User login
Spontaneous Repigmentation of Silvery Hair in an Infant With Congenital Hydrops Fetalis and Hypoproteinemia
Silvery hair is characteristic of 3 rare autosomal-recessive disorders—Chédiak-Higashi syndrome (CHS), Elejalde syndrome (ES), and Griscelli syndrome (GS)—which are associated with mutations in various genes that encode several proteins involved in the intracellular processing and movement of melanosomes. We report the case of a 2-month-old male infant with transient silvery hair and generalized hypopigmentation of the skin and eyes who did not have any genetic mutations associated with the classic syndromes that usually are characterized by transient silvery hair.
Case Report
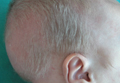
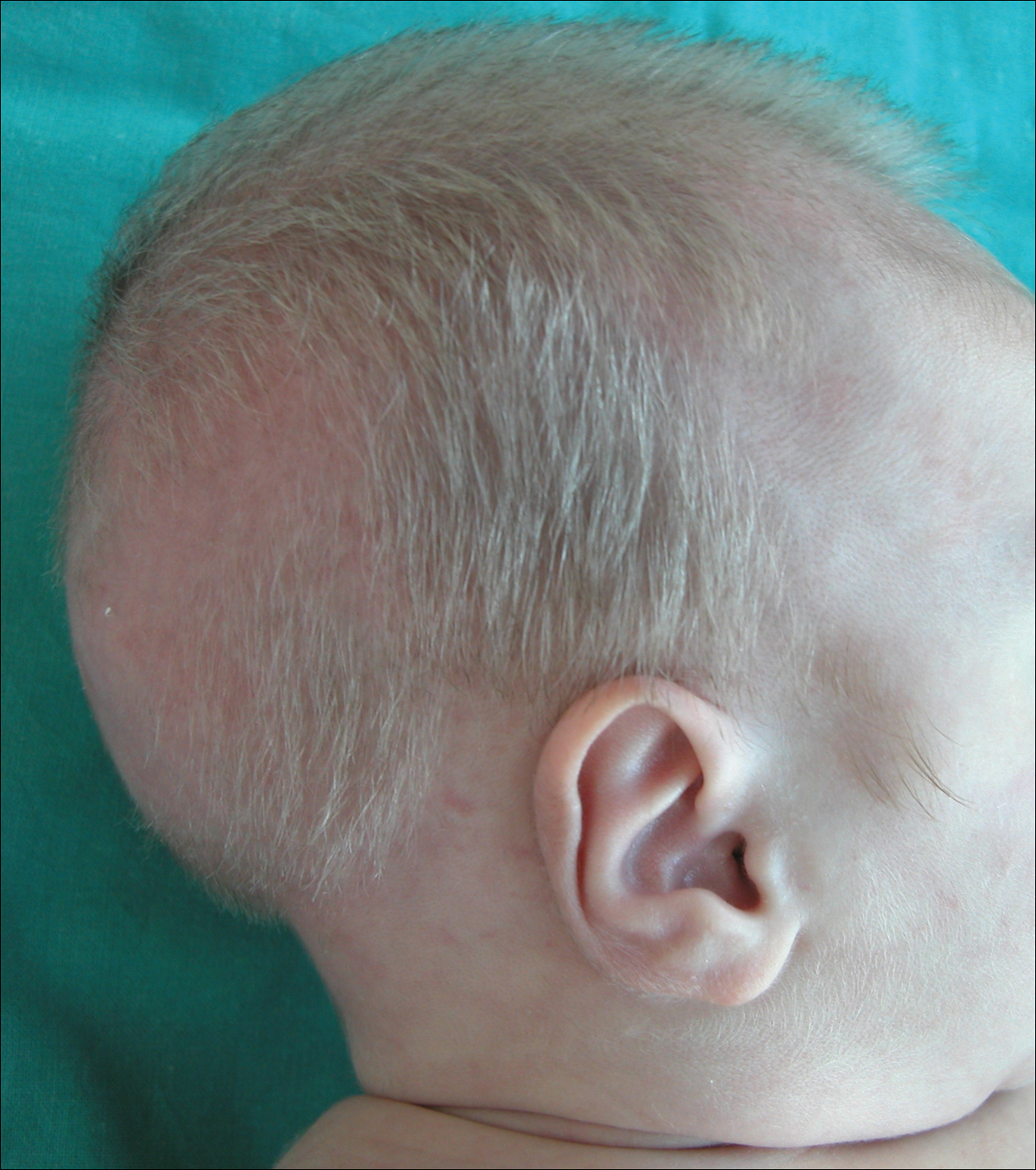
A 2-month-old male infant presented to the dermatology department for evaluation of silvery hair with generalized hypopigmentation of the skin and eyes (Figure 1) that had developed at 1 month of age. His parents were healthy, nonconsanguineous, and reported no family history of silvery hair. The patient was delivered by cesarean section at 35 weeks’ gestation. His medical history was remarkable for congenital hydrops fetalis with pleuropericardial effusion, ascites, soft-tissue edema, and hydrocele with no signs of any congenital infection. Both the patient and his mother were O Rh +.
Several studies were performed following delivery. A direct Coombs test was negative. Blood studies revealed hypothyroidism and hypoalbuminemia secondary to protein loss associated with fetal hydrops. Cerebral, abdominal, and renal ultrasound; echocardiogram; thoracic and abdominal computed tomography; and cerebral magnetic resonance imaging revealed no abnormalities.
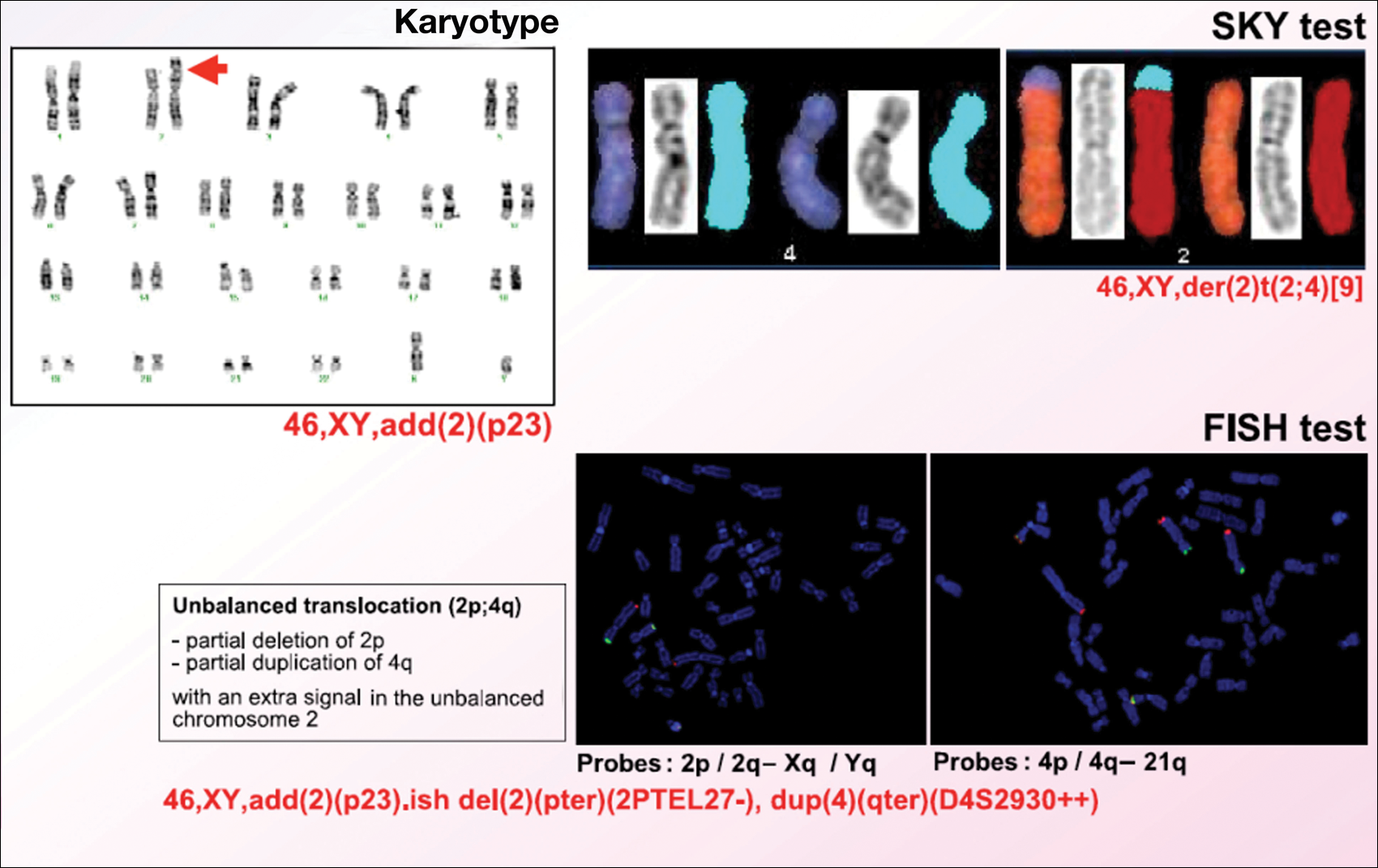
Karyotype results showed 46,XY,add(2)(p23), and subsequent spectral karyotyping and fluorescence in situ hybridization tests identified a chromosomal abnormality (46,XY,add[2][p23].ish del[2][pter][2PTEL27‒], dup[4][qter][D4S2930++])(Figure 2). Parental karyotypes were normal.
After birth, the infant was admitted to the neonatal intensive care unit for 50 days and received pleural and peritoneal drainages, mechanical ventilation, vasoactive drugs, parenteral nutrition with resolution of the hypoalbuminemia, levothyroxine, and intravenous antibiotics for central venous catheter infection. No drugs known to be associated with hypopigmentation of the hair, skin, or eyes were administered.
Two weeks after discharge from the neonatal intensive care unit, the patient was referred to our department. Physical examination revealed silvery hair on the scalp, eyebrows, and eyelashes, along with generalized hypopigmentation of the skin and eyes. Abdominal, cardiovascular, respiratory, and neurologic examination revealed no abnormalities, and no hepatosplenomegaly, lymphadenopathy, nystagmus, or strabismus was noted.
Light microscopy of the hair revealed small and regular aggregates of melanin along the hair shaft, predominantly in the medulla (Figure 3). Light microscopy of a skin biopsy specimen showed normal pigmentation in the melanocytes and no giant melanosomes. The melanocyte count was within reference range. A peripheral blood smear showed no giant granules in the granulocytes. No treatment was administered and the patient was followed closely every month. When the patient returned for follow-up at 9 months of age, physical examination revealed brown hair on the head, eyebrows, and eyelashes, as well as normal pigmentation of the skin and eyes (Figure 4). Thyroid function was normal and no recurrent infections of any type were noted. At follow-up at the age of 4 years, he showed normal neurological and psychological development with brown hair, no recurrent infections, and normal thyroid function. Given that CHS, ES, and GS had been ruled out, the clinical presentation and the genetic mutation detected may indicate that this case represents a new entity characterized by transient silvery hair.


Comment
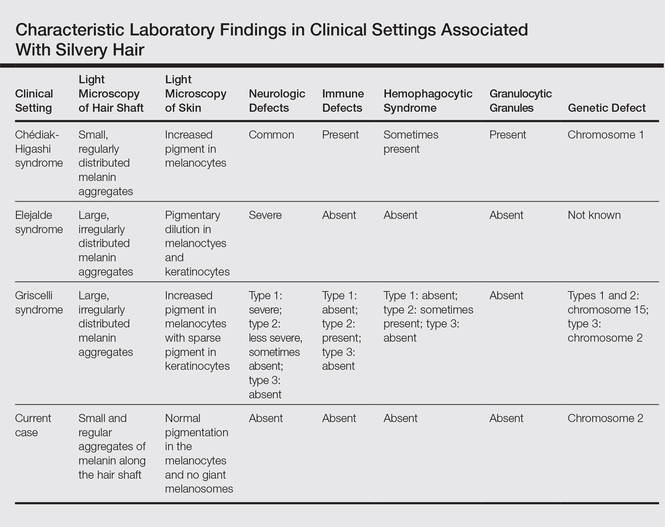
Silvery hair is a known feature of CHS, ES, and GS (Table). The characteristic hypopigmentation associated with these autosomal-recessive disorders is the result of impaired melanosome transport leading to failed transfer of melanin to keratinocytes. These disorders differ from oculocutaneous albinism in that melanin synthesis is unaffected.
Chédiak-Higashi syndrome is characterized by generalized hypopigmentation of the skin and eyes, silvery hair, neurologic and immune dysfunction, lymphoproliferative disorders, and large granules in granulocytes and other cell types.1-3 A common complication of CHS is hemophagocytic lymphohistiocytosis, which is characterized by fever, jaundice, lymphadenopathy, hepatosplenomegaly, and pancytopenia.4 Pigmentary dilution of the irises also may be present, along with photophobia, strabismus, nystagmus, and impaired visual acuity. Chédiak-Higashi syndrome is the result of a genetic defect in the lysosomal trafficking regulator gene, also known as CHS1 (located on chromosome 1q42.1‒q42.2).5 Melanin in the hair shaft is distributed uniformly in multiple small aggregates. Light microscopy of the skin typically shows giant melanosomes in melanocytes and aberrant keratinocyte maturation.
Elejalde syndrome is characterized by silvery hair (eyelashes and eyebrows), neurologic defects, and normal immunologic function.6,7 The underlying molecular basis remains unknown. It appears related to or allelic to GS type 1 and thus associated with mutations in MYO5A (myosin VA); however, the gene mutation responsible has yet to be defined.8 Light microscopy of the hair shaft usually shows an irregular distribution of large melanin aggregates, primarily in the medulla.9,10 Skin biopsy generally shows irregular distribution and irregular size of melanin granules in the basal layer.11 Leukocytes usually show no abnormal cytoplasmic granules. Ocular involvement is common and may present as nystagmus, diplopia, hypopigmented retinas, and/or papilledema.
In GS, hair microscopy generally reveals large aggregates of melanin pigment distributed irregularly along the hair shaft. Granulocytes typically show no giant granules. Light microscopy of the skin usually shows increased pigment in melanocytes with sparse pigment in keratinocytes. Griscelli syndrome is classified into 3 types.12 In GS type 1, patients have silvery gray hair, light-colored skin, severe neurologic defects,13 and normal immune status. This variant is caused by a mutation in the MYO5A gene located on chromosome 15q21. In GS type 2, patients have silvery gray hair, pyogenic infections, an accelerated phase of hemophagocytic lymphohistiocytosis, and variable neurologic defects in the absence of primary neurologic disease.14,15 This variant is caused by a mutation in the RAB27A (member RAS oncogene family) gene located on chromosome 15q21. In GS type 3, patients exhibit generalized hypopigmentation of the skin and hair with no abnormalities of the nervous or immune systems. There are 2 different mutations associated with GS type 3: the first is located on chromosome 2q37.3, causing a mutation in MLPH (melanophilin), and the second is caused by an F-exon deletion in the MYO5A gene.14
Our patient had silvery hair, generalized hypopigmentation of the skin and eyes, and normal central nervous system function with no other ocular involvement and no evidence of recurrent infections of any kind. Light microscopy showed small and regular melanin pigment aggregates in the hair shaft, which differs from the irregular pigment aggregates in GS and ES.
The regular melanin pigment aggregates observed along the hair shaft were consistent with CHS, but other manifestations of this syndrome were absent: ocular, neurologic, hematologic, and immunologic abnormalities with presence of giant intracytoplasmic granules in leukocytes, and giant melanosomes in melanocytes. In our patient, the absence of these features along with the spontaneous repigmentation of the silvery hair, improvement of thyroid function, reversal of hypoalbuminemia, and the chromosomopathy detected make a diagnosis of CHS highly improbable.
We concluded that the silvery hair noted in our patient resulted from the 46,XY,add(2)(p23) chromosomal abnormality. This mutation could affect some of the genes that control the trafficking of melanosomes or could induce hypothyroidism and hypoproteinemia associated with congenital hydrops fetalis (Figure 5).
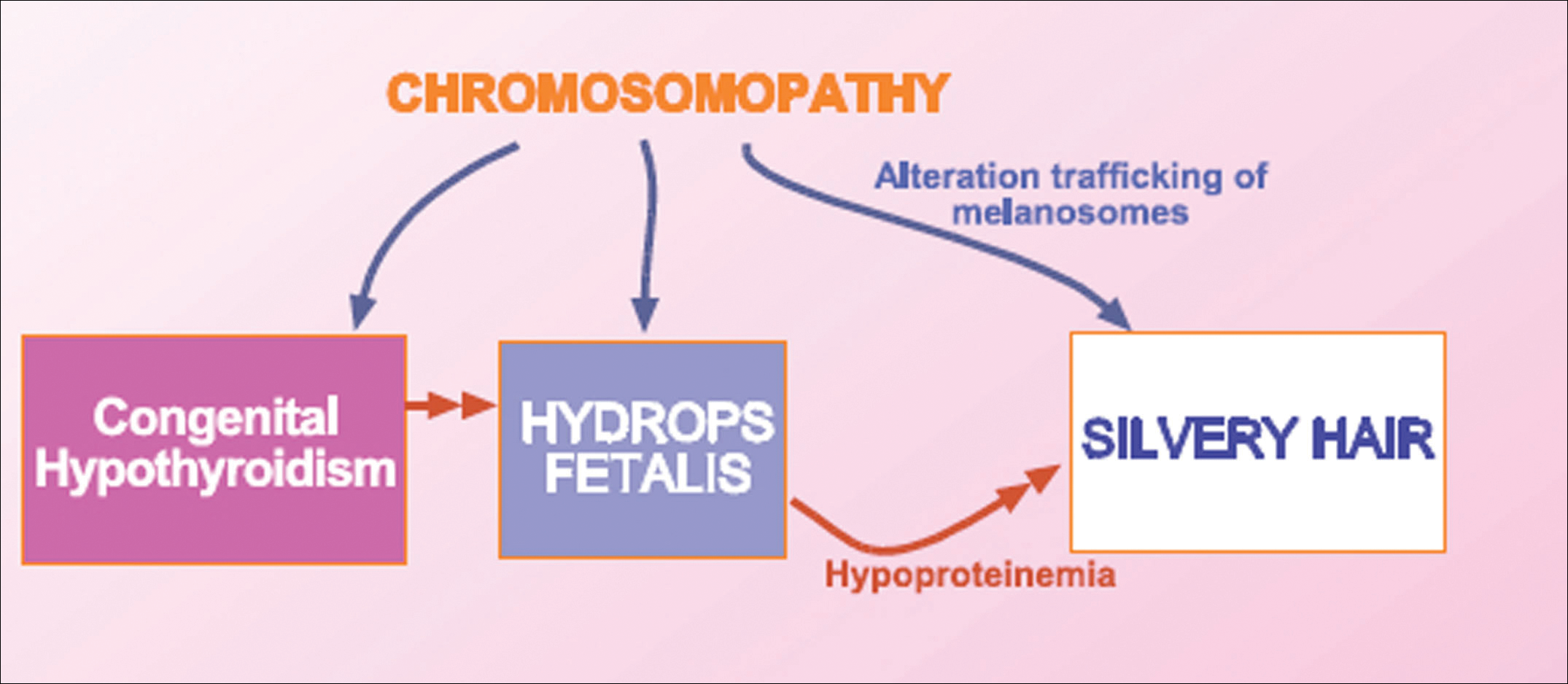
Hydrops fetalis is a potentially fatal condition characterized by severe edema (swelling) in a fetus or neonate. There are 2 types of hydrops fetalis: immune and nonimmune. Immune hydrops fetalis may develop in an Rh+ fetus with an Rh– mother, as the mother’s immune cells begin to break down the red blood cells of the fetus, resulting in anemia in the fetus with subsequent fetal heart failure, leading to an accumulation of large amounts of fluid in the tissues and organs. Nonimmune hydrops fetalis can occur secondary to diseases that interfere with the fetus’s ability to manage fluid (eg, severe anemia; congenital infections; urinary, lymphatic, heart, or thoracic defects; inborn errors of metabolism; chromosomal abnormalities). Case studies have suggested that congenital hypothyroidism could be a cause of nonimmune hydrops fetalis.16,17 Thyroid hormone deficiency reduces stimulation of adrenergic receptors in the lymphatic system and lungs, thereby decreasing lymph flow and protein efflux to the lymphatic system and decreasing clearance of liquid from the lungs. The final result is lymph vessel engorgement and subsequent leakage of lymphatic fluid to pleural spaces, causing hydrops fetalis and chylothorax.
The 46,XY,add(2)(p23) chromosomal abnormality has not been commonly associated with hypothyroidism and hydrops fetalis. The silvery hair in our patient was transient and spontaneously repigmented to brown over the course of follow-up in conjunction with improved physiologic changes. We concluded that the silvery hair in our patient was induced by his hypoproteinemic status secondary to hydrops fetalis and hypothyroidism.
Conclusion
In addition to CHS, ES, and GS, the differential diagnosis for silvery hair with abnormal skin pigmentation in children should include 46,XY,add(2)(p23) mutation, as was detected in our patient. Evaluation should include light microscopy of the hair shaft, skin biopsy, assessment of immune function, peripheral blood smear, and neurologic and eye examinations.
- White JG. The Chédiak-Higashi syndrome: a possible lysosomal disease. Blood. 1966;28:143-156.
- Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chédiak-Higashi syndrome. Mol Genet Metab. 1999;68:283-303.
- Kaplan J, De Domenico I, Ward DM. Chédiak-Higashi syndrome. Curr Opin Hematol. 2008;15:22-29.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis [published online December 7, 2006]. Eur J Pediatr. 2007;166:95-109.
- Morrone K, Wang Y, Huizing M, et al. Two novel mutations identified in an African-American child with Chédiak-Higashi syndrome [published online March 24, 2010]. Case Report Med. 2010;2010:967535.
- Ivanovich J, Mallory S, Storer T, et al. 12-year-old male with Elejalde syndrome (neuroectodermal melanolysosomal disease). Am J Med Genet. 2001;98:313-316.
- Cahali JB, Fernandez SA, Oliveira ZN, et al. Elejalde syndrome: report of a case and review of the literature. Pediatr Dermatol. 2004;21:479-482.
- Bahadoran P, Ortonne JP, Ballotti R, et al. Comment on Elejalde syndrome and relationship with Griscelli syndrome. Am J Med Genet. 2003;116:408-409.
- Duran-McKinster C, Rodriguez-Jurado R, Ridaura C, et al. Elejalde syndrome—a melanolysosomal neurocutaneous syndrome: clinical and morphological findings in 7 patients. Arch Dermatol. 1999;135:182-186.
- Happle R. Neurocutaneous diseases. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2131-2148.
- Sanal O, Yel L, Kucukali T, et al. An allelic variant of Griscelli disease: presentation with severe hypotonia, mental-motor retardation, and hypopigmentation consistent with Elejalde syndrome (neuroectodermal melanolysosomal disorder). J Neurol. 2000;247:570-572.
- Malhotra AK, Bhaskar G, Nanda M, et al. Griscelli syndrome. J Am Acad Dermatol. 2006;55:337-340.
- Al-Idrissi E, ElGhazali G, Alzahrani M, et al. Premature birth, respiratory distress, intracerebral hemorrhage, and silvery-gray hair: differential diagnosis of the 3 types of Griscelli syndrome. J Pediatr Hematol Oncol. 2010;32:494-496.
- Ménasché G, Ho CH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest. 2003;112:450-456.
- Griscelli C, Durandy A, Guy-Grand D, et al. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691-702.
- Narchi H. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;104:1416-1417.
- Kessel I, Makhoul IR, Sujov P. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;103:E9.
Silvery hair is characteristic of 3 rare autosomal-recessive disorders—Chédiak-Higashi syndrome (CHS), Elejalde syndrome (ES), and Griscelli syndrome (GS)—which are associated with mutations in various genes that encode several proteins involved in the intracellular processing and movement of melanosomes. We report the case of a 2-month-old male infant with transient silvery hair and generalized hypopigmentation of the skin and eyes who did not have any genetic mutations associated with the classic syndromes that usually are characterized by transient silvery hair.
Case Report
A 2-month-old male infant presented to the dermatology department for evaluation of silvery hair with generalized hypopigmentation of the skin and eyes (Figure 1) that had developed at 1 month of age. His parents were healthy, nonconsanguineous, and reported no family history of silvery hair. The patient was delivered by cesarean section at 35 weeks’ gestation. His medical history was remarkable for congenital hydrops fetalis with pleuropericardial effusion, ascites, soft-tissue edema, and hydrocele with no signs of any congenital infection. Both the patient and his mother were O Rh +.

Several studies were performed following delivery. A direct Coombs test was negative. Blood studies revealed hypothyroidism and hypoalbuminemia secondary to protein loss associated with fetal hydrops. Cerebral, abdominal, and renal ultrasound; echocardiogram; thoracic and abdominal computed tomography; and cerebral magnetic resonance imaging revealed no abnormalities.
Karyotype results showed 46,XY,add(2)(p23), and subsequent spectral karyotyping and fluorescence in situ hybridization tests identified a chromosomal abnormality (46,XY,add[2][p23].ish del[2][pter][2PTEL27‒], dup[4][qter][D4S2930++])(Figure 2). Parental karyotypes were normal.

After birth, the infant was admitted to the neonatal intensive care unit for 50 days and received pleural and peritoneal drainages, mechanical ventilation, vasoactive drugs, parenteral nutrition with resolution of the hypoalbuminemia, levothyroxine, and intravenous antibiotics for central venous catheter infection. No drugs known to be associated with hypopigmentation of the hair, skin, or eyes were administered.
Two weeks after discharge from the neonatal intensive care unit, the patient was referred to our department. Physical examination revealed silvery hair on the scalp, eyebrows, and eyelashes, along with generalized hypopigmentation of the skin and eyes. Abdominal, cardiovascular, respiratory, and neurologic examination revealed no abnormalities, and no hepatosplenomegaly, lymphadenopathy, nystagmus, or strabismus was noted.
Light microscopy of the hair revealed small and regular aggregates of melanin along the hair shaft, predominantly in the medulla (Figure 3). Light microscopy of a skin biopsy specimen showed normal pigmentation in the melanocytes and no giant melanosomes. The melanocyte count was within reference range. A peripheral blood smear showed no giant granules in the granulocytes. No treatment was administered and the patient was followed closely every month. When the patient returned for follow-up at 9 months of age, physical examination revealed brown hair on the head, eyebrows, and eyelashes, as well as normal pigmentation of the skin and eyes (Figure 4). Thyroid function was normal and no recurrent infections of any type were noted. At follow-up at the age of 4 years, he showed normal neurological and psychological development with brown hair, no recurrent infections, and normal thyroid function. Given that CHS, ES, and GS had been ruled out, the clinical presentation and the genetic mutation detected may indicate that this case represents a new entity characterized by transient silvery hair.


Comment
Silvery hair is a known feature of CHS, ES, and GS (Table). The characteristic hypopigmentation associated with these autosomal-recessive disorders is the result of impaired melanosome transport leading to failed transfer of melanin to keratinocytes. These disorders differ from oculocutaneous albinism in that melanin synthesis is unaffected.

Chédiak-Higashi syndrome is characterized by generalized hypopigmentation of the skin and eyes, silvery hair, neurologic and immune dysfunction, lymphoproliferative disorders, and large granules in granulocytes and other cell types.1-3 A common complication of CHS is hemophagocytic lymphohistiocytosis, which is characterized by fever, jaundice, lymphadenopathy, hepatosplenomegaly, and pancytopenia.4 Pigmentary dilution of the irises also may be present, along with photophobia, strabismus, nystagmus, and impaired visual acuity. Chédiak-Higashi syndrome is the result of a genetic defect in the lysosomal trafficking regulator gene, also known as CHS1 (located on chromosome 1q42.1‒q42.2).5 Melanin in the hair shaft is distributed uniformly in multiple small aggregates. Light microscopy of the skin typically shows giant melanosomes in melanocytes and aberrant keratinocyte maturation.
Elejalde syndrome is characterized by silvery hair (eyelashes and eyebrows), neurologic defects, and normal immunologic function.6,7 The underlying molecular basis remains unknown. It appears related to or allelic to GS type 1 and thus associated with mutations in MYO5A (myosin VA); however, the gene mutation responsible has yet to be defined.8 Light microscopy of the hair shaft usually shows an irregular distribution of large melanin aggregates, primarily in the medulla.9,10 Skin biopsy generally shows irregular distribution and irregular size of melanin granules in the basal layer.11 Leukocytes usually show no abnormal cytoplasmic granules. Ocular involvement is common and may present as nystagmus, diplopia, hypopigmented retinas, and/or papilledema.
In GS, hair microscopy generally reveals large aggregates of melanin pigment distributed irregularly along the hair shaft. Granulocytes typically show no giant granules. Light microscopy of the skin usually shows increased pigment in melanocytes with sparse pigment in keratinocytes. Griscelli syndrome is classified into 3 types.12 In GS type 1, patients have silvery gray hair, light-colored skin, severe neurologic defects,13 and normal immune status. This variant is caused by a mutation in the MYO5A gene located on chromosome 15q21. In GS type 2, patients have silvery gray hair, pyogenic infections, an accelerated phase of hemophagocytic lymphohistiocytosis, and variable neurologic defects in the absence of primary neurologic disease.14,15 This variant is caused by a mutation in the RAB27A (member RAS oncogene family) gene located on chromosome 15q21. In GS type 3, patients exhibit generalized hypopigmentation of the skin and hair with no abnormalities of the nervous or immune systems. There are 2 different mutations associated with GS type 3: the first is located on chromosome 2q37.3, causing a mutation in MLPH (melanophilin), and the second is caused by an F-exon deletion in the MYO5A gene.14
Our patient had silvery hair, generalized hypopigmentation of the skin and eyes, and normal central nervous system function with no other ocular involvement and no evidence of recurrent infections of any kind. Light microscopy showed small and regular melanin pigment aggregates in the hair shaft, which differs from the irregular pigment aggregates in GS and ES.
The regular melanin pigment aggregates observed along the hair shaft were consistent with CHS, but other manifestations of this syndrome were absent: ocular, neurologic, hematologic, and immunologic abnormalities with presence of giant intracytoplasmic granules in leukocytes, and giant melanosomes in melanocytes. In our patient, the absence of these features along with the spontaneous repigmentation of the silvery hair, improvement of thyroid function, reversal of hypoalbuminemia, and the chromosomopathy detected make a diagnosis of CHS highly improbable.
We concluded that the silvery hair noted in our patient resulted from the 46,XY,add(2)(p23) chromosomal abnormality. This mutation could affect some of the genes that control the trafficking of melanosomes or could induce hypothyroidism and hypoproteinemia associated with congenital hydrops fetalis (Figure 5).

Hydrops fetalis is a potentially fatal condition characterized by severe edema (swelling) in a fetus or neonate. There are 2 types of hydrops fetalis: immune and nonimmune. Immune hydrops fetalis may develop in an Rh+ fetus with an Rh– mother, as the mother’s immune cells begin to break down the red blood cells of the fetus, resulting in anemia in the fetus with subsequent fetal heart failure, leading to an accumulation of large amounts of fluid in the tissues and organs. Nonimmune hydrops fetalis can occur secondary to diseases that interfere with the fetus’s ability to manage fluid (eg, severe anemia; congenital infections; urinary, lymphatic, heart, or thoracic defects; inborn errors of metabolism; chromosomal abnormalities). Case studies have suggested that congenital hypothyroidism could be a cause of nonimmune hydrops fetalis.16,17 Thyroid hormone deficiency reduces stimulation of adrenergic receptors in the lymphatic system and lungs, thereby decreasing lymph flow and protein efflux to the lymphatic system and decreasing clearance of liquid from the lungs. The final result is lymph vessel engorgement and subsequent leakage of lymphatic fluid to pleural spaces, causing hydrops fetalis and chylothorax.
The 46,XY,add(2)(p23) chromosomal abnormality has not been commonly associated with hypothyroidism and hydrops fetalis. The silvery hair in our patient was transient and spontaneously repigmented to brown over the course of follow-up in conjunction with improved physiologic changes. We concluded that the silvery hair in our patient was induced by his hypoproteinemic status secondary to hydrops fetalis and hypothyroidism.
Conclusion
In addition to CHS, ES, and GS, the differential diagnosis for silvery hair with abnormal skin pigmentation in children should include 46,XY,add(2)(p23) mutation, as was detected in our patient. Evaluation should include light microscopy of the hair shaft, skin biopsy, assessment of immune function, peripheral blood smear, and neurologic and eye examinations.
Silvery hair is characteristic of 3 rare autosomal-recessive disorders—Chédiak-Higashi syndrome (CHS), Elejalde syndrome (ES), and Griscelli syndrome (GS)—which are associated with mutations in various genes that encode several proteins involved in the intracellular processing and movement of melanosomes. We report the case of a 2-month-old male infant with transient silvery hair and generalized hypopigmentation of the skin and eyes who did not have any genetic mutations associated with the classic syndromes that usually are characterized by transient silvery hair.
Case Report
A 2-month-old male infant presented to the dermatology department for evaluation of silvery hair with generalized hypopigmentation of the skin and eyes (Figure 1) that had developed at 1 month of age. His parents were healthy, nonconsanguineous, and reported no family history of silvery hair. The patient was delivered by cesarean section at 35 weeks’ gestation. His medical history was remarkable for congenital hydrops fetalis with pleuropericardial effusion, ascites, soft-tissue edema, and hydrocele with no signs of any congenital infection. Both the patient and his mother were O Rh +.

Several studies were performed following delivery. A direct Coombs test was negative. Blood studies revealed hypothyroidism and hypoalbuminemia secondary to protein loss associated with fetal hydrops. Cerebral, abdominal, and renal ultrasound; echocardiogram; thoracic and abdominal computed tomography; and cerebral magnetic resonance imaging revealed no abnormalities.
Karyotype results showed 46,XY,add(2)(p23), and subsequent spectral karyotyping and fluorescence in situ hybridization tests identified a chromosomal abnormality (46,XY,add[2][p23].ish del[2][pter][2PTEL27‒], dup[4][qter][D4S2930++])(Figure 2). Parental karyotypes were normal.

After birth, the infant was admitted to the neonatal intensive care unit for 50 days and received pleural and peritoneal drainages, mechanical ventilation, vasoactive drugs, parenteral nutrition with resolution of the hypoalbuminemia, levothyroxine, and intravenous antibiotics for central venous catheter infection. No drugs known to be associated with hypopigmentation of the hair, skin, or eyes were administered.
Two weeks after discharge from the neonatal intensive care unit, the patient was referred to our department. Physical examination revealed silvery hair on the scalp, eyebrows, and eyelashes, along with generalized hypopigmentation of the skin and eyes. Abdominal, cardiovascular, respiratory, and neurologic examination revealed no abnormalities, and no hepatosplenomegaly, lymphadenopathy, nystagmus, or strabismus was noted.
Light microscopy of the hair revealed small and regular aggregates of melanin along the hair shaft, predominantly in the medulla (Figure 3). Light microscopy of a skin biopsy specimen showed normal pigmentation in the melanocytes and no giant melanosomes. The melanocyte count was within reference range. A peripheral blood smear showed no giant granules in the granulocytes. No treatment was administered and the patient was followed closely every month. When the patient returned for follow-up at 9 months of age, physical examination revealed brown hair on the head, eyebrows, and eyelashes, as well as normal pigmentation of the skin and eyes (Figure 4). Thyroid function was normal and no recurrent infections of any type were noted. At follow-up at the age of 4 years, he showed normal neurological and psychological development with brown hair, no recurrent infections, and normal thyroid function. Given that CHS, ES, and GS had been ruled out, the clinical presentation and the genetic mutation detected may indicate that this case represents a new entity characterized by transient silvery hair.


Comment
Silvery hair is a known feature of CHS, ES, and GS (Table). The characteristic hypopigmentation associated with these autosomal-recessive disorders is the result of impaired melanosome transport leading to failed transfer of melanin to keratinocytes. These disorders differ from oculocutaneous albinism in that melanin synthesis is unaffected.

Chédiak-Higashi syndrome is characterized by generalized hypopigmentation of the skin and eyes, silvery hair, neurologic and immune dysfunction, lymphoproliferative disorders, and large granules in granulocytes and other cell types.1-3 A common complication of CHS is hemophagocytic lymphohistiocytosis, which is characterized by fever, jaundice, lymphadenopathy, hepatosplenomegaly, and pancytopenia.4 Pigmentary dilution of the irises also may be present, along with photophobia, strabismus, nystagmus, and impaired visual acuity. Chédiak-Higashi syndrome is the result of a genetic defect in the lysosomal trafficking regulator gene, also known as CHS1 (located on chromosome 1q42.1‒q42.2).5 Melanin in the hair shaft is distributed uniformly in multiple small aggregates. Light microscopy of the skin typically shows giant melanosomes in melanocytes and aberrant keratinocyte maturation.
Elejalde syndrome is characterized by silvery hair (eyelashes and eyebrows), neurologic defects, and normal immunologic function.6,7 The underlying molecular basis remains unknown. It appears related to or allelic to GS type 1 and thus associated with mutations in MYO5A (myosin VA); however, the gene mutation responsible has yet to be defined.8 Light microscopy of the hair shaft usually shows an irregular distribution of large melanin aggregates, primarily in the medulla.9,10 Skin biopsy generally shows irregular distribution and irregular size of melanin granules in the basal layer.11 Leukocytes usually show no abnormal cytoplasmic granules. Ocular involvement is common and may present as nystagmus, diplopia, hypopigmented retinas, and/or papilledema.
In GS, hair microscopy generally reveals large aggregates of melanin pigment distributed irregularly along the hair shaft. Granulocytes typically show no giant granules. Light microscopy of the skin usually shows increased pigment in melanocytes with sparse pigment in keratinocytes. Griscelli syndrome is classified into 3 types.12 In GS type 1, patients have silvery gray hair, light-colored skin, severe neurologic defects,13 and normal immune status. This variant is caused by a mutation in the MYO5A gene located on chromosome 15q21. In GS type 2, patients have silvery gray hair, pyogenic infections, an accelerated phase of hemophagocytic lymphohistiocytosis, and variable neurologic defects in the absence of primary neurologic disease.14,15 This variant is caused by a mutation in the RAB27A (member RAS oncogene family) gene located on chromosome 15q21. In GS type 3, patients exhibit generalized hypopigmentation of the skin and hair with no abnormalities of the nervous or immune systems. There are 2 different mutations associated with GS type 3: the first is located on chromosome 2q37.3, causing a mutation in MLPH (melanophilin), and the second is caused by an F-exon deletion in the MYO5A gene.14
Our patient had silvery hair, generalized hypopigmentation of the skin and eyes, and normal central nervous system function with no other ocular involvement and no evidence of recurrent infections of any kind. Light microscopy showed small and regular melanin pigment aggregates in the hair shaft, which differs from the irregular pigment aggregates in GS and ES.
The regular melanin pigment aggregates observed along the hair shaft were consistent with CHS, but other manifestations of this syndrome were absent: ocular, neurologic, hematologic, and immunologic abnormalities with presence of giant intracytoplasmic granules in leukocytes, and giant melanosomes in melanocytes. In our patient, the absence of these features along with the spontaneous repigmentation of the silvery hair, improvement of thyroid function, reversal of hypoalbuminemia, and the chromosomopathy detected make a diagnosis of CHS highly improbable.
We concluded that the silvery hair noted in our patient resulted from the 46,XY,add(2)(p23) chromosomal abnormality. This mutation could affect some of the genes that control the trafficking of melanosomes or could induce hypothyroidism and hypoproteinemia associated with congenital hydrops fetalis (Figure 5).

Hydrops fetalis is a potentially fatal condition characterized by severe edema (swelling) in a fetus or neonate. There are 2 types of hydrops fetalis: immune and nonimmune. Immune hydrops fetalis may develop in an Rh+ fetus with an Rh– mother, as the mother’s immune cells begin to break down the red blood cells of the fetus, resulting in anemia in the fetus with subsequent fetal heart failure, leading to an accumulation of large amounts of fluid in the tissues and organs. Nonimmune hydrops fetalis can occur secondary to diseases that interfere with the fetus’s ability to manage fluid (eg, severe anemia; congenital infections; urinary, lymphatic, heart, or thoracic defects; inborn errors of metabolism; chromosomal abnormalities). Case studies have suggested that congenital hypothyroidism could be a cause of nonimmune hydrops fetalis.16,17 Thyroid hormone deficiency reduces stimulation of adrenergic receptors in the lymphatic system and lungs, thereby decreasing lymph flow and protein efflux to the lymphatic system and decreasing clearance of liquid from the lungs. The final result is lymph vessel engorgement and subsequent leakage of lymphatic fluid to pleural spaces, causing hydrops fetalis and chylothorax.
The 46,XY,add(2)(p23) chromosomal abnormality has not been commonly associated with hypothyroidism and hydrops fetalis. The silvery hair in our patient was transient and spontaneously repigmented to brown over the course of follow-up in conjunction with improved physiologic changes. We concluded that the silvery hair in our patient was induced by his hypoproteinemic status secondary to hydrops fetalis and hypothyroidism.
Conclusion
In addition to CHS, ES, and GS, the differential diagnosis for silvery hair with abnormal skin pigmentation in children should include 46,XY,add(2)(p23) mutation, as was detected in our patient. Evaluation should include light microscopy of the hair shaft, skin biopsy, assessment of immune function, peripheral blood smear, and neurologic and eye examinations.
- White JG. The Chédiak-Higashi syndrome: a possible lysosomal disease. Blood. 1966;28:143-156.
- Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chédiak-Higashi syndrome. Mol Genet Metab. 1999;68:283-303.
- Kaplan J, De Domenico I, Ward DM. Chédiak-Higashi syndrome. Curr Opin Hematol. 2008;15:22-29.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis [published online December 7, 2006]. Eur J Pediatr. 2007;166:95-109.
- Morrone K, Wang Y, Huizing M, et al. Two novel mutations identified in an African-American child with Chédiak-Higashi syndrome [published online March 24, 2010]. Case Report Med. 2010;2010:967535.
- Ivanovich J, Mallory S, Storer T, et al. 12-year-old male with Elejalde syndrome (neuroectodermal melanolysosomal disease). Am J Med Genet. 2001;98:313-316.
- Cahali JB, Fernandez SA, Oliveira ZN, et al. Elejalde syndrome: report of a case and review of the literature. Pediatr Dermatol. 2004;21:479-482.
- Bahadoran P, Ortonne JP, Ballotti R, et al. Comment on Elejalde syndrome and relationship with Griscelli syndrome. Am J Med Genet. 2003;116:408-409.
- Duran-McKinster C, Rodriguez-Jurado R, Ridaura C, et al. Elejalde syndrome—a melanolysosomal neurocutaneous syndrome: clinical and morphological findings in 7 patients. Arch Dermatol. 1999;135:182-186.
- Happle R. Neurocutaneous diseases. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2131-2148.
- Sanal O, Yel L, Kucukali T, et al. An allelic variant of Griscelli disease: presentation with severe hypotonia, mental-motor retardation, and hypopigmentation consistent with Elejalde syndrome (neuroectodermal melanolysosomal disorder). J Neurol. 2000;247:570-572.
- Malhotra AK, Bhaskar G, Nanda M, et al. Griscelli syndrome. J Am Acad Dermatol. 2006;55:337-340.
- Al-Idrissi E, ElGhazali G, Alzahrani M, et al. Premature birth, respiratory distress, intracerebral hemorrhage, and silvery-gray hair: differential diagnosis of the 3 types of Griscelli syndrome. J Pediatr Hematol Oncol. 2010;32:494-496.
- Ménasché G, Ho CH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest. 2003;112:450-456.
- Griscelli C, Durandy A, Guy-Grand D, et al. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691-702.
- Narchi H. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;104:1416-1417.
- Kessel I, Makhoul IR, Sujov P. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;103:E9.
- White JG. The Chédiak-Higashi syndrome: a possible lysosomal disease. Blood. 1966;28:143-156.
- Introne W, Boissy RE, Gahl WA. Clinical, molecular, and cell biological aspects of Chédiak-Higashi syndrome. Mol Genet Metab. 1999;68:283-303.
- Kaplan J, De Domenico I, Ward DM. Chédiak-Higashi syndrome. Curr Opin Hematol. 2008;15:22-29.
- Janka GE. Familial and acquired hemophagocytic lymphohistiocytosis [published online December 7, 2006]. Eur J Pediatr. 2007;166:95-109.
- Morrone K, Wang Y, Huizing M, et al. Two novel mutations identified in an African-American child with Chédiak-Higashi syndrome [published online March 24, 2010]. Case Report Med. 2010;2010:967535.
- Ivanovich J, Mallory S, Storer T, et al. 12-year-old male with Elejalde syndrome (neuroectodermal melanolysosomal disease). Am J Med Genet. 2001;98:313-316.
- Cahali JB, Fernandez SA, Oliveira ZN, et al. Elejalde syndrome: report of a case and review of the literature. Pediatr Dermatol. 2004;21:479-482.
- Bahadoran P, Ortonne JP, Ballotti R, et al. Comment on Elejalde syndrome and relationship with Griscelli syndrome. Am J Med Genet. 2003;116:408-409.
- Duran-McKinster C, Rodriguez-Jurado R, Ridaura C, et al. Elejalde syndrome—a melanolysosomal neurocutaneous syndrome: clinical and morphological findings in 7 patients. Arch Dermatol. 1999;135:182-186.
- Happle R. Neurocutaneous diseases. In: Freedberg IM, Eisen AZ, Wolff K, et al, eds. Dermatology in General Medicine. 5th ed. New York, NY: McGraw-Hill; 1999:2131-2148.
- Sanal O, Yel L, Kucukali T, et al. An allelic variant of Griscelli disease: presentation with severe hypotonia, mental-motor retardation, and hypopigmentation consistent with Elejalde syndrome (neuroectodermal melanolysosomal disorder). J Neurol. 2000;247:570-572.
- Malhotra AK, Bhaskar G, Nanda M, et al. Griscelli syndrome. J Am Acad Dermatol. 2006;55:337-340.
- Al-Idrissi E, ElGhazali G, Alzahrani M, et al. Premature birth, respiratory distress, intracerebral hemorrhage, and silvery-gray hair: differential diagnosis of the 3 types of Griscelli syndrome. J Pediatr Hematol Oncol. 2010;32:494-496.
- Ménasché G, Ho CH, Sanal O, et al. Griscelli syndrome restricted to hypopigmentation results from a melanophilin defect (GS3) or a MYO5A F-exon deletion (GS1). J Clin Invest. 2003;112:450-456.
- Griscelli C, Durandy A, Guy-Grand D, et al. A syndrome associating partial albinism and immunodeficiency. Am J Med. 1978;65:691-702.
- Narchi H. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;104:1416-1417.
- Kessel I, Makhoul IR, Sujov P. Congenital hypothyroidism and nonimmune hydrops fetalis: associated? Pediatrics. 1999;103:E9.
Practice Points
- Silvery hair is characteristic of 3 rare autosomal-recessive disorders: Chédiak-Higashi syndrome, Elejalde syndrome, and Griscelli syndrome.
- Hypopigmentation is the result of impaired melanosome transport leading to failed transfer of melanin to keratinocytes.
- Evaluation should include light microscopy of the hair shaft, skin biopsy, assessment of immune function, peripheral blood smear, and neurologic and eye examinations.
Silicone Arthroplasty After Ankylosis of Proximal Interphalangeal Joints in Rheumatoid Arthritis: A Case Report
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
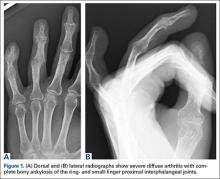
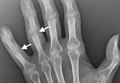
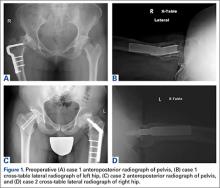
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
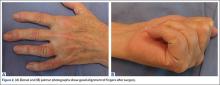
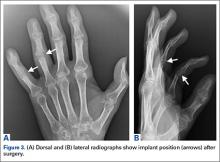
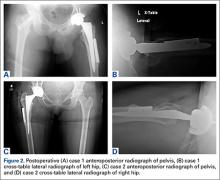
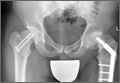
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
Rheumatoid arthritis (RA) commonly affects the hand and fingers, most often at the metacarpophalangeal and proximal interphalangeal (PIP) joints. Synovitis, tendon ruptures, Boutonnière and swan-neck deformities, and joint destruction often occur. Bony ankylosis is not commonly described yet frequently occurs in patients with RA.1
Implant arthroplasty is an established treatment for arthritis of the hand and fingers. Indications for its use include RA, osteoarthritis, and posttraumatic arthritis. Most patients treated with implant arthroplasty can expect pain relief and 40° to 65° of PIP joint motion.2,3 Silicone arthroplasty historically has been used for pain relief but not for restoration of motion in an ankylosed joint. To our knowledge, there are no reports of using implant arthroplasty in the treatment of spontaneous ankylosis in RA. Contraindications for this procedure would include infection, irreparable flexor or extensor apparatus, and severe medical comorbidities.
In this article, we report a case of PIP joint autofusion treated with silicone PIP arthroplasty in a patient with RA. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 56-year-old woman who had had RA for more than 20 years underwent left carpometacarpal arthroplasty and thumb reconstruction. She subsequently presented with complaints of progressively worsening functioning of the left ring and small fingers. On initial evaluation, her PIP joints were fused in about 15° of flexion. Radiographs (Figures 1A, 1B) showed severe diffuse arthritis of the hands and complete bony ankylosis of the ring- and small-finger PIP joints with radial deviation of the ring-finger middle phalanx. The patient had minimal pain but wanted improved hand motion and opted for takedown of the ankylosis with silicone PIP joint arthroplasty.
Radial dorsal incisions were made over the PIP joints of the ring and small fingers. As is not the case with arthroplasty for routine PIP joint arthritis, presence of bony ankylosis made identification of the native PIP joint more difficult. The transverse retinacular ligament was identified and opened, and the collateral ligament, which was not ankylosed, was dissected off the proximal phalanx. These landmarks were useful in locating the PIP joint, and proper positioning was confirmed with fluoroscopy. The ankylosed joint space was opened with an osteotome, and about 8 to 10 mm of bone was resected to create space for the instrumentation. As the amount of scarring within the flexor tendon sheath was not significant, restoration of motion did not require extensive tenolysis. The extensor mechanism was slightly contracted, but the bony resection allowed flexion to be restored. The distal portion of the proximal phalanx was then resected. The proximal and middle phalanges were reamed, and a silicone prosthesis was placed with the finger held straight. The collateral ligament was repaired back to the proximal phalanx with 4-0 polydioxanone sutures placed through a bone tunnel created with a Kirschner wire. The skin was closed with 4-0 nylon, and a postoperative splint was applied.
The initial postoperative course was unremarkable. The patient was immobilized in 10° of PIP joint flexion for 10 days, and therapy was initiated after the splint was removed. Twenty-four months after surgery, the patient was pain-free and had 60° of active PIP joint flexion, with extensor lag of only 10°. Clinically, alignment of the fingers was satisfactory; there was mild persistent radial deviation of 10° to 15° (Figures 2A, 2B). Radiographs showed good positioning of the implants (Figures 3A, 3B) and no sign of coronal instability. The patient was satisfied with her improved functioning and returned to employment as a hospital clerk, working full-time.
Discussion
RA of the hand and fingers can be painful and disabling. Although there are several treatment options for many of the most common manifestations, options are limited for bony ankylosis of the finger joints. The patient described in this case report had minimal pain, but the loss of motion of the PIP joints in her ring and small fingers created difficulties for her at work. She wanted surgery that would improve the functioning of her fingers. PIP joint arthroplasty traditionally has been the treatment of choice for PIP joint arthritis. In 1985, Swanson and colleagues2 reported on more than 400 silicone PIP arthroplasties performed over 16 years. Mean range of motion (ROM) was between 45° and 60°, with 70% of patients having ROM of more than 40°. Pain relief was complete in 98% of cases. Complications included implant fracture (5%) and recurrent or new deformities (6.5%). A 10.9% revision rate was noted at minimum 1-year follow-up. Recent implants made of improved biomaterials hold promise, but longer term follow-up is still needed.
Silicone arthroplasty has also been used as an effective treatment for non-RA of the PIP joint. Bales and colleagues4 reviewed long-term results of silicone arthroplasty for PIP joint osteoarthritis in 22 patients. At a mean of 10 years, mean QuickDASH (Disabilities of the Arm, Shoulder, and Hand) score was 17, mean visual analog scale score for pain was 0.4, and implant survivorship was 90%. Despite unchanged ROM and considerable implant deformation or fracture, patients’ pain relief and satisfaction were consistent.
Hage and colleagues5 reviewed long-term results of silicone PIP arthroplasty for posttraumatic arthritis in 14 patients. Most of the patients were satisfied: Although they had notable rotational deformity, alignment deviation, and loss of pinch strength and ROM, they were pain-free. The authors concluded that silicone arthroplasty should be used for posttraumatic arthrosis cases in which associated adhesions may be corrected with simple tenolysis, and even in these cases the objective results may not be as good as the subjective outcome.
Kaye6 used radiographs to determine the incidence of bony ankylosis in 203 patients with RA. Hand and wrist radiographs of 48 (23.6%) of these patients showed ankylosis, and 34 of the 48 patients had 2 or more joints fused. On a questionnaire, patients with ankylosis indicated more difficulty with activities of daily living and more limited activity. The authors concluded that radiographic bony ankylosis was a relatively common feature of RA and a marker of disease that was clinically, radiographically, and functionally more severe.
The patient described in this case report had a satisfactory result after PIP joint arthroplasty. At 2-year follow-up, she remained pain-free, and her previously ankylosed PIP joint had an arc of motion of 10° to 60°. Most patients with bony ankylosis of PIP joints present with minimal pain and do not seek surgical treatment. However, patients with ankylosis that limits functioning or activities of daily living may wish to pursue intervention that could be restorative. PIP joint arthroplasty may be effective in improving motion in patients with bony ankylosis of the finger joints.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
1. Kaye JJ, Callahan LF, Nance EP Jr, Brooks R, Pincus T. Bony ankylosis in rheumatoid arthritis. Associations with longer duration and greater severity of disease. Invest Radiol. 1987;22(4):303-309.
2. Swanson AB, Maupin BK, Gajjar NV, Swanson GD. Flexible implant arthroplasty in the proximal interphalangeal joint of the hand. J Hand Surg Am. 1985;10(6 pt 1):796-805.
3. Rizzo M, Beckenbaugh RD. Proximal interphalangeal joint arthroplasty. J Am Acad Orthop Surg. 2007;15(3):189-197.
4. Bales J, Wall L, Stern PJ. Long-term results of Swanson silicone arthroplasty for proximal interphalangeal joint osteoarthritis. J Hand Surg Am. 2014;39(3):455-461.
5. Hage J, Yoe E, Zering J, de Groot P. Proximal interphalangeal joint silicone arthroplasty for posttraumatic arthritis. J Hand Surg Am. 1999;24(1):73-77.
6. Kaye JJ. Radiographic assessment of rheumatoid arthritis. Rheum Dis Clin North Am. 1995;21(2):395-406.
Darkened skin, vomiting, and salt cravings in a teenager • Dx?
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
THE CASE
A 17-year-old boy presented to the emergency department (ED) with a headache, dizziness, lethargy, and weakness that he’d had for 2 weeks. The patient was taking a selective serotonin reuptake inhibitor (SSRI) for depression (sertraline 25 mg/d). He had been vomiting twice daily for the past 3 years. (Although he had been seen multiple times in urgent care clinics, he did not have regular medical care.) The boy was fatigued and had dark yellow urine. His father indicated that his son’s skin had darkened over the last 5 to 6 years and that he had been adding salt, in large quantities, to nearly all of his meals for 10 years.
The boy’s health issues were impacting his school life. He was dismissed from school often because his teachers felt he was skipping class and using the excuse of needing to urinate or vomit. He had traveled back and forth to Mexico about 2 times a year, with the last time being about 3 months before his trip to the ED.
The patient’s vitals included a temperature of 96.3º F, heart rate (HR) of 77 beats/min, respiratory rate of 16 breaths/min, and a supine blood pressure (BP) of 102/58 mm Hg. (The patient’s BP was not obtained when sitting or standing, because he felt dizzy when trying to stand or sit up and the HR monitor increased to 100 beats/min.) His weight was 106.9 pounds and height was 5 feet 8 inches. The teen was ill-appearing and somnolent. No jugular vein distention, murmurs, or gallops were noted on exam. The patient’s lips were dry and cracked, gums were darkened, and his skin was clammy to the touch. His abdomen was soft with hypoactive bowel sounds and no ascites. His extremities were non-edematous.
A chemistry panel showed a low sodium level of 99 mEq/L, a somewhat high potassium level of 5.2 mmol/L, low chloride (69 mEq/L) and CO2 (5 mEq/L) levels, a high glucose level (124 mg/dL), and normal creatinine (0.79 mg/dL), albumin (5.2 g/dL), and thyroid stimulating hormone (2.4 mIU/L) levels. A tuberculosis (TB) test, acute hepatitis panel, human immunodeficiency test, and urine drug screen were all negative. Liver enzymes and lipase levels were normal.
The patient was admitted to the pediatric intensive care unit (PICU) on 200 mL/hr normal saline (twice the normal maintenance rate) and we took over his care.
THE DIAGNOSIS
Because of the patient’s severe hyponatremia, the differential diagnosis included heart failure, cirrhosis, syndrome of inappropriate antidiuretic hormone secretion (SIADH), SSRI-induced SIADH, cerebral salt wasting, severe hypothyroidism, adrenal insufficiency, malignancies, ecstasy use, renal failure, low dietary solute intake, and psychogenic polydipsia.
A random cortisol test taken in the ED returned and was noted to be very low (<1 mcg/dL). This information, plus the signs of aldosterone deficiency (low sodium and elevated potassium levels) and adrenocorticotropic hormone (ACTH) excess (skin darkening), prompted us to perform a 250-mcg ACTH stimulation test. Results at 30 and 60 minutes both showed cortisol at <1 mcg/dL, which led us to suspect adrenal insufficiency. The diagnosis of autoimmune adrenalitis, or Addison’s disease, was confirmed after inpatient lab work returned with positive 21-hydroxylase antibodies and an elevated ACTH (1117 pg/mL; normal, 10-65 pg/mL).
We noted that the patient’s sodium level was gradually increasing while he was receiving the intravenous (IV) fluids. We were concerned, though, that too rapid a sodium correction would put the patient at risk for central pontine myelinolysis (CPM). So we held off on steroids until 24 hours after he was admitted to the PICU, when his sodium level reached 110 mEq/L.
DISCUSSION
Primary adrenal insufficiency in the developed world is commonly caused by autoimmune adrenalitis, also known as Addison’s disease. Addison’s disease is the cause of primary adrenal insufficiency in 70% to 90% of cases, with the remainder caused by TB, adrenal hemorrhage, infarction, lymphoma, cytomegalovirus, adrenoleukodystrophy, or metastatic cancer. We also considered adrenoleukodystrophy in our patient, but felt it unlikely in a 17-year-old with normal mental status and positive adrenal antibodies.
The first evidence of Addison’s disease is usually an increase in plasma renin activity with low serum aldosterone. This might explain our patient’s years of salt cravings prior to presentation. There is typically a decrease in serum cortisol response to ACTH stimulation several months to years after the onset of salt cravings. The next sign of deterioration in adrenal function is an increase in basal serum ACTH; the process concludes with a decreased basal serum cortisol level.1-3 By the time our patient presented to the ED, his ACTH was very high, his cortisol was low, and his ACTH stimulation response was low.
Acute adrenal insufficiency crisis usually occurs after a prolonged period of nonspecific complaints due to a loss of both glucocorticoids and mineralocorticoids; by the time overt symptoms occur, 90% of the adrenal gland may be destroyed.3 Patients (such as ours) may present with symptoms such as abdominal pain, weakness, vomiting, fever, and decreased responsiveness. Hyponatremia and hyperkalemia are commonly seen at initial diagnosis. BP can be compromised in some patients due to loss of vascular tone; our patient did not present with this finding.
Treatment includes hydrocortisone and fludrocortisone for life
Initial management focuses on rehydration, maintenance of BP, cardiac monitoring, and electrolyte monitoring with a focus on slow normalization of electrolyte abnormalities. Patients should be treated with hydrocortisone (approximately 10 mg/m2/d) and fludrocortisone (usually 0.1 mg/d), and they will be maintained on this regimen for life.1,3
During acute illness, the doses of hydrocortisone are usually tripled and given 3 times per day to address the increased cortisol needs of the stress response. Lack of stress dose steroids in the setting of illness can lead to repeat adrenal crisis events.
Patients should be taught about intramuscular (IM) hydrocortisone use (100 mg IM) for emergencies and should have medical identification. In many states, emergency medical technicians (EMTs) are now able to administer the patient’s own supply of hydrocortisone. EMTs have even begun carrying hydrocortisone in some states in response to a campaign by the CARES Foundation, a nonprofit organization dedicated to helping families and individuals affected by congenital adrenal hyperplasia.
We started our patient on 100 mg/m2/d hydrocortisone 24 hours after he was admitted to the PICU. (At that time, his sodium level was 110 mEq/L.) Forty-eight hours after admission, we started the patient on fludrocortisone for mineralocorticoid effect at 0.1 mg/d. (The patient’s sodium level was 122 mEq/L). At 72 hours after admission, the patient’s sodium level was 137 mEq/L and his mental status was normal. Normal saline was discontinued when sodium normalized. He was discharged 2 days later. He was informed he should continue these medications for life, though doses might be adjusted slightly with time.
Two weeks later, our patient’s sodium level had reached 141 mEq/L and his weight loss, depression, vomiting, and fatigue had resolved. He stopped taking his SSRI. He was still craving extra salt, but not as much, and his urine was no longer a very dark yellow.
In retrospect, starting this patient on steroids earlier may not have resulted in any more of a rapid sodium rise than that which occurred otherwise, but we believe that our concern for CPM at that time justified the delay in steroid use. We felt it was safe to delay steroids because the patient’s BP was stable and his clinical picture was rapidly improving. In most cases, however, delaying steroids is not advisable.
THE TAKEAWAY
Adrenal insufficiency can be clearly diagnosed via labs and clinical presentation, and is potentially lethal if unrecognized. The predominant manifestations of adrenal crisis are hypotension and shock, usually with hyponatremia and hyperkalemia. During stressful events or illness, patients should increase their glucocorticoid dose. If they are on hydrocortisone, instructions are usually to triple the dose, and give the medication 3 times a day. Patients require instruction beforehand on how and when to increase doses for illness so that they can handle this on their own. Patients should carry a medical identification card so that their condition is evident to anyone caring for them in the ED.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.
1. Husebye ES, Allolio B, Arlt W, et al. Consensus statement on the diagnosis, treatment and follow-up of patients with primary adrenal insufficiency. J Intern Med. 2014;275:104-115.
2. Betterle C, Morlin L. Autoimmune Addison’s disease. Endocr Dev. 2011;20:161-172.
3. Brandão Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014;13:408-411.
Sore throat • vaginal discharge • labial ulcer • Dx?
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
THE CASE
The mother of a 13-year-old girl brought her daughter to our family medicine clinic for follow-up after being seen in the emergency department (ED) 3 days earlier. The girl had presented to the ED with a one-day history of back, chest, and vaginal pain. She was diagnosed with a urinary tract infection and treated empirically with phenazopyridine and cephalexin pending a urine culture.
During the follow-up appointment, the patient complained of worsening vaginal pain and increased vaginal discharge, but reported resolution of her back and chest pain. She also said that a week earlier, she’d had a fever that reached 104° F and a sore throat. She denied urinary frequency/urgency, sexual activity, or sexual abuse. The result of the urine culture performed in the ED was <10,000 col/mL (normal urogenital flora).
A genitourinary (GU) exam revealed erythematous patches with small amounts of crusting at the inner labia bilaterally. The labia were also swollen and diffusely tender to palpation. The patient had a white/gray discharge, but no vesicles or papules. The physician was unable to place a speculum due to pain.
The differential diagnosis at the time included candidal vaginitis and cellulitis. Since the patient’s skin was non-erythematous and she had vaginal discharge, she was treated for presumed severe candidal vaginitis with fluconazole and clotrimazole 1% cream. (The antibiotics were stopped because the patient reported worsening symptoms after they were prescribed in the ED.) The patient was told to return to the ED if she experienced signs and symptoms such as worsening vaginal pain or discharge, fever, or chills. A repeat urine culture was performed and the results came back normal.
Worsening symptoms. Six days later, the patient returned to the ED with urinary hesitation and persistent dysuria; she was admitted for pain control. She also complained of worsening labial swelling and increased vaginal discharge despite adherence to the fluconazole and clotrimazole cream regimen, which were discontinued on admission to the ED. She continued to deny being sexually active or abused.
A GU exam showed a 1-cm shallow ulcer on the right labium and a copious amount of foul-smelling white discharge. An Ob/Gyn resident and attending physician examined the patient; their differential diagnosis at this point included herpes simplex virus (HSV), Epstein-Barr virus (EBV), gonorrhea/chlamydia, and trauma. The patient was given topical lidocaine for pain control and started on acyclovir for presumed HSV while awaiting the HSV test results. A pelvic ultrasound and laboratory work-up were ordered at this time as well.
THE DIAGNOSIS
The pelvic ultrasound showed that the uterus was a normal size and that there was no gross mass or significant pelvic fluid. The patient’s right ovary measured 2.8 × 1.6 cm; the left ovary was not seen.
The patient’s laboratory work-up included an unremarkable comprehensive metabolic panel. A complete blood count was within normal limits, except for the patient’s monocyte level, which was at 12.9% (reference range: 0%-12%). The patient had a negative urinary human chorionic gonadotropin test, and was negative for HSV, chlamydia, gonorrhea, and trichomoniasis. A rapid plasma reagin test and human immunodeficiency virus antibody (1+2) tests were nonreactive. A wet prep was negative. A mononuclear spot test (monospot), however, was positive.
Results from the monospot testing took several days to return. By the time the results arrived, the patient had been transferred to a local children’s hospital for assessment in their pediatric urology department, as she was experiencing urinary hesitation and required catheterization. The diagnosis of infectious mononucleosis presenting with genital ulcer was made. EBV cultures were never obtained, but seemed to be the likely cause of the patient’s infectious mononucleosis given her clinical symptoms and lab results.
DISCUSSION
Approximately 95% of adults worldwide are infected with EBV.1 While the infection is often asymptomatic, some patients will develop infectious mononucleosis.1 EBV is the most common cause of infectious mononucleosis, mainly affecting teenagers and young adults (especially college students). At least 25% of teenagers and young adults who become infected with EBV will develop infectious mononucleosis.2
Typical symptoms of infectious mononucleosis include extreme fatigue, fever, sore throat, and head and body aches.2 In this case, the patient did have a fever and sore throat one week prior to presentation at our clinic, but she never complained of fatigue.
The association between mononucleosis and genital ulcers is not well known,3,4 and the exact method by which EBV causes genital ulcers is unclear.5 One review found that only 13 instances of genital ulceration in females attributable to EBV infection had been reported.5 When ulceration does occur, the majority of cases have involved young females who presented with only mild symptoms of mononucleosis.3,6 EBV has been found to present in the cervix, which suggests direct inoculation.3,6
Our patient remained catheterized for 2 days while in the children’s hospital. Her ulcer started to heal and she was sent home in stable condition. No additional follow-up was required and the ulcer did not recur.
THE TAKEAWAY
Include infectious mononucleosis in the differential for patients presenting with vaginal ulcers—especially those who deny sexual activity. Including testing for EBV and mononucleosis antibodies in the work-up can aid in the diagnosis. Cases such as this one are also a good reminder of the need to question young people while their parents/guardians are not in the examroom to foster an open and honest patient-physician relationship.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
1. Womack J, Jimenez M. Common questions about infectious mononucleosis. Am Fam Physician. 2015;91:372-376.
2. Centers for Disease Control and Prevention. About infectious mononucleosis. Centers for Disease Control and Prevention Web site. Available at: http://www.cdc.gov/epstein-barr/about-mono.html. Accessed April 26, 2016.
3. Lorenzo CV, Robertson WS. Genital ulcerations as presenting symptom of infectious mononucleosis. J Am Board Fam Pract. 2005;18:67-68.
4. Sisson BA, Glick L. Genital ulceration as a presenting manifestation of infectious mononucleosis. J Pediatr Adolesc Gynecol. 1998;11:185-187.
5. Barnes CJ, Alió AB, Cunningham BB, et al. Epstein-Barr virus-associated genital ulcers: an under-recognized disorder. Pediatr Dermatol. 2007;24:130-134.
6. Wilson RW. Genital ulcers and mononucleosis. Pediatr Infect Dis J. 1993;12:418.
Paraneoplastic syndrome and underlying breast cancer: a worsening rash despite initiation of chemotherapy
DRESS Syndrome With Autoimmune Hepatitis From Strontium Ranelate
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.
A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).
A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.

A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).

A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs. The pathogenesis of DRESS syndrome is still unknown. Immunological factors such as a defect in detoxification of culprit drugs and infections seem to be involved. The most commonly associated drugs are anticonvulsants and sulfonamides, but dapsone, allopurinol, and minocycline also have been reported to be associated with DRESS syndrome.1
Although therapies for postmenopausal osteoporosis are considered to be safe from cutaneous side effects, there have been several reported cases of DRESS syndrome associated with strontium ranelate.2 Strontium ranelate is not used in the United States; nevertheless, some US patients may be taking this drug as an alternative to the current US Food and Drug Administration–approved drugs for osteoporosis. We report a case of DRESS syndrome in a woman who developed an extensive maculopapular rash, eosinophilia, dyspnea, bilateral cervical lymphadenopathy, and reactivation of Epstein-Barr virus (EBV) with liver damage 3 weeks after administration of strontium ranelate for postmenopausal osteoporosis. Approximately 6 months after total remission of skin conditions, the patient developed autoimmune hepatitis.
Case Report
A 64-year-old woman presented to the emergency department with dyspnea, fever (temperature, 38.5°C), and a generalized rash that had developed a few days prior. The patient reported that she was previously in good health and had no prior allergic episodes. She had been taking strontium ranelate for 3 weeks to treat postmenopausal osteoporosis and reported no other medication use. The patient was hospitalized because of worsening symptoms. Physical examination revealed a pruritic maculopapular rash involving the trunk, arms, and legs (Figure 1) with facial edema, mild inspiratory as well as expiratory dyspnea, and wheezing on all lung fields. An enlarged soft liver (6–7 cm from the right costal arch) and cervical bilateral lymphadenopathy were found.

A chest radiograph detected a slight increase of the peribronchial thickening with interstitial involvement at the bilateral basal and perihilar levels, and an ultrasound of the chest confirmed the presence of many enlarged cervical bilateral lymph nodes between 2 and 4 cm in diameter.
Laboratory tests revealed the following values: leukocytosis (21,390/μL [reference range, 4500–11,000/μL]) with eosinophilia (27% [reference range, 2.7%]; 5780/μL [reference range, 0–450/μL]), elevated C-reactive protein (20 mg/L [reference range, 0.08–3.1 mg/L]), elevated erythrocyte sedimentation rate (35 mm/h [reference range, 0–20 mm/h]), a reactivation of EBV confirmed by simultaneous seropositivity to early antigen IgM and EBV nuclear antigen, liver damage with notable increases in liver function tests (aspartate aminotransferase, 51 U/L [reference range, 10–30 U/L]); alanine aminotransferase, 104 U/L [reference range 10–40 U/L]); γ-glutamyltransferase, 52 U/L [reference range, 2–30 U/L]), and no thyroid dysfunction.
Blood and urine cultures; antinuclear antibodies; and serology for hepatitis A, B, and C virus, as well as herpes simplex virus type 6 (HHV-6), chlamydia, Mycoplasma, and cytomegalovirus (CMV) were all negative. Histologic examination after skin biopsy showed keratinocytes with spongiosis, intraepidermal eosinophilic infiltration, suffusion of red blood cells with perivascular granulocytes, and lymphocyte inflammatory infiltrate (Figure 2).

A diagnosis of DRESS syndrome was made on the basis of the following clinical data supported by laboratory findings: generalized maculopapular rash, eosinophilia, lung involvement with dyspnea, bilateral cervical lymphadenopathy, and liver damage, as well as an identified reactivation of EBV and onset of symptoms 3 weeks after treatment with strontium ranelate.
The patient was given intravenous methylprednisolone 120 mg once daily for 1 week in gradually decreasing doses. Three weeks of steroid therapy were necessary to obtain the first good results. Improvement of the patient’s clinical condition was considerably slow. Fever and rash gradually disappeared and the patient was discharged with oral corticosteroids. In the 2 months after starting systemic corticosteroid therapy, the lesions had not progressed and all other clinical symptoms improved. A slow but notable regression of the skin reaction was observed.
In a subsequent checkup approximately 8 months following initial presentation, the patient developed autoimmune hepatitis. There was a notable increase in liver enzymes and serum immunoglobulin content as well as positivity of antinuclear antibodies (1:160) and antimitochondrial antibodies (1:160). A liver biopsy was performed and confirmed the histologic pattern of autoimmune hepatitis. Thyroid function was reevaluated, but no other autoimmune disease was identified.
The patient was given another dose of steroids (prednisolone 25 mg daily). Liver function normalized within 1 month (aspartate aminotransferase levels went from 195 U/L to 21 U/L; alanine aminotransferase went from 324 U/L to 21 U/L; γ-glutamyltransferase went from 268 U/L to 63 U/L). The patient is currently taking a maintenance dose of prednisolone 5 mg and has normal liver function.
Comment
Uses of Strontium Ranelate
Strontium ranelate is recommended for reducing the risk for fracture in postmenopausal women 70 years and older with a bone mineral density T-score of –3.0 or lower (ie, primary prevention) as well as for the treatment of morphometric vertebral fracture in established postmenopausal osteoporosis (ie, secondary prevention). Strontium ranelate has a dual action that includes increasing bone formation and reducing bone resorption, leading to rebalancing of bone remodeling in favor of bone formation. Strontium ranelate was shown to increase the recruitment and activity of osteoblastic cells and to inhibit the recruitment and activity of osteoclasts.2 The recommended dose of oral strontium ranelate is 2 g once daily.
Side Effects of Strontium Ranelate
In a 3-year study of side effects associated with strontium ranelate, severe reactions were described in 23% of the reported adverse effects in 844 patients.3 In this study, cardiovascular effects, particularly thromboembolism, and DRESS syndrome were the most frequent side effects. Since its introduction in the market, at least 16 cases of DRESS syndrome related to strontium ranelate use have been reported in Europe, including 2 fatal cases.2 Two deaths have been reported to be associated with this drug,2 which was the basis of the warning document by the European Medicines Agency regarding the risk for strontium ranelate inducing DRESS syndrome.4
Development of DRESS Syndrome
The most common agents involved in DRESS syndrome are anticonvulsants, sulfonamides, dapsone, minocycline, allopurinol, and gold salts, as well as celecoxib, antituberculosis drugs, nonsteroidal anti-inflammatory drugs, antibiotics, calcium channel blockers, and antiretroviral drugs.5,6 The mortality rate of DRESS syndrome is 10%.6
The pathophysiology of DRESS syndrome is still unclear. Altered drug metabolism, genetic predisposition, and concomitant infection or reactivation of bacterial or viral infection (eg, HHV-6, EBV, CMV, human immunodeficiency virus, influenza, viral hepatitis) could be factors leading to development of DRESS. Autoimmune or connective-tissue diseases also have been suggested to increase the risk.7
Clinicians should suspect DRESS syndrome in any patient developing a rash 3 to 6 weeks after starting drug therapy. This disorder often starts with fever (temperature >38°C) and includes cutaneous symptoms such as generalized rash that may progress to exfoliative dermatitis. There usually is involvement of one or several internal organs with the development of hepatitis; interstitial pneumonia; interstitial nephritis; myopericarditis; myositis; pancreatitis; thyroiditis; and hematological abnormalities, primarily eosinophilia or atypical lymphocytosis. Facial edema and lymphadenopathy also may be present. A skin biopsy can confirm the clinical diagnosis of DRESS syndrome but is not specific because cutaneous histologic patterns often show a lymphocytic infiltrate that sometimes mimics cutaneous lymphoma. Other diseases that DRESS syndrome may mimic include Stevens-Johnson syndrome and toxic epidermal necrolysis as well as Kawasaki disease, Still disease, acute viral infections, idiopathic hypereosinophilic syndrome, and lymphoma, which should be excluded from the differential diagnosis.8
Diagnosis of DRESS Syndrome
There is no gold standard for the diagnosis of DRESS syndrome. In our case, the diagnosis of DRESS syndrome was based on the RegiSCAR (European Registry of Severe Cutaneous Adverse Reactions to Drugs) score as described by Kardaun et al,9 which grades DRESS syndrome cases as excluded (<2 points), possible (2–3 points), probable (4–5 points), or definite (>5 points) based on the following clinical criteria: fever (temperature >38.5°C; from a minimum of –1 point if absent to a maximum of 0 points if present); enlarged lymph nodes (from a minimum of 0 points if absent to a maximum of 1 point if present); eosinophilia (0 points if absent, 1 point if 10%–19% or 700–1500 μL, 2 points if ≥20% or >1500 μL); atypical lymphocytes (from a minimum of 0 points if absent to maximum of 1 point if present); skin involvement with rash (1 point if >50% of body surface area is involved, 1 point if there is a maculopapular rash, 1 point if skin biopsy suggests DRESS syndrome); organ involvement (1 point each for liver, kidneys, lungs, muscle/heart, pancreas, and other organs); resolution in at least 15 days (from a minimum of –1 point if absent to maximum of 0 points if present); and evaluation of other potential causes measuring antinuclear antibodies, blood culture, and serology for hepatitis virus (A–C), chlamydia, and Mycoplasma (1 point if 3 or more are negative and none positive). Virus reactivation also should be considered a main characteristic of DRESS syndrome. Therefore, its prevalence is not homogenous, so the absence of viral reactivation cannot be considered exclusion criteria. Several case reports and a few well-documented series have evidenced markers of virus reactivation in many cases of DRESS. Herpes simplex virus 6, CMV, and EBV are the most frequently reactivated.
The total RegiSCAR score of 8 in our case was taken as a definite indication of DRESS syndrome (temperature, 38.5°C [0 points]; enlarged lymph nodes [1 point]; eosinophilia, ≥20% or >1500 μL [2 points]; skin involvement with >50% body surface area involved [1 point] with a maculopapular rash [1 point] and histopathologic findings suggesting DRESS syndrome [1 point]; lung and liver involvement [2 points]). The causative drug was identified by carefully collecting the patient’s medication history and by evaluating clinical outcome characterized by improved skin and systemic symptoms after discontinuation of strontium ranelate.
Because of the high morbidity of DRESS syndrome, it needs to be diagnosed effectively and must be considered in the differential for any patient developing the triad of skin rash, hypereosinophilia, and systemic symptoms, as well as several other side effects when taking strontium ranelate.10
Therapies for DRESS Syndrome
Treatment of DRESS syndrome has not yet been standardized. Prompt withdrawal of the causative drug is the only mandatory activity in the treatment of DRESS syndrome. Systemic corticosteroids may be needed for organ or life-threatening disease, though the efficacy is controversial because it may result in activation of HHV-6, which in turn is probably involved in the pathogenesis of DRESS syndrome.
Conclusion
This case confirms that strontium ranelate should be considered a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome. Case reports underline the importance of recognition of cutaneous adverse reactions in patients undergoing treatment of postmenopausal osteoporosis. The prognosis is good with immediate recognition followed by immediate and permanent withdrawal of the drug, along with hospitalization and systemic corticosteroids when necessary. The possibility of developing autoimmune hepatitis as a part of DRESS syndrome related to strontium ranelate has been reported,11 usually months after the acute episode.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
- Tas S, Simonart T. Management of drug rash with eosinophilia and systemic symptoms (DRESS syndrome): an update. Dermatology. 2003;206:353-356.
- Le Merlouette M, Adamski H, Dinulescu M, et al. Strontium ranelate–induced DRESS syndrome. Ann Dermatol Venereol. 2011;138:124-128.
- Jonville-Bera AP, Autret-Leca E. Adverse drug reactions of strontium ranelate (Protelos®) in France. Presse Med. 2011;40:453-462.
- Assessment report for Protelos and Osseor. European Medicines Agency website. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Assessment_Report_-_Variation/human/000560/WC500131789.pdf). Published May 25, 2012. Accessed May 9, 2016.
- Breathnach S. Drug rash eosinophilia and systemic symptoms (DRESS) syndrome. types of clinical reaction: drug reaction. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 4. 8th ed. Hoboken, NJ: Oxford Wiley-Blackwell Publications; 2010:75.26.
- Lee JH, Park HK, Heo J, et al. Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome induced by celecoxib and anti-tuberculosis drugs. J Korean Med Sci. 2008;23:521-525.
- Musette P, Brandi ML, Cacoub P, et al. Treatment of osteoporosis: recognizing and managing cutaneous adverse reactions and drug-induced hypersensitivity. Osteoporos Int. 2010;21:723-732.
- Telles Rudge de Aquino R, Vieitas Vergueiro CS, Ruffolo Magliari ME, et al. Sulfasalazine-induced DRESS syndrome (drug rash with eosinophilia and systemic symptoms). Sao Paulo Med J. 2008;126:225-226.
- Kardaun SH, Sidoroff A, Valeyrie-Allanore L, et al. Variability in the clinical pattern of cutaneous side-effects of drugs with systemic symptoms: does a DRESS syndrome really exist? Br J Dermatol. 2007;156:609-611.
- Pernicova I, Middleton ET, Aye M. Rash, strontium ranelate and DRESS syndrome put into perspective. European Medicine Agency on the alert [published online September 20, 2008]. Osteoporos Int. 2008;19:1811-1812.
- Kinyó A, Belsö N, Nagy N, et al. Strontium ranelate-induced DRESS syndrome with persistent autoimmune hepatitis. Acta Derm Venereol. 2011;91:205-206.
Practice Points
- Drug rash with eosinophilia and systemic symptoms (DRESS) syndrome refers to a severe, acute, potentially fatal, multisystem adverse drug reaction characterized by skin rash, fever, hematological abnormalities, and lymphadenopathy with involvement of several internal organs.
- Strontium ranelate should be considered as a possible factor in the etiopathology of DRESS syndrome and in the development of autoimmune hepatitis as a part of DRESS syndrome.
Lupus Erythematosus and Localized Scleroderma Coexistent at the Same Sites: A Rare Presentation of Overlap Syndrome of Connective-Tissue Diseases
Although lupus erythematosus (LE) and scleroderma are regarded as 2 distinct entities, there have been multiple cases described in the literature showing an overlap between these 2 disease processes. We report the case of a 60-year-old man with clinical and histopathologic findings consistent with the presence of localized scleroderma and discoid LE (DLE) within the same lesions. We also present a review of the literature and delineate the general patterns of coexistence of these 2 diseases based on our case and other reported cases.
Case Report
A 60-year-old man presented with a progressive pruritic rash on the face, neck, and upper back of approximately 20 to 30 years’ duration. On initial evaluation, the patient was found to have indurated hypopigmented plaques with follicular plugging bilaterally on the cheeks, temples, ears, and upper back (Figure 1). Punch biopsies were performed on the left cheek and upper back. Histopathology was notable for vacuolar interface dermatitis with dermal sclerosis at both sites. Specifically, interface changes, basement membrane thickening, and periadnexal inflammation were present on histopathologic examination from both biopsies supporting a diagnosis of DLE (Figure 2A). However, there also was sclerosis present in the reticular dermis, suggesting a diagnosis of localized scleroderma (Figure 2B). Direct immunofluorescence was negative for a lupus band. Laboratory workup was positive for antinuclear antibody (titer, 1:40; speckled pattern) and anti–Sjögren syndrome antigen A but negative for double-stranded DNA antibody, anti-Smith antibody, anti–Sjögren syndrome antigen B, and Scl-70.

The patient was started on oral hydroxychloroquine 200 mg twice daily and clobetasol oint-ment 0.05% twice daily to affected areas. After 2 weeks of treatment, he developed urticaria on the trunk and the hydroxychloroquine was discontinued. He continued using only topical steroids following a regimen of applying clobetasol ointment 0.05% twice daily for 2 weeks, alternating with the use of triamcinolone ointment 0.1% twice daily for 2 weeks with improvement of the pruritus, but the induration and hypopigmentation remained unchanged. Alternative systemic medication was started with mycophenolate mofetil 1 g twice daily. The patient showed remarkable clinical improvement with a decrease in induration and partial resolution of follicular plugging after 4 months of treatment with mycophenolate mofetil in combination with the topical steroid regimen.
Comment
Autoimmune connective-tissue diseases (CTDs) often occur with a wide range of symptoms and signs. Most often patients affected by these diseases can be sorted into one of the named CTDs such as LE, rheumatoid arthritis, scleroderma, polymyositis/dermatomyositis, and Sjögren syndrome. On the other hand, it is widely recognized that patients with one classic autoimmune CTD are likely to possess multiple autoantibodies, and a small number of these patients develop symptoms and/or signs that satisfy the diagnostic criteria of a second autoimmune CTD; these latter patients are said to have an overlap syndrome.1 The development of a second identifiable CTD, hence indicating an overlap syndrome, may occur coincident to the initial CTD or may occur at a different time.1
Essentially all 5 of the CTDs mentioned above have been reported to occur in combination with one another. Most of the reports involving overlap among these 5 CTDs include patients with multiorgan systemic involvement without cutaneous involvement, leading to a fairly simple straightforward classification of overlap syndromes as viewed by rheumatologists.1
When the overlap occurs between the localized forms of scleroderma and purely cutaneous LE, the situation becomes even more complicated, as the skin lesions of the 2 diseases may occur at separate locations or coexistent disease may develop in the same location, as in our case.
More than 100 cases have been reported wherein LE and scleroderma coexist in the same patient.1 Most of these cases have been examples of type 1 overlap (Table 1), though a few have been type 2 overlap, with localized scleroderma coexisting with systemic LE or vice versa.1,2 There are rare reports of an overlap of the localized form of both of these entities (type 3 overlap), as demonstrated in our patient. According to a PubMed search of articles indexed for MEDLINE using the search terms localized scleroderma and morphea as well as discoid lupus erythematosus, we found 12 other cases describing type 3 overlap (Table 2).

The first case was described in 1976 as annular atrophic plaques on the face and neck of a 48-year-old man.3 As in our case, there were overlapping features of DLE and localized scleroderma. The investigators postulated that the entity was an atypical form of DLE.3 There were 4 more cases described in 1978, but the majority of these patients were young women with linear plaques. Instead of calling the disease a new form of DLE, the investigators considered it to be an overlap syndrome.4 Many years passed before another similar case was described in the literature in 1990.5 Interestingly, the investigators performed multiple biopsies on this patient over several years and observed that the pathology changed from subacute cutaneous LE to an overlap of subacute cutaneous LE and localized scleroderma to localized scleroderma, suggesting that localized scleroderma was the end result of persistent inflammation from the cutaneous LE lesions. The investigators compared the evolution of subacute cutaneous LE to localized scleroderma in the patient to the evolution of acute graft-versus-host disease (GVHD) to chronic GVHD. Acute GVHD has a lichenoid tissue reaction that develops into sclerosis in the chronic form.5
Additionally, there were 3 cases in the literature showing an overlap of lupus panniculitis with localized scleroderma.6,7 Stork and Vosmik6 described a case of a 22-year-old woman with lesions clinically suspicious for localized scleroderma, with lupus panniculitis demonstrated on histopathology. They discussed the difficulty in differentiating between lupus panniculitis and localized scleroderma but did not specify whether they believed the case represented a distinct entity or an overlap syndrome.6 Alternatively, Marzano et al7 reported 2 similar cases, which the investigators considered to be a specific new variant called sclerodermic linear lupus panniculitis.
In the last 10 years, there were 3 additional cases reported that described an overlap of DLE and localized scleroderma in the same anatomic location, similar to our patient.8-10 Although Julia et al8 considered their case to be an example of the distinct entity called sclerodermiform linear LE, the investigators in the other 2 cases described the possibility of an overlap syndrome.9,10
Based on reported cases, we found the following patterns in the overlap of cutaneous LE and localized scleroderma: predilection for young women, photodistributed lesions, DLE, linear morphology clinically, and positivity along the dermoepidermal junction on direct immunofluorescence. As in our case, the few affected men were older compared to affected women. Men ranged in age from 34 to 48 years compared to women who ranged in age from 7 to 29 years. We did not find a pattern in the laboratory findings in these patients. Most patients had a good response to antimalarials, topical steroids, or systemic steroids.
Conclusion
All 12 previously reported cases showed some form of overlap of cutaneous LE and localized scleroderma. As previously discussed, overlap syndromes are common in patients with CTDs. We postulate that our case represents a rare form of overlap syndrome, with the overlap occurring at the same clinical sites.
- Iaccarino L, Gatto M, Bettio S, et al. Overlap connective tissue disease syndromes [published online June 26, 2012]. Autoimmun Reviews. 2012;12:363-373.
- Balbir-Gurman A, Braun-Moscovici Y. Scleroderma overlap syndrome. Isr Med Assoc J. 2011;13:14-20.
- Chorzelski TP, Jablonska S, Blaszyczyk M, et al. Annular atrophic plaques of the face. Arch Dermatol. 1976;112:1143-1145.
- Umbert P, Winkelmann RK. Concurrent localized scleroderma and discoid lupus erythematosus. Arch Dermatol. 1978;114:1473-1478.
- Rao BK, Coldiron B, Freeman RG, et al. Subacute cutaneous lupus progressing to morphea erythematosus lesions. J Am Acad Dermatol. 1990;23(5, pt 2):1019-1022.
- Stork J, Vosmik F. Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol. 1994;19:79-82.
- Marzano AV, Tanzi C, Caputo R, et al. Sclerodermic linear lupus panniculitis: report of two cases. Dermatology. 2005;210:329-332.
- Julia M, Mascaro JM Jr, Guilaber A, et al. Sclerodermiform linear lupus erythematosus: a distinct entity or coexistence of two autoimmune diseases? J Am Acad Dermatol. 2008;58:665-667.
- Mir A, Tlougan B, O’Reilly K, et al. Morphea with discoid lupus erythematosus. Dermatol Online J. 2011;17:10.
- Khelifa E, Masouye I, Pham HC, et al. Linear sclerodermic lupus erythematosus, a distinct variant of linear morphea and chronic cutaneous lupus erythematosus. Int J Dermatol. 2011;50:1491-1495.
Although lupus erythematosus (LE) and scleroderma are regarded as 2 distinct entities, there have been multiple cases described in the literature showing an overlap between these 2 disease processes. We report the case of a 60-year-old man with clinical and histopathologic findings consistent with the presence of localized scleroderma and discoid LE (DLE) within the same lesions. We also present a review of the literature and delineate the general patterns of coexistence of these 2 diseases based on our case and other reported cases.
Case Report
A 60-year-old man presented with a progressive pruritic rash on the face, neck, and upper back of approximately 20 to 30 years’ duration. On initial evaluation, the patient was found to have indurated hypopigmented plaques with follicular plugging bilaterally on the cheeks, temples, ears, and upper back (Figure 1). Punch biopsies were performed on the left cheek and upper back. Histopathology was notable for vacuolar interface dermatitis with dermal sclerosis at both sites. Specifically, interface changes, basement membrane thickening, and periadnexal inflammation were present on histopathologic examination from both biopsies supporting a diagnosis of DLE (Figure 2A). However, there also was sclerosis present in the reticular dermis, suggesting a diagnosis of localized scleroderma (Figure 2B). Direct immunofluorescence was negative for a lupus band. Laboratory workup was positive for antinuclear antibody (titer, 1:40; speckled pattern) and anti–Sjögren syndrome antigen A but negative for double-stranded DNA antibody, anti-Smith antibody, anti–Sjögren syndrome antigen B, and Scl-70.


The patient was started on oral hydroxychloroquine 200 mg twice daily and clobetasol oint-ment 0.05% twice daily to affected areas. After 2 weeks of treatment, he developed urticaria on the trunk and the hydroxychloroquine was discontinued. He continued using only topical steroids following a regimen of applying clobetasol ointment 0.05% twice daily for 2 weeks, alternating with the use of triamcinolone ointment 0.1% twice daily for 2 weeks with improvement of the pruritus, but the induration and hypopigmentation remained unchanged. Alternative systemic medication was started with mycophenolate mofetil 1 g twice daily. The patient showed remarkable clinical improvement with a decrease in induration and partial resolution of follicular plugging after 4 months of treatment with mycophenolate mofetil in combination with the topical steroid regimen.
Comment
Autoimmune connective-tissue diseases (CTDs) often occur with a wide range of symptoms and signs. Most often patients affected by these diseases can be sorted into one of the named CTDs such as LE, rheumatoid arthritis, scleroderma, polymyositis/dermatomyositis, and Sjögren syndrome. On the other hand, it is widely recognized that patients with one classic autoimmune CTD are likely to possess multiple autoantibodies, and a small number of these patients develop symptoms and/or signs that satisfy the diagnostic criteria of a second autoimmune CTD; these latter patients are said to have an overlap syndrome.1 The development of a second identifiable CTD, hence indicating an overlap syndrome, may occur coincident to the initial CTD or may occur at a different time.1
Essentially all 5 of the CTDs mentioned above have been reported to occur in combination with one another. Most of the reports involving overlap among these 5 CTDs include patients with multiorgan systemic involvement without cutaneous involvement, leading to a fairly simple straightforward classification of overlap syndromes as viewed by rheumatologists.1
When the overlap occurs between the localized forms of scleroderma and purely cutaneous LE, the situation becomes even more complicated, as the skin lesions of the 2 diseases may occur at separate locations or coexistent disease may develop in the same location, as in our case.
More than 100 cases have been reported wherein LE and scleroderma coexist in the same patient.1 Most of these cases have been examples of type 1 overlap (Table 1), though a few have been type 2 overlap, with localized scleroderma coexisting with systemic LE or vice versa.1,2 There are rare reports of an overlap of the localized form of both of these entities (type 3 overlap), as demonstrated in our patient. According to a PubMed search of articles indexed for MEDLINE using the search terms localized scleroderma and morphea as well as discoid lupus erythematosus, we found 12 other cases describing type 3 overlap (Table 2).


The first case was described in 1976 as annular atrophic plaques on the face and neck of a 48-year-old man.3 As in our case, there were overlapping features of DLE and localized scleroderma. The investigators postulated that the entity was an atypical form of DLE.3 There were 4 more cases described in 1978, but the majority of these patients were young women with linear plaques. Instead of calling the disease a new form of DLE, the investigators considered it to be an overlap syndrome.4 Many years passed before another similar case was described in the literature in 1990.5 Interestingly, the investigators performed multiple biopsies on this patient over several years and observed that the pathology changed from subacute cutaneous LE to an overlap of subacute cutaneous LE and localized scleroderma to localized scleroderma, suggesting that localized scleroderma was the end result of persistent inflammation from the cutaneous LE lesions. The investigators compared the evolution of subacute cutaneous LE to localized scleroderma in the patient to the evolution of acute graft-versus-host disease (GVHD) to chronic GVHD. Acute GVHD has a lichenoid tissue reaction that develops into sclerosis in the chronic form.5
Additionally, there were 3 cases in the literature showing an overlap of lupus panniculitis with localized scleroderma.6,7 Stork and Vosmik6 described a case of a 22-year-old woman with lesions clinically suspicious for localized scleroderma, with lupus panniculitis demonstrated on histopathology. They discussed the difficulty in differentiating between lupus panniculitis and localized scleroderma but did not specify whether they believed the case represented a distinct entity or an overlap syndrome.6 Alternatively, Marzano et al7 reported 2 similar cases, which the investigators considered to be a specific new variant called sclerodermic linear lupus panniculitis.
In the last 10 years, there were 3 additional cases reported that described an overlap of DLE and localized scleroderma in the same anatomic location, similar to our patient.8-10 Although Julia et al8 considered their case to be an example of the distinct entity called sclerodermiform linear LE, the investigators in the other 2 cases described the possibility of an overlap syndrome.9,10
Based on reported cases, we found the following patterns in the overlap of cutaneous LE and localized scleroderma: predilection for young women, photodistributed lesions, DLE, linear morphology clinically, and positivity along the dermoepidermal junction on direct immunofluorescence. As in our case, the few affected men were older compared to affected women. Men ranged in age from 34 to 48 years compared to women who ranged in age from 7 to 29 years. We did not find a pattern in the laboratory findings in these patients. Most patients had a good response to antimalarials, topical steroids, or systemic steroids.
Conclusion
All 12 previously reported cases showed some form of overlap of cutaneous LE and localized scleroderma. As previously discussed, overlap syndromes are common in patients with CTDs. We postulate that our case represents a rare form of overlap syndrome, with the overlap occurring at the same clinical sites.
Although lupus erythematosus (LE) and scleroderma are regarded as 2 distinct entities, there have been multiple cases described in the literature showing an overlap between these 2 disease processes. We report the case of a 60-year-old man with clinical and histopathologic findings consistent with the presence of localized scleroderma and discoid LE (DLE) within the same lesions. We also present a review of the literature and delineate the general patterns of coexistence of these 2 diseases based on our case and other reported cases.
Case Report
A 60-year-old man presented with a progressive pruritic rash on the face, neck, and upper back of approximately 20 to 30 years’ duration. On initial evaluation, the patient was found to have indurated hypopigmented plaques with follicular plugging bilaterally on the cheeks, temples, ears, and upper back (Figure 1). Punch biopsies were performed on the left cheek and upper back. Histopathology was notable for vacuolar interface dermatitis with dermal sclerosis at both sites. Specifically, interface changes, basement membrane thickening, and periadnexal inflammation were present on histopathologic examination from both biopsies supporting a diagnosis of DLE (Figure 2A). However, there also was sclerosis present in the reticular dermis, suggesting a diagnosis of localized scleroderma (Figure 2B). Direct immunofluorescence was negative for a lupus band. Laboratory workup was positive for antinuclear antibody (titer, 1:40; speckled pattern) and anti–Sjögren syndrome antigen A but negative for double-stranded DNA antibody, anti-Smith antibody, anti–Sjögren syndrome antigen B, and Scl-70.


The patient was started on oral hydroxychloroquine 200 mg twice daily and clobetasol oint-ment 0.05% twice daily to affected areas. After 2 weeks of treatment, he developed urticaria on the trunk and the hydroxychloroquine was discontinued. He continued using only topical steroids following a regimen of applying clobetasol ointment 0.05% twice daily for 2 weeks, alternating with the use of triamcinolone ointment 0.1% twice daily for 2 weeks with improvement of the pruritus, but the induration and hypopigmentation remained unchanged. Alternative systemic medication was started with mycophenolate mofetil 1 g twice daily. The patient showed remarkable clinical improvement with a decrease in induration and partial resolution of follicular plugging after 4 months of treatment with mycophenolate mofetil in combination with the topical steroid regimen.
Comment
Autoimmune connective-tissue diseases (CTDs) often occur with a wide range of symptoms and signs. Most often patients affected by these diseases can be sorted into one of the named CTDs such as LE, rheumatoid arthritis, scleroderma, polymyositis/dermatomyositis, and Sjögren syndrome. On the other hand, it is widely recognized that patients with one classic autoimmune CTD are likely to possess multiple autoantibodies, and a small number of these patients develop symptoms and/or signs that satisfy the diagnostic criteria of a second autoimmune CTD; these latter patients are said to have an overlap syndrome.1 The development of a second identifiable CTD, hence indicating an overlap syndrome, may occur coincident to the initial CTD or may occur at a different time.1
Essentially all 5 of the CTDs mentioned above have been reported to occur in combination with one another. Most of the reports involving overlap among these 5 CTDs include patients with multiorgan systemic involvement without cutaneous involvement, leading to a fairly simple straightforward classification of overlap syndromes as viewed by rheumatologists.1
When the overlap occurs between the localized forms of scleroderma and purely cutaneous LE, the situation becomes even more complicated, as the skin lesions of the 2 diseases may occur at separate locations or coexistent disease may develop in the same location, as in our case.
More than 100 cases have been reported wherein LE and scleroderma coexist in the same patient.1 Most of these cases have been examples of type 1 overlap (Table 1), though a few have been type 2 overlap, with localized scleroderma coexisting with systemic LE or vice versa.1,2 There are rare reports of an overlap of the localized form of both of these entities (type 3 overlap), as demonstrated in our patient. According to a PubMed search of articles indexed for MEDLINE using the search terms localized scleroderma and morphea as well as discoid lupus erythematosus, we found 12 other cases describing type 3 overlap (Table 2).


The first case was described in 1976 as annular atrophic plaques on the face and neck of a 48-year-old man.3 As in our case, there were overlapping features of DLE and localized scleroderma. The investigators postulated that the entity was an atypical form of DLE.3 There were 4 more cases described in 1978, but the majority of these patients were young women with linear plaques. Instead of calling the disease a new form of DLE, the investigators considered it to be an overlap syndrome.4 Many years passed before another similar case was described in the literature in 1990.5 Interestingly, the investigators performed multiple biopsies on this patient over several years and observed that the pathology changed from subacute cutaneous LE to an overlap of subacute cutaneous LE and localized scleroderma to localized scleroderma, suggesting that localized scleroderma was the end result of persistent inflammation from the cutaneous LE lesions. The investigators compared the evolution of subacute cutaneous LE to localized scleroderma in the patient to the evolution of acute graft-versus-host disease (GVHD) to chronic GVHD. Acute GVHD has a lichenoid tissue reaction that develops into sclerosis in the chronic form.5
Additionally, there were 3 cases in the literature showing an overlap of lupus panniculitis with localized scleroderma.6,7 Stork and Vosmik6 described a case of a 22-year-old woman with lesions clinically suspicious for localized scleroderma, with lupus panniculitis demonstrated on histopathology. They discussed the difficulty in differentiating between lupus panniculitis and localized scleroderma but did not specify whether they believed the case represented a distinct entity or an overlap syndrome.6 Alternatively, Marzano et al7 reported 2 similar cases, which the investigators considered to be a specific new variant called sclerodermic linear lupus panniculitis.
In the last 10 years, there were 3 additional cases reported that described an overlap of DLE and localized scleroderma in the same anatomic location, similar to our patient.8-10 Although Julia et al8 considered their case to be an example of the distinct entity called sclerodermiform linear LE, the investigators in the other 2 cases described the possibility of an overlap syndrome.9,10
Based on reported cases, we found the following patterns in the overlap of cutaneous LE and localized scleroderma: predilection for young women, photodistributed lesions, DLE, linear morphology clinically, and positivity along the dermoepidermal junction on direct immunofluorescence. As in our case, the few affected men were older compared to affected women. Men ranged in age from 34 to 48 years compared to women who ranged in age from 7 to 29 years. We did not find a pattern in the laboratory findings in these patients. Most patients had a good response to antimalarials, topical steroids, or systemic steroids.
Conclusion
All 12 previously reported cases showed some form of overlap of cutaneous LE and localized scleroderma. As previously discussed, overlap syndromes are common in patients with CTDs. We postulate that our case represents a rare form of overlap syndrome, with the overlap occurring at the same clinical sites.
- Iaccarino L, Gatto M, Bettio S, et al. Overlap connective tissue disease syndromes [published online June 26, 2012]. Autoimmun Reviews. 2012;12:363-373.
- Balbir-Gurman A, Braun-Moscovici Y. Scleroderma overlap syndrome. Isr Med Assoc J. 2011;13:14-20.
- Chorzelski TP, Jablonska S, Blaszyczyk M, et al. Annular atrophic plaques of the face. Arch Dermatol. 1976;112:1143-1145.
- Umbert P, Winkelmann RK. Concurrent localized scleroderma and discoid lupus erythematosus. Arch Dermatol. 1978;114:1473-1478.
- Rao BK, Coldiron B, Freeman RG, et al. Subacute cutaneous lupus progressing to morphea erythematosus lesions. J Am Acad Dermatol. 1990;23(5, pt 2):1019-1022.
- Stork J, Vosmik F. Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol. 1994;19:79-82.
- Marzano AV, Tanzi C, Caputo R, et al. Sclerodermic linear lupus panniculitis: report of two cases. Dermatology. 2005;210:329-332.
- Julia M, Mascaro JM Jr, Guilaber A, et al. Sclerodermiform linear lupus erythematosus: a distinct entity or coexistence of two autoimmune diseases? J Am Acad Dermatol. 2008;58:665-667.
- Mir A, Tlougan B, O’Reilly K, et al. Morphea with discoid lupus erythematosus. Dermatol Online J. 2011;17:10.
- Khelifa E, Masouye I, Pham HC, et al. Linear sclerodermic lupus erythematosus, a distinct variant of linear morphea and chronic cutaneous lupus erythematosus. Int J Dermatol. 2011;50:1491-1495.
- Iaccarino L, Gatto M, Bettio S, et al. Overlap connective tissue disease syndromes [published online June 26, 2012]. Autoimmun Reviews. 2012;12:363-373.
- Balbir-Gurman A, Braun-Moscovici Y. Scleroderma overlap syndrome. Isr Med Assoc J. 2011;13:14-20.
- Chorzelski TP, Jablonska S, Blaszyczyk M, et al. Annular atrophic plaques of the face. Arch Dermatol. 1976;112:1143-1145.
- Umbert P, Winkelmann RK. Concurrent localized scleroderma and discoid lupus erythematosus. Arch Dermatol. 1978;114:1473-1478.
- Rao BK, Coldiron B, Freeman RG, et al. Subacute cutaneous lupus progressing to morphea erythematosus lesions. J Am Acad Dermatol. 1990;23(5, pt 2):1019-1022.
- Stork J, Vosmik F. Lupus erythematosus panniculitis with morphea-like lesions. Clin Exp Dermatol. 1994;19:79-82.
- Marzano AV, Tanzi C, Caputo R, et al. Sclerodermic linear lupus panniculitis: report of two cases. Dermatology. 2005;210:329-332.
- Julia M, Mascaro JM Jr, Guilaber A, et al. Sclerodermiform linear lupus erythematosus: a distinct entity or coexistence of two autoimmune diseases? J Am Acad Dermatol. 2008;58:665-667.
- Mir A, Tlougan B, O’Reilly K, et al. Morphea with discoid lupus erythematosus. Dermatol Online J. 2011;17:10.
- Khelifa E, Masouye I, Pham HC, et al. Linear sclerodermic lupus erythematosus, a distinct variant of linear morphea and chronic cutaneous lupus erythematosus. Int J Dermatol. 2011;50:1491-1495.
Practice Points
- Discoid lupus erythematosus and localized scleroderma may rarely overlap within the same lesions.
- Cutaneous overlap syndromes tend to respond well to antimalarials, topical steroids, and systemic steroids.
Total Hip Arthroplasty After Proximal Femoral Osteotomy: A Technique That Can Be Used to Address Presence of a Retained Intracortical Plate
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
Total hip arthroplasty (THA) is an effective treatment for advanced hip arthritis from a variety of causes, including osteoarthritis, inflammatory arthritis, posttraumatic arthritis, and sequelae of developmental disorders. It is not uncommon to perform THA in the presence of a previous proximal femoral osteotomy that may have been performed for slipped capital femoral epiphysis (SCFE), Legg-Calvé-Perthes disease, or developmental dysplasia of the hip, among other conditions. These osteotomies are commonly combined with internal fixation, a plate-and-screw device. These patients are at risk for developing degenerative arthritis at an earlier age than patients with other types of arthritis and subsequently may undergo THA at a younger age.1-3 Presence of a plate can pose a technical challenge during THA surgery. THA performed after intertrochanteric osteotomy has higher rates of perioperative and postoperative complications.4 Ferguson and colleagues4 noted difficulty during hardware removal in 24% of cases. Among the complications encountered were broken hardware, stripped screws, greater trochanteric fracture, stress risers from previous screw holes, canal narrowing from endosteal hypertrophy around hardware, and lateral cortical deficiency after removal of the side plate. As intertrochanteric osteotomies are often performed in patients who have yet to reach skeletal maturity, cortical hypertrophy can lead to complete coverage of the side plate and an “intracortical” position.
This article reports on 2 THA cases in which a technique was used to avoid intracortical plate removal and the resulting problems of lateral cortical deficiency. During each THA, the plate was left in place to avoid compromise of the lateral femoral cortex. The patients provided written informed consent for print and electronic publication of these case reports.
Case Reports
Case 1
An adolescent with bilateral SCFE was treated first with internal fixation of the right hip and subsequently with left proximal femoral osteotomy with internal fixation. He did well until age 31 years, when he developed progressively worsening pain about the left hip. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the left hip. Radiographs showed a sliding hip screw in place, with proximal femoral deformity consisting of femoral neck shortening and posterior angulation (Figures 1A, 1B). Preoperative Harris Hip Score was 54.5.
Case 2
A 51-year-old woman presented with a history of right hip problems dating back to age 13 years, when she sustained a fracture of the right hip and was treated with internal fixation. At age 15 years, she underwent proximal femoral osteotomy to correct residual deformity. She did well until age 45 years, when she developed worsening hip symptoms. Clinical findings and imaging studies were consistent with advanced degenerative arthritis of the right hip. Radiographs showed a fixed-angle blade plate in the proximal femur, with significant proximal femoral deformity (Figures 1C, 1D). Preoperative Harris Hip Score was 53.6.
Surgical Technique
In both cases, a standard series of radiographs was obtained—an anteroposterior (AP) radiograph of the pelvis and AP and cross-table lateral radiographs of the operative hip (Figure 1). Computed tomography (CT) with a metal-artifact-reducing technique may be useful in determining amount of cortical bone remaining under the plate. CT showed limited lateral cortex beneath the side plate and bony overgrowth covering the side plate. Preoperative templating was performed using previously described techniques.5
During THA, before removing any portion of any retained hardware, the surgeon should perform 3 important actions: Dislocate the hip, perform all appropriate capsular releases, and reduce the hip. Dislocating the hip before hardware removal significantly decreases the risk for fracture caused by stress risers, as the force required for dislocation is much more controlled because of the capsular releases. After hardware removal, the hip can be easily redislocated, and the femoral neck osteotomy can be performed.
When plate and screws are in an intracortical position, the screws can be removed only after removing the small shell of cortical bone covering them. The amount of bone to be removed is minimal. After the screws are removed, the plate remains in place. A motorized device with a metal-cutting attachment is used to transect the construct at the junction of the plate and barrel (case 1) or at the bend of a fixed-angle device (case 2). Laparotomy sponges are placed around the proximal femur to minimize the amount of soft tissue that could be exposed to metal shavings. Copious irrigation is used throughout this part of the procedure. Osteotomes are used to elevate the proximal portion of the plate and the barrel, preserving the distal portion of the plate on the lateral cortex of the femoral shaft.
After the head is removed, the rest of the THA can be performed using standard press-fit insertion technique (Figures 2A-2D). Care must be taken to ensure that the distal aspect of the femoral stem bypasses the most distal screw hole by at least 2 cortical diameters in order to reduce the risk for periprosthetic fracture.
By 2-year follow-up, both patients had regained excellent range of motion, ambulation, and overall function. Postoperative Harris Hip Scores were 86.6 and 83.8, respectively. There were no radiographic signs of complications.
Discussion
THA can be challenging in the setting of previously placed internal fixation devices, particularly devices inserted during a patient’s adolescence, as significant bony overgrowth can occur. The standard approach has been to remove the internal fixation device and then perform the THA. In most cases, and particularly when the internal fixation device is in an intracortical position, the result is significant compromise of bone. This article describes a technique in which a portion of the hardware is retained to avoid compromise of the lateral femoral cortex, thereby allowing insertion of a noncemented femoral component.
THA is the most effective procedure for reducing hip pain and disability in the setting of degenerative changes.6 Patients with SCFE, Legg-Calvé-Perthes disease, or developmental dysplasia of the hip generally are younger at the time they may be sufficiently symptomatic to consider THA.7,8 Many have had previous surgery using internal fixation devices. THAs after previous osteotomies with internal fixation devices are more technically demanding, require more operative time, are subject to more blood loss, and have a higher rate of complications, including femoral fracture. Ferguson and colleagues4 and Boos and colleagues9 found these surgeries were more difficult 33.8% and 36.8% of the time, respectively. For these reasons, some authors have recommended removing the internal fixation device as soon as the osteotomy is healed.4 However, this has not become the standard of care, and surgeons continue to perform THAs in the presence of a previous osteotomy with an internal fixation device in place.
The technique described in this article was used successfully in 2 cases. In each case, leaving the intracortical plate in place avoided compromise of the lateral femoral cortex and allowed insertion of a noncemented femoral component without complication. Of course, with the screw holes representing stress risers, careful insertion of the femoral component was required. Retaining the intracortical plate allowed it to function as part of the lateral femoral cortex, thereby maintaining the structural integrity of the femoral canal. As has been described for the 2 cases, a blade plate and plate and barrel were converted to a limited intracortical plate by removing the proximal portion of the plates—a modification that could be applied to other types of internal fixation devices that extend into the femoral neck as long as appropriate cutting tools are available.
Conclusion
THA in the setting of a retained internal fixation device is relatively common. This article describes a technique that can be used when a plate applied to the lateral femoral cortex has become intracortical as a result of extensive bony overgrowth. In using this technique to avoid plate removal, the surgeon eliminates the need for more extensive procedures aimed at compensating for deficiency of the femoral cortex in the area of plate removal. Although only 2 cases are presented here, this technique potentially can be used more broadly in these specific clinical situations.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
1. Engesæter LB, Engesæter IØ, Fenstad AM, et al. Low revision rate after total hip arthroplasty in patients with pediatric hip diseases. Acta Orthop. 2012;83(5):436-441.
2. Froberg L, Christensen F, Pedersen NW, Overgaard S. The need for total hip arthroplasty in Perthes disease: a long-term study. Clin Orthop Relat Res. 2011;469(4):1134-1140.
3. Furnes O, Lie SA, Espehaug B, Vollset SE, Engesæter LB, Havelin LI. Hip disease and the prognosis of total hip replacements. A review of 53,698 primary total hip replacements reported to the Norwegian Arthroplasty Register 1987-99. J Bone Joint Surg Br. 2001;83(4):579-586.
4. Ferguson GM, Cabanela ME, Ilstrup DM. Total hip arthroplasty after failed intertrochanteric osteotomy. J Bone Joint Surg Br. 1994;76(2):252-257.
5. Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76(4):432-442.
6. Wroblewski BM, Siney PD. Charnley low-friction arthroplasty of the hip. Long-term results. Clin Orthop Relat Res. 1993;(292):191-201.
7. Chandler HP, Reineck FT, Wixson RL, McCarthy JC. Total hip replacement in patients younger than thirty years old. A five-year follow-up study. J Bone Joint Surg Am. 1981;63(9):1426-1434.
8. Dorr LD, Luckett M, Conaty JP. Total hip arthroplasties in patients younger than 45 years. A nine- to ten-year follow-up study. Clin Orthop Relat Res. 1990;(260):215-219.
9. Boos N, Krushell R, Ganz R, Müller ME. Total hip arthroplasty after previous proximal femoral osteotomy. J Bone Joint Surg Br. 1997;79(2):247-253.
Merkel Cell Carcinoma in a Vein Graft Donor Site
Case Report
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.
Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.
The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
Case Report
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.

Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.

The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
Case Report
A 70-year-old man with history of coronary artery disease presented with a growing lesion on the right leg of 1 year’s duration. The lesion developed at a vein graft donor site for a coronary artery bypass that had been performed 18 years prior to presentation. The patient reported that the lesion was sensitive to touch. Physical examination revealed a 27-mm, firm, violaceous plaque on the medial aspect of the right upper shin (Figure 1). Mild pitting edema also was noted on both lower legs but was more prominent on the right leg. A 6-mm punch biopsy was performed.

Histology showed diffuse infiltration of the dermis and subcutaneous fat by intermediate-sized atypical blue cells with scant cytoplasm (Figure 2). The tumor exhibited moderate cytologic atypia with occasional mitotic figures, and lymphovascular invasion was present. Staining for CD3 was negative within the tumor, but a few reactive lymphocytes were highlighted at the periphery. Staining for CD20 and CD30 was negative. Strong and diffuse staining for cyto-keratin 20 and pan-cytokeratin was noted within the tumor with the distinctive perinuclear pattern characteristic of Merkel cell carcinoma (MCC). Staining for cytokeratin 7 was negative. Synaptophysin and chromogranin were strongly and diffusely positive within the tumor, consistent with a diagnosis of MCC.

The patient was found to have stage IIA (T2N0M0) MCC. Computed tomography completed for staging showed no evidence of metastasis. Wide local excision of the lesion was performed. Margins were negative, as was a right inguinal sentinel lymph node dissection. Because of the size of the tumor and the presence of lymphovascular invasion, radiation therapy at the primary tumor site was recommended. Local radiation treatment (200 cGy daily) was administered for a total dose of 5000 cGy over 5 weeks. The patient currently is free of recurrence or metastases and is being followed by the oncology, surgery, and dermatology departments.
Comment
Merkel cell carcinoma is a rare aggressive cutaneous malignancy. The exact pathogenesis is unknown, but immunosuppression and UV radiation, possibly through its immunosuppressive effects, appear to be contributing factors. More recently, the Merkel cell polyomavirus has been linked to MCC in approximately 80% of cases.1,2
Merkel cell carcinoma is more common in individuals with fair skin, and the average age at diagnosis is 69 years.1 Patients typically present with an asymptomatic, firm, erythematous or violaceous, dome-shaped nodule or a small indurated plaque, most commonly on sun-exposed areas of the head and neck followed by the upper and lower extremities including the hands, feet, ankles, and wrists. Fifteen percent to 20% of MCCs develop on the legs and feet.1 Our patient presented with an MCC that developed on the right shin at a vein graft donor site.
The development of a cutaneous malignancy in a chronic wound (also known as a Marjolin ulcer) is a rare but well-recognized process. These malignancies occur in previously traumatized or chronically inflamed wounds and have been found to occur most commonly in chronic burn wounds, especially in ungrafted full-thickness burns. Squamous cell carcinomas (SCCs) are the most common malignancies to arise in chronic wounds, but basal cell carcinomas, adenocarcinomas, melanomas, malignant fibrous histiocytomas, adenoacanthomas, liposarcomas, and osteosarcomas also have been reported.3 There also have been a few reports of MCC associated with Bowen disease that developed in burn wounds.4 These malignancies generally occur years after injury (average, 35.5 years), but there have been reports of keratoacanthomas developing as early as 3 weeks after injury.5,6
In some reports, malignancies in skin graft donor sites are differentiated from Marjolin ulcers, as the former appear in healed surgical wounds rather than in chronic unstable wounds and tend to occur sooner (ie, in weeks to months after graft harvesting).7,8 The development of these malignancies in graft donor sites is not as well recognized and has been reported in donor sites for split-thickness skin grafts (STSGs), full-thickness skin grafts, tendon grafts, and bone grafts. In addition to malignancies that arise de novo, some develop due to metastatic and iatrogenic spread. The majority of reported malignancies in tendon and bone graft donor sites have been due to metastasis or iatrogenic spread.9-14
Iatrogenic implantation of tumor cells is a well-recognized phenomenon. Hussain et al10 reported a case of implantation of SCC in an STSG donor site, most likely due to direct seeding from a hollow needle used to infiltrate local anesthetic in the tumor area and the STSG. In this case, metastasis could not be completely ruled out.10 There also have been reports of osteosarcoma, ameloblastoma, scirrhous carcinoma of the breast, and malignant fibrous histiocytoma thought to be implanted at bone graft donor sites.14-17 Iatrogenic spread of malignancies can occur through seeding from contaminated gloves or instruments such as hollow bore needles or trocar placement in laparoscopic surgery.11 Airborne spread also may be possible, as viable melanoma cells have been detected in electrocautery plume in mice.13
Metastatic malignancies including metastases from SCC, adenocarcinoma, melanoma, malignant fibrous histiocytoma, angiosarcoma, and osteosarcoma also have been reported to develop in graft donor sites.11,13,18,19 Many malignancies thought to have developed from iatrogenic seeding may actually be from metastasis either by hematogenous or lymphatic spread. A possible contributing factor may be surgery-induced immunosuppression, which has been linked to increased tumor metastasis formation.20 Surgery or trauma have been shown to have an effect on cellular components of the immune system, causing changes such as a shift in T lymphocytes toward immune-suppressive T lymphocytes and impaired function of natural killer cells, neutrophils, and macrophages.20 The suppression of cell-mediated immunity has been shown to decrease over days to weeks in the postoperative period.21 In addition to surgery- or trauma-induced immunosuppression, the risk for metastasis may increase due to increased vascular, including lymphatic, flow toward a skin graft donor site.13,16 Furthermore, trauma predisposes areas to a hypercoagulable state with increased sludging as well as increased platelet counts and fibrinogen levels, which may lead to localization of metastatic lesions.22 All of these factors could potentially work simultaneously to induce the development of metastasis in graft donor sites.
We found that SCCs and keratoacanthomas, which may be a variant of SCC, are among the only primary malignancies that have been reported to develop in skin graft donor sites.6-8 Malignancies in these donor sites appear to develop sooner than those found in chronic wounds and are reported to develop within weeks to several months postoperatively, even in as few as 2 weeks.6,8 Tamir et al6 reported 2 keratoacanthomas that developed simultaneously in a burn scar and STSG donor site. The investigators believed it could be a sign of reduced immune surveillance in the 2 affected areas.6 It has been hypothesized that one cause of local immune suppression in Marjolin ulcers could be due to poor lymphatic regeneration in scar tissue, which would prevent delivery of antigens and stimulated lymphocytes.23 Haik et al7 considered this possibility when discussing a case of SCC that developed at the site of an STSG. The authors did not feel it applied, however, as the donor site had only undergone a single skin harvesting procedure.7 Ponnuvelu et al8 felt that inflammation was the underlying etiology behind the 2 cases they reported of SCCs that developed in STSG donor sites. The inflammation associated with tumors has many of the same processes involved in wound healing (eg, cellular proliferation, angiogenesis). Ponnuvelu et al8 hypothesized that the local inflammation caused by graft harvesting produced an ideal environment for early carcinogenesis. Although in chronic wounds it is believed that continual repair and regeneration in recurrent ulceration contributes to neoplastic initiation, it is thought that even a single injury may lead to malignant change, which may be because prior actinic damage or another cause has made the area more susceptible to these changes.24,25 Surgery-induced immunosuppression also may play a role in development of primary malignancies in graft donor sites.
There have been a few reports of SCCs and basal cell carcinomas occurring in other surgical scars that healed without complications.24,26-28 Similar to the malignancies in graft donor sites, some authors differentiate malignancies that occur in surgical scars that heal without complications from Marjolin ulcers, as they do not occur in chronically irritated wounds. These malignancies have been reported in scars from sternotomies, an infertility procedure, hair transplantation, thyroidectomy, colostomy, cleft lip repair, inguinal hernia repair, and paraumbilical laparoscopic port site. The time between surgery and diagnosis of malignancy ranged from 9 months to 67 years.24,26-28 The development of malignancies in these surgical scars may be due to local immunosuppression, possibly from decreased lymphatic flow; additionally, the inflammation in wound healing may provide the ideal environment for carcinogenesis. Trauma in areas already susceptible to malignant change could be a contributing factor.
Conclusion
Our patient developed an MCC in a vein graft donor site 18 years after vein harvesting. It was likely a primary tumor, as vein harvesting was done for coronary artery bypass graft. There was no evidence of any other lesions on physical examination or computed tomography, making it doubtful that an MCC serving as a primary lesion for seeding or metastasis was present. If such a lesion had been present at that time, it would likely have spread well before the time of presentation to our clinic due to the fast doubling time and high rate of metastasis characteristic of MCCs, further lessening the possibility of metastasis or implantation.
The extended length of time from procedure to lesion development in our patient is much longer than for other reported malignancies in graft donor sites, but the reported time for malignancies in other postsurgical scars is more varied. Regardless of whether the MCC in our patient is classified as a Marjolin ulcer, the pathogenesis is unclear. It is thought that a single injury could lead to malignant change in predisposed skin. Our patient’s legs did not have any evidence of prior actinic damage; however, it is likely that he had local immune suppression, which may have made him more susceptible to these changes. It is unlikely that surgery-induced immunosuppression played a role in our patient, as specific cellular components of the immune system only appear to be affected over days to weeks in the postoperative period. Although poor lymphatic regeneration in scar tissue leading to decreased immune surveillance is not generally thought to contribute to malignancies in most surgical scars, our patient underwent vein harvesting. Chronic edema commonly occurs after vein harvesting and is believed to be due to trauma to the lymphatics. Local immune suppression also may have led to increased susceptibility to infection by the MCC polyomavirus, which has been found to be associated with many MCCs. In addition, the area may have been more susceptible to carcinogenesis due to changes from inflammation from wound healing. We suspect together these factors contributed to the development of our patient’s MCC. Although rare, graft donor sites should be examined periodically for the development of malignancy.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
- Swann MH, Yoon J. Merkel cell carcinoma. Semin Oncol. 2007;34:51-56.
- Schrama D, Ugurel S, Becker JC. Merkel cell carcinoma: recent insights and new treatment options. Curr Opin Oncol. 2012;24:141-149.
- Kadir AR. Burn scar neoplasm. Ann Burns Fire Disasters. 2007;20:185-188.
- Walsh NM. Primary neuroendocrine (Merkel cell) carcinoma of the skin: morphologic diversity and implications thereof. Hum Pathol. 2001;32:680-689.
- Guenther N, Menenakos C, Braumann C, et al. Squamous cell carcinoma arising on a skin graft 64 years after primary injury. Dermatol Online J. 2007;13:27.
- Tamir G, Morgenstern S, Ben-Amitay D, et al. Synchronous appearance of keratoacanthomas in burn scar and skin graft donor site shortly after injury. J Am Acad Dermatol. 1999;40:870-871.
- Haik J, Georgiou I, Farber N, et al. Squamous cell carcinoma arising in a split-thickness skin graft donor site. Burns. 2008;34:891-893.
- Ponnuvelu G, Ng MF, Connolly CM, et al. Inflammation to skin malignancy, time to rethink the link: SCC in skin graft donor sites. Surgeon. 2011;9:168-169.
- Bekar A, Kahveci R, Tolunay S, et al. Metastatic gliosarcoma mass extension to a donor fascia lata graft harvest site by tumor cell contamination. World Neurosurg. 2010;73:719-721.
- Hussain A, Ekwobi C, Watson S. Metastatic implantation squamous cell carcinoma in a split-thickness skin graft donor site. J Plast Reconstr Aesthet Surg. 2011;64:690-692.
- May JT, Patil YJ. Keratoacanthoma-type squamous cell carcinoma developing in a skin graft donor site after tumor extirpation at a distant site. Ear Nose Throat J. 2010;89:E11-E13.
- Serrano-Ortega S, Buendia-Eisman A, Ortega del Olmo RM, et al. Melanoma metastasis in donor site of full-thickness skin graft. Dermatology. 2000;201:377-378.
- Wright H, McKinnell TH, Dunkin C. Recurrence of cutaneous squamous cell carcinoma at remote limb donor site. J Plast Reconstr Aesthet Surg. 2012;65:1265-1266.
- Yip KM, Lin J, Kumta SM. A pelvic osteosarcoma with metastasis to the donor site of the bone graft. a case report. Int Orthop. 1996;20:389-391.
- Dias RG, Abudu A, Carter SR, et al. Tumour transfer to bone graft donor site: a case report and review of the literature of the mechanism of seeding. Sarcoma. 2000;4:57-59.
- Neilson D, Emerson DJ, Dunn L. Squamous cell carcinoma of skin developing in a skin graft donor site. Br J Plast Surg. 1988;41:417-419.
- Singh C, Ibrahim S, Pang KS, et al. Implantation metastasis in a 13-year-old girl: a case report. J Orthop Surg (Hong Kong). 2003;11:94-96.
- Enion DS, Scott MJ, Gouldesbrough D. Cutaneous metastasis from a malignant fibrous histiocytoma to a limb skin graft donor site. Br J Surg. 1993;80:366.
- Yamasaki O, Terao K, Asagoe K, et al. Koebner phenomenon on skin graft donor site in cutaneous angiosarcoma. Eur J Dermatol. 2001;11:584-586.
- Hogan BV, Peter MB, Shenoy HG, et al. Surgery induced immunosuppression. Surgeon. 2011;9:38-43.
- Neeman E, Ben-Eliyahu S. The perioperative period and promotion of cancer metastasis: new outlooks on mediating mechanisms and immune involvement. Brain Behav Immun. 2013;30(suppl):32-40.
- Agostino D, Cliffton EE. Trauma as a cause of localization of blood-borne metastases: preventive effect of heparin and fibrinolysin. Ann Surg. 1965;161:97-102.
- Hammond JS, Thomsen S, Ward CG. Scar carcinoma arising acutely in a skin graft donor site. J Trauma. 1987;27:681-683.
- Korula R, Hughes CF. Squamous cell carcinoma arising in a sternotomy scar. Ann Thorac Surg. 1991;51:667-669.
- Kennedy CTC, Burd DAR, Creamer D. Mechanical and thermal injury. In: Burns T, Breathnach S, Cox N, et al, eds. Rook’s Textbook of Dermatology. Vol 2. 8th ed. Hoboken, NJ: Wiley-Blackwell; 2010:28.1-28.94.
- Durrani AJ, Miller RJ, Davies M. Basal cell carcinoma arising in a laparoscopic port site scar at the umbilicus. Plast Reconstr Surg. 2005;116:348-350.
- Kotwal S, Madaan S, Prescott S, et al. Unusual squamous cell carcinoma of the scrotum arising from a well healed, innocuous scar of an infertility procedure: a case report. Ann R Coll Surg Engl. 2007;89:17-19.
- Ozyazgan I, Kontas O. Previous injuries or scars as risk factors for the development of basal cell carcinoma. Scand J Plast Reconstr Surg Hand Surg. 2004;38:11-15.
Practice Points
- Malignancies (both primary and metastatic) can develop in graft donor sites including donor sites for split-thickness skin, full-thickness skin, tendon, bone, and vein grafts.
- Primary malignancies that develop in graft donor sites may be distinct from malignancies that develop in chronic wounds, as the former occur in healed surgical wounds and tend to occur sooner after injury (ie, weeks to months after graft harvesting versus years).
- Although the occurrence is rare, graft donor sites should be examined periodically for development of malignancies.
Regional Lymphomatoid Papulosis of the Breast Restricted to an Area of Prior Radiotherapy
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.
Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.

Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
Lymphomatoid papulosis (LyP) is a clinicopathologic variant of CD30+ primary cutaneous T-cell lymphoproliferative disorder characterized by a chronic, recurrent, self-healing eruption of papules and small nodules. From a clinical point of view, LyP is not considered a malignant disorder despite demonstration of clonality in most cases.1 From a histopathologic point of view, there are 5 types of LyP: (1) type A, the most common type, which is characterized by a wedge-shaped infiltrate composed of clustered large atypical cells admixed with neutrophils, eosinophils, histiocytes, and small lymphocytes; (2) type B, a rare variant characterized by a bandlike infiltrate of small- to medium-sized pleomorphic and hyperchromatic lymphocytes involving the superficial dermis with epidermotropism; (3) type C, which consists of a nodular infiltrate of large atypical cells with a cohesive arrangement closely similar to anaplastic large-cell lymphoma; (4) type D, a variant with histopathologic features that resemble primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma, but neoplastic cells express CD30 and a T-cell cytotoxic phenotype (βF1+, CD3+, CD4‒, CD8+), and follow-up usually does not reveal development of systemic involvement or signs of other cutaneous lymphomas2; and (5) type E, which is characterized by oligolesional papules that rapidly ulcerate and evolve into large, necrotic, escharlike lesions with a diameter of 1 to 4 cm and an angiocentric and angiodestructive infiltrate of small- to medium-sized atypical lymphocytes expressing CD30 and frequently CD8.3
The clinical appearance of LyP usually is polymorphic, with lesions in different stages of evolution scattered all over the skin; however, the lesions are occasionally localized only to one area of the skin, the so-called regional or agminated LyP.4-14 We report a case of regional LyP that exclusively involved the skin of the left breast, which had previously received radiotherapy for treatment of breast carcinoma. Lymphomatoid papulosis with cutaneous lesions involving only an area of irradiated skin is rare.
Case Report
A 59-year-old woman presented with new-onset cutaneous lesions on the left breast. The patient had a history of invasive ductal carcinoma of the left breast, which had been treated 5 years prior with a partial mastectomy and radiotherapy (10 Gy per week for 5 consecutive weeks [50 Gy total]). Physical examination revealed a large nodular lesion with a necrotic surface on the upper half of the left breast as well as 3 small papular lesions with eroded surfaces on the lower half of the breast (Figure 1). A clinical diagnosis of cutaneous metastases from breast carcinoma was suspected.

Biopsies from one small papule and the large nodular lesion showed similar findings consisting of a necrotic epidermis covered by crusts and a wedge-shaped infiltrate involving the superficial dermis (Figure 2A). The infiltrate was mostly composed of large atypical mononuclear cells with oval to kidney-shaped nuclei, prominent nucleoli, and ample basophilic cytoplasm. Many mitotic figures were seen within the infiltrate (Figure 2B). The infiltrate of atypical cells was admixed with small lymphocytes, histiocytes, and some eosinophils. Immunohistochemically, the large atypical cells expressed CD2, CD3, CD4, CD45, CD30, and epithelial membrane antigen (Figures 2C and 2D). A few atypical cells also expressed CD8 and T-cell intracellular antigen 1. Approximately 60% of the nuclei of the atypical cells showed MIB-1 positivity, while CD20, CD56, AE1/AE3, S-100 protein, CD34, and CD31 were negative. The anaplastic lymphoma kinase was not expressed in atypical cells. Monoclonal rearrangement of the γ T-cell receptor was demonstrated on polymerase chain reaction. Physical examination showed no lymphadenopathy in any lymph node chains. Computed tomography of the chest and abdomen failed to demonstrate systemic involvement. On the basis of these clinical, histologic, immunohistochemical, and molecular results, a diagnosis of type A regional LyP was established.

The patient was treated with 2 daily applications of clobetasol propionate cream 0.5 mg/g and 10 mg of oral methotrexate per week for 4 weeks. After 4 weeks of treatment, the lesions on the left breast had resolved leaving slightly atrophic scars. Six months later, an episode of recurrent papular lesions occurred in the same area and responded to the same treatment, but no systemic involvement had been found.
Comment
Regional LyP is a rare variant, with only a few reported cases in the literature.4-18 Scarisbrick et al4 originally reported 4 patients with LyP limited to specific regions. Interestingly, one of the patients had mycosis fungoides and the LyP lesions were confined to the same region where the mycosis fungoides lesions were observed.4 In a review of LyP in patients from the Netherlands (n=118), lesions limited to a specific region of the body were observed in 13% of cases.5 Cases of LyP limited to acral skin also have been reported.6-8 Heald et al9 described 7 patients who had continuing eruptions of papulonodules with histopathologic features of LyP within well-circumscribed areas of the skin. The investigators interpreted this localized variant of LyP as an equivalent of the limited plaque stage of mycosis fungoides. Interestingly, one of the patients with LyP eventually developed plaques of mycosis fungoides in other areas of the skin not involved by LyP.9 Sharma et al10 described an additional example of regional LyP, and Nakahigashi et al11 described a patient with tumor-stage mycosis fungoides who subsequently developed regional LyP involving the right side of the chest. Kim et al12 described a patient with recurrent episodes of regional LyP exclusively involving the periorbital skin, and Torrelo et al13 reported a 12-year-old boy with persistent lesions of LyP involving the skin of the right side of the abdomen. Coelho et al14 reported a 13-year-old adolescent girl who presented with recurrent papules of LyP exclusively involving the left upper arm. Buder et al15 reported a case of LyP limited to Becker melanosis. Shang et al16 described an additional caseof regional LyP that was successfully controlled by interferon alfa-2b and nitrogen mustard solution. Haus et al17 reported type A LyP confined to the cutaneous area within a red tattoo. Finally, Wang et al18 reported a case of regional LyP in association with pseudoepitheliomatous hyperplasia
Several dermatoses may appear as specific isomorphic responses to various external stimuli, and it is possible that radiotherapy induces some damage that favors the location of the lesions because the irradiated skin behaves as a locus minoris resistentiae. Pemphigus vulgaris,19,20 Sweet syndrome,21 cutaneous angiosarcoma,22-32 and cutaneous metastases from malignant melanoma also have been reported to be confined to irradiated skin.33 However, in our PubMed search of articles indexed for MEDLINE using the terms lymphomatoid papules and regional, none of the previously reported cases of regional LyP had a history of radiotherapy, and in no instance did the lesions develop on a previously irradiated area of the skin.4-18 The localization of the lesions in our patient could have been the result of the so-called radiation recall phenomenon. Recall dermatitis is defined as a skin reaction in a previously irradiated field, usually subsequent to the administration of cytotoxic drugs or antibiotics.34 It may appear days to years after exposure to ionizing radiation and has mostly been associated with chemotherapy drugs, but recall dermatitis is neither exclusive of chemotherapy medications nor strictly radiotherapy induced. The concept of recall dermatitis has been expanded beyond radiation recall dermatitis to include dermatitis induced by other stimuli, including other drugs, contact irritants, and UV radiation, as well as residual herpes zoster. Nevertheless, in recall dermatitis the triggering drug or agent recalls a prior dermatitis in the involved area, such as sunburn or radiodermatitis. In our patient, there was no history of LyP prior to irradiation of the left breast; therefore, the most plausible interpretation of the peculiar localization of the lesions in our patient seems to be that the eruption resulted as expression of a locus minoris resistentiae.
Distinction between primary cutaneous anaplastic large-cell lymphoma and LyP may be difficult because the histopathologic and immunophenotypic features may overlap. In our case, the presence of several papular lesions and one large nodule are more consistent, from a clinical point of view, with a diagnosis of LyP rather than primary cutaneous anaplastic large-cell lymphoma, which usually presents with a solitary and often large, ulcerated, reddish brown tumor. In our patient, the absence of lymphadenopathy, negative results of the computed tomography of the chest and abdomen, and lack of expression for anaplastic lymphoma kinase in atypical cells of the infiltrate militate against a diagnosis of secondary cutaneous involvement from nodal disease.
The histopathologic differential diagnosis of the current case also included cutaneous CD30+ epithelioid angiosarcoma of the breast. Weed and Folpe35 reported the case of an 85-year-old woman who developed a CD30+ epithelioid angiosarcoma on the breast after undergoing breast-conserving surgery and adjuvant radiotherapy for treatment of an infiltrating ductal carcinoma of the breast. Histopathology showed a diffuse replacement of the dermis by a highly malignant-appearing epithelioid neoplasm growing in a solid sheet. Neoplastic cells expressed strong CD30 immunoreactivity with absence of immunoexpression for cytokeratins, S-100 protein, and CD45. Additional immunostaining demonstrated that neoplastic cells also expressed strong immunoreactivity for CD31 and the friend leukemia virus integration 1 gene, FLI-1, and focal positivity for von Willebrand factor, supporting a diagnosis of epithelioid angiosarcoma.35 In our patient, CD34 and CD31 were negative, which ruled out the endothelial nature of neoplastic cells.
Conclusion
In summary, we report an example of regional LyP limited to the left breast of a woman with a history of partial mastectomy and adjuvant radiotherapy for treatment of invasive ductal breast carcinoma. It is a rare case of regional LyP exclusively involving an irradiated area of the skin.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
- Ralfkiaer E, Willemze R, Paulli M, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphomatoid Tissues. Lyon, France: IARC Press, 2008:300-301.
- Saggini A, Gulia A, Argenyi Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. description of 9 cases. Am J Surg Pathol. 2010;34:1168-1175.
- Kempf W, Kazakov DV, Schärer L, et al. Angioinvasive lymphomatoid papulosis: a new variant simulating aggressive lymphomas. Am J Surg Pathol. 2013;37:1-13.
- Scarisbrick JJ, Evans AV, Woolford AJ, et al. Regional lymphomatoid papulosis: a report of four cases. Br J Dermatol. 1999;141:1125-1128.
- Bekkenk MW, Geelen FA, van Voorst Vader PC, et al. Primary and secondary cutaneous CD30+ lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment. Blood. 2000;95:3653-3661.
- Thomas GJ, Conejo-Mir JS, Ruiz AP, et al. Lymphomatoid papulosis in childhood with exclusive acral involvement. Pediatr Dermatol. 1998;15:146-147.
- Deroo-Berger MC, Skowson F, Roner S, et al. Lymphomatoid papulosis: a localized form with acral pustular involvement. Dermatology. 2002;205:60-62.
- Kagaya M, Kondo S, Kamada A, et al. Localized lymphomatoid papulosis. Dermatology. 2002;204:72-74.
- Heald P, Subtil A, Breneman D, et al. Persistent agmination of lymphomatoid papulosis: an equivalent of limited plaque mycosis fungoides type of cutaneous T-cell lymphoma. J Am Acad Dermatol. 2007;57:1005-1011.
- Sharma V, Xu G, Petronic-Rosic V, et al. Clinicopathologic challenge. regional lymphomatoid papulosis, type A. Int J Dermatol. 2007;46:905-909.
- Nakahigashi K, Ishida Y, Matsumura Y, et al. Large cell transformation mimicking regional lymphomatoid papulosis in a patient with mycosis fungoides. J Dermatol. 2008;35:283-288.
- Kim YJ, Rho YK, Yoo KH, et al. Case of regional lymphomatoid papulosis confined to the periorbital areas. J Dermatol. 2009;36:163-165.
- Torrelo A, Colmenero I, Hernández A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26:762-764.
- Coelho JD, Afonso A, Feio AB. Regional lymphomatoid papulosis in a child—treatment with a UVB phototherapy handpiece. J Cosmet Laser Ther. 2010;12:155-156.
- Buder K, Wendel AM, Cerroni L, et al. A case of lymphomatoid papulosis limited to Becker’s melanosis. Dermatology. 2013;226:124-127.
- Shang SX, Chen H, Sun JF, et al. Regional lymphomatoid papulosis successfully controlled by interferon α-2b and nitrogen mustard solution. Chin Med J (Engl). 2013;126:3194-3195.
- Haus G, Utikal J, Geraud C, et al. CD30-positive lymphoproliferative disorder in a red tattoo: regional lymphomatoid papulosis type C or pseudolymphoma? Br J Dermatol. 2014;171:668-670.
- Wang T, Guo CL, Xu CC, et al. Regional lymphomatoid papulosis in association with pseudoepitheliomatous hyperplasia: 13 years follow-up. J Eur Acad Dermatol Venereol. 2015;29:1853-1854.
- Davis M, Feverman EJ. Induction of pemphigus by X-ray irradiation. Clin Exp Dermatol. 1987;12:197-199.
- Crovato F, Descrello G, Nazzari G, et al. Liner pemphigus vulgaris after X-ray irradiation. Dermatologica. 1989;179:135-136.
- Vergara G, Vargas-Machuca I, Pastor MA, et al. Localized Sweet’s syndrome in radiation-induced locus minoris resistentae. J Am Acad Dermatol. 2003;49:907-909.
- Caldwell JB, Ryan MT, Benson PM, et al. Cutaneous angiosarcoma arising in the radiation site of a congenital hemangioma. J Am Acad Dermatol. 1995;33:865-870.
- Stone NM, Holden CA. Postirradiation angiosarcoma. Clin Exp Dermatol. 1997;22:46-47.
- Goette EK, Detlefs RL. Postirradiation angiosarcoma. J Am Acad Dermatol. 1985;12:922-926.
- Chen TK, Goffman KD, Hendricks EJ. Angiosarcoma following therapeutic irradiation. Cancer. 1979;44:2044-2048.
- Rubin E, Maddox WA, Mazur MT. Cutaneous angiosarcoma of the breast 7 years after lumpectomy and radiation therapy. Radiology. 1990;174:258-260.
- Stokkel MPM, Peterse HL. Angiosarcoma of the breast after lumpectomy and radiation therapy for adenocarcinoma. Cancer. 1992;69:2965-2968.
- Moskaluk CA, Merino MJ, Danforth DN, et al. Low-grade angiosarcoma of the skin of the breast: a complication of lumpectomy and radiation therapy for breast carcinoma. Hum Pathol. 1992;23:710-714.
- Parham DM, Fisher C. Angiosarcomas of the breast developing post radiotherapy. Histopathology. 1997;31:189-195.
- Rao J, DeKoven JG, Beatty JD, et al. Cutaneous angiosarcoma as a delayed complication of radiation therapy for carcinoma of the breast. J Am Acad Dermatol. 2003;49:532-538.
- Billings SD, McKenney JK, Folpe Al, et al. Cutaneous angiosarcoma following breast-conserving surgery and radiation. an analysis of 27 cases. Am J Surg Pathol. 2004;28:781-788.
- Fodor J, Orosz Z, Szabo E, et al. Angiosarcoma after conservation treatment for breast carcinoma: our experience and a review of the literature. J Am Acad Dermatol. 2006;54:499-504.
- Roses DP, Harris MN, Rigel D, et al. Local and in-transit metastases following definitive excision from primary cutaneous malignant melanoma. Ann Surg. 1983;198:65-69.
- Burris HA 3rd, Hurtig J. Radiation recall with anticancer agents. Oncologist. 2010;15:1227-1237.
- Weed BR, Folpe AL. Cutaneous CD30-positive epithelioid angiosarcoma following breast-conserving therapy and irradiation. a potential diagnostic pitfall. Am J Dermatopathol. 2008;30:370-372.
Practice Points
- Cutaneous lesions of lymphomatoid papulosis (LyP) sometimes are confined to only one area of the skin, which is known as regional LyP.
- Patients with regional LyP have the same prognosis as those with widespread LyP, and no specific association has been reported with this clinical variant.
- Lesions of regional LyP respond to the same treatments as widespread LyP.