User login
Woman, 78, With Dyspnea, Dry Cough, and Fatigue
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
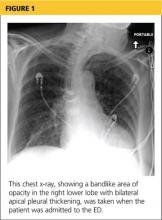
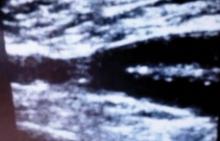
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
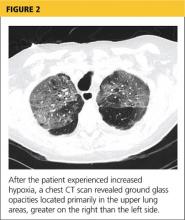
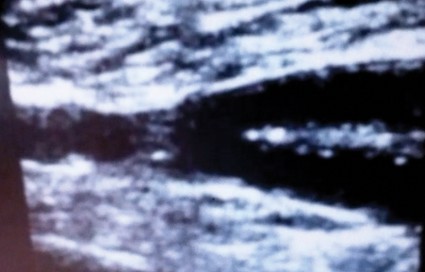
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
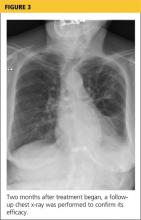
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
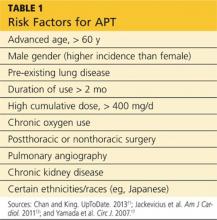
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
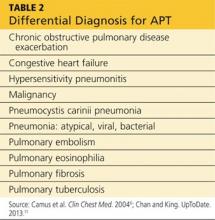
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
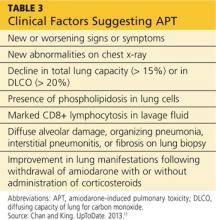
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
A 78-year-old woman presented to the emergency department (ED) complaining of shortness of breath, a dry nonproductive cough, fatigue, hypoxia, and general malaise lasting for several months and worsening over a two-week period. She denied having fever, chills, hemoptysis, weight loss, headache, rashes, or joint pain. She reported sweats, decrease in appetite, wheezing, cough without sputum production, and slight swelling of the legs. The patient complained of chest pain upon admission, but it resolved quickly.
The patient, a retired widow with five grown children, denied recent surgery or exposure to sick people, had not travelled, and reported no changes in her home environment. She claimed to have no pets but admitted to currently smoking about four cigarettes a day; she had previously smoked, on average, three packs of cigarettes per day for 60 years. She denied using alcohol or drugs, including intravenous agents.
The patient’s medical history was significant for paroxysmal atrial fibrillation. She had also been diagnosed with chronic obstructive pulmonary disease (COPD), transient ischemic attack, patent foramen ovale, hyperlipidemia, seizure disorder, and hypothyroidism. She had no known HIV risk factors and had had no exposure to asbestos or tuberculosis.
The patient’s current medications included amiodarone (200 mg/d) for four years; valproic acid (500 mg/d); aspirin (325 mg/d); levothyroxine (50 g/d); rosuvastatin (10 mg/d); daily warfarin, dosed according to the international normalized ratio (INR); and budesonide/formoterol (160/4.5 mg, one puff bid). She denied having any drug allergies.
Physical examination in the ED revealed a pulse of 63 beats/min; blood pressure, 108/50 mm Hg; and respiratory rate, 16 to 20 breaths/min. The patient’s O2 saturation was 84% on room air; 82% to 84% on 4 L to 6 L of supplemental oxygen; 87% to 92% with a venturi mask; and 95% on biphasic positive airway pressure (BiPAP) device. She was afebrile with hypoxia and able to speak in full sentences. Crackles were detected in the upper lung fields, best heard anteriorly, as well as a few scattered wheezes and rhonchi. Her heart sounds were normal with a regular rhythm; her extremities exhibited trace edema bilaterally. The remainder of the physical exam was normal.
The patient’s laboratory values included a normal white blood cell (WBC) count, elevated lactic acid dehydrogenase (LDH) at 448 IU/L (reference range, 84 to 246 IU/L), and no eosinophils. The erythrocyte sedimentation rate (ESR) was not measured on admission. Blood analysis of her N-terminal pro-brain natriuretic peptide (NT-proBNP) was 4,877 pg/mL; for women older than 75, a level higher than 1,800 pg/mL is abnormal.
A chest x-ray was performed on admission, showing hyperinflation of the lungs with mild coarsening of the lung markings. A bandlike area of opacity in the right lower lobe with bilateral apical pleural thickening was noted (see Figure 1). Noncontrast CT of the chest revealed diffuse upper lobe ground glass opacities in both lungs, extending into the right middle lobe and lingula as well the superior segments of the lower lobes, with areas of emphysema and septal thickening. Numerous nodules, some of which appeared cavitary, were apparent in the lower lobes.
A two-dimensional echocardiogram demonstrated normal left ventricular size and systolic function, mild tricuspid regurgitation without evidence of pulmonary hypertension, and mild left atrial enlargement.
The patient was admitted to the cardiac unit for evaluation. While there, she received one dose of methylprednisolone (125 mg IV), three doses of ipratropium bromide/albuterol, one dose of ceftriaxone (1 g IV), and one dose of azithromycin (500 mg po). In the absence of significant leg edema and an elevation of jugular venous distention with a normal two-dimensional echocardiogram, heart failure was ruled out. The chest pains reported on initial presentation were ultimately felt to be noncardiac in nature.
After the patient was transferred to the medical floor with an initial diagnosis of exacerbation of her COPD, she was treated with antibiotics, nebulizers, and corticosteroids. She continued to experience episodes of O2 desaturation while on 4 L to 6 L of oxygen via nasal cannula and on a venturi mask. She was then placed on a BiPAP device, set to 12/5, and 50% Fio2 (fraction of inspired oxygen), which improved her oxygenation.
Her hypoxia prompted further radiographic studies. The resulting chest CT scan showed ground glass opacities located primarily in the upper lung areas, greater on the right than on the left side (see Figure 2). The radiologist suggested that the hypoxia was caused by an infection, but because the patient’s presenting symptoms were chronic in nature, drug-induced causes were considered as well. Amiodarone was discontinued.
Cardiology was consulted and agreed that stopping amiodarone was acceptable since the patient was in sinus rhythm at the time. The patient continued to take antibiotics and prednisone. Her symptoms slowly improved during hospitalization, and she required less oxygen. Based on the patient’s presentation, physical exam findings, imaging studies, and laboratory findings, amiodarone-induced pulmonary toxicity (APT) was diagnosed.
She was discharged home on supplemental oxygen at 4 L via cannula, a tapering dosage of prednisone, and metered-dose inhalers for fluticasone/salmeterol and tiotropium bromide. She also had outpatient appointments scheduled, one with the pulmonologist to follow up on her imaging studies and to manage the prednisone taper and the other with the cardiologist to manage her atrial fibrillation.
At pulmonology two months later, she had a chest x-ray (see Figure 3) and pulmonary function tests (PFTs). The patient reported feeling progressively better in the past month. Her dyspnea on exertion had improved, and she did not require supplemental oxygen anymore. She stopped smoking cigarettes.
The patient continued to use fluticasone/salmeterol but stopped tiotropium bromide. On physical exam, her O2 saturation was 95% on room air, heart rhythm and rate were regular, and her lungs revealed very minimal crackles at the right base but were otherwise clear.
The plan specified continuing the prednisone taper. The patient was asked to call the office if she had any worsening shortness of breath, cough, and sputum production. She was also encouraged to continue refraining from smoking cigarettes. This patient had done very well, with near complete resolution of symptoms and a clear chest x-ray.
Continue reading for discussion...
DISCUSSION
Amiodarone, a highly effective antiarrhythmic drug, is FDA approved for suppressing ventricular fibrillation and ventricular tachycardia. It is also used off-label as a second- or third-line choice for atrial fibrillation.1
Standard of care requires that, prior to starting amiodarone therapy, patients have a baseline chest x-ray and PFTs with diffusing capacity performed. Thereafter, the patient should be monitored with annual chest x-rays, with one performed promptly if new symptoms develop. Serial PFTs have not offered any benefit for monitoring, but a decrease of more than 15% in total lung capacity or more than 20% in diffusing capacity from baseline is consistent with APT.2
Adverse effects, both cardiac and noncardiac, are common with amiodarone therapy. They include proarrhythmias, bradycardia, and heart block, as well as thyroid and liver dysfunctions; dermatologic conditions such as blue-gray discoloration of the skin and photosensitivity; neurologic effects such as ataxia, paresthesias, and tremor; ocular problems, including corneal microdeposits; gastrointestinal problems such as nausea, anorexia, and constipation; and lung problems such as pulmonary toxicity, pleural effusion, and pleural thickening.3-6 Of these, pulmonary toxicity is the most severe and life threatening.7
APT, also known as amiodarone pneumonitis and amiodarone lung, typically manifests from a few months to a year and a half after treatment is commenced.6 APT can occur even after the drug is discontinued, because amiodarone has a very long elimination half-life of approximately 15 to 45 days and a tendency to concentrate in organs with high blood perfusion and in adipose tissues.8 Patients taking 400 mg/d for two months or longer or 200 mg/d for more than two years are considered at higher risk for APT.9 The severity of disease appears to correlate with the cumulative dose and length of treatment.10
Numerous risk factors for pulmonary toxicity have been reported, including high drug dosage, pre-existing lung disease, patient age, and prior surgery (see Table 1).11 According to an analysis of a database of 237 patients, only age and duration of amiodarone therapy were significant risk factors for APT.9 Its incidence is not precisely known; reported rates range from 1% to 17%.6,12,13
Presentation with such nonspecific symptoms as shortness of breath, nonproductive cough, fatigue, hypoxia, and general malaise is typical for many pulmonary and cardiac illnesses (see Table 2), making APT difficult to diagnose.14 Occasionally, rapid onset with progression to pneumonitis and respiratory failure masquerades as acute respiratory distress syndrome (ARDS).15
Notable, however, is that APT can manifest with nonproductive cough and dyspnea in 50% to 75% of cases. In addition, presenting symptoms will include fever (33% to 50% of cases) with associated malaise, fatigue, chest pain, and weight loss. In patients with APT, the physical exam usually reveals bilateral crackles on inspiration, but diffuse rales may be heard as well.11
Laboratory studies are not very helpful in diagnosing APT. Patients may present with nonspecific elevated WBCs without eosinophilia and an elevated LDH level.11 An elevated ESR may be detected before symptoms of APT manifest and can be present at the time of diagnosis.6
Imaging studies are far more helpful and specific in diagnosing APT. The typical chest x-ray shows bilateral patchy diffuse infiltrates.12 CT of the chest is usually more revealing, demonstrating ground glass opacities in the periphery and subpleural thickening, especially where infiltrates are denser. This thickening may result in pleuritic chest pain.6
The right upper lobe is more often affected in these cases than the left lung.6 Numerous pulmonary nodules in the upper lobes are found rarely and can be confused with lung cancer. These nodules are likely the result of an accumulation of the drug in areas of previous inflammation; a lung mass should prompt the addition of APT in the differential.2,16
APT is a diagnosis of exclusion, requiring clinical suspicion, drug history, imaging, and consideration of the differential. The presence of three or more clinical factors supports a diagnosis of APT (see Table 3).11
Once APT is recognized, the first action is to have the patient stop taking amiodarone, followed by the administration of corticosteroids (eg, prednisone 40 to 60 mg/d11) for four to 12 months.17 Patients, especially those with underlying lung disease, will typically require temporary oxygen supplementation until hypoxia resolves. Even after the drug has been discontinued, some patients experience worsening symptoms before they see improvement simply because the drug can persist in lung tissue for up to a year following cessation of therapy.6
If APT is diagnosed early, the prognosis is favorable. In one study, a significant number of APT patients stabilized or improved after withdrawal of the drug, regardless of concurrent treatment with corticosteroids.18 Follow-up studies, both imaging and PFT, indicate complete clearing of lung opacities in the majority of patients treated for APT.19 Radiologic improvement may be seen six months after cessation of amiodarone.20 Patients who develop ARDS tend to do poorly and have a mortality rate of approximately 50%.11
Continue reading for the conclusion...
CONCLUSION
Among patients who are taking long-term or high-dose amiodarone, particularly those older than 60, new-onset nonproductive cough and dyspnea signal the need for pulmonary and cardiac work-up. Once the diagnosis of APT is made, treatment is straightforward: Withdraw the amiodarone, and initiate corticosteroid therapy.
REFERENCES
1. Fuster V, Rydén LE, Asinger RW, et al; American College of Cardiology/American Heart Association Task Force on Practice Guidelines; European Society of Cardiology Committee for Practice Guidelines and Policy Conferences (Committee to Develop Guidelines for the Management of Patients With Atrial Fibrillation); North American Society of Pacing and Electrophysiology. ACC/AHA/ESC guidelines for the management of patients with atrial fibrillation: executive summary. Circulation. 2001; 104(17):2118-2150.
2. Jarand J, Lee A, Leigh R. Amiodaronoma: an unusual form of amiodarone-induced pulmonary toxicity. CMAJ. 2007;176(10):1411-1413.
3. Connolly S. Evidence-based analysis of amiodarone efficacy and safety. Circulation. 1999;100:2025-2034.
4. Amiodarone Trials Meta-Analysis Investigators. Effect of prophylactic amiodarone on mortality after acute myocardial infarction and in congestive heart failure: meta-analysis of individual data from 6500 patients in randomised trials. Lancet. 1997;350(9089):1417-1424.
5. Pollak PT. Clinical organ toxicity of antiarrhythmic compounds: ocular and pulmonary manifestations. Am J Cardiol. 1999;84(9A):37R-45R.
6. Camus P, Martin W, Rosenow E. Amiodarone pulmonary toxicity. Clin Chest Med. 2004;25(1):65-75.
7. Rady MY, Ryan T, Starr NJ. Preoperative therapy with amiodarone and the incidence of acute organ dysfunction after cardiac surgery. Anesth Analg. 1997;85(3):489-497.
8. Canada A, Lesko L, Haffajee C, et al. Amiodarone for tachyarrhythmias: kinetics, and efficacy. Drug Intell Clin Pharm. 1983;17(2):100-104.
9. Ernawati DK, Stafford L, Hughes JD. Amiodarone-induced pulmonary toxicity. Br J Clin Pharmacol. 2008;66(1):82-87.
10. Liu FL, Cohen RD, Downar E, et al. Amiodarone pulmonary toxicity: functional and ultrastructural evaluation. Thorax. 1986;41(2):100-105.
11. Chan E, King TE. Amiodarone pulmonary toxicity. UpToDate. 2013. www.uptodate.com/contents/amiodarone-pulmonary-toxicity. Accessed January 17, 2014.
12. Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43-48.
13. Jackevicius CA, Tom A, Essebag V, et al. Population-level incidence and risk factors for pulmonary toxicity associated with amiodarone. Am J Cardiol. 2011;108:705-710.
14. Jessurun G, Crijns H. Amiodarone pulmonary toxicity [editorial]. BMJ. 1997;314(7081):619-620.
15. Nacca N, Castigliano B, Yuhico L, et al. Severe amiodarone induced pulmonary toxicity. J Thorac Dis. 2012;4(6):667-670.
16. Arnon R, Raz I, Chajek-Shaul T, et al. Amiodarone pulmonary toxicity presenting as a solitary lung mass. Chest. 1988;93(2):425-427.
17. Yamada Y, Shiga T, Matsuda N, et al. Incidence and predictors of pulmonary toxicity in Japanese patients receiving low-dose amiodarone. Circ J. 2007;71(10):1610-1616.
18. Coudert B, Bailly F, Lombard JN, et al. Amiodarone pneumonitis: bronchoalveolar lavage findings in 15 patients and review of the literature. Chest. 1992;102(4):1005-1012.
19. Vernhet H, Bousquet C, Durand G, et al. Reversible amiodarone-induced lung disease: HRCT findings. Eur Radiol. 2001;11(9):1697-1703.
20. Olson LK, Forrest JV, Friedman PJ, et al. Pneumonitis after amiodarone therapy. Radiology. 1984;150(2):327-330.
Small AAA: To treat or not to treat
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Once again our authorities choose to agree rather than disagree. Although there may be some minor differences in opinion, it appears that both suggest that careful observation is the preferred management for small abdominal aortic aneurysms. However, there may still be some controversy since I believe the data they use to support observation is confounded by including patients whose aneurysms were less than 5 cm. I think almost everyone would agree that it is safe to monitor the <5 cm AAA. But what about the 5.2 cm in a small woman or a patient with chronic obstructive pulmonary disease or a strong family history of rupture or, for that matter, in any patient? If you have an opinion one way or another I invite you to send your comments to info@vascularspecialistonline.com for inclusion in a future edition of Vascular Specialist. In the meantime if you go to www.Vascularspecialistonline.com you can respond to our "Online Question of the Month" about the treatment of these small AAA.
--Dr. Russell Samson is the medical editor of Vascular Specialist.
Procedural risks remain an issue.
By Kenneth Ouriel, M.D.
Abdominal aortic aneurysms (AAA) are treated to prevent death from aneurysm rupture. Depending on the patient’s baseline medical status, however, the risks of the procedure itself may outweigh the risks of leaving the aneurysm untreated.1 Endovascular aneurysm repair (EVAR), originally developed as a less invasive alternative to traditional open surgery,2 has incompletely addressed this issue. While prospective randomized clinical trials demonstrated reduction in early morbidity and mortality with EVAR, early benefits have not translated into long-term survival benefit over open surgical repair.3,4
Several studies have demonstrated improved results with EVAR when performed in patients with smaller aneurysms.5,6 This observation may relate to the frequency of more challenging anatomy in larger aneurysms, with a higher frequency of shorter, larger diameter, angulated, and conical proximal aortic necks. While the potential benefit of repair in patients with larger aneurysms is greatest with respect to the prevention of rupture, some of this benefit may be offset by poorer long-term outcome. These results are only in part a function of complications related to the device, such as proximal endoleaks and migration. In addition, patients with larger aneurysms are slightly older and sicker than those with smaller aneurysms, accounting for an increased frequency of non-aneurysm related events.
Noting the less challenging aortic anatomy and younger, healthier characteristics of the subpopulation with smaller AAA, two randomized studies were organized to compare the results of early endovascular repair versus ultrasound or computed tomographic (CT) surveillance.7 The PIVOTAL trial enrolled 728 subjects with AAA between 4 and 5 cm in diameter, randomizing to either early EVAR with the AneuRx or Talent endografts or to ultrasound/CT imaging studies every 6 months. The perioperative mortality rate was 0.6% in the early EVAR group. Over a mean follow-up period of 20 months, there were no differences in all-cause mortality between the two groups, each with 15 deaths (4.1%). The primary endpoint of rupture or aneurysm-related death was similar in the two groups, with a hazard ratio of 0.99 in the early EVAR group. However, at 36 months almost 50% of the surveillance group underwent aneurysm repair for sac enlargement, the development of aneurysm-related symptoms, or patient choice. Interestingly, a follow-up economic substudy documented similar health care costs in the two treatment groups at 48 months of follow-up, even though 36.3% of the surveyed patients did not undergo repair.
A second study, the CEASAR trial, randomized 360 patients with AAA 4.1-5.4 cm in diameter to early EVAR with the Cook Zenith device or to serial ultrasound surveillance. After 4.5 years of follow-up, no significant difference was detected in the primary endpoint of all-cause mortality. The Kaplan-Meier estimates of all-cause mortality was 14.5% in the early repair group versus 10.1% in the surveillance group. Aneurysm-related mortality, aneurysm rupture, and major morbidity rates were similar. Like the PIVOTAL trial, the majority of subjects underwent delayed repair, with a frequency of 60% at 3-years and 85% at 4.5 years.
The findings of these two randomized studies suggest that there is no survival advantage to early EVAR in patients with smaller AAA. With the increasing use of statins, ACE inhibitors, and better overall medical management in patients with AAA, the rate of aneurysm enlargement and risk of rupture are quite low in patients with small AAA.8 While survival benefits have not been demonstrated, however, there appear to be few quantifiable disadvantages to early repair. Moreover, the long-term financial impact to the healthcare system appears to be similar when patients are treated early compared to surveillance with serial imaging studies,9 and patient quality of life may be improved with early EVAR.10 These observations suggest that the repair of smaller AAA should be individualized, based upon the particular clinical presentation and the wishes of a patient. The summary of available data suggests that either approach protects a patient from rupture and surveillance culminates in the eventual repair of the aneurysm in the majority of patients.
Dr. Ouriel is a vascular surgeon and president and CEO of Syntactx.
References
1. J Vasc Surg, 2012. 55(5): p. 1263-7.
2. J Vasc Interv Radiol, 2012. 23(7): p. 866-72; quiz 872.
3. J Endovasc Ther, 2012. 19(2): p. 182-92.
4. J Vasc Surg, 2012. 55(1): p. 33-40.
5. Ann Vasc Surg, 2012. 26(6): p. 860 e1-7.
6. J Vasc Interv Radiol, 2013. 24(1): p. 49-55
7. J Vasc Surg, 2012. 56(3): p. 630-6.
8. J Cardiovasc Surg (Torino), 2013. 1. Br J Surg 2007;94:702-8.
2. Semin Interv Cardiol 2000;5:3-6.
3. NEnglJMed 2010;362:1863-71.
4. JAMA 2009;302:1535-42.
5.. J Vasc Surg 2003;37:1206-12.
6. J Vasc Surg 2006;44:920-29; discussion 9-31.
7. J Vasc Surg 2010;51:1081-7.
8. EurJVascEndovascSurg 2011;41:2-10.
9. J Vasc Surg 2013;58:302-10.
10. Eur J Vasc Endovasc Surg 2011;41:324-31.
Small aneurysms should be left alone.
By Karl A. Illig, M.D.
The currently accepted recommendation to delay repair of abdominal aortic aneurysms until they reach 5-5.5 cm is based on the historical mortality and morbidity of open repair.1 Endovascular aneurysm repair (EVAR) has clearly been shown to reduce the risk of operation, perhaps by as much as two-thirds. Should we now change the threshold for aneurysm repair, especially if the patient is a candidate for EVAR?
There are many arguments to repair small aneurysms. However, the answer really depends upon empirical data. At least three major papers address this question. In the U.K. Small Aneurysm Trial, 1,090 patients with abdominal aortic aneurysms measuring 4.0-5.5 cm in diameter were randomized to undergo early elective open surgery versus ultrasonic surveillance.2 There was an early survival disadvantage for those undergoing open surgery, as expected, but the curves evened out at 3 years or so, and no survival advantage occurred in either group, obviously favoring observation alone. Similarly, the ADAM group, pursuing the same protocol in 1,136 U.S. veterans, found the same thing, despite a low operative mortality of 2.7%. No advantage to early open repair could be seen.3
What of the situation after endovascular repair? The PIVOTAL trial, the lead author of which wrote the accompanying commentary, randomly assigned 728 patients with aneurysms measuring 4.0-5.0 cm to early endovascular repair with the Medtronic device versus ultrasonic surveillance.4 Aneurysm rupture or aneurysm-related death occurred in only two patients in each group (0.6%). The authors appropriately concluded that surveillance alone was equally as efficacious as early endovascular repair for patients with small aneurysms.
Finally, what of the arguments that, by waiting until the aneurysm is larger, you lose the window of opportunity for endovascular repair? We recently explored this in a cohort of 221 patients undergoing preoperative CT scanning for aneurysms of all sizes.5 With receiver operator curve analysis, a cutoff of 5.7 cm best differentiated those who were endovascular candidates from those who were not. Put another way, the rate of endovascular suitability hovered right around 80% until the aneurysm reached 6 cm, at which point it dropped off. In other words, waiting until the aneurysm reaches 5.5 cm doesn’t reduce the chance that a patient will be a candidate for EVAR.
There are many arguments for early intervention in small aneurysms. The concept that EVAR is safer than the procedure originally used to make the recommendation to wait until 5.5 cm is a valid point. However, empiric data still do not show any benefit for either open or endovascular repair as opposed to surveillance at sizes smaller than this. The operative mortality difference is only a couple of percentage points or so, and long-term survival appears to be identical after the initial risk has passed. This 2% or 3% early survival benefit does not seem to impart any long-term advantage, and long-term survival does not seem to be impaired by being a bit conservative. Until data come along that definitively show benefit for early repair, current guidelines remain valid.
Dr. Illig is the director of vascular surgery at USF Health, Tampa.
References:
1. J Vasc Surg 2009;50(8S):1S-49S.
2. Lancet 1998; 352:1649-55.
3. NEJM 2002; 346 (19): 1437-44.
4. J Vasc Surg 2010; 51:1081-7.
5. J Vasc Surg 2010;52:873-7.
Online Question of the Month
New and Noteworthy Information—February 2014
Alcohol consumption may reduce the risk of developing multiple sclerosis (MS) and attenuate the effect of smoking, according to research published online ahead of print January 6 in JAMA Neurology. Scientists examined data from the Epidemiological Investigation of MS (EIMS), which included 745 cases and 1,761 controls, and from the Genes and Environment in MS (GEMS) study, which recruited 5,874 cases and 5,246 controls. In EIMS, women who reported high alcohol consumption (>112 g/week) had an odds ratio (OR) of 0.6 of developing MS, compared with nondrinking women. Men with high alcohol consumption (>168 g/week) in EIMS had an OR of 0.5, compared with nondrinking men. The OR for the comparison in GEMS was 0.7 for women and 0.7 for men. In both studies, the detrimental effect of smoking was more pronounced among nondrinkers.
A lentiviral vector-based gene therapy may be safe and improve motor behavior in patients with Parkinson’s disease, according to a study published online ahead of print January 10 in Lancet. In a phase I–II open-label trial, 15 patients received bilateral injections of gene therapy into the putamen and were followed up for 12 months. Participants received a low dose (1.9 × 107 transducing units [TU]), medium dose (4.0 × 107 TU), or a high dose (1 × 108 TU) of gene therapy. Patients reported 51 mild adverse events, three moderate adverse events, and no serious adverse events. The investigators noted a significant improvement in mean Unified Parkinson’s Disease Rating Scale part III motor scores off medication in all patients at six months, compared with baseline.
The FDA has approved a three-times-per-week formulation of Copaxone 40 mg/mL. The new formulation will enable a less-frequent dosing regimen to be administered subcutaneously to patients with relapsing forms of multiple sclerosis (MS). The approval is based on data from the Phase III Glatiramer Acetate Low-Frequency Administration study of more than 1,400 patients. In the trial, investigators found that a 40-mg/mL dose of Copaxone administered subcutaneously three times per week significantly reduced relapse rates at 12 months and demonstrated a favorable safety and tolerability profile in patients with relapsing-remitting MS. In addition to the newly approved dose, daily Copaxone 20 mg/mL will continue to be available. The daily subcutaneous injection was approved in 1996. Both formulations are manufactured by Teva Pharmaceutical Industries, which is headquartered in Jerusalem.
When administered with amitriptyline, cognitive behavioral therapy (CBT) may result in greater reductions in days with headache and in migraine-related disability among young persons with chronic migraine, compared with headache education, according to research published December 25, 2013, in JAMA. In a randomized clinical trial, 135 children (ages 10 to 17) with chronic migraine and a Pediatric Migraine Disability Assessment Score (PedMIDAS) greater than 20 points were assigned to CBT plus amitriptyline or headache education plus amitriptyline. At the 20-week end point, days with headache were reduced by 11.5 for the CBT plus amitriptyline group, compared with 6.8 for the headache education plus amitriptyline group. The PedMIDAS decreased by 52.7 points for the CBT group and by 38.6 points for the headache education group.
Low levels of vitamin D early in the course of multiple sclerosis (MS) are a strong risk factor for long-term disease activity and progression in patients who were primarily treated with interferon beta-1b, according to a study published online January 20 in JAMA Neurology. Researchers compared early and delayed interferon beta-1b treatment in 468 patients with clinically isolated syndrome, measuring serum levels of 25-hydroxyvitamin D (25[OH]D) at baseline and at six, 12, and 24 months. “A 50-nmol/L (20-ng/mL) increment in average serum 25(OH)D levels within the first 12 months predicted a 57% lower rate of new active lesions, 57% lower relapse rate, 25% lower yearly increase in T2 lesion volume, and 0.41% lower yearly loss in brain volume from months 12 to 60,” stated the study authors.
Excessive alcohol consumption in men was associated with faster cognitive decline, compared with light to moderate alcohol consumption, researchers reported online ahead of print January 15 in Neurology. The findings are based on data from 5,054 men and 2,099 women (mean age, 56) who had their alcohol consumption analyzed three times in the 10 years preceding the first cognitive assessment. In men, the investigators observed no differences in cognitive decline among alcohol abstainers, those who quit using alcohol, and light or moderate alcohol drinkers (<20 g/day). Alcohol consumption ≥36 g/day was associated with faster decline in all cognitive domains, compared with consumption between 0.1 and 19.9 g/day. In women, 10-year abstainers had a faster decline in the global cognitive score and executive function, compared with those drinking between 0.1 and 9.9 g/day of alcohol.
Vitamin D supplements may reduce pain in patients with fibromyalgia syndrome, according to a study in the February issue of Pain. The randomized controlled trial enrolled 30 women with fibromyalgia syndrome with serum calcifediol levels <32 ng/mL (80 nmol/L), in whom the goal was to achieve serum calcifediol levels between 32 and 48 ng/mL for 20 weeks with an oral cholecalciferol supplement. Re-evaluation was performed in both groups after an additional 24 weeks without cholecalciferol supplementation. The researchers observed a marked reduction in pain during the treatment period in those who received the supplement, and optimization of calcifediol levels had a positive effect on the perception of pain. “This economical therapy with a low side effect profile may well be considered in patients with fibromyalgia syndrome,” the researchers concluded.
A simple on-field blood test may help diagnose sports concussion. Relative and absolute increases in the astroglial protein, serum S100B, can accurately distinguish sports-related concussion from sports-related exertion, according to a study published online January 8 in PLOS One. Serum S100B was measured in 46 collegiate and semiprofessional contact sport athletes at preseason baseline, within three hours of injury, and at days 2, 3, and 7 post–sports-related concussion. Twenty-two athletes had a sports-related concussion, and 17 had S100B testing within three hours postinjury. The mean three-hour post–sports-related concussion S100B level was significantly higher than at preseason baseline, while the mean postexertion S100B level was not significantly different than that from the preseason baseline. S100B levels at postinjury days 2, 3, and 7 were significantly lower than at the three-hour level and were not different than at baseline.
Herpes zoster is an independent risk factor for vascular disease, particularly for stroke, transient ischemic attack, and myocardial infarction, in patients affected before age 40, researchers reported online ahead of print January 2 in Neurology. The findings are based on a retrospective cohort of 106,601 cases of herpes zoster and 213,202 controls from a general practice database in the United Kingdom. The investigators found that risk factors for vascular disease were significantly increased in patients with herpes zoster compared with controls. In addition, adjusted hazard ratios for TIA and myocardial infarction, but not stroke, were increased in all patients with herpes zoster. Stroke, TIA, and myocardial infarction were increased in cases in which herpes zoster occurred when the participants were younger than 40.
A study appearing January 22 online in Neurology found that a higher omega-3 index was correlated with larger total normal brain volume and hippocampal volume in postmenopausal women measured eight years later. Researchers assessed RBC eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and MRI brain volumes in 1,111 postmenopausal women from the Women’s Health Initiative Memory Study. In fully adjusted models, a 1-SD greater RBC EPA + DHA (omega-3 index) level was correlated with 2.1 cm3 larger brain volume. “DHA was marginally correlated with total brain volume while EPA was less so,” reported the investigators. In fully adjusted models, a 1-SD greater omega-3 index was correlated with greater hippocampal volume. “While normal aging results in overall brain atrophy, lower omega-3 index may signal increased risk of hippocampal atrophy,” wrote the investigators.
Exposure to DDT may increase the risk of developing Alzheimer’s disease, particularly in people older than 60, according to a study published online ahead of print January 27 in JAMA Neurology. Researchers examined the level of DDE, the chemical compound produced when DDT breaks down in the body, in the blood of 86 patients with Alzheimer’s disease and 79 controls. Blood levels of DDE were almost four times higher in 74 of the patients with Alzheimer’s disease than in the controls. Patients with APOE4, which greatly increases the risk of developing Alzheimer’s disease, and high blood levels of DDE exhibited more severe cognitive impairment than patients without the gene. In addition, DDT and DDE apparently increased the amount of a protein associated with plaques believed to be a hallmark of Alzheimer’s disease.
Mortality is higher among patients with multiple sclerosis (MS) than among Americans without the disease, according to research published online ahead of print December 26, 2013, in Multiple Sclerosis and Related Disorders. Investigators extracted records from a US commercial health insurance database—the OptumInsight Research database—for 30,402 patients with MS and 89,818 healthy comparators. Patient data were recorded from 1996 to 2009. Annual mortality rates were 899/100,000 among patients with MS and 446/100,000 among comparators. Standardized mortality ratio was 1.70 for patients with MS and 0.80 for the general US population. Kaplan–Meier analysis yielded a median survival from birth that was six years lower among patients with MS than among comparators. The six-year decrement in lifespan is consistent with a decrement found in recent research conducted in Canada, said the investigators.
Chronic obstructive pulmonary disease (COPD) may increase the risk of mild cognitive impairment (MCI), researchers reported in the November 2013 issue of Mayo Clinic Proceedings. The investigators evaluated 1,927 patients (ages 70 to 89) enrolled in the population-based Mayo Clinic Study of Aging. Participants received a nurse assessment, neurologic evaluation, and neuropsychologic testing. A consensus panel diagnosed MCI according to standardized criteria. COPD was identified by the review of medical records. A total of 288 patients had COPD. Prevalence of MCI was 27% among patients with COPD and 15% among patients without COPD. The odds ratio for MCI was 1.60 in patients who had had COPD for five years or fewer and 2.10 in patients who had had COPD for more than five years.
—Erik Greb and Colby Stong
Alcohol consumption may reduce the risk of developing multiple sclerosis (MS) and attenuate the effect of smoking, according to research published online ahead of print January 6 in JAMA Neurology. Scientists examined data from the Epidemiological Investigation of MS (EIMS), which included 745 cases and 1,761 controls, and from the Genes and Environment in MS (GEMS) study, which recruited 5,874 cases and 5,246 controls. In EIMS, women who reported high alcohol consumption (>112 g/week) had an odds ratio (OR) of 0.6 of developing MS, compared with nondrinking women. Men with high alcohol consumption (>168 g/week) in EIMS had an OR of 0.5, compared with nondrinking men. The OR for the comparison in GEMS was 0.7 for women and 0.7 for men. In both studies, the detrimental effect of smoking was more pronounced among nondrinkers.
A lentiviral vector-based gene therapy may be safe and improve motor behavior in patients with Parkinson’s disease, according to a study published online ahead of print January 10 in Lancet. In a phase I–II open-label trial, 15 patients received bilateral injections of gene therapy into the putamen and were followed up for 12 months. Participants received a low dose (1.9 × 107 transducing units [TU]), medium dose (4.0 × 107 TU), or a high dose (1 × 108 TU) of gene therapy. Patients reported 51 mild adverse events, three moderate adverse events, and no serious adverse events. The investigators noted a significant improvement in mean Unified Parkinson’s Disease Rating Scale part III motor scores off medication in all patients at six months, compared with baseline.
The FDA has approved a three-times-per-week formulation of Copaxone 40 mg/mL. The new formulation will enable a less-frequent dosing regimen to be administered subcutaneously to patients with relapsing forms of multiple sclerosis (MS). The approval is based on data from the Phase III Glatiramer Acetate Low-Frequency Administration study of more than 1,400 patients. In the trial, investigators found that a 40-mg/mL dose of Copaxone administered subcutaneously three times per week significantly reduced relapse rates at 12 months and demonstrated a favorable safety and tolerability profile in patients with relapsing-remitting MS. In addition to the newly approved dose, daily Copaxone 20 mg/mL will continue to be available. The daily subcutaneous injection was approved in 1996. Both formulations are manufactured by Teva Pharmaceutical Industries, which is headquartered in Jerusalem.
When administered with amitriptyline, cognitive behavioral therapy (CBT) may result in greater reductions in days with headache and in migraine-related disability among young persons with chronic migraine, compared with headache education, according to research published December 25, 2013, in JAMA. In a randomized clinical trial, 135 children (ages 10 to 17) with chronic migraine and a Pediatric Migraine Disability Assessment Score (PedMIDAS) greater than 20 points were assigned to CBT plus amitriptyline or headache education plus amitriptyline. At the 20-week end point, days with headache were reduced by 11.5 for the CBT plus amitriptyline group, compared with 6.8 for the headache education plus amitriptyline group. The PedMIDAS decreased by 52.7 points for the CBT group and by 38.6 points for the headache education group.
Low levels of vitamin D early in the course of multiple sclerosis (MS) are a strong risk factor for long-term disease activity and progression in patients who were primarily treated with interferon beta-1b, according to a study published online January 20 in JAMA Neurology. Researchers compared early and delayed interferon beta-1b treatment in 468 patients with clinically isolated syndrome, measuring serum levels of 25-hydroxyvitamin D (25[OH]D) at baseline and at six, 12, and 24 months. “A 50-nmol/L (20-ng/mL) increment in average serum 25(OH)D levels within the first 12 months predicted a 57% lower rate of new active lesions, 57% lower relapse rate, 25% lower yearly increase in T2 lesion volume, and 0.41% lower yearly loss in brain volume from months 12 to 60,” stated the study authors.
Excessive alcohol consumption in men was associated with faster cognitive decline, compared with light to moderate alcohol consumption, researchers reported online ahead of print January 15 in Neurology. The findings are based on data from 5,054 men and 2,099 women (mean age, 56) who had their alcohol consumption analyzed three times in the 10 years preceding the first cognitive assessment. In men, the investigators observed no differences in cognitive decline among alcohol abstainers, those who quit using alcohol, and light or moderate alcohol drinkers (<20 g/day). Alcohol consumption ≥36 g/day was associated with faster decline in all cognitive domains, compared with consumption between 0.1 and 19.9 g/day. In women, 10-year abstainers had a faster decline in the global cognitive score and executive function, compared with those drinking between 0.1 and 9.9 g/day of alcohol.
Vitamin D supplements may reduce pain in patients with fibromyalgia syndrome, according to a study in the February issue of Pain. The randomized controlled trial enrolled 30 women with fibromyalgia syndrome with serum calcifediol levels <32 ng/mL (80 nmol/L), in whom the goal was to achieve serum calcifediol levels between 32 and 48 ng/mL for 20 weeks with an oral cholecalciferol supplement. Re-evaluation was performed in both groups after an additional 24 weeks without cholecalciferol supplementation. The researchers observed a marked reduction in pain during the treatment period in those who received the supplement, and optimization of calcifediol levels had a positive effect on the perception of pain. “This economical therapy with a low side effect profile may well be considered in patients with fibromyalgia syndrome,” the researchers concluded.
A simple on-field blood test may help diagnose sports concussion. Relative and absolute increases in the astroglial protein, serum S100B, can accurately distinguish sports-related concussion from sports-related exertion, according to a study published online January 8 in PLOS One. Serum S100B was measured in 46 collegiate and semiprofessional contact sport athletes at preseason baseline, within three hours of injury, and at days 2, 3, and 7 post–sports-related concussion. Twenty-two athletes had a sports-related concussion, and 17 had S100B testing within three hours postinjury. The mean three-hour post–sports-related concussion S100B level was significantly higher than at preseason baseline, while the mean postexertion S100B level was not significantly different than that from the preseason baseline. S100B levels at postinjury days 2, 3, and 7 were significantly lower than at the three-hour level and were not different than at baseline.
Herpes zoster is an independent risk factor for vascular disease, particularly for stroke, transient ischemic attack, and myocardial infarction, in patients affected before age 40, researchers reported online ahead of print January 2 in Neurology. The findings are based on a retrospective cohort of 106,601 cases of herpes zoster and 213,202 controls from a general practice database in the United Kingdom. The investigators found that risk factors for vascular disease were significantly increased in patients with herpes zoster compared with controls. In addition, adjusted hazard ratios for TIA and myocardial infarction, but not stroke, were increased in all patients with herpes zoster. Stroke, TIA, and myocardial infarction were increased in cases in which herpes zoster occurred when the participants were younger than 40.
A study appearing January 22 online in Neurology found that a higher omega-3 index was correlated with larger total normal brain volume and hippocampal volume in postmenopausal women measured eight years later. Researchers assessed RBC eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and MRI brain volumes in 1,111 postmenopausal women from the Women’s Health Initiative Memory Study. In fully adjusted models, a 1-SD greater RBC EPA + DHA (omega-3 index) level was correlated with 2.1 cm3 larger brain volume. “DHA was marginally correlated with total brain volume while EPA was less so,” reported the investigators. In fully adjusted models, a 1-SD greater omega-3 index was correlated with greater hippocampal volume. “While normal aging results in overall brain atrophy, lower omega-3 index may signal increased risk of hippocampal atrophy,” wrote the investigators.
Exposure to DDT may increase the risk of developing Alzheimer’s disease, particularly in people older than 60, according to a study published online ahead of print January 27 in JAMA Neurology. Researchers examined the level of DDE, the chemical compound produced when DDT breaks down in the body, in the blood of 86 patients with Alzheimer’s disease and 79 controls. Blood levels of DDE were almost four times higher in 74 of the patients with Alzheimer’s disease than in the controls. Patients with APOE4, which greatly increases the risk of developing Alzheimer’s disease, and high blood levels of DDE exhibited more severe cognitive impairment than patients without the gene. In addition, DDT and DDE apparently increased the amount of a protein associated with plaques believed to be a hallmark of Alzheimer’s disease.
Mortality is higher among patients with multiple sclerosis (MS) than among Americans without the disease, according to research published online ahead of print December 26, 2013, in Multiple Sclerosis and Related Disorders. Investigators extracted records from a US commercial health insurance database—the OptumInsight Research database—for 30,402 patients with MS and 89,818 healthy comparators. Patient data were recorded from 1996 to 2009. Annual mortality rates were 899/100,000 among patients with MS and 446/100,000 among comparators. Standardized mortality ratio was 1.70 for patients with MS and 0.80 for the general US population. Kaplan–Meier analysis yielded a median survival from birth that was six years lower among patients with MS than among comparators. The six-year decrement in lifespan is consistent with a decrement found in recent research conducted in Canada, said the investigators.
Chronic obstructive pulmonary disease (COPD) may increase the risk of mild cognitive impairment (MCI), researchers reported in the November 2013 issue of Mayo Clinic Proceedings. The investigators evaluated 1,927 patients (ages 70 to 89) enrolled in the population-based Mayo Clinic Study of Aging. Participants received a nurse assessment, neurologic evaluation, and neuropsychologic testing. A consensus panel diagnosed MCI according to standardized criteria. COPD was identified by the review of medical records. A total of 288 patients had COPD. Prevalence of MCI was 27% among patients with COPD and 15% among patients without COPD. The odds ratio for MCI was 1.60 in patients who had had COPD for five years or fewer and 2.10 in patients who had had COPD for more than five years.
—Erik Greb and Colby Stong
Alcohol consumption may reduce the risk of developing multiple sclerosis (MS) and attenuate the effect of smoking, according to research published online ahead of print January 6 in JAMA Neurology. Scientists examined data from the Epidemiological Investigation of MS (EIMS), which included 745 cases and 1,761 controls, and from the Genes and Environment in MS (GEMS) study, which recruited 5,874 cases and 5,246 controls. In EIMS, women who reported high alcohol consumption (>112 g/week) had an odds ratio (OR) of 0.6 of developing MS, compared with nondrinking women. Men with high alcohol consumption (>168 g/week) in EIMS had an OR of 0.5, compared with nondrinking men. The OR for the comparison in GEMS was 0.7 for women and 0.7 for men. In both studies, the detrimental effect of smoking was more pronounced among nondrinkers.
A lentiviral vector-based gene therapy may be safe and improve motor behavior in patients with Parkinson’s disease, according to a study published online ahead of print January 10 in Lancet. In a phase I–II open-label trial, 15 patients received bilateral injections of gene therapy into the putamen and were followed up for 12 months. Participants received a low dose (1.9 × 107 transducing units [TU]), medium dose (4.0 × 107 TU), or a high dose (1 × 108 TU) of gene therapy. Patients reported 51 mild adverse events, three moderate adverse events, and no serious adverse events. The investigators noted a significant improvement in mean Unified Parkinson’s Disease Rating Scale part III motor scores off medication in all patients at six months, compared with baseline.
The FDA has approved a three-times-per-week formulation of Copaxone 40 mg/mL. The new formulation will enable a less-frequent dosing regimen to be administered subcutaneously to patients with relapsing forms of multiple sclerosis (MS). The approval is based on data from the Phase III Glatiramer Acetate Low-Frequency Administration study of more than 1,400 patients. In the trial, investigators found that a 40-mg/mL dose of Copaxone administered subcutaneously three times per week significantly reduced relapse rates at 12 months and demonstrated a favorable safety and tolerability profile in patients with relapsing-remitting MS. In addition to the newly approved dose, daily Copaxone 20 mg/mL will continue to be available. The daily subcutaneous injection was approved in 1996. Both formulations are manufactured by Teva Pharmaceutical Industries, which is headquartered in Jerusalem.
When administered with amitriptyline, cognitive behavioral therapy (CBT) may result in greater reductions in days with headache and in migraine-related disability among young persons with chronic migraine, compared with headache education, according to research published December 25, 2013, in JAMA. In a randomized clinical trial, 135 children (ages 10 to 17) with chronic migraine and a Pediatric Migraine Disability Assessment Score (PedMIDAS) greater than 20 points were assigned to CBT plus amitriptyline or headache education plus amitriptyline. At the 20-week end point, days with headache were reduced by 11.5 for the CBT plus amitriptyline group, compared with 6.8 for the headache education plus amitriptyline group. The PedMIDAS decreased by 52.7 points for the CBT group and by 38.6 points for the headache education group.
Low levels of vitamin D early in the course of multiple sclerosis (MS) are a strong risk factor for long-term disease activity and progression in patients who were primarily treated with interferon beta-1b, according to a study published online January 20 in JAMA Neurology. Researchers compared early and delayed interferon beta-1b treatment in 468 patients with clinically isolated syndrome, measuring serum levels of 25-hydroxyvitamin D (25[OH]D) at baseline and at six, 12, and 24 months. “A 50-nmol/L (20-ng/mL) increment in average serum 25(OH)D levels within the first 12 months predicted a 57% lower rate of new active lesions, 57% lower relapse rate, 25% lower yearly increase in T2 lesion volume, and 0.41% lower yearly loss in brain volume from months 12 to 60,” stated the study authors.
Excessive alcohol consumption in men was associated with faster cognitive decline, compared with light to moderate alcohol consumption, researchers reported online ahead of print January 15 in Neurology. The findings are based on data from 5,054 men and 2,099 women (mean age, 56) who had their alcohol consumption analyzed three times in the 10 years preceding the first cognitive assessment. In men, the investigators observed no differences in cognitive decline among alcohol abstainers, those who quit using alcohol, and light or moderate alcohol drinkers (<20 g/day). Alcohol consumption ≥36 g/day was associated with faster decline in all cognitive domains, compared with consumption between 0.1 and 19.9 g/day. In women, 10-year abstainers had a faster decline in the global cognitive score and executive function, compared with those drinking between 0.1 and 9.9 g/day of alcohol.
Vitamin D supplements may reduce pain in patients with fibromyalgia syndrome, according to a study in the February issue of Pain. The randomized controlled trial enrolled 30 women with fibromyalgia syndrome with serum calcifediol levels <32 ng/mL (80 nmol/L), in whom the goal was to achieve serum calcifediol levels between 32 and 48 ng/mL for 20 weeks with an oral cholecalciferol supplement. Re-evaluation was performed in both groups after an additional 24 weeks without cholecalciferol supplementation. The researchers observed a marked reduction in pain during the treatment period in those who received the supplement, and optimization of calcifediol levels had a positive effect on the perception of pain. “This economical therapy with a low side effect profile may well be considered in patients with fibromyalgia syndrome,” the researchers concluded.
A simple on-field blood test may help diagnose sports concussion. Relative and absolute increases in the astroglial protein, serum S100B, can accurately distinguish sports-related concussion from sports-related exertion, according to a study published online January 8 in PLOS One. Serum S100B was measured in 46 collegiate and semiprofessional contact sport athletes at preseason baseline, within three hours of injury, and at days 2, 3, and 7 post–sports-related concussion. Twenty-two athletes had a sports-related concussion, and 17 had S100B testing within three hours postinjury. The mean three-hour post–sports-related concussion S100B level was significantly higher than at preseason baseline, while the mean postexertion S100B level was not significantly different than that from the preseason baseline. S100B levels at postinjury days 2, 3, and 7 were significantly lower than at the three-hour level and were not different than at baseline.
Herpes zoster is an independent risk factor for vascular disease, particularly for stroke, transient ischemic attack, and myocardial infarction, in patients affected before age 40, researchers reported online ahead of print January 2 in Neurology. The findings are based on a retrospective cohort of 106,601 cases of herpes zoster and 213,202 controls from a general practice database in the United Kingdom. The investigators found that risk factors for vascular disease were significantly increased in patients with herpes zoster compared with controls. In addition, adjusted hazard ratios for TIA and myocardial infarction, but not stroke, were increased in all patients with herpes zoster. Stroke, TIA, and myocardial infarction were increased in cases in which herpes zoster occurred when the participants were younger than 40.
A study appearing January 22 online in Neurology found that a higher omega-3 index was correlated with larger total normal brain volume and hippocampal volume in postmenopausal women measured eight years later. Researchers assessed RBC eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), and MRI brain volumes in 1,111 postmenopausal women from the Women’s Health Initiative Memory Study. In fully adjusted models, a 1-SD greater RBC EPA + DHA (omega-3 index) level was correlated with 2.1 cm3 larger brain volume. “DHA was marginally correlated with total brain volume while EPA was less so,” reported the investigators. In fully adjusted models, a 1-SD greater omega-3 index was correlated with greater hippocampal volume. “While normal aging results in overall brain atrophy, lower omega-3 index may signal increased risk of hippocampal atrophy,” wrote the investigators.
Exposure to DDT may increase the risk of developing Alzheimer’s disease, particularly in people older than 60, according to a study published online ahead of print January 27 in JAMA Neurology. Researchers examined the level of DDE, the chemical compound produced when DDT breaks down in the body, in the blood of 86 patients with Alzheimer’s disease and 79 controls. Blood levels of DDE were almost four times higher in 74 of the patients with Alzheimer’s disease than in the controls. Patients with APOE4, which greatly increases the risk of developing Alzheimer’s disease, and high blood levels of DDE exhibited more severe cognitive impairment than patients without the gene. In addition, DDT and DDE apparently increased the amount of a protein associated with plaques believed to be a hallmark of Alzheimer’s disease.
Mortality is higher among patients with multiple sclerosis (MS) than among Americans without the disease, according to research published online ahead of print December 26, 2013, in Multiple Sclerosis and Related Disorders. Investigators extracted records from a US commercial health insurance database—the OptumInsight Research database—for 30,402 patients with MS and 89,818 healthy comparators. Patient data were recorded from 1996 to 2009. Annual mortality rates were 899/100,000 among patients with MS and 446/100,000 among comparators. Standardized mortality ratio was 1.70 for patients with MS and 0.80 for the general US population. Kaplan–Meier analysis yielded a median survival from birth that was six years lower among patients with MS than among comparators. The six-year decrement in lifespan is consistent with a decrement found in recent research conducted in Canada, said the investigators.
Chronic obstructive pulmonary disease (COPD) may increase the risk of mild cognitive impairment (MCI), researchers reported in the November 2013 issue of Mayo Clinic Proceedings. The investigators evaluated 1,927 patients (ages 70 to 89) enrolled in the population-based Mayo Clinic Study of Aging. Participants received a nurse assessment, neurologic evaluation, and neuropsychologic testing. A consensus panel diagnosed MCI according to standardized criteria. COPD was identified by the review of medical records. A total of 288 patients had COPD. Prevalence of MCI was 27% among patients with COPD and 15% among patients without COPD. The odds ratio for MCI was 1.60 in patients who had had COPD for five years or fewer and 2.10 in patients who had had COPD for more than five years.
—Erik Greb and Colby Stong
Regimen shows promise for ENKTL
SAN FRANCISCO—Results of a single-center study suggest that a 3-drug regimen may be a safe and effective treatment option for patients with newly diagnosed or relapsed/refractory extranodal natural killer/T-cell lymphoma (ENKTL).
The combination of pegaspargase, gemcitabine, and oxaliplatin (P-Gemox) elicited a high rate of response in this cohort of 60 Chinese patients.
P-Gemox also produced higher survival rates than those previously observed with the EPOCH regimen.
Grade 1/2 myelosuppression occurred in more than half of patients in this study, and nearly three-quarters of patients experienced grade 1/2 nausea. But grade 3/4 adverse events were minimal.
Hui-qiang Huang, MD, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented these results at the 6th Annual T-cell Lymphoma Forum.
Dr Huang noted that advanced ENKTL is relatively resistant to anthracycline-based chemotherapy. And although the SMILE and AspaMetDex regimens are effective, they confer relatively severe toxicities and are inconvenient to administer.
“So chemotherapeutic combinations with high efficacy and low toxicities are urgently needed,” he said.
With this in mind, he and his colleagues assessed P-Gemox in 61 patients with ENKTL. Thirty-six patients were newly diagnosed, and 25 had relapsed/refractory disease. Roughly 69% of patients were male, and about 86% were older than 60 years of age.
Overall, 36.1% of patients had stage IE disease, 31.1% had stage IIE, 4.9% had stage IIIE, and 27.9% had stage IVE.
The relapsed/refractory patients had received a range of prior treatment regimens, including CHOP/L-ASP+CHOP, EPOCH, V-EPOCH, ICE, IMVP-16, and SMILE. And 13 patients had received radiotherapy.
For this study, all 61 patients received intravenous gemcitabine at 1000 mg/m2 on days 1 and 8, intravenous oxaliplatin at 130 mg/m2 on day 1, and intramuscular pegaspargase at 2500 U/m2 on day 1. This regimen was repeated every 3 weeks.
Patients with stage IE/IIE disease received 3 cycles followed by radiotherapy (50-56 Gy). Relapsed/refractory patients received 2 to 6 cycles, and those who responded well were recommended for autologous transplant.
Response and subsequent treatment
Sixty patients were evaluable for response. (One patient in the newly diagnosed group was not evaluable).
The overall response rate (ORR) was 90%, with 63.3% of patients achieving a complete response (CR), 26.7% achieving a partial response (PR), and 8.3% maintaining stable disease (SD).
Among newly diagnosed patients, the ORR was 94.3%. CRs occurred in 74.3% of patients, PRs in in 20%, and SD in 5.7%.
And among the relapsed/refractory patients, the ORR was 84%. CRs were seen in 48% of patients, PRs in 36%, and SD in 12%.
“For patients with early stage disease, we found P-Gemox can further improve the outcomes of radiotherapy,” Dr Huang noted.
The treatment also provided a good bridge to transplant. Eight patients underwent transplant after achieving CR. One of these patients died 9 months after the procedure, but the other 7 patients were still in CR at a median of 14.6 months (range, 4.8-19.7 months).
‘Encouraging’ survival
The median follow-up was 29.5 months. The researchers confirmed progressive disease in 18 of the 61 patients—7 in the newly diagnosed group and 11 in the relapsed/refractory group.
Nine patients died of disease progression—1 in the newly diagnosed group and 8 in the relapsed/refractory group.
The 2-year overall survival was 86%, and the 2-year progression-free survival was 75.6%. Both overall and progression-free survival were superior in the newly diagnosed patients (P=0.054 and P=0.004, respectively).
“For the relapsed/refractory cases, considering they had already received a lot of previous treatments, we thought this outcome with P-Gemox is still quite encouraging,” Dr Huang said.
When the researchers compared overall survival with P-Gemox to previous results observed with EPOCH in newly diagnosed ENKTL patients (Huang et al, Leuk & Lymph 2011), they found P-Gemox was superior.
‘Tolerable’ toxicity
Toxicity with P-Gemox was tolerable and manageable, according to Dr Huang. The main adverse events were nausea and myelosuppression. But the rate of grade 3/4 events was low, and there were no treatment-related deaths.
Specifically, the grade 1/2 adverse events included nausea (73.8%), neutropenia (58%), thrombocytopenia (52.4%), hypoprotinemia (52.4%), anemia (52.4%), vomiting (49.2%), prolonged APTT (44.2%), elevated transaminase (34.1%), elevated bilirubin (27.9%), mucositis (24.5%), decreased fibrinogen (23%), elevated BUN (4.9%), intracranial bleeding (1.6%), stomach bleeding (1.6%), pancreatitis (1.6%), and herpes (1.6%).
Grade 3/4 adverse events included neutropenia (19.7%), thrombocytopenia (16.4%), hypoprotinemia (1.6%), anemia (1.6%), vomiting (3.2%), elevated transaminase (1.6%), and decreased fibrinogen (1.6%).
“We found that P-Gemox is an effective, safe, and convenient regimen in Chinese patients with ENKTL, both treatment-naïve and relapsed/refractory,” Dr Huang concluded. “These results provide a basis for subsequent studies.”
Dr Huang and his colleagues also presented the results of this research at the ASH Annual Meeting in December as abstract 642. (Information presented at the T-cell Lymphoma Forum differs from that in the ASH abstract). ![]()
SAN FRANCISCO—Results of a single-center study suggest that a 3-drug regimen may be a safe and effective treatment option for patients with newly diagnosed or relapsed/refractory extranodal natural killer/T-cell lymphoma (ENKTL).
The combination of pegaspargase, gemcitabine, and oxaliplatin (P-Gemox) elicited a high rate of response in this cohort of 60 Chinese patients.
P-Gemox also produced higher survival rates than those previously observed with the EPOCH regimen.
Grade 1/2 myelosuppression occurred in more than half of patients in this study, and nearly three-quarters of patients experienced grade 1/2 nausea. But grade 3/4 adverse events were minimal.
Hui-qiang Huang, MD, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented these results at the 6th Annual T-cell Lymphoma Forum.
Dr Huang noted that advanced ENKTL is relatively resistant to anthracycline-based chemotherapy. And although the SMILE and AspaMetDex regimens are effective, they confer relatively severe toxicities and are inconvenient to administer.
“So chemotherapeutic combinations with high efficacy and low toxicities are urgently needed,” he said.
With this in mind, he and his colleagues assessed P-Gemox in 61 patients with ENKTL. Thirty-six patients were newly diagnosed, and 25 had relapsed/refractory disease. Roughly 69% of patients were male, and about 86% were older than 60 years of age.
Overall, 36.1% of patients had stage IE disease, 31.1% had stage IIE, 4.9% had stage IIIE, and 27.9% had stage IVE.
The relapsed/refractory patients had received a range of prior treatment regimens, including CHOP/L-ASP+CHOP, EPOCH, V-EPOCH, ICE, IMVP-16, and SMILE. And 13 patients had received radiotherapy.
For this study, all 61 patients received intravenous gemcitabine at 1000 mg/m2 on days 1 and 8, intravenous oxaliplatin at 130 mg/m2 on day 1, and intramuscular pegaspargase at 2500 U/m2 on day 1. This regimen was repeated every 3 weeks.
Patients with stage IE/IIE disease received 3 cycles followed by radiotherapy (50-56 Gy). Relapsed/refractory patients received 2 to 6 cycles, and those who responded well were recommended for autologous transplant.
Response and subsequent treatment
Sixty patients were evaluable for response. (One patient in the newly diagnosed group was not evaluable).
The overall response rate (ORR) was 90%, with 63.3% of patients achieving a complete response (CR), 26.7% achieving a partial response (PR), and 8.3% maintaining stable disease (SD).
Among newly diagnosed patients, the ORR was 94.3%. CRs occurred in 74.3% of patients, PRs in in 20%, and SD in 5.7%.
And among the relapsed/refractory patients, the ORR was 84%. CRs were seen in 48% of patients, PRs in 36%, and SD in 12%.
“For patients with early stage disease, we found P-Gemox can further improve the outcomes of radiotherapy,” Dr Huang noted.
The treatment also provided a good bridge to transplant. Eight patients underwent transplant after achieving CR. One of these patients died 9 months after the procedure, but the other 7 patients were still in CR at a median of 14.6 months (range, 4.8-19.7 months).
‘Encouraging’ survival
The median follow-up was 29.5 months. The researchers confirmed progressive disease in 18 of the 61 patients—7 in the newly diagnosed group and 11 in the relapsed/refractory group.
Nine patients died of disease progression—1 in the newly diagnosed group and 8 in the relapsed/refractory group.
The 2-year overall survival was 86%, and the 2-year progression-free survival was 75.6%. Both overall and progression-free survival were superior in the newly diagnosed patients (P=0.054 and P=0.004, respectively).
“For the relapsed/refractory cases, considering they had already received a lot of previous treatments, we thought this outcome with P-Gemox is still quite encouraging,” Dr Huang said.
When the researchers compared overall survival with P-Gemox to previous results observed with EPOCH in newly diagnosed ENKTL patients (Huang et al, Leuk & Lymph 2011), they found P-Gemox was superior.
‘Tolerable’ toxicity
Toxicity with P-Gemox was tolerable and manageable, according to Dr Huang. The main adverse events were nausea and myelosuppression. But the rate of grade 3/4 events was low, and there were no treatment-related deaths.
Specifically, the grade 1/2 adverse events included nausea (73.8%), neutropenia (58%), thrombocytopenia (52.4%), hypoprotinemia (52.4%), anemia (52.4%), vomiting (49.2%), prolonged APTT (44.2%), elevated transaminase (34.1%), elevated bilirubin (27.9%), mucositis (24.5%), decreased fibrinogen (23%), elevated BUN (4.9%), intracranial bleeding (1.6%), stomach bleeding (1.6%), pancreatitis (1.6%), and herpes (1.6%).
Grade 3/4 adverse events included neutropenia (19.7%), thrombocytopenia (16.4%), hypoprotinemia (1.6%), anemia (1.6%), vomiting (3.2%), elevated transaminase (1.6%), and decreased fibrinogen (1.6%).
“We found that P-Gemox is an effective, safe, and convenient regimen in Chinese patients with ENKTL, both treatment-naïve and relapsed/refractory,” Dr Huang concluded. “These results provide a basis for subsequent studies.”
Dr Huang and his colleagues also presented the results of this research at the ASH Annual Meeting in December as abstract 642. (Information presented at the T-cell Lymphoma Forum differs from that in the ASH abstract). ![]()
SAN FRANCISCO—Results of a single-center study suggest that a 3-drug regimen may be a safe and effective treatment option for patients with newly diagnosed or relapsed/refractory extranodal natural killer/T-cell lymphoma (ENKTL).
The combination of pegaspargase, gemcitabine, and oxaliplatin (P-Gemox) elicited a high rate of response in this cohort of 60 Chinese patients.
P-Gemox also produced higher survival rates than those previously observed with the EPOCH regimen.
Grade 1/2 myelosuppression occurred in more than half of patients in this study, and nearly three-quarters of patients experienced grade 1/2 nausea. But grade 3/4 adverse events were minimal.
Hui-qiang Huang, MD, PhD, of Sun Yat-sen University Cancer Center in Guangzhou, China, presented these results at the 6th Annual T-cell Lymphoma Forum.
Dr Huang noted that advanced ENKTL is relatively resistant to anthracycline-based chemotherapy. And although the SMILE and AspaMetDex regimens are effective, they confer relatively severe toxicities and are inconvenient to administer.
“So chemotherapeutic combinations with high efficacy and low toxicities are urgently needed,” he said.
With this in mind, he and his colleagues assessed P-Gemox in 61 patients with ENKTL. Thirty-six patients were newly diagnosed, and 25 had relapsed/refractory disease. Roughly 69% of patients were male, and about 86% were older than 60 years of age.
Overall, 36.1% of patients had stage IE disease, 31.1% had stage IIE, 4.9% had stage IIIE, and 27.9% had stage IVE.
The relapsed/refractory patients had received a range of prior treatment regimens, including CHOP/L-ASP+CHOP, EPOCH, V-EPOCH, ICE, IMVP-16, and SMILE. And 13 patients had received radiotherapy.
For this study, all 61 patients received intravenous gemcitabine at 1000 mg/m2 on days 1 and 8, intravenous oxaliplatin at 130 mg/m2 on day 1, and intramuscular pegaspargase at 2500 U/m2 on day 1. This regimen was repeated every 3 weeks.
Patients with stage IE/IIE disease received 3 cycles followed by radiotherapy (50-56 Gy). Relapsed/refractory patients received 2 to 6 cycles, and those who responded well were recommended for autologous transplant.
Response and subsequent treatment
Sixty patients were evaluable for response. (One patient in the newly diagnosed group was not evaluable).
The overall response rate (ORR) was 90%, with 63.3% of patients achieving a complete response (CR), 26.7% achieving a partial response (PR), and 8.3% maintaining stable disease (SD).
Among newly diagnosed patients, the ORR was 94.3%. CRs occurred in 74.3% of patients, PRs in in 20%, and SD in 5.7%.
And among the relapsed/refractory patients, the ORR was 84%. CRs were seen in 48% of patients, PRs in 36%, and SD in 12%.
“For patients with early stage disease, we found P-Gemox can further improve the outcomes of radiotherapy,” Dr Huang noted.
The treatment also provided a good bridge to transplant. Eight patients underwent transplant after achieving CR. One of these patients died 9 months after the procedure, but the other 7 patients were still in CR at a median of 14.6 months (range, 4.8-19.7 months).
‘Encouraging’ survival
The median follow-up was 29.5 months. The researchers confirmed progressive disease in 18 of the 61 patients—7 in the newly diagnosed group and 11 in the relapsed/refractory group.
Nine patients died of disease progression—1 in the newly diagnosed group and 8 in the relapsed/refractory group.
The 2-year overall survival was 86%, and the 2-year progression-free survival was 75.6%. Both overall and progression-free survival were superior in the newly diagnosed patients (P=0.054 and P=0.004, respectively).
“For the relapsed/refractory cases, considering they had already received a lot of previous treatments, we thought this outcome with P-Gemox is still quite encouraging,” Dr Huang said.
When the researchers compared overall survival with P-Gemox to previous results observed with EPOCH in newly diagnosed ENKTL patients (Huang et al, Leuk & Lymph 2011), they found P-Gemox was superior.
‘Tolerable’ toxicity
Toxicity with P-Gemox was tolerable and manageable, according to Dr Huang. The main adverse events were nausea and myelosuppression. But the rate of grade 3/4 events was low, and there were no treatment-related deaths.
Specifically, the grade 1/2 adverse events included nausea (73.8%), neutropenia (58%), thrombocytopenia (52.4%), hypoprotinemia (52.4%), anemia (52.4%), vomiting (49.2%), prolonged APTT (44.2%), elevated transaminase (34.1%), elevated bilirubin (27.9%), mucositis (24.5%), decreased fibrinogen (23%), elevated BUN (4.9%), intracranial bleeding (1.6%), stomach bleeding (1.6%), pancreatitis (1.6%), and herpes (1.6%).
Grade 3/4 adverse events included neutropenia (19.7%), thrombocytopenia (16.4%), hypoprotinemia (1.6%), anemia (1.6%), vomiting (3.2%), elevated transaminase (1.6%), and decreased fibrinogen (1.6%).
“We found that P-Gemox is an effective, safe, and convenient regimen in Chinese patients with ENKTL, both treatment-naïve and relapsed/refractory,” Dr Huang concluded. “These results provide a basis for subsequent studies.”
Dr Huang and his colleagues also presented the results of this research at the ASH Annual Meeting in December as abstract 642. (Information presented at the T-cell Lymphoma Forum differs from that in the ASH abstract). ![]()
System allows precise gene editing in monkeys

Credit: Yuyu Niu et al.
Although monkeys can be useful as models of human disease, precisely modifying their genes has proven difficult.
Now, investigators say they’ve achieved precise gene modification in monkeys using the CRISPR/Cas9 system.
“Our study shows that the CRISPR/Cas9 system enables simultaneous disruption of 2 target genes in 1 step, without producing off-target mutations,” said Jiahao Sha, PhD, of Nanjing Medical University in Nanjing, China.
“Considering that many human diseases are caused by genetic abnormalities, targeted genetic modification in monkeys is invaluable for the generation of human disease models.”
Dr Sha and his colleagues described this research in Cell.
The CRISPR/Cas9 system is a gene-editing tool capable of targeting specific DNA sequences in the genome. Cas9 proteins, which are directed by single-guide RNAs to specific sites in the genome, generate mutations by introducing double-stranded DNA breaks.
Until now, the CRISPR/Cas9 system and other targeted gene-editing techniques were successfully applied to mammals such as mice and rats, but not to primates.
Dr Sha and his colleagues injected messenger RNA encoding Cas9, as well as single-guide RNAs designed to target 3 specific genes, into one-cell-stage embryos of cynomolgus monkeys.
After sequencing DNA from 15 embryos, the team found that 8 of these embryos showed evidence of simultaneous mutations in 2 of the target genes.
The researchers then transferred genetically modified embryos into surrogate females, one of which gave birth to a set of twins. By sequencing the twins’ DNA, the team found mutations in 2 of the target genes.
Moreover, the CRISPR/Cas9 system did not produce mutations at genomic sites that were not targeted. And this suggests the tool will not cause undesirable effects when applied to monkeys.
“With the precise genomic targeting of the CRISPR/Cas9 system, we expect that many disease models will be generated in monkeys,” said Weizhi Ji, PhD, of the Yunnan Key Laboratory of Primate Biomedical Research in Kunming, China.
“[This] will significantly advance the development of therapeutic strategies in biomedical research.” ![]()

Credit: Yuyu Niu et al.
Although monkeys can be useful as models of human disease, precisely modifying their genes has proven difficult.
Now, investigators say they’ve achieved precise gene modification in monkeys using the CRISPR/Cas9 system.
“Our study shows that the CRISPR/Cas9 system enables simultaneous disruption of 2 target genes in 1 step, without producing off-target mutations,” said Jiahao Sha, PhD, of Nanjing Medical University in Nanjing, China.
“Considering that many human diseases are caused by genetic abnormalities, targeted genetic modification in monkeys is invaluable for the generation of human disease models.”
Dr Sha and his colleagues described this research in Cell.
The CRISPR/Cas9 system is a gene-editing tool capable of targeting specific DNA sequences in the genome. Cas9 proteins, which are directed by single-guide RNAs to specific sites in the genome, generate mutations by introducing double-stranded DNA breaks.
Until now, the CRISPR/Cas9 system and other targeted gene-editing techniques were successfully applied to mammals such as mice and rats, but not to primates.
Dr Sha and his colleagues injected messenger RNA encoding Cas9, as well as single-guide RNAs designed to target 3 specific genes, into one-cell-stage embryos of cynomolgus monkeys.
After sequencing DNA from 15 embryos, the team found that 8 of these embryos showed evidence of simultaneous mutations in 2 of the target genes.
The researchers then transferred genetically modified embryos into surrogate females, one of which gave birth to a set of twins. By sequencing the twins’ DNA, the team found mutations in 2 of the target genes.
Moreover, the CRISPR/Cas9 system did not produce mutations at genomic sites that were not targeted. And this suggests the tool will not cause undesirable effects when applied to monkeys.
“With the precise genomic targeting of the CRISPR/Cas9 system, we expect that many disease models will be generated in monkeys,” said Weizhi Ji, PhD, of the Yunnan Key Laboratory of Primate Biomedical Research in Kunming, China.
“[This] will significantly advance the development of therapeutic strategies in biomedical research.” ![]()

Credit: Yuyu Niu et al.
Although monkeys can be useful as models of human disease, precisely modifying their genes has proven difficult.
Now, investigators say they’ve achieved precise gene modification in monkeys using the CRISPR/Cas9 system.
“Our study shows that the CRISPR/Cas9 system enables simultaneous disruption of 2 target genes in 1 step, without producing off-target mutations,” said Jiahao Sha, PhD, of Nanjing Medical University in Nanjing, China.
“Considering that many human diseases are caused by genetic abnormalities, targeted genetic modification in monkeys is invaluable for the generation of human disease models.”
Dr Sha and his colleagues described this research in Cell.
The CRISPR/Cas9 system is a gene-editing tool capable of targeting specific DNA sequences in the genome. Cas9 proteins, which are directed by single-guide RNAs to specific sites in the genome, generate mutations by introducing double-stranded DNA breaks.
Until now, the CRISPR/Cas9 system and other targeted gene-editing techniques were successfully applied to mammals such as mice and rats, but not to primates.
Dr Sha and his colleagues injected messenger RNA encoding Cas9, as well as single-guide RNAs designed to target 3 specific genes, into one-cell-stage embryos of cynomolgus monkeys.
After sequencing DNA from 15 embryos, the team found that 8 of these embryos showed evidence of simultaneous mutations in 2 of the target genes.
The researchers then transferred genetically modified embryos into surrogate females, one of which gave birth to a set of twins. By sequencing the twins’ DNA, the team found mutations in 2 of the target genes.
Moreover, the CRISPR/Cas9 system did not produce mutations at genomic sites that were not targeted. And this suggests the tool will not cause undesirable effects when applied to monkeys.
“With the precise genomic targeting of the CRISPR/Cas9 system, we expect that many disease models will be generated in monkeys,” said Weizhi Ji, PhD, of the Yunnan Key Laboratory of Primate Biomedical Research in Kunming, China.
“[This] will significantly advance the development of therapeutic strategies in biomedical research.” ![]()
Inhibitor strengthens RBCs in PNH

Credit: NHLBI
The apoptosis inhibitor aurin tricarboxylic acid (ATA) is active against paroxysmal nocturnal hemoglobinemia (PNH), according to research published in PLOS ONE.
PNH is a rare condition in which red blood cells (RBCs) become vulnerable to attacks by the complement immune system and subsequently rupture.
This can lead to complications such as anemia, kidney disease, and fatal thromboses.
PNH results from a lack of 2 proteins that protect RBCs from destruction: decay-accelerating factor (CD55), an inhibitor of alternative pathway C3 convertase, and protectin (CD59), an inhibitor of membrane attack complex (MAC) formation.
Because previous studies suggested that ATA selectively blocks complement activation at the C3 convertase stage and MAC formation at the C9 insertion stage, researchers thought ATA might prove effective against PNH.
First, they compared RBCs from 5 patients with PNH (who were on long-term treatment with eculizumab) to RBCs from healthy individuals.
Despite the eculizumab, the PNH patients’ RBCs were twice as vulnerable to complement-induced lysis as the healthy subjects’ RBCs. And western blot revealed both C3 and C5 convertases on the membranes of patients’ RBCs.
However, when the researchers added ATA to patients’ blood samples, the RBCs were protected from complement attack. In fact, the drug restored the RBCs’ resistance to the same level as normal RBCs.
“Our study suggests that ATA could offer more complete protection as an oral treatment for PNH, while eliminating the need for infusions,” said study author Patrick McGeer, MD, PhD, of the University of British Columbia in Vancouver, Canada.
“PNH is a disease that may happen to anyone through a chance mutation, and, if nature were to design a perfect fix for this mutation, it would be ATA.”
Dr McGeer added that many diseases are caused or worsened by an overactive complement immune system. So his group’s findings could have implications for conditions such as Alzheimer’s disease, Parkinson’s disease, macular degeneration, amyotrophic lateral sclerosis, multiple sclerosis, and rheumatoid arthritis.
He and his colleagues are now proceeding with further testing, and Dr McGeer expects ATA could be available in clinics within a year. ![]()

Credit: NHLBI
The apoptosis inhibitor aurin tricarboxylic acid (ATA) is active against paroxysmal nocturnal hemoglobinemia (PNH), according to research published in PLOS ONE.
PNH is a rare condition in which red blood cells (RBCs) become vulnerable to attacks by the complement immune system and subsequently rupture.
This can lead to complications such as anemia, kidney disease, and fatal thromboses.
PNH results from a lack of 2 proteins that protect RBCs from destruction: decay-accelerating factor (CD55), an inhibitor of alternative pathway C3 convertase, and protectin (CD59), an inhibitor of membrane attack complex (MAC) formation.
Because previous studies suggested that ATA selectively blocks complement activation at the C3 convertase stage and MAC formation at the C9 insertion stage, researchers thought ATA might prove effective against PNH.
First, they compared RBCs from 5 patients with PNH (who were on long-term treatment with eculizumab) to RBCs from healthy individuals.
Despite the eculizumab, the PNH patients’ RBCs were twice as vulnerable to complement-induced lysis as the healthy subjects’ RBCs. And western blot revealed both C3 and C5 convertases on the membranes of patients’ RBCs.
However, when the researchers added ATA to patients’ blood samples, the RBCs were protected from complement attack. In fact, the drug restored the RBCs’ resistance to the same level as normal RBCs.
“Our study suggests that ATA could offer more complete protection as an oral treatment for PNH, while eliminating the need for infusions,” said study author Patrick McGeer, MD, PhD, of the University of British Columbia in Vancouver, Canada.
“PNH is a disease that may happen to anyone through a chance mutation, and, if nature were to design a perfect fix for this mutation, it would be ATA.”
Dr McGeer added that many diseases are caused or worsened by an overactive complement immune system. So his group’s findings could have implications for conditions such as Alzheimer’s disease, Parkinson’s disease, macular degeneration, amyotrophic lateral sclerosis, multiple sclerosis, and rheumatoid arthritis.
He and his colleagues are now proceeding with further testing, and Dr McGeer expects ATA could be available in clinics within a year. ![]()

Credit: NHLBI
The apoptosis inhibitor aurin tricarboxylic acid (ATA) is active against paroxysmal nocturnal hemoglobinemia (PNH), according to research published in PLOS ONE.
PNH is a rare condition in which red blood cells (RBCs) become vulnerable to attacks by the complement immune system and subsequently rupture.
This can lead to complications such as anemia, kidney disease, and fatal thromboses.
PNH results from a lack of 2 proteins that protect RBCs from destruction: decay-accelerating factor (CD55), an inhibitor of alternative pathway C3 convertase, and protectin (CD59), an inhibitor of membrane attack complex (MAC) formation.
Because previous studies suggested that ATA selectively blocks complement activation at the C3 convertase stage and MAC formation at the C9 insertion stage, researchers thought ATA might prove effective against PNH.
First, they compared RBCs from 5 patients with PNH (who were on long-term treatment with eculizumab) to RBCs from healthy individuals.
Despite the eculizumab, the PNH patients’ RBCs were twice as vulnerable to complement-induced lysis as the healthy subjects’ RBCs. And western blot revealed both C3 and C5 convertases on the membranes of patients’ RBCs.
However, when the researchers added ATA to patients’ blood samples, the RBCs were protected from complement attack. In fact, the drug restored the RBCs’ resistance to the same level as normal RBCs.
“Our study suggests that ATA could offer more complete protection as an oral treatment for PNH, while eliminating the need for infusions,” said study author Patrick McGeer, MD, PhD, of the University of British Columbia in Vancouver, Canada.
“PNH is a disease that may happen to anyone through a chance mutation, and, if nature were to design a perfect fix for this mutation, it would be ATA.”
Dr McGeer added that many diseases are caused or worsened by an overactive complement immune system. So his group’s findings could have implications for conditions such as Alzheimer’s disease, Parkinson’s disease, macular degeneration, amyotrophic lateral sclerosis, multiple sclerosis, and rheumatoid arthritis.
He and his colleagues are now proceeding with further testing, and Dr McGeer expects ATA could be available in clinics within a year. ![]()
Measuring Agreement After CICU Handoffs
Increasing attention has been paid to the need for effective handoffs between healthcare providers since the Joint Commission identified standardized handoff protocols as a National Patient Safety Goal in 2006.1 Aside from adverse consequences for patients, poor handoffs produce provider uncertainty about care plans.[2, 3] Agreement on clinical information after a handoff is critical because a significant proportion of data is not documented in the medical record, leaving providers reliant on verbal communication.[4, 5, 6] Providers may enter the handoff with differing opinions; however, to mitigate the potential safety consequences of discontinuity of care,[7] the goal should be to achieve consensus about proposed courses of action.
Given the recent focus on improving handoffs, rigorous, outcome‐driven measures of handoff quality are clearly needed, but measuring shift‐to‐shift handoff quality has proved challenging.[8, 9] Previous studies of physician handoffs surveyed receivers for satisfaction,[10, 11] compared reported omissions to audio recordings,[3] and developed evaluation tools for receivers to rate handoffs.[12, 13, 14, 15] None directly assess the underlying goal of a handoff: the transfer of understanding from sender to receiver, enabling safe transfer of patient care responsibility.[16] We therefore chose to measure agreement on patient condition and treatment plans following handoff as an indicator of the quality of the shared clinical understanding formed. Advantages of piloting this approach in the pediatric cardiac intensive care unit (CICU) include the relatively homogenous patient population and small number of medical providers. If effective, the strategy of tool development and evaluation could be generalized to different clinical environments and provider groups.
Our aim was to develop and validate a tool to measure the level of shared clinical understanding regarding the condition and treatment plan of a CICU patient after handoff. The tool we designed was the pediatric cardiology Patient Knowledge Assessment Tool (PKAT), a brief, multiple‐item questionnaire focused on key data elements for individual CICU patients. Although variation in provider opinion helps detect diagnostic or treatment errors,[8] the PKAT is based on the assumption that achieving consensus on clinical status and the next steps of care is the goal of the handoff.
METHODS
Setting
The CICU is a 24‐bed medical and surgical unit in a 500‐bed free standing children's hospital. CICU attending physicians work 12‐ or 24‐hour shifts and supervise front line clinicians (including subspecialty fellows, nurse practitioners, and hospitalists, referred to as clinicians in this article) who work day or night shifts. Handoffs occur twice daily, with no significant differences in handoff practices between the 2 times. Attending physicians (referred to as attendings in this article) conduct parallel but separate handoffs from clinicians. All providers work exclusively in the CICU with the exception of fellows, who rotate monthly.
This study was approved by the institutional review board at The Children's Hospital of Philadelphia. All provider subjects provided informed consent. Consent for patient subjects was waived.
Development of the PKAT
We developed the PKAT content domains based on findings from previous studies,[2, 3] unpublished survey data about handoff omissions in our CICU, and CICU attending expert opinion. Pilot testing included 39 attendings and clinicians involved in 60 handoffs representing a wide variety of admissions. Participants were encouraged to share opinions on tool content and design with study staff. The PKAT (see Supporting Information, Appendix, in the online version of this article) was refined iteratively based on this feedback.
Video Simulation Testing
We used video simulation to test the PKAT for inter‐rater reliability. Nine patient handoff scenarios were written with varying levels of patient complexity and clarity of dialogue. The scenarios were filmed using the same actors and location to minimize variability aside from content. We recruited 10 experienced provider subjects (attendings and senior fellows) to minimize the effect of knowledge deficits. For each simulated handoff, subjects were encouraged to annotate a mock sign‐out sheet, which mimicked the content and format of the CICU sign‐out sheet. After watching all 9 scenarios, subjects completed a PKAT for each handoff from the perspective of the receiver based on the videotape. These standardized conditions allowed for assessment of inter‐rater reliability.
In Situ Testing
We then tested the PKAT in situ in the CICU to assess construct validity. We chose to study the morning handoff because the timing and location are more consistent. We planned to study 90 patient handoffs because the standard practice for testing a new psychometric instrument is to collect 10 observations per item.[17] On study days, 4 providers completed a PKAT for each selected handoff: the sending attending, receiving attending, sending clinician, and receiving clinician.
Study days were scheduled over 2 months to encompass a range of providers. Given the small number of attendings, we did not exclude those who had participated in video simulation testing. On study days, 6 patients were enrolled using stratified sampling to ensure adequate representation of new admissions (ie, admitted within 24 hours). The sending attending received the PKAT forms prior to the handoff. The receiving attending and clinicians received the PKAT after handoff. This difference in administration was due to logistic concerns: sending attendings requested to receive the PKATs earlier because they had to complete all 6 PKATs, whereas other providers completed 3 or fewer per day. Thus, sending attendings could complete the PKAT before or after the handoff, whereas all other participants completed the instrument after the handoff.
To test for construct validity, we gathered data on participating providers and patients, hypothesizing that PKAT agreement levels would decrease in response to less experienced providers or more complex patients. Provider characteristics included previous handoff education and amount of time worked in our CICU. Attending CICU experience was dichotomized into first year versus second or greater year. Clinician experience was dichotomized into first or second month versus third or greater month of CICU service. Each PKAT asked the handoff receiver whether he or she had recently cared for this patient or gathered information prior to handoff (eg, speaking to bedside nurse).
Recorded patient characteristics included age, length of stay, and admission type including neonatal/preoperative observation, postoperative (first 7 days after operation), prolonged postoperative (>7 days after operation), and medical (all others). In recognition of differences in handoffs during the first 24 hours of admission and the right‐skewed length of stay in the CICU, we analyzed length of stay based on the following categories: new admission (<24 hours), days 2 to 7, days 8 to 14, days 15 to 31, and >31 days. Because the number of active medications has been shown to correlate with treatment regimen complexity[18] and physician ratings of illness severity,[19] we recorded this number as a surrogate measure of patient complexity. For analytic purposes, we categorized the number of active medications into quartiles.
Provider subject characteristics and PKAT responses were collected using paper forms and entered into REDCap (Research Electronic Data Capture; REDCap Consortium,
Statistical Analysis
The primary outcome measure was the PKAT agreement level among providers evaluating the same handoff. For the reliability assessment, we calculated agreement across all providers analyzing the simulation videos, expecting that multiple providers should have high agreement for the same scenarios if the instrument has high inter‐rater reliability. For the validity assessment, we calculated agreement for each individual handoff by item and then calculated average levels of agreement for each item across provider and patient characteristics. We analyzed handoffs between attendings and clinicians separately. For items with mutually exclusive responses, simple yes/no agreement was calculated. For items requiring at least 1 response, agreement was coded when both respondents selected at least 1 response in common. For items that did not require a selection, credit was given if both subjects agreed that none of the conditions were present or if they agreed that at least 1 condition was present. In a secondary analysis, we repeated the analyses with unique sender‐receiver pair as the unit of analysis to account for correlation in the pair interaction.
Summary statistics were used to describe provider and patient characteristics. Mean rates of agreement with 95% confidence intervals were calculated for each item. The Wilcoxon rank sum test was used to compare mean results between groups (eg, attendings vs clinicians). A nonparametric test for trend, which is an extension of the Wilcoxon rank sum test,[21] was used to compare mean results across ordered categories (eg, length of stay). All tests of significance were at P<0.05 level and 2‐tailed. All statistical analysis was done using Stata 12 (StataCorp, College Station, TX).
RESULTS
Provider subject types are represented in Table 1. Handoffs between these 29 individuals resulted in 70 unique sender and receiver combinations with a median of 2 PKATs completed per unique sender‐receiver pair (range, 115). Attendings had lower rates of handoff education than clinicians (11% vs 85% for in situ testing participants, P=0.01). Attendings participating in in situ testing had worked in the CICU for a median of 3 years (range, 116 years). Clinicians participating in in situ testing had a median of 3 months of CICU experience (range, 195 months). Providers were 100% compliant with PKAT completion.
| Simulation Testing, n=10 | In Situ Testing, n=29 | |
|---|---|---|
| ||
| Attending physicians | 40% (4) | 31% (9) |
| Clinicians | 60% (6) | 69% (20) |
| Clinician type | ||
| Cardiology | 67% (4) | 35% (7) |
| Critical care medicine | 33% (2) | 25% (5) |
| CICU nurse practitioner | 25% (5) | |
| Anesthesia | 5% (1) | |
| Neonatology | 5% (1) | |
| Hospitalist | 5% (1) | |
Video Simulation Testing
Inter‐rater agreement is shown in Figure 1. Raters achieved perfect agreement for 8/9 questions on at least 1 scenario, supporting high inter‐rater reliability for these items. Some items had particularly high reliability. For example, on item 3, subjects achieved perfect agreement for 5/9 scenarios, making 1 both the median and maximum value. Because item 7 (barriers to transfer) did not demonstrate high inter‐rater agreement, we excluded it from the in situ analysis.
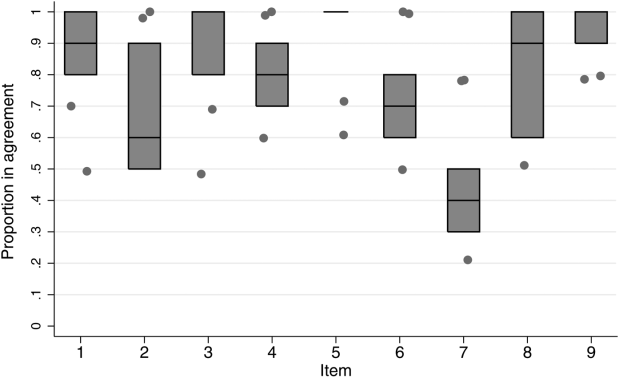
In Situ Testing
Characteristics of patients whose handoffs were selected for in situ testing are listed in Table 2. Because some patients were selected on multiple study days, these 90 handoffs represented 58 unique patients. These 58 patients are representative of the CICU population (data not shown). The number of handoffs studied per patient ranged from 1 to 7 (median 1). A total of 19 patients were included in the study more than once; 13 were included twice.
| Characteristic | Categories | Percentage |
|---|---|---|
| ||
| Age | <1 month | 30 |
| 112 months | 34 | |
| 112 years | 28 | |
| 1318 years | 6 | |
| >18 years | 2 | |
| Type of admission | Postnatal observation/preoperative | 20 |
| Postoperative | 29 | |
| Prolonged postoperative (>7 days) | 33 | |
| Other admission | 18 | |
| CICU days | 1 | 31 |
| 27 | 22 | |
| 814 | 10 | |
| 1531 | 13 | |
| >31 | 23 | |
| Active medications | <8 | 26 |
| 811 | 26 | |
| 1218 | 26 | |
| >18 | 23 | |
Rates of agreement between handoff pairs, stratified by attending versus clinician, are shown in Table 3. Overall mean levels of agreement ranged from 0.41 to 0.87 (median 0.77). Except for the ratio of pulmonary to systemic blood flow question, there were no significant differences in agreement between attendings as compared to clinicians. When this analysis was repeated with unique sender‐receiver pair as the unit of analysis to account for within‐pair clustering, we obtained qualitatively similar results (data not shown).
| PKAT Item | Agreement Level | ||||
|---|---|---|---|---|---|
| Attending Physician Pair | Clinician Pair | Pa | |||
| Mean | 95% CI | Mean | 95% CI | ||
| |||||
| Clinical condition | 0.71 | 0.620.81 | 0.78 | 0.690.87 | 0.31 |
| Cardiovascular plan | 0.76 | 0.670.85 | 0.68 | 0.580.78 | 0.25 |
| Respiratory plan | 0.67 | 0.580.78 | 0.76 | 0.670.85 | 0.26 |
| Source of pulmonary blood flow | 0.83 | 0.750.91 | 0.87 | 0.800.94 | 0.53 |
| Ratio of pulmonary to systemic flow | 0.67 | 0.570.77 | 0.41 | 0.310.51 | <0.01 |
| Anticoagulation indication | 0.79 | 0.700.87 | 0.77 | 0.680.86 | 0.72 |
| Active cardiovascular issues | 0.87 | 0.800.94 | 0.76 | 0.670.85 | 0.06 |
| Active noncardiovascular issues | 0.80 | 0.720.88 | 0.78 | 0.690.87 | 0.72 |
Both length of stay and increasing number of medications affected agreement levels for PKAT items (Table 4). Increasing length of stay correlated directly with agreement on cardiovascular plan and ratio of pulmonary to systemic flow and inversely with indication for anticoagulation. Increasing number of medications had an inverse correlation with agreement on indication for anticoagulation, active cardiovascular issues, and active noncardiovascular issues.
| Item | CICU LOS | No. of Active Medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day (n=56) | 27 Days (n=40) | 814 Days (n=18) | 1531 Days (n=24) | >31 Days (n=42) | Pa | 8 (n=46) | 811 (n=46) | 1218 (n=46) | >18 (n=42) | Pa | |
| |||||||||||
| Clinical condition | 0.75 | 0.63 | 0.78 | 0.83 | 0.79 | 0.29 | 0.71 | 0.70 | 0.78 | 0.79 | 0.32 |
| Cardiovascular plan | 0.59 | 0.73 | 0.67 | 0.79 | 0.86 | <0.01 | 0.63 | 0.72 | 0.63 | 0.81 | 0.16 |
| Respiratory plan | 0.68 | 0.78 | 0.61 | 0.83 | 0.69 | 0.79 | 0.67 | 0.72 | 0.78 | 0.69 | 0.68 |
| Source of pulmonary blood flow | 0.93 | 0.75 | 0.72 | 0.96 | 0.83 | 0.63 | 0.72 | 0.91 | 0.98 | 0.79 | 0.22 |
| Ratio of pulmonary to systemic flow | 0.45 | 0.40 | 0.67 | 0.75 | 0.62 | 0.01 | 0.46 | 0.52 | 0.52 | 0.67 | 0.06 |
| Anticoagulation indication | 0.89 | 0.83 | 0.89 | 0.67 | 0.60 | <0.01 | 0.93 | 0.78 | 0.76 | 0.62 | <0.01 |
| Active cardiovascular issues | 0.86 | 0.78 | 0.72 | 0.92 | 0.76 | 0.52 | 0.87 | 0.76 | 0.54 | 0.55 | <0.01 |
| Active noncardiovascular issues | 0.86 | 0.80 | 0.72 | 0.75 | 0.74 | 0.12 | 0.83 | 0.83 | 0.76 | 0.52 | <0.01 |
In contrast, there were no significant differences in item agreement levels based on provider characteristics, including experience, handoff education, prehandoff preparation, or continuity (data not shown).
CONCLUSIONS
Our results provide initial evidence of reliability and validity of scores for a novel tool, the PKAT, designed to assess providers' shared clinical understanding of a pediatric CICU patient's condition and treatment plan. Because this information should be mutually understood following any handoff, we believe this tool or similar agreement assessments could be used to measure handoff quality across a range of clinical settings. Under the standardized conditions of video simulation, experienced CICU providers achieved high levels of agreement on the PKAT, demonstrating inter‐rater reliability. In situ testing results suggest that the PKAT can validly identify differences in understanding between providers for both routine and complex patients.
The achievement of 100% compliance with in situ testing demonstrates that this type of tool can feasibly be used in a real‐time clinical environment. As expected, mean agreement levels in situ were lower than levels achieved in video simulation. By item, mean levels of agreement for attending and clinician pairs were similar.
Our assessment of PKAT validity demonstrated mixed results. On the one hand, PKAT agreement did not vary significantly by any measured provider characteristics. Consistent with the lack of difference between attendings and clinicians, more experienced providers in both groups did not achieve higher levels of agreement. This finding is surprising, and may illustrate that unmeasured provider characteristics, such as content knowledge, obscure the effects of experience or other measured variables on agreement levels. Alternatively, providing the PKAT to the sending attending prior to the handoff, rather than afterward as for the receiving attendings and clinicians, might have artificially lowered attending agreement levels, concealing a difference due to experience.
On the other hand, construct validity of several items was supported by the difference in agreement levels based on patient characteristics. Agreement levels varied on 5/8 questions as patients became more complex, either defined by length of stay or number of medications. These differences show that agreement on PKAT items responds to changes in handoff complexity, a form of construct validity. Furthermore, these findings suggest that handoffs of more chronic or complex patients may require more attention for components prone to disagreement in these settings. Although complexity and longer length of stay are nonmodifiable risk factors, identifying these handoffs as more susceptible to disagreement provides potential targets for intervention.
It is important to move beyond he said/she said evaluations to assess shared understanding after a handoff, because high fidelity transfer of information is necessary for safe transfer of responsibility. The PKAT addresses this key component of handoff quality in a novel fashion. Although high‐fidelity information transfer may correlate with receiving provider satisfaction, this relationship has not yet been explored. Future studies will evaluate the association between receiver evaluations of handoffs and PKAT agreement, as well as the relationship between PKAT performance and subsequent patient outcomes.
Limitations of this approach include the challenges inherent in reducing a complex understanding of a patient to a multiple‐item instrument. Furthermore, PKAT use may influence handoff content due to the Hawthorne effect. Although our analysis rests on the argument that agreement is the goal of a handoff, some differences of opinion within the care team enrich resilience. Regardless, to maintain continuity of care, providers need to reach agreement on the next steps in a patient's care during the handoff. Because we focused only on agreement, this approach does not compare respondents' answers to a verifiable source of truth, if it exists. Therefore, 2 respondents who agree on the wrong answer receive the same score as 2 who agree on the right answer. Other limitations include using the number of medications as a marker of handoff complexity. Finally, conducting this study in a single CICU limits generalizability. However, we believe that all PKAT items are generalizable to other pediatric CICUs, and that several are generalizable to other pediatric intensive care settings. The approach of measuring shared understanding could be generalized more widely with development of items specific to different clinical settings.
Because the PKAT can be completed and scored quickly, it could be used as a real‐time measure of quality improvement interventions such as the introduction of a standardized handoff protocol. Alternatively, provider pairs could use the PKAT as a final handoff safety check to confirm consensus before transfer of responsibility. The concept of measuring shared clinical understanding could be extended to develop similar instruments for different clinical settings.
Acknowledgements
The authors thank the CICU providers for their enthusiasm for and participation in this study. The authors also thank Margaret Wolff, MD, Newton Buchanan, and the Center for Simulation, Advanced Education and Innovation at The Children's Hospital of Philadelphia for assistance in filming the video scenarios.
Disclosures: Dr. Bates was supported in part by NICHD/T32 HD060550 and NHLBI/T32 HL07915 grant funding. Dr. Metlay was supported by a Mid‐Career Investigator Award in Patient Oriented Research (K24‐AI073957). The authors report no conflicts of interest.
- , . The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Quality and Safety in Health Care. 2010;19(6):493–497. doi: 10.1136/qshc.2009.033480.
- . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , , . Nursing handovers: do we really need them? J Nurs Manag. 2004;12(1):37–42.
- , , , et al. Assessing clinical handover between paramedics and the trauma team. Injury. 2010;41(5):460–464.
- , , , , . Answering questions on call: Pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , . Patient handoffs: standardized and reliable measurement tools remain elusive. Jt Comm J Qual Patient Saf. 2010;36(2):52–61.
- , , , , . Improving measurement in clinical handover. Qual Saf Health Care. 2009;18(4):272–276.
- , , , . Adequacy of information transferred at resident sign‐out (inhospital handover of care): a prospective survey. Qual Saf Health Care. 2008;17(1):6–10.
- , , . Standardized Sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2009;25(2):129–134.
- , , , , . Assessing the quality of patient handoffs at care transitions. Qual Saf Health Care. 2010;19(6):1–5.
- , , , , . Implementing peer evaluation of handoffs: associations with experience and workload. J Hosp Med. 2013;8(3):132–136.
- , , , et al. Development of a handoff evaluation tool for shift‐to‐shift physician handoffs: the handoff CEX. J Hosp Med. 2013;8(4):191–200.
- , . The effects of patient handoff characteristics on subsequent care: a systematic review and areas for future research. Acad Med. 2012;87(8):1105–1124.
- . Construct validity in organizational behavior. In: Cummings LL, Stawe BM, eds. Research in Organizational Behavior. Vol 2. Greenwich, CT: JAI Press; 1980:3–43.
- , , , , . Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–1376.
- , , . A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
Increasing attention has been paid to the need for effective handoffs between healthcare providers since the Joint Commission identified standardized handoff protocols as a National Patient Safety Goal in 2006.1 Aside from adverse consequences for patients, poor handoffs produce provider uncertainty about care plans.[2, 3] Agreement on clinical information after a handoff is critical because a significant proportion of data is not documented in the medical record, leaving providers reliant on verbal communication.[4, 5, 6] Providers may enter the handoff with differing opinions; however, to mitigate the potential safety consequences of discontinuity of care,[7] the goal should be to achieve consensus about proposed courses of action.
Given the recent focus on improving handoffs, rigorous, outcome‐driven measures of handoff quality are clearly needed, but measuring shift‐to‐shift handoff quality has proved challenging.[8, 9] Previous studies of physician handoffs surveyed receivers for satisfaction,[10, 11] compared reported omissions to audio recordings,[3] and developed evaluation tools for receivers to rate handoffs.[12, 13, 14, 15] None directly assess the underlying goal of a handoff: the transfer of understanding from sender to receiver, enabling safe transfer of patient care responsibility.[16] We therefore chose to measure agreement on patient condition and treatment plans following handoff as an indicator of the quality of the shared clinical understanding formed. Advantages of piloting this approach in the pediatric cardiac intensive care unit (CICU) include the relatively homogenous patient population and small number of medical providers. If effective, the strategy of tool development and evaluation could be generalized to different clinical environments and provider groups.
Our aim was to develop and validate a tool to measure the level of shared clinical understanding regarding the condition and treatment plan of a CICU patient after handoff. The tool we designed was the pediatric cardiology Patient Knowledge Assessment Tool (PKAT), a brief, multiple‐item questionnaire focused on key data elements for individual CICU patients. Although variation in provider opinion helps detect diagnostic or treatment errors,[8] the PKAT is based on the assumption that achieving consensus on clinical status and the next steps of care is the goal of the handoff.
METHODS
Setting
The CICU is a 24‐bed medical and surgical unit in a 500‐bed free standing children's hospital. CICU attending physicians work 12‐ or 24‐hour shifts and supervise front line clinicians (including subspecialty fellows, nurse practitioners, and hospitalists, referred to as clinicians in this article) who work day or night shifts. Handoffs occur twice daily, with no significant differences in handoff practices between the 2 times. Attending physicians (referred to as attendings in this article) conduct parallel but separate handoffs from clinicians. All providers work exclusively in the CICU with the exception of fellows, who rotate monthly.
This study was approved by the institutional review board at The Children's Hospital of Philadelphia. All provider subjects provided informed consent. Consent for patient subjects was waived.
Development of the PKAT
We developed the PKAT content domains based on findings from previous studies,[2, 3] unpublished survey data about handoff omissions in our CICU, and CICU attending expert opinion. Pilot testing included 39 attendings and clinicians involved in 60 handoffs representing a wide variety of admissions. Participants were encouraged to share opinions on tool content and design with study staff. The PKAT (see Supporting Information, Appendix, in the online version of this article) was refined iteratively based on this feedback.
Video Simulation Testing
We used video simulation to test the PKAT for inter‐rater reliability. Nine patient handoff scenarios were written with varying levels of patient complexity and clarity of dialogue. The scenarios were filmed using the same actors and location to minimize variability aside from content. We recruited 10 experienced provider subjects (attendings and senior fellows) to minimize the effect of knowledge deficits. For each simulated handoff, subjects were encouraged to annotate a mock sign‐out sheet, which mimicked the content and format of the CICU sign‐out sheet. After watching all 9 scenarios, subjects completed a PKAT for each handoff from the perspective of the receiver based on the videotape. These standardized conditions allowed for assessment of inter‐rater reliability.
In Situ Testing
We then tested the PKAT in situ in the CICU to assess construct validity. We chose to study the morning handoff because the timing and location are more consistent. We planned to study 90 patient handoffs because the standard practice for testing a new psychometric instrument is to collect 10 observations per item.[17] On study days, 4 providers completed a PKAT for each selected handoff: the sending attending, receiving attending, sending clinician, and receiving clinician.
Study days were scheduled over 2 months to encompass a range of providers. Given the small number of attendings, we did not exclude those who had participated in video simulation testing. On study days, 6 patients were enrolled using stratified sampling to ensure adequate representation of new admissions (ie, admitted within 24 hours). The sending attending received the PKAT forms prior to the handoff. The receiving attending and clinicians received the PKAT after handoff. This difference in administration was due to logistic concerns: sending attendings requested to receive the PKATs earlier because they had to complete all 6 PKATs, whereas other providers completed 3 or fewer per day. Thus, sending attendings could complete the PKAT before or after the handoff, whereas all other participants completed the instrument after the handoff.
To test for construct validity, we gathered data on participating providers and patients, hypothesizing that PKAT agreement levels would decrease in response to less experienced providers or more complex patients. Provider characteristics included previous handoff education and amount of time worked in our CICU. Attending CICU experience was dichotomized into first year versus second or greater year. Clinician experience was dichotomized into first or second month versus third or greater month of CICU service. Each PKAT asked the handoff receiver whether he or she had recently cared for this patient or gathered information prior to handoff (eg, speaking to bedside nurse).
Recorded patient characteristics included age, length of stay, and admission type including neonatal/preoperative observation, postoperative (first 7 days after operation), prolonged postoperative (>7 days after operation), and medical (all others). In recognition of differences in handoffs during the first 24 hours of admission and the right‐skewed length of stay in the CICU, we analyzed length of stay based on the following categories: new admission (<24 hours), days 2 to 7, days 8 to 14, days 15 to 31, and >31 days. Because the number of active medications has been shown to correlate with treatment regimen complexity[18] and physician ratings of illness severity,[19] we recorded this number as a surrogate measure of patient complexity. For analytic purposes, we categorized the number of active medications into quartiles.
Provider subject characteristics and PKAT responses were collected using paper forms and entered into REDCap (Research Electronic Data Capture; REDCap Consortium,
Statistical Analysis
The primary outcome measure was the PKAT agreement level among providers evaluating the same handoff. For the reliability assessment, we calculated agreement across all providers analyzing the simulation videos, expecting that multiple providers should have high agreement for the same scenarios if the instrument has high inter‐rater reliability. For the validity assessment, we calculated agreement for each individual handoff by item and then calculated average levels of agreement for each item across provider and patient characteristics. We analyzed handoffs between attendings and clinicians separately. For items with mutually exclusive responses, simple yes/no agreement was calculated. For items requiring at least 1 response, agreement was coded when both respondents selected at least 1 response in common. For items that did not require a selection, credit was given if both subjects agreed that none of the conditions were present or if they agreed that at least 1 condition was present. In a secondary analysis, we repeated the analyses with unique sender‐receiver pair as the unit of analysis to account for correlation in the pair interaction.
Summary statistics were used to describe provider and patient characteristics. Mean rates of agreement with 95% confidence intervals were calculated for each item. The Wilcoxon rank sum test was used to compare mean results between groups (eg, attendings vs clinicians). A nonparametric test for trend, which is an extension of the Wilcoxon rank sum test,[21] was used to compare mean results across ordered categories (eg, length of stay). All tests of significance were at P<0.05 level and 2‐tailed. All statistical analysis was done using Stata 12 (StataCorp, College Station, TX).
RESULTS
Provider subject types are represented in Table 1. Handoffs between these 29 individuals resulted in 70 unique sender and receiver combinations with a median of 2 PKATs completed per unique sender‐receiver pair (range, 115). Attendings had lower rates of handoff education than clinicians (11% vs 85% for in situ testing participants, P=0.01). Attendings participating in in situ testing had worked in the CICU for a median of 3 years (range, 116 years). Clinicians participating in in situ testing had a median of 3 months of CICU experience (range, 195 months). Providers were 100% compliant with PKAT completion.
| Simulation Testing, n=10 | In Situ Testing, n=29 | |
|---|---|---|
| ||
| Attending physicians | 40% (4) | 31% (9) |
| Clinicians | 60% (6) | 69% (20) |
| Clinician type | ||
| Cardiology | 67% (4) | 35% (7) |
| Critical care medicine | 33% (2) | 25% (5) |
| CICU nurse practitioner | 25% (5) | |
| Anesthesia | 5% (1) | |
| Neonatology | 5% (1) | |
| Hospitalist | 5% (1) | |
Video Simulation Testing
Inter‐rater agreement is shown in Figure 1. Raters achieved perfect agreement for 8/9 questions on at least 1 scenario, supporting high inter‐rater reliability for these items. Some items had particularly high reliability. For example, on item 3, subjects achieved perfect agreement for 5/9 scenarios, making 1 both the median and maximum value. Because item 7 (barriers to transfer) did not demonstrate high inter‐rater agreement, we excluded it from the in situ analysis.

In Situ Testing
Characteristics of patients whose handoffs were selected for in situ testing are listed in Table 2. Because some patients were selected on multiple study days, these 90 handoffs represented 58 unique patients. These 58 patients are representative of the CICU population (data not shown). The number of handoffs studied per patient ranged from 1 to 7 (median 1). A total of 19 patients were included in the study more than once; 13 were included twice.
| Characteristic | Categories | Percentage |
|---|---|---|
| ||
| Age | <1 month | 30 |
| 112 months | 34 | |
| 112 years | 28 | |
| 1318 years | 6 | |
| >18 years | 2 | |
| Type of admission | Postnatal observation/preoperative | 20 |
| Postoperative | 29 | |
| Prolonged postoperative (>7 days) | 33 | |
| Other admission | 18 | |
| CICU days | 1 | 31 |
| 27 | 22 | |
| 814 | 10 | |
| 1531 | 13 | |
| >31 | 23 | |
| Active medications | <8 | 26 |
| 811 | 26 | |
| 1218 | 26 | |
| >18 | 23 | |
Rates of agreement between handoff pairs, stratified by attending versus clinician, are shown in Table 3. Overall mean levels of agreement ranged from 0.41 to 0.87 (median 0.77). Except for the ratio of pulmonary to systemic blood flow question, there were no significant differences in agreement between attendings as compared to clinicians. When this analysis was repeated with unique sender‐receiver pair as the unit of analysis to account for within‐pair clustering, we obtained qualitatively similar results (data not shown).
| PKAT Item | Agreement Level | ||||
|---|---|---|---|---|---|
| Attending Physician Pair | Clinician Pair | Pa | |||
| Mean | 95% CI | Mean | 95% CI | ||
| |||||
| Clinical condition | 0.71 | 0.620.81 | 0.78 | 0.690.87 | 0.31 |
| Cardiovascular plan | 0.76 | 0.670.85 | 0.68 | 0.580.78 | 0.25 |
| Respiratory plan | 0.67 | 0.580.78 | 0.76 | 0.670.85 | 0.26 |
| Source of pulmonary blood flow | 0.83 | 0.750.91 | 0.87 | 0.800.94 | 0.53 |
| Ratio of pulmonary to systemic flow | 0.67 | 0.570.77 | 0.41 | 0.310.51 | <0.01 |
| Anticoagulation indication | 0.79 | 0.700.87 | 0.77 | 0.680.86 | 0.72 |
| Active cardiovascular issues | 0.87 | 0.800.94 | 0.76 | 0.670.85 | 0.06 |
| Active noncardiovascular issues | 0.80 | 0.720.88 | 0.78 | 0.690.87 | 0.72 |
Both length of stay and increasing number of medications affected agreement levels for PKAT items (Table 4). Increasing length of stay correlated directly with agreement on cardiovascular plan and ratio of pulmonary to systemic flow and inversely with indication for anticoagulation. Increasing number of medications had an inverse correlation with agreement on indication for anticoagulation, active cardiovascular issues, and active noncardiovascular issues.
| Item | CICU LOS | No. of Active Medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day (n=56) | 27 Days (n=40) | 814 Days (n=18) | 1531 Days (n=24) | >31 Days (n=42) | Pa | 8 (n=46) | 811 (n=46) | 1218 (n=46) | >18 (n=42) | Pa | |
| |||||||||||
| Clinical condition | 0.75 | 0.63 | 0.78 | 0.83 | 0.79 | 0.29 | 0.71 | 0.70 | 0.78 | 0.79 | 0.32 |
| Cardiovascular plan | 0.59 | 0.73 | 0.67 | 0.79 | 0.86 | <0.01 | 0.63 | 0.72 | 0.63 | 0.81 | 0.16 |
| Respiratory plan | 0.68 | 0.78 | 0.61 | 0.83 | 0.69 | 0.79 | 0.67 | 0.72 | 0.78 | 0.69 | 0.68 |
| Source of pulmonary blood flow | 0.93 | 0.75 | 0.72 | 0.96 | 0.83 | 0.63 | 0.72 | 0.91 | 0.98 | 0.79 | 0.22 |
| Ratio of pulmonary to systemic flow | 0.45 | 0.40 | 0.67 | 0.75 | 0.62 | 0.01 | 0.46 | 0.52 | 0.52 | 0.67 | 0.06 |
| Anticoagulation indication | 0.89 | 0.83 | 0.89 | 0.67 | 0.60 | <0.01 | 0.93 | 0.78 | 0.76 | 0.62 | <0.01 |
| Active cardiovascular issues | 0.86 | 0.78 | 0.72 | 0.92 | 0.76 | 0.52 | 0.87 | 0.76 | 0.54 | 0.55 | <0.01 |
| Active noncardiovascular issues | 0.86 | 0.80 | 0.72 | 0.75 | 0.74 | 0.12 | 0.83 | 0.83 | 0.76 | 0.52 | <0.01 |
In contrast, there were no significant differences in item agreement levels based on provider characteristics, including experience, handoff education, prehandoff preparation, or continuity (data not shown).
CONCLUSIONS
Our results provide initial evidence of reliability and validity of scores for a novel tool, the PKAT, designed to assess providers' shared clinical understanding of a pediatric CICU patient's condition and treatment plan. Because this information should be mutually understood following any handoff, we believe this tool or similar agreement assessments could be used to measure handoff quality across a range of clinical settings. Under the standardized conditions of video simulation, experienced CICU providers achieved high levels of agreement on the PKAT, demonstrating inter‐rater reliability. In situ testing results suggest that the PKAT can validly identify differences in understanding between providers for both routine and complex patients.
The achievement of 100% compliance with in situ testing demonstrates that this type of tool can feasibly be used in a real‐time clinical environment. As expected, mean agreement levels in situ were lower than levels achieved in video simulation. By item, mean levels of agreement for attending and clinician pairs were similar.
Our assessment of PKAT validity demonstrated mixed results. On the one hand, PKAT agreement did not vary significantly by any measured provider characteristics. Consistent with the lack of difference between attendings and clinicians, more experienced providers in both groups did not achieve higher levels of agreement. This finding is surprising, and may illustrate that unmeasured provider characteristics, such as content knowledge, obscure the effects of experience or other measured variables on agreement levels. Alternatively, providing the PKAT to the sending attending prior to the handoff, rather than afterward as for the receiving attendings and clinicians, might have artificially lowered attending agreement levels, concealing a difference due to experience.
On the other hand, construct validity of several items was supported by the difference in agreement levels based on patient characteristics. Agreement levels varied on 5/8 questions as patients became more complex, either defined by length of stay or number of medications. These differences show that agreement on PKAT items responds to changes in handoff complexity, a form of construct validity. Furthermore, these findings suggest that handoffs of more chronic or complex patients may require more attention for components prone to disagreement in these settings. Although complexity and longer length of stay are nonmodifiable risk factors, identifying these handoffs as more susceptible to disagreement provides potential targets for intervention.
It is important to move beyond he said/she said evaluations to assess shared understanding after a handoff, because high fidelity transfer of information is necessary for safe transfer of responsibility. The PKAT addresses this key component of handoff quality in a novel fashion. Although high‐fidelity information transfer may correlate with receiving provider satisfaction, this relationship has not yet been explored. Future studies will evaluate the association between receiver evaluations of handoffs and PKAT agreement, as well as the relationship between PKAT performance and subsequent patient outcomes.
Limitations of this approach include the challenges inherent in reducing a complex understanding of a patient to a multiple‐item instrument. Furthermore, PKAT use may influence handoff content due to the Hawthorne effect. Although our analysis rests on the argument that agreement is the goal of a handoff, some differences of opinion within the care team enrich resilience. Regardless, to maintain continuity of care, providers need to reach agreement on the next steps in a patient's care during the handoff. Because we focused only on agreement, this approach does not compare respondents' answers to a verifiable source of truth, if it exists. Therefore, 2 respondents who agree on the wrong answer receive the same score as 2 who agree on the right answer. Other limitations include using the number of medications as a marker of handoff complexity. Finally, conducting this study in a single CICU limits generalizability. However, we believe that all PKAT items are generalizable to other pediatric CICUs, and that several are generalizable to other pediatric intensive care settings. The approach of measuring shared understanding could be generalized more widely with development of items specific to different clinical settings.
Because the PKAT can be completed and scored quickly, it could be used as a real‐time measure of quality improvement interventions such as the introduction of a standardized handoff protocol. Alternatively, provider pairs could use the PKAT as a final handoff safety check to confirm consensus before transfer of responsibility. The concept of measuring shared clinical understanding could be extended to develop similar instruments for different clinical settings.
Acknowledgements
The authors thank the CICU providers for their enthusiasm for and participation in this study. The authors also thank Margaret Wolff, MD, Newton Buchanan, and the Center for Simulation, Advanced Education and Innovation at The Children's Hospital of Philadelphia for assistance in filming the video scenarios.
Disclosures: Dr. Bates was supported in part by NICHD/T32 HD060550 and NHLBI/T32 HL07915 grant funding. Dr. Metlay was supported by a Mid‐Career Investigator Award in Patient Oriented Research (K24‐AI073957). The authors report no conflicts of interest.
Increasing attention has been paid to the need for effective handoffs between healthcare providers since the Joint Commission identified standardized handoff protocols as a National Patient Safety Goal in 2006.1 Aside from adverse consequences for patients, poor handoffs produce provider uncertainty about care plans.[2, 3] Agreement on clinical information after a handoff is critical because a significant proportion of data is not documented in the medical record, leaving providers reliant on verbal communication.[4, 5, 6] Providers may enter the handoff with differing opinions; however, to mitigate the potential safety consequences of discontinuity of care,[7] the goal should be to achieve consensus about proposed courses of action.
Given the recent focus on improving handoffs, rigorous, outcome‐driven measures of handoff quality are clearly needed, but measuring shift‐to‐shift handoff quality has proved challenging.[8, 9] Previous studies of physician handoffs surveyed receivers for satisfaction,[10, 11] compared reported omissions to audio recordings,[3] and developed evaluation tools for receivers to rate handoffs.[12, 13, 14, 15] None directly assess the underlying goal of a handoff: the transfer of understanding from sender to receiver, enabling safe transfer of patient care responsibility.[16] We therefore chose to measure agreement on patient condition and treatment plans following handoff as an indicator of the quality of the shared clinical understanding formed. Advantages of piloting this approach in the pediatric cardiac intensive care unit (CICU) include the relatively homogenous patient population and small number of medical providers. If effective, the strategy of tool development and evaluation could be generalized to different clinical environments and provider groups.
Our aim was to develop and validate a tool to measure the level of shared clinical understanding regarding the condition and treatment plan of a CICU patient after handoff. The tool we designed was the pediatric cardiology Patient Knowledge Assessment Tool (PKAT), a brief, multiple‐item questionnaire focused on key data elements for individual CICU patients. Although variation in provider opinion helps detect diagnostic or treatment errors,[8] the PKAT is based on the assumption that achieving consensus on clinical status and the next steps of care is the goal of the handoff.
METHODS
Setting
The CICU is a 24‐bed medical and surgical unit in a 500‐bed free standing children's hospital. CICU attending physicians work 12‐ or 24‐hour shifts and supervise front line clinicians (including subspecialty fellows, nurse practitioners, and hospitalists, referred to as clinicians in this article) who work day or night shifts. Handoffs occur twice daily, with no significant differences in handoff practices between the 2 times. Attending physicians (referred to as attendings in this article) conduct parallel but separate handoffs from clinicians. All providers work exclusively in the CICU with the exception of fellows, who rotate monthly.
This study was approved by the institutional review board at The Children's Hospital of Philadelphia. All provider subjects provided informed consent. Consent for patient subjects was waived.
Development of the PKAT
We developed the PKAT content domains based on findings from previous studies,[2, 3] unpublished survey data about handoff omissions in our CICU, and CICU attending expert opinion. Pilot testing included 39 attendings and clinicians involved in 60 handoffs representing a wide variety of admissions. Participants were encouraged to share opinions on tool content and design with study staff. The PKAT (see Supporting Information, Appendix, in the online version of this article) was refined iteratively based on this feedback.
Video Simulation Testing
We used video simulation to test the PKAT for inter‐rater reliability. Nine patient handoff scenarios were written with varying levels of patient complexity and clarity of dialogue. The scenarios were filmed using the same actors and location to minimize variability aside from content. We recruited 10 experienced provider subjects (attendings and senior fellows) to minimize the effect of knowledge deficits. For each simulated handoff, subjects were encouraged to annotate a mock sign‐out sheet, which mimicked the content and format of the CICU sign‐out sheet. After watching all 9 scenarios, subjects completed a PKAT for each handoff from the perspective of the receiver based on the videotape. These standardized conditions allowed for assessment of inter‐rater reliability.
In Situ Testing
We then tested the PKAT in situ in the CICU to assess construct validity. We chose to study the morning handoff because the timing and location are more consistent. We planned to study 90 patient handoffs because the standard practice for testing a new psychometric instrument is to collect 10 observations per item.[17] On study days, 4 providers completed a PKAT for each selected handoff: the sending attending, receiving attending, sending clinician, and receiving clinician.
Study days were scheduled over 2 months to encompass a range of providers. Given the small number of attendings, we did not exclude those who had participated in video simulation testing. On study days, 6 patients were enrolled using stratified sampling to ensure adequate representation of new admissions (ie, admitted within 24 hours). The sending attending received the PKAT forms prior to the handoff. The receiving attending and clinicians received the PKAT after handoff. This difference in administration was due to logistic concerns: sending attendings requested to receive the PKATs earlier because they had to complete all 6 PKATs, whereas other providers completed 3 or fewer per day. Thus, sending attendings could complete the PKAT before or after the handoff, whereas all other participants completed the instrument after the handoff.
To test for construct validity, we gathered data on participating providers and patients, hypothesizing that PKAT agreement levels would decrease in response to less experienced providers or more complex patients. Provider characteristics included previous handoff education and amount of time worked in our CICU. Attending CICU experience was dichotomized into first year versus second or greater year. Clinician experience was dichotomized into first or second month versus third or greater month of CICU service. Each PKAT asked the handoff receiver whether he or she had recently cared for this patient or gathered information prior to handoff (eg, speaking to bedside nurse).
Recorded patient characteristics included age, length of stay, and admission type including neonatal/preoperative observation, postoperative (first 7 days after operation), prolonged postoperative (>7 days after operation), and medical (all others). In recognition of differences in handoffs during the first 24 hours of admission and the right‐skewed length of stay in the CICU, we analyzed length of stay based on the following categories: new admission (<24 hours), days 2 to 7, days 8 to 14, days 15 to 31, and >31 days. Because the number of active medications has been shown to correlate with treatment regimen complexity[18] and physician ratings of illness severity,[19] we recorded this number as a surrogate measure of patient complexity. For analytic purposes, we categorized the number of active medications into quartiles.
Provider subject characteristics and PKAT responses were collected using paper forms and entered into REDCap (Research Electronic Data Capture; REDCap Consortium,
Statistical Analysis
The primary outcome measure was the PKAT agreement level among providers evaluating the same handoff. For the reliability assessment, we calculated agreement across all providers analyzing the simulation videos, expecting that multiple providers should have high agreement for the same scenarios if the instrument has high inter‐rater reliability. For the validity assessment, we calculated agreement for each individual handoff by item and then calculated average levels of agreement for each item across provider and patient characteristics. We analyzed handoffs between attendings and clinicians separately. For items with mutually exclusive responses, simple yes/no agreement was calculated. For items requiring at least 1 response, agreement was coded when both respondents selected at least 1 response in common. For items that did not require a selection, credit was given if both subjects agreed that none of the conditions were present or if they agreed that at least 1 condition was present. In a secondary analysis, we repeated the analyses with unique sender‐receiver pair as the unit of analysis to account for correlation in the pair interaction.
Summary statistics were used to describe provider and patient characteristics. Mean rates of agreement with 95% confidence intervals were calculated for each item. The Wilcoxon rank sum test was used to compare mean results between groups (eg, attendings vs clinicians). A nonparametric test for trend, which is an extension of the Wilcoxon rank sum test,[21] was used to compare mean results across ordered categories (eg, length of stay). All tests of significance were at P<0.05 level and 2‐tailed. All statistical analysis was done using Stata 12 (StataCorp, College Station, TX).
RESULTS
Provider subject types are represented in Table 1. Handoffs between these 29 individuals resulted in 70 unique sender and receiver combinations with a median of 2 PKATs completed per unique sender‐receiver pair (range, 115). Attendings had lower rates of handoff education than clinicians (11% vs 85% for in situ testing participants, P=0.01). Attendings participating in in situ testing had worked in the CICU for a median of 3 years (range, 116 years). Clinicians participating in in situ testing had a median of 3 months of CICU experience (range, 195 months). Providers were 100% compliant with PKAT completion.
| Simulation Testing, n=10 | In Situ Testing, n=29 | |
|---|---|---|
| ||
| Attending physicians | 40% (4) | 31% (9) |
| Clinicians | 60% (6) | 69% (20) |
| Clinician type | ||
| Cardiology | 67% (4) | 35% (7) |
| Critical care medicine | 33% (2) | 25% (5) |
| CICU nurse practitioner | 25% (5) | |
| Anesthesia | 5% (1) | |
| Neonatology | 5% (1) | |
| Hospitalist | 5% (1) | |
Video Simulation Testing
Inter‐rater agreement is shown in Figure 1. Raters achieved perfect agreement for 8/9 questions on at least 1 scenario, supporting high inter‐rater reliability for these items. Some items had particularly high reliability. For example, on item 3, subjects achieved perfect agreement for 5/9 scenarios, making 1 both the median and maximum value. Because item 7 (barriers to transfer) did not demonstrate high inter‐rater agreement, we excluded it from the in situ analysis.

In Situ Testing
Characteristics of patients whose handoffs were selected for in situ testing are listed in Table 2. Because some patients were selected on multiple study days, these 90 handoffs represented 58 unique patients. These 58 patients are representative of the CICU population (data not shown). The number of handoffs studied per patient ranged from 1 to 7 (median 1). A total of 19 patients were included in the study more than once; 13 were included twice.
| Characteristic | Categories | Percentage |
|---|---|---|
| ||
| Age | <1 month | 30 |
| 112 months | 34 | |
| 112 years | 28 | |
| 1318 years | 6 | |
| >18 years | 2 | |
| Type of admission | Postnatal observation/preoperative | 20 |
| Postoperative | 29 | |
| Prolonged postoperative (>7 days) | 33 | |
| Other admission | 18 | |
| CICU days | 1 | 31 |
| 27 | 22 | |
| 814 | 10 | |
| 1531 | 13 | |
| >31 | 23 | |
| Active medications | <8 | 26 |
| 811 | 26 | |
| 1218 | 26 | |
| >18 | 23 | |
Rates of agreement between handoff pairs, stratified by attending versus clinician, are shown in Table 3. Overall mean levels of agreement ranged from 0.41 to 0.87 (median 0.77). Except for the ratio of pulmonary to systemic blood flow question, there were no significant differences in agreement between attendings as compared to clinicians. When this analysis was repeated with unique sender‐receiver pair as the unit of analysis to account for within‐pair clustering, we obtained qualitatively similar results (data not shown).
| PKAT Item | Agreement Level | ||||
|---|---|---|---|---|---|
| Attending Physician Pair | Clinician Pair | Pa | |||
| Mean | 95% CI | Mean | 95% CI | ||
| |||||
| Clinical condition | 0.71 | 0.620.81 | 0.78 | 0.690.87 | 0.31 |
| Cardiovascular plan | 0.76 | 0.670.85 | 0.68 | 0.580.78 | 0.25 |
| Respiratory plan | 0.67 | 0.580.78 | 0.76 | 0.670.85 | 0.26 |
| Source of pulmonary blood flow | 0.83 | 0.750.91 | 0.87 | 0.800.94 | 0.53 |
| Ratio of pulmonary to systemic flow | 0.67 | 0.570.77 | 0.41 | 0.310.51 | <0.01 |
| Anticoagulation indication | 0.79 | 0.700.87 | 0.77 | 0.680.86 | 0.72 |
| Active cardiovascular issues | 0.87 | 0.800.94 | 0.76 | 0.670.85 | 0.06 |
| Active noncardiovascular issues | 0.80 | 0.720.88 | 0.78 | 0.690.87 | 0.72 |
Both length of stay and increasing number of medications affected agreement levels for PKAT items (Table 4). Increasing length of stay correlated directly with agreement on cardiovascular plan and ratio of pulmonary to systemic flow and inversely with indication for anticoagulation. Increasing number of medications had an inverse correlation with agreement on indication for anticoagulation, active cardiovascular issues, and active noncardiovascular issues.
| Item | CICU LOS | No. of Active Medications | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 Day (n=56) | 27 Days (n=40) | 814 Days (n=18) | 1531 Days (n=24) | >31 Days (n=42) | Pa | 8 (n=46) | 811 (n=46) | 1218 (n=46) | >18 (n=42) | Pa | |
| |||||||||||
| Clinical condition | 0.75 | 0.63 | 0.78 | 0.83 | 0.79 | 0.29 | 0.71 | 0.70 | 0.78 | 0.79 | 0.32 |
| Cardiovascular plan | 0.59 | 0.73 | 0.67 | 0.79 | 0.86 | <0.01 | 0.63 | 0.72 | 0.63 | 0.81 | 0.16 |
| Respiratory plan | 0.68 | 0.78 | 0.61 | 0.83 | 0.69 | 0.79 | 0.67 | 0.72 | 0.78 | 0.69 | 0.68 |
| Source of pulmonary blood flow | 0.93 | 0.75 | 0.72 | 0.96 | 0.83 | 0.63 | 0.72 | 0.91 | 0.98 | 0.79 | 0.22 |
| Ratio of pulmonary to systemic flow | 0.45 | 0.40 | 0.67 | 0.75 | 0.62 | 0.01 | 0.46 | 0.52 | 0.52 | 0.67 | 0.06 |
| Anticoagulation indication | 0.89 | 0.83 | 0.89 | 0.67 | 0.60 | <0.01 | 0.93 | 0.78 | 0.76 | 0.62 | <0.01 |
| Active cardiovascular issues | 0.86 | 0.78 | 0.72 | 0.92 | 0.76 | 0.52 | 0.87 | 0.76 | 0.54 | 0.55 | <0.01 |
| Active noncardiovascular issues | 0.86 | 0.80 | 0.72 | 0.75 | 0.74 | 0.12 | 0.83 | 0.83 | 0.76 | 0.52 | <0.01 |
In contrast, there were no significant differences in item agreement levels based on provider characteristics, including experience, handoff education, prehandoff preparation, or continuity (data not shown).
CONCLUSIONS
Our results provide initial evidence of reliability and validity of scores for a novel tool, the PKAT, designed to assess providers' shared clinical understanding of a pediatric CICU patient's condition and treatment plan. Because this information should be mutually understood following any handoff, we believe this tool or similar agreement assessments could be used to measure handoff quality across a range of clinical settings. Under the standardized conditions of video simulation, experienced CICU providers achieved high levels of agreement on the PKAT, demonstrating inter‐rater reliability. In situ testing results suggest that the PKAT can validly identify differences in understanding between providers for both routine and complex patients.
The achievement of 100% compliance with in situ testing demonstrates that this type of tool can feasibly be used in a real‐time clinical environment. As expected, mean agreement levels in situ were lower than levels achieved in video simulation. By item, mean levels of agreement for attending and clinician pairs were similar.
Our assessment of PKAT validity demonstrated mixed results. On the one hand, PKAT agreement did not vary significantly by any measured provider characteristics. Consistent with the lack of difference between attendings and clinicians, more experienced providers in both groups did not achieve higher levels of agreement. This finding is surprising, and may illustrate that unmeasured provider characteristics, such as content knowledge, obscure the effects of experience or other measured variables on agreement levels. Alternatively, providing the PKAT to the sending attending prior to the handoff, rather than afterward as for the receiving attendings and clinicians, might have artificially lowered attending agreement levels, concealing a difference due to experience.
On the other hand, construct validity of several items was supported by the difference in agreement levels based on patient characteristics. Agreement levels varied on 5/8 questions as patients became more complex, either defined by length of stay or number of medications. These differences show that agreement on PKAT items responds to changes in handoff complexity, a form of construct validity. Furthermore, these findings suggest that handoffs of more chronic or complex patients may require more attention for components prone to disagreement in these settings. Although complexity and longer length of stay are nonmodifiable risk factors, identifying these handoffs as more susceptible to disagreement provides potential targets for intervention.
It is important to move beyond he said/she said evaluations to assess shared understanding after a handoff, because high fidelity transfer of information is necessary for safe transfer of responsibility. The PKAT addresses this key component of handoff quality in a novel fashion. Although high‐fidelity information transfer may correlate with receiving provider satisfaction, this relationship has not yet been explored. Future studies will evaluate the association between receiver evaluations of handoffs and PKAT agreement, as well as the relationship between PKAT performance and subsequent patient outcomes.
Limitations of this approach include the challenges inherent in reducing a complex understanding of a patient to a multiple‐item instrument. Furthermore, PKAT use may influence handoff content due to the Hawthorne effect. Although our analysis rests on the argument that agreement is the goal of a handoff, some differences of opinion within the care team enrich resilience. Regardless, to maintain continuity of care, providers need to reach agreement on the next steps in a patient's care during the handoff. Because we focused only on agreement, this approach does not compare respondents' answers to a verifiable source of truth, if it exists. Therefore, 2 respondents who agree on the wrong answer receive the same score as 2 who agree on the right answer. Other limitations include using the number of medications as a marker of handoff complexity. Finally, conducting this study in a single CICU limits generalizability. However, we believe that all PKAT items are generalizable to other pediatric CICUs, and that several are generalizable to other pediatric intensive care settings. The approach of measuring shared understanding could be generalized more widely with development of items specific to different clinical settings.
Because the PKAT can be completed and scored quickly, it could be used as a real‐time measure of quality improvement interventions such as the introduction of a standardized handoff protocol. Alternatively, provider pairs could use the PKAT as a final handoff safety check to confirm consensus before transfer of responsibility. The concept of measuring shared clinical understanding could be extended to develop similar instruments for different clinical settings.
Acknowledgements
The authors thank the CICU providers for their enthusiasm for and participation in this study. The authors also thank Margaret Wolff, MD, Newton Buchanan, and the Center for Simulation, Advanced Education and Innovation at The Children's Hospital of Philadelphia for assistance in filming the video scenarios.
Disclosures: Dr. Bates was supported in part by NICHD/T32 HD060550 and NHLBI/T32 HL07915 grant funding. Dr. Metlay was supported by a Mid‐Career Investigator Award in Patient Oriented Research (K24‐AI073957). The authors report no conflicts of interest.
- , . The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Quality and Safety in Health Care. 2010;19(6):493–497. doi: 10.1136/qshc.2009.033480.
- . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , , . Nursing handovers: do we really need them? J Nurs Manag. 2004;12(1):37–42.
- , , , et al. Assessing clinical handover between paramedics and the trauma team. Injury. 2010;41(5):460–464.
- , , , , . Answering questions on call: Pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , . Patient handoffs: standardized and reliable measurement tools remain elusive. Jt Comm J Qual Patient Saf. 2010;36(2):52–61.
- , , , , . Improving measurement in clinical handover. Qual Saf Health Care. 2009;18(4):272–276.
- , , , . Adequacy of information transferred at resident sign‐out (inhospital handover of care): a prospective survey. Qual Saf Health Care. 2008;17(1):6–10.
- , , . Standardized Sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2009;25(2):129–134.
- , , , , . Assessing the quality of patient handoffs at care transitions. Qual Saf Health Care. 2010;19(6):1–5.
- , , , , . Implementing peer evaluation of handoffs: associations with experience and workload. J Hosp Med. 2013;8(3):132–136.
- , , , et al. Development of a handoff evaluation tool for shift‐to‐shift physician handoffs: the handoff CEX. J Hosp Med. 2013;8(4):191–200.
- , . The effects of patient handoff characteristics on subsequent care: a systematic review and areas for future research. Acad Med. 2012;87(8):1105–1124.
- . Construct validity in organizational behavior. In: Cummings LL, Stawe BM, eds. Research in Organizational Behavior. Vol 2. Greenwich, CT: JAI Press; 1980:3–43.
- , , , , . Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–1376.
- , , . A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
- , . The published literature on handoffs in hospitals: deficiencies identified in an extensive review. Quality and Safety in Health Care. 2010;19(6):493–497. doi: 10.1136/qshc.2009.033480.
- . Communication failures in patient sign‐out and suggestions for improvement: a critical incident analysis. Qual Saf Health Care. 2005;14(6):401–407.
- , , , , . Consequences of inadequate sign‐out for patient care. Arch Intern Med. 2008;168(16):1755–1760.
- , , , , , . Nursing handovers: do we really need them? J Nurs Manag. 2004;12(1):37–42.
- , , , et al. Assessing clinical handover between paramedics and the trauma team. Injury. 2010;41(5):460–464.
- , , , , . Answering questions on call: Pediatric resident physicians' use of handoffs and other resources. J Hosp Med. 2013;8(6):328–333.
- , , , , . Managing discontinuity in academic medical centers: strategies for a safe and effective resident sign‐out. J Hosp Med. 2006;1(4):257–266.
- , . Patient handoffs: standardized and reliable measurement tools remain elusive. Jt Comm J Qual Patient Saf. 2010;36(2):52–61.
- , , , , . Improving measurement in clinical handover. Qual Saf Health Care. 2009;18(4):272–276.
- , , , . Adequacy of information transferred at resident sign‐out (inhospital handover of care): a prospective survey. Qual Saf Health Care. 2008;17(1):6–10.
- , , . Standardized Sign‐out reduces intern perception of medical errors on the general internal medicine ward. Teach Learn Med. 2009;21(2):121–126.
- , , , et al. Hand‐off education and evaluation: piloting the observed simulated hand‐off experience (OSHE). J Gen Intern Med. 2009;25(2):129–134.
- , , , , . Assessing the quality of patient handoffs at care transitions. Qual Saf Health Care. 2010;19(6):1–5.
- , , , , . Implementing peer evaluation of handoffs: associations with experience and workload. J Hosp Med. 2013;8(3):132–136.
- , , , et al. Development of a handoff evaluation tool for shift‐to‐shift physician handoffs: the handoff CEX. J Hosp Med. 2013;8(4):191–200.
- , . The effects of patient handoff characteristics on subsequent care: a systematic review and areas for future research. Acad Med. 2012;87(8):1105–1124.
- . Construct validity in organizational behavior. In: Cummings LL, Stawe BM, eds. Research in Organizational Behavior. Vol 2. Greenwich, CT: JAI Press; 1980:3–43.
- , , , , . Development and validation of the medication regimen complexity index. Ann Pharmacother. 2004;38(9):1369–1376.
- , , . A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45(2):197–203.
- , , , , , . Research electronic data capture (REDCap)—a metadata‐driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381.
- . A Wilcoxon‐type test for trend. Stat Med. 1985;4(1):87–90.
© 2014 Society of Hospital Medicine
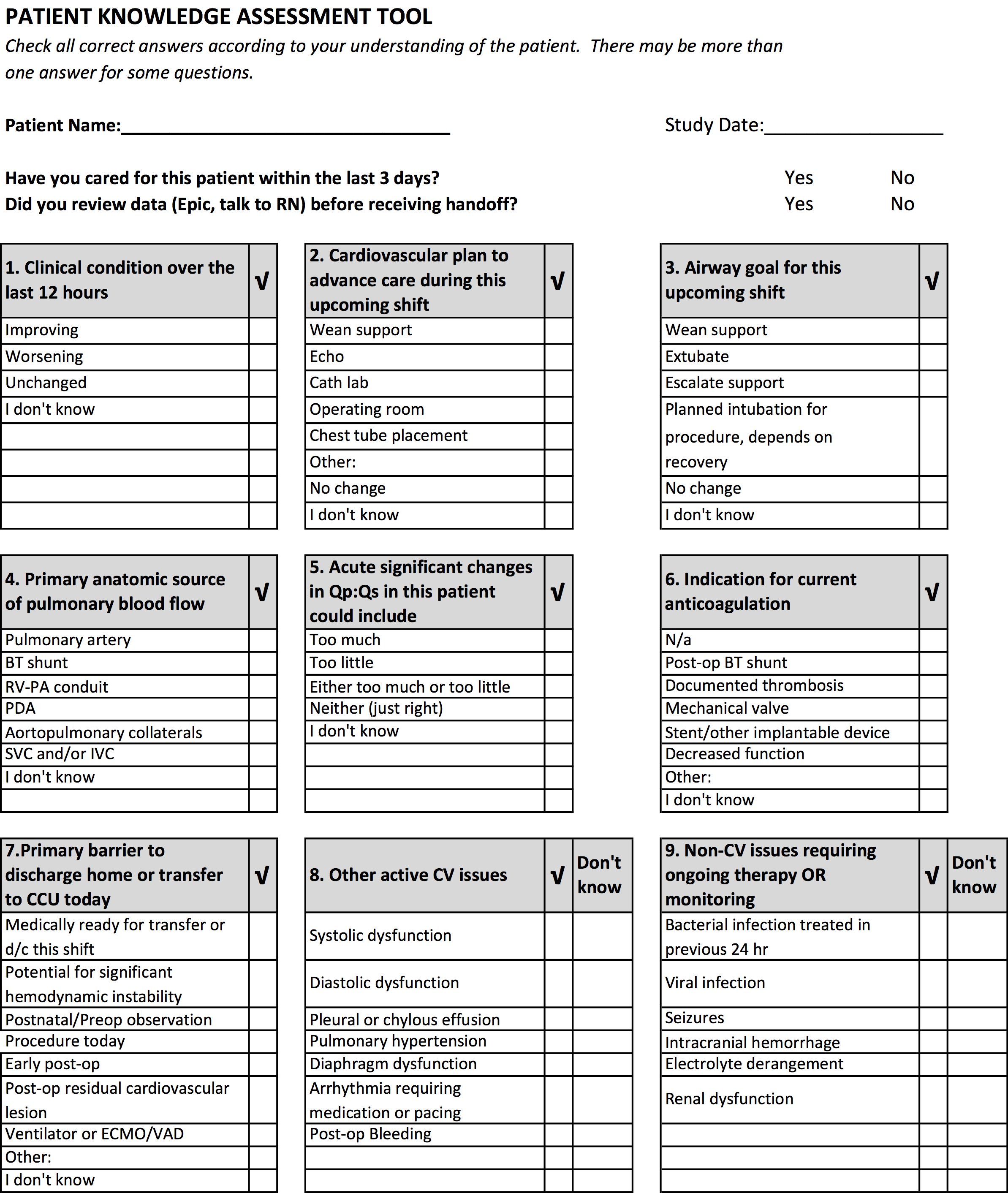
Percutaneous closure
Editor’s Note: I urge readers unfamiliar with the Perclose Proglide® device to pay special attention to the instructions for use and to follow them carefully. The device is relatively simple to use but it is a complex piece of equipment and so it is also easy to misuse it with dire consequences. I suggest that one at least read the section on "Troubleshooting," since preventing some of these problems can be lifesaving.
The key to successful percutaneous closure is selecting the appropriate site of entry into the common femoral artery (CFA). Using ultrasound (in transverse and longitudinal planes) and fluoroscopy, I gain access at a noncalcified spot 1 cm above the bifurcation of the CFA (Fig. 1). This is immediately confirmed with an oblique angiogram while pulling the 5F sheath to the ipsilateral side (Fig. 2). This small last maneuver allows me to see exactly where the puncture was and confirms that I will be able to use a closure device.
If using a sheath larger than 12F, I would dilate the track and cut any skin bridges within the puncture site using 11 blade. Doing this maneuver at the end of the procedure could result in inadvertently cutting the sutures. The Proglide device is then inserted and the sutures deployed as per the instructions for use.
At the end of the case, the sheath is pulled over a nonstiff wire while pulling on the nonrail (blue end) wire in a coaxial fashion. I can’t stress enough the need to be calm and not to pull too hard on the suture. This can result in the suture breaking or being pulled out of the artery. The knot pusher is then used on each suture sequentially. At this point, I tug on the wire to make sure it is "snug" within the arteriotomy. This signifies adequate closure but I also check briefly to ensure that there is no significant bleeding. Then I can go ahead and remove the wire and slide the knot pusher again.
The final step is to advance the knot pusher over the two sutures and cut the suture just under the skin. There is no need to slide the knot pusher all the way down to cut the suture as this may inadvertently cut the knot. Steristrips and Band-Aid are applied and I reverse the heparin after confirming the status of distal pulses
Dr. Mussa is an assistant professor of surgery at New York University School of Medicine and Langone Medical Center.
Editor’s Note: I urge readers unfamiliar with the Perclose Proglide® device to pay special attention to the instructions for use and to follow them carefully. The device is relatively simple to use but it is a complex piece of equipment and so it is also easy to misuse it with dire consequences. I suggest that one at least read the section on "Troubleshooting," since preventing some of these problems can be lifesaving.
The key to successful percutaneous closure is selecting the appropriate site of entry into the common femoral artery (CFA). Using ultrasound (in transverse and longitudinal planes) and fluoroscopy, I gain access at a noncalcified spot 1 cm above the bifurcation of the CFA (Fig. 1). This is immediately confirmed with an oblique angiogram while pulling the 5F sheath to the ipsilateral side (Fig. 2). This small last maneuver allows me to see exactly where the puncture was and confirms that I will be able to use a closure device.
If using a sheath larger than 12F, I would dilate the track and cut any skin bridges within the puncture site using 11 blade. Doing this maneuver at the end of the procedure could result in inadvertently cutting the sutures. The Proglide device is then inserted and the sutures deployed as per the instructions for use.
At the end of the case, the sheath is pulled over a nonstiff wire while pulling on the nonrail (blue end) wire in a coaxial fashion. I can’t stress enough the need to be calm and not to pull too hard on the suture. This can result in the suture breaking or being pulled out of the artery. The knot pusher is then used on each suture sequentially. At this point, I tug on the wire to make sure it is "snug" within the arteriotomy. This signifies adequate closure but I also check briefly to ensure that there is no significant bleeding. Then I can go ahead and remove the wire and slide the knot pusher again.
The final step is to advance the knot pusher over the two sutures and cut the suture just under the skin. There is no need to slide the knot pusher all the way down to cut the suture as this may inadvertently cut the knot. Steristrips and Band-Aid are applied and I reverse the heparin after confirming the status of distal pulses
Dr. Mussa is an assistant professor of surgery at New York University School of Medicine and Langone Medical Center.
Editor’s Note: I urge readers unfamiliar with the Perclose Proglide® device to pay special attention to the instructions for use and to follow them carefully. The device is relatively simple to use but it is a complex piece of equipment and so it is also easy to misuse it with dire consequences. I suggest that one at least read the section on "Troubleshooting," since preventing some of these problems can be lifesaving.
The key to successful percutaneous closure is selecting the appropriate site of entry into the common femoral artery (CFA). Using ultrasound (in transverse and longitudinal planes) and fluoroscopy, I gain access at a noncalcified spot 1 cm above the bifurcation of the CFA (Fig. 1). This is immediately confirmed with an oblique angiogram while pulling the 5F sheath to the ipsilateral side (Fig. 2). This small last maneuver allows me to see exactly where the puncture was and confirms that I will be able to use a closure device.
If using a sheath larger than 12F, I would dilate the track and cut any skin bridges within the puncture site using 11 blade. Doing this maneuver at the end of the procedure could result in inadvertently cutting the sutures. The Proglide device is then inserted and the sutures deployed as per the instructions for use.
At the end of the case, the sheath is pulled over a nonstiff wire while pulling on the nonrail (blue end) wire in a coaxial fashion. I can’t stress enough the need to be calm and not to pull too hard on the suture. This can result in the suture breaking or being pulled out of the artery. The knot pusher is then used on each suture sequentially. At this point, I tug on the wire to make sure it is "snug" within the arteriotomy. This signifies adequate closure but I also check briefly to ensure that there is no significant bleeding. Then I can go ahead and remove the wire and slide the knot pusher again.
The final step is to advance the knot pusher over the two sutures and cut the suture just under the skin. There is no need to slide the knot pusher all the way down to cut the suture as this may inadvertently cut the knot. Steristrips and Band-Aid are applied and I reverse the heparin after confirming the status of distal pulses
Dr. Mussa is an assistant professor of surgery at New York University School of Medicine and Langone Medical Center.
Predictions for 2014
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.
Are you prepared to manage the infectious disease challenges you’ll be facing in 2014? Here are my Top 5 predictions for what lies ahead in infectious diseases for the next year with pearls to help you in your practice. The first addresses a series of concerns around influenza. Others target diagnoses you might not have encountered or considered in the past. The last will hopefully improve HPV vaccination rates in your practice.
1. Expect an especially busy influenza season and the possibility that you may encounter patients with life-threatening influenza. We’ve already detected influenza in over 1,000 children at my institution, almost all 2009 pandemic H1N1 influenza A viruses, which is consistent with the national data from the Centers for Disease Control and Prevention. We are really just a month into influenza season, and we are seeing a significant number of children admitted to our pediatric intensive care unit with life-threatening disease presentations, and we’ve also seen unusual influenza complications. Talk to your ID colleagues about the potential for intravenous zanamivir in critically ill children who do not respond to oseltamivir. While pulmonary complications of influenza are most common, unusual presentations you may encounter include influenza encephalopathy (altered mental status, seizures, and mutism) and bacterial superinfection (when fever recurs or recrudesces after initial improvement, often 3-5 days into the course, think Staphylococcus aureus or Group A streptococcal disease). The CDC is alerting practitioners to the potential for increased morbidity and mortality in young/middle aged adults so the parents of your patients are at increased risk this year.
• False-negative testing can happen if the sensitivity of the rapid test is low, but a false-negative test can occur if the specimen is collected late in the clinical course. (This is especially true in the adult population in which testing may be negative at just 4-5 days into the course of disease.)
• Recognize that all hospitalized children should be treated with oseltamivir, as well as children who are immunocompromised; have chronic cardiopulmonary conditions, including hemodynamically significant heart disease and asthma; renal disease; metabolic disease, including diabetes; pregnant teens; morbidly obese patients; patients with neuromuscular/neurodevelopmental conditions (especially those with difficulty controlling airway secretions); and children under 2 years of age.
• I predict you may be hearing about oseltamivir shortages, but for now this relates to the sporadic difficulty in finding the oseltamivir suspension, in part, because of the lack of early season availability of this product at retail pharmacies, many of which are just getting in their stock. Prescribe the suspension for children aged younger than 1 year and be explicit about the mL dosage that should be dispensed. For children over 1 year of age, capsules can be opened and placed in pudding for those who cannot swallow capsules. Lexicomp Online offers guidelines for easy use of 30-mg, 45-mg and 75-mg capsules for different weight categories. If the suspension is necessary for an infant and is not available, the drug can be compounded by your pharmacy using capsules. You may find some pharmacies are reluctant to compound, so be prepared to contact your local children’s hospital for help. And keep offering vaccine throughout the season to healthy patients!
2. Most practitioners are aware of the importance of methicillin-resistant S. aureus (MRSA) as a pathogen that causes bacteremia and musculoskeletal and pulmonary disease in otherwise healthy children. I suspect there is less awareness that, in many locales, methicillin-sensitive S. aureus (MSSA) is being seen just as often, if not slightly more often than MRSA, as a bloodstream pathogen. The inclusion of vancomycin (which covers MRSA) with cefepime should be considered for empiric coverage in the otherwise healthy child with suspected sepsis. Cefepime is a fourth-generation cephalosporin with good gram-negative and gram-positive coverage and also has bactericidal activity against MSSA strains. Clindamycin should be considered as an adjunct to vancomycin and cefepime in those with toxin-mediated disease/toxic shock syndrome. Of course, modification of the empiric regimen should follow identification of the specific pathogen and the site(s) of infection.
3. E. coli remains the most common cause of urinary tract infections in children, but infections caused by multiple drug resistant (MDR) Escherichia coli strains are increasingly being seen. Consider infection caused by extended spectrum beta-lactamase–producing organisms in children with underlying renal anomalies, especially if they have been previously exposed to third-generation cephalosporins. Most strains are also resistant to fluoroquinolones, trimethoprim-sulfamethoxazole, and aminoglycosides as well as to non–carbapenem beta-lactams. Speaking of antibiotic resistance, look for many hospital microbiology laboratories to begin using advanced molecular detection methodology to more quickly identify bacterial and fungal isolates; such methods could reduce the time of identification from over 24 hours with conventional techniques to less than one hour. The use of newer systems to identify microbes and confirm susceptibility testing has the potential to transform care and improve outcomes.
4. Consider the diagnosis of human parechovirus (HPeV) infection in young febrile infants with sepsis/meningitis presentation but negative bacterial cultures. Detection of HPeV by polymerase chain reaction testing in serum or cerebrospinal fluid is diagnostic. Exclusion of herpes simplex virus and enterovirus disease is key, as similar clinical presentations may be seen. HPeV infections are more commonly noted in late spring and early summer in contrast to enteroviral infections, which tend to occur from July to September.
5. The strength of your vaccine recommendation continues to be the most important factor affecting the parental decision to vaccinate a child. Nowhere is this more obvious than with human papillomavirus vaccine (HPV), where practitioners often simply offer the vaccine rather than recommend it. In terms of teenage vaccines, when practitioners recommend Tdap (tetanus, diphtheria, and pertussis vaccine) and meningococcal conjugate vaccine as standard for their patients ("Today your child will receive whooping cough vaccine and the meningitis vaccine."), vaccine uptake is very high. But when it comes to the HPV vaccine, some practitioners feel they first must establish whether the parents are aware of HPV vaccine; then discuss their questions regarding the safety of the vaccine; and finally, explain that the vaccine prevents cancer. Some practitioners offer the option of "thinking about" the vaccine for the next visit, but in such cases, the patient generally leaves without receiving the vaccine. Add HPV vaccine into your standard teen vaccine recommendation and make it a goal to get the first vaccine initiated in all eligible patients. The three-dose HPV vaccine schedule is still recommended, but I predict that simplification of the schedule may occur as early as 2014 in the United States. We’ll keep you posted.
Dr. Jackson is director of the division of infectious disease and associate director of the infectious disease fellowship program at the University of Missouri, Kansas City.