User login
Analysis of exhaled volatile organic compounds may accurately detect NASH
The analysis of volatile organic compounds in exhaled breath may provide a noninvasive and accurate test for diagnosing nonalcoholic steatohepatitis, according to results from a pilot study published in March.
This test could reduce the number of unnecessary liver biopsies and missed diagnoses associated with assessing plasma transaminase levels, reported Dr. Froukje J. Verdam of Maastricht (the Netherlands) University Medical Center and her associates (J. Hepatol. 2013;58:543-8).
Researchers evaluated breath samples with gas chromatography–mass spectrometry from 65 consecutive overweight or obese patients before they underwent laparoscopic abdominal surgery, between October 2007 and May 2011. These results were compared with histologic analysis of liver biopsies taken intraoperatively and assessments of plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Overall, liver biopsies showed that 39 patients (60%) had nonalcoholic steatohepatitis (NASH), defined as "showing signs of steatosis and inflammation." Additionally, ALT and AST levels were significantly higher in patients with the disease than without. However, "parameters such as gender, age, BMI, and HbA1c did not differ significantly," reported the study authors.
The analysis of three volatile organic compounds (VOCs) – n-tridecane, 3-methylbutanonitrile, and 1-propanol – enabled investigators to distinguish between patients with and without NASH, with a sensitivity of 90%, a specificity of 69%, and an area under the receiver operating characteristic (ROC) curve of 0.77 plus or minus 0.07. The positive predictive value of using VOC analysis for NASH was 81%, while the negative predictive value was 82%.
In comparison, in 61 patients from whom plasma was available, the sensitivity of measuring ALT was 19%, while the specificity was 96%. The positive and negative predictive values of ALT were 88% and 43%, respectively.
Further evaluation of the AST/ALT ratio found that it was 32% sensitive and 79% specific, while positive and negative predictive values were 70% and 43%, respectively.
"It can be concluded that the diagnostic value of VOC is much higher than that of plasma transaminases, resulting in less misdiagnosed patients," wrote the study authors. Prediction of NASH using VOC, ALT, and the AST/ALT ratio did not reflect liver biopsy results in 18%, 51%, and 49% of subjects, respectively.
Using VOC evaluation rather than histologic testing has several other advantages, according to the researchers. "The analysis of exhaled breath can identify NASH presence at an early stage, and early identification in a mild stage is pivotal to enhance the chances of cure," they wrote. "Furthermore, whereas a small part of the liver is considered in the evaluation of biopsies, the breath test used in this study noninvasively reflects total liver function."
Funding for this pilot study was provided by grants from the Dutch SenterNovem Innovation Oriented Research Program on Genomics and the Transnational University Limburg, Belgium. The study authors reported no conflicts of interest.
Dr. Scott L. Friedman comments: The study findings are
"intriguing," and the performance metrics of the analysis of exhaled
VOCs "are promising but not exceptional," wrote Dr. Scott L. Friedman.
However, "they well exceed the predictive values of transaminases, so
that the technology has value and merits further refinement and
validation."
The investigators do "not indicate through what
metabolic pathways and in which cells these specific organic compounds
are generated, and why they might correlate with disease activity," he
added. "Without such insight, the test is a correlative marker rather
than a true biomarker since there is no mechanistic link to a
disease-related pathway, which is a key requirement for a biomarker."
Dr.
Friedman is professor of medicine, liver diseases, at the Mount Sinai
School of Medicine in New York. These remarks were adapted from his
editorial accompanying this article and another on fatty liver disease
and telomerase length (J. Hepatol. 2013;58:j407-8 ). He is a consultant
for Exalenz Biosciences, which produces the methacetin breath test.
Dr. Scott L. Friedman comments: The study findings are
"intriguing," and the performance metrics of the analysis of exhaled
VOCs "are promising but not exceptional," wrote Dr. Scott L. Friedman.
However, "they well exceed the predictive values of transaminases, so
that the technology has value and merits further refinement and
validation."
The investigators do "not indicate through what
metabolic pathways and in which cells these specific organic compounds
are generated, and why they might correlate with disease activity," he
added. "Without such insight, the test is a correlative marker rather
than a true biomarker since there is no mechanistic link to a
disease-related pathway, which is a key requirement for a biomarker."
Dr.
Friedman is professor of medicine, liver diseases, at the Mount Sinai
School of Medicine in New York. These remarks were adapted from his
editorial accompanying this article and another on fatty liver disease
and telomerase length (J. Hepatol. 2013;58:j407-8 ). He is a consultant
for Exalenz Biosciences, which produces the methacetin breath test.
Dr. Scott L. Friedman comments: The study findings are
"intriguing," and the performance metrics of the analysis of exhaled
VOCs "are promising but not exceptional," wrote Dr. Scott L. Friedman.
However, "they well exceed the predictive values of transaminases, so
that the technology has value and merits further refinement and
validation."
The investigators do "not indicate through what
metabolic pathways and in which cells these specific organic compounds
are generated, and why they might correlate with disease activity," he
added. "Without such insight, the test is a correlative marker rather
than a true biomarker since there is no mechanistic link to a
disease-related pathway, which is a key requirement for a biomarker."
Dr.
Friedman is professor of medicine, liver diseases, at the Mount Sinai
School of Medicine in New York. These remarks were adapted from his
editorial accompanying this article and another on fatty liver disease
and telomerase length (J. Hepatol. 2013;58:j407-8 ). He is a consultant
for Exalenz Biosciences, which produces the methacetin breath test.
The analysis of volatile organic compounds in exhaled breath may provide a noninvasive and accurate test for diagnosing nonalcoholic steatohepatitis, according to results from a pilot study published in March.
This test could reduce the number of unnecessary liver biopsies and missed diagnoses associated with assessing plasma transaminase levels, reported Dr. Froukje J. Verdam of Maastricht (the Netherlands) University Medical Center and her associates (J. Hepatol. 2013;58:543-8).
Researchers evaluated breath samples with gas chromatography–mass spectrometry from 65 consecutive overweight or obese patients before they underwent laparoscopic abdominal surgery, between October 2007 and May 2011. These results were compared with histologic analysis of liver biopsies taken intraoperatively and assessments of plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Overall, liver biopsies showed that 39 patients (60%) had nonalcoholic steatohepatitis (NASH), defined as "showing signs of steatosis and inflammation." Additionally, ALT and AST levels were significantly higher in patients with the disease than without. However, "parameters such as gender, age, BMI, and HbA1c did not differ significantly," reported the study authors.
The analysis of three volatile organic compounds (VOCs) – n-tridecane, 3-methylbutanonitrile, and 1-propanol – enabled investigators to distinguish between patients with and without NASH, with a sensitivity of 90%, a specificity of 69%, and an area under the receiver operating characteristic (ROC) curve of 0.77 plus or minus 0.07. The positive predictive value of using VOC analysis for NASH was 81%, while the negative predictive value was 82%.
In comparison, in 61 patients from whom plasma was available, the sensitivity of measuring ALT was 19%, while the specificity was 96%. The positive and negative predictive values of ALT were 88% and 43%, respectively.
Further evaluation of the AST/ALT ratio found that it was 32% sensitive and 79% specific, while positive and negative predictive values were 70% and 43%, respectively.
"It can be concluded that the diagnostic value of VOC is much higher than that of plasma transaminases, resulting in less misdiagnosed patients," wrote the study authors. Prediction of NASH using VOC, ALT, and the AST/ALT ratio did not reflect liver biopsy results in 18%, 51%, and 49% of subjects, respectively.
Using VOC evaluation rather than histologic testing has several other advantages, according to the researchers. "The analysis of exhaled breath can identify NASH presence at an early stage, and early identification in a mild stage is pivotal to enhance the chances of cure," they wrote. "Furthermore, whereas a small part of the liver is considered in the evaluation of biopsies, the breath test used in this study noninvasively reflects total liver function."
Funding for this pilot study was provided by grants from the Dutch SenterNovem Innovation Oriented Research Program on Genomics and the Transnational University Limburg, Belgium. The study authors reported no conflicts of interest.
The analysis of volatile organic compounds in exhaled breath may provide a noninvasive and accurate test for diagnosing nonalcoholic steatohepatitis, according to results from a pilot study published in March.
This test could reduce the number of unnecessary liver biopsies and missed diagnoses associated with assessing plasma transaminase levels, reported Dr. Froukje J. Verdam of Maastricht (the Netherlands) University Medical Center and her associates (J. Hepatol. 2013;58:543-8).
Researchers evaluated breath samples with gas chromatography–mass spectrometry from 65 consecutive overweight or obese patients before they underwent laparoscopic abdominal surgery, between October 2007 and May 2011. These results were compared with histologic analysis of liver biopsies taken intraoperatively and assessments of plasma levels of alanine aminotransferase (ALT) and aspartate aminotransferase (AST).
Overall, liver biopsies showed that 39 patients (60%) had nonalcoholic steatohepatitis (NASH), defined as "showing signs of steatosis and inflammation." Additionally, ALT and AST levels were significantly higher in patients with the disease than without. However, "parameters such as gender, age, BMI, and HbA1c did not differ significantly," reported the study authors.
The analysis of three volatile organic compounds (VOCs) – n-tridecane, 3-methylbutanonitrile, and 1-propanol – enabled investigators to distinguish between patients with and without NASH, with a sensitivity of 90%, a specificity of 69%, and an area under the receiver operating characteristic (ROC) curve of 0.77 plus or minus 0.07. The positive predictive value of using VOC analysis for NASH was 81%, while the negative predictive value was 82%.
In comparison, in 61 patients from whom plasma was available, the sensitivity of measuring ALT was 19%, while the specificity was 96%. The positive and negative predictive values of ALT were 88% and 43%, respectively.
Further evaluation of the AST/ALT ratio found that it was 32% sensitive and 79% specific, while positive and negative predictive values were 70% and 43%, respectively.
"It can be concluded that the diagnostic value of VOC is much higher than that of plasma transaminases, resulting in less misdiagnosed patients," wrote the study authors. Prediction of NASH using VOC, ALT, and the AST/ALT ratio did not reflect liver biopsy results in 18%, 51%, and 49% of subjects, respectively.
Using VOC evaluation rather than histologic testing has several other advantages, according to the researchers. "The analysis of exhaled breath can identify NASH presence at an early stage, and early identification in a mild stage is pivotal to enhance the chances of cure," they wrote. "Furthermore, whereas a small part of the liver is considered in the evaluation of biopsies, the breath test used in this study noninvasively reflects total liver function."
Funding for this pilot study was provided by grants from the Dutch SenterNovem Innovation Oriented Research Program on Genomics and the Transnational University Limburg, Belgium. The study authors reported no conflicts of interest.
Major finding: Analysis of volatile organic compounds (VOCs) in exhaled breath to diagnose NASH was 90% sensitive and 69% specific.
Data source: A pilot study of 65 consecutive patients comparing VOC analysis of exhaled breath with plasma transaminase levels and liver biopsy.
Disclosures: Funding for this pilot study was provided by grants from the Dutch SenterNovem Innovation Oriented Research Program on Genomics and the Transnational University Limburg, Belgium. The study authors reported no conflicts of interest.
New antiplatelet drug seems more effective than standard
Credit: Andre E.X. Brown
SAN FRANCISCO—The novel antiplatelet agent cangrelor is more effective than clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures, results of the CHAMPION PHOENIX trial suggest.
Researchers found that intravenous cangrelor reduced the overall odds of complications from stenting procedures, including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis.
Treatment with cangrelor also resulted in significantly higher rates of major and minor bleeding as compared to clopidogrel. But the rates of severe bleeding were similar between the treatment arms.
These data were presented on March 10 at the 2013 American College of Cardiology Scientific Session and simultaneously published in NEJM. The study was sponsored by The Medicines Company, the makers of cangrelor.
“We are very excited about the potential for this new medication to reduce complications in patients receiving coronary stents for a wide variety of indications,” said investigator Deepak L. Bhatt, MD, MPH, of Brigham and Women’s Hospital in Boston.
“In addition to being much quicker to take effect and more potent than currently available treatment options, this intravenous drug is reversible and has a fast offset of action, which could be an advantage if emergency surgery is needed.”
In this randomized, double-blind trial, Dr Bhatt and his colleagues compared cangrelor to clopidogrel in 11,145 patients treated at 153 centers around the world.
The study included patients who were undergoing elective or urgent percutaneous coronary intervention. Patients with a high risk of bleeding or recent exposure to other anticoagulants were excluded.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
A secondary endpoint was the rate of stent thrombosis alone. At 48 hours, 0.8% of patients in the cangrelor arm had stent thrombosis, as did 1.4% of patients in the clopidogrel arm (P=0.01). At 30 days, the rate was 1.3% in the cangrelor arm and 1.9% in the clopidogrel arm (P=0.01).
The primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, it measured 0.16% in the cangrelor arm and 0.11% in the clopidogrel arm (P=0.44).
Secondary endpoints included major and minor bleeding (not related to coronary artery bypass grafting) according to ACUITY criteria.
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Other treatment-emergent adverse events included agitation, diarrhea, chest pain, dyspnea, and procedural pain. There were significantly more cases of transient dyspnea with cangrelor
than with clopidogrel, at 1.2% and 0.3%, respectively (P<0.001). But there were no statistically significant differences with regard to adverse events other than those mentioned here.
The overall rate of treatment-related adverse events was 20.2% in the cangrelor arm and 19.1% in the clopidogrel arm (P=0.13). And these events led to treatment discontinuation in 0.5% of patients in the cangrelor arm and 0.4% of patients in the clopidogrel arm.
“The investigators feel the data are compelling,” Dr Bhatt concluded. “The data we’ve shown are clear and consistent across all relevant subgroups or patient populations. [Cangrelor] has several advantages, and nothing out there right now has quite the same biological properties.” ![]()

Credit: Andre E.X. Brown
SAN FRANCISCO—The novel antiplatelet agent cangrelor is more effective than clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures, results of the CHAMPION PHOENIX trial suggest.
Researchers found that intravenous cangrelor reduced the overall odds of complications from stenting procedures, including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis.
Treatment with cangrelor also resulted in significantly higher rates of major and minor bleeding as compared to clopidogrel. But the rates of severe bleeding were similar between the treatment arms.
These data were presented on March 10 at the 2013 American College of Cardiology Scientific Session and simultaneously published in NEJM. The study was sponsored by The Medicines Company, the makers of cangrelor.
“We are very excited about the potential for this new medication to reduce complications in patients receiving coronary stents for a wide variety of indications,” said investigator Deepak L. Bhatt, MD, MPH, of Brigham and Women’s Hospital in Boston.
“In addition to being much quicker to take effect and more potent than currently available treatment options, this intravenous drug is reversible and has a fast offset of action, which could be an advantage if emergency surgery is needed.”
In this randomized, double-blind trial, Dr Bhatt and his colleagues compared cangrelor to clopidogrel in 11,145 patients treated at 153 centers around the world.
The study included patients who were undergoing elective or urgent percutaneous coronary intervention. Patients with a high risk of bleeding or recent exposure to other anticoagulants were excluded.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
A secondary endpoint was the rate of stent thrombosis alone. At 48 hours, 0.8% of patients in the cangrelor arm had stent thrombosis, as did 1.4% of patients in the clopidogrel arm (P=0.01). At 30 days, the rate was 1.3% in the cangrelor arm and 1.9% in the clopidogrel arm (P=0.01).
The primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, it measured 0.16% in the cangrelor arm and 0.11% in the clopidogrel arm (P=0.44).
Secondary endpoints included major and minor bleeding (not related to coronary artery bypass grafting) according to ACUITY criteria.
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Other treatment-emergent adverse events included agitation, diarrhea, chest pain, dyspnea, and procedural pain. There were significantly more cases of transient dyspnea with cangrelor
than with clopidogrel, at 1.2% and 0.3%, respectively (P<0.001). But there were no statistically significant differences with regard to adverse events other than those mentioned here.
The overall rate of treatment-related adverse events was 20.2% in the cangrelor arm and 19.1% in the clopidogrel arm (P=0.13). And these events led to treatment discontinuation in 0.5% of patients in the cangrelor arm and 0.4% of patients in the clopidogrel arm.
“The investigators feel the data are compelling,” Dr Bhatt concluded. “The data we’ve shown are clear and consistent across all relevant subgroups or patient populations. [Cangrelor] has several advantages, and nothing out there right now has quite the same biological properties.” ![]()

Credit: Andre E.X. Brown
SAN FRANCISCO—The novel antiplatelet agent cangrelor is more effective than clopidogrel as thromboprophylaxis for patients undergoing coronary stent procedures, results of the CHAMPION PHOENIX trial suggest.
Researchers found that intravenous cangrelor reduced the overall odds of complications from stenting procedures, including death, myocardial infarction, ischemia-driven revascularization, and stent thrombosis.
Treatment with cangrelor also resulted in significantly higher rates of major and minor bleeding as compared to clopidogrel. But the rates of severe bleeding were similar between the treatment arms.
These data were presented on March 10 at the 2013 American College of Cardiology Scientific Session and simultaneously published in NEJM. The study was sponsored by The Medicines Company, the makers of cangrelor.
“We are very excited about the potential for this new medication to reduce complications in patients receiving coronary stents for a wide variety of indications,” said investigator Deepak L. Bhatt, MD, MPH, of Brigham and Women’s Hospital in Boston.
“In addition to being much quicker to take effect and more potent than currently available treatment options, this intravenous drug is reversible and has a fast offset of action, which could be an advantage if emergency surgery is needed.”
In this randomized, double-blind trial, Dr Bhatt and his colleagues compared cangrelor to clopidogrel in 11,145 patients treated at 153 centers around the world.
The study included patients who were undergoing elective or urgent percutaneous coronary intervention. Patients with a high risk of bleeding or recent exposure to other anticoagulants were excluded.
The study’s primary efficacy endpoint was the incidence of death, myocardial infarction, ischemia-driven revascularization, or stent thrombosis.
At 48 hours, 4.7% of patients in the cangrelor arm had met this endpoint, compared to 5.9% of patients in the clopidogrel arm (P=0.005). At 30 days, the incidence was 6.0% in the cangrelor arm and 7.0% in the clopidogrel arm (P=0.03).
A secondary endpoint was the rate of stent thrombosis alone. At 48 hours, 0.8% of patients in the cangrelor arm had stent thrombosis, as did 1.4% of patients in the clopidogrel arm (P=0.01). At 30 days, the rate was 1.3% in the cangrelor arm and 1.9% in the clopidogrel arm (P=0.01).
The primary safety endpoint was severe bleeding according to GUSTO criteria. At 48 hours, it measured 0.16% in the cangrelor arm and 0.11% in the clopidogrel arm (P=0.44).
Secondary endpoints included major and minor bleeding (not related to coronary artery bypass grafting) according to ACUITY criteria.
Major bleeding occurred in 4.3% of patients on cangrelor and 2.5% of patients on clopidogrel (P<0.001). And minor bleeding occurred in 11.8% of patients on cangrelor and 8.6% of patients on clopidogrel (P<0.001).
Other treatment-emergent adverse events included agitation, diarrhea, chest pain, dyspnea, and procedural pain. There were significantly more cases of transient dyspnea with cangrelor
than with clopidogrel, at 1.2% and 0.3%, respectively (P<0.001). But there were no statistically significant differences with regard to adverse events other than those mentioned here.
The overall rate of treatment-related adverse events was 20.2% in the cangrelor arm and 19.1% in the clopidogrel arm (P=0.13). And these events led to treatment discontinuation in 0.5% of patients in the cangrelor arm and 0.4% of patients in the clopidogrel arm.
“The investigators feel the data are compelling,” Dr Bhatt concluded. “The data we’ve shown are clear and consistent across all relevant subgroups or patient populations. [Cangrelor] has several advantages, and nothing out there right now has quite the same biological properties.” ![]()
A Sheep in Wolf's Clothing
A 51‐year‐old man presented with severe pain and swelling in the lower anterior right thigh. He stated that the symptoms limited his movement, and began 4 days prior to this presentation. He rated the pain severity a 10 on a 10‐point scale. He denied fevers, chills, or history of trauma or weight loss.
Cellulitis of the lower extremity is the most likely possibility, but the presence of severe pain and swelling of an extremity in the absence of trauma should always make the clinician consider deep‐seated infections such as myositis or necrotizing fasciitis. An early clue for necrotizing fasciitis is severe pain that is disproportionate to the physical examination findings. Erythema, bullous lesions, or crepitus can develop later in the course. The absence of fever and chills also raises the possibility of noninfectious causes such as unrecognized trauma, deep vein thrombosis, or tumor.
The patient had a 15‐year history of type 2 diabetes complicated by end‐stage renal disease secondary to diabetic nephropathy for which he had been on hemodialysis for 5 months, proliferative diabetic retinopathy that rendered him legally blind, hypertension, and anemia. He stated that his diabetes had been poorly controlled, especially after he started dialysis.
A history of poorly controlled diabetes mellitus certainly increases the risk of the infectious disorders mentioned above. The patient's long‐standing history of diabetes mellitus with secondary nephropathy and retinopathy puts him at higher risk of atherosclerosis and vascular insufficiency, which consequently increase his risk for ischemic myonecrosis. Diabetic amyotrophy (diabetic lumbosacral plexopathy) is also a possibility, as it usually manifests with acute, unilateral, and focal tenderness followed by weakness involving a proximal leg. However, it typically occurs in patients who have been recently diagnosed with type 2 diabetes mellitus or whose disease has been under fairly good control and usually is associated with significant weight loss.
The patient was on oral medications for his diabetes until 1year before his presentation, at which point he was switched to insulin therapy. His other medications were amlodipine, lisinopril, aspirin, sevelamer, calcitriol, and calcium and iron supplements. He denied using alcohol, tobacco, or illicit drugs. He lives in Chicago and denies a recent travel history. His family history was significant for type 2 diabetes in multiple family members.
The absence of drugs, tobacco, and alcohol lowers the risk of some infectious and ischemic conditions. Patients with alcoholic liver disease who live in the southern United States are predisposed to developing Vibrio vulnificus myositis and fasciitis after ingesting contaminated oysters during the summer months. However, the clinical presentation of Vibrio usually includes septic shock and bullous lesions on the lower extremity. Also, the patient denies any recent travel to the southern United States, which makes Vibrio myositis and fasciitis less likely. Tobacco abuse increases the risk of atherosclerosis, peripheral vascular insufficiency, and ischemic myonecrosis.
The patient had a temperature of 99.1F, blood pressure of 139/85 mm Hg, pulse of 97 beats/minute, and respiratory rate of 18 breaths/minute. His body mass index was 31 kg/m2. Physical examination revealed a firm, warm, severely tender area of swelling in the inferomedial aspect of the right thigh. The knee was also swollen, and effusion could not be ruled out. The range of motion of the knee was markedly limited by pain. The skin overlying the swelling was erythematous but not broken. No crepitus was noted. The strength of the right lower extremity muscles could not be accurately assessed because of the patient's excruciating pain, but the patient was able to move his foot and toes against gravity. Sensation was absent in most of the tested points in the feet but was normal in the legs. The deep tendon reflexes in both ankles were normal. The pedal pulses were mildly decreased in both feet. He also had extremely decreased visual acuity, which has been chronic. The rest of the physical examination was unremarkable.
The absence of fever does not rule out a serious infection in a diabetic patient but does raise the possibility of a noninfectious cause. Also, over‐the‐counter acetaminophen or nonsteroidal anti‐inflammatory drugs could mask a fever. The patient's physical examination was significant for obesity, a risk factor for developing deep‐seated infections, and a firm and severely tender area of swelling near the right knee that limited range of motion. Septic arthritis of the knee is one possibility; arthrocentesis should be performed as soon as possible. The absence of crepitus, because it is a late physical examination finding, does not rule out myositis or necrotizing fasciitis. The presence of unilateral lower extremity swelling also raises the suspicion for a deep vein thrombosis, which warrants compression ultrasonography. The localized tenderness and the lack of dermatological manifestations, such as Gottron's papules, makes an inflammatory myositis such as dermatomyositis much less likely.
Laboratory studies demonstrated a hemoglobin A1C of 13.0% (reference range, 4.36.1%), fasting blood glucose level of 224 mg/dL (reference range, 7099 mg/dL), white blood cell count of 8300 cells/mm3 (reference range, 450011,000 cells/mm3) without band forms, erythrocyte sedimentation rate of 81 mm/hr (reference range, <14 mm/hr), and creatinine kinase level of 582 IU/L (reference range, 30200 IU/L). Routine chemistries were normal otherwise. An x‐ray of the right knee revealed soft tissue edema. The right knee was aspirated, and fluid analysis revealed a white blood cell count of 106 cells/mm3 (reference range, <200 cell/mm3). Compression ultrasonography of the right lower extremity did not reveal thrombosis.
Poor glycemic control, as evidenced by a high hemoglobin A1C level, is associated with a higher probability of infectious complications. An elevated sedimentation rate is compatible with an infection, and an increased creatinine kinase intensifies suspicion of myositis or myonecrosis. A normal white blood cell count decreases, but does not eliminate, the likelihood of a serious bacterial infection. The fluid analysis rules out septic arthritis, and the compression ultrasonography findings make deep vein thrombosis very unlikely. However, the differential diagnosis still includes myositis, clostridial myonecrosis, cellulitis, and necrotizing fasciitis. The patient should undergo magnetic resonance imaging (MRI) of the lower extremity, and a surgical consultation should be obtained to consider the possibility of surgical exploration.
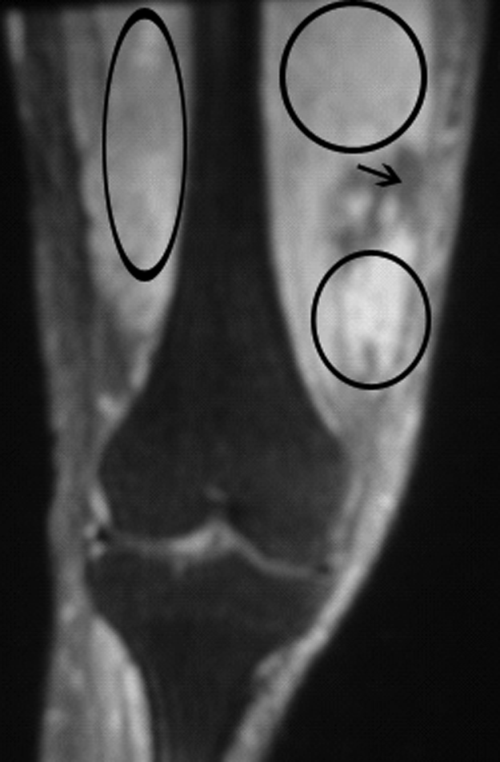
Blood and the aspirated fluids were sent for culturing, and the patient was started on empiric antibiotics. MRI of his right thigh revealed extensive edema involving the vastus medialis and lateralis of the quadriceps as well as subcutaneous edema without fascial enhancement or gas (Figure 1).
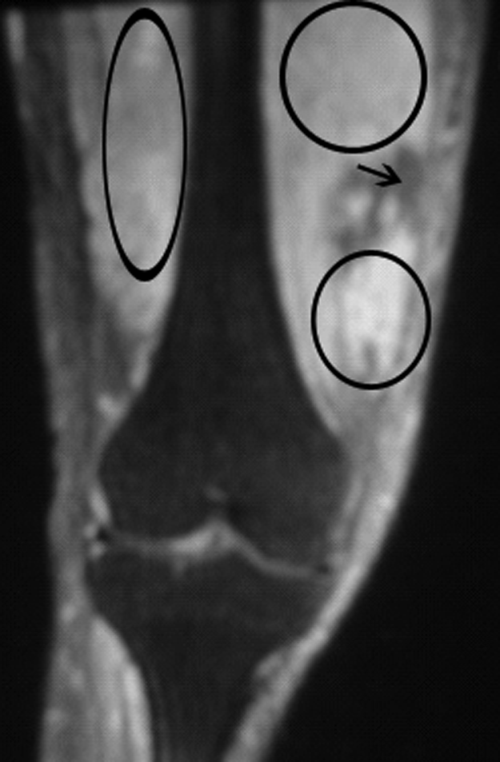
The absence of gas and fascial enhancement makes clostridial myonecrosis or necrotizing fasciitis less likely. The absence of a fluid collection in the muscle makes pyomyositis due to Staphylococci unlikely. Broad‐spectrum antibiotic coverage (usually vancomycin and either piperacillin/tazobactam or a carbapenem) targeting methicillin‐resistant Staphylococcus aureus, anaerobes, Streptococci, and Enterobacteriaceae should be empirically started as soon as cultures are obtained. Clindamycin should be part of the empiric antibiotic regimen to block toxin production in the event that Streptococcus pyogenes is responsible.
Surgical biopsy of the right vastus medialis muscle was performed, and tissue was sent for Gram staining, culture, and routine histopathological analysis. Gram staining was negative, and histopathological analysis revealed ischemic skeletal muscle fibers with areas of necrosis (Figure 2). Cultures from blood, fluid from the right knee, and muscular tissue samples did not grow any bacteria.
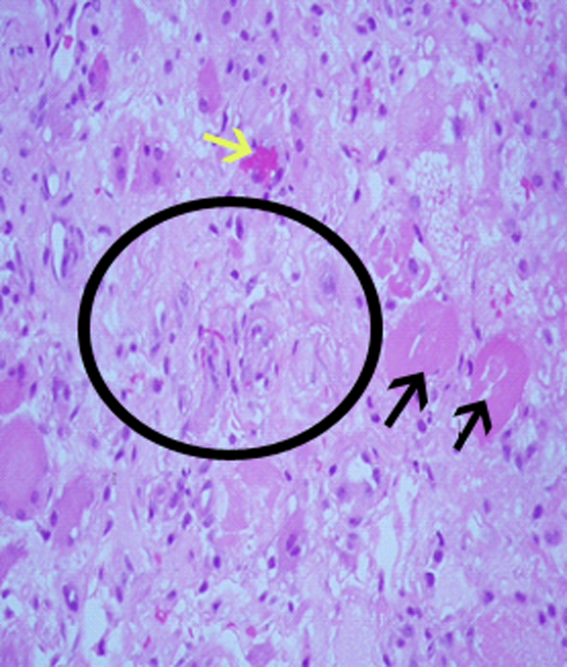
The muscle biopsy results are consistent with myonecrosis. Clostridial myonecrosis is possible but usually is associated with gas in tissues or occurs in the setting of intra‐abdominal pathology or severe trauma, and tissue culture was negative. Ischemic myonecrosis due to severe vascular insufficiency would be unlikely given the presence of pedal pulses and the absence of toes or forefoot cyanosis. A vasculitis syndrome is also unlikely because of the focal nature of the findings and the absence of weight loss, muscle weakness, and chronic joint pain in the patient's history. Calciphylaxis (calcific uremic arteriolopathy) might be considered in a patient with end‐stage renal disease who presents with a thigh pain; however, this condition is usually characterized by areas of ischemic necrosis that develop in the dermis and/or subcutaneous fat and infrequently involve muscles. The absence of the painful subcutaneous nodules typical of calciphylaxis makes it an unlikely diagnosis.
A diagnosis of diabetic myonecrosis was made. Antibiotics were discontinued, and the patient was treated symptomatically. His symptoms improved during the next few days. The patient was discharged from the hospital, and conservative management with bed rest and analgesics for 4 weeks was prescribed. Four months later, however, the patient returned with similar symptoms in the contralateral thigh. The patient was diagnosed with recurrent diabetic myonecrosis by MRI and muscle biopsy findings. Conservative management was advised, and the patient became pain‐free in a few weeks.
DISCUSSION
Diabetic myonecrosis (also known as diabetic muscle infarction) is a rare disorder initially described in 1965[1] that typically presents spontaneously as an acute, localized, severely painful swelling that limits the mobility of the affected extremity, usually without systemic signs of infection. It affects the thighs in 83% of patients and the calves in 17% of patients.[2, 3] Bilateral involvement, which is usually asynchronous, occurs in one‐third of patients.[4] The upper limbs are rarely involved. Diabetic myonecrosis affects patients who have a relatively longstanding history of diabetes. It is commonly associated with the microvascular complications of diabetes, including nephropathy (80% of patients), retinopathy (60% of patents), and/or neuropathy (64% of patients).[3, 5] The pathogenesis of diabetic myonecrosis is unclear, but the disease is likely due to a diffuse microangiopathy and atherosclerosis.[2, 5] Some authors have suggested that abnormalities in the clotting or fibrinolytic pathways play a role in the etiology of the disorder.[6]
Clinical and MRI findings can be used to make the diagnosis with reasonable certainty.[3, 5] Although both ultrasonography and MRI have been used to assess patients with diabetic myonecrosis, MRI with intravenous contrast enhancement appears to be the most useful diagnostic technique. It demonstrates extensive edema within the muscle(s), muscle enlargement, subcutaneous and interfascial edema, a patchwork pattern of involvement, and a high signal intensity on T2‐weighted images.[4, 7] Gadolinium enhancement may reveal an enhanced margin of the infarcted muscle with a central nonenhancing area of necrotic tissue.[4, 5] Muscle biopsy is not typically indicated because it may prolong recovery time and lead to infections.[8, 9, 10, 11] When performed, however, muscle biopsy reveals ischemic muscle fibers in different stages of degeneration and regeneration, with areas of necrosis and edema. Occlusion of arterioles and capillaries by fibrin could also be seen.[1] Although the patient underwent a muscle biopsy because infection could not be excluded definitively on clinical grounds, we believe that repeating the biopsy 4 months later was inappropriate.
Diabetic myonecrosis should be considered in a diabetic patient who presents with severe localized muscle pain and swelling of an extremity, especially if the clinical features favoring infection are absent. The differential diagnosis should include infection (eg, clostridial myonecrosis, myositis, cellulitis, abscess, necrotizing fasciitis, osteomyelitis), trauma (eg, hematoma, muscle rupture, myositis ossificans), peripheral neuropathy (particularly lumbosacral plexopathy), vascular disorders (deep vein thrombosis, and compartment syndrome), tumors, inflammatory muscle diseases, and drug‐related myositis.
No evidence‐based recommendations regarding the management of diabetic myonecrosis are available, although the findings of one retrospective analysis support conservative management with bed rest, leg elevation, and analgesics.[12] Physiotherapy may cause the condition to worsen,[13, 14] but routine daily activity, although often painful, is not harmful.[14] Some authors suggest a cautious use of antiplatelet or anti‐inflammatory medications.[12] We would also recommend achieving good glycemic control during the illness. Owing to the rarity of the disease, however, no studies have definitively shown that this hastens recovery or prevents recurrent diabetic myonecrosis. Surgery may prolong the recovery period; one study found that the recovery period of patients with diabetic myonecrosis who underwent surgery was longer than that of those who were treated conservatively (13 weeks vs 5.5 weeks).[12] Patients with diabetic myonecrosis have a good short‐term prognosis. Longer‐term, however, they have a poor prognosis; their recurrence rate is as high as 40%, and their 2‐year mortality rate is 10%, even after one episode of the disease. Death in these patients is mainly due to macrovascular events.[12]
TEACHING POINTS
- Diabetic myonecrosis is a rare complication of longstanding and poorly controlled diabetes. It usually presents with acute localized muscular pain in the lower extremities.
- Although a definitive diagnosis of diabetic myonecrosis is histopathologic, a clinical diagnosis can be made with reasonable certainty for patients with compatible MRI findings and no clinical or laboratory features suggesting infection.
- Conservative management with bed rest, analgesics, and antiplatelets is recommended. Surgery should be avoided, as it may prolong recovery.
Disclosure
Nothing to report.
- , . Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1:39–42.
- . Diabetic muscle infarction: an underdiagnosed complication of long‐standing diabetes. Diabetes Care. 2003;26:211–215.
- , , . Diabetic muscle infarction: case report and review. J Rheumatol. 2004;31:190–194.
- , , , , , . Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology. 1999;211:241–247.
- , , , . Skeletal muscle infarction in diabetes mellitus. J Rheumatol. 2000;27:1063–1068.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29–1997. A 54‐year‐old diabetic woman with pain and swelling of the leg. N Engl J Med. 1997;337:839–845.
- , , . Clinical and radiological aspects of idiopathic diabetic muscle infarction. Rational approach to diagnosis and treatment. J Bone Joint Surg Br. 1999;81:323–326.
- , , . Diabetic muscular infarction. Preventing morbidity by avoiding excisional biopsy. Arch Intern Med. 1997;157:1611.
- , . Case‐of‐the‐month: painful thigh mass in a young woman: diabetic muscle infarction. Muscle Nerve. 1992;15:850–855.
- . Diabetic muscle infarction: magnetic resonance imaging (MRI) avoids the need for biopsy. Muscle Nerve. 1995;18:129–130.
- , , , . Skeletal muscle infarction in diabetes: MR findings. J Comput Assist Tomogr. 1993;17:986–988.
- , . Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11:8–12.
- , , . Diabetic muscular infarction. Semin Arthritis Rheum. 1993;22:280–287.
- , . Focal infarction of muscle in diabetics. Diabetes Care. 1986;9:623–630.
A 51‐year‐old man presented with severe pain and swelling in the lower anterior right thigh. He stated that the symptoms limited his movement, and began 4 days prior to this presentation. He rated the pain severity a 10 on a 10‐point scale. He denied fevers, chills, or history of trauma or weight loss.
Cellulitis of the lower extremity is the most likely possibility, but the presence of severe pain and swelling of an extremity in the absence of trauma should always make the clinician consider deep‐seated infections such as myositis or necrotizing fasciitis. An early clue for necrotizing fasciitis is severe pain that is disproportionate to the physical examination findings. Erythema, bullous lesions, or crepitus can develop later in the course. The absence of fever and chills also raises the possibility of noninfectious causes such as unrecognized trauma, deep vein thrombosis, or tumor.
The patient had a 15‐year history of type 2 diabetes complicated by end‐stage renal disease secondary to diabetic nephropathy for which he had been on hemodialysis for 5 months, proliferative diabetic retinopathy that rendered him legally blind, hypertension, and anemia. He stated that his diabetes had been poorly controlled, especially after he started dialysis.
A history of poorly controlled diabetes mellitus certainly increases the risk of the infectious disorders mentioned above. The patient's long‐standing history of diabetes mellitus with secondary nephropathy and retinopathy puts him at higher risk of atherosclerosis and vascular insufficiency, which consequently increase his risk for ischemic myonecrosis. Diabetic amyotrophy (diabetic lumbosacral plexopathy) is also a possibility, as it usually manifests with acute, unilateral, and focal tenderness followed by weakness involving a proximal leg. However, it typically occurs in patients who have been recently diagnosed with type 2 diabetes mellitus or whose disease has been under fairly good control and usually is associated with significant weight loss.
The patient was on oral medications for his diabetes until 1year before his presentation, at which point he was switched to insulin therapy. His other medications were amlodipine, lisinopril, aspirin, sevelamer, calcitriol, and calcium and iron supplements. He denied using alcohol, tobacco, or illicit drugs. He lives in Chicago and denies a recent travel history. His family history was significant for type 2 diabetes in multiple family members.
The absence of drugs, tobacco, and alcohol lowers the risk of some infectious and ischemic conditions. Patients with alcoholic liver disease who live in the southern United States are predisposed to developing Vibrio vulnificus myositis and fasciitis after ingesting contaminated oysters during the summer months. However, the clinical presentation of Vibrio usually includes septic shock and bullous lesions on the lower extremity. Also, the patient denies any recent travel to the southern United States, which makes Vibrio myositis and fasciitis less likely. Tobacco abuse increases the risk of atherosclerosis, peripheral vascular insufficiency, and ischemic myonecrosis.
The patient had a temperature of 99.1F, blood pressure of 139/85 mm Hg, pulse of 97 beats/minute, and respiratory rate of 18 breaths/minute. His body mass index was 31 kg/m2. Physical examination revealed a firm, warm, severely tender area of swelling in the inferomedial aspect of the right thigh. The knee was also swollen, and effusion could not be ruled out. The range of motion of the knee was markedly limited by pain. The skin overlying the swelling was erythematous but not broken. No crepitus was noted. The strength of the right lower extremity muscles could not be accurately assessed because of the patient's excruciating pain, but the patient was able to move his foot and toes against gravity. Sensation was absent in most of the tested points in the feet but was normal in the legs. The deep tendon reflexes in both ankles were normal. The pedal pulses were mildly decreased in both feet. He also had extremely decreased visual acuity, which has been chronic. The rest of the physical examination was unremarkable.
The absence of fever does not rule out a serious infection in a diabetic patient but does raise the possibility of a noninfectious cause. Also, over‐the‐counter acetaminophen or nonsteroidal anti‐inflammatory drugs could mask a fever. The patient's physical examination was significant for obesity, a risk factor for developing deep‐seated infections, and a firm and severely tender area of swelling near the right knee that limited range of motion. Septic arthritis of the knee is one possibility; arthrocentesis should be performed as soon as possible. The absence of crepitus, because it is a late physical examination finding, does not rule out myositis or necrotizing fasciitis. The presence of unilateral lower extremity swelling also raises the suspicion for a deep vein thrombosis, which warrants compression ultrasonography. The localized tenderness and the lack of dermatological manifestations, such as Gottron's papules, makes an inflammatory myositis such as dermatomyositis much less likely.
Laboratory studies demonstrated a hemoglobin A1C of 13.0% (reference range, 4.36.1%), fasting blood glucose level of 224 mg/dL (reference range, 7099 mg/dL), white blood cell count of 8300 cells/mm3 (reference range, 450011,000 cells/mm3) without band forms, erythrocyte sedimentation rate of 81 mm/hr (reference range, <14 mm/hr), and creatinine kinase level of 582 IU/L (reference range, 30200 IU/L). Routine chemistries were normal otherwise. An x‐ray of the right knee revealed soft tissue edema. The right knee was aspirated, and fluid analysis revealed a white blood cell count of 106 cells/mm3 (reference range, <200 cell/mm3). Compression ultrasonography of the right lower extremity did not reveal thrombosis.
Poor glycemic control, as evidenced by a high hemoglobin A1C level, is associated with a higher probability of infectious complications. An elevated sedimentation rate is compatible with an infection, and an increased creatinine kinase intensifies suspicion of myositis or myonecrosis. A normal white blood cell count decreases, but does not eliminate, the likelihood of a serious bacterial infection. The fluid analysis rules out septic arthritis, and the compression ultrasonography findings make deep vein thrombosis very unlikely. However, the differential diagnosis still includes myositis, clostridial myonecrosis, cellulitis, and necrotizing fasciitis. The patient should undergo magnetic resonance imaging (MRI) of the lower extremity, and a surgical consultation should be obtained to consider the possibility of surgical exploration.
Blood and the aspirated fluids were sent for culturing, and the patient was started on empiric antibiotics. MRI of his right thigh revealed extensive edema involving the vastus medialis and lateralis of the quadriceps as well as subcutaneous edema without fascial enhancement or gas (Figure 1).

The absence of gas and fascial enhancement makes clostridial myonecrosis or necrotizing fasciitis less likely. The absence of a fluid collection in the muscle makes pyomyositis due to Staphylococci unlikely. Broad‐spectrum antibiotic coverage (usually vancomycin and either piperacillin/tazobactam or a carbapenem) targeting methicillin‐resistant Staphylococcus aureus, anaerobes, Streptococci, and Enterobacteriaceae should be empirically started as soon as cultures are obtained. Clindamycin should be part of the empiric antibiotic regimen to block toxin production in the event that Streptococcus pyogenes is responsible.
Surgical biopsy of the right vastus medialis muscle was performed, and tissue was sent for Gram staining, culture, and routine histopathological analysis. Gram staining was negative, and histopathological analysis revealed ischemic skeletal muscle fibers with areas of necrosis (Figure 2). Cultures from blood, fluid from the right knee, and muscular tissue samples did not grow any bacteria.

The muscle biopsy results are consistent with myonecrosis. Clostridial myonecrosis is possible but usually is associated with gas in tissues or occurs in the setting of intra‐abdominal pathology or severe trauma, and tissue culture was negative. Ischemic myonecrosis due to severe vascular insufficiency would be unlikely given the presence of pedal pulses and the absence of toes or forefoot cyanosis. A vasculitis syndrome is also unlikely because of the focal nature of the findings and the absence of weight loss, muscle weakness, and chronic joint pain in the patient's history. Calciphylaxis (calcific uremic arteriolopathy) might be considered in a patient with end‐stage renal disease who presents with a thigh pain; however, this condition is usually characterized by areas of ischemic necrosis that develop in the dermis and/or subcutaneous fat and infrequently involve muscles. The absence of the painful subcutaneous nodules typical of calciphylaxis makes it an unlikely diagnosis.
A diagnosis of diabetic myonecrosis was made. Antibiotics were discontinued, and the patient was treated symptomatically. His symptoms improved during the next few days. The patient was discharged from the hospital, and conservative management with bed rest and analgesics for 4 weeks was prescribed. Four months later, however, the patient returned with similar symptoms in the contralateral thigh. The patient was diagnosed with recurrent diabetic myonecrosis by MRI and muscle biopsy findings. Conservative management was advised, and the patient became pain‐free in a few weeks.
DISCUSSION
Diabetic myonecrosis (also known as diabetic muscle infarction) is a rare disorder initially described in 1965[1] that typically presents spontaneously as an acute, localized, severely painful swelling that limits the mobility of the affected extremity, usually without systemic signs of infection. It affects the thighs in 83% of patients and the calves in 17% of patients.[2, 3] Bilateral involvement, which is usually asynchronous, occurs in one‐third of patients.[4] The upper limbs are rarely involved. Diabetic myonecrosis affects patients who have a relatively longstanding history of diabetes. It is commonly associated with the microvascular complications of diabetes, including nephropathy (80% of patients), retinopathy (60% of patents), and/or neuropathy (64% of patients).[3, 5] The pathogenesis of diabetic myonecrosis is unclear, but the disease is likely due to a diffuse microangiopathy and atherosclerosis.[2, 5] Some authors have suggested that abnormalities in the clotting or fibrinolytic pathways play a role in the etiology of the disorder.[6]
Clinical and MRI findings can be used to make the diagnosis with reasonable certainty.[3, 5] Although both ultrasonography and MRI have been used to assess patients with diabetic myonecrosis, MRI with intravenous contrast enhancement appears to be the most useful diagnostic technique. It demonstrates extensive edema within the muscle(s), muscle enlargement, subcutaneous and interfascial edema, a patchwork pattern of involvement, and a high signal intensity on T2‐weighted images.[4, 7] Gadolinium enhancement may reveal an enhanced margin of the infarcted muscle with a central nonenhancing area of necrotic tissue.[4, 5] Muscle biopsy is not typically indicated because it may prolong recovery time and lead to infections.[8, 9, 10, 11] When performed, however, muscle biopsy reveals ischemic muscle fibers in different stages of degeneration and regeneration, with areas of necrosis and edema. Occlusion of arterioles and capillaries by fibrin could also be seen.[1] Although the patient underwent a muscle biopsy because infection could not be excluded definitively on clinical grounds, we believe that repeating the biopsy 4 months later was inappropriate.
Diabetic myonecrosis should be considered in a diabetic patient who presents with severe localized muscle pain and swelling of an extremity, especially if the clinical features favoring infection are absent. The differential diagnosis should include infection (eg, clostridial myonecrosis, myositis, cellulitis, abscess, necrotizing fasciitis, osteomyelitis), trauma (eg, hematoma, muscle rupture, myositis ossificans), peripheral neuropathy (particularly lumbosacral plexopathy), vascular disorders (deep vein thrombosis, and compartment syndrome), tumors, inflammatory muscle diseases, and drug‐related myositis.
No evidence‐based recommendations regarding the management of diabetic myonecrosis are available, although the findings of one retrospective analysis support conservative management with bed rest, leg elevation, and analgesics.[12] Physiotherapy may cause the condition to worsen,[13, 14] but routine daily activity, although often painful, is not harmful.[14] Some authors suggest a cautious use of antiplatelet or anti‐inflammatory medications.[12] We would also recommend achieving good glycemic control during the illness. Owing to the rarity of the disease, however, no studies have definitively shown that this hastens recovery or prevents recurrent diabetic myonecrosis. Surgery may prolong the recovery period; one study found that the recovery period of patients with diabetic myonecrosis who underwent surgery was longer than that of those who were treated conservatively (13 weeks vs 5.5 weeks).[12] Patients with diabetic myonecrosis have a good short‐term prognosis. Longer‐term, however, they have a poor prognosis; their recurrence rate is as high as 40%, and their 2‐year mortality rate is 10%, even after one episode of the disease. Death in these patients is mainly due to macrovascular events.[12]
TEACHING POINTS
- Diabetic myonecrosis is a rare complication of longstanding and poorly controlled diabetes. It usually presents with acute localized muscular pain in the lower extremities.
- Although a definitive diagnosis of diabetic myonecrosis is histopathologic, a clinical diagnosis can be made with reasonable certainty for patients with compatible MRI findings and no clinical or laboratory features suggesting infection.
- Conservative management with bed rest, analgesics, and antiplatelets is recommended. Surgery should be avoided, as it may prolong recovery.
Disclosure
Nothing to report.
A 51‐year‐old man presented with severe pain and swelling in the lower anterior right thigh. He stated that the symptoms limited his movement, and began 4 days prior to this presentation. He rated the pain severity a 10 on a 10‐point scale. He denied fevers, chills, or history of trauma or weight loss.
Cellulitis of the lower extremity is the most likely possibility, but the presence of severe pain and swelling of an extremity in the absence of trauma should always make the clinician consider deep‐seated infections such as myositis or necrotizing fasciitis. An early clue for necrotizing fasciitis is severe pain that is disproportionate to the physical examination findings. Erythema, bullous lesions, or crepitus can develop later in the course. The absence of fever and chills also raises the possibility of noninfectious causes such as unrecognized trauma, deep vein thrombosis, or tumor.
The patient had a 15‐year history of type 2 diabetes complicated by end‐stage renal disease secondary to diabetic nephropathy for which he had been on hemodialysis for 5 months, proliferative diabetic retinopathy that rendered him legally blind, hypertension, and anemia. He stated that his diabetes had been poorly controlled, especially after he started dialysis.
A history of poorly controlled diabetes mellitus certainly increases the risk of the infectious disorders mentioned above. The patient's long‐standing history of diabetes mellitus with secondary nephropathy and retinopathy puts him at higher risk of atherosclerosis and vascular insufficiency, which consequently increase his risk for ischemic myonecrosis. Diabetic amyotrophy (diabetic lumbosacral plexopathy) is also a possibility, as it usually manifests with acute, unilateral, and focal tenderness followed by weakness involving a proximal leg. However, it typically occurs in patients who have been recently diagnosed with type 2 diabetes mellitus or whose disease has been under fairly good control and usually is associated with significant weight loss.
The patient was on oral medications for his diabetes until 1year before his presentation, at which point he was switched to insulin therapy. His other medications were amlodipine, lisinopril, aspirin, sevelamer, calcitriol, and calcium and iron supplements. He denied using alcohol, tobacco, or illicit drugs. He lives in Chicago and denies a recent travel history. His family history was significant for type 2 diabetes in multiple family members.
The absence of drugs, tobacco, and alcohol lowers the risk of some infectious and ischemic conditions. Patients with alcoholic liver disease who live in the southern United States are predisposed to developing Vibrio vulnificus myositis and fasciitis after ingesting contaminated oysters during the summer months. However, the clinical presentation of Vibrio usually includes septic shock and bullous lesions on the lower extremity. Also, the patient denies any recent travel to the southern United States, which makes Vibrio myositis and fasciitis less likely. Tobacco abuse increases the risk of atherosclerosis, peripheral vascular insufficiency, and ischemic myonecrosis.
The patient had a temperature of 99.1F, blood pressure of 139/85 mm Hg, pulse of 97 beats/minute, and respiratory rate of 18 breaths/minute. His body mass index was 31 kg/m2. Physical examination revealed a firm, warm, severely tender area of swelling in the inferomedial aspect of the right thigh. The knee was also swollen, and effusion could not be ruled out. The range of motion of the knee was markedly limited by pain. The skin overlying the swelling was erythematous but not broken. No crepitus was noted. The strength of the right lower extremity muscles could not be accurately assessed because of the patient's excruciating pain, but the patient was able to move his foot and toes against gravity. Sensation was absent in most of the tested points in the feet but was normal in the legs. The deep tendon reflexes in both ankles were normal. The pedal pulses were mildly decreased in both feet. He also had extremely decreased visual acuity, which has been chronic. The rest of the physical examination was unremarkable.
The absence of fever does not rule out a serious infection in a diabetic patient but does raise the possibility of a noninfectious cause. Also, over‐the‐counter acetaminophen or nonsteroidal anti‐inflammatory drugs could mask a fever. The patient's physical examination was significant for obesity, a risk factor for developing deep‐seated infections, and a firm and severely tender area of swelling near the right knee that limited range of motion. Septic arthritis of the knee is one possibility; arthrocentesis should be performed as soon as possible. The absence of crepitus, because it is a late physical examination finding, does not rule out myositis or necrotizing fasciitis. The presence of unilateral lower extremity swelling also raises the suspicion for a deep vein thrombosis, which warrants compression ultrasonography. The localized tenderness and the lack of dermatological manifestations, such as Gottron's papules, makes an inflammatory myositis such as dermatomyositis much less likely.
Laboratory studies demonstrated a hemoglobin A1C of 13.0% (reference range, 4.36.1%), fasting blood glucose level of 224 mg/dL (reference range, 7099 mg/dL), white blood cell count of 8300 cells/mm3 (reference range, 450011,000 cells/mm3) without band forms, erythrocyte sedimentation rate of 81 mm/hr (reference range, <14 mm/hr), and creatinine kinase level of 582 IU/L (reference range, 30200 IU/L). Routine chemistries were normal otherwise. An x‐ray of the right knee revealed soft tissue edema. The right knee was aspirated, and fluid analysis revealed a white blood cell count of 106 cells/mm3 (reference range, <200 cell/mm3). Compression ultrasonography of the right lower extremity did not reveal thrombosis.
Poor glycemic control, as evidenced by a high hemoglobin A1C level, is associated with a higher probability of infectious complications. An elevated sedimentation rate is compatible with an infection, and an increased creatinine kinase intensifies suspicion of myositis or myonecrosis. A normal white blood cell count decreases, but does not eliminate, the likelihood of a serious bacterial infection. The fluid analysis rules out septic arthritis, and the compression ultrasonography findings make deep vein thrombosis very unlikely. However, the differential diagnosis still includes myositis, clostridial myonecrosis, cellulitis, and necrotizing fasciitis. The patient should undergo magnetic resonance imaging (MRI) of the lower extremity, and a surgical consultation should be obtained to consider the possibility of surgical exploration.
Blood and the aspirated fluids were sent for culturing, and the patient was started on empiric antibiotics. MRI of his right thigh revealed extensive edema involving the vastus medialis and lateralis of the quadriceps as well as subcutaneous edema without fascial enhancement or gas (Figure 1).

The absence of gas and fascial enhancement makes clostridial myonecrosis or necrotizing fasciitis less likely. The absence of a fluid collection in the muscle makes pyomyositis due to Staphylococci unlikely. Broad‐spectrum antibiotic coverage (usually vancomycin and either piperacillin/tazobactam or a carbapenem) targeting methicillin‐resistant Staphylococcus aureus, anaerobes, Streptococci, and Enterobacteriaceae should be empirically started as soon as cultures are obtained. Clindamycin should be part of the empiric antibiotic regimen to block toxin production in the event that Streptococcus pyogenes is responsible.
Surgical biopsy of the right vastus medialis muscle was performed, and tissue was sent for Gram staining, culture, and routine histopathological analysis. Gram staining was negative, and histopathological analysis revealed ischemic skeletal muscle fibers with areas of necrosis (Figure 2). Cultures from blood, fluid from the right knee, and muscular tissue samples did not grow any bacteria.

The muscle biopsy results are consistent with myonecrosis. Clostridial myonecrosis is possible but usually is associated with gas in tissues or occurs in the setting of intra‐abdominal pathology or severe trauma, and tissue culture was negative. Ischemic myonecrosis due to severe vascular insufficiency would be unlikely given the presence of pedal pulses and the absence of toes or forefoot cyanosis. A vasculitis syndrome is also unlikely because of the focal nature of the findings and the absence of weight loss, muscle weakness, and chronic joint pain in the patient's history. Calciphylaxis (calcific uremic arteriolopathy) might be considered in a patient with end‐stage renal disease who presents with a thigh pain; however, this condition is usually characterized by areas of ischemic necrosis that develop in the dermis and/or subcutaneous fat and infrequently involve muscles. The absence of the painful subcutaneous nodules typical of calciphylaxis makes it an unlikely diagnosis.
A diagnosis of diabetic myonecrosis was made. Antibiotics were discontinued, and the patient was treated symptomatically. His symptoms improved during the next few days. The patient was discharged from the hospital, and conservative management with bed rest and analgesics for 4 weeks was prescribed. Four months later, however, the patient returned with similar symptoms in the contralateral thigh. The patient was diagnosed with recurrent diabetic myonecrosis by MRI and muscle biopsy findings. Conservative management was advised, and the patient became pain‐free in a few weeks.
DISCUSSION
Diabetic myonecrosis (also known as diabetic muscle infarction) is a rare disorder initially described in 1965[1] that typically presents spontaneously as an acute, localized, severely painful swelling that limits the mobility of the affected extremity, usually without systemic signs of infection. It affects the thighs in 83% of patients and the calves in 17% of patients.[2, 3] Bilateral involvement, which is usually asynchronous, occurs in one‐third of patients.[4] The upper limbs are rarely involved. Diabetic myonecrosis affects patients who have a relatively longstanding history of diabetes. It is commonly associated with the microvascular complications of diabetes, including nephropathy (80% of patients), retinopathy (60% of patents), and/or neuropathy (64% of patients).[3, 5] The pathogenesis of diabetic myonecrosis is unclear, but the disease is likely due to a diffuse microangiopathy and atherosclerosis.[2, 5] Some authors have suggested that abnormalities in the clotting or fibrinolytic pathways play a role in the etiology of the disorder.[6]
Clinical and MRI findings can be used to make the diagnosis with reasonable certainty.[3, 5] Although both ultrasonography and MRI have been used to assess patients with diabetic myonecrosis, MRI with intravenous contrast enhancement appears to be the most useful diagnostic technique. It demonstrates extensive edema within the muscle(s), muscle enlargement, subcutaneous and interfascial edema, a patchwork pattern of involvement, and a high signal intensity on T2‐weighted images.[4, 7] Gadolinium enhancement may reveal an enhanced margin of the infarcted muscle with a central nonenhancing area of necrotic tissue.[4, 5] Muscle biopsy is not typically indicated because it may prolong recovery time and lead to infections.[8, 9, 10, 11] When performed, however, muscle biopsy reveals ischemic muscle fibers in different stages of degeneration and regeneration, with areas of necrosis and edema. Occlusion of arterioles and capillaries by fibrin could also be seen.[1] Although the patient underwent a muscle biopsy because infection could not be excluded definitively on clinical grounds, we believe that repeating the biopsy 4 months later was inappropriate.
Diabetic myonecrosis should be considered in a diabetic patient who presents with severe localized muscle pain and swelling of an extremity, especially if the clinical features favoring infection are absent. The differential diagnosis should include infection (eg, clostridial myonecrosis, myositis, cellulitis, abscess, necrotizing fasciitis, osteomyelitis), trauma (eg, hematoma, muscle rupture, myositis ossificans), peripheral neuropathy (particularly lumbosacral plexopathy), vascular disorders (deep vein thrombosis, and compartment syndrome), tumors, inflammatory muscle diseases, and drug‐related myositis.
No evidence‐based recommendations regarding the management of diabetic myonecrosis are available, although the findings of one retrospective analysis support conservative management with bed rest, leg elevation, and analgesics.[12] Physiotherapy may cause the condition to worsen,[13, 14] but routine daily activity, although often painful, is not harmful.[14] Some authors suggest a cautious use of antiplatelet or anti‐inflammatory medications.[12] We would also recommend achieving good glycemic control during the illness. Owing to the rarity of the disease, however, no studies have definitively shown that this hastens recovery or prevents recurrent diabetic myonecrosis. Surgery may prolong the recovery period; one study found that the recovery period of patients with diabetic myonecrosis who underwent surgery was longer than that of those who were treated conservatively (13 weeks vs 5.5 weeks).[12] Patients with diabetic myonecrosis have a good short‐term prognosis. Longer‐term, however, they have a poor prognosis; their recurrence rate is as high as 40%, and their 2‐year mortality rate is 10%, even after one episode of the disease. Death in these patients is mainly due to macrovascular events.[12]
TEACHING POINTS
- Diabetic myonecrosis is a rare complication of longstanding and poorly controlled diabetes. It usually presents with acute localized muscular pain in the lower extremities.
- Although a definitive diagnosis of diabetic myonecrosis is histopathologic, a clinical diagnosis can be made with reasonable certainty for patients with compatible MRI findings and no clinical or laboratory features suggesting infection.
- Conservative management with bed rest, analgesics, and antiplatelets is recommended. Surgery should be avoided, as it may prolong recovery.
Disclosure
Nothing to report.
- , . Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1:39–42.
- . Diabetic muscle infarction: an underdiagnosed complication of long‐standing diabetes. Diabetes Care. 2003;26:211–215.
- , , . Diabetic muscle infarction: case report and review. J Rheumatol. 2004;31:190–194.
- , , , , , . Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology. 1999;211:241–247.
- , , , . Skeletal muscle infarction in diabetes mellitus. J Rheumatol. 2000;27:1063–1068.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29–1997. A 54‐year‐old diabetic woman with pain and swelling of the leg. N Engl J Med. 1997;337:839–845.
- , , . Clinical and radiological aspects of idiopathic diabetic muscle infarction. Rational approach to diagnosis and treatment. J Bone Joint Surg Br. 1999;81:323–326.
- , , . Diabetic muscular infarction. Preventing morbidity by avoiding excisional biopsy. Arch Intern Med. 1997;157:1611.
- , . Case‐of‐the‐month: painful thigh mass in a young woman: diabetic muscle infarction. Muscle Nerve. 1992;15:850–855.
- . Diabetic muscle infarction: magnetic resonance imaging (MRI) avoids the need for biopsy. Muscle Nerve. 1995;18:129–130.
- , , , . Skeletal muscle infarction in diabetes: MR findings. J Comput Assist Tomogr. 1993;17:986–988.
- , . Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11:8–12.
- , , . Diabetic muscular infarction. Semin Arthritis Rheum. 1993;22:280–287.
- , . Focal infarction of muscle in diabetics. Diabetes Care. 1986;9:623–630.
- , . Tumoriform focal muscular degeneration in two diabetic patients. Diabetologia. 1965;1:39–42.
- . Diabetic muscle infarction: an underdiagnosed complication of long‐standing diabetes. Diabetes Care. 2003;26:211–215.
- , , . Diabetic muscle infarction: case report and review. J Rheumatol. 2004;31:190–194.
- , , , , , . Muscle infarction in patients with diabetes mellitus: MR imaging findings. Radiology. 1999;211:241–247.
- , , , . Skeletal muscle infarction in diabetes mellitus. J Rheumatol. 2000;27:1063–1068.
- Case records of the Massachusetts General Hospital. Weekly clinicopathological exercises. Case 29–1997. A 54‐year‐old diabetic woman with pain and swelling of the leg. N Engl J Med. 1997;337:839–845.
- , , . Clinical and radiological aspects of idiopathic diabetic muscle infarction. Rational approach to diagnosis and treatment. J Bone Joint Surg Br. 1999;81:323–326.
- , , . Diabetic muscular infarction. Preventing morbidity by avoiding excisional biopsy. Arch Intern Med. 1997;157:1611.
- , . Case‐of‐the‐month: painful thigh mass in a young woman: diabetic muscle infarction. Muscle Nerve. 1992;15:850–855.
- . Diabetic muscle infarction: magnetic resonance imaging (MRI) avoids the need for biopsy. Muscle Nerve. 1995;18:129–130.
- , , , . Skeletal muscle infarction in diabetes: MR findings. J Comput Assist Tomogr. 1993;17:986–988.
- , . Treatment and outcomes of diabetic muscle infarction. J Clin Rheumatol. 2005;11:8–12.
- , , . Diabetic muscular infarction. Semin Arthritis Rheum. 1993;22:280–287.
- , . Focal infarction of muscle in diabetics. Diabetes Care. 1986;9:623–630.
FDR and Telemetry Rhythm at Time of IHCA
In‐hospital cardiac arrest (IHCA) research often relies on the first documented cardiac rhythm (FDR) on resuscitation records at the time of cardiopulmonary resuscitation (CPR) initiation as a surrogate for arrest etiology.[1] Over 1000 hospitals report the FDR and associated cardiac arrest data to national registries annually.[2, 3] These data are subsequently used to report national IHCA epidemiology, as well as to develop and refine guidelines for in‐hospital resuscitation.[4]
Suspecting that the FDR might represent the later stage of a progressive cardiopulmonary process rather than a sudden dysrhythmia, we sought to compare the first rhythm documented on resuscitation records at the time of CPR initiation with the telemetry rhythm at the time of the code blue call. We hypothesized that the agreement between FDR and telemetry rhythm would be <80% beyond that predicted by chance (kappa<0.8).[5]
METHODS
Design
Between June 2008 and February 2010, we performed a cross‐sectional study at a 750‐bed adult tertiary care hospital (Christiana Hospital) and a 240‐bed adult inner city community hospital (Wilmington Hospital). Both hospitals included teaching and nonteaching inpatient services. The Christiana Care Health System Institutional Review Board approved the study.
Study Population
Eligible subjects included a convenience sample of adult inpatients aged 18 years who were monitored on the hospital's telemetry system during the 2 minutes prior to a code blue call from a nonintensive care, noncardiac care inpatient ward for IHCA. Intensive care unit (ICU) locations were excluded because they are not captured in our central telemetry recording system. We defined IHCA as a resuscitation event requiring >1 minute of chest compressions and/or defibrillation. We excluded patients with do not attempt resuscitation orders at the time of the IHCA. For patients with multiple IHCAs, only their first event was included in the analysis. International Classification of Diseases, 9th Revision admission diagnoses were categorized into infectious, oncology, endocrine/metabolic; cardiovascular, renal, or other disease categories. The decision to place patients on telemetry monitoring was not part of the study and was entirely at the discretion of the physicians caring for the patients.
Variables and Measurements
We reviewed the paper resuscitation records of each IHCA during the study period and identified the FDR. To create groups that would allow comparison between telemetry and resuscitation record rhythms, we placed each rhythm into 1 of the following 3 categories: asystole, ventricular tachyarrhythmia (VTA), or other organized rhythms (Table 1). It was not possible to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was pulseless electrical activity (PEA) or a perfusing rhythm. Therefore, we elected to take a conservative approach that would bias toward agreement (the opposite direction of our hypothesis that the rhythms are discrepant) and consider all other organized rhythms in agreement with one another. We reviewed printouts of telemetry electrocardiographic records for each patient. Minute 0 was defined as the time of the code blue call. Two physician investigators (C.C. and U.B.) independently reviewed telemetry data for each patient at minute 0 and the 2 minutes preceding the code blue call (minutes 1 and 2). Rhythms at each minute mark were assigned to 1 of the following categories according to the classification scheme in Table 1: asystole, VTA, or other organized rhythms. Leads off and uninterpretable telemetry were also noted. Discrepancies in rhythm categorization between reviewers were resolved by a third investigator (M.Z.) blinded to rhythm category assignment. We used the telemetry rhythm at minute 0 for analysis whenever possible. If the leads were off or the telemetry was uninterpretable at minute 0, we used minute 1. If minute 1 was also unusable, we used minute 2. If there were no usable data at minutes 0, 1, or 2, we excluded the patient.
| Category | Rhythm |
|---|---|
| Asystole | Asystole |
| Ventricular tachyarrhythmia | Ventricular fibrillation, ventricular tachycardia |
| Other organized rhythms | Atrial fibrillation, bradycardia, paced pulseless electrical activity, sinus, idioventricular, other |
Statistical Analysis
We determined the percent agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category. We then calculated an unweighted kappa for the agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category.
RESULTS
During the study period, there were 135 code blue calls for urgent assistance among telemetry‐monitored non‐ICU patients. Of the 135 calls, we excluded 4 events (3%) that did not meet the definition of IHCA, 9 events (7%) with missing or uninterpretable data, and 53 events (39%) with unobtainable data due to automatic purging from the telemetry server. Therefore, 69 events in 69 different patients remained for analysis. Twelve of the 69 included arrests that occurred at Wilmington Hospital and 57 at Christiana Hospital. The characteristics of the patients are shown in Table 2.
| n | % | |
|---|---|---|
| Age, y | ||
| 3039 | 1 | 1.4 |
| 4049 | 4 | 5.8 |
| 5059 | 11 | 15.9 |
| 6069 | 15 | 21.7 |
| 7079 | 16 | 23.2 |
| 8089 | 18 | 26.1 |
| 90+ | 4 | 5.8 |
| Sex | ||
| Male | 26 | 37.7 |
| Female | 43 | 62.3 |
| Race/ethnicity | ||
| White | 51 | 73.9 |
| Black | 17 | 24.6 |
| Hispanic | 1 | 1.4 |
| Admission body mass index | ||
| Underweight (<18.5) | 3 | 4.3 |
| Normal (18.5<25) | 15 | 21.7 |
| Overweight (25<30) 24 | 24 | 34.8 |
| Obese (30<35) | 17 | 24.6 |
| Very obese (35) | 9 | 13.0 |
| Unknown | 1 | 1.4 |
| Admission diagnosis category | ||
| Infectious | 29 | 42.0 |
| Oncology | 4 | 5.8 |
| Endocrine/metabolic | 22 | 31.9 |
| Cardiovascular | 7 | 10.1 |
| Renal | 2 | 2.8 |
| Other | 5 | 7.2 |
Of the 69 arrests, we used the telemetry rhythm at minute 0 in 42 patients (61%), minute 1 in 22 patients (32%), and minute 2 in 5 patients (7%). Agreement between telemetry and FDR was 65% (kappa=0.37, 95% confidence interval: 0.17‐0.56) (Table 3). Agreement did not vary significantly by sex, race, hospital, weekday, time of day, or minute used in the analysis. Agreement was not associated with survival to hospital discharge.
| Telemetry | Resuscitation Record | |||
|---|---|---|---|---|
| Asystole | Ventricular Tachyarrhythmia | Other Organized Rhythms | Total | |
| ||||
| Asystole | 3 | 0 | 2 | 5 |
| Ventricular tachyarrhythmia | 1 | 12 | 8 | 21 |
| Other organized rhythms | 8 | 5 | 30 | 43 |
| Total | 12 | 17 | 40 | 69 |
Of the 69 IHCA events, the FDRs vs telemetry rhythms at the time of IHCA were: asystole 17% vs 7%, VTA 25% vs 31%, and other organized rhythms 58% vs 62%. Among the 12 events with FDR recorded as asystole, telemetry at the time of the code call was asystole in 3 (25%), VTA in 1 (8%), and other organized rhythms in 8 (67%). Among the 17 events with FDR recorded as VTA, telemetry at the time of the code call was VTA in 12 (71%) and other organized rhythms in 5 (29%). Among the 40 events with FDR recorded as other organized rhythms, telemetry at the time of the code call was asystole in 2 (5%), VTA in 8 (20%), and other organized rhythms in 30 (75%). Among the 8 patients with VTA on telemetry and other organized rhythms on the resuscitation record, the other organized rhythms were documented as PEA (n=6), sinus (n=1), and bradycardia (n=1). Of the 12 patients with VTA on telemetry and on the resuscitation record, 8 (67%) had ventricular tachycardia on telemetry. Four of the 8 (50%) who had ventricular tachycardia on telemetry had deteriorated into ventricular fibrillation by the time the FDR was recorded. Of the 4 who had ventricular fibrillation on telemetry, all had ventricular fibrillation as the FDR on the resuscitation record.
DISCUSSION
These results establish that FDRs often differ from the telemetry rhythms at the time of the code blue call. This is important because national registries such as the American Heart Association's Get with the GuidelinesResuscitation[2] database use the FDR as a surrogate for arrest etiology, and use their findings to report national IHCA outcomes as well as to develop and refine evidence‐based guidelines for in‐hospital resuscitation. Our findings suggest that using the FDR may be an oversimplification of the complex progression of cardiac rhythms that occurs in the periarrest period. Adding preceding telemetry rhythms to the data elements collected may shed additional light on etiology. Furthermore, our results demonstrate that, among adults with VTA or asystole documented upon arrival of the code blue team, other organized rhythms are often present at the time the staff recognized a life‐threatening condition and called for immediate assistance. This suggests that the VTA and asystole FDRs may represent the later stages of progressive cardiopulmonary processes. This is in contrast to out‐of‐hospital cardiac arrests typically attributed to sudden catastrophic dysrhythmias that often progress to asystole unless rapidly defibrillated.[6, 7, 8] Out‐of‐hospital and in‐hospital arrests are likely different (but overlapping) entities that might benefit from different resuscitation strategies.[9, 10] We hypothesize that, for a subset of these patients, progressive respiratory insufficiency and circulatory shockconditions classically associated more strongly with pediatric than adult IHCAmay have been directly responsible for the event.[1] If future research supports the concept that progressive respiratory insufficiency and circulatory shock are responsible for more adult IHCA than previously recognized, more robust monitoring may be indicated for a larger subset of adult patients hospitalized on general wards. This could include pulse oximetry (wave form can be a surrogate for perfusion), respiratory rate, and/or end‐tidal CO2 monitoring. In addition, if future research confirms that there is a greater distinction between in‐hospital and out‐of‐hospital cardiac arrest etiology, the expert panels that develop resuscitation guidelines should consider including setting of resuscitation as a branch point in future algorithms.
Our study had several limitations. First, the sample size was small due to uninterpretable rhythm strips, and for 39% of the total code events, the telemetry data had already been purged from the system by the time research staff attempted to retrieve it. Although we do not believe that there was any systematic bias to the data analyzed, the possibility cannot be completely excluded. Second, we were constrained by the inability to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was PEA. Thus, we categorized rhythms into large groups. Although this limited the granularity of the rhythm groups, it was a conservative approach that likely biased toward agreement (the opposite direction of our hypothesis). Third, the lack of perfect time synchronization between the telemetry system, wall clocks in the hospital, and wrist watches that may be referenced when documenting resuscitative efforts on the resuscitation record means that the rhythms we used may have reflected physiology after interventions had already commenced. Thus, in some situations, minute 1, 2, or earlier minutes may more accurately reflect the preintervention rhythm. Highly accurate time synchronization should be a central component of future prospective work in this area.
CONCLUSIONS
The FDR had only fair agreement with the telemetry rhythm at the time of the code blue call. Among those with VTA or asystole documented on CPR initiation, telemetry often revealed other organized rhythms present at the time hospital staff recognized a life‐threatening condition. In contrast to out‐of‐hospital cardiac arrest, FDR of asystole was only rarely preceded by VTA, and FDR of VTA was often preceded by an organized rhythm.[8, 11] Future studies should examine antecedent rhythms in combination with respiratory and perfusion status to more precisely determine arrest etiology.
Acknowledgments
The authors thank the staff at Flex Monitoring at Christiana Care Health System for their vital contributions to the study.
Disclosures
Dr. Zubrow had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , , , et al. First documented rhythm and clinical outcome from in‐hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57.
- Get With The Guidelines–Resuscitation (GWTG‐R) overview. Available at: http://www.heart.org/HEARTORG/HealthcareResearch/GetWithTheGuidelines‐Resuscitation/Get‐With‐The‐Guidelines‐ResuscitationOverview_UCM_314497_Article.jsp. Accessed May 8, 2012.
- , , , et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital “Utstein Style”. Circulation. 1997;95:2213–2239.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- , , , et al. Characteristics and outcome among patients suffering in‐hospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. 2001;48:125–135.
- , , , et al. A comparison between patients suffering in‐hospital and out‐of hospital cardiac arrest in terms of treatment and outcome. J Intern Med. 2000;248:53–60.
- , , , et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcomes and outcome in relation to time of arrest. Am Heart J. 2010;159:749–756.
- , , , et al.; Resuscitation Outcomes Consortium Investigators. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313–321.
- , . In‐hospital cardiac arrest. Emerg Med Clin North Am. 2012;30:25–34.
- , , , et al. Analysis of initial rhythm, witnessed status and delay to treatment among survivors of out‐of‐hospital cardiac arrest in Sweden. Heart. 2010;96:1826–1830.
In‐hospital cardiac arrest (IHCA) research often relies on the first documented cardiac rhythm (FDR) on resuscitation records at the time of cardiopulmonary resuscitation (CPR) initiation as a surrogate for arrest etiology.[1] Over 1000 hospitals report the FDR and associated cardiac arrest data to national registries annually.[2, 3] These data are subsequently used to report national IHCA epidemiology, as well as to develop and refine guidelines for in‐hospital resuscitation.[4]
Suspecting that the FDR might represent the later stage of a progressive cardiopulmonary process rather than a sudden dysrhythmia, we sought to compare the first rhythm documented on resuscitation records at the time of CPR initiation with the telemetry rhythm at the time of the code blue call. We hypothesized that the agreement between FDR and telemetry rhythm would be <80% beyond that predicted by chance (kappa<0.8).[5]
METHODS
Design
Between June 2008 and February 2010, we performed a cross‐sectional study at a 750‐bed adult tertiary care hospital (Christiana Hospital) and a 240‐bed adult inner city community hospital (Wilmington Hospital). Both hospitals included teaching and nonteaching inpatient services. The Christiana Care Health System Institutional Review Board approved the study.
Study Population
Eligible subjects included a convenience sample of adult inpatients aged 18 years who were monitored on the hospital's telemetry system during the 2 minutes prior to a code blue call from a nonintensive care, noncardiac care inpatient ward for IHCA. Intensive care unit (ICU) locations were excluded because they are not captured in our central telemetry recording system. We defined IHCA as a resuscitation event requiring >1 minute of chest compressions and/or defibrillation. We excluded patients with do not attempt resuscitation orders at the time of the IHCA. For patients with multiple IHCAs, only their first event was included in the analysis. International Classification of Diseases, 9th Revision admission diagnoses were categorized into infectious, oncology, endocrine/metabolic; cardiovascular, renal, or other disease categories. The decision to place patients on telemetry monitoring was not part of the study and was entirely at the discretion of the physicians caring for the patients.
Variables and Measurements
We reviewed the paper resuscitation records of each IHCA during the study period and identified the FDR. To create groups that would allow comparison between telemetry and resuscitation record rhythms, we placed each rhythm into 1 of the following 3 categories: asystole, ventricular tachyarrhythmia (VTA), or other organized rhythms (Table 1). It was not possible to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was pulseless electrical activity (PEA) or a perfusing rhythm. Therefore, we elected to take a conservative approach that would bias toward agreement (the opposite direction of our hypothesis that the rhythms are discrepant) and consider all other organized rhythms in agreement with one another. We reviewed printouts of telemetry electrocardiographic records for each patient. Minute 0 was defined as the time of the code blue call. Two physician investigators (C.C. and U.B.) independently reviewed telemetry data for each patient at minute 0 and the 2 minutes preceding the code blue call (minutes 1 and 2). Rhythms at each minute mark were assigned to 1 of the following categories according to the classification scheme in Table 1: asystole, VTA, or other organized rhythms. Leads off and uninterpretable telemetry were also noted. Discrepancies in rhythm categorization between reviewers were resolved by a third investigator (M.Z.) blinded to rhythm category assignment. We used the telemetry rhythm at minute 0 for analysis whenever possible. If the leads were off or the telemetry was uninterpretable at minute 0, we used minute 1. If minute 1 was also unusable, we used minute 2. If there were no usable data at minutes 0, 1, or 2, we excluded the patient.
| Category | Rhythm |
|---|---|
| Asystole | Asystole |
| Ventricular tachyarrhythmia | Ventricular fibrillation, ventricular tachycardia |
| Other organized rhythms | Atrial fibrillation, bradycardia, paced pulseless electrical activity, sinus, idioventricular, other |
Statistical Analysis
We determined the percent agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category. We then calculated an unweighted kappa for the agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category.
RESULTS
During the study period, there were 135 code blue calls for urgent assistance among telemetry‐monitored non‐ICU patients. Of the 135 calls, we excluded 4 events (3%) that did not meet the definition of IHCA, 9 events (7%) with missing or uninterpretable data, and 53 events (39%) with unobtainable data due to automatic purging from the telemetry server. Therefore, 69 events in 69 different patients remained for analysis. Twelve of the 69 included arrests that occurred at Wilmington Hospital and 57 at Christiana Hospital. The characteristics of the patients are shown in Table 2.
| n | % | |
|---|---|---|
| Age, y | ||
| 3039 | 1 | 1.4 |
| 4049 | 4 | 5.8 |
| 5059 | 11 | 15.9 |
| 6069 | 15 | 21.7 |
| 7079 | 16 | 23.2 |
| 8089 | 18 | 26.1 |
| 90+ | 4 | 5.8 |
| Sex | ||
| Male | 26 | 37.7 |
| Female | 43 | 62.3 |
| Race/ethnicity | ||
| White | 51 | 73.9 |
| Black | 17 | 24.6 |
| Hispanic | 1 | 1.4 |
| Admission body mass index | ||
| Underweight (<18.5) | 3 | 4.3 |
| Normal (18.5<25) | 15 | 21.7 |
| Overweight (25<30) 24 | 24 | 34.8 |
| Obese (30<35) | 17 | 24.6 |
| Very obese (35) | 9 | 13.0 |
| Unknown | 1 | 1.4 |
| Admission diagnosis category | ||
| Infectious | 29 | 42.0 |
| Oncology | 4 | 5.8 |
| Endocrine/metabolic | 22 | 31.9 |
| Cardiovascular | 7 | 10.1 |
| Renal | 2 | 2.8 |
| Other | 5 | 7.2 |
Of the 69 arrests, we used the telemetry rhythm at minute 0 in 42 patients (61%), minute 1 in 22 patients (32%), and minute 2 in 5 patients (7%). Agreement between telemetry and FDR was 65% (kappa=0.37, 95% confidence interval: 0.17‐0.56) (Table 3). Agreement did not vary significantly by sex, race, hospital, weekday, time of day, or minute used in the analysis. Agreement was not associated with survival to hospital discharge.
| Telemetry | Resuscitation Record | |||
|---|---|---|---|---|
| Asystole | Ventricular Tachyarrhythmia | Other Organized Rhythms | Total | |
| ||||
| Asystole | 3 | 0 | 2 | 5 |
| Ventricular tachyarrhythmia | 1 | 12 | 8 | 21 |
| Other organized rhythms | 8 | 5 | 30 | 43 |
| Total | 12 | 17 | 40 | 69 |
Of the 69 IHCA events, the FDRs vs telemetry rhythms at the time of IHCA were: asystole 17% vs 7%, VTA 25% vs 31%, and other organized rhythms 58% vs 62%. Among the 12 events with FDR recorded as asystole, telemetry at the time of the code call was asystole in 3 (25%), VTA in 1 (8%), and other organized rhythms in 8 (67%). Among the 17 events with FDR recorded as VTA, telemetry at the time of the code call was VTA in 12 (71%) and other organized rhythms in 5 (29%). Among the 40 events with FDR recorded as other organized rhythms, telemetry at the time of the code call was asystole in 2 (5%), VTA in 8 (20%), and other organized rhythms in 30 (75%). Among the 8 patients with VTA on telemetry and other organized rhythms on the resuscitation record, the other organized rhythms were documented as PEA (n=6), sinus (n=1), and bradycardia (n=1). Of the 12 patients with VTA on telemetry and on the resuscitation record, 8 (67%) had ventricular tachycardia on telemetry. Four of the 8 (50%) who had ventricular tachycardia on telemetry had deteriorated into ventricular fibrillation by the time the FDR was recorded. Of the 4 who had ventricular fibrillation on telemetry, all had ventricular fibrillation as the FDR on the resuscitation record.
DISCUSSION
These results establish that FDRs often differ from the telemetry rhythms at the time of the code blue call. This is important because national registries such as the American Heart Association's Get with the GuidelinesResuscitation[2] database use the FDR as a surrogate for arrest etiology, and use their findings to report national IHCA outcomes as well as to develop and refine evidence‐based guidelines for in‐hospital resuscitation. Our findings suggest that using the FDR may be an oversimplification of the complex progression of cardiac rhythms that occurs in the periarrest period. Adding preceding telemetry rhythms to the data elements collected may shed additional light on etiology. Furthermore, our results demonstrate that, among adults with VTA or asystole documented upon arrival of the code blue team, other organized rhythms are often present at the time the staff recognized a life‐threatening condition and called for immediate assistance. This suggests that the VTA and asystole FDRs may represent the later stages of progressive cardiopulmonary processes. This is in contrast to out‐of‐hospital cardiac arrests typically attributed to sudden catastrophic dysrhythmias that often progress to asystole unless rapidly defibrillated.[6, 7, 8] Out‐of‐hospital and in‐hospital arrests are likely different (but overlapping) entities that might benefit from different resuscitation strategies.[9, 10] We hypothesize that, for a subset of these patients, progressive respiratory insufficiency and circulatory shockconditions classically associated more strongly with pediatric than adult IHCAmay have been directly responsible for the event.[1] If future research supports the concept that progressive respiratory insufficiency and circulatory shock are responsible for more adult IHCA than previously recognized, more robust monitoring may be indicated for a larger subset of adult patients hospitalized on general wards. This could include pulse oximetry (wave form can be a surrogate for perfusion), respiratory rate, and/or end‐tidal CO2 monitoring. In addition, if future research confirms that there is a greater distinction between in‐hospital and out‐of‐hospital cardiac arrest etiology, the expert panels that develop resuscitation guidelines should consider including setting of resuscitation as a branch point in future algorithms.
Our study had several limitations. First, the sample size was small due to uninterpretable rhythm strips, and for 39% of the total code events, the telemetry data had already been purged from the system by the time research staff attempted to retrieve it. Although we do not believe that there was any systematic bias to the data analyzed, the possibility cannot be completely excluded. Second, we were constrained by the inability to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was PEA. Thus, we categorized rhythms into large groups. Although this limited the granularity of the rhythm groups, it was a conservative approach that likely biased toward agreement (the opposite direction of our hypothesis). Third, the lack of perfect time synchronization between the telemetry system, wall clocks in the hospital, and wrist watches that may be referenced when documenting resuscitative efforts on the resuscitation record means that the rhythms we used may have reflected physiology after interventions had already commenced. Thus, in some situations, minute 1, 2, or earlier minutes may more accurately reflect the preintervention rhythm. Highly accurate time synchronization should be a central component of future prospective work in this area.
CONCLUSIONS
The FDR had only fair agreement with the telemetry rhythm at the time of the code blue call. Among those with VTA or asystole documented on CPR initiation, telemetry often revealed other organized rhythms present at the time hospital staff recognized a life‐threatening condition. In contrast to out‐of‐hospital cardiac arrest, FDR of asystole was only rarely preceded by VTA, and FDR of VTA was often preceded by an organized rhythm.[8, 11] Future studies should examine antecedent rhythms in combination with respiratory and perfusion status to more precisely determine arrest etiology.
Acknowledgments
The authors thank the staff at Flex Monitoring at Christiana Care Health System for their vital contributions to the study.
Disclosures
Dr. Zubrow had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
In‐hospital cardiac arrest (IHCA) research often relies on the first documented cardiac rhythm (FDR) on resuscitation records at the time of cardiopulmonary resuscitation (CPR) initiation as a surrogate for arrest etiology.[1] Over 1000 hospitals report the FDR and associated cardiac arrest data to national registries annually.[2, 3] These data are subsequently used to report national IHCA epidemiology, as well as to develop and refine guidelines for in‐hospital resuscitation.[4]
Suspecting that the FDR might represent the later stage of a progressive cardiopulmonary process rather than a sudden dysrhythmia, we sought to compare the first rhythm documented on resuscitation records at the time of CPR initiation with the telemetry rhythm at the time of the code blue call. We hypothesized that the agreement between FDR and telemetry rhythm would be <80% beyond that predicted by chance (kappa<0.8).[5]
METHODS
Design
Between June 2008 and February 2010, we performed a cross‐sectional study at a 750‐bed adult tertiary care hospital (Christiana Hospital) and a 240‐bed adult inner city community hospital (Wilmington Hospital). Both hospitals included teaching and nonteaching inpatient services. The Christiana Care Health System Institutional Review Board approved the study.
Study Population
Eligible subjects included a convenience sample of adult inpatients aged 18 years who were monitored on the hospital's telemetry system during the 2 minutes prior to a code blue call from a nonintensive care, noncardiac care inpatient ward for IHCA. Intensive care unit (ICU) locations were excluded because they are not captured in our central telemetry recording system. We defined IHCA as a resuscitation event requiring >1 minute of chest compressions and/or defibrillation. We excluded patients with do not attempt resuscitation orders at the time of the IHCA. For patients with multiple IHCAs, only their first event was included in the analysis. International Classification of Diseases, 9th Revision admission diagnoses were categorized into infectious, oncology, endocrine/metabolic; cardiovascular, renal, or other disease categories. The decision to place patients on telemetry monitoring was not part of the study and was entirely at the discretion of the physicians caring for the patients.
Variables and Measurements
We reviewed the paper resuscitation records of each IHCA during the study period and identified the FDR. To create groups that would allow comparison between telemetry and resuscitation record rhythms, we placed each rhythm into 1 of the following 3 categories: asystole, ventricular tachyarrhythmia (VTA), or other organized rhythms (Table 1). It was not possible to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was pulseless electrical activity (PEA) or a perfusing rhythm. Therefore, we elected to take a conservative approach that would bias toward agreement (the opposite direction of our hypothesis that the rhythms are discrepant) and consider all other organized rhythms in agreement with one another. We reviewed printouts of telemetry electrocardiographic records for each patient. Minute 0 was defined as the time of the code blue call. Two physician investigators (C.C. and U.B.) independently reviewed telemetry data for each patient at minute 0 and the 2 minutes preceding the code blue call (minutes 1 and 2). Rhythms at each minute mark were assigned to 1 of the following categories according to the classification scheme in Table 1: asystole, VTA, or other organized rhythms. Leads off and uninterpretable telemetry were also noted. Discrepancies in rhythm categorization between reviewers were resolved by a third investigator (M.Z.) blinded to rhythm category assignment. We used the telemetry rhythm at minute 0 for analysis whenever possible. If the leads were off or the telemetry was uninterpretable at minute 0, we used minute 1. If minute 1 was also unusable, we used minute 2. If there were no usable data at minutes 0, 1, or 2, we excluded the patient.
| Category | Rhythm |
|---|---|
| Asystole | Asystole |
| Ventricular tachyarrhythmia | Ventricular fibrillation, ventricular tachycardia |
| Other organized rhythms | Atrial fibrillation, bradycardia, paced pulseless electrical activity, sinus, idioventricular, other |
Statistical Analysis
We determined the percent agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category. We then calculated an unweighted kappa for the agreement between the resuscitation record rhythm category and the last interpretable telemetry rhythm category.
RESULTS
During the study period, there were 135 code blue calls for urgent assistance among telemetry‐monitored non‐ICU patients. Of the 135 calls, we excluded 4 events (3%) that did not meet the definition of IHCA, 9 events (7%) with missing or uninterpretable data, and 53 events (39%) with unobtainable data due to automatic purging from the telemetry server. Therefore, 69 events in 69 different patients remained for analysis. Twelve of the 69 included arrests that occurred at Wilmington Hospital and 57 at Christiana Hospital. The characteristics of the patients are shown in Table 2.
| n | % | |
|---|---|---|
| Age, y | ||
| 3039 | 1 | 1.4 |
| 4049 | 4 | 5.8 |
| 5059 | 11 | 15.9 |
| 6069 | 15 | 21.7 |
| 7079 | 16 | 23.2 |
| 8089 | 18 | 26.1 |
| 90+ | 4 | 5.8 |
| Sex | ||
| Male | 26 | 37.7 |
| Female | 43 | 62.3 |
| Race/ethnicity | ||
| White | 51 | 73.9 |
| Black | 17 | 24.6 |
| Hispanic | 1 | 1.4 |
| Admission body mass index | ||
| Underweight (<18.5) | 3 | 4.3 |
| Normal (18.5<25) | 15 | 21.7 |
| Overweight (25<30) 24 | 24 | 34.8 |
| Obese (30<35) | 17 | 24.6 |
| Very obese (35) | 9 | 13.0 |
| Unknown | 1 | 1.4 |
| Admission diagnosis category | ||
| Infectious | 29 | 42.0 |
| Oncology | 4 | 5.8 |
| Endocrine/metabolic | 22 | 31.9 |
| Cardiovascular | 7 | 10.1 |
| Renal | 2 | 2.8 |
| Other | 5 | 7.2 |
Of the 69 arrests, we used the telemetry rhythm at minute 0 in 42 patients (61%), minute 1 in 22 patients (32%), and minute 2 in 5 patients (7%). Agreement between telemetry and FDR was 65% (kappa=0.37, 95% confidence interval: 0.17‐0.56) (Table 3). Agreement did not vary significantly by sex, race, hospital, weekday, time of day, or minute used in the analysis. Agreement was not associated with survival to hospital discharge.
| Telemetry | Resuscitation Record | |||
|---|---|---|---|---|
| Asystole | Ventricular Tachyarrhythmia | Other Organized Rhythms | Total | |
| ||||
| Asystole | 3 | 0 | 2 | 5 |
| Ventricular tachyarrhythmia | 1 | 12 | 8 | 21 |
| Other organized rhythms | 8 | 5 | 30 | 43 |
| Total | 12 | 17 | 40 | 69 |
Of the 69 IHCA events, the FDRs vs telemetry rhythms at the time of IHCA were: asystole 17% vs 7%, VTA 25% vs 31%, and other organized rhythms 58% vs 62%. Among the 12 events with FDR recorded as asystole, telemetry at the time of the code call was asystole in 3 (25%), VTA in 1 (8%), and other organized rhythms in 8 (67%). Among the 17 events with FDR recorded as VTA, telemetry at the time of the code call was VTA in 12 (71%) and other organized rhythms in 5 (29%). Among the 40 events with FDR recorded as other organized rhythms, telemetry at the time of the code call was asystole in 2 (5%), VTA in 8 (20%), and other organized rhythms in 30 (75%). Among the 8 patients with VTA on telemetry and other organized rhythms on the resuscitation record, the other organized rhythms were documented as PEA (n=6), sinus (n=1), and bradycardia (n=1). Of the 12 patients with VTA on telemetry and on the resuscitation record, 8 (67%) had ventricular tachycardia on telemetry. Four of the 8 (50%) who had ventricular tachycardia on telemetry had deteriorated into ventricular fibrillation by the time the FDR was recorded. Of the 4 who had ventricular fibrillation on telemetry, all had ventricular fibrillation as the FDR on the resuscitation record.
DISCUSSION
These results establish that FDRs often differ from the telemetry rhythms at the time of the code blue call. This is important because national registries such as the American Heart Association's Get with the GuidelinesResuscitation[2] database use the FDR as a surrogate for arrest etiology, and use their findings to report national IHCA outcomes as well as to develop and refine evidence‐based guidelines for in‐hospital resuscitation. Our findings suggest that using the FDR may be an oversimplification of the complex progression of cardiac rhythms that occurs in the periarrest period. Adding preceding telemetry rhythms to the data elements collected may shed additional light on etiology. Furthermore, our results demonstrate that, among adults with VTA or asystole documented upon arrival of the code blue team, other organized rhythms are often present at the time the staff recognized a life‐threatening condition and called for immediate assistance. This suggests that the VTA and asystole FDRs may represent the later stages of progressive cardiopulmonary processes. This is in contrast to out‐of‐hospital cardiac arrests typically attributed to sudden catastrophic dysrhythmias that often progress to asystole unless rapidly defibrillated.[6, 7, 8] Out‐of‐hospital and in‐hospital arrests are likely different (but overlapping) entities that might benefit from different resuscitation strategies.[9, 10] We hypothesize that, for a subset of these patients, progressive respiratory insufficiency and circulatory shockconditions classically associated more strongly with pediatric than adult IHCAmay have been directly responsible for the event.[1] If future research supports the concept that progressive respiratory insufficiency and circulatory shock are responsible for more adult IHCA than previously recognized, more robust monitoring may be indicated for a larger subset of adult patients hospitalized on general wards. This could include pulse oximetry (wave form can be a surrogate for perfusion), respiratory rate, and/or end‐tidal CO2 monitoring. In addition, if future research confirms that there is a greater distinction between in‐hospital and out‐of‐hospital cardiac arrest etiology, the expert panels that develop resuscitation guidelines should consider including setting of resuscitation as a branch point in future algorithms.
Our study had several limitations. First, the sample size was small due to uninterpretable rhythm strips, and for 39% of the total code events, the telemetry data had already been purged from the system by the time research staff attempted to retrieve it. Although we do not believe that there was any systematic bias to the data analyzed, the possibility cannot be completely excluded. Second, we were constrained by the inability to retrospectively ascertain the presence of pulses to determine if an organized rhythm identified on telemetry tracings was PEA. Thus, we categorized rhythms into large groups. Although this limited the granularity of the rhythm groups, it was a conservative approach that likely biased toward agreement (the opposite direction of our hypothesis). Third, the lack of perfect time synchronization between the telemetry system, wall clocks in the hospital, and wrist watches that may be referenced when documenting resuscitative efforts on the resuscitation record means that the rhythms we used may have reflected physiology after interventions had already commenced. Thus, in some situations, minute 1, 2, or earlier minutes may more accurately reflect the preintervention rhythm. Highly accurate time synchronization should be a central component of future prospective work in this area.
CONCLUSIONS
The FDR had only fair agreement with the telemetry rhythm at the time of the code blue call. Among those with VTA or asystole documented on CPR initiation, telemetry often revealed other organized rhythms present at the time hospital staff recognized a life‐threatening condition. In contrast to out‐of‐hospital cardiac arrest, FDR of asystole was only rarely preceded by VTA, and FDR of VTA was often preceded by an organized rhythm.[8, 11] Future studies should examine antecedent rhythms in combination with respiratory and perfusion status to more precisely determine arrest etiology.
Acknowledgments
The authors thank the staff at Flex Monitoring at Christiana Care Health System for their vital contributions to the study.
Disclosures
Dr. Zubrow had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. The authors report no conflicts of interest.
- , , , et al. First documented rhythm and clinical outcome from in‐hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57.
- Get With The Guidelines–Resuscitation (GWTG‐R) overview. Available at: http://www.heart.org/HEARTORG/HealthcareResearch/GetWithTheGuidelines‐Resuscitation/Get‐With‐The‐Guidelines‐ResuscitationOverview_UCM_314497_Article.jsp. Accessed May 8, 2012.
- , , , et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital “Utstein Style”. Circulation. 1997;95:2213–2239.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- , , , et al. Characteristics and outcome among patients suffering in‐hospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. 2001;48:125–135.
- , , , et al. A comparison between patients suffering in‐hospital and out‐of hospital cardiac arrest in terms of treatment and outcome. J Intern Med. 2000;248:53–60.
- , , , et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcomes and outcome in relation to time of arrest. Am Heart J. 2010;159:749–756.
- , , , et al.; Resuscitation Outcomes Consortium Investigators. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313–321.
- , . In‐hospital cardiac arrest. Emerg Med Clin North Am. 2012;30:25–34.
- , , , et al. Analysis of initial rhythm, witnessed status and delay to treatment among survivors of out‐of‐hospital cardiac arrest in Sweden. Heart. 2010;96:1826–1830.
- , , , et al. First documented rhythm and clinical outcome from in‐hospital cardiac arrest among children and adults. JAMA. 2006;295(1):50–57.
- Get With The Guidelines–Resuscitation (GWTG‐R) overview. Available at: http://www.heart.org/HEARTORG/HealthcareResearch/GetWithTheGuidelines‐Resuscitation/Get‐With‐The‐Guidelines‐ResuscitationOverview_UCM_314497_Article.jsp. Accessed May 8, 2012.
- , , , et al. Recommended guidelines for reviewing, reporting, and conducting research on in‐hospital resuscitation: the in‐hospital “Utstein Style”. Circulation. 1997;95:2213–2239.
- , , , et al. Cardiopulmonary resuscitation of adults in the hospital: a report of 14,720 cardiac arrests from the National Registry of Cardiopulmonary Resuscitation. Resuscitation. 2003;58:297–308.
- , . The measurement of observer agreement for categorical data. Biometrics. 1977;33(1):159–174.
- , , , et al. Characteristics and outcome among patients suffering in‐hospital cardiac arrest in monitored and nonmonitored areas. Resuscitation. 2001;48:125–135.
- , , , et al. A comparison between patients suffering in‐hospital and out‐of hospital cardiac arrest in terms of treatment and outcome. J Intern Med. 2000;248:53–60.
- , , , et al. Cardiac arrest outside and inside hospital in a community: mechanisms behind the differences in outcomes and outcome in relation to time of arrest. Am Heart J. 2010;159:749–756.
- , , , et al.; Resuscitation Outcomes Consortium Investigators. Ventricular tachyarrhythmias after cardiac arrest in public versus at home. N Engl J Med. 2011;364:313–321.
- , . In‐hospital cardiac arrest. Emerg Med Clin North Am. 2012;30:25–34.
- , , , et al. Analysis of initial rhythm, witnessed status and delay to treatment among survivors of out‐of‐hospital cardiac arrest in Sweden. Heart. 2010;96:1826–1830.
Early Warning Score Qualitative Study
Thousands of hospitals have recently implemented rapid response systems (RRSs), attempting to reduce mortality outside of intensive care units (ICUs).[1, 2] These systems have 2 clinical components, a response (efferent) arm and an identification (afferent) arm.[3] The response arm is usually composed of a medical emergency team (MET) that responds to calls for urgent assistance. The identification arm includes tools to help clinicians recognize patients who require assistance from the MET. In many hospitals, the identification arm includes an early warning score (EWS). In pediatric patients, EWSs assign point values to vital signs that fall outside of age‐based ranges, among other clinical observations. They then generate a total score intended to help clinicians identify patients exhibiting early signs of deterioration.[4, 5, 6, 7, 8, 9, 10, 11]
When experimentally applied to vital sign datasets, the test characteristics of pediatric EWSs in detecting clinical deterioration are highly variable across studies, with major tradeoffs between sensitivity, specificity, and predictive values that differ by outcome, score, and cut‐point (Table 1). This reflects the difficulty of identifying deteriorating patients using only objective measures. However, in real‐world settings, EWSs are used by clinicians in conjunction with their clinical judgment. We hypothesized that EWSs have benefits that extend beyond their ability to predict deterioration, and thus have value not demonstrated by test characteristics alone. In order to further explore this issue, we aimed to qualitatively evaluate mechanisms beyond their statistical ability to predict deterioration by which physicians and nurses use EWSs to support their decision making.
| Score and Citation | Outcome Measure | Score Cut‐point | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|
| ||||||
| Brighton Paediatric Early Warning Score[5] | RRT or code blue call | 4 | 86% | NR | NR | NR |
| Bristol Paediatric Early Warning Tool[6, 11] | Escalation to higher level of care | 1 | ER | ER | 63% | NR |
| Cardiff and Vale Paediatric Early Warning System[7] | Respiratory or cardiac arrest, HDU/ICU admission, or death | 1 | 89% | 64% | 2% | >99% |
| Bedside Paediatric Early Warning System score, original version[8] | Code blue call | 5 | 78% | 95% | 4% | NR |
| Bedside Paediatric Early Warning System score, simplified version[9] | Urgent ICU admission without a code blue call | 8 | 82% | 93% | NR | NR |
| Bedside Paediatric Early Warning System score, simplified version[10] | Urgent ICU admission or code blue call | 7 | 64% | 91% | 9% | NR |
METHODS
Overview
As 1 component of a larger study, we conducted semistructured interviews with nurses and physicians at The Children's Hospital of Philadelphia (CHOP) between May and October 2011. In separate subprojects using the same participants, the larger study also aimed to identify residual barriers to calling for urgent assistance and assess the role of families in the recognition of deterioration and MET activation.
Setting
The Children's Hospital of Philadelphia is an urban, tertiary‐care pediatric hospital with 504 beds. Surgical patients hospitalized outside of ICUs are cared for by surgeons and surgical nurses without pediatrician co‐management. Implementation of a RRS was prompted by serious safety events in which clinical deterioration was either not recognized or was recognized and not escalated. Prior to RRS implementation, a code blue team could be activated for patients in immediate need of resuscitation, or, for less‐urgent needs, a pediatric ICU fellow could be paged by physicians for informal consults.
A multidisciplinary team developed and pilot‐tested the RRS, then implemented it hospital‐wide in February 2010. Representing an aspect of a multipronged approach to improve safety culture, the RRS consisted of (1) an EWS based upon Parshuram's Bedside Paediatric Early Warning System,[8, 9, 10] calculated by hand on a paper form (see online supplementary content) at the same frequency as vital signs (usually every 4 hours), and (2) a 30‐minute response MET available for activation by any clinician for any concern, 24 hours per day, 7 days per week. Escalation guidelines included a prompt to activate the MET for a score that increased to the red zone (9). For concerns that could not wait 30 minutes, any hospital employee could activate the immediate‐response code blue team.
Utilization of the RRS at CHOP is high, with 23 calls to the MET per day and a combined MET/code‐blue team call rate of 27.8 per 1000 admissions.[12] Previously reported pediatric call rates range from 2.8 to 44.0, with a median of 9.6 per 1000 admissions across 6 studies.[13, 14, 15, 16, 17, 18, 19] Since implementation, there has been a statistically significant net reduction in critical deterioration events (unpublished data).
Participants
We recruited nurses and physicians who had recently cared for children age 18 years on general medical or surgical wards with false‐negative or false‐positive EWSs (instances when the score failed to predict deterioration). Recruitment ceased when we reached thematic data saturation (a qualitative research term for the point at which no new themes emerge with additional interviews).[20]
Data Collection
Through a detailed review of the relevant literature and consultation with experts, we developed a semistructured interview guide (see online supplementary content) to elicit nurses' and physicians' viewpoints regarding the mechanisms by which they use EWSs to support their decision making.
Experienced qualitative research scientists (F.K.B. and J.H.H.) trained 2 study interviewers (B.P. and K.M.T.). In order to minimize social‐desirability bias, the interviewers were not clinicians and were not involved in RRS operations. Each interview was recorded, professionally transcribed, and imported into NVivo 8.0 software for analysis (QSR International, Melbourne, Australia).
Data Analysis
We coded the interviews inductively, without using a predetermined set of themes. This approach is known as grounded theory methodology.[21] Two team members coded each interview independently. They then reviewed their coding together and discussed discrepancies until reaching consensus. In weekly meetings while the interviews were ongoing, we compared newly collected data with themes that had previously emerged in order to guide further thematic development and refinement (the constant comparative method).[22] After all of the interviews were completed and consensus had been reached for each individual interview, the study team convened a series of additional meetings to further refine and finalize the themes.
Human Subjects
The CHOP Institutional Review Board approved this study. All participants provided written informed consent.
RESULTS
Participants
We recruited 27 nurses and 30 physicians before reaching thematic data saturation. Because surgical patients are underrepresented relative to medical patients among the population with false‐positive and false‐negative scores in our hospital, this included 3 randomly selected surgical nurses and 7 randomly selected surgical physicians recruited to ensure thematic data saturation for surgical settings. Characteristics of the participants are displayed in Table 2.
| Physicians (n=30) | Nurses (n=27) | |||
|---|---|---|---|---|
| ||||
| N | % | N | % | |
| Race | ||||
| Asian | 2 | 6.7 | 1 | 3.7 |
| Black | 0 | 0.0 | 2 | 7.4 |
| White | 26 | 86.7 | 22 | 81.5 |
| Prefer not to say | 1 | 3.3 | 1 | 3.7 |
| >1 race | 1 | 3.3 | 1 | 3.7 |
| Ethnicity | ||||
| Hispanic/Latino | 2 | 6.7 | 1 | 3.7 |
| Not Hispanic/Latino | 23 | 76.7 | 25 | 92.6 |
| Prefer not to say | 5 | 16.7 | 1 | 3.7 |
| Sex | ||||
| F | 16 | 53.3 | 25 | 92.6 |
| M | 14 | 46.7 | 2 | 7.4 |
| Practice setting | ||||
| Medical | 21 | 70.0 | 22 | 81.5 |
| Surgical | 9 | 30.0 | 5 | 18.5 |
| Among physicians only, experience level | ||||
| Intern | 7 | 23.3 | ||
| Senior resident | 7 | 23.3 | ||
| Attending physician | 16 | 53.3 | ||
| Among attending physicians only, no. of years practicing | ||||
| <5 | 8 | 50.0 | ||
| 5<10 | 3 | 18.8 | ||
| 10 | 5 | 31.3 | ||
| Among nurses only, no. of years practicing | ||||
| <1 | 5 | 18.5 | ||
| 1<2 | 5 | 18.5 | ||
| 2<5 | 9 | 33.3 | ||
| 5<10 | 4 | 14.8 | ||
| 10<20 | 1 | 3.7 | ||
| 20 | 3 | 11.1 | ||
| Recruitment method | ||||
| Cared for patient with false‐positive score | 10 | 33.3 | 14 | 51.9 |
| Cared for patient with false‐negative score | 13 | 43.3 | 10 | 37.0 |
| Randomly selected to ensure data saturation for surgical settings | 7 | 23.3 | 3 | 11.1 |
Thematic Analysis
We provide the final themes, associated subthemes, and representative quotations below, with additional supporting quotations in Table 3. Because CHOP's MET is named the Critical Assessment Team, the term CAT appears in some quotations.
|
| Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration. |
| I think [the EWS] helps us to be focused and gives us definite criteria to look for if there is an issue or change. It hopefully gives us a head start if there is going to be a change. They have a way of tracking it with the different color‐coding system they use Like, Oh geez, the heart rate is a little bit higher, that changes the color from yellow to orange, then I have to let the charge nurse know because that is a change from where they were earlier it kind of organizes it, I feel like, from where it was before. (medical nurse with 23 years of experience) |
| I think for myself, as a new clinician, one of our main goals is to help judge sick versus not sick. So to have a concrete system for thinking about that is helpful. (medical intern) |
| I think [the EWS] can help put things together for us. When you are really busy, you don't always get to focus on a lot of details. It is like another red flag to say you might have not realized that the child's heart rates went up further, but now here's some evidence that they did. (medical senior resident) |
| I think that the ability to use the EWS to watch the progression of a patient over time is really helpful. I've had a few patients that have gotten sicker from a respiratory standpoint. We can have multiple on the floor at the same time, and what's nice is that sometimes nurses have been able to come to me and we can really see through the score that we are at the point where a higher level of care is needed, whereas, in the old system, without that, we would have had to essentially wait for true clinical decompensation before the ICU would have been involved. I think that does help to deliver better care. (medical senior resident) |
| Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children. |
| Sometimes you just write down the vitals and maybe you are not really thinking, and then when you go to do the EWS you looked at the score and it's really off in their age range. It kind of gives you 1 more step to recognize that there's a problem. (medical nurse with <1 year of experience) |
| I see the role [of the EWS] more broadly as a guide of where your patient should fall with their vital signs according to their age. I think that has been the biggest help for me, to be able to visualize, I have a 3‐year‐old; this is where they should be for their respiratory rate or heart rate. I think it has been good to be able to see that they are falling within the range appropriate for their age. (surgical nurse with 9 years of experience) |
| Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients. |
| The times when I think the EWS helped me out the most are when there is a little bit of disagreement maybe the doctors and the nurses don't see eye‐to‐eye on how the patient is doing and so a higher score can sometimes be a way to say, I know there is nothing specifically going on, but if you take a look at the EWSs they are turning up very significantly. That might be enough to at least get a second opinion on that patient or to start some kind of change in their care. (medical nurse with <1 year of experience) |
| If we have the EWS to back us up, we can use that to say, Look, I don't feel comfortable with this patient, the EWS is 7 and I know you are saying they are okay, but I would feel more comfortable calling. Having that protocol in place I feel like it really gives us a voice it kind of gives us the, not that they don't trust us, but if they say, Oh, I think the child is fine but if I tell them Look, their EWS is an 8 or a 9, they are like, Oh, okay. It is not just you freaking out. There is an issue. (medical nurse with 3 years of experience) |
| I think that since it has been instituted nursing is coming to residents more than they did beforehand Can you reassess this patient? Do you think that we should call CAT? I think that it encourages residents to reevaluate patients at times when things are changing, and quicker than it did without the system in place. (medical senior resident) |
| I view [the EWS] as a tool, like if I have someone managing my patients when I am on service this would be a good tool because it mandates the nurses to notify and it also mandates the residents to understand what's going on. I think that was done on purpose. (medical attending physician in practice for 8 years) |
| Theme 4: In some patients, the EWS may not help with decision‐making. These include patients who are very stable and have a low likelihood of deterioration, and patients with abnormal physiology at baseline who consistently have very high EWSs. |
| The patient I took care of in this situation was a really sick kid to begin with, and it wasn't so much they were concerned about his EWS because, unless there was a really serious event, he would probably be staying on our floor anyway in some cases we just have some really sick kids whose scores may constantly be high all the time, so it wouldn't be helpful for the doctors or us to really bring it up. (medical nurse with 1 year of experience) |
Of note, after interviewing 9 surgeons, we found that they were not very familiar with the EWS and had little to say either positively or negatively about the system. For example, when asked what they thought about the EWS, a surgical intern said, I have no idea. I don't have enough experience with it. This is probably the first time that I ever had anybody telling me that the system is in place. Therefore, surgeons did not contribute meaningfully to the themes below.
Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration
Nurses and physicians frequently discussed the direct role of the EWS in revealing changes consistent with early signs of deterioration. A medical nurse with <1 year of experience said, The higher the number gets, the more it sets off a red flag to you to kind of keep an eye on certain things. They are just as important as taking a set of vitals. When asked if the EWS had ever helped to identify deterioration, a medical attending physician in practice for 5 years said, I think sometimes we will blow off, so to speak, certain things, but when you look at the numbers and you see a big [EWS] change versus if you were [just] looking at individual vital signs, then yeah, I think it has made a difference.
Nurses and physicians also discussed the role of the EWSs in prompting them to closely examine individual vital signs and think critically about whether or not a patient is exhibiting early signs of deterioration. A surgical nurse with <1 year of experience said, Sometimes I feel like if you want things to be okay you can kind of write them off, but when you have to write [the EWS] down it kind of jogs you to think, maybe something is going on or maybe someone else needs to know about this. A medical senior resident commented, I think it has alerted me earlier to changes in vital signs that I might not necessarily have known. I think there are nurses that use it and they see that there is an elevation and they call you about it. Then it makes me go back and look through and see what their vital signs are and if it happens in timewe only go through and look at everyone's vital signs about twice a dayit can be very helpful.
Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children
Although this theme did not appear among physicians, nurses frequently noted that they referred to the scoring sheet as a reference for vital signs appropriate for hospitalized children. A surgical nurse with <1 year of experience said, In nursing school, I mostly dealt with adults. So, to figure out the different ranges for normal vital signs, it helps to have it listed on paper so I can see, 'Oh, I didn't realize that this 10‐year‐old's heart rate is higher than it should be.' A medical nurse with 14 years of experience cited the benefits for less‐experienced nurses, noting, [The EWS helps] newer nurses who don't know the ranges. Where it's Oh, my kid's blood pressure is 81 [mm Hg] over something, then they can look at their age and say, Oh, that is completely normal for a 2‐month‐old. But [before the EWS] there was nowhere to look to see the ranges. Unless you were [Pediatric Advanced Life Support] certified where you would know that stuff, there was a lot of anxiety related to vital signs.
Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients
Nurses and physicians often described the role of the EWS as a source of objective evidence that a patient was exhibiting a concerning change. They shared the ways in which the EWS was used to convey concerns, noting most commonly that this was used as a communication tool by nurses to raise their concerns with physicians. A medical nurse with 23 years of experience said, [With the EWS] you feel like you have concrete evidence. It's not just a feeling [that] they are not looking as well as they were it feels scientific. Building upon this concept, a medical attending physician in practice for 2 years said, The EWS is a number that certainly gives people a sense of Here's the data behind why I am really coming to you and insisting on this. It is not calling and saying, I just have a bad feeling, it is, I have a bad feeling and his EWS has gone to a 9.
Theme 4: In some patients, the EWS may not help with decision making. These include patients who are very stable and have a low likelihood of deterioration, patients with abnormal physiology at baseline who consistently have very high EWSs, and patients experiencing neurologic deterioration
Nurses and physicians described some patient scenarios in which the EWS may not help with decision making. Discussing postoperative patients, a surgical nurse with 1 year of experience said, I love doing [the EWS] for some patients. I think it makes perfect sense. Then there are some patients [for whom] I am doing it just to do it because they are only here for 24 hours. They are completely stable. They never had 1 vital sign that was even a little bit off. It's kind of like we are just filling it out to fill it out. Commenting on patients at the other end of the spectrum, a medical attending physician in practice for 2 years said, [The EWS] can be a useful composite tool, but for specialty patients with abnormal baselines, I think it is much more a question of making sure you pay attention to the specific changes, whether it is the EWS or heart rate or vital signs or pain score or any of those things. A final area in which nurses and physicians identified weaknesses in the EWS surrounded neurologic deterioration. Specifically, nurses and physicians described experiences when the EWS increased minimally or not at all in patients with sudden seizures or concerning mental status changes that warranted escalation of care.
DISCUSSION
This study is the first to analyze viewpoints on the mechanisms by which EWSs impact decision making among physicians and nurses who had recently experienced score failures. Our study, performed in a children's hospital, builds upon the findings of related studies performed in hospitals that care primarily for adults.[23, 24, 25, 26, 27, 28] Andrews and Waterman found that nurses consider the utility of EWSs to extend beyond detecting deterioration by providing quantifiable evidence, packaged in the form of a score that improves communication between nurses and physicians.[23] Mackintosh and colleagues found that a RRS that included an EWS helped to formalize the way nurses and physicians understand deterioration, enable them to overcome hierarchical boundaries through structured discussions, and empower them to call for help.[24] In a quasi‐experimental study, McDonnell and colleagues found that an EWS improved self‐assessed knowledge, skills, and confidence of nursing staff to detect and manage deteriorating patients.[25] In addition, we describe novel findings, including the use of EWS parameters as reference ranges independent of the score, and specific situations when the EWS fails to support decision making. The weaknesses we identified could be used to drive EWS optimization for low‐risk patients who are stable as well as higher‐risk patients with abnormal baseline physiology and those at risk of neurologic deterioration.
This study has several limitations. Although the interviewers were not involved in RRS operations, it is possible that social desirability bias influenced responses. Next, we identified a knowledge gap among surgeons, and they contributed minimally to our findings. This is most likely because (1) surgical patients deteriorate on the wards less often than medical patients in our hospital, so surgeons are rarely presented with EWSs; (2) surgeons spend less time on the wards compared with medical physicians; and (3) surgical residents rotate in short blocks interspersed with rotations at other hospitals and may be less engaged in hospital safety initiatives.
CONCLUSIONS
Although EWSs perform only marginally well as statistical tools to predict clinical deterioration, nurses and physicians who recently experienced score failures described substantial benefits in using them to help identify deteriorating patients and transcend barriers to escalation of care by serving as objective communication tools. Combining an EWS with a clinician's judgment may result in a system better equipped to respond to deterioration than previous EWS studies focused on their test characteristics alone suggest. Future research should seek to compare and prospectively evaluate the clinical effectiveness of EWSs in real‐world settings.
Acknowledgments
Disclosures: This project was funded by the Pennsylvania Health Research Formula Fund Award (awarded to Keren and Bonafide) and the CHOP Nursing Research and Evidence‐Based Practice Award (awarded to Roberts). The funders did not influence the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication. The authors have no other conflicts to report.
- Institute for Healthcare Improvement. Overview of the Institute for Healthcare Improvement Five Million Lives Campaign. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed June 21, 2012.
- UK National Institute for Health and Clinical Excellence (NICE). Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital. Available at: http://publications.nice.org.uk/acutely‐ill‐patients‐in‐hospital‐cg50. Published July 2007. Accessed June 21, 2012.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006; 34(9):2463–2478.
- . Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32–35.
- , , , , , . Sensitivity of the Pediatric Early Warning Score to identify patient deterioration. Pediatrics. 2010;125(4):e763–e769.
- , , . Promoting care for acutely ill children—development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs. 2006;22(2):73–81.
- , , , . Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child. 2009;94(8):602–606.
- , , . The Pediatric Early Warning System Score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271–278.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13(4):R135.
- , , , et al. Multi‐centre validation of the Bedside Paediatric Early Warning System Score: a severity of illness score to detect evolving critical illness in hospitalized children. Crit Care. 2011;15(4):R184.
- , . Evaluation of a paediatric early warning tool—claims unsubstantiated. Intensive Crit Care Nurs. 2006;22(6):315–316.
- , , , et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874–e881.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128(1):72–78.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246.
- , , , et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center. Arch Pediatr Adolesc Med. 2008;162(2):117–122.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital. JAMA. 2007;298(19):2267–2274.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312.
- , , , et al. Implementation and impact of a rapid response team in a children's hospital. Jt Comm J Qual Patient Saf. 2007;33(7):418–425.
- , , . How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
- . Different approaches in grounded theory. In: Bryant A, Charmaz K, eds. The Sage Handbook of Grounded Theory. Los Angeles, CA: Sage; 2007:191–213.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine De Gruyter; 1967.
- , . Packaging: a grounded theory of how to report physiological deterioration effectively. J Adv Nurs. 2005;52(5):473–481.
- , , . Understanding how rapid response systems may improve safety for the acutely ill patient: learning from the frontline. BMJ Qual Saf. 2012;21(2):135–144.
- , , , , , . A before and after study assessing the impact of a new model for recognizing and responding to early signs of deterioration in an acute hospital. J Adv Nurs. 2013;69(1):41–52.
- , . Overcoming gendered and professional hierarchies in order to facilitate escalation of care in emergency situations: the role of standardised communication protocols. Soc Sci Med. 2010;71(9):1683–1686.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , , , . Leading successful rapid response teams: a multisite implementation evaluation. J Nurs Adm. 2009;39(4):176–181.
Thousands of hospitals have recently implemented rapid response systems (RRSs), attempting to reduce mortality outside of intensive care units (ICUs).[1, 2] These systems have 2 clinical components, a response (efferent) arm and an identification (afferent) arm.[3] The response arm is usually composed of a medical emergency team (MET) that responds to calls for urgent assistance. The identification arm includes tools to help clinicians recognize patients who require assistance from the MET. In many hospitals, the identification arm includes an early warning score (EWS). In pediatric patients, EWSs assign point values to vital signs that fall outside of age‐based ranges, among other clinical observations. They then generate a total score intended to help clinicians identify patients exhibiting early signs of deterioration.[4, 5, 6, 7, 8, 9, 10, 11]
When experimentally applied to vital sign datasets, the test characteristics of pediatric EWSs in detecting clinical deterioration are highly variable across studies, with major tradeoffs between sensitivity, specificity, and predictive values that differ by outcome, score, and cut‐point (Table 1). This reflects the difficulty of identifying deteriorating patients using only objective measures. However, in real‐world settings, EWSs are used by clinicians in conjunction with their clinical judgment. We hypothesized that EWSs have benefits that extend beyond their ability to predict deterioration, and thus have value not demonstrated by test characteristics alone. In order to further explore this issue, we aimed to qualitatively evaluate mechanisms beyond their statistical ability to predict deterioration by which physicians and nurses use EWSs to support their decision making.
| Score and Citation | Outcome Measure | Score Cut‐point | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|
| ||||||
| Brighton Paediatric Early Warning Score[5] | RRT or code blue call | 4 | 86% | NR | NR | NR |
| Bristol Paediatric Early Warning Tool[6, 11] | Escalation to higher level of care | 1 | ER | ER | 63% | NR |
| Cardiff and Vale Paediatric Early Warning System[7] | Respiratory or cardiac arrest, HDU/ICU admission, or death | 1 | 89% | 64% | 2% | >99% |
| Bedside Paediatric Early Warning System score, original version[8] | Code blue call | 5 | 78% | 95% | 4% | NR |
| Bedside Paediatric Early Warning System score, simplified version[9] | Urgent ICU admission without a code blue call | 8 | 82% | 93% | NR | NR |
| Bedside Paediatric Early Warning System score, simplified version[10] | Urgent ICU admission or code blue call | 7 | 64% | 91% | 9% | NR |
METHODS
Overview
As 1 component of a larger study, we conducted semistructured interviews with nurses and physicians at The Children's Hospital of Philadelphia (CHOP) between May and October 2011. In separate subprojects using the same participants, the larger study also aimed to identify residual barriers to calling for urgent assistance and assess the role of families in the recognition of deterioration and MET activation.
Setting
The Children's Hospital of Philadelphia is an urban, tertiary‐care pediatric hospital with 504 beds. Surgical patients hospitalized outside of ICUs are cared for by surgeons and surgical nurses without pediatrician co‐management. Implementation of a RRS was prompted by serious safety events in which clinical deterioration was either not recognized or was recognized and not escalated. Prior to RRS implementation, a code blue team could be activated for patients in immediate need of resuscitation, or, for less‐urgent needs, a pediatric ICU fellow could be paged by physicians for informal consults.
A multidisciplinary team developed and pilot‐tested the RRS, then implemented it hospital‐wide in February 2010. Representing an aspect of a multipronged approach to improve safety culture, the RRS consisted of (1) an EWS based upon Parshuram's Bedside Paediatric Early Warning System,[8, 9, 10] calculated by hand on a paper form (see online supplementary content) at the same frequency as vital signs (usually every 4 hours), and (2) a 30‐minute response MET available for activation by any clinician for any concern, 24 hours per day, 7 days per week. Escalation guidelines included a prompt to activate the MET for a score that increased to the red zone (9). For concerns that could not wait 30 minutes, any hospital employee could activate the immediate‐response code blue team.
Utilization of the RRS at CHOP is high, with 23 calls to the MET per day and a combined MET/code‐blue team call rate of 27.8 per 1000 admissions.[12] Previously reported pediatric call rates range from 2.8 to 44.0, with a median of 9.6 per 1000 admissions across 6 studies.[13, 14, 15, 16, 17, 18, 19] Since implementation, there has been a statistically significant net reduction in critical deterioration events (unpublished data).
Participants
We recruited nurses and physicians who had recently cared for children age 18 years on general medical or surgical wards with false‐negative or false‐positive EWSs (instances when the score failed to predict deterioration). Recruitment ceased when we reached thematic data saturation (a qualitative research term for the point at which no new themes emerge with additional interviews).[20]
Data Collection
Through a detailed review of the relevant literature and consultation with experts, we developed a semistructured interview guide (see online supplementary content) to elicit nurses' and physicians' viewpoints regarding the mechanisms by which they use EWSs to support their decision making.
Experienced qualitative research scientists (F.K.B. and J.H.H.) trained 2 study interviewers (B.P. and K.M.T.). In order to minimize social‐desirability bias, the interviewers were not clinicians and were not involved in RRS operations. Each interview was recorded, professionally transcribed, and imported into NVivo 8.0 software for analysis (QSR International, Melbourne, Australia).
Data Analysis
We coded the interviews inductively, without using a predetermined set of themes. This approach is known as grounded theory methodology.[21] Two team members coded each interview independently. They then reviewed their coding together and discussed discrepancies until reaching consensus. In weekly meetings while the interviews were ongoing, we compared newly collected data with themes that had previously emerged in order to guide further thematic development and refinement (the constant comparative method).[22] After all of the interviews were completed and consensus had been reached for each individual interview, the study team convened a series of additional meetings to further refine and finalize the themes.
Human Subjects
The CHOP Institutional Review Board approved this study. All participants provided written informed consent.
RESULTS
Participants
We recruited 27 nurses and 30 physicians before reaching thematic data saturation. Because surgical patients are underrepresented relative to medical patients among the population with false‐positive and false‐negative scores in our hospital, this included 3 randomly selected surgical nurses and 7 randomly selected surgical physicians recruited to ensure thematic data saturation for surgical settings. Characteristics of the participants are displayed in Table 2.
| Physicians (n=30) | Nurses (n=27) | |||
|---|---|---|---|---|
| ||||
| N | % | N | % | |
| Race | ||||
| Asian | 2 | 6.7 | 1 | 3.7 |
| Black | 0 | 0.0 | 2 | 7.4 |
| White | 26 | 86.7 | 22 | 81.5 |
| Prefer not to say | 1 | 3.3 | 1 | 3.7 |
| >1 race | 1 | 3.3 | 1 | 3.7 |
| Ethnicity | ||||
| Hispanic/Latino | 2 | 6.7 | 1 | 3.7 |
| Not Hispanic/Latino | 23 | 76.7 | 25 | 92.6 |
| Prefer not to say | 5 | 16.7 | 1 | 3.7 |
| Sex | ||||
| F | 16 | 53.3 | 25 | 92.6 |
| M | 14 | 46.7 | 2 | 7.4 |
| Practice setting | ||||
| Medical | 21 | 70.0 | 22 | 81.5 |
| Surgical | 9 | 30.0 | 5 | 18.5 |
| Among physicians only, experience level | ||||
| Intern | 7 | 23.3 | ||
| Senior resident | 7 | 23.3 | ||
| Attending physician | 16 | 53.3 | ||
| Among attending physicians only, no. of years practicing | ||||
| <5 | 8 | 50.0 | ||
| 5<10 | 3 | 18.8 | ||
| 10 | 5 | 31.3 | ||
| Among nurses only, no. of years practicing | ||||
| <1 | 5 | 18.5 | ||
| 1<2 | 5 | 18.5 | ||
| 2<5 | 9 | 33.3 | ||
| 5<10 | 4 | 14.8 | ||
| 10<20 | 1 | 3.7 | ||
| 20 | 3 | 11.1 | ||
| Recruitment method | ||||
| Cared for patient with false‐positive score | 10 | 33.3 | 14 | 51.9 |
| Cared for patient with false‐negative score | 13 | 43.3 | 10 | 37.0 |
| Randomly selected to ensure data saturation for surgical settings | 7 | 23.3 | 3 | 11.1 |
Thematic Analysis
We provide the final themes, associated subthemes, and representative quotations below, with additional supporting quotations in Table 3. Because CHOP's MET is named the Critical Assessment Team, the term CAT appears in some quotations.
|
| Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration. |
| I think [the EWS] helps us to be focused and gives us definite criteria to look for if there is an issue or change. It hopefully gives us a head start if there is going to be a change. They have a way of tracking it with the different color‐coding system they use Like, Oh geez, the heart rate is a little bit higher, that changes the color from yellow to orange, then I have to let the charge nurse know because that is a change from where they were earlier it kind of organizes it, I feel like, from where it was before. (medical nurse with 23 years of experience) |
| I think for myself, as a new clinician, one of our main goals is to help judge sick versus not sick. So to have a concrete system for thinking about that is helpful. (medical intern) |
| I think [the EWS] can help put things together for us. When you are really busy, you don't always get to focus on a lot of details. It is like another red flag to say you might have not realized that the child's heart rates went up further, but now here's some evidence that they did. (medical senior resident) |
| I think that the ability to use the EWS to watch the progression of a patient over time is really helpful. I've had a few patients that have gotten sicker from a respiratory standpoint. We can have multiple on the floor at the same time, and what's nice is that sometimes nurses have been able to come to me and we can really see through the score that we are at the point where a higher level of care is needed, whereas, in the old system, without that, we would have had to essentially wait for true clinical decompensation before the ICU would have been involved. I think that does help to deliver better care. (medical senior resident) |
| Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children. |
| Sometimes you just write down the vitals and maybe you are not really thinking, and then when you go to do the EWS you looked at the score and it's really off in their age range. It kind of gives you 1 more step to recognize that there's a problem. (medical nurse with <1 year of experience) |
| I see the role [of the EWS] more broadly as a guide of where your patient should fall with their vital signs according to their age. I think that has been the biggest help for me, to be able to visualize, I have a 3‐year‐old; this is where they should be for their respiratory rate or heart rate. I think it has been good to be able to see that they are falling within the range appropriate for their age. (surgical nurse with 9 years of experience) |
| Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients. |
| The times when I think the EWS helped me out the most are when there is a little bit of disagreement maybe the doctors and the nurses don't see eye‐to‐eye on how the patient is doing and so a higher score can sometimes be a way to say, I know there is nothing specifically going on, but if you take a look at the EWSs they are turning up very significantly. That might be enough to at least get a second opinion on that patient or to start some kind of change in their care. (medical nurse with <1 year of experience) |
| If we have the EWS to back us up, we can use that to say, Look, I don't feel comfortable with this patient, the EWS is 7 and I know you are saying they are okay, but I would feel more comfortable calling. Having that protocol in place I feel like it really gives us a voice it kind of gives us the, not that they don't trust us, but if they say, Oh, I think the child is fine but if I tell them Look, their EWS is an 8 or a 9, they are like, Oh, okay. It is not just you freaking out. There is an issue. (medical nurse with 3 years of experience) |
| I think that since it has been instituted nursing is coming to residents more than they did beforehand Can you reassess this patient? Do you think that we should call CAT? I think that it encourages residents to reevaluate patients at times when things are changing, and quicker than it did without the system in place. (medical senior resident) |
| I view [the EWS] as a tool, like if I have someone managing my patients when I am on service this would be a good tool because it mandates the nurses to notify and it also mandates the residents to understand what's going on. I think that was done on purpose. (medical attending physician in practice for 8 years) |
| Theme 4: In some patients, the EWS may not help with decision‐making. These include patients who are very stable and have a low likelihood of deterioration, and patients with abnormal physiology at baseline who consistently have very high EWSs. |
| The patient I took care of in this situation was a really sick kid to begin with, and it wasn't so much they were concerned about his EWS because, unless there was a really serious event, he would probably be staying on our floor anyway in some cases we just have some really sick kids whose scores may constantly be high all the time, so it wouldn't be helpful for the doctors or us to really bring it up. (medical nurse with 1 year of experience) |
Of note, after interviewing 9 surgeons, we found that they were not very familiar with the EWS and had little to say either positively or negatively about the system. For example, when asked what they thought about the EWS, a surgical intern said, I have no idea. I don't have enough experience with it. This is probably the first time that I ever had anybody telling me that the system is in place. Therefore, surgeons did not contribute meaningfully to the themes below.
Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration
Nurses and physicians frequently discussed the direct role of the EWS in revealing changes consistent with early signs of deterioration. A medical nurse with <1 year of experience said, The higher the number gets, the more it sets off a red flag to you to kind of keep an eye on certain things. They are just as important as taking a set of vitals. When asked if the EWS had ever helped to identify deterioration, a medical attending physician in practice for 5 years said, I think sometimes we will blow off, so to speak, certain things, but when you look at the numbers and you see a big [EWS] change versus if you were [just] looking at individual vital signs, then yeah, I think it has made a difference.
Nurses and physicians also discussed the role of the EWSs in prompting them to closely examine individual vital signs and think critically about whether or not a patient is exhibiting early signs of deterioration. A surgical nurse with <1 year of experience said, Sometimes I feel like if you want things to be okay you can kind of write them off, but when you have to write [the EWS] down it kind of jogs you to think, maybe something is going on or maybe someone else needs to know about this. A medical senior resident commented, I think it has alerted me earlier to changes in vital signs that I might not necessarily have known. I think there are nurses that use it and they see that there is an elevation and they call you about it. Then it makes me go back and look through and see what their vital signs are and if it happens in timewe only go through and look at everyone's vital signs about twice a dayit can be very helpful.
Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children
Although this theme did not appear among physicians, nurses frequently noted that they referred to the scoring sheet as a reference for vital signs appropriate for hospitalized children. A surgical nurse with <1 year of experience said, In nursing school, I mostly dealt with adults. So, to figure out the different ranges for normal vital signs, it helps to have it listed on paper so I can see, 'Oh, I didn't realize that this 10‐year‐old's heart rate is higher than it should be.' A medical nurse with 14 years of experience cited the benefits for less‐experienced nurses, noting, [The EWS helps] newer nurses who don't know the ranges. Where it's Oh, my kid's blood pressure is 81 [mm Hg] over something, then they can look at their age and say, Oh, that is completely normal for a 2‐month‐old. But [before the EWS] there was nowhere to look to see the ranges. Unless you were [Pediatric Advanced Life Support] certified where you would know that stuff, there was a lot of anxiety related to vital signs.
Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients
Nurses and physicians often described the role of the EWS as a source of objective evidence that a patient was exhibiting a concerning change. They shared the ways in which the EWS was used to convey concerns, noting most commonly that this was used as a communication tool by nurses to raise their concerns with physicians. A medical nurse with 23 years of experience said, [With the EWS] you feel like you have concrete evidence. It's not just a feeling [that] they are not looking as well as they were it feels scientific. Building upon this concept, a medical attending physician in practice for 2 years said, The EWS is a number that certainly gives people a sense of Here's the data behind why I am really coming to you and insisting on this. It is not calling and saying, I just have a bad feeling, it is, I have a bad feeling and his EWS has gone to a 9.
Theme 4: In some patients, the EWS may not help with decision making. These include patients who are very stable and have a low likelihood of deterioration, patients with abnormal physiology at baseline who consistently have very high EWSs, and patients experiencing neurologic deterioration
Nurses and physicians described some patient scenarios in which the EWS may not help with decision making. Discussing postoperative patients, a surgical nurse with 1 year of experience said, I love doing [the EWS] for some patients. I think it makes perfect sense. Then there are some patients [for whom] I am doing it just to do it because they are only here for 24 hours. They are completely stable. They never had 1 vital sign that was even a little bit off. It's kind of like we are just filling it out to fill it out. Commenting on patients at the other end of the spectrum, a medical attending physician in practice for 2 years said, [The EWS] can be a useful composite tool, but for specialty patients with abnormal baselines, I think it is much more a question of making sure you pay attention to the specific changes, whether it is the EWS or heart rate or vital signs or pain score or any of those things. A final area in which nurses and physicians identified weaknesses in the EWS surrounded neurologic deterioration. Specifically, nurses and physicians described experiences when the EWS increased minimally or not at all in patients with sudden seizures or concerning mental status changes that warranted escalation of care.
DISCUSSION
This study is the first to analyze viewpoints on the mechanisms by which EWSs impact decision making among physicians and nurses who had recently experienced score failures. Our study, performed in a children's hospital, builds upon the findings of related studies performed in hospitals that care primarily for adults.[23, 24, 25, 26, 27, 28] Andrews and Waterman found that nurses consider the utility of EWSs to extend beyond detecting deterioration by providing quantifiable evidence, packaged in the form of a score that improves communication between nurses and physicians.[23] Mackintosh and colleagues found that a RRS that included an EWS helped to formalize the way nurses and physicians understand deterioration, enable them to overcome hierarchical boundaries through structured discussions, and empower them to call for help.[24] In a quasi‐experimental study, McDonnell and colleagues found that an EWS improved self‐assessed knowledge, skills, and confidence of nursing staff to detect and manage deteriorating patients.[25] In addition, we describe novel findings, including the use of EWS parameters as reference ranges independent of the score, and specific situations when the EWS fails to support decision making. The weaknesses we identified could be used to drive EWS optimization for low‐risk patients who are stable as well as higher‐risk patients with abnormal baseline physiology and those at risk of neurologic deterioration.
This study has several limitations. Although the interviewers were not involved in RRS operations, it is possible that social desirability bias influenced responses. Next, we identified a knowledge gap among surgeons, and they contributed minimally to our findings. This is most likely because (1) surgical patients deteriorate on the wards less often than medical patients in our hospital, so surgeons are rarely presented with EWSs; (2) surgeons spend less time on the wards compared with medical physicians; and (3) surgical residents rotate in short blocks interspersed with rotations at other hospitals and may be less engaged in hospital safety initiatives.
CONCLUSIONS
Although EWSs perform only marginally well as statistical tools to predict clinical deterioration, nurses and physicians who recently experienced score failures described substantial benefits in using them to help identify deteriorating patients and transcend barriers to escalation of care by serving as objective communication tools. Combining an EWS with a clinician's judgment may result in a system better equipped to respond to deterioration than previous EWS studies focused on their test characteristics alone suggest. Future research should seek to compare and prospectively evaluate the clinical effectiveness of EWSs in real‐world settings.
Acknowledgments
Disclosures: This project was funded by the Pennsylvania Health Research Formula Fund Award (awarded to Keren and Bonafide) and the CHOP Nursing Research and Evidence‐Based Practice Award (awarded to Roberts). The funders did not influence the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication. The authors have no other conflicts to report.
Thousands of hospitals have recently implemented rapid response systems (RRSs), attempting to reduce mortality outside of intensive care units (ICUs).[1, 2] These systems have 2 clinical components, a response (efferent) arm and an identification (afferent) arm.[3] The response arm is usually composed of a medical emergency team (MET) that responds to calls for urgent assistance. The identification arm includes tools to help clinicians recognize patients who require assistance from the MET. In many hospitals, the identification arm includes an early warning score (EWS). In pediatric patients, EWSs assign point values to vital signs that fall outside of age‐based ranges, among other clinical observations. They then generate a total score intended to help clinicians identify patients exhibiting early signs of deterioration.[4, 5, 6, 7, 8, 9, 10, 11]
When experimentally applied to vital sign datasets, the test characteristics of pediatric EWSs in detecting clinical deterioration are highly variable across studies, with major tradeoffs between sensitivity, specificity, and predictive values that differ by outcome, score, and cut‐point (Table 1). This reflects the difficulty of identifying deteriorating patients using only objective measures. However, in real‐world settings, EWSs are used by clinicians in conjunction with their clinical judgment. We hypothesized that EWSs have benefits that extend beyond their ability to predict deterioration, and thus have value not demonstrated by test characteristics alone. In order to further explore this issue, we aimed to qualitatively evaluate mechanisms beyond their statistical ability to predict deterioration by which physicians and nurses use EWSs to support their decision making.
| Score and Citation | Outcome Measure | Score Cut‐point | Sens | Spec | PPV | NPV |
|---|---|---|---|---|---|---|
| ||||||
| Brighton Paediatric Early Warning Score[5] | RRT or code blue call | 4 | 86% | NR | NR | NR |
| Bristol Paediatric Early Warning Tool[6, 11] | Escalation to higher level of care | 1 | ER | ER | 63% | NR |
| Cardiff and Vale Paediatric Early Warning System[7] | Respiratory or cardiac arrest, HDU/ICU admission, or death | 1 | 89% | 64% | 2% | >99% |
| Bedside Paediatric Early Warning System score, original version[8] | Code blue call | 5 | 78% | 95% | 4% | NR |
| Bedside Paediatric Early Warning System score, simplified version[9] | Urgent ICU admission without a code blue call | 8 | 82% | 93% | NR | NR |
| Bedside Paediatric Early Warning System score, simplified version[10] | Urgent ICU admission or code blue call | 7 | 64% | 91% | 9% | NR |
METHODS
Overview
As 1 component of a larger study, we conducted semistructured interviews with nurses and physicians at The Children's Hospital of Philadelphia (CHOP) between May and October 2011. In separate subprojects using the same participants, the larger study also aimed to identify residual barriers to calling for urgent assistance and assess the role of families in the recognition of deterioration and MET activation.
Setting
The Children's Hospital of Philadelphia is an urban, tertiary‐care pediatric hospital with 504 beds. Surgical patients hospitalized outside of ICUs are cared for by surgeons and surgical nurses without pediatrician co‐management. Implementation of a RRS was prompted by serious safety events in which clinical deterioration was either not recognized or was recognized and not escalated. Prior to RRS implementation, a code blue team could be activated for patients in immediate need of resuscitation, or, for less‐urgent needs, a pediatric ICU fellow could be paged by physicians for informal consults.
A multidisciplinary team developed and pilot‐tested the RRS, then implemented it hospital‐wide in February 2010. Representing an aspect of a multipronged approach to improve safety culture, the RRS consisted of (1) an EWS based upon Parshuram's Bedside Paediatric Early Warning System,[8, 9, 10] calculated by hand on a paper form (see online supplementary content) at the same frequency as vital signs (usually every 4 hours), and (2) a 30‐minute response MET available for activation by any clinician for any concern, 24 hours per day, 7 days per week. Escalation guidelines included a prompt to activate the MET for a score that increased to the red zone (9). For concerns that could not wait 30 minutes, any hospital employee could activate the immediate‐response code blue team.
Utilization of the RRS at CHOP is high, with 23 calls to the MET per day and a combined MET/code‐blue team call rate of 27.8 per 1000 admissions.[12] Previously reported pediatric call rates range from 2.8 to 44.0, with a median of 9.6 per 1000 admissions across 6 studies.[13, 14, 15, 16, 17, 18, 19] Since implementation, there has been a statistically significant net reduction in critical deterioration events (unpublished data).
Participants
We recruited nurses and physicians who had recently cared for children age 18 years on general medical or surgical wards with false‐negative or false‐positive EWSs (instances when the score failed to predict deterioration). Recruitment ceased when we reached thematic data saturation (a qualitative research term for the point at which no new themes emerge with additional interviews).[20]
Data Collection
Through a detailed review of the relevant literature and consultation with experts, we developed a semistructured interview guide (see online supplementary content) to elicit nurses' and physicians' viewpoints regarding the mechanisms by which they use EWSs to support their decision making.
Experienced qualitative research scientists (F.K.B. and J.H.H.) trained 2 study interviewers (B.P. and K.M.T.). In order to minimize social‐desirability bias, the interviewers were not clinicians and were not involved in RRS operations. Each interview was recorded, professionally transcribed, and imported into NVivo 8.0 software for analysis (QSR International, Melbourne, Australia).
Data Analysis
We coded the interviews inductively, without using a predetermined set of themes. This approach is known as grounded theory methodology.[21] Two team members coded each interview independently. They then reviewed their coding together and discussed discrepancies until reaching consensus. In weekly meetings while the interviews were ongoing, we compared newly collected data with themes that had previously emerged in order to guide further thematic development and refinement (the constant comparative method).[22] After all of the interviews were completed and consensus had been reached for each individual interview, the study team convened a series of additional meetings to further refine and finalize the themes.
Human Subjects
The CHOP Institutional Review Board approved this study. All participants provided written informed consent.
RESULTS
Participants
We recruited 27 nurses and 30 physicians before reaching thematic data saturation. Because surgical patients are underrepresented relative to medical patients among the population with false‐positive and false‐negative scores in our hospital, this included 3 randomly selected surgical nurses and 7 randomly selected surgical physicians recruited to ensure thematic data saturation for surgical settings. Characteristics of the participants are displayed in Table 2.
| Physicians (n=30) | Nurses (n=27) | |||
|---|---|---|---|---|
| ||||
| N | % | N | % | |
| Race | ||||
| Asian | 2 | 6.7 | 1 | 3.7 |
| Black | 0 | 0.0 | 2 | 7.4 |
| White | 26 | 86.7 | 22 | 81.5 |
| Prefer not to say | 1 | 3.3 | 1 | 3.7 |
| >1 race | 1 | 3.3 | 1 | 3.7 |
| Ethnicity | ||||
| Hispanic/Latino | 2 | 6.7 | 1 | 3.7 |
| Not Hispanic/Latino | 23 | 76.7 | 25 | 92.6 |
| Prefer not to say | 5 | 16.7 | 1 | 3.7 |
| Sex | ||||
| F | 16 | 53.3 | 25 | 92.6 |
| M | 14 | 46.7 | 2 | 7.4 |
| Practice setting | ||||
| Medical | 21 | 70.0 | 22 | 81.5 |
| Surgical | 9 | 30.0 | 5 | 18.5 |
| Among physicians only, experience level | ||||
| Intern | 7 | 23.3 | ||
| Senior resident | 7 | 23.3 | ||
| Attending physician | 16 | 53.3 | ||
| Among attending physicians only, no. of years practicing | ||||
| <5 | 8 | 50.0 | ||
| 5<10 | 3 | 18.8 | ||
| 10 | 5 | 31.3 | ||
| Among nurses only, no. of years practicing | ||||
| <1 | 5 | 18.5 | ||
| 1<2 | 5 | 18.5 | ||
| 2<5 | 9 | 33.3 | ||
| 5<10 | 4 | 14.8 | ||
| 10<20 | 1 | 3.7 | ||
| 20 | 3 | 11.1 | ||
| Recruitment method | ||||
| Cared for patient with false‐positive score | 10 | 33.3 | 14 | 51.9 |
| Cared for patient with false‐negative score | 13 | 43.3 | 10 | 37.0 |
| Randomly selected to ensure data saturation for surgical settings | 7 | 23.3 | 3 | 11.1 |
Thematic Analysis
We provide the final themes, associated subthemes, and representative quotations below, with additional supporting quotations in Table 3. Because CHOP's MET is named the Critical Assessment Team, the term CAT appears in some quotations.
|
| Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration. |
| I think [the EWS] helps us to be focused and gives us definite criteria to look for if there is an issue or change. It hopefully gives us a head start if there is going to be a change. They have a way of tracking it with the different color‐coding system they use Like, Oh geez, the heart rate is a little bit higher, that changes the color from yellow to orange, then I have to let the charge nurse know because that is a change from where they were earlier it kind of organizes it, I feel like, from where it was before. (medical nurse with 23 years of experience) |
| I think for myself, as a new clinician, one of our main goals is to help judge sick versus not sick. So to have a concrete system for thinking about that is helpful. (medical intern) |
| I think [the EWS] can help put things together for us. When you are really busy, you don't always get to focus on a lot of details. It is like another red flag to say you might have not realized that the child's heart rates went up further, but now here's some evidence that they did. (medical senior resident) |
| I think that the ability to use the EWS to watch the progression of a patient over time is really helpful. I've had a few patients that have gotten sicker from a respiratory standpoint. We can have multiple on the floor at the same time, and what's nice is that sometimes nurses have been able to come to me and we can really see through the score that we are at the point where a higher level of care is needed, whereas, in the old system, without that, we would have had to essentially wait for true clinical decompensation before the ICU would have been involved. I think that does help to deliver better care. (medical senior resident) |
| Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children. |
| Sometimes you just write down the vitals and maybe you are not really thinking, and then when you go to do the EWS you looked at the score and it's really off in their age range. It kind of gives you 1 more step to recognize that there's a problem. (medical nurse with <1 year of experience) |
| I see the role [of the EWS] more broadly as a guide of where your patient should fall with their vital signs according to their age. I think that has been the biggest help for me, to be able to visualize, I have a 3‐year‐old; this is where they should be for their respiratory rate or heart rate. I think it has been good to be able to see that they are falling within the range appropriate for their age. (surgical nurse with 9 years of experience) |
| Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients. |
| The times when I think the EWS helped me out the most are when there is a little bit of disagreement maybe the doctors and the nurses don't see eye‐to‐eye on how the patient is doing and so a higher score can sometimes be a way to say, I know there is nothing specifically going on, but if you take a look at the EWSs they are turning up very significantly. That might be enough to at least get a second opinion on that patient or to start some kind of change in their care. (medical nurse with <1 year of experience) |
| If we have the EWS to back us up, we can use that to say, Look, I don't feel comfortable with this patient, the EWS is 7 and I know you are saying they are okay, but I would feel more comfortable calling. Having that protocol in place I feel like it really gives us a voice it kind of gives us the, not that they don't trust us, but if they say, Oh, I think the child is fine but if I tell them Look, their EWS is an 8 or a 9, they are like, Oh, okay. It is not just you freaking out. There is an issue. (medical nurse with 3 years of experience) |
| I think that since it has been instituted nursing is coming to residents more than they did beforehand Can you reassess this patient? Do you think that we should call CAT? I think that it encourages residents to reevaluate patients at times when things are changing, and quicker than it did without the system in place. (medical senior resident) |
| I view [the EWS] as a tool, like if I have someone managing my patients when I am on service this would be a good tool because it mandates the nurses to notify and it also mandates the residents to understand what's going on. I think that was done on purpose. (medical attending physician in practice for 8 years) |
| Theme 4: In some patients, the EWS may not help with decision‐making. These include patients who are very stable and have a low likelihood of deterioration, and patients with abnormal physiology at baseline who consistently have very high EWSs. |
| The patient I took care of in this situation was a really sick kid to begin with, and it wasn't so much they were concerned about his EWS because, unless there was a really serious event, he would probably be staying on our floor anyway in some cases we just have some really sick kids whose scores may constantly be high all the time, so it wouldn't be helpful for the doctors or us to really bring it up. (medical nurse with 1 year of experience) |
Of note, after interviewing 9 surgeons, we found that they were not very familiar with the EWS and had little to say either positively or negatively about the system. For example, when asked what they thought about the EWS, a surgical intern said, I have no idea. I don't have enough experience with it. This is probably the first time that I ever had anybody telling me that the system is in place. Therefore, surgeons did not contribute meaningfully to the themes below.
Theme 1: The EWS facilitates patient safety by alerting nurses and physicians to concerning vital sign changes and prompting them to think critically about the possibility of deterioration
Nurses and physicians frequently discussed the direct role of the EWS in revealing changes consistent with early signs of deterioration. A medical nurse with <1 year of experience said, The higher the number gets, the more it sets off a red flag to you to kind of keep an eye on certain things. They are just as important as taking a set of vitals. When asked if the EWS had ever helped to identify deterioration, a medical attending physician in practice for 5 years said, I think sometimes we will blow off, so to speak, certain things, but when you look at the numbers and you see a big [EWS] change versus if you were [just] looking at individual vital signs, then yeah, I think it has made a difference.
Nurses and physicians also discussed the role of the EWSs in prompting them to closely examine individual vital signs and think critically about whether or not a patient is exhibiting early signs of deterioration. A surgical nurse with <1 year of experience said, Sometimes I feel like if you want things to be okay you can kind of write them off, but when you have to write [the EWS] down it kind of jogs you to think, maybe something is going on or maybe someone else needs to know about this. A medical senior resident commented, I think it has alerted me earlier to changes in vital signs that I might not necessarily have known. I think there are nurses that use it and they see that there is an elevation and they call you about it. Then it makes me go back and look through and see what their vital signs are and if it happens in timewe only go through and look at everyone's vital signs about twice a dayit can be very helpful.
Theme 2: The EWS provides less‐experienced nurses with helpful age‐based reference ranges for vital signs that they use when caring for hospitalized children
Although this theme did not appear among physicians, nurses frequently noted that they referred to the scoring sheet as a reference for vital signs appropriate for hospitalized children. A surgical nurse with <1 year of experience said, In nursing school, I mostly dealt with adults. So, to figure out the different ranges for normal vital signs, it helps to have it listed on paper so I can see, 'Oh, I didn't realize that this 10‐year‐old's heart rate is higher than it should be.' A medical nurse with 14 years of experience cited the benefits for less‐experienced nurses, noting, [The EWS helps] newer nurses who don't know the ranges. Where it's Oh, my kid's blood pressure is 81 [mm Hg] over something, then they can look at their age and say, Oh, that is completely normal for a 2‐month‐old. But [before the EWS] there was nowhere to look to see the ranges. Unless you were [Pediatric Advanced Life Support] certified where you would know that stuff, there was a lot of anxiety related to vital signs.
Theme 3: The EWS provides concrete evidence of clinical changes in the form of a score. This empowers nurses to overcome escalation barriers and communicate their concerns, helping them take action to rescue their deteriorating patients
Nurses and physicians often described the role of the EWS as a source of objective evidence that a patient was exhibiting a concerning change. They shared the ways in which the EWS was used to convey concerns, noting most commonly that this was used as a communication tool by nurses to raise their concerns with physicians. A medical nurse with 23 years of experience said, [With the EWS] you feel like you have concrete evidence. It's not just a feeling [that] they are not looking as well as they were it feels scientific. Building upon this concept, a medical attending physician in practice for 2 years said, The EWS is a number that certainly gives people a sense of Here's the data behind why I am really coming to you and insisting on this. It is not calling and saying, I just have a bad feeling, it is, I have a bad feeling and his EWS has gone to a 9.
Theme 4: In some patients, the EWS may not help with decision making. These include patients who are very stable and have a low likelihood of deterioration, patients with abnormal physiology at baseline who consistently have very high EWSs, and patients experiencing neurologic deterioration
Nurses and physicians described some patient scenarios in which the EWS may not help with decision making. Discussing postoperative patients, a surgical nurse with 1 year of experience said, I love doing [the EWS] for some patients. I think it makes perfect sense. Then there are some patients [for whom] I am doing it just to do it because they are only here for 24 hours. They are completely stable. They never had 1 vital sign that was even a little bit off. It's kind of like we are just filling it out to fill it out. Commenting on patients at the other end of the spectrum, a medical attending physician in practice for 2 years said, [The EWS] can be a useful composite tool, but for specialty patients with abnormal baselines, I think it is much more a question of making sure you pay attention to the specific changes, whether it is the EWS or heart rate or vital signs or pain score or any of those things. A final area in which nurses and physicians identified weaknesses in the EWS surrounded neurologic deterioration. Specifically, nurses and physicians described experiences when the EWS increased minimally or not at all in patients with sudden seizures or concerning mental status changes that warranted escalation of care.
DISCUSSION
This study is the first to analyze viewpoints on the mechanisms by which EWSs impact decision making among physicians and nurses who had recently experienced score failures. Our study, performed in a children's hospital, builds upon the findings of related studies performed in hospitals that care primarily for adults.[23, 24, 25, 26, 27, 28] Andrews and Waterman found that nurses consider the utility of EWSs to extend beyond detecting deterioration by providing quantifiable evidence, packaged in the form of a score that improves communication between nurses and physicians.[23] Mackintosh and colleagues found that a RRS that included an EWS helped to formalize the way nurses and physicians understand deterioration, enable them to overcome hierarchical boundaries through structured discussions, and empower them to call for help.[24] In a quasi‐experimental study, McDonnell and colleagues found that an EWS improved self‐assessed knowledge, skills, and confidence of nursing staff to detect and manage deteriorating patients.[25] In addition, we describe novel findings, including the use of EWS parameters as reference ranges independent of the score, and specific situations when the EWS fails to support decision making. The weaknesses we identified could be used to drive EWS optimization for low‐risk patients who are stable as well as higher‐risk patients with abnormal baseline physiology and those at risk of neurologic deterioration.
This study has several limitations. Although the interviewers were not involved in RRS operations, it is possible that social desirability bias influenced responses. Next, we identified a knowledge gap among surgeons, and they contributed minimally to our findings. This is most likely because (1) surgical patients deteriorate on the wards less often than medical patients in our hospital, so surgeons are rarely presented with EWSs; (2) surgeons spend less time on the wards compared with medical physicians; and (3) surgical residents rotate in short blocks interspersed with rotations at other hospitals and may be less engaged in hospital safety initiatives.
CONCLUSIONS
Although EWSs perform only marginally well as statistical tools to predict clinical deterioration, nurses and physicians who recently experienced score failures described substantial benefits in using them to help identify deteriorating patients and transcend barriers to escalation of care by serving as objective communication tools. Combining an EWS with a clinician's judgment may result in a system better equipped to respond to deterioration than previous EWS studies focused on their test characteristics alone suggest. Future research should seek to compare and prospectively evaluate the clinical effectiveness of EWSs in real‐world settings.
Acknowledgments
Disclosures: This project was funded by the Pennsylvania Health Research Formula Fund Award (awarded to Keren and Bonafide) and the CHOP Nursing Research and Evidence‐Based Practice Award (awarded to Roberts). The funders did not influence the study design; the collection, analysis, or interpretation of data; the writing of the report; or the decision to submit the article for publication. The authors have no other conflicts to report.
- Institute for Healthcare Improvement. Overview of the Institute for Healthcare Improvement Five Million Lives Campaign. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed June 21, 2012.
- UK National Institute for Health and Clinical Excellence (NICE). Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital. Available at: http://publications.nice.org.uk/acutely‐ill‐patients‐in‐hospital‐cg50. Published July 2007. Accessed June 21, 2012.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006; 34(9):2463–2478.
- . Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32–35.
- , , , , , . Sensitivity of the Pediatric Early Warning Score to identify patient deterioration. Pediatrics. 2010;125(4):e763–e769.
- , , . Promoting care for acutely ill children—development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs. 2006;22(2):73–81.
- , , , . Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child. 2009;94(8):602–606.
- , , . The Pediatric Early Warning System Score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271–278.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13(4):R135.
- , , , et al. Multi‐centre validation of the Bedside Paediatric Early Warning System Score: a severity of illness score to detect evolving critical illness in hospitalized children. Crit Care. 2011;15(4):R184.
- , . Evaluation of a paediatric early warning tool—claims unsubstantiated. Intensive Crit Care Nurs. 2006;22(6):315–316.
- , , , et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874–e881.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128(1):72–78.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246.
- , , , et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center. Arch Pediatr Adolesc Med. 2008;162(2):117–122.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital. JAMA. 2007;298(19):2267–2274.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312.
- , , , et al. Implementation and impact of a rapid response team in a children's hospital. Jt Comm J Qual Patient Saf. 2007;33(7):418–425.
- , , . How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
- . Different approaches in grounded theory. In: Bryant A, Charmaz K, eds. The Sage Handbook of Grounded Theory. Los Angeles, CA: Sage; 2007:191–213.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine De Gruyter; 1967.
- , . Packaging: a grounded theory of how to report physiological deterioration effectively. J Adv Nurs. 2005;52(5):473–481.
- , , . Understanding how rapid response systems may improve safety for the acutely ill patient: learning from the frontline. BMJ Qual Saf. 2012;21(2):135–144.
- , , , , , . A before and after study assessing the impact of a new model for recognizing and responding to early signs of deterioration in an acute hospital. J Adv Nurs. 2013;69(1):41–52.
- , . Overcoming gendered and professional hierarchies in order to facilitate escalation of care in emergency situations: the role of standardised communication protocols. Soc Sci Med. 2010;71(9):1683–1686.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , , , . Leading successful rapid response teams: a multisite implementation evaluation. J Nurs Adm. 2009;39(4):176–181.
- Institute for Healthcare Improvement. Overview of the Institute for Healthcare Improvement Five Million Lives Campaign. Available at: http://www.ihi.org/offerings/Initiatives/PastStrategicInitiatives/5MillionLivesCampaign/Pages/default.aspx. Accessed June 21, 2012.
- UK National Institute for Health and Clinical Excellence (NICE). Acutely Ill Patients in Hospital: Recognition of and Response to Acute Illness in Adults in Hospital. Available at: http://publications.nice.org.uk/acutely‐ill‐patients‐in‐hospital‐cg50. Published July 2007. Accessed June 21, 2012.
- , , , et al. Findings of the first consensus conference on medical emergency teams. Crit Care Med. 2006; 34(9):2463–2478.
- . Detecting and managing deterioration in children. Paediatr Nurs. 2005;17(1):32–35.
- , , , , , . Sensitivity of the Pediatric Early Warning Score to identify patient deterioration. Pediatrics. 2010;125(4):e763–e769.
- , , . Promoting care for acutely ill children—development and evaluation of a paediatric early warning tool. Intensive Crit Care Nurs. 2006;22(2):73–81.
- , , , . Prospective cohort study to test the predictability of the Cardiff and Vale paediatric early warning system. Arch Dis Child. 2009;94(8):602–606.
- , , . The Pediatric Early Warning System Score: a severity of illness score to predict urgent medical need in hospitalized children. J Crit Care. 2006;21(3):271–278.
- , , . Development and initial validation of the Bedside Paediatric Early Warning System score. Crit Care. 2009;13(4):R135.
- , , , et al. Multi‐centre validation of the Bedside Paediatric Early Warning System Score: a severity of illness score to detect evolving critical illness in hospitalized children. Crit Care. 2011;15(4):R184.
- , . Evaluation of a paediatric early warning tool—claims unsubstantiated. Intensive Crit Care Nurs. 2006;22(6):315–316.
- , , , et al. Development of a pragmatic measure for evaluating and optimizing rapid response systems. Pediatrics. 2012;129(4):e874–e881.
- , , , et al. Implementation of a multicenter rapid response system in pediatric academic hospitals is effective. Pediatrics. 2011;128(1):72–78.
- , , , , . Rapid response teams: a systematic review and meta‐analysis. Arch Intern Med. 2010;170(1):18–26.
- , , , et al. Implementation of a medical emergency team in a large pediatric teaching hospital prevents respiratory and cardiopulmonary arrests outside the intensive care unit. Pediatr Crit Care Med. 2007;8(3):236–246.
- , , , et al. Transition from a traditional code team to a medical emergency team and categorization of cardiopulmonary arrests in a children's center. Arch Pediatr Adolesc Med. 2008;162(2):117–122.
- , , , et al. Effect of a rapid response team on hospital‐wide mortality and code rates outside the ICU in a children's hospital. JAMA. 2007;298(19):2267–2274.
- , . Reduction of hospital mortality and of preventable cardiac arrest and death on introduction of a pediatric medical emergency team. Pediatr Crit Care Med. 2009;10(3):306–312.
- , , , et al. Implementation and impact of a rapid response team in a children's hospital. Jt Comm J Qual Patient Saf. 2007;33(7):418–425.
- , , . How many interviews are enough? An experiment with data saturation and variability. Field Methods. 2006;18(1):59–82.
- . Different approaches in grounded theory. In: Bryant A, Charmaz K, eds. The Sage Handbook of Grounded Theory. Los Angeles, CA: Sage; 2007:191–213.
- , . The Discovery of Grounded Theory: Strategies for Qualitative Research. New York, NY: Aldine De Gruyter; 1967.
- , . Packaging: a grounded theory of how to report physiological deterioration effectively. J Adv Nurs. 2005;52(5):473–481.
- , , . Understanding how rapid response systems may improve safety for the acutely ill patient: learning from the frontline. BMJ Qual Saf. 2012;21(2):135–144.
- , , , , , . A before and after study assessing the impact of a new model for recognizing and responding to early signs of deterioration in an acute hospital. J Adv Nurs. 2013;69(1):41–52.
- , . Overcoming gendered and professional hierarchies in order to facilitate escalation of care in emergency situations: the role of standardised communication protocols. Soc Sci Med. 2010;71(9):1683–1686.
- , , , , . Defining impact of a rapid response team: qualitative study with nurses, physicians and hospital administrators. BMJ Qual Saf. 2012;21(5):391–398.
- , , , , . Leading successful rapid response teams: a multisite implementation evaluation. J Nurs Adm. 2009;39(4):176–181.
Copyright © 2013 Society of Hospital Medicine
Bumps
People hate bumps.
Bumps are ugly. Bumps are nasty. Bumps bother.
Seeing bumps makes people frown. Touching bumps makes them shudder.
Bumps on toads. Bumps on potatoes. Bumps on trees.
But especially bumps on skin – on faces, on lips, on tongues, on genitals. Bumps almost anywhere.
Bumps bother the people who have them. They especially agitate other people who have to look at them, so they point out the bumps and make it perfectly plain how bumps make them feel:
• "My wife says, ‘When are you going to get that disgusting red spot off your neck?’" (About a hemangioma.)
• "My kids say, ‘Dad, when are you getting that gross thing off your back?’" (About an epidermoid cyst.)
• "That black spot on your back – have you had that looked at?" (A doctor – not a dermatologist, of course – asks a patient about a dermal nevus you’ve been reassuring that patient about for years.)
"Just leave those skin tags on your neck alone," you say.
"But they’re nasty! Can’t you take them off?"
"I suggest you leave the cyst alone. Removing it would require surgery."
"But I hate it!"
Even nonverbal observers can call attention to bumps. More than one nursing mother has had me remove a mole from her breast, even though it’s been there without changing for a long time, because "the baby keeps grabbing at it."
But once the people who see bumps can talk, it’s open bump season. My wife had a blue nevus removed from her cheek many years ago. She recalls that she did it because our youngest son, about 8 years old at the time, kept pointing to her cheek and saying, "Blue nevus! Blue nevus!" (Yes, he could be irritating then, but no, he wasn’t diagnostically precocious – I’d told him what it was.)
That son now has three children of his own, so he can look to his own parenting challenges, not to mention his own blemishes.
The loaded words people apply to their bumps – ugly, disgusting, gross, nasty – are not the ones you’d expect, and they have nothing to do with histology or malignant potential. But if you listen for these words, you’ll hear them as often as I do.
Some of my bumpy conversations are droll in unexpected ways. Last week, for instance, Seth came in for his annual physical.
"I have these two things under my left arm," he said, pointing to a pair of skin tags.
"Do they bother you?" I asked.
"They bother my kids," he said. "Adam and Melissa keep pointing at them. They call them Fred."
"Fred? What do they call the other one?"
"Also Fred."
"Did you know," I asked him, "that all little thingies hanging off the body are male? People always say, ‘Can’t you get rid of those little guys?’ "
"I didn’t know that," said Seth.
"You see what you can learn at the dermatologist’s office?" I said. "If you want, I can make your kids happy and get rid of Fred. Both of him."
"Sure," said Seth. I loaded up my electric needle. I don’t play video games. Who needs when you have a Hyfrecator? Soon Fred was vaporized. BLAMMM! So was Fred. KAPOWW!
"Seth," I said, "if Dr. Seuss had written a book about dermatology, he might have called it ‘Bye, Bye, Fred’ and it may have gone like this":
See Fred.
Fred bled.
Fred bled red.
Fred bled red in bed.
Zap, Fred! Pow, Fred!
Now Fred is dead.
Sayonara, Fred.
Go ahead, moles, warts, skin tags, bumps of all kinds. Make my day.
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at sknews@elsevier.com.
People hate bumps.
Bumps are ugly. Bumps are nasty. Bumps bother.
Seeing bumps makes people frown. Touching bumps makes them shudder.
Bumps on toads. Bumps on potatoes. Bumps on trees.
But especially bumps on skin – on faces, on lips, on tongues, on genitals. Bumps almost anywhere.
Bumps bother the people who have them. They especially agitate other people who have to look at them, so they point out the bumps and make it perfectly plain how bumps make them feel:
• "My wife says, ‘When are you going to get that disgusting red spot off your neck?’" (About a hemangioma.)
• "My kids say, ‘Dad, when are you getting that gross thing off your back?’" (About an epidermoid cyst.)
• "That black spot on your back – have you had that looked at?" (A doctor – not a dermatologist, of course – asks a patient about a dermal nevus you’ve been reassuring that patient about for years.)
"Just leave those skin tags on your neck alone," you say.
"But they’re nasty! Can’t you take them off?"
"I suggest you leave the cyst alone. Removing it would require surgery."
"But I hate it!"
Even nonverbal observers can call attention to bumps. More than one nursing mother has had me remove a mole from her breast, even though it’s been there without changing for a long time, because "the baby keeps grabbing at it."
But once the people who see bumps can talk, it’s open bump season. My wife had a blue nevus removed from her cheek many years ago. She recalls that she did it because our youngest son, about 8 years old at the time, kept pointing to her cheek and saying, "Blue nevus! Blue nevus!" (Yes, he could be irritating then, but no, he wasn’t diagnostically precocious – I’d told him what it was.)
That son now has three children of his own, so he can look to his own parenting challenges, not to mention his own blemishes.
The loaded words people apply to their bumps – ugly, disgusting, gross, nasty – are not the ones you’d expect, and they have nothing to do with histology or malignant potential. But if you listen for these words, you’ll hear them as often as I do.
Some of my bumpy conversations are droll in unexpected ways. Last week, for instance, Seth came in for his annual physical.
"I have these two things under my left arm," he said, pointing to a pair of skin tags.
"Do they bother you?" I asked.
"They bother my kids," he said. "Adam and Melissa keep pointing at them. They call them Fred."
"Fred? What do they call the other one?"
"Also Fred."
"Did you know," I asked him, "that all little thingies hanging off the body are male? People always say, ‘Can’t you get rid of those little guys?’ "
"I didn’t know that," said Seth.
"You see what you can learn at the dermatologist’s office?" I said. "If you want, I can make your kids happy and get rid of Fred. Both of him."
"Sure," said Seth. I loaded up my electric needle. I don’t play video games. Who needs when you have a Hyfrecator? Soon Fred was vaporized. BLAMMM! So was Fred. KAPOWW!
"Seth," I said, "if Dr. Seuss had written a book about dermatology, he might have called it ‘Bye, Bye, Fred’ and it may have gone like this":
See Fred.
Fred bled.
Fred bled red.
Fred bled red in bed.
Zap, Fred! Pow, Fred!
Now Fred is dead.
Sayonara, Fred.
Go ahead, moles, warts, skin tags, bumps of all kinds. Make my day.
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at sknews@elsevier.com.
People hate bumps.
Bumps are ugly. Bumps are nasty. Bumps bother.
Seeing bumps makes people frown. Touching bumps makes them shudder.
Bumps on toads. Bumps on potatoes. Bumps on trees.
But especially bumps on skin – on faces, on lips, on tongues, on genitals. Bumps almost anywhere.
Bumps bother the people who have them. They especially agitate other people who have to look at them, so they point out the bumps and make it perfectly plain how bumps make them feel:
• "My wife says, ‘When are you going to get that disgusting red spot off your neck?’" (About a hemangioma.)
• "My kids say, ‘Dad, when are you getting that gross thing off your back?’" (About an epidermoid cyst.)
• "That black spot on your back – have you had that looked at?" (A doctor – not a dermatologist, of course – asks a patient about a dermal nevus you’ve been reassuring that patient about for years.)
"Just leave those skin tags on your neck alone," you say.
"But they’re nasty! Can’t you take them off?"
"I suggest you leave the cyst alone. Removing it would require surgery."
"But I hate it!"
Even nonverbal observers can call attention to bumps. More than one nursing mother has had me remove a mole from her breast, even though it’s been there without changing for a long time, because "the baby keeps grabbing at it."
But once the people who see bumps can talk, it’s open bump season. My wife had a blue nevus removed from her cheek many years ago. She recalls that she did it because our youngest son, about 8 years old at the time, kept pointing to her cheek and saying, "Blue nevus! Blue nevus!" (Yes, he could be irritating then, but no, he wasn’t diagnostically precocious – I’d told him what it was.)
That son now has three children of his own, so he can look to his own parenting challenges, not to mention his own blemishes.
The loaded words people apply to their bumps – ugly, disgusting, gross, nasty – are not the ones you’d expect, and they have nothing to do with histology or malignant potential. But if you listen for these words, you’ll hear them as often as I do.
Some of my bumpy conversations are droll in unexpected ways. Last week, for instance, Seth came in for his annual physical.
"I have these two things under my left arm," he said, pointing to a pair of skin tags.
"Do they bother you?" I asked.
"They bother my kids," he said. "Adam and Melissa keep pointing at them. They call them Fred."
"Fred? What do they call the other one?"
"Also Fred."
"Did you know," I asked him, "that all little thingies hanging off the body are male? People always say, ‘Can’t you get rid of those little guys?’ "
"I didn’t know that," said Seth.
"You see what you can learn at the dermatologist’s office?" I said. "If you want, I can make your kids happy and get rid of Fred. Both of him."
"Sure," said Seth. I loaded up my electric needle. I don’t play video games. Who needs when you have a Hyfrecator? Soon Fred was vaporized. BLAMMM! So was Fred. KAPOWW!
"Seth," I said, "if Dr. Seuss had written a book about dermatology, he might have called it ‘Bye, Bye, Fred’ and it may have gone like this":
See Fred.
Fred bled.
Fred bled red.
Fred bled red in bed.
Zap, Fred! Pow, Fred!
Now Fred is dead.
Sayonara, Fred.
Go ahead, moles, warts, skin tags, bumps of all kinds. Make my day.
Dr. Rockoff practices dermatology in Brookline, Mass. To respond to this column, e-mail him at our editorial offices at sknews@elsevier.com.
You need a time clock
Every medical office, even the smallest, should have a time clock, and there are two very good reasons why. The obvious one is for stamping employee time cards. This is essential, even if all your employees are paid weekly or semiweekly rather than by the hour.
In most states, any employee who works more than 40 hours in any given week must be paid overtime wages. Employees know this, and disgruntled ones have been known to file complaints stating that they had worked hundreds of hours of unpaid overtime. This may be completely untrue, but labor boards almost invariably side with employees in such disputes – unless the employer can produce time records to disprove the claim. A time clock is cheap insurance against such headaches.
For hourly wage employees, time records are even more important, obviously because you only want to pay them for the hours they work. If you are paying your part-timers for the number of hours they should be working, without documenting how many hours they actually work, you could be paying for a lot of nonwork. Employees have little incentive to arrive on time or to stay the entire length of their shift, if they know they are being paid for a set number of hours anyway. And they certainly will balk at staying late if they can’t count on being paid for the extra time.
Time clocks also work to the advantage of your employees, since they will be paid for all the time they work. In fact, if any employees object to being asked to punch in every day, point out that they will be assured of payment for fractional time worked past their usual hours – time which until now may have gone unpaid.
The second – possibly more important – reason to have a time clock is to punch in your patients. A time clock is a great tool in the endless struggle to run your practice on time.
As each patient arrives, have your receptionist time-stamp the "encounter form" that goes back with the patient’s chart. As you take each chart off the door and enter the room, one glance at the time stamp will tell you exactly how long that patient has been waiting.
Now you no longer have to guess how far behind you are – and you’ll have an answer for the curmudgeon who walked in 15 minutes ago, but insists he’s been sitting there for 2 hours.
Time/attendance systems range from simple and cheap to complex and expensive. Many of the newer mechanical clocks will automatically calculate time between punches and total work time, and these can be configured for weekly, biweekly, semimonthly or monthly pay periods. Some will automatically deduct meal breaks from the totals. However, remember that you can only exclude meal breaks from compensable time when an employee is completely relieved of work duties for at least 1 uninterrupted half-hour.
If you have a problem with "buddy punching" (employees punching in or out for each other), some clocks are equipped to recognize fingerprints or hand contours.
There are also electronic timing systems, both web-based and in-house, which can be deployed across a local computer network. These systems will print time sheets with employee hours and earnings calculated, and some will even interface with financial software such as QuickBooks and other third party payroll services.
One popular Web-based system is Count Me In, which has the fingerprint option, and also allows you to restrict clocking in or out to those IP addresses that you authorize. The system prevents employees from punching in from home, or a vacation house, or a distant casino. Other examples of cloud-based systems: Time Card Manager, Time Force, and Time America. PHP Timeclock is a free, open-source download – though setting it up on your server will require some technical expertise.
As always, I have no financial interest in any product or service discussed in this column. Whether you go the mechanical or electronic route, make sure that the system you choose has security measures in place to prevent anyone from altering the displayed time at will. You need to be reasonably certain that your time stamps have not been fudged.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Every medical office, even the smallest, should have a time clock, and there are two very good reasons why. The obvious one is for stamping employee time cards. This is essential, even if all your employees are paid weekly or semiweekly rather than by the hour.
In most states, any employee who works more than 40 hours in any given week must be paid overtime wages. Employees know this, and disgruntled ones have been known to file complaints stating that they had worked hundreds of hours of unpaid overtime. This may be completely untrue, but labor boards almost invariably side with employees in such disputes – unless the employer can produce time records to disprove the claim. A time clock is cheap insurance against such headaches.
For hourly wage employees, time records are even more important, obviously because you only want to pay them for the hours they work. If you are paying your part-timers for the number of hours they should be working, without documenting how many hours they actually work, you could be paying for a lot of nonwork. Employees have little incentive to arrive on time or to stay the entire length of their shift, if they know they are being paid for a set number of hours anyway. And they certainly will balk at staying late if they can’t count on being paid for the extra time.
Time clocks also work to the advantage of your employees, since they will be paid for all the time they work. In fact, if any employees object to being asked to punch in every day, point out that they will be assured of payment for fractional time worked past their usual hours – time which until now may have gone unpaid.
The second – possibly more important – reason to have a time clock is to punch in your patients. A time clock is a great tool in the endless struggle to run your practice on time.
As each patient arrives, have your receptionist time-stamp the "encounter form" that goes back with the patient’s chart. As you take each chart off the door and enter the room, one glance at the time stamp will tell you exactly how long that patient has been waiting.
Now you no longer have to guess how far behind you are – and you’ll have an answer for the curmudgeon who walked in 15 minutes ago, but insists he’s been sitting there for 2 hours.
Time/attendance systems range from simple and cheap to complex and expensive. Many of the newer mechanical clocks will automatically calculate time between punches and total work time, and these can be configured for weekly, biweekly, semimonthly or monthly pay periods. Some will automatically deduct meal breaks from the totals. However, remember that you can only exclude meal breaks from compensable time when an employee is completely relieved of work duties for at least 1 uninterrupted half-hour.
If you have a problem with "buddy punching" (employees punching in or out for each other), some clocks are equipped to recognize fingerprints or hand contours.
There are also electronic timing systems, both web-based and in-house, which can be deployed across a local computer network. These systems will print time sheets with employee hours and earnings calculated, and some will even interface with financial software such as QuickBooks and other third party payroll services.
One popular Web-based system is Count Me In, which has the fingerprint option, and also allows you to restrict clocking in or out to those IP addresses that you authorize. The system prevents employees from punching in from home, or a vacation house, or a distant casino. Other examples of cloud-based systems: Time Card Manager, Time Force, and Time America. PHP Timeclock is a free, open-source download – though setting it up on your server will require some technical expertise.
As always, I have no financial interest in any product or service discussed in this column. Whether you go the mechanical or electronic route, make sure that the system you choose has security measures in place to prevent anyone from altering the displayed time at will. You need to be reasonably certain that your time stamps have not been fudged.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Every medical office, even the smallest, should have a time clock, and there are two very good reasons why. The obvious one is for stamping employee time cards. This is essential, even if all your employees are paid weekly or semiweekly rather than by the hour.
In most states, any employee who works more than 40 hours in any given week must be paid overtime wages. Employees know this, and disgruntled ones have been known to file complaints stating that they had worked hundreds of hours of unpaid overtime. This may be completely untrue, but labor boards almost invariably side with employees in such disputes – unless the employer can produce time records to disprove the claim. A time clock is cheap insurance against such headaches.
For hourly wage employees, time records are even more important, obviously because you only want to pay them for the hours they work. If you are paying your part-timers for the number of hours they should be working, without documenting how many hours they actually work, you could be paying for a lot of nonwork. Employees have little incentive to arrive on time or to stay the entire length of their shift, if they know they are being paid for a set number of hours anyway. And they certainly will balk at staying late if they can’t count on being paid for the extra time.
Time clocks also work to the advantage of your employees, since they will be paid for all the time they work. In fact, if any employees object to being asked to punch in every day, point out that they will be assured of payment for fractional time worked past their usual hours – time which until now may have gone unpaid.
The second – possibly more important – reason to have a time clock is to punch in your patients. A time clock is a great tool in the endless struggle to run your practice on time.
As each patient arrives, have your receptionist time-stamp the "encounter form" that goes back with the patient’s chart. As you take each chart off the door and enter the room, one glance at the time stamp will tell you exactly how long that patient has been waiting.
Now you no longer have to guess how far behind you are – and you’ll have an answer for the curmudgeon who walked in 15 minutes ago, but insists he’s been sitting there for 2 hours.
Time/attendance systems range from simple and cheap to complex and expensive. Many of the newer mechanical clocks will automatically calculate time between punches and total work time, and these can be configured for weekly, biweekly, semimonthly or monthly pay periods. Some will automatically deduct meal breaks from the totals. However, remember that you can only exclude meal breaks from compensable time when an employee is completely relieved of work duties for at least 1 uninterrupted half-hour.
If you have a problem with "buddy punching" (employees punching in or out for each other), some clocks are equipped to recognize fingerprints or hand contours.
There are also electronic timing systems, both web-based and in-house, which can be deployed across a local computer network. These systems will print time sheets with employee hours and earnings calculated, and some will even interface with financial software such as QuickBooks and other third party payroll services.
One popular Web-based system is Count Me In, which has the fingerprint option, and also allows you to restrict clocking in or out to those IP addresses that you authorize. The system prevents employees from punching in from home, or a vacation house, or a distant casino. Other examples of cloud-based systems: Time Card Manager, Time Force, and Time America. PHP Timeclock is a free, open-source download – though setting it up on your server will require some technical expertise.
As always, I have no financial interest in any product or service discussed in this column. Whether you go the mechanical or electronic route, make sure that the system you choose has security measures in place to prevent anyone from altering the displayed time at will. You need to be reasonably certain that your time stamps have not been fudged.
Dr. Eastern practices dermatology and dermatologic surgery in Belleville, N.J.
Interferon-free regimen cures 100% of hard-to-treat hepatitis C
ATLANTA – Adding ledipasvir to sofosbuvir and ribavirin produced sustained virological responses 12 weeks after therapy in 100% of treatment-naive and prior nonresponder patients with chronic hepatitis C genotype 1 in the ELECTRON trial.
"Certainly adding a second direct-acting antiviral agent, ledipasvir, increases the efficacy of sofosbuvir plus ribavirin," Dr. Edward Gane said at the Conference on Retroviruses and Opportunistic Infections.
Three-quarters of the roughly 170 million people infected with hepatitis C virus (HCV) worldwide have genotype 1, the most difficult strain to treat.
Current treatment includes triple therapy with a protease inhibitor plus peginterferon and ribavirin for 24-48 weeks, but PI-based therapy is limited by complex dosing regimens, the potential for resistance, and lower responses in prior nonresponders, explained Dr. Gane of Auckland Clinical Studies in New Zealand.
The investigators hypothesized that combining two direct-acting antivirals with a different mechanism would enhance response.
At last year’s CROI meeting, Dr. Gane reported that treatment with the nucleotide NS5B inhibitor sofosbuvir (formerly known as GS-7977) and ribavirin alone led to early viral load suppression, but relapses within 4 weeks of stopping treatment resulted in 12-week posttreatment sustained virological response (SVR12) rates of 84% among treatment-naive patients and only 10% among previous interferon-based therapy null responders.
In the current arm of the trial, the NS5A inhibitor ledipasvir (formerly known as GS-5885) was added to sofosbuvir and weight-based ribavirin, all for 12 weeks, in 25 noncirrhotic treatment-naive and 9 null responders, defined by less than a 2-log reduction in HCV RNA after 12 weeks of peginterferon and ribavirin.
The majority of treatment-naive and null responders were genotype 1a (80% and 89%) and had high baseline HCV RNA loads (mean 5.9 log10 IU/mL and 6.9 log10 IU/mL). The more favorable IL28B genotype CC genotype was present in 36% of treatment-naive patients, but in no null responders. The patients median age was 48; 94% were white.
Early on–treatment viral suppression was very rapid, with all treatment-naive patients and all but one prior null responder having an undetectable viral load at week 4, Dr. Gane said. This patient’s load was on the threshold at week 4 and became undetectable by week 5, resulting in SVR12 rates of 100% in both groups.
No viral breakthroughs were observed, and all patients achieved an end-of-treatment response.
Unlike the earlier arm of the trial, however, both groups maintained undetectable HCV viral loads at 4 and 12 weeks after therapy, he said.
The triple combination was well tolerated and safe. Three serious adverse events occurred, but none were treatment related. One patient had to stop therapy at week 8 due to the event, but subsequently achieved SVR24. The most common adverse events were anemia (20%), depression (8%), and headache (4%), and all were in treatment-naive patients.
Grade 3 laboratory abnormalities occurred in 52% of the treatment-naive and 22% of null responders. No grade 4 abnormalities were seen, Dr. Gane said.
Ledipasvir and sofosbuvir have been combined into a single fixed-dose tablet and is being evaluated in phase III studies in patients with cirrhosis and to determine whether there is a need for ribavirin, he said. Additional studies are also underway to explore shorter durations of therapy.
ELECTRON was sponsored by Gilead Sciences. Dr. Gane reported ties with Gilead, Janssen-Cilag, Novartis, Pharmasset, Roche and Vertex.
 |
| Dr. Steven L. Flamm |
These results of the phaseII ELECTRON trial arm testing the all-oral regimen of sofosbuvir (NS5b polymerase inhibitor), ledipasvir (NS5a inhibitor) and weight-based ribavirin in patients with chronic HCV genotype 1 (without cirrhosis) adds to the recent number of stunning reports describing all-oral treatment regimens for chronic HCV. Such therapeutic approaches offer the promise of tolerable regimens that have outstanding efficacy and may have few or no contraindications to therapy.
It is unclear if patients with cirrhosis will respond as well as noncirrhotic patients did in ELECTRON and whether or not ribavirin is needed; phase III trials are currently underway to address these issues. If the phase II results are replicated in these phase III trials and if the medications are available to all patients who have chronic HCV genotype 1, this type of a regimen could represent the “apocalypse moment” for HCV, an age during which the most common type of HCV worldwide (genotype 1) is largely eliminated.
Steven L. Flamm, M.D., is chief of transplant hepatology and is professor of medicine in the division of gastroenterology and hepatology at Northwestern University Feinberg School of Medicine, Chicago. He disclosed receiving research support from Gilead and serving as an advisor to the company.
 |
| Dr. Steven L. Flamm |
These results of the phaseII ELECTRON trial arm testing the all-oral regimen of sofosbuvir (NS5b polymerase inhibitor), ledipasvir (NS5a inhibitor) and weight-based ribavirin in patients with chronic HCV genotype 1 (without cirrhosis) adds to the recent number of stunning reports describing all-oral treatment regimens for chronic HCV. Such therapeutic approaches offer the promise of tolerable regimens that have outstanding efficacy and may have few or no contraindications to therapy.
It is unclear if patients with cirrhosis will respond as well as noncirrhotic patients did in ELECTRON and whether or not ribavirin is needed; phase III trials are currently underway to address these issues. If the phase II results are replicated in these phase III trials and if the medications are available to all patients who have chronic HCV genotype 1, this type of a regimen could represent the “apocalypse moment” for HCV, an age during which the most common type of HCV worldwide (genotype 1) is largely eliminated.
Steven L. Flamm, M.D., is chief of transplant hepatology and is professor of medicine in the division of gastroenterology and hepatology at Northwestern University Feinberg School of Medicine, Chicago. He disclosed receiving research support from Gilead and serving as an advisor to the company.
 |
| Dr. Steven L. Flamm |
These results of the phaseII ELECTRON trial arm testing the all-oral regimen of sofosbuvir (NS5b polymerase inhibitor), ledipasvir (NS5a inhibitor) and weight-based ribavirin in patients with chronic HCV genotype 1 (without cirrhosis) adds to the recent number of stunning reports describing all-oral treatment regimens for chronic HCV. Such therapeutic approaches offer the promise of tolerable regimens that have outstanding efficacy and may have few or no contraindications to therapy.
It is unclear if patients with cirrhosis will respond as well as noncirrhotic patients did in ELECTRON and whether or not ribavirin is needed; phase III trials are currently underway to address these issues. If the phase II results are replicated in these phase III trials and if the medications are available to all patients who have chronic HCV genotype 1, this type of a regimen could represent the “apocalypse moment” for HCV, an age during which the most common type of HCV worldwide (genotype 1) is largely eliminated.
Steven L. Flamm, M.D., is chief of transplant hepatology and is professor of medicine in the division of gastroenterology and hepatology at Northwestern University Feinberg School of Medicine, Chicago. He disclosed receiving research support from Gilead and serving as an advisor to the company.
ATLANTA – Adding ledipasvir to sofosbuvir and ribavirin produced sustained virological responses 12 weeks after therapy in 100% of treatment-naive and prior nonresponder patients with chronic hepatitis C genotype 1 in the ELECTRON trial.
"Certainly adding a second direct-acting antiviral agent, ledipasvir, increases the efficacy of sofosbuvir plus ribavirin," Dr. Edward Gane said at the Conference on Retroviruses and Opportunistic Infections.
Three-quarters of the roughly 170 million people infected with hepatitis C virus (HCV) worldwide have genotype 1, the most difficult strain to treat.
Current treatment includes triple therapy with a protease inhibitor plus peginterferon and ribavirin for 24-48 weeks, but PI-based therapy is limited by complex dosing regimens, the potential for resistance, and lower responses in prior nonresponders, explained Dr. Gane of Auckland Clinical Studies in New Zealand.
The investigators hypothesized that combining two direct-acting antivirals with a different mechanism would enhance response.
At last year’s CROI meeting, Dr. Gane reported that treatment with the nucleotide NS5B inhibitor sofosbuvir (formerly known as GS-7977) and ribavirin alone led to early viral load suppression, but relapses within 4 weeks of stopping treatment resulted in 12-week posttreatment sustained virological response (SVR12) rates of 84% among treatment-naive patients and only 10% among previous interferon-based therapy null responders.
In the current arm of the trial, the NS5A inhibitor ledipasvir (formerly known as GS-5885) was added to sofosbuvir and weight-based ribavirin, all for 12 weeks, in 25 noncirrhotic treatment-naive and 9 null responders, defined by less than a 2-log reduction in HCV RNA after 12 weeks of peginterferon and ribavirin.
The majority of treatment-naive and null responders were genotype 1a (80% and 89%) and had high baseline HCV RNA loads (mean 5.9 log10 IU/mL and 6.9 log10 IU/mL). The more favorable IL28B genotype CC genotype was present in 36% of treatment-naive patients, but in no null responders. The patients median age was 48; 94% were white.
Early on–treatment viral suppression was very rapid, with all treatment-naive patients and all but one prior null responder having an undetectable viral load at week 4, Dr. Gane said. This patient’s load was on the threshold at week 4 and became undetectable by week 5, resulting in SVR12 rates of 100% in both groups.
No viral breakthroughs were observed, and all patients achieved an end-of-treatment response.
Unlike the earlier arm of the trial, however, both groups maintained undetectable HCV viral loads at 4 and 12 weeks after therapy, he said.
The triple combination was well tolerated and safe. Three serious adverse events occurred, but none were treatment related. One patient had to stop therapy at week 8 due to the event, but subsequently achieved SVR24. The most common adverse events were anemia (20%), depression (8%), and headache (4%), and all were in treatment-naive patients.
Grade 3 laboratory abnormalities occurred in 52% of the treatment-naive and 22% of null responders. No grade 4 abnormalities were seen, Dr. Gane said.
Ledipasvir and sofosbuvir have been combined into a single fixed-dose tablet and is being evaluated in phase III studies in patients with cirrhosis and to determine whether there is a need for ribavirin, he said. Additional studies are also underway to explore shorter durations of therapy.
ELECTRON was sponsored by Gilead Sciences. Dr. Gane reported ties with Gilead, Janssen-Cilag, Novartis, Pharmasset, Roche and Vertex.
ATLANTA – Adding ledipasvir to sofosbuvir and ribavirin produced sustained virological responses 12 weeks after therapy in 100% of treatment-naive and prior nonresponder patients with chronic hepatitis C genotype 1 in the ELECTRON trial.
"Certainly adding a second direct-acting antiviral agent, ledipasvir, increases the efficacy of sofosbuvir plus ribavirin," Dr. Edward Gane said at the Conference on Retroviruses and Opportunistic Infections.
Three-quarters of the roughly 170 million people infected with hepatitis C virus (HCV) worldwide have genotype 1, the most difficult strain to treat.
Current treatment includes triple therapy with a protease inhibitor plus peginterferon and ribavirin for 24-48 weeks, but PI-based therapy is limited by complex dosing regimens, the potential for resistance, and lower responses in prior nonresponders, explained Dr. Gane of Auckland Clinical Studies in New Zealand.
The investigators hypothesized that combining two direct-acting antivirals with a different mechanism would enhance response.
At last year’s CROI meeting, Dr. Gane reported that treatment with the nucleotide NS5B inhibitor sofosbuvir (formerly known as GS-7977) and ribavirin alone led to early viral load suppression, but relapses within 4 weeks of stopping treatment resulted in 12-week posttreatment sustained virological response (SVR12) rates of 84% among treatment-naive patients and only 10% among previous interferon-based therapy null responders.
In the current arm of the trial, the NS5A inhibitor ledipasvir (formerly known as GS-5885) was added to sofosbuvir and weight-based ribavirin, all for 12 weeks, in 25 noncirrhotic treatment-naive and 9 null responders, defined by less than a 2-log reduction in HCV RNA after 12 weeks of peginterferon and ribavirin.
The majority of treatment-naive and null responders were genotype 1a (80% and 89%) and had high baseline HCV RNA loads (mean 5.9 log10 IU/mL and 6.9 log10 IU/mL). The more favorable IL28B genotype CC genotype was present in 36% of treatment-naive patients, but in no null responders. The patients median age was 48; 94% were white.
Early on–treatment viral suppression was very rapid, with all treatment-naive patients and all but one prior null responder having an undetectable viral load at week 4, Dr. Gane said. This patient’s load was on the threshold at week 4 and became undetectable by week 5, resulting in SVR12 rates of 100% in both groups.
No viral breakthroughs were observed, and all patients achieved an end-of-treatment response.
Unlike the earlier arm of the trial, however, both groups maintained undetectable HCV viral loads at 4 and 12 weeks after therapy, he said.
The triple combination was well tolerated and safe. Three serious adverse events occurred, but none were treatment related. One patient had to stop therapy at week 8 due to the event, but subsequently achieved SVR24. The most common adverse events were anemia (20%), depression (8%), and headache (4%), and all were in treatment-naive patients.
Grade 3 laboratory abnormalities occurred in 52% of the treatment-naive and 22% of null responders. No grade 4 abnormalities were seen, Dr. Gane said.
Ledipasvir and sofosbuvir have been combined into a single fixed-dose tablet and is being evaluated in phase III studies in patients with cirrhosis and to determine whether there is a need for ribavirin, he said. Additional studies are also underway to explore shorter durations of therapy.
ELECTRON was sponsored by Gilead Sciences. Dr. Gane reported ties with Gilead, Janssen-Cilag, Novartis, Pharmasset, Roche and Vertex.
AT CROI 2013
Major finding: Sustained virological response rates 12 weeks posttreatment were 100% in treatment-naive and prior null responders.
Data source: In an arm of the ELECTRON trial, the NS5A inhibitor ledipasvir (formerly known as GS-5885) was added to sofosbuvir and weight-based ribavirin, all for 12 weeks, in 25 noncirrhotic treatment-naive and 9 null responders.
Disclosures: ELECTRON was sponsored by Gilead Sciences. Dr. Gane reported ties with Gilead, Janssen-Cilag, Novartis, Pharmasset, Roche and Vertex.
FDA again rejects rivaroxaban for ACS

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()

Credit: CDC
For a second time, the FDA has decided against approving rivaroxaban (Xarelto) to reduce the risk of cardiovascular events in patients with acute coronary syndrome (ACS).
The agency has issued a complete response letter to the drug’s makers, Janssen Pharmaceuticals.
The contents of the letter are not publicly known, but representatives at Janssen have said they “are evaluating the letter and will respond to the agency’s questions.”
This is not the first time the FDA has raised questions about the use of rivaroxaban in ACS. Last June, the agency issued a complete response letter requesting additional information on the drug.
A month before that, an FDA review committee had expressed concerns about missing follow-up data from the ATLAS ACS 2 TIMI 51 trial (JL Mega et al, NEJM, 2012).
So in September, Janssen supplied the FDA with data on the patients who had withdrawn from trial. The company was able to confirm the vital status information for 843 (63%) of the 1338 trial participants who previously had unknown vital status.
Of those 843 patients, 37 had died. The company said the deaths were equally distributed among the treatment groups—rivaroxaban at 2.5 mg, rivaroxaban at 5 mg, and placebo.
“We remain confident in the robustness and results of the ATLAS ACS 2 TIMI 51 trial, evidenced by a significant reduction in cardiovascular events, including a clinically important decrease in cardiovascular death . . . ,” said Christopher Nessel, Vice President at Janssen.
“While we saw an increase in major bleeding, there was no increase in fatal bleeding. We will continue to work with the FDA to address their questions.”
Rivaroxaban is already approved by the FDA to treat and prevent the recurrence of deep vein thrombosis and pulmonary embolism, as thromboprophylaxis in patients who have undergone knee or hip replacement surgery, as well as to reduce the risk of stroke in patients with non-valvular atrial fibrillation. ![]()
The Heart Team
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.
Although to many, the concept of Heart Teams, as examined in Mitchel L. Zoler’s article, "Heart teams inch into routine cardiac practice," seem novel, such collaborations were the norm at the dawn of cardiac surgery.
Beginning with the surgical approach to valvular and, later, coronary vascular surgery, the interaction between cardiac physiologists (as they were called then), coronary angiographers, and cardiac surgeons in deciding where and when to operate was often difficult and contentious. Cardiac surgery was a high-risk procedure, and the outcomes were uncertain. Over the last 50 years we have come a long way and much of what we do is almost commonplace, as frequently performed as a cholecystectomy or appendectomy and with similar risks. Over time, we have become casual with our decision-making process. Both cardiologists and cardiac surgeons have staked out their own therapeutic parameters. Specialty society guidelines have provided important boundaries within which we can and should operate.
At the same time, we continue to push the envelope to identify therapeutic targets and technologies. We have developed complex interventional and surgical procedures and have applied them to older and sicker patient populations. New technology has opened avenues of therapy that we could not have imagined at the inception of interventional cardiology and cardiac surgery.
The advanced interventional surgical approach now requires even greater interaction with more special players in both cardiology and surgery. Although the modern cardiology practice is built on everyday procedures that provide the platform on which we treat a variety of cardiac issues that commonly do not require ongoing group interactions, the new treatment options require a more interactive and collegial environment. It is in this domain that the Heart Team has an important role and has found success. It was re-initiated as a result of the development of the transcatheter aortic valve implantation, which requires close cardiology and surgical interaction. It has expanded as a team approach to the treatment choices in the care of patients with structural heart disease.
Definitions of the boundaries of the new therapies raise important economic and professional challenges. The Heart Team as currently organized provides the framework of that discourse. To some, it will represent an inconvenience and an obstruction to their individual professional performance: The requirement to participate in a structured interaction is just one more barrier to the daily performance of their skills. To others, it will provide an important process that will improve performance: It is an opportunity to coordinate the different skills required for the advance treatments and, more importantly, it represents a forum to educate not only the current participants but also the physician, nurses, and technicians for the future. The discussion and planning for the surgical approach for a particular patient provides a dynamic discussion of the therapeutic options and the important decisions about appropriateness of the procedure. This interactive learning process is critical to the interdisciplinary training of all present and future players.
The growth of cardiovascular therapy has led to the construction of large stand-alone units or sections within hospitals identified as heart centers or institutes. The creation of these facilities provides the professional structure and financial environment to create the Heart Team and answer some of the issues raised in the article in this issue. Initially devised as a combination of marketing and professional associations, they now can provide the educational and scientific structure of the Heart Team.
Dr. Goldstein, medical editor of Cardiology News, is professor of medicine at Wayne State University and division head emeritus of cardiovascular medicine at Henry Ford Hospital, both in Detroit. He is on data safety monitoring committees for the National Institutes of Health and several pharmaceutical companies.