User login
Unusual Presentation of Ectopic Extramammary Paget Disease
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
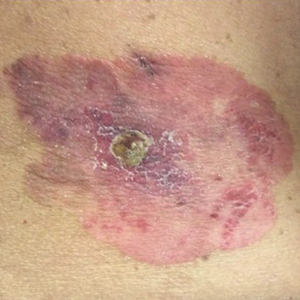
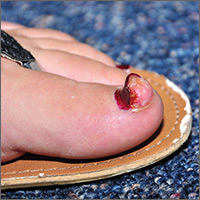
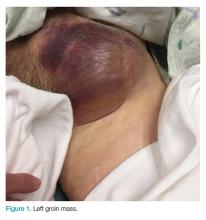
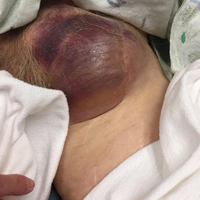
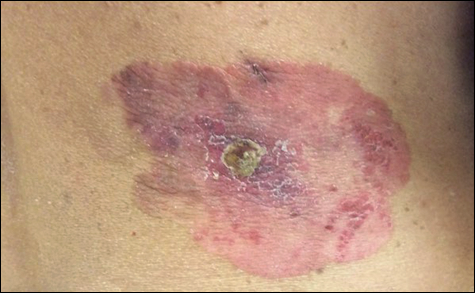
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.
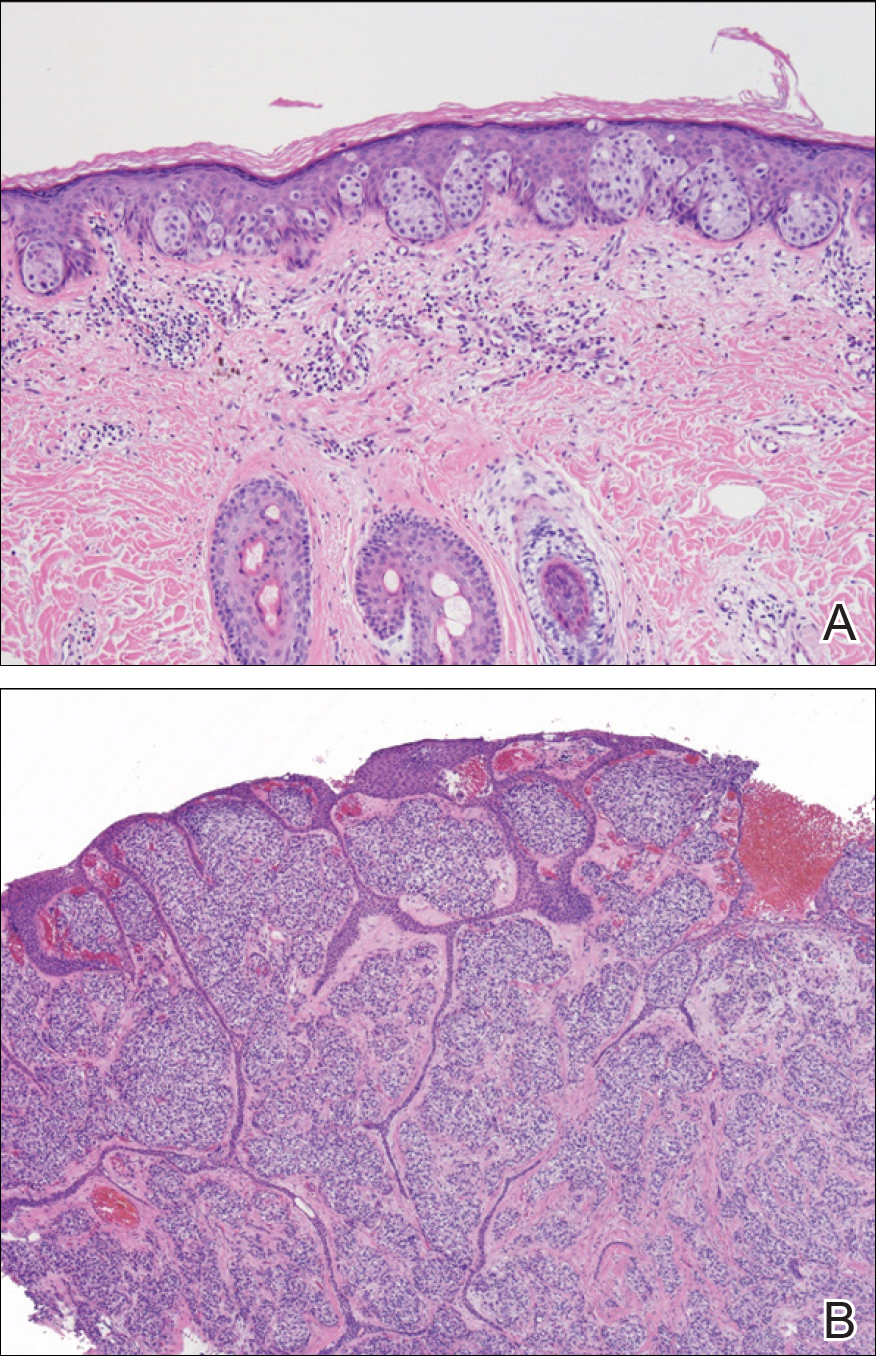
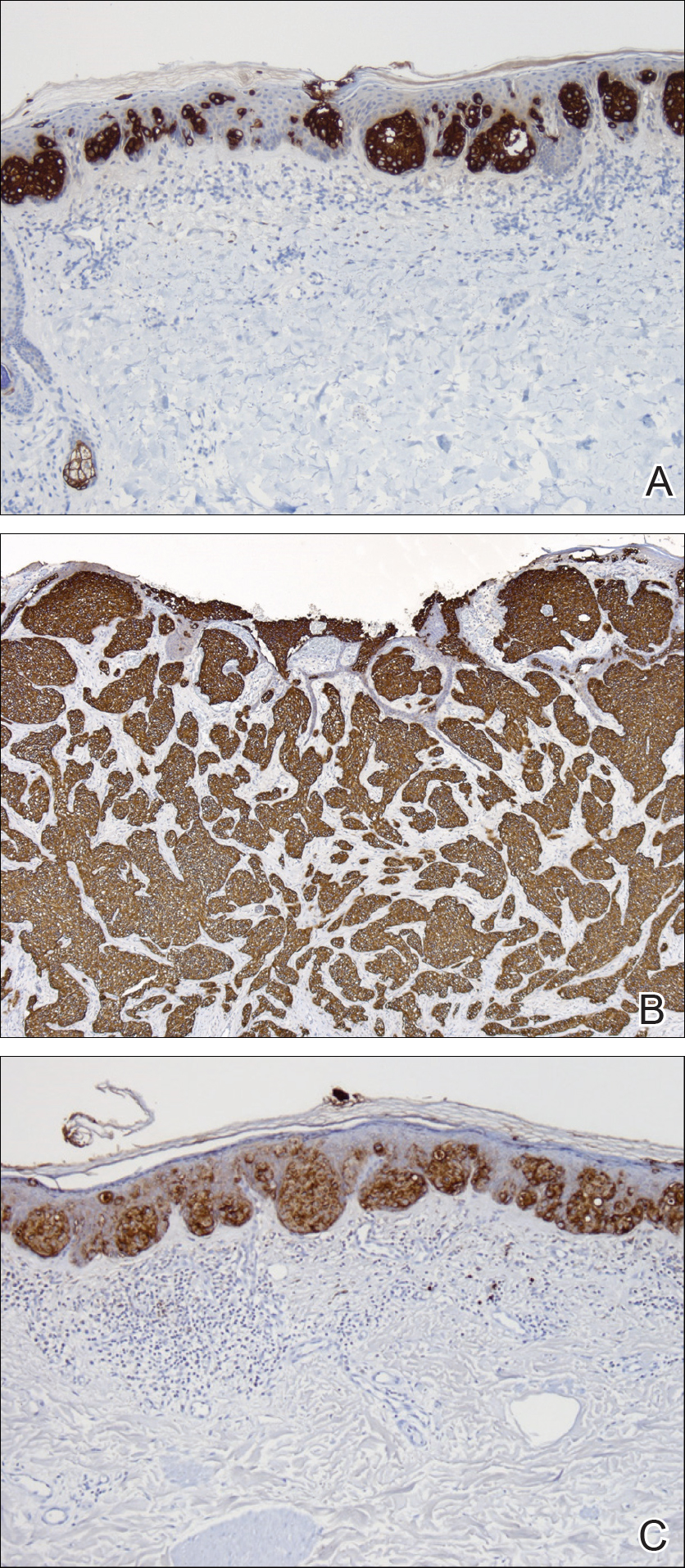
Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.
Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.

Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.


Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
Extramammary Paget disease (EMPD) is a malignant epithelial tumor that most commonly affects the anogenital region and less frequently arises in the axillae. Most cases occur in locations where apocrine glands predominate.1 Few cases of EMPD arising in nonapocrine-bearing regions, or ectopic EMPD, have been reported.2 We describe a case of primary ectopic EMPD with an infiltrative growth pattern arising on the back of a 67-year-old Thai man.
Case Report
A 67-year-old Thai man presented to the dermatology clinic for evaluation of a persistent rash on the right lower back of approximately 30 years’ duration. He reported that the eruption had started out as a small coin-shaped area but had slowly increased in size. Over the last 2 years, the area had grown more rapidly and became pruritic. His medical history was remarkable for hypertension treated with losartan, but he was otherwise healthy. He had no history of cancer or gastrointestinal tract or genitourinary symptoms, and he had no recent fever, weight loss, or night sweats.
On physical examination a well-demarcated, asymmetric, erythematous to brown plaque was noted on the right lower back. The plaque was surfaced by scale and contained a central hyperkeratotic papule (Figure 1). The skin examination was otherwise unremarkable. The patient had no lymphadenopathy.

Two punch biopsies were performed. On low power, acanthosis and hyperkeratosis of the epidermis were noted. The epidermis contained a proliferation of large (tumor) cells with pleomorphic nuclei, prominent nucleoli, and abundant pale to clear cytoplasm. The cells were present singularly as well as in clusters and were most prominent along the basal layer but many were also seen extending to more superficial levels of the epidermis (Figure 2A). In one biopsy, the tumor cells were found in the dermis with an infiltrative growth pattern (Figure 2B). Immunohistochemistry (IHC) studies for cytokeratin 7 (Figure 3A and 3B) and carcinoembryonic antigen (Figure 3C) labeled the tumor cells. An IHC study for gross cystic disease fluid protein 15 labeled some of the tumor cells. Immunohistochemistry studies for S-100, human melanoma black 45 (HMB-45), p16, and renal cell carcinoma did not label the tumor cells. An IHC study for MIB-1 labeled many of the tumor cells, indicating a notably increased mitotic index. The patient was diagnosed with ectopic EMPD. He underwent an endoscopy, colonoscopy, and cystoscopy, all of which were normal.


Comment
Extramammary Paget disease is a malignant tumor typically found in apocrine-rich areas of the skin, particularly the anogenital skin. It is categorized as primary or secondary EMPD. Primary EMPD arises as an intraepithelial adenocarcinoma, with the Toker cell as the cell of origin.3 Secondary EMPD represents a cutaneous extension of an underlying malignancy (eg, colorectal, urothelial, prostatic, gynecologic).4
Ectopic EMPD arises in nonapocrine-bearing areas, specifically the nongerminative milk line. A review of the literature using Google Scholar and the search term ectopic extramammary Paget disease showed that there have been at least 30 cases of ectopic EMPD reported. Older men are more commonly affected, with a mean age at diagnosis of approximately 68 years. Although the tumor is most commonly seen on the trunk, cases on the head, arms, and legs have been reported.5
This tumor is most frequently seen in Asian individuals, as in our patient, with a ratio of approximately 3:1.5 Interestingly, triple or quadruple EMPD was reported in 68 Japanese patients but rarely has been reported outside of Japan.6 It is thought that some germinative apocrine-differentiating cells might preferentially exist on the trunk of Asians, leading to an increased incidence of EMPD in this population5; however, the exact reason for this racial preference is not completely understood, and more studies are needed to investigate this association.
Diagnosis of ectopic EMPD is made histologically. Tumor cells have abundant pale cytoplasm and large pleomorphic nuclei with prominent nucleoli. The cells are arranged in small groups or singly within the basal regions of the epidermis. In longstanding lesions, the entire thickness of the epidermis may be involved. Uncommonly, the tumor cells may invade the dermis, such as in our patient. On immunohistochemistry, the tumor cells stain positive for carcinoembryonic antigen, epithelial membrane antigen, and low-molecular-weight cytokeratins (eg, cytokeratin 7). Many of the tumor cells also express gross cystic disease fluid protein 15, which helps exclude cutaneous invasion of secondary EMPD.7-9 Cases of primary cutaneous apocrine carcinoma can have similar histologic and immunohistochemical findings to invasive EMPD, which further supports the possible apocrine derivation of Paget disease. In our patient, we considered the diagnosis of primary cutaneous apocrine adenocarcinoma with epidermotropism; however, we favored the diagnosis of ectopic EMPD with dermal invasion given the extensive epidermal-only involvement seen in one of the biopsies, which would be unusual for primary cutaneous apocrine adenocarcinoma.
Our patient had no identified underlying malignancy upon further workup; however, many cases of EMPD have been associated with an underlying malignancy.9-15 Several authors have reported a range of underlying malignancies associated with EMPD, with the incidence ranging from 11% to 45%.9-15 The location of the underlying internal malignancy appears to be closely related to the location of the EMPD.11 It is recommended that a thorough workup for internal malignancies be performed, including a full skin examination, lymph node examination, colonoscopy, cystoscopy, and gynecologic/prostate examination, among others.
No known differences in the prognosis or associated underlying malignancies between ectopic and ordinary EMPD have been reported; however, it has been noted that EMPD with invasion into the dermis does correlate with a more aggressive course and worse prognosis.8 Treatment includes surgical removal by Mohs micrographic surgery or wide local excision. Long-term follow-up is required since recurrences can be frequent.11-15
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
- Mazoujian G, Pinkus GS, Haagensen DE Jr. Extramammary Paget’s disease—evidence for an apocrine origin: an immunoperoxidase study of gross cystic disease fluid protein-15, carcinoembryonic antigen, and keratin proteins. Am J Surg Pathol. 1984;8:43-50.
- Saida T, Iwata M. “Ectopic” extramammary Paget’s disease affecting the lower anterior aspect of the chest. J Am Acad Dermatol. 1987;17(5, pt 2):910-913.
- Willman JH, Golitz LE, Fitzpatrick JE. Vulvar clear cells of Toker: precursors of extramammary Paget’s disease. Am J Dermatopathol. 2005;27:185-188.
- Lloyd J, Flanagan AM. Mammary and extramammary Paget’s disease.J Clin Pathol. 2000;53:742-749.
- Sawada Y, Bito T, Kabashima R, et al. Ectopic extramammary Paget’s disease: case report and literature review. Acta Derm Venereol. 2010;90:502-505.
- Abe S, Kabashima K, Nishio D, et al. Quadruple extramammary Paget’s disease. Acta Derm Venereol. 2007;87:80-81.
- Kanitakis J. Mammary and extramammary Paget’s disease. J Eur Acad Dermatol Venereol. 2007;21:581-590.
- Goldblum JR, Hart WR. Vulvar Paget’s disease: a clinicopathologic and immunohistochemical study of 19 cases. Am J Surg Pathol. 1997;21:1178-1187.
- Goldblum JR, Hart WR. Perianal Paget’s disease: a histologic and immunohistochemical study of 11 cases with and without associated rectal adenocarcinoma. Am J Surg Pathol. 1998;22:170-179.
- Shepherd V, Davidson EJ, Davies‐Humphreys J. Extramammary Paget’s disease. BJOG. 2005;112:273-279.
- Chanda JJ. Extramammary Paget’s disease: prognosis and relationship to internal malignancy. J Am Acad Dermatol. 1985;13:1009-1014.
- Besa P, Rich TA, Delclos L, et al. Extramammary Paget’s disease of the perineal skin: role of radiotherapy. Int J Radiat Oncol Biol Phys. 1992;24:73-78.
- Fanning J, Lambert HC, Hale TM, et al. Paget’s disease of the vulva: prevalence of associated vulvar adenocarcinoma, invasive Paget’s disease, and recurrence after surgical excision. Am J Obstet Gynecol. 1999;180:24-27.
- Parker LP, Parker JR, Bodurka-Bevers D, et al. Paget’s disease of the vulva: pathology, pattern of involvement, and prognosis. Gynecol Oncol. 2000;77:183-189.
- Marchesa P, Fazio VW, Oliart S, et al. Long-term outcome of patients with perianal Paget’s disease. Ann Surg Oncol. 1997;4:475-480.
Practice Points
- Ectopic extramammary Paget disease (EMPD) is a rare presentation of EMPD that is histologically identical to EMPD.
- Ectopic EMPD can be associated with underlying malignancy and therefore warrants a thorough workup.
Electrocardiography: Flecainide Toxicity
Case
An 86-year-old woman, who recently had been seen in the same facility after a ground level fall, presented to the ED with to a 2- to 3-day history of vague abdominal pain, increasing weakness, nausea, and dry heaves.
Upon examination, the patient was unable to stand due to generalized weakness She arrived at the ED via emergency medical services. Her vital signs at presentation were significant for a systolic blood pressure (BP) of 90 mm Hg with a wide complex tachycardia concerning for ventricular tachycardia. The patient’s other vital signers were: heart rate, 136 beats/min; respiratory rate 20 breaths/min; and pulse oximetry was 94% on 4 liters/min of oxygen via nasal cannula.
The patient’s medical history was significant for atrial fibrillation and an indwelling pacemaker, for which she was chronically on flecai
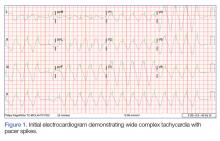
The initial electrocardiogram (ECG) revealed a wide complex rhythm with pacemaker spikes (Figure 1). Based on these findings, electrodes were placed on the patient in the event she required cardioversion. The patient was started on an amiodarone intravenous (IV) drip for presumptive ventricular tachycardia.
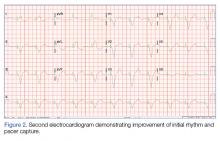
During the patient’s evaluation in the ED, she experienced transient drops in BP, which were responsive to an IV fluid bolus of normal saline, and the amiodarone drip was discontinued. The patient’s ECG findings were compared to previous ECG studies, as was her current medication list and prior health issues. After ruling-out other causes, flecainide toxicity was considered high in the differential, and she was given 1 ampule of bicarbonate IV, after which a second ECG showed heart rhythm converted from a wide-complex tachycardia to a paced rhythm, markedly improved from the initial ECG (Figure 2). Similarly, there was a marked improvement in BP.
An interrogation of the patient’s pacemaker revealed an atrial flutter with a rate below detection for mode switch, with one-to-one tracking/pacing. The pacemaker was reprogrammed to divide the DDIR mode with detection rate at 120 mm Hg with mode switch activated. This was felt to be consistent with flecainide toxicity precipitating the cardiac conduction issues.
Laboratory studies showed an elevated flecainide level at 1.39 mcg/mL (upper limits of normal of 1 mcg/mL). Other studies showed worsening congestive heart failure, with a brain natriuretic peptide of 8,057 pg/mL and mild dehydration, with serum creatinine increased from her baseline of 0.9 to 1.38 mg/dL.
The patient’s abdominal pain was further evaluated and she was found to have acute cholecystitis. She was admitted to the intensive care unit with cardiology and general surgery consulting.
Discussion
Flecainide acetate was approved by the Food and Drug Administration in 1984.1It is a Vaughan-Williams class IC antiarrhythmic with a sodium channel blocker action used to treat supra ventricular arrhythmias. The CAST trial in 1989 investigated the efficacy of this class of antiarrhythmics, which resulted in a revision of its role.2 Based on this study, flecainide is not recommended for patients with structural heart disease or coronary artery disease.2,3 However, it is recommended as a first-line therapy for pharmacologic cardioversion and maintenance of normal sinus rhythm in patients with atrial fibrillation and supraventricular tachycardia4,5 without the above caveats.
Class IC agents produce a selective block at the sodium (Na+) channels, resulting in the slowing of cardiac conduction.6,7 This high affinity for Na+ channels combined with slow unbinding kinetics during diastole explain the slowing of recovery time and prolongation of the refractory period.6,8,9 These electrophysiologic properties all can increase the PR, QRS, and QT interval duration. The QT interval is not significantly affected, as most of the QT prolongation is due to the QRS widening.6,10,11 Widening of the QRS by greater than 25% as compared to the baseline value is used as the threshold to decrease dosing or discontinue the use of flecainide.3The toxic effects of flecainide on cardiac conduction can produce prolonged QRS duration of up to 50%, and PR interval up to 30%, especially in rapid heart rates. Signs of intoxication are difficult to discern owing to its nonspecific presentation. A well-documented, but under-recognized, presentation of flecainide toxicity is the transformation of atrial fibrillation to atrial flutter.5,7,9,11-13 The reported rate of this pro arrhythmic effect can be as high as 3.5% to 5%.14,15Flecainide toxicity can occur secondary to chronic ingestion and may be precipitated in mild renal failure. The majority of flecainide is renally excreted and the half-life is 20 hours. Maximum therapeutic effect is seen between levels of 0.2 to 1 mcg/mL with levels greater than 0.7 to 1 mcg/mL associated with adverse effects.9 Systemic effects include dizziness and visual disturbances. A high degree of suspicion for flecainide toxicity is required when the patient’s initial presentation is nonspecific. In this circumstance, real-time bedside interrogation of the pacemaker is invaluable. Early diagnosis and treatment minimizes the risk for adverse sequelae, including death. Treatment includes increasing the excretion of flecainide, symptomatic support (including pacemaker placement, intravenous fat emulsion, or extracorporeal circulatory support) and administration of sodium bicarbonate, to transiently reverse the effect of the sodium channel blockade, in severe cases.15-17
1. Hudak JM, Banitt EH, Schmid JR. Discovery and development of flecainide. Am J Cardiol. 1984;53(5):17B-20B.
2. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST). N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629.
3. Andrikopoulos GK, Pastromas S, Tzeis S. Flecainide: Current status and perspectives in arrhythmia management. World J Cardiol. 2015;7(2):76-85. doi:10.4330/wjc.v7.i2.76.
4. Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747. doi:10.1093/eurheartj/ehs253.
5. Courand PY, Sibellas F, Ranc S, Mullier A, Kirkorian G, Bonnefoy E. Arrhythmogenic effect of flecainide toxicity. Cardiol J. 2013;20:203-205. doi:10.5603/CJ.2013.0035.
6. Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985;29(1):1-33.
7. Taylor R, Gandhi MM, Lloyd G. Tachycardia due to atrial flutter with rapid 1:1 conduction following treatment of atrial fibrillation with flecainide. Br Med J. 2010;340:b4684.
8. Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315(1):36-41.
9. Levis JT. ECG diagnosis: flecainide toxicity. Perm J. 2012;16(4):53.
10. Hellestrand KJ, Bexton RS, Nathan AW, Spurrell RA, Camm AJ. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48(2):140-148.
11. Rognoni A, Bertolazzi M, Peron M, et al. Electrocardiographic changes in a rare case of flecainide poisoning: a case report. Cases J. 2009;2:9137. doi:10.1186/1757-1626-2-9137.
12. Nabar A, Rodriguez LM, Timmermans C, Smeets JL, Wellens HJ. Radiofrequency ablation of “class IC atrial flutter” in patients with resistant atrial fibrillation. Am J Cardiol. 1999;83(5):785-787, A10.
13. Kola S, Mahata I, Kocheril AG. A case of flecainide toxicity. EP Lab Digest. 2015;15(5).
14. Falk RH. Proarrhythmia in patients treated for atrial fibrillation or flutter. Ann Intern Med. 1992;117(2):141-150.
15. Lloyd T, Zimmerman J, Griffin GD. Irreversible third-degree heart block and pacemaker implant in a case of flecainide toxicity. Am J Emerg Med. 2013;31(9):1418.e1-e2. doi:10.1016/j.ajem.2013.04.025.
16. Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27(4):405-408.
17. Ellsworth H, Stellpflug SJ, Cole JB, Dolan JA, Harris CR. A life-threatening flecainide overdose treated with intravenous fat emulsion. Pacing Clin Electrophysiol. 2013;36(3):e87-e89. doi:10.1111/j.1540-8159.2012.03485.x.
Case
An 86-year-old woman, who recently had been seen in the same facility after a ground level fall, presented to the ED with to a 2- to 3-day history of vague abdominal pain, increasing weakness, nausea, and dry heaves.
Upon examination, the patient was unable to stand due to generalized weakness She arrived at the ED via emergency medical services. Her vital signs at presentation were significant for a systolic blood pressure (BP) of 90 mm Hg with a wide complex tachycardia concerning for ventricular tachycardia. The patient’s other vital signers were: heart rate, 136 beats/min; respiratory rate 20 breaths/min; and pulse oximetry was 94% on 4 liters/min of oxygen via nasal cannula.
The patient’s medical history was significant for atrial fibrillation and an indwelling pacemaker, for which she was chronically on flecai
The initial electrocardiogram (ECG) revealed a wide complex rhythm with pacemaker spikes (Figure 1). Based on these findings, electrodes were placed on the patient in the event she required cardioversion. The patient was started on an amiodarone intravenous (IV) drip for presumptive ventricular tachycardia.
During the patient’s evaluation in the ED, she experienced transient drops in BP, which were responsive to an IV fluid bolus of normal saline, and the amiodarone drip was discontinued. The patient’s ECG findings were compared to previous ECG studies, as was her current medication list and prior health issues. After ruling-out other causes, flecainide toxicity was considered high in the differential, and she was given 1 ampule of bicarbonate IV, after which a second ECG showed heart rhythm converted from a wide-complex tachycardia to a paced rhythm, markedly improved from the initial ECG (Figure 2). Similarly, there was a marked improvement in BP.
An interrogation of the patient’s pacemaker revealed an atrial flutter with a rate below detection for mode switch, with one-to-one tracking/pacing. The pacemaker was reprogrammed to divide the DDIR mode with detection rate at 120 mm Hg with mode switch activated. This was felt to be consistent with flecainide toxicity precipitating the cardiac conduction issues.
Laboratory studies showed an elevated flecainide level at 1.39 mcg/mL (upper limits of normal of 1 mcg/mL). Other studies showed worsening congestive heart failure, with a brain natriuretic peptide of 8,057 pg/mL and mild dehydration, with serum creatinine increased from her baseline of 0.9 to 1.38 mg/dL.
The patient’s abdominal pain was further evaluated and she was found to have acute cholecystitis. She was admitted to the intensive care unit with cardiology and general surgery consulting.
Discussion
Flecainide acetate was approved by the Food and Drug Administration in 1984.1It is a Vaughan-Williams class IC antiarrhythmic with a sodium channel blocker action used to treat supra ventricular arrhythmias. The CAST trial in 1989 investigated the efficacy of this class of antiarrhythmics, which resulted in a revision of its role.2 Based on this study, flecainide is not recommended for patients with structural heart disease or coronary artery disease.2,3 However, it is recommended as a first-line therapy for pharmacologic cardioversion and maintenance of normal sinus rhythm in patients with atrial fibrillation and supraventricular tachycardia4,5 without the above caveats.
Class IC agents produce a selective block at the sodium (Na+) channels, resulting in the slowing of cardiac conduction.6,7 This high affinity for Na+ channels combined with slow unbinding kinetics during diastole explain the slowing of recovery time and prolongation of the refractory period.6,8,9 These electrophysiologic properties all can increase the PR, QRS, and QT interval duration. The QT interval is not significantly affected, as most of the QT prolongation is due to the QRS widening.6,10,11 Widening of the QRS by greater than 25% as compared to the baseline value is used as the threshold to decrease dosing or discontinue the use of flecainide.3The toxic effects of flecainide on cardiac conduction can produce prolonged QRS duration of up to 50%, and PR interval up to 30%, especially in rapid heart rates. Signs of intoxication are difficult to discern owing to its nonspecific presentation. A well-documented, but under-recognized, presentation of flecainide toxicity is the transformation of atrial fibrillation to atrial flutter.5,7,9,11-13 The reported rate of this pro arrhythmic effect can be as high as 3.5% to 5%.14,15Flecainide toxicity can occur secondary to chronic ingestion and may be precipitated in mild renal failure. The majority of flecainide is renally excreted and the half-life is 20 hours. Maximum therapeutic effect is seen between levels of 0.2 to 1 mcg/mL with levels greater than 0.7 to 1 mcg/mL associated with adverse effects.9 Systemic effects include dizziness and visual disturbances. A high degree of suspicion for flecainide toxicity is required when the patient’s initial presentation is nonspecific. In this circumstance, real-time bedside interrogation of the pacemaker is invaluable. Early diagnosis and treatment minimizes the risk for adverse sequelae, including death. Treatment includes increasing the excretion of flecainide, symptomatic support (including pacemaker placement, intravenous fat emulsion, or extracorporeal circulatory support) and administration of sodium bicarbonate, to transiently reverse the effect of the sodium channel blockade, in severe cases.15-17
Case
An 86-year-old woman, who recently had been seen in the same facility after a ground level fall, presented to the ED with to a 2- to 3-day history of vague abdominal pain, increasing weakness, nausea, and dry heaves.
Upon examination, the patient was unable to stand due to generalized weakness She arrived at the ED via emergency medical services. Her vital signs at presentation were significant for a systolic blood pressure (BP) of 90 mm Hg with a wide complex tachycardia concerning for ventricular tachycardia. The patient’s other vital signers were: heart rate, 136 beats/min; respiratory rate 20 breaths/min; and pulse oximetry was 94% on 4 liters/min of oxygen via nasal cannula.
The patient’s medical history was significant for atrial fibrillation and an indwelling pacemaker, for which she was chronically on flecai
The initial electrocardiogram (ECG) revealed a wide complex rhythm with pacemaker spikes (Figure 1). Based on these findings, electrodes were placed on the patient in the event she required cardioversion. The patient was started on an amiodarone intravenous (IV) drip for presumptive ventricular tachycardia.
During the patient’s evaluation in the ED, she experienced transient drops in BP, which were responsive to an IV fluid bolus of normal saline, and the amiodarone drip was discontinued. The patient’s ECG findings were compared to previous ECG studies, as was her current medication list and prior health issues. After ruling-out other causes, flecainide toxicity was considered high in the differential, and she was given 1 ampule of bicarbonate IV, after which a second ECG showed heart rhythm converted from a wide-complex tachycardia to a paced rhythm, markedly improved from the initial ECG (Figure 2). Similarly, there was a marked improvement in BP.
An interrogation of the patient’s pacemaker revealed an atrial flutter with a rate below detection for mode switch, with one-to-one tracking/pacing. The pacemaker was reprogrammed to divide the DDIR mode with detection rate at 120 mm Hg with mode switch activated. This was felt to be consistent with flecainide toxicity precipitating the cardiac conduction issues.
Laboratory studies showed an elevated flecainide level at 1.39 mcg/mL (upper limits of normal of 1 mcg/mL). Other studies showed worsening congestive heart failure, with a brain natriuretic peptide of 8,057 pg/mL and mild dehydration, with serum creatinine increased from her baseline of 0.9 to 1.38 mg/dL.
The patient’s abdominal pain was further evaluated and she was found to have acute cholecystitis. She was admitted to the intensive care unit with cardiology and general surgery consulting.
Discussion
Flecainide acetate was approved by the Food and Drug Administration in 1984.1It is a Vaughan-Williams class IC antiarrhythmic with a sodium channel blocker action used to treat supra ventricular arrhythmias. The CAST trial in 1989 investigated the efficacy of this class of antiarrhythmics, which resulted in a revision of its role.2 Based on this study, flecainide is not recommended for patients with structural heart disease or coronary artery disease.2,3 However, it is recommended as a first-line therapy for pharmacologic cardioversion and maintenance of normal sinus rhythm in patients with atrial fibrillation and supraventricular tachycardia4,5 without the above caveats.
Class IC agents produce a selective block at the sodium (Na+) channels, resulting in the slowing of cardiac conduction.6,7 This high affinity for Na+ channels combined with slow unbinding kinetics during diastole explain the slowing of recovery time and prolongation of the refractory period.6,8,9 These electrophysiologic properties all can increase the PR, QRS, and QT interval duration. The QT interval is not significantly affected, as most of the QT prolongation is due to the QRS widening.6,10,11 Widening of the QRS by greater than 25% as compared to the baseline value is used as the threshold to decrease dosing or discontinue the use of flecainide.3The toxic effects of flecainide on cardiac conduction can produce prolonged QRS duration of up to 50%, and PR interval up to 30%, especially in rapid heart rates. Signs of intoxication are difficult to discern owing to its nonspecific presentation. A well-documented, but under-recognized, presentation of flecainide toxicity is the transformation of atrial fibrillation to atrial flutter.5,7,9,11-13 The reported rate of this pro arrhythmic effect can be as high as 3.5% to 5%.14,15Flecainide toxicity can occur secondary to chronic ingestion and may be precipitated in mild renal failure. The majority of flecainide is renally excreted and the half-life is 20 hours. Maximum therapeutic effect is seen between levels of 0.2 to 1 mcg/mL with levels greater than 0.7 to 1 mcg/mL associated with adverse effects.9 Systemic effects include dizziness and visual disturbances. A high degree of suspicion for flecainide toxicity is required when the patient’s initial presentation is nonspecific. In this circumstance, real-time bedside interrogation of the pacemaker is invaluable. Early diagnosis and treatment minimizes the risk for adverse sequelae, including death. Treatment includes increasing the excretion of flecainide, symptomatic support (including pacemaker placement, intravenous fat emulsion, or extracorporeal circulatory support) and administration of sodium bicarbonate, to transiently reverse the effect of the sodium channel blockade, in severe cases.15-17
1. Hudak JM, Banitt EH, Schmid JR. Discovery and development of flecainide. Am J Cardiol. 1984;53(5):17B-20B.
2. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST). N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629.
3. Andrikopoulos GK, Pastromas S, Tzeis S. Flecainide: Current status and perspectives in arrhythmia management. World J Cardiol. 2015;7(2):76-85. doi:10.4330/wjc.v7.i2.76.
4. Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747. doi:10.1093/eurheartj/ehs253.
5. Courand PY, Sibellas F, Ranc S, Mullier A, Kirkorian G, Bonnefoy E. Arrhythmogenic effect of flecainide toxicity. Cardiol J. 2013;20:203-205. doi:10.5603/CJ.2013.0035.
6. Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985;29(1):1-33.
7. Taylor R, Gandhi MM, Lloyd G. Tachycardia due to atrial flutter with rapid 1:1 conduction following treatment of atrial fibrillation with flecainide. Br Med J. 2010;340:b4684.
8. Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315(1):36-41.
9. Levis JT. ECG diagnosis: flecainide toxicity. Perm J. 2012;16(4):53.
10. Hellestrand KJ, Bexton RS, Nathan AW, Spurrell RA, Camm AJ. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48(2):140-148.
11. Rognoni A, Bertolazzi M, Peron M, et al. Electrocardiographic changes in a rare case of flecainide poisoning: a case report. Cases J. 2009;2:9137. doi:10.1186/1757-1626-2-9137.
12. Nabar A, Rodriguez LM, Timmermans C, Smeets JL, Wellens HJ. Radiofrequency ablation of “class IC atrial flutter” in patients with resistant atrial fibrillation. Am J Cardiol. 1999;83(5):785-787, A10.
13. Kola S, Mahata I, Kocheril AG. A case of flecainide toxicity. EP Lab Digest. 2015;15(5).
14. Falk RH. Proarrhythmia in patients treated for atrial fibrillation or flutter. Ann Intern Med. 1992;117(2):141-150.
15. Lloyd T, Zimmerman J, Griffin GD. Irreversible third-degree heart block and pacemaker implant in a case of flecainide toxicity. Am J Emerg Med. 2013;31(9):1418.e1-e2. doi:10.1016/j.ajem.2013.04.025.
16. Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27(4):405-408.
17. Ellsworth H, Stellpflug SJ, Cole JB, Dolan JA, Harris CR. A life-threatening flecainide overdose treated with intravenous fat emulsion. Pacing Clin Electrophysiol. 2013;36(3):e87-e89. doi:10.1111/j.1540-8159.2012.03485.x.
1. Hudak JM, Banitt EH, Schmid JR. Discovery and development of flecainide. Am J Cardiol. 1984;53(5):17B-20B.
2. Cardiac Arrhythmia Suppression Trial (CAST) Investigators. Preliminary report: effect of encainide and flecainide on mortality in a randomized trial of arrhythmia suppression after myocardial infarction. The Cardiac Arrhythmia Suppression Trial (CAST). N Engl J Med. 1989;321(6):406-412. doi:10.1056/NEJM198908103210629.
3. Andrikopoulos GK, Pastromas S, Tzeis S. Flecainide: Current status and perspectives in arrhythmia management. World J Cardiol. 2015;7(2):76-85. doi:10.4330/wjc.v7.i2.76.
4. Camm AJ, Lip GY, De Caterina R, et al; ESC Committee for Practice Guidelines (CPG). 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719-2747. doi:10.1093/eurheartj/ehs253.
5. Courand PY, Sibellas F, Ranc S, Mullier A, Kirkorian G, Bonnefoy E. Arrhythmogenic effect of flecainide toxicity. Cardiol J. 2013;20:203-205. doi:10.5603/CJ.2013.0035.
6. Holmes B, Heel RC. Flecainide. A preliminary review of its pharmacodynamic properties and therapeutic efficacy. Drugs. 1985;29(1):1-33.
7. Taylor R, Gandhi MM, Lloyd G. Tachycardia due to atrial flutter with rapid 1:1 conduction following treatment of atrial fibrillation with flecainide. Br Med J. 2010;340:b4684.
8. Roden DM, Woosley RL. Drug therapy. Flecainide. N Engl J Med. 1986;315(1):36-41.
9. Levis JT. ECG diagnosis: flecainide toxicity. Perm J. 2012;16(4):53.
10. Hellestrand KJ, Bexton RS, Nathan AW, Spurrell RA, Camm AJ. Acute electrophysiological effects of flecainide acetate on cardiac conduction and refractoriness in man. Br Heart J. 1982;48(2):140-148.
11. Rognoni A, Bertolazzi M, Peron M, et al. Electrocardiographic changes in a rare case of flecainide poisoning: a case report. Cases J. 2009;2:9137. doi:10.1186/1757-1626-2-9137.
12. Nabar A, Rodriguez LM, Timmermans C, Smeets JL, Wellens HJ. Radiofrequency ablation of “class IC atrial flutter” in patients with resistant atrial fibrillation. Am J Cardiol. 1999;83(5):785-787, A10.
13. Kola S, Mahata I, Kocheril AG. A case of flecainide toxicity. EP Lab Digest. 2015;15(5).
14. Falk RH. Proarrhythmia in patients treated for atrial fibrillation or flutter. Ann Intern Med. 1992;117(2):141-150.
15. Lloyd T, Zimmerman J, Griffin GD. Irreversible third-degree heart block and pacemaker implant in a case of flecainide toxicity. Am J Emerg Med. 2013;31(9):1418.e1-e2. doi:10.1016/j.ajem.2013.04.025.
16. Corkeron MA, van Heerden PV, Newman SM, Dusci L. Extracorporeal circulatory support in near-fatal flecainide overdose. Anaesth Intensive Care. 1999;27(4):405-408.
17. Ellsworth H, Stellpflug SJ, Cole JB, Dolan JA, Harris CR. A life-threatening flecainide overdose treated with intravenous fat emulsion. Pacing Clin Electrophysiol. 2013;36(3):e87-e89. doi:10.1111/j.1540-8159.2012.03485.x.
Sarcoidosis Resulting in Exsanguinating Esophageal Variceal Hemorrhage
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
Sarcoidosis is a systemic disorder of unknown etiology and is characterized by the formation of granulomas throughout various organs in the body. The most common form is pulmonary sarcoidosis, which affects 90% of patients; the second most common form is oculocutaneous sarcoidosis;1 and the third most common form is hepatic sarcoidosis, which affects 63% to 90% of patients.2 Although the liver is frequently involved in all forms of sarcoidosis, only a fraction of patients present with clinically evident liver disease.1 Approximately 20% to 30% of patients have abnormalities on liver function tests, whereas only about 1% of patients show evidence of portal hypertension and cirrhosis.3 In fact, in the English literature, there were 35 reported cases of portal hypertension due to sarcoidosis between 1949 to 2001, of which 16 of the patients had no evidence of cirrhosis.4
The diagnosis of sarcoidosis is usually made by a compilation of clinical signs and symptoms, imaging studies, and biopsies demonstrating noncaseating granulomas. This case report describes a patient who presented with portal hypertension and esophageal variceal bleeding secondary to sarcoidosis of the liver without cirrhotic changes.
Case
A 47-year-old woman presented to the ED via emergency medical services with a 1-hour history of hematemesis and melena. The patient stated that she felt fatigued, nauseated, and light-headed, but had no pain or focal weakness. Her medical history was significant for pulmonary and renal sarcoidosis. She underwent a liver biopsy 1 week prior to presentation, with a 6-day hospitalization period, due to new ascites found on examination.
The patient’s vital signs at presentation were: blood pressure (BP), 72/56 mm Hg; heart rate (HR), 133 beats/min, respiratory rate, 24 breaths/min; and temperature, 97.0oF. Oxygen saturation was 99% on room air. Physical examination revealed an alert and oriented middle-aged woman in extremis who was vomiting dark-colored blood. The cardiac and pulmonary examination revealed no extraneous sounds; the abdominal examination showed ascites with a liver edge palpable 4 cm beneath the right costal margin. The patient had no scleral icterus, palmar erythema, spider angiomata, fetor hepaticus, caput medusa, cutaneous ecchymoses, or any other stigmata of cirrhosis.
Two large-bore peripheral intravenous (IV) catheters were placed and a massive blood transfusion protocol was initiated. Packed red blood cells (PRBCs) from the resuscitation-area refrigerator were infused immediately via a pressurized fluid warmer.
After consultation with gastroenterology and general surgery services, the patient was given 1 g ceftriaxone IV, 1 g tranexamic acid IV, 20 mcg desmopressin IV, 50 mcg octreotide IV, 40 mg pantoprazole IV, 8 mg ondansetron IV, 4 g calcium gluconate IV, and 100 mg hydrocortisone IV.
Throughout the patient’s first 10 minutes in the ED, she remained persistently hypotensive and continued to vomit. Since the patient’s sensorium was intact, the team quickly discussed goals of care with her. The patient’s wishes were for maximal life-sustaining therapy, including endotracheal intubation and chest compressions, if necessary.
After this discussion, the patient was given IV etomidate and rocuronium and was intubated using video-assisted laryngoscopy. Following intubation, she was sedated with an infusion of fentanyl and underwent orogastric tube placement to aspirate stomach contents. A total of 2.5 L of frank blood were drained from the patient’s stomach.
A size 9 French single lumen left-femoral central venous catheter also was placed, through which additional blood products were infused. The patient received a total of 28 U PRBCs, fresh frozen plasma, and platelets over a 3-hour period. During transfusion, the patient’s vital signs improved to a systolic BP ranging between 110 to 120 mm Hg and an HR ranging between 90 to 110 beats/min; she did not experience any further hypotensive episodes throughout her stay in the ED.
Laboratory studies were significant for metabolic acidosis, hyperkalemia, acute on chronic anemia, leukocytosis, and acute on chronic renal failure. Synthetic function of the liver and transaminases appeared normal (Table).
The patient’s hyperkalemia was treated with 1 g calcium chloride IV, 50 g dextrose IV, and 10 U regular insulin IV. A portable chest radiograph showed an appropriately positioned endotracheal tube, and an electrocardiogram revealed sinus tachycardia without signs of hyperkalemia. A computed tomography (CT) scan of the abdomen and pelvis from the patient’s recent hospitalization, 1 week prior to presentation, showed hepatomegaly, liver granulomas, ascites, and periportal lymphadenopathy (Figure 1).
A review of the patient’s recent liver biopsy and ascitic fluid analysis revealed noncaseating granulomas compressing the hepatic sinusoids, and a serum ascites albumin gradient greater than 1.1 g/dL, implying portal hypertension without cirrhosis. The surgical team attempted to place a Sengstaken-Blakemore tube, but the device could not be positioned properly due to the patient’s narrowed esophagus.
The ED nurses cleaned the patient, preserving her dignity; thereafter the patient’s adult children visited with her briefly before she was taken for an upper endoscopy, which was performed in the ED. The endoscopy revealed actively hemorrhaging esophageal varices at the gastroesophageal junction (Figure 2). The varices were treated with endoscopic ligation; the gastroenterologist placed a total of 11 bands, resulting in cessation of bleeding.
After the endoscopy, the patient was admitted to the medical intensive care unit (ICU). Approximately 1.5 hours after arriving at the ICU, she developed renewed hematemesis. Despite efforts to control bleeding and provide hemodynamic support, the patient died 1 hour later.
Discussion
Etiology
Esophageal variceal hemorrhage is caused by pressure elevation in the portal venous system, leading to engorged esophageal veins that can bleed spontaneously. Approximately 90% of portal hypertension is due to liver cirrhosis.5 The remaining 10% of cases are primarily vascular in etiology, with endothelial dysfunction and thrombosis leading to increased portal resistance. Noncirrhotic causes of portal hypertension include malignancy, congenital diseases, viral hepatitides, vascular thromboses or fistulae, constrictive pericarditis, fatty liver of pregnancy, drugs, radiation injury, and infiltrative diseases.5
Sarcoidosis may cause noncaseating granulomas to form in the liver, leading to portal hypertension and fatal exsanguination from esophageal variceal hemorrhage. Although the lesions of sarcoidosis classically form in the lungs, any organ system may be affected.6,7 Frank cirrhosis of the liver occurs in only 1% of sarcoidosis patients; however, radiographic involvement of the liver is seen in 5% to 15% of patients.8
There are several mechanisms which may be responsible for portal hypertension in patients with sarcoidosis, including granulomas causing mass effect on the hepatic sinusoids; arteriovenous shunts within the granuloma; granulomatous phlebitis within the sinusoids; or compressive periportal lymphadenopathy.9 Regardless of the mechanism, a review of the literature demonstrates an association between sarcoidosis and symptomatic portal hypertension.2,4,10,11Although our patient ultimately died, early initiation of massive blood transfusion protocol, airway protection, attention to electrolytes, and endoscopic control of the hemorrhage source provided the best chance for survival.
Medical Therapy
The first priority in managing and treating esophageal varices is to secure the patient’s airways to prevent aspiration. Two large bore IV lines should be placed to permit rapid infusion of crystalloid fluids or blood products. Initiating antibiotics, specifically IV ceftriaxone, to patients with variceal bleeding is a class I recommendation, as this is the only intervention shown to increase patient survival.12 Although proton pump inhibitors (PPI) and somatostatin analogues (typically octreotide) are frequently given, they are both class II recommendations because there is limited evidence supporting the benefit of their use.12 However, current guidelines recommend treating patients for variceal bleeding with an initial bolus of a PPI, followed by a continuous infusion of PPI for 72 hours. As previously noted, multiple studies, have failed to show any decrease in mortality associated with this treatment.12
Other agents that are used to treat variceal bleeding include octreotide and vasopressin. Octreotide, a somatostatin analog, is generally given as an initial IV bolus followed by continuous infusion, and has been shown to decrease transfusion requirements without mortality benefit.12 Vasopressin is generally given to critically ill patients, and is considered a third-line treatment for variceal bleeding.
Since our patient had a history of chronic kidney disease, desmopressin was empirically administered in the event platelet dysfunction was a contributing factor to bleeding.13 The absence of cirrhosis was significant because our patient was unlikely to have a bleeding diathesis caused by coagulation factor deficiency. Therefore, the goal transfusion ratio of blood products should be balanced, similar to that in traumatic exsanguination, rather than favoring an increased ratio of plasma to other blood products. Similarly, tranexamic acid was administered because insufficient tamponade rather than coagulopathy was the presumed cause of sustained hemorrhage.
An additional complicating factor in our patient’s care was the potential effect of the massive transfusion on electrolytes. Packed RBCs have a pH of approximately 6.8 and may carry up to 25 mmol/L of potassium, which may have exacerbated our patient’s underlying hyperkalemia.14 Rapid blood transfusion also places patients at risk for acute hypocalcemia secondary to citrate toxicity; this did not occur in our patient in part because the metabolic function of her liver was preserved and citrate could be broken down in the hepatocyte Krebs cycle.15 Calcium therapy doubled as treatment for the hyperkalemia and as prophylaxis against further hypocalcemia. No dysrhythmias were observed.
Surgical Intervention
Emergency physicians should consult with gastroenterology services so that an endoscopy can be performed as soon as possible to evaluate for and control bleeding. When an endoscopy cannot be performed rapidly, there are multiple balloon tamponade devices available that can be used to temporize the bleeding, such as the Sengstaken-Blakemore tube.12
Although balloon tamponade devices are typically reserved for the last line of therapy, endoscopy rather than transjugular intrahepatic portosystemic shunt (TIPS) was the preferred method of hemorrhage source control in our patient for several reasons. First, although the working diagnosis of varices was based on the patient’s history, we wanted to evaluate for other causes of upper gastrointestinal bleeding since our patient had no history of endoscopy. Therefore, endoscopy had both a therapeutic and diagnostic value. Secondly, though TIPS may decrease pressure within the bleeding varix, only endoscopy permits direct hemostasis. Also, endoscopy also was preferred over TIPS because our patient was too unstable to move to the interventional radiology suite.16
Conclusion
Although life-threatening esophageal variceal hemorrhage is a rare manifestation of an uncommon disease, it should be considered in the differential diagnosis of a patient who has sarcoidosis and presents with gastrointestinal bleeding. Additionally, when caring for a patient with massive hematemesis without evidence of liver cirrhosis, other etiologies of portal hypertension and esophageal varices, such as sarcoidosis, should be considered.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.
1. Rao DA, Dellaripa PF. Extrapulmonary manifestations of sarcoidosis. Rheum Dis Clin North Am. 2013;39(2):277-297. doi:10.1016/j.rdc.2013.02.007.
2. Mistilis SP, Green JR, Schiff L. Hepatic sarcoidosis with portal hypertension. Am J Med. 1964;36(3):470-475. doi:10.1016/0002-9343(64)90175-5.
3. Tekeste H, Latour F, Levitt RE. Portal hypertension complicating sarcoid liver disease: case report and review of the literature. Am J Gastroenterol. 1984;79(5):389-396.
4. Ivonye C, Elhammali B, Henriques-Forsythe M, Bennett-Gittens R, Oderinde A. Disseminated sarcoidosis resulting in portal hypertension and gastrointestinal bleeding: a rare presentation. Can J Gastroenterol. 2012;26(8):508-509. http://www.ncbi.nlm.nih.gov/pubmed/22891173. Accessed May 16, 2018.
5. Tetangco EP, Silva RG, Lerma EV. Portal hypertension: etiology, evaluation, and management. Dis Mon. 2016;62(12):411-426. doi:10.1016/j.disamonth.2016.08.001.
6. Valeyre D, Prasse A, Nunes H, Uzunhan Y, Brillet PY, Müller-Quernheim J. Sarcoidosis. Lancet. 2014;383(9923):1155-1167. doi:10.1016/S0140-6736(13)60680-7.
7. Al-Kofahi K, Korsten P, Ascoli C, et al. Management of extrapulmonary sarcoidosis: challenges and solutions. Ther Clin Risk Manag. 2016;12:1623-1634. doi:10.2147/TCRM.S74476.
8. Iannuzzi MC, Rybicki BA, Teirstein AS. Sarcoidosis. N Engl J Med. 2007;357(21):2153-2165. doi:10.1056/NEJMra071714.
9. Ebert EC, Kierson M, Hagspiel KD. Gastrointestinal and hepatic manifestations of sarcoidosis. Am J Gastroenterol. 2008;103(12):3184-3192. doi:10.1111/j.1572-0241.2008.02202.x.
10. Fraimow W, Myerson RM. Portal hypertension and bleeding esophageal varices secondary to sarcoidosis of the liver. Am J Med. 1957;23(6):995-998.
11. Saito H, Ohmori M, Iwamuro M, et al. Hepatic and gastric involvement in a case of systemic sarcoidosis presenting with rupture of esophageal varices. Intern Med. 2018;56(19):2583-2588. doi:10.2169/internalmedicine.8768-16.
12. DeLaney M, Greene CJ. Emergency Department evaluation and management of patients with upper gastrointestinal bleeding. Emerg Med Pract. 2015;17(4):1-18; quiz 19.
13. Ozgönenel B, Rajpurkar M, Lusher JM. How do you treat bleeding disorders with desmopressin? Postgrad Med J. 2007;83(977):159-163. doi:10.1136/pgmj.2006.052118.
14. Sümpelmann R, Schürholz T, Thorns E, Hausdörfer J. Acid-base, electrolyte and metabolite concentrations in packed red blood cells for major transfusion in infants. Paediatr Anaesth. 2001;11(2):169-173. doi:10.1046/j.1460-9592.2001.00637.x.
15. Monchi M. Citrate pathophysiology and metabolism. Transfus Apher Sci. 2018;56(1):28-30. doi:10.1016/j.transci.2016.12.013.
16. Shah RP, Sze DY. Complications during transjugular intrahepatic portosystemic shunt creation. Tech Vasc Interv Radiol. 2016;19(1):61-73. doi:10.1053/j.tvir.2016.01.007.
Enlarging nodule under the toenail • no history of trauma • unremarkable medical history • Dx?
THE CASE
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; Michael.m.barna.mil@mail.mil.
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
THE CASE
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; Michael.m.barna.mil@mail.mil.
THE CASE
A 28-year-old woman with an unremarkable medical history presented with an enlarging nodule that had been growing under her left great toenail for 6 months. The patient monitored the nodule, hoping that it would resolve on its own, but found that it steadily increased in size and began to displace the nail, causing pain. At the time of presentation, the nodule measured approximately 10 mm in diameter, and there was significant (~80°) superior displacement of the nail (FIGURE 1).
An initial radiograph identified a 5.5-mm bony density arising from the dorsal surface of the left first distal phalanx with no significant degenerative changes (FIGURE 2). A subsequent magnetic resonance image confirmed the bony excrescence and noted marrow continuity. A thin amount of T2 bright signal was also observed, suggesting either a cartilaginous cap or soft tissue edema secondary to pressure on the nail bed (FIGURE 3).
THE DIAGNOSIS
Histologic examination demonstrated a thin (3 mm) cartilaginous cap overlying an area of mature fibrocartilage with no definite periosteum. The osseous component appeared to mature from the cartilage, and the marrow was focally fatty and fibrosed (FIGURES 4A and 4B). Expert consultation with the Joint Pathology Center confirmed a benign osteochondromatous lesion.
The histologic differential diagnosis of this patient’s lesion included subungual exostosis and osteochondroma. Based on the patient’s age, location of the lesion, and histologic findings, the final diagnosis was subungual exostosis.
DISCUSSION
Subungual exostoses are benign osteocartilaginous tumors that most commonly affect children and young adults. They predominantly manifest on the dorsomedial aspect of the tip of the great toe (~80%), but can occur on other digits of the foot or hand.1 They are caused by a proliferation of fibrous tissue under the nail bed. The fibrocartilage cap then undergoes endochondral ossification to woven bone and lamellar bone trabeculae. As these lesions mature, they establish continuity with the underlying bone in the phalanx.2 Subungual exostoses were once thought to represent a proliferative response to trauma, but further research has identified a recurrent t(X;6) (q22;q13-14) translocation, suggesting a neoplastic origin.3
Osteochondromas are also common benign tumors formed by endochondral ossification, although secondary transformation into low-grade chondrosarcomas is well-documented.1 Osteochondromas commonly affect younger patients. They occur at epiphyseal areas of developing bone and have a hyaline matrix and chondrocyte pattern similar to that of a normal epiphyseal area, with confluence to the underlying trabecular and cortical bone. They are not caused by previous trauma and generally only become symptomatic after they have grown large enough to cause mechanical problems.1
Continue to: More diagnoses to consider
More diagnoses to consider
Other potential diagnoses for benign osteochondromatous lesions include bizarre parosteal osteochondromatous proliferations (BPOP) and digital mucous cysts.
BPOPs, also known as Nora’s lesions (crediting preliminary research performed by Nora and colleagues in 19834), are irregular formations of hypercellular cartilage, bone, and large chondrocytes. They predominantly occur in the small bones of the hands and feet, but may involve the skull and long bones.3 Unlike subungual exostoses and osteochondromas, BPOPs tend to occur in the third and fourth decades of life and generally do not alter, or have continuity with, the underlying bone.4
Histologically, BPOPs undergo irregular maturation, leaving a characteristic blue tint at the border of the newly formed trabecular bone. As with subungual exostoses, these lesions were traditionally believed to be reactive in nature. However, cytogenetic studies have identified variant translocations involving 1q32 (most commonly t[1;17] [q32;q21]) that are unique and common to these lesions.5
Digital mucous cysts are benign ganglion cysts that typically appear in the distal interphalangeal joints or at the proximal nail fold. They are believed to result from mucoid degeneration of connective tissue. Although generally associated with the hands, these cysts can also occur on the feet.6
Continue to: Our patient's outcome
Our patient’s outcome
After orthopedic consultation, the lesion and a 5 × 5-mm portion of the adherent germinal nail matrix were resected operatively through a medial excision. A small flap of the lateral nail matrix was rotated to cover the matrix defect, and the wound was closed. Postoperatively, the patient experienced slow wound healing (a total of 3 weeks), but there was no recurrence of the lesion at the 2-month follow-up.
THE TAKEAWAY
Osteocartilaginous tumors present as rapidly growing lesions on the distal tips of fingers and toes, but they may also occur on long bones and on the skull. Rarely malignant in nature, most of these lesions can be differentiated by location, histopathologic features, and patient age at onset. Consider surgical consultation and excision for relief of pain and/or cosmetic reasons. Recurrence is rare.
CORRESPONDENCE
Michael Barna, MD, Naval Hospital Camp Lejeune, Department of Family Medicine, 100 Brewster Blvd, Camp Lejeune, NC 28547; Michael.m.barna.mil@mail.mil.
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
1. Miller-Breslow A, Dorfman HD. Dupuytren’s (subungual) exostosis. Am J Surg Pathol. 1988;12:368-378.
2. DaCambra MP, Gupta SK, Ferri-de-Barros F. Subungual exostosis of the toes: a systematic review. Clin Orthop Relat Res. 2014;472:1251-1259.
3. Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion). Am J Surg Pathol. 1993;17:691-697.
4. Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hand and feet. Am J Surg Pathol. 1983;7:245-250.
5. Zambrano E, Nosé V, Perez-Atayde AR, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion). Am J Surg Pathol. 2004;28:1033-1039.
6. Salerni G, Alonso C. Images in clinical medicine. Digital mucous cyst. N Engl J Med. 2012;366:1335.
History of posttraumatic stress disorder • priapism • Dx?
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; caleb.g.dickison.mil@mail.mil.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; caleb.g.dickison.mil@mail.mil.
THE CASE
A 35-year-old African-American man, who was an active duty service member, presented to the Troop Medical Clinic with a 4-hour history of priapism. He had been taking sertraline 100 mg and prazosin 10 mg nightly for 4 months to treat his posttraumatic stress disorder (PTSD) with no reported adverse effects. These doses were titrated 2 months prior to presentation. The patient reported that he took his usual medication doses before bed and awoke at 3 am with a penile erection. At 7 am, he presented to the clinic because of pain from the continued erection.
THE DIAGNOSIS
A penile erection was present on physical exam. All medications were reviewed for adverse effects. A work-up for anemia, sickle cell disease, thalassemia, and platelet abnormalities was negative. A blood gas analysis performed on blood aspirated from the corpus cavernosum showed hypoxemia, hypercarbia, and acidosis, confirming a diagnosis of ischemic priapism.
DISCUSSION
Priapism is a prolonged erection of the penis that is usually not associated with sexual activity or stimulation. It is considered a urologic emergency and requires prompt treatment to prevent long-term complications, such as permanent erectile dysfunction.
Priapism is classified as one of 2 types: ischemic (“low flow”) or nonischemic (“high flow”).
Ischemic priapism is the most common type. It is caused by dysfunctional cavernosal smooth muscle, which creates a compartment-like syndrome in the cavernous tissue that leads to hypoxia and acidosis.1 Nonischemic priapism is often caused by a fistula between the cavernosal artery and corpus cavernosum and is common with traumatic injuries. Nonischemic priapism has a lower risk for long-term complications (due to the blood being well-oxygenated) and often resolves spontaneously without treatment.2,3
Certain medications can cause priapism
Our patient’s ischemic priapism was most likely caused by the combined antagonistic properties of prazosin and sertraline on alpha-1 adrenergic receptors.3,4 Adrenergic alpha-blockers block the sympathetic system, which can in turn inhibit penile detumescence and cause priapism.4
An increasingly common Tx combination. Selective serotonin reuptake inhibitors (SSRIs) such as sertraline are considered first-line treatment for the symptoms of PTSD, and prazosin has been found to be effective in the treatment of nightmares associated with PTSD. (Treatment of PTSD-related nightmares with prazosin is an off-label but frequent use of the medication.) This combination of medications is becoming increasingly common for the treatment of PTSD and its associated symptoms.5-7
Continue to: Cases to date provide interesting insight into this adverse effect
Cases to date provide interesting insight into this adverse effect
In our literature review, no documented cases of priapism were attributed to prazosin when it was used for the treatment of nightmares, but there are multiple case reports of priapism linked to the drug’s use for hypertension.
In the majority of these case reports, the dosage exceeded 10 mg/d and was much higher than the dosage typically used to treat nightmares.7 Many of the affected patients also had associated comorbidities such as diabetes or chronic kidney disease.4
Sertraline has been associated with priapism when used as monotherapy and in combination therapy with antipsychotics. All SSRIs have antagonistic properties to alpha-1 adrenergic receptors, but sertraline appears to have more than a 10-fold increase in affinity when compared to other SSRIs.3
Treatment: An injection and aspiration
Our patient was treated with phenylephrine injection and aspiration, which resolved the priapism. Prazosin was stopped, and the patient was weaned off of sertraline. He continued to follow up closely with Behavioral Health for further management of his PTSD and associated symptoms.
Continue to: THE TAKEAWAY
THE TAKEAWAY
PTSD is being diagnosed more frequently, especially in active duty soldiers, veterans, members of the National Guard, and reservists.8 Because nightmares are a common symptom of PTSD and SSRIs are first-line treatment for PTSD, the combination of prazosin and an SSRI for the treatment of PTSD is frequently encountered.5-7 Providers who prescribe and/or care for patients treated with these medications need to counsel patients on the risk of priapism and the risks associated with a delay in seeking medical care.
If a patient who is taking these medications presents with priapism, contact Urology immediately for acute management. Both medications must be stopped to prevent future episodes; prazosin can be stopped immediately, but patients must be weaned off of sertraline to avoid experiencing withdrawal symptoms. Patients will need to follow up with a behavioral health team for continued management of their PTSD symptoms.
CORRESPONDENCE
Caleb Dickison, DO, Fort Belvoir Community Hospital, 9300 Dewitt Loop, Fort Belvoir, VA 22060; caleb.g.dickison.mil@mail.mil.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
1. Pryor J, Akkus E, Alter G, et al. Priapism. J Sex Med. 2004;1:116-120.
2. Broderick GA, Gordon D, Hypolite J, et al. Anoxia and corporal smooth muscle dysfunction: a model for ischemic priapism. J Urol. 1994;151:259-262.
3. Choua, R, Lee HC, Castro J, et al. Priapism associated with multiple psychotropics: a case report and review of the literature. 2007. Available at: http://primarypsychiatry.com/priapism-associated-with-multiple-psychotropics-a-case-report-and-review-of-the-literature/. Accessed on May 7, 2018.
4. Spagnul SJ, Cabral PH, Verndl DO, et al. Adrenergic alpha-blockers: an infrequent and overlooked cause of priapism. Int J Impot Res. 2011;23:95-98.
5. Stein DJ, Ipser JC, Seedat S. Pharmacotherapy for posttraumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006;CD002795.
6. Taylor FB, Martin P, Thompson C, et al. Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma PTSD: a placebo-controlled study. Biol Psychiatry. 2008;63:629-632.
7. Raskind MA, Peskind ER, Hoff DJ, et al. A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry. 2007;61:928-934.
8. Grieger TA, Cozza SJ, Ursano RJ, et al. Posttraumatic stress disorder and depression in battle-injured soldiers. Am J Psychiatry. 2006;163:1777-1783.
Soft Tissue Reconstruction of the Proximal Tibiofibular Joint by Using Split Biceps Femoris Graft with 5-Year Clinical Follow-up
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
ABSTRACT
Instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that presents unique challenges to treatment. We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations, treated surgically at our institution using a split biceps femoris tendon graft for PTFJ reconstruction. She underwent several attempts at nonoperative management until we decided to proceed with surgical intervention. A split biceps femoris graft was used to restore stability of the PTFJ. Approximately 5 years postoperatively, she achieved full range of motion as well as functional and clinical Knee Society Scores of 94 and 90 points, respectively. To the best of our knowledge, this is the first case report of PTFJ instability treated surgically with long-term follow-up. Future studies should focus on the long-term satisfactory outcomes of soft tissue stabilization of a chronically unstable PTFJ.
The instability of the proximal tibiofibular joint (PTFJ) is a rare clinical condition that commonly occurs secondary to an initial pivoting or twisting event of a flexed knee. Although acute PTFJ dislocations respond well to closed reduction and casting, the treatment of chronic PTFJ instability presents a unique challenge.1 Surgical fixation methods include tibiofibular joint recreation using either a split semitendinosus or biceps femoris graft, as well as a Tightrope device.2-6 Older surgical options for chronic PTFJ instability include fibular head resection or PTFJ arthrodesis.7 However, these older techniques have fallen out of favor, and the optimal surgical technique for the treatment of this injury remains a point of contention.
We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations. The patient was surgically treated at our institution by using a split biceps femoris tendon graft for PTFJ reconstruction. This article specifically details the surgical technique used, provides data obtained at the 5-year clinical follow-up, and reviews prior publications on this injury. The patient provided written informed consent for print and electronic publication of this case report.
CASE
A 26-year-old woman presented with a 4-year history of lateral right knee pain with any physical activity. She stated that her pain began immediately following a fall, which was initially treated with casting and immobilization for approximately 6 weeks. After treatment, she began to develop symptoms of “popping on the outside of the knee.” In the 8 months prior to her presentation to our practice, these symptoms had intensified in pain severity and frequency. She reported that the popping events occurred most often with deep squatting.
No gross deformity was observed upon physical examination, and both knees were visibly symmetric. Evidence of effusion was absent. The patient felt no pain with the passive motion of her knee, and she presented the full range of motion (ROM) from 0° to 120°. Anterior drawer, McMurray, Lachman, and pivot shift tests were all negative. Upon the application of manual pressure, the fibular head could be dislocated anteriorly (Video 1). This dislocation recreated the patient’s symptoms. The fibular head could not be subluxed or dislocated posteriorly. Flexing the knee to 90° facilitated reproducing manual anterior dislocation. The contralateral knee was examined and demonstrated no appreciable PTFJ instability. The patient exhibited no other signs of generalized ligamentous laxity. Her sensation in the lower leg was intact, and she reported no tingling or numbness in the peroneal nerve distribution. Tinel’s test of the peroneal nerve was negative.
Continue to: X-ray imaging revealed...
X-ray imaging revealed symmetrically aligned knees with the fibular head in place within the PTFJ. Magnetic resonance imaging (MRI) and computed tomography demonstrated no evidence of soft tissue posterolateral corner injury, meniscal damage, bony fracture, or PTFJ arthrosis.
When the patient presented to our office, she reported having undergone several failed efforts of nonoperative treatment, including bracing and activity modification. On the basis of the chronicity of the reported symptoms, level of pain, and the desire of the patient to return to full activity, we recommended the surgical reconstruction of the PTFJ by using a split biceps femoris tendon graft.
OPERATIVE TECHNIQUE
The patient was positioned supine on a Jackson table. General anesthesia was utilized. Biplanar fluoroscopic imaging of the fibula was obtained with the fibular head manually dislocated and reduced. A bump was placed beneath the right thigh to create resting knee flexion. The patient was prepped and draped in sterile fashion, and a tourniquet was applied.
A 10-cm curvilinear surgical incision was made centered over the fibular neck and extending proximally within the interval between the iliotibial band and the biceps femoris tendon. Dissection was performed. The peroneal nerve was identified, carefully dissected out, and then isolated with a vessel loop. The biceps femoris tendon insertion on the fibular head was dissected while ensuring that the nerve was isolated, and the anterior half of the tendon was marked approximately 14 cm proximally using a surgical marker. A 15-blade was then used to split the tendon proximally along the marked path while taking care to preserve the tendinous insertion on the fibular head. The split portion of the tendon was freed from all underlying tissue, and the most distal 2 cm was tubularized using a running baseball stitch and No. 2 Ethibond.
The anterior and posterior aspects of the fibular head were then débrided of tissue, and a guidewire was placed anteriorly-to-posterior. After the position of the guidewire was confirmed with fluoroscopy, a 5-0 cannulated reamer was used to drill through the fibular head. Next, the interval between the biceps femoris and iliotibial band was found, and the lateral head of the gastrocnemius was retracted posteriorly within this interval. A portion of the soleus muscle was also elevated off of the posterior capsule and posterior tibia. The iliotibial band insertion at Gerdy’s tubercle was then identified, and a guidewire was placed from anterior-to-posterior within the tibia, with the starting point just posterior to Gerdy’s tubercle. The wire was advanced under direct visualization with an ACL tibial guide and confirmed fluoroscopically. A 5-mm cannulated reamer was then used to drill over the guidewire through the anterior and posterior cortex of the tibia. A suture passer was passed anterior-to-posterior through this tunnel to retrieve the tubularized portion of the biceps femoris graft, which was then shuttled through the tibial tunnel. This same tubularized graft segment was then shuttled anteriorly-to-posteriorly through the fibular tunnel. At this point, approximately 3 cm of the graft protruded from the posterior aspect of the fibular tunnel.
Continue to: The remaining graft was held...
The remaining graft was held taut, and the knee was cycled through flexion and extension. The knee was then placed in approximately 30° of flexion, and the fibular head was noted to be well reduced within the tibiofibular joint. This was confirmed visually and fluoroscopically. A 4.75-mm biotenodesis interference screw was then placed from anterior-to-posterior in the fibular tunnel. The remaining tendon exiting posteriorly from the tunnel was then over-sewn onto the remaining native biceps femoris tendon attached to the fibular head. The knee was stable through flexion and extension, and gentle pressure on the fibular head demonstrated no subluxation motion (Video 2). The wound was copiously irrigated with normal saline. The tourniquet was then taken down, and following the reapproximation of the deep fascia, the wound was closed in standard subcutaneous fashion.
POSTOPERATIVE COURSE
The patient was initially kept in a knee immobilizer following surgery and instructed to use touch-down weight-bearing for 3 weeks. She was switched to a hinged brace at 1 week postoperatively. Physical therapy began with range of motion exercises, and an active flexion was withheld until 6 weeks postoperatively. After 6 weeks, the patient was allowed to progress to an active ROM and increase to weight-bearing as tolerated. Strengthening was started at 12 weeks.
MRI was performed at 4 months postoperatively because the patient reported pain with running. The MRI demonstrated no evidence of stress reaction or fracture in the area of reconstruction. She was advised to continue with physical therapy and stop running. At 5-month post-reconstruction, the patient reported that her pain had resolved and that she had no complaints of any peroneal nerve neuropraxia. At 6 months she had returned to normal activity without complaints. At this point, she was instructed to follow-up as needed.
The patient was seen in office 5.5 years after the initial surgery for an unrelated orthopedic issue. At this time, follow-up data were obtained for her PTFJ reconstruction. She stated that she was very satisfied with the results of her surgery. She claimed to be pain free and had been performing normal activities without any difficulty. Upon physical examination, she achieved full range of motion. She had no extension lag or flexion contracture. She achieved functional and clinical Knee Society Scores of 94 and 90 points, respectively.
DISCUSSION
This article details a soft tissue PTFJ reconstruction using a split biceps femoris graft with over 5 years of clinical follow-up. Chronic PTFJ instability is a rare clinical entity, and unless gross instability is evident upon physical examination, its diagnosis may be confused with the diagnosis of more common complaints, such as meniscal tears or iliotibial band syndrome.
Continue to: Ogden first described...
Ogden8 first described the classification system for PTFJ dislocations. The classification system is based on dislocation direction and whether the joint is partially subluxed or dislocated. The classification system is as follows: type 1, atraumatic subluxation; type 2, anterolateral dislocation; type 3, posteromedial dislocation; and type 4, superior dislocation. Anterolateral PTFJ dislocation is the most commonly reported PTFJ dislocation in published literature. This case was classified as a type 2 dislocation given that the patient’s fibular head can be dislocated with manual pressure following an initial traumatic event.
Past instances of PTFJ instability have been managed with closed reduction and protected weight-bearing, as well as with various open reduction techniques.2-7 Surgical reconstruction is commonly considered in chronic cases or if nonoperative modalities have failed. Although PTFJ arthrodesis or fibular head resection has been used as a prior treatment option, the postoperative complications associated with each of these techniques have since caused them to fall out of favor.
The split biceps femoris graft has been successfully used in the soft tissue reconstruction of PTFJ.3,5-7 The soft tissue reconstruction of the PTFJ provides advantages over arthrodesis or fibular head resection because it preserves normal anatomy and avoids secondary stresses to the ankle encountered in the latter procedure. Fibular head resection also presents secondary complications, such as the loss of the biceps femoris and posterolateral corner ligament insertion points.9 Similar to this study, prior works have reported returns to functionality. However, this study represents the longest clinical postoperative follow-up of PTFJ ligament reconstruction. By using a split biceps graft, the insertion point of the biceps on the fibular head is preserved, thus maintaining normal function while still allowing for an easily tubularized graft for anatomic PTFJ ligament reconstruction.
CONSLUSION
We present data for over 5 years of follow-up for our surgical approach to this rare pathology. To the best of our knowledge, this is the first case report of PTFJ instability that was treated surgically and with a long-term follow-up. The patient did not demonstrate loss of knee motion, pain, or peroneal nerve symptoms. Moreover, she was very satisfied with the procedure at the most recent follow-up and had returned to unrestricted activity. The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results, and future studies should confirm these satisfactory outcomes in the long-term.
This paper will be judged for the Resident Writer’s Award.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
1. Nieuwe Weme RA, Somford MP, Schepers T. Proximal tibiofibular dislocation: a case report and review of literature. Strategies Trauma Limb Reconstr. 2014;9(3):185-189. doi:10.1007/s11751-014-0209-8.
2. Tafazal SI, Flowers MJ. Proximal tibiofibular joint instability in a child: stabilization with Tightrope. J Pediatr Orthop B. 2013;22(4):363-366. doi:10.1097/BPB.0b013e32836026b1.
3. Kobbe P, Flohe S, Wellmann M, Russe K. Stabilization of chronic proximal tibiofibular joint instability with a semitendinosus graft. Acta Orthop Belg. 2010;76(6):830-833.
4. Miettinen H, Kettunen J, Vaatainen U. Dislocation of the proximal tibiofibular joint.A new method for fixation. Arch Orthop Trauma Surg. 1999;119(5-6):358-359.
5. Mena H, Brautigan B, Johnson DL. Split biceps femoris tendon reconstruction for proximal tibiofibular joint instability. Arthroscopy. 2001;17(6):668-671.
6. Weinert CR Jr, Raczka R. Recurrent dislocation of the superior tibiofibular joint. Surgical stabilization by ligament reconstruction. J Bone Joint Surg Am. 1986;68(1):126-128.
7. Giachino AA. Recurrent dislocations of the proximal tibiofibular joint. Report of two cases. J Bone Joint Surg Am. 1986;68(7):1104-1106.
8. Ogden JA. Subluxation and dislocation of the proximal tibiofibular joint. J Bone Joint Surg Am. 1974;56(1):145-154.
9. Shapiro GS, Fanton GS, Dillingham MF. Reconstruction for recurrent dislocation of the proximal tibiofibular joint. A new technique. Orthop Rev. 1993;22(11):1229-1232.
TAKE-HOME POINTS
- We present the case of an active 26-year-old woman with a 4-year history of recurrent PTFJ subluxations.
- We chose to treat this patient surgically using split biceps femoris tendon graft for PTFJ reconstruction after failed nonoperative management.
- Surgical correction should be considered for those who fail several courses of nonoperative management.
- In our practice, we prefer reconstruction over arthrodesis as it preserves normal anatomy and avoids secondary stresses to the ankle.
- The soft tissue stabilization of a chronically unstable PTFJ is a viable treatment modality that provides good results
Digital Ischemia From Accidental Epinephrine Injection
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
Patients presenting to the ED with injuries due to accidental self-injection with an epinephrine pen typically receive treatment to alleviate symptoms and reduce the potential of digital ischemia leading to gangrene and loss of tissue and function. Although there is no consensus or set guidelines in the literature regarding the management protocol of such cases, many reports support pharmacological intervention. There are, however, other reports that advocate conservative, nonpharmaceutical management (eg, immersing the affected digit in warm water) or an observation-only approach.
We present the first case report in Saudi Arabia of digital ischemia due to accidental injection of an epinephrine autoinjector, along with a review of the literature and management recommendations.
Case
A 28-year-old woman presented to the ED in significant pain and discomfort 20 minutes after she accidentally injected the entire contents of her aunt’s epinephrine autoinjector (0.3 mg of 1:1000) into her right thumb. The patient, who was in significant pain and discomfort, stated that she was unable to remove the injector needle, which was firmly embedded in the bone of the palmer aspect of the distal phalanx in a manner similar to that of an intraosseous injection (Figure 1).
The patient’s vital signs and oxygen saturation on presentation were within normal limits. The emergency physician successfully removed the embedded needle through moderate countertraction. On examination, the patient’s right thumb was pale and cold, and had poor capillary refill (Figure 2). Due to concerns of the potential for digital tissue ischemia leading to tissue loss and gangrene, warm, moist compresses were applied to the affected thumb, followed by 2% topical nitroglycerin paste, after which the thumb was covered with an occlusive dressing. Since there was no improvement in circulation after 20 minutes, an infiltrate of 5 mg (0.5 mL of 10 mg/mL) of phentolamine (α-agonist) mixed with 2.5 mL of 2% lidocaine was injected at the puncture site and base of the right thumb.1 Hyperemia developed immediately at both injection sites, and the patient’s right thumb returned to a normal color and sensation 1 hour later, with a return to normal capillary refill. She remained in stable condition and was discharged home. Prior to discharge, the patient was educated on the proper handling and administration of an epinephrine autoinjector.
Discussion
Epinephrine is an ὰ- and β-adrenergic agonist that binds to the ὰ-adrenergic receptors of blood vessels, causing an increase in vascular resistance and vasoconstriction. Although the plasma half-life of epinephrine is approximately 2 to 3 minutes, subcutaneous or intramuscular injection resulting in local vasoconstriction may delay absorption; therefore, the effects of epinephrine may last much longer than its half-life.
The incidence of accidental injection from an epinephrine autoinjector is estimated to be 1 per 50,000 units dispensed.2 To date, there are no established treatment guidelines on managing cases of digital injection. An online PubMed and Google Scholar search of the literature found one systematic review,3 four observational studies,4-7 seven case series,8-14 and several case reports1,15-33 on the subject. Most of the patients in the published retrospective studies (71%) were treated conservatively with warming of the affected hand and observation, and the majority of patients in the case reports (87%) were treated pharmacologically, most commonly with topical nitroglycerin and phentolamine.1,3-34 All of the patients in both the retrospective studies and case reports had restoration of perfusion without necrosis, irrespective of treatment modality. However, patients who were managed conservatively or who were treated with topical nitroglycerin required a longer duration of stay in the ED, suffered from severe reperfusion pain, and in some cases, had a longer time to complete recovery (≥10 weeks).8
Pharmaceutical and Nonpharmaceutical Management
Phentolamine. Phentolamine is a nonselective ὰ-adrenergic antagonist that binds to ὰ1 and ὰ2 receptors of blood vessels, resulting in a decrease in peripheral vascular resistance and vasodilation. Phentolamine directly antagonizes the effect of epinephrine by blocking the ὰ-adrenergic receptors, which in our patient resulted in immediate return of digital circulation and full resolution of symptoms.
Topical Nitroglycerin. Nitroglycerin is a nitrate vasodilator that when metabolically converted to nitric oxide, results in smooth muscle relaxation, venodilation, and arteriodilation. Patients suffering from digital ischemia and vasoconstriction may be treated with topical nitroglycerin paste to reverse ischemia by causing smooth muscle relaxation of digital blood vessels. Conservative Management. As previously noted, not all cases of digital epinephrine injection are treated pharmacologically. Some patients are not treated, but kept in observation until the ischemic effects of epinephrine have resolved. Likewise, some patients are treated conservatively with warm water compresses or by fully immersing the affected digit in warm water to facilitate reversal of vasoconstriction and ischemia.3,8
Treatment Efficacy
In 2007, Fitzcharles-Bowe et al8 published a review of 59 cases of digital injection with high-dose epinephrine from 1989 to 2005. In this review, 32 of the 59 patients received no treatment, 25 patients received pharmacological treatment and in two patients, the treatment was unknown. Phentolamine was the most commonly used pharmacological agent (15 of 25 cases or 60%). Although none of the patients experienced digital necrosis, those treated with a local infiltration of phentolamine experienced a faster resolution of symptoms and normalization of perfusion. In 2004, Turner1 reported a case of a 10-year-old boy who was treated with phentolamine following an accidental injection of epinephrine into his left hand. While circulation returned to the affected digit within 5 minutes of receiving the phentolamine injection, the patient continued to experience reduced sensation in the digit 6 weeks later.8
Interestingly, one of the coauthors of the Fitzcharles-Bowe et al8 report intentionally injected three of the digits of his left hand (middle, ring, and small fingers) at the same time with high-dose epinephrine to carefully observe and document the outcomes. All three of the digits became very pale and cool, with decreased sensation. The author treated himself conservatively (observation-only). He experienced spontaneous return of circulation in two of the digits within 6 to 10 hours. Although there was some spontaneous return of circulation to the third digit after 13 hours, the author noted prolonged, intense reperfusion pain 4 hours after return of circulation. He also suffered from neuropraxia in the third digit, which did not fully resolve until 10 weeks after the injury.8
A review of the literature shows phentolamine to be a safe and effective treatment for patients presenting with digital ischemia, with no long-term adverse effects or complications. Moreover, phentolamine appears to be safe and effective for use in both adult and pediatric patients.3,8,35-38
Accidental Injection Prevention
Some of the cases of accidental epinephrine injection are due to user error. For example, a novice user may be holding the incorrect end of the injector in his or her hand when attempting to administer/deploy the device, resulting in premature dislodgement of the needle.39
Although, most of the autoinjector devices available today are user-friendly, we believe the addition of a safety feature such as a trigger or safety-lock may further help to reduce accidents. The European Medicines Agency recommends that all patients and caregivers receive training on the proper handling and administration of epinephrine autoinjectors, citing this as the most important factor to ensure successful use of an epinephrine autoinjector and reduce accidental injury.40 The patient in this case had not received any formal education or training regarding autoinjector use prior to this incident.
Safety of Lidocaine-Containing Epinephrine in Digital Anesthesia
Aside from cases of accidental digital epinephrine injection, clinicians have traditionally been taught to avoid using lidocaine with epinephrine for digital anesthesia. However, since the introduction of commercial lidocaine with epinephrine in 1948, there are no case reports of digital gangrene from commercially available lidocaine-epinephrine formulations.41,42 In a multicenter prospective study by Lalonde et al43 of 3,110 consecutive cases of elective injection of low-dose epinephrine in the hand, the authors concluded the likelihood of finger infarction is remote, particularly with possible phentolamine rescue therapy. Moreover, lidocaine-containing epinephrine (1%-2%) has a much lower concentration of epinephrine per mL of solution (5-10 mcg/mL) and appears to be safe for digital use.
Conclusion
This case describes the presentation and treatment of accidental digital injection of epinephrine, highlighting and supporting the benefits of local infiltration with phentolamine and observation until full recovery of perfusion. Local treatment with phentolamine not only facilitates recovery and return of capillary refill, but also shortens the duration of symptoms and alleviates vasoconstriction. In less severe cases, watchful waiting and observation may be appropriate and effective.
This case also underscores the importance of patient and caregiver education on the proper handling and administration of epinephrine autoinjectors to decrease the incidence of accidental injection.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
1. Turner MJ. Accidental Epipen injection into a digit - the value of a Google search. Ann R Coll Surg Engl. 2004;86(3):218-219. doi:10.1308/003588404323043391.
2. McGovern SJ. Treatment of accidental digital injection of adrenaline from an auto-injector device. J Accid Emerg Med. 1997;14(6):379-380.
3. Wright M. Treatment after accidental injection with epinephrine autoinjector: a systematic review. J Allergy & Therapy. 2014;5(3):1000175. doi:10.4172/2155-6121.1000175.
4. Mrvos R, Anderson BD, Krenzelok EP. Accidental injection of epinephrine from an autoinjector: invasive treatment not always required. South Med J. 2002;95(3):318-320.
5. Muck AE, Bebarta VS, Borys DJ, Morgan DL. Six years of epinephrine digital injections: absence of significant local or systemic effects. Ann Emerg Med. 2010;56(3):270-274. doi:10.1016/j.annemergmed.2010.02.019.
6. Simons FE, Edwards ES, Read EJ Jr, Clark S, Liebelt EL. Voluntarily reported unintentional injections from epinephrine auto-injectors. J Allergy Clin Immunol. 2010;125(2):419-423. doi:10.1016/j.jaci.2009.10.056.
7. Blume-Odom CM, Scalzo AJ, Weber JA. EpiPen accidental injection-134 cases over 10 years. Clin Toxicol. 2010;48:651.
8. Fitzcharles-Bowe C, Denkler K, Lalonde D. Finger injection with high-dose (1:1,000) epinephrine: Does it cause finger necrosis and should it be treated? Hand. 2007;2(1):5-11. doi:10.1007/s11552-006-9012-4.
9. Velissariou I, Cottrell S, Berry K, Wilson B. Management of adrenaline (epinephrine) induced digital ischaemia in children after accidental injection from an EpiPen. Emerg Med J. 2004;21(3):387-388.
10. ElMaraghy MW, ElMaraghy AW, Evans HB. Digital adrenaline injection injuries: a case series and review. Can J Plast Surg. 1998;6:196-200.
11. Skorpinski EW, McGeady SJ, Yousef E. Two cases of accidental epinephrine injection into a finger. J Allergy Clin Immunol. 2006;117(2):463-464.
12. Nagaraj J, Reddy S, Murray R, Murphy N. Use of glyceryl trinitrate patches in the treatment of accidental digital injection of epinephrine from an autoinjector. Eur J Emerg Med. 2009;16(4):227-228. doi:10.1097/MEJ.0b013e328306f0ee.
13. Stier PA, Bogner MP, Webster K, Leikin JB, Burda A. Use of subcutaneous terbutaline to reverse peripheral ischemia. Am J Emerg Med. 1999;17(1):91-94.
14. Lee G, Thomas PC. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
15. Baris S, Saricoban HE, Ak K, Ozdemir C. Papaverine chloride as a topical vasodilator in accidental injection of adrenaline into a digital finger. Allergy. 2011;66(11):1495-1496. doi:10.1111/j.1398-9995.2011.02664.x.
16. Buse K, Hein W, Drager N. Making Sense of Global Health Governance: A Policy Perspective. Basingstoke, England: Palgrave Macmillan UK; 2009.
17. Sherman SC. Digital Epipen® injection: a case of conservative management. J Emerg Med. 2011;41(6):672-674. doi:10.1016/j.jemermed.2009.07.027.
18. Janssen RL, Roeleveld-Versteegh AB, Wessels-Basten SJ, Hendriks T. [Auto-injection with epinephrine in the finger of a 5-year-old child]. Ned Tijdschr Geneeskd. 2008;152(17):1005-1008.
19. Singh T, Randhawa S, Khanna R. The EpiPen and the ischaemic finger. Eur J Emerg Med. 2007;14(4):222-223.
20. Barkhordarian AR, Wakelin SH, Paes TR. Accidental digital injection of adrenaline from an autoinjector device. Br J Dermatol. 2000;143(6):1359.
21. Deshmukh N, Tolland JT. Treatment of accidental epinephrine injection in a finger. J Emerg Med. 1989;7(4):408.
22. Hinterberger JW, Kintzi HE. Phentolamine reversal of epinephrine-induced digital vasospasm. How to save an ischemic finger. Arch Fam Med. 1994;3(2):193-195.
23. Peyko V, Cohen V, Jellinek-Cohen SP, Pearl-Davis M. Evaluation and treatment of accidental autoinjection of epinephrine. Am J Health Syst Pharm. 2013;70(9):778-781. doi:10.2146/ajhp120316.
24. Hardy SJ, Agostini DE. Accidental epinephrine auto-injector-induced digital ischemia reversed by phentolamine digital block. J Am Osteopath Assoc. 1995;95(6):377-378.
25. Kaspersen J, Vedsted P. [Accidental injection of adrenaline in a finger with EpiPen]. Ugeskr Laeger. 1998;160(45):6531-6532.
26. Schintler MV, Arbab E, Aberer W, Spendel S, Scharnagl E. Accidental perforating bone injury using the EpiPen autoinjection device. Allergy. 2005;60(2):259-260.
27. Khairalla E. Epinephrine-induced digital ischemia relieved by phentolamine. Plast Reconstr Surg. 2001;108(6):1831-1832.
28. Murali KS, Nayeem N. Accidental digital injection of adrenaline from an autoinjector device. J Accid Emerg Med. 1998;15(4):287.
29. Sellens C, Morrison L. Accidental injection of epinephrine by a child: a unique approach to treatment. CJEM. 1999;1(1):34-36.
30. Klemawesch P. Hyperbaric oxygen relieves severe digital ischaemia from accidental EpiPen injection. 2009 American Academy of Allergy, Asthma and Immunology Annual Meeting.
31. McCauley WA, Gerace RV, Scilley C. Treatment of accidental digital injection of epinephrine. Ann Emerg Med. 1991;20(6):665-668.
32. Mathez C, Favrat B, Staeger P. Management options for accidental injection of epinephrine from an autoinjector: a case report. J Med Case Rep. 2009;3:7268. doi:10.4076/1752-1947-3-7268.
33. Molony D. Adrenaline-induced digital ischaemia reversed with phentolamine. ANZ J Surg. 2006;76(12):1125-1126.
34. Carrascosa MF, Gallastegui-Menéndez A, Teja-Santamaría C, Caviedes JR. Accidental finger ischaemia induced by epinephrine autoinjector. BMJ Case Rep. 2013;2013. pii:bcr2013200783. doi:10.1136/bcr-2013-200783.
35. Patel R, Kumar H. Epinephrine induced digital ischemia after accidental injection from an auto-injector device. Indian Pediatr. 2013;50(2):247.
36. Xu J, Holt A. Use of Phentolamine in the treatment of Epipen induced digital ischemia. BMJ Case Rep. 2012;2012. doi:10.1136/bcr.12.2011.5450.
37. McNeil C, Copeland J. Accidental digital epinephrine injection: to treat or not to treat? Can Fam Physician. 2014;60(8):726-728.
38. Bodkin RP, Acquisto NM, Gunyan H, Wiegand TJ. Two cases of accidental injection of epinephrine into a digit treated with subcutaneous phentolamine injections. Case Rep Emerg Med. 2013;2013:586207. doi:10.1155/2013/586207.
39. Simons FE, Lieberman PL, Read EJ Jr, Edwards ES. Hazards of unintentional injection of epinephrine from autoinjectors: a systematic review. Ann Allergy Asthma Immunol. 2009;102(4):282-287. doi:10.1016/S1081-1206(10)60332-8.
40. European Medicines Agency. Better training tools recommended to support patients using adrenaline auto-injectors. European Medicines Agency, 2015.
41. Denkler K. A comprehensive review of epinephrine in the finger: to do or not to do.
42. Thomson CJ, Lalonde DH, Denkler KA, Feicht AJ. A critical look at the evidence for and against elective epinephrine use in the finger. Plast Reconstr Surg. 2007;119(1):260-266.
43. Lalonde D, Bell M, Benoit P, Sparkes G, Denkler K, Chang P. A multicenter prospective study of 3,110 consecutive cases of elective epinephrine use in the fingers and hand: the Dalhousie Project clinical phase. J Hand Surg Am. 2005;30(5):1061-1067. doi:10.1016/j.jhsa.2005.05.006.
A Forgotten Cause of Cardiac Tamponade
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
Purulent pericarditis is an infection within the pericardial space rarely seen in the modern antibiotic era. Most cases are secondary to another infectious process of bacterial, viral, fungal, or parasitic origin.1,2 Predisposing factors include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse disorder.1 Although purulent pericarditis has been described extensively in the literature, it is a challenging diagnosis if it is not initially considered within the differential diagnosis repertoire.1-4 Most authors agree that this may be because it has become an infrequent diagnosis.1,2 In addition, purulent pericarditis may have an atypical presentation when compared with a classic case of pericarditis.2,3 The authors believe that this forgotten entity will be revisited through this case.
Case Presentation
A 66-year-old-man was transferred to Veterans Affairs Caribbean Healthcare System (VACHS) from a community hospital with a diagnosis of community-acquired pneumonia (CAP) and bilateral pleural effusions. Four days prior to arrival at the community hospital, the patient had developed diffuse, watery diarrhea, which resolved in 3 days. After resolution of diarrhea, he began experiencing shortness of breath on exertion that progressed to onset at rest. The patient reported no fever, chills, nausea, vomiting, cough, or contact with others who were not healthy. He had a history of alcohol misuse without liver cirrhosis and reported no chronic diseases or use of medications. The patient had no history of tuberculosis exposure or pneumococcal vaccination, and had a negative interferon gamma release assay.
On admission to the community hospital, the patient was treated for CAP with ceftriaxone and azithromycin. On hospital day 3, the patient developed hypoxemia and an altered mental status. He was started on supplemental oxygen and transferred to the intensive care unit (ICU). Antibiotic therapy consequently was changed to levofloxacin and meropenem. However, no clinical improvement was noted on the following days.
On hospital day 7, the patient developed acute respiratory failure that required mechanical ventilation while being transferred to VACHS via air ambulance. His vital signs on arrival were the following: temperature, 97° F; heart rate, 86 beats/min; blood pressure, 103/61 mm Hg; respiratory rate, 14 breaths/min and SaO2 of 97%, measured while he breathed supplemental oxygen at an FiO2 of 0.4.
Hours after arrival, the patient developed sinus tachycardia and hypotension. A bedside 2D echocardiogram demonstrated a large pericardial effusion with diastolic collapse of the right atrium (Figure 2).
The patient’s clinical condition improved following drainage of pericardial fluid, with no further need for inotropic support. Antibiotic therapy was changed to vancomycin and meropenem. Initial microbiologic samples from pericardial fluid demonstrated Gram-positive diplococci, suggestive of Streptococcus pneumoniae (S pneumoniae) (Figure 4). Other diagnostic pericardial fluid test results included: WBC count 25,330 cmm, with 99% neutrophils and 1% lymphocytes; total protein, 3.8 mg/dL; glucose, < 2.0 mg/dL,LDH, > 2,500 U/L, potassium hydroxide preparation. The tests found no fungus, and the acid fast bacilli smear revealed no Bacillus. However, the pericardial fluid culture failed to demonstrate growth of any organism. Blood cultures also were negative.
The patient underwent anterior thoracotomy with partial pericardiectomy, and a pericardial tube was left in place connected to drainage. During the procedure, an abundant amount of fibrinous tissue was evacuated from the pericardial space (Figure 5).
The patient was extubated, pericardial and pleural tubes were removed, and he was transferred to the internal medicine ward 24 days after admission to the ICU. He received in-patient physical rehabilitation while completing a 6-week course of IV antibiotics (vancomycin and meropenem). After completion of therapy, the patient received the pneumococcal polysaccharide vaccination, and an echocardiography was repeated. No significant re-accumulation of pericardial effusion or constrictive pattern was evidenced. The patient was discharged to his out-of-state home, and follow-up was consequently lost.
Discussion
Purulent pericarditis is an infection localized within the pericardial space. Most cases are secondary to an infectious process elsewhere, which could be of bacterial, viral, fungal, or parasitic etiology.1 Five mechanisms could lead the infecting organism to infect the pericardial space; contiguous spread from intrathoracic site, hematogenous spread, extension from myocardial site, perforating injury or surgery, and extension from a subdiaphragmatic site.1 Predisposing factors for the development of this condition include malignancy, chronic kidney disease, immunosuppression, diabetes mellitus, and alcohol misuse. Pericarditis is an infection localized within the pericardial space.
Purulent pericarditis has become a rare entity in the antibiotic era.2 Prior to the development of antibiotics, most cases were secondary to S pneumoniae.1,2,5,6 As per Cilloniz and colleagues, about 40% to 50% of all cases of purulent pericarditis are caused by Gram-positive bacteria, mostly S pneumoniae.5 In this case study, bacterial culture did not reveal growth of an organism—most likely because the patient had received antibiotics elsewhere. However, Gram-positive cocci were seen within the initial pericardial aspirate. This organism was suspected to have spread contiguously from a pulmonary focus, which also led to pleural effusions.
Since the patient in this case study had no history of thoracic surgery, malignancy, or other immunosuppression, the patient’s history of alcohol misuse was the only predisposing factor for development of purulent pericarditis. Contrary to the common presentation of pericarditis, purulent pericarditis may not have the common clinical findings, such as chest pain, pericardial friction rub, and distended neck veins.2,3 Furthermore, according to Parikh and colleagues, about 35% of affected patients may have a normal electrocardiogram.2 Hence, the diagnosis of purulent pericarditis often is missed because the classic signs of pericarditis are often absent, and other nonspecific symptoms are attributed to initial underlying infection.7
A high index of suspicion is needed to diagnose purulent pericarditis. Once a diagnosis is made, initial treatment should consist of prompt drainage of pericardial fluid combined with systemic antibiotic therapy. Vancomycin and a third-generation cephalosporin may be started empirically until results of pericardial fluid cultures become available.3 Drainage can be achieved by pericardiocentesis, pericardiotomy, or pericardiectomy (partial or total).1 In cases of hemodynamic instability due to cardiac tamponade, sonographically guided pericardiocentesis should be undertaken and an indwelling pericardial catheter left in place.1 Although this is the simplest and fastest method of evacuation, it may not be effective when dealing with thick, fibrinous fluid. In such cases, intrapericardial fibrinolysis may be considered. This approach may be undertaken early in the process, after drainage insertion, or as salvage therapy, when there has been incomplete evacuation of purulent material or open surgical drainage is not available.
Streptokinase, urokinase, and tissue plasminogen activator have been used for intrapericadial fibrinolysis.1 However, there is no definite data on dosage or frequency at which these medications should be administered. No matter the therapeutic approach, effective drainage of the pericardial fluid is crucial to avoid the development of pericardial constriction. Constrictive pericarditis occurs when fibrosis and adhesions create a dense pericardium that encases the heart. This causes impaired ventricular filling that can lead eventually to heart failure.4 Pericardiectomy is the definitive treatment for constrictive pericarditis.
Conclusion
Although purulent pericarditis has become a rare diagnosis since the development of antibiotics, knowledge of how to identify it is essential since mortality reaches 100% if the diagnosis is missed.4 Even when the condition is promptly diagnosed and treated, mortality is 40%, mainly due to cardiac tamponade, septic shock, or constriction.1 The case presented here illustrates the clinical features associated with this condition. Knowing these features can translate in a successful patient outcome.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
1. Ferreira dos Santos L, Moreira D, Ribeiro P, et al. Purulent pericarditis: a rare diagnosis [in Portuguese]. Rev Port Cardiol. 2013;32(9):721-727.
2. Parikh SV, Memon N, Echols M, Shah J, McGuire DK, Keeley EC. Purulent pericarditis: report of 2 cases and review of the literature. Medicine (Baltimore). 2009;88(1):52–65.
3. Go C, Asnis DS, Saltzman H. Pneumococcal pericarditis since 1980. Clin Infect Dis. 1998;27(5):1338-1340.
4. Wada A, Craft J, Mazzaferri EL. Purulent pericarditis leading to constriction. Cardiol Res. 2014;5(6):188-190.
5. Cillóniz C, Rangel E, Barlascini C, Piroddi IMG, Torres A, Nicolini A. Streptococcus pneumoniae-associated pneumonia complicated by purulent pericarditis: case series [in English, Portuguese]. J Bras Pneumol. 2015;41(4):389-394.
6. Saenz RE, Sanders CV, Aldridge KE, Patel MM. Purulent pericarditis with associated cardiac tamponade caused by a Streptococcus pneumoniae strain highly resistant to penicillin, cefotaxime, and ceftriaxone. Clin Infect Dis. 1998;26(3):762–763.
7. Sagristà-Sauleda J, Barrabés JA, Permanyer-Miralda G, Soler-Soler J. Purulent pericarditis: review of a 20-year experience in a general hospital. J Am Coll Cardiol. 1993; 22(6):1661-1665.
Emergency Imaging: Femoral Pseudoaneurysm
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
Case
An 84-year-old man, who was a resident at a local nursing home, presented for evaluation after the nursing staff noticed an increasingly swollen mass on the patient’s left groin. The patient’s medical history was significant for bilateral aortofemoral graft surgery, dementia, hypertension, and severe peripheral artery disease (PAD). He was not on any anticoagulation or antiplatelet agents. Due to the patient’s dementia, he was unable to provide a history regarding the onset of the swelling or any other signs or symptoms.
On examination, the patient did not appear in distress. His son, who was the patient’s durable power of attorney, was likewise unable to provide a clear timeframe regarding onset of the mass. The patient had no recent history of trauma and had not undergone any recent medical procedures. Vital signs at presentation were: blood pressure, 110/70 mm Hg; heart rate, 84 beats/min; respiratory rate, 13 breaths/min; and temperature, 98.6°F. Oxygen saturation was 94% on room air.
Clinical examination revealed a pulsatile, purple left groin mass and bruit. The mass was located around the left inguinal ligament and extended down the proximal, inner thigh (Figure 1). There was no drainage or lesions from the mass. Inspection of the patient’s hip demonstrated decreased adduction, limited by the mass; otherwise, there was normal range of motion. The dorsalis pedis and posterior tibial pulses were equal and intact, and the rest of the physical examination was unremarkable.
The patient tolerated the examination without focal signs of discomfort. A Doppler ultrasound revealed findings consistent with a common femoral pseudoaneurysm (PSA) (Figure 2). For better visualization and extension, a computed tomography angiogram (CTA) was obtained, which demonstrated a PSA measuring 11.7 x 10.7 x 7.3 cm; there was no active extravasation (Figure 3).
The patient was started on intravenous normal saline while vascular surgery services was consulted for management and repair. After a discussion with the son regarding the patient’s wishes, surgical intervention was refused and the patient was conservatively managed and transitioned to hospice care.
Discussion
A true aneurysm differs from a PSA in that true aneurysms involve all three layers of the vessel wall. A PSA consists partly of the vessel wall and partly of encapsulating fibrous tissue or surrounding tissue.
Etiology
Femoral artery PSAs can be iatrogenic, for example, develop following cardiac catheterization or at the anastomotic site of previous surgery.1 The incidence of diagnostic postcatheterization PSA ranges from 0.05% to 2%, whereas interventional postcatheterization PSA ranges from 2% to 6%.2
With the increasing number of peripheral coronary diagnostics and interventions, emergency physicians should include PSA in the differential diagnosis of patients with a recent or remote history of catheterization or bypass grafts. Less commonly, femoral PSAs are caused by non-surgical trauma or infection (ie, mycotic PSA). Patient risk factors for development of PSA include obesity, hypertension, PAD, and anticoagulation.3 Patients with femoral artery PSAs may present with a painful or painless pulsatile mass. Mass effect of the PSA can compress nearby neurovascular structures, leading to femoral neuropathies or limb edema secondary to venous obstruction.4 Complications of embolization or thrombosis can cause limb ischemia, neuropathy, and claudication, while rupture may present with a rapidly expanding groin hematoma. Additionally, sizeable PSAs can cause overlying skin necrosis.5
Imaging Studies
Diagnosis of a PSA can be made through Doppler ultrasound, which is the preferred imaging modality due to its accuracy, noninvasive nature, and low cost. Doppler ultrasound has been found to have a sensitivity of 94% and specificity of 97% in detecting PSAs. Additional imaging with CTA can provide further definition of vasculopathy.6 Treatment should be considered for patients with a symptomatic femoral PSA, a PSA measuring more than 3 cm, or patients who are on anticoagulation therapy. Studies have shown that observation-only and follow-up may be appropriate for patients with a PSA measuring less than 3 cm. A study by Toursarkissian et al7 found that the majority of PSAs smaller than 3 cm spontaneously resolved in a mean of 23 days without limb-threatening complications.
Treatment
Traditionally, open surgical repair techniques were the only treatment option for PSAs. However, in the early 1990s, the advent of new techniques such as stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection, have developed as alternatives to open surgical repair; there has been variable success to these minimally invasive approaches.5,8
Ultrasound-Guided Compression. A conservative approach to treating PSAs, ultrasound-guided compression requires sustained compression by a skilled physician. This technique is associated with significant discomfort to the patient.5 Ultrasound-Guided Thrombin Injection. This technique is the treatment of choice for postcatheterization PSA. However, this intervention is contraindicated in patients who have concerning features such as an infected PSA, rapid expansion, skin necrosis, or signs of limb ischemia. Additionally, ultrasound-guided thrombin injection is not appropriate for use in patients with a PSA occurring at anastomosis of a synthetic graft and native artery.5
Conclusion
Based on our patient’s clinical presentation and history of aortofemoral bypass surgery, we suspected a femoral PSA. While the PSA noted in our patient was sizeable, imaging studies and clinical examination showed no sign of limb ischemia or rupture.
Femoral PSAs are usually iatrogenic in nature, typically developing shortly after catheterization or a previous bypass surgery. The most serious complication of a PSA is rupture, but a thorough examination of the distal extremity is warranted to assess for limb ischemia as well. Ultrasound imaging is considered the modality of choice based on its high sensitivity and sensitivity for detecting PSAs.
Small PSAs (<3 cm) can be managed medically, but larger PSAs (>3 cm) require treatment. Newer techniques, including stenting, coil insertion, ultrasound-guided compression, and ultrasound-guided thrombin injection are alternatives to open surgical repair of larger, uncomplicated PSAs. However, urgent open surgical repair is the only option when there is evidence of a ruptured PSA, ischemia, or skin necrosis.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.
1. Faggioli GL, Stella A, Gargiulo M, Tarantini S, D’Addato M, Ricotta JJ. Morphology of small aneurysms: definition and impact on risk of rupture. Am J Surg. 1994;168(2):131-135.
2. Hessel SJ, Adams DF, Abrams HL. Complications of angiography. Radiology. 1981;138(2):273-281. doi:10.1148/radiology.138.2.7455105.
3. Petrou E, Malakos I, Kampanarou S, Doulas N, Voudris V. Life-threatening rupture of a femoral pseudoaneurysm after cardiac catheterization. Open Cardiovasc Med J. 2016;10:201-204. doi:10.2174/1874192401610010201.
4. Mees B, Robinson D, Verhagen H, Chuen J. Non-aortic aneurysms—natural history and recommendations for referral and treatment. Aust Fam Physician. 2013;42(6):370-374.
5. Webber GW, Jang J, Gustavson S, Olin JW. Contemporary management of postcatheterization pseudoaneurysms. Circulation. 2007;115(20):2666-2674. doi:10.1161/CIRCULATIONAHA.106.681973.
6. Coughlin BF, Paushter DM. Peripheral pseudoaneurysms: evaluation with duplex US. Radiology. 1988;168(2):339-342. doi:10.1148/radiology.168.2.3293107.
7. Toursarkissian B, Allen BT, Petrinec D, et al. Spontaneous closure of selected iatrogenic pseudoaneurysms and arteriovenous fistulae. J Vasc Surg. 1997;25(5):803-809; discussion 808-809.
8. Corriere MA, Guzman RJ. True and false aneurysms of the femoral artery. Semin Vasc Surg. 2005;18(4):216-223. doi:10.1053/j.semvascsurg.2005.09.008.