User login
The 2014 CPT and Medicare code changes affecting ObGyn practice
The code set of the 2014 Current Procedural Terminology (CPT), which took effect on January 1, includes several changes that affect all women’s health-care providers, including:
a clarification of who should bill discharge-day management
the addition of interprofessional telephone and Internet consultations
new codes for image-guided fluid drainage
new codes for fibroid embolization and laparoscopic ablation of fibroids.
There are also some new laboratory codes: one that captures the work of the noninvasive prenatal DNA test Harmony, and one to test for Trichomonas vaginalis. Finally, the code for anogenital examinations was revised to reflect current practice.
Medicare also has made some changes you should note, related to the levonorgestrel-releasing intrauterine system Skyla and billing for “incident to” services, and the type of provider who can order a fecal occult blood test. In addition, Medicare changes to some of the practice expense relative value units (RVUs) and geographic payment adjustor values will have an impact on some frequently used ObGyn services.
The changes to the CPT code set took effect January 1. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on that date.
CPT CODE CHANGES
Discharge-day management coding clarified
Codes 99238 and 99239 should be reported by the admitting provider for all services rendered on the date of discharge as long as the admission and discharge were not on the same date of service. Concurrent hospital services performed by the nonadmitting clinician on the date of discharge should be billed instead as a subsequent inpatient hospital encounter (codes 99231–99233).
Interprofessional phone and Web consultations now reimbursed
Most clinicians at one time or another end up giving advice to another health-care provider about the care of a patient he or she never sees and, up until 2014, there was no way to ask for reimbursement for this additional work. Starting on January 1, however, there were four new codes to allow a consultant clinician to report this work. These services, of a consulting physician who has specific specialty expertise, typically will be provided in complex or urgent situations where a timely face-to-face service with the patient may not be feasible.
The new codes are billed based on total documented cumulative time spent (to account for more than one telephone/Internet contact to complete the consultation request). The codes are for interprofessional telephone/Internet assessment and management service provided by a consultative physician including a verbal and written report to the patient’s treating/requesting physician or other qualified health-care professional, with varying time intervals for medical consultative discussion and review:
99446 5–10 minutes
99447 11–20 minutes
99448 21–30 minutes
99449 31 minutes or more
Like all new codes, these have some very specific requirements:
The billing physician cannot have had a face-to-face encounter with the patient within the past 14 days. If the consultation leads to scheduling a face-to-face appointment or surgery within 14 days, these codes cannot be reported.
If the consultation is to accept transfer of care or arrange for an immediate face-to-face encounter with the consulting physician, these codes should not be billed.
The documentation must include a review of all pertinent medical records, studies, medications, etc., that may be required to render an opinion on how to proceed with care of the patient, and reviewing of any data is not reported separately.
The patient either can be new to the consultant or can be established (with a new or an exacerbated problem).
The majority of the service (more than 50%) must be devoted to the medical consultative verbal/Internet real-time discussion, and not be reported more than once within a 7-day interval.
The request for advice by the qualified health-care professional must be documented in the patient’s medical record, including the reason for the request.
There must be a verbal opinion report and written report from the consultant to the treating physician.
The treating physician who asks for the telephone/Internet advice can report a prolonged services, non–face-to-face code if the time exceeds the typical time of a problem E/M service by 30 minutes to get credit for the discussion with the consultant.
CASE
As an example, Dr. Moody, Mary’s primary care physician, has ordered a computed tomography scan for her due to reports of sharp epigastric pain. A large mass in the area of the right ovary is detected. Dr. Moody phones Dr. Gerard, the patient’s ObGyn of record, for an opinion about additional testing for this mass. Mary was last seen by Dr. Gerard at her well-woman visit 8 months ago; there were no complaints reported or problems detected.
Dr. Gerard recommends that additional views of the mass be obtained and that a CA 125 test be performed due to Mary’s family history of ovarian cancer. He also recommends that Mary be sent for a consultation with a gynecologic oncologist as soon as possible. The total time spent on this consultation is 15 minutes, and Dr. Gerard reports Mary’s consultative session to her insurance company with CPT code 99447.
Image-guided drainage of a fluid collection
CPT code 10030 has been added to report image-guided drainage of a fluid collection using a catheter for areas just under the skin. This code would be used if the patient had an abscess, hematoma, seroma, lymphocele, or cyst that was drained percutaneously. For instance, this code could be reported for a hematoma located in the abdominal wall or just under the skin. The code bundles image guidance, but it can be reported more than once if there is more than one collection drained with a separate catheter.
CPT also has added additional codes for image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst) of visceral, peritoneal, or retroperitoneal collections. The codes for these procedures are:
49405...; visceral (eg, kidney, liver, spleen, lung/mediastinum), percutaneous
49406...; peritoneal or retroperitoneal, percutaneous
49407...; peritoneal or retroperitoneal, transvaginal or transrectal
With the addition of these new codes, the old code 58823 has been eliminated.
Uterine fibroid treatment
There are two changes with regard to the treatment of uterine fibroids. First, CPT code 37210 (Uterine fibroid embolization [UFE, embolization of the uterine arteries to treat uterine fibroids, leiomyomata], percutaneous approach inclusive of vascular access, vessel selection, embolization, and all radiologic supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the procedure) has been eliminated and replaced by a more general code that will apply to any tumor or organ. This new code is 37243 (Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; for tumors, organ ischemia, or infarction).
Second, there is now a Category III code for the laparoscopic ablation of uterine fibroids: 0336T (Laparoscopy, surgical, ablation of uterine fibroid[s], including intraoperative ultrasound guidance and monitoring, radiofrequency). Clinical research has shown that radiofrequency ablation (RFA) is effective in treating fibroids, resolving associated symptoms in more than 80% of treated patients. Because RFA is not yet a standard of care, this Category III code must be reported in order for data on its use to be collected. Under CPT rules, you may not use an unlisted code in place of the Category III code for this procedure. If you are performing RFA, it may be considered experimental by some payers, but you can still make a case for payment with the submission of adequate documentation with the claim in the form of peer-reviewed articles and the patient’s circumstances that preclude more standard surgeries.
Anogenital examination coding
Code 99170 was revised to reflect current practice. The procedure is not always performed with a colposcope, but usually requires digital imaging for legal recoding and documentation. The revised code reads “anogenital examination, magnified, in childhood for suspected trauma, including image recording when performed.” Moderate sedation, if performed, may be billed separately using code 99143-99150.
LABORATORY CODE CHANGES
Cell-free DNA testing code added
As of January 1, there is a new code to report cell-free prenatal DNA testing to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy).
Related Article: Update on Obstetrics Jaimey Pauli, MD, and John T. Repke, MD (January 2014)
In addition, the code 84112, which used to be defined as “placental alpha microglobulin-1 (PAMG-1), cervicovaginal secretion, qualitative,” has been revised. The revision was done to make it clear that it can be ordered for other proteins that are tested in amniotic fluid. Code 84112 is now defined as follows: Evaluation of cervicovaginal fluid for specific amniotic fluid protein(s) (eg, placental alpha microglobulin-1 [PAMG-1], placental protein 12 [PP12], alpha-fetoprotein), qualitative, each specimen.
This test is normally ordered to determine whether the fetal membranes have ruptured, but this is not a Clinical Laboratory Improvement Amendments (CLIA) waived or Provider Performed Microscopy Procedures (PPMP) test. Therefore, only the laboratory with the applicable CLIA certificate can bill for it.
There are now two code options for T vaginalis testing
To the existing code 87660 (direct probe technique) is added the new code 87661, T vaginalis, amplified probe technique.
Three new codes for the flu vaccine:
90673, Flublok (effective January 2013)
90686, Fluzone, preservative-free (effective December 2012)
90688, FluLaval (effective August 2013)
In addition, Medicare has deleted code G2033, which was used to report Flublok. It will now accept the CPT code 90673 for this influenza product.
Keep in mind that reporting the administration of the influenza vaccine is different for Medicare than private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration.
MEDICARE CODING CHANGES
Skyla. The new code is J7301, levonorgestrel-releasing intrauterine contraceptive, 13.5 mg. This replaces the temporary code Q0090, which was added by Medicare on July 1, 2013.
Related Article: 5 IUD myths dispelled Anne A. Moore, DNP, APN (September 2013)
More providers can order fecal occult blood tests. To expand access to screening fecal occult blood testing, Medicare has revised the rules on who can order these tests. Effective January 1, 2014, not only a physician but also the billing physician’s assistant (PA), certified nurse specialist (CNS), or nurse practitioner (NP) can order the test. But as before January 1, the physician, PA, CNS, or NP is responsible for using the results of the screening test in the overall management of the patient’s medical care.
“Incident to” providers must be state-licensed. Medicare recently became aware that it was being billed in several situations for ‘‘incident to’’ services that were provided by auxiliary personnel (rather than the physician or practitioner billing for the services) who did not meet the state standards for those services. For this reason, Medicare has revised the “incident to” rules to make it clear that the person who is assigned to provide the aspect of the service must be licensed within their state to provide the services performed.
SGR fate, and your reimbursement, unknown at this time
At the time this article was finalized, there was no information about the fate of the Medicare payment mechanism for 2014. If the sustained growth formula used to calculate the Medicare conversion factor for physician reimbursement is not fixed by Congress, the projected 2014 conversion factor will be $27.2006, a decrease from the current conversion factor of $34.023.
But even without concrete, final information on this complicating factor, changes to the geographic adjustment units (which in turn determine the payment allowance for physicians based on their practice location), as well as changes to the practice expense RVUs for such office procedures as urodynamic testing, may spell decreased payments in 2014 from Medicare or payers who use Medicare as the basis for reimbursement.
Some states will fare better than others. The geographic payment cost index for all but a handful of states will be adjusted downward. The good news is that if you practice in Alabama, Alaska, Colorado, Connecticut, Delaware, Louisiana, Minnesota, New Hampshire, New Mexico, New York, Virginia, certain areas of California (San Francisco, Los Angeles, Marin County), and the Washington DC area, your geographic factors will increase. This increase may offset any decrease in the RVUs.
ObGyn reimbursements hardest hit by decreased RVUs. The RVUs for 2014 for the technical component of all the urodynamic testing codes will be reduced by 6% to 40%, with the biggest hit coming to codes 51726-51727 (complex cystometrogram with urethral and voiding pressure studies). In-office procedures such as endometrial ablation, endometrial cryoablation, and hysteroscopic sterilization will see around an 8% decrease to the practice expense RVUs. This same reduction will be noticed in the technical-component reimbursement for gynecologic and obstetric ultrasounds, with the notable exception that the RVUs were increased for umbilical artery Doppler.
The final result for increased or decreased payments via the relative value system will therefore depend on your practice location, and whether you are billing the technical component only for many of these procedures (and, of course, the final outcome of the SGR). WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
The code set of the 2014 Current Procedural Terminology (CPT), which took effect on January 1, includes several changes that affect all women’s health-care providers, including:
a clarification of who should bill discharge-day management
the addition of interprofessional telephone and Internet consultations
new codes for image-guided fluid drainage
new codes for fibroid embolization and laparoscopic ablation of fibroids.
There are also some new laboratory codes: one that captures the work of the noninvasive prenatal DNA test Harmony, and one to test for Trichomonas vaginalis. Finally, the code for anogenital examinations was revised to reflect current practice.
Medicare also has made some changes you should note, related to the levonorgestrel-releasing intrauterine system Skyla and billing for “incident to” services, and the type of provider who can order a fecal occult blood test. In addition, Medicare changes to some of the practice expense relative value units (RVUs) and geographic payment adjustor values will have an impact on some frequently used ObGyn services.
The changes to the CPT code set took effect January 1. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on that date.
CPT CODE CHANGES
Discharge-day management coding clarified
Codes 99238 and 99239 should be reported by the admitting provider for all services rendered on the date of discharge as long as the admission and discharge were not on the same date of service. Concurrent hospital services performed by the nonadmitting clinician on the date of discharge should be billed instead as a subsequent inpatient hospital encounter (codes 99231–99233).
Interprofessional phone and Web consultations now reimbursed
Most clinicians at one time or another end up giving advice to another health-care provider about the care of a patient he or she never sees and, up until 2014, there was no way to ask for reimbursement for this additional work. Starting on January 1, however, there were four new codes to allow a consultant clinician to report this work. These services, of a consulting physician who has specific specialty expertise, typically will be provided in complex or urgent situations where a timely face-to-face service with the patient may not be feasible.
The new codes are billed based on total documented cumulative time spent (to account for more than one telephone/Internet contact to complete the consultation request). The codes are for interprofessional telephone/Internet assessment and management service provided by a consultative physician including a verbal and written report to the patient’s treating/requesting physician or other qualified health-care professional, with varying time intervals for medical consultative discussion and review:
99446 5–10 minutes
99447 11–20 minutes
99448 21–30 minutes
99449 31 minutes or more
Like all new codes, these have some very specific requirements:
The billing physician cannot have had a face-to-face encounter with the patient within the past 14 days. If the consultation leads to scheduling a face-to-face appointment or surgery within 14 days, these codes cannot be reported.
If the consultation is to accept transfer of care or arrange for an immediate face-to-face encounter with the consulting physician, these codes should not be billed.
The documentation must include a review of all pertinent medical records, studies, medications, etc., that may be required to render an opinion on how to proceed with care of the patient, and reviewing of any data is not reported separately.
The patient either can be new to the consultant or can be established (with a new or an exacerbated problem).
The majority of the service (more than 50%) must be devoted to the medical consultative verbal/Internet real-time discussion, and not be reported more than once within a 7-day interval.
The request for advice by the qualified health-care professional must be documented in the patient’s medical record, including the reason for the request.
There must be a verbal opinion report and written report from the consultant to the treating physician.
The treating physician who asks for the telephone/Internet advice can report a prolonged services, non–face-to-face code if the time exceeds the typical time of a problem E/M service by 30 minutes to get credit for the discussion with the consultant.
CASE
As an example, Dr. Moody, Mary’s primary care physician, has ordered a computed tomography scan for her due to reports of sharp epigastric pain. A large mass in the area of the right ovary is detected. Dr. Moody phones Dr. Gerard, the patient’s ObGyn of record, for an opinion about additional testing for this mass. Mary was last seen by Dr. Gerard at her well-woman visit 8 months ago; there were no complaints reported or problems detected.
Dr. Gerard recommends that additional views of the mass be obtained and that a CA 125 test be performed due to Mary’s family history of ovarian cancer. He also recommends that Mary be sent for a consultation with a gynecologic oncologist as soon as possible. The total time spent on this consultation is 15 minutes, and Dr. Gerard reports Mary’s consultative session to her insurance company with CPT code 99447.
Image-guided drainage of a fluid collection
CPT code 10030 has been added to report image-guided drainage of a fluid collection using a catheter for areas just under the skin. This code would be used if the patient had an abscess, hematoma, seroma, lymphocele, or cyst that was drained percutaneously. For instance, this code could be reported for a hematoma located in the abdominal wall or just under the skin. The code bundles image guidance, but it can be reported more than once if there is more than one collection drained with a separate catheter.
CPT also has added additional codes for image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst) of visceral, peritoneal, or retroperitoneal collections. The codes for these procedures are:
49405...; visceral (eg, kidney, liver, spleen, lung/mediastinum), percutaneous
49406...; peritoneal or retroperitoneal, percutaneous
49407...; peritoneal or retroperitoneal, transvaginal or transrectal
With the addition of these new codes, the old code 58823 has been eliminated.
Uterine fibroid treatment
There are two changes with regard to the treatment of uterine fibroids. First, CPT code 37210 (Uterine fibroid embolization [UFE, embolization of the uterine arteries to treat uterine fibroids, leiomyomata], percutaneous approach inclusive of vascular access, vessel selection, embolization, and all radiologic supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the procedure) has been eliminated and replaced by a more general code that will apply to any tumor or organ. This new code is 37243 (Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; for tumors, organ ischemia, or infarction).
Second, there is now a Category III code for the laparoscopic ablation of uterine fibroids: 0336T (Laparoscopy, surgical, ablation of uterine fibroid[s], including intraoperative ultrasound guidance and monitoring, radiofrequency). Clinical research has shown that radiofrequency ablation (RFA) is effective in treating fibroids, resolving associated symptoms in more than 80% of treated patients. Because RFA is not yet a standard of care, this Category III code must be reported in order for data on its use to be collected. Under CPT rules, you may not use an unlisted code in place of the Category III code for this procedure. If you are performing RFA, it may be considered experimental by some payers, but you can still make a case for payment with the submission of adequate documentation with the claim in the form of peer-reviewed articles and the patient’s circumstances that preclude more standard surgeries.
Anogenital examination coding
Code 99170 was revised to reflect current practice. The procedure is not always performed with a colposcope, but usually requires digital imaging for legal recoding and documentation. The revised code reads “anogenital examination, magnified, in childhood for suspected trauma, including image recording when performed.” Moderate sedation, if performed, may be billed separately using code 99143-99150.
LABORATORY CODE CHANGES
Cell-free DNA testing code added
As of January 1, there is a new code to report cell-free prenatal DNA testing to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy).
Related Article: Update on Obstetrics Jaimey Pauli, MD, and John T. Repke, MD (January 2014)
In addition, the code 84112, which used to be defined as “placental alpha microglobulin-1 (PAMG-1), cervicovaginal secretion, qualitative,” has been revised. The revision was done to make it clear that it can be ordered for other proteins that are tested in amniotic fluid. Code 84112 is now defined as follows: Evaluation of cervicovaginal fluid for specific amniotic fluid protein(s) (eg, placental alpha microglobulin-1 [PAMG-1], placental protein 12 [PP12], alpha-fetoprotein), qualitative, each specimen.
This test is normally ordered to determine whether the fetal membranes have ruptured, but this is not a Clinical Laboratory Improvement Amendments (CLIA) waived or Provider Performed Microscopy Procedures (PPMP) test. Therefore, only the laboratory with the applicable CLIA certificate can bill for it.
There are now two code options for T vaginalis testing
To the existing code 87660 (direct probe technique) is added the new code 87661, T vaginalis, amplified probe technique.
Three new codes for the flu vaccine:
90673, Flublok (effective January 2013)
90686, Fluzone, preservative-free (effective December 2012)
90688, FluLaval (effective August 2013)
In addition, Medicare has deleted code G2033, which was used to report Flublok. It will now accept the CPT code 90673 for this influenza product.
Keep in mind that reporting the administration of the influenza vaccine is different for Medicare than private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration.
MEDICARE CODING CHANGES
Skyla. The new code is J7301, levonorgestrel-releasing intrauterine contraceptive, 13.5 mg. This replaces the temporary code Q0090, which was added by Medicare on July 1, 2013.
Related Article: 5 IUD myths dispelled Anne A. Moore, DNP, APN (September 2013)
More providers can order fecal occult blood tests. To expand access to screening fecal occult blood testing, Medicare has revised the rules on who can order these tests. Effective January 1, 2014, not only a physician but also the billing physician’s assistant (PA), certified nurse specialist (CNS), or nurse practitioner (NP) can order the test. But as before January 1, the physician, PA, CNS, or NP is responsible for using the results of the screening test in the overall management of the patient’s medical care.
“Incident to” providers must be state-licensed. Medicare recently became aware that it was being billed in several situations for ‘‘incident to’’ services that were provided by auxiliary personnel (rather than the physician or practitioner billing for the services) who did not meet the state standards for those services. For this reason, Medicare has revised the “incident to” rules to make it clear that the person who is assigned to provide the aspect of the service must be licensed within their state to provide the services performed.
SGR fate, and your reimbursement, unknown at this time
At the time this article was finalized, there was no information about the fate of the Medicare payment mechanism for 2014. If the sustained growth formula used to calculate the Medicare conversion factor for physician reimbursement is not fixed by Congress, the projected 2014 conversion factor will be $27.2006, a decrease from the current conversion factor of $34.023.
But even without concrete, final information on this complicating factor, changes to the geographic adjustment units (which in turn determine the payment allowance for physicians based on their practice location), as well as changes to the practice expense RVUs for such office procedures as urodynamic testing, may spell decreased payments in 2014 from Medicare or payers who use Medicare as the basis for reimbursement.
Some states will fare better than others. The geographic payment cost index for all but a handful of states will be adjusted downward. The good news is that if you practice in Alabama, Alaska, Colorado, Connecticut, Delaware, Louisiana, Minnesota, New Hampshire, New Mexico, New York, Virginia, certain areas of California (San Francisco, Los Angeles, Marin County), and the Washington DC area, your geographic factors will increase. This increase may offset any decrease in the RVUs.
ObGyn reimbursements hardest hit by decreased RVUs. The RVUs for 2014 for the technical component of all the urodynamic testing codes will be reduced by 6% to 40%, with the biggest hit coming to codes 51726-51727 (complex cystometrogram with urethral and voiding pressure studies). In-office procedures such as endometrial ablation, endometrial cryoablation, and hysteroscopic sterilization will see around an 8% decrease to the practice expense RVUs. This same reduction will be noticed in the technical-component reimbursement for gynecologic and obstetric ultrasounds, with the notable exception that the RVUs were increased for umbilical artery Doppler.
The final result for increased or decreased payments via the relative value system will therefore depend on your practice location, and whether you are billing the technical component only for many of these procedures (and, of course, the final outcome of the SGR). WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
The code set of the 2014 Current Procedural Terminology (CPT), which took effect on January 1, includes several changes that affect all women’s health-care providers, including:
a clarification of who should bill discharge-day management
the addition of interprofessional telephone and Internet consultations
new codes for image-guided fluid drainage
new codes for fibroid embolization and laparoscopic ablation of fibroids.
There are also some new laboratory codes: one that captures the work of the noninvasive prenatal DNA test Harmony, and one to test for Trichomonas vaginalis. Finally, the code for anogenital examinations was revised to reflect current practice.
Medicare also has made some changes you should note, related to the levonorgestrel-releasing intrauterine system Skyla and billing for “incident to” services, and the type of provider who can order a fecal occult blood test. In addition, Medicare changes to some of the practice expense relative value units (RVUs) and geographic payment adjustor values will have an impact on some frequently used ObGyn services.
The changes to the CPT code set took effect January 1. Because of Health Insurance Portability and Accountability Act (HIPAA) requirements, insurers were required to accept new codes on that date.
CPT CODE CHANGES
Discharge-day management coding clarified
Codes 99238 and 99239 should be reported by the admitting provider for all services rendered on the date of discharge as long as the admission and discharge were not on the same date of service. Concurrent hospital services performed by the nonadmitting clinician on the date of discharge should be billed instead as a subsequent inpatient hospital encounter (codes 99231–99233).
Interprofessional phone and Web consultations now reimbursed
Most clinicians at one time or another end up giving advice to another health-care provider about the care of a patient he or she never sees and, up until 2014, there was no way to ask for reimbursement for this additional work. Starting on January 1, however, there were four new codes to allow a consultant clinician to report this work. These services, of a consulting physician who has specific specialty expertise, typically will be provided in complex or urgent situations where a timely face-to-face service with the patient may not be feasible.
The new codes are billed based on total documented cumulative time spent (to account for more than one telephone/Internet contact to complete the consultation request). The codes are for interprofessional telephone/Internet assessment and management service provided by a consultative physician including a verbal and written report to the patient’s treating/requesting physician or other qualified health-care professional, with varying time intervals for medical consultative discussion and review:
99446 5–10 minutes
99447 11–20 minutes
99448 21–30 minutes
99449 31 minutes or more
Like all new codes, these have some very specific requirements:
The billing physician cannot have had a face-to-face encounter with the patient within the past 14 days. If the consultation leads to scheduling a face-to-face appointment or surgery within 14 days, these codes cannot be reported.
If the consultation is to accept transfer of care or arrange for an immediate face-to-face encounter with the consulting physician, these codes should not be billed.
The documentation must include a review of all pertinent medical records, studies, medications, etc., that may be required to render an opinion on how to proceed with care of the patient, and reviewing of any data is not reported separately.
The patient either can be new to the consultant or can be established (with a new or an exacerbated problem).
The majority of the service (more than 50%) must be devoted to the medical consultative verbal/Internet real-time discussion, and not be reported more than once within a 7-day interval.
The request for advice by the qualified health-care professional must be documented in the patient’s medical record, including the reason for the request.
There must be a verbal opinion report and written report from the consultant to the treating physician.
The treating physician who asks for the telephone/Internet advice can report a prolonged services, non–face-to-face code if the time exceeds the typical time of a problem E/M service by 30 minutes to get credit for the discussion with the consultant.
CASE
As an example, Dr. Moody, Mary’s primary care physician, has ordered a computed tomography scan for her due to reports of sharp epigastric pain. A large mass in the area of the right ovary is detected. Dr. Moody phones Dr. Gerard, the patient’s ObGyn of record, for an opinion about additional testing for this mass. Mary was last seen by Dr. Gerard at her well-woman visit 8 months ago; there were no complaints reported or problems detected.
Dr. Gerard recommends that additional views of the mass be obtained and that a CA 125 test be performed due to Mary’s family history of ovarian cancer. He also recommends that Mary be sent for a consultation with a gynecologic oncologist as soon as possible. The total time spent on this consultation is 15 minutes, and Dr. Gerard reports Mary’s consultative session to her insurance company with CPT code 99447.
Image-guided drainage of a fluid collection
CPT code 10030 has been added to report image-guided drainage of a fluid collection using a catheter for areas just under the skin. This code would be used if the patient had an abscess, hematoma, seroma, lymphocele, or cyst that was drained percutaneously. For instance, this code could be reported for a hematoma located in the abdominal wall or just under the skin. The code bundles image guidance, but it can be reported more than once if there is more than one collection drained with a separate catheter.
CPT also has added additional codes for image-guided fluid collection drainage by catheter (eg, abscess, hematoma, seroma, lymphocele, cyst) of visceral, peritoneal, or retroperitoneal collections. The codes for these procedures are:
49405...; visceral (eg, kidney, liver, spleen, lung/mediastinum), percutaneous
49406...; peritoneal or retroperitoneal, percutaneous
49407...; peritoneal or retroperitoneal, transvaginal or transrectal
With the addition of these new codes, the old code 58823 has been eliminated.
Uterine fibroid treatment
There are two changes with regard to the treatment of uterine fibroids. First, CPT code 37210 (Uterine fibroid embolization [UFE, embolization of the uterine arteries to treat uterine fibroids, leiomyomata], percutaneous approach inclusive of vascular access, vessel selection, embolization, and all radiologic supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the procedure) has been eliminated and replaced by a more general code that will apply to any tumor or organ. This new code is 37243 (Vascular embolization or occlusion, inclusive of all radiological supervision and interpretation, intraprocedural roadmapping, and imaging guidance necessary to complete the intervention; for tumors, organ ischemia, or infarction).
Second, there is now a Category III code for the laparoscopic ablation of uterine fibroids: 0336T (Laparoscopy, surgical, ablation of uterine fibroid[s], including intraoperative ultrasound guidance and monitoring, radiofrequency). Clinical research has shown that radiofrequency ablation (RFA) is effective in treating fibroids, resolving associated symptoms in more than 80% of treated patients. Because RFA is not yet a standard of care, this Category III code must be reported in order for data on its use to be collected. Under CPT rules, you may not use an unlisted code in place of the Category III code for this procedure. If you are performing RFA, it may be considered experimental by some payers, but you can still make a case for payment with the submission of adequate documentation with the claim in the form of peer-reviewed articles and the patient’s circumstances that preclude more standard surgeries.
Anogenital examination coding
Code 99170 was revised to reflect current practice. The procedure is not always performed with a colposcope, but usually requires digital imaging for legal recoding and documentation. The revised code reads “anogenital examination, magnified, in childhood for suspected trauma, including image recording when performed.” Moderate sedation, if performed, may be billed separately using code 99143-99150.
LABORATORY CODE CHANGES
Cell-free DNA testing code added
As of January 1, there is a new code to report cell-free prenatal DNA testing to screen for fetal aneuploidy. This new code is 81507 (Fetal aneuploidy [trisomy 21, 18, and 13] DNA sequence analysis of selected regions using maternal plasma, algorithm reported as a risk score for each trisomy).
Related Article: Update on Obstetrics Jaimey Pauli, MD, and John T. Repke, MD (January 2014)
In addition, the code 84112, which used to be defined as “placental alpha microglobulin-1 (PAMG-1), cervicovaginal secretion, qualitative,” has been revised. The revision was done to make it clear that it can be ordered for other proteins that are tested in amniotic fluid. Code 84112 is now defined as follows: Evaluation of cervicovaginal fluid for specific amniotic fluid protein(s) (eg, placental alpha microglobulin-1 [PAMG-1], placental protein 12 [PP12], alpha-fetoprotein), qualitative, each specimen.
This test is normally ordered to determine whether the fetal membranes have ruptured, but this is not a Clinical Laboratory Improvement Amendments (CLIA) waived or Provider Performed Microscopy Procedures (PPMP) test. Therefore, only the laboratory with the applicable CLIA certificate can bill for it.
There are now two code options for T vaginalis testing
To the existing code 87660 (direct probe technique) is added the new code 87661, T vaginalis, amplified probe technique.
Three new codes for the flu vaccine:
90673, Flublok (effective January 2013)
90686, Fluzone, preservative-free (effective December 2012)
90688, FluLaval (effective August 2013)
In addition, Medicare has deleted code G2033, which was used to report Flublok. It will now accept the CPT code 90673 for this influenza product.
Keep in mind that reporting the administration of the influenza vaccine is different for Medicare than private payers. Administration code G0008 and diagnosis code V04.81 would be reported in conjunction with the appropriate vaccine code for Medicare, while CPT instructs you to report 90471 instead for the administration.
MEDICARE CODING CHANGES
Skyla. The new code is J7301, levonorgestrel-releasing intrauterine contraceptive, 13.5 mg. This replaces the temporary code Q0090, which was added by Medicare on July 1, 2013.
Related Article: 5 IUD myths dispelled Anne A. Moore, DNP, APN (September 2013)
More providers can order fecal occult blood tests. To expand access to screening fecal occult blood testing, Medicare has revised the rules on who can order these tests. Effective January 1, 2014, not only a physician but also the billing physician’s assistant (PA), certified nurse specialist (CNS), or nurse practitioner (NP) can order the test. But as before January 1, the physician, PA, CNS, or NP is responsible for using the results of the screening test in the overall management of the patient’s medical care.
“Incident to” providers must be state-licensed. Medicare recently became aware that it was being billed in several situations for ‘‘incident to’’ services that were provided by auxiliary personnel (rather than the physician or practitioner billing for the services) who did not meet the state standards for those services. For this reason, Medicare has revised the “incident to” rules to make it clear that the person who is assigned to provide the aspect of the service must be licensed within their state to provide the services performed.
SGR fate, and your reimbursement, unknown at this time
At the time this article was finalized, there was no information about the fate of the Medicare payment mechanism for 2014. If the sustained growth formula used to calculate the Medicare conversion factor for physician reimbursement is not fixed by Congress, the projected 2014 conversion factor will be $27.2006, a decrease from the current conversion factor of $34.023.
But even without concrete, final information on this complicating factor, changes to the geographic adjustment units (which in turn determine the payment allowance for physicians based on their practice location), as well as changes to the practice expense RVUs for such office procedures as urodynamic testing, may spell decreased payments in 2014 from Medicare or payers who use Medicare as the basis for reimbursement.
Some states will fare better than others. The geographic payment cost index for all but a handful of states will be adjusted downward. The good news is that if you practice in Alabama, Alaska, Colorado, Connecticut, Delaware, Louisiana, Minnesota, New Hampshire, New Mexico, New York, Virginia, certain areas of California (San Francisco, Los Angeles, Marin County), and the Washington DC area, your geographic factors will increase. This increase may offset any decrease in the RVUs.
ObGyn reimbursements hardest hit by decreased RVUs. The RVUs for 2014 for the technical component of all the urodynamic testing codes will be reduced by 6% to 40%, with the biggest hit coming to codes 51726-51727 (complex cystometrogram with urethral and voiding pressure studies). In-office procedures such as endometrial ablation, endometrial cryoablation, and hysteroscopic sterilization will see around an 8% decrease to the practice expense RVUs. This same reduction will be noticed in the technical-component reimbursement for gynecologic and obstetric ultrasounds, with the notable exception that the RVUs were increased for umbilical artery Doppler.
The final result for increased or decreased payments via the relative value system will therefore depend on your practice location, and whether you are billing the technical component only for many of these procedures (and, of course, the final outcome of the SGR). WE WANT TO HEAR FROM YOU!
Drop us a line and let us know what you think about current articles, which topics you'd like to see covered in future issues, and what challenges you face in daily practice. Tell us what you think by emailing us at: obg@frontlinemedcom.com
WATCH for Melanie Witt’s update on ICD-10 conversion ahead of its official release date later this year.
Levamisole contamination of cocaine resulting in neutropenia and thrombovasculopathy: a report from the Southern Network on Adverse Reactions (SONAR)
Background
Levamisole is a pharmaceutical with anthelminthic and immunomodulatory properties that received approval from the Food and Drug Administration in 1991 as part of adjuvant chemotherapy regimens for colorectal cancer. The addition of levamisole to 5-flouroruacil (5-FU) was first evaluated by the North Central Cancer Treatment Group in a 3-arm clinical trial that found that 5-FUlevamisole for 12 months was superior to either surgery alone or surgery followed by levamisole alone (recurrence rate was reduced by 40% and the death rate by 33% in Dukes’ C colon cancer).1 A subsequent trial intergroup trial randomized patients with Dukes’ B2 and C colon cancer to surgery alone or 1 year of adjuvant levamisole or 5FUlevamisole and confirmed the efficacy of 5FUlevamisole with respect to disease free survival and overall survival.2 As a result, adjuvant chemotherapy became the standard for stage III colon cancer as reported by an National Cancer Institute consensus development panel.3 Subsequently, primarily because of toxicity reasons, leucovorin replaced levamisole in most adjuvant chemotheraoy regimens for stage III colorectal cancer. Clinical toxicity of levamisole was noted as early as 1976 when several cases of leukopenia and agranulocytosis were reported. Recurrence with re-exposure was well described and agranulocytosis spontaneously reversed upon discontinuation of therapy. Vasculitis secondary to levamisole treatment was first reported in 1978, presenting primarily as leukocytoclastic vasculitis, cutaneous necrotising vasculitis and thrombotic vasculopathy without vasculitis. These findings typically, but not invariably, involve the ear lobes. In the early 1990s, levamisole became unavailable for human use in the United States due to toxicity concerns. Various neurological side effects were described with levamisole therapy, the most concerning complication being multifocal inflammatory leukoencephalopathy. Recently, several persons have developed a novel syndrome characterized by necrotic noses and ears, leg ulcers, agranulocytosis, thrombovasculopathy, and positive antineutrophil cytoplasmic antibodies (ANCAs) as a result of the drug cocaine being adulterated with levamisole. We describe the drug below.
Background
Levamisole is a pharmaceutical with anthelminthic and immunomodulatory properties that received approval from the Food and Drug Administration in 1991 as part of adjuvant chemotherapy regimens for colorectal cancer. The addition of levamisole to 5-flouroruacil (5-FU) was first evaluated by the North Central Cancer Treatment Group in a 3-arm clinical trial that found that 5-FUlevamisole for 12 months was superior to either surgery alone or surgery followed by levamisole alone (recurrence rate was reduced by 40% and the death rate by 33% in Dukes’ C colon cancer).1 A subsequent trial intergroup trial randomized patients with Dukes’ B2 and C colon cancer to surgery alone or 1 year of adjuvant levamisole or 5FUlevamisole and confirmed the efficacy of 5FUlevamisole with respect to disease free survival and overall survival.2 As a result, adjuvant chemotherapy became the standard for stage III colon cancer as reported by an National Cancer Institute consensus development panel.3 Subsequently, primarily because of toxicity reasons, leucovorin replaced levamisole in most adjuvant chemotheraoy regimens for stage III colorectal cancer. Clinical toxicity of levamisole was noted as early as 1976 when several cases of leukopenia and agranulocytosis were reported. Recurrence with re-exposure was well described and agranulocytosis spontaneously reversed upon discontinuation of therapy. Vasculitis secondary to levamisole treatment was first reported in 1978, presenting primarily as leukocytoclastic vasculitis, cutaneous necrotising vasculitis and thrombotic vasculopathy without vasculitis. These findings typically, but not invariably, involve the ear lobes. In the early 1990s, levamisole became unavailable for human use in the United States due to toxicity concerns. Various neurological side effects were described with levamisole therapy, the most concerning complication being multifocal inflammatory leukoencephalopathy. Recently, several persons have developed a novel syndrome characterized by necrotic noses and ears, leg ulcers, agranulocytosis, thrombovasculopathy, and positive antineutrophil cytoplasmic antibodies (ANCAs) as a result of the drug cocaine being adulterated with levamisole. We describe the drug below.
Background
Levamisole is a pharmaceutical with anthelminthic and immunomodulatory properties that received approval from the Food and Drug Administration in 1991 as part of adjuvant chemotherapy regimens for colorectal cancer. The addition of levamisole to 5-flouroruacil (5-FU) was first evaluated by the North Central Cancer Treatment Group in a 3-arm clinical trial that found that 5-FUlevamisole for 12 months was superior to either surgery alone or surgery followed by levamisole alone (recurrence rate was reduced by 40% and the death rate by 33% in Dukes’ C colon cancer).1 A subsequent trial intergroup trial randomized patients with Dukes’ B2 and C colon cancer to surgery alone or 1 year of adjuvant levamisole or 5FUlevamisole and confirmed the efficacy of 5FUlevamisole with respect to disease free survival and overall survival.2 As a result, adjuvant chemotherapy became the standard for stage III colon cancer as reported by an National Cancer Institute consensus development panel.3 Subsequently, primarily because of toxicity reasons, leucovorin replaced levamisole in most adjuvant chemotheraoy regimens for stage III colorectal cancer. Clinical toxicity of levamisole was noted as early as 1976 when several cases of leukopenia and agranulocytosis were reported. Recurrence with re-exposure was well described and agranulocytosis spontaneously reversed upon discontinuation of therapy. Vasculitis secondary to levamisole treatment was first reported in 1978, presenting primarily as leukocytoclastic vasculitis, cutaneous necrotising vasculitis and thrombotic vasculopathy without vasculitis. These findings typically, but not invariably, involve the ear lobes. In the early 1990s, levamisole became unavailable for human use in the United States due to toxicity concerns. Various neurological side effects were described with levamisole therapy, the most concerning complication being multifocal inflammatory leukoencephalopathy. Recently, several persons have developed a novel syndrome characterized by necrotic noses and ears, leg ulcers, agranulocytosis, thrombovasculopathy, and positive antineutrophil cytoplasmic antibodies (ANCAs) as a result of the drug cocaine being adulterated with levamisole. We describe the drug below.
CAR-T cells drive ALL into remission
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
NEW ORLEANS – Modified T cells continue to show their mettle against treatment-refractory leukemias, based on study results presented at the annual meeting of the American Society of Hematology.
Among children and young adults with heavily pretreated relapsed or refractory acute lymphoblastic leukemia (ALL), chimeric antigen receptor (CAR) T cells targeted against the CD19 receptor produced complete responses in 10 of 16 patients, including 3 patients with primary, treatment-refractory ALL who had never previously been in remission, reported Dr. Daniel W. Lee III of the National Cancer Institute in Bethesda, Md.
"We were able to clear CNS [central nervous system] leukemia using CAR-T cells alone," Dr. Lee said.
In a second study, CD19-targeted T cells induced molecular remissions in adults with B-lineage ALL refractory to chemotherapy, said Dr. Marco L. Davila from the cellular therapeutics center at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York City.
The immunotherapy produced a complete response (CR) in 12 of 16 patients, and a complete molecular response (CRm) in 12.
Dr. Davila commented that CAR-T cell therapy appears to be a good therapeutic choice for relapsed/refractory B-ALL.
"Especially in light of the data that we see in indolent lymphomas, where the response rates have not been nearly as great, I would speculate that there may be something about this disease that makes it particularly well suited to the second-generation CAR-T cell therapy," he said.
In both studies, therapy with CAR-T cells served as a bridge to stem cell transplantation for several patients.
Different CAR-T flavors
Each research team used its own variation on CAR-T cell therapy. The NCI investigators collected peripheral blood mononuclear cells (PBMCs) from patients via apheresis and used a gamma retrovirus to introduce into effector cells a genetic sequence targeting the CD19 receptor on malignant cells. The NCI version also uses CD28 costimulatory signaling to boost cell-killing effects.
The patients are conditioned with fludarabine and cyclophosphamide, and the treated T-cells are reinfused into the patients 11 days after harvesting.
In the phase I study, 15 patients with relapsed or refractory ALL and 1 with diffuse large B-cell lymphoma were treated. Eight of the patients had undergone at least one hematopoietic stem cell transplant, and all had received total body irradiation. Four had previously received another form of immunotherapy. The patients had to have been at least 100 days post transplant, with no graft-vs.-host disease.
T-cell expansion and transduction was feasible in this heavily pretreated population. All but two patients had an adequate or good expression of CAR-T cells. These patients were treated nonetheless, and one went on to have a minimal-residual disease (MRD) negative response, Dr. Lee noted.
In all, 10 of the patients had complete responses: All of these patients had ALL, and three had never previously achieved a remission. The patient with non-Hodgkin’s lymphoma did not have a significant treatment response.
Of eight patients who were negative for MRD after therapy, six went on to have stem cell transplants, with no unexpected toxicities.
As in other CAR-T cell studies, the chief toxicities seen included grade 4 neutropenia lasting longer than 2 weeks in nine patients, and the cytokine-release syndrome in four patients (grade 3 in two patients and grade 4 in two patients). One patient with the cytokine-release syndrome had cardiac arrest but was successfully resuscitated.
The cytokine-release syndrome was found to be associated with interleukin-6 (IL-6) and could be ameliorated with the IL-6 blocking agent tocilizumab (Actemra). The severity of cytokine-release syndrome did not correlate with tumor burden, the researchers noted.
MSKCC Study
Dr. Davila and his colleagues used a slightly different CAR-T cell construction, also with CD28 costimulation, to treat B-ALL in adults who either had refractory or relapsed disease (MRD-positive) or who were in their first complete remission. Patients who were positive for the Philadelphia chromosome (Ph+) and those who had extramedullary disease, CNS leukemia, or were in relapse after allogeneic stem cell transplant were all eligible.
He presented data on 16 patients with B-ALL with long-term follow-up: 14 patients had a complete response, with an average time to complete response of 24.5 days.
Seven patients in the MSKCC study have gone on to allogeneic stem cell transplants; three patients in complete remission were not eligible for transplant because of medical contraindications, and one additional patient was being evaluated for transplant. There have been no post-transplant relapses to date, with the longest follow-up out to 2 years, Dr. Davila said.
As in the NCI study, the cytokine-release syndrome was a common toxicity. The investigators initially tried to manage it with steroids but found that it came at the cost of lymphotoxicity that caused a marked decline in serum T-cells. They subsequently switched to tocilizumab, which was effective at treating the syndrome without lymphotoxicity.
Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by MSKCC. He reported having no conflicts of interest.
AT ASH 2013
Major finding: Anti-CD19 chimeric antigen receptor T cells induced complete responses in 10 of 16 children and young adults with relapsed/refractory acute lymphoblastic leukemia. In a second study, CD19-targeted T cells induced complete molecular responses in 12 of 16 adults with B-lineage ALL refractory to chemotherapy.
Data source: Phase I studies of 2 novel CAR-T cell therapeutic strategies in a total of 32 patients.
Disclosures: Dr. Lee’s study was supported by the National Cancer Institute and St. Baldrick’s Foundation. He discussed off-label use of CAR-T cells. Dr. Lee reported having no conflicts of interest. Dr. Davila’s study was supported by Memorial Sloan-Kettering Cancer Center. He reported having no conflicts of interest.
Brentuximab vedotin proves active in DLBCL

Credit: Linda Bartlett
NEW ORLEANS—Brentuximab vedotin has demonstrated “compelling” antitumor activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
The investigators were also surprised
to find that the activity of this anti-CD30 monoclonal antibody
conjugate did not seem to
correlate with a patient’s level of CD30 expression.
In fact, some of
the patients with the weakest CD30 expression had the best responses to
the drug.
Eric Jacobsen, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues presented these results at the 2013 ASH Annual Meeting as abstract 848.
Thus far, the researchers have enrolled 62 patients with B-cell lymphomas, including 44 with DLBCL, on this phase 2 study.
Sixty-five percent of patients had primary refractory disease, 76% were refractory to their most recent prior therapy, and 23% had never responded to any treatment.
However, 40% of the 43 evaluable DLBCL patients had an objective response to brentuximab vedotin. The median response duration was 36 weeks, and some responses lasted more than 8 months.
Seven patients had complete remissions, and 10 had partial remissions. In the other B-cell lymphoma patients, 22 had an objective response.
The researchers called this compelling antitumor activity in a highly refractory population.
“[Brentuximab vedotin] was more active than many expected,” Dr Jacobsen said. “In my opinion, these results are encouraging enough to take the drug forward in diffuse large B-cell lymphoma.”
The researchers said the drug’s safety profile was consistent with previous results. Six patients stopped treatment due to adverse events, including 2 who developed peripheral neuropathy.
Treatment-emergent adverse events included fatigue (40%), nausea (37%), neutropenia (37%), fever (32%), diarrhea (31%), peripheral sensory neuropathy (26%), vomiting (23%), anemia (21%), and constipation (21%).
Role of CD30
Brentuximab vedotin is a monoclonal antibody that binds to CD30. This molecule’s expression varies, but researchers have estimated that CD30 is present in a quarter to a third of B-cell non-Hodgkin lymphoma cells.
In this study, some of the patients’ lymphoma cells strongly expressed CD30. But, in other patients, the investigators were unable to detect any CD30 expression at all. And the patients’ level of CD30 expression bore no relationship to how they responded to the drug.
“In fact, although the trend was not statistically significant, there was almost an inverse correlation,” Dr Jacobsen said. “Some patients with the weakest CD30 expression had the most positive responses.”
One possible explanation for this is that the drug bound to another target, but preclinical tests suggested this was not the case. Other possibilities are that brentuximab vedotin binds more effectively to CD30 than the antibody used to detect CD30 in the lab or that different cells have differing abilities to ingest brentuximab once the antibody binds to the cell.
There is no clear answer from the study, Dr Jacobsen said, but lab tests are ongoing. He and his colleagues are beginning to evaluate the drug’s activity in a cohort of patients whose lymphomas have no measurable CD30 expression.
This study was supported by Seattle Genetics. ![]()

Credit: Linda Bartlett
NEW ORLEANS—Brentuximab vedotin has demonstrated “compelling” antitumor activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
The investigators were also surprised
to find that the activity of this anti-CD30 monoclonal antibody
conjugate did not seem to
correlate with a patient’s level of CD30 expression.
In fact, some of
the patients with the weakest CD30 expression had the best responses to
the drug.
Eric Jacobsen, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues presented these results at the 2013 ASH Annual Meeting as abstract 848.
Thus far, the researchers have enrolled 62 patients with B-cell lymphomas, including 44 with DLBCL, on this phase 2 study.
Sixty-five percent of patients had primary refractory disease, 76% were refractory to their most recent prior therapy, and 23% had never responded to any treatment.
However, 40% of the 43 evaluable DLBCL patients had an objective response to brentuximab vedotin. The median response duration was 36 weeks, and some responses lasted more than 8 months.
Seven patients had complete remissions, and 10 had partial remissions. In the other B-cell lymphoma patients, 22 had an objective response.
The researchers called this compelling antitumor activity in a highly refractory population.
“[Brentuximab vedotin] was more active than many expected,” Dr Jacobsen said. “In my opinion, these results are encouraging enough to take the drug forward in diffuse large B-cell lymphoma.”
The researchers said the drug’s safety profile was consistent with previous results. Six patients stopped treatment due to adverse events, including 2 who developed peripheral neuropathy.
Treatment-emergent adverse events included fatigue (40%), nausea (37%), neutropenia (37%), fever (32%), diarrhea (31%), peripheral sensory neuropathy (26%), vomiting (23%), anemia (21%), and constipation (21%).
Role of CD30
Brentuximab vedotin is a monoclonal antibody that binds to CD30. This molecule’s expression varies, but researchers have estimated that CD30 is present in a quarter to a third of B-cell non-Hodgkin lymphoma cells.
In this study, some of the patients’ lymphoma cells strongly expressed CD30. But, in other patients, the investigators were unable to detect any CD30 expression at all. And the patients’ level of CD30 expression bore no relationship to how they responded to the drug.
“In fact, although the trend was not statistically significant, there was almost an inverse correlation,” Dr Jacobsen said. “Some patients with the weakest CD30 expression had the most positive responses.”
One possible explanation for this is that the drug bound to another target, but preclinical tests suggested this was not the case. Other possibilities are that brentuximab vedotin binds more effectively to CD30 than the antibody used to detect CD30 in the lab or that different cells have differing abilities to ingest brentuximab once the antibody binds to the cell.
There is no clear answer from the study, Dr Jacobsen said, but lab tests are ongoing. He and his colleagues are beginning to evaluate the drug’s activity in a cohort of patients whose lymphomas have no measurable CD30 expression.
This study was supported by Seattle Genetics. ![]()

Credit: Linda Bartlett
NEW ORLEANS—Brentuximab vedotin has demonstrated “compelling” antitumor activity in patients with relapsed or refractory diffuse large B-cell lymphoma (DLBCL), according to researchers.
The investigators were also surprised
to find that the activity of this anti-CD30 monoclonal antibody
conjugate did not seem to
correlate with a patient’s level of CD30 expression.
In fact, some of
the patients with the weakest CD30 expression had the best responses to
the drug.
Eric Jacobsen, MD, of the Dana-Farber Cancer Institute in Boston, Massachusetts, and his colleagues presented these results at the 2013 ASH Annual Meeting as abstract 848.
Thus far, the researchers have enrolled 62 patients with B-cell lymphomas, including 44 with DLBCL, on this phase 2 study.
Sixty-five percent of patients had primary refractory disease, 76% were refractory to their most recent prior therapy, and 23% had never responded to any treatment.
However, 40% of the 43 evaluable DLBCL patients had an objective response to brentuximab vedotin. The median response duration was 36 weeks, and some responses lasted more than 8 months.
Seven patients had complete remissions, and 10 had partial remissions. In the other B-cell lymphoma patients, 22 had an objective response.
The researchers called this compelling antitumor activity in a highly refractory population.
“[Brentuximab vedotin] was more active than many expected,” Dr Jacobsen said. “In my opinion, these results are encouraging enough to take the drug forward in diffuse large B-cell lymphoma.”
The researchers said the drug’s safety profile was consistent with previous results. Six patients stopped treatment due to adverse events, including 2 who developed peripheral neuropathy.
Treatment-emergent adverse events included fatigue (40%), nausea (37%), neutropenia (37%), fever (32%), diarrhea (31%), peripheral sensory neuropathy (26%), vomiting (23%), anemia (21%), and constipation (21%).
Role of CD30
Brentuximab vedotin is a monoclonal antibody that binds to CD30. This molecule’s expression varies, but researchers have estimated that CD30 is present in a quarter to a third of B-cell non-Hodgkin lymphoma cells.
In this study, some of the patients’ lymphoma cells strongly expressed CD30. But, in other patients, the investigators were unable to detect any CD30 expression at all. And the patients’ level of CD30 expression bore no relationship to how they responded to the drug.
“In fact, although the trend was not statistically significant, there was almost an inverse correlation,” Dr Jacobsen said. “Some patients with the weakest CD30 expression had the most positive responses.”
One possible explanation for this is that the drug bound to another target, but preclinical tests suggested this was not the case. Other possibilities are that brentuximab vedotin binds more effectively to CD30 than the antibody used to detect CD30 in the lab or that different cells have differing abilities to ingest brentuximab once the antibody binds to the cell.
There is no clear answer from the study, Dr Jacobsen said, but lab tests are ongoing. He and his colleagues are beginning to evaluate the drug’s activity in a cohort of patients whose lymphomas have no measurable CD30 expression.
This study was supported by Seattle Genetics. ![]()
Corneal nerve fiber loss may predict diabetic neuropathy
MELBOURNE – Corneal nerve fiber length, measured using corneal confocal microscopy, is significantly reduced in individuals with type 1 diabetes who go on to develop diabetic neuropathy at 3 years, according to data from the longitudinal LANDMark study.
Researchers found that corneal nerve fibre length was significantly lower at baseline in individuals who developed neuropathy than in those who did not over the 3-year follow up (13.3 vs. 17.4 mm/mm2, respectively; P = 0.036).
Corneal nerve fiber length, which is a measure of amount of nerve tissue per unit area in the cornea, may be an useful, noninvasive adjunct to diabetic neuropathy screening, Nicola Pritchard, a researcher for the Institute of Health and Biomedical Innovation at Queensland University of Technology, Brisbane, suggested in her presentation of the results at the World Diabetes Congress.
"In animal models, we know that the dropout of nerves in the cornea actually does precede the dropout of nerves in the foot," Ms. Pritchard said in an interview.
"Our hope is that this technique will be useful to pick up very, very early signs of neuropathy, way before people are getting symptoms and before things develop to a stage where there’s damage," she said.
LANDMark (Longitudinal Assessment of Neuropathy in Diabetes Using Novel Ophthalmic Markers) is a 5-year observational study of 242 individuals with type 1 diabetes.
The 3-year analysis included data from 64 participants without baseline neuropathy, seven (11%) of whom had developed neuropathy by 3 years, as defined by the Toronto criteria.
Study participants undergo annual neuropathy assessments, including measurement of corneal nerve parameters using corneal confocal microscopy and measurements of corneal sensitivity using noncontact corneal esthesiometry.
The study showed that reduced peroneal conduction velocity and cold sensation and increased vibration threshold also were associated with development of diabetic neuropathy.
However, although corneal nerve fiber length was significantly reduced at baseline in individuals who developed neuropathy, compared with those who did not, at the 3-year mark there was no significant difference in corneal nerve fiber length between the two groups.
Ms. Pritchard said that it was unclear why the nerve fibre parameters improved over time, suggesting that perhaps the nerves were growing to fill in the gaps.
Nathan Efron, D.Sc., research leader of the LANDMark study, said corneal confocal microscopy had the potential to be a very simple screening technique for diabetic neuropathy that could be applied at the same time as patients come in for their annual fundus photographs.
"At the very least, it’s a viable alternative technique to the range of techniques neurologists and diabetic specialists already have at their disposal, but the potential advantage of this technique is that it might be a very early marker of diabetic neuropathy," said Dr. Efron, professor in the School of Optometry and Vision Science at Queensland University of Technology.
"We’re not there yet, but down the line, that’s where this all could come to, as long as we can get more firm data and validate it a bit better," he said.
There were no relevant conflicts of interest declared.
MELBOURNE – Corneal nerve fiber length, measured using corneal confocal microscopy, is significantly reduced in individuals with type 1 diabetes who go on to develop diabetic neuropathy at 3 years, according to data from the longitudinal LANDMark study.
Researchers found that corneal nerve fibre length was significantly lower at baseline in individuals who developed neuropathy than in those who did not over the 3-year follow up (13.3 vs. 17.4 mm/mm2, respectively; P = 0.036).
Corneal nerve fiber length, which is a measure of amount of nerve tissue per unit area in the cornea, may be an useful, noninvasive adjunct to diabetic neuropathy screening, Nicola Pritchard, a researcher for the Institute of Health and Biomedical Innovation at Queensland University of Technology, Brisbane, suggested in her presentation of the results at the World Diabetes Congress.
"In animal models, we know that the dropout of nerves in the cornea actually does precede the dropout of nerves in the foot," Ms. Pritchard said in an interview.
"Our hope is that this technique will be useful to pick up very, very early signs of neuropathy, way before people are getting symptoms and before things develop to a stage where there’s damage," she said.
LANDMark (Longitudinal Assessment of Neuropathy in Diabetes Using Novel Ophthalmic Markers) is a 5-year observational study of 242 individuals with type 1 diabetes.
The 3-year analysis included data from 64 participants without baseline neuropathy, seven (11%) of whom had developed neuropathy by 3 years, as defined by the Toronto criteria.
Study participants undergo annual neuropathy assessments, including measurement of corneal nerve parameters using corneal confocal microscopy and measurements of corneal sensitivity using noncontact corneal esthesiometry.
The study showed that reduced peroneal conduction velocity and cold sensation and increased vibration threshold also were associated with development of diabetic neuropathy.
However, although corneal nerve fiber length was significantly reduced at baseline in individuals who developed neuropathy, compared with those who did not, at the 3-year mark there was no significant difference in corneal nerve fiber length between the two groups.
Ms. Pritchard said that it was unclear why the nerve fibre parameters improved over time, suggesting that perhaps the nerves were growing to fill in the gaps.
Nathan Efron, D.Sc., research leader of the LANDMark study, said corneal confocal microscopy had the potential to be a very simple screening technique for diabetic neuropathy that could be applied at the same time as patients come in for their annual fundus photographs.
"At the very least, it’s a viable alternative technique to the range of techniques neurologists and diabetic specialists already have at their disposal, but the potential advantage of this technique is that it might be a very early marker of diabetic neuropathy," said Dr. Efron, professor in the School of Optometry and Vision Science at Queensland University of Technology.
"We’re not there yet, but down the line, that’s where this all could come to, as long as we can get more firm data and validate it a bit better," he said.
There were no relevant conflicts of interest declared.
MELBOURNE – Corneal nerve fiber length, measured using corneal confocal microscopy, is significantly reduced in individuals with type 1 diabetes who go on to develop diabetic neuropathy at 3 years, according to data from the longitudinal LANDMark study.
Researchers found that corneal nerve fibre length was significantly lower at baseline in individuals who developed neuropathy than in those who did not over the 3-year follow up (13.3 vs. 17.4 mm/mm2, respectively; P = 0.036).
Corneal nerve fiber length, which is a measure of amount of nerve tissue per unit area in the cornea, may be an useful, noninvasive adjunct to diabetic neuropathy screening, Nicola Pritchard, a researcher for the Institute of Health and Biomedical Innovation at Queensland University of Technology, Brisbane, suggested in her presentation of the results at the World Diabetes Congress.
"In animal models, we know that the dropout of nerves in the cornea actually does precede the dropout of nerves in the foot," Ms. Pritchard said in an interview.
"Our hope is that this technique will be useful to pick up very, very early signs of neuropathy, way before people are getting symptoms and before things develop to a stage where there’s damage," she said.
LANDMark (Longitudinal Assessment of Neuropathy in Diabetes Using Novel Ophthalmic Markers) is a 5-year observational study of 242 individuals with type 1 diabetes.
The 3-year analysis included data from 64 participants without baseline neuropathy, seven (11%) of whom had developed neuropathy by 3 years, as defined by the Toronto criteria.
Study participants undergo annual neuropathy assessments, including measurement of corneal nerve parameters using corneal confocal microscopy and measurements of corneal sensitivity using noncontact corneal esthesiometry.
The study showed that reduced peroneal conduction velocity and cold sensation and increased vibration threshold also were associated with development of diabetic neuropathy.
However, although corneal nerve fiber length was significantly reduced at baseline in individuals who developed neuropathy, compared with those who did not, at the 3-year mark there was no significant difference in corneal nerve fiber length between the two groups.
Ms. Pritchard said that it was unclear why the nerve fibre parameters improved over time, suggesting that perhaps the nerves were growing to fill in the gaps.
Nathan Efron, D.Sc., research leader of the LANDMark study, said corneal confocal microscopy had the potential to be a very simple screening technique for diabetic neuropathy that could be applied at the same time as patients come in for their annual fundus photographs.
"At the very least, it’s a viable alternative technique to the range of techniques neurologists and diabetic specialists already have at their disposal, but the potential advantage of this technique is that it might be a very early marker of diabetic neuropathy," said Dr. Efron, professor in the School of Optometry and Vision Science at Queensland University of Technology.
"We’re not there yet, but down the line, that’s where this all could come to, as long as we can get more firm data and validate it a bit better," he said.
There were no relevant conflicts of interest declared.
AT THE WORLD DIABETES CONGRESS
Major finding: Corneal nerve fiber length was significantly lower at baseline in individuals who developed neuropathy at 3 years, compared with those who did not develop neuropathy (13.3 vs. 17.4 mm/mm2, respectively; P = 0.036).
Data source: Analysis of baseline and 3-year data from the LANDMark trial looking at novel ophthalmic markers of neuropathy in 242 individuals with type 1 diabetes.
Disclosures: No financial conflicts of interest declared.
New and Noteworthy Information—January 2014
The Bacille Calmette-Guérin (BCG) vaccine may benefit patients with clinically isolated syndrome (CIS), according to research published online ahead of print December 4, 2013, in Neurology. A total of 82 participants with CIS were randomized to BCG or placebo and monitored monthly with brain MRI for six months. All patients subsequently received IM interferon β-1a for 12 months. In an open-label extension phase, patients received disease-modifying therapies (DMTs) recommended by their neurologists. During the initial six months, the number of cumulative lesions was significantly lower among vaccinated subjects. The number of total T1-hypointense lesions was lower in the BCG group at months 6, 12, and 18. After 60 months, the probability of clinically definite multiple sclerosis was lower in the BCG plus DMT arm, and more vaccinated people remained DMT-free.
Exercise programs may significantly improve the ability of people with dementia to perform activities of daily living, according to a study published online ahead of print December 4, 2013, in the Cochrane Library. Exercise also may improve cognition in these patients, but may not affect depression. Investigators reviewed randomized controlled trials in which older people diagnosed with dementia were allocated to exercise programs or to control groups, which received standard care or social contact. Sixteen trials with 937 participants met the inclusion criteria. The trials were highly heterogeneous in terms of subtype and severity of participants’ dementia, and type, duration, and frequency of exercise. The researchers found that informal caregivers’ burden may be reduced when the family member with dementia participates in an exercise program.
Thrombin activity may enable neurologists to detect multiple sclerosis (MS) before clinical signs of the disease are present, according to research published online ahead of print November 29, 2013, in Annals of Neurology. Using a novel molecular probe, investigators characterized the activity pattern of thrombin, the central protease of the coagulation cascade, in experimental autoimmune encephalomyelitis. Thrombin activity preceded the onset of neurologic signs; increased at disease peak; and correlated with fibrin deposition, microglial activation, demyelination, axonal damage, and clinical severity. Mice with a genetic deficit in prothrombin confirmed the specificity of the thrombin probe. Scientists may be able to use thrombin activity to develop sensitive probes for the preclinical detection and monitoring of neuroinflammation and MS progression, according to the investigators.
An athlete with concussion symptoms should not be allowed to return to play on the same day, according to the latest consensus statement on sports-related concussion, which was summarized in the December 2013 issue of Neurosurgery. The Concussion in Sport Group (CISG 4) based its recommendations on the advice of an expert panel that was sponsored by five international sports governing bodies. Between 80% and 90% of concussions resolve within seven to 10 days, but recovery may take longer in children and adolescents, according to the consensus statement. The updated statement emphasizes the distinction between concussion and mild traumatic brain injury. The CISG 4 suggests that patients with concussion have normal findings on brain neuroimaging studies (eg, CT scan), but those with traumatic brain injury have abnormal imaging findings.
Vitamin D may prevent multiple sclerosis (MS) by blocking T helper (TH) cells from migrating into the CNS, according to research published online ahead of print December 9, 2013, in Proceedings of the National Academy of Sciences. Investigators administered 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the bioactive form of vitamin D, to animals with experimental autoimmune encephalomyelitis, a mouse model of MS. Myelin-reactive TH cells were generated in the presence of 1,25(OH)2D3, secreted proinflammatory cytokines, and did not preferentially differentiate into suppressor T cells. The cells left the lymph node, entered the peripheral circulation, and migrated to the immunization sites. TH cells from 1,25(OH)2D3-treated mice were unable to enter the CNS parenchyma, however. Instead, the cells were maintained in the periphery. The mice developed experimental autoimmune encephalomyelitis when treatment ceased.
Among people with type 2 diabetes, dementia incidence may be highest among Native Americans and African Americans and lowest among Asians, according to a study published online ahead of print November 22, 2013, in Diabetes Care. Scientists identified 22,171 patients age 60 or older with diabetes and without preexisting dementia in the Kaiser Permanente Northern California Diabetes Registry. The investigators abstracted prevalent medical history and dementia incidence from medical records and calculated age-adjusted incidence densities. Dementia was diagnosed in 17.1% of patients. Age-adjusted dementia incidence densities were 34/1,000 person-years among Native Americans, 27/1,000 person-years among African Americans, and 19/1,000 person-years among Asians. Hazard ratios (relative to Asians) were 1.64 for Native Americans, 1.44 for African Americans, 1.30 for non-Hispanic whites, and 1.19 for Latinos.
Veterans with blast injuries have changes in brain tissue that may be apparent on imaging years later, according to data presented at the 99th Annual Meeting of the Radiological Society of North America. Researchers compared diffusion tensor imaging (DTI)-derived fractional anisotropy (FA) values in 10 veterans of Operations Iraqi Freedom and Enduring Freedom who had been diagnosed with mild traumatic brain injury with those of 10 healthy controls. The average time elapsed between the blast-induced injury and DTI scan among the patients was 51.3 months. FA values were significantly different between the two groups, and the researchers found significant correlations between FA values and attention, delayed memory, and psychomotor test scores. The results suggest that blast injury may have a long-term impact on the brain.
Among college athletes, head impact exposure may be related to white matter diffusion measures and cognition during the course of one playing season, even in the absence of diagnosed concussion, according to data published online ahead of print December 11, 2013, in Neurology. Researchers prospectively studied 79 noncontact sport athletes and 80 nonconcussed varsity football and ice hockey players who wore helmets that recorded the acceleration-time history of the head following impact. Mean diffusivity (MD) in the corpus callosum was significantly different between groups. Measures of head impact exposure correlated with white matter diffusivity measures in the corpus callosum, amygdala, cerebellar white matter, hippocampus, and thalamus. The magnitude of change in corpus callosum MD postseason was associated with poorer performance on a measure of verbal learning and memory.
Among veterans, traumatic brain injury (TBI) during the most recent deployment is the strongest predictor of postdeployment symptoms of post-traumatic stress disorder (PTSD), even when accounting for predeployment symptoms, prior TBI, and combat intensity, according to research published online ahead of print December 11, 2013, in JAMA Psychiatry. A total of 1,648 active-duty Marine and Navy servicemen underwent clinical interviews and completed self-assessments approximately one month before a seven-month deployment and three to six months after deployment. At the predeployment assessment, 56.8% of participants reported prior TBI. At postdeployment assessment, 19.8% reported sustaining TBI between predeployment and postdeployment assessments. Probability of PTSD was highest for participants with severe predeployment symptoms, high combat intensity, and deployment-related TBI. TBI doubled the PTSD rates for participants with less severe predeployment PTSD symptoms.
Fidgetin inhibition could promote tissue regeneration and repair the broken cell connections that occur in spinal cord injury and other conditions, according to research presented at the 2013 Annual Meeting of the American Society for Cell Biology. Fidgetin prunes unstable microtubule scaffolding in cells, as well as unneeded connections in the neuronal network as the latter grows. Researchers used a novel nanoparticle technology to block fidgetin in the injured nerves of adult rats. The nanoparticles were infused with small interfering RNA that bound the messenger RNA (mRNA) transcribed from the fidgetin gene. The mRNA for fidgetin was not translated, and the cell did not produce fidgetin. Blocking fidgetin restarted tissue growth in the animals. The technique could benefit patients with myocardial infarction or chronic cutaneous wounds.
Deep brain stimulation may improve driving ability for people with Parkinson’s disease, according to a study published online ahead of print December 18, 2013, in Neurology. Investigators studied 23 people who had deep brain stimulators, 21 people with Parkinson’s disease without stimulators, and 21 healthy individuals. Participants were tested with a driving simulator. Individuals with stimulators completed the test once with the stimulator on, once with it off, and once with the stimulator off after receiving levodopa. People with Parkinson’s disease without stimulators performed worse than controls in almost every category. People with stimulators did not perform significantly worse than the controls. Participants with stimulators had an average of 3.8 slight driving errors on the test, compared with 7.5 for the controls and 11.4 for people with Parkinson’s disease without stimulators.
Gadolinium-based contrast medium (Gd-CM) may be associated with abnormalities on brain MRI, according to research published online ahead of print December 17, 2013, in Radiology. Researchers compared unenhanced T1-weighted MR images of 19 patients who had undergone six or more contrast-enhanced brain scans with images of 16 people who had received six or fewer unenhanced scans. The hyperintensity of the dentate nucleus and globus pallidus correlated with the number of Gd-CM administrations. Hyperintensity in the dentate nucleus and globus pallidus on unenhanced MRI may be a consequence of the number of previous Gd-CM administrations, according to the researchers. Because gadolinium has a high signal intensity in the body, the data suggest that the toxic gadolinium component remains in the body in patients with normal renal function.
—Erik Greb
The Bacille Calmette-Guérin (BCG) vaccine may benefit patients with clinically isolated syndrome (CIS), according to research published online ahead of print December 4, 2013, in Neurology. A total of 82 participants with CIS were randomized to BCG or placebo and monitored monthly with brain MRI for six months. All patients subsequently received IM interferon β-1a for 12 months. In an open-label extension phase, patients received disease-modifying therapies (DMTs) recommended by their neurologists. During the initial six months, the number of cumulative lesions was significantly lower among vaccinated subjects. The number of total T1-hypointense lesions was lower in the BCG group at months 6, 12, and 18. After 60 months, the probability of clinically definite multiple sclerosis was lower in the BCG plus DMT arm, and more vaccinated people remained DMT-free.
Exercise programs may significantly improve the ability of people with dementia to perform activities of daily living, according to a study published online ahead of print December 4, 2013, in the Cochrane Library. Exercise also may improve cognition in these patients, but may not affect depression. Investigators reviewed randomized controlled trials in which older people diagnosed with dementia were allocated to exercise programs or to control groups, which received standard care or social contact. Sixteen trials with 937 participants met the inclusion criteria. The trials were highly heterogeneous in terms of subtype and severity of participants’ dementia, and type, duration, and frequency of exercise. The researchers found that informal caregivers’ burden may be reduced when the family member with dementia participates in an exercise program.
Thrombin activity may enable neurologists to detect multiple sclerosis (MS) before clinical signs of the disease are present, according to research published online ahead of print November 29, 2013, in Annals of Neurology. Using a novel molecular probe, investigators characterized the activity pattern of thrombin, the central protease of the coagulation cascade, in experimental autoimmune encephalomyelitis. Thrombin activity preceded the onset of neurologic signs; increased at disease peak; and correlated with fibrin deposition, microglial activation, demyelination, axonal damage, and clinical severity. Mice with a genetic deficit in prothrombin confirmed the specificity of the thrombin probe. Scientists may be able to use thrombin activity to develop sensitive probes for the preclinical detection and monitoring of neuroinflammation and MS progression, according to the investigators.
An athlete with concussion symptoms should not be allowed to return to play on the same day, according to the latest consensus statement on sports-related concussion, which was summarized in the December 2013 issue of Neurosurgery. The Concussion in Sport Group (CISG 4) based its recommendations on the advice of an expert panel that was sponsored by five international sports governing bodies. Between 80% and 90% of concussions resolve within seven to 10 days, but recovery may take longer in children and adolescents, according to the consensus statement. The updated statement emphasizes the distinction between concussion and mild traumatic brain injury. The CISG 4 suggests that patients with concussion have normal findings on brain neuroimaging studies (eg, CT scan), but those with traumatic brain injury have abnormal imaging findings.
Vitamin D may prevent multiple sclerosis (MS) by blocking T helper (TH) cells from migrating into the CNS, according to research published online ahead of print December 9, 2013, in Proceedings of the National Academy of Sciences. Investigators administered 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the bioactive form of vitamin D, to animals with experimental autoimmune encephalomyelitis, a mouse model of MS. Myelin-reactive TH cells were generated in the presence of 1,25(OH)2D3, secreted proinflammatory cytokines, and did not preferentially differentiate into suppressor T cells. The cells left the lymph node, entered the peripheral circulation, and migrated to the immunization sites. TH cells from 1,25(OH)2D3-treated mice were unable to enter the CNS parenchyma, however. Instead, the cells were maintained in the periphery. The mice developed experimental autoimmune encephalomyelitis when treatment ceased.
Among people with type 2 diabetes, dementia incidence may be highest among Native Americans and African Americans and lowest among Asians, according to a study published online ahead of print November 22, 2013, in Diabetes Care. Scientists identified 22,171 patients age 60 or older with diabetes and without preexisting dementia in the Kaiser Permanente Northern California Diabetes Registry. The investigators abstracted prevalent medical history and dementia incidence from medical records and calculated age-adjusted incidence densities. Dementia was diagnosed in 17.1% of patients. Age-adjusted dementia incidence densities were 34/1,000 person-years among Native Americans, 27/1,000 person-years among African Americans, and 19/1,000 person-years among Asians. Hazard ratios (relative to Asians) were 1.64 for Native Americans, 1.44 for African Americans, 1.30 for non-Hispanic whites, and 1.19 for Latinos.
Veterans with blast injuries have changes in brain tissue that may be apparent on imaging years later, according to data presented at the 99th Annual Meeting of the Radiological Society of North America. Researchers compared diffusion tensor imaging (DTI)-derived fractional anisotropy (FA) values in 10 veterans of Operations Iraqi Freedom and Enduring Freedom who had been diagnosed with mild traumatic brain injury with those of 10 healthy controls. The average time elapsed between the blast-induced injury and DTI scan among the patients was 51.3 months. FA values were significantly different between the two groups, and the researchers found significant correlations between FA values and attention, delayed memory, and psychomotor test scores. The results suggest that blast injury may have a long-term impact on the brain.
Among college athletes, head impact exposure may be related to white matter diffusion measures and cognition during the course of one playing season, even in the absence of diagnosed concussion, according to data published online ahead of print December 11, 2013, in Neurology. Researchers prospectively studied 79 noncontact sport athletes and 80 nonconcussed varsity football and ice hockey players who wore helmets that recorded the acceleration-time history of the head following impact. Mean diffusivity (MD) in the corpus callosum was significantly different between groups. Measures of head impact exposure correlated with white matter diffusivity measures in the corpus callosum, amygdala, cerebellar white matter, hippocampus, and thalamus. The magnitude of change in corpus callosum MD postseason was associated with poorer performance on a measure of verbal learning and memory.
Among veterans, traumatic brain injury (TBI) during the most recent deployment is the strongest predictor of postdeployment symptoms of post-traumatic stress disorder (PTSD), even when accounting for predeployment symptoms, prior TBI, and combat intensity, according to research published online ahead of print December 11, 2013, in JAMA Psychiatry. A total of 1,648 active-duty Marine and Navy servicemen underwent clinical interviews and completed self-assessments approximately one month before a seven-month deployment and three to six months after deployment. At the predeployment assessment, 56.8% of participants reported prior TBI. At postdeployment assessment, 19.8% reported sustaining TBI between predeployment and postdeployment assessments. Probability of PTSD was highest for participants with severe predeployment symptoms, high combat intensity, and deployment-related TBI. TBI doubled the PTSD rates for participants with less severe predeployment PTSD symptoms.
Fidgetin inhibition could promote tissue regeneration and repair the broken cell connections that occur in spinal cord injury and other conditions, according to research presented at the 2013 Annual Meeting of the American Society for Cell Biology. Fidgetin prunes unstable microtubule scaffolding in cells, as well as unneeded connections in the neuronal network as the latter grows. Researchers used a novel nanoparticle technology to block fidgetin in the injured nerves of adult rats. The nanoparticles were infused with small interfering RNA that bound the messenger RNA (mRNA) transcribed from the fidgetin gene. The mRNA for fidgetin was not translated, and the cell did not produce fidgetin. Blocking fidgetin restarted tissue growth in the animals. The technique could benefit patients with myocardial infarction or chronic cutaneous wounds.
Deep brain stimulation may improve driving ability for people with Parkinson’s disease, according to a study published online ahead of print December 18, 2013, in Neurology. Investigators studied 23 people who had deep brain stimulators, 21 people with Parkinson’s disease without stimulators, and 21 healthy individuals. Participants were tested with a driving simulator. Individuals with stimulators completed the test once with the stimulator on, once with it off, and once with the stimulator off after receiving levodopa. People with Parkinson’s disease without stimulators performed worse than controls in almost every category. People with stimulators did not perform significantly worse than the controls. Participants with stimulators had an average of 3.8 slight driving errors on the test, compared with 7.5 for the controls and 11.4 for people with Parkinson’s disease without stimulators.
Gadolinium-based contrast medium (Gd-CM) may be associated with abnormalities on brain MRI, according to research published online ahead of print December 17, 2013, in Radiology. Researchers compared unenhanced T1-weighted MR images of 19 patients who had undergone six or more contrast-enhanced brain scans with images of 16 people who had received six or fewer unenhanced scans. The hyperintensity of the dentate nucleus and globus pallidus correlated with the number of Gd-CM administrations. Hyperintensity in the dentate nucleus and globus pallidus on unenhanced MRI may be a consequence of the number of previous Gd-CM administrations, according to the researchers. Because gadolinium has a high signal intensity in the body, the data suggest that the toxic gadolinium component remains in the body in patients with normal renal function.
—Erik Greb
The Bacille Calmette-Guérin (BCG) vaccine may benefit patients with clinically isolated syndrome (CIS), according to research published online ahead of print December 4, 2013, in Neurology. A total of 82 participants with CIS were randomized to BCG or placebo and monitored monthly with brain MRI for six months. All patients subsequently received IM interferon β-1a for 12 months. In an open-label extension phase, patients received disease-modifying therapies (DMTs) recommended by their neurologists. During the initial six months, the number of cumulative lesions was significantly lower among vaccinated subjects. The number of total T1-hypointense lesions was lower in the BCG group at months 6, 12, and 18. After 60 months, the probability of clinically definite multiple sclerosis was lower in the BCG plus DMT arm, and more vaccinated people remained DMT-free.
Exercise programs may significantly improve the ability of people with dementia to perform activities of daily living, according to a study published online ahead of print December 4, 2013, in the Cochrane Library. Exercise also may improve cognition in these patients, but may not affect depression. Investigators reviewed randomized controlled trials in which older people diagnosed with dementia were allocated to exercise programs or to control groups, which received standard care or social contact. Sixteen trials with 937 participants met the inclusion criteria. The trials were highly heterogeneous in terms of subtype and severity of participants’ dementia, and type, duration, and frequency of exercise. The researchers found that informal caregivers’ burden may be reduced when the family member with dementia participates in an exercise program.
Thrombin activity may enable neurologists to detect multiple sclerosis (MS) before clinical signs of the disease are present, according to research published online ahead of print November 29, 2013, in Annals of Neurology. Using a novel molecular probe, investigators characterized the activity pattern of thrombin, the central protease of the coagulation cascade, in experimental autoimmune encephalomyelitis. Thrombin activity preceded the onset of neurologic signs; increased at disease peak; and correlated with fibrin deposition, microglial activation, demyelination, axonal damage, and clinical severity. Mice with a genetic deficit in prothrombin confirmed the specificity of the thrombin probe. Scientists may be able to use thrombin activity to develop sensitive probes for the preclinical detection and monitoring of neuroinflammation and MS progression, according to the investigators.
An athlete with concussion symptoms should not be allowed to return to play on the same day, according to the latest consensus statement on sports-related concussion, which was summarized in the December 2013 issue of Neurosurgery. The Concussion in Sport Group (CISG 4) based its recommendations on the advice of an expert panel that was sponsored by five international sports governing bodies. Between 80% and 90% of concussions resolve within seven to 10 days, but recovery may take longer in children and adolescents, according to the consensus statement. The updated statement emphasizes the distinction between concussion and mild traumatic brain injury. The CISG 4 suggests that patients with concussion have normal findings on brain neuroimaging studies (eg, CT scan), but those with traumatic brain injury have abnormal imaging findings.
Vitamin D may prevent multiple sclerosis (MS) by blocking T helper (TH) cells from migrating into the CNS, according to research published online ahead of print December 9, 2013, in Proceedings of the National Academy of Sciences. Investigators administered 1,25-dihydroxyvitamin D3 [1,25(OH)2D3], the bioactive form of vitamin D, to animals with experimental autoimmune encephalomyelitis, a mouse model of MS. Myelin-reactive TH cells were generated in the presence of 1,25(OH)2D3, secreted proinflammatory cytokines, and did not preferentially differentiate into suppressor T cells. The cells left the lymph node, entered the peripheral circulation, and migrated to the immunization sites. TH cells from 1,25(OH)2D3-treated mice were unable to enter the CNS parenchyma, however. Instead, the cells were maintained in the periphery. The mice developed experimental autoimmune encephalomyelitis when treatment ceased.
Among people with type 2 diabetes, dementia incidence may be highest among Native Americans and African Americans and lowest among Asians, according to a study published online ahead of print November 22, 2013, in Diabetes Care. Scientists identified 22,171 patients age 60 or older with diabetes and without preexisting dementia in the Kaiser Permanente Northern California Diabetes Registry. The investigators abstracted prevalent medical history and dementia incidence from medical records and calculated age-adjusted incidence densities. Dementia was diagnosed in 17.1% of patients. Age-adjusted dementia incidence densities were 34/1,000 person-years among Native Americans, 27/1,000 person-years among African Americans, and 19/1,000 person-years among Asians. Hazard ratios (relative to Asians) were 1.64 for Native Americans, 1.44 for African Americans, 1.30 for non-Hispanic whites, and 1.19 for Latinos.
Veterans with blast injuries have changes in brain tissue that may be apparent on imaging years later, according to data presented at the 99th Annual Meeting of the Radiological Society of North America. Researchers compared diffusion tensor imaging (DTI)-derived fractional anisotropy (FA) values in 10 veterans of Operations Iraqi Freedom and Enduring Freedom who had been diagnosed with mild traumatic brain injury with those of 10 healthy controls. The average time elapsed between the blast-induced injury and DTI scan among the patients was 51.3 months. FA values were significantly different between the two groups, and the researchers found significant correlations between FA values and attention, delayed memory, and psychomotor test scores. The results suggest that blast injury may have a long-term impact on the brain.
Among college athletes, head impact exposure may be related to white matter diffusion measures and cognition during the course of one playing season, even in the absence of diagnosed concussion, according to data published online ahead of print December 11, 2013, in Neurology. Researchers prospectively studied 79 noncontact sport athletes and 80 nonconcussed varsity football and ice hockey players who wore helmets that recorded the acceleration-time history of the head following impact. Mean diffusivity (MD) in the corpus callosum was significantly different between groups. Measures of head impact exposure correlated with white matter diffusivity measures in the corpus callosum, amygdala, cerebellar white matter, hippocampus, and thalamus. The magnitude of change in corpus callosum MD postseason was associated with poorer performance on a measure of verbal learning and memory.
Among veterans, traumatic brain injury (TBI) during the most recent deployment is the strongest predictor of postdeployment symptoms of post-traumatic stress disorder (PTSD), even when accounting for predeployment symptoms, prior TBI, and combat intensity, according to research published online ahead of print December 11, 2013, in JAMA Psychiatry. A total of 1,648 active-duty Marine and Navy servicemen underwent clinical interviews and completed self-assessments approximately one month before a seven-month deployment and three to six months after deployment. At the predeployment assessment, 56.8% of participants reported prior TBI. At postdeployment assessment, 19.8% reported sustaining TBI between predeployment and postdeployment assessments. Probability of PTSD was highest for participants with severe predeployment symptoms, high combat intensity, and deployment-related TBI. TBI doubled the PTSD rates for participants with less severe predeployment PTSD symptoms.
Fidgetin inhibition could promote tissue regeneration and repair the broken cell connections that occur in spinal cord injury and other conditions, according to research presented at the 2013 Annual Meeting of the American Society for Cell Biology. Fidgetin prunes unstable microtubule scaffolding in cells, as well as unneeded connections in the neuronal network as the latter grows. Researchers used a novel nanoparticle technology to block fidgetin in the injured nerves of adult rats. The nanoparticles were infused with small interfering RNA that bound the messenger RNA (mRNA) transcribed from the fidgetin gene. The mRNA for fidgetin was not translated, and the cell did not produce fidgetin. Blocking fidgetin restarted tissue growth in the animals. The technique could benefit patients with myocardial infarction or chronic cutaneous wounds.
Deep brain stimulation may improve driving ability for people with Parkinson’s disease, according to a study published online ahead of print December 18, 2013, in Neurology. Investigators studied 23 people who had deep brain stimulators, 21 people with Parkinson’s disease without stimulators, and 21 healthy individuals. Participants were tested with a driving simulator. Individuals with stimulators completed the test once with the stimulator on, once with it off, and once with the stimulator off after receiving levodopa. People with Parkinson’s disease without stimulators performed worse than controls in almost every category. People with stimulators did not perform significantly worse than the controls. Participants with stimulators had an average of 3.8 slight driving errors on the test, compared with 7.5 for the controls and 11.4 for people with Parkinson’s disease without stimulators.
Gadolinium-based contrast medium (Gd-CM) may be associated with abnormalities on brain MRI, according to research published online ahead of print December 17, 2013, in Radiology. Researchers compared unenhanced T1-weighted MR images of 19 patients who had undergone six or more contrast-enhanced brain scans with images of 16 people who had received six or fewer unenhanced scans. The hyperintensity of the dentate nucleus and globus pallidus correlated with the number of Gd-CM administrations. Hyperintensity in the dentate nucleus and globus pallidus on unenhanced MRI may be a consequence of the number of previous Gd-CM administrations, according to the researchers. Because gadolinium has a high signal intensity in the body, the data suggest that the toxic gadolinium component remains in the body in patients with normal renal function.
—Erik Greb
Ponatinib may be returning to market with new safety measures
The leukemia drug ponatinib is expected to return to market once the drug’s manufacturer has implemented measures to address the recently discovered safety risks.
The US Food and Drug Administration (FDA) is requiring that a number of safety measures be adopted, including changes to ponatinib’s label to narrow the drug’s indication and the addition of warnings about the increased risk of thrombosis and venous occlusion associated with ponatinib use.
In addition, dosing and administration recommendations must be revised, the patient medication guide must be updated, a risk evaluation and mitigation strategy (REMS) must be implemented, and postmarket investigations must be conducted to further characterize the drug’s safety and dosing.
Ponatinib was approved by the FDA in December 2012 to treat adults with chronic myeloid leukemia (CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) that is resistant to or intolerant of other tyrosine kinase inhibitors (TKIs).
But in October 2013, follow-up results of the phase 2 PACE trial suggested ponatinib can increase a patient’s risk of arterial and venous thrombotic events. So all trials of the drug were placed on partial clinical hold, with the exception of the phase 3 EPIC trial, which was discontinued.
Then, the FDA suspended sales and marketing of ponatinib, pending results of a safety evaluation. Now, the agency has decided ponatinib can return to the market if the new safety measures are implemented.
Safety measures in detail
The FDA is requiring that ponatinib use be restricted to:
- Adults with T315I-positive CML (chronic phase, accelerated phase, or blast phase)
- Adults with T315I-positive Ph+ ALL
- Adults with CML (chronic phase, accelerated phase, or blast phase) who cannot receive another TKI
- Adults with Ph+ ALL who cannot receive another TKI.
The Warnings and Precautions section of the drug’s label must be revised to include a description of the arterial and venous thrombosis and occlusions that have occurred in at least 27%—more than 1 in every 4—of patients treated with ponatinib.
The Dosage and Administration recommendations must be revised to state that the optimal dose of ponatinib has not been identified. The recommended starting dose remains 45 mg administered orally once daily, with or without food.
The patient Medication Guide must be revised to include additional safety information consistent with the safety information in the revised drug label.
The ponatinib REMS must inform prescribers about the approved indications for use and the serious risk of vascular occlusion and thromboembolism associated with the drug. The REMS must include the following:
- REMS letter to healthcare professionals who are known or likely to prescribe ponatinib
- REMS letter for professional societies to be distributed to their members
- REMS fact sheet for healthcare professionals
- Public statement to be published quarterly for 1 year in several professional journals
- Information to be prominently displayed at scientific meetings
- Ponatinib REMS website to provide access to all REMS materials for the duration of the REMS.
And postmarket investigations must further evaluate the dose selection, drug exposure, treatment response, and toxicity of ponatinib.
For more information, see the FDA’s safety communication. ![]()
The leukemia drug ponatinib is expected to return to market once the drug’s manufacturer has implemented measures to address the recently discovered safety risks.
The US Food and Drug Administration (FDA) is requiring that a number of safety measures be adopted, including changes to ponatinib’s label to narrow the drug’s indication and the addition of warnings about the increased risk of thrombosis and venous occlusion associated with ponatinib use.
In addition, dosing and administration recommendations must be revised, the patient medication guide must be updated, a risk evaluation and mitigation strategy (REMS) must be implemented, and postmarket investigations must be conducted to further characterize the drug’s safety and dosing.
Ponatinib was approved by the FDA in December 2012 to treat adults with chronic myeloid leukemia (CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) that is resistant to or intolerant of other tyrosine kinase inhibitors (TKIs).
But in October 2013, follow-up results of the phase 2 PACE trial suggested ponatinib can increase a patient’s risk of arterial and venous thrombotic events. So all trials of the drug were placed on partial clinical hold, with the exception of the phase 3 EPIC trial, which was discontinued.
Then, the FDA suspended sales and marketing of ponatinib, pending results of a safety evaluation. Now, the agency has decided ponatinib can return to the market if the new safety measures are implemented.
Safety measures in detail
The FDA is requiring that ponatinib use be restricted to:
- Adults with T315I-positive CML (chronic phase, accelerated phase, or blast phase)
- Adults with T315I-positive Ph+ ALL
- Adults with CML (chronic phase, accelerated phase, or blast phase) who cannot receive another TKI
- Adults with Ph+ ALL who cannot receive another TKI.
The Warnings and Precautions section of the drug’s label must be revised to include a description of the arterial and venous thrombosis and occlusions that have occurred in at least 27%—more than 1 in every 4—of patients treated with ponatinib.
The Dosage and Administration recommendations must be revised to state that the optimal dose of ponatinib has not been identified. The recommended starting dose remains 45 mg administered orally once daily, with or without food.
The patient Medication Guide must be revised to include additional safety information consistent with the safety information in the revised drug label.
The ponatinib REMS must inform prescribers about the approved indications for use and the serious risk of vascular occlusion and thromboembolism associated with the drug. The REMS must include the following:
- REMS letter to healthcare professionals who are known or likely to prescribe ponatinib
- REMS letter for professional societies to be distributed to their members
- REMS fact sheet for healthcare professionals
- Public statement to be published quarterly for 1 year in several professional journals
- Information to be prominently displayed at scientific meetings
- Ponatinib REMS website to provide access to all REMS materials for the duration of the REMS.
And postmarket investigations must further evaluate the dose selection, drug exposure, treatment response, and toxicity of ponatinib.
For more information, see the FDA’s safety communication. ![]()
The leukemia drug ponatinib is expected to return to market once the drug’s manufacturer has implemented measures to address the recently discovered safety risks.
The US Food and Drug Administration (FDA) is requiring that a number of safety measures be adopted, including changes to ponatinib’s label to narrow the drug’s indication and the addition of warnings about the increased risk of thrombosis and venous occlusion associated with ponatinib use.
In addition, dosing and administration recommendations must be revised, the patient medication guide must be updated, a risk evaluation and mitigation strategy (REMS) must be implemented, and postmarket investigations must be conducted to further characterize the drug’s safety and dosing.
Ponatinib was approved by the FDA in December 2012 to treat adults with chronic myeloid leukemia (CML) or Philadelphia chromosome-positive acute lymphoblastic leukemia (Ph+ ALL) that is resistant to or intolerant of other tyrosine kinase inhibitors (TKIs).
But in October 2013, follow-up results of the phase 2 PACE trial suggested ponatinib can increase a patient’s risk of arterial and venous thrombotic events. So all trials of the drug were placed on partial clinical hold, with the exception of the phase 3 EPIC trial, which was discontinued.
Then, the FDA suspended sales and marketing of ponatinib, pending results of a safety evaluation. Now, the agency has decided ponatinib can return to the market if the new safety measures are implemented.
Safety measures in detail
The FDA is requiring that ponatinib use be restricted to:
- Adults with T315I-positive CML (chronic phase, accelerated phase, or blast phase)
- Adults with T315I-positive Ph+ ALL
- Adults with CML (chronic phase, accelerated phase, or blast phase) who cannot receive another TKI
- Adults with Ph+ ALL who cannot receive another TKI.
The Warnings and Precautions section of the drug’s label must be revised to include a description of the arterial and venous thrombosis and occlusions that have occurred in at least 27%—more than 1 in every 4—of patients treated with ponatinib.
The Dosage and Administration recommendations must be revised to state that the optimal dose of ponatinib has not been identified. The recommended starting dose remains 45 mg administered orally once daily, with or without food.
The patient Medication Guide must be revised to include additional safety information consistent with the safety information in the revised drug label.
The ponatinib REMS must inform prescribers about the approved indications for use and the serious risk of vascular occlusion and thromboembolism associated with the drug. The REMS must include the following:
- REMS letter to healthcare professionals who are known or likely to prescribe ponatinib
- REMS letter for professional societies to be distributed to their members
- REMS fact sheet for healthcare professionals
- Public statement to be published quarterly for 1 year in several professional journals
- Information to be prominently displayed at scientific meetings
- Ponatinib REMS website to provide access to all REMS materials for the duration of the REMS.
And postmarket investigations must further evaluate the dose selection, drug exposure, treatment response, and toxicity of ponatinib.
For more information, see the FDA’s safety communication. ![]()
Emerging therapies for melanoma
Metastatic melanoma is a highly challenging cancer to treat. Like other solid tumors, it is a very heterogeneous disease both clinically and biologically. Consequently, the first decision point in its management is to assess the severity of an individual patient’s disease. This can be done based on the patient’s symptoms and how they have evolved over the preceding 1-2 months, performance status, the extent of disease as determined by physical examination, and staging workup, which should include either computed tomography scans of the body or a positron emission tomography/CT study as well as a brain magnetic resonance imaging scan. Patients with brain metastases as a subset (which is sizable – 20%-25% have brain metastases) require special attention because they may not respond to systemic therapies and will thus have to be managed with brain-targeted treatment options. Tumor testing for BRAF mutations is necessary in all patients with metastatic melanoma because the BRAF inhibitors (vemurafenib or dabrafenib) are a preferred choice of targeted therapy for this subset of patients, which constitutes about 50% of all melanoma patients. Immunotherapy plays an important role in nearly all patients with metastatic melanoma including those who have progressed after anti-BRAF therapy. Chemotherapy still has a significant (yet diminishing) role for patients who are no longer suitable for immunotherapy.
Metastatic melanoma is a highly challenging cancer to treat. Like other solid tumors, it is a very heterogeneous disease both clinically and biologically. Consequently, the first decision point in its management is to assess the severity of an individual patient’s disease. This can be done based on the patient’s symptoms and how they have evolved over the preceding 1-2 months, performance status, the extent of disease as determined by physical examination, and staging workup, which should include either computed tomography scans of the body or a positron emission tomography/CT study as well as a brain magnetic resonance imaging scan. Patients with brain metastases as a subset (which is sizable – 20%-25% have brain metastases) require special attention because they may not respond to systemic therapies and will thus have to be managed with brain-targeted treatment options. Tumor testing for BRAF mutations is necessary in all patients with metastatic melanoma because the BRAF inhibitors (vemurafenib or dabrafenib) are a preferred choice of targeted therapy for this subset of patients, which constitutes about 50% of all melanoma patients. Immunotherapy plays an important role in nearly all patients with metastatic melanoma including those who have progressed after anti-BRAF therapy. Chemotherapy still has a significant (yet diminishing) role for patients who are no longer suitable for immunotherapy.
Metastatic melanoma is a highly challenging cancer to treat. Like other solid tumors, it is a very heterogeneous disease both clinically and biologically. Consequently, the first decision point in its management is to assess the severity of an individual patient’s disease. This can be done based on the patient’s symptoms and how they have evolved over the preceding 1-2 months, performance status, the extent of disease as determined by physical examination, and staging workup, which should include either computed tomography scans of the body or a positron emission tomography/CT study as well as a brain magnetic resonance imaging scan. Patients with brain metastases as a subset (which is sizable – 20%-25% have brain metastases) require special attention because they may not respond to systemic therapies and will thus have to be managed with brain-targeted treatment options. Tumor testing for BRAF mutations is necessary in all patients with metastatic melanoma because the BRAF inhibitors (vemurafenib or dabrafenib) are a preferred choice of targeted therapy for this subset of patients, which constitutes about 50% of all melanoma patients. Immunotherapy plays an important role in nearly all patients with metastatic melanoma including those who have progressed after anti-BRAF therapy. Chemotherapy still has a significant (yet diminishing) role for patients who are no longer suitable for immunotherapy.
Nilotinib beats imatinib in newly diagnosed CML

Credit: CDC
NEW ORLEANS—New data indicate a trend for longer overall survival and event-free survival in newly diagnosed chronic myeloid leukemia (CML) patients on nilotinib versus imatinib.
Five-year data from the phase 3 ENESTnd study demonstrate higher rates of early and deeper molecular response in newly diagnosed CML patients taking nilotinib, as well as a reduced risk of progression compared to imatinib.
These results were presented at the 2013 ASH Annual Meeting as abstract 92.
“These new, updated data reaffirm the superiority of nilotinib over imatinib at achieving deeper molecular responses and provide even more evidence supporting nilotinib as an appropriate treatment of choice in newly diagnosed patients,” said Giuseppe Saglio, MD, of the University of Turin in Italy.
“Now, we are looking at how deeper molecular responses may help guide our approach towards how we treat CML in the future.”
The 5-year ENESTnd data showed that nilotinib can produce superior responses across various Philadelphia chromosome-positive CML patient populations, including newly diagnosed patients. Results showed higher rates of early and deeper sustained molecular response, known as MR4.5.
The difference in the rates of MR4.5 continued to be higher when nilotinib was given at 300 mg or 400 mg twice daily, when compared to imatinib (MR4.5: 6%-10% difference by 1 year, 21%-23% difference by 5 years).
“The most important endpoint is cumulative incidence of MR4.5,” Dr Saglio said. “At 5 years, this is achieved by 54% of those in nilotinib-300-mg group, 52% in the nilotinib-400-mg group, and 31% in the imatinib group. And the curves are still diverging.”
The data also indicate a trend for higher overall survival and event-free survival rates in patients treated with nilotinib.
Fifteen patients treated with imatinib had CML-related deaths, compared to 6 patients in the arm receiving nilotinib at 300 mg twice daily and 4 patients in the arm receiving nilotinib at 400 mg twice daily.
Few new adverse events and laboratory abnormalities were observed between year 4 and year 5. Rates of patients with adverse events leading to discontinuation were 11.1% in the 300-mg nilotinib group, 17.7% in the 400-mg nilotinib group, and 13.2% in the imatinib group.
Dr Saglio noted that select cardiac and vascular events are slightly more frequent on nilotinib versus imatinib. But there has been no increase in the annual incidence of these events over time.
Therefore, Dr Saglio concluded, “Nilotinib, a standard-of-care frontline therapy option for newly diagnosed, chronic-phase CML patients, affords superior efficacy compared with imatinib, including higher rates of early molecular response (which is associated with improved long-term outcomes), higher rates of deep molecular response, and a lower risk of disease progression. Nilotinib continues to show good tolerability with long-term follow-up.” ![]()

Credit: CDC
NEW ORLEANS—New data indicate a trend for longer overall survival and event-free survival in newly diagnosed chronic myeloid leukemia (CML) patients on nilotinib versus imatinib.
Five-year data from the phase 3 ENESTnd study demonstrate higher rates of early and deeper molecular response in newly diagnosed CML patients taking nilotinib, as well as a reduced risk of progression compared to imatinib.
These results were presented at the 2013 ASH Annual Meeting as abstract 92.
“These new, updated data reaffirm the superiority of nilotinib over imatinib at achieving deeper molecular responses and provide even more evidence supporting nilotinib as an appropriate treatment of choice in newly diagnosed patients,” said Giuseppe Saglio, MD, of the University of Turin in Italy.
“Now, we are looking at how deeper molecular responses may help guide our approach towards how we treat CML in the future.”
The 5-year ENESTnd data showed that nilotinib can produce superior responses across various Philadelphia chromosome-positive CML patient populations, including newly diagnosed patients. Results showed higher rates of early and deeper sustained molecular response, known as MR4.5.
The difference in the rates of MR4.5 continued to be higher when nilotinib was given at 300 mg or 400 mg twice daily, when compared to imatinib (MR4.5: 6%-10% difference by 1 year, 21%-23% difference by 5 years).
“The most important endpoint is cumulative incidence of MR4.5,” Dr Saglio said. “At 5 years, this is achieved by 54% of those in nilotinib-300-mg group, 52% in the nilotinib-400-mg group, and 31% in the imatinib group. And the curves are still diverging.”
The data also indicate a trend for higher overall survival and event-free survival rates in patients treated with nilotinib.
Fifteen patients treated with imatinib had CML-related deaths, compared to 6 patients in the arm receiving nilotinib at 300 mg twice daily and 4 patients in the arm receiving nilotinib at 400 mg twice daily.
Few new adverse events and laboratory abnormalities were observed between year 4 and year 5. Rates of patients with adverse events leading to discontinuation were 11.1% in the 300-mg nilotinib group, 17.7% in the 400-mg nilotinib group, and 13.2% in the imatinib group.
Dr Saglio noted that select cardiac and vascular events are slightly more frequent on nilotinib versus imatinib. But there has been no increase in the annual incidence of these events over time.
Therefore, Dr Saglio concluded, “Nilotinib, a standard-of-care frontline therapy option for newly diagnosed, chronic-phase CML patients, affords superior efficacy compared with imatinib, including higher rates of early molecular response (which is associated with improved long-term outcomes), higher rates of deep molecular response, and a lower risk of disease progression. Nilotinib continues to show good tolerability with long-term follow-up.” ![]()

Credit: CDC
NEW ORLEANS—New data indicate a trend for longer overall survival and event-free survival in newly diagnosed chronic myeloid leukemia (CML) patients on nilotinib versus imatinib.
Five-year data from the phase 3 ENESTnd study demonstrate higher rates of early and deeper molecular response in newly diagnosed CML patients taking nilotinib, as well as a reduced risk of progression compared to imatinib.
These results were presented at the 2013 ASH Annual Meeting as abstract 92.
“These new, updated data reaffirm the superiority of nilotinib over imatinib at achieving deeper molecular responses and provide even more evidence supporting nilotinib as an appropriate treatment of choice in newly diagnosed patients,” said Giuseppe Saglio, MD, of the University of Turin in Italy.
“Now, we are looking at how deeper molecular responses may help guide our approach towards how we treat CML in the future.”
The 5-year ENESTnd data showed that nilotinib can produce superior responses across various Philadelphia chromosome-positive CML patient populations, including newly diagnosed patients. Results showed higher rates of early and deeper sustained molecular response, known as MR4.5.
The difference in the rates of MR4.5 continued to be higher when nilotinib was given at 300 mg or 400 mg twice daily, when compared to imatinib (MR4.5: 6%-10% difference by 1 year, 21%-23% difference by 5 years).
“The most important endpoint is cumulative incidence of MR4.5,” Dr Saglio said. “At 5 years, this is achieved by 54% of those in nilotinib-300-mg group, 52% in the nilotinib-400-mg group, and 31% in the imatinib group. And the curves are still diverging.”
The data also indicate a trend for higher overall survival and event-free survival rates in patients treated with nilotinib.
Fifteen patients treated with imatinib had CML-related deaths, compared to 6 patients in the arm receiving nilotinib at 300 mg twice daily and 4 patients in the arm receiving nilotinib at 400 mg twice daily.
Few new adverse events and laboratory abnormalities were observed between year 4 and year 5. Rates of patients with adverse events leading to discontinuation were 11.1% in the 300-mg nilotinib group, 17.7% in the 400-mg nilotinib group, and 13.2% in the imatinib group.
Dr Saglio noted that select cardiac and vascular events are slightly more frequent on nilotinib versus imatinib. But there has been no increase in the annual incidence of these events over time.
Therefore, Dr Saglio concluded, “Nilotinib, a standard-of-care frontline therapy option for newly diagnosed, chronic-phase CML patients, affords superior efficacy compared with imatinib, including higher rates of early molecular response (which is associated with improved long-term outcomes), higher rates of deep molecular response, and a lower risk of disease progression. Nilotinib continues to show good tolerability with long-term follow-up.” ![]()
Pediatric Discharge Systematic Review
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).
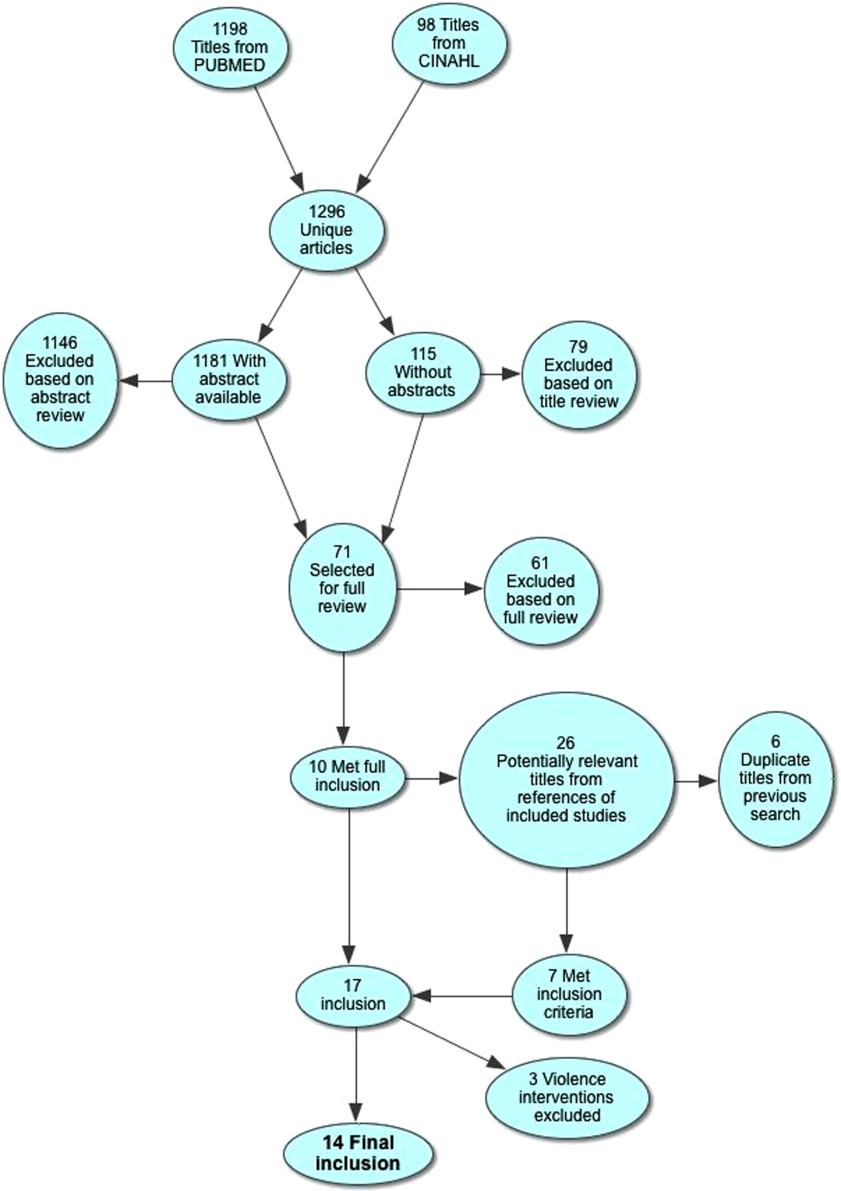
Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]
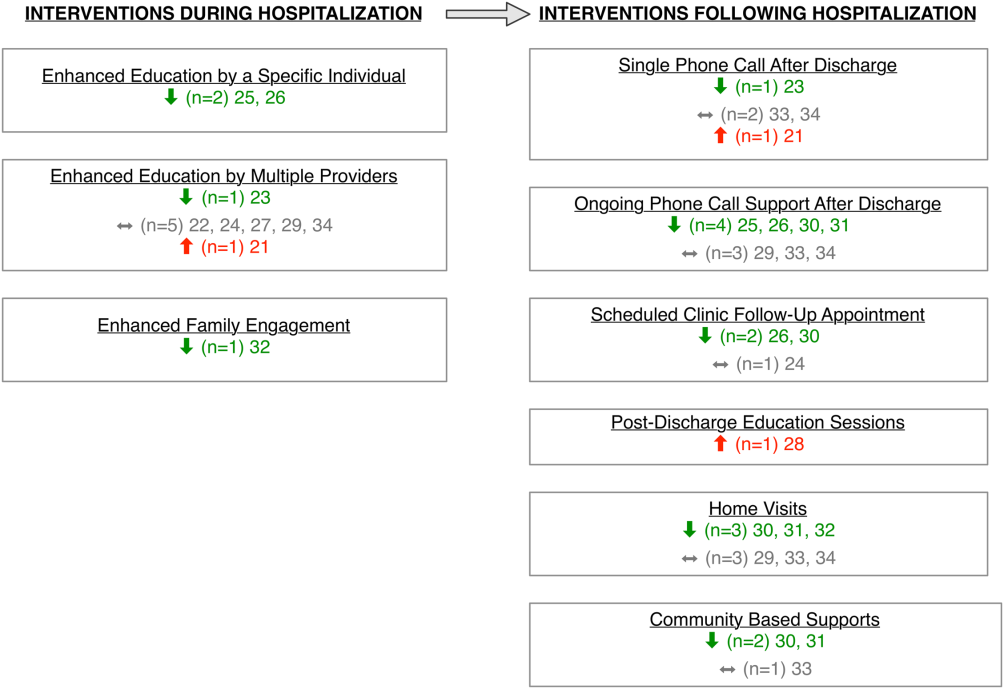
Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).

Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]

Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
The process of discharging a pediatric patient from an acute care facility is currently fraught with difficulties. More than 20% of parents report problems in the transition of care from the hospital to the home and ambulatory care setting.[1] Clinical providers likewise note communication challenges around the time of discharge,[2, 3] especially when inpatient and outpatient providers are different, as with the hospitalist model.[4] Poor communication and problems in discharge transition and continuity of care often culminate in adverse events,[5, 6] including return to emergency department (ED) care and hospital readmission.[7]
Thirty‐day readmissions are common for certain pediatric conditions, such as oncologic diseases, transplantation, and sickle cell anemia and vary significantly across children's hospitals.[8] Discharge planning may decrease 30‐day readmissions in hospitalized adults[9]; however, it is not clear that the same is true in children. Both the preventability of pediatric readmissions[10] and the extent to which readmissions reflect suboptimal care[11] are subjects of debate. Despite these uncertainties, collaborative efforts intended to decrease pediatric readmissions[12] and improve discharge transitions[13, 14] are underway.
To inform these debates and efforts, we undertook a systematic review of the evidence of hospital‐initiated interventions to reduce repeat utilization of the ED and hospital. Acknowledging that existing evidence for condition‐specific discharge interventions in pediatrics might be limited, we sought to identify common elements of successful interventions across pediatric conditions.
METHODS
Search Strategy
With the assistance of a research librarian, we searched MEDLINE and CINAHL (Cumulative Index to Nursing and Allied Health Literature) from the inception of these databases through to March 28, 2012 (for search strategies, see the Supporting Information, Appendix, Part 1, in the online version of this article).
Study Selection
Two authors (K.A. and C.K.) independently reviewed abstracts identified by the initial search, as well as abstracts of references of included articles. Eligibility criteria for inclusion in full review included: (1) discharge‐oriented process or intervention initiated in the inpatient setting, (2) study outcomes related to subsequent utilization including hospital readmission or emergency department visit after hospitalization, (3) child‐ or adolescent‐focused or child‐specific results presented separately, and (4) written or available in English. If abstract review did not sufficiently clarify whether all eligibility criteria were met, the article was included in the full review. Two authors (K.A. and C.K.) independently reviewed articles meeting criteria for full review to determine eligibility. Disagreements regarding inclusion in the final analysis were discussed with all 4 authors. We excluded studies in countries with low or lower‐middle incomes,[15] as discharge interventions in these countries may not be broadly applicable.
Data Abstraction, Quality Assessment, and Data Synthesis
Two authors (K.A. and C.K.) independently abstracted data using a modified Cochrane Collaboration data collection form.[16] We independently scored the included studies using the Downs and Black checklist, which assesses the risk of bias and the quality of both randomized and nonrandomized studies.[17] This checklist yields a composite score of 0 to 28 points, excluding the item assessing power. As many studies either lacked power calculations or included power calculations based on outcomes not included in our review, we performed calculations to determine the sample size needed to detect a decrease in readmission or ED utilization by 20% from baseline or control rates. Due to the heterogeneous nature of included studies in terms of population, interventions, study design, and outcomes, meta‐analysis was not performed.
RESULTS
Electronic search yielded a total of 1296 unique citations. Review of abstracts identified 40 studies for full article review. We identified 10 articles that met all inclusion criteria. Subsequent review of references of included articles identified 20 additional articles for full review, 7 of which met all inclusion criteria. However, 3 articles[18, 19, 20] assessed the impact of violence interventions primarily on preventing reinjury and recidivism and thus were excluded (see Supporting Information, Appendix, Part 2, in the online version of this article for findings of the 3 articles). In total, we included 14 articles in our review[21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34] (Figure 1).

Patient Populations and Intervention Timing and Components
Studies varied regarding the specific medical conditions they evaluated. Eight of the papers reported discharge interventions for children with asthma, 5 papers focused on discharge from the neonatal intensive care unit (NICU), and a final study discussed a discharge intervention for children with cancer (Table 1). Although our primary goal was to synthesize discharge interventions across pediatric conditions, we provide a summary of discharge interventions by condition (see Supporting Information, Appendix, Part 3, in the online version of this article).
| Author, Year | Study Design | Age | Inclusion | Exclusion | Intervention | Control |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 12 months18 years | Admitted for asthma at a single hospital in California. | 45 minutes of enhanced asthma education and phone call 3 weeks after discharge (n=698) | Patients were matched on age and past utilization who received standard education/care (n=698) | |
| Espinoza‐Palma, 2009[22] | RCT | 515 years | Admitted for asthma at a single hospital in Chile. | Chronic lung disease or neurologic alteration. | Self‐management education program with a postdischarge game to reinforce educational concepts (n=42) | Standard education (n=46) |
| Ng, 2006[23] | RCT | 215 years | Admitted for asthma in a pediatric ward at a single hospital in China. | Admitted to PICU or non‐Chinese speaking. | Evaluation by asthma nurse, animated asthma education booklet, 50‐minute discharge teaching session, follow‐up by phone at 1 week (n=55) | Evaluation by asthma nurse by physician referral, a written asthma education booklet, 30‐minute discharge teaching session (n=45) |
| Stevens, 2002[24] | RCT | 18 months5 years | In ED or admitted with primary diagnosis of asthma/wheezing at 2 hospitals in the United Kingdom. | Admitted when no researcher available. | Enhanced asthma education and follow‐up in a clinic 1 month after encounter (n=101) | Usual care (n=99) |
| Wesseldine, 1999[25] | RCT | 216 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted when no researcher available. | 20 minutes of enhanced asthma education including: guided self‐management plan, booklet, asthma hotline contact, and sometimes oral steroids (n=80) | Standard discharge that varied by provider (n=80) |
| Madge, 1997[26] | RCT | 214 years | Admitted for asthma at a single hospital in the United Kingdom. | Admitted on weekend. | 45 minutes of enhanced asthma education with written asthma plan, a nurse follow‐up visit 23 weeks postdischarge, telephone support, and a course of oral steroids (n=96) | Standard education (did not include written asthma plan) (n=105) |
| Taggart, 1991[27] | Pre‐post | 612 years | Admitted for asthma at single institution in Washington, DC with history of at least one ED visit in prior 6 months. | If resided outside of metro area. | Received written educational materials, adherence assistance, discussed emotions of asthma, video education provided, and tailored nursing interactions (n=40) | Enrolled patient's prior utilization |
| Mitchell, 1986[28] | RCT | >2 years | Admitted for asthma at single institution in New Zealand. | Having a previous life‐threatening attack. | 6 monthly postdischarge education sessions on lung anatomy/physiology, triggers and avoidance, asthma medication, advice on when and where to seek care (n=94 children of European descent, n=84 children of Polynesian descent) | Standard discharge (n=106 children of European descent; n=84 children of Polynesian descent) |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | <18 years | New oncologic diagnoses in hospital in Turkey. | Children who died during follow‐up. | Frequent needs assessment, education, home visits, fever guidance, telephone consultation, and manual for home care; patients lived in Izmir (n=25) | Routine hospital services without formal education; patients lived outside of Izmir (n=24) |
| NICU | ||||||
| Broyles, 2000[30] | RCT | Neonate | Infants with birth weight <1500 g with mechanical vent use in 48 hours of life, born at single NICU in Texas. | Infant death, infant adopted or moved out of enrollment county. | Specialized follow‐up available 5 days a week for well or sick visits; access to medical advice via phone 24 hours a day, transportation to ED provided when needed; home visitation, parent education, and "foster grandmother" offered (n=446) | Specialized follow‐up available 2 mornings a week for well or sick visits; all other sick visits to be made through acute care clinic or ED (n=441) |
| Finello, 1998[31] | RCT | Neonate | Infants with birth weight between 750 and1750 g; discharged from 2 NICUs in California. | Infants with gross abnormalities. | Three separate intervention groups (n=20 in each): (1) home healthhome visits during the first 4 weeks after discharge, with physician consultation available at all times; (2) home visitinghealth and development support, parental support, support with referral services for 2 years after discharge; (3) home health and home visiting arms combined | Standard discharge (n=20). |
| Kotagal, 1995[32] | Pre‐post | Neonate | Infants discharged from a single NICU in Ohio. | Patients (n=257) discharged after restructuring of discharge practices including: removal of discharge weight criteria, engagement of family prior to discharge, evaluation of home environment prior to discharge, and arrangement of home health visits and follow‐up | Patients discharged before discharge restructuring (n=483) | |
| Casiro, 1993[33] | RCT | Neonate | Infants meeting discharge criteria from 1 of 2 NICUs in Canada. | Congenital anomalies, chronic neonatal illness, parent refusal, family complications, and death. | Early discharge based on prespecified criteria with 8 weeks of services including: assistance with infant care, sibling care and housekeeping; nurse availability via phone; follow‐up phone calls and home visitation tailored to family need (n=50) | Discharged at the discretion of their attending physicians; standard newborn public health referral for routine follow‐up (n=50) |
| Brooten, 1986[34] | RCT | Neonate | Infants born <1500 g at a single NICU in Pennsylvania. | Death, life‐threatening congenital anomalies, grade 4 IVH, surgical history, O2 requirement >10 weeks, family complications. | Early discharge based on prespecified criteria with weekly education prior to discharge, postdischarge follow‐up phone call, and home nurse visitation; consistent nurse availability via phone (n=39) | Standard discharge practices with a discharge weight minimum of 2.2 kg (n=40) |
Studies varied regarding the timing and nature of the intervention components. Eight discharge interventions included a major inpatient component, in addition to outpatient support or follow‐up.[21, 23, 24, 25, 26, 29, 32, 34] Two studies included an inpatient education component only.[22, 27] The remainder were initiated during index hospitalization but focused primarily on home visitation, enhanced follow‐up, and support after discharge (Figure 2).[28, 30, 31, 33]

Outcome Assessment Methods
Readmission and subsequent ED utilization events were identified using multiple techniques. Some authors accessed claims records to capture all outcomes.[30, 33] Others relied on chart review.[21, 25, 26, 27, 28, 31, 32] One study supplemented hospital records with outpatient records.[24] Some investigators used parental reports.[22, 23, 31] Two studies did not describe methods for identifying postdischarge events.[29, 34]
Study Quality
The quality of the included studies varied (Table 2). Many of the studies had inadequate sample size to detect a difference in either readmission or ED visit subsequent to discharge. Eight studies found differences in either subsequent ED utilization, hospitalization, or both and were considered adequately powered for these specific outcomes.[21, 23, 25, 26, 28, 30, 31, 32] In contrast, among studies with readmission as an outcome, 6 were not adequately powered to detect a difference in this particular outcome.[24, 30, 31, 32, 33, 34] In these 6 studies, all except 1 study30 had <10% of the sample size required to detect differences in readmission. Further, 2 studies that examined ED utilization were underpowered to detect differences between intervention and control groups.[24, 26] We were unable to perform power calculations for 3 studies,[22, 27, 29] as the authors presented the number of events without clear denominators.
| Author, Year | Study Design | D&B Score* | Adequately Powered (Yes/No)** | Timing of Outcome | Major Findings | Major Limitations |
|---|---|---|---|---|---|---|
| ||||||
| Asthma | ||||||
| Davis, 2011[21] | Retrospective matched case control | 14 | Readmission: N/A; ED: yes | 1 year | Patients with enhanced education had higher hazards of return to ED visit. | Intervention not randomized; only 29% of eligible children enrolled with unclear selection decisions due to lack of study personnel or caregiver presence in hospital; only 67% completed the intervention; 50% of patients were not local; follow‐up was not well described. |
| Espinoza‐Palma, 2009[22] | RCT | 19 | Readmission: b; ED:b | 1 year | No difference between the intervention and control in hospitalizations or ED visits. ED visits and hospitalizations decreased in year after compared to the year prior for both intervention and control. | Pre‐post analysis with similar effects in cases and controls, results may reflect regression to mean; follow‐up was not well described, and 12.5% who were lost to follow‐up were excluded from analysis; study was in Chile with different demographics than in the United States. |
| Ng, 2006[23] | RCT | 20 | Readmission: yes; ED: yes | 3 months | Patients in the intervention group were less likely to be readmitted or visit the ED. | Recruitment/refusal was not well described; number lost to follow‐up was not reported; study was in China with different demographics than the United States. |
| Stevens, 2002[24] | RCT | 20 | Readmission: no ED: no | 1 year | No differences between intervention and control for any outcomes. | 11% were lost to follow‐up; number of patients who refused was not reported; analysis did not adjust for site of recruitment (ED vs inpatient); 30% of children did not have a prior diagnosis of asthma; study was in England with different demographics than in the United States. |
| Wesseldine, 1999[25] | RCT | 20 | Readmission: yes; ED: yes | 6 months | Patients in intervention group less likely to be readmitted or visit ED. | Unclear if intervention group received oral steroids that might drive effect; number lost to follow‐up was not reported; high miss rate for recruitment; study was in England with different demographics than the United States. |
| Madge, 1997 [26] | RCT | 22 | Readmission: yes; ED: no | 214 months | Patients in intervention group were less likely to be readmitted compared to controls. No differences in repeat ED visits. | Unclear if education or oral steroids drove effect; number of patients who refused or were lost to follow‐not reported; time to outcome (214 months) varied for different patients, which may introduce bias given the seasonality of asthma; study was in Scotland with different demographics than the United States. |
| Taggart, 1991[27] | Pre‐post | 12 | Readmission:b; ED:b | 15 months | Overall there was no change in ED or hospitalization utilization from pre to post. When limited to children with severe asthma, there was a decrease in ED utilization after the intervention compared to prior ED use. | Use of historical utilization as a comparison does not account for potential effects of regression to mean or improvement with age; over one‐half of eligible patients were excluded due to lack of consent or inability to collect baseline data; inclusion criterion did not specify that prior utilization was necessarily for asthma exacerbation; number lost to follow‐up was not reported. |
| Mitchell, 1986[28] | RCT | 14 | Readmission: yesc; ED: N/A | 6 months and 618 months | Increase in percentage of readmission between 6 and 18 months for children of European descent. | Unclear exclusion criterion; full compliance with intervention only 52%; number of patients lost to follow‐up (outcome) was not reported; statistical analysis was not clearly described. |
| Cancer | ||||||
| Caliskan Yilmaz, 2009[29] | Quasiexperimental | 10 | Readmission:b; ED: N/A | Not specified | For the first readmission to the hospital, more of the readmissions were planned in the intervention group compared to the control group. Number of readmissions was not assessed. | Intervention was not randomized; children who died were excluded (4%); planned vs unplanned distinction not validated; unclear cointerventions regarding chemotherapy administration; recruitment and follow‐up was not well described; not all comparisons were described in methods. |
| NICU | ||||||
| Broyles, 2000[30] | RCT | 23 | Readmission: no; ED: yes | At 1 year adjusted age | Overall hospitalization rates were similar but there were fewer admissions to the ICU. Intervention group had fewer ED visits. Total costs were less in intervention group. | 10% refused to participate or consent was not sought, and 12% were excluded after randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries). |
| Finello, 1998[31] | RCT | 11 | Readmission: nod; ED: yes | At 6 months adjusted age and between 6 and 12 months adjusted age | No changes in hospitalization rates.d The home health+home visit arm had fewer ED visits between 6 and 12 months of life. Intervention was reported as saving money by decreasing initial length of stay. | Inclusion and exclusion criteria, recruitment/refusal, outcomes, and analysis plan were not clearly described; sample size was too small for effective randomization; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment; 15% of outcomes were missing. |
| Kotagal, 1995[32] | Pre‐post | 15 | Readmission: no; ED: yes | 14 days | Decreased number of ED visits in patients in intervention. No difference in readmission. Costs and length of stay were less in intervention. | Designed to decrease length of stay; pre‐post nature of study allows for possibility of other changes to practices other than the intervention. |
| Casiro, 1993[33] | RCT | 18 | Readmission: no; ED: N/A | 1 year of life | There were no differences in the readmissions or number of ambulatory care visits after discharge. Infants were discharged earlier in the intervention group, which resulted in cost savings. | Designed to decrease length of stay; 13% refused or were excluded due to family complications; and 8% were lost to follow‐up; different periods of follow‐up (outcomes observed at 1 year of life regardless of discharge timing); analysis did not adjust for site of recruitment (1 of 2 nurseries); 81% of infants were born to Caucasian women, which may limit generalizability. |
| Brooten, 1986[34] | RCT | 15 | Readmission: no; ED: N/A | 14 days and 18 months | No difference in readmission. Significantly lower charges during initial hospitalization for intervention group. | Designed to decrease length of stay; unclear when randomization occurred and exclusions unclear; 12.5% were excluded due to refusal or family issues; follow‐up not well described, and loss to follow‐up was unknown. |
Excluding the assessment of statistical power, Downs and Black scores ranged from 10 to 23 (maximum 28 possible points) indicating varying quality. As would be expected with discharge interventions, studies did not blind participants; 2 studies did, however, appropriately blind the outcome evaluators to intervention assignment.[22, 30] Even though 10 out of the 14 studies were randomized controlled trials, randomization may not have been completely effective due to sample size being too small for effective randomization,[31] large numbers of excluded subjects after randomization,[30] and unclear randomization process.[34] Several studies had varying follow‐up periods for patients within a given study. For example, 3 NICU studies assessed readmission at 1‐year corrected age,[30, 31, 33] creating the analytic difficulty that the amount of time a given patient was at risk for readmission was dependent on when the patient was discharged, yet this was not accounted for in the analyses. Only 2 studies demonstrated low rates of loss to follow‐up (<10%).[30, 33] The remainder of the studies either had high incompletion/loss to follow‐up rates (>10%)[22, 24, 31] or did not report rates.[21, 23, 25, 26, 27, 28, 29, 32, 34] Finally, 3 studies recruited patients from multiple sites,[24, 31, 33] and none adjusted for potential differences in effect based on enrollment site.
Findings Across Patient Populations Regarding Readmission
Of the 4 studies that demonstrated change in overall readmission,[23, 25, 26, 28] all were asthma focused; 3 demonstrated a decrease in readmissions,[23, 25, 26] and 1 an increase in readmissions.[28] The 3 effective interventions included 1‐on‐1 inpatient education delivered by an asthma nurse, in addition to postdischarge follow‐up support, either by telephone or clinic visit. Two of these interventions provided rescue oral steroids to some patients on discharge.[25, 26] In contrast, a study from New Zealand evaluated a series of postdischarge visits using an existing public health nurse infrastructure and demonstrated an increase in readmission between 6 to 18 months after admission in European children.[28] An additional study focused on outpatient support after discharge from the NICU, and demonstrated a lower frequency of readmission to the intensive care unit without overall reduction of hospital readmission (Tables 1 and 2).[30]
Findings Across Patient Populations Regarding Subsequent ED Visits
Of all the discharge interventions, 6 demonstrated differences in return to the ED after discharge. Five studies described a decrease in ED visits after hospitalization,[23, 25, 30, 31, 32] and 1 showed an increase.[21] Three studies in the NICU population demonstrated decreased ED utilization through a combination of augmented family engagement during hospitalization and/or enhanced support after discharge. Two inpatient asthma education interventions with structured postdischarge follow‐up decreased return visitation to the ED.[23, 26] The intervention that worsened subsequent ED utilization (ie, increased ED visit hazard compared to matched controls) provided enhanced inpatient education to a nonrandom group of children hospitalized with asthma and provided a follow‐up phone call 3 weeks after discharge (Tables 1 and 2).[21]
DISCUSSION
In this review, we synthesized evidence regarding pediatric hospital discharge‐focused interventions intended to reduce subsequent utilization through decreased readmission and ED visits. Our review identified 14 studies clustered in 3 clinical areas: asthma, NICU care (chiefly prematurity), and cancer. Overall, 6 interventions demonstrated a reduction either in subsequent hospitalization or ED use. Four of the 6 positive interventions included both an enhanced inpatient education and engagement component as well as enhanced follow‐up after discharge. Importantly, all of the interventions were multifaceted; thus, we could not ascertain which specific aspects of the interventions mediated the change. Many of the included studies had significant methodological limitations.
Current Conceptual Framework
There are a number of existing discharge transitional care frameworks from prior studies[35, 36] and professional societies.[37] The Stepping Up to the Plate (SUTTP) alliance, a collaborative of 9 professional organizations, including the American Academy of Pediatrics, introduced 1 such framework in 2007. SUTTP sought to enhance care transitions by outlining principles of discharge transitional care including: (1) enhanced accountability, (2) creation of a central coordination hub charged with communicating expectations for care, (3) clear and direct communication of treatment plans and follow‐up, (4) timely feedback/feed‐forward of relevant information, and (5) involvement of family member at every stage.[38] In the context of the SUTTP framework, we present 3 hypotheses based on our findings to guide future work.
Hypothesis: Appointing a Dedicated Individual or Coordinating Hub Reduces Subsequent Utilization
Ostensibly, each discharge intervention included in this review sought to enhance accountability of providers or their health systems for discharge transitional care. Two of the asthma interventions appointed a particular provider to coordinate the discharge transition and demonstrated reductions in readmission.[25, 26] The successful NICU discharge interventions provided an integrated accountability structure across the health system, with a transition of accountability to an outpatient provider or central coordinating hub available to provide assistance and resources for an extended period following discharge.
By contrast, interventions with more than 1 individual intervener or without a centrally coordinated system for discharge transitional care tended not to demonstrate reduction in subsequent utilization.[21, 24, 27, 28] In fact, the 1 asthma intervention that utilized a previously existing public health nurse infrastructure demonstrated an increase in readmission.[28] Future efforts to enhance transitional care might investigate directly the impact of accountability structure on subsequent utilization by varying the number of effector individuals or the organization to which they report (eg, hospital system vs public health department).
Hypothesis: Individualized Task Learning and Feedback Enhances Effectiveness
Studies varied with respect to the extent they incorporated the principles of enhanced communication of the treatment and follow‐up plan and timely feedback/feed‐forward of relevant information. Successful efforts, however, seemed to embrace these strategies. Each of the 3 interventions that demonstrated readmission reduction[23, 25, 26] developed an individualized treatment plan during hospitalization, with either a specific follow‐up plan or resources for outpatient support. Two of these interventions assessed asthma inhaler technique prior to discharge, creating an inpatient audit and feedback loop allowing for assessment of competence prior to discharge. Audit and feedback has demonstrated promise modifying provider behavior[39] and is a plausible approach to enhancing patient and family self‐care.
Hypothesis: Timing of Intervention Enhances Effectiveness
Discrete sentinel events such as inpatient admission, may serve as a teachable moment[40, 41] or a tipping point[42] for some patients/families to initiate behavior change. Four of the 6 positive studies had a robust inpatient education component. By providing enhanced inpatient support, providers may be engaging the family at a timely opportunity to improve care. Both timing of the intervention (at admission vs discharge) and content (education‐ vs family‐engagement focused) are likely important to their effect and should be further explored with prospective study.
Persistent Literature Gaps
Follow‐up with a primary care provider after discharge is another intervention that might decrease postdischarge utilization. We did not identify any studies that specifically examined primary care follow‐up. However, 2 studies[43, 44] that did not meet our inclusion criteria (because they included adults and did not stratify by age group in the analysis) examined any outpatient follow‐up after discharge using state‐specific Medicaid claims. One study found that outpatient follow‐up after sickle cell hospitalization was associated with lower rates of readmission.[43] The other found no difference in readmission across multiple conditions.[44] One recent review of outpatient follow‐up from the ED for asthma found that even when increases in follow‐up were achieved, no reduction in the subsequent utilization was observed.[45]
Additional important questions remain underexplored. First, are condition‐specific interventions superior to those that span conditions? All of the interventions that demonstrated reductions in readmission were condition‐specific, yet no generic interventions met our inclusion criteria. Importantly, only 1 study[29] in our review examined discharge processes from 1 of the pediatric conditions with the most variation[8] in readmission. Further, no studies focused on children with complex medical conditions, who are known to be at increased risk of readmission,[46] indicating a sizable knowledge gap persists in understanding how to prevent readmissions in the most vulnerable pediatric populations.
Lastly, who are the most appropriate effector individuals for discharge‐focused transitional care interventions? Demographically matched effector individuals have shown promise in improving care using community health workers.[47, 48] The degree to which the identity of the intervener mediates subsequent ED and hospital utilization warrants further investigation.
Limitations of This Systematic Review
The studies included in this review assessed different outcomes at different intervals, precluding meta‐analysis. With greater consistency in the collection of data on the quality of discharge processes and their subsequent outcomes, future studies may offer further clarity as to which discharge‐oriented practices are more effective than others. Because we only identified literature in 3 pediatric conditions, generalizability beyond these conditions may be limited. The settings of the interventions also occurred in multiple countries; we excluded countries from low or low‐middle incomes to facilitate generalizability. As many of the discharge processes contained multiple interventions, it is not possible to ascertain which, if any, singular action may decrease posthospitalization utilization. Additionally, some of the included interventions are older, and it is plausible that discharge processes have evolved with the expansion of the hospitalist model.
Methods of data collection influence the quality of results in the included studies. Most of the studies included in this review used either medical record review or parental self‐report of utilization. Parental report may be sufficient for hospitalizations and ED utilization; however, it is subject to recall bias. Chart review likely underestimates the number of postdischarge events, depending on the individual institution's proportion of the market and the tendency of individuals to seek care at multiple institutions. Claims data may offer the most accurate assessments of ED and hospital utilization and cost, but can be more difficult to obtain and do not provide the same potential for granularity as parent report or medical records review.
Finally, subsequent ED visits, readmissions, and cost may not be the best measures of the quality of discharge transitional care. A number of tools have been developed to more specifically evaluate the quality of transitional care in adults,[49, 50] including a validated instrument that consists of only 3 items,[50] which primarily assesses the extent to which patients are prepared for self‐care upon discharge. For pediatric populations, validated tools assessing caregiver experience with discharge[51] and discharge readiness[52] are also available. These instruments may assist those interested in assessing process‐related outcomes that specifically assess discharge transitional care elements and may mediate subsequent ED visits or hospitalizations.
CONCLUSION
Successful discharge interventions to reduce pediatric readmission and ED have some common features, including an individual or team with specialized knowledge of the condition that assumed responsibility for the inpatient‐to‐outpatient transition and offered ongoing support to the family following discharge. All studies included in our review examined multiple discharge interventions; however, many did not have enough participants to detect differences in the outcomes of interest. Future studies might adapt common features of effective interventions, which are consistent with professional societies' recommendations.
Acknowledgements
The authors thank Marisa Conte for her help with developing the search algorithms for the review.
Disclosures: Drs. Auger and Kenyon received salary support from the Robert Wood Johnson Foundation Clinical Scholars program. Dr. Feudtner does not have any funding sources to disclose. Dr. Davis is funded in part by the Michigan Department of Community Health to serve as the Chief Medical Executive. The views expressed herein are not necessarily the views of the Department of Community Health. The authors have no conflicts of interest to report.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.
- , , , , . Are hospital characteristics associated with parental views of pediatric inpatient care quality? Pediatrics. 2003;111(2):308–314.
- , , , , , . Deficits in communication and information transfer between hospital‐based and primary care physicians: implications for patient safety and continuity of care. JAMA. 2007;297(8):831–841.
- , , , et al. Improving transitions of care at hospital discharge‐‐implications for pediatric hospitalists and primary care providers. J Healthc Qual. 2010;32(5):51–60.
- , . Hospitalists in children's hospitals: what we know now and what we need to know. J Pediatr. 2006;148(3):296–299.
- , , , , . The incidence and severity of adverse events affecting patients after discharge from the hospital. Ann Intern Med. 2003;138(3):161–167.
- , , , et al. Adverse events among medical patients after discharge from hospital. CMAJ. 2004;170(3):345–349.
- , , , . Medical errors related to discontinuity of care from an inpatient to an outpatient setting. J Gen Intern Med. 2003;18(8):646–651.
- , , , et al. Pediatric readmission prevalence and variability across hospitals. JAMA. 2013;309:372–380.
- , , , , , . Discharge planning from hospital to home. Cochrane Database Syst Rev. 2013;(1):CD000313.
- , , , , , . Preventability of early readmissions at a children's hospital. Pediatrics. 2012;131(1):e171–e181.
- , , , et al. State‐level child health system performance and the likelihood of readmission to children's hospitals. J Pediatr. 2010;157(1):98–102.e1.
- Ohio Children's Hospitals' solutions for patient safety. Available at: http://solutionsforpatientsafety.org/files/sps‐fact‐sheet.pdf. Accessed July 24, 2013.
- American Academy of Pediatrics. Value in inpatient pediatrics (VIP) network projects. Available at: http://www.aap.org/en‐us/professional‐resources/practice‐support/quality‐improvement/Quality‐Improvement‐Innovation‐Networks/Pages/Value‐in‐Inpatient‐Pediatrics‐Network‐Projects.aspx. Accessed July 24, 2013.
- Child Health Corporation of America. Resources for managing the patient discharge process. Available at: http://www.chca.com/news/index.html. Accessed October 31, 2013.
- The World Bank. World Development Indicators 2012. Available at: http://data.worldbank.org/sites/default/files/wdi‐2012‐ebook.pdf. Accessed July 5, 2013.
- The Cochrane Collaboration. Data collection form: Intervention review—RCTs and non‐RCTs. Available at: http://hiv.cochrane.org/sites/hiv.cochrane.org/files/uploads/Data%20extraction%20form_all%20studies.docx. Accessed July 24, 2013.
- , . The feasibility of creating a checklist for the assessment of the methodological quality both of randomised and non‐randomised studies of health care interventions. J Epidemiol Community Health. 1998;52(6):377–384.
- , , , et al. Brief violence interventions with community case management services are effective for high‐risk trauma patients. J Trauma. 2011;71(1):228–237.
- , , , , , . Benefits of a hospital‐based peer intervention program for violently injured youth. J Am Coll Surg. 2007;205(5):684–689.
- , , , , . Caught in the crossfire: the effects of a peer‐based intervention program for violently injured youth. J Adolesc Health. 2004;34(3):177–183.
- , , , , . A matched‐cohort evaluation of a bedside asthma intervention for patients hospitalized at a large urban children's hospital. J Urban Health. 2011;88(suppl 1):49–60.
- , , , et al. Effectiveness of asthma education with and without a self‐management plan in hospitalized children. J Asthma. 2009;46(9):906–910.
- , , , , And . Effect of a structured asthma education program on hospitalized asthmatic children: a randomized controlled study. Pediatr Int. 2006;48(2):158–162.
- , , , , , Parental education and guided self‐management of asthma and wheezing in the pre‐school child: a randomised controlled trial. Thorax. 2002;57(1):39–44.
- , , . Structured discharge procedure for children admitted to hospital with acute asthma: a randomised controlled trial of nursing practice. Arch Dis Child. 1999;80(2):110–114.
- , , . Impact of a nurse‐led home management training programme in children admitted to hospital with acute asthma: a randomised controlled study. Thorax. 1997;52(3):223–228.
- , , , et al. You Can Control Asthma: evaluation of an asthma education program for hospitalized inner‐city children. Patient Educ Couns. 1991;17(1):35–47.
- , , . Asthma education by community child health nurses. Arch Dis Child. 1986;61(12):1184–1189.
- , . Effectiveness of a discharge‐planning program and home visits for meeting the physical care needs of children with cancer. Support Care Cancer. 2009;18(2):243–253.
- , , , et al. Comprehensive follow‐up care and life‐threatening illnesses among high‐risk infants: a randomized controlled trial. JAMA. 2000;284(16):2070–2076.
- , , , . Very low birth weight infants and their families during the first year of life: comparisons of medical outcomes based on after care services. J Perinatol. 1998;18(5):365–371.
- , , , , . Description and evaluation of a program for the early discharge of infants from a neonatal intensive care unit. J Pediatr. 1995;127(2):285–290.
- , , , et al. Earlier discharge with community‐based intervention for low birth weight infants: a randomized trial. Pediatrics. 1993;92(1):128–134.
- , , , et al. A randomized clinical trial of early hospital discharge and home follow‐up of very‐low‐birth‐weight infants. N Engl J Med. 1986;315(15):934–939.
- , , . Care transitions from inpatient to outpatient settings: ongoing challenges and emerging best practices. Hosp Pract (1995). 2011;39(3):128–139.
- , , , , . Interventions to reduce 30‐day rehospitalization: a systematic review. Ann Intern Med. 2011;155(8):520–528.
- . Clinical report—physicians' roles in coordinating care of hospitalized children. Pediatrics. 2010;126(4):829–832.
- . White space or black hole: what can we do to improve care transitions? ABIM Foundation. Available at: http://www.abimfoundation.org/∼/media/Files/Publications/F06‐05‐2007_6.ashx. Accessed September 5, 2012.
- , , , et al. Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev. 2012;(6):CD000259.
- , , , , . A smoking cessation intervention for parents of children who are hospitalized for respiratory illness: the stop tobacco outreach program. Pediatrics. 2003;111(1):140–145.
- , . A randomized, controlled trial of smoking cessation counseling provided during child hospitalization for respiratory illness. Pediatr Pulmonol. 2008;43(6):561–566.
- , . Embracing chaos and complexity: a quantum change for public health. Am J Public Health. 2008;98(8):1382–1389.
- , , , , , . Outpatient follow‐up and rehospitalizations for sickle cell disease patients. Pediatr Blood Cancer. 2012;58(3):406–409.
- , , . Does having an outpatient visit after hospital discharge reduce the likelihood of readmission? Del Med J. 2003;75(8):291–298.
- , , . Follow‐up after acute asthma episodes. Proc Am Thorac Soc. 2009;6(4):386–393.
- , , , et al. Hospital utilization and characteristics of patients experiencing recurrent readmissions within children's hospitals. JAMA. 2011;305(7):682–690.
- , , , et al. A randomized controlled evaluation of the effect of community health workers on hospitalization for asthma: the asthma coach. Arch Pediatr Adolesc Med. 2009;163(3):225–232.
- , , , . The Seattle‐King County Healthy Homes Project: a randomized, controlled trial of a community health worker intervention to decrease exposure to indoor asthma triggers. Am J Public Health. 2005;95(4):652–659.
- , , , , , . Development and testing of a measure designed to assess the quality of care transitions. Int J Integr Care. 2002;2:e02.
- , , , . Assessing the quality of transitional care: further applications of the care transitions measure. Med Care. 2008;46(3):317–322.
- , , , et al. Hospital readmission and parent perceptions of their child's hospital discharge. Int J Qual Health Care. 2013;25(5):573–581.
- , . Psychometric properties of the Readiness for Hospital Discharge Scale. J Nurs Meas. 2006;14(3):163–180.