User login
The Utility and Interpretation of Ambulatory Glucose Profiles
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.
Achieving the glycemic target—a key goal of diabetes management—remains elusive despite pharmacological and technological advances in insulin delivery and glucose monitoring
FACULTY
Davida F. Kruger, MSN, APN-BC, BC-ADM
Certified Nurse Practitioner
Henry Ford Medical Group
Division of Endocrinology, Diabetes, Bone, and Mineral
Disease
Detroit, Michigan
DISCLOSURES
Davida Kruger discloses that she is on the advisory boards for Abbott; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and sanofi-aventis U.S. LLC; and on the speakers’ bureaus for Animas Corporation, AstraZeneca; Boehringer Ingelheim/Eli Lilly and Company; Dexcom, Inc.; Janssen Pharmaceuticals, Inc.; Novo Nordisk; and Valeritas, Inc. She owns stock in Dexcom, Inc.
Stephen Brunton, MD, discloses that he is on the advisory boards and speakers’ bureaus for AstraZeneca; Boehringer Ingelheim; Becton, Dickinson and Company; Eli Lilly and Company; Janssen Pharmaceuticals, Inc.; and Novo Nordisk. He is also on the advisory board for Abbott Diabetes.
Click here to read the supplement.
To receive CME credit, please read the article and on completion, go to www.pcmg-us.org/agpCME to complete the online evaluation and receive your certificate of completion.
September 2016: Click for Credit
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Here are 5 articles in the August issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Women With BRCA1 Mutations at Higher Risk for Endometrial Cancers
To take the posttest, go to: http://bit.ly/2t6SPIY
Expires June 30, 2017
VITALSKey clinical point: Clinicians may wish to discuss the option of hysterectomy at the time of salpingo-oophorectomy in women with deleterious BRCA1 mutations.
Major finding: Among women with BRCA1 but not BRCA2 mutations there was increased risk for serous/serous-like endometrial carcinomas.
Data source: Prospective multicenter follow-up study of 1,083 women with BRCA mutations who underwent salpingo-oophorectomy without hysterectomy.
Disclosures: The study was supported by grants from the Department of Defense, National Institutes of Health, and public and private foundations. Coauthor Robert Soslow, MD, disclosed consulting for EMD Serono. No others reported conflicts of interest. The editorialists reported no conflicts of interest related to the study.
2. Cochrane Review: Topical Steroid—Vitamin D Combo Best for Scalp Psoriasis
To take the posttest, go to: http://bit.ly/2sIyLNI
Expires July 14, 2017
VITALSKey clinical point: The combination of a topical steroid and topical vitamin D is marginally better but with a similar safety profile to steroids alone as a treatment for psoriasis on the scalp.
Major finding: The combination of a topical steroid and vitamin D showed a small but statistically significant advantage over steroids alone, and a greater advantage over vitamin D alone.
Data source: A systematic review of 59 randomized controlled studies in 11,561 patients.
Disclosures: The study was supported by the Universidade Federal de São Paulo, Brazil; the Universidade Federal do Rio Grande do Norte, Brazil; and the National Institute for Health Research, United Kingdom. Six authors and one clinical referee declared speakers' fees, research grants, and funding from the pharmaceutical industry. One author had no conflicts of interest to disclose.
3. Study Finds Emergence of Azithromycin-resistant Gonorrhea
To take the posttest, go to: http://bit.ly/2u1nMmb
Expires July 16, 2017
VITALSKey clinical point:Resistance to azithromycin is emerging among patients diagnosed with gonorrhea.
Major finding: Among patients with gonorrhea, resistance to azithromycin increased from 0.6% in 2013 to 2.5% in 2014, predominantly in the Midwest.
Data source: An analysis of 5,093 Neisseria gonorrhoeae isolates from 27 clinics as part of the CDC's Gonococcal Isolate Surveillance Project.
Disclosures: The researchers had no financial disclosures.
4. Statins Improve Ovarian Cancer Survival
To take the posttest, go to: http://bit.ly/2t6swCF
Expires June 16, 2017
VITALSKey clinical point: The risk of all-cause mortality in ovarian cancer patients on statin therapy was reduced by one-third.
Major finding: Mean survival in a large cohort of women with stage III ovarian cancer was 5.8 months longer among those on statin therapy.
Data source: A retrospective study of 1,510 women diagnosed with epithelial ovarian cancer during 2007-2009.
Disclosures: Dr. Vogel reported having no financial conflicts regarding this study, conducted without commercial support.
5. Common Surgeries Linked to Chronic Opioid Use Among Opioid-naive Patients
To take the posttest, go to: http://bit.ly/2ub9fFg
Expires June 18, 2017
VITALSKey clinical point: Common surgeries increase the risk of chronic opioid use in opioid-naive adults, especially among those using antidepressants or benzodiazepines before their operations, and those with substance abuse histories.
Major finding: After adjustment for potential confounders, knee replacement increased the risk fivefold; open cholecystectomy almost fourfold; and total hip replacement and simple mastectomy almost threefold.
Data source: Insurance claims of more than 18 million people.
Disclosures: The authors had no disclosures. The work was funded in part by the Foundation for Anesthesia Education and Research and the Anesthesia Quality Institute. Claims data came from MarketScan (Truven Health Analytics).
Scaling Up Efforts to Bring Weight Down: An Update on Recommendations, Techniques, and Pharmacotherapies for Adult Weight Management
Obesity meets 3 standard defining criteria of a disease: it is associated with impairment of normal bodily function, has characteristic signs and symptoms, and results in bodily harm.1 Accordingly, authoritative organizations, including the American Medical Association, formally recognize obesity as a disease—more specifically, a chronic, relapsing, neurobehavioral disease.1-6
Click here to read the activity.
Click here to complete the posttest and evaluation.
Obesity meets 3 standard defining criteria of a disease: it is associated with impairment of normal bodily function, has characteristic signs and symptoms, and results in bodily harm.1 Accordingly, authoritative organizations, including the American Medical Association, formally recognize obesity as a disease—more specifically, a chronic, relapsing, neurobehavioral disease.1-6
Click here to read the activity.
Click here to complete the posttest and evaluation.
Obesity meets 3 standard defining criteria of a disease: it is associated with impairment of normal bodily function, has characteristic signs and symptoms, and results in bodily harm.1 Accordingly, authoritative organizations, including the American Medical Association, formally recognize obesity as a disease—more specifically, a chronic, relapsing, neurobehavioral disease.1-6
Click here to read the activity.
Click here to complete the posttest and evaluation.
Systemic Lupus Erythematosus: The Devastatingly Deceptive Disease
CE/CME No: CR-1608
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and explain the various clinical manifestations of systemic lupus erythematosus (SLE).
• Define the differential diagnosis for SLE.
• List the elements of the laboratory work-up used in the diagnosis of lupus.
• Describe the therapeutic options for patients with SLE.
FACULTY
Michael Felz is an Assistant Professor at Augusta University (formerly Georgia Regents University) in Augusta, Georgia. Mary Bailey Wickham is a PA student in her final year at Augusta University.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2016.
Article begins on next page >>
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that often goes undiagnosed initially. Timely detection of SLE is important, because prompt treatment can prevent its many major complications—notably, end organ damage. Here’s how to distinguish SLE from other illnesses with similar presentations and how to recognize the complications of undiagnosed SLE, which can progress rapidly and fatally.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disorder that can involve multiple organ systems. The presence of antinuclear antibodies (ANA) is a common marker for this disease. In autoimmune diseases such as SLE, the immune system attacks the cells of healthy tissues throughout the body. Genetic, hormonal, and environmental factors (eg, ultraviolet light, infectious viruses, and even use of certain medications) have been implicated in the pathogenesis.1-3
It is estimated that 1.5 million people in the United States and up to 5 million people worldwide have SLE.4 It is nine to 10 times more prevalent in women—especially those of reproductive age—than menand occurs more frequently in African-American, Hispanic, and Asian women than in non-Hispanic Caucasian women.1,2,4-6 Siblings of SLE patients are 30 times more likely to develop the disease, compared to individuals without an affected relative.2 Increased mortality in persons with SLE is attributed to accelerated atherosclerosis, infection, malignancy, and target organ damage, particularly end-stage renal disease.3 Women ages 33 to 45 with SLE are at increased risk (50x greater) for myocardial infarction due to premature atherosclerosis than age-matched women in the general population.7 The life expectancy of SLE patients with renal damage is 23.7 years less than that of the general population.8
Increased awareness of SLE has led to drastic improvements in associated mortality over the past five decades. The survival rate in the 1950s was 50% at 2 years, while current rates are about 95% at 5 years and about 90% at 10 years.3,9 These improvements likely reflect earlier diagnosis and treatment on the part of well-informed clinicians, as well as more effective treatment.
SLE MANIFESTATIONS
SLE can affect any organ in the body with a broad spectrum of clinical manifestations, making it a devastatingly deceptive disease. Disease severity may vary by age, by organ involvement, and over time. Onset may be gradual and mild or rapidly progressive with severe organ involvement. Constitutional manifestations such as fatigue, weight loss, anorexia, and low-grade fever often serve as initial complaints. However, these features are common to a variety of infectious and inflammatory conditions, making early SLE easily overlooked and frequently misdiagnosed. 2
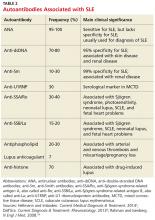
A mix of manifestations involving the joints, skin, mouth, kidneys, lungs, heart, and nervous system offers clues to the diagnosis of SLE (see Table 1). Arthritis is the most common symptom, occurring in 85% to 90% of SLE cases.1,10 It is typically nonerosive, inflammatory, symmetric or asymmetric, and polyarticular (involving five or more joints)and may be accompanied by constitutional symptoms.1,2,11 The joints most commonly affected are the proximal interphalangeals, metacarpophalangeals (MCP), knees, and wrists.2 Morning stiffness is a common complaint.1,11 Jaccoud arthropathy, which is characterized by reducible, nonerosive joint subluxations (eg, swan neck deformities, ulnar deviation, boutonniere deformities, and z-shaped thumbs), can be seen in SLE patients.3 When patients present with articular and constitutional symptoms but lack other typical manifestations of SLE, such as skin rash, appropriate measures—for example, arthrocentesis—should be taken to evaluate for infection.11
Cutaneous manifestations are the second most common feature at disease onset, with photosensitivity and malar rash being the most prevalent.10 Nearly all patients experience skin lesions at some point during the disease course.1 Diagnostic, or lupus-specific, lesions can be classified into three types: acute, subacute, and chronic.
Acute cutaneous lupus erythematosus (ACLE) is almost always associated with SLE, while subacute cutaneous lupus erythematosus (SCLE) is seen in about 50% of SLE patients.12 ACLE is usually precipitated by sunlight exposure and includes the classic erythematous, macular, “butterfly” rash located on the malar regions of the face, which may remain for days to weeks.2,12 Diffuse or discoid alopecia also may develop in ACLE, along with oral ulcers arising in purpuric necrotic lesions on the palate, buccal mucosa, or gums. Generalized erythematous, papular, or urticarial lesions may affect the face, arms, dorsa of the hands, or “V” of the neck.12
SCLE tends to be sudden in onset, with annular lesions or psoriasiform plaques on the upper trunk, arms, and dorsa of the hands that often coalesce into polycyclic lesions.12 These subacute rashes are often associated with anti-SSA/Ro antibodies.
Chronic cutaneous lupus erythematosus is usually characterized by skin disease alone.12 Discoid lupus is the most common type, with circular scaly plaques with erythematous, hyperpigmented rims and atrophic hypopigmented centers that leave scars.2,12 It is commonly seen on the face, neck, and scalp.
During the course of SLE, mucous membrane involvement—typically painless oral or nasal ulcers—occurs in 25% to 45% of patients.2 Oral lesions are most commonly found on the hard palate and buccal mucosa.3,12
Lupus nephritis, perhaps the most dangerous manifestation of SLE, conveys high risk for organ failure, a higher mortality rate compared to patients without renal involvement, and lower life expectancy.8,11 Up to 60% of Asians, African Americans, and Hispanics develop renal disease during the course of their illness.8 The dominant feature is proteinuria, typically accompanied by microscopic hematuria.2
Neuropsychiatric SLE (NPSLE) is a clinical manifestation that is poorly understood.13 An estimated 28% to 40% of NPSLE manifestations develop prior to or synchronous with the diagnosis, and 63% arise within the first year of diagnosis.13 Mild cognitive impairment is the most common manifestation,reported in up to 20% to 30% of SLE patients.2,13 Seizures and psychosis are reported in 7% to 10% of SLE patients, and psychosis—characterized by hallucinations or delusions—in 3.5%.2
Cardiac findings are common among SLE patients, with an estimated prevalence of 50%, but are rarely the presenting manifestation.14 Pericarditis with effusion is the most common cardiac manifestation, occurring in 25% of SLE patients.2 Advancing atherosclerosis due to chronic inflammation becomes a major cause of mortality in the later years for SLE patients.1 Compared to the general population, the incidence of myocardial infarction in SLE patients is increased fivefold.1 Pleuritis is the most common pleuropulmonary manifestation in SLE.11 Pleuritic chest pain with or without a pleural effusion occurs in 45% to 60% of SLE patients.2
Continue for differential diagnoses >>
DIFFERENTIAL DIAGNOSES
The differential diagnosis for SLE includes rheumatoid arthritis (RA), septic arthritis, mixed connective tissue disease (MCTD), Sjögren syndrome, systemic sclerosis (SSc), polymyositis (PM), fibromyalgia, and drug-induced lupus. Symmetrical, inflammatory, polyarticular arthritis with a predilection for the wrist and MCP joints occurs in both RA and SLE.1,15 And, because the initial articular features of SLE are symmetric arthralgias, patients with SLE are frequently misdiagnosed with RA. The absence of destructive bony erosions on radiographs and large joint effusions, along with the joint reducibility in SLE, can help distinguish it from RA.16 Asymmetric arthritis, which can be a presenting feature in both RA and SLE, is more commonly seen in the latter. ANA and rheumatoid factor test results can be positive in both disorders, but antibodies to anti-cyclic citrullinated peptides, with a 95% specificity for RA but absent in SLE, distinguish RA from SLE.1,16
Patients with MCTD display an array of overlapping features of SLE, PM, and SSc, making the diagnosis difficult.17 Although MCTD can evolve into other connective tissue diseases, such as SLE, it is nonetheless considered a distinct entity.17 High titers of anti-U1 ribonucleoprotein (anti-U1RNP) antibodies are indicative of MCTD. Anti-U1RNP is rarely detected in SLE and almost never seen in other rheumatic diseases.17 Typical manifestations of MCTD are Raynaud phenomenon, swollen fingers (referred to as “sausage digits”), and protuberant polyarthritis.17
Anti-SSA/Ro and anti-SSB/La antibodies, although detectable in SLE patients, are more commonly associated with Sjögren syndrome. In addition, patients with Sjögren syndrome frequently demonstrate signs of keratoconjunctivitis sicca and xerostomia.16
The clinical features of fibromyalgia include diffuse musculoskeletal pain that readily mimics SLE arthralgias. The 2011 modification of the 2010 American College of Rheumatology (ACR) preliminary diagnostic criteria for fibromyalgia serves as a reliable tool for diagnosing patients with nonspecific, diffuse pain.18 This 2011 modification includes 19 pain locations and the six self-reported symptoms: fatigue, impaired sleep, headaches, depression, poor cognition, and abdominal pain.18
SSc, also known as scleroderma, is characterized by skin thickening and/or CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia). The presence of anti-Scl-70 and anti-centromere antibodies are noted as well.16
Finally, a suspicion of SLE mandates an evaluation for drug-induced lupus by assessing the patient’s exposure to culprit medications, such as hydralazine, procainamide, isoniazid, methyldopa, chlorpromazine, quinidine, minocycline, and tumor necrosis factor inhibitiors.1,11 Four key features point toward drug-induced lupus:
• The female-to-male ratio is nearly equivalent.
• Nephritis and central nervous system (CNS) manifestations are not commonly present.
• Anti–double-stranded DNA (anti-dsDNA) antibodies and hypocomplementemia are absent.
• The clinical features and laboratory abnormalities return to baseline once the offending agent is removed.1
Anti-histone antibodies are present in approximately 75% of patients with drug-induced lupus but can also be seen in patients with SLE.11
Continue for laboratory work-up >>
LABORATORY WORK-UP
Laboratory abnormalities associated with SLE include anemia, leukopenia, lymphopenia, thrombocytopenia, hypocomplementemia, and proteinuria. A typical work-up includes a routine complete blood count (CBC) with differential, serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), urinalysis with microscopy, and serologic ANA titer.1,16,19 A CBC with differential may reveal hematologic abnormalities, such as anemia of chronic disease (most commonly) or autoimmune hemolytic anemia, as well as leukopenia and thrombocytopenia due to circulating autoantibodies.3 An elevated ESR and CRP indicate the severity of the systemic inflammation and/or infection. Urinalysis is effective for detecting lupus with renal diseaseand may reveal proteinuria due to renal dysfunction.2
A positive ANA titer indicates widespread activation of the immune system targeted against nuclear and cytoplasmic subparticles. The vast majority of patients with SLE will develop a positive ANA with a high titer at some point during the course of their disease.16 The ANA is highly sensitive for SLE (93% to 95%) but lacks specificity (57%).20The most common tests for ANA are enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence assay (IFA). ELISA is more sensitive in detecting ANA, while IFA is the gold standard due to its high specificity.21 Some laboratories may use immunoassay as a screening tool for ANA and then use IFA to confirm positive or equivocal results.21 Positive ANA results can be seen in patients with other rheumatologic diseases and in up to 15% of all healthy persons, but with low or borderline titers.22 For these reasons, ANA testing alone is a poor predictor of SLE.
When either the ANA test results are positive or are negative but a strong clinical suspicion for SLE remains, clinicians should order tests for antibodies to extractable nuclear antigens (ENA panel; see Table 2).3,16 Anti-dsDNA and anti-Smith (anti-Sm) antibodies are both specific for SLE, and levels of anti-dsDNA reflect disease activity in many patients.1,19 In contrast, anti-dsDNA antibodies are found in fewer than 0.5% of healthy individuals and patients with other autoimmune conditions.19 Among patients with high levels of anti-dsDNA antibodies and clinically inactive disease, 80% will have active disease within five years after elevated antibodies are detected.19
Autoantibodies, including ANA, anti-SSA/Ro, anti-SSB/La, and antiphospholipid antibodies, are usually detectable for many years prior to the onset of symptomatic SLE, while others, such as anti-Sm and anti-U1RNP, appear just months before the diagnosis.23 Patients with positive ANA results who do not meet criteria for SLE are still at risk for lupus and other autoimmune diseases, because complex autoimmune changes occur years before the diagnosis of SLE.23 These patients should be followed closely.
Continue for making the diagnosis >>
MAKING THE DIAGNOSIS
Diagnosing SLE may prove problematic because of the remarkable variety of relapsing and remitting clinical features, mimicry of similar conditions, and lack of a simple, definitive diagnostic test. Initial diagnosis of SLE depends on the disease manifestation, published criteria, and exclusion of alternative diagnoses. Confirmation requires careful clinical assessment, based on a thorough medical history and complete physical examination, along with specific laboratory testing.1,16 Biopsy results indicative of lupus nephritis in the presence of ANA or anti-dsDNA antibodies also confirm the diagnosis of SLE.24
Although created for research purposes, ACR classification criteria for SLE, published in 1982 and revised in 1997, have been used for more than 30 years to diagnose lupus (see www.rheumatology.org/Practice-Quality/Clinical-Support/Criteria/ACR-Endorsed-Criteria). In 2012, the Systemic Lupus International Collaborating Clinics (SLICC) group revised the 1997 ACR classification criteria to address major flaws and to improve clinical precision.24 According to SLICC, a definitive diagnosis requires the presence of at least four of 17 criteria, including at least one clinical and one immunologic criterion.24 The SLICC revisions have resulted in fewer misclassifications and provide greater sensitivity but lower specificity in the identification of SLE in comparison to the 1997 ACR criteria.24 To date, no one set of criteria allows for early diagnosis of SLE.
MANAGEMENT OPTIONS
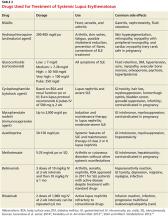
Treatment must be tailored to the patient’s specific organ system involvement. Effective therapy hinges on controlling symptoms and reducing underlying inflammation.25 Four classes of drugs are used: NSAIDs, antimalarial drugs, corticosteroids, and cytotoxic drugs (see Table 3). Most patients benefit from NSAIDs to alleviate minor arthritis and arthralgia symptoms, but the risk for peptic ulcers and nephrotoxicity should be addressed; this may require the concomitant use of gastroprotective agents such as proton pump inhibitors.25 Antimalarials are effective for musculoskeletal symptoms that do not respond to NSAIDs and for cutaneous rashes.1 The current antimalarial drug of choice is hydroxychloroquine (200 to 400 mg/d po), which has been shown to control SLE manifestations by reducing and preventing disease flares.1,11,26 It is well tolerated and can be used for the duration of treatment.11,26 Patients should be informed that this drug’s onset of action is one month.26 In rare cases, this drug can cause retinal toxicity; therefore, SLE patients receiving hydroxychloroquine should be referred to an ophthalmologist for a baseline eye examination and yearly assessments to monitor for this rare adverse effect.25,26
Low-dose corticosteroids, such as oral prednisolone or methylprednisolone, are employed when NSAIDs and antimalarials fail to control arthritis or cutaneous SLE eruptions.25 Major systemic manifestations that occur during a disease flare—such as severe arthritis, hemolytic anemia, glomerulonephritis, alveolar hemorrhage, pericarditis, pleurisy, or CNS involvement—necessitate high-dose IV corticosteroids in conjunction with immunosuppressive agents.1,11,25 These high-dose glucocorticoids should be gradually withdrawn as soon as remission is achieved.11 Long-term suppressive therapy with oral corticosteroids in addition to other agents is often needed to preserve organ function.25
The major adverse effects of long-term glucocorticoids are osteoporosis, hypertension, hyperlipidemia, glucose intolerance, and susceptibility to infection. It is recommended that patients taking prednisolone 7.5 mg/d or more undergo a bone mineral density scan every two years.25 Those with T scores below –2.5 should be prescribed bisphosphonates.25
Immunosuppressive agents, such as cyclophosphamide, mycophenolate mofetil, and azathioprine, are used in conjunction with corticosteroids or when syndromes are resistant to corticosteroids.1 Collaboration between primary care, rheumatology, and nephrology is advisable for patients requiring immunosuppressive or disease-modifying pharmacologic agents.
Two new treatments for SLE are the immunologic agents belimumab and rituximab.7 Belimumab, a monoclonal human antibody, is the first medication in the past 50 years that has been approved by the FDA for antibody-positive SLE patients with active lupus unresponsive to standard treatment.7,27 Rituximab is an anti-CD20 monoclonal antibody, approved by the FDA for non-Hodgkin lymphoma, chronic lymphocytic leukemia, and RA, and is now considered an option for SLE refractory to conventional treatment regimens.7,27 The efficacy of belimumab and rituximab, and the spectrum of indications for their use, are still under study, but these new therapeutic agents hold promise for the treatment of patients with refractory SLE.
Continue for helping patients live with SLE >>
HELPING PATIENTs LIVE WITH SLE
Patients with SLE have a higher mortality rate, as well as a lower quality of life, compared to the general population.28 The major contributors to a decreased quality of life are fatigue, mood disturbances (eg, depression), and chronic pain.28 Practitioners should advise SLE patients to participate in support groups and psychotherapy to alleviate the anxiety and depression associated with this chronic disease.
For patients with long-standing disease, accelerated atherosclerotic cardiovascular disease adds to morbidity and mortality. For this reason, obesity, hypertension, hyperlipidemia, and smoking are targets for intervention. Lifestyle modifications—such as exercise, smoking cessation, a healthy diet with low saturated fat, stress avoidance, and adequate rest—are recommended.26
Avoiding overexposure to sunlight, by using sunscreen with an SPF of at least 30 and wearing sun-protective clothing, is essential for management of cutaneous lupus.25,26 Yearly influenza vaccination is appropriate, as are other immunizations (eg, pneumococcal vaccine).26
Advise women of childbearing age with SLE that lupus flares result in a high risk for miscarriage. All women should undergo yearly cervical cancer screening.26
Patients taking long-term glucocorticoids should adopt bone-protective behaviors, including quitting smoking, limiting alcohol intake, partaking in weight-bearing exercise, and consuming dietary calcium and vitamin D.25 Patients taking these drugs should avoid live virus vaccines. Those on immunosuppressive therapy should be warned about the hazardous adverse effects of glucocorticoids.
MONITORING AND FOLLOW-UP
Collaborative efforts between primary care providers and several types of specialty providers can facilitate coordinated interventions in the long-term management of lupus. Rheumatologists are experts in making therapeutic decisions for SLE.
Patients being treated for SLE require routine monitoring to assess disease activity and detect flares. The European League Against Rheumatism (EULAR) guidelines recommend that monitoring include assessment for new clinical manifestations, routine laboratory tests, and immunologic assays, chiefly anti-dsDNA, anti-Sm, and serum complement levels, coupled with one of the validated global activity indices, such as the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).29
A routine office visit with a physical examination and laboratory testing for CBC with differential, basic metabolic panel, and urinalysis every three months is recommended for patients with stable disease; patients with uncontrolled SLE may require weekly visits.11,29 Patients taking immunosuppressive drugs should be provided with adverse-effect profiles alerting them to toxicity symptoms and require frequent laboratory monitoring for potential toxicity.11
CONCLUSION
Advances in immunologically targeted serologic tests have shed more light on the underlying pathogenesis of SLE, which in turn has led to improvements in disease detection and monitoring of complications, as well as advances in therapy. Although SLE cannot be cured, emerging therapies targeting different mechanisms of SLE offer hope for patients diagnosed with this complex disease.
1. Hellmann DB, Imboden JB. Rheumatologic & immunologic disorders. In: Papadakis M, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment. 53rd ed. New York, NY: McGraw-Hill; 2014:786-836.
2. Bertsias G, Cervera R, Boumpas DT. Systemic lupus erythematosus: pathogenesis and clinical features. In: Bijlsma JWJ, ed. EULAR Textbook on Rheumatic Diseases. London: BMJ Group; 2012:476-505.
3. Dall’Era M. Chapter 21. Systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
4. Lupus Foundation of America. What is lupus? www.lupus.org/answers/entry/what-is-lupus. Accessed July 19, 2016.
5. Furst DE, Clarke AE, Fernandes AW, et al. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2012;22(1):99-105.
6. Pons-Estel GL, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257-268.
7. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878-1888.
8. Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(8):2154-2160.
9. Merola JF, Bermas B, Lu B, et al. Clinical manifestations and survival among adults with SLE according to age at diagnosis. Lupus. 2014;23(8):778-784.
10. Font J, Cervera R, Ramos-Casals M, et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33(4):217-230.
11. Kiriakidou M, Cotton D, Taichman D, Williams S. Systemic lupus erythematosus.Ann Intern Med. 2013;159(7):2-16.
12. Wolff K, Johnson R, Saavedra A. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013:334-342.
13. Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol. 2011;9(3):449-457.
14. Chen PY, Chang CH, Hsu CC, et al. Systemic lupus erythematosus presenting with cardiac symptoms. Am J Emerg Med. 2014;32(9):1117-1119.
15. Hahn BHH. Chapter 378: Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
16. Wallace DJ. Diagnosis and differential diagnosis of systemic lupus erythematosus in adults. UpToDate. www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-systemic-lupus-erythematosus-in-adults. Accessed July 19, 2016.
17. Cappelli S, Bellando Randone S, Martinovic D, et al. “To be or not to be,” ten years after: evidence for mixed connective tissue disease as a distinct entity. Semin Arthritis Rheum. 2012;41(4):589-598.
18. Bennett RM, Friend R, Marcus D, et al. Criteria for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American College of Rheumatology criteria and the development of alternative criteria. Arthritis Care Res (Hoboken). 2014;66(9):1364-1373.
19. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929-939.
20. Magrey M, Abelson A. Laboratory evaluation of rheumatic diseases. Cleveland Clinic Center for Continuing Education 2010. www.cleveland clinicmeded.com/medicalpubs/diseasemanagement/rheumatology/laboratory-evaluation-rheumatic-diseases/. Accessed July 19, 2016.
21. Copple SS, Sawitzke AD, Wilson AM, et al. Enzyme-linked immunosorbent assay screening then indirect immunofluorescence confirmation of antinuclear antibodies: a statistical analysis. Am J Clin Pathol. 2011;135(5):678-684.
22. Von Feld JM; American College of Rheumatology. Antinuclear antibodies (ANA). 2015. www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Antinuclear-Antibodies-ANA. Accessed July 19, 2016.
23. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533.
24. Petri M, Orbai A, Alarcón G, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677-2686.
25. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: a therapeutic challenge. Postgrad Med J. 2002;78:599-606.
26. Dall’Era M, Wofsy D. Treatment of systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
27. Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS–lupus connection. Nat Biotechnol. 2012;30(1):69-77.
28. Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Research & Ther. 2012;14(suppl 4):S4.
29. Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67(2):195-205.
CE/CME No: CR-1608
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and explain the various clinical manifestations of systemic lupus erythematosus (SLE).
• Define the differential diagnosis for SLE.
• List the elements of the laboratory work-up used in the diagnosis of lupus.
• Describe the therapeutic options for patients with SLE.
FACULTY
Michael Felz is an Assistant Professor at Augusta University (formerly Georgia Regents University) in Augusta, Georgia. Mary Bailey Wickham is a PA student in her final year at Augusta University.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2016.
Article begins on next page >>
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that often goes undiagnosed initially. Timely detection of SLE is important, because prompt treatment can prevent its many major complications—notably, end organ damage. Here’s how to distinguish SLE from other illnesses with similar presentations and how to recognize the complications of undiagnosed SLE, which can progress rapidly and fatally.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disorder that can involve multiple organ systems. The presence of antinuclear antibodies (ANA) is a common marker for this disease. In autoimmune diseases such as SLE, the immune system attacks the cells of healthy tissues throughout the body. Genetic, hormonal, and environmental factors (eg, ultraviolet light, infectious viruses, and even use of certain medications) have been implicated in the pathogenesis.1-3
It is estimated that 1.5 million people in the United States and up to 5 million people worldwide have SLE.4 It is nine to 10 times more prevalent in women—especially those of reproductive age—than menand occurs more frequently in African-American, Hispanic, and Asian women than in non-Hispanic Caucasian women.1,2,4-6 Siblings of SLE patients are 30 times more likely to develop the disease, compared to individuals without an affected relative.2 Increased mortality in persons with SLE is attributed to accelerated atherosclerosis, infection, malignancy, and target organ damage, particularly end-stage renal disease.3 Women ages 33 to 45 with SLE are at increased risk (50x greater) for myocardial infarction due to premature atherosclerosis than age-matched women in the general population.7 The life expectancy of SLE patients with renal damage is 23.7 years less than that of the general population.8
Increased awareness of SLE has led to drastic improvements in associated mortality over the past five decades. The survival rate in the 1950s was 50% at 2 years, while current rates are about 95% at 5 years and about 90% at 10 years.3,9 These improvements likely reflect earlier diagnosis and treatment on the part of well-informed clinicians, as well as more effective treatment.
SLE MANIFESTATIONS
SLE can affect any organ in the body with a broad spectrum of clinical manifestations, making it a devastatingly deceptive disease. Disease severity may vary by age, by organ involvement, and over time. Onset may be gradual and mild or rapidly progressive with severe organ involvement. Constitutional manifestations such as fatigue, weight loss, anorexia, and low-grade fever often serve as initial complaints. However, these features are common to a variety of infectious and inflammatory conditions, making early SLE easily overlooked and frequently misdiagnosed. 2
A mix of manifestations involving the joints, skin, mouth, kidneys, lungs, heart, and nervous system offers clues to the diagnosis of SLE (see Table 1). Arthritis is the most common symptom, occurring in 85% to 90% of SLE cases.1,10 It is typically nonerosive, inflammatory, symmetric or asymmetric, and polyarticular (involving five or more joints)and may be accompanied by constitutional symptoms.1,2,11 The joints most commonly affected are the proximal interphalangeals, metacarpophalangeals (MCP), knees, and wrists.2 Morning stiffness is a common complaint.1,11 Jaccoud arthropathy, which is characterized by reducible, nonerosive joint subluxations (eg, swan neck deformities, ulnar deviation, boutonniere deformities, and z-shaped thumbs), can be seen in SLE patients.3 When patients present with articular and constitutional symptoms but lack other typical manifestations of SLE, such as skin rash, appropriate measures—for example, arthrocentesis—should be taken to evaluate for infection.11
Cutaneous manifestations are the second most common feature at disease onset, with photosensitivity and malar rash being the most prevalent.10 Nearly all patients experience skin lesions at some point during the disease course.1 Diagnostic, or lupus-specific, lesions can be classified into three types: acute, subacute, and chronic.
Acute cutaneous lupus erythematosus (ACLE) is almost always associated with SLE, while subacute cutaneous lupus erythematosus (SCLE) is seen in about 50% of SLE patients.12 ACLE is usually precipitated by sunlight exposure and includes the classic erythematous, macular, “butterfly” rash located on the malar regions of the face, which may remain for days to weeks.2,12 Diffuse or discoid alopecia also may develop in ACLE, along with oral ulcers arising in purpuric necrotic lesions on the palate, buccal mucosa, or gums. Generalized erythematous, papular, or urticarial lesions may affect the face, arms, dorsa of the hands, or “V” of the neck.12
SCLE tends to be sudden in onset, with annular lesions or psoriasiform plaques on the upper trunk, arms, and dorsa of the hands that often coalesce into polycyclic lesions.12 These subacute rashes are often associated with anti-SSA/Ro antibodies.
Chronic cutaneous lupus erythematosus is usually characterized by skin disease alone.12 Discoid lupus is the most common type, with circular scaly plaques with erythematous, hyperpigmented rims and atrophic hypopigmented centers that leave scars.2,12 It is commonly seen on the face, neck, and scalp.
During the course of SLE, mucous membrane involvement—typically painless oral or nasal ulcers—occurs in 25% to 45% of patients.2 Oral lesions are most commonly found on the hard palate and buccal mucosa.3,12
Lupus nephritis, perhaps the most dangerous manifestation of SLE, conveys high risk for organ failure, a higher mortality rate compared to patients without renal involvement, and lower life expectancy.8,11 Up to 60% of Asians, African Americans, and Hispanics develop renal disease during the course of their illness.8 The dominant feature is proteinuria, typically accompanied by microscopic hematuria.2
Neuropsychiatric SLE (NPSLE) is a clinical manifestation that is poorly understood.13 An estimated 28% to 40% of NPSLE manifestations develop prior to or synchronous with the diagnosis, and 63% arise within the first year of diagnosis.13 Mild cognitive impairment is the most common manifestation,reported in up to 20% to 30% of SLE patients.2,13 Seizures and psychosis are reported in 7% to 10% of SLE patients, and psychosis—characterized by hallucinations or delusions—in 3.5%.2
Cardiac findings are common among SLE patients, with an estimated prevalence of 50%, but are rarely the presenting manifestation.14 Pericarditis with effusion is the most common cardiac manifestation, occurring in 25% of SLE patients.2 Advancing atherosclerosis due to chronic inflammation becomes a major cause of mortality in the later years for SLE patients.1 Compared to the general population, the incidence of myocardial infarction in SLE patients is increased fivefold.1 Pleuritis is the most common pleuropulmonary manifestation in SLE.11 Pleuritic chest pain with or without a pleural effusion occurs in 45% to 60% of SLE patients.2
Continue for differential diagnoses >>
DIFFERENTIAL DIAGNOSES
The differential diagnosis for SLE includes rheumatoid arthritis (RA), septic arthritis, mixed connective tissue disease (MCTD), Sjögren syndrome, systemic sclerosis (SSc), polymyositis (PM), fibromyalgia, and drug-induced lupus. Symmetrical, inflammatory, polyarticular arthritis with a predilection for the wrist and MCP joints occurs in both RA and SLE.1,15 And, because the initial articular features of SLE are symmetric arthralgias, patients with SLE are frequently misdiagnosed with RA. The absence of destructive bony erosions on radiographs and large joint effusions, along with the joint reducibility in SLE, can help distinguish it from RA.16 Asymmetric arthritis, which can be a presenting feature in both RA and SLE, is more commonly seen in the latter. ANA and rheumatoid factor test results can be positive in both disorders, but antibodies to anti-cyclic citrullinated peptides, with a 95% specificity for RA but absent in SLE, distinguish RA from SLE.1,16
Patients with MCTD display an array of overlapping features of SLE, PM, and SSc, making the diagnosis difficult.17 Although MCTD can evolve into other connective tissue diseases, such as SLE, it is nonetheless considered a distinct entity.17 High titers of anti-U1 ribonucleoprotein (anti-U1RNP) antibodies are indicative of MCTD. Anti-U1RNP is rarely detected in SLE and almost never seen in other rheumatic diseases.17 Typical manifestations of MCTD are Raynaud phenomenon, swollen fingers (referred to as “sausage digits”), and protuberant polyarthritis.17
Anti-SSA/Ro and anti-SSB/La antibodies, although detectable in SLE patients, are more commonly associated with Sjögren syndrome. In addition, patients with Sjögren syndrome frequently demonstrate signs of keratoconjunctivitis sicca and xerostomia.16
The clinical features of fibromyalgia include diffuse musculoskeletal pain that readily mimics SLE arthralgias. The 2011 modification of the 2010 American College of Rheumatology (ACR) preliminary diagnostic criteria for fibromyalgia serves as a reliable tool for diagnosing patients with nonspecific, diffuse pain.18 This 2011 modification includes 19 pain locations and the six self-reported symptoms: fatigue, impaired sleep, headaches, depression, poor cognition, and abdominal pain.18
SSc, also known as scleroderma, is characterized by skin thickening and/or CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia). The presence of anti-Scl-70 and anti-centromere antibodies are noted as well.16
Finally, a suspicion of SLE mandates an evaluation for drug-induced lupus by assessing the patient’s exposure to culprit medications, such as hydralazine, procainamide, isoniazid, methyldopa, chlorpromazine, quinidine, minocycline, and tumor necrosis factor inhibitiors.1,11 Four key features point toward drug-induced lupus:
• The female-to-male ratio is nearly equivalent.
• Nephritis and central nervous system (CNS) manifestations are not commonly present.
• Anti–double-stranded DNA (anti-dsDNA) antibodies and hypocomplementemia are absent.
• The clinical features and laboratory abnormalities return to baseline once the offending agent is removed.1
Anti-histone antibodies are present in approximately 75% of patients with drug-induced lupus but can also be seen in patients with SLE.11
Continue for laboratory work-up >>
LABORATORY WORK-UP
Laboratory abnormalities associated with SLE include anemia, leukopenia, lymphopenia, thrombocytopenia, hypocomplementemia, and proteinuria. A typical work-up includes a routine complete blood count (CBC) with differential, serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), urinalysis with microscopy, and serologic ANA titer.1,16,19 A CBC with differential may reveal hematologic abnormalities, such as anemia of chronic disease (most commonly) or autoimmune hemolytic anemia, as well as leukopenia and thrombocytopenia due to circulating autoantibodies.3 An elevated ESR and CRP indicate the severity of the systemic inflammation and/or infection. Urinalysis is effective for detecting lupus with renal diseaseand may reveal proteinuria due to renal dysfunction.2
A positive ANA titer indicates widespread activation of the immune system targeted against nuclear and cytoplasmic subparticles. The vast majority of patients with SLE will develop a positive ANA with a high titer at some point during the course of their disease.16 The ANA is highly sensitive for SLE (93% to 95%) but lacks specificity (57%).20The most common tests for ANA are enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence assay (IFA). ELISA is more sensitive in detecting ANA, while IFA is the gold standard due to its high specificity.21 Some laboratories may use immunoassay as a screening tool for ANA and then use IFA to confirm positive or equivocal results.21 Positive ANA results can be seen in patients with other rheumatologic diseases and in up to 15% of all healthy persons, but with low or borderline titers.22 For these reasons, ANA testing alone is a poor predictor of SLE.
When either the ANA test results are positive or are negative but a strong clinical suspicion for SLE remains, clinicians should order tests for antibodies to extractable nuclear antigens (ENA panel; see Table 2).3,16 Anti-dsDNA and anti-Smith (anti-Sm) antibodies are both specific for SLE, and levels of anti-dsDNA reflect disease activity in many patients.1,19 In contrast, anti-dsDNA antibodies are found in fewer than 0.5% of healthy individuals and patients with other autoimmune conditions.19 Among patients with high levels of anti-dsDNA antibodies and clinically inactive disease, 80% will have active disease within five years after elevated antibodies are detected.19
Autoantibodies, including ANA, anti-SSA/Ro, anti-SSB/La, and antiphospholipid antibodies, are usually detectable for many years prior to the onset of symptomatic SLE, while others, such as anti-Sm and anti-U1RNP, appear just months before the diagnosis.23 Patients with positive ANA results who do not meet criteria for SLE are still at risk for lupus and other autoimmune diseases, because complex autoimmune changes occur years before the diagnosis of SLE.23 These patients should be followed closely.
Continue for making the diagnosis >>
MAKING THE DIAGNOSIS
Diagnosing SLE may prove problematic because of the remarkable variety of relapsing and remitting clinical features, mimicry of similar conditions, and lack of a simple, definitive diagnostic test. Initial diagnosis of SLE depends on the disease manifestation, published criteria, and exclusion of alternative diagnoses. Confirmation requires careful clinical assessment, based on a thorough medical history and complete physical examination, along with specific laboratory testing.1,16 Biopsy results indicative of lupus nephritis in the presence of ANA or anti-dsDNA antibodies also confirm the diagnosis of SLE.24
Although created for research purposes, ACR classification criteria for SLE, published in 1982 and revised in 1997, have been used for more than 30 years to diagnose lupus (see www.rheumatology.org/Practice-Quality/Clinical-Support/Criteria/ACR-Endorsed-Criteria). In 2012, the Systemic Lupus International Collaborating Clinics (SLICC) group revised the 1997 ACR classification criteria to address major flaws and to improve clinical precision.24 According to SLICC, a definitive diagnosis requires the presence of at least four of 17 criteria, including at least one clinical and one immunologic criterion.24 The SLICC revisions have resulted in fewer misclassifications and provide greater sensitivity but lower specificity in the identification of SLE in comparison to the 1997 ACR criteria.24 To date, no one set of criteria allows for early diagnosis of SLE.
MANAGEMENT OPTIONS
Treatment must be tailored to the patient’s specific organ system involvement. Effective therapy hinges on controlling symptoms and reducing underlying inflammation.25 Four classes of drugs are used: NSAIDs, antimalarial drugs, corticosteroids, and cytotoxic drugs (see Table 3). Most patients benefit from NSAIDs to alleviate minor arthritis and arthralgia symptoms, but the risk for peptic ulcers and nephrotoxicity should be addressed; this may require the concomitant use of gastroprotective agents such as proton pump inhibitors.25 Antimalarials are effective for musculoskeletal symptoms that do not respond to NSAIDs and for cutaneous rashes.1 The current antimalarial drug of choice is hydroxychloroquine (200 to 400 mg/d po), which has been shown to control SLE manifestations by reducing and preventing disease flares.1,11,26 It is well tolerated and can be used for the duration of treatment.11,26 Patients should be informed that this drug’s onset of action is one month.26 In rare cases, this drug can cause retinal toxicity; therefore, SLE patients receiving hydroxychloroquine should be referred to an ophthalmologist for a baseline eye examination and yearly assessments to monitor for this rare adverse effect.25,26
Low-dose corticosteroids, such as oral prednisolone or methylprednisolone, are employed when NSAIDs and antimalarials fail to control arthritis or cutaneous SLE eruptions.25 Major systemic manifestations that occur during a disease flare—such as severe arthritis, hemolytic anemia, glomerulonephritis, alveolar hemorrhage, pericarditis, pleurisy, or CNS involvement—necessitate high-dose IV corticosteroids in conjunction with immunosuppressive agents.1,11,25 These high-dose glucocorticoids should be gradually withdrawn as soon as remission is achieved.11 Long-term suppressive therapy with oral corticosteroids in addition to other agents is often needed to preserve organ function.25
The major adverse effects of long-term glucocorticoids are osteoporosis, hypertension, hyperlipidemia, glucose intolerance, and susceptibility to infection. It is recommended that patients taking prednisolone 7.5 mg/d or more undergo a bone mineral density scan every two years.25 Those with T scores below –2.5 should be prescribed bisphosphonates.25
Immunosuppressive agents, such as cyclophosphamide, mycophenolate mofetil, and azathioprine, are used in conjunction with corticosteroids or when syndromes are resistant to corticosteroids.1 Collaboration between primary care, rheumatology, and nephrology is advisable for patients requiring immunosuppressive or disease-modifying pharmacologic agents.
Two new treatments for SLE are the immunologic agents belimumab and rituximab.7 Belimumab, a monoclonal human antibody, is the first medication in the past 50 years that has been approved by the FDA for antibody-positive SLE patients with active lupus unresponsive to standard treatment.7,27 Rituximab is an anti-CD20 monoclonal antibody, approved by the FDA for non-Hodgkin lymphoma, chronic lymphocytic leukemia, and RA, and is now considered an option for SLE refractory to conventional treatment regimens.7,27 The efficacy of belimumab and rituximab, and the spectrum of indications for their use, are still under study, but these new therapeutic agents hold promise for the treatment of patients with refractory SLE.
Continue for helping patients live with SLE >>
HELPING PATIENTs LIVE WITH SLE
Patients with SLE have a higher mortality rate, as well as a lower quality of life, compared to the general population.28 The major contributors to a decreased quality of life are fatigue, mood disturbances (eg, depression), and chronic pain.28 Practitioners should advise SLE patients to participate in support groups and psychotherapy to alleviate the anxiety and depression associated with this chronic disease.
For patients with long-standing disease, accelerated atherosclerotic cardiovascular disease adds to morbidity and mortality. For this reason, obesity, hypertension, hyperlipidemia, and smoking are targets for intervention. Lifestyle modifications—such as exercise, smoking cessation, a healthy diet with low saturated fat, stress avoidance, and adequate rest—are recommended.26
Avoiding overexposure to sunlight, by using sunscreen with an SPF of at least 30 and wearing sun-protective clothing, is essential for management of cutaneous lupus.25,26 Yearly influenza vaccination is appropriate, as are other immunizations (eg, pneumococcal vaccine).26
Advise women of childbearing age with SLE that lupus flares result in a high risk for miscarriage. All women should undergo yearly cervical cancer screening.26
Patients taking long-term glucocorticoids should adopt bone-protective behaviors, including quitting smoking, limiting alcohol intake, partaking in weight-bearing exercise, and consuming dietary calcium and vitamin D.25 Patients taking these drugs should avoid live virus vaccines. Those on immunosuppressive therapy should be warned about the hazardous adverse effects of glucocorticoids.
MONITORING AND FOLLOW-UP
Collaborative efforts between primary care providers and several types of specialty providers can facilitate coordinated interventions in the long-term management of lupus. Rheumatologists are experts in making therapeutic decisions for SLE.
Patients being treated for SLE require routine monitoring to assess disease activity and detect flares. The European League Against Rheumatism (EULAR) guidelines recommend that monitoring include assessment for new clinical manifestations, routine laboratory tests, and immunologic assays, chiefly anti-dsDNA, anti-Sm, and serum complement levels, coupled with one of the validated global activity indices, such as the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).29
A routine office visit with a physical examination and laboratory testing for CBC with differential, basic metabolic panel, and urinalysis every three months is recommended for patients with stable disease; patients with uncontrolled SLE may require weekly visits.11,29 Patients taking immunosuppressive drugs should be provided with adverse-effect profiles alerting them to toxicity symptoms and require frequent laboratory monitoring for potential toxicity.11
CONCLUSION
Advances in immunologically targeted serologic tests have shed more light on the underlying pathogenesis of SLE, which in turn has led to improvements in disease detection and monitoring of complications, as well as advances in therapy. Although SLE cannot be cured, emerging therapies targeting different mechanisms of SLE offer hope for patients diagnosed with this complex disease.
CE/CME No: CR-1608
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the pathophysiology and explain the various clinical manifestations of systemic lupus erythematosus (SLE).
• Define the differential diagnosis for SLE.
• List the elements of the laboratory work-up used in the diagnosis of lupus.
• Describe the therapeutic options for patients with SLE.
FACULTY
Michael Felz is an Assistant Professor at Augusta University (formerly Georgia Regents University) in Augusta, Georgia. Mary Bailey Wickham is a PA student in her final year at Augusta University.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of August 2016.
Article begins on next page >>
Systemic lupus erythematosus (SLE) is a chronic autoimmune disease that often goes undiagnosed initially. Timely detection of SLE is important, because prompt treatment can prevent its many major complications—notably, end organ damage. Here’s how to distinguish SLE from other illnesses with similar presentations and how to recognize the complications of undiagnosed SLE, which can progress rapidly and fatally.
Systemic lupus erythematosus (SLE) is a chronic inflammatory disorder that can involve multiple organ systems. The presence of antinuclear antibodies (ANA) is a common marker for this disease. In autoimmune diseases such as SLE, the immune system attacks the cells of healthy tissues throughout the body. Genetic, hormonal, and environmental factors (eg, ultraviolet light, infectious viruses, and even use of certain medications) have been implicated in the pathogenesis.1-3
It is estimated that 1.5 million people in the United States and up to 5 million people worldwide have SLE.4 It is nine to 10 times more prevalent in women—especially those of reproductive age—than menand occurs more frequently in African-American, Hispanic, and Asian women than in non-Hispanic Caucasian women.1,2,4-6 Siblings of SLE patients are 30 times more likely to develop the disease, compared to individuals without an affected relative.2 Increased mortality in persons with SLE is attributed to accelerated atherosclerosis, infection, malignancy, and target organ damage, particularly end-stage renal disease.3 Women ages 33 to 45 with SLE are at increased risk (50x greater) for myocardial infarction due to premature atherosclerosis than age-matched women in the general population.7 The life expectancy of SLE patients with renal damage is 23.7 years less than that of the general population.8
Increased awareness of SLE has led to drastic improvements in associated mortality over the past five decades. The survival rate in the 1950s was 50% at 2 years, while current rates are about 95% at 5 years and about 90% at 10 years.3,9 These improvements likely reflect earlier diagnosis and treatment on the part of well-informed clinicians, as well as more effective treatment.
SLE MANIFESTATIONS
SLE can affect any organ in the body with a broad spectrum of clinical manifestations, making it a devastatingly deceptive disease. Disease severity may vary by age, by organ involvement, and over time. Onset may be gradual and mild or rapidly progressive with severe organ involvement. Constitutional manifestations such as fatigue, weight loss, anorexia, and low-grade fever often serve as initial complaints. However, these features are common to a variety of infectious and inflammatory conditions, making early SLE easily overlooked and frequently misdiagnosed. 2
A mix of manifestations involving the joints, skin, mouth, kidneys, lungs, heart, and nervous system offers clues to the diagnosis of SLE (see Table 1). Arthritis is the most common symptom, occurring in 85% to 90% of SLE cases.1,10 It is typically nonerosive, inflammatory, symmetric or asymmetric, and polyarticular (involving five or more joints)and may be accompanied by constitutional symptoms.1,2,11 The joints most commonly affected are the proximal interphalangeals, metacarpophalangeals (MCP), knees, and wrists.2 Morning stiffness is a common complaint.1,11 Jaccoud arthropathy, which is characterized by reducible, nonerosive joint subluxations (eg, swan neck deformities, ulnar deviation, boutonniere deformities, and z-shaped thumbs), can be seen in SLE patients.3 When patients present with articular and constitutional symptoms but lack other typical manifestations of SLE, such as skin rash, appropriate measures—for example, arthrocentesis—should be taken to evaluate for infection.11
Cutaneous manifestations are the second most common feature at disease onset, with photosensitivity and malar rash being the most prevalent.10 Nearly all patients experience skin lesions at some point during the disease course.1 Diagnostic, or lupus-specific, lesions can be classified into three types: acute, subacute, and chronic.
Acute cutaneous lupus erythematosus (ACLE) is almost always associated with SLE, while subacute cutaneous lupus erythematosus (SCLE) is seen in about 50% of SLE patients.12 ACLE is usually precipitated by sunlight exposure and includes the classic erythematous, macular, “butterfly” rash located on the malar regions of the face, which may remain for days to weeks.2,12 Diffuse or discoid alopecia also may develop in ACLE, along with oral ulcers arising in purpuric necrotic lesions on the palate, buccal mucosa, or gums. Generalized erythematous, papular, or urticarial lesions may affect the face, arms, dorsa of the hands, or “V” of the neck.12
SCLE tends to be sudden in onset, with annular lesions or psoriasiform plaques on the upper trunk, arms, and dorsa of the hands that often coalesce into polycyclic lesions.12 These subacute rashes are often associated with anti-SSA/Ro antibodies.
Chronic cutaneous lupus erythematosus is usually characterized by skin disease alone.12 Discoid lupus is the most common type, with circular scaly plaques with erythematous, hyperpigmented rims and atrophic hypopigmented centers that leave scars.2,12 It is commonly seen on the face, neck, and scalp.
During the course of SLE, mucous membrane involvement—typically painless oral or nasal ulcers—occurs in 25% to 45% of patients.2 Oral lesions are most commonly found on the hard palate and buccal mucosa.3,12
Lupus nephritis, perhaps the most dangerous manifestation of SLE, conveys high risk for organ failure, a higher mortality rate compared to patients without renal involvement, and lower life expectancy.8,11 Up to 60% of Asians, African Americans, and Hispanics develop renal disease during the course of their illness.8 The dominant feature is proteinuria, typically accompanied by microscopic hematuria.2
Neuropsychiatric SLE (NPSLE) is a clinical manifestation that is poorly understood.13 An estimated 28% to 40% of NPSLE manifestations develop prior to or synchronous with the diagnosis, and 63% arise within the first year of diagnosis.13 Mild cognitive impairment is the most common manifestation,reported in up to 20% to 30% of SLE patients.2,13 Seizures and psychosis are reported in 7% to 10% of SLE patients, and psychosis—characterized by hallucinations or delusions—in 3.5%.2
Cardiac findings are common among SLE patients, with an estimated prevalence of 50%, but are rarely the presenting manifestation.14 Pericarditis with effusion is the most common cardiac manifestation, occurring in 25% of SLE patients.2 Advancing atherosclerosis due to chronic inflammation becomes a major cause of mortality in the later years for SLE patients.1 Compared to the general population, the incidence of myocardial infarction in SLE patients is increased fivefold.1 Pleuritis is the most common pleuropulmonary manifestation in SLE.11 Pleuritic chest pain with or without a pleural effusion occurs in 45% to 60% of SLE patients.2
Continue for differential diagnoses >>
DIFFERENTIAL DIAGNOSES
The differential diagnosis for SLE includes rheumatoid arthritis (RA), septic arthritis, mixed connective tissue disease (MCTD), Sjögren syndrome, systemic sclerosis (SSc), polymyositis (PM), fibromyalgia, and drug-induced lupus. Symmetrical, inflammatory, polyarticular arthritis with a predilection for the wrist and MCP joints occurs in both RA and SLE.1,15 And, because the initial articular features of SLE are symmetric arthralgias, patients with SLE are frequently misdiagnosed with RA. The absence of destructive bony erosions on radiographs and large joint effusions, along with the joint reducibility in SLE, can help distinguish it from RA.16 Asymmetric arthritis, which can be a presenting feature in both RA and SLE, is more commonly seen in the latter. ANA and rheumatoid factor test results can be positive in both disorders, but antibodies to anti-cyclic citrullinated peptides, with a 95% specificity for RA but absent in SLE, distinguish RA from SLE.1,16
Patients with MCTD display an array of overlapping features of SLE, PM, and SSc, making the diagnosis difficult.17 Although MCTD can evolve into other connective tissue diseases, such as SLE, it is nonetheless considered a distinct entity.17 High titers of anti-U1 ribonucleoprotein (anti-U1RNP) antibodies are indicative of MCTD. Anti-U1RNP is rarely detected in SLE and almost never seen in other rheumatic diseases.17 Typical manifestations of MCTD are Raynaud phenomenon, swollen fingers (referred to as “sausage digits”), and protuberant polyarthritis.17
Anti-SSA/Ro and anti-SSB/La antibodies, although detectable in SLE patients, are more commonly associated with Sjögren syndrome. In addition, patients with Sjögren syndrome frequently demonstrate signs of keratoconjunctivitis sicca and xerostomia.16
The clinical features of fibromyalgia include diffuse musculoskeletal pain that readily mimics SLE arthralgias. The 2011 modification of the 2010 American College of Rheumatology (ACR) preliminary diagnostic criteria for fibromyalgia serves as a reliable tool for diagnosing patients with nonspecific, diffuse pain.18 This 2011 modification includes 19 pain locations and the six self-reported symptoms: fatigue, impaired sleep, headaches, depression, poor cognition, and abdominal pain.18
SSc, also known as scleroderma, is characterized by skin thickening and/or CREST syndrome (calcinosis, Raynaud phenomenon, esophageal dysmotility, sclerodactyly, telangiectasia). The presence of anti-Scl-70 and anti-centromere antibodies are noted as well.16
Finally, a suspicion of SLE mandates an evaluation for drug-induced lupus by assessing the patient’s exposure to culprit medications, such as hydralazine, procainamide, isoniazid, methyldopa, chlorpromazine, quinidine, minocycline, and tumor necrosis factor inhibitiors.1,11 Four key features point toward drug-induced lupus:
• The female-to-male ratio is nearly equivalent.
• Nephritis and central nervous system (CNS) manifestations are not commonly present.
• Anti–double-stranded DNA (anti-dsDNA) antibodies and hypocomplementemia are absent.
• The clinical features and laboratory abnormalities return to baseline once the offending agent is removed.1
Anti-histone antibodies are present in approximately 75% of patients with drug-induced lupus but can also be seen in patients with SLE.11
Continue for laboratory work-up >>
LABORATORY WORK-UP
Laboratory abnormalities associated with SLE include anemia, leukopenia, lymphopenia, thrombocytopenia, hypocomplementemia, and proteinuria. A typical work-up includes a routine complete blood count (CBC) with differential, serum creatinine, erythrocyte sedimentation rate (ESR), C-reactive protein (CRP), urinalysis with microscopy, and serologic ANA titer.1,16,19 A CBC with differential may reveal hematologic abnormalities, such as anemia of chronic disease (most commonly) or autoimmune hemolytic anemia, as well as leukopenia and thrombocytopenia due to circulating autoantibodies.3 An elevated ESR and CRP indicate the severity of the systemic inflammation and/or infection. Urinalysis is effective for detecting lupus with renal diseaseand may reveal proteinuria due to renal dysfunction.2
A positive ANA titer indicates widespread activation of the immune system targeted against nuclear and cytoplasmic subparticles. The vast majority of patients with SLE will develop a positive ANA with a high titer at some point during the course of their disease.16 The ANA is highly sensitive for SLE (93% to 95%) but lacks specificity (57%).20The most common tests for ANA are enzyme-linked immunosorbent assay (ELISA) and indirect immunofluorescence assay (IFA). ELISA is more sensitive in detecting ANA, while IFA is the gold standard due to its high specificity.21 Some laboratories may use immunoassay as a screening tool for ANA and then use IFA to confirm positive or equivocal results.21 Positive ANA results can be seen in patients with other rheumatologic diseases and in up to 15% of all healthy persons, but with low or borderline titers.22 For these reasons, ANA testing alone is a poor predictor of SLE.
When either the ANA test results are positive or are negative but a strong clinical suspicion for SLE remains, clinicians should order tests for antibodies to extractable nuclear antigens (ENA panel; see Table 2).3,16 Anti-dsDNA and anti-Smith (anti-Sm) antibodies are both specific for SLE, and levels of anti-dsDNA reflect disease activity in many patients.1,19 In contrast, anti-dsDNA antibodies are found in fewer than 0.5% of healthy individuals and patients with other autoimmune conditions.19 Among patients with high levels of anti-dsDNA antibodies and clinically inactive disease, 80% will have active disease within five years after elevated antibodies are detected.19
Autoantibodies, including ANA, anti-SSA/Ro, anti-SSB/La, and antiphospholipid antibodies, are usually detectable for many years prior to the onset of symptomatic SLE, while others, such as anti-Sm and anti-U1RNP, appear just months before the diagnosis.23 Patients with positive ANA results who do not meet criteria for SLE are still at risk for lupus and other autoimmune diseases, because complex autoimmune changes occur years before the diagnosis of SLE.23 These patients should be followed closely.
Continue for making the diagnosis >>
MAKING THE DIAGNOSIS
Diagnosing SLE may prove problematic because of the remarkable variety of relapsing and remitting clinical features, mimicry of similar conditions, and lack of a simple, definitive diagnostic test. Initial diagnosis of SLE depends on the disease manifestation, published criteria, and exclusion of alternative diagnoses. Confirmation requires careful clinical assessment, based on a thorough medical history and complete physical examination, along with specific laboratory testing.1,16 Biopsy results indicative of lupus nephritis in the presence of ANA or anti-dsDNA antibodies also confirm the diagnosis of SLE.24
Although created for research purposes, ACR classification criteria for SLE, published in 1982 and revised in 1997, have been used for more than 30 years to diagnose lupus (see www.rheumatology.org/Practice-Quality/Clinical-Support/Criteria/ACR-Endorsed-Criteria). In 2012, the Systemic Lupus International Collaborating Clinics (SLICC) group revised the 1997 ACR classification criteria to address major flaws and to improve clinical precision.24 According to SLICC, a definitive diagnosis requires the presence of at least four of 17 criteria, including at least one clinical and one immunologic criterion.24 The SLICC revisions have resulted in fewer misclassifications and provide greater sensitivity but lower specificity in the identification of SLE in comparison to the 1997 ACR criteria.24 To date, no one set of criteria allows for early diagnosis of SLE.
MANAGEMENT OPTIONS
Treatment must be tailored to the patient’s specific organ system involvement. Effective therapy hinges on controlling symptoms and reducing underlying inflammation.25 Four classes of drugs are used: NSAIDs, antimalarial drugs, corticosteroids, and cytotoxic drugs (see Table 3). Most patients benefit from NSAIDs to alleviate minor arthritis and arthralgia symptoms, but the risk for peptic ulcers and nephrotoxicity should be addressed; this may require the concomitant use of gastroprotective agents such as proton pump inhibitors.25 Antimalarials are effective for musculoskeletal symptoms that do not respond to NSAIDs and for cutaneous rashes.1 The current antimalarial drug of choice is hydroxychloroquine (200 to 400 mg/d po), which has been shown to control SLE manifestations by reducing and preventing disease flares.1,11,26 It is well tolerated and can be used for the duration of treatment.11,26 Patients should be informed that this drug’s onset of action is one month.26 In rare cases, this drug can cause retinal toxicity; therefore, SLE patients receiving hydroxychloroquine should be referred to an ophthalmologist for a baseline eye examination and yearly assessments to monitor for this rare adverse effect.25,26
Low-dose corticosteroids, such as oral prednisolone or methylprednisolone, are employed when NSAIDs and antimalarials fail to control arthritis or cutaneous SLE eruptions.25 Major systemic manifestations that occur during a disease flare—such as severe arthritis, hemolytic anemia, glomerulonephritis, alveolar hemorrhage, pericarditis, pleurisy, or CNS involvement—necessitate high-dose IV corticosteroids in conjunction with immunosuppressive agents.1,11,25 These high-dose glucocorticoids should be gradually withdrawn as soon as remission is achieved.11 Long-term suppressive therapy with oral corticosteroids in addition to other agents is often needed to preserve organ function.25
The major adverse effects of long-term glucocorticoids are osteoporosis, hypertension, hyperlipidemia, glucose intolerance, and susceptibility to infection. It is recommended that patients taking prednisolone 7.5 mg/d or more undergo a bone mineral density scan every two years.25 Those with T scores below –2.5 should be prescribed bisphosphonates.25
Immunosuppressive agents, such as cyclophosphamide, mycophenolate mofetil, and azathioprine, are used in conjunction with corticosteroids or when syndromes are resistant to corticosteroids.1 Collaboration between primary care, rheumatology, and nephrology is advisable for patients requiring immunosuppressive or disease-modifying pharmacologic agents.
Two new treatments for SLE are the immunologic agents belimumab and rituximab.7 Belimumab, a monoclonal human antibody, is the first medication in the past 50 years that has been approved by the FDA for antibody-positive SLE patients with active lupus unresponsive to standard treatment.7,27 Rituximab is an anti-CD20 monoclonal antibody, approved by the FDA for non-Hodgkin lymphoma, chronic lymphocytic leukemia, and RA, and is now considered an option for SLE refractory to conventional treatment regimens.7,27 The efficacy of belimumab and rituximab, and the spectrum of indications for their use, are still under study, but these new therapeutic agents hold promise for the treatment of patients with refractory SLE.
Continue for helping patients live with SLE >>
HELPING PATIENTs LIVE WITH SLE
Patients with SLE have a higher mortality rate, as well as a lower quality of life, compared to the general population.28 The major contributors to a decreased quality of life are fatigue, mood disturbances (eg, depression), and chronic pain.28 Practitioners should advise SLE patients to participate in support groups and psychotherapy to alleviate the anxiety and depression associated with this chronic disease.
For patients with long-standing disease, accelerated atherosclerotic cardiovascular disease adds to morbidity and mortality. For this reason, obesity, hypertension, hyperlipidemia, and smoking are targets for intervention. Lifestyle modifications—such as exercise, smoking cessation, a healthy diet with low saturated fat, stress avoidance, and adequate rest—are recommended.26
Avoiding overexposure to sunlight, by using sunscreen with an SPF of at least 30 and wearing sun-protective clothing, is essential for management of cutaneous lupus.25,26 Yearly influenza vaccination is appropriate, as are other immunizations (eg, pneumococcal vaccine).26
Advise women of childbearing age with SLE that lupus flares result in a high risk for miscarriage. All women should undergo yearly cervical cancer screening.26
Patients taking long-term glucocorticoids should adopt bone-protective behaviors, including quitting smoking, limiting alcohol intake, partaking in weight-bearing exercise, and consuming dietary calcium and vitamin D.25 Patients taking these drugs should avoid live virus vaccines. Those on immunosuppressive therapy should be warned about the hazardous adverse effects of glucocorticoids.
MONITORING AND FOLLOW-UP
Collaborative efforts between primary care providers and several types of specialty providers can facilitate coordinated interventions in the long-term management of lupus. Rheumatologists are experts in making therapeutic decisions for SLE.
Patients being treated for SLE require routine monitoring to assess disease activity and detect flares. The European League Against Rheumatism (EULAR) guidelines recommend that monitoring include assessment for new clinical manifestations, routine laboratory tests, and immunologic assays, chiefly anti-dsDNA, anti-Sm, and serum complement levels, coupled with one of the validated global activity indices, such as the Systemic Lupus Erythematosus Disease Activity Index (SLEDAI).29
A routine office visit with a physical examination and laboratory testing for CBC with differential, basic metabolic panel, and urinalysis every three months is recommended for patients with stable disease; patients with uncontrolled SLE may require weekly visits.11,29 Patients taking immunosuppressive drugs should be provided with adverse-effect profiles alerting them to toxicity symptoms and require frequent laboratory monitoring for potential toxicity.11
CONCLUSION
Advances in immunologically targeted serologic tests have shed more light on the underlying pathogenesis of SLE, which in turn has led to improvements in disease detection and monitoring of complications, as well as advances in therapy. Although SLE cannot be cured, emerging therapies targeting different mechanisms of SLE offer hope for patients diagnosed with this complex disease.
1. Hellmann DB, Imboden JB. Rheumatologic & immunologic disorders. In: Papadakis M, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment. 53rd ed. New York, NY: McGraw-Hill; 2014:786-836.
2. Bertsias G, Cervera R, Boumpas DT. Systemic lupus erythematosus: pathogenesis and clinical features. In: Bijlsma JWJ, ed. EULAR Textbook on Rheumatic Diseases. London: BMJ Group; 2012:476-505.
3. Dall’Era M. Chapter 21. Systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
4. Lupus Foundation of America. What is lupus? www.lupus.org/answers/entry/what-is-lupus. Accessed July 19, 2016.
5. Furst DE, Clarke AE, Fernandes AW, et al. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2012;22(1):99-105.
6. Pons-Estel GL, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257-268.
7. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878-1888.
8. Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(8):2154-2160.
9. Merola JF, Bermas B, Lu B, et al. Clinical manifestations and survival among adults with SLE according to age at diagnosis. Lupus. 2014;23(8):778-784.
10. Font J, Cervera R, Ramos-Casals M, et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33(4):217-230.
11. Kiriakidou M, Cotton D, Taichman D, Williams S. Systemic lupus erythematosus.Ann Intern Med. 2013;159(7):2-16.
12. Wolff K, Johnson R, Saavedra A. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013:334-342.
13. Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol. 2011;9(3):449-457.
14. Chen PY, Chang CH, Hsu CC, et al. Systemic lupus erythematosus presenting with cardiac symptoms. Am J Emerg Med. 2014;32(9):1117-1119.
15. Hahn BHH. Chapter 378: Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
16. Wallace DJ. Diagnosis and differential diagnosis of systemic lupus erythematosus in adults. UpToDate. www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-systemic-lupus-erythematosus-in-adults. Accessed July 19, 2016.
17. Cappelli S, Bellando Randone S, Martinovic D, et al. “To be or not to be,” ten years after: evidence for mixed connective tissue disease as a distinct entity. Semin Arthritis Rheum. 2012;41(4):589-598.
18. Bennett RM, Friend R, Marcus D, et al. Criteria for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American College of Rheumatology criteria and the development of alternative criteria. Arthritis Care Res (Hoboken). 2014;66(9):1364-1373.
19. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929-939.
20. Magrey M, Abelson A. Laboratory evaluation of rheumatic diseases. Cleveland Clinic Center for Continuing Education 2010. www.cleveland clinicmeded.com/medicalpubs/diseasemanagement/rheumatology/laboratory-evaluation-rheumatic-diseases/. Accessed July 19, 2016.
21. Copple SS, Sawitzke AD, Wilson AM, et al. Enzyme-linked immunosorbent assay screening then indirect immunofluorescence confirmation of antinuclear antibodies: a statistical analysis. Am J Clin Pathol. 2011;135(5):678-684.
22. Von Feld JM; American College of Rheumatology. Antinuclear antibodies (ANA). 2015. www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Antinuclear-Antibodies-ANA. Accessed July 19, 2016.
23. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533.
24. Petri M, Orbai A, Alarcón G, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677-2686.
25. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: a therapeutic challenge. Postgrad Med J. 2002;78:599-606.
26. Dall’Era M, Wofsy D. Treatment of systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
27. Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS–lupus connection. Nat Biotechnol. 2012;30(1):69-77.
28. Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Research & Ther. 2012;14(suppl 4):S4.
29. Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67(2):195-205.
1. Hellmann DB, Imboden JB. Rheumatologic & immunologic disorders. In: Papadakis M, McPhee SJ, Rabow MW, eds. Current Medical Diagnosis & Treatment. 53rd ed. New York, NY: McGraw-Hill; 2014:786-836.
2. Bertsias G, Cervera R, Boumpas DT. Systemic lupus erythematosus: pathogenesis and clinical features. In: Bijlsma JWJ, ed. EULAR Textbook on Rheumatic Diseases. London: BMJ Group; 2012:476-505.
3. Dall’Era M. Chapter 21. Systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
4. Lupus Foundation of America. What is lupus? www.lupus.org/answers/entry/what-is-lupus. Accessed July 19, 2016.
5. Furst DE, Clarke AE, Fernandes AW, et al. Incidence and prevalence of adult systemic lupus erythematosus in a large US managed-care population. Lupus. 2012;22(1):99-105.
6. Pons-Estel GL, Alarcón GS, Scofield L, et al. Understanding the epidemiology and progression of systemic lupus erythematosus. Semin Arthritis Rheum. 2010;39(4):257-268.
7. Lisnevskaia L, Murphy G, Isenberg D. Systemic lupus erythematosus. Lancet. 2014;384(9957):1878-1888.
8. Mok CC, Kwok RC, Yip PS. Effect of renal disease on the standardized mortality ratio and life expectancy of patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(8):2154-2160.
9. Merola JF, Bermas B, Lu B, et al. Clinical manifestations and survival among adults with SLE according to age at diagnosis. Lupus. 2014;23(8):778-784.
10. Font J, Cervera R, Ramos-Casals M, et al. Clusters of clinical and immunologic features in systemic lupus erythematosus: analysis of 600 patients from a single center. Semin Arthritis Rheum. 2004;33(4):217-230.
11. Kiriakidou M, Cotton D, Taichman D, Williams S. Systemic lupus erythematosus.Ann Intern Med. 2013;159(7):2-16.
12. Wolff K, Johnson R, Saavedra A. Fitzpatrick’s Color Atlas and Synopsis of Clinical Dermatology. 7th ed. New York, NY: McGraw-Hill; 2013:334-342.
13. Popescu A, Kao AH. Neuropsychiatric systemic lupus erythematosus. Curr Neuropharmacol. 2011;9(3):449-457.
14. Chen PY, Chang CH, Hsu CC, et al. Systemic lupus erythematosus presenting with cardiac symptoms. Am J Emerg Med. 2014;32(9):1117-1119.
15. Hahn BHH. Chapter 378: Systemic lupus erythematosus. In: Kasper DL, Fauci AS, Hauser SL, et al, eds. Harrison’s Principles of Internal Medicine. 19th ed. New York, NY: McGraw-Hill; 2015.
16. Wallace DJ. Diagnosis and differential diagnosis of systemic lupus erythematosus in adults. UpToDate. www.uptodate.com/contents/diagnosis-and-differential-diagnosis-of-systemic-lupus-erythematosus-in-adults. Accessed July 19, 2016.
17. Cappelli S, Bellando Randone S, Martinovic D, et al. “To be or not to be,” ten years after: evidence for mixed connective tissue disease as a distinct entity. Semin Arthritis Rheum. 2012;41(4):589-598.
18. Bennett RM, Friend R, Marcus D, et al. Criteria for the diagnosis of fibromyalgia: validation of the modified 2010 preliminary American College of Rheumatology criteria and the development of alternative criteria. Arthritis Care Res (Hoboken). 2014;66(9):1364-1373.
19. Rahman A, Isenberg DA. Systemic lupus erythematosus. N Engl J Med. 2008;358(9):929-939.
20. Magrey M, Abelson A. Laboratory evaluation of rheumatic diseases. Cleveland Clinic Center for Continuing Education 2010. www.cleveland clinicmeded.com/medicalpubs/diseasemanagement/rheumatology/laboratory-evaluation-rheumatic-diseases/. Accessed July 19, 2016.
21. Copple SS, Sawitzke AD, Wilson AM, et al. Enzyme-linked immunosorbent assay screening then indirect immunofluorescence confirmation of antinuclear antibodies: a statistical analysis. Am J Clin Pathol. 2011;135(5):678-684.
22. Von Feld JM; American College of Rheumatology. Antinuclear antibodies (ANA). 2015. www.rheumatology.org/I-Am-A/Patient-Caregiver/Diseases-Conditions/Antinuclear-Antibodies-ANA. Accessed July 19, 2016.
23. Arbuckle MR, McClain MT, Rubertone MV, et al. Development of autoantibodies before the clinical onset of systemic lupus erythematosus. N Engl J Med. 2003;349(16):1526-1533.
24. Petri M, Orbai A, Alarcón G, et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64(8):2677-2686.
25. Ioannou Y, Isenberg DA. Current concepts for the management of systemic lupus erythematosus in adults: a therapeutic challenge. Postgrad Med J. 2002;78:599-606.
26. Dall’Era M, Wofsy D. Treatment of systemic lupus erythematosus. In: Imboden JB, Hellmann DB, Stone JH, eds. Current Diagnosis & Treatment: Rheumatology. 3rd ed. New York, NY: McGraw-Hill; 2013.
27. Stohl W, Hilbert DM. The discovery and development of belimumab: the anti-BLyS–lupus connection. Nat Biotechnol. 2012;30(1):69-77.
28. Lateef A, Petri M. Unmet medical needs in systemic lupus erythematosus. Arthritis Research & Ther. 2012;14(suppl 4):S4.
29. Bertsias G, Ioannidis JP, Boletis J, et al. EULAR recommendations for the management of systemic lupus erythematosus. Report of a task force of the EULAR standing committee for international clinical studies including therapeutics. Ann Rheum Dis. 2008;67(2):195-205.
July 2016: Click for Credit
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
Here are 4 articles in the July issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Pregnancy Alters Pharmacodynamics of Anti-TNF Agents in Women With IBD
To take the posttest, go to: http://bit.ly/1VQFIHf
Expires May 24, 2017
VITALS
Key clinical point: Blood levels of infliximab rose during pregnancy, while adalimumab levels remained stable, even after researchers accounted for changes in albumin, body mass index, and C-reactive protein levels.
Major finding: Median infliximab concentrations rose from 8.5 mcg/mL in the first trimester to a peak of 21 mcg/mL during the middle of the third trimester (P = .04). Median adalimumab levels ranged between 8.6 and 12.2 mcg/mL during pregnancy.
Data source: A prospective study of 25 pregnant women with ulcerative colitis or Crohn's disease.
Disclosures: Dr. Seow disclosed ties with Janssen, AbbVie, Takeda, Shire, and Actavis.
2. Vascular Disease Linked to Sight Loss in Giant Cell Arteritis
To take the posttest, go to: http://bit.ly/1UqLuu5
Expires May 10, 2017
VITALS
Key clinical point: Patients with vascular disease who develop giant cell arteritis may require careful monitoring for sight loss.
Major finding: Overall, 42.9% of patients had some visual disturbance at first clinic review; 7.9% were blind at 6 months.
Data source: Analysis of 433 patients newly diagnosed with GCA participating in the Diagnostic and Classification Criteria in Vasculitis Study (DCVAS).
Disclosures: The DCVAS study is supported by the American College of Rheumatology and is funded by the European League Against Rheumatism and the Vasculitis Foundation. Dr. Yates reported that he had no relevant disclosures.
3. Pediatric and Adolescent Mental Health
Part 1: Diagnoses, drug prescribing vary widely
To take the posttest, go to: http://bit.ly/24FHTxY
Expires April 1, 2017
VITALS
Key clinical point: A lack of psychiatrists only partially accounted for substantial variations in rates of mental illness diagnosis and prescriptions for psychotropic medications given in practices nationwide, a study has shown.
Major finding: Nationwide, 15% of pediatric patients received a mental health diagnosis, and 14% were prescribed psychotropic medications in primary care, regardless of colocated mental health services.
Data source: A retrospective study of electronic health records for 294,748 patients aged 4-18 years.
Disclosures: Dr. Alexander G. Fiks is an investigator for Pfizer; the other researchers said they had no relevant financial disclosures. This study was funded by the National Institutes of Health and the National Institute of Child Health and Human Development under the Best Pharmaceuticals for Children Act.
Part 2: Disorders prevalent in young transgender women
To take the posttest, go to: http://bit.ly/24FCDdq
Expires March 21, 2017
VITALS
Key clinical point: Young transgender women have a high prevalence of psychiatric disorders that is two to four times higher than that in the general population.
Major finding: 41.5% of the study participants had at least one psychiatric disorder, such as major depressive disorder, suicidality, generalized anxiety, PTSD, and alcohol or substance dependence.
Data source: An observational cohort study involving 298 transgender women aged 16-29 years residing in Chicago and Boston.
Disclosures: This study was supported by the National Institute of Mental Health. Dr. Reisner and his associates reported having no relevant financial disclosures.
Caregivers of Dementia Patients: Mental Health Screening & Support
CE/CME No: CR-1606
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the adverse consequences that caregivers of persons with Alzheimer disease or other dementias commonly experience.
• Identify reliable and validated tools in the public domain available for use in primary care settings to assess a caregiver's well-being.
• List interventions that are known to support and improve the lives of caregivers seeking care.
• Discuss the impact of support groups on depression and burden of care experienced by caregivers.
• Define the role of primary care providers in reducing the negative aspects of caregiving.
FACULTY
Nancy Langman is a mental health and public health nurse practitioner on Martha’s Vineyard and an Adjunct Clinical Instructor at the University of Massachusetts Amherst.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of June 2016.
Article begins on next page >>
Caregivers, mostly family and friends, play an important role in the complex care of persons with Alzheimer disease and other dementias. Primary care providers are uniquely positioned to assess for the negative consequences of caregiving, including depression, anxiety, and caregivers' failure to care for their own health needs. This article provides you with reliable, valid screening tools and recommendations for evidence-based interventions to increase the caregiver’s and patient’s quality of life and care.
Henry, a retired health care administrator, received a diagnosis of Alzheimer disease in his early 80s. Given his career experience, he knew where this disease might take him. His wife, Joann, worked in admissions in a nursing home prior to retirement and was equally informed of the course of the disease. They were among the more fortunate ones struggling with this life-changing diagnosis, in that they had a great primary care provider, access to some of the best neuropsychologists and neurologists in the country, financial stability, and adult children nearby.
Caregivers are a rapidly growing segment of the system of care in the United States, with more than 15 million providing care for those with Alzheimer disease (AD) and other dementias, according to the Alzheimer’s Association.1 Lack of training and support puts caregivers at risk for depression, anxiety, and failure to take care of their own health.2
The incidence and prevalence of dementia continue to increase as the population ages, placing an enormous emotional, physical, and economic burden on caregivers as well as families and society. Given our rapidly growing elderly population and the important role caregivers play, providing evidence-based care and support for caregivers of dementia patients should be a priority for primary care providers.3 Progress in this area requires primary care practitioners to take a lead role in addressing the complex issues that adversely affect caregivers and their loved ones.3 Nurse practitioners (NPs) and physician assistants (PAs) are in a pivotal position to implement caregiver screening and provide referrals to evidence-based interventions.
Who are the caregivers?
Caregivers in the US are predominantly women, and they provide 75% to 80% of long-term care in the community.4 They are largely untrained, unsupervised, unpaid, and undersupported in our society. There are many faces of caregivers: elderly spouses who themselves have health care challenges; adult children, often referred to as the “sandwich generation” as they care for their own families as well as their aging parents; nieces, nephews, and other relatives who find themselves in the position of being the only family left to care for a loved one; and paid caregivers, who also experience the stress of caregiving. Caregivers face many challenges that create both psychological and physical stress, as they are increasingly expected to provide more demanding and complex care, including medication management.4
The MetLife Mature Market Institute reports that 20% of working female caregivers older than 50 experience depression, compared to 8% of peers who are not caregivers.5 Depression among caregivers is well documented, with evidence showing that clinically significant symptoms of depression occur in 40% to 70% of caregivers and that 25% to 50% of these caregivers meet criteria for major depression.6
Continue for assessment tools >>
Assessment Tools
Assessment tools are commonly used to screen for known negative effects of caregiving and to monitor these effects following targeted interventions. Many caregiver assessment tools exist. According to the Family Caregiver Alliance’s Selected Caregiver Assessment Measures, recent efforts have focused on revising available tools to make them shorter and easier to use.7 Newer assessment models attempted to blend content areas (depression, burden, health behaviors, and quality of life) to establish a single screening instrument, in contrast to the stand-alone tools that measure only one domain.8
It is important to select an assessment tool that is easy to administer, reliable and valid, and in the public domain.9-17 In its 2002 consensus project, the Family Caregiver Alliance recommended that assessments be multidimensional in approach, periodically updated, and reflective of culturally competent practice.18 In addition, they believe that those doing the assessments should have relevant training on the role of caregivers and the impact of caregiving.
The Caregiver Assessment Grid was developed by the Michigan Dementia Coalition following a review of 19 scales that measure caregiver burden, stress, quality of life, memory, behavior, and perceptions of caregiving tasks (among others).19 Tools range from simple to complex, with some having Yes or No answers and others using four- or five-point Likert scales. Tools listed in the Caregiver Assessment Grid that meet the criteria for brevity and are in the public domain are discussed here.
The Zarit Burden Interview (ZBI) was initially a 29-item tool that was reduced first to 22 items and then further to 12 items, with a brief screening version containing only four items.10,20 Correlations between the reduced-length versions were .92 to .97 for the short version and .83 to .93 for the screening version.7 The Zarit screening version has a sensitivity of 98.5% and a specificity of 94.7%.10 The ZBI is frequently applied to assess burden, has been cited in many studies, and has been validated for use in other languages.21 This interview tool measures subjective burden, distress, perceptions of social and physical health, financial and emotional burden, and relationship with care recipient. The ZBI has been embedded in other blended assessment tools; for example, it is part of the California Caregiver Resource Centers Uniform Assessment Tool.19
The Pearlin Caregivers’ Stress Scales are based on a conceptual model of the Alzheimer’s Caregiver Stress tool, an eight-item scale developed by the Alzheimer’s Association that links “yes” answers to helpful websites.22 This 15-item instrument addresses cognitive status, problem behaviors, overload, relational deprivation, family conflict, job/caregiving conflict, and economic strain, among others.22
The American Medical Association (AMA) published an 18-item caregiver assessment tool for health care professionals in 2002, encouraging them to identify the needs of caregivers. This tool includes 16 Yes or No questions and two global scale items.19,23
The Risk Appraisal Measure (RAM) developed by Czaja et al is a 16-item assessment that takes 5 to 7 minutes to administer and identifies risk areas for caregivers. This instrument explores six domains of caregiver risk that are potentially amenable to intervention: depression, burden, self-care and health behaviors, social support, safety, and patient problem behaviors. The RAM was developed and validated using data from REACH II (Resources for Enhancing Alzheimer’s Caregiver Health)11 in a study involving 642 participants (219 white; 211 black; 212 Hispanic).11 The authors reported acceptable concurrent validity and internal consistency for the entire scale for the overall sample (Cronbach alpha = .65) and across racial and ethnic groups.11 The authors acknowledge that the Cronbach alpha (a measure of internal consistency, or how closely related a set of items are as a group) is relatively low but explain that this is expected due to the six distinct domains the instrument attempts to measure. The findings from this study highlight the challenge of maintaining reliability and validity in blended screening tools.
The Geriatric Depression Scale (GDS) is a broadly used, well-known tool that has been used extensively with the elderly population.24 The GDS has both a long (30 questions) and short (15 questions) version. In the shorter version, five of the Yes or No questions indicate depression if answered negatively and 10 indicate depression if answered positively. The long and short forms were compared in a validation study and found to be successful in differentiating depressed from nondepressed adults (r = .84, P < .001).25
The Center for Epidemiology Studies Depression Scale (CES-D) is a 20-item self-report scale that takes 5 minutes to administer and measures depressive feelings and behaviors over the previous week.16 It is commonly used to assess depression in caregivers.26 Matschinger and colleagues have expressed concerns about the CES-D being administered to caregivers, many of whom are elderly, because the questions in this instrument are oppositely worded: One part asserts and the other denies the content to avoid a tendency for respondents to give positive answers to questions (known as acquiescence).27 These researchers raise the concern that opposite wording may affect the reliability of the scale and recommend against its use in elderly persons.
The Caregiver Burden Scale, adapted version from the Family Practice Notebook, is a 22-item version of the Caregiver Burden Interview.28 A 12-item version was developed by Bèdard et al, along with a four-question screening version.10
There are several easily accessible online self-assessment tools. The AMA Caregiver Self-Assessment is self-scored and offers help interpreting the scores, suggestions for next steps, and resource information.23 The Caregiver Stress Self-assessment offered by Mass.gov is a modified version of Dr. Steven Zarit’s work and is also self-scored.29 The Veterans Administration (VA) has a more complex Self-Assessment Worksheet that focuses on roles and responsibilities as well as stress; a list of “next step” actions is offered, along with information about VA resources.30 While these tools allow users to assess their well-being in privacy, they do not offer the support and interventions that face-to-face screening can include.
Following Henry’s confirmed diagnosis of AD, his NP screened him for depression using the Geriatric Depression Scale and prescribed an antidepressant. She recommended that he take donepezil in the hope of slowing the progression of memory loss in the early to middle stages of the disease. She also screened his wife for her level of caregiver stress using the Zarit Burden Interview, shortened version, and referred her to a caregiver support group, informing her of respite services, including a supportive day program offered at the local Council on Aging. She also referred Henry to a memory loss support group in the community. Despite the available support, the stress in their life was palpable.
Continue for interventions >>
Interventions
Assessing caregiver challenges is only half the task of seeking to improve their lives. The next step is to provide advice and referral for supportive services including structured, valid, and reliable interventions. Structured support groups have been studied locally, nationally, and internationally.26,31,32 Burns and colleagues developed a study to test two 24-month primary care interventions for caregivers of those with AD, focused on alleviating caregivers' distress.33 In this randomized clinical trial, subjects were assigned to one of two groups: one received behavior management alone and the other added stress coping to the behavior management. Those who received only behavior management had worse outcomes for general well-being and depression. The researchers concluded that “brief primary care interventions may be effective in reducing caregiver distress and burden in the long-term.” This study underscores the need for a multifaceted approach to supporting caregivers in their complex and demanding role.
For depression
Depression is a common caregiver complaint; however, measuring incidence and prevalence of mental health issues is a challenge. Studies evaluating the effectiveness of interventions have had mixed results. The REACH II study showed that although caregivers do not usually meet criteria for clinical depression, they nonetheless experience depressive symptoms.34 But, although depression is the most widely studied health consequence of being a caregiver for someone with AD,35 experts report that there is little consistent evidence about the effectiveness of caregiver interventions.
A prospective single-blind randomized controlled trial with a three-month follow-up evaluated whether a cognitive–behavioral family intervention, consisting of education, stress management, and coping skills training, was effective in reducing the burden of care among caregivers of persons with AD.36 Caregiver burden was assessed at pretreatment, posttreatment (nine months after trial entry), and three-month follow-up (12 months after trial entry), using measures of psychological distress and depression and general health. The results showed that the intervention resulted in a significant reduction in distress and depression for caregivers and had a positive effect on modifying patient behaviors. Unfortunately, the intervention is lengthy and requires special training for the interventionist.
A study by Chu and colleagues explored providing a 12-week structured support group to Taiwanese caregivers of those with dementia and found that the support group reduced caregivers’ depression but did not have an effect on burden of care.37 Two other studies showed that support groups have a significant effect on depression and decrease caregiver burden and bother.38,39 Another study of spouses of persons with AD (n = 406) randomly assigned them to either a support and counseling intervention (comprised of six counseling sessions followed by a support group) or to a control group that received routine care.40 The authors assessed all participants before and after the intervention using the Geriatric Depression Scale and found significantly fewer symptoms of depression in the intervention group; these effects were sustained for 3.1 years postintervention. They also found that only group interventions based on psychoeducational theory had a positive effect on depression of caregivers. In a subsequent analysis, the researchers concluded that additional studies of psychosocial interventions for caregivers are warranted and should incorporate biological measures of physical health outcomes.41
Lavretsky, Siddarth, and Irwin conducted the first randomized placebo-controlled double-blind trial of the use of an antidepressant to reduce depression and improve resilience and quality of life among caregivers of persons with dementia.42 Their study demonstrated the efficacy of antidepressant therapy for caregiver depression: 86% of caregivers in the intervention group achieved remission, compared to 44% in the placebo group. Caregivers treated with antidepressants reported reduced anxiety, improved resilience, and decreased burden and stress. The authors also found that the level of depression and burden correlate to the severity of the care recipient’s dementia, related disability, and behavioral problems.42 However, these findings are limited due to the study’s small sample size (n = 28).
Elliott and colleagues report that depression serves a mediating function between the health of caregivers and their experience of burden.38Mediating variables play an important role in governing the relationship between caregiver burden and the health of the caregiver, while moderating variables change the effect between them when increased or decreased. In the first case, caregiver education would likely mediate burden of care; in the second, caregiver sleep (or lack thereof) would moderate the impact of caregiving on depression.
For caregiver burden
Three areas that cause burden for caregivers are activities of daily living, such as eating, bathing, and toileting; instrumental activities of daily living, encompassing shopping, food preparation, and financial management; and behavior and safety, including falls, fires, and driving.43 Caregivers experience physical symptoms, depression, and feelings of burden when faced with a greater number of tasks, more problematic behaviors, and/or more family disagreements.44
The concept of caregiver burden is the focus of many studies, all of them seeking answers for how best to support caregivers caring for a loved one with AD.37,43,45,46 In a multicenter prospective randomized study conducted in 11 hospital and nonhospital psychiatric outpatient clinics in southern Europe (N = 115), the intervention group participated in eight individual sessions over four months that focused on learning strategies for managing AD patient care.46 Data were collected on caregiver stress, quality of life, and perceived health to determine the impact on caregiver burden. The intervention was found to minimize caregiver burden as measured by the ZBI.
Lai and Thomson evaluated a random sample of family caregivers (n = 340) and concluded that providing tangible services and resources should be the first step in reducing burden of care. They report that caregivers’ perceived adequacy of support services predicts caregiver burden. They noted that emotional support results in only marginal benefits.45 Other studies have concurred with the finding that support groups provide emotional support, information, and problem-solving skills to caregivers but do not reduce caregiver burden.31
For caregivers of those with dementia, the strongest predictor of distress is care recipients’ problem behaviors.26 Some theorize that the distress that caregivers experience has multiple components, including belligerence, lack of cooperation, oppositional behaviors, and disruption of sleep patterns of the care recipient.13 Support groups should address behavioral interventions that caregivers can use when faced with the challenging behaviors that often concur with neurocognitive disorders, including confusion, wandering, agitation, crying, swearing, and combativeness.
Caregivers may experience increased stress as their loved one’s dementia progresses. The New York University (NYU) Caregiver Intervention is a long-running randomized controlled study of counseling and support interventions for caregivers of spouses with dementia, conducted by the NYU School of Medicine. Among the array of published analyses of data from this study was a trial reporting that counseling and support interventions reduced the rate of nursing home placement by 28.3%, compared with usual care, and delayed nursing home placement; 61.2% of this delay was attributed to social support, response to patient behaviors, and reduced depression.47 Comprehensive counseling and support provided during the progression of AD patients transitioning to institutionalization can be beneficial to spouses and may translate more broadly to caregivers in general.48
Equipping caregivers to manage the most difficult aspect of their role should be a priority. Many towns and cities offer day programs for those with memory disorders, allowing for respite for caregivers. Councils on aging are a great resource for service information and specialized programs. For those who meet the income guidelines, there may even be financial support for family caregivers. To seek out services, contact the national Alzheimer’s Association (Alz.org) or a local branch. For information on behavioral interventions, Caregiver.org has many available resources on a variety of topics. (Additional resources are listed in “Caregiving Resources.”)
Continue for caregiver stress and self-care >>
Caregiver stress and SELF-CARE
Primary care providers can be instrumental in not only offering referral to services to reduce caregiver stress but also encouraging self-care behaviors for caregivers. They have the opportunity, early on, to anticipate the stresses that caregivers may experience as their loved one’s disease progresses and to provide helpful referral for services (including psychotherapy and support groups), information, and support to preventively address functional ability and self-care behaviors.
The relationship between caregiver stress and self-care behavior has been widely studied. Using the Caregiving Hassles Scale, Kinney and Stephens examined the mediating function of the relationship between caregiving stress and self-care behavior. They found caregiver stress to correlate to self-rated health at a statistically significant level (r = .30, P = .003).13 On a positive note, the investigators also found that the more symptoms family members reported (depression, poor health), the more self-care behaviors they used.
Lu and Wykle also used the Caregiving Hassles Scale in their correlational crossfunctional study. Caregivers (n = 99) were assessed for the mediating function of the relationship between caregiving stress and self-care behavior in response to symptoms.49 Those who reported higher levels of caregiving stress also reported poorer self-rated health, poorer physical function, high levels of depressed mood, and more self-care behaviors at a statistically significant level (r =.30, P = .003, Cronbach’s alpha = .95). The researchers determined that depressed mood was a strong mediator between caregiver stress and response to the symptoms with self-care behaviors.
Unsteady on his feet, Henry became a fall risk. His growing confusion and cognitive decline led to increased depression and agitation, which reduced his socialization and physical activity. Over time, this resulted in a disturbing chain of events, including urinary tract infections, hospitalization, and behavior changes that were upsetting to his wife and children as well as to Henry. Joann was not sleeping well and contracted a cold that turned into pneumonia, leading to a hospitalization. Through all of this, Joann knew that she could call Henry’s NP for advice and support. She found solace in knowing that the caregiver support group she attended regularly would always be there to encourage and inform her and that Henry’s support group would not only give her respite as a caregiver but also provide Henry with cognitive stimulation shown to enhance the well-being of those experiencing memory loss. These groups soon became her lifeline. Without vital early screenings, this family would not have adequately managed the difficulties brought on by Henry’s unexpected diagnosis.
Continue for summary and conclusions >>
Summary and conclusions
Extensive research on caregivers has focused on depression, burden of care, and self-care issues, with mixed findings. Gottlieb and colleagues report that caregivers struggle with the “apparent sadness, listlessness, and vegetative behavior” of their loved one struggling with AD.35 Primary care providers are in a pivotal position to improve caregivers’ health status using reliable and valid assessment tools and offerring referral to services that have been shown to help caregivers in their complex and challenging role.
Support groups, assistance with behavioral interventions, information about the disease process and effective interventions, medication for clinical depression, respite and day programs for persons with neurocognitive disorders, and encouragement of self-care all reduce caregiver stress. Offering effective interventions can improve the physical and mental health of burdened caregivers and positively impact the lives of their loved ones.
Given the growing number of caregivers and the significant effect of caregiving on their health, staying alert should be a priority in all practices. Simply adding two questions to those we regularly ask—Do you care for a loved one struggling with memory loss? How many hours a week do you provide care?—can illuminate the challenges caregivers face, so we can monitor their health status appropriately.
1. 2015 Alzheimer’s Disease Facts and Figures. Chicago: Alzheimer’s Association; 2015. www.alz.org/facts/downloads/facts_figures_2015.pdf. Accessed May 20, 2016.
2. Wallis L. Reach VA helps family caregivers of dementia patients. Am J Nurs. 2011;111(6):18.
3. Office of Disease Prevention and Health Promotion. 2020 Topics and objectives—objectives A–Z. Healthy People 2020. www.healthypeople.gov/2020/topics-objectives. Accessed May 20, 2016.
4. Levine C, Halper D, Peist A, Gould D. Bridging troubled waters: family caregivers, transitions, and long-term care. Health Aff. 2010;29(1):116-124.
5. MetLife Mature Market Institute, National Alliance for Caregiving, and the University of Pittsburgh Institute on Aging. The MetLife Study of Working Caregivers and Employer Health Care Costs. February 2010. www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-care-costs.pdf. Accessed May 20, 2016.
6. Zarit SH. Assessment of family caregivers: A research perspective. In: Family Caregiver Alliance. Caregiver Assessment: Voices and Views From the Field. Report from a National Consensus Development Conference. Volume II. San Francisco: Family Caregiver Alliance; 2006:14. https://caregiver.org/sites/caregiver.org/files/pdfs/v2_consensus.pdf. Accessed May 20, 2016.
7. Family Caregiver Alliance National Center on Caregiving. (2012). Selected Caregiver Assessment Measures: A Resource Inventory for Practitioners. 2nd ed. https://www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012. Accessed May 20, 2016.
8. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. San Francisco: Family Caregiver Alliance; 2006. www.caregiver.org/sites/caregiver.org/files/pdfs/Assessment_Toolkit_20060802.pdf. Accessed May 20, 2016.
9. Antonovsky A. The structure and properties of the Sense of Coherence Scale. Soc Sci Med. 1993;36(6):725-733.
10. Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652-657.
11. Czaja SJ, Gitlin LN, Schulz R, et al. Development of the Risk Appraisal Measure (RAM): a brief screen to identify risk areas and guide interventions for dementia caregivers. J Am Geriatr Soc. 2009;57(6):1064-1072.
12. Gort AM, Mingot M, March J, et al. Short Zarit scale in dementias. Med Clin (Barc). 2010;35(10):447-449.
13. Kinney JM, Stephens MA. Caregiving Hassles Scale: assessing the daily hassles of caring for a family member with dementia. Gerontologist. 1989;29(3):328-332.
14. Locke DEC, Dassel KB, Hall G, et al. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire. Dement Geriatr Cogn Disord. 2009;27:260-272.
15. Picot SJ, Youngblut J, Zeller R. Development and testing of a measure of perceived caregiver rewards in adults. J Nurs Meas. 1997;5(1):33-52.
16. Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 1986;5:119-136.
17. Seng BK, Luo N, Ng WY, et al. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singapore. 2010;39(10):758-763.
18. Family Caregiver Alliance. Caregiver Assessment: Principles, Guidelines and Strategies for Change. Report from a National Consensus Development Conference (Vol. I). San Francisco: Family Caregiver Alliance; 2006:12. https://www.caregiver.org/sites/caregiver.org/files/pdfs/v1_consensus.pdf. Accessed May 20, 2016.
19. Michigan Dementia Coalition. Caregiver Assessment Tool Grid. The Rosalynn Carter Institute. www.rosalynncarter.org/UserFiles/Michigan%20Assessment%20Grid.pdf. Accessed May 20, 2016.
20. Zarit SH, Reever KE, Bach-Peterson J. Relatives of impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
21. Miyamoto Y, Tachimo H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253.
22. Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583-594.
23. American Medical Association. Caregiver Self-Assessment Questionnaire. National Caregivers Library. www.caregiverslibrary.org/Portals/0/CaringforYourself_CaregiverSelfAssessmentQuestionaire.pdf. Accessed May 20, 2016.
24. Greenberg SA. The Geriatric Depression Scale (GDS). In: Try This: Best Practices in Nursing Care to Older Adults. Issue 4. New York, NY: Hartford Institute for Geriatric Nursing, NYU College of Nursing; 2012. https://consultgeri.org/try-this/general-assessment/issue-4. Accessed May 20, 2016.
25. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986:165-173.
26. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577-595.
27. Matschinger H, Schork A, Riedel-Heller S, Angemeyer M. On the application of the CES-D with the elderly: dimensional structure and artifacts resulting from oppositely worded items. Int J Methods Psychiatr Res. 2006;9(4):199-209.
28. Caregiver Burden Scale. Family Practice Notebook. www.fpnotebook.com/Geri/Exam/CrgvrBrdnScl.htm. Accessed May 20, 2016.
29. Caregiver Stress Self-Assessment. www.mass.gov/elders/docs/caregiver-stress-self-assessment.pdf. Accessed May 20, 2016.
30. US Department of Veterans Affairs. Caregiver Self-Assessment Worksheet. http://www.va.gov/GERIATRICS/guide/longtermcare/Caregiver_Self_Assessment.pdf. Accessed May 20, 2016.
31. Thompson CA, Spilsbury K, Hall J, et al. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr. 2007;7:18-29.
32. Chien LY, Chu H, Guo JL, et al. Caregiver support groups in patients with dementia: a meta-analysis. Int J Geriatr Psychiatry. 2011;26:1089-1098.
33. Burns R, Nichols LO, Martindale-Adams J, et al. Primary care interventions for dementia caregivers: 2-year outcomes for the REACH study. Gerontologist. 2003;43(4):547-555.
34. Schulz R, Burgio L, Burns R, et al. Resources for Enhancing Alzheimer’s Caregivers Health (REACH): overview, site-specific outcomes, and future directions. Gerontologist. 2003;43(4):514-520.
35. Gottlieb BH, Thompson LW, Bourgeois M. Monitoring and evaluating interventions. In: Coon DW, Gallagher-Thompson D, Thompson LW, eds. Innovative Interventions to Reduce Dementia Caregiver Distress: A Clinical Guide. New York, NY: Springer; 2003:28-49.
36. Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive-behavioral family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry. 2000;176:557-562.
37. Chu H, Yang CY, Liao YH, et al. The effects of a support group on dementia caregivers’ burden and depression. J Aging Health. 2011;23(2): 228-241.
38. Elliott AF, Burgio LD, DeCoster J. Enhancing caregiver health: findings from the Resources for Enhancing Alzheimer’s disease Health II intervention. J Am Geriatr Soc. 2010;58:30-37.
39. Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3): 356-372.
40. Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms of caregivers in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(5):850-856.
41. Mittelman M, Roth D, Clay O, Haley W. Preserving health of Alzheimer caregivers: impact of a spouse caregiver intervention. Am J Geriatr Psychiatry. 2007;15(9):780-789.
42. Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: a pilot randomized placebo-controlled trial of escitalopram. Am J Geriatr Psychiatry. 2010;18(2):154-164.
43. Wimo A, von Strauss E, Nordberg G, et al. Time spent on informal and formal caregiving for persons with dementia in Sweden. Health Policy. 2002;61(3):255-268.
44. Koerner SS, Shirai Y, Kenyon DB. Sociocontextual circumstances in daily stress reactivity among caregivers for elder relatives. J Gerontol B Psychol Sci Soc Sci. 2010;65(5):561-572.
45. Lai DW, Thomson C. The impact of perceived adequacy of social support on caregiving burden of family caregivers. Fam in Soc: J Contemp Social Serv. 2011;92(1):99-106.
46. Martin-Carrasco M, Martin M, Valero C, et al. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in Alzheimer’s disease patients’ caregivers. Int J Geriatr Psychiatry. 2009;24:489-499.
47. Mittelman MS, Haley WE, Clay OJ, Roth RL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
48. Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counseling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer’s disease during the transition to institutionalization? Results from the New York University caregiver intervention study. J Am Geriatr Soc. 2008;56(3):421-428.
49. Lu YFY, Wykle M. Relationships between caregiver stress and self-care behaviors in response to symptoms. Clin Nurs Res. 2007;16:29-43.
CE/CME No: CR-1606
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the adverse consequences that caregivers of persons with Alzheimer disease or other dementias commonly experience.
• Identify reliable and validated tools in the public domain available for use in primary care settings to assess a caregiver's well-being.
• List interventions that are known to support and improve the lives of caregivers seeking care.
• Discuss the impact of support groups on depression and burden of care experienced by caregivers.
• Define the role of primary care providers in reducing the negative aspects of caregiving.
FACULTY
Nancy Langman is a mental health and public health nurse practitioner on Martha’s Vineyard and an Adjunct Clinical Instructor at the University of Massachusetts Amherst.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of June 2016.
Article begins on next page >>
Caregivers, mostly family and friends, play an important role in the complex care of persons with Alzheimer disease and other dementias. Primary care providers are uniquely positioned to assess for the negative consequences of caregiving, including depression, anxiety, and caregivers' failure to care for their own health needs. This article provides you with reliable, valid screening tools and recommendations for evidence-based interventions to increase the caregiver’s and patient’s quality of life and care.
Henry, a retired health care administrator, received a diagnosis of Alzheimer disease in his early 80s. Given his career experience, he knew where this disease might take him. His wife, Joann, worked in admissions in a nursing home prior to retirement and was equally informed of the course of the disease. They were among the more fortunate ones struggling with this life-changing diagnosis, in that they had a great primary care provider, access to some of the best neuropsychologists and neurologists in the country, financial stability, and adult children nearby.
Caregivers are a rapidly growing segment of the system of care in the United States, with more than 15 million providing care for those with Alzheimer disease (AD) and other dementias, according to the Alzheimer’s Association.1 Lack of training and support puts caregivers at risk for depression, anxiety, and failure to take care of their own health.2
The incidence and prevalence of dementia continue to increase as the population ages, placing an enormous emotional, physical, and economic burden on caregivers as well as families and society. Given our rapidly growing elderly population and the important role caregivers play, providing evidence-based care and support for caregivers of dementia patients should be a priority for primary care providers.3 Progress in this area requires primary care practitioners to take a lead role in addressing the complex issues that adversely affect caregivers and their loved ones.3 Nurse practitioners (NPs) and physician assistants (PAs) are in a pivotal position to implement caregiver screening and provide referrals to evidence-based interventions.
Who are the caregivers?
Caregivers in the US are predominantly women, and they provide 75% to 80% of long-term care in the community.4 They are largely untrained, unsupervised, unpaid, and undersupported in our society. There are many faces of caregivers: elderly spouses who themselves have health care challenges; adult children, often referred to as the “sandwich generation” as they care for their own families as well as their aging parents; nieces, nephews, and other relatives who find themselves in the position of being the only family left to care for a loved one; and paid caregivers, who also experience the stress of caregiving. Caregivers face many challenges that create both psychological and physical stress, as they are increasingly expected to provide more demanding and complex care, including medication management.4
The MetLife Mature Market Institute reports that 20% of working female caregivers older than 50 experience depression, compared to 8% of peers who are not caregivers.5 Depression among caregivers is well documented, with evidence showing that clinically significant symptoms of depression occur in 40% to 70% of caregivers and that 25% to 50% of these caregivers meet criteria for major depression.6
Continue for assessment tools >>
Assessment Tools
Assessment tools are commonly used to screen for known negative effects of caregiving and to monitor these effects following targeted interventions. Many caregiver assessment tools exist. According to the Family Caregiver Alliance’s Selected Caregiver Assessment Measures, recent efforts have focused on revising available tools to make them shorter and easier to use.7 Newer assessment models attempted to blend content areas (depression, burden, health behaviors, and quality of life) to establish a single screening instrument, in contrast to the stand-alone tools that measure only one domain.8
It is important to select an assessment tool that is easy to administer, reliable and valid, and in the public domain.9-17 In its 2002 consensus project, the Family Caregiver Alliance recommended that assessments be multidimensional in approach, periodically updated, and reflective of culturally competent practice.18 In addition, they believe that those doing the assessments should have relevant training on the role of caregivers and the impact of caregiving.
The Caregiver Assessment Grid was developed by the Michigan Dementia Coalition following a review of 19 scales that measure caregiver burden, stress, quality of life, memory, behavior, and perceptions of caregiving tasks (among others).19 Tools range from simple to complex, with some having Yes or No answers and others using four- or five-point Likert scales. Tools listed in the Caregiver Assessment Grid that meet the criteria for brevity and are in the public domain are discussed here.
The Zarit Burden Interview (ZBI) was initially a 29-item tool that was reduced first to 22 items and then further to 12 items, with a brief screening version containing only four items.10,20 Correlations between the reduced-length versions were .92 to .97 for the short version and .83 to .93 for the screening version.7 The Zarit screening version has a sensitivity of 98.5% and a specificity of 94.7%.10 The ZBI is frequently applied to assess burden, has been cited in many studies, and has been validated for use in other languages.21 This interview tool measures subjective burden, distress, perceptions of social and physical health, financial and emotional burden, and relationship with care recipient. The ZBI has been embedded in other blended assessment tools; for example, it is part of the California Caregiver Resource Centers Uniform Assessment Tool.19
The Pearlin Caregivers’ Stress Scales are based on a conceptual model of the Alzheimer’s Caregiver Stress tool, an eight-item scale developed by the Alzheimer’s Association that links “yes” answers to helpful websites.22 This 15-item instrument addresses cognitive status, problem behaviors, overload, relational deprivation, family conflict, job/caregiving conflict, and economic strain, among others.22
The American Medical Association (AMA) published an 18-item caregiver assessment tool for health care professionals in 2002, encouraging them to identify the needs of caregivers. This tool includes 16 Yes or No questions and two global scale items.19,23
The Risk Appraisal Measure (RAM) developed by Czaja et al is a 16-item assessment that takes 5 to 7 minutes to administer and identifies risk areas for caregivers. This instrument explores six domains of caregiver risk that are potentially amenable to intervention: depression, burden, self-care and health behaviors, social support, safety, and patient problem behaviors. The RAM was developed and validated using data from REACH II (Resources for Enhancing Alzheimer’s Caregiver Health)11 in a study involving 642 participants (219 white; 211 black; 212 Hispanic).11 The authors reported acceptable concurrent validity and internal consistency for the entire scale for the overall sample (Cronbach alpha = .65) and across racial and ethnic groups.11 The authors acknowledge that the Cronbach alpha (a measure of internal consistency, or how closely related a set of items are as a group) is relatively low but explain that this is expected due to the six distinct domains the instrument attempts to measure. The findings from this study highlight the challenge of maintaining reliability and validity in blended screening tools.
The Geriatric Depression Scale (GDS) is a broadly used, well-known tool that has been used extensively with the elderly population.24 The GDS has both a long (30 questions) and short (15 questions) version. In the shorter version, five of the Yes or No questions indicate depression if answered negatively and 10 indicate depression if answered positively. The long and short forms were compared in a validation study and found to be successful in differentiating depressed from nondepressed adults (r = .84, P < .001).25
The Center for Epidemiology Studies Depression Scale (CES-D) is a 20-item self-report scale that takes 5 minutes to administer and measures depressive feelings and behaviors over the previous week.16 It is commonly used to assess depression in caregivers.26 Matschinger and colleagues have expressed concerns about the CES-D being administered to caregivers, many of whom are elderly, because the questions in this instrument are oppositely worded: One part asserts and the other denies the content to avoid a tendency for respondents to give positive answers to questions (known as acquiescence).27 These researchers raise the concern that opposite wording may affect the reliability of the scale and recommend against its use in elderly persons.
The Caregiver Burden Scale, adapted version from the Family Practice Notebook, is a 22-item version of the Caregiver Burden Interview.28 A 12-item version was developed by Bèdard et al, along with a four-question screening version.10
There are several easily accessible online self-assessment tools. The AMA Caregiver Self-Assessment is self-scored and offers help interpreting the scores, suggestions for next steps, and resource information.23 The Caregiver Stress Self-assessment offered by Mass.gov is a modified version of Dr. Steven Zarit’s work and is also self-scored.29 The Veterans Administration (VA) has a more complex Self-Assessment Worksheet that focuses on roles and responsibilities as well as stress; a list of “next step” actions is offered, along with information about VA resources.30 While these tools allow users to assess their well-being in privacy, they do not offer the support and interventions that face-to-face screening can include.
Following Henry’s confirmed diagnosis of AD, his NP screened him for depression using the Geriatric Depression Scale and prescribed an antidepressant. She recommended that he take donepezil in the hope of slowing the progression of memory loss in the early to middle stages of the disease. She also screened his wife for her level of caregiver stress using the Zarit Burden Interview, shortened version, and referred her to a caregiver support group, informing her of respite services, including a supportive day program offered at the local Council on Aging. She also referred Henry to a memory loss support group in the community. Despite the available support, the stress in their life was palpable.
Continue for interventions >>
Interventions
Assessing caregiver challenges is only half the task of seeking to improve their lives. The next step is to provide advice and referral for supportive services including structured, valid, and reliable interventions. Structured support groups have been studied locally, nationally, and internationally.26,31,32 Burns and colleagues developed a study to test two 24-month primary care interventions for caregivers of those with AD, focused on alleviating caregivers' distress.33 In this randomized clinical trial, subjects were assigned to one of two groups: one received behavior management alone and the other added stress coping to the behavior management. Those who received only behavior management had worse outcomes for general well-being and depression. The researchers concluded that “brief primary care interventions may be effective in reducing caregiver distress and burden in the long-term.” This study underscores the need for a multifaceted approach to supporting caregivers in their complex and demanding role.
For depression
Depression is a common caregiver complaint; however, measuring incidence and prevalence of mental health issues is a challenge. Studies evaluating the effectiveness of interventions have had mixed results. The REACH II study showed that although caregivers do not usually meet criteria for clinical depression, they nonetheless experience depressive symptoms.34 But, although depression is the most widely studied health consequence of being a caregiver for someone with AD,35 experts report that there is little consistent evidence about the effectiveness of caregiver interventions.
A prospective single-blind randomized controlled trial with a three-month follow-up evaluated whether a cognitive–behavioral family intervention, consisting of education, stress management, and coping skills training, was effective in reducing the burden of care among caregivers of persons with AD.36 Caregiver burden was assessed at pretreatment, posttreatment (nine months after trial entry), and three-month follow-up (12 months after trial entry), using measures of psychological distress and depression and general health. The results showed that the intervention resulted in a significant reduction in distress and depression for caregivers and had a positive effect on modifying patient behaviors. Unfortunately, the intervention is lengthy and requires special training for the interventionist.
A study by Chu and colleagues explored providing a 12-week structured support group to Taiwanese caregivers of those with dementia and found that the support group reduced caregivers’ depression but did not have an effect on burden of care.37 Two other studies showed that support groups have a significant effect on depression and decrease caregiver burden and bother.38,39 Another study of spouses of persons with AD (n = 406) randomly assigned them to either a support and counseling intervention (comprised of six counseling sessions followed by a support group) or to a control group that received routine care.40 The authors assessed all participants before and after the intervention using the Geriatric Depression Scale and found significantly fewer symptoms of depression in the intervention group; these effects were sustained for 3.1 years postintervention. They also found that only group interventions based on psychoeducational theory had a positive effect on depression of caregivers. In a subsequent analysis, the researchers concluded that additional studies of psychosocial interventions for caregivers are warranted and should incorporate biological measures of physical health outcomes.41
Lavretsky, Siddarth, and Irwin conducted the first randomized placebo-controlled double-blind trial of the use of an antidepressant to reduce depression and improve resilience and quality of life among caregivers of persons with dementia.42 Their study demonstrated the efficacy of antidepressant therapy for caregiver depression: 86% of caregivers in the intervention group achieved remission, compared to 44% in the placebo group. Caregivers treated with antidepressants reported reduced anxiety, improved resilience, and decreased burden and stress. The authors also found that the level of depression and burden correlate to the severity of the care recipient’s dementia, related disability, and behavioral problems.42 However, these findings are limited due to the study’s small sample size (n = 28).
Elliott and colleagues report that depression serves a mediating function between the health of caregivers and their experience of burden.38Mediating variables play an important role in governing the relationship between caregiver burden and the health of the caregiver, while moderating variables change the effect between them when increased or decreased. In the first case, caregiver education would likely mediate burden of care; in the second, caregiver sleep (or lack thereof) would moderate the impact of caregiving on depression.
For caregiver burden
Three areas that cause burden for caregivers are activities of daily living, such as eating, bathing, and toileting; instrumental activities of daily living, encompassing shopping, food preparation, and financial management; and behavior and safety, including falls, fires, and driving.43 Caregivers experience physical symptoms, depression, and feelings of burden when faced with a greater number of tasks, more problematic behaviors, and/or more family disagreements.44
The concept of caregiver burden is the focus of many studies, all of them seeking answers for how best to support caregivers caring for a loved one with AD.37,43,45,46 In a multicenter prospective randomized study conducted in 11 hospital and nonhospital psychiatric outpatient clinics in southern Europe (N = 115), the intervention group participated in eight individual sessions over four months that focused on learning strategies for managing AD patient care.46 Data were collected on caregiver stress, quality of life, and perceived health to determine the impact on caregiver burden. The intervention was found to minimize caregiver burden as measured by the ZBI.
Lai and Thomson evaluated a random sample of family caregivers (n = 340) and concluded that providing tangible services and resources should be the first step in reducing burden of care. They report that caregivers’ perceived adequacy of support services predicts caregiver burden. They noted that emotional support results in only marginal benefits.45 Other studies have concurred with the finding that support groups provide emotional support, information, and problem-solving skills to caregivers but do not reduce caregiver burden.31
For caregivers of those with dementia, the strongest predictor of distress is care recipients’ problem behaviors.26 Some theorize that the distress that caregivers experience has multiple components, including belligerence, lack of cooperation, oppositional behaviors, and disruption of sleep patterns of the care recipient.13 Support groups should address behavioral interventions that caregivers can use when faced with the challenging behaviors that often concur with neurocognitive disorders, including confusion, wandering, agitation, crying, swearing, and combativeness.
Caregivers may experience increased stress as their loved one’s dementia progresses. The New York University (NYU) Caregiver Intervention is a long-running randomized controlled study of counseling and support interventions for caregivers of spouses with dementia, conducted by the NYU School of Medicine. Among the array of published analyses of data from this study was a trial reporting that counseling and support interventions reduced the rate of nursing home placement by 28.3%, compared with usual care, and delayed nursing home placement; 61.2% of this delay was attributed to social support, response to patient behaviors, and reduced depression.47 Comprehensive counseling and support provided during the progression of AD patients transitioning to institutionalization can be beneficial to spouses and may translate more broadly to caregivers in general.48
Equipping caregivers to manage the most difficult aspect of their role should be a priority. Many towns and cities offer day programs for those with memory disorders, allowing for respite for caregivers. Councils on aging are a great resource for service information and specialized programs. For those who meet the income guidelines, there may even be financial support for family caregivers. To seek out services, contact the national Alzheimer’s Association (Alz.org) or a local branch. For information on behavioral interventions, Caregiver.org has many available resources on a variety of topics. (Additional resources are listed in “Caregiving Resources.”)
Continue for caregiver stress and self-care >>
Caregiver stress and SELF-CARE
Primary care providers can be instrumental in not only offering referral to services to reduce caregiver stress but also encouraging self-care behaviors for caregivers. They have the opportunity, early on, to anticipate the stresses that caregivers may experience as their loved one’s disease progresses and to provide helpful referral for services (including psychotherapy and support groups), information, and support to preventively address functional ability and self-care behaviors.
The relationship between caregiver stress and self-care behavior has been widely studied. Using the Caregiving Hassles Scale, Kinney and Stephens examined the mediating function of the relationship between caregiving stress and self-care behavior. They found caregiver stress to correlate to self-rated health at a statistically significant level (r = .30, P = .003).13 On a positive note, the investigators also found that the more symptoms family members reported (depression, poor health), the more self-care behaviors they used.
Lu and Wykle also used the Caregiving Hassles Scale in their correlational crossfunctional study. Caregivers (n = 99) were assessed for the mediating function of the relationship between caregiving stress and self-care behavior in response to symptoms.49 Those who reported higher levels of caregiving stress also reported poorer self-rated health, poorer physical function, high levels of depressed mood, and more self-care behaviors at a statistically significant level (r =.30, P = .003, Cronbach’s alpha = .95). The researchers determined that depressed mood was a strong mediator between caregiver stress and response to the symptoms with self-care behaviors.
Unsteady on his feet, Henry became a fall risk. His growing confusion and cognitive decline led to increased depression and agitation, which reduced his socialization and physical activity. Over time, this resulted in a disturbing chain of events, including urinary tract infections, hospitalization, and behavior changes that were upsetting to his wife and children as well as to Henry. Joann was not sleeping well and contracted a cold that turned into pneumonia, leading to a hospitalization. Through all of this, Joann knew that she could call Henry’s NP for advice and support. She found solace in knowing that the caregiver support group she attended regularly would always be there to encourage and inform her and that Henry’s support group would not only give her respite as a caregiver but also provide Henry with cognitive stimulation shown to enhance the well-being of those experiencing memory loss. These groups soon became her lifeline. Without vital early screenings, this family would not have adequately managed the difficulties brought on by Henry’s unexpected diagnosis.
Continue for summary and conclusions >>
Summary and conclusions
Extensive research on caregivers has focused on depression, burden of care, and self-care issues, with mixed findings. Gottlieb and colleagues report that caregivers struggle with the “apparent sadness, listlessness, and vegetative behavior” of their loved one struggling with AD.35 Primary care providers are in a pivotal position to improve caregivers’ health status using reliable and valid assessment tools and offerring referral to services that have been shown to help caregivers in their complex and challenging role.
Support groups, assistance with behavioral interventions, information about the disease process and effective interventions, medication for clinical depression, respite and day programs for persons with neurocognitive disorders, and encouragement of self-care all reduce caregiver stress. Offering effective interventions can improve the physical and mental health of burdened caregivers and positively impact the lives of their loved ones.
Given the growing number of caregivers and the significant effect of caregiving on their health, staying alert should be a priority in all practices. Simply adding two questions to those we regularly ask—Do you care for a loved one struggling with memory loss? How many hours a week do you provide care?—can illuminate the challenges caregivers face, so we can monitor their health status appropriately.
CE/CME No: CR-1606
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Describe the adverse consequences that caregivers of persons with Alzheimer disease or other dementias commonly experience.
• Identify reliable and validated tools in the public domain available for use in primary care settings to assess a caregiver's well-being.
• List interventions that are known to support and improve the lives of caregivers seeking care.
• Discuss the impact of support groups on depression and burden of care experienced by caregivers.
• Define the role of primary care providers in reducing the negative aspects of caregiving.
FACULTY
Nancy Langman is a mental health and public health nurse practitioner on Martha’s Vineyard and an Adjunct Clinical Instructor at the University of Massachusetts Amherst.
The author has no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of June 2016.
Article begins on next page >>
Caregivers, mostly family and friends, play an important role in the complex care of persons with Alzheimer disease and other dementias. Primary care providers are uniquely positioned to assess for the negative consequences of caregiving, including depression, anxiety, and caregivers' failure to care for their own health needs. This article provides you with reliable, valid screening tools and recommendations for evidence-based interventions to increase the caregiver’s and patient’s quality of life and care.
Henry, a retired health care administrator, received a diagnosis of Alzheimer disease in his early 80s. Given his career experience, he knew where this disease might take him. His wife, Joann, worked in admissions in a nursing home prior to retirement and was equally informed of the course of the disease. They were among the more fortunate ones struggling with this life-changing diagnosis, in that they had a great primary care provider, access to some of the best neuropsychologists and neurologists in the country, financial stability, and adult children nearby.
Caregivers are a rapidly growing segment of the system of care in the United States, with more than 15 million providing care for those with Alzheimer disease (AD) and other dementias, according to the Alzheimer’s Association.1 Lack of training and support puts caregivers at risk for depression, anxiety, and failure to take care of their own health.2
The incidence and prevalence of dementia continue to increase as the population ages, placing an enormous emotional, physical, and economic burden on caregivers as well as families and society. Given our rapidly growing elderly population and the important role caregivers play, providing evidence-based care and support for caregivers of dementia patients should be a priority for primary care providers.3 Progress in this area requires primary care practitioners to take a lead role in addressing the complex issues that adversely affect caregivers and their loved ones.3 Nurse practitioners (NPs) and physician assistants (PAs) are in a pivotal position to implement caregiver screening and provide referrals to evidence-based interventions.
Who are the caregivers?
Caregivers in the US are predominantly women, and they provide 75% to 80% of long-term care in the community.4 They are largely untrained, unsupervised, unpaid, and undersupported in our society. There are many faces of caregivers: elderly spouses who themselves have health care challenges; adult children, often referred to as the “sandwich generation” as they care for their own families as well as their aging parents; nieces, nephews, and other relatives who find themselves in the position of being the only family left to care for a loved one; and paid caregivers, who also experience the stress of caregiving. Caregivers face many challenges that create both psychological and physical stress, as they are increasingly expected to provide more demanding and complex care, including medication management.4
The MetLife Mature Market Institute reports that 20% of working female caregivers older than 50 experience depression, compared to 8% of peers who are not caregivers.5 Depression among caregivers is well documented, with evidence showing that clinically significant symptoms of depression occur in 40% to 70% of caregivers and that 25% to 50% of these caregivers meet criteria for major depression.6
Continue for assessment tools >>
Assessment Tools
Assessment tools are commonly used to screen for known negative effects of caregiving and to monitor these effects following targeted interventions. Many caregiver assessment tools exist. According to the Family Caregiver Alliance’s Selected Caregiver Assessment Measures, recent efforts have focused on revising available tools to make them shorter and easier to use.7 Newer assessment models attempted to blend content areas (depression, burden, health behaviors, and quality of life) to establish a single screening instrument, in contrast to the stand-alone tools that measure only one domain.8
It is important to select an assessment tool that is easy to administer, reliable and valid, and in the public domain.9-17 In its 2002 consensus project, the Family Caregiver Alliance recommended that assessments be multidimensional in approach, periodically updated, and reflective of culturally competent practice.18 In addition, they believe that those doing the assessments should have relevant training on the role of caregivers and the impact of caregiving.
The Caregiver Assessment Grid was developed by the Michigan Dementia Coalition following a review of 19 scales that measure caregiver burden, stress, quality of life, memory, behavior, and perceptions of caregiving tasks (among others).19 Tools range from simple to complex, with some having Yes or No answers and others using four- or five-point Likert scales. Tools listed in the Caregiver Assessment Grid that meet the criteria for brevity and are in the public domain are discussed here.
The Zarit Burden Interview (ZBI) was initially a 29-item tool that was reduced first to 22 items and then further to 12 items, with a brief screening version containing only four items.10,20 Correlations between the reduced-length versions were .92 to .97 for the short version and .83 to .93 for the screening version.7 The Zarit screening version has a sensitivity of 98.5% and a specificity of 94.7%.10 The ZBI is frequently applied to assess burden, has been cited in many studies, and has been validated for use in other languages.21 This interview tool measures subjective burden, distress, perceptions of social and physical health, financial and emotional burden, and relationship with care recipient. The ZBI has been embedded in other blended assessment tools; for example, it is part of the California Caregiver Resource Centers Uniform Assessment Tool.19
The Pearlin Caregivers’ Stress Scales are based on a conceptual model of the Alzheimer’s Caregiver Stress tool, an eight-item scale developed by the Alzheimer’s Association that links “yes” answers to helpful websites.22 This 15-item instrument addresses cognitive status, problem behaviors, overload, relational deprivation, family conflict, job/caregiving conflict, and economic strain, among others.22
The American Medical Association (AMA) published an 18-item caregiver assessment tool for health care professionals in 2002, encouraging them to identify the needs of caregivers. This tool includes 16 Yes or No questions and two global scale items.19,23
The Risk Appraisal Measure (RAM) developed by Czaja et al is a 16-item assessment that takes 5 to 7 minutes to administer and identifies risk areas for caregivers. This instrument explores six domains of caregiver risk that are potentially amenable to intervention: depression, burden, self-care and health behaviors, social support, safety, and patient problem behaviors. The RAM was developed and validated using data from REACH II (Resources for Enhancing Alzheimer’s Caregiver Health)11 in a study involving 642 participants (219 white; 211 black; 212 Hispanic).11 The authors reported acceptable concurrent validity and internal consistency for the entire scale for the overall sample (Cronbach alpha = .65) and across racial and ethnic groups.11 The authors acknowledge that the Cronbach alpha (a measure of internal consistency, or how closely related a set of items are as a group) is relatively low but explain that this is expected due to the six distinct domains the instrument attempts to measure. The findings from this study highlight the challenge of maintaining reliability and validity in blended screening tools.
The Geriatric Depression Scale (GDS) is a broadly used, well-known tool that has been used extensively with the elderly population.24 The GDS has both a long (30 questions) and short (15 questions) version. In the shorter version, five of the Yes or No questions indicate depression if answered negatively and 10 indicate depression if answered positively. The long and short forms were compared in a validation study and found to be successful in differentiating depressed from nondepressed adults (r = .84, P < .001).25
The Center for Epidemiology Studies Depression Scale (CES-D) is a 20-item self-report scale that takes 5 minutes to administer and measures depressive feelings and behaviors over the previous week.16 It is commonly used to assess depression in caregivers.26 Matschinger and colleagues have expressed concerns about the CES-D being administered to caregivers, many of whom are elderly, because the questions in this instrument are oppositely worded: One part asserts and the other denies the content to avoid a tendency for respondents to give positive answers to questions (known as acquiescence).27 These researchers raise the concern that opposite wording may affect the reliability of the scale and recommend against its use in elderly persons.
The Caregiver Burden Scale, adapted version from the Family Practice Notebook, is a 22-item version of the Caregiver Burden Interview.28 A 12-item version was developed by Bèdard et al, along with a four-question screening version.10
There are several easily accessible online self-assessment tools. The AMA Caregiver Self-Assessment is self-scored and offers help interpreting the scores, suggestions for next steps, and resource information.23 The Caregiver Stress Self-assessment offered by Mass.gov is a modified version of Dr. Steven Zarit’s work and is also self-scored.29 The Veterans Administration (VA) has a more complex Self-Assessment Worksheet that focuses on roles and responsibilities as well as stress; a list of “next step” actions is offered, along with information about VA resources.30 While these tools allow users to assess their well-being in privacy, they do not offer the support and interventions that face-to-face screening can include.
Following Henry’s confirmed diagnosis of AD, his NP screened him for depression using the Geriatric Depression Scale and prescribed an antidepressant. She recommended that he take donepezil in the hope of slowing the progression of memory loss in the early to middle stages of the disease. She also screened his wife for her level of caregiver stress using the Zarit Burden Interview, shortened version, and referred her to a caregiver support group, informing her of respite services, including a supportive day program offered at the local Council on Aging. She also referred Henry to a memory loss support group in the community. Despite the available support, the stress in their life was palpable.
Continue for interventions >>
Interventions
Assessing caregiver challenges is only half the task of seeking to improve their lives. The next step is to provide advice and referral for supportive services including structured, valid, and reliable interventions. Structured support groups have been studied locally, nationally, and internationally.26,31,32 Burns and colleagues developed a study to test two 24-month primary care interventions for caregivers of those with AD, focused on alleviating caregivers' distress.33 In this randomized clinical trial, subjects were assigned to one of two groups: one received behavior management alone and the other added stress coping to the behavior management. Those who received only behavior management had worse outcomes for general well-being and depression. The researchers concluded that “brief primary care interventions may be effective in reducing caregiver distress and burden in the long-term.” This study underscores the need for a multifaceted approach to supporting caregivers in their complex and demanding role.
For depression
Depression is a common caregiver complaint; however, measuring incidence and prevalence of mental health issues is a challenge. Studies evaluating the effectiveness of interventions have had mixed results. The REACH II study showed that although caregivers do not usually meet criteria for clinical depression, they nonetheless experience depressive symptoms.34 But, although depression is the most widely studied health consequence of being a caregiver for someone with AD,35 experts report that there is little consistent evidence about the effectiveness of caregiver interventions.
A prospective single-blind randomized controlled trial with a three-month follow-up evaluated whether a cognitive–behavioral family intervention, consisting of education, stress management, and coping skills training, was effective in reducing the burden of care among caregivers of persons with AD.36 Caregiver burden was assessed at pretreatment, posttreatment (nine months after trial entry), and three-month follow-up (12 months after trial entry), using measures of psychological distress and depression and general health. The results showed that the intervention resulted in a significant reduction in distress and depression for caregivers and had a positive effect on modifying patient behaviors. Unfortunately, the intervention is lengthy and requires special training for the interventionist.
A study by Chu and colleagues explored providing a 12-week structured support group to Taiwanese caregivers of those with dementia and found that the support group reduced caregivers’ depression but did not have an effect on burden of care.37 Two other studies showed that support groups have a significant effect on depression and decrease caregiver burden and bother.38,39 Another study of spouses of persons with AD (n = 406) randomly assigned them to either a support and counseling intervention (comprised of six counseling sessions followed by a support group) or to a control group that received routine care.40 The authors assessed all participants before and after the intervention using the Geriatric Depression Scale and found significantly fewer symptoms of depression in the intervention group; these effects were sustained for 3.1 years postintervention. They also found that only group interventions based on psychoeducational theory had a positive effect on depression of caregivers. In a subsequent analysis, the researchers concluded that additional studies of psychosocial interventions for caregivers are warranted and should incorporate biological measures of physical health outcomes.41
Lavretsky, Siddarth, and Irwin conducted the first randomized placebo-controlled double-blind trial of the use of an antidepressant to reduce depression and improve resilience and quality of life among caregivers of persons with dementia.42 Their study demonstrated the efficacy of antidepressant therapy for caregiver depression: 86% of caregivers in the intervention group achieved remission, compared to 44% in the placebo group. Caregivers treated with antidepressants reported reduced anxiety, improved resilience, and decreased burden and stress. The authors also found that the level of depression and burden correlate to the severity of the care recipient’s dementia, related disability, and behavioral problems.42 However, these findings are limited due to the study’s small sample size (n = 28).
Elliott and colleagues report that depression serves a mediating function between the health of caregivers and their experience of burden.38Mediating variables play an important role in governing the relationship between caregiver burden and the health of the caregiver, while moderating variables change the effect between them when increased or decreased. In the first case, caregiver education would likely mediate burden of care; in the second, caregiver sleep (or lack thereof) would moderate the impact of caregiving on depression.
For caregiver burden
Three areas that cause burden for caregivers are activities of daily living, such as eating, bathing, and toileting; instrumental activities of daily living, encompassing shopping, food preparation, and financial management; and behavior and safety, including falls, fires, and driving.43 Caregivers experience physical symptoms, depression, and feelings of burden when faced with a greater number of tasks, more problematic behaviors, and/or more family disagreements.44
The concept of caregiver burden is the focus of many studies, all of them seeking answers for how best to support caregivers caring for a loved one with AD.37,43,45,46 In a multicenter prospective randomized study conducted in 11 hospital and nonhospital psychiatric outpatient clinics in southern Europe (N = 115), the intervention group participated in eight individual sessions over four months that focused on learning strategies for managing AD patient care.46 Data were collected on caregiver stress, quality of life, and perceived health to determine the impact on caregiver burden. The intervention was found to minimize caregiver burden as measured by the ZBI.
Lai and Thomson evaluated a random sample of family caregivers (n = 340) and concluded that providing tangible services and resources should be the first step in reducing burden of care. They report that caregivers’ perceived adequacy of support services predicts caregiver burden. They noted that emotional support results in only marginal benefits.45 Other studies have concurred with the finding that support groups provide emotional support, information, and problem-solving skills to caregivers but do not reduce caregiver burden.31
For caregivers of those with dementia, the strongest predictor of distress is care recipients’ problem behaviors.26 Some theorize that the distress that caregivers experience has multiple components, including belligerence, lack of cooperation, oppositional behaviors, and disruption of sleep patterns of the care recipient.13 Support groups should address behavioral interventions that caregivers can use when faced with the challenging behaviors that often concur with neurocognitive disorders, including confusion, wandering, agitation, crying, swearing, and combativeness.
Caregivers may experience increased stress as their loved one’s dementia progresses. The New York University (NYU) Caregiver Intervention is a long-running randomized controlled study of counseling and support interventions for caregivers of spouses with dementia, conducted by the NYU School of Medicine. Among the array of published analyses of data from this study was a trial reporting that counseling and support interventions reduced the rate of nursing home placement by 28.3%, compared with usual care, and delayed nursing home placement; 61.2% of this delay was attributed to social support, response to patient behaviors, and reduced depression.47 Comprehensive counseling and support provided during the progression of AD patients transitioning to institutionalization can be beneficial to spouses and may translate more broadly to caregivers in general.48
Equipping caregivers to manage the most difficult aspect of their role should be a priority. Many towns and cities offer day programs for those with memory disorders, allowing for respite for caregivers. Councils on aging are a great resource for service information and specialized programs. For those who meet the income guidelines, there may even be financial support for family caregivers. To seek out services, contact the national Alzheimer’s Association (Alz.org) or a local branch. For information on behavioral interventions, Caregiver.org has many available resources on a variety of topics. (Additional resources are listed in “Caregiving Resources.”)
Continue for caregiver stress and self-care >>
Caregiver stress and SELF-CARE
Primary care providers can be instrumental in not only offering referral to services to reduce caregiver stress but also encouraging self-care behaviors for caregivers. They have the opportunity, early on, to anticipate the stresses that caregivers may experience as their loved one’s disease progresses and to provide helpful referral for services (including psychotherapy and support groups), information, and support to preventively address functional ability and self-care behaviors.
The relationship between caregiver stress and self-care behavior has been widely studied. Using the Caregiving Hassles Scale, Kinney and Stephens examined the mediating function of the relationship between caregiving stress and self-care behavior. They found caregiver stress to correlate to self-rated health at a statistically significant level (r = .30, P = .003).13 On a positive note, the investigators also found that the more symptoms family members reported (depression, poor health), the more self-care behaviors they used.
Lu and Wykle also used the Caregiving Hassles Scale in their correlational crossfunctional study. Caregivers (n = 99) were assessed for the mediating function of the relationship between caregiving stress and self-care behavior in response to symptoms.49 Those who reported higher levels of caregiving stress also reported poorer self-rated health, poorer physical function, high levels of depressed mood, and more self-care behaviors at a statistically significant level (r =.30, P = .003, Cronbach’s alpha = .95). The researchers determined that depressed mood was a strong mediator between caregiver stress and response to the symptoms with self-care behaviors.
Unsteady on his feet, Henry became a fall risk. His growing confusion and cognitive decline led to increased depression and agitation, which reduced his socialization and physical activity. Over time, this resulted in a disturbing chain of events, including urinary tract infections, hospitalization, and behavior changes that were upsetting to his wife and children as well as to Henry. Joann was not sleeping well and contracted a cold that turned into pneumonia, leading to a hospitalization. Through all of this, Joann knew that she could call Henry’s NP for advice and support. She found solace in knowing that the caregiver support group she attended regularly would always be there to encourage and inform her and that Henry’s support group would not only give her respite as a caregiver but also provide Henry with cognitive stimulation shown to enhance the well-being of those experiencing memory loss. These groups soon became her lifeline. Without vital early screenings, this family would not have adequately managed the difficulties brought on by Henry’s unexpected diagnosis.
Continue for summary and conclusions >>
Summary and conclusions
Extensive research on caregivers has focused on depression, burden of care, and self-care issues, with mixed findings. Gottlieb and colleagues report that caregivers struggle with the “apparent sadness, listlessness, and vegetative behavior” of their loved one struggling with AD.35 Primary care providers are in a pivotal position to improve caregivers’ health status using reliable and valid assessment tools and offerring referral to services that have been shown to help caregivers in their complex and challenging role.
Support groups, assistance with behavioral interventions, information about the disease process and effective interventions, medication for clinical depression, respite and day programs for persons with neurocognitive disorders, and encouragement of self-care all reduce caregiver stress. Offering effective interventions can improve the physical and mental health of burdened caregivers and positively impact the lives of their loved ones.
Given the growing number of caregivers and the significant effect of caregiving on their health, staying alert should be a priority in all practices. Simply adding two questions to those we regularly ask—Do you care for a loved one struggling with memory loss? How many hours a week do you provide care?—can illuminate the challenges caregivers face, so we can monitor their health status appropriately.
1. 2015 Alzheimer’s Disease Facts and Figures. Chicago: Alzheimer’s Association; 2015. www.alz.org/facts/downloads/facts_figures_2015.pdf. Accessed May 20, 2016.
2. Wallis L. Reach VA helps family caregivers of dementia patients. Am J Nurs. 2011;111(6):18.
3. Office of Disease Prevention and Health Promotion. 2020 Topics and objectives—objectives A–Z. Healthy People 2020. www.healthypeople.gov/2020/topics-objectives. Accessed May 20, 2016.
4. Levine C, Halper D, Peist A, Gould D. Bridging troubled waters: family caregivers, transitions, and long-term care. Health Aff. 2010;29(1):116-124.
5. MetLife Mature Market Institute, National Alliance for Caregiving, and the University of Pittsburgh Institute on Aging. The MetLife Study of Working Caregivers and Employer Health Care Costs. February 2010. www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-care-costs.pdf. Accessed May 20, 2016.
6. Zarit SH. Assessment of family caregivers: A research perspective. In: Family Caregiver Alliance. Caregiver Assessment: Voices and Views From the Field. Report from a National Consensus Development Conference. Volume II. San Francisco: Family Caregiver Alliance; 2006:14. https://caregiver.org/sites/caregiver.org/files/pdfs/v2_consensus.pdf. Accessed May 20, 2016.
7. Family Caregiver Alliance National Center on Caregiving. (2012). Selected Caregiver Assessment Measures: A Resource Inventory for Practitioners. 2nd ed. https://www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012. Accessed May 20, 2016.
8. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. San Francisco: Family Caregiver Alliance; 2006. www.caregiver.org/sites/caregiver.org/files/pdfs/Assessment_Toolkit_20060802.pdf. Accessed May 20, 2016.
9. Antonovsky A. The structure and properties of the Sense of Coherence Scale. Soc Sci Med. 1993;36(6):725-733.
10. Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652-657.
11. Czaja SJ, Gitlin LN, Schulz R, et al. Development of the Risk Appraisal Measure (RAM): a brief screen to identify risk areas and guide interventions for dementia caregivers. J Am Geriatr Soc. 2009;57(6):1064-1072.
12. Gort AM, Mingot M, March J, et al. Short Zarit scale in dementias. Med Clin (Barc). 2010;35(10):447-449.
13. Kinney JM, Stephens MA. Caregiving Hassles Scale: assessing the daily hassles of caring for a family member with dementia. Gerontologist. 1989;29(3):328-332.
14. Locke DEC, Dassel KB, Hall G, et al. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire. Dement Geriatr Cogn Disord. 2009;27:260-272.
15. Picot SJ, Youngblut J, Zeller R. Development and testing of a measure of perceived caregiver rewards in adults. J Nurs Meas. 1997;5(1):33-52.
16. Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 1986;5:119-136.
17. Seng BK, Luo N, Ng WY, et al. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singapore. 2010;39(10):758-763.
18. Family Caregiver Alliance. Caregiver Assessment: Principles, Guidelines and Strategies for Change. Report from a National Consensus Development Conference (Vol. I). San Francisco: Family Caregiver Alliance; 2006:12. https://www.caregiver.org/sites/caregiver.org/files/pdfs/v1_consensus.pdf. Accessed May 20, 2016.
19. Michigan Dementia Coalition. Caregiver Assessment Tool Grid. The Rosalynn Carter Institute. www.rosalynncarter.org/UserFiles/Michigan%20Assessment%20Grid.pdf. Accessed May 20, 2016.
20. Zarit SH, Reever KE, Bach-Peterson J. Relatives of impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
21. Miyamoto Y, Tachimo H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253.
22. Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583-594.
23. American Medical Association. Caregiver Self-Assessment Questionnaire. National Caregivers Library. www.caregiverslibrary.org/Portals/0/CaringforYourself_CaregiverSelfAssessmentQuestionaire.pdf. Accessed May 20, 2016.
24. Greenberg SA. The Geriatric Depression Scale (GDS). In: Try This: Best Practices in Nursing Care to Older Adults. Issue 4. New York, NY: Hartford Institute for Geriatric Nursing, NYU College of Nursing; 2012. https://consultgeri.org/try-this/general-assessment/issue-4. Accessed May 20, 2016.
25. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986:165-173.
26. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577-595.
27. Matschinger H, Schork A, Riedel-Heller S, Angemeyer M. On the application of the CES-D with the elderly: dimensional structure and artifacts resulting from oppositely worded items. Int J Methods Psychiatr Res. 2006;9(4):199-209.
28. Caregiver Burden Scale. Family Practice Notebook. www.fpnotebook.com/Geri/Exam/CrgvrBrdnScl.htm. Accessed May 20, 2016.
29. Caregiver Stress Self-Assessment. www.mass.gov/elders/docs/caregiver-stress-self-assessment.pdf. Accessed May 20, 2016.
30. US Department of Veterans Affairs. Caregiver Self-Assessment Worksheet. http://www.va.gov/GERIATRICS/guide/longtermcare/Caregiver_Self_Assessment.pdf. Accessed May 20, 2016.
31. Thompson CA, Spilsbury K, Hall J, et al. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr. 2007;7:18-29.
32. Chien LY, Chu H, Guo JL, et al. Caregiver support groups in patients with dementia: a meta-analysis. Int J Geriatr Psychiatry. 2011;26:1089-1098.
33. Burns R, Nichols LO, Martindale-Adams J, et al. Primary care interventions for dementia caregivers: 2-year outcomes for the REACH study. Gerontologist. 2003;43(4):547-555.
34. Schulz R, Burgio L, Burns R, et al. Resources for Enhancing Alzheimer’s Caregivers Health (REACH): overview, site-specific outcomes, and future directions. Gerontologist. 2003;43(4):514-520.
35. Gottlieb BH, Thompson LW, Bourgeois M. Monitoring and evaluating interventions. In: Coon DW, Gallagher-Thompson D, Thompson LW, eds. Innovative Interventions to Reduce Dementia Caregiver Distress: A Clinical Guide. New York, NY: Springer; 2003:28-49.
36. Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive-behavioral family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry. 2000;176:557-562.
37. Chu H, Yang CY, Liao YH, et al. The effects of a support group on dementia caregivers’ burden and depression. J Aging Health. 2011;23(2): 228-241.
38. Elliott AF, Burgio LD, DeCoster J. Enhancing caregiver health: findings from the Resources for Enhancing Alzheimer’s disease Health II intervention. J Am Geriatr Soc. 2010;58:30-37.
39. Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3): 356-372.
40. Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms of caregivers in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(5):850-856.
41. Mittelman M, Roth D, Clay O, Haley W. Preserving health of Alzheimer caregivers: impact of a spouse caregiver intervention. Am J Geriatr Psychiatry. 2007;15(9):780-789.
42. Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: a pilot randomized placebo-controlled trial of escitalopram. Am J Geriatr Psychiatry. 2010;18(2):154-164.
43. Wimo A, von Strauss E, Nordberg G, et al. Time spent on informal and formal caregiving for persons with dementia in Sweden. Health Policy. 2002;61(3):255-268.
44. Koerner SS, Shirai Y, Kenyon DB. Sociocontextual circumstances in daily stress reactivity among caregivers for elder relatives. J Gerontol B Psychol Sci Soc Sci. 2010;65(5):561-572.
45. Lai DW, Thomson C. The impact of perceived adequacy of social support on caregiving burden of family caregivers. Fam in Soc: J Contemp Social Serv. 2011;92(1):99-106.
46. Martin-Carrasco M, Martin M, Valero C, et al. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in Alzheimer’s disease patients’ caregivers. Int J Geriatr Psychiatry. 2009;24:489-499.
47. Mittelman MS, Haley WE, Clay OJ, Roth RL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
48. Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counseling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer’s disease during the transition to institutionalization? Results from the New York University caregiver intervention study. J Am Geriatr Soc. 2008;56(3):421-428.
49. Lu YFY, Wykle M. Relationships between caregiver stress and self-care behaviors in response to symptoms. Clin Nurs Res. 2007;16:29-43.
1. 2015 Alzheimer’s Disease Facts and Figures. Chicago: Alzheimer’s Association; 2015. www.alz.org/facts/downloads/facts_figures_2015.pdf. Accessed May 20, 2016.
2. Wallis L. Reach VA helps family caregivers of dementia patients. Am J Nurs. 2011;111(6):18.
3. Office of Disease Prevention and Health Promotion. 2020 Topics and objectives—objectives A–Z. Healthy People 2020. www.healthypeople.gov/2020/topics-objectives. Accessed May 20, 2016.
4. Levine C, Halper D, Peist A, Gould D. Bridging troubled waters: family caregivers, transitions, and long-term care. Health Aff. 2010;29(1):116-124.
5. MetLife Mature Market Institute, National Alliance for Caregiving, and the University of Pittsburgh Institute on Aging. The MetLife Study of Working Caregivers and Employer Health Care Costs. February 2010. www.metlife.com/assets/cao/mmi/publications/studies/2010/mmi-working-caregivers-employers-health-care-costs.pdf. Accessed May 20, 2016.
6. Zarit SH. Assessment of family caregivers: A research perspective. In: Family Caregiver Alliance. Caregiver Assessment: Voices and Views From the Field. Report from a National Consensus Development Conference. Volume II. San Francisco: Family Caregiver Alliance; 2006:14. https://caregiver.org/sites/caregiver.org/files/pdfs/v2_consensus.pdf. Accessed May 20, 2016.
7. Family Caregiver Alliance National Center on Caregiving. (2012). Selected Caregiver Assessment Measures: A Resource Inventory for Practitioners. 2nd ed. https://www.caregiver.org/selected-caregiver-assessment-measures-resource-inventory-practitioners-2012. Accessed May 20, 2016.
8. Family Caregiver Alliance. Caregivers Count Too! A Toolkit to Help Practitioners Assess the Needs of Family Caregivers. San Francisco: Family Caregiver Alliance; 2006. www.caregiver.org/sites/caregiver.org/files/pdfs/Assessment_Toolkit_20060802.pdf. Accessed May 20, 2016.
9. Antonovsky A. The structure and properties of the Sense of Coherence Scale. Soc Sci Med. 1993;36(6):725-733.
10. Bédard M, Molloy DW, Squire L, et al. The Zarit Burden Interview: a new short version and screening version. Gerontologist. 2001;41(5):652-657.
11. Czaja SJ, Gitlin LN, Schulz R, et al. Development of the Risk Appraisal Measure (RAM): a brief screen to identify risk areas and guide interventions for dementia caregivers. J Am Geriatr Soc. 2009;57(6):1064-1072.
12. Gort AM, Mingot M, March J, et al. Short Zarit scale in dementias. Med Clin (Barc). 2010;35(10):447-449.
13. Kinney JM, Stephens MA. Caregiving Hassles Scale: assessing the daily hassles of caring for a family member with dementia. Gerontologist. 1989;29(3):328-332.
14. Locke DEC, Dassel KB, Hall G, et al. Assessment of patient and caregiver experiences of dementia-related symptoms: development of the Multidimensional Assessment of Neurodegenerative Symptoms Questionnaire. Dement Geriatr Cogn Disord. 2009;27:260-272.
15. Picot SJ, Youngblut J, Zeller R. Development and testing of a measure of perceived caregiver rewards in adults. J Nurs Meas. 1997;5(1):33-52.
16. Radloff LS, Teri L. Use of the Center for Epidemiological Studies-Depression Scale with older adults. Clin Gerontol. 1986;5:119-136.
17. Seng BK, Luo N, Ng WY, et al. Validity and reliability of the Zarit Burden Interview in assessing caregiving burden. Ann Acad Med Singapore. 2010;39(10):758-763.
18. Family Caregiver Alliance. Caregiver Assessment: Principles, Guidelines and Strategies for Change. Report from a National Consensus Development Conference (Vol. I). San Francisco: Family Caregiver Alliance; 2006:12. https://www.caregiver.org/sites/caregiver.org/files/pdfs/v1_consensus.pdf. Accessed May 20, 2016.
19. Michigan Dementia Coalition. Caregiver Assessment Tool Grid. The Rosalynn Carter Institute. www.rosalynncarter.org/UserFiles/Michigan%20Assessment%20Grid.pdf. Accessed May 20, 2016.
20. Zarit SH, Reever KE, Bach-Peterson J. Relatives of impaired elderly: correlates of feelings of burden. Gerontologist. 1980;20(6):649-655.
21. Miyamoto Y, Tachimo H, Ito H. Formal caregiver burden in dementia: impact of behavioral and psychological symptoms of dementia and activities of daily living. Geriatr Nurs. 2010;31(4):246-253.
22. Pearlin LI, Mullan JT, Semple SJ, Skaff MM. Caregiving and the stress process: an overview of concepts and their measures. Gerontologist. 1990;30(5):583-594.
23. American Medical Association. Caregiver Self-Assessment Questionnaire. National Caregivers Library. www.caregiverslibrary.org/Portals/0/CaringforYourself_CaregiverSelfAssessmentQuestionaire.pdf. Accessed May 20, 2016.
24. Greenberg SA. The Geriatric Depression Scale (GDS). In: Try This: Best Practices in Nursing Care to Older Adults. Issue 4. New York, NY: Hartford Institute for Geriatric Nursing, NYU College of Nursing; 2012. https://consultgeri.org/try-this/general-assessment/issue-4. Accessed May 20, 2016.
25. Sheikh JI, Yesavage JA. Geriatric Depression Scale (GDS): recent evidence and development of a shorter version. In: Brink TL, ed. Clinical Gerontology: A Guide to Assessment and Intervention. New York, NY: The Haworth Press; 1986:165-173.
26. Pinquart M, Sörensen S. Helping caregivers of persons with dementia: which interventions work and how large are their effects? Int Psychogeriatr. 2006;18(4):577-595.
27. Matschinger H, Schork A, Riedel-Heller S, Angemeyer M. On the application of the CES-D with the elderly: dimensional structure and artifacts resulting from oppositely worded items. Int J Methods Psychiatr Res. 2006;9(4):199-209.
28. Caregiver Burden Scale. Family Practice Notebook. www.fpnotebook.com/Geri/Exam/CrgvrBrdnScl.htm. Accessed May 20, 2016.
29. Caregiver Stress Self-Assessment. www.mass.gov/elders/docs/caregiver-stress-self-assessment.pdf. Accessed May 20, 2016.
30. US Department of Veterans Affairs. Caregiver Self-Assessment Worksheet. http://www.va.gov/GERIATRICS/guide/longtermcare/Caregiver_Self_Assessment.pdf. Accessed May 20, 2016.
31. Thompson CA, Spilsbury K, Hall J, et al. Systematic review of information and support interventions for caregivers of people with dementia. BMC Geriatr. 2007;7:18-29.
32. Chien LY, Chu H, Guo JL, et al. Caregiver support groups in patients with dementia: a meta-analysis. Int J Geriatr Psychiatry. 2011;26:1089-1098.
33. Burns R, Nichols LO, Martindale-Adams J, et al. Primary care interventions for dementia caregivers: 2-year outcomes for the REACH study. Gerontologist. 2003;43(4):547-555.
34. Schulz R, Burgio L, Burns R, et al. Resources for Enhancing Alzheimer’s Caregivers Health (REACH): overview, site-specific outcomes, and future directions. Gerontologist. 2003;43(4):514-520.
35. Gottlieb BH, Thompson LW, Bourgeois M. Monitoring and evaluating interventions. In: Coon DW, Gallagher-Thompson D, Thompson LW, eds. Innovative Interventions to Reduce Dementia Caregiver Distress: A Clinical Guide. New York, NY: Springer; 2003:28-49.
36. Marriott A, Donaldson C, Tarrier N, Burns A. Effectiveness of cognitive-behavioral family intervention in reducing the burden of care in carers of patients with Alzheimer’s disease. Br J Psychiatry. 2000;176:557-562.
37. Chu H, Yang CY, Liao YH, et al. The effects of a support group on dementia caregivers’ burden and depression. J Aging Health. 2011;23(2): 228-241.
38. Elliott AF, Burgio LD, DeCoster J. Enhancing caregiver health: findings from the Resources for Enhancing Alzheimer’s disease Health II intervention. J Am Geriatr Soc. 2010;58:30-37.
39. Sörensen S, Pinquart M, Duberstein P. How effective are interventions with caregivers? An updated meta-analysis. Gerontologist. 2002;42(3): 356-372.
40. Mittelman MS, Roth DL, Coon DW, Haley WE. Sustained benefit of supportive intervention for depressive symptoms of caregivers in patients with Alzheimer’s disease. Am J Psychiatry. 2004;161(5):850-856.
41. Mittelman M, Roth D, Clay O, Haley W. Preserving health of Alzheimer caregivers: impact of a spouse caregiver intervention. Am J Geriatr Psychiatry. 2007;15(9):780-789.
42. Lavretsky H, Siddarth P, Irwin MR. Improving depression and enhancing resilience in family dementia caregivers: a pilot randomized placebo-controlled trial of escitalopram. Am J Geriatr Psychiatry. 2010;18(2):154-164.
43. Wimo A, von Strauss E, Nordberg G, et al. Time spent on informal and formal caregiving for persons with dementia in Sweden. Health Policy. 2002;61(3):255-268.
44. Koerner SS, Shirai Y, Kenyon DB. Sociocontextual circumstances in daily stress reactivity among caregivers for elder relatives. J Gerontol B Psychol Sci Soc Sci. 2010;65(5):561-572.
45. Lai DW, Thomson C. The impact of perceived adequacy of social support on caregiving burden of family caregivers. Fam in Soc: J Contemp Social Serv. 2011;92(1):99-106.
46. Martin-Carrasco M, Martin M, Valero C, et al. Effectiveness of a psychoeducational intervention program in the reduction of caregiver burden in Alzheimer’s disease patients’ caregivers. Int J Geriatr Psychiatry. 2009;24:489-499.
47. Mittelman MS, Haley WE, Clay OJ, Roth RL. Improving caregiver well-being delays nursing home placement of patients with Alzheimer disease. Neurology. 2006;67(9):1592-1599.
48. Gaugler JE, Roth DL, Haley WE, Mittelman MS. Can counseling and support reduce burden and depressive symptoms in caregivers of people with Alzheimer’s disease during the transition to institutionalization? Results from the New York University caregiver intervention study. J Am Geriatr Soc. 2008;56(3):421-428.
49. Lu YFY, Wykle M. Relationships between caregiver stress and self-care behaviors in response to symptoms. Clin Nurs Res. 2007;16:29-43.
Low Back Pain: Evidence-based Diagnosis and Treatment
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
CE/CME No: CR-1605
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Identify "red flag" items in the history and physical exam that make low back pain (LBP) "complicated."
• Stratify patients into three categories: simple back pain, complicated back pain, and back pain with sciatica.
• Discuss when appropriate additional testing/imaging is needed based on LBP categories.
• Discuss patient perceptions and costs associated with imaging and LBP.
• Describe basic treatment options for noncomplicated acute LBP.
FACULTY
Mike Roscoe is the PA Program Director at the University of Evansville, Indiana. Alyssa Nishihira is in her final year of the PA program at Butler University, Indianapolis; after graduation, she will be practicing at Advanced Neurosurgery in Reno, Nevada.
The authors have no financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of May 2016.
Article begins on next page >>
Low back pain (LBP) is one of the most common reasons for an office visit, but most cases—at least 95%—have a benign underlying cause. Evaluation of LBP patients in the primary care setting, therefore, must focus on identifying “red flags” in the history and physical exam that suggest a significant underlying process requiring further work-up, including imaging. This evidence-based approach helps control costs and prevents the detrimental effects of unnecessary testing.
Low back pain (LBP) plagues many Americans and is a common reason for office visits in the United States. In 2010, back symptoms were the principal reason for 1.3% of office visits in the US.1 Recent data suggest that 75% to 85% of all Americans will experience an episode of LBP at least once in their lifetime.2 It is the leading cause of years lived with disability in the US3 and is a common reason for work disability. From a health care system standpoint, LBP imposes a considerable burden, accounting for more than $85 billion annually in direct costs.2
The etiology of LBP can be related to several anatomic and physiologic changes. Potential origins of LBP include, but are not limited to, pathology of the vertebrospinal ligaments, musculature, facet joints, fascia, vertebra and vertebral disks, and the extensive neurovascular components of the lumbar region. Although the potential causes of LBP are many, the majority of patients presenting with acute LBP usually improve with minimal clinical intervention within the first month. This is true even for patients who report limitations in daily activities and those with severe, acute cases of LBP.
A single standard of care for patients presenting with LBP has not been established. The wide array of choices for diagnosis and treatment of LBP is one factor that hinders the development of a standard diagnostic protocol. The challenge to clinicians when diagnosing LBP is to differentiate the patients with benign, self-limiting LBP (simple), who comprise the vast majority of LBP patients, from the 1% to 5% with a serious underlying pathology (complicated).4
Continue for stratification of low back pain >>
STRATIFICATION OF LOW BACK PAIN
Koes and colleagues analyzed 13 different national guidelines and two international guidelines for the management of LBP.5 They found that the guidelines consistently recommend focusing the history and physical exam (HPE) on identifying features suggestive of underlying serious pathology, or “red flags,” and excluding specific diseases.5 They also found that none of the guidelines recommends the routine use of imaging in patients without suspected serious pathology.5 The American College of Radiology simplified this approach to patients with LBP by creating a list of red flags to look for during the HPE.3 The presence of red flags indicates a case of complicated LBP, and patients who present with them should undergo additional diagnostic studies to screen for serious underlying conditions (see the Table).
The HPE should ultimately separate patients into three categories to determine the need for imaging (and course of treatment): (1) simple acute back pain, (2) complicated back pain with red flag (ie, a potential underlying systemic disease), and (3) LBP with neurologic deficits potentially requiring surgery.5
Simple acute low back pain
Up to 85% of patients presenting with LBP may never receive a definitive diagnosis due to lack of specific symptoms and ambiguous imaging results.6 Clinicians can assume that LBP in these patients is due to a mechanical cause, by far the most common cause of LBP.7 It is therefore more useful to rule out serious or potentially fatal causes of LBP (complicated LBP) rather than rule in a cause for patients presenting with LBP.
It is generally accepted among practitioners that a thorough HPE alone is sufficient for evaluating most patients presenting with acute LBP lasting less than four weeks.5 Patients presenting without red flags should be assured that improvement of acute LBP is typical, and that no diagnostic intervention is needed unless they do not improve as expected per patient or provider (eg, in terms of activities of daily living or work restrictions). The Figure depicts an appropriate approach to diagnosis and treatment in patients presenting with LBP.8 Clinicians should also offer patient education for self-care and discuss noninvasive treatment options, including pharmacologic and nonpharmacologic therapy.9
Low back pain with red flags (complicated)
Patient history is more useful than the physical exam in screening for spinal malignancies. In one particular combination (age > 50, history of cancer, unexplained weight loss, and failure to improve with conservative therapy), red flag symptoms are 100% sensitive for detecting malignancy.10 However, malignant neoplasms of the spine make up less than 1% of the diagnoses of patients presenting with LBP in primary care.4 Additionally, Deyo and Diehl reviewed five studies of a large series of consecutive spine films with large sample sizes and found the incidence of tumors, infections, and inflammatory spondyloarthropathies together were present in less than 2%.11 This low prevalence underscores the challenge of diagnosing serious pathology of the spine in the primary care setting.
Patients with complicated back pain presenting with red flags should always be examined for an underlying systemic disease. There is one red flag that, seen in isolation, meaningfully increases the likelihood of cancer: a previous history of cancer.4 Otherwise, inflammatory markers (eg, erythrocyte sedimentation rate) can be used to determine the need for advanced imaging (see the Figure).10
Low back pain with neurologic findings (sciatica)
Screening (HPE) for neurologic damage is difficult because traditional findings of neurologic injury (paresis or muscle weakness, impaired reflexes, sensory deficits, and decreased range of motion) all have low sensitivity with higher specificity.12 For this reason, these tests are of limited value as screening tools during the HPE. Specific exams, such as the straight leg raise and crossed straight leg tests, are also of limited value, especially in the primary care setting, because of inconsistent sensitivity and specificity.
This is the primary reason that the HPE in patients with LBP who have neurologic findings must include evaluation for urgent findings (see the Figure). If any red flags are present, advanced imaging is immediately warranted. Otherwise, inflammatory markers and plain radiography may be obtained, and advanced imaging may be considered if the plain radiography and/or inflammatory markers are abnormal.
There is also an approach that advocates the use of advanced imaging in patients with significant functional disability due to their LBP. Two questionnaires, the Oswestry Low Back Pain Disability Index and the Roland-Morris Disability Questionnaire, evaluate subjective data to determine a patient’s functional disability due to LBP.The validity of both tests has been confirmed.13
Continue for diagnostic imaging >>
DIAGNOSTIC IMAGING
The majority of patients presenting with LBP without concerning symptoms can be assumed to have nonspecific mechanical back pain. These patients do not need radiography unless the pain has not improved after four to six weeks of conservative care, because plain radiographs often detect findings (degenerative joint disease, bone spurs, spondylosis) that are unrelated to symptoms.9 Advanced imaging is generally recommended only for LBP patients with red flags due to the potentially critical nature of these cases.5 Patients with LBP presenting with any of these factors require further testing, even if the duration of their pain is less than four weeks.
If a patient’s LBP persists beyond four weeks, the clinician must decide which diagnostic test to order. General medical knowledge suggests that MRI is superior to plain radiography because it shows soft tissue and can detect more concerning abnormalities, such as infections, cancer, and metastatic tumors. CT is better for showing bony abnormalities, but these rarely correlate with a patient’s LBP, and CT subjects patients to levels of radiation that can increase cancer risks.14 Plain radiography in this cohort (LBP > 4 wk) is not generally recommended as it cannot show intervertebral discs or evaluate the degree of spinal stenosis as accurately as MRI. Additionally, these lumbar radiographs expose patients to more than 35 times the radiation delivered in a single chest radiograph.15
COSTS AND PATIENT OUTCOMES
The estimated cost of unnecessary imaging for LBP is $300 million per year.16 There is evidence of a strong association between advanced lumbar spine imaging and increased rates of surgery and significantly higher total medical expenditures.17,18 One study examined patients with nonspecific LBP who either received MRI within 30 days post-onset (defined as “early MRI”) or did not receive MRI. Early-MRI patients had significantly higher total medical expenses ($12,948, P < .0001) than the no-MRI group.17 The early-MRI group also had significantly longer periods of disability and were less likely to go off disability than the no-MRI group (P < .0001).
Cost-effectiveness studies of plain radiographs, dating back to 1982, have yielded similar findings. Liang et al suggested that if radiography was done routinely at the initial visit in patients with acute LBP but no red flags, the cost would be more than $2,000 (in 1982 dollars) to avert one day of pain.19 A more recent study examined patients with acute LBP who received MRI, with one group blinded (both patients and physicians) to their MRI results for six months while the other group received their results within 48 hours.20 All patients underwent a physical exam by a study coordinator, and treatment was assigned prior to imaging. At six weeks and one year, there was no significant difference in treatment assignments or self-reported surveys between groups, indicating that the MRI results had no significant influence on patient outcomes.
Despite the large increase in the use of advanced diagnostic imaging aimed at improving patient care and outcomes, there is a lack of data showing any correlative or causative connection between the two. Given this lack of evidence, and the potentially detrimental radiation exposure and increased costs to patients, clinicians should follow evidence-based guidelines when considering diagnostic imaging in patients presenting with LBP.
Continue for patient perception >>
PATIENT PERCEPTION
Patient satisfaction plays a very important role in health care and may correlate with compliance and other outcomes. One study showed that while radiography in patients with LBP was not associated with improved clinical outcomes, it did increase patients’ satisfaction with the care they received.21 A study that grouped patients requiring imaging for LBP into rapid MRI and plain film radiography cohorts found that patients who received rapid MRI were more assured by their results than were patients in the radiography group (74% vs 58%, P = .002).22 Both groups showed significant clinical improvement in the first three months, but there was no difference between groups at either the three- or 12-month mark. In both groups, reassurance was positively correlated with patient satisfaction (Pearson correlation coefficients, 0.55-0.59, P < .001).
Patients may be reassured by imaging, even when it is unnecessary. Effectively explaining symptomatology during the HPE to patients with LBP should be of high priority to clinicians. A study found that when patients with mechanical LBP did not receive an adequate explanation of the problem, they were less satisfied with their visit and wanted more diagnostic tests.11 Another study found that when low-risk patients were randomly assigned to a control group and received an educational intervention only, they reported equal satisfaction with their care and had clinical outcomes equal to those of the treatment group that received a plain radiograph.11
Given the costs, radiation risks, and other negative aspects of unnecessary imaging, additional diagnostic tests may not be in a patient’s best interest. A careful physical exam should be performed, with the clinician providing ongoing commentary to reassure patients that the clinician is neither dismissing the patient’s symptoms nor inappropriately avoiding further tests.
Often, medical providers order imaging with the intention to reassure patients with the results and thus ultimately increase the patient’s sense of well-being. However, the opposite effect may occur, with patients actually developing a decreased sense of wellness with no alteration of outcomes. A study evaluated general health (GH) scores (based on results from several screening questionnaires that assessed the patient’s current physical and mental health state) in patients receiving MRI results.20 The patients were divided into those who received results (within 48 hours), and those who did not unless it was critical to patient management (blinded group). At six weeks, the blinded group’s GH score was significantly higher than the early-informed group’s GH score. This suggests that receiving MRI results may negatively influence patients’ perception of their general health.20
The same meta-analysis that reviewed patient outcomes also evaluated mental health and quality-of-life scores of LBP patients who received either MRI, CT, or radiography.23 There was no short-term (< 3 mo) or long-term (6-12 mo) difference between patients who received radiography versus advanced imaging. This indicates that using imaging of any kind in patients with LBP but without indications of serious underlying conditions does not improve clinical outcomes and is negatively correlated with quality-of-life measures at short- and long-term intervals.23
Continue for treatment >>
TREATMENT
The prognosis of simple acute mechanical LBP is excellent. Although back pain is a leading reason for visiting health care providers, many affected individuals never seek medical care and apparently improve on their own. In a random telephone survey of North Carolina residents, only 39% of persons with LBP sought medical care.24 Therefore, patients who do seek treatment should be given reassurance, and therapies should be tailored to the individual in the least invasive and most cost-effective manner. Many treatment options are available for LBP, but often strong evidence of benefit is lacking.
Pharmacologic therapy
Anti-inflammatories. It can be assumed that when a patient comes to the practitioner for evaluation of LBP, there is an expectation that some type of medication will be recommended or prescribed for pain relief. Unless there is a contraindication, NSAIDs are often first-line therapy, and they are effective for short-term symptom relief when compared with placebo.25 A mild pain medication, such as acetaminophen, is also a common treatment. The 2007 joint practice guideline from the American Pain Society (APS) and the American College of Physicians (ACP) recommends acetaminophen or NSAIDs as first-line therapy for acute LBP.3 Neither agent—NSAIDs or acetaminophen—has shown superiority, and combining the two has shown no additional benefits.26 Caution must be used, however, as NSAIDs have a risk for gastrointestinal toxicity and nephrotoxicity, and acetaminophen has a dose- and patient-dependent risk for hepatotoxicity.
Muscle relaxants. Muscle relaxants are another pharmacologic treatment option for LBP. Most pain reduction from this class of medication occurs in the first one to two weeks of therapy, although benefit may continue for up to four weeks.27 There is also evidence that a combination of an NSAID and a muscle relaxer has added benefits.27 These medications are centrally acting, so sedation and dizziness are common; all medications in this class have these adverse effects to some degree. Carisoprodol has as its first metabolite meprobamate, which is a tranquilizer used to treat anxiety disorders; it has a potential for abuse and should be used with caution in certain populations.
Opioids. Opioids are commonly prescribed to patients with LBP, though there are limited data regarding efficacy. One trial compared an NSAID alone versus an NSAID plus oxycodone/acetaminophen and found no significant difference in pain or disability after seven days.28 In addition, the adverse effects of opioids, which include sedation, constipation, nausea, and confusion, may be amplified in the elderly population; therefore, opioids should be prescribed with caution in these patients. If prescribed to treat acute LBP, opioids should be used in short, scheduled dosing regimens since NSAIDs or acetaminophen suffice for most patients.
Corticosteroids. Oral glucocorticoids are sometimes given to patients with acute LBP, and they likely are used more frequently in patients with radicular symptoms. However, the APS/ACP 2007 joint guidelines recommend against use of systemic glucocorticoids for acute LBP due to lack of proven benefit.3 Epidural steroid injections are not generally beneficial for isolated acute LBP, but there is evidence that they are helpful with persistent radicular pain.29 Zarghooni and colleagues found significant reductions in pain and use of pain medication after single-shot epidural injections.29
Other pharmacologic therapies, acupuncture, sclerotherapy, and other methods are used to treat back pain, but these are typically reserved for chronic, not acute, LBP.
Nonpharmacologic therapy
Physical therapy. Physical therapy is a commonly prescribed treatment for LBP. Systematic literature reviews indicate that for patients with acute LBP (< 6 wk), there is no difference in the effectiveness of exercise therapy compared to no treatment and care provided by a general practitioner or to manipulations.30 For patients with subacute (6-12 wk) and chronic (≥ 12 wk) LBP, exercise therapy is effective compared to no treatment.30 There is debate, however, over which exercise activities should be used. Research supports strength/resistance and coordination/stabilization exercises.
Most therapists recommend the McKenzie method or spine stabilization exercises.31 The McKenzie method is used for LBP with sciatica; the patient moves through exercises within the prone position and focuses on extension of the spine. Spine stabilization is an active form of exercise based on a “neutral spine” position and helps strengthen muscles to maintain this position (core stabilization). The McKenzie method, when added to first-line care for LBP, does not produce significant improvements in pain or other clinical outcomes, although it may reduce health care utilization.32 Spine stabilization exercises have been shown to decrease pain, disability, and risk for recurrence after a first episode of back pain.33 The apparent success of physical therapy is attributed to compliance with directed home exercise programs, which have been shown to reduce the rate of recurrence, decrease episodes of acute LBP, and decrease the need for health services.34
Spinal traction. Traction or nonsurgical spinal decompression has emerged as a treatment for LBP. Unfortunately, there are little data to support its use as a treatment for acute LBP. Only a few randomized trials showed benefit, and these were small studies with a high risk for bias. A Cochrane review published in 2013 looked at 32 studies involving 2,762 patients with acute, subacute, and chronic LBP.35 The review did not find any evidence that traction alone or in combination with other therapy was any better than placebo treatment.35
Spinal manipulation. Spinal manipulation may be more effective than placebo treatment in reducing pain when the pain has been present for less than six weeks, but it is not more effective in reducing disability.36 There is little or no high-level evidence about spinal manipulation for acute LBP. However, there is some evidence of cost-effectiveness when using spinal manipulation in subacute to chronic pain.37 Chiropractic techniques are considered safe (when performed by a trained provider), but a systematic review found that these techniques provide no clinically relevant improvement in pain or disability when compared to other treatments.38
Bed rest. Bed rest has not been shown to improve outcomes, and in fact patients who had bed rest had less favorable outcomes than those who stayed active.39 Bed rest is less effective at reducing pain and improving function when compared to staying active.39
Continue for recommended management >>
Recommended management
A patient who presents with nonspecific acute LBP should have a thorough HPE to evaluate for the presence of red flags. If no concerning findings are present, the initial visit should focus on patient education based on the following items: (1) good prognosis with little intervention, (2) staying active and avoiding bed rest as much as possible, and (3) avoiding pain-causing movements when possible. The second step is to initiate a trial of an NSAID or acetaminophen and consider a muscle relaxant based on pain severity. Avoid opioid therapy if possible, but use conservative dosing if required for severe pain. Patients should be advised to return in two to four weeks if they do not experience significant improvement. At this time, the clinician may consider referring the patient for physical therapy, changing NSAIDs, ordering inflammatory markers, and/or referring to a specialist.
CONCLUSION
Although no single diagnostic protocol for LBP exists, the clinician must be able to distinguish simple from complex types. A thorough HPE is useful for categorizing the patient’s pain, with diagnostic imaging reserved for those patients with severe or progressive neurologic deficits, suspicion of serious underlying conditions, or LBP lasting more than four weeks without improvement. MRI, if available, is generally preferred over CT because it does not use ionizing radiation and provides better visualization of soft tissue, vertebral marrow, and the spinal cord. Symptomatology should be explained to patients with LBP during the HPE, with ongoing commentary to increase patient satisfaction and compliance. About two-thirds of patients with LBP do not seek evaluation from a health care provider; therefore, those who do seek treatment should be reassured, and therapies tailored to the individual in the least invasive and most cost-effective manner possible.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
1. CDC. National Ambulatory Medical Care Survey: 2010 Summary Tables. Table 9. www.cdc.gov/nchs/data/ahcd/namcs_summary/2010_namcs_web_tables.pdf. Accessed March 29, 2016.
2. Davies C, Nitz AJ, Mattacola CG, et al. Practice patterns when treating patients with low back pain: a survey of physical therapists. Physiother Theor Pract. 2014;30(6):399-408.
3. American College of Radiology. ACR Appropriateness Criteria. Low back pain. 2015. www.acr.org/~/media/ACR/Documents/AppCriteria/Diagnostic/LowBackPain.pdf. Accessed March 10, 2016.
4. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low back pain. Cochrane Database Syst Rev. 2013;2:CD008686.
5. Koes BW, Tulder M, Lin CW, et al. An updated overview of clinical guidelines for the management of non-specific low back pain in primary care. Eur Spine J. 2010;19(12):2075-2094.
6. Deyo RA, Rainville J, Kent DL. What can the history and physical examination tell us about low back pain? JAMA. 1992;268(6):760-765.
7. Jarvik JG. Diagnostic evaluation of low back pain with emphasis on imaging. Ann Intern Med. 2002;137:586-597.
8. Diagnostic testing for low back pain. In: Post TW (ed), UpToDate, Waltham, MA. www.uptodate.com. Accessed March 16, 2016.
9. Chou R, Qaseem A, Snow V, et al; Clinical Efficacy Assessment Subcommittee of the American College of Physicians; American College of Physicians; American Pain Society Low Back Pain Guidelines Panel. Diagnosis and treatment of low back pain: a joint clinical practice guideline from the American College of Physicians and the American Pain Society. Ann Intern Med. 2007;147(7):478-491.
10. Deyo RA, Diehl AK. Cancer as a cause of back pain: frequency, clinical presentation, and diagnostic strategies. J Gen Intern Med. 1988;3(3):230-238.
11. Deyo RA, Diehl AK. Patient satisfaction with medical care for low-back pain. Spine. 1986;11(1):28-30.
12. Pradeep S, Rainville J, Katz JN, et al. The accuracy of the physical examination for the diagnosis of midlumbar and low lumbar nerve root impingement. Spine. 2011;36(1):63-73.
13. Leclaire R, Blier F, Fortin L, Proulx R. A cross-sectional study comparing the Oswestry and Roland-Morris Functional Disability Scales in two populations of patients with low back pain of different levels of severity. Spine. 1997;22(1):68-71
14. FDA. Radiation emitting products. www.fda.gov/Radiation-EmittingProducts/RadiationEmittingProductsandProcedures/MedicalImaging/MedicalX-Rays/ucm115317.htm. Accessed March 29, 2016.
15. Simpson AK, Whang PG, Jonisch A, et al. The radiation exposure associated with cervical and lumbar spine radiographs. J Spinal Disord Tech. 2008;21(6):409-412.
16. Srinivas S, Deyo R, Berger Z. Application of “less is more” to lower back pain. Arch Intern Med. 2012;172(13):1016-1020.
17. Webster BS, Bauer AZ, Choi Y, et al. Iatrogenic consequences of early magnetic resonance imaging in acute, work-related, disabling back pain. Spine. 2013;38(22):1939-1946.
18. Webster BS, Bauer AZ, Choi Y, et al. The cascade of medical services and associated longitudinal costs due to nonadherent magnetic resonance imaging for low back pain. Spine. 2014;39(17):1433-1440.
19. Liang M, Komaroff AL. Roentgenograms in primary care patients with acute low back pain: a cost-effectiveness analysis. Arch Intern Med. 1982;142(6):1108-1112.
20. Ash LM, Modic MT, Obuchowski NA, et al. Effects of diagnostic information, per se, on patient outcomes in acute radiculopathy and low back pain. AJNR Am J Neuroradiol. 2008;29(6):1098-1103.
21. Kendrick D, Fielding K, Bentley E, et al. Radiography of the lumbar spine in primary care patients with low back pain: randomized controlled trial. BMJ. 2001;322(7283):400-405.
22. Jarvik JG, Hollingworth W, Martin B, et al. Rapid magnetic resonance imaging vs radiographs for patients with low back pain. JAMA. 2003;289(21):2810-2818.
23. Chou R, Fu R, Carrino JA, Deyo RA. Imaging strategies for low-back pain: systematic review and meta-analysis. Lancet. 2009;373(9662):463-472.
24. Carey TS, Evans AT, Hadler NM, et al. Acute severe low back pain: a population-based study of prevalence and care-seeking. Spine. 1996;21(3):339-344.
25. Roelofs PD, Deyo RA, Koes BW, et al. Nonsteroidal anti-inflammatory drugs for low back pain. Spine. 2008;33(16):1766-1774.
26. Hancock MJ, Maher CG, Latimer J, et al. Assessment of diclofenac or spinal manipulative therapy, or both, in addition to recommended first-line treatment for acute low back pain: a randomized controlled trial. Lancet. 2007;370(10):1638-1643.
27. Van Tulder MW, Touray T, Furlan AD, et al. Muscle relaxants for non-specific low-back pain. Cochrane Database Syst Rev. 2003;(4):CD004252.
28. Friedman BW, Dym AA, Davitt M, et al. Naproxen with cyclobenzaprine, oxycodone/acetaminophen, or placebo for treating acute low back pain: a randomized clinical trial. JAMA. 2015;314(15):1572-1580.
29. Zarghooni K, Rashidi A, Siewe, J, et al. Single-shot epidural injections in the management of radicular pain. Orthop Rev (Pavia). 2015;7(4):5985.
30. Smidt N, deVet HC, Bouter LM, et al. Effectiveness of exercise therapy: A best-evidence summary of systematic reviews. Aust J Physiother. 2005;51(2):71-85.
31. Casazza BA. Diagnosis and treatment of acute low back pain. Am Fam Physician. 2012;85(4):343-350.
32. Machado LA, Maher CG, Herbert RD, et al. The effectiveness of the McKenzie method in addition to first-line care for acute low back pain: a randomized controlled trial. BMC Med. 2010;8(10):1-10.
33. Cho I, Jeon C, Lee S, et al. Effects of lumbar stabilization exercise on functional disability and lumbar lordosis angle in patients with chronic low back pain. J Phys Ther Sci. 2015;27(6):1983-1985.
34. Choi BK, Verbeek JH, Tam WW, Jiang JY. Exercises for prevention of recurrences of low-back pain (review). Cochrane Database Syst Rev. 2010;(1):CD006555.
35. Wegner I, Widyahening IS, van Tulder MW, et al. Traction for low-back pain with or without sciatica (review). Cochrane Database Syst Rev. 2013;(8):CD003010.
36. Hoiriis KT, Pfleger B, McDuffie FC, et al. A randomized clinical trial comparing chiropractic adjustments to muscle relaxants for subacute low back pain. J Manipulative Physiol Ther. 2004;27(6):388-398.
37. Lin CC, Haas M, Maher CG, et al. Cost-effectiveness of guideline-endorsed treatments for low back pain: a systematic review. Eur Spine J. 2011;20:1024-1038.
38. Walker BF, French SD, Grant W, Green S. A Cochrane Review of combined chiropractic interventions for low-back pain. Spine. 2011;36(3): 230-242.
39. Dahm KT, Brurberg KG, Jamtvedt G, Hagen KB. Advice to rest in bed versus advice to stay active for acute low-back pain and sciatica. Cochrane Database Syst Rev. 2010;(6):CD007612.
40. Staiger T, Paauw D, Deyo A, Jarvik JG. Imaging studies for acute low back pain. When and when not to order them. Postgrad Med. 1999;105(4):161-162,165-166,171-172.
April 2016: Click for Credit
Here are 4 articles in the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Later Menopause Lowers Risk for Later Depression
To take the posttest, go to: http://bit.ly/1U7I7f3
Expires January 6, 2017
VITALS
Key clinical point: Later menopause, with its longer estrogen exposure, appears tied to a lower risk of postmenopausal depression.
Major finding: The risk of depression decreased by 2% for each 2 premenopausal years after age 40.
Data source: The meta-analysis comprised 14 studies with more than 67,700 women.
Disclosures: Neither Dr. Georgakis nor any of the coauthors declared any financial conflicts.
2. Preschool ASD Prevalence Estimates Lower Than Grade School Estimates
To take the posttest, go to: http://bit.ly/24Mec0X
Expires January 5, 2017
VITALS
Key clinical point: The prevalence of autism spectrum disorders among 4-year-olds is about 30% lower than among 8-year-olds.
Major finding: Prevalence of ASD among 4-year-olds was 13/1,000 children across five U.S. states.
Data source: A comparison of health and medical records for nationally representative cohorts involving 58,467 4-year-olds and 56,727 8-year-olds in five U.S. states in 2010.
Disclosures: The Centers for Disease Control and Prevention funded the research. Dr. Christensen and her associates reported no disclosures.
3. Long-term PPI Use Linked to Increased Risk for Dementia
To take the posttest, go to: http://bit.ly/1nrCdsb
Expires February 24, 2017
VITALS
Key clinical point: Proton pump inhibitors may add to the risk of dementia in older adults.
Major finding: The risk of incident dementia was 44% higher in adults who used PPIs long term, compared with those who did not.
Data source: The prospective cohort study included 73,679 adults aged 75 years and older.
Disclosures: The researchers had no financial conflicts to disclose.
4. Elevated Cardiovascular Risks Linked to Hidradenitis Suppurativa
To take the posttest, go to: http://bit.ly/1nrEFz3
Expires February 17, 2017
VITALS
Key clinical point: Hidradenitis suppurativa is associated with a significantly increased risk of adverse cardiovascular events and all-cause mortality.
Major finding: Individuals with hidradenitis suppurativa had a 57% greater risk of myocardial infarction and 33% greater risk of ischemic stroke, compared with the general population.
Data source: A population-based cohort study in 5,964 patients with hidradenitis suppurativa.
Disclosures: No conflicts of interest were declared.
Here are 4 articles in the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Later Menopause Lowers Risk for Later Depression
To take the posttest, go to: http://bit.ly/1U7I7f3
Expires January 6, 2017
VITALS
Key clinical point: Later menopause, with its longer estrogen exposure, appears tied to a lower risk of postmenopausal depression.
Major finding: The risk of depression decreased by 2% for each 2 premenopausal years after age 40.
Data source: The meta-analysis comprised 14 studies with more than 67,700 women.
Disclosures: Neither Dr. Georgakis nor any of the coauthors declared any financial conflicts.
2. Preschool ASD Prevalence Estimates Lower Than Grade School Estimates
To take the posttest, go to: http://bit.ly/24Mec0X
Expires January 5, 2017
VITALS
Key clinical point: The prevalence of autism spectrum disorders among 4-year-olds is about 30% lower than among 8-year-olds.
Major finding: Prevalence of ASD among 4-year-olds was 13/1,000 children across five U.S. states.
Data source: A comparison of health and medical records for nationally representative cohorts involving 58,467 4-year-olds and 56,727 8-year-olds in five U.S. states in 2010.
Disclosures: The Centers for Disease Control and Prevention funded the research. Dr. Christensen and her associates reported no disclosures.
3. Long-term PPI Use Linked to Increased Risk for Dementia
To take the posttest, go to: http://bit.ly/1nrCdsb
Expires February 24, 2017
VITALS
Key clinical point: Proton pump inhibitors may add to the risk of dementia in older adults.
Major finding: The risk of incident dementia was 44% higher in adults who used PPIs long term, compared with those who did not.
Data source: The prospective cohort study included 73,679 adults aged 75 years and older.
Disclosures: The researchers had no financial conflicts to disclose.
4. Elevated Cardiovascular Risks Linked to Hidradenitis Suppurativa
To take the posttest, go to: http://bit.ly/1nrEFz3
Expires February 17, 2017
VITALS
Key clinical point: Hidradenitis suppurativa is associated with a significantly increased risk of adverse cardiovascular events and all-cause mortality.
Major finding: Individuals with hidradenitis suppurativa had a 57% greater risk of myocardial infarction and 33% greater risk of ischemic stroke, compared with the general population.
Data source: A population-based cohort study in 5,964 patients with hidradenitis suppurativa.
Disclosures: No conflicts of interest were declared.
Here are 4 articles in the April issue of Clinician Reviews (individual articles are valid for one year from date of publication—expiration dates below):
1. Later Menopause Lowers Risk for Later Depression
To take the posttest, go to: http://bit.ly/1U7I7f3
Expires January 6, 2017
VITALS
Key clinical point: Later menopause, with its longer estrogen exposure, appears tied to a lower risk of postmenopausal depression.
Major finding: The risk of depression decreased by 2% for each 2 premenopausal years after age 40.
Data source: The meta-analysis comprised 14 studies with more than 67,700 women.
Disclosures: Neither Dr. Georgakis nor any of the coauthors declared any financial conflicts.
2. Preschool ASD Prevalence Estimates Lower Than Grade School Estimates
To take the posttest, go to: http://bit.ly/24Mec0X
Expires January 5, 2017
VITALS
Key clinical point: The prevalence of autism spectrum disorders among 4-year-olds is about 30% lower than among 8-year-olds.
Major finding: Prevalence of ASD among 4-year-olds was 13/1,000 children across five U.S. states.
Data source: A comparison of health and medical records for nationally representative cohorts involving 58,467 4-year-olds and 56,727 8-year-olds in five U.S. states in 2010.
Disclosures: The Centers for Disease Control and Prevention funded the research. Dr. Christensen and her associates reported no disclosures.
3. Long-term PPI Use Linked to Increased Risk for Dementia
To take the posttest, go to: http://bit.ly/1nrCdsb
Expires February 24, 2017
VITALS
Key clinical point: Proton pump inhibitors may add to the risk of dementia in older adults.
Major finding: The risk of incident dementia was 44% higher in adults who used PPIs long term, compared with those who did not.
Data source: The prospective cohort study included 73,679 adults aged 75 years and older.
Disclosures: The researchers had no financial conflicts to disclose.
4. Elevated Cardiovascular Risks Linked to Hidradenitis Suppurativa
To take the posttest, go to: http://bit.ly/1nrEFz3
Expires February 17, 2017
VITALS
Key clinical point: Hidradenitis suppurativa is associated with a significantly increased risk of adverse cardiovascular events and all-cause mortality.
Major finding: Individuals with hidradenitis suppurativa had a 57% greater risk of myocardial infarction and 33% greater risk of ischemic stroke, compared with the general population.
Data source: A population-based cohort study in 5,964 patients with hidradenitis suppurativa.
Disclosures: No conflicts of interest were declared.
Are Cognitive Biases Influencing Your Clinical Decisions?
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
CE/CME No: CR-1603
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• List the characteristics of System 1 and System 2 thinking.
• Explain how System 1 and System 2 thinking affects clinical decisions.
• Define the characteristics of no-fault, system, and cognitive errors and how they affect health care delivery.
• Describe how biases and cognitive dispositions to respond cause health care providers to make clinical decision errors.
• List some effective debiasing techniques to improve clinical decisions and patient safety.
FACULTY
David J. Klocko is an Associate Professor and Academic Coordinator in the Department of Physician Assistant Studies at the University of Texas Southwestern Medical Center, School of Health Professions, Dallas.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of March 2016.
Article begins on next page >>
Diagnostic errors occur for many reasons, some of which are based in cognitive biases. Also called cognitive dispositions to respond (CDR), these can result from failures in perception, faulty mental shortcuts, or unconscious biases, and clinicians are usually unaware they exist. This article discusses the influence CDRs have on clinical decisions and walks you through methods for purposeful debiasing.
Diagnosis is the foundation of medicine ... [and] diagnostic reasoning is a critical aspect of clinical performance.1
— Pat Croskerry, MD, PhD
Diagnostic errors compromise patient safety and the quality of health care and account for the majority of paid malpractice claims. They are especially common in family medicine, internal medicine, emergency medicine, and urgent care, wherethe error rate can be as high as 15%.2 However, all health care providers are subject to errors in clinical judgment, regardless of the setting or specialty in which they practice.3
Clinical disciplines such as internal medicine and emergency medicine have higher error rates than the perceptual disciplines, radiology and pathology. Higher diagnostic error rates in the clinical disciplines are due to the elevated case complexity and the need for rapid interpretation of diagnostic studies. In the perceptual disciplines such as pathology and radiology, fewer time pressures and the ability to obtain a second opinion before making a diagnosis decrease error rates.3 In a National Practitioner Data Bank analysis, more diagnostic error claims occurred in the outpatient setting than in the inpatient setting.4
Quality assurance and performance improvement have become paramount for all health care providers. The modern patient safety movement began in 1999 with the Institute of Medicine (IOM) report To Err Is Human, which highlighted how a faulty health care system causes people to make mistakes and negatively impacts patient safety.5 Some examples of errors arising from imperfections in the health system include medication errors, patient falls, wrong-site surgeries, and improper patient identification. Despite an increased emphasis on patient safety and quality improvement, diagnostic error had not been a focus of attention for policy makers and institutions. Only since the IOM report was released have the medical profession and health policy makers begun to pay attention to diagnostic errors as a serious patient safety issue.5
Cognitive biases, or cognitive dispositions to respond (CDR), can influence clinical decision-making and lead to diagnostic errors. By understanding the thinking processes involved in diagnostic reasoning and the interaction between these processes and cognitive biases, clinicians can take steps to counteract the influence of cognitive biases on their clinical decisions. Here, a brief introduction to dual processing theory is provided, along with information to help clinicians identify potential cognitive biases. Workplace and educational debiasing techniques to counter biases that lead to cognitive decision errors are presented as well.
DIAGNOSTIC ERRORS
All advanced practice providers are at risk for making a clinical decision error. The diagnostic errors that are made in clinical practice can be classified into three broad etiologic categories6:
No-fault errors occur when a rare disease is misdiagnosed as something more common or a disease is silent or presents in an atypical manner. An example of an error that falls into this category is a delayed diagnosis of ischemic bowel in a diabetic patient with no abdominal pain. Another example is a patient with a language barrier who is not able to describe his or her symptoms clearly, leading the clinician to misinterpret the history. Patient nonadherence to recommended care can also be viewed as no-fault, as in the case of a patient diagnosed with colon cancer who did not obtain a recommended screening colonoscopy.6 In one study, no-fault errors accounted for 7% of diagnostic errors.7
System errors occur as a result of “latent” faults in the process of delivering care and can be technical or organizational in nature.6 Examples of diagnostic errors related to technical issues are misdiagnosis or delayed diagnosis resulting from lack of appropriate testing or equipment or from incorrect laboratory results caused by technical problems with equipment. Organizational shortcomings that contribute to diagnostic errors include imperfections in department policies, error tolerance culture, poor patient care coordination, communication problems, inadequate staff training, poor working conditions, unavailability of acute specialty care, and failing to follow up with patients having abnormal diagnostic study results.6 Excessive workload and heavy administrative responsibilities also can contribute to clinician decision errors.
An example of a specific clinical organizational system error would be a missed or delayed diagnosis of a cancer on a chest x-ray due to lack of an “over-read” by a radiologist. Due to cost, many private practices do not send all radiographs for a radiologist’s interpretation. Another example is a patient with a severe eye injury who develops complications after being transferred to another hospital because there is not an on-call ophthalmologist at the presenting hospital.6 Delays in reviewing patient laboratory results are a significant system-based source of medical errors. In one study, 83% of the physician respondents reported at least one delay in reviewing test results in the past two months, with 18% reporting five or more delays in reviewing test results over the same time period.8
Cognitive errors are caused by gaps in knowledge or experience, inadequate interpretation of diagnostic studies, or succumbing to faulty heuristics and biases.6 With cognitive errors, incorrect perception or interpretation of a clinical situation results in faulty differential diagnosis development. Confirmation bias is one type of cognitive error—once supporting information is found for a diagnosis, the search for information to rule out the diagnosis stops.6
An example of this would be a patient with an ankle fracture who is discharged with a missed proximal fibula fracture after the clinician performs a physical exam only on the ankle and orders an ankle x-ray. A cognitive error like this would occur due to inadvertent omission of an important physical exam component or the clinician not knowing the importance of examining the knee when evaluating an ankle fracture.
It is important to note that clinical decision errors are usually multifactorial. In a study involving 100 cases of diagnostic error in internal medicine, Graber and colleagues determined that in 46% of the cases errors were caused by a combination of system-related and cognitive factors.7
Continue for decision making >>
Decision Making: Dual Process Theory
Over the past two decades, dual process theory (DPT) has been recognized as a reliable model of the decision-making process in the psychology literature.9 DPT proposes two unique processes of thinking during decision making, referred to as System 1 and System 2, or Type 1 and Type 2, processes. A brief introduction to DPT is given here for practicing clinicians, but a detailed discussion of the literature pertaining to this concept is beyond the scope of this review.
System 1 processes are “intuitive,” utilize pattern recognition and heuristics, and rely heavily on the context or conditions in which the decision is made. The intuitive System 1 mode of thinking uses a pattern recognition or “gut reaction” approach.10 It is fast and reflexive but can be subject to deficits in predictive power and reliability.10 Experienced clinicians use pattern recognition in conditions presenting with classic signs and symptoms.10 For example, the clinician who evaluates a 12-year-old child with an annular, erythemic patch with central clearing on the forearm and immediately diagnoses ringworm is thinking in the intuitive mode. Generally, human beings are most comfortable in this decision mode because it involves intuition and requires less mental effort and concentration. For clinicians, System 1 thinking is the default defense mechanism against “decision fatigue” and “cognitive overload” during a busy shift, and it is the thinking mode used when clinicians are stressed, hurried, tired, and working with a lack of resources.9,10 Croskerry maintains, however, that such clinical situations, and the reliance on System 1 thinking that such situations entail, can make clinicians more vulnerable to certain biases.9
System 2 thinking is analytic, deductive, slow, and deliberate. This mode of thinking has high predictive power with high reliability, and it is less influenced by the context or conditions in which the decision is being made.10 Clinicians use this mode of thinking when patients present with vague signs and symptoms and a diagnosis is not instantly recognized.10 System 2 decision making would be required, for example, when evaluating a 55-year-old woman with chest pain. The clinical condition requires the clinician to acquire more data and make a conscious effort to analyze results, and arriving at a clinical decision in this situation takes more time. Shortcuts due to time pressures can have devastating outcomes in this setting. It should be mentioned, however, that psychology research has shown that the System 2 analytic approach is mentally taxing and may also result in poor decisions (“thinking too much”).11
Intuitive and analytic thinking are not independent of each other. During a clinical encounter, there is unconscious switching back and forth between the two modes as the clinician evaluates the information at hand in order to produce a decision.12 A patient presenting with a chief complaint may trigger a System 1 decision, but due to uncertainty there may be a “System 2 override”where the clinician consciously forces herself to reassess and perform further analysis.10 System 1 intuitive decision processes become more dominant with experience. Many encounters requiring System 2 thinking early in a clinician’s career may become System 1 decisions as the clinician gains expertise.10 This results as the clinician develops a “mental library” of previous encounters with commonly seen medical conditions.13 It is important to note that clinical decision errors often result from a combination of knowledge gaps and processing malfunctions and not from one process alone.14
Similarly, diagnostic errors are not purely a result of cognitive biases or reliance on System 1 or System 2 thinking, but rather are a result of multiple factors.In a study that looked at provider time to diagnosis and accuracy of diagnosis, results indicated that System 1 reasoning was not more error prone than System 2 thinking.15 Experienced clinicians emphasize that errors can occur at any time or in any context in both System 1 and 2 modes of thinking.16
The vast majority of human decisions—95%—are made in System 1 mode, while only 5% of our “thinking” is conscious analytic thought.17 Croskerry suggests that clinical reasoning defaults to the faster, more mentally economic System 1 thinking, which can make clinicians prone to error by allowing intuition, heuristics, and processes that are most vulnerable to mistakes—stereotyping, prejudices, and biases—to influence a decision.9,18 Both novice and expert clinicians should be encouraged to develop insight into their intuitive and analytic decision-making processes and become aware of which thinking mode they are using in a specific clinical situation.
Continue for cognitive dispositions to respond >>
Cognitive Dispositions to Respond
Diagnostic errors are often associated with cognitive errors such as failures in perception, failed heuristics, and biases; as a group, these cognitive errors have been labeled cognitive dispositions to respond.1 In the medical and psychology literature, more than 100 CDRs have been identified.19 Common CDR/bias definitions are provided in the graphic.
In everyday practice, clinicians encounter clinical scenarios or situations where CDRs can affect decision making. The following brief clinical examples further illustrate the defining characteristics of the CDRs. Cognitive errors related to these CDRs can occur if a clinician does not remain completely objective.
Availability is a bias that applies the saying “more common diseases are common.” An example of this bias in practice would be a provider who has seen three patients with abdominal pain and diagnosed gastritis for each. A fourth patient presents with abdominal pain, is diagnosed with gastritis, but actually has appendicitis.
Search satisficing, or premature closure, occurs when one has found enough information to make a diagnosis and then stops looking for further causes or additional problems. For example, a PA rounds on a patient who is post-op day 1 from coronary bypass surgery and develops decreasing oxygen saturation. A chest x-ray reveals right lower lobe opacity consistent with either pneumonia or pleural effusion; antibiotics are started and oxygen concentration is increased on the ventilator. The radiologist later informs the PA that the patient also has a left-sided pneumothorax. The PA did not treat that because he stopped looking for other causes of the oxygen desaturation once the right lower lobe pneumonia was found.
Continue for confirmation >>
Confirmation bias occurs when clinicians seek to confirm a diagnosis rather than rule it out. For example, a patient presents with first-time, new-onset “classic” migraine symptoms, characterized as “the worst headache of her life.” The provider asks patient history questions to confirm the initial impression of a migraine headache and does not order a CT scan.
Posterior probability is a bias whereby the clinician gives excessive weight to a patient’s previous medical history. It occurs, for example, when a patient with chronic back pain is diagnosed with musculoskeletal back pain without considering other causes, such as urinary tract infection or pyelonephritis.
Diagnosis momentum bias occurs when a clinician relies on information handed down from numerous parties involved with the patient. An example is a patient who has a syncopal episode in church and several tonic-clonic movements while briefly unconscious. Nearby witnesses describe the event as a “seizure,” and paramedics relaying information to the emergency department indicate that the patient had a “seizure.” Ultimately, the triage information records “seizure” as the diagnosis. A cognitive error can occur if the treating clinician does not take a thorough history to consider an alternative diagnosis.
Fundamental attribution error bias occurs when a provider is judgmental and blames the patient for their disease. A provider who quips, “No wonder that patient has diabetes and hypertension; she weighs 325 lb,” is exhibiting fundamental attribution error bias.
Ascertainment bias allows preconceived notions, including stereotypes, to influence a clinician’s thinking. A provider who determines that all female patients with multiple somatic complaints have anxiety and depression is subject to this bias.
Triage cueing occurs when some aspect of the triage process influences the clinician’s thinking, such as when the clinician assumes that patients who are placed in the fast track are low acuity and therefore gives no consideration to higher acuity diagnoses.
Playing the odds assumes that a patient with a vague presentation has a benign condition rather than a serious one because the odds favor that. An example of this bias occurs when a 65-year-old woman with vomiting during flu season is quickly diagnosed with gastroenteritis. Fortunately, the patient is on a telemetry monitor while getting IV fluids and antinausea medication. The monitor results indicate that her vomiting episodes are occurring during long periods of sinus arrest.
Psych-out bias applies when signs or symptoms in a patient with a psychiatric diagnosis are ascribed to the underlying psychiatric condition and other serious possibilities are quickly dismissed. For example, a provider who assumes that an unstable psychiatric patient is nonadherent with her prescribed medication or is abusing substances rather than considering an underlying medical illness is demonstrating psych-out bias.
Illusory correlation bias occurs, for example, when the provider makes the assumption that the emergency department will be busy because there is a full moon.
Continue to find out if you are at risk for being wrong >>
AM I AT RISK FOR BEING WRONG?
Autonomous advanced practice clinicians in high-risk practice settings have an immense responsibility to ensure that their patients are getting the best possible care. It is documented that as expertise develops, knowledge and decision processes change. Ordinarily, highly experienced clinicians use the more time-efficient System 1 process when faced with common disorders; for more complex disorders, they change to System 2 thinking to facilitate a more comprehensive evaluation.13 In many instances, however, a provider may inadvertently take shortcuts to conclude the clinical encounter, including relying on intuitive thinking—which can be prone to bias—when analytic thinking is necessary.
Clinicians are usually unaware of the influence that biases may have on their decision making and should reflect on their behavior to determine if any biases exist. To improve patient safety and facilitate better care, all providers should perform a personal inventory to identify CDRs they may have developed. Questions that will help to reveal CDRs include
- Am I rushing to get off my shift on time?
- Was the patient “turned over” to me at the shift change?
- Have I allowed a previously negative experience with this patient to influence my objectivity and clinical decision-making?
- Am I tired?
- Has the diagnosis been suggested by the nurse, paramedic, or the patient’s family?9
- Has the diagnosis been suggested by the nurse,
If one or more biases are found, a purposeful effort to mentally “uncouple” from a bias should be done. This process is referred to as metacognition, or thinking about one’s own thought processes.9 Paramount among the thinking processes that may be at play is an awareness of how System 1 and System 2 thinking interact and affect clinical decision making, as this enables the clinician to recognize which mode of thinking they use to arrive at a decision and when they need to shift from intuitive to analytic thinking.
Another factor to consider is overconfidence: Berner and Graber note that a provider’s overconfidence3 in his or her own knowledge and experience and lack of awareness of when an “override” is needed can be a cause of diagnostic errors.18 The tendency to shore up existing beliefs rather than force a new cognitive strategy is a sign of a rigid thinking process that may ultimately result in a poor clinical decision.9 Finally, providers should be aware of their surroundings and practice environments. As noted earlier, emergency medicine, family medicine, internal medicine, and urgent care have high diagnostic error rates due, in part, to high patient volumes.1
Once a tendency for a certain cognitive bias is recognized, the next step is to develop a sustainable method to counteract it, a process referred to as debiasing, to prevent cognitive errors. The table lists some workplace and educational debiasing techniques that have been described in the literature.20,21 Critics of cognitive debiasing argue that CDRs are preconscious, that awareness of CDRs is not enough to counteract their effects, and that there is no ability for one to develop “generic” conscious efforts to counter them.14 Their concern here is that a clinician may be able to counter a bias in one clinical context but not in another.14 It is clear that clinical reasoning is complex and involves many interrelated elements, such as clinical knowledge and critical thinking, with System 1 and 2 thinking working in tandem and metacognition overarching the whole process.21 Errors in diagnosis can have multiple causes and no single cognitive approach can be effective in addressing all of these causes. Knowing about cognitive bias helps clinicians address one possible element underlying diagnostic errors. Efforts to eliminate bias in clinical reasoning should begin early in clinical education; this can be done by incorporating instruction on clinical reasoning, including the relationship between intuitive and analytic decisions, metacognition, and awareness of the strengths and weaknesses of heuristics.22
In summary, in clinical situations where bias or uncertainty might exist, a clinician can make an effort to avoid a bad decision by
- Stepping back and reflecting to consider if a bias exists.
- Developing rules and mental procedures to reject a reflexive automatic response and force a “System 2 override.”9
- Developing “mental-ware” (mental techniques) to uncouple from a recognized or recurring cognitive bias.9
Continue to conclusion >>
Conclusion
This article reminds health care providers that cognitive biases can influence clinical decision-making. Clinicians should be aware of how System 1 and System 2 thinking couple with unconscious cognitive biases to affect clinical decisions and patient safety. Once a provider identifies a bias, he or she should attempt to employ one or more debiasing techniques. Medical decision errors usually occur due to multiple factors, and one thinking mode is not more error prone than the other (analytic versus intuitive). Cognitive errors are also caused by knowledge gaps and faulty patient data processing. Future research is needed to assess outcomes of quality improvement projects that include these components.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
1. Croskerry P. Diagnostic failure: a cognitive and affective approach. In: Henriksen K, Battles JB, Marks ES, Lewin DI, eds. Advances in Patient Safety: From Research to Implementation (Volume 2: Concepts and Methodology). Rockville, MD: Agency for Healthcare Research and Quality; 2005:241-254.
2. Croskerry P. The importance of cognitive errors in diagnosis and strategies to minimize them. Acad Med. 2003;78:775-780.
3. Berner ES, Graber ML. Overconfidence as a cause of diagnostic error in medicine. Am J Med. 2008;121(5A):S2-S23.
4. Saber Tehrani AS, Lee H, Mathews SC, et al. 25-year summary of US malpractice claims for diagnostic errors 1986-2010: an analysis from the National Practitioner Data Bank. BMJ Qual Saf. 2013;22(8):672-680.
5. Kohn LT, Corrigan JM, Donaldson MS, eds. To Err Is Human: Building a Safer Health System. Washington, DC: National Academy of Sciences; 1999.
6. Graber M, Gordon R, Franklin N. Reducing diagnostic errors in medicine: what’s the goal? Acad Med. 2002;77:981-992.
7. Graber ML, Franklin N, Gordon R. Diagnostic error in internal medicine. Arch Intern Med. 2005;165(13):1493-1499.
8. Poon EG, Gandhi TK, Sequist TD, et al. “I wish I had seen this test result earlier!”: dissatisfaction with test result management systems in primary care. Arch Intern Med. 2004;164(20):2223-2228.
9. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 1: origins of bias and theory of debiasing. BMJ Qual Saf. 2013;22(suppl 2):ii58-ii64.
10. Croskerry P, Nimmo GR. Better clinical decision making and reducing diagnostic error. J R Coll Physicians Edinb. 2011;41(2):155-162.
11. Wilson TD, Schooler JW. Thinking too much: introspection can reduce the quality of p and decisions. J Pers Soc Psychol. 1991;60(2): 181-192.
12. Croskerry P. A universal model of diagnostic reasoning. Acad Med. 2009; 84(8):1022-1028.
13. Norman G, Young M, Brooks L. Non-analytical models of clinical reasoning: the role of experience. Med Educ. 2007;41(12):1140-1145.
14. Norman GR, Eva KW. Diagnostic error and clinical reasoning. Med Educ. 2010;44(1):94-100.
15. Sherbino J, Dore KL, Wood TJ, et al. The relationship between response time and diagnostic accuracy. Acad Med. 2012;87(6):785-791.
16. Petrie D, Campbell S. Clinical decision making, fast and slow. Acad Med. 2013;88(5):557.
17. Lakoff G, Johnson M. Philosophy in the Flesh: The Embodied Mind and Its Challenge to Western Thought. New York, NY: Basic Books; 1999.
18. Sinclair D, Croskerry P. Patient safety and diagnostic error: tips for your next shift. Can Fam Physician. 2010;56(1):28-30.
19. Croskerry P. From mindless to mindful practice—cognitive bias and clinical decision making. N Engl J Med. 2013;368(26):2445-2448.
20. Croskerry P, Singhal G, Mamede S. Cognitive debiasing 2: impediments to and strategies for change. BMJ Qual Saf. 2013;22(suppl 2):ii65-ii72.
21. Groves M. Understanding clinical reasoning: the next step in working out how it really works. Med Educ. 2012;46(5):444-446.
22. Trowbridge RL, Dhaliwal G, Cosby KS. Educational agenda for diagnostic error reduction. BMJ Qual Saf. 2013;22(suppl 2):ii28-ii32.
College Health May Be Full of Surprises: International Travelers and Tropical Diseases
CE/CME No: CR-1602
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain how accessibility to travel affects the etiology of illness.
• Understand the typical and atypical signs and symptoms of malaria, dengue fever, and chikungunya.
• Identify the proper laboratory workup and treatment for malaria, dengue fever, and chikungunya.
• Discuss multiple ways to prevent mosquito-borne illness in your patients and the importance of a pretravel consultation.
FACULTY
Eve B. Hoover is completing a postgraduate academic fellowship at Midwestern University at Glendale, Arizona, and practices at Logistics Health, Inc, in Phoenix.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2016.
Article begins on next page >>
As the number of international travelers increases, so does the likelihood of transmission of illnesses to locations where they were previously rarely diagnosed. Clinicians at college health centers must be aware of tropical medicine diagnoses, especially in returning international students who have fever and other constitutional symptoms. This article provides a refresher regarding the diagnoses of malaria, dengue fever, and chikungunya.
Travel, whether for work, education, or pleasure, continues to increase, with the number of international travelers exceeding 1.1 billion in 2014.1 International travelers may unknowingly expose themselves and others to multiple health hazards previously thought to be foreign to the United States. Jane Zuckerman, who works with the World Health Organization (WHO), has noted that just over a century ago, the first human flew in an aircraft.2 Now, the sky is no longer the limit, and genes and micro-organisms travel as freely as their human hosts.
The international student population at American universities is at an all-time high (see Figure 1). Study abroad programs, which include American students who travel to developed and underdeveloped countries, also continue to increase. According to the CDC, the number of American students studying abroad has increased more than threefold in the past 20 years.3 According to the Institute of International Education, 304,467 American college students studied abroad in 2013/2014.4 Historically, most American students studied abroad in European countries, but in recent years the list of destinations has expanded, with increases in the percentage of students who travel to Africa, Asia, and the Middle East, and decreases in the percentage choosing Europe and Oceania.3
The sizable number of international students at universities, combined with the study abroad programs, have broadened the scope of the campus health care provider’s differential diagnosis. Diseases and infections that occur in developing countries can differ from those commonly seen in the US and Europe. It is important for health care providers to be reminded of conditions they seldom see and for a tropical medicine zebra to be considered in the appropriate patient population.
Continue for three causes of fever in returning travelers >>
THREE CAUSES OF FEVER IN RETURNING TRAVELERS
There are numerous etiologies for fever in the returning traveler. Factors such as location of travel, length of stay, dates of travel, date of symptom onset, risk activities undertaken, and reason for visit help determine the cause of illness.5 Two of the most commonly encountered conditions causing illness in the febrile traveler are dengue fever and malaria. Additionally, chikungunya is an emerging health concern in the US that has received increased attention following a massive outbreak in the Caribbean (which affected many American travelers) in 2013. In a retrospective study of patient records from 462 febrile adults who traveled to malaria-endemic areas, Siikamäki and colleagues found that every fourth febrile returning traveler had an illness that was potentially life-threatening.6 Understanding the possible causes of febrile illness in travelers can aid the clinician in diagnosing and correctly treating potentially life-threatening conditions.
Malaria
Malaria is a mosquito-borne illness transmitted in humans by female Anopheles mosquitoes.7 It is caused by infection with the protozoal parasites Plasmodium falciparum, P vivax, P ovale, P malariae, and occasionally other Plasmodium species.8 An infected female Anopheles mosquito transmits the parasite into a human host through a bite. The most severe form of human malaria, which can be fatal, is caused by P falciparum. Falciparum and vivax malaria are the most common forms of malaria worldwide.8
According to the WHO, there were an estimated 214 million clinical episodes of malaria worldwide, and malaria was the cause of 438,000 deaths, in 2015.9 In 2012, the CDC received reports of 1,687 cases of malaria in the US.8 The number of malaria cases has been steadily increasing since 1973.8 Figure 2 shows the number of malaria cases diagnosed in each state in 2012. The data demonstrate that malaria is the primary cause of death in travel-related fever. Malaria is also the most common single reason for travel-related fever without findings on exam or workup.
Dengue fever
Dengue is a mosquito-borne disease (transmitted by an infected Aedes mosquito) and is caused by four types of flaviviruses (DENV-1, DENV-2, DENV-3, DENV-4).10 It is the most common arboviral disease in humans.11 In 2009, the WHO revised its dengue categories to include dengue, dengue with warning signs, and severe dengue.12,13 Previously, the categories included dengue fever, dengue hemorrhagic fever, and dengue shock syndrome.
Dengue is endemic throughout the tropics and subtropics and is a leading cause of febrile illness among travelers returning from the Caribbean, South America, and South and Southeast Asia.14 There has been a 30-fold increase in dengue fever in the past 50 years.10 This illness is present in more than 100 countries; in the US, outbreaks have occurred in Florida, Hawaii, and along the Texas-Mexico border.14
Chikungunya
Chikungunya virus (CHIKV) is an arbovirus that is transmitted by Aedes mosquitoes (Aedes aegypti and Aedes albopictus).15 The term chikungunya is derived from a word in the Swahili and Maconde language that means “the one that is folded.”16 This description refers to the severe arthralgias that can cause a hunched-over gait in the patient with chikungunya.
Chikungunya historically has not had a significant impact in the Americas or Europe. However, more than one million suspected cases of chikungunya have been reported in the Americas since October 2013.16 Most cases of CHIKV infection diagnosed in the US have occurred in travelers; however, there have also been documented cases of local transmission of the virus.17 Local transmission occurs when the ill returning traveler unknowingly spreads disease, with the aid of the mosquito vector, upon return to the US.
Continue for patient presentation >>
PATIENT PRESENTATION
A 19-year-old previously healthy male student presented to the university health clinic for evaluation. During the exam, he lay on the examination table, covered with a blanket and shaking uncontrollably with intense rigors. Although he was hesitant to answer questions due to feeling so ill, he reported that he had returned from India two weeks prior and his symptoms—fever, rigors, ache, fatigue, headache, and nausea—began abruptly, hours before his arrival at the clinic.
The patient was diaphoretic and taking rapid, shallow inspirations. Assessment of vital signs revealed a blood pressure of 148/86 mm Hg; respiratory rate, 24 breaths/min; temperature, 103°F; and heart rate, 112 beats/min.
HEENT evaluation showed dry mucous membranes but no other abnormality. Neck was supple with no lymphadenopathy or nuchal rigidity. On cardiac exam, there were no murmurs or rubs. Lungs were clear to auscultation. Abdomen was soft and nontender, and bowel sounds were present in all four quadrants. There was no costovertebral angle tenderness. Skin was warm, clammy, and without rash. There were no focal neurologic deficits.
Complete blood count, comprehensive metabolic panel, and urinalysis were without abnormality. Examination of thick and thin blood smears revealed multiple red blood cells (RBCs) infected with malaria parasites and the appearance of the classic “headphone” form within the cells. Based on the in-office laboratory results of the blood smear, the patient was diagnosed with malaria.
The patient was not surprised by the diagnosis, as he had experienced these same symptoms with previous bouts of malaria. He and his family were from India, and the patient was an international college student. He had not taken malaria chemoprophylaxis prior to his most recent trip. After a short hospital admission for hydration, observation, treatment, and consultation by an infectious disease specialist, the patient was released back to the demands of college life.
Continue for signs and symptoms >>
SIGNS AND SYMPTOMS
Malaria
Signs and symptoms of malaria can vary greatly from none to illness causing death. The classic clinical features of malaria (fever, headache, back pain, chills, sweating, myalgia, nausea, vomiting, and cough) are caused by the parasite developing in RBCs, causing toxins to accumulate.18 Following the mosquito bite, there is typically an incubation period of seven to 30 days.
When first diagnosing malaria, the clinician needs to determine if the cause is P falciparum (the most severe form of malaria). If so, the clinician then must determine if the case is severe or nonsevere.7 In P falciparum infections, symptom onset can be later, especially if the patient took prophylaxis.7 Longer incubation periods are often seen with P vivax, P malariae, and P ovale infections. P vivax and P ovale can lie dormant in hepatic cells and reactivate after months or even years.7
Adults and children may experience different malaria symptoms, particularly with severe forms of the disease. Pediatric patients with severe falciparum malaria may experience respiratory distress, convulsions, and hypoglycemia more commonly than adults. More than half of adults with severe falciparum experience acute respiratory distress syndrome and acute renal failure.7 Pregnant women are at increased risk for complications.
Dengue fever
Approximately 75% of patients infected with DENV are asymptomatic.14 However, if symptomatic, the most common symptoms (fever, myalgia, headache, rash, arthralgia, abdominal pain, and nausea) begin abruptly after an incubation period of four to seven days.
Dengue can also present with atypical manifestations. In a prospective study by Nimmagadda and colleagues involving 150 participants with confirmed dengue fever, more than half of subjects had at least one atypical symptom along with more typical symptoms.10 The most common atypical manifestation was abnormal liver function, which was present in 40.6% of participants. Other atypical symptoms seen were febrile diarrhea (12%), renal failure (8%), acalculous cholecystitis (6.6%), and conduction abnormalities of the heart (6%). Less common atypical manifestations observed in this study included encephalitis, seizures, acute respiratory distress syndrome, disseminated intravascular coagulation, acute pancreatitis, myositis, and atrial fibrillation. The authors recommended that clinicians maintain a high level of vigilance for atypical manifestations of dengue fever, noting that most of the severe complications of dengue can be avoided if the disease is diagnosed correctly early in the course of illness.
Chikungunya
The typical presentation of CHIKV infection is a patient who abruptly develops fever, headache, polyarthralgia, and myalgia. The joint pain most frequently affects the small joints, such as the interphalangeal joints of the hands as well as the ankles and wrists.15 Back pain is also common, and rash is present in more than half of cases. The rash in adults can be maculopapular and in children is more often bullous. Fever, rash, and headache typically last seven to 10 days, while the arthralgia can last much longer—three to four months in a third of patients and three to five years in 10%.15
CHIKV infection that presents with typical clinical manifestations is usually self-limited; however, more severe atypical symptoms can occur and may lead to long-term morbidity. These atypical manifestations of chikungunya, which are rare, include acute disseminated encephalomyelitis, aseptic meningitis, meningoencephalitis, sensorineural hearing loss, myelitis, myeloradiculopathy, and Guillain-Barré syndrome.19 Chikungunya also has been associated with bleeding manifestations, acute renal failure, and electrolyte disturbance.19
Continue for the diagnosis >>
DIAGNOSIS
Malaria
The clinical presentation of malaria is nonspecific, so it is important to identify patients with a travel history and perform testing when this diagnosis is suspected.7,8 The gold standard for diagnosing malaria remains microscopy of thick and thin films of the patient’s peripheral blood.7 In patients with blood-stage malaria, a blood slide will show multiple infected RBCs and the appearance of the classic “headphone” form within the cells (see Figure 3). This test allows for efficient detection of malaria parasites, determination of parasite species, and calculation of percent parasitemia. Early differentiation between falciparum and nonfalciparum malaria is required, since P falciparum is the most life-threatening form of malaria.7 All of these factors are key to determining the best treatment plan for each patient.8
Rapid diagnostic tests (RDTs) and polymerase chain reaction (PCR) for malaria are increasingly available for use in US laboratories. In comparison to microscopy, these newer diagnostic tools are slower, more costly, and less readily available. For this reason, microscopy remains the most common means of diagnosis.7 PCR is helpful for species confirmation of malaria parasites and can be used to confirm a positive result on microscopy.8
It is possible for the first malaria test to be negative, and performing a repeat test the following day in a stable patient is recommended.7 However, more than three tests are not needed as long as the patient’s symptoms are not changing.
Dengue fever
Laboratory diagnosis of dengue can be confirmed with detection of DENV genomic sequences through PCR or nonstructural protein 1 antigen by immunoassay.14 Virus isolation in cell culture, detection of viral RNA by nucleic acid amplification tests, and detection of viral antigens by rapid tests or enzyme-linked immunosorbent assay (ELISA) are most useful if a patient presents within five days of fever onset.13
After five days of febrile illness, dengue viruses and antigens disappear from the blood as the specific antibody levels rise.13 Therefore, ELISA testing for immunoglobulin (Ig) M anti-DENV is a more effective lab study for dengue in patients presenting after one week. Testing for IgG anti-DENV is not recommended for making a diagnosis, however, because this antibody remains elevated for life after any DENV infection, leading to many false-positive test results. 14
A useful diagnostic aid for detecting severe dengue is the tourniquet test, which assesses for microvascular fragility. To perform the test, inflate a blood pressure cuff on the arm to midway between systolic and diastolic blood pressures, and maintain pressure for five minutes. After releasing the pressure, count the number of petechea in one square inch of skin; if 20 or more are found, the test is positive.12
Chikungunya
Chikungunya laboratory testing is limited in the US due to lack of availability. Testing is available only at the CDC, one commercial laboratory, and a few state health departments.17
The only reliable method for diagnosing CHIKV infection is through testing of blood samples. Chikungunya should not be diagnosed clinically because of the difficulty in differentiating it from dengue fever and other viral illnesses. The laboratory diagnosis of CHIKV infection can be obtained through detection of the virus, viral RNA, or specific antibodies related to chikungunya.20 Serologic detection of IgM or IgG antibodies is the most common method of diagnosis and is recommended by the CDC.20 If initial IgM and IgG testing is negative but clinical suspicion remains high, repeat testing should be done during the convalescent phase of the illness (≥ 7 days after symptom onset).21 Reverse transcriptase PCR is an effective diagnostic laboratory method for chikungunya and can be used in the first seven days of illness.20 ELISA and the hemagglutination inhibition assay can also provide diagnostic information.20
Lab findings associated with malaria, dengue fever, and chikungunya are summarized in the Table.
Continue for differentiating between dengue, malaria, and chikungunya early in presentation >>
Differentiating between dengue, malaria, and chikungunya early in presentation
Hematologic parameters can be used as a diagnostic aid when differentiating among certain causes of fever, as noted in a study by Joshi and Shah.22 In the setting of a febrile illness, thrombocytopenia (platelet count < 150,000/µL) is a predictor of malaria, especially in combination with anemia (hemoglobin < 10 g/dL). Thrombocytopenia is also common with dengue fever, but patients with dengue typically have normal hemoglobin. According to Joshi and Shah, patients having the combination of anemia and thrombocytopenia were 22 times more likely to have malaria than patients without these laboratory findings.22
Kutsuna and colleagues also found disease-specific clues in laboratory data when differentiating between dengue fever and malaria. Patients with dengue fever had significantly lower white blood cell counts than patients with malaria.23 In addition, although thrombocytopenia is seen in both dengue fever and malaria, platelet counts are lower in patients with malaria at first presentation. However, with dengue fever the platelet count can decrease three to six days into the illness when fever abates.23 Furthermore, total bilirubin tends to increase in malaria but is unaffected in dengue fever. Last, C-reactive protein can be helpful in assessing malaria severity and clinical improvement at follow-up, as well as for differentiating malaria from other conditions (eg, dengue), especially if the value is greater than 10 mg/L.23
Distinguishing chikungunya from dengue fever in the early stages of illness is difficult, and there is no pathognomonic hematologic laboratory study that helps with this task. With both diseases, the patient may have leukopenia, elevated erythrocyte sedimentation rate, and (rarely) thrombocytopenia. Chipwaza and colleagues discuss the significant overlap in symptoms in nonmalaria febrile illness. The overlap makes clinical diagnosis difficult; lab testing is essential for establishing the diagnosis.21 Once CHIKV is confirmed, the clinician is typically reassured of a more benign, self-limited course.
Continue for treatment/management >>
TREATMENT / MANAGEMENT
Malaria
The treatment of malaria varies depending on the severity of disease and the probability the organism is resistant to antimalarial drugs. The likelihood of drug resistance is determined based on the species of malaria parasite and the location where the infection occurred.24
Malaria is considered severe if one or more of the following are present: neurologic sequelae, renal failure, severe anemia, ARDS, jaundice, or parasite burden greater than 5%.8 Patients with severe malaria are treated with parenteral (IV) antimalarials; the two options for parenteral medication are quinine and artesunate.7 All patients treated with parenteral antimalarial agents should take a full course of oral medication for malaria as well. Oral antimalarial medications include, but are not limited to, quinine sulfate, atovaquone/proguanil, artemether-lumefantrine, doxycycline, clindamycin, sulfadoxine/pyrimethamine, chloroquine, and primaquine. The oral medications most commonly used to treat nonfalciparum malaria are chloroquine followed by primaquine.7 Chloroquine is not typically used for falciparum malaria due to widespread resistance.7
In cases of malaria caused by P vivax or P ovale infection, the likelihood of parasitic infection lying dormant in the liver must be considered. Additional treatment is often needed to eradicate this type of infection.7 The relapse of symptoms can occur years after the acute attack.25 Primaquine is the only approved medication for preventing and treating parasitic relapse associated with dormant infection.25
Dengue fever
The typical course of dengue follows three phases: febrile, critical, and convalescent.14 Dengue is usually a self-limiting febrile illness and typically resolves within one week after symptom onset without major complications.11 During the critical phase, most patients begin to improve, but up to 5% of cases develop concerning warning signs and symptoms that could represent a life-threatening condition that requires intense treatment and close monitoring.14 Warning signs for worsening disease are caused by marked increase in vascular permeability and include narrow pulse pressure, pleural effusions, ascites, and hemorrhagic manifestations (hematemesis, melena, menorrhagia).14
Treatment of most cases of dengue involves use of acetaminophen for comfort and fever reduction, hydration, and rest.26 Treatment of worsening dengue includes inpatient admission and possibly ICU admission for close observation and frequent monitoring.14 It is important to avoid aspirin and other NSAIDs due to the risk for bleeding complications in severe dengue.14 There are no approved antivirals for treatment of dengue. To reduce the risk for transmission of dengue, febrile patients should avoid further mosquito bites.14
Clinicians should be aware of warning signs of worsening illness with dengue fever. Signs of worsening dengue fever include postural hypotension, thrombocytopenia, decrease in serum albumin, and rising hematocrit.10
Chikungunya
CHIKV illness is usually a self-limiting condition. Diagnosis of chikungunya may take time, and providers should assume the chikungunya patient may have dengue, which has the potential to be more pathologic. Accordingly, they must watch for warning signs of dengue until CHIKV is confirmed.27
Management focuses on supportive care, including hydration and rest. Typically, the medications used are antipyretics (acetaminophen and ibuprofen) and analgesics; no antiviral medication for chikungunya is available.22 Aspirin is avoided due to the risk for Reye syndrome. Antihistamines may be helpful for patients who have an associated pruritic rash. Cold compresses can be beneficial for joint pain and swelling. Additionally, it is important to keep patients under mosquito nets during the febrile phase to decrease the risk for disease transmission.
Continue for patient education >>
PATIENT EDUCATION
Vaccination
Vaccines are not available for dengue fever, malaria, or chikungunya. However, researchers have been working on a vaccine for malaria for decades, and presently, more than 20 vaccine constructs are being tested and researched in preclinical trials.28 Similarly, efforts to develop an effective vaccine for chikungunya have been under way since the 1970s.15
Chemoprophylaxis
There are no chemoprophylaxis options available for dengue or chikungunya. The most effective measures to prevent dengue are strategies aimed at avoiding mosquito bites (ie, vector control and individual protections like repellants).16 One group of authors notes that developing a larger network of research laboratories capable of prompt diagnosis of arbovirus infections would help to better control chikungunya.
Multiple options for malaria chemoprophylaxis are available, and these vary by country of travel. Chemoprophylaxis options include doxycycline, mefloquine, atovaquone/proguanil, chloroquine, and primaquine.8 For dormant forms of malaria residing in the liver, primaquine is the only effective treatment.8
It is important to remember that no antimalarial drug is 100% protective. Malaria chemoprophylaxis reduces the risk for malaria, but it is often taken inadequately, which can delay symptom onset and lead to a false-negative result on initial blood films.5 All patients with fever who have visited a tropical country within one year of presentation should be screened for malaria.5 It is incorrect and dangerous to assume that a patient who received malaria chemoprophylaxis does not have malaria. Diagnosis of malaria requires a high index of suspicion, and clinicians must remember that malaria can occur even with perfect prophylaxis.7 Unfortunately, malaria parasites are becoming resistant to some commonly used antimalarial drugs. Resistance patterns are being tracked by the CDC.8
Pretravel visit with clinician
Many illnesses related to travel can be prevented with vaccination, chemoprophylaxis, and patient education. However, many travelers do not visit a health care professional before travel (even among those traveling to perceived “risky” destinations, such as Sub-Saharan Africa).29 In a study of ill returned travelers, Leder and colleagues found that only 40% had sought pretravel advice from providers. Interestingly, many of those ill patients who obtained a pretravel consult did not receive appropriate vaccines, such as hepatitis A or influenza vaccine. Some of these patients were diagnosed with preventable conditions.29
Open and thorough communication between clinicians and patients is paramount to protect travelers from disease and illness. Clinicians have the opportunity to greatly impact the health of their patients by recommending a pretravel consultation. What may take extra time and effort on the front end of the trip may save significant time and energy on the back end, and even save lives.
Mosquito bite avoidance
One of the most important patient education discussions in pretravel consultation is mosquito bite avoidance. Travelers should be advised to find accommodations with air conditioning and screened windows and doors.14 To avoid mosquito bites, they should cover their arms and legs adequately with proper clothing. Standing water (such as in flower pots), which can encourage mosquito breeding, should be avoided as well. Travelers should use insect repellents and insecticides, especially in cool, dark areas (eg, closets and bathrooms) where mosquitoes hide.14
Continue for follow-up >>
FOLLOW-UP
Malaria
Appropriate follow-up, need for hospitalization, and choice of medical treatment are determined by disease cause, severity of illness, and patient demographics. Follow-up is necessary to ensure improvement and no development of atypical symptoms. Additionally, the clinician needs to keep in mind the risk for malaria strains that can have a dormant stage.
Dengue fever
Clinicians must remind convalescing patients to watch for severe abdominal pain, vomiting, difficulty breathing, and signs of bleeding (epistaxis, bruising, bloody stool, and menorrhagia). Clinicians must also be attentive to changing lab values, including a decrease in platelet count and an increase in hematocrit, along with signs of hypovolemic shock, ascites, pleural effusions, and narrow pulse pressure.14
Chikungunya
Patients are reminded to keep themselves comfortable by rehydrating and treating the discomfort associated with arthralgias. In a longitudinal study of chikungunya patients, 60% experienced continued arthralgias three years after diagnosis.23,30 Patient education regarding the potential for long-term arthralgia is important, as it may impact activities of daily living and work.
Long-term NSAIDs have been used for patients with recurrent or even chronic arthralgia.31 There are limited data available on beneficial treatments, such as chloroquine sulfate or disease-modifying antirheumatic drugs, for chronic arthralgia associated with chikungunya.23 Depression and recurrent cutaneous lesions also are possible in patients with long-term symptoms.
Continue for the conclusion >>
CONCLUSION
“People, as well as pathogens, travel from all around the world in all directions.”32 With the ever-increasing mobility of populations around the world, transmission of illness and medical norms are constantly changing. All clinicians should keep in mind the less commonly seen diagnostic entities and remember the importance of obtaining a complete travel history in the febrile patient.
Early detection and appropriate supportive care of patients with dengue fever and malaria can be lifesaving. In addition, proper pretravel consultations can provide a wealth of patient education for at-risk travelers and help prevent a number of debilitating infectious diseases.
1. World Tourism Organization. Over 1.1 billion tourists travelled abroad in 2014 [press release]. January 27, 2015. http://media.unwto.org/press-release/2015-01-27/over-11-billion-tourists-travelled-abroad-2014. Accessed January 21, 2016.
2. Zuckerman JN. Public health and travel medicine: intricately intertwined. Perspect Public Health. 2012;132(5):206.
3. Rhodes G, DeRomaña I, Ebner J. Advising travelers with special needs: study abroad & other international student travel. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellow book/2016/advising-travelers-with-specific-needs/study-abroad-other-international-student-travel. Accessed January 21, 2016.
4. Institute of International Education. US study abroad: all destinations. www.iie.org/Research-and-Publications/Open-Doors/Data/US-Study-Abroad/All-Destinations/2012-14. Accessed January 21, 2016.
5. Hearn P, Johnston V. Assessment of returning travelers with fever. Medicine. 2014;42(2):66-72.
6. Siikamäki HM, Kivelä PS, Sipilä PN, et al. Fever in travelers returning from malaria-endemic areas: don’t look for malaria only. J Travel Med. 2011;18(4):239-244.
7. Walker NF, Nadjm B, Whitty CJ. Malaria. Medicine. 2014;42(2):100-106.
8. Cullen KA, Arguin PM. Malaria surveillance—United States, 2012. MMWR Surveill Summ. 2014;63(12):1-22.
9. World Health Organization. Malaria. Fact sheet no. 94. www.who.int/mediacentre/factsheets/fs094/en/. Accessed January 21, 2016.
10. Nimmagadda SS, Mahabala C, Boloor A, et al. Atypical manifestations of dengue fever (DF)—where do we stand today? J Clin Diagn Res. 2014;8(1):71-73.
11. Whitehorn J, Yacoub S, Anders KL, et al. Dengue therapeutics, chemoprophylaxis, and allied tools: state of the art and future directions. PLoS Negl Trop Dis. 2014;8(8):e3025.
12. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
13. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1. www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed January 21, 2016.
14. Tomashek KM, Sharp TM, Margolis HS. Infectious diseases related to travel: dengue. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/dengue. Accessed January 21, 2016.
15. Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9(5):1-18.
16. Hrnjakovi`c Cvjetkovi`c IB, Cvjetkovi`c D, Pati`c A, et al. Chikungunya—a serious threat for public health. Med Pregl. 2015;68(3/4):122-125.
17. Lindsey NP, Prince HE, Kosoy O, et al. Chikungunya virus infections among travelers-United States, 2010-2013. Am J Trop Med Hyg. 2015;92(1):82-87.
18. CDC. Malaria. www.cdc.gov/malaria/about/disease.html. Accessed January 21, 2016.
19. Das S, Sarkar N, Majumder J, et al. Acute disseminated encephalomyelitis in a child with chikungunya virus infection. J Pediatr Infect Dis. 2014;9(1):37-41.
20. Schwartz KL, Giga A, Boggild AK. Chikungunya fever in Canada: fever and polyarthritis in a returned traveller. CMAJ. 2014;186(10):772-774.
21. Chipwaza B, Mugasa JP, Selemani M, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8(11):e3335.
22. Joshi HA, Shah SS. Platelet count—a diagnostic aid in fever. Natl J Integrated Res Med. 2013;4(3):128-132.
23. Kutsuna S, Hayakawa K, Kato Y, et al. Comparison of clinical characteristics and laboratory findings of malaria, dengue, and enteric fever in returning travelers: 8-year experience at a referral center in Tokyo, Japan. J Infect Chemother. 2015;21(4):272-276.
24. CDC. Malaria diagnosis & treatment in the United States. www.cdc.gov/malaria/diagnosis_treatment/index.html. Accessed January 21, 2016.
25. Roy M, Bouma MJ, Ionides EL, et al. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7(1):e1979.
26. Tither PH. Preventing dengue and chikungunya fever among international travelers. J Am Assoc Nurse Pract. 2014;26(11):584-594.
27. Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. Biomed Res Int. 2015;2015:834371.
28. World Health Organization. Malaria vaccine development. www.who.int/malaria/areas/vaccine/en/#. Accessed January 21, 2016.
29. Leder K, Torresi J, Libman MD, et al. GeoSentinel Surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158(6):456-468.
30. Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137.
31. Essackjee K, Goorah S, Ramchurn SK, et al. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad Med. 2013;89:440-447.
32. Piyaphanee W, Steffen R, Shlim DR, et al. Travel medicine for Asian travelers—do we need new approaches? J Travel Med. 2012;19(6):335-337.
CE/CME No: CR-1602
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain how accessibility to travel affects the etiology of illness.
• Understand the typical and atypical signs and symptoms of malaria, dengue fever, and chikungunya.
• Identify the proper laboratory workup and treatment for malaria, dengue fever, and chikungunya.
• Discuss multiple ways to prevent mosquito-borne illness in your patients and the importance of a pretravel consultation.
FACULTY
Eve B. Hoover is completing a postgraduate academic fellowship at Midwestern University at Glendale, Arizona, and practices at Logistics Health, Inc, in Phoenix.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2016.
Article begins on next page >>
As the number of international travelers increases, so does the likelihood of transmission of illnesses to locations where they were previously rarely diagnosed. Clinicians at college health centers must be aware of tropical medicine diagnoses, especially in returning international students who have fever and other constitutional symptoms. This article provides a refresher regarding the diagnoses of malaria, dengue fever, and chikungunya.
Travel, whether for work, education, or pleasure, continues to increase, with the number of international travelers exceeding 1.1 billion in 2014.1 International travelers may unknowingly expose themselves and others to multiple health hazards previously thought to be foreign to the United States. Jane Zuckerman, who works with the World Health Organization (WHO), has noted that just over a century ago, the first human flew in an aircraft.2 Now, the sky is no longer the limit, and genes and micro-organisms travel as freely as their human hosts.
The international student population at American universities is at an all-time high (see Figure 1). Study abroad programs, which include American students who travel to developed and underdeveloped countries, also continue to increase. According to the CDC, the number of American students studying abroad has increased more than threefold in the past 20 years.3 According to the Institute of International Education, 304,467 American college students studied abroad in 2013/2014.4 Historically, most American students studied abroad in European countries, but in recent years the list of destinations has expanded, with increases in the percentage of students who travel to Africa, Asia, and the Middle East, and decreases in the percentage choosing Europe and Oceania.3
The sizable number of international students at universities, combined with the study abroad programs, have broadened the scope of the campus health care provider’s differential diagnosis. Diseases and infections that occur in developing countries can differ from those commonly seen in the US and Europe. It is important for health care providers to be reminded of conditions they seldom see and for a tropical medicine zebra to be considered in the appropriate patient population.
Continue for three causes of fever in returning travelers >>
THREE CAUSES OF FEVER IN RETURNING TRAVELERS
There are numerous etiologies for fever in the returning traveler. Factors such as location of travel, length of stay, dates of travel, date of symptom onset, risk activities undertaken, and reason for visit help determine the cause of illness.5 Two of the most commonly encountered conditions causing illness in the febrile traveler are dengue fever and malaria. Additionally, chikungunya is an emerging health concern in the US that has received increased attention following a massive outbreak in the Caribbean (which affected many American travelers) in 2013. In a retrospective study of patient records from 462 febrile adults who traveled to malaria-endemic areas, Siikamäki and colleagues found that every fourth febrile returning traveler had an illness that was potentially life-threatening.6 Understanding the possible causes of febrile illness in travelers can aid the clinician in diagnosing and correctly treating potentially life-threatening conditions.
Malaria
Malaria is a mosquito-borne illness transmitted in humans by female Anopheles mosquitoes.7 It is caused by infection with the protozoal parasites Plasmodium falciparum, P vivax, P ovale, P malariae, and occasionally other Plasmodium species.8 An infected female Anopheles mosquito transmits the parasite into a human host through a bite. The most severe form of human malaria, which can be fatal, is caused by P falciparum. Falciparum and vivax malaria are the most common forms of malaria worldwide.8
According to the WHO, there were an estimated 214 million clinical episodes of malaria worldwide, and malaria was the cause of 438,000 deaths, in 2015.9 In 2012, the CDC received reports of 1,687 cases of malaria in the US.8 The number of malaria cases has been steadily increasing since 1973.8 Figure 2 shows the number of malaria cases diagnosed in each state in 2012. The data demonstrate that malaria is the primary cause of death in travel-related fever. Malaria is also the most common single reason for travel-related fever without findings on exam or workup.
Dengue fever
Dengue is a mosquito-borne disease (transmitted by an infected Aedes mosquito) and is caused by four types of flaviviruses (DENV-1, DENV-2, DENV-3, DENV-4).10 It is the most common arboviral disease in humans.11 In 2009, the WHO revised its dengue categories to include dengue, dengue with warning signs, and severe dengue.12,13 Previously, the categories included dengue fever, dengue hemorrhagic fever, and dengue shock syndrome.
Dengue is endemic throughout the tropics and subtropics and is a leading cause of febrile illness among travelers returning from the Caribbean, South America, and South and Southeast Asia.14 There has been a 30-fold increase in dengue fever in the past 50 years.10 This illness is present in more than 100 countries; in the US, outbreaks have occurred in Florida, Hawaii, and along the Texas-Mexico border.14
Chikungunya
Chikungunya virus (CHIKV) is an arbovirus that is transmitted by Aedes mosquitoes (Aedes aegypti and Aedes albopictus).15 The term chikungunya is derived from a word in the Swahili and Maconde language that means “the one that is folded.”16 This description refers to the severe arthralgias that can cause a hunched-over gait in the patient with chikungunya.
Chikungunya historically has not had a significant impact in the Americas or Europe. However, more than one million suspected cases of chikungunya have been reported in the Americas since October 2013.16 Most cases of CHIKV infection diagnosed in the US have occurred in travelers; however, there have also been documented cases of local transmission of the virus.17 Local transmission occurs when the ill returning traveler unknowingly spreads disease, with the aid of the mosquito vector, upon return to the US.
Continue for patient presentation >>
PATIENT PRESENTATION
A 19-year-old previously healthy male student presented to the university health clinic for evaluation. During the exam, he lay on the examination table, covered with a blanket and shaking uncontrollably with intense rigors. Although he was hesitant to answer questions due to feeling so ill, he reported that he had returned from India two weeks prior and his symptoms—fever, rigors, ache, fatigue, headache, and nausea—began abruptly, hours before his arrival at the clinic.
The patient was diaphoretic and taking rapid, shallow inspirations. Assessment of vital signs revealed a blood pressure of 148/86 mm Hg; respiratory rate, 24 breaths/min; temperature, 103°F; and heart rate, 112 beats/min.
HEENT evaluation showed dry mucous membranes but no other abnormality. Neck was supple with no lymphadenopathy or nuchal rigidity. On cardiac exam, there were no murmurs or rubs. Lungs were clear to auscultation. Abdomen was soft and nontender, and bowel sounds were present in all four quadrants. There was no costovertebral angle tenderness. Skin was warm, clammy, and without rash. There were no focal neurologic deficits.
Complete blood count, comprehensive metabolic panel, and urinalysis were without abnormality. Examination of thick and thin blood smears revealed multiple red blood cells (RBCs) infected with malaria parasites and the appearance of the classic “headphone” form within the cells. Based on the in-office laboratory results of the blood smear, the patient was diagnosed with malaria.
The patient was not surprised by the diagnosis, as he had experienced these same symptoms with previous bouts of malaria. He and his family were from India, and the patient was an international college student. He had not taken malaria chemoprophylaxis prior to his most recent trip. After a short hospital admission for hydration, observation, treatment, and consultation by an infectious disease specialist, the patient was released back to the demands of college life.
Continue for signs and symptoms >>
SIGNS AND SYMPTOMS
Malaria
Signs and symptoms of malaria can vary greatly from none to illness causing death. The classic clinical features of malaria (fever, headache, back pain, chills, sweating, myalgia, nausea, vomiting, and cough) are caused by the parasite developing in RBCs, causing toxins to accumulate.18 Following the mosquito bite, there is typically an incubation period of seven to 30 days.
When first diagnosing malaria, the clinician needs to determine if the cause is P falciparum (the most severe form of malaria). If so, the clinician then must determine if the case is severe or nonsevere.7 In P falciparum infections, symptom onset can be later, especially if the patient took prophylaxis.7 Longer incubation periods are often seen with P vivax, P malariae, and P ovale infections. P vivax and P ovale can lie dormant in hepatic cells and reactivate after months or even years.7
Adults and children may experience different malaria symptoms, particularly with severe forms of the disease. Pediatric patients with severe falciparum malaria may experience respiratory distress, convulsions, and hypoglycemia more commonly than adults. More than half of adults with severe falciparum experience acute respiratory distress syndrome and acute renal failure.7 Pregnant women are at increased risk for complications.
Dengue fever
Approximately 75% of patients infected with DENV are asymptomatic.14 However, if symptomatic, the most common symptoms (fever, myalgia, headache, rash, arthralgia, abdominal pain, and nausea) begin abruptly after an incubation period of four to seven days.
Dengue can also present with atypical manifestations. In a prospective study by Nimmagadda and colleagues involving 150 participants with confirmed dengue fever, more than half of subjects had at least one atypical symptom along with more typical symptoms.10 The most common atypical manifestation was abnormal liver function, which was present in 40.6% of participants. Other atypical symptoms seen were febrile diarrhea (12%), renal failure (8%), acalculous cholecystitis (6.6%), and conduction abnormalities of the heart (6%). Less common atypical manifestations observed in this study included encephalitis, seizures, acute respiratory distress syndrome, disseminated intravascular coagulation, acute pancreatitis, myositis, and atrial fibrillation. The authors recommended that clinicians maintain a high level of vigilance for atypical manifestations of dengue fever, noting that most of the severe complications of dengue can be avoided if the disease is diagnosed correctly early in the course of illness.
Chikungunya
The typical presentation of CHIKV infection is a patient who abruptly develops fever, headache, polyarthralgia, and myalgia. The joint pain most frequently affects the small joints, such as the interphalangeal joints of the hands as well as the ankles and wrists.15 Back pain is also common, and rash is present in more than half of cases. The rash in adults can be maculopapular and in children is more often bullous. Fever, rash, and headache typically last seven to 10 days, while the arthralgia can last much longer—three to four months in a third of patients and three to five years in 10%.15
CHIKV infection that presents with typical clinical manifestations is usually self-limited; however, more severe atypical symptoms can occur and may lead to long-term morbidity. These atypical manifestations of chikungunya, which are rare, include acute disseminated encephalomyelitis, aseptic meningitis, meningoencephalitis, sensorineural hearing loss, myelitis, myeloradiculopathy, and Guillain-Barré syndrome.19 Chikungunya also has been associated with bleeding manifestations, acute renal failure, and electrolyte disturbance.19
Continue for the diagnosis >>
DIAGNOSIS
Malaria
The clinical presentation of malaria is nonspecific, so it is important to identify patients with a travel history and perform testing when this diagnosis is suspected.7,8 The gold standard for diagnosing malaria remains microscopy of thick and thin films of the patient’s peripheral blood.7 In patients with blood-stage malaria, a blood slide will show multiple infected RBCs and the appearance of the classic “headphone” form within the cells (see Figure 3). This test allows for efficient detection of malaria parasites, determination of parasite species, and calculation of percent parasitemia. Early differentiation between falciparum and nonfalciparum malaria is required, since P falciparum is the most life-threatening form of malaria.7 All of these factors are key to determining the best treatment plan for each patient.8
Rapid diagnostic tests (RDTs) and polymerase chain reaction (PCR) for malaria are increasingly available for use in US laboratories. In comparison to microscopy, these newer diagnostic tools are slower, more costly, and less readily available. For this reason, microscopy remains the most common means of diagnosis.7 PCR is helpful for species confirmation of malaria parasites and can be used to confirm a positive result on microscopy.8
It is possible for the first malaria test to be negative, and performing a repeat test the following day in a stable patient is recommended.7 However, more than three tests are not needed as long as the patient’s symptoms are not changing.
Dengue fever
Laboratory diagnosis of dengue can be confirmed with detection of DENV genomic sequences through PCR or nonstructural protein 1 antigen by immunoassay.14 Virus isolation in cell culture, detection of viral RNA by nucleic acid amplification tests, and detection of viral antigens by rapid tests or enzyme-linked immunosorbent assay (ELISA) are most useful if a patient presents within five days of fever onset.13
After five days of febrile illness, dengue viruses and antigens disappear from the blood as the specific antibody levels rise.13 Therefore, ELISA testing for immunoglobulin (Ig) M anti-DENV is a more effective lab study for dengue in patients presenting after one week. Testing for IgG anti-DENV is not recommended for making a diagnosis, however, because this antibody remains elevated for life after any DENV infection, leading to many false-positive test results. 14
A useful diagnostic aid for detecting severe dengue is the tourniquet test, which assesses for microvascular fragility. To perform the test, inflate a blood pressure cuff on the arm to midway between systolic and diastolic blood pressures, and maintain pressure for five minutes. After releasing the pressure, count the number of petechea in one square inch of skin; if 20 or more are found, the test is positive.12
Chikungunya
Chikungunya laboratory testing is limited in the US due to lack of availability. Testing is available only at the CDC, one commercial laboratory, and a few state health departments.17
The only reliable method for diagnosing CHIKV infection is through testing of blood samples. Chikungunya should not be diagnosed clinically because of the difficulty in differentiating it from dengue fever and other viral illnesses. The laboratory diagnosis of CHIKV infection can be obtained through detection of the virus, viral RNA, or specific antibodies related to chikungunya.20 Serologic detection of IgM or IgG antibodies is the most common method of diagnosis and is recommended by the CDC.20 If initial IgM and IgG testing is negative but clinical suspicion remains high, repeat testing should be done during the convalescent phase of the illness (≥ 7 days after symptom onset).21 Reverse transcriptase PCR is an effective diagnostic laboratory method for chikungunya and can be used in the first seven days of illness.20 ELISA and the hemagglutination inhibition assay can also provide diagnostic information.20
Lab findings associated with malaria, dengue fever, and chikungunya are summarized in the Table.
Continue for differentiating between dengue, malaria, and chikungunya early in presentation >>
Differentiating between dengue, malaria, and chikungunya early in presentation
Hematologic parameters can be used as a diagnostic aid when differentiating among certain causes of fever, as noted in a study by Joshi and Shah.22 In the setting of a febrile illness, thrombocytopenia (platelet count < 150,000/µL) is a predictor of malaria, especially in combination with anemia (hemoglobin < 10 g/dL). Thrombocytopenia is also common with dengue fever, but patients with dengue typically have normal hemoglobin. According to Joshi and Shah, patients having the combination of anemia and thrombocytopenia were 22 times more likely to have malaria than patients without these laboratory findings.22
Kutsuna and colleagues also found disease-specific clues in laboratory data when differentiating between dengue fever and malaria. Patients with dengue fever had significantly lower white blood cell counts than patients with malaria.23 In addition, although thrombocytopenia is seen in both dengue fever and malaria, platelet counts are lower in patients with malaria at first presentation. However, with dengue fever the platelet count can decrease three to six days into the illness when fever abates.23 Furthermore, total bilirubin tends to increase in malaria but is unaffected in dengue fever. Last, C-reactive protein can be helpful in assessing malaria severity and clinical improvement at follow-up, as well as for differentiating malaria from other conditions (eg, dengue), especially if the value is greater than 10 mg/L.23
Distinguishing chikungunya from dengue fever in the early stages of illness is difficult, and there is no pathognomonic hematologic laboratory study that helps with this task. With both diseases, the patient may have leukopenia, elevated erythrocyte sedimentation rate, and (rarely) thrombocytopenia. Chipwaza and colleagues discuss the significant overlap in symptoms in nonmalaria febrile illness. The overlap makes clinical diagnosis difficult; lab testing is essential for establishing the diagnosis.21 Once CHIKV is confirmed, the clinician is typically reassured of a more benign, self-limited course.
Continue for treatment/management >>
TREATMENT / MANAGEMENT
Malaria
The treatment of malaria varies depending on the severity of disease and the probability the organism is resistant to antimalarial drugs. The likelihood of drug resistance is determined based on the species of malaria parasite and the location where the infection occurred.24
Malaria is considered severe if one or more of the following are present: neurologic sequelae, renal failure, severe anemia, ARDS, jaundice, or parasite burden greater than 5%.8 Patients with severe malaria are treated with parenteral (IV) antimalarials; the two options for parenteral medication are quinine and artesunate.7 All patients treated with parenteral antimalarial agents should take a full course of oral medication for malaria as well. Oral antimalarial medications include, but are not limited to, quinine sulfate, atovaquone/proguanil, artemether-lumefantrine, doxycycline, clindamycin, sulfadoxine/pyrimethamine, chloroquine, and primaquine. The oral medications most commonly used to treat nonfalciparum malaria are chloroquine followed by primaquine.7 Chloroquine is not typically used for falciparum malaria due to widespread resistance.7
In cases of malaria caused by P vivax or P ovale infection, the likelihood of parasitic infection lying dormant in the liver must be considered. Additional treatment is often needed to eradicate this type of infection.7 The relapse of symptoms can occur years after the acute attack.25 Primaquine is the only approved medication for preventing and treating parasitic relapse associated with dormant infection.25
Dengue fever
The typical course of dengue follows three phases: febrile, critical, and convalescent.14 Dengue is usually a self-limiting febrile illness and typically resolves within one week after symptom onset without major complications.11 During the critical phase, most patients begin to improve, but up to 5% of cases develop concerning warning signs and symptoms that could represent a life-threatening condition that requires intense treatment and close monitoring.14 Warning signs for worsening disease are caused by marked increase in vascular permeability and include narrow pulse pressure, pleural effusions, ascites, and hemorrhagic manifestations (hematemesis, melena, menorrhagia).14
Treatment of most cases of dengue involves use of acetaminophen for comfort and fever reduction, hydration, and rest.26 Treatment of worsening dengue includes inpatient admission and possibly ICU admission for close observation and frequent monitoring.14 It is important to avoid aspirin and other NSAIDs due to the risk for bleeding complications in severe dengue.14 There are no approved antivirals for treatment of dengue. To reduce the risk for transmission of dengue, febrile patients should avoid further mosquito bites.14
Clinicians should be aware of warning signs of worsening illness with dengue fever. Signs of worsening dengue fever include postural hypotension, thrombocytopenia, decrease in serum albumin, and rising hematocrit.10
Chikungunya
CHIKV illness is usually a self-limiting condition. Diagnosis of chikungunya may take time, and providers should assume the chikungunya patient may have dengue, which has the potential to be more pathologic. Accordingly, they must watch for warning signs of dengue until CHIKV is confirmed.27
Management focuses on supportive care, including hydration and rest. Typically, the medications used are antipyretics (acetaminophen and ibuprofen) and analgesics; no antiviral medication for chikungunya is available.22 Aspirin is avoided due to the risk for Reye syndrome. Antihistamines may be helpful for patients who have an associated pruritic rash. Cold compresses can be beneficial for joint pain and swelling. Additionally, it is important to keep patients under mosquito nets during the febrile phase to decrease the risk for disease transmission.
Continue for patient education >>
PATIENT EDUCATION
Vaccination
Vaccines are not available for dengue fever, malaria, or chikungunya. However, researchers have been working on a vaccine for malaria for decades, and presently, more than 20 vaccine constructs are being tested and researched in preclinical trials.28 Similarly, efforts to develop an effective vaccine for chikungunya have been under way since the 1970s.15
Chemoprophylaxis
There are no chemoprophylaxis options available for dengue or chikungunya. The most effective measures to prevent dengue are strategies aimed at avoiding mosquito bites (ie, vector control and individual protections like repellants).16 One group of authors notes that developing a larger network of research laboratories capable of prompt diagnosis of arbovirus infections would help to better control chikungunya.
Multiple options for malaria chemoprophylaxis are available, and these vary by country of travel. Chemoprophylaxis options include doxycycline, mefloquine, atovaquone/proguanil, chloroquine, and primaquine.8 For dormant forms of malaria residing in the liver, primaquine is the only effective treatment.8
It is important to remember that no antimalarial drug is 100% protective. Malaria chemoprophylaxis reduces the risk for malaria, but it is often taken inadequately, which can delay symptom onset and lead to a false-negative result on initial blood films.5 All patients with fever who have visited a tropical country within one year of presentation should be screened for malaria.5 It is incorrect and dangerous to assume that a patient who received malaria chemoprophylaxis does not have malaria. Diagnosis of malaria requires a high index of suspicion, and clinicians must remember that malaria can occur even with perfect prophylaxis.7 Unfortunately, malaria parasites are becoming resistant to some commonly used antimalarial drugs. Resistance patterns are being tracked by the CDC.8
Pretravel visit with clinician
Many illnesses related to travel can be prevented with vaccination, chemoprophylaxis, and patient education. However, many travelers do not visit a health care professional before travel (even among those traveling to perceived “risky” destinations, such as Sub-Saharan Africa).29 In a study of ill returned travelers, Leder and colleagues found that only 40% had sought pretravel advice from providers. Interestingly, many of those ill patients who obtained a pretravel consult did not receive appropriate vaccines, such as hepatitis A or influenza vaccine. Some of these patients were diagnosed with preventable conditions.29
Open and thorough communication between clinicians and patients is paramount to protect travelers from disease and illness. Clinicians have the opportunity to greatly impact the health of their patients by recommending a pretravel consultation. What may take extra time and effort on the front end of the trip may save significant time and energy on the back end, and even save lives.
Mosquito bite avoidance
One of the most important patient education discussions in pretravel consultation is mosquito bite avoidance. Travelers should be advised to find accommodations with air conditioning and screened windows and doors.14 To avoid mosquito bites, they should cover their arms and legs adequately with proper clothing. Standing water (such as in flower pots), which can encourage mosquito breeding, should be avoided as well. Travelers should use insect repellents and insecticides, especially in cool, dark areas (eg, closets and bathrooms) where mosquitoes hide.14
Continue for follow-up >>
FOLLOW-UP
Malaria
Appropriate follow-up, need for hospitalization, and choice of medical treatment are determined by disease cause, severity of illness, and patient demographics. Follow-up is necessary to ensure improvement and no development of atypical symptoms. Additionally, the clinician needs to keep in mind the risk for malaria strains that can have a dormant stage.
Dengue fever
Clinicians must remind convalescing patients to watch for severe abdominal pain, vomiting, difficulty breathing, and signs of bleeding (epistaxis, bruising, bloody stool, and menorrhagia). Clinicians must also be attentive to changing lab values, including a decrease in platelet count and an increase in hematocrit, along with signs of hypovolemic shock, ascites, pleural effusions, and narrow pulse pressure.14
Chikungunya
Patients are reminded to keep themselves comfortable by rehydrating and treating the discomfort associated with arthralgias. In a longitudinal study of chikungunya patients, 60% experienced continued arthralgias three years after diagnosis.23,30 Patient education regarding the potential for long-term arthralgia is important, as it may impact activities of daily living and work.
Long-term NSAIDs have been used for patients with recurrent or even chronic arthralgia.31 There are limited data available on beneficial treatments, such as chloroquine sulfate or disease-modifying antirheumatic drugs, for chronic arthralgia associated with chikungunya.23 Depression and recurrent cutaneous lesions also are possible in patients with long-term symptoms.
Continue for the conclusion >>
CONCLUSION
“People, as well as pathogens, travel from all around the world in all directions.”32 With the ever-increasing mobility of populations around the world, transmission of illness and medical norms are constantly changing. All clinicians should keep in mind the less commonly seen diagnostic entities and remember the importance of obtaining a complete travel history in the febrile patient.
Early detection and appropriate supportive care of patients with dengue fever and malaria can be lifesaving. In addition, proper pretravel consultations can provide a wealth of patient education for at-risk travelers and help prevent a number of debilitating infectious diseases.
CE/CME No: CR-1602
PROGRAM OVERVIEW
Earn credit by reading this article and successfully completing the posttest and evaluation. Successful completion is defined as a cumulative score of at least 70% correct.
EDUCATIONAL OBJECTIVES
• Explain how accessibility to travel affects the etiology of illness.
• Understand the typical and atypical signs and symptoms of malaria, dengue fever, and chikungunya.
• Identify the proper laboratory workup and treatment for malaria, dengue fever, and chikungunya.
• Discuss multiple ways to prevent mosquito-borne illness in your patients and the importance of a pretravel consultation.
FACULTY
Eve B. Hoover is completing a postgraduate academic fellowship at Midwestern University at Glendale, Arizona, and practices at Logistics Health, Inc, in Phoenix.
The author has no significant financial relationships to disclose.
ACCREDITATION STATEMENT
This program has been reviewed and is approved for a maximum of 1.0 hour of American Academy of Physician Assistants (AAPA) Category 1 CME credit by the Physician Assistant Review Panel. [NPs: Both ANCC and the AANP Certification Program recognize AAPA as an approved provider of Category 1 credit.] Approval is valid for one year from the issue date of February 2016.
Article begins on next page >>
As the number of international travelers increases, so does the likelihood of transmission of illnesses to locations where they were previously rarely diagnosed. Clinicians at college health centers must be aware of tropical medicine diagnoses, especially in returning international students who have fever and other constitutional symptoms. This article provides a refresher regarding the diagnoses of malaria, dengue fever, and chikungunya.
Travel, whether for work, education, or pleasure, continues to increase, with the number of international travelers exceeding 1.1 billion in 2014.1 International travelers may unknowingly expose themselves and others to multiple health hazards previously thought to be foreign to the United States. Jane Zuckerman, who works with the World Health Organization (WHO), has noted that just over a century ago, the first human flew in an aircraft.2 Now, the sky is no longer the limit, and genes and micro-organisms travel as freely as their human hosts.
The international student population at American universities is at an all-time high (see Figure 1). Study abroad programs, which include American students who travel to developed and underdeveloped countries, also continue to increase. According to the CDC, the number of American students studying abroad has increased more than threefold in the past 20 years.3 According to the Institute of International Education, 304,467 American college students studied abroad in 2013/2014.4 Historically, most American students studied abroad in European countries, but in recent years the list of destinations has expanded, with increases in the percentage of students who travel to Africa, Asia, and the Middle East, and decreases in the percentage choosing Europe and Oceania.3
The sizable number of international students at universities, combined with the study abroad programs, have broadened the scope of the campus health care provider’s differential diagnosis. Diseases and infections that occur in developing countries can differ from those commonly seen in the US and Europe. It is important for health care providers to be reminded of conditions they seldom see and for a tropical medicine zebra to be considered in the appropriate patient population.
Continue for three causes of fever in returning travelers >>
THREE CAUSES OF FEVER IN RETURNING TRAVELERS
There are numerous etiologies for fever in the returning traveler. Factors such as location of travel, length of stay, dates of travel, date of symptom onset, risk activities undertaken, and reason for visit help determine the cause of illness.5 Two of the most commonly encountered conditions causing illness in the febrile traveler are dengue fever and malaria. Additionally, chikungunya is an emerging health concern in the US that has received increased attention following a massive outbreak in the Caribbean (which affected many American travelers) in 2013. In a retrospective study of patient records from 462 febrile adults who traveled to malaria-endemic areas, Siikamäki and colleagues found that every fourth febrile returning traveler had an illness that was potentially life-threatening.6 Understanding the possible causes of febrile illness in travelers can aid the clinician in diagnosing and correctly treating potentially life-threatening conditions.
Malaria
Malaria is a mosquito-borne illness transmitted in humans by female Anopheles mosquitoes.7 It is caused by infection with the protozoal parasites Plasmodium falciparum, P vivax, P ovale, P malariae, and occasionally other Plasmodium species.8 An infected female Anopheles mosquito transmits the parasite into a human host through a bite. The most severe form of human malaria, which can be fatal, is caused by P falciparum. Falciparum and vivax malaria are the most common forms of malaria worldwide.8
According to the WHO, there were an estimated 214 million clinical episodes of malaria worldwide, and malaria was the cause of 438,000 deaths, in 2015.9 In 2012, the CDC received reports of 1,687 cases of malaria in the US.8 The number of malaria cases has been steadily increasing since 1973.8 Figure 2 shows the number of malaria cases diagnosed in each state in 2012. The data demonstrate that malaria is the primary cause of death in travel-related fever. Malaria is also the most common single reason for travel-related fever without findings on exam or workup.
Dengue fever
Dengue is a mosquito-borne disease (transmitted by an infected Aedes mosquito) and is caused by four types of flaviviruses (DENV-1, DENV-2, DENV-3, DENV-4).10 It is the most common arboviral disease in humans.11 In 2009, the WHO revised its dengue categories to include dengue, dengue with warning signs, and severe dengue.12,13 Previously, the categories included dengue fever, dengue hemorrhagic fever, and dengue shock syndrome.
Dengue is endemic throughout the tropics and subtropics and is a leading cause of febrile illness among travelers returning from the Caribbean, South America, and South and Southeast Asia.14 There has been a 30-fold increase in dengue fever in the past 50 years.10 This illness is present in more than 100 countries; in the US, outbreaks have occurred in Florida, Hawaii, and along the Texas-Mexico border.14
Chikungunya
Chikungunya virus (CHIKV) is an arbovirus that is transmitted by Aedes mosquitoes (Aedes aegypti and Aedes albopictus).15 The term chikungunya is derived from a word in the Swahili and Maconde language that means “the one that is folded.”16 This description refers to the severe arthralgias that can cause a hunched-over gait in the patient with chikungunya.
Chikungunya historically has not had a significant impact in the Americas or Europe. However, more than one million suspected cases of chikungunya have been reported in the Americas since October 2013.16 Most cases of CHIKV infection diagnosed in the US have occurred in travelers; however, there have also been documented cases of local transmission of the virus.17 Local transmission occurs when the ill returning traveler unknowingly spreads disease, with the aid of the mosquito vector, upon return to the US.
Continue for patient presentation >>
PATIENT PRESENTATION
A 19-year-old previously healthy male student presented to the university health clinic for evaluation. During the exam, he lay on the examination table, covered with a blanket and shaking uncontrollably with intense rigors. Although he was hesitant to answer questions due to feeling so ill, he reported that he had returned from India two weeks prior and his symptoms—fever, rigors, ache, fatigue, headache, and nausea—began abruptly, hours before his arrival at the clinic.
The patient was diaphoretic and taking rapid, shallow inspirations. Assessment of vital signs revealed a blood pressure of 148/86 mm Hg; respiratory rate, 24 breaths/min; temperature, 103°F; and heart rate, 112 beats/min.
HEENT evaluation showed dry mucous membranes but no other abnormality. Neck was supple with no lymphadenopathy or nuchal rigidity. On cardiac exam, there were no murmurs or rubs. Lungs were clear to auscultation. Abdomen was soft and nontender, and bowel sounds were present in all four quadrants. There was no costovertebral angle tenderness. Skin was warm, clammy, and without rash. There were no focal neurologic deficits.
Complete blood count, comprehensive metabolic panel, and urinalysis were without abnormality. Examination of thick and thin blood smears revealed multiple red blood cells (RBCs) infected with malaria parasites and the appearance of the classic “headphone” form within the cells. Based on the in-office laboratory results of the blood smear, the patient was diagnosed with malaria.
The patient was not surprised by the diagnosis, as he had experienced these same symptoms with previous bouts of malaria. He and his family were from India, and the patient was an international college student. He had not taken malaria chemoprophylaxis prior to his most recent trip. After a short hospital admission for hydration, observation, treatment, and consultation by an infectious disease specialist, the patient was released back to the demands of college life.
Continue for signs and symptoms >>
SIGNS AND SYMPTOMS
Malaria
Signs and symptoms of malaria can vary greatly from none to illness causing death. The classic clinical features of malaria (fever, headache, back pain, chills, sweating, myalgia, nausea, vomiting, and cough) are caused by the parasite developing in RBCs, causing toxins to accumulate.18 Following the mosquito bite, there is typically an incubation period of seven to 30 days.
When first diagnosing malaria, the clinician needs to determine if the cause is P falciparum (the most severe form of malaria). If so, the clinician then must determine if the case is severe or nonsevere.7 In P falciparum infections, symptom onset can be later, especially if the patient took prophylaxis.7 Longer incubation periods are often seen with P vivax, P malariae, and P ovale infections. P vivax and P ovale can lie dormant in hepatic cells and reactivate after months or even years.7
Adults and children may experience different malaria symptoms, particularly with severe forms of the disease. Pediatric patients with severe falciparum malaria may experience respiratory distress, convulsions, and hypoglycemia more commonly than adults. More than half of adults with severe falciparum experience acute respiratory distress syndrome and acute renal failure.7 Pregnant women are at increased risk for complications.
Dengue fever
Approximately 75% of patients infected with DENV are asymptomatic.14 However, if symptomatic, the most common symptoms (fever, myalgia, headache, rash, arthralgia, abdominal pain, and nausea) begin abruptly after an incubation period of four to seven days.
Dengue can also present with atypical manifestations. In a prospective study by Nimmagadda and colleagues involving 150 participants with confirmed dengue fever, more than half of subjects had at least one atypical symptom along with more typical symptoms.10 The most common atypical manifestation was abnormal liver function, which was present in 40.6% of participants. Other atypical symptoms seen were febrile diarrhea (12%), renal failure (8%), acalculous cholecystitis (6.6%), and conduction abnormalities of the heart (6%). Less common atypical manifestations observed in this study included encephalitis, seizures, acute respiratory distress syndrome, disseminated intravascular coagulation, acute pancreatitis, myositis, and atrial fibrillation. The authors recommended that clinicians maintain a high level of vigilance for atypical manifestations of dengue fever, noting that most of the severe complications of dengue can be avoided if the disease is diagnosed correctly early in the course of illness.
Chikungunya
The typical presentation of CHIKV infection is a patient who abruptly develops fever, headache, polyarthralgia, and myalgia. The joint pain most frequently affects the small joints, such as the interphalangeal joints of the hands as well as the ankles and wrists.15 Back pain is also common, and rash is present in more than half of cases. The rash in adults can be maculopapular and in children is more often bullous. Fever, rash, and headache typically last seven to 10 days, while the arthralgia can last much longer—three to four months in a third of patients and three to five years in 10%.15
CHIKV infection that presents with typical clinical manifestations is usually self-limited; however, more severe atypical symptoms can occur and may lead to long-term morbidity. These atypical manifestations of chikungunya, which are rare, include acute disseminated encephalomyelitis, aseptic meningitis, meningoencephalitis, sensorineural hearing loss, myelitis, myeloradiculopathy, and Guillain-Barré syndrome.19 Chikungunya also has been associated with bleeding manifestations, acute renal failure, and electrolyte disturbance.19
Continue for the diagnosis >>
DIAGNOSIS
Malaria
The clinical presentation of malaria is nonspecific, so it is important to identify patients with a travel history and perform testing when this diagnosis is suspected.7,8 The gold standard for diagnosing malaria remains microscopy of thick and thin films of the patient’s peripheral blood.7 In patients with blood-stage malaria, a blood slide will show multiple infected RBCs and the appearance of the classic “headphone” form within the cells (see Figure 3). This test allows for efficient detection of malaria parasites, determination of parasite species, and calculation of percent parasitemia. Early differentiation between falciparum and nonfalciparum malaria is required, since P falciparum is the most life-threatening form of malaria.7 All of these factors are key to determining the best treatment plan for each patient.8
Rapid diagnostic tests (RDTs) and polymerase chain reaction (PCR) for malaria are increasingly available for use in US laboratories. In comparison to microscopy, these newer diagnostic tools are slower, more costly, and less readily available. For this reason, microscopy remains the most common means of diagnosis.7 PCR is helpful for species confirmation of malaria parasites and can be used to confirm a positive result on microscopy.8
It is possible for the first malaria test to be negative, and performing a repeat test the following day in a stable patient is recommended.7 However, more than three tests are not needed as long as the patient’s symptoms are not changing.
Dengue fever
Laboratory diagnosis of dengue can be confirmed with detection of DENV genomic sequences through PCR or nonstructural protein 1 antigen by immunoassay.14 Virus isolation in cell culture, detection of viral RNA by nucleic acid amplification tests, and detection of viral antigens by rapid tests or enzyme-linked immunosorbent assay (ELISA) are most useful if a patient presents within five days of fever onset.13
After five days of febrile illness, dengue viruses and antigens disappear from the blood as the specific antibody levels rise.13 Therefore, ELISA testing for immunoglobulin (Ig) M anti-DENV is a more effective lab study for dengue in patients presenting after one week. Testing for IgG anti-DENV is not recommended for making a diagnosis, however, because this antibody remains elevated for life after any DENV infection, leading to many false-positive test results. 14
A useful diagnostic aid for detecting severe dengue is the tourniquet test, which assesses for microvascular fragility. To perform the test, inflate a blood pressure cuff on the arm to midway between systolic and diastolic blood pressures, and maintain pressure for five minutes. After releasing the pressure, count the number of petechea in one square inch of skin; if 20 or more are found, the test is positive.12
Chikungunya
Chikungunya laboratory testing is limited in the US due to lack of availability. Testing is available only at the CDC, one commercial laboratory, and a few state health departments.17
The only reliable method for diagnosing CHIKV infection is through testing of blood samples. Chikungunya should not be diagnosed clinically because of the difficulty in differentiating it from dengue fever and other viral illnesses. The laboratory diagnosis of CHIKV infection can be obtained through detection of the virus, viral RNA, or specific antibodies related to chikungunya.20 Serologic detection of IgM or IgG antibodies is the most common method of diagnosis and is recommended by the CDC.20 If initial IgM and IgG testing is negative but clinical suspicion remains high, repeat testing should be done during the convalescent phase of the illness (≥ 7 days after symptom onset).21 Reverse transcriptase PCR is an effective diagnostic laboratory method for chikungunya and can be used in the first seven days of illness.20 ELISA and the hemagglutination inhibition assay can also provide diagnostic information.20
Lab findings associated with malaria, dengue fever, and chikungunya are summarized in the Table.
Continue for differentiating between dengue, malaria, and chikungunya early in presentation >>
Differentiating between dengue, malaria, and chikungunya early in presentation
Hematologic parameters can be used as a diagnostic aid when differentiating among certain causes of fever, as noted in a study by Joshi and Shah.22 In the setting of a febrile illness, thrombocytopenia (platelet count < 150,000/µL) is a predictor of malaria, especially in combination with anemia (hemoglobin < 10 g/dL). Thrombocytopenia is also common with dengue fever, but patients with dengue typically have normal hemoglobin. According to Joshi and Shah, patients having the combination of anemia and thrombocytopenia were 22 times more likely to have malaria than patients without these laboratory findings.22
Kutsuna and colleagues also found disease-specific clues in laboratory data when differentiating between dengue fever and malaria. Patients with dengue fever had significantly lower white blood cell counts than patients with malaria.23 In addition, although thrombocytopenia is seen in both dengue fever and malaria, platelet counts are lower in patients with malaria at first presentation. However, with dengue fever the platelet count can decrease three to six days into the illness when fever abates.23 Furthermore, total bilirubin tends to increase in malaria but is unaffected in dengue fever. Last, C-reactive protein can be helpful in assessing malaria severity and clinical improvement at follow-up, as well as for differentiating malaria from other conditions (eg, dengue), especially if the value is greater than 10 mg/L.23
Distinguishing chikungunya from dengue fever in the early stages of illness is difficult, and there is no pathognomonic hematologic laboratory study that helps with this task. With both diseases, the patient may have leukopenia, elevated erythrocyte sedimentation rate, and (rarely) thrombocytopenia. Chipwaza and colleagues discuss the significant overlap in symptoms in nonmalaria febrile illness. The overlap makes clinical diagnosis difficult; lab testing is essential for establishing the diagnosis.21 Once CHIKV is confirmed, the clinician is typically reassured of a more benign, self-limited course.
Continue for treatment/management >>
TREATMENT / MANAGEMENT
Malaria
The treatment of malaria varies depending on the severity of disease and the probability the organism is resistant to antimalarial drugs. The likelihood of drug resistance is determined based on the species of malaria parasite and the location where the infection occurred.24
Malaria is considered severe if one or more of the following are present: neurologic sequelae, renal failure, severe anemia, ARDS, jaundice, or parasite burden greater than 5%.8 Patients with severe malaria are treated with parenteral (IV) antimalarials; the two options for parenteral medication are quinine and artesunate.7 All patients treated with parenteral antimalarial agents should take a full course of oral medication for malaria as well. Oral antimalarial medications include, but are not limited to, quinine sulfate, atovaquone/proguanil, artemether-lumefantrine, doxycycline, clindamycin, sulfadoxine/pyrimethamine, chloroquine, and primaquine. The oral medications most commonly used to treat nonfalciparum malaria are chloroquine followed by primaquine.7 Chloroquine is not typically used for falciparum malaria due to widespread resistance.7
In cases of malaria caused by P vivax or P ovale infection, the likelihood of parasitic infection lying dormant in the liver must be considered. Additional treatment is often needed to eradicate this type of infection.7 The relapse of symptoms can occur years after the acute attack.25 Primaquine is the only approved medication for preventing and treating parasitic relapse associated with dormant infection.25
Dengue fever
The typical course of dengue follows three phases: febrile, critical, and convalescent.14 Dengue is usually a self-limiting febrile illness and typically resolves within one week after symptom onset without major complications.11 During the critical phase, most patients begin to improve, but up to 5% of cases develop concerning warning signs and symptoms that could represent a life-threatening condition that requires intense treatment and close monitoring.14 Warning signs for worsening disease are caused by marked increase in vascular permeability and include narrow pulse pressure, pleural effusions, ascites, and hemorrhagic manifestations (hematemesis, melena, menorrhagia).14
Treatment of most cases of dengue involves use of acetaminophen for comfort and fever reduction, hydration, and rest.26 Treatment of worsening dengue includes inpatient admission and possibly ICU admission for close observation and frequent monitoring.14 It is important to avoid aspirin and other NSAIDs due to the risk for bleeding complications in severe dengue.14 There are no approved antivirals for treatment of dengue. To reduce the risk for transmission of dengue, febrile patients should avoid further mosquito bites.14
Clinicians should be aware of warning signs of worsening illness with dengue fever. Signs of worsening dengue fever include postural hypotension, thrombocytopenia, decrease in serum albumin, and rising hematocrit.10
Chikungunya
CHIKV illness is usually a self-limiting condition. Diagnosis of chikungunya may take time, and providers should assume the chikungunya patient may have dengue, which has the potential to be more pathologic. Accordingly, they must watch for warning signs of dengue until CHIKV is confirmed.27
Management focuses on supportive care, including hydration and rest. Typically, the medications used are antipyretics (acetaminophen and ibuprofen) and analgesics; no antiviral medication for chikungunya is available.22 Aspirin is avoided due to the risk for Reye syndrome. Antihistamines may be helpful for patients who have an associated pruritic rash. Cold compresses can be beneficial for joint pain and swelling. Additionally, it is important to keep patients under mosquito nets during the febrile phase to decrease the risk for disease transmission.
Continue for patient education >>
PATIENT EDUCATION
Vaccination
Vaccines are not available for dengue fever, malaria, or chikungunya. However, researchers have been working on a vaccine for malaria for decades, and presently, more than 20 vaccine constructs are being tested and researched in preclinical trials.28 Similarly, efforts to develop an effective vaccine for chikungunya have been under way since the 1970s.15
Chemoprophylaxis
There are no chemoprophylaxis options available for dengue or chikungunya. The most effective measures to prevent dengue are strategies aimed at avoiding mosquito bites (ie, vector control and individual protections like repellants).16 One group of authors notes that developing a larger network of research laboratories capable of prompt diagnosis of arbovirus infections would help to better control chikungunya.
Multiple options for malaria chemoprophylaxis are available, and these vary by country of travel. Chemoprophylaxis options include doxycycline, mefloquine, atovaquone/proguanil, chloroquine, and primaquine.8 For dormant forms of malaria residing in the liver, primaquine is the only effective treatment.8
It is important to remember that no antimalarial drug is 100% protective. Malaria chemoprophylaxis reduces the risk for malaria, but it is often taken inadequately, which can delay symptom onset and lead to a false-negative result on initial blood films.5 All patients with fever who have visited a tropical country within one year of presentation should be screened for malaria.5 It is incorrect and dangerous to assume that a patient who received malaria chemoprophylaxis does not have malaria. Diagnosis of malaria requires a high index of suspicion, and clinicians must remember that malaria can occur even with perfect prophylaxis.7 Unfortunately, malaria parasites are becoming resistant to some commonly used antimalarial drugs. Resistance patterns are being tracked by the CDC.8
Pretravel visit with clinician
Many illnesses related to travel can be prevented with vaccination, chemoprophylaxis, and patient education. However, many travelers do not visit a health care professional before travel (even among those traveling to perceived “risky” destinations, such as Sub-Saharan Africa).29 In a study of ill returned travelers, Leder and colleagues found that only 40% had sought pretravel advice from providers. Interestingly, many of those ill patients who obtained a pretravel consult did not receive appropriate vaccines, such as hepatitis A or influenza vaccine. Some of these patients were diagnosed with preventable conditions.29
Open and thorough communication between clinicians and patients is paramount to protect travelers from disease and illness. Clinicians have the opportunity to greatly impact the health of their patients by recommending a pretravel consultation. What may take extra time and effort on the front end of the trip may save significant time and energy on the back end, and even save lives.
Mosquito bite avoidance
One of the most important patient education discussions in pretravel consultation is mosquito bite avoidance. Travelers should be advised to find accommodations with air conditioning and screened windows and doors.14 To avoid mosquito bites, they should cover their arms and legs adequately with proper clothing. Standing water (such as in flower pots), which can encourage mosquito breeding, should be avoided as well. Travelers should use insect repellents and insecticides, especially in cool, dark areas (eg, closets and bathrooms) where mosquitoes hide.14
Continue for follow-up >>
FOLLOW-UP
Malaria
Appropriate follow-up, need for hospitalization, and choice of medical treatment are determined by disease cause, severity of illness, and patient demographics. Follow-up is necessary to ensure improvement and no development of atypical symptoms. Additionally, the clinician needs to keep in mind the risk for malaria strains that can have a dormant stage.
Dengue fever
Clinicians must remind convalescing patients to watch for severe abdominal pain, vomiting, difficulty breathing, and signs of bleeding (epistaxis, bruising, bloody stool, and menorrhagia). Clinicians must also be attentive to changing lab values, including a decrease in platelet count and an increase in hematocrit, along with signs of hypovolemic shock, ascites, pleural effusions, and narrow pulse pressure.14
Chikungunya
Patients are reminded to keep themselves comfortable by rehydrating and treating the discomfort associated with arthralgias. In a longitudinal study of chikungunya patients, 60% experienced continued arthralgias three years after diagnosis.23,30 Patient education regarding the potential for long-term arthralgia is important, as it may impact activities of daily living and work.
Long-term NSAIDs have been used for patients with recurrent or even chronic arthralgia.31 There are limited data available on beneficial treatments, such as chloroquine sulfate or disease-modifying antirheumatic drugs, for chronic arthralgia associated with chikungunya.23 Depression and recurrent cutaneous lesions also are possible in patients with long-term symptoms.
Continue for the conclusion >>
CONCLUSION
“People, as well as pathogens, travel from all around the world in all directions.”32 With the ever-increasing mobility of populations around the world, transmission of illness and medical norms are constantly changing. All clinicians should keep in mind the less commonly seen diagnostic entities and remember the importance of obtaining a complete travel history in the febrile patient.
Early detection and appropriate supportive care of patients with dengue fever and malaria can be lifesaving. In addition, proper pretravel consultations can provide a wealth of patient education for at-risk travelers and help prevent a number of debilitating infectious diseases.
1. World Tourism Organization. Over 1.1 billion tourists travelled abroad in 2014 [press release]. January 27, 2015. http://media.unwto.org/press-release/2015-01-27/over-11-billion-tourists-travelled-abroad-2014. Accessed January 21, 2016.
2. Zuckerman JN. Public health and travel medicine: intricately intertwined. Perspect Public Health. 2012;132(5):206.
3. Rhodes G, DeRomaña I, Ebner J. Advising travelers with special needs: study abroad & other international student travel. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellow book/2016/advising-travelers-with-specific-needs/study-abroad-other-international-student-travel. Accessed January 21, 2016.
4. Institute of International Education. US study abroad: all destinations. www.iie.org/Research-and-Publications/Open-Doors/Data/US-Study-Abroad/All-Destinations/2012-14. Accessed January 21, 2016.
5. Hearn P, Johnston V. Assessment of returning travelers with fever. Medicine. 2014;42(2):66-72.
6. Siikamäki HM, Kivelä PS, Sipilä PN, et al. Fever in travelers returning from malaria-endemic areas: don’t look for malaria only. J Travel Med. 2011;18(4):239-244.
7. Walker NF, Nadjm B, Whitty CJ. Malaria. Medicine. 2014;42(2):100-106.
8. Cullen KA, Arguin PM. Malaria surveillance—United States, 2012. MMWR Surveill Summ. 2014;63(12):1-22.
9. World Health Organization. Malaria. Fact sheet no. 94. www.who.int/mediacentre/factsheets/fs094/en/. Accessed January 21, 2016.
10. Nimmagadda SS, Mahabala C, Boloor A, et al. Atypical manifestations of dengue fever (DF)—where do we stand today? J Clin Diagn Res. 2014;8(1):71-73.
11. Whitehorn J, Yacoub S, Anders KL, et al. Dengue therapeutics, chemoprophylaxis, and allied tools: state of the art and future directions. PLoS Negl Trop Dis. 2014;8(8):e3025.
12. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
13. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1. www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed January 21, 2016.
14. Tomashek KM, Sharp TM, Margolis HS. Infectious diseases related to travel: dengue. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/dengue. Accessed January 21, 2016.
15. Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9(5):1-18.
16. Hrnjakovi`c Cvjetkovi`c IB, Cvjetkovi`c D, Pati`c A, et al. Chikungunya—a serious threat for public health. Med Pregl. 2015;68(3/4):122-125.
17. Lindsey NP, Prince HE, Kosoy O, et al. Chikungunya virus infections among travelers-United States, 2010-2013. Am J Trop Med Hyg. 2015;92(1):82-87.
18. CDC. Malaria. www.cdc.gov/malaria/about/disease.html. Accessed January 21, 2016.
19. Das S, Sarkar N, Majumder J, et al. Acute disseminated encephalomyelitis in a child with chikungunya virus infection. J Pediatr Infect Dis. 2014;9(1):37-41.
20. Schwartz KL, Giga A, Boggild AK. Chikungunya fever in Canada: fever and polyarthritis in a returned traveller. CMAJ. 2014;186(10):772-774.
21. Chipwaza B, Mugasa JP, Selemani M, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8(11):e3335.
22. Joshi HA, Shah SS. Platelet count—a diagnostic aid in fever. Natl J Integrated Res Med. 2013;4(3):128-132.
23. Kutsuna S, Hayakawa K, Kato Y, et al. Comparison of clinical characteristics and laboratory findings of malaria, dengue, and enteric fever in returning travelers: 8-year experience at a referral center in Tokyo, Japan. J Infect Chemother. 2015;21(4):272-276.
24. CDC. Malaria diagnosis & treatment in the United States. www.cdc.gov/malaria/diagnosis_treatment/index.html. Accessed January 21, 2016.
25. Roy M, Bouma MJ, Ionides EL, et al. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7(1):e1979.
26. Tither PH. Preventing dengue and chikungunya fever among international travelers. J Am Assoc Nurse Pract. 2014;26(11):584-594.
27. Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. Biomed Res Int. 2015;2015:834371.
28. World Health Organization. Malaria vaccine development. www.who.int/malaria/areas/vaccine/en/#. Accessed January 21, 2016.
29. Leder K, Torresi J, Libman MD, et al. GeoSentinel Surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158(6):456-468.
30. Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137.
31. Essackjee K, Goorah S, Ramchurn SK, et al. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad Med. 2013;89:440-447.
32. Piyaphanee W, Steffen R, Shlim DR, et al. Travel medicine for Asian travelers—do we need new approaches? J Travel Med. 2012;19(6):335-337.
1. World Tourism Organization. Over 1.1 billion tourists travelled abroad in 2014 [press release]. January 27, 2015. http://media.unwto.org/press-release/2015-01-27/over-11-billion-tourists-travelled-abroad-2014. Accessed January 21, 2016.
2. Zuckerman JN. Public health and travel medicine: intricately intertwined. Perspect Public Health. 2012;132(5):206.
3. Rhodes G, DeRomaña I, Ebner J. Advising travelers with special needs: study abroad & other international student travel. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellow book/2016/advising-travelers-with-specific-needs/study-abroad-other-international-student-travel. Accessed January 21, 2016.
4. Institute of International Education. US study abroad: all destinations. www.iie.org/Research-and-Publications/Open-Doors/Data/US-Study-Abroad/All-Destinations/2012-14. Accessed January 21, 2016.
5. Hearn P, Johnston V. Assessment of returning travelers with fever. Medicine. 2014;42(2):66-72.
6. Siikamäki HM, Kivelä PS, Sipilä PN, et al. Fever in travelers returning from malaria-endemic areas: don’t look for malaria only. J Travel Med. 2011;18(4):239-244.
7. Walker NF, Nadjm B, Whitty CJ. Malaria. Medicine. 2014;42(2):100-106.
8. Cullen KA, Arguin PM. Malaria surveillance—United States, 2012. MMWR Surveill Summ. 2014;63(12):1-22.
9. World Health Organization. Malaria. Fact sheet no. 94. www.who.int/mediacentre/factsheets/fs094/en/. Accessed January 21, 2016.
10. Nimmagadda SS, Mahabala C, Boloor A, et al. Atypical manifestations of dengue fever (DF)—where do we stand today? J Clin Diagn Res. 2014;8(1):71-73.
11. Whitehorn J, Yacoub S, Anders KL, et al. Dengue therapeutics, chemoprophylaxis, and allied tools: state of the art and future directions. PLoS Negl Trop Dis. 2014;8(8):e3025.
12. Mayxay M, Phetsouvanh R, Moore CE, et al. Predictive diagnostic value of the tourniquet test for the diagnosis of dengue infection in adults. Trop Med Int Health. 2011;16(1):127-133.
13. World Health Organization. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control. WHO/HTM/NTD/DEN/2009.1. www.who.int/tdr/publications/documents/dengue-diagnosis.pdf. Accessed January 21, 2016.
14. Tomashek KM, Sharp TM, Margolis HS. Infectious diseases related to travel: dengue. In: CDC Health Information for International Travel. wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/dengue. Accessed January 21, 2016.
15. Vega-Rúa A, Lourenço-de-Oliveira R, Mousson L, et al. Chikungunya virus transmission potential by local Aedes mosquitoes in the Americas and Europe. PLoS Negl Trop Dis. 2015;9(5):1-18.
16. Hrnjakovi`c Cvjetkovi`c IB, Cvjetkovi`c D, Pati`c A, et al. Chikungunya—a serious threat for public health. Med Pregl. 2015;68(3/4):122-125.
17. Lindsey NP, Prince HE, Kosoy O, et al. Chikungunya virus infections among travelers-United States, 2010-2013. Am J Trop Med Hyg. 2015;92(1):82-87.
18. CDC. Malaria. www.cdc.gov/malaria/about/disease.html. Accessed January 21, 2016.
19. Das S, Sarkar N, Majumder J, et al. Acute disseminated encephalomyelitis in a child with chikungunya virus infection. J Pediatr Infect Dis. 2014;9(1):37-41.
20. Schwartz KL, Giga A, Boggild AK. Chikungunya fever in Canada: fever and polyarthritis in a returned traveller. CMAJ. 2014;186(10):772-774.
21. Chipwaza B, Mugasa JP, Selemani M, et al. Dengue and chikungunya fever among viral diseases in outpatient febrile children in Kilosa district hospital, Tanzania. PLoS Negl Trop Dis. 2014;8(11):e3335.
22. Joshi HA, Shah SS. Platelet count—a diagnostic aid in fever. Natl J Integrated Res Med. 2013;4(3):128-132.
23. Kutsuna S, Hayakawa K, Kato Y, et al. Comparison of clinical characteristics and laboratory findings of malaria, dengue, and enteric fever in returning travelers: 8-year experience at a referral center in Tokyo, Japan. J Infect Chemother. 2015;21(4):272-276.
24. CDC. Malaria diagnosis & treatment in the United States. www.cdc.gov/malaria/diagnosis_treatment/index.html. Accessed January 21, 2016.
25. Roy M, Bouma MJ, Ionides EL, et al. The potential elimination of Plasmodium vivax malaria by relapse treatment: insights from a transmission model and surveillance data from NW India. PLoS Negl Trop Dis. 2013;7(1):e1979.
26. Tither PH. Preventing dengue and chikungunya fever among international travelers. J Am Assoc Nurse Pract. 2014;26(11):584-594.
27. Mardekian SK, Roberts AL. Diagnostic options and challenges for dengue and chikungunya viruses. Biomed Res Int. 2015;2015:834371.
28. World Health Organization. Malaria vaccine development. www.who.int/malaria/areas/vaccine/en/#. Accessed January 21, 2016.
29. Leder K, Torresi J, Libman MD, et al. GeoSentinel Surveillance of illness in returned travelers, 2007-2011. Ann Intern Med. 2013;158(6):456-468.
30. Schilte C, Staikowsky F, Couderc T, et al. Chikungunya virus-associated long-term arthralgia: a 36-month prospective longitudinal study. PLoS Negl Trop Dis. 2013;7:e2137.
31. Essackjee K, Goorah S, Ramchurn SK, et al. Prevalence of and risk factors for chronic arthralgia and rheumatoid-like polyarthritis more than 2 years after infection with chikungunya virus. Postgrad Med. 2013;89:440-447.
32. Piyaphanee W, Steffen R, Shlim DR, et al. Travel medicine for Asian travelers—do we need new approaches? J Travel Med. 2012;19(6):335-337.