User login
Thrower’s Fracture of the Humerus
Case
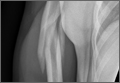
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
Case
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
Case
An otherwise healthy 29-year-old man presented to the ED for evaluation of right arm pain. He had been throwing a baseball when he felt acute onset of severe pain in his right shoulder and became unable to use his arm. Radiographs of the humerus were obtained (Figure a and b).
Fracture of the Humerus
A thrower’s fracture is a rare fracture pattern characterized by a spontaneous fracture of the mid to distal third of the humeral diaphysis during an attempted throwing motion. It was first described by Wilmoth in a case report published in 1930.1 Understanding the proposed mechanism and complications of injury are important for proper work-up and management in the ED.
Fractures of the humerus in young adults are typically the result of high-energy direct trauma. So how does the humerus fracture from throwing a baseball? The most commonly proposed mechanism is an excessive torque during the cocking and acceleration phases of the throwing motion.2-5 This can be visualized as a pitcher’s arm maximally cocked back prior to forward acceleration. During the transition into the acceleration phase, internal rotation is abruptly initiated by the subscapularis, pectoralis major, and latissimus dorsi.6,7 The distal humerus continues to externally rotate due to the momentum generated by the cocking phase, while the proximal humerus violently internally rotates, creating a torsional force on the humerus at the insertion of these muscles and a fulcrum for potential fracture.8 Spiral fractures are the most commonly seen fracture pattern, which correlates with this proposed mechanism.9
Thrower’s fractures are most commonly reported in men in their 20s and 30s who are less seasoned athletes.10,11 These individuals are potentially at greater risk due to the lack of compensatory humeral cortical hypertrophy from repetitive throwing10,12 coupled with a less refined throwing motion.13 Additionally, up to 75% of patients experience prodromal throwing pain at the impending fracture site,11 which suggests that a primary insult such as a stress fracture may also predispose patients to this fracture pattern.
Once a fracture is suspected, a neurovascular assessment should immediately be performed, because concurrent radial nerve injuries have been reported in an average of 11.8% of mid-distal humeral fractures.14 Fractures with associated radial nerve deficits should not be reduced without an orthopedic consultation. Most radial nerve injuries are the result of neuropraxia, which usually resolves spontaneously, and attempted reduction may result in worsening nerve damage.14,15 Additionally, the orthopedist may consider late exploration if no spontaneous nerve recovery occurs within 3 to 6 months.16 Thrower’s fractures with or without associated radial nerve palsies are typically treated conservatively with a hanging cast, which has shown similar results to orthopedic fixation.10,17 The emergency physician should feel comfortable not ordering additional imaging to search for a pathological fracture, unless plain films suggest otherwise.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
1. Wilmoth CL. Recurrent fracture of the humerus due to sudden extreme muscular action. J Bone Joint Surg.1930;12(1):168-169.
2. Miller A, Dodson CC, Ilyas AM. Thrower’s fracture of the humerus. Orthop Clin North Am. 2014;45(4):565-569.
3. Weseley MS, Barenfeld PA. Ball throwers’ fracture of the humerus. Six case reports. Clin Orthop Relat Res. 1969;64:153-156.
4. Chao SL, Miller M,Teng SW. A mechanism of spiral fracture of the humerus: a report of 129 cases following the throwing of hand grenades. J Trauma. 1971;11(7):602-605.
5. Polu KR, Schenck RC Jr, Wirth MA, Greeson J, Cone RO 3rd, Rockwood CA Jr. Stress fracture of the humerus in a collegiate baseball pitcher. A case report. Am J Sports Med. 1999;27(6):813-816.
6. Jobe FW, Moynes DR, Tibone JE, Perry J. An EMG analysis of the shoulder in pitching. A second report. Am J Sports Med. 1984;12(3):218-220.
7. Pappas AM, Zawacki RM, Sullivan TJ. Biomechanics of baseball pitching. A preliminary report. Am J Sports Med. 1985;13(4):216-222.
8. Sabick MB, Torry MR, Kim YK, Hawkins RJ. Humeral torque in professional baseball pitchers. Am J Sports Med. 2004;32(4):892-898.
9. Klenerman L. Fractures of the shaft of the humerus. J Bone Joint Surg Br. 1966;48(1):105-111.
10. Ogawa K, Yoshida A. Throwing fracture of the humeral shaft. An analysis of 90 patients. Am J Sports Med. 1998;26(2):242-246.
11. Branch T, Partin C, Chamberland P, Emeterio E, Sabetelle M. Spontaneous fractures of the humerus during pitching. A series of 12 cases. Am J Sports Med. 1992;20(4):468-470.
12. Tullos HS, Erwin WD, Woods GW, Wukasch DC, Cooley DA, King JW. Unusual lesions of the pitching arm. Clin Orthop Relat Res. 1972;88:169-182.
13. Bingham EL. Fractures of the humerus from muscular violence. U S Armed Forces Med J. 1959;10(1):22-25.
14. Shao YC, Harwood P, Grotz MR, Limb D, Giannoudis PV. Radial nerve palsy associated with fractures of the shaft of the humerus: a systematic review. J Bone Joint Surg Br. 2005;87(12):1647-1652.
15. Bishop J, Ring D. Management of radial nerve palsy associated with humeral shaft fracture: a decision analysis model. J Hand Surg Am. 2009;34(6)991-996.
16. Niver GE, Ilyas AM. Management of radial nerve palsy following fractures of the humerus. Orthop Clin North Am. 2013;44(3):419-424.
17. Kaplan H, Kiral A, Kuskucu M, Arpacioglu MO, Sarioglu A, Rodop O. Report of eight cases of humeral fracture following the throwing of hand grenades. Arch Orthop Trauma Surg. 1998;117(1-2):50-52.
Spontaneous Cervical Spinal Epidural Hematoma
Unilateral weakness is a common ED presentation with a diverse etiology, including stroke.1,2 Studies have reported a misdiagnosis rate of stroke and transient ischemic attack of approximately 10%.3 This case presents an unusual stroke mimic where treatment with an anticoagulant could have led to adverse outcomes. It also highlights the importance of considering a spontaneous cervical spinal epidural hematoma (SCSEH) as a stroke mimic. This is especially pertinent when a patient’s symptoms are not fully consistent with an acute stroke, in order to avoid potentially dangerous anticoagulation and allow for prompt treatment of the hematoma.
Case
A 68-year-old woman presented to the ED via ambulance with the complaint of stroke-like symptoms. She had a 2-hour history of sudden-onset right arm and leg weakness with loss of sensation, and right-sided neck pain. She also complained of a mild right-sided headache, which had an insidious onset and had been present for the past 2 days. She had recently finished a course of antibiotics for a urinary tract infection. There was no history of trauma. The patient had no significant medical history, was taking no medications, and was a nonsmoker with a normal body mass index. Her family history was significant for cerebral vascular accidents.
On arrival at the ED, the patient had a blood pressure of 179/95 mm Hg; her other vital signs were normal. On examination, she had a right-sided hemiplegia, with a 0/5 power grading observed for motor strength for both her right arm and leg. She reported paresthesia in dermatomes C4 to C5 and L3 to L5. There was extreme tenderness when her right trapezius and upper paraspinal muscles were palpated, but she had no midline cervical spine tenderness and had full, though painful, range of movement of her neck. Her left side was unaffected. She had normal cranial nerves, no higher cortical dysfunction, a Glasgow Coma Scale (GCS) score of 15, and complete control of her bladder and bowel.
A computed tomography angiogram (CTA) of the carotid arteries and a CT scan of the head were ordered to rule out acute stroke and carotid artery dissection. The CT scan of the head showed no acute bleeding or evidence of infarction. However, the CTA raised suspicion of an SCSEH.
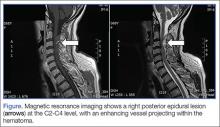
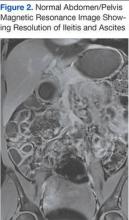
Subsequent magnetic resonance imaging showed a right posterior epidural lesion measuring 3 to 3.5 cm in length and 8 to 9 mm in maximal thickness, extending from the midpoint of C2 to the C3-C4 disc level. The lesion compressed the spinal cord, and this stenosis was made worse by posterior disc osteophytes. There was a single strongly enhancing vessel projecting within the hematoma, raising the suspicion of an active bleed (Figure).
Blood tests, including a coagulation screen, were normal and an electrocardiogram showed normal sinus rhythm at 57 beats/minute.
The patient was transferred to the neurosurgery team at the tertiary center, where she underwent an emergency C2-C3 laminectomy that day. She was discharged 6 days later and has made a full recovery with physiotherapy, regaining full function of her right arm and leg.
Discussion
Spontaneous cervical spinal epidural hematomas are rare, with an estimated annual incidence of 0.1 cases per 100,000 people.4 The etiology is largely unknown, but SCSEH has been attributed to a venous source.5 Multiple predisposing factors have been reported, including coagulopathies, anticoagulation, disc herniation, vascular malformations, neoplasms, and idiopathic causes.3,4
The most common presentation of an SCSEH is sudden onset of interscapular or cervical pain with paresthesia and even paralysis. Paraplegia and quadriplegia are common; however, hemiplegia as a presentation of an SCSEH is rarely reported in the literature.6-8
In a case report, Wang et al6 described a similar presentation and management of an SCSEH in the ED as our case. However, the patient in that report was initially treated for a stroke with heparin, and the authors commented that the unnecessary anticoagulation could have caused expansion of the hematoma and subsequent spinal cord compression.6
Both our patient and the patient described by Wang et al6 presented with hemiplegia, which is more commonly seen as a presenting feature in stroke and is a rarely reported presentation of an SCSEH. However, neck pain is not a classical presenting feature in stroke, and this prompted us to order the CTA of the carotid arteries and CT scan of the head. This ultimately led to the correct diagnosis and prompt management, avoiding unnecessary and potentially dangerous anticoagulation.
Conclusion
Hemiplegia is an important, though rarely reported, presentation of an SCSEH and should not be misdiagnosed as an acute stroke. Neck pain in a patient presenting with unilateral weakness should be a red flag that prompts the emergency physician (EP) to search for alternative diagnoses to stroke. If a patient with an SCSEH presents to the ED, prompt and accurate recognition by an EP allows for early surgical intervention, which improves clinical outcome, aids neurological recovery, and minimizes long-term sequales.6
1. Nickel C, Nemec M, Bingisser R. Weakness as presenting symptom in emergency department. Swiss Med Wkly. 2009;139(17-18):271-272.
2. Asimos AW, Hockberger RS, Grayzel J. Evaluation of the adult with acute weakness in the emergency department. UpToDate web site. Available at: http://www.uptodate.com/contents/evaluation-of-the-adult-with-acute-weakness-in-the-emergency-department. Accessed April 12, 2016.
3. Pope JV, Edlow JA. Avoiding misdiagnosis in patients with neurological emergencies. Emerg Med Int. 2012;2012:949275.
4. Baek B, Hur J, Kwon Ki, Lee HK. Spontaneous spinal epidural hematoma. J Korean Neurosurg Soc. 2008; 44(1):40-42.
5. Fukui M, Swarnkar A, Williams R. Acute spontaneous spinal epidural hematomas. AJNR Am J Neuroradiol.1999;20(7):1365-1372.
6. Wang CC, Chang CH, Lin HJ, Lin KC, Kuo JR. Misdiagnosis of spontaneous cervical epidural haemorrhage. Eur Spine J. 2009;18(Suppl 2):210-212.
7. Hsieh CF, Lin HJ, Chen KT, Foo NP, Te AL. Acute spontaneous cervical spinal epidural hematoma with hemiparesis as the initial presentation. Eur J Emerg Med. 2006;13(1):36-38.
8. Dimou J, Jithoo R, Bush S. A patient with delayed traumatic cervical spinal epidural hematoma presenting with hemiparesis. J Clin Neurosci. 2010;17(3):404-405.
Unilateral weakness is a common ED presentation with a diverse etiology, including stroke.1,2 Studies have reported a misdiagnosis rate of stroke and transient ischemic attack of approximately 10%.3 This case presents an unusual stroke mimic where treatment with an anticoagulant could have led to adverse outcomes. It also highlights the importance of considering a spontaneous cervical spinal epidural hematoma (SCSEH) as a stroke mimic. This is especially pertinent when a patient’s symptoms are not fully consistent with an acute stroke, in order to avoid potentially dangerous anticoagulation and allow for prompt treatment of the hematoma.
Case
A 68-year-old woman presented to the ED via ambulance with the complaint of stroke-like symptoms. She had a 2-hour history of sudden-onset right arm and leg weakness with loss of sensation, and right-sided neck pain. She also complained of a mild right-sided headache, which had an insidious onset and had been present for the past 2 days. She had recently finished a course of antibiotics for a urinary tract infection. There was no history of trauma. The patient had no significant medical history, was taking no medications, and was a nonsmoker with a normal body mass index. Her family history was significant for cerebral vascular accidents.
On arrival at the ED, the patient had a blood pressure of 179/95 mm Hg; her other vital signs were normal. On examination, she had a right-sided hemiplegia, with a 0/5 power grading observed for motor strength for both her right arm and leg. She reported paresthesia in dermatomes C4 to C5 and L3 to L5. There was extreme tenderness when her right trapezius and upper paraspinal muscles were palpated, but she had no midline cervical spine tenderness and had full, though painful, range of movement of her neck. Her left side was unaffected. She had normal cranial nerves, no higher cortical dysfunction, a Glasgow Coma Scale (GCS) score of 15, and complete control of her bladder and bowel.
A computed tomography angiogram (CTA) of the carotid arteries and a CT scan of the head were ordered to rule out acute stroke and carotid artery dissection. The CT scan of the head showed no acute bleeding or evidence of infarction. However, the CTA raised suspicion of an SCSEH.
Subsequent magnetic resonance imaging showed a right posterior epidural lesion measuring 3 to 3.5 cm in length and 8 to 9 mm in maximal thickness, extending from the midpoint of C2 to the C3-C4 disc level. The lesion compressed the spinal cord, and this stenosis was made worse by posterior disc osteophytes. There was a single strongly enhancing vessel projecting within the hematoma, raising the suspicion of an active bleed (Figure).
Blood tests, including a coagulation screen, were normal and an electrocardiogram showed normal sinus rhythm at 57 beats/minute.
The patient was transferred to the neurosurgery team at the tertiary center, where she underwent an emergency C2-C3 laminectomy that day. She was discharged 6 days later and has made a full recovery with physiotherapy, regaining full function of her right arm and leg.
Discussion
Spontaneous cervical spinal epidural hematomas are rare, with an estimated annual incidence of 0.1 cases per 100,000 people.4 The etiology is largely unknown, but SCSEH has been attributed to a venous source.5 Multiple predisposing factors have been reported, including coagulopathies, anticoagulation, disc herniation, vascular malformations, neoplasms, and idiopathic causes.3,4
The most common presentation of an SCSEH is sudden onset of interscapular or cervical pain with paresthesia and even paralysis. Paraplegia and quadriplegia are common; however, hemiplegia as a presentation of an SCSEH is rarely reported in the literature.6-8
In a case report, Wang et al6 described a similar presentation and management of an SCSEH in the ED as our case. However, the patient in that report was initially treated for a stroke with heparin, and the authors commented that the unnecessary anticoagulation could have caused expansion of the hematoma and subsequent spinal cord compression.6
Both our patient and the patient described by Wang et al6 presented with hemiplegia, which is more commonly seen as a presenting feature in stroke and is a rarely reported presentation of an SCSEH. However, neck pain is not a classical presenting feature in stroke, and this prompted us to order the CTA of the carotid arteries and CT scan of the head. This ultimately led to the correct diagnosis and prompt management, avoiding unnecessary and potentially dangerous anticoagulation.
Conclusion
Hemiplegia is an important, though rarely reported, presentation of an SCSEH and should not be misdiagnosed as an acute stroke. Neck pain in a patient presenting with unilateral weakness should be a red flag that prompts the emergency physician (EP) to search for alternative diagnoses to stroke. If a patient with an SCSEH presents to the ED, prompt and accurate recognition by an EP allows for early surgical intervention, which improves clinical outcome, aids neurological recovery, and minimizes long-term sequales.6
Unilateral weakness is a common ED presentation with a diverse etiology, including stroke.1,2 Studies have reported a misdiagnosis rate of stroke and transient ischemic attack of approximately 10%.3 This case presents an unusual stroke mimic where treatment with an anticoagulant could have led to adverse outcomes. It also highlights the importance of considering a spontaneous cervical spinal epidural hematoma (SCSEH) as a stroke mimic. This is especially pertinent when a patient’s symptoms are not fully consistent with an acute stroke, in order to avoid potentially dangerous anticoagulation and allow for prompt treatment of the hematoma.
Case
A 68-year-old woman presented to the ED via ambulance with the complaint of stroke-like symptoms. She had a 2-hour history of sudden-onset right arm and leg weakness with loss of sensation, and right-sided neck pain. She also complained of a mild right-sided headache, which had an insidious onset and had been present for the past 2 days. She had recently finished a course of antibiotics for a urinary tract infection. There was no history of trauma. The patient had no significant medical history, was taking no medications, and was a nonsmoker with a normal body mass index. Her family history was significant for cerebral vascular accidents.
On arrival at the ED, the patient had a blood pressure of 179/95 mm Hg; her other vital signs were normal. On examination, she had a right-sided hemiplegia, with a 0/5 power grading observed for motor strength for both her right arm and leg. She reported paresthesia in dermatomes C4 to C5 and L3 to L5. There was extreme tenderness when her right trapezius and upper paraspinal muscles were palpated, but she had no midline cervical spine tenderness and had full, though painful, range of movement of her neck. Her left side was unaffected. She had normal cranial nerves, no higher cortical dysfunction, a Glasgow Coma Scale (GCS) score of 15, and complete control of her bladder and bowel.
A computed tomography angiogram (CTA) of the carotid arteries and a CT scan of the head were ordered to rule out acute stroke and carotid artery dissection. The CT scan of the head showed no acute bleeding or evidence of infarction. However, the CTA raised suspicion of an SCSEH.
Subsequent magnetic resonance imaging showed a right posterior epidural lesion measuring 3 to 3.5 cm in length and 8 to 9 mm in maximal thickness, extending from the midpoint of C2 to the C3-C4 disc level. The lesion compressed the spinal cord, and this stenosis was made worse by posterior disc osteophytes. There was a single strongly enhancing vessel projecting within the hematoma, raising the suspicion of an active bleed (Figure).
Blood tests, including a coagulation screen, were normal and an electrocardiogram showed normal sinus rhythm at 57 beats/minute.
The patient was transferred to the neurosurgery team at the tertiary center, where she underwent an emergency C2-C3 laminectomy that day. She was discharged 6 days later and has made a full recovery with physiotherapy, regaining full function of her right arm and leg.
Discussion
Spontaneous cervical spinal epidural hematomas are rare, with an estimated annual incidence of 0.1 cases per 100,000 people.4 The etiology is largely unknown, but SCSEH has been attributed to a venous source.5 Multiple predisposing factors have been reported, including coagulopathies, anticoagulation, disc herniation, vascular malformations, neoplasms, and idiopathic causes.3,4
The most common presentation of an SCSEH is sudden onset of interscapular or cervical pain with paresthesia and even paralysis. Paraplegia and quadriplegia are common; however, hemiplegia as a presentation of an SCSEH is rarely reported in the literature.6-8
In a case report, Wang et al6 described a similar presentation and management of an SCSEH in the ED as our case. However, the patient in that report was initially treated for a stroke with heparin, and the authors commented that the unnecessary anticoagulation could have caused expansion of the hematoma and subsequent spinal cord compression.6
Both our patient and the patient described by Wang et al6 presented with hemiplegia, which is more commonly seen as a presenting feature in stroke and is a rarely reported presentation of an SCSEH. However, neck pain is not a classical presenting feature in stroke, and this prompted us to order the CTA of the carotid arteries and CT scan of the head. This ultimately led to the correct diagnosis and prompt management, avoiding unnecessary and potentially dangerous anticoagulation.
Conclusion
Hemiplegia is an important, though rarely reported, presentation of an SCSEH and should not be misdiagnosed as an acute stroke. Neck pain in a patient presenting with unilateral weakness should be a red flag that prompts the emergency physician (EP) to search for alternative diagnoses to stroke. If a patient with an SCSEH presents to the ED, prompt and accurate recognition by an EP allows for early surgical intervention, which improves clinical outcome, aids neurological recovery, and minimizes long-term sequales.6
1. Nickel C, Nemec M, Bingisser R. Weakness as presenting symptom in emergency department. Swiss Med Wkly. 2009;139(17-18):271-272.
2. Asimos AW, Hockberger RS, Grayzel J. Evaluation of the adult with acute weakness in the emergency department. UpToDate web site. Available at: http://www.uptodate.com/contents/evaluation-of-the-adult-with-acute-weakness-in-the-emergency-department. Accessed April 12, 2016.
3. Pope JV, Edlow JA. Avoiding misdiagnosis in patients with neurological emergencies. Emerg Med Int. 2012;2012:949275.
4. Baek B, Hur J, Kwon Ki, Lee HK. Spontaneous spinal epidural hematoma. J Korean Neurosurg Soc. 2008; 44(1):40-42.
5. Fukui M, Swarnkar A, Williams R. Acute spontaneous spinal epidural hematomas. AJNR Am J Neuroradiol.1999;20(7):1365-1372.
6. Wang CC, Chang CH, Lin HJ, Lin KC, Kuo JR. Misdiagnosis of spontaneous cervical epidural haemorrhage. Eur Spine J. 2009;18(Suppl 2):210-212.
7. Hsieh CF, Lin HJ, Chen KT, Foo NP, Te AL. Acute spontaneous cervical spinal epidural hematoma with hemiparesis as the initial presentation. Eur J Emerg Med. 2006;13(1):36-38.
8. Dimou J, Jithoo R, Bush S. A patient with delayed traumatic cervical spinal epidural hematoma presenting with hemiparesis. J Clin Neurosci. 2010;17(3):404-405.
1. Nickel C, Nemec M, Bingisser R. Weakness as presenting symptom in emergency department. Swiss Med Wkly. 2009;139(17-18):271-272.
2. Asimos AW, Hockberger RS, Grayzel J. Evaluation of the adult with acute weakness in the emergency department. UpToDate web site. Available at: http://www.uptodate.com/contents/evaluation-of-the-adult-with-acute-weakness-in-the-emergency-department. Accessed April 12, 2016.
3. Pope JV, Edlow JA. Avoiding misdiagnosis in patients with neurological emergencies. Emerg Med Int. 2012;2012:949275.
4. Baek B, Hur J, Kwon Ki, Lee HK. Spontaneous spinal epidural hematoma. J Korean Neurosurg Soc. 2008; 44(1):40-42.
5. Fukui M, Swarnkar A, Williams R. Acute spontaneous spinal epidural hematomas. AJNR Am J Neuroradiol.1999;20(7):1365-1372.
6. Wang CC, Chang CH, Lin HJ, Lin KC, Kuo JR. Misdiagnosis of spontaneous cervical epidural haemorrhage. Eur Spine J. 2009;18(Suppl 2):210-212.
7. Hsieh CF, Lin HJ, Chen KT, Foo NP, Te AL. Acute spontaneous cervical spinal epidural hematoma with hemiparesis as the initial presentation. Eur J Emerg Med. 2006;13(1):36-38.
8. Dimou J, Jithoo R, Bush S. A patient with delayed traumatic cervical spinal epidural hematoma presenting with hemiparesis. J Clin Neurosci. 2010;17(3):404-405.
Multiple Morphologically Distinct Cutaneous Granular Cell Tumors Occurring in a Single Patient
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.
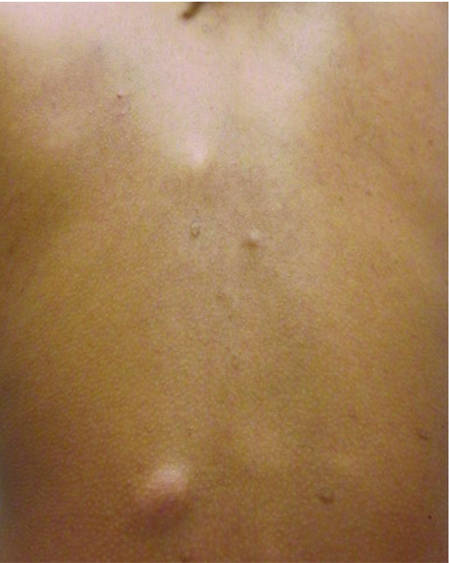 | ||
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||
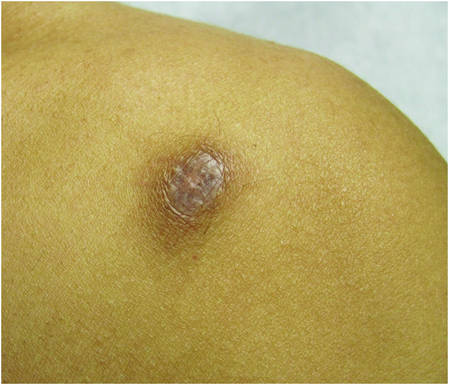 | ||
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
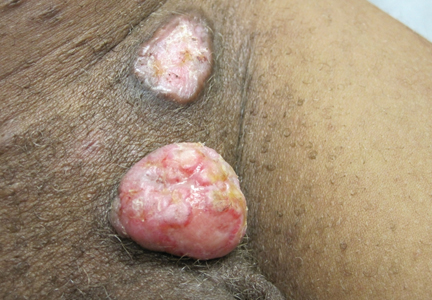
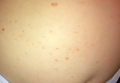
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
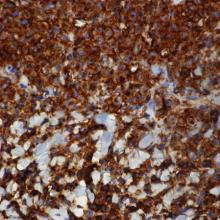
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.
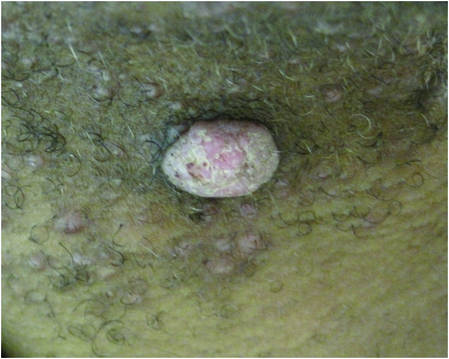 | 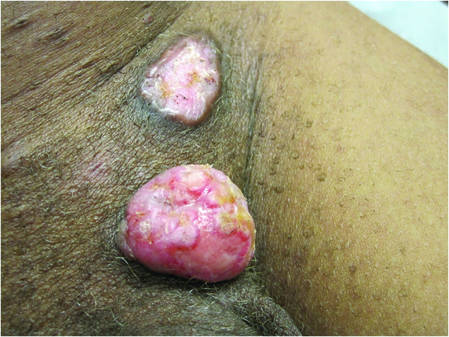 | |
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |
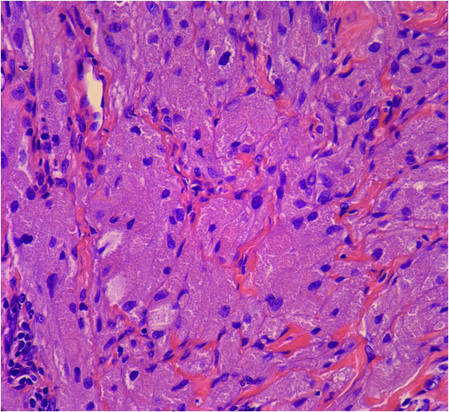 | 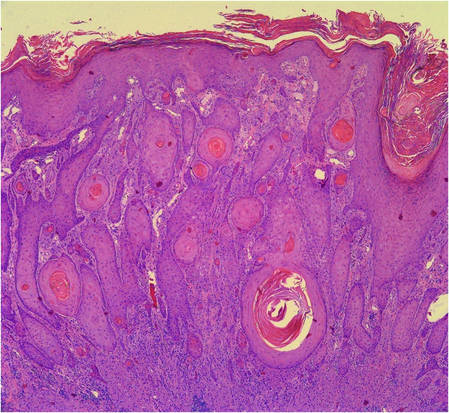 | |
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.
 | ||
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||
 | ||
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.
 |  | |
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |
 |  | |
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
Case Report
A 27-year-old black man was admitted to the hospital with chills; night sweats; unintentional 25-lb weight loss; and multiple widespread, painful, progressively enlarging skin nodules of 3 months’ duration. The lesions had first developed on the back and later appeared on the face, trunk, arms, thighs, and genital region. He denied dysuria or urethral discharge. He had a remote history of adequately treated chlamydia infection but no other remarkable personal or family history.
 | ||
| Figure 1. Firm subcutaneous nodules on the back with no epidermal change. | ||
 | ||
| Figure 2. Firm dermal papule on the anterior aspect of the left shoulder with violaceous hyperpigmentation (dermatofibromalike). |
Physical examination revealed a thin man with more than 20 lesions on the face, trunk, arms, thighs, and genital region ranging in size from 1 to 4 cm. Lesion morphologies varied greatly and included subcutaneous firm nodules with no epidermal change (Figure 1); dermatofibromalike nodules with overlying erythema and hyperpigmentation (Figure 2); condylomalike, verrucous, pink papulonodules (Figure 3); ulcerated angular plaques with rolled borders and palpable tumor extension deep (1–2 cm) to the subcutis (Figure 4); and a vegetative, eroded, exophytic tumor with palpable deep extension (Figure 4). A diffuse, erythematous, macular eruption also was noted on the trunk and bilateral arms and legs including the soles of both feet along with nontender cervical, axillary, and inguinal lymphadenopathy. The ocular, oral, and nasal mucosae were not affected.
The differential diagnosis for each lesion differed based on morphology. Infectious, inflammatory, and neoplastic processes were considered, including syphilis, dermatofibroma, dermatofibrosarcoma protuberans, metastatic disease, leukemia cutis, sarcoidosis, panniculitis, condyloma acuminatum, and vegetative herpes simplex virus infection (inguinal lesion).
Laboratory data revealed a reactive rapid plasma reagin with treponemal IgG titers of 1:64. Urine chlamydia RNA probe and lymphogranuloma venereum (LGV) serum antibodies also were positive. Human immunodeficiency virus screening was negative. Positron emission tomography–computerized tomography revealed enlarged and hypermetabolic lymphadenopathy above and below the diaphragm.
After therapy with intravenous penicillin G and oral doxycycline for concurrent secondary syphilis and LGV, the patient’s macular eruption and constitutional symptoms resolved within weeks of the initial presentation. His lymphadenopathy improved, his rapid plasma reagin titer decreased, and his chlamydia RNA became undetectable. However, the skin lesions remained unchanged.
Incisional biopsies of 4 clinically distinct skin lesions revealed well-delineated dermal proliferations of cells with eosinophilic granular cytoplasm and indistinct cell borders (Figure 5). Two specimens displayed marked epidermal hyperplasia (Figure 6).
No atypical mitotic figures were identified. Immunohistochemistry for S-100 protein was diffusely positive in the neoplastic cells. Immunohistochemistry for Treponema pallidum was negative.
No mycobacterial or fungal organisms were identified in acid-fast bacillus, periodic acid–Schiff, or Gomori methenamine-silver–stained sections. All 4 lesions had histopathologic findings characteristic of granular cell tumors (GCTs). A lesion in the left inguinal region (Figure 4 [medial lesion]), which initially was thought to be condyloma latum or a squamous cell carcinoma (SCC), also was later confirmed to be a GCT.
Repeat positron emission tomography–computerized tomography several weeks later confirmed resolution of the previously noted lymphadenopathy. Although 2 GCTs have not recurred after biopsy, the other 2, which the patient refused to have completely excised, continued to grow. Follow-up 2.5 years after hospitalization revealed persistence of the lesions with no remarkable morphological changes.
 |  | |
| Figure 3. Verrucous pink papule on the right side of the neck. | Figure 4. Ulcerated angular plaque in the left inguinal/genital area with rolled borders and tumor extension deep to the subcutis adjacent to a vegetative, eroded, exophytic tumor with palpable deep extension. | |
 |  | |
Figure 5. Large polygonal cells with eosinophilic granular cytoplasm, prominent bland nuclei, and indistinct cell borders (H&E, original magnification ×40). | Figure 6. Marked pseudoepitheliomatous hyperplasia (H&E, original magnification ×10). |
Comment
First described in 1854, GCTs are uncommon neoplasms of probable Schwann cell origin that can arise in almost any location of the body but most often appear on the skin and in the subcutaneous tissues and oral cavity.1,2 The commonly regarded rule of thirds describes its most favored locations: one-third on the tongue, one-third on the skin, and one-third in internal organs.3,4 Granular cell tumors occur with greater frequency in adults, females, and black individuals.1-5
Cutaneous GCTs usually present as solitary asymptomatic masses; however, multiple tumors have been noted in up to 25% of reported cases.4,6 In children, multiple cutaneous GCTs have been reported in the setting of neurofibromatosis type I as well as with other disorders.2,5,7-9
Cutaneous GCTs have been reported to range from sessile, pedunculated, or verrucous nodules to subcutaneous papules and nodules with no epidermal change. Our case not only illustrated the diverse clinical appearance of cutaneous GCTs but also demonstrated multiple morphologically distinct cutaneous GCTs occurring in a single patient. Of particular interest is our patient’s coexisting secondary syphilis and LGV infections, which can pose a diagnostic dilemma to the unsuspecting clinician. The manifold appearances of this patient’s GCTs resulted in a broad differential diagnosis. Syphilis (condyloma latum), condyloma acuminatum, LGV, metastatic disease, Kaposi sarcoma, lymphoma, dermatofibrosarcoma protuberans, leiomyoma, SCC, and deep fungal and atypical mycobacterial infection were all considerations. In 1981, Apisarnthanarax1 reviewed 88 cases of GCTs seen over a 15-year period and discovered that the preoperative clinical diagnoses were incorrect in all cases. Skin biopsy is necessary to diagnose GCT, and our patient’s case underscores the need for a thorough history, physical examination, and laboratory evaluation to rule out coexisting diseases.
Histopathology of cutaneous GCTs shows an unencapsulated dermal proliferation of large monotonous polygonal cells with blurred cell borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests. Nuclei are small, uniform, round, centrally located, and rarely contain mitoses.3 The presence of mitotic activity on histopathology does not necessarily portend malignant biological behavior.5 Overlying pseudoepitheliomatous hyperplasia has been reported in as many as 85% of GCTs and may mimic SCC.10 The neoplastic cells stain positively with S-100 protein, neuron-specific enolase, and peripheral nerve myelin proteins.3,4 The cytoplasmic granules are positive on periodic acid–Schiff staining and diastase resistant and will sometimes stain for CD68.1 Electron microscopy shows degraded myelinated axons intracellularly.4
Malignancy is rare and reportedly occurs in 1% to 3% of cases.4,5 Consideration of both clinical behavior and histopathology is important in distinguishing benign from malignant lesions. According to published reports, in GCTs that were regarded as malignant, size tended to be greater than 4 cm, growth was rapid, and metastases to regional lymph nodes were observed.4,5 Histologically, nuclear pleomorphism and atypia, cell spindling, vesicular nuclei with prominent nucleoli, necrosis, and high mitotic activity favor malignancy.1,3
Treatment is complete surgical excision. Observation is acceptable if tumors are asymptomatic and do not impede function. Regression of some GCTs has been induced with use of intralesional corticosteroids.5 Spontaneous regression is rare. Prior reports have emphasized the importance of long-term follow-up in patients with multiple GCTs to monitor for development of systemic lesions.4
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
1. Apisarnthanarax P. Granular cell tumor. an analysis of 16 cases and review of the literature. J Am Acad Dermatol. 1981;5:171-182.
2. Guiglia MC, Prendiville JS. Multiple granular cell tumors associated with giant speckled lentiginous nevus and nevus flammeus in a child. J Am Acad Dermatol. 1991;24(2, pt 2):359-363.
3. Hazan C, Fangman W. Multiple cutaneous granular-cell tumors. Dermatol Online J. 2007;13:4.
4. Gross VL, Lynfield Y. Multiple cutaneous granular cell tumors: a case report and review of the literature. Cutis. 2002;69:343-346.
5. Martin RW 3rd, Neldner KH, Boyd AS, et al. Multiple cutaneous granular cell tumors and neurofibromatosis in childhood. a case report and review of the literature. Arch Dermatol. 1990;126:1051-1056.
6. Janousková G, Campr V, Konkol’ová R, et al. Multiple granular cell tumour. J Eur Acad Dermatol Venereol. 2004;18:347-349.
7. Gunson TH, Hashim N, Sharpe GR. Generalized lentiginosis, short stature, and multiple cutaneous nodules—quiz case. LEOPARD syndrome (LS) associated with multiple granular cell tumors (GCTs). Arch Dermatol. 2010;146:337-342.
8. De Raeve L, Roseeuw D, Otten J. Multiple cutaneous granular cell tumors in a child in remission for Hodgkin’s disease. J Am Acad Dermatol. 2002;47(2 suppl):S180-S182.
9. Ramaswamy PV, Storm CA, Filiano JJ, et al. Multiple granular cell tumors in a child with Noonan syndrome. Pediatr Dermatol. 2010;27:209-211.
10. Bangle R Jr. A morphological and histochemical study of the granular-cell myoblastoma. Cancer. 1952;5:950-965.
Practice Points
- Granular cell tumors (GCTs) typically present as solitary lesions; however, multiple lesions occur in approximately 25% of cases.
- Granular cell tumors have a variable clinical appearance and may mimic malignant neoplasms (eg, squamous cell carcinoma) as well as infectious diseases (eg, condyloma, syphilis).
- The histological features of GCTs are distinctive, including an unencapsulated dermal proliferation of monotonous polygonal cells with indistinct borders and fine, granular, eosinophilic cytoplasm arranged in irregular sheets and nests.
Pityriasis Lichenoides Chronica Presenting With Bilateral Palmoplantar Involvement
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
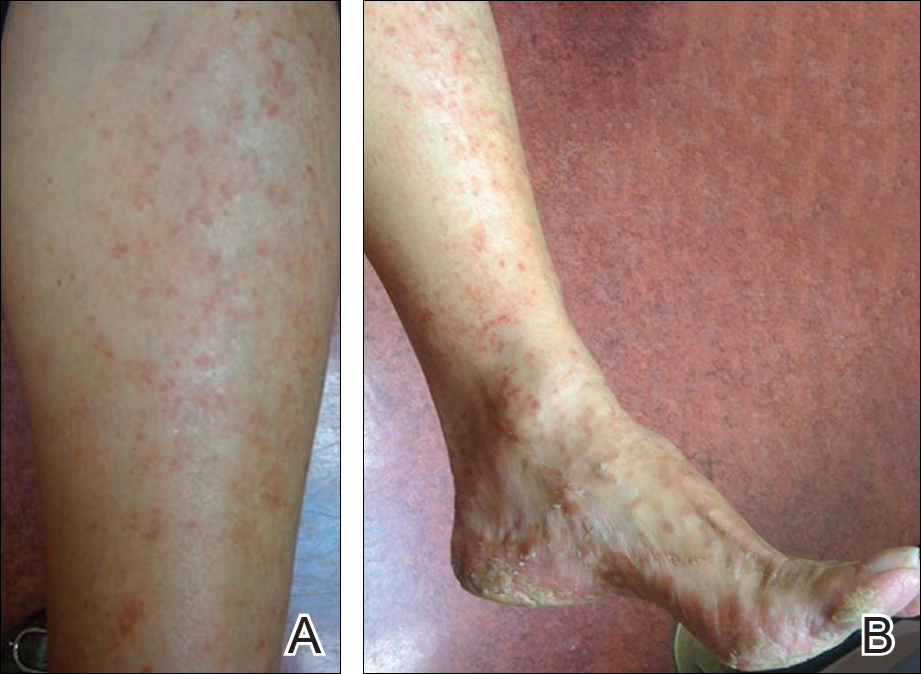
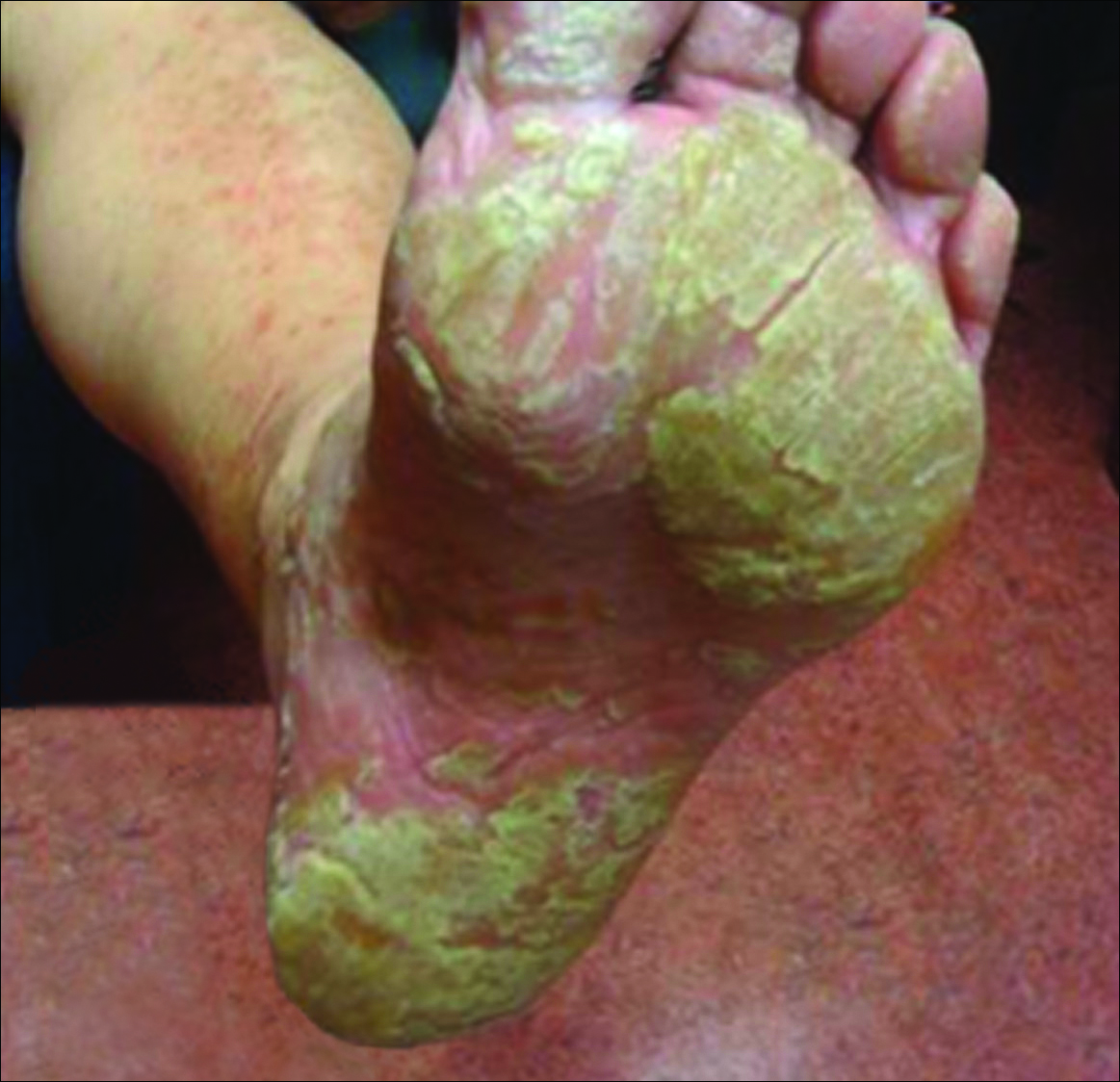
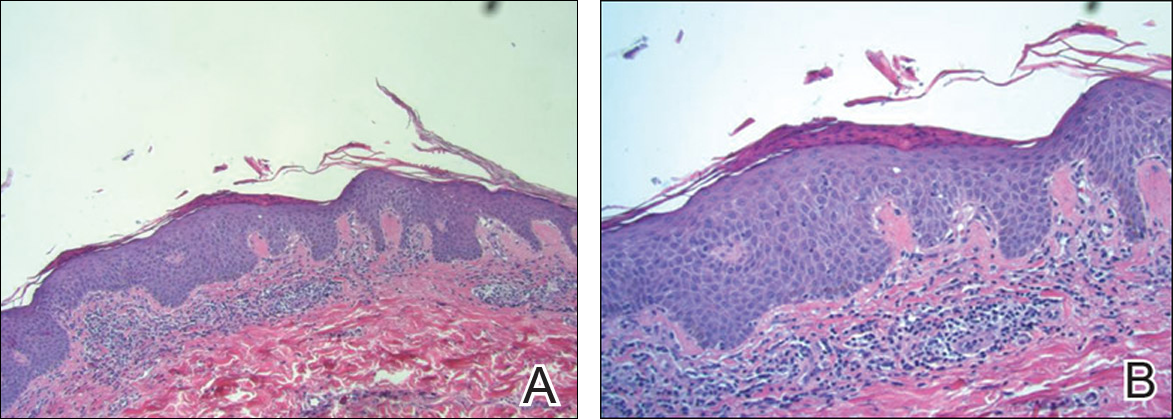
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).
The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
Pityriasis lichenoides is an uncommon, acquired, idiopathic, self-limiting skin disease that poses a challenge to patients and clinicians to diagnose and treat. Several variants exist including pityriasis lichenoides et varioliformis acuta (PLEVA), pityriasis lichenoides chronica (PLC), and febrile ulceronecrotic Mucha-Habermann disease. Precise classification can be difficult due to an overlap of clinical and histologic features. The spectrum of this inflammatory skin disorder is characterized by recurrent crops of spontaneously regressing papulosquamous, polymorphic, and ulceronecrotic papules affecting the trunk and extremities. Pityriasis lichenoides is a monoclonal T-cell disorder that needs careful follow-up because it can progress, though rarely, to cutaneous T-cell lymphoma. In this case report we describe a patient with a rare presentation of PLC exhibiting bilateral palmoplantar involvement and mimicking psoriasis. We review the literature and discuss the clinical course, pathogenesis, and current treatment modalities of PLC.
Case Report
A 61-year-old woman presented with a recurrent itchy rash on the legs, feet, hands, and trunk of several months’ duration. Her medical history included Helicobacter pylori–associated peptic ulcer disease and hypertension. She was not taking any prescription medications. She reported no alcohol or tobacco use or any personal or family history of skin disease. For many years she had lived part-time in Hong Kong, and she was concerned that her skin condition might be infectious or allergic in nature because she had observed similar skin lesions in Hong Kong natives who attributed the outbreaks of rash to “bad water.”
Physical examination revealed reddish brown crusted papules and plaques scattered bilaterally over the legs and feet (Figure 1); serpiginous scaly patches on the hips, thighs, and back; and thick hyperkeratotic psoriasiform plaques with yellow scale and crust on the palms and soles (Figure 2). The nails and oral mucosa were unaffected. Histopathologic evaluation of the lesions obtained from the superior aspect of the thigh showed parakeratotic scale and a lichenoid lymphocytic infiltrate in the papillary dermis consistent with PLC (Figure 3).



The patient was started on tetracycline 500 mg twice daily for 10 days and on narrowband UVB (NB-UVB) therapy at 350 J/cm2 with incremental increases of 60 J/cm2 at each treatment for a maximum dose of 770 J/cm2. She received 9 treatments in total over 1 month and noted some improvement in overall appearance of the lesions, mostly over the trunk and extremities. Palmoplantar lesions were resistant to treatment. Therapy with NB-UVB was discontinued, as the patient had to return to Hong Kong. Given the brief course of NB-UVB therapy, it was hard to assess why the palmoplantar lesions failed to respond to treatment.
Comment
Subtypes
Pityriasis lichenoides is a unique inflammatory disorder that usually presents with guttate papules in various stages of evolution ranging from acute hemorrhagic, vesicular, or ulcerated lesions to chronic pink papules with adherent micalike scale. Two ends of the spectrum are PLEVA and PLC. Papule distribution often is diffuse, affecting both the trunk and extremities, but involvement can be confined to the trunk producing a central distribution or restricted to the extremities giving a peripheral pattern. A purely acral localization is uncommon and rarely has been documented in the literature.1
Pityriasis lichenoides et varioliformis acuta typically presents with an acute polymorphous eruption of 2- to 3-mm erythematous macules that evolve into papules with a fine, micaceous, centrally attached scale. The center of the papule then undergoes hemorrhagic necrosis, becomes ulcerated with reddish brown crust, and may heal with a varioliform scar. Symptoms may include a burning sensation and pruritus. Successive crops may persist for weeks, months, and sometimes years.2
Febrile ulceronecrotic Mucha-Habermann disease is an acute and severe generalized eruption of ulceronecrotic plaques. Extensive painful necrosis of the skin may follow and there is an increased risk for secondary infection.2 Systemic symptoms may include fever, sore throat, diarrhea, and abdominal pain. Febrile ulceronecrotic Mucha-Habermann disease has a mortality rate of 25% and should be treated as a dermatologic emergency.2
Pityriasis lichenoides chronica has a more gradual presentation and indolent course than PLEVA. It most commonly presents as small asymptomatic polymorphous red-brown maculopapules with micaceous scale.3 Papules spontaneously flatten over a few weeks. Postinflammatory hypopigmentation or hyperpigmentation may persist once the lesions resolve. Similar to PLEVA, PLC has a relapsing course but with longer periods of remission. Pityriasis lichenoides chronica usually involves the trunk and proximal extremities, but acral distributions, as in our case, have been described. This rare variant of pityriasis lichenoides may be underrecognized and underdiagnosed due to its resemblance to psoriasis.1
The prevalence and incidence of PLC in the general population is unknown. There appears to be no predominance based on gender, ethnicity, or geographical location, and it occurs in both children and adults. One study showed the average age to be 29 years.2
Etiology
The cause of pityriasis lichenoides is unknown, but there are 3 popular theories regarding its pathogenesis: a hypersensitivity response due to an infectious agent, an inflammatory response to a T-cell dyscrasia, or an immune complex–mediated hypersensitivity vasculitis.2 The theory of an infectious cause has been proposed due to reports of disease clustering in families and communities.2,3 Elevated titers of certain pathogens and clearing of the disease after pathogen-specific treatment also have been reported. Possible triggers cited in the literature include the Epstein-Barr virus, Toxoplasma gondii, parvovirus B19, adenovirus, human immunodeficiency virus, freeze-dried live attenuated measles vaccine, Staphylococcus aureus, and group A β-hemolytic streptococci.2,3
Some reported cases of pityriasis lichenoides have demonstrated T-cell clonality. Weinberg et al4 found a significantly higher number of clonal T cells in PLEVA than in PLC (P=.008) and hypothesized that PLEVA is actually a benign clonal T-cell disorder arising from a specific subset of T cells in PLC. Malignant transformation of pityriasis lichenoides has been reported but is rare.3
Differential Diagnosis
Historically, pityriasis lichenoides has been confused with many other dermatoses. With palmoplantar involvement, consider other papulosquamous disorders such as palmoplantar psoriasis, lichen planus, cutaneous T-cell lymphoma, lymphomatoid papulosis, vasculitis, and secondary syphilis. Rule out alternative diagnoses with histologic examination; assessments of nails, oral mucosa, joints, and constitutional symptoms; and laboratory testing.
Histopathology
Pityriasis lichenoides et varioliformis acuta and PLC are similar with subtle and gradually evolving differences, supporting the notion that these disorders are polar ends of the same disease spectrum.2 Pityriasis lichenoides et varioliformis acuta typically produces a dense wedge-shaped dermal infiltrate composed of CD8+ T cells and histiocytes most concentrated along the basal layer with lymphocytic exocytosis into the epidermis and perivascular inflammation. The epidermis also demonstrates spongiosis, necrosis and apoptosis of keratinocytes, neutrophilic inclusions, vacuolar degeneration, intraepidermal vesicles and ulceration, and focal parakeratosis with scale and crust. In contrast, PLC is less exaggerated than PLEVA with a superficial bandlike lymphocytic infiltrate in which CD4+ T cells predominate with minimal perivascular involvement. Immunohistochemical studies reveal that CD8+ cells predominate in PLEVA, while CD4+ cells predominate in PLC. Staining for HLA-DR–positive keratinocytes yields stronger and more diffuse findings in PLEVA than in PLC and is considered a marker for the former.2
Treatment
There is no standard treatment of pityriasis lichenoides. However, combination therapy is considered the best approach. To date, phototherapy has been the most effective modality and is considered a first-line treatment of PLC. Variants of phototherapy include UVB, NB-UVB, psoralen plus UVA, and UVA1.5 One study showed UVA1 (340–400 nm) treatment to be effective and well tolerated at a medium dose of 60 J/cm2.6 Narrowband UVB has become a well-used phototherapy for a variety of skin conditions including pityriasis lichenoides. In a study by Aydogan et al,5 NB-UVB was safe and effective for the management of PLEVA and PLC. The authors also argue that it has added advantages over other phototherapies, including a more immunosuppressive effect on lymphoproliferation that causes a greater depletion of T cells in skin lesions, possibly due to its deeper dermal penetration compared with broadband UVB. Narrowband UVB also is safe in children.5 Tapering of phototherapy has been recommended to prevent relapses.3
If infection is a suspected contributor to the problem, treat as needed. The antibiotics tetracycline, erythromycin, and dapsone have been used with success, as well as the antiviral acyclovir. Tetracycline and erythromycin also may confer anti-inflammatory benefits. A gradual taper of these agents is advised to prevent recurrences. Topical corticosteroids and coal tar may help alleviate pruritus and inflammation; however, they do not affect the course of the disease.3 In one report, the topical immunomodulator tacrolimus markedly reduced lesions, most likely due to its anti-inflammatory effect. After discontinuation of the medication, lesions recurred but were less severe.7
Clinical Recommendations
Early diagnosis and management of pityriasis lichenoides is essential. At this time, screening for pathogens is not advised unless the patient has specific symptoms of infection. Due to the history of recurrence with this disease, combination therapy is recommended with a gradual taper of all modalities. Because of the rare but possible transformation to malignancy, careful follow-up and repeated biopsies have been advised in chronic intermittent disease.3
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
- Kossard S. Acral pityriasis lichenoides. Australas J Dermatol. 2002;43:68-71.
- Bowers S, Warshaw EM. Pityriasis lichenoides and its subtypes. J Am Acad Dermatol. 2006;55:557-568; quiz 573-576.
- Khachemoune A, Blyumin ML. Pityriasis lichenoides: pathophysiology, classification, and treatment. Am J Clin Dermatol. 2007;8:29-36.
- Weinberg JM, Kristal L, Chooback L, et al. The clonal nature of pityriasis lichenoides. Arch Dematol. 2002;138:1063-1067.
- Aydogan K, Saricaoglu H, Turan H. Narrowband UVB (311nm, TL01) phototherapy for pityriasis lichenoides. Photodermatol Photoimmunol Photomed. 2008;24:128-133.
- Pinton P, Capezzera R, Zane C, et al. Medium-dose ultraviolet A1 therapy for pityriasis lichenoides et varioliformis acuta and pityriasis lichenoides chronic. J Am Acad Dermatol. 2002;47:401-414.
- Simon D, Boudny C, Nievergelt H, et al. Successful treatment of pityriasis lichenoides with topical tacrolimus. Br J Dermatol. 2004;150:1033-1035.
Practice Points
- Diagnosis of pityriasis lichenoides may be difficult due to a wide spectrum of clinical presentations.
- Pityriasis lichenoides chronica (PLC) with palmoplantar involvement may mimic psoriasis.
- Screening for infections is not recommended in patients with PLC unless the patient has other symptoms pointing to a specific infection.
- Phototherapy currently is the most effective treatment modality for PLC.
Acute benzodiazepine toxicity exacerbated by concomitant oral olanzapine in a patient with pancreatic cancer
Severe eosinophilia associated with cholangiocarcinoma
It is widely recognized that eosinophils are found in tumor infiltrates and that their mechanism of action is associated with particular symptoms and prognosis. However, the causes of and reasons for this process remain unclear, as does the exact mechanism by which it occurs. We report on the case of a 71-year-old woman with cholangiocellar carcinoma (CCC) with a marked eosinophilia. When the patient presented at the hospital, she said she was suffering from fatigue, depression, and pain.
Click on the PDF icon at the top of this introduction to read the full article.
It is widely recognized that eosinophils are found in tumor infiltrates and that their mechanism of action is associated with particular symptoms and prognosis. However, the causes of and reasons for this process remain unclear, as does the exact mechanism by which it occurs. We report on the case of a 71-year-old woman with cholangiocellar carcinoma (CCC) with a marked eosinophilia. When the patient presented at the hospital, she said she was suffering from fatigue, depression, and pain.
Click on the PDF icon at the top of this introduction to read the full article.
It is widely recognized that eosinophils are found in tumor infiltrates and that their mechanism of action is associated with particular symptoms and prognosis. However, the causes of and reasons for this process remain unclear, as does the exact mechanism by which it occurs. We report on the case of a 71-year-old woman with cholangiocellar carcinoma (CCC) with a marked eosinophilia. When the patient presented at the hospital, she said she was suffering from fatigue, depression, and pain.
Click on the PDF icon at the top of this introduction to read the full article.
An unusual case of non-small-cell lung cancer presenting as spontaneous cardiac tamponade
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Hemorrhagic pericardial effusion with associated cardiac tamponade as a de novo sign of malignancy is seen in about 2% of patients.1 Consequently, cardiac tamponade is an oncologic emergency and considered a unique presentation of a malignancy.2 Cancer emergency is defined as an acute condition that is caused directly by the cancer itself or its treatment and requires intervention to avoid death or significant morbidity.3 The mechanism by which cardiac tamponade is classified as a life-threatening emergency stems from its impairment of right ventricular filling, resulting in ventricular diastolic collapse and decreased cardiac output, which can ultimately lead to death.4
Click on the PDF icon at the top of this introduction to read the full article.
Increased syncopal episodes post surgery • Dx?
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
THE CASE
A 58-year-old woman sought care at our clinic for recurrent syncopal and near-syncopal events following surgical repair of a left hip fracture. The first syncopal event occurred one day post-surgery shortly after standing and was attributed to orthostatic hypotension. Subsequently, the patient experienced 2 events during her hospital stay. Both events occurred in the upright position and were preceded by lightheadedness, warmth, and diaphoresis. They were short in duration (<30 seconds) with spontaneous and complete recovery. The patient had no associated chest pain or palpitations.
The patient’s past medical history included osteopenia, dyslipidemia, and vasovagal syncope, averaging one to 2 events per year. Given her past history, the physicians caring for her assumed that she was having recurrences of her vasovagal syncope. She was discharged home on fludrocortisone 0.1 mg/d, sodium chloride 1 g tid, enoxaparin 40 mg/d, and acetaminophen and oxycodone as needed for pain.
One week later, the patient experienced another syncopal event at home, prompting her to visit our clinic for further evaluation. On arrival, her vital signs were stable. Her oxygen saturation level was 98%, she was not orthostatic, and her physical exam and blood studies were unremarkable. An echocardiogram showed preserved left ventricular function with no evidence of right ventricular dilatation or strain.
THE DIAGNOSIS
The patient’s revised Geneva Score for pulmonary embolism (PE) was 2 to 5 depending on the heart rate used (66-80 beats per minute), putting her in a low-to-intermediate risk group with an estimated PE prevalence between 8% and 28%.1 Given her recent surgery and the increase in the frequency of her vasovagal events, a computed tomography pulmonary angiogram (CT-PA) was performed. The CT-PA showed a PE in the lateral and posterior basal subsegmental branches of the right lower lobe. Doppler ultrasound revealed no evidence of acute deep vein thrombosis.
DISCUSSION
Syncope may develop in 9% to 19% of patients with PE.2-6 While syncope in patients with PE is often attributed to reduced cardiac filling secondary to massive emboli, it is important to recognize that patients can also present with vasovagal syncope in the absence of massive emboli.
One mechanism for the development of syncope is right ventricular failure with subsequent impairment of left ventricular filling, leading to arterial hypotension. Indeed, the majority of patients with PE and syncope have a massive embolism defined as greater than a 50% reduction in the pulmonary circulation.7 In one study, 60% of patients with PE who presented with syncope had a massive PE compared to 39% of patients presenting without syncope (P=.036).8
Another reported mechanism for syncope in a patient with PE is transient high-degree atrioventricular (AV) block.9 Sudden increases in right-sided pressure can lead to transient right bundle branch block, which may result in complete heart block in the setting of baseline left bundle branch block.
Lastly, patients with PE may develop a vasovagal-like reaction, such as the Bezold-Jarisch reflex, which results in transient arterial hypotension and cerebral hypoperfusion.10 In such instances, the postulated mechanism is activation of cardiac vagal afferents, which results in an increase in vagal tone and peripheral sympathetic withdrawal leading to hypotension and syncope. It is important to note that this mechanism can occur in the absence of massive PE. In one study, up to 40% of patients with PE and syncope did not have a massive PE, and almost 6% had thrombi only in small branches of the pulmonary artery.8
This patient had isolated subsegmental defects, identified on the CT-PA. The sensitivity of CT-PA to detect subsegmental PE ranges from 53% to 100%.11 While this test has its limitations, the introduction of the multi-detector CT technique has significantly increased the rate of detection with a specificity of 96%.12,13
Was PE the cause of the syncope, or just an incidental finding?
In this case, we believe the CT-PA findings were diagnostic for PE. What is less clear is whether the PE was the cause of the syncope.
Asymptomatic post-operative PE with isolated subsegmental defects has been reported.14-16 When compared to patients with a defect at a segmental or more proximal level, these patients often have less dyspnea, are less likely to be classified as having a high clinical probability of PE, and have a lower prevalence of proximal deep vein thrombosis (3.3% vs 43.8%; P<.0001).17 Therefore, one could argue that the PE finding in our case was incidental. While this is a possibility, we believe the patient’s syncope was due to PE for the following reasons.
First, several investigators have reported transient increases in vagal tone and syncope following PE consistent with a vasovagal-like response.7,18 Therefore, it is possible that the reduction in preload associated with PE triggered a Bezold-Jarisch-like reflex leading to syncope. The patient’s history of vasovagal syncope was certainly indicative of increased susceptibility to reflex-mediated events, thus supporting our hypothesis.
Second, our patient had a cluster of events following surgery compared to the one to 2 events she experienced per year prior to surgery. The increased incidence of events would be an unusual progression of her syncope in the absence of clear triggers, again rendering our hypothesis more plausible.
The patient was admitted to our hospital and started on a higher dose of enoxaparin (60 mg twice daily). She was subsequently discharged home on rivaroxaban 15 mg twice daily and midodrine 2.5 mg twice daily in addition to the medications she was already taking. At her 6-week follow-up visit, she reported no recurrences.
THE TAKEAWAY
This case demonstrates that non-massive PE can present as vasovagal syncope. Recognizing that PE could lead to reflex-mediated syncope in the absence of massive emboli, it is important to rule it out in the evaluation of patients with vasovagal syncope when risk factors for PE are present.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
1. Le Gal G, Righini M, Roy PM, et al. Prediction of pulmonary embolism in the emergency department: the revised Geneva score. Ann Intern Med. 2006;144:165-171.
2. Calvo-Romero JM, Pérez-Miranda M, Bureo-Dacal P. Syncope in acute pulmonary embolism. Eur J Emerg Med. 2004;11:208-209.
3. Castelli R, Tarsia P, Tantardini C, et al. Syncope in patients with pulmonary embolism: comparison between patients with syncope as the presenting symptom of pulmonary embolism and patients with pulmonary embolism without syncope. Vasc Med. 2003;8:257-261.
4. Kasper W, Konstantinides S, Geibel A, et al. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171.
5. Koutkia P, Wachtel TJ. Pulmonary embolism presenting as syncope: case report and review of the literature. Heart Lung. 1999;28:342-347.
6. Torbicki A, Perrier A, Konstantinides S, et al; ESC Committee for Practice Guidelines (CPG). Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Eur Heart J. 2008;29:2276-2315.
7. Thames MD, Alpert JS, Dalen JE. Syncope in patients with pulmonary embolism. JAMA. 1977;238:2509-2511.
8. Duplyakov D, Kurakina E, Pavlova T, et al. Value of syncope in patients with high-to-intermediate risk pulmonary artery embolism. Eur Heart J Acute Cardiovasc Care. 2015;4:353-358.
9. Wilner C, Garnier-Crussard JP, Huygue De Mahenge A, et al. [Paroxysmal atrioventricular block, cause of syncope in pulmonary embolism. 2 cases]. Presse Med. 1983;12:2987-2989.
10. Frink RJ, James TN. Intracardiac route of the Bezold-Jarisch reflex. Am J Physiol. 1971;221:1464-1469.
11. Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: A systematic review. Ann Intern Med. 2000;132:227-232.
12. Stein PD, Fowler SE, Goodman LR, et al; PIOPED II Investigators. Multidetector computed tomography for acute pulmonary embolism. N Engl J Med. 2006;354:2317-2327.
13. Vedovati MC, Becattini C, Agnelli G, et al. Multidetector CT scan for acute pulmonary embolism: embolic burden and clinical outcome. Chest. 2012;142:1417-1424.
14. Musset D, Parent F, Meyer G, et al; Evaluation du Scanner Spiralé dans l’Embolie Pulmonaire study group. Diagnostic strategy for patients with suspected pulmonary embolism: a prospective multicentre outcome study. Lancet. 2002;360:1914-1920.
15. Simpson RJ Jr, Podolak R, Mangano CA Jr, et al. Vagal syncope during recurrent pulmonary embolism. JAMA. 1983;249:390-393.
16. Perrier A, Roy PM, Sanchez O, et al. Multidetector-row computed tomography in suspected pulmonary embolism. N Engl J Med. 2005;352:1760-1768.
17. Le Gal G, Righini M, Parent F, et al. Diagnosis and management of subsegmental pulmonary embolism. J Thromb Haemost. 2006;4:724-731.
18. Eldadah ZA, Najjar SS, Ziegelstein RC. A patient with syncope, only “vagally” related to the heart. Chest. 2000;117:1801-1803.
Congenital Self-healing Reticulohistiocytosis: An Underreported Entity
Langerhans cell histiocytosis (LCH), also known as histiocytosis X, is a general term that describes a group of rare disorders characterized by the proliferation of Langerhans cells.1 Central to immune surveillance and the elimination of foreign substances from the body, Langerhans cells are derived from bone marrow progenitor cells and found in the epidermis but are capable of migrating from the skin to the lymph nodes. In LCH, these cells congregate on bone tissue, particularly in the head and neck region, causing a multitude of problems.2
The spectrum of LCH includes 4 variants: congenital self-healing reticulohistiocytosis (CSHR)(also known as Hashimoto-Pritzker disease), Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma (also known as pulmonary histiocytosis X)(Table). Despite the various clinical presentations and levels of severity, all variants are caused by the proliferation of Langerhans cells. We present a case of CSHR in a 6-month-old male infant that was initially diagnosed as molluscum contagiosum. We believe the actual incidence of CSHR may be underreported due to its spontaneous regression and low rate of clinical recognition.
Case Report
A 6-month-old male infant was referred to our clinic by his pediatrician with a generalized cutaneous eruption of 3 weeks’ duration. The eruption, which followed a recent viral upper respiratory tract infection, was characterized by multiple flesh-colored to erythematous, umbilicated papules distributed along the postauricular region, scalp (Figure 1A), abdomen (Figure 1B), and anterior aspect of the neck. Due to his recent illness, the patient was diagnosed with molluscum contagiosum by the referring pediatrician that was treated symptomatically with hydrocortisone lotion, Schamberg’s cream formulated in our office (a compound mixture of zinc oxide, menthol, calcium hydroxide solution, and olive oil), and pediatric diphenhydramine as needed. During a subsequent visit 2 weeks later, a more potent topical corticosteroid and a low-dose systemic corticosteroid was prescribed for 1 week due to development of new lesions and exacerbation of existing lesions. On follow-up 1 week later, the lesions on the trunk had improved, but the patient had developed new lesions on the scalp that differed from prior findings in that they were darker (more erythematous to brown) and firmer (papules and nodules).
 | 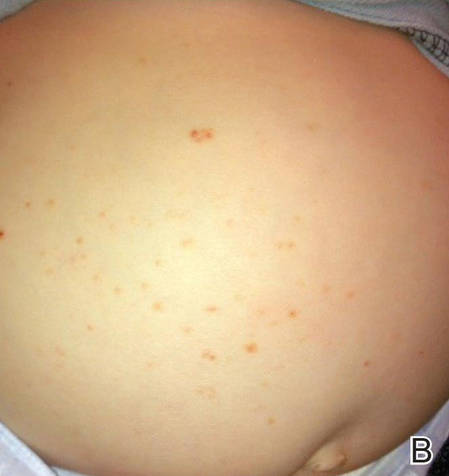 | |
Figure 1. Multiple fleshcolored to erythematous, umbilicated papules on the frontal scalp (A) and erythematous papules on the abdomen (B). | ||
A shave biopsy was obtained from the frontal scalp to rule out LCH. Histologic examination and culture of the biopsy specimen revealed an atypical cellular infiltrate effacing the dermoepidermal junction and extensive epidermotropism. Focal erosion of the epidermis and an acute inflammatory exudate were visible. The nuclei of the cellular infiltrate were enlarged and hyperchromatic with a characteristic reniform appearance and indistinct nucleoli (Figure 2). The cells were admixed with scattered eosinophils and extravasated red blood cells.
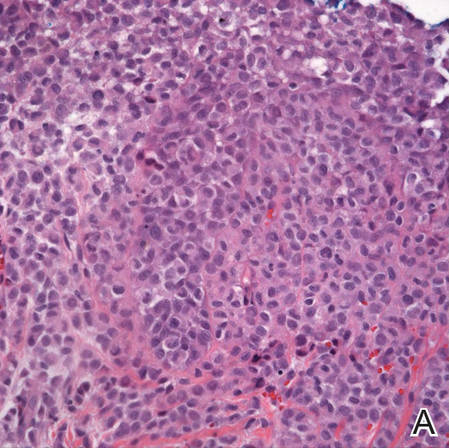 | 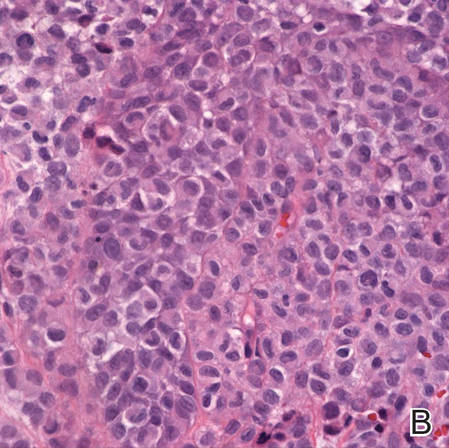 | |
Figure 2. Low-power view of dermal mononuclear cells with reniform nuclei (A)(H&E, original magnification ×100), and high-power view of enlarged and hyperchromatic nuclei with a characteristic reniform appearance admixed with eosinophils and extravasated red blood cells (B) (H&E, original magnification ×400). | ||
Immunohistochemical staining of the biopsy specimen was strongly positive for both CD1a and S-100 expression (Figure 3). Histopathologic findings were consistent with LCH. Clinicopathologic correlation strongly favored the diagnosis of CSHR.
Comment
Congenital self-healing reticulohistiocytosis is a rare, benign, congenital variant of LCH that spontaneously resolves with no systemic involvement. The more aggressive forms typically manifest at birth or during the first 2 months of life and regress within 3 to 4 months.5 Since CSHR was first described in 1973 by Hashimoto and Pritzker,5 more than 100 cases have been reported, but the true incidence is believed to be higher than reported given the high rate of spontaneous resolution and the low rate of clinical recognition.2 The first reported case of CSHR occurred in a female infant who presented at birth with multiple, diffusely distributed, red-brown papules that were 2 to 4 mm in diameter. Although the patient received no treatment, the exanthem completely resolved within 3.5 months without recurrence at 14-year follow-up.5 Most often, CSHR presents as multiple papules or nodules with occasional disseminated crusting and is followed within a few months by a dramatic and spontaneous regression. Lesions may heal with mild postinflammatory hyperpigmentation. Pseudo-Darier sign, the propensity to urticate from physical manipulation, has been reported in some lesions with an increased number of mast cells.6 Extensive superficial nasal and oral mucosal erosions have been reported in 2 cases.7 Solitary lesions have been reported in 25% of cases.8
The etiology of CSHR remains unknown, though neoplastic, viral, and immunologic origins have been suggested. There have been reports that human herpesvirus 6 may contribute to the development of LCH.9 It may be postulated that our patient’s presentation of CSHR was potentiated by his recent upper respiratory tract illness. In the literature, CSHR is distributed equally among males and females. Prevalence is higher in the white population than in other racial groups.5
Although CSHR is a benign cutaneous variant of LCH, there have been reports of patients with disseminated and extracutaneous involvement. In 1 rare case, CSHR reportedly involved the eyes, producing multiple, bilateral, well-circumscribed, diffuse, yellow-white lesions of the retinal pigment epithelium throughout the posterior pole of the eyes.10 The retinal lesions spontaneously regressed along with the skin manifestations. Additionally, it was reported that a neonate in Thailand presented with CSHR at birth and 1 month later developed multiple lung cysts that had completely regressed 11 months later.11 One study reported that initial diagnoses of LCH in 18 patients with only cutaneous involvement eventually progressed to systemic LCH, requiring further management.12 When LCH is suspected, a thorough physical examination, including hematologic and coagulation evaluation, liver function tests, musculoskeletal examination, and consultation with specialists if necessary, is recommended.13
There are 3 additional variants of LCH. Letterer-Siwe disease is an acute form of LCH that accounts for 10% of all LCH cases and typically presents in children younger than 2 years. It involves multiple organs, including the bones, lungs, liver, and lymph nodes.14 Affected patients usually present with fever; hepatosplenomegaly; anemia; lymphadenopathy; extensive lytic skull lesions; and a generalized cutaneous eruption, appearing as a maculopapular scaling rash with underlying purpura on the scalp, neck, axilla, and trunk.3 Letterer-Siwe disease is inherited in an autosomal-recessive pattern. Diagnosis is confirmed by skin biopsy demonstrating a thinning of the epidermis and a collection of reticulum cells in the dermis.3 Letterer-Siwe disease is treated with radiation and chemotherapy; if left untreated, the disease is fatal.4
Hand-Schüller-Christian disease, a chronic form of LCH, is most commonly seen in children aged 2 to 6 years and accounts for 15% to 20% of all LCH cases. This LCH variant presents with a classic triad of diabetes insipidus (resulting from erosion into the sella turcica), lytic bone lesions, and exophthalmos.15 Hand-Schüller-Christian disease also affects the oral cavity, producing nodular ulcerations of the hard palate, trouble swallowing, and halitosis.4 The involvement of lytic bone lesions of the mastoid process and petrous portions of the temporal bones may cause recurrent or chronic otitis media and otitis externa. Hand-Schüller-Christian disease is treated with a combination of chemotherapy, radiation, and surgical excision. The mortality rate is 30%.4
Eosinophilic granuloma is the most prevalent variant of LCH, accounting for 60% to 80% of all cases. Characterized by Langerhans cell granulomatous infiltration of the lungs and painful cystic bone lesions, eosinophilic granuloma primarily presents in the third or fourth decades of life.16 Some studies suggest an epidemiologic association with tobacco use.17 In the preliminary stages of this disease, Langerhans cells, eosinophils, lymphocytes, and fibroblasts infiltrate and form nodules on the terminal bronchioles in the upper and middle lung zones, damaging the airway walls.18 Fibrotic scarring progresses, ultimately resulting in alveolar destruction.10 The common signs and symptoms of eosinophilic granuloma are a nonproductive cough, dyspnea, weight loss, spontaneous pneumothorax, fever, peripheral edema, and a tricuspid regurgitation murmur.14 The prognosis of eosinophilic granuloma is variable. Although some patients progress to end-stage fibrotic lung disease requiring lung transplant, there have been reports of complete remission following cessation of cigarette smoking.17
Langerhans cells travel from the bone marrow to the epidermis where they express the CD1a protein on the surface of the antigen-presenting cell. Elevated levels of cytokines, such as tumor necrosis factor α, IFN-γ, granulocyte-macrophage colony-stimulating factor, and interleukins have been seen in patients with LCH.1 Their role in the pathogenesis of this disease remains unknown, but the elevated levels of cytokines may indicate the lack of an efficient immune system.
Histologically, hematoxylin and eosin–stained sections demonstrate an infiltrate of histiocytes, neutrophils, eosinophils, and an increased number of mast cells involving the papillary and reticular dermis. Infiltrating Langerhans cells have concave reniform nuclei18 and stain positive for CD1a, S-100, and CD68 antigens.15 In 10% to 30% of CSHR cases, Birbeck granules can be seen on electron microscopy and tend to transform into laminated dense bodies, signifying the degenerative changes seen in CSHR.15 The various forms of LCH exhibit no significant differences in the expression of the epithelial cadherin, the phosphorylated histone H3, and the Ki-67 proteins, indicating that they are simply different forms of the same disease represented on a spectrum.15
Conclusion
The actual incidence of CSHR may be notably underreported due to its spontaneous regression and low rate of clinical recognition. A subtype of LCH, CSHR is a diagnosis of exclusion. Although CSHR generally follows a benign clinical course, a thorough workup and evaluation for systemic disease with close follow-up is recommended after diagnosis due to the potential of LCH to involve multiple organs and to relapse at a later date after apparent regression.
1. Hussein MR. Skin-limited Langerhans’ cell histiocytosis in children. Cancer Invest. 2009;27:504-511.
2. Nakahigashi K, Ohta M, Sakai R, et al. Late-onset self-healing reticulohistiocytosis: pediatric case of Hashimoto-Pritzker type Langerhans cell histiocytosis. J Dermatol. 2007;34:205-209.
3. Pant C, Madonia P, Bahna SL, et al. Langerhans cell histiocytosis, a case of Letterer Siwe disease. J La State Med Soc. 2009;161:211-212.
4. Ferreira LM, Emerich PS, Diniz LM, et al. Langerhans cell histiocytosis: Letterer-Siwe disease–the importance of dermatological diagnosis in two cases [in Portuguese]. An Bras Dermatol. 2009;84:405-409.
5. Hashimoto K, Pritzker MS. Electron microscopic study of reticulohistiocytoma. an unusual case of congenital, self-healing reticulohistiocytosis. Arch Dermatol. 1973;107:263-270.
6. Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children’s Medical Center. J Am Acad Dermatol. 2007;56:290-294.
7. Le Bidre E, Lorette G, Delage M, et al. Extensive, erosive congenital self-healing cell histiocytosis [published online December 22, 2008]. J Eur Acad Dermatol Venereol. 2009;23:835-836.
8. Weiss T, Weber L, Scharffetter-Kochanek K, et al. Solitary cutaneous dendritic cell tumor in a child: role of dendritic cell markers for the diagnosis of skin Langerhans cell histiocytosis. J Am Acad Dermatol. 2005;53:838-844.
9. Csire M, Mikala G, Jákó J, et al. Persistent long-term human herpesvirus 6 (HHV-6) infection in a patient with Langerhans cell histiocytosis [published online July 3, 2007]. Pathol Oncol Res. 2007;13:157-160.
10. Zaenglein AL, Steele MA, Kamino H, et al. Congenital self-healing reticulohistiocytosis with eye involvement. Pediatr Dermatol. 2001;18:135-137.
11. Chunharas A, Pabunruang W, Hongeng S. Congenital self-healing Langerhans cell histiocytosis with pulmonary involvement: spontaneous regression. J Med Assoc Thai. 2002;85(suppl 4):S1309-S1313.
12. Minkov M, Prosch H, Steiner M, et al. Langerhans cell histiocytosis in neonates. Pediatr Blood Cancer. 2005;45:802-807.
13. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008;25:291-295.
14. Stacher E, Beham-Schmid C, Terpe HJ, et al. Pulmonary histiocytic sarcoma mimicking pulmonary Langerhans cell histiocytosis in a young adult presenting with spontaneous pneumothorax: a potential diagnostic pitfall [published online June 27, 2009]. Virchows Arch. 2009;455:187-190.
15. Scolozzi P, Lombardi T, Monnier P, et al. Multisystem Langerhans’ cell histiocytosis (Hand-Schüller-Christian disease) in an adult: a case report and review of the literature [published online October 10, 2003]. Eur Arch Otorhinolaryngol. 2004;261:326-330.
16. Noonan V, Kabani S, Alibhai K. Langerhans cell histiocytosis (eosinophilic granuloma). J Mass Dent Soc. 2011;60:35.
17. Podbielski FJ, Worley TA, Korn JM, et al. Eosinophilic granuloma of the lung and rib. Asian Cardiovasc Thorac Ann. 2009;17:194-195.
18. Rosso DA, Ripoli MF, Roy A, et al. Serum levels of interleukin-1 receptor antagonist and tumor necrosis factor-alpha are elevated in children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2003;25:480-483.
Langerhans cell histiocytosis (LCH), also known as histiocytosis X, is a general term that describes a group of rare disorders characterized by the proliferation of Langerhans cells.1 Central to immune surveillance and the elimination of foreign substances from the body, Langerhans cells are derived from bone marrow progenitor cells and found in the epidermis but are capable of migrating from the skin to the lymph nodes. In LCH, these cells congregate on bone tissue, particularly in the head and neck region, causing a multitude of problems.2
The spectrum of LCH includes 4 variants: congenital self-healing reticulohistiocytosis (CSHR)(also known as Hashimoto-Pritzker disease), Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma (also known as pulmonary histiocytosis X)(Table). Despite the various clinical presentations and levels of severity, all variants are caused by the proliferation of Langerhans cells. We present a case of CSHR in a 6-month-old male infant that was initially diagnosed as molluscum contagiosum. We believe the actual incidence of CSHR may be underreported due to its spontaneous regression and low rate of clinical recognition.
Case Report
A 6-month-old male infant was referred to our clinic by his pediatrician with a generalized cutaneous eruption of 3 weeks’ duration. The eruption, which followed a recent viral upper respiratory tract infection, was characterized by multiple flesh-colored to erythematous, umbilicated papules distributed along the postauricular region, scalp (Figure 1A), abdomen (Figure 1B), and anterior aspect of the neck. Due to his recent illness, the patient was diagnosed with molluscum contagiosum by the referring pediatrician that was treated symptomatically with hydrocortisone lotion, Schamberg’s cream formulated in our office (a compound mixture of zinc oxide, menthol, calcium hydroxide solution, and olive oil), and pediatric diphenhydramine as needed. During a subsequent visit 2 weeks later, a more potent topical corticosteroid and a low-dose systemic corticosteroid was prescribed for 1 week due to development of new lesions and exacerbation of existing lesions. On follow-up 1 week later, the lesions on the trunk had improved, but the patient had developed new lesions on the scalp that differed from prior findings in that they were darker (more erythematous to brown) and firmer (papules and nodules).
 |  | |
Figure 1. Multiple fleshcolored to erythematous, umbilicated papules on the frontal scalp (A) and erythematous papules on the abdomen (B). | ||
A shave biopsy was obtained from the frontal scalp to rule out LCH. Histologic examination and culture of the biopsy specimen revealed an atypical cellular infiltrate effacing the dermoepidermal junction and extensive epidermotropism. Focal erosion of the epidermis and an acute inflammatory exudate were visible. The nuclei of the cellular infiltrate were enlarged and hyperchromatic with a characteristic reniform appearance and indistinct nucleoli (Figure 2). The cells were admixed with scattered eosinophils and extravasated red blood cells.
 |  | |
Figure 2. Low-power view of dermal mononuclear cells with reniform nuclei (A)(H&E, original magnification ×100), and high-power view of enlarged and hyperchromatic nuclei with a characteristic reniform appearance admixed with eosinophils and extravasated red blood cells (B) (H&E, original magnification ×400). | ||
Immunohistochemical staining of the biopsy specimen was strongly positive for both CD1a and S-100 expression (Figure 3). Histopathologic findings were consistent with LCH. Clinicopathologic correlation strongly favored the diagnosis of CSHR.
Comment
Congenital self-healing reticulohistiocytosis is a rare, benign, congenital variant of LCH that spontaneously resolves with no systemic involvement. The more aggressive forms typically manifest at birth or during the first 2 months of life and regress within 3 to 4 months.5 Since CSHR was first described in 1973 by Hashimoto and Pritzker,5 more than 100 cases have been reported, but the true incidence is believed to be higher than reported given the high rate of spontaneous resolution and the low rate of clinical recognition.2 The first reported case of CSHR occurred in a female infant who presented at birth with multiple, diffusely distributed, red-brown papules that were 2 to 4 mm in diameter. Although the patient received no treatment, the exanthem completely resolved within 3.5 months without recurrence at 14-year follow-up.5 Most often, CSHR presents as multiple papules or nodules with occasional disseminated crusting and is followed within a few months by a dramatic and spontaneous regression. Lesions may heal with mild postinflammatory hyperpigmentation. Pseudo-Darier sign, the propensity to urticate from physical manipulation, has been reported in some lesions with an increased number of mast cells.6 Extensive superficial nasal and oral mucosal erosions have been reported in 2 cases.7 Solitary lesions have been reported in 25% of cases.8
The etiology of CSHR remains unknown, though neoplastic, viral, and immunologic origins have been suggested. There have been reports that human herpesvirus 6 may contribute to the development of LCH.9 It may be postulated that our patient’s presentation of CSHR was potentiated by his recent upper respiratory tract illness. In the literature, CSHR is distributed equally among males and females. Prevalence is higher in the white population than in other racial groups.5
Although CSHR is a benign cutaneous variant of LCH, there have been reports of patients with disseminated and extracutaneous involvement. In 1 rare case, CSHR reportedly involved the eyes, producing multiple, bilateral, well-circumscribed, diffuse, yellow-white lesions of the retinal pigment epithelium throughout the posterior pole of the eyes.10 The retinal lesions spontaneously regressed along with the skin manifestations. Additionally, it was reported that a neonate in Thailand presented with CSHR at birth and 1 month later developed multiple lung cysts that had completely regressed 11 months later.11 One study reported that initial diagnoses of LCH in 18 patients with only cutaneous involvement eventually progressed to systemic LCH, requiring further management.12 When LCH is suspected, a thorough physical examination, including hematologic and coagulation evaluation, liver function tests, musculoskeletal examination, and consultation with specialists if necessary, is recommended.13
There are 3 additional variants of LCH. Letterer-Siwe disease is an acute form of LCH that accounts for 10% of all LCH cases and typically presents in children younger than 2 years. It involves multiple organs, including the bones, lungs, liver, and lymph nodes.14 Affected patients usually present with fever; hepatosplenomegaly; anemia; lymphadenopathy; extensive lytic skull lesions; and a generalized cutaneous eruption, appearing as a maculopapular scaling rash with underlying purpura on the scalp, neck, axilla, and trunk.3 Letterer-Siwe disease is inherited in an autosomal-recessive pattern. Diagnosis is confirmed by skin biopsy demonstrating a thinning of the epidermis and a collection of reticulum cells in the dermis.3 Letterer-Siwe disease is treated with radiation and chemotherapy; if left untreated, the disease is fatal.4
Hand-Schüller-Christian disease, a chronic form of LCH, is most commonly seen in children aged 2 to 6 years and accounts for 15% to 20% of all LCH cases. This LCH variant presents with a classic triad of diabetes insipidus (resulting from erosion into the sella turcica), lytic bone lesions, and exophthalmos.15 Hand-Schüller-Christian disease also affects the oral cavity, producing nodular ulcerations of the hard palate, trouble swallowing, and halitosis.4 The involvement of lytic bone lesions of the mastoid process and petrous portions of the temporal bones may cause recurrent or chronic otitis media and otitis externa. Hand-Schüller-Christian disease is treated with a combination of chemotherapy, radiation, and surgical excision. The mortality rate is 30%.4
Eosinophilic granuloma is the most prevalent variant of LCH, accounting for 60% to 80% of all cases. Characterized by Langerhans cell granulomatous infiltration of the lungs and painful cystic bone lesions, eosinophilic granuloma primarily presents in the third or fourth decades of life.16 Some studies suggest an epidemiologic association with tobacco use.17 In the preliminary stages of this disease, Langerhans cells, eosinophils, lymphocytes, and fibroblasts infiltrate and form nodules on the terminal bronchioles in the upper and middle lung zones, damaging the airway walls.18 Fibrotic scarring progresses, ultimately resulting in alveolar destruction.10 The common signs and symptoms of eosinophilic granuloma are a nonproductive cough, dyspnea, weight loss, spontaneous pneumothorax, fever, peripheral edema, and a tricuspid regurgitation murmur.14 The prognosis of eosinophilic granuloma is variable. Although some patients progress to end-stage fibrotic lung disease requiring lung transplant, there have been reports of complete remission following cessation of cigarette smoking.17
Langerhans cells travel from the bone marrow to the epidermis where they express the CD1a protein on the surface of the antigen-presenting cell. Elevated levels of cytokines, such as tumor necrosis factor α, IFN-γ, granulocyte-macrophage colony-stimulating factor, and interleukins have been seen in patients with LCH.1 Their role in the pathogenesis of this disease remains unknown, but the elevated levels of cytokines may indicate the lack of an efficient immune system.
Histologically, hematoxylin and eosin–stained sections demonstrate an infiltrate of histiocytes, neutrophils, eosinophils, and an increased number of mast cells involving the papillary and reticular dermis. Infiltrating Langerhans cells have concave reniform nuclei18 and stain positive for CD1a, S-100, and CD68 antigens.15 In 10% to 30% of CSHR cases, Birbeck granules can be seen on electron microscopy and tend to transform into laminated dense bodies, signifying the degenerative changes seen in CSHR.15 The various forms of LCH exhibit no significant differences in the expression of the epithelial cadherin, the phosphorylated histone H3, and the Ki-67 proteins, indicating that they are simply different forms of the same disease represented on a spectrum.15
Conclusion
The actual incidence of CSHR may be notably underreported due to its spontaneous regression and low rate of clinical recognition. A subtype of LCH, CSHR is a diagnosis of exclusion. Although CSHR generally follows a benign clinical course, a thorough workup and evaluation for systemic disease with close follow-up is recommended after diagnosis due to the potential of LCH to involve multiple organs and to relapse at a later date after apparent regression.
Langerhans cell histiocytosis (LCH), also known as histiocytosis X, is a general term that describes a group of rare disorders characterized by the proliferation of Langerhans cells.1 Central to immune surveillance and the elimination of foreign substances from the body, Langerhans cells are derived from bone marrow progenitor cells and found in the epidermis but are capable of migrating from the skin to the lymph nodes. In LCH, these cells congregate on bone tissue, particularly in the head and neck region, causing a multitude of problems.2
The spectrum of LCH includes 4 variants: congenital self-healing reticulohistiocytosis (CSHR)(also known as Hashimoto-Pritzker disease), Letterer-Siwe disease, Hand-Schüller-Christian disease, and eosinophilic granuloma (also known as pulmonary histiocytosis X)(Table). Despite the various clinical presentations and levels of severity, all variants are caused by the proliferation of Langerhans cells. We present a case of CSHR in a 6-month-old male infant that was initially diagnosed as molluscum contagiosum. We believe the actual incidence of CSHR may be underreported due to its spontaneous regression and low rate of clinical recognition.
Case Report
A 6-month-old male infant was referred to our clinic by his pediatrician with a generalized cutaneous eruption of 3 weeks’ duration. The eruption, which followed a recent viral upper respiratory tract infection, was characterized by multiple flesh-colored to erythematous, umbilicated papules distributed along the postauricular region, scalp (Figure 1A), abdomen (Figure 1B), and anterior aspect of the neck. Due to his recent illness, the patient was diagnosed with molluscum contagiosum by the referring pediatrician that was treated symptomatically with hydrocortisone lotion, Schamberg’s cream formulated in our office (a compound mixture of zinc oxide, menthol, calcium hydroxide solution, and olive oil), and pediatric diphenhydramine as needed. During a subsequent visit 2 weeks later, a more potent topical corticosteroid and a low-dose systemic corticosteroid was prescribed for 1 week due to development of new lesions and exacerbation of existing lesions. On follow-up 1 week later, the lesions on the trunk had improved, but the patient had developed new lesions on the scalp that differed from prior findings in that they were darker (more erythematous to brown) and firmer (papules and nodules).
 |  | |
Figure 1. Multiple fleshcolored to erythematous, umbilicated papules on the frontal scalp (A) and erythematous papules on the abdomen (B). | ||
A shave biopsy was obtained from the frontal scalp to rule out LCH. Histologic examination and culture of the biopsy specimen revealed an atypical cellular infiltrate effacing the dermoepidermal junction and extensive epidermotropism. Focal erosion of the epidermis and an acute inflammatory exudate were visible. The nuclei of the cellular infiltrate were enlarged and hyperchromatic with a characteristic reniform appearance and indistinct nucleoli (Figure 2). The cells were admixed with scattered eosinophils and extravasated red blood cells.
 |  | |
Figure 2. Low-power view of dermal mononuclear cells with reniform nuclei (A)(H&E, original magnification ×100), and high-power view of enlarged and hyperchromatic nuclei with a characteristic reniform appearance admixed with eosinophils and extravasated red blood cells (B) (H&E, original magnification ×400). | ||
Immunohistochemical staining of the biopsy specimen was strongly positive for both CD1a and S-100 expression (Figure 3). Histopathologic findings were consistent with LCH. Clinicopathologic correlation strongly favored the diagnosis of CSHR.
Comment
Congenital self-healing reticulohistiocytosis is a rare, benign, congenital variant of LCH that spontaneously resolves with no systemic involvement. The more aggressive forms typically manifest at birth or during the first 2 months of life and regress within 3 to 4 months.5 Since CSHR was first described in 1973 by Hashimoto and Pritzker,5 more than 100 cases have been reported, but the true incidence is believed to be higher than reported given the high rate of spontaneous resolution and the low rate of clinical recognition.2 The first reported case of CSHR occurred in a female infant who presented at birth with multiple, diffusely distributed, red-brown papules that were 2 to 4 mm in diameter. Although the patient received no treatment, the exanthem completely resolved within 3.5 months without recurrence at 14-year follow-up.5 Most often, CSHR presents as multiple papules or nodules with occasional disseminated crusting and is followed within a few months by a dramatic and spontaneous regression. Lesions may heal with mild postinflammatory hyperpigmentation. Pseudo-Darier sign, the propensity to urticate from physical manipulation, has been reported in some lesions with an increased number of mast cells.6 Extensive superficial nasal and oral mucosal erosions have been reported in 2 cases.7 Solitary lesions have been reported in 25% of cases.8
The etiology of CSHR remains unknown, though neoplastic, viral, and immunologic origins have been suggested. There have been reports that human herpesvirus 6 may contribute to the development of LCH.9 It may be postulated that our patient’s presentation of CSHR was potentiated by his recent upper respiratory tract illness. In the literature, CSHR is distributed equally among males and females. Prevalence is higher in the white population than in other racial groups.5
Although CSHR is a benign cutaneous variant of LCH, there have been reports of patients with disseminated and extracutaneous involvement. In 1 rare case, CSHR reportedly involved the eyes, producing multiple, bilateral, well-circumscribed, diffuse, yellow-white lesions of the retinal pigment epithelium throughout the posterior pole of the eyes.10 The retinal lesions spontaneously regressed along with the skin manifestations. Additionally, it was reported that a neonate in Thailand presented with CSHR at birth and 1 month later developed multiple lung cysts that had completely regressed 11 months later.11 One study reported that initial diagnoses of LCH in 18 patients with only cutaneous involvement eventually progressed to systemic LCH, requiring further management.12 When LCH is suspected, a thorough physical examination, including hematologic and coagulation evaluation, liver function tests, musculoskeletal examination, and consultation with specialists if necessary, is recommended.13
There are 3 additional variants of LCH. Letterer-Siwe disease is an acute form of LCH that accounts for 10% of all LCH cases and typically presents in children younger than 2 years. It involves multiple organs, including the bones, lungs, liver, and lymph nodes.14 Affected patients usually present with fever; hepatosplenomegaly; anemia; lymphadenopathy; extensive lytic skull lesions; and a generalized cutaneous eruption, appearing as a maculopapular scaling rash with underlying purpura on the scalp, neck, axilla, and trunk.3 Letterer-Siwe disease is inherited in an autosomal-recessive pattern. Diagnosis is confirmed by skin biopsy demonstrating a thinning of the epidermis and a collection of reticulum cells in the dermis.3 Letterer-Siwe disease is treated with radiation and chemotherapy; if left untreated, the disease is fatal.4
Hand-Schüller-Christian disease, a chronic form of LCH, is most commonly seen in children aged 2 to 6 years and accounts for 15% to 20% of all LCH cases. This LCH variant presents with a classic triad of diabetes insipidus (resulting from erosion into the sella turcica), lytic bone lesions, and exophthalmos.15 Hand-Schüller-Christian disease also affects the oral cavity, producing nodular ulcerations of the hard palate, trouble swallowing, and halitosis.4 The involvement of lytic bone lesions of the mastoid process and petrous portions of the temporal bones may cause recurrent or chronic otitis media and otitis externa. Hand-Schüller-Christian disease is treated with a combination of chemotherapy, radiation, and surgical excision. The mortality rate is 30%.4
Eosinophilic granuloma is the most prevalent variant of LCH, accounting for 60% to 80% of all cases. Characterized by Langerhans cell granulomatous infiltration of the lungs and painful cystic bone lesions, eosinophilic granuloma primarily presents in the third or fourth decades of life.16 Some studies suggest an epidemiologic association with tobacco use.17 In the preliminary stages of this disease, Langerhans cells, eosinophils, lymphocytes, and fibroblasts infiltrate and form nodules on the terminal bronchioles in the upper and middle lung zones, damaging the airway walls.18 Fibrotic scarring progresses, ultimately resulting in alveolar destruction.10 The common signs and symptoms of eosinophilic granuloma are a nonproductive cough, dyspnea, weight loss, spontaneous pneumothorax, fever, peripheral edema, and a tricuspid regurgitation murmur.14 The prognosis of eosinophilic granuloma is variable. Although some patients progress to end-stage fibrotic lung disease requiring lung transplant, there have been reports of complete remission following cessation of cigarette smoking.17
Langerhans cells travel from the bone marrow to the epidermis where they express the CD1a protein on the surface of the antigen-presenting cell. Elevated levels of cytokines, such as tumor necrosis factor α, IFN-γ, granulocyte-macrophage colony-stimulating factor, and interleukins have been seen in patients with LCH.1 Their role in the pathogenesis of this disease remains unknown, but the elevated levels of cytokines may indicate the lack of an efficient immune system.
Histologically, hematoxylin and eosin–stained sections demonstrate an infiltrate of histiocytes, neutrophils, eosinophils, and an increased number of mast cells involving the papillary and reticular dermis. Infiltrating Langerhans cells have concave reniform nuclei18 and stain positive for CD1a, S-100, and CD68 antigens.15 In 10% to 30% of CSHR cases, Birbeck granules can be seen on electron microscopy and tend to transform into laminated dense bodies, signifying the degenerative changes seen in CSHR.15 The various forms of LCH exhibit no significant differences in the expression of the epithelial cadherin, the phosphorylated histone H3, and the Ki-67 proteins, indicating that they are simply different forms of the same disease represented on a spectrum.15
Conclusion
The actual incidence of CSHR may be notably underreported due to its spontaneous regression and low rate of clinical recognition. A subtype of LCH, CSHR is a diagnosis of exclusion. Although CSHR generally follows a benign clinical course, a thorough workup and evaluation for systemic disease with close follow-up is recommended after diagnosis due to the potential of LCH to involve multiple organs and to relapse at a later date after apparent regression.
1. Hussein MR. Skin-limited Langerhans’ cell histiocytosis in children. Cancer Invest. 2009;27:504-511.
2. Nakahigashi K, Ohta M, Sakai R, et al. Late-onset self-healing reticulohistiocytosis: pediatric case of Hashimoto-Pritzker type Langerhans cell histiocytosis. J Dermatol. 2007;34:205-209.
3. Pant C, Madonia P, Bahna SL, et al. Langerhans cell histiocytosis, a case of Letterer Siwe disease. J La State Med Soc. 2009;161:211-212.
4. Ferreira LM, Emerich PS, Diniz LM, et al. Langerhans cell histiocytosis: Letterer-Siwe disease–the importance of dermatological diagnosis in two cases [in Portuguese]. An Bras Dermatol. 2009;84:405-409.
5. Hashimoto K, Pritzker MS. Electron microscopic study of reticulohistiocytoma. an unusual case of congenital, self-healing reticulohistiocytosis. Arch Dermatol. 1973;107:263-270.
6. Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children’s Medical Center. J Am Acad Dermatol. 2007;56:290-294.
7. Le Bidre E, Lorette G, Delage M, et al. Extensive, erosive congenital self-healing cell histiocytosis [published online December 22, 2008]. J Eur Acad Dermatol Venereol. 2009;23:835-836.
8. Weiss T, Weber L, Scharffetter-Kochanek K, et al. Solitary cutaneous dendritic cell tumor in a child: role of dendritic cell markers for the diagnosis of skin Langerhans cell histiocytosis. J Am Acad Dermatol. 2005;53:838-844.
9. Csire M, Mikala G, Jákó J, et al. Persistent long-term human herpesvirus 6 (HHV-6) infection in a patient with Langerhans cell histiocytosis [published online July 3, 2007]. Pathol Oncol Res. 2007;13:157-160.
10. Zaenglein AL, Steele MA, Kamino H, et al. Congenital self-healing reticulohistiocytosis with eye involvement. Pediatr Dermatol. 2001;18:135-137.
11. Chunharas A, Pabunruang W, Hongeng S. Congenital self-healing Langerhans cell histiocytosis with pulmonary involvement: spontaneous regression. J Med Assoc Thai. 2002;85(suppl 4):S1309-S1313.
12. Minkov M, Prosch H, Steiner M, et al. Langerhans cell histiocytosis in neonates. Pediatr Blood Cancer. 2005;45:802-807.
13. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008;25:291-295.
14. Stacher E, Beham-Schmid C, Terpe HJ, et al. Pulmonary histiocytic sarcoma mimicking pulmonary Langerhans cell histiocytosis in a young adult presenting with spontaneous pneumothorax: a potential diagnostic pitfall [published online June 27, 2009]. Virchows Arch. 2009;455:187-190.
15. Scolozzi P, Lombardi T, Monnier P, et al. Multisystem Langerhans’ cell histiocytosis (Hand-Schüller-Christian disease) in an adult: a case report and review of the literature [published online October 10, 2003]. Eur Arch Otorhinolaryngol. 2004;261:326-330.
16. Noonan V, Kabani S, Alibhai K. Langerhans cell histiocytosis (eosinophilic granuloma). J Mass Dent Soc. 2011;60:35.
17. Podbielski FJ, Worley TA, Korn JM, et al. Eosinophilic granuloma of the lung and rib. Asian Cardiovasc Thorac Ann. 2009;17:194-195.
18. Rosso DA, Ripoli MF, Roy A, et al. Serum levels of interleukin-1 receptor antagonist and tumor necrosis factor-alpha are elevated in children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2003;25:480-483.
1. Hussein MR. Skin-limited Langerhans’ cell histiocytosis in children. Cancer Invest. 2009;27:504-511.
2. Nakahigashi K, Ohta M, Sakai R, et al. Late-onset self-healing reticulohistiocytosis: pediatric case of Hashimoto-Pritzker type Langerhans cell histiocytosis. J Dermatol. 2007;34:205-209.
3. Pant C, Madonia P, Bahna SL, et al. Langerhans cell histiocytosis, a case of Letterer Siwe disease. J La State Med Soc. 2009;161:211-212.
4. Ferreira LM, Emerich PS, Diniz LM, et al. Langerhans cell histiocytosis: Letterer-Siwe disease–the importance of dermatological diagnosis in two cases [in Portuguese]. An Bras Dermatol. 2009;84:405-409.
5. Hashimoto K, Pritzker MS. Electron microscopic study of reticulohistiocytoma. an unusual case of congenital, self-healing reticulohistiocytosis. Arch Dermatol. 1973;107:263-270.
6. Kapur P, Erickson C, Rakheja D, et al. Congenital self-healing reticulohistiocytosis (Hashimoto-Pritzker disease): ten-year experience at Dallas Children’s Medical Center. J Am Acad Dermatol. 2007;56:290-294.
7. Le Bidre E, Lorette G, Delage M, et al. Extensive, erosive congenital self-healing cell histiocytosis [published online December 22, 2008]. J Eur Acad Dermatol Venereol. 2009;23:835-836.
8. Weiss T, Weber L, Scharffetter-Kochanek K, et al. Solitary cutaneous dendritic cell tumor in a child: role of dendritic cell markers for the diagnosis of skin Langerhans cell histiocytosis. J Am Acad Dermatol. 2005;53:838-844.
9. Csire M, Mikala G, Jákó J, et al. Persistent long-term human herpesvirus 6 (HHV-6) infection in a patient with Langerhans cell histiocytosis [published online July 3, 2007]. Pathol Oncol Res. 2007;13:157-160.
10. Zaenglein AL, Steele MA, Kamino H, et al. Congenital self-healing reticulohistiocytosis with eye involvement. Pediatr Dermatol. 2001;18:135-137.
11. Chunharas A, Pabunruang W, Hongeng S. Congenital self-healing Langerhans cell histiocytosis with pulmonary involvement: spontaneous regression. J Med Assoc Thai. 2002;85(suppl 4):S1309-S1313.
12. Minkov M, Prosch H, Steiner M, et al. Langerhans cell histiocytosis in neonates. Pediatr Blood Cancer. 2005;45:802-807.
13. Satter EK, High WA. Langerhans cell histiocytosis: a review of the current recommendations of the Histiocyte Society. Pediatr Dermatol. 2008;25:291-295.
14. Stacher E, Beham-Schmid C, Terpe HJ, et al. Pulmonary histiocytic sarcoma mimicking pulmonary Langerhans cell histiocytosis in a young adult presenting with spontaneous pneumothorax: a potential diagnostic pitfall [published online June 27, 2009]. Virchows Arch. 2009;455:187-190.
15. Scolozzi P, Lombardi T, Monnier P, et al. Multisystem Langerhans’ cell histiocytosis (Hand-Schüller-Christian disease) in an adult: a case report and review of the literature [published online October 10, 2003]. Eur Arch Otorhinolaryngol. 2004;261:326-330.
16. Noonan V, Kabani S, Alibhai K. Langerhans cell histiocytosis (eosinophilic granuloma). J Mass Dent Soc. 2011;60:35.
17. Podbielski FJ, Worley TA, Korn JM, et al. Eosinophilic granuloma of the lung and rib. Asian Cardiovasc Thorac Ann. 2009;17:194-195.
18. Rosso DA, Ripoli MF, Roy A, et al. Serum levels of interleukin-1 receptor antagonist and tumor necrosis factor-alpha are elevated in children with Langerhans cell histiocytosis. J Pediatr Hematol Oncol. 2003;25:480-483.
Practice Points
- Langerhans cell histiocytosis (LCH) is believed to occur in 1:200,000 children and tends to be underdiagnosed, as some patients may have no symptoms while others have symptoms that are misdiagnosed as other conditions.
- Patients with LCH usually should have long-term follow-up care to detect progression or complications of the disease or treatment.
Recurrent Abdominal Pain and Bowel Edema in a Middle-Aged Woman
A 34-year-old African American woman presented to the emergency department (ED) after several hours of sharp lower abdominal pain and cramping followed by nausea and vomiting. The pain initially began in the periumbilical region and migrated to the bilateral lower quadrants. The patient reported no fevers, chills, diarrhea, hematemesis, or hematochezia associated with these symptoms. She also reported no unusual food exposures or sick contacts.
The patient’s medical history was notable only for hypertension; her surgical history included 2 cesarean section births several years prior to presentation. Her father was diagnosed with stomach cancer in his 40s. The patient’s only medications were an oral contraceptive, lisinopril, and an antihistamine taken as needed for seasonal allergies. She had no history of tobacco, alcohol, or illicit drug use and no known drug allergies.
While in the ED, the patient’s physical exam revealed mild tachycardia (104 bpm on arrival, which improved with fluid resuscitation) and diffuse abdominal tenderness. Laboratory evaluation revealed a mild leukocytosis (10.7 x 103/L) but normal liver-associated enzymes, lipase, and urinalysis. A computed tomography (CT) scan of the abdomen and pelvis with oral and IV contrast revealed diffuse ileal wall thickening with significant perihepatic and perisplenic ascites with pelvic free fluid suspicious for an inflammatory vs infectious enteritis (Figure 1).
The patient was treated supportively with IV fluids, antiemetics, and pain medication. Her symptoms generally improved over several days, though she did develop loose stools that prompted infectious stool studies, which were negative for typical pathogens. Follow-up laboratory testing revealed resolution of her leukocytosis.
About 2 weeks later, the patient had another acute attack of abdominal pain, again associated with nausea and vomiting.
Six weeks after her initial presentation, the patient presented to the ED for the third time with the same symptoms. A CT scan again displayed diffuse ileal wall thickening with significant ascites; slightly worse than the image from initial presentation (Figure 3).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Diagnosis
This patient presented with recurrent episodes of diffuse small bowel wall thickening and ascites associated with diffuse abdominal pain, nausea, and vomiting. Her symptoms resolved spontaneously, correlating with normalization of bowel wall appearance on imaging studies. Initially, the patient’s symptoms were most concerning for infectious or inflammatory enteritis. Infection became lower on the differential as the patient’s symptoms continued to recur and then resolve spontaneously without antiviral or antibiotic treatment. She also had no fevers, and stools samples were negative for infectious causes of her symptoms.
Inflammatory bowel disease (IBD) was considered, but direct visualization of the small bowel and colonic mucosa was unremarkable. In addition, the sporadic nature of her symptoms did not fit the typical IBD presentation. The patient had no risk factors or history that would suggest ischemic disease, vasculitis, or radiation-induced enteritis. Hereditary angioedema and acquired C1 esterase deficiency were considered given the intermittent nature and characteristic quality of her symptoms. However, serum C4 and C1 esterase inhibitor levels returned within normal limits when measured during these episodes. Finally, visceral angioedema was considered.
Visceral angioedema may be related to medications and is specifically associated with angiotensin-converting-enzyme (ACE) inhibitors as well as β-lactams and high doses of nonsteroidal anti-inflammatory drugs (NSAIDs). Given the characteristic presentation with no other inciting cause, the patient’s lisinopril was felt to be the causative agent and was discontinued. Her symptoms resolved completely and never returned. The patient’s final diagnosis was ACE inhibitor-induced visceral angioedema.
About This Condition
Angiotensin-converting enzyme inhibitors were first introduced in the early 1980s and have been prescribed more frequently as the indications for their use have increased. Some estimate that ACE inhibitors are used by more than 40 million people worldwide.1 Angioedema has been reported to occur in 0.1% to 0.2% of patients taking ACE inhibitorsand accounts for 20% to 30% of all angioedema cases presenting to EDs.2,3 However, ACE inhibitors recently have been recognized as a rare cause of angioedema of the gastrointestinal tract. One of the largest literature reviews on ACE inhibitor-induced gastrointestinal angioedema describes only 27 cases.3
Prevalence seems to be highest among middle-aged or older women, particularly among African Americans.3 The interval between medication initiation and onset of symptoms can vary, ranging from 24 hours to 9 years.3,4 In many cases, lack of recognition of this condition early in the disease course led to costly and invasive procedures, such as abdominal laparotomy, before reaching a diagnosis. Other literature reviews report similar patient characteristics and initial disease manifestations: female predominance, often middle-aged, presenting with abdominal pain and emesis associated with bowel wall thickening and ascites on CT.4,5 Additionally, in the majority of cases, visceral angioedema occurred in the absence of oropharyngeal angioedema. Unlike allergic angioedema or NSAID-induced angioedema, ACE inhibitor-induced angioedema is not associated with urticaria.6
The exact pathway of ACE inhibitor-induced angioedema is not completely understood but is thought to be bradykinin mediated. Angiotensin-converting enzyme inhibitors decrease the degradation of bradykinin, which ultimately leads to an increase in vascular permeability and results in an increased plasma extravasation into the interstitial space of subcutaneous or submucosal tissue.1 However, many experts believe that the exclusive role of bradykinin is unlikely. Some suggest that patients with ACE inhibitor-induced angioedema are more likely to have decreased levels or defects in other enzymes such as carboxypeptidase N and aminopeptidase P, which are involved in the breakdown of bradykinin and its metabolites.6 Given the female predominance in this patient population, it also seems reasonable to consider the role of estrogens in the pathogenesis of this disease, although none have been identified to the knowledge of the authors.
Treatment of ACE inhibitor-induced angioedema is largely supportive following discontinuation of the offending medication. There have been case reports of infrequent, mild, recurrent episodes of angioedema, even after ACE inhibitor discontinuation, so these should be anticipated.7
Conclusions
Angiotensin-converting enzyme inhibitor-induced gastrointestinal angioedema is a rare condition. It generally presents as recurrent abdominal pain and nausea with CT findings of intestinal edema and ascites. It is more common among the middle-aged, women, and minorities. ACE inhibitor-induced angioedema should be kept on the differential for patients with the aforementioned characteristics, especially if infection, inflammatory bowel disease, ischemic disease, or vasculitis is deemed unlikely. Identifying this condition early can save patients from unnecessary hospitalizations, physical and emotional discomfort, and further health care costs.
1. Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin-converting enzyme inhibitor. Curr Opin Allergy Clin Immunol. 2013;13(4):337-344.
2. Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol. 2000;31(3):254-257.
3. Benson BC, Smith C, Laczek JT. Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: a case series and literature review. J Clin Gastroenterol. 2013;47(10):844-849.
4. Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci. 2002;324(2):106-108.
5. Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS. Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: clinical and imaging findings in 20 patients. AJR Am R Roentgenol. 2011;197(2):393-398.
6. Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61(4):545-557.
7. Cicardi MC, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004;164(8):910-913.
A 34-year-old African American woman presented to the emergency department (ED) after several hours of sharp lower abdominal pain and cramping followed by nausea and vomiting. The pain initially began in the periumbilical region and migrated to the bilateral lower quadrants. The patient reported no fevers, chills, diarrhea, hematemesis, or hematochezia associated with these symptoms. She also reported no unusual food exposures or sick contacts.
The patient’s medical history was notable only for hypertension; her surgical history included 2 cesarean section births several years prior to presentation. Her father was diagnosed with stomach cancer in his 40s. The patient’s only medications were an oral contraceptive, lisinopril, and an antihistamine taken as needed for seasonal allergies. She had no history of tobacco, alcohol, or illicit drug use and no known drug allergies.
While in the ED, the patient’s physical exam revealed mild tachycardia (104 bpm on arrival, which improved with fluid resuscitation) and diffuse abdominal tenderness. Laboratory evaluation revealed a mild leukocytosis (10.7 x 103/L) but normal liver-associated enzymes, lipase, and urinalysis. A computed tomography (CT) scan of the abdomen and pelvis with oral and IV contrast revealed diffuse ileal wall thickening with significant perihepatic and perisplenic ascites with pelvic free fluid suspicious for an inflammatory vs infectious enteritis (Figure 1).
The patient was treated supportively with IV fluids, antiemetics, and pain medication. Her symptoms generally improved over several days, though she did develop loose stools that prompted infectious stool studies, which were negative for typical pathogens. Follow-up laboratory testing revealed resolution of her leukocytosis.
About 2 weeks later, the patient had another acute attack of abdominal pain, again associated with nausea and vomiting.
Six weeks after her initial presentation, the patient presented to the ED for the third time with the same symptoms. A CT scan again displayed diffuse ileal wall thickening with significant ascites; slightly worse than the image from initial presentation (Figure 3).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Diagnosis
This patient presented with recurrent episodes of diffuse small bowel wall thickening and ascites associated with diffuse abdominal pain, nausea, and vomiting. Her symptoms resolved spontaneously, correlating with normalization of bowel wall appearance on imaging studies. Initially, the patient’s symptoms were most concerning for infectious or inflammatory enteritis. Infection became lower on the differential as the patient’s symptoms continued to recur and then resolve spontaneously without antiviral or antibiotic treatment. She also had no fevers, and stools samples were negative for infectious causes of her symptoms.
Inflammatory bowel disease (IBD) was considered, but direct visualization of the small bowel and colonic mucosa was unremarkable. In addition, the sporadic nature of her symptoms did not fit the typical IBD presentation. The patient had no risk factors or history that would suggest ischemic disease, vasculitis, or radiation-induced enteritis. Hereditary angioedema and acquired C1 esterase deficiency were considered given the intermittent nature and characteristic quality of her symptoms. However, serum C4 and C1 esterase inhibitor levels returned within normal limits when measured during these episodes. Finally, visceral angioedema was considered.
Visceral angioedema may be related to medications and is specifically associated with angiotensin-converting-enzyme (ACE) inhibitors as well as β-lactams and high doses of nonsteroidal anti-inflammatory drugs (NSAIDs). Given the characteristic presentation with no other inciting cause, the patient’s lisinopril was felt to be the causative agent and was discontinued. Her symptoms resolved completely and never returned. The patient’s final diagnosis was ACE inhibitor-induced visceral angioedema.
About This Condition
Angiotensin-converting enzyme inhibitors were first introduced in the early 1980s and have been prescribed more frequently as the indications for their use have increased. Some estimate that ACE inhibitors are used by more than 40 million people worldwide.1 Angioedema has been reported to occur in 0.1% to 0.2% of patients taking ACE inhibitorsand accounts for 20% to 30% of all angioedema cases presenting to EDs.2,3 However, ACE inhibitors recently have been recognized as a rare cause of angioedema of the gastrointestinal tract. One of the largest literature reviews on ACE inhibitor-induced gastrointestinal angioedema describes only 27 cases.3
Prevalence seems to be highest among middle-aged or older women, particularly among African Americans.3 The interval between medication initiation and onset of symptoms can vary, ranging from 24 hours to 9 years.3,4 In many cases, lack of recognition of this condition early in the disease course led to costly and invasive procedures, such as abdominal laparotomy, before reaching a diagnosis. Other literature reviews report similar patient characteristics and initial disease manifestations: female predominance, often middle-aged, presenting with abdominal pain and emesis associated with bowel wall thickening and ascites on CT.4,5 Additionally, in the majority of cases, visceral angioedema occurred in the absence of oropharyngeal angioedema. Unlike allergic angioedema or NSAID-induced angioedema, ACE inhibitor-induced angioedema is not associated with urticaria.6
The exact pathway of ACE inhibitor-induced angioedema is not completely understood but is thought to be bradykinin mediated. Angiotensin-converting enzyme inhibitors decrease the degradation of bradykinin, which ultimately leads to an increase in vascular permeability and results in an increased plasma extravasation into the interstitial space of subcutaneous or submucosal tissue.1 However, many experts believe that the exclusive role of bradykinin is unlikely. Some suggest that patients with ACE inhibitor-induced angioedema are more likely to have decreased levels or defects in other enzymes such as carboxypeptidase N and aminopeptidase P, which are involved in the breakdown of bradykinin and its metabolites.6 Given the female predominance in this patient population, it also seems reasonable to consider the role of estrogens in the pathogenesis of this disease, although none have been identified to the knowledge of the authors.
Treatment of ACE inhibitor-induced angioedema is largely supportive following discontinuation of the offending medication. There have been case reports of infrequent, mild, recurrent episodes of angioedema, even after ACE inhibitor discontinuation, so these should be anticipated.7
Conclusions
Angiotensin-converting enzyme inhibitor-induced gastrointestinal angioedema is a rare condition. It generally presents as recurrent abdominal pain and nausea with CT findings of intestinal edema and ascites. It is more common among the middle-aged, women, and minorities. ACE inhibitor-induced angioedema should be kept on the differential for patients with the aforementioned characteristics, especially if infection, inflammatory bowel disease, ischemic disease, or vasculitis is deemed unlikely. Identifying this condition early can save patients from unnecessary hospitalizations, physical and emotional discomfort, and further health care costs.
A 34-year-old African American woman presented to the emergency department (ED) after several hours of sharp lower abdominal pain and cramping followed by nausea and vomiting. The pain initially began in the periumbilical region and migrated to the bilateral lower quadrants. The patient reported no fevers, chills, diarrhea, hematemesis, or hematochezia associated with these symptoms. She also reported no unusual food exposures or sick contacts.
The patient’s medical history was notable only for hypertension; her surgical history included 2 cesarean section births several years prior to presentation. Her father was diagnosed with stomach cancer in his 40s. The patient’s only medications were an oral contraceptive, lisinopril, and an antihistamine taken as needed for seasonal allergies. She had no history of tobacco, alcohol, or illicit drug use and no known drug allergies.
While in the ED, the patient’s physical exam revealed mild tachycardia (104 bpm on arrival, which improved with fluid resuscitation) and diffuse abdominal tenderness. Laboratory evaluation revealed a mild leukocytosis (10.7 x 103/L) but normal liver-associated enzymes, lipase, and urinalysis. A computed tomography (CT) scan of the abdomen and pelvis with oral and IV contrast revealed diffuse ileal wall thickening with significant perihepatic and perisplenic ascites with pelvic free fluid suspicious for an inflammatory vs infectious enteritis (Figure 1).
The patient was treated supportively with IV fluids, antiemetics, and pain medication. Her symptoms generally improved over several days, though she did develop loose stools that prompted infectious stool studies, which were negative for typical pathogens. Follow-up laboratory testing revealed resolution of her leukocytosis.
About 2 weeks later, the patient had another acute attack of abdominal pain, again associated with nausea and vomiting.
Six weeks after her initial presentation, the patient presented to the ED for the third time with the same symptoms. A CT scan again displayed diffuse ileal wall thickening with significant ascites; slightly worse than the image from initial presentation (Figure 3).
- What is your diagnosis?
- How would you treat this patient?
[Click through to the next page to see the answer.]
Diagnosis
This patient presented with recurrent episodes of diffuse small bowel wall thickening and ascites associated with diffuse abdominal pain, nausea, and vomiting. Her symptoms resolved spontaneously, correlating with normalization of bowel wall appearance on imaging studies. Initially, the patient’s symptoms were most concerning for infectious or inflammatory enteritis. Infection became lower on the differential as the patient’s symptoms continued to recur and then resolve spontaneously without antiviral or antibiotic treatment. She also had no fevers, and stools samples were negative for infectious causes of her symptoms.
Inflammatory bowel disease (IBD) was considered, but direct visualization of the small bowel and colonic mucosa was unremarkable. In addition, the sporadic nature of her symptoms did not fit the typical IBD presentation. The patient had no risk factors or history that would suggest ischemic disease, vasculitis, or radiation-induced enteritis. Hereditary angioedema and acquired C1 esterase deficiency were considered given the intermittent nature and characteristic quality of her symptoms. However, serum C4 and C1 esterase inhibitor levels returned within normal limits when measured during these episodes. Finally, visceral angioedema was considered.
Visceral angioedema may be related to medications and is specifically associated with angiotensin-converting-enzyme (ACE) inhibitors as well as β-lactams and high doses of nonsteroidal anti-inflammatory drugs (NSAIDs). Given the characteristic presentation with no other inciting cause, the patient’s lisinopril was felt to be the causative agent and was discontinued. Her symptoms resolved completely and never returned. The patient’s final diagnosis was ACE inhibitor-induced visceral angioedema.
About This Condition
Angiotensin-converting enzyme inhibitors were first introduced in the early 1980s and have been prescribed more frequently as the indications for their use have increased. Some estimate that ACE inhibitors are used by more than 40 million people worldwide.1 Angioedema has been reported to occur in 0.1% to 0.2% of patients taking ACE inhibitorsand accounts for 20% to 30% of all angioedema cases presenting to EDs.2,3 However, ACE inhibitors recently have been recognized as a rare cause of angioedema of the gastrointestinal tract. One of the largest literature reviews on ACE inhibitor-induced gastrointestinal angioedema describes only 27 cases.3
Prevalence seems to be highest among middle-aged or older women, particularly among African Americans.3 The interval between medication initiation and onset of symptoms can vary, ranging from 24 hours to 9 years.3,4 In many cases, lack of recognition of this condition early in the disease course led to costly and invasive procedures, such as abdominal laparotomy, before reaching a diagnosis. Other literature reviews report similar patient characteristics and initial disease manifestations: female predominance, often middle-aged, presenting with abdominal pain and emesis associated with bowel wall thickening and ascites on CT.4,5 Additionally, in the majority of cases, visceral angioedema occurred in the absence of oropharyngeal angioedema. Unlike allergic angioedema or NSAID-induced angioedema, ACE inhibitor-induced angioedema is not associated with urticaria.6
The exact pathway of ACE inhibitor-induced angioedema is not completely understood but is thought to be bradykinin mediated. Angiotensin-converting enzyme inhibitors decrease the degradation of bradykinin, which ultimately leads to an increase in vascular permeability and results in an increased plasma extravasation into the interstitial space of subcutaneous or submucosal tissue.1 However, many experts believe that the exclusive role of bradykinin is unlikely. Some suggest that patients with ACE inhibitor-induced angioedema are more likely to have decreased levels or defects in other enzymes such as carboxypeptidase N and aminopeptidase P, which are involved in the breakdown of bradykinin and its metabolites.6 Given the female predominance in this patient population, it also seems reasonable to consider the role of estrogens in the pathogenesis of this disease, although none have been identified to the knowledge of the authors.
Treatment of ACE inhibitor-induced angioedema is largely supportive following discontinuation of the offending medication. There have been case reports of infrequent, mild, recurrent episodes of angioedema, even after ACE inhibitor discontinuation, so these should be anticipated.7
Conclusions
Angiotensin-converting enzyme inhibitor-induced gastrointestinal angioedema is a rare condition. It generally presents as recurrent abdominal pain and nausea with CT findings of intestinal edema and ascites. It is more common among the middle-aged, women, and minorities. ACE inhibitor-induced angioedema should be kept on the differential for patients with the aforementioned characteristics, especially if infection, inflammatory bowel disease, ischemic disease, or vasculitis is deemed unlikely. Identifying this condition early can save patients from unnecessary hospitalizations, physical and emotional discomfort, and further health care costs.
1. Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin-converting enzyme inhibitor. Curr Opin Allergy Clin Immunol. 2013;13(4):337-344.
2. Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol. 2000;31(3):254-257.
3. Benson BC, Smith C, Laczek JT. Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: a case series and literature review. J Clin Gastroenterol. 2013;47(10):844-849.
4. Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci. 2002;324(2):106-108.
5. Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS. Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: clinical and imaging findings in 20 patients. AJR Am R Roentgenol. 2011;197(2):393-398.
6. Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61(4):545-557.
7. Cicardi MC, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004;164(8):910-913.
1. Campo P, Fernandez TD, Canto G, Mayorga C. Angioedema induced by angiotensin-converting enzyme inhibitor. Curr Opin Allergy Clin Immunol. 2013;13(4):337-344.
2. Chase MP, Fiarman GS, Scholz FJ, MacDermott RP. Angioedema of the small bowel due to an angiotensin-converting enzyme inhibitor. J Clin Gastroenterol. 2000;31(3):254-257.
3. Benson BC, Smith C, Laczek JT. Angiotensin converting enzyme inhibitor-induced gastrointestinal angioedema: a case series and literature review. J Clin Gastroenterol. 2013;47(10):844-849.
4. Schmidt TD, McGrath KM. Angiotensin-converting enzyme inhibitor angioedema of the intestine: a case report and review of the literature. Am J Med Sci. 2002;324(2):106-108.
5. Scheirey CD, Scholz FJ, Shortsleeve MJ, Katz DS. Angiotensin-converting enzyme inhibitor-induced small-bowel angioedema: clinical and imaging findings in 20 patients. AJR Am R Roentgenol. 2011;197(2):393-398.
6. Inomata N. Recent advances in drug-induced angioedema. Allergol Int. 2012;61(4):545-557.
7. Cicardi MC, Zingale LC, Bergamaschini L, Agostoni A. Angioedema associated with angiotensin-converting enzyme inhibitor use: outcome after switching to a different treatment. Arch Intern Med. 2004;164(8):910-913.