User login
Native tissue is superior to vaginal mesh for prolapse repair, two studies report
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P <.0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P <.0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
Have you read recent articles in OBG Management about the surgical use of mesh?
Click here to access the list.
- Michele Jonsson Funk, PhD, and colleagues from University of North Carolina (UNC) at Chapel Hill concluded that using vaginal mesh versus native tissue for anterior prolapse repair is associated with 5-year increased risk of any repeat surgery, especially surgery for mesh removal.1
- Shunaha Kim-Fine, MD, and colleagues from Mayo Clinic, Rochester, Minnesota, believe that traditional native tissue repair is the best procedure for most women undergoing vaginal POP repair.2
UNC study details
Investigators from the Gillings School of Global Public Health at UNC studied health-care claims from 2005 to 2010. They identified women who, after undergoing anterior wall prolapse repair, experienced repeat surgery for recurrent prolapse or mesh removal. Of the initial 27,809 anterior prolapse surgeries, 6,871 (24.7%) included the use of vaginal mesh.1
5-year risk of repeat surgery. The authors determined that1:
- the 5-year cumulative risk of any repeat surgery was significantly higher with the use of vaginal mesh than with the use of native tissue (15.2% vs 9.8%, respectively; P <.0001 with a risk of mesh revision or removal>
- the 5-year risk for recurrent prolapse surgery between both groups was comparable (10.4% vs 9.3%, P = .70).
Mayo Clinic study details
Researchers from Female Pelvic Medicine and Reconstructive Surgery, Division of Gynecologic Surgery at Mayo Clinic reviewed the literature and compared vaginal native tissue repair with vaginal mesh–augmented repair of pelvic organ prolapse. Their report was published online ahead of print on January 17, 2013, in Current Bladder Dysfunction Reports.
The authors discuss POP; the procedures available to treat symptomatic POP; the Public Heath Notifications issued in 2008 and 2011 from U.S. Food and Drug Administration (FDA) regarding the use of transvaginal mesh in POP repair; and success, failure, and complication rates from both techniques.2
“Given the lack of robust and long-term data in these relatively new procedures for [mesh-augmentation] repair, we agree with the caution and prudence communication in the recent FDA warning,” state the authors.2 However, a caveat is offered that native tissue repair must utilize best principles of surgical technique and incorporate a multicompartment repair to achieve optimal outcome. The authors strongly advise that appropriate surgical technique, obtained only through adequate surgical training, can be improved for both repair procedures.2
Risks and complications from mesh. Mesh introduces unique risks related to the mesh itself, including mesh erosion, and complications, including new onset pain and dyspareunia following mesh-augmented repair. Complications are possibly related to the intrinsic properties of the mesh, (ie, shrinkage); to the patient (ie, scarring); or to the operative technique (ie, the placement/location of the mesh and increased tension on the mesh). The authors conclude that additional studies are needed, given the lack of robust and long-term data on mesh-augmentation repair of POP.2
“The evidence thus far has not shown that the benefits of mesh outweigh the added risks in vaginal prolapse repairs,” write the authors.2 Therefore, although patient-centered success rates for both techniques of POP repair are equivalent, the authors conclude: “there does not appear to be a clear advantage of mesh augmentation repair over native tissue in terms of anatomic success.”2
To access the Jonsson Funk abstract, click here.
To access the Kim-Fine abstract, click here.
We want to hear from you! Tell us what you think.
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
1. Jonsson Funk M, Visco AG, Weidner AC, Pate V, Wu JM. Long-term outcomes of vaginal mesh versus native tissue repair for anterior vaginal wall prolapse [published online ahead of print February 12, 2013]. Int Urogynecol J. doi:10.1007/s00192-013-2043-9.
2. Kim-Fine S, Occhino JA, Gebhart JB. Vaginal prolapse repair—Native tissue repair versus mesh augmentation: Newer isn’t always better [published online ahead of print January 17, 2013]. Curr Bladder Dysfunct Rep. 2013;8(1):25-31doi:10.1007/s11884-012-0170-7.
More NEWS FOR YOUR PRACTICE…
appropriate for?
Help patients control their asthma
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
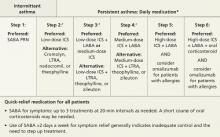
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
• Classify and treat asthma based on the patient’s worst symptom, whether or not it is the symptom that occurs most frequently. C
• Treat patients with poorly controlled asthma aggressively to gain quick control, then scale back slowly to the fewest medications and lowest doses needed to maintain control. A
• Reserve long-acting beta-agonists for use as an adjunct to inhaled corticosteroids for adults with poor baseline pulmonary function tests. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
CASE Angela D, a 34-year-old patient, has asthma with recurrent exacerbations. She uses a low-dose inhaled corticosteroid (ICS) daily and an albuterol inhaler, as needed, for shortness of breath or wheezing. She also has allergic rhinitis, for which she uses nasal fluticasone. Yet despite this regimen, Ms. D reports she still experiences wheezing, chest tightness, and shortness of breath 3 to 4 times a week and is awak-ened by coughing at least twice a week. In the past 6 months, she has had one emergency department (ED) visit and completed 2 courses of oral steroids.
Ms. D has gained weight since her last visit 3 months ago; her body mass index has gone from 27.5 to 29 kg/m2. And, while she has always been somewhat anxious, Ms. D notes that her anxiety has gotten progressively worse, as well.
About 25 million Americans—approximately one in 12—suffer from asthma1 and, despite improvements in asthma guidelines and treatment in the last 20 years,2 many still struggle with uncontrolled symptoms.3 The consequences can be severe.
Suboptimal control of asthma is associated with a significant decrease in quality of life, a greater likelihood of absence from work or school, and an increased risk for life-threatening events, trips to the ED, hospital admissions, and death.1 A multifaceted approach, including regular assessment, aggressive medication management, and attention to comorbidities, is needed to alleviate the suffering of patients with persistent asthma. This evidence-based review can help you provide such broad-based treatment.
Diagnosis and classification go hand in hand
The cornerstones of asthma management are accurate diagnosis and assessment of disease severity, based on both qualitative and quantitative measures. Start with a patient history, eliciting information about symptoms, triggers, risk factors, and most importantly, how often symptoms occur. Classic high-pitched wheezing sounds during exhalation, a cough that often worsens at night, shortness of breath, and chest tightness should raise suspicion for an asthma diagnosis.2 But frequency (and timing) of symptoms and exacerbations, as well as changes in the patient’s ability to function normally, help to determine whether asthma is classified as mild intermittent, mild persistent, moderate persistent, or severe persistent (TABLE).2
TABLE
Classifying asthma severity2
| Findings | Mild intermittent | Mild persistent | Moderate persistent | Severe persistent |
|---|---|---|---|---|
| Frequency | ≤2/wk | >2/wk, but <1/d | Daily | Continuous |
| Exacerbations | Rare | <2/wk | ≥2/wk | Frequent |
| Activity level | Normal | May decrease with exacerbation | Frequently limited | Significantly limited |
| Nighttime symptoms | ≤2/mo | >2/mo | >1/wk | Frequent |
| FEV1 (or PEF) predicted | >80% | >80% | >60% to <80% | ≤60% |
| PEF variability | <20% | 20%-30% | >30% | >30% |
| FEV1, forced expiratory volume in one second; PEF, peak expiratory flow. | ||||
Because asthma treatment should be based on its classification, an accurate assessment of disease severity is especially important for patients like Ms. D, who have been treated for asthma but continue to have unresolved symptoms. Keep in mind that asthma classification should be based on the worst symptom a patient has, not necessarily the symptom that occurs most frequently. Thus, a patient who has daytime symptoms requiring use of a rescue inhaler 2 to 3 times a week but is awakened at night with shortness of breath 2 times a week would receive a diagnosis of moderate persistent asthma on the basis of the night-time symptoms.
In assessing asthma severity, it is also important to ask specifically about recent events, including ED visits, hospitalizations, and intubations. This information, as well as answers to questions about smoking status, mental health problems, quality of life, and treatment compliance—and whether the patient can afford to purchase the asthma medications you’ve prescribed—can be used to assess the likelihood of poor outcomes.2
Factor in spirometry findings
History and physical examination alone cannot adequately diagnose and classify asthma severity.4,5 Spirometry, a reimbursable office test that can be administered by trained staff members, can be beneficial for any patient older than 5 years for whom a diagnosis of asthma is being considered or disease severity being determined.2 Other objective measures, such as the Mini Asthma Quality of Life questionnaire (http://erj.ersjournals.com/content/14/1/32.full.pdf+html) and peak expiratory flow measurement, may be helpful, as well.2,6
Spirometry measures forced expiratory volume in one second (FEV1) and forced vital capacity (FVC) and calculates the FEV1/FVC ratio. Reference spirometry values vary according to patient characteristics, such as age, height, sex, and race, as well as the positioning of the patient during the test.7 (A seated position is optimal to reduce the risk of falls as a result of the light-headedness some patients may experience.) The American Thoracic Society provides a set of criteria (available at http://www.gp-training.net/protocol/respiratory/copd/spirometry.htm) that should be considered in interpreting test results.8
The 3 main spirometry patterns you’ll see are:
- Normal (FEV1 >80% predicted; FVC >80% predicted; FEV1/FVC >70%)
- Obstructive (FEV1 <80% predicted; FVC normal or mildly reduced; FEV1/FVC <70%)
- Restrictive (FEV1 normal or mildly reduced; FVC <80% predicted; FEV1/FVC >70%).
Because asthma is a chronic disease with fluctuating symptomatology and severity, spirometry testing should be repeated and results compared on several occasions as a guide to treatment.9 When an obstructive pattern is found, the patient should receive a bronchodilator treatment, then undergo spirometry 15 to 20 minutes later to determine reversibility. A reversible obstructive pattern, defined as an increase in FEV1 by 12% (≥200 mL), is consistent with an asthma diagnosis. If spirometry results are consistently normal but a high clinical suspicion for obstructive disease remains, the patient should be evaluated with a methacholine or histamine challenge test to definitively rule out asthma.10
Rule out asthma mimics. Many medical conditions can mimic symptoms of asthma and result in misdiagnosis or incorrect severity classification and unnecessary treatment. Patients should be evaluated for alternate or coexisting pulmonary conditions, including restrictive lung disease, vocal cord dysfunction, cough-variant asthma, malignancy, and allergies. For a patient whose asthma diagnosis is in doubt or who has a restrictive pattern on spirometry, additional evaluation based on signs and symptoms may require comprehensive pulmonary function testing, chest x-ray, bronchoscopy, laryngoscopy, computed tomography, and/or allergy testing.2
Peak expiratory flow (PEF). While measuring PEF should not replace spirometry or formal pulmonary function testing, it can be helpful for evaluating disease severity and monitoring treatment. Patients should use their own peak flow meters, and results compared with their personal best measurements. An improvement of 60 L/min or >20% after treatment with a bronchodilator is suggestive of asthma.9 There are a number of free or low-cost apps that patients can use to track their PEF measurements and response to treatment, such as Asthma MD, Huff and Puff (for children), and the Peak Flow Calculator.11-13
An evidence-based approach to asthma treatment
The first step in treating newly diagnosed asthma is to advise the patient to avoid known triggers, such as allergens, stressors, and particular odors or activities, to the extent possible, and, most importantly, to avoid exposure to smoke. If the patient smokes—cigarettes, marijuana, hookah, or pipe—stress the importance of quitting and living in a home that is smoke free. The link between asthma exacerbations and cockroaches is also well documented, particularly affecting those in urban areas. Avoidance of cockroaches and their droppings is critical, and may require the use of pest control services.14,15
A general principle of asthma management is to treat it aggressively initially to help the patient achieve quick control, then gradually cut back to the fewest medications and lowest effective doses required to maintain control.2 The National Heart, Lung, and Blood Institute (NHLBI)’s 2007 Expert Panel Report: Guidelines for the Diagnosis and Management of Asthma (FIGURE)2 call for a stepwise approach.
Short-acting beta-agonists (SABAs) and ICS—first-line asthma therapy—have minimal risks or adverse effects. SABAs help reverse acute shortness of breath and wheezing, and ICS can reduce the frequency of exacerbations.2
FIGURE
Stepwise approach to asthma management for patients ≥12 years
*Consult with an asthma specialist if Step 4 care or higher is required; consider consultation at Step 3.
†Consider subcutaneous allergen immunotherapy for patients with allergic asthma.
ICS, inhaled corticosteroid; LABA, long-acting beta-agonist; LTRA, leukotriene receptor agonist; SABA, short-acting beta-agonist.
Adapted from: National Asthma Education and Prevention Program. J Allergy Clin Immunol. 2007.2
Second-line therapy is less clearcut
There are several options for patients whose symptoms are not well controlled with first-line treatment: (1) Add a long-acting beta-agonist (LABA); (2) add a leukotriene receptor antagonist (LTRA); or (3) increase the ICS dose, the most straightforward approach.
A dose increase avoids both the additional risk of adverse drug reactions and the added cost associated with another medication. But the easiest solution is not necessarily the best. Consider the evidence detailed below, which includes findings from studies published after the NHLBI’s guidelines.
The research on LABAs
LABAs have been widely used as adjunctive therapy for adults with asthma. However, a 2006 study raised safety concerns.16
The Salmeterol Multicenter Asthma Research Trial (SMART) compared the safety of the LABA salmeterol with a placebo added to usual asthma care over a 28-week treatment period. Overall, the primary composite end point—the number of respiratory-related deaths or life-threatening events—was low, and not statistically significant for salmeterol (50 vs 36; relative risk [RR]=1.40; 95% confidence interval [CI], 0.91-2.14).16 However, individual outcomes—respiratory-related deaths, asthma-related deaths, and asthma-related deaths or life-threatening episodes—were significantly more likely in the salmeterol group compared with the placebo group. In subgroup analysis, African American patients were found to be at greatest risk.16
It is hard to draw general conclusions from these data because the study was terminated early and poor outcomes were limited to a particular study year. Nonetheless, many physicians remain wary of LABAs as adjunctive therapy because of these findings and the media publicity they generated.
A 2010 Cochrane review provided additional data on the safety and efficacy of the combination of a LABA and ICS compared with a higher dose of ICS.17 The review, which included 48 randomized controlled trials, found that combination therapy had a lower risk of exacerbations for which oral corticosteroids were required than a higher dose of ICS (RR=0.88; 95% CI, 0.78-0.98; P=.02). The median number needed to treat (NNT) was 73. No significant difference in the risk of overall adverse events (RR=0.99; 95% CI, 0.95-1.03) was found, but there was an increase in the risk of tremor (RR=1.84; 95% CI, 1.20-2.82) and a decrease in risk for oral thrush (RR=0.58; 95% CI, 0.40-0.86) in the combination therapy group.
While the Cochrane review did not show a combination of LABA and ICS to be less safe overall than higher doses of ICS alone, the findings were less favorable for children and patients with higher baseline lung function, in circumstances in which the combination therapy was taken for a longer duration, and when the LABA being studied was formoterol.17
Overall, it is when a LABA is delivered via separate inhaler that adverse outcomes have been reported. Findings have been positive when the LABA is combined with ICS, and this combination is recommended as maintenance therapy for moderate to severe asthma.
Two new studies, published in March 2013, reported successful use of a LABA-ICS combination not only for maintenance via scheduled dosing, but also for early phases of exacerbation via extra dosing—an approach called Single inhaler Maintenance and Reliever Therapy (SMART).18,19 In both studies, SMART resulted in less excessive use of SABAs and less need for oral steroids, fewer hospitalizations for asthma, and fewer cases of progression to a full-blown exacerbation.
The takeaway: LABAs should be reserved for use as an adjunct to ICS in adults with poor baseline pulmonary function tests or severe asthma, and delivered as a combination product with ICS, not as a separate inhaled medication. SMART is a safe and effective means of administering LABA-ICS therapy for some patients at risk for frequent severe exacerbations.
When to consider LTRAs
LTRAs can be valuable medications in asthma management and there are extensive data on their use, particularly in the treatment of children with asthma. A Cochrane review published in 2012, however, supported current guideline recommendations, finding that as monotherapy, ICS are superior to LTRAs.20
When LTRAs as an adjunctive therapy to ICS were compared with ICS monotherapy, researchers found a modest improvement in PEF (weighted mean difference [WMD] =7.7 L/min; 95% CI, 3.6-11.8) in the group receiving combination therapy and a decrease in the need for a SABA as rescue therapy (WMD=1 puff/week; 95% CI, 0.5-2.0).21 There was no significant reduction in the risk of exacerbations requiring systemic steroids (RR=0.64; 95% CI, 0.38-1.07).
LABAs and LTRAs go head to head. A 2010 Cochrane review compared the efficacy and safety of a daily LABA vs a LTRA as add-on therapy for patients whose asthma was not well controlled with ICS monotherapy.22 The LABA/ICS combination was significantly better at reducing the risk of exacerbations requiring systemic corticosteroids than monotherapy with either a LTRA or ICS, reducing the risk from 11% to 9% (RR=0.83; 95% CI, 0.71-0.97). The NNT to prevent one exacerbation over 48 weeks was 38 (95% CI, 22-244).22
The safety of LABAs continues to be a concern, however, as serious adverse events were more common in the LABA group. The number needed to harm (NNH) with LABA therapy vs LTRA over 48 weeks was 78; 95% CI, 33 to infinity.22 (The width of the CI indicates that while harm is possible in as few as 33 patients, it is also possible that an infinite number of patients would need to be treated for one individual to incur harm.) Overall, the evidence suggests that LABAs are superior add-on therapy to ICS for the treatment of uncontrolled asthma compared with LTRAs, but their use nonetheless requires caution and close monitoring in African American and pediatric patients.17
Is there a role for a long-acting anticholinergic inhaler?
Long-acting anticholinergic medication (LAAM)—tiotropium is the only drug in this class on the market, but there are others in clinical trials—is the mainstay of therapy for chronic obstructive pulmonary disease. This drug class was not widely available or studied as an asthma treatment when the NHLBI guidelines were drafted.
A 2010 study of tiotropium challenged the notion that there is no place for LAAMs in asthma therapy. Using a 3-way crossover design, the study compared the addition of tiotropium to ICS with a double dose of ICS or a LABA/ICS combination.23
The results suggest that LAAMs could be useful in treating uncontrolled asthma. Compared with the double dose of ICS, the tiotropium/ICS combination increased PEF by a mean difference of 25.8 L/min (P<.001) and resulted in a statistically significant improvement in the proportion of asthma control days, FEV1, and daily symptom scores.23 As an adjunctive treatment to ICS, tiotropium was not inferior to a LABA.
CASE After a detailed history, physical exam, and diagnostic testing, Ms. D was given a diagnosis of moderate persistent asthma. We recognized the need to step up her treatment. Prior to making any changes in her medication regimen, however, our team, which included a clinical pharmacist, observed her use of inhaled medications and verified that she was using the inhaler properly. We then initiated combination therapy, pairing a LABA and ICS.
Comorbidities complicate asthma management
Asthma management is often complicated by other uncontrolled coexisting medical problems. Common comorbidities that can affect asthma severity include allergic rhinitis, chronic sinusitis, gastroesophageal reflux disease (GERD), obesity, obstructive sleep apnea (OSA), mental health disorders, tobacco use, and hormonal disturbances.2
Allergic rhinitis. Allergic rhinitis has been associated with worse asthma control and a negative impact on quality of life, and the upper airway inflammation associated with it should be treated.24
Antihistamines and nasal steroids are the most effective medical management. Some patients with allergic rhinitis benefit from blood or skin allergy testing for confirmation or to aid in avoidance. Referral to an allergist may be necessary if symptoms are recalcitrant, a food allergy is in question, or the diagnosis is unclear.
GERD. Compared with the general population, patients with asthma have a much higher risk of GERD, although it is not always symptomatic. While results are inconsistent and difficult to predict, treating symptomatic GERD with acid-blocking medications can result in better asthma control for some patients. However, proton pump inhibitors should not be used to treat asthma symptoms in patients with asymptomatic GERD.25,26
Obesity and OSA. Weight loss can significantly improve asthma control, decrease medication use, and improve quality of life.27,28 Obese patients are less likely to respond to treatment with ICS.2 Weight loss also benefits those who suffer from OSA, which may contribute to airway hyperresponsiveness.29
Mental health disorders. Compared with the general population, patients with asthma are more likely to have depression, anxiety, and panic disorders.30 Diagnosis and treatment of these comorbid conditions can lead to better asthma management, increased medication adherence, decreased health care utilization—including fewer ED visits and hospitalizations—and a better quality of life.30
CASE We also addressed our patient’s comorbidities—weight gain, allergic rhinitis, and anxiety. The allergic rhinitis was already well-controlled with a nasal steroid, but we suspected a relationship between Ms. D’s weight gain and increasing anxiety associated with some recent life events. We suggested she see a counselor, and she agreed.
When the patient returned in 12 weeks, she reported that she hardly needed her rescue inhaler anymore and that she was managing her anxiety more effectively. She also told us that she had begun a low-fat dietary regimen, and we confirmed that she had already lost 5 pounds.
CORRESPONDENCE
Stephen A. Wilson, MD, MPH, FAAFP, UPMC St. Margaret, 815 Freeport Road, Pittsburgh, PA 15215; wilsons2@upmc.edu
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
1. American Academy of Allergy, Asthma, and Immunology. Asthma statistics. Available at: http://www.aaaai.org/about-the-aaaai/newsroom/asthma-statistics.aspx. Accessed March 7, 2012.
2. National Asthma Education and Prevention Program. Expert Panel Report 3: guidelines for diagnosis and management of asthma. J Allergy Clin Immunol. 2007;120(5 suppl):S94-S138.
3. Centers for Disease Control and Prevention. National surveillance for asthma—United States, 1980-2004. MMWR Surveill Summ. 2007;56(8):1-54.
4. Stout JW, Visness CM, Enright P, et al. Classification of asthma severity in children. Arch Pediatr Adolesc Med. 2006;160:844-850.
5. Bacharier LB, Strunk RC, Mauger D, et al. Classifying asthma severity in children. Am J Respir Crit Care Med. 2004;170:426-432.
6. Juniper EF, Guyatt GH, Cox FM, et al. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J. 1999;14:32-38.
7. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179-187.
8. Miller MR, Hankinson J, Brusasco V, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319-338.
9. Bateman ED, Hurd SS, Barnes PJ, et al. Global Strategy for Asthma Management and Prevention. Eur Respir J. 2008;31:143-178.
10. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of COPD. February 2013. Available at: http://www.goldcopd.org/guidelines-global-strategy-for-diagnosis-management.html. Accessed March 7, 2013.
11. AsthmaMD. Available at: http://www.asthmamd.org/#resources/iphone_chart.jpg. Accessed March 7, 2013.
12. Indiegogo. Huff & Puff. Available at: http://www.indiegogo.com/projects/the-best-asthma-education-app-in-the-world-period. Accessed March 7, 2013.
13. Vimukti Technologies Pvt Ltd. Peak flow calculator. Available at: http://appworld.blackberry.com/webstore/content/7615. Accessed March 7, 2013,
14. Morgan WJ, Crain EF, Gruchalla RS, et al. Results of a home-based environmental intervention among urban children with asthma. N Engl J Med. 2004;35:1068-1080.
15. Phipatanakul W, Matsui E, Portnoy J, et al. Environmental assessment and exposure reduction of rodents: a practice parameter. Ann Allergy Asthma Immunol. 2012;109:375-387.
16. Nelson HS, Weiss ST, Bleeker ER, et al. The Salmeterol Multicenter Asthma Research Trial. Chest. 2006;129:15-26.
17. Ducharme FM, Ni Chroinin M, Greenstone I, et al. Addition of long-acting beta2-agonists to inhaled steroids versus higher dose inhaled steroids in adults and children with persistent asthma. Cochrane Database Syst Rev. 2010;(4):CD005533.-
18. Papi A, Corradi M, Pigeon-Francisco C, et al. Beclometasone-formoterol as maintenance and reliever treatment in patients with asthma. Lancet Respir Med. 2013;1:23-31.
19. Patel M, Pilcher J, Pritchard A, et al. Efficacy and safety of maintenance and reliever combination budesonide-formoterol inhaler in patients with asthma at risk of severe exacerba-tions. Lancet Respir Med. 2013;1:32-42.
20. Chauhan BF, Ducharme FM. Anti-leukotriene agents compared to inhaled corticosteroids in the management of recurrent and/or chronic asthma in adults and children. Cochrane Database Syst Rev. 2012;(5):CD002314.-
21. Ducharme FM. Addition of anti-leukotriene agents to inhaled corticosteroids for chronic asthma. Cochrane Database Syst Rev. 2004;(1):CD003133.-
22. Ducharme FM, Lasserson TJ, Cates CJ. Addition to inhaled corticosteroids of long-acting beta2-agonists versus anti-leukotrienes for chronic asthma. Cochrane Database Syst Rev. 2011;(5):CD003137.-
23. Peters SP, Kunselman SJ, Icitovic N, et al. Tiotropium bromide step-up therapy for adults with uncontrolled asthma. N Engl J Med. 2010;363:1715-1726.
24. Vandenplas O, Dramaix M, Joos G, et al. The impact of concomitant rhinitis on asthma-related quality of life and asthma control. Allergy. 2010;65:1290-1297.
25. Gibson PG, Henry RL, Coughlan JL. Gastroesophageal reflux treatment for asthma in adults and children. Cochrane Database Syst Rev. 2003;(2):CD001496.-
26. The American Lung Association Asthma Clinical Research Centers. Efficacy of esomeprazole for treatment of poorly controlled asthma. N Engl J Med. 2009;360:1487-1499.
27. Eneli IU, Skybo T, Camargo CA, Jr. Weight loss and asthma. Thorax. 2008;63:671-676.
28. Stenius-Aarniala B, Poussa T, Kvarnstrom J, et al. Immediate and long term effects of weight reduction in obese people with asthma. BMJ. 2000;320:827-832.
29. Sariman N, Levent E, Cubuk R, et al. Bronchial hyperreactivity and airway wall thickening in obstructive sleep apnea patients. Sleep Breath. 2011;15:341-50.
30. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104:22-28.
A practical guide to shoulder injuries in the throwing athlete
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; gpujalte@hmc.psu.edu
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; gpujalte@hmc.psu.edu
• Manage most throwing injuries with relative rest and physical therapy. A
• Evaluate patients for total loss of range of motion, which is a predictor of increased injury. B
Strength of recommendation (SOR)
A Good-quality patient-oriented evidence
B Inconsistent or limited-quality patient-oriented evidence
C Consensus, usual practice, opinion, disease-oriented evidence, case series
Baseball players and other athletes who spend much of their time throwing a ball risk a variety of shoulder injuries because the repetitive motion causes repeated microtraumatic stress in the area. Injuries result from overuse of the muscles involved, improper technique—or both.
The review that follows will help you zero in on the correct diagnosis and identify the treatment that’s best for your patient.
First step: Cover these points in the history
In order to gather a detailed history of a patient with shoulder pain, you’ll want to do the following:
Ask about the location of the pain. Anterior shoulder pain is associated with subluxation, multidirectional instability, subacromial bursitis, and injury to the biceps, supraspinatus or subscapularis.1 Posterior shoulder pain has been linked to infraspinatus injuries.
Assess the severity of the pain. Ask patients: “On a scale of one to 10, where 10 is the worst pain you have ever felt, how would you rate the pain you are feeling?”
Pinpoint the timing of the pain. Determine the phase of the throwing process that reproduces the primary symptoms. The 6 phases are wind up, early cocking, late cocking, acceleration, deceleration, and follow-through (FIGURE 1). So if, for instance, the patient tells you that his arm “went dead” during the late cocking, or early acceleration phase, it should prompt you to suspect subluxation.2
See how the Neer’s and Hawkin’s tests are done
Christopher Faubel, MD, Thepainsource.com
Neer’s Impingement test
Hawkins test
FIGURE 1
The 6 phases of throwing
Ascertain the nature of the patient’s pain after activity. Does the patient experience the pain at night? If he answers Yes, you’ll want to consider the possibility of a rotator cuff tear.
Ask these targeted questions:
- When you raise your arm, do you feel a pinching pain in your shoulder? This may suggest the presence of impingement.
- Is your shoulder “catching” or “locking up”? If so, consider a labral tear or loose body, eg, a piece of cartilage or bone floating around in the joint.
- Does your shoulder feel like it is coming out of its socket—either partially or completely? This suggests shoulder instability.
- Is it difficult for you to reach behind your back, or do you have shoulder pain when you try to do this? This may indicate glenohumeral internal rotation deficit.
- Do you feel like you are throwing the ball slower, or with less accuracy? This may be an indication that there is something wrong with the rotator cuff muscles, the innervation around the shoulders, or the labrum that partly holds the shoulder together. Sometimes, a tear of the labrum presents simply as a “loss of power” in throwing, as defined by the athlete who is used to throwing the ball faster or farther.
Diagnoses to consider
Based on the patient’s history and responses to your questions, you’ll likely consider one of the following diagnoses as part of the differential.
External or internal impingement syndromes
What you’ll see. External or “subacromial” impingement syndrome results from compression of the rotator cuff between the coracoacromial arch and the humeral head. A sloping or hooked acromion or osteophyte may contribute to the syndrome.3 Neer’s and Hawkin’s tests are often positive, and there may be pain with the arc of motion. (For more on these and the other tests mentioned here, see “Athlete has shoulder problems? Consider these tests”.)
While a full review of provocative shoulder testing is beyond the scope of this article, specific tests for impingement, labral tears, instability, and rotator cuff tears should be included when examining the throwing athlete.
Impingement
Neer’s test. The clinician uses one hand to passively flex the arm of a patient whose thumb is pointing down, as the clinician’s other hand stabilizes the patient’s scapula. The test is positive for impingement if the patient feels pain in the shoulder with this maneuver.
Hawkin’s test. This test involves stabilizing the scapula, passively abducting the shoulder to 90°, flexing the shoulder to 30°, flexing the elbow to 90°, and internally rotating the shoulder. Pain with this maneuver suggests rotator cuff impingement.
Labral tears
O’Brien’s test. The physician asks the patient to adduct his arm across the midline of his body while keeping his shoulder flexed at 90° and his thumb down. As he does this, the physician pushes downward to resist the patient’s shoulder flexion and to see if the patient feels pain. Then, the same motion is done by the patient, but this time with the thumb up. If the pain is not present—or diminishes—with the thumb up, the test is considered positive for a labral tear.
Instability
Load and shift test. The physician uses force to push the humeral head centrally onto the glenoid fossa and then attempts to move the humeral head backward and forward, while keeping the scapula stable, to see how far it can go. Displacement <1 cm is mild; 1 to 2 cm is moderate; and >2 cm is severe.
Sulcus sign. With the patient’s arm in a relaxed position at his side, the physician pulls it downward. If a gap more than 1 cm wide develops between the humeral head and the acromion, the test is positive for inferior glenohumeral instability.
Apprehension-relocation test. The physician asks the patient to lie down on his back and abduct his shoulder at 90°. The physician then externally rotates the patient’s arm and places stress on the glenohumeral joint. A patient with shoulder instability will often stop the physician and say that he feels as if his shoulder is going to “pop out.”
The relocation part of the test is done by the physician applying a posteriorly directed force on the front of the shoulder. If the patient says that the almost popping out feeling of his shoulder has disappeared (and experiences a sense of relief), the test is considered positive.
Rotator cuff tears
Drop arm test. The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. The test is considered positive for a supraspinatus muscle tear if the patient is unable to keep the arm elevated after the physician releases the arm.
Empty can test (Jobe test). The shoulder is passively abducted to 90° and flexed to 30° while the thumb is pointing down. In this position, resistance is provided as the patient tries to lift the arm upward. Pain with weakness suggests a tear of the supraspinatus muscle.
Push-off test. The clinician asks the patient to adduct and internally rotate his arm behind the back. The examiner provides resistance as the patient tries to push the arm away from the body. Pain with weakness suggests a tear of the subscapularis muscle.
Internal impingement results from pinching of the rotator cuff between the posterosuperior labrum and the greater tuberosity. The pain usually occurs with repetitive maximal shoulder internal rotation and abduction, which leads to cumulative microtrauma and eventual articular-sided rotator cuff pathology.4 The patient will complain of pain with shoulder internal rotation and abduction. Neer’s and Hawkin’s tests are helpful in detecting internal impingement.
Shoulder girdle fatigue from lack of conditioning and overthrowing—or the tight posterior capsule often seen in throwing athletes—may also contribute to the disorder.4
Treatment. Proper management involves relative rest from overhead activities, and an individualized rehabilitation program that includes dynamic stretching/strengthening through the rotator cuff, posterior capsule, and scapular stabilizers. Injecting a corticosteroid-analgesic solution into the subacromial space may help you arrive at a diagnosis and also offers symptomatic relief.1,3 Consider bursectomy, arthroscopic acromioplasty, capsulotomy and/or debridement for recalcitrant cases.4,5
Shoulder labrum pathology
What you’ll see. Overhead-throwing athletes are at risk of labral tears. The externally rotated, abducted arm of a thrower causes posterior rotation of the biceps anchor, peeling the biceps from its superior labrum attachment,6 a superior labrum anterior and posterior (SLAP) tear, or a type II tear from anterior to posterior. SLAP tears may lead to shoulder catching and locking. The patient may complain of vague shoulder pain,7 which is worse in the late cocking phase. O’Brien’s test will be positive.
Magnetic resonance imaging (MRI) with arthrogram can reveal a labral tear. Consider ordering an MRI when the athlete’s pain is accompanied by mechanical symptoms, such as locking, catching, or instability, or if the shoulder signs and symptoms do not appear to be responding to appropriate physical therapy interventions after a period of time—usually 4 to 6 weeks.
Treatment. For small tears, conservative management includes relative rest and physical therapy.3 Depending on the tear morphology, consider arthroscopic labral debridement or repair if conservative measures fail. The literature offers mixed conclusions on the benefits of surgery, with varying rates of full return to play.8-10
Shoulder instability
What you’ll see. Instability in throwing athletes is multifactorial, and rarely due to an isolated shoulder structure injury.11 Patients will complain that their shoulder feels as if it is going to come out of its socket, even when they are not throwing. To help detect instability, look for the sulcus sign, and do a load and shift test and an apprehension-relocation test.
Two categories of injury. Instability injuries fall into 2 primary categories: TUBS (Traumatic, Unilateral, associated with Bankart lesion, treated with Surgery) and AMBRI (Atraumatic, Multidirectional, Bilateral, treated with Rehabilitation, Inferior capsular shift).
As its name makes clear, TUBS is associated with a Bankart lesion (an avulsion of the anteroinferior glenoid labrum to its attachment to the humerus). Shoulder x-rays, including outlet, axillary lateral, and anteroposterior views,3 may reveal a bony Bankart lesion. You may also see a Hill-Sachs lesion here, which is noted on the humeral posterolateral head as a depression in the bony cortex.
AMBRI is more common than TUBS in throwers. Athletes often gain a competitive edge by increasing external rotation. However, when overdone, this results in the excessive laxity seen in AMBRI. While rare, acute traumatic dislocation can occur in those with AMBRI-type instability.
Treatment. Scapular stabilization exercises, dynamic rotator cuff strengthening, relative rest, and a short course (7-10 days) of nonsteroidal anti-inflammatory drugs are the mainstays of shoulder instability treatment in the throwing athlete.1,3 A throwing program may be started when the athlete is asymptomatic and has rested. You may also need to prescribe a longer rest period of 4 to 6 weeks if the symptoms return after commencing activity. For recalcitrant cases, consider surgery (via open or arthroscopic approaches6) to treat the associated underlying pathology.
Glenohumeral internal rotation deficit
What you’ll see. Posterior capsular contracture, common in the throwing athlete’s shoulder, causes decreased internal rotation and posterior shift of the total arc of glenohumeral motion.3 The patient may complain of decreased ability to reach backwards or pain when attempting to do so.
The anterior aspect of the shoulder’s capsule also lengthens, allowing anterior capsular laxity that causes additional problems, including internal impingement, SLAP tears, articular-sided, partial-thickness rotator cuff tears, and posterosuperior rotator cuff impingement. The risk for this cascade of complications increases in patients with throwing-shoulder internal rotation deficits ≥25° compared with the nonthrowing side, and a total arc of motion <180°.12
Treatment. Stretching the tight posterior capsule using the sleeper stretch (FIGURE 2) or the cross-body stretch (FIGURE 3) has proven very successful, with 90% of athletes seeing their symptoms resolve within 2 weeks.13,14 If conservative treatment is ineffective, consider selective arthroscopic capsular release of the posterior inferior glenohumeral ligament.
FIGURE 2
The sleeper stretch
FIGURE 3
The cross-body stretch
Rotator cuff tears
What you’ll see. Partial-thickness, articular-sided tears of the supraspinatus, infraspinatus, or both—found posterosuperiorly at the posterior rotator interval—are common in throwing athletes. The patient may complain of weakness when trying to do overhead tasks or movements requiring shoulder abduction. The supraspinatus is usually the muscle affected, and so testing of this muscle with the “empty can test” will show pain with weakness if there is a tear. However, full-thickness rotator cuff tears are rare;3 consider a diagnosis of instability or a partial tear in such cases. An MRI can reveal a rotator cuff tear. In fact, the imaging may be necessary for any suspicion of a tear in an athlete.
Treatment. Recommend strengthening exercises to patients before considering surgery. Nonoperative treatment is preferred, and should be given a fair trial before surgery; studies have not consistently supported the operative approach to rotator cuff tears.5,15 However, if conservative management fails, arthroscopic debridement of torn tissues is recommended over open procedures.3
Scapular dyskinesis and “SICK syndrome”
What you’ll see. Poor development of, or fatigue in, the scapular stabilizers leads to scapular dyskinesis (poor scapular control and motion). Scapulothoracic dyskinesis can progress to an overuse muscular fatigue syndrome called the “SICK syndrome” (Scapular malposition, Inferior medial border prominence, Coracoid pain and malposition, and dysKinesis of scapular movement).16 The most common symptoms include anterior shoulder pain, posterior/superior scapular pain with or without radiation,16 and a “dead arm” sensation. If not treated, this can result in rotator cuff lesions, impingement, and labral pathology.
Treatment. Both treatment and prevention are dependent on the proper biomechanics to retract and rotate the scapula correctly during throwing.1 Strengthening the scapular stabilizers and stretching tight posterior structures help to promote proper biomechanics, and enable a successful return to throwing.14
Help patients prevent injuries in the first place
To reduce the risk of shoulder injuries, athletes need to maintain an appropriate “thrower’s motion” at the glenohumeral joint.17 Overhead throwing athletes often exhibit excessive external rotation in their dominant shoulders,18 while internal rotation is reduced.19
Frequent gentle stretching may help maintain equal total motion in both the throwing shoulder and the nondominant shoulder. However, warn patients to avoid overaggressive stretching to gain mobility; the goal should be to maintain mobility.17
Strengthening of the entire upper extremity (shoulder, scapula, elbow, and wrist) is essential. While the individual needs of each athlete must be addressed, electromyographic studies of the throwing motion suggest that stretching, strengthening, and retraining of the muscles that allow the shoulders to rotate upwards and backwards help the shoulder blade keep close to the rib cage at the back. These are the most important initial steps in rehabilitating shoulder injuries in a throwing athlete.
Prevention and treatment programs for the throwing athlete should always incorporate dynamic stabilization and neuromuscular control.17 Additionally, the transfer of kinetic energy, as well as proximal stability with distal mobility of the upper extremity, are enhanced by core stabilization drills, including planks and side planks, as well as lower body training. As such, core strengthening is a very important component of injury prevention exercise regimens for throwing athletes.
Lastly, throwing programs incorporating maximum pitch counts per day, rest days, and gentle throwing are key to injury prevention. Direct young throwing athletes and their parents to resources such as http://pediatrics.aappublications.org/content/129/3/e842.full.pdf+html. (Tell them to see the recommendations at the end of the document.) Keep in mind, however, that there are no clear recommendations for college and professional pitching.
Young athletes. It is important to note that athletes with immature skeletons are at particular risk of injury due to the relative weakness of the open growth plate and the development of muscle imbalance. It is essential to appropriately apply the principles discussed here to young athletes to prevent injury.
CORRESPONDENCE
George Guntur A. Pujalte, MD, Penn State Milton S. Hershey Medical Center, 500 Hershey Center Drive, Hershey, PA 17033; gpujalte@hmc.psu.edu
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
1. Altcheck DW, Dines DM. Shoulder injuries in the throwing athlete. J Am Acad Orthop Surg. 1995;3:159-165.
2. Rowe CR, Zarins B. Recurrent transient subluxation of the shoulder. J Bone Joint Surg Am. 1981;63:863-872.
3. Jancosko JJ, Kazanjian JE. Shoulder injuries in the throwing athlete. Phys Sportsmed. 2012;40:84-90.
4. Jobe CM. Posterior superior glenoid impingement: expanded spectrum. Arthroscopy. 1995;11:530-536.
5. Riand N, Boulahia A, Walch G. Posterosuperior impingement of the shoulder in the athlete: results of arthroscopic debridement in 75 patients. Rev Chir Orthop Reparatrice Appar Mot. 2002;88:19-27.
6. Bottoni CR, Smith EL, Berkowitz MJ, et al. Arthroscopic versus open shoulder stabilization for recurrent anterior instability: a prospective randomized controlled trial. Am J Sports Med. 2006;34:1730-1737.
7. Burkhart SS, Morgan CD. The peel-back mechanism: its role in producing and extending posterior type II SLAP lesions and its effect on SLAP repair rehabilitation. Arthroscopy. 1998;14:637-640.
8. Glascow SG, Bruce RA, Yacobucci GN, et al. Arthroscopic resection of glenoid labral tears in the athlete: a report of 29 cases. Arthroscopy. 1992;8:48-54.
9. Altcheck DW, Warren RF, Wickiewicz TL, et al. Arthroscopic labral debridement: a three-year follow-up study. Am J Sports Med. 1992;20:702-706.
10. Kim SH, Ha KI, Kim SH, et al. Results of arthroscopic treatment of superior labral lesions. J Bone Joint Surg Am. 2002;84:981-985.
11. Pouliart N, Marmor S, Gagey O. Simulated capsulolabral lesion in cadavers: dislocation does not result from a Bankart lesion only. Arthroscopy. 2006;22:748-754.
12. Verna C. Shoulder flexibility to reduce impingement. Paper presented at: 3rd Annual Professional Baseball Athletic Trainers Society Meeting; March 1991; Mesa, Ariz.
13. Lintner D, Mayol M, Uzodinma O, et al. Glenohumeral internal rotation deficits in professional pitchers enrolled in an internal rotation stretching program. Am J Sports Med. 2007;35:617-621.
14. Burkhart SS, Morgan CD, Kibler WB. The disabled throwing shoulder: spectrum of pathology. Part I: pathoanatomy and biomechanics. Arthroscopy. 2003;19:404-420.
15. Mazoue CG, Andrews JR. Repair of full thickness rotator cuff tears in professional baseball players. Am J Sports Med. 2006;34:182-189.
16. Cheung S. Shoulder injuries in the throwing athlete. Orth Sports Med. 2011;4:173-184.
17. Reinold MM, Gill TJ, Wilk KE, et al. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 2: injury prevention and treatment. Sports Health. 2010;2:101-115.
18. Reinold MM, Gill TJ. Current concepts in the evaluation and treatment of the shoulder in overhead throwing athletes, part 1: physical characteristics and clinical examination. Sports Health. 2010;2:39-50.
19. Reinold MM, Wilk KE, Macrina LC, et al. Changes in shoulder and elbow passive range of motion after pitching in professional baseball players. Am J Sports Med. 2008;36:523-527.
MEAN: How to manage a child who bullies
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
A survey from the National Institute of Child Health and Human Development estimated that 20% of 6th through 10th graders admitted to bullying their classmates.1 In addition to an increased risk for personal injury, bullied children are more likely to report low self-esteem and emotional problems2 and often experience loneliness.1 In contrast, children who bully suffer in their school performance1 and are more likely to engage in drug use3 and violence4 later in life. Child psychiatrists often see both bullies and their victims.
Evidence-based recommendations are available to help educators improve the school climate5 and identify children who are at an increased risk for bullying,6 but research supporting specific clinical strategies for managing a child who bullies is limited. Establishing rapport and engaging a bully often is challenging; these difficulties further complicate assessment and successful management of such children.
We present the mnemonic MEAN to help clinicians assess and understand children who bully.
Model. Discuss, demonstrate, and practice models of alternative social skills and behaviors, including active listening, being open to others’ views, accepting failure, controlling impulses, developing problem-solving techniques, and treating others with respect.
Empathize. Encourage children who bully to explore their feelings about themselves—which may uncover poor self-esteem, anger, or guilt—and acknowledge the hurt they cause others by bullying. Focusing on the pain they inflict on others in the context of personal experiences of pain that likely is driving their aggression may enable bullies to empathize with their victims.
Assess. Help the bully assess the costs and benefits of his or her behavior. Point out what the bully stands to gain from ending his or her aggressive behavior, which likely already has resulted in lost recesses, after school detentions, missed sports practices, and the loss of privileges at home. Most importantly, assess and treat any underlying psychopathology, including mood and anxiety disorders.
Nurture. Aid the bully in identifying his or her prosocial strengths to build self-esteem and thereby reduce the need to commit aggressive acts as a means of gaining a sense of control or personal security. Disarm the child with your genuine concern for his or her well-being.
Using these psychotherapeutic techniques may enhance establishing rapport with a child who bullies and may improve outcomes.
Disclosures
Dr. Kepple reports no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
Dr. Madaan receives grant or research support from Eli Lilly and Company, Forest Pharmaceuticals, Merck, Otsuka, Pfizer Inc., and Shire.
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
1. Nansel TR, Overpeck M, Pilla RS, et al. Bullying behaviors among US youth: prevalence and association with psychosocial adjustment. JAMA. 2001;285(16):2094-2100.
2. Guerra NG, Williams KR, Sadek S. Understanding bullying and victimization during childhood and adolescence: a mixed methods study. Child Dev. 2011;82(1):295-310.
3. Tharp-Taylor S, Haviland A, D’Amico EJ. Victimization from mental and physical bullying and substance use in early adolescence. Addict Behav. 2009;34(6-7):561-567.
4. Duke NN, Pettingell SL, McMorris BJ, et al. Adolescent violence perpetration: associations with multiple types of adverse childhood experiences. Pediatrics. 2010;125(4):e778-e786.
5. Olweus D, Limber SP. Bullying in school: evaluation and dissemination of the Olweus Bullying Prevention Program. Am J Orthopsychiatry. 2010;80(1):124-134.
6. Jansen DE, Veenstra R, Ormel J, et al. Early risk factors for being a bully, victim, or bully/victim in late elementary and early secondary education. The longitudinal TRAILS study. BMC Public Health. 2011;11:440.-
8 tips for talking to parents and children about school shootings
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
In the aftermath of a school shooting, parents and teachers may seek a psychiatrist’s advice on how to best discuss these incidents with children. We offer guidelines on what to tell concerned parents, educators, and other adults who may interact with children affected by a school shooting.
6 tips for interacting with children
1. Talk about the event. Instruct adults to ask children to share their feelings about the incident and to show genuine interest in listening to the child’s thoughts and point of view. Adults shouldn’t pretend the event hasn’t occurred or isn’t serious. Children may be more worried if they think adults are too afraid to tell them what is happening. It is important to gently correct any misinformation older students may have received via social media.1
2. Reinforce that home is a safe haven. Overwhelming emotions and uncertainty can bring about a sense of insecurity in children. Children may come home seeking a safe environment. Advise parents to plan a night where family members participate in a favorite family activity.1 Tell parents to remind their children that trust-worthy adults—parents, emergency workers, police, firefighters, doctors, and the military—are helping provide safety, comfort, and support.2
3. Limit television time. If children are exposed to the news, parents should watch it with them briefly, but avoid letting children rewatch the same event repetitively. Constant exposure to the event may heighten a child’s anxiety and fears.
4. Maintain a normal routine. Tell parents they should maintain, as best they can, their normal routine for dinner, homework, chores, and bedtime, but to remain flexible.2 Children may have a hard time concentrating on schoolwork or falling asleep. Advise parents to spend extra time reading or playing quiet games with their children, particularly at bedtime. These activities are calming, foster a sense of closeness and security, and reinforce a feeling of normalcy.
5. Encourage emotions. Instruct parents to explain to their children that all feelings are okay and normal, and to let children talk about their feelings and help put them into perspective.1 Children may need help in expressing these feelings, so be patient. If an incident happened at the child’s school, teachers and administrators may conduct group sessions to help children express their concerns about being back in school.
6. Seek creativity or spirituality. Encourage parents and other adults to provide a creative outlet for children, such as making get well cards or sending letters to the survivors and their families. Writing thank you letters to doctors, nurses, fire-fighters, and police officers also may be comforting.1,2 Suggest that parents encourage their children to pray or think hopeful thoughts for the victims and their families.
2 tips for interacting with adults
7. Recommend they take care of themselves. Explain to adult caregivers that because children learn by observing, they shouldn’t ignore their own feelings of anxiety, grief, and anger. By expressing their emotions in a productive manner, adults will be better able to support their children. Encourage adults to talk to friends, family, religious leaders, or mental health counselors.
8. Advise adults to be alert for children who may need professional help. Tell them to be vigilant when monitoring a child’s emotional state. Children who may benefit from mental health counseling after a tragedy may exhibit warning signs, such as changes in behavior, appetite, and sleep patterns, which may indicate the child is experiencing grief, anxiety, or discomfort.
Remind adults to be aware of children who are at greater risk for mental health issues, including those who are already struggling with other recent traumatic experiences—past traumatic experiences, personal loss, depression, or other mental illness.1 Be particularly observant for children who may be at risk of suicide.1,2 Professional counseling may be needed for a child who is experiencing an emotional reaction that lasts >1 month and is impacting his or her daily functioning.1
Disclosure
The authors report no financial relationship with any company whose products are mentioned in this article or with manufacturers of competing products.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
1. American Psychological Association. Helping your children manage distress in the aftermath of a shooting. http://www.apa.org/helpcenter/aftermath.aspx. Updated April 2011. Accessed February 15, 2013.
2. National Association of School Psychologists resources. A national tragedy: helping children cope. http://www.nasponline.org/resources/crisis_safety/terror_general.aspx. Published September 2001. Accessed February 15, 2013.
Recent advances in the management of advanced non–small-cell lung cancer
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.
Lung cancer is the leading cause of cancer-related mortality among men and women in the United States. Non–small-cell lung cancer (NSCLC) accounts for about 85% of all lung cancers. Most patients with NSCLC present with advanced disease and median overall survival in this incurable setting remain dismal. Accumulating evidence suggests that both histology and molecular signature have prognostic and predictive value for NSCLC. Recent advances in the molecular characterization of NSCLC tumors have made individualized treatment approaches feasible. Personalized chemotherapy and targeted biological therapy based on a tumor’s individual biologic and molecular profile can optimize efficacy while minimizing toxicity. Molecular testing for activating mutations in the epidermal growth factor receptor (EGFR) domain and EML4-ALK translocation are routinely used to guide therapeutic decisions. Several new treatments that irreversibly target EGFR family members are in development for patients with NSCLC. Novel EML4-ALK inhibitors such as LDK378 are promising agents with encouraging early efficacy data. KRAS mutations are the most common mutation in adenocarcinomas. Although no agents for this subset of NSCLC have been approved, there are several agents in clinical development, including selumetinib, an MEK inhibitor, that seem promising. A growing body of evidence suggests that NSCLC is subject to immune surveillance. Immunotherapeutic interventions, including vaccine therapy and antigen-independent immunomodulatory strategies, may improve outcomes in NSCLC. In this review, we summarize recent advances in non–small-cell lung cancer, with an emphasis on investigational strategies for individualized treatment.
*Click on the link to the left for a PDF of the full article.
Chest Pain and Vomiting with Prior Heart Attack
ANSWER
The correct interpretation includes sinus tachycardia with occasional premature ventricular complexes (PVCs), left atrial enlargement, evidence of a previous inferior MI, and an acute anterior MI. Sinus tachycardia is evidenced by a rate ≥ 100 beats/min and the presence of a P wave for every QRS complex with a constant PR interval. A single PVC is evident (12th beat of the rhythm strips V1, II, and V5).
Left atrial enlargement is evidenced by a large P wave in lead II and a biphasic P wave with the terminal portion larger than the initial portion in lead V1. An old inferior MI is evidenced by the presence of Q waves in leads II, III, and aVF.
An evolving anterior MI is diagnosed by the presence of poor R-wave progression with ST-segment elevations and T-wave inversion in leads V2, V3, and V4. This was subsequently confirmed by clinically significant elevations of serum troponin levels and by cardiac catheterization, which revealed occlusion of the left anterior descending artery distal to the internal mammary artery anastomosis.
ANSWER
The correct interpretation includes sinus tachycardia with occasional premature ventricular complexes (PVCs), left atrial enlargement, evidence of a previous inferior MI, and an acute anterior MI. Sinus tachycardia is evidenced by a rate ≥ 100 beats/min and the presence of a P wave for every QRS complex with a constant PR interval. A single PVC is evident (12th beat of the rhythm strips V1, II, and V5).
Left atrial enlargement is evidenced by a large P wave in lead II and a biphasic P wave with the terminal portion larger than the initial portion in lead V1. An old inferior MI is evidenced by the presence of Q waves in leads II, III, and aVF.
An evolving anterior MI is diagnosed by the presence of poor R-wave progression with ST-segment elevations and T-wave inversion in leads V2, V3, and V4. This was subsequently confirmed by clinically significant elevations of serum troponin levels and by cardiac catheterization, which revealed occlusion of the left anterior descending artery distal to the internal mammary artery anastomosis.
ANSWER
The correct interpretation includes sinus tachycardia with occasional premature ventricular complexes (PVCs), left atrial enlargement, evidence of a previous inferior MI, and an acute anterior MI. Sinus tachycardia is evidenced by a rate ≥ 100 beats/min and the presence of a P wave for every QRS complex with a constant PR interval. A single PVC is evident (12th beat of the rhythm strips V1, II, and V5).
Left atrial enlargement is evidenced by a large P wave in lead II and a biphasic P wave with the terminal portion larger than the initial portion in lead V1. An old inferior MI is evidenced by the presence of Q waves in leads II, III, and aVF.
An evolving anterior MI is diagnosed by the presence of poor R-wave progression with ST-segment elevations and T-wave inversion in leads V2, V3, and V4. This was subsequently confirmed by clinically significant elevations of serum troponin levels and by cardiac catheterization, which revealed occlusion of the left anterior descending artery distal to the internal mammary artery anastomosis.
Three hours ago, this 69-year-old man started to experience substernal chest pain, nausea, and vomiting. He had stopped for lunch after spending the morning raking leaves; his chest pain began while he was at the drive-through window at a local fast food restaurant. By the time he got home, he was nauseous and had an episode of emesis. The pain is described as a “dull, heavy ache” with radiation to the left neck and arm. The patient states he is diaphoretic, but attributes it to the labor associated with his yardwork. He recalls that his first heart attack started in a similar way and fears he is having another. Following the emesis, his nausea stopped, and he rested in his recliner, hoping his symptoms would resolve. Unfortunately, the chest pain worsened, and he drove himself to the clinic rather than calling 911. (He didn’t want to go to the ED or get billed for the ambulance ride.) Review of his medical history reveals coronary artery disease, evidenced by an inferior MI in 2008. This was treated with coronary artery bypass surgery with reversed saphenous vein grafts placed to the first and second obtuse marginal branches of the circumflex coronary artery, reversed saphenous vein graft to the posterior descending artery, and an internal mammary artery graft placed to the proximal left anterior descending artery. His history is also remarkable for hyperlipidemia, hypertension, and obesity. He is a retired postal worker who has lived alone since his wife died three years ago. He drinks a six-pack of beer daily and continues to smoke one to two packs of cigarettes per day, depending on his activities. His family history includes coronary artery disease, hypertension, diabetes, and stroke. His current medications include aspirin, atorvastatin, clopidogrel, furosemide, isosorbide dinitrate, metoprolol, nicotine patch, potassium chloride, and valsartan. He is intolerant of ACE inhibitors, due to the resulting chronic, dry cough. The review of systems reveals that he has had headaches over the past week and that he recently recovered from the flu. He has had no changes in bowel or bladder function, shortness of breath, or recent weight loss or gain. Vital signs include a blood pressure of 168/100 mm Hg; pulse, 110 beats/min; respiratory rate, 18 breaths/min-1; O2 saturation, 98% on room air; and temperature, 37.2°C. His weight is 108 kg, and his height is 170 cm. Physical findings show that the lungs are clear to auscultation; his rhythm is regular with no murmurs, rubs, or gallops; and the point of maximum impulse is not displaced. The neck veins are flat, and there is no peripheral edema. A well-healed median sternotomy scar is evident, as are bilateral lower extremity saphenous vein harvest scars. The abdomen is soft and nontender, and peripheral pulses are strong and equal bilaterally. There are no neurologic abnormalities noted. Laboratory samples and an ECG are obtained. The following findings are noted on the ECG: a ventricular rate of 108 beats/min; PR interval, 140 ms; QRS duration, 116 ms; QT/QTc interval, 372/498 ms; P axis, 70°; R axis, 75°; and T axis, 167°. What is your interpretation of this ECG?
Thumb: Scaling with Pitted Nail Plate
ANSWER
The most likely diagnosis is psoriasis (choice “d”), which can manifest with localized involvement (see discussion below).
Fungal infection (choice “a”) is unlikely, given the negative results of the KOH prep and total lack of response to treatment for that diagnosis.
Eczema (choice “b”) does not manifest as such thick, adherent scale. If any nail changes were involved, they would likely consist of transverse nail ridges.
A variant of squamous cell carcinoma (SCC; choice “c”)—caused by human papillomavirus (HPV), for example—can produce somewhat similar changes in the skin. But it would be unlikely to lead to nail changes, and it would not be intermittent, as this rash was in apparent response to OTC cream.
DISCUSSION
A punch biopsy is sometimes required to confirm the diagnosis of psoriasis, but this combination of skin and nail changes is quite suggestive of that entity. In this case, the confirmation was made by a different route: The rheumatologist judged that the patient’s arthritis was psoriatic in nature. The arthritis was treated with methotrexate, which soon cleared the skin disease without any help from dermatology.
This case serves to reinforce the possible mind-body role of stress in the genesis of this disease; however, at least 30% of the time, there is a positive family history. It also demonstrates the seemingly contradictory fact that the severity of the psoriasis doesn’t always correlate with the presence or absence of psoriatic arthritis.
Adding to the difficulty of making the diagnosis of psoriasis is the localized distribution of the scales, since it can usually be corroborated by finding lesions in their usual haunts—such as the extensor surfaces of arms, legs, on the trunk, or in the scalp. Cases such as this one force the provider to carefully consider the differential, which includes fungal infection. But the dermatophytes that cause ordinary fungal infections have to come from predictable sources (eg, children, pets, or farm animals), all missing from this patient’s history.
Had the rash been “fixed” (unchanging), the diagnosis of SCC would have to be considered more carefully. Superficial SCC is called Bowen’s disease, which, in cases such as this, is usually caused by HPV, not by the more typical overexposure to UV light. Though superficial, Bowen’s disease can become focally invasive and can even metastasize.
Had this patient not been treated with methotrexate, we would probably have used topical class 1 corticosteroid creams, which would have had a good chance to improve his skin but not his nail. The patient was also counseled regarding the role of stress and increased alcohol intake in the worsening of his disease. His prognosis for skin and joint disease is decent, though he will probably experience recurrences.
ANSWER
The most likely diagnosis is psoriasis (choice “d”), which can manifest with localized involvement (see discussion below).
Fungal infection (choice “a”) is unlikely, given the negative results of the KOH prep and total lack of response to treatment for that diagnosis.
Eczema (choice “b”) does not manifest as such thick, adherent scale. If any nail changes were involved, they would likely consist of transverse nail ridges.
A variant of squamous cell carcinoma (SCC; choice “c”)—caused by human papillomavirus (HPV), for example—can produce somewhat similar changes in the skin. But it would be unlikely to lead to nail changes, and it would not be intermittent, as this rash was in apparent response to OTC cream.
DISCUSSION
A punch biopsy is sometimes required to confirm the diagnosis of psoriasis, but this combination of skin and nail changes is quite suggestive of that entity. In this case, the confirmation was made by a different route: The rheumatologist judged that the patient’s arthritis was psoriatic in nature. The arthritis was treated with methotrexate, which soon cleared the skin disease without any help from dermatology.
This case serves to reinforce the possible mind-body role of stress in the genesis of this disease; however, at least 30% of the time, there is a positive family history. It also demonstrates the seemingly contradictory fact that the severity of the psoriasis doesn’t always correlate with the presence or absence of psoriatic arthritis.
Adding to the difficulty of making the diagnosis of psoriasis is the localized distribution of the scales, since it can usually be corroborated by finding lesions in their usual haunts—such as the extensor surfaces of arms, legs, on the trunk, or in the scalp. Cases such as this one force the provider to carefully consider the differential, which includes fungal infection. But the dermatophytes that cause ordinary fungal infections have to come from predictable sources (eg, children, pets, or farm animals), all missing from this patient’s history.
Had the rash been “fixed” (unchanging), the diagnosis of SCC would have to be considered more carefully. Superficial SCC is called Bowen’s disease, which, in cases such as this, is usually caused by HPV, not by the more typical overexposure to UV light. Though superficial, Bowen’s disease can become focally invasive and can even metastasize.
Had this patient not been treated with methotrexate, we would probably have used topical class 1 corticosteroid creams, which would have had a good chance to improve his skin but not his nail. The patient was also counseled regarding the role of stress and increased alcohol intake in the worsening of his disease. His prognosis for skin and joint disease is decent, though he will probably experience recurrences.
ANSWER
The most likely diagnosis is psoriasis (choice “d”), which can manifest with localized involvement (see discussion below).
Fungal infection (choice “a”) is unlikely, given the negative results of the KOH prep and total lack of response to treatment for that diagnosis.
Eczema (choice “b”) does not manifest as such thick, adherent scale. If any nail changes were involved, they would likely consist of transverse nail ridges.
A variant of squamous cell carcinoma (SCC; choice “c”)—caused by human papillomavirus (HPV), for example—can produce somewhat similar changes in the skin. But it would be unlikely to lead to nail changes, and it would not be intermittent, as this rash was in apparent response to OTC cream.
DISCUSSION
A punch biopsy is sometimes required to confirm the diagnosis of psoriasis, but this combination of skin and nail changes is quite suggestive of that entity. In this case, the confirmation was made by a different route: The rheumatologist judged that the patient’s arthritis was psoriatic in nature. The arthritis was treated with methotrexate, which soon cleared the skin disease without any help from dermatology.
This case serves to reinforce the possible mind-body role of stress in the genesis of this disease; however, at least 30% of the time, there is a positive family history. It also demonstrates the seemingly contradictory fact that the severity of the psoriasis doesn’t always correlate with the presence or absence of psoriatic arthritis.
Adding to the difficulty of making the diagnosis of psoriasis is the localized distribution of the scales, since it can usually be corroborated by finding lesions in their usual haunts—such as the extensor surfaces of arms, legs, on the trunk, or in the scalp. Cases such as this one force the provider to carefully consider the differential, which includes fungal infection. But the dermatophytes that cause ordinary fungal infections have to come from predictable sources (eg, children, pets, or farm animals), all missing from this patient’s history.
Had the rash been “fixed” (unchanging), the diagnosis of SCC would have to be considered more carefully. Superficial SCC is called Bowen’s disease, which, in cases such as this, is usually caused by HPV, not by the more typical overexposure to UV light. Though superficial, Bowen’s disease can become focally invasive and can even metastasize.
Had this patient not been treated with methotrexate, we would probably have used topical class 1 corticosteroid creams, which would have had a good chance to improve his skin but not his nail. The patient was also counseled regarding the role of stress and increased alcohol intake in the worsening of his disease. His prognosis for skin and joint disease is decent, though he will probably experience recurrences.
About a year ago, this 40-year-old man developed scaling on the distal one-third of his thumbnail. After an ini-tial apparent response to OTC hydrocortisone 1% cream, the condition began to worsen, spreading to more of the thumb. The patient’s primary care provider diagnosed fungal infection and prescribed a combination clotrima-zole/betamethasone cream, which had no effect. A subsequent four-month course of terbinafine (250 mg/d) also yielded no improvement. The patient then requested referral to dermatology. The patient considers himself “quite healthy,” aside from having moderately severe arthritis, for which he takes nabumetone (750 mg bid). His arthritis recently worsened, and he is scheduled to see a rheumatologist in two months. He admits to being under a great deal of stress shortly before his skin condition developed. As a result, he in-creased his alcohol intake for a few months, but he quit drinking altogether soon afterward. He denies any family history of skin disease and has no children or contact with animals. On examination, most of his thumb is covered by thick adherent white scales on a pinkish base. The margins are sharply defined. A KOH prep is performed, but no fungal elements are seen. The adjacent thumbnail has focal areas of pitting in the nail plate, as well as yellowish discoloration on the dis-tal edge. No such changes are seen on his other nails, and examination of his elbows, knees, trunk, and scalp fails to reveal any significant abnormalities.
Board Certification Requirements Changes
In 2005, we conducted a study of the prevalence of board certification requirements for hospital privileging of pediatricians.[1] Since that time, there have been many changes in the landscape of both physician and healthcare‐system quality assessment. New developments include greater utilization of physician quality‐of‐care assessment tools, a change from recertification for time‐limited board certification to Maintenance of Certification (MOC) in 2010, and an increasing commitment on the part of hospitals and state licensing officials to patient safety and quality‐of‐care issues, due in part to the continued interest by governmental and private payors and the public on external measurement of healthcare quality.[2, 3, 4, 5, 6]
MOC is an ongoing process of lifelong learning and self‐assessment to continuously improve knowledge and clinical performance. It has been adopted by all 24 member boards of the American Boards of Medical Specialties. MOC is focused on the 6 core competencies of quality medical care as outlined by the Accreditation Council for Graduate Medical Education (ACGME): (1) patient care, (2) medical knowledge, (3) practice‐based learning, (4) systems‐based practice, (5) professionalism, and (6) interpersonal and communication skills. To address, these competencies, MOC involves a 4‐part process for continuous learning that is required to keep certification current: (1) licensure and professional standing, (2) lifelong learning and self‐assessment, (3) cognitive expertise, and (4) practice performance assessment.[7, 8]
Our previous study found that many hospitals utilize specialty certification as a marker of quality for privileging.[1] To explore changes in the policies of hospitals regarding requirements for board certification and the incorporation of MOC into those requirements, we conducted a 5‐year follow‐up study of a national random sample of hospitals in 2010.
METHODS
Sample
All hospitals identified in the American Hospital Association's 2009 Annual Survey of Hospitals as providing care to pediatric patients were included in the sampling frame (N=2136). We then selected a stratified random sample of 10% of the total (N=220) hospitals weighted to provide nationally representative estimates. The sample was stratified by Council of Teaching Hospitals (COTH) designation (teaching vs nonteaching) and National Association of Children's Hospitals and Related Institutions (NACHRI) membership. In contrast to our previous study, in this study we did not stratify according to the designation of freestanding children's hospital (vs part of a hospital system) or metropolitan statistical area size (urban vs rural), as comparisons across these designations were not found to be significant in 2005.
Hospitals were sampled with varying probabilities from each stratum. Weights were applied to create a representative sample of the overall hospital population. The total sampling weight (TSW) calculated for each hospital was based on the probability of selection into the study (P) and the response rate (RR). The following formula was used: TSW: (1/P) (1/RR).
Survey Instrument
In collaboration with the American Board of Pediatrics Research Advisory Committee, we developed a 24‐item, fixed‐choice, structured questionnaire to be administered by phone. The survey was designed to be completed in 15 minutes or less and focused on board certification requirements at initial privileging, recredentialing, and MOC requirements.
The survey focused on the following descriptive research questions: Do hospitals require board certification for pediatricians at the time of initial privileging? Do they ever require board certification for privileging? Are there different certification requirements for generalists vs subspecialists? Are pediatricians with permanent certificates required to enroll in MOC?
Other questions focused on such issues such as whether the hospital was familiar with the requirements of MOC, whether MOC was required of all pediatricians, and whether the institution of MOC changed certification requirements at the hospital.
The instrument was pilot tested for clarity and ease of use with representatives from a convenience sample of hospitals within the state of Michigan and revised to clarify potentially ambiguous questions. Pilot surveys were not included in the analyses.
Questionnaire Administration
Data collection took place between April 2010 and June 2010. Interviewers requested to speak with the department responsible for credentialing or privileging at the hospital, typically the Medical Staff Office, the Office of Clinical Affairs, or the Credentialing or Privileging Department. When the appropriate person was identified and located, interviewers explained the purpose of the study and obtained verbal consent to participate.
Data Analysis
Initially, frequency distributions were calculated for all survey items to create descriptive statistics. Next, we performed a cross‐tabulation of responses by the specific hospital classifications listed above (COTH and NACHRI status) and computed the 2 statistics. Finally, we conducted bivariate analyses on the 2005 and 2010 results. SAS version 9.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. P<0.05 was considered statistically significant.
Although this study is similar to the study that was completed in 2005,[1] we have reanalyzed those data to more specifically assess certification policy. All results are now weighted in contrast to the 2005 study, which only weighted the results by hospital classification. Thus, the numbers in some cases may be slightly different from those reported in 2006. We believe that this has resulted in a more robust analysis of hospital use of board certification in privileging.
Comparisons
Where possible, results were compared with those found in a 2005 study of hospital privileging.[1] The sampling frame for that study was identical to the current study, but the specific hospitals may or may not be included in the current study.
The study was approved by the University of Michigan Medical School Institutional Review Board.
RESULTS
Response Rate and Respondent Demographics
Of the 220 hospitals surveyed, 23 were ineligible because they did not have at least 1 pediatrician on staff. Of the remaining 197 hospitals, 154 completed the survey, resulting in a 78% participation rate.
Response rates did not differ significantly by NACHRI or COTH hospital status; therefore, there was no impact on the analytic power of the weighting. Approximately half (54%, n=82) of the respondents were NACHRI member hospitals, and 49% (n=75) were COTH hospitals.
Because not every hospital responded to every question, the total number for each question response may differ slightly.
2005 VS 2010 COMPARISONS
Board Certification Requirements
Compared with our findings in 2005, in 2010 a greater proportion of hospitals now require board certification for general pediatricians (80% vs 67%, P=0.141). Among these hospitals, a much larger proportion (24% vs 4%) now require board certification for all pediatricians at the point of initial privileging (Table 1). Similarly, a greater proportion of hospitals now require board certification for pediatric subspecialists (86% vs 71%, P=0.048). The percentage of hospitals that require subspecialists to be board certified at the point of initial privileging also increased from 10% in 2005 to 34% in 2010.
| General Pediatricians | Pediatric Subspecialists | |||
|---|---|---|---|---|
| 2005 (N=159) | 2010 (N=154) | 2005 (N=153) | 2010 (N=147) | |
| ||||
| Certification never required | 33%a | 20%a | 29%b | 14%b |
| Certification ever required | 67%a | 80%a | 71%b | 86%b |
| At time of initial privileging for all pediatricians | 4% | 24% | 10% | 34% |
| Within a specified time frame of initial privileging | 50% | 29% | 41% | 32% |
| At time of initial privileging but only for some pediatricians | 11% | 24% | 16% | 17% |
| Only recertification required | 2% | 3% | 4% | 3% |
The proportion of teaching (COTH) hospitals that require general pediatricians to be board certified at some point in time increased from 63% in 2005 to 89% in 2010 (P=0.001), and the percentage that require board certification for all pediatricians at initial privileging increased from 2% in 2005 to 25% in 2010. Similarly, the proportion of teaching hospitals that require pediatric subspecialists to be board certified increased from 66% in 2005 to 89% in 2010 (P=0.003).
There were small changes between 2005 and 2010 in the proportion of nonteaching (68% vs 79%, P=0.231), NACHRI‐member (76% vs 82%, P=0.366), and non‐NACHRI member (67% vs 80%, P=0.156) hospitals that require pediatricians to be board certified at some point in time. The proportion of nonteaching (4% vs 24%), NACHRI‐member (5% vs 32%), and non‐NACHRI (4% vs 23%) hospitals that require board certification at the point of initial privileging also increased between 2005 and 2010.
Certification Policies at Initial Privileging
Although in 2010, a greater proportion of hospitals reported that they require board certification for general pediatricians and pediatric subspecialists at the point of initial privileging, a much larger proportion of hospitals reported that they make exceptions to their board certification policies for both general pediatricians (99% vs 41%) (Table 2) and pediatric subspecialists (98% vs 14%) (Table 3). Among hospitals that do not require board certification at the point of initial privileging, only small differences were seen in requirements around completion of residency or fellowship training and time frame after which certification must be achieved (Tables 2 and 3).
| 2005 (N=159) | 2010 (N=154) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 4% | 24% |
| Mixed policy | 11% | 24% |
| No | 85% | 52% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 41% | 99% |
| Required certification to be current | 99% | 99% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete residency | 85% | 84% |
| Established time frame after which certification must be achieved | 48% | 51% |
| 2005 (N=153) | 2010 (N=147) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 10% | 34% |
| Mixed policy | 5% | 17% |
| No | 85% | 49% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 14% | 98% |
| Required certification to be current | 83% | 100% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete fellowship | 86% | 86% |
| Established time frame after which certification must be achieved | 47% | 52% |
There were no meaningful differences between board certification policies for general pediatricians and pediatric subspecialists in 2010.
Comparing Recertification and MOC Policies
Few hospitals required permanent certificate holders to recertify (2005) or enroll in MOC (2010) in 2005 (5%) or 2010 (6%). The proportion of hospitals that required recertification or MOC enrollment for general pediatricians increased from 33% in 2005 to 42% in 2010. Similarly, the percentage of hospitals that required recertification or MOC enrollment for pediatric subspecialists increased from 25% in 2005 to 35% in 2010.
Between 2005 and 2010, there was no significant change in the proportion of hospitals that reported revoking or denying privileges to a pediatrician due to failure to recertify or enroll in MOC (3% vs 6%).
SPECIFIC MAINTENANCE OF CERTIFICATION POLICIES IN 2010
Board Certification Requirements
Respondents from 29% of hospitals reported that they were not at all familiar with the American Board of Pediatrics' (ABP) MOC program. Most respondents (58%) were familiar with MOC, with 37% reporting that they were somewhat familiar, and 12% reporting that they were very familiar with the program.
Three‐fourths of hospitals (76%) reported that their MOC requirements do not differ from their recertification requirements held prior to the institution of MOC, and 14% reported that their hospital had not yet established specific MOC requirements.
The majority of respondents (62%) had verified the board certification of some physicians since the institution of the ABP's MOC program on January 1, 2010. A majority (53%) of hospitals track MOC data for all pediatricians, whereas 3% of respondents track MOC data only for those pediatricians whose initial certification was time limited.
Of those hospitals that require pediatricians with permanent certificates to enroll in MOC, 9% allow them to retain their privileges for a period of time if they are not meeting the requirements for MOC. Among hospitals that require pediatricians with time‐limited certificates to enroll in MOC, fewer than half allow general pediatricians (37%) and pediatric subspecialists (40%) to retain their privileges if they are not meeting the requirements for MOC.
The majority of respondents (89%) reported that the initiation of MOC had not changed board certification requirements at their hospital. However, respondents from over one‐quarter of hospitals (27%) reported that they expect changes in their hospital's certification or MOC requirements in the next 2 years. Those hospitals that reported changes moved to more stringent requirements for certification at initial privileging and requirements for permanent certificate holders to meet MOC requirements.
DISCUSSION
In the 5 years since our previous study, a larger proportion of hospitals now require pediatricians to become board certified to obtain hospital privileges. Of note is that a larger proportion of hospitals also now require board certification at the time of initial privileging for both generalist and subspecialist pediatricians.
Hospitals face increasing pressure to differentiate themselves from their peers through better patient outcomes.[9, 10] The increase from 67% to 80% of hospitals requiring board certification may be a result of hospitals utilizing certification as a proxy for assessment of physician quality or as a way to engage physicians in quality improvement through the MOC program.[11] Hospitals may also be responding to greater interest in MOC from regulatory agencies such as the Centers for Medicare and Medicaid Services Maintenance of Certification Program Incentive, which rewards physicians with an additional incentive payment beyond the Physician Quality Reporting System incentive for their participation in the MOC program.[12]
Interestingly, although a greater proportion of hospitals reported that they require certification, a much larger proportion of hospitals make exceptions to the policy. The exceptions could include grandfathering physicians who had hospital privileges prior to the policy change, or giving recent graduates additional time to obtain board certification. It is unknown whether or not all of these physicians would be required to obtain board certification or participate in MOC after some provisional time frame.
Hospitals in our study appear to be incorporating the MOC program into their policies. However, fewer than half of the hospitals studied require pediatricians with time‐limited certificates to enroll in MOC if their certificates have expired. In addition, some hospitals are still establishing their MOC requirements for those pediatricians with time‐limited and permanent certificates. It is likely that the majority of hospitals retained their previous board certification requirements, and that the current flux in hospital requirements is not unique to pediatrics, as all American Board of Medical Specialties' specialties have recently implemented MOC requirements.[13] Hospitals will likely adjust their credentialing policies as their familiarity and experience with MOC grows.
The primary purpose of the specialty certification process is to provide to the public, which includes both individual consumers and regulatory agencies, an assessment of the competency of individual physicians. Self‐regulation through certification is a privilege of trust granted to the medical profession by the public. This is an essential concept that underlies the concept of specialty certification.[14] As the public has continued to adopt a greater focus, and additional demands, on safety and quality assessment in healthcare, the medical profession must in turn be responsive.[13, 15, 16, 17] Failure in this regard would run the risk of losing that trust with the public, with the resultant loss of the ability to self‐regulate.
Studies have indicated a positive relationship between board certification and quality of care, yet this area remains hampered by a paucity of data.[18, 19, 20, 21, 22] Pham et al. found that board certified physicians were more likely to provide preventative care services to Medicare patients.[22] In 2008, Turchin et al. showed that recertification made a small, yet meaningful, difference in physician treatment of hypertension.[18] This area of research is especially important, as the MOC program is more comprehensive and utilizes an ongoing system of assessment and physician engagement. As such, it has been criticized by some for being complicated, burdensome, and irrelevant to the manner in which physicians actually practice.[23, 24] However, previous methods of certification were limited to assessing physicians at 1 point in time during their entire careers (eg, permanent certification) or at specific intervals (eg, time‐limited certification). With recent increased attention to improving the quality of patient care, these methods were unable to assure the public that physicians maintained their knowledge and skills over time in an environment of increasing rapid incorporation of new knowledge into clinical practice. Recent reports have also shown that (years of) practice does not make perfect with regard to physician performance. In fact, there may be deterioration of performance over long periods of practice.[25] Furthermore, although physicians commonly believe they are able to assess their own performance, available evidence does not support that contention.[26, 27] Thus, there is a need for an objective ongoing assessment of physician performance that also has the capacity to continuously improve the quality of care provided.
The comprehensive nature of the MOC program is a result of efforts to meaningfully incorporate the 6 competencies defined by the ACGME into the certification process. Although MOC is still relatively new and maturing, a growing body of evidence is demonstrating effectiveness of specific components of the program.[28, 29, 30, 31] In the field of pediatrics, several programs approved for MOC credit have already demonstrated their effectiveness in improving the quality of care in clinical practice.[32, 33, 34, 35, 36] However, additional efforts are needed to evaluate more of the part 4 (Assessment of Practice Performance) modules to assess their impact on patient care. The continued commitment to quality of care and quality improvement in hospitals will likely result in a further adoption of MOC requirements as the process matures and demonstrable impacts on patient outcomes are assessed. Furthermore, greater coordination of MOC with quality assessments in health plans and in the changes taking place in the process of licensure will likely help to streamline the paperwork and documentation burden placed on physicians by multiple assessment efforts.
This study has several limitations. Because the MOC program was initiated by the ABP in January 2010, there may be a lag in uptake of this particular requirement by hospitals. In some cases, this may have been the first time that members of the credentialing staff had considered the MOC program. It is probable that staff awareness will increase over time, as hospital policies are further developed and greater exposure to the specifics of the MOC program occurs. Additionally, although we compared stratified random samples of hospitals in 2005 and 2010, we did not follow the same group of hospitals over time.
As with all changes to the certification program over the years, there is a period of time required for new requirements to be understood and accepted by both those in regulatory positions and those in the medical profession. The demands of the public for increasingly comprehensive assessments of healthcare quality will continue into the future.
Acknowledgments
Disclosures: Funding was provided by a grant from the American Board of Pediatrics Foundation. The authors have no other disclosures or conflicts of interest to report.
- , , , , , . Policies and practices related to the role of board certification and recertification of pediatricians in hospital privileging. JAMA. 2006;295(8):905–912.
- American Board of Pediatrics. Maintenance of Certification: MOC requirements. 2011. Available at: https://www.abp.org/ABPWeb Static/#murl%3D%2FABPWebStatic%2Fmoc.html%26surl%3D%2 Fabpwebsite%2Fmoc%2Fphysicianrequirements%2Fphysreq.htm. Accessed May 23, 2011.
- , , , , , . Maintenance of licensure: protecting the public, promoting quality health care. J Med Regul. 2010;96(2):13–20.
- , , , , . Setting a fair performance standard for physicians' quality of patient care. J Gen Intern Med. 2011;26(5):467–473.
- , . Payer trend: “tiering” physicians and “steering” patients. Fam Pract Manag. 2007;14(10):24–26.
- , , , et al. Association between maintenance of certification examination scores and quality of care for medicare beneficiaries. Arch Intern Med. 2008;168(13):1396–1403.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/Maintenance_of_Certification/ABMS_MOC.aspx. Accessed January 23, 2012.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/maintenance_of_certification/MOC_competencies.aspx. Accessed January 24, 2012.
- , , . Hospital performance reports: impact on quality, market share, and reputation. Health Aff (Millwood). 2005;24(4):1150–1160.
- , , , et al. Impact of public reporting of coronary artery bypass graft surgery performance data on market share, mortality, and patient selection. Med Care. 2011;49(12):1118–1125.
- , , . Hospital strategies to engage physicians in quality improvement. Available at: www.hschange.org/CONTENT/1087. Accessed June 4, 2012.
- The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements of Self‐Nomination for 2012. http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instru ments/PQRS/downloads/2012_MaintenanceofCertificationProgram_ mmrvsd01162012.pdf. Accessed June 4, 2012.
- , , . Maintenance of Certification, maintenance of public trust. Plast Reconstr Surg. 2011;127(2):967–973.
- , . Credentialing and public accountability: a central role for board certification. JAMA. 2006;295(8):939–940.
- , , , . Perspectives and preferences among the general public regarding physician selection and board certification. J Pediatr. 2010;156(5):841–845, 845.e1.
- , . Public perceptions of quality care and provider profiling in New York: implications for improving quality care and public health. J Public Health Manag Pract. 2004;10(3):241–250.
- . Future of board certification in a new era of public accountability. J Am Board Fam Med. 2010;23(suppl 1):S32–S39.
- , , , , . Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation. 2008;117(5):623–628.
- , , , , . Physician board certification and the care and outcomes of elderly patients with acute myocardial infarction. J Gen Intern Med. 2006;21(3):238–244.
- , , . Certifying examination performance and patient outcomes following acute myocardial infarction. Med Educ. 2002;36(9):853–859.
- , , , , . Specialty board certification and clinical outcomes: the missing link. Acad Med. 2002;77(6):534–542.
- , , , . Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294(4):473–481.
- . Are you ready for maintenance of certification? Fam Pract Manag. 2005;12(1):42–48.
- , , , , . Clinical decisions. American Board of Internal Medicine maintenance of certification program. N Engl J Med. 2010;362(10):948–952.
- . As doctors age, worries about their abilities grow. New York Times. January 24, 2011:D.1.
- , , . Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260–273.
- , , , , , . Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094–1102.
- , , . The impact of a preventive cardiology quality improvement intervention on residents and clinics: a qualitative exploration. Am J Med Qual. 2009;24(2):99–107.
- , , , , , . Promoting physicians' self‐assessment and quality improvement: the ABIM diabetes practice improvement module. J Contin Educ Health Prof. 2006;26(2):109–119.
- , , , et al. Self‐assessment of practice performance: development of the ABIM Practice Improvement Module (PIM). J Contin Educ Health Prof. 2008;28(1):38–46.
- , , , , , . Variation in internal medicine residency clinic practices: assessing practice environments and quality of care. J Gen Intern Med. 2008;23(7):914–920.
- , , , et al. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444.
- , , , et al. ImproveCareNow: the development of a pediatric inflammatory bowel disease improvement network. Inflamm Bowel Dis. 2011;17(1):450–457.
- , . Pay for performance alone cannot drive quality. Arch Pediatr Adolesc Med. 2007;161(7):650–655.
- , , , et al. National pediatric cardiology quality improvement collaborative: lessons learned from development and early years. Prog Pediatr Cardiol. 2011;32(2):103–109.
- , , , et al. Reducing PICU central line‐associated bloodstream infections: 3‐year results. Pediatrics. 2011;128(5):e1077–e1083.
In 2005, we conducted a study of the prevalence of board certification requirements for hospital privileging of pediatricians.[1] Since that time, there have been many changes in the landscape of both physician and healthcare‐system quality assessment. New developments include greater utilization of physician quality‐of‐care assessment tools, a change from recertification for time‐limited board certification to Maintenance of Certification (MOC) in 2010, and an increasing commitment on the part of hospitals and state licensing officials to patient safety and quality‐of‐care issues, due in part to the continued interest by governmental and private payors and the public on external measurement of healthcare quality.[2, 3, 4, 5, 6]
MOC is an ongoing process of lifelong learning and self‐assessment to continuously improve knowledge and clinical performance. It has been adopted by all 24 member boards of the American Boards of Medical Specialties. MOC is focused on the 6 core competencies of quality medical care as outlined by the Accreditation Council for Graduate Medical Education (ACGME): (1) patient care, (2) medical knowledge, (3) practice‐based learning, (4) systems‐based practice, (5) professionalism, and (6) interpersonal and communication skills. To address, these competencies, MOC involves a 4‐part process for continuous learning that is required to keep certification current: (1) licensure and professional standing, (2) lifelong learning and self‐assessment, (3) cognitive expertise, and (4) practice performance assessment.[7, 8]
Our previous study found that many hospitals utilize specialty certification as a marker of quality for privileging.[1] To explore changes in the policies of hospitals regarding requirements for board certification and the incorporation of MOC into those requirements, we conducted a 5‐year follow‐up study of a national random sample of hospitals in 2010.
METHODS
Sample
All hospitals identified in the American Hospital Association's 2009 Annual Survey of Hospitals as providing care to pediatric patients were included in the sampling frame (N=2136). We then selected a stratified random sample of 10% of the total (N=220) hospitals weighted to provide nationally representative estimates. The sample was stratified by Council of Teaching Hospitals (COTH) designation (teaching vs nonteaching) and National Association of Children's Hospitals and Related Institutions (NACHRI) membership. In contrast to our previous study, in this study we did not stratify according to the designation of freestanding children's hospital (vs part of a hospital system) or metropolitan statistical area size (urban vs rural), as comparisons across these designations were not found to be significant in 2005.
Hospitals were sampled with varying probabilities from each stratum. Weights were applied to create a representative sample of the overall hospital population. The total sampling weight (TSW) calculated for each hospital was based on the probability of selection into the study (P) and the response rate (RR). The following formula was used: TSW: (1/P) (1/RR).
Survey Instrument
In collaboration with the American Board of Pediatrics Research Advisory Committee, we developed a 24‐item, fixed‐choice, structured questionnaire to be administered by phone. The survey was designed to be completed in 15 minutes or less and focused on board certification requirements at initial privileging, recredentialing, and MOC requirements.
The survey focused on the following descriptive research questions: Do hospitals require board certification for pediatricians at the time of initial privileging? Do they ever require board certification for privileging? Are there different certification requirements for generalists vs subspecialists? Are pediatricians with permanent certificates required to enroll in MOC?
Other questions focused on such issues such as whether the hospital was familiar with the requirements of MOC, whether MOC was required of all pediatricians, and whether the institution of MOC changed certification requirements at the hospital.
The instrument was pilot tested for clarity and ease of use with representatives from a convenience sample of hospitals within the state of Michigan and revised to clarify potentially ambiguous questions. Pilot surveys were not included in the analyses.
Questionnaire Administration
Data collection took place between April 2010 and June 2010. Interviewers requested to speak with the department responsible for credentialing or privileging at the hospital, typically the Medical Staff Office, the Office of Clinical Affairs, or the Credentialing or Privileging Department. When the appropriate person was identified and located, interviewers explained the purpose of the study and obtained verbal consent to participate.
Data Analysis
Initially, frequency distributions were calculated for all survey items to create descriptive statistics. Next, we performed a cross‐tabulation of responses by the specific hospital classifications listed above (COTH and NACHRI status) and computed the 2 statistics. Finally, we conducted bivariate analyses on the 2005 and 2010 results. SAS version 9.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. P<0.05 was considered statistically significant.
Although this study is similar to the study that was completed in 2005,[1] we have reanalyzed those data to more specifically assess certification policy. All results are now weighted in contrast to the 2005 study, which only weighted the results by hospital classification. Thus, the numbers in some cases may be slightly different from those reported in 2006. We believe that this has resulted in a more robust analysis of hospital use of board certification in privileging.
Comparisons
Where possible, results were compared with those found in a 2005 study of hospital privileging.[1] The sampling frame for that study was identical to the current study, but the specific hospitals may or may not be included in the current study.
The study was approved by the University of Michigan Medical School Institutional Review Board.
RESULTS
Response Rate and Respondent Demographics
Of the 220 hospitals surveyed, 23 were ineligible because they did not have at least 1 pediatrician on staff. Of the remaining 197 hospitals, 154 completed the survey, resulting in a 78% participation rate.
Response rates did not differ significantly by NACHRI or COTH hospital status; therefore, there was no impact on the analytic power of the weighting. Approximately half (54%, n=82) of the respondents were NACHRI member hospitals, and 49% (n=75) were COTH hospitals.
Because not every hospital responded to every question, the total number for each question response may differ slightly.
2005 VS 2010 COMPARISONS
Board Certification Requirements
Compared with our findings in 2005, in 2010 a greater proportion of hospitals now require board certification for general pediatricians (80% vs 67%, P=0.141). Among these hospitals, a much larger proportion (24% vs 4%) now require board certification for all pediatricians at the point of initial privileging (Table 1). Similarly, a greater proportion of hospitals now require board certification for pediatric subspecialists (86% vs 71%, P=0.048). The percentage of hospitals that require subspecialists to be board certified at the point of initial privileging also increased from 10% in 2005 to 34% in 2010.
| General Pediatricians | Pediatric Subspecialists | |||
|---|---|---|---|---|
| 2005 (N=159) | 2010 (N=154) | 2005 (N=153) | 2010 (N=147) | |
| ||||
| Certification never required | 33%a | 20%a | 29%b | 14%b |
| Certification ever required | 67%a | 80%a | 71%b | 86%b |
| At time of initial privileging for all pediatricians | 4% | 24% | 10% | 34% |
| Within a specified time frame of initial privileging | 50% | 29% | 41% | 32% |
| At time of initial privileging but only for some pediatricians | 11% | 24% | 16% | 17% |
| Only recertification required | 2% | 3% | 4% | 3% |
The proportion of teaching (COTH) hospitals that require general pediatricians to be board certified at some point in time increased from 63% in 2005 to 89% in 2010 (P=0.001), and the percentage that require board certification for all pediatricians at initial privileging increased from 2% in 2005 to 25% in 2010. Similarly, the proportion of teaching hospitals that require pediatric subspecialists to be board certified increased from 66% in 2005 to 89% in 2010 (P=0.003).
There were small changes between 2005 and 2010 in the proportion of nonteaching (68% vs 79%, P=0.231), NACHRI‐member (76% vs 82%, P=0.366), and non‐NACHRI member (67% vs 80%, P=0.156) hospitals that require pediatricians to be board certified at some point in time. The proportion of nonteaching (4% vs 24%), NACHRI‐member (5% vs 32%), and non‐NACHRI (4% vs 23%) hospitals that require board certification at the point of initial privileging also increased between 2005 and 2010.
Certification Policies at Initial Privileging
Although in 2010, a greater proportion of hospitals reported that they require board certification for general pediatricians and pediatric subspecialists at the point of initial privileging, a much larger proportion of hospitals reported that they make exceptions to their board certification policies for both general pediatricians (99% vs 41%) (Table 2) and pediatric subspecialists (98% vs 14%) (Table 3). Among hospitals that do not require board certification at the point of initial privileging, only small differences were seen in requirements around completion of residency or fellowship training and time frame after which certification must be achieved (Tables 2 and 3).
| 2005 (N=159) | 2010 (N=154) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 4% | 24% |
| Mixed policy | 11% | 24% |
| No | 85% | 52% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 41% | 99% |
| Required certification to be current | 99% | 99% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete residency | 85% | 84% |
| Established time frame after which certification must be achieved | 48% | 51% |
| 2005 (N=153) | 2010 (N=147) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 10% | 34% |
| Mixed policy | 5% | 17% |
| No | 85% | 49% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 14% | 98% |
| Required certification to be current | 83% | 100% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete fellowship | 86% | 86% |
| Established time frame after which certification must be achieved | 47% | 52% |
There were no meaningful differences between board certification policies for general pediatricians and pediatric subspecialists in 2010.
Comparing Recertification and MOC Policies
Few hospitals required permanent certificate holders to recertify (2005) or enroll in MOC (2010) in 2005 (5%) or 2010 (6%). The proportion of hospitals that required recertification or MOC enrollment for general pediatricians increased from 33% in 2005 to 42% in 2010. Similarly, the percentage of hospitals that required recertification or MOC enrollment for pediatric subspecialists increased from 25% in 2005 to 35% in 2010.
Between 2005 and 2010, there was no significant change in the proportion of hospitals that reported revoking or denying privileges to a pediatrician due to failure to recertify or enroll in MOC (3% vs 6%).
SPECIFIC MAINTENANCE OF CERTIFICATION POLICIES IN 2010
Board Certification Requirements
Respondents from 29% of hospitals reported that they were not at all familiar with the American Board of Pediatrics' (ABP) MOC program. Most respondents (58%) were familiar with MOC, with 37% reporting that they were somewhat familiar, and 12% reporting that they were very familiar with the program.
Three‐fourths of hospitals (76%) reported that their MOC requirements do not differ from their recertification requirements held prior to the institution of MOC, and 14% reported that their hospital had not yet established specific MOC requirements.
The majority of respondents (62%) had verified the board certification of some physicians since the institution of the ABP's MOC program on January 1, 2010. A majority (53%) of hospitals track MOC data for all pediatricians, whereas 3% of respondents track MOC data only for those pediatricians whose initial certification was time limited.
Of those hospitals that require pediatricians with permanent certificates to enroll in MOC, 9% allow them to retain their privileges for a period of time if they are not meeting the requirements for MOC. Among hospitals that require pediatricians with time‐limited certificates to enroll in MOC, fewer than half allow general pediatricians (37%) and pediatric subspecialists (40%) to retain their privileges if they are not meeting the requirements for MOC.
The majority of respondents (89%) reported that the initiation of MOC had not changed board certification requirements at their hospital. However, respondents from over one‐quarter of hospitals (27%) reported that they expect changes in their hospital's certification or MOC requirements in the next 2 years. Those hospitals that reported changes moved to more stringent requirements for certification at initial privileging and requirements for permanent certificate holders to meet MOC requirements.
DISCUSSION
In the 5 years since our previous study, a larger proportion of hospitals now require pediatricians to become board certified to obtain hospital privileges. Of note is that a larger proportion of hospitals also now require board certification at the time of initial privileging for both generalist and subspecialist pediatricians.
Hospitals face increasing pressure to differentiate themselves from their peers through better patient outcomes.[9, 10] The increase from 67% to 80% of hospitals requiring board certification may be a result of hospitals utilizing certification as a proxy for assessment of physician quality or as a way to engage physicians in quality improvement through the MOC program.[11] Hospitals may also be responding to greater interest in MOC from regulatory agencies such as the Centers for Medicare and Medicaid Services Maintenance of Certification Program Incentive, which rewards physicians with an additional incentive payment beyond the Physician Quality Reporting System incentive for their participation in the MOC program.[12]
Interestingly, although a greater proportion of hospitals reported that they require certification, a much larger proportion of hospitals make exceptions to the policy. The exceptions could include grandfathering physicians who had hospital privileges prior to the policy change, or giving recent graduates additional time to obtain board certification. It is unknown whether or not all of these physicians would be required to obtain board certification or participate in MOC after some provisional time frame.
Hospitals in our study appear to be incorporating the MOC program into their policies. However, fewer than half of the hospitals studied require pediatricians with time‐limited certificates to enroll in MOC if their certificates have expired. In addition, some hospitals are still establishing their MOC requirements for those pediatricians with time‐limited and permanent certificates. It is likely that the majority of hospitals retained their previous board certification requirements, and that the current flux in hospital requirements is not unique to pediatrics, as all American Board of Medical Specialties' specialties have recently implemented MOC requirements.[13] Hospitals will likely adjust their credentialing policies as their familiarity and experience with MOC grows.
The primary purpose of the specialty certification process is to provide to the public, which includes both individual consumers and regulatory agencies, an assessment of the competency of individual physicians. Self‐regulation through certification is a privilege of trust granted to the medical profession by the public. This is an essential concept that underlies the concept of specialty certification.[14] As the public has continued to adopt a greater focus, and additional demands, on safety and quality assessment in healthcare, the medical profession must in turn be responsive.[13, 15, 16, 17] Failure in this regard would run the risk of losing that trust with the public, with the resultant loss of the ability to self‐regulate.
Studies have indicated a positive relationship between board certification and quality of care, yet this area remains hampered by a paucity of data.[18, 19, 20, 21, 22] Pham et al. found that board certified physicians were more likely to provide preventative care services to Medicare patients.[22] In 2008, Turchin et al. showed that recertification made a small, yet meaningful, difference in physician treatment of hypertension.[18] This area of research is especially important, as the MOC program is more comprehensive and utilizes an ongoing system of assessment and physician engagement. As such, it has been criticized by some for being complicated, burdensome, and irrelevant to the manner in which physicians actually practice.[23, 24] However, previous methods of certification were limited to assessing physicians at 1 point in time during their entire careers (eg, permanent certification) or at specific intervals (eg, time‐limited certification). With recent increased attention to improving the quality of patient care, these methods were unable to assure the public that physicians maintained their knowledge and skills over time in an environment of increasing rapid incorporation of new knowledge into clinical practice. Recent reports have also shown that (years of) practice does not make perfect with regard to physician performance. In fact, there may be deterioration of performance over long periods of practice.[25] Furthermore, although physicians commonly believe they are able to assess their own performance, available evidence does not support that contention.[26, 27] Thus, there is a need for an objective ongoing assessment of physician performance that also has the capacity to continuously improve the quality of care provided.
The comprehensive nature of the MOC program is a result of efforts to meaningfully incorporate the 6 competencies defined by the ACGME into the certification process. Although MOC is still relatively new and maturing, a growing body of evidence is demonstrating effectiveness of specific components of the program.[28, 29, 30, 31] In the field of pediatrics, several programs approved for MOC credit have already demonstrated their effectiveness in improving the quality of care in clinical practice.[32, 33, 34, 35, 36] However, additional efforts are needed to evaluate more of the part 4 (Assessment of Practice Performance) modules to assess their impact on patient care. The continued commitment to quality of care and quality improvement in hospitals will likely result in a further adoption of MOC requirements as the process matures and demonstrable impacts on patient outcomes are assessed. Furthermore, greater coordination of MOC with quality assessments in health plans and in the changes taking place in the process of licensure will likely help to streamline the paperwork and documentation burden placed on physicians by multiple assessment efforts.
This study has several limitations. Because the MOC program was initiated by the ABP in January 2010, there may be a lag in uptake of this particular requirement by hospitals. In some cases, this may have been the first time that members of the credentialing staff had considered the MOC program. It is probable that staff awareness will increase over time, as hospital policies are further developed and greater exposure to the specifics of the MOC program occurs. Additionally, although we compared stratified random samples of hospitals in 2005 and 2010, we did not follow the same group of hospitals over time.
As with all changes to the certification program over the years, there is a period of time required for new requirements to be understood and accepted by both those in regulatory positions and those in the medical profession. The demands of the public for increasingly comprehensive assessments of healthcare quality will continue into the future.
Acknowledgments
Disclosures: Funding was provided by a grant from the American Board of Pediatrics Foundation. The authors have no other disclosures or conflicts of interest to report.
In 2005, we conducted a study of the prevalence of board certification requirements for hospital privileging of pediatricians.[1] Since that time, there have been many changes in the landscape of both physician and healthcare‐system quality assessment. New developments include greater utilization of physician quality‐of‐care assessment tools, a change from recertification for time‐limited board certification to Maintenance of Certification (MOC) in 2010, and an increasing commitment on the part of hospitals and state licensing officials to patient safety and quality‐of‐care issues, due in part to the continued interest by governmental and private payors and the public on external measurement of healthcare quality.[2, 3, 4, 5, 6]
MOC is an ongoing process of lifelong learning and self‐assessment to continuously improve knowledge and clinical performance. It has been adopted by all 24 member boards of the American Boards of Medical Specialties. MOC is focused on the 6 core competencies of quality medical care as outlined by the Accreditation Council for Graduate Medical Education (ACGME): (1) patient care, (2) medical knowledge, (3) practice‐based learning, (4) systems‐based practice, (5) professionalism, and (6) interpersonal and communication skills. To address, these competencies, MOC involves a 4‐part process for continuous learning that is required to keep certification current: (1) licensure and professional standing, (2) lifelong learning and self‐assessment, (3) cognitive expertise, and (4) practice performance assessment.[7, 8]
Our previous study found that many hospitals utilize specialty certification as a marker of quality for privileging.[1] To explore changes in the policies of hospitals regarding requirements for board certification and the incorporation of MOC into those requirements, we conducted a 5‐year follow‐up study of a national random sample of hospitals in 2010.
METHODS
Sample
All hospitals identified in the American Hospital Association's 2009 Annual Survey of Hospitals as providing care to pediatric patients were included in the sampling frame (N=2136). We then selected a stratified random sample of 10% of the total (N=220) hospitals weighted to provide nationally representative estimates. The sample was stratified by Council of Teaching Hospitals (COTH) designation (teaching vs nonteaching) and National Association of Children's Hospitals and Related Institutions (NACHRI) membership. In contrast to our previous study, in this study we did not stratify according to the designation of freestanding children's hospital (vs part of a hospital system) or metropolitan statistical area size (urban vs rural), as comparisons across these designations were not found to be significant in 2005.
Hospitals were sampled with varying probabilities from each stratum. Weights were applied to create a representative sample of the overall hospital population. The total sampling weight (TSW) calculated for each hospital was based on the probability of selection into the study (P) and the response rate (RR). The following formula was used: TSW: (1/P) (1/RR).
Survey Instrument
In collaboration with the American Board of Pediatrics Research Advisory Committee, we developed a 24‐item, fixed‐choice, structured questionnaire to be administered by phone. The survey was designed to be completed in 15 minutes or less and focused on board certification requirements at initial privileging, recredentialing, and MOC requirements.
The survey focused on the following descriptive research questions: Do hospitals require board certification for pediatricians at the time of initial privileging? Do they ever require board certification for privileging? Are there different certification requirements for generalists vs subspecialists? Are pediatricians with permanent certificates required to enroll in MOC?
Other questions focused on such issues such as whether the hospital was familiar with the requirements of MOC, whether MOC was required of all pediatricians, and whether the institution of MOC changed certification requirements at the hospital.
The instrument was pilot tested for clarity and ease of use with representatives from a convenience sample of hospitals within the state of Michigan and revised to clarify potentially ambiguous questions. Pilot surveys were not included in the analyses.
Questionnaire Administration
Data collection took place between April 2010 and June 2010. Interviewers requested to speak with the department responsible for credentialing or privileging at the hospital, typically the Medical Staff Office, the Office of Clinical Affairs, or the Credentialing or Privileging Department. When the appropriate person was identified and located, interviewers explained the purpose of the study and obtained verbal consent to participate.
Data Analysis
Initially, frequency distributions were calculated for all survey items to create descriptive statistics. Next, we performed a cross‐tabulation of responses by the specific hospital classifications listed above (COTH and NACHRI status) and computed the 2 statistics. Finally, we conducted bivariate analyses on the 2005 and 2010 results. SAS version 9.1 (SAS Institute Inc., Cary, NC) was used for all statistical analyses. P<0.05 was considered statistically significant.
Although this study is similar to the study that was completed in 2005,[1] we have reanalyzed those data to more specifically assess certification policy. All results are now weighted in contrast to the 2005 study, which only weighted the results by hospital classification. Thus, the numbers in some cases may be slightly different from those reported in 2006. We believe that this has resulted in a more robust analysis of hospital use of board certification in privileging.
Comparisons
Where possible, results were compared with those found in a 2005 study of hospital privileging.[1] The sampling frame for that study was identical to the current study, but the specific hospitals may or may not be included in the current study.
The study was approved by the University of Michigan Medical School Institutional Review Board.
RESULTS
Response Rate and Respondent Demographics
Of the 220 hospitals surveyed, 23 were ineligible because they did not have at least 1 pediatrician on staff. Of the remaining 197 hospitals, 154 completed the survey, resulting in a 78% participation rate.
Response rates did not differ significantly by NACHRI or COTH hospital status; therefore, there was no impact on the analytic power of the weighting. Approximately half (54%, n=82) of the respondents were NACHRI member hospitals, and 49% (n=75) were COTH hospitals.
Because not every hospital responded to every question, the total number for each question response may differ slightly.
2005 VS 2010 COMPARISONS
Board Certification Requirements
Compared with our findings in 2005, in 2010 a greater proportion of hospitals now require board certification for general pediatricians (80% vs 67%, P=0.141). Among these hospitals, a much larger proportion (24% vs 4%) now require board certification for all pediatricians at the point of initial privileging (Table 1). Similarly, a greater proportion of hospitals now require board certification for pediatric subspecialists (86% vs 71%, P=0.048). The percentage of hospitals that require subspecialists to be board certified at the point of initial privileging also increased from 10% in 2005 to 34% in 2010.
| General Pediatricians | Pediatric Subspecialists | |||
|---|---|---|---|---|
| 2005 (N=159) | 2010 (N=154) | 2005 (N=153) | 2010 (N=147) | |
| ||||
| Certification never required | 33%a | 20%a | 29%b | 14%b |
| Certification ever required | 67%a | 80%a | 71%b | 86%b |
| At time of initial privileging for all pediatricians | 4% | 24% | 10% | 34% |
| Within a specified time frame of initial privileging | 50% | 29% | 41% | 32% |
| At time of initial privileging but only for some pediatricians | 11% | 24% | 16% | 17% |
| Only recertification required | 2% | 3% | 4% | 3% |
The proportion of teaching (COTH) hospitals that require general pediatricians to be board certified at some point in time increased from 63% in 2005 to 89% in 2010 (P=0.001), and the percentage that require board certification for all pediatricians at initial privileging increased from 2% in 2005 to 25% in 2010. Similarly, the proportion of teaching hospitals that require pediatric subspecialists to be board certified increased from 66% in 2005 to 89% in 2010 (P=0.003).
There were small changes between 2005 and 2010 in the proportion of nonteaching (68% vs 79%, P=0.231), NACHRI‐member (76% vs 82%, P=0.366), and non‐NACHRI member (67% vs 80%, P=0.156) hospitals that require pediatricians to be board certified at some point in time. The proportion of nonteaching (4% vs 24%), NACHRI‐member (5% vs 32%), and non‐NACHRI (4% vs 23%) hospitals that require board certification at the point of initial privileging also increased between 2005 and 2010.
Certification Policies at Initial Privileging
Although in 2010, a greater proportion of hospitals reported that they require board certification for general pediatricians and pediatric subspecialists at the point of initial privileging, a much larger proportion of hospitals reported that they make exceptions to their board certification policies for both general pediatricians (99% vs 41%) (Table 2) and pediatric subspecialists (98% vs 14%) (Table 3). Among hospitals that do not require board certification at the point of initial privileging, only small differences were seen in requirements around completion of residency or fellowship training and time frame after which certification must be achieved (Tables 2 and 3).
| 2005 (N=159) | 2010 (N=154) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 4% | 24% |
| Mixed policy | 11% | 24% |
| No | 85% | 52% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 41% | 99% |
| Required certification to be current | 99% | 99% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete residency | 85% | 84% |
| Established time frame after which certification must be achieved | 48% | 51% |
| 2005 (N=153) | 2010 (N=147) | |
|---|---|---|
| Certification required at initial privileging | ||
| Yes | 10% | 34% |
| Mixed policy | 5% | 17% |
| No | 85% | 49% |
| If hospital required certification at initial privileging: | ||
| Allowed exceptions to policy at initial privileging | 14% | 98% |
| Required certification to be current | 83% | 100% |
| If hospital did not require certification at initial privileging: | ||
| Required to complete fellowship | 86% | 86% |
| Established time frame after which certification must be achieved | 47% | 52% |
There were no meaningful differences between board certification policies for general pediatricians and pediatric subspecialists in 2010.
Comparing Recertification and MOC Policies
Few hospitals required permanent certificate holders to recertify (2005) or enroll in MOC (2010) in 2005 (5%) or 2010 (6%). The proportion of hospitals that required recertification or MOC enrollment for general pediatricians increased from 33% in 2005 to 42% in 2010. Similarly, the percentage of hospitals that required recertification or MOC enrollment for pediatric subspecialists increased from 25% in 2005 to 35% in 2010.
Between 2005 and 2010, there was no significant change in the proportion of hospitals that reported revoking or denying privileges to a pediatrician due to failure to recertify or enroll in MOC (3% vs 6%).
SPECIFIC MAINTENANCE OF CERTIFICATION POLICIES IN 2010
Board Certification Requirements
Respondents from 29% of hospitals reported that they were not at all familiar with the American Board of Pediatrics' (ABP) MOC program. Most respondents (58%) were familiar with MOC, with 37% reporting that they were somewhat familiar, and 12% reporting that they were very familiar with the program.
Three‐fourths of hospitals (76%) reported that their MOC requirements do not differ from their recertification requirements held prior to the institution of MOC, and 14% reported that their hospital had not yet established specific MOC requirements.
The majority of respondents (62%) had verified the board certification of some physicians since the institution of the ABP's MOC program on January 1, 2010. A majority (53%) of hospitals track MOC data for all pediatricians, whereas 3% of respondents track MOC data only for those pediatricians whose initial certification was time limited.
Of those hospitals that require pediatricians with permanent certificates to enroll in MOC, 9% allow them to retain their privileges for a period of time if they are not meeting the requirements for MOC. Among hospitals that require pediatricians with time‐limited certificates to enroll in MOC, fewer than half allow general pediatricians (37%) and pediatric subspecialists (40%) to retain their privileges if they are not meeting the requirements for MOC.
The majority of respondents (89%) reported that the initiation of MOC had not changed board certification requirements at their hospital. However, respondents from over one‐quarter of hospitals (27%) reported that they expect changes in their hospital's certification or MOC requirements in the next 2 years. Those hospitals that reported changes moved to more stringent requirements for certification at initial privileging and requirements for permanent certificate holders to meet MOC requirements.
DISCUSSION
In the 5 years since our previous study, a larger proportion of hospitals now require pediatricians to become board certified to obtain hospital privileges. Of note is that a larger proportion of hospitals also now require board certification at the time of initial privileging for both generalist and subspecialist pediatricians.
Hospitals face increasing pressure to differentiate themselves from their peers through better patient outcomes.[9, 10] The increase from 67% to 80% of hospitals requiring board certification may be a result of hospitals utilizing certification as a proxy for assessment of physician quality or as a way to engage physicians in quality improvement through the MOC program.[11] Hospitals may also be responding to greater interest in MOC from regulatory agencies such as the Centers for Medicare and Medicaid Services Maintenance of Certification Program Incentive, which rewards physicians with an additional incentive payment beyond the Physician Quality Reporting System incentive for their participation in the MOC program.[12]
Interestingly, although a greater proportion of hospitals reported that they require certification, a much larger proportion of hospitals make exceptions to the policy. The exceptions could include grandfathering physicians who had hospital privileges prior to the policy change, or giving recent graduates additional time to obtain board certification. It is unknown whether or not all of these physicians would be required to obtain board certification or participate in MOC after some provisional time frame.
Hospitals in our study appear to be incorporating the MOC program into their policies. However, fewer than half of the hospitals studied require pediatricians with time‐limited certificates to enroll in MOC if their certificates have expired. In addition, some hospitals are still establishing their MOC requirements for those pediatricians with time‐limited and permanent certificates. It is likely that the majority of hospitals retained their previous board certification requirements, and that the current flux in hospital requirements is not unique to pediatrics, as all American Board of Medical Specialties' specialties have recently implemented MOC requirements.[13] Hospitals will likely adjust their credentialing policies as their familiarity and experience with MOC grows.
The primary purpose of the specialty certification process is to provide to the public, which includes both individual consumers and regulatory agencies, an assessment of the competency of individual physicians. Self‐regulation through certification is a privilege of trust granted to the medical profession by the public. This is an essential concept that underlies the concept of specialty certification.[14] As the public has continued to adopt a greater focus, and additional demands, on safety and quality assessment in healthcare, the medical profession must in turn be responsive.[13, 15, 16, 17] Failure in this regard would run the risk of losing that trust with the public, with the resultant loss of the ability to self‐regulate.
Studies have indicated a positive relationship between board certification and quality of care, yet this area remains hampered by a paucity of data.[18, 19, 20, 21, 22] Pham et al. found that board certified physicians were more likely to provide preventative care services to Medicare patients.[22] In 2008, Turchin et al. showed that recertification made a small, yet meaningful, difference in physician treatment of hypertension.[18] This area of research is especially important, as the MOC program is more comprehensive and utilizes an ongoing system of assessment and physician engagement. As such, it has been criticized by some for being complicated, burdensome, and irrelevant to the manner in which physicians actually practice.[23, 24] However, previous methods of certification were limited to assessing physicians at 1 point in time during their entire careers (eg, permanent certification) or at specific intervals (eg, time‐limited certification). With recent increased attention to improving the quality of patient care, these methods were unable to assure the public that physicians maintained their knowledge and skills over time in an environment of increasing rapid incorporation of new knowledge into clinical practice. Recent reports have also shown that (years of) practice does not make perfect with regard to physician performance. In fact, there may be deterioration of performance over long periods of practice.[25] Furthermore, although physicians commonly believe they are able to assess their own performance, available evidence does not support that contention.[26, 27] Thus, there is a need for an objective ongoing assessment of physician performance that also has the capacity to continuously improve the quality of care provided.
The comprehensive nature of the MOC program is a result of efforts to meaningfully incorporate the 6 competencies defined by the ACGME into the certification process. Although MOC is still relatively new and maturing, a growing body of evidence is demonstrating effectiveness of specific components of the program.[28, 29, 30, 31] In the field of pediatrics, several programs approved for MOC credit have already demonstrated their effectiveness in improving the quality of care in clinical practice.[32, 33, 34, 35, 36] However, additional efforts are needed to evaluate more of the part 4 (Assessment of Practice Performance) modules to assess their impact on patient care. The continued commitment to quality of care and quality improvement in hospitals will likely result in a further adoption of MOC requirements as the process matures and demonstrable impacts on patient outcomes are assessed. Furthermore, greater coordination of MOC with quality assessments in health plans and in the changes taking place in the process of licensure will likely help to streamline the paperwork and documentation burden placed on physicians by multiple assessment efforts.
This study has several limitations. Because the MOC program was initiated by the ABP in January 2010, there may be a lag in uptake of this particular requirement by hospitals. In some cases, this may have been the first time that members of the credentialing staff had considered the MOC program. It is probable that staff awareness will increase over time, as hospital policies are further developed and greater exposure to the specifics of the MOC program occurs. Additionally, although we compared stratified random samples of hospitals in 2005 and 2010, we did not follow the same group of hospitals over time.
As with all changes to the certification program over the years, there is a period of time required for new requirements to be understood and accepted by both those in regulatory positions and those in the medical profession. The demands of the public for increasingly comprehensive assessments of healthcare quality will continue into the future.
Acknowledgments
Disclosures: Funding was provided by a grant from the American Board of Pediatrics Foundation. The authors have no other disclosures or conflicts of interest to report.
- , , , , , . Policies and practices related to the role of board certification and recertification of pediatricians in hospital privileging. JAMA. 2006;295(8):905–912.
- American Board of Pediatrics. Maintenance of Certification: MOC requirements. 2011. Available at: https://www.abp.org/ABPWeb Static/#murl%3D%2FABPWebStatic%2Fmoc.html%26surl%3D%2 Fabpwebsite%2Fmoc%2Fphysicianrequirements%2Fphysreq.htm. Accessed May 23, 2011.
- , , , , , . Maintenance of licensure: protecting the public, promoting quality health care. J Med Regul. 2010;96(2):13–20.
- , , , , . Setting a fair performance standard for physicians' quality of patient care. J Gen Intern Med. 2011;26(5):467–473.
- , . Payer trend: “tiering” physicians and “steering” patients. Fam Pract Manag. 2007;14(10):24–26.
- , , , et al. Association between maintenance of certification examination scores and quality of care for medicare beneficiaries. Arch Intern Med. 2008;168(13):1396–1403.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/Maintenance_of_Certification/ABMS_MOC.aspx. Accessed January 23, 2012.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/maintenance_of_certification/MOC_competencies.aspx. Accessed January 24, 2012.
- , , . Hospital performance reports: impact on quality, market share, and reputation. Health Aff (Millwood). 2005;24(4):1150–1160.
- , , , et al. Impact of public reporting of coronary artery bypass graft surgery performance data on market share, mortality, and patient selection. Med Care. 2011;49(12):1118–1125.
- , , . Hospital strategies to engage physicians in quality improvement. Available at: www.hschange.org/CONTENT/1087. Accessed June 4, 2012.
- The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements of Self‐Nomination for 2012. http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instru ments/PQRS/downloads/2012_MaintenanceofCertificationProgram_ mmrvsd01162012.pdf. Accessed June 4, 2012.
- , , . Maintenance of Certification, maintenance of public trust. Plast Reconstr Surg. 2011;127(2):967–973.
- , . Credentialing and public accountability: a central role for board certification. JAMA. 2006;295(8):939–940.
- , , , . Perspectives and preferences among the general public regarding physician selection and board certification. J Pediatr. 2010;156(5):841–845, 845.e1.
- , . Public perceptions of quality care and provider profiling in New York: implications for improving quality care and public health. J Public Health Manag Pract. 2004;10(3):241–250.
- . Future of board certification in a new era of public accountability. J Am Board Fam Med. 2010;23(suppl 1):S32–S39.
- , , , , . Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation. 2008;117(5):623–628.
- , , , , . Physician board certification and the care and outcomes of elderly patients with acute myocardial infarction. J Gen Intern Med. 2006;21(3):238–244.
- , , . Certifying examination performance and patient outcomes following acute myocardial infarction. Med Educ. 2002;36(9):853–859.
- , , , , . Specialty board certification and clinical outcomes: the missing link. Acad Med. 2002;77(6):534–542.
- , , , . Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294(4):473–481.
- . Are you ready for maintenance of certification? Fam Pract Manag. 2005;12(1):42–48.
- , , , , . Clinical decisions. American Board of Internal Medicine maintenance of certification program. N Engl J Med. 2010;362(10):948–952.
- . As doctors age, worries about their abilities grow. New York Times. January 24, 2011:D.1.
- , , . Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260–273.
- , , , , , . Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094–1102.
- , , . The impact of a preventive cardiology quality improvement intervention on residents and clinics: a qualitative exploration. Am J Med Qual. 2009;24(2):99–107.
- , , , , , . Promoting physicians' self‐assessment and quality improvement: the ABIM diabetes practice improvement module. J Contin Educ Health Prof. 2006;26(2):109–119.
- , , , et al. Self‐assessment of practice performance: development of the ABIM Practice Improvement Module (PIM). J Contin Educ Health Prof. 2008;28(1):38–46.
- , , , , , . Variation in internal medicine residency clinic practices: assessing practice environments and quality of care. J Gen Intern Med. 2008;23(7):914–920.
- , , , et al. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444.
- , , , et al. ImproveCareNow: the development of a pediatric inflammatory bowel disease improvement network. Inflamm Bowel Dis. 2011;17(1):450–457.
- , . Pay for performance alone cannot drive quality. Arch Pediatr Adolesc Med. 2007;161(7):650–655.
- , , , et al. National pediatric cardiology quality improvement collaborative: lessons learned from development and early years. Prog Pediatr Cardiol. 2011;32(2):103–109.
- , , , et al. Reducing PICU central line‐associated bloodstream infections: 3‐year results. Pediatrics. 2011;128(5):e1077–e1083.
- , , , , , . Policies and practices related to the role of board certification and recertification of pediatricians in hospital privileging. JAMA. 2006;295(8):905–912.
- American Board of Pediatrics. Maintenance of Certification: MOC requirements. 2011. Available at: https://www.abp.org/ABPWeb Static/#murl%3D%2FABPWebStatic%2Fmoc.html%26surl%3D%2 Fabpwebsite%2Fmoc%2Fphysicianrequirements%2Fphysreq.htm. Accessed May 23, 2011.
- , , , , , . Maintenance of licensure: protecting the public, promoting quality health care. J Med Regul. 2010;96(2):13–20.
- , , , , . Setting a fair performance standard for physicians' quality of patient care. J Gen Intern Med. 2011;26(5):467–473.
- , . Payer trend: “tiering” physicians and “steering” patients. Fam Pract Manag. 2007;14(10):24–26.
- , , , et al. Association between maintenance of certification examination scores and quality of care for medicare beneficiaries. Arch Intern Med. 2008;168(13):1396–1403.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/Maintenance_of_Certification/ABMS_MOC.aspx. Accessed January 23, 2012.
- American Board of Medical Specialties. ABMS Maintenance of Certification. Available at: http://www.abms.org/maintenance_of_certification/MOC_competencies.aspx. Accessed January 24, 2012.
- , , . Hospital performance reports: impact on quality, market share, and reputation. Health Aff (Millwood). 2005;24(4):1150–1160.
- , , , et al. Impact of public reporting of coronary artery bypass graft surgery performance data on market share, mortality, and patient selection. Med Care. 2011;49(12):1118–1125.
- , , . Hospital strategies to engage physicians in quality improvement. Available at: www.hschange.org/CONTENT/1087. Accessed June 4, 2012.
- The Physician Quality Reporting System Maintenance of Certification Program Incentive Requirements of Self‐Nomination for 2012. http://www.cms.gov/Medicare/Quality‐Initiatives‐Patient‐Assessment‐Instru ments/PQRS/downloads/2012_MaintenanceofCertificationProgram_ mmrvsd01162012.pdf. Accessed June 4, 2012.
- , , . Maintenance of Certification, maintenance of public trust. Plast Reconstr Surg. 2011;127(2):967–973.
- , . Credentialing and public accountability: a central role for board certification. JAMA. 2006;295(8):939–940.
- , , , . Perspectives and preferences among the general public regarding physician selection and board certification. J Pediatr. 2010;156(5):841–845, 845.e1.
- , . Public perceptions of quality care and provider profiling in New York: implications for improving quality care and public health. J Public Health Manag Pract. 2004;10(3):241–250.
- . Future of board certification in a new era of public accountability. J Am Board Fam Med. 2010;23(suppl 1):S32–S39.
- , , , , . Effect of board certification on antihypertensive treatment intensification in patients with diabetes mellitus. Circulation. 2008;117(5):623–628.
- , , , , . Physician board certification and the care and outcomes of elderly patients with acute myocardial infarction. J Gen Intern Med. 2006;21(3):238–244.
- , , . Certifying examination performance and patient outcomes following acute myocardial infarction. Med Educ. 2002;36(9):853–859.
- , , , , . Specialty board certification and clinical outcomes: the missing link. Acad Med. 2002;77(6):534–542.
- , , , . Delivery of preventive services to older adults by primary care physicians. JAMA. 2005;294(4):473–481.
- . Are you ready for maintenance of certification? Fam Pract Manag. 2005;12(1):42–48.
- , , , , . Clinical decisions. American Board of Internal Medicine maintenance of certification program. N Engl J Med. 2010;362(10):948–952.
- . As doctors age, worries about their abilities grow. New York Times. January 24, 2011:D.1.
- , , . Systematic review: the relationship between clinical experience and quality of health care. Ann Intern Med. 2005;142(4):260–273.
- , , , , , . Accuracy of physician self‐assessment compared with observed measures of competence: a systematic review. JAMA. 2006;296(9):1094–1102.
- , , . The impact of a preventive cardiology quality improvement intervention on residents and clinics: a qualitative exploration. Am J Med Qual. 2009;24(2):99–107.
- , , , , , . Promoting physicians' self‐assessment and quality improvement: the ABIM diabetes practice improvement module. J Contin Educ Health Prof. 2006;26(2):109–119.
- , , , et al. Self‐assessment of practice performance: development of the ABIM Practice Improvement Module (PIM). J Contin Educ Health Prof. 2008;28(1):38–46.
- , , , , , . Variation in internal medicine residency clinic practices: assessing practice environments and quality of care. J Gen Intern Med. 2008;23(7):914–920.
- , , , et al. Statewide NICU central‐line‐associated bloodstream infection rates decline after bundles and checklists. Pediatrics. 2011;127(3):436–444.
- , , , et al. ImproveCareNow: the development of a pediatric inflammatory bowel disease improvement network. Inflamm Bowel Dis. 2011;17(1):450–457.
- , . Pay for performance alone cannot drive quality. Arch Pediatr Adolesc Med. 2007;161(7):650–655.
- , , , et al. National pediatric cardiology quality improvement collaborative: lessons learned from development and early years. Prog Pediatr Cardiol. 2011;32(2):103–109.
- , , , et al. Reducing PICU central line‐associated bloodstream infections: 3‐year results. Pediatrics. 2011;128(5):e1077–e1083.
Copyright © 2013 Society of Hospital Medicine
Improvement Is Independent of Dementia
Loss of functional independence is a serious complication of delirium,[1, 2] with functional consequences often persisting long after the index hospital admission.[3] Preexisting dementia is a major risk factor for delirium in hospitalized older patients,[4, 5] and the occurrence of delirium may alter the clinical course of an underlying dementia with negative prognostic implications, including further functional and cognitive decline,[3] increased rehospitalization rates,[6] institutionalization,[3] and death.[7] Despite the adverse functional outcomes of delirium, there remains a scarcity of well‐designed intervention trials for the rehabilitation of older patients recovering from delirium.
Studies investigating the influence of cognitive impairment on rehabilitation outcomes have yielded conflicting results. Landi and colleagues identified cognitive impairment as a negative predictor of functional recovery among older patients in a rehabilitation unit.[8] Yet, other studies had reported functional improvements with rehabilitation regardless of cognition.[9, 10, 11] However, it is not possible to determine if cognitive impairment in the earlier studies had been consequent to delirium, dementia, or both. Although there has been emerging evidence for the impact of delirium on disease trajectory among patients with dementia, it is less clear whether interventions for delirium prevention and management will yield comparable outcomes with the presence of preexisting dementia.
The geriatric monitoring unit (GMU) is a specialized 5‐bed unit developed for the care of delirious older patients and is modeled after the delirium room,[12] with adoption of core interventions from the Hospital Elder Life Program[13] and use of evening bright light therapy to consolidate circadian rhythm and improve sleep in older inpatients.[14] The core interventions in this multicomponent delirium management program focused on early mobilization and rehabilitation, occurring concurrently with medical management, to address all precipitating and predisposing factors for delirium.[15] As functional decline in an older patient can develop as early as 2 days into a hospital admission,[16] early intervention once the patient has been admitted is essential to prevent the cascade to irreversible functional loss. Hence, we sought to determine the functional progress of delirious older patients exposed to a multicomponent delirium management program and whether the presence of underlying dementia impacted the functional recovery of older patients with delirium. The secondary objective was to identify predictors of functional recovery in older hospitalized patients with delirium.
METHODS
Setting and Participants
This prospective cohort study recruited patients who had been admitted to the GMU of Tan Tock Seng Hospital, Singapore, during the period of November 2010 to November 2011. The admission criteria for the GMU included patients aged 65 years and older who were admitted to the geriatric medicine department and assessed to have delirium, either on admission to the general ward or incident delirium that developed during the hospital stay. The diagnosis of delirium was established in accordance with the Confusion Assessment Method (CAM) diagnostic criteria.[17] Patients were excluded if they met any of the following criteria: (1) presence of medical illnesses that required special monitoring (eg, telemetry for arrhythmias or acute myocardial infarction); (2) critically ill, in coma, or with terminal illness; (3) uncommunicative or with severe aphasia; (4) severely combative behavior with high risk of harm to self, staff, or other patients; (5) contraindications to bright light therapy (manic disorders, severe eye disorders, photosensitive skin disorders, or use of photosensitizing medications); (6) being on respiratory or contact precautions; and (7) refusal of GMU admission by patient, family, or physician‐in‐charge.[15]
The core interventions adopted in the GMU facilitate early mobilization through strict avoidance of mechanical restraints (and refraining from pharmacological restraints where possible), encouraging patients to mobilize early with the support of therapists and trained nurses, and daily review of the continued need for intravenous drip, urinary catheter, or supplemental oxygen to minimize immobilizing equipment. There is a strong emphasis on rehabilitation as part of the multicomponent delirium program; patients participate in daily orientation 3 times a day by a trained nurse using a reality orientation board, engage in therapeutic activities 3 times a day for cognitive stimulation and socialization, and attend daily physiotherapy and occupational therapy sessions. Additionally, we actively seek to correct any sensory impairment with visual aids (such as eye glasses), earwax disimpaction where necessary, and provision of hearing aids or portable audio amplifier. Nonpharmacological measures implemented to improve sleep at night for delirious older patients include evening bright light therapy and a sleep enhancement protocol of warm milk and relaxation music. These interventions are practiced for all patients through their stay in the GMU, with compliance ensured via a structured protocol in the daily nursing workflow and documentation sheet.
All patients fulfilling CAM criteria for delirium and admitted to the GMU were eligible for this study. However, patients who were prematurely transferred out of the GMU (for reasons such as instability of medical conditions requiring intensive monitoring or patients requiring contact precautions) were excluded from subsequent analysis. Patients with repeated GMU admissions had only their first admission included for analysis.
Ethics approval for conduct of this study was obtained from the National Healthcare Group Domain Specific Review Board.
Assessments
All patients underwent a detailed cognitive evaluation by the consultant geriatrician on admission to the GMU. A family member or other designated caregiver was routinely interviewed to establish the patient's baseline cognitive functioning prior to the current admission. The medical records of all patients were reviewed to ascertain whether a diagnosis of dementia had been previously established. In patients yet to be diagnosed, a diagnosis of dementia was made in the current admission if the corroborative history suggested presence of cognitive symptoms consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria for dementia[18] of at least 6 months' duration, in accordance with the standardized process for cognitive evaluation.[19]
Delirium subtypehyperactive, hypoactive, or mixed deliriumwas determined by the consultant geriatrician at admission to the GMU based on clinical assessment of the patient's mental state and behavior. All patients underwent baseline and daily cognitive status assessment using the locally validated 10‐point Abbreviated Mental Test (AMT) and 28‐point Chinese Mini‐Mental State Examination (CMMSE), with higher scores reflective of better cognitive performance.[20] Delirium severity was assessed daily and scored on the Delirium Rating Scale‐98 (DRS‐sev, maximum severity score of 39 points)[21] and CAM severity (CAM‐sev).[17] The cognitive tests and delirium severity scoring were administered by a trained assessor from the time of admission to the GMU until patient's discharge from the GMU. A comprehensive history taking (including medication reconciliation), detailed physical examination, and review of all laboratory/emmaging investigations were performed routinely at admission to identify all potential precipitating factors for delirium, and the Charlson's co‐morbidity[22] and modified Severity of Illness Index scored.[23] The modified Barthel Index (MBI),[24] which measures activities of daily living (ADL), was used to monitor functional progress from time of admission until discharge from the GMU, and was rated by an occupational therapist observing the patient at ADL tasks. A patient was deemed to have recovered from delirium if the CAM criteria for delirium was no longer met, with diagnosis of recovery being supported by improvement in cognitive and/or delirium severity scores as well as input from the multidisciplinary team. Patients were discharged from the GMU once assessed to have recovered from delirium, but may continue inpatient treatment in a general ward for other outstanding medical issues (such as continuation of intravenous antibiotics) or while awaiting transfer to a post‐acute care facility for continued rehabilitation.
Primary Outcome
The primary outcome was recovery of physical function and the difference in total MBI score of each patient at the GMU discharge from that at the GMU admission provided an estimate of the extent of functional recovery achieved. To define clinically meaningful functional improvement, we categorized MBI scores into the following: (1) total MBI score 0 to 20 indicates total dependence, (2) 21 to 60 severe dependence, (3) 61 to 90 moderate dependence, (4) 91 to 99 slight dependence, and (5) 100 full independence.[24] The total MBI gain at discharge was considered clinically meaningful if accompanied by patient transcending into a less dependent category.
Statistical Analysis
Summary measures of baseline characteristics are presented as means ( standard deviations) and proportions. The difference between admission and discharge AMT, CMMSE, and MBI scores was calculated for each patient and represented the extent of cognitive and functional recovery achieved at the time of discharge from the GMU. Paired sample t test was used to evaluate differences between admission and discharge cognitive and functional scores, as well as changes in delirium severity as measured on CAM‐sev and DRS‐sev, for the entire cohort of GMU patients.
To determine whether preexisting dementia impacts on cognitive and functional recovery of delirious patients, independent sample t test was performed to compare mean changes in AMT, CMMSE, and MBI scores for the demented vs nondemented groups. The proportion of patients achieving clinically meaningful MBI gain in each group was compared using Pearson 2 test.
To identify predictors of functional recovery, univariate analyses were first performed to examine the relationship between predictor variables and MBI gain. The candidate predictors were defined a priori and included (1) age, (2) gender, (3) Charlson's comorbidity score, (4) Severity of Illness Index, (5) number of precipitating causes of delirium (categoricalsingle, 2 or >2 precipitating causes), (6) presentation of delirium (categoricalhypoactive, hyperactive, mixed), (7) delirium severity on admission (CAM‐sev and DRS‐sev scores), (8) duration of delirium, and (9) presence of underlying dementia. Predictors with a univariate P value <0.20 were entered into a multiple linear regression model, with the dependent variable being change in MBI score. Only significant predictors (P0.05) were retained in the final model. All models controlled for admission MBI score.
Statistical analyses were performed using SPSS software (version 16.0; IBM, Armonk, NY). All statistical tests were 2‐tailed, with P value0.05 considered statistically significant.
RESULTS
Patient Characteristics
One hundred forty‐six elderly patients with delirium were admitted to the GMU during the 1‐year study period. One hundred twenty‐two patients were analyzed after excluding 24 patients (17 patients [mean age 83.28.7 years, 47.1% females] were transferred out prematurely due to infection control precautions or were critically ill requiring intensive monitoring; 7 were repeat admissions). The mean age of patients admitted to the GMU was 84.17.6 years, with a predominance of females (60.7%). Most patients presented with either hyperactive (49.2%) or mixed (35.2%) delirium, with hypoactive delirium being the least common presentation (15.6%). Sepsis, notably urinary tract infection or pneumonia, was the predominant principal precipitating cause, contributing to 68.0% of delirium cases within the GMU. At admission, the GMU cohort had a mean CMMSE score of 5.305.53, and mean MBI score of 31.6126.61. Eighty‐two patients (67.2%) had delirium superimposed on dementia, whereas 40 patients (32.8%) did not have underlying dementia.
Baseline characteristics of patients with and without underlying dementia are shown in Table 1. There were no significant differences in age, gender, ethnicity, and illness severity between groups with and without dementia, although patients with dementia had higher comorbidity (Charlson's comorbidity score 2.27 vs 1.75, P=0.054). The presentation and severity of delirium at admission to the GMU was similar in both groups.
| Underlying Dementia | P Value | ||
|---|---|---|---|
| Absent (n=40) | Present (n=82) | ||
| |||
| Demographics | |||
| Age, meanSD, y | 84.08.1 | 84.27.4 | 0.88 |
| Male gender, n (%) | 18 (45.0) | 29 (35.4) | 0.45 |
| Chinese, n (%) | 34 (85.0) | 70 (85.4) | 0.67 |
| Presentation of delirium | 0.99 | ||
| Hyperactive, n (%) | 20 (50.0) | 40 (48.8) | |
| Hypoactive, n (%) | 6 (15.0) | 13 (15.9) | |
| Mixed, n (%) | 14 (35.0) | 29 (35.4) | |
| Delirium severity on admissiona | |||
| CAM‐sev, meanSD | 5.231.17 | 4.74 1.47 | 0.07 |
| DRS‐sev, meanSD | 27.306.37 | 26.326.70 | 0.43 |
| Cognitive status on admissiona | |||
| AMT, meanSD | 2.051.97 | 1.682.22 | 0.38 |
| CMMSE, meanSD | 5.185.13 | 5.355.75 | 0.87 |
| Functional status on admission, MBI score, meanSDa | 29.4825.90 | 32.6627.04 | 0.54 |
| Comorbidities | |||
| Charlson score, meanSD | 1.751.63 | 2.271.25 | 0.054 |
| Severity of Illness, meanSD | 2.000.32 | 2.100.40 | 0.15 |
| Precipitating causes of delirium | |||
| Number of precipitants, n (%) | 0.41 | ||
| Single precipitating cause | 12 (30.0) | 31 (37.8) | |
| 2 precipitating causes | 12 (30.0) | 28 (34.1) | |
| >2 precipitating causes | 16 (40.0) | 23 (28.1) | |
| Principal precipitating cause, n (%) | 0.050 | ||
| UTI | 17 (42.5) | 27 (32.9) | |
| Pneumonia | 3 (7.5) | 23 (28.0) | |
| Combined UTI and pneumonia or sepsis from >1 source | 3 (7.5) | 9 (11.0) | |
| Stroke | 0 (0) | 3 (3.7) | |
| Biochemical abnormalities | 2 (5.0) | 4 (4.9) | |
| Intracranial hemorrhage | 3 (7.5) | 1(1.2) | |
| Fracture | 3 (7.5) | 2 (2.4) | |
| Postoperative state | 2 (5.0) | 3 (3.7) | |
| Others | 7 (17.5) | 10 (12.2) | |
Patients without underlying dementia more often required multiple insults to precipitate delirium compared with patients with dementia, although this difference did not fulfill statistical significance. Although sepsis remained the primary precipitating cause of delirium in patients with and without dementia, urinary tract infection was more common among patients without dementia, whereas pneumonia was a more common precipitant in patients with dementia (P=0.050). The cognitive performance and functional status were similar at GMU admission for both groups.
Cognitive and Functional Outcomes on Discharge
The average duration of delirium for the GMU cohort was 8.2 days, with the mean length of total hospital stay being 17.0 days. The length of GMU stay for each patient was equivalent to the duration of delirium as patients were transferred out of the GMU once assessed to have recovered from delirium. Significant cognitive improvement was observed with recovery from delirium, with AMT and CMMSE scores improving by a mean of 1.442.38 and 3.545.61, respectively (paired t test, P<0.001). Patients demonstrated significant functional recovery at discharge from the GMU compared with their functional performance at admission to the GMU, with a mean MBI gain of 19.4217.11 (P<0.001), and 59 patients (48.4%) had progressed to a less‐dependent category.
Table 2 compares the cognitive and functional progress of patients with and without dementia. There was no difference in duration of delirium or length of total hospital stay between the demented and nondemented groups. Within‐group comparison showed patients with and without dementia managing significant improvement in cognitive scores at GMU discharge compared with GMU admission, although the magnitude of improvement was greater for nondemented patients (CMMSE improvement +6.73 vs +1.99, P<0.001). The mean MBI gain at GMU discharge compared with GMU admission was 20.4316.99 (P<0.001) for patients with dementia and 17.3517.39 (P<0.001) for patients without underlying dementia. There was no significant difference in the extent of functional improvement achieved between the demented and nondemented groups. Nineteen patients (47.5%) without dementia and 40 patients (48.8%) with preexisting dementia were in a less‐dependent category at GMU discharge compared with GMU admission.
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Duration of delirium, meanSD, d | 8.2 | 7.47.6 | 8.65.7 | 0.35 |
| Length of hospital stay, meanSD, da | 17.0 | 18.69.7 | 16.38.7 | 0.18 |
| Cognitive scores on dischargeb | ||||
| AMT, mean (SD) | 3.25 (3.00) | 5.20 (2.88) | 2.29 (2.58) | <0.001 |
| CMMSE, mean (SD) | 8.84 (6.81) | 11.90 (6.16) | 7.34 (6.64) | <0.001 |
| Improvement in cognitive scores | ||||
| AMT, mean (SD)c | +1.44 (2.38) | +3.15 (2.68) | +0.61 (1.70) | <0.001 |
| CMMSE, mean (SD)c | +3.54 (5.61) | +6.73 (5.74) | +1.99 (4.87) | <0.001 |
| Delirium severity on discharge | ||||
| CAM‐sev, mean (SD) | 2.43 (1.44) | 2.15 (1.46) | 2.57 (1.42) | 0.13 |
| DRS‐sev, mean (SD) | 16.83 (6.97) | 14.45 (6.90) | 18.00 (6.74) | 0.008 |
| Change in delirium severity scores | ||||
| CAM‐sev, mean (SD)c | 2.47 (1.73) | 3.08 (1.67) | 2.17 (1.68) | 0.006 |
| DRS‐sev, mean (SD)c | 9.72 (7.30) | 12.85 (6.43) | 8.17 (7.25) | 0.001 |
| Functional status | ||||
| MBI discharge, mean (SD) | 51.03 (26.20) | 46.83 (24.09) | 53.09 (27.07) | 0.22 |
| MBI, mean (SD)c | +19.42 (17.11) | +17.35 (17.39) | +20.43 (16.99) | 0.35 |
| Progress to less‐dependent category at discharge, n (%)b | 59 (48.4%) | 19 (47.5%) | 40 (48.8%) | 1.00 |
Seventy‐nine patients (64.8% of the GMU cohort) were discharged back to their own home following the index hospitalization, 22 patients (18.0%) required further rehabilitation in a community hospital or subacute ward before returning home, and 19 patients (15.6%) were admitted to long‐term care. There was no significant difference in discharge destination between patients with and without dementia (Table 3).
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Discharge destination, n (%) | 0.14 | |||
| Own home | 79 (64.8) | 24 (60.0) | 55 (67.9) | |
| Community hospital or subacute ward (with eventual discharge home after rehabilitation) | 22 (18.0) | 10 (25.0) | 12 (14.6) | |
| Long‐term care | 19 (15.6) | 6 (15.0) | 13 (16.0) | |
| Inpatient hospice | 1 (0.8) | 0 (0) | 1 (1.2) | |
Predictors of Functional Recovery on Discharge
Age, gender, number of precipitating causes, severity of illness, and type of delirium had a univariate P value <0.20 and were entered into the multiple regression model. After controlling for admission MBI score in multivariate analysis, gender, presentation of delirium, and severity of illness remained independent factors for improvement in MBI score (Table 4). Female patients exhibited greater functional recovery than males. Hypoactive delirium was associated with the worst prognosis for functional recovery, with improvement in MBI score being 14.47 points lower than that achieved by patients with hyperactive delirium. Severity of illness at admission was another negative predictor of functional recovery, with each unit increase in Severity of Illness Index (higher score indicating more severe illness) associated with 10.59 points lower gain in MBI.
| Predictor | Model 1 | Final Model | ||
|---|---|---|---|---|
| B | P Value | B | P Value | |
| ||||
| Age | 0.245 | 0.184 | ||
| Female (vs male) | 6.421 | 0.023 | 7.393 | 0.009 |
| Number of precipitating causes | 2.044 | 0.229 | ||
| Hypoactive delirium (vs hyperactive delirium) | 15.383 | <0.001 | 14.472 | 0.001 |
| Severity of illness | 10.596 | 0.003 | 10.591 | 0.003 |
DISCUSSION
Our results support a delirium management unit focused on geriatric nursing care and rehabilitation in promoting positive cognitive and functional gains in older patients with delirium. In addition, we have documented that patients with dementia and recovering from delirium have comparable potential for functional recovery as their cognitively intact counterparts, despite there being less improvement in cognitive test scores and higher comorbidity compared with patients without dementia.
Persistent delirium and failure to recognize the condition have been associated with poor functional recovery.[25, 26] Early recognition of delirium and actively addressing all predisposing and precipitating factors, along with emphasis on rehabilitation in a multidisciplinary unit, appear to be important factors contributing to the positive functional outcomes in our patients. In addition, none of the patients admitted to the GMU had been subject to physical restraint, and this, along with the higher nursing ratio, facilitated early mobilization essential to prevent functional decline. The overall length of hospital stay, averaging 17.0 days, compared favorably with a pre‐GMU cohort, where the average length of hospital stay for delirious patients was 20.9 days. The length of stay in an acute hospital ward included waiting time for transfer to appropriate postacute facility (community hospital, subacute ward, or nursing home).
In a study of older hospital inpatients, delirium independent of dementia was predictive of sustained poor cognitive and functional status in the year following hospitalization.[3] Thus, although delirium has been generally regarded as a transient and reversible condition, it is also increasingly recognized that recovery may not always be complete. This is a likely explanation for the low cognitive test scores at discharge from the GMU, albeit improved compared with admission, even among patients without dementia. The present study lacks data on longer‐term cognitive outcomes following delirium resolution. However, delirium is reportedly a risk factor for subsequent development of dementia,[27] and contributes to accelerated cognitive decline in patients with existing dementia.[28]
Despite reports of adverse outcomes of delirium superimposed on dementia, few intervention studies have specifically examined the management of delirium in older adults with dementia. In our study, preexisting dementia did not preclude delirious patients from functional improvement, adding to the still‐limited literature reporting positive rehabilitation outcomes in patients with cognitive impairment.[10, 29] In addition, patients with dementia did not take longer to recover from delirium than those without dementia, nor did they appear to require a longer duration for making similar functional gains given that length of hospital stay did not differ between the 2 groups. Illness severity and presentation of delirium, rather than preexisting dementia, predicted poor functional recovery. The complex etiology of delirium, representing an interaction between baseline patient vulnerability (predisposing factors) and exposure to noxious insults or precipitating factors, suggests that highly vulnerable patients (eg, dementia) may develop delirium with a relatively innocuous insult, as opposed to the multiple noxious insults anticipated in nonvulnerable patients.[30] Although Severity of Illness Index was similar, patients without dementia had a trend for more than 1 precipitating factor, and serious medical conditions, such as intracranial hemorrhage, fracture, and need for surgical intervention were more likely to be present, factors that can expectedly influence functional recovery. Patients with hypoactive delirium had the worst functional outcome, consistent with previously observed poor prognosis in hypoactive delirium.[31, 32] Our findings, if corroborated by future studies, may offer a means to attenuate the trajectory of functional decline observed in patients with delirium superimposed on dementia. This will be of particular relevance as earlier studies had observed a high risk of institutionalization in patients with delirium superimposed on dementia compared with dementia alone.[3, 33]
One limitation of this study was the failure to adjust for premorbid functional status due to the lack of information on baseline function prior to the current hospital admission. Even though we demonstrated functional improvement at discharge compared with admitting functional status, we were not able to ascertain if the patients had returned to their premorbid level of cognitive and physical functioning when discharged from the GMU. This study was also limited by its observational nature, the small sample size, and short‐term outcomes. However, the beneficial impact of the GMU on functional improvement in patients with delirium was supported by observed higher MBI gain compared with a pre‐GMU cohort (mean MBI gain, 7.511.2; discharge MBI, 45.832.9). Data collection for 6‐month and 1‐year outcomes is presently in progress to determine sustainability of any improvement achieved. Our exclusion criteria limits generalizability of the benefits of the GMU to patients considered medically unstable, in whom delirium is prevalent. However, such patients are likely too ill to participate in the intense rehabilitation practiced in the GMU.
We have shown that patients with delirium can engage and benefit from a rehabilitation program, their low cognitive test scores at admission notwithstanding, suggesting that early initiation of rehabilitation should be encouraged regardless of the cognitive and functional performance at presentation. In addition, we found that patients with delirium superimposed on dementia benefit from a multicomponent delirium treatment program to the same extent as delirious patients without dementia, which may contribute to improved quality of life and reduction in burden of care. As delirium is a complex medical problem with multiple predisposing and precipitating factors, a multicomponent intervention program as practiced in the GMU is likely to yield more consistent outcomes than a single intervention strategy. With the widely reported poor prognostic significance of delirium superimposed on dementia, more well‐designed controlled trials are urgently needed to identify intervention strategies that will aid the management of this group of hospitalized elders.
Acknowledgments
Disclosures: This work was supported by FY2010 Ministry of Health Quality Improvement Funding (MOH HQIF) Optimising Acute Delirium Care in Tan Tock Seng Hospital (Reference: HQIF 2010/17). The authors report no conflicts of interest.
- , , , et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–M186.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , , . Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–583.
- , , , . Delirium risk factors in elderly hospitalized patients. J General Intern Med. 1998;13:204–212.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–1732.
- , . Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Predictors of rehabilitation outcomes in frail patients treated in a geriatric hospital. J Am Geriatr Soc. 2002;50:679–684.
- , , , , . Effect of cognitive impairment on rehabilitation outcome. Am J Phys Med Rehabil. 1996;75:40–43.
- , , , , . Rehabilitation outcomes in cognitively impaired patients admitted to skilled nursing facilities from the community. Arch Phys Med Rehabil. 2004;85:1602–1607.
- , , , . Does cognitive impairment affect rehabilitation outcome? J Am Geriatr Soc. 2011;59:2108–2111.
- , , , , , . A model for managing delirious older inpatients. J Am Geriatr Soc. 2003;51:1031–1035.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Light therapy for insomnia in older adults. Clin Geriatr Med. 2008;24(1):139–149.
- , , , , , . A new model of delirium care in the acute geriatric setting: geriatric monitoring unit. BMC Geriatr. 2011;11:41.
- , , , , . The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303.
- , , , , , . Clarifying confusion: The Confusion Assessment Method. A new method for detecting delirium. Ann Intern Med. 1990;113:941–948.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
- , . An evidence‐based clinical approach to the diagnosis of dementia. Ann Acad Med Singapore. 2003;32:740–748.
- , , , . Diagnostic performance of two mental status tests in the older Chinese: influence of education and age on cut‐off values. Int J Geriatr Psychiatry. 2000;15:234–241.
- , , , , , . Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242.
- , , , . A new method of classifying prognositic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39(11):830–836.
- , , . Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709.
- , , . Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatr. 2005;5:5.
- , , , , , . Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–208.
- , , , , . The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575.
- , , , , . Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321:1107–1111.
- . Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14(4):745–764.
- , . Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28:115–119.
- , , , , , . Severity and course of delirium in medically hospitalised nursing facility residents. Am J Geriatr Psychiatry. 2001;9:72–77.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. A meta‐analysis. JAMA. 2010;304(4):443–451.
Loss of functional independence is a serious complication of delirium,[1, 2] with functional consequences often persisting long after the index hospital admission.[3] Preexisting dementia is a major risk factor for delirium in hospitalized older patients,[4, 5] and the occurrence of delirium may alter the clinical course of an underlying dementia with negative prognostic implications, including further functional and cognitive decline,[3] increased rehospitalization rates,[6] institutionalization,[3] and death.[7] Despite the adverse functional outcomes of delirium, there remains a scarcity of well‐designed intervention trials for the rehabilitation of older patients recovering from delirium.
Studies investigating the influence of cognitive impairment on rehabilitation outcomes have yielded conflicting results. Landi and colleagues identified cognitive impairment as a negative predictor of functional recovery among older patients in a rehabilitation unit.[8] Yet, other studies had reported functional improvements with rehabilitation regardless of cognition.[9, 10, 11] However, it is not possible to determine if cognitive impairment in the earlier studies had been consequent to delirium, dementia, or both. Although there has been emerging evidence for the impact of delirium on disease trajectory among patients with dementia, it is less clear whether interventions for delirium prevention and management will yield comparable outcomes with the presence of preexisting dementia.
The geriatric monitoring unit (GMU) is a specialized 5‐bed unit developed for the care of delirious older patients and is modeled after the delirium room,[12] with adoption of core interventions from the Hospital Elder Life Program[13] and use of evening bright light therapy to consolidate circadian rhythm and improve sleep in older inpatients.[14] The core interventions in this multicomponent delirium management program focused on early mobilization and rehabilitation, occurring concurrently with medical management, to address all precipitating and predisposing factors for delirium.[15] As functional decline in an older patient can develop as early as 2 days into a hospital admission,[16] early intervention once the patient has been admitted is essential to prevent the cascade to irreversible functional loss. Hence, we sought to determine the functional progress of delirious older patients exposed to a multicomponent delirium management program and whether the presence of underlying dementia impacted the functional recovery of older patients with delirium. The secondary objective was to identify predictors of functional recovery in older hospitalized patients with delirium.
METHODS
Setting and Participants
This prospective cohort study recruited patients who had been admitted to the GMU of Tan Tock Seng Hospital, Singapore, during the period of November 2010 to November 2011. The admission criteria for the GMU included patients aged 65 years and older who were admitted to the geriatric medicine department and assessed to have delirium, either on admission to the general ward or incident delirium that developed during the hospital stay. The diagnosis of delirium was established in accordance with the Confusion Assessment Method (CAM) diagnostic criteria.[17] Patients were excluded if they met any of the following criteria: (1) presence of medical illnesses that required special monitoring (eg, telemetry for arrhythmias or acute myocardial infarction); (2) critically ill, in coma, or with terminal illness; (3) uncommunicative or with severe aphasia; (4) severely combative behavior with high risk of harm to self, staff, or other patients; (5) contraindications to bright light therapy (manic disorders, severe eye disorders, photosensitive skin disorders, or use of photosensitizing medications); (6) being on respiratory or contact precautions; and (7) refusal of GMU admission by patient, family, or physician‐in‐charge.[15]
The core interventions adopted in the GMU facilitate early mobilization through strict avoidance of mechanical restraints (and refraining from pharmacological restraints where possible), encouraging patients to mobilize early with the support of therapists and trained nurses, and daily review of the continued need for intravenous drip, urinary catheter, or supplemental oxygen to minimize immobilizing equipment. There is a strong emphasis on rehabilitation as part of the multicomponent delirium program; patients participate in daily orientation 3 times a day by a trained nurse using a reality orientation board, engage in therapeutic activities 3 times a day for cognitive stimulation and socialization, and attend daily physiotherapy and occupational therapy sessions. Additionally, we actively seek to correct any sensory impairment with visual aids (such as eye glasses), earwax disimpaction where necessary, and provision of hearing aids or portable audio amplifier. Nonpharmacological measures implemented to improve sleep at night for delirious older patients include evening bright light therapy and a sleep enhancement protocol of warm milk and relaxation music. These interventions are practiced for all patients through their stay in the GMU, with compliance ensured via a structured protocol in the daily nursing workflow and documentation sheet.
All patients fulfilling CAM criteria for delirium and admitted to the GMU were eligible for this study. However, patients who were prematurely transferred out of the GMU (for reasons such as instability of medical conditions requiring intensive monitoring or patients requiring contact precautions) were excluded from subsequent analysis. Patients with repeated GMU admissions had only their first admission included for analysis.
Ethics approval for conduct of this study was obtained from the National Healthcare Group Domain Specific Review Board.
Assessments
All patients underwent a detailed cognitive evaluation by the consultant geriatrician on admission to the GMU. A family member or other designated caregiver was routinely interviewed to establish the patient's baseline cognitive functioning prior to the current admission. The medical records of all patients were reviewed to ascertain whether a diagnosis of dementia had been previously established. In patients yet to be diagnosed, a diagnosis of dementia was made in the current admission if the corroborative history suggested presence of cognitive symptoms consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria for dementia[18] of at least 6 months' duration, in accordance with the standardized process for cognitive evaluation.[19]
Delirium subtypehyperactive, hypoactive, or mixed deliriumwas determined by the consultant geriatrician at admission to the GMU based on clinical assessment of the patient's mental state and behavior. All patients underwent baseline and daily cognitive status assessment using the locally validated 10‐point Abbreviated Mental Test (AMT) and 28‐point Chinese Mini‐Mental State Examination (CMMSE), with higher scores reflective of better cognitive performance.[20] Delirium severity was assessed daily and scored on the Delirium Rating Scale‐98 (DRS‐sev, maximum severity score of 39 points)[21] and CAM severity (CAM‐sev).[17] The cognitive tests and delirium severity scoring were administered by a trained assessor from the time of admission to the GMU until patient's discharge from the GMU. A comprehensive history taking (including medication reconciliation), detailed physical examination, and review of all laboratory/emmaging investigations were performed routinely at admission to identify all potential precipitating factors for delirium, and the Charlson's co‐morbidity[22] and modified Severity of Illness Index scored.[23] The modified Barthel Index (MBI),[24] which measures activities of daily living (ADL), was used to monitor functional progress from time of admission until discharge from the GMU, and was rated by an occupational therapist observing the patient at ADL tasks. A patient was deemed to have recovered from delirium if the CAM criteria for delirium was no longer met, with diagnosis of recovery being supported by improvement in cognitive and/or delirium severity scores as well as input from the multidisciplinary team. Patients were discharged from the GMU once assessed to have recovered from delirium, but may continue inpatient treatment in a general ward for other outstanding medical issues (such as continuation of intravenous antibiotics) or while awaiting transfer to a post‐acute care facility for continued rehabilitation.
Primary Outcome
The primary outcome was recovery of physical function and the difference in total MBI score of each patient at the GMU discharge from that at the GMU admission provided an estimate of the extent of functional recovery achieved. To define clinically meaningful functional improvement, we categorized MBI scores into the following: (1) total MBI score 0 to 20 indicates total dependence, (2) 21 to 60 severe dependence, (3) 61 to 90 moderate dependence, (4) 91 to 99 slight dependence, and (5) 100 full independence.[24] The total MBI gain at discharge was considered clinically meaningful if accompanied by patient transcending into a less dependent category.
Statistical Analysis
Summary measures of baseline characteristics are presented as means ( standard deviations) and proportions. The difference between admission and discharge AMT, CMMSE, and MBI scores was calculated for each patient and represented the extent of cognitive and functional recovery achieved at the time of discharge from the GMU. Paired sample t test was used to evaluate differences between admission and discharge cognitive and functional scores, as well as changes in delirium severity as measured on CAM‐sev and DRS‐sev, for the entire cohort of GMU patients.
To determine whether preexisting dementia impacts on cognitive and functional recovery of delirious patients, independent sample t test was performed to compare mean changes in AMT, CMMSE, and MBI scores for the demented vs nondemented groups. The proportion of patients achieving clinically meaningful MBI gain in each group was compared using Pearson 2 test.
To identify predictors of functional recovery, univariate analyses were first performed to examine the relationship between predictor variables and MBI gain. The candidate predictors were defined a priori and included (1) age, (2) gender, (3) Charlson's comorbidity score, (4) Severity of Illness Index, (5) number of precipitating causes of delirium (categoricalsingle, 2 or >2 precipitating causes), (6) presentation of delirium (categoricalhypoactive, hyperactive, mixed), (7) delirium severity on admission (CAM‐sev and DRS‐sev scores), (8) duration of delirium, and (9) presence of underlying dementia. Predictors with a univariate P value <0.20 were entered into a multiple linear regression model, with the dependent variable being change in MBI score. Only significant predictors (P0.05) were retained in the final model. All models controlled for admission MBI score.
Statistical analyses were performed using SPSS software (version 16.0; IBM, Armonk, NY). All statistical tests were 2‐tailed, with P value0.05 considered statistically significant.
RESULTS
Patient Characteristics
One hundred forty‐six elderly patients with delirium were admitted to the GMU during the 1‐year study period. One hundred twenty‐two patients were analyzed after excluding 24 patients (17 patients [mean age 83.28.7 years, 47.1% females] were transferred out prematurely due to infection control precautions or were critically ill requiring intensive monitoring; 7 were repeat admissions). The mean age of patients admitted to the GMU was 84.17.6 years, with a predominance of females (60.7%). Most patients presented with either hyperactive (49.2%) or mixed (35.2%) delirium, with hypoactive delirium being the least common presentation (15.6%). Sepsis, notably urinary tract infection or pneumonia, was the predominant principal precipitating cause, contributing to 68.0% of delirium cases within the GMU. At admission, the GMU cohort had a mean CMMSE score of 5.305.53, and mean MBI score of 31.6126.61. Eighty‐two patients (67.2%) had delirium superimposed on dementia, whereas 40 patients (32.8%) did not have underlying dementia.
Baseline characteristics of patients with and without underlying dementia are shown in Table 1. There were no significant differences in age, gender, ethnicity, and illness severity between groups with and without dementia, although patients with dementia had higher comorbidity (Charlson's comorbidity score 2.27 vs 1.75, P=0.054). The presentation and severity of delirium at admission to the GMU was similar in both groups.
| Underlying Dementia | P Value | ||
|---|---|---|---|
| Absent (n=40) | Present (n=82) | ||
| |||
| Demographics | |||
| Age, meanSD, y | 84.08.1 | 84.27.4 | 0.88 |
| Male gender, n (%) | 18 (45.0) | 29 (35.4) | 0.45 |
| Chinese, n (%) | 34 (85.0) | 70 (85.4) | 0.67 |
| Presentation of delirium | 0.99 | ||
| Hyperactive, n (%) | 20 (50.0) | 40 (48.8) | |
| Hypoactive, n (%) | 6 (15.0) | 13 (15.9) | |
| Mixed, n (%) | 14 (35.0) | 29 (35.4) | |
| Delirium severity on admissiona | |||
| CAM‐sev, meanSD | 5.231.17 | 4.74 1.47 | 0.07 |
| DRS‐sev, meanSD | 27.306.37 | 26.326.70 | 0.43 |
| Cognitive status on admissiona | |||
| AMT, meanSD | 2.051.97 | 1.682.22 | 0.38 |
| CMMSE, meanSD | 5.185.13 | 5.355.75 | 0.87 |
| Functional status on admission, MBI score, meanSDa | 29.4825.90 | 32.6627.04 | 0.54 |
| Comorbidities | |||
| Charlson score, meanSD | 1.751.63 | 2.271.25 | 0.054 |
| Severity of Illness, meanSD | 2.000.32 | 2.100.40 | 0.15 |
| Precipitating causes of delirium | |||
| Number of precipitants, n (%) | 0.41 | ||
| Single precipitating cause | 12 (30.0) | 31 (37.8) | |
| 2 precipitating causes | 12 (30.0) | 28 (34.1) | |
| >2 precipitating causes | 16 (40.0) | 23 (28.1) | |
| Principal precipitating cause, n (%) | 0.050 | ||
| UTI | 17 (42.5) | 27 (32.9) | |
| Pneumonia | 3 (7.5) | 23 (28.0) | |
| Combined UTI and pneumonia or sepsis from >1 source | 3 (7.5) | 9 (11.0) | |
| Stroke | 0 (0) | 3 (3.7) | |
| Biochemical abnormalities | 2 (5.0) | 4 (4.9) | |
| Intracranial hemorrhage | 3 (7.5) | 1(1.2) | |
| Fracture | 3 (7.5) | 2 (2.4) | |
| Postoperative state | 2 (5.0) | 3 (3.7) | |
| Others | 7 (17.5) | 10 (12.2) | |
Patients without underlying dementia more often required multiple insults to precipitate delirium compared with patients with dementia, although this difference did not fulfill statistical significance. Although sepsis remained the primary precipitating cause of delirium in patients with and without dementia, urinary tract infection was more common among patients without dementia, whereas pneumonia was a more common precipitant in patients with dementia (P=0.050). The cognitive performance and functional status were similar at GMU admission for both groups.
Cognitive and Functional Outcomes on Discharge
The average duration of delirium for the GMU cohort was 8.2 days, with the mean length of total hospital stay being 17.0 days. The length of GMU stay for each patient was equivalent to the duration of delirium as patients were transferred out of the GMU once assessed to have recovered from delirium. Significant cognitive improvement was observed with recovery from delirium, with AMT and CMMSE scores improving by a mean of 1.442.38 and 3.545.61, respectively (paired t test, P<0.001). Patients demonstrated significant functional recovery at discharge from the GMU compared with their functional performance at admission to the GMU, with a mean MBI gain of 19.4217.11 (P<0.001), and 59 patients (48.4%) had progressed to a less‐dependent category.
Table 2 compares the cognitive and functional progress of patients with and without dementia. There was no difference in duration of delirium or length of total hospital stay between the demented and nondemented groups. Within‐group comparison showed patients with and without dementia managing significant improvement in cognitive scores at GMU discharge compared with GMU admission, although the magnitude of improvement was greater for nondemented patients (CMMSE improvement +6.73 vs +1.99, P<0.001). The mean MBI gain at GMU discharge compared with GMU admission was 20.4316.99 (P<0.001) for patients with dementia and 17.3517.39 (P<0.001) for patients without underlying dementia. There was no significant difference in the extent of functional improvement achieved between the demented and nondemented groups. Nineteen patients (47.5%) without dementia and 40 patients (48.8%) with preexisting dementia were in a less‐dependent category at GMU discharge compared with GMU admission.
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Duration of delirium, meanSD, d | 8.2 | 7.47.6 | 8.65.7 | 0.35 |
| Length of hospital stay, meanSD, da | 17.0 | 18.69.7 | 16.38.7 | 0.18 |
| Cognitive scores on dischargeb | ||||
| AMT, mean (SD) | 3.25 (3.00) | 5.20 (2.88) | 2.29 (2.58) | <0.001 |
| CMMSE, mean (SD) | 8.84 (6.81) | 11.90 (6.16) | 7.34 (6.64) | <0.001 |
| Improvement in cognitive scores | ||||
| AMT, mean (SD)c | +1.44 (2.38) | +3.15 (2.68) | +0.61 (1.70) | <0.001 |
| CMMSE, mean (SD)c | +3.54 (5.61) | +6.73 (5.74) | +1.99 (4.87) | <0.001 |
| Delirium severity on discharge | ||||
| CAM‐sev, mean (SD) | 2.43 (1.44) | 2.15 (1.46) | 2.57 (1.42) | 0.13 |
| DRS‐sev, mean (SD) | 16.83 (6.97) | 14.45 (6.90) | 18.00 (6.74) | 0.008 |
| Change in delirium severity scores | ||||
| CAM‐sev, mean (SD)c | 2.47 (1.73) | 3.08 (1.67) | 2.17 (1.68) | 0.006 |
| DRS‐sev, mean (SD)c | 9.72 (7.30) | 12.85 (6.43) | 8.17 (7.25) | 0.001 |
| Functional status | ||||
| MBI discharge, mean (SD) | 51.03 (26.20) | 46.83 (24.09) | 53.09 (27.07) | 0.22 |
| MBI, mean (SD)c | +19.42 (17.11) | +17.35 (17.39) | +20.43 (16.99) | 0.35 |
| Progress to less‐dependent category at discharge, n (%)b | 59 (48.4%) | 19 (47.5%) | 40 (48.8%) | 1.00 |
Seventy‐nine patients (64.8% of the GMU cohort) were discharged back to their own home following the index hospitalization, 22 patients (18.0%) required further rehabilitation in a community hospital or subacute ward before returning home, and 19 patients (15.6%) were admitted to long‐term care. There was no significant difference in discharge destination between patients with and without dementia (Table 3).
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Discharge destination, n (%) | 0.14 | |||
| Own home | 79 (64.8) | 24 (60.0) | 55 (67.9) | |
| Community hospital or subacute ward (with eventual discharge home after rehabilitation) | 22 (18.0) | 10 (25.0) | 12 (14.6) | |
| Long‐term care | 19 (15.6) | 6 (15.0) | 13 (16.0) | |
| Inpatient hospice | 1 (0.8) | 0 (0) | 1 (1.2) | |
Predictors of Functional Recovery on Discharge
Age, gender, number of precipitating causes, severity of illness, and type of delirium had a univariate P value <0.20 and were entered into the multiple regression model. After controlling for admission MBI score in multivariate analysis, gender, presentation of delirium, and severity of illness remained independent factors for improvement in MBI score (Table 4). Female patients exhibited greater functional recovery than males. Hypoactive delirium was associated with the worst prognosis for functional recovery, with improvement in MBI score being 14.47 points lower than that achieved by patients with hyperactive delirium. Severity of illness at admission was another negative predictor of functional recovery, with each unit increase in Severity of Illness Index (higher score indicating more severe illness) associated with 10.59 points lower gain in MBI.
| Predictor | Model 1 | Final Model | ||
|---|---|---|---|---|
| B | P Value | B | P Value | |
| ||||
| Age | 0.245 | 0.184 | ||
| Female (vs male) | 6.421 | 0.023 | 7.393 | 0.009 |
| Number of precipitating causes | 2.044 | 0.229 | ||
| Hypoactive delirium (vs hyperactive delirium) | 15.383 | <0.001 | 14.472 | 0.001 |
| Severity of illness | 10.596 | 0.003 | 10.591 | 0.003 |
DISCUSSION
Our results support a delirium management unit focused on geriatric nursing care and rehabilitation in promoting positive cognitive and functional gains in older patients with delirium. In addition, we have documented that patients with dementia and recovering from delirium have comparable potential for functional recovery as their cognitively intact counterparts, despite there being less improvement in cognitive test scores and higher comorbidity compared with patients without dementia.
Persistent delirium and failure to recognize the condition have been associated with poor functional recovery.[25, 26] Early recognition of delirium and actively addressing all predisposing and precipitating factors, along with emphasis on rehabilitation in a multidisciplinary unit, appear to be important factors contributing to the positive functional outcomes in our patients. In addition, none of the patients admitted to the GMU had been subject to physical restraint, and this, along with the higher nursing ratio, facilitated early mobilization essential to prevent functional decline. The overall length of hospital stay, averaging 17.0 days, compared favorably with a pre‐GMU cohort, where the average length of hospital stay for delirious patients was 20.9 days. The length of stay in an acute hospital ward included waiting time for transfer to appropriate postacute facility (community hospital, subacute ward, or nursing home).
In a study of older hospital inpatients, delirium independent of dementia was predictive of sustained poor cognitive and functional status in the year following hospitalization.[3] Thus, although delirium has been generally regarded as a transient and reversible condition, it is also increasingly recognized that recovery may not always be complete. This is a likely explanation for the low cognitive test scores at discharge from the GMU, albeit improved compared with admission, even among patients without dementia. The present study lacks data on longer‐term cognitive outcomes following delirium resolution. However, delirium is reportedly a risk factor for subsequent development of dementia,[27] and contributes to accelerated cognitive decline in patients with existing dementia.[28]
Despite reports of adverse outcomes of delirium superimposed on dementia, few intervention studies have specifically examined the management of delirium in older adults with dementia. In our study, preexisting dementia did not preclude delirious patients from functional improvement, adding to the still‐limited literature reporting positive rehabilitation outcomes in patients with cognitive impairment.[10, 29] In addition, patients with dementia did not take longer to recover from delirium than those without dementia, nor did they appear to require a longer duration for making similar functional gains given that length of hospital stay did not differ between the 2 groups. Illness severity and presentation of delirium, rather than preexisting dementia, predicted poor functional recovery. The complex etiology of delirium, representing an interaction between baseline patient vulnerability (predisposing factors) and exposure to noxious insults or precipitating factors, suggests that highly vulnerable patients (eg, dementia) may develop delirium with a relatively innocuous insult, as opposed to the multiple noxious insults anticipated in nonvulnerable patients.[30] Although Severity of Illness Index was similar, patients without dementia had a trend for more than 1 precipitating factor, and serious medical conditions, such as intracranial hemorrhage, fracture, and need for surgical intervention were more likely to be present, factors that can expectedly influence functional recovery. Patients with hypoactive delirium had the worst functional outcome, consistent with previously observed poor prognosis in hypoactive delirium.[31, 32] Our findings, if corroborated by future studies, may offer a means to attenuate the trajectory of functional decline observed in patients with delirium superimposed on dementia. This will be of particular relevance as earlier studies had observed a high risk of institutionalization in patients with delirium superimposed on dementia compared with dementia alone.[3, 33]
One limitation of this study was the failure to adjust for premorbid functional status due to the lack of information on baseline function prior to the current hospital admission. Even though we demonstrated functional improvement at discharge compared with admitting functional status, we were not able to ascertain if the patients had returned to their premorbid level of cognitive and physical functioning when discharged from the GMU. This study was also limited by its observational nature, the small sample size, and short‐term outcomes. However, the beneficial impact of the GMU on functional improvement in patients with delirium was supported by observed higher MBI gain compared with a pre‐GMU cohort (mean MBI gain, 7.511.2; discharge MBI, 45.832.9). Data collection for 6‐month and 1‐year outcomes is presently in progress to determine sustainability of any improvement achieved. Our exclusion criteria limits generalizability of the benefits of the GMU to patients considered medically unstable, in whom delirium is prevalent. However, such patients are likely too ill to participate in the intense rehabilitation practiced in the GMU.
We have shown that patients with delirium can engage and benefit from a rehabilitation program, their low cognitive test scores at admission notwithstanding, suggesting that early initiation of rehabilitation should be encouraged regardless of the cognitive and functional performance at presentation. In addition, we found that patients with delirium superimposed on dementia benefit from a multicomponent delirium treatment program to the same extent as delirious patients without dementia, which may contribute to improved quality of life and reduction in burden of care. As delirium is a complex medical problem with multiple predisposing and precipitating factors, a multicomponent intervention program as practiced in the GMU is likely to yield more consistent outcomes than a single intervention strategy. With the widely reported poor prognostic significance of delirium superimposed on dementia, more well‐designed controlled trials are urgently needed to identify intervention strategies that will aid the management of this group of hospitalized elders.
Acknowledgments
Disclosures: This work was supported by FY2010 Ministry of Health Quality Improvement Funding (MOH HQIF) Optimising Acute Delirium Care in Tan Tock Seng Hospital (Reference: HQIF 2010/17). The authors report no conflicts of interest.
Loss of functional independence is a serious complication of delirium,[1, 2] with functional consequences often persisting long after the index hospital admission.[3] Preexisting dementia is a major risk factor for delirium in hospitalized older patients,[4, 5] and the occurrence of delirium may alter the clinical course of an underlying dementia with negative prognostic implications, including further functional and cognitive decline,[3] increased rehospitalization rates,[6] institutionalization,[3] and death.[7] Despite the adverse functional outcomes of delirium, there remains a scarcity of well‐designed intervention trials for the rehabilitation of older patients recovering from delirium.
Studies investigating the influence of cognitive impairment on rehabilitation outcomes have yielded conflicting results. Landi and colleagues identified cognitive impairment as a negative predictor of functional recovery among older patients in a rehabilitation unit.[8] Yet, other studies had reported functional improvements with rehabilitation regardless of cognition.[9, 10, 11] However, it is not possible to determine if cognitive impairment in the earlier studies had been consequent to delirium, dementia, or both. Although there has been emerging evidence for the impact of delirium on disease trajectory among patients with dementia, it is less clear whether interventions for delirium prevention and management will yield comparable outcomes with the presence of preexisting dementia.
The geriatric monitoring unit (GMU) is a specialized 5‐bed unit developed for the care of delirious older patients and is modeled after the delirium room,[12] with adoption of core interventions from the Hospital Elder Life Program[13] and use of evening bright light therapy to consolidate circadian rhythm and improve sleep in older inpatients.[14] The core interventions in this multicomponent delirium management program focused on early mobilization and rehabilitation, occurring concurrently with medical management, to address all precipitating and predisposing factors for delirium.[15] As functional decline in an older patient can develop as early as 2 days into a hospital admission,[16] early intervention once the patient has been admitted is essential to prevent the cascade to irreversible functional loss. Hence, we sought to determine the functional progress of delirious older patients exposed to a multicomponent delirium management program and whether the presence of underlying dementia impacted the functional recovery of older patients with delirium. The secondary objective was to identify predictors of functional recovery in older hospitalized patients with delirium.
METHODS
Setting and Participants
This prospective cohort study recruited patients who had been admitted to the GMU of Tan Tock Seng Hospital, Singapore, during the period of November 2010 to November 2011. The admission criteria for the GMU included patients aged 65 years and older who were admitted to the geriatric medicine department and assessed to have delirium, either on admission to the general ward or incident delirium that developed during the hospital stay. The diagnosis of delirium was established in accordance with the Confusion Assessment Method (CAM) diagnostic criteria.[17] Patients were excluded if they met any of the following criteria: (1) presence of medical illnesses that required special monitoring (eg, telemetry for arrhythmias or acute myocardial infarction); (2) critically ill, in coma, or with terminal illness; (3) uncommunicative or with severe aphasia; (4) severely combative behavior with high risk of harm to self, staff, or other patients; (5) contraindications to bright light therapy (manic disorders, severe eye disorders, photosensitive skin disorders, or use of photosensitizing medications); (6) being on respiratory or contact precautions; and (7) refusal of GMU admission by patient, family, or physician‐in‐charge.[15]
The core interventions adopted in the GMU facilitate early mobilization through strict avoidance of mechanical restraints (and refraining from pharmacological restraints where possible), encouraging patients to mobilize early with the support of therapists and trained nurses, and daily review of the continued need for intravenous drip, urinary catheter, or supplemental oxygen to minimize immobilizing equipment. There is a strong emphasis on rehabilitation as part of the multicomponent delirium program; patients participate in daily orientation 3 times a day by a trained nurse using a reality orientation board, engage in therapeutic activities 3 times a day for cognitive stimulation and socialization, and attend daily physiotherapy and occupational therapy sessions. Additionally, we actively seek to correct any sensory impairment with visual aids (such as eye glasses), earwax disimpaction where necessary, and provision of hearing aids or portable audio amplifier. Nonpharmacological measures implemented to improve sleep at night for delirious older patients include evening bright light therapy and a sleep enhancement protocol of warm milk and relaxation music. These interventions are practiced for all patients through their stay in the GMU, with compliance ensured via a structured protocol in the daily nursing workflow and documentation sheet.
All patients fulfilling CAM criteria for delirium and admitted to the GMU were eligible for this study. However, patients who were prematurely transferred out of the GMU (for reasons such as instability of medical conditions requiring intensive monitoring or patients requiring contact precautions) were excluded from subsequent analysis. Patients with repeated GMU admissions had only their first admission included for analysis.
Ethics approval for conduct of this study was obtained from the National Healthcare Group Domain Specific Review Board.
Assessments
All patients underwent a detailed cognitive evaluation by the consultant geriatrician on admission to the GMU. A family member or other designated caregiver was routinely interviewed to establish the patient's baseline cognitive functioning prior to the current admission. The medical records of all patients were reviewed to ascertain whether a diagnosis of dementia had been previously established. In patients yet to be diagnosed, a diagnosis of dementia was made in the current admission if the corroborative history suggested presence of cognitive symptoms consistent with Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM‐IV) criteria for dementia[18] of at least 6 months' duration, in accordance with the standardized process for cognitive evaluation.[19]
Delirium subtypehyperactive, hypoactive, or mixed deliriumwas determined by the consultant geriatrician at admission to the GMU based on clinical assessment of the patient's mental state and behavior. All patients underwent baseline and daily cognitive status assessment using the locally validated 10‐point Abbreviated Mental Test (AMT) and 28‐point Chinese Mini‐Mental State Examination (CMMSE), with higher scores reflective of better cognitive performance.[20] Delirium severity was assessed daily and scored on the Delirium Rating Scale‐98 (DRS‐sev, maximum severity score of 39 points)[21] and CAM severity (CAM‐sev).[17] The cognitive tests and delirium severity scoring were administered by a trained assessor from the time of admission to the GMU until patient's discharge from the GMU. A comprehensive history taking (including medication reconciliation), detailed physical examination, and review of all laboratory/emmaging investigations were performed routinely at admission to identify all potential precipitating factors for delirium, and the Charlson's co‐morbidity[22] and modified Severity of Illness Index scored.[23] The modified Barthel Index (MBI),[24] which measures activities of daily living (ADL), was used to monitor functional progress from time of admission until discharge from the GMU, and was rated by an occupational therapist observing the patient at ADL tasks. A patient was deemed to have recovered from delirium if the CAM criteria for delirium was no longer met, with diagnosis of recovery being supported by improvement in cognitive and/or delirium severity scores as well as input from the multidisciplinary team. Patients were discharged from the GMU once assessed to have recovered from delirium, but may continue inpatient treatment in a general ward for other outstanding medical issues (such as continuation of intravenous antibiotics) or while awaiting transfer to a post‐acute care facility for continued rehabilitation.
Primary Outcome
The primary outcome was recovery of physical function and the difference in total MBI score of each patient at the GMU discharge from that at the GMU admission provided an estimate of the extent of functional recovery achieved. To define clinically meaningful functional improvement, we categorized MBI scores into the following: (1) total MBI score 0 to 20 indicates total dependence, (2) 21 to 60 severe dependence, (3) 61 to 90 moderate dependence, (4) 91 to 99 slight dependence, and (5) 100 full independence.[24] The total MBI gain at discharge was considered clinically meaningful if accompanied by patient transcending into a less dependent category.
Statistical Analysis
Summary measures of baseline characteristics are presented as means ( standard deviations) and proportions. The difference between admission and discharge AMT, CMMSE, and MBI scores was calculated for each patient and represented the extent of cognitive and functional recovery achieved at the time of discharge from the GMU. Paired sample t test was used to evaluate differences between admission and discharge cognitive and functional scores, as well as changes in delirium severity as measured on CAM‐sev and DRS‐sev, for the entire cohort of GMU patients.
To determine whether preexisting dementia impacts on cognitive and functional recovery of delirious patients, independent sample t test was performed to compare mean changes in AMT, CMMSE, and MBI scores for the demented vs nondemented groups. The proportion of patients achieving clinically meaningful MBI gain in each group was compared using Pearson 2 test.
To identify predictors of functional recovery, univariate analyses were first performed to examine the relationship between predictor variables and MBI gain. The candidate predictors were defined a priori and included (1) age, (2) gender, (3) Charlson's comorbidity score, (4) Severity of Illness Index, (5) number of precipitating causes of delirium (categoricalsingle, 2 or >2 precipitating causes), (6) presentation of delirium (categoricalhypoactive, hyperactive, mixed), (7) delirium severity on admission (CAM‐sev and DRS‐sev scores), (8) duration of delirium, and (9) presence of underlying dementia. Predictors with a univariate P value <0.20 were entered into a multiple linear regression model, with the dependent variable being change in MBI score. Only significant predictors (P0.05) were retained in the final model. All models controlled for admission MBI score.
Statistical analyses were performed using SPSS software (version 16.0; IBM, Armonk, NY). All statistical tests were 2‐tailed, with P value0.05 considered statistically significant.
RESULTS
Patient Characteristics
One hundred forty‐six elderly patients with delirium were admitted to the GMU during the 1‐year study period. One hundred twenty‐two patients were analyzed after excluding 24 patients (17 patients [mean age 83.28.7 years, 47.1% females] were transferred out prematurely due to infection control precautions or were critically ill requiring intensive monitoring; 7 were repeat admissions). The mean age of patients admitted to the GMU was 84.17.6 years, with a predominance of females (60.7%). Most patients presented with either hyperactive (49.2%) or mixed (35.2%) delirium, with hypoactive delirium being the least common presentation (15.6%). Sepsis, notably urinary tract infection or pneumonia, was the predominant principal precipitating cause, contributing to 68.0% of delirium cases within the GMU. At admission, the GMU cohort had a mean CMMSE score of 5.305.53, and mean MBI score of 31.6126.61. Eighty‐two patients (67.2%) had delirium superimposed on dementia, whereas 40 patients (32.8%) did not have underlying dementia.
Baseline characteristics of patients with and without underlying dementia are shown in Table 1. There were no significant differences in age, gender, ethnicity, and illness severity between groups with and without dementia, although patients with dementia had higher comorbidity (Charlson's comorbidity score 2.27 vs 1.75, P=0.054). The presentation and severity of delirium at admission to the GMU was similar in both groups.
| Underlying Dementia | P Value | ||
|---|---|---|---|
| Absent (n=40) | Present (n=82) | ||
| |||
| Demographics | |||
| Age, meanSD, y | 84.08.1 | 84.27.4 | 0.88 |
| Male gender, n (%) | 18 (45.0) | 29 (35.4) | 0.45 |
| Chinese, n (%) | 34 (85.0) | 70 (85.4) | 0.67 |
| Presentation of delirium | 0.99 | ||
| Hyperactive, n (%) | 20 (50.0) | 40 (48.8) | |
| Hypoactive, n (%) | 6 (15.0) | 13 (15.9) | |
| Mixed, n (%) | 14 (35.0) | 29 (35.4) | |
| Delirium severity on admissiona | |||
| CAM‐sev, meanSD | 5.231.17 | 4.74 1.47 | 0.07 |
| DRS‐sev, meanSD | 27.306.37 | 26.326.70 | 0.43 |
| Cognitive status on admissiona | |||
| AMT, meanSD | 2.051.97 | 1.682.22 | 0.38 |
| CMMSE, meanSD | 5.185.13 | 5.355.75 | 0.87 |
| Functional status on admission, MBI score, meanSDa | 29.4825.90 | 32.6627.04 | 0.54 |
| Comorbidities | |||
| Charlson score, meanSD | 1.751.63 | 2.271.25 | 0.054 |
| Severity of Illness, meanSD | 2.000.32 | 2.100.40 | 0.15 |
| Precipitating causes of delirium | |||
| Number of precipitants, n (%) | 0.41 | ||
| Single precipitating cause | 12 (30.0) | 31 (37.8) | |
| 2 precipitating causes | 12 (30.0) | 28 (34.1) | |
| >2 precipitating causes | 16 (40.0) | 23 (28.1) | |
| Principal precipitating cause, n (%) | 0.050 | ||
| UTI | 17 (42.5) | 27 (32.9) | |
| Pneumonia | 3 (7.5) | 23 (28.0) | |
| Combined UTI and pneumonia or sepsis from >1 source | 3 (7.5) | 9 (11.0) | |
| Stroke | 0 (0) | 3 (3.7) | |
| Biochemical abnormalities | 2 (5.0) | 4 (4.9) | |
| Intracranial hemorrhage | 3 (7.5) | 1(1.2) | |
| Fracture | 3 (7.5) | 2 (2.4) | |
| Postoperative state | 2 (5.0) | 3 (3.7) | |
| Others | 7 (17.5) | 10 (12.2) | |
Patients without underlying dementia more often required multiple insults to precipitate delirium compared with patients with dementia, although this difference did not fulfill statistical significance. Although sepsis remained the primary precipitating cause of delirium in patients with and without dementia, urinary tract infection was more common among patients without dementia, whereas pneumonia was a more common precipitant in patients with dementia (P=0.050). The cognitive performance and functional status were similar at GMU admission for both groups.
Cognitive and Functional Outcomes on Discharge
The average duration of delirium for the GMU cohort was 8.2 days, with the mean length of total hospital stay being 17.0 days. The length of GMU stay for each patient was equivalent to the duration of delirium as patients were transferred out of the GMU once assessed to have recovered from delirium. Significant cognitive improvement was observed with recovery from delirium, with AMT and CMMSE scores improving by a mean of 1.442.38 and 3.545.61, respectively (paired t test, P<0.001). Patients demonstrated significant functional recovery at discharge from the GMU compared with their functional performance at admission to the GMU, with a mean MBI gain of 19.4217.11 (P<0.001), and 59 patients (48.4%) had progressed to a less‐dependent category.
Table 2 compares the cognitive and functional progress of patients with and without dementia. There was no difference in duration of delirium or length of total hospital stay between the demented and nondemented groups. Within‐group comparison showed patients with and without dementia managing significant improvement in cognitive scores at GMU discharge compared with GMU admission, although the magnitude of improvement was greater for nondemented patients (CMMSE improvement +6.73 vs +1.99, P<0.001). The mean MBI gain at GMU discharge compared with GMU admission was 20.4316.99 (P<0.001) for patients with dementia and 17.3517.39 (P<0.001) for patients without underlying dementia. There was no significant difference in the extent of functional improvement achieved between the demented and nondemented groups. Nineteen patients (47.5%) without dementia and 40 patients (48.8%) with preexisting dementia were in a less‐dependent category at GMU discharge compared with GMU admission.
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Duration of delirium, meanSD, d | 8.2 | 7.47.6 | 8.65.7 | 0.35 |
| Length of hospital stay, meanSD, da | 17.0 | 18.69.7 | 16.38.7 | 0.18 |
| Cognitive scores on dischargeb | ||||
| AMT, mean (SD) | 3.25 (3.00) | 5.20 (2.88) | 2.29 (2.58) | <0.001 |
| CMMSE, mean (SD) | 8.84 (6.81) | 11.90 (6.16) | 7.34 (6.64) | <0.001 |
| Improvement in cognitive scores | ||||
| AMT, mean (SD)c | +1.44 (2.38) | +3.15 (2.68) | +0.61 (1.70) | <0.001 |
| CMMSE, mean (SD)c | +3.54 (5.61) | +6.73 (5.74) | +1.99 (4.87) | <0.001 |
| Delirium severity on discharge | ||||
| CAM‐sev, mean (SD) | 2.43 (1.44) | 2.15 (1.46) | 2.57 (1.42) | 0.13 |
| DRS‐sev, mean (SD) | 16.83 (6.97) | 14.45 (6.90) | 18.00 (6.74) | 0.008 |
| Change in delirium severity scores | ||||
| CAM‐sev, mean (SD)c | 2.47 (1.73) | 3.08 (1.67) | 2.17 (1.68) | 0.006 |
| DRS‐sev, mean (SD)c | 9.72 (7.30) | 12.85 (6.43) | 8.17 (7.25) | 0.001 |
| Functional status | ||||
| MBI discharge, mean (SD) | 51.03 (26.20) | 46.83 (24.09) | 53.09 (27.07) | 0.22 |
| MBI, mean (SD)c | +19.42 (17.11) | +17.35 (17.39) | +20.43 (16.99) | 0.35 |
| Progress to less‐dependent category at discharge, n (%)b | 59 (48.4%) | 19 (47.5%) | 40 (48.8%) | 1.00 |
Seventy‐nine patients (64.8% of the GMU cohort) were discharged back to their own home following the index hospitalization, 22 patients (18.0%) required further rehabilitation in a community hospital or subacute ward before returning home, and 19 patients (15.6%) were admitted to long‐term care. There was no significant difference in discharge destination between patients with and without dementia (Table 3).
| Overall GMU Cohort (n=122) | Underlying Dementia | P Value | ||
|---|---|---|---|---|
| Absent (n=40) | Present (n=82) | |||
| ||||
| Discharge destination, n (%) | 0.14 | |||
| Own home | 79 (64.8) | 24 (60.0) | 55 (67.9) | |
| Community hospital or subacute ward (with eventual discharge home after rehabilitation) | 22 (18.0) | 10 (25.0) | 12 (14.6) | |
| Long‐term care | 19 (15.6) | 6 (15.0) | 13 (16.0) | |
| Inpatient hospice | 1 (0.8) | 0 (0) | 1 (1.2) | |
Predictors of Functional Recovery on Discharge
Age, gender, number of precipitating causes, severity of illness, and type of delirium had a univariate P value <0.20 and were entered into the multiple regression model. After controlling for admission MBI score in multivariate analysis, gender, presentation of delirium, and severity of illness remained independent factors for improvement in MBI score (Table 4). Female patients exhibited greater functional recovery than males. Hypoactive delirium was associated with the worst prognosis for functional recovery, with improvement in MBI score being 14.47 points lower than that achieved by patients with hyperactive delirium. Severity of illness at admission was another negative predictor of functional recovery, with each unit increase in Severity of Illness Index (higher score indicating more severe illness) associated with 10.59 points lower gain in MBI.
| Predictor | Model 1 | Final Model | ||
|---|---|---|---|---|
| B | P Value | B | P Value | |
| ||||
| Age | 0.245 | 0.184 | ||
| Female (vs male) | 6.421 | 0.023 | 7.393 | 0.009 |
| Number of precipitating causes | 2.044 | 0.229 | ||
| Hypoactive delirium (vs hyperactive delirium) | 15.383 | <0.001 | 14.472 | 0.001 |
| Severity of illness | 10.596 | 0.003 | 10.591 | 0.003 |
DISCUSSION
Our results support a delirium management unit focused on geriatric nursing care and rehabilitation in promoting positive cognitive and functional gains in older patients with delirium. In addition, we have documented that patients with dementia and recovering from delirium have comparable potential for functional recovery as their cognitively intact counterparts, despite there being less improvement in cognitive test scores and higher comorbidity compared with patients without dementia.
Persistent delirium and failure to recognize the condition have been associated with poor functional recovery.[25, 26] Early recognition of delirium and actively addressing all predisposing and precipitating factors, along with emphasis on rehabilitation in a multidisciplinary unit, appear to be important factors contributing to the positive functional outcomes in our patients. In addition, none of the patients admitted to the GMU had been subject to physical restraint, and this, along with the higher nursing ratio, facilitated early mobilization essential to prevent functional decline. The overall length of hospital stay, averaging 17.0 days, compared favorably with a pre‐GMU cohort, where the average length of hospital stay for delirious patients was 20.9 days. The length of stay in an acute hospital ward included waiting time for transfer to appropriate postacute facility (community hospital, subacute ward, or nursing home).
In a study of older hospital inpatients, delirium independent of dementia was predictive of sustained poor cognitive and functional status in the year following hospitalization.[3] Thus, although delirium has been generally regarded as a transient and reversible condition, it is also increasingly recognized that recovery may not always be complete. This is a likely explanation for the low cognitive test scores at discharge from the GMU, albeit improved compared with admission, even among patients without dementia. The present study lacks data on longer‐term cognitive outcomes following delirium resolution. However, delirium is reportedly a risk factor for subsequent development of dementia,[27] and contributes to accelerated cognitive decline in patients with existing dementia.[28]
Despite reports of adverse outcomes of delirium superimposed on dementia, few intervention studies have specifically examined the management of delirium in older adults with dementia. In our study, preexisting dementia did not preclude delirious patients from functional improvement, adding to the still‐limited literature reporting positive rehabilitation outcomes in patients with cognitive impairment.[10, 29] In addition, patients with dementia did not take longer to recover from delirium than those without dementia, nor did they appear to require a longer duration for making similar functional gains given that length of hospital stay did not differ between the 2 groups. Illness severity and presentation of delirium, rather than preexisting dementia, predicted poor functional recovery. The complex etiology of delirium, representing an interaction between baseline patient vulnerability (predisposing factors) and exposure to noxious insults or precipitating factors, suggests that highly vulnerable patients (eg, dementia) may develop delirium with a relatively innocuous insult, as opposed to the multiple noxious insults anticipated in nonvulnerable patients.[30] Although Severity of Illness Index was similar, patients without dementia had a trend for more than 1 precipitating factor, and serious medical conditions, such as intracranial hemorrhage, fracture, and need for surgical intervention were more likely to be present, factors that can expectedly influence functional recovery. Patients with hypoactive delirium had the worst functional outcome, consistent with previously observed poor prognosis in hypoactive delirium.[31, 32] Our findings, if corroborated by future studies, may offer a means to attenuate the trajectory of functional decline observed in patients with delirium superimposed on dementia. This will be of particular relevance as earlier studies had observed a high risk of institutionalization in patients with delirium superimposed on dementia compared with dementia alone.[3, 33]
One limitation of this study was the failure to adjust for premorbid functional status due to the lack of information on baseline function prior to the current hospital admission. Even though we demonstrated functional improvement at discharge compared with admitting functional status, we were not able to ascertain if the patients had returned to their premorbid level of cognitive and physical functioning when discharged from the GMU. This study was also limited by its observational nature, the small sample size, and short‐term outcomes. However, the beneficial impact of the GMU on functional improvement in patients with delirium was supported by observed higher MBI gain compared with a pre‐GMU cohort (mean MBI gain, 7.511.2; discharge MBI, 45.832.9). Data collection for 6‐month and 1‐year outcomes is presently in progress to determine sustainability of any improvement achieved. Our exclusion criteria limits generalizability of the benefits of the GMU to patients considered medically unstable, in whom delirium is prevalent. However, such patients are likely too ill to participate in the intense rehabilitation practiced in the GMU.
We have shown that patients with delirium can engage and benefit from a rehabilitation program, their low cognitive test scores at admission notwithstanding, suggesting that early initiation of rehabilitation should be encouraged regardless of the cognitive and functional performance at presentation. In addition, we found that patients with delirium superimposed on dementia benefit from a multicomponent delirium treatment program to the same extent as delirious patients without dementia, which may contribute to improved quality of life and reduction in burden of care. As delirium is a complex medical problem with multiple predisposing and precipitating factors, a multicomponent intervention program as practiced in the GMU is likely to yield more consistent outcomes than a single intervention strategy. With the widely reported poor prognostic significance of delirium superimposed on dementia, more well‐designed controlled trials are urgently needed to identify intervention strategies that will aid the management of this group of hospitalized elders.
Acknowledgments
Disclosures: This work was supported by FY2010 Ministry of Health Quality Improvement Funding (MOH HQIF) Optimising Acute Delirium Care in Tan Tock Seng Hospital (Reference: HQIF 2010/17). The authors report no conflicts of interest.
- , , , et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–M186.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , , . Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–583.
- , , , . Delirium risk factors in elderly hospitalized patients. J General Intern Med. 1998;13:204–212.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–1732.
- , . Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Predictors of rehabilitation outcomes in frail patients treated in a geriatric hospital. J Am Geriatr Soc. 2002;50:679–684.
- , , , , . Effect of cognitive impairment on rehabilitation outcome. Am J Phys Med Rehabil. 1996;75:40–43.
- , , , , . Rehabilitation outcomes in cognitively impaired patients admitted to skilled nursing facilities from the community. Arch Phys Med Rehabil. 2004;85:1602–1607.
- , , , . Does cognitive impairment affect rehabilitation outcome? J Am Geriatr Soc. 2011;59:2108–2111.
- , , , , , . A model for managing delirious older inpatients. J Am Geriatr Soc. 2003;51:1031–1035.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Light therapy for insomnia in older adults. Clin Geriatr Med. 2008;24(1):139–149.
- , , , , , . A new model of delirium care in the acute geriatric setting: geriatric monitoring unit. BMC Geriatr. 2011;11:41.
- , , , , . The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303.
- , , , , , . Clarifying confusion: The Confusion Assessment Method. A new method for detecting delirium. Ann Intern Med. 1990;113:941–948.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
- , . An evidence‐based clinical approach to the diagnosis of dementia. Ann Acad Med Singapore. 2003;32:740–748.
- , , , . Diagnostic performance of two mental status tests in the older Chinese: influence of education and age on cut‐off values. Int J Geriatr Psychiatry. 2000;15:234–241.
- , , , , , . Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242.
- , , , . A new method of classifying prognositic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39(11):830–836.
- , , . Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709.
- , , . Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatr. 2005;5:5.
- , , , , , . Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–208.
- , , , , . The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575.
- , , , , . Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321:1107–1111.
- . Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14(4):745–764.
- , . Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28:115–119.
- , , , , , . Severity and course of delirium in medically hospitalised nursing facility residents. Am J Geriatr Psychiatry. 2001;9:72–77.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. A meta‐analysis. JAMA. 2010;304(4):443–451.
- , , , et al. Acute delirium and functional decline in the hospitalized elderly patient. J Gerontol. 1993;48:M181–M186.
- , , , , . Does delirium contribute to poor hospital outcomes? A three‐site epidemiologic study. J Gen Intern Med. 1998;13:234–242.
- , , , , . Delirium in older medical inpatients and subsequent cognitive and functional status: a prospective study. CMAJ. 2001;165(5):575–583.
- , , , . Delirium risk factors in elderly hospitalized patients. J General Intern Med. 1998;13:204–212.
- , , . Delirium superimposed on dementia: a systematic review. J Am Geriatr Soc. 2002;50:1723–1732.
- , . Consequences of not recognizing delirium superimposed on dementia in hospitalized elderly individuals. J Gerontol Nurs. 2000;26:30–40.
- , , , , . Delirium predicts 12‐month mortality. Arch Intern Med. 2002;162:457–463.
- , , , et al. Predictors of rehabilitation outcomes in frail patients treated in a geriatric hospital. J Am Geriatr Soc. 2002;50:679–684.
- , , , , . Effect of cognitive impairment on rehabilitation outcome. Am J Phys Med Rehabil. 1996;75:40–43.
- , , , , . Rehabilitation outcomes in cognitively impaired patients admitted to skilled nursing facilities from the community. Arch Phys Med Rehabil. 2004;85:1602–1607.
- , , , . Does cognitive impairment affect rehabilitation outcome? J Am Geriatr Soc. 2011;59:2108–2111.
- , , , , , . A model for managing delirious older inpatients. J Am Geriatr Soc. 2003;51:1031–1035.
- , , , , . The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. J Am Geriatr Soc. 2000;48:1697–1706.
- . Light therapy for insomnia in older adults. Clin Geriatr Med. 2008;24(1):139–149.
- , , , , , . A new model of delirium care in the acute geriatric setting: geriatric monitoring unit. BMC Geriatr. 2011;11:41.
- , , , , . The natural history of functional morbidity in hospitalized older patients. J Am Geriatr Soc. 1990;38:1296–1303.
- , , , , , . Clarifying confusion: The Confusion Assessment Method. A new method for detecting delirium. Ann Intern Med. 1990;113:941–948.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association; 1994.
- , . An evidence‐based clinical approach to the diagnosis of dementia. Ann Acad Med Singapore. 2003;32:740–748.
- , , , . Diagnostic performance of two mental status tests in the older Chinese: influence of education and age on cut‐off values. Int J Geriatr Psychiatry. 2000;15:234–241.
- , , , , , . Validation of the Delirium Rating Scale‐revised‐98: comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci. 2001;13:229–242.
- , , , . A new method of classifying prognositic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383.
- , , , , . Resource consumption in hospitalised, frail older patients. Ann Acad Med Singapore. 2010;39(11):830–836.
- , , . Improving the sensitivity of the Barthel Index for stroke rehabilitation. J Clin Epidemiol. 1989;42(8):703–709.
- , , . Incomplete functional recovery after delirium in elderly people: a prospective cohort study. BMC Geriatr. 2005;5:5.
- , , , , , . Association between delirium resolution and functional recovery among newly admitted postacute facility patients. J Gerontol A Biol Sci Med Sci. 2006;61(2):204–208.
- , , , , . The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychol Rev. 2004;14(2):87–98.
- , , , et al. Delirium accelerates cognitive decline in Alzheimer disease. Neurology. 2009;72:1570–1575.
- , , , , . Randomised, clinically controlled trial of intensive geriatric rehabilitation in patients with hip fracture: subgroup analysis of patients with dementia. BMJ. 2000;321:1107–1111.
- . Delirium in hospitalized older patients. Clin Geriatr Med. 1998;14(4):745–764.
- , . Clinical significance of delirium subtypes in older people. Age Ageing. 1999;28:115–119.
- , , , , , . Severity and course of delirium in medically hospitalised nursing facility residents. Am J Geriatr Psychiatry. 2001;9:72–77.
- , , , , , . Delirium in elderly patients and the risk of postdischarge mortality, institutionalization and dementia. A meta‐analysis. JAMA. 2010;304(4):443–451.
Copyright © 2013 Society of Hospital Medicine