User login
Isolated Brachialis Muscle Atrophy
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
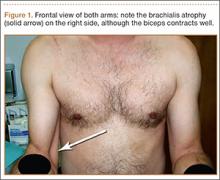
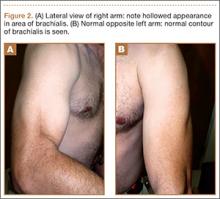
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
Isolated brachialis muscle atrophy has been rarely reported. Among the few cases in the literature, 1 was attributed to a presumed compartment syndrome,1 1 to a displaced clavicle fracture,2 and 3 to neuralgic amyotrophy.3,4 We present a case of isolated brachialis muscle atrophy of unknown etiology, the presentation of which is consistent with neuralgic amyotrophy, also known as Parsonage-Turner syndrome or brachial plexitis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 37-year-old right-handed highway worker presented for evaluation of right-arm muscle atrophy. One year earlier, while lifting heavy bags at work, he felt a painful strain in his right arm, although there was no bruising or swelling. Approximately 4 weeks after this incident, he developed right shoulder pain and began to notice a slight decrease in the muscle mass of his right anterior arm. On evaluation at an outside facility, the physician noted some brachialis muscle atrophy. His shoulder pain was attributed to acromioclavicular joint problems. After an initial trial of physical therapy that did not alleviate this joint pain, an acromioclavicular joint resection was performed, and his pain improved. The brachialis muscle atrophy continued to progress, however. Over the course of the next 6 months, the patient noticed a continually decreasing muscle mass in his right arm, as well as arm fatigue with routine recreational activities. On follow-up, again at an outside institution, the treating physicians noted continued atrophy of the distal arm corresponding to the region of the brachialis musculature. Magnetic resonance imaging showed continuity of the brachialis muscle and tendon, with muscle atrophy. The patient was able to return to work, although with a subjective decrease in right elbow flexion strength.
On presentation at our institution, the patient complained of right arm weakness with heavy use but did not have pain or sensory complaints. His medical history was otherwise unremarkable. Physical examination revealed obvious wasting of the right brachialis muscle, most notable on the lateral aspect of the distal arm (Figures 1, 2A, 2B). His biceps muscle was functioning with full strength and had a normal bulk. He had a normal range of active and passive motion, including full extension and flexion of both elbows, as well as complete pronosupination of the forearms. There was no focal tenderness. Manual muscle testing of both upper extremities was completely normal except for 4/5 flexion strength of the right elbow. Neurovascular examination also revealed normal findings, including intact sensation over the radiolateral forearm. A second magnetic resonance image showed that the brachialis muscle had completely atrophied. Because the clinical examination and imaging studies both indicated isolated brachialis atrophy without deficit elsewhere along the musculocutaneous nerve, electromyography was not performed. The patient was fully functional and working at his usual occupation, and no further intervention was recommended.
Discussion
Isolated wasting of the brachialis muscle is extremely rare with few reports in the literature. Farmer and colleagues1 reported a case of brachialis atrophy that was presumed to have resulted from exercise-induced chronic compartment syndrome. In that case, the patient developed a prodrome of arm pain followed by brachialis muscle atrophy. This patient was treated with oral anti-inflammatory agents with improvement in pain but without recovery of the brachialis muscle. While this case was attributed to compartment syndrome, it is likely that it represented neuralgic amyotrophy because there was no evidence of elbow flexion contracture, which would have accompanied true necrosis of the brachialis muscle as seen in compartment syndrome. However, acute compartment syndrome of the brachialis muscle after minor trauma has been reported.5 In that case, full-scale compartment syndrome was treated with rapid fasciotomy, with complete recovery of the brachialis.
Isolated brachialis atrophy has also been described in the setting of a displaced midshaft clavicle fracture in an elite athlete.2 Two fracture fragments were thought to have injured the brachial plexus, separately causing brachialis atrophy and altered sensation over the clavicular head of the deltoid muscle. Atrophy remained 1 year after injury.
Although it had been occasionally reported, the first large series of patients with sporadic neuralgic amyotrophy in the upper extremity was reported by Parsonage and Turner6 in 1948. They described 136 patients who developed flaccid paralysis and atrophy of various muscles of the shoulder girdle and/or upper extremity. This was generally preceded by acute pain in the shoulder girdle, often associated with antecedent viral infection, stress, illness, or other precipitating factors.
To our knowledge, there have been 3 other reported cases of neuralgic amyotrophy of the brachialis muscle. Watson and colleagues3 presented 2 patients with nonspecific, neurogenic shoulder pain after which an indolent, progressive atrophy of the brachialis muscle ensued.3 Van Tongel and colleagues4 described a more traditional case of Parsonage-Turner syndrome, with bilateral wasting of the shoulder girdle that also exhibited unilateral brachialis atrophy without affecting other muscles in the arm.4 Our case, with shoulder pain followed by muscle atrophy, fits the pattern of neuralgic amyotrophy.
Others have similarly described isolated wasting of 1 muscle with the sparing of other muscles with a common innervation. Isolated atrophy of the extensor or flexor pollicis longus has been reported as variants of either posterior or anterior interosseous neuropathy, respectively.7,8 Nerve fibers in the brachial plexus destined to innervate muscles supplied by the anterior interosseous nerve may be the cause of the motor deficit in cases of anterior interosseous nerve palsy, which seem to be associated with brachial plexitis.9
We present a case of isolated brachialis muscle atrophy after a minor trauma that may have resulted from Parsonage-Turner syndrome or a variant of brachial plexitis. The constellation of shoulder and arm pain, with subsequent muscle atrophy, makes this diagnosis likely.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
1. Farmer KW, McFarland EG, Sonin A, Cosgarea AJ, Roehrig GJ. Isolated necrosis of the brachialis muscle due to exercise. Orthopedics. 2002;25(6):682-684.
2. Rüst CA, Knechtle B, Knechtle P, Rosemann T. Atrophy of the brachialis muscle after a displaced clavicle fracture in an Ironman triathlete: case report. J Brachial Plex Periph Nerve Inj. 2011;6(1):e44-e47.
3. Watson BV, Rose-Innes A, Engstrom JW, Brown JD. Isolated brachialis wasting: an unusual presentation of neuralgic amyotrophy. Muscle Nerve. 2001;24(12):1699-1702.
4. Van Tongel A, Schreurs M, Bruyninckx F, Debeer P. Bilateral Parsonage-Turner syndrome with unilateral brachialis muscle wasting: a case report. J Shoulder Elbow Surg. 2010;19(8):e14-e16.
5. Jenkins NH, Mintowt-Czyz WJ. Compression of the biceps-brachialis compartment after trivial trauma. J Bone Joint Surg Br. 1986;68(3):374.
6. Parsonage MJ, Turner JW. Neuralgic amyotrophy; the shoulder-girdle syndrome. Lancet. 1948;1(6513):973-978.
7. Horton TC. Isolated paralysis of the extensor pollicis longus muscle: a further variation of posterior interosseous nerve palsy. J Hand Surg Br. 2000;25(2):225-226.
8. Hill NA, Howard FM, Huffer BR. The incomplete anterior interosseous nerve syndrome. J Hand Surg Am. 1985;10(1):4-16.
9. Rennels GD, Ochoa J. Neuralgic amyotrophy manifesting as anterior interosseous nerve palsy. Muscle Nerve. 1980;3(2):160-164.
Giant Bone Island of the Tibia in a Child
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
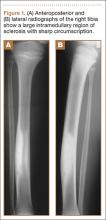
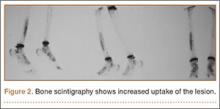
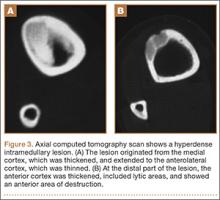
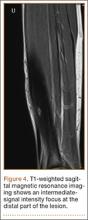
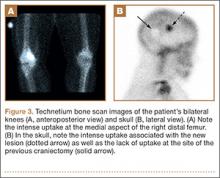
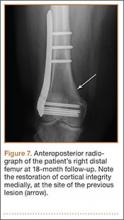
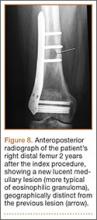
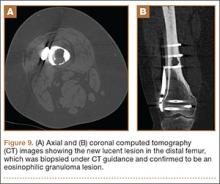
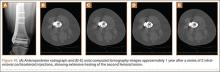
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
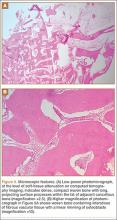
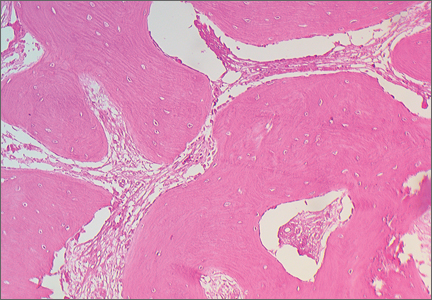
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
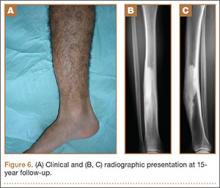
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
A bone island is a focus of normal cortical bone located within the medullary cavity. The vast majority of bone islands are small, measuring from 1 mm to 2 cm in size. They are found more frequently in adults than in children. The lesion can be virtually diagnosed on the basis of its characteristic clinical and imaging features. Differential diagnosis may be difficult when the lesion manifests itself uncharacteristically by being symptomatic, very large, and hot on bone scan.1-4
The term giant bone island has been used to describe a large lesion1 that measures more than 2 cm in any dimension.5 Giant bone islands have been described only in adults,1,5-15 and the longest bone island length reported is 10.5 cm.10 They are usually symptomatic and associated with increased radionuclide uptake on bone scintigraphy.14
The history and the clinical and imaging presentation of an even longer, symptomatic, and scintigraphically hot lesion in the tibial diaphysis of a 10-year-old boy is reported. The lesion further exhibited several atypical imaging features necessitating an open biopsy, which confirmed the diagnosis of a giant bone island. The pertinent differential diagnosis and the clinical and radiographic findings after 15-year follow-up are also presented and discussed. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 10-year-old boy was admitted for surgical repair of an inguinal hernia. Physical examination revealed a painless but tender anterior bowing of the right tibial diaphysis. The patient was a healthy-appearing white male with normal vital signs, gait, and posture. His parents noticed a slight protuberance of the tibia at age 2.5 years. No medical advice was asked for the bone swelling after that time. After recovery from the inguinal hernia repair 3 weeks later, the bone lesion was thoroughly examined. Radiographs showed an oblong, homogenous region of dense sclerosis in the diaphysis of the right tibia. The lesion had relatively well-defined margins and was located in the medullary cavity. Speculations were not obvious in the periphery of the lesion, which exhibited a sharp circumscription (Figures 1A, 1B). A well-defined lytic area was evident at the distal part of the lesion (Figure 1B). There was no periosteal reaction. Blood and serum chemistries were within normal limits, including serum calcium, phosphorus, and alkaline phosphatase. A conventional 3-phase bone scintigraphy (300 MBq) with technetium-99m HDP (hydroxydiphosphonate) indicated increased uptake in the area of the lesion but no other skeletal abnormality (Figure 2). Computed tomography (CT) showed that the lesion was purely intramedullary and densely blastic. The lesion originated from the medial cortex, which was thickened (Figure 3A). The lesion extended to the anterolateral cortex, which was thinned and included a lytic area. In the distal part of the lesion, the anterolateral cortex was thickened, included lytic areas, and exhibited an anterior portion of cortical destruction (Figure 3B). The fatty marrow adjacent to the region of sclerosis appeared normal. There was no evidence of extraosseous soft-tissue changes. On both T1- and T2-weighted magnetic resonance imaging (MRI), the lesion exhibited low-signal intensity. The lesion measured 10.8×2.2×1 cm. It originated from the medial cortical bone of the tibia, blended into the medullary cavity, and extended anteriorly towards and through the anterior cortex. The area of cortical destruction was clearly evident on the axial MRI. The periosteum was displaced and eroded anteriorly by focal radiating bony streaks. No enhancement was seen after the intravenous administration of gadolinium-diethylenetriamine pentaacetic acid (Gd-DTPA) as a contrast medium. There were no extraosseous soft-tissue changes. In the distal part of the lesion, sagittal and axial MRI showed a 1.2×0.8×0.7-cm well-defined ovoid focus, with characteristics of cystic degeneration that exhibited intermediate-signal intensity on T1-weighted MRI (Figure 4) and high-signal intensity on T2-weighted MRI.
An open biopsy was performed. Macroscopically, a wedge of compact bone measuring 3×1.7×0.6 cm was taken. Microscopic examination showed a thinned periphery of lamellar (mature) bone with haversian canals and, beneath it, woven (immature) bone with long-surface processes projecting within adjacent cancellous bone (Figure 5A). The woven bone contained loose vascular fibrous tissue. No osteoclasts were noted, and the very few osteoblasts lining the bone trabeculae were small, single-layered, and flat (Figure 5B). There was no evidence of neoplastic cells. There was no abnormality of the periosteum and the surrounding soft tissues.
The histology was pathognomonic of a giant bone island. No additional surgical intervention was recommended.
The postoperative course was uncomplicated, and the patient was discharged 2 weeks later. An above-the-knee plaster was recommended for 3 months and a below-the-knee splint for an additional 2-month period. Full weight-bearing was allowed only after the postsurgical sixth month to prevent an impending fracture. The tibial bowing was tender to pressure or palpation, and the patient reported mild spontaneous pain during follow-up. Radiographs 1 year after surgery indicated that the bone area removed for biopsy was replaced by compact bone. MRI performed 4 years after surgery showed that the volume of the lesion in relation to the host bone was not changed.
At the last follow-up 15 years after surgery, the anterior tibial bowing was not changed (Figure 6A), but the patient additionally complained of skin irritation after intense training wearing boots during military service. The radiographic appearance of the lesion was also not changed, while the periphery of the lesion exhibited scarce radiating bony streaks with rounded contours (Figures 6B, 6C). The clinical symptoms and signs from wearing military boots completely subsided after a couple of weeks’ rest from daily army activities, but the mild spontaneous pain and the local tenderness over the tibial bowing persisted.
Discussion
Giant bone islands are more likely to be associated with clinical symptoms than the usual small-sized bone island. Some degree of pain was detected in 8 of 10 patients with a giant bone island presented in the literature, but it was induced by trauma in 3 of them.14
Radiographic appearance is among the distinguishing diagnostic features of a giant bone island. It appears as an ovoid, round, or oblong, homogenously dense, single or multiple focus of sclerosis within the medullary cavity; it is oriented along the long axis of the host bone, and it exhibits peripheral pseudopodia or radiating spicules producing the typical “thorny” or “paintbrush” appearance.8,16,17 It does not exhibit cortical penetration and it is not associated with periosteal reaction.10
The CT findings include a sclerotic and hyperdense focus with spiculated margins extending into the adjacent cancellous bone. The lack of bone destruction and soft-tissue mass are also diagnostic.3,7 MRI findings will reflect the low-signal intensity characteristics of cortical bone on all pulse sequences.18
Enostoses usually exhibit no activity on skeletal scintigraphy, while giant lesions generally show increased radiotracer uptake.5,9-11,14,19-27 The latter may result from the increased amount of bone turnover, which is seen more often with larger lesions because of active bone deposition and remodeling.20,21,23,28 Histopathology of a giant bone island appears identical to the well-described pathologic appearance of smaller bone islands. The lesion is composed of compact lamellar bone and haversian systems, which blend with the adjacent spongiosa. The surrounding cancellous bone forms thorn-like trabeculae radiating from the lesion and merging with the cancellous bone.1,4,5,8,28
The presumptive diagnosis of a bone island is based on the clinical findings, imaging features, and follow-up examinations. An asymptomatic, isolated, sclerotic bone lesion showing the typical features of a bone island on plain radiography, CT, and MRI, whatever its size, that is nonactive on bone scan may be easily diagnosed. However, a symptomatic patient with a hot lesion on scintigraphy should be carefully observed. In addition, larger lesions may raise the suspicion of a neoplasm, such as a sclerotic variant of osteosarcoma. In such cases, an open biopsy may be undertaken. No specific treatment is required after the diagnosis has been confirmed. There is no literature to suggest that, after adequate biopsy confirmation, excision or resection is necessary. Follow-up radiographic examination of the lesion should be suggested to monitor for any potential growth.2,10,23
The first giant bone island appearing in a child is presented in this report. The lack of a causative factor leading to the anterior tibial bowing indicated that the bone deformity was caused primarily by the lesion. The present case is unusual for the appearance of several atypical features, some of which have not been previously described. Peripheral radiating spiculated margin was absent on the patient’s initial radiographs and CT imaging. MRI indicated only the presence of radiating bony streaks that displaced and eroded the periosteum on the anterior border of the lesion. The CT findings that the lesion likely originated or was in close proximity with the medial cortex of the tibia were also atypical. It has been previously reported that spinal lesions located immediately below the cortex tend to fuse with the endosteal surface, while similar features may also be seen in the appendicular enostoses.4,29 Other CT findings, such as the thinning of the overlying anterolateral cortical bone, as well as the cortical thickening at the periphery of the lesion associated with areas of soft-tissue attenuation and anterior cortical destruction, have not been described even in the atypical features of a giant bone island. The lytic area resembling a nidus that was evident at the distal part of the lesion was more likely consistent with an area of resorption, which, although rare, has been described on giant lesions.2,9,29 The substantial amount of woven bone transforming to lamellar bone that was evident in the present patient’s microscopic features is also an atypical finding, although it may be expected to some degree in scintigraphically hot, large lesions.28 The clinical and imaging progress of the lesion supported the diagnosis of a giant bone island. The degree of the anterior tibial bowing and the volume of the lesion in relation to the host bone were not changed throughout the follow-up period, indicating that the growth of the lesion followed the growth of the normal bone.
The differential diagnosis of a giant bone island includes a variety of benign tumors and tumor-like lesions, as well as malignant bone lesions.2,4,23,28,30,31 In the patient presented in this report, the diagnosis of an atypical sclerotic presentation of a nonossifying fibroma or healing stage of this lesion could be consistent with some of the CT findings, including the eccentric origin from the cortex associated with medial cortical thickening, the anterolateral cortical thinning, and the soft-tissue attenuation of cortical areas. In addition, unifocal osteofibrous dysplasia may also present with a long intracortical diaphyseal lucency within an area of marked cortical sclerosis and cause a bowing deformity. Both diagnoses were excluded, since no fibrous stroma was evident on the histologic examination of the lesion. A large or giant long-bone osteoma would be associated with the outer cortical margin of bone but would not involve the intramedullary space. The scintigraphically increased uptake of radioisotope, as well as the CT and MRI findings, were not consistent with the diagnosis of osteoid osteoma, osteoblastoma, or osteomyelitis. Although most imaging findings were consistent with a benign lesion, and contrast-enhanced MRI showed no increased vascularity, anterior cortical disruption necessitated a bone biopsy to rule out any potential malignancy.
The histopathology in association with the clinical and imaging findings indicated the diagnosis of a giant bone island. The increased proportion of maturing woven bone over lamellar bone indicated an active remodeling lesion that could be related to the patient’s age, since the clinical and radiographic features of the lesion were not changed after 15-year follow-up.
Conclusion
This is the first giant bone island diagnosed in a patient before puberty. Its greatest length was 10.8 cm, which is the longest reported in the literature. The imaging appearance included several atypical features that are very rare or have not been reported. Microscopic features indicated less mature lamellar bone and a prominent proportion of maturing woven bone. The clinical and the radiographic appearance of the lesion were not changed after 15-year follow-up.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
1. Smith J. Giant bone islands. Radiology. 1973;7(1):35-36.
2. Mirra JM. Bone Tumors: Clinical, Radiologic and Pathologic Correlations. Philadelphia, PA: Lea & Febiger; 1989.
3. Greenspan A. Bone island (enostosis): current concept - a review. Skeletal Radiol. 1995;24(2):111-115.
4. Kransdorf MJ, Peterson JJ, Bancroft LW. MR imaging of the knee: incidental osseous lesions. Radiol Clin North Am. 2007;45(6):943-954.
5. Gold RH, Mirra JM, Remotti F, Pignatti G. Case report 527: Giant bone island of tibia. Skeletal Radiol. 1989;18(2):129-132.
6. Onitsuka H. Roentgenologic aspects of bone islands. Radiology. 1977;123(3):607-612.
7. Ehara S, Kattapuram SV, Rosenberg AE. Giant bone island. Computed tomography findings. Clin Imaging. 1989;13(3):231-233.
8. Greenspan A, Steiner G, Knutzon R. Bone island (enostosis): clinical significance and radiologic and pathologic correlations. Skeletal Radiol. 1991;20(2):85-90.
9. Avery GR, Wilsdon JB, Malcolm AJ. Giant bone island with some central resorption. Skeletal Radiol. 1995;24(1):59-60.
10. Brien EW, Mirra JM, Latanza L, Fedenko A, Luck J Jr. Giant bone island of femur. Case report, literature review, and its distinction from low grade osteosarcoma. Skeletal Radiol. 1995;24(7):546-550.
11. Greenspan A, Klein MJ. Giant bone island. Skeletal Radiol. 1996;25(1):67-69.
12. Trombetti A, Noël E. Giant bone islands: a case with 31 years of follow-up. Joint Bone Spine. 2002;69(1):81-84.
13. Dhaon BK, Gautam VK, Jain P, Jaiswal A, Nigam V. Giant bone island of femur complicating replacement arthroplasty: a report of two cases. J Surg Orthop Adv. 2004;13(4):220-223.
14. Park HS, Kim JR, Lee SY, Jang KY. Symptomatic giant (10-cm) bone island of the tibia. Skeletal Radiol. 2005;34(6):347-350.
15. Ikeuchi M, Komatsu M, Tani T. Giant bone island of femur with femoral head necrosis: a case report. Arch Orthop Trauma Surg. 2010;130(4):447-450.
16. Kim SK, Barry WF Jr. Bone island. Am J Roentgenol Radium Ther Nucl Med. 1964;92:1301-1306.
17. Kim SK, Barry WF Jr. Bone islands. Radiology. 1968;90(1):77-78.
18. Cerase A, Priolo F. Skeletal benign bone-forming lesions. Eur J Radiol. 1998;27:S91–S97.
19. Go RT, El-Khoury GY, Wehbe MA. Radionuclide bone image in growing and stable bone island. Skeletal Radiol. 1980;5(1):15-18.
20. Hall FM, Goldberg RP, Davies JA, Fainsinger MH. Scintigraphic assessment of bone islands. Radiology. 1980;135(3):737-742.
21. Greenspan A, Stadalnik RC. Bone island: scintigraphic findings and their clinical application. Can Assoc Radiol J. 1995;46(5):368-379.
22. Sickles EA, Genant HK, Hoffer PB. Increased localization of 99mTc-pyrophosphate in a bone island: case report. J Nucl Med. 1976;17(2):113-115.
23. Dorfman HD, Czerniak B. Bone Tumors. St Louis: Mosby; 1998.
24. Ngan H. Growing bone islands. Clin Radiol. 1972;23(2):199-201.
25. Davies JA, Hall FM, Goldberg RP, Kasdon EJ. Positive bone scan in a bone island. Case report. J Bone Joint Surg Am. 1979;61(6):943-945.
26. Simon K, Mulligan ME. Growing bone islands revisited. A case report. J Bone Joint Surg Am. 1985;67(5):809-811.
27. Blank N, Lieber A. The significance of growing bone islands. Radiology. 1965;85(3):508-511.
28. Greenspan A, Gernot J, Wolfgang R. Differential Diagnosis of Orthopaedic Oncology. Philadelphia, PA: Lippincott Williams & Wilkins; 2007.
29. Kransdorf MJ, Murphey MD. Osseous tumors. In: Davies AM, Sundaram M, James SLJ, eds. Imaging of Bone Tumors and Tumor-Like Lesions. Berlin, Germany: Springer-Verlag; 2009.
30. Mödder B, Guhl B, Schaefer HE. Growing bone islands as differential diagnosis of osteoplastic metastases. Rontgenblatter. 1980;33(6):286-288.
31. Flechner RE, Mills SE. Atlas of Tumor Pathology: Tumors of the Bones and Joints. Washington, DC: Armed Forces Institute of Pathology; 1993.
Acquired Port-wine Stain With Superimposed Eczema Following Penetrating Abdominal Trauma
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
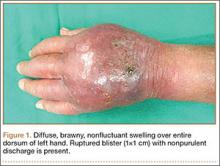
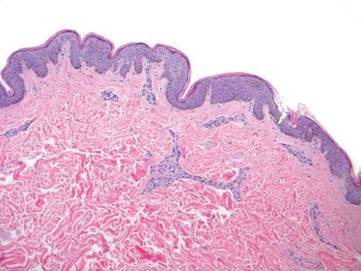
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.
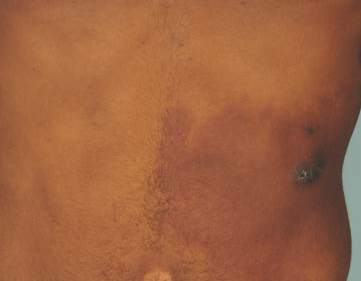
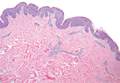
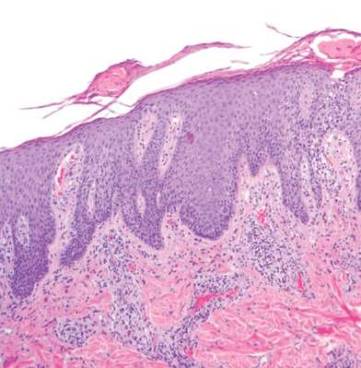
Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.
The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.

Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.


The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
Port-wine stains (PWSs) are common congenital capillary vascular malformations with an incidence of 3 per 1000 neonates.1 Rarely, acquired PWSs are seen, sometimes appearing following trauma.2-5 Port-wine stains are diagnosed clinically and present as painless, partially or entirely blanchable pink patches that respect the median (midline) plane.6 Although histopathologic examination is not necessary for diagnosis of PWS, typical findings include dilated, ectatic capillaries.7,8 Since it was first reported by Traub9 in 1939, more than 60 cases of acquired PWSs have been reported.10 A PubMed search of articles indexed for MEDLINE using the search terms acquired port-wine stain and port-wine stain and eczema yielded no cases of acquired PWS with associated eczematous changes and only 30 cases of congenital PWS with superimposed eczema.11-18 We report the case of an acquired PWS with superimposed eczema in an 18-year-old man following penetrating abdominal trauma.
Case Report
An otherwise healthy 18-year-old man presented to our dermatology office for evaluation of an eruption that had developed at the site of an abdominal stab wound he sustained 2 to 3 years prior. One year after he was stabbed, the patient developed a nonpruritic, painless red patch located 1 cm anterior to the healed wound on the left abdomen. The patch gradually grew larger to involve the entire left abdomen, extending to the left lower back. The site of the healed stab wound also became raised and pruritic, and the patient noted another pruritic plaque that formed within the larger patch. The patient reported no other skin conditions prior to the current eruption. His medical history was notable for seasonal allergies and asthma, but no childhood eczema.
Physical examination revealed a healthy, well-nourished man with Fitzpatrick skin type IV. A red, purpuric, coalescent patch with slightly arcuate borders extending from the mid abdomen to the left posterior flank was noted. The left lateral aspect of the patch blanched with pressure and respected the median plane. Within the larger patch, a 4-cm×2-cm lichenified, slightly macerated, hyperpigmented plaque was noted at the site of the stab wound (Figure 1). Based on these clinical findings, a presumptive diagnosis of an acquired PWS with superimposed eczema was made.

Punch biopsy specimens were taken from the large vascular patch and the smaller lichenified plaque. Histopathologic examination of the vascular patch showed an increased number of small vessels in the superficial dermis with thickened vessel walls, ectatic lumens, and no vasculopathy, consistent with a vascular malformation or a reactive vascular proliferation (Figure 2). On histopathology, the plaque showed epidermal spongiosis and hyperplasia with serum crust and a papillary dermis containing a mixed inflammatory infiltrate with occasional eosinophils, consistent with an eczematous dermatitis (Figure 3). The histologic findings confirmed the clinical diagnosis.


The pruritic, lichenified plaque improved with application of triamcinolone ointment 0.1% twice daily for 2 weeks. Magnetic resonance imaging to rule out an underlying arteriovenous malformation was recommended, but the patient declined.
Comment
The exact cause of PWS is unknown. There have been a multitude of genomic suspects for congenital lesions, including a somatic activating mutation (ie, a mutation acquired during fetal development) of the GNAQ (guanine nucleotide binding protein [G protein], q polypeptide) gene, which may contribute to abnormal cell proliferation including the regulation of blood vessels, and inactivating mutations in the RASA1 (RAS p21 protein activator [GTPase activating protein] 1) gene, which controls endothelial cell organization.19-22 Later mutations (ie, those occurring after the first trimester) may be more likely to result in isolated PWSs as opposed to syndromic PWSs.19 Whatever the source of genetic misinformation, it is thought that the diminished neuronal control of blood flow and the resulting alterations in dermal structure contribute to the pathogenesis of PWS and its associated histologic features.7,23
The clinical and histopathologic features of acquired PWSs are indistinguishable from those of congenital lesions, indicating that different processes may lead to the same presentation.4 Abnormal innervation and decreased supportive stroma have both been identified as contributing factors in the development of congenital and acquired PWSs.7,23-25 Rosen and Smoller23 found that diminished nerve density affects vascular tone and caliber in PWSs and had hypothesized in a prior report that decreased perivascular Schwann cells may indicate abnormal sympathetic innervation.7 Since then, PWS has been shown to lack both somatic and sensory innervation.24 Tsuji and Sawabe25 indicated that alterations to the perivascular stroma, whether congenital or as a result of trauma, decrease support for vessels, leading to ectasia.
In addition to an acquired PWS, our patient also had associated eczema within the PWS. Eczematous lesions were absent elsewhere, and he did not have a history of childhood eczema. Our review of the literature yielded 8 studies since 1996 that collectively described 30 cases of eczema within PWSs.11-18 Only 2 of these reports described adult patients with concomitant eczema and PWS and none described acquired PWS.13,18
Few studies have addressed the relationship between PWSs and eczema. It is unclear if concomitant PWS and localized eczema are collision dermatoses or if a PWS may predispose the affected skin to eczema.11-13 It has been hypothesized that the increased dermal vasculature in PWSs predisposes the skin to the development of eczema—more specifically, that ectasia may lead to increased inflammation.12,17 The concept of the “immunocompromised district” proposed by Ruocco et al26 is a unifying theory that may underlie the association noted between cases of trauma and later development of a PWS and superimposed eczematous dermatitis, such as in our case. Trauma is noted as one of a number of possible disruptive forces affecting both immunomodulation and neuromodulation within a local area of skin, leading to increased susceptibility of that district to various cutaneous diseases.26
Although our patient’s eczema responded to conservative treatment with a topical steroid, several case series have reported success with laser therapy in the treatment of PWS while preventing recurrence of associated eczematous dermatitis.12,17 Following the cessation of eczema treatment with topical steroid, which causes vasoconstriction, we suggest postponing laser therapy several weeks to allow resolution of vasoconstriction, thus providing enhanced therapeutic targeting with a vascular laser. Of particular relevance to our case, a recent study showed efficacy of the pulsed dye laser in treating PWSs in Fitzpatrick skin types IV and V.27
Conclusion
Although acquired PWS is rare, it can present later in life as an acquired lesion at a site of previous trauma.1-5 Congenital capillary malformations also can be associated with superimposed, localized eczema.11-18 We present a rarely reported case of an acquired PWS with superimposed, localized eczema. As in cases of congenital PWS with concomitant eczema, the associated eczema in our case was responsive to topical corticosteroid therapy. Additionally, pulsed dye laser has been shown to treat PWSs while preventing the recurrence of eczema, and it has been deemed effective for individuals with darker skin types.12,17, 27 Further studies are needed to explore the relationship between PWS and eczema.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
- Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218-222.
- Fegeler F. Naevus flammeus im trigeminusgebiet nach trauma im rahmen eines posttraumatisch-vegetativen syndroms. Arch Dermatol Syphilol. 1949;188:416-422.
- Kirkland CR, Mutasim DF. Acquired port-wine stain following repetitive trauma. J Am Acad Dermatol. 2011;65:462-463.
- Adams BB, Lucky AW. Acquired port-wine stains and antecedent trauma: case report and review of the literature. Arch Dermatol. 2000;136:897-899.
- Colver GB, Ryan TJ. Acquired port-wine stain. Arch Dermatol. 1986;122:1415-1416.
- Nigro J, Swerlick RA, Sepp NT, et al. Angiogenesis, vascular malformations and proliferations. In: Arndt KA, LeBoit PE, Robinson JK, Wintroub BU, eds. Cutaneous Medicine and Surgery: An Integrated Program in Dermatology. Philadelphia, PA: WB Saunders Co; 1996:1492-1521.
- Smoller BR, Rosen S. Port-wine stains. a disease of altered neural modulation of blood vessels? Arch Dermatol. 1986;122:177-179.
- Chang CJ, Yu JS, Nelson JS. Confocal microscopy study of neurovascular distribution in facial port wine stains(capillary malformation). J Formos Med Assoc. 2008;107:559-666.
- Traub EF. Naevus flammeus appearing at the age of twenty three. Arch Dermatol. 1939;39:752.
- Freysz M, Cribier B, Lipsker, D. Fegelers syndrome, acquired port-wine stain or acquired capillary malformation: three cases and a literature review [article in French]. Ann Dermatol Venereol. 2013;140:341-346.
- Tay YK, Morelli J, Weston WL. Inflammatory nuchal-occipital port-wine stains. J Am Acad Dermatol. 1996;35:811-813.
- Sidwell RU, Syed S, Harper JI. Port-wine stains and eczema. Br J Dermatol. 2001;144:1269-1270.
- Hofer T. Meyerson phenomenon within a nevus flammeus. Dermatology. 2002;205:180-183.
- Raff K, Landthaler M, Hoheleutner U. Port-wine stains with eczema. Phlebologie. 2003;32:15-17.
- Tsuboi H, Miyata T, Katsuoka K. Eczema in a port-wine stain. Clin Exp Dermatol. 2003;28:322-323.
- Rajan N, Natarahan S. Impetiginized eczema arising within a port-wine stain of the arm. J Eur Acad Dermatol Venereol. 2006;20:1009-1010.
- Fonder MA, Mamelak AJ, Kazin RA, et al. Port-wine-stain-associated dermatitis: implications for cutaneous vascular laser therapy. Pediatr Dermatol. 2007;24:376-379.
- Simon V, Wolfgan H, Katharina F. Meyerson-Phenomenon hides a nevus flammeus. J Dtsch Dermatol Ges. 2011;9:305-307.
- Shirley MD, Tang H, Gallione CJ, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971-1979.
- Hershkovitz D, Bercovich D, Sprecher E, et al. RASA1 mutations may cause hereditary capillary malformations without arteriovenous malformations. Br J Dermatol. 2008;158:1035-1040.
- Eerola I, Boon LM, Mulliken JB, et al. Capillary malformation-arteriovenous malformation, a new clinical and genetic disorder caused by RASA1 mutations. Am J Hum Genet. 2003;73:1240-1249.
- Henkemeyer M, Rossi DJ, Holmyard DP, et al. Vascular system defects and neuronal apoptosis in mice lacking ras GTPase-activating protein. Nature. 1995;377:695-701.
- Rosen S, Smoller BR. Port-wine stains: a new hypothesis. J Am Acad Dermatol. 1987;17:164-166.
- Rydh M, Malm BM, Jernmeck J, et al. Ectatic blood vessels in port-wine stains lack innervation: possible role in pathogenesis. Plast Reconstr Surg. 1991;87:419-422.
- Tsuji T, Sawabe M. A new type of telangiectasia following trauma. J Cutan Pathol. 1988;15:22-26.
- Ruocco V, Ruocco E, Brunnetti G, et al. Opportunistic localization of skin lesions on vulnerable areas. Clin Dermatol. 2011;29:483-488.
- Thajudeheen CP, Jyothy K, Pryadarshi A. Treatment of port-wine stains with flash lamp pumped pulsed dye laser on Indian skin: a six year study. J Cutan Aesthet Surg. 2014;7:32-36.
Practice Points
- Port-wine stains (PWSs) most often are congenital lesions but can present later in life as acquired lesions with the same clinical and histologic findings.
- Magnetic resonance imaging of acquired PWSs should be considered to rule out underlying vascular anomalies (eg, deeper arteriovenous malformations).
- Pulsed dye laser therapy is safe for darker skin types and is the treatment of choice for acquired PWSs.
Nonconsecutive Pars Interarticularis Defects
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
Spondylolysis is a bone defect of the pars interarticularis. It is usually seen in adolescents who participate in sporting activities that involve repetitive axial loads to a hyperextended lower back, such as football offensive lineman, throwing athletes, and gymnasts. It occurs frequently in the L5 pars and can be unilateral or bilateral. The majority of reported multiple-level spondylolysis is at consecutive lumbar segments.1-6 Rarely, it affects noncontiguous levels. Most patients respond well to conservative treatment in the form of activity modification and orthosis.7 Surgical intervention is considered if 6 months of conservative management fails, spondylolisthesis develops, or intractable neurologic symptoms arise.
This case report presents an 18-year-old man with noncontiguous spondylolysis at L2 and L5 who was successfully treated with a 1-level pars repair at L2 after failed conservative management. This unique case highlights the importance of using single-photon emission computed tomography (SPECT) scan and diagnostic pars block when planning for surgical treatment in the rare cases of noncontiguous spondylolysis. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
An 18-year-old man presented to the clinic with worsening lower back pain for the previous 4 weeks. He was playing high school baseball and stated the pain was worse when he swung his bat. He had no history of trauma or back pain. Rest was the only alleviating factor, and he was beginning to experience pain when he stood after sitting. He denied any radicular pain. On examination, he had midline tenderness along the upper lumbar spine and pain with lumbar spine extension. His neurologic examination showed normal sensation with 5/5 strength in all key muscle groups. Plain radiograph of the lumbar spine showed an L5 pars defect (Figures 1A, 1B). A SPECT scan showed increased uptake at L2 pars bilaterally; the L5 pars did not show increased uptake (Figures 2A, 2B). A computed tomography (CT) scan confirmed bilateral L2 pars fractures and a left L5 pars fracture (Figures 3A, 3B). Bony changes in the form of marginal sclerosis at the L5, but not the L2, pars suggested that the L2 fracture was acute while the L5 fracture was chronic (Figures 4A, 4B).
The patient had conservative management for 6 months in the form of lumbosacral orthosis (LSO), cessation of sports activities, and physical therapy. The patient was initially treated with an LSO brace for 3 months, after which he had physical therapy. At 6 month follow-up, he reported continuing, significant back pain. A repeat CT scan of the lumbar spine showed no interval healing of the bilateral L2 or the unilateral L5 pars fractures. As a result of the patient’s noncontiguous pars fractures, a diagnostic CT-guided block of L2 pars was performed to identify which level was his main pain generator (Figure 5). He reported a brief period of complete pain relief after the L2 pars block. With failure of 6 months’ conservative management and positive SPECT scan and diagnostic block, surgical treatment was recommended. Prior to surgical intervention, magnetic resonance imaging was obtained to rule out pathology (eg, disc degeneration, infection, or tumor) other than the pars defect that could require fusion instead of pars repair (Figures 6A, 6B). Because of the patient’s young age, bilateral L2 pars repair rather than fusion was indicated. After 8 months of persistent back pain, he underwent bilateral L2 pars repair with iliac crest autograft, pedicle screws, and sublaminar hook fixation (Figures 7A, 7B). The patient had an uneventful immediate postoperative course. A 6-month postoperative CT scan showed bridging callus at the L2 pars; however, the left L5 pars fracture was still visible (Figures 8A-8C). Over a 6-month postoperative period, the patient had continued improvement in his back pain, advanced his activity as tolerated without problem, and was allowed to resume his sports activities. At 2-year follow-up, he was playing baseball and basketball, and denied any back pain.
Discussion
Lumbar spondylolysis is commonly seen at the fourth and fifth lumbar vertebrae, and accounts for more than 95% of spondylolysis cases.8 Multiple-level spondylolysis is a relatively rare finding with an incidence varying between 1.2% and 5.6%. The majority of the reported multiple-level cases are adjacent.1-3,6 Adolescents often present with a history of insidious-onset low back pain without radicular symptoms that is exacerbated by activity. Occasionally, an acute injury may elicit the onset of pain. A thorough history with emphasis on pain in relation to activity and sports involvement is beneficial. The patient in the current study was a throwing athlete and presented with 4 weeks of back pain that worsened with activity; he had no history of trauma.
Radiographic assessment using standing anteroposterior, lateral, and oblique radiographs of the thoracolumbar spine is useful in the initial assessment. A SPECT scan of the lumbosacral spine is highly sensitive for identifying spondylolytic defects when plain radiographs are within normal limits, yet a high index of suspicion remains given the patient’s history and physical examination findings.9,10 Increased radionuclide uptake within the pars indicates a stress reaction and, possibly, a more acute pathology. The plain radiographs of the patient showed only L5 spondylolysis. However, a SPECT scan showed only increased uptake in L2 pars on both sides. These findings suggested chronic L5 and acute L2 pars defects. A thin-cut CT scan gives the best visualization of pars defect and can help in differentiating chronic defect with sclerotic margins versus acute defect with hazy irregular margins. In the current case, the CT scan showed changes consistent with unilateral chronic L5 and bilateral acute L2 pars defects.
The origin of the pain in spondylolysis is from the tissues rich in nociceptive nerve endings in the loose posterior arch. A CT-guided pars block is a very useful diagnostic preoperative tool that confirms the symptomatic level in cases of multilevel pars defect, especially if they are noncontiguous. In this case, the diagnostic preoperative bilateral L2 pars block confirmed that the pain generator was the acute L2 rather than the chronic L5 pars defect. This step assured that surgical treatment involving only the L2 level would be beneficial in alleviating the patient’s back pain after the failure of 6 months of conservative treatment.
Most patients with single-level spondylolysis respond to conservative treatment, especially after early diagnosis and treatment. The traditional nonoperative treatment of children and adolescents with a symptomatic spondylolytic lesion was a period of rest and progressive increased activity with physical therapy. Immobilization with an LSO was reserved for individuals who did not respond to rest and physical therapy.11 However, multiple studies revealed early immobilization achieved results superior to activity restriction alone, and individuals who underwent a period of activity restriction prior to bracing were more likely to experience persistent symptoms.12-14 Our patient underwent conservative treatment for 6 months, in the form of LSO, cessation of sport activities, and physical therapy, which failed to give him relief of his back pain.
Surgical intervention is warranted for adolescents with persistent, debilitating pain intractable to at least a 6-month period of nonoperative management. Additional indications for surgical management are those individuals who present with neurologic deficits and isthmic spondylolisthesis. Surgical treatment involves direct pars repair with iliac crest bone graft and use of a sublaminar hook/pedicle screw construct, cerclage wire, or pars screw.15-18
In contrast to single-level pars defects that respond well to conservative treatment, there are conflicting reports regarding the management of multiple-level pars fractures; a few reports suggest good outcome with conservative management, but the majority state that surgery is often required and conservative measures are rarely useful.1-4,6 Nayeemuddin and colleagues19 reported a case of a 16-year-old football player who presented with a 4-month history of constant low back pain related to bilateral L3 and L5 pars defects that responded to 1 year of conservative management, when the more acute fractures at L3 showed complete bony union and the patient had symptomatic pain relief and was able to return to full sporting activity.
Chang and colleagues2 reported 10 patients with adjacent 2-level bilateral spondylolysis treated successfully using a pedicle screw–hook construct with autogenous bone grafting. Ogawa and colleagues5 reported adjacent 2-level spondylolysis in 5 patients and 3-level spondylolysis in 2 patients, who were treated successfully by segmental wire fixation and bone grafting. Ivanic and colleagues15 retrospectively reviewed 113 patients with spondylolysis who were treated with direct repair using a hook-screw construct and showed a pseudoarthrosis rate of 13.3%. Superior fusion rates were observed in patients 14 years and younger compared with older patients, particularly those 20 years and older.15 Roca and colleagues16 prospectively analyzed 19 consecutive cases of spondylolysis that were repaired using a hook-screw construct. Twelve of 13 patients (92%) who were 20 years or younger at the time of the study (average age, 17.2 years) had fusion, whereas, in 6 patients 21 years and older (average age, 27.5 years), no cases of fusion were observed. The patients 20 years or younger had significantly better clinical results than those obtained in the patients 21 years and older. The authors concluded that pedicle screw–hook fixation is a useful alternative in the treatment of spondylolysis in adolescents, but did not recommend this procedure in patients older than 20 years.16
Conclusion
The current case demonstrates a unique example of rare noncontiguous pars defects successfully treated with primary repair of 1 level when conservative management failed and the symptomatic defect was isolated. It also highlights the importance of investigating the entirety of the lumbar spine when diagnosis of L5 spondylolysis rules out noncontiguous pars defects. The treatment of noncontiguous pars defects is not well defined; this case showed the importance of using a SPECT scan and a diagnostic pars block to help isolate the symptomatic level when surgical management is considered after a failure of conservative treatment. This case shows 2 possible results: the chronic unilateral L5 defect responded to nonsurgical treatment with asymptomatic fibrous nonunion, while the more acute bilateral L2 defect responded to pars repair with pedicle screw–hook fixation and iliac crest bone graft.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
1. Al-Sebai MW, Al-Khawashki H. Spondyloptosis and multiple-level spondylolysis. Eur Spine J. 1999;8(1):75-77.
2. Chang JH, Lee CH, Wu SS, Lin LC, et al. Management of multiple level spondylolysis of the lumbar spine in young males: a report of six cases. J Formos Med Assoc. 2001;100(7)2:497-502.
3. Eingorn D, Pizzutillo PD. Pars interarticularis fusion of multiple levels of lumbar spondylolysis. A case report. Spine. 1985;10(3):250-252.
4. Nozawa S, Shimizu K, Miyamoto K, Tanaka M. Repair of pars interarticularis defect by segmental wire fixation in young athletes with spondylolysis. Am J Sports Med. 2003;31(3):359-364.
5. Ogawa H, Nishimoto H, Hosoe H, Suzuki N, Kanamori Y, Shimizu K. Clinical outcome after segmental wire fixation and bone grafting for repair of the defects in multiple level lumbar spondylolysis. J Spinal Disord Tech. 2007;20(7):521-525.
6. Ravichandran G. Multiple lumbar spondylolyses. Spine. 1980;5(6):552-557.
7. Sys J, Michielsen J, Bracke P, Martens M, Verstreken J. Nonoperative treatment of active spondylolysis in elite athletes with normal X-ray findings: literature review and results of conservative treatment. Eur Spine J. 2001;10(6):498-504.
8. Saraste H. Spondylolysis and spondylolisthesis. Acta Orthop Scand Suppl. 1993;251:84-86.
9. Anderson K, Sarwark JF, Conway JJ, Logue ES, Schafer MS. Quantitative assessment with SPECT imaging of stress injuries of the pars interarticularis and response to bracing. J Pediatr Orthop. 2000;20(1):28-33.
10. Bodner RJ, Heyman S, Drummond DS, Gregg JR. The use of single photon emission computed tomography (SPECT) in the diagnosis of low-back pain in young patients. Spine. 1988;13(10):1155-1160.
11. Steiner ME, Micheli LJ. Treatment of symptomatic spondylolysis and spondylolisthesis with the modified Boston brace. Spine. 1985;10(10):937-943.
12. Blanda J, Bethem D, Moats W, Lew M. Defects of pars interarticularis in athletes: a protocol for nonoperative treatment. J Spinal Disord. 1993;6(5):406-411.
13. Kurd MF, Patel D, Norton R, Picetti G, Friel B, Vaccaro AR. Nonoperative treatment of symptomatic spondylolysis. J Spinal Disord Tech. 2007;20(8):560-564.
14. Pizzutillo PD, Hummer CD 3rd. Nonoperative treatment for painful adolescent spondylolysis or spondylolisthesis. J Pediatr Orthop. 1989;9(5):538-540.
15. Ivanic GM, Pink TP, Achatz W, Ward JC, Homann NC, May M. Direct stabilization of lumbar spondylolysis with a hook screw: mean 11-year follow-up period for 113 patients. Spine. 2003;28(3):255-259.
16. Roca J, Iborra M, Cavanilles-Walker JM, Alberti G. Direct repair of spondylolysis using a new pedicle screw hook fixation: clinical and CT-assessed study: an analysis of 19 patients. J Spinal Disord Tech. 2005;18(suppl):S82-S89.
17. Schlenzka D, Remes V, Helenius I, et al. Direct repair for treatment of symptomatic spondylolysis and low-grade isthmic spondylolisthesis in young patients: no benefit in comparison to segmental fusion after a mean follow-up of 14.8 years. Eur Spine J. 2006;15(10):1437-1447.
18. Buck JE. Direct repair of the defect in spondylolisthesis. Preliminary report. J Bone Joint Surg Br. 1970;52(3):432-437.
19. Nayeemuddin M, Richards PJ, Ahmed EB. The imaging and management of nonconsecutive pars interarticularis defects: a case report and review of literature. Spine J. 2011;11(12):1157-1163.
Acute Onset of Vancomycin Anaphylaxis With Disseminated Intravascular Coagulation in an Orthopedic Patient Despite Prior Repeated Exposure
Vancomycin is a glycopeptide antibiotic that exhibits bactericidal activity against gram-positive cocci. It is commonly recommended for surgical prophylaxis in cases of suspected bacterial resistance or penicillin allergy.1 Two main types of hypersensitivity reactions associated with vancomycin can have similar presentations. Red man syndrome is an anaphylactoid reaction caused by direct release of histamine from mast cells via a nonimmunologic mechanism, and is the more common of the 2 reactions. The second type is an anaphylactic reaction, which is an immunoglobulin E (IgE)–mediated systemic event and requires exposure to become sensitized.2,3
We present a patient who had received vancomycin on at least 12 occasions without incident. On this occasion, however, she developed a true anaphylactic reaction causing acute hemodynamic collapse that she survived after extensive resuscitation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 55-year-old woman had a history of metastatic giant cell tumor of the right proximal tibia. She was originally treated 27 years ago for proximal tibial resection and reconstruction with a custom proximal tibial prosthesis. Four months later, she underwent resection of multiple pulmonary metastases via bilateral thoracotomies in a single surgical setting. After this, the patient had no evidence of recurrent metastatic disease. In subsequent years, the patient underwent multiple revision surgeries for problems such as hardware failure, patellar maltracking, and infection. The patient underwent 19 operations, including several nonorthopedic procedures. Because the patient had a rash after receiving penicillin as a child, she was thought to be allergic to penicillin. Consequently, she received vancomycin as antibiotic prophylaxis for the majority of these procedures. She also received extended courses of vancomycin of at least 6 weeks on 2 separate occasions. During her most recent revision procedure, 6 weeks prior to the procedure under discussion, the patient took vancomycin without incident. She was then found to have a prosthetic infection with Staphylococcus epidermidis, the same organism isolated in her previous infections, and she was advised to undergo a staged revision.
After a preoperative medical evaluation by her primary care physician, the patient was taken to the operating room for prosthesis removal and antibiotic spacer placement. She was anemic with a hemoglobin level of 8.8 g/dL; her erythrocyte sedimentation rate (ESR) was 102 mm/h (normal, <22 mm/h) and her C-reactive protein (CRP) was 38 mg/L (normal, <3 mg/L), but, otherwise, her laboratory values were normal, including a white blood cell count (WBC) of 8100/µL. Her electrocardiogram showed a normal sinus rhythm with nonspecific ST- and T-wave changes. Antibiotics were held until after cultures were taken. General endotracheal tube anesthesia was induced with 2 mg midazolam, 100 µg fentanyl, 180 mg propofol, and 140 mg succinylcholine, followed by 10 mg vecuronium, and maintained with desflurane. A tourniquet was not used per the surgeon’s routine. Dissection was carried down to the prosthesis and showed a small amount of purulent fluid. Transfusion of 1 unit of packed red blood cells (pRBC) was started during the approach owing to relatively low preoperative hemoglobin and significant blood loss. Approximately 500 mL of blood was lost during the approach secondary to the extensive dissection and the local inflammatory response from infection and recent surgery. After cultures were taken, and approximately 10 minutes after blood transfusion began, infusion of 1 g vancomycin in 250 mL normal saline was started via an infusion pump to run over 1 hour.
After infusion of 5 mL vancomycin, the patient’s blood pressure dropped from 117/63 mm Hg to 63/30 mm Hg; her pulse concurrently dropped from 90 to 50 beats/min. Vancomycin infusion was immediately stopped, anesthesia gasses were turned off, and patient received a bolus of normal saline with a second unit of pRBC. Patient received boluses of 0.5 mg to 1.0 mg epinephrine and 100 µg phenylephrine without sustained increase in blood pressure, which had dropped to 54/24 mm Hg, although the patient became tachycardic to ~120 beats/min after epinephrine. A sudden drop in end-tidal CO2 from 40s mm Hg to 20s mm Hg was also noted, indicating continuous but significantly decreased perfusion of the lungs.
We elected to abort the procedure, and a vacuum-assisted closure (VAC) dressing was applied to the open wound. After 15 minutes, the patient’s pulses, which had been faint, became impalpable, and cardiopulmonary resuscitation was initiated for about 7 minutes. The patient received 40 units vasopressin with repeated boluses of 0.5 mg epinephrine; a norepinephrine continuous infusion was started with the return of pulses. The patient also received 50 mg diphenhydramine, 125 mg methylprednisolone, and 20 mg famotidine for suspected anaphylaxis. A central venous line and arterial line were placed, and blood was drawn for laboratory analysis. The patient was noted to have clear breath sounds with no obvious rash, and her urine remained clear. Blood gas showed a profound metabolic acidosis, with pH of 7.09, base deficit of 5.9, and lactate of 8.9. The patient was treated with bicarbonate infusion. The patient was noted to ooze significantly during central venous line and arterial line placement, despite apparently normal coagulation during the surgical approach. Coagulation values were consistent with disseminated intravascular coagulation (DIC): prothrombin time, 57 s (international normalized ratio, 6.7); partial thromboplastin time, >200 s; thrombin time, 110 s; D-dimer, >10,000 ng/mL (normal, 0-200 ng/mL); and fibrinogen, <60 mg/dL (normal, 222-475 mg/dL). The patient’s thromboelastogram showed a flat line indicating an absence of clotting. Interestingly, the platelet count remained near the preoperative level at 338×103/µL. The patient’s blood pressure remained labile and was responsive primarily to epinephrine boluses, of which she received a total of 5 mg. After 1 hour of resuscitation, during which time the patient received a total of 5 L crystalloid and 3 units pRBC, the patient was transferred to the intensive care unit (ICU), intubated, and started on a titrated epinephrine infusion.
Upon arrival in the ICU, the patient quickly stabilized hemodynamically. She was weaned from all inotropic support within 2 hours of arrival. The patient lost 800 mL of blood through wound VAC over the first 12 hours postoperatively and required a total of 11 units of pRBC, 6 units fresh frozen plasma, and 3 units of pooled cryoprecipitate, all of which were compatible. Laboratory values, including arterial pH, lactic acid, and coagulation studies, normalized on the evening of surgery, and, by the next morning, the patient was alert and was extubated without difficulty. Steroids were tapered without hemodynamic compromise while the patient was in the ICU. Cardiology examination revealed no abnormalities. Because of the temporal association of blood transfusion with cardiovascular collapse, pRBC units were retested for antibodies and cultured. Both of these investigations were negative. Wound cultures again were positive for Staphylococcus epidermidis, and blood cultures were negative. The patient was started on daptomycin based on susceptibility profiles. Serum histamine levels taken during initial resuscitation in the operating room were normal. The serum tryptase level obtained at the same time was markedly elevated at >700 ng/mL (normal, <11.5 ng/mL), although this information was not available until several days later.
The patient underwent 2 additional surgeries during the same admission, including the prosthesis removal and tobramycin cement spacer placement, without incident. She was discharged home, again without incident. The patient was later evaluated by an outside allergist and underwent skin puncture and intradermal allergy testing. The results were consistent with a strong IgE-mediated hypersensitivity. Interestingly, she was found not to have a penicillin allergy.
Discussion
Vancomycin hypersensitivity reactions include the anaphylactoid reaction red man syndrome and a true IgE-mediated anaphylactic reaction. Red man syndrome is much more common, with reported rates in infected patients from 3.7% to 47%,4,5 when vancomycin is given at the suggested rate of 1 g over 1 hour. The reaction occurs because of histamine release from mast cells and basophils, and does not require previous sensitization.3 The rate of infusion is directly related to the development of symptoms, with 100% of patients developing symptoms in 1 study with rapid infusion (1 g over 10 min).6 Red man syndrome can typically be prevented by slowing the rate of infusion or by giving an H1 blocker.3 Anaphylaxis is more rare but can occur.7 Anaphylaxis is mediated by vancomycin-specific IgE, which requires previous exposure, as was the case with our patient. Interestingly, the patient had received vancomycin many times without any signs of a hypersensitivity reaction. Antihistamines are not effective in treating anaphylaxis, and epinephrine is the first-line agent.3 This was clearly demonstrated in this case, as there was a significant hemodynamic response to epinephrine and a negligible response to other vasopressors, specifically norepinephrine and vasopressin.
Most hypersensitivity reactions during the course of a surgical procedure occur with induction of anesthesia, with neuromuscular blocking agents and antibiotics being the most common causes.8 In our case, antibiotics were held until after deep cultures were taken. Given the time from induction to the anaphylactic reaction, it is unlikely the reaction resulted from the induction agents or the neuromuscular blocking agent. The possibility of a transfusion reaction was also investigated, since a unit of pRBC was still being transfused when symptoms began. An acute hemolytic transfusion reaction has the classic triad of fever, flank pain, and hemoglobinuria, and can also present as DIC.9 Under anesthesia, DIC can often be the presenting sign. In this case, a hemolytic transfusion reaction appeared very unlikely. All of the blood components the patient received were rechecked and found to be compatible, posttransfusion analysis showed no evidence of hemolysis in any sample, and the direct antiglobulin test was negative in all components.
To our knowledge, there are no reported cases of vancomycin-induced anaphylaxis with concomitant DIC. Symptoms of anaphylaxis after exposure to a possible antigen include rapid onset of hypotension or rapid onset of signs in at least 2 organ systems, including cutaneous, gastrointestinal, respiratory, and cardiovascular.10 Anaphylaxis with DIC is rare after exposure to any substance but has been reported.11 In fact, induction of systemic anaphylaxis in mice is known to cause DIC, with platelet-activating factor suggested as an important common mediator. A similar mechanism is suspected in humans.12
Confirmation of, and, certainly, prediction of, a vancomycin hypersensitivity reaction is difficult. Histamine levels can be used as a measure of mast-cell degranulation, but serum levels peak within 5 minutes and quickly return to baseline, limiting its diagnostic usefulness.3 Tryptase is an enzyme found in the secretory granules of mast cells. It has become an accepted marker of acute anaphylaxis, and, in vancomycin hypersensitivity reactions, can also distinguish between anaphylactic and anaphylactoid reactions.13 Tryptase levels peak 1 to 2 hours after the reaction, making this easier to measure than histamine, but results may not be available for several days, making it useful only in retrospect, as in our case. Skin testing is probably the best way to confirm a hypersensitivity reaction, although even this has been questioned with vancomycin because some find a high false-positive rate3, while others think the false-negative rate is likely too high.7 In this case, we were able to confirm our initial clinical suspicion with both an elevated tryptase level and a positive skin test.
Conclusion
We present a rare case of vancomycin anaphylaxis with DIC after repeated and prolonged previous exposure, which was treated acutely with hemodynamic resuscitation, replacement of blood components, steroids, and, most importantly, repeated boluses of epinephrine. Although several papers have described successful vancomycin desensitization7, this was fortunately not necessary in this case because the causative organism was sensitive to other acceptable antibiotics. The patient has been treated with systemic daptomycin and a tobramycin cement spacer without further incident.
1. Recommendation for the use of intravenous antibiotic prophylaxis in primary total joint arthroplasty. AAOS Information Statement 1027. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/advistmt/1027.asp. Published June 2004. Accessed October 28, 2015.
2. Duffy BL. Vancomycin reaction during spinal anesthesia. Anaesth Intensive Case. 2002;30(3):364-366.
3. Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001;35(11):1458-1464.
4. O’Sullivan TL, Ruffing MJ, Lamp KC, Warbasse LH, Rybak MJ. Prospective evaluation of red man syndrome in patients receiving vancomycin. J Infect Dis. 1993;168(3):773-776.
5. Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991;164(6):1180-1185.
6. Renz CL, Thurn JD, Finn HA, Lynch JP, Moss J. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med. 1999;27(9):1732-1737.
7. Kupstaite R, Baranauskaite A, Pileckyte M, Sveikata A, Kadusevicius E, Muckiene G. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas). 2010;46(1):30-33.
8. Lobera T, Audicana MT, Pozo MD, et al. Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain. J Investig Allergol Clin Immunol. 2008;18(5):350-356.
9. Berséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(suppl 1):114S-123S.
10. Schwartz LB. Systemic anaphylaxis, food allergy, and insect sting allergy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier; 2011:1633-1638.
11. Jung JW, Jeon EJ, Kim JW, et al. A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res. 2012;4(2):107-109.
12. Choi IH, Ha TY, Lee DG, et al. Occurrence of disseminated intravascular coagulation (DIC) in active systemic anaphylaxis: role of platelet-activating factor. Clin Exp Immunol. 1995;100(3):390-394.
13. Renz CL, Laroche D, Thurn JD, et al. Tryptase levels are not increased during vancomycin-induced anaphylactoid reactions. Anesthesiology. 1998;89(3):620-625.
Vancomycin is a glycopeptide antibiotic that exhibits bactericidal activity against gram-positive cocci. It is commonly recommended for surgical prophylaxis in cases of suspected bacterial resistance or penicillin allergy.1 Two main types of hypersensitivity reactions associated with vancomycin can have similar presentations. Red man syndrome is an anaphylactoid reaction caused by direct release of histamine from mast cells via a nonimmunologic mechanism, and is the more common of the 2 reactions. The second type is an anaphylactic reaction, which is an immunoglobulin E (IgE)–mediated systemic event and requires exposure to become sensitized.2,3
We present a patient who had received vancomycin on at least 12 occasions without incident. On this occasion, however, she developed a true anaphylactic reaction causing acute hemodynamic collapse that she survived after extensive resuscitation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 55-year-old woman had a history of metastatic giant cell tumor of the right proximal tibia. She was originally treated 27 years ago for proximal tibial resection and reconstruction with a custom proximal tibial prosthesis. Four months later, she underwent resection of multiple pulmonary metastases via bilateral thoracotomies in a single surgical setting. After this, the patient had no evidence of recurrent metastatic disease. In subsequent years, the patient underwent multiple revision surgeries for problems such as hardware failure, patellar maltracking, and infection. The patient underwent 19 operations, including several nonorthopedic procedures. Because the patient had a rash after receiving penicillin as a child, she was thought to be allergic to penicillin. Consequently, she received vancomycin as antibiotic prophylaxis for the majority of these procedures. She also received extended courses of vancomycin of at least 6 weeks on 2 separate occasions. During her most recent revision procedure, 6 weeks prior to the procedure under discussion, the patient took vancomycin without incident. She was then found to have a prosthetic infection with Staphylococcus epidermidis, the same organism isolated in her previous infections, and she was advised to undergo a staged revision.
After a preoperative medical evaluation by her primary care physician, the patient was taken to the operating room for prosthesis removal and antibiotic spacer placement. She was anemic with a hemoglobin level of 8.8 g/dL; her erythrocyte sedimentation rate (ESR) was 102 mm/h (normal, <22 mm/h) and her C-reactive protein (CRP) was 38 mg/L (normal, <3 mg/L), but, otherwise, her laboratory values were normal, including a white blood cell count (WBC) of 8100/µL. Her electrocardiogram showed a normal sinus rhythm with nonspecific ST- and T-wave changes. Antibiotics were held until after cultures were taken. General endotracheal tube anesthesia was induced with 2 mg midazolam, 100 µg fentanyl, 180 mg propofol, and 140 mg succinylcholine, followed by 10 mg vecuronium, and maintained with desflurane. A tourniquet was not used per the surgeon’s routine. Dissection was carried down to the prosthesis and showed a small amount of purulent fluid. Transfusion of 1 unit of packed red blood cells (pRBC) was started during the approach owing to relatively low preoperative hemoglobin and significant blood loss. Approximately 500 mL of blood was lost during the approach secondary to the extensive dissection and the local inflammatory response from infection and recent surgery. After cultures were taken, and approximately 10 minutes after blood transfusion began, infusion of 1 g vancomycin in 250 mL normal saline was started via an infusion pump to run over 1 hour.
After infusion of 5 mL vancomycin, the patient’s blood pressure dropped from 117/63 mm Hg to 63/30 mm Hg; her pulse concurrently dropped from 90 to 50 beats/min. Vancomycin infusion was immediately stopped, anesthesia gasses were turned off, and patient received a bolus of normal saline with a second unit of pRBC. Patient received boluses of 0.5 mg to 1.0 mg epinephrine and 100 µg phenylephrine without sustained increase in blood pressure, which had dropped to 54/24 mm Hg, although the patient became tachycardic to ~120 beats/min after epinephrine. A sudden drop in end-tidal CO2 from 40s mm Hg to 20s mm Hg was also noted, indicating continuous but significantly decreased perfusion of the lungs.
We elected to abort the procedure, and a vacuum-assisted closure (VAC) dressing was applied to the open wound. After 15 minutes, the patient’s pulses, which had been faint, became impalpable, and cardiopulmonary resuscitation was initiated for about 7 minutes. The patient received 40 units vasopressin with repeated boluses of 0.5 mg epinephrine; a norepinephrine continuous infusion was started with the return of pulses. The patient also received 50 mg diphenhydramine, 125 mg methylprednisolone, and 20 mg famotidine for suspected anaphylaxis. A central venous line and arterial line were placed, and blood was drawn for laboratory analysis. The patient was noted to have clear breath sounds with no obvious rash, and her urine remained clear. Blood gas showed a profound metabolic acidosis, with pH of 7.09, base deficit of 5.9, and lactate of 8.9. The patient was treated with bicarbonate infusion. The patient was noted to ooze significantly during central venous line and arterial line placement, despite apparently normal coagulation during the surgical approach. Coagulation values were consistent with disseminated intravascular coagulation (DIC): prothrombin time, 57 s (international normalized ratio, 6.7); partial thromboplastin time, >200 s; thrombin time, 110 s; D-dimer, >10,000 ng/mL (normal, 0-200 ng/mL); and fibrinogen, <60 mg/dL (normal, 222-475 mg/dL). The patient’s thromboelastogram showed a flat line indicating an absence of clotting. Interestingly, the platelet count remained near the preoperative level at 338×103/µL. The patient’s blood pressure remained labile and was responsive primarily to epinephrine boluses, of which she received a total of 5 mg. After 1 hour of resuscitation, during which time the patient received a total of 5 L crystalloid and 3 units pRBC, the patient was transferred to the intensive care unit (ICU), intubated, and started on a titrated epinephrine infusion.
Upon arrival in the ICU, the patient quickly stabilized hemodynamically. She was weaned from all inotropic support within 2 hours of arrival. The patient lost 800 mL of blood through wound VAC over the first 12 hours postoperatively and required a total of 11 units of pRBC, 6 units fresh frozen plasma, and 3 units of pooled cryoprecipitate, all of which were compatible. Laboratory values, including arterial pH, lactic acid, and coagulation studies, normalized on the evening of surgery, and, by the next morning, the patient was alert and was extubated without difficulty. Steroids were tapered without hemodynamic compromise while the patient was in the ICU. Cardiology examination revealed no abnormalities. Because of the temporal association of blood transfusion with cardiovascular collapse, pRBC units were retested for antibodies and cultured. Both of these investigations were negative. Wound cultures again were positive for Staphylococcus epidermidis, and blood cultures were negative. The patient was started on daptomycin based on susceptibility profiles. Serum histamine levels taken during initial resuscitation in the operating room were normal. The serum tryptase level obtained at the same time was markedly elevated at >700 ng/mL (normal, <11.5 ng/mL), although this information was not available until several days later.
The patient underwent 2 additional surgeries during the same admission, including the prosthesis removal and tobramycin cement spacer placement, without incident. She was discharged home, again without incident. The patient was later evaluated by an outside allergist and underwent skin puncture and intradermal allergy testing. The results were consistent with a strong IgE-mediated hypersensitivity. Interestingly, she was found not to have a penicillin allergy.
Discussion
Vancomycin hypersensitivity reactions include the anaphylactoid reaction red man syndrome and a true IgE-mediated anaphylactic reaction. Red man syndrome is much more common, with reported rates in infected patients from 3.7% to 47%,4,5 when vancomycin is given at the suggested rate of 1 g over 1 hour. The reaction occurs because of histamine release from mast cells and basophils, and does not require previous sensitization.3 The rate of infusion is directly related to the development of symptoms, with 100% of patients developing symptoms in 1 study with rapid infusion (1 g over 10 min).6 Red man syndrome can typically be prevented by slowing the rate of infusion or by giving an H1 blocker.3 Anaphylaxis is more rare but can occur.7 Anaphylaxis is mediated by vancomycin-specific IgE, which requires previous exposure, as was the case with our patient. Interestingly, the patient had received vancomycin many times without any signs of a hypersensitivity reaction. Antihistamines are not effective in treating anaphylaxis, and epinephrine is the first-line agent.3 This was clearly demonstrated in this case, as there was a significant hemodynamic response to epinephrine and a negligible response to other vasopressors, specifically norepinephrine and vasopressin.
Most hypersensitivity reactions during the course of a surgical procedure occur with induction of anesthesia, with neuromuscular blocking agents and antibiotics being the most common causes.8 In our case, antibiotics were held until after deep cultures were taken. Given the time from induction to the anaphylactic reaction, it is unlikely the reaction resulted from the induction agents or the neuromuscular blocking agent. The possibility of a transfusion reaction was also investigated, since a unit of pRBC was still being transfused when symptoms began. An acute hemolytic transfusion reaction has the classic triad of fever, flank pain, and hemoglobinuria, and can also present as DIC.9 Under anesthesia, DIC can often be the presenting sign. In this case, a hemolytic transfusion reaction appeared very unlikely. All of the blood components the patient received were rechecked and found to be compatible, posttransfusion analysis showed no evidence of hemolysis in any sample, and the direct antiglobulin test was negative in all components.
To our knowledge, there are no reported cases of vancomycin-induced anaphylaxis with concomitant DIC. Symptoms of anaphylaxis after exposure to a possible antigen include rapid onset of hypotension or rapid onset of signs in at least 2 organ systems, including cutaneous, gastrointestinal, respiratory, and cardiovascular.10 Anaphylaxis with DIC is rare after exposure to any substance but has been reported.11 In fact, induction of systemic anaphylaxis in mice is known to cause DIC, with platelet-activating factor suggested as an important common mediator. A similar mechanism is suspected in humans.12
Confirmation of, and, certainly, prediction of, a vancomycin hypersensitivity reaction is difficult. Histamine levels can be used as a measure of mast-cell degranulation, but serum levels peak within 5 minutes and quickly return to baseline, limiting its diagnostic usefulness.3 Tryptase is an enzyme found in the secretory granules of mast cells. It has become an accepted marker of acute anaphylaxis, and, in vancomycin hypersensitivity reactions, can also distinguish between anaphylactic and anaphylactoid reactions.13 Tryptase levels peak 1 to 2 hours after the reaction, making this easier to measure than histamine, but results may not be available for several days, making it useful only in retrospect, as in our case. Skin testing is probably the best way to confirm a hypersensitivity reaction, although even this has been questioned with vancomycin because some find a high false-positive rate3, while others think the false-negative rate is likely too high.7 In this case, we were able to confirm our initial clinical suspicion with both an elevated tryptase level and a positive skin test.
Conclusion
We present a rare case of vancomycin anaphylaxis with DIC after repeated and prolonged previous exposure, which was treated acutely with hemodynamic resuscitation, replacement of blood components, steroids, and, most importantly, repeated boluses of epinephrine. Although several papers have described successful vancomycin desensitization7, this was fortunately not necessary in this case because the causative organism was sensitive to other acceptable antibiotics. The patient has been treated with systemic daptomycin and a tobramycin cement spacer without further incident.
Vancomycin is a glycopeptide antibiotic that exhibits bactericidal activity against gram-positive cocci. It is commonly recommended for surgical prophylaxis in cases of suspected bacterial resistance or penicillin allergy.1 Two main types of hypersensitivity reactions associated with vancomycin can have similar presentations. Red man syndrome is an anaphylactoid reaction caused by direct release of histamine from mast cells via a nonimmunologic mechanism, and is the more common of the 2 reactions. The second type is an anaphylactic reaction, which is an immunoglobulin E (IgE)–mediated systemic event and requires exposure to become sensitized.2,3
We present a patient who had received vancomycin on at least 12 occasions without incident. On this occasion, however, she developed a true anaphylactic reaction causing acute hemodynamic collapse that she survived after extensive resuscitation. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 55-year-old woman had a history of metastatic giant cell tumor of the right proximal tibia. She was originally treated 27 years ago for proximal tibial resection and reconstruction with a custom proximal tibial prosthesis. Four months later, she underwent resection of multiple pulmonary metastases via bilateral thoracotomies in a single surgical setting. After this, the patient had no evidence of recurrent metastatic disease. In subsequent years, the patient underwent multiple revision surgeries for problems such as hardware failure, patellar maltracking, and infection. The patient underwent 19 operations, including several nonorthopedic procedures. Because the patient had a rash after receiving penicillin as a child, she was thought to be allergic to penicillin. Consequently, she received vancomycin as antibiotic prophylaxis for the majority of these procedures. She also received extended courses of vancomycin of at least 6 weeks on 2 separate occasions. During her most recent revision procedure, 6 weeks prior to the procedure under discussion, the patient took vancomycin without incident. She was then found to have a prosthetic infection with Staphylococcus epidermidis, the same organism isolated in her previous infections, and she was advised to undergo a staged revision.
After a preoperative medical evaluation by her primary care physician, the patient was taken to the operating room for prosthesis removal and antibiotic spacer placement. She was anemic with a hemoglobin level of 8.8 g/dL; her erythrocyte sedimentation rate (ESR) was 102 mm/h (normal, <22 mm/h) and her C-reactive protein (CRP) was 38 mg/L (normal, <3 mg/L), but, otherwise, her laboratory values were normal, including a white blood cell count (WBC) of 8100/µL. Her electrocardiogram showed a normal sinus rhythm with nonspecific ST- and T-wave changes. Antibiotics were held until after cultures were taken. General endotracheal tube anesthesia was induced with 2 mg midazolam, 100 µg fentanyl, 180 mg propofol, and 140 mg succinylcholine, followed by 10 mg vecuronium, and maintained with desflurane. A tourniquet was not used per the surgeon’s routine. Dissection was carried down to the prosthesis and showed a small amount of purulent fluid. Transfusion of 1 unit of packed red blood cells (pRBC) was started during the approach owing to relatively low preoperative hemoglobin and significant blood loss. Approximately 500 mL of blood was lost during the approach secondary to the extensive dissection and the local inflammatory response from infection and recent surgery. After cultures were taken, and approximately 10 minutes after blood transfusion began, infusion of 1 g vancomycin in 250 mL normal saline was started via an infusion pump to run over 1 hour.
After infusion of 5 mL vancomycin, the patient’s blood pressure dropped from 117/63 mm Hg to 63/30 mm Hg; her pulse concurrently dropped from 90 to 50 beats/min. Vancomycin infusion was immediately stopped, anesthesia gasses were turned off, and patient received a bolus of normal saline with a second unit of pRBC. Patient received boluses of 0.5 mg to 1.0 mg epinephrine and 100 µg phenylephrine without sustained increase in blood pressure, which had dropped to 54/24 mm Hg, although the patient became tachycardic to ~120 beats/min after epinephrine. A sudden drop in end-tidal CO2 from 40s mm Hg to 20s mm Hg was also noted, indicating continuous but significantly decreased perfusion of the lungs.
We elected to abort the procedure, and a vacuum-assisted closure (VAC) dressing was applied to the open wound. After 15 minutes, the patient’s pulses, which had been faint, became impalpable, and cardiopulmonary resuscitation was initiated for about 7 minutes. The patient received 40 units vasopressin with repeated boluses of 0.5 mg epinephrine; a norepinephrine continuous infusion was started with the return of pulses. The patient also received 50 mg diphenhydramine, 125 mg methylprednisolone, and 20 mg famotidine for suspected anaphylaxis. A central venous line and arterial line were placed, and blood was drawn for laboratory analysis. The patient was noted to have clear breath sounds with no obvious rash, and her urine remained clear. Blood gas showed a profound metabolic acidosis, with pH of 7.09, base deficit of 5.9, and lactate of 8.9. The patient was treated with bicarbonate infusion. The patient was noted to ooze significantly during central venous line and arterial line placement, despite apparently normal coagulation during the surgical approach. Coagulation values were consistent with disseminated intravascular coagulation (DIC): prothrombin time, 57 s (international normalized ratio, 6.7); partial thromboplastin time, >200 s; thrombin time, 110 s; D-dimer, >10,000 ng/mL (normal, 0-200 ng/mL); and fibrinogen, <60 mg/dL (normal, 222-475 mg/dL). The patient’s thromboelastogram showed a flat line indicating an absence of clotting. Interestingly, the platelet count remained near the preoperative level at 338×103/µL. The patient’s blood pressure remained labile and was responsive primarily to epinephrine boluses, of which she received a total of 5 mg. After 1 hour of resuscitation, during which time the patient received a total of 5 L crystalloid and 3 units pRBC, the patient was transferred to the intensive care unit (ICU), intubated, and started on a titrated epinephrine infusion.
Upon arrival in the ICU, the patient quickly stabilized hemodynamically. She was weaned from all inotropic support within 2 hours of arrival. The patient lost 800 mL of blood through wound VAC over the first 12 hours postoperatively and required a total of 11 units of pRBC, 6 units fresh frozen plasma, and 3 units of pooled cryoprecipitate, all of which were compatible. Laboratory values, including arterial pH, lactic acid, and coagulation studies, normalized on the evening of surgery, and, by the next morning, the patient was alert and was extubated without difficulty. Steroids were tapered without hemodynamic compromise while the patient was in the ICU. Cardiology examination revealed no abnormalities. Because of the temporal association of blood transfusion with cardiovascular collapse, pRBC units were retested for antibodies and cultured. Both of these investigations were negative. Wound cultures again were positive for Staphylococcus epidermidis, and blood cultures were negative. The patient was started on daptomycin based on susceptibility profiles. Serum histamine levels taken during initial resuscitation in the operating room were normal. The serum tryptase level obtained at the same time was markedly elevated at >700 ng/mL (normal, <11.5 ng/mL), although this information was not available until several days later.
The patient underwent 2 additional surgeries during the same admission, including the prosthesis removal and tobramycin cement spacer placement, without incident. She was discharged home, again without incident. The patient was later evaluated by an outside allergist and underwent skin puncture and intradermal allergy testing. The results were consistent with a strong IgE-mediated hypersensitivity. Interestingly, she was found not to have a penicillin allergy.
Discussion
Vancomycin hypersensitivity reactions include the anaphylactoid reaction red man syndrome and a true IgE-mediated anaphylactic reaction. Red man syndrome is much more common, with reported rates in infected patients from 3.7% to 47%,4,5 when vancomycin is given at the suggested rate of 1 g over 1 hour. The reaction occurs because of histamine release from mast cells and basophils, and does not require previous sensitization.3 The rate of infusion is directly related to the development of symptoms, with 100% of patients developing symptoms in 1 study with rapid infusion (1 g over 10 min).6 Red man syndrome can typically be prevented by slowing the rate of infusion or by giving an H1 blocker.3 Anaphylaxis is more rare but can occur.7 Anaphylaxis is mediated by vancomycin-specific IgE, which requires previous exposure, as was the case with our patient. Interestingly, the patient had received vancomycin many times without any signs of a hypersensitivity reaction. Antihistamines are not effective in treating anaphylaxis, and epinephrine is the first-line agent.3 This was clearly demonstrated in this case, as there was a significant hemodynamic response to epinephrine and a negligible response to other vasopressors, specifically norepinephrine and vasopressin.
Most hypersensitivity reactions during the course of a surgical procedure occur with induction of anesthesia, with neuromuscular blocking agents and antibiotics being the most common causes.8 In our case, antibiotics were held until after deep cultures were taken. Given the time from induction to the anaphylactic reaction, it is unlikely the reaction resulted from the induction agents or the neuromuscular blocking agent. The possibility of a transfusion reaction was also investigated, since a unit of pRBC was still being transfused when symptoms began. An acute hemolytic transfusion reaction has the classic triad of fever, flank pain, and hemoglobinuria, and can also present as DIC.9 Under anesthesia, DIC can often be the presenting sign. In this case, a hemolytic transfusion reaction appeared very unlikely. All of the blood components the patient received were rechecked and found to be compatible, posttransfusion analysis showed no evidence of hemolysis in any sample, and the direct antiglobulin test was negative in all components.
To our knowledge, there are no reported cases of vancomycin-induced anaphylaxis with concomitant DIC. Symptoms of anaphylaxis after exposure to a possible antigen include rapid onset of hypotension or rapid onset of signs in at least 2 organ systems, including cutaneous, gastrointestinal, respiratory, and cardiovascular.10 Anaphylaxis with DIC is rare after exposure to any substance but has been reported.11 In fact, induction of systemic anaphylaxis in mice is known to cause DIC, with platelet-activating factor suggested as an important common mediator. A similar mechanism is suspected in humans.12
Confirmation of, and, certainly, prediction of, a vancomycin hypersensitivity reaction is difficult. Histamine levels can be used as a measure of mast-cell degranulation, but serum levels peak within 5 minutes and quickly return to baseline, limiting its diagnostic usefulness.3 Tryptase is an enzyme found in the secretory granules of mast cells. It has become an accepted marker of acute anaphylaxis, and, in vancomycin hypersensitivity reactions, can also distinguish between anaphylactic and anaphylactoid reactions.13 Tryptase levels peak 1 to 2 hours after the reaction, making this easier to measure than histamine, but results may not be available for several days, making it useful only in retrospect, as in our case. Skin testing is probably the best way to confirm a hypersensitivity reaction, although even this has been questioned with vancomycin because some find a high false-positive rate3, while others think the false-negative rate is likely too high.7 In this case, we were able to confirm our initial clinical suspicion with both an elevated tryptase level and a positive skin test.
Conclusion
We present a rare case of vancomycin anaphylaxis with DIC after repeated and prolonged previous exposure, which was treated acutely with hemodynamic resuscitation, replacement of blood components, steroids, and, most importantly, repeated boluses of epinephrine. Although several papers have described successful vancomycin desensitization7, this was fortunately not necessary in this case because the causative organism was sensitive to other acceptable antibiotics. The patient has been treated with systemic daptomycin and a tobramycin cement spacer without further incident.
1. Recommendation for the use of intravenous antibiotic prophylaxis in primary total joint arthroplasty. AAOS Information Statement 1027. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/advistmt/1027.asp. Published June 2004. Accessed October 28, 2015.
2. Duffy BL. Vancomycin reaction during spinal anesthesia. Anaesth Intensive Case. 2002;30(3):364-366.
3. Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001;35(11):1458-1464.
4. O’Sullivan TL, Ruffing MJ, Lamp KC, Warbasse LH, Rybak MJ. Prospective evaluation of red man syndrome in patients receiving vancomycin. J Infect Dis. 1993;168(3):773-776.
5. Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991;164(6):1180-1185.
6. Renz CL, Thurn JD, Finn HA, Lynch JP, Moss J. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med. 1999;27(9):1732-1737.
7. Kupstaite R, Baranauskaite A, Pileckyte M, Sveikata A, Kadusevicius E, Muckiene G. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas). 2010;46(1):30-33.
8. Lobera T, Audicana MT, Pozo MD, et al. Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain. J Investig Allergol Clin Immunol. 2008;18(5):350-356.
9. Berséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(suppl 1):114S-123S.
10. Schwartz LB. Systemic anaphylaxis, food allergy, and insect sting allergy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier; 2011:1633-1638.
11. Jung JW, Jeon EJ, Kim JW, et al. A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res. 2012;4(2):107-109.
12. Choi IH, Ha TY, Lee DG, et al. Occurrence of disseminated intravascular coagulation (DIC) in active systemic anaphylaxis: role of platelet-activating factor. Clin Exp Immunol. 1995;100(3):390-394.
13. Renz CL, Laroche D, Thurn JD, et al. Tryptase levels are not increased during vancomycin-induced anaphylactoid reactions. Anesthesiology. 1998;89(3):620-625.
1. Recommendation for the use of intravenous antibiotic prophylaxis in primary total joint arthroplasty. AAOS Information Statement 1027. American Academy of Orthopaedic Surgeons website. http://www.aaos.org/about/papers/advistmt/1027.asp. Published June 2004. Accessed October 28, 2015.
2. Duffy BL. Vancomycin reaction during spinal anesthesia. Anaesth Intensive Case. 2002;30(3):364-366.
3. Wazny LD, Daghigh B. Desensitization protocols for vancomycin hypersensitivity. Ann Pharmacother. 2001;35(11):1458-1464.
4. O’Sullivan TL, Ruffing MJ, Lamp KC, Warbasse LH, Rybak MJ. Prospective evaluation of red man syndrome in patients receiving vancomycin. J Infect Dis. 1993;168(3):773-776.
5. Wallace MR, Mascola JR, Oldfield EC 3rd. Red man syndrome: incidence, etiology, and prophylaxis. J Infect Dis. 1991;164(6):1180-1185.
6. Renz CL, Thurn JD, Finn HA, Lynch JP, Moss J. Antihistamine prophylaxis permits rapid vancomycin infusion. Crit Care Med. 1999;27(9):1732-1737.
7. Kupstaite R, Baranauskaite A, Pileckyte M, Sveikata A, Kadusevicius E, Muckiene G. Severe vancomycin-induced anaphylactic reaction. Medicina (Kaunas). 2010;46(1):30-33.
8. Lobera T, Audicana MT, Pozo MD, et al. Study of hypersensitivity reactions and anaphylaxis during anesthesia in Spain. J Investig Allergol Clin Immunol. 2008;18(5):350-356.
9. Berséus O, Boman K, Nessen SC, Westerberg LA. Risks of hemolysis due to anti-A and anti-B caused by the transfusion of blood or blood components containing ABO-incompatible plasma. Transfusion. 2013;53(suppl 1):114S-123S.
10. Schwartz LB. Systemic anaphylaxis, food allergy, and insect sting allergy. In: Goldman L, Schafer AI, eds. Goldman’s Cecil Medicine. 24th ed. Philadelphia, PA: Elsevier; 2011:1633-1638.
11. Jung JW, Jeon EJ, Kim JW, et al. A fatal case of intravascular coagulation after bee sting acupuncture. Allergy Asthma Immunol Res. 2012;4(2):107-109.
12. Choi IH, Ha TY, Lee DG, et al. Occurrence of disseminated intravascular coagulation (DIC) in active systemic anaphylaxis: role of platelet-activating factor. Clin Exp Immunol. 1995;100(3):390-394.
13. Renz CL, Laroche D, Thurn JD, et al. Tryptase levels are not increased during vancomycin-induced anaphylactoid reactions. Anesthesiology. 1998;89(3):620-625.
Necrotizing Fasciitis Caused by Cryptococcus gattii
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
Necrotizing fasciitis (NF) is a severe, rapidly spreading soft-tissue infection with high morbidity and mortality. Bacteriology in NF may be varied, and the etiology is often polymicrobial. It is important to consider the potential for fungal involvement despite its rarity. Cryptococcal NF has been reported in immunocompromised patients, with Cryptococcus neoformans being the most common offending organism.1-4
C neoformans is a basidiomycotic yeast that was previously considered a homogenous species.5,6 From the antigenic properties of its polysaccharide capsule, 3 main variants were described: C neoformans var. grubii, C neoformans var. neoformans, and C neoformans var. gattii. Subsequently, C neoformans var. gattii was found to be genetically and biochemically different from C neoformans. This discovery led to the distinction of C neoformans var. gattii as a separate species and it being renamed C gattii.6
C gattii was first recognized on Vancouver Island in 2001.7 Although C gattii is predominantly restricted to tropical and subtropical climates, its true epidemiology has been limited by diagnostic methods. C gattii can be diagnosed with laboratory culture media such as birdseed agars and L-canavanine-glycine-bromothymol (CGB) agar.6 However, most reports of Cryptococcus NF do not specify the culture media used to isolate Cryptococcus. In addition to culture media, molecular genotyping studies also allow for confirmation of the diagnosis of C gattii and have the added benefit of enabling identification of the molecular genotype. Nonetheless, in many clinical microbiology laboratories, Cryptococcus is not identified to the species level, much less to the molecular genotype.7 Given these diagnostic limitations and the fact that C gattii was only recently identified as a separate species, it is possible that any pre-2006 cases of NF attributed to C neoformans could in fact have been caused by C gattii.
In this article, we review the literature and report a case of NF of the hand that was caused by C gattii in a patient with diabetes. To our knowledge, this is the first reported case of NF caused by C gattii. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 73-year-old man was admitted with a 1-week history of swelling and pain in the dorsum of the left hand. He had been sitting in an outdoor eatery in Singapore when an insect bit the hand over the dorsum. Two days later, he consulted his family physician, who began treatment with oral amoxicillin/clavulanic acid. After 4 days of treatment, there was clinical progression of increased swelling and pain in the hand. Six days after initial injury, the patient presented to the department of orthopedic surgery.
Physical examination revealed diffuse, brawny, nonfluctuant swelling over the entire dorsum of the left hand (Figure 1). There was a 1×1-cm ruptured blister with some nonpurulent discharge just distal to the wrist joint. Neurovascular status and the extensor mechanism of the fingers were intact. The wrist joint had full range of motion. There was no fever.
Laboratory testing revealed an elevated white blood cell count (16.6×109/L), a C-reactive protein (CRP) level of 237 nmol/L, a random blood glucose level of 12.6 mmol/L, and a LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score of 7.8
Given the severe swelling, intravenous amoxicillin/clavulanic acid was started. The patient received a total of 3 doses before operative débridement of the left hand. Operative findings were NF of the hand, grayish necrotic fascia, and foul-smelling “dishwater” fluid. A single specimen of fascia from the surgical site was sent for examination. Histopathologic examination of formalin-fixed, paraffin-embedded tissue revealed necrotizing suppurative inflammation with fungal organisms present (Figures 2, 3).
Tissue cultures were obtained during surgery. The organism grew as scanty, small, wet-looking colonies on sheep blood agar after 48 hours of incubation. Microscopy revealed an oval yeast. The organism was identified and reported as C gattii by matrix-assisted laser desorption ionization–time of flight mass spectrometry (MALDI-TOF MS; Biotyper 2.0.1 software; Bruker Daltonics), with a score of 1.914.9 All other intraoperative cultures for aerobic and anaerobic bacteria were negative. Molecular genotyping was performed with polymerase chain reaction assay to identify the molecular subtype.10C gattii genotype VGII was isolated. A cryptococcal serum antigen assay was positive at 1:256.
A series of tests was performed to screen for disseminated disease. Blood cultures were negative for fungus. Chest radiography and computed tomography of the brain did not show any pulmonary or cerebral involvement. Cerebrospinal fluid was not available for examination, as the patient declined lumbar puncture. Blood tests included a negative result for human immunodeficiency virus (HIV). The patient was found to have previously undiagnosed diabetes mellitus (hemoglobin A1c, 7.9%). T-cell counts and ratios were normal.
The patient was started on intravenous amphotericin B 60 mg/d and flucytosine 500 mg every 6 hours for 3 weeks. Oral fluconazole 400 mg every morning was also given (intended duration, 6 mo). Given that diabetes was newly diagnosed, the patient was treated with metformin; his capillary blood glucose level remained stable during his inpatient stay.
Four débridements of the dorsal hand wound were performed—the first on day of admission and the other 3 on hospitalization days 3, 7, and 18 (Figure 4). Subsequent wound resurfacing with a split skin graft harvested from the forearm was performed on hospitalization day 22. After surgery, the hand was dressed with a bulky cotton dressing. Five days after the patient was discharged, during review in the outpatient clinic, the skin graft was noted to be taking well. The patient did not attend postoperative physical therapy. He was maintained on metformin and given a follow-up clinic appointment for his diabetes. Four months after surgery, the wound was completely healed, and normal functional use of the hand recovered.
Discussion
NF is a severe soft-tissue infection with potential for rapid progression. Surgical débridement should be performed urgently to reduce the chance of morbidity and mortality.11 The initial classification by Giuliano and colleagues12 was based on bacteriology and included type I (anaerobic species in combination with a facultative species) and type II (monomicrobial usually involving group A β-hemolytic Streptococcus). This classification was modified by Morgan13 to include gram-negative organisms as well as fungal organisms (Table 1).
Fungal NF is rare, with Candida, Apophysomyces, and Cryptococcus described in the literature.1,14,15 Fungal infections tend to occur in immunocompromised patients; risk factors are steroid immunosuppression, poorly controlled diabetes, and peripheral vascular disease.16 Some zygomycetes may also affect immunocompetent patients.15
C gattii is an encapsulated yeast organism that is genetically and biochemically distinct from C neoformans. It is endemic to tropical parts of Africa and Australia. Its main environmental sources are eucalyptus trees (Eucalyptus camaldulensis, Eucalyptus tereticornis) and decaying hollows in living trees.17 In addition, there have been reports of isolation of C gattii from insect frass,18 which would make infection by an insect bite a possible transmission route. Worldwide distribution of this pathogen has increased recently, with outbreaks noted on Vancouver Island and in areas in Canada and the northwest United States.7
The true incidence of NF secondary to C gattii is difficult to determine. C gattii was only recently identified as a separate species, and pre-2006 cases of NF attributed to C neoformans may instead have been caused by C gattii. Misidentification has been compounded by the fact that the tests required for accurate diagnosis of C gattii infection may not be readily available in many clinical microbiology laboratories. Cryptococcus can be identified with various methods, including direct microscopy, culturing of tissue or fluid samples, and measurement of cryptococcal serum antigen. However, tests such as specific culture media, mass spectrometry, and molecular typing studies are required to determine cryptococcal species. L-canavanine-glycine-bromothymol blue (CGB) agar is a medium that is often used to differentiate C gattii from C neoformans because of the ability of C gattii to produce a blue appearance.6 Modern techniques, such as MALDI-TOF MS, have also been used to successfully distinguish between C gattii and C neoformans.9 MALDI-TOF MS identifies species on the basis of characteristic protein spectra extracted from whole cells. Using commercial and supplemental reference libraries, the system compares signal matches in the reference spectrum with Cryptococcus entries in the library—allowing rapid and accurate identification of cryptococcal species. However, this diagnostic method is limited by availability of adequate Cryptococcus entries in the reference library and by the high cost of acquiring the machine.
Serotyping is based on the antigenic property of the capsule and was once used to differentiate C neoformans into its 3 main varieties: var. neoformans, var. grubii, var. gattii. However, when it was realized that the antigenic property of the strain can be unstable and that there are hybrids containing more than 1 serotype, serotyping was abandoned as a species-differentiation test.6 The current gold standard for species differentiation is molecular genotyping. Molecular genotyping studies can confirm the diagnosis of C gattii infection and allow differentiation of C gattii into its 4 main molecular types: VGI, VGII, VGIII, VGIV. Using methods such as polymerase chain reaction (PCR) and restriction fragment length polymorphism (RFLP) analysis, molecular typing allows for specific epidemiology charting of C gattii genotypes.7
Although the transmission route for cryptococcal infection is mainly respiratory, direct inoculation has been reported as well.19 Cutaneous lesions, which occur in 5% to 20% of cryptococcal infections, often present in the head and neck.2,20,21 Primary cutaneous infections from cryptococcosis are rare, and cutaneous manifestations are often a sign of disseminated disease. Disseminated disease is defined as the involvement of 2 or more noncontiguous sites or evidence of high fungal burden based on cryptococcal antigen titer of more than 1:512.12 It is important to exclude disseminated disease in all cases of cryptococcosis, as it may be fatal.20 The neural and pulmonary systems should be screened.22 Cellulitis from cryptococcosis is almost always limited to immunocompromised patients, though there are reports of crytococcal cutaneous disease in immunocompetent patients.3,15 Interestingly, though C neoformans often affects immunocompromised patients, the emerging pathogen of C gattii affects immunocompetent patients.7,17,23 Our patient’s undiagnosed diabetes may have been a risk factor for cryptococcal infection. His cryptococcal antigen titer was 1:256, with no evidence of other sites of involvement. We therefore believe this to be a rare case of direct inoculation secondary to an insect bite.
The literature includes 12 reported cases of NF secondary to Cryptococcus (Table 2), all C neoformans. Of these cases, 9 involved immunosuppression, and most of these patients were on long-term steroid treatment after organ transplantation. The most common infection site was the lower extremity. These cases of cryptococcal NF show that immunosuppression, and long-term steroid use in particular, is an important risk factor. The mortality rate for these reviewed cases was 41.6% (5/12). According to the literature, the mortality rates for patients with cryptococcal soft-tissue infections24 and posttransplant patients with cryptococcal NF21 were 37.5% and 60%, respectively. We believe the mortality rate in our reviewed cases likely was confounded by the fact that most of the patients were posttransplant patients on long-term immunosuppression.
Of the 12 patients, 5 had primary cutaneous disease. There seems to be no relationship between outcome and dissemination of disease. In addition, there is a paucity of literature on the effect of disseminated disease and cryptococcal soft-tissue infections. Therefore, no firm conclusions can be drawn regarding the effects of disseminated disease on severity of cryptococcal soft-tissue infection.
Treatment of cryptococcal NF involves a combination of surgical débridement and long-term antifungal therapy. Surgical débridement of NF includes delineating the extent of infection with complete surgical excision of the affected tissue.25 The aims of surgery should be to remove all unhealthy tissue, identify the offending organism, and plan for resurfacing or reconstruction of the afflicted extremity. Intraoperative-tissue histology should be performed to confirm the diagnosis of NF. Histology can be used to demonstrate cryptococcal infection. The diagnosis of cryptococcal infection can be aided with fungal cultures, and therefore we recommend that tissue cultures be sent not only for routine aerobic/anaerobic bacteria but also for mycobacteria and fungal organisms. Laboratory tests that aid in diagnosis include serum cryptococcal antigen titer.
The current treatment recommendation for cryptococcal disease in patients who are not HIV-positive or transplant hosts is amphotericin B deoxycholate 0.7 to 1.0 mg/kg/d plus flucytosine 100 mg/kg/d for at least 4 weeks.22 The regimen period may be shortened to 14 days for patients at low risk of treatment failure. Fluconazole should be given as maintenance therapy (200 mg/d) for 6 to 12 months. There is no compelling evidence for immunoglobulin therapy for cryptococcal disease.22
Conclusion
NF caused by Cryptococcus is rare. A high level of suspicion, and intraoperative specimens for histology and fungal microscopy and culture, can help in establishing the diagnosis. Molecular genotyping remains the diagnostic method of choice for NF secondary to Cryptococcus. Effective treatment consists of aggressive surgical débridement and antifungal therapy.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
1. Marcus JR, Hussong JW, Gonzalez C, Dumanian GA. Risk factors in necrotizing fasciitis: a case involving Cryptococcus neoformans. Ann Plast Surg. 1998;40(1):80-83.
2. Huang KC, Tu YK, Lee KF, Huang TJ, Wen-Wei Hsu R. Disseminated cryptococcosis presented as necrotizing fasciitis of a limb. J Trauma. 2007;63(2):E44-E46.
3. Capoor MR, Khanna G, Malhotra R. Disseminated cryptococcosis with necrotizing fasciitis in an apparently immunocompetent host: a case report. Med Mycol. 2008;46:269-273.
4. Adachi M, Tsurata D, Imanishi H, Ishii M, Kobayashi H. Necrotizing fasciitis caused by Cryptococcus neoformans in a patient with pemphigus vegetans. Clin Exp Dermatol. 2009;34(8):e751-e753.
5. Enache-Angoulvant A, Chandenier J, Symoens F, et al. Molecular identification of Cryptococcus neoformans serotypes. J Clin Microbiol. 2007;45(4):1261-1265.
6. Kwon-Chung KJ, Varma A. Do major species concepts support one, two or more species within Cryptococcus neoformans? FEMS Yeast Res. 2006;6(4):657-687.
7. Datta K, Bartlett KH, Baer R, et al; Cryptococcus gattii Working Group of the Pacific Northwest. Spread of Cryptococcus gattii into Pacific Northwest region of the United States. Emerg Infect Dis. 2009;15(8):1185-1191.
8. Wong CH, Khin LW, Heng KS, Tan KC, Low CO. The LRINEC (Laboratory Risk Indicator for Necrotizing Fasciitis) score: a tool for distinguishing necrotizing fasciitis from other soft tissue infections. Crit Care Med. 2004;32(7):1535-1541.
9. McTaggart LR, Lei E, Richardson SE, Hoang L, Fothergill A, Zhang SX. Rapid identification of Cryptococcus neoformans and Cryptococcus gattii by matrix-assisted laser desorption ionization-time of flight mass spectrometry. J Clin Microbiol. 2011;49(8):3050-3053.
10. Meyer W, Castañeda A, Jackson S, Huynh M, Castañeda E; IberoAmerican Cryptococcal Study Group. Molecular typing of IberoAmerican Cryptococcus neoformans isolates. Emerg Infect Dis. 2003;9(2):189-195.
11. Wong CH, Chang HC, Pasupathy S, Khin LW, Tan JL, Low CO. Necrotizing fasciitis: clinical presentation, microbiology and determinants of mortality. J Bone Joint Surg Am. 2003;85(8):1454-1460.
12. Giuliano A, Lewis F Jr, Hadley K, Blaisdell FW. Bacteriology of necrotizing fasciitis. Am J Surg. 1977;134(1):52-57.
13. Morgan MS. Diagnosis and management of necrotising fasciitis: a multiparametric approach. J Hosp Infect. 2010;75(4):249-257.
14. Buchanan PJ, Mast BA, Lottenberg L, Kim T, Efron PA, Ang DN. Candida albicans necrotizing soft tissue infection: a case report and literature review of fungal necrotizing soft tissue infections. Ann Plastic Surg. 2013;70(6):739-741.
15. Jain D, Kumar Y, Vasishta RK, Rajesh L, Pattari SK, Chakrabarti A. Zygomycotic necrotizing fasciitis in immunocompetent patients: a series of 18 cases. Modern Pathol. 2006;19(9):1221-1226.
16. Fontes RA Jr, Ogilvie CM, Miclau T. Necrotizing soft-tissue infections. J Am Acad Orthop Surg. 2000;8(3):151-158.
17. Sorrell TC. Cryptococcus neoformans variety gattii. Med Mycol. 2001;39(2):155-168.
18. Kidd SE, Sorrell TC, Meyer W. Isolation of two molecular types of Cryptococcus neoformans var. gattii from insect frass. Med Mycol. 2003;41(2):171-176.
19. Neuville S, Dromer F, Morin O, Dupont B, Ronin O, Lortholary O; French Cryptococcosis Study Group. Primary cutaneous cryptococcosis: a distinct clinical entity. Clin Infect Dis. 2003;36(3):337-347.
20. Basaran O, Emiroglu R, Arikan U, Karakayali H, Haberal M. Cryptococcal necrotizing fasciitis with multiple sites of involvement in the lower extremities. Dermatol Surg. 2003;29(11):1158-1160.
21. Baer S, Baddley JW, Gnann JW, Pappas PG. Cryptococcal disease presenting as necrotizing cellulitis in transplant recipients. Transpl Infect Dis. 2009;11(4):353-358.
22. Perfect JR, Dismukes WE, Dromer F, et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis. 2010;50(3):291-322.
23. Chan M, Lye D, Win MK, Chow A, Barkham T. Clinical and microbiological characteristics of cryptococcosis in Singapore: predominance of Cryptococcus neoformans compared with Cryptococcus gattii. Int J Infect Dis. 2014;26:110-115.
24. Gave AA, Torres R, Kaplan L. Cryptococcal myositis and vasculitis: an unusual necrotizing soft tissue infection. Surg Infect. 2004;5(3):309-313.
25. Wong CH, Yam AK, Tan AB, Song C. Approach to debridement in necrotizing fasciitis. Am J Surg. 2008;196(3):e19-e24.
26. Bégon E, Bachmeyer C, Thibault M, et al. Necrotizing fasciitis due to Cryptococcus neoformans in a diabetic patient with chronic renal insufficiency. Clin Exp Dermatol. 2009;34(8):935-936.
27. Doorenbos-Bot AC, Hooymans JM, Blanksma LJ. Periorbital necrotising fasciitis due to Cryptococcus neoformans in a healthy young man. Doc Ophthalmol. 1990;75(3-4):315-320.
28. Yoneda T, Itami Y, Hirayama A, Saka T, Yoshida K, Fujimoto K. Cryptococcal necrotizing fasciitis in a patient after renal transplantation—a case report. Transplant Proc. 2014;46(2):620-622.
Multifocal Langerhans Cell Histiocytosis in an Adult
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
Eosinophilic granuloma (EG) is the most common benign form of Langerhans cell histiocytosis (LCH). Initially described by Lichtenstein in 1953, LCH encompasses a triad of proliferative granulomatous disorders primarily affecting children: EG, Hand-Schüller-Christian disease, and Letterer-Siwe disease.1 Lichtenstein first termed the disease histiocytosis X, after recognizing that the 3 syndromes had the same histology.1 The term was updated after the clonal proliferation of Langerhans cells in the pathogenesis of the disease was discovered.
As LCH is generally considered a pediatric disease, there is little in the literature regarding adult-onset LCH. The incidence of LCH in adults is reported as 1 to 2 cases per million, significantly lower than that in children.2,3 Two studies have reported the mean age at diagnosis in adults as the fourth decade of life, and have suggested a male predominance.4,5 The vast majority of adult LCH cases described are simple EG, with very few cases of multisystem disseminated disease reported.5
Adult patients with LCH typically present with solitary lesions in bone. Approximately 10% of cases have extraosseous involvement, with the lung being the most common site.6 Lesions tend to be unifocal, with fewer than 10 reports describing multifocal EG.1,7-13 The axial skeleton is most frequently involved, with the majority of lesions occurring in the skull, ribs, vertebrae, or mandible.14 While less common, the femur, humerus, and clavicle are most often involved when the appendicular skeleton is affected.5
In a literature review, a few case reports describe adult-onset EG of the skull. Only 5 case reports since the 1970s describe adult patients with EG of the femur. We present a rare case of multifocal EG in a 48-year-old woman with lesions of the femur and skull, as well as a review of the literature. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 48-year-old woman presented with progressive right knee pain that was exacerbated by weight-bearing. She denied trauma, fevers, fatigue, or weight change. Her history was significant for an EG of the skull, excised at an outside institution 2 years prior to presentation. The patient also admitted to recent onset of right-sided skull pain, near the region of her previous surgery.
Physical examination demonstrated tenderness to palpation and fullness over the right medial distal femur and a normal neurovascular examination of the right lower extremity. Radiographs of the knee showed a cortically based, lytic, destructive lesion involving the medial femoral condyle, with soft-tissue extension (Figures 1A, 1B). Magnetic resonance imaging (MRI) of the right knee showed the lesion, with extraosseous soft-tissue extension (Figures 2A, 2B). The mass was isointense to muscle on T1-weighted images and hyperintense on T2-weighted images. Technetium bone scanning showed increased uptake in the right femur and the right skull (Figures 3A, 3B). MRI of the brain confirmed a new lesion in the right diploic space, distinct from the previous EG lesion site (Figures 4A-4D). An ultrasound-guided biopsy of the femur was performed and was consistent with EG.
After reevaluation and clearance by her neurosurgeon, the patient underwent curettage and allografting of the femoral lesion, with prophylactic internal fixation using a titanium distal femoral locking plate (Figure 5). Intraoperative frozen section was consistent with EG, which was confirmed with additional immunohistochemical workup (Figures 6A-6D).
The patient recovered uneventfully and follow-up radiographs showed restoration of the bony cortex of the medial femoral condyle (Figure 7). The second skull lesion, which was also consistent with EG, was excised by her neurosurgeon.
The patient remained asymptomatic until 2 years later, when she began experiencing mild pain in her right distal thigh and knee. Radiographs showed a new lytic focus in the right distal metadiaphysis (Figure 8) which was not present on her last radiograph 6 months prior. A computed tomography (CT) scan showed a lytic lesion involving the right distal femur medullary canal with cortical thinning and destruction, most pronounced posteriorly (Figures 9A, 9B). There was also an extraosseous soft-tissue component to the lesion. Bone scan showed increased uptake in the area of the new lesion. There was no increased uptake elsewhere, including the medial distal femur at the site of the old lesion, to suggest other lesions, and no increased uptake in the skull.
Given that the location of the lesion was distinct from the prior site of curettage and bone grafting, it was thought to be consistent with a new EG lesion. The patient underwent CT-guided biopsy, with simultaneous intralesional corticosteroid injection to treat the lesion when on-site pathology confirmed the etiology. Further surgical management was deemed unnecessary because internal fixation was present and spanned the new lesion. Final analysis of the fine-needle aspirate of the new lesion was positive for numerous eosinophils and histiocytes, consistent with EG.
At 6-week follow-up after the intralesional steroid injection, the patient’s pain continued to abate, and she was ambulating with crutches. Repeat CT scan of the right distal femur showed improvement of the extraosseous soft-tissue component, while the lucency in the femur itself remained unchanged. The decision was made to proceed with a second intralesional corticosteroid injection under CT guidance. The patient’s symptoms continued to improve, and repeat imaging 1 year after her steroid injections showed substantial bony healing with reconstitution of her cortical bone (Figures 10A-10E).
The patient had had 4 distinct tumors consistent with EG and was referred to a medical oncologist for further workup. The patient began treatment with zoledronic acid to prevent development of further lesions. At most recent follow-up, the patient was 18 months out from her second intralesional corticosteroid injection and was doing very well. She reported being pain-free and was walking 3 to 4 miles per week without gait aids. There was no evidence of new disease. The medial distal femur lesion was completely healed, and the distal metaphyseal lesion was nearly healed, with very little residual evidence of lesions.
Discussion
Adult-onset multifocal EG is a rare entity. Most affected patients develop lesions in the axial skeleton, with the skull, mandible, and vertebrae most commonly involved.14 Only 5 cases of femoral EG have been reported, one of which was multifocal.11,14-17
Of these patients, 3 were between the ages of 33 and 53 years and had insidious onset of hip pain that failed conservative management.14,15,17 Further imaging and biopsy revealed unifocal EG in the proximal femur in each case. Each patient received a different form of treatment, including curettage and radiation, radiofrequency ablation, and/or physical therapy. At the time of publication, all patients had reported improvement in their clinical symptoms.14,15,17 The fourth patient was a man with human immunodeficiency virus (HIV) with 3 months of progressive thigh pain. Further evaluation found an isolated EG of the femoral diaphysis that progressed to pathologic fracture. He was treated with curettage and intramedullary nailing, and had improved symptoms and radiographic signs of healing at 30-month follow-up.16
An interesting case by Kerzl and colleagues11 reported a 63-year-old woman with a 24-year history of multiple symmetric lesions of the femora, leading to multiple pathologic fractures. Like our patient, her initial lesion was in the skull. Initial pathology specimens led to the diagnosis of EG. However, as the patient aged, she developed symptoms of diabetes insipidus and xanthelasma, which led to reevaluation of histology from 3 bony lesions. The patient was determined to have multifocal EG of the skull and femur, with simultaneous occurrence of Erdheim-Chester disease, which also causes bone lesions in addition to diabetes insipidus and xanthelasma.11
Though LCH was initially described more than 50 years ago, many aspects of LCH remain an enigma, especially in adults. The etiology of the disease is poorly understood. Controversy exists regarding whether LCH is primarily an immunoregulatory, neoplastic, or reactive disorder. The vast majority of adult cases described in the literature are EG, with very few cases of multisystem disseminated disease reported.5
The spectrum of disorders constituting LCH is heterogenous. Eosinophilic granuloma is the most common form, reportedly accounting for 60% to 70% of all cases, usually presenting as solitary bone lesions.6 Eosinophilic granuloma refers to the localized form of LCH, in which the disease is limited to bone or lung.18 This is the least aggressive form of the disease, with the most favorable prognosis. Hand- Schüller-Christian disease is a chronic, recurring form of LCH, with disseminated disease, affecting both bone and extraskeletal sites. Hand-Schüller-Christian disease is known for the classic triad of diabetes insipidus, exophthalmos, and destructive bone lesions. Patients may also present with otitis media or neurologic complaints from pathologic vertebral fractures. Letterer-Siwe disease refers to the acute, disseminated, fulminant form of LCH. This is the least common form of LCH and is predominately described in young children. Patients present with hepatosplenomegaly, lymphadenopathy, skin rash, fever, anemia, and thrombocytopenia.19 It is rapidly progressive, leading to multiorgan dysfunction and death within 1 to 2 years.18
The classification of LCH follows the Histiocyte Society guidelines developed from multicenter randomized trials in children.3 Classification is based on affected organs and is divided into 2 categories: single-system disease or multisystem disease. Single-system disease may be single site (bone, skin, or solitary lymph node) or multisite (multifocal bone disease or multiple lymph nodes). Multisystem disease is further classified into low-risk or risk groups. The low-risk group involves disseminated disease without involvement of risk organs (lungs, liver, spleen, and hematopoietic system). Involvement of 1 or more risk organs places the patient in the risk group, associated with the least favorable prognosis.3
In adults, the most common presenting symptoms are local pain from bony involvement, weight loss, and fever. Bony lesions most often occur in the skull, especially in the jaw. Long bones are less frequently involved, with lesions occurring in the long bones in approximately 17% of patients.3 The rib has also been reported as a common site of involvement in adults.5 Similar to children, diabetes insipidus remains a classic manifestation of LCH because of pituitary gland involvement. Other common symptoms of LCH in adults are cough, dyspnea, and chest pain from pulmonary involvement. Up to 20% to 30% of adult LCH patients have isolated pulmonary lesions, although pulmonary LCH may also occur as part of multisystem disease (risk group).3,4,20
Eosinophilic granuloma bone lesions have a variety of radiographic appearances but most commonly appear as lytic lesions. They often mimic aggressive lesions with permeative bone destruction, periostitis, ill-defined borders, and cortical erosion. Most lesions arise in the medullary space but can present as a destructive, cortically based lesion, as it did in our patient’s first femoral lesion. The differential diagnosis for a lytic medullary bone lesion includes benign entities, such as nonossifying fibromas, bone cysts, or osteomyelitis, but also includes malignant tumors, such as metastases, Ewing sarcoma, and lymphoma. A destructive, cortically based lesion in an adult should raise a very high suspicion for metastatic carcinoma until proven otherwise. Other diagnostic considerations for a cortically based lesion include chondromyxoid fibroma and surface bone lesions, such as surface chondroma and osteoma, or osteosarcoma (parosteal and periosteal). In the skull, lesions commonly erode the outer table more than the inner table (the typical “beveled-edge” appearance). Skull lesions also may have a small, central, dense focus within the lytic lesion (“button sequestrum”).
Bone scanning is often not as sensitive in detecting EG lesions compared with other bone tumors, although in our patient the bone scan was positive. In patients with a negative bone scan but a high index of suspicion, a radiographic skeletal survey should be obtained to rule out other lesions. MRI typically shows T2-hyperintense, T1-hypointense lesions with surrounding bone marrow edema and variable contrast enhancement, which is relatively nonspecific. The high sensitivity of MRI allows accurate delineation of the extent of the lesions and evaluates for the presence of an extraosseous soft-tissue component. Biopsy is generally necessary to establish a definitive histologic diagnosis. In our patient, despite her history of biopsy-proven EG, the aggressive appearance of a destructive, cortically based lesion made obtaining a biopsy critical to establish a definitive diagnosis in this case.
The histopathologic examination of the tissue from our patient was typical of that seen in patients with EG. It revealed tissue fragments with diffuse sheets of histiocytes displaying nuclear grooves, admixed numerous eosinophils with eosinophilic microabscesses, and scattered lymphocytes (Figures 6A, 6B). There were areas of necrosis, raising the possibility of osteomyelitis. However, the presence of classic histomorphologic features of LCH in the majority of the tissue fragments, along with CD1a- and S100-positivity in the histiocytes, confirmed the diagnosis of LCH (Figures 6C, 6D). Although not highly specific, a positive CD1a immunostain with the described histomorphologic findings in the proper clinical setting is often considered sufficient for LCH diagnosis. S100 is an important adjunct immunostain in the evaluation of histiocytic disorders. A positive S100 immunostain helps identify histiocytes, which are also CD1a-positive, because the latter immunostain can also be positive in some lymphomas and thymomas.21
After diagnosis of LCH has been confirmed, staging includes radiographs of any suspicious bone lesions, chest radiograph, bone scan, abdominal ultrasound, routine laboratory studies, and chest CT if pulmonary LCH is suspected.
The optimal treatment strategy for adult patients has not been clearly defined, and current strategies for LCH vary depending on organ involvement and extent of disease. Therapeutic options include observation, local treatment with steroids, local excision with curettage with or without bone grafting, chemotherapy, immunomodulation, irradiation, and stem cell transplantation in advanced disease. In general, patients who benefit from systemic therapy, such as chemotherapy or immunomodulation, include those with multisystem disease, refractory or recurrent lesions, and multifocal skeletal involvement.22
Patients with more limited disease, such as EG of bone, may undergo observation or local intralesional treatment. Eosinophilic granuloma of bone may resolve spontaneously and commonly does so when it is located in the pediatric spine. However, the therapeutic approach in adults with EG is controversial, given that spontaneous resolution is less likely to occur in the skeletally mature. Plasschaert and colleagues23 reported a recurrence rate of 26% in skeletally mature patients with EG of bone treated with biopsy followed by curettage with or without grafting. In the skeletally immature group, there were no clinical or radiographic signs of recurrence in the 2-year follow-up period.23 Thus, treatment in the adult population must be considered separate from the skeletally immature and in the appropriate clinical context. Depending on the location of the lesion, patients may become symptomatic or be at risk for pathologic fracture. In such circumstances, curettage with or without bone grafting and prophylactic internal fixation may be indicated. Other treatments, such as intralesional infiltration with corticosteroids, have been reported, but the role of such treatment in adults is undetermined.24,25 Radiation is typically not recommended in single-system disease unless a vital organ is threatened.26 Overall, patients with single-system disease have an excellent prognosis, and treatment should be determined on an individual basis.3
Eosinophilic granuloma represents less than 1% of all bone tumors, and adult presentation is very rare. The differential diagnosis of lytic bone lesions is broad and includes metastatic carcinoma, lymphoma/myeloma, osteomyelitis, osteoblastoma, aneurysmal bone cyst, and Ewing sarcoma. While EG is more common and easily diagnosed in children, it should be considered in the differential diagnosis in adults, so that the appropriate diagnostic workup and treatment can be performed.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
1. Lahiani D, Hammami BK, Maâloul I, et al. Multifocal Langerhans cell histiocytosis of bone: late revelation in a 76-year-old woman. Rev Med Interne. 2008;29(3):249-251.
2. Baumgartner I, von Hochstetter A, Baumert B, Luetolf U, Follath F. Langerhans’-cell histiocytosis in adults. Med Pediatr Oncol. 1997;28(1):9-14.
3. Stockschlaeder M, Sucker C. Adult Langerhans cell histiocytosis. Eur J Haematol. 2006;76(5):363-368.
4. Aricò M, Girschikofsky M, Généreau T, et al. Langerhans cell histiocytosis in adults. Report from the International Registry of the Histiocyte Society. Eur J Cancer. 2003;39(16):2341-2348.
5. Islinger RB, Kuklo TR, Owens BD, et al. Langerhans’ cell histiocytosis in patients older than 21 years. Clin Orthop Relat Res. 2000;379:231-235.
6. Key SJ, O’Brien CJ, Silvester KC, Crean SJ. Eosinophilic granuloma: resolution of maxillofacial bony lesions following minimal intervention. Report of three cases and a review of the literature. J Craniomaxillofac Surg. 2004;32(3):170-175.
7. Bodner G, Kreczy A, Rachbauer F, Baechter O, Peer S. Eosinophilic granuloma of the bone: ultrasonographic imaging. Australas Radiol. 2002;46(4):418-421.
8. Boutsen Y, Esselinckx W, Delos M, Nisolle JF. Adult onset of multifocal eosinophilic granuloma of bone: a long-term follow-up with evaluation of various treatment options and spontaneous healing. Clin Rheumatol. 1999;18(1):69-73.
9. Corti F, Valicenti A, Bertolucci D, Bruno J, Gustinucci R. Multifocal Langerhans cell granulomatosis. Report of a clinical case. Minerva Med. 1994;85(7-8):413-416.
10. Demirci I. Adult eosinophilic granuloma of the lumbar spine with atypical dissemination. Case report: a long-term follow-up. Zentralbl Neurochir. 2004;65(2):84-87.
11. Kerzl R, Eyerich K, Eberlein B, et al. Parallel occurrence of Erdheim-Chester disease and eosinophilic granuloma in the same patient. J Eur Acad Dermatol Venereol. 2009;23(2):224-226.
12. Nguyen BD, Roarke MC, Chivers SF. Multifocal Langerhans cell histiocytosis with infiltrative pelvic lesions: PET/CT imaging. Clin Nucl Med. 2010;35(10): 824-826.
13. Scolozzi P, Lombardi T, Monnier P, Jaques B. Multisystem Langerhans’ cell histiocytosis (Hand-Schuller-Christian disease) in an adult: a case report and review of the literature. Eur Arch Otorhinolaryngol. 2004;261(6):326-330.
14. King JJ, Melvin JS, Iwenofu OH, Fox EJ. Thigh pain in a 53-year-old woman. Clin Orthop Relat Res. 2009;467(6):1652-1657.
15. Hair LC, Deyle GD. Eosinophilic granuloma in a patient with hip pain. J Orthop Sports Phys Ther. 2011;41(2):119.
16. Panayiotakopoulos GD, Sipsas NV, Kontos A, et al. Eosinophilic granuloma of the femur in an HIV-1 positive patient. AIDS Patient Care STDS. 2002;16(3):103-106.
17. Rodrigues RJ, Lewis HH. Eosinophilic granuloma of bone. Review of literature and case presentation. Clin Orthop Relat Res. 1971;77:183-192.
18. Stull MA, Kransdorf MJ, Devaney KO. Langerhans cell histiocytosis of bone. Radiographics. 1992;12(4):801-823.
19. Lichtenstein L. Histiocytosis X (eosinophilic granuloma of bone, Letterer-Siwe disease, and Schueller-Christian disease). Further observations of pathological and clinical importance. J Bone Joint Surg Am. 1964;46:76-90.
20. Götz G, Fichter J. Langerhans’-cell histiocytosis in 58 adults. Eur J Med Res. 2004;9(11):510-514.
21. Cheng KL, Glu PG, Weiss LM. Hematopoeitic tumors. In: Peiguo C, Weiss L, eds. Modern Immunohistochemistry. New York, NY: Cambridge University Press; 2009:503.
22. Broadbent V, Gadner H. Current therapy for Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12(2):327-338.
23. Plasschaert F, Craig C, Bell R, Cole WG, Wunder JS, Alman BA. Eosinophilic granuloma. A different behaviour in children than in adults. J Bone Joint Surg Br. 2002;84(6):870-872.
24. Capanna R, Springfield DS, Ruggieri P, et al. Direct cortisone injection in osinophilic granuloma of bone: a preliminary report on 11 patients. J Pediatr Orthop. 1985;5(3):339-342.
25. Egeler RM, Thompson RC Jr, Voûte PA, Nesbit ME Jr. Intralesional infiltration of corticosteroids in localized Langerhans’ cell histiocytosis. J Pediatr Orthop. 1992;12(6):811-814.
26. Ladisch S, Gadner H. Treatment of Langerhans cell histiocytosis–evolution and current approaches. Br J Cancer Suppl. 1994;23:S41-S46.
Lipoma of the Tendon Sheath in the Fourth Extensor Compartment of the Hand
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
Lipomas are relatively common benign tumors composed primarily of adipose tissue. They can occur anywhere on the body and are seen often in the hands and forearm. Typically localized to the subcutaneous fat layer, a lipoma is rarely associated with a tendon sheath or tendon compartment.1,2 When this uncommon event occurs, the lipoma is appropriately labeled lipoma of the tendon sheath.
While there are numerous case reports of lipomas of the tendon sheath occurring in association with tendons in the lower extremity, there are no reports, to our knowledge, of their occurrence in the extensor compartments of the hand.1 We report a rare case of lipoma of the tendon sheath localized to the fourth dorsal compartment of the hand, which was successfully treated with surgical excision. The patient provided written informed consent for print and electronic publication of this case report.
Case Report
A 33-year-old right hand–dominant waitress presented with a chief complaint of a painful, slowly enlarging right dorsal hand mass of 5 years’ duration. The mass was particularly bothersome with activities involving grip and finger extension. Physical examination revealed a mobile, rubbery mass on the dorsum of the hand that moved slightly with fist formation. There were no signs of neurovascular compromise. She had normal hand and wrist range of motion.
Plain radiographs were unremarkable (Figures 1A, 1B). Magnetic resonance imaging (MRI) with and without contrast revealed a 4×2-cm mass consistent with a diagnosis of lipoma. However, it was unique in that it appeared to extend from the long- and ring-finger extensor tendon sheaths in the fourth dorsal compartment of the hand (Figures 2A, 2B) and was deemed a lipoma of the tendon sheath. Representative MRI also showed the lipoma to be present within the fourth extensor compartment of the hand (Figure 2B). Because of the mass’s increasing size and interference with hand function, the patient elected to have the mass excised.
Surgical Technique
A 3-cm longitudinal incision was made over the dorsum of the hand centered directly over the mass. Dissection was carried through the subcutaneous tissue to the distal margin of the extensor retinaculum. The fourth dorsal compartment was entered and the tendons of the fourth extensor compartment were identified. Immediately beneath the extensor tendons to the long and ring fingers was a yellow, rubbery mass consistent with lipoma (Figure 3). This mass was strongly adherent to the underlying tendons and had to be dissected carefully with tenotomy scissors. Fortunately, the mass could be excised as a single unit (Figure 4). It was sent to the pathology department for histologic examination, which revealed mature adipose tissue and confirmed the diagnosis of lipoma. The wound was closed with absorbable suture, and a soft, sterile dressing was applied.
Postoperative Care
The patient was seen in follow-up 2 weeks later for routine evaluation. She had an intact wound with minimal hand pain, and full wrist and hand range of motion. She returned to work as a waitress approximately 3 weeks after surgery without difficulty. At her 6-week postoperative mark, she had a pain-free wrist with a well-healed incision and no signs of recurrence.
Discussion
Tendon sheath lipomas, whether in the upper or lower extremities, are exceedingly rare entities. Further, lipomas of an individual extensor compartment of the hand (as in our case) have yet to be described, in contrast to lipomas of flexor tendon sheaths.3 There are only a handful of case reports in the literature of lipomas of the tendon sheath, and none to our knowledge of their existence in the extensor compartments of the hand. Nevertheless, it is important for the treating surgeon to be aware of their existence and know some basics about them and their treatment.
There are 2 types of tendon sheath lipomas: discrete solid masses of adipose tissue (which we encountered) and adipose tissue coupled with hypertrophic synovial villi (or, lipoma arborescens).4,5 Of note, the latter is significantly more common than the former, which makes our case even more uncommon. Although both types of lipoma of the tendon sheath are benign, they can cause symptoms such as pain, finger stiffness, and nerve compression.6 Thus, they frequently merit surgical removal, as in our case.
The appropriate workup for lipoma of the tendon sheath generally includes thorough history, physical examination, and advanced imaging, such as MRI. MRI is usually diagnostic of such a lesion and can aid in surgical planning.1 Regarding their overall prognosis, all lipomas (even large ones) are benign by definition but can transform into liposarcomas in rare cases.4 Lipomas are typically treated surgically by simple excision, and lipoma of the tendon sheath is no different. As long as complete excision of a tendon sheath lipoma is performed, recurrence rates are less than 5%.2,3
Surgeons should also be aware that, with long-standing lipomas of the tendon sheath, weakening of a tendon secondary to irritation from the mass is a possibility, especially in the lower extremities. All tendons should be inspected carefully at the time of surgery to ensure that other procedures, such as tendon grafting or side-to-side tenodesis, are not required. Although lipomas of the tendon sheath and extensor compartments are quite rare, all surgeons evaluating masses for possible surgical excision should be aware of their existence and know how to manage them appropriately.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
1. Khan AZ, Shafafy M, Latimer MD, Crosby J. A lipoma within the Achilles tendon sheath. Foot Ankle Surg. 2012;18(1):e16-e17.
2. Bryan RS, Dahlin DC, Sullivan CR. Lipoma of the tendon sheath. J Bone Joint Surg Am. 1956;38(6):1275-1280.
3. Kremchek TE, Kremchek EJ. Carpal tunnel syndrome caused by flexor tendon sheath lipoma. Orthop Rev. 1998;17(11):1083-1085.
4. Murphey MD, Caroll JF, Flemming DJ, Pope TL, Gannon FH, Kransdorf MJ. From the archives of AFIP: benign musculoskeletal lipomatous lesions. Radiographics. 2004;24(5):1433-1466.
5. Chronopoulous E, Nicholas P, Karanikas C, et al. Patient presenting with lipoma of the index finger: a case report. Cases J. 2010;3:20.
6. Elbardouni A, Kharmaz M, Salah Berrada M, Mahfoud M, Eylaacoubi M. Well-circumscribed deep-seated lesions of the upper extremity. A report of 13 cases. Orthop Traumatol: Surg Res. 2011;97(2):152-158.
Current Management of Acute Bronchiolitis: An Evidence-Based Approach
Case
An 8-week-old male infant was brought to the ED by his parents after an episode in which it appeared the baby had stopped breathing. The parents stated that while lying on his mother’s lap at home, the patient stopped breathing for approximately 10 to 15 seconds, during which time his face exhibited a bluish color. They further noted that the patient began breathing again after gentle stimulation and had been acting normally since.
The patient was born at 39 weeks gestation via normal vaginal delivery and without any complications. His parents further stated that prior to the cessation of breathing incident, his symptoms of nasal congestion, decreased energy level, and fast breathing had gradually worsened over the past 2 days. The parents also noted that the infant had not been feeding as well over the past 2 days.
Upon arrival, the patient’s vital signs were: heart rate, 140 beats/minute; respiratory rate (RR), 72 beats/minute; and temperature 101.3°F. Oxygen saturation was 92% on room air. On physical examination, the infant had significant rhinorrhea, moderate intercostal and supraclavicular retractions, ausculatory wheezes, and transmitted upper airway noises throughout.
Overview
Bronchiolitis, a disorder caused by a viral lower respiratory tract infection, is the most common lower respiratory infection in children younger than age 2 years.1 In 2014, the American Academy of Pediatrics (AAP) characterized bronchiolitis as “rhinitis, cough, tachypnea, wheezing, rales, use of accessory muscles, and/or nasal flaring in children under 24 months of age.”2 This condition is the most common cause of hospitalization in the first 12 months of life. It is responsible for over 100,000 admissions annually at an estimated cost to the healthcare system of $1.73 billion.3
Etiology and Pathophysiology
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis. In the United States, the highest incidence of infection occurs during the months of December through March, with some degree of regional variability.4 A number of other viruses that can cause bronchiolitis include human metapneumovirus, parainfluenza virus, and influenza virus.1 Infection with RSV does not grant permanent immunity, and reinfection is common throughout life.2
Pathophysiologically, bronchiolitis is characterized by an invasion of bronchial epithelial cells that lead to to cell death and sloughing into the bronchial lumen. This, coupled with increased mucous production and submucosal edema, leads to a narrowing of the bronchial lumen and obstruction of airflow.5
Clinical Manifestations
Bronchiolitis represents a constellation of signs and symptoms beginning with those of an upper respiratory tract infection, including nasal congestion and rhinorrhea with mild cough. On days 3 to 5, the following symptoms develop: tachypnea, wheezing, rales, and signs of respiratory distress (eg, grunting, nasal flaring, inter-/subcostal retractions). Approximately two-thirds of patients will develop a fever.2 Recovery tends to begin around days 5 to 7, with the median duration of illness being 12 days.1 It should be noted that bronchiolitis represents a highly variable and dynamic disease state. Transient episodes of improvement and worsening are common, emphasizing the importance of serial examinations and assessments. Though rare, progression to respiratory failure and death do occur.2
History and Risk Stratification
The focus of the initial history by the clinician should serve two primary purposes. First, it is important to differentiate infants with probable bronchiolitis from those with other disease states having similar clinical manifestations. One of the most challenging diseases to differentiate from bronchiolitis is that of reactive airway disease (RAD). Eliciting a history of allergic rhinitis, eczema, or a family history of asthma may be helpful in determining the precise etiology of the patient’s symptoms. Although no longer recommended for children with bronchiolitis (as will be later discussed), a trial of a bronchodilation may be beneficial in the setting of familial atopy.
The second—and perhaps most important—aspect of patient history is to determine the presence of risk factors for both apnea and the development of severe bronchiolitis. Regarding the risk factors for apnea, Willwerth et al6 developed a set of criteria to identify patients at high risk for apnea in the inpatient setting. Patients were considered high risk if they were born at full term and were younger than 1 month of age; if they were born preterm (<37 weeks gestation) and were younger than 48 weeks postconception; and/or if the infant’s parents or a clinician had already witnessed an episode of apnea during the patient’s illness. In this study, all patients who developed apnea were correctly identified by the risk criteria.6 Risk factors for severe bronchiolitis include the following: patient age younger than 12 weeks; patient prematurity younger than 37 weeks gestation; and an underlying hemodynamically significant congenital heart disease, chronic lung disease/bronchopulmonary dysplasia, or an immunocompromised state.1
Diagnosis
In 2014, the AAP updated its guidelines on the diagnosis, management, and prevention of bronchiolitis. One of the strongest statements in these guidelines emphasize that the diagnosis of bronchiolitis should be based almost exclusively on the history and physical examination.2 In children younger than age 2 years, historical features such as a viral prodrome, followed by progressively worsening increased respiratory effort and signs and symptoms of lower respiratory-tract disease (eg, wheezing), should guide clinicians to the diagnosis of bronchiolitis. Although nonspecific, physical examination findings such as rhinorrhea, cough, tachypnea, wheezing, rales, and increased respiratory effort—when coupled with a good history—can be beneficial in the diagnosis of bronchiolitis.
Pulse Oximetry
Pulse oximetry has become a standard part of the clinical assessment of patients with bronchiolitis. This is based on data suggesting that pulse oximetry detects hypoxia in cases where it was not suspected on physical examination alone.7 However, the effectiveness of pulse oximetry in predicting clinical outcomes is limited. Pulse oximetry should not be used as a proxy for respiratory distress, as studies have shown poor correlation between respiratory distress and oxygen saturations in infants with lower respiratory tract infection.8
Radiographic Evaluation
Regarding the diagnosis of bronchiolitis, the AAP notes, “radiographic and laboratory studies should not be obtained routinely.”2 While many children with bronchiolitis may have abnormalities on radiographs, there is insufficient data to suggest that chest radiographs correlate with disease severity. In addition, several studies, including a prospective cohort study by Schuh et al,9 have shown that infants with suspected lower respiratory tract infections who undergo radiography are more likely to receive antibiotics without any difference in outcomes.
Laboratory Studies
As stated in the AAP guidelines, routine laboratory testing, particularly virologic studies for RSV, have little role in the diagnosis of bronchiolitis. Since numerous viruses can cause bronchiolitis and have similar clinical presentations, the absence of identification of a particular virologic agent does not exclude the diagnosis of bronchiolitis and is moreover unlikely to alter management.
Although routine laboratory evaluation is not recommended in infants with bronchiolitis, one subgroup in which it may be beneficial is in the assessment of serious bacterial infections (SBIs) in febrile infants with bronchiolitis who are younger than 60 days old. Levine et al10 conducted a large, multicenter, prospective, cross-sectional study of young, febrile infants to determine the risk of SBI in those with RSV bronchiolitis versus those without RSV bronchiolitis. They found that overall febrile infants younger than age 60 days with RSV bronchiolitis have a lower rate of SBI than those without RSV (7% v 12.5%, respectively).10 In infants between age 28 and 60 days with RSV bronchiolitis, the origin of all SBIs in the study were urinary tract infections. In patients younger than 28 days of age, the risk of developing an SBI was found to be no different between the RSV-positive and RSV-negative groups.10
Based on the findings in this study, it is recommended that, at the very least, urinalysis for bacterial infection be performed in all infants with RSV bronchiolitis who are younger than age 60 days. Furthermore, since there was no difference in the rates of SBI in patients younger than age 28 days, infants in this age range should undergo a full septic work-up (blood, urine, and cerebrospinal fluid)—regardless of RSV infection status. For infants between ages 28 and 60 days, there is not enough evidence to recommend for or against further laboratory evaluation other than urinalysis.
Treatment
Nasal Suctioning
Nasal suctioning has become the first-line treatment for infants with bronchiolitis. It is used to clear secretions from the nasal passages to aid in respiration, which is particularly important in younger infants—who are obligate nose breathers. Current recommendations are to perform suctioning with increasing respiratory effort, before feeding and before laying the infant down to sleep.1
Bronchodilators
In the past, bronchodilators such as the β-agonist albuterol have been used to treat bronchiolitis with the idea that bronchial smooth muscle relaxation would improve clinical symptoms. In its 2006 guidelines, the AAP had recommended a trial of albuterol and continuation only if there was a documented objective response. In the 2014 updated guidelines, however, the AAP no longer recommends the use of albuterol in any capacity.
Although several meta-analyses and systematic reviews have demonstrated that bronchodilators may improve clinical symptoms scores, they did not affect disease resolution, need for hospitalization, or length of hospital stay.2 In addition, a recent Cochrane systematic review noted no benefit in the clinical course of infants with bronchiolitis treated with bronchodilators, and cited the potential adverse events (tachycardia and tremors) as outweighing any potential benefit.11 In addition to albuterol, the AAP no longer recommends the use of nebulized epinephrine in the treatment of bronchiolitis.2
Hypertonic Saline
Although hypertonic saline (HTS) has been increasingly studied for the treatment of bronchiolitis, the AAP does not recommend its use in the ED. Despite evidence that HTS may reduce hospital length of stay after 24 hours of use in settings where the typical duration of hospitalization exceeds 3 days, it has not been shown to reduce the rate of hospitalization when used in an emergency setting.2
Corticosteroids
While there is good evidence that corticosteroids are beneficial in treating some respiratory diseases, such as asthma and croup, numerous studies have repeatedly failed to show a benefit in treating bronchiolitis. One of the largest studies, a multicenter, randomized, controlled trial of dexamethasone for bronchiolitis by the Pediatric Emergency Care Applied Research Network, did not show any alteration in admission rates, respiratory status after 4 hours of observation, or length of hospital stay.12 Accordingly, the AAP strongly recommends against the administration of corticosteroids for bronchiolitis in any setting.2
Oxygen Therapy
Oxygen therapy is often necessary in patients with bronchiolitis who demonstrate hypoxia. The definition of hypoxia in this patient population has remained variable. The AAP has established a threshold of oxyhemoglobin saturation (SpO2) of less than 90% to define hypoxia and has empowered clinicians to not administer oxygen if the SpO2 exceeds 90%. Based on the oxyhemoglobin dissociation curve, the authors of the AAP guidelines note that when the SpO2 is less than 90%, small decreases in the arterial partial pressure of oxygen (PaO2) result in large decreases in the SpO2. When SpO2 is greater than 90%, however, large increases in PaO2 are associated with only small increased in SpO2. The AAP guidelines note, “In infants and children with bronchiolitis, no data exist to suggest that such increases [in PaO2 and SpO2] result in any clinically significant differences in physiologic function, patient symptoms, or clinical outcomes.”2
A relatively new method of administration of oxygen to infants with bronchiolitis is via a humidified, heated, high-flow nasal cannula (HHHFNC). This therapy has been shown to generate continuous positive airway pressure, which improves respiratory effort, reduces the work of breathing, and may decrease the need for intubation.2
Patient Disposition
One of the most challenging tasks for emergency physicians (EPs) is determining the appropriate disposition of infants with bronchiolitis. The variable presentation and dynamic nature of the disease make this particularly difficult. Patients at high risk for apnea should be admitted to the hospital for observation and further care as needed. Admission also should be strongly considered for those with significantly increased work of breathing and tachypnea that does not improve with suctioning—especially when these interfere with feeding. Infants with poor feeding or evidence of dehydration should be admitted to the hospital for intravenous (IV) fluid hydration or nasogastric feedings. Patients with hypoxia (SpO2 saturations <90%) should also be admitted for supplemental oxygen therapy. It should be noted, however, the AAP recommends “spot-checks” over continuous pulse oximetry in patients who do not require oxygen therapy.2
Another important factor affecting patient disposition is the ability of the caregiver to provide basic patient care and ensure close outpatient follow-up. Prior to discharge, caregivers should be educated on the highly dynamic nature of bronchiolitis and the signs and symptoms that would require prompt return to the ED—especially if the infant has risk factors for the development of severe disease.
Case Conclusion
Based on the patient’s symptoms, history (most notably, the recent incident of sleep apnea at home), and physical examination, the EP quickly identified this infant was at a high risk for both severe bronchiolitis and apnea and required aggressive management. Nasal suctioning was immediately performed to help clear the patient’s secretions; this, however, only slightly improved his RR and work of breathing. Although the infant’s SpO2 was greater than 90% on room air, the EP administered oxygen via HHHFNC at 6 L per minute, which produced a significant improvement in both RR and effort.
Given the patient’s age and the presence of a fever, a urinalysis was also obtained, the results of which showed no evidence of infection. Since the patient was only able to bottle-feed for a few minutes at a time, the EP initiated IV fluid hydration and contacted the hospitalist team for inpatient admission.
The infant was gradually weaned from HHHFNC on hospital day 2 but remained with suboptimal oral intake for another 24 hours. By hospital day 4, his work of breathing had improved significantly, and he was feeding well with through the assistance of pre-feeding nasal syringe suctioning. The patient was discharged home in the care of his parents later that same day with only mild tachypnea over baseline. At discharge, the EP emphasized the importance of providing close follow-up with their son’s pediatrician. The infant continued to gradually improve as an outpatient, with resolution of nasal congestion by day 12 of his illness; he returned to his baseline breathing and feeding pattern on day 14.
Dr Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of The King’s Daughters, Norfolk. Dr Clingenpeel is a fellowship director of pediatric emergency medicine, and an associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Joseph M. Evidence-based assessment and management of acute bronchiolitis in the emergency department. Pediatr Emerg Med Pract. 2011;8(3):1-20.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guidelines: the diagnosis, management, and prevention of bronchiolitis [Published correction appears in Pediatrics. 2014;134(5):e1474-e1502]. Pediatrics. 2014;134:5 e1474-e1502.
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-20009. Pediatrics. 2013;32(1):28-36.
- Centers for Disease Control and Prevention (CDC). Respiratory syncytial virus activity—United States, July 2011-January 2013. MMWR Morb Mortal Wkly Rep. 2013;62(8):141-144.
- Harper MB, Fleisher GR. Infectious emergencies. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2010:916-917.
- Willwerth BM, Harper MB, Greenes DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48(4):441-447.
- Shaw KN, Bell LM, Sherman NH. Outpatient assessment of infants with bronchiolitis. Am J Dis Child. 1991;145(2):151-155.
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145(1):106-109.
- Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433.
- Levine DA, Platt SL, Dayan PS, et al; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infection. Pediatrics. 2004;113(6):1728-1734.
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;(6):CD001266.
- Corneli HM, Zorc JJ, Majahan P, et al; Bronchiolitis Study Group of the Pediatric Emergency Care Applied Research Network (PECARN). A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357(4):331-339.
Case
An 8-week-old male infant was brought to the ED by his parents after an episode in which it appeared the baby had stopped breathing. The parents stated that while lying on his mother’s lap at home, the patient stopped breathing for approximately 10 to 15 seconds, during which time his face exhibited a bluish color. They further noted that the patient began breathing again after gentle stimulation and had been acting normally since.
The patient was born at 39 weeks gestation via normal vaginal delivery and without any complications. His parents further stated that prior to the cessation of breathing incident, his symptoms of nasal congestion, decreased energy level, and fast breathing had gradually worsened over the past 2 days. The parents also noted that the infant had not been feeding as well over the past 2 days.
Upon arrival, the patient’s vital signs were: heart rate, 140 beats/minute; respiratory rate (RR), 72 beats/minute; and temperature 101.3°F. Oxygen saturation was 92% on room air. On physical examination, the infant had significant rhinorrhea, moderate intercostal and supraclavicular retractions, ausculatory wheezes, and transmitted upper airway noises throughout.
Overview
Bronchiolitis, a disorder caused by a viral lower respiratory tract infection, is the most common lower respiratory infection in children younger than age 2 years.1 In 2014, the American Academy of Pediatrics (AAP) characterized bronchiolitis as “rhinitis, cough, tachypnea, wheezing, rales, use of accessory muscles, and/or nasal flaring in children under 24 months of age.”2 This condition is the most common cause of hospitalization in the first 12 months of life. It is responsible for over 100,000 admissions annually at an estimated cost to the healthcare system of $1.73 billion.3
Etiology and Pathophysiology
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis. In the United States, the highest incidence of infection occurs during the months of December through March, with some degree of regional variability.4 A number of other viruses that can cause bronchiolitis include human metapneumovirus, parainfluenza virus, and influenza virus.1 Infection with RSV does not grant permanent immunity, and reinfection is common throughout life.2
Pathophysiologically, bronchiolitis is characterized by an invasion of bronchial epithelial cells that lead to to cell death and sloughing into the bronchial lumen. This, coupled with increased mucous production and submucosal edema, leads to a narrowing of the bronchial lumen and obstruction of airflow.5
Clinical Manifestations
Bronchiolitis represents a constellation of signs and symptoms beginning with those of an upper respiratory tract infection, including nasal congestion and rhinorrhea with mild cough. On days 3 to 5, the following symptoms develop: tachypnea, wheezing, rales, and signs of respiratory distress (eg, grunting, nasal flaring, inter-/subcostal retractions). Approximately two-thirds of patients will develop a fever.2 Recovery tends to begin around days 5 to 7, with the median duration of illness being 12 days.1 It should be noted that bronchiolitis represents a highly variable and dynamic disease state. Transient episodes of improvement and worsening are common, emphasizing the importance of serial examinations and assessments. Though rare, progression to respiratory failure and death do occur.2
History and Risk Stratification
The focus of the initial history by the clinician should serve two primary purposes. First, it is important to differentiate infants with probable bronchiolitis from those with other disease states having similar clinical manifestations. One of the most challenging diseases to differentiate from bronchiolitis is that of reactive airway disease (RAD). Eliciting a history of allergic rhinitis, eczema, or a family history of asthma may be helpful in determining the precise etiology of the patient’s symptoms. Although no longer recommended for children with bronchiolitis (as will be later discussed), a trial of a bronchodilation may be beneficial in the setting of familial atopy.
The second—and perhaps most important—aspect of patient history is to determine the presence of risk factors for both apnea and the development of severe bronchiolitis. Regarding the risk factors for apnea, Willwerth et al6 developed a set of criteria to identify patients at high risk for apnea in the inpatient setting. Patients were considered high risk if they were born at full term and were younger than 1 month of age; if they were born preterm (<37 weeks gestation) and were younger than 48 weeks postconception; and/or if the infant’s parents or a clinician had already witnessed an episode of apnea during the patient’s illness. In this study, all patients who developed apnea were correctly identified by the risk criteria.6 Risk factors for severe bronchiolitis include the following: patient age younger than 12 weeks; patient prematurity younger than 37 weeks gestation; and an underlying hemodynamically significant congenital heart disease, chronic lung disease/bronchopulmonary dysplasia, or an immunocompromised state.1
Diagnosis
In 2014, the AAP updated its guidelines on the diagnosis, management, and prevention of bronchiolitis. One of the strongest statements in these guidelines emphasize that the diagnosis of bronchiolitis should be based almost exclusively on the history and physical examination.2 In children younger than age 2 years, historical features such as a viral prodrome, followed by progressively worsening increased respiratory effort and signs and symptoms of lower respiratory-tract disease (eg, wheezing), should guide clinicians to the diagnosis of bronchiolitis. Although nonspecific, physical examination findings such as rhinorrhea, cough, tachypnea, wheezing, rales, and increased respiratory effort—when coupled with a good history—can be beneficial in the diagnosis of bronchiolitis.
Pulse Oximetry
Pulse oximetry has become a standard part of the clinical assessment of patients with bronchiolitis. This is based on data suggesting that pulse oximetry detects hypoxia in cases where it was not suspected on physical examination alone.7 However, the effectiveness of pulse oximetry in predicting clinical outcomes is limited. Pulse oximetry should not be used as a proxy for respiratory distress, as studies have shown poor correlation between respiratory distress and oxygen saturations in infants with lower respiratory tract infection.8
Radiographic Evaluation
Regarding the diagnosis of bronchiolitis, the AAP notes, “radiographic and laboratory studies should not be obtained routinely.”2 While many children with bronchiolitis may have abnormalities on radiographs, there is insufficient data to suggest that chest radiographs correlate with disease severity. In addition, several studies, including a prospective cohort study by Schuh et al,9 have shown that infants with suspected lower respiratory tract infections who undergo radiography are more likely to receive antibiotics without any difference in outcomes.
Laboratory Studies
As stated in the AAP guidelines, routine laboratory testing, particularly virologic studies for RSV, have little role in the diagnosis of bronchiolitis. Since numerous viruses can cause bronchiolitis and have similar clinical presentations, the absence of identification of a particular virologic agent does not exclude the diagnosis of bronchiolitis and is moreover unlikely to alter management.
Although routine laboratory evaluation is not recommended in infants with bronchiolitis, one subgroup in which it may be beneficial is in the assessment of serious bacterial infections (SBIs) in febrile infants with bronchiolitis who are younger than 60 days old. Levine et al10 conducted a large, multicenter, prospective, cross-sectional study of young, febrile infants to determine the risk of SBI in those with RSV bronchiolitis versus those without RSV bronchiolitis. They found that overall febrile infants younger than age 60 days with RSV bronchiolitis have a lower rate of SBI than those without RSV (7% v 12.5%, respectively).10 In infants between age 28 and 60 days with RSV bronchiolitis, the origin of all SBIs in the study were urinary tract infections. In patients younger than 28 days of age, the risk of developing an SBI was found to be no different between the RSV-positive and RSV-negative groups.10
Based on the findings in this study, it is recommended that, at the very least, urinalysis for bacterial infection be performed in all infants with RSV bronchiolitis who are younger than age 60 days. Furthermore, since there was no difference in the rates of SBI in patients younger than age 28 days, infants in this age range should undergo a full septic work-up (blood, urine, and cerebrospinal fluid)—regardless of RSV infection status. For infants between ages 28 and 60 days, there is not enough evidence to recommend for or against further laboratory evaluation other than urinalysis.
Treatment
Nasal Suctioning
Nasal suctioning has become the first-line treatment for infants with bronchiolitis. It is used to clear secretions from the nasal passages to aid in respiration, which is particularly important in younger infants—who are obligate nose breathers. Current recommendations are to perform suctioning with increasing respiratory effort, before feeding and before laying the infant down to sleep.1
Bronchodilators
In the past, bronchodilators such as the β-agonist albuterol have been used to treat bronchiolitis with the idea that bronchial smooth muscle relaxation would improve clinical symptoms. In its 2006 guidelines, the AAP had recommended a trial of albuterol and continuation only if there was a documented objective response. In the 2014 updated guidelines, however, the AAP no longer recommends the use of albuterol in any capacity.
Although several meta-analyses and systematic reviews have demonstrated that bronchodilators may improve clinical symptoms scores, they did not affect disease resolution, need for hospitalization, or length of hospital stay.2 In addition, a recent Cochrane systematic review noted no benefit in the clinical course of infants with bronchiolitis treated with bronchodilators, and cited the potential adverse events (tachycardia and tremors) as outweighing any potential benefit.11 In addition to albuterol, the AAP no longer recommends the use of nebulized epinephrine in the treatment of bronchiolitis.2
Hypertonic Saline
Although hypertonic saline (HTS) has been increasingly studied for the treatment of bronchiolitis, the AAP does not recommend its use in the ED. Despite evidence that HTS may reduce hospital length of stay after 24 hours of use in settings where the typical duration of hospitalization exceeds 3 days, it has not been shown to reduce the rate of hospitalization when used in an emergency setting.2
Corticosteroids
While there is good evidence that corticosteroids are beneficial in treating some respiratory diseases, such as asthma and croup, numerous studies have repeatedly failed to show a benefit in treating bronchiolitis. One of the largest studies, a multicenter, randomized, controlled trial of dexamethasone for bronchiolitis by the Pediatric Emergency Care Applied Research Network, did not show any alteration in admission rates, respiratory status after 4 hours of observation, or length of hospital stay.12 Accordingly, the AAP strongly recommends against the administration of corticosteroids for bronchiolitis in any setting.2
Oxygen Therapy
Oxygen therapy is often necessary in patients with bronchiolitis who demonstrate hypoxia. The definition of hypoxia in this patient population has remained variable. The AAP has established a threshold of oxyhemoglobin saturation (SpO2) of less than 90% to define hypoxia and has empowered clinicians to not administer oxygen if the SpO2 exceeds 90%. Based on the oxyhemoglobin dissociation curve, the authors of the AAP guidelines note that when the SpO2 is less than 90%, small decreases in the arterial partial pressure of oxygen (PaO2) result in large decreases in the SpO2. When SpO2 is greater than 90%, however, large increases in PaO2 are associated with only small increased in SpO2. The AAP guidelines note, “In infants and children with bronchiolitis, no data exist to suggest that such increases [in PaO2 and SpO2] result in any clinically significant differences in physiologic function, patient symptoms, or clinical outcomes.”2
A relatively new method of administration of oxygen to infants with bronchiolitis is via a humidified, heated, high-flow nasal cannula (HHHFNC). This therapy has been shown to generate continuous positive airway pressure, which improves respiratory effort, reduces the work of breathing, and may decrease the need for intubation.2
Patient Disposition
One of the most challenging tasks for emergency physicians (EPs) is determining the appropriate disposition of infants with bronchiolitis. The variable presentation and dynamic nature of the disease make this particularly difficult. Patients at high risk for apnea should be admitted to the hospital for observation and further care as needed. Admission also should be strongly considered for those with significantly increased work of breathing and tachypnea that does not improve with suctioning—especially when these interfere with feeding. Infants with poor feeding or evidence of dehydration should be admitted to the hospital for intravenous (IV) fluid hydration or nasogastric feedings. Patients with hypoxia (SpO2 saturations <90%) should also be admitted for supplemental oxygen therapy. It should be noted, however, the AAP recommends “spot-checks” over continuous pulse oximetry in patients who do not require oxygen therapy.2
Another important factor affecting patient disposition is the ability of the caregiver to provide basic patient care and ensure close outpatient follow-up. Prior to discharge, caregivers should be educated on the highly dynamic nature of bronchiolitis and the signs and symptoms that would require prompt return to the ED—especially if the infant has risk factors for the development of severe disease.
Case Conclusion
Based on the patient’s symptoms, history (most notably, the recent incident of sleep apnea at home), and physical examination, the EP quickly identified this infant was at a high risk for both severe bronchiolitis and apnea and required aggressive management. Nasal suctioning was immediately performed to help clear the patient’s secretions; this, however, only slightly improved his RR and work of breathing. Although the infant’s SpO2 was greater than 90% on room air, the EP administered oxygen via HHHFNC at 6 L per minute, which produced a significant improvement in both RR and effort.
Given the patient’s age and the presence of a fever, a urinalysis was also obtained, the results of which showed no evidence of infection. Since the patient was only able to bottle-feed for a few minutes at a time, the EP initiated IV fluid hydration and contacted the hospitalist team for inpatient admission.
The infant was gradually weaned from HHHFNC on hospital day 2 but remained with suboptimal oral intake for another 24 hours. By hospital day 4, his work of breathing had improved significantly, and he was feeding well with through the assistance of pre-feeding nasal syringe suctioning. The patient was discharged home in the care of his parents later that same day with only mild tachypnea over baseline. At discharge, the EP emphasized the importance of providing close follow-up with their son’s pediatrician. The infant continued to gradually improve as an outpatient, with resolution of nasal congestion by day 12 of his illness; he returned to his baseline breathing and feeding pattern on day 14.
Dr Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of The King’s Daughters, Norfolk. Dr Clingenpeel is a fellowship director of pediatric emergency medicine, and an associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
Case
An 8-week-old male infant was brought to the ED by his parents after an episode in which it appeared the baby had stopped breathing. The parents stated that while lying on his mother’s lap at home, the patient stopped breathing for approximately 10 to 15 seconds, during which time his face exhibited a bluish color. They further noted that the patient began breathing again after gentle stimulation and had been acting normally since.
The patient was born at 39 weeks gestation via normal vaginal delivery and without any complications. His parents further stated that prior to the cessation of breathing incident, his symptoms of nasal congestion, decreased energy level, and fast breathing had gradually worsened over the past 2 days. The parents also noted that the infant had not been feeding as well over the past 2 days.
Upon arrival, the patient’s vital signs were: heart rate, 140 beats/minute; respiratory rate (RR), 72 beats/minute; and temperature 101.3°F. Oxygen saturation was 92% on room air. On physical examination, the infant had significant rhinorrhea, moderate intercostal and supraclavicular retractions, ausculatory wheezes, and transmitted upper airway noises throughout.
Overview
Bronchiolitis, a disorder caused by a viral lower respiratory tract infection, is the most common lower respiratory infection in children younger than age 2 years.1 In 2014, the American Academy of Pediatrics (AAP) characterized bronchiolitis as “rhinitis, cough, tachypnea, wheezing, rales, use of accessory muscles, and/or nasal flaring in children under 24 months of age.”2 This condition is the most common cause of hospitalization in the first 12 months of life. It is responsible for over 100,000 admissions annually at an estimated cost to the healthcare system of $1.73 billion.3
Etiology and Pathophysiology
Respiratory syncytial virus (RSV) is the most common cause of bronchiolitis. In the United States, the highest incidence of infection occurs during the months of December through March, with some degree of regional variability.4 A number of other viruses that can cause bronchiolitis include human metapneumovirus, parainfluenza virus, and influenza virus.1 Infection with RSV does not grant permanent immunity, and reinfection is common throughout life.2
Pathophysiologically, bronchiolitis is characterized by an invasion of bronchial epithelial cells that lead to to cell death and sloughing into the bronchial lumen. This, coupled with increased mucous production and submucosal edema, leads to a narrowing of the bronchial lumen and obstruction of airflow.5
Clinical Manifestations
Bronchiolitis represents a constellation of signs and symptoms beginning with those of an upper respiratory tract infection, including nasal congestion and rhinorrhea with mild cough. On days 3 to 5, the following symptoms develop: tachypnea, wheezing, rales, and signs of respiratory distress (eg, grunting, nasal flaring, inter-/subcostal retractions). Approximately two-thirds of patients will develop a fever.2 Recovery tends to begin around days 5 to 7, with the median duration of illness being 12 days.1 It should be noted that bronchiolitis represents a highly variable and dynamic disease state. Transient episodes of improvement and worsening are common, emphasizing the importance of serial examinations and assessments. Though rare, progression to respiratory failure and death do occur.2
History and Risk Stratification
The focus of the initial history by the clinician should serve two primary purposes. First, it is important to differentiate infants with probable bronchiolitis from those with other disease states having similar clinical manifestations. One of the most challenging diseases to differentiate from bronchiolitis is that of reactive airway disease (RAD). Eliciting a history of allergic rhinitis, eczema, or a family history of asthma may be helpful in determining the precise etiology of the patient’s symptoms. Although no longer recommended for children with bronchiolitis (as will be later discussed), a trial of a bronchodilation may be beneficial in the setting of familial atopy.
The second—and perhaps most important—aspect of patient history is to determine the presence of risk factors for both apnea and the development of severe bronchiolitis. Regarding the risk factors for apnea, Willwerth et al6 developed a set of criteria to identify patients at high risk for apnea in the inpatient setting. Patients were considered high risk if they were born at full term and were younger than 1 month of age; if they were born preterm (<37 weeks gestation) and were younger than 48 weeks postconception; and/or if the infant’s parents or a clinician had already witnessed an episode of apnea during the patient’s illness. In this study, all patients who developed apnea were correctly identified by the risk criteria.6 Risk factors for severe bronchiolitis include the following: patient age younger than 12 weeks; patient prematurity younger than 37 weeks gestation; and an underlying hemodynamically significant congenital heart disease, chronic lung disease/bronchopulmonary dysplasia, or an immunocompromised state.1
Diagnosis
In 2014, the AAP updated its guidelines on the diagnosis, management, and prevention of bronchiolitis. One of the strongest statements in these guidelines emphasize that the diagnosis of bronchiolitis should be based almost exclusively on the history and physical examination.2 In children younger than age 2 years, historical features such as a viral prodrome, followed by progressively worsening increased respiratory effort and signs and symptoms of lower respiratory-tract disease (eg, wheezing), should guide clinicians to the diagnosis of bronchiolitis. Although nonspecific, physical examination findings such as rhinorrhea, cough, tachypnea, wheezing, rales, and increased respiratory effort—when coupled with a good history—can be beneficial in the diagnosis of bronchiolitis.
Pulse Oximetry
Pulse oximetry has become a standard part of the clinical assessment of patients with bronchiolitis. This is based on data suggesting that pulse oximetry detects hypoxia in cases where it was not suspected on physical examination alone.7 However, the effectiveness of pulse oximetry in predicting clinical outcomes is limited. Pulse oximetry should not be used as a proxy for respiratory distress, as studies have shown poor correlation between respiratory distress and oxygen saturations in infants with lower respiratory tract infection.8
Radiographic Evaluation
Regarding the diagnosis of bronchiolitis, the AAP notes, “radiographic and laboratory studies should not be obtained routinely.”2 While many children with bronchiolitis may have abnormalities on radiographs, there is insufficient data to suggest that chest radiographs correlate with disease severity. In addition, several studies, including a prospective cohort study by Schuh et al,9 have shown that infants with suspected lower respiratory tract infections who undergo radiography are more likely to receive antibiotics without any difference in outcomes.
Laboratory Studies
As stated in the AAP guidelines, routine laboratory testing, particularly virologic studies for RSV, have little role in the diagnosis of bronchiolitis. Since numerous viruses can cause bronchiolitis and have similar clinical presentations, the absence of identification of a particular virologic agent does not exclude the diagnosis of bronchiolitis and is moreover unlikely to alter management.
Although routine laboratory evaluation is not recommended in infants with bronchiolitis, one subgroup in which it may be beneficial is in the assessment of serious bacterial infections (SBIs) in febrile infants with bronchiolitis who are younger than 60 days old. Levine et al10 conducted a large, multicenter, prospective, cross-sectional study of young, febrile infants to determine the risk of SBI in those with RSV bronchiolitis versus those without RSV bronchiolitis. They found that overall febrile infants younger than age 60 days with RSV bronchiolitis have a lower rate of SBI than those without RSV (7% v 12.5%, respectively).10 In infants between age 28 and 60 days with RSV bronchiolitis, the origin of all SBIs in the study were urinary tract infections. In patients younger than 28 days of age, the risk of developing an SBI was found to be no different between the RSV-positive and RSV-negative groups.10
Based on the findings in this study, it is recommended that, at the very least, urinalysis for bacterial infection be performed in all infants with RSV bronchiolitis who are younger than age 60 days. Furthermore, since there was no difference in the rates of SBI in patients younger than age 28 days, infants in this age range should undergo a full septic work-up (blood, urine, and cerebrospinal fluid)—regardless of RSV infection status. For infants between ages 28 and 60 days, there is not enough evidence to recommend for or against further laboratory evaluation other than urinalysis.
Treatment
Nasal Suctioning
Nasal suctioning has become the first-line treatment for infants with bronchiolitis. It is used to clear secretions from the nasal passages to aid in respiration, which is particularly important in younger infants—who are obligate nose breathers. Current recommendations are to perform suctioning with increasing respiratory effort, before feeding and before laying the infant down to sleep.1
Bronchodilators
In the past, bronchodilators such as the β-agonist albuterol have been used to treat bronchiolitis with the idea that bronchial smooth muscle relaxation would improve clinical symptoms. In its 2006 guidelines, the AAP had recommended a trial of albuterol and continuation only if there was a documented objective response. In the 2014 updated guidelines, however, the AAP no longer recommends the use of albuterol in any capacity.
Although several meta-analyses and systematic reviews have demonstrated that bronchodilators may improve clinical symptoms scores, they did not affect disease resolution, need for hospitalization, or length of hospital stay.2 In addition, a recent Cochrane systematic review noted no benefit in the clinical course of infants with bronchiolitis treated with bronchodilators, and cited the potential adverse events (tachycardia and tremors) as outweighing any potential benefit.11 In addition to albuterol, the AAP no longer recommends the use of nebulized epinephrine in the treatment of bronchiolitis.2
Hypertonic Saline
Although hypertonic saline (HTS) has been increasingly studied for the treatment of bronchiolitis, the AAP does not recommend its use in the ED. Despite evidence that HTS may reduce hospital length of stay after 24 hours of use in settings where the typical duration of hospitalization exceeds 3 days, it has not been shown to reduce the rate of hospitalization when used in an emergency setting.2
Corticosteroids
While there is good evidence that corticosteroids are beneficial in treating some respiratory diseases, such as asthma and croup, numerous studies have repeatedly failed to show a benefit in treating bronchiolitis. One of the largest studies, a multicenter, randomized, controlled trial of dexamethasone for bronchiolitis by the Pediatric Emergency Care Applied Research Network, did not show any alteration in admission rates, respiratory status after 4 hours of observation, or length of hospital stay.12 Accordingly, the AAP strongly recommends against the administration of corticosteroids for bronchiolitis in any setting.2
Oxygen Therapy
Oxygen therapy is often necessary in patients with bronchiolitis who demonstrate hypoxia. The definition of hypoxia in this patient population has remained variable. The AAP has established a threshold of oxyhemoglobin saturation (SpO2) of less than 90% to define hypoxia and has empowered clinicians to not administer oxygen if the SpO2 exceeds 90%. Based on the oxyhemoglobin dissociation curve, the authors of the AAP guidelines note that when the SpO2 is less than 90%, small decreases in the arterial partial pressure of oxygen (PaO2) result in large decreases in the SpO2. When SpO2 is greater than 90%, however, large increases in PaO2 are associated with only small increased in SpO2. The AAP guidelines note, “In infants and children with bronchiolitis, no data exist to suggest that such increases [in PaO2 and SpO2] result in any clinically significant differences in physiologic function, patient symptoms, or clinical outcomes.”2
A relatively new method of administration of oxygen to infants with bronchiolitis is via a humidified, heated, high-flow nasal cannula (HHHFNC). This therapy has been shown to generate continuous positive airway pressure, which improves respiratory effort, reduces the work of breathing, and may decrease the need for intubation.2
Patient Disposition
One of the most challenging tasks for emergency physicians (EPs) is determining the appropriate disposition of infants with bronchiolitis. The variable presentation and dynamic nature of the disease make this particularly difficult. Patients at high risk for apnea should be admitted to the hospital for observation and further care as needed. Admission also should be strongly considered for those with significantly increased work of breathing and tachypnea that does not improve with suctioning—especially when these interfere with feeding. Infants with poor feeding or evidence of dehydration should be admitted to the hospital for intravenous (IV) fluid hydration or nasogastric feedings. Patients with hypoxia (SpO2 saturations <90%) should also be admitted for supplemental oxygen therapy. It should be noted, however, the AAP recommends “spot-checks” over continuous pulse oximetry in patients who do not require oxygen therapy.2
Another important factor affecting patient disposition is the ability of the caregiver to provide basic patient care and ensure close outpatient follow-up. Prior to discharge, caregivers should be educated on the highly dynamic nature of bronchiolitis and the signs and symptoms that would require prompt return to the ED—especially if the infant has risk factors for the development of severe disease.
Case Conclusion
Based on the patient’s symptoms, history (most notably, the recent incident of sleep apnea at home), and physical examination, the EP quickly identified this infant was at a high risk for both severe bronchiolitis and apnea and required aggressive management. Nasal suctioning was immediately performed to help clear the patient’s secretions; this, however, only slightly improved his RR and work of breathing. Although the infant’s SpO2 was greater than 90% on room air, the EP administered oxygen via HHHFNC at 6 L per minute, which produced a significant improvement in both RR and effort.
Given the patient’s age and the presence of a fever, a urinalysis was also obtained, the results of which showed no evidence of infection. Since the patient was only able to bottle-feed for a few minutes at a time, the EP initiated IV fluid hydration and contacted the hospitalist team for inpatient admission.
The infant was gradually weaned from HHHFNC on hospital day 2 but remained with suboptimal oral intake for another 24 hours. By hospital day 4, his work of breathing had improved significantly, and he was feeding well with through the assistance of pre-feeding nasal syringe suctioning. The patient was discharged home in the care of his parents later that same day with only mild tachypnea over baseline. At discharge, the EP emphasized the importance of providing close follow-up with their son’s pediatrician. The infant continued to gradually improve as an outpatient, with resolution of nasal congestion by day 12 of his illness; he returned to his baseline breathing and feeding pattern on day 14.
Dr Schneider is a pediatric emergency medicine fellow, Eastern Virginia Medical School, Children’s Hospital of The King’s Daughters, Norfolk. Dr Clingenpeel is a fellowship director of pediatric emergency medicine, and an associate professor of pediatrics, Eastern Virginia Medical School, Norfolk.
- Joseph M. Evidence-based assessment and management of acute bronchiolitis in the emergency department. Pediatr Emerg Med Pract. 2011;8(3):1-20.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guidelines: the diagnosis, management, and prevention of bronchiolitis [Published correction appears in Pediatrics. 2014;134(5):e1474-e1502]. Pediatrics. 2014;134:5 e1474-e1502.
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-20009. Pediatrics. 2013;32(1):28-36.
- Centers for Disease Control and Prevention (CDC). Respiratory syncytial virus activity—United States, July 2011-January 2013. MMWR Morb Mortal Wkly Rep. 2013;62(8):141-144.
- Harper MB, Fleisher GR. Infectious emergencies. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2010:916-917.
- Willwerth BM, Harper MB, Greenes DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48(4):441-447.
- Shaw KN, Bell LM, Sherman NH. Outpatient assessment of infants with bronchiolitis. Am J Dis Child. 1991;145(2):151-155.
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145(1):106-109.
- Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433.
- Levine DA, Platt SL, Dayan PS, et al; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infection. Pediatrics. 2004;113(6):1728-1734.
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;(6):CD001266.
- Corneli HM, Zorc JJ, Majahan P, et al; Bronchiolitis Study Group of the Pediatric Emergency Care Applied Research Network (PECARN). A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357(4):331-339.
- Joseph M. Evidence-based assessment and management of acute bronchiolitis in the emergency department. Pediatr Emerg Med Pract. 2011;8(3):1-20.
- Ralston SL, Lieberthal AS, Meissner HC, et al; American Academy of Pediatrics. Clinical practice guidelines: the diagnosis, management, and prevention of bronchiolitis [Published correction appears in Pediatrics. 2014;134(5):e1474-e1502]. Pediatrics. 2014;134:5 e1474-e1502.
- Hasegawa K, Tsugawa Y, Brown DF, Mansbach JM, Camargo CA Jr. Trends in bronchiolitis hospitalizations in the United States, 2000-20009. Pediatrics. 2013;32(1):28-36.
- Centers for Disease Control and Prevention (CDC). Respiratory syncytial virus activity—United States, July 2011-January 2013. MMWR Morb Mortal Wkly Rep. 2013;62(8):141-144.
- Harper MB, Fleisher GR. Infectious emergencies. In: Fleisher GR, Ludwig S, eds. Textbook of Pediatric Emergency Medicine. 6th ed. Philadelphia, PA: Lippincott Williams & Wilkins;2010:916-917.
- Willwerth BM, Harper MB, Greenes DS. Identifying hospitalized infants who have bronchiolitis and are at high risk for apnea. Ann Emerg Med. 2006;48(4):441-447.
- Shaw KN, Bell LM, Sherman NH. Outpatient assessment of infants with bronchiolitis. Am J Dis Child. 1991;145(2):151-155.
- Wang EE, Milner RA, Navas L, Maj H. Observer agreement for respiratory signs and oximetry in infants hospitalized with lower respiratory infections. Am Rev Respir Dis. 1992;145(1):106-109.
- Schuh S, Lalani A, Allen U, et al. Evaluation of the utility of radiography in acute bronchiolitis. J Pediatr. 2007;150(4):429-433.
- Levine DA, Platt SL, Dayan PS, et al; Multicenter RSV-SBI Study Group of the Pediatric Emergency Medicine Collaborative Research Committee of the American Academy of Pediatrics. Risk of serious bacterial infection in young febrile infants with respiratory syncytial virus infection. Pediatrics. 2004;113(6):1728-1734.
- Gadomski AM, Scribani MB. Bronchodilators for bronchiolitis. Cochrane Database Syst Rev. 2014;(6):CD001266.
- Corneli HM, Zorc JJ, Majahan P, et al; Bronchiolitis Study Group of the Pediatric Emergency Care Applied Research Network (PECARN). A multicenter, randomized, controlled trial of dexamethasone for bronchiolitis. N Engl J Med. 2007;357(4):331-339.
Case Studies in Toxicology: The Perilous Pursuit of Perfection
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
Case
A 37-year-old woman had undergone outpatient tumescent liposuction of her abdominal adipose tissue. Upon initiation of the procedure, she developed acute confusion followed by agitation and hallucinations and was transported by emergency medical services to the nearest hospital.
Upon arrival to the ED, the patient was mildly agitated. Her initial vital signs were: blood pressure, 122/79 mm Hg; heart rate, 57 beats/minute; respiratory rate, 18 breaths/minute; and temperature, 96.8°F. Oxygen saturation was 100% on room air.
On examination, the patient was awake and oriented, but was restless and perseverating. She described the sensation of “dying and coming back to life.” The patient’s pupils were equal, round, and normally reactive; her skin was neither diaphoretic nor dry; her heart rate and rhythm were normal; and her lungs were clear to auscultation bilaterally. The skin of the patient’s abdomen was pale, cool, and clammy, but otherwise soft with normoactive bowel sounds. An electrocardiogram revealed sinus rhythm with normal QRS and QTc intervals.
What is tumescent liposuction?
The tumescent technique (the word tumescent derived from the Latin word meaning “to swell”) involves the infiltration of a large volume of a dilute solution of lidocaine and epinephrine into the subcutaneous fat until it becomes distended and tense. The administration of this solution allows for the removal of significant adipose tissue with minimal blood loss and without general anesthesia. Advocates of tumescent liposuction argue that this dilution and subcutaneous infiltration alter the pharmacokinetics of lidocaine, providing a safe delivery of large doses of the drug. In addition, a substantial quantity of the infiltrated lidocaine is suctioned out with the fat removal. Klein3 specifically recommends administering a dilution of 1 g lidocaine in 1,000 mL of saline (0.1%) to the patient in aliquots of up to 2 g lidocaine. Such a high dose may approach or surpass 35 mg/kg, far exceeding the recommended lidocaine infiltration dose of 7 mg/kg when mixed with epinephrine.
Several studies have investigated the safety of the tumescent technique and the risk of lidocaine toxicity. In a review of pharmacokinetic studies, patients received between 10.5 and 67.7 mg/kg of lidocaine with a reported maximum serum lidocaine concentration in all patients of only 2.93 mcg/mL (therapeutic, 1-5 mcg/mL).4 However, peak serum lidocaine concentrations may not occur for up to 28 hours following tumescent infiltration.4 Despite the purported safety findings of this and other studies, there are also reports of tumescent anesthesia-associated toxicity and fatalities.5,6
Case Continuation
The patient’s mental status slowly improved throughout her hospital stay and she was reportedly at baseline by the following morning. Her serum lidocaine concentration drawn at presentation to the ED was 9.7 mcg/mL.
What are the clinical effects of lidocaine toxicity?
The clinical effects of lidocaine overdose are dependent on both the magnitude of the exposure and the rate at which it occurs. The central nervous system (CNS) and heart are the organ systems primarily affected by lidocaine. As a local anesthetic, lidocaine inhibits the action potential formation in electrically excitable cells. This reversible inhibition of the voltage-gated sodium channels prevents the influx of positively charged sodium ions and the resultant depolarization of the cell. Lidocaine, initially or at low concentrations, has a quiescent effect on neurons, which explains its therapeutic use as a local anesthetic.
Similarly, lidocaine initially reduces impulse propagation through the cardiac conduction system and can be used to treat rhythm disturbances. When used for the suppression of cardiac dysrhythmias, the therapeutic serum concentration of lidocaine is 1 to 5 mcg/mL. Subjective symptoms of lidocaine toxicity arising from therapeutic use (primarily when it is used to manage dysrhythmia) include light-headedness, disorientation, confusion, and psychosis, and are associated with serum concentrations of 3 to 6 mcg/mL. As the concentration increases, the clinical effects of lidocaine appear to shift from inhibitory to excitatory. At serum concentrations of 5 to 9 mcg/mL, objective symptoms, such as excitation, tremor, and seizure predominate. As the concentration continues to rise, coma, respiratory depression, cardiovascular (CV) collapse, and death may occur.7
The exact mechanism of this toxicity transition from inhibitory effect of sodium channel blockade to excitatory effect is not well understood. Some suggest a preferential inhibition of inhibitory interneurons in the CNS is responsible.8 Another potential mechanism is a concentration-dependent inhibition of the potassium rectifier channel.9 Inhibition of the efflux of positively charged potassium ions would result in slowing cellular repolarization, leaving the cells in a relatively excitable state. In the CNS, this produces seizures; in the heart, it may result in dysrhythmia. Cardiovascular collapse may occur with very high serum concentrations of lidocaine or following a rapid serum increase—eg, after a large intravenous (IV) bolus dose,7 which can potentially result from an unintentional intravascular injection during tumescent liposuction.
What is the treatment for lidocaine toxicity?
The first step in the treatment of lidocaine-associated CNS toxicity is the discontinuation of the drug. Failure to appropriately recognize the symptoms of early lidocaine toxicity may result in the progression to severe CNS effects and eventual CV collapse. Benzodiazepines should be used as needed for mild symptoms. Seizures should be treated aggressively with benzodiazepines or barbiturates, while ensuring maintenance of oxygenation, ventilation, and perfusion.7
In cases of lidocaine-associated CV toxicity, treatment begins with airway management, oxygen administration, and life support. Potential antidotal treatment of severe local anesthetic-associated CV toxicity involves the rapid administration of IV fat emulsion, or “lipid rescue.” Although best studied for bupivacaine toxicity, the exact mechanism of IV fat emulsion as an antidote is not completely understood. However, in the treatment of local anesthetic toxicity, lipid rescue is believed to offer a “sink” to remove the lipid-soluble anesthetics from their site of action and trap them within the vascular space. Suggested dosing of 20% lipid solution is a bolus of 1.5 mL/kg over a 1-minute period, followed by 0.25mL/kg per minute or 15 mL/kg per hour to run over 30 to 60 minutes.10
Case Conclusion
The patient made a full recovery and was discharged home in normal condition. Her healthcare provider was informed about the complication of the procedure.
Dr Hines is a senior toxicology fellow, department of emergency medicine, New York University School of Medicine. Dr Nelson, editor of “Case Studies in Toxicology,” is a professor in the department of emergency medicine and director of the medical toxicology fellowship program at the New York University School of Medicine and the New York City Poison Control Center. He is also associate editor, toxicology, of the EMERGENCY MEDICINE editorial board.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.
- Lozinski A, Huq NS. Tumescent liposuction. Clin Plastic Surg. 2013;40(4):593-613.
- Klein JA. Tumescent technique chronicles: local anesthesia, liposuction, and beyond. Dermatol Surg. 1995;21(5):449-457.
- Klein JA. Tumescent technique for regional anesthesia permits lidocaine doses of 35 mg/kg for liposuction. J Dermatol Surg Oncol. 1990;16(3):248-263.
- Conroy PH, O’Rourke J. Tumescent anesthesia. The Surgeon. 2012;210-201.
- Rao RR, Fly SF, Hoffman RS. Deaths related to liposuction. N Engl J Med. 1999;340(19):1471-1475.
- Martinez MA, Ballesteros S, Segura LJ, Garcia M. Reporting a fatality during tumescent liposuction. Forensic Sci Int. 2008;178(1):e11-e-16.
- Schwartz DR, Kaufman B. Local anesthestics. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:921-930.
- Tanaka K, Yamasaki M. Blocking of cortical inhibitory synapses by intravenous lidoaine. Nature. 1966;209(5019):207-208.
- Friederich P, Benzenberg D, Urban BW. Bupivacaine inhibits human neuronal Kv3 channels in SH-SY5Y human neuroblastoma cells. Br J Anaesth. 2002;88(6):864-866.
- Bania TC. Antidotes in depth, intravenous fat emulsions. In: Hoffman RS, Howland MA, Lewin NA, Nelson LS, Goldfrank LR. Goldfrank’s Toxicologic Emergencies. 10th ed. New York, NY: McGraw Hill; 2015:931-937.