User login
Ancillary Testing for Rotavirus
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
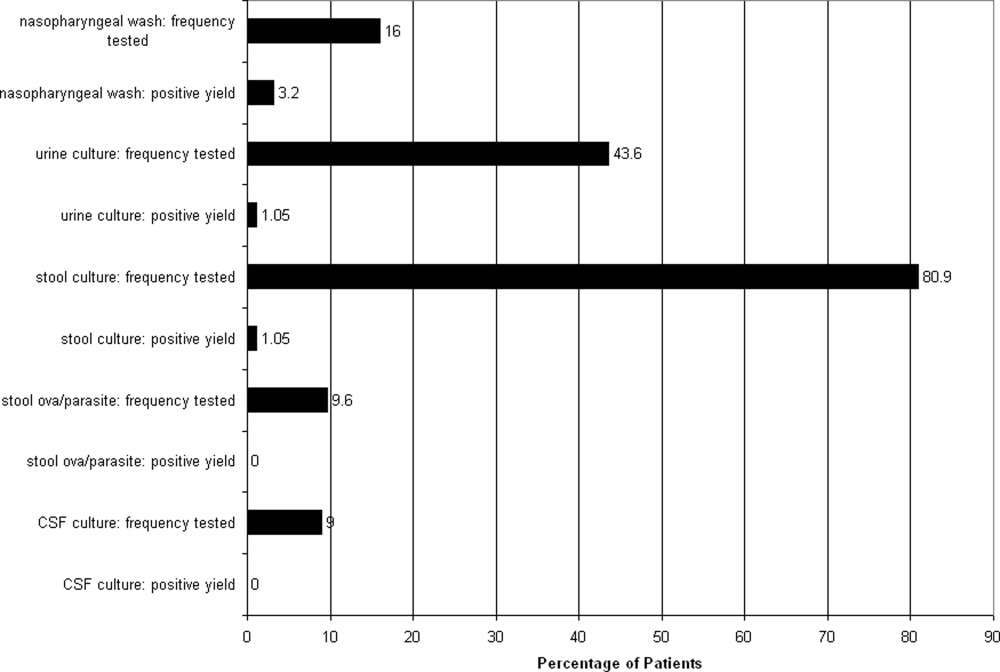
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
Rotavirus gastroenteritis (RGE) accounts for approximately 70,000 pediatric hospitalizations annually in the United States.1 Costly microbiological assays are frequently performed in these patients to exclude concurrent serious bacterial infection (SBI), though the actual incidence of SBI is quite low.28 Our objectives were to describe the incidence of SBI in children evaluated at a community hospital and subsequently diagnosed with laboratory‐confirmed RGE and to determine whether ancillary testing was associated with prolonged length of stay (LOS) in hospitalized patients.
Materials and Methods
Study Design and Setting
This retrospective cohort study was conducted at the Albert Einstein Medical Center (AEMC, Philadelphia, PA) and approved by the AEMC institutional review board. During the study period, there were approximately 20,000 pediatric outpatient evaluations and 2000 pediatric hospitalizations per year.
Participants, Study Protocol, and Data Collection
Children under 18 years of age were included if they were evaluated in the pediatric clinic, emergency department (ED), or admitted to the pediatric floor at AEMC between January 1, 1998 and May 31, 2003 and tested positive for stool rotavirus antigen. Study patients were identified using 3 methods: first, International Classification of Diseases, ninth revision, Clinical Modification (ICD‐9‐CM) discharge diagnosis code for enteritis due to rotavirus (ICD‐9‐CM, 008.61); then, pediatric ward admission logs identified gastroenteritis patients; and finally, review of microbiology laboratory records confirmed the presence of a positive stool rotavirus antigen test. Patients with nosocomial RGE, defined by gastroenteritis symptoms manifesting 3 or more days after hospitalization, were excluded.
Study Definitions
Prolonged LOS was defined as hospitalization of 3 days as this value represented the 75th percentile for LOS in our cohort. Patients discharged directly from the ED were classified as not having a prolonged LOS. Bacteremia was defined as isolation of a known bacterial pathogen from blood culture, excluding isolates that reflected commensal skin flora. Fever was defined as temperature >38.0C. Tachypnea and tachycardia were defined using previously published age‐specific definitions.9 Bacterial meningitis required isolation of a bacterial pathogen from the cerebrospinal fluid (CSF) or, in patients who received antibiotics prior to evaluation, the combination of CSF pleocytosis (defined as white blood cell count 8/mm3) and bacteria detectable on CSF Gram stain. Urinary tract infection was defined as growth of a single pathogen yielding 50,000 colony forming units (cfu)/mL from a catheterized specimen. Significant past medical history constituted any preexisting medical diagnosis.
Stool samples were assayed for rotaviral antigen by means of ImmunoCard STAT! Rotavirus (Meridian Bioscience, Cincinnati, OH). Abstracted data was entered onto standardized data collection forms and included demographic identifiers, clinical presentation, past medical history, laboratory investigations, and subsequent hospital course.
Data Analysis
Data were analyzed using STATA version 9.2 (Stata Corporation, College Station, TX). Categorical variables were described using counts and percentages. Continuous variables were described using median and interquartile range (IQR) values. Bivariate analyses were conducted to determine the association between potential risk factors and prolonged LOS. Categorical values were compared using either the 2 or the Fisher exact test. Continuous variables were compared with the Wilcoxon rank‐sum test. Adjusted analyses, using logistic regression, were then performed to identify factors independently associated with prolonged LOS. Variables with a P‐value <0.2 were considered for inclusion in the multivariable model. Candidate variables were entered into the model using a purposeful selection approach and included in the final multivariable model if they remained significant on adjusted analysis or if they were involved in confounding. Confounding was assumed to be present if adjustment for a variable produced an odds ratio (OR) that was >15% different than the unadjusted OR. Since prolonged LOS was defined as LOS >75th percentile for the cohort, we had 80% power (alpha = 0.05) to detect an OR of 4 or more for variables with a prevalence of 40% or greater in the study cohort.
Results
One hundred cases of RGE were initially identified; 6 patients were excluded4 with negative rotavirus stool antigen tests and 2 because the infection was nosocomially‐acquired. The remaining 94 cases were included in the analysis. Fifty‐eight (61.7%) of the patients were male, and 80 (85.1%) were African‐American. The median age was 8 months (IQR, 1 month to 16 years) and 83 patients (88.3%) were admitted to hospital. Fifty patients (53.2%) were febrile at presentation. The median length of stay was 2 days (IQR, 1‐3 days).
There were no patients with SBI (95% confidence interval [CI], 0%‐3.8%). Ten patients (12%) had received antibiotics in the 72 hours prior to evaluation; 6 of these 10 patients had blood cultures obtained. Peripheral blood cultures were drawn from 47 patients (50%). Of these, 43 (91.5%) were negative. Three cultures yielded viridans group streptococci, and 1 culture yielded vancomycin‐resistant Enterococcus species (VRE). The cultures yielding viridans group streptococci were drawn from 3 infants aged 42 days, 4 months, and 12 months. All 3 infants were febrile at presentation. In 2 of the 3 infants, 2 sets of blood cultures were drawn and viridans group streptococci was isolated from only 1 of the 2 cultures. The third infant made a rapid clinical recovery without antibiotic intervention and was discharged in less than 48 hours, belying microbiological evidence of bacteremia. Therefore, we classified all 3 viridans group streptococci cultures as contaminated specimens. The difference in the frequency with which blood cultures were performed in children younger than (59%) or older than (44%) 6 months of age was not statistically significant (2, P = 0.143).
The patient with VRE isolated from blood culture was a 4‐month‐old male who presented with 2 days of vomiting and diarrhea and a fever to 38.7C. The VRE culture, while potentially representing bacterial translocation in the setting of RGE, was presumed to be a contaminant when a repeat peripheral culture was negative. The patient had received amoxicillin for the treatment of otitis media prior to presentation and acquisition of cultures. The susceptibility testing results for ampicillin or amoxicillin were not available; however, the patient did not receive antibiotics for treatment of the VRE blood culture isolate.
Multiple microbiological assays were performed (Figure 1). Many of the detected organisms were considered nonpathogenic. Stool bacterial cultures were obtained in 76 patients (80.9%) with only 1 (1.3%) positive isolate, Proteus mirabilis, considered nonpathogenic. Urine cultures from 41 patients (43.6%) yielded only 1 (2.4%) positive result, Staphylococcus aureus, deemed a contaminant. Nasopharyngeal washes from 15 patients (16%) revealed 3 (20%) positive results (respiratory syncytial virus in 2 patients and influenza virus in 1). Stool assayed for ova and parasites in 9 patients (9.6%) was negative. CSF cultured in 9 patients was also negative, although 3 samples demonstrated pleocytosis. Nonmicrobiological assays included 4 normal chest radiographs, 2 normal urinalyses, and 3 arterial blood gases revealing metabolic acidosis.

A complete blood count was obtained in 77 patients (81.9%). The median peripheral white blood cell count was 8800/mm3 (IQR, 6800 to 11,800). There were no differences between those with and without prolonged LOS on univariate analysis with regard to vital signs or initial symptoms such as tachypnea, fever, tachycardia, or other features associated with illness severity (eg, extent of dehydration). There were no differences in hematological or chemical parameters or with the performance of any other testing. In bivariate analyses, age 6 months (unadjusted OR, 3.43; 95% CI, 1.26‐9.50; P < 0.01) and collection of peripheral blood culture (OR, 3.12; 95% CI, 1.13‐8.98; P < 0.01) were associated with prolonged LOS. Other variables considered for inclusion in the multivariable model included duration of symptoms, presence of a preexisting medical condition, and performance of a nasopharyngeal wash for respiratory virus detection. In multivariable analysis, age <6 months (adjusted OR, 3.01; 95% CI, 1.17‐7.74; P = 0.022) and the performance of a blood culture (adjusted OR, 2.71; 95% CI, 1.03‐7.13; P = 0.043) were independently associated with a prolonged LOS.
Discussion
The absence of SBI in our relatively small cohort of children admitted to a community hospital with laboratory‐confirmed RGE supports earlier estimates of an incidence of less than 1%,5, 7 an incidence similar to that of occult bacteremia in febrile children 2 to 36 months of age following introduction of the heptavalent pneumococcal conjugate vaccine in 2000.10, 11 We found 13 cases reported in the English literature (Table 1). Several salient features are noted when comparing these case reports. All cases of SBI following laboratory‐confirmed RGE were characterized by the development of a second fever after the resolution of initial symptoms. These fevers presented at a mean day of hospitalization of 2.8 (range, 2‐5). Second fevers were high (mean, 39.2C; range, 38.2C to 40C). Cultures obtained other than peripheral blood cultures were only positive in 1 patient; this patient also had cellulitis and Escherichia coli was isolated from both blood and wound cultures.3 One of the reported children with bacteremia died, 2 cases of SBI following RGE were complicated by disseminated intravascular coagulopathy, and 1 case by acute renal failure. Enterobacter cloacae (n = 4) and Klebsiella pneumoniae (n = 3) were the most commonly isolated organisms from peripheral blood culture.
| References | Age (months)/Sex | Hospital day of bacteremia | Second fever (C)* | Organism Cultured from Peripheral Blood | Other Culture Results | Outcome |
|---|---|---|---|---|---|---|
| ||||||
| Adler et al.2 | 9/♂ | 3 | 39.5 | Klebsiella pneumoniae | None | Full recovery after uncomplicated course |
| Adler et al.2 | 9/♂ | 2 | 40 | Escherichia coli | None | Full recovery after uncomplicated course |
| Adler et al.2 | 0.74/♀ | 3 | 39 | Klebsiella pneumoniae | Urine, CSF cultures negative | ARF, resolved to full recovery |
| Carneiro et al.4 | 10/♀ | 3 | 39.1 | ESBL‐producing Escherichia coli | Wound culture (cellulitis) from day 3 in PICU yielded ESBL‐producing Escherichia coli | Full recovery after DIC and transfer to PICU |
| Cicchetti et al.3 | 18/♂ | 2 | high | Pantoea agglomerans | None | DIC resolved with Protein C concentrate infusions |
| Gonzalez‐Carretero et al.5 | 1.5/♂ | 3 | 39.3 | Streptococcus viridans | Urine, CSF cultures negative | Full recovery after uncomplicated course |
| Gonzalez‐Carretero et al.5 | 10/♂ | 5 | 38.3 | Enterobacter cloacae | Stool culture negative | Full recovery after uncomplicated course |
| Kashiwagi et al.6 | 12/♂ | 7 | 38.0 | Klebsiella oxytoca | Not reported | Died |
| Lowenthal et. al7 | 6/♂ | 3 | 40 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 4/♀ | 2 | 39.5 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course without antibiotic therapy |
| Lowenthal et. al7 | 0.5/♀ | 3 | 38.2 | Klebsiella pneumoniae | CSF and urine cultures negative | Full recovery after uncomplicated course |
| Lowenthal et. al7 | 13/♀ | 2 | 39.3 | Enterobacter cloacae | Urine culture negative | Full recovery after uncomplicated course |
| Mel et. al8 | 16/♀ | 5 | 39.8 | ESBL‐producing Escherichia coli | Urine culture negative | Full recovery after uncomplicated course |
Many children in our study had ancillary laboratory testing performed. The results of these tests were typically normal and rarely affected clinical management in a positive manner. Bacteria and parasites are relatively rare causes of gastroenteritis in the United States in comparison with rotavirus, particularly during the winter months. However, stool was sent for bacterial culture in over 80% of patients and for ova and parasite detection in almost 10% of patients ultimately diagnosed with RGE. Furthermore, despite the relatively low prevalence of bacteremia since licensure of the Haemophilus influenzae type b vaccine, a majority of children had a complete blood count performed while one‐half also had blood obtained for culture. In our cohort, children 6 months and younger and those from whom a blood culture was collected were at an increased risk for prolonged LOS. It was not clear from medical record review whether children with prolonged LOS were kept in the hospital longer for the sole purpose of awaiting the results of blood cultures.
SBI rarely occurs in the context of RGE. While secondary fever seems to be a common manifestation, the sensitivity of secondary fever as a marker for SBI after RGE in this population is unknown. However, given the very low incidence, the potentially serious complications of SBI following laboratory confirmed RGE, and the likely successful management of these complications in the hospital setting, slightly longer hospitalizations for children under 1 year of age must be weighed against earlier discharges with instructions from clinicians to caregivers for careful monitoring of fever and outpatient follow‐up shortly after discharge.
This study has several limitations. First, the timing of the availability of the results of rotavirus antigen testing is not known. It is possible that the rapid availability of rotavirus test results in some circumstances encouraged clinicians to abandon tests seeking other sources of infection. Conversely, children with gastroenteritis in the context of a concurrent bacterial infection may have been less likely to undergo rotavirus stool antigen testing. This latter possibility would bias our findings toward underestimating the prevalence of concurrent bacterial infection among children with RGE. Second, this study was performed prior to licensure and widespread use of the currently‐licensed vaccine against rotavirus (Rotateq; Merck and Company, Whitehouse Station, NJ). Reductions in the burden of gastroenteritis caused by rotavirus may have a much more dramatic impact on resource utilization in the treatment of gastroenteritis than reductions in ancillary testing. Finally, this study was performed at a single urban community hospital and therefore cannot be generalized to other settings such as academic tertiary care centers. Furthermore, test ordering patterns may be local or regional and other community hospitals may exhibit different patterns. Further clarification of the role of ancillary testing in children presenting with diarrhea during the winter months is warranted since reducing the extent of such testing would dramatically reduce resource utilization for this illness. Finally, a blood culture was not obtained from all patients. Therefore, occult bacteremia attributable to RGE could not be detected. Since no patient in our study underwent subsequent clinical deterioration, we presume that any case of occult bacteremia resolved spontaneously and was not of clinical consequence, although such occurrences would cause us to underestimate the prevalence of SBI in this population.
Resource utilization in our cohort was high, while yield from microbiological investigations was low. This finding challenges the need to perform invasive, costly assays to exclude concurrent SBI in this population. It is possible that children with viral gastroenteritis caused by pathogens other than rotavirus are also at low risk of SBI. However, the diagnostic strategy that best identifies patients at risk for SBI following acute gastroenteritis is unknown. Further studies are needed to determine an ideal clinical approach to the infant with RGE.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
- ,,,,,.Hospitalizations associated with rotavirus gastroenteritis in the United States, 1993‐2002.Pediatr Infect Dis J.2006;25(6):489–493.
- ,,,.Enteric gram‐negative sepsis complicating rotavirus gastroenteritis in previously healthy infants.Clin Pediatr (Phila).2005;44(4):351–354.
- ,,, et al.Septic shock complicating acute rotavirus‐associated diarrhea.Pediatr Infect Dis J.2006;25(6):571–572.
- ,,,,,.Pantoea agglomerans sepsis after rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(3):280–281.
- ,,.Rotavirus gastroenteritis leading to secondary bacteremia in previously healthy infants.Pediatrics.2006;118(5):2255–2256; author reply2256–2257.
- ,,, et al.Klebsiella oxytoca septicemia complicating rotavirus‐associated acute diarrhea.Pediatr Infect Dis J.2007;26(2):191–192.
- ,,,,.Secondary bacteremia after rotavirus gastroenteritis in infancy.Pediatrics.2006;117(1):224–226.
- ,,,.Extended spectrum beta‐lactamase‐positive Escherichia coli bacteremia complicating rotavirus gastroenteritis.Pediatr Infect Dis J.2006;25(10):962.
- ,,,.The Philadelphia Guide: Inpatient Pediatrics.Philadelphia:Lippincott Williams and Wilkins;2005.
- ,,, et al.Changing epidemiology of outpatient bacteremia in 3‐ to 36‐month‐old children after the introduction of the heptavalent‐conjugated pneumococcal vaccine.Pediatr Infect Dis J.2006;25(4):293–300.
- ,.Incidence of occult bacteremia among highly febrile young children in the era of the pneumococcal conjugate vaccine: a study from a Children's Hospital Emergency Department and Urgent Care Center.Arch Pediatr Adolesc Med.2004;158(7):671–675.
Copyright © 2009 Society of Hospital Medicine
New Therapies for UGH
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
Upper gastrointestinal hemorrhage (UGH) is a common cause of acute admission for hospitalization.13 However, recent advances in our understanding of erosive disease (ED) and peptic ulcer disease (PUD), 2 of the most common etiologies of UGH, have led to effective strategies to reduce the risk of UGH. Successful implementation of these strategies, such as treatment of Helicobacter pylori (H. pylori) and the use of proton pump inhibitors (PPIs) and selective cyclooxygenase‐2 inhibitors (COX‐2s) in place of traditional nonselective nonsteroidal antiinflammatory drugs (NSAIDs), may be able to significantly reduce rates of UGH caused by ED and PUD.47
Prior to these preventive treatments, PUD and ED, both acid‐related disorders, were the most common causes of UGH requiring admission to the hospital, accounting for 62% and 14% of all UGHs, respectively.2 Given the widespread treatment of H. pylori and use of PPIs and COX‐2s, we might expect that the distribution of etiologies of UGH may have changed. However, there are limited data on the distribution of etiologies of UGH in the era of effective preventive therapy.8 If the distribution of etiologies causing patients to present with UGH has fundamentally changed with these new treatments, established strategies of managing acute UGH may need to be reevaluated. Given that well‐established guidelines exist and that many hospitals use a protocol‐driven management strategy to decide on the need for admission and/or intensive care unit (ICU) admission, changes in the distribution of etiologies since the widespread use of these new pharmacologic approaches may affect the appropriateness of these protocols.9, 10 For example, if the eradication of H. pylori has dramatically reduced the proportion of UGH caused by PUD, then risk stratification studies developed when PUD was far more common may need to be revisited. This would be particularly important if bleeding from PUD was of significantly different severity than bleeding from other causes.
While patients with H. pylori‐related UGH from PUD should be treated for H. pylori eradication, several important questions remain surrounding the use of newer therapeutics that may mitigate the risk of UGH in some patients. It is unclear what proportion of patients admitted with UGH in this new era developed bleeding despite using preventive therapy. These treatment failures are known to occur, but it is not well known how much of the burden of UGH today is due to this breakthrough bleeding.5, 6, 11, 12 Contrastingly, there are also patients who are admitted with UGH who are not on preventive treatment. Current guidelines suggest that high‐risk patients requiring NSAIDs be given COX‐2s or traditional NSAIDs with a PPI.1315 However, there is significant disagreement between these national guidelines about what constitutes a high‐risk profile.1315 For example, some guidelines recommend that elderly patients requiring NSAIDs should be on a PPI while others do not make that recommendation. Similarly, while prior UGH is a well‐recognized risk factor for future bleeding risk even without NSAIDs, current guidelines do not provide guidance toward the use of preventive therapy in these patients. If there are few patients who present with UGH related to acid disease that are not on a preventive therapy, then these unanswered questions or conflicts within current guidelines become less important. However, if a large portion of UGH is due to acid‐related disease in patients not on preventive therapy, then these unanswered questions may become important for future research.
In contrast to previous studies, the current study examines the distribution of etiologies of UGH in the era of widespread use of effective preventive therapy for ED and PUD in 2 U.S. academic medical centers. Prior studies were done before the advent of new therapeutics and did not compare different sites, which may be important.16, 17
PATIENTS AND METHODS
Patients
Consecutive patients admitted with UGH were identified at 2 academic medical centers as part of a larger observational study examining the impact of hospitalist physicians on the care of acute medical patients.18 The sample was selected from the 12,091 consecutive general medical patients admitted from July 2001 to June 2003 with UGH identified by International Classification of Diseases, Ninth revision, Clinical Modification (ICD‐9 CM) codes from administrative data and confirmed by chart abstraction. ICD‐9 CM codes for UGH included: esophageal varices with hemorrhage (456.0 and 456.20), Mallory‐Weiss syndrome (530.7), gastric ulcer with hemorrhage (531.00‐531.61), duodenal ulcer with hemorrhage (532.00‐532.61), peptic ulcer, site unspecified, with hemorrhage (533.00‐533.61), gastrojejunal ulcer with hemorrhage (534.00‐534.61), gastritis with hemorrhage (535.61), angiodysplasia of stomach/duodenum with hemorrhage (537.83), and hematemesis (578.0 and 578.9).19 Finally, the admission diagnoses for all patients in the larger cohort were reviewed and any with gastrointestinal hemorrhage were screened for possible inclusion to account for any missed ICD‐9 codes. Subjects were then included in this analysis if they had observed hematemesis, nasogastric (NG) tube aspirate with gross or hemoccult blood, or history of hematemesis, bloody diarrhea, or melena upon chart review.
Data
The inpatient medical records were abstracted by trained researchers. Etiologies of UGH were assessed by esophagogastroduodenoscopy (EGD) report, which listed findings and etiologies as assessed by the endoscopist. Multiple etiologies were allowed if more than 1 source of bleeding was identified. Prior medical history and preadmission medication use were obtained from 3 sources: (1) the emergency department medical record; (2) nursing admission documentation; and (3) the admission history and physical documentation. Risk factors and preadmission medication use were considered present if documented in any of the 3 sources. Relevant past medical history included known risk factors for UGH, including: end‐stage renal disease, alcohol abuse, prior history of UGH, and steroid use. Prior H. pylori status/testing could not reliably be obtained from these data sources. Preadmission medication use of interest included aspirin, NSAIDS, anticoagulants, antiplatelet agents, as well as PPIs and COX‐2s. Demographics, including age, race, and gender, were obtained from administrative databases.
We defined subjects as at‐risk if they had any of the following risk factors: prior UGH (at any time), use of an NSAID (traditional or selective COX‐2), or use of an aspirin prior to admission. Patients taking COX‐2s were included for 2 reasons. First, while COX‐2 inhibitors are associated with a lower risk of UGH than traditional NSAIDs, it is likely that they still lead to an increased risk of UGH compared to placebo. Second, if a patient required NSAIDs of some type (traditional or selective), preadmission use of a COX‐2 rather than a traditional NSAID may reflect the intention of decreasing the risk of UGH compared to using traditional NSAIDs. In order to use the most conservative estimate of potential missed opportunities for prevention, preadmission use of a PPI or COX‐2 was considered preventive therapy. All preadmission medication use was obtained from chart review. Therefore, duration of and purpose for medication use were not available.
Development of the abstraction tool was performed by the authors. Testing of the tool was performed on a learning set of 20 charts at each center. All additional abstractors were trained with a learning set of at least 20 charts to assure uniform abstraction techniques.
Analysis
For each risk factor and etiology, we calculated the proportion of patients with the risk factor or etiology both overall and by site. Differences in risk factors between sites were assessed using chi‐square tests of association. Differences in etiologies between sites were assessed using unadjusted odds ratios (ORs) as well as ORs from logistic regression models controlling for age, gender, and race (black versus not black). Center 1 was the urban center and center 2 was the rural site.
This study was approved by the Institutional Review Board at the University of Iowa Carver College of Medicine and the University of Chicago.
RESULTS
From the entire cohort of 12,091 admitted to the 2 inpatient medical services, 227 (1.9%) patients were identified as having UGH; 138 (61%) were from center 1, where 87% of patients were black and 89 (39%) were from center 2, where 89% of patients were white. Overall, the mean age was 59 years, 45% were female, and 41% were white (Table 1).
| Characteristic | Total (n = 227) | Center 1 (n = 138) | Center 2 (n = 89) | P Value Center 1 versus 2 |
|---|---|---|---|---|
| ||||
| Mean age (years) | 58.6 | 59.5 | 57.1 | 0.317 |
| % Female | 44.5 | 48.6 | 38.2 | 0.126 |
| % White | 41.2 | 10.2 | 88.8 | <0.001 |
| % African American | 54.0 | 86.9 | 3.4 | <0.001 |
| % Other | 4.9 | 2.9 | 7.9 | <0.001 |
The most common etiologies of UGH were ED (44%), PUD (33%), and varices (17%) in the overall population. These same 3 etiologies were also the most common in both of the medical centers, although there were significant differences in the rates of etiologies between the 2 centers. ED was more common among subjects from center 1 (59%) than from center 2 (19%) (P < 0.001), while variceal bleeding was more common among subjects from center 2 (34%) than from center 1 (6.5%) (P = 0.009) (Table 2).
| Etiology | All n = 227 (%) | Center 1 n = 138 (%) | Center 2 n = 89 (%) | Unadjusted OR (95% CI): Center 1 versus 2 | P Value for Unadjusted OR | Adjusted* OR (95% CI): Center 1 versus 2 | P Value (for Adjusted OR) |
|---|---|---|---|---|---|---|---|
| |||||||
| ED | 43.6 | 59.4 | 19.1 | 6.20 (3.3111.62) | <0.001 | 7.10 (2.4820.31) | <0.001 |
| PUD | 33.0 | 37.0 | 27.0 | 1.59 (0.892.84) | 0.119 | 1.33 (0.483.67) | 0.578 |
| Varices | 17.2 | 6.5 | 33.7 | 0.14 (0.060.31) | <0.001 | 0.12 (0.030.60) | 0.009 |
| AVM | 5.3 | 2.9 | 9.0 | 0.30 (0.091.04) | 0.057 | 0.21 (0.031.69) | 0.141 |
| Mallory Weiss Tear | 4.9 | 4.4 | 5.6 | 0.76 (0.232.58) | 0.664 | 0.34 (0.024.85) | 0.425 |
| Cancer/masses | 2.6 | 2.9 | 2.3 | 1.30 (0.237.24) | 0.766 | 0.62 (0.0312.12) | 0.751 |
In multivariate logistic regression analyses, only age and site remained independent predictors of etiologies. Advancing age was associated with a higher risk of arteriovenous malformations (AVMs) with the odds of AVMs increasing 6% for every additional year of life (P = 0.007). Site was associated with both ED and variceal bleeding. Patients from center 1 were significantly more likely to have UGH caused by ED, with an OR = 7.10 (P < 0.001), compared to subjects from center 2. However, subjects from center 1 had a significantly lower OR (OR = 0.12) than those subjects at center 2 (P = 0.009) of having UGH caused by a variceal bleed (Table 2).
Risk factors for UGH were common among the patients, including use of aspirin (25.1%), NSAIDs (22.9%), COX‐2s (4.9%), or prior history of UGH (43%). Additionally, 6.6% of patients were taking both an NSAID and aspirin. Differences between the 2 sites were seen only in aspirin use, with 34.8% of patients in the center 1 population using aspirin compared to 10.1% in center 2 (P < 0.001) (Table 3).
| Risk Factor | All (%) | Center 1 (%) | Center 2 (%) | P Value |
|---|---|---|---|---|
| ||||
| Previous UGH | 42.7 | 41.3 | 45.2 | 0.586 |
| NSAID use | 22.9 | 21.7 | 24.7 | 0.602 |
| ASA use | 25.1 | 34.8 | 10.1 | <0.001 |
| NSAID + ASA | 6.6 | 6.5 | 6.7 | 0.948 |
| COX‐2 use | 4.9 | 6.5 | 2.3 | 0.143 |
| PPI use | 18.5 | 18.1 | 19.1 | 0.852 |
Among the overall population, 68.7% of patients had identifiable risk factors (prior history of UGH or preadmission use of aspirin, NSAIDs, or COX‐2s). Of all subjects, 18.5% were on PPIs and 4.9% were taking COX‐2s while 21.1% of at risk subjects were on PPIs and 6.5% of these subjects were on a COX‐2.
Finally, we examined the effects of variations in preadmission medication use between the sites on the etiologies of UGH. None of the site‐based differences in etiologies could be explained by differences in preadmission medication patterns.
DISCUSSION
Despite the emergence of effective therapies for lowering the risk of ED and PUD, these remain the most common etiologies of UGH in our cohort of patients. In a dramatic change from historically reported patterns, ED was more common than PUD. In prior studies, PUD accounted for almost two‐thirds of all UGH.2 While some of the newer therapeutics such as PPIs and COX‐2s reduce the risk for acid‐related bleeding of all types, H. pylori eradication is effective primarily for PUD. Therefore, it may be that widespread testing and treatment of H. pylori have dramatically decreased rates of PUD. Unfortunately, this study does not allow us to directly evaluate the effect of H. pylori treatment on the changing epidemiology of UGH, as that would require a population‐based study.
While decreasing rates of PUD could explain a portion of the change in the distribution of etiologies, increasing rates of ED could also be playing a role. Prior studies have suggested that African Americans and the elderly are more susceptible to ED, particularly in the setting of NSAIDs and/or aspirin use, and less susceptible to cirrhosis.13, 16, 17, 2023 Our finding of a higher rate of ED and lower rates of cirrhosis in center 1 with a higher proportion of African Americans and greater aspirin use is consistent with these prior findings. However, in multivariate analyses, neither race nor preadmission medication use patterns explained the differences in etiologies seen. This suggests that some other factors must play a role in the differences between the 2 centers studied. These results emphasize the importance of local site characteristics in the interpretation and implementation of national guidelines and recommendations. This finding may be particularly important in diseases and clinical presentations that rely on protocol‐driven pathways, such as UGH. Current recommendations on implementing clinical pathways derived from national guidelines emphasize the fact that national development and local implementation optimization is probably the best approach for effective pathway utilization.24
It is important to understand why ED and PUD, for which we now have effective pharmacologic therapies, continue to account for such a large percentage of the burden of UGH. In this study, we found that a majority of subjects were known to have significant risk factors for UGH (aspirin use, NSAID use, COX‐2s, or prior UGH) and only 31% of the subjects could not have been identified as at‐risk prior to admission. PPIs or COX‐2s should not be used universally as preventive therapy, and they are not completely effective at preventing UGH in at‐risk patients. In this study, two‐thirds of patients with risk factors were not on preventive therapy, but almost one‐third of patients with risk factors had bleeding despite being on preventive therapy. A better understanding of why these treatment failures (bleeding despite preventive therapy) occur may be helpful in our future ability to prevent UGH. This study was not designed to determine if the two‐thirds of patients not taking preventive therapy were being treated consistent with established guidelines. However, current guidelines have significant variation in recommendations as to which patients are at high enough risk to warrant preventive therapy,1315 and there is no consensus as to which patients are at high enough risk to warrant preventive therapy. Our data suggest that additional studies will be required to determine the optimal recommendations for preventive therapy among at‐risk patients.
There are several limitations to this study. First, it only included 2 academic institutions. However, these institutions represented very different patient populations. Second, the study design is not a population‐based study. This limitation prevents us from addressing questions such as the effectiveness or cost‐effectiveness of interventions to prevent admission for UGH. Although we analyzed preadmission PPI or COX‐2 use in at‐risk patients as preventive therapy, we are unable to determine the actual intent of the physician in prescribing these drugs. Finally, although the mechanisms by which PPIs and COX‐2 affect the risk of UGH are fundamentally different and should not be considered equivalent choices, we chose to analyze either option as representing a preventive strategy in order to provide the most conservative estimate possible of preventive therapy utilization rates. However, our assumptions would generally overestimate the use of preventive therapy (as opposed to PPI use for symptom control), as we assumed all potentially preventive therapy was intended as such.
This study highlights several unanswered questions that may be important in the management of UGH. First, identifying factors that affect local patters of UGH may better inform local implementation of nationally developed guidelines. Second, a more complete understanding of the impact positive and negative risk factors for UGH have on specific patient populations may allow for a more consistent targeted approach to using preventive therapy in at‐risk patients.
Finally, and perhaps most importantly, is to determine if the change in distribution of etiologies is in fact related to a decline in bleeding related to PUD. In addition to this being a marker of the success of the H. pylori story, it may have important implications on our understanding of the acute management of UGH. If PUD is of a different severity than other common causes of UGH, such as ED, current risk stratification prediction models may need to be revalidated. For example, if UGH secondary to PUD results in greater morbidity and mortality than UGH secondary to ED, our current models identifying who requires ICU admission, urgent endoscopy, and other therapeutic interventions may result in overutilization of these resource intensive interventions. However, if larger studies do not confirm this decline in PUD it suggests the need for additional studies to identify why PUD remains so prevalent despite the major advances in treatment and prevention of PUD through H. pylori identification and eradication.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
- ,,, et al.Effects of physician experience on costs and outcomes on an academic general medicine service: results of a trial of hospitalists.Ann Int Med.2002;137(11):866–874.
- .Epidemiology of hospitalization for acute upper gastrointestinal hemorrhage: a population‐based study.Am J Gastroenterol.1995;90(2):206–210.
- ,,, et al.Epidemiology and course of acute upper gastro‐intestinal haemorrhage in four French geographical areas.Eur J Gastroenterol Hepatol.2000;12:175–181.
- ,,, et al.Prevention of ulcer recurrence after eradication of Helicobacter pylore: a prospective long‐term follow‐up study.Gastroenterology.1997;113:1082–1086.
- ,,, et al.Treatment of Helicobacter pylore in patients with duodenal ulcer hemorrhage‐a long‐term randomized, controlled study.Am J Gasterenterol.2000;95:2225–2232.
- ,,, et al.Preventing recurrent upper gastrointestinal bleeding in patients with Helicobacter pylori infection who are taking low‐dose aspirin or naproxen.N Engl J Med.2001;344:967–973.
- ,,, et al.Lansoprazole for the prevention of recurrences of ulcer complications from long‐term low‐dose aspirin use.N Engl J Med.2002;346:2033–2038.
- ,,, et al.Acute upper GI bleeding: did anything change?: time trend analysis of incidence and outcome of acute upper GI bleeding between 1993/1994 and 2000.Am J Gastroenterol.2003;98:1494–1499.
- ,,, et al.Upper gastrointestinal hemorrhage clinical guideline‐determining the optimal length of stay.Am J Med.1996;100:313–322.
- ,,.Consensus recommendations for managing patients with nonvariceal upper gastrointestinal bleeding.Ann Intern Med.2003;139:843–857.
- ,,, et al.Comparison of upper gastrointestinal toxicity of rofecoxib and naproxen in patients with rheumatoid arthritis. VIGOR Study Group.N Engl J Med.2000;343:1520–1528.
- ,, et al.Gastrointestinal toxicity with celecoxib vs nonsteroidal anti‐inflammatory drugs for osteoarthritis and rheumatoid arthritis: the CLASS study: a randomized controlled trial. Celecoxib Long‐term Arthritis Safety Study.JAMA.2000;284:1247–1255.
- AGS Panel on Persistent Pain in Older Persons.The management of persistent pain in older persons.J Am Geriatr Soc.2002;50(6 Suppl):S205–S224.
- ,,, et al.Pain in osteoarthritis, rheumatoid arthritis and juvenile chronic arthritis.2nd ed.Clinical practice guideline no. 2.Glenview, IL:American Pain Society (APS);2002:179 p.
- .Recommendations for the medical management of osteoarthritis of the hip and knee.Arthritis Rheum.2000;43:1905–1915.
- ,,, et al.Incidence of and mortality from acute upper gastrointestinal haemorrhage in the United Kingdom.BMJ.1995;311:222–226.
- ,,, et al.Risk factors for hospitalized gastrointestinal bleeding among older persons.J Am Geriatr Soc.2001;49:126–133.
- ,,, et al.Effects of inpatient experience on outcomes and costs in a multicenter trial of academic hospitalists.Society of General Internal Medicine Annual Meeting2005.
- ,,,,,.Early endoscopy in upper gastrointestinal hemorrhage: association with recurrent bleeding, surgery, and length of hospital stay.Gastrointest Endosc.1999;49(2):145–152.
- ,,, et al.A comparison of the spectrum of chronic hepatitis C virus between Caucasians and African Americans.Clin Gastroenterol Hepatol.2004;2:469–473.
- ,,, et al.Gastroesophageal reflux among different racial groups in the United States.Gastroenterology.2004;126:1692–1699.
- ,,,.Risk factors for erosive reflux esophagitis: a case‐control study.Am J Gastroenterol.2001;96:41–46.
- ,.Upper gastrointestinal toxicity of nonsteroidal anti‐inflammatory drugs in African‐American and Hispanic elderly patients.Ethn Dis.2003;13:528–533.
- ,.Evidence‐based quality improvement: the state of the science.Health Aff (Millwood).2005;24(1):138–150.
Copyright © 2009 Society of Hospital Medicine
Personalized Vascular Access Training
The Accreditation Counsel for Graduate Medical Education (ACGME) states in its Program Requirements for Residency Education in Internal Medicine that all residents must develop technical proficiency in several procedures, including central venous line placement.1 Developing competency in common procedural skills has long been a part of medical training. The philosophy of see‐1, do‐1, teach‐1 is still the most common means by which most residents seek to obtain this proficiency, even though serious concerns have been raised about this approach.2 A typical first experience in central line placement usually involves an eager (or terrified) trainee making several clumsy attempts on an actual patient, under the hurried guidance of a senior resident who themselves received an unknown degree of training. In this scenario, rarely does standardized instruction, formal evaluation, or structured follow‐up occur.
A revitalized emphasis is now being placed on patient safety in healthcare, including an industry‐wide commitment to minimizing procedural complications. The most common complications associated to central line placement include vascular damage and catheter‐related bloodstream infections. A number of creative approaches are being developed to improve the quality of instruction on proper procedural techniques, all varying considerably in sophistication, scope, and rigor. Examples include the use of computer‐assisted methods for training ultrasound‐guided needle insertion techniques and ureteroscopy training, hands‐on training with synthetic models for thoracentesis training, video training, and uterine aspiration using papayas.311 Implicit in this trend is recognition that we, as educators, healthcare providers, and patient advocates, must design more cost effective and efficient ways to teach medical and surgical procedural techniques to clinicians.
Our approach was previously described in phase I of the Procedural Patient Safety Initiative (PPSI).12 In PPSI‐I, we introduced a nonhuman tissue model (NHTM; Figure 1) as the basis for teaching physicians a more rigorous curriculum of essential central line placement skills. By way of brief review, the NHTMs were constructed by tunneling 0.2‐mm‐thick rubber tubing (vessels) lengthwise through raw, whole chickens purchased at the grocery store. The vessels were filled with colored water to simulate blood. The NHTM has several unique features, including: (1) realistic‐appearing vessels when viewed under ultrasound, which mimic the appearance of human internal jugular veins and carotid arteries (Figure 2); (2) tissue turgor and vessel composition that produce realistic pops and flashes upon puncture and allow for multiple cannulations; (3) the ability to perform a complete central line placement (including wire advancement, dilation, line insertion, suturing, and sterile dressing placement); (4) cost effectiveness relative to other commercially‐available products (each NHTM costs $120 and can withstand multiple cannulations over 2 days).1317 During the training sessions of Phase I, participants were oriented to the ultrasound machines, shown the contents of our central line kit, and taught the principles of wide sterile barriers (WSBs), sharps safety, and vascular access under real‐time ultrasound guidance. A self‐completed survey tool was filled out by the participants before and after the session that contained questions about their precourse baseline procedural experience, and their subjective comfort level with specific skills after the course. The results of our intervention, as measured by the responses to the survey, were significantly positive. We recognized the limitations of these results based on using subjective criteria to measure efficacy, a lack of follow‐up on participants' skill retention, and with no ultimate evaluation of procedural competency evaluations on actual patients (compared to an untrained control group).
Our ultimate goal is to validate a curriculum that will give trainees the necessary education and skills that enables them to make a smooth, competent, and complication‐free transition to live patient procedures. Phase II of PPSI is our next step toward this goal. In this study, we sought to measure the impact of intensive, 1:1 central line placement training with a proceduralist, objectively validate the efficacy of the NHTM and our training curriculum using a standardized 6‐point scoring scale and skilled evaluators, and to evaluate the degree of skill retention over time (decay). Our hypothesis was that the depth of skills' imprinting from a single, standardized training session would result in a significant improvement in measured procedure skills immediately after the trainee is taught the skills, and that the retention of these skills would be demonstrable when participants were reevaluated at a future date.
Methods
PPSI‐II was an observational, prospective study conducted by The Procedure Center at Cedars‐Sinai Medical Center, a 900+‐bed, community‐based teaching hospital. The Procedure Center is staffed by dedicated Proceduralists who perform a number of common medical procedures on a daily basis and are facile with both real‐time ultrasound guidance and proper procedural techniques.18, 19 Our target population was the incoming Internal Medicine residents. Subjects were recruited by email prior to orientation week and were offered the option of participating in our study. Our only exclusion criterion was the prior observation or placement of 10 or more central lines. The study was approved by our hospital's Institutional Review Board prior to initiating recruitment. Those who chose not to participate underwent the standard orientation training required by our institution, which included a brief overview lecture on the topic of central lines and ultrasound‐guidance, a group viewing of the New England Journal of Medicine (NEJM) video on central line placement,20 and small‐group (4 participants/group) hands‐on practice sessions lasting 45 minutes with NHTMs and a trained Proceduralist.
All of the evaluations for Phase II were done using the Central Line Placement Skill Assessment Tool depicted in Figure 3. This tool, which was developed by Cedars‐Sinai Proceduralists solely for the purposes of this study, is a comprehensive step‐by‐step checklist delineating the specific steps necessary to place a sterile, ultrasound‐guided central venous catheter. It was closely derived from a central line insertion checklist that was created by the Procedure Center 3 years ago to help guide novice clinicians through the procedure, and has since been widely used throughout the institution during the placement of hundreds of central lines. The scoring system, also devised by Cedars‐Sinai Proceduralists, was based on over 15 years of experience supervising and teaching hundreds of residents on proper central line insertion techniques. It consists of clear definitions for each score that were agreed upon via consensus amongst study coordinators. Prior to any evaluations being conducted, we put our 2 evaluators (both senior medicine residents) through identical and simultaneous scoring training with the Proceduralist trainer to standardize procedural knowledge and scoring methodology.
A total of 20 incoming interns (trainees) out of a possible 54 invitations (37%) volunteered to participate. Each trainee was randomly assigned a number from 1 to 20. The study began for each trainee with a brief, 5‐question survey to determine their prior procedural experience (Table 1). Next, each trainee watched the NEJM online training video on central line placement.20 They were then brought into a training room that contained an NHTM sitting on a Mayo stand, an ultrasound machine, and all the materials required to place a central line insertion under ultrasound‐guidance. The trainee's baseline central line insertion skills were evaluated on 22 unique procedure steps, with each score being given by 1 of the 2 evaluators (initial evaluation). The trainee did not receive any guidance or suggestions during this initial evaluation unless the trainee reached an impasse. In these cases, the evaluator completed that single step on the trainee's behalf and then allowed the session to resume. The identity of the evaluator was indicated on each evaluation form, and after each of the evaluations the completed assessment tool was given to our blinded assistant for data recording.
| Number of trainees | 20 |
| How many prior central lines have you inserted independently? (exclusion criteria: >10) | 20 answered 0 |
| How many prior central line insertions have you assisted with? (exclusion criteria: >10) | 13 answered 0; 7 had assisted between 1 and 4 lines |
| How many prior central line insertions have you observed? (exclusion criteria: >10) | 3 answered 0; 17 had observed between 1 and 5 lines |
| Have you had any prior exposure to the use of ultrasound for central line insertion? | 13 no; 7 yes |
| Have you had any prior exposure to the use of wide sterile barriers for procedures? | 11 no; 9 yes |
Each trainee was then given a personalized, hands‐on training session by a proceduralist, using the checklist as a guide to take them through all the steps of a central line insertion. The trainee was allowed to observe and practice each skill for an unlimited period of time with the proceduralist present, until he or she demonstrated competency and felt confident enough with their independent skills (in both trainee's and proceduralist's judgment) to move forward. The entire session ended only when all steps had been taught and practiced to the proceduralist's satisfaction, the trainee felt comfortable independently performing each step (and in proper sequence), and all questions had been answered. At no time was there an imposition of time constraints or external pressure from study coordinators.
Immediately following this training session the proceduralist and trainee left the room, the procedure room was reset by an evaluator (taking approximately 5‐10 minutes), and then the trainee submitted themselves to an immediate posttraining evaluation (immediate evaluation). As with the initial assessment, the evaluator did not interfere or make any comments or suggestions during the evaluation periods, unless the trainee reached an impasse at any step. In that case, the trainee would receive a 0 for that step, the evaluator would assist them to complete that step only, and then the session would continue. No time limits were imposed.
The final part of the study required each trainee to return for follow‐up assessment (delayed evaluation), a process that was identical to the immediate posttraining evaluation. This delayed evaluation was intended to occur between 3 to 4 weeks after the immediate posttraining session, based on trainees' schedules and availability. No refresher or practice time was permitted prior to the delayed evaluation: upon arrival, trainees wrote down on a separate piece of paper (not seen by the evaluator) the number of interim line experiences they had experienced, then they were brought directly into a fully‐prepared room, and instructed to begin. The evaluator was also blind to the trainee's scores from the 2 previous evaluation sessions.
The primary endpoints were the degree of changes in overall average scores (from the 22 steps on the assessment tool) from the initial to the immediate evaluations and from the immediate to delayed evaluations. The secondary endpoints were also based on changes in average scores from the initial to immediate and immediate to delayed evaluations, and looked at 5 essential elements (steps in the assessment tool that we deemed critical to the safe and successful placement of a central line). These essential elements included (1) hand washing; (2) creation of a WSB; (3) ultrasound‐guided vessel cannulation; (4) proper catheter placement; and (5) sharps safety. Of note, the creation of a WSB element consisted of 4 steps, each of which was analyzed separately. The average scores are reported as means standard deviations (SDs).
To determine the type of analysis that would be performed, we started by assessing the changes using paired t tests. The Kolmogorov‐Smirnov and Anderson‐Darling normality tests revealed no evidence of violations of the normality assumption, confirming that using paired t tests was valid.
To address potential contamination from residents' real experiences on the rate of their knowledge decay between the immediate evaluation and delayed follow‐up, each participant completed a brief survey before their delayed evaluation asking about interim experiences. All calculations were performed including and excluding from participants' scores with affirmative answers to control for this contamination. Last, a post‐hoc analysis was performed on participants' scores using a scatterplot and statistical analyses to control for the varying time‐to‐follow‐up.
Results
All 20 individuals completed the study, for a total of 60 evaluations (20 each of initial, immediate, and delayed). The actual training time (not including the viewing of the video) ranged between 45 to 120 minutes, depending on the trainee. Our primary endpoints are depicted in Table 2. The mean overall score on the initial evaluation was 1.0 0.8. The mean overall score for the immediate posttraining evaluation was 4.4 0.3. This improvement of 3.4 points was significant (P < 0.001; 95% CI, 3.0‐3.7). The delayed evaluations took place an average of 22 days after the training session (range, 5‐101 days), and produced an overall mean score of 4.2 0.3. This decay of 0.2 was not significant (P = 0.14; 95% CI, 0.31 to 0.05). With regard to the amount of skills decay, additional calculations were performed from the scatterplot that depicted scores and the variability in time‐to‐follow‐up. We found that even after controlling this variable, the amount of decay for the overall score remained insignificant.
| ||
| Mean (SD) score of initial (baseline) evaluation | 1.0 (0.80) | |
| Mean (SD) score of immediate posttraining (baseline) evaluation | 4.4 (0.30) | |
| Average change between initial and immediate posttraining scores | +3.4 | P < 0.001; CI, 3.0‐3.7 |
| Mean (SD) score of delayed posttraining evaluation | 4.2 (0.32) | |
| Change between immediate and delayed posttraining scores | 0.2 | P = 0.144; CI, 0.31‐0.05 |
The results of the secondary endpoint calculations (essential elements) are depicted in Table 3. Ultrasound‐guided vessel cannulations improved from an initial average score of 0.9 1.0 to an immediate average score of 4.2 0.5 (P < 0.001; 95% CI, 3.0‐3.7); the delayed score of 4.3 0.6 was statistically unchanged from immediate (P = 0.77; 95% CI, 0.4 to 0.3). Catheter placement skills improved from 1.1 1.1 to 4.2 0.5 (P < 0.001; 95% CI, 2.6‐3.7), and the delayed score of 4.3 0.7 was unchanged from immediate (P < 0.58; 95% CI, 0.5 to 0.3). Sharps safety also improved significantly from initial (2.0 2.3) to immediate (4.9 0.5) (P < 0.0001; 95% CI, 1.9‐3.9), and the delayed scores dropped insignificantly to 4.6 0.8 (P = 0.08; 95% CI, 0.0‐0.6). Hand washing improved significantly from an initial score of 0.9 1.9 to an immediate score of 3.5 2.2 (P < 0.001; 95% CI, 1.4‐3.7), and decayed insignificantly on the delayed evaluation to 3.0 2.3 (P = 0.53; 95% CI, 0.9 to 1.7). WSB skills consisted of 4 individual steps, all of which all improved significantly from initial to immediate scores, and had insignificant decays on the delayed evaluations (see Table 3 WSB for details).
| Initial Evaluation | Immediate Follow‐Up | P Value (Initial to Immediate) | Delayed Follow‐Up | P Value (Immediate to Delayed) | |
|---|---|---|---|---|---|
| |||||
| Ultrasound‐guided insertion of needle into vein (step 15) | 0.9 (1.0) | 4.2 (0.5) | P < 0.001; CI, 3.0‐3.7 | 4.3 ( 0.6) | P = 0.77; CI, 0.4 to 0.3 |
| Catheter placement (step 18) | 1.1 (1.1) | 4.2 (0.5) | P < 0.0001; CI, 2.6‐3.7 | 4.3 ( 0.7) | P = 0.58; CI, 0.5 to 0.3 |
| Sharps safety (step 20) | 2.0 (2.3) | 4.9 (0.5) | P < 0.0001; CI, 1.9‐3.9 | 4.6 ( 0.8) | P = 0.08; CI = 0 to 0.6 |
| Hand washing (step 2) | 0.9 (1.9) | 3.5 (2.2) | P < 0.001; CI, 1.4‐3.7 | 3.0 ( 2.3) | P = 0.53; CI, 0.9 to 1.7 |
| WSBs | |||||
| MD prep (step 3) | 1.8 (1.5) | 4.3 (0.7) | P < 0.0001; CI, 1.7‐3.3 | 4.2 ( 0.6) | P = 0.30; CI, 0.2 to 0.6 |
| Site sterilization (step 7) | 1.1 (1.1) | 4.3 (0.9) | P < 0.0001; CI, 2.7‐3.7 | 4.5 ( 0.5) | P = 0.45; CI, 0.6 to 0.3 |
| WSB creation (step 8) | 0.6 ( 0.6) | 4.1 ( 0.9) | P < 0.0001; CI, 3.0‐4.0 | 4.4 ( 0.6) | P = 0.26; CI, 0.7 to 0.2 |
| Ultrasound probe cover application (step 9) | 0.4 ( 0.9) | 4.1 ( 0.8) | P < 0.0001; CI, 3.2‐4.1 | 4.4 ( 0.8) | P = 0.23; CI, 0.8 to 0.2 |
We performed validation exercises to determine the degree of interrater agreement. Of the 60 total evaluations that were eventually performed, 11 evaluations had been performed simultaneously and independently by evaluators A and B. An analysis of the scores assigned by each evaluator to these 11 trainees revealed a high level of interrater agreement (96%). Further, we performed independent analyses of the trainees' scores as assessed by evaluator A (22 sessions) or evaluator B (27 sessions) across the initial, immediate, and delayed sessions, and we detected no statistical differences in the changes in scores (which mirrored the overall results above).
With regards to real‐life contamination between immediate scores and delayed scores, we identified 3 trainees who had placed central lines on actual patients during the interim period (2 trainees placed 1 line each, and 1 trainee placed 2 lines). We repeated all of the calculations without these participants' delayed scores and determined that the removal of their scores did not change the statistical significance of any of the study results. With regard to knowledge decay, the scatterplot comparing delayed scores to varying time‐to‐follow up revealed no correlation.
Discussion
Our study was designed to determine whether novice trainees could learn and retain proper central line placement skills on the NHTM by receiving personalized training in a relaxed, 1‐on‐1 learning environment. Success was measured by trained evaluators using a detailed evaluation tool with a 6‐point scoring scale. The results of our primary endpoints (changes in overall average scores across the 3 evaluation periods) confirmed that this type of training could quickly improve novice practitioners' skill levels from very low (initial evaluation) to significantly higher (immediate posttraining). The dropoff (decay) in skill levels was found to not be statistically significant over a period of several weeks, although we recognize that further study should be performed to establish the degree of skill decay over a longer period of time.
Because some steps in a central line insertion are more critical to the procedure's success than others (ie, a skin nick with a scalpel is less critical than vessel cannulation under ultrasound‐guidance), we analyzed 5 essential elements individually as secondary endpoints. This secondary analysis was designed to unmask any critical skill deficiencies that might otherwise have been lost in the overall analysis. For each individual essential elements step, this subanalysis similarly revealed a significant improvement from initial to immediate posttraining, and an insignificant score decay on the delayed evaluation.
We recognize a number of limitations to this study. First, the n is relatively small. A larger sample size would have allowed for greater statistical power. In addition, the scoring system used for this study was created by our Procedure Center staff and had never been truly validated elsewhere. The scoring system was transparent and logical, but we recognize that any attempt to use an interval scoring system to quantify procedural skills will be inherently imperfect; the difference between 1 and 2 is not necessarily the same as a difference between 4 and 5. Great efforts were taken to mitigate the impact of this limitation: explicit definitions were established for each score, and we put our evaluators through a rigorous scoring orientation at the outset to standardize their interpretation and use of the scoring system and assessment tool.
The variability in the amount of training time spent in each session could be considered to be a confounder. Our prior experiences training interns in small groups, however, suggested that individuals learn these skills at different paces and in different ways, and so we consider our customized approach to be an essential part of this training experience. We do recognize the practical limitations inherent in rolling out such an open‐ended approach, and program directors may face time and/or resource limitations if attempting to replicate this training strategy.
We were also aware of potential interrater variability between the evaluators. Our approach to addressing this was multifactorial: we went to great lengths to standardize evaluators' understanding of the intended scoring methodology prior to the initiation of the study. We also assessed the degree of interrater reliability once all data was collected. This analysis reinforced that both evaluators were scoring trainees in a virtually identical fashion. We attribute this consistency to the quality of the scoring system, the effectiveness of the prestudy evaluator orientation with a proceduralist, and the high degree of teamwork between the 2 evaluators that kept them closely in sync with one another throughout the study.
Evaluator bias was also a concern. While each evaluator was blinded to the trainees' prior scores, the setup associated with the different training sessions, as well as the obvious differences in performance between the trainees' initial and immediate/delayed performances, made full blinding of the evaluators difficult. The theoretical risk of evaluator bias in this study would have led to evaluators rating trainees higher in the immediate and delayed performances in order to demonstrate more dramatic results. We believe that, since the evaluators themselves did not perform the actual training, and since they did not know the previous scores for the trainee, they were less inclined to skew the scores. Video recording each performance and submitting this recording to a fully‐blinded, third‐party evaluator would have more rigorously ensured blinding than we were able to accomplish. This approach could be considered in future studies of this type.
An addition limitation involved the time‐to‐follow‐up. While a longer time interval between the immediate and delayed evaluations may have better evaluated the impact of the training and potential decay, we sought to balance this with the growing risk of contamination from real central line placement experiences as more time passed. With this issue in mind, the removal of the delayed scores from the 3 trainees who had placed central lines on actual patients in between the immediate and delayed evaluations (2 trainees placed 1 line each, and 1 trainee placed 2 lines) did not change the statistical significance of any of the study results.
One practical concern has to do with the reproducibility of this approach at other institutions. Each trainee received up to 2 hours of individualized attention, and each session consumed fresh supplies and required a proceduralist's and an evaluator's time. This represents a significant commitment of materials and manpower. A careful cost/benefit analysis is therefore warranted before implementing this kind of rigorous training program. As mentioned, the cost of the NHTM is approximately $120 and can withstand several cannulations over a 2‐day period; the sterile supplies and central line add up to approximately another $75/evaluation. Depending on the number of interns and residents at a given institution, these costs could prove prohibitive to cash‐poor residency training programs. In the larger picture, however, catheter‐related bloodstream infections have been estimated to result in a mortality rate of 4% to 20%, and a single catheter‐related bloodstream infection can cost up to $45,000.2124 In addition, new Medicare reimbursement policies are now beginning to limit hospital reimbursement for these types of iatrogenic events; hence, narrowing the margin of error and putting even greater financial pressures on hospitals.25 It is our belief, therefore, that an up‐front investment in NHTMs (or an alternative simulator), basic supplies, and the necessary trainer time will prove to be cost‐effective and enlightened investments from forward‐thinking leadership.
Last, we are also aware that our study did not look at whether our trainees' improved performance on the NHTM actually translated into better patient outcomes. Since patient safety is our ultimate goal, and this phase of PPSI limited all of our training and evaluations to the NHTMs, future studies must ultimately evaluate how well these learned skills translate into procedure performance on actual patients. This controlled study (possibly with a see‐1 do‐1 teach‐1 control group) will be logistically challenging, but will be the most definitive manner with which to demonstrate the true value of personalized training sessions using the NHTM (or another nonhuman simulator).
PPSI‐II demonstrated that using the NHTM as the basis for training novice practitioners in a personalized, 1‐on‐1 training session led to significant improvements in measured procedural skills. Further, these skills were retained over time. This positive study contributes to the growing body of literature pointing towards the role of intensive 1‐on‐1 training with simulators to advance procedural education for clinicians. Ultimately, we aim to demonstrate that providing trainees this type of training prior to having them perform procedures on actual patients will translate into superior patient care, greater success rates, fewer minor and major complications, and lower overall patient care costs. Rather than clinging to the classic but never‐validated see‐1, do‐1, teach‐1 approach, we believe that procedural training must adapt new curricula and technologies that will help us achieve the goals of maximizing the safety and quality of care for our patients.
Acknowledgements
The authors recognize and appreciate the entire staff of The Procedure Center at Cedars‐Sinai Medical Center for their support of this research project. The authors give special thanks to Obed Martinez for his tireless assistance with the scheduling and coordination of training activities, and to Jim Mirocha for statistical analysis and editorial contributions.
- Accreditation Council for Graduate Medical Education (ACGME). Home page. Available at: http://www.acgme.org. Accessed June2009.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety and quality.J Hosp Med.2007;2(3):123–125.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2(3):135–142.
- ,,,,,Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3(1):48–54.
- ,,.Central line simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,, et al.Developing technical expertise in emergency medicine—the role of simulation in procedural skill acquisition.Acad Emerg Med.2008;15:1046–1057.
- ,,,.A training system for ultrasound‐guided needle insertion procedures.Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv.2007;10(1):566–574.
- ,,, et al.Video‐based training increases sterile‐technique compliance during central venous catheter insertion.Crit Care Med.2007;35:1302–1306.
- ,,,,Comparison of results of virtual‐reality simulator and training model for basic ureteroscopy training.J Endourol.2006;20(4):266–271.
- ,.Papaya: a simulation model for training in uterine aspiration.Fam Med.2005;37(4):242–244.
- ,,,,.An intervention to improve procedure education for internal medicine residents.J Gen Intern Med.2008;23(3):288–293.
- ,,.The use of tissue models for vascular access training: phase 1 of the procedural patient safety initiative.J Gen Intern Med.2006;21(5):514–517.
- Blue phantom: CVC hands‐on trainer, items # BPH600f, BPH604HP, BPH600AP. Available at: http://www.bluephantom.com/desktopdefault.aspx?tabid=232. Accessed June2009.
- Simulab Corporation: Central Line Man System. Available at: http://www.simulab.com/product/surgery/open/centralineman‐system. Accessed June2009.
- KyotoKagaku Co., Ltd.: CVC Insertion Simulator. Available at: http://www.kyotokagaku.com/products/detail01/m93u.html. Accessed June2009.
- First Aid Manufacturer CVC Simulator. Available at: http://www.first‐aid‐manufacturer.com/CVC‐Simulator.aspx. Accessed June2009.
- Limbs and Things: Central Venous Catheter Insertion Simulator, part #KKM93UB. Available at: http://www.golimbs.com/products/products.php?sectid=5356(17):1789–1790.
- ,,.Practice #20: proceduralists. The Advisory Board Annual Report.2007:162–169.
- NEJM video. Available at: http://content.nejm.org/cgi/content/short/356/21/e21. Accessed June2009.
- .Prevention of intravascular catheter‐related infections.Ann Intern Med.2000;132(5):391–402.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Recomm Rep.2002;51(RR‐10):1–29.
- ,,, et al.Clinical and economic outcomes in critically ill patients with nosocomial catheter‐related bloodstream infections.Clin Infect Dis.2005;41:1591–1598.
- ,,,,,.Attributable morbidity and mortality of catheter‐related septicemia in critically ill patients: a matched, risk‐adjusted, cohort study.Infect Control Hosp Epidemiol.1999;20(6):396–401.
- Centers for Medicaid and Medicare Services. U.S. Department of Health and Human Services. Hospital‐Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp#TopOfPage. Accessed June2009.
The Accreditation Counsel for Graduate Medical Education (ACGME) states in its Program Requirements for Residency Education in Internal Medicine that all residents must develop technical proficiency in several procedures, including central venous line placement.1 Developing competency in common procedural skills has long been a part of medical training. The philosophy of see‐1, do‐1, teach‐1 is still the most common means by which most residents seek to obtain this proficiency, even though serious concerns have been raised about this approach.2 A typical first experience in central line placement usually involves an eager (or terrified) trainee making several clumsy attempts on an actual patient, under the hurried guidance of a senior resident who themselves received an unknown degree of training. In this scenario, rarely does standardized instruction, formal evaluation, or structured follow‐up occur.
A revitalized emphasis is now being placed on patient safety in healthcare, including an industry‐wide commitment to minimizing procedural complications. The most common complications associated to central line placement include vascular damage and catheter‐related bloodstream infections. A number of creative approaches are being developed to improve the quality of instruction on proper procedural techniques, all varying considerably in sophistication, scope, and rigor. Examples include the use of computer‐assisted methods for training ultrasound‐guided needle insertion techniques and ureteroscopy training, hands‐on training with synthetic models for thoracentesis training, video training, and uterine aspiration using papayas.311 Implicit in this trend is recognition that we, as educators, healthcare providers, and patient advocates, must design more cost effective and efficient ways to teach medical and surgical procedural techniques to clinicians.
Our approach was previously described in phase I of the Procedural Patient Safety Initiative (PPSI).12 In PPSI‐I, we introduced a nonhuman tissue model (NHTM; Figure 1) as the basis for teaching physicians a more rigorous curriculum of essential central line placement skills. By way of brief review, the NHTMs were constructed by tunneling 0.2‐mm‐thick rubber tubing (vessels) lengthwise through raw, whole chickens purchased at the grocery store. The vessels were filled with colored water to simulate blood. The NHTM has several unique features, including: (1) realistic‐appearing vessels when viewed under ultrasound, which mimic the appearance of human internal jugular veins and carotid arteries (Figure 2); (2) tissue turgor and vessel composition that produce realistic pops and flashes upon puncture and allow for multiple cannulations; (3) the ability to perform a complete central line placement (including wire advancement, dilation, line insertion, suturing, and sterile dressing placement); (4) cost effectiveness relative to other commercially‐available products (each NHTM costs $120 and can withstand multiple cannulations over 2 days).1317 During the training sessions of Phase I, participants were oriented to the ultrasound machines, shown the contents of our central line kit, and taught the principles of wide sterile barriers (WSBs), sharps safety, and vascular access under real‐time ultrasound guidance. A self‐completed survey tool was filled out by the participants before and after the session that contained questions about their precourse baseline procedural experience, and their subjective comfort level with specific skills after the course. The results of our intervention, as measured by the responses to the survey, were significantly positive. We recognized the limitations of these results based on using subjective criteria to measure efficacy, a lack of follow‐up on participants' skill retention, and with no ultimate evaluation of procedural competency evaluations on actual patients (compared to an untrained control group).


Our ultimate goal is to validate a curriculum that will give trainees the necessary education and skills that enables them to make a smooth, competent, and complication‐free transition to live patient procedures. Phase II of PPSI is our next step toward this goal. In this study, we sought to measure the impact of intensive, 1:1 central line placement training with a proceduralist, objectively validate the efficacy of the NHTM and our training curriculum using a standardized 6‐point scoring scale and skilled evaluators, and to evaluate the degree of skill retention over time (decay). Our hypothesis was that the depth of skills' imprinting from a single, standardized training session would result in a significant improvement in measured procedure skills immediately after the trainee is taught the skills, and that the retention of these skills would be demonstrable when participants were reevaluated at a future date.
Methods
PPSI‐II was an observational, prospective study conducted by The Procedure Center at Cedars‐Sinai Medical Center, a 900+‐bed, community‐based teaching hospital. The Procedure Center is staffed by dedicated Proceduralists who perform a number of common medical procedures on a daily basis and are facile with both real‐time ultrasound guidance and proper procedural techniques.18, 19 Our target population was the incoming Internal Medicine residents. Subjects were recruited by email prior to orientation week and were offered the option of participating in our study. Our only exclusion criterion was the prior observation or placement of 10 or more central lines. The study was approved by our hospital's Institutional Review Board prior to initiating recruitment. Those who chose not to participate underwent the standard orientation training required by our institution, which included a brief overview lecture on the topic of central lines and ultrasound‐guidance, a group viewing of the New England Journal of Medicine (NEJM) video on central line placement,20 and small‐group (4 participants/group) hands‐on practice sessions lasting 45 minutes with NHTMs and a trained Proceduralist.
All of the evaluations for Phase II were done using the Central Line Placement Skill Assessment Tool depicted in Figure 3. This tool, which was developed by Cedars‐Sinai Proceduralists solely for the purposes of this study, is a comprehensive step‐by‐step checklist delineating the specific steps necessary to place a sterile, ultrasound‐guided central venous catheter. It was closely derived from a central line insertion checklist that was created by the Procedure Center 3 years ago to help guide novice clinicians through the procedure, and has since been widely used throughout the institution during the placement of hundreds of central lines. The scoring system, also devised by Cedars‐Sinai Proceduralists, was based on over 15 years of experience supervising and teaching hundreds of residents on proper central line insertion techniques. It consists of clear definitions for each score that were agreed upon via consensus amongst study coordinators. Prior to any evaluations being conducted, we put our 2 evaluators (both senior medicine residents) through identical and simultaneous scoring training with the Proceduralist trainer to standardize procedural knowledge and scoring methodology.

A total of 20 incoming interns (trainees) out of a possible 54 invitations (37%) volunteered to participate. Each trainee was randomly assigned a number from 1 to 20. The study began for each trainee with a brief, 5‐question survey to determine their prior procedural experience (Table 1). Next, each trainee watched the NEJM online training video on central line placement.20 They were then brought into a training room that contained an NHTM sitting on a Mayo stand, an ultrasound machine, and all the materials required to place a central line insertion under ultrasound‐guidance. The trainee's baseline central line insertion skills were evaluated on 22 unique procedure steps, with each score being given by 1 of the 2 evaluators (initial evaluation). The trainee did not receive any guidance or suggestions during this initial evaluation unless the trainee reached an impasse. In these cases, the evaluator completed that single step on the trainee's behalf and then allowed the session to resume. The identity of the evaluator was indicated on each evaluation form, and after each of the evaluations the completed assessment tool was given to our blinded assistant for data recording.
| Number of trainees | 20 |
| How many prior central lines have you inserted independently? (exclusion criteria: >10) | 20 answered 0 |
| How many prior central line insertions have you assisted with? (exclusion criteria: >10) | 13 answered 0; 7 had assisted between 1 and 4 lines |
| How many prior central line insertions have you observed? (exclusion criteria: >10) | 3 answered 0; 17 had observed between 1 and 5 lines |
| Have you had any prior exposure to the use of ultrasound for central line insertion? | 13 no; 7 yes |
| Have you had any prior exposure to the use of wide sterile barriers for procedures? | 11 no; 9 yes |
Each trainee was then given a personalized, hands‐on training session by a proceduralist, using the checklist as a guide to take them through all the steps of a central line insertion. The trainee was allowed to observe and practice each skill for an unlimited period of time with the proceduralist present, until he or she demonstrated competency and felt confident enough with their independent skills (in both trainee's and proceduralist's judgment) to move forward. The entire session ended only when all steps had been taught and practiced to the proceduralist's satisfaction, the trainee felt comfortable independently performing each step (and in proper sequence), and all questions had been answered. At no time was there an imposition of time constraints or external pressure from study coordinators.
Immediately following this training session the proceduralist and trainee left the room, the procedure room was reset by an evaluator (taking approximately 5‐10 minutes), and then the trainee submitted themselves to an immediate posttraining evaluation (immediate evaluation). As with the initial assessment, the evaluator did not interfere or make any comments or suggestions during the evaluation periods, unless the trainee reached an impasse at any step. In that case, the trainee would receive a 0 for that step, the evaluator would assist them to complete that step only, and then the session would continue. No time limits were imposed.
The final part of the study required each trainee to return for follow‐up assessment (delayed evaluation), a process that was identical to the immediate posttraining evaluation. This delayed evaluation was intended to occur between 3 to 4 weeks after the immediate posttraining session, based on trainees' schedules and availability. No refresher or practice time was permitted prior to the delayed evaluation: upon arrival, trainees wrote down on a separate piece of paper (not seen by the evaluator) the number of interim line experiences they had experienced, then they were brought directly into a fully‐prepared room, and instructed to begin. The evaluator was also blind to the trainee's scores from the 2 previous evaluation sessions.
The primary endpoints were the degree of changes in overall average scores (from the 22 steps on the assessment tool) from the initial to the immediate evaluations and from the immediate to delayed evaluations. The secondary endpoints were also based on changes in average scores from the initial to immediate and immediate to delayed evaluations, and looked at 5 essential elements (steps in the assessment tool that we deemed critical to the safe and successful placement of a central line). These essential elements included (1) hand washing; (2) creation of a WSB; (3) ultrasound‐guided vessel cannulation; (4) proper catheter placement; and (5) sharps safety. Of note, the creation of a WSB element consisted of 4 steps, each of which was analyzed separately. The average scores are reported as means standard deviations (SDs).
To determine the type of analysis that would be performed, we started by assessing the changes using paired t tests. The Kolmogorov‐Smirnov and Anderson‐Darling normality tests revealed no evidence of violations of the normality assumption, confirming that using paired t tests was valid.
To address potential contamination from residents' real experiences on the rate of their knowledge decay between the immediate evaluation and delayed follow‐up, each participant completed a brief survey before their delayed evaluation asking about interim experiences. All calculations were performed including and excluding from participants' scores with affirmative answers to control for this contamination. Last, a post‐hoc analysis was performed on participants' scores using a scatterplot and statistical analyses to control for the varying time‐to‐follow‐up.
Results
All 20 individuals completed the study, for a total of 60 evaluations (20 each of initial, immediate, and delayed). The actual training time (not including the viewing of the video) ranged between 45 to 120 minutes, depending on the trainee. Our primary endpoints are depicted in Table 2. The mean overall score on the initial evaluation was 1.0 0.8. The mean overall score for the immediate posttraining evaluation was 4.4 0.3. This improvement of 3.4 points was significant (P < 0.001; 95% CI, 3.0‐3.7). The delayed evaluations took place an average of 22 days after the training session (range, 5‐101 days), and produced an overall mean score of 4.2 0.3. This decay of 0.2 was not significant (P = 0.14; 95% CI, 0.31 to 0.05). With regard to the amount of skills decay, additional calculations were performed from the scatterplot that depicted scores and the variability in time‐to‐follow‐up. We found that even after controlling this variable, the amount of decay for the overall score remained insignificant.
| ||
| Mean (SD) score of initial (baseline) evaluation | 1.0 (0.80) | |
| Mean (SD) score of immediate posttraining (baseline) evaluation | 4.4 (0.30) | |
| Average change between initial and immediate posttraining scores | +3.4 | P < 0.001; CI, 3.0‐3.7 |
| Mean (SD) score of delayed posttraining evaluation | 4.2 (0.32) | |
| Change between immediate and delayed posttraining scores | 0.2 | P = 0.144; CI, 0.31‐0.05 |
The results of the secondary endpoint calculations (essential elements) are depicted in Table 3. Ultrasound‐guided vessel cannulations improved from an initial average score of 0.9 1.0 to an immediate average score of 4.2 0.5 (P < 0.001; 95% CI, 3.0‐3.7); the delayed score of 4.3 0.6 was statistically unchanged from immediate (P = 0.77; 95% CI, 0.4 to 0.3). Catheter placement skills improved from 1.1 1.1 to 4.2 0.5 (P < 0.001; 95% CI, 2.6‐3.7), and the delayed score of 4.3 0.7 was unchanged from immediate (P < 0.58; 95% CI, 0.5 to 0.3). Sharps safety also improved significantly from initial (2.0 2.3) to immediate (4.9 0.5) (P < 0.0001; 95% CI, 1.9‐3.9), and the delayed scores dropped insignificantly to 4.6 0.8 (P = 0.08; 95% CI, 0.0‐0.6). Hand washing improved significantly from an initial score of 0.9 1.9 to an immediate score of 3.5 2.2 (P < 0.001; 95% CI, 1.4‐3.7), and decayed insignificantly on the delayed evaluation to 3.0 2.3 (P = 0.53; 95% CI, 0.9 to 1.7). WSB skills consisted of 4 individual steps, all of which all improved significantly from initial to immediate scores, and had insignificant decays on the delayed evaluations (see Table 3 WSB for details).
| Initial Evaluation | Immediate Follow‐Up | P Value (Initial to Immediate) | Delayed Follow‐Up | P Value (Immediate to Delayed) | |
|---|---|---|---|---|---|
| |||||
| Ultrasound‐guided insertion of needle into vein (step 15) | 0.9 (1.0) | 4.2 (0.5) | P < 0.001; CI, 3.0‐3.7 | 4.3 ( 0.6) | P = 0.77; CI, 0.4 to 0.3 |
| Catheter placement (step 18) | 1.1 (1.1) | 4.2 (0.5) | P < 0.0001; CI, 2.6‐3.7 | 4.3 ( 0.7) | P = 0.58; CI, 0.5 to 0.3 |
| Sharps safety (step 20) | 2.0 (2.3) | 4.9 (0.5) | P < 0.0001; CI, 1.9‐3.9 | 4.6 ( 0.8) | P = 0.08; CI = 0 to 0.6 |
| Hand washing (step 2) | 0.9 (1.9) | 3.5 (2.2) | P < 0.001; CI, 1.4‐3.7 | 3.0 ( 2.3) | P = 0.53; CI, 0.9 to 1.7 |
| WSBs | |||||
| MD prep (step 3) | 1.8 (1.5) | 4.3 (0.7) | P < 0.0001; CI, 1.7‐3.3 | 4.2 ( 0.6) | P = 0.30; CI, 0.2 to 0.6 |
| Site sterilization (step 7) | 1.1 (1.1) | 4.3 (0.9) | P < 0.0001; CI, 2.7‐3.7 | 4.5 ( 0.5) | P = 0.45; CI, 0.6 to 0.3 |
| WSB creation (step 8) | 0.6 ( 0.6) | 4.1 ( 0.9) | P < 0.0001; CI, 3.0‐4.0 | 4.4 ( 0.6) | P = 0.26; CI, 0.7 to 0.2 |
| Ultrasound probe cover application (step 9) | 0.4 ( 0.9) | 4.1 ( 0.8) | P < 0.0001; CI, 3.2‐4.1 | 4.4 ( 0.8) | P = 0.23; CI, 0.8 to 0.2 |
We performed validation exercises to determine the degree of interrater agreement. Of the 60 total evaluations that were eventually performed, 11 evaluations had been performed simultaneously and independently by evaluators A and B. An analysis of the scores assigned by each evaluator to these 11 trainees revealed a high level of interrater agreement (96%). Further, we performed independent analyses of the trainees' scores as assessed by evaluator A (22 sessions) or evaluator B (27 sessions) across the initial, immediate, and delayed sessions, and we detected no statistical differences in the changes in scores (which mirrored the overall results above).
With regards to real‐life contamination between immediate scores and delayed scores, we identified 3 trainees who had placed central lines on actual patients during the interim period (2 trainees placed 1 line each, and 1 trainee placed 2 lines). We repeated all of the calculations without these participants' delayed scores and determined that the removal of their scores did not change the statistical significance of any of the study results. With regard to knowledge decay, the scatterplot comparing delayed scores to varying time‐to‐follow up revealed no correlation.
Discussion
Our study was designed to determine whether novice trainees could learn and retain proper central line placement skills on the NHTM by receiving personalized training in a relaxed, 1‐on‐1 learning environment. Success was measured by trained evaluators using a detailed evaluation tool with a 6‐point scoring scale. The results of our primary endpoints (changes in overall average scores across the 3 evaluation periods) confirmed that this type of training could quickly improve novice practitioners' skill levels from very low (initial evaluation) to significantly higher (immediate posttraining). The dropoff (decay) in skill levels was found to not be statistically significant over a period of several weeks, although we recognize that further study should be performed to establish the degree of skill decay over a longer period of time.
Because some steps in a central line insertion are more critical to the procedure's success than others (ie, a skin nick with a scalpel is less critical than vessel cannulation under ultrasound‐guidance), we analyzed 5 essential elements individually as secondary endpoints. This secondary analysis was designed to unmask any critical skill deficiencies that might otherwise have been lost in the overall analysis. For each individual essential elements step, this subanalysis similarly revealed a significant improvement from initial to immediate posttraining, and an insignificant score decay on the delayed evaluation.
We recognize a number of limitations to this study. First, the n is relatively small. A larger sample size would have allowed for greater statistical power. In addition, the scoring system used for this study was created by our Procedure Center staff and had never been truly validated elsewhere. The scoring system was transparent and logical, but we recognize that any attempt to use an interval scoring system to quantify procedural skills will be inherently imperfect; the difference between 1 and 2 is not necessarily the same as a difference between 4 and 5. Great efforts were taken to mitigate the impact of this limitation: explicit definitions were established for each score, and we put our evaluators through a rigorous scoring orientation at the outset to standardize their interpretation and use of the scoring system and assessment tool.
The variability in the amount of training time spent in each session could be considered to be a confounder. Our prior experiences training interns in small groups, however, suggested that individuals learn these skills at different paces and in different ways, and so we consider our customized approach to be an essential part of this training experience. We do recognize the practical limitations inherent in rolling out such an open‐ended approach, and program directors may face time and/or resource limitations if attempting to replicate this training strategy.
We were also aware of potential interrater variability between the evaluators. Our approach to addressing this was multifactorial: we went to great lengths to standardize evaluators' understanding of the intended scoring methodology prior to the initiation of the study. We also assessed the degree of interrater reliability once all data was collected. This analysis reinforced that both evaluators were scoring trainees in a virtually identical fashion. We attribute this consistency to the quality of the scoring system, the effectiveness of the prestudy evaluator orientation with a proceduralist, and the high degree of teamwork between the 2 evaluators that kept them closely in sync with one another throughout the study.
Evaluator bias was also a concern. While each evaluator was blinded to the trainees' prior scores, the setup associated with the different training sessions, as well as the obvious differences in performance between the trainees' initial and immediate/delayed performances, made full blinding of the evaluators difficult. The theoretical risk of evaluator bias in this study would have led to evaluators rating trainees higher in the immediate and delayed performances in order to demonstrate more dramatic results. We believe that, since the evaluators themselves did not perform the actual training, and since they did not know the previous scores for the trainee, they were less inclined to skew the scores. Video recording each performance and submitting this recording to a fully‐blinded, third‐party evaluator would have more rigorously ensured blinding than we were able to accomplish. This approach could be considered in future studies of this type.
An addition limitation involved the time‐to‐follow‐up. While a longer time interval between the immediate and delayed evaluations may have better evaluated the impact of the training and potential decay, we sought to balance this with the growing risk of contamination from real central line placement experiences as more time passed. With this issue in mind, the removal of the delayed scores from the 3 trainees who had placed central lines on actual patients in between the immediate and delayed evaluations (2 trainees placed 1 line each, and 1 trainee placed 2 lines) did not change the statistical significance of any of the study results.
One practical concern has to do with the reproducibility of this approach at other institutions. Each trainee received up to 2 hours of individualized attention, and each session consumed fresh supplies and required a proceduralist's and an evaluator's time. This represents a significant commitment of materials and manpower. A careful cost/benefit analysis is therefore warranted before implementing this kind of rigorous training program. As mentioned, the cost of the NHTM is approximately $120 and can withstand several cannulations over a 2‐day period; the sterile supplies and central line add up to approximately another $75/evaluation. Depending on the number of interns and residents at a given institution, these costs could prove prohibitive to cash‐poor residency training programs. In the larger picture, however, catheter‐related bloodstream infections have been estimated to result in a mortality rate of 4% to 20%, and a single catheter‐related bloodstream infection can cost up to $45,000.2124 In addition, new Medicare reimbursement policies are now beginning to limit hospital reimbursement for these types of iatrogenic events; hence, narrowing the margin of error and putting even greater financial pressures on hospitals.25 It is our belief, therefore, that an up‐front investment in NHTMs (or an alternative simulator), basic supplies, and the necessary trainer time will prove to be cost‐effective and enlightened investments from forward‐thinking leadership.
Last, we are also aware that our study did not look at whether our trainees' improved performance on the NHTM actually translated into better patient outcomes. Since patient safety is our ultimate goal, and this phase of PPSI limited all of our training and evaluations to the NHTMs, future studies must ultimately evaluate how well these learned skills translate into procedure performance on actual patients. This controlled study (possibly with a see‐1 do‐1 teach‐1 control group) will be logistically challenging, but will be the most definitive manner with which to demonstrate the true value of personalized training sessions using the NHTM (or another nonhuman simulator).
PPSI‐II demonstrated that using the NHTM as the basis for training novice practitioners in a personalized, 1‐on‐1 training session led to significant improvements in measured procedural skills. Further, these skills were retained over time. This positive study contributes to the growing body of literature pointing towards the role of intensive 1‐on‐1 training with simulators to advance procedural education for clinicians. Ultimately, we aim to demonstrate that providing trainees this type of training prior to having them perform procedures on actual patients will translate into superior patient care, greater success rates, fewer minor and major complications, and lower overall patient care costs. Rather than clinging to the classic but never‐validated see‐1, do‐1, teach‐1 approach, we believe that procedural training must adapt new curricula and technologies that will help us achieve the goals of maximizing the safety and quality of care for our patients.
Acknowledgements
The authors recognize and appreciate the entire staff of The Procedure Center at Cedars‐Sinai Medical Center for their support of this research project. The authors give special thanks to Obed Martinez for his tireless assistance with the scheduling and coordination of training activities, and to Jim Mirocha for statistical analysis and editorial contributions.
The Accreditation Counsel for Graduate Medical Education (ACGME) states in its Program Requirements for Residency Education in Internal Medicine that all residents must develop technical proficiency in several procedures, including central venous line placement.1 Developing competency in common procedural skills has long been a part of medical training. The philosophy of see‐1, do‐1, teach‐1 is still the most common means by which most residents seek to obtain this proficiency, even though serious concerns have been raised about this approach.2 A typical first experience in central line placement usually involves an eager (or terrified) trainee making several clumsy attempts on an actual patient, under the hurried guidance of a senior resident who themselves received an unknown degree of training. In this scenario, rarely does standardized instruction, formal evaluation, or structured follow‐up occur.
A revitalized emphasis is now being placed on patient safety in healthcare, including an industry‐wide commitment to minimizing procedural complications. The most common complications associated to central line placement include vascular damage and catheter‐related bloodstream infections. A number of creative approaches are being developed to improve the quality of instruction on proper procedural techniques, all varying considerably in sophistication, scope, and rigor. Examples include the use of computer‐assisted methods for training ultrasound‐guided needle insertion techniques and ureteroscopy training, hands‐on training with synthetic models for thoracentesis training, video training, and uterine aspiration using papayas.311 Implicit in this trend is recognition that we, as educators, healthcare providers, and patient advocates, must design more cost effective and efficient ways to teach medical and surgical procedural techniques to clinicians.
Our approach was previously described in phase I of the Procedural Patient Safety Initiative (PPSI).12 In PPSI‐I, we introduced a nonhuman tissue model (NHTM; Figure 1) as the basis for teaching physicians a more rigorous curriculum of essential central line placement skills. By way of brief review, the NHTMs were constructed by tunneling 0.2‐mm‐thick rubber tubing (vessels) lengthwise through raw, whole chickens purchased at the grocery store. The vessels were filled with colored water to simulate blood. The NHTM has several unique features, including: (1) realistic‐appearing vessels when viewed under ultrasound, which mimic the appearance of human internal jugular veins and carotid arteries (Figure 2); (2) tissue turgor and vessel composition that produce realistic pops and flashes upon puncture and allow for multiple cannulations; (3) the ability to perform a complete central line placement (including wire advancement, dilation, line insertion, suturing, and sterile dressing placement); (4) cost effectiveness relative to other commercially‐available products (each NHTM costs $120 and can withstand multiple cannulations over 2 days).1317 During the training sessions of Phase I, participants were oriented to the ultrasound machines, shown the contents of our central line kit, and taught the principles of wide sterile barriers (WSBs), sharps safety, and vascular access under real‐time ultrasound guidance. A self‐completed survey tool was filled out by the participants before and after the session that contained questions about their precourse baseline procedural experience, and their subjective comfort level with specific skills after the course. The results of our intervention, as measured by the responses to the survey, were significantly positive. We recognized the limitations of these results based on using subjective criteria to measure efficacy, a lack of follow‐up on participants' skill retention, and with no ultimate evaluation of procedural competency evaluations on actual patients (compared to an untrained control group).


Our ultimate goal is to validate a curriculum that will give trainees the necessary education and skills that enables them to make a smooth, competent, and complication‐free transition to live patient procedures. Phase II of PPSI is our next step toward this goal. In this study, we sought to measure the impact of intensive, 1:1 central line placement training with a proceduralist, objectively validate the efficacy of the NHTM and our training curriculum using a standardized 6‐point scoring scale and skilled evaluators, and to evaluate the degree of skill retention over time (decay). Our hypothesis was that the depth of skills' imprinting from a single, standardized training session would result in a significant improvement in measured procedure skills immediately after the trainee is taught the skills, and that the retention of these skills would be demonstrable when participants were reevaluated at a future date.
Methods
PPSI‐II was an observational, prospective study conducted by The Procedure Center at Cedars‐Sinai Medical Center, a 900+‐bed, community‐based teaching hospital. The Procedure Center is staffed by dedicated Proceduralists who perform a number of common medical procedures on a daily basis and are facile with both real‐time ultrasound guidance and proper procedural techniques.18, 19 Our target population was the incoming Internal Medicine residents. Subjects were recruited by email prior to orientation week and were offered the option of participating in our study. Our only exclusion criterion was the prior observation or placement of 10 or more central lines. The study was approved by our hospital's Institutional Review Board prior to initiating recruitment. Those who chose not to participate underwent the standard orientation training required by our institution, which included a brief overview lecture on the topic of central lines and ultrasound‐guidance, a group viewing of the New England Journal of Medicine (NEJM) video on central line placement,20 and small‐group (4 participants/group) hands‐on practice sessions lasting 45 minutes with NHTMs and a trained Proceduralist.
All of the evaluations for Phase II were done using the Central Line Placement Skill Assessment Tool depicted in Figure 3. This tool, which was developed by Cedars‐Sinai Proceduralists solely for the purposes of this study, is a comprehensive step‐by‐step checklist delineating the specific steps necessary to place a sterile, ultrasound‐guided central venous catheter. It was closely derived from a central line insertion checklist that was created by the Procedure Center 3 years ago to help guide novice clinicians through the procedure, and has since been widely used throughout the institution during the placement of hundreds of central lines. The scoring system, also devised by Cedars‐Sinai Proceduralists, was based on over 15 years of experience supervising and teaching hundreds of residents on proper central line insertion techniques. It consists of clear definitions for each score that were agreed upon via consensus amongst study coordinators. Prior to any evaluations being conducted, we put our 2 evaluators (both senior medicine residents) through identical and simultaneous scoring training with the Proceduralist trainer to standardize procedural knowledge and scoring methodology.

A total of 20 incoming interns (trainees) out of a possible 54 invitations (37%) volunteered to participate. Each trainee was randomly assigned a number from 1 to 20. The study began for each trainee with a brief, 5‐question survey to determine their prior procedural experience (Table 1). Next, each trainee watched the NEJM online training video on central line placement.20 They were then brought into a training room that contained an NHTM sitting on a Mayo stand, an ultrasound machine, and all the materials required to place a central line insertion under ultrasound‐guidance. The trainee's baseline central line insertion skills were evaluated on 22 unique procedure steps, with each score being given by 1 of the 2 evaluators (initial evaluation). The trainee did not receive any guidance or suggestions during this initial evaluation unless the trainee reached an impasse. In these cases, the evaluator completed that single step on the trainee's behalf and then allowed the session to resume. The identity of the evaluator was indicated on each evaluation form, and after each of the evaluations the completed assessment tool was given to our blinded assistant for data recording.
| Number of trainees | 20 |
| How many prior central lines have you inserted independently? (exclusion criteria: >10) | 20 answered 0 |
| How many prior central line insertions have you assisted with? (exclusion criteria: >10) | 13 answered 0; 7 had assisted between 1 and 4 lines |
| How many prior central line insertions have you observed? (exclusion criteria: >10) | 3 answered 0; 17 had observed between 1 and 5 lines |
| Have you had any prior exposure to the use of ultrasound for central line insertion? | 13 no; 7 yes |
| Have you had any prior exposure to the use of wide sterile barriers for procedures? | 11 no; 9 yes |
Each trainee was then given a personalized, hands‐on training session by a proceduralist, using the checklist as a guide to take them through all the steps of a central line insertion. The trainee was allowed to observe and practice each skill for an unlimited period of time with the proceduralist present, until he or she demonstrated competency and felt confident enough with their independent skills (in both trainee's and proceduralist's judgment) to move forward. The entire session ended only when all steps had been taught and practiced to the proceduralist's satisfaction, the trainee felt comfortable independently performing each step (and in proper sequence), and all questions had been answered. At no time was there an imposition of time constraints or external pressure from study coordinators.
Immediately following this training session the proceduralist and trainee left the room, the procedure room was reset by an evaluator (taking approximately 5‐10 minutes), and then the trainee submitted themselves to an immediate posttraining evaluation (immediate evaluation). As with the initial assessment, the evaluator did not interfere or make any comments or suggestions during the evaluation periods, unless the trainee reached an impasse at any step. In that case, the trainee would receive a 0 for that step, the evaluator would assist them to complete that step only, and then the session would continue. No time limits were imposed.
The final part of the study required each trainee to return for follow‐up assessment (delayed evaluation), a process that was identical to the immediate posttraining evaluation. This delayed evaluation was intended to occur between 3 to 4 weeks after the immediate posttraining session, based on trainees' schedules and availability. No refresher or practice time was permitted prior to the delayed evaluation: upon arrival, trainees wrote down on a separate piece of paper (not seen by the evaluator) the number of interim line experiences they had experienced, then they were brought directly into a fully‐prepared room, and instructed to begin. The evaluator was also blind to the trainee's scores from the 2 previous evaluation sessions.
The primary endpoints were the degree of changes in overall average scores (from the 22 steps on the assessment tool) from the initial to the immediate evaluations and from the immediate to delayed evaluations. The secondary endpoints were also based on changes in average scores from the initial to immediate and immediate to delayed evaluations, and looked at 5 essential elements (steps in the assessment tool that we deemed critical to the safe and successful placement of a central line). These essential elements included (1) hand washing; (2) creation of a WSB; (3) ultrasound‐guided vessel cannulation; (4) proper catheter placement; and (5) sharps safety. Of note, the creation of a WSB element consisted of 4 steps, each of which was analyzed separately. The average scores are reported as means standard deviations (SDs).
To determine the type of analysis that would be performed, we started by assessing the changes using paired t tests. The Kolmogorov‐Smirnov and Anderson‐Darling normality tests revealed no evidence of violations of the normality assumption, confirming that using paired t tests was valid.
To address potential contamination from residents' real experiences on the rate of their knowledge decay between the immediate evaluation and delayed follow‐up, each participant completed a brief survey before their delayed evaluation asking about interim experiences. All calculations were performed including and excluding from participants' scores with affirmative answers to control for this contamination. Last, a post‐hoc analysis was performed on participants' scores using a scatterplot and statistical analyses to control for the varying time‐to‐follow‐up.
Results
All 20 individuals completed the study, for a total of 60 evaluations (20 each of initial, immediate, and delayed). The actual training time (not including the viewing of the video) ranged between 45 to 120 minutes, depending on the trainee. Our primary endpoints are depicted in Table 2. The mean overall score on the initial evaluation was 1.0 0.8. The mean overall score for the immediate posttraining evaluation was 4.4 0.3. This improvement of 3.4 points was significant (P < 0.001; 95% CI, 3.0‐3.7). The delayed evaluations took place an average of 22 days after the training session (range, 5‐101 days), and produced an overall mean score of 4.2 0.3. This decay of 0.2 was not significant (P = 0.14; 95% CI, 0.31 to 0.05). With regard to the amount of skills decay, additional calculations were performed from the scatterplot that depicted scores and the variability in time‐to‐follow‐up. We found that even after controlling this variable, the amount of decay for the overall score remained insignificant.
| ||
| Mean (SD) score of initial (baseline) evaluation | 1.0 (0.80) | |
| Mean (SD) score of immediate posttraining (baseline) evaluation | 4.4 (0.30) | |
| Average change between initial and immediate posttraining scores | +3.4 | P < 0.001; CI, 3.0‐3.7 |
| Mean (SD) score of delayed posttraining evaluation | 4.2 (0.32) | |
| Change between immediate and delayed posttraining scores | 0.2 | P = 0.144; CI, 0.31‐0.05 |
The results of the secondary endpoint calculations (essential elements) are depicted in Table 3. Ultrasound‐guided vessel cannulations improved from an initial average score of 0.9 1.0 to an immediate average score of 4.2 0.5 (P < 0.001; 95% CI, 3.0‐3.7); the delayed score of 4.3 0.6 was statistically unchanged from immediate (P = 0.77; 95% CI, 0.4 to 0.3). Catheter placement skills improved from 1.1 1.1 to 4.2 0.5 (P < 0.001; 95% CI, 2.6‐3.7), and the delayed score of 4.3 0.7 was unchanged from immediate (P < 0.58; 95% CI, 0.5 to 0.3). Sharps safety also improved significantly from initial (2.0 2.3) to immediate (4.9 0.5) (P < 0.0001; 95% CI, 1.9‐3.9), and the delayed scores dropped insignificantly to 4.6 0.8 (P = 0.08; 95% CI, 0.0‐0.6). Hand washing improved significantly from an initial score of 0.9 1.9 to an immediate score of 3.5 2.2 (P < 0.001; 95% CI, 1.4‐3.7), and decayed insignificantly on the delayed evaluation to 3.0 2.3 (P = 0.53; 95% CI, 0.9 to 1.7). WSB skills consisted of 4 individual steps, all of which all improved significantly from initial to immediate scores, and had insignificant decays on the delayed evaluations (see Table 3 WSB for details).
| Initial Evaluation | Immediate Follow‐Up | P Value (Initial to Immediate) | Delayed Follow‐Up | P Value (Immediate to Delayed) | |
|---|---|---|---|---|---|
| |||||
| Ultrasound‐guided insertion of needle into vein (step 15) | 0.9 (1.0) | 4.2 (0.5) | P < 0.001; CI, 3.0‐3.7 | 4.3 ( 0.6) | P = 0.77; CI, 0.4 to 0.3 |
| Catheter placement (step 18) | 1.1 (1.1) | 4.2 (0.5) | P < 0.0001; CI, 2.6‐3.7 | 4.3 ( 0.7) | P = 0.58; CI, 0.5 to 0.3 |
| Sharps safety (step 20) | 2.0 (2.3) | 4.9 (0.5) | P < 0.0001; CI, 1.9‐3.9 | 4.6 ( 0.8) | P = 0.08; CI = 0 to 0.6 |
| Hand washing (step 2) | 0.9 (1.9) | 3.5 (2.2) | P < 0.001; CI, 1.4‐3.7 | 3.0 ( 2.3) | P = 0.53; CI, 0.9 to 1.7 |
| WSBs | |||||
| MD prep (step 3) | 1.8 (1.5) | 4.3 (0.7) | P < 0.0001; CI, 1.7‐3.3 | 4.2 ( 0.6) | P = 0.30; CI, 0.2 to 0.6 |
| Site sterilization (step 7) | 1.1 (1.1) | 4.3 (0.9) | P < 0.0001; CI, 2.7‐3.7 | 4.5 ( 0.5) | P = 0.45; CI, 0.6 to 0.3 |
| WSB creation (step 8) | 0.6 ( 0.6) | 4.1 ( 0.9) | P < 0.0001; CI, 3.0‐4.0 | 4.4 ( 0.6) | P = 0.26; CI, 0.7 to 0.2 |
| Ultrasound probe cover application (step 9) | 0.4 ( 0.9) | 4.1 ( 0.8) | P < 0.0001; CI, 3.2‐4.1 | 4.4 ( 0.8) | P = 0.23; CI, 0.8 to 0.2 |
We performed validation exercises to determine the degree of interrater agreement. Of the 60 total evaluations that were eventually performed, 11 evaluations had been performed simultaneously and independently by evaluators A and B. An analysis of the scores assigned by each evaluator to these 11 trainees revealed a high level of interrater agreement (96%). Further, we performed independent analyses of the trainees' scores as assessed by evaluator A (22 sessions) or evaluator B (27 sessions) across the initial, immediate, and delayed sessions, and we detected no statistical differences in the changes in scores (which mirrored the overall results above).
With regards to real‐life contamination between immediate scores and delayed scores, we identified 3 trainees who had placed central lines on actual patients during the interim period (2 trainees placed 1 line each, and 1 trainee placed 2 lines). We repeated all of the calculations without these participants' delayed scores and determined that the removal of their scores did not change the statistical significance of any of the study results. With regard to knowledge decay, the scatterplot comparing delayed scores to varying time‐to‐follow up revealed no correlation.
Discussion
Our study was designed to determine whether novice trainees could learn and retain proper central line placement skills on the NHTM by receiving personalized training in a relaxed, 1‐on‐1 learning environment. Success was measured by trained evaluators using a detailed evaluation tool with a 6‐point scoring scale. The results of our primary endpoints (changes in overall average scores across the 3 evaluation periods) confirmed that this type of training could quickly improve novice practitioners' skill levels from very low (initial evaluation) to significantly higher (immediate posttraining). The dropoff (decay) in skill levels was found to not be statistically significant over a period of several weeks, although we recognize that further study should be performed to establish the degree of skill decay over a longer period of time.
Because some steps in a central line insertion are more critical to the procedure's success than others (ie, a skin nick with a scalpel is less critical than vessel cannulation under ultrasound‐guidance), we analyzed 5 essential elements individually as secondary endpoints. This secondary analysis was designed to unmask any critical skill deficiencies that might otherwise have been lost in the overall analysis. For each individual essential elements step, this subanalysis similarly revealed a significant improvement from initial to immediate posttraining, and an insignificant score decay on the delayed evaluation.
We recognize a number of limitations to this study. First, the n is relatively small. A larger sample size would have allowed for greater statistical power. In addition, the scoring system used for this study was created by our Procedure Center staff and had never been truly validated elsewhere. The scoring system was transparent and logical, but we recognize that any attempt to use an interval scoring system to quantify procedural skills will be inherently imperfect; the difference between 1 and 2 is not necessarily the same as a difference between 4 and 5. Great efforts were taken to mitigate the impact of this limitation: explicit definitions were established for each score, and we put our evaluators through a rigorous scoring orientation at the outset to standardize their interpretation and use of the scoring system and assessment tool.
The variability in the amount of training time spent in each session could be considered to be a confounder. Our prior experiences training interns in small groups, however, suggested that individuals learn these skills at different paces and in different ways, and so we consider our customized approach to be an essential part of this training experience. We do recognize the practical limitations inherent in rolling out such an open‐ended approach, and program directors may face time and/or resource limitations if attempting to replicate this training strategy.
We were also aware of potential interrater variability between the evaluators. Our approach to addressing this was multifactorial: we went to great lengths to standardize evaluators' understanding of the intended scoring methodology prior to the initiation of the study. We also assessed the degree of interrater reliability once all data was collected. This analysis reinforced that both evaluators were scoring trainees in a virtually identical fashion. We attribute this consistency to the quality of the scoring system, the effectiveness of the prestudy evaluator orientation with a proceduralist, and the high degree of teamwork between the 2 evaluators that kept them closely in sync with one another throughout the study.
Evaluator bias was also a concern. While each evaluator was blinded to the trainees' prior scores, the setup associated with the different training sessions, as well as the obvious differences in performance between the trainees' initial and immediate/delayed performances, made full blinding of the evaluators difficult. The theoretical risk of evaluator bias in this study would have led to evaluators rating trainees higher in the immediate and delayed performances in order to demonstrate more dramatic results. We believe that, since the evaluators themselves did not perform the actual training, and since they did not know the previous scores for the trainee, they were less inclined to skew the scores. Video recording each performance and submitting this recording to a fully‐blinded, third‐party evaluator would have more rigorously ensured blinding than we were able to accomplish. This approach could be considered in future studies of this type.
An addition limitation involved the time‐to‐follow‐up. While a longer time interval between the immediate and delayed evaluations may have better evaluated the impact of the training and potential decay, we sought to balance this with the growing risk of contamination from real central line placement experiences as more time passed. With this issue in mind, the removal of the delayed scores from the 3 trainees who had placed central lines on actual patients in between the immediate and delayed evaluations (2 trainees placed 1 line each, and 1 trainee placed 2 lines) did not change the statistical significance of any of the study results.
One practical concern has to do with the reproducibility of this approach at other institutions. Each trainee received up to 2 hours of individualized attention, and each session consumed fresh supplies and required a proceduralist's and an evaluator's time. This represents a significant commitment of materials and manpower. A careful cost/benefit analysis is therefore warranted before implementing this kind of rigorous training program. As mentioned, the cost of the NHTM is approximately $120 and can withstand several cannulations over a 2‐day period; the sterile supplies and central line add up to approximately another $75/evaluation. Depending on the number of interns and residents at a given institution, these costs could prove prohibitive to cash‐poor residency training programs. In the larger picture, however, catheter‐related bloodstream infections have been estimated to result in a mortality rate of 4% to 20%, and a single catheter‐related bloodstream infection can cost up to $45,000.2124 In addition, new Medicare reimbursement policies are now beginning to limit hospital reimbursement for these types of iatrogenic events; hence, narrowing the margin of error and putting even greater financial pressures on hospitals.25 It is our belief, therefore, that an up‐front investment in NHTMs (or an alternative simulator), basic supplies, and the necessary trainer time will prove to be cost‐effective and enlightened investments from forward‐thinking leadership.
Last, we are also aware that our study did not look at whether our trainees' improved performance on the NHTM actually translated into better patient outcomes. Since patient safety is our ultimate goal, and this phase of PPSI limited all of our training and evaluations to the NHTMs, future studies must ultimately evaluate how well these learned skills translate into procedure performance on actual patients. This controlled study (possibly with a see‐1 do‐1 teach‐1 control group) will be logistically challenging, but will be the most definitive manner with which to demonstrate the true value of personalized training sessions using the NHTM (or another nonhuman simulator).
PPSI‐II demonstrated that using the NHTM as the basis for training novice practitioners in a personalized, 1‐on‐1 training session led to significant improvements in measured procedural skills. Further, these skills were retained over time. This positive study contributes to the growing body of literature pointing towards the role of intensive 1‐on‐1 training with simulators to advance procedural education for clinicians. Ultimately, we aim to demonstrate that providing trainees this type of training prior to having them perform procedures on actual patients will translate into superior patient care, greater success rates, fewer minor and major complications, and lower overall patient care costs. Rather than clinging to the classic but never‐validated see‐1, do‐1, teach‐1 approach, we believe that procedural training must adapt new curricula and technologies that will help us achieve the goals of maximizing the safety and quality of care for our patients.
Acknowledgements
The authors recognize and appreciate the entire staff of The Procedure Center at Cedars‐Sinai Medical Center for their support of this research project. The authors give special thanks to Obed Martinez for his tireless assistance with the scheduling and coordination of training activities, and to Jim Mirocha for statistical analysis and editorial contributions.
- Accreditation Council for Graduate Medical Education (ACGME). Home page. Available at: http://www.acgme.org. Accessed June2009.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety and quality.J Hosp Med.2007;2(3):123–125.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2(3):135–142.
- ,,,,,Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3(1):48–54.
- ,,.Central line simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,, et al.Developing technical expertise in emergency medicine—the role of simulation in procedural skill acquisition.Acad Emerg Med.2008;15:1046–1057.
- ,,,.A training system for ultrasound‐guided needle insertion procedures.Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv.2007;10(1):566–574.
- ,,, et al.Video‐based training increases sterile‐technique compliance during central venous catheter insertion.Crit Care Med.2007;35:1302–1306.
- ,,,,Comparison of results of virtual‐reality simulator and training model for basic ureteroscopy training.J Endourol.2006;20(4):266–271.
- ,.Papaya: a simulation model for training in uterine aspiration.Fam Med.2005;37(4):242–244.
- ,,,,.An intervention to improve procedure education for internal medicine residents.J Gen Intern Med.2008;23(3):288–293.
- ,,.The use of tissue models for vascular access training: phase 1 of the procedural patient safety initiative.J Gen Intern Med.2006;21(5):514–517.
- Blue phantom: CVC hands‐on trainer, items # BPH600f, BPH604HP, BPH600AP. Available at: http://www.bluephantom.com/desktopdefault.aspx?tabid=232. Accessed June2009.
- Simulab Corporation: Central Line Man System. Available at: http://www.simulab.com/product/surgery/open/centralineman‐system. Accessed June2009.
- KyotoKagaku Co., Ltd.: CVC Insertion Simulator. Available at: http://www.kyotokagaku.com/products/detail01/m93u.html. Accessed June2009.
- First Aid Manufacturer CVC Simulator. Available at: http://www.first‐aid‐manufacturer.com/CVC‐Simulator.aspx. Accessed June2009.
- Limbs and Things: Central Venous Catheter Insertion Simulator, part #KKM93UB. Available at: http://www.golimbs.com/products/products.php?sectid=5356(17):1789–1790.
- ,,.Practice #20: proceduralists. The Advisory Board Annual Report.2007:162–169.
- NEJM video. Available at: http://content.nejm.org/cgi/content/short/356/21/e21. Accessed June2009.
- .Prevention of intravascular catheter‐related infections.Ann Intern Med.2000;132(5):391–402.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Recomm Rep.2002;51(RR‐10):1–29.
- ,,, et al.Clinical and economic outcomes in critically ill patients with nosocomial catheter‐related bloodstream infections.Clin Infect Dis.2005;41:1591–1598.
- ,,,,,.Attributable morbidity and mortality of catheter‐related septicemia in critically ill patients: a matched, risk‐adjusted, cohort study.Infect Control Hosp Epidemiol.1999;20(6):396–401.
- Centers for Medicaid and Medicare Services. U.S. Department of Health and Human Services. Hospital‐Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp#TopOfPage. Accessed June2009.
- Accreditation Council for Graduate Medical Education (ACGME). Home page. Available at: http://www.acgme.org. Accessed June2009.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety and quality.J Hosp Med.2007;2(3):123–125.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2(3):135–142.
- ,,,,,Mastery learning of thoracentesis skills by internal medicine residents using simulation technology and deliberate practice.J Hosp Med.2008;3(1):48–54.
- ,,.Central line simulation: a new training algorithm.Am Surg.2007;73:680–682.
- ,,, et al.Developing technical expertise in emergency medicine—the role of simulation in procedural skill acquisition.Acad Emerg Med.2008;15:1046–1057.
- ,,,.A training system for ultrasound‐guided needle insertion procedures.Med Image Comput Comput Assist Interv Int Conf Med Image Comput Comput Assist Interv.2007;10(1):566–574.
- ,,, et al.Video‐based training increases sterile‐technique compliance during central venous catheter insertion.Crit Care Med.2007;35:1302–1306.
- ,,,,Comparison of results of virtual‐reality simulator and training model for basic ureteroscopy training.J Endourol.2006;20(4):266–271.
- ,.Papaya: a simulation model for training in uterine aspiration.Fam Med.2005;37(4):242–244.
- ,,,,.An intervention to improve procedure education for internal medicine residents.J Gen Intern Med.2008;23(3):288–293.
- ,,.The use of tissue models for vascular access training: phase 1 of the procedural patient safety initiative.J Gen Intern Med.2006;21(5):514–517.
- Blue phantom: CVC hands‐on trainer, items # BPH600f, BPH604HP, BPH600AP. Available at: http://www.bluephantom.com/desktopdefault.aspx?tabid=232. Accessed June2009.
- Simulab Corporation: Central Line Man System. Available at: http://www.simulab.com/product/surgery/open/centralineman‐system. Accessed June2009.
- KyotoKagaku Co., Ltd.: CVC Insertion Simulator. Available at: http://www.kyotokagaku.com/products/detail01/m93u.html. Accessed June2009.
- First Aid Manufacturer CVC Simulator. Available at: http://www.first‐aid‐manufacturer.com/CVC‐Simulator.aspx. Accessed June2009.
- Limbs and Things: Central Venous Catheter Insertion Simulator, part #KKM93UB. Available at: http://www.golimbs.com/products/products.php?sectid=5356(17):1789–1790.
- ,,.Practice #20: proceduralists. The Advisory Board Annual Report.2007:162–169.
- NEJM video. Available at: http://content.nejm.org/cgi/content/short/356/21/e21. Accessed June2009.
- .Prevention of intravascular catheter‐related infections.Ann Intern Med.2000;132(5):391–402.
- ,,, et al.Guidelines for the prevention of intravascular catheter‐related infections.MMWR Recomm Rep.2002;51(RR‐10):1–29.
- ,,, et al.Clinical and economic outcomes in critically ill patients with nosocomial catheter‐related bloodstream infections.Clin Infect Dis.2005;41:1591–1598.
- ,,,,,.Attributable morbidity and mortality of catheter‐related septicemia in critically ill patients: a matched, risk‐adjusted, cohort study.Infect Control Hosp Epidemiol.1999;20(6):396–401.
- Centers for Medicaid and Medicare Services. U.S. Department of Health and Human Services. Hospital‐Acquired Conditions. Available at: http://www.cms.hhs.gov/HospitalAcqCond/06_Hospital‐Acquired_Conditions.asp#TopOfPage. Accessed June2009.
Copyright © 2009 Society of Hospital Medicine
Advanced Measures in Palliative Care
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Resuscitation status and patient wishes in terms of advanced cardiopulmonary support must be addressed during inpatient hospital admissions. However, the lack of clarity of the patients' wishes and the variability in physicians' comfort addressing these issues often leads to ambiguity in an emergency setting. This may result in inappropriately aggressive management, and conversely, it may also lead to withholding potentially lifesaving therapy due to Do Not Resuscitate (DNR) designation. We report a case of hemodynamic instability due to acute supraventricular tachycardia (SVT) in a patient with a DNR designation. He was successfully treated according to the advanced cardiac life support (ACLS) protocol for SVT. We also discuss some of the ethical challenges of providing potential life‐sustaining interventions in palliative medicine, as well as the dilemma of whether or not to provide such interventions to patients who have DNR status.
Case Presentation
A 45‐year‐old man with advanced tonsillar cancer was admitted to an inpatient palliative care unit for evaluation and treatment of anorexia, progressive pain, and asthenia. He had undergone tumor debulking and neck dissection followed by adjuvant chemotherapy and external beam radiation therapy. Despite maximal therapy, the patient developed locally recurrent disease (leading to more surgery) and later, progressive metastatic disease (treated with palliative radiation therapy). With ongoing weight loss and failure to thrive, a percutaneous gastrostomy tube was placed for nutritional support. Still, the patient suffered from significant stomatitis, esophagitis, and diarrhea consistent with radiation‐induced injury, and had several admissions for dehydration and pain control.
During this and prior admissions, the patient clearly articulated his preference for DNR status. The patient was clinically declining, but was still functional, with and estimated survival of weeks to a few months. As with previous admissions, he was given intravenous fluids and parenteral opioids, and his electrolytes and vital signs normalized to his baseline. On the day of anticipated discharge, the patient was at his hemodynamic baseline (pulse of 100 beats per minute, blood pressure of 98/60 mmHg). Upon returning to bed after a shower, the patient developed acute dyspnea, weakness, and diaphoresis. Heart rate was 170 beats per minute and blood pressure was 70/50 mmHg. Intravenous normal saline boluses were given while electrocardiogram (EKG) was obtained. EKG revealed SVT with changes suggestive of demand myocardial ischemia. Carotid massage and Valsalva maneuvers were unsuccessful in converting the rhythm to sinus.
At that point, consideration was given to his DNR designation. The treating physician and patient briefly discussed the alternatives of no treatment of his arrhythmia, or alternatively, more aggressive treatment options on the Palliative Care Unit, including intravenous (IV) adenosine and direct current cardioversion. He did not have a detailed advanced directive discussing similar scenarios; he had only completed a commonly‐used, state‐issued Durable DNR form. All decided the SVT was potentially reversible and appeared to be causing many of the patient's acute symptoms; hence, aggressive treatment of the arrhythmia was in his best interest.
Despite absence of telemetry monitoring, consideration was given to IV diltiazem or metoprolol, either of which could precipitate worsening hypotension. However, the goals were to restore his previous rhythm, to relieve symptoms with a minimum of side effects and unintended effects, and to avoid intensive care unit (ICU) transfer. Intravenous adenosine and esmolol were also considered, given their shorter half‐life, potentially lower side effect profile, and ability to produce relief of the patient's distress without further complication. The pros and cons of the situation were discussed with the patient. While he desperately wanted to feel better, he wished to stay with his family where he was. He consented to a trial of adenosine, and agreed to remain on the Palliative Care Unit. The therapeutic plan was a trial of IV adenosine, and then metoprolol if necessary. He was assured that if this was unsuccessful, we would do all we could to keep him comfortable without ICU transfer. While the patient was monitored with a portable 12‐lead EKG machine, the Palliative Medicine fellow administered adenosine 6 mg IV. Predictably, the patient noted flushing, a sense of impending doom, and a short pause of asystole. This was followed by electrocardiographic conversion to sinus tachycardia at a rate of 100 beats per minute and hemodynamic and symptomatic improvement. The patient noted that his dyspnea and generalized sense of not feeling well resolved, and he was monitored for about 30 minutes without return of the SVT. The remainder of his hospitalization was uneventful, and he was discharged to home hospice the following day. He survived for another 3 weeks without return of symptoms of arrhythmia.
Discussion
Patient preferences in terms of advanced cardiopulmonary support must be addressed during hospital admission. This is in accord with recommendations from the Patient Self‐Determination Act of 1990, as well as the Joint Commission on Accreditation of Healthcare Organizations.1 Nevertheless, the number of U.S. adults with completed advance directives to guide care providers and families with preferences if personally unable to articulate them is estimated at 5% to 25%.2 Clearly‐documented wishes are particularly important in patients with advanced cancer; however, early studies show that this happens as little as 27% of the time3 in seriously ill cancer patients. In fact, oncology physicians report direct discussions about death with only 37% of their dying patients4 and cancer patients are found to have discussions at far lower rates than patients with amyotrophic lateral sclerosis despite worse survival.5
Cardiopulmonary resuscitation (CPR) and the advanced cardiac life support (ACLS) algorithms were established to treat life‐threatening arrhythmias (namely ventricular tachycardia/fibrillation) in otherwise healthy patients who experienced witnessed intraoperative arrest. Original reports of closed chest compressions were in the intraoperative or perioperative setting.6 However, benefits of rapid initiation of CPR in witnessed out‐of‐hospital cardiac arrest were later noted as providing the only reasonable hope for reduced mortality and improved neurologic outcomes.7, 8
While CPR has shown this marginal but significant difference in outcomes of witnessed out‐of‐hospital cardiac arrest, patient with advanced life‐limiting or life threatening illness tend to have even worse outcomes even if cardiac arrest is witnessed. Survival of all cardiac arrest patients to discharge has been estimated at 3% to 14% if cardiac arrest occurs outside of the hospital and 10% to 20% for witnessed, in‐hospital cardiac arrest.912 However, a recent meta‐analysis of resuscitation for cancer patients estimates overall survival to discharge at 6.2%, and less when factoring in metastatic disease (5.6%), or ICU care at time of arrest (2.2%).13
Multiple reasons have been cited regarding why patients choose to forego resuscitation or proceed with full resuscitation status despite advanced life‐threatening illness. Factors associated with refusal of CPR include being older, female, living in a nursing home and having a worsening functional status, depression, and/or an expected poor outcome.14, 15 One can speculate that fear of no longer being cared for or being abandoned may be inferred or directly stated, and this may or may not be related to socioeconomic factors, stressors outside of the medical system, or underlying depressive symptomatology, especially hopelessness. Alternatively, 1 study revealed that an unclear expectation of outcome and prognosis after cardiopulmonary arrest led some to proceed with full resuscitative measures.15
Reports differ regarding the advanced care trajectory based on patient wishes. One study of 872 critically ill cancer patients found no significant difference in application of life‐sustaining therapies regardless of presence of an advance directive.3 The SUPPORT study mentioned above was specifically designed to understand preferences for CPR.14 While SUPPORT found that foregoing CPR may be associated with a small reduction in intensity of care, there was no difference in overall hospital survival.14 Last, although advance directives are static in terms of patient's stated wishes, a patient with decision‐making capacity is able to request a shift in goals of care at any time. However, a case‐based survey of 241 responding physicians concluded that a DNR order may indeed be associated with less aggressive and/or life‐prolonging interventions, CPR notwithstanding.16 This concept of treating those with DNR status less aggressively is often born out in terms of popular perception.17 A recent study has demonstrated that patients who discuss these issues with physicians and elect a DNR status not only have fewer aggressive interventions, but also report a higher quality of life.4
A particular nidus for this confusion may be how one interprets the DNR directive. Although DNR is specifically associated with 3 basic tenets (no endotracheal intubation, no chest compressions, and no defibrillation in the setting of cardiopulmonary arrest), this designation does not substitute for intact patient decision‐making capacity in considering other supportive measures. Intermediate steps such as limited aggressive therapy orders have been suggested to provide time‐limited and goal‐limited advanced care.9 While this offers a broader array of scenarios to be considered prior to and during clinical encounters, this may also muddy the picture with impractical options and further lack of clarity in already complex situations. The Physician Orders for Life‐Sustaining Treatment (POLST) movement has taken roots in several states, targeting seriously ill patients such as the frail and elderly. The POLST provides more explicit information regarding limited advanced measures such as nutrition or antibiotics, and may be particularly useful as a prehospital decision aid.18 While the POLST, just as the traditional advance directive, may provide clinical guidance outside of situations described explicitly therein,, it may not provide further information about goals of care, (ie, Is there a situation when 1 of these measures may be acceptable?). To reiterate what was stated about traditional directives, the POLST also applies only in situations where a patient is lacking decision‐making capacity at the time of an acute event.
The designation of DNR may indeed allow for introduction of advanced care measures that may be in accord with the patient's overall wishes and clinical prognosis. Several interventions may be appropriate on a time‐limited basis. In addition to administration of adenosine or antiarrhythmics, as in the case of our patient, the use of broad‐spectrum antimicrobial therapy, vasoactive medications, and consideration for intensive monitoring may all be appropriate on a time‐limited basis. Nevertheless, without a clear understanding of the goals of limited aggressive therapy, some would argue there is always a slippery slope in terms of technology and the implementation of advanced care measures. Hence, expectations regarding perceived outcomes, goals to be achieved by the therapy, and reasonable time lines may further clarify the patient's wishes.
In this patient scenario, the administration of adenosine is generally safe, but may lead to prolonged asystole, atrial fibrillation, and ventricular tachyarrhythmias.1921 This may lead one to consider further downstream ACLS interventions, including defibrillation or atropine. From an ethical standpoint, it is valuable to consider what would have been the next step beyond this step, in terms of advanced care measures. In the case of our patient, these measures were considered, and all accepted the goals of our intervention and its limitations. While virtually all treatments provided by physicians may predispose patients to iatrogenesis, the risks and benefits of interventions are particularly important considerations in the seriously ill patient with limited life expectancy.
Iatrogenic adverse events can be serious and fatal, and occur in 4% to 9% of hospitalized patients.2224 There has been much debate about what to do for iatrogenic adverse events, particularly when patients have clearly articulated advanced directives and DNR requests. While some argue there is a higher moral duty to reverse complications resulting from physician error or treatment‐induced complication, others would feel that the fiduciary obligation is to the patient's request.25, 26 Again, in the setting of our clinical scenario, having clear, up‐front expectations about goals of care and limitation inherent were articulated as much as able.
With increasing complexity of inpatient care and team‐based models of care becoming the norm, discerning patient's wishes continuously throughout a hospital course is critical. While this responsibility previously would have fallen to the 1 coordinating clinician (ie, the primary care physician, or the patient's subspecialist), it is increasingly becoming the responsibility of all members of the team. While provider's level of prior education, exposure, and comfort may vary, several resources have attempted to address these concerns and attempted to lay a framework for overcoming barriers to these discussion and tips on empathetic and effective communication.17, 2729
Skills notwithstanding, hospitalists particularly face a challenge in communicating these tenuous issues with patients. While there is intrinsic value in having an standardized approach to these situations, hospitalists are often thrown into these difficult situations in a fragmented, nonlongitudinal fashion, further heightening the clinical and ethical tension.28, 30 However, hospitalists are also is an area where they can truly make an impact in these patients' lives at a critical juncture. Evidence suggests that regardless of the provider who broaches the subject, patients have a desire to talk about these issues.4, 14 Hospitalists may be in an advantageous position compared to their primary care or subspecialist colleagues, in that they can offer a fresh perspective and the ability to have a dialog with the patient about these issues.
Implications
While patients are entitled to die free from the intrusion of chest compression and endotracheal tubes, they are also entitled to have symptoms aggressively managed. Advanced care measures may be appropriate for symptom palliation in complex clinical situations. A careful understanding of the patient's wishes and goals of care, after thoughtful exploration, may include therapies that in isolation, appear to be extraordinary or excessive. SVT is often quickly and successfully treated at the bedside. Despite a firm DNR status, treatment with IV adenosine allowed our patient time to return home with his family.
Acknowledgements
Special thanks to Dr. Paul S. Mueller for his thoughtful review and commentary regarding this manuscript.
- 2006 Comprehensive Accreditation Manual for Hospitals: The Official Handbook (CAMH).Oak Brook Terrace, IL:Joint Commission Resources;2006.
- ,,.Advance directives and do‐not‐resuscitate orders on general medical wards versus the intensive care unit.Mil Med.204;169:433–436.
- ,,.Advance directives in critically ill cancer patients.Crit Care Nurs Clin North Am.2000;12:373–383.
- ,,, et al.Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment.JAMA.2008;300:1665–1673.
- ,,, et al.Decision‐making in patients with advanced cancer compared with amyotrophic lateral sclerosis.J Med Ethics.2008;34:664–668.
- ,,.Closed‐chest cardiac massage.JAMA.1960;173:1064–1067.
- ,,, et al.Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS Study Phase I results. Ontario prehospital advanced life support.Ann Emerg Med.1999;33:44–50.
- ,,, et al.Effect of bystander initiated cardiopulmonary resuscitation on ventricular fibrillation and survival after witnessed cardiac arrest outside hospital.Br Heart J.1994;72:408–412.
- ,,.CPR for patients labeled DNR: the role of the limited aggressive therapy order.Ann Intern Med.2003;138:65–68.
- ,,, et al.A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group.N Engl J Med.1998;339:1595–1601.
- ,,,.Does age affect outcomes of out‐of‐hospital cardiopulmonary resuscitation?JAMA.1990;264:2109–2110.
- ,,, et al.A comparison of standard cardiopulmonary resuscitation and active compression‐decompression resuscitation for out‐of‐hospital cardiac arrest. French Active Compression‐Decompression Cardiopulmonary Resuscitation Study Group.N Engl J Med.1999;341:569–575.
- ,,,,,.Survival in cancer patients undergoing in‐hospital cardiopulmonary resuscitation: a meta‐analysis.Resuscitation.2006;71:152–160.
- ,,, et al.Choices of seriously ill patients about cardiopulmonary resuscitation: correlates and outcomes. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments.Am J Med.1996:128–137.
- ,,,,,.Preferences for life‐sustaining treatments in advance care planning and surrogate decision making.J Palliat Med.2000;3(1):37–48.
- ,.The effect of do‐not‐resuscitate orders on physician‐making.J Am Geriatr Soc.2002;50:2057–2061.
- ,,, et al.A staff dialogue on do not resuscitate orders: psychosocial issues faced by patients, their families, and caregivers.Oncologist.1999;4:256–262.
- ,,,.Hope for the future: achieving the original intent of advance directives.Hastings Cent Rep.2005;12S:S26–S30.
- ,.Adenosine‐induced non‐sustained polymorphic ventricular tachycardia.Eur Heart J.1994;15:281–282.
- ,,,.Adenosine induced ventricular arrhythmias in the emergency room.Pacing Clin Electrophysiol.2001;24:450–455.
- ,.Torsades de pointes after intravenous adenosine in the presence of prolonged QT syndrome.Am Heart J.1992;123:794–796.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients—results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- .The hazards of hospitalization.Ann Intern Med.1964;60:100–110.
- ,,,.Iatrogenic illness on a general medical service at a university hospital.N Engl J Med.1981;304:638–642.
- ,.Overriding a patient's refusal of treatment after an iatrogenic complication.N Engl J Med.1997;336:1908–1910.
- ,,.Would physicians override a do‐not‐resuscitate order when a cardiac arrest is iatrogenic?J Gen Intern Med.1999;14:35–38.
- ,,.Discussing resuscitation preferences with patients: challenges and rewards.J Hosp Med.2006;1:231–240.
- .Decision making at a time of crisis near the end of life.JAMA.2004;292:1738–1743.
- ,,,.Advance care planning as a process: structuring the discussions in practice.J Am Geriatr Soc.1995;43:440–446.
- .Addressing end‐of‐life issues.JAMA.2005;293:162.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Resuscitation status and patient wishes in terms of advanced cardiopulmonary support must be addressed during inpatient hospital admissions. However, the lack of clarity of the patients' wishes and the variability in physicians' comfort addressing these issues often leads to ambiguity in an emergency setting. This may result in inappropriately aggressive management, and conversely, it may also lead to withholding potentially lifesaving therapy due to Do Not Resuscitate (DNR) designation. We report a case of hemodynamic instability due to acute supraventricular tachycardia (SVT) in a patient with a DNR designation. He was successfully treated according to the advanced cardiac life support (ACLS) protocol for SVT. We also discuss some of the ethical challenges of providing potential life‐sustaining interventions in palliative medicine, as well as the dilemma of whether or not to provide such interventions to patients who have DNR status.
Case Presentation
A 45‐year‐old man with advanced tonsillar cancer was admitted to an inpatient palliative care unit for evaluation and treatment of anorexia, progressive pain, and asthenia. He had undergone tumor debulking and neck dissection followed by adjuvant chemotherapy and external beam radiation therapy. Despite maximal therapy, the patient developed locally recurrent disease (leading to more surgery) and later, progressive metastatic disease (treated with palliative radiation therapy). With ongoing weight loss and failure to thrive, a percutaneous gastrostomy tube was placed for nutritional support. Still, the patient suffered from significant stomatitis, esophagitis, and diarrhea consistent with radiation‐induced injury, and had several admissions for dehydration and pain control.
During this and prior admissions, the patient clearly articulated his preference for DNR status. The patient was clinically declining, but was still functional, with and estimated survival of weeks to a few months. As with previous admissions, he was given intravenous fluids and parenteral opioids, and his electrolytes and vital signs normalized to his baseline. On the day of anticipated discharge, the patient was at his hemodynamic baseline (pulse of 100 beats per minute, blood pressure of 98/60 mmHg). Upon returning to bed after a shower, the patient developed acute dyspnea, weakness, and diaphoresis. Heart rate was 170 beats per minute and blood pressure was 70/50 mmHg. Intravenous normal saline boluses were given while electrocardiogram (EKG) was obtained. EKG revealed SVT with changes suggestive of demand myocardial ischemia. Carotid massage and Valsalva maneuvers were unsuccessful in converting the rhythm to sinus.
At that point, consideration was given to his DNR designation. The treating physician and patient briefly discussed the alternatives of no treatment of his arrhythmia, or alternatively, more aggressive treatment options on the Palliative Care Unit, including intravenous (IV) adenosine and direct current cardioversion. He did not have a detailed advanced directive discussing similar scenarios; he had only completed a commonly‐used, state‐issued Durable DNR form. All decided the SVT was potentially reversible and appeared to be causing many of the patient's acute symptoms; hence, aggressive treatment of the arrhythmia was in his best interest.
Despite absence of telemetry monitoring, consideration was given to IV diltiazem or metoprolol, either of which could precipitate worsening hypotension. However, the goals were to restore his previous rhythm, to relieve symptoms with a minimum of side effects and unintended effects, and to avoid intensive care unit (ICU) transfer. Intravenous adenosine and esmolol were also considered, given their shorter half‐life, potentially lower side effect profile, and ability to produce relief of the patient's distress without further complication. The pros and cons of the situation were discussed with the patient. While he desperately wanted to feel better, he wished to stay with his family where he was. He consented to a trial of adenosine, and agreed to remain on the Palliative Care Unit. The therapeutic plan was a trial of IV adenosine, and then metoprolol if necessary. He was assured that if this was unsuccessful, we would do all we could to keep him comfortable without ICU transfer. While the patient was monitored with a portable 12‐lead EKG machine, the Palliative Medicine fellow administered adenosine 6 mg IV. Predictably, the patient noted flushing, a sense of impending doom, and a short pause of asystole. This was followed by electrocardiographic conversion to sinus tachycardia at a rate of 100 beats per minute and hemodynamic and symptomatic improvement. The patient noted that his dyspnea and generalized sense of not feeling well resolved, and he was monitored for about 30 minutes without return of the SVT. The remainder of his hospitalization was uneventful, and he was discharged to home hospice the following day. He survived for another 3 weeks without return of symptoms of arrhythmia.
Discussion
Patient preferences in terms of advanced cardiopulmonary support must be addressed during hospital admission. This is in accord with recommendations from the Patient Self‐Determination Act of 1990, as well as the Joint Commission on Accreditation of Healthcare Organizations.1 Nevertheless, the number of U.S. adults with completed advance directives to guide care providers and families with preferences if personally unable to articulate them is estimated at 5% to 25%.2 Clearly‐documented wishes are particularly important in patients with advanced cancer; however, early studies show that this happens as little as 27% of the time3 in seriously ill cancer patients. In fact, oncology physicians report direct discussions about death with only 37% of their dying patients4 and cancer patients are found to have discussions at far lower rates than patients with amyotrophic lateral sclerosis despite worse survival.5
Cardiopulmonary resuscitation (CPR) and the advanced cardiac life support (ACLS) algorithms were established to treat life‐threatening arrhythmias (namely ventricular tachycardia/fibrillation) in otherwise healthy patients who experienced witnessed intraoperative arrest. Original reports of closed chest compressions were in the intraoperative or perioperative setting.6 However, benefits of rapid initiation of CPR in witnessed out‐of‐hospital cardiac arrest were later noted as providing the only reasonable hope for reduced mortality and improved neurologic outcomes.7, 8
While CPR has shown this marginal but significant difference in outcomes of witnessed out‐of‐hospital cardiac arrest, patient with advanced life‐limiting or life threatening illness tend to have even worse outcomes even if cardiac arrest is witnessed. Survival of all cardiac arrest patients to discharge has been estimated at 3% to 14% if cardiac arrest occurs outside of the hospital and 10% to 20% for witnessed, in‐hospital cardiac arrest.912 However, a recent meta‐analysis of resuscitation for cancer patients estimates overall survival to discharge at 6.2%, and less when factoring in metastatic disease (5.6%), or ICU care at time of arrest (2.2%).13
Multiple reasons have been cited regarding why patients choose to forego resuscitation or proceed with full resuscitation status despite advanced life‐threatening illness. Factors associated with refusal of CPR include being older, female, living in a nursing home and having a worsening functional status, depression, and/or an expected poor outcome.14, 15 One can speculate that fear of no longer being cared for or being abandoned may be inferred or directly stated, and this may or may not be related to socioeconomic factors, stressors outside of the medical system, or underlying depressive symptomatology, especially hopelessness. Alternatively, 1 study revealed that an unclear expectation of outcome and prognosis after cardiopulmonary arrest led some to proceed with full resuscitative measures.15
Reports differ regarding the advanced care trajectory based on patient wishes. One study of 872 critically ill cancer patients found no significant difference in application of life‐sustaining therapies regardless of presence of an advance directive.3 The SUPPORT study mentioned above was specifically designed to understand preferences for CPR.14 While SUPPORT found that foregoing CPR may be associated with a small reduction in intensity of care, there was no difference in overall hospital survival.14 Last, although advance directives are static in terms of patient's stated wishes, a patient with decision‐making capacity is able to request a shift in goals of care at any time. However, a case‐based survey of 241 responding physicians concluded that a DNR order may indeed be associated with less aggressive and/or life‐prolonging interventions, CPR notwithstanding.16 This concept of treating those with DNR status less aggressively is often born out in terms of popular perception.17 A recent study has demonstrated that patients who discuss these issues with physicians and elect a DNR status not only have fewer aggressive interventions, but also report a higher quality of life.4
A particular nidus for this confusion may be how one interprets the DNR directive. Although DNR is specifically associated with 3 basic tenets (no endotracheal intubation, no chest compressions, and no defibrillation in the setting of cardiopulmonary arrest), this designation does not substitute for intact patient decision‐making capacity in considering other supportive measures. Intermediate steps such as limited aggressive therapy orders have been suggested to provide time‐limited and goal‐limited advanced care.9 While this offers a broader array of scenarios to be considered prior to and during clinical encounters, this may also muddy the picture with impractical options and further lack of clarity in already complex situations. The Physician Orders for Life‐Sustaining Treatment (POLST) movement has taken roots in several states, targeting seriously ill patients such as the frail and elderly. The POLST provides more explicit information regarding limited advanced measures such as nutrition or antibiotics, and may be particularly useful as a prehospital decision aid.18 While the POLST, just as the traditional advance directive, may provide clinical guidance outside of situations described explicitly therein,, it may not provide further information about goals of care, (ie, Is there a situation when 1 of these measures may be acceptable?). To reiterate what was stated about traditional directives, the POLST also applies only in situations where a patient is lacking decision‐making capacity at the time of an acute event.
The designation of DNR may indeed allow for introduction of advanced care measures that may be in accord with the patient's overall wishes and clinical prognosis. Several interventions may be appropriate on a time‐limited basis. In addition to administration of adenosine or antiarrhythmics, as in the case of our patient, the use of broad‐spectrum antimicrobial therapy, vasoactive medications, and consideration for intensive monitoring may all be appropriate on a time‐limited basis. Nevertheless, without a clear understanding of the goals of limited aggressive therapy, some would argue there is always a slippery slope in terms of technology and the implementation of advanced care measures. Hence, expectations regarding perceived outcomes, goals to be achieved by the therapy, and reasonable time lines may further clarify the patient's wishes.
In this patient scenario, the administration of adenosine is generally safe, but may lead to prolonged asystole, atrial fibrillation, and ventricular tachyarrhythmias.1921 This may lead one to consider further downstream ACLS interventions, including defibrillation or atropine. From an ethical standpoint, it is valuable to consider what would have been the next step beyond this step, in terms of advanced care measures. In the case of our patient, these measures were considered, and all accepted the goals of our intervention and its limitations. While virtually all treatments provided by physicians may predispose patients to iatrogenesis, the risks and benefits of interventions are particularly important considerations in the seriously ill patient with limited life expectancy.
Iatrogenic adverse events can be serious and fatal, and occur in 4% to 9% of hospitalized patients.2224 There has been much debate about what to do for iatrogenic adverse events, particularly when patients have clearly articulated advanced directives and DNR requests. While some argue there is a higher moral duty to reverse complications resulting from physician error or treatment‐induced complication, others would feel that the fiduciary obligation is to the patient's request.25, 26 Again, in the setting of our clinical scenario, having clear, up‐front expectations about goals of care and limitation inherent were articulated as much as able.
With increasing complexity of inpatient care and team‐based models of care becoming the norm, discerning patient's wishes continuously throughout a hospital course is critical. While this responsibility previously would have fallen to the 1 coordinating clinician (ie, the primary care physician, or the patient's subspecialist), it is increasingly becoming the responsibility of all members of the team. While provider's level of prior education, exposure, and comfort may vary, several resources have attempted to address these concerns and attempted to lay a framework for overcoming barriers to these discussion and tips on empathetic and effective communication.17, 2729
Skills notwithstanding, hospitalists particularly face a challenge in communicating these tenuous issues with patients. While there is intrinsic value in having an standardized approach to these situations, hospitalists are often thrown into these difficult situations in a fragmented, nonlongitudinal fashion, further heightening the clinical and ethical tension.28, 30 However, hospitalists are also is an area where they can truly make an impact in these patients' lives at a critical juncture. Evidence suggests that regardless of the provider who broaches the subject, patients have a desire to talk about these issues.4, 14 Hospitalists may be in an advantageous position compared to their primary care or subspecialist colleagues, in that they can offer a fresh perspective and the ability to have a dialog with the patient about these issues.
Implications
While patients are entitled to die free from the intrusion of chest compression and endotracheal tubes, they are also entitled to have symptoms aggressively managed. Advanced care measures may be appropriate for symptom palliation in complex clinical situations. A careful understanding of the patient's wishes and goals of care, after thoughtful exploration, may include therapies that in isolation, appear to be extraordinary or excessive. SVT is often quickly and successfully treated at the bedside. Despite a firm DNR status, treatment with IV adenosine allowed our patient time to return home with his family.
Acknowledgements
Special thanks to Dr. Paul S. Mueller for his thoughtful review and commentary regarding this manuscript.
The approach to clinical conundrums by an expert clinician is revealed through the presentation of an actual patient's case in an approach typical of a morning report. Similarly to patient care, sequential pieces of information are provided to the clinician, who is unfamiliar with the case. The focus is on the thought processes of both the clinical team caring for the patient and the discussant.
Resuscitation status and patient wishes in terms of advanced cardiopulmonary support must be addressed during inpatient hospital admissions. However, the lack of clarity of the patients' wishes and the variability in physicians' comfort addressing these issues often leads to ambiguity in an emergency setting. This may result in inappropriately aggressive management, and conversely, it may also lead to withholding potentially lifesaving therapy due to Do Not Resuscitate (DNR) designation. We report a case of hemodynamic instability due to acute supraventricular tachycardia (SVT) in a patient with a DNR designation. He was successfully treated according to the advanced cardiac life support (ACLS) protocol for SVT. We also discuss some of the ethical challenges of providing potential life‐sustaining interventions in palliative medicine, as well as the dilemma of whether or not to provide such interventions to patients who have DNR status.
Case Presentation
A 45‐year‐old man with advanced tonsillar cancer was admitted to an inpatient palliative care unit for evaluation and treatment of anorexia, progressive pain, and asthenia. He had undergone tumor debulking and neck dissection followed by adjuvant chemotherapy and external beam radiation therapy. Despite maximal therapy, the patient developed locally recurrent disease (leading to more surgery) and later, progressive metastatic disease (treated with palliative radiation therapy). With ongoing weight loss and failure to thrive, a percutaneous gastrostomy tube was placed for nutritional support. Still, the patient suffered from significant stomatitis, esophagitis, and diarrhea consistent with radiation‐induced injury, and had several admissions for dehydration and pain control.
During this and prior admissions, the patient clearly articulated his preference for DNR status. The patient was clinically declining, but was still functional, with and estimated survival of weeks to a few months. As with previous admissions, he was given intravenous fluids and parenteral opioids, and his electrolytes and vital signs normalized to his baseline. On the day of anticipated discharge, the patient was at his hemodynamic baseline (pulse of 100 beats per minute, blood pressure of 98/60 mmHg). Upon returning to bed after a shower, the patient developed acute dyspnea, weakness, and diaphoresis. Heart rate was 170 beats per minute and blood pressure was 70/50 mmHg. Intravenous normal saline boluses were given while electrocardiogram (EKG) was obtained. EKG revealed SVT with changes suggestive of demand myocardial ischemia. Carotid massage and Valsalva maneuvers were unsuccessful in converting the rhythm to sinus.
At that point, consideration was given to his DNR designation. The treating physician and patient briefly discussed the alternatives of no treatment of his arrhythmia, or alternatively, more aggressive treatment options on the Palliative Care Unit, including intravenous (IV) adenosine and direct current cardioversion. He did not have a detailed advanced directive discussing similar scenarios; he had only completed a commonly‐used, state‐issued Durable DNR form. All decided the SVT was potentially reversible and appeared to be causing many of the patient's acute symptoms; hence, aggressive treatment of the arrhythmia was in his best interest.
Despite absence of telemetry monitoring, consideration was given to IV diltiazem or metoprolol, either of which could precipitate worsening hypotension. However, the goals were to restore his previous rhythm, to relieve symptoms with a minimum of side effects and unintended effects, and to avoid intensive care unit (ICU) transfer. Intravenous adenosine and esmolol were also considered, given their shorter half‐life, potentially lower side effect profile, and ability to produce relief of the patient's distress without further complication. The pros and cons of the situation were discussed with the patient. While he desperately wanted to feel better, he wished to stay with his family where he was. He consented to a trial of adenosine, and agreed to remain on the Palliative Care Unit. The therapeutic plan was a trial of IV adenosine, and then metoprolol if necessary. He was assured that if this was unsuccessful, we would do all we could to keep him comfortable without ICU transfer. While the patient was monitored with a portable 12‐lead EKG machine, the Palliative Medicine fellow administered adenosine 6 mg IV. Predictably, the patient noted flushing, a sense of impending doom, and a short pause of asystole. This was followed by electrocardiographic conversion to sinus tachycardia at a rate of 100 beats per minute and hemodynamic and symptomatic improvement. The patient noted that his dyspnea and generalized sense of not feeling well resolved, and he was monitored for about 30 minutes without return of the SVT. The remainder of his hospitalization was uneventful, and he was discharged to home hospice the following day. He survived for another 3 weeks without return of symptoms of arrhythmia.
Discussion
Patient preferences in terms of advanced cardiopulmonary support must be addressed during hospital admission. This is in accord with recommendations from the Patient Self‐Determination Act of 1990, as well as the Joint Commission on Accreditation of Healthcare Organizations.1 Nevertheless, the number of U.S. adults with completed advance directives to guide care providers and families with preferences if personally unable to articulate them is estimated at 5% to 25%.2 Clearly‐documented wishes are particularly important in patients with advanced cancer; however, early studies show that this happens as little as 27% of the time3 in seriously ill cancer patients. In fact, oncology physicians report direct discussions about death with only 37% of their dying patients4 and cancer patients are found to have discussions at far lower rates than patients with amyotrophic lateral sclerosis despite worse survival.5
Cardiopulmonary resuscitation (CPR) and the advanced cardiac life support (ACLS) algorithms were established to treat life‐threatening arrhythmias (namely ventricular tachycardia/fibrillation) in otherwise healthy patients who experienced witnessed intraoperative arrest. Original reports of closed chest compressions were in the intraoperative or perioperative setting.6 However, benefits of rapid initiation of CPR in witnessed out‐of‐hospital cardiac arrest were later noted as providing the only reasonable hope for reduced mortality and improved neurologic outcomes.7, 8
While CPR has shown this marginal but significant difference in outcomes of witnessed out‐of‐hospital cardiac arrest, patient with advanced life‐limiting or life threatening illness tend to have even worse outcomes even if cardiac arrest is witnessed. Survival of all cardiac arrest patients to discharge has been estimated at 3% to 14% if cardiac arrest occurs outside of the hospital and 10% to 20% for witnessed, in‐hospital cardiac arrest.912 However, a recent meta‐analysis of resuscitation for cancer patients estimates overall survival to discharge at 6.2%, and less when factoring in metastatic disease (5.6%), or ICU care at time of arrest (2.2%).13
Multiple reasons have been cited regarding why patients choose to forego resuscitation or proceed with full resuscitation status despite advanced life‐threatening illness. Factors associated with refusal of CPR include being older, female, living in a nursing home and having a worsening functional status, depression, and/or an expected poor outcome.14, 15 One can speculate that fear of no longer being cared for or being abandoned may be inferred or directly stated, and this may or may not be related to socioeconomic factors, stressors outside of the medical system, or underlying depressive symptomatology, especially hopelessness. Alternatively, 1 study revealed that an unclear expectation of outcome and prognosis after cardiopulmonary arrest led some to proceed with full resuscitative measures.15
Reports differ regarding the advanced care trajectory based on patient wishes. One study of 872 critically ill cancer patients found no significant difference in application of life‐sustaining therapies regardless of presence of an advance directive.3 The SUPPORT study mentioned above was specifically designed to understand preferences for CPR.14 While SUPPORT found that foregoing CPR may be associated with a small reduction in intensity of care, there was no difference in overall hospital survival.14 Last, although advance directives are static in terms of patient's stated wishes, a patient with decision‐making capacity is able to request a shift in goals of care at any time. However, a case‐based survey of 241 responding physicians concluded that a DNR order may indeed be associated with less aggressive and/or life‐prolonging interventions, CPR notwithstanding.16 This concept of treating those with DNR status less aggressively is often born out in terms of popular perception.17 A recent study has demonstrated that patients who discuss these issues with physicians and elect a DNR status not only have fewer aggressive interventions, but also report a higher quality of life.4
A particular nidus for this confusion may be how one interprets the DNR directive. Although DNR is specifically associated with 3 basic tenets (no endotracheal intubation, no chest compressions, and no defibrillation in the setting of cardiopulmonary arrest), this designation does not substitute for intact patient decision‐making capacity in considering other supportive measures. Intermediate steps such as limited aggressive therapy orders have been suggested to provide time‐limited and goal‐limited advanced care.9 While this offers a broader array of scenarios to be considered prior to and during clinical encounters, this may also muddy the picture with impractical options and further lack of clarity in already complex situations. The Physician Orders for Life‐Sustaining Treatment (POLST) movement has taken roots in several states, targeting seriously ill patients such as the frail and elderly. The POLST provides more explicit information regarding limited advanced measures such as nutrition or antibiotics, and may be particularly useful as a prehospital decision aid.18 While the POLST, just as the traditional advance directive, may provide clinical guidance outside of situations described explicitly therein,, it may not provide further information about goals of care, (ie, Is there a situation when 1 of these measures may be acceptable?). To reiterate what was stated about traditional directives, the POLST also applies only in situations where a patient is lacking decision‐making capacity at the time of an acute event.
The designation of DNR may indeed allow for introduction of advanced care measures that may be in accord with the patient's overall wishes and clinical prognosis. Several interventions may be appropriate on a time‐limited basis. In addition to administration of adenosine or antiarrhythmics, as in the case of our patient, the use of broad‐spectrum antimicrobial therapy, vasoactive medications, and consideration for intensive monitoring may all be appropriate on a time‐limited basis. Nevertheless, without a clear understanding of the goals of limited aggressive therapy, some would argue there is always a slippery slope in terms of technology and the implementation of advanced care measures. Hence, expectations regarding perceived outcomes, goals to be achieved by the therapy, and reasonable time lines may further clarify the patient's wishes.
In this patient scenario, the administration of adenosine is generally safe, but may lead to prolonged asystole, atrial fibrillation, and ventricular tachyarrhythmias.1921 This may lead one to consider further downstream ACLS interventions, including defibrillation or atropine. From an ethical standpoint, it is valuable to consider what would have been the next step beyond this step, in terms of advanced care measures. In the case of our patient, these measures were considered, and all accepted the goals of our intervention and its limitations. While virtually all treatments provided by physicians may predispose patients to iatrogenesis, the risks and benefits of interventions are particularly important considerations in the seriously ill patient with limited life expectancy.
Iatrogenic adverse events can be serious and fatal, and occur in 4% to 9% of hospitalized patients.2224 There has been much debate about what to do for iatrogenic adverse events, particularly when patients have clearly articulated advanced directives and DNR requests. While some argue there is a higher moral duty to reverse complications resulting from physician error or treatment‐induced complication, others would feel that the fiduciary obligation is to the patient's request.25, 26 Again, in the setting of our clinical scenario, having clear, up‐front expectations about goals of care and limitation inherent were articulated as much as able.
With increasing complexity of inpatient care and team‐based models of care becoming the norm, discerning patient's wishes continuously throughout a hospital course is critical. While this responsibility previously would have fallen to the 1 coordinating clinician (ie, the primary care physician, or the patient's subspecialist), it is increasingly becoming the responsibility of all members of the team. While provider's level of prior education, exposure, and comfort may vary, several resources have attempted to address these concerns and attempted to lay a framework for overcoming barriers to these discussion and tips on empathetic and effective communication.17, 2729
Skills notwithstanding, hospitalists particularly face a challenge in communicating these tenuous issues with patients. While there is intrinsic value in having an standardized approach to these situations, hospitalists are often thrown into these difficult situations in a fragmented, nonlongitudinal fashion, further heightening the clinical and ethical tension.28, 30 However, hospitalists are also is an area where they can truly make an impact in these patients' lives at a critical juncture. Evidence suggests that regardless of the provider who broaches the subject, patients have a desire to talk about these issues.4, 14 Hospitalists may be in an advantageous position compared to their primary care or subspecialist colleagues, in that they can offer a fresh perspective and the ability to have a dialog with the patient about these issues.
Implications
While patients are entitled to die free from the intrusion of chest compression and endotracheal tubes, they are also entitled to have symptoms aggressively managed. Advanced care measures may be appropriate for symptom palliation in complex clinical situations. A careful understanding of the patient's wishes and goals of care, after thoughtful exploration, may include therapies that in isolation, appear to be extraordinary or excessive. SVT is often quickly and successfully treated at the bedside. Despite a firm DNR status, treatment with IV adenosine allowed our patient time to return home with his family.
Acknowledgements
Special thanks to Dr. Paul S. Mueller for his thoughtful review and commentary regarding this manuscript.
- 2006 Comprehensive Accreditation Manual for Hospitals: The Official Handbook (CAMH).Oak Brook Terrace, IL:Joint Commission Resources;2006.
- ,,.Advance directives and do‐not‐resuscitate orders on general medical wards versus the intensive care unit.Mil Med.204;169:433–436.
- ,,.Advance directives in critically ill cancer patients.Crit Care Nurs Clin North Am.2000;12:373–383.
- ,,, et al.Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment.JAMA.2008;300:1665–1673.
- ,,, et al.Decision‐making in patients with advanced cancer compared with amyotrophic lateral sclerosis.J Med Ethics.2008;34:664–668.
- ,,.Closed‐chest cardiac massage.JAMA.1960;173:1064–1067.
- ,,, et al.Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS Study Phase I results. Ontario prehospital advanced life support.Ann Emerg Med.1999;33:44–50.
- ,,, et al.Effect of bystander initiated cardiopulmonary resuscitation on ventricular fibrillation and survival after witnessed cardiac arrest outside hospital.Br Heart J.1994;72:408–412.
- ,,.CPR for patients labeled DNR: the role of the limited aggressive therapy order.Ann Intern Med.2003;138:65–68.
- ,,, et al.A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group.N Engl J Med.1998;339:1595–1601.
- ,,,.Does age affect outcomes of out‐of‐hospital cardiopulmonary resuscitation?JAMA.1990;264:2109–2110.
- ,,, et al.A comparison of standard cardiopulmonary resuscitation and active compression‐decompression resuscitation for out‐of‐hospital cardiac arrest. French Active Compression‐Decompression Cardiopulmonary Resuscitation Study Group.N Engl J Med.1999;341:569–575.
- ,,,,,.Survival in cancer patients undergoing in‐hospital cardiopulmonary resuscitation: a meta‐analysis.Resuscitation.2006;71:152–160.
- ,,, et al.Choices of seriously ill patients about cardiopulmonary resuscitation: correlates and outcomes. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments.Am J Med.1996:128–137.
- ,,,,,.Preferences for life‐sustaining treatments in advance care planning and surrogate decision making.J Palliat Med.2000;3(1):37–48.
- ,.The effect of do‐not‐resuscitate orders on physician‐making.J Am Geriatr Soc.2002;50:2057–2061.
- ,,, et al.A staff dialogue on do not resuscitate orders: psychosocial issues faced by patients, their families, and caregivers.Oncologist.1999;4:256–262.
- ,,,.Hope for the future: achieving the original intent of advance directives.Hastings Cent Rep.2005;12S:S26–S30.
- ,.Adenosine‐induced non‐sustained polymorphic ventricular tachycardia.Eur Heart J.1994;15:281–282.
- ,,,.Adenosine induced ventricular arrhythmias in the emergency room.Pacing Clin Electrophysiol.2001;24:450–455.
- ,.Torsades de pointes after intravenous adenosine in the presence of prolonged QT syndrome.Am Heart J.1992;123:794–796.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients—results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- .The hazards of hospitalization.Ann Intern Med.1964;60:100–110.
- ,,,.Iatrogenic illness on a general medical service at a university hospital.N Engl J Med.1981;304:638–642.
- ,.Overriding a patient's refusal of treatment after an iatrogenic complication.N Engl J Med.1997;336:1908–1910.
- ,,.Would physicians override a do‐not‐resuscitate order when a cardiac arrest is iatrogenic?J Gen Intern Med.1999;14:35–38.
- ,,.Discussing resuscitation preferences with patients: challenges and rewards.J Hosp Med.2006;1:231–240.
- .Decision making at a time of crisis near the end of life.JAMA.2004;292:1738–1743.
- ,,,.Advance care planning as a process: structuring the discussions in practice.J Am Geriatr Soc.1995;43:440–446.
- .Addressing end‐of‐life issues.JAMA.2005;293:162.
- 2006 Comprehensive Accreditation Manual for Hospitals: The Official Handbook (CAMH).Oak Brook Terrace, IL:Joint Commission Resources;2006.
- ,,.Advance directives and do‐not‐resuscitate orders on general medical wards versus the intensive care unit.Mil Med.204;169:433–436.
- ,,.Advance directives in critically ill cancer patients.Crit Care Nurs Clin North Am.2000;12:373–383.
- ,,, et al.Associations between end‐of‐life discussions, patient mental health, medical care near death, and caregiver bereavement adjustment.JAMA.2008;300:1665–1673.
- ,,, et al.Decision‐making in patients with advanced cancer compared with amyotrophic lateral sclerosis.J Med Ethics.2008;34:664–668.
- ,,.Closed‐chest cardiac massage.JAMA.1960;173:1064–1067.
- ,,, et al.Modifiable factors associated with improved cardiac arrest survival in a multicenter basic life support/defibrillation system: OPALS Study Phase I results. Ontario prehospital advanced life support.Ann Emerg Med.1999;33:44–50.
- ,,, et al.Effect of bystander initiated cardiopulmonary resuscitation on ventricular fibrillation and survival after witnessed cardiac arrest outside hospital.Br Heart J.1994;72:408–412.
- ,,.CPR for patients labeled DNR: the role of the limited aggressive therapy order.Ann Intern Med.2003;138:65–68.
- ,,, et al.A comparison of repeated high doses and repeated standard doses of epinephrine for cardiac arrest outside the hospital. European Epinephrine Study Group.N Engl J Med.1998;339:1595–1601.
- ,,,.Does age affect outcomes of out‐of‐hospital cardiopulmonary resuscitation?JAMA.1990;264:2109–2110.
- ,,, et al.A comparison of standard cardiopulmonary resuscitation and active compression‐decompression resuscitation for out‐of‐hospital cardiac arrest. French Active Compression‐Decompression Cardiopulmonary Resuscitation Study Group.N Engl J Med.1999;341:569–575.
- ,,,,,.Survival in cancer patients undergoing in‐hospital cardiopulmonary resuscitation: a meta‐analysis.Resuscitation.2006;71:152–160.
- ,,, et al.Choices of seriously ill patients about cardiopulmonary resuscitation: correlates and outcomes. SUPPORT Investigators. Study to understand prognoses and preferences for outcomes and risks of treatments.Am J Med.1996:128–137.
- ,,,,,.Preferences for life‐sustaining treatments in advance care planning and surrogate decision making.J Palliat Med.2000;3(1):37–48.
- ,.The effect of do‐not‐resuscitate orders on physician‐making.J Am Geriatr Soc.2002;50:2057–2061.
- ,,, et al.A staff dialogue on do not resuscitate orders: psychosocial issues faced by patients, their families, and caregivers.Oncologist.1999;4:256–262.
- ,,,.Hope for the future: achieving the original intent of advance directives.Hastings Cent Rep.2005;12S:S26–S30.
- ,.Adenosine‐induced non‐sustained polymorphic ventricular tachycardia.Eur Heart J.1994;15:281–282.
- ,,,.Adenosine induced ventricular arrhythmias in the emergency room.Pacing Clin Electrophysiol.2001;24:450–455.
- ,.Torsades de pointes after intravenous adenosine in the presence of prolonged QT syndrome.Am Heart J.1992;123:794–796.
- ,,, et al.Incidence of adverse events and negligence in hospitalized patients—results of the Harvard Medical Practice Study I.N Engl J Med.1991;324:370–376.
- .The hazards of hospitalization.Ann Intern Med.1964;60:100–110.
- ,,,.Iatrogenic illness on a general medical service at a university hospital.N Engl J Med.1981;304:638–642.
- ,.Overriding a patient's refusal of treatment after an iatrogenic complication.N Engl J Med.1997;336:1908–1910.
- ,,.Would physicians override a do‐not‐resuscitate order when a cardiac arrest is iatrogenic?J Gen Intern Med.1999;14:35–38.
- ,,.Discussing resuscitation preferences with patients: challenges and rewards.J Hosp Med.2006;1:231–240.
- .Decision making at a time of crisis near the end of life.JAMA.2004;292:1738–1743.
- ,,,.Advance care planning as a process: structuring the discussions in practice.J Am Geriatr Soc.1995;43:440–446.
- .Addressing end‐of‐life issues.JAMA.2005;293:162.
Simulator Training of Future Hospitalists
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
Internal medicine residency programs, the major pipeline for incoming hospitalists, often provide little hands‐on experience in bedside procedures. Some residents may only insert 1 central venous catheter every 4 months on the general medicine wards,1 and others may gain little more experience during intensive care unit rotations. As seen in the survey presented by Grover et al.2 in this issue of the Journal, after 3 years of training in all types of patient care units, residents often count their accumulated experience on their fingers and toes. Such sparse experience hardly leads to expertise. Recognizing this pervasive lack of training the American Board of Internal Medicine narrowed its certification requirements for bedside procedures in 2006.3 Residents are no longer expected to perform bedside procedures but instead to know them. This important revision acknowledges that manual skills training should neither be assumed nor expendablecontinuing to do so is too risky.4 Yet as internal medicine residency programs focus their bedside procedure training on cognitive competence, the ongoing exodus of bedside procedures to the up‐market hands of subspecialists, surgeons, anesthesiologists, and interventional radiologists5 will likely accelerate.
But why should hospitalists disrupt this trend? Bedside procedures are common and not always conveniently needed during daytime hours. Roughly one‐tenth of general medicine inpatients receive a central venous catheter (CVC) insertion, a lumbar puncture, an abdominal paracentesis, or a thoracentesis.6 Among these patients, about one‐half will urgently need procedures during off‐hours. Outside of the emergency department, hospitalists will likely remain the only group of physicians available at the bedsides of general medicine inpatients 7 days a week, 24 hours per day. Thus, in developing our particular practice system to best serve our patients,7 we believe that hospitalists ought to remain principals in ensuring that inpatients have ready access to expertly performed bedside procedures.
Yet unfortunately, given the limited training in manual skills that today's internal medicine residents receive, hospitalists are increasingly less prepared to provide this access themselves.8 State‐of‐the‐art training methods developed by medical specialties that depend largely on manual skills provide promising potential solutions for both future and practicing hospitalists.9 In particular, patient simulators can provide trainees with the essential hands‐on experience they often lack. In contrast to the ad hoc see‐one, do‐one, teach‐one method in current widespread use, training with simulators has distinct advantages. First, simulators obviate the increasingly awkward consent as patients grow savvier about safety concerns and (understandably) less tolerant of a novice's need to acquire experience.10 Second, training with simulators is controlled so that anatomic variations, comorbidities, patient discomfort, and time pressuresthough important real‐world factorscan be artificially removed in the earlier cognitive and integrative stages of training.11 Third, immediate feedback, which at the bedside of real patients is often empathetically avoided or delivered in cryptic hand signals, can be unmistakably unmuted and honest in the simulator setting. Fourth, and most important to the development of expertise, simulators can be used repeatedly, allowing trainees first to become facile in the mechanics of their performance (eg, holding an ultrasound probe for real‐time guidance or knowing how it feels to enter a vein) before attempting a procedure on a patient.
Three examples of patient simulators used to train internal medicine residents in CVC insertion are presented in this issue of the Journal.1214 Using observers who adhered to objective, a priori assessment criteria, both Rosen et al.13 and Millington et al.14 carefully demonstrate that internal medicine residents' manual skills can improve with patient simulators. Given the understood importance of hands‐on experience in manual skills training,15 these anticipated findings are important validations of simulator theory. The work by Barsuk et al.12 goes further to begin to examine whether or not simulator training actually leads to improved patient outcomesthe holy grail of such research. In this observational study, compared to residents who did not undergo simulator training, those who did undergo such training had 1 fewer needle passes during successful CVC insertions. Given the relative infrequency of periprocedural complications, this study was understandably underpowered to measure true complications, relying instead on the often‐used surrogate of needle passes. Nonetheless, this work will serve as an important initial example of why simulator training may be worth the effort.
To direct participation in simulator training, we endorse selecting trainees who will perform bedside procedures in their future practice.16 Given the trend in manual skills training among internal medicine residency training programs, hospitalist programs may need to shoulder this effort themselves. Thankfully, simulator training need not be expensive. Based on transfer‐of‐learning research,17 the fidelity of the simulator is less important than the accumulated experience it can afford. Even low‐fidelity simulators, such as the store‐bought whole chicken used by Rosen et al.,13 may preserve trainees' manual skills just as effectively as the expensive, bionic, high‐fidelity simulators used by Barsuk et al.12 and Millington et al.14
Beyond the costs of training, however, hospital administrators and hospitalist group leaders have more complex externalities and opportunity costs to weigh when evaluating which physician groups should perform bedside procedures. The intuitively lower‐cost strategy for hospitals, we believe, would be to ask hospitalists to perform bedside procedures at patients' bedsides instead of asking, say, highly‐paid interventional radiologists to perform the same procedures in fully‐staffed fluoroscopy suites. There is, however, very little research to help inform these decisions. As hospitalists, we know firsthand that modern healthcare remuneration is based more on doing than on knowing. Yet, whether or not bedside procedures afford financial incentives for hospitalists is unclearmuch will depend on local factors. Regardless of the finances, we believe that hospitalists skilled in performing common bedside procedures can improve the quality and efficiency of care delivery at patients' bedsides. So, instead of a call to arms for yet another turf battle, let's continue development of state‐of‐the‐art training methods like simulators to ensure that future hospitalists can expertly perform bedside procedures. After all, fighting for improvements in patient safety is a battle that we hospitalists know how to win.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
- ,,,,,.Firm‐based trial to improve central venous catheter insertion practices.J Hosp Med.2007;2:135–142.
- ,,,,,.Development of a test to evaluate residents' knowledge of medical procedures.J Hosp Med.2009;XX:XXX–XXX.
- American Board of Internal Medicine. Policies and procedures for certification, May 2009. Available at: http://www.abim.org/default.aspx; Accessed August2009.
- .Procedural competence of internal medicine residents: time to address the gap.J Gen Intern Med.2000;15:432–433.
- ,.The declining number and variety of procedures done by general internists: a resurvey of members of the American College of Physicians.Ann Intern Med.2007;146:355–360.
- ,,, et al.Impact of a bedside procedure service on general medicine inpatients: a firm‐based trial.J Hosp Med.2006;2:143–149.
- ,.What procedures should internists do?Ann Intern Med.2007;146:392–394.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24
- ,.Teaching surgical skills—changes in the wind.N Engl J Med.2006;355:2664–2669.
- ,,,.Patients' willingness to allow residents to learn to practice medical procedures.Acad Med.2004;79:144–147.
- ,.Human performance.Belmont, CA:Brooks/Cole;1967.
- ,,,,.Use of simulation‐based mastery learning to improve the quality of central venous catheter placement in a medical intensive care unit.J Hosp Med.2009;4(7):397–403.
- ,,,,.Does personalized vascular access training on a non‐human tissue model allow for learning and retention of central line placement skills? Phase II of the procedural patient safety initiative (PPSI‐II).J Hosp Med.2009;4(7):423–429.
- ,,,,.Improving internal medicine residents' performance, knowledge, and confidence in central venous catheterization using simulators.J Hosp Med.2009;4(7):410–414.
- ,,,.The Cambridge handbook of expertise and expert performance.New York, NY:Cambridge University Press;2006.
- ,,.Procedural training at a crossroads: striking a balance between education, patient safety, and quality.J Hosp Med.2007;2:123–125.
- ,,, et al.The educational impact of bench model fidelity on the acquisition of technical skill: the use of clinically relevant outcome measures.Ann Surg.2004;240:374–381.
Elephantiasis Nostras Verrucosa
A 79‐year‐old woman presented from a nursing home with unusual lower extremity skin changes. Her medical history included congestive heart failure, morbid obesity, chronic lymphedema, and deep vein thrombosis with inferior vena cava filter placement. There was no history of cellulitis, filariasis, or travel to endemic areas. The patient was afebrile without adenopathy and had bilateral lower extremity edema with hyperpigmented, cobble‐stoned, hyperkeratotic skin and verrucous nodules on the inner thighs (Figures 1 and 2). Elephantiasis nostras verrucosa secondary to longstanding lymphedema and obesity was diagnosed by the dermatology consultant. The patient was treated with compression stockings and topical emollients. Elephantiasis nostras verrucosa is a rare disorder secondary to chronic noninfectious or recurrent cellulitic lymphedema that results in hyperplastic fibrotic dermal changes.1 Diagnosis is clinical, but biopsy to exclude malignancies such as Stewart‐Treves syndrome is needed in atypical cases.2 Treatment options include compression stockings, limb elevation, topical keratolytics, emollients, retinoids, and surgical debridement.2, 3
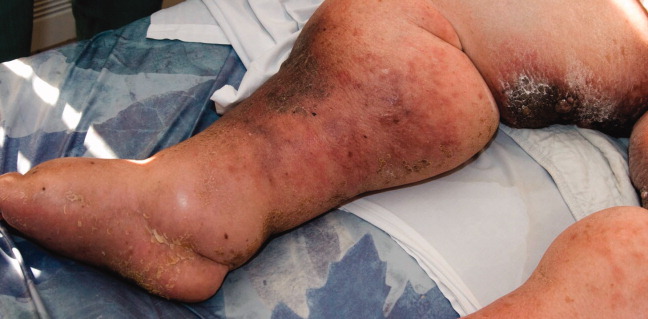
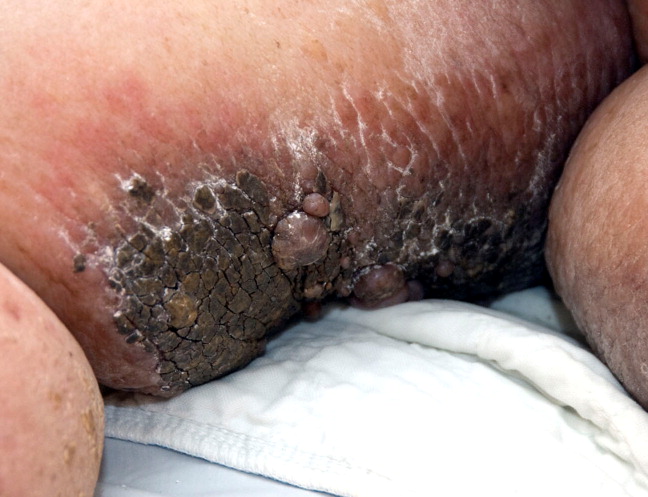
- ,,.Elephantiasis nostras verrucosa.Cutis.1998;62:77–80.
- ,.Elephantiasis nostras verrucosa: a review.Am J Clin Dermatol.2008;9:141–146.
- ,,,.Elephantiasis nostras verrucosa successfully treated by surgical debridement.Dermatol Surg.2004;30:939–941.
A 79‐year‐old woman presented from a nursing home with unusual lower extremity skin changes. Her medical history included congestive heart failure, morbid obesity, chronic lymphedema, and deep vein thrombosis with inferior vena cava filter placement. There was no history of cellulitis, filariasis, or travel to endemic areas. The patient was afebrile without adenopathy and had bilateral lower extremity edema with hyperpigmented, cobble‐stoned, hyperkeratotic skin and verrucous nodules on the inner thighs (Figures 1 and 2). Elephantiasis nostras verrucosa secondary to longstanding lymphedema and obesity was diagnosed by the dermatology consultant. The patient was treated with compression stockings and topical emollients. Elephantiasis nostras verrucosa is a rare disorder secondary to chronic noninfectious or recurrent cellulitic lymphedema that results in hyperplastic fibrotic dermal changes.1 Diagnosis is clinical, but biopsy to exclude malignancies such as Stewart‐Treves syndrome is needed in atypical cases.2 Treatment options include compression stockings, limb elevation, topical keratolytics, emollients, retinoids, and surgical debridement.2, 3


A 79‐year‐old woman presented from a nursing home with unusual lower extremity skin changes. Her medical history included congestive heart failure, morbid obesity, chronic lymphedema, and deep vein thrombosis with inferior vena cava filter placement. There was no history of cellulitis, filariasis, or travel to endemic areas. The patient was afebrile without adenopathy and had bilateral lower extremity edema with hyperpigmented, cobble‐stoned, hyperkeratotic skin and verrucous nodules on the inner thighs (Figures 1 and 2). Elephantiasis nostras verrucosa secondary to longstanding lymphedema and obesity was diagnosed by the dermatology consultant. The patient was treated with compression stockings and topical emollients. Elephantiasis nostras verrucosa is a rare disorder secondary to chronic noninfectious or recurrent cellulitic lymphedema that results in hyperplastic fibrotic dermal changes.1 Diagnosis is clinical, but biopsy to exclude malignancies such as Stewart‐Treves syndrome is needed in atypical cases.2 Treatment options include compression stockings, limb elevation, topical keratolytics, emollients, retinoids, and surgical debridement.2, 3


- ,,.Elephantiasis nostras verrucosa.Cutis.1998;62:77–80.
- ,.Elephantiasis nostras verrucosa: a review.Am J Clin Dermatol.2008;9:141–146.
- ,,,.Elephantiasis nostras verrucosa successfully treated by surgical debridement.Dermatol Surg.2004;30:939–941.
- ,,.Elephantiasis nostras verrucosa.Cutis.1998;62:77–80.
- ,.Elephantiasis nostras verrucosa: a review.Am J Clin Dermatol.2008;9:141–146.
- ,,,.Elephantiasis nostras verrucosa successfully treated by surgical debridement.Dermatol Surg.2004;30:939–941.
Benign Pneumatosis Intestinalis
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
A 9‐year‐old male, with a history of immune thrombocytopenic purpura (ITP) and hypoplastic left heart syndrome (HLHS) repaired by total cavopulmonary shunt, presented to the Emergency Department with a 4‐day history of crampy abdominal pain with defecation and a 4‐day history of nonbloody diarrhea with intermittent nonbilious vomiting. The abdominal pain was diffuse and nonspecific without any radiation. He had a history of encopresis and constipation over the past 3 months. No anorexia was noted and the pain did not keep him from his activities of daily living. Review of all other systems was noncontributory.
His past medical history consisted of HLHS repaired by total cavopulmonary shunt with excellent results. He was diagnosed with ITP about 6 weeks prior to this presentation and had been treated with intravenous immunoglobulin and oral prednisone. His home medications included prednisone (1.5 mg/kg/day), lansoprazole, digoxin, enalapril, furosemide, and warfarin.
On physical examination, his temperature was 37C, pulse rate 97, respiratory rate 16, unlabored blood pressure was 112/74 mm Hg, oxygen saturation 91% on room air, and weight was 22.8 kg. He had a grade 4/6 holosystolic murmur across the precordium and multiple healed surgical incisions. His abdomen was soft without tenderness or distention with normoactive bowel sounds. The rest of his physical exam was unremarkable. An acute abdominal series was obtained, which showed pneumatosis intestinalis (PI) of the right colon, pneumoperitoneum, and possible portal venous gas (Figure 1A). Initial laboratory evaluation including a complete blood count and a comprehensive metabolic panel; amylase, lipase, lactate, and venous blood gas were all within normal limits, with the exception of a platelet level of 75,000/L (normal, 150,000450,000 cells/mL). Stool was negative for Rotavirus antigen, Clostridium difficile toxin, Helicobacter pylori antigen, and Shiga toxin 1 and 2. Bacterial cultures and trichrome stain for ova and parasites were both negative. Stool analysis for occult blood was negative on admission and became positive during his hospital course. A contrast computed tomography (CT) of the abdomen and pelvis confirmed the findings of pneumatosis intestinalis in the cecum and ascending colon with intraperitoneal and retroperitoneal air, but did not reveal any portal venous gas (Figure 1B).

The patient was admitted to the Children's Hospital and made nil per os (NPO; ie, nothing by mouth), placed on ampicillin/sulbactam and metronidazole prophylaxis, and observed with serial abdominal examinations. Total parenteral nutrition (TPN) was begun on hospital day 2 and continued for 10 days. Surgical intervention was not required at the initial presentation secondary to his clinical and hemodynamic stability. Since immunosuppression from chronic steroid therapy is a known risk factor for the development of PI,1 a slow steroid taper with intravenous methylprednisolone was initiated and was transitioned to oral prednisone after he resumed oral nutrition. He remained NPO for 10 days until the PI radiographically resolved. Oral feeds were reintroduced slowly without complications or recurrence of PI.
Discussion
Three major hypotheses for the origin of bowel wall gas have been proposed: intraluminal gastrointestinal (GI) gas; bacterial production of gas; and pulmonary gas.1 The intralumenal GI gas hypothesis states that intralumenal gas translocates to the bowel wall due to increased intralumenal pressure, mucosal injury from direct trauma, reduction is size of Peyer's patches from immunosuppressive medications, or a combination of factors.1, 2 The bacterial theory proposes direct invasion of the bowel wall by gas‐producing bacteria; this hypothesis is not adequately supported by bacteriologic data.2 The pulmonary gas hypothesis states that alveolar rupture could result in dissection of air through the mediastinum to the retroperitoneum and eventually along vascular channels to the gut.2 Increased intralumenal gut pressure due to coughing also drives this dissection of gas into the bowel wall.2
Chronic immunosuppression with steroids and congenital heart disease are both known risk factors for the development of PI.3 Our patient presented with common complaints of abdominal pain, encopresis, and vomiting, with a benign exam. However, he had a radiographic finding of PI. With bowel rest, antibiotics, and TPN, our patient made a full recovery without requiring surgical intervention. With patients at higher risk for PI, there needs to be a higher index of suspicion for PI in the setting of GI complaints and hemodynamic instability. It has been reported in the literature that patients at higher risk can include those with inflammatory bowel disease, chronic pulmonary disease, immunosuppressive states such as leukemia or acquired immunodeficiency syndrome, short gut syndrome, and malignancies.1, 3 Kurbegov and Sondheimer4 published a series of 32 nonneonatal cases of PI, looking for characteristics that predicted higher risk of poor outcome. Their findings showed low serum bicarbonate and PI with free air and portal venous gas as significant predictors of poor outcome.4 A recent study by Morris et al.5 showed similar results: lactic acidosis is a predictor of poor patient outcome. The clinical examination of this patient, both initially and longitudinally, and the lack of laboratory abnormalities were the key factors in the disposition of this patient in the setting of alarming abdominal radiographs.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.
- ,,.The spectrum of pneumatosis intestinalis.Arch Surg.2003;138(1):68–75.
- ,,, et al.Pneumatosis intestinalis with pneumoperitoneum mimicking intestinal perforation in a patient with myelodysplastic syndrome after hematopoietic stem cell transplantation.Korean J Intern Med.2007;22(1):40–44.
- ,.Benign pneumatosis in children.Pediatr Radiol.2000;30(11):786–793.
- ,.Pneumatosis intestinalis in non‐neonatal pediatric patients.Pediatrics.2001;108(2):402–406.
- ,,, et al.Management and outcome of pneumatosis intestinalis.Am J Surg.2008;195(5):679–682; discussion 682–683.
VTE Risk in Patients with PICCs
The use of peripherally inserted central catheters (PICCs) to facilitate the administration of intravenous medications and fluids has become commonplace in hospitalized patients. Clinicians often prefer PICCs over other central venous catheters due to their ease of insertion and the perception that PICCs may have lower risks than other central venous catheters. However, recent studies1, 2 have begun to suggest that the benefit derived from these devices can be offset by the development of complications such as upper extremity deep vein thrombosis (UEDVT). Since venous thromboembolism (VTE) in hospitalized patients is associated with increased morbidity, mortality, length of stay, and costs, we sought to determine the rate of VTE in a population of patients who received a PICC solely during their hospital stay.
Methods
Data Collection
This study was a retrospective, electronic chart review of patients who received a PICC while hospitalized between August 1, 2005 and November 1, 2005 at the Methodist University Hospital (MUH), a 652‐bed, urban, university‐affiliated, community hospital in Memphis, TN. Patients were identified through the use of a PICC database that is maintained by the nurses who routinely place the PICCs. The data collected included the date of insertion, the diameter of the catheter, the vein accessed, the position of the catheter tip, and the reason for PICC insertion. These factors as well as demographics were examined to determine whether they were associated with thrombosis. These data were linked with data from the ultrasound laboratory and nuclear medicine/radiology laboratory and with hospital discharge data. Data were recorded by trained research assistants and verified for accuracy by the study investigators. The institutional review board approved the study protocol prior to data collection.
Patients and Outcomes
All adult consecutive patients who had a PICC inserted during the study period and who did not have a UEDVT or pulmonary embolism (PE) at the time of PICC insertion were included in the study.
PICCs were placed using a modified Seldinger technique at the bedside with portable ultrasound guidance. The vessel of choice for insertion was the basilic vein. Confirmation of catheter tip placement in the lower third of the superior vena cava (SVC) was done with chest x‐ray prior to use of the PICC. The PICC manufacturer was Boston Scientific (Vaxcel with PASV; Natick, MA) and normal saline was used for routine flushing of the PICC.
Study Outcomes
Symptomatic UEDVT
A UEDVT was defined as a symptomatic event in the ipsilateral extremity leading to the performance of duplex ultrasonography, which confirmed the diagnosis of UEDVT. Systematic screening for UEDVT was not performed on any patients during the study period. Sonographic diagnosis of UEDVT was based on noncompressibility of a venous segment of the upper arm or the internal jugular vein; absent or reduced flow on Doppler imaging with failure to augment on compression of the arm; or the presence of echogenic material compatible with thrombus in the arm or central venous vasculature on real‐time imaging. Superficial thrombosis was not counted as a UEDVT event.
Symptomatic PE
PE was defined as a symptomatic event prompting the performance of ventilation‐perfusion lung scan or spiral computed tomography (CT). Systematic screening for PE was not done on any patients during the study period. Radiologic diagnosis of PE was not standardized, but intraluminal filling defect of a lobar artery or more proximal pulmonary arterial vasculature on spiral CT, or an abnormal ventilation‐perfusion (V2) scan with a high clinical suspicion for PE must have been noted on the radiology report along with the physician's clinical diagnosis of PE.
Statistical Analyses
The incidence of VTE was reported as the proportion of patients who had a documented event during hospitalization, and also as the number of events per 1000 PICC‐days. Baseline characteristics were assessed as potential risk factors. Differences in proportions were tested with the chi square or Fisher exact test, and differences in means of the continuous variables were tested using the t‐test. Univariate analysis of symptomatic VTE by each potential risk factor was performed using logistic regression. Logistic regression models were used to simultaneously assess the relationship between the baseline factors and the probability of developing a thrombotic event. Using a backward elimination modeling strategy, only factors that maintained a P value of <0.05 were retained in the final model. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. SAS version 8.2 (SAS Institute, Inc., Cary, NC) was used for data analysis.
Results
Patient Demographics and Baseline Characteristics
From August 1, 2005 to November 1, 2005, 954 PICCs were inserted in 777 patients. The demographics and baseline characteristics of the 777 patients are outlined in Table 1. History of cancer was present in 20.21% of the patients. History of VTE was present in 7.02% of the patients. The most common reason for PICC insertion was poor access in 90.6% of the PICCs. The most common medication infused through the PICC was antibiotics in 69.2% and intravenous hydration in 50.1%. The basilic vein was accessed in 90%. The tip location, as determined by chest X‐ray at the time of insertion, was the SVC in 85.3%. PICCs were in situ for an average of 9 days and most were 5‐French (Fr) catheters. The average duration of PICC placement was over 9 days, and the average length of stay slightly exceeded 16 days.
| Patient Characteristics | Study Population |
|---|---|
| |
| Age (years), mean (SD); range | 60.8 (17.8); range, 1999 |
| Gender, n (%) | |
| Women | 465 (59.8) |
| Ethnicity, n (%) | |
| White | 223 (28.6) |
| African American | 543 (69.9) |
| Hispanic | 4 (0.5) |
| Weight (kg), mean (SD); range | 81.0 (25.8); range, 30207 |
| History of cancer, n (%) | 157 (20.21) |
| History of venous thromboembolism, n (%) | 55 (7.07) |
| PICC location, n (%) | |
| Right vein | 538 (69.2) |
| Vein accessed, n (%) | |
| Basilic | 695 (90.0) |
| Cephalic | 39 (5.1) |
| Median | 38 (4.9) |
| PICC reason, n (%)* | |
| Poor access | 704 (90.6) |
| Antibiotic iv. | 377 (69.2) |
| Hydration iv. | 389 (50.1) |
| Irritant drug | 19 (2.5) |
| TPN | 47 (6.1) |
| Chemotherapy | 23 (3.0) |
| Pain medication | 22 (2.8) |
| Blood and blood products | 16 (2.1) |
| Other nonblood | 30 (3.9) |
| PICC tip location, n (%) | |
| Central location | 643 (85.3) |
| Noncentral location | 111 (14.7) |
| Catheter lumen, n (%) | |
| 4Fr | 10 (1.3) |
| 5Fr | 758 (97.5) |
| Other | 9 (1.1) |
| Length of stay (days), mean (SD); range | 16.3 (17.2); range, 1224 |
| PICC days; range | 9.6 (9.0); range, 164 |
Outcomes: VTE
During their hospital stay, 38 patients experienced 1 or more VTEs, yielding an incidence of 4.89% (Table 2). A total of 7444 PICC‐days were recorded for 777 patients. This yields a rate of 5.10 VTEs/1000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 3.65 UEDVT/1000 PICC days. The mean length of stay was 26 days in those with VTE versus 15.8 days in those who did not develop VTE (P < 0.001). Average PICC‐days were also longer in those who developed VTE compared to those who did not (13 days vs. 9; P < 0.001).
| Outcome | n | % |
|---|---|---|
| ||
| Total VTE | 38* | 4.89 |
| Upper extremity thromboses | 31 | 3.99 |
| UEDVT | 27 | 3.47 |
| Superficial upper extremity thrombosis | 4 | 0.51 |
| PE | 8 | 1.03 |
VTE prophylaxis (using enoxaparin or heparin) was administered to 26% of patients from the time of PICC insertion until UEDVT occurred. Only 12.5% of those who developed PE were given VTE prophylaxis. All patients who developed a UEDVT or PE were treated with full anticoagulation except those with contraindications and patients with superficial upper extremity thrombosis. Five of the patients with UEDVT and 2 of the patients with PE died during their hospitalization.
Four of the 8 patients with PICC‐associated PE had bilateral lower extremity ultrasounds performed, and 3 of these were negative. The other 4 patients did not have ultrasounds performed.
Risk Factors
History of VTE was the strongest risk factor for VTE in univariate analysis, patients with a history of VTE being 10 times more likely to develop a PICC‐related VTE event (Table 3). PICC tip location was strongly associated with VTE. A noncentral location of the tip at the time of insertion was associated with a 2.34 (95% CI, 1.15‐4.75) higher risk of developing VTE during the hospitalization stay, compared to a central location (SVC or RA). The duration of PICC use was also associated with an increased risk of developing VTE (OR for a 10‐day increase in duration, 1.40 (95% CI, 1.07‐1.84), as it was the length of hospital stay (OR for a 10‐day increase in duration, 1.18 (95% CI, 1.05‐1.33). The duration of hospital stay was correlated with the duration of PICC use; however, its temporal relationship with a VTE was uncertain, as a prolonged hospital stay could be the consequence of a VTE event in some cases. In the multivariate analysis, history of VTE, PICC tip location, and length of stay retained statistical significance (Table 4); while our data are suggestive for an association with VTE events, the duration of PICC use did not maintain statistical significance, in the presence of PICC tip location. History of VTE remained the strongest risk factor for subsequent VTE (OR, 10.83; 95% CI, 4.89‐23.95); the location of the PICC tip was location highly associated with subsequent VTE (OR, 2.61; 95% CI, 1.28‐5.35).
| Characteristic | Univariate Model OR (95% CI) |
|---|---|
| |
| Age | 1.01 (0.991.03) |
| Gender (male versus female) | 1.06 (0.562.01) |
| Race (nonwhite versus white) | 1.07 (0.532.18) |
| History of cancer | 1.72 (0.823.61) |
| History of VTE | 10.36 (4.8122.34) |
| PICC location: right versus left vein | 1.62 (0.763.44) |
| PICC location | |
| Cephalic | Referent |
| Basilic | 2.29 (0.3117.07) |
| Median | 4.91 (0.5345.97) |
| PICC tip location: (noncentral versus central)* | 2.43 (1.154.75) |
| PICC days (10 days unit) | 1.40 (1.071.84) |
| Length of stay (10 days unit) | 1.18 (1.051.33) |
| Characteristic | Multivariate Model OR (95% CI) |
|---|---|
| |
| History of VTE | 10.83 (4.8923.95) |
| PICC tip location (noncentral versus central)* | 2.61 (1.285.35) |
| Length of stay (10 days unit) | 1.21 (1.071.37) |
Discussion
Overall Findings
Our study is the first to examine both UEDVT and PE rates in hospitalized adult patients with PICC lines. We found that 4.89% of our patients experienced a thromboembolic complication during their hospitalization stay, yielding a rate of 5.1 VTE/1,000 PICC‐days and 3.63 UEDVT/1,000 PICC‐days. These figures are similar to or higher than those reported by other retrospective studies using symptom‐driven ultrasound diagnosis of UEDVT, reporting incidence figures ranging from 2 per 100 patients,3 to 2.47 per 100 patients,4 to 3.7 per 100 patients.5
There are several factors to consider when comparing our findings to previously published studies. First, our study population differs by being older and by including hospitalized patients with both acute and chronic medical illnesses, and with a wider spectrum of PICC indications. Second, the duration of PICC use is known to be associated with the development of symptomatic UEDVT.6 Several published studies do not report the duration of PICC use, thus making the comparison of incidence difficult to interpret. Third, the duration of patient follow‐up influences the likelihood that a symptomatic UEDVT is diagnosed; patient follow‐up time is oftentimes unspecified in some studies, thus making comparisons difficult. In contrast, our study reports the incidence of VTE during a clearly specified time window; ie, hospital stay.
Several studies that reported the incidence per 100 PICCs found incidences ranging from 3.9 per 100 PICCs7 and 4.66 per 100 PICCs.8 Similarly, studies of different patient populations and the use of systematic diagnostic and follow‐up methods have found very high rates of thrombosis (15.4% in a randomized clinical trial [RCT] of total parenteral nutrition [TPN],9 38% with systematic venography to diagnose UEDVT,10 and 64.52% in an RCT of intensive care unit [ICU] patients11).
PE
In the largest study to date of 2063 patients who received a PICC for intravenous antibiotic therapy, it was found that 2.5% developed upper extremity thrombosis; of these, 3.8% also had a positive VQ scan for PE.4 However, this study did not systematically review records for PE in those without upper extremity thrombosis. Our study showed that approximately 1% of those who received a PICC developed a PE during their hospitalization. In 3 of our 8 patients who developed a PE, bilateral lower extremity ultrasounds were negative for PE. None of the subjects who developed PE received ultrasounds of the upper extremities in order to look for deep vein thrombosis (DVT), possibly due to a lack of awareness of PICC‐associated VTE.
Risk Factors
Among the risk factors explored, we found that patients with previous history of a venous thromboembolic event are much more likely to develop a PICC‐related VTE. This is not surprising, as previous VTE has been identified a risk factor.1214 We also found that patients whose PICC was not confirmed to be in the SVC or at the junction of the SVC and the RA, were twice as likely to experience VTE. An increased risk associated with noncentral tip location was reported in 2 previous studies using venography to assess vein condition among infectious disease patients,15 and oncology patients.16 This is biologically plausible, since the smaller diameter vessels may be more conducive to thrombus formation than the SVC or RA. Other factors such as blood flow rate, turbulent flow, and endothelial injury may also play a role. As recognized by the National Association of Vascular Access Networks,17 tip positioning is influenced by catheter length, anthropometric measurements, and anatomical pathways. Central tip location, whenever possible, may be 1 of the few controllable risk factors for UEDVT. Decreasing the hospital‐acquired DVT events has been recognized as an important quality improvement, requiring an increased identification and treatment of high‐risk groups. Such hospital‐based approaches have been suggested as effective.18, 19 Both tip location and history of VTE are identifiable risk factors that may be readily incorporated into specific strategies to decrease hospital‐acquired DVT events.
While previously reported as a risk factor, left‐sided catheter location was not significantly associated with VTE in our study. Our data suggest that the duration of PICC use is associated with the risk of developing a VTE event during hospital stay, yet without reaching statistical significance. In the absence of information regarding the underlying comorbidities and/or reasons for hospitalization, interpretation of PICC line duration remains elusive.
Anticoagulant Prophylaxis
Most of the subjects in our cohort did not receive VTE prophylaxis. The efficacy of anticoagulant prophylaxis for preventing catheter‐associated VTE is controversial. In fact, current guidelines do not support VTE prophylaxis as a means to reduce rates of VTE associated with central venous catheters. Furthermore, recent controlled clinical trials of low‐molecular weight heparins at standard prophylactic doses and low doses of warfarin for VTE prevention in patients with central venous catheters undergoing cancer chemotherapy have not demonstrated reductions in VTE rates.2022 In contrast, a recent meta‐analysis suggests that anticoagulant prophylaxis is effective for preventing all catheter‐associated DVT in patients with central venous catheters, but the effectiveness for preventing symptomatic VTE, including PE, remains uncertain.23
Limitations
The small number of VTE and the lack of control for the underlying disease, concomitant therapy, and anticoagulant prophylaxis limited our ability to identify independent risk factors. Further studies are needed to confirm our finding that 1% of patients who receive a PICC will develop PE.
VTE were identified only when symptoms led to diagnostic testing, so our event rates likely underestimate the true rate of PICC‐associated VTE. It is estimated that subclinical thromboses can be found in 30% to 60% of all central catheters.10, 24 The clinical significance of silent thromboses remains to be established.
Strengths
The Society of Interventional Radiology recommends uniform reporting requirements to assist in study design and outcomes reporting on central venous access devices.25 We adhered to many of these guidelines which may facilitate comparison among studies from different institutions.
Our study is one of the largest studies to asses the rate of thrombosis among inpatients. We used broad eligibility criteria, included consecutive, unselected patients, in a large cohort of patients who were all hospitalized for acute medical illnesses. Furthermore, we used data collection that corroborated data across the entire range of hospital records, and as such we were able to determine accurately the incidence of thrombosis. UEDVT and PE were diagnosed objectively and, unlike previous studies, we also collected information on prophylaxis and treatment of VTE. To our knowledge, our study is the first to document an increased risk of symptomatic UEDVT and PE among acutely ill hospitalized patients who receive PICC.
Prior studies may be less applicable to PICC‐associated VTE in hospitalized patients because the populations included a mix of inpatients and outpatients. Our results may be more applicable to hospitalized patients who receive a PICC because more severely ill patients who are hospitalized may be at higher risk of thrombosis than outpatients. Also, previous studies included some patients who received relatively small, 3‐Fr to 4‐Fr single‐lumen PICCs, whereas the current practice is to insert larger, double‐lumen, 5‐Fr catheters. Today's PICCs may be more likely to result in vessel occlusion, venous stasis, and thrombosis.
In conclusion, we have shown that the incidence of VTE in our study population was 4.89% with a rate of 5.10 VTE/1,000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 365 UEVDVT/1000 PICC days. The most significant predictors of VTE were history of previous VTE and the location of the PICC tip at the time of insertion.
- ,,,.High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours.Intern Med J.2004;34:234–238.
- ,,.Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward.World J Surg.2006;30:1605–1619.
- ,,,,.Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients.South Med J.2006;99:1073–1077.
- ,,, et al.Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience.Clin Infect Dis.2002;34:1179–1183.
- ,,.Peripherally inserted central catheters in the intensive care unit.J Intensive Care Med.1996;11:49–54.
- ,,,,,.The incidence of upper extremity deep venous thrombosis associated with peripherally inserted central catheters in an intensive care unit. [Abstract]. Chest 2003: 69th Annual Meeting of the American College of Chest Physicians; October 25–30, 2003, Orlando, FL;2003.
- ,.Venous thrombosis related to peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:837–840.
- ,,,.Peripherally inserted central catheters: outcome as a function of the operator.J Vasc Interv Radiol.2001;12:723–729.
- ,,,,,.Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally‐inserted central catheters.Clin Nutr.2000;19:237–243.
- ,,, et al.Venous thrombosis associated with the placement of peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:1309–1314.
- ,,,,,.Peripherally inserted central catheter (PICC) associated upper extremity deep venous thrombosis (UEDVT) in critical care setting (abstract). CHEST 2005: 71st Annual Meeting of the American College of Chest Physicians. October 29, 2005 to November 3, 2005, Montreal, Quebec, Canada.
- ,,,.A prospective study of the incidence of deep‐vein thrombosis within a defined urban population.J Intern Med.1992;232:155–160.
- ,,, et al.Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications.Arch Intern Med.1997;157:57–62.
- .Risk factors for venous thrombotic disease.Thromb Haemost.1999;82:610–619.
- ,,.Complications of long arm‐catheters: a randomized trial of central vs peripheral tip location.JPEN J Parenter Enteral Nutr.1996;20:20–24.
- ,,.Tunneled catheter thrombosis: factors related to incidence.Oncol Nurs Forum.1990;17:543–549.
- National Association of Vascular Access Networks.Tip location of peripherally inserted central catheters. NVAS position statement.J Vasc Access Devices.1998;3(2):8–10.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3:148–155.
- ,,, et al.Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double‐blind, placebo‐controlled, randomized study in cancer patients.J Clin Oncol.2005;23:4057–4062.
- ,,, et al.Dalteparin for prevention of catheter‐related complications in cancer patients with central venous catheters: final results of a double‐blind, placebo‐controlled phase III trial.Ann Oncol.2006;17:289–296.
- ,,, et al.Randomized placebo‐controlled study of low‐dose warfarin for the prevention of central venous catheter‐associated thrombosis in patients with cancer.J Clin Oncol.2005;23:4063–4069.
- ,,,.Prevention of central venous catheter‐associated thrombosis: a meta‐analysis.Am J Med.2007;120:901e1–e13.
- .Deep venous thrombosis of the upper extremity.Am Fam Physician.1997;55:533–539.
- ,,,.Reporting standards for central venous access.J Vasc Interv Radiol.2003;14:S443–S452.
The use of peripherally inserted central catheters (PICCs) to facilitate the administration of intravenous medications and fluids has become commonplace in hospitalized patients. Clinicians often prefer PICCs over other central venous catheters due to their ease of insertion and the perception that PICCs may have lower risks than other central venous catheters. However, recent studies1, 2 have begun to suggest that the benefit derived from these devices can be offset by the development of complications such as upper extremity deep vein thrombosis (UEDVT). Since venous thromboembolism (VTE) in hospitalized patients is associated with increased morbidity, mortality, length of stay, and costs, we sought to determine the rate of VTE in a population of patients who received a PICC solely during their hospital stay.
Methods
Data Collection
This study was a retrospective, electronic chart review of patients who received a PICC while hospitalized between August 1, 2005 and November 1, 2005 at the Methodist University Hospital (MUH), a 652‐bed, urban, university‐affiliated, community hospital in Memphis, TN. Patients were identified through the use of a PICC database that is maintained by the nurses who routinely place the PICCs. The data collected included the date of insertion, the diameter of the catheter, the vein accessed, the position of the catheter tip, and the reason for PICC insertion. These factors as well as demographics were examined to determine whether they were associated with thrombosis. These data were linked with data from the ultrasound laboratory and nuclear medicine/radiology laboratory and with hospital discharge data. Data were recorded by trained research assistants and verified for accuracy by the study investigators. The institutional review board approved the study protocol prior to data collection.
Patients and Outcomes
All adult consecutive patients who had a PICC inserted during the study period and who did not have a UEDVT or pulmonary embolism (PE) at the time of PICC insertion were included in the study.
PICCs were placed using a modified Seldinger technique at the bedside with portable ultrasound guidance. The vessel of choice for insertion was the basilic vein. Confirmation of catheter tip placement in the lower third of the superior vena cava (SVC) was done with chest x‐ray prior to use of the PICC. The PICC manufacturer was Boston Scientific (Vaxcel with PASV; Natick, MA) and normal saline was used for routine flushing of the PICC.
Study Outcomes
Symptomatic UEDVT
A UEDVT was defined as a symptomatic event in the ipsilateral extremity leading to the performance of duplex ultrasonography, which confirmed the diagnosis of UEDVT. Systematic screening for UEDVT was not performed on any patients during the study period. Sonographic diagnosis of UEDVT was based on noncompressibility of a venous segment of the upper arm or the internal jugular vein; absent or reduced flow on Doppler imaging with failure to augment on compression of the arm; or the presence of echogenic material compatible with thrombus in the arm or central venous vasculature on real‐time imaging. Superficial thrombosis was not counted as a UEDVT event.
Symptomatic PE
PE was defined as a symptomatic event prompting the performance of ventilation‐perfusion lung scan or spiral computed tomography (CT). Systematic screening for PE was not done on any patients during the study period. Radiologic diagnosis of PE was not standardized, but intraluminal filling defect of a lobar artery or more proximal pulmonary arterial vasculature on spiral CT, or an abnormal ventilation‐perfusion (V2) scan with a high clinical suspicion for PE must have been noted on the radiology report along with the physician's clinical diagnosis of PE.
Statistical Analyses
The incidence of VTE was reported as the proportion of patients who had a documented event during hospitalization, and also as the number of events per 1000 PICC‐days. Baseline characteristics were assessed as potential risk factors. Differences in proportions were tested with the chi square or Fisher exact test, and differences in means of the continuous variables were tested using the t‐test. Univariate analysis of symptomatic VTE by each potential risk factor was performed using logistic regression. Logistic regression models were used to simultaneously assess the relationship between the baseline factors and the probability of developing a thrombotic event. Using a backward elimination modeling strategy, only factors that maintained a P value of <0.05 were retained in the final model. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. SAS version 8.2 (SAS Institute, Inc., Cary, NC) was used for data analysis.
Results
Patient Demographics and Baseline Characteristics
From August 1, 2005 to November 1, 2005, 954 PICCs were inserted in 777 patients. The demographics and baseline characteristics of the 777 patients are outlined in Table 1. History of cancer was present in 20.21% of the patients. History of VTE was present in 7.02% of the patients. The most common reason for PICC insertion was poor access in 90.6% of the PICCs. The most common medication infused through the PICC was antibiotics in 69.2% and intravenous hydration in 50.1%. The basilic vein was accessed in 90%. The tip location, as determined by chest X‐ray at the time of insertion, was the SVC in 85.3%. PICCs were in situ for an average of 9 days and most were 5‐French (Fr) catheters. The average duration of PICC placement was over 9 days, and the average length of stay slightly exceeded 16 days.
| Patient Characteristics | Study Population |
|---|---|
| |
| Age (years), mean (SD); range | 60.8 (17.8); range, 1999 |
| Gender, n (%) | |
| Women | 465 (59.8) |
| Ethnicity, n (%) | |
| White | 223 (28.6) |
| African American | 543 (69.9) |
| Hispanic | 4 (0.5) |
| Weight (kg), mean (SD); range | 81.0 (25.8); range, 30207 |
| History of cancer, n (%) | 157 (20.21) |
| History of venous thromboembolism, n (%) | 55 (7.07) |
| PICC location, n (%) | |
| Right vein | 538 (69.2) |
| Vein accessed, n (%) | |
| Basilic | 695 (90.0) |
| Cephalic | 39 (5.1) |
| Median | 38 (4.9) |
| PICC reason, n (%)* | |
| Poor access | 704 (90.6) |
| Antibiotic iv. | 377 (69.2) |
| Hydration iv. | 389 (50.1) |
| Irritant drug | 19 (2.5) |
| TPN | 47 (6.1) |
| Chemotherapy | 23 (3.0) |
| Pain medication | 22 (2.8) |
| Blood and blood products | 16 (2.1) |
| Other nonblood | 30 (3.9) |
| PICC tip location, n (%) | |
| Central location | 643 (85.3) |
| Noncentral location | 111 (14.7) |
| Catheter lumen, n (%) | |
| 4Fr | 10 (1.3) |
| 5Fr | 758 (97.5) |
| Other | 9 (1.1) |
| Length of stay (days), mean (SD); range | 16.3 (17.2); range, 1224 |
| PICC days; range | 9.6 (9.0); range, 164 |
Outcomes: VTE
During their hospital stay, 38 patients experienced 1 or more VTEs, yielding an incidence of 4.89% (Table 2). A total of 7444 PICC‐days were recorded for 777 patients. This yields a rate of 5.10 VTEs/1000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 3.65 UEDVT/1000 PICC days. The mean length of stay was 26 days in those with VTE versus 15.8 days in those who did not develop VTE (P < 0.001). Average PICC‐days were also longer in those who developed VTE compared to those who did not (13 days vs. 9; P < 0.001).
| Outcome | n | % |
|---|---|---|
| ||
| Total VTE | 38* | 4.89 |
| Upper extremity thromboses | 31 | 3.99 |
| UEDVT | 27 | 3.47 |
| Superficial upper extremity thrombosis | 4 | 0.51 |
| PE | 8 | 1.03 |
VTE prophylaxis (using enoxaparin or heparin) was administered to 26% of patients from the time of PICC insertion until UEDVT occurred. Only 12.5% of those who developed PE were given VTE prophylaxis. All patients who developed a UEDVT or PE were treated with full anticoagulation except those with contraindications and patients with superficial upper extremity thrombosis. Five of the patients with UEDVT and 2 of the patients with PE died during their hospitalization.
Four of the 8 patients with PICC‐associated PE had bilateral lower extremity ultrasounds performed, and 3 of these were negative. The other 4 patients did not have ultrasounds performed.
Risk Factors
History of VTE was the strongest risk factor for VTE in univariate analysis, patients with a history of VTE being 10 times more likely to develop a PICC‐related VTE event (Table 3). PICC tip location was strongly associated with VTE. A noncentral location of the tip at the time of insertion was associated with a 2.34 (95% CI, 1.15‐4.75) higher risk of developing VTE during the hospitalization stay, compared to a central location (SVC or RA). The duration of PICC use was also associated with an increased risk of developing VTE (OR for a 10‐day increase in duration, 1.40 (95% CI, 1.07‐1.84), as it was the length of hospital stay (OR for a 10‐day increase in duration, 1.18 (95% CI, 1.05‐1.33). The duration of hospital stay was correlated with the duration of PICC use; however, its temporal relationship with a VTE was uncertain, as a prolonged hospital stay could be the consequence of a VTE event in some cases. In the multivariate analysis, history of VTE, PICC tip location, and length of stay retained statistical significance (Table 4); while our data are suggestive for an association with VTE events, the duration of PICC use did not maintain statistical significance, in the presence of PICC tip location. History of VTE remained the strongest risk factor for subsequent VTE (OR, 10.83; 95% CI, 4.89‐23.95); the location of the PICC tip was location highly associated with subsequent VTE (OR, 2.61; 95% CI, 1.28‐5.35).
| Characteristic | Univariate Model OR (95% CI) |
|---|---|
| |
| Age | 1.01 (0.991.03) |
| Gender (male versus female) | 1.06 (0.562.01) |
| Race (nonwhite versus white) | 1.07 (0.532.18) |
| History of cancer | 1.72 (0.823.61) |
| History of VTE | 10.36 (4.8122.34) |
| PICC location: right versus left vein | 1.62 (0.763.44) |
| PICC location | |
| Cephalic | Referent |
| Basilic | 2.29 (0.3117.07) |
| Median | 4.91 (0.5345.97) |
| PICC tip location: (noncentral versus central)* | 2.43 (1.154.75) |
| PICC days (10 days unit) | 1.40 (1.071.84) |
| Length of stay (10 days unit) | 1.18 (1.051.33) |
| Characteristic | Multivariate Model OR (95% CI) |
|---|---|
| |
| History of VTE | 10.83 (4.8923.95) |
| PICC tip location (noncentral versus central)* | 2.61 (1.285.35) |
| Length of stay (10 days unit) | 1.21 (1.071.37) |
Discussion
Overall Findings
Our study is the first to examine both UEDVT and PE rates in hospitalized adult patients with PICC lines. We found that 4.89% of our patients experienced a thromboembolic complication during their hospitalization stay, yielding a rate of 5.1 VTE/1,000 PICC‐days and 3.63 UEDVT/1,000 PICC‐days. These figures are similar to or higher than those reported by other retrospective studies using symptom‐driven ultrasound diagnosis of UEDVT, reporting incidence figures ranging from 2 per 100 patients,3 to 2.47 per 100 patients,4 to 3.7 per 100 patients.5
There are several factors to consider when comparing our findings to previously published studies. First, our study population differs by being older and by including hospitalized patients with both acute and chronic medical illnesses, and with a wider spectrum of PICC indications. Second, the duration of PICC use is known to be associated with the development of symptomatic UEDVT.6 Several published studies do not report the duration of PICC use, thus making the comparison of incidence difficult to interpret. Third, the duration of patient follow‐up influences the likelihood that a symptomatic UEDVT is diagnosed; patient follow‐up time is oftentimes unspecified in some studies, thus making comparisons difficult. In contrast, our study reports the incidence of VTE during a clearly specified time window; ie, hospital stay.
Several studies that reported the incidence per 100 PICCs found incidences ranging from 3.9 per 100 PICCs7 and 4.66 per 100 PICCs.8 Similarly, studies of different patient populations and the use of systematic diagnostic and follow‐up methods have found very high rates of thrombosis (15.4% in a randomized clinical trial [RCT] of total parenteral nutrition [TPN],9 38% with systematic venography to diagnose UEDVT,10 and 64.52% in an RCT of intensive care unit [ICU] patients11).
PE
In the largest study to date of 2063 patients who received a PICC for intravenous antibiotic therapy, it was found that 2.5% developed upper extremity thrombosis; of these, 3.8% also had a positive VQ scan for PE.4 However, this study did not systematically review records for PE in those without upper extremity thrombosis. Our study showed that approximately 1% of those who received a PICC developed a PE during their hospitalization. In 3 of our 8 patients who developed a PE, bilateral lower extremity ultrasounds were negative for PE. None of the subjects who developed PE received ultrasounds of the upper extremities in order to look for deep vein thrombosis (DVT), possibly due to a lack of awareness of PICC‐associated VTE.
Risk Factors
Among the risk factors explored, we found that patients with previous history of a venous thromboembolic event are much more likely to develop a PICC‐related VTE. This is not surprising, as previous VTE has been identified a risk factor.1214 We also found that patients whose PICC was not confirmed to be in the SVC or at the junction of the SVC and the RA, were twice as likely to experience VTE. An increased risk associated with noncentral tip location was reported in 2 previous studies using venography to assess vein condition among infectious disease patients,15 and oncology patients.16 This is biologically plausible, since the smaller diameter vessels may be more conducive to thrombus formation than the SVC or RA. Other factors such as blood flow rate, turbulent flow, and endothelial injury may also play a role. As recognized by the National Association of Vascular Access Networks,17 tip positioning is influenced by catheter length, anthropometric measurements, and anatomical pathways. Central tip location, whenever possible, may be 1 of the few controllable risk factors for UEDVT. Decreasing the hospital‐acquired DVT events has been recognized as an important quality improvement, requiring an increased identification and treatment of high‐risk groups. Such hospital‐based approaches have been suggested as effective.18, 19 Both tip location and history of VTE are identifiable risk factors that may be readily incorporated into specific strategies to decrease hospital‐acquired DVT events.
While previously reported as a risk factor, left‐sided catheter location was not significantly associated with VTE in our study. Our data suggest that the duration of PICC use is associated with the risk of developing a VTE event during hospital stay, yet without reaching statistical significance. In the absence of information regarding the underlying comorbidities and/or reasons for hospitalization, interpretation of PICC line duration remains elusive.
Anticoagulant Prophylaxis
Most of the subjects in our cohort did not receive VTE prophylaxis. The efficacy of anticoagulant prophylaxis for preventing catheter‐associated VTE is controversial. In fact, current guidelines do not support VTE prophylaxis as a means to reduce rates of VTE associated with central venous catheters. Furthermore, recent controlled clinical trials of low‐molecular weight heparins at standard prophylactic doses and low doses of warfarin for VTE prevention in patients with central venous catheters undergoing cancer chemotherapy have not demonstrated reductions in VTE rates.2022 In contrast, a recent meta‐analysis suggests that anticoagulant prophylaxis is effective for preventing all catheter‐associated DVT in patients with central venous catheters, but the effectiveness for preventing symptomatic VTE, including PE, remains uncertain.23
Limitations
The small number of VTE and the lack of control for the underlying disease, concomitant therapy, and anticoagulant prophylaxis limited our ability to identify independent risk factors. Further studies are needed to confirm our finding that 1% of patients who receive a PICC will develop PE.
VTE were identified only when symptoms led to diagnostic testing, so our event rates likely underestimate the true rate of PICC‐associated VTE. It is estimated that subclinical thromboses can be found in 30% to 60% of all central catheters.10, 24 The clinical significance of silent thromboses remains to be established.
Strengths
The Society of Interventional Radiology recommends uniform reporting requirements to assist in study design and outcomes reporting on central venous access devices.25 We adhered to many of these guidelines which may facilitate comparison among studies from different institutions.
Our study is one of the largest studies to asses the rate of thrombosis among inpatients. We used broad eligibility criteria, included consecutive, unselected patients, in a large cohort of patients who were all hospitalized for acute medical illnesses. Furthermore, we used data collection that corroborated data across the entire range of hospital records, and as such we were able to determine accurately the incidence of thrombosis. UEDVT and PE were diagnosed objectively and, unlike previous studies, we also collected information on prophylaxis and treatment of VTE. To our knowledge, our study is the first to document an increased risk of symptomatic UEDVT and PE among acutely ill hospitalized patients who receive PICC.
Prior studies may be less applicable to PICC‐associated VTE in hospitalized patients because the populations included a mix of inpatients and outpatients. Our results may be more applicable to hospitalized patients who receive a PICC because more severely ill patients who are hospitalized may be at higher risk of thrombosis than outpatients. Also, previous studies included some patients who received relatively small, 3‐Fr to 4‐Fr single‐lumen PICCs, whereas the current practice is to insert larger, double‐lumen, 5‐Fr catheters. Today's PICCs may be more likely to result in vessel occlusion, venous stasis, and thrombosis.
In conclusion, we have shown that the incidence of VTE in our study population was 4.89% with a rate of 5.10 VTE/1,000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 365 UEVDVT/1000 PICC days. The most significant predictors of VTE were history of previous VTE and the location of the PICC tip at the time of insertion.
The use of peripherally inserted central catheters (PICCs) to facilitate the administration of intravenous medications and fluids has become commonplace in hospitalized patients. Clinicians often prefer PICCs over other central venous catheters due to their ease of insertion and the perception that PICCs may have lower risks than other central venous catheters. However, recent studies1, 2 have begun to suggest that the benefit derived from these devices can be offset by the development of complications such as upper extremity deep vein thrombosis (UEDVT). Since venous thromboembolism (VTE) in hospitalized patients is associated with increased morbidity, mortality, length of stay, and costs, we sought to determine the rate of VTE in a population of patients who received a PICC solely during their hospital stay.
Methods
Data Collection
This study was a retrospective, electronic chart review of patients who received a PICC while hospitalized between August 1, 2005 and November 1, 2005 at the Methodist University Hospital (MUH), a 652‐bed, urban, university‐affiliated, community hospital in Memphis, TN. Patients were identified through the use of a PICC database that is maintained by the nurses who routinely place the PICCs. The data collected included the date of insertion, the diameter of the catheter, the vein accessed, the position of the catheter tip, and the reason for PICC insertion. These factors as well as demographics were examined to determine whether they were associated with thrombosis. These data were linked with data from the ultrasound laboratory and nuclear medicine/radiology laboratory and with hospital discharge data. Data were recorded by trained research assistants and verified for accuracy by the study investigators. The institutional review board approved the study protocol prior to data collection.
Patients and Outcomes
All adult consecutive patients who had a PICC inserted during the study period and who did not have a UEDVT or pulmonary embolism (PE) at the time of PICC insertion were included in the study.
PICCs were placed using a modified Seldinger technique at the bedside with portable ultrasound guidance. The vessel of choice for insertion was the basilic vein. Confirmation of catheter tip placement in the lower third of the superior vena cava (SVC) was done with chest x‐ray prior to use of the PICC. The PICC manufacturer was Boston Scientific (Vaxcel with PASV; Natick, MA) and normal saline was used for routine flushing of the PICC.
Study Outcomes
Symptomatic UEDVT
A UEDVT was defined as a symptomatic event in the ipsilateral extremity leading to the performance of duplex ultrasonography, which confirmed the diagnosis of UEDVT. Systematic screening for UEDVT was not performed on any patients during the study period. Sonographic diagnosis of UEDVT was based on noncompressibility of a venous segment of the upper arm or the internal jugular vein; absent or reduced flow on Doppler imaging with failure to augment on compression of the arm; or the presence of echogenic material compatible with thrombus in the arm or central venous vasculature on real‐time imaging. Superficial thrombosis was not counted as a UEDVT event.
Symptomatic PE
PE was defined as a symptomatic event prompting the performance of ventilation‐perfusion lung scan or spiral computed tomography (CT). Systematic screening for PE was not done on any patients during the study period. Radiologic diagnosis of PE was not standardized, but intraluminal filling defect of a lobar artery or more proximal pulmonary arterial vasculature on spiral CT, or an abnormal ventilation‐perfusion (V2) scan with a high clinical suspicion for PE must have been noted on the radiology report along with the physician's clinical diagnosis of PE.
Statistical Analyses
The incidence of VTE was reported as the proportion of patients who had a documented event during hospitalization, and also as the number of events per 1000 PICC‐days. Baseline characteristics were assessed as potential risk factors. Differences in proportions were tested with the chi square or Fisher exact test, and differences in means of the continuous variables were tested using the t‐test. Univariate analysis of symptomatic VTE by each potential risk factor was performed using logistic regression. Logistic regression models were used to simultaneously assess the relationship between the baseline factors and the probability of developing a thrombotic event. Using a backward elimination modeling strategy, only factors that maintained a P value of <0.05 were retained in the final model. Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated. SAS version 8.2 (SAS Institute, Inc., Cary, NC) was used for data analysis.
Results
Patient Demographics and Baseline Characteristics
From August 1, 2005 to November 1, 2005, 954 PICCs were inserted in 777 patients. The demographics and baseline characteristics of the 777 patients are outlined in Table 1. History of cancer was present in 20.21% of the patients. History of VTE was present in 7.02% of the patients. The most common reason for PICC insertion was poor access in 90.6% of the PICCs. The most common medication infused through the PICC was antibiotics in 69.2% and intravenous hydration in 50.1%. The basilic vein was accessed in 90%. The tip location, as determined by chest X‐ray at the time of insertion, was the SVC in 85.3%. PICCs were in situ for an average of 9 days and most were 5‐French (Fr) catheters. The average duration of PICC placement was over 9 days, and the average length of stay slightly exceeded 16 days.
| Patient Characteristics | Study Population |
|---|---|
| |
| Age (years), mean (SD); range | 60.8 (17.8); range, 1999 |
| Gender, n (%) | |
| Women | 465 (59.8) |
| Ethnicity, n (%) | |
| White | 223 (28.6) |
| African American | 543 (69.9) |
| Hispanic | 4 (0.5) |
| Weight (kg), mean (SD); range | 81.0 (25.8); range, 30207 |
| History of cancer, n (%) | 157 (20.21) |
| History of venous thromboembolism, n (%) | 55 (7.07) |
| PICC location, n (%) | |
| Right vein | 538 (69.2) |
| Vein accessed, n (%) | |
| Basilic | 695 (90.0) |
| Cephalic | 39 (5.1) |
| Median | 38 (4.9) |
| PICC reason, n (%)* | |
| Poor access | 704 (90.6) |
| Antibiotic iv. | 377 (69.2) |
| Hydration iv. | 389 (50.1) |
| Irritant drug | 19 (2.5) |
| TPN | 47 (6.1) |
| Chemotherapy | 23 (3.0) |
| Pain medication | 22 (2.8) |
| Blood and blood products | 16 (2.1) |
| Other nonblood | 30 (3.9) |
| PICC tip location, n (%) | |
| Central location | 643 (85.3) |
| Noncentral location | 111 (14.7) |
| Catheter lumen, n (%) | |
| 4Fr | 10 (1.3) |
| 5Fr | 758 (97.5) |
| Other | 9 (1.1) |
| Length of stay (days), mean (SD); range | 16.3 (17.2); range, 1224 |
| PICC days; range | 9.6 (9.0); range, 164 |
Outcomes: VTE
During their hospital stay, 38 patients experienced 1 or more VTEs, yielding an incidence of 4.89% (Table 2). A total of 7444 PICC‐days were recorded for 777 patients. This yields a rate of 5.10 VTEs/1000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 3.65 UEDVT/1000 PICC days. The mean length of stay was 26 days in those with VTE versus 15.8 days in those who did not develop VTE (P < 0.001). Average PICC‐days were also longer in those who developed VTE compared to those who did not (13 days vs. 9; P < 0.001).
| Outcome | n | % |
|---|---|---|
| ||
| Total VTE | 38* | 4.89 |
| Upper extremity thromboses | 31 | 3.99 |
| UEDVT | 27 | 3.47 |
| Superficial upper extremity thrombosis | 4 | 0.51 |
| PE | 8 | 1.03 |
VTE prophylaxis (using enoxaparin or heparin) was administered to 26% of patients from the time of PICC insertion until UEDVT occurred. Only 12.5% of those who developed PE were given VTE prophylaxis. All patients who developed a UEDVT or PE were treated with full anticoagulation except those with contraindications and patients with superficial upper extremity thrombosis. Five of the patients with UEDVT and 2 of the patients with PE died during their hospitalization.
Four of the 8 patients with PICC‐associated PE had bilateral lower extremity ultrasounds performed, and 3 of these were negative. The other 4 patients did not have ultrasounds performed.
Risk Factors
History of VTE was the strongest risk factor for VTE in univariate analysis, patients with a history of VTE being 10 times more likely to develop a PICC‐related VTE event (Table 3). PICC tip location was strongly associated with VTE. A noncentral location of the tip at the time of insertion was associated with a 2.34 (95% CI, 1.15‐4.75) higher risk of developing VTE during the hospitalization stay, compared to a central location (SVC or RA). The duration of PICC use was also associated with an increased risk of developing VTE (OR for a 10‐day increase in duration, 1.40 (95% CI, 1.07‐1.84), as it was the length of hospital stay (OR for a 10‐day increase in duration, 1.18 (95% CI, 1.05‐1.33). The duration of hospital stay was correlated with the duration of PICC use; however, its temporal relationship with a VTE was uncertain, as a prolonged hospital stay could be the consequence of a VTE event in some cases. In the multivariate analysis, history of VTE, PICC tip location, and length of stay retained statistical significance (Table 4); while our data are suggestive for an association with VTE events, the duration of PICC use did not maintain statistical significance, in the presence of PICC tip location. History of VTE remained the strongest risk factor for subsequent VTE (OR, 10.83; 95% CI, 4.89‐23.95); the location of the PICC tip was location highly associated with subsequent VTE (OR, 2.61; 95% CI, 1.28‐5.35).
| Characteristic | Univariate Model OR (95% CI) |
|---|---|
| |
| Age | 1.01 (0.991.03) |
| Gender (male versus female) | 1.06 (0.562.01) |
| Race (nonwhite versus white) | 1.07 (0.532.18) |
| History of cancer | 1.72 (0.823.61) |
| History of VTE | 10.36 (4.8122.34) |
| PICC location: right versus left vein | 1.62 (0.763.44) |
| PICC location | |
| Cephalic | Referent |
| Basilic | 2.29 (0.3117.07) |
| Median | 4.91 (0.5345.97) |
| PICC tip location: (noncentral versus central)* | 2.43 (1.154.75) |
| PICC days (10 days unit) | 1.40 (1.071.84) |
| Length of stay (10 days unit) | 1.18 (1.051.33) |
| Characteristic | Multivariate Model OR (95% CI) |
|---|---|
| |
| History of VTE | 10.83 (4.8923.95) |
| PICC tip location (noncentral versus central)* | 2.61 (1.285.35) |
| Length of stay (10 days unit) | 1.21 (1.071.37) |
Discussion
Overall Findings
Our study is the first to examine both UEDVT and PE rates in hospitalized adult patients with PICC lines. We found that 4.89% of our patients experienced a thromboembolic complication during their hospitalization stay, yielding a rate of 5.1 VTE/1,000 PICC‐days and 3.63 UEDVT/1,000 PICC‐days. These figures are similar to or higher than those reported by other retrospective studies using symptom‐driven ultrasound diagnosis of UEDVT, reporting incidence figures ranging from 2 per 100 patients,3 to 2.47 per 100 patients,4 to 3.7 per 100 patients.5
There are several factors to consider when comparing our findings to previously published studies. First, our study population differs by being older and by including hospitalized patients with both acute and chronic medical illnesses, and with a wider spectrum of PICC indications. Second, the duration of PICC use is known to be associated with the development of symptomatic UEDVT.6 Several published studies do not report the duration of PICC use, thus making the comparison of incidence difficult to interpret. Third, the duration of patient follow‐up influences the likelihood that a symptomatic UEDVT is diagnosed; patient follow‐up time is oftentimes unspecified in some studies, thus making comparisons difficult. In contrast, our study reports the incidence of VTE during a clearly specified time window; ie, hospital stay.
Several studies that reported the incidence per 100 PICCs found incidences ranging from 3.9 per 100 PICCs7 and 4.66 per 100 PICCs.8 Similarly, studies of different patient populations and the use of systematic diagnostic and follow‐up methods have found very high rates of thrombosis (15.4% in a randomized clinical trial [RCT] of total parenteral nutrition [TPN],9 38% with systematic venography to diagnose UEDVT,10 and 64.52% in an RCT of intensive care unit [ICU] patients11).
PE
In the largest study to date of 2063 patients who received a PICC for intravenous antibiotic therapy, it was found that 2.5% developed upper extremity thrombosis; of these, 3.8% also had a positive VQ scan for PE.4 However, this study did not systematically review records for PE in those without upper extremity thrombosis. Our study showed that approximately 1% of those who received a PICC developed a PE during their hospitalization. In 3 of our 8 patients who developed a PE, bilateral lower extremity ultrasounds were negative for PE. None of the subjects who developed PE received ultrasounds of the upper extremities in order to look for deep vein thrombosis (DVT), possibly due to a lack of awareness of PICC‐associated VTE.
Risk Factors
Among the risk factors explored, we found that patients with previous history of a venous thromboembolic event are much more likely to develop a PICC‐related VTE. This is not surprising, as previous VTE has been identified a risk factor.1214 We also found that patients whose PICC was not confirmed to be in the SVC or at the junction of the SVC and the RA, were twice as likely to experience VTE. An increased risk associated with noncentral tip location was reported in 2 previous studies using venography to assess vein condition among infectious disease patients,15 and oncology patients.16 This is biologically plausible, since the smaller diameter vessels may be more conducive to thrombus formation than the SVC or RA. Other factors such as blood flow rate, turbulent flow, and endothelial injury may also play a role. As recognized by the National Association of Vascular Access Networks,17 tip positioning is influenced by catheter length, anthropometric measurements, and anatomical pathways. Central tip location, whenever possible, may be 1 of the few controllable risk factors for UEDVT. Decreasing the hospital‐acquired DVT events has been recognized as an important quality improvement, requiring an increased identification and treatment of high‐risk groups. Such hospital‐based approaches have been suggested as effective.18, 19 Both tip location and history of VTE are identifiable risk factors that may be readily incorporated into specific strategies to decrease hospital‐acquired DVT events.
While previously reported as a risk factor, left‐sided catheter location was not significantly associated with VTE in our study. Our data suggest that the duration of PICC use is associated with the risk of developing a VTE event during hospital stay, yet without reaching statistical significance. In the absence of information regarding the underlying comorbidities and/or reasons for hospitalization, interpretation of PICC line duration remains elusive.
Anticoagulant Prophylaxis
Most of the subjects in our cohort did not receive VTE prophylaxis. The efficacy of anticoagulant prophylaxis for preventing catheter‐associated VTE is controversial. In fact, current guidelines do not support VTE prophylaxis as a means to reduce rates of VTE associated with central venous catheters. Furthermore, recent controlled clinical trials of low‐molecular weight heparins at standard prophylactic doses and low doses of warfarin for VTE prevention in patients with central venous catheters undergoing cancer chemotherapy have not demonstrated reductions in VTE rates.2022 In contrast, a recent meta‐analysis suggests that anticoagulant prophylaxis is effective for preventing all catheter‐associated DVT in patients with central venous catheters, but the effectiveness for preventing symptomatic VTE, including PE, remains uncertain.23
Limitations
The small number of VTE and the lack of control for the underlying disease, concomitant therapy, and anticoagulant prophylaxis limited our ability to identify independent risk factors. Further studies are needed to confirm our finding that 1% of patients who receive a PICC will develop PE.
VTE were identified only when symptoms led to diagnostic testing, so our event rates likely underestimate the true rate of PICC‐associated VTE. It is estimated that subclinical thromboses can be found in 30% to 60% of all central catheters.10, 24 The clinical significance of silent thromboses remains to be established.
Strengths
The Society of Interventional Radiology recommends uniform reporting requirements to assist in study design and outcomes reporting on central venous access devices.25 We adhered to many of these guidelines which may facilitate comparison among studies from different institutions.
Our study is one of the largest studies to asses the rate of thrombosis among inpatients. We used broad eligibility criteria, included consecutive, unselected patients, in a large cohort of patients who were all hospitalized for acute medical illnesses. Furthermore, we used data collection that corroborated data across the entire range of hospital records, and as such we were able to determine accurately the incidence of thrombosis. UEDVT and PE were diagnosed objectively and, unlike previous studies, we also collected information on prophylaxis and treatment of VTE. To our knowledge, our study is the first to document an increased risk of symptomatic UEDVT and PE among acutely ill hospitalized patients who receive PICC.
Prior studies may be less applicable to PICC‐associated VTE in hospitalized patients because the populations included a mix of inpatients and outpatients. Our results may be more applicable to hospitalized patients who receive a PICC because more severely ill patients who are hospitalized may be at higher risk of thrombosis than outpatients. Also, previous studies included some patients who received relatively small, 3‐Fr to 4‐Fr single‐lumen PICCs, whereas the current practice is to insert larger, double‐lumen, 5‐Fr catheters. Today's PICCs may be more likely to result in vessel occlusion, venous stasis, and thrombosis.
In conclusion, we have shown that the incidence of VTE in our study population was 4.89% with a rate of 5.10 VTE/1,000 PICC‐days. There were 27 patients who had a UEDVT over the 7444 PICC days yielding a rate of 365 UEVDVT/1000 PICC days. The most significant predictors of VTE were history of previous VTE and the location of the PICC tip at the time of insertion.
- ,,,.High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours.Intern Med J.2004;34:234–238.
- ,,.Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward.World J Surg.2006;30:1605–1619.
- ,,,,.Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients.South Med J.2006;99:1073–1077.
- ,,, et al.Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience.Clin Infect Dis.2002;34:1179–1183.
- ,,.Peripherally inserted central catheters in the intensive care unit.J Intensive Care Med.1996;11:49–54.
- ,,,,,.The incidence of upper extremity deep venous thrombosis associated with peripherally inserted central catheters in an intensive care unit. [Abstract]. Chest 2003: 69th Annual Meeting of the American College of Chest Physicians; October 25–30, 2003, Orlando, FL;2003.
- ,.Venous thrombosis related to peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:837–840.
- ,,,.Peripherally inserted central catheters: outcome as a function of the operator.J Vasc Interv Radiol.2001;12:723–729.
- ,,,,,.Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally‐inserted central catheters.Clin Nutr.2000;19:237–243.
- ,,, et al.Venous thrombosis associated with the placement of peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:1309–1314.
- ,,,,,.Peripherally inserted central catheter (PICC) associated upper extremity deep venous thrombosis (UEDVT) in critical care setting (abstract). CHEST 2005: 71st Annual Meeting of the American College of Chest Physicians. October 29, 2005 to November 3, 2005, Montreal, Quebec, Canada.
- ,,,.A prospective study of the incidence of deep‐vein thrombosis within a defined urban population.J Intern Med.1992;232:155–160.
- ,,, et al.Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications.Arch Intern Med.1997;157:57–62.
- .Risk factors for venous thrombotic disease.Thromb Haemost.1999;82:610–619.
- ,,.Complications of long arm‐catheters: a randomized trial of central vs peripheral tip location.JPEN J Parenter Enteral Nutr.1996;20:20–24.
- ,,.Tunneled catheter thrombosis: factors related to incidence.Oncol Nurs Forum.1990;17:543–549.
- National Association of Vascular Access Networks.Tip location of peripherally inserted central catheters. NVAS position statement.J Vasc Access Devices.1998;3(2):8–10.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3:148–155.
- ,,, et al.Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double‐blind, placebo‐controlled, randomized study in cancer patients.J Clin Oncol.2005;23:4057–4062.
- ,,, et al.Dalteparin for prevention of catheter‐related complications in cancer patients with central venous catheters: final results of a double‐blind, placebo‐controlled phase III trial.Ann Oncol.2006;17:289–296.
- ,,, et al.Randomized placebo‐controlled study of low‐dose warfarin for the prevention of central venous catheter‐associated thrombosis in patients with cancer.J Clin Oncol.2005;23:4063–4069.
- ,,,.Prevention of central venous catheter‐associated thrombosis: a meta‐analysis.Am J Med.2007;120:901e1–e13.
- .Deep venous thrombosis of the upper extremity.Am Fam Physician.1997;55:533–539.
- ,,,.Reporting standards for central venous access.J Vasc Interv Radiol.2003;14:S443–S452.
- ,,,.High rate of complications associated with peripherally inserted central venous catheters in patients with solid tumours.Intern Med J.2004;34:234–238.
- ,,.Peripherally inserted central venous catheters are not superior to central venous catheters in the acute care of surgical patients on the ward.World J Surg.2006;30:1605–1619.
- ,,,,.Peripherally inserted central venous catheter‐associated thrombosis: retrospective analysis of clinical risk factors in adult patients.South Med J.2006;99:1073–1077.
- ,,, et al.Venous thrombosis associated with peripherally inserted central catheters: a retrospective analysis of the Cleveland Clinic experience.Clin Infect Dis.2002;34:1179–1183.
- ,,.Peripherally inserted central catheters in the intensive care unit.J Intensive Care Med.1996;11:49–54.
- ,,,,,.The incidence of upper extremity deep venous thrombosis associated with peripherally inserted central catheters in an intensive care unit. [Abstract]. Chest 2003: 69th Annual Meeting of the American College of Chest Physicians; October 25–30, 2003, Orlando, FL;2003.
- ,.Venous thrombosis related to peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:837–840.
- ,,,.Peripherally inserted central catheters: outcome as a function of the operator.J Vasc Interv Radiol.2001;12:723–729.
- ,,,,,.Complications and cost associated with parenteral nutrition delivered to hospitalized patients through either subclavian or peripherally‐inserted central catheters.Clin Nutr.2000;19:237–243.
- ,,, et al.Venous thrombosis associated with the placement of peripherally inserted central catheters.J Vasc Interv Radiol.2000;11:1309–1314.
- ,,,,,.Peripherally inserted central catheter (PICC) associated upper extremity deep venous thrombosis (UEDVT) in critical care setting (abstract). CHEST 2005: 71st Annual Meeting of the American College of Chest Physicians. October 29, 2005 to November 3, 2005, Montreal, Quebec, Canada.
- ,,,.A prospective study of the incidence of deep‐vein thrombosis within a defined urban population.J Intern Med.1992;232:155–160.
- ,,, et al.Upper‐extremity deep vein thrombosis. Risk factors, diagnosis, and complications.Arch Intern Med.1997;157:57–62.
- .Risk factors for venous thrombotic disease.Thromb Haemost.1999;82:610–619.
- ,,.Complications of long arm‐catheters: a randomized trial of central vs peripheral tip location.JPEN J Parenter Enteral Nutr.1996;20:20–24.
- ,,.Tunneled catheter thrombosis: factors related to incidence.Oncol Nurs Forum.1990;17:543–549.
- National Association of Vascular Access Networks.Tip location of peripherally inserted central catheters. NVAS position statement.J Vasc Access Devices.1998;3(2):8–10.
- ,.Improving safety with information technology.N Engl J Med.2003;348:2526–2534.
- ,,.Innovative approaches to increase deep vein thrombosis prophylaxis rate resulting in a decrease in hospital‐acquired deep vein thrombosis at a tertiary‐care teaching hospital.J Hosp Med.2008;3:148–155.
- ,,, et al.Enoxaparin for the prevention of venous thromboembolism associated with central vein catheter: a double‐blind, placebo‐controlled, randomized study in cancer patients.J Clin Oncol.2005;23:4057–4062.
- ,,, et al.Dalteparin for prevention of catheter‐related complications in cancer patients with central venous catheters: final results of a double‐blind, placebo‐controlled phase III trial.Ann Oncol.2006;17:289–296.
- ,,, et al.Randomized placebo‐controlled study of low‐dose warfarin for the prevention of central venous catheter‐associated thrombosis in patients with cancer.J Clin Oncol.2005;23:4063–4069.
- ,,,.Prevention of central venous catheter‐associated thrombosis: a meta‐analysis.Am J Med.2007;120:901e1–e13.
- .Deep venous thrombosis of the upper extremity.Am Fam Physician.1997;55:533–539.
- ,,,.Reporting standards for central venous access.J Vasc Interv Radiol.2003;14:S443–S452.
Copyright © 2009 Society of Hospital Medicine
Retrievable Vena Cava Filters
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21
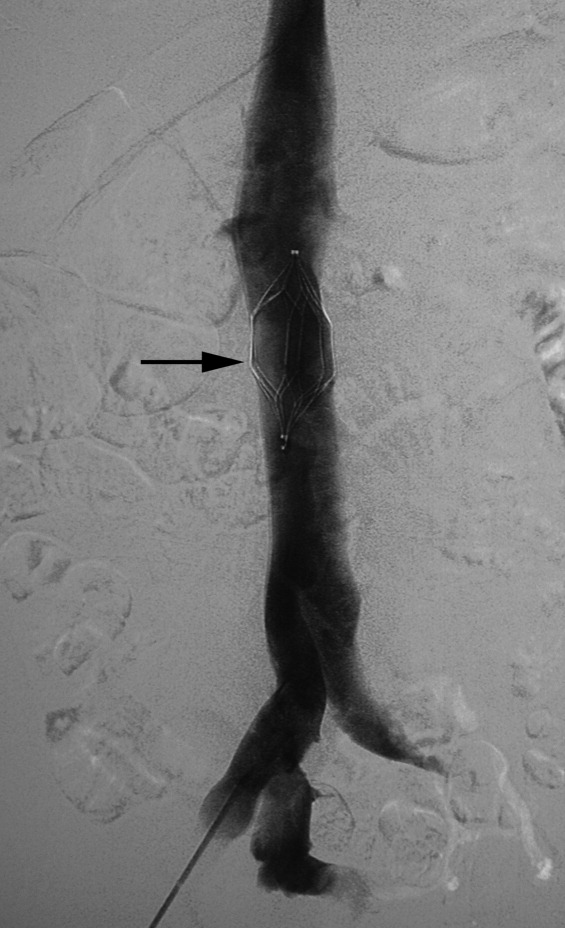
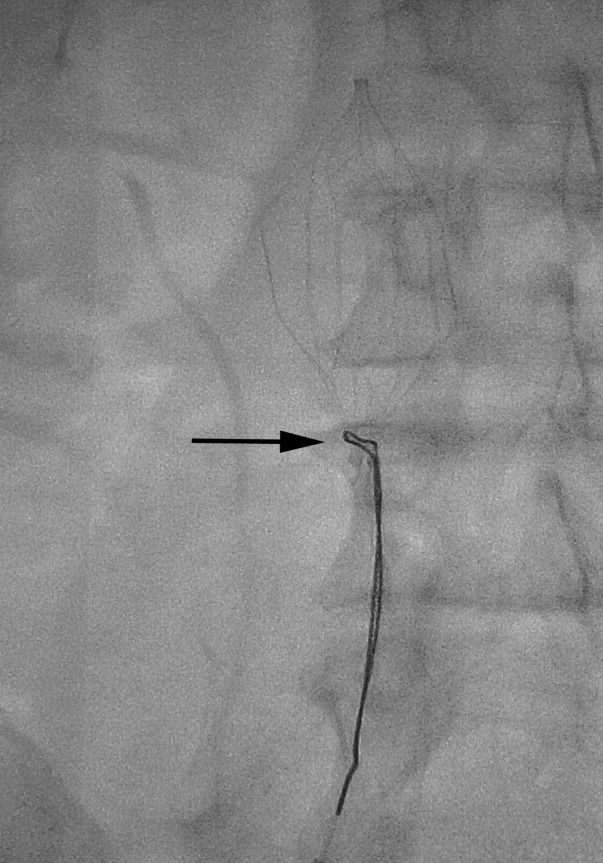
| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure <90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34
VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21


| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure <90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34

VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
Vena cava filters were introduced in the 1960s as a mechanical means to prevent pulmonary embolism (PE).1 Since that time, the number of filters placed has grown steadily, to over 49,000 annually in the United States alone.2 However, patients with vena cava filters can develop complications from the filter itself, which can lead to significant morbidity and, rarely, mortality. In particular, the interruption of venous flow caused by the filter can precipitate lower extremity deep vein thrombosis (DVT),3 as well as vena caval thrombosis involving the filter itself. This has led some experts to recommend indefinite anticoagulation in patients with vena caval filters,4, 5 potentially exposing many patients to the risks of anticoagulation. Given these long‐term safety concerns, there has been recent enthusiasm for the development of optional filters. Optional vena cava filters can be classified into 2 types: temporary and retrievable. Temporary filters, which are not currently available in the United States, are held in place by a tether or catheter5 and cannot be used as permanent devices. Retrievable filters, on the other hand, maintain their position by hooks, radial pressure, or barbs and can either be removed within a prescribed time period after placement or remain in place permanently. In this way, optional filters offer the possibility of avoiding long‐term filter complications in patients with temporary contraindications to anticoagulation. Not surprisingly, the use of retrievable filters has increased dramatically, with many filters being placed for prophylactic indications in patients without known venous thromboembolism (VTE).6 In this work we review the different types of retrievable vena cava filters, current indications for placement, complications, and areas for future research.
Filter Design and Efficacy
Currently, there are 5 U.S. Food and Drug Administration (FDA)‐approved filters in the United States that can be used as retrievable filters: ALN (ALN Implants Chirurgicaux, Ghisonaccia, France); Celect (Cook Medical Incorporated, Bloomington, IN); Gunther‐Tulip (Cook Medical Incorporated, Bloomington, IN); G2 (Bard Peripheral Vascular, Tempe, AZ); and OptEase (Cordis Corporation, Miami Lakes, FL) (Table 1). Three more devices are in U.S. clinical trials: SafeFlo (Rafael Medical Technologies, Hasselt, Belgium); Crux (Crux Biomedical, Portola Valley, CA); and Option (Rex Medical, Conshohocken, PA). Filters are constructed from magnetic resonance imaging (MRI)‐compatible, nonferromagnetic alloys and are produced in either a hexagonal or conical shape. There are potential advantages and disadvantages to both designs. A hexagonal design is thought to be better for trapping small thrombi, but conical filters may have a decreased propensity toward thrombosis.7 When a hexagonal filter becomes partially occluded in vitro, flow disturbances can lead to turbulence, stasis, and progressive clot formation.7 Some clinical studies have demonstrated an increased incidence of thrombosis with hexagonal filters,8 but further investigation is needed to determine if a true correlation exists. Comparisons of the 2 types of filter design are limited but have shown no difference in their efficacy in the prevention of PE.9 Therefore, filter choice is usually dependent upon the physician performing the procedure, although other factors, such as caval size, clot extent, available venous access, and route of retrieval also may affect this decision. Furthermore, retrospective reviews have shown no difference in efficacy between retrievable and permanent filters.10
| Filter | Image | Insertion Site | Retrieval Site | Maximum Successful Documented Dwell Time |
|---|---|---|---|---|
| Gunther‐Tulip (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 204 days42 | |
| Optease (photo courtesy of Cordis Corporation, Miami Lakes, FL) | Femoral or jugular | Femoral | 48 days43 | |
| ALN (photo courtesy of ALN Implants Chirurgicaux, Ghisonaccia, France) | Femoral, jugular, or brachial | Jugular | 352 days44 | |
| Celect (photo courtesy of Cook Medical Incorporated, Bloomington, IN) | Femoral or jugular | Jugular | 357 days45 | |
| G2 (photo courtesy of Bard Peripheral Vascular, Tempe, AZ) | Femoral or jugular | Jugular | 300 days46 |
Insertion of filters is typically performed under fluoroscopy in the operating room or interventional radiology suite. Placement can also occur at the bedside using intravascular ultrasound. This option is particularly useful for critically ill patients who are not stable enough to leave the intensive care unit (ICU) for insertion. The safety of this approach has been documented for both retrievable and permanent filters.11, 12 Duplex ultrasonography has been used to allow bedside placement of permanent filters, but published experience with this modality in placement of retrievable filters is lacking.13, 14
There are no set time limits for retrieving filters, although the retrieval success rate decreases as the time postplacement increases. Rather, the decision to remove them is based on the clinical situation. Table 1 shows data on some of the longest documented successful dwell times for the various retrievable filters. Prior to filter retrieval, a venogram is performed to ensure that there is no clot in the inferior vena cava (IVC) or common iliac veins (Figure 1). Removal of a retrievable filter involves snaring one end of the filter with a hook and then slipping a sheath over the filter, which retracts the filter from the vessel wall as it is being pulled into the sheath (Figure 2). Retrieval rates from various studies are listed in Table 2. Common reasons for nonretrieval include loss to follow up,15 ongoing contraindications to anticoagulation,11, 1618 presence of large thrombi in the filter,16, 1820 poor patient prognosis,16, 18 unrelated death,1618 and filter tilting or embedment.19, 21


| Study | Total Number of Patients | Study Type | Filter Type | Follow‐Up Duration (months) | PE [number (%)] | IVC Thrombosis [number (%)] | DVT [number (%)] | Retrieval Attempted/ Successful Retrieval [number (%)] | Mean Duration Between Filter Placement and Retrieval (days) |
|---|---|---|---|---|---|---|---|---|---|
| |||||||||
| Millward et al., 200116 | 90 | RO/PO | G | 3.4 | 0 | 1/39 (2.6) | 1/39 (2.6) | 53 (59)/52 (98) | 9 |
| de Gregorio et al., 200319 | 87 | RO | G | N/R | 0 | 0 | 0 | 69 (79)/68 (99) | 13 |
| Wicky et al., 200317 | 71 | RO | G | 30 | 0 | 0 | 0 | 47 (66)/33 (70) | 8.2 |
| Rosenthal et al., 200411 | 94 | PO | O | N/R | 0 | 0 | 1 (1.1) | 34 (36)/31 (91) | 19 |
| Grande et al., 200515 | 106 | RO | R | N/R | 3 (2.8) | 0 | 0 | 15 (14)/14 (93) | 150 |
| Oliva et al., 200547 | 27 | PO | O | N/R | 0 | 0 | 1/27 (3.7) | 21 (78)/21 (100) | 11.1 |
| Hoppe et al., 200618 | 41 | PO | G | 3 | 1 (2.4) | 1 (2.4) | 1 (2.4) | 23 (57)/23 (100) | 11.1 |
| Kalva et al., 200648 | 96 | RO | R | 5.3 | 1 (1.0) | 0 | 10/53 (18) | 11 (12)/9 (82) | 117 |
| Meier et al., 200635 | 37 | PO | O | 5 | 0 | 1/5 (20) | 1/5 (20) | 32 (86)/32 (100) | 16 |
| Ray et al., 200649 | 197 | RO | G, R | N/R | 1 (0.5)‐G | 2 (1.0)‐G | 0 | 94 (48)/80 (85) | 11 (G)/28 (R) |
| Rosenthal et al., 200650 | 127 | RO | G, R, O | N/R | 0 | 0 | 0 | 70 (52)/66 (94) | 71 |
| Looby et al., 200721 | 147 | RO | G | N/R | 1 (0.7) | 0 | 0 | 45 (31)/36 (80) | 33.6 |
| Yamagami et al., 200751 | 86 | RO | G | N/R | 0 | N/R | N/R | 80 (93)/77 (96) | 13.4 |
| Kim et al, 200852 | 427 | RO | G, P, R, G2 | 10.4 | 20 (4.7) | 2 (0.5) | 54 (12.6) | 60 (15.5)/46 (69.7) | 20.4 |
Indications for Filter Placement
Patients with Known VTE
Suggested indications for the use of vena cava filters in patients with proven VTE are listed in Table 3. For patients at risk for either recurrent or severe bleeding (eg, multiple falls, recurrent gastrointestinal or intracranial hemorrhage) or most patients who have failed treatment with therapeutic anticoagulation, a permanent filter is usually the preferred mechanical option. However, for certain conditions (such as Trousseau's syndrome, heparin‐induced thrombocytopenia, antiphospholipid syndrome, or anatomic abnormalities such as thoracic outlet syndrome‐Paget‐von Schroetter syndrome, or May‐Thurner syndrome‐iliac vein compression syndrome), vena cava filters have been shown either to be ineffective or to worsen thrombosis. In these cases, alternative therapies must be used, based on the underlying disorder and the clinical situation.
| Anticipated Transient Need for Anticoagulation | Anticipated Long‐Term Need for Anticoagulation* | |
|---|---|---|
| ||
| Transient bleeding risk in a patient at high risk for recurrent thromboembolism | Retrievable filter appropriate | Retrievable filter appropriate |
| Permanent, or likely recurrent, bleeding risk | Retrievable filter with extended dwell time | Permanent filter appropriate |
| No unusual bleeding risk | No filter indicated | No filter indicated |
A retrievable filter should only be considered in patients who have a transient contraindication to anticoagulation (Table 5). Such contraindications include isolated but treatable episodes of hemorrhage, urgent surgeries, or procedures associated with a high risk of bleeding, and trauma. The risk of recurrent VTE in the absence of anticoagulation has been estimated at 40% in the first month after VTE and then 10% during the second and third months.22 Therefore, it is reasonable to place a retrievable filter in perioperative patients who cannot be treated with therapeutic anticoagulation during the first 30 days after an acute VTE. If more than 30 days have passed since the thrombotic event, a filter is probably not necessary for patients who will have temporary interruptions in anticoagulation therapy. Instead, bridging anticoagulation (eg, unfractionated heparin [UFH] or low molecular weight heparin [LMWH]) can be given while warfarin is being held prior to surgery. Then, the patient can be transitioned back to warfarin therapy with prophylactic and then therapeutic LMWH or UFH in the postoperative period.
|
| Recurrent VTE |
| Idiopathic VTE |
| Near‐fatal thrombosis |
| Thrombosis at an unusual site (eg, mesenteric vein) |
| VTE in high‐risk thrombophilic disorders: |
| Antiphospholipid antibody syndrome |
| Protein C or S deficiency |
| Antithrombin III deficiency |
| Heterozygous mutations for both the Factor V Leiden and the Prothrombin gene mutation (compound heterozygosity) |
| Homozygous Factor V Leiden mutation |
| Cancer‐associated VTE |
| Major trauma |
| Peripartum |
| Isolated and treatable causes of hemorrhage (eg, peptic ulcer) |
| Bleeding complications after procedures or surgeries53 |
| Liver or kidney biopsy |
| Urgent surgery associated with a high bleeding risk54 |
| Cardiac (coronary artery bypass or valve replacement) |
| Vascular (aortic aneurysm repair, peripheral artery bypass) |
| Neurosurgical (intracranial or spinal) |
| Urologic (prostate and bladder) |
| Major cancer surgery |
Controversy remains regarding the use of retrievable filters in patients with calf vein DVT. It also exists for patients with massive or submassive PE who are receiving anticoagulation therapy but are at high risk for poor outcomes should another PEeven if smalloccur while they are on anticoagulation therapy. Vena cava filters are generally not recommended for patients with distal VTE unless they have a persistent contraindication to anticoagulation therapy and have shown clot propagation on serial duplex studies. At least 1 institution, however, has noted an increased use of filter placement in this population since the advent of retrievable filters.23 Randomized controlled trials and practice guidelines are still lacking in this area. Therefore, there is currently insufficient evidence to recommend retrievable filters for distal VTE.
There is also insufficient evidence to recommend filters for patients with massive or submassive PE who can tolerate anticoagulation therapy. Only 1 registry study has compared patients with massive PE (defined by a systolic blood pressure <90 mmHg at presentation) who were treated with vena cava filters to those who were not.24 Though there was a reduction in recurrent PE and mortality at 90 days in patients who received filters, this result requires further confirmation due to the small number of patients who received filters (11 patients) and a possible selection bias (patients who received filters were, on average, 16 years younger than those who did not). More evidence will be needed to weigh not only the cost but the risks of filter insertion (such as insertion site hematoma, increased incidence of DVT, or contrast nephropathy) against any benefit. Until then, routine filter use in patients with massive or submassive PE cannot be routinely recommended, but may be considered in those with massive PE and impending hemodynamic collapse.
Prophylaxis in High‐Risk Patients
Controversy also exists in the use of retrievable filters in patients without VTE who are at high risk for thromboembolic events. Currently, there are no randomized controlled trials that have established the efficacy of retrievable filters as prophylaxis in these patients. However, there are a number of prospective and retrospective studies that examine this topic, particularly in trauma patients.
Trauma
The Eastern Association for the Surgery of Trauma currently recommends that prophylactic filters be considered in trauma patients who are at increased risk for bleeding and prolonged immobilization (level III).25 These patients include those with severe closed head injury, incomplete spinal cord injury with paraplegia or quadriplegia, multiple long bone fractures, and complex pelvic fractures with multiple long bone fractures. The largest study to date on retrievable filters in trauma patients was done by the American Association for the Surgery of Trauma.26 The incidence of new PE after filter placement was 0.5%, which compares favorably with permanent filter recipients (PE 0.7%) and historical controls (2.1%).27 OptEase filters were more commonly associated with caval thrombosis. The majority of filters (78%) were not retrieved, primarily because patients were lost to follow up. Failure to retrieve filters has become a major issue as these devices grow in popularity.28, 29 In this situation, the benefit of using retrievable filters could be mitigated by the same long‐term complications associated with permanent filters. Therefore, well‐coordinated patient follow‐up is essential to ensure optimal use of retrievable filters. Furthermore, randomized studies of retrievable filters are urgently needed to confirm that vena cava filters are associated with net benefit compared with conventional approaches to VTE prophylaxis (enoxaparin, sequential compression devices) in trauma patients.
Other High‐Risk Situations
The use of permanent filters has been studied in neurosurgical, bariatric, orthopedic, and pregnant patients. However, there are very few studies that look at the use of retrievable filters specifically in these populations. One such study was done in obese (body mass index [BMI] > 55 kg/m2) patients undergoing gastric bypass surgery.30 Filter retrieval rates were high (87%), and there were no DVTs or PEs prior to or after removal. The authors attributed their high removal rates to a dedicated follow‐up program and close collaboration with the interventional radiologists. More research needs to be done comparing outcomes with filters to conventional pharmacologic VTE prophylaxis before these devices can be recommended in these patients.
Filter Complications
During Filter Placement
Complications related to both retrievable and nonretrievable filter placement are rare but have been documented in several studies. Failure of the filter to deploy properly has been reported.21 The same study also noted pneumothorax as a complication in some patients whose filters were inserted via the jugular vein.21 Therefore, location of access and retrieval should be an important consideration for patients with significant underlying pulmonary disease. Insertion site thrombosis and arteriovenous fistula formation have been reported primarily with permanent filters31, 32; that risk could be extrapolated to retrievable filters given that the method of placement is the same. Iodine contrast‐induced nephropathy is of concern for high‐risk patients, although the procedure can be performed using gadolinium‐based contrast, carbon dioxide contrast, or without contrast (under ultrasound guidance).
During Filter Retrieval
Filter tilting and clot trapping under the filter that occurs during the filter removal process are infrequent causes of non‐retrieval. Tilting of the filter sometimes can pose problems, but if this occurs, the filter can be repositioned so that the degree of tilt no longer precludes removal. Severe cases of tilting that lead to nonretrieval are very rare. When thrombus is trapped in the filter (Figure 3), retrieval often depends on the amount of thrombus. A visual scale to assist in judgment of thrombus volume has been developed to assist in retrieval decision‐making.33 In some cases, catheter‐directed thrombolysis has been used to facilitate thrombus dissolution.34

VTE After Placement
Table 2 lists the incidence of VTE after retrievable filter placement. The overall incidence of PE is low, but that of DVT varies widely. These data raise the possibility that some filters may not be removed due to the occurrence of a new DVT, thereby becoming permanent filters with the associated risks of recurrent DVT, caval thrombosis, and PE. Only a few studies have investigated the differences in the rate of PE between permanent and retrievable filters and have shown no differences.29 The long‐term complication rates of retrievable filters and how they may differ from permanent filters warrants further investigation.
Some studies have also noted the development of PE after filter retrieval.35, 36 It is possible that a subclinical DVT was present at the time of removal or that the filter was retrieved before the risk of thrombosis had resolved. Therefore, consideration should be given to the use of duplex ultrasound evaluation for DVT prior to filter removal to ensure that patients with active thrombosis receive therapeutic anticoagulation for an appropriate duration.
Because of the concern for DVT and PE associated with retrievable filters, anticoagulation should ideally occur before and after retrieval, once the bleeding risk has become acceptable. Consensus guidelines support this practice,5, 37 though one systematic review has found insufficient evidence regarding the use of anticoagulation in patients with vena cava filters.4 Retrospective reviews have shown that filters can be both placed and removed without bleeding complications, even in patients who are therapeutically anticoagulated with warfarin and/or LMWH.38, 39 Further investigation would be useful to confirm whether this is an effective approach to VTE prevention at the time of retrieval.
Other Adverse Events
Other complications that have been associated with retrievable filters include migration, fracture, infection, and perforation. It may be difficult to estimate the true incidence of these complications, as most of the literature on this topic comes from case reports. Vena cava perforation with hooks may be not uncommon but in most cases is not clinically significant.40 Filter fracture is more common but rarely reported. Filter migration toward the heart is a very rare but potentially life‐threatening complication. The Recovery filter was taken off the market due in part to concerns about migration.26 As the use of retrievable filters increases, complications related to filters will need to be monitored.
Ongoing and Future Research
Other types of removable filters are currently in development. Convertible filters that can be converted into a stent once they are no longer needed are under investigation. Other devices, such as absorbable or drug‐eluting filters, are also being studied.5 In addition, there is ongoing research to better characterize the safety and efficacy of available filters. The Prevention du Risque d'Embolie Pulmonaire par Interruption Cave (PREPIC) 2 will assess their use in the first prospective, randomized, controlled trial of retrievable filters in patients with acute VTE receiving anticoagulation (
Conclusions
There is growing concern over the increased use of vena caval filters for the prevention of PE.41 Retrievable filters offer the possibility of protection without the risk of long‐term complications attributable to permanent filters. The advent of these devices has lead to an increase in overall filter use but also could result in filter placement without adequate consideration of the potential complications or consequences of nonretrieval. More evidence is needed in order to establish best practice guidelines for retrievable filter use. Until these data are available, these devices should be used only in patients with acute VTE who are at risk for recurrent thromboembolism and have a transient risk for bleeding.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.
- ,,, et al.A vena caval filter for the prevention of pulmonary embolus.Surg Forum.1967;18.
- ,,.Twenty‐one‐year trends in the use of inferior vena cava filters.Arch Intern Med.2004;164:1541–1545.
- ,,, et al.A clinical trial of vena caval filters in the prevention of pulmonary embolism in patients with proximal deep‐vein thrombosis. Prevention du Risque d'Embolie Pulmonaire par Interruption Cave Study Group.N Engl J Med.1998;338:409–415.
- ,.The need for anticoagulation following inferior vena cava filter placement: systematic review.Cardiovasc Intervent Radiol.2008;31:316–324.
- ,,, et al.Guidelines for the use of retrievable and convertible vena cava filters: report from the Society of Interventional Radiology multidisciplinary consensus conference.J Vasc Interv Radiol.2006;17:449–459.
- ,,, et al.Changing patterns in the use of inferior vena cava filters: review of a single center experience.J Am Coll Surg.2007;205:564–569.
- ,,.Hemodynamic effects of clot entrapment in the TrapEase inferior vena cava filter.J Vasc Interv Radiol.2004;15:485–490.
- ,,, et al.Vena cava filters and inferior vena cava thrombosis.J Vasc Surg.2007;45:789–794.
- ,,, et al.Clinical comparison of two optional vena cava filters.J Vasc Interv Radiol.2007;18:505–511.
- ,,, et al.Use of retrievable compared to permanent inferior vena cava filters: a single‐institution experience.Cardiovasc Intervent Radiol.2008;31:308–315.
- ,,, et al.Role of prophylactic temporary inferior vena cava filters placed at the ICU bedside under intravascular ultrasound guidance in patients with multiple trauma.J Vasc Surg.2004;40:958–964.
- ,,.Bedside vena cava filter placement with intravascular ultrasound: a simple, accurate, single venous access method.J Vasc Surg.2007;46:1284–1286.
- ,,.The bedside insertion of inferior vena cava filters using ultrasound guidance.Perspect Vasc Surg Endovasc Ther.2007;19:78–84.
- ,,, et al.Comparison of bedside transabdominal duplex ultrasound versus contrast venography for inferior vena cava filter placement: what is the best imaging modality?Ann Vasc Surg.2005;19:229–234.
- ,,, et al.Experience with the recovery filter as a retrievable inferior vena cava filter.J Vasc Interv Radiol.2005;16:1189–1193.
- ,,, et al.Gunther Tulip retrievable vena cava filter: results from the Registry of the Canadian Interventional Radiology Association.J Vasc Interv Radiol.2001;12:1053–1058.
- ,,, et al.Clinical experience with retrievable Gunther Tulip vena cava filters.J Endovasc Ther.2003;10:994–1000.
- ,,, et al.Gunther Tulip filter retrievability multicenter study including CT follow‐up: final report.J Vasc Interv Radiol.2006;17:1017–1023.
- ,,, et al.The Gunther Tulip retrievable filter: prolonged temporary filtration by repositioning within the inferior vena cava.J Vasc Interv Radiol.2003;14:1259–1265.
- ,,, et al.Retrievable inferior vena cava filters: early clinical experience.J Cardiovasc Surg (Torino).2005;46:163–169.
- ,,, et al.Gunther Tulip retrievable inferior vena caval filters: indications, efficacy, retrieval, and complications.Cardiovasc Intervent Radiol.2007;30:59–65.
- ,.Management of anticoagulation before and after elective surgery.N Engl J Med.1997;336:1506–1511.
- ,,, et al.Changes in inferior vena cava filter placement over the past decade at a large community‐based academic health center.J Vasc Surg.2008;47:157–165.
- ,,, et al.Massive pulmonary embolism.Circulation.2006;113:577–582.
- ,,, et al.Practice management guidelines for the prevention of venous thromboembolism in trauma patients: the EAST practice management guidelines work group.J Trauma.2002;53:142–164.
- ,,, et al.Practice patterns and outcomes of retrievable vena cava filters in trauma patients: an AAST multicenter study.J Trauma.2007;62:17–24; discussion 24‐25.
- ,.Inferior vena cava interruption. In: Crowther M, et al., eds.Evidence‐Based Hematology.West Sussex, UK:Wiley‐Blackwell Publishing;2008:99–109.
- ,,, et al.Are temporary inferior vena cava filters really temporary?Am J Surg.2005;190:858–863.
- ,,, et al.Retrievable vena cava filters for preventing pulmonary embolism in trauma patients: a cautionary tale.J Trauma.2006;60:35–40.
- ,,, et al.Safety, feasibility, and outcome of retrievable vena cava filters in high‐risk surgical patients.J Vasc Surg.2007;45:784–788; discussion 788.
- ,,, et al.Femoral arteriovenous fistula after placement of a Kimray‐Greenfield filter.AJR Am J Roentgenol.1988;151:681–682.
- ,,, et al.Prophylactic Greenfield filters: acute complications and long‐term follow‐up.J Trauma.1996;41:231–236; discussion 236‐237.
- ,,.Estimation of trapped thrombus volumes in retrievable inferior vena cava filters: a visual scale.J Vasc Interv Radiol.2007;18:273–276.
- ,,, et al.Endovascular recanalization of the thrombosed filter‐bearing inferior vena cava.J Vasc Interv Radiol.2003;14:893–903.
- ,,, et al.Early experience with the retrievable OptEase vena cava filter in high‐risk trauma patients.Eur J Vasc Endovasc Surg.2006;32:589–595.
- ,,, et al.Current trends in vena caval filtration with the introduction of a retrievable filter at a level I trauma center.J Trauma.2004;57:32–36.
- ,,, et al.Antithrombotic therapy for venous thromboembolic disease: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy.Chest.2004;126:401S–428S.
- ,,, et al.Safety of inferior vena cava filter retrieval in anticoagulated patients.Chest.2007;132:31–36.
- ,,, et al.Should anticoagulant therapy be stopped or reversed before venous intervention?Can Assoc Radiol J.1999;50:306–309.
- ,,.Retrievable vena cava filters: clinical experience.Curr Opin Pulm Med.2006;12:304–309.
- .Use of emboli‐blocking filters increases, but rigorous data are lacking.JAMA.2006;295:989–990.
- ,,, et al.Extended interval for retrieval of vena cava filters is safe and may maximize protection against pulmonary embolism.Am J Surg.2006;192:789–794.
- ,,, et al.Long‐term retrievability of IVC filters: should we abandon permanent devices?Cardiovasc Intervent Radiol.2007;30:820–827.
- ,,, et al.A prospective long‐term study of 220 patients with a retrievable vena cava filter for secondary prevention of venous thromboembolism.Chest.2007;131:223–229.
- ,,, et al.The Cook Celect filter: the UK and global experience so far. In:European Congress of Radiology.2008;European Society of Radiology:Vienna, Austria.
- ,,, et al.Multicenter retrievability trial of the recovery G2 filter.J Vasc Interv Radiol.2008;19:S28.
- ,,, et al.The Jonas study: evaluation of the retrievability of the Cordis OptEase inferior vena cava filter.J Vasc Interv Radiol.2005;16:1439–1445.
- ,,, et al.“Recovery” vena cava filter: experience in 96 patients.Cardiovasc Intervent Radiol.2006;29:559–564.
- ,,, et al.Outcomes with retrievable inferior vena cava filters: a multicenter study.J Vasc Interv Radiol.2006;17:1595–1604.
- ,,, et al.Retrievable inferior vena cava filters: initial clinical results.Ann Vasc Surg.2006;20:157–165.
- ,,, et al.Evaluation of retrievability of the Günther Tulip vena cava filter.Cardiovasc Intervent Radiol.2007;30:226–231.
- ,,, et al.A comparison of clinical outcomes with retrievable and permanent inferior vena cava filters.J Vasc Interv Radiol.2008;19:393–399.
- .The management of anticoagulation before and after procedures.Med Clin North Am.2001;85:1109–1116.
- ,,, et al.The perioperative management of antithrombotic therapy: American College of Chest Physicians Evidence‐Based Clinical Practice Guidelines, 8th ed.Chest.2008;133:299S–339S.
Asking for Help
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
There is little scientific evidence about professional help‐seeking behavior among resident physicians. Although junior physicians have many sources of information available to them in the course of clinical practiceprint materials, internet resources, curbside consultations, and advice from senior residents and facultywe have little empirical knowledge about when, why, and how physician trainees ask for help.
To study this phenomenon, we examined the use of a medical procedure service (MPS) by resident physicians. The MPS is an inpatient service at a Boston teaching hospital that provides education, supervision, and evaluation of internal medicine residents who perform common bedside procedures; it has been described previously.1 Residents who call the MPS review an online curriculum with self‐assessment quizzes, perform procedures with faculty supervision and feedback, and assess their own performance using online checklists. This program has been available to internal medicine residents since 2002. In a previous study, we found that residents reported greater comfort performing bedside procedures when they used the procedure service, when the operator was a postgraduate year (PGY)2 or PGY3 resident (compared to PGY1 residents), and while placing central venous catheters (CVCs) (compared to thoracenteses).2
The goal of the current study was to examine help‐seeking behavior among resident physicians as they placed CVCs and performed thoracenteses. We interpreted the decision to use the MPS to indicate that the resident successfully sought and received assistance from pulmonary attending physicians or interventional pulmonary fellows. We hypothesized that: (1) residents earlier in their training would choose to use the procedure service due to their relative lack of experience; (2) they would seek consultation when the procedure was performed in high‐risk patients, as indicated by the number of comorbidities, presence of medications that increase the risk of bleeding, and treatment in an intensive care unit; and (3) residents would be less likely to call the MPS for urgent or emergent situations, when timely assistance may be difficult to obtain. To examine the potentially confounding influence of procedures supervised by non‐MPS physicians, we also investigated differences between informally supervised procedures (i.e. by a non‐MPS attending or fellow) and unsupervised procedures (i.e. no attending or fellow supervision) to determine whether any significant differences in their characteristics existed.
Methods
Study Site
We studied CVC placement and thoracenteses performed by internal medicine residents at a 556‐bed Boston teaching hospital in 2003‐2004. During the 9‐month study period, 63 PGY1 residents (16 in a 1‐year preliminary program) and 95 PGY2 and PGY3 residents were enrolled in the program.
The MPS was staffed by hospitalists and pulmonologists skilled in teaching and performing 4 common inpatient procedures: CVC placement, thoracentesis, lumbar puncture, and paracentesis. We chose to study only the first 2 procedures because supervision of CVCs and thoracenteses by pulmonologists was available 24 hours a day in this initial year of the MPS. The other procedures were supervised by hospitalists during business hours only at the time. Ultrasound guidance was available for all procedures, supervised or not. At the time of the study, the residency program recommended consulting the MPS for procedures, but this was not mandatory. A resident electing to use the MPS to supervise a procedure on her own patient would page the MPS physician. If she were performing a procedure for the first time, she was required to review an online multimedia curriculum and complete a 5‐question cognitive test. She would then perform the procedure while supervised by the MPS physician, who would complete a checklist evaluation of the resident's performance online. All residents performing procedures, regardless of use of the MPS, would also complete procedure logs online to document procedural experience for the American Board of Internal Medicine requirements.
Study Design and Data Sources
We prospectively collected data from resident procedure logs from July 2003 through April 2004. We elicited the following information from the residents for each procedure: name of operator, year of training, date of procedure, patient's medical record number, name of attending or fellow supervisor, procedure, immediate complications (pneumothorax, bleeding, other, or none), self‐reported level of urgency (emergent, urgent, elective), time of day, procedure location, and the number of such procedures completed previously. We categorized level of supervision as: (1) MPS‐supervised if a pulmonary attending or interventional pulmonary fellow were listed as the supervisor (entailing formal faculty development as MPS faculty, resident use of the curriculum, and completion of faculty evaluations with structured feedback); (2) informally supervised if nonpulmonary attendings or fellows were involved (who may not supervise the entire procedure and would not complete a faculty evaluation); and (3) unsupervised if a resident physician or no supervisor was identified. Faculty development involved a single training session with the interventional pulmonary fellows and attendings and focused on optimal procedural teaching. During the session, we described the structure of the MPS, provided the curricular materials available to the residents, and reviewed the faculty evaluation forms in depth.
We abstracted patient characteristics (age, race/ethnicity, type of insurance, length of stay) from the electronic medical record. We performed retrospective chart reviews to record patient comorbidities (as defined by modified Deyo criteria3), to determine the number of medications associated with the risk of bleeding (such as anticoagulants and antiplatelet agents), and to discover complications that arose after the procedure was logged, including delayed bleeding, pneumothorax, or infection (localized site infection or line‐related bloodstream infection).
Data Analyses
We tabulated characteristics of residents (training level, gender, and self‐reported number of procedures), procedures (procedure type, procedure location, level of urgency, time of day), and patients (number of comorbidities and number of medications that promote bleeding) by use of the MPS. We combined resident‐reported (ie, immediate) complications and delayed complications (identified on retrospective chart review), stratified by use of the MPS. We also performed a subgroup analysis of non‐MPS procedures by comparing resident, procedure, and patient characteristics by presence or absence of informal supervision.
We created a univariable logistic regression model to examine factors associated with elective use of the MPS. We dichotomized the following independent variables: resident characteristics (PGY status, female gender, first time performing the procedure), patient characteristics (nonwhite race, female gender, Medicaid recipient, 3 or more comorbidities, any bleeding medication), and procedure characteristics (intensive care unit procedures, nonelective procedures, procedures performed between 11 PM and 8 AM). We also included 2 patient‐related interval variables (age and length of stay) in the univariable logistic regression model. We created a multivariable logistic regression model with backward elimination (P < 0.05) using the same independent variables as in the univariable analyses, to identify factors associated with use of the MPS, clustering by resident. We repeated this method to create a multivariable model to examine factors associated with the use of informal supervision among non‐MPS procedures. Analyses used Stata 7.0 (StataCorp, College Station, TX).
The study protocol was approved in advance by the hospital investigational review board.
Results
Resident Characteristics
Sixty‐nine residents reported procedures during the 9‐month study period (Table 1). Thirty (43%) residents were PGY1 and 36 (52%) were female. Twelve (17%) residents performed the procedure for the first time.
| Total residents, n (%) | 69 (100) |
|---|---|
| |
| Training year, n (%) | |
| PGY1 | 30 (43) |
| PGY2 | 23 (33) |
| PGY3 | 16 (23) |
| Gender, n (%) | |
| Female | 36 (52) |
| Male | 33 (48) |
| Self‐reported number of prior procedures, n (%)* | |
| 0 | 12 (17) |
| 1‐5 | 26 (38) |
| >6 | 31 (45) |
Patient Characteristics
The 134 patients in the study had a mean age of 65.6 years. One‐half of patients were female, and 34% were nonwhite. The principal insurer was Medicare (57%); 24% were privately insured, and 17% received Medicaid. The mean length of stay was 18.4 days (range, 0‐98 days).
MPS and Non‐MPS Procedures
As detailed in the bivariate analyses in Table 2, residents performed 191 procedures (156 CVCs and 35 thoracenteses). PGY1 residents performed approximately one‐half of the 79 MPS procedures. Fifty‐one (65%) of the 79 MPS procedures were CVC placements and 28 (35%) were thoracenteses (P < 0.001). MPS procedures were less often performed in the emergency department than non‐MPS procedures (1% versus 21%, P < 0.001). There was no significant difference in the percentage of MPS and non‐MPS procedures by time of day. Patients whose procedures were supervised by the MPS had on average 3.0 comorbidities, while patients who underwent non‐MPS procedures had 2.6 comorbidities (P = 0.02). Complications occurred in 11 (14%) of MPS and 22 (20%) of non‐MPS procedures, a statistically nonsignificant difference.
| MPS | No MPS | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 79 (100) | 112 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.77 | ||
| PGY1 | 42 (53) | 60 (54) | |
| PGY2 | 22 (28) | 34 (30) | |
| PGY3 | 15 (19) | 18 (16) | |
| Gender, n (%) | 0.50 | ||
| Female | 42 (53) | 54 (48) | |
| Male | 37 (47) | 58 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.11 | ||
| 0 | 14 (18) | 13 (12) | |
| 1‐5 | 40 (51) | 47 (42) | |
| >6 | 25 (32) | 52 (46) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.001 | ||
| Central venous catheter | 51 (65) | 105 (94) | |
| Thoracentesis | 28 (35) | 7 (6) | |
| Location, n (%) | 0.001 | ||
| Ward | 18 (23) | 17 (15) | |
| Emergency department | 1 (1) | 23 (21) | |
| Intensive care unit | 53 (67) | 71 (63) | |
| Other | 7 (9) | 1 (1) | |
| Urgency, n (%) | 0.001 | ||
| Elective | 32 (41) | 22 (20) | |
| Urgent | 42 (53) | 78 (70) | |
| Emergent | 5 (6) | 12 (11) | |
| Time of day, n (%) | 0.33 | ||
| 8 AM to 5 PM | 50 (63) | 65 (58) | |
| 5 PM to 11 PM | 15 (19) | 20 (18) | |
| 11 PM to 8 AM | 14 (18) | 27 (24) | |
| Complications or problems, n (%) | 0.54 | ||
| Bleeding | 1 (1) | 6 (5) | |
| Pneumothorax | 1 (1) | 2 (2) | |
| Infection | 5 (6) | 5 (4) | |
| Other∥ | 4 (5) | 9 (8) | |
| None | 68 (86) | 90 (80) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 3.0 (1.4, 0‐7) | 2.6 (1.6, 0‐7) | 0.02 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 1.1 (1.0, 0‐3) | 1.1 (0.9, 0‐3) | 0.90 |
In the univariable analysis, the only variable associated with elective use of the MPS was the presence of 3 or more comorbidities (oodds ratio [OR], 2.3; 95% confidence interval [CI], 1.2‐4.1). In the multivariable analysis, residents were more likely to use the MPS when patients had 3 or more comorbidities (OR, 2.1; 95% CI, 1.2‐3.5) and less likely to use the MPS when procedures were either urgent or emergent (OR, 0.4; 95% CI, 0.2‐0.8).
Unsupervised and Informally Supervised Procedures
Table 3 shows the results of the bivariate analyses of the characteristics of the 112 procedures that were unsupervised or supervised by non‐MPS physicians. Twenty‐seven (24%) were informally supervised by nonpulmonary attendings. Residents who had performed more than 6 procedures previously were more likely to be informally supervised than not supervised at all (P = 0.001). More informally supervised procedures were performed in the emergency department (41%) than in other settings (P = 0.01). There were no significant differences in year of training, gender, urgency, time of day, complications, comorbidities, or bleeding medications.
| Informal Supervision | No Supervision | P Value* | |
|---|---|---|---|
| |||
| Total procedures, n (%) | 27 (100) | 85 (100) | |
| Resident characteristics | |||
| Resident level, n (%) | 0.13 | ||
| PGY1 | 10 (37) | 50 (59) | |
| PGY2 | 12 (44) | 22 (26) | |
| PGY3 | 5 (19) | 13 (15) | |
| Gender, n (%) | 0.99 | ||
| Female | 13 (48) | 41 (48) | |
| Male | 14 (52) | 44 (52) | |
| Self‐reported number of prior procedures, n (%) | 0.001 | ||
| 0 | 2 (7) | 11 (13) | |
| 1‐5 | 4 (15) | 43 (51) | |
| >6 | 21 (78) | 31 (36) | |
| Procedure characteristics | |||
| Procedure, n (%) | 0.53 | ||
| Central venous catheter | 26 (96) | 79 (93) | |
| Thoracentesis | 1 (4) | 6 (7) | |
| Location, n (%) | 0.01 | ||
| Ward | 1 (4) | 16 (19) | |
| Emergency department | 11 (41) | 12 (14) | |
| Intensive care unit | 15 (56) | 56 (66) | |
| Other | 0 (0) | 1 (1) | |
| Urgency, n (%) | 0.33 | ||
| Elective | 4 (15) | 18 (21) | |
| Urgent | 19 (70) | 59 (69) | |
| Emergent | 4 (15) | 8 (9) | |
| Time of Day, n (%) | 0.11 | ||
| 8 AM to 5 PM | 13 (48) | 52 (61) | |
| 5 PM to 11 PM | 4 (15) | 16 (19) | |
| 11 PM to 8 AM | 10 (37) | 17 (20) | |
| Complications or problems, n (%) | 0.45 | ||
| Bleeding | 0 (0) | 6 (7) | |
| Pneumothorax | 0 (0) | 2 (2) | |
| Infection | 0 (0) | 5 (6) | |
| Other∥ | 2 (7) | 7 (8) | |
| None | 25 (93) | 65 (76) | |
| Patient characteristics | |||
| Number of comorbidities, mean (SD, range) | 2.2 (1.3, 1‐5) | 2.7 (1.7, 0‐7) | 0.22 |
| Number of medications associated with bleeding risk, mean (SD, range)# | 0.9 (.93, 0‐3) | 1.1 (0.9, 0‐3) | 0.24 |
In the multivariable analysis, the only factor associated with the use of informal supervision (rather than absent supervision) was patient gender; informal supervision was less likely with female patients (OR, 0.3; 95% CI, 0.1‐0.8).
Discussion
To understand professional help‐seeking behavior by internal medicine resident physicians, we studied factors associated with the use of a MPS for performing 2 common bedside procedures. We found that residents used the MPS more often when they performed procedures on patients with more comorbidities and less often during urgent or emergent procedures.
These results are consistent with our hypothesis that residents use formal supervision when caring for high‐risk patients. We had also hypothesized that they would seek the MPS for patients on medications that increase the risk of bleeding, but this was not borne out. One possible explanation is that invasive procedures on anticoagulated patients may be deferred or avoided. Additionally, we did not collect prothrombin times nor platelet count, which may represent better proxies for coagulopathy. Our hypothesis that residents would not seek the MPS for urgent and emergent procedures was confirmed; the time delay between contacting the faculty member and performing the procedure may have inhibited or obviated consultation of the MPS. We hypothesized that interns would use the MPS preferentially; we found instead that level of training did not influence use of the MPS. A resident early in training may struggle with the balance between autonomy and supervision, wanting instead to establish himself as able to solve clinical problems independently and by seeking consultation only as a last resort. Alternatively, interns may be primarily supervised by their residents and may seek expert assistance only for particularly challenging or high‐risk cases. Additionally, as newcomers to the training program, they may not be well acquainted with the role and availability of the service (although periodic announcements were made throughout the year). Our examination of procedures not supervised by the MPS showed that informally supervised and unsupervised procedures are quite similar to each other; the inverse relationship between informal supervision and patient gender is difficult to explain and may be spurious.
To our knowledge, only 1 author has postulated a theoretical foundation for help‐seeking in trainees, depicted in the context of the patient‐resident‐attending triadic relationship.4 The mature help‐seeker, whether patient, resident, or attending, is willing to confront problems, receptive to new information, able to acknowledge dependence on expertise, and able to apply new input with self‐reliance. However, little is known about how this model manifests itself empirically in professional help‐seeking or what the optimal conditions of faculty supervision are. One observational study suggested that faculty who spent more time on hospital floors created environments with higher resident satisfaction scores, higher perceived quality of patient care, and, paradoxically, increased perceptions of autonomy.5 These results are consistent with our previous work showing that residents' comfort with bedside procedures increased with use of the MPS.2 In the related field of consultation medicine, 2 studies6, 7 showed that family practitioners prefer to consult internal medicine subspecialists over general internists. One of these studies7 demonstrated that the primary need was for a consultant with technical (ie, procedural) skills. Our use of MPS faculty who are specifically skilled in performing medical procedures appears to be consistent with this observation that specific technical expertise is valued over general supervision or guidance.
How can we best design formal procedural supervision programs that allow residents to obtain help when they need it? In addition to fostering mature help‐seeking behavior, help‐giving requires: (1) an environment that encourages help‐seeking; (2) a mechanism to provide assistance when and where it is needed; (3) supervisors with technical expertise; and (4) supervision that supports learning, skill acquisition, and graduated autonomy. It is difficult to devise mechanisms that include all of these elements. For instance, 24‐hour per day faculty coverage may be logistically challenging and expensive. Physicians with technical expertise may not be good teachers despite faculty development on procedural teaching. Obstacles to successful help‐seeking may include differences in residents' and supervisors' perceptions about the need for supervision. For example, a supervisor may be available and willing to assist, but the resident may feel capable of performing independently. When assistance is provided, residents and supervisors may differ in their perceptions of the quality of supervision.8 Ultimately, any educational intervention to increase supervision must confront a cultural norm of self‐sufficiency among many residency programs, in which managing a situation without assistance is equated with competence. To address this issue, our hospital has mandated the use of the MPS for all bedside procedures since 2005 and staffed the program 24 hours a day, in recognition of the potential risk of procedural complications9, 10 among inexperienced trainees.
This study has several limitations. We had a small number of thoracenteses. The study was not designed or powered to examine differences in complication rates among MPS and non‐MPS procedures. Because we represent a single institution, our findings may not be generalizable to other teaching hospitals or nonteaching settings. Our data on procedure characteristics were ascertained through resident self‐reports and, though typically submitted in a timely way, are subject to recall bias. In particular, discrepancies in the reported level of urgency may have affected our results about the time‐dependent nature of help‐seeking. Additionally, our findings about the types of patients about which residents seek consultation are somewhat at odds; use of the modified Deyo criteria to adjust for clinical severity weighs chronic conditions heavily and may translate into complication risk, but the level of urgency may better reflect the acuity of the clinical presentation. We could not distinguish between resident‐supervised procedures and those performed without supervision because of limited data. We also acknowledge the possibility that some non‐MPS faculty (classified for the study as informal supervisors) may serendipitously provide an equal quality of supervision that our MPS faculty did, by being present throughout the procedure and giving structured and valuable feedback.
Nevertheless, our results suggest that many residents do seek formal help appropriately when they perform procedures on the sickest patients, recognizing the risk and technical difficulty associated with bedside procedures in these patients. Our results also point to a greater area of inquiry: how do we optimally address the help‐seeking needs among trainee physicians? How do we create an environment in which help‐seeking is encouraged? How do we overcome the logistical barriers of providing timely assistance to residents, particularly at times of greatest need (urgent or emergent procedures)? How do we confront a longstanding culture in which independence is equated with competence, especially as it relates to procedural skills? A better understanding of how the widespread availability of programs like our MPS would affect the residents' use of supervision in general may guide the design of resident curricula and the development of mechanisms to ensure safe and effective clinical care.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
- ,,, et al.Creation of an innovative inpatient medical procedure service and a method to evaluate house staff competency.J Gen Intern Med.2004;19:510–513.
- ,,, et al.Beyond the comfort zone: residents assess their comfort performing inpatient medical procedures.Am J Med.2006;119:71.e17–e24.
- ,,.Adapting a clinical comorbidity index for use with ICD‐9‐CM administrative databases.J Clin Epidemiol.1992;45:613–619.
- .Parallel process in the family medicine system: issues and challenges for resident training.Fam Med.1990;22:312–319.
- ,,,,.Increased faculty presence on inpatient teaching services.Mayo Clin Proc.2004;79:332–336.
- ,,,.Principles of effective consultation: an update for the 21st‐century consultant.Arch Intern Med.2007;167:271–275.
- ,.Family physician consultation/referral patterns.J Am Board Fam Pract.1988;1:106–111.
- ,,, et al.Operating room teamwork among physicians and nurses: teamwork in the eye of the beholder.J Am Coll Surg.2006;202:746–752.
- ,,, et al.The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II.N Engl J Med.1991;324:377–384.
- ,,,.Impact of procedure‐related complications on patient outcome on a general medicine service.J Gen Intern Med.1994;9:66–70.
Copyright © 2009 Society of Hospital Medicine