User login
Symptoms Mimicking Those of Hypokalemic Periodic Paralysis Induced by Soluble Barium Poisoning
Hypokalemic periodic paralysis (HPP) is a relatively common and potentially life-threating condition that can be either sporadic or recurring and has both inherited and acquired causes.1 Familial HPP, on the other hand, is a rare condition (1:100,000) caused by loss of function mutations leading to the disruption of membrane potential consequently making them inexcitable.2 Appearance of symptoms is typically in the first or second decade of life (60% of cases have onset aged < 16 years) with susceptible individuals experiencing sudden onset of perioral numbness; weakness; centrifugal paralysis, often with nausea; vomiting and diarrhea; and prostration, usually triggered by highcarbohydrate meals and rest following sustained muscle-group use.3
These symptoms are common to all forms of HPP, making the differential diagnosis wide and confusing. Rhabdomyolysis is occasionally associated with many severe hypokalemic episodes.4 Myopathy and permanent muscle weakness have been reported in HPP.5,6 Other reported inciting factors include a drop in serum potassium caused by β-adrenergic bronchodilator treatment.7 Clinical attacks also have been associated with diabetic ketoacidosis and combined hypokalemia and hypophosphatemia.8 Thyrotoxicosis also causes similar muscle action potential changes but only when hyperthyroidism is uncorrected. 9-12 Less commonly, hypothyroidism has been reported to be associated with hypokalemic paralysis.3
Pa Ping, a condition involving hypokalemic paralysis of uncertain etiology, is geographically centered in the Szechuan region of China.13 Cases of Bartter, Liddle, and Gitelman syndromes also have been associated with hypokalemic paralysis.3,14 There is an association with malignant hyperthermia following or during systemic anesthesia. Patients presenting as Guillain-Barré syndrome have been found to have periodic paralysis triggered by hypokalemia from any cause.15 Sjögren syndrome and renal tubular acidosis also are reported to have triggered symptoms of hypokalemic paralysis.16,17
True type 1 HPP is caused by channelopathies resulting from mutations in the calcium channel gene CACN1AS (HypoPP1), which accounts for 70% of the cases, whereas type 2 HPP is cause by sodium channel gene SCN4A (HypoPP2) mutations, which accounts for 10% to 20% of cases.18,19 An association with a voltage-gated potassium channel KCNE3 mutation has been made but is disputed.20,21 Females typically have less severe and less frequent attacks, and attacks lessen or disappear during pregnancy.22
In a small controlled trial, acetazolamide has been reported to have prophylactic benefit, although a more powerful carbonic anhydrase inhibitor, dichlorophenamide, was reported to be effective in a study after acetazolamide had become ineffective.23,24 These treatments would not be expected to be of clinical use in hypokalemia due to barium poisoning.
Barium poisoning has been reported as a result of accidental contamination of foodstuffs with soluble barium.25 Onset of symptoms is rapid, with nausea, vomiting, diarrhea, and malaise followed rapidly by weakness, which can include the muscles of respiration. This littleconsidered but rapidly lethal poisoning event can be accidental as a result of environmental exposure due to unintentional ingestion of the toxin or deliberate criminal poisoning as in this case. Because deliberate poisoning rarely crosses the mind of the clinician, awareness of the potential similarity of barium poisoning to other forms of HPP and even familial HPP is important.
Case Presentation
A male veteran aged 45 years when treated by the authors was well until moving into a new rural home when he began to experience acute episodes of variable perioral numbness, diarrhea, paresthesias, abdominal cramping, and weakness, which ranged from mild, self-terminating extremity weakness to 3 episodes of respiratory failure that required intubation and mechanical ventilation.
All episodes were accompanied by hypokalemia in the range of 2 to 3 mEq/L, but levels varied erratically during admissions from severe hypokalemia to normo- and hyperkalemia. Over 3 years, the patient was admitted to the hospital 19 times, underwent extensive workup, and was referred to endocrinology services at Duke University, Vanderbilt University, and the Cleveland Clinic. Diagnostic efforts centered on establishing whether he had a latepresenting variant of familial HPP.
Genetic evaluations could not identify known single-nucleotide polymorphisms associated with that condition. The consensus was that he had a potassium leak somewhere between his kidneys and bladder. Recommended management was a high baseline oral potassium supplementation and spironolactone. He had a brief period of improvement after moving to a different house, but the episodes returned once he moved back to his old house despite adherence to recommended treatment. In December 2012, he experienced his worst episode, with potassium 1.8 mEq/L on admission, resulting in admission to the intensive care unit (ICU).
Following a precipitous clinical decline, the patient was intubated and mechanically ventilated. Nephrology was consulted and given the recurrent life-threatening pattern, an intensive chart review was undertaken. It was noted that a urine arsenic level that had been normal several admissions previously at 18 μg/L was elevated during a subsequent admission at 59 μg/L, and several weeks later during a later admission the level had fallen to 15 μg/L. Urine lead was undetectable on 3 occasions, and urine mercury was within normal limits.
Arsenic toxicity did not match the patient’s clinical syndrome, but the pattern seemed to be consistent with the possibility of unexplained toxic exposure and subsequent clearance. Therefore, an intensive literature search for syndromes of environmental exposure or poisoning resembling HPP was undertaken. The search revealed several references in the literature to paralysis similar to HPP that involved ingestion of hair-removing soap and rat poison containing barium sulfide and carbonate. References also pointed to the similarity of the symptoms to Guillain-Barre syndrome.
As a result of that literature search, a blood barium level was collected in the ICU that revealed 14,550 ng/mL. A scalp hair sample showed 6.1 μg barium per gram of hair (reference, 0.53 μg/g to 2 μg/g). Neither the patient nor his wife reported being involved in painting, ceramic work, decorating glassware or fabric with dyes, working with stained glass, smelting, metal welding, or use of vermicides.
A U.S. Environmental Protection Agency team was sent to the house, and a detailed toxic survey of the house and the surrounding grounds was conducted with no excess barium found. Barium levels were checked by a private physician on the wife and 2 minor children. The wife’s barium levels came back undetectable in a blood sample and elevated in a hair sample. One child had a very low detected level in her blood and slightly elevated in her hair, and the other child had a low level in her blood and her hair. Because the circumstances of the wife’s and children’s exposure could not be explained environmentally nor could the veteran’s exposure source be identified, the VA Police Service contacted the Tennessee Bureau of Investigation, and they questioned the veteran and his wife.
Shortly after that the veteran received a paralyzing gunshot wound to the back, and the ensuing investigation resulted in incarceration of his wife for both attempted murder by firearm and serial poisoning after soluble barium-containing materials were found hidden in the house.
Discussion
Human barium poisoning is a rarely reported toxic exposure that results in rapid onset of nausea, vomiting, diarrhea, progressive weakness that may end in respiratory paralysis and death if intubation and mechanical ventilation are not promptly initiated. Although the barium found in radiographic contrast media is highly insoluble, ingested barium carbonate and sulfide are rapidly absorbed into the bloodstream, reaching high levels quickly and altering the conductance of potassium channels. The result is erratic variation in blood potassium and prolonged paralysis unless it is immediately suspected and hemodialysis is initiated. In this case, the suspicion level at the time of intubation was insufficient to justify initiating acute hemodialysis.
Soluble barium is available from a number of open sources. Depilatory powders and several rat poisons list barium sulfide or carbonate, both soluble forms of barium rapidly absorbed through the gastrointestinal mucosa, as a major ingredient. One celebrated 2012 case in a city near Chattanooga, Tennessee, involved allegations of barium carbonate poisoning involving rat poison mixed into coffee creamer, but no charges could be filed because the sample handling precluded definitive linkage. Another deliberate toxic poisoning in Texas was traced to soluble barium introduced into a father’s food by his daughter.
The patient reported here experienced 3 years and 19 admissions with 3 episodes of mechanical intubation before his suspected variant HPP was recognized as actually being due to soluble barium poisoning.
Barium does not appear in usual heavy metal urine and blood screens and as a result may not be asked for if not thought of in the differential diagnosis. Physicians dealing with instances of recurrent suspected HPP that do not fit usual age and clinical characteristics for HPP, lack the single-nucleotide polymorphisms associated with the disease, and are not associated with other conditions causing severe hypokalemia, such as renal tubular acidosis, Bartter, Liddle or Gitelman syndrome or severe diuretic or licorice-induced hypokalemia should have soluble barium poisoning included in the differential diagnosis. Appropriately drawn blood specimens in special metal-free sampling tubes and hair barium levels should be included in the diagnostic workup. If poisoning is suspected, a chain of evidence should be obtained to protect possible future criminal investigation against compromise.
Acknowledgments
The authors thanks Tennessee 2nd District Attorney General Barry P. Staubus, 2nd District Assistant Attorney General Teresa A. Nelson, the VA Police Service, and the Tennessee Bureau of Investigation for their help.
1. Ahlawat SK, Sachdev A. Hypokalaemic paralysis. Postgrad Med J. 1999;75(882):193-197.
2. Fontaine B. Periodic paralysis. Adv Genet.2008;63:3-23.
3. Kayal AK, Goswami M, Das M, Jain R. Clinical and biochemical spectrum of hypokalemic paralysis in North: East India. Ann Indian Acad Neurol.2013;16(2):211-217.
4. Johnson CH, VanTassell VJ. Acute barium poisoning with respiratory failure and rhabdomyolysis. Ann Emerg Med. 1991;20(10):1138-1142.
5. Gold R, Reichmann H. Muscle pathology correlates with permanent weakness in hypokalemic periodic paralysis: a case report. Acta Neuropathol. 1992;84(2):202-206.
6. Links TP, Zwarts MJ, Wilmink JT, Molenaar WM, Oosterhuis HJ. Permanent muscle weakness in familial hypokalaemic periodic paralysis. Clinical, radiological and pathological aspects. Brain. 1990;113(pt 6):1873-1889.
7. Tucker C, Villanueva L. Acute hypokalemic periodic paralysis possibly precipitated by albuterol. Am J Health Syst Pharm. 2013;70(18):1588-1591.
8. Liu PY, Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc. 2004;67(7):355-359.
9. Sigue G, Gamble L, Pelitere M, et al. From profound hypokalemia to life-threatening hyperkalemia: a case of barium sulfide poisoning. Arch Intern Med. 2000;160(4):548-541.
10. Kuntzer T, Flocard F, Vial C, et al. Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve. 2000;23(7):1089-1094.
11. Tengan CH, Antunes AC, Gabbai AA, Manzano GM. The exercise test as a monitor of disease status in hypokalaemic periodic paralysis. J Neurol Neurosurg Psychiatry. 2004;75(3):497-499.
12. McManis PG, Lambert EH, Daube JR. The exercise test in periodic paralysis. Muscle Nerve. 1986;9(8):704-710.
13. Huang K-W. Pa ping (transient paralysis simulating family periodic paralysis). Chin Med J. 1943;61(4):305-312.
14. Ng HY, Lin SH, Hsu CY, Tsai YZ, Chen HC, Lee CT. Hypokalemic paralysis due to Gitelman syndrome:a family study. Neurology. 2006;67(6):1080-1082.
15. Mohta M, Kalra B, Shukla R, Sethi AK. An unusual presentation of hypokalemia. J Anesth Clin Res. 2014;5(3):389.
16. Fujimoto T, Shiiki H, Takahi Y, Dohi K. Primary Sjögren’s Syndrome presenting as hypokalaemic periodic paralysis and respiratory arrest. Clin Rheumatol. 2001;20(5):365-368.
17. Chang YC, Huang CC, Chiou YY, Yu CY. Renal tubular acidosis complicated with hypokalemic periodic paralysis. Pediatr Neurol. 1995;13(1):52-54.
18. Lehmann-Horn F, Jurkat-Rott K, Rüdel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep. 2002;2(1):61-69.
19. Venance SL, Cannon SC, Fialho D, et al; CINCH investigators. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129(pt 1):8-17.
20. Sharma C, Nath K, Parekh J. Reversible electrophysiological abnormalities in hypokalemic paralysis: case report of two cases. Ann Indian Acad Neurol. 2014;17(1):100-102.
21. Sternberg D, Tabti N, Fournier E, Hainque B, Fontaine B. Lack of association of the potassium channel-associated peptide MiRP2-R83H variant with periodic paralysis. Neurology. 2003;61(6):857-859.
22. Ke Q, Luo B, Qi M, Du Y, Wu W. Gender differences in penetrance and phenotype in hypokalemic periodic paralysis. Muscle Nerve. 2013;47(1):41-45.
23. Griggs RC, Engel WK, Resnick JS. Acetazolamide treatment of hypokalemic periodic paralysis. Prevention of attacks and improvement of persistent weakness. Ann Intern Med. 1970;73(1):39-48.
24. Dalakas MC, Engel WK. Treatment of “permanent” muscle weakness in familial hypokalemic periodic paralysis. Muscle Nerve. 1983;6(3):182-186.
25. Ghose A, Sayeed AA, Hossain A, Rahman R, Faiz A, Haque G. Mass barium carbonate poisoning with fatal outcome, lessons learned: a case series. Cases J. 2009;2:9327.
Hypokalemic periodic paralysis (HPP) is a relatively common and potentially life-threating condition that can be either sporadic or recurring and has both inherited and acquired causes.1 Familial HPP, on the other hand, is a rare condition (1:100,000) caused by loss of function mutations leading to the disruption of membrane potential consequently making them inexcitable.2 Appearance of symptoms is typically in the first or second decade of life (60% of cases have onset aged < 16 years) with susceptible individuals experiencing sudden onset of perioral numbness; weakness; centrifugal paralysis, often with nausea; vomiting and diarrhea; and prostration, usually triggered by highcarbohydrate meals and rest following sustained muscle-group use.3
These symptoms are common to all forms of HPP, making the differential diagnosis wide and confusing. Rhabdomyolysis is occasionally associated with many severe hypokalemic episodes.4 Myopathy and permanent muscle weakness have been reported in HPP.5,6 Other reported inciting factors include a drop in serum potassium caused by β-adrenergic bronchodilator treatment.7 Clinical attacks also have been associated with diabetic ketoacidosis and combined hypokalemia and hypophosphatemia.8 Thyrotoxicosis also causes similar muscle action potential changes but only when hyperthyroidism is uncorrected. 9-12 Less commonly, hypothyroidism has been reported to be associated with hypokalemic paralysis.3
Pa Ping, a condition involving hypokalemic paralysis of uncertain etiology, is geographically centered in the Szechuan region of China.13 Cases of Bartter, Liddle, and Gitelman syndromes also have been associated with hypokalemic paralysis.3,14 There is an association with malignant hyperthermia following or during systemic anesthesia. Patients presenting as Guillain-Barré syndrome have been found to have periodic paralysis triggered by hypokalemia from any cause.15 Sjögren syndrome and renal tubular acidosis also are reported to have triggered symptoms of hypokalemic paralysis.16,17
True type 1 HPP is caused by channelopathies resulting from mutations in the calcium channel gene CACN1AS (HypoPP1), which accounts for 70% of the cases, whereas type 2 HPP is cause by sodium channel gene SCN4A (HypoPP2) mutations, which accounts for 10% to 20% of cases.18,19 An association with a voltage-gated potassium channel KCNE3 mutation has been made but is disputed.20,21 Females typically have less severe and less frequent attacks, and attacks lessen or disappear during pregnancy.22
In a small controlled trial, acetazolamide has been reported to have prophylactic benefit, although a more powerful carbonic anhydrase inhibitor, dichlorophenamide, was reported to be effective in a study after acetazolamide had become ineffective.23,24 These treatments would not be expected to be of clinical use in hypokalemia due to barium poisoning.
Barium poisoning has been reported as a result of accidental contamination of foodstuffs with soluble barium.25 Onset of symptoms is rapid, with nausea, vomiting, diarrhea, and malaise followed rapidly by weakness, which can include the muscles of respiration. This littleconsidered but rapidly lethal poisoning event can be accidental as a result of environmental exposure due to unintentional ingestion of the toxin or deliberate criminal poisoning as in this case. Because deliberate poisoning rarely crosses the mind of the clinician, awareness of the potential similarity of barium poisoning to other forms of HPP and even familial HPP is important.
Case Presentation
A male veteran aged 45 years when treated by the authors was well until moving into a new rural home when he began to experience acute episodes of variable perioral numbness, diarrhea, paresthesias, abdominal cramping, and weakness, which ranged from mild, self-terminating extremity weakness to 3 episodes of respiratory failure that required intubation and mechanical ventilation.
All episodes were accompanied by hypokalemia in the range of 2 to 3 mEq/L, but levels varied erratically during admissions from severe hypokalemia to normo- and hyperkalemia. Over 3 years, the patient was admitted to the hospital 19 times, underwent extensive workup, and was referred to endocrinology services at Duke University, Vanderbilt University, and the Cleveland Clinic. Diagnostic efforts centered on establishing whether he had a latepresenting variant of familial HPP.
Genetic evaluations could not identify known single-nucleotide polymorphisms associated with that condition. The consensus was that he had a potassium leak somewhere between his kidneys and bladder. Recommended management was a high baseline oral potassium supplementation and spironolactone. He had a brief period of improvement after moving to a different house, but the episodes returned once he moved back to his old house despite adherence to recommended treatment. In December 2012, he experienced his worst episode, with potassium 1.8 mEq/L on admission, resulting in admission to the intensive care unit (ICU).
Following a precipitous clinical decline, the patient was intubated and mechanically ventilated. Nephrology was consulted and given the recurrent life-threatening pattern, an intensive chart review was undertaken. It was noted that a urine arsenic level that had been normal several admissions previously at 18 μg/L was elevated during a subsequent admission at 59 μg/L, and several weeks later during a later admission the level had fallen to 15 μg/L. Urine lead was undetectable on 3 occasions, and urine mercury was within normal limits.
Arsenic toxicity did not match the patient’s clinical syndrome, but the pattern seemed to be consistent with the possibility of unexplained toxic exposure and subsequent clearance. Therefore, an intensive literature search for syndromes of environmental exposure or poisoning resembling HPP was undertaken. The search revealed several references in the literature to paralysis similar to HPP that involved ingestion of hair-removing soap and rat poison containing barium sulfide and carbonate. References also pointed to the similarity of the symptoms to Guillain-Barre syndrome.
As a result of that literature search, a blood barium level was collected in the ICU that revealed 14,550 ng/mL. A scalp hair sample showed 6.1 μg barium per gram of hair (reference, 0.53 μg/g to 2 μg/g). Neither the patient nor his wife reported being involved in painting, ceramic work, decorating glassware or fabric with dyes, working with stained glass, smelting, metal welding, or use of vermicides.
A U.S. Environmental Protection Agency team was sent to the house, and a detailed toxic survey of the house and the surrounding grounds was conducted with no excess barium found. Barium levels were checked by a private physician on the wife and 2 minor children. The wife’s barium levels came back undetectable in a blood sample and elevated in a hair sample. One child had a very low detected level in her blood and slightly elevated in her hair, and the other child had a low level in her blood and her hair. Because the circumstances of the wife’s and children’s exposure could not be explained environmentally nor could the veteran’s exposure source be identified, the VA Police Service contacted the Tennessee Bureau of Investigation, and they questioned the veteran and his wife.
Shortly after that the veteran received a paralyzing gunshot wound to the back, and the ensuing investigation resulted in incarceration of his wife for both attempted murder by firearm and serial poisoning after soluble barium-containing materials were found hidden in the house.
Discussion
Human barium poisoning is a rarely reported toxic exposure that results in rapid onset of nausea, vomiting, diarrhea, progressive weakness that may end in respiratory paralysis and death if intubation and mechanical ventilation are not promptly initiated. Although the barium found in radiographic contrast media is highly insoluble, ingested barium carbonate and sulfide are rapidly absorbed into the bloodstream, reaching high levels quickly and altering the conductance of potassium channels. The result is erratic variation in blood potassium and prolonged paralysis unless it is immediately suspected and hemodialysis is initiated. In this case, the suspicion level at the time of intubation was insufficient to justify initiating acute hemodialysis.
Soluble barium is available from a number of open sources. Depilatory powders and several rat poisons list barium sulfide or carbonate, both soluble forms of barium rapidly absorbed through the gastrointestinal mucosa, as a major ingredient. One celebrated 2012 case in a city near Chattanooga, Tennessee, involved allegations of barium carbonate poisoning involving rat poison mixed into coffee creamer, but no charges could be filed because the sample handling precluded definitive linkage. Another deliberate toxic poisoning in Texas was traced to soluble barium introduced into a father’s food by his daughter.
The patient reported here experienced 3 years and 19 admissions with 3 episodes of mechanical intubation before his suspected variant HPP was recognized as actually being due to soluble barium poisoning.
Barium does not appear in usual heavy metal urine and blood screens and as a result may not be asked for if not thought of in the differential diagnosis. Physicians dealing with instances of recurrent suspected HPP that do not fit usual age and clinical characteristics for HPP, lack the single-nucleotide polymorphisms associated with the disease, and are not associated with other conditions causing severe hypokalemia, such as renal tubular acidosis, Bartter, Liddle or Gitelman syndrome or severe diuretic or licorice-induced hypokalemia should have soluble barium poisoning included in the differential diagnosis. Appropriately drawn blood specimens in special metal-free sampling tubes and hair barium levels should be included in the diagnostic workup. If poisoning is suspected, a chain of evidence should be obtained to protect possible future criminal investigation against compromise.
Acknowledgments
The authors thanks Tennessee 2nd District Attorney General Barry P. Staubus, 2nd District Assistant Attorney General Teresa A. Nelson, the VA Police Service, and the Tennessee Bureau of Investigation for their help.
Hypokalemic periodic paralysis (HPP) is a relatively common and potentially life-threating condition that can be either sporadic or recurring and has both inherited and acquired causes.1 Familial HPP, on the other hand, is a rare condition (1:100,000) caused by loss of function mutations leading to the disruption of membrane potential consequently making them inexcitable.2 Appearance of symptoms is typically in the first or second decade of life (60% of cases have onset aged < 16 years) with susceptible individuals experiencing sudden onset of perioral numbness; weakness; centrifugal paralysis, often with nausea; vomiting and diarrhea; and prostration, usually triggered by highcarbohydrate meals and rest following sustained muscle-group use.3
These symptoms are common to all forms of HPP, making the differential diagnosis wide and confusing. Rhabdomyolysis is occasionally associated with many severe hypokalemic episodes.4 Myopathy and permanent muscle weakness have been reported in HPP.5,6 Other reported inciting factors include a drop in serum potassium caused by β-adrenergic bronchodilator treatment.7 Clinical attacks also have been associated with diabetic ketoacidosis and combined hypokalemia and hypophosphatemia.8 Thyrotoxicosis also causes similar muscle action potential changes but only when hyperthyroidism is uncorrected. 9-12 Less commonly, hypothyroidism has been reported to be associated with hypokalemic paralysis.3
Pa Ping, a condition involving hypokalemic paralysis of uncertain etiology, is geographically centered in the Szechuan region of China.13 Cases of Bartter, Liddle, and Gitelman syndromes also have been associated with hypokalemic paralysis.3,14 There is an association with malignant hyperthermia following or during systemic anesthesia. Patients presenting as Guillain-Barré syndrome have been found to have periodic paralysis triggered by hypokalemia from any cause.15 Sjögren syndrome and renal tubular acidosis also are reported to have triggered symptoms of hypokalemic paralysis.16,17
True type 1 HPP is caused by channelopathies resulting from mutations in the calcium channel gene CACN1AS (HypoPP1), which accounts for 70% of the cases, whereas type 2 HPP is cause by sodium channel gene SCN4A (HypoPP2) mutations, which accounts for 10% to 20% of cases.18,19 An association with a voltage-gated potassium channel KCNE3 mutation has been made but is disputed.20,21 Females typically have less severe and less frequent attacks, and attacks lessen or disappear during pregnancy.22
In a small controlled trial, acetazolamide has been reported to have prophylactic benefit, although a more powerful carbonic anhydrase inhibitor, dichlorophenamide, was reported to be effective in a study after acetazolamide had become ineffective.23,24 These treatments would not be expected to be of clinical use in hypokalemia due to barium poisoning.
Barium poisoning has been reported as a result of accidental contamination of foodstuffs with soluble barium.25 Onset of symptoms is rapid, with nausea, vomiting, diarrhea, and malaise followed rapidly by weakness, which can include the muscles of respiration. This littleconsidered but rapidly lethal poisoning event can be accidental as a result of environmental exposure due to unintentional ingestion of the toxin or deliberate criminal poisoning as in this case. Because deliberate poisoning rarely crosses the mind of the clinician, awareness of the potential similarity of barium poisoning to other forms of HPP and even familial HPP is important.
Case Presentation
A male veteran aged 45 years when treated by the authors was well until moving into a new rural home when he began to experience acute episodes of variable perioral numbness, diarrhea, paresthesias, abdominal cramping, and weakness, which ranged from mild, self-terminating extremity weakness to 3 episodes of respiratory failure that required intubation and mechanical ventilation.
All episodes were accompanied by hypokalemia in the range of 2 to 3 mEq/L, but levels varied erratically during admissions from severe hypokalemia to normo- and hyperkalemia. Over 3 years, the patient was admitted to the hospital 19 times, underwent extensive workup, and was referred to endocrinology services at Duke University, Vanderbilt University, and the Cleveland Clinic. Diagnostic efforts centered on establishing whether he had a latepresenting variant of familial HPP.
Genetic evaluations could not identify known single-nucleotide polymorphisms associated with that condition. The consensus was that he had a potassium leak somewhere between his kidneys and bladder. Recommended management was a high baseline oral potassium supplementation and spironolactone. He had a brief period of improvement after moving to a different house, but the episodes returned once he moved back to his old house despite adherence to recommended treatment. In December 2012, he experienced his worst episode, with potassium 1.8 mEq/L on admission, resulting in admission to the intensive care unit (ICU).
Following a precipitous clinical decline, the patient was intubated and mechanically ventilated. Nephrology was consulted and given the recurrent life-threatening pattern, an intensive chart review was undertaken. It was noted that a urine arsenic level that had been normal several admissions previously at 18 μg/L was elevated during a subsequent admission at 59 μg/L, and several weeks later during a later admission the level had fallen to 15 μg/L. Urine lead was undetectable on 3 occasions, and urine mercury was within normal limits.
Arsenic toxicity did not match the patient’s clinical syndrome, but the pattern seemed to be consistent with the possibility of unexplained toxic exposure and subsequent clearance. Therefore, an intensive literature search for syndromes of environmental exposure or poisoning resembling HPP was undertaken. The search revealed several references in the literature to paralysis similar to HPP that involved ingestion of hair-removing soap and rat poison containing barium sulfide and carbonate. References also pointed to the similarity of the symptoms to Guillain-Barre syndrome.
As a result of that literature search, a blood barium level was collected in the ICU that revealed 14,550 ng/mL. A scalp hair sample showed 6.1 μg barium per gram of hair (reference, 0.53 μg/g to 2 μg/g). Neither the patient nor his wife reported being involved in painting, ceramic work, decorating glassware or fabric with dyes, working with stained glass, smelting, metal welding, or use of vermicides.
A U.S. Environmental Protection Agency team was sent to the house, and a detailed toxic survey of the house and the surrounding grounds was conducted with no excess barium found. Barium levels were checked by a private physician on the wife and 2 minor children. The wife’s barium levels came back undetectable in a blood sample and elevated in a hair sample. One child had a very low detected level in her blood and slightly elevated in her hair, and the other child had a low level in her blood and her hair. Because the circumstances of the wife’s and children’s exposure could not be explained environmentally nor could the veteran’s exposure source be identified, the VA Police Service contacted the Tennessee Bureau of Investigation, and they questioned the veteran and his wife.
Shortly after that the veteran received a paralyzing gunshot wound to the back, and the ensuing investigation resulted in incarceration of his wife for both attempted murder by firearm and serial poisoning after soluble barium-containing materials were found hidden in the house.
Discussion
Human barium poisoning is a rarely reported toxic exposure that results in rapid onset of nausea, vomiting, diarrhea, progressive weakness that may end in respiratory paralysis and death if intubation and mechanical ventilation are not promptly initiated. Although the barium found in radiographic contrast media is highly insoluble, ingested barium carbonate and sulfide are rapidly absorbed into the bloodstream, reaching high levels quickly and altering the conductance of potassium channels. The result is erratic variation in blood potassium and prolonged paralysis unless it is immediately suspected and hemodialysis is initiated. In this case, the suspicion level at the time of intubation was insufficient to justify initiating acute hemodialysis.
Soluble barium is available from a number of open sources. Depilatory powders and several rat poisons list barium sulfide or carbonate, both soluble forms of barium rapidly absorbed through the gastrointestinal mucosa, as a major ingredient. One celebrated 2012 case in a city near Chattanooga, Tennessee, involved allegations of barium carbonate poisoning involving rat poison mixed into coffee creamer, but no charges could be filed because the sample handling precluded definitive linkage. Another deliberate toxic poisoning in Texas was traced to soluble barium introduced into a father’s food by his daughter.
The patient reported here experienced 3 years and 19 admissions with 3 episodes of mechanical intubation before his suspected variant HPP was recognized as actually being due to soluble barium poisoning.
Barium does not appear in usual heavy metal urine and blood screens and as a result may not be asked for if not thought of in the differential diagnosis. Physicians dealing with instances of recurrent suspected HPP that do not fit usual age and clinical characteristics for HPP, lack the single-nucleotide polymorphisms associated with the disease, and are not associated with other conditions causing severe hypokalemia, such as renal tubular acidosis, Bartter, Liddle or Gitelman syndrome or severe diuretic or licorice-induced hypokalemia should have soluble barium poisoning included in the differential diagnosis. Appropriately drawn blood specimens in special metal-free sampling tubes and hair barium levels should be included in the diagnostic workup. If poisoning is suspected, a chain of evidence should be obtained to protect possible future criminal investigation against compromise.
Acknowledgments
The authors thanks Tennessee 2nd District Attorney General Barry P. Staubus, 2nd District Assistant Attorney General Teresa A. Nelson, the VA Police Service, and the Tennessee Bureau of Investigation for their help.
1. Ahlawat SK, Sachdev A. Hypokalaemic paralysis. Postgrad Med J. 1999;75(882):193-197.
2. Fontaine B. Periodic paralysis. Adv Genet.2008;63:3-23.
3. Kayal AK, Goswami M, Das M, Jain R. Clinical and biochemical spectrum of hypokalemic paralysis in North: East India. Ann Indian Acad Neurol.2013;16(2):211-217.
4. Johnson CH, VanTassell VJ. Acute barium poisoning with respiratory failure and rhabdomyolysis. Ann Emerg Med. 1991;20(10):1138-1142.
5. Gold R, Reichmann H. Muscle pathology correlates with permanent weakness in hypokalemic periodic paralysis: a case report. Acta Neuropathol. 1992;84(2):202-206.
6. Links TP, Zwarts MJ, Wilmink JT, Molenaar WM, Oosterhuis HJ. Permanent muscle weakness in familial hypokalaemic periodic paralysis. Clinical, radiological and pathological aspects. Brain. 1990;113(pt 6):1873-1889.
7. Tucker C, Villanueva L. Acute hypokalemic periodic paralysis possibly precipitated by albuterol. Am J Health Syst Pharm. 2013;70(18):1588-1591.
8. Liu PY, Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc. 2004;67(7):355-359.
9. Sigue G, Gamble L, Pelitere M, et al. From profound hypokalemia to life-threatening hyperkalemia: a case of barium sulfide poisoning. Arch Intern Med. 2000;160(4):548-541.
10. Kuntzer T, Flocard F, Vial C, et al. Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve. 2000;23(7):1089-1094.
11. Tengan CH, Antunes AC, Gabbai AA, Manzano GM. The exercise test as a monitor of disease status in hypokalaemic periodic paralysis. J Neurol Neurosurg Psychiatry. 2004;75(3):497-499.
12. McManis PG, Lambert EH, Daube JR. The exercise test in periodic paralysis. Muscle Nerve. 1986;9(8):704-710.
13. Huang K-W. Pa ping (transient paralysis simulating family periodic paralysis). Chin Med J. 1943;61(4):305-312.
14. Ng HY, Lin SH, Hsu CY, Tsai YZ, Chen HC, Lee CT. Hypokalemic paralysis due to Gitelman syndrome:a family study. Neurology. 2006;67(6):1080-1082.
15. Mohta M, Kalra B, Shukla R, Sethi AK. An unusual presentation of hypokalemia. J Anesth Clin Res. 2014;5(3):389.
16. Fujimoto T, Shiiki H, Takahi Y, Dohi K. Primary Sjögren’s Syndrome presenting as hypokalaemic periodic paralysis and respiratory arrest. Clin Rheumatol. 2001;20(5):365-368.
17. Chang YC, Huang CC, Chiou YY, Yu CY. Renal tubular acidosis complicated with hypokalemic periodic paralysis. Pediatr Neurol. 1995;13(1):52-54.
18. Lehmann-Horn F, Jurkat-Rott K, Rüdel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep. 2002;2(1):61-69.
19. Venance SL, Cannon SC, Fialho D, et al; CINCH investigators. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129(pt 1):8-17.
20. Sharma C, Nath K, Parekh J. Reversible electrophysiological abnormalities in hypokalemic paralysis: case report of two cases. Ann Indian Acad Neurol. 2014;17(1):100-102.
21. Sternberg D, Tabti N, Fournier E, Hainque B, Fontaine B. Lack of association of the potassium channel-associated peptide MiRP2-R83H variant with periodic paralysis. Neurology. 2003;61(6):857-859.
22. Ke Q, Luo B, Qi M, Du Y, Wu W. Gender differences in penetrance and phenotype in hypokalemic periodic paralysis. Muscle Nerve. 2013;47(1):41-45.
23. Griggs RC, Engel WK, Resnick JS. Acetazolamide treatment of hypokalemic periodic paralysis. Prevention of attacks and improvement of persistent weakness. Ann Intern Med. 1970;73(1):39-48.
24. Dalakas MC, Engel WK. Treatment of “permanent” muscle weakness in familial hypokalemic periodic paralysis. Muscle Nerve. 1983;6(3):182-186.
25. Ghose A, Sayeed AA, Hossain A, Rahman R, Faiz A, Haque G. Mass barium carbonate poisoning with fatal outcome, lessons learned: a case series. Cases J. 2009;2:9327.
1. Ahlawat SK, Sachdev A. Hypokalaemic paralysis. Postgrad Med J. 1999;75(882):193-197.
2. Fontaine B. Periodic paralysis. Adv Genet.2008;63:3-23.
3. Kayal AK, Goswami M, Das M, Jain R. Clinical and biochemical spectrum of hypokalemic paralysis in North: East India. Ann Indian Acad Neurol.2013;16(2):211-217.
4. Johnson CH, VanTassell VJ. Acute barium poisoning with respiratory failure and rhabdomyolysis. Ann Emerg Med. 1991;20(10):1138-1142.
5. Gold R, Reichmann H. Muscle pathology correlates with permanent weakness in hypokalemic periodic paralysis: a case report. Acta Neuropathol. 1992;84(2):202-206.
6. Links TP, Zwarts MJ, Wilmink JT, Molenaar WM, Oosterhuis HJ. Permanent muscle weakness in familial hypokalaemic periodic paralysis. Clinical, radiological and pathological aspects. Brain. 1990;113(pt 6):1873-1889.
7. Tucker C, Villanueva L. Acute hypokalemic periodic paralysis possibly precipitated by albuterol. Am J Health Syst Pharm. 2013;70(18):1588-1591.
8. Liu PY, Jeng CY. Severe hypophosphatemia in a patient with diabetic ketoacidosis and acute respiratory failure. J Chin Med Assoc. 2004;67(7):355-359.
9. Sigue G, Gamble L, Pelitere M, et al. From profound hypokalemia to life-threatening hyperkalemia: a case of barium sulfide poisoning. Arch Intern Med. 2000;160(4):548-541.
10. Kuntzer T, Flocard F, Vial C, et al. Exercise test in muscle channelopathies and other muscle disorders. Muscle Nerve. 2000;23(7):1089-1094.
11. Tengan CH, Antunes AC, Gabbai AA, Manzano GM. The exercise test as a monitor of disease status in hypokalaemic periodic paralysis. J Neurol Neurosurg Psychiatry. 2004;75(3):497-499.
12. McManis PG, Lambert EH, Daube JR. The exercise test in periodic paralysis. Muscle Nerve. 1986;9(8):704-710.
13. Huang K-W. Pa ping (transient paralysis simulating family periodic paralysis). Chin Med J. 1943;61(4):305-312.
14. Ng HY, Lin SH, Hsu CY, Tsai YZ, Chen HC, Lee CT. Hypokalemic paralysis due to Gitelman syndrome:a family study. Neurology. 2006;67(6):1080-1082.
15. Mohta M, Kalra B, Shukla R, Sethi AK. An unusual presentation of hypokalemia. J Anesth Clin Res. 2014;5(3):389.
16. Fujimoto T, Shiiki H, Takahi Y, Dohi K. Primary Sjögren’s Syndrome presenting as hypokalaemic periodic paralysis and respiratory arrest. Clin Rheumatol. 2001;20(5):365-368.
17. Chang YC, Huang CC, Chiou YY, Yu CY. Renal tubular acidosis complicated with hypokalemic periodic paralysis. Pediatr Neurol. 1995;13(1):52-54.
18. Lehmann-Horn F, Jurkat-Rott K, Rüdel R. Periodic paralysis: understanding channelopathies. Curr Neurol Neurosci Rep. 2002;2(1):61-69.
19. Venance SL, Cannon SC, Fialho D, et al; CINCH investigators. The primary periodic paralyses: diagnosis, pathogenesis and treatment. Brain. 2006;129(pt 1):8-17.
20. Sharma C, Nath K, Parekh J. Reversible electrophysiological abnormalities in hypokalemic paralysis: case report of two cases. Ann Indian Acad Neurol. 2014;17(1):100-102.
21. Sternberg D, Tabti N, Fournier E, Hainque B, Fontaine B. Lack of association of the potassium channel-associated peptide MiRP2-R83H variant with periodic paralysis. Neurology. 2003;61(6):857-859.
22. Ke Q, Luo B, Qi M, Du Y, Wu W. Gender differences in penetrance and phenotype in hypokalemic periodic paralysis. Muscle Nerve. 2013;47(1):41-45.
23. Griggs RC, Engel WK, Resnick JS. Acetazolamide treatment of hypokalemic periodic paralysis. Prevention of attacks and improvement of persistent weakness. Ann Intern Med. 1970;73(1):39-48.
24. Dalakas MC, Engel WK. Treatment of “permanent” muscle weakness in familial hypokalemic periodic paralysis. Muscle Nerve. 1983;6(3):182-186.
25. Ghose A, Sayeed AA, Hossain A, Rahman R, Faiz A, Haque G. Mass barium carbonate poisoning with fatal outcome, lessons learned: a case series. Cases J. 2009;2:9327.
Cerebral Venous Thrombosis
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
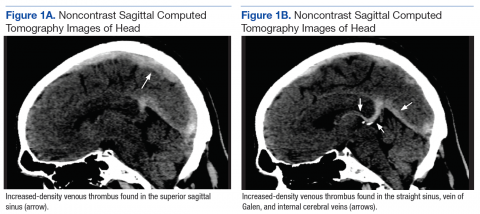
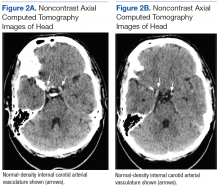
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
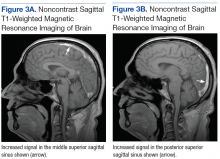
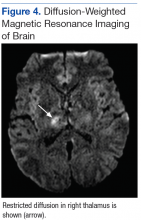
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
Cerebral venous thrombosis (CVT) is a rare cerebrovascular disease that affects about 5 in 1 million people each year and accounts for 0.5% of all strokes.1 Previously it was thought to be caused most commonly by infections (eg, mastoiditis, otitis, meningitis) affecting the superior sagittal sinus and often resulting in focal neurologic deficits, seizures, coma, and death. Although local and systemic infections are still prominent risk factors in its development, CVT is now primarily recognized as a nonseptic disease with a wide spectrum of clinical presentations.
Cerebral venous thrombosis causes reduced outflow of blood and cerebrospinal fluid, which in half of affected patients leads to significant venous infarct. As opposed to arterial infarctions, CVT mainly affects children and young adults; it is an important cause of stroke in the younger population.2 There is significant overlap of the many risk factors for CVT and those for venous thromboembolism (VTE): cancer, obesity, genetic thrombophilia, trauma, infection, and prior neurosurgery. However, the most common acquired risk factors for CVT are oral contraceptive use and pregnancy, which explains why CVT is 3 times more likely to occur in young and middle-aged women.3
Cerebral venous thrombosis was first recognized by a French physician in the 19th century, a time when the condition was diagnosed at autopsy, which typically showed hemorrhagic lesions at the thrombus site.4 For many years heparin was contraindicated in the treatment of CVT, and only within the past 25 years did advances in neuroimaging allow for earlier diagnosis and change perspectives on the management of this disease.
Cerebral venous thrombosis occurs from formation of a thrombus within the cerebral venous sinuses, leading to elevated intracranial pressure and eventually ischemia or intracranial hemorrhage. Improved imaging techniques, notably magnetic resonance imaging (MRI) and computed tomography (CT) venography, allow physicians to identify thrombus formation earlier and begin anticoagulation therapy with heparin before infarction. A meta-analysis of studies found that heparin was safer and more efficacious in treating CVT compared with placebo.5 Furthermore, several small randomized trials found treatment with unfractionated heparin (UFH) or low-molecularweight heparin (LMWH) was not associated with higher risk of hemorrhagic stroke in these patients.6-8
Despite improvements in imaging modalities, in many cases diagnosis is delayed, as most patients with CVT have a wide spectrum of presentations with nonspecific symptoms, such as headache, seizure, focal sensorimotor deficits, and papilledema.9 Clinical presentations of CVT depend on a variety of factors, including age, time of onset, CVT location, and presence of parenchymal lesions. Isolated headache is the most common initial symptom, and in many cases is the only presenting manifestation of CVT.1 Encephalopathy with multifocal signs, delirium, or dysfunction in executive functions most commonly occurs in elderly populations.
Cavernous sinus thrombosis most commonly produces generalized headaches, orbital pain, proptosis, and diplopia, whereas cortical vein thrombosis often produces seizures and focal sensorimotor deficits. Aphasia may be present in patients with isolated left transverse sinus thrombosis. In the presence of deep cerebral venous occlusion, patients can present in coma or with severe cognitive deficits and widespread paresis.10 Thrombosis of different veins and sinuses results in a wide spectrum of diverse clinical pictures, posing a diagnostic challenge and affecting clinical outcomes.
Given the variable and often nonspecific clinical presentations of these cases, unenhanced CT typically is the first imaging study ordered. According to the literature, noncontrast CT is not sensitive (25%-56%) in detecting CVT, and findings are normal in up to 26% of patients, rarely providing a specific diagnosis.11 Furthermore, visualization on MRI can be difficult during the acute phase of CVT, as the thrombus initially appears isointense on T1-weighted images and gradually becomes hyperintense over the course of the disease process.12 These difficulties with the usual first-choice imaging examinations often result in a delay in diagnosing CVT. However, several points on close examination of these imaging studies may help physicians establish a high index of clinical suspicion and order the appropriate follow-up studies for CVT.
The authors report the case of a patient who presented with a 1-week history of confusion, headaches, and dizziness. His nonspecific presentation along with the absence of obvious signs of CVT on imaging prolonged his diagnosis and the initiation of an appropriate treatment plan.
Case Report
A 57-year-old white air-conditioning mechanic presented to the emergency department (ED) with a 1-week history of gradual-onset confusion, severe headaches, dizziness, light-headedness, poor memory, and increased sleep. Two days earlier, he presented with similar symptoms to an outside facility, where he was admitted and underwent a workup for stroke, hemorrhage, and cardiac abnormalities—including noncontrast CT of the head. With nothing of clinical significance found, the patient was discharged and was advised to follow up on an outpatient basis.
Persisting symptoms brought the patient to the ED 1 day later, and he was admitted. He described severe, progressive, generalized headaches that were more severe when he was lying down at night and waking in the morning. He did not report that the headaches worsened with coughing or straining, and he reported no history of trauma, neck stiffness, vision change, seizures, and migraines. His medical history was significant for hypertension, dyslipidemia, and in 2011, an unprovoked deep vein thrombosis (DVT) in the right leg. He reported no history of tobacco, alcohol, or illicit drug use. He had served in the U.S. Navy, working in electronics, intelligence, data systems, and satellite communications.
On initial physical examination, the patient was afebrile and lethargic, and his blood pressure was mildly elevated (144/83 mm Hg). Cardiopulmonary examination was normal. Neurologic examination revealed no severe focal deficits, and cranial nerves II to XII were intact. Funduscopic examination was normal, with no papilledema noted. Motor strength was 5/5 bilaterally in the deltoids, biceps, triceps, radial and ulnar wrist extensors, iliopsoas, quadriceps, hamstrings, tibialis anterior and posterior, fibulares, and gastrocnemius. Pinprick sensation and light-touch sensation were decreased within the lateral bicipital region of the left upper extremity. Pinprick sensation was intact bilaterally in 1-inch increments at all other distributions along the medial and lateral aspects of the upper and lower extremities. Muscle tone and bulk were normal in all extremities. Reflexes were 2+ bilaterally in the biceps, brachioradialis, triceps, quadriceps, and Achilles. The Babinski sign was absent bilaterally, the finger-tonose and heel-to-shin tests were normal, the Romberg sign was absent, and there was no evidence of pronator drift. Laboratory test results were normal except for slightly elevated hemoglobin (17.5 g/dL) and D-dimer (588 ng/mL) levels.
Although noncontrast CT of the head initially showed no acute intracranial abnormalities, retrospective close comparison with the arterial system revealed slightly increased attenuation in the superior sagittal sinus, straight sinus, vein of Galen, and internal cerebral veins (Figures 1A and 1B) relative to the arterial carotid anterior circulation (Figures 2A and 2B).
Subsequent brain MRI without contrast showed a hyperintense T1 signal involving the superior sagittal sinus (Figures 3A and 3B), extending into the straight sinus and the vein of Galen. Magnetic resonance imaging with contrast demonstrated a prominent filling defect primarily in the superior sagittal sinus, in the right transverse sinus, and in the vein of Galen. Diffusion-weighted brain MRI sequence showed restricted diffusion localized to the right thalamic area (Figure 4) and no evidence of hemorrhage.
Treatment
International guidelines recommend using heparin to achieve rapid anticoagulation, stop the thrombotic process, and prevent extension of the thrombus.13 Theoretically, more rapid recanalization may have been achieved by performing endovascular thrombectomy in the present case. However, severe bleeding complications, combined with higher cost and the limited availability of clinicians experienced in treating this rare disease, convince physicians to rely on heparin as first-line treatment for CVT.14 A small randomized clinical trial found LMWH safer and more efficacious than UFH in treating CVT.15 After stabilization, oral anticoagulation therapy is used to maintain an international normalized ratio (INR) between 2.0 and 3.0 for at least 3 months.14
Given these findings, the patient was initially treated with LMWH. Eventually he was switched to oral warfarin and showed signs of clinical improvement. A hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation, and he was discharged and instructed to continue the oral warfarin therapy.
On follow-up, the hematology and neurology team initiated indefinite treatment with warfarin for his genetic hypercoagulability state. Monitoring of the dose of anticoagulation therapy was started to maintain INR between 2.0 and 3.0. The patient began coming to the office for INR monitoring every 2 to 3 weeks, and his most recent INR, in May 2017, was 2.66. He is taking 7.5 mg of warfarin on Wednesdays and Sundays and 5 mg on all other days and currently does not report any progressive neurologic deficits.
Discussion
The clinical findings of CVT and the hypercoagulability state workup revealed that the patient was heterozygous for the prothrombin G20210A mutation. Prothrombin is the precursor to thrombin, which is a key regulator of the clotting cascade and a promoter of coagulation. Carriers of the mutation have elevated levels of blood plasma prothrombin and have been associated with a 4 times higher risk for VTE.16
Several large studies and systematic reviews have confirmed that the prothrombin G20210A mutation is associated with higher rates of VTE, leading to an increased risk for DVT of the leg or pulmonary embolism.17-19 More specifically, a metaanalysis of 15 case–control studies found strong associations between the mutation and CVT.20 Despite this significant association, studies are inconclusive about whether heterozygosity for the mutation is associated with increased rates of recurrent CVT or other VTE in the absence of other risk factors, such as oral contraceptive use, trauma, malignancy, and infection.21-23 Therefore, the optimal duration of anticoagulation therapy for CVT is not well established in patients with the mutation. However, the present patient was started on indefinite anticoagulation therapy because the underlying etiology of the CVT was not reversible or transient, and this CVT was his second episode of VTE, following a 2011 DVT in the right leg.
The case discussed here illustrates the clinical presentation and diagnostic complexities of CVT. Two days before coming to the ED, the patient presented to an outside facility and underwent a workup for nonspecific symptoms (eg, confusion, headaches). Due to the nonspecific presentation associated with CVT, a detailed history is imperative to distinguish symptoms suggesting increased intracranial pressure, such as headaches worse when lying down or present in the morning, with a high clinical suspicion of CVT. The ability to attain these specific details leads clinicians toward obtaining the necessary imaging studies for potential CVT patients, and may prevent delay in diagnosis and treatment. The thrombus in CVT initially consists of deoxyhemoglobin and appears on MRI as an isointense signal on T1-weighted images and a hypointense signal on T2-weighted images; over subsequent days, the thrombus changes to methemoglobin and appears as a slightly hyperintense signal on both T1- and T2-weighted images.24
During this phase, there are some false negatives, as the thrombus can be mistaken for imaging artifacts, hematocrit elevations, or low flow of normal venous blood. Given the clinical findings and imaging studies, it is essential to distinguish CVT from other benign etiologies. Earlier diagnosis and initiation of anticoagulation therapy may have precluded the small amount of localized ischemic changes in this patient’s right thalamus, thus preventing the mild sensory loss in the left upper extremity. With the variable and nonspecific clinical presentations and the difficulties in identifying CVT with first-line imaging, progression of thrombus formation may lead to severe focal neurologic deficits, coma, or death.
Using CT imaging studies to compare the blood in the draining cerebral sinuses with the blood in the arterial system can help distinguish CVT from other etiologies of hyperdense abnormalities, such as increased hematocrit or decreased flow. Retrospective close examination of the present patient’s noncontrast CT images of the head and brain revealed slight hyperdensity in the cerebral sinuses compared with the arterial blood, suggesting the presence of thrombus formation in the cerebral veins. As CT is often the first study used to evaluate the nonspecific clinical presentations of these patients, identifying subtle signaldensity differences between the arterial and venous systems could guide physicians in identifying CVT earlier.
The authors reiterate the importance of meticulous imaging interpretation in light of the entire clinical picture: In these patients, it is imperative to have a high index of clinical suspicion for CVT in order to prevent more serious complications, such as ischemic or hemorrhagic stroke.
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
1. Bousser MG, Ferro JM. Cerebral venous thrombosis: an update. Lancet Neurol. 2007;6(2):162-170.
2. Coutinho JM. Cerebral venous thrombosis. J Thromb Haemost. 2015;13(suppl 1):S238-S244.
3. Coutinho JM, Ferro JM, Canhão P, et al. Cerebral venous and sinus thrombosis in women. Stroke. 2009;40(7):2356-2361.
4. Zuurbier SM, Coutinho JM. Cerebral venous thrombosis. Adv Exp Med Biol. 2017;906:183-193.
5. Einhäupl KM, Villringer A, Meister W, et al. Heparin treatment in sinus venous thrombosis. Lancet. 1991;338(8767):597-600.
6. de Bruijn SF, Stam J. Randomized, placebocontrolled trial of anticoagulant treatment with lowmolecular-weight heparin for cerebral sinus thrombosis. Stroke. 1999;30(3):484-488.
7. Nagaraja D, Haridas T, Taly AB, Veerendrakumar M, SubbuKrishna DK. Puerperal cerebral venous thrombosis: therapeutic benefit of low dose heparin. Neurol India. 1999;47(1):43-46.
8. Coutinho JM, de Bruijn SF, deVeber G, Stam J. Anticoagulation for cerebral venous sinus thrombosis. Stroke. 2012;43(4):e41-e42.
9. Sassi SB, Touati N, Baccouche H, Drissi C, Romdhane NB, Hentati F. Cerebral venous thrombosis. Clin Appl Thromb Hemost. 2016:1076029616665168. [Epub ahead of print.]
10. Ferro JM, Canhão P. Cerebral venous sinus thrombosis: update on diagnosis and management. Curr Cardiol Rep. 2014;16(9):523.
11. Albright KC, Freeman WD, Kruse BT. Cerebral venous thrombosis. J Emerg Med. 2010;38(2):238-239.
12. Lafitte F, Boukobza M, Guichard JP, et al. MRI and MRA for diagnosis and follow-up of cerebral venous thrombosis (CVT). Clin Radiol. 1997;52(9):672-679.
13. Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42(4):1158-1192.
14. Coutinho JM, Middeldorp S, Stam J. Advances in the treatment of cerebral venous thrombosis. Curr Treat Options Neurol. 2014;16(7):299.
15. Coutinho JM, Ferro JM, Canhão P, Barinagarrementeria F, Bousser MG, Stam J; ISCVT Investigators. Unfractionated or low-molecular weight heparin for the treatment of cerebral venous thrombosis. Stroke. 2010;41(11):2575-2580.
16. Rosendaal FR. Venous thrombosis: the role of genes, environment, and behavior. Hematology Am Soc Hematol Educ Program. 2005:1-12.
17. Dentali F, Crowther M, Ageno W. Thrombophilic abnormalities, oral contraceptives, and risk of cerebral vein thrombosis: a meta-analysis. Blood.2006;107(7):2766-2773.
18. Salomon O, Steinberg DM, Zivelin A, et al. Single and combined prothrombotic factors in patients with idiopathic venous thromboembolism: prevalence and risk assessment. Arterioscler Thromb Vasc Biol. 1999;19(3):511-518.
19. Emmerich J, Rosendaal FR, Cattaneo M, et al. Combined effect of factor V Leiden and prothrombin 20210A on the risk of venous thromboembolism— pooled analysis of 8 case–control studies including 2310 cases and 3204 controls. Study Group for Pooled-Analysis in Venous Thromboembolism. Thromb Haemost. 2001;86(3):809-816.
20. Lauw MN, Barco S, Coutinho JM, Middeldorp S. Cerebral venous thrombosis and thrombophilia: a systematic review and meta-analysis. Semin Thromb Hemost. 2013;39(8):913-927.
21. Dentali F, Poli D, Scoditti U, et al. Long-term outcomes of patients with cerebral vein thrombosis: a multicenter study. J Thromb Haemost. 2012;10(7):1297-1302.
22. Martinelli I, Bucciarelli P, Passamonti SM, Battaglioli T, Previtali E, Mannucci PM. Long-term evaluation of the risk of recurrence after cerebral sinus-venous thrombosis. Circulation. 2010;121(25):2740-2746.
23. Gosk-Bierska I, Wysokinski W, Brown RD Jr, et al. Cerebral venous sinus thrombosis: incidence of venous thrombosis recurrence and survival. Neurology. 2006;67(5):814-819.
24. Galidie G, Le Gall R, Cordoliani YS, Pharaboz C, Le Marec E, Cosnard G. Thrombosis of the cerebral veins. X-ray computed tomography and MRI imaging. 11 cases [in French]. J Radiol. 1992;73(3):175-190
Case Studies in Toxicology: An Unlikely Cause of Paralysis
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
Case
An Asian man in his third decade, with a medical history of hypertension and hyperlipidemia, and who had recently been involved in a motor vehicle collision (MVC), presented to the ED with a chief complaint of severe bilateral upper and lower extremity weakness. The patient noted that the weakness had begun the previous evening and became progressively worse throughout the night, to the point that he was unable to move any of his extremities on the morning of presentation.
Upon arrival at the ED, the patient was awake, alert, and oriented to self, time, and place; he also spoke in full sentences without distress. He denied fever, chills, difficulty breathing, or preceding viral illness. The patient stated that he was not taking any medications and denied a history of alcohol, tobacco, or drug abuse.
Initial vital signs at presentation were: blood pressure, 141/50 mm Hg; heart rate, 90 beats/min; respiratory rate, 16 breaths/min; and temperature, 97.4°F. Oxygen saturation was 100% on room air. On physical examination, the patient was in no acute distress and had a normal mental status. His pupils were normally reactive and his other cranial nerves were normal. Muscle strength in the upper and lower extremities was 1/5 with 1+ reflexes bilaterally, and there was no sensory deficit. The patient was placed on continuous cardiac monitoring with pulse oximetry.
What is the differential diagnosis for acute extremity weakness or paralysis?
The differential diagnosis for acute symmetrical extremity weakness or paralysis is broad and includes conditions of neurological, inflammatory, and toxic/metabolic etiologies.1 Neurological diagnoses to consider include acute stroke, specifically of the anterior cerebral or middle cerebral artery territories; Guillain-Barré syndrome; myasthenia gravis; spinal cord compression; and tick paralysis. Acute ischemic or hemorrhagic stroke most frequently presents with unilateral upper or lower extremity weakness accompanied by garbled speech and sensory deficits. Patients who have suffered a brainstem or cerebellar stroke commonly present with alterations of consciousness, visual changes, and ataxia. Posterior circulation strokes are also characterized by crossed neurological deficits, such as motor deficits on one side of the body and sensory deficits on the other.
Spinal Cord Pathology. Signs and symptoms of spinal cord compression or inflammation vary widely depending on the level affected. Motor and sensory findings of spinal cord pathology include muscle weakness, spasticity, hyper- or hyporeflexia, and a discrete level below which sensation is absent or reduced.
Guillain-Barré Syndrome. Patients who have Guillain-Barré syndrome (a disease of the myelin sheaths of the peripheral nerves) often present with complaints of numbness or paresthesias in the extremities.2 The condition is characterized by progressive symmetric muscle weakness accompanied by absent or depressed deep tendon reflexes and is typically associated with a recent exposure to an infectious agent such as a viral upper respiratory infection, bacterial infection, or vaccine.
Myasthenia Gravis. Myasthenia gravis is a disease of the neuromuscular junction. It presents with weakness in any muscle group, and the muscles are easily fatigued by repetitive use.
Toxic Exposures. Toxins, such as botulinum, ixovotoxin, nicotine, succinylcholine, and tetrodotoxin, are prominent, though less common, causes of muscular weakness or paralysis. Botulinum toxin acts at the neuromuscular junction. Patients with botulism typically present with a gastrointestinal prodrome of nausea, vomiting, and diarrhea followed by cranial nerve dysfunction and descending muscle weakness.3
Tetrodotoxin, nicotine, and curare-like paralytics act at the motor end plate of the neuromuscular junction to produce neuromuscular blockade with subsequent muscular weakness or paralysis. Similarly, ixovotoxin, the toxin responsible for tick paralysis, causes ascending flaccid paralysis by decreasing the release of acetylcholine at the neuromuscular junction.3
Metabolic and Endocrine Disorders. Conditions such as hypokalemia, hypomagnesemia, and periodic paralysis can also present with neurological complaints such as generalized weakness and paresthesias. Of note, it is important to differentiate true neuromuscular weakness from weakness secondary to limited effort.
Case Continuation
Because of the patient’s history of an MVC, cervical cord compression was considered concerning enough to require exclusion through magnetic resonance imaging (MRI) of the cervical spine. However, upon arrival at the MRI suite, the patient became severely tachypneic and tachycardic, and was unable to tolerate lying flat. He was intubated for impending respiratory failure. Laboratory results from blood drawn prior to transport to MRI were reported immediately after the resuscitation and were notable for the following: potassium, <1.5 mEq/L; bicarbonate, 20 mEq/L; creatine kinase, 889 U/L; ethanol, not detected.
What is hypokalemic periodic paralysis?
Hypokalemic periodic paralysis (HypoKPP) is a syndrome of episodic muscle weakness with concomitant hypokalemia. Familial forms of HypoKPP have been attributed to mutations in genes coding for either calcium or sodium channels.
The nonfamilial form of HypoKPP is attributed to hyperthyroidism and is most often seen in Asian men in the second and third decades of life. The disorder is characterized by acute onset hypokalemia and extremity paralysis with simultaneous hyperthyroid state. It is believed that hypokalemia occurs as a result of intracellular shift of potassium from thyroid-induced hormone sensitization of the Na+/K+-ATPase rather than a depletion of total body potassium. Acute episodes of paralysis are triggered by high-carbohydrate meals, alcohol consumption, emotional stress, and infection. Paralysis can last from 3 to 96 hours and is accompanied by decreased or absent deep tendon reflexes with normal sensation and mental status.
In the nonfamilial form of HypoKPP, signs of thyrotoxicosis are often present and include tachycardia, moist skin, and hyperthermia, but it may be difficult to specifically recognize this etiology given the patient’s grave clinical condition.4 Similar to many significant metabolic and electrolyte disturbances, complications of HypoKPP include dysrhythmia, respiratory failure, and sometimes death.5
How should HypoKPP be managed in the ED?
Management of HypoKPP begins with careful assessment of the patient’s airway, breathing, and circulation. Once the patient is stabilized, management of consequential effects of hypokalemia, such as respiratory distress and muscular paralysis, should focus on correcting the electrolyte and endocrine derangements.
Propranolol. If the patient exhibits signs of thyrotoxicosis, initial treatment includes propranolol, a nonselective beta-blocker, which both prevents the intracellular shift of potassium and assists in correcting the underlying hyperthyroid and hypermetabolic state. Although there is no standard propranolol dosing protocol for HypoKPP, some authors suggest that an aggressive dose of 2 mg intravenously (IV) every 10 minutes can shorten the patient’s episode of paralysis to 6 hours.6
Potassium Chloride. Administration of potassium chloride to raise the serum potassium to life-sustaining concentrations should be done cautiously through IV infusion of standard doses.7 In correcting hypokalemia with potassium, care should be taken to avoid overcorrection, which may subsequently result in rebound hyperkalemia as the total body potassium redistributes. Lower doses of potassium (ie, <50 mEq per dose), are preferred to achieve adequate repletion while avoiding rebound hyperkalemia.8
Case Conclusion
The results of thyroid studies that had been added on to the original set of laboratory studies revealed profound hyperthyroidism, with an essentially absent concentration of thyroid-stimulating hormone.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
1. Morchi RS. Weakness. In: Rosen P, ed. Rosen’s Emergency Medicine. 8th ed. Philadelphia, PA: Elsevier; 2014:124-128.
2. McGillicuddy DC, Walker O, Shapiro NI, Edlow JA. Guillain-Barré syndrome in the emergency department. Ann Emerg Med. 2006;47(4):390-393. doi:10.1016/j.annemergmed.2005.05.008.
3. Rao RB. Neurological principles. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR, eds. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw-Hill; 2015:315-323.
4. Lam L, Nair RJ, Tingle L. Thyrotoxic periodic paralysis. Proc (Bayl Univ Med Cent). 2006;19(2):126-129.
5. Li X, Yao S, Xiang Y, et al. The clinical and genetic features in a cohort of mainland Chinese patients with thyrotoxic periodic paralysis. BMC Neurol. 2015;15:38. doi:10.1186/s12883-015-0290-8.
6. Birkhahn RH, Gaeta TJ, Melniker L. Thyrotoxic periodic paralysis and intravenous propranolol in the emergency setting. J Emerg Med. 2000;18(2):199-202.
7. Lu KC, Hsu YJ, Chiu JS, Hsu YD, Lin SH. Effects of potassium supplementation on the recovery of thyrotoxic periodic paralysis. Am J Emerg Med. 2004;22(7):544-547.
8. Tassone H, Moulin A, Henderson SO. The pitfalls of potassium replacement in thyrotoxic periodic paralysis: a case report and review of the literature. J Emerg Med. 2004;26(2):157-161. doi:10.1016/j.jemermed.2003.05.004.
Vascular Injury Following a Fall Onto an Outstretched Hand
A 46-year-old man with a remote history of general tonic-clonic seizures, for which he was taking phenytoin, presented to the ED 30 minutes after sustaining a witnessed mechanical fall. The patient had fallen onto his nondominant left hand, which resulted in an injury to his elbow. He reported neither losing consciousness nor experiencing any seizures following the incident. He denied dislocating the joint or sustaining any other injuries from the fall. He also denied a history of past left elbow injury.
The patient was alert, oriented, and provided a full history of the incident. Regarding medical history, he stated that his last seizure had occurred 10 years prior. Except for the left elbow pain, a review of his systems was negative. The patient appeared in no acute distress, and supported his left upper extremity with a bandana and his right hand.
The patient’s vital signs were normal. The physical examination was negative except for the left elbow, which had significant swelling and limited range of motion without skin break, leading to suspicion for a prehospital dislocation with self-reduction. The joints above, below, and at the injury site were assessed for neurovascular injury.
Computed tomography angiography of the left upper extremity showed a brachial artery occlusion above the elbow, with reconstitution below the joint (Figure 2).
Discussion
There is a paucity of information on vascular injury from elbow dislocation in the emergency medicine literature. A recent literature search referenced orthopedic pitfalls in the ED,1 but most data appear in the orthopedic and vascular literature. A case report from the orthopedic literature in Brazil cites a vascular injury after ED relocation of a dislocated elbow following an assault.2
The elbow is the second most commonly dislocated joint (not including the patella) after the shoulder.3 Posterior dislocations make up the majority of these injuries. Simple versus complex injuries can be differentiated by the presence or absence of fracture.4 Simple complications include stiffness; loss of mobility, especially with full extension; neurovascular injuries; and compartment syndrome. Complex injuries involve fractures and potential neurovascular injuries, stiffness, pain, and loss of mobility.
Soft tissue injuries, fractures, and neurovascular complaints represent the majority of ED encounters, and are commonly related to falls. The elbow is the articulation of the humerus, ulna, and radius bones. Range of motion includes, but is not limited to, flexion, extension, supination, and pronation. Tears in the lateral ulnar ligament, joint capsule, and medial collateral ligament lead to instability of the joint and increase risk of dislocation.
Fractures make up to 20% of injuries to the elbow. These include fractures of the radial head and neck (most common), olecranon, and distal humerus.5 Open elbow fractures are rare, as are vascular injuries (5%-13% of cases).6 When present, vascular elbow injuries usually involve the brachial artery, and display abnormal palpable and Doppler assessment of the brachial and radial arteries.6
Nerve injuries may include injury to the radial nerve. Manifestations of radial nerve injury include abnormal sensation to the dorsum of the hand, trouble straightening the arm, and wrist-drop. Ulnar nerve injury typically presents with abnormal sensation to the fourth and fifth digits and decreased grip strength.
Conclusion
Vascular abnormalities are rare complications following elbow injuries. Our patient sustained a lacerated brachial artery, which was repaired via saphenous graft; brachial and basilic vein lacerations, which were ligated; and an avulsion fracture with an unstable joint, which was stabilized with external fixation and stabilization. He was discharged the following day with full neurovascular function.
A methodical approach to assessing patients presenting with elbow injury is essential to making the correct diagnosis. This should include a careful evaluation of the joints above and below the area of injury, as well as attention to the neurovascular examination, with a heightened suspicion for a vascular abnormality in complex injuries. Doppler and ultrasound evaluation with multiple rechecks can assist with the diagnosis. Our patient was rapidly assessed with a concern for a vascular injury, and was emergently referred to vascular surgery for repair of the brachial artery and stabilization of the joint.
1. Carter SJ, Germann CA, Dacus AA, Sweeney TW, Perron AD. Orthopedic pitfalls in the ED: neurovascular injury associated with posterior elbow dislocations. Am J Emerg Med. 2010;28(8):960-965. doi:10.1016/j.ajem.2009.05.024.
2. Miyazaki AN, Fregoneze M, Santos PD, do Val Sella G, Checchia CS, Checchia SL. Brachial artery injury due to closed posterior elbow dislocation: case report. Rev Bras Ortop. 2016;51(2):239-243. doi:10.1016/j.rboe.2016.02.007.
3. Beingessner J, Pollock W, King GJW. Elbow fractures and dislocations. In: Court-Brown CM, Heckman JD, McQueen MM, Ricci WM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. Vol 1. 8th ed. Philadelphia, PA: Wolters Kluwer Health; 2015:1179-1228.
4. McCabe MP, Savoie FH 3rd. Simple elbow dislocations: evaluation, management, and outcomes. Phys Sportsmed. 2012;40(1):62-71. doi:10.3810/psm.2012.02.1952.
5. Jungbluth P, Hakimi M, Linhart W, Windolf J. Current concepts: simple and complex elbow dislocations—acute and definitive treatment. Eur J Trauma Emerg Surg. 2008;34(2):120-130. doi:10.1007/s00068-008-8033-9.
6. Marcheix B, Chaufour X, Ayel J, et al. Transection of the brachial artery after closed posterior elbow dislocation. J Vasc Surg. 2005;42(6):1230-1232. doi:10.1016/j.jvs.2005.07.046.
A 46-year-old man with a remote history of general tonic-clonic seizures, for which he was taking phenytoin, presented to the ED 30 minutes after sustaining a witnessed mechanical fall. The patient had fallen onto his nondominant left hand, which resulted in an injury to his elbow. He reported neither losing consciousness nor experiencing any seizures following the incident. He denied dislocating the joint or sustaining any other injuries from the fall. He also denied a history of past left elbow injury.
The patient was alert, oriented, and provided a full history of the incident. Regarding medical history, he stated that his last seizure had occurred 10 years prior. Except for the left elbow pain, a review of his systems was negative. The patient appeared in no acute distress, and supported his left upper extremity with a bandana and his right hand.
The patient’s vital signs were normal. The physical examination was negative except for the left elbow, which had significant swelling and limited range of motion without skin break, leading to suspicion for a prehospital dislocation with self-reduction. The joints above, below, and at the injury site were assessed for neurovascular injury.
Computed tomography angiography of the left upper extremity showed a brachial artery occlusion above the elbow, with reconstitution below the joint (Figure 2).
Discussion
There is a paucity of information on vascular injury from elbow dislocation in the emergency medicine literature. A recent literature search referenced orthopedic pitfalls in the ED,1 but most data appear in the orthopedic and vascular literature. A case report from the orthopedic literature in Brazil cites a vascular injury after ED relocation of a dislocated elbow following an assault.2
The elbow is the second most commonly dislocated joint (not including the patella) after the shoulder.3 Posterior dislocations make up the majority of these injuries. Simple versus complex injuries can be differentiated by the presence or absence of fracture.4 Simple complications include stiffness; loss of mobility, especially with full extension; neurovascular injuries; and compartment syndrome. Complex injuries involve fractures and potential neurovascular injuries, stiffness, pain, and loss of mobility.
Soft tissue injuries, fractures, and neurovascular complaints represent the majority of ED encounters, and are commonly related to falls. The elbow is the articulation of the humerus, ulna, and radius bones. Range of motion includes, but is not limited to, flexion, extension, supination, and pronation. Tears in the lateral ulnar ligament, joint capsule, and medial collateral ligament lead to instability of the joint and increase risk of dislocation.
Fractures make up to 20% of injuries to the elbow. These include fractures of the radial head and neck (most common), olecranon, and distal humerus.5 Open elbow fractures are rare, as are vascular injuries (5%-13% of cases).6 When present, vascular elbow injuries usually involve the brachial artery, and display abnormal palpable and Doppler assessment of the brachial and radial arteries.6
Nerve injuries may include injury to the radial nerve. Manifestations of radial nerve injury include abnormal sensation to the dorsum of the hand, trouble straightening the arm, and wrist-drop. Ulnar nerve injury typically presents with abnormal sensation to the fourth and fifth digits and decreased grip strength.
Conclusion
Vascular abnormalities are rare complications following elbow injuries. Our patient sustained a lacerated brachial artery, which was repaired via saphenous graft; brachial and basilic vein lacerations, which were ligated; and an avulsion fracture with an unstable joint, which was stabilized with external fixation and stabilization. He was discharged the following day with full neurovascular function.
A methodical approach to assessing patients presenting with elbow injury is essential to making the correct diagnosis. This should include a careful evaluation of the joints above and below the area of injury, as well as attention to the neurovascular examination, with a heightened suspicion for a vascular abnormality in complex injuries. Doppler and ultrasound evaluation with multiple rechecks can assist with the diagnosis. Our patient was rapidly assessed with a concern for a vascular injury, and was emergently referred to vascular surgery for repair of the brachial artery and stabilization of the joint.
A 46-year-old man with a remote history of general tonic-clonic seizures, for which he was taking phenytoin, presented to the ED 30 minutes after sustaining a witnessed mechanical fall. The patient had fallen onto his nondominant left hand, which resulted in an injury to his elbow. He reported neither losing consciousness nor experiencing any seizures following the incident. He denied dislocating the joint or sustaining any other injuries from the fall. He also denied a history of past left elbow injury.
The patient was alert, oriented, and provided a full history of the incident. Regarding medical history, he stated that his last seizure had occurred 10 years prior. Except for the left elbow pain, a review of his systems was negative. The patient appeared in no acute distress, and supported his left upper extremity with a bandana and his right hand.
The patient’s vital signs were normal. The physical examination was negative except for the left elbow, which had significant swelling and limited range of motion without skin break, leading to suspicion for a prehospital dislocation with self-reduction. The joints above, below, and at the injury site were assessed for neurovascular injury.
Computed tomography angiography of the left upper extremity showed a brachial artery occlusion above the elbow, with reconstitution below the joint (Figure 2).
Discussion
There is a paucity of information on vascular injury from elbow dislocation in the emergency medicine literature. A recent literature search referenced orthopedic pitfalls in the ED,1 but most data appear in the orthopedic and vascular literature. A case report from the orthopedic literature in Brazil cites a vascular injury after ED relocation of a dislocated elbow following an assault.2
The elbow is the second most commonly dislocated joint (not including the patella) after the shoulder.3 Posterior dislocations make up the majority of these injuries. Simple versus complex injuries can be differentiated by the presence or absence of fracture.4 Simple complications include stiffness; loss of mobility, especially with full extension; neurovascular injuries; and compartment syndrome. Complex injuries involve fractures and potential neurovascular injuries, stiffness, pain, and loss of mobility.
Soft tissue injuries, fractures, and neurovascular complaints represent the majority of ED encounters, and are commonly related to falls. The elbow is the articulation of the humerus, ulna, and radius bones. Range of motion includes, but is not limited to, flexion, extension, supination, and pronation. Tears in the lateral ulnar ligament, joint capsule, and medial collateral ligament lead to instability of the joint and increase risk of dislocation.
Fractures make up to 20% of injuries to the elbow. These include fractures of the radial head and neck (most common), olecranon, and distal humerus.5 Open elbow fractures are rare, as are vascular injuries (5%-13% of cases).6 When present, vascular elbow injuries usually involve the brachial artery, and display abnormal palpable and Doppler assessment of the brachial and radial arteries.6
Nerve injuries may include injury to the radial nerve. Manifestations of radial nerve injury include abnormal sensation to the dorsum of the hand, trouble straightening the arm, and wrist-drop. Ulnar nerve injury typically presents with abnormal sensation to the fourth and fifth digits and decreased grip strength.
Conclusion
Vascular abnormalities are rare complications following elbow injuries. Our patient sustained a lacerated brachial artery, which was repaired via saphenous graft; brachial and basilic vein lacerations, which were ligated; and an avulsion fracture with an unstable joint, which was stabilized with external fixation and stabilization. He was discharged the following day with full neurovascular function.
A methodical approach to assessing patients presenting with elbow injury is essential to making the correct diagnosis. This should include a careful evaluation of the joints above and below the area of injury, as well as attention to the neurovascular examination, with a heightened suspicion for a vascular abnormality in complex injuries. Doppler and ultrasound evaluation with multiple rechecks can assist with the diagnosis. Our patient was rapidly assessed with a concern for a vascular injury, and was emergently referred to vascular surgery for repair of the brachial artery and stabilization of the joint.
1. Carter SJ, Germann CA, Dacus AA, Sweeney TW, Perron AD. Orthopedic pitfalls in the ED: neurovascular injury associated with posterior elbow dislocations. Am J Emerg Med. 2010;28(8):960-965. doi:10.1016/j.ajem.2009.05.024.
2. Miyazaki AN, Fregoneze M, Santos PD, do Val Sella G, Checchia CS, Checchia SL. Brachial artery injury due to closed posterior elbow dislocation: case report. Rev Bras Ortop. 2016;51(2):239-243. doi:10.1016/j.rboe.2016.02.007.
3. Beingessner J, Pollock W, King GJW. Elbow fractures and dislocations. In: Court-Brown CM, Heckman JD, McQueen MM, Ricci WM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. Vol 1. 8th ed. Philadelphia, PA: Wolters Kluwer Health; 2015:1179-1228.
4. McCabe MP, Savoie FH 3rd. Simple elbow dislocations: evaluation, management, and outcomes. Phys Sportsmed. 2012;40(1):62-71. doi:10.3810/psm.2012.02.1952.
5. Jungbluth P, Hakimi M, Linhart W, Windolf J. Current concepts: simple and complex elbow dislocations—acute and definitive treatment. Eur J Trauma Emerg Surg. 2008;34(2):120-130. doi:10.1007/s00068-008-8033-9.
6. Marcheix B, Chaufour X, Ayel J, et al. Transection of the brachial artery after closed posterior elbow dislocation. J Vasc Surg. 2005;42(6):1230-1232. doi:10.1016/j.jvs.2005.07.046.
1. Carter SJ, Germann CA, Dacus AA, Sweeney TW, Perron AD. Orthopedic pitfalls in the ED: neurovascular injury associated with posterior elbow dislocations. Am J Emerg Med. 2010;28(8):960-965. doi:10.1016/j.ajem.2009.05.024.
2. Miyazaki AN, Fregoneze M, Santos PD, do Val Sella G, Checchia CS, Checchia SL. Brachial artery injury due to closed posterior elbow dislocation: case report. Rev Bras Ortop. 2016;51(2):239-243. doi:10.1016/j.rboe.2016.02.007.
3. Beingessner J, Pollock W, King GJW. Elbow fractures and dislocations. In: Court-Brown CM, Heckman JD, McQueen MM, Ricci WM, Tornetta P, eds. Rockwood and Green’s Fractures in Adults. Vol 1. 8th ed. Philadelphia, PA: Wolters Kluwer Health; 2015:1179-1228.
4. McCabe MP, Savoie FH 3rd. Simple elbow dislocations: evaluation, management, and outcomes. Phys Sportsmed. 2012;40(1):62-71. doi:10.3810/psm.2012.02.1952.
5. Jungbluth P, Hakimi M, Linhart W, Windolf J. Current concepts: simple and complex elbow dislocations—acute and definitive treatment. Eur J Trauma Emerg Surg. 2008;34(2):120-130. doi:10.1007/s00068-008-8033-9.
6. Marcheix B, Chaufour X, Ayel J, et al. Transection of the brachial artery after closed posterior elbow dislocation. J Vasc Surg. 2005;42(6):1230-1232. doi:10.1016/j.jvs.2005.07.046.
Elevated levels of AST, ALT, and CPK • no family history of liver disease • Dx?
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
THE CASE
A 26-year-old healthy male veteran with bipolar disorder and post-traumatic stress disorder was referred for a gastroenterology consultation after a routine laboratory evaluation revealed elevated levels of aspartate aminotransferase (AST), 1040 IU/L (normal range, 10-40 IU/L), and alanine aminotransferase (ALT), 334 IU/L (normal range, 7-56 IU/L). He had been taking divalproex and ziprasidone for the previous 2 years, during which time liver test results had been normal.
The patient reported no symptoms in the course of a detailed history. He had no family history of liver disease, drank alcohol infrequently, and didn’t use tobacco. He hadn’t received any blood transfusions and didn’t have tattoos.
The patient indicated that he had recently returned from military deployment and that a week before his laboratory tests, he’d resumed weight training. To boost his workout, he’d begun taking a nutritional supplement supplied by a friend. Further questioning revealed that the supplement was MuscleMeds’ Code Red, which contains 1,3-dimethylamylamine (DMAA). He denied using any other dietary supplements.
The physical examination was unremarkable and additional lab work was unrevealing. Lab results included normal levels of ceruloplasmin, alpha-1 antitrypsin, ferritin, iron, and transferrin. Viral hepatitis serologies revealed immunity to the hepatitis A and B virus. The patient tested negative for Epstein-Barr virus, cytomegalovirus, herpes simplex virus, human immunodeficiency virus, antinuclear antibody, anti-smooth muscle antibody, and antimitochondrial antibody. A toxicology screen was remarkable for cannabinoids. The remainder of the basic metabolic panel and complete blood count were within normal limits.
THE DIAGNOSIS
The patient’s AST and ALT levels prompted measurement of creatine phosphokinase (CPK), which was elevated at 34,270 IU/L (normal range, 22-198 IU/L). We diagnosed rhabdomyolysis in this patient, which can be associated with elevated levels of AST and ALT. When we contacted the patient about the diagnosis, he reported no muscle aches or pains, or other symptoms.
We instructed the patient to increase his fluid intake and refrain from further use of Code Red. Repeat liver tests one month after the initial consultation revealed significant improvement in AST (29 IU/L) and ALT (68 IU/L), as well as a decline in CPK to 743 IU/L.
DISCUSSION
Much debate has surrounded the safety and use of DMAA, also known as methylhexamine or Geranamine, in dietary supplements such as Code Red. Eli Lilly and Company developed and patented DMAA in the 1940s, then trademarked it under the name Forthane as an inhaled nasal decongestant in 1971.1-3 United States Food and Drug Administration (FDA) approval for Forthane was withdrawn in 1983 at Lilly’s request.4 DMAA was reintroduced as a dietary supplement more than a decade ago after the FDA, in 2004, banned supplements containing ephedrine alkaloids, which have effects similar to DMAA.5
DMAA has been used to increase muscle mass, promote weight loss, and improve physical performance; it’s also been used as a recreational drug.6-8 Several case reports have described poor outcomes in patients who consumed DMAA products. In 2012, the deaths of 2 military personnel who used DMAA prompted the FDA to warn manufacturers of DMAA-containing supplements to stop production, but such supplements remain easily available in the United States.6
DMAA’s validity as a dietary supplement is controversial. The claim that DMAA is naturally present in geraniums hasn’t been verified, leading some to question whether an inaccurate description of DMAA as a natural substance was employed to justify its use as a nutritional supplement.9 No published evidence exists to establish DMAA as a dietary ingredient.10,11
A long list of potential adverse effects
DMAA is an indirect sympathomimetic with vasoconstricting and cardiovascular effects.12 Animal studies have shown effects similar to ephedrine and amphetamines.12-15 Marsh and colleagues reported that a single oral dose of 3 mg/kg in a human (210 mg/70 kg) moderately increases heart rate and blood pressure and can lead to confusion and concentration problems.16
Oral intake of DMAA affects the lungs at doses above 4 to 15 mg, the heart after 50 to 75 mg, and blood pressure after 100 mg.17 Because of the drug’s long half-life—24 hours based on urinary excretion rates—Venhuis and Kaste reported that there is a risk from repeated doses within 24 to 36 hours that can lead to steadily stronger pharmacologic effects.17
The use of DMAA has been cited in 5 cases of hemorrhagic stroke, a case of acute heart failure, and the deaths of 2 military personnel who experienced asystole during aerobic exercise.7,8,18-20 These individuals ranged in age from 22 to 41 years.
Initial symptoms included severe headaches, palpitations, dizziness, twitching of extremities, nausea, vomiting, confusion, agitation, and chest pain. The 2 military personnel suffered leg cramps and dyspnea followed by loss of consciousness. Several individuals were hypertensive on presentation to the emergency department with blood pressures as high as 240/120 mm Hg.
THE TAKEAWAY
Our patient presented with transaminitis and was found to have rhabdomyolysis after using DMAA. A few case reports have associated rhabdomyolysis with elevated liver function tests.21,22 We suspect that DMAA use, which has been linked to adverse effects such as hypertension, tachycardia, and muscle aches, may also cause leakage of muscle enzymes and the development of rhabdomyolysis.
Although a single instance can’t prove causation, this case may illustrate additional adverse effects of DMAA beyond the already long list of risks, including hypertension, seizures, cerebral hemorrhage, arrhythmias, myocardial infarction, cardiomyopathy, and death.7,8,18-20,23 It’s important for physicians to recognize that their patients may be using dietary supplements to increase strength, energy, or weight loss and to be aware of the potential adverse effects.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
1. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Aminoalkanes. Patent US2350318A. May 30, 1944.
2. Shonle HA, Rohrmann E, inventors; Eli Lilly and Company, assignee. Carbonates of 1-R-1 aminoethanes. Patent US2386273. October 9, 1945.
3. Eli Lilly and Company. Forthane. Registration 0925396, February 1, 1971. United States Patent and Trademark Office.
4. Federal Register. Vol. 48, No. 218/Notices. November 9, 1983.
5. Shipley A. Chemist’s new product contains hidden substance. Washington Post. May 8, 2006:Sports. Available at: http://www.washingtonpost.com/wp-dyn/content/article/2006/05/07/AR2006050700913.html. Accessed June 5, 2017.
6. Gregory PJ. Availability of DMAA supplements despite US Food and Drug Administration action. JAMA Intern Med. 2013;173:164-165.
7. Gee P, Jackson S, Easton J. Another bitter pill: a case of toxicity from DMAA party pills. N Z Med J. 2010;123:124-127.
8. Gee P, Tallon C, Long N, et al. Use of recreational drug 1,3 Dimethylamylamine (DMAA) [corrected] associated with cerebral hemorrhage. Ann Emerg Med. 2012;60:431-434.
9. Ping Z, Jun Q, Qing L. A study on the chemical constituents of geranium oil. Journal of Guizhou Institute of Technology. 1996;25:82-85.
10. Lisi A, Hasick N, Kazlauskas R, et al. Studies of methylhexaneamine in supplements and geranium oil. Drug Test Anal. 2011;3:873-876.
11. Elsohly MA, Gul W, Elsohly KM, et al. Pelargonium oil and methyl hexaneamine (MHA): analytical approaches supporting the absence of MHA in authenticated Pelargonium graveolens plant material and oil. J Anal Toxicol. 2012;36:457-471.
12. Charlier R. [Pharmacology of 2-amino-4-methylhexane]. Arch Int Pharmacodyn Ther. 1950;83:573-584.
13. Ahlquist R. A contribution to the pharmacology of the aliphatic amines. J Pharmacol Exp Ther. 1944;81:235-239.
14. Swanson EE, Chen KK. Comparison of pressor action of aliphatic amines. J Pharmacol Exp Ther. 1946;88:10-13.
15. Swanson EE, Chen KK. Comparison of pressor action of alicyclic derivatives of aliphatic amines. J Pharmacol Exp Ther. 1948;93:423-429.
16. Marsh DF, Howard A, Herring DA. The comparative pharmacology of the isomeric nitrogen methyl substituted heptylamines. J Pharmacol Exp Ther. 1951;103:325-329.
17. Venhuis BJ, Kaste D. Scientific opinion on the regulatory status of 1,3-dimethylamylamine (DMAA). European Journal of Food Research and Review. 2012;2:93-100.
18. Eliason MJ, Eichner A, Cancio A, et al. Case reports: Death of active duty soldiers following ingestion of dietary supplements containing 1,3-dimethylamylamine (DMAA). Mil Med. 2012;177:1455-1459.
19. Young C, Oladipo O, Frasier S, et al. Hemorrhagic stroke in young healthy male following use of sports supplement Jack3d. Mil Med. 2012;177:1450-1454.
20. Salinger L, Daniels B, Sangalli B, et al. Recreational use of a bodybuilding supplement resulting in severe cardiotoxicity. Clin Toxicol (Philadelphia). 2011;49:573-574.
21. Lee GY, Lee H, Kim YJ. Rhabdomyolysis recognized after elevation of liver enzymes following prolonged urologic surgery with lateral decubitus position: a case report. Korean J Anesthesiol. 2011;61:341-343.
22. Karcher C, Dieterich HJ, Schroeder TH. Rhabdomyolysis in an obese patient after total knee arthroplasty. Br J Anaesth. 2006;97:822-824.
23. Karnatovskaia LV, Leoni JC, Freeman ML. Cardiac arrest in a 21-year-old man after ingestion of 1,3-DMAA-containing workout supplement. Clin J Sport Med. 2015;25:e23-e25.
Bilateral chylothorax in an AIDS patient with newly diagnosed Kaposi sarcoma
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
Kaposi sarcoma is an angioproliferative tumor that is associated with human herpes virus-8 (HHV-8). Mucocutaneous disease is the most common site for manifestation of AIDS-related Kaposi sarcoma, commonly affecting the lower extremities, oral mucosa, face, and genitalia. Pleural effusions can occur in 36%-60% of patients with Kaposi sarcoma, and it has been documented that chylothorax is a rare, but plausible presentation in patients with Kaposi sarcoma.1 We present here a case of bilateral chylothorax in a patient with AIDS-related Kaposi sarcoma.
Case presentation and summary
A 52-year-old MSM male with AIDS (CD4, <20 mm3; viral load, 58 copies/ml) presented to the emergency department with complaints of shortness of breath, productive cough, and diarrhea for 2 days prior to presentation. His medical history also included chronic obstructive pulmonary disease, coronary artery disease, and hyperlipidemia. The patient was not on HAART because of his history of noncompliance. The results of a chest X-ray and computed-tomography (CT) scan showed that the patient had bilateral pleural effusion and a spiculated 14-mm nodule in the left upper lobe.
The patient underwent ultrasound-guided placement of a 12-French left-sided chest catheter, and a milky white fluid was aspirated from the left pleural space. Laboratory analysis of the pleural fluid confirmed an exudate with an elevated triglyceride level of 120 mg/dL (chylous, >110 mg/dL) indicating chylothorax.
On close physical examination, the patient was found to have multiple irregular plaques on the back and lower extremities. As described by dermatology, there was a violaceous indurated plaque on the left axillae, violaceous indurated plaques with superficial scale grouped on the left midlateral back, and hyperpigmented lichenified plaques and papules on bilateral shins, with some with plate-like scale. Two punch biopsies were taken of the skin lesions, which confirmed Kaposi sarcoma, plaque stage from the lesion biopsied on the back, and patch stage from the lesion biopsied in the left axilla. Cytology of the pleural fluid was negative for malignant cells. On review by the radiologist of the CT scan of the chest, there was no indication of gross distention of the thoracic duct. Treatment options were offered to the patient, and the patient was considering options for chemotherapy and home hospice given his advanced disease state at the time of discharge.
Discussion
Chylothorax occurs with a thoracic duct obstruction, which results in leakage of lymphatic fluid into the pleural cavity. The two leading causes of chylothorax are trauma and malignancy, with lymphoma being the most common cause of chylothorax among those with malignancy.2 Chylothorax, however, is a rare but documented complication of Kaposi sarcoma. Marais and colleagues reported the case of a 3-year-old HIV-positive patient with newly diagnosed Kaposi sarcoma who was found to have tumor infiltration in the thoracic duct leading to bilateral chylothorax.3 Maradona and colleagues described a 40-year-old man with AIDS-related Kaposi sarcoma who was found to have pleural and pericardial Kaposi sarcoma with chylothorax.4 Priest and colleagues wrote about a 32-year-old patient with AIDS with biopsy-proven Kaposi sarcoma who required multiple therapeutic thoracenteses for rapidly recurrent left chylothorax effusions.5
There are two leading discussions as to the pathophysiology of chylothorax that is related to Kaposi sarcoma: chylothorax developing secondary to metastatic disease or the development of chylothorax secondary to primary Kaposi sarcoma arising from the pleural region.6 One case report examined pleural and lung biopsies in a 34-year-old patient with AIDS-related Kaposi sarcoma that showed immunohistochemical staining that was suggestive of early-stage Kaposi sarcoma of lymphatic endothelial origin. The authors were attempting to illustrate that Kaposi sarcoma may have a stem-cell origin which can differentiate into lymph cells. Kontantinopoulos and colleagues postulated that in situ Kaposi sarcoma can arise from the lymphatic system with a resultant clinical presentation of chylothorax.7 The more mainstream thought however, is that chylothorax has been found to develop secondary to metastatic disease. The present case, therefore, illustrates an unusual presentation of cytology negative chylothorax in a patient with AIDS-related Kaposi sarcoma.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
1. Sridar S, Garza EG, Cox J, Rumbak MJ. Serosanguineous pleural effusions in a patient with HIV and Kaposi sarcoma: pleuroscopic findings. J Bronchology Interv Pulmonol. 2011;18(4):337-339.
2. Light RW. Chylothorax and pseudochylothorax. In: Light RW, ed. Pleural diseases. 6th ed. Philadelphia: Lippincott Williams & Wilkins, 2013:412-426.
3. Marais BJ, Pienaar J, Gie RP. Kaposi sarcoma with upper airway obstruction and bilateral chylothoraces. Pediatr Infect Dis J. 2003;22:926-928.
4. Maradona JA, Carton JA, Asensi V, Rodriguez-Guardado A. AIDS-related Kaposi sarcoma with chylothorax and pericardial involvement satisfactorily treated with liposomal doxorubicin. AIDS. 2002;16(5):806.
5. Priest ER, Weiss R. Chylothorax with Kaposi sarcoma. South Med J. 1991;84:806-807.
6. Pantanowitz L, Dezube BJ. Kaposi sarcoma in unusual locations. BMC Cancer. 2008;8:190.
7. Konstantinopoulos PA, Dezube BJ, Pantanowitz L. Morphologic and immunophenotypic evidence of in situ Kaposi sarcoma. BMC Clin Pathol. 2006;30:6:7.
Metastatic Kaposi sarcoma with osseous involvement in a patient with AIDS
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
Kaposi sarcoma is an AIDS-defining illness associated with human herpes virus-8 (HHV-8) co-infection. It was described in 1872 by the Hungarian dermatologist Mortiz Kaposi, and was an isolated and sporadic occurrence before the emergence of HIV infection and AIDS.1 It was first affiliated as an AIDS-associated neoplasm in 1981.1 Kaposi sarcoma is a systemic disease that can present with cutaneous lesions with or without internal involvement. There are four subtypes: Classic, African endemic, AIDS-related (CD4 count, <200), and Kaposi sarcoma in iatrogenically immunosuppressed patients. The disease has the propensity to manifest in the skin and gastro-intestinal and respiratory tracts, and osseous involvement is rarely encountered. We present here the case of an AIDS-positive man with generalized bone pain as a result of metastasis from Kaposi sarcoma. Our discussion includes the epidemiological, clinical, pathological, and radiological facets of AIDS-related Kaposi sarcoma, and the anomaly of osseous involvement.
Case presentation and summary
He restarted his previous HAART regimen in March 2016, and was subsequently started on chemotherapy with liposomal doxorubicin (50 mg [20 mg/m2] in 250 ml D5W IV every 2 weeks) because of his extensive disease.2 He completed 6 cycles by June 2016. However, he returned in July 2016 with worsening back pain. A repeat CT scan revealed significant improvement in the disseminated lymphadenopathy, but worsening osseous metastatic disease was seen in the lumbar, thoracic, and pelvic regions. A pelvic lytic lesion biopsy revealed Kaposi sarcoma; pathology showed spindle cells positive for CD34, CD31, and HHV-8 (Figure 2). The patient received palliative radiation to the spine, aiding in pain management and ambulatory dysfunction. He continued with his noncompliance with all medications and outpatient follow-ups, and succumbed to his disease burden.
Discussion
Kaposi sarcoma is a low-grade mesenchymal tumor that involves the blood and lymphatic vessels.3 Its association with AIDS was revealed in the early 1980s at the start of the HIV epidemic in the United States. In 1994, Chang and colleagues discovered the association between Karposi sarcoma and HHV-8 by isolating DNA fragments of HHV in Kaposi sarcoma tumors from AIDS patients.4 The mode of transmission of HHV-8 has not been fully decoded. It has been presumed that adult homosexual contact continues to be an important route of transmission, inferring a common route of infection. In 1990, the overall risk of developing Kaposi sarcoma in AIDS patients was 20,000 times greater than it was in the general population, and 300 times greater than in other immunosuppressed patients.5 This suggests an increase in incidence, in direct relation, with a decrease in the CD4 count.
Kaposi sarcoma can present with a range of clinical features, from negligible cutaneous lesions to a hastily progressing neoplasm. Involvement in the musculoskeletal system is infrequent, but encountered increasingly in the AIDS-related subtype. Moreover, it is recurrently observed in the African population.6 In one of the largest reviews to date exploring Kaposi sarcoma involving the musculoskeletal system, Caponetti and colleagues observed the greatest osseous involvement distinctly in patients with CD4 and T-cell counts below 100 cells/mm3.6
Kaposi sarcoma musculoskeletal involvement, specifically bone, is atypical. If it does occur, it usually manifests as a result of contiguous invasion from an adjacent nonosseous lesion. Caponetti and colleagues that isolated osseous Kaposi sarcoma lesions (with no overlying skin lesion) were found to be more likely to be associated with AIDS in the review by Caponetti and colleagues.6 As in our patient, it is also typically a manifestation of more widely disseminated disease.7
Most of the osseous lytic lesions in AIDS patients are located in the axial skeleton. Radiological features of musculoskeletal Kaposi sarcoma are variable. As observed by Caponetti and colleagues, Kaposi sarcoma lesions can appear as a periosteal reaction, cortical erosions, osteolysis, or osseous destruction, with irregular-shaped cortical erosions being most typical.6 Despite their osteolytic features, Kaposi sarcoma lesions are often not visualized by conventional radiography.6 The preferred imaging for identification of lytic bone changes is CT (Figure 3). Magnetic resonance imaging can also help distinguish marrow abnormalities as well as adjacent soft tissues masses. Radiologically, Kaposi sarcoma osseous lesions have parallel features to bacillary angiomatosis, tuberculosis, or lymphoma.8 Therefore, biopsy of the lesion is essential in establishing the diagnosis of Kaposi sarcoma.
In theory, there should be clinical improvement in Kaposi sarcoma when immunity is restored. Cancers caused by the Epstein-Barr virus and Kaposi sarcoma-associated herpes virus may eventually also be preventable with vaccines.10
There is rarely bone involvement without the foreshadowing of a poor prognosis. Erroneous patient care may inevitably arise from Kaposi sarcoma in uncharacteristic sites. A differential of Kaposi sarcoma should be included if a patient with AIDS presents with osteolytic lesions on imaging. Biopsying the lesion cements the diagnosis and eliminates the possibility of mimicry conditions such as bacillary angiomatosis, benign vascular lesions, and angiosarcoma. As of today, a HAART regimen remains the standard initial care for patients with Kaposi sarcoma.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
1. Radu O, Pantanowitz L. Kaposi sarcoma. Arch Pathol Lab Med. 2013;137:289-294.
2. Northfelt DW, Dezube BJ, Thommes JA, et al. Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi sarcoma: results of a randomized phase III clinical trial. J Clin Oncol. 1998;16(7):2445-2451.
3. Restrepo CS, Martinez S, Lemos JA, et al. Imaging manifestations of Kaposi sarcoma. RadioGraphics. 2006;26:1169-1185.
4. Chang Y, Cesarman E, Pessin MS, et al. Identification of herpes virus-like DNA sequences in AIDS-associated Kaposi sarcoma. Science. 1994;266:1865-1869.
5. Beral V, Peterman TA, Berkelman RL, Jaffe HW. Kaposi sarcoma among persons with AIDS: a sexually transmitted infection? Lancet. 1990;335:123-128.
6. Caponetti G, Dezube BJ, Restrepo CS, Pantanowitz I. Kaposi sarcoma of the musculoskeletal system: a review of 66 patients. Cancer. 2007;109(6):1040-1052.
7. Krishna G, Chitkara RK. Osseous Kaposi sarcoma. JAMA. 2003;286(9):1106.
8. Thanos L, Mylona S, Kalioras V, Pomoni M, Batakis N. Osseous Kaposi sarcoma in an HIV-positive patient. Skeletal Radiol. 2004;33(4):241-243.
9. Guiholt A, Dupin N, Marcelin AG, et al. Low T-cell response to human herpesvirus 8 in patients with AIDS-related and classic Kaposi sarcoma. J Infect Dis. 2006;194(8):1078-1088.
10. Gopal S, Achenbach CJ, Yanik EL, Dither DP, Eron JJ, Engels EA. Moving forward in HIV-associated cancer. J Clin Oncol. 2014;32(9):876-880.
A rare case of hypoglycemia induced by a classic gastrointestinal stromal tumor
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
Hypoglycemia, a frequently encountered medical emergency, is usually seen in patients with diabetes, most commonly as a result of iatrogenesis. However, it can also be encountered in nondiabetic patients. Various causes, such as pancreatic islet cell tumors producing insulin, primary or secondary adrenal insufficiency, advanced liver disease, pheochromocytoma and hypothyroidism, have been found to contribute to the condition in the nondiabetic population.1 In rare cases, an excessive production of insulin-like growth factor (IGF-2) – a condition known as nonislet cell tumor-induced hypoglycemia (NICTH) – has also been found to cause hypoglycemia. Hypoinsulinemic hypoglycemia, with low IGF-1 levels and an IGF-2-IgF1 ratio of greater than 10, is found to be suggestive of NICTH.
Case presentation and summary
An 81-year-old man with a history of diabetes mellitus, systolic heart failure, chronic kidney disease, and metastatic classical gastrointestinal spindle cell sarcoma presented to the emergency department with an acute change in mental status resulting from a new onset hypoglycemia. He was admitted, and during his hospital stay, he experienced severe hypoglycemic episodes with symptomatic presentations of diaphoresis on multiple occasions. A detailed history revealed that for diabetes, the patient had been on insulin for the first 12 years after his diagnosis, after which he was switched to metformin 500 mg twice daily for about 2 years, and as a satisfactory glycemic control was attained, eventually metformin had also been stopped 3 years prior to the current presentation.
The patient’s past medical records were obtained from the hospital at which he had been diagnosed gastrointestinal spindle cell sarcoma. Patient had not received treatment for the cancer as the disease was too widespread to be treated. The gastrointestinal spindle cell sarcoma, which had initially been surgically resected 7 years before the current presentation, had a recurrence 3 years later with abdominal and pulmonary metastasis, but no liver metastasis. No further intervention was carried out because the widely metastasized disease would not have benefited from any more surgical intervention and chemotherapy was not initiated because of the patient’s comorbid illnesses.
A blood sample drawn from the patient at the time of one hypoglycemic event, revealed low serum insulin <0.1 U/ml (normal, 2-19.6 U/ml); low C-peptide level, 0.59 ng/ml (0.8-3.85 ng/ml); low IGF-1, 16 ng/ml (5-4 ng/ml); and IGF-3, 0.9 ng/ml (2.2-4.5 ng/ml). IGF-2 levels were found to be markedly elevated at 945 ng/ml (47-350 ng/ml). The calculated IGF-2-IGF-1 ratio was 59.06 (normal, <10), suggesting NICTH as the etiology for the patient’s hypoglycemia.
The hypoglycemic episodes were initially treated with a continuous dextrose infusion followed by diazoxide treatment. However, diazoxide did not prevent his hypoglycemic episodes, so dexamethasone was considered as an alternative for his condition. The dexamethasone treatment resulted in the normalization of the patient’s serum glucose levels and resolution of his symptoms. The patient was discharged in a satisfactory state few days later and followed up thereafter. No recurrence of hypoglycemic episodes was found, and he was continued on dexamethasone therapy.
Discussion
Hypoglycemia due to NICTH is rare, with a prevalence of four times less than that of insulinoma.3 In most cases, NICTH occurs in patients with solid tumors of mesenchymal and epithelial origins such as hepatocellular carcinoma, gastric carcinoma or mesothelioma.4 In NICTH, the serum levels of insulin, C-peptide, and IGF-1 are usually decreased or undetectable. However, the circulating levels of total IGF2 may be increased, decreased, or normal. Concurrent normal to high morning cortisol and normal response to cosyntropin stimulation can rule out adrenal insufficiency and suggest NICTH. An IGF-2: IGF-1 ratio of >10 is considered to be clinically significant and highly suggestive of NICTH.5 Hypoglycemia in NICTH can be managed by administration of oral glucose, intravenous dextrose or glucagon. In some cases, diazoxide, a potent inhibitor of insulin secretion, has been found to be useful.6 Diazoxide directly inhibits the release of insulin through stimulation of adrenergic receptors and also has an extra pancreatic hyperglycemic effect, probably by inhibiting cyclic adenosine monophosphate phosphodiesterase, resulting in higher plasma levels of cyclic AMP and enhanced glycogenolysis.
Glucocorticoid therapy has been shown to suppress IGF-2 in a dose dependent manner and also by increasing gluconeogenesis.7 Surgical resection of the tumor whenever possible is the treatment of choice followed by radiotherapy and chemotherapy for inoperable disease and if successful, usually results in resolution of hypoglycemia. Imatinib, is the chemotherapeutic drug of choice for metastatic GIST, but many case reports have suggested worsening of hypoglycemia in advanced GIST with the use of the drug.8 The patient described in our report was not on any chemotherapy, hence hypoglycemia could not be attributed to it. On the basis of findings among 24 patients with GIST, Rikhof and colleagues have recommended monitoring plasma levels of pro-IGF-IIE to identify patients at high risk for developing hypoglycemia, especially those with progressive disease.9 Furthermore, over expression of IGF-2 as a predictor of potential relapse may be an area for potential research and further study.10
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
1. Marks V, Teale JD. Tumours producing hypoglycaemia. Diabetes Metab Rev. 1991;7:79-91.
2. Dutta P, Aggarwal A, Gogate Y, Nahar U, Shah VN, Singla M. Non-islet cell tumor-induced hypoglycemia: a report of five cases and brief review of the literature. Endocrinol Diabetes Metab Case Rep. 2013;2013:130046
3. de Groot JW, Rikhof B, van Doorn J, et al. Non-islet cell tumour-induced hypoglycaemia: a review of the literature including two new cases. Endocr Relat Cancer. 2007;14:979-93.
4. Fukuda I, Hizuka N, Ishikawa Y, et al. Clinical features of insulin-like growth factor II producing non-islet-cell tumor hypoglycemia
5. Marks V, Teale JD: Tumours producing hypoglycaemia. Endocr Relat Cancer. 1998;5:111-129.
6. Le Roith D. Tumor-induced hypoglycemia. N Engl J Med. 1999;341:757-758.
7. Teale JD, Marks V. Glucocorticoid therapy suppresses abnormal secretion of big IGF-II by non-islet cell tumours inducing hypoglycaemia (NICTH). Clin Endocrinol .1998;49:491-498.
8. Hamberg P, De Jong FA, Boonstra JG, et al. Non-islet-cell tumor induced hypoglycemia in patients with advanced gastrointestinal stromal tumor possibly worsened by imatinib. J Clin Oncol. 2006;24:e30-e31.
9. Rikhof B, van Doorn J, Suurmeijer AJ, et al. Insulin-like growth factors and insulin-like growth factor-binding proteins in relation to disease status and incidence of hypoglycaemia in patients with a gastrointestinal stromal tumour. Ann Oncol. 2009;20:1582-1588.
10. Braconi C, Bracci R, Bearzi I, et al. Insulin-like growth factor (IGF) 1 and 2 help to predict disease outcome in GIST patients. Ann Oncol. 2008;19:1293-1298.
Pregnant Patient Develops a Rare Case of Multiple Sclerosis
Pregnancy is generally found to offer a respite from multiple sclerosis (MS). Pregnant women rarely develop MS or to have relapses. But in a unique and challenging case, a woman in her 14th week of her second pregnancy developed signs and symptoms of tumefactive multiple sclerosis (TMS), a rare subtype of MS. The TMS was only one of several unexpected clinical puzzles, according to the clinicians reporting on the case.
The patient, who had been healthy, was admitted with acute onset of paresthesias and word-finding difficulty. She had just had a long drive from Florida, and the the clinicians first assumed that she was fatigued from the trip and from the pregnancy. A magnetic resonance imaging (MRI) scan of the brain, however, suggested an ischemic event.
While hospitalized, the patient’s condition rapidly worsened. More scan and test findings proved consistent with TMS. A repeat MRI scan showed interval progression with a growing tumefactive demyelinating lesion (TDL) with diffuse surrounding edema and new periventricular signal changes. Although rare, TDLs often represent fulminant forms of MS, the clinicians note. Because the lesions mimic strokes, tumors, and abscesses, diagnosis is difficult. Moreover, the gadolinium (which was avoided because it can cause birth defects) might have helped them visualize lesions sooner.
The patient was started on high-dose IV methylprednisolone and plasma exchange, but the response was mild. The poor response to both treatment modalities is infrequent in TMS, the clinicians say—yet another unforeseen obstacle.
In addition to counseling the patient about the usual protective effects of pregnancy, her clinicians counseled her “extensively” about natalizumab and the possible beneficial effects of disease-modifying therapies. But the patient made the difficult decision to terminate the pregnancy, in part because she felt it was better to focus on her existing child rather than on caring for 2 young children while having a chronic progressive disease with uncertain recovery.
Another surprise was in store. Within 12 hours after an uncomplicated dilatation and curettage, the patient was able to move her right arm. That “drastic improvement” was followed by moderate improvement in her right leg. Her “paradoxical” improvement after the termination might indicate a “different from expected” hormonal influence in the pathogenesis of TMS, the clinicians say, but more likely represents a delayed corroborating effect of steroids and plasma exchange.
In the following weeks, the patient’s recovery was “satisfying” with gradual improvement and partial return of expressive language. Eighteen months later, the patient was clinically stable on natalizumab.
Source:
Pakneshan S, Bernitsas E. BMJ Case Rep. 2017. pii: bcr-2017-219534.
doi: 10.1136/bcr-2017-219534.
Pregnancy is generally found to offer a respite from multiple sclerosis (MS). Pregnant women rarely develop MS or to have relapses. But in a unique and challenging case, a woman in her 14th week of her second pregnancy developed signs and symptoms of tumefactive multiple sclerosis (TMS), a rare subtype of MS. The TMS was only one of several unexpected clinical puzzles, according to the clinicians reporting on the case.
The patient, who had been healthy, was admitted with acute onset of paresthesias and word-finding difficulty. She had just had a long drive from Florida, and the the clinicians first assumed that she was fatigued from the trip and from the pregnancy. A magnetic resonance imaging (MRI) scan of the brain, however, suggested an ischemic event.
While hospitalized, the patient’s condition rapidly worsened. More scan and test findings proved consistent with TMS. A repeat MRI scan showed interval progression with a growing tumefactive demyelinating lesion (TDL) with diffuse surrounding edema and new periventricular signal changes. Although rare, TDLs often represent fulminant forms of MS, the clinicians note. Because the lesions mimic strokes, tumors, and abscesses, diagnosis is difficult. Moreover, the gadolinium (which was avoided because it can cause birth defects) might have helped them visualize lesions sooner.
The patient was started on high-dose IV methylprednisolone and plasma exchange, but the response was mild. The poor response to both treatment modalities is infrequent in TMS, the clinicians say—yet another unforeseen obstacle.
In addition to counseling the patient about the usual protective effects of pregnancy, her clinicians counseled her “extensively” about natalizumab and the possible beneficial effects of disease-modifying therapies. But the patient made the difficult decision to terminate the pregnancy, in part because she felt it was better to focus on her existing child rather than on caring for 2 young children while having a chronic progressive disease with uncertain recovery.
Another surprise was in store. Within 12 hours after an uncomplicated dilatation and curettage, the patient was able to move her right arm. That “drastic improvement” was followed by moderate improvement in her right leg. Her “paradoxical” improvement after the termination might indicate a “different from expected” hormonal influence in the pathogenesis of TMS, the clinicians say, but more likely represents a delayed corroborating effect of steroids and plasma exchange.
In the following weeks, the patient’s recovery was “satisfying” with gradual improvement and partial return of expressive language. Eighteen months later, the patient was clinically stable on natalizumab.
Source:
Pakneshan S, Bernitsas E. BMJ Case Rep. 2017. pii: bcr-2017-219534.
doi: 10.1136/bcr-2017-219534.
Pregnancy is generally found to offer a respite from multiple sclerosis (MS). Pregnant women rarely develop MS or to have relapses. But in a unique and challenging case, a woman in her 14th week of her second pregnancy developed signs and symptoms of tumefactive multiple sclerosis (TMS), a rare subtype of MS. The TMS was only one of several unexpected clinical puzzles, according to the clinicians reporting on the case.
The patient, who had been healthy, was admitted with acute onset of paresthesias and word-finding difficulty. She had just had a long drive from Florida, and the the clinicians first assumed that she was fatigued from the trip and from the pregnancy. A magnetic resonance imaging (MRI) scan of the brain, however, suggested an ischemic event.
While hospitalized, the patient’s condition rapidly worsened. More scan and test findings proved consistent with TMS. A repeat MRI scan showed interval progression with a growing tumefactive demyelinating lesion (TDL) with diffuse surrounding edema and new periventricular signal changes. Although rare, TDLs often represent fulminant forms of MS, the clinicians note. Because the lesions mimic strokes, tumors, and abscesses, diagnosis is difficult. Moreover, the gadolinium (which was avoided because it can cause birth defects) might have helped them visualize lesions sooner.
The patient was started on high-dose IV methylprednisolone and plasma exchange, but the response was mild. The poor response to both treatment modalities is infrequent in TMS, the clinicians say—yet another unforeseen obstacle.
In addition to counseling the patient about the usual protective effects of pregnancy, her clinicians counseled her “extensively” about natalizumab and the possible beneficial effects of disease-modifying therapies. But the patient made the difficult decision to terminate the pregnancy, in part because she felt it was better to focus on her existing child rather than on caring for 2 young children while having a chronic progressive disease with uncertain recovery.
Another surprise was in store. Within 12 hours after an uncomplicated dilatation and curettage, the patient was able to move her right arm. That “drastic improvement” was followed by moderate improvement in her right leg. Her “paradoxical” improvement after the termination might indicate a “different from expected” hormonal influence in the pathogenesis of TMS, the clinicians say, but more likely represents a delayed corroborating effect of steroids and plasma exchange.
In the following weeks, the patient’s recovery was “satisfying” with gradual improvement and partial return of expressive language. Eighteen months later, the patient was clinically stable on natalizumab.
Source:
Pakneshan S, Bernitsas E. BMJ Case Rep. 2017. pii: bcr-2017-219534.
doi: 10.1136/bcr-2017-219534.