions.html. Accessed April 7, 2014.
- Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309:355–363.
- Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med 2013; 28:269–282.
- Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med 2011; 11:49.
- Weissman JS, Stern RS, Epstein AM. The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals. Inquiry 1994; 31:163–172.
- Proctor EK, Morrow-Howell N, Li H, Dore P. Adequacy of home care and hospital readmission for elderly congestive heart failure patients. Health Soc Work 2000; 25:87–96.
- Kansagara D, Ramsay RS, Labby D, Saha S. Post-discharge intervention in vulnerable, chronically ill patients. J Hosp Med 2012; 7:124–130.
- Englander H, Kansagara D. Planning and designing the care transitions innovation (C-Train) for uninsured and Medicaid patients. J Hosp Med 2012; 7:524–529.
- Brown R, Peikes D, Chen A, Schore J. 15-site randomized trial of coordinated care in Medicare FFS. Health Care Financ Rev 2008; 30:5–25.
- Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Post-discharge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist 2008; 48:495–504.
- Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health 2009; 27:287–302.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. www.hospitalmedicine.org/BOOST. Accessed April 7, 2014.
- Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013; 8:421–427.
- Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf 2012; 38:301–310.
- Boston University Medical Center. Project RED: Re-Engineered Discharge. www.bu.edu/fammed/projectred/. Accessed April 7, 2014.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
- Berkowitz RE, Fang Z, Helfand BK, Jones RN, Schreiber R, Paasche-Orlow MK. Project ReEngineered Discharge (RED) lowers hospital readmissions of patients discharged from a skilled nursing facility. J Am Med Dir Assoc 2013; 14:736–740.
- Institute for Healthcare Improvement. STAAR: STate Action on Avoidable Re-hospitalizations. www.ihi.org/offerings/Initiatives/STAAR/Pages/default.aspx. Accessed April 7, 2014.
- Boutwell AE, Johnson MB, Rutherford P, et al. An early look at a four-state initiative to reduce avoidable hospital readmissions. Health Aff (Millwood) 2011; 30:1272–1280.
- University of Colorado Denver. The Care Transitions Program. www.caretransitions.org/. Accessed April 7, 2014.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Penn Nursing Science. Transitional Care Model. www.transitional-care.info/. Accessed April 7, 2014.
- Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med 1994; 120:999–1006.
- Stauffer BD, Fullerton C, Fleming N, et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med 2011; 171:1238–1243.
- The Illinois Transitional Care Consortium. The Bridge Model. www.transitionalcare.org/the-bridge-model. Accessed April 7, 2014.
- Altfeld SJ, Shier GE, Rooney M, et al. Effects of an enhanced discharge planning intervention for hospitalized older adults: a randomized trial. Gerontologist 2013; 53:430–440.
- Johns Hopkins Bloomberg School of Public Health. Guided Care. www.guidedcare.org. Accessed April 7, 2014.
- Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med 2011; 171:460–466.
- Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc 2006; 54:1136–1141.
- Bielaszka-DuVernay C. The ‘GRACE’ model: in-home assessments lead to better care for dual eligibles. Health Aff (Millwood) 2011; 30:431–434.
- Florida Atlantic University. INTERACT: Interventions to Reduce Acute Care Transfers. http://interact2.net/. Accessed April 7, 2014.
- Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. J Am Geriatr Soc 2011; 59:745–753.
- West Virginia Medical Institute. HHQI-BPIPs (Home Health Quality Improvement - Best Practices Intervention Packages). www.home-healthquality.org/Education/BPIPS.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Independence at Home Demonstration. http://innovation.cms.gov/initiatives/Independence-at-Home/. Accessed April 7, 2014.
- Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med 2011; 155:520–528.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009; 4:211–218.
- Garåsen H, Windspoll R, Johnsen R. Intermediate care at a community hospital as an alternative to prolonged general hospital care for elderly patients: a randomised controlled trial. BMC Public Health 2007; 7:68.
- Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc 2009; 57:395–402.
- Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc 2004; 52:1817–1825.
- Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood) 2011; 30:746–754.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med 2011; 171:678–684.
- Agency for Healthcare Research and Quality (AHRQ). Health Literacy Universal Precautions Toolkit. www.ahrq.gov/legacy/qual/literacy/. Accessed April 7, 2014.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Mittler JN, O’Hora JL, Harvey JB, Press MJ, Volpp KG, Scanlon DP. Turning readmission reduction policies into results: some lessons from a multistate initiative to reduce readmissions. Popul Health Manag 2013; 16:255–260.
- Coleman EA. Falling through the cracks: challenges and opportunities for improving transitional care for persons with continuous complex care needs. J Am Geriatr Soc 2003; 51:549–555.
- Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med 2009; 360:1418–1428.
- Rosenthal JM, Miller DB. Providers have failed to work for continuity. Hospitals 1979; 53:79–83.
- Gabow P, Halvorson G, Kaplan G. Marshaling leadership for high-value health care: an Institute of Medicine discussion paper. JAMA 2012; 308:239–240.
- Bonner A, Schneider CD, Weissman JS. Massachusetts State Quality Improvement Institute. Massachusetts Strategic Plan for Care Transitions. Massachusetts Executive Office of Health and Human Services, 2010. http://www.patientcarelink.org/uploadDocs/1/Strategic-Plan-for-Care-Transitions_2-11-2010-(2).pdf. Accessed April 7, 2014.
- Mor V, Intrator O, Feng Z, Grabowski DC. The revolving door of rehospitalization from skilled nursing facilities. Health Aff (Millwood) 2010; 29:57–64.
- Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Diagnosis, 2010. HCUP Statistical Brief #153. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb153.pdf. Accessed April 7, 2014.
- Weiss AJ (Truven Health Analytics), Elixhauser A (AHRQ), Steiner C (AHRQ). Readmissions to US Hospitals by Procedure, 2010. HCUP Statistical Brief #154. April 2013. Agency for Healthcare Research and Quality, Rockville, MD. http://www.hcup-us.ahrq.gov/reports/statbriefs/sb154.pdf. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Readmissions Reduction Program. http://www.cms.gov/Medicare/Medicare-Fee-for-Service-Payment/AcuteInpatientPPS/Readmissions-Reduction-Program.html. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Medicare To Penalize 2,217 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2012/August/13/medicare-hospitals-readmissions-penalties.aspx. Accessed April 7, 2014.
- Kaiser Health News (KHN); Rau J. Armed With Bigger Fines, Medicare To Punish 2,225 Hospitals For Excess Readmissions. http://www.kaiserhealthnews.org/Stories/2013/August/02/readmission-penalties-medicare-hospitals-year-two.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Community-based Care Transitions Program. http://innovation.cms.gov/initiatives/CCTP/. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Hospital Engagement Networks (HENs). http://partnershipforpatients.cms.gov/about-the-partnership/hospital-engagement-networks/thehospitalengagementnetworks.html. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). About the Partnership for Patients. http://partnershipforpatients.cms.gov/about-the-partnership/about-thepartnershipforpatients.html. Accessed April 7, 2014.
- Jha AK, Joynt KE, Orav EJ, Epstein AM. The long-term effect of premier pay for performance on patient outcomes. N Engl J Med 2012; 366:1606–615.
- Gerhardt G, Yemane A, Hickman P, Oelschlaeger A, Rollins E, Brennan N; Centers for Medicare & Medicaid Services (CMS). Medicare Readmission Rates Showed Meaningful Decline in 2012. http://www.cms.gov/mmrr/Briefs/B2013/mmrr-2013-003-02-b01.html. Accessed April 7, 2014.
- Office of Inspector General; US Department of Health and Human Services. Hospitals’ Use of Observation Stays and Short Inpatient Stays for Medicare Beneficiaries. Report (OEI-02-12-00040). http://oig.hhs.gov/oei/reports/oei-02-12-00040.asp. Accessed April 7, 2014.
- van Walraven C, Bennett C, Jennings A, Austin PC, Forster AJ. Proportion of hospital readmissions deemed avoidable: a systematic review. CMAJ 2011; 183:E391–E402.
- Forster AJ, Murff HJ, Peterson JF, Gandhi TK, Bates DW. The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med 2003; 138:161–167.
- Moore C, McGinn T, Halm E. Tying up loose ends: discharging patients with unresolved medical issues. Arch Intern Med 2007; 167:1305–1311.
- Roy CL, Poon EG, Karson AS, et al. Patient safety concerns arising from test results that return after hospital discharge. Ann Intern Med 2005; 143:121–128.
- Coleman EA, Fox PD; HMO Workgroup on Care Management. Managing patient care transitions: a report of the HMO Care Management Workgroup. Health-plan 2004; 45:36–39.
- Coleman EA, Berenson RA. Lost in transition: challenges and opportunities for improving the quality of transitional care. Ann Intern Med 2004; 141:533–536.
- Snow V, Beck D, Budnitz T, et al; American College of Physicians; Society of General Internal Medicine; Society of Hospital Medicine; American Geriatrics Society; American College of Emergency Physicians; Society of Academic Emergency Medicine. Transitions of Care Consensus Policy Statement American College of Physicians-Society of General Internal Medicine-Society of Hospital Medicine-American Geriatrics Society-American College of Emergency Physicians-Society of Academic Emergency Medicine. J Gen Intern Med 2009; 24:971–976.
- Pham HH, Schrag D, O’Malley AS, Wu B, Bach PB. Care patterns in Medicare and their implications for pay for performance. N Engl J Med 2007; 356:1130–1139.
- Kripalani S, LeFevre F, Phillips CO, Williams MV, Basaviah P, Baker DW. Deficits in communication and information transfer between hospital-based and primary care physicians: implications for patient safety and continuity of care. JAMA 2007; 297:831–841.
- Solet DJ, Norvell JM, Rutan GH, Frankel RM. Lost in translation: challenges and opportunities in physician-to-physician communication during patient handoffs. Acad Med 2005; 80:1094–1099.
- Kripalani S, Jackson AT, Schnipper JL, Coleman EA. Promoting effective transitions of care at hospital discharge: a review of key issues for hospitalists. J Hosp Med 2007; 2:314–323.
- National Research Council. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: The National Academies Press, 2001.
- O’Leary KJ, Kulkarni N, Landler MP, et al. Hospitalized patients’ understanding of their plan of care. Mayo Clin Proc 2010; 85:47–52.
- Coleman EA, Chugh A, Williams MV, et al. Understanding and execution of discharge instructions. Am J Med Qual 2013; 28:383–391.
- Berkman ND, Sheridan SL, Donahue KE, Halpern DJ, Crotty K. Low health literacy and health outcomes: an updated systematic review. Ann Intern Med 2011; 155:97–107.
- Kutner M, Greenberg E, Jin Y, Paulsen C. The Health Literacy of America’s Adults: Results From the 2003 National Assessment of Adult Literacy (NCES 2006–483). US Department of Education. Washington, DC: National Center for Education Statistics, 2006. http://nces.ed.gov/pubs2006/2006483.pdf. Accessed April 7, 2014.
- Hibbard JH, Greene J. What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs. Health Aff (Millwood) 2013; 32:207–214.
- Lin CY, Barnato AE, Degenholtz HB. Physician follow-up visits after acute care hospitalization for elderly Medicare beneficiaries discharged to noninstitutional settings. J Am Geriatr Soc 2011; 59:1947–1954.
- Sharma G, Kuo YF, Freeman JL, Zhang DD, Goodwin JS. Outpatient follow-up visit and 30-day emergency department visit and readmission in patients hospitalized for chronic obstructive pulmonary disease. Arch Intern Med 2010; 170:1664–1670.
- Hernandez AF, Greiner MA, Fonarow GC, et al. Relationship between early physician follow-up and 30-day readmission among Medicare beneficiaries hospitalized for heart failure. JAMA 2010; 303:1716–1722.
- van Walraven C, Taljaard M, Etchells E, et al. The independent association of provider and information continuity on outcomes after hospital discharge: implications for hospitalists. J Hosp Med 2010; 5:398–405.
- Sommers A, Cunningham PJ. Physician Visits After Hospital Discharge: Implications for Reducing Readmissions. Research Brief No. 6. National Institute for Health Care Reform (NIHCR), 2011. www.nihcr.org/Reducing_Readmissions.html. Accessed April 7, 2014.
- Dharmarajan K, Hsieh AF, Lin Z, et al. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA 2013; 309:355–363.
- Calvillo-King L, Arnold D, Eubank KJ, et al. Impact of social factors on risk of readmission or mortality in pneumonia and heart failure: systematic review. J Gen Intern Med 2013; 28:269–282.
- Coventry PA, Gemmell I, Todd CJ. Psychosocial risk factors for hospital readmission in COPD patients on early discharge services: a cohort study. BMC Pulm Med 2011; 11:49.
- Weissman JS, Stern RS, Epstein AM. The impact of patient socioeconomic status and other social factors on readmission: a prospective study in four Massachusetts hospitals. Inquiry 1994; 31:163–172.
- Proctor EK, Morrow-Howell N, Li H, Dore P. Adequacy of home care and hospital readmission for elderly congestive heart failure patients. Health Soc Work 2000; 25:87–96.
- Kansagara D, Ramsay RS, Labby D, Saha S. Post-discharge intervention in vulnerable, chronically ill patients. J Hosp Med 2012; 7:124–130.
- Englander H, Kansagara D. Planning and designing the care transitions innovation (C-Train) for uninsured and Medicaid patients. J Hosp Med 2012; 7:524–529.
- Brown R, Peikes D, Chen A, Schore J. 15-site randomized trial of coordinated care in Medicare FFS. Health Care Financ Rev 2008; 30:5–25.
- Arbaje AI, Wolff JL, Yu Q, Powe NR, Anderson GF, Boult C. Post-discharge environmental and socioeconomic factors and the likelihood of early hospital readmission among community-dwelling Medicare beneficiaries. Gerontologist 2008; 48:495–504.
- Peek CJ, Baird MA, Coleman E. Primary care for patient complexity, not only disease. Fam Syst Health 2009; 27:287–302.
- Society of Hospital Medicine. Project BOOST: Better Outcomes by Optimizing Safe Transitions. www.hospitalmedicine.org/BOOST. Accessed April 7, 2014.
- Hansen LO, Greenwald JL, Budnitz T, et al. Project BOOST: effectiveness of a multihospital effort to reduce rehospitalization. J Hosp Med. 2013; 8:421–427.
- Maynard GA, Budnitz TL, Nickel WK, et al. 2011 John M. Eisenberg Patient Safety and Quality Awards. Mentored implementation: building leaders and achieving results through a collaborative improvement model. Innovation in patient safety and quality at the national level. Jt Comm J Qual Patient Saf 2012; 38:301–310.
- Boston University Medical Center. Project RED: Re-Engineered Discharge. www.bu.edu/fammed/projectred/. Accessed April 7, 2014.
- Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med 2009; 150:178–187.
- Berkowitz RE, Fang Z, Helfand BK, Jones RN, Schreiber R, Paasche-Orlow MK. Project ReEngineered Discharge (RED) lowers hospital readmissions of patients discharged from a skilled nursing facility. J Am Med Dir Assoc 2013; 14:736–740.
- Institute for Healthcare Improvement. STAAR: STate Action on Avoidable Re-hospitalizations. www.ihi.org/offerings/Initiatives/STAAR/Pages/default.aspx. Accessed April 7, 2014.
- Boutwell AE, Johnson MB, Rutherford P, et al. An early look at a four-state initiative to reduce avoidable hospital readmissions. Health Aff (Millwood) 2011; 30:1272–1280.
- University of Colorado Denver. The Care Transitions Program. www.caretransitions.org/. Accessed April 7, 2014.
- Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med 2006; 166:1822–1828.
- Voss R, Gardner R, Baier R, Butterfield K, Lehrman S, Gravenstein S. The care transitions intervention: translating from efficacy to effectiveness. Arch Intern Med 2011; 171:1232–1237.
- Penn Nursing Science. Transitional Care Model. www.transitional-care.info/. Accessed April 7, 2014.
- Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med 1994; 120:999–1006.
- Stauffer BD, Fullerton C, Fleming N, et al. Effectiveness and cost of a transitional care program for heart failure: a prospective study with concurrent controls. Arch Intern Med 2011; 171:1238–1243.
- The Illinois Transitional Care Consortium. The Bridge Model. www.transitionalcare.org/the-bridge-model. Accessed April 7, 2014.
- Altfeld SJ, Shier GE, Rooney M, et al. Effects of an enhanced discharge planning intervention for hospitalized older adults: a randomized trial. Gerontologist 2013; 53:430–440.
- Johns Hopkins Bloomberg School of Public Health. Guided Care. www.guidedcare.org. Accessed April 7, 2014.
- Boult C, Reider L, Leff B, et al. The effect of guided care teams on the use of health services: results from a cluster-randomized controlled trial. Arch Intern Med 2011; 171:460–466.
- Counsell SR, Callahan CM, Buttar AB, Clark DO, Frank KI. Geriatric Resources for Assessment and Care of Elders (GRACE): a new model of primary care for low-income seniors. J Am Geriatr Soc 2006; 54:1136–1141.
- Bielaszka-DuVernay C. The ‘GRACE’ model: in-home assessments lead to better care for dual eligibles. Health Aff (Millwood) 2011; 30:431–434.
- Florida Atlantic University. INTERACT: Interventions to Reduce Acute Care Transfers. http://interact2.net/. Accessed April 7, 2014.
- Ouslander JG, Lamb G, Tappen R, et al. Interventions to reduce hospitalizations from nursing homes: evaluation of the INTERACT II collaborative quality improvement project. J Am Geriatr Soc 2011; 59:745–753.
- West Virginia Medical Institute. HHQI-BPIPs (Home Health Quality Improvement - Best Practices Intervention Packages). www.home-healthquality.org/Education/BPIPS.aspx. Accessed April 7, 2014.
- Centers for Medicare & Medicaid Services (CMS). Independence at Home Demonstration. http://innovation.cms.gov/initiatives/Independence-at-Home/. Accessed April 7, 2014.
- Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med 2011; 155:520–528.
- Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. JAMA 1999; 281:613–620.
- Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. J Hosp Med 2009; 4:211–218.
- Garåsen H, Windspoll R, Johnsen R. Intermediate care at a community hospital as an alternative to prolonged general hospital care for elderly patients: a randomised controlled trial. BMC Public Health 2007; 7:68.
- Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. J Am Geriatr Soc 2009; 57:395–402.
- Coleman EA, Smith JD, Frank JC, Min SJ, Parry C, Kramer AM. Preparing patients and caregivers to participate in care delivered across settings: the Care Transitions Intervention. J Am Geriatr Soc 2004; 52:1817–1825.
- Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: the importance of transitional care in achieving health reform. Health Aff (Millwood) 2011; 30:746–754.
- O’Leary KJ, Buck R, Fligiel HM, et al. Structured interdisciplinary rounds in a medical teaching unit: improving patient safety. Arch Intern Med 2011; 171:678–684.
- Agency for Healthcare Research and Quality (AHRQ). Health Literacy Universal Precautions Toolkit. www.ahrq.gov/legacy/qual/literacy/. Accessed April 7, 2014.
- Schillinger D, Piette J, Grumbach K, et al. Closing the loop: physician communication with diabetic patients who have low health literacy. Arch Intern Med 2003; 163:83–90.
- Mittler JN, O’Hora JL, Harvey JB, Press MJ, Volpp KG, Scanlon DP. Turning readmission reduction policies into results: some lessons from a multistate initiative to reduce readmissions. Popul Health Manag 2013; 16:255–260.
KEY POINTS
- Traditional health care delivery models typically do not have mechanisms in place for coordinating care across settings, such as when a patient goes from the hospital to a skilled nursing facility or to home.
- Transitions can fail, leading to hospital readmission, because of ineffective patient and caregiver education, discharge summaries that are incomplete or not communicated to the patient and the next care setting, lack of follow-up with primary care providers, and poor patient social support.
- A number of programs are trying to improve transitions of care, with some showing reductions in hospital readmission rates and emergency department visits.
- Successful programs use multiple interventions simultaneously, including improved communication among health care providers, better patient and caregiver education, and coordination of social and health care services.
Heart failure in African Americans: Disparities can be overcome
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
African Americans are disproportionately affected by heart failure and have not experienced the same benefit from treatment as white patients have. Much of the disparity can be blamed on modifiable risk factors such as uncontrolled hypertension and on suboptimal health care. When African Americans are treated according to guidelines, discrepant outcomes can be minimized.
In this article, we review the processes contributing to heart failure in African Americans, its management, and challenges with regard to disparities.
HEART FAILURE IS INCREASING
Despite 20 years of progress in understanding the pathophysiology of heart failure and developing medical and surgical therapies for it, its prevalence and associated morbidity are increasing in the United States. In 2010, 6.6 million (2.8%) of the adults in the United States had heart failure,1 and the prevalence is expected to increase by about 25% by 2030.
DISPARITIES IN INCIDENCE, OUTCOMES
Heart failure is more prevalent in African Americans than in whites, imposes higher rates of death and morbidity, and has a more malignant course.1–6
According to American Heart Association statistics, the annual incidence of heart failure in whites is approximately 6 per 1,000 person-years, while in African Americans it is 9.1 per 1,000 person-years.1 In the Atherosclerosis Risk in Communities study, the incidence of new heart failure was 1.0 per 1,000 person-years in Chinese Americans, 2.4 in whites, 3.5 in Hispanics, and 4.6 in African Americans.2
Moreover, when hospitalized for heart failure, African Americans have a 45% greater risk of death or decline in functional status than whites.7
Heart failure also occurs earlier in African Americans. Bibbins-Domingo et al8 reported that heart failure before age 50 was 20 times more frequent in African Americans than in whites. Functional and structural cardiac changes appeared an average of 10 years before the onset of symptoms and were strongly associated with the development of subsequent heart failure.8
In the Women’s Health Initiative, African American women had higher rates of heart failure than white women, perhaps in part because of higher rates of diabetes.9
Heart failure with preserved ejection fraction
About half of patients who have signs and symptoms of heart failure have a normal (“preserved”) ejection fraction. The incidence of this condition, previously called diastolic heart failure, appears to be similar between African Americans and whites. However, African Americans appear to have a greater incidence of factors that predispose to it and tend to present later in the course.10 For example, African Americans have higher left ventricular mass and wall thickness and a higher incidence of left ventricular hypertrophy than white patients.11–13 In addition, those with heart failure with preserved ejection fraction tend to be younger, female, more likely to have hypertension and diabetes, and less likely to have coronary artery disease, and tend to have worse renal function than their white counterparts.14,15 The predisposition to diastolic impairment persists even after adjusting for risk factors.11–15 The mortality rate in African Americans with heart failure with preserved ejection fraction and without coronary artery disease may also be higher than that of comparable white patients.16
WHY DO AFRICAN AMERICANS HAVE MORE HEART FAILURE?
Modifiable risk factors
In African Americans, the higher percentage of cases of heart failure is attributable to modifiable risk factors such as hypertension, hyperglycemia, left ventricular hypertrophy, and smoking, and fewer cases are due to ischemic heart disease.2,3 Nonischemic cardiomyopathy predominates in African Americans, whereas ischemic cardiomyopathy predominates in whites.
Hypertension, diabetes, obesity, and chronic kidney disease all portend subsequent heart failure and are common in African Americans, but hypertension is the main culprit.3,5,8,17–21 The prevalence of hypertension in African Americans is among the highest in the world, and because African Americans are more likely to have poorer control of their hypertension, they consequently have more target-organ damage.22 Indeed, in many hypertensive African Americans who develop heart failure, the hypertension is poorly controlled. However, even after adjusting for risk factors, and particularly blood pressure control, African Americans remain at higher risk of heart failure.23
The specific mechanistic links between hypertension and heart failure remain to be identified. Despite having a higher prevalence of left ventricular hypertrophy and left ventricular remodeling, African Americans with heart failure tend toward systolic heart failure, as opposed to heart failure with preserved ejection fraction.
Neurohormonal imbalances and endothelial dysfunction
Derangements in the renin-angiotensin-aldosterone and adrenergic axes are likely the main pathophysiologic mechanisms in the genesis of heart failure in all populations. However, other factors may underlie the enhanced disease burden in African Americans.
Impaired endothelial function, as evidenced by impaired digital and brachial artery vasomotion, is very common in African Americans.24–26 The small arteries of African Americans are less elastic than those of whites and Chinese.27 The underlying mechanism may be related to increased oxidative stress, decreased nitric oxide availability, exaggerated vasoconstrictor response, and attenuated responsiveness to vasodilators and nitric oxide.28–31
Genetic polymorphisms
An important caveat in discussing racial differences in heart failure is that “race” is completely arbitrary and is based on sociopolitical rather than scientific or physiologic definitions. Perceived genetic influences are likely to represent complex gene-gene, gene-environment, and gene-drug interactions.
This is especially true for African Americans, who are a markedly heterogeneous group. The US Office of Management and Budget defines “black” or “African American” as having origins in any of the black racial groups of Africa (www.census.gov/2010census/data). Thus, “African American” includes sixth-generation descendants of African slaves, recently immigrated Jamaicans, and black descendants of French and Spanish people.
Most African Americans have some European ancestry. In one study, the estimated proportion of European ancestry ranged from 7% in Jamaicans of African descent to approximately 23% in African Americans in New Orleans.32
Nevertheless, several polymorphisms associated with the risk of heart failure may provide insight into some of the “race-based” differences in pathophysiology and response to medications and, it is hoped, may eventually serve as the basis for tailored therapy. Genes of interest include those for:
- Beta 1 adrenergic receptor
- Alpha 2c receptor33
- Aldosterone synthase34
- G protein
- Transforming growth factor beta
- Nitric oxide synthase35
- Transthyrectin.36,37
Socioeconomic factors and quality of care
Heart failure patients—and especially African Americans—have high rates of hospital readmission, and socioeconomic factors have been implicated. In more than 40,000 patients with heart failure, lower income was a significant predictor of hospital readmission.38 Socioeconomic factors in turn could account for delay in seeking treatment for worsening symptoms, failure to recognize symptoms, limited disease awareness, inadequate access to health care, noncompliance with follow-up appointments, and poor adherence to recommended treatment, all of which are common in African American patients.38,39
African Americans also report more discrimination from health care providers, have more concerns about blood pressure medications, and are more likely to have misperceptions about high blood pressure (eg, that it is not serious), all of which may interfere with optimal blood pressure control.40 Managing heart failure in African Americans should include trying to identify and eliminate barriers to attaining treatment goals.
PREVENTING HEART FAILURE BY REDUCING RISK FACTORS
The American College of Cardiology Foundation and American Heart Association, in their 2013 guidelines, underscored the progressive nature of heart failure by defining four stages of the disease, from stage A (at risk) through stage D (refractory heart failure) (Figure 1).41 They also emphasized the importance of preventing it.
A thorough clinical assessment, with appropriate assessment for risk factors and intervention at stage A, is critical in preventing left ventricular remodeling and heart failure. These risk factors include hypertension, hyperlipidemia, atherosclerosis, diabetes mellitus, valvular disease, obesity, physical inactivity, excessive alcohol intake, poor diet, and smoking.
Hypertension is especially important in African Americans and requires vigorous screening and aggressive treatment. Antihypertensive drugs should be prescribed early, with a lower threshold for escalating therapy with combinations of drugs, as most patients require more than one.
There is considerable debate about the appropriate blood pressure thresholds for diagnosing hypertension and the optimal target blood pressures in African Americans. The 2014 report of the Joint National Committee recommends a similar hypertension treatment target of 140/90 mm Hg for all patients except older adults (for whom 150/90 mm Hg is acceptable), and no separate target for African Americans.42 Previous guidelines from this committee recommended thiazide-type diuretics as first-line therapy for hypertension in African Americans43; the new ones recommend thiazide-type diuretics or calcium channel blockers. However, in those with left ventricular systolic dysfunction, hypertension treatment should include drugs shown to reduce the risk of death in heart failure—ie, angiotensin-converting enzyme (ACE) inhibitors, beta-blockers, hydralazine, nitrates, and aldosterone receptor antagonists.
Salt intake should be reduced to less than 3 g per day (1,200 mg of sodium per day), which has been shown to substantially reduce rates of cardiovascular morbidity and mortality and health care costs.44 Since most Americans consume 7 to 10 g of salt per day, strict salt restriction should be encouraged as a preventive measure.
Diabetes should be screened for and treated in African Americans per current American Diabetes Association guidelines.
Dyslipidemia should also be screened for and treated per guidelines.45
Smoking cessation, moderation of alcohol intake, and avoidance of illicit drugs should be encouraged. Given that African Americans develop heart failure at a relatively early age, the level of vigilance should be high and the threshold for screening should be low.
Healthy neighborhoods, healthy people
Neighborhoods can be designed and built with wellness in mind, incorporating features such as access to healthy food and walkability. Living in such neighborhoods leads to more physical activity and less obesity, although this relationship may be less robust in African Americans.46–49
Environmental factors are multifactorial in African Americans and extend beyond those afforded by the built environment. For instance, lack of safety may hinder the potential benefit of an otherwise walkable neighborhood. These interactions are highly complex, and more investigation is needed to determine the effect of built environments on risk factors in African Americans.
DRUG THERAPY FOR HEART FAILURE IN AFRICAN AMERICANS
Use standard therapies
ACE inhibitors, beta-blockers, and aldosterone antagonists are the standard of care in heart failure, with digoxin (Lanoxin) and diuretics used as adjuncts to control symptoms.
African Americans may respond differently than whites to some of these drugs (Table 1). However, these findings should be interpreted with caution, since most of them came from subgroup analyses of trials in which African Americans accounted for as many as 28% to as few as 1%.50 To date, no data unequivocally show that we should use standard heart failure therapies any differently in African Americans than in whites.
Digoxin: Limited role to control symptoms
Post hoc analysis of the Digitalis Investigation Group trial, in which 14% of the patients were nonwhite, revealed that compared with placebo, digitalis (and achieving a serum digitalis concentration of 0.5 to 0.9 ng/mL) was associated with lower rates of all-cause mortality in most subgroups—except nonwhites.51
In general, digoxin has a limited role in heart failure, since other drugs are available that substantially modify outcomes. However, it can be considered in patients who have persistent heart failure symptoms.
ACE inhibitors, ARBs are recommended
ACE inhibitors are recommended for patients with New York Heart Association (NYHA) class I, II, III, or IV heart failure (class I recommendation, ie, “recommended”; level of evidence A on a scale of A, B, and C) and as part of standard therapy for African American patients with heart failure with symptomatic or asymptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence C).41
Although African American patients did not appear to derive any benefit from enalapril (Vasotec) in the Studies of Left Ventricular Dysfunction (SOLVD) trial,52 a subsequent analysis that involved the SOLVD Prevention Trial did not find any differences between African Americans and whites in response to this agent.6 Similarly, a meta-analysis did not suggest differences in ACE-inhibitor efficacy in reducing adverse cardiovascular outcomes in heart failure between African Americans and non–African Americans.53
Of note: African Americans have a 3% to 4% higher incidence of angioedema from ACE inhibitors than whites.54,55
Angiotensin receptor blockers (ARBs) can be used as substitute therapy in African Americans who cannot tolerate ACE inhibitors (class IIa recommendation, ie, “reasonable”; level of evidence B).41
Beta-blockers also recommended
Beta-blockers are recommended in NYHA class I, II, III, and IV heart failure (class I recommendation; level of evidence A) and as part of standard therapy for African Americans with heart failure due to symptomatic left ventricular systolic dysfunction (class I recommendation; level of evidence B) and asymptomatic left ventricular systolic dysfunction (level of evidence C).41
Carvedilol (Coreg) and metoprolol (Lopressor) are the standard beta-blockers used to treat heart failure, and these drugs should be used in African Americans as well as in whites.15,53,56–59 Of interest, however, race-specific differences may exist in the beta-adrenergic pathway.60,61
Aldosterone antagonists: More study needed
Aldosterone antagonists, also called mineralocorticoid antagonists, ie, spironolactone (Aldactone) and eplerenone (Inspra), are recommended in addition to beta-blockers and ACE inhibitors for NYHA class II–IV heart failure, unless contraindicated (class I recommendation; level of evidence A).
However, trials of aldosterone antagonists to date have enrolled few African Americans.62–64 The limited data suggest that African Americans with heart failure may be less responsive to the renal effects of spironolactone, demonstrating less of an increase in serum potassium levels, and there are essentially no data to guide the use of these drugs in African Americans with heart failure.65 Further study is needed. But in the absence of data to the contrary, these agents, should also be used in African American patients with class III or IV heart failure.
Hydralazine plus nitrates: Recommended for African Americans
Hydralazine plus isosorbide dinitrate (available as BiDil) is recommended as part of standard therapy, in addition to beta-blockers and ACE inhibitors specifically for African Americans with left ventricular systolic dysfunction and NYHA class III or IV heart failure (class I recommendation; level of evidence A), as well as NYHA class II heart failure (class I recommendation; level of evidence B).41
Preliminary evidence for this combination came from the Department of Veterans Affairs Cooperative Vasodilator-Heart Failure Trials.66
Subsequently, the African-American Heart Failure Trial67 was conducted in self-identified African American patients with NYHA class III or IV heart failure on standard heart failure therapy, including an ACE inhibitor if tolerated. Patients were randomly assigned to receive a fixed combination of isosorbide 20 mg and hydralazine 37.5 mg, one or two tablets three times a day, or placebo. The target dose of isosorbide dinitrate was 120 mg, and the target dose of hydralazine was 225 mg daily. Follow-up was up to 18 months. The study was terminated early because of a significant 43% improvement in overall survival for the patients in the isosorbide-hydralazine group. In addition, the rate of first hospitalization was 39% lower and the mean improvement in quality-of-life scores was 52% greater with isosorbide-hydralazine than with placebo.67
There has been much debate about whether the benefit seen in this trial was the result of a hemodynamic effect, blood pressure response, or neurohormonal modulation. The benefit is less likely from a reduction in blood pressure, as the patients who had low blood pressure derived a mortality benefit similar to those with higher blood pressure, despite no further reduction in their blood pressure.68
Treatment for heart failure with preserved ejection fraction
Although there are no data on how to manage heart failure with preserved ejection fraction that are specific to African Americans, the ACCF/AHA guideline41 recommends treating systolic and diastolic hypertension (class I, level of evidence B) according to published clinical practice guidelines and using diuretics to alleviate volume overload (class I; level of evidence C). Revascularization and management of atrial fibrillation are also “reasonable,” as are the use of ARBs, ACE inhibitors, and beta-blockers in the management of hypertension (class IIa; level of evidence C). ARBs may also be considered to reduce hospitalization in symptomatic patients with heart failure with preserved ejection fraction (class IIb, ie, “may be considered”; level of evidence B).
For acute decompensated heart failure
One of the greatest challenges in heart failure is treating patients who present with acute decompensated heart failure.
As in the general population, the major precipitating factor for hospitalization with decompensated heart failure in African Americans is nonadherence to prescribed dietary and medication regimens.35 African Americans with acute decompensated heart failure tend to be younger and to have nonischemic cardiomyopathy, hypertension, diabetes, and obesity, but a lower risk of death.35,69,70 Up to 44% have uncontrolled hypertension.35
Inotropes and vasodilators have undergone multiple trials in the acutely decompensated state in the general population, but no trial has demonstrated a reduction in the mortality rate, and some showed a higher mortality rate. Thus, the treatment of acute decompensated heart failure remains primarily consensus-guided and symptom-focused.
Loop diuretics have been the mainstay in managing fluid retention and congestion in heart failure. The Diuretic Optimization Strategies Evaluation trial tested low-dose vs high-dose intravenous furosemide (Lasix) given either as a continuous infusion or as intermittent intravenous boluses. All strategies were safe and effective.71
Although ultrafiltration is an effective method of decongestion in heart failure and has been associated with a reduction in hospitalization, it is also associated with worsening renal function.72 The Cardiorenal Rescue Study in Acute Decompensated Heart Failure73 compared ultrafiltration vs stepped diuretic therapy. In this trial, which enrolled approximately 26% nonwhites, stepped diuretic therapy was superior to ultrafiltration in preserving renal function in acute decompensated heart failure, although the efficacy of fluid removal was similar.
Both studies were small, and subgroup analyses are not likely to yield useful information. Nevertheless, these data support the use of intravenous diuretics, by continuous infusion or bolus, in acute decompensated heart failure.
Despite no benefit in terms of the mortality rate, inotropes continue to be used in some cases of acute decompensated heart failure, and African Americans appear to have a response to milrinone (Primacor IV) similar to that in whites.69
In a nonrandomized study in which most patients were black, high-dose intravenous nitroglycerin appeared to be safe and associated with less need for ventilator support and intensive care unit admission, compared retrospectively with a population that did not receive high-dose nitroglycerin.74
Given the different profile of the African American patient with acute decompensated heart failure, prospective studies would be useful in determining the best management strategy.
TREATMENTS FOR ADVANCED HEART FAILURE
Cardiac resynchronization and implantable cardioverter-defibrillators
Cardiac resynchronization therapy is indicated for patients with NYHA class II, III, and ambulatory class IV heart failure and left ventricular ejection fraction less than or equal to 35%, sinus rhythm, left bundle branch block, and a QRS duration greater than or equal to 150 ms (class I recommendation; level of evidence A for class NYHA III and IV; level of evidence B for NYHA class II).41
An implantable cardioverter-defibrillator is recommended in patients with NYHA class II or III heart failure for primary prevention of sudden cardiac death in selected patients with nonischemic dilated cardiomyopathy or ischemic heart disease (class I recommendation; level of evidence A).
However, few members of racial and ethnic minorities were included in trials of implantable cardioverter-defibrillators75,76 or cardiac resynchronization,7,77,78 so that subgroup analysis is limited. Use of an implantable cardioverter-defibrillator showed similar reduction in mortality between African Americans and whites, and compliance with device implantation and medical therapy was comparable.79
Among patients discharged from hospitals in the American Heart Association’s Get With the Guidelines–Heart Failure Quality Improvement Program, fewer than 40% of potentially eligible patients received an implantable cardioverter-defibrillator, and rates were significantly lower for African Americans.80 When they can get cardiac resynchronization therapy, African Americans appear to experience similar benefit from it.81
Heart transplantation: Poorer outcomes in African Americans?
Heart transplantation remains the most effective and durable therapy for advanced heart failure. Median survival approaches 14 years.82
However, a retrospective study found that African American recipients had an 11.5% lower 10-year survival rate than whites, which persisted after adjusting for risk, donor-recipient matching by race, and censoring of deaths in the first year.83 Although socioeconomic factors and poor human leukocyte antigen matching have been implicated, a retrospective cohort study showed that African American recipients had a higher risk of death than white recipients even after adjustment for recipient, transplant, and socioeconomic factors.84–87 African Americans were more likely to die of graft failure or of a cardiovascular cause than white patients, but were less likely to die of infection or malignancy. Although mortality rates decreased over time for all transplant recipients, the disparity in mortality rates between African Americans and whites remained essentially unchanged.84
Among all donor-recipient combinations, African American recipients of hearts from African American donors had the highest risk of death.88
Limited access to transplantation persists, particularly for African Americans of lower socioeconomic status. African Americans are more likely than whites to be uninsured, and the funding requirement to be placed on the transplantation list disproportionately affects African Americans.89,90
Left-ventricular assist devices
Left-ventricular assist devices (LVADs) improve survival in heart transplantation candidates and heart failure patients who do not qualify for transplantation. After LVAD implantation, African American patients have similar 1- and 2-year survival rates and no difference in readmission rates compared with whites.91,92
Access to LVAD implantation, however, is significantly influenced by race, and African Americans are significantly less likely to receive one (OR = 0.29).93 Further investigation is required to identify disparities in outcome, access, and contributing factors.
DISPARITIES CAN BE MINIMIZED
In general, heart failure in African Americans is characterized by a high prevalence of hypertension as a major risk factor and potentially different pathogenesis than in the general population. Furthermore, heart failure in African Americans is more prevalent, occurs at an early age, and has a more severe course than in whites, perhaps because of a higher prevalence of risk factors such as diabetes mellitus, obesity, and again, hypertension. These disparities are multifactorial and involve a complex interplay between genes, environment, and socioeconomic factors.
For now, heart failure in African Americans should be treated according to standard evidenced-based strategies, which include a combination of isosorbide dinitrate and hydralazine in addition to other neurohormonal modifying agents (ACE inhibitors, beta-blockers, aldosterone antagonists), a strategy demonstrated to reduce mortality rates in African Americans. When treated according to guidelines, disparities in outcomes can be minimized.
However, many questions about managing heart failure remain unanswered, since African Americans have been markedly underrepresented in clinical trials. Clinical trials need to enroll enough African Americans to answer the questions of interest. Disparities in outcomes must be investigated in a scientific and hypothesis-driven manner. The effect of the built environment on African Americans needs more study as well, as success with these strategies may be impeded by unrecognized factors.
Preventing heart failure should be a priority. Efforts should be directed toward detecting and modifying risk factors early, managing hypertension aggressively, and identifying left ventricular dysfunction early.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
- Go AS, Mozaffarian D, Roger VL, et al; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2013 update: a report from the American Heart Association. Circulation 2013; 127:e6–e245.
- Bahrami H, Kronmal R, Bluemke DA, et al. Differences in the incidence of congestive heart failure by ethnicity: the multi-ethnic study of atherosclerosis. Arch Intern Med 2008; 168:2138–2145.
- Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol 2008; 101:1016–1022.
- Kalogeropoulos A, Georgiopoulou V, Kritchevsky SB, et al. Epidemiology of incident heart failure in a contemporary elderly cohort: the health, aging, and body composition study. Arch Intern Med 2009; 169:708–715.
- Dries DL, Exner DV, Gersh BJ, Cooper HA, Carson PE, Domanski MJ. Racial differences in the outcome of left ventricular dysfunction. N Engl J Med 1999; 340:609–616.
- Exner DV, Dries DL, Domanski MJ, Cohn JN. Lesser response to angiotensin-converting-enzyme inhibitor therapy in black as compared with white patients with left ventricular dysfunction. N Engl J Med 2001; 344:1351–1357.
- Bardy GH, Lee KL, Mark DB, et al; Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) Investigators. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med 2005; 352:225–237.
- Bibbins-Domingo K, Pletcher MJ, Lin F, et al. Racial differences in incident heart failure among young adults. N Engl J Med 2009; 360:1179–1190.
- Eaton CB, Abdulbaki AM, Margolis KL, et al. Racial and ethnic differences in incident hospitalized heart failure in postmenopausal women: the Women’s Health Initiative. Circulation 2012; 126:688–696.
- Ilksoy N, Hoffman M, Moore RH, Easley K, Jacobson TA. Comparison of African-American patients with systolic heart failure versus preserved ejection fraction. Am J Cardiol 2006; 98:806–808.
- Sharp A, Tapp R, Francis DP, et al. Ethnicity and left ventricular diastolic function in hypertension an ASCOT (Anglo-Scandinavian Cardiac Outcomes Trial) substudy. J Am Coll Cardiol 2008; 52:1015–1021.
- Kizer JR, Arnett DK, Bella JN, et al. Differences in left ventricular structure between black and white hypertensive adults: the Hypertension Genetic Epidemiology Network study. Hypertension 2004; 43:1182–1188.
- Drazner MH, Dries DL, Peshock RM, et al. Left ventricular hypertrophy is more prevalent in blacks than whites in the general population: the Dallas Heart Study. Hypertension 2005; 46:124–129.
- East MA, Peterson ED, Shaw LK, Gattis WA, O’Connor CM. Racial differences in the outcomes of patients with diastolic heart failure. Am Heart J 2004; 148:151–156.
- Klapholz M, Maurer M, Lowe AM, et al; New York Heart Failure Consortium. Hospitalization for heart failure in the presence of a normal left ventricular ejection fraction: results of the New York Heart Failure Registry. J Am Coll Cardiol 2004; 43:1432–1438.
- Agoston I, Cameron CS, Yao D, Dela Rosa A, Mann DL, Deswal A. Comparison of outcomes of white versus black patients hospitalized with heart failure and preserved ejection fraction. Am J Cardiol 2004; 94:1003–1007.
- Packer M, Bristow MR, Cohn JN, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. US Carvedilol Heart Failure Study Group. N Engl J Med 1996; 334:1349–1355.
- Yancy CW, Strong M. The natural history, epidemiology, and prognosis of heart failure in African Americans. Congest Heart Fail 2004; 10:15–18.
- Velagaleti RS, Gona P, Larson MG, et al. Multimarker approach for the prediction of heart failure incidence in the community. Circulation 2010; 122:1700–1706.
- Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth R. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J 2006; 152:348–354.
- Howard G, Prineas R, Moy C, et al. Racial and geographic differences in awareness, treatment, and control of hypertension: the REasons for Geographic And Racial Differences in Stroke study. Stroke 2006; 37:1171–1178.
- Hertz RP, Unger AN, Cornell JA, Saunders E. Racial disparities in hypertension prevalence, awareness, and management. Arch Intern Med 2005; 165:2098–2104.
- Okin PM, Kjeldsen SE, Dahlöf B, Devereux RB. Racial differences in incident heart failure during antihypertensive therapy. Circ Cardiovasc Qual Outcomes 2011; 4:157–164.
- Mulukutla SR, Venkitachalam L, Bambs C, et al. Black race is associated with digital artery endothelial dysfunction: results from the Heart SCORE study. Eur Heart J 2010; 31:2808–2815.
- Campia U, Choucair WK, Bryant MB, Waclawiw MA, Cardillo C, Panza JA. Reduced endothelium-dependent and -independent dilation of conductance arteries in African Americans. J Am Coll Cardiol 2002; 40:754–760.
- Perregaux D, Chaudhuri A, Rao S, et al. Brachial vascular reactivity in blacks. Hypertension 2000; 36:866–871.
- Duprez DA, Jacobs DR, Lutsey PL, et al. Race/ethnic and sex differences in large and small artery elasticity—results of the multiethnic study of atherosclerosis (MESA). Ethn Dis 2009; 19:243–250.
- Kalinowski L, Dobrucki IT, Malinski T. Race-specific differences in endothelial function: predisposition of African Americans to vascular diseases. Circulation 2004; 109:2511–2517.
- Kojda G, Harrison D. Interactions between NO and reactive oxygen species: pathophysiological importance in atherosclerosis, hypertension, diabetes and heart failure. Cardiovasc Res 1999; 43:562–571.
- Gattás GJ, Kato M, Soares-Vieira JA, et al. Ethnicity and glutathione S-transferase (GSTM1/GSTT1) polymorphisms in a Brazilian population. Braz J Med Biol Res 2004; 37:451–458.
- Li R, Lyn D, Lapu-Bula R, et al. Relation of endothelial nitric oxide synthase gene to plasma nitric oxide level, endothelial function, and blood pressure in African Americans. Am J Hypertens 2004; 17:560–567.
- Parra EJ, Marcini A, Akey J, et al. Estimating African American admixture proportions by use of population-specific alleles. Am J Hum Genet 1998; 63:1839–1851.
- Small KM, Wagoner LE, Levin AM, Kardia SL, Liggett SB. Synergistic polymorphisms of beta1- and alpha2C-adrenergic receptors and the risk of congestive heart failure. N Engl J Med 2002; 347:1135–1142.
- McNamara DM, Tam SW, Sabolinski ML, et al. Aldosterone synthase promoter polymorphism predicts outcome in African Americans with heart failure: results from the A-HeFT Trial. J Am Coll Cardiol 2006; 48:1277–1282.
- McNamara DM, Tam SW, Sabolinski ML, et al. Endothelial nitric oxide synthase (NOS3) polymorphisms in African Americans with heart failure: results from the A-HeFT trial. J Card Fail 2009; 15:191–198.
- Jacobson DR, Pastore RD, Yaghoubian R, et al. Variant-sequence transthyretin (isoleucine 122) in late-onset cardiac amyloidosis in black Americans. N Engl J Med 1997; 336:466–473.
- Buxbaum J, Alexander A, Koziol J, Tagoe C, Fox E, Kitzman D. Significance of the amyloidogenic transthyretin Val 122 Ile allele in African Americans in the Arteriosclerosis Risk in Communities (ARIC) and Cardiovascular Health (CHS) Studies. Am Heart J 2010; 159:864–870.
- Philbin EF, Dec GW, Jenkins PL, DiSalvo TG. Socioeconomic status as an independent risk factor for hospital readmission for heart failure. Am J Cardiol 2001; 87:1367–1371.
- Evangelista LS, Dracup K, Doering LV. Racial differences in treatment-seeking delays among heart failure patients. J Card Fail 2002; 8:381–386.
- Kressin NR, Orner MB, Manze M, Glickman ME, Berlowitz D. Understanding contributors to racial disparities in blood pressure control. Circ Cardiovasc Qual Outcomes 2010; 3:173–180.
- Yancy CW, Jessup M, Bozkurt B, et al; ACCF/AHA Task Force Members. 2013 ACCF/AHA guideline for the management of heart failure. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 62:e147–e329.
- James PA, Oparil S, Carter BL, et al. 2014 evidence-based guideline for the management of high blood pressure in adults. Report from the panel members appointed to the Eighth Joint National Committee (JNC 8). JAMA 2013; doi: 10.1001/jama.2013.284427. E-pub ahead of print.
- Chobanian AV, Bakris GL, Black HR, et al; Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure; National Heart, Lung, and Blood Institute; National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension 2003; 42:1206–1252.
- Bibbins-Domingo K, Chertow GM, Coxson PG, et al. Projected effect of dietary salt reductions on future cardiovascular disease. N Engl J Med 2010; 362:590–599.
- Stone NJ, Robinson J, Lichtenstein AH, et al. 2013 ACC/AHA guideline on the treatment of blood cholesterol to reduce atherosclerotic cardiovascular risk in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; published online Nov 13. DOI: 10.1016/j.jacc.2013.11.002.
- Casagrande SS, Whitt-Glover MC, Lancaster KJ, Odoms-Young AM, Gary TL. Built environment and health behaviors among African Americans: a systematic review. Am J Prev Med 2009; 36:174–181.
- Gustat J, Rice J, Parker KM, Becker AB, Farley TA. Effect of changes to the neighborhood built environment on physical activity in a low-income African American neighborhood. Prev Chronic Dis 2012; 9:E57.
- Casagrande SS, Franco M, Gittelsohn J, et al. Healthy food availability and the association with BMI in Baltimore, Maryland. Public Health Nutr 2011; 14:1001–1007.
- Stewart JE, Battersby SE, Lopez-De Fede A, Remington KC, Hardin JW, Mayfield-Smith K. Diabetes and the socioeconomic and built environment: geovisualization of disease prevalence and potential contextual associations using ring maps. Int J Health Geogr 2011; 10:18.
- Franciosa JA, Ferdinand KC, Yancy CW; Consensus Statement on Heart Failure in African Americans Writing Group. Treatment of heart failure in African Americans: a consensus statement. Congest Heart Fail 2010; 16:27–38.
- Ahmed A, Rich MW, Love TE, et al. Digoxin and reduction in mortality and hospitalization in heart failure: a comprehensive post hoc analysis of the DIG trial. Eur Heart J 2006; 27:178–186.
- Effect of enalapril on survival in patients with reduced left ventricular ejection fractions and congestive heart failure.The SOLVD Investigators. N Engl J Med 1991; 325:293–302.
- Shekelle PG, Rich MW, Morton SC, et al. Efficacy of angiotensin-converting enzyme inhibitors and beta-blockers in the management of left ventricular systolic dysfunction according to race, gender, and diabetic status: a meta-analysis of major clinical trials. J Am Coll Cardiol 2003; 41:1529–1538.
- Gibbs CR, Lip GY, Beevers DG. Angioedema due to ACE inhibitors: increased risk in patients of African origin. Br J Clin Pharmacol 1999; 48:861–865.
- Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther 1996; 60:8–13.
- Beta-Blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001; 344:1659–1667.
- Packer M, Coats AJ, Fowler MB, et al; Carvedilol Prospective Randomized Cumulative Survival Study Group. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001; 344:1651–1658.
- Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF). Lancet 1999; 353:2001–2007.
- Goldstein S, Deedwania P, Gottlieb S, Wikstrand J; MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the Metoprolol CR/XL randomized intervention trial in chronic heart failure). Am J Cardiol 2003; 92:478–480.
- Bristow MR, Murphy GA, Krause-Steinrauf H, et al. An alpha2C-adrenergic receptor polymorphism alters the norepinephrine-lowering effects and therapeutic response of the beta-blocker bucindolol in chronic heart failure. Circ Heart Fail 2010; 3:21–28.
- Bristow MR, Krause-Steinrauf H, Nuzzo R, et al. Effect of baseline or changes in adrenergic activity on clinical outcomes in the beta-blocker evaluation of survival trial. Circulation 2004; 110:1437–1442.
- Pitt B, Zannad F, Remme WJ, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. Randomized Aldactone Evaluation Study Investigators. N Engl J Med 1999; 341:709–717.
- Pitt B, Remme W, Zannad F, et al; Eplerenone Post-Acute Myocardial Infarction Heart Failure Efficacy and Survival Study Investigators. Eplerenone, a selective aldosterone blocker, in patients with left ventricular dysfunction after myocardial infarction. N Engl J Med 2003; 348:1309–1321.
- Zannad F, McMurray JJ, Krum H, et al; EMPHASIS-HF Study Group. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med 2011; 364:11–21.
- Cavallari LH, Groo VL, Momary KM, Fontana D, Viana MA, Vaitkus P. Racial differences in potassium response to spironolactone in heart failure. Congest Heart Fail 2006; 12:200–205.
- Cohn JN, Johnson G, Ziesche S, et al. A comparison of enalapril with hydralazine-isosorbide dinitrate in the treatment of chronic congestive heart failure. N Engl J Med 1991; 325:303–310.
- Taylor AL, Ziesche S, Yancy C, et al; African-American Heart Failure Trial Investigators. Combination of isosorbide dinitrate and hydralazine in blacks with heart failure. N Engl J Med 2004; 351:2049–2057.
- Anand IS, Tam SW, Rector TS, et al. Influence of blood pressure on the effectiveness of a fixed-dose combination of isosorbide dinitrate and hydralazine in the African-American Heart Failure Trial. J Am Coll Cardiol 2007; 49:32–39.
- Echols MR, Felker GM, Thomas KL, et al. Racial differences in the characteristics of patients admitted for acute decompensated heart failure and their relation to outcomes: results from the OPTIME-CHF trial. J Card Fail 2006; 12:684–688.
- Kamath SA, Drazner MH, Wynne J, Fonarow GC, Yancy CW. Characteristics and outcomes in African American patients with decompensated heart failure. Arch Intern Med 2008; 168:1152–1158.
- Felker GM, Lee KL, Bull DA, et al; NHLBI Heart Failure Clinical Research Network. Diuretic strategies in patients with acute decompensated heart failure. N Engl J Med 2011; 364:797–805.
- Costanzo MR, Guglin ME, Saltzberg MT, et al; UNLOAD Trial Investigators. Ultrafiltration versus intravenous diuretics for patients hospitalized for acute decompensated heart failure. J Am Coll Cardiol 2007; 49:675–683.
- Bart BA, Goldsmith SR, Lee KL, et al; Heart Failure Clinical Research Network. Ultrafiltration in decompensated heart failure with cardiorenal syndrome. N Engl J Med 2012; 367:2296–2304.
- Levy P, Compton S, Welch R, et al. Treatment of severe decompensated heart failure with high-dose intravenous nitroglycerin: a feasibility and outcome analysis. Ann Emerg Med 2007; 50:144–152.
- Cleland JG, Daubert JC, Erdmann E, et al; Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med 2005; 352:1539–1549.
- Young JB, Abraham WT, Smith AL, et al; Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA 2003; 289:2685–2694.
- Moss AJ, Zareba W, Hall WJ, et al; Multicenter Automatic Defibrillator Implantation Trial II Investigators. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med 2002; 346:877–883.
- Bristow MR, Saxon LA, Boehmer J, et al; Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med 2004; 350:2140–2150.
- Mitchell JE, Hellkamp AS, Mark DB, et al; SCD-HeFT Investigators. Outcome in African Americans and other minorities in the Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT). Am Heart J 2008; 155:501–506.
- Hernandez AF, Fonarow GC, Liang L, et al. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA 2007; 298:1525–1532.
- Farmer SA, Kirkpatrick JN, Heidenreich PA, Curtis JP, Wang Y, Groeneveld PW. Ethnic and racial disparities in cardiac resynchronization therapy. Heart Rhythm 2009; 6:325–331.
- Colvin-Adams M, Smith JM, Heubner BM, et al. OPTN/SRTR 2011 Annual Data Report: heart. Am J Transplant 2013; 13(suppl 1):119–148.
- Allen JG, Weiss ES, Arnaoutakis GJ, et al. The impact of race on survival after heart transplantation: an analysis of more than 20,000 patients. Ann Thorac Surg 2010; 89:1956–1964.
- Liu V, Bhattacharya J, Weill D, Hlatky MA. Persistent racial disparities in survival after heart transplantation. Circulation 2011; 123:1642–1649.
- Mahle WT, Kanter KR, Vincent RN. Disparities in outcome for black patients after pediatric heart transplantation. J Pediatr 2005; 147:739–743.
- Park MH, Tolman DE, Kimball PM. Disproportionate HLA matching may contribute to racial disparity in patient survival following cardiac transplantation. Clin Transplant 1996; 10(6 Pt 2):625–628.
- Park MH, Tolman DE, Kimball PM. The impact of race and HLA matching on long-term survival following cardiac transplantation. Transplant Proc 1997; 29:1460–1463.
- Callender CO, Cherikh WS, Miles PV, et al. Blacks as donors for transplantation: suboptimal outcomes overcome by transplantation into other minorities. Transplant Proc 2008; 40:995–1000.
- King LP, Siminoff LA, Meyer DM, et al. Health insurance and cardiac transplantation: a call for reform. J Am Coll Cardiol 2005; 45:1388–1391.
- Ozminkowski RJ, White AJ, Hassol A, Murphy M. Minimizing racial disparity regarding receipt of a cadaver kidney transplant. Am J Kidney Dis 1997; 30:749–759.
- Aggarwal A, Gupta A, Pappas PS, Tatooles A, Bhat G. Racial differences in patients with left ventricular assist devices. ASAIO J 2012; 58:499–502.
- Tsiouris A, Brewer RJ, Borgi J, Nemeh H, Paone G, Morgan JA. Continuous-flow left ventricular assist device implantation as a bridge to transplantation or destination therapy: racial disparities in outcomes. J Heart Lung Transplant 2013; 32:299–304.
- Joyce DL, Conte JV, Russell SD, Joyce LD, Chang DC. Disparities in access to left ventricular assist device therapy. J Surg Res 2009; 152:111–117.
KEY POINTS
- The natural history, epidemiology, and outcomes of heart failure in African Americans differ from those in whites.
- Hypertension is the predominant risk factor for heart failure in African Americans, and aggressive management of hypertension may substantially reduce the incidence and consequences of heart failure in this population.
- Heart failure in African Americans should be treated according to the same evidenced-based strategies as in the general population. In addition, a combination of isosorbide dinitrate and hydralazine is recommended in African Americans.
- Many questions remain unanswered, since African Americans have been markedly underrepresented in clinical trials.
A 20-year-old woman with fatigue and palpitations
A 20-year-old woman presents to the emergency department with fatigue and the sudden onset of palpitations. She reports no history of significant illness or surgery. She says she is not currently taking prescription or over-the-counter medications. She does not smoke, drink alcohol, or use illicit drugs.
Her weight is 52 kg (115 lb), her height is 170 cm (67 in), and her body mass index (BMI) is 18 kg/m2. Vital signs: temperature 35.7°C (96.4°F), blood pressure 92/48 mm Hg, heart rate 73 bpm, respiratory rate 5 breaths per minute, and oxygen saturation 98% on room air.
She appears tired but is alert, conversant, and cooperative. Her skin is normal, and dentition is fair. Her pulse is regular, and respirations are slow. The abdomen is soft, non-tender, and flat. Strength is 4 on a scale of 5 in all extremities. Deep-tendon reflexes are 2+ and symmetric.
Electrocardiography (Figure 1) in the emergency department shows ST-segment depression, a prolonged corrected QT interval of 665 msec, T-wave inversion, PR prolongation, increased P-wave amplitude, and U waves.
1. Which electrolyte abnormality is associated with this electrocardiographic picture?
- Hypercalcemia
- Hyperkalemia
- Hypocalcemia
- Hypokalemia
Hypokalemia is the likely cause of these findings. The finding of U waves is considered significant when they are inverted, merged with the T wave, or have an amplitude greater than the T wave.1 U waves are best seen in the precordial leads. When severe, hypokalemia can lead to potentially fatal arrhythmias such as high-grade atrioventricular block, ventricular tachycardia, and ventricular fibrillation.2
Hyperkalemia is associated with peaked T waves, a prolonged PR interval, decreased P wave amplitude, and a widened QRS complex.2 When acute and severe, hyperkalemia is associated with ventricular arrhythmia.
Hypocalcemia is associated with a prolonged QT interval and ventricular dysrhythmia, but not U waves.2
Hypercalcemia is associated with bradydysrhythmia, as well as with a shortened QT interval.2
LABORATORY TESTING
Laboratory testing shows the following:
- Sodium 126 mmol/L (reference range 135–145)
- Potassium 1.5 mmol/L (3.5–5.1)
- Chloride 58 mmol/L (100–110)
- Bicarbonate 62 mmol/L (20–30)
- Blood urea nitrogen 16 mg/dL (7–18)
- Creatinine 0.8 mg/dL (0.5–1.0)
- Glucose 106 mg/dL (70–110)
- Ionized calcium 4.4 mg/dL (4.5–5.3)
- Magnesium 1.8 mg/dL (1.7–2.3)
- Phosphorus 4.1 mg/dL (2.5–4.5)
- Venous blood gases pH 7.56 (7.35–7.45), Pco2 69 mm Hg (35–45).
POTASSIUM HOMEOSTASIS
Ninety-eight percent of potassium is intracellular and only 2% is extracellular.3 The main cellular stores are myocytes and hepatocytes. Patients with decreased muscle mass may be at a higher risk of hypokalemia as a result of decreased skeletal muscle stores.4
The acute development of hypokalemia occurs from transcellular shifts. Alkalosis, insulin secretion, and beta-adrenergic stimulation promote the intracellular uptake of potassium. The major hormonal regulator of potassium excretion is aldosterone, which is stimulated by renal hypoperfusion and promotes potassium-ion secretion in the distal convoluted tubule.
Chronic hypokalemia develops in patients with ongoing renal or gastrointestinal potassium loss. If the cause of potassium loss is not elucidated by the history, the physical, and a review of medications, then one of two things is possible: either the patient has renal tubular disease affecting acid-base and potassium regulation, causing excessive mineralocorticoid secretion, which is associated with an abnormal response to aldosterone; or the patient is not being forthcoming in the history.
2. Which is the most likely cause of hypokalemia in this patient?
- Vomiting
- Liddle syndrome
- Bartter syndrome
- Gitelman syndrome
- Diuretic use
Her laboratory tests reveal hypokalemia and hyponatremic-hypochloremic metabolic alkalosis with compensatory respiratory acidosis. In metabolic alkalosis, the expected respiratory compensation is an increase of 0.7 mm Hg in Pco2 for each 1-mEq/L increase in bicarbonate. Therefore, the expected Pco2 is 67, close to the patient’s actual value of 69.
Protracted vomiting with loss of gastric acid juices could be a cause of the metabolic disturbances in this young woman, although she did not mention vomiting during the history.
Liddle syndrome, or pseudoaldosteronism, is a rare autosomal dominant disorder characterized by altered renal epithelial sodium channels, excessive sodium retention, and resultant hypertension. Hypokalemia and alkalosis are seen in Liddle syndrome, but the absence of hypertension in our patient makes Liddle syndrome unlikely.
Bartter syndrome is an inherited autosomal recessive disorder of the sodium-potassium-chloride cotransporter in the thick ascending loop of Henle, resulting in impaired reabsorption of chloride and sodium. Bartter syndrome mimics chronic loop-diuretic use and is associated with hypercalciuria. Bartter syndrome is possible in this patient; however, patients with Bartter syndrome are usually diagnosed in infancy or childhood and have evidence of growth impairment.
Gitelman syndrome is an autosomal recessive disorder of the thiazide-sensitive sodium-chloride cotransporter. Although Gitelman syndrome is more common than Bartter syndrome and presents at older ages, it is not usually associated with such profound metabolic alkalosis. Gitelman syndrome mimics chronic use of thiazide diuretics and is associated with hypocalciuria.
Diuretic use could also cause the metabolic disturbances described; however, the patient denied taking diuretics.
The most common cause of hypokalemia in clinical practice is diuretic use.4 In this young woman with unexplained hypokalemia, the most likely cause is either undisclosed self-induced vomiting or diuretic abuse. The degree of metabolic alkalosis suggests vomiting, since metabolic alkalosis this severe is usually seen only with protracted vomiting. Bartter and Gitelman syndromes are included in the differential diagnosis, but they are much less common than hypokalemia associated with diuretics or self-induced vomiting.5
3. Which test could help elucidate the cause of hypokalemia in this patient?
- Ratio of plasma aldosterone to rennin
- Urine chloride
- Ratio of urinary potassium to creatinine
- Urinary anion gap and urinary pH
APPROACH TO HYPOKALEMIA
Determining the cause of hypokalemia starts with a thorough history and physical examination. The history should focus on drugs such as diuretics and laxatives. Women should be asked about their menstrual history since irregular periods may suggest an eating disorder. The physical examination should focus on signs that suggest self-induced vomiting, such as dry skin, dental erosions, enlarged parotid glands, and calluses or scars on the knuckles.
Patients with an unclear cause of hypokalemia after a thorough history and physical examination can be categorized into one of three groups based on blood pressure and acid-base status:
- Hypokalemia, hypertension, metabolic alkalosis
- Hypokalemia, normal blood pressure, metabolic acidosis
- Hypokalemia, normal blood pressure, metabolic alkalosis.
Hypokalemia, hypertension, metabolic alkalosis
The blood pressure provides an important clue in the evaluation of hypokalemia. The combination of hypertension, hypokalemia, and alkalosis should raise concern for hyperaldosteronism or pseudoaldosteronism. Primary hyperaldosteronism from an adrenal adenoma (Conn syndrome) is characterized by a plasma aldosterone-renin ratio of greater than 20.6,7 In contrast, patients with secondary hyperaldosteronism due to renovascular disease have a plasma aldosterone-renin ratio of less than 10. Patients with pseudoaldosteronism have low aldosterone and renin levels and hypertension. Since our patient has a normal blood pressure, testing the plasma aldosterone and renin levels would not help determine the cause of her hypokalemia.
Hypokalemia, normal blood pressure, metabolic acidosis
Patients with normal blood pressure, hypokalemia, and normal plasma anion gap acidosis either have renal tubular acidosis or have lost potassium because of diarrhea or laxative abuse. In a patient who denies taking laxatives or denies a history of diarrhea, checking the urinary anion gap and urinary pH may help differentiate the cause of acidosis and hypokalemia.
The urinary anion gap, calculated by the equation sodium + potassium − chloride, is an indirect estimate of hydrogen excretion in the form of ammonium ion8; the normal value is 0 to 10 mEq/L. A negative value represents increased hydrogen excretion in response to systemic acidosis from gastrointestinal or renal loss of bicarbonate (proximal renal tubular acidosis). A urinary pH greater than 5.5 in the setting of systemic acidosis suggests impaired ability of the kidneys to acidify urine and raises the possibility of renal tubular acidosis.
This patient has metabolic alkalosis, so calculation of the urinary anion gap would not be helpful.
Hypokalemia, normal blood pressure, metabolic alkalosis
Patients such as ours, with normal blood pressure, hypokalemia, and alkalosis, have been vomiting, have used diuretics, or have an inherited renal tubulopathy such as Bartter or Gitelman syndrome. Usually, differentiating Bartter and Gitelman syndromes from chronic vomiting or diuretic use is done with the history and physical examination. However, in patients with a questionable history and a lack of findings on physical examination, checking the urinary chloride, potassium, calcium, and creatinine may be helpful.
A urinary potassium-creatinine ratio greater than 15 suggests renal loss, whereas a ratio less than 15 suggests extrarenal loss.9
Patients who are taking a diuretic or who have Bartter or Gitelman syndrome have a high urinary chloride concentration, ie, greater than 20 mmol/L, whereas patients with hypokalemia and alkalosis from chronic vomiting tend to have a concentration less than 10 mmol/L.10
Table 1 summarizes an approach to the evaluation of unexplained hypokalemia based on blood pressure and acid-base status.
A HIDDEN HISTORY
On further questioning, the patient admits to an 8-year history of daily self-induced vomiting in an attempt to lose weight, in addition to multiple hospitalizations for hypokalemia and a previous diagnosis of an eating disorder.
INITIAL MANAGEMENT OF HYPOKALEMIA
The initial management of hypokalemia should focus on life-threatening emergencies. While patients with potassium levels greater than 3 mmol/L are usually asymptomatic, those with levels below 3 mmol/L present with muscle weakness and rhabdomyolysis.4 An acute drop in serum potassium to less than 2 mmol/L is associated with respiratory muscle weakness and ventricular arrhythmias.4 If the patient has cardiac symptoms or hypoventilation due to respiratory muscle weakness, continuous monitoring in the intensive care unit and aggressive therapy are warranted.
4. Which potassium formulation is most appropriate for the treatment of hypokalemia in this patient?
- Potassium chloride
- Potassium phosphate
- Potassium acetate
Oral potassium is preferable in patients with a serum potassium above 2.5 mmol/L.4,11 Potassium phosphate should be used when supplementation with both potassium and phosphorus is needed. Potassium acetate should be reserved for patients with acidosis and hypokalemia. Otherwise, potassium chloride is typically preferred.4,12 It comes in liquid and tablet forms. Liquid forms have an unpleasant taste, whereas tablets are usually well tolerated. No more than 20 to 40 mEq of potassium chloride tablets should be given at a time, since higher doses are associated with gastrointestinal mucosal injury.12
Potassium chloride is particularly preferred in patients with metabolic alkalosis, since increased chloride intake and delivery to the distal tubule increases the expression of pendrin, a luminal chloride and bicarbonate exchanger in the cortical collecting duct.13 With metabolic alkalosis, increased excretion of bicarbonate occurs through up-regulation of pendrin. Potassium depletion down-regulates pendrin.13 Additionally, correction of metabolic alkalosis increases serum potassium by movement of potassium from the intracellular to the extracellular space.
Intravenous potassium should be reserved for patients with severe hypokalemia (< 2.5 mmol/L) or significant arrhythmias.11 Oral and intravenous potassium can safely be given simultaneously.11 The intravenous rate should not exceed more than 10 to 20 mEq of potassium chloride per hour unless the patient has a life-threatening arrhythmia, respiratory failure, or severe hypokalemia.14,15 In life-threatening situations, a femoral line should be placed, and potassium should be given as rapidly as 20 mEq over 15 to 20 minutes.14 Cannulation of the subclavian and internal jugular veins should be avoided in severe hypokalemia since mechanical irritation from guidewire placement can provoke ventricular arrhythmias.14
During intravenous administration of potassium, laboratory monitoring after every 20 mEq of potassium chloride is advised because of the possibility of rebound hyperkalemia. In patients with severe hypokalemia, avoidance of factors that can worsen intracellular shift of potassium is also important. Avoid dextrose-containing fluids to prevent insulin-induced shifting of potassium into cells. Restore intravascular volume to blunt hypovolemia-induced renin and aldosterone secretion. If a patient presents with severe hypokalemia and acidosis, correct the hypokalemia before the acidosis to avoid intracellular shift of potassium.
OUR PATIENT’S MANAGEMENT AND FOLLOW-UP PLAN
Given the severity of our patient’s hypokalemia and her complaint of palpitations, she was admitted to the hospital for monitoring. She required 180 mEq of intravenous potassium chloride and 140 mEq of oral potassium chloride during the first 24 hours in order to achieve a serum potassium level above 3 mmol/L. Electrocardiographic U waves resolved once the level was above 2 mmol/L, and ST depressions resolved once it was above 3 mmol/L. The QT interval normalized after 24 hours of hospitalization.
On discharge, she was prescribed oral potassium chloride 40 mEq daily and magnesium sulfate 400 mg twice daily, with plans for a followup visit with her outpatient therapy team, which includes a psychiatrist, a social worker, and her primary care provider. She declined a referral for inpatient therapy but agreed to a goal of decreasing the frequency of induced vomiting and outpatient visits. She was also educated on how and when to access emergency medical care.16
- Rautaharju PM, Surawicz B, Gettes LS, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT Interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:982–991.
- Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med 2004; 27:153–160.
- Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 2011; 7:75–84.
- Gennari FJ. Hypokalemia. N Engl J Med 1998; 339:451–458.
- Mehler PS. Clinical practice. Bulimia nervosa. N Engl J Med 2003; 349:875–881.
- Tzanela M, Effraimidis G, Vassiliadi D, et al. The aldosterone to renin ratio in the evaluation of patients with incidentally detected adrenal masses. Endocrine 2007; 32:136–142.
- Diederich S, Mai K, Bähr V, Helffrich S, Pfeiffer A, Perschel FH. The simultaneous measurement of plasma-aldosterone- and -renin-concentration allows rapid classification of all disorders of the renin-aldosterone system. Exp Clin Endocrinol Diabetes 2007; 115:433–438.
- Goldstein MB, Bear R, Richardson RM, Marsden PA, Halperin ML. The urine anion gap: a clinically useful index of ammonium excretion. Am J Med Sci 1986; 292:198–202.
- Groeneveld JH, Sijpkens YW, Lin SH, Davids MR, Halperin ML. An approach to the patient with severe hypokalaemia: the potassium quiz. QJM 2005; 98:305–316.
- Galla JH. Metabolic alkalosis. J Am Soc Nephrol 2000; 11:369–375.
- Asmar A, Mohandas R, Wingo CS. A physiologic-based approach to the treatment of a patient with hypokalemia. Am J Kidney Dis 2012; 60:492–497.
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med 2000; 160:2429–2436.
- Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 2012; 23:204–207.
- Kruse JA, Carlson RW. Rapid correction of hypokalemia using concentrated intravenous potassium chloride infusions. Arch Intern Med 1990; 150:613–617.
- Weiner ID, Wingo CS. Hypokalemia—consequences, causes, and correction. J Am Soc Nephrol 1997; 8:1179–1188.
- AED Medical Care Standards Task Force. Eating disorders: Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders. AED Report 2012. www.aedweb.org/web/downloads/Guide-English.pdf. Accessed April 4, 2014.
A 20-year-old woman presents to the emergency department with fatigue and the sudden onset of palpitations. She reports no history of significant illness or surgery. She says she is not currently taking prescription or over-the-counter medications. She does not smoke, drink alcohol, or use illicit drugs.
Her weight is 52 kg (115 lb), her height is 170 cm (67 in), and her body mass index (BMI) is 18 kg/m2. Vital signs: temperature 35.7°C (96.4°F), blood pressure 92/48 mm Hg, heart rate 73 bpm, respiratory rate 5 breaths per minute, and oxygen saturation 98% on room air.
She appears tired but is alert, conversant, and cooperative. Her skin is normal, and dentition is fair. Her pulse is regular, and respirations are slow. The abdomen is soft, non-tender, and flat. Strength is 4 on a scale of 5 in all extremities. Deep-tendon reflexes are 2+ and symmetric.
Electrocardiography (Figure 1) in the emergency department shows ST-segment depression, a prolonged corrected QT interval of 665 msec, T-wave inversion, PR prolongation, increased P-wave amplitude, and U waves.
1. Which electrolyte abnormality is associated with this electrocardiographic picture?
- Hypercalcemia
- Hyperkalemia
- Hypocalcemia
- Hypokalemia
Hypokalemia is the likely cause of these findings. The finding of U waves is considered significant when they are inverted, merged with the T wave, or have an amplitude greater than the T wave.1 U waves are best seen in the precordial leads. When severe, hypokalemia can lead to potentially fatal arrhythmias such as high-grade atrioventricular block, ventricular tachycardia, and ventricular fibrillation.2
Hyperkalemia is associated with peaked T waves, a prolonged PR interval, decreased P wave amplitude, and a widened QRS complex.2 When acute and severe, hyperkalemia is associated with ventricular arrhythmia.
Hypocalcemia is associated with a prolonged QT interval and ventricular dysrhythmia, but not U waves.2
Hypercalcemia is associated with bradydysrhythmia, as well as with a shortened QT interval.2
LABORATORY TESTING
Laboratory testing shows the following:
- Sodium 126 mmol/L (reference range 135–145)
- Potassium 1.5 mmol/L (3.5–5.1)
- Chloride 58 mmol/L (100–110)
- Bicarbonate 62 mmol/L (20–30)
- Blood urea nitrogen 16 mg/dL (7–18)
- Creatinine 0.8 mg/dL (0.5–1.0)
- Glucose 106 mg/dL (70–110)
- Ionized calcium 4.4 mg/dL (4.5–5.3)
- Magnesium 1.8 mg/dL (1.7–2.3)
- Phosphorus 4.1 mg/dL (2.5–4.5)
- Venous blood gases pH 7.56 (7.35–7.45), Pco2 69 mm Hg (35–45).
POTASSIUM HOMEOSTASIS
Ninety-eight percent of potassium is intracellular and only 2% is extracellular.3 The main cellular stores are myocytes and hepatocytes. Patients with decreased muscle mass may be at a higher risk of hypokalemia as a result of decreased skeletal muscle stores.4
The acute development of hypokalemia occurs from transcellular shifts. Alkalosis, insulin secretion, and beta-adrenergic stimulation promote the intracellular uptake of potassium. The major hormonal regulator of potassium excretion is aldosterone, which is stimulated by renal hypoperfusion and promotes potassium-ion secretion in the distal convoluted tubule.
Chronic hypokalemia develops in patients with ongoing renal or gastrointestinal potassium loss. If the cause of potassium loss is not elucidated by the history, the physical, and a review of medications, then one of two things is possible: either the patient has renal tubular disease affecting acid-base and potassium regulation, causing excessive mineralocorticoid secretion, which is associated with an abnormal response to aldosterone; or the patient is not being forthcoming in the history.
2. Which is the most likely cause of hypokalemia in this patient?
- Vomiting
- Liddle syndrome
- Bartter syndrome
- Gitelman syndrome
- Diuretic use
Her laboratory tests reveal hypokalemia and hyponatremic-hypochloremic metabolic alkalosis with compensatory respiratory acidosis. In metabolic alkalosis, the expected respiratory compensation is an increase of 0.7 mm Hg in Pco2 for each 1-mEq/L increase in bicarbonate. Therefore, the expected Pco2 is 67, close to the patient’s actual value of 69.
Protracted vomiting with loss of gastric acid juices could be a cause of the metabolic disturbances in this young woman, although she did not mention vomiting during the history.
Liddle syndrome, or pseudoaldosteronism, is a rare autosomal dominant disorder characterized by altered renal epithelial sodium channels, excessive sodium retention, and resultant hypertension. Hypokalemia and alkalosis are seen in Liddle syndrome, but the absence of hypertension in our patient makes Liddle syndrome unlikely.
Bartter syndrome is an inherited autosomal recessive disorder of the sodium-potassium-chloride cotransporter in the thick ascending loop of Henle, resulting in impaired reabsorption of chloride and sodium. Bartter syndrome mimics chronic loop-diuretic use and is associated with hypercalciuria. Bartter syndrome is possible in this patient; however, patients with Bartter syndrome are usually diagnosed in infancy or childhood and have evidence of growth impairment.
Gitelman syndrome is an autosomal recessive disorder of the thiazide-sensitive sodium-chloride cotransporter. Although Gitelman syndrome is more common than Bartter syndrome and presents at older ages, it is not usually associated with such profound metabolic alkalosis. Gitelman syndrome mimics chronic use of thiazide diuretics and is associated with hypocalciuria.
Diuretic use could also cause the metabolic disturbances described; however, the patient denied taking diuretics.
The most common cause of hypokalemia in clinical practice is diuretic use.4 In this young woman with unexplained hypokalemia, the most likely cause is either undisclosed self-induced vomiting or diuretic abuse. The degree of metabolic alkalosis suggests vomiting, since metabolic alkalosis this severe is usually seen only with protracted vomiting. Bartter and Gitelman syndromes are included in the differential diagnosis, but they are much less common than hypokalemia associated with diuretics or self-induced vomiting.5
3. Which test could help elucidate the cause of hypokalemia in this patient?
- Ratio of plasma aldosterone to rennin
- Urine chloride
- Ratio of urinary potassium to creatinine
- Urinary anion gap and urinary pH
APPROACH TO HYPOKALEMIA
Determining the cause of hypokalemia starts with a thorough history and physical examination. The history should focus on drugs such as diuretics and laxatives. Women should be asked about their menstrual history since irregular periods may suggest an eating disorder. The physical examination should focus on signs that suggest self-induced vomiting, such as dry skin, dental erosions, enlarged parotid glands, and calluses or scars on the knuckles.
Patients with an unclear cause of hypokalemia after a thorough history and physical examination can be categorized into one of three groups based on blood pressure and acid-base status:
- Hypokalemia, hypertension, metabolic alkalosis
- Hypokalemia, normal blood pressure, metabolic acidosis
- Hypokalemia, normal blood pressure, metabolic alkalosis.
Hypokalemia, hypertension, metabolic alkalosis
The blood pressure provides an important clue in the evaluation of hypokalemia. The combination of hypertension, hypokalemia, and alkalosis should raise concern for hyperaldosteronism or pseudoaldosteronism. Primary hyperaldosteronism from an adrenal adenoma (Conn syndrome) is characterized by a plasma aldosterone-renin ratio of greater than 20.6,7 In contrast, patients with secondary hyperaldosteronism due to renovascular disease have a plasma aldosterone-renin ratio of less than 10. Patients with pseudoaldosteronism have low aldosterone and renin levels and hypertension. Since our patient has a normal blood pressure, testing the plasma aldosterone and renin levels would not help determine the cause of her hypokalemia.
Hypokalemia, normal blood pressure, metabolic acidosis
Patients with normal blood pressure, hypokalemia, and normal plasma anion gap acidosis either have renal tubular acidosis or have lost potassium because of diarrhea or laxative abuse. In a patient who denies taking laxatives or denies a history of diarrhea, checking the urinary anion gap and urinary pH may help differentiate the cause of acidosis and hypokalemia.
The urinary anion gap, calculated by the equation sodium + potassium − chloride, is an indirect estimate of hydrogen excretion in the form of ammonium ion8; the normal value is 0 to 10 mEq/L. A negative value represents increased hydrogen excretion in response to systemic acidosis from gastrointestinal or renal loss of bicarbonate (proximal renal tubular acidosis). A urinary pH greater than 5.5 in the setting of systemic acidosis suggests impaired ability of the kidneys to acidify urine and raises the possibility of renal tubular acidosis.
This patient has metabolic alkalosis, so calculation of the urinary anion gap would not be helpful.
Hypokalemia, normal blood pressure, metabolic alkalosis
Patients such as ours, with normal blood pressure, hypokalemia, and alkalosis, have been vomiting, have used diuretics, or have an inherited renal tubulopathy such as Bartter or Gitelman syndrome. Usually, differentiating Bartter and Gitelman syndromes from chronic vomiting or diuretic use is done with the history and physical examination. However, in patients with a questionable history and a lack of findings on physical examination, checking the urinary chloride, potassium, calcium, and creatinine may be helpful.
A urinary potassium-creatinine ratio greater than 15 suggests renal loss, whereas a ratio less than 15 suggests extrarenal loss.9
Patients who are taking a diuretic or who have Bartter or Gitelman syndrome have a high urinary chloride concentration, ie, greater than 20 mmol/L, whereas patients with hypokalemia and alkalosis from chronic vomiting tend to have a concentration less than 10 mmol/L.10
Table 1 summarizes an approach to the evaluation of unexplained hypokalemia based on blood pressure and acid-base status.
A HIDDEN HISTORY
On further questioning, the patient admits to an 8-year history of daily self-induced vomiting in an attempt to lose weight, in addition to multiple hospitalizations for hypokalemia and a previous diagnosis of an eating disorder.
INITIAL MANAGEMENT OF HYPOKALEMIA
The initial management of hypokalemia should focus on life-threatening emergencies. While patients with potassium levels greater than 3 mmol/L are usually asymptomatic, those with levels below 3 mmol/L present with muscle weakness and rhabdomyolysis.4 An acute drop in serum potassium to less than 2 mmol/L is associated with respiratory muscle weakness and ventricular arrhythmias.4 If the patient has cardiac symptoms or hypoventilation due to respiratory muscle weakness, continuous monitoring in the intensive care unit and aggressive therapy are warranted.
4. Which potassium formulation is most appropriate for the treatment of hypokalemia in this patient?
- Potassium chloride
- Potassium phosphate
- Potassium acetate
Oral potassium is preferable in patients with a serum potassium above 2.5 mmol/L.4,11 Potassium phosphate should be used when supplementation with both potassium and phosphorus is needed. Potassium acetate should be reserved for patients with acidosis and hypokalemia. Otherwise, potassium chloride is typically preferred.4,12 It comes in liquid and tablet forms. Liquid forms have an unpleasant taste, whereas tablets are usually well tolerated. No more than 20 to 40 mEq of potassium chloride tablets should be given at a time, since higher doses are associated with gastrointestinal mucosal injury.12
Potassium chloride is particularly preferred in patients with metabolic alkalosis, since increased chloride intake and delivery to the distal tubule increases the expression of pendrin, a luminal chloride and bicarbonate exchanger in the cortical collecting duct.13 With metabolic alkalosis, increased excretion of bicarbonate occurs through up-regulation of pendrin. Potassium depletion down-regulates pendrin.13 Additionally, correction of metabolic alkalosis increases serum potassium by movement of potassium from the intracellular to the extracellular space.
Intravenous potassium should be reserved for patients with severe hypokalemia (< 2.5 mmol/L) or significant arrhythmias.11 Oral and intravenous potassium can safely be given simultaneously.11 The intravenous rate should not exceed more than 10 to 20 mEq of potassium chloride per hour unless the patient has a life-threatening arrhythmia, respiratory failure, or severe hypokalemia.14,15 In life-threatening situations, a femoral line should be placed, and potassium should be given as rapidly as 20 mEq over 15 to 20 minutes.14 Cannulation of the subclavian and internal jugular veins should be avoided in severe hypokalemia since mechanical irritation from guidewire placement can provoke ventricular arrhythmias.14
During intravenous administration of potassium, laboratory monitoring after every 20 mEq of potassium chloride is advised because of the possibility of rebound hyperkalemia. In patients with severe hypokalemia, avoidance of factors that can worsen intracellular shift of potassium is also important. Avoid dextrose-containing fluids to prevent insulin-induced shifting of potassium into cells. Restore intravascular volume to blunt hypovolemia-induced renin and aldosterone secretion. If a patient presents with severe hypokalemia and acidosis, correct the hypokalemia before the acidosis to avoid intracellular shift of potassium.
OUR PATIENT’S MANAGEMENT AND FOLLOW-UP PLAN
Given the severity of our patient’s hypokalemia and her complaint of palpitations, she was admitted to the hospital for monitoring. She required 180 mEq of intravenous potassium chloride and 140 mEq of oral potassium chloride during the first 24 hours in order to achieve a serum potassium level above 3 mmol/L. Electrocardiographic U waves resolved once the level was above 2 mmol/L, and ST depressions resolved once it was above 3 mmol/L. The QT interval normalized after 24 hours of hospitalization.
On discharge, she was prescribed oral potassium chloride 40 mEq daily and magnesium sulfate 400 mg twice daily, with plans for a followup visit with her outpatient therapy team, which includes a psychiatrist, a social worker, and her primary care provider. She declined a referral for inpatient therapy but agreed to a goal of decreasing the frequency of induced vomiting and outpatient visits. She was also educated on how and when to access emergency medical care.16
A 20-year-old woman presents to the emergency department with fatigue and the sudden onset of palpitations. She reports no history of significant illness or surgery. She says she is not currently taking prescription or over-the-counter medications. She does not smoke, drink alcohol, or use illicit drugs.
Her weight is 52 kg (115 lb), her height is 170 cm (67 in), and her body mass index (BMI) is 18 kg/m2. Vital signs: temperature 35.7°C (96.4°F), blood pressure 92/48 mm Hg, heart rate 73 bpm, respiratory rate 5 breaths per minute, and oxygen saturation 98% on room air.
She appears tired but is alert, conversant, and cooperative. Her skin is normal, and dentition is fair. Her pulse is regular, and respirations are slow. The abdomen is soft, non-tender, and flat. Strength is 4 on a scale of 5 in all extremities. Deep-tendon reflexes are 2+ and symmetric.
Electrocardiography (Figure 1) in the emergency department shows ST-segment depression, a prolonged corrected QT interval of 665 msec, T-wave inversion, PR prolongation, increased P-wave amplitude, and U waves.
1. Which electrolyte abnormality is associated with this electrocardiographic picture?
- Hypercalcemia
- Hyperkalemia
- Hypocalcemia
- Hypokalemia
Hypokalemia is the likely cause of these findings. The finding of U waves is considered significant when they are inverted, merged with the T wave, or have an amplitude greater than the T wave.1 U waves are best seen in the precordial leads. When severe, hypokalemia can lead to potentially fatal arrhythmias such as high-grade atrioventricular block, ventricular tachycardia, and ventricular fibrillation.2
Hyperkalemia is associated with peaked T waves, a prolonged PR interval, decreased P wave amplitude, and a widened QRS complex.2 When acute and severe, hyperkalemia is associated with ventricular arrhythmia.
Hypocalcemia is associated with a prolonged QT interval and ventricular dysrhythmia, but not U waves.2
Hypercalcemia is associated with bradydysrhythmia, as well as with a shortened QT interval.2
LABORATORY TESTING
Laboratory testing shows the following:
- Sodium 126 mmol/L (reference range 135–145)
- Potassium 1.5 mmol/L (3.5–5.1)
- Chloride 58 mmol/L (100–110)
- Bicarbonate 62 mmol/L (20–30)
- Blood urea nitrogen 16 mg/dL (7–18)
- Creatinine 0.8 mg/dL (0.5–1.0)
- Glucose 106 mg/dL (70–110)
- Ionized calcium 4.4 mg/dL (4.5–5.3)
- Magnesium 1.8 mg/dL (1.7–2.3)
- Phosphorus 4.1 mg/dL (2.5–4.5)
- Venous blood gases pH 7.56 (7.35–7.45), Pco2 69 mm Hg (35–45).
POTASSIUM HOMEOSTASIS
Ninety-eight percent of potassium is intracellular and only 2% is extracellular.3 The main cellular stores are myocytes and hepatocytes. Patients with decreased muscle mass may be at a higher risk of hypokalemia as a result of decreased skeletal muscle stores.4
The acute development of hypokalemia occurs from transcellular shifts. Alkalosis, insulin secretion, and beta-adrenergic stimulation promote the intracellular uptake of potassium. The major hormonal regulator of potassium excretion is aldosterone, which is stimulated by renal hypoperfusion and promotes potassium-ion secretion in the distal convoluted tubule.
Chronic hypokalemia develops in patients with ongoing renal or gastrointestinal potassium loss. If the cause of potassium loss is not elucidated by the history, the physical, and a review of medications, then one of two things is possible: either the patient has renal tubular disease affecting acid-base and potassium regulation, causing excessive mineralocorticoid secretion, which is associated with an abnormal response to aldosterone; or the patient is not being forthcoming in the history.
2. Which is the most likely cause of hypokalemia in this patient?
- Vomiting
- Liddle syndrome
- Bartter syndrome
- Gitelman syndrome
- Diuretic use
Her laboratory tests reveal hypokalemia and hyponatremic-hypochloremic metabolic alkalosis with compensatory respiratory acidosis. In metabolic alkalosis, the expected respiratory compensation is an increase of 0.7 mm Hg in Pco2 for each 1-mEq/L increase in bicarbonate. Therefore, the expected Pco2 is 67, close to the patient’s actual value of 69.
Protracted vomiting with loss of gastric acid juices could be a cause of the metabolic disturbances in this young woman, although she did not mention vomiting during the history.
Liddle syndrome, or pseudoaldosteronism, is a rare autosomal dominant disorder characterized by altered renal epithelial sodium channels, excessive sodium retention, and resultant hypertension. Hypokalemia and alkalosis are seen in Liddle syndrome, but the absence of hypertension in our patient makes Liddle syndrome unlikely.
Bartter syndrome is an inherited autosomal recessive disorder of the sodium-potassium-chloride cotransporter in the thick ascending loop of Henle, resulting in impaired reabsorption of chloride and sodium. Bartter syndrome mimics chronic loop-diuretic use and is associated with hypercalciuria. Bartter syndrome is possible in this patient; however, patients with Bartter syndrome are usually diagnosed in infancy or childhood and have evidence of growth impairment.
Gitelman syndrome is an autosomal recessive disorder of the thiazide-sensitive sodium-chloride cotransporter. Although Gitelman syndrome is more common than Bartter syndrome and presents at older ages, it is not usually associated with such profound metabolic alkalosis. Gitelman syndrome mimics chronic use of thiazide diuretics and is associated with hypocalciuria.
Diuretic use could also cause the metabolic disturbances described; however, the patient denied taking diuretics.
The most common cause of hypokalemia in clinical practice is diuretic use.4 In this young woman with unexplained hypokalemia, the most likely cause is either undisclosed self-induced vomiting or diuretic abuse. The degree of metabolic alkalosis suggests vomiting, since metabolic alkalosis this severe is usually seen only with protracted vomiting. Bartter and Gitelman syndromes are included in the differential diagnosis, but they are much less common than hypokalemia associated with diuretics or self-induced vomiting.5
3. Which test could help elucidate the cause of hypokalemia in this patient?
- Ratio of plasma aldosterone to rennin
- Urine chloride
- Ratio of urinary potassium to creatinine
- Urinary anion gap and urinary pH
APPROACH TO HYPOKALEMIA
Determining the cause of hypokalemia starts with a thorough history and physical examination. The history should focus on drugs such as diuretics and laxatives. Women should be asked about their menstrual history since irregular periods may suggest an eating disorder. The physical examination should focus on signs that suggest self-induced vomiting, such as dry skin, dental erosions, enlarged parotid glands, and calluses or scars on the knuckles.
Patients with an unclear cause of hypokalemia after a thorough history and physical examination can be categorized into one of three groups based on blood pressure and acid-base status:
- Hypokalemia, hypertension, metabolic alkalosis
- Hypokalemia, normal blood pressure, metabolic acidosis
- Hypokalemia, normal blood pressure, metabolic alkalosis.
Hypokalemia, hypertension, metabolic alkalosis
The blood pressure provides an important clue in the evaluation of hypokalemia. The combination of hypertension, hypokalemia, and alkalosis should raise concern for hyperaldosteronism or pseudoaldosteronism. Primary hyperaldosteronism from an adrenal adenoma (Conn syndrome) is characterized by a plasma aldosterone-renin ratio of greater than 20.6,7 In contrast, patients with secondary hyperaldosteronism due to renovascular disease have a plasma aldosterone-renin ratio of less than 10. Patients with pseudoaldosteronism have low aldosterone and renin levels and hypertension. Since our patient has a normal blood pressure, testing the plasma aldosterone and renin levels would not help determine the cause of her hypokalemia.
Hypokalemia, normal blood pressure, metabolic acidosis
Patients with normal blood pressure, hypokalemia, and normal plasma anion gap acidosis either have renal tubular acidosis or have lost potassium because of diarrhea or laxative abuse. In a patient who denies taking laxatives or denies a history of diarrhea, checking the urinary anion gap and urinary pH may help differentiate the cause of acidosis and hypokalemia.
The urinary anion gap, calculated by the equation sodium + potassium − chloride, is an indirect estimate of hydrogen excretion in the form of ammonium ion8; the normal value is 0 to 10 mEq/L. A negative value represents increased hydrogen excretion in response to systemic acidosis from gastrointestinal or renal loss of bicarbonate (proximal renal tubular acidosis). A urinary pH greater than 5.5 in the setting of systemic acidosis suggests impaired ability of the kidneys to acidify urine and raises the possibility of renal tubular acidosis.
This patient has metabolic alkalosis, so calculation of the urinary anion gap would not be helpful.
Hypokalemia, normal blood pressure, metabolic alkalosis
Patients such as ours, with normal blood pressure, hypokalemia, and alkalosis, have been vomiting, have used diuretics, or have an inherited renal tubulopathy such as Bartter or Gitelman syndrome. Usually, differentiating Bartter and Gitelman syndromes from chronic vomiting or diuretic use is done with the history and physical examination. However, in patients with a questionable history and a lack of findings on physical examination, checking the urinary chloride, potassium, calcium, and creatinine may be helpful.
A urinary potassium-creatinine ratio greater than 15 suggests renal loss, whereas a ratio less than 15 suggests extrarenal loss.9
Patients who are taking a diuretic or who have Bartter or Gitelman syndrome have a high urinary chloride concentration, ie, greater than 20 mmol/L, whereas patients with hypokalemia and alkalosis from chronic vomiting tend to have a concentration less than 10 mmol/L.10
Table 1 summarizes an approach to the evaluation of unexplained hypokalemia based on blood pressure and acid-base status.
A HIDDEN HISTORY
On further questioning, the patient admits to an 8-year history of daily self-induced vomiting in an attempt to lose weight, in addition to multiple hospitalizations for hypokalemia and a previous diagnosis of an eating disorder.
INITIAL MANAGEMENT OF HYPOKALEMIA
The initial management of hypokalemia should focus on life-threatening emergencies. While patients with potassium levels greater than 3 mmol/L are usually asymptomatic, those with levels below 3 mmol/L present with muscle weakness and rhabdomyolysis.4 An acute drop in serum potassium to less than 2 mmol/L is associated with respiratory muscle weakness and ventricular arrhythmias.4 If the patient has cardiac symptoms or hypoventilation due to respiratory muscle weakness, continuous monitoring in the intensive care unit and aggressive therapy are warranted.
4. Which potassium formulation is most appropriate for the treatment of hypokalemia in this patient?
- Potassium chloride
- Potassium phosphate
- Potassium acetate
Oral potassium is preferable in patients with a serum potassium above 2.5 mmol/L.4,11 Potassium phosphate should be used when supplementation with both potassium and phosphorus is needed. Potassium acetate should be reserved for patients with acidosis and hypokalemia. Otherwise, potassium chloride is typically preferred.4,12 It comes in liquid and tablet forms. Liquid forms have an unpleasant taste, whereas tablets are usually well tolerated. No more than 20 to 40 mEq of potassium chloride tablets should be given at a time, since higher doses are associated with gastrointestinal mucosal injury.12
Potassium chloride is particularly preferred in patients with metabolic alkalosis, since increased chloride intake and delivery to the distal tubule increases the expression of pendrin, a luminal chloride and bicarbonate exchanger in the cortical collecting duct.13 With metabolic alkalosis, increased excretion of bicarbonate occurs through up-regulation of pendrin. Potassium depletion down-regulates pendrin.13 Additionally, correction of metabolic alkalosis increases serum potassium by movement of potassium from the intracellular to the extracellular space.
Intravenous potassium should be reserved for patients with severe hypokalemia (< 2.5 mmol/L) or significant arrhythmias.11 Oral and intravenous potassium can safely be given simultaneously.11 The intravenous rate should not exceed more than 10 to 20 mEq of potassium chloride per hour unless the patient has a life-threatening arrhythmia, respiratory failure, or severe hypokalemia.14,15 In life-threatening situations, a femoral line should be placed, and potassium should be given as rapidly as 20 mEq over 15 to 20 minutes.14 Cannulation of the subclavian and internal jugular veins should be avoided in severe hypokalemia since mechanical irritation from guidewire placement can provoke ventricular arrhythmias.14
During intravenous administration of potassium, laboratory monitoring after every 20 mEq of potassium chloride is advised because of the possibility of rebound hyperkalemia. In patients with severe hypokalemia, avoidance of factors that can worsen intracellular shift of potassium is also important. Avoid dextrose-containing fluids to prevent insulin-induced shifting of potassium into cells. Restore intravascular volume to blunt hypovolemia-induced renin and aldosterone secretion. If a patient presents with severe hypokalemia and acidosis, correct the hypokalemia before the acidosis to avoid intracellular shift of potassium.
OUR PATIENT’S MANAGEMENT AND FOLLOW-UP PLAN
Given the severity of our patient’s hypokalemia and her complaint of palpitations, she was admitted to the hospital for monitoring. She required 180 mEq of intravenous potassium chloride and 140 mEq of oral potassium chloride during the first 24 hours in order to achieve a serum potassium level above 3 mmol/L. Electrocardiographic U waves resolved once the level was above 2 mmol/L, and ST depressions resolved once it was above 3 mmol/L. The QT interval normalized after 24 hours of hospitalization.
On discharge, she was prescribed oral potassium chloride 40 mEq daily and magnesium sulfate 400 mg twice daily, with plans for a followup visit with her outpatient therapy team, which includes a psychiatrist, a social worker, and her primary care provider. She declined a referral for inpatient therapy but agreed to a goal of decreasing the frequency of induced vomiting and outpatient visits. She was also educated on how and when to access emergency medical care.16
- Rautaharju PM, Surawicz B, Gettes LS, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT Interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:982–991.
- Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med 2004; 27:153–160.
- Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 2011; 7:75–84.
- Gennari FJ. Hypokalemia. N Engl J Med 1998; 339:451–458.
- Mehler PS. Clinical practice. Bulimia nervosa. N Engl J Med 2003; 349:875–881.
- Tzanela M, Effraimidis G, Vassiliadi D, et al. The aldosterone to renin ratio in the evaluation of patients with incidentally detected adrenal masses. Endocrine 2007; 32:136–142.
- Diederich S, Mai K, Bähr V, Helffrich S, Pfeiffer A, Perschel FH. The simultaneous measurement of plasma-aldosterone- and -renin-concentration allows rapid classification of all disorders of the renin-aldosterone system. Exp Clin Endocrinol Diabetes 2007; 115:433–438.
- Goldstein MB, Bear R, Richardson RM, Marsden PA, Halperin ML. The urine anion gap: a clinically useful index of ammonium excretion. Am J Med Sci 1986; 292:198–202.
- Groeneveld JH, Sijpkens YW, Lin SH, Davids MR, Halperin ML. An approach to the patient with severe hypokalaemia: the potassium quiz. QJM 2005; 98:305–316.
- Galla JH. Metabolic alkalosis. J Am Soc Nephrol 2000; 11:369–375.
- Asmar A, Mohandas R, Wingo CS. A physiologic-based approach to the treatment of a patient with hypokalemia. Am J Kidney Dis 2012; 60:492–497.
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med 2000; 160:2429–2436.
- Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 2012; 23:204–207.
- Kruse JA, Carlson RW. Rapid correction of hypokalemia using concentrated intravenous potassium chloride infusions. Arch Intern Med 1990; 150:613–617.
- Weiner ID, Wingo CS. Hypokalemia—consequences, causes, and correction. J Am Soc Nephrol 1997; 8:1179–1188.
- AED Medical Care Standards Task Force. Eating disorders: Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders. AED Report 2012. www.aedweb.org/web/downloads/Guide-English.pdf. Accessed April 4, 2014.
- Rautaharju PM, Surawicz B, Gettes LS, et al; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society. AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part IV: the ST segment, T and U waves, and the QT Interval: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society. Endorsed by the International Society for Computerized Electrocardiology. J Am Coll Cardiol 2009; 53:982–991.
- Diercks DB, Shumaik GM, Harrigan RA, Brady WJ, Chan TC. Electrocardiographic manifestations: electrolyte abnormalities. J Emerg Med 2004; 27:153–160.
- Unwin RJ, Luft FC, Shirley DG. Pathophysiology and management of hypokalemia: a clinical perspective. Nat Rev Nephrol 2011; 7:75–84.
- Gennari FJ. Hypokalemia. N Engl J Med 1998; 339:451–458.
- Mehler PS. Clinical practice. Bulimia nervosa. N Engl J Med 2003; 349:875–881.
- Tzanela M, Effraimidis G, Vassiliadi D, et al. The aldosterone to renin ratio in the evaluation of patients with incidentally detected adrenal masses. Endocrine 2007; 32:136–142.
- Diederich S, Mai K, Bähr V, Helffrich S, Pfeiffer A, Perschel FH. The simultaneous measurement of plasma-aldosterone- and -renin-concentration allows rapid classification of all disorders of the renin-aldosterone system. Exp Clin Endocrinol Diabetes 2007; 115:433–438.
- Goldstein MB, Bear R, Richardson RM, Marsden PA, Halperin ML. The urine anion gap: a clinically useful index of ammonium excretion. Am J Med Sci 1986; 292:198–202.
- Groeneveld JH, Sijpkens YW, Lin SH, Davids MR, Halperin ML. An approach to the patient with severe hypokalaemia: the potassium quiz. QJM 2005; 98:305–316.
- Galla JH. Metabolic alkalosis. J Am Soc Nephrol 2000; 11:369–375.
- Asmar A, Mohandas R, Wingo CS. A physiologic-based approach to the treatment of a patient with hypokalemia. Am J Kidney Dis 2012; 60:492–497.
- Cohn JN, Kowey PR, Whelton PK, Prisant LM. New guidelines for potassium replacement in clinical practice: a contemporary review by the National Council on Potassium in Clinical Practice. Arch Intern Med 2000; 160:2429–2436.
- Luke RG, Galla JH. It is chloride depletion alkalosis, not contraction alkalosis. J Am Soc Nephrol 2012; 23:204–207.
- Kruse JA, Carlson RW. Rapid correction of hypokalemia using concentrated intravenous potassium chloride infusions. Arch Intern Med 1990; 150:613–617.
- Weiner ID, Wingo CS. Hypokalemia—consequences, causes, and correction. J Am Soc Nephrol 1997; 8:1179–1188.
- AED Medical Care Standards Task Force. Eating disorders: Critical Points for Early Recognition and Medical Risk Management in the Care of Individuals with Eating Disorders. AED Report 2012. www.aedweb.org/web/downloads/Guide-English.pdf. Accessed April 4, 2014.
An 18-year-old woman with hepatic cysts
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
An 18-year-old woman presents with 3 days of epigastric abdominal pain, with no fever or constitutional symptoms. She was born in the United States and reports yearly trips since age 3 to her family’s farm in a rural area of Mexico, where she is exposed to dogs and horses.
Ultrasonography reveals two large hepatic cysts measuring 5.8 × 6.8 × 5.4 cm and 5.3 × 4.9 × 7 cm, with thickened walls and internal debris (Figure 1). The debris moves to dependent areas when the patient is asked to move onto her side.
Laboratory values at the time of presentation are as follows:
- White blood cell count 11.9 × 109/L (reference range 4.5–11.0), with 20% eosinophils
- Alkaline phosphatase 116 U/L (30–100)
- Total protein 7.3 g/dL (6.0–8.0)
- Albumin 4.3 g/dL (3.5–5.0)
- Aspartate aminotransferase (AST) 19 U/L (10–40)
- Alanine aminotransferase (ALT) 18 U/L (5–40)
- Total bilirubin 0.2 mg/dL (0.3–1.2)
- Direct bilirubin 0.1 mg/dL (0.1–0.3)
- Echinococcus antibody (IgG) testing is positive.
CYSTIC ECHINOCOCCOSIS
The two clinically relevant species of Echinococcus that cause human infection are E granulosus (in cystic echinococcosis) and E multilocularis (in alveolar echinococcosis). Based on clinical and radiographic findings, hepatic hydatid disease from cystic echinococcosis can usually be differentiated from the alveolar form.
E granulosus is a parasitic tapeworm that requires an intermediate host (sheep, goats, cows) and a definite host (dogs, foxes, and related species) for its life cycle. Humans become infected when they ingest food contaminated with feces that contain the eggs of the tapeworm or when they handle carnivorous animals, usually dogs, and accidentally ingest the tapeworm eggs. Once ingested, the egg releases an oncosphere that penetrates the intestinal wall, enters the circulation, and develops into a cyst, most often in the liver and the lungs.1 Human-to-human transmission does not occur.2
Hydatid cysts grow slowly, at a rate of 1 to 50 mm per year,3 so most patients remain asymptomatic for several years. Symptoms occur when a cyst ruptures or impinges on structures.3 Fever and constitutional symptoms usually occur only if there is rupture or bacterial superinfection of the cyst. Tests of liver function tend to be normal unless a cyst obstructs biliary flow. Eosinophilia occurs in 25% of patients.1 Eosinophilia along with the abrupt onset of abdominal pain suggests cyst rupture.
Making the diagnosis
Diagnosis is made by characteristic ultrasonographic findings and by serologic testing. Antibody assays for Echinococcus include indirect hemagglutination, enzyme-linked immunosorbent assay, and latex agglutination. However, these serologic antibody assays for immunoglobulin G cross-react to different echinococcal species as well as to other helminthic infections. Specific serologic studies such as an enzyme-linked immunosorbent assay for E multilocularis are available to confirm the species of Echinococcus but are only used to distinguish cystic echinococcosis from alveolar echinococcosis.
Treatment options
Treatment options include surgery, percutaneous procedures, drug therapy, and observation.
Currently, there is no clear consensus on treatment. To guide treatment decisions, the World Health Organization Informal Working Group on Echinococcosis (WHO-IWGE) recommends management of hepatic hydatid cysts based on classification, size, symptoms, location, and available resources.3
Two percutaneous treatments are aspiration, injection, and re-aspiration to destroy the germinal matrix, and percutaneous therapy to destroy the endocyst. Percutaneous aspiration, injection, and re-aspiration is increasingly used as the first-line treatment for single or easily accessible cysts and for patients who cannot undergo surgery. Surgery is considered for multiple cysts, large cysts, and cysts not easily accessible with a percutaneous technique.3 Complication rates and length of hospital stay with percutaneous aspiration are lower than with surgery.4 Observation is recommended for small, asymptomatic, inactive cysts.
Leakage of cyst contents during surgical or percutaneous intervention or spontaneous rupture can cause a recurrence,5 and anaphylaxis is a potential complication of cyst rupture.1 For this reason, giving oral albendazole (Albenza) is recommended before any intervention. Sterilization of the cyst contents with a protoscolicidal agent (20% NaCl) before evacuation of cyst contents is also standard practice.
The rate of cyst recurrence is 16.2% with open surgery and 3.5% with percutaneous intervention.6 A higher incidence of recurrence in patients who undergo surgical cystectomy likely reflects the more complicated and active nature of the cysts in patients who undergo surgery.6
Albendazole is the drug of choice for hepatic hydatid disease.3 The optimal duration of treatment is unclear but should be guided by a combination of clinical response, medication side effects, serologic titers, and imaging. The most common adverse effects of albendazole are hepatotoxicity, abdominal pain, and nausea.
OUR PATIENT’S DIAGNOSIS AND TREATMENT
In our patient, ultrasonography confirmed the diagnosis of cystic echinococcosis by the finding of active anechoic cysts with echogenic internal debris and with a well-delineated cyst wall. The WHO-IWGE classification was CE1, ie, active anechoic cysts with internal echogenic debris.
Our patient underwent surgical rather than percutaneous cystectomy because of concern about possible cyst leakage, since she had presented with the acute onset of pain and eosinophilia. We were also concerned about the subdiaphragmatic location of the larger cyst, which could have been difficult to reach percutaneously.
Open total pericystectomy involved opening the cyst cavity, sterilizing the contents with 20% NaCl, evacuating the cyst contents, and removing the cyst tissue. Two large cysts were excised and sent for histologic examination, which confirmed E granulosus. Percutaneous aspiration was necessary 4 months later because of a recurrence, and albendazole 400 mg twice daily was continued for another 5 months. Ultrasonography 3 years later showed no evidence of echinococcal cysts, and her antibody titers remain undetectable.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
- McManus DP, Gray DJ, Zhang W, Yang Y. Diagnosis, treatment, and management of echinococcosis. BMJ 2012; 344:e3866.
- McManus DP, Zhang W, Li J, Bartley PB. Echinococcosis. Lancet 2003; 362:1295–1304.
- Brunetti E, Kern P, Vuitton DA; Writing Panel for the WHO-IWGE. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop 2010; 114:1–16.
- Khuroo MS, Wani NA, Javid G, et al. Percutaneous drainage compared with surgery for hepatic hydatid cysts. N Engl J Med 1997; 337:881–887.
- Kayaalp C, Sengul N, Akoglu M. Importance of cyst content in hydatid liver surgery. Arch Surg 2002; 137:159–163.
- Yagci G, Ustunsoz B, Kaymakcioglu N, et al. Results of surgical, laparoscopic, and percutaneous treatment for hydatid disease of the liver: 10 years experience with 355 patients. World J Surg 2005; 29:1670–1679.
Syncope during a pharmacologic nuclear stress test
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
A 60-year-old woman was referred for pharmacologic nuclear stress testing before treatment for breast cancer. She had hypertension, diabetes mellitus, coronary artery disease, and a remote history of stroke, and she was taking clonidine (Catapres), labetalol (Normodyne, Trandate), furosemide (Lasix), hydralazine, valsartan (Diovan), insulin, and the aspirin-dipyridamole combination Aggrenox. Her vital signs and electrocardiogram before the stress test were normal.
The stress test was started with a standard protocol of adenosine (Adenoscan) infused intravenously over 4 minutes. For the first 2 minutes, she was stable and had no symptoms, but then sinus pauses and second-degree atrioventricular block type 2 developed, after which her heart stopped beating (Figure 1). The infusion was immediately stopped, but she became unresponsive and remained pulseless.
Cardiopulmonary resuscitation was started, aminophylline 100 mg was given intravenously, and she regained a pulse and blood pressure within a few minutes. She was then transferred to the emergency room, where she returned to her baseline clinical and neurologic status without symptoms.
AN UNRECOGNIZED DRUG INTERACTION
Asystole occurred in this patient because of the interaction of intravenous adenosine with the dipyridamole in the medication Aggrenox. Although adenosine, given during pharmacologic stress testing, is known to interact with various medications, the potential for this interaction may be overlooked if the culprit is present in a combination drug. Aggrenox is commonly given for secondary stroke prevention and should be discontinued before pharmacologic nuclear stress testing.
Pharmacologic stress testing involves two commonly used stress agents, adenosine and regadenoson (Lexiscan), which cause coronary vasodilation through their action on A2A receptors in the heart. Coronary vasodilation results in flow heterogeneity in the region of a stenotic artery, which can be detected with nuclear perfusion agents. In addition, adenosine has a short-lived effect on the A1 receptors that block atrioventricular conduction.1
Dipyridamole (Persantine) is contraindicated when either adenosine or regadenoson is used. Dipyridamole enhances the effect of exogenous and endogenous adenosine by inhibiting its uptake by cardiac cells, thus enhancing the action of these coronary vasodilators.2 Atrioventricular block is common during adenosine stress testing but is transient because adenosine has a short half-life (< 10 seconds), and complete heart block or asystole, as seen in this patient, is rare. Giving intravenous adenosine or regadenoson to patients on dipyridamole may have a marked effect on adenosine receptors, so that profound bradycardia and even asystole leading to cardiac collapse may occur. No data are available on the specific interaction of dipyridamole and regadenoson.
Even though the pharmacodynamics of the interaction between dipyridamole and adenosine are known,3 few reports are available detailing serious adverse events. The contraindication to pharmacologic stress testing in patients taking dipyridamole is noted in the American Society of Nuclear Cardiology Guidelines for stress protocols,4 which advise discontinuing dipyridamole-containing drugs at least 48 hours before the use of adenosine or regadenoson. Similarly, the American Heart Association guidelines5 for the management of supraventricular tachycardia recommend an initial dose of 3 mg of adenosine rather than 6 mg in patients who have been taking dipyridamole.
The dose of aminophylline for reversing the adverse effects of adenosine or regadenoson is 50 to 250 mg intravenously over 30 to 60 seconds. But since these adverse effects are short-lived once the infusion is stopped, aminophylline is usually given only if the adverse effects are severe, as in this patient.
Pharmacologic nuclear stress testing with adenosine receptor agonists (eg, adenosine or regadenoson) is contraindicated in patients taking dipyridamole or the combination pill Aggrenox because of the potential for profound bradyarrhythmias or asystole.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
- Zoghbi GJ, Iskandrian AE. Selective adenosine agonists and myocardial perfusion imaging. J Nucl Cardiol 2012; 19:126–141.
- Lerman BB, Wesley RC, Belardinelli L. Electrophysiologic effects of dipyridamole on atrioventricular nodal conduction and supraventricular tachycardia. Role of endogenous adenosine. Circulation 1989; 80:1536–1543.
- Biaggioni I, Onrot J, Hollister AS, Robertson D. Cardiovascular effects of adenosine infusion in man and their modulation by dipyridamole. Life Sci 1986; 39:2229–2236.
- Henzlova MJ, Cerqueira MD, Mahmarian JJ, Yao SS; Quality Assurance Committee of the American Society of Nuclear Cardiology. Stress protocols and tracers. J Nucl Cardiol 2006; 13:e80–e90.
- ECC Committee, Subcommittees and Task Forces of the American Heart Association. 2005 American Heart Association guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Part 7.3: management of symptomatic bradycardia and tachycardia. Circulation 2005; 112(suppl 24):IV67–IV77.
Niacin's effect on cardiovascular risk: Have we finally learned our lesson?
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
Randomized controlled trials have unequivocally shown that lowering levels of low-density lipoprotein cholesterol (LDL-C) with statins reduces the rate of cardiovascular events.1–3 Yet many patients still have heart attacks even though they are on statins, so the search continues for other agents to lower cardiovascular risk.4
Niacin has been used for its lipid-modifying effects for more than 50 years. In addition to being the most potent agent for raising the level of high-density lipoprotein cholesterol (HDL-C), niacin decreases the atherogenic lipids triglyceride, LDL-C, and lipoprotein (a)5 and can be very effective in treating mixed dyslipidemias such as hypertriglyceridemia and low HDL-C. This is particularly important for the challenging patients seen in preventive cardiology clinics.
In 1986, before statins were available, the Coronary Drug Project6 showed that immediate-release forms of niacin lowered the rates of nonfatal myocardial infarction and long-term mortality. Later, imaging studies demonstrated that niacin slows progression of carotid intima-medial thickness and coronary atherosclerosis.7–9 Furthermore, meta-analyses of these studies suggest cardiovascular benefit for patients at high vascular risk.10
However, niacin is difficult to use in clinical practice. The near-ubiquitous experience of flushing has limited our ability to give doses high enough to modify plasma lipid levels and rates of clinical events.
To try to mitigate this side effect, investigators developed extended-release formulations and agents such as laropiprant, a chemical antagonist of the interaction between niacin and epidermal prostanoid receptors implicated as the mechanism behind flushing. Although these innovations do not eliminate flushing, they reduce it, and thus have prompted hopes of using niacin more widely in statin-treated patients. However, whether widespread use of niacin on a background of statin therapy would have an impact on cardiovascular events remained to be established.
WHAT WE HAVE LEARNED LATELY ABOUT NIACIN?
More-tolerable formulations of niacin prompted interest in its potential to lower the residual cardiovascular risk observed in statin-treated patients. Two large clinical trials attempted to determine its impact on cardiovascular events in the contemporary era.
The AIM-HIGH study
In the Atherothrombosis Intervention in Metabolic Syndrome With Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) study,11 3,414 patients at high vascular risk with low HDL-C were treated with niacin or placebo. The trial was stopped early because of no evidence of clinical benefit with niacin and because of concern about an increased risk of stroke, a finding ultimately not observed on a complete review of the data.
I reviewed the limitations of this study earlier in this journal.12 The study was small, use of low-dose niacin was allowed in the placebo group, and physicians could treat high LDL-C as they saw fit during the study, so that more patients in the placebo group received high-dose statin therapy and ezetimibe. All of this likely limited the study’s ability to measure the clinical impact of niacin. As a result, this study was not a pure evaluation of the benefits of niacin vs placebo in addition to standard medical therapy. Hope remained that a much larger study with greater statistical power and a simpler design would provide a definitive answer.
HPS2–THRIVE
The Heart Protection Study 2–Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE), with more than 40,000 patients, was the largest cardiovascular outcomes trial of lipid-modifying therapy to date.13 Its purpose was to determine whether extended-release niacin plus the prostanoid receptor antagonist laropiprant would reduce the rate of cardiovascular events in patients with clinically established vascular disease.
Patients age 50 to 80 with a history of myocardial infarction, ischemic stroke, transient ischemic attack, peripheral arterial disease, or diabetes with other forms of coronary heart disease received a standardized LDL-C-lowering regimen with simvastatin 40 mg daily, with or without ezetimibe 10 mg daily, to achieve a total cholesterol target of 135 mg/dL or below. All were treated with extended-release niacin 2 g daily plus laropiprant 40 mg daily for 1 month to assess compliance. They were then randomized to treatment with extended-release niacin 2 g plus laropiprant 40 mg or placebo daily. At baseline, the mean lipid values were LDL-C 63 mg/dL, HDL-C 44 mg/dL, and triglyceride 125 mg/dL.
Before the end of the trial, the investigators reported a high rate of myopathy-related adverse events in the niacin group, particularly in Chinese patients.13 This contributed to a high dropout rate in the niacin group, in which one quarter of patients stopped taking the study drug.
During the study, niacin lowered the LDL-C level by a mean of 10 mg/dL, lowered triglycerides by 33 mg/dL, and raised HDL-C by 6 mg/dL. On the basis of previous observational studies and randomized clinical trials, the authors calculated that such lipid changes should translate to a 10% to 15% reduction in vascular events. However, no reduction was observed in the primary end point of major vascular events, which included nonfatal myocardial infarction, coronary death, any nonfatal or fatal stroke, and any arterial revascularization, including amputation. The rates were 15% in the placebo group vs 14.5% in the niacin group (P = .96).
A statistically significant 10% reduction in the rate of arterial revascularization was observed in the niacin group, perhaps consistent with earlier observations of an antiatherosclerotic effect.
Subgroup analyses, while always to be interpreted with caution, also provide some interesting findings for consideration. A significant interaction was observed between treatment and baseline LDL-C, with those in the highest LDL-C tertile (> 77 mg/dL) demonstrating a potential reduction in the primary end point with niacin treatment. In addition, a trend toward potential benefit with niacin in patients in Europe, but not in China, was also observed; however, this just failed to meet statistical significance.
HPS2-THRIVE provided important information about the safety of extended-release niacin in combination with laropiprant. The niacin group experienced higher rates not only of myopathy but also of diabetic complications, new diagnosis of diabetes, serious infections, and bleeding. Whether these observations were related to niacin or to laropiprant is unknown. In fact, recent reports suggest laropiprant has adverse effects that may have substantially reduced the potential benefits of niacin.
The overall conclusion of HPS2-THRIVE was that there was no widespread clinical benefit from the combination of niacin and laropiprant in statin-treated patients with vascular disease, and that there was a potential increase in adverse events. Accordingly, the combination treatment will not be integrated into clinical practice.
WHERE DO WE GO FROM HERE?
Despite their limitations, these two large trials suggest that niacin does not reduce cardiovascular risk in patients already receiving a statin.
Might some subgroups be more likely to benefit from niacin? The finding of potential benefit in patients with higher baseline LDL-C suggests this may be true. At baseline, the HPS2-THRIVE patients had very good LDL-C control and had HDL-C levels within the normal range, not necessarily reflecting the patients we see in daily practice, who require more effective reductions in vascular risk. Furthermore, failure of both fibrates and niacin to reduce risk may have reflected the attempt to study these agents in broad patient populations as opposed to focusing on specific cohorts, such as patients with mixed dyslipidemia, for which there is suggestion of benefit.14 It seems unlikely that such a study will be performed in a clinical setting in which niacin may be of greater utility. The experience of adverse events would appear to make that a certainty.
For now, niacin will remain useful in lipid clinics for managing refractory dyslipidemia. Specifically, its ability to lower triglyceride and lipoprotein (a) and to raise HDL-C will continue to be of interest in the clinical management of patients and in the formulation of treatment guidelines. Another reason to use it is to lower LDL-C in patients who cannot tolerate statins. However, there is currently no evidence from randomized controlled trials to support its broader use.
While registry information could provide some sense of real-world effects of niacin’s use, this is a suboptimal way to evaluate the potential efficacy of a therapy—randomized controlled trials are the gold standard. The major flaws of both of the large trials of niacin point out the need for thoughtful study design to avoid incorrectly dismissing potentially useful therapies. But for now, the renaissance of niacin as a means of lowering cardiovascular risk is only wishful thinking.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
- Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet 1994; 344:1383–1389.
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002; 360:7–22.
- Ridker PM, Danielson E, Fonseca FA, et al; JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med 2008; 359:2195–2207.
- Libby P. The forgotten majority: unfinished business in cardiovascular risk reduction. J Am Coll Cardiol 2005; 46:1225–1228.
- deLemos AS, Wolfe ML, Long CJ, Sivapackianathan R, Rader DJ. Identification of genetic variants in endothelial lipase in persons with elevated high-density lipoprotein cholesterol. Circulation 2002; 106:1321–1326.
- Canner PL, Berge KG, Wenger NK, et al. Fifteen year mortality in Coronary Drug Project patients: long-term benefit with niacin. J Am Coll Cardiol 1986; 8:1245–1255.
- Taylor AJ, Sullenberger LE, Lee HJ, Lee JK, Grace KA. Arterial Biology for the Investigation of the Treatment Effects of Reducing Cholesterol (ARBITER) 2: a double-blind, placebo-controlled study of extended-release niacin on atherosclerosis progression in secondary prevention patients treated with statins. Circulation 2004; 110:3512–3517.
- Taylor AJ, Lee HJ, Sullenberger LE. The effect of 24 months of combination statin and extended-release niacin on carotid intima-media thickness: ARBITER 3. Curr Med Res Opin 2006; 22:2243–2250.
- Brown BG, Zhao XQ, Chait A, et al. Simvastatin and niacin, antioxidant vitamins, or the combination for the prevention of coronary disease. N Engl J Med 2001; 345:1583–1592.
- Lavigne PM, Karas RH. The current state of niacin in cardiovascular disease prevention: a systematic review and meta-regression. J Am Coll Cardiol 2013; 61:440–446.
- AIM-HIGH Investigators; Boden WE, Probstfield JL, Anderson T, et al. Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med 2011; 365:2255–2267.
- Nicholls SJ. Is niacin ineffective? Or did AIM-HIGH miss its target? Cleve Clin J Med 2012; 79:38–43.
- HPS2-THRIVE Collaborative Group. HPS2-THRIVE randomized placebo-controlled trial in 25 673 high-risk patients of ER niacin/laropiprant: trial design, pre-specified muscle and liver outcomes, and reasons for stopping study treatment. Eur Heart J 2013; 34:1279–1291.
- Jun M, Foote C, Lv J, et al. Effects of fibrates on cardiovascular outcomes: a systematic review and meta-analysis. Lancet 2010; 375:1875–1884.
The generalist, the specialist, and the patient with chronic kidney disease
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
A key part of medical practice is managing professional relationships. This includes effective communication with each other: primary care provider, specialist, and patient in all permutations. I have previously written about how technologic advances both facilitate and hamper interphysician communication. But as payment models morph, as health systems become more complex and insulated, and as the medicine subspecialty workforce changes, the relationship between generalist and nonprocedural specialist will continue to evolve. I can offer personal testimony to the enormous value of sharing our electronic medical record with my nephrology colleagues within the institution; online (nondisruptive) management “conversation” is common in real time while I am with a patient in the office.
Gone is the time when referral was a necessary mechanism to build a practice, when a primary care physician would send everyone with an elevated alkaline phosphatase to the neighboring gastroenterologist, who in turn would send everyone without a primary care doctor to him or her. But there has always been the potential for professional, ego-based tension between primary care and nonprocedural specialist physicians, although this tension is rarely discussed. When does referral to a specialist by a general internist imply a lack of appropriate knowledge or an unwillingness to do an appropriate literature review? When should a specialist be concerned about “interfering” in primary care—by initiating more aggressive blood pressure control, or by giving the patient a needed vaccination? And what should be done if the patient decides to change the captain of the medical team? Maybe in the new medical care arena we will indeed function and be judged as a team, physician communication and transitions will be seamless, and all that matters will be the patient. Time will tell.
For now, the comanagement of patients with a chronic disease is often a challenge. The discussion by Sakhuja et al of patients with chronic kidney disease (CKD) highlights important clinical issues faced by primary care providers and nephrologists. With the increased diagnosis of early CKD, there may not be enough consulting nephrologists to see all these patients. And when CKD is diagnosed at an early stage, not all patients may warrant a specialist consultation. Yet the gaps in clinical care are clear. Too many patients with “a little” proteinuria or microhematuria do not get an adequate microscopic urinalysis to look for a treatable inflammatory renal disorder. Too many patients with a “slightly” elevated creatinine and blood pressure do not have their pressure aggressively treated, despite evidence that a systolic blood pressure in the high 130s is associated with more rapid progression of CKD. Should we establish expectations for ourselves, or should we just take a step back and refer all these patients to a nephrologist and await guidance? This is where I believe that a few clearly written and widely disseminated guidelines would help. Knowledge of appropriate and basic guidelines for diagnosing and managing common disorders (not just CKD) should be the focus of continuing medical education and should be required for maintaining certification for all internists, including specialists. But, as always, guidelines often need to be tailored for the patient in our examining room.
There are nuances in the care of patients with CKD that, as a nonspecialist, I will not automatically know need to be implemented. As an internist, I should know the value of starting inhibition of the angiotensin pathway in patients with proteinuria, but as CKD progresses in a specific patient, should this be decreased? Should I initiate urate-lowering therapy,1 hoping to slow the rate of my patient’s renal demise?
When do we know enough to know that we do not need to ask for a specialist’s input? How well do we self-assess our clinical knowledge and skills? How can we achieve the right balance between referral and self-management? We try to save our patient the cost of the time and the copayment to see a specialist, and with bundled care we try to minimize consultant fees and time. But in the meantime, are we ordering unnecessary tests or delaying appropriate therapy?
As we think about the comanagement of patients with CKD, we need to recognize and utilize the nuanced improvements in care that our nephrology colleagues can provide. As non-nephrologists, we should be able to start a thoughtful diagnostic evaluation. For example, an antinuclear antibody test in the absence of evidence of glomerulonephritis is not likely to be informative in determining the cause of an isolated elevated creatinine; a urinalysis is. We should be able to recognize potential renal injury (proteinuria, decreased glomerular filtration rate, microhematuria, hypertension), and initiate aggressive mitigation of factors that are known to enhance progression of the CKD (proteinuria, hypertension) and contribute to the significant morbidity and mortality of CKD-associated cardiovascular disease.
We should already be managing hypertension, diabetes, and hyperlipidemia, but CKD should be a red flag, driving us to more aggressively control these comorbidities, and driving us to do better than control only the estimated 46.4% of hypertensive patients in 2009 and 2010 whose hypertension was adequately controlled.2 There is no reason for us to step back and wait for direction in addressing these most common issues. And our specialist colleagues will be there to efficiently assist in refining the nuances of care.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.
- Levy GD, Rashid N, Niu F, Cheetham TC. Effect of urate-lowering therapies on renal disease progression in patients with hyperuricemia. J Rheumatol 2014; Apr 1, doi: 10.3899/jrheum.131159. Epub ahead of print.
- Guo F, He D, Zhang W, Walton RG. Trends in prevalence, awareness, management, and control of hypertension among United States adults, 1999 to 2010. J Am Coll Cardiol 2012; 60:599–606.