User login
Social Distortion?
A presentation at last week's 13th annual Management of the Hospitalized Patient conference in San Francisco described various ways that hospitals, hospitalists, and HM groups can incorporate new social media into their practice routines.
Hospitalist Russell Cucina, MD, MS, associate medical director of information technology at the University of California at San Francisco, says some physicians mistakenly disclose unprofessional content through social networks. He points to a recent article in the Journal of the American Medical Association (2009;302(12):1309-1315), which shows some physicians inadvertently violate Health Insurance Portability and Accountability Act privacy rules by accepting e-mails from patients that contain protected personal health information.
But in many cases, hospitals and physicians use blogs, Twitter, Facebook, LinkedIn, and other networking sites to exchange information with colleagues, promote their practice in their communities, or recruit new physicians. Such organizations as the Mayo Clinic and SHM use Facebook to reach targeted audiences, while the Centers for Disease Control and Prevention (CDC) uses Twitter to quickly disseminate influenza updates. Dr. Cucina says he knows of 167 U.S. hospitals using the much-hyped Twitter, but he could not find an HM group that uses the quick-hit network. He also reports that Ozmosis and Sermo, networking sites reserved for physicians, have yet to catch on in a big way.
Christine Roed, MD, a hospitalist at El Camino Hospital in Mountain View, Calif., says she sees great potential for communicating within her small medical group and for tapping into public health information. "I also feel it might be quite overwhelming. I think we have to look quite carefully at which information sources are reliable and, in turn, advise the public," Dr. Roed says. "I think a lot of physicians don't really have time to sit down and figure out what they're going to do with these things."
A presentation at last week's 13th annual Management of the Hospitalized Patient conference in San Francisco described various ways that hospitals, hospitalists, and HM groups can incorporate new social media into their practice routines.
Hospitalist Russell Cucina, MD, MS, associate medical director of information technology at the University of California at San Francisco, says some physicians mistakenly disclose unprofessional content through social networks. He points to a recent article in the Journal of the American Medical Association (2009;302(12):1309-1315), which shows some physicians inadvertently violate Health Insurance Portability and Accountability Act privacy rules by accepting e-mails from patients that contain protected personal health information.
But in many cases, hospitals and physicians use blogs, Twitter, Facebook, LinkedIn, and other networking sites to exchange information with colleagues, promote their practice in their communities, or recruit new physicians. Such organizations as the Mayo Clinic and SHM use Facebook to reach targeted audiences, while the Centers for Disease Control and Prevention (CDC) uses Twitter to quickly disseminate influenza updates. Dr. Cucina says he knows of 167 U.S. hospitals using the much-hyped Twitter, but he could not find an HM group that uses the quick-hit network. He also reports that Ozmosis and Sermo, networking sites reserved for physicians, have yet to catch on in a big way.
Christine Roed, MD, a hospitalist at El Camino Hospital in Mountain View, Calif., says she sees great potential for communicating within her small medical group and for tapping into public health information. "I also feel it might be quite overwhelming. I think we have to look quite carefully at which information sources are reliable and, in turn, advise the public," Dr. Roed says. "I think a lot of physicians don't really have time to sit down and figure out what they're going to do with these things."
A presentation at last week's 13th annual Management of the Hospitalized Patient conference in San Francisco described various ways that hospitals, hospitalists, and HM groups can incorporate new social media into their practice routines.
Hospitalist Russell Cucina, MD, MS, associate medical director of information technology at the University of California at San Francisco, says some physicians mistakenly disclose unprofessional content through social networks. He points to a recent article in the Journal of the American Medical Association (2009;302(12):1309-1315), which shows some physicians inadvertently violate Health Insurance Portability and Accountability Act privacy rules by accepting e-mails from patients that contain protected personal health information.
But in many cases, hospitals and physicians use blogs, Twitter, Facebook, LinkedIn, and other networking sites to exchange information with colleagues, promote their practice in their communities, or recruit new physicians. Such organizations as the Mayo Clinic and SHM use Facebook to reach targeted audiences, while the Centers for Disease Control and Prevention (CDC) uses Twitter to quickly disseminate influenza updates. Dr. Cucina says he knows of 167 U.S. hospitals using the much-hyped Twitter, but he could not find an HM group that uses the quick-hit network. He also reports that Ozmosis and Sermo, networking sites reserved for physicians, have yet to catch on in a big way.
Christine Roed, MD, a hospitalist at El Camino Hospital in Mountain View, Calif., says she sees great potential for communicating within her small medical group and for tapping into public health information. "I also feel it might be quite overwhelming. I think we have to look quite carefully at which information sources are reliable and, in turn, advise the public," Dr. Roed says. "I think a lot of physicians don't really have time to sit down and figure out what they're going to do with these things."
Baucus Plan Lends Clarity to Healthcare Debate
Last week’s release of the “chairman’s mark” of the America’s Healthy Future Act from Senate Finance Committee Chairman Max Baucus (D-Mont.) opened the latest chapter in the debate over healthcare reform. Beyond the hot-button issues, several Medicare-related proposals could directly impact hospitalists. Here’s a look at four of them, with observations from Eric Siegal, MD, FHM, chair of SHM’s Public Policy Committee.
Addition of a hospital value-based purchasing (VBP) program to Medicare beginning in 2012. The program would tie incentive payments to performance on quality measures related to such conditions as heart failure, pneumonia, surgical care, and patient perceptions of care. So far, the program’s rough outlines have been well received. “We fundamentally support hospital value-based purchasing,” Dr. Siegal says. “We think it’s a necessary step in the evolution to higher-value health care in general.”
Expansion of the Physician’s Quality Reporting Initiative, with a 1% payment penalty by 2012 for nonparticipants. The bill also would direct the Centers for Medicare and Medicaid Services (CMS) to improve the appeals process and feedback mechanism. Although the Baucus plan’s “mark” doesn’t discuss transitioning to pay-for-performance, Dr. Siegal says the shift likely is inevitable. In the meantime, pay-for-reporting can encourage better outcomes through a public reporting mechanism and “grease the skids” for a pay-for-performance initiative.
Creation of a CMS Payment Innovation Center “authorized to test, evaluate, and expand different payment structures and methodologies,” with a goal of improving quality and reducing Medicare costs. Dr. Siegal says the proposal is consistent with SHM’s aims. “We have for a long time advocated for a robust capability to test new payment models and to figure out what works better than what we have right now,” he says.
Establishment of a three-year Medicare pilot called the Community Care Transitions Program. The program would spend $500 million over 10 years on efforts to reduce preventable rehospitalizations. SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) likely would qualify. “We’re very positive about that,” Dr. Siegal says. “I think there is a huge amount of scrutiny now on avoidable rehospitalizations. We think BOOST is a step in the right direction, and we’d love to see greater funding to roll this out on a much larger basis.”
For more information on the current healthcare reform debate, visit SHM’s advocacy portal.
Last week’s release of the “chairman’s mark” of the America’s Healthy Future Act from Senate Finance Committee Chairman Max Baucus (D-Mont.) opened the latest chapter in the debate over healthcare reform. Beyond the hot-button issues, several Medicare-related proposals could directly impact hospitalists. Here’s a look at four of them, with observations from Eric Siegal, MD, FHM, chair of SHM’s Public Policy Committee.
Addition of a hospital value-based purchasing (VBP) program to Medicare beginning in 2012. The program would tie incentive payments to performance on quality measures related to such conditions as heart failure, pneumonia, surgical care, and patient perceptions of care. So far, the program’s rough outlines have been well received. “We fundamentally support hospital value-based purchasing,” Dr. Siegal says. “We think it’s a necessary step in the evolution to higher-value health care in general.”
Expansion of the Physician’s Quality Reporting Initiative, with a 1% payment penalty by 2012 for nonparticipants. The bill also would direct the Centers for Medicare and Medicaid Services (CMS) to improve the appeals process and feedback mechanism. Although the Baucus plan’s “mark” doesn’t discuss transitioning to pay-for-performance, Dr. Siegal says the shift likely is inevitable. In the meantime, pay-for-reporting can encourage better outcomes through a public reporting mechanism and “grease the skids” for a pay-for-performance initiative.
Creation of a CMS Payment Innovation Center “authorized to test, evaluate, and expand different payment structures and methodologies,” with a goal of improving quality and reducing Medicare costs. Dr. Siegal says the proposal is consistent with SHM’s aims. “We have for a long time advocated for a robust capability to test new payment models and to figure out what works better than what we have right now,” he says.
Establishment of a three-year Medicare pilot called the Community Care Transitions Program. The program would spend $500 million over 10 years on efforts to reduce preventable rehospitalizations. SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) likely would qualify. “We’re very positive about that,” Dr. Siegal says. “I think there is a huge amount of scrutiny now on avoidable rehospitalizations. We think BOOST is a step in the right direction, and we’d love to see greater funding to roll this out on a much larger basis.”
For more information on the current healthcare reform debate, visit SHM’s advocacy portal.
Last week’s release of the “chairman’s mark” of the America’s Healthy Future Act from Senate Finance Committee Chairman Max Baucus (D-Mont.) opened the latest chapter in the debate over healthcare reform. Beyond the hot-button issues, several Medicare-related proposals could directly impact hospitalists. Here’s a look at four of them, with observations from Eric Siegal, MD, FHM, chair of SHM’s Public Policy Committee.
Addition of a hospital value-based purchasing (VBP) program to Medicare beginning in 2012. The program would tie incentive payments to performance on quality measures related to such conditions as heart failure, pneumonia, surgical care, and patient perceptions of care. So far, the program’s rough outlines have been well received. “We fundamentally support hospital value-based purchasing,” Dr. Siegal says. “We think it’s a necessary step in the evolution to higher-value health care in general.”
Expansion of the Physician’s Quality Reporting Initiative, with a 1% payment penalty by 2012 for nonparticipants. The bill also would direct the Centers for Medicare and Medicaid Services (CMS) to improve the appeals process and feedback mechanism. Although the Baucus plan’s “mark” doesn’t discuss transitioning to pay-for-performance, Dr. Siegal says the shift likely is inevitable. In the meantime, pay-for-reporting can encourage better outcomes through a public reporting mechanism and “grease the skids” for a pay-for-performance initiative.
Creation of a CMS Payment Innovation Center “authorized to test, evaluate, and expand different payment structures and methodologies,” with a goal of improving quality and reducing Medicare costs. Dr. Siegal says the proposal is consistent with SHM’s aims. “We have for a long time advocated for a robust capability to test new payment models and to figure out what works better than what we have right now,” he says.
Establishment of a three-year Medicare pilot called the Community Care Transitions Program. The program would spend $500 million over 10 years on efforts to reduce preventable rehospitalizations. SHM’s Project BOOST (Better Outcomes for Older Adults through Safe Transitions) likely would qualify. “We’re very positive about that,” Dr. Siegal says. “I think there is a huge amount of scrutiny now on avoidable rehospitalizations. We think BOOST is a step in the right direction, and we’d love to see greater funding to roll this out on a much larger basis.”
For more information on the current healthcare reform debate, visit SHM’s advocacy portal.
1,200 Satisfied Customers and Counting
Another SHM Leadership Academy just ended, with introductory and advanced courses presented at the Fontainebleau resort, complete with the backdrop of Miami Beach. These four-day courses are aimed at hospitalist leaders, and have trained more than 1,200 participants to date. The faculty includes nationally recognized experts in their respective fields, as well as experienced HM leaders.
Level I courses are designed to help new leaders in HM, and focus on communication skills, hospital business drivers, leadership, strategic planning, and conflict resolution. Harjit Bhogal, MD, a hospitalist at Johns Hopkins University School of Medicine in Baltimore, found the Level I course addressed many of the roles she has as a new hospitalist. “The session on strategic planning was interactive and extremely informative,” Dr. Bhogal says. She also says the academy is an ideal place for networking, as attendees have access to more than 100 practicing hospitalist leaders with whom to connect.
Level II takes concepts introduced in Level I and refines them. Communication and negotiation, “meta-leadership,” financial storytelling, and a leadership roundtable help more advanced leaders tackle more complex issues. The session on meta-leadership was “terrific,” according to hospitalist Darlene Tad-y, MD, of Johns Hopkins. “One of the best days I have spent as an adult learner,” she says. “Lenny [Marcus] was absolutely amazing; his walk-in-the-woods approach was a terrific method to discuss leadership.”
Many see hospitalists as the future leaders of the hospital and throughout healthcare. “In addition to helping train leaders for hospital medicine, we believe that SHM has the obligation to train hospitalists to be the next hospital CMOs and CEOs,” says Larry Wellikson, MD, FHM, CEO of SHM.
Apparently, 1,200 academy graduates agree with him.
Another SHM Leadership Academy just ended, with introductory and advanced courses presented at the Fontainebleau resort, complete with the backdrop of Miami Beach. These four-day courses are aimed at hospitalist leaders, and have trained more than 1,200 participants to date. The faculty includes nationally recognized experts in their respective fields, as well as experienced HM leaders.
Level I courses are designed to help new leaders in HM, and focus on communication skills, hospital business drivers, leadership, strategic planning, and conflict resolution. Harjit Bhogal, MD, a hospitalist at Johns Hopkins University School of Medicine in Baltimore, found the Level I course addressed many of the roles she has as a new hospitalist. “The session on strategic planning was interactive and extremely informative,” Dr. Bhogal says. She also says the academy is an ideal place for networking, as attendees have access to more than 100 practicing hospitalist leaders with whom to connect.
Level II takes concepts introduced in Level I and refines them. Communication and negotiation, “meta-leadership,” financial storytelling, and a leadership roundtable help more advanced leaders tackle more complex issues. The session on meta-leadership was “terrific,” according to hospitalist Darlene Tad-y, MD, of Johns Hopkins. “One of the best days I have spent as an adult learner,” she says. “Lenny [Marcus] was absolutely amazing; his walk-in-the-woods approach was a terrific method to discuss leadership.”
Many see hospitalists as the future leaders of the hospital and throughout healthcare. “In addition to helping train leaders for hospital medicine, we believe that SHM has the obligation to train hospitalists to be the next hospital CMOs and CEOs,” says Larry Wellikson, MD, FHM, CEO of SHM.
Apparently, 1,200 academy graduates agree with him.
Another SHM Leadership Academy just ended, with introductory and advanced courses presented at the Fontainebleau resort, complete with the backdrop of Miami Beach. These four-day courses are aimed at hospitalist leaders, and have trained more than 1,200 participants to date. The faculty includes nationally recognized experts in their respective fields, as well as experienced HM leaders.
Level I courses are designed to help new leaders in HM, and focus on communication skills, hospital business drivers, leadership, strategic planning, and conflict resolution. Harjit Bhogal, MD, a hospitalist at Johns Hopkins University School of Medicine in Baltimore, found the Level I course addressed many of the roles she has as a new hospitalist. “The session on strategic planning was interactive and extremely informative,” Dr. Bhogal says. She also says the academy is an ideal place for networking, as attendees have access to more than 100 practicing hospitalist leaders with whom to connect.
Level II takes concepts introduced in Level I and refines them. Communication and negotiation, “meta-leadership,” financial storytelling, and a leadership roundtable help more advanced leaders tackle more complex issues. The session on meta-leadership was “terrific,” according to hospitalist Darlene Tad-y, MD, of Johns Hopkins. “One of the best days I have spent as an adult learner,” she says. “Lenny [Marcus] was absolutely amazing; his walk-in-the-woods approach was a terrific method to discuss leadership.”
Many see hospitalists as the future leaders of the hospital and throughout healthcare. “In addition to helping train leaders for hospital medicine, we believe that SHM has the obligation to train hospitalists to be the next hospital CMOs and CEOs,” says Larry Wellikson, MD, FHM, CEO of SHM.
Apparently, 1,200 academy graduates agree with him.
Sleep Disruptions and Sedative Use
Adequate sleep is important for health, yet the hospital environment commonly disrupts sleep.13 Sleep improves after several days in the hospital.3, 4 Sleep deprivation increases cortisol levels5 and sleep loss of greater than 4 hours may be hyperalgesic.6 Even a few days' suppression of slow‐wave sleep worsens glucose tolerance.7 Sleep disruption may cause irritability and aggressiveness,8 impaired memory consolidation, and delirium.2
Noise may disrupt sleep. The World Health Organization recommends a maximum of 30 to 40 dBA in patients' rooms at night.9, 10 Normal conversation occurs at 60 dBA. Medical equipment alarms are about 80 dBA.
Sedative use is common in the hospital.3 Sedatives typically shorten sleep latency and suppress rapid eye movement (REM) sleep. However, some sedatives cause delirium, falls, amnesia, and confusion, particularly in the elderly.1113
Most research on sleep in hospitalized patients has been done in the critical care setting, often in sedated ventilated patients, where sleep disruption is well‐described.1416 Only a few small studies have assessed the sleep of hospitalized patients outside critical care.17, 18
A single blinded interventional trial assessed sedative use, but was a nonrandomized study.19, 20 As‐needed sedative use was measured among hospitalized elderly patients as a secondary endpoint. The intervention, known as the Hospital Elder Life Program (HELP), included a protocol with noise reduction, massage, music, and warm drinks, as well as rescheduling of medications and procedures; it resulted in a 24% reduction in as‐needed sedative use. Another trial decreased noise and reduced overnight X‐rays on a surgical unit, then measured staff and patient attitudes.21 Two interventional studies in nursing homes reduced noise and light, and/or increased daytime activity and found no effect on most objective measures of sleep.22, 23 One descriptive study found most sleep disturbances in medical‐surgical patients came from noise and sleeping in an unfamiliar bed.4
We hypothesized that an intervention designed to improve patient sleep through changes in staff behavior would decrease sedative use among unselected patients in a medical‐surgical unit. We measured sedative use as our primary endpoint as a marker for effective sleep, and because decreased sedative use is desirable. We also hypothesized that the intervention would lead to improved sleep experiences, as measured by a questionnaire and Verran Snyder‐Halpern (VSH) sleep scores as secondary endpoints.24
Materials And Methods
Study Design
This was a pre‐post study assessing the effect of the intervention on as‐needed sedative use, questionnaire responses, and sleep quality. It was an intention‐to‐treat analysis, and was blinded in terms of measurement of sedative use. The Institutional Review Board of Cambridge Health Alliance approved the study.
Setting and Patients
The site was the only medical‐surgical unit of Somerville Hospital, a small urban community teaching hospital that is part of Cambridge Health Alliance. The hospital unit was chosen for its architectural characteristics, and is organized spatially as 3 U‐shaped pods surrounding nursing workstations. Hence, patient rooms were nearly equidistant from the nurses' stations, unlike a hallway design where distant rooms are quieter. Six rooms were private; 11 were semiprivate. Most of the unit's 28 beds are used for medical patients covered by the hospitalist service. Residents see a minority of patients. A hospitalist is available around the clock. Few agency nurses are used.
Preintervention patients were recruited between April and August 2007. The intervention was planned and implemented from September 2007 to January 2008. Intervention patients were recruited between February and June 2008. The most common principle diagnoses on the unit were chest pain (11%), pneumonia (8%), congestive heart failure (CHF) (5.1%), and chronic obstructive pulmonary disease (COPD) flare (3%). Exclusion criteria ensured that no patient was ill enough to require intensive care unit (ICU)‐level care or was actively dying. All consecutive hospitalized patients on the unit on Tuesdays through Fridays were potentially eligible and invited to participate unless they met exclusion criteria. The limited days of the week ensured that technical support would be available during the intervention phase.
Exclusion criteria were: known sleep disorders; language other than English, Spanish, Portuguese, or Haitian Creole; surgery the prior day; arrival on the floor after 10 PM the prior evening; residence on the unit for more than 4 days; alcohol or drug withdrawal; end‐of‐life morphine drip; significant hearing loss; and blindness.
Study Protocol
A single investigator surveyed patients in the morning about the prior night's sleep experience. The surveys consisted of the VSH sleep scale, as well as an 8‐item questionnaire developed from informal pilot interviews with about 18 patients conducted by 1 of the investigators (M.B.) (Supporting Information Figure 1). The VSH scale is a visual analog scale using a 100‐cm line,24 which we modified with a 100‐mm line to make it easier to collect data. The questionnaire and VSH scores of patients with cognitive impairment were not included in the final analysis. Cognitive impairment was determined by diagnoses present in chart review. Surveys and consent forms were available in 4 languages and trained interpreters were used as needed. Nurses, providers, and patients were blinded to the measurement of as‐needed sedative use, and staff were unaware of which patients were study subjects.

Measurements
Nighttime administration of any medication ordered prn sleep or insomnia was measured using the pharmacy dispensing equipment (Pyxis; Cardinal Health, Dublin, OH), then verified by reviewing the patients' medication administration records. VSH sleep scores were created by measuring the distance in millimeters from the lower end of the scale (0) to the location marked.
We also tracked adherence to some aspects of the intervention. The questionnaire recorded door closing. Chart audits measured the numbers of different prescribers, and the frequency of medication orders using flexible timing.
Data Analysis
Medication use was analyzed as any as‐needed sedative use vs. none. The proportions of patients who used sedatives preintervention and postintervention were compared using a 2‐sample Z statistic, as were survey items. Mean VSH scores were compared with 2‐sample t tests. The study had greater than 80% power to detect a difference in proportion of at least 0.14 at alpha = 0.05.
Design and Implementation of the Intervention
Preintervention, routine vital signs were taken every 8 hours: 8 AM, 4 PM, and midnight. Night nurses arrived at 11 PM, and typically turned off the hallway lights, but the practice was variable and occurred at no set time.
Patients in our informal pilot interviews identified vital signs, medication administration, noise, and evening diuretic administration as disrupting their sleep. After the preintervention phase, we spent 4 months designing and implementing the intervention. We solicited opinions from staff, who identified inflexible timing of medications as disruptive. The plan was discussed at routine staff meetings of all shifts.
The intervention, called the Somerville Protocol (Figure 1) created an 8‐hour Quiet Time from 10 PM to 6 AM, when disruptions were minimized. Vital signs were taken 2 hours earlier (6 AM, 2 PM, and 10 PM); routine medication administration was avoided; and noise was reduced. As before, telemetry patients required vital signs every 4 hours. At 10 PM, hallway lights were turned off by a timer while the Lullaby by Brahms played overhead, signaling the start of Quiet Time to staff and patients. Inexpensive sound meters were installed in each nursing area. They flashed warning lights when 60 dBA was exceeded.
A physician and nurse served as champions. Educational signs were posted in the hospitalists' call room and in the nursing areas. The champions used e‐mail and detailed the intervention to staff. Because the staff played an active role in intervention planning, implementation went smoothly.
Results
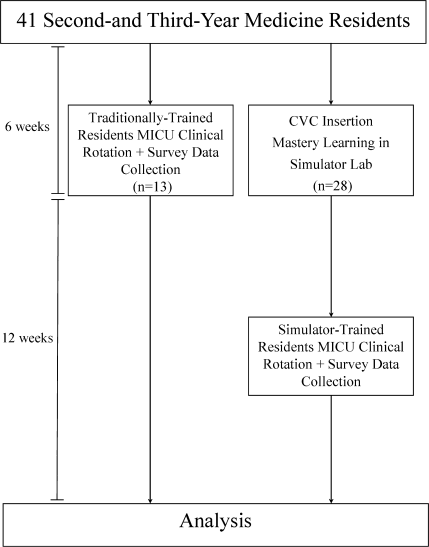
During the preintervention phase, 334 patients were screened, 294 were eligible, and 54.7% of eligible subjects were enrolled (n = 161). During the intervention phase, 211 patients were screened, 188 were eligible, and 56.3% of eligible patients were enrolled (n = 106). The mean patient age was 60.6 years. The preintervention and intervention groups did not differ significantly in enrollment rate, age, gender, cognitive impairment, surgical status, or hearing deficiencies (Table 1). Over 93% of patients were nonsurgical.
| Preintervention Patients (n = 161) | Intervention Patients (n = 106) | P Values for Difference | |
|---|---|---|---|
| Mean age (years) | 59.1 | 62.95 | P = 0.146 |
| Males, n (%) | 79 (49.1%) | 46 (43.4%) | P = 0.38 |
| Hard of hearing, n (%) (self‐report) | 33/157 (21.0%) | 14/103 (13.6%) | P = 0.128 |
| English‐speaking, n (%) | 134 (83%) | 83 (78.3%) | P = 0.34 |
| Cognitive impairment, n (%) | 4 (2.5%) | 3 (2.8%) | P = 0.88 |
| Surgical patients, n (%) | 10 (6.2%) | 2 (1.8%) | P = 0.089 |
Sedative Use
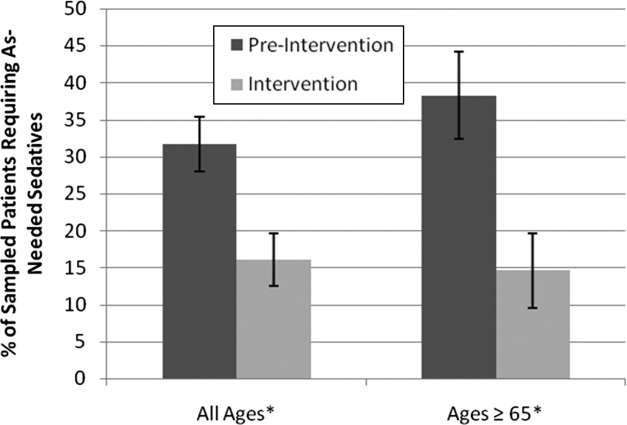
Preintervention, 31.7% of patients received nighttime as‐needed sedatives, versus 16.0% of the intervention group, a 49.4% reduction (P = 0.0041; 95% confidence interval [CI]: 0.056‐0.26) (Figure 2). In patients aged 65 years or older, 38.2% received nighttime as‐needed sedatives preintervention, and 14.6% did postintervention, a 61.2% reduction (P = 0.0054; 95% CI: 0.084‐0.39).
Questionnaire Results
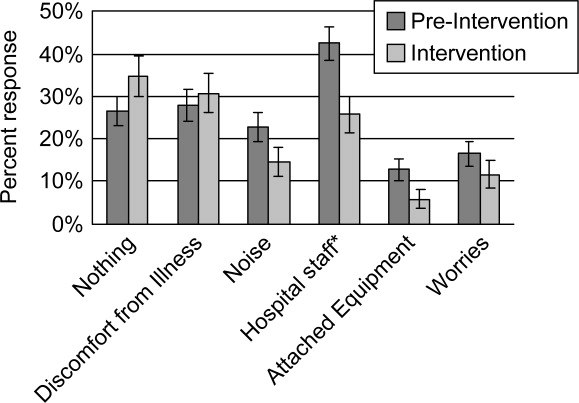
Preintervention, hospital staff was by far the biggest factor keeping patients awake, with 42.4% of patients reporting it (Figure 3). This dropped to only 25.7% with the intervention, a 39.3% decrease (P = 0.009; 95% CI: 0.0452‐0.2765). Preintervention, 19.2% of patients selected voices as the noise most likely to bother them at night, and this dropped to 9.9% with the intervention, a 48% decrease (P = 0.045; 95% CI: 0.0074‐0.1787). No other significant differences were found.
VSH Sleep Score Results
We found no improvement in any measure of the VSH sleep scale. However, 75% of our patients were unable to use the modified VSH scale, generally because they felt too ill, and were then prompted by the surveyor to choose a number between 1 and 10 that reflected their experience.
Protocol Adherence
Changes in unit routines resulted in complete adherence to the new vital signs schedule and avoidance of routine evening diuretics. The closing of patients' doors did not change. An audit of 40 charts found that the percentage of medication orders written with appropriate flexible timing increased from 82% (n = 228) to 95.5% (n = 200) (P = 0.001; 95% CI: 0.077‐0.192). From 20 to 30 different providers wrote orders during each phase.
Discussion
Our trial found that hospital staff was the factor most responsible for patient sleep disruption, and that behavioral interventions on hospital staff can reduce use of as‐needed sedatives. The only previously reported intervention to reduce sedative use, the HELP strategy, involved a complex intervention requiring extra staff, with adherence ranging from 10% to 75%.19, 20, 25 In contrast, our protocol can be easily replicated at minimal cost.
Our results are consistent with those of Freedman et al.,26 who found that noise was not the primary factor responsible for sleep disruption in ICU patients, and that staff activities were at least as important a factor. The study is also consistent with the nursing home studies in which decreases in noise and light did not improve sleep.22, 23 It refutes the study that showed that most sleep disturbance in medical‐surgical patients comes from noise and sleeping in an unfamiliar bed.4 Our results call into question the use of the VSH scale in hospitalized patients, which was designed for use in healthy subjects.
Limitations of this study were as follows: moderate size, lack of refined measures of disease severity, and, as in previous studies,19, 2123 the lack of randomized concurrent controls. Evaluation of secondary endpoints was limited by lack of validation of the questionnaire with objective observations, and inability to use the modified VSH scale. Self‐reports of sleep may correlate imperfectly with objective measures, such as polysomnography.27
A larger concurrent trial randomizing similar units at multiple hospitals would be ideal. Future research is needed to determine whether improving sleep in the hospital improves other outcomes, such as recovery times, delirium, falls, or cost.
The need to reduce as‐needed sedatives is an important safety issue and similar interventions in other hospitals may be helpful. Simple changes in staff routines and provider prescribing habits can yield significant reductions in sedative use.
Acknowledgements
The authors thank Gertrude Gavin, Steffie Woolhandler, MD, Linda Borodkin, John Brusch, MD, Patricia Crombie, Priscilla Dasse, Glen Dawson, Ben Davenny, Linda Kasten, Judith Krempin, Mark Letzeisen, Carmen Mohan, and Arun Mohan. Linda Kasten, Timothy Schmidt, and Glen Dawson provided statistical analysis. The sound meters (Yacker Trackers, Creative Toys of Colorado) were donated by John Brusch, who has no financial conflict of interest.
- ,,,.Sleep in hospitalized medical patients, Part 1: Factors affecting sleep.J Hosp Med.2008;3:473–482.
- ,.Sleep‐dependent learning and memory consolidation.Neuron.2004;44:121–133.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,.The sleep experience of medical and surgical patients.Clin Nurs Res.2003;12:159–173.
- .Metabolic and endocrine effects of sleep deprivation.Essent Psychopharmacol.2005;6:341–347.
- ,,,,.Sleep loss and REM sleep loss are hyperalgesic.Sleep.2006;29:145–151.
- ,,,.Slow‐wave sleep and the risk of type 2 diabetes in humans.Proc Natl Acad Sci USA.2008;105:1044–1049.
- .Sleep inquiry: a look with fresh eyes.Image J Nurs Sch.1993;25:249–256.
- Berglund B, Lindvall T, Schwela D, eds.Guidelines for Community Noise.World Health Organization;1999:47.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118:3629–3645.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- ,.Sleep in the critically ill patient.Sleep.2006;29:707–716.
- ,,.Sleep quality in hospitalized patients.J Clin Nurs.2005;14:107–113.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30:237–243.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,,.The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program.J Am Geriatr Soc.2000;48:1697–1706.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104:40–48; quiz 48‐49.
- ,,,,,.A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial.J Am Geriatr Soc.2006;54:38–47.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47:430–438.
- ,.Instrumentation to describe subjective sleep characteristics in healthy subjects.Res Nurs Health.1987;10:155–163.
- ,,,,.The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial.Arch Intern Med.2003;163:958–964.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,.When sleep is perceived as wakefulness: an experimental study on state perception during physiological sleep.J Sleep Res.2007;16:346–353.
Adequate sleep is important for health, yet the hospital environment commonly disrupts sleep.13 Sleep improves after several days in the hospital.3, 4 Sleep deprivation increases cortisol levels5 and sleep loss of greater than 4 hours may be hyperalgesic.6 Even a few days' suppression of slow‐wave sleep worsens glucose tolerance.7 Sleep disruption may cause irritability and aggressiveness,8 impaired memory consolidation, and delirium.2
Noise may disrupt sleep. The World Health Organization recommends a maximum of 30 to 40 dBA in patients' rooms at night.9, 10 Normal conversation occurs at 60 dBA. Medical equipment alarms are about 80 dBA.
Sedative use is common in the hospital.3 Sedatives typically shorten sleep latency and suppress rapid eye movement (REM) sleep. However, some sedatives cause delirium, falls, amnesia, and confusion, particularly in the elderly.1113
Most research on sleep in hospitalized patients has been done in the critical care setting, often in sedated ventilated patients, where sleep disruption is well‐described.1416 Only a few small studies have assessed the sleep of hospitalized patients outside critical care.17, 18
A single blinded interventional trial assessed sedative use, but was a nonrandomized study.19, 20 As‐needed sedative use was measured among hospitalized elderly patients as a secondary endpoint. The intervention, known as the Hospital Elder Life Program (HELP), included a protocol with noise reduction, massage, music, and warm drinks, as well as rescheduling of medications and procedures; it resulted in a 24% reduction in as‐needed sedative use. Another trial decreased noise and reduced overnight X‐rays on a surgical unit, then measured staff and patient attitudes.21 Two interventional studies in nursing homes reduced noise and light, and/or increased daytime activity and found no effect on most objective measures of sleep.22, 23 One descriptive study found most sleep disturbances in medical‐surgical patients came from noise and sleeping in an unfamiliar bed.4
We hypothesized that an intervention designed to improve patient sleep through changes in staff behavior would decrease sedative use among unselected patients in a medical‐surgical unit. We measured sedative use as our primary endpoint as a marker for effective sleep, and because decreased sedative use is desirable. We also hypothesized that the intervention would lead to improved sleep experiences, as measured by a questionnaire and Verran Snyder‐Halpern (VSH) sleep scores as secondary endpoints.24
Materials And Methods
Study Design
This was a pre‐post study assessing the effect of the intervention on as‐needed sedative use, questionnaire responses, and sleep quality. It was an intention‐to‐treat analysis, and was blinded in terms of measurement of sedative use. The Institutional Review Board of Cambridge Health Alliance approved the study.
Setting and Patients
The site was the only medical‐surgical unit of Somerville Hospital, a small urban community teaching hospital that is part of Cambridge Health Alliance. The hospital unit was chosen for its architectural characteristics, and is organized spatially as 3 U‐shaped pods surrounding nursing workstations. Hence, patient rooms were nearly equidistant from the nurses' stations, unlike a hallway design where distant rooms are quieter. Six rooms were private; 11 were semiprivate. Most of the unit's 28 beds are used for medical patients covered by the hospitalist service. Residents see a minority of patients. A hospitalist is available around the clock. Few agency nurses are used.
Preintervention patients were recruited between April and August 2007. The intervention was planned and implemented from September 2007 to January 2008. Intervention patients were recruited between February and June 2008. The most common principle diagnoses on the unit were chest pain (11%), pneumonia (8%), congestive heart failure (CHF) (5.1%), and chronic obstructive pulmonary disease (COPD) flare (3%). Exclusion criteria ensured that no patient was ill enough to require intensive care unit (ICU)‐level care or was actively dying. All consecutive hospitalized patients on the unit on Tuesdays through Fridays were potentially eligible and invited to participate unless they met exclusion criteria. The limited days of the week ensured that technical support would be available during the intervention phase.
Exclusion criteria were: known sleep disorders; language other than English, Spanish, Portuguese, or Haitian Creole; surgery the prior day; arrival on the floor after 10 PM the prior evening; residence on the unit for more than 4 days; alcohol or drug withdrawal; end‐of‐life morphine drip; significant hearing loss; and blindness.
Study Protocol
A single investigator surveyed patients in the morning about the prior night's sleep experience. The surveys consisted of the VSH sleep scale, as well as an 8‐item questionnaire developed from informal pilot interviews with about 18 patients conducted by 1 of the investigators (M.B.) (Supporting Information Figure 1). The VSH scale is a visual analog scale using a 100‐cm line,24 which we modified with a 100‐mm line to make it easier to collect data. The questionnaire and VSH scores of patients with cognitive impairment were not included in the final analysis. Cognitive impairment was determined by diagnoses present in chart review. Surveys and consent forms were available in 4 languages and trained interpreters were used as needed. Nurses, providers, and patients were blinded to the measurement of as‐needed sedative use, and staff were unaware of which patients were study subjects.

Measurements
Nighttime administration of any medication ordered prn sleep or insomnia was measured using the pharmacy dispensing equipment (Pyxis; Cardinal Health, Dublin, OH), then verified by reviewing the patients' medication administration records. VSH sleep scores were created by measuring the distance in millimeters from the lower end of the scale (0) to the location marked.
We also tracked adherence to some aspects of the intervention. The questionnaire recorded door closing. Chart audits measured the numbers of different prescribers, and the frequency of medication orders using flexible timing.
Data Analysis
Medication use was analyzed as any as‐needed sedative use vs. none. The proportions of patients who used sedatives preintervention and postintervention were compared using a 2‐sample Z statistic, as were survey items. Mean VSH scores were compared with 2‐sample t tests. The study had greater than 80% power to detect a difference in proportion of at least 0.14 at alpha = 0.05.
Design and Implementation of the Intervention
Preintervention, routine vital signs were taken every 8 hours: 8 AM, 4 PM, and midnight. Night nurses arrived at 11 PM, and typically turned off the hallway lights, but the practice was variable and occurred at no set time.
Patients in our informal pilot interviews identified vital signs, medication administration, noise, and evening diuretic administration as disrupting their sleep. After the preintervention phase, we spent 4 months designing and implementing the intervention. We solicited opinions from staff, who identified inflexible timing of medications as disruptive. The plan was discussed at routine staff meetings of all shifts.
The intervention, called the Somerville Protocol (Figure 1) created an 8‐hour Quiet Time from 10 PM to 6 AM, when disruptions were minimized. Vital signs were taken 2 hours earlier (6 AM, 2 PM, and 10 PM); routine medication administration was avoided; and noise was reduced. As before, telemetry patients required vital signs every 4 hours. At 10 PM, hallway lights were turned off by a timer while the Lullaby by Brahms played overhead, signaling the start of Quiet Time to staff and patients. Inexpensive sound meters were installed in each nursing area. They flashed warning lights when 60 dBA was exceeded.
A physician and nurse served as champions. Educational signs were posted in the hospitalists' call room and in the nursing areas. The champions used e‐mail and detailed the intervention to staff. Because the staff played an active role in intervention planning, implementation went smoothly.
Results
During the preintervention phase, 334 patients were screened, 294 were eligible, and 54.7% of eligible subjects were enrolled (n = 161). During the intervention phase, 211 patients were screened, 188 were eligible, and 56.3% of eligible patients were enrolled (n = 106). The mean patient age was 60.6 years. The preintervention and intervention groups did not differ significantly in enrollment rate, age, gender, cognitive impairment, surgical status, or hearing deficiencies (Table 1). Over 93% of patients were nonsurgical.
| Preintervention Patients (n = 161) | Intervention Patients (n = 106) | P Values for Difference | |
|---|---|---|---|
| Mean age (years) | 59.1 | 62.95 | P = 0.146 |
| Males, n (%) | 79 (49.1%) | 46 (43.4%) | P = 0.38 |
| Hard of hearing, n (%) (self‐report) | 33/157 (21.0%) | 14/103 (13.6%) | P = 0.128 |
| English‐speaking, n (%) | 134 (83%) | 83 (78.3%) | P = 0.34 |
| Cognitive impairment, n (%) | 4 (2.5%) | 3 (2.8%) | P = 0.88 |
| Surgical patients, n (%) | 10 (6.2%) | 2 (1.8%) | P = 0.089 |
Sedative Use
Preintervention, 31.7% of patients received nighttime as‐needed sedatives, versus 16.0% of the intervention group, a 49.4% reduction (P = 0.0041; 95% confidence interval [CI]: 0.056‐0.26) (Figure 2). In patients aged 65 years or older, 38.2% received nighttime as‐needed sedatives preintervention, and 14.6% did postintervention, a 61.2% reduction (P = 0.0054; 95% CI: 0.084‐0.39).

Questionnaire Results
Preintervention, hospital staff was by far the biggest factor keeping patients awake, with 42.4% of patients reporting it (Figure 3). This dropped to only 25.7% with the intervention, a 39.3% decrease (P = 0.009; 95% CI: 0.0452‐0.2765). Preintervention, 19.2% of patients selected voices as the noise most likely to bother them at night, and this dropped to 9.9% with the intervention, a 48% decrease (P = 0.045; 95% CI: 0.0074‐0.1787). No other significant differences were found.

VSH Sleep Score Results
We found no improvement in any measure of the VSH sleep scale. However, 75% of our patients were unable to use the modified VSH scale, generally because they felt too ill, and were then prompted by the surveyor to choose a number between 1 and 10 that reflected their experience.
Protocol Adherence
Changes in unit routines resulted in complete adherence to the new vital signs schedule and avoidance of routine evening diuretics. The closing of patients' doors did not change. An audit of 40 charts found that the percentage of medication orders written with appropriate flexible timing increased from 82% (n = 228) to 95.5% (n = 200) (P = 0.001; 95% CI: 0.077‐0.192). From 20 to 30 different providers wrote orders during each phase.
Discussion
Our trial found that hospital staff was the factor most responsible for patient sleep disruption, and that behavioral interventions on hospital staff can reduce use of as‐needed sedatives. The only previously reported intervention to reduce sedative use, the HELP strategy, involved a complex intervention requiring extra staff, with adherence ranging from 10% to 75%.19, 20, 25 In contrast, our protocol can be easily replicated at minimal cost.
Our results are consistent with those of Freedman et al.,26 who found that noise was not the primary factor responsible for sleep disruption in ICU patients, and that staff activities were at least as important a factor. The study is also consistent with the nursing home studies in which decreases in noise and light did not improve sleep.22, 23 It refutes the study that showed that most sleep disturbance in medical‐surgical patients comes from noise and sleeping in an unfamiliar bed.4 Our results call into question the use of the VSH scale in hospitalized patients, which was designed for use in healthy subjects.
Limitations of this study were as follows: moderate size, lack of refined measures of disease severity, and, as in previous studies,19, 2123 the lack of randomized concurrent controls. Evaluation of secondary endpoints was limited by lack of validation of the questionnaire with objective observations, and inability to use the modified VSH scale. Self‐reports of sleep may correlate imperfectly with objective measures, such as polysomnography.27
A larger concurrent trial randomizing similar units at multiple hospitals would be ideal. Future research is needed to determine whether improving sleep in the hospital improves other outcomes, such as recovery times, delirium, falls, or cost.
The need to reduce as‐needed sedatives is an important safety issue and similar interventions in other hospitals may be helpful. Simple changes in staff routines and provider prescribing habits can yield significant reductions in sedative use.
Acknowledgements
The authors thank Gertrude Gavin, Steffie Woolhandler, MD, Linda Borodkin, John Brusch, MD, Patricia Crombie, Priscilla Dasse, Glen Dawson, Ben Davenny, Linda Kasten, Judith Krempin, Mark Letzeisen, Carmen Mohan, and Arun Mohan. Linda Kasten, Timothy Schmidt, and Glen Dawson provided statistical analysis. The sound meters (Yacker Trackers, Creative Toys of Colorado) were donated by John Brusch, who has no financial conflict of interest.
Adequate sleep is important for health, yet the hospital environment commonly disrupts sleep.13 Sleep improves after several days in the hospital.3, 4 Sleep deprivation increases cortisol levels5 and sleep loss of greater than 4 hours may be hyperalgesic.6 Even a few days' suppression of slow‐wave sleep worsens glucose tolerance.7 Sleep disruption may cause irritability and aggressiveness,8 impaired memory consolidation, and delirium.2
Noise may disrupt sleep. The World Health Organization recommends a maximum of 30 to 40 dBA in patients' rooms at night.9, 10 Normal conversation occurs at 60 dBA. Medical equipment alarms are about 80 dBA.
Sedative use is common in the hospital.3 Sedatives typically shorten sleep latency and suppress rapid eye movement (REM) sleep. However, some sedatives cause delirium, falls, amnesia, and confusion, particularly in the elderly.1113
Most research on sleep in hospitalized patients has been done in the critical care setting, often in sedated ventilated patients, where sleep disruption is well‐described.1416 Only a few small studies have assessed the sleep of hospitalized patients outside critical care.17, 18
A single blinded interventional trial assessed sedative use, but was a nonrandomized study.19, 20 As‐needed sedative use was measured among hospitalized elderly patients as a secondary endpoint. The intervention, known as the Hospital Elder Life Program (HELP), included a protocol with noise reduction, massage, music, and warm drinks, as well as rescheduling of medications and procedures; it resulted in a 24% reduction in as‐needed sedative use. Another trial decreased noise and reduced overnight X‐rays on a surgical unit, then measured staff and patient attitudes.21 Two interventional studies in nursing homes reduced noise and light, and/or increased daytime activity and found no effect on most objective measures of sleep.22, 23 One descriptive study found most sleep disturbances in medical‐surgical patients came from noise and sleeping in an unfamiliar bed.4
We hypothesized that an intervention designed to improve patient sleep through changes in staff behavior would decrease sedative use among unselected patients in a medical‐surgical unit. We measured sedative use as our primary endpoint as a marker for effective sleep, and because decreased sedative use is desirable. We also hypothesized that the intervention would lead to improved sleep experiences, as measured by a questionnaire and Verran Snyder‐Halpern (VSH) sleep scores as secondary endpoints.24
Materials And Methods
Study Design
This was a pre‐post study assessing the effect of the intervention on as‐needed sedative use, questionnaire responses, and sleep quality. It was an intention‐to‐treat analysis, and was blinded in terms of measurement of sedative use. The Institutional Review Board of Cambridge Health Alliance approved the study.
Setting and Patients
The site was the only medical‐surgical unit of Somerville Hospital, a small urban community teaching hospital that is part of Cambridge Health Alliance. The hospital unit was chosen for its architectural characteristics, and is organized spatially as 3 U‐shaped pods surrounding nursing workstations. Hence, patient rooms were nearly equidistant from the nurses' stations, unlike a hallway design where distant rooms are quieter. Six rooms were private; 11 were semiprivate. Most of the unit's 28 beds are used for medical patients covered by the hospitalist service. Residents see a minority of patients. A hospitalist is available around the clock. Few agency nurses are used.
Preintervention patients were recruited between April and August 2007. The intervention was planned and implemented from September 2007 to January 2008. Intervention patients were recruited between February and June 2008. The most common principle diagnoses on the unit were chest pain (11%), pneumonia (8%), congestive heart failure (CHF) (5.1%), and chronic obstructive pulmonary disease (COPD) flare (3%). Exclusion criteria ensured that no patient was ill enough to require intensive care unit (ICU)‐level care or was actively dying. All consecutive hospitalized patients on the unit on Tuesdays through Fridays were potentially eligible and invited to participate unless they met exclusion criteria. The limited days of the week ensured that technical support would be available during the intervention phase.
Exclusion criteria were: known sleep disorders; language other than English, Spanish, Portuguese, or Haitian Creole; surgery the prior day; arrival on the floor after 10 PM the prior evening; residence on the unit for more than 4 days; alcohol or drug withdrawal; end‐of‐life morphine drip; significant hearing loss; and blindness.
Study Protocol
A single investigator surveyed patients in the morning about the prior night's sleep experience. The surveys consisted of the VSH sleep scale, as well as an 8‐item questionnaire developed from informal pilot interviews with about 18 patients conducted by 1 of the investigators (M.B.) (Supporting Information Figure 1). The VSH scale is a visual analog scale using a 100‐cm line,24 which we modified with a 100‐mm line to make it easier to collect data. The questionnaire and VSH scores of patients with cognitive impairment were not included in the final analysis. Cognitive impairment was determined by diagnoses present in chart review. Surveys and consent forms were available in 4 languages and trained interpreters were used as needed. Nurses, providers, and patients were blinded to the measurement of as‐needed sedative use, and staff were unaware of which patients were study subjects.

Measurements
Nighttime administration of any medication ordered prn sleep or insomnia was measured using the pharmacy dispensing equipment (Pyxis; Cardinal Health, Dublin, OH), then verified by reviewing the patients' medication administration records. VSH sleep scores were created by measuring the distance in millimeters from the lower end of the scale (0) to the location marked.
We also tracked adherence to some aspects of the intervention. The questionnaire recorded door closing. Chart audits measured the numbers of different prescribers, and the frequency of medication orders using flexible timing.
Data Analysis
Medication use was analyzed as any as‐needed sedative use vs. none. The proportions of patients who used sedatives preintervention and postintervention were compared using a 2‐sample Z statistic, as were survey items. Mean VSH scores were compared with 2‐sample t tests. The study had greater than 80% power to detect a difference in proportion of at least 0.14 at alpha = 0.05.
Design and Implementation of the Intervention
Preintervention, routine vital signs were taken every 8 hours: 8 AM, 4 PM, and midnight. Night nurses arrived at 11 PM, and typically turned off the hallway lights, but the practice was variable and occurred at no set time.
Patients in our informal pilot interviews identified vital signs, medication administration, noise, and evening diuretic administration as disrupting their sleep. After the preintervention phase, we spent 4 months designing and implementing the intervention. We solicited opinions from staff, who identified inflexible timing of medications as disruptive. The plan was discussed at routine staff meetings of all shifts.
The intervention, called the Somerville Protocol (Figure 1) created an 8‐hour Quiet Time from 10 PM to 6 AM, when disruptions were minimized. Vital signs were taken 2 hours earlier (6 AM, 2 PM, and 10 PM); routine medication administration was avoided; and noise was reduced. As before, telemetry patients required vital signs every 4 hours. At 10 PM, hallway lights were turned off by a timer while the Lullaby by Brahms played overhead, signaling the start of Quiet Time to staff and patients. Inexpensive sound meters were installed in each nursing area. They flashed warning lights when 60 dBA was exceeded.
A physician and nurse served as champions. Educational signs were posted in the hospitalists' call room and in the nursing areas. The champions used e‐mail and detailed the intervention to staff. Because the staff played an active role in intervention planning, implementation went smoothly.
Results
During the preintervention phase, 334 patients were screened, 294 were eligible, and 54.7% of eligible subjects were enrolled (n = 161). During the intervention phase, 211 patients were screened, 188 were eligible, and 56.3% of eligible patients were enrolled (n = 106). The mean patient age was 60.6 years. The preintervention and intervention groups did not differ significantly in enrollment rate, age, gender, cognitive impairment, surgical status, or hearing deficiencies (Table 1). Over 93% of patients were nonsurgical.
| Preintervention Patients (n = 161) | Intervention Patients (n = 106) | P Values for Difference | |
|---|---|---|---|
| Mean age (years) | 59.1 | 62.95 | P = 0.146 |
| Males, n (%) | 79 (49.1%) | 46 (43.4%) | P = 0.38 |
| Hard of hearing, n (%) (self‐report) | 33/157 (21.0%) | 14/103 (13.6%) | P = 0.128 |
| English‐speaking, n (%) | 134 (83%) | 83 (78.3%) | P = 0.34 |
| Cognitive impairment, n (%) | 4 (2.5%) | 3 (2.8%) | P = 0.88 |
| Surgical patients, n (%) | 10 (6.2%) | 2 (1.8%) | P = 0.089 |
Sedative Use
Preintervention, 31.7% of patients received nighttime as‐needed sedatives, versus 16.0% of the intervention group, a 49.4% reduction (P = 0.0041; 95% confidence interval [CI]: 0.056‐0.26) (Figure 2). In patients aged 65 years or older, 38.2% received nighttime as‐needed sedatives preintervention, and 14.6% did postintervention, a 61.2% reduction (P = 0.0054; 95% CI: 0.084‐0.39).

Questionnaire Results
Preintervention, hospital staff was by far the biggest factor keeping patients awake, with 42.4% of patients reporting it (Figure 3). This dropped to only 25.7% with the intervention, a 39.3% decrease (P = 0.009; 95% CI: 0.0452‐0.2765). Preintervention, 19.2% of patients selected voices as the noise most likely to bother them at night, and this dropped to 9.9% with the intervention, a 48% decrease (P = 0.045; 95% CI: 0.0074‐0.1787). No other significant differences were found.

VSH Sleep Score Results
We found no improvement in any measure of the VSH sleep scale. However, 75% of our patients were unable to use the modified VSH scale, generally because they felt too ill, and were then prompted by the surveyor to choose a number between 1 and 10 that reflected their experience.
Protocol Adherence
Changes in unit routines resulted in complete adherence to the new vital signs schedule and avoidance of routine evening diuretics. The closing of patients' doors did not change. An audit of 40 charts found that the percentage of medication orders written with appropriate flexible timing increased from 82% (n = 228) to 95.5% (n = 200) (P = 0.001; 95% CI: 0.077‐0.192). From 20 to 30 different providers wrote orders during each phase.
Discussion
Our trial found that hospital staff was the factor most responsible for patient sleep disruption, and that behavioral interventions on hospital staff can reduce use of as‐needed sedatives. The only previously reported intervention to reduce sedative use, the HELP strategy, involved a complex intervention requiring extra staff, with adherence ranging from 10% to 75%.19, 20, 25 In contrast, our protocol can be easily replicated at minimal cost.
Our results are consistent with those of Freedman et al.,26 who found that noise was not the primary factor responsible for sleep disruption in ICU patients, and that staff activities were at least as important a factor. The study is also consistent with the nursing home studies in which decreases in noise and light did not improve sleep.22, 23 It refutes the study that showed that most sleep disturbance in medical‐surgical patients comes from noise and sleeping in an unfamiliar bed.4 Our results call into question the use of the VSH scale in hospitalized patients, which was designed for use in healthy subjects.
Limitations of this study were as follows: moderate size, lack of refined measures of disease severity, and, as in previous studies,19, 2123 the lack of randomized concurrent controls. Evaluation of secondary endpoints was limited by lack of validation of the questionnaire with objective observations, and inability to use the modified VSH scale. Self‐reports of sleep may correlate imperfectly with objective measures, such as polysomnography.27
A larger concurrent trial randomizing similar units at multiple hospitals would be ideal. Future research is needed to determine whether improving sleep in the hospital improves other outcomes, such as recovery times, delirium, falls, or cost.
The need to reduce as‐needed sedatives is an important safety issue and similar interventions in other hospitals may be helpful. Simple changes in staff routines and provider prescribing habits can yield significant reductions in sedative use.
Acknowledgements
The authors thank Gertrude Gavin, Steffie Woolhandler, MD, Linda Borodkin, John Brusch, MD, Patricia Crombie, Priscilla Dasse, Glen Dawson, Ben Davenny, Linda Kasten, Judith Krempin, Mark Letzeisen, Carmen Mohan, and Arun Mohan. Linda Kasten, Timothy Schmidt, and Glen Dawson provided statistical analysis. The sound meters (Yacker Trackers, Creative Toys of Colorado) were donated by John Brusch, who has no financial conflict of interest.
- ,,,.Sleep in hospitalized medical patients, Part 1: Factors affecting sleep.J Hosp Med.2008;3:473–482.
- ,.Sleep‐dependent learning and memory consolidation.Neuron.2004;44:121–133.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,.The sleep experience of medical and surgical patients.Clin Nurs Res.2003;12:159–173.
- .Metabolic and endocrine effects of sleep deprivation.Essent Psychopharmacol.2005;6:341–347.
- ,,,,.Sleep loss and REM sleep loss are hyperalgesic.Sleep.2006;29:145–151.
- ,,,.Slow‐wave sleep and the risk of type 2 diabetes in humans.Proc Natl Acad Sci USA.2008;105:1044–1049.
- .Sleep inquiry: a look with fresh eyes.Image J Nurs Sch.1993;25:249–256.
- Berglund B, Lindvall T, Schwela D, eds.Guidelines for Community Noise.World Health Organization;1999:47.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118:3629–3645.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- ,.Sleep in the critically ill patient.Sleep.2006;29:707–716.
- ,,.Sleep quality in hospitalized patients.J Clin Nurs.2005;14:107–113.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30:237–243.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,,.The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program.J Am Geriatr Soc.2000;48:1697–1706.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104:40–48; quiz 48‐49.
- ,,,,,.A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial.J Am Geriatr Soc.2006;54:38–47.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47:430–438.
- ,.Instrumentation to describe subjective sleep characteristics in healthy subjects.Res Nurs Health.1987;10:155–163.
- ,,,,.The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial.Arch Intern Med.2003;163:958–964.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,.When sleep is perceived as wakefulness: an experimental study on state perception during physiological sleep.J Sleep Res.2007;16:346–353.
- ,,,.Sleep in hospitalized medical patients, Part 1: Factors affecting sleep.J Hosp Med.2008;3:473–482.
- ,.Sleep‐dependent learning and memory consolidation.Neuron.2004;44:121–133.
- ,,,,,.An assessment of quality of sleep and the use of drugs with sedating properties in hospitalized adult patients.Health Qual Life Outcomes.2004;2:17.
- ,,,.The sleep experience of medical and surgical patients.Clin Nurs Res.2003;12:159–173.
- .Metabolic and endocrine effects of sleep deprivation.Essent Psychopharmacol.2005;6:341–347.
- ,,,,.Sleep loss and REM sleep loss are hyperalgesic.Sleep.2006;29:145–151.
- ,,,.Slow‐wave sleep and the risk of type 2 diabetes in humans.Proc Natl Acad Sci USA.2008;105:1044–1049.
- .Sleep inquiry: a look with fresh eyes.Image J Nurs Sch.1993;25:249–256.
- Berglund B, Lindvall T, Schwela D, eds.Guidelines for Community Noise.World Health Organization;1999:47.
- ,,,,,.Noise levels in Johns Hopkins Hospital.J Acoust Soc Am.2005;118:3629–3645.
- .Explicit criteria for determining potentially inappropriate medication use by the elderly. An update.Arch Intern Med.1997;157:1531–1536.
- .Delirium in older persons.N Engl J Med.2006;354:1157–1165.
- ,,,,.Sedative hypnotics in older people with insomnia: meta‐analysis of risks and benefits.BMJ.2005;331:1169.
- .Sleep in acute care units.Sleep Breath.2006;10:6–15.
- ,,,,.Quantity and quality of sleep in the surgical intensive care unit: are our patients sleeping?J Trauma.2007;63:1210–1214.
- ,.Sleep in the critically ill patient.Sleep.2006;29:707–716.
- ,,.Sleep quality in hospitalized patients.J Clin Nurs.2005;14:107–113.
- ,.Interactive relationships between hospital patients' noise‐induced stress and other stress with sleep.Heart Lung.2001;30:237–243.
- ,,, et al.A multicomponent intervention to prevent delirium in hospitalized older patients.N Engl J Med.1999;340:669–676.
- ,,,,.The Hospital Elder Life Program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program.J Am Geriatr Soc.2000;48:1697–1706.
- ,,,,.Noise control: a nursing team's approach to sleep promotion.Am J Nurs.2004;104:40–48; quiz 48‐49.
- ,,,,,.A nonpharmacological intervention to improve sleep in nursing home patients: results of a controlled clinical trial.J Am Geriatr Soc.2006;54:38–47.
- ,,,,.The nursing home at night: effects of an intervention on noise, light, and sleep.J Am Geriatr Soc.1999;47:430–438.
- ,.Instrumentation to describe subjective sleep characteristics in healthy subjects.Res Nurs Health.1987;10:155–163.
- ,,,,.The role of adherence on the effectiveness of nonpharmacologic interventions: evidence from the delirium prevention trial.Arch Intern Med.2003;163:958–964.
- ,,.Patient perception of sleep quality and etiology of sleep disruption in the intensive care unit.Am J Respir Crit Care Med.1999;159:1155–1162.
- ,,.When sleep is perceived as wakefulness: an experimental study on state perception during physiological sleep.J Sleep Res.2007;16:346–353.
New Jersey Cracks Down on Never Events
A landmark New Jersey law that increases public disclosure of major preventable medical errors and prohibits charges for certain medical expenses related to those mistakes has hospitalists poised to take a leadership role in patient-safety efforts.
The legislation allows the state to release hospital-specific data on 14 medical mistakes considered by the federal government to be most preventable. It also bans hospitals from charging patients or insurers for follow-up medical costs related to the errors, including pressure ulcers, DVT, and catheter-associated urinary tract infections.
“It’s hard to argue the New Jersey legislation doesn’t make sense,” says hospitalist Niraj Sehgal, MD, MPH, associate chair for quality and safety in the Department of Medicine at the University of California at San Francisco. “After all, if I had the wrong limb operated on, I certainly wouldn’t expect to pay for the subsequent care needs, and nor should our system.”
Dr. Sehgal expects more states to follow New Jersey’s lead, a likely outcome given the fact the AARP is calling the Garden State measure “a national landmark.” The legislation also dovetails with the “no pay for errors” initiative from the Centers for Medicare & Medicaid Services.
Many hospitalists think HM groups are best positioned to spearhead quality and patient-safety efforts tied to the legislation. Hospitalist Vincent Barba, MD, FACP, FHM, medical director for quality improvement with University of Medicine and Dentistry of New Jersey in Newark, says HM leaders just have to seize the opportunity.
“Be the home team,” Dr. Barba says. “Step up. Take a leading role.”
A landmark New Jersey law that increases public disclosure of major preventable medical errors and prohibits charges for certain medical expenses related to those mistakes has hospitalists poised to take a leadership role in patient-safety efforts.
The legislation allows the state to release hospital-specific data on 14 medical mistakes considered by the federal government to be most preventable. It also bans hospitals from charging patients or insurers for follow-up medical costs related to the errors, including pressure ulcers, DVT, and catheter-associated urinary tract infections.
“It’s hard to argue the New Jersey legislation doesn’t make sense,” says hospitalist Niraj Sehgal, MD, MPH, associate chair for quality and safety in the Department of Medicine at the University of California at San Francisco. “After all, if I had the wrong limb operated on, I certainly wouldn’t expect to pay for the subsequent care needs, and nor should our system.”
Dr. Sehgal expects more states to follow New Jersey’s lead, a likely outcome given the fact the AARP is calling the Garden State measure “a national landmark.” The legislation also dovetails with the “no pay for errors” initiative from the Centers for Medicare & Medicaid Services.
Many hospitalists think HM groups are best positioned to spearhead quality and patient-safety efforts tied to the legislation. Hospitalist Vincent Barba, MD, FACP, FHM, medical director for quality improvement with University of Medicine and Dentistry of New Jersey in Newark, says HM leaders just have to seize the opportunity.
“Be the home team,” Dr. Barba says. “Step up. Take a leading role.”
A landmark New Jersey law that increases public disclosure of major preventable medical errors and prohibits charges for certain medical expenses related to those mistakes has hospitalists poised to take a leadership role in patient-safety efforts.
The legislation allows the state to release hospital-specific data on 14 medical mistakes considered by the federal government to be most preventable. It also bans hospitals from charging patients or insurers for follow-up medical costs related to the errors, including pressure ulcers, DVT, and catheter-associated urinary tract infections.
“It’s hard to argue the New Jersey legislation doesn’t make sense,” says hospitalist Niraj Sehgal, MD, MPH, associate chair for quality and safety in the Department of Medicine at the University of California at San Francisco. “After all, if I had the wrong limb operated on, I certainly wouldn’t expect to pay for the subsequent care needs, and nor should our system.”
Dr. Sehgal expects more states to follow New Jersey’s lead, a likely outcome given the fact the AARP is calling the Garden State measure “a national landmark.” The legislation also dovetails with the “no pay for errors” initiative from the Centers for Medicare & Medicaid Services.
Many hospitalists think HM groups are best positioned to spearhead quality and patient-safety efforts tied to the legislation. Hospitalist Vincent Barba, MD, FACP, FHM, medical director for quality improvement with University of Medicine and Dentistry of New Jersey in Newark, says HM leaders just have to seize the opportunity.
“Be the home team,” Dr. Barba says. “Step up. Take a leading role.”
In the Literature: Research You Need to Know
Clinical question: Does aspirin prevent cardiovascular events in patients with peripheral artery disease (PAD)?
Background: Evidence that aspirin decreases risk of cardiovascular events in patients with symptomatic coronary artery disease and cerebrovascular disease has led to a recommendation of aspirin as secondary prevention in PAD. Evidence for its efficacy in this context is not established.
Study design: Meta-analysis.
Setting: Multiple study sites.
Synopsis: The investigators looked at 18 randomized controlled trials involving 5,269 participants, 2,823 of whom received aspirin, including 1,516 as monotherapy and 2,446 controls. The primary endpoint investigated was cardiovascular events.
This meta-analysis did not show a statistical benefit in cardiovascular event reduction (8.9% vs. 11%) in aspirin therapy in patients with peripheral artery disease, but it did show a decrease in the secondary endpoint of nonfatal strokes. A subset analysis of aspirin monotherapy versus placebo showed a nonsignificant decrease in the primary endpoint.
The studies had a short timeline with few cardiovascular events, so conclusions cannot be drawn about longer timeframes. Furthermore, some of the included studies were not designed to measure cardiovascular events. Finally, the study was not powered to detect differences less than 25%.
Bottom line: In patients with PAD, aspirin might not decrease the incidence of cardiovascular events.
Citation: Berger JT, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301(18):1909-1919.
—Reviewed for The Hospitalist by Steven Deitelzweig, MD, MMM, FHM; Frank Wharton, MD, FACP; Renee Meadows, MD, FHM; Srinivas Vuppala, MD; Kevin Hude, MD; Doris Lin, MD; Damodar Kumbala, MD, Department of Hospital Medicine, Ochsner Medical Center, New Orleans
Clinical question: Does aspirin prevent cardiovascular events in patients with peripheral artery disease (PAD)?
Background: Evidence that aspirin decreases risk of cardiovascular events in patients with symptomatic coronary artery disease and cerebrovascular disease has led to a recommendation of aspirin as secondary prevention in PAD. Evidence for its efficacy in this context is not established.
Study design: Meta-analysis.
Setting: Multiple study sites.
Synopsis: The investigators looked at 18 randomized controlled trials involving 5,269 participants, 2,823 of whom received aspirin, including 1,516 as monotherapy and 2,446 controls. The primary endpoint investigated was cardiovascular events.
This meta-analysis did not show a statistical benefit in cardiovascular event reduction (8.9% vs. 11%) in aspirin therapy in patients with peripheral artery disease, but it did show a decrease in the secondary endpoint of nonfatal strokes. A subset analysis of aspirin monotherapy versus placebo showed a nonsignificant decrease in the primary endpoint.
The studies had a short timeline with few cardiovascular events, so conclusions cannot be drawn about longer timeframes. Furthermore, some of the included studies were not designed to measure cardiovascular events. Finally, the study was not powered to detect differences less than 25%.
Bottom line: In patients with PAD, aspirin might not decrease the incidence of cardiovascular events.
Citation: Berger JT, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301(18):1909-1919.
—Reviewed for The Hospitalist by Steven Deitelzweig, MD, MMM, FHM; Frank Wharton, MD, FACP; Renee Meadows, MD, FHM; Srinivas Vuppala, MD; Kevin Hude, MD; Doris Lin, MD; Damodar Kumbala, MD, Department of Hospital Medicine, Ochsner Medical Center, New Orleans
Clinical question: Does aspirin prevent cardiovascular events in patients with peripheral artery disease (PAD)?
Background: Evidence that aspirin decreases risk of cardiovascular events in patients with symptomatic coronary artery disease and cerebrovascular disease has led to a recommendation of aspirin as secondary prevention in PAD. Evidence for its efficacy in this context is not established.
Study design: Meta-analysis.
Setting: Multiple study sites.
Synopsis: The investigators looked at 18 randomized controlled trials involving 5,269 participants, 2,823 of whom received aspirin, including 1,516 as monotherapy and 2,446 controls. The primary endpoint investigated was cardiovascular events.
This meta-analysis did not show a statistical benefit in cardiovascular event reduction (8.9% vs. 11%) in aspirin therapy in patients with peripheral artery disease, but it did show a decrease in the secondary endpoint of nonfatal strokes. A subset analysis of aspirin monotherapy versus placebo showed a nonsignificant decrease in the primary endpoint.
The studies had a short timeline with few cardiovascular events, so conclusions cannot be drawn about longer timeframes. Furthermore, some of the included studies were not designed to measure cardiovascular events. Finally, the study was not powered to detect differences less than 25%.
Bottom line: In patients with PAD, aspirin might not decrease the incidence of cardiovascular events.
Citation: Berger JT, Krantz MJ, Kittelson JM, Hiatt WR. Aspirin for the prevention of cardiovascular events in patients with peripheral artery disease: a meta-analysis of randomized trials. JAMA. 2009;301(18):1909-1919.
—Reviewed for The Hospitalist by Steven Deitelzweig, MD, MMM, FHM; Frank Wharton, MD, FACP; Renee Meadows, MD, FHM; Srinivas Vuppala, MD; Kevin Hude, MD; Doris Lin, MD; Damodar Kumbala, MD, Department of Hospital Medicine, Ochsner Medical Center, New Orleans
Progesterone use in management of secondary amenorrhea
An e-newsletter focusing on challenging case studies
Progesterone use in management of secondary amenorrhea
Read the following case study and earn
FREE 0.5 CME/CE CREDIT
Expiration date: July 31, 2009
Estimated time to complete activity: 0.5 hour
Target: Physicians, physician assistants and nurse practitioners
Supported by an educational grant from Solvay Pharmaceuticals, Inc.
Sponsored by the American Society for Reproductive Medicine.
Click here to take the FREE CME/CE test online for 0.5 credits
Nicole is 27 years old, gravida 0, with a long history of obesity and great difficulty losing weight. She reports having irregular menstrual cycles ranging from 45 days to 6 months apart. Her last menstrual period was 5 months ago, when she had a heavy menses that lasted 2 weeks. Her body mass index is 42 kg/m2, which is considered morbid obesity. Nicole has a large abdominal pannus and normal blood pressure. She has scattered acne lesions on her face and upper back and acanthosis nigricans, a dark pigmentation around the neck and the axilla. Acanthosis nigricans is a biomarker for insulin resistance and high circulating insulin levels.
Nicole’s pelvic exam showed a normal vagina and cervix, but it was not possible to palpate her uterus or ovaries because of her abdominal girth. Transvaginal ultrasound found an endometrial thickness of 22 mm. Her ovaries appeared to be multicystic but had normal ovarian volumes. We performed an endometrial biopsy because of concerns about the long duration of unopposed estrogen exposure. The biopsy revealed complex hyperplasia with no atypia.
A lipid profile demonstrated cholesterol 245 mg/dL low-density lipoprotein cholesterol of 180 mg/dL and triglyceride levels of 259 mg/dL. This patient demonstrates many features we see with increasing frequency in reproductive-aged women as we deal with the obesity epidemic.
A complicated diagnosis
Nicole’s abdominal obesity, high triglycerides, and evidence of insulin-resistance constituted metabolic syndrome. Although there are many different criteria for this, we used those of the National Cholesterol Education Program (TABLE).1
| Components of Metabolic Syndrome Related to Cardiovascular Disease
Source: Grundy SM, et al. Circulation. 2002;106:3143-3421. |
Long-term prognosis?
If she is not treated, Nicole would be unable to achieve pregnancy because she does not ovulate and she may have an increased risk of endometrial cancer. Her obesity would eventually cause increasing insulin levels, exhaustion of her pancreatic insulin secretion, and type 2 diabetes. Her obesity might also result in osteoarthritis and difficulty with mobility. Her lipid levels confer increased risk of hypertension and cardiovascular events.
Selecting a treatment strategy: Considerations
If this patient does not desire pregnancy, short-term treatment for endometrial hyperplasia involves administration of progesterone or progestins for at least 2 weeks per month for 3 months, with a follow-up endometrial biopsy.
Nicole also needs protection against endometrial hyperplasia even after this episode is addressed. Oral contraceptives are frequently used for long-term treatment, although there might be concern about the risk of deep vein thrombosis (DVT), particularly in obese or morbidly obese patients and older patients who smoke.
If this patient were not a candidate for oral contraceptives, progesterone or a progestin would typically be administered for approximately 2 weeks each month to prevent recurrence of the hyperplasia. Patients who do not desire a monthly withdrawal bleed can take progesterone every 2 to 3 months. Patients who are being treated with a progestogen for amenorrhea and hyperplasia may occasionally ovulate. They should be counseled that an alternative form of contraception, such as condoms, is needed to avoid pregnancy.
An intrauterine device (IUD) for this patient could also be considered for this patient. We have individual patients using a levonorgestrel-containing IUD. One challenge we have faced with some of our obese patients is difficultly accessing the cervix for placement.
Case study follow-up
Nicole received norethindrone,2,3 5 mg, a strong progestin, for 2 weeks and had a withdrawal flow for 3 months in a row. Her repeat endometrial biopsy showed a normal endometrium. She still did not ovulate, and since she was not sexually active at that time, she elected to use progesterone, 200 mg, for 14 days every 2 months. We treated her acne with topical clindamycin gel and she joined our supervised diet and exercise program. She is considering bariatric surgery but must participate in the lifestyle program for at least 6 months in order to qualify.
| Click here to take the FREE CME/CE test online for 0.5 credits References 1. Grundy SM, Becker D, Clark LT, et al, for the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation.2002;106:3143-3421. 2. King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril.1986;46(6):1062-1066. 3. Whitehead MI, Hillard TC, Crook D. The role and use of progestogens. Obstet Gynecol.1990;75(4)suppl):59S-76S. Additional newsletters with free CME/CE: Progesterone use in assisted reproductive technology Sexuality, Reproduction & Menopause © 2009 Quadrant HealthCom Inc. |
An e-newsletter focusing on challenging case studies
Progesterone use in management of secondary amenorrhea
Read the following case study and earn
FREE 0.5 CME/CE CREDIT
Expiration date: July 31, 2009
Estimated time to complete activity: 0.5 hour
Target: Physicians, physician assistants and nurse practitioners
Supported by an educational grant from Solvay Pharmaceuticals, Inc.
Sponsored by the American Society for Reproductive Medicine.
Click here to take the FREE CME/CE test online for 0.5 credits
Nicole is 27 years old, gravida 0, with a long history of obesity and great difficulty losing weight. She reports having irregular menstrual cycles ranging from 45 days to 6 months apart. Her last menstrual period was 5 months ago, when she had a heavy menses that lasted 2 weeks. Her body mass index is 42 kg/m2, which is considered morbid obesity. Nicole has a large abdominal pannus and normal blood pressure. She has scattered acne lesions on her face and upper back and acanthosis nigricans, a dark pigmentation around the neck and the axilla. Acanthosis nigricans is a biomarker for insulin resistance and high circulating insulin levels.
Nicole’s pelvic exam showed a normal vagina and cervix, but it was not possible to palpate her uterus or ovaries because of her abdominal girth. Transvaginal ultrasound found an endometrial thickness of 22 mm. Her ovaries appeared to be multicystic but had normal ovarian volumes. We performed an endometrial biopsy because of concerns about the long duration of unopposed estrogen exposure. The biopsy revealed complex hyperplasia with no atypia.
A lipid profile demonstrated cholesterol 245 mg/dL low-density lipoprotein cholesterol of 180 mg/dL and triglyceride levels of 259 mg/dL. This patient demonstrates many features we see with increasing frequency in reproductive-aged women as we deal with the obesity epidemic.
A complicated diagnosis
Nicole’s abdominal obesity, high triglycerides, and evidence of insulin-resistance constituted metabolic syndrome. Although there are many different criteria for this, we used those of the National Cholesterol Education Program (TABLE).1
| Components of Metabolic Syndrome Related to Cardiovascular Disease
Source: Grundy SM, et al. Circulation. 2002;106:3143-3421. |
Long-term prognosis?
If she is not treated, Nicole would be unable to achieve pregnancy because she does not ovulate and she may have an increased risk of endometrial cancer. Her obesity would eventually cause increasing insulin levels, exhaustion of her pancreatic insulin secretion, and type 2 diabetes. Her obesity might also result in osteoarthritis and difficulty with mobility. Her lipid levels confer increased risk of hypertension and cardiovascular events.
Selecting a treatment strategy: Considerations
If this patient does not desire pregnancy, short-term treatment for endometrial hyperplasia involves administration of progesterone or progestins for at least 2 weeks per month for 3 months, with a follow-up endometrial biopsy.
Nicole also needs protection against endometrial hyperplasia even after this episode is addressed. Oral contraceptives are frequently used for long-term treatment, although there might be concern about the risk of deep vein thrombosis (DVT), particularly in obese or morbidly obese patients and older patients who smoke.
If this patient were not a candidate for oral contraceptives, progesterone or a progestin would typically be administered for approximately 2 weeks each month to prevent recurrence of the hyperplasia. Patients who do not desire a monthly withdrawal bleed can take progesterone every 2 to 3 months. Patients who are being treated with a progestogen for amenorrhea and hyperplasia may occasionally ovulate. They should be counseled that an alternative form of contraception, such as condoms, is needed to avoid pregnancy.
An intrauterine device (IUD) for this patient could also be considered for this patient. We have individual patients using a levonorgestrel-containing IUD. One challenge we have faced with some of our obese patients is difficultly accessing the cervix for placement.
Case study follow-up
Nicole received norethindrone,2,3 5 mg, a strong progestin, for 2 weeks and had a withdrawal flow for 3 months in a row. Her repeat endometrial biopsy showed a normal endometrium. She still did not ovulate, and since she was not sexually active at that time, she elected to use progesterone, 200 mg, for 14 days every 2 months. We treated her acne with topical clindamycin gel and she joined our supervised diet and exercise program. She is considering bariatric surgery but must participate in the lifestyle program for at least 6 months in order to qualify.
| Click here to take the FREE CME/CE test online for 0.5 credits References 1. Grundy SM, Becker D, Clark LT, et al, for the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation.2002;106:3143-3421. 2. King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril.1986;46(6):1062-1066. 3. Whitehead MI, Hillard TC, Crook D. The role and use of progestogens. Obstet Gynecol.1990;75(4)suppl):59S-76S. Additional newsletters with free CME/CE: Progesterone use in assisted reproductive technology Sexuality, Reproduction & Menopause © 2009 Quadrant HealthCom Inc. |
An e-newsletter focusing on challenging case studies
Progesterone use in management of secondary amenorrhea
Read the following case study and earn
FREE 0.5 CME/CE CREDIT
Expiration date: July 31, 2009
Estimated time to complete activity: 0.5 hour
Target: Physicians, physician assistants and nurse practitioners
Supported by an educational grant from Solvay Pharmaceuticals, Inc.
Sponsored by the American Society for Reproductive Medicine.
Click here to take the FREE CME/CE test online for 0.5 credits
Nicole is 27 years old, gravida 0, with a long history of obesity and great difficulty losing weight. She reports having irregular menstrual cycles ranging from 45 days to 6 months apart. Her last menstrual period was 5 months ago, when she had a heavy menses that lasted 2 weeks. Her body mass index is 42 kg/m2, which is considered morbid obesity. Nicole has a large abdominal pannus and normal blood pressure. She has scattered acne lesions on her face and upper back and acanthosis nigricans, a dark pigmentation around the neck and the axilla. Acanthosis nigricans is a biomarker for insulin resistance and high circulating insulin levels.
Nicole’s pelvic exam showed a normal vagina and cervix, but it was not possible to palpate her uterus or ovaries because of her abdominal girth. Transvaginal ultrasound found an endometrial thickness of 22 mm. Her ovaries appeared to be multicystic but had normal ovarian volumes. We performed an endometrial biopsy because of concerns about the long duration of unopposed estrogen exposure. The biopsy revealed complex hyperplasia with no atypia.
A lipid profile demonstrated cholesterol 245 mg/dL low-density lipoprotein cholesterol of 180 mg/dL and triglyceride levels of 259 mg/dL. This patient demonstrates many features we see with increasing frequency in reproductive-aged women as we deal with the obesity epidemic.
A complicated diagnosis
Nicole’s abdominal obesity, high triglycerides, and evidence of insulin-resistance constituted metabolic syndrome. Although there are many different criteria for this, we used those of the National Cholesterol Education Program (TABLE).1
| Components of Metabolic Syndrome Related to Cardiovascular Disease
Source: Grundy SM, et al. Circulation. 2002;106:3143-3421. |
Long-term prognosis?
If she is not treated, Nicole would be unable to achieve pregnancy because she does not ovulate and she may have an increased risk of endometrial cancer. Her obesity would eventually cause increasing insulin levels, exhaustion of her pancreatic insulin secretion, and type 2 diabetes. Her obesity might also result in osteoarthritis and difficulty with mobility. Her lipid levels confer increased risk of hypertension and cardiovascular events.
Selecting a treatment strategy: Considerations
If this patient does not desire pregnancy, short-term treatment for endometrial hyperplasia involves administration of progesterone or progestins for at least 2 weeks per month for 3 months, with a follow-up endometrial biopsy.
Nicole also needs protection against endometrial hyperplasia even after this episode is addressed. Oral contraceptives are frequently used for long-term treatment, although there might be concern about the risk of deep vein thrombosis (DVT), particularly in obese or morbidly obese patients and older patients who smoke.
If this patient were not a candidate for oral contraceptives, progesterone or a progestin would typically be administered for approximately 2 weeks each month to prevent recurrence of the hyperplasia. Patients who do not desire a monthly withdrawal bleed can take progesterone every 2 to 3 months. Patients who are being treated with a progestogen for amenorrhea and hyperplasia may occasionally ovulate. They should be counseled that an alternative form of contraception, such as condoms, is needed to avoid pregnancy.
An intrauterine device (IUD) for this patient could also be considered for this patient. We have individual patients using a levonorgestrel-containing IUD. One challenge we have faced with some of our obese patients is difficultly accessing the cervix for placement.
Case study follow-up
Nicole received norethindrone,2,3 5 mg, a strong progestin, for 2 weeks and had a withdrawal flow for 3 months in a row. Her repeat endometrial biopsy showed a normal endometrium. She still did not ovulate, and since she was not sexually active at that time, she elected to use progesterone, 200 mg, for 14 days every 2 months. We treated her acne with topical clindamycin gel and she joined our supervised diet and exercise program. She is considering bariatric surgery but must participate in the lifestyle program for at least 6 months in order to qualify.
| Click here to take the FREE CME/CE test online for 0.5 credits References 1. Grundy SM, Becker D, Clark LT, et al, for the National Cholesterol Education Program Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation.2002;106:3143-3421. 2. King RJ, Whitehead MI. Assessment of the potency of orally administered progestins in women. Fertil Steril.1986;46(6):1062-1066. 3. Whitehead MI, Hillard TC, Crook D. The role and use of progestogens. Obstet Gynecol.1990;75(4)suppl):59S-76S. Additional newsletters with free CME/CE: Progesterone use in assisted reproductive technology Sexuality, Reproduction & Menopause © 2009 Quadrant HealthCom Inc. |
Trivialization of Diagnosis
Historically, diagnosis has been central to patient care. Making the correct diagnosis serves as a guide to the choice of treatment, permits assessment of prognosis, and indicates what complications to expect. Arriving at the correct diagnosis has been a major goalthe Holy Grail, as it were. Accurate diagnosis continues to be a major focus of medical practice, and accurate diagnoses are routinely made every day. Still, many experienced clinicians have the impression that in recent years the primacy of diagnosis has been coming under attack from several sources.
A decade ago, Thomas Szasz pointed out that disease is a fact of nature, while diagnosis is man made.1 The noun diagnosis is derived from the Greek verb diagignoskeinindicating knowledge attained through analysis. As defined in Merriam‐Webster's Collegiate Dictionary, the diagnosis essentially means the conclusion arrived at by the art of identifying a disease. It is the product of an intellectual effort of a particular analytic type. The response to the question What is the diagnosis? has been the name of the specific disease entity with which the patient is afflicted.
Disease entities represent coherent, organizing concepts.2 A specific disease is a condition with characteristic manifestationsclinical, histologic, or pathophysiologic. If untreated, it results in dysfunction or, in some cases, death. Differentiation of one disease from another is enhanced when there is some sort of understanding, even if incomplete, of the specific pathophysiology at play. Admittedly, concepts of what constitute specific disease entities are not fixed; they evolve with time. Not all diseases have been identified. The underlying etiology may or may not be known. Nonetheless, diseases are recognized as specific entities, distinct from other diseases. Thus, anemia is not regarded as a disease, while pernicious anemia and iron deficiency anemia are diseases. Fever is not a disease, while typhoid fever is. Arthritis is not a disease, while gonococcal arthritis is.
Billable Terms Are Replacing Traditional Medical Diagnoses
The term diagnosis has been redefined to comply with the need to enter a diagnosis for billing purposes. Use of this term for this purpose has confused the issue. Diagnoses entered for such purposes are largely derived from International Classification of Diseases (ICD) lists.3 However, the ICD was not intended to definitively identify underlying diseases, nor to serve as a guide to management and prognostication. The 6th revision of the ICD in 1948, the first revision to be widely employed, was designed for epidemiologic purposes and achieved widespread use to obtain mortality and morbidity statistics.4 It was subsequently also used as a tool to index hospital medical records.
Significantly, it was also employed for billing purposes, with far‐reaching pernicious consequences. Although the ICD purports to be a list of diseases, it actually includes symptoms and signs. Consequently, in the billing context, diagnosis no longer necessarily refers to specific disease states; it now refers to billable termsoften the manifestation that was responsible for the patient seeking medical assistance. Far from being the product of an intellectual effort, it is often merely a justification for submitting a bill. Examples of such diagnoses are shown in Table 1. Many of them represent symptoms, signs, or laboratory abnormalities. The importance of accurate medical diagnosis has been cheapened by this change. The effect is to devalue diagnosisto lessen its status as the Holy Grail.
| Abdominal pain | General symptoms | Special symptom |
| Abnormal blood test | Immune disorders | Splenomegaly |
| Back disorder | Joint disorder | Throat pain |
| Coagulation defects | Myoneural disorder | Urinary symptoms |
| Diseases of esophagus | Otalgia | Visual disturbance |
| Eye disorders | Pain in joint | Vomiting |
| Fluid/electrolyte disorders | Right lower quadrant mass | Wheezing |
The effect of this on trainees is invidious, and predictable. The traditional meaning of diagnosis is being replaced in our minds. Physicians in training are tempted to deceive themselves into believing that they have arrived at an understanding of what they are dealing with when they enter such a diagnosis. After all, have they not responded to the question: what is the diagnosis?
We do not mean to imply that physicians are doing anything wrong by entering ICD terms for billing purposes. What must be done for billing purposes must be done. It is important to be aware, however, and to continually remind ourselves, that what has been entered for this purpose is often not a true medical diagnosis.
Further, when the diagnosis is not yet known, it is not possible to enter a true diagnosis. There is no way to say I don't know. It would be preferable to simply admit that the diagnosis is not yet established, as a medical resident has recently emphasized.5
Diagnosis Often Gets Short Shrift Because of the Perceived Urgency of Discharge
The emphasis on diagnosis several generations ago may have resulted, at least in part, from the relative paucity of effective therapeutic interventions before the 1930s. Things have changed; therapeutic capabilities are much more powerful now. Making the correct diagnosis seems to have lost its urgency. Instead of the major question being what is the diagnosis? it now is often what do we do now? The diagnosis is often an afterthought. Indeed, it is sometimes not even mentioned in discharge summaries, where, not uncommonly, one sees nondiagnoses such as blood in stool or polyarthritis.
In addition, we are under pressure to shorten the inpatient stay of hospitalized patients. At least a portion of the public is aware of this; thus, it has been noted in the New York Times that: The pressure to get patients out of the hospital rapidly can focus medical attention on treatment rather than diagnosis.6 We commonly seek to ameliorate the patients' status to permit discharge before (or often without) learning what we are dealing with. Sometimes one senses that the primary question has become how soon can we discharge this patient?
A price is paid for this. In the absence of a valid diagnosis, patients may be subjected to a broad array of nonessential investigations and therapeutic interventions, each with its own possible complications. Patients are often discharged without a diagnosis having been made, presenting a serious challenge to outpatient physicians who are left to manage them without a clear idea of what they are dealing with. It often falls to the outpatient physicians to make the diagnosis. This is somewhat problematic, since they themselves are under harsh time pressure. Patients often require rehospitalization for the same as‐yet‐undiagnosed condition.
The Problem‐Oriented Record Poses Problems
The widespread use of the problem‐oriented record, originated by Lawrence Weed,7 has led to problems of its own.8 It has evolved, away from its original intent. In practice, its major emphasis often seems to be on identification of problems and tracking their progress, rather than on synthesis. This often leads to muddy rather than clear diagnostic thinking. Assessments and progress notes frequently consist of lists of symptoms, organs, abnormal laboratory findings, or even medical specialties. The net effect is often fragmented thinkingas Weed7 put it, failure to integrate findings into a single entity. Synthesizing diverse findings into a single entity, when possible, is necessary to define a diagnosis. Failure to do so may have serious consequences. In a recent study of diagnostic errors in internal medicine, cognitive errors were frequently found to contribute to such errors.9 The most common cognitive problem was faulty synthesis. How much worse than faulty synthesis is failure to synthesize at all!
Presumptive Diagnoses, Even if Incorrect, Metamorphose into Established Diagnoses
We must often treat empirically. When there is no firm diagnosis, presumptive diagnoses must be made and acted upon. Unfortunately, there are not always mechanisms for the physician to make it clear that his or her diagnosis is only presumptive. (A common example is acute viral syndrome, generally an educated guess.) All too often, presumptive diagnoses are entered, without qualification, as definitive diagnoses, and then achieve immortality. Thus, if a patient is incorrectly diagnosed as having rheumatoid arthritis, all subsequent presentations will start: A so‐and‐so year‐old woman with rheumatoid arthritis for many years Presumptive diagnoses are frequently not questioned. It is easier to assume that they were arrived at after due consideration. Once entered in the medical record, they may be difficult to remove.
It is true that the need to arrive at a precise diagnosis is less pressing for some medical specialties than for others. Emergency physicians, critical care physicians, and frequently, surgeons, must commonly act on the basis of presumptive diagnoses. In contrast, internists, family physicians, psychiatrists, and indeed all physicians who care for patients with chronic illnesses can, with time, be expected to sort out accurate from inaccurate presumptive diagnoses.
A specific example of the problem of presumptive diagnosis is of interest. It is not uncommon, following a first encounter, for a diagnosis to be entered based on the patient's history alone. While such diagnoses are frequently correct, they are not invariably correct. The patient may have arrived at the conclusion herself; she may have misunderstood what she was told by her physician, or her physician may have been in error. Such inaccurate diagnoses also often achieve immortality in the medical record.
Apparent Disparaging of the Importance of Diagnosis
Further trivialization has come from a number of publications expressing concerns about the importance of diagnosis. Thus we read that there are negative consequences of emphasis on diagnosis. When we know what is wrong, we focus less on the individual and more on the disease.10 In his recent book Our Present Complaint. American Medicine, Then and Now, the scholar C.E. Rosenberg11 includes a chapter with the provocative title The Tyranny of Diagnosis. He points out that even a century ago the fear was expressed that burgeoning scientific medicine would lead to denigration of physicians' holistic and intuitive skills.11 Other authors maintain that firm diagnoses may be misleading, since many diseases are a matter of degree in a continuuma spectrumthat are best defined employing a statistical model of risk prediction.12 The suggestion is made that the usefulness of diagnostic tests should not be related to the presence or absence of a disease, but rather to whether they influence outcome.13
Scientific medicine is focused on diagnosis. Denigration of diagnosis has often come, as a philosophical posture, from opponents of reductionist thinking. As Rosenberg11 points out: It has become fashionable among humanistic and social science‐oriented commentators to dwell on the distinction between illness and disease, between the patient's felt experience and the constructions placed on that experience by the world of medicine. Their opposition, he feels, reflects the value‐laden mutual incompatibility (real or apparent) of art and science, of holism and reductionism.2
It is true that medicine is more than just biology. There is a great deal to be said for the view that scientific medicine tends to deemphasize the humanistic, holistic aspects of medical practice. However, despite all these concerns, most physiciansand, to be fair, most criticsagree that making an accurate diagnosis is important. Thus, though the title of his relevant chapter is The Tyranny of Diagnosis, Rosenberg11 states: I might just as well have used the term indispensability. Indeed, the opening words of that chapter are: Diagnosis has always played a pivotal role in medicine.11 Other authors cited above issue this disclaimer: We are not against diagnosis. Diagnosis does and always will play a central role in clinical medicine.12
The importance of diagnosis is underscored by the vigorous debate about how to assess diagnostic tests;14 apparently, diagnosis does indeed matter. While it is true that diagnoses are not always precise, objective, and quantifiable,10 abundant evidence points to the unavoidable conclusion that identifying the patient's disease is heuristically useful; that is, it works.2 The track record of modern scientific medicine in improving mortality and morbidity speaks for itself. It hardly seems necessary to defend it. In addition to representing a valuable intellectual challenge in its own right, diagnosis is pivotal to the scientific mission of medicine.
What Can Be Done?
The net effect of all these forces: the use of billable terms as diagnoses, the pressures of managed care, fragmented problem lists, persistence of incorrect presumptive diagnoses in medical records, and antireductionist criticisms is to encourage sloppy diagnostic thinking in some physicians. What can be done to emphasize the proper use of differential diagnosis in arriving at a definitive diagnosis? What can be done to underscore the importance of differentiating between presumptive and definitive diagnoses? Most importantly, how can we instill the respect for the intellectual honesty necessary to acquire and retain these skills?
Above all, we should relentlessly impress on our students and trainees the importance of arriving at an accurate definitive diagnosis. They should be aware that the job is only half done if the diagnosis has not been made. We should do this repeatedly, both by word and by example. We ourselves must display intellectual honesty.
In addition, we ought to be able to enter diagnosis uncertain, so coded, or to append the phrase cause unknown after the manifestation of concern, when we don't really know what is going on. We should routinely indicate when a diagnosis is merely presumptive. Perhaps we need a way to indicate: This diagnosis is definitive or to indicate the specific evidence that led to the diagnosis (eg, biopsy, laboratory result, radiographic finding). Finally, we need to correct the current confusion between diseases and billable terms, to differentiate the disease from the symptom, perhaps by labeling ICD‐9‐CM codes simply as billing codes, with a separate entry for actual medical diagnoses.
Although powerful historical forces have brought us to this state, we believe that arriving at the correct diagnosis is at least as important now as it has been in the past, and that its primacy should be recognized, celebrated, and fought for. We owe our patients no less.
Acknowledgements
The authors thank Drs. James Pile, Neal Dawson, and David Samols for their helpful suggestions.
- .What counts as disease? Rationales and rationalizations for treatment.Forsch Komplementarmed.1998;5(suppl S1):40–46.
- .What is disease? In memory of Owsei Temkin.Bull Hist Med.2003;77:491–505.
- World Health Organization (WHO). International Classification of Diseases (ICD). Available at: http://www.who.int/classifications/icd/en. Accessed June2009.
- World Health Organization (WHO). History of the development of the ICD. Available at: http://www.who.int/classifications/icd/en/HistoryOfICD. pdf. Accessed June2009.
- .Living unlabeled—diagnosis and disorder.N Engl J Med.2008;359:1650–1653.
- . Poison Pill. New York Times Magazine. 2008: 24–26. Available at: http://www.nytimes.com/2008/04/13/magazine/13wwln‐diagnosis‐t.html. Accessed June2009.
- .Medical records that guide and teach.N Engl J Med.1968;278:593–600.
- .Clear writing, clear thinking and the disappearing art of the problem list.J Hosp Med.2007;2:199–202.
- , , .Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- . The Tyranny of Diagnosis. New York Times. Available at: http://www.nytimes.com/2008/09/19/health/chen9‐18.html?partner=rssnyt2007.
- , , .Against diagnosis.Ann Intern Med.2008;149:200–203.
- , , .A philosophical perspective supports the need for patient‐outcome studies in diagnostic test evaluation.J Clin Epidemiol.2009;62:58–61.
- , .Evidence based diagnostics.BMJ.2005;330:724–726.
Historically, diagnosis has been central to patient care. Making the correct diagnosis serves as a guide to the choice of treatment, permits assessment of prognosis, and indicates what complications to expect. Arriving at the correct diagnosis has been a major goalthe Holy Grail, as it were. Accurate diagnosis continues to be a major focus of medical practice, and accurate diagnoses are routinely made every day. Still, many experienced clinicians have the impression that in recent years the primacy of diagnosis has been coming under attack from several sources.
A decade ago, Thomas Szasz pointed out that disease is a fact of nature, while diagnosis is man made.1 The noun diagnosis is derived from the Greek verb diagignoskeinindicating knowledge attained through analysis. As defined in Merriam‐Webster's Collegiate Dictionary, the diagnosis essentially means the conclusion arrived at by the art of identifying a disease. It is the product of an intellectual effort of a particular analytic type. The response to the question What is the diagnosis? has been the name of the specific disease entity with which the patient is afflicted.
Disease entities represent coherent, organizing concepts.2 A specific disease is a condition with characteristic manifestationsclinical, histologic, or pathophysiologic. If untreated, it results in dysfunction or, in some cases, death. Differentiation of one disease from another is enhanced when there is some sort of understanding, even if incomplete, of the specific pathophysiology at play. Admittedly, concepts of what constitute specific disease entities are not fixed; they evolve with time. Not all diseases have been identified. The underlying etiology may or may not be known. Nonetheless, diseases are recognized as specific entities, distinct from other diseases. Thus, anemia is not regarded as a disease, while pernicious anemia and iron deficiency anemia are diseases. Fever is not a disease, while typhoid fever is. Arthritis is not a disease, while gonococcal arthritis is.
Billable Terms Are Replacing Traditional Medical Diagnoses
The term diagnosis has been redefined to comply with the need to enter a diagnosis for billing purposes. Use of this term for this purpose has confused the issue. Diagnoses entered for such purposes are largely derived from International Classification of Diseases (ICD) lists.3 However, the ICD was not intended to definitively identify underlying diseases, nor to serve as a guide to management and prognostication. The 6th revision of the ICD in 1948, the first revision to be widely employed, was designed for epidemiologic purposes and achieved widespread use to obtain mortality and morbidity statistics.4 It was subsequently also used as a tool to index hospital medical records.
Significantly, it was also employed for billing purposes, with far‐reaching pernicious consequences. Although the ICD purports to be a list of diseases, it actually includes symptoms and signs. Consequently, in the billing context, diagnosis no longer necessarily refers to specific disease states; it now refers to billable termsoften the manifestation that was responsible for the patient seeking medical assistance. Far from being the product of an intellectual effort, it is often merely a justification for submitting a bill. Examples of such diagnoses are shown in Table 1. Many of them represent symptoms, signs, or laboratory abnormalities. The importance of accurate medical diagnosis has been cheapened by this change. The effect is to devalue diagnosisto lessen its status as the Holy Grail.
| Abdominal pain | General symptoms | Special symptom |
| Abnormal blood test | Immune disorders | Splenomegaly |
| Back disorder | Joint disorder | Throat pain |
| Coagulation defects | Myoneural disorder | Urinary symptoms |
| Diseases of esophagus | Otalgia | Visual disturbance |
| Eye disorders | Pain in joint | Vomiting |
| Fluid/electrolyte disorders | Right lower quadrant mass | Wheezing |
The effect of this on trainees is invidious, and predictable. The traditional meaning of diagnosis is being replaced in our minds. Physicians in training are tempted to deceive themselves into believing that they have arrived at an understanding of what they are dealing with when they enter such a diagnosis. After all, have they not responded to the question: what is the diagnosis?
We do not mean to imply that physicians are doing anything wrong by entering ICD terms for billing purposes. What must be done for billing purposes must be done. It is important to be aware, however, and to continually remind ourselves, that what has been entered for this purpose is often not a true medical diagnosis.
Further, when the diagnosis is not yet known, it is not possible to enter a true diagnosis. There is no way to say I don't know. It would be preferable to simply admit that the diagnosis is not yet established, as a medical resident has recently emphasized.5
Diagnosis Often Gets Short Shrift Because of the Perceived Urgency of Discharge
The emphasis on diagnosis several generations ago may have resulted, at least in part, from the relative paucity of effective therapeutic interventions before the 1930s. Things have changed; therapeutic capabilities are much more powerful now. Making the correct diagnosis seems to have lost its urgency. Instead of the major question being what is the diagnosis? it now is often what do we do now? The diagnosis is often an afterthought. Indeed, it is sometimes not even mentioned in discharge summaries, where, not uncommonly, one sees nondiagnoses such as blood in stool or polyarthritis.
In addition, we are under pressure to shorten the inpatient stay of hospitalized patients. At least a portion of the public is aware of this; thus, it has been noted in the New York Times that: The pressure to get patients out of the hospital rapidly can focus medical attention on treatment rather than diagnosis.6 We commonly seek to ameliorate the patients' status to permit discharge before (or often without) learning what we are dealing with. Sometimes one senses that the primary question has become how soon can we discharge this patient?
A price is paid for this. In the absence of a valid diagnosis, patients may be subjected to a broad array of nonessential investigations and therapeutic interventions, each with its own possible complications. Patients are often discharged without a diagnosis having been made, presenting a serious challenge to outpatient physicians who are left to manage them without a clear idea of what they are dealing with. It often falls to the outpatient physicians to make the diagnosis. This is somewhat problematic, since they themselves are under harsh time pressure. Patients often require rehospitalization for the same as‐yet‐undiagnosed condition.
The Problem‐Oriented Record Poses Problems
The widespread use of the problem‐oriented record, originated by Lawrence Weed,7 has led to problems of its own.8 It has evolved, away from its original intent. In practice, its major emphasis often seems to be on identification of problems and tracking their progress, rather than on synthesis. This often leads to muddy rather than clear diagnostic thinking. Assessments and progress notes frequently consist of lists of symptoms, organs, abnormal laboratory findings, or even medical specialties. The net effect is often fragmented thinkingas Weed7 put it, failure to integrate findings into a single entity. Synthesizing diverse findings into a single entity, when possible, is necessary to define a diagnosis. Failure to do so may have serious consequences. In a recent study of diagnostic errors in internal medicine, cognitive errors were frequently found to contribute to such errors.9 The most common cognitive problem was faulty synthesis. How much worse than faulty synthesis is failure to synthesize at all!
Presumptive Diagnoses, Even if Incorrect, Metamorphose into Established Diagnoses
We must often treat empirically. When there is no firm diagnosis, presumptive diagnoses must be made and acted upon. Unfortunately, there are not always mechanisms for the physician to make it clear that his or her diagnosis is only presumptive. (A common example is acute viral syndrome, generally an educated guess.) All too often, presumptive diagnoses are entered, without qualification, as definitive diagnoses, and then achieve immortality. Thus, if a patient is incorrectly diagnosed as having rheumatoid arthritis, all subsequent presentations will start: A so‐and‐so year‐old woman with rheumatoid arthritis for many years Presumptive diagnoses are frequently not questioned. It is easier to assume that they were arrived at after due consideration. Once entered in the medical record, they may be difficult to remove.
It is true that the need to arrive at a precise diagnosis is less pressing for some medical specialties than for others. Emergency physicians, critical care physicians, and frequently, surgeons, must commonly act on the basis of presumptive diagnoses. In contrast, internists, family physicians, psychiatrists, and indeed all physicians who care for patients with chronic illnesses can, with time, be expected to sort out accurate from inaccurate presumptive diagnoses.
A specific example of the problem of presumptive diagnosis is of interest. It is not uncommon, following a first encounter, for a diagnosis to be entered based on the patient's history alone. While such diagnoses are frequently correct, they are not invariably correct. The patient may have arrived at the conclusion herself; she may have misunderstood what she was told by her physician, or her physician may have been in error. Such inaccurate diagnoses also often achieve immortality in the medical record.
Apparent Disparaging of the Importance of Diagnosis
Further trivialization has come from a number of publications expressing concerns about the importance of diagnosis. Thus we read that there are negative consequences of emphasis on diagnosis. When we know what is wrong, we focus less on the individual and more on the disease.10 In his recent book Our Present Complaint. American Medicine, Then and Now, the scholar C.E. Rosenberg11 includes a chapter with the provocative title The Tyranny of Diagnosis. He points out that even a century ago the fear was expressed that burgeoning scientific medicine would lead to denigration of physicians' holistic and intuitive skills.11 Other authors maintain that firm diagnoses may be misleading, since many diseases are a matter of degree in a continuuma spectrumthat are best defined employing a statistical model of risk prediction.12 The suggestion is made that the usefulness of diagnostic tests should not be related to the presence or absence of a disease, but rather to whether they influence outcome.13
Scientific medicine is focused on diagnosis. Denigration of diagnosis has often come, as a philosophical posture, from opponents of reductionist thinking. As Rosenberg11 points out: It has become fashionable among humanistic and social science‐oriented commentators to dwell on the distinction between illness and disease, between the patient's felt experience and the constructions placed on that experience by the world of medicine. Their opposition, he feels, reflects the value‐laden mutual incompatibility (real or apparent) of art and science, of holism and reductionism.2
It is true that medicine is more than just biology. There is a great deal to be said for the view that scientific medicine tends to deemphasize the humanistic, holistic aspects of medical practice. However, despite all these concerns, most physiciansand, to be fair, most criticsagree that making an accurate diagnosis is important. Thus, though the title of his relevant chapter is The Tyranny of Diagnosis, Rosenberg11 states: I might just as well have used the term indispensability. Indeed, the opening words of that chapter are: Diagnosis has always played a pivotal role in medicine.11 Other authors cited above issue this disclaimer: We are not against diagnosis. Diagnosis does and always will play a central role in clinical medicine.12
The importance of diagnosis is underscored by the vigorous debate about how to assess diagnostic tests;14 apparently, diagnosis does indeed matter. While it is true that diagnoses are not always precise, objective, and quantifiable,10 abundant evidence points to the unavoidable conclusion that identifying the patient's disease is heuristically useful; that is, it works.2 The track record of modern scientific medicine in improving mortality and morbidity speaks for itself. It hardly seems necessary to defend it. In addition to representing a valuable intellectual challenge in its own right, diagnosis is pivotal to the scientific mission of medicine.
What Can Be Done?
The net effect of all these forces: the use of billable terms as diagnoses, the pressures of managed care, fragmented problem lists, persistence of incorrect presumptive diagnoses in medical records, and antireductionist criticisms is to encourage sloppy diagnostic thinking in some physicians. What can be done to emphasize the proper use of differential diagnosis in arriving at a definitive diagnosis? What can be done to underscore the importance of differentiating between presumptive and definitive diagnoses? Most importantly, how can we instill the respect for the intellectual honesty necessary to acquire and retain these skills?
Above all, we should relentlessly impress on our students and trainees the importance of arriving at an accurate definitive diagnosis. They should be aware that the job is only half done if the diagnosis has not been made. We should do this repeatedly, both by word and by example. We ourselves must display intellectual honesty.
In addition, we ought to be able to enter diagnosis uncertain, so coded, or to append the phrase cause unknown after the manifestation of concern, when we don't really know what is going on. We should routinely indicate when a diagnosis is merely presumptive. Perhaps we need a way to indicate: This diagnosis is definitive or to indicate the specific evidence that led to the diagnosis (eg, biopsy, laboratory result, radiographic finding). Finally, we need to correct the current confusion between diseases and billable terms, to differentiate the disease from the symptom, perhaps by labeling ICD‐9‐CM codes simply as billing codes, with a separate entry for actual medical diagnoses.
Although powerful historical forces have brought us to this state, we believe that arriving at the correct diagnosis is at least as important now as it has been in the past, and that its primacy should be recognized, celebrated, and fought for. We owe our patients no less.
Acknowledgements
The authors thank Drs. James Pile, Neal Dawson, and David Samols for their helpful suggestions.
Historically, diagnosis has been central to patient care. Making the correct diagnosis serves as a guide to the choice of treatment, permits assessment of prognosis, and indicates what complications to expect. Arriving at the correct diagnosis has been a major goalthe Holy Grail, as it were. Accurate diagnosis continues to be a major focus of medical practice, and accurate diagnoses are routinely made every day. Still, many experienced clinicians have the impression that in recent years the primacy of diagnosis has been coming under attack from several sources.
A decade ago, Thomas Szasz pointed out that disease is a fact of nature, while diagnosis is man made.1 The noun diagnosis is derived from the Greek verb diagignoskeinindicating knowledge attained through analysis. As defined in Merriam‐Webster's Collegiate Dictionary, the diagnosis essentially means the conclusion arrived at by the art of identifying a disease. It is the product of an intellectual effort of a particular analytic type. The response to the question What is the diagnosis? has been the name of the specific disease entity with which the patient is afflicted.
Disease entities represent coherent, organizing concepts.2 A specific disease is a condition with characteristic manifestationsclinical, histologic, or pathophysiologic. If untreated, it results in dysfunction or, in some cases, death. Differentiation of one disease from another is enhanced when there is some sort of understanding, even if incomplete, of the specific pathophysiology at play. Admittedly, concepts of what constitute specific disease entities are not fixed; they evolve with time. Not all diseases have been identified. The underlying etiology may or may not be known. Nonetheless, diseases are recognized as specific entities, distinct from other diseases. Thus, anemia is not regarded as a disease, while pernicious anemia and iron deficiency anemia are diseases. Fever is not a disease, while typhoid fever is. Arthritis is not a disease, while gonococcal arthritis is.
Billable Terms Are Replacing Traditional Medical Diagnoses
The term diagnosis has been redefined to comply with the need to enter a diagnosis for billing purposes. Use of this term for this purpose has confused the issue. Diagnoses entered for such purposes are largely derived from International Classification of Diseases (ICD) lists.3 However, the ICD was not intended to definitively identify underlying diseases, nor to serve as a guide to management and prognostication. The 6th revision of the ICD in 1948, the first revision to be widely employed, was designed for epidemiologic purposes and achieved widespread use to obtain mortality and morbidity statistics.4 It was subsequently also used as a tool to index hospital medical records.
Significantly, it was also employed for billing purposes, with far‐reaching pernicious consequences. Although the ICD purports to be a list of diseases, it actually includes symptoms and signs. Consequently, in the billing context, diagnosis no longer necessarily refers to specific disease states; it now refers to billable termsoften the manifestation that was responsible for the patient seeking medical assistance. Far from being the product of an intellectual effort, it is often merely a justification for submitting a bill. Examples of such diagnoses are shown in Table 1. Many of them represent symptoms, signs, or laboratory abnormalities. The importance of accurate medical diagnosis has been cheapened by this change. The effect is to devalue diagnosisto lessen its status as the Holy Grail.
| Abdominal pain | General symptoms | Special symptom |
| Abnormal blood test | Immune disorders | Splenomegaly |
| Back disorder | Joint disorder | Throat pain |
| Coagulation defects | Myoneural disorder | Urinary symptoms |
| Diseases of esophagus | Otalgia | Visual disturbance |
| Eye disorders | Pain in joint | Vomiting |
| Fluid/electrolyte disorders | Right lower quadrant mass | Wheezing |
The effect of this on trainees is invidious, and predictable. The traditional meaning of diagnosis is being replaced in our minds. Physicians in training are tempted to deceive themselves into believing that they have arrived at an understanding of what they are dealing with when they enter such a diagnosis. After all, have they not responded to the question: what is the diagnosis?
We do not mean to imply that physicians are doing anything wrong by entering ICD terms for billing purposes. What must be done for billing purposes must be done. It is important to be aware, however, and to continually remind ourselves, that what has been entered for this purpose is often not a true medical diagnosis.
Further, when the diagnosis is not yet known, it is not possible to enter a true diagnosis. There is no way to say I don't know. It would be preferable to simply admit that the diagnosis is not yet established, as a medical resident has recently emphasized.5
Diagnosis Often Gets Short Shrift Because of the Perceived Urgency of Discharge
The emphasis on diagnosis several generations ago may have resulted, at least in part, from the relative paucity of effective therapeutic interventions before the 1930s. Things have changed; therapeutic capabilities are much more powerful now. Making the correct diagnosis seems to have lost its urgency. Instead of the major question being what is the diagnosis? it now is often what do we do now? The diagnosis is often an afterthought. Indeed, it is sometimes not even mentioned in discharge summaries, where, not uncommonly, one sees nondiagnoses such as blood in stool or polyarthritis.
In addition, we are under pressure to shorten the inpatient stay of hospitalized patients. At least a portion of the public is aware of this; thus, it has been noted in the New York Times that: The pressure to get patients out of the hospital rapidly can focus medical attention on treatment rather than diagnosis.6 We commonly seek to ameliorate the patients' status to permit discharge before (or often without) learning what we are dealing with. Sometimes one senses that the primary question has become how soon can we discharge this patient?
A price is paid for this. In the absence of a valid diagnosis, patients may be subjected to a broad array of nonessential investigations and therapeutic interventions, each with its own possible complications. Patients are often discharged without a diagnosis having been made, presenting a serious challenge to outpatient physicians who are left to manage them without a clear idea of what they are dealing with. It often falls to the outpatient physicians to make the diagnosis. This is somewhat problematic, since they themselves are under harsh time pressure. Patients often require rehospitalization for the same as‐yet‐undiagnosed condition.
The Problem‐Oriented Record Poses Problems
The widespread use of the problem‐oriented record, originated by Lawrence Weed,7 has led to problems of its own.8 It has evolved, away from its original intent. In practice, its major emphasis often seems to be on identification of problems and tracking their progress, rather than on synthesis. This often leads to muddy rather than clear diagnostic thinking. Assessments and progress notes frequently consist of lists of symptoms, organs, abnormal laboratory findings, or even medical specialties. The net effect is often fragmented thinkingas Weed7 put it, failure to integrate findings into a single entity. Synthesizing diverse findings into a single entity, when possible, is necessary to define a diagnosis. Failure to do so may have serious consequences. In a recent study of diagnostic errors in internal medicine, cognitive errors were frequently found to contribute to such errors.9 The most common cognitive problem was faulty synthesis. How much worse than faulty synthesis is failure to synthesize at all!
Presumptive Diagnoses, Even if Incorrect, Metamorphose into Established Diagnoses
We must often treat empirically. When there is no firm diagnosis, presumptive diagnoses must be made and acted upon. Unfortunately, there are not always mechanisms for the physician to make it clear that his or her diagnosis is only presumptive. (A common example is acute viral syndrome, generally an educated guess.) All too often, presumptive diagnoses are entered, without qualification, as definitive diagnoses, and then achieve immortality. Thus, if a patient is incorrectly diagnosed as having rheumatoid arthritis, all subsequent presentations will start: A so‐and‐so year‐old woman with rheumatoid arthritis for many years Presumptive diagnoses are frequently not questioned. It is easier to assume that they were arrived at after due consideration. Once entered in the medical record, they may be difficult to remove.
It is true that the need to arrive at a precise diagnosis is less pressing for some medical specialties than for others. Emergency physicians, critical care physicians, and frequently, surgeons, must commonly act on the basis of presumptive diagnoses. In contrast, internists, family physicians, psychiatrists, and indeed all physicians who care for patients with chronic illnesses can, with time, be expected to sort out accurate from inaccurate presumptive diagnoses.
A specific example of the problem of presumptive diagnosis is of interest. It is not uncommon, following a first encounter, for a diagnosis to be entered based on the patient's history alone. While such diagnoses are frequently correct, they are not invariably correct. The patient may have arrived at the conclusion herself; she may have misunderstood what she was told by her physician, or her physician may have been in error. Such inaccurate diagnoses also often achieve immortality in the medical record.
Apparent Disparaging of the Importance of Diagnosis
Further trivialization has come from a number of publications expressing concerns about the importance of diagnosis. Thus we read that there are negative consequences of emphasis on diagnosis. When we know what is wrong, we focus less on the individual and more on the disease.10 In his recent book Our Present Complaint. American Medicine, Then and Now, the scholar C.E. Rosenberg11 includes a chapter with the provocative title The Tyranny of Diagnosis. He points out that even a century ago the fear was expressed that burgeoning scientific medicine would lead to denigration of physicians' holistic and intuitive skills.11 Other authors maintain that firm diagnoses may be misleading, since many diseases are a matter of degree in a continuuma spectrumthat are best defined employing a statistical model of risk prediction.12 The suggestion is made that the usefulness of diagnostic tests should not be related to the presence or absence of a disease, but rather to whether they influence outcome.13
Scientific medicine is focused on diagnosis. Denigration of diagnosis has often come, as a philosophical posture, from opponents of reductionist thinking. As Rosenberg11 points out: It has become fashionable among humanistic and social science‐oriented commentators to dwell on the distinction between illness and disease, between the patient's felt experience and the constructions placed on that experience by the world of medicine. Their opposition, he feels, reflects the value‐laden mutual incompatibility (real or apparent) of art and science, of holism and reductionism.2
It is true that medicine is more than just biology. There is a great deal to be said for the view that scientific medicine tends to deemphasize the humanistic, holistic aspects of medical practice. However, despite all these concerns, most physiciansand, to be fair, most criticsagree that making an accurate diagnosis is important. Thus, though the title of his relevant chapter is The Tyranny of Diagnosis, Rosenberg11 states: I might just as well have used the term indispensability. Indeed, the opening words of that chapter are: Diagnosis has always played a pivotal role in medicine.11 Other authors cited above issue this disclaimer: We are not against diagnosis. Diagnosis does and always will play a central role in clinical medicine.12
The importance of diagnosis is underscored by the vigorous debate about how to assess diagnostic tests;14 apparently, diagnosis does indeed matter. While it is true that diagnoses are not always precise, objective, and quantifiable,10 abundant evidence points to the unavoidable conclusion that identifying the patient's disease is heuristically useful; that is, it works.2 The track record of modern scientific medicine in improving mortality and morbidity speaks for itself. It hardly seems necessary to defend it. In addition to representing a valuable intellectual challenge in its own right, diagnosis is pivotal to the scientific mission of medicine.
What Can Be Done?
The net effect of all these forces: the use of billable terms as diagnoses, the pressures of managed care, fragmented problem lists, persistence of incorrect presumptive diagnoses in medical records, and antireductionist criticisms is to encourage sloppy diagnostic thinking in some physicians. What can be done to emphasize the proper use of differential diagnosis in arriving at a definitive diagnosis? What can be done to underscore the importance of differentiating between presumptive and definitive diagnoses? Most importantly, how can we instill the respect for the intellectual honesty necessary to acquire and retain these skills?
Above all, we should relentlessly impress on our students and trainees the importance of arriving at an accurate definitive diagnosis. They should be aware that the job is only half done if the diagnosis has not been made. We should do this repeatedly, both by word and by example. We ourselves must display intellectual honesty.
In addition, we ought to be able to enter diagnosis uncertain, so coded, or to append the phrase cause unknown after the manifestation of concern, when we don't really know what is going on. We should routinely indicate when a diagnosis is merely presumptive. Perhaps we need a way to indicate: This diagnosis is definitive or to indicate the specific evidence that led to the diagnosis (eg, biopsy, laboratory result, radiographic finding). Finally, we need to correct the current confusion between diseases and billable terms, to differentiate the disease from the symptom, perhaps by labeling ICD‐9‐CM codes simply as billing codes, with a separate entry for actual medical diagnoses.
Although powerful historical forces have brought us to this state, we believe that arriving at the correct diagnosis is at least as important now as it has been in the past, and that its primacy should be recognized, celebrated, and fought for. We owe our patients no less.
Acknowledgements
The authors thank Drs. James Pile, Neal Dawson, and David Samols for their helpful suggestions.
- .What counts as disease? Rationales and rationalizations for treatment.Forsch Komplementarmed.1998;5(suppl S1):40–46.
- .What is disease? In memory of Owsei Temkin.Bull Hist Med.2003;77:491–505.
- World Health Organization (WHO). International Classification of Diseases (ICD). Available at: http://www.who.int/classifications/icd/en. Accessed June2009.
- World Health Organization (WHO). History of the development of the ICD. Available at: http://www.who.int/classifications/icd/en/HistoryOfICD. pdf. Accessed June2009.
- .Living unlabeled—diagnosis and disorder.N Engl J Med.2008;359:1650–1653.
- . Poison Pill. New York Times Magazine. 2008: 24–26. Available at: http://www.nytimes.com/2008/04/13/magazine/13wwln‐diagnosis‐t.html. Accessed June2009.
- .Medical records that guide and teach.N Engl J Med.1968;278:593–600.
- .Clear writing, clear thinking and the disappearing art of the problem list.J Hosp Med.2007;2:199–202.
- , , .Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- . The Tyranny of Diagnosis. New York Times. Available at: http://www.nytimes.com/2008/09/19/health/chen9‐18.html?partner=rssnyt2007.
- , , .Against diagnosis.Ann Intern Med.2008;149:200–203.
- , , .A philosophical perspective supports the need for patient‐outcome studies in diagnostic test evaluation.J Clin Epidemiol.2009;62:58–61.
- , .Evidence based diagnostics.BMJ.2005;330:724–726.
- .What counts as disease? Rationales and rationalizations for treatment.Forsch Komplementarmed.1998;5(suppl S1):40–46.
- .What is disease? In memory of Owsei Temkin.Bull Hist Med.2003;77:491–505.
- World Health Organization (WHO). International Classification of Diseases (ICD). Available at: http://www.who.int/classifications/icd/en. Accessed June2009.
- World Health Organization (WHO). History of the development of the ICD. Available at: http://www.who.int/classifications/icd/en/HistoryOfICD. pdf. Accessed June2009.
- .Living unlabeled—diagnosis and disorder.N Engl J Med.2008;359:1650–1653.
- . Poison Pill. New York Times Magazine. 2008: 24–26. Available at: http://www.nytimes.com/2008/04/13/magazine/13wwln‐diagnosis‐t.html. Accessed June2009.
- .Medical records that guide and teach.N Engl J Med.1968;278:593–600.
- .Clear writing, clear thinking and the disappearing art of the problem list.J Hosp Med.2007;2:199–202.
- , , .Diagnostic error in internal medicine.Arch Intern Med.2005;165:1493–1499.
- . The Tyranny of Diagnosis. New York Times. Available at: http://www.nytimes.com/2008/09/19/health/chen9‐18.html?partner=rssnyt2007.
- , , .Against diagnosis.Ann Intern Med.2008;149:200–203.
- , , .A philosophical perspective supports the need for patient‐outcome studies in diagnostic test evaluation.J Clin Epidemiol.2009;62:58–61.
- , .Evidence based diagnostics.BMJ.2005;330:724–726.
Simulation Improves CVC Placement
Central venous catheter (CVC) insertions are commonly performed at the bedside in medical intensive care unit (MICU) settings. Internal medicine residents are required to demonstrate knowledge regarding CVC indications, complications, and sterile technique,1 and often perform the procedure during training. Education in CVC insertion is needed because many internal medicine residents are uncomfortable performing this procedure.2 CVC insertion also carries the risk of potentially life‐threatening complications including infection, pneumothorax, arterial puncture, deep vein thrombosis, and bleeding. Education and training may also contribute to improved patient care because increased physician experience with CVC insertion reduces complication risk.3, 4 Similarly, a higher number of needle passes or attempts during CVC insertion correlates with mechanical complications such as pneumothorax or arterial punctures.48 Pneumothorax rates for internal jugular (IJ) CVCs have been reported to range from 0% to 0.2% and for subclavian (SC) CVCs from 1.5% to 3.1%.4, 5 The arterial puncture rate for IJ CVCs ranges from 5.0% to 9.4% and for SC CVCs from 3.1% to 4.9%.4, 5 Proper use of ultrasound to assist with IJ CVC insertion has been shown to decrease these mechanical complications.4, 5 However, studies of ultrasound use with SC CVC insertion have mixed results.4
Simulation‐based training has been used in medical education to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.9, 10 We previously used simulation‐based mastery learning to improve the thoracentesis and advanced cardiac life support (ACLS) skills of internal medicine residents.11, 12 Although a few small studies have linked simulation‐based interventions to improved quality of care,1319 more work is needed to show that results from a simulated environment transfer to actual patient care.
This study had 2 aims. The first was to expand our simulation‐based mastery learning to CVC insertion using a CVC simulator and ultrasound device. The second was to assess quality indicators (number of needle passes, pneumothorax, arterial punctures, and need for catheter adjustment) and resident confidence related to actual CVC insertions in the MICU before and after an educational intervention.
Materials and Methods
Design
This was a cohort study20 of IJ and SC CVC insertions by 41 second‐ and third‐year internal medicine residents rotating through the MICU in a university‐affiliated program from October 2006 to February 2007. The Northwestern University Institutional Review Board approved the study. All study participants were required to give informed consent prior to participation.
Thirteen residents rotated through the MICU during a 6‐week preintervention phase. These residents served as a traditionally trained group that did not receive CVC insertion simulator training. Simultaneously, 28 residents who rotated through the MICU later in the study period received simulation‐based training in CVC insertion and served as the simulator‐trained group (Figure 1). Demographic data were obtained from the participants including age, gender, ethnicity, year of training, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2.
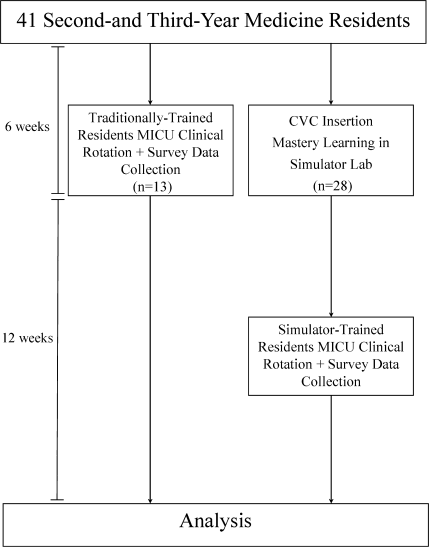
Simulator‐trained residents underwent baseline skill assessment (pretest) using a 27‐item checklist in IJ and SC CVC insertions (see Appendix). Checklists were developed by one author (J.H.B.) using appropriate references4, 5 and a step‐by‐step process,21 and reviewed for completeness by another author with expertise in checklist development (D.B.W.). Each skill or other action was listed in order and given equal weight. A dichotomous scoring scale of 1 = done correctly and 0 = done incorrectly/not done was imposed for each item. Assessments were performed using Simulab's CentralLineMan. This model features realistic tissue with ultrasound compatibility, an arterial pulse, and self‐sealing veins and skins. Needles, dilators, and guidewires can be inserted and realistic venous and arterial pressures demonstrated (Figure 2).
Residents in the simulator‐trained group received two, 2‐hour education sessions featuring a lecture, ultrasound training, deliberate practice with the CVC simulator, and feedback.22 Education sessions contained standardized didactic material on CVC indications and complications, as well as a stepwise demonstration of IJ and SC CVC insertions using ultrasound and landmark techniques. These sessions were supervised by a senior hospitalist faculty member with expertise in CVC insertions (J.H.B.). Residents were expected to use the ultrasound device for all IJ CVC insertions. However, its use was optional for SC CVC insertion. After training, residents were retested (posttest) and required to meet or exceed a minimum passing score (MPS) set by an expert panel for both IJ and SC procedures.23 This 11 member expert panel provided item‐based (Angoff) and group‐based (Hofstee) judgments on the 27‐item checklists as described previously.23
Residents who did not achieve the MPS had more deliberate practice and were retested until the MPS was reached; the key feature of mastery learning.24 After completing simulation‐based mastery learning in CVC insertion, the 28 simulator‐trained residents rotated through the MICU.
Data Collection
All pretests and posttests (using the 27‐item checklist) were graded by a single unblinded instructor (J.H.B.) and were videotaped. Another faculty instructor with expertise in scoring clinical skills examinations and blind to pre‐post status (D.B.W.) rescored a random 50% sample of the tests to assess interrater reliability.
Data regarding actual CVC insertions in the MICU were collected by contacting all MICU residents daily during the study period. This allowed for CVC insertions to be identified within 24 hours. All survey data were collected anonymously. The primary inserter of each CVC was questioned about quality indicators and procedural self‐confidence concerning CVC placement. CVCs primarily inserted by nonstudy subjects (first‐year residents, emergency medicine residents, pulmonary‐critical care medicine faculty members, and subspecialty fellows) or CVC placements that were supervised, but not directly placed by study participants, were excluded.
Outcome Measures
Pretest and posttest checklist scores from simulator‐trained residents were compared to measure the impact of training sessions. Residents rotating through the MICU were asked about several quality indicators related to actual CVC insertions. Quality indicators include: (1) number of needle passes required during the procedure (skin punctures); (2) presence of complications including pneumothorax and arterial puncture; and (3) need for CVC adjustment after chest x‐ray. Participants were also questioned regarding their confidence in CVC insertion using a 100 point scale (0 = not confident and 100 = very confident). Survey results from the 28 simulator‐trained residents were compared to results from the 13 traditionally‐trained residents.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges, using the kappa () coefficient adjusted25, 26 using the formula of Brennan and Prediger.27 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t‐tests.
MICU survey results were compared using t‐tests. Traditionally‐trained and simulator‐trained groups were assessed for demographic differences using t‐tests and the chi‐square statistic. Spearman's rank correlation coefficient was used to assess for relationships between resident self‐confidence and quality indicators. All analyses were preformed using SPSS statistical software, version 16.0 (SPSS, Inc., Chicago, IL).
Results
All eligible residents participated in the study and completed the entire protocol. There was no significant difference in age, gender, ethnicity, year of training, or USMLE Step 1 and 2 scores between the traditionally‐trained and simulator‐trained groups.
Interrater reliability measured by the mean kappa coefficient was very high (n = 0.94) across the 27 IJ and SC checklist items. No resident met the MPS (79.1%) for CVC insertion at baseline testing. In the simulator‐trained group, 25 of 28 (89%) residents achieved SC skill mastery and 27 of 28 (96%) achieved IJ skill mastery within the standard four hour curriculum. All residents subsequently reached the MPS with less than one hour of additional practice time. A graphic portrait of the residents' pretest and posttest performance on the simulated CVC clinical skills examination with descriptive statistics is shown in Figure 3. After the educational intervention, posttest scores significantly improved (p < 0.001), to meet or exceed the MPS.
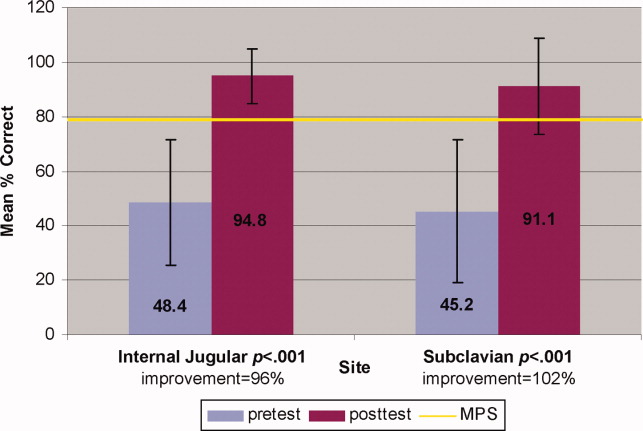
Traditionally trained and simulator‐trained residents independently inserted 46 CVCs during the study period. Simulator‐trained residents required significantly fewer needle passes to insert all actual CVCs in the MICU compared to traditionally trained residents: mean (M) = 1.79, standard deviation (SD) = 1.03 versus M = 2.78, SD = 1.77 (p = 0.04). As shown in Table 1, the groups did not differ in pneumothorax, arterial puncture, or mean number of CVC adjustments. In addition, the groups did not differ in use of ultrasound for IJ or SC CVC insertions. One IJ CVC was inserted without ultrasound in the traditionally‐trained group; 2 were inserted without ultrasound in the simulator‐trained group. Ultrasound was not used during any SC CVC insertions in the traditionally‐trained group and was used for 1 SC CVC insertion in the simulator‐trained group.
| Internal Jugular and Subclavian CVCs | |||
|---|---|---|---|
| Traditionally Trained Residents | Simulator Trained Residents | P value | |
| |||
| Number of attempts during insertion [mean (SD)] | 2.78 (1.77) | 1.79 (1.03) | 0.04* |
| Pneumothorax (number) | 0 | 0 | n/a |
| Arterial puncture (%) | 11 | 7 | 0.65 |
| CVC adjustment (%) | 15 | 8 | 0.52 |
| Confidence (%) [mean (SD)] | 68 (20) | 81 (11) | 0.02* |
Simulator‐trained residents displayed more self‐confidence about their procedural skills than traditionally‐trained residents (M = 81, SD = 11 versus M = 68, SD = 20, p = 0.02). Spearman correlations showed no practical association between resident self‐confidence and performance on CVC insertion quality indicators.
Discussion
This study demonstrates the use of a mastery learning model to develop CVC insertion skills to a high achievement level among internal medicine residents. Our data support prior work showing that procedural skills that are poor at baseline can be increased significantly using simulation‐based training and deliberate practice.1118, 28 This report on CVC insertion adds to the growing body of literature showing that simulation training complements standard medical education,1119, 28 and expands the clinical application of the mastery model beyond thoracentesis and ACLS.11, 12 Use of the mastery model described in this study also has important implications for patients. In our training program, residents are required to demonstrate procedural mastery in a simulated environment before independently performing a CVC insertion on an actual patient. This is in sharp contrast to the traditional clinical model of procedural training at the bedside, and may be used in other training programs and with other invasive procedures.
The second aim of our study was to determine the impact of simulation‐based training on actual clinical practice by residents in the MICU. To our knowledge, no prior study has demonstrated that simulation‐based training in CVC insertion improves patient outcomes. We believe our results advance what is known about the impact of simulation‐based training because simulator‐trained residents in this study performed actual CVC insertions in the MICU using significantly fewer needle passes. Needle passes have been used by other investigators as a surrogate measure for reduced CVC‐associated complications because mechanical complications rise exponentially with more than two insertion attempts.47, 29 We believe this finding demonstrates transfer of skill acquired from simulation‐based training to the actual clinical environment. It is possible that ultrasound training accounts for the improvement in the simulator‐trained group. However, we do not believe that ultrasound training is entirely responsible as prior work has shown that deliberate practice using mastery learning without ultrasound significantly improved resident performance of thoracentesis11 and ACLS12, 19 procedures. We did not show a significant reduction in complications such as pneumothorax or arterial puncture. This is likely due to the small sample size and the low number of procedures and complications during the study period.
Our results also show that resident self‐confidence regarding actual CVC insertions improved after simulation training. These findings are similar to prior reports linking improved confidence among trainees after simulation‐based training in CVC insertion.29, 30 Our results did not reveal a correlation between improved self‐confidence and clinical skill acquisition. Linking improved self‐confidence to improved clinical skill is important because self‐assessment does not always correlate with performance ability.31, 32
More study is needed to evaluate the impact of simulation‐based training on the quality of CVC insertions by trainees. Mechanisms shown to decrease complications of CVC placement include use of ultrasound,4, 7, 3336 full sterile barrier technique,3739 chlorhexidine skin preparations,4042 and nurse‐physician education.43 Our simulation‐training program incorporates each of these elements. We plan to expand our simulation‐based training intervention to a larger sample size to determine its impact on mechanical and infectious complication rates linked to CVC insertion.
This study has several limitations. It was performed at a single institution over a short time period. However, demonstration of significantly fewer needle passes and improved resident self‐confidence after simulator training are important findings that warrant further study. It was impossible to blind raters during the skills assessment examination about whether the resident was performing a pretest or posttest. This was accounted for by using a second rater, who was blind to the pretest and posttest status of the examinee. The arterial puncture rate of 7% among simulator‐trained residents was higher than expected, although it remains within published ranges.4, 5 Also, a low total number of CVCs were evaluated during the study. This is likely due to strict exclusion criteria employed in order to study the impact of simulation training. For example, CVC insertions were only evaluated if they were actually performed by study residents (supervised insertions were excluded) and femoral catheters were not evaluated. We did not track clinical experience with CVC insertion by residents before the study. Residents who were simulator‐trained may have had more clinical experience with CVC insertion and this may have impacted their performance. However, residents did not differ in year of training or clinical rotations, and there is clear evidence that clinical training is not a proxy for skill acquisition.44 Finally, outcome data were measured via resident questionnaires that relied on resident recall about CVC insertion rather than observer ratings. This method was selected because observer ratings could not be standardized given the large number of clinical supervisors in the MICU over the study period. Information about needle passes and arterial puncture also may not be documented in procedural notes and could not be obtained by medical record review. We attempted to minimize recall bias by surveying residents within 24 hours of CVC placement.
In conclusion, this study demonstrates that simulation‐based training and deliberate practice in a mastery learning setting improves performance of both simulated and actual CVC insertions by internal medicine residents. Procedural training remains an important component of internal medicine training, although internists are performing fewer invasive procedures now than in years past.45, 46 Use of a mastery model of CVC insertion requires that trainees demonstrate skill in a simulated environment before independently performing this invasive procedure on patients. Further study is needed to assess clinical outcomes such as reduced CVC‐related infections and mechanical complications after simulation‐based training.
Acknowledgements
The authors thank the Northwestern University internal medicine residents for their dedication to education and patient care. They acknowledge Drs. J. Larry Jameson and Charles Watts for their support and encouragement of this work.
Appendix
Central Venous Catheter Insertion Checklists for Simulation‐based Education 0, 0
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to keep brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| The ultrasound (US) probe is properly set up with sterile sheath and sonographic gel | A | B | C |
| The vein is localized using anatomical landmarks with the US machine | A | B | C |
| If no US is used this is wrong | |||
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter‐ syringe complex, cannulate the vein while aspirating (must be done with US) | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to leave brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| **The US probe is properly set up with sterile sheath and sonographic gel . (MUST DO if use US) | A | B | C |
| The vein is localized using US machine or anatomical landmarks are verbalized | A | B | C |
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized using a larger needle (must verbalize they anesthetize the clavicle) | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter syringe complex cannulate the vein while aspirating (optional confirmed by US) | A | B | C |
| If US was not used then expected to state they are directing the needle to the sternal notch | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
- American Board of Internal Medicine. Procedures Required for Internal Medicine. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed January 28, 2009.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,, et al.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,, et al.Mechanical complications of central venous catheters.J Intensive Care Med.2006;21:40–46.
- ,,, et al.Risk factors of failure and immediate complication of subclavian vein catheterization in critically ill patients.Intensive Care Med.2002;28:1036–1041.
- ,,, et al.Complications and failures of subclavian‐vein catheterization.N Engl J Med.1994;331:1735–1738.
- .Central venous catheterization: better and worse.J Intensive Care Med.2006;21:51–53.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3:48–54.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- ,,, et al.Laparoscopic skills are improved with LapMentor training: results of a randomized, double‐blinded study.Ann Surg.2006;243:854–860.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Multicenter, randomized, controlled trial of virtual‐reality simulator training in acquisition of competency in colonoscopy.Gastrointest Endosc.2006;64:361–368.
- ,,, et al.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,.Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study.Am J Gastroenterol.2004;99:33–37.
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–463.
- ,,, et al.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.CHEST.2008;133:56–61.
- ,.Clinical Epidemiology: the Essentials.4th ed.Philadelphia:Lippincott Williams 2005.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July2000. Available at: http://www. wmich.edu/evalctr/checklists/cdc.htm. Accessed May 15, 2006.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79:S70–S81.
- ,,, et al.Do baseline data influence standard setting for a clinical skills examination?Acad Med.2007;82:S105–S108.
- ,,, et al.Lessons for Continuing Medical Education from simulation research in undergraduate and graduate medical education.CHEST.2009;135.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 41:687–699.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:202–208.
- ,,.Central catheter simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,.The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative.J Gen Intern Med.2006;21:514–517.
- ,,, et al.The use of simulation in emergency medicine: a research agenda.Acad Emerg Med.2007;14:353–363.
- ,,, et al.Graduating internal medicine residents' self‐assessment and performance of advanced cardiac life support skills.Med Teach.2006;28:365–369.
- ,.Bedside ultrasonography in the ICU: Part 2.CHEST.2005;128:1766–1781.
- ,,, et al.Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein: a randomized study.Anesthesiology.1998;88:1195–1201.
- ,,, et al.Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department.Acad Emerg Med.2002;9:800–805.
- ,,, et al.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24:2053–2058.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,,, et al.Chlorhexidine compared with povidone‐iodine solution for vascular catheter‐site care: a meta‐analysis.Ann Intern Med.2002;136:792–801.
- ,,.Prospective randomised trial of povidone‐iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters.Lancet.1991;338:339–343.
- ,,, et al.Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients.Crit Care Med.1996;24:1818–1823.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.CHEST.2004;126:1612–1618.
- ,,.Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142:260–273.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–393.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
Central venous catheter (CVC) insertions are commonly performed at the bedside in medical intensive care unit (MICU) settings. Internal medicine residents are required to demonstrate knowledge regarding CVC indications, complications, and sterile technique,1 and often perform the procedure during training. Education in CVC insertion is needed because many internal medicine residents are uncomfortable performing this procedure.2 CVC insertion also carries the risk of potentially life‐threatening complications including infection, pneumothorax, arterial puncture, deep vein thrombosis, and bleeding. Education and training may also contribute to improved patient care because increased physician experience with CVC insertion reduces complication risk.3, 4 Similarly, a higher number of needle passes or attempts during CVC insertion correlates with mechanical complications such as pneumothorax or arterial punctures.48 Pneumothorax rates for internal jugular (IJ) CVCs have been reported to range from 0% to 0.2% and for subclavian (SC) CVCs from 1.5% to 3.1%.4, 5 The arterial puncture rate for IJ CVCs ranges from 5.0% to 9.4% and for SC CVCs from 3.1% to 4.9%.4, 5 Proper use of ultrasound to assist with IJ CVC insertion has been shown to decrease these mechanical complications.4, 5 However, studies of ultrasound use with SC CVC insertion have mixed results.4
Simulation‐based training has been used in medical education to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.9, 10 We previously used simulation‐based mastery learning to improve the thoracentesis and advanced cardiac life support (ACLS) skills of internal medicine residents.11, 12 Although a few small studies have linked simulation‐based interventions to improved quality of care,1319 more work is needed to show that results from a simulated environment transfer to actual patient care.
This study had 2 aims. The first was to expand our simulation‐based mastery learning to CVC insertion using a CVC simulator and ultrasound device. The second was to assess quality indicators (number of needle passes, pneumothorax, arterial punctures, and need for catheter adjustment) and resident confidence related to actual CVC insertions in the MICU before and after an educational intervention.
Materials and Methods
Design
This was a cohort study20 of IJ and SC CVC insertions by 41 second‐ and third‐year internal medicine residents rotating through the MICU in a university‐affiliated program from October 2006 to February 2007. The Northwestern University Institutional Review Board approved the study. All study participants were required to give informed consent prior to participation.
Thirteen residents rotated through the MICU during a 6‐week preintervention phase. These residents served as a traditionally trained group that did not receive CVC insertion simulator training. Simultaneously, 28 residents who rotated through the MICU later in the study period received simulation‐based training in CVC insertion and served as the simulator‐trained group (Figure 1). Demographic data were obtained from the participants including age, gender, ethnicity, year of training, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2.

Simulator‐trained residents underwent baseline skill assessment (pretest) using a 27‐item checklist in IJ and SC CVC insertions (see Appendix). Checklists were developed by one author (J.H.B.) using appropriate references4, 5 and a step‐by‐step process,21 and reviewed for completeness by another author with expertise in checklist development (D.B.W.). Each skill or other action was listed in order and given equal weight. A dichotomous scoring scale of 1 = done correctly and 0 = done incorrectly/not done was imposed for each item. Assessments were performed using Simulab's CentralLineMan. This model features realistic tissue with ultrasound compatibility, an arterial pulse, and self‐sealing veins and skins. Needles, dilators, and guidewires can be inserted and realistic venous and arterial pressures demonstrated (Figure 2).

Residents in the simulator‐trained group received two, 2‐hour education sessions featuring a lecture, ultrasound training, deliberate practice with the CVC simulator, and feedback.22 Education sessions contained standardized didactic material on CVC indications and complications, as well as a stepwise demonstration of IJ and SC CVC insertions using ultrasound and landmark techniques. These sessions were supervised by a senior hospitalist faculty member with expertise in CVC insertions (J.H.B.). Residents were expected to use the ultrasound device for all IJ CVC insertions. However, its use was optional for SC CVC insertion. After training, residents were retested (posttest) and required to meet or exceed a minimum passing score (MPS) set by an expert panel for both IJ and SC procedures.23 This 11 member expert panel provided item‐based (Angoff) and group‐based (Hofstee) judgments on the 27‐item checklists as described previously.23
Residents who did not achieve the MPS had more deliberate practice and were retested until the MPS was reached; the key feature of mastery learning.24 After completing simulation‐based mastery learning in CVC insertion, the 28 simulator‐trained residents rotated through the MICU.
Data Collection
All pretests and posttests (using the 27‐item checklist) were graded by a single unblinded instructor (J.H.B.) and were videotaped. Another faculty instructor with expertise in scoring clinical skills examinations and blind to pre‐post status (D.B.W.) rescored a random 50% sample of the tests to assess interrater reliability.
Data regarding actual CVC insertions in the MICU were collected by contacting all MICU residents daily during the study period. This allowed for CVC insertions to be identified within 24 hours. All survey data were collected anonymously. The primary inserter of each CVC was questioned about quality indicators and procedural self‐confidence concerning CVC placement. CVCs primarily inserted by nonstudy subjects (first‐year residents, emergency medicine residents, pulmonary‐critical care medicine faculty members, and subspecialty fellows) or CVC placements that were supervised, but not directly placed by study participants, were excluded.
Outcome Measures
Pretest and posttest checklist scores from simulator‐trained residents were compared to measure the impact of training sessions. Residents rotating through the MICU were asked about several quality indicators related to actual CVC insertions. Quality indicators include: (1) number of needle passes required during the procedure (skin punctures); (2) presence of complications including pneumothorax and arterial puncture; and (3) need for CVC adjustment after chest x‐ray. Participants were also questioned regarding their confidence in CVC insertion using a 100 point scale (0 = not confident and 100 = very confident). Survey results from the 28 simulator‐trained residents were compared to results from the 13 traditionally‐trained residents.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges, using the kappa () coefficient adjusted25, 26 using the formula of Brennan and Prediger.27 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t‐tests.
MICU survey results were compared using t‐tests. Traditionally‐trained and simulator‐trained groups were assessed for demographic differences using t‐tests and the chi‐square statistic. Spearman's rank correlation coefficient was used to assess for relationships between resident self‐confidence and quality indicators. All analyses were preformed using SPSS statistical software, version 16.0 (SPSS, Inc., Chicago, IL).
Results
All eligible residents participated in the study and completed the entire protocol. There was no significant difference in age, gender, ethnicity, year of training, or USMLE Step 1 and 2 scores between the traditionally‐trained and simulator‐trained groups.
Interrater reliability measured by the mean kappa coefficient was very high (n = 0.94) across the 27 IJ and SC checklist items. No resident met the MPS (79.1%) for CVC insertion at baseline testing. In the simulator‐trained group, 25 of 28 (89%) residents achieved SC skill mastery and 27 of 28 (96%) achieved IJ skill mastery within the standard four hour curriculum. All residents subsequently reached the MPS with less than one hour of additional practice time. A graphic portrait of the residents' pretest and posttest performance on the simulated CVC clinical skills examination with descriptive statistics is shown in Figure 3. After the educational intervention, posttest scores significantly improved (p < 0.001), to meet or exceed the MPS.

Traditionally trained and simulator‐trained residents independently inserted 46 CVCs during the study period. Simulator‐trained residents required significantly fewer needle passes to insert all actual CVCs in the MICU compared to traditionally trained residents: mean (M) = 1.79, standard deviation (SD) = 1.03 versus M = 2.78, SD = 1.77 (p = 0.04). As shown in Table 1, the groups did not differ in pneumothorax, arterial puncture, or mean number of CVC adjustments. In addition, the groups did not differ in use of ultrasound for IJ or SC CVC insertions. One IJ CVC was inserted without ultrasound in the traditionally‐trained group; 2 were inserted without ultrasound in the simulator‐trained group. Ultrasound was not used during any SC CVC insertions in the traditionally‐trained group and was used for 1 SC CVC insertion in the simulator‐trained group.
| Internal Jugular and Subclavian CVCs | |||
|---|---|---|---|
| Traditionally Trained Residents | Simulator Trained Residents | P value | |
| |||
| Number of attempts during insertion [mean (SD)] | 2.78 (1.77) | 1.79 (1.03) | 0.04* |
| Pneumothorax (number) | 0 | 0 | n/a |
| Arterial puncture (%) | 11 | 7 | 0.65 |
| CVC adjustment (%) | 15 | 8 | 0.52 |
| Confidence (%) [mean (SD)] | 68 (20) | 81 (11) | 0.02* |
Simulator‐trained residents displayed more self‐confidence about their procedural skills than traditionally‐trained residents (M = 81, SD = 11 versus M = 68, SD = 20, p = 0.02). Spearman correlations showed no practical association between resident self‐confidence and performance on CVC insertion quality indicators.
Discussion
This study demonstrates the use of a mastery learning model to develop CVC insertion skills to a high achievement level among internal medicine residents. Our data support prior work showing that procedural skills that are poor at baseline can be increased significantly using simulation‐based training and deliberate practice.1118, 28 This report on CVC insertion adds to the growing body of literature showing that simulation training complements standard medical education,1119, 28 and expands the clinical application of the mastery model beyond thoracentesis and ACLS.11, 12 Use of the mastery model described in this study also has important implications for patients. In our training program, residents are required to demonstrate procedural mastery in a simulated environment before independently performing a CVC insertion on an actual patient. This is in sharp contrast to the traditional clinical model of procedural training at the bedside, and may be used in other training programs and with other invasive procedures.
The second aim of our study was to determine the impact of simulation‐based training on actual clinical practice by residents in the MICU. To our knowledge, no prior study has demonstrated that simulation‐based training in CVC insertion improves patient outcomes. We believe our results advance what is known about the impact of simulation‐based training because simulator‐trained residents in this study performed actual CVC insertions in the MICU using significantly fewer needle passes. Needle passes have been used by other investigators as a surrogate measure for reduced CVC‐associated complications because mechanical complications rise exponentially with more than two insertion attempts.47, 29 We believe this finding demonstrates transfer of skill acquired from simulation‐based training to the actual clinical environment. It is possible that ultrasound training accounts for the improvement in the simulator‐trained group. However, we do not believe that ultrasound training is entirely responsible as prior work has shown that deliberate practice using mastery learning without ultrasound significantly improved resident performance of thoracentesis11 and ACLS12, 19 procedures. We did not show a significant reduction in complications such as pneumothorax or arterial puncture. This is likely due to the small sample size and the low number of procedures and complications during the study period.
Our results also show that resident self‐confidence regarding actual CVC insertions improved after simulation training. These findings are similar to prior reports linking improved confidence among trainees after simulation‐based training in CVC insertion.29, 30 Our results did not reveal a correlation between improved self‐confidence and clinical skill acquisition. Linking improved self‐confidence to improved clinical skill is important because self‐assessment does not always correlate with performance ability.31, 32
More study is needed to evaluate the impact of simulation‐based training on the quality of CVC insertions by trainees. Mechanisms shown to decrease complications of CVC placement include use of ultrasound,4, 7, 3336 full sterile barrier technique,3739 chlorhexidine skin preparations,4042 and nurse‐physician education.43 Our simulation‐training program incorporates each of these elements. We plan to expand our simulation‐based training intervention to a larger sample size to determine its impact on mechanical and infectious complication rates linked to CVC insertion.
This study has several limitations. It was performed at a single institution over a short time period. However, demonstration of significantly fewer needle passes and improved resident self‐confidence after simulator training are important findings that warrant further study. It was impossible to blind raters during the skills assessment examination about whether the resident was performing a pretest or posttest. This was accounted for by using a second rater, who was blind to the pretest and posttest status of the examinee. The arterial puncture rate of 7% among simulator‐trained residents was higher than expected, although it remains within published ranges.4, 5 Also, a low total number of CVCs were evaluated during the study. This is likely due to strict exclusion criteria employed in order to study the impact of simulation training. For example, CVC insertions were only evaluated if they were actually performed by study residents (supervised insertions were excluded) and femoral catheters were not evaluated. We did not track clinical experience with CVC insertion by residents before the study. Residents who were simulator‐trained may have had more clinical experience with CVC insertion and this may have impacted their performance. However, residents did not differ in year of training or clinical rotations, and there is clear evidence that clinical training is not a proxy for skill acquisition.44 Finally, outcome data were measured via resident questionnaires that relied on resident recall about CVC insertion rather than observer ratings. This method was selected because observer ratings could not be standardized given the large number of clinical supervisors in the MICU over the study period. Information about needle passes and arterial puncture also may not be documented in procedural notes and could not be obtained by medical record review. We attempted to minimize recall bias by surveying residents within 24 hours of CVC placement.
In conclusion, this study demonstrates that simulation‐based training and deliberate practice in a mastery learning setting improves performance of both simulated and actual CVC insertions by internal medicine residents. Procedural training remains an important component of internal medicine training, although internists are performing fewer invasive procedures now than in years past.45, 46 Use of a mastery model of CVC insertion requires that trainees demonstrate skill in a simulated environment before independently performing this invasive procedure on patients. Further study is needed to assess clinical outcomes such as reduced CVC‐related infections and mechanical complications after simulation‐based training.
Acknowledgements
The authors thank the Northwestern University internal medicine residents for their dedication to education and patient care. They acknowledge Drs. J. Larry Jameson and Charles Watts for their support and encouragement of this work.
Appendix
Central Venous Catheter Insertion Checklists for Simulation‐based Education 0, 0
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to keep brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| The ultrasound (US) probe is properly set up with sterile sheath and sonographic gel | A | B | C |
| The vein is localized using anatomical landmarks with the US machine | A | B | C |
| If no US is used this is wrong | |||
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter‐ syringe complex, cannulate the vein while aspirating (must be done with US) | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to leave brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| **The US probe is properly set up with sterile sheath and sonographic gel . (MUST DO if use US) | A | B | C |
| The vein is localized using US machine or anatomical landmarks are verbalized | A | B | C |
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized using a larger needle (must verbalize they anesthetize the clavicle) | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter syringe complex cannulate the vein while aspirating (optional confirmed by US) | A | B | C |
| If US was not used then expected to state they are directing the needle to the sternal notch | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
Central venous catheter (CVC) insertions are commonly performed at the bedside in medical intensive care unit (MICU) settings. Internal medicine residents are required to demonstrate knowledge regarding CVC indications, complications, and sterile technique,1 and often perform the procedure during training. Education in CVC insertion is needed because many internal medicine residents are uncomfortable performing this procedure.2 CVC insertion also carries the risk of potentially life‐threatening complications including infection, pneumothorax, arterial puncture, deep vein thrombosis, and bleeding. Education and training may also contribute to improved patient care because increased physician experience with CVC insertion reduces complication risk.3, 4 Similarly, a higher number of needle passes or attempts during CVC insertion correlates with mechanical complications such as pneumothorax or arterial punctures.48 Pneumothorax rates for internal jugular (IJ) CVCs have been reported to range from 0% to 0.2% and for subclavian (SC) CVCs from 1.5% to 3.1%.4, 5 The arterial puncture rate for IJ CVCs ranges from 5.0% to 9.4% and for SC CVCs from 3.1% to 4.9%.4, 5 Proper use of ultrasound to assist with IJ CVC insertion has been shown to decrease these mechanical complications.4, 5 However, studies of ultrasound use with SC CVC insertion have mixed results.4
Simulation‐based training has been used in medical education to increase knowledge, provide opportunities for deliberate and safe practice, and shape the development of clinical skills.9, 10 We previously used simulation‐based mastery learning to improve the thoracentesis and advanced cardiac life support (ACLS) skills of internal medicine residents.11, 12 Although a few small studies have linked simulation‐based interventions to improved quality of care,1319 more work is needed to show that results from a simulated environment transfer to actual patient care.
This study had 2 aims. The first was to expand our simulation‐based mastery learning to CVC insertion using a CVC simulator and ultrasound device. The second was to assess quality indicators (number of needle passes, pneumothorax, arterial punctures, and need for catheter adjustment) and resident confidence related to actual CVC insertions in the MICU before and after an educational intervention.
Materials and Methods
Design
This was a cohort study20 of IJ and SC CVC insertions by 41 second‐ and third‐year internal medicine residents rotating through the MICU in a university‐affiliated program from October 2006 to February 2007. The Northwestern University Institutional Review Board approved the study. All study participants were required to give informed consent prior to participation.
Thirteen residents rotated through the MICU during a 6‐week preintervention phase. These residents served as a traditionally trained group that did not receive CVC insertion simulator training. Simultaneously, 28 residents who rotated through the MICU later in the study period received simulation‐based training in CVC insertion and served as the simulator‐trained group (Figure 1). Demographic data were obtained from the participants including age, gender, ethnicity, year of training, and scores on the United States Medical Licensing Examination (USMLE) Steps 1 and 2.

Simulator‐trained residents underwent baseline skill assessment (pretest) using a 27‐item checklist in IJ and SC CVC insertions (see Appendix). Checklists were developed by one author (J.H.B.) using appropriate references4, 5 and a step‐by‐step process,21 and reviewed for completeness by another author with expertise in checklist development (D.B.W.). Each skill or other action was listed in order and given equal weight. A dichotomous scoring scale of 1 = done correctly and 0 = done incorrectly/not done was imposed for each item. Assessments were performed using Simulab's CentralLineMan. This model features realistic tissue with ultrasound compatibility, an arterial pulse, and self‐sealing veins and skins. Needles, dilators, and guidewires can be inserted and realistic venous and arterial pressures demonstrated (Figure 2).

Residents in the simulator‐trained group received two, 2‐hour education sessions featuring a lecture, ultrasound training, deliberate practice with the CVC simulator, and feedback.22 Education sessions contained standardized didactic material on CVC indications and complications, as well as a stepwise demonstration of IJ and SC CVC insertions using ultrasound and landmark techniques. These sessions were supervised by a senior hospitalist faculty member with expertise in CVC insertions (J.H.B.). Residents were expected to use the ultrasound device for all IJ CVC insertions. However, its use was optional for SC CVC insertion. After training, residents were retested (posttest) and required to meet or exceed a minimum passing score (MPS) set by an expert panel for both IJ and SC procedures.23 This 11 member expert panel provided item‐based (Angoff) and group‐based (Hofstee) judgments on the 27‐item checklists as described previously.23
Residents who did not achieve the MPS had more deliberate practice and were retested until the MPS was reached; the key feature of mastery learning.24 After completing simulation‐based mastery learning in CVC insertion, the 28 simulator‐trained residents rotated through the MICU.
Data Collection
All pretests and posttests (using the 27‐item checklist) were graded by a single unblinded instructor (J.H.B.) and were videotaped. Another faculty instructor with expertise in scoring clinical skills examinations and blind to pre‐post status (D.B.W.) rescored a random 50% sample of the tests to assess interrater reliability.
Data regarding actual CVC insertions in the MICU were collected by contacting all MICU residents daily during the study period. This allowed for CVC insertions to be identified within 24 hours. All survey data were collected anonymously. The primary inserter of each CVC was questioned about quality indicators and procedural self‐confidence concerning CVC placement. CVCs primarily inserted by nonstudy subjects (first‐year residents, emergency medicine residents, pulmonary‐critical care medicine faculty members, and subspecialty fellows) or CVC placements that were supervised, but not directly placed by study participants, were excluded.
Outcome Measures
Pretest and posttest checklist scores from simulator‐trained residents were compared to measure the impact of training sessions. Residents rotating through the MICU were asked about several quality indicators related to actual CVC insertions. Quality indicators include: (1) number of needle passes required during the procedure (skin punctures); (2) presence of complications including pneumothorax and arterial puncture; and (3) need for CVC adjustment after chest x‐ray. Participants were also questioned regarding their confidence in CVC insertion using a 100 point scale (0 = not confident and 100 = very confident). Survey results from the 28 simulator‐trained residents were compared to results from the 13 traditionally‐trained residents.
Data Analysis
Checklist score reliability was estimated by calculating interrater reliability, the preferred method for assessments that depend on human judges, using the kappa () coefficient adjusted25, 26 using the formula of Brennan and Prediger.27 Within‐group differences from pretest (baseline) to posttest (outcome) were analyzed using paired t‐tests.
MICU survey results were compared using t‐tests. Traditionally‐trained and simulator‐trained groups were assessed for demographic differences using t‐tests and the chi‐square statistic. Spearman's rank correlation coefficient was used to assess for relationships between resident self‐confidence and quality indicators. All analyses were preformed using SPSS statistical software, version 16.0 (SPSS, Inc., Chicago, IL).
Results
All eligible residents participated in the study and completed the entire protocol. There was no significant difference in age, gender, ethnicity, year of training, or USMLE Step 1 and 2 scores between the traditionally‐trained and simulator‐trained groups.
Interrater reliability measured by the mean kappa coefficient was very high (n = 0.94) across the 27 IJ and SC checklist items. No resident met the MPS (79.1%) for CVC insertion at baseline testing. In the simulator‐trained group, 25 of 28 (89%) residents achieved SC skill mastery and 27 of 28 (96%) achieved IJ skill mastery within the standard four hour curriculum. All residents subsequently reached the MPS with less than one hour of additional practice time. A graphic portrait of the residents' pretest and posttest performance on the simulated CVC clinical skills examination with descriptive statistics is shown in Figure 3. After the educational intervention, posttest scores significantly improved (p < 0.001), to meet or exceed the MPS.

Traditionally trained and simulator‐trained residents independently inserted 46 CVCs during the study period. Simulator‐trained residents required significantly fewer needle passes to insert all actual CVCs in the MICU compared to traditionally trained residents: mean (M) = 1.79, standard deviation (SD) = 1.03 versus M = 2.78, SD = 1.77 (p = 0.04). As shown in Table 1, the groups did not differ in pneumothorax, arterial puncture, or mean number of CVC adjustments. In addition, the groups did not differ in use of ultrasound for IJ or SC CVC insertions. One IJ CVC was inserted without ultrasound in the traditionally‐trained group; 2 were inserted without ultrasound in the simulator‐trained group. Ultrasound was not used during any SC CVC insertions in the traditionally‐trained group and was used for 1 SC CVC insertion in the simulator‐trained group.
| Internal Jugular and Subclavian CVCs | |||
|---|---|---|---|
| Traditionally Trained Residents | Simulator Trained Residents | P value | |
| |||
| Number of attempts during insertion [mean (SD)] | 2.78 (1.77) | 1.79 (1.03) | 0.04* |
| Pneumothorax (number) | 0 | 0 | n/a |
| Arterial puncture (%) | 11 | 7 | 0.65 |
| CVC adjustment (%) | 15 | 8 | 0.52 |
| Confidence (%) [mean (SD)] | 68 (20) | 81 (11) | 0.02* |
Simulator‐trained residents displayed more self‐confidence about their procedural skills than traditionally‐trained residents (M = 81, SD = 11 versus M = 68, SD = 20, p = 0.02). Spearman correlations showed no practical association between resident self‐confidence and performance on CVC insertion quality indicators.
Discussion
This study demonstrates the use of a mastery learning model to develop CVC insertion skills to a high achievement level among internal medicine residents. Our data support prior work showing that procedural skills that are poor at baseline can be increased significantly using simulation‐based training and deliberate practice.1118, 28 This report on CVC insertion adds to the growing body of literature showing that simulation training complements standard medical education,1119, 28 and expands the clinical application of the mastery model beyond thoracentesis and ACLS.11, 12 Use of the mastery model described in this study also has important implications for patients. In our training program, residents are required to demonstrate procedural mastery in a simulated environment before independently performing a CVC insertion on an actual patient. This is in sharp contrast to the traditional clinical model of procedural training at the bedside, and may be used in other training programs and with other invasive procedures.
The second aim of our study was to determine the impact of simulation‐based training on actual clinical practice by residents in the MICU. To our knowledge, no prior study has demonstrated that simulation‐based training in CVC insertion improves patient outcomes. We believe our results advance what is known about the impact of simulation‐based training because simulator‐trained residents in this study performed actual CVC insertions in the MICU using significantly fewer needle passes. Needle passes have been used by other investigators as a surrogate measure for reduced CVC‐associated complications because mechanical complications rise exponentially with more than two insertion attempts.47, 29 We believe this finding demonstrates transfer of skill acquired from simulation‐based training to the actual clinical environment. It is possible that ultrasound training accounts for the improvement in the simulator‐trained group. However, we do not believe that ultrasound training is entirely responsible as prior work has shown that deliberate practice using mastery learning without ultrasound significantly improved resident performance of thoracentesis11 and ACLS12, 19 procedures. We did not show a significant reduction in complications such as pneumothorax or arterial puncture. This is likely due to the small sample size and the low number of procedures and complications during the study period.
Our results also show that resident self‐confidence regarding actual CVC insertions improved after simulation training. These findings are similar to prior reports linking improved confidence among trainees after simulation‐based training in CVC insertion.29, 30 Our results did not reveal a correlation between improved self‐confidence and clinical skill acquisition. Linking improved self‐confidence to improved clinical skill is important because self‐assessment does not always correlate with performance ability.31, 32
More study is needed to evaluate the impact of simulation‐based training on the quality of CVC insertions by trainees. Mechanisms shown to decrease complications of CVC placement include use of ultrasound,4, 7, 3336 full sterile barrier technique,3739 chlorhexidine skin preparations,4042 and nurse‐physician education.43 Our simulation‐training program incorporates each of these elements. We plan to expand our simulation‐based training intervention to a larger sample size to determine its impact on mechanical and infectious complication rates linked to CVC insertion.
This study has several limitations. It was performed at a single institution over a short time period. However, demonstration of significantly fewer needle passes and improved resident self‐confidence after simulator training are important findings that warrant further study. It was impossible to blind raters during the skills assessment examination about whether the resident was performing a pretest or posttest. This was accounted for by using a second rater, who was blind to the pretest and posttest status of the examinee. The arterial puncture rate of 7% among simulator‐trained residents was higher than expected, although it remains within published ranges.4, 5 Also, a low total number of CVCs were evaluated during the study. This is likely due to strict exclusion criteria employed in order to study the impact of simulation training. For example, CVC insertions were only evaluated if they were actually performed by study residents (supervised insertions were excluded) and femoral catheters were not evaluated. We did not track clinical experience with CVC insertion by residents before the study. Residents who were simulator‐trained may have had more clinical experience with CVC insertion and this may have impacted their performance. However, residents did not differ in year of training or clinical rotations, and there is clear evidence that clinical training is not a proxy for skill acquisition.44 Finally, outcome data were measured via resident questionnaires that relied on resident recall about CVC insertion rather than observer ratings. This method was selected because observer ratings could not be standardized given the large number of clinical supervisors in the MICU over the study period. Information about needle passes and arterial puncture also may not be documented in procedural notes and could not be obtained by medical record review. We attempted to minimize recall bias by surveying residents within 24 hours of CVC placement.
In conclusion, this study demonstrates that simulation‐based training and deliberate practice in a mastery learning setting improves performance of both simulated and actual CVC insertions by internal medicine residents. Procedural training remains an important component of internal medicine training, although internists are performing fewer invasive procedures now than in years past.45, 46 Use of a mastery model of CVC insertion requires that trainees demonstrate skill in a simulated environment before independently performing this invasive procedure on patients. Further study is needed to assess clinical outcomes such as reduced CVC‐related infections and mechanical complications after simulation‐based training.
Acknowledgements
The authors thank the Northwestern University internal medicine residents for their dedication to education and patient care. They acknowledge Drs. J. Larry Jameson and Charles Watts for their support and encouragement of this work.
Appendix
Central Venous Catheter Insertion Checklists for Simulation‐based Education 0, 0
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to keep brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| The ultrasound (US) probe is properly set up with sterile sheath and sonographic gel | A | B | C |
| The vein is localized using anatomical landmarks with the US machine | A | B | C |
| If no US is used this is wrong | |||
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter‐ syringe complex, cannulate the vein while aspirating (must be done with US) | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
| |||
| Informed consent obtained: must do all | A | B | C |
| Benefits | |||
| Risks | |||
| Consent given | |||
| Place the patient in slight Trendelenburg position | A | B | C |
| Flush the ports on the catheter with sterile saline | A | B | C |
| Clamp each port (ok to leave brown port open) | A | B | C |
| Remove brown port from end of catheter to accommodate wire | A | B | C |
| Area is cleaned with chlorhexadine | A | B | C |
| Resident gets in sterile gown, gloves, hat and mask | A | B | C |
| Area is draped in usual sterile fashion (must be full body drape) | A | B | C |
| **The US probe is properly set up with sterile sheath and sonographic gel . (MUST DO if use US) | A | B | C |
| The vein is localized using US machine or anatomical landmarks are verbalized | A | B | C |
| The skin is anesthetized with 1% lidocaine in a small wheal | A | B | C |
| The deeper structures are anesthetized using a larger needle (must verbalize they anesthetize the clavicle) | A | B | C |
| Localize the vein with this needle (optional) | A | B | C |
| Using the large needle or catheter syringe complex cannulate the vein while aspirating (optional confirmed by US) | A | B | C |
| If US was not used then expected to state they are directing the needle to the sternal notch | A | B | C |
| Remove the syringe from the needle or advance the catheter into the vein removing both the syringe and needle | A | B | C |
| Advance the guidewire into the vein no more than approximately 1215 cm | A | B | C |
| Knick the skin with the scalpel to advance the dilator | A | B | C |
| Advance the dilator over the guidewire and dilate the vein | A | B | C |
| Advance the triple lumen over the guidewire | A | B | C |
| Never let go of the guidewire | A | B | C |
| Once the catheter is inserted remove the guidewire in its entirety | A | B | C |
| Advance the catheter to approx to 1416cm on the right side, 1618 cm on the left side | A | B | C |
| Ensure there is blood flow/flush each port | A | B | C |
| Secure the catheter in place (suture or staple) | A | B | C |
| Place dressing over catheter | A | B | C |
| Get a chest x‐ray | A | B | C |
| Notify that the catheter is ok to use | A | B | C |
| Maintain sterile technique | A | B | C |
- American Board of Internal Medicine. Procedures Required for Internal Medicine. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed January 28, 2009.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,, et al.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,, et al.Mechanical complications of central venous catheters.J Intensive Care Med.2006;21:40–46.
- ,,, et al.Risk factors of failure and immediate complication of subclavian vein catheterization in critically ill patients.Intensive Care Med.2002;28:1036–1041.
- ,,, et al.Complications and failures of subclavian‐vein catheterization.N Engl J Med.1994;331:1735–1738.
- .Central venous catheterization: better and worse.J Intensive Care Med.2006;21:51–53.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3:48–54.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- ,,, et al.Laparoscopic skills are improved with LapMentor training: results of a randomized, double‐blinded study.Ann Surg.2006;243:854–860.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Multicenter, randomized, controlled trial of virtual‐reality simulator training in acquisition of competency in colonoscopy.Gastrointest Endosc.2006;64:361–368.
- ,,, et al.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,.Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study.Am J Gastroenterol.2004;99:33–37.
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–463.
- ,,, et al.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.CHEST.2008;133:56–61.
- ,.Clinical Epidemiology: the Essentials.4th ed.Philadelphia:Lippincott Williams 2005.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July2000. Available at: http://www. wmich.edu/evalctr/checklists/cdc.htm. Accessed May 15, 2006.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79:S70–S81.
- ,,, et al.Do baseline data influence standard setting for a clinical skills examination?Acad Med.2007;82:S105–S108.
- ,,, et al.Lessons for Continuing Medical Education from simulation research in undergraduate and graduate medical education.CHEST.2009;135.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 41:687–699.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:202–208.
- ,,.Central catheter simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,.The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative.J Gen Intern Med.2006;21:514–517.
- ,,, et al.The use of simulation in emergency medicine: a research agenda.Acad Emerg Med.2007;14:353–363.
- ,,, et al.Graduating internal medicine residents' self‐assessment and performance of advanced cardiac life support skills.Med Teach.2006;28:365–369.
- ,.Bedside ultrasonography in the ICU: Part 2.CHEST.2005;128:1766–1781.
- ,,, et al.Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein: a randomized study.Anesthesiology.1998;88:1195–1201.
- ,,, et al.Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department.Acad Emerg Med.2002;9:800–805.
- ,,, et al.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24:2053–2058.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,,, et al.Chlorhexidine compared with povidone‐iodine solution for vascular catheter‐site care: a meta‐analysis.Ann Intern Med.2002;136:792–801.
- ,,.Prospective randomised trial of povidone‐iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters.Lancet.1991;338:339–343.
- ,,, et al.Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients.Crit Care Med.1996;24:1818–1823.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.CHEST.2004;126:1612–1618.
- ,,.Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142:260–273.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–393.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- American Board of Internal Medicine. Procedures Required for Internal Medicine. Available at: http://www.abim.org/certification/policies/imss/im.aspx. Accessed January 28, 2009.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,, et al.Central vein catheterization. Failure and complication rates by three percutaneous approaches.Arch Intern Med.1986;146:259–261.
- ,.Preventing complications of central venous catheterization.N Engl J Med.2003;348:1123–1133.
- ,,, et al.Mechanical complications of central venous catheters.J Intensive Care Med.2006;21:40–46.
- ,,, et al.Risk factors of failure and immediate complication of subclavian vein catheterization in critically ill patients.Intensive Care Med.2002;28:1036–1041.
- ,,, et al.Complications and failures of subclavian‐vein catheterization.N Engl J Med.1994;331:1735–1738.
- .Central venous catheterization: better and worse.J Intensive Care Med.2006;21:51–53.
- ,,, et al.Reliability and validity of a simulation‐based acute care skills assessment for medical students and residents.Anesthesiology.2003;99:1270–1280.
- ,,, et al.Simulation technology for health care professional skills training and assessment.JAMA.1999;282:861–866.
- ,,, et al.Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3:48–54.
- ,,, et al.Mastery learning of advanced cardiac life support skills by internal medicine residents using simulation technology and deliberate practice.J Gen Intern Med.2006;21:251–256.
- ,,, et al.Laparoscopic skills are improved with LapMentor training: results of a randomized, double‐blinded study.Ann Surg.2006;243:854–860.
- ,,.Bronchoscopy simulator effectively prepares junior residents to competently perform basic clinical bronchoscopy.Ann Thorac Surg.2004;78:287–291.
- ,,, et al.Multicenter, randomized, controlled trial of virtual‐reality simulator training in acquisition of competency in colonoscopy.Gastrointest Endosc.2006;64:361–368.
- ,,, et al.Achieving house staff competence in emergency airway management: results of a teaching program using a computerized patient simulator.Crit Care Med.2004;32:2422–2427.
- ,.Computer simulator training enhances the competency of gastroenterology fellows at colonoscopy: results of a pilot study.Am J Gastroenterol.2004;99:33–37.
- ,,, et al.Virtual reality training improves operating room performance: results of a randomized, double‐blinded study.Ann Surg.2002;236:458–463.
- ,,, et al.Simulation‐based education improves quality of care during cardiac arrest team responses at an academic teaching hospital: a case‐control study.CHEST.2008;133:56–61.
- ,.Clinical Epidemiology: the Essentials.4th ed.Philadelphia:Lippincott Williams 2005.
- . The Checklists Development Checklist. Western Michigan University Evaluation Center, July2000. Available at: http://www. wmich.edu/evalctr/checklists/cdc.htm. Accessed May 15, 2006.
- .Deliberate practice and the acquisition and maintenance of expert performance in medicine and related domains.Acad Med.2004;79:S70–S81.
- ,,, et al.Do baseline data influence standard setting for a clinical skills examination?Acad Med.2007;82:S105–S108.
- ,,, et al.Lessons for Continuing Medical Education from simulation research in undergraduate and graduate medical education.CHEST.2009;135.
- .Reliability: on the reproducibility of assessment data.Med Educ.2004;38:1006–1012.
- ,,.Statistical Methods for Rates and Proportions.3rd ed.New York:John Wiley 41:687–699.
- ,,, et al.Simulation‐based training of internal medicine residents in advanced cardiac life support protocols: a randomized trial.Teach Learn Med.2005;17:202–208.
- ,,.Central catheter simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,.The use of tissue models for vascular access training. Phase I of the procedural patient safety initiative.J Gen Intern Med.2006;21:514–517.
- ,,, et al.The use of simulation in emergency medicine: a research agenda.Acad Emerg Med.2007;14:353–363.
- ,,, et al.Graduating internal medicine residents' self‐assessment and performance of advanced cardiac life support skills.Med Teach.2006;28:365–369.
- ,.Bedside ultrasonography in the ICU: Part 2.CHEST.2005;128:1766–1781.
- ,,, et al.Pulsed Doppler ultrasonography guidance for catheterization of the subclavian vein: a randomized study.Anesthesiology.1998;88:1195–1201.
- ,,, et al.Ultrasound guidance versus the landmark technique for the placement of central venous catheters in the emergency department.Acad Emerg Med.2002;9:800–805.
- ,,, et al.Ultrasound guidance for placement of central venous catheters: a meta‐analysis of the literature.Crit Care Med.1996;24:2053–2058.
- ,,, et al.Eliminating catheter‐related bloodstream infections in the intensive care unit.Crit Care Med.2004;32:2014–2020.
- ,,, et al.An intervention to decrease catheter‐related bloodstream infections in the ICU.N Engl J Med.2006;355:2725–2732.
- ,,, et al.Education of physicians‐in‐training can decrease the risk for vascular catheter infection.Ann Intern Med.2000;132:641–648.
- ,,, et al.Chlorhexidine compared with povidone‐iodine solution for vascular catheter‐site care: a meta‐analysis.Ann Intern Med.2002;136:792–801.
- ,,.Prospective randomised trial of povidone‐iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters.Lancet.1991;338:339–343.
- ,,, et al.Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients.Crit Care Med.1996;24:1818–1823.
- ,,, et al.The effect of an education program on the incidence of central venous catheter‐associated bloodstream infection in a medical ICU.CHEST.2004;126:1612–1618.
- ,,.Systematic review: the relationship between clinical experience and quality of health care.Ann Intern Med.2005;142:260–273.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–393.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
Copyright © 2009 Society of Hospital Medicine
Bleeding Factors
An 80‐year‐old man with coronary artery disease and chronic obstructive pulmonary disease (COPD) was admitted to an outside hospital after a mechanical fall. On presentation to the emergency room his systolic blood pressure was found to be 86/62 mm Hg. He complained of right flank, groin, and thigh pain. On physical exam, a hematoma extending from his right groin down to his right knee was found, as well as scattered ecchymoses involving his trunk and all 4 extremities. His hemoglobin was low, at 5.6 g/dL (14‐17 g/dL). A computed tomography (CT) scan revealed a right‐sided retroperitoneal bleed extending from the iliopsoas into his right thigh. The patient received 13 transfusions of packed red blood cells over the course of 9 days as he continued to bleed. Transfer to our facility for further workup and management ensued.
On serial testing at our institution his activated partial thromboplastin time (aPTT) was elevated at >160 seconds (normal range, 24‐36 seconds). Further coagulation parameters were found as follows: platelets 182.000/L; prothrombin time 17.6 seconds; international normalized ratio (INR) 1.4; thrombin 18 seconds; fibrinogen 778 mg/dL; and D‐dimer 3866 ng/mL. Of note, the patient had not received any medications known to potentially interfere with the measured aPTT. Because the source of his bleeding was not apparent at this point, disorders of primary hemostasis, including hereditary disease states (eg, von Willebrand disease), iatrogenic disorders (eg, drug‐induced), or acquired disorders, such as immune thrombocytopenia, were considered and ruled out. At this point the differential diagnoses had to be expanded, and secondary disorders of hemostasis were considered. A deficiency or decreased activity of coagulation factors was suspected. Whereas factor IX and XI were found to be normal, the factor VIII level was significantly decreased at 3% (50%‐150% being normal). This prompted an assay to check for the presence of a factor VIII inhibitor. It proved to be significantly elevated at 25.6 Bethesda Units (BU) (normal, 0.00‐0.04 BU). On that basis we arrived at the diagnosis of acquired factor VIII deficiency, but the etiology of such remained unclear to this point. Hence a search for the specific etiology of acquired factor IIII deficiency was launched, and connective tissue disease, as well as malignancy, was ruled out. While inflammatory bowel disease is a known potential cause for this condition, the clinical picture was not consistent with such and this diagnosis was not considered further. The patient received immunosuppressive therapy with prednisone 1 mg/kg orally per day. Rituximab and cyclophosphamide were considered, but due to bacteremia from bilateral parotitis, this was deferred. Of note, his bleeding abnormality was apparent prior to initiation of antibiotic therapy.
The bleeding stopped 2 days after initiation of treatment. At the time of discharge, 2 weeks after presentation, factor VIII inhibitor levels had decreased to 13 BU and his partial thromboplastin time (PTT) was 100 seconds.
Discussion
Acquired factor VIII inhibitor, also called acquired hemophilia A, is a rare, potentially life‐threatening bleeding disorder. It is caused by autoantibodies directed against coagulation factor VIII.1
The estimated incidence in the general population is 1 in 4 million/year. Risk factors include advanced age, pregnancy and the postpartum period, rheumatoid disease/connective tissue disease, inflammatory bowel disease, medications (especially antibiotics and psychiatric drugs), and malignancy. Both solid tumors as well as hematologic malignancies have been associated with acquired hemophilia A.2
Patients older than 85 years are more frequently affected. The annual incidence is 14.7 in 1 million in this age group. Hence, it is found rarely in young patients, but pregnancy and the postpartum period represent the exception.
Patients with acquired factor VIII inhibitor tend to bleed into the skin, soft tissue, muscle, brain, and mucous membranes. Most of the time, they present with epistaxis, retroperitoneal hematomas, or gastrointestinal bleeds, while patients with congenital factor VIII deficiency3 are more likely to bleed into the large joints. Acquired factor VIII inhibitor is associated with a high morbidity and mortality.
In the presence of an isolated elevated aPTT, once heparin has been ruled out, specific factor deficiencies and/or inhibitors need to be considered. The inhibitor assay helps to establish the diagnosis of acquired factor VIII deficiency and allows the quantification of factor VIII inhibitor. A search for specific etiologies of acquired factor VIII inhibitors should be undertaken; however, in 50% of cases no concomitant condition is found. The differential diagnoses should be expanded within the appropriate framework and tailored to the individual patient.
Control of bleeding might be achieved by factor VIII concentrate if the bleeding is mild. However, if the hemorrhage is life‐threatening, recombinant factor VII is frequently required to stop the bleeding.4 One has to be aware that recombinant factor VII may precipitate thromboembolic events and as such might pose a dilemma, as the degree of bleeding has to be balanced with the risk of unintended side effects. Therapy to eliminate factor VIII inhibitor is the combination of prednisone and cyclophosphamide, though monoclonal CD20 antibody (Rituximab) has become the first‐line agent in the appropriate setting.5 Risk and benefit of therapy have to be balanced with the severity of the bleed and potential unintended side effects of immunosuppression, especially in the presence of infection.
As hospitalists, we are challenged daily by a high degree of complexities in inpatient care. Hospitalists are well trained to manage a wide variety of conditions, and coagulopathies are no exception. They are so common in the inpatient setting that every hospitalist should be familiar with the basic principles of diagnosing and managing bleeding disorders. Because of the hospitalist's ability to promptly react, the consulting role of the hematologist can be reserved for the more unusual blood dyscrasias.
This article is intended to raise physician awareness for the discussed condition because early recognition and treatment are of paramount importance in patient outcome.
- ,.Acquired inhibitors.Bailleres Clin Haematol.1996:9:331–354.
- ,,,.Acquired hemophilia A: a concise review.Am J Hematol.2005;80:50–63.
- ,.Acquired hemophilia.Rev Clin Exp Hematol.2005;5:389–404.
- ,,,.Treatment of acquired haemophilia with recombinant activated VII: a critical appraisal.Haemophilia.2007;13:451–461.
- ,,,.Selective B‐cell depletion with rituximab for the treatment of patients with acquired hemophilia.Blood.2004:103:4424–4428.
An 80‐year‐old man with coronary artery disease and chronic obstructive pulmonary disease (COPD) was admitted to an outside hospital after a mechanical fall. On presentation to the emergency room his systolic blood pressure was found to be 86/62 mm Hg. He complained of right flank, groin, and thigh pain. On physical exam, a hematoma extending from his right groin down to his right knee was found, as well as scattered ecchymoses involving his trunk and all 4 extremities. His hemoglobin was low, at 5.6 g/dL (14‐17 g/dL). A computed tomography (CT) scan revealed a right‐sided retroperitoneal bleed extending from the iliopsoas into his right thigh. The patient received 13 transfusions of packed red blood cells over the course of 9 days as he continued to bleed. Transfer to our facility for further workup and management ensued.
On serial testing at our institution his activated partial thromboplastin time (aPTT) was elevated at >160 seconds (normal range, 24‐36 seconds). Further coagulation parameters were found as follows: platelets 182.000/L; prothrombin time 17.6 seconds; international normalized ratio (INR) 1.4; thrombin 18 seconds; fibrinogen 778 mg/dL; and D‐dimer 3866 ng/mL. Of note, the patient had not received any medications known to potentially interfere with the measured aPTT. Because the source of his bleeding was not apparent at this point, disorders of primary hemostasis, including hereditary disease states (eg, von Willebrand disease), iatrogenic disorders (eg, drug‐induced), or acquired disorders, such as immune thrombocytopenia, were considered and ruled out. At this point the differential diagnoses had to be expanded, and secondary disorders of hemostasis were considered. A deficiency or decreased activity of coagulation factors was suspected. Whereas factor IX and XI were found to be normal, the factor VIII level was significantly decreased at 3% (50%‐150% being normal). This prompted an assay to check for the presence of a factor VIII inhibitor. It proved to be significantly elevated at 25.6 Bethesda Units (BU) (normal, 0.00‐0.04 BU). On that basis we arrived at the diagnosis of acquired factor VIII deficiency, but the etiology of such remained unclear to this point. Hence a search for the specific etiology of acquired factor IIII deficiency was launched, and connective tissue disease, as well as malignancy, was ruled out. While inflammatory bowel disease is a known potential cause for this condition, the clinical picture was not consistent with such and this diagnosis was not considered further. The patient received immunosuppressive therapy with prednisone 1 mg/kg orally per day. Rituximab and cyclophosphamide were considered, but due to bacteremia from bilateral parotitis, this was deferred. Of note, his bleeding abnormality was apparent prior to initiation of antibiotic therapy.
The bleeding stopped 2 days after initiation of treatment. At the time of discharge, 2 weeks after presentation, factor VIII inhibitor levels had decreased to 13 BU and his partial thromboplastin time (PTT) was 100 seconds.
Discussion
Acquired factor VIII inhibitor, also called acquired hemophilia A, is a rare, potentially life‐threatening bleeding disorder. It is caused by autoantibodies directed against coagulation factor VIII.1
The estimated incidence in the general population is 1 in 4 million/year. Risk factors include advanced age, pregnancy and the postpartum period, rheumatoid disease/connective tissue disease, inflammatory bowel disease, medications (especially antibiotics and psychiatric drugs), and malignancy. Both solid tumors as well as hematologic malignancies have been associated with acquired hemophilia A.2
Patients older than 85 years are more frequently affected. The annual incidence is 14.7 in 1 million in this age group. Hence, it is found rarely in young patients, but pregnancy and the postpartum period represent the exception.
Patients with acquired factor VIII inhibitor tend to bleed into the skin, soft tissue, muscle, brain, and mucous membranes. Most of the time, they present with epistaxis, retroperitoneal hematomas, or gastrointestinal bleeds, while patients with congenital factor VIII deficiency3 are more likely to bleed into the large joints. Acquired factor VIII inhibitor is associated with a high morbidity and mortality.
In the presence of an isolated elevated aPTT, once heparin has been ruled out, specific factor deficiencies and/or inhibitors need to be considered. The inhibitor assay helps to establish the diagnosis of acquired factor VIII deficiency and allows the quantification of factor VIII inhibitor. A search for specific etiologies of acquired factor VIII inhibitors should be undertaken; however, in 50% of cases no concomitant condition is found. The differential diagnoses should be expanded within the appropriate framework and tailored to the individual patient.
Control of bleeding might be achieved by factor VIII concentrate if the bleeding is mild. However, if the hemorrhage is life‐threatening, recombinant factor VII is frequently required to stop the bleeding.4 One has to be aware that recombinant factor VII may precipitate thromboembolic events and as such might pose a dilemma, as the degree of bleeding has to be balanced with the risk of unintended side effects. Therapy to eliminate factor VIII inhibitor is the combination of prednisone and cyclophosphamide, though monoclonal CD20 antibody (Rituximab) has become the first‐line agent in the appropriate setting.5 Risk and benefit of therapy have to be balanced with the severity of the bleed and potential unintended side effects of immunosuppression, especially in the presence of infection.
As hospitalists, we are challenged daily by a high degree of complexities in inpatient care. Hospitalists are well trained to manage a wide variety of conditions, and coagulopathies are no exception. They are so common in the inpatient setting that every hospitalist should be familiar with the basic principles of diagnosing and managing bleeding disorders. Because of the hospitalist's ability to promptly react, the consulting role of the hematologist can be reserved for the more unusual blood dyscrasias.
This article is intended to raise physician awareness for the discussed condition because early recognition and treatment are of paramount importance in patient outcome.
An 80‐year‐old man with coronary artery disease and chronic obstructive pulmonary disease (COPD) was admitted to an outside hospital after a mechanical fall. On presentation to the emergency room his systolic blood pressure was found to be 86/62 mm Hg. He complained of right flank, groin, and thigh pain. On physical exam, a hematoma extending from his right groin down to his right knee was found, as well as scattered ecchymoses involving his trunk and all 4 extremities. His hemoglobin was low, at 5.6 g/dL (14‐17 g/dL). A computed tomography (CT) scan revealed a right‐sided retroperitoneal bleed extending from the iliopsoas into his right thigh. The patient received 13 transfusions of packed red blood cells over the course of 9 days as he continued to bleed. Transfer to our facility for further workup and management ensued.
On serial testing at our institution his activated partial thromboplastin time (aPTT) was elevated at >160 seconds (normal range, 24‐36 seconds). Further coagulation parameters were found as follows: platelets 182.000/L; prothrombin time 17.6 seconds; international normalized ratio (INR) 1.4; thrombin 18 seconds; fibrinogen 778 mg/dL; and D‐dimer 3866 ng/mL. Of note, the patient had not received any medications known to potentially interfere with the measured aPTT. Because the source of his bleeding was not apparent at this point, disorders of primary hemostasis, including hereditary disease states (eg, von Willebrand disease), iatrogenic disorders (eg, drug‐induced), or acquired disorders, such as immune thrombocytopenia, were considered and ruled out. At this point the differential diagnoses had to be expanded, and secondary disorders of hemostasis were considered. A deficiency or decreased activity of coagulation factors was suspected. Whereas factor IX and XI were found to be normal, the factor VIII level was significantly decreased at 3% (50%‐150% being normal). This prompted an assay to check for the presence of a factor VIII inhibitor. It proved to be significantly elevated at 25.6 Bethesda Units (BU) (normal, 0.00‐0.04 BU). On that basis we arrived at the diagnosis of acquired factor VIII deficiency, but the etiology of such remained unclear to this point. Hence a search for the specific etiology of acquired factor IIII deficiency was launched, and connective tissue disease, as well as malignancy, was ruled out. While inflammatory bowel disease is a known potential cause for this condition, the clinical picture was not consistent with such and this diagnosis was not considered further. The patient received immunosuppressive therapy with prednisone 1 mg/kg orally per day. Rituximab and cyclophosphamide were considered, but due to bacteremia from bilateral parotitis, this was deferred. Of note, his bleeding abnormality was apparent prior to initiation of antibiotic therapy.
The bleeding stopped 2 days after initiation of treatment. At the time of discharge, 2 weeks after presentation, factor VIII inhibitor levels had decreased to 13 BU and his partial thromboplastin time (PTT) was 100 seconds.
Discussion
Acquired factor VIII inhibitor, also called acquired hemophilia A, is a rare, potentially life‐threatening bleeding disorder. It is caused by autoantibodies directed against coagulation factor VIII.1
The estimated incidence in the general population is 1 in 4 million/year. Risk factors include advanced age, pregnancy and the postpartum period, rheumatoid disease/connective tissue disease, inflammatory bowel disease, medications (especially antibiotics and psychiatric drugs), and malignancy. Both solid tumors as well as hematologic malignancies have been associated with acquired hemophilia A.2
Patients older than 85 years are more frequently affected. The annual incidence is 14.7 in 1 million in this age group. Hence, it is found rarely in young patients, but pregnancy and the postpartum period represent the exception.
Patients with acquired factor VIII inhibitor tend to bleed into the skin, soft tissue, muscle, brain, and mucous membranes. Most of the time, they present with epistaxis, retroperitoneal hematomas, or gastrointestinal bleeds, while patients with congenital factor VIII deficiency3 are more likely to bleed into the large joints. Acquired factor VIII inhibitor is associated with a high morbidity and mortality.
In the presence of an isolated elevated aPTT, once heparin has been ruled out, specific factor deficiencies and/or inhibitors need to be considered. The inhibitor assay helps to establish the diagnosis of acquired factor VIII deficiency and allows the quantification of factor VIII inhibitor. A search for specific etiologies of acquired factor VIII inhibitors should be undertaken; however, in 50% of cases no concomitant condition is found. The differential diagnoses should be expanded within the appropriate framework and tailored to the individual patient.
Control of bleeding might be achieved by factor VIII concentrate if the bleeding is mild. However, if the hemorrhage is life‐threatening, recombinant factor VII is frequently required to stop the bleeding.4 One has to be aware that recombinant factor VII may precipitate thromboembolic events and as such might pose a dilemma, as the degree of bleeding has to be balanced with the risk of unintended side effects. Therapy to eliminate factor VIII inhibitor is the combination of prednisone and cyclophosphamide, though monoclonal CD20 antibody (Rituximab) has become the first‐line agent in the appropriate setting.5 Risk and benefit of therapy have to be balanced with the severity of the bleed and potential unintended side effects of immunosuppression, especially in the presence of infection.
As hospitalists, we are challenged daily by a high degree of complexities in inpatient care. Hospitalists are well trained to manage a wide variety of conditions, and coagulopathies are no exception. They are so common in the inpatient setting that every hospitalist should be familiar with the basic principles of diagnosing and managing bleeding disorders. Because of the hospitalist's ability to promptly react, the consulting role of the hematologist can be reserved for the more unusual blood dyscrasias.
This article is intended to raise physician awareness for the discussed condition because early recognition and treatment are of paramount importance in patient outcome.
- ,.Acquired inhibitors.Bailleres Clin Haematol.1996:9:331–354.
- ,,,.Acquired hemophilia A: a concise review.Am J Hematol.2005;80:50–63.
- ,.Acquired hemophilia.Rev Clin Exp Hematol.2005;5:389–404.
- ,,,.Treatment of acquired haemophilia with recombinant activated VII: a critical appraisal.Haemophilia.2007;13:451–461.
- ,,,.Selective B‐cell depletion with rituximab for the treatment of patients with acquired hemophilia.Blood.2004:103:4424–4428.
- ,.Acquired inhibitors.Bailleres Clin Haematol.1996:9:331–354.
- ,,,.Acquired hemophilia A: a concise review.Am J Hematol.2005;80:50–63.
- ,.Acquired hemophilia.Rev Clin Exp Hematol.2005;5:389–404.
- ,,,.Treatment of acquired haemophilia with recombinant activated VII: a critical appraisal.Haemophilia.2007;13:451–461.
- ,,,.Selective B‐cell depletion with rituximab for the treatment of patients with acquired hemophilia.Blood.2004:103:4424–4428.