User login
M. Alexander Otto began his reporting career early in 1999 covering the pharmaceutical industry for a national pharmacists' magazine and freelancing for the Washington Post and other newspapers. He then joined BNA, now part of Bloomberg News, covering health law and the protection of people and animals in medical research. Alex next worked for the McClatchy Company. Based on his work, Alex won a year-long Knight Science Journalism Fellowship to MIT in 2008-2009. He joined the company shortly thereafter. Alex has a newspaper journalism degree from Syracuse (N.Y.) University and a master's degree in medical science -- a physician assistant degree -- from George Washington University. Alex is based in Seattle.
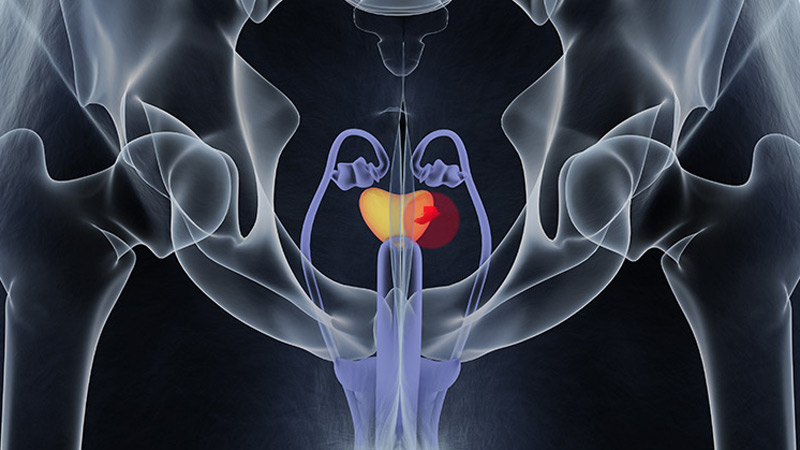
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
The FDA has granted Cleveland Diagnostics' IsoPSA test premarket approval (PMA) to help detect prostate cancer in men aged ≥ 50 years with elevated PSA levels.
IsoPSA is a blood assay that detects variations of the PSA protein that signal a higher likelihood of high-grade tumors. It is one of several biomarker tests included in the National Comprehensive Cancer Network's guidelines on early detection of prostate cancer.
Cleveland Diagnostics noted that 75% of prostate biopsies are negative for high-grade disease. IsoPSA and similar tests aim to help identify men who need a biopsy while allowing others avoid an unnecessary procedure.
IsoPSA has been available since 2020 under the FDA's Laboratory-Developed Test rubric, meaning that blood samples had to be shipped for analysis to Cleveland Diagnostics' lab. With the PMA, testing can now be done at CLIA-certified labs across the country.
The company expects the approval should increase access to IsoPSA and reduce turnaround time. "We remain focused on executing our commercial strategy and expanding access to IsoPSA," company President and CEO Arnon Chait, PhD, said in a press release.
The approval was based, in part, on a prospective validation study of 888 men scheduled for prostate biopsy. IsoPSA demonstrated an AUC of 0.783 for high-grade tumors, with a sensitivity of 90.2% and a specificity of 45.5%. In a real-world clinical utility study with 900 patients, IsoPSA testing led to a 55% decrease in biopsy recommendations.
The test is covered by Medicare and a growing number of commercial payers, Cleveland Diagnostics said.
M. Alexander Otto is a physician assistant with a master's degree in medical science and a journalism degree from Newhouse. He is an award-winning medical journalist who worked for several major news outlets before joining Medscape Medical News. Alex is also an MIT Knight Science Journalism Fellow. Email: aotto@mdedge.com.
A version of this article first appeared on Medscape.com.
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
FDA OKs Blood-Based Test to Help Diagnose Prostate Cancer
Revumenib Approved for R/R Acute Leukemia With KMT2A Translocation
The approval makes the oral small-molecule menin inhibitor the first pharmaceutical to carry the indication. It blocks the binding of menin to mutated KMT2A fusion proteins, tamping down the process that leads to the disease.
Although a relatively uncommon form of leukemia, KMT2A rearrangements are a major driver of acute leukemia in infants.
Approval was based on a single-arm of the open-label AUGMENT-101 trial with 104 adult and pediatric patients with the mutation. Pediatric patients were at least 30 days old.
The rate of complete remission (CR) plus CR with partial hematologic recovery was 21.2% (22 patients) with a median duration of 6.4 months. The median time to remission was 1.9 months.
Eighty-three patients required blood cell and/or platelet transfusions at baseline; 12 (14%) did not need transfusions for 56 days afterward. Of the 21 who were transfusion free at baseline, 10 (48%) remained so over the same period.
The most common adverse reactions in 20% or more of patients were hemorrhage, nausea, increased phosphate, musculoskeletal pain, infection, increased aspartate aminotransferase, febrile neutropenia, increased alanine aminotransferase, increased intact parathyroid hormone, bacterial infection, diarrhea, differentiation syndrome, electrocardiogram QT prolonged, decreased phosphate, increased triglycerides, decreased potassium, decreased appetite, constipation, edema, viral infection, fatigue, and increased alkaline phosphatase.
The recommended dose varies by weight and concomitant use of strong CYP3A4 inhibitors. Because of an anticipated delay in commercial availability, the lowest strength dose of revumenib will be available through an expanded access program for patients who weigh < 40 kg.
A version of this article appeared on Medscape.com.
The approval makes the oral small-molecule menin inhibitor the first pharmaceutical to carry the indication. It blocks the binding of menin to mutated KMT2A fusion proteins, tamping down the process that leads to the disease.
Although a relatively uncommon form of leukemia, KMT2A rearrangements are a major driver of acute leukemia in infants.
Approval was based on a single-arm of the open-label AUGMENT-101 trial with 104 adult and pediatric patients with the mutation. Pediatric patients were at least 30 days old.
The rate of complete remission (CR) plus CR with partial hematologic recovery was 21.2% (22 patients) with a median duration of 6.4 months. The median time to remission was 1.9 months.
Eighty-three patients required blood cell and/or platelet transfusions at baseline; 12 (14%) did not need transfusions for 56 days afterward. Of the 21 who were transfusion free at baseline, 10 (48%) remained so over the same period.
The most common adverse reactions in 20% or more of patients were hemorrhage, nausea, increased phosphate, musculoskeletal pain, infection, increased aspartate aminotransferase, febrile neutropenia, increased alanine aminotransferase, increased intact parathyroid hormone, bacterial infection, diarrhea, differentiation syndrome, electrocardiogram QT prolonged, decreased phosphate, increased triglycerides, decreased potassium, decreased appetite, constipation, edema, viral infection, fatigue, and increased alkaline phosphatase.
The recommended dose varies by weight and concomitant use of strong CYP3A4 inhibitors. Because of an anticipated delay in commercial availability, the lowest strength dose of revumenib will be available through an expanded access program for patients who weigh < 40 kg.
A version of this article appeared on Medscape.com.
The approval makes the oral small-molecule menin inhibitor the first pharmaceutical to carry the indication. It blocks the binding of menin to mutated KMT2A fusion proteins, tamping down the process that leads to the disease.
Although a relatively uncommon form of leukemia, KMT2A rearrangements are a major driver of acute leukemia in infants.
Approval was based on a single-arm of the open-label AUGMENT-101 trial with 104 adult and pediatric patients with the mutation. Pediatric patients were at least 30 days old.
The rate of complete remission (CR) plus CR with partial hematologic recovery was 21.2% (22 patients) with a median duration of 6.4 months. The median time to remission was 1.9 months.
Eighty-three patients required blood cell and/or platelet transfusions at baseline; 12 (14%) did not need transfusions for 56 days afterward. Of the 21 who were transfusion free at baseline, 10 (48%) remained so over the same period.
The most common adverse reactions in 20% or more of patients were hemorrhage, nausea, increased phosphate, musculoskeletal pain, infection, increased aspartate aminotransferase, febrile neutropenia, increased alanine aminotransferase, increased intact parathyroid hormone, bacterial infection, diarrhea, differentiation syndrome, electrocardiogram QT prolonged, decreased phosphate, increased triglycerides, decreased potassium, decreased appetite, constipation, edema, viral infection, fatigue, and increased alkaline phosphatase.
The recommended dose varies by weight and concomitant use of strong CYP3A4 inhibitors. Because of an anticipated delay in commercial availability, the lowest strength dose of revumenib will be available through an expanded access program for patients who weigh < 40 kg.
A version of this article appeared on Medscape.com.
Michigan Oncologist Charged in Scheme to Illegally Sell Cancer Drugs
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
In late October, a federal grand jury charged a Detroit-area medical oncologist Naveed Aslam, MD, in an indictment for his part in a scheme to illegally sell cancer drugs.
According to the indictment, Aslam acquired and sold more than $17 million in cancer drugs and personally netted more than $2.5 million during the scheme.
The charges against Aslam, filed on October 23 in the US District Court for the Eastern District of Michigan, include 10 counts of illegally selling or trading prescription drugs and one count of conspiring to do so.
“Dr. Aslam’s alleged participation in this scheme not only allowed him to profit unlawfully from the sale of cancer drugs but it also posed a serious threat by potentially placing these medications into the wrong hands,” Cheyvoryea Gibson, special agent in charge of the FBI in Michigan, said in a press release announcing the indictment.
The investigation is being conducted jointly by the FBI, the US Food and Drug Administration (FDA), the US Department of Health and Human Services Office of Inspector General, and Homeland Security Investigations.
The indictment alleges that Aslam was recruited by an unnamed operator of a Michigan corporation that engaged in business as a retail pharmacy and in the wholesale distribution of expensive prescription drugs, largely oncology drugs.
According to the indictment, Aslam and the operator came to an agreement where Aslam would purchase these expensive drugs from an authorized distributor under the false pretense that he was going to prescribe them to patients.
Instead, Aslam allegedly “sold and transferred the prescription drugs” to or through the Michigan business, with involvement from the unnamed operator and a second unnamed individual.
The unnamed individuals “identified customers interested in buying prescription cancer drugs” and “communicated with Dr. Aslam about what cancer drugs were requested,” according to the press release. “Dr. Aslam used his access to certain cancer drugs through his medical practice, Somerset Hematology and Oncology, P.C., to order and purchase the cancer drugs from his supplier.”
The indictment lays out that Aslam allegedly profited from this scheme in several ways, which included charging the Michigan business more than he paid the distributor for the drugs, sharing the profits when the business resold the drugs at a markup, and receiving rebates and discounts from the distributor “based on the amount of qualifying drugs he purchased and resold.”
According to the indictment, the scheme ran from early 2019 to mid-2023 and included four antibody drug conjugates — trastuzumab deruxtecan (Enhertu), enfortumab vedotin (Padcev), tisotumab vedotin (Tivdak), and sacituzumab govitecan (Trodelvy) — and the monoclonal antibody mogamulizumab (Poteligeo) for cutaneous T-cell lymphoma.
By working with Aslam, the operatives “obtained prescription drugs from an authorized distributor that they would not otherwise have been permitted to purchase, and which they were able to sell at a profit,” according to the indictment.
Both the prosecuting assistant US attorney, Andrew Lievense, and Aslam’s defense lawyer, Daniel Dena, declined to comment for this news organization.
The prosecutor is seeking to recoup the more than $2.5 million Aslam allegedly pocketed, according to the indictment. The press release also noted that an “indictment is only a charge and is not evidence of guilt.”
A version of this article first appeared on Medscape.com.
FDA Approves Lymphir for R/R Cutaneous T-Cell Lymphoma
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
The immunotherapy is a reformulation of denileukin diftitox (Ontak), initially approved in 1999 for certain patients with persistent or recurrent cutaneous T-cell lymphoma. In 2014, the original formulation was voluntarily withdrawn from the US market. Citius acquired rights to market a reformulated product outside of Asia in 2021.
This is the first indication for Lymphir, which targets interleukin-2 receptors on malignant T cells.
This approval marks “a significant milestone” for patients with cutaneous T-cell lymphoma, a rare cancer, company CEO Leonard Mazur said in a press release announcing the approval. “The introduction of Lymphir, with its potential to rapidly reduce skin disease and control symptomatic itching without cumulative toxicity, is expected to expand the [cutaneous T-cell lymphoma] treatment landscape and grow the overall market, currently estimated to be $300-$400 million.”
Approval was based on the single-arm, open-label 302 study in 69 patients who had a median of four prior anticancer therapies. Patients received 9 mcg/kg daily from day 1 to day 5 of 21-day cycles until disease progression or unacceptable toxicity.
The objective response rate was 36.2%, including complete responses in 8.7% of patients. Responses lasted 6 months or longer in 52% of patients. Over 80% of subjects had a decrease in skin tumor burden, and almost a third had clinically significant improvements in pruritus.
Adverse events occurring in 20% or more of patients include increased transaminases, decreased albumin, decreased hemoglobin, nausea, edema, fatigue, musculoskeletal pain, rash, chills, constipation, pyrexia, and capillary leak syndrome.
Labeling carries a boxed warning of capillary leak syndrome. Other warnings include visual impairment, infusion reactions, hepatotoxicity, and embryo-fetal toxicity. Citius is under a postmarketing requirement to characterize the risk for visual impairment.
The company expects to launch the agent within 5 months.
A version of this article first appeared on Medscape.com.
What Does Hormone Receptor Mean in BRCA-Associated BC?
CHICAGO — Being hormone receptor positive is generally a favorable prognostic factor in breast cancer, but that doesn’t seem to be the case in women with BRCA-associated tumors, according to a study presented at the American Society of Clinical Oncology annual meeting.
The conclusion is based on a large international study on how hormone receptor status impacts breast cancer outcomes in young women with germline BRCA pathological variants (PVs).
Overall, “hormone receptor positivity did not seem to have a strong positive prognostic value in young BRCA carriers” with early breast cancer, lead investigator Luca Arecco, MD, an oncology resident at the University of Genoa, Italy, said at the meeting.
Investigators reviewed the records of 4709 women ages 40 years or younger with stage 1-3 BRCA-associated invasive breast cancer treated from 2000 to 2020 at 78 centers in 28 countries across four continents. Median follow-up was about 8 years.
Weaker Prognostic Value in Hormone Receptor Status
They found, in general, that hormone receptor–positive breast cancer appears to be biologically more aggressive in patients with BRCA PVs than in the general breast cancer population, generating outcomes similar to those with hormone receptor-negative BRCA tumors.
Specifically, among patients with germline BRCA PVs, while hormone receptor–positive patients had a higher distant recurrence rate (13.1% vs. 9.6%) than hormone receptor–negative patients, 8-year disease free survival (65.8% and 63.4% respectively) and overall survival (a bit under 90% in both groups) were similar.
Hormone receptor–positive patients did have a lower rate of second primary breast cancers (9.1% versus 14.7%).
In the formal write-up of the results published shortly after the meeting in Annals of Oncology, the investigators concluded that “in young BRCA carriers, differences in recurrence pattern and second primary breast cancer among hormone receptor–positive versus negative disease warrant consideration in counseling patients on treatment, follow-up, and risk-reducing surgery.”
The team also found other differences between BRCA-associated breast cancer and sporadic disease. For instance, in the BRCA cohort, luminal A-like breast cancer had a worse long-term prognosis in their BRCA cohort than triple-negative or HER2-positive disease. Luminal A-like tumors are generally considered less aggressive, but in patients with BRCA PVs, “improving neoadjuvant chemotherapy … could be worthwhile,” the investigators said.
Also, although the risk of recurrence for sporadic hormone receptor–negative tumors is highest in the first few years, the team found that the risk in the hormone negative BRCA cohort progressively increased with longer follow-up, driven by the occurrence of second primary breast cancers, especially in patients with BRCA 1 PVs.
Greater Clarity in Prognosis in BRCA-Associated Breast Cancer
Overall, study discussant Lisa A. Carey, MD, a breast cancer specialist at the University of North Carolina at Chapel Hill, said, “we now know much more clearly the issues of prognosis in women who are very young and have germline BRCA-associated breast cancer,” about 12% of newly diagnosed cases.
“Young patients with germline BRCA-associated breast cancers have high relapse and high new primary risks, warranting comprehensive multimodality therapy,” she said.
A bit fewer than half of women in the study were hormone receptor–positive, and they tended to be patients with BRCA 2 PVs. The rest were hormone receptor–negative and tended to have BRCA 1 PVs.
Patients with hormone receptor–positive disease had grade 3 cancers in about 50% of cases, while patients with hormone receptor–negative disease had a grade 3 disease in over 80%.
Hormone receptor–positive patients were more likely to have nodal involvement and undergo mastectomies but less likely to receive chemotherapy than hormone receptor–negative patients. It’s likely that few patients in the review received PARP inhibitors, Dr. Carey noted.
Although overall survival at 8 years was similar in both groups, after that point “the prognosis of patients with hormone receptor–positive disease appeared to be worse … This appeared to occur earlier than that described in sporadic disease,” in which the worsening of survival in hormone receptor–positive disease occurs after a follow-up of at least 14-15 years, the investigators noted in their journal report.
The work was funded by the Italian Association for Cancer Research, Institut Jules Bordet, Korea Health Industry Development Institute, Australian National Health and Medical Council, Cancer Australia, US National Institute of Health, and others. Dr. Arecco had no disclosures. Dr. Carey and other coauthors disclosed research funding, speaker honoraria, and other financial relationships with AstraZeneca, Genentech/Roche, Lilly, and other pharmaceutical companies.
CHICAGO — Being hormone receptor positive is generally a favorable prognostic factor in breast cancer, but that doesn’t seem to be the case in women with BRCA-associated tumors, according to a study presented at the American Society of Clinical Oncology annual meeting.
The conclusion is based on a large international study on how hormone receptor status impacts breast cancer outcomes in young women with germline BRCA pathological variants (PVs).
Overall, “hormone receptor positivity did not seem to have a strong positive prognostic value in young BRCA carriers” with early breast cancer, lead investigator Luca Arecco, MD, an oncology resident at the University of Genoa, Italy, said at the meeting.
Investigators reviewed the records of 4709 women ages 40 years or younger with stage 1-3 BRCA-associated invasive breast cancer treated from 2000 to 2020 at 78 centers in 28 countries across four continents. Median follow-up was about 8 years.
Weaker Prognostic Value in Hormone Receptor Status
They found, in general, that hormone receptor–positive breast cancer appears to be biologically more aggressive in patients with BRCA PVs than in the general breast cancer population, generating outcomes similar to those with hormone receptor-negative BRCA tumors.
Specifically, among patients with germline BRCA PVs, while hormone receptor–positive patients had a higher distant recurrence rate (13.1% vs. 9.6%) than hormone receptor–negative patients, 8-year disease free survival (65.8% and 63.4% respectively) and overall survival (a bit under 90% in both groups) were similar.
Hormone receptor–positive patients did have a lower rate of second primary breast cancers (9.1% versus 14.7%).
In the formal write-up of the results published shortly after the meeting in Annals of Oncology, the investigators concluded that “in young BRCA carriers, differences in recurrence pattern and second primary breast cancer among hormone receptor–positive versus negative disease warrant consideration in counseling patients on treatment, follow-up, and risk-reducing surgery.”
The team also found other differences between BRCA-associated breast cancer and sporadic disease. For instance, in the BRCA cohort, luminal A-like breast cancer had a worse long-term prognosis in their BRCA cohort than triple-negative or HER2-positive disease. Luminal A-like tumors are generally considered less aggressive, but in patients with BRCA PVs, “improving neoadjuvant chemotherapy … could be worthwhile,” the investigators said.
Also, although the risk of recurrence for sporadic hormone receptor–negative tumors is highest in the first few years, the team found that the risk in the hormone negative BRCA cohort progressively increased with longer follow-up, driven by the occurrence of second primary breast cancers, especially in patients with BRCA 1 PVs.
Greater Clarity in Prognosis in BRCA-Associated Breast Cancer
Overall, study discussant Lisa A. Carey, MD, a breast cancer specialist at the University of North Carolina at Chapel Hill, said, “we now know much more clearly the issues of prognosis in women who are very young and have germline BRCA-associated breast cancer,” about 12% of newly diagnosed cases.
“Young patients with germline BRCA-associated breast cancers have high relapse and high new primary risks, warranting comprehensive multimodality therapy,” she said.
A bit fewer than half of women in the study were hormone receptor–positive, and they tended to be patients with BRCA 2 PVs. The rest were hormone receptor–negative and tended to have BRCA 1 PVs.
Patients with hormone receptor–positive disease had grade 3 cancers in about 50% of cases, while patients with hormone receptor–negative disease had a grade 3 disease in over 80%.
Hormone receptor–positive patients were more likely to have nodal involvement and undergo mastectomies but less likely to receive chemotherapy than hormone receptor–negative patients. It’s likely that few patients in the review received PARP inhibitors, Dr. Carey noted.
Although overall survival at 8 years was similar in both groups, after that point “the prognosis of patients with hormone receptor–positive disease appeared to be worse … This appeared to occur earlier than that described in sporadic disease,” in which the worsening of survival in hormone receptor–positive disease occurs after a follow-up of at least 14-15 years, the investigators noted in their journal report.
The work was funded by the Italian Association for Cancer Research, Institut Jules Bordet, Korea Health Industry Development Institute, Australian National Health and Medical Council, Cancer Australia, US National Institute of Health, and others. Dr. Arecco had no disclosures. Dr. Carey and other coauthors disclosed research funding, speaker honoraria, and other financial relationships with AstraZeneca, Genentech/Roche, Lilly, and other pharmaceutical companies.
CHICAGO — Being hormone receptor positive is generally a favorable prognostic factor in breast cancer, but that doesn’t seem to be the case in women with BRCA-associated tumors, according to a study presented at the American Society of Clinical Oncology annual meeting.
The conclusion is based on a large international study on how hormone receptor status impacts breast cancer outcomes in young women with germline BRCA pathological variants (PVs).
Overall, “hormone receptor positivity did not seem to have a strong positive prognostic value in young BRCA carriers” with early breast cancer, lead investigator Luca Arecco, MD, an oncology resident at the University of Genoa, Italy, said at the meeting.
Investigators reviewed the records of 4709 women ages 40 years or younger with stage 1-3 BRCA-associated invasive breast cancer treated from 2000 to 2020 at 78 centers in 28 countries across four continents. Median follow-up was about 8 years.
Weaker Prognostic Value in Hormone Receptor Status
They found, in general, that hormone receptor–positive breast cancer appears to be biologically more aggressive in patients with BRCA PVs than in the general breast cancer population, generating outcomes similar to those with hormone receptor-negative BRCA tumors.
Specifically, among patients with germline BRCA PVs, while hormone receptor–positive patients had a higher distant recurrence rate (13.1% vs. 9.6%) than hormone receptor–negative patients, 8-year disease free survival (65.8% and 63.4% respectively) and overall survival (a bit under 90% in both groups) were similar.
Hormone receptor–positive patients did have a lower rate of second primary breast cancers (9.1% versus 14.7%).
In the formal write-up of the results published shortly after the meeting in Annals of Oncology, the investigators concluded that “in young BRCA carriers, differences in recurrence pattern and second primary breast cancer among hormone receptor–positive versus negative disease warrant consideration in counseling patients on treatment, follow-up, and risk-reducing surgery.”
The team also found other differences between BRCA-associated breast cancer and sporadic disease. For instance, in the BRCA cohort, luminal A-like breast cancer had a worse long-term prognosis in their BRCA cohort than triple-negative or HER2-positive disease. Luminal A-like tumors are generally considered less aggressive, but in patients with BRCA PVs, “improving neoadjuvant chemotherapy … could be worthwhile,” the investigators said.
Also, although the risk of recurrence for sporadic hormone receptor–negative tumors is highest in the first few years, the team found that the risk in the hormone negative BRCA cohort progressively increased with longer follow-up, driven by the occurrence of second primary breast cancers, especially in patients with BRCA 1 PVs.
Greater Clarity in Prognosis in BRCA-Associated Breast Cancer
Overall, study discussant Lisa A. Carey, MD, a breast cancer specialist at the University of North Carolina at Chapel Hill, said, “we now know much more clearly the issues of prognosis in women who are very young and have germline BRCA-associated breast cancer,” about 12% of newly diagnosed cases.
“Young patients with germline BRCA-associated breast cancers have high relapse and high new primary risks, warranting comprehensive multimodality therapy,” she said.
A bit fewer than half of women in the study were hormone receptor–positive, and they tended to be patients with BRCA 2 PVs. The rest were hormone receptor–negative and tended to have BRCA 1 PVs.
Patients with hormone receptor–positive disease had grade 3 cancers in about 50% of cases, while patients with hormone receptor–negative disease had a grade 3 disease in over 80%.
Hormone receptor–positive patients were more likely to have nodal involvement and undergo mastectomies but less likely to receive chemotherapy than hormone receptor–negative patients. It’s likely that few patients in the review received PARP inhibitors, Dr. Carey noted.
Although overall survival at 8 years was similar in both groups, after that point “the prognosis of patients with hormone receptor–positive disease appeared to be worse … This appeared to occur earlier than that described in sporadic disease,” in which the worsening of survival in hormone receptor–positive disease occurs after a follow-up of at least 14-15 years, the investigators noted in their journal report.
The work was funded by the Italian Association for Cancer Research, Institut Jules Bordet, Korea Health Industry Development Institute, Australian National Health and Medical Council, Cancer Australia, US National Institute of Health, and others. Dr. Arecco had no disclosures. Dr. Carey and other coauthors disclosed research funding, speaker honoraria, and other financial relationships with AstraZeneca, Genentech/Roche, Lilly, and other pharmaceutical companies.
FROM ASCO 2024
Circulating Tumor DNA Hints at BC Recurrence Risk
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
CHICAGO — Circulating tumor DNA (ctDNA) can predict relapse risk in some cases of early, high-risk breast cancer, but it’s too soon to use it to guide adjuvant therapy decisions, according to a study presented at the American Society of Clinical Oncology annual meeting.
Detectable ctDNA is “highly prognostic of worse outcomes, particularly in patients who [remain] persistently positive,” but the correlation isn’t perfect, said lead investigator Sherene Loi, MMBS, PhD, a breast cancer specialist at the Peter MacCallum Cancer Centre in Melbourne, Australia.
Although less likely, relapses also occurred in the study among women without ctDNA elevation. Conversely, there were women with elevated ctDNA who did not relapse, she said. The study was a subanalysis of the monarchE trial of adjuvant abemaciclib, a CDK 4/6 inhibitor.
Eventually, “we would like to use” ctDNA to guide adjuvant treatment decisions, but the research isn’t there yet, Dr. Loi said. It’s possible, for instance, that persistently detectable ctDNA indicates early treatment failure and the need for treatment intensification. Future research should tackle the issue.
Study discussant Francois-Clement Bidard, MD, PhD, a breast cancer specialist at Institut Curie, Paris, agreed that ctDNA isn’t ready for primetime in adjuvant early, high-risk breast cancer.
“There is no clinical evidence to suggest that there is clinical utility in this setting. There are several trials that are ongoing,” he said, but for now “you shouldn’t,” for example, “use ctDNA to de-escalate adjuvant CDK4/6 [inhibitors]. It could be in the future that we could have data on this, but at the moment, [the] clear clinical message [is] no way.”
At 5-year follow-up, the monarchE trial found a 7.6% invasive disease-free survival (IDFS) improvement when abemaciclib was added to the first 2 years of endocrine therapy in women with HR+, HER2-, node positive, high-risk early breast cancer. The combination is now a standard adjuvant option for the disease.
The ctDNA study focused on a subset of 910 subjects with adequate ctDNA testing to run the analysis. The study population was also selected to be enriched for overall IDFS events (27% versus 18% across the trial’s 5,637 subjects). An IDFS event was defined as a local, regional, contralateral or distant invasive recurrence; a new primary tumor; or death from any cause.
Testing was performed using the Signatera ctDNA assay. Baseline samples were taken after completion of adjuvant chemotherapy, then again at 3, 6, or 24 months.
Overall, ctDNA detection was infrequent. Just 8% of patients were positive at baseline and 17% were positive at any point during the trial. Even so, ctDNA detection at any point was adversely prognostic.
Patients who were ctDNA positive at baseline were more likely to experience an IDFS event, compared with those who were ctDNA negative at baseline (80% at 4 years follow-up versus 23%).
Similarly, those who remained positive or became positive during testing were more likely to experience an IDFS event compared with those who became negative or remained negative throughout testing.
For instance, all 34 patients who were positive at baseline and remained positive had an IDFS event by year 4, versus just 40% who started positive but then cleared their ctDNA.
Among women who were negative at baseline and remained negative, 13% had an IDFS event versus 89% who started negative but then turned positive. Subjects who turned positive also had the shortest time to an IDFS event, a median of 7 months.
Among women who recurred, those who were ctDNA negative tended to have local, regional, or contralateral recurrences, while ctDNA positive patients tended to have distant recurrences.
The finding “really highlights that ctDNA antedates the metastatic clinical relapse. What the ctDNA is telling you is that the metastatic process has been completed, and metastases are about to grow,” Dr. Bidard said.
The work was funded by Eli Lilly, maker of abemaciclib, with collaboration from Natera, maker of the Signatera assay. Dr. Loi is an adviser and researcher for Lilly, among other industry ties. Dr. Bidard is a speaker and consultant for Lilly, among other ties.
FROM ASCO 2024
‘Double-Expresser’ DLBCL: Tucidinostat Improved R-CHOP Outcomes
At the annual meeting of the American Society of Clinical Oncology (ASCO), they announced that combining the histone deacetylase inhibitor tucidinostat with standard R-CHOP chemotherapy in previously untreated “double-expresser” patients improved complete response and event-free survival (EFS) rates over R-CHOP alone.
The trial, dubbed DEB, is the first phase 3 investigation to confirm the benefit of combination treatment with an epigenetic agent for such patients, lead investigator and study presenter Weili Zhao, MD, PhD, a hematologist at the Shanghai Institute of Hematology, told her audience.
“Tucidinostat plus R-CHOP could be a new frontline treatment option in this patient population,” Dr. Zhao said.
However, the agent is not available in the United States, at least for now. It is approved in China for peripheral T-cell lymphoma (PTCL) and HER2-negative breast cancer and in Japan for PTCL and adult T-cell leukemia/lymphoma.
Dr. Zhao said that tucidinostat was also conditionally approved for DLBCL in China recently, based on the strength of the DEB results.
Study discussant Peter Riedell, MD, a hematologist at the University of Chicago, Illinois, was cautious on several points.
First, there’s been no overall survival benefit in the trial, but follow-up so far has been short, at a median of 13.9 months, and Dr. Zhao reported interim, not final, results.
Dr. Riedell also noted that there were more grade 3 or worse adverse events, particularly hematologic side effects, hypokalemia, and pneumonia, with tucidinostat add-on in DEB.
Other outstanding questions include the applicability of the findings to non-Chinese patients and the effect of adding tucidinostat to another common DLCBL chemotherapy regimen, pola-R-CHP, which is being increasingly used in the United States for higher-risk disease, which includes double-expressers of the MYC and BCLC oncogenes.
DEB equally randomized 423 patients at 40 centers in China to either six cycles of R-CHOP with tucidinostat 20 μg twice weekly or R-CHOP with placebo. Complete responders went on to either tucidinostat or placebo maintenance treatment for up to 24 weeks. Subjects had an International Prognostic Index score of at least 2.
Out of a total of 152 EFS events, 64 (30.3%) were in the tucidinostat group and 88 (41.5%) were in the placebo group; 24-month EFS was 58.9% with tucidinostat and 46.2% with R-CHOP alone (hazard ratio, 0.68; P = .018).
Meanwhile, the complete response rate with tucidinostat was 73.0% versus 61.8% (P = .014).
Although there were more higher-grade adverse events in the experimental arm, most patients were able to tolerate and complete the planned treatment cycles.
The study was funded by tucidinostat maker Chipscreen Biosciences. Dr. Zhao reported no disclosures. Dr. Riedell disclosed ties with numerous companies, including BeiGene (a partner of Chipscreen) and Novartis.
At the annual meeting of the American Society of Clinical Oncology (ASCO), they announced that combining the histone deacetylase inhibitor tucidinostat with standard R-CHOP chemotherapy in previously untreated “double-expresser” patients improved complete response and event-free survival (EFS) rates over R-CHOP alone.
The trial, dubbed DEB, is the first phase 3 investigation to confirm the benefit of combination treatment with an epigenetic agent for such patients, lead investigator and study presenter Weili Zhao, MD, PhD, a hematologist at the Shanghai Institute of Hematology, told her audience.
“Tucidinostat plus R-CHOP could be a new frontline treatment option in this patient population,” Dr. Zhao said.
However, the agent is not available in the United States, at least for now. It is approved in China for peripheral T-cell lymphoma (PTCL) and HER2-negative breast cancer and in Japan for PTCL and adult T-cell leukemia/lymphoma.
Dr. Zhao said that tucidinostat was also conditionally approved for DLBCL in China recently, based on the strength of the DEB results.
Study discussant Peter Riedell, MD, a hematologist at the University of Chicago, Illinois, was cautious on several points.
First, there’s been no overall survival benefit in the trial, but follow-up so far has been short, at a median of 13.9 months, and Dr. Zhao reported interim, not final, results.
Dr. Riedell also noted that there were more grade 3 or worse adverse events, particularly hematologic side effects, hypokalemia, and pneumonia, with tucidinostat add-on in DEB.
Other outstanding questions include the applicability of the findings to non-Chinese patients and the effect of adding tucidinostat to another common DLCBL chemotherapy regimen, pola-R-CHP, which is being increasingly used in the United States for higher-risk disease, which includes double-expressers of the MYC and BCLC oncogenes.
DEB equally randomized 423 patients at 40 centers in China to either six cycles of R-CHOP with tucidinostat 20 μg twice weekly or R-CHOP with placebo. Complete responders went on to either tucidinostat or placebo maintenance treatment for up to 24 weeks. Subjects had an International Prognostic Index score of at least 2.
Out of a total of 152 EFS events, 64 (30.3%) were in the tucidinostat group and 88 (41.5%) were in the placebo group; 24-month EFS was 58.9% with tucidinostat and 46.2% with R-CHOP alone (hazard ratio, 0.68; P = .018).
Meanwhile, the complete response rate with tucidinostat was 73.0% versus 61.8% (P = .014).
Although there were more higher-grade adverse events in the experimental arm, most patients were able to tolerate and complete the planned treatment cycles.
The study was funded by tucidinostat maker Chipscreen Biosciences. Dr. Zhao reported no disclosures. Dr. Riedell disclosed ties with numerous companies, including BeiGene (a partner of Chipscreen) and Novartis.
At the annual meeting of the American Society of Clinical Oncology (ASCO), they announced that combining the histone deacetylase inhibitor tucidinostat with standard R-CHOP chemotherapy in previously untreated “double-expresser” patients improved complete response and event-free survival (EFS) rates over R-CHOP alone.
The trial, dubbed DEB, is the first phase 3 investigation to confirm the benefit of combination treatment with an epigenetic agent for such patients, lead investigator and study presenter Weili Zhao, MD, PhD, a hematologist at the Shanghai Institute of Hematology, told her audience.
“Tucidinostat plus R-CHOP could be a new frontline treatment option in this patient population,” Dr. Zhao said.
However, the agent is not available in the United States, at least for now. It is approved in China for peripheral T-cell lymphoma (PTCL) and HER2-negative breast cancer and in Japan for PTCL and adult T-cell leukemia/lymphoma.
Dr. Zhao said that tucidinostat was also conditionally approved for DLBCL in China recently, based on the strength of the DEB results.
Study discussant Peter Riedell, MD, a hematologist at the University of Chicago, Illinois, was cautious on several points.
First, there’s been no overall survival benefit in the trial, but follow-up so far has been short, at a median of 13.9 months, and Dr. Zhao reported interim, not final, results.
Dr. Riedell also noted that there were more grade 3 or worse adverse events, particularly hematologic side effects, hypokalemia, and pneumonia, with tucidinostat add-on in DEB.
Other outstanding questions include the applicability of the findings to non-Chinese patients and the effect of adding tucidinostat to another common DLCBL chemotherapy regimen, pola-R-CHP, which is being increasingly used in the United States for higher-risk disease, which includes double-expressers of the MYC and BCLC oncogenes.
DEB equally randomized 423 patients at 40 centers in China to either six cycles of R-CHOP with tucidinostat 20 μg twice weekly or R-CHOP with placebo. Complete responders went on to either tucidinostat or placebo maintenance treatment for up to 24 weeks. Subjects had an International Prognostic Index score of at least 2.
Out of a total of 152 EFS events, 64 (30.3%) were in the tucidinostat group and 88 (41.5%) were in the placebo group; 24-month EFS was 58.9% with tucidinostat and 46.2% with R-CHOP alone (hazard ratio, 0.68; P = .018).
Meanwhile, the complete response rate with tucidinostat was 73.0% versus 61.8% (P = .014).
Although there were more higher-grade adverse events in the experimental arm, most patients were able to tolerate and complete the planned treatment cycles.
The study was funded by tucidinostat maker Chipscreen Biosciences. Dr. Zhao reported no disclosures. Dr. Riedell disclosed ties with numerous companies, including BeiGene (a partner of Chipscreen) and Novartis.
FROM ASCO 2024
Subcutaneous Immunotherapy Promises Better Life For Cancer Patients
The possibility is being driven by the development of subcutaneous formulations of commonly used immune checkpoint inhibitors for non–small cell lung cancer (NSCLC) and other indications, including pembrolizumab, nivolumab, durvalumab, atezolizumab, and amivantamab.
Instead of waiting anywhere from 30 minutes to several hours for infusions into their veins, patients would spend just a few minutes being injected under the loose skin of their abdomens or thighs. Clinicians would save time and money, and patients would leave the clinic much sooner than normal. The ease of subcutaneous injections also opens up an opportunity for home treatment, a potential boon for people who don’t want to spend their remaining time on hospital visits.
“In the future, I hope we can deliver these medicines at home,” said Hazel O’Sullivan, MBBCh, a medical lung cancer oncologist at Cork University, Ireland, who explained the issues during a session at the 2024 European Lung Cancer Congress.
She was the discussant on two studies at the meeting that highlighted the latest developments in the field, the IMscin002 study of subcutaneous atezolizumab and the PALOMA study of subcutaneous amivantamab, both mostly in NSCLC patients.
Subcutaneous atezolizumab was approved recently in Europe after its maker, Genentech/Roche, made a convincing case that its pharmacokinetics, efficacy, and safety are comparable to the intravenous (IV) version. The U.S. Food and Drug Administration is considering approval; Genentech/Roche anticipates a decision in 2024.
IMscin002 randomized 179 stage 2-4 NSCLC patients evenly to IV or subcutaneous atezolizumab for the first three cycles, then switched them for three more cycles.
Participants were then asked what version they preferred and what they wanted to continue with.
Seventy-one percent said they liked the subcutaneous version better and 80% opted to continue with it. Their main reasons were because they spent less time in the clinic and it was more comfortable.
When asked about the potential for home administration, presenter Federico Cappuzzo, MD, PhD, a medical lung cancer oncologist in Rome, said that it could be “an important option in the future,” particularly in isolated areas far away from hospitals.
The authors of new research are currently evaluating whether home administration is possible. Nurses are administering atezolizumab to patients in their homes with telemedicine monitoring.
The other subcutaneous study presented at the meeting, the PALOMA trial with amivantamab, had only 19 subjects. Administration took no more than 10 minutes, versus potentially hours, especially for the first dose. Subcutaneous amivantamab was given once a month, versus every 2 weeks for the IV formulation, during the maintenance phase of treatment.
The take-home from PALOMA is that the risk of infusion reactions is lower with subcutaneous administration (16% versus 67%) but the risk of mostly mild skin rashes is higher (79% versus 36%).
Investigation is ongoing to confirm safety, pharmacokinetic, and efficacy equivalence with the IV formulation, including in combination with other medications.
When asked about home administration of amivantamab, PALOMA lead investigator Natasha Leighl, MD, a lung, and breast cancer medical oncologist at the University of Toronto, stated that patients probably need to be watched in the clinic for the first 4 months.
The atezolizumab study was funded by maker Genentech/Roche. The amivantamab study was funded by its maker, Janssen. The amivantamab investigator, Dr. Leighl, reported grants, honoraria, and travel payments from Janssen. Dr. Cappuzzo, the investigator on the atezolizumab study, reported speaker and adviser payments from Genentech/Roche. The discussant, Dr. O’Sullivan, wasn’t involved with either company but reported payments from Amgen and AstraZeneca and travel costs covered by Takeda.
The possibility is being driven by the development of subcutaneous formulations of commonly used immune checkpoint inhibitors for non–small cell lung cancer (NSCLC) and other indications, including pembrolizumab, nivolumab, durvalumab, atezolizumab, and amivantamab.
Instead of waiting anywhere from 30 minutes to several hours for infusions into their veins, patients would spend just a few minutes being injected under the loose skin of their abdomens or thighs. Clinicians would save time and money, and patients would leave the clinic much sooner than normal. The ease of subcutaneous injections also opens up an opportunity for home treatment, a potential boon for people who don’t want to spend their remaining time on hospital visits.
“In the future, I hope we can deliver these medicines at home,” said Hazel O’Sullivan, MBBCh, a medical lung cancer oncologist at Cork University, Ireland, who explained the issues during a session at the 2024 European Lung Cancer Congress.
She was the discussant on two studies at the meeting that highlighted the latest developments in the field, the IMscin002 study of subcutaneous atezolizumab and the PALOMA study of subcutaneous amivantamab, both mostly in NSCLC patients.
Subcutaneous atezolizumab was approved recently in Europe after its maker, Genentech/Roche, made a convincing case that its pharmacokinetics, efficacy, and safety are comparable to the intravenous (IV) version. The U.S. Food and Drug Administration is considering approval; Genentech/Roche anticipates a decision in 2024.
IMscin002 randomized 179 stage 2-4 NSCLC patients evenly to IV or subcutaneous atezolizumab for the first three cycles, then switched them for three more cycles.
Participants were then asked what version they preferred and what they wanted to continue with.
Seventy-one percent said they liked the subcutaneous version better and 80% opted to continue with it. Their main reasons were because they spent less time in the clinic and it was more comfortable.
When asked about the potential for home administration, presenter Federico Cappuzzo, MD, PhD, a medical lung cancer oncologist in Rome, said that it could be “an important option in the future,” particularly in isolated areas far away from hospitals.
The authors of new research are currently evaluating whether home administration is possible. Nurses are administering atezolizumab to patients in their homes with telemedicine monitoring.
The other subcutaneous study presented at the meeting, the PALOMA trial with amivantamab, had only 19 subjects. Administration took no more than 10 minutes, versus potentially hours, especially for the first dose. Subcutaneous amivantamab was given once a month, versus every 2 weeks for the IV formulation, during the maintenance phase of treatment.
The take-home from PALOMA is that the risk of infusion reactions is lower with subcutaneous administration (16% versus 67%) but the risk of mostly mild skin rashes is higher (79% versus 36%).
Investigation is ongoing to confirm safety, pharmacokinetic, and efficacy equivalence with the IV formulation, including in combination with other medications.
When asked about home administration of amivantamab, PALOMA lead investigator Natasha Leighl, MD, a lung, and breast cancer medical oncologist at the University of Toronto, stated that patients probably need to be watched in the clinic for the first 4 months.
The atezolizumab study was funded by maker Genentech/Roche. The amivantamab study was funded by its maker, Janssen. The amivantamab investigator, Dr. Leighl, reported grants, honoraria, and travel payments from Janssen. Dr. Cappuzzo, the investigator on the atezolizumab study, reported speaker and adviser payments from Genentech/Roche. The discussant, Dr. O’Sullivan, wasn’t involved with either company but reported payments from Amgen and AstraZeneca and travel costs covered by Takeda.
The possibility is being driven by the development of subcutaneous formulations of commonly used immune checkpoint inhibitors for non–small cell lung cancer (NSCLC) and other indications, including pembrolizumab, nivolumab, durvalumab, atezolizumab, and amivantamab.
Instead of waiting anywhere from 30 minutes to several hours for infusions into their veins, patients would spend just a few minutes being injected under the loose skin of their abdomens or thighs. Clinicians would save time and money, and patients would leave the clinic much sooner than normal. The ease of subcutaneous injections also opens up an opportunity for home treatment, a potential boon for people who don’t want to spend their remaining time on hospital visits.
“In the future, I hope we can deliver these medicines at home,” said Hazel O’Sullivan, MBBCh, a medical lung cancer oncologist at Cork University, Ireland, who explained the issues during a session at the 2024 European Lung Cancer Congress.
She was the discussant on two studies at the meeting that highlighted the latest developments in the field, the IMscin002 study of subcutaneous atezolizumab and the PALOMA study of subcutaneous amivantamab, both mostly in NSCLC patients.
Subcutaneous atezolizumab was approved recently in Europe after its maker, Genentech/Roche, made a convincing case that its pharmacokinetics, efficacy, and safety are comparable to the intravenous (IV) version. The U.S. Food and Drug Administration is considering approval; Genentech/Roche anticipates a decision in 2024.
IMscin002 randomized 179 stage 2-4 NSCLC patients evenly to IV or subcutaneous atezolizumab for the first three cycles, then switched them for three more cycles.
Participants were then asked what version they preferred and what they wanted to continue with.
Seventy-one percent said they liked the subcutaneous version better and 80% opted to continue with it. Their main reasons were because they spent less time in the clinic and it was more comfortable.
When asked about the potential for home administration, presenter Federico Cappuzzo, MD, PhD, a medical lung cancer oncologist in Rome, said that it could be “an important option in the future,” particularly in isolated areas far away from hospitals.
The authors of new research are currently evaluating whether home administration is possible. Nurses are administering atezolizumab to patients in their homes with telemedicine monitoring.
The other subcutaneous study presented at the meeting, the PALOMA trial with amivantamab, had only 19 subjects. Administration took no more than 10 minutes, versus potentially hours, especially for the first dose. Subcutaneous amivantamab was given once a month, versus every 2 weeks for the IV formulation, during the maintenance phase of treatment.
The take-home from PALOMA is that the risk of infusion reactions is lower with subcutaneous administration (16% versus 67%) but the risk of mostly mild skin rashes is higher (79% versus 36%).
Investigation is ongoing to confirm safety, pharmacokinetic, and efficacy equivalence with the IV formulation, including in combination with other medications.
When asked about home administration of amivantamab, PALOMA lead investigator Natasha Leighl, MD, a lung, and breast cancer medical oncologist at the University of Toronto, stated that patients probably need to be watched in the clinic for the first 4 months.
The atezolizumab study was funded by maker Genentech/Roche. The amivantamab study was funded by its maker, Janssen. The amivantamab investigator, Dr. Leighl, reported grants, honoraria, and travel payments from Janssen. Dr. Cappuzzo, the investigator on the atezolizumab study, reported speaker and adviser payments from Genentech/Roche. The discussant, Dr. O’Sullivan, wasn’t involved with either company but reported payments from Amgen and AstraZeneca and travel costs covered by Takeda.
FROM ELCC 2024
Lung Cancer Screening Unveils Hidden Health Risks
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
The reason is because the low-dose CT scans used for screening cover the lower neck down to the upper abdomen, revealing far more anatomy than simply the lungs.
In fact, lung cancer screening can provide information on three of the top 10 causes of death worldwide: ischemic heart disease, chronic obstructive pulmonary disease, and, of course, lung cancer.
With lung cancer screening, “we are basically targeting many birds with one low-dose stone,” explained Jelena Spasic MD, PhD, at the European Lung Cancer Congress (ELCC) 2024.
Dr. Spasic, a medical oncologist at the Institute for Oncology and Radiology of Serbia in Belgrade, was the discussant on a study that gave an indication on just how useful screening can be for other diseases.
The study, dubbed 4-IN-THE-LUNG-RUN trial (4ITLR), is an ongoing prospective trial in six European countries that is using lung cancer screening scans to also look for coronary artery calcifications, a marker of atherosclerosis.
Usually, coronary calcifications are considered incidental findings on lung cancer screenings and reported to subjects’ physicians for heart disease risk assessment.
The difference in 4ITLR is that investigators are actively looking for the lesions and quantifying the extent of calcifications.
It’s made possible by the artificial intelligence-based software being used to read the scans. In addition to generating reports on lung nodules, it also automatically calculates an Agatston score, a quantification of the degree of coronary artery calcification for each subject.
At the meeting, which was organized by the European Society for Clinical Oncology, 4ITLR investigator Daiwei Han, MD, PhD, a research associate at the Institute for Diagnostic Accuracy in Groningen, the Netherlands, reported outcomes in the first 2487 of the 24,000 planned subjects.
To be eligible for screening, participants had to be 60-79 years old and either current smokers, past smokers who had quit within 10 years, or people with a 35 or more pack-year history. The median age in the study was 68.1 years.
Overall, 53% of subjects had Agatston scores of 100 or more, indicating the need for treatment to prevent active coronary artery disease, Dr. Han said.
Fifteen percent were at high risk for heart disease with scores of 400-999, indicating extensive coronary artery calcification, and 16.2% were at very high risk, with scores of 1000 or higher. The information is being shared with participants’ physicians.
The risk of heart disease was far higher in men, who made up 56% of the study population. While women had a median Agatston score of 61, the median score for men was 211.1.
The findings illustrate the potential of dedicated cardiovascular screening within lung cancer screening programs, Dr. Han said, noting that 4ITLR will also incorporate COPD risk assessment.
The study also shows the increased impact lung cancer screening programs could have if greater use were made of the CT images to look for other diseases, Dr. Spasic said.
4ITLR is funded by the European Union’s Horizon 2020 Program. Dr. Spasic and Dr. Han didn’t have any relevant disclosures.
FROM ELCC 2024
BC axillary dissection may be unnecessary for isolated tumor cells after NAC
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
SAN ANTONIO — Axillary lymph node dissection is the current standard of care in breast cancer when metastases are found in sentinel lymph nodes after neoadjuvant chemotherapy.
However, what to do when isolated tumor cells instead of outright metastases are found in sentinel nodes is an open question. Some surgeons opt for a full axillary dissection while others do not, and there is no standard of care, explained Giacomo Montagna, MD, a breast cancer surgeon at Memorial Sloan Kettering Cancer Center, New York City.
The study led and presented by Dr. Montagna at the San Antonio Breast Cancer Symposium brings some much-needed clarity to the issue.
The findings argue strongly against “routine axillary lymph node dissection” — with its considerable morbidities — “in patients with residual isolated tumor cells after neoadjuvant chemotherapy,” Dr. Montagna said.
Study discussant Elizabeth Mittendorf, MD, PhD, a breast cancer surgeon at Brigham and Women’s Hospital, Boston, agreed.
“It appears that the presence of isolated tumor cells in the sentinel nodes does not negatively impact oncologic outcomes. These additional data allow us to debunk some of the surgical dogma we grew up with, specifically that lymph node dissection is required for either survival or local control,” Dr. Mittendorf said.
However, there was concern among audience members that the information gleaned from a full dissection might still be needed to guide follow-on adjuvant therapy decisions.
Dr. Mittendorf didn’t think so. Although additional positive lymph nodes were found in almost a third of women who had axillary dissections in the review, the majority of involved nodes simply had more isolated tumor cells; macrometastases were found in just 5% of cases.
So, for most patients, additional information from axillary dissections is “unlikely needed to inform adjuvant therapy, and in fact,” based on the 5% figure, “we are thinking we would have to do well over a hundred lymph node dissections in such patients to inform treatment recommendations for fewer than five. This comes at the cost of fair morbidity,” she said.
Study details
The retrospective study, dubbed OPBC05/EUBREAST-14R/ICARO, included 583 women with cT1-4 N0-3 breast cancer treated at 62 centers in 18 countries. The majority of subjects were from the United States and Europe.
Every patient was found to have isolated tumor cells (ITCs) in their sentinel lymph nodes after neoadjuvant chemotherapy (NAC), which generally included anthracycline and taxane-based regimens. The majority of patients did not have a pathologic complete response to NAC.
Overall, 182 patients (31%) had a subsequent axillary lymph node dissection; the rest did not.
Dissections were more common in the presence of lymphovascular invasion and N2/N3 disease as well as when fewer lymph nodes were removed and when ITCs were found during surgery on frozen section, which was the case in a quarter of patients.
Additional positive nodes were found in 30% of patients in the dissection group and consisted of more nodes with ITCs in 18%, micrometastases in 7%, and macrometastases in 5%. Receptor status and nodal status at presentation did not have an impact on the likelihood of finding macrometastases.
The main finding of the study was that there were no statistically significant differences in recurrence outcomes between the two groups.
The 5-year rate of isolated axillary recurrence was 1.7% with axillary lymph node dissection (ALND) versus 1.1% without it. The 5-year rate of any invasive recurrence was 16% in the ALND arm and 19% in the no-dissection group.
The median age in the study was 48 years. The majority of patients (57%) had clinical T2 tumors. Most were HR positive and either HER2 negative (41%) or HER2 positive (28%).
Regional nodal radiation was more common in the ALND group, 82% versus 75%. The dissection arm had a mean of 2.8 sentinel lymph nodes removed versus 3.5 in the no-dissection group.
“The likelihood of finding additional positive lymph nodes in patients with residual ITCs after NAC is lower than in patients with residual micro- and macrometastases. In the majority of cases, they contain ITCs. Nodal recurrence after omission of ALND is rare in this population,” the investigators concluded in their abstract.
The work was funded by EUBREAST. Dr. Montagna doesn’t have any disclosures. Dr. Mittendorf has several industry ties, including being an advisor for Roche, AstraZeneca, and Moderna and a speaker for Merck.
AT SABCS 2023