User login
Unusual Form and Location of a Tumor: Multiosseous Ewing Sarcoma in the Foot
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
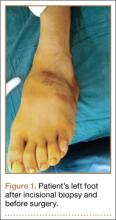
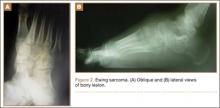
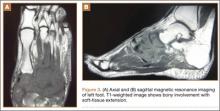
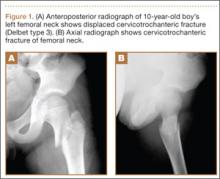
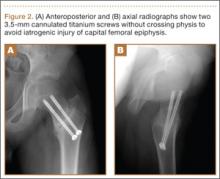
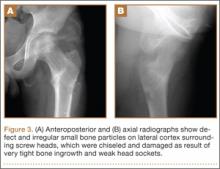
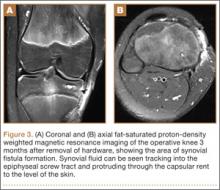
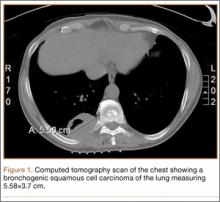
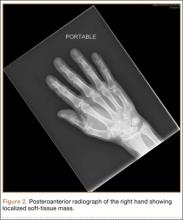
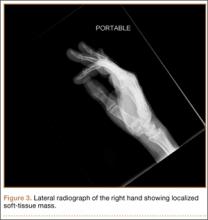
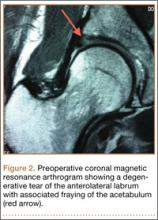
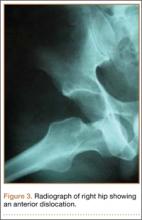
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
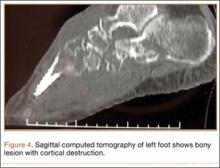
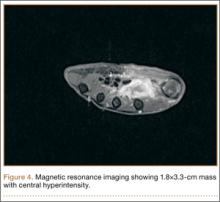
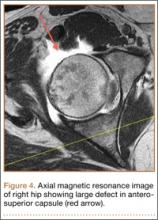
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
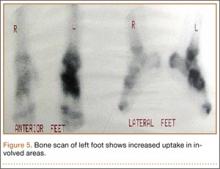
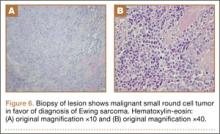
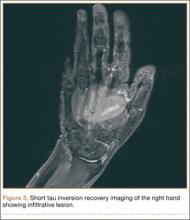
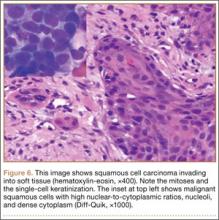
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
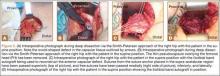
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
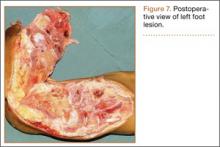
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
Ewing sarcomas are characterized as primitive malignant round cell tumors.1 These tumors are diagnosed by neuroectodermal differentiation and by their common histologic and immunohistochemical properties.2 Ewing sarcoma is the second most common malignant bone tumor in adolescents and young adults. It is the fourth most common primary malignant tumor, accounting for about 9% of all malignant tumors of bone. The most common primary bone tumors are multiple myeloma, osteosarcoma, and chondrosarcoma.3
The diaphyses of long bones (eg, femur, tibia, humerus) and flat bones (eg, pelvis, scapula) are the most commonly involved sites. Involvement of bones in the hands and feet is uncommon (3%-5% of reported cases).4 The foot bones most commonly involved include the calcaneus and the metatarsals, in the series by Casadei and colleagues.5
About 90% of Ewing sarcoma cases present before age 20 years (mean age, 13 years).6 Typical presentation is that of localized pain at the involved site. Some patients have systemic symptoms, such as fever, malaise, weight loss, leukocytosis, and increased erythrocyte sedimentation rate (ESR) mimicking infection. Radiographically, Ewing sarcoma appears as a permeative destructive bone lesion with a moth-eaten appearance (almost 76% of cases).7 This is usually associated with lamellated periosteal new bone formation or an “onion skin” appearance. Less commonly, a sunburst configuration with an associated soft-tissue mass can be seen. Computed tomography (CT) and magnetic resonance imaging (MRI) show the osseous extent of the tumor and the presence or absence of the soft-tissue component of the tumor. Radionuclide bone scans show increased technetium-99m methylene diphosphonate accumulation and are typically hot.6
Histopathologically, the tumor is composed of small, uniformly sized cells characterized by an almost clear eosinophilic cytoplasm and very little intercellular matrix. There are lobules and strands divided by prominent septa. Macroscopically, appearance can range from a soft, fleshy solid mass to an almost liquid form, as the lesion does not produce any matrix. At time of surgery, the tumor may have a liquefied component and the appearance of pus.6 Prognostic factors are tumor site in foot and treatment according to the series by Casadei and colleagues.5 Patients with large central tumors, especially in the pelvis, have worse outcomes than patients with distal tumors.8
In this article, we report a case of multifocal Ewing sarcoma involving multiple bones in the foot. Given the multifocal nature of the disease confined to the foot, the initial impression was that of osteomyelitis. We describe the histologic, radiologic, and diagnostic features of the tumor and outline treatment and prognosis. To our knowledge, this is the first report of multifocal Ewing sarcoma involving multiple bones in the foot. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 19-year-old man visited our clinic with the chief complaints of left foot pain and swelling. The pain started 10 months earlier and was followed by swelling. Complaints started after a minor local trauma. The man sought outside medical attention 8 months after pain onset. At his first visit at another institution, an initial radiograph was reported as normal, and all laboratory measures, including complete blood cell count (CBC) differential, ESR, and C-reactive protein (CRP) level, were within normal limits. Under the erroneous diagnosis of infection, the patient was treated with cloxacillin 500 mg 4 times a day for 4 weeks.
The patient’s pain had started 10 months before presentation (2 months after antibiotic therapy was initiated) (Figure 1). Physical examination at our institution revealed a palpable mass on the dorsum of the left foot. Anteroposterior and lateral plain radiographs showed a permeative lytic lesion with cortical destruction in the left calcaneus, navicular, cuboid, and cuneiform bones and in all metatarsal bones except the first (Figure 2). A soft-tissue mass around the involved bones was noted as well. The talus was not involved (Figure 3).
CT showed permeative destruction of left foot bones, including the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first. Invasion through the overlying cortex of the involved bones indicated aggressive biological activity of the tumor (Figure 4). MRI showed a destructive bony lesion of the mentioned bones associated with the soft-tissue mass (Figure 3).
Bone scan showed increased uptake in the involved areas (Figure 5). Chest plain radiographs and CT showed no distant metastasis. An incisional biopsy was performed, and histopathology showed a malignant small round cell tumor, identified as Ewing sarcoma (Figure 6). An immunohistochemistry study demonstrated positive CD99 and negative cytokeratin, leukocyte common antigen, desmin, and synaptophysin.
The patient was started on 4 cycles of adjuvant chemotherapy. Cycles 1 and 3 involved cyclophosphamide 2 g, vincristine 2 g, and doxorubicin 50 mg; cycles 2 and 4 involved ifosfamide 3.5 g and etoposide 200 mg. Tumor shrinkage occurred after chemotherapy. Clinical response to preoperative chemotherapy was documented by a decrease in tumor size at follow-ups. The patient underwent below-knee amputation.
Postoperative histopathology confirmed the diagnosis of Ewing sarcoma of the calcaneus, navicular, cuboid, and cuneiform bones and all metatarsal bones except the first (Figure 7). At 2-year follow-up, the patient had no evidence of local recurrence or distant metastasis based on chest CT and clinical examination of the affected site.
Discussion
Ewing sarcoma is the prototype of round small cell malignancies that arise from the long bones and the flat bones. It seldom involves the hands or feet. To our knowledge, this is the first report of Ewing sarcoma of the foot with multiple-bone involvement. Our literature review found a case of Ewing sarcoma of the first phalanx of the third toe, the second metatarsal bone, the cuneiform, the cuboid, and the talus, with lesser soft-tissue extension compared with our patient’s case.9
As this foot tumor is rare, there are few reports on its clinical aspects, appropriate treatment, and long-term outcome. For treatment of nonmetastatic Ewing sarcoma, limb-salvage surgery is advised if response has been good and there is a reasonable expectation of negative margins and good functional results.
Radiation and surgery may be part of the overall treatment plan. Radiation without surgery has a unique role in pelvic Ewing sarcoma, in contrast to extremity Ewing sarcoma. In our opinion, margins and histologic necrosis in the resection specimen are examined after surgery, and, if the margins are widely negative and histologic response is good, no further local control is advised. If the margin is positive, postoperative radiation therapy is recommended.1 Amputation has gradually become a (rare) choice in the treatment of extremity sarcomas.9 In our patient’s case, surgery was preferred over radiation therapy after chemotherapy because of the low risk of local side effects and the expected high efficacy. In addition, radiation at such high doses for Ewing sarcoma in the foot causes functional impairment. Because of the multiple-bone involvement, a salvage procedure was not possible for our patient. Given the calcaneal involvement, however, below-knee amputation was considered safer than ankle disarticulation.
Multiple-bone involvement occurs in the advanced stage of Ewing sarcoma, usually after visceral and pulmonary metastases are detected.9 The case reported by Rammal and colleagues9 had both multiple-bone involvement in the foot and pulmonary metastasis. The authors indicated that hematogenous spread of the tumor was discerned because the lesions were noncontiguous.9 Our patient had no distant metastases. We think his tumor originated in a tarsal or midtarsal bone and extended to adjacent bones. Therefore, it probably spread through its capsular and ligamentous attachment among tarsal and midtarsal bones, as the involvement was contiguous rather than distinct.
Average delay from symptom onset to diagnosis was reported to be 34 weeks.3 Average physician delay from initial visit to correct diagnosis was reported to be 19 weeks.3 Patients may have erythema, fever, and swelling, suggestive of osteomyelitis.3 Laboratory results may show increased white blood cell count and elevated ESR and CRP level.3 In addition, needle biopsy of the tumor may reveal an appearance grossly similar to that of pus.3 Therefore, physicians may send all the tissue out for microbiological analysis (according to the erroneous diagnosis of infection) and none out for pathologic analysis. The situation can be further complicated when Ewing sarcoma occurs in the foot, an uncommon site. In this special case, multiple-bone involvement can present a misleading clinical picture of infection.10 In other words, infection is one of the best choices in the differential diagnosis.7 Also to be considered are multicentric giant cell tumor, fibrosarcoma,11 and osteosarcoma.12
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
1. Herring JA. Malignant tumors of bone. In: Herring JA, ed. Tachdjian’s Pediatric Orthopaedics. Philadelphia, PA: Saunders Elsevier; 2008:2324-2327.
2. Cavazzana AO, Miser JS, Jefferson J, Triche TJ. Experimental evidence for a neural origin of Ewing’s sarcoma of bone. Am J Pathol. 1987;127(3):507-518.
3. Canale ST, Beaty JH. Malignant tumors of bone. In: Canale ST, ed. Campbell’s Operative Orthopaedics. Philadelphia, PA: Mosby Elsevier; 2008:910-913.
4. Unni KK. Ewing sarcoma. In: Unni KK, ed. Dahlin’s Bone Tumor: General Aspects and Data on 11087 Cases. Philadelphia, PA: Lippincott-Raven; 1996:121-142.
5. Casadei R, Magnani M, Biagini R, Mercuri M. Prognostic factors in Ewing’s sarcoma of the foot. Clin Orthop. 2004;(420):230-238.
6. Greenspan A, Jundt G, Remagen W. Bone-forming (osteogenic) lesions. In: Greenspan A, Jundt G, Remagen W, eds. Differential Diagnosis in Orthopaedic Oncology. 2nd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2007:114.
7. Metcalfe JE, Grimer RJ. Ewing’s sarcoma of the foot masquerading as osteomyelitis. Foot Ankle Surg. 2004;10(1):29-33.
8. Hoffmann C, Ahrens S, Dunst J, et al. Pelvis Ewing sarcoma: a retrospective analysis of 241 cases. Cancer. 1999;85(4):869-877.
9. Rammal H, Ghanem I, Torbey PH, Dagher F, Kharrat K. Multifocal Ewing sarcoma of the foot. J Pediatr Hematol Oncol. 2008;30(4):298-300.
10. Ledermann HP, Morrison WB, Schweitzer ME. MR image analysis of pedal osteomyelitis: distribution, patterns of spread, and frequency of associated ulceration and septic arthritis. Radiology. 2002;223(3):747-755.
11. Dhillon MS, Prabhudev Prasad AP, Virk MS, Aggarwal S. Multicentric giant cell tumor involving the same foot: a case report and review of literature. Indian J Orthop. 2007;41(2):154-157.
12. Baraga JJ, Amarami KK, Swee RG, Wold L, Unni KK. Radiographic features of Ewing’s sarcoma of the bones of the hand and feet. Skeletal Radiol. 2001;30(3):121-126.
Spontaneous, Chronic Expanding Posterior Thigh Hematoma Mimicking Soft-Tissue Sarcoma in a Morbidly Obese Pregnant Woman
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
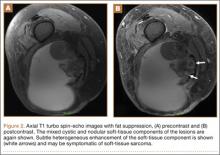
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
Soft-tissue sarcomas are quite rare, with an annual incidence of 20 to 30 per 1,000,000 persons in the United States.1 Because of their heterogeneous presentation, they remain a diagnostic challenge and are often initially confused for more common, benign disorders.2 Chronic expanding hematoma, first described by Friedlander and colleagues3 in 1968, is a rare entity that is particularly difficult to distinguish from soft-tissue malignancy.3-5 Chronic expanding hematoma is defined as a hematoma that gradually expands over 1 month or longer, is absent of neoplastic change on histologic sections, and does not occur in the setting of coagulopathy.6
Typically associated with remote trauma, these lesions often present as a slowly growing mass on the anterior or lateral thigh, calf, or buttock.3-4,7-9 They have been reported to persist as long as 46 years, with sizes ranging from 3 to 55 cm in maximum diameter.7 On imaging, they have a cystic appearance with a dense fibrous capsule.7-8 Most cases resolve uneventfully after drainage or marginal excision, although some cases require repeated intervention.7 This case report describes a morbidly obese patient with a chronic expanding hematoma in the distal posterior thigh whose definitive treatment was delayed 6 months because of her pregnancy status and inability to lie prone for open biopsy. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 27-year-old morbidly obese woman, who was pregnant at 12 weeks gestation, was seen in an orthopedic oncology clinic with a 1-month history of a slowly growing, painful posterior thigh mass. She had no history of cancer or bleeding disorder, and denied a history of trauma or constitutional symptoms consistent with malignancy. Coagulation studies were normal. Magnetic resonance imaging (MRI) obtained 2 weeks prior in the emergency room showed a cystic lesion with mass-like components in the posterior compartment of the distal right thigh, measuring 17 cm longitudinally. The lesion was located adjacent to, but not involving, the sciatic nerve and femoral vasculature. On initial examination, the large soft-tissue mass was evident and moderately painful to palpation; no skin changes were noted, and the patient had a normal sensorimotor examination. Fine-needle aspiration was performed, which resulted in amorphous debris consistent with hematoma.
Repeat MRI 2 months later showed increased size of the lesion (9.5×10.5 cm axial, 22.0 cm craniocaudal). Although most findings of a more extensive imaging protocol, including precontrast and postcontrast sequences, were consistent with hematoma, the lesion also had several characteristics that indicated soft-tissue sarcoma. Specifically, findings suggestive of chronic hematoma included the hyperintense short tau inversion recovery (STIR) T1/T2 signal of the cystic component consistent with proteinaceous fluid and the low STIR TI/T2 signal of the periphery consistent with a rim of hemosiderin (Figure 1). Additionally, the cystic component of the lesion had multiple fine septations that are atypical for a hematoma (Figure 1), and several lymph nodes greater than 1.7 cm in short axis were noted in the anterior thigh and hemipelvis that were suspicious of metastatic lymphadenopathy. The encapsulated appearance of the lesion with a sharply defined margin and short transition zone were also reassuring findings for a benign lesion (Figures 1, 2A, 2B). However, several findings were identified that suggested soft-tissue sarcoma, including a nodular soft-tissue component on the medial wall of the lesion that had heterogeneous enhancement with contrast (Figure 2B). We, therefore, proceeded with ultrasound-guided core needle biopsy of the mass and cytologic sampling of the fluid components, which were again consistent with hematoma; no evidence of internal vascular flow was noted on Doppler ultrasound. Ultrasound-guided right inguinal lymph node biopsy was also performed and was negative for malignancy. Because of her large body habitus and pregnancy status, it was agreed that open biopsy should be delayed until after delivery to avoid placing the patient in a prone position.
The patient visited the emergency room several times during the following months because of intermittent exacerbations of her lower extremity pain, swelling, and occasional paresthesias. About 6 months after initial presentation, repeat MRI again showed increased size of the mass (13.5×13.5 cm axial, 28 cm craniocaudal). There was also increased displacement of the adjacent neurovascular structures but no evidence of deep vein thrombosis. Because of concerns about the increased symptomatology of her thigh mass and possible sampling error of the previous biopsies, an elective cesarean section was performed at 35 weeks gestation. One week later, after clearance by her obstetrician, we proceeded with open biopsy of the mass in prone position. Initial sampling was negative for malignancy on frozen section; then, we expressed 1.75 L of brown fluid and solidified blood products, irrigated copiously, and placed a surgical drain. The permanent histologic specimens were again consistent with hematoma, and microbial cultures were negative. A week later, the patient accidentally removed her drain, and she presented with a fever (101°F) on postoperative day (POD) 15. Computed tomography showed reaccumulation of fluid; duplex ultrasound was negative. She was placed on cephalexin and underwent ultrasound-guided replacement of the drain with removal of an additional 750 mL fluid on POD 20. She drained an additional 150 to 200 mL/d for 1 month, with marked improvement in her leg swelling and knee range of motion. The drainage decreased during the next 3 weeks, and the drain was removed on POD 75.
Discussion
The presence of a hematoma in the extremities is usually a straightforward diagnosis. However, the unusual circumstances of this case highlight all the indications for investigation for possible soft-tissue sarcoma when a patient presents with what appears to be a benign condition.
Hematomas are rare in the absence of trauma or coagulopathy, with chronic expansion of hematomas rarer still.4,7,10-11 The patient had no evidence of coagulopathy because of her ability to have an uncomplicated pregnancy and elective cesarean section. She denied a history of trauma, and the location of her hematoma at the posterior distal thigh is an uncommon site of injury. In this setting, fine-needle aspiration and serial imaging to assess for progressive increase in lesion size were indicated to rule out malignancy.2
MRI is the gold-standard imaging modality for distinguishing soft-tissue masses from hematomas.5,12-14 Unlike the typical appearance of a hematoma, sarcomas of the soft-tissue extremities are often complex cystic lesions with multiple septations, internal soft-tissue components, and relatively ill-defined margins.15-17 However, as a hematoma becomes chronic, it can develop a fibrinous capsule, and the contents can manifest an atypical, heterogeneous appearance from scattered, progressive accumulation of blood products that is essentially indistinguishable from sarcomas on imaging.5
Because of the expansion of the hematoma and the atypical appearance of the mass on imaging, repeated core biopsy and, eventually, open biopsy were indicated, despite a preliminary negative diagnosis based on fine-needle aspiration. This resulted from the possibility of sampling error that is particularly relevant to cystic sarcomas, because only portions of the mass may be composed of malignant cells.2 An unusual aspect of this case is the regional lymphadenopathy noted on MRI, because regional lymphatic spread is a known mechanism of metastasis in soft-tissue sarcomas.18 However, the inguinal biopsies showed a chronic inflammatory infiltrate and were negative for malignancy, and enlarged nodes were not seen on imaging several months later. It is possible that the lymphadenopathy resulted from an unrelated process; alternatively, it may have been secondary to impaired lymphatic drainage because of mass effect from the hematoma, which also caused temporary lower extremity swelling.
The distal posterior thigh is an unreported location for a chronic expanding hematoma. Our patient developed slowly progressive lower-limb swelling and, eventually, paresthesias because of displacement of the neurovasculature, an unusual sequela that was recently reported in a similar case of an acute spontaneous hematoma in a patient on warfarin.19 Rupture of a Baker cyst is a possible inciting factor in our patient, although the proximal location of the lesion and the clearly defined tissue plane on MRI between the hematoma and the popliteal region make this unlikely. Finally, the patient’s lesion showed no evidence of vascular flow on Doppler ultrasonography, although giant hematomas secondary to popliteal aneurysm rupture have been reported.20-22
Conclusion
This case highlights the features of a chronic expanding hematoma that can suggest soft-tissue sarcoma and shows the recommended diagnostic steps to differentiate the 2 conditions. This case also describes an unreported location for a chronic expanding hematoma with resulting progressive neurovascular displacement caused by mass effect. We recommend careful monitoring of patients with similarly expansile lesions in this region for signs of neurovascular compromise.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
1. O’Sullivan B, Pisters PW. Staging and prognostic factor evaluation in soft tissue sarcoma. Surg Oncol Clin N Am. 2003;12(2):333-353.
2. Rougraff B. The diagnosis and management of soft tissue sarcomas of the extremities in the adult. Curr Probl Cancer. 1999;23(1):1-50.
3. Friedlander HL, Bump RG. Chronic expanding hematoma of the calf. A case report. J Bone Joint Surg Am. 1968;50(6):1237-1241.
4. Liu CW, Kuo CL, Tsai TY, Lin LC, Wu CC. Massive gluteal mass mimicking sarcoma: chronic expanding hematoma. Formosan J Musculoskeletal Disord. 2011;2(3):106-108.
5. Taieb S, Penel N, Vanseymortier L, Ceugnart L. Soft tissue sarcomas or intramuscular haematomas? Eur J Radiol. 2009;72(1):44-49.
6. Reid JD, Kommareddi S, Lankerani M, Park MC. Chronic expanding hematomas. A clinicopathologic entity. JAMA. 1980;244(21):2441-2442.
7. Okada K, Sugiyama T, Kato H, Tani T. Chronic expanding hematoma mimicking soft tissue neoplasm. J Clin Oncol. 2001;19(11):2971-2972.
8. Negoro K, Uchida K, Yayama T, Kokubo Y, Baba H. Chronic expanding hematoma of the thigh. Joint Bone Spine. 2012;79(2):192-194.
9. Goddard MS, Vakil JJ, McCarthy EF, Khanuja HS. Chronic expanding hematoma of the lateral thigh and massive bony destruction after a failed total hip arthroplasty. J Arthroplasty. 2011;26(2):338.e13-.e15.
10. Radford DM, Schuh ME, Nambisan RN, Karakousis CP. Pseudo-tumor of the calf. Eur J Surg Oncol. 1993;19(3):300-301.
11. Mann HA, Hilton A, Goddard NJ, Smith MA, Holloway B, Lee CA. Synovial sarcoma mimicking haemophilic pseudotumour. Sarcoma. 2006;2006:27212.
12. Kransdorf MJ, Murphey MD. Radiologic evaluation of soft-tissue masses: a current perspective. AJR Am J Roentgenol. 2000;175(3):575-587.
13. Vanel D, Verstraete KL, Shapeero LG. Primary tumors of the musculoskeletal system. Radiol Clin North Am. 1997;35(1):213-237.
14. Siegel MJ. Magnetic resonance imaging of musculoskeletal soft tissue masses. Radiol Clin North Am. 2001;39(4):701-720.
15. O’Connor EE, Dixon LB, Peabody T, Stacy GS. MRI of cystic and soft-tissue masses of the shoulder joint. AJR Am J Roentgenol. 2004;183(1):39-47.
16. Bermejo A, De Bustamante TD, Martinez A, Carrera R, Zabia E, Manjon P. MR imaging in the evaluation of cystic-appearing soft-tissue masses of the extremities. Radiographics. 2013;33(3):833-855.
17. Morrison C, Wakely PE Jr, Ashman CJ, Lemley D, Theil K. Cystic synovial sarcoma. Ann Diagn Pathol. 2001;5(1):48-56.
18. Eilber FC, Rosen G, Nelson SD, et al. High-grade extremity soft tissue sarcomas: factors predictive of local recurrence and its effect on morbidity and mortality. Ann Surg. 2003;237(2):218-226.
19. Kuo CH. Peripheral neuropathy and lower limb swelling caused by a giant popliteal fossa hematoma. Neurol Sci. 2012;33(2):475-476.
20. Reijnen MM, de Rhoter W, Zeebregts CJ. Treatment of a symptomatic popliteal pseudoaneurysm using a stent-graft and ultrasound-guided evacuation of the haematoma. Emerg Radiol. 2009;16(2):167-169.
21. Rossi FH, Veith FJ, Lipsitz EC, Izukawa NM, Oliveira LA, Silva DG. Giant femoropopliteal artery aneurysm and vein rupture. Vascular. 2004;12(4):263-265.
22. Lamoca LM, Alerany MB, Hernando LL. Endovascular therapy for a ruptured popliteal aneurysm. Catheter Cardiovasc Interv. 2010;75(3):427-429.
Severe Neurologic Manifestations of Fat Embolism Syndrome in a Polytrauma Patient
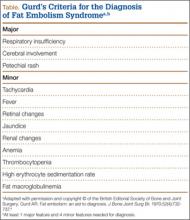
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
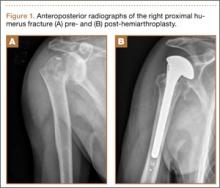
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
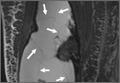
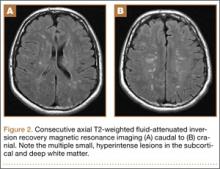
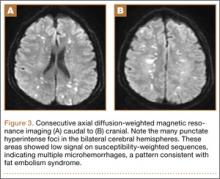
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
Fat embolism syndrome (FES) was first described by Von Bergmann in 1873 in a patient with a fractured femur.1 While fat within the circulation (fat embolism) is relatively common following long-bone fracture, the clinical pattern of symptoms that make up FES is less so, occurring in 1% to 3% of isolated long-bone fractures and 5% to 10% of patients with multiple skeletal trauma.1 A variety of clinical, laboratory, and imaging criteria has been described, classically by Gurd in 1970 (Table).1-6 Most commonly, however, it is a diagnosis of exclusion when the classic triad of respiratory difficulty, neurologic abnormalities, and a characteristic petechial rash are present in the appropriate clinical setting.6
The neurologic sequelae of this syndrome can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of these symptoms usually occurs between 24 hours and 48 hours (mean, 40 hours) after trauma.1 While these neurologic manifestations occur in up to 86% of patients with FES, it is rare for them to be present without the pulmonary symptoms of dyspnea, hypoxemia, and tachypnea, which are the most common presenting symptoms of the disease.1-6 In this case report, we describe severe, rapid-onset neurologic manifestations, without the typical pulmonary involvement, as the primary clinical presentation of FES in a polytrauma patient. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A previously healthy 50-year-old man presented to the emergency room in transfer from an outside hospital after a rollover motor vehicle collision in which he was ejected approximately 50 feet. Injuries included a right proximal humerus fracture/dislocation (Figure 1), right ulnar styloid fracture, L1 compression fracture, and multiple rib fractures. On admission, the patient had an ethanol level of 969 mg/L (.097%) and a urine drug screen positive only for opioids, presumably because of pain medication given that day. He denied a history of alcohol abuse and reported consuming 2 to 3 beers per week. The patient was awake, alert, and oriented with a Glasgow Coma Scale (GCS) of 15. He was tachycardic (heart rate, 126), tachypneic (respiratory rate, 24), and febrile (temperature, 38.6°C [101.5°F]), and his white blood cell count was elevated at 29.5×109/L. On examination, his right arm was found to be neurovascularly intact; it was placed in a sling with a forearm splint, and the patient was admitted to the intermediate special care unit on spine precautions with a plan for right shoulder hemiarthroplasty the following day.
Overnight the patient’s mental status began to deteriorate, and approximately 10 hours after initial assessment, he was not answering questions but was able to respond to some commands. On hospital day 2, approximately 20 hours after initial assessment, the patient had a GCS of 8, was not responding to commands, and moved only in response to painful stimuli. The patient had been prescribed morphine by patient-controlled analgesia and had received intravenous hydromorphone on the day of admission, although the amount of medication delivered was not thought adequate to explain this deterioration. On the morning of hospital day 2, noncontrast brain computed tomography (CT) was normal with no evidence of intracranial hemorrhage or infarct. This was followed by brain magnetic resonance imaging (MRI), with the T2-weighted images showing numerous, small hyperintense lesions in subcortical and periventricular white matter, corpus callosum, basal ganglia, brain stem, and cerebellar hemispheres (Figure 2). The lesions also showed hyperintensity on diffusion-weighted MRI and were interpreted to be consistent with multiple, tiny infarcts (Figure 3). In addition, susceptibility-weighted sequences showed low signal in the same areas, suggesting multiple microhemorrhages, a pattern consistent with FES. Oxygen saturations remained 95% to 99%, and chest radiograph revealed clear lung fields without infiltrate. On hospital day 2, the patient was transferred to the intensive care unit and intubated for airway protection owing to an inability to clear secretions, although arterial blood gas levels remained normal. An echocardiogram revealed no right-to-left shunt, such as a patent foramen ovale (PFO); an electroencephalogram showed no seizure-like activity. No petechial rash was noted on skin examination. The patient was treated with supportive care. Right shoulder hemiarthroplasty was performed on hospital day 7 without complications (Figure 1). On hospital day 13, the patient was following commands and on day 14 he was extubated. His mental status continued to improve, and he was discharged to a rehabilitation facility after 36 days. On last follow-up, 6 months after initial injury, the patient was recovering well with no residual neurologic deficits and only minor limitation in range of motion of the right shoulder.
Discussion
This case presented an interesting diagnostic challenge regarding the patient’s rapid decline in mental status, with a differential diagnosis including diffuse axonal injury (DAI), anoxic brain injury, posttraumatic seizure, other intracranial pathology, such as stroke or hemorrhage, and FES. FES was diagnosed, when other possibilities were ruled out, given the characteristic findings on brain MRI described above in the context of multiple fractures.
Pathophysiology
Despite its recognition in 1873, there is no consensus on the pathophysiological mechanism that causes the clinical symptoms of FES. In the setting of trauma, there are 2 predominant theories. The mechanical theory postulates that fat globules enter the circulation through disrupted venules after the fracture of marrow-containing bones, passing to the arterial circulation through pulmonary vasculature, or paradoxically, by way of a right-to-left shunt, such as a PFO.1,3 The presence of fat in the heart, visualized as echogenic material in the right and left atria on transesophageal echocardiography, has been confirmed in multiple studies during orthopedic procedures, including total knee arthroplasty and femoral reaming.8,9 These fat particles can lodge as microembolisms in target organs such as the skin and brain. However, autopsy studies have shown a lack of correlation of the severity of symptoms and the quantity of intravascular fat.1 In addition, the typical 24- to 72-hour delay in the onset of symptoms after initial trauma would argue against a solely mechanical explanation.10
Alternatively or concomitantly, the biochemical theory proposes that embolized fat may be degraded to toxic intermediaries, such as free fatty acids and C-reactive protein, which cause end-organ damage.3 This has been shown in an animal model, in which intravascular injection of free fatty acids was associated with endothelial damage and increased capillary permeability in the lung, leading to acute respiratory distress syndrome (ARDS).11 The same mechanism could explain injury to other end organs and is consistent with the delay in onset of symptoms after acute injury. In our patient’s case, the absence of pulmonary involvement, lack of a right-to-left vascular shunt such as a PFO, and presence of a systemic inflammatory response on admission may implicate the production of toxic intermediaries from the metabolism of embolized fat as the source of this patient’s FES.
Clinical Presentation
The initial presentation of FES usually manifests as respiratory distress and hypoxia.10 Chest radiographs are often normal, as in our patient, but can show bilateral diffuse interstitial or alveolar infiltrates.2,6 CT more often has findings, including bilateral ground-glass opacities with interlobar septal thickening.12 A petechial rash can be found on the head, neck, anterior thorax, axillae, subconjunctiva, and oral mucous membranes, although it occurs in only 20% to 50% of cases.1,2,13 Neurologic sequelae are present in up to 80% of patients,7 with onset typically following pulmonary symptoms.1,10 These sequelae can range from headache, confusion, and agitation to stupor, focal neurologic signs, and, less commonly, coma.7 Onset of symptoms generally occurs between 24 and 48 hours after trauma,1 although they have been reported as early as 12 hours.10 This case is an example of an atypical course, with the initial presentation of neurologic symptoms at approximately 14 hours after trauma with rapid progression to coma without classic pulmonary symptoms.
Diagnosis
Owing to the nonspecific clinical features of FES, a variety of clinical, laboratory, and imaging criteria has been described. Of these criteria, the most frequently referenced is by Gurd in 1970,4,5 who divided the features into major and minor, with 1 major and 4 minor features required to make the diagnosis (Table). In applying these criteria to our patient, we found that he exhibited the major criteria of cerebral involvement and minor criteria of tachycardia, fever, and thrombocytopenia. Respiratory insufficiency and petechial rash, as well as jaundice, renal changes, and anemia were negative features. Retinal changes, elevated erythrocyte sedimentation rate, and fat macroglobulinemia were not tested or examined. Although in our case the clinical and laboratory criteria for the diagnosis of FES as defined by Gurd were not met, the sensitivity of Gurd’s and other criteria is debated.10
Laboratory tests specific for the disease have not been developed. Although elevated serum levels of lipase, increased blood lipid levels, and fat globules in the urine, sputum, and blood have all been proposed, they are found in trauma patients with and without FES.2,5,6
The nonspecific nature of the signs and symptoms of FES and the lack of reliable laboratory tests for diagnosis of the syndrome highlight the importance of radiographic evaluation in patients with neurologic symptoms. Brain CT scans are usually negative,14 although, in some cases, they may show diffuse edema with scattered low attenuating areas and hemorrhage.15 MRI is more sensitive, and T2-weighted images typically reveal multiple small, nonconfluent hyperintense lesions, usually in the periventricular, subcortical, and deep white matter, sometimes referred to as the “starfield” pattern.14,16 The differential diagnosis for these findings is broad and, in addition to FES, includes DAI, vasogenic edema with microinfarcts, and demyelinating disease.14 Sensitivity and specificity may be increased with the addition of diffusion-weighted MRI, which shows scattered bright spots on a dark background in a similar “starfield” pattern as on T2-weighted images.15 Susceptibility-weighted MRI has recently been introduced as having utility in the diagnosis of FES, with areas of low-signal intensity indicating diffuse microhemorrhages.17 DAI can show a similar pattern; however, the autopsy-confirmed locations of the abnormalities are distinct, with those of FES being found in cerebral and cerebellar white matter and splenium of the corpus callosum and radiographic abnormalities of DAI being found in the gray-white matter junction, dorsolateral brainstem, and splenium of corpus callosum.17
Prevention and Treatment
Of primary importance in the prevention of FES is early stabilization of fractures. Several studies have shown a decreased incidence of FES when long-bone fractures are treated with immediate operative fixation.18,19 However, in the setting of polytrauma, the desire for early definitive treatment must be balanced against the risks for the exaggerated immune response from prolonged surgery.20 The timing of fracture fixation to prevent sequelae of the inflammatory response, such as ARDS and multiple organ dysfunction syndrome, is still debated. In a review article, Pape and colleagues20 suggest classifying the multiply injured patient as stable, borderline, unstable, and in extremis based on clinical and laboratory criteria. They recommend early definitive fixation for stable patients and those patients who are borderline or unstable and responsive to resuscitation, whereas damage-control orthopedics and staged fracture fixation should be considered in the other groups.
Several pharmacologic interventions have been described, although their effects are highly variable and none have clear indications.1-3,6 The most heavily researched is corticosteroids, with the proposed mechanisms of action including blunting of the inflammatory response, stabilizing the pulmonary capillary membrane to reduce interstitial edema, preventing activation of the complement system, and retarding platelet aggregation.21 A recent meta-analysis to assess this intervention examined 6 studies with a total of 386 patients with long-bone fractures who were randomized to treatment with corticosteroids or supportive care only.22 They found a reduced risk for FES in those patients who received corticosteroids, but there was no difference in mortality between groups. Given these results, the utility of corticosteroids is still debated.
Once FES has occurred, treatment options usually focus on supportive care, with most patients having a full recovery.1,3 No specific treatments are available, and symptomatic treatment is the suggested approach, including ensuring adequate oxygenation and ventilation and providing hemodynamic support and volume and blood-product resuscitation as needed.1-3,6
Conclusion
We have presented a case of FES unique in its rapid onset, an initial presentation with neurologic manifestations without typical pulmonary involvement, and the mechanism of end-organ damage without a right-to-left shunt. This case emphasizes the importance of considering FES in the patient with deteriorating mental status in the setting of multiple fractures, particularly in the absence of other characteristic clinical findings, such as pulmonary distress and the pathognomonic petechial rash. Brain MRI can play an important role in diagnosing those patients presenting with predominantly neurological symptoms. Early recognition of this condition allows for the anticipation of complications of the disease process, such as respiratory distress, and the potential need for mechanical ventilation and hemodynamic support.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
1. Johnson MJ, Lucas GL. Fat embolism syndrome. Orthopedics. 1996;19(1):41-49.
2. Levy D. The fat embolism syndrome. A review. Clin Orthop. 1990;261:281-286.
3. Mellor A, Soni N. Fat embolism. Anaesthesia. 2001;56(2):145-154.
4. Gurd AR. Fat embolism: an aid to diagnosis. J Bone Joint Surg Br. 1970:52(4):732-737.
5. Gurd AR, Wilson RI. The fat embolism syndrome. J Bone Joint Surg Br. 1974;56(3):408-416.
6. Bulger EM, Smith DG, Maier RV, Jurkovich GJ. Fat embolism syndrome. A 10-year review. Arch Surg. 1997;132(4):435-439.
7. Jacobson DM, Terrence CF, Reinmuth OM. The neurologic manifestations of fat embolism. Neurology. 1986;36(6):847-851.
8. Sulek CA, Davies LK, Enneking FK, Gearen PA, Lobato EB. Cerebral microembolism diagnosed by transcranial Doppler during total knee arthroplasty: correlation with transesophageal echocardiography. Anesthesiology. 1999;91(3):672-676.
9. Volgas DA, Burch T, Stannard JP, Ellis T, Bilotta J, Alonso JE. Fat embolus in femur fractures: a comparison of two reaming systems. Injury. 2010;41(Suppl 2):S90-S93.
10. Gupta B, D’souza N, Sawhney C, et al. Analyzing fat embolism syndrome in trauma patients at AIIMS Apex Trauma Center, New Delhi, India. J Emerg Trauma Shock. 2011;4(3):337–341.
11. King EG, Wagner WW Jr, Ashbaugh DG, Latham LP, Halsey DR. Alterations in pulmonary microanatomy after fat embolism. In vivo observations via thoracic window of the oleic acid-embolized canine lung. Chest. 1971:59(5):524-530.
12. Malagari K, Economopoulos N, Stoupis C, et al. High-resolution CT findings in mild pulmonary fat embolism. Chest. 2003:123(4):1196-1201.
13. King MB, Harmon KR. Unusual forms of pulmonary embolism. Clin Chest Med. 1994;15(3):561-580.
14. Parizel PM, Demey HE, Veeckmans G, et al. Early diagnosis of cerebral fat embolism syndrome by diffusion-weighted MRI (starfield pattern). Stroke. 2001;32(12):2942-2944.
15. Simon AD, Ulmer JL, Strottmann JM. Contrast-enhanced MR imaging of cerebral fat embolism: case report and review of the literature. AJNR Am J Neuroradiol. 2003;24(1):97-101.
16. Butteriss DJ, Mahad D, Soh C, Walls T, Weir D, Birchall D. Reversible cytotoxic cerebral edema in cerebral fat embolism. AJNR Am J Neuroradiol. 2006;27(3):620-623.
17. Zaitsu Y, Terae S, Kudo K, et al. Susceptibility-weighted imaging of cerebral fat embolism. J Comput Assist Tomogr. 2010;34(1):107-112.
18. Riska EB, Myllynen P. Fat embolism in patients with multiple injuries. J Trauma. 1982;22(11):891-894.
19. Svenningsen S, Nesse O, Finsen V, Hole A, Benum P. Prevention of fat embolism syndrome in patients with femoral fractures–immediate or delayed operative fixation? Ann Chir Gynaecol. 1987;76(3):163-166.
20. Pape HC, Tornetta P, Tarkin I, Tzioupis C, Sabeson V, Olson SA. Timing of fracture fixation in multitrauma patients: the role of early total care and damage control surgery. J Am Acad Orthop Surg. 2009;17(9):541-549.
21. Gosseling HR, Pellegrini VD Jr. Fat embolism syndrome: a review of the pathophysiology and physiological basis of treatment. Clin Orthop. 1982;165:68-82.
22. Bederman SS, Bhandari M, McKee MD, Schemitsch EH. Do corticosteroids reduce the risk of fat embolism syndrome in patients with long-bone fractures? A meta-analysis. Can J Surg. 2009:52(5):386-393.
Subtrochanteric Femur Fracture After Removal of Screws for Femoral Neck Fracture in a Child
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
Subtrochanteric fractures and other complications related to hardware removal in children with slipped capital femoral epiphysis (SCFE) have been well documented.1-3 Subtrochanteric fractures after cannulated screw fixation of femoral neck fractures in adults have also been well recognized,4 and there are several reports on the topic.4,5 However, there are no reports on subtrochanteric fractures after removal of the screws for femoral neck fractures in children.
In this article, we report the case of a child who sustained a subtrochanteric fracture after the screw removal and healing that followed a femoral neck fracture. The patient’s parent provided written informed consent for print and electronic publication of this case report. In addition, our institutional review board approved this case report.
Case Report
A 10-year-old boy was brought to our emergency department with the chief complaint of left hip pain after a car accident. Anteroposterior and axial lateral radiographs showed a displaced cervicotrochanteric femoral neck fracture (Figures 1A, 1B). The patient was admitted to the hospital and underwent closed reduction and internal fixation with two 3.5-mm cannulated titanium screws within 12 hours of arrival. The screws did not cross the physis to avoid iatrogenic injury of the capital femoral epiphysis (Figures 2A, 2B). The entry point was located at the lower level of the lesser trochanter. The lateral cortex was penetrated only once by the guide wire for the placement of each screw.
The patient was discharged to home care with a crutch and an ischial weight-bearing long leg brace for protection from unexpected external force. Two months after surgery, we allowed the patient to walk with the brace and without the crutch. Full-weight-bearing ambulation was allowed 3 months after surgery.
About 9 months after initial surgery, we removed 2 titanium screws, which were completely covered with growing new bone. The lateral cortex surrounding the screw heads was chiseled from the lower level of the lesser trochanter to remove the completely immersed screw heads (Figures 3A, 3B).
After screw removal, we recommended non-weight-bearing crutch-walking for 2 weeks followed by partial weight-bearing with crutch for another month. However, the patient started full weight-bearing 2 weeks after screw removal. One month after screw removal, he was brought to the emergency department with severe left hip pain after missing a step on a path. Anteroposterior and lateral radiographs showed an oblique subtrochanteric fracture at the empty screw holes (Figures 4A, 4B). A plate and 4 screws were placed to stabilize the subtrochanteric fracture, and a hip spica cast was applied and was to be worn for 3 weeks (Figures 5A, 5B).
At final follow-up, 6 months after the second surgery, the fracture was healed, and there had been no complications, such as avascular necrosis of the femoral head and leg-length discrepancy (Figures 6A, 6B).
Discussion
Although in situ pinning of SCFE is a common procedure with good results, the rate of complications of hardware removal can be as high as 34%; these complications are well documented.5 Subtrochanteric fracture as a complication of proximal femoral neck pinning in adults is also well documented.4,5 However, there are no reports on subtrochanteric fractures after screw removal in the treatment of femoral neck fractures in children.
Brooks and colleagues6 emphasized the point that multiple passes weakened the lateral cortex, decreased the energy-absorbing capacity by 55.2%, and increased local stress. Even if a screw is placed in a relatively safe zone above the lesser trochanter, pie-crusting of the cortex can weaken it enough to predispose it to failure under a relatively normal load.7 We inserted 2 cannulated titanium screws without repositioning or multiple drilling, and the femoral neck fracture was united.
The common denominator for subtrochanteric fractures after screw or pin fixation of femoral neck fractures in adults seemed to be the entry point of the lateral cortex below the level of the most inferior edge of the lesser trochanter.4 The pin should have its entry site proximal to the level of the lesser trochnater. Paloski and colleagues7 and Canale and colleagues8 hypothesized that this screw acted as a stress riser to the normal bone, which underwent abnormal loads caused by the patient’s habitus and later mechanism of injury. In our patient’s case, the appropriate starting point for perpendicular penetration of the femoral neck fracture line was on the lateral femoral cortex at the level of the lesser trochanter. We thought this entry on the lateral cortex might predispose the patient to a subtrochanteric fracture. The starting point of the screw is considered the most important factor in preventing fracture after screw removal.
As titanium pins cause very tight bone ingrowth,9,10 the surface of titanium screws used for femoral neck fractures in children are smoothed to reduce turning force.1 The hexagonal sockets wore off rapidly and proved to be too weak to overcome the necessary torque for loosening the pin from the bone.
Lee and colleagues10 found that significantly more operative time was needed to remove titanium pins (vs steel pins) after 12 months or longer. When Asnis III pins (Howmedica, Rutherford, New Jersey) were used in the treatment of femoral neck fractures in aged patients, similar problems did not occur. One possible explanation is that bone density is higher in adolescents than in adults. In addition, more bone ingrowth and higher bone compression might occur in adolescent bones.1 Given the considerable disadvantages noted in their series, Ilchmann and Parsch1 concluded that use of cannulated titanium screws should be suspended and that stainless steel pins are safe to use in SCFE.
In our patient’s case, we also struggled to remove titanium screws. Subtrochanteric fractures can be complications after removal of screws for femoral neck fractures in children. If there are no specific screw-related symptoms, one should consider leaving the screw in place and avoiding screw removal.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
1. Ilchmann T, Parsch K. Complications at screw removal in slipped capital femoral epiphysis treated by cannulated titanium screws. Arch Orthop Trauma Surg. 2006;126(6):359-363.
2. Raney EM, Freccero LA, Dolan DE, Lighter R, Fillman L, Chambers HG. Evidence-based analysis of removal of orthopaedic implants in the pediatric population. J Pediatr Orthop. 2008;28(7):701-704.
3. Karagkevrekis CB, Rahman H. Subtrochanteric femoral fracture following removal of screw for slipped capital femoral epiphysis. Injury. 2003;38(4):320-321.
4. Kloen P, Rubel IF, Lyden JP, Helfet DL. Subtrochanteric fracture after cannulated screw fixation of femoral neck fractures: a report of four cases. J Orthop Trauma. 2003;17(3):225-229.
5. Karr RK, Schwab JP. Subtrochanteric fracture as complication of proximal femoral pinning. Clin Orthop. 1985;(194):214-217.
6. Brooks DB, Burstein AH, Frankel VH. The biomechanics of torsional fractures. The stress concentration effect of a drill hole. J Bone Joint Surg Am. 1970;52(3):507-514.
7. Paloski M, Taylor BC, Willits M. Subtrochanteric femur fracture after slipped capital femoral epiphysis pinning: a novel treatment. Adv Orthop. 2011;2011:809136.
8. Canale ST, Casillas M, Banta JV. Displaced femoral neck fractures at the bone–screw interface after in situ fixation of slipped capital femoral epiphysis. J Pediatr Orthop. 1997;17(2):212-215.
9. Vresilovic EJ, Spindler KP, Robertson WW Jr, Davidson RS, Drummond DS. Failure of pin removal after in situ pinning of slipped capital femoral epiphysis: a comparison of different pin types. J Pediatr Orthop. 1990;10(6):764-768.
10. Lee TK, Haynes RJ, Longo JA, Chu JR. Pin removal in slipped capital femoral epiphysis: the unsuitability of titanium devices. J Pediatr Orthop. 1996;16(1):49-52.
Synovial Fistula After Tension Band Plating for Genu Valgum Correction
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
Children often present to orthopedic surgeons with angular deformities about the knee. Temporary hemiepiphysiodesis, which is a frequently performed procedure to address such deformities, is safe and reversible. Specifically, tension band plating has become one of the most commonly performed techniques, especially given its low complication rates and minimally invasive nature.1-4 Complications reported with this method include mechanical hardware failure,5 implant migration,4 and recurvatum.3
We present an unreported complication of a synovial fistula formation after the removal of a tension band plate in a child who had achieved appropriate correction of her genu valgum. The patient and her family provided written informed consent for print and electronic publication of this case report.
Case Report
An 11-year-old girl presented to the pediatric orthopedics clinic with concern for genu valgum of the right lower extremity. She underwent a right proximal tibia medial hemiepiphysiodesis via tension band plating technique. Her clinic visit 4 weeks after surgery showed well-healed incisions and no signs of infection. She achieved appropriate correction and underwent hardware removal approximately 6 months after her initial surgery.
One month after hardware removal, the patient began to notice increased swelling and erythema around her incision site with associated pain. No fluid or drainage was seen at that time. She underwent irrigation and débridement shortly thereafter, and the wound was left open for wet-to-dry dressing changes (Figure 1). Intraoperative cultures were negative, but the patient received empiric antibiotic therapy. She continued to have difficulty with wound healing for the next month and was referred to plastic surgery. She underwent repeat irrigation and débridement, followed by coverage with a split-thickness skin graft by the plastic surgery service. Intraoperative cultures were again negative. During both irrigation and débridement procedures, care was taken to remain superficial and not violate the knee capsule.
At her 2-week postoperative check, the bolster covering the split thickness skin graft was removed, which revealed a 2×2-mm area of clear erosion near the central portion of her wound with synovial fluid drainage (Figure 2). Because of concern for a synovial fistula, magnetic resonance imaging (MRI) of the right knee was obtained, which confirmed the synovial fistula (Figures 3A, 3B). The coronal cut on MRI clearly showed the fistula with synovial fluid tracking into the epiphyseal screw tract through the breached capsule and to the level of the skin. She was immobilized in a long leg cast with the knee in extension for 6 weeks. Upon return, her fistula had closed, and she has not had any more wound issues.
Discussion
To our knowledge, this is the first report of a synovial fistula after temporary hemiepiphysiodesis performed via tension band plating. Capsular knee anatomy may explain the etiology of the synovial fistula after hardware removal. The medial knee capsule composition and attachment sites have been extensively studied.6 In contrast to other joints, such as the shoulder, elbow, ankle, and hip, the metaphysis of the knee lies outside the capsule because the capsule inserts proximal at the level of the physis.7 During tension band plating, the epiphyseal screw breaches the capsule but serves as a plug while in place, which prevents the formation of a synovial fistula. When the screw is removed, the capsular rent spontaneously closes in almost all cases. However, the opportunity exists for a synovial fistula to form while the capsule heals, as evidenced by the current case. Such an issue does not apply to the metaphyseal screw because it is inserted outside the capsule.
Although it is possible that the synovial fistula was inadvertently created during one of the irrigation and débridement procedures, this is very unlikely. The surgeons who performed these washout procedures are knowledgeable and familiar with knee anatomy. Both irrigation and débridement procedures were superficial, and care was taken not to violate the knee capsule.
A synovial fistula after knee surgery is rare. Larsen8 described the fistula as a phenomenon that develops when excessive synovial fluid forces its way through a synovial incision with knee flexion and muscle contraction. Such a complication is most routinely described after knee arthroscopy. Proffer and colleagues9 reported an incidence of 6.1 per 1000 after knee arthroscopies. The average number of days until fistula diagnosis was 6 days (range, 3-10 days). All fistulae were treated with immobilization and closed after an average of 9 days (range, 7-14 days). There were no associated infections, although prophylactic antibiotics were given. A national survey found that knee fistulae accounted for only 3.2% of all complications of knee arthroscopy.10
The treatment for a synovial fistula is largely nonoperative. Most will resolve with a brief period of immobilization, which allows the fistula to close.9-10 Literature addressing fistulae that fail to heal with nonoperative treatment is limited. Excision and direct closure of the fistula, especially when chronic, often proves futile and leads to a high recurrence rate.11 An alternative but more extensive treatment involves excision and coverage with a myofascial flap.12
Complications reported after tension band plating are uncommon. Two studies reported no complications regarding the use of the tension band plate.1-2 Burghardt and colleagues,5 in reporting the results of a multicenter survey, found that 15% of surgeons who had used tension band plating had seen a total of 65 cases of mechanical failure. In all cases, the screws, not the plate, failed. Another study reported implant migration in 1 patient but attributed the complication to a technical error from placing the distal screw too close to the physis.4 A third study documented that 2 patients developed clinically significant recurvatum, most likely because of anterior placement of the plate.3 It is important to identify a synovial fistula postoperatively because it provides a direct route for pathogens from the external environment to enter the intra-articular space and the opportunity for a septic joint to develop. Infection should always be ruled out and, if present, appropriately treated.
Conclusion
Physicians performing tension band plating in the knee should be aware of the possible complication of a synovial fistula, which has traditionally been reported only in relation to knee arthroscopy. Given the proposed etiology of the synovial fistula, we recommend a brief period of immobilization of 3 to 5 days after tension band plate removal, allowing the capsular rent to heal and minimizing the risk of a synovial fistula.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
1. Burghardt RD, Herzenberg JE, Standard SC, Paley D. Temporary hemiepiphyseal arrest using a screw and plate device to treat knee and ankle deformities in children: a preliminary report. J Child Orthop. 2008;2(3):187-197.
2. Boero S, Michelis MB, Riganti S. Use of the eight-plate for angular correction of knee deformities due to idiopathic and pathologic physis: initiating treatment according to etiology. J Child Orthop. 2011;5(3):209-216.
3. Guzman H, Yaszay B, Scott VP, Bastrom TP, Mubarak SJ. Early experience with medial femoral tension band plating in idiopathic genu valgum. J Child Orthop. 2011;5(1):11-17.
4. Ballal MS, Bruce CE, Nayagam S. Correcting genu varum and genu valgum in children by guided growth: temporary hemiepiphysiodesis using tension band plates. J Bone Joint Surg Br. 2010; 92(2):273-276.
5. Burghardt RD, Specht SC, Herzenberg JE. Mechanical failures of eight-plate guided growth system for temporary hemiepiphysiodesis. J Pediatr Orthop. 2010;30(6):594-597.
6. LaPrade RF, Engebretsen AH, Ly TV, Johansen S, Wentorf FA, Engebretsen L. The anatomy of the medial part of the knee. J Bone Joint Surg Am. 2007;89(9):2000-2010.
7. Montgomery CO, Siegel E, Blasier RD, Suva LJ. Concurrent septic arthritis and osteomyelitis in children. J Pediatr Orthop. 2013;33(4):464-467.
8. Larsen RL. Synovial sinus. In: Epps CH Jr, ed. Complications in Orthopaedic Surgery. 2nd ed. Philadelphia, PA: JB Lippincott; 1978:5-11.
9. Proffer DS, Drez D Jr, Daus GP. Synovial fistula of the knee: a complication of arthroscopy. Arthroscopy. 1991;7(1):98-100.
10. Committee on Complications of Arthroscopy Association of North America. Complications of arthroscopy and arthroscopic surgery: results of a national survey. Arthroscopy. 1985;1(4):214-220.
11. Yiannakopoulos CK. Diagnosis and treatment of postarthroscopic synovial knee fistulae: a report of four cases and review of the literature. J Knee Surg. 2007;20(1):34-38.
12. Méndez-Fernández MA. Treatment of chronic recurrent fistulae with myofascial flaps. Br J Plast Surg. 1993;46(4):303-306.
Acute Upper Abdominal Pain in Early Pregnancy
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
A 32-year-old G1P0 woman at 7 4/7 weeks’ gestation (intrauterine pregnancy confirmed by ultrasound) with a history of hypertension and anxiety presented to the emergency department reporting right upper quadrant and right flank pain. The patient did not report taking any medications. Her symptoms had begun about 6 hours prior to presentation. She did not report fever, chills, nausea, vomiting, anorexia, or urinary or bowels symptoms. She was afebrile, and her initial blood pressure was 174/84 mm Hg, but within an hour of presentation had decreased to 137/82 mm Hg.
On exam she was in moderate-to-severe discomfort. She was tender to palpation in the right flank as well as the right upper quadrant and had a positive Murphy sign. Her white blood cell (WBC) count was 18,800/μL, hemoglobin and hematocrit were normal, liver function tests were within normal limits, and sodium was 133 mmol/L; otherwise, electrolytes were normal. The urinalysis showed a specific gravity of 1.022 with 3 red blood cells per high powered field, but was otherwise normal. A right upper quadrant ultrasound showed a normal gallbladder without cholelithiasis. Subsequently, a magnetic resonance imaging (MRI) of the abdomen was obtained (Figure 1).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Our Treatment
An MRI of the abdomen revealed a right adrenal hematoma (Figure 2). This diagnosis was made after common causes of the right upper quadrant and right flank pain were excluded and was confirmed with an MRI of the abdomen.
Discussion
Common causes of right flank and right upper quadrant pain include urolithiasis, pyelonephritis, and acute cholecystitis (Table). Patients with urolithiasis will typically present with sudden onset of colicky flank pain, which may radiate to the inguinal region, with or without nausea and vomiting.1,2 Microhematuria may be absent in as many as 10% to 20% of patients.2 Computed tomography is nearly 97% sensitive and 96% specific for urolithiasis.2 Although only 37% to 64% sensitive in detecting urolithiasis, abdominal ultrasound is 85% to 94% specific for detecting hydronephrosis.2
Symptoms of pyelonephritis range from mild to severe costovertebral angle pain with or without fever and/or lower urinary tract symptoms to sepsis.3 Urinalysis typically shows leukocyte esterase (72%-97% sensitive, 41%-86% specific for culture-confirmed urinary tract infection) and microscopic pyuria (90%-96% sensitive, 47%-50% specific for culture-confirmed urinary tract infection).3,4 Urine culture will reveal 105 colony-forming units in 95% of patients with acute uncomplicated pyelonephritis.3 Imaging is typically reserved for atypical presentations or in cases of nonresolution of symptoms despite treatment in order to rule out structural anomalies, an obstructive process, or abscess formation.3
Acute cholecystitis is suggested by right upper quadrant pain, often following a meal. Symptoms include fever, leukocytosis, and an elevated serum bilirubin level.5 Ultrasound findings of acute cholecystitis are gallbladder wall thickening, pericholecystic fluid, or sonographic Murphy sign.6 Ultrasound has a sensitivity of 81% and a specificity of 83% for detecting acute cholecystitis, whereas hepatobiliary iminodiacetic acid scan (cholescintigraphy) is 96% sensitive and 90% specific and is used in cases where ultrasound is indeterminate.6
Spontaneous adrenal hemorrhage (SAH) typically presents with symptoms of upper abdominal pain and/or flank pain. If hemorrhage is significant, hypotension and a drop in hematocrit may occur. Alternatively, if SAH is bilateral and results in a deficiency of adrenal hormones, hypotension may be secondary to adrenal insufficiency. Due to its ready availability, most SAHs are diagnosed by CT scan ordered for alternate reasons.7
In the pregnant patient, to avoid exposing the developing fetus to radiation, ultrasound is typically the first imaging modality to investigate right upper quadrant or flank pain. However, ultrasound is less sensitive for evaluating anomalies of the adrenal gland.8 An MRI is the most accurate imaging modality for diagnosing adrenal hemorrhage and avoids exposing the developing fetus to radiation.9 In this case, the absence of an etiology of the patient’s symptoms on right upper quadrant ultrasound led to further evaluation, with abdominal MRI revealing the diagnosis.
In a review of 141 cases of adrenal hemorrhage at the Mayo Clinic in Rochester, Minnesota, 16 patients had a spontaneous adrenal hemorrhage, which presented with severe abdominal and flank pain of sudden onset.7 Seven of these patients required surgery to control the bleeding.7 None of the 13 patients with a functioning contralateral adrenal gland required adrenal replacement therapy.7 Gavrilova-Jordan and colleagues reported a case of a spontaneous unilateral adrenal hemorrhage in the third trimester of pregnancy that was managed conservatively with favorable outcomes.10 Potential causes of spontaneous adrenal hemorrhage include:
• Antiphospholipid antibody syndrome
• Heparin-induced thrombocytopenia
• Trauma
• Physiologic stress (eg, sepsis)
• Adrenal mass (pheochromocytoma, angiomyolipoma, or metastatic cancer).6,11
Case Outcome
Initial right upper quadrant ultrasound in this patient revealed a normal gallbladder and a small amount of perinephric fluid surrounding the right kidney. On the night of admission she experienced an elevated temperature of 101.9°F. Due to the fever and leukocytosis, she was initiated on antibiotics (ampicillin-sulbactam 3 g IV every 6 hours) covering a urinary source. On hospital day 2, right lower quadrant and right upper quadrant ultrasounds were performed, revealing decreased fluid surrounding her right kidney and without evidence of appendicitis. Due to persistent leukocytosis (WBC count peaked at 26,400/μL), essentially normal urinalysis, and negative urine culture, an abdominal MRI was obtained to evaluate for a perinephric abscess. The MRI revealed the spontaneous adrenal hemorrhage.
The patient was observed as an inpatient for the following 48 hours. Her electrolytes remained normal and hemoglobin decreased to 9.5 g/dL. She remained hemodynamically stable. Serum cortisol and angiotensin levels were normal, antibiotics were discontinued, and the patient was discharged to outpatient follow-up.
A repeat MRI of the abdomen obtained 6 weeks later revealed a resolving right adrenal hematoma. At 39 weeks’ gestation, she delivered a vigorous female infant weighing 3,200 g (7 lb 1 oz) with an Apgar score at birth of 8/9.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
1. Wasserstein AG. Nephrolithiasis. Am J Kidney Dis. 2005;45(2):422-428.
2. Ban KM, Easter JS. Selected urologic problems. In: Marx JA, Hockberger RS, Walls, RM, eds. Rosen’s Emergency Medicine: Concepts and Clinical Practice. 7th ed. Philadelphia, PA: Elsevier; 2010:1297-1324.
3. Colgan R, Williams M, Johnson JR. Diagnosis and treatment of acute pyelonephritis in women. Am Fam Physician. 2011;84(5):519-526.
4. Simerville JA, Maxted WC, Pahira JJ. Urinalysis: A comprehensive review. Am Fam Physician. 2005;71(6):1153-1162.
5. Friedman LS. Liver, biliary tract & pancreas disorders. In: McPhee SJ, Papadakis MA, eds. Current Medical Diagnosis and Treatment, 2012. 51st ed. New York, NY: Lange Medical Books/McGraw-Hill; 2012:644-698.
6. Kiewiet JJ, Leeuwenburgh MM, Bipat S, Bossuyt PM, Stoker J, Boermeester MA. A systematic review and meta-analysis of diagnostic performance of imaging in acute cholecystitis. Radiology. 2012;264(3):708-720.
7. Vella A, Nippoldt TB, Morris JC 3rd. Adrenal hemorrhage: A 25-year experience at the Mayo Clinic. Mayo Clin Proc. 2001;76(2):161-168.
8. Kawashima A, Sandler CM, Ernst RD, et al. Imaging of nontraumatic hemorrhage of the adrenal gland. Radiographics. 1999;19(4):949-963.
9. Hoeffel C, Legmann P, Luton JP, Chapuis Y, Fayet-Bonnin P. Spontaneous unilateral adrenal hemorrhage: Computerized tomography and magnetic resonance imaging findings in 8 cases. J Urol. 1995;154(5):1647-1651.
10. Gavrilova-Jordan L, Edmister WB, Farrell MA, Watson WJ. Spontaneous adrenal hemorrhage during pregnancy: A review of the literature and a case report of successful conservative management. Obstet Gynecol Surv. 2005;60(3):191-195.
11. Marti JL, Millet J, Sosa JA, Roman SA, Carling T, Udelsman R. Spontaneous adrenal hemorrhage with associated masses: Etiology and management in 6 cases and a review of 133 reported cases. World J Surg. 2012;36(1):75-82.
Pulmonary Vein Thrombosis Associated With Metastatic Carcinoma
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
Pulmonary vein thrombosis (PVT) is rare and underdiagnosed in clinical practice. It has been described following lobectomy, lung transplant, and in association with metastatic carcinoma.1-4 Some cases have been described as idiopathic.5-7 Its exact incidence is unknown, and treatment depends on etiology. On the other hand, pulmonary artery thromboembolism is a well-known entity with identified risk factors as well as clearly defined modalities of management. The following is a case of PVT, which occurred in the setting of small cell carcinoma of the lung (SCLC) and mantle cell lymphoma of the small intestine.
CASE PRESENTATION
A 66-year-old male veteran with a past medical history of type 2 diabetes mellitus, hypertension, and chronic obstructive pulmonary disease, who had a 40 pack-year history of cigarette smoking, was admitted to the hospital for severe, sudden onset abdominal pain. The pain was localized in the right lower quadrant and then became generalized. It was sharp, aggravated by movement, and relieved by rest. The patient reported being constipated for the past couple of days.
A review of systems revealed that he had been coughing for about 3 days prior to admission. A computed tomography (CT) scan of the abdomen showed pneumoperitoneum and a mass with mural thickening around the distal ileum/cecal area (Figure 1). There was also a partially visualized mass in the infrahilar area of the right lower lobe and bilateral adrenal masses seen on the scan. A chest CT with contrast was then performed, which showed a 7.5 x 6.6 x 6.6-cm mass in the right lower lobe posterior to the right hilum. The mass encased the right mainstem bronchus, and there was a low-density-filling defect in the inferior branch of the right pulmonary vein (Figure 2). An echocardiography did not show any thrombus within the atria or ventricles.
The patient underwent emergent exploratory laparotomy for bowel perforation. The operative finding was a small perforation of the small intestine with an associated mass. There were metastatic lesions throughout the abdomen. A partial small bowel resection was performed. Post exploratory laparotomy, a fiberoptic bronchoscopy was performed, which revealed a 1-cm fungating lesion at the takeoff of the superior segment of the right lower lobe. Brushings were obtained from the mass. The pathology of the lung mass was small cell carcinoma, whereas that of the bowel mass was mantle cell lymphoma. Brain magnetic resonance imaging revealed that he had metastasis to the brain with a 4-cm mass in the cerebellum. He was anticoagulated with heparin for the PVT. Based on his poor functional status and his overall clinical condition, his prognosis was poor. He received hospice care and died 3 months later.
DISCUSSION
Pulmonary vein thrombosis is a rare condition. The incidence is unclear, as most of the literature includes case reports. The majority of PVT cases are reported following lobectomy for malignancy and lung transplantation.1-3 The incidence following lung transplant was reported in the early postoperative period to be 15% in a center during the first 2 years of the study.3 Pulmonary vein thrombosis has also been described following metastatic cancer, such as liposarcoma.4
This patient’s case was discovered in the setting of SCLC and mantle cell lymphoma of the small intestine. Small cell carcinoma of the lung was reported to invade the pulmonary vein into the left atrium.8 In this patient, the left atrium was not invaded. There have been cases of spontaneous or idiopathic PVT described, presenting as abdominal pain, hemoptysis, and chest pain.5-7 No precipitating causes were detected in these patients.
The pathogenesis of PVT from a tumor is unclear, although several theories have been postulated: It could result from direct extension of the tumor into the vein, from compression of the vein by the tumor, or from epithelial damage as a result of tumor invasion. The tumor thrombus has been described to extend into the right atrium.6,8 The mechanism of thrombosis remains unclear in the patient postlobectomy or postlung transplantation, although intraoperative torsion and injury of vessels are implicated. Similar to deep vein thrombosis, PVT could also result from intimal damage or sluggish flow in the pulmonary stump in the postoperative patient.2,9,10
The presentation of PVT is usually nonspecific, including dyspnea, cough, pleuritic chest pain, and hemoptysis. It has been reported as causing massive hemoptysis due to acute pulmonary infarction.7 Acute PVT occurring postoperatively in the lung transplant patient may be disastrous and lead to early postoperative allograft failure.11 Pulmonary vein thrombosis may also present more insidiously with recurrent pulmonary edema and pulmonary fibrosis.12 This patient presented with abdominal pain; further workup led to the finding of a lung mass. Pulmonary vein thrombosis has been reported to result in systemic emboli, resulting in cerebrovascular accidents, or it can manifest as aseptic and tumor emboli.2,5,10,13,14
Newer CT techniques have made identifying PVT possible in a similar manner to which pulmonary arterial emboli are detected by using the pulmonary venous phase of a contrast CT of the chest.5 Echocardiography may demonstrate the extension of the thrombus into the atrium; a transesophageal echocardiogram would be preferable over a transthoracic echocardiogram. Magnetic resonance imaging of the chest is another useful modality for diagnosis, because it is able to distinguish between a bland thrombus and a tumor thrombus in the pulmonary vein.15
Treatment of PVT depends on the overall clinical condition of the patient. Irrespective of the etiology, a review of the literature does not indicate the preferred duration of anticoagulation or preference for modality of anticoagulation between oral vitamin K antagonists or heparin—low molecular or unfractionated.1,3-6 Patients who develop PVT following malignancy are usually anticoagulated with therapy for the cancer. The treatment of PVT in the setting of lung transplant is more challenging and includes systemic heparinization, thrombolytics, and surgical thrombectomy.3,11,16 The majority of the literature includes case reports with varying morbidity and mortality, depending on the etiology. Ninety-day mortality of 38% was reported following lung transplant.3
CONCLUSION
Pulmonary vein thrombosis presents in a nonspecific manner. The diagnosis is now more readily made with the advent of a variety of diagnostic modalities, especially with transesophageal echocardiography, which may be performed at the bedside in the intensive care unit. The treatment remains challenging with mortality dependent on the etiology. A diagnosis of PVT needs to be considered in patients with appropriate risk factors. A high index of suspicion will enable the diagnosis in the proper clinical scenario.
Author disclosures
The authors report no actual or potential conflicts of interest with regard to this article.
Disclaimer
The opinions expressed herein are those of the authors and do not necessarily reflect those of Federal Practitioner, Frontline Medical Communications Inc., the U.S. Government, or any of its agencies. This article may discuss unlabeled or investigational use of certain drugs. Please review complete prescribing information for specific drugs or drug combinations—including indications, contraindications, warnings, and adverse effects—before administering pharmacologic therapy to patients.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
1. Genta PR, Ho N, Beyruti R, Takagaki TY, Terra-Filho M. Pulmonary vein thrombosis after bilobectomy and development of collateral circulation. Thorax. 2003;58(6):550-551.
2. Ohtaka K, Hida Y, Kaga K, et al. Pulmonary vein thrombosis after video-assisted thoracoscopic left upper lobectomy. J Thorac Cardiovasc Surg. 2012;143(1):e3-e5.
3. Schulman LL, Anandarangam T, Leibowitz DW, et al. Four-year prospective study of pulmonary venous thrombosis after lung transplantation. J Am Soc Echocardiogr. 2001;14(8):806-812.
4. Tamizifar B, Zadeh MR, Foroghi E. Pulmonary vein thrombosis after metastatic liposarcoma. Med Arh. 2012;66(1):68-69.
5. Selvidge SD, Gavant ML. Idiopathic pulmonary vein thrombosis: Detection by CT and MR imaging. AJR Am J Roentgenol. 1999;172(6):1639-1641.
6. Wu JP, Wu Q, Yang Y, DU ZZ, Sun HF. Idiopathic pulmonary vein thrombosis extending to left atrium: A case report with a literature review. Chin Med J (Engl). 2012;125(6):1197-1200.
7. Alexander GR, Reddi A, Reddy D. Idiopathic pulmonary vein thrombosis: A rare cause of massive hemoptysis. Ann Thorac Surg. 2009;88(1):281-283.
8. Chan V, Neumann D. Small cell lung carcinoma invading the pulmonary vein and left atrium as imaged by PET/CT. Eur J Nucl Med Mol Imaging. 2005;32(12):1493.
9. Burri E, Duwe J, Kull C, Glaser C, Maurer CA. Pulmonary vein thrombosis after lower lobectomy of the left lung. J Cardiovasc Surg (Torino). 2006;47(5):609-612.
10. Schwalm S, Ward RP, Spencer KT. Transient ischemic attack in a patient with pulmonary vein thrombosis after left upper lobectomy for squamous cell lung cancer. J Am Soc Echocardiogr. 2004;17(5):487-488.
11. Cywinski JB, Wallace L, Parker BM. Pulmonary vein thrombosis after sequential double-lung transplantation. J Cardiothorac Vasc Anesth. 2005;19(2):225-227.
12. Cavaco RA, Kaul S, Chapman T, et al. Idiopathic pulmonary fibrosis associated with pulmonary vein thrombosis: A case report. Cases J. 2009;2:9156.
13. Kim NH, Roldan CA, Shively BK. Pulmonary vein thrombosis. Chest. 1993;104(2):624-626.
14. Uhlmann EJ, Dunitz JM, Fiol ME. Pulmonary vein thrombosis after lung transplantation presenting as stroke. J Heart Lung Transplant. 2009;28(2):209-210.
15. Hricak H, Amparo E, Fisher MR, Crooks L, Higgins CB. Abdominal venous system: Assessment using MR. Radiology. 1985;156(2):415-422.
16. Nagahiro I, Horton M, Wilson M, Bennetts J, Spratt P, Glanville AR. Pulmonary vein thrombosis treated successfully by thrombectomy after bilateral sequential lung transplantation: Report of a case. Surg Today. 2003;33(4):282-284.
Bronchogenic Squamous Cell Carcinoma With Soft-Tissue Metastasis to the Hand: An Unusual Case Presentation and Review of the Literature
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
Carcinoma of the lung is the most common lethal form of cancer in both men and women worldwide.1 It accounts for more deaths than the next 3 most common cancers combined. In 2012, 160,000 Americans are estimated to have died from lung cancer.1 Lung cancer is known to have a high metastatic potential for the brain, bones, adrenal glands, lungs, and liver.2 Orthopedic manifestations frequently include bony metastasis, most commonly the vertebrae (42%), ribs (20%), and pelvis (18%).3 Acral metastatic disease is defined as metastasis distal to the elbow or the knee. Bony acral metastases from lung carcinoma to the upper and lower extremities are extremely uncommon, accounting for only 1% each of total bone metastases from carcinoma of the lung.3 Metastases to the bones of the hand are even rarer. Only 0.1% of metastatic disease from any type of carcinoma or sarcoma manifests as metastasis in the hand.4 There are only a few reports in the literature of soft-tissue or muscular metastasis to the hand from a carcinoma. Of these cases, the majority are caused by metastatic lung carcinoma.5-9 There are no reports in the literature of metastatic disease of squamous cell origin affecting the soft tissues of the hand.
We present a case of a man with known metastatic squamous cell carcinoma of the lung who presented with acral soft-tissue metastatic disease. This report highlights a rare clinical scenario that has not been reported in the literature. The report also emphasizes a rare but important consideration for clinicians who encounter acral soft-tissue lesions in patients with a history of a primary carcinoma. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old man presented with right-sided pleuritic flank pain, along with a 30-lb weight loss over a 6-month period. A computed tomographic scan revealed a 5.58×3.7-cm cavitary lesion in the right lower lobe with abutment of the posterior chest wall (Figure 1). He underwent biopsy and staging, and was found to be T3N1, with biopsy-proven well-differentiated bronchogenic squamous cell carcinoma. The patient then underwent right lower and middle lobectomy with concomitant en-bloc resection of the posterior portion of ribs 7 to 11, along with mediastinal lymph-node dissection with negative margins. After surgery, he was treated with 4 cycles of adjuvant chemotherapy with cisplatin and docetaxel.
Six months after surgery, the patient began to complain of right-hand pain isolated to the thenar eminence. He also described swelling and significant pain with active or passive movement of the thumb and with relatively mild-to-moderate palpation of the area. The patient reported that the functioning of his thumb deteriorated rapidly over the course of about 1 month. On physical examination, he was neurovascularly intact with no apparent deficit in sensation of his right hand. There was no erythema or overlying skin changes. His right thenar eminence was mildly enlarged as compared with the left, and a firm, focal mass was readily palpated. Range of motion at the metacarpophalangeal joint of the thumb and index finger was limited because of pain. Thumb opposition was markedly limited. After a detailed history and physical examination, we were concerned about possible deep space infection, old hematoma, or possible metastatic disease. Magnetic resonance imaging (MRI) was ordered to evaluate the palpable mass.
Radiographically, localized soft-tissue swelling was present on the palmar surface of the hand obliquely overlying the index finger metacarpal (Figures 2, 3). On MRI, the lesion measured approximately 1.8×3.3 cm and was isointense to slightly hyperintense diffusely with central hyperintensity on T1 images (Figure 4). On T2 and short tau inversion recovery images, the lesion was more strikingly hyperintense and infiltrative in appearance (Figure 5). Postcontrast images showed avid enhancement peripherally, with central nonenhancement consistent with necrosis in the adductor pollicis.
We performed a biopsy of the lesion with the aid of immediate adequacy by fine needle aspiration cytology. We saw mitotically active malignant cells with large nuclei, high nuclear-to-cytoplasmic ratios, nucleoli, and dense cytoplasm, suggesting a metastatic squamous cell carcinoma. Because infection was part of the differential, it is pertinent to note that there was no significant inflammatory infiltrate. The core biopsy was consistent with metastatic lung cancer (Figure 6).
Discussion
This patient presented an interesting diagnostic challenge, particularly because of his previous malignancy. The differential diagnosis of acute onset thenar pain without history of trauma would include encompassing soft-tissue abscess, osteomyelitis, and infectious myositis. Soft-tissue hematoma is also in the differential for this patient, especially given the malignancy. Bony metastasis should be considered in this patient given the propensity of lung carcinoma to metastasize to bone. The location would certainly be atypical, with metastasis to the bones of the forearm or hand representing only 0.1% of all metastasis of any type of primary carcinoma or sarcoma.4 Primary bone or soft-tissue sarcoma should also be considered. Some authors have also suggested that necrosis, peritumoral edema-like signal, and lobulation are more common with skeletal muscle metastasis than with a primary sarcoma.10 In this case, the degree of surrounding postcontrast enhancement made simple muscle tear with hematoma unlikely, despite the presence of increased T1 signal. The lack of evidence for localized infection and the presence of a firm focal mass on physical examination made tumor more likely than infection.
Acrometastasis
Metastatic disease distal to the elbow and knee is very rare; specifically, metastatic disease of the hands or feet accounts for approximately 0.1% of all metastases.4 Carcinoma of the lung accounts for 44% to 47% of all acrometastasis.11,12 When hand acrometastasis is considered, the right hand accounts for 55% of bony cases, likely because of hand dominance, although approximately 10% of patients had bilateral acral metastatic disease.12 The underlying mechanism of acrometastasis remains unclear; however, some authors have postulated that it may result from an increase in vascularity or a trauma to the affected extremity.12,13 Flynn and colleagues12 reviewed the literature and reported a total of 257 cases of acral metastasis to the hand; they found that the median age at presentation was 58 years. Men were more than twice as likely to be affected when compared with women. Most commonly, the primary malignancies were in the lung (44%), kidney (12%), and breast (10%). The authors also reported less common cases of acral metastasis with primary malignancies located in the stomach, liver, rectum, prostate, and colon. Most commonly, these metastases were found in the distal phalynx, followed by the metacarpals, proximal phalynx, and middle phalynx.12
Soft-Tissue Metastasis
Skeletal muscle metastasis occurs in 0.8% to 17.5% of metastatic neoplasms.14-17 Studies in lung cancer patients have also revealed a low prevalence of muscular metastasis (0% to 0.8%).16 The rarity of muscular metastatic disease has been attributed to local inhibition of tumor survival secondary to muscle contraction, increased diffusing capacity of enzymes and immune cells, and extreme variability in blood flow and pH, lactate, and oxygen concentration. Skeletal muscular metastases most commonly arise from the lung, kidneys, colon, or melanoma.16 In a recent large series of more than 1400 patients imaged for soft-tissue masses, 2.5% were metastatic.18 There are only 2 reports of soft-tissue metastatic disease involving the hand: one from a patient with a thyroid carcinoma and the other from a patient with a lung adenocarcinoma.18 Soft-tissue metastatic disease from squamous cell carcinoma distal to the wrist has never been reported in the literature.
Acral Soft-Tissue Metastasis
A review from 2012 found 264 cases of skeletal muscle metastasis from 151 articles.6 Only 2 (0.75%) of these patients, as reported above, had a soft-tissue metastasis distal to the wrist.6,17
Conclusion
We report the first known case of a soft-tissue metastasis distal to the wrist from a primary bronchogenic squamous cell carcinoma. This report highlights the extremely uncommon presentation of soft-tissue acral metastatic disease of a bronchogenic squamous cell carcinoma of the lung. Although exceedingly rare, oncologists and physicians who manage pathology of the hand should consider metastatic disease when evaluating a patient with complaints of hand pain and a soft-tissue mass, especially in a patient with a known primary malignancy.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
1. American Cancer Society. Lung Cancer (Non-Small Cell). http://www.cancer.org/acs/groups/cid/documents/webcontent/003115-pdf.pdf. Revised April 30, 2014. Accessed July 22, 2014.
2. Willis RA. Pathology of Tumors. London, England: Butterworth; 1960.
3. Sugiura H, Yamada K, Sugiura T, Hida T, Mitsudomi T. Predictors of survival in patients with bone metastasis of lung cancer. Clin Orthop. 2008;466(3):729-736.
4. Kerin R. Metastatic tumors of the hand. A review of the literature. J Bone Joint Surg Am. 1983;65(9):1331-1335.
5. Alpar S. Muscle metastasis in a patient with squamous cell lung cancer. Turkish Respiratory Journal. 2002;3(2):75-78.
6. Haygood TM, Wong J, Lin JC, et al. Skeletal muscle metastases: a three-part study of a not-so-rare entity. Skeletal Radiol. 2012;41(8):899-909.
7. Tuoheti Y, Okada K, Osanai T, et al. Skeletal muscle metastases of carcinoma: a clinicopathological study of 12 cases. Jpn J Clin Oncol. 2004;34(4):210-214.
8. Chan NP, Yeo W, Ahuja AT, King AD. Multiple skeletal muscle metastases. Hong Kong Med J. 1999;5(4):410.
9. Molina-Garrido MJ, Guillen-Ponce C. Muscle metastasis of carcinoma. Clin Transl Oncol. 2011;13(2):98-101.
10. Williams JB, Youngberg RA, Bui-Mansfield LT, Pitcher JD. MR imaging of skeletal muscle metastases. AJR Am J Roentgenol. 1997;168(2):555-557.
11. Libson E, Bloom RA, Husband JE, Stoker DJ. Metastatic tumours of bones of the hand and foot. A comparative review and report of 43 additional cases. Skeletal Radiol. 1987;16(5):387-392.
12. Flynn CJ, Danjoux C, Wong J, et al. Two cases of acrometastasis to the hands and review of the literature. Curr Oncol. 2008;15(5):51-58.
13. Healey JH, Turnbull AD, Miedema B, Lane JM. Acrometastases. A study of twenty-nine patients with osseous involvement of the hands and feet. J Bone Joint Surg Am. 1986;68(5):743-746.
14. Sudo A, Ogihara Y, Shiokawa Y, Fujinami S, Sekiguchi S. Intramuscular metastasis of carcinoma. Clin Orthop. 1993(296):213-217.
15. Surov A, Hainz M, Holzhausen HJ, et al. Skeletal muscle metastases: primary tumours, prevalence, and radiological features. Eur Radiol. 2010;20(3):649-658.
16. Pearson CM. Incidence and type of pathologic alterations observed in muscle in a routine autopsy survey. Neurology. 1959;9:757-766.
17. Acinas Garcia O, Fernández FA, Satué EG, Beulta L, Val-Bernal JF. Metastasis of malignant neoplasms to skeletal muscle. Rev Esp Oncol. 1984;31(1):57-67.
18. Glockner JF, White LM, Sundaram M, McDonald DJ. Unsuspected metastases presenting as solitary soft tissue lesions: a fourteen-year review. Skeletal Radiol. 2000;29(5):270-274.
Anterior Hip Capsuloligamentous Reconstruction for Recurrent Instability After Hip Arthroscopy
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
Hip arthroscopy has experienced a dramatic increase in popularity, largely resulting from improvements in techniques and technology.1,2 As with any procedure, there are complications associated with arthroscopy of the hip. These include neurapraxia, iatrogenic cartilage and labral injuries, postoperative bleeding, perineal skin necrosis, infection, intra-articular instrument breakage, intra-abdominal fluid extravasation, avascular necrosis, and femoral neck fracture.1-4 Many of these have been attributed to the expected learning curve seen with any new procedure, and are less likely to occur as surgeons become more familiar with the procedure.1 One rare but serious complication is anterior dislocation of the hip.5-7
We present a patient who experienced an anterior hip dislocation and instability after hip arthroscopy, and was successfully treated with an anterior capsuloligamentous reconstruction. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An otherwise healthy 37-year-old woman presented to our clinic with a 6-month history of right groin pain and an occasional popping sensation during activity, which was unresponsive to hip-specific physical therapy. On physical examination, she was 5 ft 10 in tall, weighed 150 lbs, and appeared in excellent physical condition. She had no signs of systemic ligamentous laxity. She had an otherwise normal musculoskeletal, neurologic, and vascular examination in her bilateral lower extremities. She had a mild antalgic gait on the right leg.
The affected right hip could be flexed painfully to 120º, extended to 0º, adducted 20º, and abducted 45º. At 90º of flexion, her right hip could be externally rotated 30º and internally rotated 20º. Internal rotation during hip flexion beyond 90º caused sharp pain in the groin. Her normal left hip could be flexed to 120º, extended to 0º, adducted 30º, and abducted 60º. At 90º of flexion, her left hip could be externally rotated 50º and internally rotated 30º. She had negative Ober tests bilaterally but had tenderness along the right iliotibial band. She had negative Patrick and Gaenslen tests bilaterally. She had no tenderness in the area of either greater trochanter.
Imaging evaluation included plain radiographs and a magnetic resonance arthrogram (MRA) of the right hip. The plain radiographs showed signs of femoroacetabular impingement, but no joint space narrowing, no dysplasia, and no retroversion of the acetabulum (Figures 1A, 1B). The MRA showed a degenerative peripheral tear of the anterosuperior labrum without significant cartilage wear (Figure 2).
Based upon her findings on physical examination and imaging, we recommended arthroscopic treatment of her right hip pathology. Thirteen months after initial presentation, we performed a right hip arthroscopy with the patient in the supine position. Through modified anterior and anterolateral portals, we used electrocautery to perform a capsulotomy from the 9 o’clock to 12 o’clock positions. A central compartment diagnostic arthroscopy showed mild degenerative fraying of the labrum from the 9 o’clock to 12 o’clock positions without signs of detachment. There was grade III chondral fraying near the articular margin in that same arc. The femoral articular cartilage appeared normal, as did the ligamentum teres. We used a shaver to gently débride the torn labrum down to stable tissue. The frayed cartilage on the acetabulum was also gently débrided.
Traction was released and the hip was flexed. Minimal capsular release and débridement were performed for adequate visualization of the peripheral compartment. A diagnostic examination revealed a significant cam-type impingement lesion from the 12 o’clock to 6 o’clock positions. We performed a femoral neck resection, with a proximal-distal dimension of 15 mm and a depth of 7 mm. A dynamic fluoroscopic examination of the hip joint showed no signs of impingement. In accordance with our standard protocol, the anterior capsulotomy was not repaired.
Postoperatively, the patient was instructed to perform toe-touch weight-bearing with crutches for 2 weeks and to advance to full weight-bearing over the next 2 weeks. She did not use a hip orthosis. She was also advised to avoid combined hip extension/external rotation maneuvers for the first 4 weeks. She took part in a formal hip-specific physical therapy program for a total of 12 weeks. She was seen in clinic at 2, 6, and 12 weeks postoperatively and appeared to have had a typical, uneventful course. We advised her to gradually return to normal activities as tolerated at the 12-week visit.
Four months after the procedure, the patient returned to our clinic for evaluation after a right hip dislocation. Two days prior, she was at a school function with her child and experienced sudden pain and inability to bear weight after she extended and externally rotated her right hip in a low-energy manner. She was taken to an emergency room and found to have an anterior dislocation of the right hip (Figure 3), which was concentrically reduced under anesthesia.
Upon questioning, she reported having had feelings of mild instability of the right hip during demanding activities (jogging, yoga) after sustaining a low-energy fall 1 month prior to her dislocation. On examination, she had significant apprehension about the right hip during gentle external rotation maneuvers. An MRA 2 weeks after the dislocation showed a large defect of the anterosuperior capsuloligamentous complex measuring 4 cm from medial to lateral and 2.5 cm superior to inferior (Figure 4). No loose bodies, chondral injuries, or recurrent tears of the labrum were seen. Typical postoperative changes were observed at the femoral head-neck junction.
Initially, we recommended nonoperative management with 6 weeks of toe-touch weight-bearing and strict avoidance of hip extension–external rotation maneuvers. No hip orthosis was used. After this period, the patient advanced to full weight-bearing and continued in hip-specific physical therapy. Despite continued therapy and avoidance of provocative maneuvers, the patient reported persistent feelings of right hip instability with significant apprehension during extension and external rotation of the right hip. A repeat MRA 4 months after the hip dislocation showed a persistent defect in the anterosuperior capsuloligamentous complex and no signs of avascular necrosis. After 6 months of conservative treatment, we recommended an open capsulorrhaphy of the right hip with autograft iliotibial band reconstruction of the iliofemoral ligament and capsule.
Six months after the dislocation, the patient underwent the recommended procedure. After induction of general anesthesia, she was placed in the supine position on a standard operating table. A Smith-Petersen approach was used to visualize the anterior hip structures. During deep dissection, we observed a large defect, measuring 2.5×4 cm (Figure 5A), in the anterior hip capsule, with only a thin pseudocapsule covering the femoral head. Extensive mobilization of the anterior capsule was unsuccessful.
The decision was made to harvest a graft from the patient’s ipsilateral iliotibial band. A skin incision was made over the iliotibial band in the distal midthigh region, and a 2.5×4-cm graft was harvested from the central portion of the iliotibial band. An arthrotomy was performed on the hip joint (Figure 5B). The labrum appeared healthy without recurrent tearing or fraying, and other than focal thinning on the superior acetabulum, the cartilage appeared healthy. A double-loaded anchor was placed in the supra-acetabular region, and the sutures were passed through the graft. Then, No. 2 nonabsorbable sutures were sequentially placed between the capsular remnant and the graft medially, inferiorly, and laterally. The graft was placed into position (Figure 5C) and the sutures were tied (Figure 5D).
Postoperatively, the patient was allowed toe-touch weight-bearing for 6 weeks, with strict avoidance of extension–external rotation maneuvers. She participated in a 12-week course of physical therapy with gradual advancement of activities. About a year after the capsulorrhaphy, she was able to resume all previous activities with only occasional low-level discomfort. She returned to the clinic 16 months after the capsulorrhaphy complaining of increased pain with long-distance running but denied feelings of instability. We performed an intra-articular hip injection under ultrasound guidance, which provided 100% relief of her symptoms. We obtained an MRA to evaluate for any recurrent capsular or labral injury (Figure 6). The previous anterosuperior capsular defect was not visible, and no signs of recurrent labral or cartilage injury were seen.
Discussion
With the increasing popularity of hip arthroscopy, more complications are being reported as well, including postoperative hip instability. Three separate cases of anterior hip instability have been published in the past several years.5-7
Ranawat and colleagues5 were the first to report a case of postoperative anterior hip dislocation after arthroscopy. Their patient was a 52-year-old woman with right hip pain and generalized ligamentous laxity. Her preoperative radiographs showed no evidence of degenerative changes, dysplasia, or femoroacetabular impingement. An MRA showed a peripheral tear of the anterosuperior labrum. At arthroscopy, her right hip was easily distracted 2 to 3 cm with what they described as “minimal traction.” A small 1- to 2-cm capsulotomy was performed about the anterior portal. A detached labral tear was identified and repaired with an anchor, and no rim resection was performed. To improve visualization of the peripheral compartment, they extended the previous capsulotomy 1 to 2 cm and débrided the edges. A cam-type lesion was identified and resected. Lastly, they performed an anterior capsular plication, specifically including the iliofemoral ligament. Postoperatively, the patient wore a hip orthosis for 6 weeks to prevent extension and external rotation of the hip as well as a foot brace at night for 3 weeks. The patient was allowed to partially bear weight for the first 6 weeks with use of crutches. Approximately 2 months postoperatively, she slipped and fell down a short flight of stairs. She was diagnosed with an anterior hip dislocation. After successful closed reduction, she was treated conservatively with the same regimen used earlier. She remained symptomatic over the next several months with signs of instability and apprehension, and she eventually underwent a repeat hip arthroscopy. A 1- to 2-cm tear of the anterior capsule and iliofemoral ligament was treated with a revision arthroscopic capsular plication. A postoperative regimen similar to that used at the index procedure was instituted and, at most recent follow-up, she was found to have occasional pain without instability.
Matsuda6 reported a case of acute iatrogenic hip dislocation after arthroscopic surgery. His patient was a 39-year-old woman with a mildly retroverted acetabulum leading to impingement about the hip. She had no signs of generalized ligamentous laxity. A hip arthroscopy in the lateral position was performed, with no comment about the extent of the capsulotomy. During the procedure, about 5 mm of anterosuperior acetabulum were removed as part of arthroscopic rim trimming for treatment of the pincer lesion. A femoral osteochondroplasty was also performed (unspecified size) to restore more normal anterolateral offset. One confounding factor was that supranormal hip distraction was needed for 20 minutes to aid in removal of a metallic piece from a radiofrequency ablator, which inadvertently detached. The patient experienced an anterior hip dislocation in the recovery room and was found to be unstable during closed reduction under general anesthesia. A mini-open capsular repair was performed, which showed a 1×1.5-cm defect in the anterolateral capsule. After closure of the defect, the hip was found to be stable under fluoroscopic examination. Postoperatively, the patient was allowed to perform partial weight-bearing in a hip-knee-ankle-foot orthosis for 2 months and then a flexible hip brace for 1 month. At 15-month follow-up, her hip was stable and she was pain-free.
Benali and Katthagen7 highlighted the significant contribution of the labrum to hip stability in a dysplastic hip. Their patient was a 49-year-old woman with mild hip dysplasia and a degenerative bucket-handle tear of the ventrolateral labrum. The patient underwent a near-complete labral resection and rim trimming at an outside institution. The patient began full weight-bearing at 3 weeks postoperatively and noticed considerable groin and back pain (no hip orthosis use was mentioned). After failed treatment for suspected lumbar pathology, she was referred to the authors’ clinic for further evaluation. Plain radiographs showed subluxation of the left hip with degenerative changes. The patient had an uneventful left total hip arthroplasty (THA).
After reviewing the 3 reported cases of hip instability after arthroscopy, we suggest that surgeons fully recognize and appreciate the delicate balance of stability and motion provided by the static and dynamic stabilizers of the hip joint, and be cognizant of potential imbalance created by surgical intervention.8,9 Postarthroscopic hip instability appears to be multifactorial in nature, because all of the reported cases detailed different factors, both patient- and surgeon-related, contributing to instability.
Ranawat and colleagues5 identified several factors that may have contributed to the anterior hip dislocation sustained by their patient, including the patient’s generalized ligamentous laxity, performance of a capsulectomy (with repair of iliofemoral ligament), and a traumatic fall. Benali and Katthagen7 (although they did not perform the index procedure) described the disastrous complication of overzealous labral resection and rim trimming in a patient with hip dysplasia. Matsuda6 performed a labral resection and rim trimming, an extended (unspecified size) capsulotomy, and also used supranormal traction for 20 minutes to remove an iatrogenic foreign body. Surgeons performing hip arthroscopies should be aware of all these factors, because many are directly controlled by the surgeon.
The only factor we feel may have contributed to hip instability in our patient was the performance of a capsulotomy without closure. Our patient was an otherwise healthy woman with no signs of ligamentous laxity, hip dysplasia, or retroversion of the acetabulum. We did not perform a labral resection or rim trimming. We use modified anterior and anterolateral portals, and electrocautery to connect the portals. This typically leads to a release of a thin strip (less than 5 mm wide) of 3 cm of capsule. Based upon findings at rare second-look arthroscopy for recurrent symptoms, Dr. Guanche has observed that the capsulotomy from the initial procedure heals with normal-appearing tissue. Also, during peripheral compartment arthroscopy, we do not routinely release the iliofemoral ligament, and the orbicular ligament is left intact. Instead, we prefer to flex the hip and débride only enough capsular tissue to allow for adequate visualization.
Little has been published on capsulotomy closure after hip arthroscopy, and no consensus exists. Our standard practice is to not close the capsulotomy, which accords with the practice of other surgeons.9 There is concern, however, that extensive capsulotomy leading to injury or disruption of the iliofemoral ligament may cause anterior hip instability, driving other prominent hip arthroscopists to routinely close the capsulotomy.9,10 Myers and colleagues10 published a recent biomechanical study on the role of the labrum and the capsular ligaments in hip stability. They concluded that the iliofemoral ligament plays a significant role in limiting external rotation and anterior translation of the femoral head, and recommended closure of the capsulotomy after arthroscopy. Of note, Dr. Guanche has performed more than 1500 hip arthroscopic procedures in the past 5 years, and we are aware of only 2 patients who have sustained anterior hip dislocations, in spite of our not closing the capsulotomy defect. This highlights an important clinical question in need of further investigation.
Our case also raises questions about the ideal postoperative regimen after standard hip arthroscopy. Although we do not routinely prescribe hip orthoses for our patients, others do.5 We are unaware of any proven benefit to the standard use of hip orthoses, and are concerned over the possible lack of patient compliance and of adequate restraint. We prefer to educate our patients on avoiding the “at-risk” position of hip extension and external rotation and to counsel them on gradual return to activities. Studies are needed to determine the role of these devices in hip arthroscopy, as well as the ideal postoperative activity regimen.
Our patient failed 6 months of conservative treatment after her dislocation and continued to have feelings of hip instability even during light activities. As a result of this failure and given an anatomical defect in the anterior capsuloligamentous complex, we decided our patient would be best treated with reconstruction of the defect. We did not think a revision capsular plication, as done by Ranawat and colleagues,5 was a reasonable option for our patient because of a large defect in the capsular tissue. Even in smaller defects, plication could potentially lead to overtightening of the capsule and subsequent overconstraint of the joint. Also, plication of defects may place excessive strain on the suture, which may fail if the repair is even mildly stressed.
Recurrent anterior hip dislocations, although rare in their own right, are much more common after THA than after hip arthroscopy.11 Fujishiro and colleagues12 described a similar technique to ours developed to treat a patient with recurrent anterior hip instability from anterior capsular insufficiency after multiple revision THA procedures. They used a Leeds-Keio artificial ligament to reconstruct the iliofemoral ligament, and this successfully treated their patient’s instability.
Conclusion
We believe this is the first report of recurrent instability after hip arthroscopy, necessitating reconstruction of the anterior capsuloligamentous complex. This case shows that reconstruction of the iliofemoral ligament with iliotibial band autograft is safe, restores hip stability without compromising function, and should be considered by any hip arthroscopist encountering a similar scenario. It also highlights the importance of the capsuloligamentous complex surrounding the hip joint for its stability and the need for further research to better delineate the indications for capsular repair/closure after capsulotomy.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
1. Ilizaliturri VM Jr. Complications of arthroscopic femoroacetabular impingement treatment: a review. Clin Orthop. 2009;467(3):760-768.
2. Clarke MT, Villar RN. Hip arthroscopy: complications in 1054 cases. Clin Orthop. 2003;406:84-88.
3. Smart LR, Oetgen M, Noonan B, Medvecky M. Beginning hip arthroscopy: indications, positioning, portals, basic techniques, and complications. Arthroscopy. 2007;23(12):1348-1353.
4. Sampson TG. Complications of hip arthroscopy. Tech Orthop. 2005;20:63-66.
5. Ranawat AS, McClincy M, Sekiya JK. Anterior dislocation of the hip after arthroscopy in a patient with capsular laxity of the hip. A case report. J Bone Joint Surg Am. 2009;91(1):192-197.
6. Matsuda DK. Acute iatrogenic dislocation following hip impingement arthroscopic surgery. Arthroscopy. 2009;25(4):400-404.
7. Benali Y, Katthagen BD. Hip subluxation as a complication of arthroscopic debridement. Arthroscopy. 2009;25(4):405-407.
8. Shindle MK, Voos JE, Nho SJ, Heyworth BE, Kelly BT. Arthroscopic management of labral tears in the hip. J Bone Joint Surg Am. 2008;90(suppl 4):2-19.
9. Bedi A, Galano G, Walsh C, Kelly BT. Capsular management during hip arthroscopy: from femoroacetabular impingement to instability. Arthroscopy. 2011;27(12):1720-1731.
10. Myers CA, Register BC, Lertwanich P, et al. Role of the acetabular labrum and the iliofemoral ligament in hip stability: an in vitro biplane fluoroscopy study. Am J Sports Med. 2011;39(suppl):85S-91S.
11. Sariali E, Leonard P, Mamoudy P. Dislocation after total hip arthroplasty using Hueter anterior approach. J Arthroplasty. 2008;23(2):266-272.
12. Fujishiro T, Nishikawa T, Takikawa S, Saegusa Y, Yoshiya S, Kurosaka M. Reconstruction of the iliofemoral ligament with an artificial ligament for recurrent anterior dislocation of total hip arthroplasty. J Arthroplasty. 2003;18(4):524-527.
Bilateral hand cramping and weakness • broad fingers • coarse facial features • Dx?
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
THE CASE
A 37-year-old right-hand dominant woman came to our clinic seeking treatment for bilateral generalized hand cramping and weakness that she had been experiencing for approximately 2 to 3 years. She was dropping objects and had finger locking, yet had no numbness, tingling, or morning stiffness.
Ten months earlier, she had given birth to a healthy 3715 g girl. Our patient’s prenatal glucose tolerance test had been normal. Her pregnancy and delivery had been significant for oligohydramnios, failed post-term (41 weeks 4 days) induction, and emergent low transverse cesarean section due to fetal bradycardia. Since giving birth, our patient had 3 menstrual periods while breastfeeding. She had a copper intrauterine device inserted at her 6-week postpartum visit. She also had 2 truncal acrochordons removed 3 months postpartum. She had no history of neck trauma, overuse injury, or occupational exposures.
Her blood pressure and vital signs were within normal limits. Physical exam was notable for subtly coarse facial features and broad fingers (FIGURE 1).
She had normal wrist and hand joint range of motion; her wrist and hand strengths, including grip strength, were 5 out of 5. Tinel’s sign, Phalen’s maneuver, and Finkelstein’s test were negative.
Her upper extremity neurovascular exams were completely normal. Initial laboratory studies—including a comprehensive metabolic panel—were normal. The only exception was her creatine kinase, which was 265 U/L (normal, 24-195 U/L).
At a follow-up appointment 7 weeks later, we gathered a more detailed history and learned that over the past 2 to 3 years, the patient had noticed that her shoe and ring sizes had been increasing. She also mentioned some mild weight gain following her pregnancy.
Occasionally, she had generalized hand swelling, headaches, and saw floaters, but she denied losing peripheral vision. Additional lab work at this time revealed a fasting growth hormone (GH) level of 27.3 ng/mL (normal, 0.05-8 ng/mL) and an insulin-like growth factor 1 (IGF-1) level of 848 ng/mL (normal, 106-368 ng/mL). An anterior pituitary hormone panel and cortisol level were normal. A urine pregnancy test was negative.
THE DIAGNOSIS
Magnetic resonance imaging (MRI) of our patient’s brain revealed a pituitary adenoma (FIGURE 2). Based on that and the patient’s elevated GH and IGF-1 levels, we diagnosed acromegaly due to a pituitary adenoma.
DISCUSSION
Acromegaly is a rare, progressively disfiguring disease with a prevalence of 40 cases per million people.1 It affects middle-aged adults, with no gender difference.2 In most cases, the cause is a benign pituitary adenoma.1-4
Physical changes include coarse facial features, generalized expansion of the skull, brow protrusion, ocular distension, prognathism, macroglossia, acral overgrowth, and dental malocclusion; these changes typically occur slowly over a long time period.1-5 For example, when we looked at the 3-year-old photo on our patient’s driver’s license, we noticed only subtle changes from her current appearance. Common clinical manifestations include headache, hyperpigmentation, hypertrichosis, hyperhidrosis, goiter, arthropathy, carpal tunnel syndrome, visual disturbances, and acrochordons.1,5
Acromegaly is associated with an increased risk of cardiovascular disease, metabolic disorders, infertility, sleep apnea, arthritis, thyroid tumors, colon adenomas, and carcinoma.1,2,4,5 Due to the insidious progression of acromegaly’s clinical manifestations, diagnosis is delayed for 4 to 10 years, on average.1 The diagnosis of acromegaly is typically based on an elevation of GH and IGF-1 levels.1,5 A brain MRI is essential in the diagnosis of a pituitary adenoma.1
Pregnancy among patients with acromegaly is uncommon. In fact, fewer than 150 cases have been reported in the literature.2,6 In most cases, it appears that pregnancy among patients with acromegaly is safe for mothers and newborns.6,7
The goals of treatment for acromegaly caused by a pituitary adenoma are to remove/ reduce the tumor and its mechanical effects, relieve symptoms, reduce serum GH and IGF-1, and restore pituitary function. Transsphenoidal surgical resection is the preferred treatment for pituitary adenomas.1,2,4 Radiation therapy and pharmacologic treatment may be necessary as adjuncts to surgery or for patients for whom surgery is contraindicated.1,4,5
Pharmacologic management of acromegaly includes dopamine agonists (cabergoline), somatostatin analogues (octreotide, lanreotide), and GH receptor antagonists (pegvisomant).1,3 Patients who receive effective early treatment of acromegaly have a life expectancy similar to that of the general population.1,5
Our patient
Our patient was referred to Neurosurgery and underwent transnasal transsphenoidal resection of the pituitary adenoma. Two weeks postop, her GH level had decreased to 0.66 ng/mL and her IGF-1 level was down to 386 ng/mL. Four months later, her GH (2.32 ng/mL) and IGF-1 levels (277 ng/mL) were within normal range and our patient reported improvement in all of her symptoms.
THE TAKEAWAY
Because it may take years for the classical clinical features of acromegaly such as coarse facial features, protruding jaw, and broad fingers to become apparent, diligent history taking is essential to diagnose the condition early. Patients may present with nonspecific and confusing symptoms such as muscle weakness.8 Early nonspecific symptoms and signs in the presence of normal basic laboratory tests should warrant an evaluation of fasting GH and IGF-1. Early treatment with surgery, radiation therapy, or pharmacotherapy may prevent or decrease the intensity of rheumatologic, cardiovascular, respiratory, and metabolic complications of acromegaly.1
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.
1. Scacchi M, Cavagnini F. Acromegaly. Pituitary. 2006;9: 297-303.
2. Hossain B, Drake WM. Acromegaly. Medicine. 2009;37: 407-410.
3. Chan MR, Ziebert M, Maas DL, et al. “My rings won’t fit anymore”. Ectopic growth hormone-secreting tumor. Am Fam Physician. 2005;71:1766-1767.
4. Lake MG, Krook LS, Cruz SV. Pituitary adenomas: an overview. Am Fam Physician. 2013;88:319-327.
5. Vilar L, Valenzuela A, Ribeiro-Oliveira A Jr, et al. Multiple facets in the control of acromegaly. Pituitary. 2014;17 suppl 1:S11-S17.
6. Cheng V, Faiman C, Kennedy L, et al. Pregnancy and acromegaly: a review. Pituitary. 2012;15:59-63.
7. Caron P, Broussaud S, Bertherat J, et al. Acromegaly and pregnancy: a retrospective multicenter study of 59 pregnancies in 46 women. J Clin Endocrinol Metab. 2010;95:4680-4687.
8. Saguil A. Evaluation of the patient with muscle weakness. Am Fam Physician. 2005;71:1327-1336.