User login
CASE REPORT: Altered Mental Status in an Elderly Woman
Case
A 100-year-old woman with a history of hypertension, hypothyroidism, and moderate Alzheimer dementia was brought to the ED by emergency medical services (EMS) for altered mental status after her home health aide (HHA) noted a change in the patient’s behavior. For the past few days, the patient’s appetite waned, and she became progressively more lethargic, not eating for over 24 hours. The aide activated 911 on the direction of the patient’s primary care physician. There were no reported changes to the patient’s medications which included aspirin, levothyroxine, and hydrochlorothiazide. She was unable to provide any meaningful history.
On arrival to the ED, the patient appeared comfortable in bed. She was sleepy, but easily aroused. Initial vital signs were: heart rate, 110 beats/minute; respiratory rate, 12 breaths/minute; blood pressure, 163/103 mm Hg; oral temperature, 98.2˚F. Oxygen (O2) saturation was 96% on room air. She was oriented to person only and responded appropriately to simple questions, intermittently following one-step commands. She was unable to attend and required redirection throughout the interview. (According to the aide, this behavior was different than her baseline.)
The patient’s head and neck examination were notable for some mild, boggy, periorbital edema and dry mucous membranes. Her thyroid examination was normal; her lungs were clear; and her cardiac examination revealed a 2/6 systolic ejection murmur over the second right intercostal space. Examination of the abdomen, extremities, and skin was unrevealing, and there were no gross focal neurological deficits. Her reflexes were normal throughout.
Initial assessment of this patient suggested a diagnosis of dementia and hypoactive delirium—the latter due to one or more of several possible etiologies.
Altered Mental Status
While altered mental status is a billable medical ICD-9-CM code1 used to specify a diagnosis on a reimbursement claim, it is not a disease state itself. Instead, it is a catchall phrase that incorporates any change in mental status, encompassing symptomatology that may have the largest differential diagnosis encountered in emergency medicine.
Delirium
An important category of altered mental status is delirium. The diagnostic criteria2 for delirium in DSM-V have remained essentially unchanged from DSM-IV; however, the prevalence of delirium as one of the key geriatric syndromes has grown as a result of increased research and education, particularly in the emergency medical setting. Distilled down, delirium can be defined as an acute change in mental status not caused by underlying dementia. Its cause is often multifactorial, and it is frequently an underappreciated consequence of both critical illness and the hospital environment.3
Delirium is an emergency unto itself, with an in-hospital mortality rate mirroring that of sepsis or acute myocardial infarction.4 The older-adult population is especially at risk of delirium and can present with one of three clinical subtypes: hyperactive (ie, agitated, etc), hypoactive (ie, somnolent, lethargic, stuporous, etc), and mixed type.5
The composition of factors precipitating the onset of delirium includes age, dementia, alcohol use, depression, illness severity, and drug exposure—notably benzodiazepines, opiates, and medications with anticholinergic properties.8 While major precipitants or causes of delirium, traditionally considered as potentially life-threatening acute events, are well known (Table 1), others are often overlooked (Table 2). Yet, ironically, these more frequently bypassed causes are more readily reversible, once discovered, such as inadequate pain control, urinary retention, constipation, dehydration, polypharmacy, and negative environmental conditions in the patient’s immediate surroundings. These findings have recently been corroborated.9
The Confusion Assessment Method
Identifying delirium can be a particular challenge for emergency physicians (EPs), especially when the patient has an underlying diagnosis of dementia and the specific degree of cognitive impairment is not known. The Confusion Assessment Method (CAM)10 is the most commonly used tool in the critical care setting11 and is the only validated tool for the ED, with an 86% sensitivity and 100% specificity.12 It evaluates four elements: (1) acute onset and fluctuating course; (2) inattention; (3) disorganized thinking; and (4) altered level of consciousness. A patient must demonstrate the first two elements in addition to either the third or fourth element to be considered to have delirium.10
The CAM intensive care unit scale has the potential to be even more applicable in the ED. Recent findings support its validation.9
Case Continued
With respect to the elderly patient in this case with dementia and multiple potential causes for hypoactive delirium, the life-saving measure was the New York City (NYC) mandate that all 911 responding EMS workers wear ambient carbon monoxide (CO) detectors. Though the value of these detectors is controversial due to their low sensitivity, the emergency medical technicians (EMTs) detected an elevated CO level of 300 ppm when they arrived at the patient’s home.
Without this information, the patient’s age and clinical presentation would almost certainly have prompted an extensive evaluation to determine the etiology of her change in mental status and most likely would have missed the true cause of CO toxicity, which was confirmed by venous co-oximetry showing the patient to have a carboxyhemoglobin (HbCO) level of 19.5%.
Carbon Monoxide Toxicity
Carbon monoxide affects multiple cell types. It binds to myoglobin and in high concentrations depresses myocardial contractility. In platelets, CO displaces nitric oxide potentially resulting in vasodilation. Life-threatening CO poisoning causes hypotension, syncope, tachycardia, and an altered mental status. Delayed neuropsychiatric sequelae also may occur as the result of free radical injury to the brain.13
Symptoms
Patients with chronic CO poisoning who can adequately communicate may report nausea, headache, lightheadedness, and lethargy mimicking other seasonal illnesses. In debilitated or cognitively impaired patients who are unable to communicate, findings may include tachycardia, a mild change in mental status, and little else. Prolonged exposure and physiologic accumulation of CO may cause depressed mental status, coma, or death.
Although HbCO levels are confirmatory of exposure, venous levels do not necessarily reflect tissue concentrations or outcomes. Patients with a similar level to that of this patient (19.5%) may present with no symptoms, mild headache, or a deep coma depending on the duration of exposure to CO.
Definitive treatment is removal from the toxic environment and prompt administration of O2. In some cases, hyperbaric therapy may be beneficial.14
Diagnosis
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
It is rarely easy to diagnose the first case of an illness of which one is unfamiliar or not accustomed to treating. Likewise, it is very difficult to consider, diagnose and, as a result, effectively manage the first presentation of a known condition that is typically seasonal or linked to a different geographic location. Acute presentations of environmental exposures, illicit drug poisonings, and communicable infectious diseases are increasingly the purview of emergency medicine. Whether it is the first case of Ebola, of severe acute respiratory syndrome, the influenza virus, a new lethal street drug overdose, or CO poisoning prior to the onset of winter, maintaining a high index of suspicion for the “index case” is of paramount importance. The patient presented here, the first CO poisoning of the season at the authors’ institution, illustrates the responsibility the EP to consider, diagnose, and prevent a wide-range of deadly consequences—injury prevention as the result of vigilance. Moreover, the consequences of missing the diagnosis would have placed others at risk for continued poisoning and possibly death.
Portable and Ambient Carboxyhemoglobin Monitors
The NYC Department of Health (NYCDOH) requires that all EMTs and paramedics wear CO detectors and all residential housing contain CO monitors. The NYCDOH also mandates that all identified cases of CO poisoning be reported to the NYC Poison Control Center. This centralization of data on any and all patients exposed to CO can result in an investigation of the source of CO by the fire department and capture symptomatic patients who present for care outside of the 911 response system. The source of CO in this patient was ultimately traced to a faulty furnace that was repaired to prevent others in the building from becoming victims of CO poisoning.
It should be noted that portable noninvasive HbCO monitors may be inadequate to rule out CO poisoning as the sensitivity of such devices can be as low as 48%.15 Carbon monoxide poisoning can result from brief exposure to a high ambient concentration, such as a fire in which environmental concentrations may exceed 500 ppm or more insidiously, in a setting of a chronic exposure. Faulty furnaces—a common seasonal cause of CO poisoning—may continue to produce adequate heat and fail to prompt any concerns.
Since CO is colorless and odorless, ambient CO detectors stationed in the home are the best means of alerting one to exposure. In this case, though mandated by NYCDOH, a CO detector was not present in the patient’s home.
Case Conclusion
Through the rapid identification of CO poisoning in this elderly patient with altered mental status, EMS was able to evacuate the building while bringing the elderly tenant and her home attendant to the ED.
Based on the elderly patient’s elevated HbCO level, she was treated with O2 and discharged from the hospital the following day feeling well. In addition to the patient’s symptoms, when the aide was interviewed, she reported that she had been experiencing daily headaches, which she said soon resolved on departure from her client’s house. Her symptoms had been bothersome, but not so severe as to prompt her to seek medical attention. The aide was found to have an HbCO level of 12.5% and was discharged from the ED after 6 hours of observation and O2 therapy. The third occupant of the building, a tenant, was also brought to the ED and found to have an HbCO level of 12%. The tenant was treated with O2 therapy and discharged to home.
Dr Caldwell is an assistant professor of medicine in the department of emergency medicine, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Rao is an assistant professor of emergency medicine; and the chief in the division of medical Toxicology, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Stern is an assistant professor of medicine, department of emergency medicine; chief of geriatric emergency medicine; and codirector of geriatric emergency medicine fellowship at New York Presbyterian Hospital/Weill Cornell Medical Center, New York.
- ICD-9Data.com Web site. 2014 ICD-9-CM Diagnosis Codes. http://www.icd9data.com/2014/Volume1/default.htm. Accessed December 4, 2014.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Han JH, Wilson A, Ely EW. Delirium in the older emergency department patient: a quiet epidemic. Emerg Med Clin North Am. 2010;28(3):611-631.
- Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
- Mulcare MR, Halpern A, Stern ME. The geriatric patient. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:741-753.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
- Han JH, Vasilevskis EE, Ely EW. Sedation and delirium. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:704-717.
- Rosen T, Connors S, Halpern A, et al. Improving emergency department identification and management of agitated delirium in older adults: Implementation and impact assessment of a comprehensive clinical protocol using an A-B-C-D-E-F mnemonic. Ann Emerg Med. 2013;62(4)(Supp 4):S53-54.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
- Monette J, Galbaud du Fort G, Fung SH, et al. Evaluation of the Confusion Assessment Method (CAM) as a screening tool for delirium in the emergency room. Gen Hosp Psychiatry. 2001;23(1):20-25.
- Weaver LK. Carbon monoxide poisoning. New Engl J Med. 2009;360(12):1217-1225.
- Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric therapy for acute carbon monoxide poisoning. New Engl J Med. 2002;347(14):1057-1067.
- Touger M, Birnbaum A, Wang J, Chou K, Pearson D, Bijur P. Performance of the RAD-57 pulse CO-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56(4):382-388.
Case
A 100-year-old woman with a history of hypertension, hypothyroidism, and moderate Alzheimer dementia was brought to the ED by emergency medical services (EMS) for altered mental status after her home health aide (HHA) noted a change in the patient’s behavior. For the past few days, the patient’s appetite waned, and she became progressively more lethargic, not eating for over 24 hours. The aide activated 911 on the direction of the patient’s primary care physician. There were no reported changes to the patient’s medications which included aspirin, levothyroxine, and hydrochlorothiazide. She was unable to provide any meaningful history.
On arrival to the ED, the patient appeared comfortable in bed. She was sleepy, but easily aroused. Initial vital signs were: heart rate, 110 beats/minute; respiratory rate, 12 breaths/minute; blood pressure, 163/103 mm Hg; oral temperature, 98.2˚F. Oxygen (O2) saturation was 96% on room air. She was oriented to person only and responded appropriately to simple questions, intermittently following one-step commands. She was unable to attend and required redirection throughout the interview. (According to the aide, this behavior was different than her baseline.)
The patient’s head and neck examination were notable for some mild, boggy, periorbital edema and dry mucous membranes. Her thyroid examination was normal; her lungs were clear; and her cardiac examination revealed a 2/6 systolic ejection murmur over the second right intercostal space. Examination of the abdomen, extremities, and skin was unrevealing, and there were no gross focal neurological deficits. Her reflexes were normal throughout.
Initial assessment of this patient suggested a diagnosis of dementia and hypoactive delirium—the latter due to one or more of several possible etiologies.
Altered Mental Status
While altered mental status is a billable medical ICD-9-CM code1 used to specify a diagnosis on a reimbursement claim, it is not a disease state itself. Instead, it is a catchall phrase that incorporates any change in mental status, encompassing symptomatology that may have the largest differential diagnosis encountered in emergency medicine.
Delirium
An important category of altered mental status is delirium. The diagnostic criteria2 for delirium in DSM-V have remained essentially unchanged from DSM-IV; however, the prevalence of delirium as one of the key geriatric syndromes has grown as a result of increased research and education, particularly in the emergency medical setting. Distilled down, delirium can be defined as an acute change in mental status not caused by underlying dementia. Its cause is often multifactorial, and it is frequently an underappreciated consequence of both critical illness and the hospital environment.3
Delirium is an emergency unto itself, with an in-hospital mortality rate mirroring that of sepsis or acute myocardial infarction.4 The older-adult population is especially at risk of delirium and can present with one of three clinical subtypes: hyperactive (ie, agitated, etc), hypoactive (ie, somnolent, lethargic, stuporous, etc), and mixed type.5
The composition of factors precipitating the onset of delirium includes age, dementia, alcohol use, depression, illness severity, and drug exposure—notably benzodiazepines, opiates, and medications with anticholinergic properties.8 While major precipitants or causes of delirium, traditionally considered as potentially life-threatening acute events, are well known (Table 1), others are often overlooked (Table 2). Yet, ironically, these more frequently bypassed causes are more readily reversible, once discovered, such as inadequate pain control, urinary retention, constipation, dehydration, polypharmacy, and negative environmental conditions in the patient’s immediate surroundings. These findings have recently been corroborated.9
The Confusion Assessment Method
Identifying delirium can be a particular challenge for emergency physicians (EPs), especially when the patient has an underlying diagnosis of dementia and the specific degree of cognitive impairment is not known. The Confusion Assessment Method (CAM)10 is the most commonly used tool in the critical care setting11 and is the only validated tool for the ED, with an 86% sensitivity and 100% specificity.12 It evaluates four elements: (1) acute onset and fluctuating course; (2) inattention; (3) disorganized thinking; and (4) altered level of consciousness. A patient must demonstrate the first two elements in addition to either the third or fourth element to be considered to have delirium.10
The CAM intensive care unit scale has the potential to be even more applicable in the ED. Recent findings support its validation.9
Case Continued
With respect to the elderly patient in this case with dementia and multiple potential causes for hypoactive delirium, the life-saving measure was the New York City (NYC) mandate that all 911 responding EMS workers wear ambient carbon monoxide (CO) detectors. Though the value of these detectors is controversial due to their low sensitivity, the emergency medical technicians (EMTs) detected an elevated CO level of 300 ppm when they arrived at the patient’s home.
Without this information, the patient’s age and clinical presentation would almost certainly have prompted an extensive evaluation to determine the etiology of her change in mental status and most likely would have missed the true cause of CO toxicity, which was confirmed by venous co-oximetry showing the patient to have a carboxyhemoglobin (HbCO) level of 19.5%.
Carbon Monoxide Toxicity
Carbon monoxide affects multiple cell types. It binds to myoglobin and in high concentrations depresses myocardial contractility. In platelets, CO displaces nitric oxide potentially resulting in vasodilation. Life-threatening CO poisoning causes hypotension, syncope, tachycardia, and an altered mental status. Delayed neuropsychiatric sequelae also may occur as the result of free radical injury to the brain.13
Symptoms
Patients with chronic CO poisoning who can adequately communicate may report nausea, headache, lightheadedness, and lethargy mimicking other seasonal illnesses. In debilitated or cognitively impaired patients who are unable to communicate, findings may include tachycardia, a mild change in mental status, and little else. Prolonged exposure and physiologic accumulation of CO may cause depressed mental status, coma, or death.
Although HbCO levels are confirmatory of exposure, venous levels do not necessarily reflect tissue concentrations or outcomes. Patients with a similar level to that of this patient (19.5%) may present with no symptoms, mild headache, or a deep coma depending on the duration of exposure to CO.
Definitive treatment is removal from the toxic environment and prompt administration of O2. In some cases, hyperbaric therapy may be beneficial.14
Diagnosis
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
It is rarely easy to diagnose the first case of an illness of which one is unfamiliar or not accustomed to treating. Likewise, it is very difficult to consider, diagnose and, as a result, effectively manage the first presentation of a known condition that is typically seasonal or linked to a different geographic location. Acute presentations of environmental exposures, illicit drug poisonings, and communicable infectious diseases are increasingly the purview of emergency medicine. Whether it is the first case of Ebola, of severe acute respiratory syndrome, the influenza virus, a new lethal street drug overdose, or CO poisoning prior to the onset of winter, maintaining a high index of suspicion for the “index case” is of paramount importance. The patient presented here, the first CO poisoning of the season at the authors’ institution, illustrates the responsibility the EP to consider, diagnose, and prevent a wide-range of deadly consequences—injury prevention as the result of vigilance. Moreover, the consequences of missing the diagnosis would have placed others at risk for continued poisoning and possibly death.
Portable and Ambient Carboxyhemoglobin Monitors
The NYC Department of Health (NYCDOH) requires that all EMTs and paramedics wear CO detectors and all residential housing contain CO monitors. The NYCDOH also mandates that all identified cases of CO poisoning be reported to the NYC Poison Control Center. This centralization of data on any and all patients exposed to CO can result in an investigation of the source of CO by the fire department and capture symptomatic patients who present for care outside of the 911 response system. The source of CO in this patient was ultimately traced to a faulty furnace that was repaired to prevent others in the building from becoming victims of CO poisoning.
It should be noted that portable noninvasive HbCO monitors may be inadequate to rule out CO poisoning as the sensitivity of such devices can be as low as 48%.15 Carbon monoxide poisoning can result from brief exposure to a high ambient concentration, such as a fire in which environmental concentrations may exceed 500 ppm or more insidiously, in a setting of a chronic exposure. Faulty furnaces—a common seasonal cause of CO poisoning—may continue to produce adequate heat and fail to prompt any concerns.
Since CO is colorless and odorless, ambient CO detectors stationed in the home are the best means of alerting one to exposure. In this case, though mandated by NYCDOH, a CO detector was not present in the patient’s home.
Case Conclusion
Through the rapid identification of CO poisoning in this elderly patient with altered mental status, EMS was able to evacuate the building while bringing the elderly tenant and her home attendant to the ED.
Based on the elderly patient’s elevated HbCO level, she was treated with O2 and discharged from the hospital the following day feeling well. In addition to the patient’s symptoms, when the aide was interviewed, she reported that she had been experiencing daily headaches, which she said soon resolved on departure from her client’s house. Her symptoms had been bothersome, but not so severe as to prompt her to seek medical attention. The aide was found to have an HbCO level of 12.5% and was discharged from the ED after 6 hours of observation and O2 therapy. The third occupant of the building, a tenant, was also brought to the ED and found to have an HbCO level of 12%. The tenant was treated with O2 therapy and discharged to home.
Dr Caldwell is an assistant professor of medicine in the department of emergency medicine, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Rao is an assistant professor of emergency medicine; and the chief in the division of medical Toxicology, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Stern is an assistant professor of medicine, department of emergency medicine; chief of geriatric emergency medicine; and codirector of geriatric emergency medicine fellowship at New York Presbyterian Hospital/Weill Cornell Medical Center, New York.
Case
A 100-year-old woman with a history of hypertension, hypothyroidism, and moderate Alzheimer dementia was brought to the ED by emergency medical services (EMS) for altered mental status after her home health aide (HHA) noted a change in the patient’s behavior. For the past few days, the patient’s appetite waned, and she became progressively more lethargic, not eating for over 24 hours. The aide activated 911 on the direction of the patient’s primary care physician. There were no reported changes to the patient’s medications which included aspirin, levothyroxine, and hydrochlorothiazide. She was unable to provide any meaningful history.
On arrival to the ED, the patient appeared comfortable in bed. She was sleepy, but easily aroused. Initial vital signs were: heart rate, 110 beats/minute; respiratory rate, 12 breaths/minute; blood pressure, 163/103 mm Hg; oral temperature, 98.2˚F. Oxygen (O2) saturation was 96% on room air. She was oriented to person only and responded appropriately to simple questions, intermittently following one-step commands. She was unable to attend and required redirection throughout the interview. (According to the aide, this behavior was different than her baseline.)
The patient’s head and neck examination were notable for some mild, boggy, periorbital edema and dry mucous membranes. Her thyroid examination was normal; her lungs were clear; and her cardiac examination revealed a 2/6 systolic ejection murmur over the second right intercostal space. Examination of the abdomen, extremities, and skin was unrevealing, and there were no gross focal neurological deficits. Her reflexes were normal throughout.
Initial assessment of this patient suggested a diagnosis of dementia and hypoactive delirium—the latter due to one or more of several possible etiologies.
Altered Mental Status
While altered mental status is a billable medical ICD-9-CM code1 used to specify a diagnosis on a reimbursement claim, it is not a disease state itself. Instead, it is a catchall phrase that incorporates any change in mental status, encompassing symptomatology that may have the largest differential diagnosis encountered in emergency medicine.
Delirium
An important category of altered mental status is delirium. The diagnostic criteria2 for delirium in DSM-V have remained essentially unchanged from DSM-IV; however, the prevalence of delirium as one of the key geriatric syndromes has grown as a result of increased research and education, particularly in the emergency medical setting. Distilled down, delirium can be defined as an acute change in mental status not caused by underlying dementia. Its cause is often multifactorial, and it is frequently an underappreciated consequence of both critical illness and the hospital environment.3
Delirium is an emergency unto itself, with an in-hospital mortality rate mirroring that of sepsis or acute myocardial infarction.4 The older-adult population is especially at risk of delirium and can present with one of three clinical subtypes: hyperactive (ie, agitated, etc), hypoactive (ie, somnolent, lethargic, stuporous, etc), and mixed type.5
The composition of factors precipitating the onset of delirium includes age, dementia, alcohol use, depression, illness severity, and drug exposure—notably benzodiazepines, opiates, and medications with anticholinergic properties.8 While major precipitants or causes of delirium, traditionally considered as potentially life-threatening acute events, are well known (Table 1), others are often overlooked (Table 2). Yet, ironically, these more frequently bypassed causes are more readily reversible, once discovered, such as inadequate pain control, urinary retention, constipation, dehydration, polypharmacy, and negative environmental conditions in the patient’s immediate surroundings. These findings have recently been corroborated.9
The Confusion Assessment Method
Identifying delirium can be a particular challenge for emergency physicians (EPs), especially when the patient has an underlying diagnosis of dementia and the specific degree of cognitive impairment is not known. The Confusion Assessment Method (CAM)10 is the most commonly used tool in the critical care setting11 and is the only validated tool for the ED, with an 86% sensitivity and 100% specificity.12 It evaluates four elements: (1) acute onset and fluctuating course; (2) inattention; (3) disorganized thinking; and (4) altered level of consciousness. A patient must demonstrate the first two elements in addition to either the third or fourth element to be considered to have delirium.10
The CAM intensive care unit scale has the potential to be even more applicable in the ED. Recent findings support its validation.9
Case Continued
With respect to the elderly patient in this case with dementia and multiple potential causes for hypoactive delirium, the life-saving measure was the New York City (NYC) mandate that all 911 responding EMS workers wear ambient carbon monoxide (CO) detectors. Though the value of these detectors is controversial due to their low sensitivity, the emergency medical technicians (EMTs) detected an elevated CO level of 300 ppm when they arrived at the patient’s home.
Without this information, the patient’s age and clinical presentation would almost certainly have prompted an extensive evaluation to determine the etiology of her change in mental status and most likely would have missed the true cause of CO toxicity, which was confirmed by venous co-oximetry showing the patient to have a carboxyhemoglobin (HbCO) level of 19.5%.
Carbon Monoxide Toxicity
Carbon monoxide affects multiple cell types. It binds to myoglobin and in high concentrations depresses myocardial contractility. In platelets, CO displaces nitric oxide potentially resulting in vasodilation. Life-threatening CO poisoning causes hypotension, syncope, tachycardia, and an altered mental status. Delayed neuropsychiatric sequelae also may occur as the result of free radical injury to the brain.13
Symptoms
Patients with chronic CO poisoning who can adequately communicate may report nausea, headache, lightheadedness, and lethargy mimicking other seasonal illnesses. In debilitated or cognitively impaired patients who are unable to communicate, findings may include tachycardia, a mild change in mental status, and little else. Prolonged exposure and physiologic accumulation of CO may cause depressed mental status, coma, or death.
Although HbCO levels are confirmatory of exposure, venous levels do not necessarily reflect tissue concentrations or outcomes. Patients with a similar level to that of this patient (19.5%) may present with no symptoms, mild headache, or a deep coma depending on the duration of exposure to CO.
Definitive treatment is removal from the toxic environment and prompt administration of O2. In some cases, hyperbaric therapy may be beneficial.14
Diagnosis
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
Although CO exposure is the most common cause of poisoning death worldwide, its detection requires a high index of suspicion, especially in areas where public-health protection measures are absent.
It is rarely easy to diagnose the first case of an illness of which one is unfamiliar or not accustomed to treating. Likewise, it is very difficult to consider, diagnose and, as a result, effectively manage the first presentation of a known condition that is typically seasonal or linked to a different geographic location. Acute presentations of environmental exposures, illicit drug poisonings, and communicable infectious diseases are increasingly the purview of emergency medicine. Whether it is the first case of Ebola, of severe acute respiratory syndrome, the influenza virus, a new lethal street drug overdose, or CO poisoning prior to the onset of winter, maintaining a high index of suspicion for the “index case” is of paramount importance. The patient presented here, the first CO poisoning of the season at the authors’ institution, illustrates the responsibility the EP to consider, diagnose, and prevent a wide-range of deadly consequences—injury prevention as the result of vigilance. Moreover, the consequences of missing the diagnosis would have placed others at risk for continued poisoning and possibly death.
Portable and Ambient Carboxyhemoglobin Monitors
The NYC Department of Health (NYCDOH) requires that all EMTs and paramedics wear CO detectors and all residential housing contain CO monitors. The NYCDOH also mandates that all identified cases of CO poisoning be reported to the NYC Poison Control Center. This centralization of data on any and all patients exposed to CO can result in an investigation of the source of CO by the fire department and capture symptomatic patients who present for care outside of the 911 response system. The source of CO in this patient was ultimately traced to a faulty furnace that was repaired to prevent others in the building from becoming victims of CO poisoning.
It should be noted that portable noninvasive HbCO monitors may be inadequate to rule out CO poisoning as the sensitivity of such devices can be as low as 48%.15 Carbon monoxide poisoning can result from brief exposure to a high ambient concentration, such as a fire in which environmental concentrations may exceed 500 ppm or more insidiously, in a setting of a chronic exposure. Faulty furnaces—a common seasonal cause of CO poisoning—may continue to produce adequate heat and fail to prompt any concerns.
Since CO is colorless and odorless, ambient CO detectors stationed in the home are the best means of alerting one to exposure. In this case, though mandated by NYCDOH, a CO detector was not present in the patient’s home.
Case Conclusion
Through the rapid identification of CO poisoning in this elderly patient with altered mental status, EMS was able to evacuate the building while bringing the elderly tenant and her home attendant to the ED.
Based on the elderly patient’s elevated HbCO level, she was treated with O2 and discharged from the hospital the following day feeling well. In addition to the patient’s symptoms, when the aide was interviewed, she reported that she had been experiencing daily headaches, which she said soon resolved on departure from her client’s house. Her symptoms had been bothersome, but not so severe as to prompt her to seek medical attention. The aide was found to have an HbCO level of 12.5% and was discharged from the ED after 6 hours of observation and O2 therapy. The third occupant of the building, a tenant, was also brought to the ED and found to have an HbCO level of 12%. The tenant was treated with O2 therapy and discharged to home.
Dr Caldwell is an assistant professor of medicine in the department of emergency medicine, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Rao is an assistant professor of emergency medicine; and the chief in the division of medical Toxicology, New York Presbyterian Hospital/Weill Cornell Medical Center, New York. Dr Stern is an assistant professor of medicine, department of emergency medicine; chief of geriatric emergency medicine; and codirector of geriatric emergency medicine fellowship at New York Presbyterian Hospital/Weill Cornell Medical Center, New York.
- ICD-9Data.com Web site. 2014 ICD-9-CM Diagnosis Codes. http://www.icd9data.com/2014/Volume1/default.htm. Accessed December 4, 2014.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Han JH, Wilson A, Ely EW. Delirium in the older emergency department patient: a quiet epidemic. Emerg Med Clin North Am. 2010;28(3):611-631.
- Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
- Mulcare MR, Halpern A, Stern ME. The geriatric patient. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:741-753.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
- Han JH, Vasilevskis EE, Ely EW. Sedation and delirium. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:704-717.
- Rosen T, Connors S, Halpern A, et al. Improving emergency department identification and management of agitated delirium in older adults: Implementation and impact assessment of a comprehensive clinical protocol using an A-B-C-D-E-F mnemonic. Ann Emerg Med. 2013;62(4)(Supp 4):S53-54.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
- Monette J, Galbaud du Fort G, Fung SH, et al. Evaluation of the Confusion Assessment Method (CAM) as a screening tool for delirium in the emergency room. Gen Hosp Psychiatry. 2001;23(1):20-25.
- Weaver LK. Carbon monoxide poisoning. New Engl J Med. 2009;360(12):1217-1225.
- Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric therapy for acute carbon monoxide poisoning. New Engl J Med. 2002;347(14):1057-1067.
- Touger M, Birnbaum A, Wang J, Chou K, Pearson D, Bijur P. Performance of the RAD-57 pulse CO-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56(4):382-388.
- ICD-9Data.com Web site. 2014 ICD-9-CM Diagnosis Codes. http://www.icd9data.com/2014/Volume1/default.htm. Accessed December 4, 2014.
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Washington, DC: American Psychiatric Association; 2013.
- Han JH, Wilson A, Ely EW. Delirium in the older emergency department patient: a quiet epidemic. Emerg Med Clin North Am. 2010;28(3):611-631.
- Inouye SK. Delirium in older persons. N Engl J Med. 2006;354(11):1157-1165.
- Mulcare MR, Halpern A, Stern ME. The geriatric patient. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:741-753.
- Han JH, Zimmerman EE, Cutler N, et al. Delirium in older emergency department patients: recognition, risk factors, and psychomotor subtypes. Acad Emerg Med. 2009;16(3):193-200.
- Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. Predictive model and interrelationship with baseline vulnerability. JAMA. 1996;275(11):852-857.
- Han JH, Vasilevskis EE, Ely EW. Sedation and delirium. In: Arbo JE, Ruoss SJ, Lighthall GK, Jones MP, eds. Decision Making in Emergency Critical Care: An Evidence-Based Handbook. Philadelphia, PA: Wolters Kluwer; 2015:704-717.
- Rosen T, Connors S, Halpern A, et al. Improving emergency department identification and management of agitated delirium in older adults: Implementation and impact assessment of a comprehensive clinical protocol using an A-B-C-D-E-F mnemonic. Ann Emerg Med. 2013;62(4)(Supp 4):S53-54.
- Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI. Clarifying confusion: the confusion assessment method. A new method for detection of delirium. Ann Intern Med. 1990;113(12):941-948.
- Wei LA, Fearing MA, Sternberg EJ, Inouye SK. The Confusion Assessment Method: a systematic review of current usage. J Am Geriatr Soc. 2008;56(5):823-830.
- Monette J, Galbaud du Fort G, Fung SH, et al. Evaluation of the Confusion Assessment Method (CAM) as a screening tool for delirium in the emergency room. Gen Hosp Psychiatry. 2001;23(1):20-25.
- Weaver LK. Carbon monoxide poisoning. New Engl J Med. 2009;360(12):1217-1225.
- Weaver LK, Hopkins RO, Chan KJ, et al. Hyperbaric therapy for acute carbon monoxide poisoning. New Engl J Med. 2002;347(14):1057-1067.
- Touger M, Birnbaum A, Wang J, Chou K, Pearson D, Bijur P. Performance of the RAD-57 pulse CO-oximeter compared with standard laboratory carboxyhemoglobin measurement. Ann Emerg Med. 2010;56(4):382-388.
My Most Unusual Case: Asphyxiation by Cake: An Interesting Case of Dyspnea
Case
A 58-year-old man presented to the ED via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a 2-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
The patient’s vital signs at examination were: oral temperature, 97.5oF; pulse, 62 beats/minute; respiratory rate (RR), 18 breaths/minute; and blood pressure, 133/83 mm Hg. Oxygen (O2) saturation was 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical examination was completely normal.
Ancillary studies revealed a normal chest X-ray. An electrocardiogram demonstrated sinus bradycardia with a rate of 56, but no evidence of ischemia or right heart strain. A complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late model rental in good condition per report. The patient informed the treating EP that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO, but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]), sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, but he was switched to 2 L of O2 via nasal cannula shortly afterward. He was observed for a total of 4 hours after arrival; as he remained symptom-free, he was discharged home. The EP was not able to obtain postdischarge follow up information.
Discussion
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Carbon dioxide is a prevalent gas that is part of everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas. Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicological effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and even death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an 8-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Other Case Presentations
Similar cases as the one presented in this article have been described in the literature. In one such case, following Hurricane Ivan, a 34-year-old-man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found the initial O2 concentration to be 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural Phenomenon
Differential Diagnosis
When CO2 toxicity is suspected, other conditions should be considered as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and Treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or computed tomography scanning, as indicated.
Initial management of suspected CO2 toxicity includes first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal. If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher than normal RR will help remove excessive CO2 if mechanical ventilation is required. If a respiratory acidosis is present, intravenous sodium bicarbonate should be avoided as this may increase the level of serum CO2. Intravenous fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
Dr Schreckengaust is an emergency physician in the department of emergency medicine at Camp Lejune, North Carolina. Dr Lang is an assistant professor in the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
- Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
- Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
- Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed November 12, 2014.
- Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
- Dunford JV, Lucas J, Vent N, Clark RF, Cantrell FL. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
- Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
Case
A 58-year-old man presented to the ED via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a 2-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
The patient’s vital signs at examination were: oral temperature, 97.5oF; pulse, 62 beats/minute; respiratory rate (RR), 18 breaths/minute; and blood pressure, 133/83 mm Hg. Oxygen (O2) saturation was 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical examination was completely normal.
Ancillary studies revealed a normal chest X-ray. An electrocardiogram demonstrated sinus bradycardia with a rate of 56, but no evidence of ischemia or right heart strain. A complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late model rental in good condition per report. The patient informed the treating EP that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO, but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]), sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, but he was switched to 2 L of O2 via nasal cannula shortly afterward. He was observed for a total of 4 hours after arrival; as he remained symptom-free, he was discharged home. The EP was not able to obtain postdischarge follow up information.
Discussion
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Carbon dioxide is a prevalent gas that is part of everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas. Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicological effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and even death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an 8-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Other Case Presentations
Similar cases as the one presented in this article have been described in the literature. In one such case, following Hurricane Ivan, a 34-year-old-man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found the initial O2 concentration to be 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural Phenomenon
Differential Diagnosis
When CO2 toxicity is suspected, other conditions should be considered as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and Treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or computed tomography scanning, as indicated.
Initial management of suspected CO2 toxicity includes first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal. If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher than normal RR will help remove excessive CO2 if mechanical ventilation is required. If a respiratory acidosis is present, intravenous sodium bicarbonate should be avoided as this may increase the level of serum CO2. Intravenous fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
Dr Schreckengaust is an emergency physician in the department of emergency medicine at Camp Lejune, North Carolina. Dr Lang is an assistant professor in the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
Case
A 58-year-old man presented to the ED via emergency medical services (EMS) with shortness of breath, lightheadedness, and nausea. Upon arrival to the ED, most of his symptoms had resolved. The patient reported that he had taken a 2-hour flight into town the previous day and had spent an uneventful evening at a local hotel. He said that he began experiencing shortness of breath and lightheadedness soon after entering his rental vehicle an hour prior to presentation, explaining that he felt as if he “could not get any air.”
The patient’s vital signs at examination were: oral temperature, 97.5oF; pulse, 62 beats/minute; respiratory rate (RR), 18 breaths/minute; and blood pressure, 133/83 mm Hg. Oxygen (O2) saturation was 100% on room air. He was alert and oriented, in no distress, easily conversational, and without diaphoresis. The lungs were clear to auscultation bilaterally, and there was no calf swelling, tenderness, or palpable cords. The remainder of the physical examination was completely normal.
Ancillary studies revealed a normal chest X-ray. An electrocardiogram demonstrated sinus bradycardia with a rate of 56, but no evidence of ischemia or right heart strain. A complete blood count, troponin I, D-dimer, and creatine phosphokinase (CPK) with MB fraction levels were all within normal limits. A serum chemistry panel was also within normal limits, except for a serum glucose level of 181 mg/dL. Venous co-oximetry showed a carboxyhemoglobin level of 0.0, and methemoglobin level of 0.5 gm% (normal range, 0.4-1.5).
Since both the patient and hotel clerk’s symptoms started when each was in the rental car, the patient was questioned about the vehicle and its contents. The car was a late model rental in good condition per report. The patient informed the treating EP that he worked as a decorative cake salesman and had brought cake samples with him to display at a trade show. He further stated that he had left these samples in the car overnight, packed in dry ice.
Upon learning this information, EMS was contacted and instructed to return to the hotel and rental vehicle. The hotel room was noted to have normal levels of O2 and carbon monoxide (CO) on measurement. Investigation of the car revealed normal levels of CO, but O2 levels too low to read on the sensor. The emergency team concluded that the dry ice (the solid form of carbon dioxide [CO2]), sublimed to CO2 gas overnight. This displaced the O2 in the vehicle, resulting in severe hypoxia and the symptoms of both the patient and hotel clerk.
The patient was initially placed on 15 L of O2 via a nonrebreather mask, but he was switched to 2 L of O2 via nasal cannula shortly afterward. He was observed for a total of 4 hours after arrival; as he remained symptom-free, he was discharged home. The EP was not able to obtain postdischarge follow up information.
Discussion
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Carbon dioxide is a prevalent gas that is part of everyday life, from an agent in fire extinguishers and carbonation in beverages to byproducts of cellular metabolism. Similar to CO, it is a colorless and odorless gas. Carbon dioxide is commonly used in the food industry as dry ice to keep items cold. In its solid state, CO2 can cause severe frostbite with direct contact, similar to a burn. However, when dry ice is warmed and sublimated to a gaseous state, large amounts of CO2 are generated, and this heavy gas can accumulate and displace air (ie, atmospheric O2), especially in confined spaces. In low concentrations, gaseous CO2 appears to have minimal toxicological effects, but at higher concentrations it can cause tachycardia, tachypnea, dyspnea, visual disturbances, arrhythmias, impaired levels of consciousness, and even death.
Carbon dioxide primarily acts as a simple asphyxiant, but it also dissolves in serum as carbonic acid, resulting in a metabolic acidosis. Compensation for this acidosis is accomplished by an increased RR (ie, respiratory alkalosis), which further worsens the intake of CO2.1,2
The normal concentration of CO2 in the atmosphere is approximately 0.04% (396 ppm). The Occupational Safety and Health Administration (OSHA) has set a maximum safe exposure level of CO2 at 0.5% (5,000 ppm) over an 8-hour day.3 Concentrations as low as 1% (10,000 ppm) may cause drowsiness. Exposure to concentrations of 7% to 10% for several minutes to an hour results in headache, tachycardia, dyspnea, and hyperventilation. At levels of 10% to 15%, dizziness, severe muscle twitching, and loss of consciousness can occur after only a few minutes. Death occurs within minutes at concentrations greater than 30%.2
Carbon dioxide also acts as a potent cerebral vasodilator, which may explain symptoms such as headache and dizziness.2 The severity of symptoms is dependent on the concentration of CO2, the length of the exposure, and the underlying health of the patient. Elevated concentrations of CO2 can occur in areas where there is limited or poor ventilation, such as in a mine (where it is known as blackdamp, stythe, or choke damp),4 submarine, grain silo, or a sealed building without mechanical ventilation.
Other Case Presentations
Similar cases as the one presented in this article have been described in the literature. In one such case, following Hurricane Ivan, a 34-year-old-man placed four 25-pound blocks of dry ice wrapped in paper in the front seat of his truck with the windows closed.5 After driving less than one quarter of a mile, he developed dyspnea and telephoned for help before losing consciousness. Fortunately, he was found in time and recovered soon after the doors to his truck were opened.5
In another case, a 59-year-old man entered a walk-in freezer that contained dry ice wrapped loosely in plastic. He was found inside the freezer 20 minutes later in cardiac arrest; resuscitation efforts were unsuccessful. Investigation of the freezer found the initial O2 concentration to be 13% (normal level, 20.93%) and an estimated CO2 level of 40%.5
Similarly, a 35-year-old woman was inadvertently locked in a bank vault while storing receipts. In a bid for help, she pulled the fire alarm, which triggered a CO2-based fire-extinguishing system. The fire department responded and found the woman dead in the vault 30 minutes later. The cause of death was labeled as CO2 intoxication.6
Natural Phenomenon
Differential Diagnosis
When CO2 toxicity is suspected, other conditions should be considered as there may be more than one process involved. For example, other causes of coma or dyspnea should be investigated, including trauma, hypoglycemia, CO, methemoglobinemia, or other metabolic processes. In addition, a patient may have a pre-existing condition, such as a trauma or an altered mental status due to drugs or alcohol, all of which can increase his or her susceptibility to the effects of CO2.
Evaluation and Treatment
Useful laboratory testing includes arterial blood gas, venous co-oximetry for carboxyhemoglobin, chemistry panels, ethanol testing, and radiographs or computed tomography scanning, as indicated.
Initial management of suspected CO2 toxicity includes first removing the patient from the source of the gas. Rescuers must exercise caution so as to prevent a mass casualty incident. Once out of the dangerous environment, as long as the patient is conscious and spontaneously breathing, supportive measures are generally all that are necessary. Oxygen should be applied, after which the spontaneously breathing patient without underlying lung disease should rapidly return to normal. If there is marked decrease in mental status or poor respiratory drive despite O2 administration, intubation with mechanical ventilation may be required. A higher than normal RR will help remove excessive CO2 if mechanical ventilation is required. If a respiratory acidosis is present, intravenous sodium bicarbonate should be avoided as this may increase the level of serum CO2. Intravenous fluids and other supportive measures, including treatment for any concurrent conditions, may be indicated.
Dr Schreckengaust is an emergency physician in the department of emergency medicine at Camp Lejune, North Carolina. Dr Lang is an assistant professor in the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. Dr Counselman is the distinguished professor and chairman of the department of emergency medicine at Eastern Virginia Medical School, Norfolk; and a physician at Emergency Physicians of Tidewater, Norfolk, Virginia. He is also the associate editor in chief of EMERGENCY MEDICINE editorial board.
- Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
- Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
- Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed November 12, 2014.
- Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
- Dunford JV, Lucas J, Vent N, Clark RF, Cantrell FL. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
- Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
- Nelson LS, Odujebe OA. Simple asphyxiants and pulmonary irritants. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies, 9th ed. New York, NY: McGraw-Hill; 2011:1644-1645.
- Langford NJ. Carbon dioxide poisoning. Toxicol Rev. 2005;24(4):229-235.
- Occupational Health and Safety Standards. Table Z-1, Limits for air contaminants. Occupational Safety and Health Administration Web site. https://www.osha.gov/pls/oshaweb/owadisp.show_document?p_table=standards&p_id=9992. Accessed November 12, 2014.
- Hedlund FH. The extreme carbon dioxide outburst at the Menzengraben potash mine 7 July 1953. Safety Sci. 2012;50(3):537-553.
- Dunford JV, Lucas J, Vent N, Clark RF, Cantrell FL. Asphyxiation due to dry ice in a walk-in freezer. J Emerg Med. 2009;36(4):353-356.
- Gill JR, Ely SF, Hua Z. Environmental gas displacement: three accidental deaths in the workplace. Am J Forensic Med Pathol. 2002;23(1):26-30.
Case Studies in Toxicology: An Amazonian Herb Goes Mainstream
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
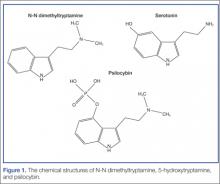
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
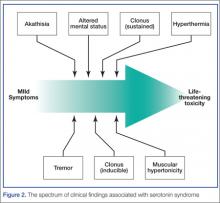
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 23-year-old Hispanic woman with no past medical history is brought to the ED for the second time in one day. On her first presentation, which was for a fever and a headache, meningitis was excluded with normal laboratory tests that included a lumbar puncture. She was administered acetaminophen for fever and pain control, and was discharged with a diagnosis of viral illness. On this second visit, 10 hours after being discharged, she presented because her family noted convulsions that began 3 hours after taking an herbal headache remedy given to her by a naturopath.
The patient arrived to the ED with a persistent seizure that terminated following administration of 2 mg of lorazepam. Her initial vital signs were: blood pressure, 115/51 mm Hg; heart rate, 121 beats/minute; respiratory rate, 24 breaths/minute; temperature, 97.6oF. Oxygen (O2) saturation was 100% with 2 L of O2 administered via nasal cannula. Her neurological examination was significant for a depressed mental status, pupils that were 6 mm and minimally reactive, clonus, and hyperreflexia. Repeat laboratory evaluation found a leukocytosis of 22.0 x 103/µL, serum bicarbonate of 9 mEq/L, and an anion gap of 22 with a normal serum lactate.
What is the differential diagnosis of this patient?
The history of medicinal plant ingestion raises the possibility of a toxicologic etiology. However, because the patient took the “medication” to treat another disorder, a search for an alternate cause should be performed. The differential diagnosis of a toxin-induced seizure is broad and includes pharmaceuticals (eg, tramadol, antihistamines), which may be surreptitiously added to herbal medication to assure efficacy. Plants associated with seizures include those containing antimuscarinic tropane alkaloids such as Jimsonweed (though a rare side effect from this plant product) or the water hemlock (Cicuta maculata). Contaminants of the plant itself may include pesticides such as organophosphates.
Although unlikely in a 21 year old, withdrawal from benzodiazepines, ethanol, baclofen, or gamma hydroxybutyrate are other possible etiologies. In addition to pharmaceutical and plant-derived causes, carbon monoxide poisoning should be a consideration in any patient with headache and flu-like illness.
This patient also presented with a constellation of other findings that included hyperreflexia, clonus, tachycardia, and altered mental status. Together these signs are expected in patients with serotonin toxicity (also referred to as serotonin syndrome), neuroleptic malignant syndrome, exogenous thyrotoxicosis, and lithium poisoning.
Case Continuation
The naturopathic practitioner arrived at the ED concerned about the patient, informing the ED team that she had given the patient 2 ounces of ayahuasca tea.
What is ayahuasca? What is the mechanism by which it exerts toxic effects?
Ayahuasca is a plant-derived psychotropic beverage that is used for religious purposes by members of two Brazilian churches—Centro Espírita Beneficente União do Vegetal (UDV) and Santo Daime. The ayahuasca beverage consists of two pharmacologically active compounds that together, but not individually, are psychoactive. The desired active effects for church participants include hallucinations, and vomiting to bring about a “religious purge.”1
Ayahuasca is prepared by combining two plants indigenous to the Amazon Basin area: Banisteriopsis caapi and either Psychotria viridis or Diplopterys cabrerana. B caapi contains the β-carboline alkaloids harmine, harmaline, and tetrahydroharmine. These alkaloids act as reversible inhibitors of the monoamine oxidase A (MAO-A) enzyme. The bark and stems of B caapi are boiled along with either P viridis or D cabrerana, both of which contain the potent hallucinogen N-N dimethyltryptamine (DMT).2 Normally, DMT is not active orally because it is enzymatically metabolized by MAO-A. However, when taken in the presence of the B caapi-derived MAO-A–inhibiting harmine alkaloids, DMT reaches the systemic circulation and produces its clinical effects.3
What are the clinical findings of serotonin toxicity?
Serotonin toxicity is a collection of clinical findings that fall under three main categories: autonomic hyperactivity, altered mental status, and muscle rigidity.5 The autonomic findings may include tachycardia, hypertension, hyperthermia, shivering, diaphoresis, or mydriasis. Altered mental status ranges from mild agitation and hypervigilance to agitated delirium to obtundation. Other neurological findings may include tremor, myoclonus, hyperreflexia, or seizures. The onset of these signs is rapid, usually occurring within minutes after exposure to one or more serotonergic compounds. Although rare, severe serotonin toxicity may be associated with hypotension and shock, leading to death.4
The diagnosis of serotonin toxicity is based on the history and physical examination of the patient. Diagnostic criteria that have been suggested include the following: (1) a recent addition or increase in a known serotonergic agent; (2) absence of other possible etiologies; (3) no recent increase or addition of a neuroleptic agent (suggesting neuroleptic malignant syndrome); and/or (4) at least 3 of the following symptoms—mental status changes, myoclonus, agitation, hyperreflexia, diaphoresis, shivering, tremor, diarrhea, incoordination, fever5 (Figure 2).
How should this patient be managed?
The management of serotonin toxicity is primarily supportive with aggressive control of hyperthermia and autonomic instability. The precipitating xenobiotic agent should be immediately discontinued. In general, treatment with intravenous fluids, cooling measures, benzodiazepines, and a nonspecific 5-HT antagonist such as cyproheptadine should greatly improve the patient’s clinical status. Patients with severe toxicity may require induced paralysis and intubation.4 It is not clear in this case if the serotonin hyperactivation was due to the DMT (5-HT2A is associated with serotonin toxicity) or another serotonergic agent (eg, dextromethorphan from a cough and cold preparation) in combination with the MAO-inhibiting harmine alkaloids.
What is the availability of ayahuasca in the United States? How is it used in its nonherbal form?
...[Ayahuasca] is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches. Many clinicians are becoming increasingly familiar with this herbal preparation since the recreational use of ayahuasca is gaining popularity in the United States. Internet fora with information on how to safely use ayahuasca, such as avoiding aged cheeses, are becoming more prevalent.7 A recent article in the New York Times described an ayahuasca gathering in Brooklyn, New York, where participants use the herb in a communal fashion.8 This herbal product is also associated with the Hollywood social scene and has received celebrity endorsements.8
The National Survey on Drug Use and Health found that the number of people in the United States who have used DMT has gone up almost every year since 2006, from an estimated 688,000 in 2006 to 1,475,000 in 2012.9 When used alone (not as ayahuasca), DMT is almost exclusively insufflated as a nasal snuff, bypassing hepatic elimination. It has an onset of around 45 seconds and a duration of 5 to 10 minutes. Insufflating DMT was historically referred to as a “businessman’s trip” because users were able to have a brief hallucinogenic experience on a lunch break and recover rapidly to perform their normal work.10
International law declares that DMT is an illegal substance and its importation is banned. However, its use for religious purposes, as is allowed for mescaline found in peyote, remains controversial.7 The UDV brought suit in United States federal court to prevent interference with the church’s use of ayahuasca during religious ceremonies based on the Religious Freedom Restoration Act. This act states that the government should not cause substantial imposition on religious practices in the absence of a compelling government interest. The court sided with the UDV, finding that the government had not sufficiently proved the alleged health risks posed by ayahuasca and could not show a substantial risk that the drug would be abused recreationally.11 Thus it is currently available in the United States and is legal for use by members of the UDV and Santo Daime churches.
Ayahuasca is not regulated by the US Food and Drug Administration. Many different types of preparations with different ingredients as well as different concentrations may exist, and clinical variability should be expected. Understanding that ayahuasca is capable of inhibiting MAO is important in order to avoid foods and medications, such as dextromethorphan, that may trigger adverse effects.
Case Conclusion
The patient’s hospital course was complicated by an additional seizure 12 hours after her initial presentation. By 36 hours she was back to her baseline mental status with a normal neurological examination.
Dr Fil is a senior fellow in medical toxicology at North Shore University Hospital, Manhasset, New York. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
- Gable RS. Risk assessment of ritual use of oral dimethyltryptamine (DMT) and harmala alkaloids. Addiction. 2007;102(1):24-34.
- Riba J, McIlhenny EH, Valle M, Bouso JC, Barker SA. Metabolism and Disposition of N,N-dimethyltryptamine and harmala alkaloids after oral administration of ayahuasca. Drug Test Anal. 2012;4(7-8):610-616.
- Riba J, Valle M, Urbano G, Yritia M, Morte A, Barbanoj MJ. Human Pharmacology of Ayahuasca: Subjective and Cardiovascular Effects, Monoamine Metabolite Excretion and Pharmacokinetics. J Pharmacol Exp Ther. 2003;306(1):73-83
- Boyer EW, Shannon M. The serotonin syndrome. N Engl J Med. 2005;352(11);1112-1120.
- Sternbach H. The serotonin syndrome. Am J Psychiatry. 1991;148(6):6;705-713.
- Dunkley EJ, Isbister GK, Sibbritt D, Dawson AH, Whyte IM. The Hunter Serotonin Toxicity Criteria: simple and accurate diagnostic decision rules for serotonin toxicity. QJM. 2003;96(9):635-642.
- Erowid. Ayahuasca Vault. https://www.erowid.org/chemicals/ayahuasca/ayahuasca.shtml. Accessed November 25, 2014.
- Morris B. Ayahuasca: a strong cup of tea. New York Times. June 13, 2014. http://www.nytimes.com/2014/06/15/fashion/ayahuasca-a-strong-cup-of-tea.html. Accessed November 25, 2014.
- Quintanilla D. DMT: Hallucinogenic Drug Used in Shamanic Rituals Goes Mainstream. 10 Dec 2013. Available: http://www.opposingviews.com/i/health/dmt-hallucinogenic-drug-used-shamanic-rituals-goes-mainstream. Last accessed 11/14/14.
- Haroz R, Greenberg MI. Emerging drugs of abuse. Med Clin North Am. 2005;89(6):1259-1276.
- Gonzales v. O Centro Espirita Beneficente Uniao do Vegetal, 546 US 418 (2006). Available at http://scholar.google.com/scholar_case?case=7036734975431570669&hl=en&as_sdt=6&as_vis=1&oi=scholarr. Accessed November 25, 2014.
Large Solitary Glomus Tumor of the Wrist Involving the Radial Artery
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
Glomus tumors are neoplasms that originate from normal glomus bodies in the skin and are most commonly found in the subungual areas of the digits.1 Glomus bodies are neuromyoarterial structures in the reticular dermis that serve as specialized arteriovenous anastomoses. These bodies contain afferent arterioles and efferent veins with multiple connections, and glomus cells have contractile properties because of their similarity to smooth muscle cells.1,2 Glomus bodies help regulate blood flow and temperature of the skin and are found in their largest concentration in the fingertips, palms of the hands, and soles of the feet.3,4
Glomus tumors represent hyperplastic glomus bodies and make up 1% to 4.5% of upper extremity neoplasms, with approximately 75% in the hand and 50% in the subungual area.1,5,6 These tumors can also present in multiple locations at once and can occur in atypical and ectopic locations.3 Although generally benign, glomus tumors can also exhibit malignant and metastatic potential in rare cases.7,8 They can also be locally aggressive with bony destruction of the distal phalynx.2,9,10 Tumors typically present as painful solitary soft-tissue lesions that are exquisitely tender to palpation, dark red-purple or bluish, and hypersensitive to cold.5,10 Van Geertruyden and colleagues10 reported that the diagnosis of glomus tumor can be made clinically in 90% of cases. However, glomus tumors can easily be mistaken for other lesions, such as hemangiomas, angiomas, neuromas, neurofibromas, lipomas, and ganglion cysts. An inaccurate or incomplete workup can result in persistent pain and symptoms along with intraoperative complications.3 Magnetic resonance imaging (MRI), the most sensitive imaging modality for detecting glomus tumors of the hand, can assist in the workup.3,11,12
Extradigital glomus tumors are difficult to diagnose because of their rarity and unspecific symptoms and presentation.13 Misdiagnosis and delayed diagnosis can result in significant chronic pain, disuse syndromes, and disability.1,10 Correct diagnosis and surgical resection are generally curative with complete resolution of symptoms.
In this article, we report a case of a large atypical glomus tumor that occurred on the wrist and involved the radial artery. This tumor was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 63-year-old man presented to clinic with an extremely tender soft-tissue mass on his nondominant, left wrist. The mass had been increasing in size for a year. It was painless at rest but very painful to light palpation, with referred pain proximally up to the shoulder.
The patient did not recall any traumatic or inciting event, had not undergone any prior workup or treatment for symptoms, and had no history of masses elsewhere on the body. Past medical history was significant for type 2 diabetes and colon and prostate cancer, which had been treated with chemotherapy and was now in remission.
Physical examination revealed a 2×2.5-cm well-circumscribed soft-tissue mass on the volar-radial aspect of the left wrist proximal to the thenar eminence and radial to the flexor carpi radialis tendon (Figure 1). The mass was soft, mobile, and nonfluctuant and did not transilluminate. The overlying skin was normal in color and appearance—no discoloration, erythema, wounds, or drainage. The radial artery was palpable, and the mass did not pulsate or have a bruit. The patient had normal wrist range of motion limited by pain on compression of the mass with motor and sensation intact throughout the hand. Plain radiographs of the wrist showed no bony pathology or involvement from the mass. A soft-tissue shadow was visible around the wrist without calcifications. A wrist MRI was performed to better evaluate the mass, and the T2-weighted images showed a heterogeneous subcutaneous mass adjacent to the radial artery with increased signal intensity from surrounding feeding vessels (Figure 2).
Given the clinical and imaging findings, there was concern for a possible vascular tumor. Therefore, excisional biopsy was recommended over needle biopsy because of the bleeding risk. With the patient under general anesthesia, and a tourniquet used without exsanguination, a Brunner-type zigzag incision was made centered over the mass with elevated skin flaps. The 2.7×2.6×1.1-cm mass was superficial and involved the radial artery (Figure 3). After the radial artery was dissected proximally and distally, 2 perforating vessels were found entering the mass. These vessels were ligated, which allowed the mass to be peeled completely off the artery. Histology with hematoxylin-eosin staining showed solid sheets of uniform round cells with interspersed capillaries and centrally placed nuclei without evidence of malignancy (Figure 4).
The tourniquet was released before skin closure, and adequate hemostasis was obtained. The wound was closed, and the patient was placed in a volar wrist splint for immobilization. Pain relief after excision of the mass was immediate, and the postoperative course uneventful. After surgery, immunohistochemistry of the mass showed minimal mitotic activity, with a positive immunoperoxidase stain for smooth muscle actin confirming a diagnosis of glomus tumor (Figure 5). At 3-year follow-up, the patient had no pain, symptoms, or tumor recurrence.
Discussion
Glomus tumors are an established cause of pain in the subungual areas of the hand; numerous cases have been reported.1,5,10,14 However, extradigital glomus tumors, particularly those involving the wrist, are rare, and only a few have been described. Given the lack of consistent findings and presentations, diagnosis is difficult. Case series have documented an overall 2:1 female-to-male predominance of glomus tumors,6 but extradigital tumors are more common in men (4.6:1 male-to-female ratio).3 Extradigital glomus tumors are commonly diagnosed between ages 40 and 80 years. Classic symptoms of subungual tumors include pain, localized tenderness, and cold hypersensitivity,1,10 but symptoms are much more variable with extradigital locations. Previous trauma or injury to the lesion area is reported in 20% to 30% of cases before symptom onset.3,15 Intravascular locations of glomus tumors are extremely rare; only 4 cases of tumors involving venous structures have been reported.16-19 In the present case, the patient’s main complaints were pain and localized tenderness associated with a progressively increasing mass without any history of trauma. The large size of his mass (~2.5 cm in diameter) on examination was unique, as was involvement of the radial artery.
Misdiagnosis and delayed diagnosis of extradigital glomus tumors are common, and symptoms such as chronic pain typically persist for 7 to 11 years before the correct diagnosis is made.1,10 On average, 2.5 physician consultants (including psychiatrists) evaluate the patient before glomus tumor is identified.10 There are other reports of atypical or ectopic glomus tumors taking 5 to 25 years to be diagnosed.20-22 The differential diagnosis for glomus tumors includes hemangiomas, cellular or cavernous hemangiomas, vascular tumors, neuromas, neurofibromas, lipomas, paragangliomas, ganglion cysts, pigmented nevi, Pacinian corpuscle hyperplasia, and foreign bodies. A key element of clinical diagnosis is the disproportionate amount of pain and localized tenderness caused by the lesion relative to its size. The hypersensitivity of this tumor is thought to result from enlargement of the tumor and impingement on nearby Pacinian corpuscles, nerve endings in the skin that are responsible for sensitivity to vibration and pressure.2,9
Plain radiographs can be useful in detecting glomus tumors of the hand but are less helpful with extradigital tumors, with identification rates of 24% in certain series.3 MRI is the most sensitive imaging modality for diagnosing glomus tumors of the hand; a detection rate of 80% to 100% has been reported in various case series.3,11,12 Specificity of MRI for glomus tumors has been reported at 50%.11,23 Placement of a radiographic marker directly over the area of most pain can assist in tumor localization.3 Glomus tumors typically have decreased signal intensity on T1-weighted images and increased intensity on T2-weighted images, but signal patterns are variable and particularly difficult to differentiate with small tumors. MRI is useful in the setting of recurrent glomus tumors, where incomplete excision is possible. In 24 cases of continued pain after glomus tumor excision, Theumann and colleagues24 used MRI to identify a nodule consistent with recurrent glomus tumor in all patients. Three-dimensional contrast-enhanced magnetic resonance angiography (MRA) can also help diagnose glomus tumors while providing valuable information regarding size and location for surgical planning.25,26 With MRA, it is crucial to evaluate the arterial or arteriovenous phase of imaging, as the glomus tumor is richly vascularized and shows contrast enhancement after intravenous injection of gadolinium.27 Angiography, ultrasonography, thermography, and scintigraphy have all been used to diagnose glomus tumors but have shown limited utility and accuracy.11
Treatment of glomus tumors is complete surgical excision because of their relatively small size and subcutaneous location. Resection success rates are consistently higher than 95%, with resolution of all symptoms.1,10,14 Local recurrence of tumors after excision occurs in 1% to 33% of cases, depending on series, and may be immediate or delayed, with immediate recurrence commonly caused by inadequate excision.1,10,15,28 Delayed recurrence is less common and presents several years after excision, typically with a new growth near the previous excision.10 Recurrence years after surgery may also represent multiple tumors unrecognized during initial workup and can be treated with repeat excision or radiotherapy.
Robert and colleagues29 recently reported the case of a glomus tumor, on the dorsal aspect of the wrist, discovered incidentally in a 71-year-old patient and treated with surgical excision. Several years earlier, Chim and colleagues30 described a similar case, of a large wrist glomus tumor worked up with MRI. In a retrospective review of all extradigital glomus tumors seen over a 20-year period, Schiefer and colleagues3 reported 4 glomus tumors of the wrist out of 56 tumors total. The most common sites were forearm (11 cases) and knee (10 cases), and the majority of patients presented with pain and localized tenderness. Mean tumor size was 0.66 cm (range, 0.1-0.3 cm), with 77% of tumors less than 1 cm. Our patient’s 2.7×2.6×1.1-cm tumor was large for a glomus tumor. Its involvement with the radial artery feeding vessels likely contributed to its large and progressively increasing size. It is worth noting that, in the series by Schiefer and colleagues,3 the only patient with symptoms persisting after excision had a large (3 cm in diameter) deep tumor of the foot; the entire tumor was removed, and there was no recurrence by 10-year follow-up. Folpe and colleagues7 suggested that deep tumors larger than 2 cm should be at higher suspicion for malignancy. Joseph and Posner21 reported 3 cases of glomus tumors, on the ulnar side of the wrist, diagnosed with help of a provocative test using ethyl chloride spray.
Conclusion
Overall, glomus tumors are rare and challenging to diagnosis and should be in the differential in any symptomatic patient with a painful soft-tissue mass of the wrist. Advanced imaging studies, such as MRI, can assist in localization, diagnosis, and preoperative planning. Histology and immunohistochemistry are essential to differentiate glomus tumor from other vascular tumors, and complete excision is necessary to prevent local recurrence.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
1. Carroll RE, Berman AT. Glomus tumors of the hand: review of the literature and report on twenty-eight cases. J Bone Joint Surg Am. 1972;54(4):691-703.
2. Riddell DH, Martin RS. Glomus tumor of unusual size; case report. Ann Surg. 1951;133(3):401-403.
3. Schiefer TK, Parker WL, Anakwenze OA, Amadio PC, Inwards CY, Spinner RJ. Extradigital glomus tumors: a 20-year experience. Mayo Clin Proc. 2006;81(10):1337-1344.
4. Tuncali D, Yilmaz AC, Terzioglu A, Aslan G. Multiple occurrences of different histologic types of the glomus tumor. J Hand Surg Am. 2005;30(1):161-164.
5. Greene RG. Soft tissue tumors of the hand and wrist. A 10 year survey. J Med Soc N J. 1964;61:495-498.
6. Maxwell GP, Curtis RM, Wilgis EF. Multiple digital glomus tumors. J Hand Surg Am. 1979;4(4):363-367.
7. Folpe AL, Fanburg-Smith JC, Miettinen M, Weiss SW. Atypical and malignant glomus tumors: analysis of 52 cases, with a proposal for the reclassification of glomus tumors. Am J Surg Pathol. 2001;25(1):1-12.
8. De Chiara A, Apice G, Mori S, et al. Malignant glomus tumour: a case report and review of the literature. Sarcoma. 2003;7(2):87-91.
9. Riveros M, Pack GT. The glomus tumor; report of 20 cases. Ann Surg. 1951;133(3):394-400.
10. Van Geertruyden J, Lorea P, Goldschmidt D, et al. Glomus tumours of the hand. A retrospective study of 51 cases. J Hand Surg Br. 1996;21(2):257-260.
11. Al-Qattan MM, Al-Namla A, Al-Thunayan A, Al-Subhi F, El-Shayeb AF. Magnetic resonance imaging in the diagnosis of glomus tumours of the hand. J Hand Surg Br. 2005;30(5):535-540.
12. Drape JL, Idy-Peretti I, Goettmann S, et al. Subungual glomus tumors: evaluation with MR imaging. Radiology. 1995;195(2):507-515.
13. Heys SD, Brittenden J, Atkinson P, Eremin O. Glomus tumour: an analysis of 43 patients and review of the literature. Br J Surg. 1992;79(4):345-347.
14. Bhaskaranand K, Navadgi BC. Glomus tumour of the hand. J Hand Surg Br. 2002;27(3):229-231.
15. Rettig AC, Strickland JW. Glomus tumor of the digits. J Hand Surg Am. 1977;2(4):261-265.
16. Beham A, Fletcher CD. Intravascular glomus tumour: a previously undescribed phenomenon. Virchows Arch A Pathol Anat Histopathol. 1991;418(2):175-177.
17. Googe PB, Griffin WC. Intravenous glomus tumor of the forearm. J Cutan Pathol. 1993;20(4):359-363.
18. Koibuchi H, Fujii Y, Taniguchi N. An unusual case of a glomus tumor developing in a subcutaneous vein of the wrist. J Clin Ultrasound. 2008;36(6):369-370.
19. Acebo E, Val-Bernal JF, Arce F. Giant intravenous glomus tumor. J Cutan Pathol. 1997;24(6):384-389.
20. Ghaly RF, Ring AM. Supraclavicular glomus tumor, 20 year history of undiagnosed shoulder pain: a case report. Pain. 1999;83(2):379-382.
21. Joseph FR, Posner MA. Glomus tumors of the wrist. J Hand Surg Am. 1983;8(6):918-920.
22. Abou Jaoude JF, Roula Farah A, Sargi Z, Khairallah S, Fakih C. Glomus tumors: report on eleven cases and a review of the literature. Chir Main. 2000;19(4):243-252.
23. Jablon M, Horowitz A, Bernstein DA. Magnetic resonance imaging of a glomus tumor of the fingertip. J Hand Surg Am. 1990;15(3):507-509.
24. Theumann NH, Goettmann S, Le Viet D, et al. Recurrent glomus tumors of fingertips: MR imaging evaluation. Radiology. 2002;223(1):143-151.
25. Boudghene FP, Gouny P, Tassart M, Callard P, Le Breton C, Vayssairat M. Subungual glomus tumor: combined use of MRI and three-dimensional contrast MR angiography. J Magn Reson Imaging. 1998;8(6):1326-1328.
26. Van Ruyssevelt CE, Vranckx P. Subungual glomus tumor: emphasis on MR angiography. AJR Am J Roentgenol. 2004;182(1):263-264.
27. Connell DA, Koulouris G, Thorn DA, Potter HG. Contrast-enhanced MR angiography of the hand. Radiographics. 2002;22(3):583-599.
28. Varian JP, Cleak DK. Glomus tumours in the hand. Hand. 1980;12(3):293-299.
29. Robert G, Sawaya E, Pelissier P. Glomus tumor of the dorsal aspect of the wrist: a case report [in French]. Chir Main. 2012;31(4):214-216.
30. Chim H, Lahiri A, Chew WY. Atypical glomus tumour of the wrist: a case report. Hand Surg. 2009;14(2-3):121-123.
Superior vena cava syndrome as an initial presentation of low-grade follicular lymphoma
Superior vena cava (SVC) syndrome refers to a constellation of symptoms produced by the obstruction of blood flow through the SVC, resulting in symptoms of dyspnea, facial and upper-extremity edema, cough, chest pain, and dysphagia.1 Malignancies represent 60%-85% of the etiologies of SVC syndrome. Cumulatively, lymphoma and lung cancer represent 95% of malignancy-related SVC syndrome etiologies, with non-small-cell lung cancer (NSCLC) reported in about 50% of cases, small-cell lung cancer (SCLC) in about 25%, and non-Hodgkin lymphoma (NHL) in 10 % of all cases.1,2
Click on the PDF icon at the top of this introduction to read the full article.
Superior vena cava (SVC) syndrome refers to a constellation of symptoms produced by the obstruction of blood flow through the SVC, resulting in symptoms of dyspnea, facial and upper-extremity edema, cough, chest pain, and dysphagia.1 Malignancies represent 60%-85% of the etiologies of SVC syndrome. Cumulatively, lymphoma and lung cancer represent 95% of malignancy-related SVC syndrome etiologies, with non-small-cell lung cancer (NSCLC) reported in about 50% of cases, small-cell lung cancer (SCLC) in about 25%, and non-Hodgkin lymphoma (NHL) in 10 % of all cases.1,2
Click on the PDF icon at the top of this introduction to read the full article.
Superior vena cava (SVC) syndrome refers to a constellation of symptoms produced by the obstruction of blood flow through the SVC, resulting in symptoms of dyspnea, facial and upper-extremity edema, cough, chest pain, and dysphagia.1 Malignancies represent 60%-85% of the etiologies of SVC syndrome. Cumulatively, lymphoma and lung cancer represent 95% of malignancy-related SVC syndrome etiologies, with non-small-cell lung cancer (NSCLC) reported in about 50% of cases, small-cell lung cancer (SCLC) in about 25%, and non-Hodgkin lymphoma (NHL) in 10 % of all cases.1,2
Click on the PDF icon at the top of this introduction to read the full article.
Radioactive Iodine Scintiphotos of a Man With Thyroid Cancer
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
The contemporary management of differentiated thyroid cancer includes posttreatment monitoring for recurrence or metastasis.1 This monitoring includes clinical, biochemical, and imaging evaluation. Follow-up treatment can then be tailored based on the results of this monitoring.
Our patient was a 61-year-old man with a history of papillary thyroid carcinoma, including lymph node involvement and an extension of the primary focus into skeletal muscle (pT3N1bMX, stage IVa). The patient’s status was posttotal thyroidectomy and radioiodine ablation therapy (196.2 mCi iodine-131) in April 2009. The patient underwent follow-up thyrotropin alpha stimulated whole-body radioiodine surveillance scanning in May 2010.
Images demonstrated residual thyroid tissue/carcinoma regional to the thyroid bed, corresponding to prior posttherapy images. Whole body scintiphotos also demonstrated abnormal iodine localization that raised the possibility of distant bony metastasis in the region of the right hip (see Figures 1A and 1B). Current treatment standards for isolated bony metastases recommend repeated radioactive iodine therapy and potential external beam radiation. Imaging is required for accurate verification.1 This abnormal osseous finding was questionable on initial review, as it was present on the posterior, not anterior, view. The patient was instructed to continue hydration and return for additional delayed scintiphotos for further evaluation.
The patient returned 4 days later for delayed scintiphotos, which again demonstrated abnormal iodine localization near the right hip. However, iodine distribution was different, including now being visible on both the anterior and posterior views (see Figures 2A and 2B on the next page).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
The patient had no pain in the area and, upon further questioning, reported that he returned wearing the same athletic shorts. Given that radioiodine is excreted in the urine, this atypical distribution was thought to reflect urinary contamination. When images were taken again with the shorts removed, no abnormal radioiodine activity was present (see Figures 2C and 2D). Additional findings with thyrotropin alfa stimulation included increased quantitative thyroglobulin values of 20.2 ng/mL with antithyroglobulin antibody < 20.0 U/mL. Radioiodine ablation therapy using thyrotropin alfa was repeated. Iodine localization also was not present in the hip on posttherapy imaging (not shown).
Despite advances in imaging techniques, radioiodine scanning remains an imperfect science. Artifacts and pitfalls have been identified; in part, these are related to the accumulation of iodide in organs other than the thyroid, such as the nasopharynx and stomach, as well as the apparent accumulation due to excretion in the gut and bladder.2-4 These variations can be divided into ectopic normal thyroid tissue, physiologic accumulation in nonthyroidal tissue, and contamination by physiologic secretions. Recent case reports have confirmed this classification. Abnormal radioiodine uptake has been described in vertebral hemangioma,5 liver abscess6 and hydatid cyst,7 bronchiectasis,8 bronchogenic cyst and mucinous cystadenoma (2 fluid-filled cavities),9 chronic submandibular sialadenitis,10 esophageal diverticulum,11 hiatal hernia,12 appendix,13 indwelling Hickman catheter,14 renal cyst,15 and, similar to this case, contamination of the hair.16
Contaminated clothing is not uncommon; however, a persistent abnormality from contaminated clothing on repeat follow-up is unusual and could easily be misinterpreted.2 It would be valuable for all providers to be aware of the pitfalls of imaging before embarking on an unnecessary and potentially hazardous—not to mention costly—treatment course.
Acknowledgments
The authors acknowledge the assistance of Richard Cacciato, MLIS, Medical Librarian, who assisted in the literature review.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review the complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
1. Cooper, DS, Doherty GM, Haugen BR, et al; American Thyroid Association (ATA) Guidelines Taskforce on Thyroid Nodules and Differentiated Thyroid Cancer. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid. 2009;19(11):1167-1214.
2. Carlisle MR, Lu C, McDougall IR. The interpretation of 131I scans in the evaluation of thyroid cancer, with an emphasis on false positive findings. Nucl Med Commun. 2003;24(6):715-735.
3. Shapiro B, Rufini V, Jarwan A, et al. Artifacts, anatomical and physiological variants, and unrelated diseases that might cause false-positive whole-body 131-I scans in patients with thyroid cancer. Semin Nucl Med. 2000;30(2):115-132.
4. Mitchell G, Pratt BE, Vini L, McCready VR, Harmer CL. False positive 131I whole body scans in thyroid cancer. Br J Radiol. 2000;73(870):627-635.
5. Khan S, Dunn J, Strickland N, Al-Nahhas A. Iodine-123 uptake in vertebral haemangiomas in a patient with papillary thyroid carcinoma. Nucl Med Rev Cent East Eur. 2008;11(1):30-33.
6. Pena Pardo FJ, Crespo de la Jara A, Fernández Morejón FJ, Sureda González M, Forteza Vila J, Brugarolas Masllorens A. Solitary focus in the liver in a thyroid cancer patient after a whole body scan with 131 iodine. Rev Esp Med Nucl. 2007;26(5):294-296.
7. Omür O, Ozbek SS, Akgün A, Yazici B, Mutlukoca N, Ozcan Z. False-positive I-131 accumulation in a hepatic hydatid cyst. Clin Nucl Med. 2007;32(12):930-932.
8. Jong I, Taubman K, Schlicht S. Bronchiectasis simulating pulmonary metastases on iodine-131 scintigraphy in well-differentiated thyroid carcinoma. Clin Nucl Med. 2005;30(10):688-689.
9. Agriantonis DJ, Hall L, Wilson MA. Pitfalls of I-131 whole body scan interpretation: Bronchogenic cyst and mucinous cystadenoma. Clin Nucl Med. 2008;33(5):325-327.
10. Ozguven M, Ilgan S, Karacalioglu AO, Arslan N, Ozturk E. Unusual patterns of I-131 accumulation. Clin Nucl Med. 2004;29(11):738-740.
11. Rashid K, Johns W, Chasse K, Walker M, Gupta SM. Esophageal diverticulum presenting as metastatic thyroid mass on iodine-131 scintigraphy. Clin Nucl Med. 2006;31(7):405-408.
12. Ceylan Gunay E, Erdogan A. Mediastinal radioiodine uptake due to hiatal hernia: A false-positive reaction in 131I scan. Rev Esp Med Nucl. 2010;29(2):95.
13. Borkar S, Grewal R, Schoder H. I-131 uptake demonstrated in the appendix on a posttreatment scan in a patient with thyroid cancer. Clin Nucl Med. 2008;33(8):551-552.
14. Groskin SA, McCrohan G. Pseudometastasis of the chest wall resulting from a Hickman catheter. J Thorac Imaging. 1994;9(3):169-171.
15. Thust S, Fernando R, Barwick T, Mohan H, Clarke SE. SPECT/CT identification of post-radioactive iodine treatment false-positive uptake in a simple renal cyst. Thyroid. 2009;19(1):75-76.
16. Sinha A, Bradley KM, Steatham J, Weaver A. Asymmetric breast uptake of radioiodine in a patient with thyroid malignancy: Metastases or not? J R Soc Med. 2008;101(6):319-320.
My Most Unusual Case: Painful Blistering on the Dorsal Hands
Patients with pseudoporphyria, an uncommon blistering skin disease, may initially present in the ED as the onset of this photosensitive condition can be acute—inciting patients to seek care urgently. The causes of pseudoporphyria may be drug-induced or related to hemodialysis, and it can develop even months to years after a patient with end-stage renal disease (ESRD) has been undergoing hemodialysis.1 Alternatively, patients can develop the condition weeks to months after starting certain medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], tyrosine kinase receptor inhibitors, hormonal contraceptives, diuretics, antiandrogens, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors). Recent case reports associate finasteride, torsemide, and β-lactam antibiotics with pseudoporphyria.2,3
With the increasing number of older Americans, as well as approximately one-third of seniors taking more than 5 drugs, the incidence of drug-induced pseudoporphyria is likely to increase in the near future.4 Additionally, ESRD is more common in patients older than age 70 years,5 increasing the risk of hemodialysis-related pseudoporphyria in this population.
Case
A 58-year-old black man presented to the ED with an 8-month history of nail changes and painful blisters on the dorsal hands. He stated that he initially noticed these findings after doing yardwork. Regarding history, he further noted that he smokes 3 cigarette packs daily and receives hemodialysis three times weekly for ESRD secondary to polycystic kidney disease. His medications included simvastatin, omeprazole, minoxidil, calcium, and a vitamin B complex.
On physical examination, the patient was well-appearing and in no acute distress with stable vital signs. His dermatologic examination revealed shallow, well-defined erosions, some with central crusting; and adjacent 1- to 2-mm scattered white papules on the dorsum of his hands bilaterally, with more appearing on his left hand than his right hand. The patient’s digits were tender to palpation, and symmetric tapering of the digits was present. Onycholysis and onychodystrophy were present in the majority of his nails.
The laboratory workup included a complete blood count and differential, which demonstrated a stable anemia of chronic disease when compared to prior labwork; a complete metabolic panel revealed expected elevated blood urea nitrogen and creatinine. Liver function and iron studies were normal.
Punch biopsies were performed on perilesional skin with direct immunofluorescence revealing linear granular deposits of immune complexes (IgG and C3), and direct microscopy noting fibrin at the dermoepidermal junction and perivascular location. These clinical and laboratory findings are consistent with porphyria cutanea tarda (PCT), pseudoporphyria, or variegate porphyria. However, his serum porphyrin studies were normal, thus supporting the diagnosis of pseudoporphyria.
Porphyria Cutanea Tarda
Porphyria cutanea tarda is the most common disorder of heme biosynthesis. In this condition, hepatic uroporphyrinogen decarboxylase is deficient, leading to the accumulation of porphyrins in the serum and blood.6 Patients typically present in adulthood complaining of painful blisters and milia on the dorsal hands, and usually do not recognize the component of sunlight exposure in the subsequent appearance of lesions.7 Genetic, environmental, and infectious etiologies contribute to its onset, acting singly or in concert.8-10 Up to half of patients with PCT are infected with hepatitis C; other associations include tobacco smoking, alcoholism, HIV infection, and hereditary hemochromatosis.10-12
As seen in this patient, no accumulation of photosensitive porphyrins is evident in pseudoporphyria through current laboratory testing. While the pathogenesis of pseudoporphyria is unknown, it may be linked to the generation of free radicals.
It is estimated that pseudoporphyria occurs in about 1.2% to 18% of patients undergoing hemodialysis treatment secondary to ESRD.13,14 Massone et al13 demonstrated that dialysis patients with ESRD are more susceptible to free-radical injury due to a reduction of the antioxidant glutathione in both plasma and erythrocytes. Additionally, ultraviolet (UV)-induced free radical formation in the skin has been well documented in the literature.15 This is further supported by a number of pseudoporphyria cases successfully treated with N-acetylcysteine, which replenishes glutathione. However, use of this medication in the treatment of pseudoporphyria is not common.
Treatment
Treatment of patients with pseudoporphyria requires a multifaceted approach. Removing the causative medication when possible may resolve the eruption. Unfortunately in our patient’s case, simvastatin, a possible cause of pseudoporphyria, was withdrawn for several months without improvement. This implicates hemodialysis as the probable cause of his pseudoporphyria. In a case of hemodialysis-induced pseudoporphyria, hemodialysis is life-saving and unable to be discontinued.
It is also important to counsel patients extensively on the importance of UV avoidance, including the use of photoprotective clothing, mineral-based sunscreen creams, and avoiding outside activity during peak daylight hours (ie, between 10:00 AM and 4:00 PM). A short course (2-4 weeks) of potent topical corticosteroids such as clobetasol 0.05% ointment can be prescribed for twice daily application to active lesions on the hands. Patients should also avoid any photosensitizing medications such as NSAIDs and tetracyclines.
Case Conclusion
On follow-up visits to the dermatology clinic, this patient continued to report improvement when compliant with the prescribed treatment plan, which included avoidance of direct sunlight, use of photoprotective clothing, and avoidance of photosensitizing medications, as well as daily use of clobetasol to lesions as needed. He noted skin flares with unprotected UV exposure.
Do you have an unusual case that you would like to share with your EM colleagues? Submissions should be cases that presented at and were managed within your ED. Please limit case reports to 1,000 words with a title of no more than 100 characters. Include a brief summary or unstructured abstract before you present the case details and include no more than two tables or figures, which should be submitted as separate, high-resolution files. Please de-identify all patient information. For full author guidelines please go to “author guidelines” at emed-journal.com. You can submit your case at editorialmanager.com/emedjournal or mdales@frontlinemedcom.com.
- Cordova KB, Oberg TJ, Malik M, Robinson-Bostom L. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22(1):45-55.
- Santo Domingo D, Stevenson ML, Auerbach J, Lerman J. Finasteride-induced pseudoporphyria. Arch Dermatol. 2011;147(6):747-748.
- Pérez-Bustillo A, Sánchez-Sambucety P, Suárez-Amor O, Rodríiguez-Prieto MA. Torsemide-induced pseudoporphyria. Arch Dermatol. 2008;144(6):812-813.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529-537.
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2014. http://www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed October 24, 2014.
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735-745.
- Poh-Fitzpatrick MB. Porphyria cutanea tarda clinical presentation. Medscape Web site. http://emedicine.medscape.com/article/1103643-clinical#a0216. Updated February 24, 2014. Accessed October 24, 2014.
- Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47(2):419-426.
- Cruz-Rojo J, Fontanellas A, Morán-Jiménez MJ. Precipitating/aggravating factors of porphyria cutanea tarda in Spanish patients. Cell Mol Biol (Noisy-le-grand). 2002;48(8):845-852.
- Jalil S, Grady JJ, Lee C, Anderson KE. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8(3):297-302.
- Elder GH. Alcohol intake and porphyria cutanea tarda. Clin Dermatol. 1999;17(4):431-436.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27(6):1661-1669.
- Massone C, Ambros-Rudolph CM, et al: Successful outcome of haemodialysis-induced pseudoporphyria after short-term oral N-acetylcysteine and switch to high-flux technique dialysis. Acta Derm Venereol. 2006;86(6):538-540.
- Cooke NS, McKenna K. A case of haemodialysis-associated pseudoporphyria successfully treated with oral N-acetylcysteine. Clin Exp Dermatol. 2007;32(1):64-66.
- Jurkiewicz BA, Buettner GR. Ultraviolet light-induced free radical formation in skin: an electron paramagnetic resonance study.
Patients with pseudoporphyria, an uncommon blistering skin disease, may initially present in the ED as the onset of this photosensitive condition can be acute—inciting patients to seek care urgently. The causes of pseudoporphyria may be drug-induced or related to hemodialysis, and it can develop even months to years after a patient with end-stage renal disease (ESRD) has been undergoing hemodialysis.1 Alternatively, patients can develop the condition weeks to months after starting certain medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], tyrosine kinase receptor inhibitors, hormonal contraceptives, diuretics, antiandrogens, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors). Recent case reports associate finasteride, torsemide, and β-lactam antibiotics with pseudoporphyria.2,3
With the increasing number of older Americans, as well as approximately one-third of seniors taking more than 5 drugs, the incidence of drug-induced pseudoporphyria is likely to increase in the near future.4 Additionally, ESRD is more common in patients older than age 70 years,5 increasing the risk of hemodialysis-related pseudoporphyria in this population.
Case
A 58-year-old black man presented to the ED with an 8-month history of nail changes and painful blisters on the dorsal hands. He stated that he initially noticed these findings after doing yardwork. Regarding history, he further noted that he smokes 3 cigarette packs daily and receives hemodialysis three times weekly for ESRD secondary to polycystic kidney disease. His medications included simvastatin, omeprazole, minoxidil, calcium, and a vitamin B complex.
On physical examination, the patient was well-appearing and in no acute distress with stable vital signs. His dermatologic examination revealed shallow, well-defined erosions, some with central crusting; and adjacent 1- to 2-mm scattered white papules on the dorsum of his hands bilaterally, with more appearing on his left hand than his right hand. The patient’s digits were tender to palpation, and symmetric tapering of the digits was present. Onycholysis and onychodystrophy were present in the majority of his nails.
The laboratory workup included a complete blood count and differential, which demonstrated a stable anemia of chronic disease when compared to prior labwork; a complete metabolic panel revealed expected elevated blood urea nitrogen and creatinine. Liver function and iron studies were normal.
Punch biopsies were performed on perilesional skin with direct immunofluorescence revealing linear granular deposits of immune complexes (IgG and C3), and direct microscopy noting fibrin at the dermoepidermal junction and perivascular location. These clinical and laboratory findings are consistent with porphyria cutanea tarda (PCT), pseudoporphyria, or variegate porphyria. However, his serum porphyrin studies were normal, thus supporting the diagnosis of pseudoporphyria.
Porphyria Cutanea Tarda
Porphyria cutanea tarda is the most common disorder of heme biosynthesis. In this condition, hepatic uroporphyrinogen decarboxylase is deficient, leading to the accumulation of porphyrins in the serum and blood.6 Patients typically present in adulthood complaining of painful blisters and milia on the dorsal hands, and usually do not recognize the component of sunlight exposure in the subsequent appearance of lesions.7 Genetic, environmental, and infectious etiologies contribute to its onset, acting singly or in concert.8-10 Up to half of patients with PCT are infected with hepatitis C; other associations include tobacco smoking, alcoholism, HIV infection, and hereditary hemochromatosis.10-12
As seen in this patient, no accumulation of photosensitive porphyrins is evident in pseudoporphyria through current laboratory testing. While the pathogenesis of pseudoporphyria is unknown, it may be linked to the generation of free radicals.
It is estimated that pseudoporphyria occurs in about 1.2% to 18% of patients undergoing hemodialysis treatment secondary to ESRD.13,14 Massone et al13 demonstrated that dialysis patients with ESRD are more susceptible to free-radical injury due to a reduction of the antioxidant glutathione in both plasma and erythrocytes. Additionally, ultraviolet (UV)-induced free radical formation in the skin has been well documented in the literature.15 This is further supported by a number of pseudoporphyria cases successfully treated with N-acetylcysteine, which replenishes glutathione. However, use of this medication in the treatment of pseudoporphyria is not common.
Treatment
Treatment of patients with pseudoporphyria requires a multifaceted approach. Removing the causative medication when possible may resolve the eruption. Unfortunately in our patient’s case, simvastatin, a possible cause of pseudoporphyria, was withdrawn for several months without improvement. This implicates hemodialysis as the probable cause of his pseudoporphyria. In a case of hemodialysis-induced pseudoporphyria, hemodialysis is life-saving and unable to be discontinued.
It is also important to counsel patients extensively on the importance of UV avoidance, including the use of photoprotective clothing, mineral-based sunscreen creams, and avoiding outside activity during peak daylight hours (ie, between 10:00 AM and 4:00 PM). A short course (2-4 weeks) of potent topical corticosteroids such as clobetasol 0.05% ointment can be prescribed for twice daily application to active lesions on the hands. Patients should also avoid any photosensitizing medications such as NSAIDs and tetracyclines.
Case Conclusion
On follow-up visits to the dermatology clinic, this patient continued to report improvement when compliant with the prescribed treatment plan, which included avoidance of direct sunlight, use of photoprotective clothing, and avoidance of photosensitizing medications, as well as daily use of clobetasol to lesions as needed. He noted skin flares with unprotected UV exposure.
Do you have an unusual case that you would like to share with your EM colleagues? Submissions should be cases that presented at and were managed within your ED. Please limit case reports to 1,000 words with a title of no more than 100 characters. Include a brief summary or unstructured abstract before you present the case details and include no more than two tables or figures, which should be submitted as separate, high-resolution files. Please de-identify all patient information. For full author guidelines please go to “author guidelines” at emed-journal.com. You can submit your case at editorialmanager.com/emedjournal or mdales@frontlinemedcom.com.
Patients with pseudoporphyria, an uncommon blistering skin disease, may initially present in the ED as the onset of this photosensitive condition can be acute—inciting patients to seek care urgently. The causes of pseudoporphyria may be drug-induced or related to hemodialysis, and it can develop even months to years after a patient with end-stage renal disease (ESRD) has been undergoing hemodialysis.1 Alternatively, patients can develop the condition weeks to months after starting certain medications (eg, nonsteroidal anti-inflammatory drugs [NSAIDs], tyrosine kinase receptor inhibitors, hormonal contraceptives, diuretics, antiandrogens, 3-hydroxy-3-methylglutaryl-coenzyme-A reductase inhibitors). Recent case reports associate finasteride, torsemide, and β-lactam antibiotics with pseudoporphyria.2,3
With the increasing number of older Americans, as well as approximately one-third of seniors taking more than 5 drugs, the incidence of drug-induced pseudoporphyria is likely to increase in the near future.4 Additionally, ESRD is more common in patients older than age 70 years,5 increasing the risk of hemodialysis-related pseudoporphyria in this population.
Case
A 58-year-old black man presented to the ED with an 8-month history of nail changes and painful blisters on the dorsal hands. He stated that he initially noticed these findings after doing yardwork. Regarding history, he further noted that he smokes 3 cigarette packs daily and receives hemodialysis three times weekly for ESRD secondary to polycystic kidney disease. His medications included simvastatin, omeprazole, minoxidil, calcium, and a vitamin B complex.
On physical examination, the patient was well-appearing and in no acute distress with stable vital signs. His dermatologic examination revealed shallow, well-defined erosions, some with central crusting; and adjacent 1- to 2-mm scattered white papules on the dorsum of his hands bilaterally, with more appearing on his left hand than his right hand. The patient’s digits were tender to palpation, and symmetric tapering of the digits was present. Onycholysis and onychodystrophy were present in the majority of his nails.
The laboratory workup included a complete blood count and differential, which demonstrated a stable anemia of chronic disease when compared to prior labwork; a complete metabolic panel revealed expected elevated blood urea nitrogen and creatinine. Liver function and iron studies were normal.
Punch biopsies were performed on perilesional skin with direct immunofluorescence revealing linear granular deposits of immune complexes (IgG and C3), and direct microscopy noting fibrin at the dermoepidermal junction and perivascular location. These clinical and laboratory findings are consistent with porphyria cutanea tarda (PCT), pseudoporphyria, or variegate porphyria. However, his serum porphyrin studies were normal, thus supporting the diagnosis of pseudoporphyria.
Porphyria Cutanea Tarda
Porphyria cutanea tarda is the most common disorder of heme biosynthesis. In this condition, hepatic uroporphyrinogen decarboxylase is deficient, leading to the accumulation of porphyrins in the serum and blood.6 Patients typically present in adulthood complaining of painful blisters and milia on the dorsal hands, and usually do not recognize the component of sunlight exposure in the subsequent appearance of lesions.7 Genetic, environmental, and infectious etiologies contribute to its onset, acting singly or in concert.8-10 Up to half of patients with PCT are infected with hepatitis C; other associations include tobacco smoking, alcoholism, HIV infection, and hereditary hemochromatosis.10-12
As seen in this patient, no accumulation of photosensitive porphyrins is evident in pseudoporphyria through current laboratory testing. While the pathogenesis of pseudoporphyria is unknown, it may be linked to the generation of free radicals.
It is estimated that pseudoporphyria occurs in about 1.2% to 18% of patients undergoing hemodialysis treatment secondary to ESRD.13,14 Massone et al13 demonstrated that dialysis patients with ESRD are more susceptible to free-radical injury due to a reduction of the antioxidant glutathione in both plasma and erythrocytes. Additionally, ultraviolet (UV)-induced free radical formation in the skin has been well documented in the literature.15 This is further supported by a number of pseudoporphyria cases successfully treated with N-acetylcysteine, which replenishes glutathione. However, use of this medication in the treatment of pseudoporphyria is not common.
Treatment
Treatment of patients with pseudoporphyria requires a multifaceted approach. Removing the causative medication when possible may resolve the eruption. Unfortunately in our patient’s case, simvastatin, a possible cause of pseudoporphyria, was withdrawn for several months without improvement. This implicates hemodialysis as the probable cause of his pseudoporphyria. In a case of hemodialysis-induced pseudoporphyria, hemodialysis is life-saving and unable to be discontinued.
It is also important to counsel patients extensively on the importance of UV avoidance, including the use of photoprotective clothing, mineral-based sunscreen creams, and avoiding outside activity during peak daylight hours (ie, between 10:00 AM and 4:00 PM). A short course (2-4 weeks) of potent topical corticosteroids such as clobetasol 0.05% ointment can be prescribed for twice daily application to active lesions on the hands. Patients should also avoid any photosensitizing medications such as NSAIDs and tetracyclines.
Case Conclusion
On follow-up visits to the dermatology clinic, this patient continued to report improvement when compliant with the prescribed treatment plan, which included avoidance of direct sunlight, use of photoprotective clothing, and avoidance of photosensitizing medications, as well as daily use of clobetasol to lesions as needed. He noted skin flares with unprotected UV exposure.
Do you have an unusual case that you would like to share with your EM colleagues? Submissions should be cases that presented at and were managed within your ED. Please limit case reports to 1,000 words with a title of no more than 100 characters. Include a brief summary or unstructured abstract before you present the case details and include no more than two tables or figures, which should be submitted as separate, high-resolution files. Please de-identify all patient information. For full author guidelines please go to “author guidelines” at emed-journal.com. You can submit your case at editorialmanager.com/emedjournal or mdales@frontlinemedcom.com.
- Cordova KB, Oberg TJ, Malik M, Robinson-Bostom L. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22(1):45-55.
- Santo Domingo D, Stevenson ML, Auerbach J, Lerman J. Finasteride-induced pseudoporphyria. Arch Dermatol. 2011;147(6):747-748.
- Pérez-Bustillo A, Sánchez-Sambucety P, Suárez-Amor O, Rodríiguez-Prieto MA. Torsemide-induced pseudoporphyria. Arch Dermatol. 2008;144(6):812-813.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529-537.
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2014. http://www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed October 24, 2014.
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735-745.
- Poh-Fitzpatrick MB. Porphyria cutanea tarda clinical presentation. Medscape Web site. http://emedicine.medscape.com/article/1103643-clinical#a0216. Updated February 24, 2014. Accessed October 24, 2014.
- Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47(2):419-426.
- Cruz-Rojo J, Fontanellas A, Morán-Jiménez MJ. Precipitating/aggravating factors of porphyria cutanea tarda in Spanish patients. Cell Mol Biol (Noisy-le-grand). 2002;48(8):845-852.
- Jalil S, Grady JJ, Lee C, Anderson KE. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8(3):297-302.
- Elder GH. Alcohol intake and porphyria cutanea tarda. Clin Dermatol. 1999;17(4):431-436.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27(6):1661-1669.
- Massone C, Ambros-Rudolph CM, et al: Successful outcome of haemodialysis-induced pseudoporphyria after short-term oral N-acetylcysteine and switch to high-flux technique dialysis. Acta Derm Venereol. 2006;86(6):538-540.
- Cooke NS, McKenna K. A case of haemodialysis-associated pseudoporphyria successfully treated with oral N-acetylcysteine. Clin Exp Dermatol. 2007;32(1):64-66.
- Jurkiewicz BA, Buettner GR. Ultraviolet light-induced free radical formation in skin: an electron paramagnetic resonance study.
- Cordova KB, Oberg TJ, Malik M, Robinson-Bostom L. Dermatologic conditions seen in end-stage renal disease. Semin Dial. 2009;22(1):45-55.
- Santo Domingo D, Stevenson ML, Auerbach J, Lerman J. Finasteride-induced pseudoporphyria. Arch Dermatol. 2011;147(6):747-748.
- Pérez-Bustillo A, Sánchez-Sambucety P, Suárez-Amor O, Rodríiguez-Prieto MA. Torsemide-induced pseudoporphyria. Arch Dermatol. 2008;144(6):812-813.
- Scott IA, Gray LC, Martin JH, Mitchell CA. Minimizing inappropriate medications in older populations: a 10-step conceptual framework. Am J Med. 2012;125(6):529-537.
- Centers for Disease Control and Prevention. National Chronic Kidney Disease Fact Sheet, 2014. http://www.cdc.gov/diabetes/pubs/pdf/kidney_Factsheet.pdf. Accessed October 24, 2014.
- Frank J, Poblete-Gutiérrez P. Porphyria cutanea tarda—when skin meets liver. Best Pract Res Clin Gastroenterol. 2010;24(5):735-745.
- Poh-Fitzpatrick MB. Porphyria cutanea tarda clinical presentation. Medscape Web site. http://emedicine.medscape.com/article/1103643-clinical#a0216. Updated February 24, 2014. Accessed October 24, 2014.
- Egger NG, Goeger DE, Payne DA, Miskovsky EP, Weinman SA, Anderson KE. Porphyria cutanea tarda: multiplicity of risk factors including HFE mutations, hepatitis C, and inherited uroporphyrinogen decarboxylase deficiency. Dig Dis Sci. 2002;47(2):419-426.
- Cruz-Rojo J, Fontanellas A, Morán-Jiménez MJ. Precipitating/aggravating factors of porphyria cutanea tarda in Spanish patients. Cell Mol Biol (Noisy-le-grand). 2002;48(8):845-852.
- Jalil S, Grady JJ, Lee C, Anderson KE. Associations among behavior-related susceptibility factors in porphyria cutanea tarda. Clin Gastroenterol Hepatol. 2010;8(3):297-302.
- Elder GH. Alcohol intake and porphyria cutanea tarda. Clin Dermatol. 1999;17(4):431-436.
- Bonkovsky HL, Poh-Fitzpatrick M, Pimstone N, et al. Porphyria cutanea tarda, hepatitis C, and HFE gene mutations in North America. Hepatology. 1998;27(6):1661-1669.
- Massone C, Ambros-Rudolph CM, et al: Successful outcome of haemodialysis-induced pseudoporphyria after short-term oral N-acetylcysteine and switch to high-flux technique dialysis. Acta Derm Venereol. 2006;86(6):538-540.
- Cooke NS, McKenna K. A case of haemodialysis-associated pseudoporphyria successfully treated with oral N-acetylcysteine. Clin Exp Dermatol. 2007;32(1):64-66.
- Jurkiewicz BA, Buettner GR. Ultraviolet light-induced free radical formation in skin: an electron paramagnetic resonance study.
Aortic Dissection
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
Case
A 72-year-old man with a past medical history of hypertension and social history of tobacco use presented to the ED with chest pain and abdominal pain. His vital signs at presentation were: heart rate, 110 beats/minute; blood pressure, 80/40 mm Hg; respiratory rate, 22 breaths/minute; temperature, afebrile. His oxygen saturation was 98% on room air. The patient was alert and oriented; his abdomen was soft with no reproducible tenderness to palpation and without a palpable mass. The remainder of the physical examination was otherwise unremarkable. An electrocardiogram revealed sinus tachycardia with left ventricular hypertrophy, and a chest X-ray was read as no acute process by radiology services. Since the patient’s creatinine level was elevated at 3.5 mg/dL, the use of radiocontrast media relatively contraindicated.
To quickly assess the patient, the treating emergency physician (EP) performed a limited transabdominal ultrasound at the bedside, which revealed an intimal flap in the abdominal aorta in the transverse plane visible at the subcostal margin (Figure 1). The longitudinal view demonstrated the intimal flap clearly, but with no clear point of origin in the abdominal portion of the aorta (Figure 2).
The subcostal cardiac view also revealed the cause for the patient’s hemodynamic instability: a large pericardial effusion with evidence of early acute pericardial tamponade via right atrial collapse (Figure 3).
The patient was taken to the operating room within 20 minutes of arrival to the ED. Expedient diagnosis of both the presence and extent of his aortic dissection and its complications by bedside ultrasound facilitated early and aggressive management of this life-threatening disease process.
Imaging Techniques
Abdominal Aorta
Ultrasound of the abdominal aorta begins with the use of a curvilinear probe, starting in the transverse plane with the probe marker pointing toward the patient’s right side (ie, the 9-o’clock position). The probe should scan the epigastrium, which is located just below the xiphoid process (Figure 4).
For orientation, the clinician should identify the vertebral body, which will appear as a dark and rounded object at the bottom center of the screen with a dark shadow behind it. Both the aorta and inferior vena cava (IVC) will be visualized just superficial to the vertebral body; the aorta typically appears anterior to the vertebral body, with the IVC slightly to the right of it. The amount of pressure applied for visualization of these structures will vary depending on the patient’s body habitus and volume status (Figure 5).
Once orientation is established and there is a clear transverse view of the aorta, calipers are used to measure the diameter of the aorta from superficial to deep, measuring from outer wall to outer wall (Figure 6).
Next, the clinician should scan the probe caudally as he or she follows the aorta down to the level of the bifurcation into the iliac vessels, near the level of the umbilicus (Figure 7).
Then, rotating the probe clockwise 90˚ to place the probe marker in the 12-o’clock position, the abdominal aorta can be measured in the long axis, giving a broad overview of the entire structure (Figure 8).
Identification of an undulating intimal flap is highly specific for the diagnosis of aortic dissection.
Thoracic Aorta
After imaging of the abdominal aorta is complete, a phased array probe is used to scan the thoracic aorta, beginning with a subxiphoid view of the heart. The probe should be placed in the transverse plane, just inferior to the xiphoid process and with the probe marker aimed toward the patient’s right side (ie, the 9-o’clock position). Next, the probe is angled cephalad and slightly toward the patient’s left shoulder, nearly laying it flat on the abdomen, using the liver as the acoustic window. As with abdominal ultrasound, depending on the patient’s body habitus and anatomy, the depth may need to be adjusted for optimal view. This is one of the best views when evaluating for pericardial effusion, which will appear as a dark or anechoic stripe surrounding the heart (Figure 3).
After this view is complete, the clinician should proceed to scan the parasternal long axis view to evaluate the aortic outflow track and descending aorta. The cardiac probe should be placed in the left third or fourth intercostal space with the probe marker angled toward the patient’s right shoulder (ie, the 10- to 11-o’clock position). Proceeding from superficial to deep on the screen, the right ventricle, left ventricle and aortic outflow tract, left atrium, and then the descending aorta (Figure 9) will be visualized.
The two main areas to assess closely are the aortic outflow tract and the descending aorta. While looking at the aortic outflow tract, evidence of aortic regurgitation or a linear echodensity within the aortic root may be seen, suggestive of the intimal flap occurring in aortic dissection. Focusing on the descending aorta, the clinician should again look for a linear echodensity across the aorta, which represents the intimal flap (red highlighted area, Figure 9).
Discussion
Acute aortic dissection is an emergent, life-threatening condition with a high morbidity and mortality rate and a wide range of clinical manifestations and atypical presentations—all of which benefit from rapid identification. The combination of these factors makes diagnosis difficult, but all the more essential, especially considering the time-sensitivity of initiating treatment with intravenous antihypertensive agents and operative intervention.1
Bedside ultrasound provides a rapid and reliable method of making the diagnosis at the point of care, thus positively affecting patient care and outcome. Although existing research is limited, available data indicate that the EP can accurately diagnose acute aortic dissection and its complications using this modality.
Rapid diagnosis of aortic catastrophes at the bedside is not a novel concept. Shuman et al2 studied bedside transabdominal ultrasound on initial presentation of patients with severe abdominal or back pain, suspicious for abdominal aortic aneurysm (AAA). In this study of 60 patients, 31 of 32 AAAs were identified; these diagnoses were made prior to a surgeon’s evaluation.
More recently, Kuhn et al3 completed a similar study of EP use of beside ultrasound in the ED. Although their study lacked strength secondary to small sample size, it did indicate the ability to accurately determine the presence of an AAA with minimal training and experience.
Bedside Ultrasound Versus Other Imaging Modalities
There are multiple imaging modalities to consider when evaluating a patient with a possible aortic dissection, the decision of which should also take into account the ability to determine alternative diagnoses.
Bedside Ultrasound. This modality provides the EP with a quick, easy tool to evaluate multiple, potential life-threatening emergencies immediately at the bedside in a patient with suspected aortic dissection.4 Numerous studies have documented a sensitivity of 78% to 87% and a specificity of 99% to 100% for the diagnosis of aortic dissection by transthoracic and transabdominal ultrasound when an undulating intimal flap is visualized.5
Computed Tomography Angiography. In comparison to beside ultrasound, computed tomography angiography (CTA) has a sensitivity of 96% to 100% and a specificity of 96% to 100%.6,7 However, since CTA requires the use of iodinated contrast material, it is relatively contraindicated in the setting of acute kidney injury, a condition not uncommon in patients with acute aortic dissection.
Magnetic Resonance Imaging. Currently the gold standard for the identification of aortic dissection, magnetic resonance imaging has both a sensitivity and specificity of 98%.7,8 The major disadvantages of this test are the lack of availability and the length of the study itself.
Chest X-ray. A chest X-ray is commonly used as a screening test for aortic dissection, despite 12% to 20% of patients with aortic dissection having a “normal” X-ray.6
Transesophageal echocardiography. Another good modality for diagnosing aortic dissection is transesophageal echocardiography (TEE), which has a sensitivity of up to 98% and a specificity of up to 97%.8 This test, however, requires an experienced operator at the bedside, typically a cardiologist, and is an invasive study that requires the use of sedation and occasionally general anesthesia. A TEE is limited by its inability to visualize the descending aorta below the stomach.6-8
Conclusion
There are several benefits to using bedside ultrasound at the point of care to diagnose aortic dissection. This modality provides not only a rapid, noninvasive, and painless study requiring no radiocontrast media, but also has a high specificity for detection of aortic dissection. Moreover, it also allows the provider to evaluate for other potential life-threatening emergencies such as concomitant abdominal aortic aneurysm, intraperitoneal hemorrhage, pericardial effusion, and cardiac tamponade.9,10
Dr Venezia is a resident in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Sawyer is a clinical instructor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk. Dr Byars is an associate professor in the department of emergency medicine, Eastern Virginia Medical School, Norfolk.
For a video clip showing a transverse view of the abdominal aorta with the dissection flap clearly visible mid-lumen of the proximal aorta, visit https://vimeo.com/111462170.
For a video clip showing a longitudinal view of the abdominal aorta with the dissection flap clearly visible in the mid-lumen of the aorta, visit https://vimeo.com/111462168.
For a video clip of a cardiac view demonstrating large pericardial effusion in the patient with aortic dissection, visit https://vimeo.com/111462169.
For a video clip demonstrating ultrasound of the parasternal long axis view with a phased array probe, visit https://vimeo.com/111462167.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.
- Lo, BM. An evidence-based approach to acute aortic syndromes. Emerg Med Pract. 2013;15(12):1-23.
- Shuman WP, Hastrup W Jr, Kohler TR, et al. Suspected leaking abdominal aortic aneurysm: use of sonography in the emergency room. Radiology. 1988;168(1):117-119.
- Kuhn M, Bonnin RL, Davey MJ, Rowland JL, Langlois SL. Emergency department ultrasound scanning for abdominal aortic aneurysm: accessible, accurate, and advantageous. Ann Emerg Med. 2000;36(3):219-223.
- Fojtik JP, Costantino TG, Dean AJ. The diagnosis of aortic dissection by emergency medicine ultrasound. J Emerg Med. 2007;32(2):191-196.
- Brunson JM, Fine RL, Schussler JM. Acute ascending aortic dissection diagnosed with transthoracic echocardiography. J Am Soc Echo. 2009;22(9):1086.e5–1086.e7.
- Erbel R, Alfonso F, Boileau C, et al; Task Force on Aortic Dissection, European Society of Cardiology. Diagnosis and management of aortic dissection. Eur Heart J. 2001;22(18):1642-1681.
- Nienaber CA, von Kodolitsch Y, Nicolas V, et al. The diagnosis of thoracic aortic dissection by noninvasive imaging procedures. N Engl J Med. 1993;328(1):1-
- Nienaber CA, Eagle KA. Aortic dissection: new frontiers in diagnosis and management: Part I: from etiology to diagnostic strategies. Circulation. 2003;108(5):628-635.
- Goodman A, Perera P, Mailhot T, Mandavia D. The role of bedside ultrasound in the diagnosis of pericardial effusion and cardiac tamponade. J Emerg Trauma Shock. 2012;5(1):72-75.
- Perera P, Mailhot T, Riley D, Mandavia D. The RUSH exam: Rapid ultrasound in shock in the evaluation of the critically ill. Emerg Med Clin North Am. 2010;28(1):29-56.
Case Studies in Toxicology: Sippin’ on Some “Sizzurp”
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 19-year-old man was found unresponsive by his girlfriend. They both attended a party the previous night where a number of people were drinking alcohol and cough syrup to get “high.” When emergency medical technicians arrived at the patient’s house, they administered naloxone, which somewhat improved the patient’s level of consciousness; oxygen was also delivered via facemask.
Upon arrival to the ED, the patient complained of hearing loss and tinnitus. His initial vital signs were: blood pressure, 99/60 mm Hg; heart rate, 110 beats/minute; respiratory rate, 20 breaths/minute; temperature, 96.8°F. Oxygen saturation was 80% on room air. On examination, he was lethargic but responsive to voice and oriented to time, place, and person. His pupils were pinpoint; his hearing was decreased bilaterally; his breathing was shallow, with rales audible at both lung bases; his bowel sounds were hypoactive; and his skin was warm and moist. The rest of the examination was otherwise unremarkable.
What cough and cold products are commonly abused with the intent to get high?
Hundreds of nonprescription pharmaceutical products—each with the potential for misuse or abuse—are available to consumers in retail stores and online. These products can be classified by expected clinical effect, which helps clinicians with the diagnosis and management of these patients (Table).
Of the antitussive products currently available over the counter (OTC), those that contain dextromethorphan have the widest abuse potential. Referred to as “dex,” “DMX,” or “tuss,” this drug is widely abused among adolescents and young adults due to its easy availability. In therapeutic doses, dextromethorphan suppresses cough via the medullary cough center. Ingesting dextromethorphan at higher doses, a practice referred to as “Robo tripping,” can produce hallucinations and a dissociative state marked by alterations in consciousness and impaired motor control. Dextromethorphan is a structural analog of ketamine and phencyclidine, which accounts for their similar clinical effects.
Codeine
Codeine is another drug added to various cough medications for its antitussive properties. An opioid, it acts centrally to suppress cough and has mild analgesic properties. It is available only by prescription in the United States, but can be purchased as an OTC product in other countries. Recently, it has come into the media spotlight as the starting product to make “Krokodil” (see Emerg Med. 2014;46[2]:76-78).
Case Continuation
While undergoing his workup in the ED, the patient became increasingly lethargic with persistent hypoxia. Although initially
responsive to naloxone, his respirations became more labored, requiring intubation. Prior to intubation and while awake, the patient mentioned that he was drinking “sizzurp” the evening prior. He denied the use of other drugs or of having any suicidal intent. A postintubation chest X-ray revealed a left-sided retrocardiac infiltrate consistent with aspiration pneumonitis.
What is sizzurp?
Sizzurp is a slang term used to describe a beverage that is most frequently comprised of fruit-flavored soda, codeine/promethazine hydrochloride cough syrup (CPHCS), and hard candy (classically a Jolly Rancher).1 This combination is ingested by the user with the intent of achieving a unique high—attributable to the combined effects of codeine, an opioid, and promethazine, an antihistamine (with antipsychotic properties). According to user reports, CPHCS induces a deep sense of euphoria, relaxation, and a slowed sense of time.2 Additional slang terms used to describe this product include “lean,” “purple drank,” “purp,” “drank,” “syrup,” “barre,” and “Texas tea.”
According to one source, purple drank originated in Houston, Texas around the 1960s, when blues musicians would combine dextromethorphan with beer.3 Over time, the recipe was modified, and by the 1980s, when purple drank was adopted by hip-hop musicians from the same Houston neighborhoods, the name sizzurp took hold.
In the 1990s, one Houston-based hiphop artist, DJ Screw, developed a genre of music called “chopped and screwed,” inspired by the CPHCS high and notable for its slowed-down tempo that fit the sedation and decreased motor activity induced by the drug. As chopped and screwed music became popularized, so too did the recreational use of CPHCS. In 2000, “Sippin’ on Some Sizzurp,” a hit song by southern hip-hop group Three Six Mafia, introduced CPHCS to more mainstream hip-hop audiences.
Despite the CPHCS-related deaths of a number of hip-hop musicians, including DJ Screw, as well as the arrests of professional
football players linked to abusing the drug, CPHCS continues to be glorified by a number of hip-hop and pop musicians.
Unfortunately, media attention of these events often has the paradoxical effect of promoting use among adolescents and young adults, and CPHCS has become a drug of choice for black adolescents in many Texas communities.4 However, one study attempting to define a purple drank user profile among college students at a large public university in the southeastern United States revealed that use was most prevalent among urban male youth primarily from Hispanic, Native American, and white ethnic backgrounds—challenging the notion that it is confined to the black community.5
Although CPHCS is only available by prescription in the United States, its widespread abuse suggests easy access to this drug. In April 2014, Actavis, the pharmaceutical company that produces a promethazine/codeine product known as the “champagne of sizzurp,” made a bold decision to cease all production and sales of the product in direct response to the widespread media attention and glamorization of CPHCS. In its announcement, the company cited its “commitment to being a partner in the fight against prescription-drug abuse.”6 Despite Actavis’ cessation of manufacturing CPHC, at least four other companies continue to sell similar formulations.
What are the dangers of CPHCS use?
The effects produced by CPHCS are described as euphoric, which may be attributable to both codeine and promethazine. Codeine, or 3-methyl morphine, is an inactive opioid agonist and prodrug that requires metabolic activation via O-demethylation to morphine by CYP2D6. Onset of action occurs 30 to 45 minutes after ingestion, while peak effects are reached within 1 to 2 hours and last approximately 4 to 6 hours.7 Since approximately 5% to 7% of the white population lack CPY2D6 function, these individuals will experience no analgesic or euphoric effects from codeine.8 However, ultra-rapid CYP2D6 metabolizers can produce significant and potentially life-threatening concentrations of morphine.
Adverse effects of recreational codeine use are similar to that of any opioid and include central nervous system (CNS) depression, miosis, and hypoactive bowel sounds, with severe toxicity marked by coma, respiratory depression, hypotension, bradycardia, and/or death due to respiratory arrest. Aspiration pneumonitis and rhabdomyolysis are complications of impaired airway protection and prolonged immobility. Opioid-induced ototoxicity, resulting in either temporary or permanent hearing loss, is a rare complication, described largely in case reports.9 (See Emerg Med. 2012;44[11]:4-6).
Promethazine hydrochloride contributes to the unique effects experienced by the recreational user and likely acts synergistically with codeine to augment CNS depression. Both a histamine H1-receptor antagonist and the muscarinic dopamine (D2)-receptor antagonist promethazine is included in prescription cough syrups to produce its antihistamine, antiemetic, and sedative properties.7 It is well absorbed from the gastrointestinal (GI) tract with more limited oral bioavailability due to the first-pass effect. Onset of action occurs within 20 minutes of administration, and the duration of effect is approximately 4 to 6 hours. Adverse effects of promethazine include variable CNS effects, from obtundation to agitated delirium, and are often accompanied by anticholinergic effects such as hyperthermia, dry flushed skin, mydriasis, hypoactive bowel sounds, and urinary retention. Neurological manifestations, likely mediated by dopamine blockade, include muscle rigidity, athetosis, hyperreflexia, and other upper motor neuron signs. Severe toxicity can produce coma, respiratory depression, seizure, and/or death.
What are the treatment strategies?
Management of patients with CPHCS toxicity, as with all poisoned patients, begins with rapid evaluation and stabilization of the airway, breathing, and circulation. The benefits of GI decontamination are likely to be outweighed by the risks engendered by CNS depression. While supportive care is the mainstay, targeted therapies may include naloxone for the treatment of opioid-induced respiratory depression and physostigmine, when contraindications have been ruled out, for the reversal of the anticholinergic toxidrome.
Conclusion
The patient was admitted to the intensive care unit where he was treated for aspiration pneumonitis, acute respiratory distress syndrome, rhabdomyolysis, and acute renal failure. His hearing loss and tinnitus resolved. He was extubated on hospital day 9 and discharged from the hospital on day 14.
Dr Laskowski is a medical toxicology fellow in the department of emergency medicine at New York University Langone Medical Center. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
- Sizzurp. Urban Dictionary Web site. http://www.urbandictionary.com/define.php?term=sizzurp. Accessed October 15, 2014.
- Jodeine. Sippin’ purple drank: an experience with promethazine with codeine & cannabis. Erowid Web site. https://www.erowid.org/experiences/exp.php?ID=54165. Accessed October 15, 2014.
- Fergusen G. Sizzurp. KCRW Radio Web site. http://www.kcrw.com/news-culture/shows/good-food/butter-carving-the-last-supper-sizzurp-cheftestants. March 23, 2013. Accessed October 15, 2014.
- Elwood WN. Sticky business: patterns of procurement and misuse of prescription cough syrup in Houston. J Psychoactive Drugs. 2001;33(2):121-133.
- Agnich LE, Stogner JM, Miller BL, Marcum CD. Purple drank prevalence and characteristics of misusers of codeine cough syrup mixtures. Addict Behav. 2013;38(9):2445-2449.
- Hlavaty C. Drug company cites abuse, pop culture hype in ending cough syrup production. Houston Chronicle. April 24, 2014. http://blog.chron.com/thetexican/2014/04/drug-company-cites-abuse-pop-culture-hype-in-ending-cough-syrup-production/. Accessed October 15, 2014.
- Burns JM, Boyer EW. Antitussives and substance abuse. Subst Abuse Rehabil. 2013;4:75-82.
- Nelson LS, Olsen D. Opioids. In: Nelson LS, Lewin NA, Howland MA, Hoffman RS, Goldfrank LR, Flomenbaum NE, eds. Goldfrank’s Toxicologic Emergencies. 9th ed. New York, NY: McGraw Hill; 2011:559-578.
- Freeman SR, Bray ME, Amos CS, Gibson WP. The association of codeine, macrocytosis and bilateral sudden or rapidly progressive profound sensorineural deafness. Acta Otolaryngol. 2009;129(1):1061-1066.
4 pregnant women with an unusual finding at delivery
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
THE CASES
CASE 1 › A 32-year-old G2P1 with an uncomplicated prenatal course presented for induction at 41 weeks and 2 days of gestation. Fetal heart tracing showed no abnormalities. A compound presentation and a prolonged second stage of labor made vacuum assistance necessary. The infant had both a true umbilical cord knot (TUCK) (FIGURE 1A) and double nuchal cord.
CASE 2 › A 46-year-old G3P0 at 38 weeks of gestation by in vitro fertilization underwent an uncomplicated primary low transverse cesarean (C-section) delivery of dichorionic/diamniotic twins. The C-section had been necessary because baby A had been in the breech position. Fetal heart tracing showed no abnormalities. Baby A had a velamentous cord insertion, and baby B had a succenturiate lobe and a TUCK.
CASE 3 › A 23-year-old G2P1 with an uncomplicated prenatal course chose to have a repeat C-section and delivered at 41 weeks in active labor. Fetal heart monitoring showed no abnormalities. Umbilical artery pH and venous pH were normal. A TUCK was noted at time of delivery.
CASE 4 › A 30-year-old G1P0 with an uncomplicated prenatal course presented in active labor at 40 weeks and 4 days of gestation. At 7 cm cervical dilation, monitoring showed repeated deep variable fetal heart rate decelerations. The patient underwent an uncomplicated primary C-section. Umbilical artery pH and venous pH were normal. A TUCK (FIGURE 1B) and double nuchal cord were found at time of delivery.
DISCUSSION
TUCKs are thought to occur when a fetus passes through a loop in the umbilical cord. They occur in <2% of term deliveries.1,2 TUCKs differ from false knots. False knots are exaggerated loops of cord vasculature.
Risk factors that have been independently associated with TUCK include advanced maternal age (AMA; >35 years), multiparity, diabetes mellitus, gestational diabetes, polyhydramnios, and previous spontaneous abortion.1-3 In one study, 72% of women with a TUCK were multiparous.3 Hershkovitz et al2 suggested that laxity of uterine and abdominal musculature in multiparous patients may contribute to increased room for TUCK formation.
The adjusted odds ratio of having a TUCK is 2.53 in women with diabetes mellitus.3 Hyperglycemia can contribute to increased fetal movements, thereby increasing the risk of TUCK development.2 Polyhydramnios is often found in patients with diabetes mellitus and gestational diabetes.3 The incidence is higher in monoamniotic twins.4
Being a male and having a longer umbilical cord may also increase the risk of TUCK. On average, male infants have longer cords than females, which may predispose them to TUCKs.3 Räisänen et al3 found that the mean cord length in TUCK infants was 16.9 cm longer than in infants without a TUCK.
Of our 4 patients, one was of AMA, 2 were multiparous, and 3 of the 4 infants who developed TUCK were male.
TUCK is usually diagnosed at delivery
Most cases of TUCK are found incidentally at the time of delivery. Antenatal diagnosis is difficult, because loops of cord lying together are easily mistaken for knots on ultrasound.5 Sepulveda et al6 evaluated the use of 3D power Doppler in 8 cases of suspected TUCK; only 63% were confirmed at delivery. Some researchers have found improved detection of TUCK with color Doppler and 4D ultrasound, which have demonstrated a “hanging noose sign” (a transverse section of umbilical cord surrounded by a loop of cord) as well as views of the cord under pressure.7-10
Outcomes associated with TUCK vary greatly. Neonates affected by TUCK have a 4% to 10% increased risk of stillbirth, usually attributed to knot tightening.2,4,11,12
In addition, there is an increased incidence of fetal heart rate abnormalities during labor.1,3,12,13
There is no increase in the incidence of assisted vaginal or C-section delivery.12 And as for whether TUCK affects an infant’s size or weight, one study found TUCK infants had a 3.2-fold higher risk of measuring small for gestational age, potentially due to chronic umbilical cord compromise; however, mean birth weight between study and control groups did not differ significantly.3
Outcomes for our patients and their infants. All 4 cases had good outcomes (TABLE). The umbilical cord knot produced no detectable fetal compromise in cases 1 through 3. In Case 4, electronic fetal monitoring showed repeated variable fetal heart rate decelerations, presumably associated with cord compression.
THE TAKEAWAY
Pregnant women who may be at risk for experiencing a TUCK include those who are older than age 35, multiparous, carrying a boy, or have diabetes mellitus, gestational diabetes, or polyhydramnios. While it is good to be aware of these risk factors, there are no recommended changes in management based on risk or ultrasound findings unless there is additional concern for fetal compromise.
Antenatal diagnosis of TUCK is challenging, but Doppler ultrasound may be able to identify the condition. Most cases of TUCK are noted on delivery, and outcomes are generally positive, although infants in whom the TUCK tightens may have an increased risk of heart rate abnormalities or stillbirth.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.
1. Joura EA, Zeisler H, Sator MO. Epidemiology and clinical value of true umbilical cord knots [in German]. Wien Klin Wochenschr. 1998;110:232-235.
2. Hershkovitz R, Silberstein T, Sheiner E, et al. Risk factors associated with true knots of the umbilical cord. Eur J Obstet Gynecol Reprod Biol. 2001;98:36-39.
3. Räisänen S, Georgiadis L, Harju M, et al. True umbilical cord knot and obstetric outcome. Int J Gynaecol Obstet. 2013;122: 18-21.
4. Maher JT, Conti JA. A comparison of umbilical cord blood gas values between newborns with and without true knots. Obstet Gynecol. 1996;88:863-866.
5. Clerici G, Koutras I, Luzietti R, et al. Multiple true umbilical knots: a silent risk for intrauterine growth restriction with anomalous hemodynamic pattern. Fetal Diagn Ther. 2007;22:440-443.
6. Sepulveda W, Shennan AH, Bower S, et al. True knot of the umbilical cord: a difficult prenatal ultrasonographic diagnosis. Ultrasound Obstet Gynecol. 1995;5:106-108.
7. Hasbun J, Alcalde JL, Sepulveda W. Three-dimensional power Doppler sonography in the prenatal diagnosis of a true knot of the umbilical cord: value and limitations. J Ultrasound Med. 2007;26:1215-1220.
8. Rodriguez N, Angarita AM, Casasbuenas A, et al. Three-dimensional high-definition flow imaging in prenatal diagnosis of a true umbilical cord knot. Ultrasound Obstet Gynecol. 2012;39:245-246.
9. Scioscia M, Fornalè M, Bruni F, et al. Four-dimensional and Doppler sonography in the diagnosis and surveillance of a true cord knot. J Clin Ultrasound. 2011;39: 157-159.
10. Sherer DM, Dalloul M, Zigalo A, et al. Power Doppler and 3-dimensional sonographic diagnosis of multiple separate true knots of the umbilical cord. J Ultrasound Med. 2005;24: 1321-1323.
11. Sørnes T. Umbilical cord knots. Acta Obstet Gynecol Scand. 2000;79:157-159.
12. Airas U, Heinonen S. Clinical significance of true umbilical knots: a population-based analysis. Am J Perinatol. 2002;19:127-132.
13. Szczepanik ME, Wittich AC. True knot of the umbilical cord: a report of 13 cases. Mil Med. 2007;172:892-894.