User login
A planning and evaluation program for assessing telecommunications applications in community radiation oncology programs
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Management-focused scientific evaluation is a useful administrative tool especially when hospitals implement a new technology. This paper describes the components of a scientific evaluation framework and then illustrates the application and the utility of the framework in a hospital-based community oncology setting. The clinical technology, Telesynergy, is an advanced telecommunications and remote medical consultation system which has been developed by the National Cancer Institute to support community hospital-based radiation oncology programs.
Click on the PDF icon at the top of this introduction to read the full article.
Inexpensive solutions to enhance remote cancer care in community hospitals
Rapidly increasing volume and complexity of information used for multidisciplinary cancer treatment requires carefully evolving communications with programmatic planning, detailed evaluation, and new methodologies and technical approaches to enhance the impact and efficacy of medical conferencing systems. We designed, implemented, and evaluated cost-effective and appropriate remote learning optimize oncology practice techniques in community hospitals. Our experience over the course of more than 7 years demonstrated simple and inexpensive communication solutions for both professional and lay education, satisfying information-dense needs of multimodality cancer care. We describe how potential complexities may be resolved with inexpensive devices and software programs. Staff teamwork and creativity are always required to implement constantly evolving technologies. We provide both quantitative and qualitative data describing activities and resulting staff responses resulting in 6,520 personnel with more than 391 aggregate credit hours of continuing medical education and continuing education credit activities with enhanced collegial participant satisfaction levels and heightened interactions/professionalism among regional oncology staff. We noted significant cost reductions for communications in all our three partnered hospitals. We demonstrated both increased satisfaction levels and heightened levels of behavioral changes (Impacts) in participants. Always, activities must be cost effective and responsive to changing medical needs. Community focused efforts with regional partners should be similar, assuring evolving successes.
Click on the PDF icon at the top of this introduction to read the full article.
Rapidly increasing volume and complexity of information used for multidisciplinary cancer treatment requires carefully evolving communications with programmatic planning, detailed evaluation, and new methodologies and technical approaches to enhance the impact and efficacy of medical conferencing systems. We designed, implemented, and evaluated cost-effective and appropriate remote learning optimize oncology practice techniques in community hospitals. Our experience over the course of more than 7 years demonstrated simple and inexpensive communication solutions for both professional and lay education, satisfying information-dense needs of multimodality cancer care. We describe how potential complexities may be resolved with inexpensive devices and software programs. Staff teamwork and creativity are always required to implement constantly evolving technologies. We provide both quantitative and qualitative data describing activities and resulting staff responses resulting in 6,520 personnel with more than 391 aggregate credit hours of continuing medical education and continuing education credit activities with enhanced collegial participant satisfaction levels and heightened interactions/professionalism among regional oncology staff. We noted significant cost reductions for communications in all our three partnered hospitals. We demonstrated both increased satisfaction levels and heightened levels of behavioral changes (Impacts) in participants. Always, activities must be cost effective and responsive to changing medical needs. Community focused efforts with regional partners should be similar, assuring evolving successes.
Click on the PDF icon at the top of this introduction to read the full article.
Rapidly increasing volume and complexity of information used for multidisciplinary cancer treatment requires carefully evolving communications with programmatic planning, detailed evaluation, and new methodologies and technical approaches to enhance the impact and efficacy of medical conferencing systems. We designed, implemented, and evaluated cost-effective and appropriate remote learning optimize oncology practice techniques in community hospitals. Our experience over the course of more than 7 years demonstrated simple and inexpensive communication solutions for both professional and lay education, satisfying information-dense needs of multimodality cancer care. We describe how potential complexities may be resolved with inexpensive devices and software programs. Staff teamwork and creativity are always required to implement constantly evolving technologies. We provide both quantitative and qualitative data describing activities and resulting staff responses resulting in 6,520 personnel with more than 391 aggregate credit hours of continuing medical education and continuing education credit activities with enhanced collegial participant satisfaction levels and heightened interactions/professionalism among regional oncology staff. We noted significant cost reductions for communications in all our three partnered hospitals. We demonstrated both increased satisfaction levels and heightened levels of behavioral changes (Impacts) in participants. Always, activities must be cost effective and responsive to changing medical needs. Community focused efforts with regional partners should be similar, assuring evolving successes.
Click on the PDF icon at the top of this introduction to read the full article.
Virtual tumor boards: community–university collaboration to improve quality of care
Objective To develop and implement virtual interactive multidisciplinary cancer tumor boards (VTBs), created throughtelemedicine links between the University of California, Davis Cancer Center and community-based cancer care providers. Thegoal of this project was to facilitate communication among community and academic cancer specialists.
Materials and methods Four geographically remote sites were selected to participate with established disease-specific tumorboards of the UC Davis Cancer Center. Telemedicine links were created using dedicated T1 lines, and PolyCom HDX 9000 was used by the center for teleconference hosting. Participants were then surveyed on their perception of the benefit of VTBs.
Results The results across disease-specific virtual tumor boards show that most of the participants reported that the right amountof clinical information on the cases was presented and that new information was discussed that helped providers manage thecare of the patients.
Conclusions Teleconferencing of disease-specific tumor boards allowed providers in a geographically remote group ofproviders to make prospective, case-based treatment decisions that increased their knowledge of treatment options and facilitatedtheir decision making. This transfer of knowledge and experience speeds up the dissemination of rapidly evolving cancer care,which could lead to higher quality patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Objective To develop and implement virtual interactive multidisciplinary cancer tumor boards (VTBs), created throughtelemedicine links between the University of California, Davis Cancer Center and community-based cancer care providers. Thegoal of this project was to facilitate communication among community and academic cancer specialists.
Materials and methods Four geographically remote sites were selected to participate with established disease-specific tumorboards of the UC Davis Cancer Center. Telemedicine links were created using dedicated T1 lines, and PolyCom HDX 9000 was used by the center for teleconference hosting. Participants were then surveyed on their perception of the benefit of VTBs.
Results The results across disease-specific virtual tumor boards show that most of the participants reported that the right amountof clinical information on the cases was presented and that new information was discussed that helped providers manage thecare of the patients.
Conclusions Teleconferencing of disease-specific tumor boards allowed providers in a geographically remote group ofproviders to make prospective, case-based treatment decisions that increased their knowledge of treatment options and facilitatedtheir decision making. This transfer of knowledge and experience speeds up the dissemination of rapidly evolving cancer care,which could lead to higher quality patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Objective To develop and implement virtual interactive multidisciplinary cancer tumor boards (VTBs), created throughtelemedicine links between the University of California, Davis Cancer Center and community-based cancer care providers. Thegoal of this project was to facilitate communication among community and academic cancer specialists.
Materials and methods Four geographically remote sites were selected to participate with established disease-specific tumorboards of the UC Davis Cancer Center. Telemedicine links were created using dedicated T1 lines, and PolyCom HDX 9000 was used by the center for teleconference hosting. Participants were then surveyed on their perception of the benefit of VTBs.
Results The results across disease-specific virtual tumor boards show that most of the participants reported that the right amountof clinical information on the cases was presented and that new information was discussed that helped providers manage thecare of the patients.
Conclusions Teleconferencing of disease-specific tumor boards allowed providers in a geographically remote group ofproviders to make prospective, case-based treatment decisions that increased their knowledge of treatment options and facilitatedtheir decision making. This transfer of knowledge and experience speeds up the dissemination of rapidly evolving cancer care,which could lead to higher quality patient outcomes.
Click on the PDF icon at the top of this introduction to read the full article.
Sickle cell crises curtailed with experimental cellular adhesion inhibitor
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
NEW ORLEANS – An experimental cellular adhesion inhibitor was successful at reducing the severity and duration of vaso-occlusive crises in patients with sickle cell disease.
In a phase II trial of 76 patients with sickle cell disease, patients randomized to receive the pan-selectin inhibitor GMI 1070 early in their hospitalization for a vaso-occlusive crisis (VOC) had shorter lengths of stay and needed significantly lower cumulative doses of narcotics for pain control than did patients randomized to placebo, reported Dr. Marilyn J. Telen, chief of the hematology division at Duke University, Durham, N.C.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
"We see somewhere between 75,000 and 90,000 admissions [annually] for acute painful vaso-occlusive crisis among this patient population. Indeed, these crises are the most common and essentially the archetypal presentation of sickle cell disease. Nevertheless, up till this time, treatment for these crises or VOC in sickle cell disease, remain only supportive, focusing largely on using narcotics for symptom relief, and then other measures, some of which are used to counteract the ill effects of narcotics," said Dr. Telen at the annual meeting of the American Society of Hematology.
GMI 1070 (being developed by GlycoMimetics, in partnership with Pfizer) is a synthetic molecule designed to inhibit the glycoprotein cellular-adhesion molecules involved in inflammation. In previous studies, the drug has been shown to be safe, and in a mouse model of VOC, was successful at restoring blood flow, Dr. Telen said. The drug has received both orphan drug and fast-track status from the Food and Drug Administration, according to GlycoMimetics.
Dr. Telen and her colleagues enrolled 76 patients aged 12-51 years with sickle cell disease and randomized them to receive a loading dose of GMI 1070 delivered intravenously (43 patients), followed by up to 14 subsequent doses delivered every 12 hours, or placebo (33 patients), with other treatment left to the discretion of the participating institutions. After an interim pharmacokinetic analysis showed that the drug did not reach target nadir levels, the dose was doubled.
All 76 patients reached the primary endpoint of VOC resolution, defined as a composite of decreased pain, termination of the need for intravenous opioids, patient and physician agreement on the ability to discharge the patient, and actual hospital discharge.
A total of 58 patients continued on the assigned drug until they either reached the primary endpoint criteria or received the maximum number of doses allowed. The remaining 18 patients discontinued the drug either for adverse events, no improvement by day 5 on the assigned drug, or other reasons.
In an analysis pooling all patients assigned to GMI 1070, including those who started out on the lower dose, there was a consistent reduction over placebo in the mean time to resolution of VOC: 103 hours vs. 144 hours for patients treated with placebo. This difference was not statistically significant, however.
A Kaplan-Meier analysis showed a median time to resolution of 69.6 hours for GMI 1070, compared with 139 hours with placebo, a difference that was not significant.
There was an 83% reduction in the secondary endpoint of cumulative opioid analgesics administered during hospitalization, a difference that was statistically significant (P =.010). There was also a reduction by 84 hours in the median time to discharge, and by 55 hours in the mean time to discharge, among patients treated with the active drug, compared with those on placebo. These differences, while large, were not significant, Dr. Telen said.
She noted that although most of the endpoints in this study failed to reach statistical significance, the separation of the curves between the placebo- and GMI 1070–treated patients began early, usually within 2 days of the start of treatment.
Total adverse event rates, including serious events and those deemed to be treatment related, were comparable between the two study arms for all subgroups.
Dr. Telen noted that because the population of patients enrolled was more clinically diverse than the available literature would suggest, the study was underpowered to detect differences, given the size of the sample. She predicted that given the size of the effects seen, statistical significance would emerge in a larger study.
GlycoMimetics is currently working with Pfizer to develop a phase III trial of GM 1070 for this indication.
The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
AT ASH 2013
Major finding: The mean time to resolution of vaso-occlusive crisis in patients with sickle cell disease was 103 hours for patients treated with GMI 1070 vs. 144 for those treated with placebo.
Data source: A randomized, double blind multicenter study of 76 patients aged 12-51.
Disclosures: The study was supported by GlycoMimetics. Dr. Telen is a consultant to the company, and several coauthors are employees of the company.
VIDEO: Idelalisib shows promise in refractory non-Hodgkin lymphoma
NEW ORLEANS – When indolent B-cell non-Hodgkin lymphoma becomes refractory to rituximab and alkylating agents, few therapeutic options remain. But the PI3kd inhibitor idelalisib may someday offer a new treatment choice. Dr. Ajay Gopal discusses the promising findings from a phase II trial of idelalisib, including a 57% response rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – When indolent B-cell non-Hodgkin lymphoma becomes refractory to rituximab and alkylating agents, few therapeutic options remain. But the PI3kd inhibitor idelalisib may someday offer a new treatment choice. Dr. Ajay Gopal discusses the promising findings from a phase II trial of idelalisib, including a 57% response rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
NEW ORLEANS – When indolent B-cell non-Hodgkin lymphoma becomes refractory to rituximab and alkylating agents, few therapeutic options remain. But the PI3kd inhibitor idelalisib may someday offer a new treatment choice. Dr. Ajay Gopal discusses the promising findings from a phase II trial of idelalisib, including a 57% response rate.
The video associated with this article is no longer available on this site. Please view all of our videos on the MDedge YouTube channel
Gene therapy for SCID-X1 may successfully reset immune system
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
NEW ORLEANS – Tweaking experimental gene therapy for X-linked severe combined immunodeficiency may help to restore patient immune function while reducing the risk for subsequent leukemias.
In a small, multinational phase I/II trial, seven of nine children with SCID-X1 showed evidence of T-cell recovery and function, as well as a lower risk for promoting growth of leukemic cells, when a self-inactivating gamma-retroviral vector (SCID-2) was used to promote reconstitution of the child’s immune system without insertional oncogenesis, reported Dr. Sung-Yun Pai at the annual meeting of the American Society of Hematology.
"Outcomes for boys who do not have well-matched donors are suboptimal, and it’s particularly for these boys that we are targeting gene therapy," said Dr. Pai, of Boston Children’s Hospital and the Dana-Farber Cancer Institute, Boston.
In previous gene therapy trials, investigators used the Moloney leukemia virus (MLV)-based gamma-retroviral vector (SCID-1) with strong promoters and enhancers to express an IL-2 receptor that reconstituted the immune system successfully in 18 of 20 boys.
However, 5 of the 20 boys developed T-cell acute lymphoblastic leukemia; 1 of the children died, and the remaining 4 were successfully treated.
The investigators found that in the patients with leukemia, the SCID-1 vector had inserted into a chromosomal region close to proto-oncogenes such as LMO2, and the enhancers were driving expression of the neighboring oncogene, promoting expression of aberrant T cells. The vector was subsequently modified with the goal of improved safety but similar efficacy to the original, said Dr. Pai. The strong viral enhancers were removed to prevent accident enhancement should the inserted genes manage to find their way into oncogenes.
The phase I/II study is being conducted in London, Paris, Boston, Cincinnati, and Los Angeles, and to date has enrolled 9 male children of a planned 20.
Of the 9, one child died from a preexisting adenoviral infection before immune recovery was complete, and one child did not have engraftment of the gene-marked cells and went on to transplant.
"The other patients have 9 months to 36 months of follow-up, they have evidence of T-cell recovery, of T-cell function, have cleared SCID-related infections, and are all out of hospital, healthy at home, [and] leading essentially normal lives."
When the investigators looked at the comparative efficacy of the SCID-1 and SCID-2 vectors, they saw that 6 months after gene therapy, there was no significant difference in the median number of T cells generated.
"It’s far too early to comment on whether this vector will truly be safer in terms of leukemia," Dr. Pai said, noting that in the SCID-1 trial the leukemias developed 3-5 years after gene therapy, and the longest follow-up in the SCID-2 study is only 3 years.
The investigators are, however, conducting molecular surrogate safety analyses looking at gene insertion sites from the blood of patients treated with SCID-1 and are comparing those sites with the vector-insertion sites in cells from patients in SCID-2.
Looking at a global genomewide map of integrations, they found no significant differences between SCID-1 and SCID-2. However, when they focused on 38 genes that are to be proto-oncogenes in lymphoid cancer, they found that significantly more integration of the modified genes occurred in proximity to the oncogenes in SCID-1 than in SCID-2 (P = .003).
"We hope that these data suggest that the modified SCID vector will show less capacity to drive aberrant cell growth and lead to less leukemogenesis," said Dr. Pai.
SCID-X1 is caused by inherited mutations in the gamma subunit of the interleukin (IL)-2 receptor. As a result, males are born without T lymphocytes or natural killer cells. Without a bone marrow or stem cell transplantation, children with the disease die early from opportunistic or community-acquired infections.
"These are really paradigm-changing results for mortally wounded children," said Dr. Laurence James Neil Cooper of the University of Texas M.D. Anderson Cancer Center in Houston, who moderated the briefing but was not involved in the study.
The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
AT ASH 2013
Major finding: Of nine boys with X-linked severe combined immunodeficiency who were treated with gene therapy, seven patients have evidence of T-cell function, have cleared SCID-related infections, and are out of hospital.
Data source: Preliminary results of a prospective phase I/II clinical trial of nine children.
Disclosures: The trial is being sponsored by Children’s Hospital Boston, Cincinnati Children’s Hospital Medical Center, and the University of California, Los Angeles. Dr. Pai and Dr. Cooper reported having no relevant conflicts of interest.
Receptor may play key role in sepsis

Staphylococcus infection
Credit: Bill Branson
Researchers have identified a receptor that may be instrumental in the body’s response to sepsis. And they believe this discovery could be the key to unlocking new treatments for the disease.
The nociceptin receptor activates the chemical nociceptin. Previous research revealed that nociceptin is involved in inflammation; it is known to affect white blood cell function.
This suggests nociceptin has an important role in the body’s response to inflammation and sepsis, according to David Lambert, PhD, of the University of Leicester in the UK, and his colleagues.
The group’s theory, which they have explored in 2 papers published in PLOS ONE, is that nociceptin makes inflammation or sepsis worse. And by blocking the nociceptin system, the symptoms of sepsis could be reduced, which could lead to new treatments.
“We have found that nociceptin, a chemical similar to endorphins produced in the body, is increased in inflammation and sepsis,” said study author Jonathan Thompson, MD, MB ChB, also of the University of Leicester.
“This suggests that drugs which block the nociceptin receptor could dampen the widespread inflammation that occurs in sepsis, and improve outcome. More work is needed, but these drugs are being developed. If they are effective, then we could potentially save many lives.”
In their first paper, the researchers described how they used fluorescent chemistry to find nociceptin receptors on blood vessels with no nerve supply. The team also showed, in a lab model of sepsis, that blocking these receptors has a protective effect.
In the second paper, the researchers recounted their discovery that bloodstream nociceptin levels are elevated in sepsis patients in intensive care. This suggests nociceptin activation might be important in critically ill patients suffering from sepsis.
Dr Lambert and his colleagues noted that sepsis remains a leading cause of admission to intensive care units, with high mortality, costs, and long-term morbidity in those who survive. The incidence of severe sepsis has increased in the last decade, making the discovery of new treatments highly desirable.
“Sepsis is a major health problem . . . that has often been under-recognized,” Dr Thompson said. “It can be rapidly fatal, especially if not diagnosed and treated early, because inflammation can spread and affect many different organs in the body.”
Dr Lambert added, “I am particularly excited by these findings, as they translate many years of laboratory work into a possible target for this disease.” ![]()

Staphylococcus infection
Credit: Bill Branson
Researchers have identified a receptor that may be instrumental in the body’s response to sepsis. And they believe this discovery could be the key to unlocking new treatments for the disease.
The nociceptin receptor activates the chemical nociceptin. Previous research revealed that nociceptin is involved in inflammation; it is known to affect white blood cell function.
This suggests nociceptin has an important role in the body’s response to inflammation and sepsis, according to David Lambert, PhD, of the University of Leicester in the UK, and his colleagues.
The group’s theory, which they have explored in 2 papers published in PLOS ONE, is that nociceptin makes inflammation or sepsis worse. And by blocking the nociceptin system, the symptoms of sepsis could be reduced, which could lead to new treatments.
“We have found that nociceptin, a chemical similar to endorphins produced in the body, is increased in inflammation and sepsis,” said study author Jonathan Thompson, MD, MB ChB, also of the University of Leicester.
“This suggests that drugs which block the nociceptin receptor could dampen the widespread inflammation that occurs in sepsis, and improve outcome. More work is needed, but these drugs are being developed. If they are effective, then we could potentially save many lives.”
In their first paper, the researchers described how they used fluorescent chemistry to find nociceptin receptors on blood vessels with no nerve supply. The team also showed, in a lab model of sepsis, that blocking these receptors has a protective effect.
In the second paper, the researchers recounted their discovery that bloodstream nociceptin levels are elevated in sepsis patients in intensive care. This suggests nociceptin activation might be important in critically ill patients suffering from sepsis.
Dr Lambert and his colleagues noted that sepsis remains a leading cause of admission to intensive care units, with high mortality, costs, and long-term morbidity in those who survive. The incidence of severe sepsis has increased in the last decade, making the discovery of new treatments highly desirable.
“Sepsis is a major health problem . . . that has often been under-recognized,” Dr Thompson said. “It can be rapidly fatal, especially if not diagnosed and treated early, because inflammation can spread and affect many different organs in the body.”
Dr Lambert added, “I am particularly excited by these findings, as they translate many years of laboratory work into a possible target for this disease.” ![]()

Staphylococcus infection
Credit: Bill Branson
Researchers have identified a receptor that may be instrumental in the body’s response to sepsis. And they believe this discovery could be the key to unlocking new treatments for the disease.
The nociceptin receptor activates the chemical nociceptin. Previous research revealed that nociceptin is involved in inflammation; it is known to affect white blood cell function.
This suggests nociceptin has an important role in the body’s response to inflammation and sepsis, according to David Lambert, PhD, of the University of Leicester in the UK, and his colleagues.
The group’s theory, which they have explored in 2 papers published in PLOS ONE, is that nociceptin makes inflammation or sepsis worse. And by blocking the nociceptin system, the symptoms of sepsis could be reduced, which could lead to new treatments.
“We have found that nociceptin, a chemical similar to endorphins produced in the body, is increased in inflammation and sepsis,” said study author Jonathan Thompson, MD, MB ChB, also of the University of Leicester.
“This suggests that drugs which block the nociceptin receptor could dampen the widespread inflammation that occurs in sepsis, and improve outcome. More work is needed, but these drugs are being developed. If they are effective, then we could potentially save many lives.”
In their first paper, the researchers described how they used fluorescent chemistry to find nociceptin receptors on blood vessels with no nerve supply. The team also showed, in a lab model of sepsis, that blocking these receptors has a protective effect.
In the second paper, the researchers recounted their discovery that bloodstream nociceptin levels are elevated in sepsis patients in intensive care. This suggests nociceptin activation might be important in critically ill patients suffering from sepsis.
Dr Lambert and his colleagues noted that sepsis remains a leading cause of admission to intensive care units, with high mortality, costs, and long-term morbidity in those who survive. The incidence of severe sepsis has increased in the last decade, making the discovery of new treatments highly desirable.
“Sepsis is a major health problem . . . that has often been under-recognized,” Dr Thompson said. “It can be rapidly fatal, especially if not diagnosed and treated early, because inflammation can spread and affect many different organs in the body.”
Dr Lambert added, “I am particularly excited by these findings, as they translate many years of laboratory work into a possible target for this disease.” ![]()
Quick Diagnosis Units
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
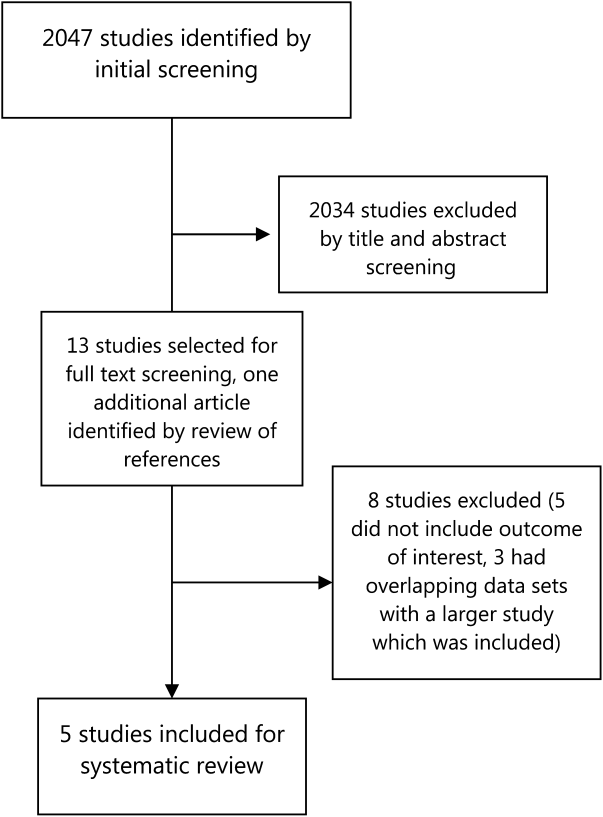
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.
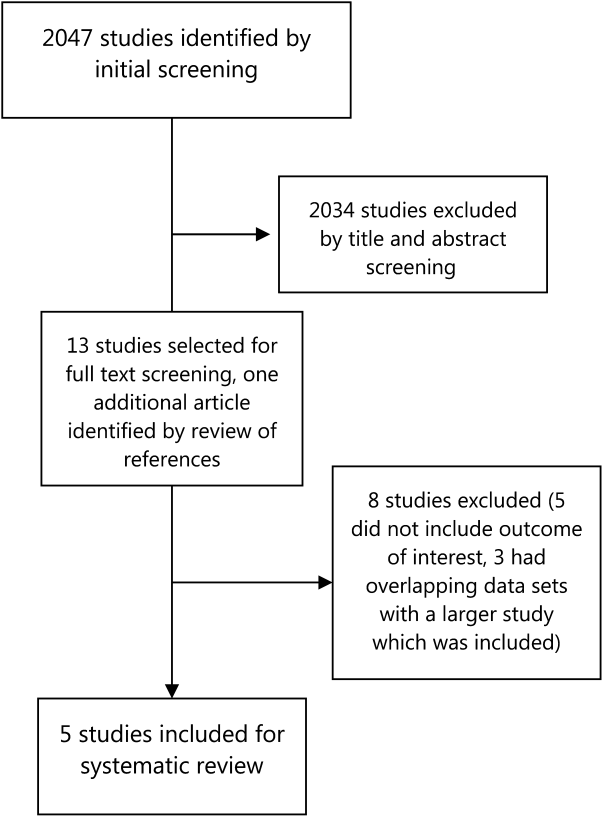
Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.

Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
Inpatient admissions are a major component of healthcare costs in the United States,[1] where the number of annual inpatient hospital admissions has increased by 15% from 34.3 million in 1993 to 39.5 million in 2006.2 Studies performed predominantly in Europe have shown that inappropriate use of hospital beds exceeds 20% across various specialties.[3] A study by Campbell et al. showed that if given the choice, 60% of physicians would consider an alternative to admission for such patients, if such an option were available, and 70% of patients would prefer not to be admitted for workup.[4] Based on similar findings, various hospitals across the world have tried to make organizational changes to allocate healthcare resources more efficiently. The concept of quick and early diagnosis was first introduced in 1996 by Kendall et al., and it included a hospital unit in the United Kingdom managed by consultants receiving referrals from primary care doctors and led to early diagnostic workup without hospitalization.[5] A more refined version of this concept, a potentially cost‐saving and efficient alternative to inpatient hospitalization for diagnostic purposes, was described by Bosch et al., and named the quick diagnosis unit (QDU).[1]
The basic objectives of QDUs include early diagnosis of potentially severe diseases such as cancer, avoiding unnecessary hospitalization, minimizing hospital morbidity, reducing costs, and improving patient satisfaction. The first described QDU was managed by internists, where patients with specific symptoms such as undiagnosed lumps or masses, anemia, hematuria, or gastrointestinal symptoms could be referred for a diagnostic evaluation. Patients were required to be well enough to travel to the QDU on an outpatient basis, and patients unable to do so were thought to be better suited for hospitalization.[1]
In the present study, we conduct a systematic review, the first one on this subject to our knowledge, of studies that tested established QDUs or similar units in hospital settings. The majority of established units were tested and exist in Europe.[1, 5, 6, 7, 8, 9, 10, 11, 12, 13, 14] They have been studied in Spain, from where much of these data have been obtained.[1]
METHODS
Study Selection
We searched MEDLINE (January 1946 to November 2012) via OVID and EMBASE (January 1974 to November 2012) via SCOPUS using keywords and Medical Subject Heading terms for quick diagnosis units and rapid diagnosis units. The detailed search strategy can be found in Table 1. A screening of titles and abstracts was done by 2 independent reviewers and followed by full‐text screening. We screened for additional articles by reviewing the bibliography of the articles selected for full‐text screening. We included in our review all studies that (1) were published in any language, (2) focused on the design and implementation of a quick diagnosis unit or a rapid diagnosis unit in a hospital setting, and (3) included at least 2 of the primary outcomes, as described below.
| No. | Searches |
|---|---|
| 1 | Quick diagnosis units.mp. |
| 2 | Quick diagnosis unit.mp. |
| 3 | (Quick adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 4 | (Quick adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 5 | (Quick adj diagnosis).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 6 | (Diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 7 | (Diagnosis adj units).mp [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 8 | Rapid diagnosis units.mp. |
| 9 | Rapid diagnosis unit.mp. |
| 10 | (Rapid adj diagnosis adj units).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 11 | (Rapid adj diagnosis adj unit).mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier] |
| 12 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 |
Outcome Measures
Our primary outcome measures were categories of final diagnosis, mean time to final diagnosis in an outpatient setting, inpatient bed‐days per patient saved, and costs saved per patient for QDUs versus in‐hospital stay. Secondary outcomes included disposition of patients after completion of this initial evaluation (whether admitted to the hospital or discharged to clinics) and the patients' care preferences, if available. For cost outcomes, currency exchange rates used for conversion were provided by Citibank National Bank Association, powered by Google online currency converter service (accessed June 16, 2013).
Data Extraction
We extracted data on the specifics of the early diagnostic unit setup including staffing and hours of operation, hospital setting, sources of referral, referring diagnosis, patient population, and the role of the diagnostic units in expediting workup and duration of study. For multiple studies done in the same institution by the same principal author, we used the study with the largest patient population to avoid duplication of data. The primary outcome measures for comparing costs were calculated by different methods and in different currencies by different investigators, which we have attempted to reconcile by using current currency conversion rates. We also evaluated patient preferences (if available) via patient surveys. The data were extracted by 2 independent reviewers, and disagreements were resolved by consensus.
RESULTS
Study Selection
Our literature search initially yielded 2047 publications, out of which 2034 were excluded after title and abstract screening. Thirteen studies were selected for full‐text review, out of which 5 were selected for detailed review based on our inclusion criteria (Figure 1). Three of the studies were in Spanish, and the results were analyzed with the help of a Spanish translator. The other 2 studies were in English.

Study Characteristics
Four studies that were included were descriptive longitudinal studies,[6, 7, 9, 10] and 1 was a retrospective study[8] (Table 2). There were a total of 8895 patients included in all of the studies. All of the studies except 1 described a similar organizational arrangement for the QDU, with 1 internist and 1 registered nurse, administrative support, and the ability to expedite the scheduling of diagnostic tests. The exception was a dedicated lung cancer rapid diagnostic unit (RDU) set up by Sanz‐Santos et al.[9] The study durations ranged from 6 months to 5 years. Patients were referred from local emergency rooms, primary care clinics, and specialty care clinics. The most common reasons for referral were anemia, adenopathy, visceromegaly, febrile syndromes, and incidentally detected masses or nodules on imaging. Two studies included some form of cost analysis,[6, 7] and 3 included patient surveys on satisfaction with patient care.[6, 7, 10]
| Author | Methods | Setup of Rapid Diagnosis Units | Sources of Referrals to the Unit | Reasons for Referrals to the Unit | Cases | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Prospective descriptive study in 4,170 patients evaluated by a dedicated QDU in a university hospital in Barcelona, Spain, between December 2007 to December 2009 and January 2010 to January 2012. QDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | Quick diagnostic unit consisting of an internist, and a registered nurse. Single consulting room with a family waiting room. Assisted by specialists from other specialties. | Local primary health center (40%), emergency room (56%), other sources (4%). | Anemia, anorexia‐cachexia syndrome, febrile syndrome, adenopathies, abdominal pain, chronic diarrhea, lung abnormalities. | 4,170 | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Prospective descriptive study with retrospective controls in 2,748 patients evaluated by a QEDU in a university hospital in Barcelona, Spain, between September 1996 and 2001. QEDU costs compared with costs for randomly selected, retrospectively reviewed hospital admissions for similar diagnosis. Care preferences studied with random surveys. | UDR made up of an internist and a nurse, a consultation and waiting room. | Referrals from emergency rooms (64%), primary care (28.6%), specialty clinics (6.4%). | Abdominal pain (12%), focal neurological symptoms (11.5%), constitutional symptoms (11%), anemia (6%), abnormal chest radiology (5.8%), palpable tumors (5.3%), adenopathies (4.7%), rectal bleeding (4.6%), febrile syndrome (4.6%), hemoptysis (3.5%), others (30%). | 2,748 | 5 years | Preferential scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Retrospective, descriptive study for 1,132 patients evaluated by a dedicated RDU in a university hospital in Barcelona, Spain from October 2005 to March 2007. | RDU consisted of an internist, a radiologist, and a nurse. | Local primary health centers (71%), emergency rooms (26%), and others (3%). | FUO, adenopathies, visceromegalies, chronic diarrhea, rectal bleeding, dysphagia, jaundice, hypercalcemia. | 1,132 | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Prospective observational study in 678 patients referred to an LC RDU, at a tertiary care center in Barcelona, Spain from October 2005 to September 2009. | An LC‐RDU, with nursing staff, 3 pulmonologists, bronchoscopy suites with EBUS‐TBNA, facilities for mediastinoscopy, CT‐guided FNAC, thoracoscopy, and surgery. | Referrals from specialty clinics (59.4%), primary care (20.2%), and local emergency rooms (20.4%). | Cough, dyspnea, hemoptysis, weight loss, imaging evidence of lung masses. | 678 | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Prospective descriptive study on 167 patients, evaluated by an RDU in a tertiary care hospital in Palencia, Spain between November 2008 and April 2009. Care preferences studied with random surveys. | An RDU run by an internist and nursing staff with administrative support. Has a consulting room and a waiting room. | Referrals from primary care (70.7%), emergency room (21.6%), specialty clinics (7.8%). | Abdominal masses and visceromegalies, chronic diarrhea, dysphagia, ascites, icterus, transaminitis, heart failure, abnormal chest imaging, suspicion of pulmonary TB, or neoplasia, | 167 | 6 months | Early scheduling and urgent specialized workup. |
Outcomes
The most common final diagnosis was malignancy in 18% to 30% of the cases[6, 7, 8, 10] and in 55% of the lung cancer RDU cases[9] (Table 3). The time from initial contact to final diagnosis ranged from 6 to 11 days. Only 3% to 10% of the patients were admitted to the hospital from the QDUs; most patients were discharged to specialty‐care clinics or to primary care centers. Capell et al.[7] estimated that such a unit could save 7 inpatient bed‐days per patient, whereas Rubio‐Rivas et al.[8] estimated that value to be 4.5 bed‐days per patient. Bosch et al.[6] calculated that they saved 8.76 bed‐days per patient.
| Author | Final Diagnosis | Time to Diagnosis | Final Disposition | Benefit Analysis | Care Preference Survey | Duration | Intervention |
|---|---|---|---|---|---|---|---|
| |||||||
| Bosch et al., 2012 | Malignancy (30%), IDA (19%), other benign GI disorders (12%), others (39%). | Mean=8.9 days (cases) (3.13 QDU visits) | Hospital for admission: 3%, primary health centers: 62%, outpatient follow‐up: 35%. | Estimated hospital days saved: mean length of stay 8.76 days. Average cost saved per process (admission to discharge): 2,514.64. | 88% preferred QDU care model over hospital stay. | 4 years | Outpatient workup with urgent first visit, preferential scheduling of diagnostic tests, and follow‐up until diagnosis is made. |
| Capell et al., 2004 | Malignancy (15%), GI disorders (24%), neurological disorders (14%). | Mean=5.7 days | Hospital for admission: 7%, primary care: 51%, outpatient hospital follow‐up: 38%,specialty clinics: 4%. | Estimated 7 inpatient bed/days per year during the period of study. Cost saved per encounter: 1,764. | 95% reported high satisfaction with QEDU. | 5 years | Prioritized scheduling and urgent workup. |
| Rubio‐Rivas et al., 2008 | Malignancy (18%). | Mean=9 days | Hospital for admission: 10%, outpatient follow‐up: 56%, discharged from follow‐up: 38%. | Hospitalizations avoided: 4.5 bed/days over the study period. Cost analysis not available. | None | 11.5 years | Prioritized scheduling and urgent workup. |
| Sanz‐Santos et al., 2010 | Lung cancer (55%). | Mean=11 days | Not available. | No available data on cost analysis or hospitalizations avoided. | None | 4 years | Specialized outpatient noninvasive and invasive workup. |
| Franco‐Hidalgo et al., 2012 | Neoplastic (19%), nonmalignant digestive diseases (23%,), infection 13%, and rheumatic (11%). | Mean=8 days | Not available. | No available data on cost analysis or hospitalizations avoided. | 97% reported high/very high satisfaction with the UDR. | 6 months | Early scheduling and urgent specialized workup. |
Two studies included a cost comparison between a conventional inpatient evaluation and a QDU evaluation. Bosch et al.[6] and Capell et al.[7] found an average saving of $3304 (2514) and $2353 (1764) per patient, respectively. Bosch et al.[6] calculated these savings by comparing QDU patients to randomly selected control patients with similar referring complaints, who had reached their final diagnosis during a conventional inpatient evaluation. Capell et al.[7] compared their QDU patient costs to estimated in‐hospital costs for similar diagnoses.
Safety data were reported in detail only by Bosch et al.[6] who showed that 125 (3%) patients who initially were stable for QDU evaluation were referred to the emergency department. A total of 15 patients required admission and 12 died, with an overall mortality for the QDU cohort of 0.3%. Causes of death in this group included sudden unexplained death in 8 patients, pulmonary embolism in 2, aspiration pneumonia in 1, and shock of unknown origin in 1. Capell et al.[7] described a 7% admission rate, and Rubio‐Rivas et al.[8] noted that number to be 10%. No mortality was reported in these 2 studies.
In terms of preference for care, an overwhelming majority (88%) of patients in 1 study[6] preferred the QDU care model over hospitalization, and 95% to 97% of patients in 2 other studies[7, 10] reported very high satisfaction rates.
DISCUSSION
Our systematic review evaluated the effectiveness of QDUs for the diagnostic evaluation of patients with potentially severe disease and showed that such units, where established, are cost‐effective, prevent unnecessary hospitalizations, and diagnose potentially severe diseases, particularly malignant conditions, in a timely manner.
QDUs can evaluate medically stable patients with a variety of complaints such as anemia, lymphadenopathy, undiagnosed lumps and masses, and gastrointestinal symptoms and accelerate the diagnostic evaluation without requiring inpatient hospitalization. Many times patients are admitted to the hospital for a diagnostic evaluation without actual treatment.[12] These patients may not be sick enough to warrant hospitalization and may be able to return to the clinic for an outpatient workup. The QDU approach can complete the evaluation in such patients with the added advantages of saving money and higher patient satisfaction, due to diminished disruption of the patient's daily life.[1, 12] As most primary care physicians are unlikely to provide regular and frequent access for unscheduled care and EDs are more likely to admit patients for diagnostic workup,[2] a QDU approach seems a reasonable alternative for making a quick diagnosis and at the same time avoiding unnecessary hospitalization. Bosch et al. have also evaluated the impact of the QDUs in the diagnosis of specific diseases such as cancer in 169 patients diagnosed at the QDU, and compared them to 53 patients who were diagnosed with cancer during an inpatient evaluation.[11] They found that although QDU patients were significantly younger than hospitalized patients, there was no difference in diagnoses established and the time to diagnosis at the QDU and length of stay in the hospital.
There is a significant cost saving associated with QDUs. The cost savings calculated by Bosch et al. and Capell et al. were for each patient enrolled in this protocol from index encounter to final diagnosis.[6, 7] These 2 studies describe primarily fixed costs saved per patient treated in the QDU versus an inpatient admission. Fixed costs in hospital care include personnel cost, buildings, and equipment, whereas variable costs include medication, test reagents, and disposable supplies.[15] In comparison with the US healthcare system, fixed costs in Europe are considerably lower, and certain variable costs (like medications and procedures) are significantly higher in the United States.[16] This suggests a greater opportunity for healthcare savings for carefully selected patients in the United States, where costs related to inpatient admissions are significantly higher.[16]
Another limitation of our analysis is the paucity of studies on this topic. Many of the publications are from Bosch et al.,[1, 6, 11, 12, 13, 14] a single group in Spain, and these show considerable cost savings, patient satisfaction, and patient safety. However, most of their data are either retrospective or from nonrandomized, prospective cohort studies. The only report describing a similar approach in the United States was by Paschal in the city of New Orleans.[17] After Charity Hospital and the Veterans Affairs Hospital in New Orleans were lost to hurricane Katrina, an urgent care clinic was set up where potentially severe diseases such as cancer, leukemia, and autoimmune and endocrine disorders were diagnosed efficiently, although safety data were not reported.
The reported studies used different study designs and evaluated different primary outcomes. These limitations can be overcome with a well‐designed prospective trial, which could also evaluate the actual impact on patient care, safety, and healthcare savings in the United States.
Safety data were reported in detail only in 1 study,[6] and the rates of admissions were reported by 2 other studies.[7, 8] These suggest that QDUs may be safe for a selected group of patients. Patients evaluated in these units preferred this approach as shown by the overwhelming majority of the patients who chose QDU care over inpatient admissions when patient surveys were performed.
CONCLUSION
In this era of healthcare reform and emphasis on value‐based care, we must optimize the efficiency of our care delivery systems and challenge our preexisting resource‐intensive healthcare models. One source of potential savings is avoiding hospitalizations for purely diagnostic purposes, utilizing quick diagnostic units for patients who are able to return for outpatient evaluations. Such units are established, have been studied in Europe, and our systematic review shows that they are cost‐effective, time‐ and resource‐efficient, and preferred by patients. In our healthcare system, with the high cost of inpatient care, the QDU can yield large savings of healthcare dollars while expediting diagnostic workup, increasing patient satisfaction, and preventing lost productivity from hospital stays. Further exploration and study of alternative care delivery models, such as quick diagnostic units, is required to achieve the goal of cost‐effective high‐quality care for all.
Disclosure: Nothing to report.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
- , , , , . Quick diagnosis units: a potentially useful alternative to conventional hospitalization. Med J Aust. 2009;191:496–498.
- , . The growing role of emergency departments in hospital admissions. N Engl J Med. 2012;5:391–393.
- , , . Measuring appropriate use of acute beds. A Systematic review of methods and results. Health Policy. 2000;3:157–184.
- . Inappropriate admissions: thoughts of patients and referring doctors. J R Soc Med. 2001;12:628–631.
- , , . QED: quick and early diagnosis. Lancet. 1996;348:528–529.
- , , . Quick diagnosis units: avoiding referrals from primary care to the ED and hospitalizations. Am J Emerg Med. 2013;31(1):114–123.
- , , , et al. Quick and early diagnostic outpatient unit: an effective and efficient assistential model. Five years experience. Med Clin (Barc). 2004;123(7):247–250.
- , , , . Rapid diagnosis unit in a third level hospital. Descriptive study of the first year and a half. Rev Clin Esp. 2008;208(11):561–563.
- , , , et al. Usefulness of a lung cancer rapid diagnosis specialist clinic. Contribution of ultrasound bronchoscopy. Arch Bronconeumol. 2010;46(12):640–645.
- , , , . Rapid diagnosis units or immediate health care clinics in internal medicine. Analysis of the first six months of operation in Palencia (Spain). Semergen. 2012;38(2):126–130.
- , , , , . Comparison of quick diagnosis units and conventional hospitalization for the diagnosis of cancer in Spain: a descriptive cohort study. Oncology (Switzerland). 2012;83(5):283–291.
- , , , . Quick diagnosis units versus hospitalization for the diagnosis of potentially severe diseases in Spain. J Hosp Med. 2012;7(1):41–47.
- , , , , . Outpatient quick diagnosis units for the evaluation of suspected severe diseases: an observational study. Clinics. 2011;66(5):737–741.
- , , , et al. Quick diagnosis units or conventional hospitalization for the diagnostic evaluation of severe anemia: a paradigm shift in public health systems? Eur J Int Med. 2012;23(2):159–164.
- , , , et al. Distribution of variable vs fixed costs of hospital care. JAMA. 1999;281(7):644–649.
- . Explaining high healthcare spending in the united states: an international comparison of supply, utilization, prices and quality. Issue Brief (Commonw Fund). 2012;10:1–14.
- . Launching complex medical workups from an urgent care platform. Ann Int Med. 2012;156:232–233.
Vascular Catheter and Lumen Adequacy
Catheter‐related bloodstream infections (CRBSIs) are among the most common forms of hospital‐acquired infection and increase both length of stay and cost of hospitalization.[1, 2] Notable efforts are being devoted to reduce the rate of CRBSI, usually by implementing a bundle of measures.[3, 4, 5] One measure focuses on reducing to a minimum the exposure to vascular catheters.[3] In addition, various studies have shown that multilumen central venous catheters (CVCs) are associated with a higher risk of CRBSI than are single‐lumen catheters.[6, 7, 8, 9, 10] Accordingly, the Healthcare Infection Control Practices Advisory Committee (HICPAC) guideline recommends that clinicians use a CVC with the minimum number of ports or lumens essential for the management of the patient.[3]
Despite the fact that most CRBSIs occur in conventional wards,[1, 11] only a few studies have been focused on the potential magnitude of reducing the number of unnecessary vascular catheters and catheter lumens in the noncritical‐care setting.[12, 13, 14, 15, 16] The adequacy of vascular‐catheter use has been predominantly assessed for nontunneled CVCs.[14, 16, 17, 18] An institutional program aimed at reducing the overall rate of CRBSI should also include other sources, such as conventional peripheral venous catheters (PVCs), peripherally inserted central catheters (PICCs), and arterial catheters (ACs).[3] The need to extend surveillance to other types of catheters has been identified by the Infectious Diseases Society of America (IDSA) as an unresolved issue.[19]
We sought to investigate the rate and appropriateness of use of vascular catheters in the entire population of inpatients at a tertiary‐care center on a single day, as well as the adequacy of the number of catheter lumens harbored by each patient, by using a set of preestablished objective criteria.
METHODS
Setting and Study Population
We performed a 1‐day cross‐sectional study in March 2012 at the University Hospital 12 de Octubre in Madrid, Spain, a 1368‐bed tertiary‐care institution with a catchment area of 412,930 inhabitants in 2011 and 5 different adult intensive care units (ICUs; medical, trauma, coronary, general surgery, and cardiac surgery). In 2009, our center joined a national program aimed at implementing a catheter‐care bundle in adult ICUs with the intention of achieving zero incidence of CVC‐related bloodstream infections. This bundle consisted of a number of evidence‐based practices[4] (eg, avoiding the femoral site if possible and removing unnecessary CVCs).[20]
Study Design and Data Collection
All inpatient beds were reviewed, even if they were unoccupied on the day of the survey. The only exclusions were pediatric wards and the hospital facility for imprisoned patients. All inpatients with 1 vascular catheters in place on the day of survey were subsequently included. We analyzed ACs, PVCs, and the following types of CVCs: nontunneled (temporary) catheters, skin‐tunneled catheters (Hickman type), totally implantable catheters (Port‐A‐Cath), Swan‐Ganz thermodilution catheters, dialysis catheters (Shaldon type), and PICCs.
The following data were abstracted using a standardized sheet from each patient's medical and nursing records and by direct inspection: basic patient demographics; type of ward (medical/surgical [hereinafter, conventional wards] or ICU); type of vascular catheter; anatomic site of catheter insertion; medical or nursing team responsible for catheter placement; catheter insertion‐site dressing regimen; overall number of vascular catheters per patient; and overall number of venous or arterial catheter lumens per patient (resulting from adding up all the lumens present in each patient, including 3‐way stopcocks and noncoring needles in Port‐A‐Cath devices; each of the inflow ports in 3‐way stopcocks attached to a vascular catheter was counted as a separate lumen). Those patients who were not in their wards on the day of survey for any reason (eg, an ongoing surgical procedure) were excluded.
The current indication to maintain ongoing catheterization was recorded by means of the following variables: overall number of intravenous (IV) medications administered during the previous 24 hours; type of medication (antimicrobial therapy, fluid therapy, vasoactive and inotropic drugs, chemotherapy, blood products, or others [eg, analgesics or diuretics]); type of IV administration regimen; and other indications for catheter use (need for monitoring hemodynamic status, renal replacement therapy, or need for preemptive vascular access in patients expected to be at risk of hemodynamic deterioration potentially requiring fluid resuscitation or inotropic support over the next days [eg, septic shock, acute decompensation of heart failure, or gastrointestinal bleeding within the previous week]).[21] After thorough scrutiny of prescription orders, we assigned each medication to one of 3 different IV administration regimens: (1) rapid infusion (over <1 hour); (2) infusion over 1 to 24 hours; and (3) continuous infusion over a 24‐hour period. In doubtful cases, nursing staff was directly asked about the regimen of infusion.
No formal informed consent was obtained from the participants, as the present study was strictly observational and part of the institutional quality initiatives. The local Clinical Research Ethics Committee approved the study protocol.
Assessment Criteria
The adequacy of use of vascular catheters and catheter lumens was assessed by one of 4 researchers not associated with day‐to‐day patient care by using a set of a priori determined criteria. To determine appropriateness, a maximum theoretical number of vascular lumens was assigned to each specific indication for catheterization (Table 1). We considered that all IV medications administered by rapid infusion could be delivered consecutively through 1 single catheter lumen. Those medications administered by infusion over 1 to 24 hours, or by continuous infusion over a 24‐hour period, would require an exclusive lumen. The nature of the infusate, the potential incompatibility between infused drugs, and the method of infusion (gravity drip or pump) were not taken into account in this assignment process. Hemodynamic monitoring and renal replacement therapy also required an exclusive catheter lumen. When the vascular catheterization was retained only for preemptive reasons, we considered as justified the use of a maximum of 2 single‐lumen catheters.
| Indication | No. of Catheter Lumens Deemed Necessary |
|---|---|
| |
| Administration of IV medications | |
| Rapid infusion over <1 hour | 1 common lumen (for all medications) |
| Infusion over 124 hours | 1 exclusive lumen (for each medication) |
| Continuous infusion over a 24‐hour period | 1 exclusive lumen (for each medication) |
| Hemodynamic monitoring | 1 exclusive lumen |
| Renal replacement therapy | 1 exclusive lumen |
| Preemptive catheterization (ie, patients at risk of hemodynamic deterioration over the next days) | Maximum of 2 single‐lumen catheters |
Appropriateness of the Use of Vascular Catheters
The presence of a conventional PVC or a nontunneled (temporary) CVC was considered justifiable if 1 of its lumens was indicated according to the above‐mentioned criteria. We applied the principle that the requirements of catheter lumens should be met by keeping the number of catheters as low as possible. For instance, if a given patient had 2 catheters with an overall number of 3 lumens (ie, 1 single‐lumen catheter and 1 double‐lumen catheter), and only 2 catheter lumens were actually deemed necessary, we considered that the overall number of catheters was inappropriate. In view of their particular characteristics, the following types of catheters were by definition deemed to be appropriate: Swan‐Ganz catheters and ACs (as nearly exclusively used for hemodynamic monitoring in critically ill patients), dialysis catheters (as solely used for this specific purpose), and PICCs and nontemporary CVCs (as most of them had been placed prior to the current hospitalization episode for the periodic administration of chemotherapy or domiciliary parenteral nutrition). Because no IV medications are delivered through Swan‐Ganz catheters, ACs, and dialysis catheters, we did not take into account the presence of these devices when assessing the appropriateness of other vascular catheters present in a given patient.
Appropriateness of the Use of Catheter Lumens
First, we added up all the catheter lumens present in each patient (regardless of the type of device), and then we established the theoretical number of catheter lumens that the patient would have actually required, according to the above‐mentioned criteria. The difference between both figures gave the number of unnecessary catheter lumens. Only the potentially removable lumens were included in this analysis (those of PVCs and nontunneled CVCs, as well as each of the noncoring needles inserted in Port‐A‐Cath catheters). The lumens of skin‐tunneled CVCs, Swan‐Ganz catheters, and PICCs were considered nonremovable and, therefore, always justified. We excluded ACs from this specific analysis.
Statistical Analysis
Quantitative data were shown as the meanstandard deviation, whereas qualitative variables were expressed as absolute and relative frequencies with 95% confidence intervals (CIs). Categorical and continuous variables were compared using [2] and unpaired Student t tests, respectively. We calculated 3 different ratios: patients with 1 inappropriate catheter to overall number of inpatients; patients with 1 inappropriate catheter to patients with 1 vascular catheter in place on the day of survey; and overall number of unnecessary catheter lumens to overall number of catheter lumens. All the significance tests were 2‐tailed. Statistics were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
RESULTS
Out of 1134 reviewed inpatient beds, 834 (73.5%) were occupied on the day of the survey. The mean age of the included patients was 64.518.8 years, and 415 (49.8%) were male. Of these patients, 575 (68.9%) had 1 vascular catheter in place. The proportion of patients with a vascular catheter was significantly higher in ICUs compared with conventional wards (100% vs 66.7%, P<0.0001; Table 2). The overall numbers of vascular catheters and catheter lumens analyzed were 703 and 1448, respectively. Regarding the type of device, 567 (80.6%) were PVCs, 111 (15.8%) were CVCs (including 65 nontunneled CVCs, 16 dialysis catheters, 15 PICCs, 7 skin‐tunneled CVCs, 5 totally implantable CVCs, and 3 Swan‐Ganz catheters), and 25 (3.5%) were ACs. The distribution according to hospital ward and anatomic site of insertion is detailed in Table 2. The use of CVCs and ACs was higher in ICUs (42.0% and 28.4% of all catheters in place, respectively) compared with conventional wards (12.0% and 0.0%, P<0.0001). The use of the subclavian vein insertion site was more common in medical wards (65.7% of all CVCs, excluding PICCs) than in surgical wards or ICUs (26.9%, P=0.0002). Most of the catheters had been inserted by nursing staff members (75.2%), followed by anesthesia physicians (13.4%) and critical‐care medicine physicians (4.8%). An opaque gauze or transparent polyurethane insertion‐site dressing was present in 378 (53.8%) and 319 (45.3%) catheters, respectively, with no significant differences according to the type of device or hospital ward.
| No. of Patients | ||||
|---|---|---|---|---|
| Overall, N=834 | Medical Wards, n=498 | Surgical Wards, n=279 | ICUs, n=57 | |
| No. of Catheters | ||||
| Overall, N=703 | Medical Wards, n=391 | Surgical Wards, n=224 | ICUs, n=88 | |
| ||||
| No. of catheters in place, n (%) | 259 (31.1) | 168 (33.7) | 91 (32.6) | 0 (0.0) |
| 1 | 575 (68.9) | 330 (66.3) | 188 (67.4) | 57 (100.0)a |
| 1 | 477 (57.2) | 299 (60.0) | 158 (56.6) | 20 (35.1) |
| 2 | 72 (8.6) | 26 (5.2) | 24 (8.6) | 22 (38.6) |
| 3 | 22 (2.6) | 5 (1.0) | 6 (2.2) | 11 (19.3) |
| 4 | 4 (0.5) | 0 (0.0) | 0 (0.0) | 4 (7.0) |
| Type of catheter, n (%) | ||||
| PVC | 567 (80.6) | 345 (88.2) | 196 (87.5) | 26 (29.6)a |
| CVC | 111 (15.8) | 46 (11.8) | 28 (12.5) | 37 (42.0)a |
| AC | 25 (3.6) | 0 (0.0) | 0 (0.0) | 25 (28.4)a |
| Insertion site, n (%)b | ||||
| PVCs | ||||
| Hand and forearm | 425 (74.9) | 245 (71.0) | 156 (79.6) | 24 (92.3) |
| Antecubital fossa | 105 (18.5) | 73 (21.2) | 31 (15.8) | 1 (3.8) |
| Arm | 36 (6.3) | 26 (7.5) | 9 (4.6) | 1 (3.8) |
| Lower extremity | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| CVCs | ||||
| Arm (PICC) | 13 (11.7) | 11 (23.9) | 2 (7.1) | 0 (0.0) |
| Subclavian vein | 40 (36.0) | 23 (50.0) | 7 (25.0) | 10 (27.0) |
| IJ vein | 47 (42.3) | 9 (19.6) | 18 (64.3) | 20 (54.1) |
| Femoral vein | 11 (9.9) | 3 (6.5) | 1 (3.6) | 7 (18.9) |
| ACs | ||||
| Upper extremity | 19 (76.0) | 0 (0.0) | 0 (0.0) | 19 (76.0) |
| Lower extremity | 6 (24.0) | 0 (0.0) | 0 (0.0) | 6 (24.0) |
| No. of lumens per catheter, meanSD | ||||
| All nonarterial catheters | 2.060.82 | 1.880.57 | 1.980.56 | 2.981.42c |
| PVCs | 1.860.45 | 1.810.44 | 1.880.39 | 2.120.66c |
| CVCs | 3.091.39 | 2.450.99 | 2.680.98 | 4.021.40c |
After excluding ACs, we found an overall mean number of 2.060.82 lumens per catheter (1.860.45 per PVC and 3.091.39 per CVC), with significant differences between ICUs and conventional wards (P<0.0001; Table 2). There was a mean of 0.860.57 3‐way stopcocks per catheter. The mean number of concurrent IV medications per patient was 2.82.7 (ranging from 2.32.1 in those with a single catheter to 10.52.6 in those with 4 catheters). The most commonly administered medications were antimicrobials (46.6% of patients with a vascular catheter), fluid therapy (33.4%), chemotherapy (2.3%), and vasoactive and inotropic drugs (1.0%). According to the administration regimen, 455 (79.1%), 30 (5.2%), and 182 (31.6%) patients were receiving medications by rapid infusion, infusion over 124 hours, or continuous infusion over a 24‐hour period, respectively. In 57 patients (9.9%), the catheter was used only as preemptive vascular access. No apparent indication for the use of a vascular catheter was found in 63 patients (10.9%).
Based on our criteria, 126 out of 834 inpatients (15.1%, 95% CI: 12.817.7) had 1 inappropriate catheter, with significant differences between conventional wards and ICUs (13.2% vs 26.3%, P=0.014). This prevalence rate increases to 21.9% (95% CI: 18.725.5) when only patients with 1 vascular catheter in place were analyzed.
Focusing on the number of catheter lumens, 631 out of 1448 (43.6%, 95% CI: 41.046.1) were considered unnecessary. There was a nonsignificant trend toward a higher rate of unnecessary lumens in conventional wards compared with ICUs (44.8% vs 39.4%, P=0.086; Table 3). Because some centers have policies requiring all inpatients to harbor 1 PVC in place throughout the entire hospitalization period, we performed a first sensitivity analysis in which we assumed that having a single functional vascular lumen was appropriate in all cases, regardless of any other criteria. Under this assumption, only 248 lumens (17.1%, 95% CI: 15.319.2) could be regarded as unnecessary. We conducted a second sensitivity analysis by including in the rate denominator only those catheter lumens potentially removable (eg, PVCs, nontunneled CVCs, and noncoring needles inserted in Port‐A‐Cath catheters). By applying this method, 48.6% of lumens (631 out of 1298, 95% CI: 45.951.3) could be considered inappropriate.
| Rate | Overall | Medical Wards | Surgical Wards | ICUs |
|---|---|---|---|---|
| ||||
| Patients with 1 inappropriate catheter/overall no. of inpatients, n (%) | 126/834 (15.1) | 66/498 (13.2) | 45/279 (16.1) | 15/57 (26.3)a |
| Patients with 1 inappropriate catheter/patients with 1 vascular catheter, n (%) | 126/575 (21.9) | 66/330 (20.0) | 45/188 (23.9) | 15/57 (26.3) |
| No. of unnecessary vascular catheter lumens/overall no. of vascular catheter lumens, n (%) | 631/1448 (43.6) | 298/684 (43.6) | 207/444 (46.6) | 126/320 (39.4) b |
DISCUSSION
In this cross‐sectional survey, we found that 1 out of every 5 (20%) adult inpatients with a vascular catheter in place in our tertiary‐care center had an inappropriate number of catheters. This figure increased to 43.6% when the number of catheter lumens was analyzed (or 17.1% if we assumed that all patients should have at least 1 vascular access during their hospitalization period solely on the basis of preemptive reasons). Such rates of unnecessary catheter use throughout an entire institution offer an opportunity for improvement in clinical practice and, eventually, for reducing catheter‐related morbidity.
Other authors have also assessed the adequacy of CVC use in either ICU[16, 17] or non‐ICU settings.[14, 15, 16, 18, 21] A recent hospital‐wide survey found that 4.8% of catheter‐days were unnecessary, with a higher proportion in conventional wards than in the ICU,[16] mirroring the results from previous studies.[14] Another prospective study, limited to conventional wards, reported that almost half of the patients had 1 day with inappropriate vascular‐device use; age, total number of catheters used, and duration of catheterization were significantly associated with this event.[21] On the contrary, compliance with the criteria drawn up by the HICPAC and the Infusion Nurses Society for PICC use was found to be high overall in a medium‐sized community hospital.[22] Interestingly, we found a differential pattern in the adequacy of catheter use between hospital areas in function of the variable analyzed: number of inappropriate vascular catheters (higher rate in ICUs) or number of unnecessary vascular lumens (higher rate in conventional wards). Although our assessment criteria may partially account for such differences (ie, drug infusions for >1 hour justified the use of an exclusive catheter lumen), this finding raises the question of whether future interventions should be aimed at modifying specific catheter practices according to the type of hospital ward.
In contrast to the amount of literature on CVC, there is a scarcity of studies evaluating the appropriateness of PVC use in clinical practice. Lederle et al. reported that 17% of patients admitted in conventional wards of a university hospital had an idle PVC, with 20% of patient‐days of catheter exposure considered unnecessary.[12] The same authors subsequently demonstrated a significant decrease in these figures by implementing a multidisciplinary quality‐improvement intervention.[13] A previous cross‐sectional survey in our center revealed a PVC use rate as high as 46.2% among non‐critically ill adult inpatients.[23] Phlebitis is a common complication of PVC use, occurring in about 7% of inpatients and usually leading to catheter removal and replacement.[24] Although at a much lower incidence, peripheral catheterization also represents a non‐negligible source of CRBSI.[25] In our institution, in which a recently implemented specific bundle has resulted in a clear improvement in CVC care,[4, 20] about 60% of CRBSI occurring during the first 3 months of 2013 were due to PVCs (unpublished data). Therefore, this type of device should be routinely included in future surveys seeking to investigate the local epidemiology of catheter use at each institution. In that sense, it should be noted that a recent clinical trial showed no benefit of routine third‐day replacement vs clinically indicated replacement for phlebitis or CRBSI.[24]
Our study suggests that the daily review of the need for maintaining the vascular catheter should take into account the number of vascular lumens, as >40% of them were deemed unnecessary. To our knowledge, this area for potential intervention has not been addressed in previous surveys. Numerous studies have long demonstrated that the use of double‐lumen or triple‐lumen CVCs is associated with a higher rate of CRBSI than single‐lumen devices.[6, 7, 8] Even though a meta‐analysis concluded that this relationship diminishes when only high‐quality studies were included,[9] a more recent prospective study reported a hazard ratio for infection of 4.4 for each additional lumen.[10] However, it might be argued against our decision to count each inflow port in multiway stopcocks as a separate vascular lumen. Because the present survey was ultimately aimed at identifying opportunities to reduce the risk of CRBSI by decreasing catheter exposure, such an approach was chosen to properly capture and quantify every single potential source of infection in catheterized patients. We hypothesize that the use of 3‐lumen stopcocks could involve an increased number of manipulations, thus jeopardizing the integrity of the insertion‐site dressing and subsequently favoring the intraluminal bacterial colonization of the common catheter. Although the current guidelines do not provide specific recommendations regarding the number of lumens in devices other than CVCs,[3, 19] the potential benefit of reducing this figure to the minimum in PVCs and ACs should also be assumed. In our opinion, specific efforts have to be focused on improving the use of 3‐way stopcocks, as we found a mean of 1.86 lumens per PVC and >3 lumens per CVC in our study. Maybe the need for 3‐way stopcocks should be reassessed on a daily basis in a similar way as that recommended for temporary CVCs.[3, 19] By eliminating unnecessary vascular lumens, the risk of CRBSI could be diminished without compromising the availability of vascular access for preemptive purposes.
The present surveillance also provides an accurate insight into the real‐life vascular catheter practices in a hospital‐wide setting, in contrast with most of the previous studies, which have been conducted in specific wards or units.[13, 15, 17] One relevant finding was the relatively low use of the subclavian vein site for central venous access (only 40.8% of all CVCs inserted), with significant differences between medical wards and the remaining hospital areas. Various studies have shown that the subclavian site is associated with a lower risk of infectious and thrombotic complications.[4, 26, 27, 28] Therefore, the HICPAC and IDSA guidelines strongly recommend using a subclavian site, rather than a jugular or a femoral site, to minimize infection risk for nontunneled CVCs.[3, 19] Nevertheless, recent studies have suggested that internal jugular and femoral sites could be acceptable when a subclavian approach is not feasible, particularly if chlorhexidine‐impregnated dressings are used and catheters are left in place for <4 days.[29]
The current study has a number of inherent limitations; the most significant is its cross‐sectional design, which precludes direct comparison of the rates of catheter use with other prospective cohort surveys.[14, 16, 21] In addition, we were not able to assess the changing dynamics of catheter use over time. In other words, the lack of use of a given device on the day of the survey does not necessarily imply inappropriateness. The criteria used to determine appropriateness of vascular catheterization were consensus opinion and not evidence‐based, a weakness shared by previous studies,[21] as current guidelines only address the indications for certain devices (ie, HICPAC and Infusion Nurses Society recommendations for PICC use).[3, 30] Although we have attempted to be liberal in accepting indications for catheter use (ie, preemptive access in patients deemed at risk of hemodynamic instability), some misclassification bias cannot be ruled out. In evaluating the adequacy of catheter lumens, we did not take into account the simultaneous delivery of incompatible infusateswhich must be infused through separate linesor other relevant variables (eg, nursing availability). Because the aim of our study was simply to determine whether a patient had an appropriate number of vascular catheters and vascular lumens in overall terms, all vascular lumens in each subject were individually counted and added regardless of the nature of the device, and therefore we were not able to disaggregate the adequacy rate by specific catheter types. Finally, the generalizability of the results may be hampered by their single‐center nature, and this limitation applies particularly to institutions with different policies than ours regarding preemptive vascular catheterization (ie, those requiring that all inpatients have at least 1 vascular lumen at any time during hospitalization).
On the other hand, some strengths of this survey merit consideration, namely its comprehensive design, capturing the entire population of adult inpatients in different hospital areas and every type of vascular catheter. Moreover, we addressed the adequacy of maintaining catheterization not only in terms of idle catheters in place, but also in terms of unnecessary lumens. In conclusion, there remains room for improvement in daily practice regarding the prompt removal of vascular catheters and vascular lumens that are no longer medically necessary. Further educational efforts among physicians and nursing staff should be targeted toward achieving this simple but effective measure to reduce the incidence of CRBSI.
Disclosures: M.F.R. holds a research‐training contract Ro Hortega from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. CM11/00187). F.L.M. is partially supported by a grant from the Research Intensification Programme in the National Health Care System (I3SNS) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. INT11/174). All authors report no conflicts of interest relevant to this article. This study was partially presented at the 52nd Annual Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC), September 912, 2012, San Francisco, California.
- , , , ; VINCat Program. Laboratory‐based surveillance of hospital‐acquired catheter‐related bloodstream infections in Catalonia: results of the VINCat Program (2007–2010). Enferm Infecc Microbiol Clin. 2012;30(suppl 3):13–19.
- , ; ICU‐Bacteremia Study Group. Outcomes of primary and catheter‐related bacteremia: a cohort and case‐control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584–1590.
- , , , et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52:e162–e193.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU [published correction appears in N Engl J Med. 2007;356:2660]. N Engl J Med. 2006;355:2725–2732.
- , , , et al; Prevention Epicenter Program. A multicenter intervention to prevent catheter‐associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27:662–669.
- , , . Infection rate for single‐lumen v triple‐lumen subclavian catheters. Infect Control Hosp Epidemiol. 1988;9:154–158.
- , , , , . Increased infection rate in double‐lumen versus single‐lumen Hickman catheters in cancer patients. South Med J. 1990;83:34–36.
- , , , , . Use of triple‐lumen subclavian catheters for administration of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1992;16:403–407.
- , , , , . Rates of infection for single‐lumen versus multilumen central venous catheters: a meta‐analysis. Crit Care Med. 2003;31:2385–2390.
- , , , et al. Multilumen central venous catheters increase risk for catheter‐related bloodstream infection: prospective surveillance study. Infection. 2008;36:322–327.
- , , . Preventing catheter‐related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin Infect Dis. 2010;51:335–341.
- , , , . The idle intravenous catheter. Ann Intern Med. 1992;116:737–738.
- , , , . Reduction of unnecessary intravenous catheter use: internal medicine house staff participate in a successful quality improvement project. Arch Intern Med. 1994;154:1829–1832.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25:266–268.
- , , , , , . Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control. 2006;34:636–641.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77:304–308.
- , , . Evaluation of unnecessary central venous catheters in critically ill patients: a prospective observational study. Can J Anaesth. 2010;57:830–835.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33:50–57.
- , , , et al. Strategies to prevent central line‐associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S22–S30.
- Spanish Ministry of Health, Social Services and Equality. Quality Agency of the National Health Service. Bacteriemia Zero Project. Available at: http://www.seguridaddelpaciente.es/index.php/lang‐en/projects/financiacion‐estudios/bacteriemia‐zero‐project.html. Accessed June 4, 2013.
- , , , , . Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78:128–132.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36:291–296.
- , , , et al. Use and abuse of intravenous catheters in conventional hospital wards [article in Spanish]. An Med Interna. 2006;23:475–477.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380:1066–1074.
- , , , et al. Clinical epidemiology and outcomes of peripheral venous catheter–related bloodstream infections at a university‐affiliated hospital. J Hosp Infect. 2007;67:22–29.
- , , , . Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19:842–845.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–707.
- , . Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long‐term intravenous therapy. Cochrane Database Syst Rev. 2007;(3):CD004084.
- , , , et al. Jugular vs. femoral short‐term catheterization and risk of infection in ICU patients: causal analysis of 2 randomized trials. Am J Respir Crit Care Med. 2013;188:1232–1239.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1 suppl):S38.
Catheter‐related bloodstream infections (CRBSIs) are among the most common forms of hospital‐acquired infection and increase both length of stay and cost of hospitalization.[1, 2] Notable efforts are being devoted to reduce the rate of CRBSI, usually by implementing a bundle of measures.[3, 4, 5] One measure focuses on reducing to a minimum the exposure to vascular catheters.[3] In addition, various studies have shown that multilumen central venous catheters (CVCs) are associated with a higher risk of CRBSI than are single‐lumen catheters.[6, 7, 8, 9, 10] Accordingly, the Healthcare Infection Control Practices Advisory Committee (HICPAC) guideline recommends that clinicians use a CVC with the minimum number of ports or lumens essential for the management of the patient.[3]
Despite the fact that most CRBSIs occur in conventional wards,[1, 11] only a few studies have been focused on the potential magnitude of reducing the number of unnecessary vascular catheters and catheter lumens in the noncritical‐care setting.[12, 13, 14, 15, 16] The adequacy of vascular‐catheter use has been predominantly assessed for nontunneled CVCs.[14, 16, 17, 18] An institutional program aimed at reducing the overall rate of CRBSI should also include other sources, such as conventional peripheral venous catheters (PVCs), peripherally inserted central catheters (PICCs), and arterial catheters (ACs).[3] The need to extend surveillance to other types of catheters has been identified by the Infectious Diseases Society of America (IDSA) as an unresolved issue.[19]
We sought to investigate the rate and appropriateness of use of vascular catheters in the entire population of inpatients at a tertiary‐care center on a single day, as well as the adequacy of the number of catheter lumens harbored by each patient, by using a set of preestablished objective criteria.
METHODS
Setting and Study Population
We performed a 1‐day cross‐sectional study in March 2012 at the University Hospital 12 de Octubre in Madrid, Spain, a 1368‐bed tertiary‐care institution with a catchment area of 412,930 inhabitants in 2011 and 5 different adult intensive care units (ICUs; medical, trauma, coronary, general surgery, and cardiac surgery). In 2009, our center joined a national program aimed at implementing a catheter‐care bundle in adult ICUs with the intention of achieving zero incidence of CVC‐related bloodstream infections. This bundle consisted of a number of evidence‐based practices[4] (eg, avoiding the femoral site if possible and removing unnecessary CVCs).[20]
Study Design and Data Collection
All inpatient beds were reviewed, even if they were unoccupied on the day of the survey. The only exclusions were pediatric wards and the hospital facility for imprisoned patients. All inpatients with 1 vascular catheters in place on the day of survey were subsequently included. We analyzed ACs, PVCs, and the following types of CVCs: nontunneled (temporary) catheters, skin‐tunneled catheters (Hickman type), totally implantable catheters (Port‐A‐Cath), Swan‐Ganz thermodilution catheters, dialysis catheters (Shaldon type), and PICCs.
The following data were abstracted using a standardized sheet from each patient's medical and nursing records and by direct inspection: basic patient demographics; type of ward (medical/surgical [hereinafter, conventional wards] or ICU); type of vascular catheter; anatomic site of catheter insertion; medical or nursing team responsible for catheter placement; catheter insertion‐site dressing regimen; overall number of vascular catheters per patient; and overall number of venous or arterial catheter lumens per patient (resulting from adding up all the lumens present in each patient, including 3‐way stopcocks and noncoring needles in Port‐A‐Cath devices; each of the inflow ports in 3‐way stopcocks attached to a vascular catheter was counted as a separate lumen). Those patients who were not in their wards on the day of survey for any reason (eg, an ongoing surgical procedure) were excluded.
The current indication to maintain ongoing catheterization was recorded by means of the following variables: overall number of intravenous (IV) medications administered during the previous 24 hours; type of medication (antimicrobial therapy, fluid therapy, vasoactive and inotropic drugs, chemotherapy, blood products, or others [eg, analgesics or diuretics]); type of IV administration regimen; and other indications for catheter use (need for monitoring hemodynamic status, renal replacement therapy, or need for preemptive vascular access in patients expected to be at risk of hemodynamic deterioration potentially requiring fluid resuscitation or inotropic support over the next days [eg, septic shock, acute decompensation of heart failure, or gastrointestinal bleeding within the previous week]).[21] After thorough scrutiny of prescription orders, we assigned each medication to one of 3 different IV administration regimens: (1) rapid infusion (over <1 hour); (2) infusion over 1 to 24 hours; and (3) continuous infusion over a 24‐hour period. In doubtful cases, nursing staff was directly asked about the regimen of infusion.
No formal informed consent was obtained from the participants, as the present study was strictly observational and part of the institutional quality initiatives. The local Clinical Research Ethics Committee approved the study protocol.
Assessment Criteria
The adequacy of use of vascular catheters and catheter lumens was assessed by one of 4 researchers not associated with day‐to‐day patient care by using a set of a priori determined criteria. To determine appropriateness, a maximum theoretical number of vascular lumens was assigned to each specific indication for catheterization (Table 1). We considered that all IV medications administered by rapid infusion could be delivered consecutively through 1 single catheter lumen. Those medications administered by infusion over 1 to 24 hours, or by continuous infusion over a 24‐hour period, would require an exclusive lumen. The nature of the infusate, the potential incompatibility between infused drugs, and the method of infusion (gravity drip or pump) were not taken into account in this assignment process. Hemodynamic monitoring and renal replacement therapy also required an exclusive catheter lumen. When the vascular catheterization was retained only for preemptive reasons, we considered as justified the use of a maximum of 2 single‐lumen catheters.
| Indication | No. of Catheter Lumens Deemed Necessary |
|---|---|
| |
| Administration of IV medications | |
| Rapid infusion over <1 hour | 1 common lumen (for all medications) |
| Infusion over 124 hours | 1 exclusive lumen (for each medication) |
| Continuous infusion over a 24‐hour period | 1 exclusive lumen (for each medication) |
| Hemodynamic monitoring | 1 exclusive lumen |
| Renal replacement therapy | 1 exclusive lumen |
| Preemptive catheterization (ie, patients at risk of hemodynamic deterioration over the next days) | Maximum of 2 single‐lumen catheters |
Appropriateness of the Use of Vascular Catheters
The presence of a conventional PVC or a nontunneled (temporary) CVC was considered justifiable if 1 of its lumens was indicated according to the above‐mentioned criteria. We applied the principle that the requirements of catheter lumens should be met by keeping the number of catheters as low as possible. For instance, if a given patient had 2 catheters with an overall number of 3 lumens (ie, 1 single‐lumen catheter and 1 double‐lumen catheter), and only 2 catheter lumens were actually deemed necessary, we considered that the overall number of catheters was inappropriate. In view of their particular characteristics, the following types of catheters were by definition deemed to be appropriate: Swan‐Ganz catheters and ACs (as nearly exclusively used for hemodynamic monitoring in critically ill patients), dialysis catheters (as solely used for this specific purpose), and PICCs and nontemporary CVCs (as most of them had been placed prior to the current hospitalization episode for the periodic administration of chemotherapy or domiciliary parenteral nutrition). Because no IV medications are delivered through Swan‐Ganz catheters, ACs, and dialysis catheters, we did not take into account the presence of these devices when assessing the appropriateness of other vascular catheters present in a given patient.
Appropriateness of the Use of Catheter Lumens
First, we added up all the catheter lumens present in each patient (regardless of the type of device), and then we established the theoretical number of catheter lumens that the patient would have actually required, according to the above‐mentioned criteria. The difference between both figures gave the number of unnecessary catheter lumens. Only the potentially removable lumens were included in this analysis (those of PVCs and nontunneled CVCs, as well as each of the noncoring needles inserted in Port‐A‐Cath catheters). The lumens of skin‐tunneled CVCs, Swan‐Ganz catheters, and PICCs were considered nonremovable and, therefore, always justified. We excluded ACs from this specific analysis.
Statistical Analysis
Quantitative data were shown as the meanstandard deviation, whereas qualitative variables were expressed as absolute and relative frequencies with 95% confidence intervals (CIs). Categorical and continuous variables were compared using [2] and unpaired Student t tests, respectively. We calculated 3 different ratios: patients with 1 inappropriate catheter to overall number of inpatients; patients with 1 inappropriate catheter to patients with 1 vascular catheter in place on the day of survey; and overall number of unnecessary catheter lumens to overall number of catheter lumens. All the significance tests were 2‐tailed. Statistics were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
RESULTS
Out of 1134 reviewed inpatient beds, 834 (73.5%) were occupied on the day of the survey. The mean age of the included patients was 64.518.8 years, and 415 (49.8%) were male. Of these patients, 575 (68.9%) had 1 vascular catheter in place. The proportion of patients with a vascular catheter was significantly higher in ICUs compared with conventional wards (100% vs 66.7%, P<0.0001; Table 2). The overall numbers of vascular catheters and catheter lumens analyzed were 703 and 1448, respectively. Regarding the type of device, 567 (80.6%) were PVCs, 111 (15.8%) were CVCs (including 65 nontunneled CVCs, 16 dialysis catheters, 15 PICCs, 7 skin‐tunneled CVCs, 5 totally implantable CVCs, and 3 Swan‐Ganz catheters), and 25 (3.5%) were ACs. The distribution according to hospital ward and anatomic site of insertion is detailed in Table 2. The use of CVCs and ACs was higher in ICUs (42.0% and 28.4% of all catheters in place, respectively) compared with conventional wards (12.0% and 0.0%, P<0.0001). The use of the subclavian vein insertion site was more common in medical wards (65.7% of all CVCs, excluding PICCs) than in surgical wards or ICUs (26.9%, P=0.0002). Most of the catheters had been inserted by nursing staff members (75.2%), followed by anesthesia physicians (13.4%) and critical‐care medicine physicians (4.8%). An opaque gauze or transparent polyurethane insertion‐site dressing was present in 378 (53.8%) and 319 (45.3%) catheters, respectively, with no significant differences according to the type of device or hospital ward.
| No. of Patients | ||||
|---|---|---|---|---|
| Overall, N=834 | Medical Wards, n=498 | Surgical Wards, n=279 | ICUs, n=57 | |
| No. of Catheters | ||||
| Overall, N=703 | Medical Wards, n=391 | Surgical Wards, n=224 | ICUs, n=88 | |
| ||||
| No. of catheters in place, n (%) | 259 (31.1) | 168 (33.7) | 91 (32.6) | 0 (0.0) |
| 1 | 575 (68.9) | 330 (66.3) | 188 (67.4) | 57 (100.0)a |
| 1 | 477 (57.2) | 299 (60.0) | 158 (56.6) | 20 (35.1) |
| 2 | 72 (8.6) | 26 (5.2) | 24 (8.6) | 22 (38.6) |
| 3 | 22 (2.6) | 5 (1.0) | 6 (2.2) | 11 (19.3) |
| 4 | 4 (0.5) | 0 (0.0) | 0 (0.0) | 4 (7.0) |
| Type of catheter, n (%) | ||||
| PVC | 567 (80.6) | 345 (88.2) | 196 (87.5) | 26 (29.6)a |
| CVC | 111 (15.8) | 46 (11.8) | 28 (12.5) | 37 (42.0)a |
| AC | 25 (3.6) | 0 (0.0) | 0 (0.0) | 25 (28.4)a |
| Insertion site, n (%)b | ||||
| PVCs | ||||
| Hand and forearm | 425 (74.9) | 245 (71.0) | 156 (79.6) | 24 (92.3) |
| Antecubital fossa | 105 (18.5) | 73 (21.2) | 31 (15.8) | 1 (3.8) |
| Arm | 36 (6.3) | 26 (7.5) | 9 (4.6) | 1 (3.8) |
| Lower extremity | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| CVCs | ||||
| Arm (PICC) | 13 (11.7) | 11 (23.9) | 2 (7.1) | 0 (0.0) |
| Subclavian vein | 40 (36.0) | 23 (50.0) | 7 (25.0) | 10 (27.0) |
| IJ vein | 47 (42.3) | 9 (19.6) | 18 (64.3) | 20 (54.1) |
| Femoral vein | 11 (9.9) | 3 (6.5) | 1 (3.6) | 7 (18.9) |
| ACs | ||||
| Upper extremity | 19 (76.0) | 0 (0.0) | 0 (0.0) | 19 (76.0) |
| Lower extremity | 6 (24.0) | 0 (0.0) | 0 (0.0) | 6 (24.0) |
| No. of lumens per catheter, meanSD | ||||
| All nonarterial catheters | 2.060.82 | 1.880.57 | 1.980.56 | 2.981.42c |
| PVCs | 1.860.45 | 1.810.44 | 1.880.39 | 2.120.66c |
| CVCs | 3.091.39 | 2.450.99 | 2.680.98 | 4.021.40c |
After excluding ACs, we found an overall mean number of 2.060.82 lumens per catheter (1.860.45 per PVC and 3.091.39 per CVC), with significant differences between ICUs and conventional wards (P<0.0001; Table 2). There was a mean of 0.860.57 3‐way stopcocks per catheter. The mean number of concurrent IV medications per patient was 2.82.7 (ranging from 2.32.1 in those with a single catheter to 10.52.6 in those with 4 catheters). The most commonly administered medications were antimicrobials (46.6% of patients with a vascular catheter), fluid therapy (33.4%), chemotherapy (2.3%), and vasoactive and inotropic drugs (1.0%). According to the administration regimen, 455 (79.1%), 30 (5.2%), and 182 (31.6%) patients were receiving medications by rapid infusion, infusion over 124 hours, or continuous infusion over a 24‐hour period, respectively. In 57 patients (9.9%), the catheter was used only as preemptive vascular access. No apparent indication for the use of a vascular catheter was found in 63 patients (10.9%).
Based on our criteria, 126 out of 834 inpatients (15.1%, 95% CI: 12.817.7) had 1 inappropriate catheter, with significant differences between conventional wards and ICUs (13.2% vs 26.3%, P=0.014). This prevalence rate increases to 21.9% (95% CI: 18.725.5) when only patients with 1 vascular catheter in place were analyzed.
Focusing on the number of catheter lumens, 631 out of 1448 (43.6%, 95% CI: 41.046.1) were considered unnecessary. There was a nonsignificant trend toward a higher rate of unnecessary lumens in conventional wards compared with ICUs (44.8% vs 39.4%, P=0.086; Table 3). Because some centers have policies requiring all inpatients to harbor 1 PVC in place throughout the entire hospitalization period, we performed a first sensitivity analysis in which we assumed that having a single functional vascular lumen was appropriate in all cases, regardless of any other criteria. Under this assumption, only 248 lumens (17.1%, 95% CI: 15.319.2) could be regarded as unnecessary. We conducted a second sensitivity analysis by including in the rate denominator only those catheter lumens potentially removable (eg, PVCs, nontunneled CVCs, and noncoring needles inserted in Port‐A‐Cath catheters). By applying this method, 48.6% of lumens (631 out of 1298, 95% CI: 45.951.3) could be considered inappropriate.
| Rate | Overall | Medical Wards | Surgical Wards | ICUs |
|---|---|---|---|---|
| ||||
| Patients with 1 inappropriate catheter/overall no. of inpatients, n (%) | 126/834 (15.1) | 66/498 (13.2) | 45/279 (16.1) | 15/57 (26.3)a |
| Patients with 1 inappropriate catheter/patients with 1 vascular catheter, n (%) | 126/575 (21.9) | 66/330 (20.0) | 45/188 (23.9) | 15/57 (26.3) |
| No. of unnecessary vascular catheter lumens/overall no. of vascular catheter lumens, n (%) | 631/1448 (43.6) | 298/684 (43.6) | 207/444 (46.6) | 126/320 (39.4) b |
DISCUSSION
In this cross‐sectional survey, we found that 1 out of every 5 (20%) adult inpatients with a vascular catheter in place in our tertiary‐care center had an inappropriate number of catheters. This figure increased to 43.6% when the number of catheter lumens was analyzed (or 17.1% if we assumed that all patients should have at least 1 vascular access during their hospitalization period solely on the basis of preemptive reasons). Such rates of unnecessary catheter use throughout an entire institution offer an opportunity for improvement in clinical practice and, eventually, for reducing catheter‐related morbidity.
Other authors have also assessed the adequacy of CVC use in either ICU[16, 17] or non‐ICU settings.[14, 15, 16, 18, 21] A recent hospital‐wide survey found that 4.8% of catheter‐days were unnecessary, with a higher proportion in conventional wards than in the ICU,[16] mirroring the results from previous studies.[14] Another prospective study, limited to conventional wards, reported that almost half of the patients had 1 day with inappropriate vascular‐device use; age, total number of catheters used, and duration of catheterization were significantly associated with this event.[21] On the contrary, compliance with the criteria drawn up by the HICPAC and the Infusion Nurses Society for PICC use was found to be high overall in a medium‐sized community hospital.[22] Interestingly, we found a differential pattern in the adequacy of catheter use between hospital areas in function of the variable analyzed: number of inappropriate vascular catheters (higher rate in ICUs) or number of unnecessary vascular lumens (higher rate in conventional wards). Although our assessment criteria may partially account for such differences (ie, drug infusions for >1 hour justified the use of an exclusive catheter lumen), this finding raises the question of whether future interventions should be aimed at modifying specific catheter practices according to the type of hospital ward.
In contrast to the amount of literature on CVC, there is a scarcity of studies evaluating the appropriateness of PVC use in clinical practice. Lederle et al. reported that 17% of patients admitted in conventional wards of a university hospital had an idle PVC, with 20% of patient‐days of catheter exposure considered unnecessary.[12] The same authors subsequently demonstrated a significant decrease in these figures by implementing a multidisciplinary quality‐improvement intervention.[13] A previous cross‐sectional survey in our center revealed a PVC use rate as high as 46.2% among non‐critically ill adult inpatients.[23] Phlebitis is a common complication of PVC use, occurring in about 7% of inpatients and usually leading to catheter removal and replacement.[24] Although at a much lower incidence, peripheral catheterization also represents a non‐negligible source of CRBSI.[25] In our institution, in which a recently implemented specific bundle has resulted in a clear improvement in CVC care,[4, 20] about 60% of CRBSI occurring during the first 3 months of 2013 were due to PVCs (unpublished data). Therefore, this type of device should be routinely included in future surveys seeking to investigate the local epidemiology of catheter use at each institution. In that sense, it should be noted that a recent clinical trial showed no benefit of routine third‐day replacement vs clinically indicated replacement for phlebitis or CRBSI.[24]
Our study suggests that the daily review of the need for maintaining the vascular catheter should take into account the number of vascular lumens, as >40% of them were deemed unnecessary. To our knowledge, this area for potential intervention has not been addressed in previous surveys. Numerous studies have long demonstrated that the use of double‐lumen or triple‐lumen CVCs is associated with a higher rate of CRBSI than single‐lumen devices.[6, 7, 8] Even though a meta‐analysis concluded that this relationship diminishes when only high‐quality studies were included,[9] a more recent prospective study reported a hazard ratio for infection of 4.4 for each additional lumen.[10] However, it might be argued against our decision to count each inflow port in multiway stopcocks as a separate vascular lumen. Because the present survey was ultimately aimed at identifying opportunities to reduce the risk of CRBSI by decreasing catheter exposure, such an approach was chosen to properly capture and quantify every single potential source of infection in catheterized patients. We hypothesize that the use of 3‐lumen stopcocks could involve an increased number of manipulations, thus jeopardizing the integrity of the insertion‐site dressing and subsequently favoring the intraluminal bacterial colonization of the common catheter. Although the current guidelines do not provide specific recommendations regarding the number of lumens in devices other than CVCs,[3, 19] the potential benefit of reducing this figure to the minimum in PVCs and ACs should also be assumed. In our opinion, specific efforts have to be focused on improving the use of 3‐way stopcocks, as we found a mean of 1.86 lumens per PVC and >3 lumens per CVC in our study. Maybe the need for 3‐way stopcocks should be reassessed on a daily basis in a similar way as that recommended for temporary CVCs.[3, 19] By eliminating unnecessary vascular lumens, the risk of CRBSI could be diminished without compromising the availability of vascular access for preemptive purposes.
The present surveillance also provides an accurate insight into the real‐life vascular catheter practices in a hospital‐wide setting, in contrast with most of the previous studies, which have been conducted in specific wards or units.[13, 15, 17] One relevant finding was the relatively low use of the subclavian vein site for central venous access (only 40.8% of all CVCs inserted), with significant differences between medical wards and the remaining hospital areas. Various studies have shown that the subclavian site is associated with a lower risk of infectious and thrombotic complications.[4, 26, 27, 28] Therefore, the HICPAC and IDSA guidelines strongly recommend using a subclavian site, rather than a jugular or a femoral site, to minimize infection risk for nontunneled CVCs.[3, 19] Nevertheless, recent studies have suggested that internal jugular and femoral sites could be acceptable when a subclavian approach is not feasible, particularly if chlorhexidine‐impregnated dressings are used and catheters are left in place for <4 days.[29]
The current study has a number of inherent limitations; the most significant is its cross‐sectional design, which precludes direct comparison of the rates of catheter use with other prospective cohort surveys.[14, 16, 21] In addition, we were not able to assess the changing dynamics of catheter use over time. In other words, the lack of use of a given device on the day of the survey does not necessarily imply inappropriateness. The criteria used to determine appropriateness of vascular catheterization were consensus opinion and not evidence‐based, a weakness shared by previous studies,[21] as current guidelines only address the indications for certain devices (ie, HICPAC and Infusion Nurses Society recommendations for PICC use).[3, 30] Although we have attempted to be liberal in accepting indications for catheter use (ie, preemptive access in patients deemed at risk of hemodynamic instability), some misclassification bias cannot be ruled out. In evaluating the adequacy of catheter lumens, we did not take into account the simultaneous delivery of incompatible infusateswhich must be infused through separate linesor other relevant variables (eg, nursing availability). Because the aim of our study was simply to determine whether a patient had an appropriate number of vascular catheters and vascular lumens in overall terms, all vascular lumens in each subject were individually counted and added regardless of the nature of the device, and therefore we were not able to disaggregate the adequacy rate by specific catheter types. Finally, the generalizability of the results may be hampered by their single‐center nature, and this limitation applies particularly to institutions with different policies than ours regarding preemptive vascular catheterization (ie, those requiring that all inpatients have at least 1 vascular lumen at any time during hospitalization).
On the other hand, some strengths of this survey merit consideration, namely its comprehensive design, capturing the entire population of adult inpatients in different hospital areas and every type of vascular catheter. Moreover, we addressed the adequacy of maintaining catheterization not only in terms of idle catheters in place, but also in terms of unnecessary lumens. In conclusion, there remains room for improvement in daily practice regarding the prompt removal of vascular catheters and vascular lumens that are no longer medically necessary. Further educational efforts among physicians and nursing staff should be targeted toward achieving this simple but effective measure to reduce the incidence of CRBSI.
Disclosures: M.F.R. holds a research‐training contract Ro Hortega from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. CM11/00187). F.L.M. is partially supported by a grant from the Research Intensification Programme in the National Health Care System (I3SNS) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. INT11/174). All authors report no conflicts of interest relevant to this article. This study was partially presented at the 52nd Annual Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC), September 912, 2012, San Francisco, California.
Catheter‐related bloodstream infections (CRBSIs) are among the most common forms of hospital‐acquired infection and increase both length of stay and cost of hospitalization.[1, 2] Notable efforts are being devoted to reduce the rate of CRBSI, usually by implementing a bundle of measures.[3, 4, 5] One measure focuses on reducing to a minimum the exposure to vascular catheters.[3] In addition, various studies have shown that multilumen central venous catheters (CVCs) are associated with a higher risk of CRBSI than are single‐lumen catheters.[6, 7, 8, 9, 10] Accordingly, the Healthcare Infection Control Practices Advisory Committee (HICPAC) guideline recommends that clinicians use a CVC with the minimum number of ports or lumens essential for the management of the patient.[3]
Despite the fact that most CRBSIs occur in conventional wards,[1, 11] only a few studies have been focused on the potential magnitude of reducing the number of unnecessary vascular catheters and catheter lumens in the noncritical‐care setting.[12, 13, 14, 15, 16] The adequacy of vascular‐catheter use has been predominantly assessed for nontunneled CVCs.[14, 16, 17, 18] An institutional program aimed at reducing the overall rate of CRBSI should also include other sources, such as conventional peripheral venous catheters (PVCs), peripherally inserted central catheters (PICCs), and arterial catheters (ACs).[3] The need to extend surveillance to other types of catheters has been identified by the Infectious Diseases Society of America (IDSA) as an unresolved issue.[19]
We sought to investigate the rate and appropriateness of use of vascular catheters in the entire population of inpatients at a tertiary‐care center on a single day, as well as the adequacy of the number of catheter lumens harbored by each patient, by using a set of preestablished objective criteria.
METHODS
Setting and Study Population
We performed a 1‐day cross‐sectional study in March 2012 at the University Hospital 12 de Octubre in Madrid, Spain, a 1368‐bed tertiary‐care institution with a catchment area of 412,930 inhabitants in 2011 and 5 different adult intensive care units (ICUs; medical, trauma, coronary, general surgery, and cardiac surgery). In 2009, our center joined a national program aimed at implementing a catheter‐care bundle in adult ICUs with the intention of achieving zero incidence of CVC‐related bloodstream infections. This bundle consisted of a number of evidence‐based practices[4] (eg, avoiding the femoral site if possible and removing unnecessary CVCs).[20]
Study Design and Data Collection
All inpatient beds were reviewed, even if they were unoccupied on the day of the survey. The only exclusions were pediatric wards and the hospital facility for imprisoned patients. All inpatients with 1 vascular catheters in place on the day of survey were subsequently included. We analyzed ACs, PVCs, and the following types of CVCs: nontunneled (temporary) catheters, skin‐tunneled catheters (Hickman type), totally implantable catheters (Port‐A‐Cath), Swan‐Ganz thermodilution catheters, dialysis catheters (Shaldon type), and PICCs.
The following data were abstracted using a standardized sheet from each patient's medical and nursing records and by direct inspection: basic patient demographics; type of ward (medical/surgical [hereinafter, conventional wards] or ICU); type of vascular catheter; anatomic site of catheter insertion; medical or nursing team responsible for catheter placement; catheter insertion‐site dressing regimen; overall number of vascular catheters per patient; and overall number of venous or arterial catheter lumens per patient (resulting from adding up all the lumens present in each patient, including 3‐way stopcocks and noncoring needles in Port‐A‐Cath devices; each of the inflow ports in 3‐way stopcocks attached to a vascular catheter was counted as a separate lumen). Those patients who were not in their wards on the day of survey for any reason (eg, an ongoing surgical procedure) were excluded.
The current indication to maintain ongoing catheterization was recorded by means of the following variables: overall number of intravenous (IV) medications administered during the previous 24 hours; type of medication (antimicrobial therapy, fluid therapy, vasoactive and inotropic drugs, chemotherapy, blood products, or others [eg, analgesics or diuretics]); type of IV administration regimen; and other indications for catheter use (need for monitoring hemodynamic status, renal replacement therapy, or need for preemptive vascular access in patients expected to be at risk of hemodynamic deterioration potentially requiring fluid resuscitation or inotropic support over the next days [eg, septic shock, acute decompensation of heart failure, or gastrointestinal bleeding within the previous week]).[21] After thorough scrutiny of prescription orders, we assigned each medication to one of 3 different IV administration regimens: (1) rapid infusion (over <1 hour); (2) infusion over 1 to 24 hours; and (3) continuous infusion over a 24‐hour period. In doubtful cases, nursing staff was directly asked about the regimen of infusion.
No formal informed consent was obtained from the participants, as the present study was strictly observational and part of the institutional quality initiatives. The local Clinical Research Ethics Committee approved the study protocol.
Assessment Criteria
The adequacy of use of vascular catheters and catheter lumens was assessed by one of 4 researchers not associated with day‐to‐day patient care by using a set of a priori determined criteria. To determine appropriateness, a maximum theoretical number of vascular lumens was assigned to each specific indication for catheterization (Table 1). We considered that all IV medications administered by rapid infusion could be delivered consecutively through 1 single catheter lumen. Those medications administered by infusion over 1 to 24 hours, or by continuous infusion over a 24‐hour period, would require an exclusive lumen. The nature of the infusate, the potential incompatibility between infused drugs, and the method of infusion (gravity drip or pump) were not taken into account in this assignment process. Hemodynamic monitoring and renal replacement therapy also required an exclusive catheter lumen. When the vascular catheterization was retained only for preemptive reasons, we considered as justified the use of a maximum of 2 single‐lumen catheters.
| Indication | No. of Catheter Lumens Deemed Necessary |
|---|---|
| |
| Administration of IV medications | |
| Rapid infusion over <1 hour | 1 common lumen (for all medications) |
| Infusion over 124 hours | 1 exclusive lumen (for each medication) |
| Continuous infusion over a 24‐hour period | 1 exclusive lumen (for each medication) |
| Hemodynamic monitoring | 1 exclusive lumen |
| Renal replacement therapy | 1 exclusive lumen |
| Preemptive catheterization (ie, patients at risk of hemodynamic deterioration over the next days) | Maximum of 2 single‐lumen catheters |
Appropriateness of the Use of Vascular Catheters
The presence of a conventional PVC or a nontunneled (temporary) CVC was considered justifiable if 1 of its lumens was indicated according to the above‐mentioned criteria. We applied the principle that the requirements of catheter lumens should be met by keeping the number of catheters as low as possible. For instance, if a given patient had 2 catheters with an overall number of 3 lumens (ie, 1 single‐lumen catheter and 1 double‐lumen catheter), and only 2 catheter lumens were actually deemed necessary, we considered that the overall number of catheters was inappropriate. In view of their particular characteristics, the following types of catheters were by definition deemed to be appropriate: Swan‐Ganz catheters and ACs (as nearly exclusively used for hemodynamic monitoring in critically ill patients), dialysis catheters (as solely used for this specific purpose), and PICCs and nontemporary CVCs (as most of them had been placed prior to the current hospitalization episode for the periodic administration of chemotherapy or domiciliary parenteral nutrition). Because no IV medications are delivered through Swan‐Ganz catheters, ACs, and dialysis catheters, we did not take into account the presence of these devices when assessing the appropriateness of other vascular catheters present in a given patient.
Appropriateness of the Use of Catheter Lumens
First, we added up all the catheter lumens present in each patient (regardless of the type of device), and then we established the theoretical number of catheter lumens that the patient would have actually required, according to the above‐mentioned criteria. The difference between both figures gave the number of unnecessary catheter lumens. Only the potentially removable lumens were included in this analysis (those of PVCs and nontunneled CVCs, as well as each of the noncoring needles inserted in Port‐A‐Cath catheters). The lumens of skin‐tunneled CVCs, Swan‐Ganz catheters, and PICCs were considered nonremovable and, therefore, always justified. We excluded ACs from this specific analysis.
Statistical Analysis
Quantitative data were shown as the meanstandard deviation, whereas qualitative variables were expressed as absolute and relative frequencies with 95% confidence intervals (CIs). Categorical and continuous variables were compared using [2] and unpaired Student t tests, respectively. We calculated 3 different ratios: patients with 1 inappropriate catheter to overall number of inpatients; patients with 1 inappropriate catheter to patients with 1 vascular catheter in place on the day of survey; and overall number of unnecessary catheter lumens to overall number of catheter lumens. All the significance tests were 2‐tailed. Statistics were performed using SPSS version 15.0 (SPSS Inc, Chicago, IL).
RESULTS
Out of 1134 reviewed inpatient beds, 834 (73.5%) were occupied on the day of the survey. The mean age of the included patients was 64.518.8 years, and 415 (49.8%) were male. Of these patients, 575 (68.9%) had 1 vascular catheter in place. The proportion of patients with a vascular catheter was significantly higher in ICUs compared with conventional wards (100% vs 66.7%, P<0.0001; Table 2). The overall numbers of vascular catheters and catheter lumens analyzed were 703 and 1448, respectively. Regarding the type of device, 567 (80.6%) were PVCs, 111 (15.8%) were CVCs (including 65 nontunneled CVCs, 16 dialysis catheters, 15 PICCs, 7 skin‐tunneled CVCs, 5 totally implantable CVCs, and 3 Swan‐Ganz catheters), and 25 (3.5%) were ACs. The distribution according to hospital ward and anatomic site of insertion is detailed in Table 2. The use of CVCs and ACs was higher in ICUs (42.0% and 28.4% of all catheters in place, respectively) compared with conventional wards (12.0% and 0.0%, P<0.0001). The use of the subclavian vein insertion site was more common in medical wards (65.7% of all CVCs, excluding PICCs) than in surgical wards or ICUs (26.9%, P=0.0002). Most of the catheters had been inserted by nursing staff members (75.2%), followed by anesthesia physicians (13.4%) and critical‐care medicine physicians (4.8%). An opaque gauze or transparent polyurethane insertion‐site dressing was present in 378 (53.8%) and 319 (45.3%) catheters, respectively, with no significant differences according to the type of device or hospital ward.
| No. of Patients | ||||
|---|---|---|---|---|
| Overall, N=834 | Medical Wards, n=498 | Surgical Wards, n=279 | ICUs, n=57 | |
| No. of Catheters | ||||
| Overall, N=703 | Medical Wards, n=391 | Surgical Wards, n=224 | ICUs, n=88 | |
| ||||
| No. of catheters in place, n (%) | 259 (31.1) | 168 (33.7) | 91 (32.6) | 0 (0.0) |
| 1 | 575 (68.9) | 330 (66.3) | 188 (67.4) | 57 (100.0)a |
| 1 | 477 (57.2) | 299 (60.0) | 158 (56.6) | 20 (35.1) |
| 2 | 72 (8.6) | 26 (5.2) | 24 (8.6) | 22 (38.6) |
| 3 | 22 (2.6) | 5 (1.0) | 6 (2.2) | 11 (19.3) |
| 4 | 4 (0.5) | 0 (0.0) | 0 (0.0) | 4 (7.0) |
| Type of catheter, n (%) | ||||
| PVC | 567 (80.6) | 345 (88.2) | 196 (87.5) | 26 (29.6)a |
| CVC | 111 (15.8) | 46 (11.8) | 28 (12.5) | 37 (42.0)a |
| AC | 25 (3.6) | 0 (0.0) | 0 (0.0) | 25 (28.4)a |
| Insertion site, n (%)b | ||||
| PVCs | ||||
| Hand and forearm | 425 (74.9) | 245 (71.0) | 156 (79.6) | 24 (92.3) |
| Antecubital fossa | 105 (18.5) | 73 (21.2) | 31 (15.8) | 1 (3.8) |
| Arm | 36 (6.3) | 26 (7.5) | 9 (4.6) | 1 (3.8) |
| Lower extremity | 1 (0.2) | 1 (0.3) | 0 (0.0) | 0 (0.0) |
| CVCs | ||||
| Arm (PICC) | 13 (11.7) | 11 (23.9) | 2 (7.1) | 0 (0.0) |
| Subclavian vein | 40 (36.0) | 23 (50.0) | 7 (25.0) | 10 (27.0) |
| IJ vein | 47 (42.3) | 9 (19.6) | 18 (64.3) | 20 (54.1) |
| Femoral vein | 11 (9.9) | 3 (6.5) | 1 (3.6) | 7 (18.9) |
| ACs | ||||
| Upper extremity | 19 (76.0) | 0 (0.0) | 0 (0.0) | 19 (76.0) |
| Lower extremity | 6 (24.0) | 0 (0.0) | 0 (0.0) | 6 (24.0) |
| No. of lumens per catheter, meanSD | ||||
| All nonarterial catheters | 2.060.82 | 1.880.57 | 1.980.56 | 2.981.42c |
| PVCs | 1.860.45 | 1.810.44 | 1.880.39 | 2.120.66c |
| CVCs | 3.091.39 | 2.450.99 | 2.680.98 | 4.021.40c |
After excluding ACs, we found an overall mean number of 2.060.82 lumens per catheter (1.860.45 per PVC and 3.091.39 per CVC), with significant differences between ICUs and conventional wards (P<0.0001; Table 2). There was a mean of 0.860.57 3‐way stopcocks per catheter. The mean number of concurrent IV medications per patient was 2.82.7 (ranging from 2.32.1 in those with a single catheter to 10.52.6 in those with 4 catheters). The most commonly administered medications were antimicrobials (46.6% of patients with a vascular catheter), fluid therapy (33.4%), chemotherapy (2.3%), and vasoactive and inotropic drugs (1.0%). According to the administration regimen, 455 (79.1%), 30 (5.2%), and 182 (31.6%) patients were receiving medications by rapid infusion, infusion over 124 hours, or continuous infusion over a 24‐hour period, respectively. In 57 patients (9.9%), the catheter was used only as preemptive vascular access. No apparent indication for the use of a vascular catheter was found in 63 patients (10.9%).
Based on our criteria, 126 out of 834 inpatients (15.1%, 95% CI: 12.817.7) had 1 inappropriate catheter, with significant differences between conventional wards and ICUs (13.2% vs 26.3%, P=0.014). This prevalence rate increases to 21.9% (95% CI: 18.725.5) when only patients with 1 vascular catheter in place were analyzed.
Focusing on the number of catheter lumens, 631 out of 1448 (43.6%, 95% CI: 41.046.1) were considered unnecessary. There was a nonsignificant trend toward a higher rate of unnecessary lumens in conventional wards compared with ICUs (44.8% vs 39.4%, P=0.086; Table 3). Because some centers have policies requiring all inpatients to harbor 1 PVC in place throughout the entire hospitalization period, we performed a first sensitivity analysis in which we assumed that having a single functional vascular lumen was appropriate in all cases, regardless of any other criteria. Under this assumption, only 248 lumens (17.1%, 95% CI: 15.319.2) could be regarded as unnecessary. We conducted a second sensitivity analysis by including in the rate denominator only those catheter lumens potentially removable (eg, PVCs, nontunneled CVCs, and noncoring needles inserted in Port‐A‐Cath catheters). By applying this method, 48.6% of lumens (631 out of 1298, 95% CI: 45.951.3) could be considered inappropriate.
| Rate | Overall | Medical Wards | Surgical Wards | ICUs |
|---|---|---|---|---|
| ||||
| Patients with 1 inappropriate catheter/overall no. of inpatients, n (%) | 126/834 (15.1) | 66/498 (13.2) | 45/279 (16.1) | 15/57 (26.3)a |
| Patients with 1 inappropriate catheter/patients with 1 vascular catheter, n (%) | 126/575 (21.9) | 66/330 (20.0) | 45/188 (23.9) | 15/57 (26.3) |
| No. of unnecessary vascular catheter lumens/overall no. of vascular catheter lumens, n (%) | 631/1448 (43.6) | 298/684 (43.6) | 207/444 (46.6) | 126/320 (39.4) b |
DISCUSSION
In this cross‐sectional survey, we found that 1 out of every 5 (20%) adult inpatients with a vascular catheter in place in our tertiary‐care center had an inappropriate number of catheters. This figure increased to 43.6% when the number of catheter lumens was analyzed (or 17.1% if we assumed that all patients should have at least 1 vascular access during their hospitalization period solely on the basis of preemptive reasons). Such rates of unnecessary catheter use throughout an entire institution offer an opportunity for improvement in clinical practice and, eventually, for reducing catheter‐related morbidity.
Other authors have also assessed the adequacy of CVC use in either ICU[16, 17] or non‐ICU settings.[14, 15, 16, 18, 21] A recent hospital‐wide survey found that 4.8% of catheter‐days were unnecessary, with a higher proportion in conventional wards than in the ICU,[16] mirroring the results from previous studies.[14] Another prospective study, limited to conventional wards, reported that almost half of the patients had 1 day with inappropriate vascular‐device use; age, total number of catheters used, and duration of catheterization were significantly associated with this event.[21] On the contrary, compliance with the criteria drawn up by the HICPAC and the Infusion Nurses Society for PICC use was found to be high overall in a medium‐sized community hospital.[22] Interestingly, we found a differential pattern in the adequacy of catheter use between hospital areas in function of the variable analyzed: number of inappropriate vascular catheters (higher rate in ICUs) or number of unnecessary vascular lumens (higher rate in conventional wards). Although our assessment criteria may partially account for such differences (ie, drug infusions for >1 hour justified the use of an exclusive catheter lumen), this finding raises the question of whether future interventions should be aimed at modifying specific catheter practices according to the type of hospital ward.
In contrast to the amount of literature on CVC, there is a scarcity of studies evaluating the appropriateness of PVC use in clinical practice. Lederle et al. reported that 17% of patients admitted in conventional wards of a university hospital had an idle PVC, with 20% of patient‐days of catheter exposure considered unnecessary.[12] The same authors subsequently demonstrated a significant decrease in these figures by implementing a multidisciplinary quality‐improvement intervention.[13] A previous cross‐sectional survey in our center revealed a PVC use rate as high as 46.2% among non‐critically ill adult inpatients.[23] Phlebitis is a common complication of PVC use, occurring in about 7% of inpatients and usually leading to catheter removal and replacement.[24] Although at a much lower incidence, peripheral catheterization also represents a non‐negligible source of CRBSI.[25] In our institution, in which a recently implemented specific bundle has resulted in a clear improvement in CVC care,[4, 20] about 60% of CRBSI occurring during the first 3 months of 2013 were due to PVCs (unpublished data). Therefore, this type of device should be routinely included in future surveys seeking to investigate the local epidemiology of catheter use at each institution. In that sense, it should be noted that a recent clinical trial showed no benefit of routine third‐day replacement vs clinically indicated replacement for phlebitis or CRBSI.[24]
Our study suggests that the daily review of the need for maintaining the vascular catheter should take into account the number of vascular lumens, as >40% of them were deemed unnecessary. To our knowledge, this area for potential intervention has not been addressed in previous surveys. Numerous studies have long demonstrated that the use of double‐lumen or triple‐lumen CVCs is associated with a higher rate of CRBSI than single‐lumen devices.[6, 7, 8] Even though a meta‐analysis concluded that this relationship diminishes when only high‐quality studies were included,[9] a more recent prospective study reported a hazard ratio for infection of 4.4 for each additional lumen.[10] However, it might be argued against our decision to count each inflow port in multiway stopcocks as a separate vascular lumen. Because the present survey was ultimately aimed at identifying opportunities to reduce the risk of CRBSI by decreasing catheter exposure, such an approach was chosen to properly capture and quantify every single potential source of infection in catheterized patients. We hypothesize that the use of 3‐lumen stopcocks could involve an increased number of manipulations, thus jeopardizing the integrity of the insertion‐site dressing and subsequently favoring the intraluminal bacterial colonization of the common catheter. Although the current guidelines do not provide specific recommendations regarding the number of lumens in devices other than CVCs,[3, 19] the potential benefit of reducing this figure to the minimum in PVCs and ACs should also be assumed. In our opinion, specific efforts have to be focused on improving the use of 3‐way stopcocks, as we found a mean of 1.86 lumens per PVC and >3 lumens per CVC in our study. Maybe the need for 3‐way stopcocks should be reassessed on a daily basis in a similar way as that recommended for temporary CVCs.[3, 19] By eliminating unnecessary vascular lumens, the risk of CRBSI could be diminished without compromising the availability of vascular access for preemptive purposes.
The present surveillance also provides an accurate insight into the real‐life vascular catheter practices in a hospital‐wide setting, in contrast with most of the previous studies, which have been conducted in specific wards or units.[13, 15, 17] One relevant finding was the relatively low use of the subclavian vein site for central venous access (only 40.8% of all CVCs inserted), with significant differences between medical wards and the remaining hospital areas. Various studies have shown that the subclavian site is associated with a lower risk of infectious and thrombotic complications.[4, 26, 27, 28] Therefore, the HICPAC and IDSA guidelines strongly recommend using a subclavian site, rather than a jugular or a femoral site, to minimize infection risk for nontunneled CVCs.[3, 19] Nevertheless, recent studies have suggested that internal jugular and femoral sites could be acceptable when a subclavian approach is not feasible, particularly if chlorhexidine‐impregnated dressings are used and catheters are left in place for <4 days.[29]
The current study has a number of inherent limitations; the most significant is its cross‐sectional design, which precludes direct comparison of the rates of catheter use with other prospective cohort surveys.[14, 16, 21] In addition, we were not able to assess the changing dynamics of catheter use over time. In other words, the lack of use of a given device on the day of the survey does not necessarily imply inappropriateness. The criteria used to determine appropriateness of vascular catheterization were consensus opinion and not evidence‐based, a weakness shared by previous studies,[21] as current guidelines only address the indications for certain devices (ie, HICPAC and Infusion Nurses Society recommendations for PICC use).[3, 30] Although we have attempted to be liberal in accepting indications for catheter use (ie, preemptive access in patients deemed at risk of hemodynamic instability), some misclassification bias cannot be ruled out. In evaluating the adequacy of catheter lumens, we did not take into account the simultaneous delivery of incompatible infusateswhich must be infused through separate linesor other relevant variables (eg, nursing availability). Because the aim of our study was simply to determine whether a patient had an appropriate number of vascular catheters and vascular lumens in overall terms, all vascular lumens in each subject were individually counted and added regardless of the nature of the device, and therefore we were not able to disaggregate the adequacy rate by specific catheter types. Finally, the generalizability of the results may be hampered by their single‐center nature, and this limitation applies particularly to institutions with different policies than ours regarding preemptive vascular catheterization (ie, those requiring that all inpatients have at least 1 vascular lumen at any time during hospitalization).
On the other hand, some strengths of this survey merit consideration, namely its comprehensive design, capturing the entire population of adult inpatients in different hospital areas and every type of vascular catheter. Moreover, we addressed the adequacy of maintaining catheterization not only in terms of idle catheters in place, but also in terms of unnecessary lumens. In conclusion, there remains room for improvement in daily practice regarding the prompt removal of vascular catheters and vascular lumens that are no longer medically necessary. Further educational efforts among physicians and nursing staff should be targeted toward achieving this simple but effective measure to reduce the incidence of CRBSI.
Disclosures: M.F.R. holds a research‐training contract Ro Hortega from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. CM11/00187). F.L.M. is partially supported by a grant from the Research Intensification Programme in the National Health Care System (I3SNS) from the Spanish Ministry of Economy and Competitiveness (Instituto de Salud Carlos III; grant no. INT11/174). All authors report no conflicts of interest relevant to this article. This study was partially presented at the 52nd Annual Interscience Congress on Antimicrobial Agents and Chemotherapy (ICAAC), September 912, 2012, San Francisco, California.
- , , , ; VINCat Program. Laboratory‐based surveillance of hospital‐acquired catheter‐related bloodstream infections in Catalonia: results of the VINCat Program (2007–2010). Enferm Infecc Microbiol Clin. 2012;30(suppl 3):13–19.
- , ; ICU‐Bacteremia Study Group. Outcomes of primary and catheter‐related bacteremia: a cohort and case‐control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584–1590.
- , , , et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52:e162–e193.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU [published correction appears in N Engl J Med. 2007;356:2660]. N Engl J Med. 2006;355:2725–2732.
- , , , et al; Prevention Epicenter Program. A multicenter intervention to prevent catheter‐associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27:662–669.
- , , . Infection rate for single‐lumen v triple‐lumen subclavian catheters. Infect Control Hosp Epidemiol. 1988;9:154–158.
- , , , , . Increased infection rate in double‐lumen versus single‐lumen Hickman catheters in cancer patients. South Med J. 1990;83:34–36.
- , , , , . Use of triple‐lumen subclavian catheters for administration of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1992;16:403–407.
- , , , , . Rates of infection for single‐lumen versus multilumen central venous catheters: a meta‐analysis. Crit Care Med. 2003;31:2385–2390.
- , , , et al. Multilumen central venous catheters increase risk for catheter‐related bloodstream infection: prospective surveillance study. Infection. 2008;36:322–327.
- , , . Preventing catheter‐related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin Infect Dis. 2010;51:335–341.
- , , , . The idle intravenous catheter. Ann Intern Med. 1992;116:737–738.
- , , , . Reduction of unnecessary intravenous catheter use: internal medicine house staff participate in a successful quality improvement project. Arch Intern Med. 1994;154:1829–1832.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25:266–268.
- , , , , , . Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control. 2006;34:636–641.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77:304–308.
- , , . Evaluation of unnecessary central venous catheters in critically ill patients: a prospective observational study. Can J Anaesth. 2010;57:830–835.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33:50–57.
- , , , et al. Strategies to prevent central line‐associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S22–S30.
- Spanish Ministry of Health, Social Services and Equality. Quality Agency of the National Health Service. Bacteriemia Zero Project. Available at: http://www.seguridaddelpaciente.es/index.php/lang‐en/projects/financiacion‐estudios/bacteriemia‐zero‐project.html. Accessed June 4, 2013.
- , , , , . Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78:128–132.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36:291–296.
- , , , et al. Use and abuse of intravenous catheters in conventional hospital wards [article in Spanish]. An Med Interna. 2006;23:475–477.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380:1066–1074.
- , , , et al. Clinical epidemiology and outcomes of peripheral venous catheter–related bloodstream infections at a university‐affiliated hospital. J Hosp Infect. 2007;67:22–29.
- , , , . Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19:842–845.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–707.
- , . Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long‐term intravenous therapy. Cochrane Database Syst Rev. 2007;(3):CD004084.
- , , , et al. Jugular vs. femoral short‐term catheterization and risk of infection in ICU patients: causal analysis of 2 randomized trials. Am J Respir Crit Care Med. 2013;188:1232–1239.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1 suppl):S38.
- , , , ; VINCat Program. Laboratory‐based surveillance of hospital‐acquired catheter‐related bloodstream infections in Catalonia: results of the VINCat Program (2007–2010). Enferm Infecc Microbiol Clin. 2012;30(suppl 3):13–19.
- , ; ICU‐Bacteremia Study Group. Outcomes of primary and catheter‐related bacteremia: a cohort and case‐control study in critically ill patients. Am J Respir Crit Care Med. 2001;163:1584–1590.
- , , , et al; Healthcare Infection Control Practices Advisory Committee. Guidelines for the prevention of intravascular catheter‐related infections. Clin Infect Dis. 2011;52:e162–e193.
- , , , et al. An intervention to decrease catheter‐related bloodstream infections in the ICU [published correction appears in N Engl J Med. 2007;356:2660]. N Engl J Med. 2006;355:2725–2732.
- , , , et al; Prevention Epicenter Program. A multicenter intervention to prevent catheter‐associated bloodstream infections. Infect Control Hosp Epidemiol. 2006;27:662–669.
- , , . Infection rate for single‐lumen v triple‐lumen subclavian catheters. Infect Control Hosp Epidemiol. 1988;9:154–158.
- , , , , . Increased infection rate in double‐lumen versus single‐lumen Hickman catheters in cancer patients. South Med J. 1990;83:34–36.
- , , , , . Use of triple‐lumen subclavian catheters for administration of total parenteral nutrition. JPEN J Parenter Enteral Nutr. 1992;16:403–407.
- , , , , . Rates of infection for single‐lumen versus multilumen central venous catheters: a meta‐analysis. Crit Care Med. 2003;31:2385–2390.
- , , , et al. Multilumen central venous catheters increase risk for catheter‐related bloodstream infection: prospective surveillance study. Infection. 2008;36:322–327.
- , , . Preventing catheter‐related bloodstream infections outside the intensive care unit: expanding prevention to new settings. Clin Infect Dis. 2010;51:335–341.
- , , , . The idle intravenous catheter. Ann Intern Med. 1992;116:737–738.
- , , , . Reduction of unnecessary intravenous catheter use: internal medicine house staff participate in a successful quality improvement project. Arch Intern Med. 1994;154:1829–1832.
- , , , , , . Unnecessary use of central venous catheters: the need to look outside the intensive care unit. Infect Control Hosp Epidemiol. 2004;25:266–268.
- , , , , , . Prospective cohort study of central venous catheters among internal medicine ward patients. Am J Infect Control. 2006;34:636–641.
- , , , et al. Hospital‐wide survey of the use of central venous catheters. J Hosp Infect. 2011;77:304–308.
- , , . Evaluation of unnecessary central venous catheters in critically ill patients: a prospective observational study. Can J Anaesth. 2010;57:830–835.
- , , , et al. Temporary central venous catheter utilization patterns in a large tertiary care center: tracking the “idle central venous catheter.” Infect Control Hosp Epidemiol. 2012;33:50–57.
- , , , et al. Strategies to prevent central line‐associated bloodstream infections in acute care hospitals. Infect Control Hosp Epidemiol. 2008;29(suppl 1):S22–S30.
- Spanish Ministry of Health, Social Services and Equality. Quality Agency of the National Health Service. Bacteriemia Zero Project. Available at: http://www.seguridaddelpaciente.es/index.php/lang‐en/projects/financiacion‐estudios/bacteriemia‐zero‐project.html. Accessed June 4, 2013.
- , , , , . Inappropriate intravascular device use: a prospective study. J Hosp Infect. 2011;78:128–132.
- , . Peripherally inserted central catheter: compliance with evidence‐based indications for insertion in an inpatient setting. J Infus Nurs. 2013;36:291–296.
- , , , et al. Use and abuse of intravenous catheters in conventional hospital wards [article in Spanish]. An Med Interna. 2006;23:475–477.
- , , , et al. Routine versus clinically indicated replacement of peripheral intravenous catheters: a randomised controlled equivalence trial. Lancet. 2012;380:1066–1074.
- , , , et al. Clinical epidemiology and outcomes of peripheral venous catheter–related bloodstream infections at a university‐affiliated hospital. J Hosp Infect. 2007;67:22–29.
- , , , . Risk of infection due to central venous catheters: effect of site of placement and catheter type. Infect Control Hosp Epidemiol. 1998;19:842–845.
- , , , et al. Complications of femoral and subclavian venous catheterization in critically ill patients: a randomized controlled trial. JAMA. 2001;286:700–707.
- , . Central venous access sites for the prevention of venous thrombosis, stenosis and infection in patients requiring long‐term intravenous therapy. Cochrane Database Syst Rev. 2007;(3):CD004084.
- , , , et al. Jugular vs. femoral short‐term catheterization and risk of infection in ICU patients: causal analysis of 2 randomized trials. Am J Respir Crit Care Med. 2013;188:1232–1239.
- Infusion Nurses Society. Infusion nursing standards of practice. J Infus Nurs. 2011;34(1 suppl):S38.
© 2013 Society of Hospital Medicine